Abstract
Knee osteoarthritis is the leading cause of functional disability in adults. The goals of knee osteoarthritis management are directed toward symptomatic pain relief along with the attainment of the functional quality of life. The treatment strategy ranges from conservative to surgical management with reparative and restorative techniques. The emergence of cell-based therapies has paved the way for the usage of mesenchymal stem cells (MSCs) in cartilage disorders. Currently, global researchers are keen on their research on nanomedicine and targeted drug delivery. MSC-derived exosomes act as a directed therapy to halt the disease progression and to provide a pain-free range of movements with increased quality of cartilage on regeneration. International Society for Extracellular Vesicles and the European Network on Microvesicles and Exosomes in Health and Disease have formed guidelines to foster the use of the growing therapeutic potential of exosomal therapy in osteoarthritis. Although regenerative therapies with MSC are being seen to hold a future in the management of osteoarthritis, extracellular vesicle–based technology holds the key to unlock the potential toward knee preservation and regeneration. The intricate composition and uncertain functioning of exosomes are inquisitive facets warranting further exploration.
Keywords: mesenchymal stem cells, exosomes, microvesicles, cartilage
Introduction
Globally, 15% population is affected by knee osteoarthritis (OA), presenting as the major morbidity and leading cause of the functional disability. 1 The probability of developing symptomatic knee OA in an entire lifetime is approximately 45%. 2 OA of the knee, the most common degenerative joint disorder, is characterized by synovial inflammation, subchondral bony sclerosis and osteophyte formation. Biochemically, knee OA represents disequilibrium between rate of the cartilage degeneration and rate of the cartilage repair. When cartilage sustains any injury, then its limited intrinsic capacity to repair and regeneration results in the supervening of knee OA. 3 The goals of knee OA management are directed toward symptomatic pain relief along with the attainment of the functional quality of life. The treatment strategy ranges from conservative to surgical management with reparative and restorative techniques.
The robust development of technologies in regenerative orthopedics has opened the doors for various researchers for targeting the molecular pathogenesis of the disease and redirecting the pathogenesis toward cartilage regeneration. The emergence of cell-based therapies has paved the way for the usage of mesenchymal stem cells (MSCs) in cartilage disorders. 4 MSCs work on the principle of paracrine effects with their anti-inflammatory, antimicrobial, analgesic, regenerating, immunomodulatory, and immune-evasive properties.4,5 Among all the available MSCs, bone marrow–derived MSCs have been proved to have the higher cartilage regenerating potentiality than MSCs from adipose tissue or synovium. 5 The various phases of clinical trials demonstrated the therapeutic and safety aspects of the usage of MSC-based therapy in knee OA. These trials reported the functional improvements in terms of pain and joint function, as well as the quality of the cartilage regenerated during the follow-up. 5
Currently, global researchers are keen on their research on nanomedicine and targeted drug delivery. With a better understanding of the mechanism of action of MSCs, further research was directed toward the identification of the key regulatory mediators of their function. With the recognition of exosomes as the carriers of such critical mediators, a new gateway to cell-free therapeutics was laid down. Utilizing exosomes as a drug delivery tool is advantageous due to their naïve characteristics derived from the parent or host cells.6-8 Albeit exosomes contribute to the normal cellular homeostasis, their crucial role in intercellular signaling can be extrapolated to the pathobiology of the disease process.9-13 These exosomes act as a cell-free mediator modulating the natural course of the disease. These exosomes function by acting on tissue repair and regeneration, intracellular communication, bioenergetics, immunoregulation, and tissue metabolism. 14 This article renders insight into pathophysiology, diagnostic, and therapeutic role of exosomes in the knee OA highlighting the role of MSC-derived exosomal therapy as a potential therapeutic avenue in the management of knee OA.
Exosomes
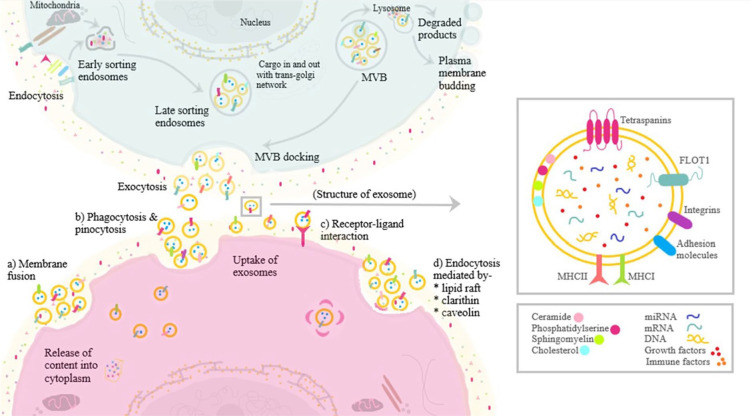
Despite a constant evolution in the categorization of these extracellular vesicles (EVs), based on the size of the EVs, exosomes are defined as a subcategory of EVs that are endosome derived lipid bilayered spherical vesicles of 40 to 150 nm in size called small EVs (sEVs) distinguishing them from the apoptotic bodies and microvesicles as shown in Table 1 .15,16 These sEVs have flotation density of 1.1 to 1.18 g/mL and express markers such as ALIX, CD81, and TSG101. 17 Almost all cells, tissues, and body fluids (plasma, urine, saliva, tears, gastrointestinal secretions, semen, and breast milk) secrete exosomes.18,19 Exosomal cargo carries an array of microbiomolecules that consists of proteins, lipids, ribonucleic acid, and deoxyribonucleic acid from the secreting parent cells.20-22 Exosomes form as a result of (a) cell membrane involution to form endosomes, (b) inward sprouting of luminal budding into multivesicular bodies, and (c) fusion of multivesicular bodies and plasma membrane and secreted into extracellular space. 23 The biosynthesis of exosomes is given in Fig. 1 . Moreover, the characteristics and behavior of the exosomes closely relate to the parent cell of origin.24-26 Considering the suitable size and property of these exosomes with their established role in various pathobiological processes, exosomal therapy has become an inquisitive issue among various researchers across a variety of fields aiming to develop a natural engineered defense system for combating the pathological process at a cellular level.
Table 1.
Forms of Extracellular Vesicles (EVs).
| Form of EVs | Size Range (nm) | Origin | Markers | Lipids |
|---|---|---|---|---|
| Apoptotic bodies | 1-5000 | Outpouching of apoptotic cell membrane | CD133; integrins; GRP94 | ?? |
| Microvesicles | 50-1000 | Outpouching of cell membrane from the parent cell of origin | CD40; CD62; integrins | Phosphatidylserine |
| Exosomes | 50-150 | Luminal budding into multivesicular bodies | CD63; CD9; ALIX; TSG101; tetraspanins; MHC1; HSP70 | Sphingomyelin, phosphatidylserine |
Figure 1.
Biogenesis and composition of exosomes.
Exosomes in the Pathophysiology of Knee OA
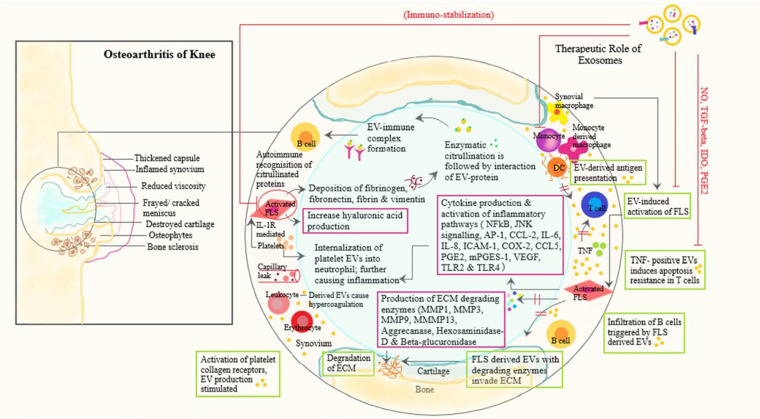
OA of the knee results due to the molecular interaction and cross-talks among the secreted pro-inflammatory cytokines and chemokines with cartilage, bone, tendon, infrapatellar fat pad, synovium, ligaments, and bursae around the knee joint. Subchondral bone (cortical bone beneath the articular cartilage) has an instrumental role in the natural course of the OA disease process. These subchondral bony changes occur due to the interaction between the paracrine mediators of bone and cartilage. 27 The cytokines and chemokines released from the surrounding structures enhance the process of degeneration of cartilage and degradation of cartilaginous matrix materials and the induction of osteophytes due to interleukin-1β (IL-1β), IL-6, IL-10, tumor necrosis factor-α (TNF-α), and bone morphogenetic proteins (BMPs). In knee OA, the upregulation of pro-inflammatory cytokine expression and matrix metalloproteinase (MMP) expression occurs.28,29 The downregulation of levels of cyclooxygenase-2 (COX-2) and microsomal PGE synthase-1 expression were observed, and eventually, the production of prostaglandin E2 (PGE2) was reduced.30,31 The role of exosomes in the pathophysiology of knee OA is shown in Fig. 2 .
