Abstract
BACKGROUND AND AIMS:
Vitamin D exerts a regulatory role over mucosal immunity via the vitamin D receptor (VDR). Although Paneth cells and their products are known to regulate the commensal and pathogenic microbiota, the role that VDRs in Paneth cells play in these responses is unknown.
METHODS:
We identified the decreased intestinal VDR significantly correlated with reduction of an inflammatory bowel disease risk gene ATG16L1 and Paneth cell lysozymes in patients with Crohn’s disease. We generated Paneth cell–specific VDR knockout (VDRΔPC) mice to investigate the molecular mechanisms.
RESULTS:
Lysozymes in the Paneth cells were significantly decreased in the VDRΔPC mice. Isolated VDRΔPC Paneth cells exhibited weakened inhibition of pathogenic bacterial growth and displayed reduced autophagic responses. VDRΔPC mice had significantly higher inflammation after Salmonella infections. VDRΔPC mice also showed high susceptibility to small intestinal injury induced by indomethacin, a nonsteroidal anti-inflammatory drug. Co-housing of VDRΔPC and VDRlox mice made the VDRΔPC less vulnerable to dextran sulfate sodium colitis, suggesting the transmission of protective bacterial from the VDRlox mice. Thus, a lack of VDR in Paneth cells leads to impaired antibacterial activities and consequently increased inflammatory responses. Genetically and environmentally regulated VDRs in the Paneth cells may set the threshold for the development of chronic inflammation, as observed in inflammatory bowel diseases.
CONCLUSIONS:
We provide new insights into the tissue-specific functions of VDRs in maintaining Paneth cell alertness to pathogens in intestinal disorders. Targeting the VDR affects multiple downstream events within Paneth cells that inhibit intestinal inflammation and establish host defense against enteropathogens.
Keywords: Autophagy, Bacteria, Crohn’s Disease (CD), Defensin
Graphical Abstract
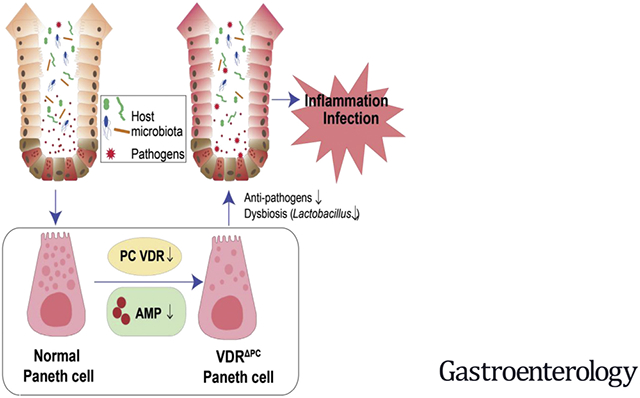
Vitamin D deficiency is associated with various diseases, including infections and inflammatory bowel disease (IBD).1-6 This is in part because vitamin D exerts a regulatory role in mucosal immunity and host defenses via vitamin D receptors (VDRs).6 VDRs belong to the nuclear receptor superfamily and regulate the transcription of many target genes, including ATG16L1, an IBD susceptibility gene involved in autophagy.7-11 Impaired ATG16L1-dependent autophagy drives ileal inflammation in Crohn’s disease (CD).12 We have previously demonstrated that intestinal epithelial VDR deficiencies lead to impaired function of the autophagy pathway because of the reduced expression of ATG16L1 and an abnormal morphology of Paneth cells.8
Paneth cells are important in both initiating and preventing inflammatory disorders. Paneth cell–specific abnormalities in the unfolded protein response may serve as the origin for intestinal inflammation.13 Paneth cells also through their secretion of antimicrobial factors regulate commensal microbe composition and protect the host from enteropathogens.14 The mechanisms that underlie Paneth cell regulation in the latter context are still unknown. We considered the potential role played by VDRs in Paneth cells, given the importance of vitamin D in host responses involving the epithelium. Importantly, VDR target genes include antimicrobial peptides (AMPs) cathelicidin15 and β defensin.16 Several IBD polymorphisms are phenotypically manifested in Paneth cells, altering AMPs and autophagy. Paneth cell abnormalities in human subjects are associated with mucosal dysbiosis in CD.17 Deletion of the intestinal epithelial VDRs contributes to abnormal Paneth cells and reduced autophagy responses.8 We thus hypothesized that the Paneth cell dysfunction induced by VDR deletion would lead to abnormal antibacterial functions and a loss of intestinal mucosal and microbial homeostasis and aimed to elucidate these mechanisms.
We identified the decreased intestinal VDR significantly correlated with reduction of ATG16L1 and Paneth cell lysozymes in patients with CD. To investigate the molecular mechanism of VDR regulation of Paneth cells, we generated Paneth cell VDR specific knockout (VDRΔPC) mice. Furthermore, we established a new method to isolate the intestinal Paneth cells by flow cytometry. The antibacterial ability of the isolated Paneth cells and their expression of the VDR and lysozymes were evaluated. In the Salmonella colitis model, VDRΔPC mice had significantly higher levels of inflammation post-Salmonella infection. The lack of VDR in the Paneth cells led to impaired antibacterial abilities and inflammatory responses. VDRΔPC mice also showed high susceptibility to small intestinal injury induced by indomethacin, a nonsteroidal anti-inflammatory drug. Furthermore, we examined the dysbiosis in the VDRΔPC mice. Co-housing of VDRΔPC and VDRlox mice made the VDRΔPC less vulnerable to dextran sulfate sodium (DSS) colitis, suggesting the transmission of protective bacterial from the VDRlox mice. Thus, our study fills an existing gap in the knowledge by characterizing the precise role of tissue-specific VDRs in regulating Paneth cells in host defenses from enteric pathogens.
Methods
More details can be found in the supplementary documents.
Human Intestinal Biopsies
Slides containing paraffin-embedded small intestinal biopsy samples of patients with CD and healthy control subjects were obtained from Dr Kaser, University of Cambridge. All protocols were approved by Cambridgeshire 4 Research Ethics Committee (reference 03/5/012). Patient characteristics are outlined in a previous publication.12
Gene Expression Datasets
For expression analyses we used microarray data registered in the Gene Expression Omnibus18 repository (https://www.ncbi.nlm.nih.gov/geo/; Gene Expression Omnibus accession number GSE102134) reported on the ileum of patients with CD and normal ileum from control individuals.19
Experimental Animals
VDRΔPC mice were obtained by crossing VDRloxP/loxP mice20 with DEFA6-cre mice.13 Mice were provided with water ad libitum and maintained in a room with a 12-hour dark–light cycle. Multiple breeding pairs were set up within a specific vivarium room where environment, cage changes, and dietary schedules are more uniform. All animal work complied with ARRIVE guidelines and was approved by the University of Illinois at Chicago Committee on Animal Resources; ethical guidelines were followed for treatment.
