Abstract
Objective
To evaluate effectiveness, in terms of patient-reported outcome measures, of platelet-rich plasma (PRP) injections for knee osteoarthritis compared to placebo and other intraarticular treatments.
Design
PubMed, Cochrane Library, Scopus, Embase, Web of Science, as well as the gray literature were searched on January 17, 2020. Randomized controlled trials (RCTs) comparing PRP injections with placebo or other injectable treatments, in any language, on humans, were included. Risk of bias was assessed following the Cochrane guidelines; quality of evidence was graded using the GRADE guidelines.
Results
Thirty-four RCTs, including 1403 knees in PRP groups and 1426 in control groups, were selected. WOMAC (Western Ontario and McMaster Universities Osteoarthritis Index) score favored PRP, with a statistically and clinically significant difference versus placebo at 12-month follow-up (P = 0.02) and versus HA (hyaluronic acid) at 6-month (P < 0.001) and 12-month (P < 0.001) follow-ups. A clinically significant difference favoring PRP versus steroids was documented for VAS (Visual Analogue Scale) pain (P < 0.001), KOOS (Knee Injury and Osteoarthritis Outcome Score) pain (P < 0.001), function in daily activities (P = 0.001), and quality of life (P < 0.001) at 6-month follow-up. However, superiority of PRP did not reach the minimal clinically important difference for all outcomes, and quality of evidence was low.
Conclusions
The effect of platelet concentrates goes beyond its mere placebo effect, and PRP injections provide better results than other injectable options. This benefit increases over time, being not significant at earlier follow-ups but becoming clinically significant after 6 to 12 months. However, although substantial, the improvement remains partial and supported by low level of evidence. This finding urges further research to confirm benefits and identify the best formulation and indications for PRP injections in knee OA.
Keywords: knee osteoarthritis, platelet-rich plasma, PRP, placebo, hyaluronic acid, steroids, meta-analysis
Introduction
Blood derivatives are increasingly used to modulate the intraarticular environment, aiming at reducing inflammatory distress and stimulating anabolism in different tissues. 1 In particular, products with an increased concentration of platelets have been developed to address osteoarthritis (OA), based on positive in vitro findings suggesting the potential of platelet-derived growth factors (GFs).2-5 In fact, platelets’ alpha granules constitute a reservoir of critical GFs, as well as cytokines, chemokines, and many other proteins; their dense granules store ADP, ATP, calcium ions, histamine, serotonin, and dopamine, and platelets also release antibacterial and fungicidal proteins that protect against infections.6-8 Platelet concentrates (platelet-rich plasma [PRP]) are therefore a simple, low-cost, and minimally invasive way to obtain a natural concentration of these GFs and bioactive molecules. 9
The theoretical benefits due to the cocktail of these molecules, their autologous nature, the lack of side-effect typical of other common on-the-shelf pharmaceuticals, 10 as well as the possibility to delay the progression of OA with positive results, as supported by preclinical evidence and promising clinical findings, have stimulated many physicians to include PRP in their practice as an alternative option to more traditional intraarticular products, such as corticosteroids and hyaluronic acid (HA).1,11,12 However, although positive results have been documented in several trials, a recent randomized controlled trial (RCT) questioned the real benefit of PRP and its claimed superiority compared to other intraarticular treatment, with a modest clinical improvement largely overlapping the one offered by viscosupplementation. 13 Moreover, while some outcomes have shown statistical superiority, it is not clear if the benefit reaches a minimal clinically important difference (MCID), 14 and therefore a real improvement perceived by patients, compared to the benefit of placebo or of other intraarticular options.
Thus, the aim of this meta-analysis was to evaluate the effectiveness of PRP compared with saline and other intraarticular treatments in knee OA patients in terms of patient-reported outcome measures. Moreover, further analysis investigated these results in terms of MCID to give clear indications on the benefits of PRP injections in the management of knee OA. The authors’ hypothesis was that PRP injection could provide better results compared with other injective treatments for knee OA.