Figure 2.
Schematic representation of role of exosomes in pathophysiology of knee osteoarthritis and key regulatory mechanisms of mesenchymal stem cell–derived exosomes.
Under physiological conditions, exosomes exhibit very low immunogenicity and cross physiological blood-brain barrier. 32 Exosomal cargoes are protected from immunological cells and circulating digestive enzymes due to their stable lipid bilayer. They elicit cargo delivery through endocytosis or membrane fusion.33,34 Kolhe et al. 35 demonstrated the communication and signaling between synovial fluid–derived sEVs and damaged articular cartilage cells. These synovial fluid–derived exosomes downregulated the expression of anti-inflammatory molecules and upregulated the expression of immune cells and pro-inflammatory molecules. They reported sEVs derived from females to have a more detrimental effect than sEVs derived from males and concluded that sEVs from the female are estrogen-responsive, which plays a pivotal role in toll-like receptor signaling in OA knee. 35
Synovial fibroblast (SF)–derived exosomal miRNA produces the inflammatory and degenerative process of articular cartilage. A few studies proved that SF-derived exosomal miRNA of female origin is closely associated with estrogen exposure and toll-like receptor signaling pathways. 36 miRNA-146a-5p derived from adipose tissue–derived sEVs demonstrate the progression of knee OA. 37 Various studies have reported the increased levels of catabolic gene expression (MMP-13 and ADAMTS-5) and decreased levels of anabolic gene expression (COL2A1 and ACAN) on IL-1β-stimulated exosomes derived from synovial fluid.38,39
The signaling pathways involved in OA knee involves transforming growth factor-β (TGF-β), SMADs, BMPs, MMPs, ADAMTS, inducible nitric oxide synthase (iNOS), IL-1, IL-6, and TNF-α, which are regulated by miRNA genes. 40 miRNAs regulate aberrant autophagy in OA chondrocytes by regulating apoptosis and reactive oxygen species molecules. 41 miRNA enhances histone deacetylation and DNA methylation of promoter sites, which affects the target gene expression in OA knee. In OA knee, WNT-5A signaling pathway regulates both cartilage differentiation and degeneration via MSC derived miRNA-92a-3p overexpression reported by Mao et al. 42 Further they found a decreased expression of miRNA-95-5p in degenerated cartilage. 43 The cartilage homeostasis can be regulated by histone deacetylase 2/8, which impairs cartilage development by inhibiting the cartilage-specific gene expression. Domenis et al. 44 investigated and treated OA patients with SF-derived exosomes and found the profound increase in MMP-7, MMP-12, IL-1β, CCL-8, CCL-15, CCL-20, and CXCL1, which lead to cartilage inflammation and degradation in joints. 10 sEVs maintain joint homeostasis by balancing the immunological signals from various cells. Once the pathogenic signals outrages the exosomal balancing, evasion of joint homeostasis results and thus the aggravation of the OA pathological process occurs.
Sources and Separation of Exosomes
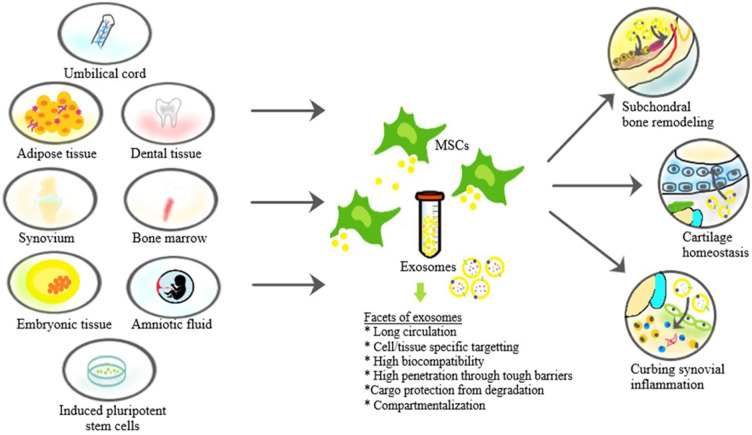
Exosomes are found in all cells and body fluids. 45 Various sources utilized for harvesting exosomes, separation techniques involved with their biological effects are given in Table 2 . Due to the challenges faced in isolating exosomes from various body fluids, regenerative and translational medicine experts used MSC-derived exosomes for treating various disorders. The exosomes derived from MSCs are of prime importance due to the greater therapeutic and regenerative potential. MSC-derived exosomes are easily extracted from bone marrow, adipose tissue, umbilical cells, endometrial fluid, amniotic fluid, and placental cells. 46 Amniotic fluid MSC-derived exosomes are more preferred for clinical applications than bone marrow–derived exosomes. 47 Various sources of MSC utilized to separate their sEVs are shown in Fig. 3 .
Table 2.
Sources and Isolation of Exosomes for Knee OA.
| Source | Exosomes | Isolation | Biological effects |
|---|---|---|---|
| Systemic sources | |||
| Bone marrow–derived MSC29,41 | miRNA-92a-3p; miRNA-26a-5p |
Ultracentrifugation Ultrafiltration |
Promotes chondrocytes migration, proliferation and
differentiation Enhanced cartilaginous matrix synthesis Promotes synovial fibroblast survival and hence reduces the occurrence of synovitis |
| Embryo-derived MSC 42 | miRNA-135b | Immunoaffinity Ultracentrifugation |
Induces in vitro chondrocyte
proliferation Enhances in vivo repair and regeneration of cartilage |
| Adipose tissue–derived MSC43,44 | miRNA-100-5p | Ultracentrifugation Ultrafiltration |
Enhanced periosteal cellular based
chondrogenesis Enhanced chondroprotective and anti-inflammatory effects |
| Synovium-derived MSC45,46 | miR-140-5p | Ultrafiltration | Halts the progression of OA to advanced stages Maintains the integrity of microstructures of trabecular bony structures Enhances the bone mineral density |
| Amniotic fluid–derived MSC 47 | AF-Exos | Precipitation | Ameliorates the pain mechanism in knee OA Complete recovery of hyaline cartilage restoration Maintains cartilage surface integrity |
| Human exfoliated deciduous teeth–derived MSC 44 | SHED-Exos | Ultracentrifugation | Enhances anabolic reaction and inhibits catabolic reaction in OA
pathogenesis; Maintains chondrocyte and joint homeostasis |
| Induced pluripotent–derived MSC 46 | iP-Exos | Ultrafiltration | Promotes chondrocytes migration, proliferation, and differentiation |
| Local sources | |||
| Synovial fluid23,48 | SF-Exos | Precipitation Affinity-based capture |
Differentiates early and late stages of OA knee Maintains joint and cartilage homeostasis |
| Cartilage49,50 | C-Exos | Ultrafiltration Precipitation |
Delays OA progression Induces ectopic chondrogenesis of cartilage progenitor cell constructs in chondrocyte-deficient areas |
| Synovium24,45 | S-Exos | Ultracentrifugation | Enhanced chondrocyte migration and proliferation via Wnt-5a and
-5b signaling Maintains the joint tribology in a rat OA model Overexpression of catabolic regulatory genes and underexpression of anabolic regulatory genes |
| Subchondral bone51,52 | SB-Exos | Immunoaffinity | Halts the severity of OA knee progression Attenuates the pain response Regulates joint homeostasis via TGF-β |
| Infrapatellar fat pad43,53 | IFP-Exos | Ultrafiltration | Enhanced regenerating potential of cartilage both in
vitro and in vivo
studies Promotes normal gait pattern in OA in rat model |
| Tendon54,55 | T-Exos | Density gradient and ultrahigh-speed centrifugation | Enhances tenogenic differentiation of MSCs Enhanced production of tendon matrix in vitro Maintenance of biomechanical strength of tendons |
| Ligaments56,57 | L-Exos | Ultracentrifugation Western blotting |
Promotion of ligamental cyclic stretch force Retards the progression to advanced stages of OA ??Unknown (to be explored) |
OA = osteoarthritis; MSC = mesenchymal stem cell.