Bacterial Strains and Growth Conditions
The Salmonella strain used in this study was S typhimurium 14028.21 Bacterial cultures were prepared by inoculating 10 mL Lysogeny broth broth with 0.01 mL of a stationary-phase culture, followed by overnight incubation at 37°C.22
Salmonella-infected Mouse Model
Animal experiments were performed using VDRloxP/loxP and VDRΔPC mice (male and female, 2–3 months old). Water and food were withdrawn 4 hours before oral gavage, with 7.5 mg per mouse of streptomycin. Afterward, the animals were supplied with water and food ad libitum. Twenty hours after the streptomycin treatment, water and food were withdrawn again for 4 hours before the mice were infected with 1 × 106 colony-forming units of Salmonella (100-mL suspension in Hank’s balanced salt solution by gavage).22
Co-housing of VDRlox and VDRΔPC Mice and Induction of DSS Colitis
Two- to 3-month-old female VDRlox and VDRΔPC mice were co-housed in new cages according to previously published methods.8 One cage contained 3 VDRlox and 2 VDRΔPC mice, another cage contained 2 mice each. The mice were fed with the same food and water. After 4 weeks of co-housing, 5% DSS (molecular eight, 40–50 kDa; USB Corp, Cleveland, OH) dissolved in filter-purified water was administered to the mice. Animals were weighed daily. At day 7 after 5% DSS administration mice were killed.8
Induction of Small-intestinal Lesions
To induce small-intestinal injury, 10 mg/kg indomethacin (Sigma Chemical, St Louis, MO) was subcutaneously given to nonfasted animals. The animals were killed 24 hours after anesthetization. The jejunum and ileum were then removed, opened along the antimesenteric attachment, and examined for lesions under a dissecting microscope with square grids. The area (mm2) of visible lesions was macroscopically measured, summed per small intestine, and expressed as an ulcers core.23
Statistical Analyses
More details are also provided in the supplement document. The statistical analyses of experimental data were performed with GraphPad Prism 5. The regression and scatter plot of VDR against ATG16L1 were performed using SAS version 9.4 (SAS Institute). Microbiome data were analyzed by using R packages ampvis2, microbiome, phyloseq, and vegan, as previously reported.24
Results
Reduced VDR Positively Correlated With the Reduction of ATG16L1 and Paneth Cell Lysozyme in Human CD
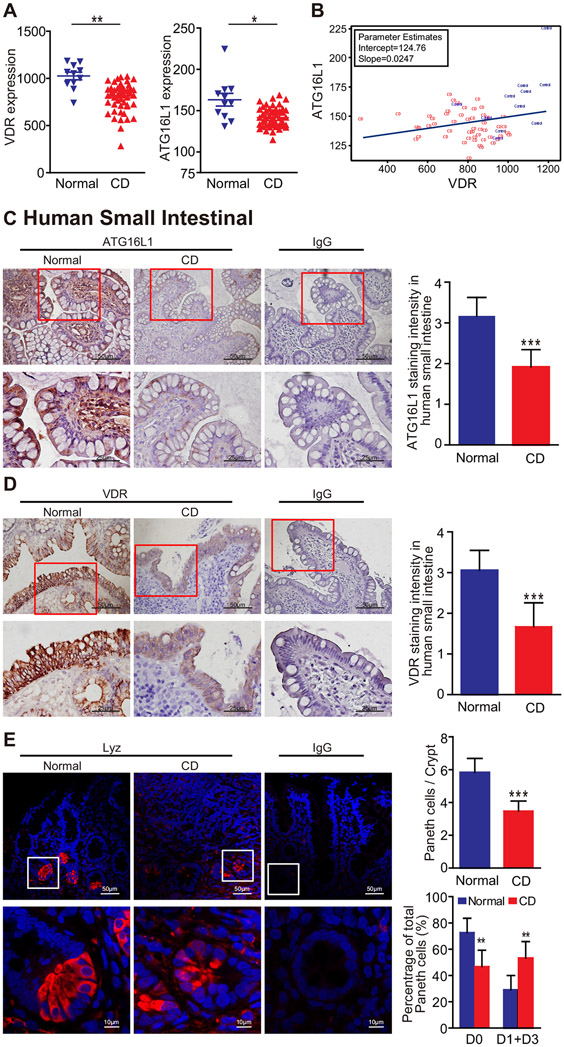
We found that the messenger RNA expression levels of VDR and ATG16L1 genes were both significantly reduced in human CD, using the Gene Expression Omnibus database GSE10213418 (Figure 1A). The regression line indicated the positive correlation of VDR and ATG16L1 expression (Figure 1B). Thus, we identified a significantly coordinated downregulated gene expression of VDR and ATG16L1 in patients with CD.
Figure 1.
The expression of VDR, ATG16L1, and lysozymes is downregulated in CD patients. (A) Reduced VDR and ATG16L1 expression in patients with CD. Data are expressed as mean ± SEM; normal, n = 11; CD, n = 51; Welch’s t test, *P < .05, **P < .01. (B) Significantly coordinated expression of VDR and ATG16L1 in CD patients. We performed a regression of VDR against ATGT6L1 and conducted a scatter plot with a regression line. Values for healthy control subjects are in blue and values for CD patients in red. Gene Expression Omnibus database GSE102134 normal, n = 11; CD, n = 51; the coefficient is 0.0247 with P = .0498 in the linear regression model. (C) Lower ATG16L1 expression in the small intestine tissues of the CD patients than that of normal tissues, as determined by immunohistochemical staining. Images are representative of experiments in triplicate. Data are expressed as mean ± SD, normal, n = 6; CD, n = 8; Welch’s t test; ***P < .001. (D) Significantly lower VDR expression in the small intestine of CD patients, compared with normal small intestine. Images are representative of experiments in triplicate. Data are expressed as mean ± SD, normal, n = 6; CD, n = 8; Welch’s t test; ***P < .001. (E) Numbers of Paneth cells per crypt and percentage of normal Paneth cells (D0) were lower in CD patients compared with normal small intestine. The percentage of Paneth cells that displayed normal (D0) and abnormal (D1–D3) patterns of lysozyme expression. Images are representative of experiments in triplicate. Data are expressed as mean ± SD, normal, n = 6; CD, n = 8; Welch’s t test or 2-way analysis of variance test, respectively; **P < .01, ***P < .001.
To investigate the changes of VDR and ATG16L1 at the protein level, we did immunohistochemical staining using small intestine tissues from healthy control subjects and CD patients. As shown in Figure 1C, a lower ATG16L1 expression was found in CD patients than that in the normal small intestine. We found significantly lower VDR expression in the small intestine of CD patients compared with the normal small intestine (Figure 1D). Reduction of VDR may lead to impaired Paneth cells and consequently increased inflammatory responses.
We then evaluated the status of the Paneth cells using a previously reported method for lysozyme detection.8 We found that the percentage of normal Paneth cells were lower in CD patients compared with the normal small intestine (Figure 1E). Abnormal Paneth cells were grouped as D1 (disordered), D2 (depleted), and D3 (diffuse).8,9 Paneth cells in the small intestine of CD patients also displayed a higher percentage of abnormal patterns of lysozyme expression (Figure 1E). Thus, we showed that VDR and ATG16L1 were markedly downregulated in patients with CD. The reduction of ATG16L1 and abnormal Paneth cells is correlated with the reduction of VDR in small intestinal samples from human colitis.
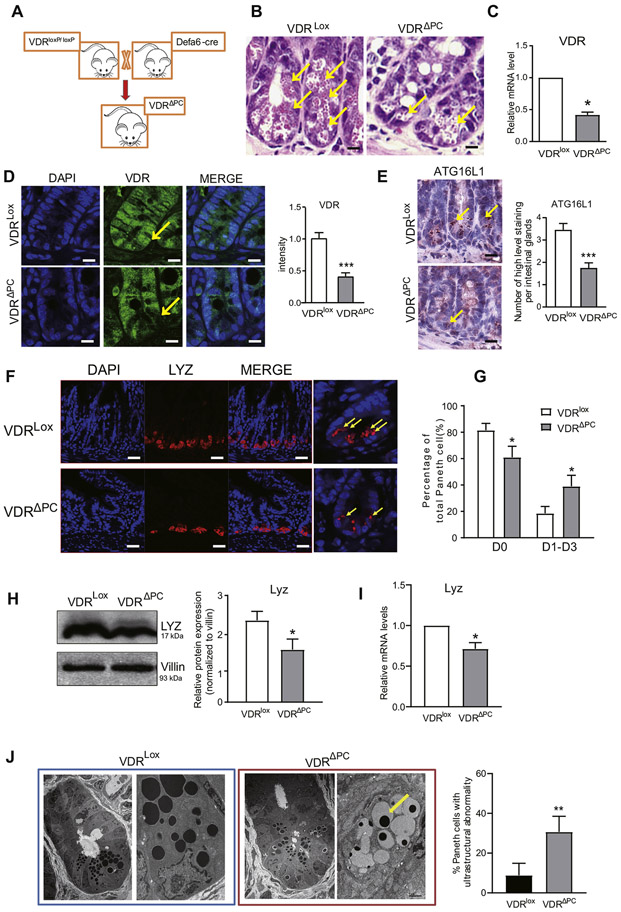
Establishing an Intestinal VDRΔPC Model
To investigate the molecular regulation of VDR in Paneth cells, we established a VDRΔPC model by crossing VDRloxP/loxP (VDRlox) mice with DEFA6-cre mice (Figure 2A). We found more granules in Paneth cells in the small intestine tissues of the VDRlox mice than in the VDRΔPC mice using hematoxylin and eosin staining (Figure 2B). To verily that the VDR gene was deleted in Paneth cells, the Paneth cells from the ileum tissue were laser captured and microdissected for quantitative polymerase chain reaction. The VDR expression in the VDRΔPC Paneth cells was significantly reduced compared with that in the VDRlox Paneth cells (Figure 2C). Furthermore, there was no detectable VDR expression in the intestinal Paneth cells of the VDRΔPC mice by immunofluorescence staining (Figure 2D). These data confirmed that the Paneth cell VDR knockout was established. We also tested the serum level of 1,25-vitamin D. However, we found that the lack of VDR in Paneth cells did not cause the changes of serum 1,25-vitamin D in vivo (data not shown).