Materials and Methods
Data Source and Searches
After the registration of the protocol on PROSPERO (CRD42019145409), the following databases were systematically searched on January 17, 2020, with no time limits and without any filters: PubMed, Cochrane Library, Scopus, Embase, Web of Science, and gray literature (isrctn.org, clinicaltrials.gov, and greylit.org) using the following string: ((PRP OR platelet rich plasma OR plasma rich in growth factors OR PRGF OR platelet derived growth factor OR platelet derived OR platelet gel OR platelet concentrate OR PRF OR platelet rich fibrin OR ACP OR autologous conditioned plasma OR APS OR autologous protein solution OR platelet lysate) AND (knee osteoarthritis)).
Study Selection
Duplicates were removed and, subsequently, all records were checked for eligibility by titles and abstracts. The full-text article was read in case not enough information could be retrieved from the abstract. The following inclusion criteria were used: RCTs (level I or II) comparing PRP injections with other intraarticular treatments or placebo, published in any language, and on humans. The Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines were used. 15 The article selection process was independently performed by 2 authors (DP, NF) with disagreement on study eligibility solved by a third author (GF).
Data Extraction and Quality Assessment
Information on trial methodology from all eligible trials included level of evidence, study design, PRP manufacturing method and characteristics, number of injections, comparative treatment, and follow-up. Information from all eligible trials on characteristics of the study population included the number of patients, OA level, sex, age, body mass index, inclusion/exclusion criteria, activity level, previous surgical treatments on the index knee, associated lesions, clinical scores, adverse events, and radiological results. Two authors independently extracted the trial information using a standardized extraction form (DP, FN). When possible, data were collected from the records; otherwise corresponding authors were contacted. The risk of bias was assessed using the revised tool for risk of bias in randomized trials (RoB 2.0) approved by the Cochrane collaboration group, which defines 3 categories: low risk, some concerns, and high risk. 16 The overall quality of evidence for each outcome was graded according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) guidelines for high, moderate, low, and very low levels of evidence. 17
Study Outcome and Statistical Analysis
The primary outcome of this meta-analysis was the overall Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score 6 and 12 months after the injections. Secondary outcomes were overall WOMAC score 1 month and 3 months after the injections, WOMAC subscores (pain, stiffness, function), pain measured on the visual analogue scale (VAS), International Knee Documentation Committee (IKDC) score, Knee injury and Osteoarthritis Outcome Score (KOOS), and responders’ rate at 1-, 3-, 6-, and 12-month follow-up. All the outcomes reported in at least 3 studies were plotted. Separate analyses were performed for each intraarticular approach compared with PRP. Sensitivity analyses including only double-blinded studies were performed. The mean differences between treatment groups were compared with the MCID reported in the literature for each score: 6.4/96 for overall WOMAC score, 1.5/20 for WOMAC pain score, 0.6/8 for WOMAC stiffness subscore, 4.6/68 for WOMAC function score, 1.37/10 for VAS pain score, 11.5/100 for IKDC score, and 10/100 for KOOS subscales.18-21
The effect of PRP and other intraarticular treatments was assessed by a z test on the pooled mean difference for continuous variables and on the pooled risk ratio for dichotomous variables. Heterogeneity was tested using Cochran’s Q statistic and I2 metric with I2 > 25% as the cutoff for the presence of significant heterogeneity: a fixed-effect model was used when I2 < 25%; otherwise, a random-effect model was preferred. The risk for publication bias was assessed using Begg’s funnel plot. A P value of 0.05 was considered significant. Sensitivity analyses were performed considering only double-blind trials. Analyses were performed using RevMan 5.3.