Figure 3.
Sources of mesenchymal stem cell (MSC)–derived exosomes and their therapeutic actions.
Exosomes as a Diagnostic Tool
Extracellular vesicles produced by cells and body fluids are released into extracellular space to modulate the disease process in various stages of the disease. Hence they act as diagnostic markers. 48 Shan et al. 49 reported elevated follicular T helper cells and serum IL-21, IL-17A, and interferon-γ (INF-γ) in knee OA patients. Kolhe et al. 35 reported that the gender-specific changes observed in synovial fluid miRNA in knee OA patients suggested the prospect of using extracellular vehicles to identify tissue-specific biomarkers in OA knee. Skriner et al. 50 stated that citrullinated peptide proteins are ubiquitous and unique. They are associated with different types of joint disorders but not in osteoarthritis. Due to elevated levels of exosomal lncRNA PCGEM1 were observed by Zhao et al. 51 in the progressive stages of OA knee, exosomal lncRNA PCGEM1 has become a powerful indicator and biomarker to differentiate between early and late stages of knee OA. Murata et al. 52 observed decreased levels of miRNA 16 and miRNA 132 in patients with knee OA than in healthy individuals and decreased levels of synovial fluid–derived miRNA-16, -146a, and -223 in patients with knee OA than in those with knee rheumatoid arthritis. In patients with knee OA, Borgonio et al. 53 found overexpression of 12 miRNAs in the plasma (miRNA-16, -20b, -29c, -30b, -93, -126, -146a, -184, -186, -195, -345, and -885-5p), when compared with 380 miRNAs. Beyer et al. 54 confirmed that decreased levels of plasma let-7e exosomes are associated with progression of hip and knee OA to a severe disease, which requires total hip/knee arthroplasty.
Exosomes in Therapeutics
In knee OA, exosomal cargo acts as a double-edged sword. Apart from being the key mediator of cartilage degradation involved in the pathogenesis of OA as detailed above, exosomes have also been used as the targeted drug therapy for joint injury and osteoarthritis over the past 5 years. Though tiny in size, exosomes are biologically active with a stable structure devoid of degradation and better serve as a targeted delivery system against the diseases. 55 The treatment of knee OA with sEVs reduced the DNA binding affinity of c-jun activating protein-1 and nuclear factor-κB (NF-κB). 56 Hence, the transcription of matric metalloproteinases was downregulated. Exosomes derived from the embryonic MSCs attenuates inflammatory response and promotes healing of subchondral bone defect in rat OA model of temporomandibular joint.57-60 On evaluating exosomes from the induced pluripotent–derived stem cells and synovial membrane, the latter halted the progression of knee OA in a mouse model but the former exhibited a superior therapeutic response and a stronger chondrocyte migration and proliferation. 61 In the past decade, the usage of MSCs plays a significant role in cartilage regeneration and focal chondral defects. Similarly, the therapeutic role of mesenchymal stromal cell–derived sEVs in knee OA is detailed below.
MSC-Derived Exosomes
The specificity of sEVs depends on its dimensions, structure, membrane markers, and biogenesis. MSC-derived sEVs promote the reparative and regenerative processes of cartilage by suppressing the immune mechanism involved in the disease process of OA knee. Rani et al., 62 Colombo et al., 63 and Thery et al. 24 proved that MSC-derived sEVs promote the chondrogenesis in the cartilage defects. The molecular composition of these extracellular vehicles includes endosome-associated proteins (Rab GTPase, SNAREs, annexins, flotillin, Tsg101), membrane proteins (CD-63, -81, -82, -53, -37), lipid raft protein (glycosylphosphatidylinositol-anchored protein) and RNA (sRNAs, miRNA, fragments of tRNA, Y-RNA, and siRNAs). The cross-talk between MSCs and the neighboring diseased micromolecular tissue environment is the zone of MSC-sEV based therapeutics. sEV formation is regulated by the tumor suppressor–activated pathway 6 and its enhanced production is regulated by p53.64,65 Recent literature reported that MSC-derived sEVs regulates cell migration, proliferation and differentiated along with the production of the extracellular matrix, which supports the cellular meshwork. 66
MSC-Derived Exosomes in Knee OA
Exosomes derived from MScs contain bioactive macromolecules with the highest therapeutic potential.67,68 Various studies have demonstrated the cartilage repair and regeneration through MSC-derived exosomes via immunomodulatory and evasion of apoptosis mechanisms. They support neoangiogenesis and cellular proliferation.69-73 These exosomes demonstrate the homing effect of parental MSCs. 74 MSC-derived exosomes also possess surface molecules such as CD-29, -44, and -73. 14
The administration of human embryonic MSC-derived exosomes as intra-articular injection has shown the regenerating potential in osteochondral defects and eliminated cartilage destruction with enhanced matrix production in the OA model.59,75 EVs isolated from the human adipose tissue–derived MSCs exerted enhanced chondroprotection through diminished pro-inflammatory mediators production and increased anti-inflammatory cytokine production. Exosomes from adipose-derived MSCs upregulates chondrogenic potential in periosteal cells via miRNA145 and miRNA 221.76,77
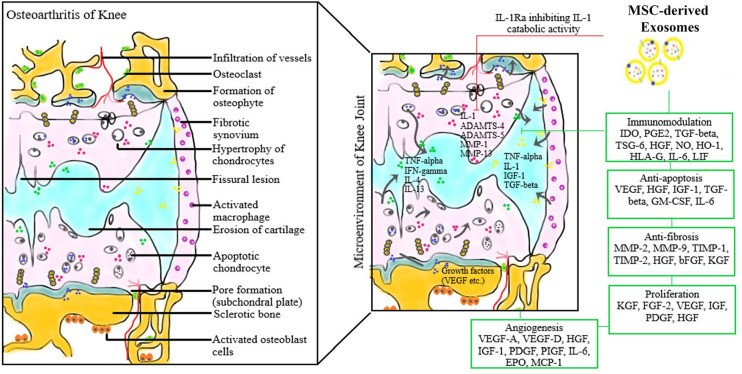
sEVs target-specific signals in subchondral bone, such as TGF-β, IL-1β, and attenuate the pathogenesis of OA and reduce the pain response.4,17,78 It was also noted in various studies that sEVs could function by TGF-β regulation in their targeted cells and downregulating MMP13 and upregulating calcitonin gene-related peptide (CGRP) and iNOS in the dorsal root ganglion of animal models.5,79-81 Moreover, bone marrow stromal cell–derived exosomes prevent the cartilage degeneration and downregulated the expression of tartrate-resistant acid phosphatase (TRAP) expression and RANKL-RANK-TRAF6 signaling activation to promote remodeling of subchondral bone.82-86 Hence these MSC-derived exosomal products can be used to ameliorate the acute pain due to the upregulated inflammatory cascade involved in the pathogenesis of the disease. The various immunomodulatory mechanisms involved in the therapeutic effects of MSC-sEVs in knee OA are given in Fig. 4 .
Figure 4.
Therapeutic immunomodulation of mesenchymal stem cell–derived exosomes in the pathogenesis of knee osteoarthritis.
Therapeutic Immunomodulatory Pathways of MSC-sEVs
Out of hundreds of sEV components, exosomal miRNA, exosomal lncRNAs, exosomal proteins and exosomal lipids play a significant role in the course of the knee OA disease process. The key exosomal components that participate in therapeutic immunomodulation against OA are the miRNAs and lncRNAs. We explain the possible immunomodulatory mechanism involved in bringing out the desired outcome.