Figure 2.
The expression of VDR was decreased in VDRΔPC mice. (A) By crossing VDRlox mice with DEFA6-cre mice, we generated Paneth cell VDR specific knockouts (VDRΔPC). (B) Hematoxylin and eosin staining shows more granules in the small-intestine tissues of VDRlox mice than in VDRΔPC mice. (C) RNAs of Paneth cells from the laser-captured micro-dissected ileum were collected for quantitative polymerase chain reaction. VDR expression in VDRΔPC mice was significantly reduced compared with VDRlox mice. Each single experiment was performed in triplicate. Data are expressed as mean ± SEM, n = 6, Student’s t test, *P < .05. (D) There is lower VDR expression in the small intestines of VDRΔPC mice than that of VDRlox mice. Immunostaining images are from a single experiment and representative of 6 mice per group. Data are expressed as means ± SEM, n = 6, Student t test. ***P < .001. (E) Lower ATG16L1 expression in the small-intestine tissues of the VDRΔPC mice than in the VDRlox mice. Images are from a single experiment and are representative of 6 mice per group. Data are expressed as means ± SEM, n = 6, Student t test. ***P < .001. (F) Lower expression of the lysozymes in the VDRΔPC mice by immunofluorescence staining. Yellow arrows represent normal lysozyme expression in the Paneth cells. Images are from a single experiment and are representative of 6 mice per group. (G) The percentage of normal Paneth cells was lower in VDRΔPC mice. Paneth cells displayed normal (D0) and abnormal (D1–D3) patterns of lysozyme (10 per group). Data are expressed as mean ± SEM. Student’s t test, *P < .05. (H) Lower expression of lysozymes in VDRΔPC mice by Western blotting. Each single experiment was performed in triplicate. Data are expressed as the mean ± SEM. Student’s t test, *P < .05. (I) RNA of the ileum tissues was collected for quantitative polymerase chain reaction. The lysozyme expression in the VDRΔPC mice was significantly reduced compared with VDRlox mice. Each single experiment was performed in triplicate. Data are expressed as the mean ± SEM, n = 6, Student’s t test, *P < .05. (J) More abnormal Paneth cells in VDRΔPC mice compared with VDRlox mice by transmission electron microscopy. Paneth cells were identified by the presence of cytoplasmic granules. Data are expressed as the mean ± SEM, n = 6, Student’s t test, **P < .01.
Absence of VDR Expression Leads to Abnormal Paneth Cells
The IBD susceptibility gene ATG16L1 is involved in autophagy and contributes to inflammation and dysbiosis.7-9 We found lower levels of ATG16L1 expression in the small intestine tissues of the VDRΔPC mice compared with those of the VDRlox mice with immunohistochemical staining (Figure 2E). Paneth cells are specialized intestinal epithelial cells that secrete AMPs, sense commensal bacteria, and maintain homeostasis at the intestinal–microbial interface.25 We evaluated the status of the Paneth cells by lysozyme detection.8 Lower expression levels of the lysozymes in the VDRΔPC mice were found by immunofluorescence staining when compared with the VDRlox mice (Figure 2F). We found the percentage of the normal Paneth cells (D0) was much lower in the VDRΔPC mice (Figure 2G). Based on the lysozymes patterns, abnormal Paneth cells were grouped as D1–D3.8,9 Abnormal Paneth cells were significantly higher in the VDRΔPC mice. These results were further confirmed by testing the lysozyme expression by Western blotting and real-time polymerase chain reaction (Figure 2H and I). In the VDRΔPC mice, lysozyme was significantly reduced at the protein and messenger RNA levels. The structure of the Paneth cells observed by a transmission electron microscopy also revealed more abnormal cells (eg, less granules) in the VDRΔPC mice when compared with the VDRlox mice (Figure 2J).
Paneth cells may support some aspects of the stem cell niche. We then examined the cell proliferation of small intestinal epithelial cells. Previous studies have identified serine/threonine protein kinase Akt26 signaling cooperates with Wingless to activate β-catenin in intestinal stem and progenitor cells through phosphorylation at Ser552 (P-β-catenin552).27 We found that P-β-catenin552 was decreased in the small intestine of VDRΔPC mice when compared with VDRlox mice (Supplementary Figure 1). We also examined the stem cell markers (lgr5 and bmi1). However, there was no significant change between VDRΔPC and VDRlox mice (data not shown).
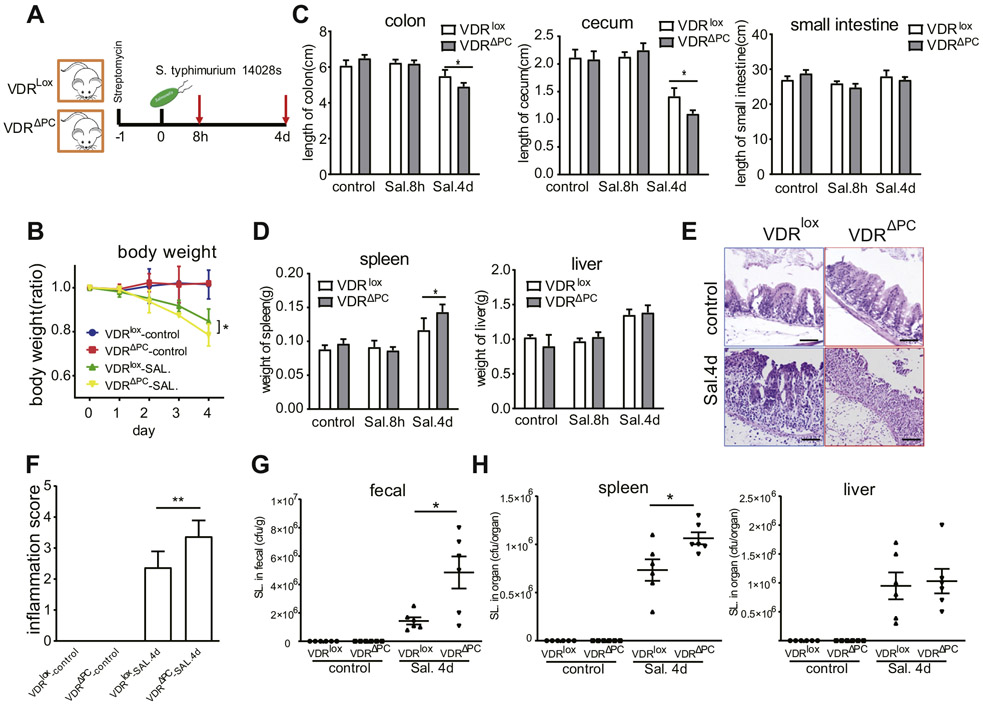
Salmonella Infections Were Worse in VDRΔPC Mice
Salmonella infections, known to induce intestinal damage28 and involve Paneth cells,29 were conducted using VDRlox and VDRΔPC mice (Figure 3A). Body weights were decreased after 4 days of the Salmonella treatment, significantly in the VDRΔPC mice compared with the VDRlox mice (Figure 3B). Cecal shortening and inflammation are key features in the Salmonella colitis mouse model.30 The length of the cecum and colon were shorter in the VDRΔPC mice than the VDRlox mice 4 days after infection (Figure 3C). Higher inflammation scores were also found in the cecum tissues of the VDRΔPC mice 4 days after infection by hematoxylin and eosin staining (Figure 3E and F). The lengths of the small intestine were measured, but no significant differences were found between the 2 mouse models. Both the liver and spleen were involved in the Salmonella infections, and so we measured their postinfection weights. After 4 days the Salmonella-treated mice had heavier spleens than the control group, especially for the VDRΔPC mice (Figure 3D). The numbers of colony-forming units were determined by plating on agar plates to determine the Salmonella colonies. The fecal and spleen samples, 4 days after infection, showed more Salmonella in the VDRΔPC mice compared with the VDRlox mice (Figure 3G and H).