Results
Characteristics of Included Studies and Patients
A total of 34 RCTs were included out of 3277 records retrieved ( Fig. 1 ). The studies were published between 2011 and 2019 and consisted of 21 double-blind studies, 5 single-blind studies, 6 un-blinded studies, and 2 articles without a clear indication of their blinding procedure. PRP injections were compared with HA injections in 21 studies, saline in 8 studies (PRP vs. HA vs. saline in 2 studies), steroids in 6 studies (PRP vs. HA vs. steroids in 1 study), ozone in 2 studies (PRP vs. HA vs. ozone in 1 study), and prolotherapy (dextrose) in 1 study. The gray literature search retrieved 28 ongoing trials, 4 apparently completed but still unpublished trials, and 1 stopped trial.
Figure 1.
PRISMA flowchart of the study selection process.
In the included studies, 1403 patients underwent PRP injections, whereas 1426 were included in the control groups. Among these, 1314 and 1314 were followed until the last follow-up of the related studies in the PRP and control groups, respectively. The male/female ratio was 0.64 in the PRP groups and 0.60 in the control groups. The patients’ mean age ranged from 49.8 to 65.5 years in the PRP groups and from 46.6 to 68.0 years in the control groups. The mean body mass index ranged from 24.0 to 31.4 in the PRP groups and from 24.1 to 31.1 in the control groups. Differences in the baseline characteristics of the groups were present in the studies of Duymus et al. (higher baseline VAS in the HA group), 22 Filardo et al. (older patients in the HA group), 23 Lin et al. (higher body mass index in the HA group), 24 and Raeissadat et al. (older patient with a better baseline WOMAC score in the HA group). 25 Further details of included studies and patients are reported in Table 1 and Supplemental Table S1.
Table 1.
Patients Characteristics of the Included Studies.
| Study | Type of Control | Patients (Knees)
Included |
Patients (Knees)
Follow-up |
Sex |
Age |
BMI |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PRP | Control | PRP | Control | PRP | Control | PRP | Control | PRP | Control | ||
| Ahmad, 2018 59 | HA | 45 (45) | 45 (45) | 45 (45) | 44 (44) | M14, F31 | M15, F30 | 56.2 ± 6.8 | 56.8 ± 7.4 | 26.7 ± 3.6 | 26.5 ± 3.5 |
| Buendía-López, 2019 60 | HA | 35 (35) | 36 (36) | 33 (33) | 32 (32) | M16, F17 | M15, F17 | 56.2 ± 3 | 56.6 ± 2.9 | 24.9 ± 0.3 | 24.9 ± 0.4 |