MSC-sEV miRNA-Mediated Pathway
A significant reduction in miRNA-92a-39 and miRNA-95-5p was observed in OA chondrocytes.42,77 An increased miRNA-145 and -221 derived from adipose tissue–derived stem cells favor chondrogenesis and suppress the expression of pro-inflammatory cytokines and promote the degenerated cartilage for repair and regeneration. Thus adipose tissue–derived exosomes favor a stimulatory effect on chondrocyte migration, proliferation, and differentiation. 77 In the knee OA rat model, Jin et al. 87 demonstrated that the overexpression of human bone marrow MSC–derived exosome (miRNA-26a-5p) retards the damage of synovial fibroblasts in vitro and enhances the longevity of SF by underexpression of PTGS2 in vitro and halts the disease progression. The enhanced cartilage matrix production and chondrocyte proliferation are demonstrated by administration of miRNA-92a-3p-transfected MSCs. 43 The administration of anti-miRNA-449a-5p reverses osteoarthritic chondrocyte–mediated proinflammatory effects and cartilage destruction in knee OA. 88
MSC-sEV lncRNA-Mediated Pathway
Exosomal lncRNA PCGEM1 demonstrated a positive correlation with the WOMAC score and exhibit a significant difference between the early and late stages of OA knee. These sEVs modulate the repair and regeneration of denuded cartilage. 51 Exosomal lncRNA-KLF3-AS1 derived from MSC exhibited overexpression of COL2A1 and aggrecan levels, underexpression of pro-inflammatory mediators and enhances the survival of chondrocytes in knee OA. 89 Exosomal lncRNA-KLF3-AS1 target miR-206/GIT1 axis, which promotes cartilage regeneration in OA. Such coordination inhibits chondrocyte apoptosis and enhances proliferation and differentiation. 90
Fibulin-3 is widely expressed in humans for skeletal development, which is an extracellular glycoprotein. Runhaar et al. 91 demonstrated a positive correlation with elevated fibulin-3 serum concentrations with the histological joint degeneration in the rat knee OA model. Kim et al. 92 proved that the elevated NADPH oxidase due to increasing age has a positive correlation with advanced stages of knee OA. Collagen X is the measure of chondrocyte hypertrophy in OA knee. 93 There is a temporal association between inflammation (high-sensitivity C-reactive protein) and chondrocyte hypertrophy (COL-X) through HIF-2α. 94 A negative correlation was observed between COMP (cartilage oligomeric matrix protein) and duration of OA and a positive correlation between COMP and age. MSC-sEV derived lncRNAs upregulate COMP, thereby facilitating the longevity and regenerative capabilities of chondrocytes by inhibiting the apoptotic pathways and pro-inflammatory cytokines.
Advantages of Cell-Free Therapeutics
Exosomal cargos are clinically and therapeutically superior to stem cell in following aspects namely (a) less inherent risk than stem cell or cell-based therapies, (b) nonreplicability of exosomes hence no risk of malignant transformation, (c) less immunogenic response toward infections and cancers, and (d) act exactly in the site of diseased tissue. 95 The versatility of sEVs enhances intracellular signaling and shuttling and proceeds to maintain micromolecular homeostasis. Exosomes offer neuroprotection and neuroplasticity by crossing the blood-brain barrier in neurodegenerative diseases. 96 Although stem cells remain the powerhouse to manufacture the MSC-sEVs, by engineering their intercellular messengers through controlled micro-environment, we can harness their regenerative potential to varied disease conditions without the need for a cellular source to exert the desired paracrine effect in the vicinity.
Regulations for sEV Usage
The International Society for Extracellular Vesicles (ISEV) and the European Network on Microvesicles and Exosomes in Health and Disease (ME-HaD) have formed guidelines to foster the use of the growing therapeutic potential of exosomal therapy in osteoarthritis. 97 The process of collection, processing, testing, quality control, and manufacturing in the production of exosomes have been addressed in their regulations. Through these policies; the guidelines address the regulatory framework that will be required for harnessing the true potential of sEVs in therapeutic applications. There are currently no Food and Drug Administration (FDA)–approved exosome products for human use in the United States. 98 Therapies using the exosomes are under the Investigational New Drug (IND) developmental phase and need the approval of the regulatory agencies before initiating the clinical trial. 99
According to the Center for Biologics Evaluation and Research (CBER), the exosomes are regulated as biological products. 100 Based on the individual types, the framework that was laid down for products in this category applies to the Exosomes. The functional moiety in sEV-based therapy determines its medicinal type. 101 As the biological medicinal products include a span of various pharmaceuticals, these were classified as Advanced Therapy Medicinal Products (ATMPs) in 2007. It was further subgrouped to conventional biological medicinal products due to the biological, physicochemical, and immunochemical properties. 102 At this moment the sEVs do not have a standardized protocol for isolation and storage; and include homemade cocktails as protocols with no standardization for reagents, storage containers and storage time for each desired sEV-based product.99,101,103
Future Research and Scope
MSC-derived exosomes act mainly by suppressing the pro-inflammatory mediators and activating the anti-inflammatory factors. With their immunomodulatory properties, they influence the role of T cells. 104 This feature remains a potential topic for investigating their role in various autoimmune and inflammatory conditions. Extensive studies are to be made to develop biomarkers for early identification of joint diseases. 69 Stem cell–derived exosomes have the potential with their biologically active contents to halt the pathogenesis of various diseases.67,68 Moreover, stem cells secrete exosomes in large numbers which are easier to culture and collect. 70 Hence, stem cell–derived sEVs hold huge potential as parental cells in the future.
The emerging role of exosomal therapy in orthopedics remains unexplored. With the ongoing research with increased interest in exosomes, sEV-based therapeutics is not far from reach.95,96 The 2 major domains of future research on exosomes involve its diagnostic and therapeutic applications.
Exosomes are a potential candidate as early diagnostic markers to identify clinical osteoarthritis and cancer based on the exosome-specific proteins.71,72 Moreover, miRNAs and lncRNAs of the exosomal structure have been considered as potential diagnostic markers for diseases like rheumatoid arthritis and osteoarthritis.35,45,73,77,105-107 Studies have detailed that exosomal components of urine were altered in the disease and could be used to predict cartilage degradation and cancer as a noninvasive biochemical marker.108,109
Exosomes can also be used as infection biomarkers in the early postoperative stages or other complex disease conditions in orthopedics from the circulating pool of sEVs in blood.9,46,102,108,110-114 Paracrine effect of exosomes from stem cells can be used to delay diseases and repair, regenerate or rejuvenate organs.33,34,62,115-121 Exosomes are deemed anti-inflammatory for various orthopedic conditions involving joints. 122 They can also be used to understand the pathophysiology of a spectrum of diseases and can propel the scope of therapies by the knowledge acquired from the current technologies in hand. 123 Therapeutic application of exosomes from defined cell sources have diverse therapeutic applications including immune-modulatory and regenerative therapies.14,75,96,124,125 Apart from orthopedics, there is a huge potential for sEV-based therapeutics in auto-immune diseases, neurodegenerative diseases, infectious diseases, and diagnosis of rare diseases and cancers, which is being actively investigated by the various registered trials worldwide.96,122,124
There is a paradigm shift with continual breakthroughs in exosome research resulting in novel therapeutic options reshaping the landscape of the14,72,77,106 market accordingly. Although research on exosomes was started on scientific interests the potential of the exosome platforms show immense promise in future therapeutics. 126
Although there are about 79 clinical trials registered to evaluate the potential of sEV-based diagnostic and therapeutic potential in varied fields such as cancer diagnosis and therapy, infection, including SARS-CoV-2, psychiatry, dermatologic applications and neurodegenerative conditions including Parkinson’s and Alzheimer’s disease, none of the trials have registered to look into their role in osteoarthritis of the knee. 124 Although regenerative therapies with MSC are being seen to hold a future in the management of OA, sEV-based technology holds the key to unlock the potential toward knee preservation and regeneration. A systematic review of preclinical studies by Tan et al. 127 has demonstrated the therapeutic efficacy of MSC exosomes in the regeneration of bone in their study. The aforementioned showed improvised biochemical, morphological and histological outcomes in adjunct to the regeneration of bone and surrounding vasculature. 107 Study on exosomes derived from platelet-rich plasma (PRP-Exos) showed that they can be used to significantly protect cartilage from degradation through the Wnt/β-catenin signaling pathway with enhanced action in comparison to activated PRP.127,128 Further research is warranted to analyze the therapeutic effects of exosomes from varied sources.
Nevertheless, challenges in this field need to be overcome. Evidence are yet to be documented for identifying the major target cell following the transfer of sEVs from cell to cell within the joint. Moreover, methods of exosome production and release inside the joint remain unclear which limits the exosome-based targeted intervention strategies. Besides, for the MSC-derived exosomes to have action on the chondrocytes in the deeper layer it has to permeate through the cartilage matrix and outer later, which is relatively complete at the early OA stage, hence engineering MSC-derived exosomes for use in the early OA might have to focus on other joint cells like synovial cells that are readily accessible to exosomes for maintenance of cartilage matrix.