Figure 3.
Salmonella infections were worse in VDRΔPC mice. (A) A Salmonella infection model. (B) Changes in the relative body weights after Salmonella infections. Data are expressed as mean ± SEM, n = 10, 2-way analysis of variance (ANOVA) test, *P < .05. (C) The length of cecum and colon for the VDRΔPC group were shorter, compared with the VDRlox mice, 4 days after infection. Data are expressed as mean ± SEM, n = 10, 2-way ANOVA test, *P < .05. (D) Large spleens were found in VDRΔPC mice 4 days postinfection. Data are expressed as mean ± SEM, n = 10, 2-way ANOVA test, *P < .05. (E and F) Higher inflammation scores in the cecum of the VDRΔPC mice 4 days after infection by hematoxylin and eosin staining. Data are expressed as mean ± SEM, n = 10, 1-way ANOVA test, **P < .01. (G) Quantification of Salmonella in feces 4 days postinfection. Data are expressed as mean ± SEM, n = 10, 1-way ANOVA test, *P < .05. (H) Quantification of the Salmonella in the spleen and liver 4 days postinfection. Data are expressed as mean ± SEM, n = 10, 1-way ANOVA test, *P < .05. Each single experiment was assayed in triplicate.
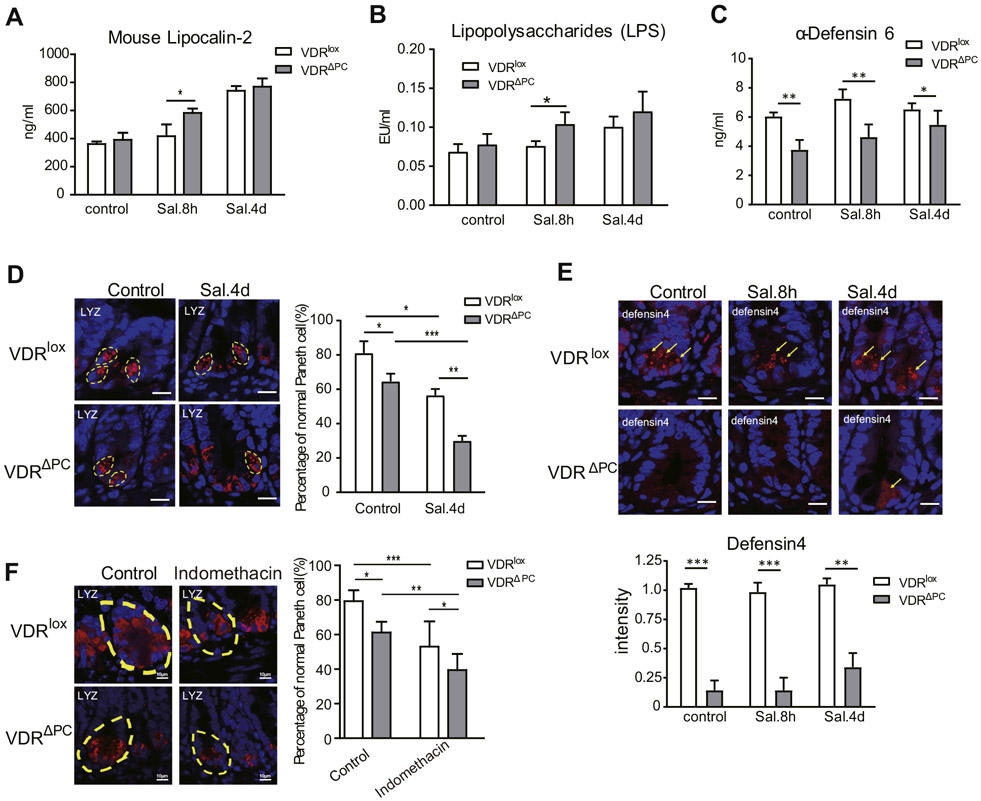
Lipocalin-2 is a pleiotropic mediator of various inflammatory processes.31 We found an increased expression level of lipocalin-2, and its expression was higher in the VDRΔPC group 8 hours after infection compared with VDRlox mice (Figure 4A). Lipopolysaccharides are characteristic components of the cell walls of gram-negative bacteria. The lipopolysaccharide increased in VDRΔPC mice compared with VDRlox mice 8 hours after infection (Figure 4B).
Figure 4.
Lack of VDR in Paneth cells leads to increased inflammatory responses. (A) Expression of lipocalin-2 was higher in feces of VDRΔPC mice. Each enzyme-linked immunosorbent assay (ELISA) was assayed in triplicate. Data are expressed as mean ± SEM, n = 10, 2-way analysis of variance (ANOVA) test, *P < .05. (B) More serum lipopolysaccharide from VDRΔPC mice 8 hours after infection. Each single experiment was assayed in triplicate. Data are expressed as the mean ± SEM, n = 10, 2-way ANOVA test, *P < .05. (C) Expression of α-defensin6 in the ileum tissue in VDRΔPC mice was lower than that in VDRlox mice by ELISA. Each single experiment was assayed in triplicate. Data are expressed as mean ± SEM, n = 10, 2-way ANOVA test, *P < .05, **P < .01. (D) The percentage of normal Paneth cells was reduced after Salmonella infection, especially in VDRΔPC mice. Data are expressed as mean ± SEM, n = 20, 2-way ANOVA test, *P < .05, **P < .01, ***P < .001. (E) Lower expression of the defensin4 in VDRΔPC mice after Salmonella infection, n = 6–9, 1-way ANOVA test, **P < .01, ***P < .001. (F) The percentage of normal Paneth cells was reduced after indomethacin treatment, especially in VDRΔPC mice. Data are expressed as mean ± SD, n = 6–9, 1-way ANOVA test, *P < .05, **P < .01, ***P < .001.
α-Defensin6 is expressed in the Paneth cells of the ileum. Its expression in the ileum tissues of VDRΔPC mice was significantly lower than that in VDRlox mice, even before Salmonella infection (Figure 4C). The percentage of normal Paneth cells was further reduced in VDRΔPC mice postinfection (Figure 4D). With the decreased number of normal Paneth cells, the expression of defensin4 was significantly downregulated in VDRΔPC mice after Salmonella treatments (Figure 4E). Taken together, the biological significance of VDRΔPC mice in a Salmonella colitis model was measured by several readouts, including body weight, intestinal inflammation, bacterial burden, bacterial translocation, lipocalin-2, lipopolysaccharide, and cellular changes of Paneth cells.
VDRΔPC Mice Showed Reduced Paneth Cells During Small-Intestinal Injuries
To further test the effects of VDRΔPC mice in response to small-intestinal injury, we treated mice with indomethacin, a nonsteroidal anti-inflammatory drug. It is a relevant CD environmental trigger that induces small-intestinal injury. We found that the percentage of normal Paneth cells was significantly reduced after the indomethacin treatment, especially in VDRΔPC mice (Figure 4F). This observation suggested that VDR deletion in Paneth cells made the mice susceptible to small-intestinal injury induced by indomethacin.