| Cerza, 2012 61 | HA | 60 (60) | 60 (60) | 60 (60) | 60 (60) | M25, F35 | M28, F32 | 66.5 ± 11.3 | 66.2 ± 10.6 | NR | NR |
| Cole, 2016 53 | HA | 52 (52) | 59 (59) | 49 (49) | 50 (50) | M28, F21 | M20, F30 | 55.9 ± 10.4 | 56.8 ± 10.5 | 27.4 ± 3.9 | 29 ± 6.4 |
| Di Martino, 2019 13 | HA | 96 (96) | 93 (93) | 85 (85) | 82 (82) | M53, F32 | M47, F35 | 52.7 ± 13.2 | 57.5 ± 11.7 | 27.2 ± 7.6 | 26.8 ± 4.3 |
| Duymus, 2017 22 | HA or ozone | 41 (41) | HA 40 (40) Ozone 39 (39) |
33 (33) | HA 34 (34) Ozone 35 (35) |
M1, F32 | HA M1, F33 Ozone M4, F31 |
60.4 ± 5.1 | HA 60.3 ± 9.1 Ozone 59.4 ± 5.7 |
27.6 ± 4.6 | HA 28.4 ± 3.6 Ozone 27.6 ± 4.4 |
| Elik, 2019 62 | Saline | 30 (30) | 30 (30) | 30 (30) | 27 (27) | M1, F29 | M3, F24 | 61.3 ± 7.91 | 60.19 ± 6.8 | 30.37 ± 4.5 | 30.7 ± 4.0 |
| Filardo, 2015 23 | HA | 96 (96) | 96 (96) | 94 (94) | 89 (89) | M60, F34 | M52, F37 | 53.32 ± 13.2 | 57.55 ± 11.8 | 26.6 ± 4 | 26.9 ± 4.4 |
| Forogh, 2016 63 | CS | 24 (24) | 24 (24) | 23 (23) | 16 (16) | M7, F17 | M9, F15 | 59.13 ± 7.03 | 61.13 ± 6.7 | 28.9 ± 2.9 | 29.2 ± 3.4 |
| de Menezes Freire, 2018 64 | CS | 25 (25) | 25 (25) | 25 (25) | 25 (25) | NR | NR | 64.15 ± 8.02 | 60.2 ± 5.9 | 76% overweight | 88% overweight |
| Gaballa, 2019 28 | Ozone | 20 (20) | 20 (20) | 20 (20) | 20 (20) | M5, F15 | M4, F16 | 53.6 ± 4.6 | 56.3 ± 4.4 | NR | NR |
| Ghai, 2019 65 | Saline | 20 (20) | 20 (20) | 20 (20) | 20 (20) | M5, F15 | M5, F15 | 49.8 ± 9.4 | 49.8 ± 9.4 | NR | NR |
| Görmeli, 2017 66 | HA or saline | 46 (46) | Saline 45 (45) 3× HA 46 (46) |
39 (39) | Saline 40 (40) 3× HA 39 (39) |
M16, F23 | S M20, F20 HA M17, F22 |
53.7 ± 13.1 | S 52.8 ± 12.8 HA 53.5 ± 14 |
28.7 ± 4.8 | S 29.5 ± 3.2 HA 29.7 ± 3.7 |
| Montañez-Heredia, 2016 67 | HA | 28 (28) | 27 (27) | 27 (27) | 26 (26) | M12, F15 | M9, F17 | 66.3 ± 8.3 | 61.5 ± 8.6 | 29 ± 5.5 | 30.4 ± 4.9 |
| Huang, 2019 26 | HA or CS | 40 (40) | HA 40 (40) CS 40 (40) |
40 (40) | HA 40 (40) CS 40 (40) |
M25, F15 | HA M19, F21 CS M21, F19 |
54.5 ± 1.2 | HA 54.8 ± 1.1 CS 54.3 ± 1.4 |
25.2 ± 4.2 | HA 25.4 ± 3.1 CS 24.6 ± 3.6 |
| Joshi Jubert, 2017 68 | CS + anesthetic | 35 (35) | 30 (30) | 34 (34) | 30 (30) | M12, F23 | M6, F24 | 65.56 ± 8.6 | 68 ± 7.2 | 31.2 ± 4.4 | 31.0 ± 4.2 |
| Kon, 2017 33 | Saline | 31 (31) | 15 (15) | 29 (29) | 14 (14) | M18, F13 | M9, F6 | 57 (41-68) | 54 (44-67) | NR | NR |
| Lana, 2016 69 | HA | 36 (36) | 36 (36) | 36 (36) | 36 (36) | M7, F29 | M3, F33 | 60.9 ± 7 | 60 ± 6.6 | 27.4 ± 6.9 | 28.2 ± 8.8 |