Conclusion
Exosomes enact as a natural vehicle for the transfer of biological substances between cells and thereby contributing to the onset and progression of OA with strong potential as a treatment of OA. However, the intricate composition and uncertain functioning are inquisitive facets warranting further exploration. Given making the exosome-based therapy a reality in the management of OA, studies investigating their mechanism of action and identification of the potential therapeutic targets hold promise.
Footnotes
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs: Madhan Jeyaraman  https://orcid.org/0000-0002-9045-9493
https://orcid.org/0000-0002-9045-9493
Sathish Muthu  https://orcid.org/0000-0002-7143-4354
https://orcid.org/0000-0002-7143-4354
References
- 1. Bjørge IM, Kim SY, Mano JF, Kalionis B, Chrzanowski W. Extracellular vesicles, exosomes and shedding vesicles in regenerative medicine - a new paradigm for tissue repair. Biomater Sci. 2017;6(1_suppl):60-78. doi: 10.1039/c7bm00479f [DOI] [PubMed] [Google Scholar]
- 2. Murphy LB, Helmick CG, Schwartz TA, Renner JB, Tudor G, Koch GG, et al. One in four people may develop symptomatic hip osteoarthritis in his or her lifetime. Osteoarthritis Cartilage. 2010;18(11):1372-9. doi: 10.1016/j.joca.2010.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vincent KR, Conrad BP, Fregly BJ, Vincent HK. The pathophysiology of osteoarthritis: a mechanical perspective on the knee joint. PM R. 2012;4(5 Suppl):S3-S9. doi: 10.1016/j.pmrj.2012.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McIntyre JA, Jones IA, Han B, Vangsness CT. Intra-articular mesenchymal stem cell therapy for the human joint: a systematic review. Am J Sports Med. 2018;46(14):3550-63. doi: 10.1177/0363546517735844 [DOI] [PubMed] [Google Scholar]
- 5. Yubo M, Yanyan L, Li L, Tao S, Bo L, Lin C. Clinical efficacy and safety of mesenchymal stem cell transplantation for osteoarthritis treatment: a meta-analysis. PloS One. 2017;12(4):e0175449. doi: 10.1371/journal.pone.0175449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schulz M, Binder WH. Mixed hybrid lipid/polymer vesicles as a novel membrane platform. Macromol Rapid Commun. 2015;36(23):2031-41. doi: 10.1002/marc.201500344 [DOI] [PubMed] [Google Scholar]
- 7. Pata V, Dan N. The effect of chain length on protein solubilization in polymer-based vesicles (polymersomes). Biophys J. 2003;85(4):2111-8. doi: 10.1016/S0006-3495(03)74639-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang X, Zhang P. Polymersomes in nanomedicine—a review. Curr Med Chem. 2017;13(2):124-9. doi: 10.2174/1573413712666161018144519 [DOI] [Google Scholar]
- 9. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. doi: 10.1126/science.aau6977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88(1_suppl):487-514. doi: 10.1146/annurev-biochem-013118-111902 [DOI] [PubMed] [Google Scholar]
- 11. Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell. 2016;30(6):836-48. doi: 10.1016/j.ccell.2016.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. 2016;126(4):1208-15. doi: 10.1172/JCI81135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14(3):195-208. doi: 10.1038/nri3622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lou G, Chen Z, Zheng M, Liu Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp Mol Med. 2017;49(6):e346. doi: 10.1038/emm.2017.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015;25(6):364-72. doi: 10.1016/j.tcb.2015.01.004 [DOI] [PubMed] [Google Scholar]
- 16. O’Brien K, Breyne K, Ughetto S, Laurent LC, Breakefield XO. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat Rev Mol Cell Biol. 2020;1-22. doi: 10.1038/s41580-020-0251-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17(7):879-87. doi: 10.1093/intimm/dxh267 [DOI] [PubMed] [Google Scholar]
- 18. Pisitkun T, Shen R-F, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A. 2004;101(36):13368-73. doi: 10.1073/pnas.0403453101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Michael A, Bajracharya SD, Yuen PST, Zhou H, Star RA, Illei GG, et al. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 2010;16(1_suppl):34-8. doi: 10.1111/j.1601-0825.2009.01604.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Admyre C, Johansson SM, Qazi KR, Fiken JJ, Lahesmaa R, Norman M, et al. Exosomes with immune modulatory features are present in human breast milk. J Immunol. 2007;179(3):1969-78. doi: 10.4049/jimmunol.179.3.1969 [DOI] [PubMed] [Google Scholar]
- 21. Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73(10):1907-20. doi: 10.1016/j.jprot.2010.06.006 [DOI] [PubMed] [Google Scholar]
- 22. Sokolova V, Ludwig AK, Hornung S, Rotan O, Horn PA, Epple M, et al. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surf B Biointerfaces. 2011;87(1_suppl):146-50. doi: 10.1016/j.colsurfb.2011.05.013 [DOI] [PubMed] [Google Scholar]
- 23. Kalra H, Adda CG, Liem M, Ang CS, Mechler A, Simpson RJ, et al. Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. Proteomics. 2013;13(22):3354-64. doi: 10.1002/pmic.201300282 [DOI] [PubMed] [Google Scholar]
- 24. Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581-93. doi: 10.1038/nri2567 [DOI] [PubMed] [Google Scholar]
- 25. Daaboul GG, Gagni P, Benussi L, Bettotti P, Ciani M, Cretich M, et al. Digital detection of exosomes by interferometric imaging. Sci Rep. 2016;6:37246. doi: 10.1038/srep37246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pitt JM, Kroemer G, Zitvogel L. Extracellular vesicles: masters of intercellular communication and potential clinical interventions. J Clin Invest. 2016;126(4):1139-43. doi: 10.1172/JCI87316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sharma AR, Jagga S, Lee SS, Nam JS. Interplay between cartilage and subchondral bone contributing to pathogenesis of osteoarthritis. Int J Mol Sci. 2013;14(10):19805-30. doi: 10.3390/ijms141019805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Little CB, Barai A, Burkhardt D, Smith SM, Fosang AJ, Werb Z, et al. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009;60(12):3723-33. doi: 10.1002/art.25002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Farahat MN, Yanni G, Poston R, Panayi GS. Cytokine expression in synovial membranes of patients with rheumatoid arthritis and osteoarthritis. Ann Rheum Dis. 1993;52(12):870-5. doi: 10.1136/ard.52.12.870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hardy MM, Seibert K, Manning PT, Currie MG, Woerner BM, Edwards D, et al. Cyclooxygenase 2-dependent prostaglandin E2 modulates cartilage proteoglycan degradation in human osteoarthritis explants. Arthritis Rheum. 2002;46(7):1789-803. doi: 10.1002/art.10356 [DOI] [PubMed] [Google Scholar]
- 31. Li X, Ellman M, Muddasani P, Wang JHC, Cs-Szabo G, van Wijnen AJ, et al. Prostaglandin E2 and its cognate EP receptors control human adult articular cartilage homeostasis and are linked to the pathophysiology of osteoarthritis. Arthritis Rheum. 2009;60(2):513-23. doi: 10.1002/art.24258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cho E, Nam GH, Hong Y, Kim YK, Kim DH, Yang Y, et al. Comparison of exosomes and ferritin protein nanocages for the delivery of membrane protein therapeutics. J Control Release. 2018;279:326-35. doi: 10.1016/j.jconrel.2018.04.037 [DOI] [PubMed] [Google Scholar]