Lack of VDR in the Paneth cells leads to impaired anti-bacterial abilities
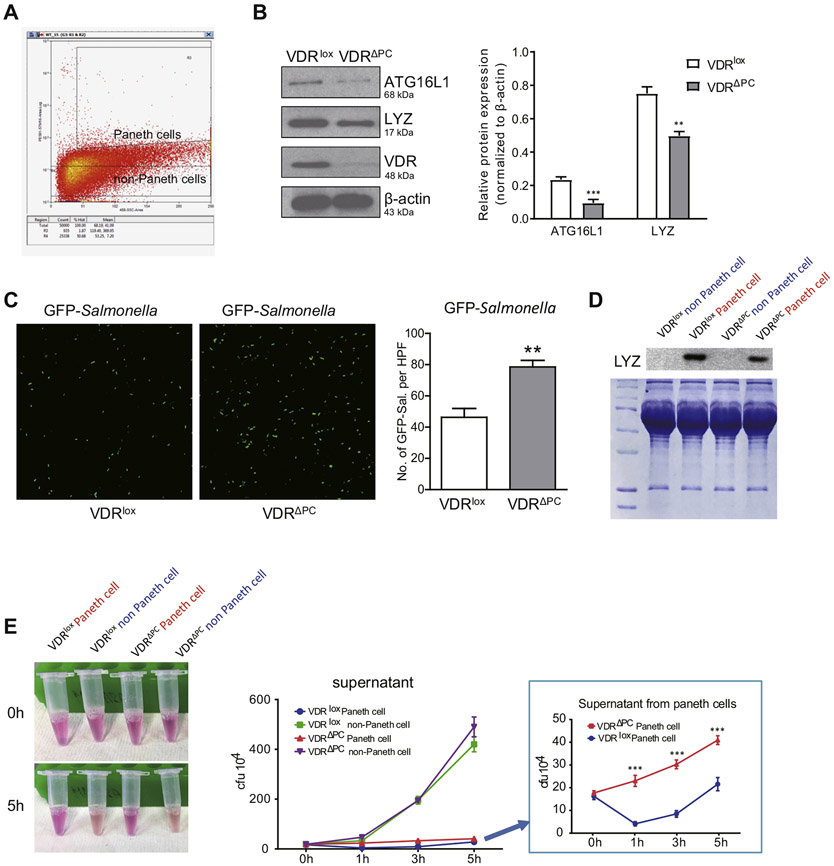
Paneth cells can control the intestinal growth of bacterial pathogens through the secretion of AMPs.32 To test their antimicrobial activity in a well-controlled condition, Paneth cells were isolated from the small intestine. We collected small-intestine tissues and digested them into single cells. After staining with anti-CD24 Ab, CD24+ Paneth cells and CD24™ non-Paneth cells were purified by flow cytometry (Figure 5A). We were able to test the protein expression in the isolated Paneth cells by Western blots. We found that the lack of VDR in Paneth cells led to significantly decreased expressions of ATG16L1 and LYZ (Figure 5B). These data also validated the successful deletion of VDR in Paneth cells in the VDRΔPC mice. Then, we tested the antibacterial functions of the purified Paneth cells. Isolated Paneth cells were incubated with green fluorescent protein Salmonella for 5 hours. The Paneth cells in the VDRΔPC group reduced the bacterial clearance ability and had more growth of Salmonella compared with the VDRlox Paneth cells (Figure 5C). The lack of VDR genes caused the VDRΔPC Paneth cells to lose their antibacterial qualities.
Figure 5.
Lack of VDR in Paneth cells leads to impaired antibacterial abilities. (A) Small intestine was collected and digested into single cells. After staining with the anti-CD24 Ab, cells were detected by flow cytometry. Paneth cells and non-Paneth cells were isolated. Each single experiment was performed in triplicate. (B) Lack of VDR in Paneth cells led to decreased expression of ATG16L1 and LYZ. Western blots were done using isolated Paneth cells from VDRlox and VDRΔPC mice. Each single experiment was performed in triplicate. Data are expressed as mean ± SEM, n = 6, Student’s t test, **P < .01, ***P < .001. (C) Isolated Paneth cells incubated with green fluorescent protein Salmonella for 5 hours. Paneth cells in the VDRlox group could inhibit Salmonella growth. Each single experiment was performed in triplicate. Data are expressed as mean ± SEM, n = 20, Student’s t test, **P < .01. (D) Expression of the lysozyme was lower in the VDRΔPC supernatant than the VDRlox supernatant. Paneth cells and non-Paneth cells were incubated overnight and the supernatant collected. Each experiment was performed in triplicate. (E) The supernatant from VDRlox Paneth cells had stronger antibacterial abilities than that of the VDRΔPC group. Salmonella was added into the supernatant. After 1, 3, and 5 hours, the medium was collected for counting the number of Salmonella. The supernatant from the Paneth cells stayed clear for 5 hours because of the antibacterial ability. Each single experiment was performed in triplicate. Data are expressed as mean ± SEM, n = 20, 2-way analysis of variance test, ***P < .001.
The antibacterial components secreted by Paneth cells also exist in the supernatants. The Paneth and non-Paneth cells were therefore cultured overnight and the supernatant collected. We determined that the expression of the lysozyme gene was lower in the VDRΔPC supernatant than in the VDRlox supernatant (Figure 5D). We then added Salmonella into these supernatant samples. After 5 hours of incubation, we collected the medium to determine the number of colony-forming units. At 0 hours the supernatant samples were clear, but after 5 hours we found that only 2 samples remained clear, and these 2 samples were from the supernatant of the Paneth cells (Figure 5E). Paneth cells can secrete AMPs and proteins to inhibit the growth of Salmonella. The VDRlox group had a stronger antibacterial ability than the VDRΔPC group (Figure 5E). However, both tubes with the non-Paneth cell fraction were cloudy after incubation for 5 hours, indicating bacterial growth. The green line (VDRlox non-Paneth cells) and purple line (VDRΔPC non-Paneth cells) show the similar trend, with no significant difference of colony-forming units (Figure 5E). Taken together, these experiments confirmed the decreased anti-bacterial ability of VDRΔPC Paneth cells without VDR.
Lack of VDR in Intestinal Paneth Cells Leads to Increased Inflammatory Responses in the Setting of Salmonella Infection
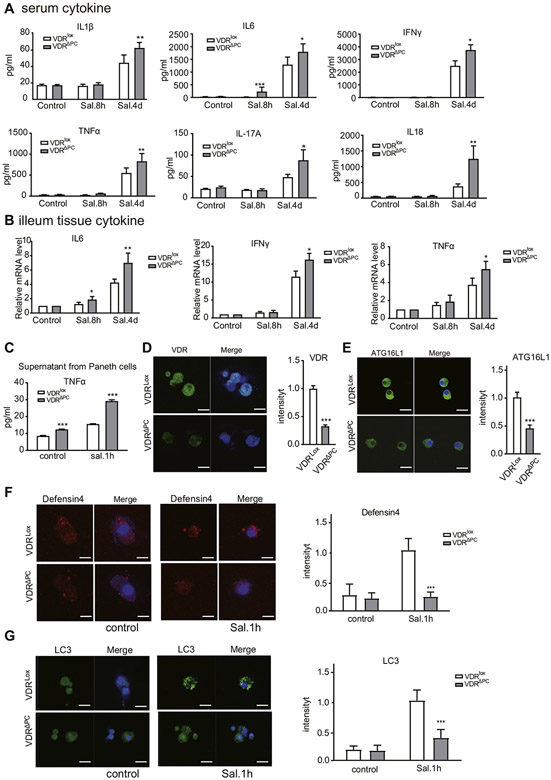
Inflammatory cytokines play a complex role in intestinal inflammation.33 We thus investigated the profile of cytokines in the serum of the mice. The cytokines we tested included interleukin (IL)1b, IL6, IL17A, IL18, interferon gamma, and tumor necrosis factor (TNF)-α. For IL6, significant differences were also found between the VDRΔPC and VDRlox mice 8 hours and 4 days after infection. Four days after infection, significant differences in the IL1β, IL17A, IL18, interferon gamma, and TNF-α were found in the Salmonella treated mice, especially VDRΔPC mice (Figure 6A). The increased expressions of the interferon gamma, IL6, and TNF-α in the ileum tissue of VDRΔPC mice after Salmonella treatments were also detected by real-time polymerase chain reaction (Figure 6B), indicating the enhanced local inflammation in the small intestine of VDRΔPC mice. TNF-α is a multifunctional proinflammatory cytokine. Paneth cells were treated with Salmonella for 1 hour, and the supernatant was collected to detect TNF-α by enzyme-linked immunosorbent assay. The expression of TNF-α was higher in the VDRΔPC supernatant than that in the VDRlox supernatant (Figure 6C).
Figure 6.
Lack of VDR in Paneth cells leads to impaired inflammatory responses. (A) Serum inflammatory cytokines were significantly increased, especially in VDRΔPC mice with the infection. Each single experiment was assayed in triplicate. Data are expressed as mean ± SEM, n = 6, 1-way analysis of variance (ANOVA) test, *P < .05, **P < .01, ***P < .001. (B) Expression of interferon gamma, IL1β, and TNF-β were increased in the VDRΔPC ileum postinfection. Each single experiment was assayed in triplicate. Data are expressed as mean ± SEM, n = 6, 1-way ANOVA test, *P < .05, **P < .01. (C) The expression of TNF-α was increased in VDRΔPC supernatant after Salmonella infection, as determined by enzyme-linked immunosorbent assay. Each single experiment was assayed in triplicate. Data are expressed as mean ± SEM, n = 10, 1-way ANOVA test, ***P < .001. (D) Expression of the VDR was decreased in VDRΔPC Paneth cells. Data are expressed as mean ± SEM, n = 10, Student’s t test, ***P < .001. (E) Expression of ATG16L1 was decreased in VDRΔPC Paneth cells. Data are expressed as mean ± SEM, n = 10, Student’s t test, ***P < .001. (F) Defensin4 expression in VDRΔPC Paneth cells was low. Each single experiment was assayed in triplicate. Data are expressed as mean ± SEM, n = 10, 1-way ANOVA test, ***P < .001. (G) After Salmonella treatment for 1 hour, stronger autophagy signals were found in VDRlox cells compared with VDRΔPC cells. Each single experiment was assayed in triplicate. Data are expressed as mean ± SEM, n = 10, 1-way ANOVA test, ***P < .001.
In the VDRΔPC Paneth cells, the expression of VDR decreased with the lower expression levels of ATG16L1 (Figure 6D and E). Defensin4 expression in VDRΔPC Paneth cells was also lower than that of the VDRlox cells after Salmonella infection (Figure 6F). We detected the autophagy marker of the Paneth cells with the CYTO-ID Autophagy detection kit (Enzo Life Sciences). After 1 hour of Salmonella treatment, we found stronger LC3 expression in the VDRlox cells compared with the VDRΔPC cells (Figure 6G), suggesting reduced autophagic responses because of the lack of VDR in Paneth cells.
Lack of VDR in Intestinal Paneth Cells Affects Intestinal Microbiota and Sensitivity to Chemical Damage
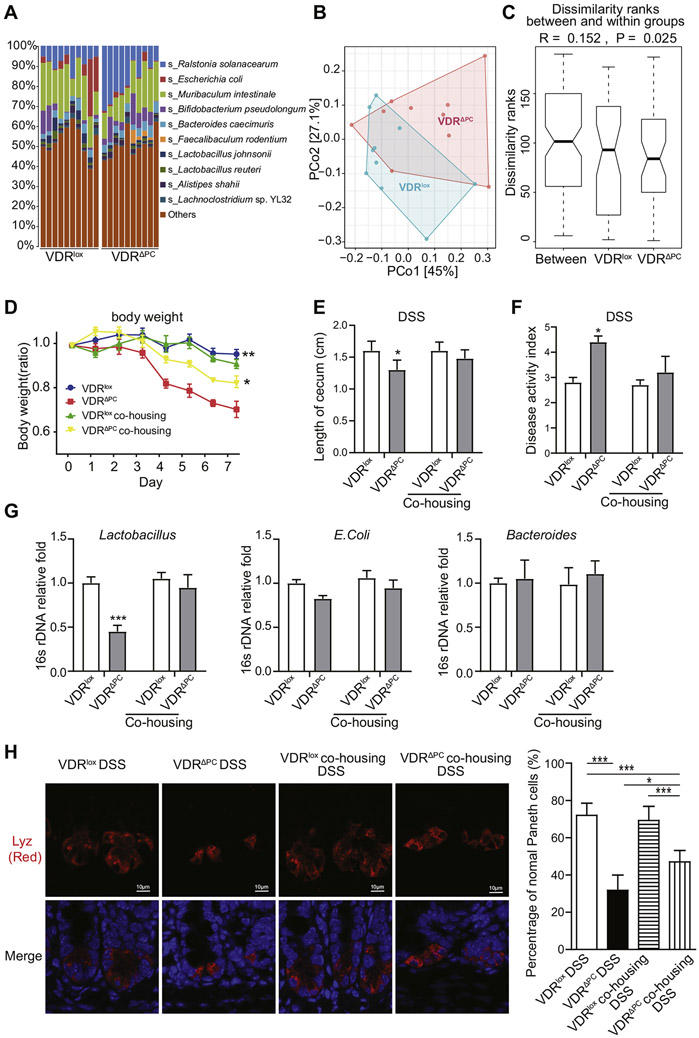
Paneth cell abnormalities in human subjects are associated with mucosal dysbiosis.17 We examined the bacterial abundance in feces of VDRΔPC and VDRlox mice using shotgun metagenomic sequencing. We found that bacterial homeostasis in VDRΔPC mice was changed compared with VDRlox mice without any treatment (Figure 7A and B). Taxa abundance and diversity of bacteria of VDRΔPC and VDRlox mice are shown in Figure 7A. The relative bacterial abundance in species levels was shown with the top 10 species. Ralstonia solanacearum and Faecalibaculum rodentium were enriched and Lactobacillus depleted in the VDRΔPC mice. Principal coordinate analysis showed that overall VDRlox and VDRΔPC mice were separated from each other with only partial overlap, and 2 principal coordinate axes could explain 72.1% of variances (Figure 7B). Furthermore, we performed the permutational multivariate analysis of variance of Bray-Curtis dissimilarity, which confirmed that VDRlox and VDRΔPC mice have different dissimilarities (P = .027). We also confirmed that the dissimilarity of bacteria in VDRΔPC mice was significantly decreased compared with VDRlox mice (Figure 7C).
Figure 7.
Lack of VDR in Paneth cells affects gut microbiota and sensitivity to chemical damage. (A) Bacterial abundance in feces of VDRΔPC and VDRlox mice. The relative bacterial abundance of top 10 species (s_) is shown. All unidentified and other identified species were grouped into “others”. Species were colored using the key as list seen on the right side. Each bar represents individual mouse, n = 10 per group. (B) Principal coordinate analysis visualized the sample differences between VDRΔPC and VDRlox mice. Samples were collected from VDRΔPC (red) and VDRlox mice (light blue). Two axes explain 72.1% (45% + 27.1%) of total sample variations, n = 10 per group. (C) Plots of between- and within-mean ranks of Bray-Curtis dissimilarity. The analysis of similarity was performed to test the Bray-Curtis dissimilarity on between- and within-groups of VDRlox and VDRΔPC. n = 10 per group, P = .025. (D) Relative body weight changes in mice with DSS. VDRΔPC mice had worse outcomes with DSS-induced colitis. Data are expressed as mean ± SEM, n = 6, 2-way analysis of variance (ANOVA) test, **P < .01, *P < .05. (E) A shortened cecum was found in VDRΔPC mice with colitis but not for co-housing mice. After 4 weeks of co-housing, mice were administered 5% DSS for 7 days to induce colitis. Data are expressed as the mean ± SEM, n = 6, Student’s t test compared between VDRLox and VDRΔPC mice, *P < .05. (F) Disease Activity Index of colon from mice with DSS treatment. Data are expressed as the mean ± SEM, n = 6, Student’s t test compared between VDRLox and VDRΔPC mice, *P < .05. (G) Lactobacillus in VDRΔPC mice was enhanced after co-housing with VDRLox mice. Data are expressed as means ± SEM. Each experiment was assayed in triplicate, n = 6, 1-way ANOVA test, ***P < .001. (H) Percentage of normal Paneth cells in VDRΔPC mice was increased after co-housing with VDRLox mice. Immunofluorescence images are representative of experiments in triplicate. Data are expressed as mean ± SD, n = 5–6, 1 way ANOVA test, *P < .05, ***P < .001.