- 33. György B, Hung ME, Breakefield XO, Leonard JN. Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annu Rev Pharmacol Toxicol. 2015;55:439-64. doi: 10.1146/annurev-pharmtox-010814-124630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tian T, Zhang HX, He CP, Fan S, Zhu YL, Qi C, et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137-49. doi: 10.1016/j.biomaterials.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 35. Kolhe R, Hunter M, Liu S, Jadeja R, Pundkar C, Mondal AK, et al. Gender-specific differential expression of exosomal miRNA in synovial fluid of patients with osteoarthritis. Sci Rep. 2017;7(1_suppl):2029. doi: 10.1038/s41598-017-01905-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ragni E, Perucca Orfei C, De Luca P, Colombini A, Vigano M, Lugano G, et al. Identification of miRNA reference genes in extracellular vesicles from adipose derived mesenchymal stem cells for studying osteoarthritis. Int J Mol Sci. 2019;20(5):1108. doi: 10.3390/ijms20051108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kato T, Miyaki S, Ishitobi H, Nakamura Y, Nakasa T, Lotz MK, et al. Exosomes from IL-1β stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res Ther. 2014;16(4):R163. doi: 10.1186/ar4679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mengshol JA, Vincenti MP, Coon CI, Barchowsky A, Brinckerhoff CE. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor κB: differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum. 2000;43(4):801-11. doi: [DOI] [PubMed] [Google Scholar]
- 39. Li YP, Wei XC, Li PC, Chen CW, Wang XH, Jiao Q, et al. The role of miRNAs in cartilage homeostasis. Curr Genomics. 2015;16(6):393-404. doi: 10.2174/1389202916666150817203144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li YS, Zhang FJ, Zeng C, Luo W, Xiao WF, Gao SG, et al. Autophagy in osteoarthritis. Joint Bone Spine. 2016;83(2):143-8. doi: 10.1016/j.jbspin.2015.06.009 [DOI] [PubMed] [Google Scholar]
- 41. Mao G, Zhang Z, Hu S, Zhang Z, Chang Z, Huang Z, et al. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res Ther. 2018;9(1_suppl):247. doi: 10.1186/s13287-018-1004-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mao G, Hu S, Zhang Z, Wu P, Zhao X, Lin R, et al. Exosomal miR-95-5p regulates chondrogenesis and cartilage degradation via histone deacetylase 2/8. J Cell Mol Med. 2018;22(11):5354-66. doi: 10.1111/jcmm.13808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mao G, Zhang Z, Huang Z, Chen W, Huang G, Meng F, et al. MicroRNA-92a-3p regulates the expression of cartilage-specific genes by directly targeting histone deacetylase 2 in chondrogenesis and degradation. Osteoarthritis Cartilage. 2017;25(4):521-32. doi: 10.1016/j.joca.2016.11.006 [DOI] [PubMed] [Google Scholar]
- 44. Domenis R, Zanutel R, Caponnetto F, Toffoletto B, Cifu A, Pistis C, et al. Characterization of the proinflammatory profile of synovial fluid-derived exosomes of patients with osteoarthritis. Mediators Inflamm. 2017;2017:4814987. doi: 10.1155/2017/4814987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van Spil WE, DeGroot J, Lems WF, Oostveen JCM, Lafeber FPJG. Serum and urinary biochemical markers for knee and hip-osteoarthritis: a systematic review applying the consensus BIPED criteria. Osteoarthritis Cartilage. 2010;18(5):605-12. doi: 10.1016/j.joca.2010.01.012 [DOI] [PubMed] [Google Scholar]
- 46. Kahlert C, Melo SA, Protopopov A, Tang J, Seth S, Koch M, et al. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J Biol Chem. 2014;289(7):3869-75. doi: 10.1074/jbc.C113.532267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Barile L, Vassalli G. Exosomes: therapy delivery tools and biomarkers of diseases. Pharmacol Ther. 2017;174:63-78. doi: 10.1016/j.pharmthera.2017.02.020 [DOI] [PubMed] [Google Scholar]
- 48. Revenfeld ALS, Bæk R, Nielsen MH, Stensballe A, Varming K, Jørgensen M. Diagnostic and prognostic potential of extracellular vesicles in peripheral blood. Clin Ther. 2014;36(6):830-46. doi: 10.1016/j.clinthera.2014.05.008 [DOI] [PubMed] [Google Scholar]
- 49. Shan Y, Qi C, Liu Y, Gao H, Zhao D, Jiang Y. Increased frequency of peripheral blood follicular helper T cells and elevated serum IL‑21 levels in patients with knee osteoarthritis. Mol Med Rep. 2017;15(3):1095-102. doi: 10.3892/mmr.2017.6132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Skriner K, Adolph K, Jungblut PR, Burmester GR. Association of citrullinated proteins with synovial exosomes. Arthritis Rheum. 2006;54(12):3809-14. doi: 10.1002/art.22276 [DOI] [PubMed] [Google Scholar]
- 51. Zhao Y, Xu J. Synovial fluid-derived exosomal lncRNA PCGEM1 as biomarker for the different stages of osteoarthritis. Int Orthop. 2018;42(12):2865-72. doi: 10.1007/s00264-018-4093-6 [DOI] [PubMed] [Google Scholar]
- 52. Murata K, Yoshitomi H, Tanida S, Ishikawa M, Nishitani K, Ito H, et al. Plasma and synovial fluid microRNAs as potential biomarkers of rheumatoid arthritis and osteoarthritis. Arthritis Res Ther. 2010;12(3):R86. doi: 10.1186/ar3013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cuadra VMB, González-Huerta NC, Romero-Córdoba S, Hidalgo-Miranda A, Miranda-Duarte A. Altered expression of circulating microRNA in plasma of patients with primary osteoarthritis and in silico analysis of their pathways. PLoS One. 2014;9(6):e97690. doi: 10.1371/journal.pone.0097690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Beyer C, Zampetaki A, Lin NY, Kleyer A, Perricone C, Iagnocco A, et al. Signature of circulating microRNAs in osteoarthritis. Ann Rheum Dis. 2015;74(3):e18. doi: 10.1136/annrheumdis-2013-204698 [DOI] [PubMed] [Google Scholar]
- 55. Mehrotra N, Tripathi RM. Short interfering RNA therapeutics: nanocarriers, prospects and limitations. IET Nanobiotechnol. 2015;9(6):386-95. doi: 10.1049/iet-nbt.2015.0018 [DOI] [PubMed] [Google Scholar]
- 56. Choi MC, Jo J, Park J, Kang HK, Park Y. NF-κB signaling pathways in osteoarthritic cartilage destruction. Cells. 2019;8(7):734. doi: 10.3390/cells8070734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yang CC, Lin CY, Wang HS, Lyu SR. Matrix metalloproteases and tissue inhibitors of metalloproteinases in medial plica and pannus-like tissue contribute to knee osteoarthritis progression. PloS One. 2013;8(11):e79662. doi: 10.1371/journal.pone.0079662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang S, Chuah SJ, Lai RC, Hui JHP, Lim SK, Toh WS. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16-27. doi: 10.1016/j.biomaterials.2017.11.028 [DOI] [PubMed] [Google Scholar]
- 59. Zhang S, Chu WC, Lai RC, Lim SK, Hui JHP, Toh WS. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage. 2016;24(12):2135-40. doi: 10.1016/j.joca.2016.06.022 [DOI] [PubMed] [Google Scholar]
- 60. Vonk LA, van Dooremalen SFJ, Liv N, Klumperman J, Coffer PJ, Saris DBF, et al. Mesenchymal stromal/stem cell-derived extracellular vesicles promote human cartilage regeneration in vitro. Theranostics. 2018;8(4):906-20. doi: 10.7150/thno.20746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhu Y, Wang Y, Zhao B, Niu X, Hu B, LI Q, et al. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res Ther. 2017;8(1_suppl):64. doi: 10.1186/s13287-017-0510-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther. 2015;23(5):812-23. doi: 10.1038/mt.2015.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255-89. doi: 10.1146/annurev-cellbio-101512-122326 [DOI] [PubMed] [Google Scholar]
- 64. Lespagnol A, Duflaut D, Beekman C, Blanc L, Fiucci G, Marine JC, et al. Exosome secretion, including the DNA damage-induced p53-dependent secretory pathway, is severely compromised in TSAP6/Steap3-null mice. Cell Death Differ. 2008;15(11):1723-33. doi: 10.1038/cdd.2008.104 [DOI] [PubMed] [Google Scholar]
- 65. Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66(9):4795-801. doi: 10.1158/0008-5472.CAN-05-4579 [DOI] [PubMed] [Google Scholar]
- 66. Heldring N, Mäger I, Wood MJA, Le Blanc K, Andaloussi SEL. Therapeutic potential of multipotent mesenchymal stromal cells and their extracellular vesicles. Hum Gene Ther. 2015;26(8):506-17. doi: 10.1089/hum.2015.072 [DOI] [PubMed] [Google Scholar]
- 67. Liu L, Jin X, Hu CF, Li R, Zhou Z, Shen CX. Exosomes derived from mesenchymal stem cells rescue myocardial ischaemia/reperfusion injury by inducing cardiomyocyte autophagy via AMPK and Akt pathways. Cell Physiol Biochem. 2017;43(1_suppl):52-68. doi: 10.1159/000480317 [DOI] [PubMed] [Google Scholar]