To investigate the biologic effects of VDR in response to injury, we used a DSS colitis model. Our data showed that VDRΔPC mice were susceptible to DSS-mediated inflammation. The VDRΔPC mice had significant loss of body weight after DSS treatment for 7 days (Figure 7D). The cecum length was significantly reduced in the VDRΔPC mice with DSS compared with VDRlox mice (Figure 7E). In VDRΔPC mice with DSS, fecal blood was more obvious and stools less formed. Accordingly, the Disease Activity Index was significantly increased compared with the VDRlox group (Figure 7F).
Because microbiota may play a role in the vulnerability of VDRΔPC mice to DSS colitis, we examined the transmissibility of the phenotype by performing a co-housing experiment and then challenging the mice with DSS. We found that co-housing decreased the Disease Activity Index of VDRΔPC mice to a level similar to that seen in VDRlox mice (Figure 7E and F). Inflammation scores in the colon tissues of VDRΔPC mice were reduced after co-housing with VDRLox mice (Supplementary Figure 2A and B). Lactobacillus in VDRLox mice increased after co-housing treatment (Figure 7G), and Lactobacillus strains have been shown to decrease the inflammatory response in the intestine.34 Interestingly, the numbers of Paneth cells per crypt were restored in the VDRΔPC mice after co-housing with the VDRLox mice (Supplementary Figure 2C). The percentage of normal Paneth cells (D0) in the VDRΔPC mice was significantly increased after co-housing with the VDRLox mice (Figure 7H). These suggest that lack of VDR in Paneth cells affects gut microbiota and sensitivity to colitis.
Discussion
Genetically regulated VDR in the Paneth cells may set the threshold for maintaining the microbial homeostasis and alertness to pathogens. Here, we identified decreased intestinal VDR significantly correlated with reduction of ATG16L1 and Paneth cell lysozymes in patients with CD. We demonstrated that a lack of VDR in Paneth cells leads to impaired antibacterial activities and increased inflammatory responses in the setting of an enteropathogenic exposure. The lysozymes in Paneth cells were significantly decreased in VDRΔPC mice compared with VDRlox mice. The inhibition of bacterial growth by VDRΔPC/CD24+ cells was significantly weakened, and VDRΔPC mice had high inflammation levels after Salmonella infection. We found dysbiosis in VDRΔPC mice. Interestingly, co-housing of VDRΔPC and VDRlox mice made the VDRΔPC less vulnerable to DSS colitis, suggesting the transmission of protective bacteria community from the VDRlox mice. Thus, our data suggest that VDR is critical in maintaining the alertness of the Paneth cells in response to pathogens.
Paneth cells play a key role in host innate immune responses and in shaping the gut microbiome.13,14,35 Paneth cells are found throughout the small intestine at the base of the intestinal glands and in the colon during infection and inflammation in a process called intestinal metaplasia. In patients with ileal CD, there is lower expression of Paneth cell defensins, increased inflammation, and reduced cell defense. Several IBD polymorphisms are phenotypically manifested in Paneth cells, altering AMPs and autophagy. VDR target genes include AMP cathelicidin,15 β defensin,16 and ATG16L1.8 Our data indicate a critical role of VDR in shaping these activities. In IBD patients, it is critical to maintain the level of VDR to suppress inflammation diseases.1-6 Here, we have shown that lacking VDR leads to decreased numbers of Paneth cells, abnormal Paneth cells in differentiation, defects in AMP secretion, and reduced pathogen clearance. VDRΔPC mice also showed susceptibility to small-intestinal injury induced by a nonsteroidal anti-inflammatory drug. Our study further indicates the importance of VDR in supporting host defenses and protecting from intestinal injury through effects on Paneth cells.
VDR deletion in Paneth cells may reduce cell development and proliferation of small intestinal epithelial cells. P-β-catenin552 was decreased in the small intestine of VDRΔPC mice. Previous studies have shown that Wingless activate β-catenin in intestinal stem cells through P-β-catenin552.27 Salmonella infection is also known to involve the β-catenin pathway.36 In future studies we will investigate the effect and mechanism of VDR deletion in Paneth cell differentiation and intestinal stem cells.
We report a significant correlation of reduced VDR and ATG16L1 in the CD patients. VDR directly regulates its target gene ATG16L1 in colitis.8 In the pathogenesis of CD, the ATG16L1 variant plays a crucial role in pathogen clearance, resulting in imbalanced cytokines, and is linked to endoplasmic reticulum stress.37 Thus, we could consider VDR deficiency as a multifunctional susceptibility factor in CD. Evidence has demonstrated that vitamin D deficiency is a critical factor in the pathology associated with infection, IBD, and other diseases.1-6 Vitamin D administration leads to a shift of the intestinal bacterial composition in CD patients but not in healthy control subjects.38 To move forward, we should have well-designed therapeutic studies to examine if enhanced vitamin D/VDR will restore functions of Paneth cells and protect again chronic inflammation and intestinal injury.
Here, we established a unique experimental model with the Paneth cell VDR deletion. In vitro, we established a system to study purified Paneth cells in an antibacterial condition. These tools allowed us to establish the mechanisms by which Paneth cell VDR regulates the host–microbial interactions in vitro and in vivo. We rarely detected the entrance of the Salmonella into the Paneth cells in vivo using small-intestinal samples. Salmonella typically targets intestinal epithelial cells but not Paneth cells, presumably because of their significant levels of AMP secretion and autophagy.14,35 Because autophagy is linked to defensin secretion,14 the diminished autophagy observed may contribute to diminished AMP secretion and increased Salmonella that resulted. Thus, both diminished AMP production and secretion because of diminished autophagy probably explains our findings as a consequence of VDR deletion.
In summary, we characterize the precise role of VDR in regulating intestinal homeostasis and microbiome by changing the biologic function of the Paneth cells. We propose that targeting the VDR affects multiple downstream events within Paneth cells that establishes increased host defense against enteropathogens. Insights gained from the understanding of how the VDR pathway is integrally involved in regulating Paneth cells and responses to microbes may serve as a novel paradigm for understanding the mechanisms of host–bacterial interactions in IBD and infection.
Supplementary Material
WHAT YOU NEED TO KNOW:
BACKGROUND AND CONTEXT
While Paneth cells are known to regulate the commensal and pathogenic microbiota, the role that vitamin D receptor (VDR) in Paneth cells play in bacterial infections and Crohn’s disease (CD) is unknown.
NEW FINDINGS
Decreased intestinal VDR significantly correlates with reduction of levels of ATG16L1 and Paneth cell Lysozymes in patients with CD. The lack of VDR in the Paneth cells lead to impaired anti-bacterial abilities and inflammatory responses, dysbiosis, and high susceptibility to small intestinal injury induced by indomethacin.
LIMITATIONS
No human therapeutic studies to examine if enhanced vitamin D/VDR will restore Paneth cells and protect again chronic inflammation and intestinal injury.
IMPACT
We provide new evidence of the tissue-specific roles of the VDR in intestinal and microbial homeostasis. Targeting the VDR affects multiple downstream events within Paneth cells in host defense and anti-inflammation.
Acknowledgements
The authors thank Figen Seiler at the University of Illinois at Chicago Electron Microscopy Core for assistance with transmission electron microscopy, the University of Illinois at Chicago DNAS facility for assistance with shotgun metagenomic sequencing, and Lorraine Holland and Svetlana Saveljeva for helping with human small intestinal samples.
Funding
This study was funded by the University of Illinois at Chicago Cancer Center and the National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health grants R01 DK105118, R01DK114126, DOD CDMRP BC191198, and DOD BC160450P1 to Jun Sun and DK088199 to Richard Blumberg. The study sponsors played no role in the study design, data collection, analysis, and interpretation of data.
Abbreviations used in this paper:
- AMP
antimicrobial peptide
- CD
Crohn’s disease
- DSS
dextran sulfate sodium
- IBD
inflammatory bowel disease
- VDR
vitamin D receptor
Footnotes
Conflicts of interest
The authors disclose no conflicts.
CRediT Authorship Contributions
Rong Lu, MD, PhD (Data curation: Lead; Formal analysis: Lead; Investigation: Lead; Methodology: Lead; Resources: Supporting; Validation: Lead; Visualization: Lead; Writing – original draft: Equal)
Yong-guo Zhang, PhD (Formal analysis: Equal; Investigation: Equal; Methodology: Equal; Resources: Supporting; Validation: Supporting; Visualization: Supporting; Writing – original draft: Supporting; Writing – review & editing: Supporting)
Yinglin Xia, PhD (Formal analysis: Supporting; Methodology: Supporting; Resources: Equal; Validation: Supporting; Writing – original draft: Supporting; Writing – review & editing: Supporting)
Jilei Zhang, PhD (Data curation: Supporting; Formal analysis: Supporting; Investigation: Supporting; Methodology: Equal; Validation: Supporting; Visualization: Supporting; Writing – review & editing: Supporting)
Arthur Kaser, PhD (Resources: Supporting; Writing – review & editing: Supporting)
Richard Blumberg, MD (Funding acquisition: Supporting; Methodology: Supporting; Resources: Supporting; Validation: Supporting; Writing – review & editing: Supporting)
Jun Sun, PhD (Conceptualization: Lead; Formal analysis: Equal; Methodology: Equal; Project administration: Lead; Resources: Lead; Supervision: Lead; Validation: Supporting; Writing – original draft: Lead; Writing – review & editing: Lead)
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org and at https://doi.org/10.1053/j.gastro.2020.11.015.
References
- 1.Lloyd-Price J, Arze C, Ananthakrishnan AN, et al. Multi-genomics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019;569:655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakke D, Sun J. Ancient nuclear receptor VDR with new functions: microbiome and inflammation. Inflamm Bowel Dis 2018;24:1149–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakke D, Chatterjee I, Agrawal A, et al. Regulation of microbiota by vitamin D receptor: a nuclear weapon in metabolic diseases. Nucl Receptor Res 2018;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbachano A, Fernandez-Barral A, Ferrer-Mayorga G, et al. The endocrine vitamin D system in the gut. Mol Cell Endocrinol 2017;453:79–87. [DOI] [PubMed] [Google Scholar]
- 5.Del Pinto R, Ferri C, Cominelli F. Vitamin D axis in inflammatory bowel diseases: role, current uses and future perspectives. Int J Mol Sci 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimitrov V, White JH. Vitamin D signaling in intestinal innate immunity and homeostasis. Mol Cell Endocrinol 2017;453:68–78. [DOI] [PubMed] [Google Scholar]
- 7.Kabi A, Nickerson KP, Homer CR, et al. Digesting the genetics of IBD: insights from studies of autophagy risk genes. Inflamm Bowel Dis 2012;18:782–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu S, Zhang YG, Lu R, et al. Intestinal epithelial vitamin D receptor deletion leads to defective autophagy in colitis. Gut 2015;64:1082–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cadwell K, Liu JY, Brown SL, et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 2008;456:259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deuring JJ, Fuhler GM, Konstantinov SR, et al. Genomic ATG16L1 risk allele-restricted Paneth cell ER stress in quiescent Crohn’s disease. Gut 2014;63:1081–1091. [DOI] [PubMed] [Google Scholar]
- 11.Kaser A, Blumberg RS. ATG16L1 Crohn’s disease risk stresses the endoplasmic reticulum of Paneth cells. Gut 2014;63:1038–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tschurtschenthaler M, Adolph TE, Ashcroft JW, et al. Defective ATG16L1-mediated removal of IRE1alpha drives Crohn’s disease-like ileitis. J Exp Med 2017;214:401–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adolph TE, Tomczak MF, Niederreiter L, et al. Paneth cells as a site of origin for intestinal inflammation. Nature 2013;503:272–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salzman NH, Underwood MA, Bevins CL. Paneth cells, defensins, and the commensal microbiota: a hypothesis on intimate interplay at the intestinal mucosa. Semin Immunol 2007;19:70–83. [DOI] [PubMed] [Google Scholar]
- 15.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J 2005;19:1067–1077. [DOI] [PubMed] [Google Scholar]
- 16.Wang TT, Nestel FP, Bourdeau V, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol 2004;173:2909–2912. [DOI] [PubMed] [Google Scholar]
- 17.Liu TC, Gurram B, Baldridge MT, et al. Paneth cell defects in Crohn’s disease patients promote dysbiosis. JCI Insight 2016;1:e86907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrett JC, Hansoul S, Nicolae DL, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet 2008;40:955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verstockt S, De Hertogh G, Van der Goten J, et al. Gene and mirna regulatory networks during different stages of Crohn’s disease. J Crohns Colitis 2019;13:916–930. [DOI] [PubMed] [Google Scholar]
- 20.Van Cromphaut SJ, Dewerchin M, Hoenderop JG, et al. Duodenal calcium absorption in vitamin D receptor-knockout mice: functional and molecular aspects. Proc Natl Acad Sci USA 2001;98:13324–13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang YG, Wu S, Xia Y, et al. Axin1 prevents Salmonella invasiveness and inflammatory response in intestinal epithelial cells. PLoS One 2012;7:e34942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu R, Liu X, Wu S, et al. Consistent activation of the betacatenin pathway by Salmonella type-three secretion effector protein AvrA in chronically infected intestine. Am J Physiol 2012;303:G1113–G1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamada S, Naito Y, Takagi T,et al. Reduced small-intestinal injury induced by indomethacin in interleukin-17A-deficient mice. J Gastroenterol Hepatol 2011;26:398–404. [DOI] [PubMed] [Google Scholar]
- 24.Xia Y, Sun J, Chen D-G. Statistical analysis of microbiome data with R. Singapore: Springer, 2018. [Google Scholar]
- 25.Koslowski MJ, Beisner J, Stange EF, et al. Innate antimicrobial host defense in small intestinal Crohn’s disease. Int J Med Microbiol 2010;300:34–40. [DOI] [PubMed] [Google Scholar]
- 26.Martin JC, Chang C, Boschetti G, et al. Single-cell analysis of Crohn’s disease lesions identifies a pathogenic cellular module associated with resistance to anti-TNF therapy. Cell 2019;178:1493–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He XC, Yin T, Grindley JC, et al. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet 2007;39:189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu S, Liao AP, Xia Y, et al. Vitamin D receptor negatively regulates bacterial-stimulated NF-kappaB activity in intestine. Am J Pathol 2010;177:686–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salzman NH, Chou MM, de Jong H, et al. Enteric salmonella infection inhibits Paneth cell antimicrobial peptide expression. Infect Immun 2003;71:1109–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu R, Wu S, Liu X, et al. Chronic effects of a Salmonella type III secretion effector protein AvrA in vivo. PLoS One 2010;5:e10505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moschen AR, Adolph TE, Gerner RR, et al. Lipocalin-2: a master mediator of intestinal and metabolic inflammation. Trends Endocrinol Metab 2017;28:388–397. [DOI] [PubMed] [Google Scholar]
- 32.Sherman MP, Bennett SH, Hwang FF, et al. Paneth cells and antibacterial host defense in neonatal small intestine. Infect Immun 2005;73:6143–6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchez-Munoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World J Gastroenterol 2008;14:4280–4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu S, Yoon S, Zhang YG, et al. Vitamin D receptor pathway is required for probiotic protection in colitis. Am J Physiol 2015;309:G341–G349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bevins CL. Paneth cells, defensins, and IBD. J Pediatr Gastroenterol Nutr 2008;46(Suppl 1):E14–E15. [DOI] [PubMed] [Google Scholar]
- 36.Liu X, Lu R, Wu S, et al. Salmonella regulation of intestinal stem cells through the Wnt/beta-catenin pathway. FEBS Lett 2010;584:911–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salem M, Ammitzboell M, Nys K, et al. ATG16L1: a multifunctional susceptibility factor in Crohn disease. Autophagy 2015;11:585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schaffler H, Herlemann DP, Klinitzke P, et al. Vitamin D administration leads to a shift of the intestinal bacterial composition in Crohn’s disease patients, but not in healthy controls. J Dig Dis 2018;19:225–234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.