- 68. Luo Q, Guo D, Liu G, Chen G, Hang M, Jin M. Exosomes from MiR-126-overexpressing Adscs are therapeutic in relieving acute myocardial ischaemic injury. Cell Physiol Biochem. 2017;44(6):2105-16. doi: 10.1159/000485949 [DOI] [PubMed] [Google Scholar]
- 69. Hon KW, Abu N, Ab Mutalib NS, Jamal R. Exosomes as potential biomarkers and targeted therapy in colorectal cancer: a mini-review. Front Pharmacol. 2017;8:583. doi: 10.3389/fphar.2017.00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Han C, Sun X, Liu L, Jiang H, Shen Y, Xu X, et al. Exosomes and their therapeutic potentials of stem cells. Stem Cells Int. 2016;2016:7653489. doi: 10.1155/2016/7653489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523(7559):177-82. doi: 10.1038/nature14581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kawakami K, Fujita Y, Matsuda Y, Arai T, Horie K, Kameyama K, et al. Gamma-glutamyltransferase activity in exosomes as a potential marker for prostate cancer. BMC Cancer. 2017;17(1_suppl):316. doi: 10.1186/s12885-017-3301-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Işın M, Uysaler E, Özgür E, Koseoglu H, Sanli O, Yucel OB, et al. Exosomal lncRNA-p21 levels may help to distinguish prostate cancer from benign disease. Front Genet. 2015;6:168. doi: 10.3389/fgene.2015.00168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wang K, Jiang Z, Webster KA, Chen J, Hu H, Zhou Y, et al. Enhanced cardioprotection by human endometrium mesenchymal stem cells driven by exosomal microRNA-21. Stem Cells Transl Med. 2017;6(1_suppl):209-22. doi: 10.5966/sctm.2015-0386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wang Y, Yu D, Liu Z, Zhou F, Dai J, Wu B, et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res Ther. 2017;8(1_suppl):189. doi: 10.1186/s13287-017-0632-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tofiño-Vian M, Guillén MI, Pérez Del Caz MD, Silvestre A, Alcaraz MJ. Microvesicles from human adipose tissue-derived mesenchymal stem cells as a new protective strategy in osteoarthritic chondrocytes. Cell Physiol Biochem. 2018;47(1_suppl):11-25. doi: 10.1159/000489739 [DOI] [PubMed] [Google Scholar]
- 77. Zhao C, Chen JY, Peng WM, Yuan B, Bi Q, Xu YJ. Exosomes from adipose-derived stem cells promote chondrogenesis and suppress inflammation by upregulating miR-145 and miR-221. Mol Med Rep. 2020;21(4):1881-9. doi: 10.3892/mmr.2020.10982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chahla J, Piuzzi NS, Mitchell JJ, Dean CS, Pascual-Garrido C, LaPrade FR, et al. Intra-articular cellular therapy for osteoarthritis and focal cartilage defects of the knee: a systematic review of the literature and study quality analysis. J Bone Joint Surg Am. 2016;98(18):1511-21. doi: 10.2106/JBJS.15.01495 [DOI] [PubMed] [Google Scholar]
- 79. He L, He T, Xing J, Zhou Q, Fan L, Liu C, et al. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res Ther. 2020;11(1_suppl):276. doi: 10.1186/s13287-020-01781-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Li J, Ding Z, Li Y, Wang W, Wang J, Yu H, et al. BMSCs-derived exosomes ameliorate pain via abrogation of aberrant nerve invasion in subchondral bone in lumbar facet joint osteoarthritis. J Orthop Res. 2020;38(3):670-9. doi: 10.1002/jor.24497 [DOI] [PubMed] [Google Scholar]
- 81. Cui Z, Crane J, Xie H, Jin X, Zhen G, Li C, et al. Halofuginone attenuates osteoarthritis by inhibition of TGF-β activity and H-type vessel formation in subchondral bone. Ann Rheum Dis. 2016;75(9):1714-21. doi: 10.1136/annrheumdis-2015-207923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhang S, Teo KYW, Chuah SJ, Lai RC, Lim SK, Toh WS. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials. 2019;200:35-47. doi: 10.1016/j.biomaterials.2019.02.006 [DOI] [PubMed] [Google Scholar]
- 83. Zhu S, Zhu J, Zhen G, Hu Y, An S, LI Y, et al. Subchondral bone osteoclasts induce sensory innervation and osteoarthritis pain. J Clin Invest. 2019;129(3):1076-93. doi: 10.1172/JCI121561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chen Y, Lin S, Sun Y, Guo J, Lu Y, Suen CW, et al. Attenuation of subchondral bone abnormal changes in osteoarthritis by inhibition of SDF-1 signaling. Osteoarthritis Cartilage. 2017;25(6):986-94. doi: 10.1016/j.joca.2017.01.008 [DOI] [PubMed] [Google Scholar]
- 85. Xu T, Xu M, Bai J, Lin J, Yu B, Liu Y, et al. Tenocyte-derived exosomes induce the tenogenic differentiation of mesenchymal stem cells through TGF-β. Cytotechnology. 2019;71(1_suppl):57-65. doi: 10.1007/s10616-018-0264-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yao Y, Chen R, Wang G, Zhang Y, Liu F. Exosomes derived from mesenchymal stem cells reverse EMT via TGF-β1/Smad pathway and promote repair of damaged endometrium. Stem Cell Res Ther. 2019;10(1_suppl):225. doi: 10.1186/s13287-019-1332-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jin Z, Ren J, Qi S. Human bone mesenchymal stem cells-derived exosomes overexpressing microRNA-26a-5p alleviate osteoarthritis via down-regulation of PTGS2. Int Immunopharmacol. 2020;78:105946. doi: 10.1016/j.intimp.2019.105946 [DOI] [PubMed] [Google Scholar]
- 88. Ni Z, Kuang L, Chen H, Xie Y, Zhang B, Ouyang J, et al. The exosome-like vesicles from osteoarthritic chondrocyte enhanced mature IL-1β production of macrophages and aggravated synovitis in osteoarthritis. Cell Death Dis. 2019;10(7):522. doi: 10.1038/s41419-019-1739-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Liu Y, Zou R, Wang Z, Wen C, Zhang F, Lin F. Exosomal KLF3-AS1 from hMSCs promoted cartilage repair and chondrocyte proliferation in osteoarthritis. Biochem J. 2018;475(22):3629-38. doi: 10.1042/BCJ20180675 [DOI] [PubMed] [Google Scholar]
- 90. Liu Y, Lin L, Zou R, Wen C, Wang Z, Lin F. MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in osteoarthritis. Cell Cycle Georget Tex. 2018;17(21-22):2411-22. doi: 10.1080/15384101.2018.1526603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. de Visser HM, Sanchez C, Mastbergen SC, Lafeber FPJG, Henrotin YE, Weinans H. Fib3-3 as a biomarker for osteoarthritis in a rat model with metabolic dysregulation. Cartilage. 2019;10(3):329-34. doi: 10.1177/1947603518754629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kim MJ, Kim HJ, Hong YH, Lee CK, Kim YW, Shon OJ, et al. Age-related NADPH oxidase (arNOX) activity correlated with cartilage degradation and bony changes in age-related osteoarthritis. J Korean Med Sci. 2015;30(9):1246. doi: 10.3346/jkms.2015.30.9.1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. He Y, Siebuhr AS, Brandt-Hansen NU, Wang J, Su D, Zheng Q, et al. Type X collagen levels are elevated in serum from human osteoarthritis patients and associated with biomarkers of cartilage degradation and inflammation. BMC Musculoskelet Disord. 2014;15:309. doi: 10.1186/1471-2474-15-309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Yang S, Kim J, Ryu JH, Oh H, Chun CH, Kim BJ, et al. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med. 2010;16(6):687-93. doi: 10.1038/nm.2153 [DOI] [PubMed] [Google Scholar]
- 95. Bunggulawa EJ, Wang W, Yin T, Wang N, Durkan C, Wang Y, et al. Recent advancements in the use of exosomes as drug delivery systems. J Nanobiotechnology. 2018;16(1_suppl):81. doi: 10.1186/s12951-018-0403-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Jan AT, Malik MA, Rahman S, Yeo HR, Lee EJ, Abdullah TS, et al. Perspective insights of exosomes in neurodegenerative diseases: a critical appraisal. Front Aging Neurosci. 2017;9;317. doi: 10.3389/fnagi.2017.00317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1_suppl):1535750. doi: 10.1080/20013078.2018.1535750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ilic N, Savic S, Siegel E, Atkinson K, Tasic L. Examination of the regulatory frameworks applicable to biologic drugs (including stem cells and their progeny) in Europe, the US, and Australia: part II—a method of software documentary analysis. Stem Cells Transl Med. 2012;1(12):909-20. doi: 10.5966/sctm.2012-0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. European Commission. EudraLex-Volume 4—good manufacturing practice (GMP) guidelines. Published December 15, 2017. Accessed September 3, 2020. https://ec.europa.eu/health/documents/eudralex/vol-4_en
- 100. Ancans J. Cell therapy medicinal product regulatory framework in Europe and its application for MSC-based therapy development. Front Immunol. 2012;3:253. doi: 10.3389/fimmu.2012.00253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. European Medicines Agency. Regulation on advanced therapy medicinal products. Published March 16, 2010. Accessed September 3, 2020. https://www.ema.europa.eu/en/documents/scientific-guideline/draft-reflection-paper-stem-cell-based-medicinal-products_en.pdf
- 102. Lian J, Lin SH, Ye Y, Chang DW, Huang M, Dinney CP, et al. Serum microRNAs as predictors of risk for non-muscle invasive bladder cancer. Oncotarget. 2018;9(19):14895-908. doi: 10.18632/oncotarget.24473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kriebardis A, Antonelou M, Stamoulis K, Papassideri I. Cell-derived microparticles in stored blood products: innocent-bystanders or effective mediators of post-transfusion reactions? Blood Transfus. 2012;10(Suppl 2):s25-s38. doi: 10.2450/2012.006S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chen W, Huang Y, Han J, Yu L, Li Y, Lu Z, et al. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol Res. 2016;64(4):831-40. doi: 10.1007/s12026-016-8798-6 [DOI] [PubMed] [Google Scholar]
- 105. Rabinowits G, Gerçel-Taylor C, Day JM, Taylor DD, Kloecker GH. Exosomal microRNA: a diagnostic marker for lung cancer. Clin Lung Cancer. 2009;10(1_suppl):42-6. doi: 10.3816/CLC.2009.n.006 [DOI] [PubMed] [Google Scholar]
- 106. Lv LL, Cao YH, Ni HF, Xu M, Liu D, Liu H, et al. MicroRNA-29c in urinary exosome/microvesicle as a biomarker of renal fibrosis. Am J Physiol Renal Physiol. 2013;305(8):F1220-F1227. doi: 10.1152/ajprenal.00148.2013 [DOI] [PubMed] [Google Scholar]
- 107. Song J, Kim D, Han J, Kim Y, Lee M, Jin EJ. PBMC and exosome-derived Hotair is a critical regulator and potent marker for rheumatoid arthritis. Clin Exp Med. 2015;15(1_suppl):121-6. doi: 10.1007/s10238-013-0271-4 [DOI] [PubMed] [Google Scholar]
- 108. Charni N, Juillet F, Garnero P. Urinary type II collagen helical peptide (HELIX-II) as a new biochemical marker of cartilage degradation in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 2005;52(4):1081-90. doi: 10.1002/art.20930 [DOI] [PubMed] [Google Scholar]
- 109. Garnero P, Charni N, Juillet F, Conrozier T, Vignon E. Increased urinary type II collagen helical and C telopeptide levels are independently associated with a rapidly destructive hip osteoarthritis. Ann Rheum Dis. 2006;65(12):1639-44. doi: 10.1136/ard.2006.052621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Cai J, Han Y, Ren H, Chen C, He D, Zhou L, et al. Extracellular vesicle-mediated transfer of donor genomic DNA to recipient cells is a novel mechanism for genetic influence between cells. J Mol Cell Biol. 2013;5(4):227-38. doi: 10.1093/jmcb/mjt011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Thakur BK, Zhang H, Becker A, Matei I, Huang Y, Costa-Silva B, et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014;24(6):766-9. doi: 10.1038/cr.2014.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Salehi M, Sharifi M. Exosomal miRNAs as novel cancer biomarkers: challenges and opportunities. J Cell Physiol. 2018;233(9):6370-80. doi: 10.1002/jcp.26481 [DOI] [PubMed] [Google Scholar]
- 113. Thind A, Wilson C. Exosomal miRNAs as cancer biomarkers and therapeutic targets. J Extracell Vesicles. 2016;5:31292. doi: 10.3402/jev.v5.31292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hallal S, Azimi A, Wei H, Ho N, Lee MYT, Sim HW, et al. A comprehensive proteomic SWATH-MS workflow for profiling blood extracellular vesicles: a new avenue for glioma tumour surveillance. Int J Mol Sci. 2020;21(13):4754. doi: 10.3390/ijms21134754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Halvaei S, Daryani S, Eslami-S Z, Samadi T, Jafarbeik-Iravani N, Bakhshayesh TO, et al. Exosomes in cancer liquid biopsy: a focus on breast cancer. Mol Ther Nucleic Acids. 2018;10:131-41. doi: 10.1016/j.omtn.2017.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Zhang B, Yin Y, Lai RC, Tan SS, Choo ABH, Lim SK. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014;23(11):1233-44. doi: 10.1089/scd.2013.0479 [DOI] [PubMed] [Google Scholar]
- 117. Lee C, Alex MS, Aslam M, Vitali SH, Vergadi E, Konstantinou G, et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126(22):2601-11. doi: 10.1161/CIRCULATIONAHA.112.114173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kanazawa H, Fujimoto Y, Teratani T, Iwasaki J, Kasahara N, Negishi K, et al. Bone marrow-derived mesenchymal stem cells ameliorate hepatic ischemia reperfusion injury in a rat model. PLoS One. 2011;6(4):e19195. doi: 10.1371/journal.pone.0019195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lai RC, Arslan F, Lee MM, Choo A, Chen TS, Salto-Tellez M, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4(3):214-22. doi: 10.1016/j.scr.2009.12.003 [DOI] [PubMed] [Google Scholar]
- 120. Salomon C, Ryan J, Sobrevia L, Kobayashi M, Ashman K, Mitchell M, et al. Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PloS One. 2013;8(7):e68451. doi: 10.1371/journal.pone.0068451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Chen J, Liu Z, Hong MM, Zhang H, Chen C, Xiao M, et al. Proangiogenic compositions of microvesicles derived from human umbilical cord mesenchymal stem cells. PloS One. 2014;9(12):e115316. doi: 10.1371/journal.pone.0115316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Burke J, Kolhe R, Hunter M, Isales C, Hamrick M, Fulzele S. Stem cell-derived exosomes: a potential alternative therapeutic agent in orthopaedics. Stem Cells Int. 2016;2016:5802529. doi: 10.1155/2016/5802529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Zhang J, Guan J, Niu X, Hu G, Guo S, Li Q, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promotingcollagen synthesis and angiogenesis. J Transl Med. 2015;13:49. doi: 10.1186/s12967-015-0417-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. –US National Library of Medicine, ClinicalTrials.gov. Search results. Accessed September 3, 2020. https://clinicaltrials.gov/ct2/results?cond=exosomes&term=&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=
- 125. Kim HS, Choi DY, Yun SJ, Choi SM, Kang JW, Jung JW, et al. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J Proteome Res. 2012;11(2):839-49. doi: 10.1021/pr200682z [DOI] [PubMed] [Google Scholar]
- 126. Joo HS, Suh JH, Lee HJ, Bang ES, Lee JM. Current knowledge and future perspectives on mesenchymal stem cell-derived exosomes as a new therapeutic agent. Int J Mol Sci. 2020;21(3):727. doi: 10.3390/ijms21030727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Tan SHS, Wong JRY, Sim SJY, Tjio CKE, Wong KL, Chew JRJ, et al. Mesenchymal stem cell exosomes in bone regenerative strategies-a systematic review of preclinical studies. Mater Today Bio. 2020;7:100067. doi: 10.1016/j.mtbio.2020.100067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Liu X, Wang L, Ma C, Wang G, Zhang Y, Sun S. Exosomes derived from platelet-rich plasma present a novel potential in alleviating knee osteoarthritis by promoting proliferation and inhibiting apoptosis of chondrocyte via Wnt/β-catenin signaling pathway. J Orthop Surg. 2019;14(1_suppl):470. doi: 10.1186/s13018-019-1529-7 [DOI] [PMC free article] [PubMed] [Google Scholar]