| Lin, 2019 24 | HA or saline | 31 (31) | HA 29 (29) Saline 27 (27) |
30 (30) | HA 27 (27) Saline 26 (26) |
M9, F22 | HA M10, F19 S M10, F17 |
61.2 ± 13.1 | HA 62.5 ± 3.0 S 62.2 ± 3.1 |
24.0 ± 2.6 | HA 26.3 ± 3.0 S 25.0 ± 3.1 |
| Lisi, 2018 70 | HA | 31 (31) | 31 (31) | 31 (31) | 31 (31) | M20, F10 | M16, F12 | 53.5 ± 15.1 | 57.1 ± 10.0 | NR | NR |
| Louis, 2017 52 | HA | 28 (NR) | 28 (NR) | 17 (NR) | 17 (NR) | M14, F10 | M11, F13 | 53.2 ± 11.7 | 48.5 ± 11.5 | 25.6 ± 2.9 | 27.0 ± 2.9 |
| Nabi, 2018 71 | CS | 36 (36) | 36 (36) | 33 (33) | 34 (34) | M5, F28 | M7, F27 | 50.09 ± 7.79 | 58.6 ± 8.8 | 28.4 ± 2.8 | 27.8 ± 3.3 |
| Papalia, 2016 72 | HA | 24 (NR) | 24 (NR) | 23 (NR) | 24 (NR) | NR | NR | NR | NR | NR | NR |
| Patel, 2013 73 | Saline | 27 (54) | 26 (52) | 26 (52) | 23 (46) | M11, F16 | M6, F17 | 53.1 ± 11.6 | 53.7 ± 8.2 | 26.3 ± 3.2 | 26.2 ± 2.9 |
| Paterson, 2016 74 | HA | 12 (12) | 11 (11) | 10 (10) | 9 (9) | M8, F3 | M7, F3 | 49.9 ± 13.7 | 52.7 ± 10.3 | 27.9 ± 11.9 | 30.9 ± 5.6 |
| Raeissadat, 2015 25 | HA | 87 (87) | 73 (73) | 77 (77) | 62 (62) | M8, F69 | M15, F47 | 56.9 ± 9.1 | 61.1 ± 7.5 | 28.2 ± 4.6 | 27.0 ± 4.2 |
| Raeissadat, 2017 75 | HA | 41 (41) | 36 (36) | 36 (36) | 33 (33) | M7, F29 | M6, F27 | 57.0 ± 7.2 | 59.5 ± 7.5 | 28.6 ± 2.8 | 27.5 ± 2.9 |
| Rahimzadeh, 2018 29 | Dextrose | 21 (21) | 21 (21) | 21 (21) | 21 (21) | M10, F11 | M11, F10 | 65.5 ± 6.6 | 64.3 ± 5.3 | 28.6 ± 1.8 | 28.3 ± 1.9 |
| Sánchez, 2012 76 | HA | 89 (89) | 87 (87) | 79 (79) | 74 (74) | M43, F46 | M29, F45 | 60.5 ± 7.9 | 58.9 ± 8.2 | 27.9 ± 2.9 | 28.2 ± 2.7 |
| Smith, 2016 77 | Saline | 15 (15) | 15 (15) | 15 (15) | 15 (15) | M5, F10 | M6, F9 | 53.5 ± 8.2 | 46.6 ± 9.4 | 29.5 ± 6.9 | 27.5 ± 4.8 |
| Su, 2018 78 | HA | 26 (26) | 32 (32) | 25 (25) | 30 (30) | M11, F14 | M12, F18 | 54.2 ± 6.6 | 53.13 ± 6.4 | 28.17 ± 1.4 | 28.7 ± 1.1 |
| Güvendi, 2018 27 | CS | 19 (19) | 19 (19) | 19 (19) | 17 (17) | M1, F18 | M2, F15 | 62.3 ± 1.6 | 62.8 ± 1.7 | 31.4 ± 0.7 | 31.1 ± 1.0 |
| Vaquerizo, 2013 79 | HA | 48 (48) | 48 (48) | 48 (48) | 42 (42) | M16, F32 | M22, F26 | 62.4 ± 6.6 | 64.8 ± 7.7 | 30.7 ± 3.6 | 31.0 ± 4.6 |
| Wu, 2018 80 | Saline | 20 (20) | 20 (20) | 20 (20) | 20 (20) | M5, F15 | M1, F15 | 63.3 ± 6.8 | 63.3 ± 6.8 | 24.1 ± 2.9 | 24.1 ± 2.9 |
BMI = body mass index; PRP = platelet-rich plasma; HA = hyaluronic acid; M = males; F = females; NR = non-reported; CS = corticosteroid.
PRP versus Placebo
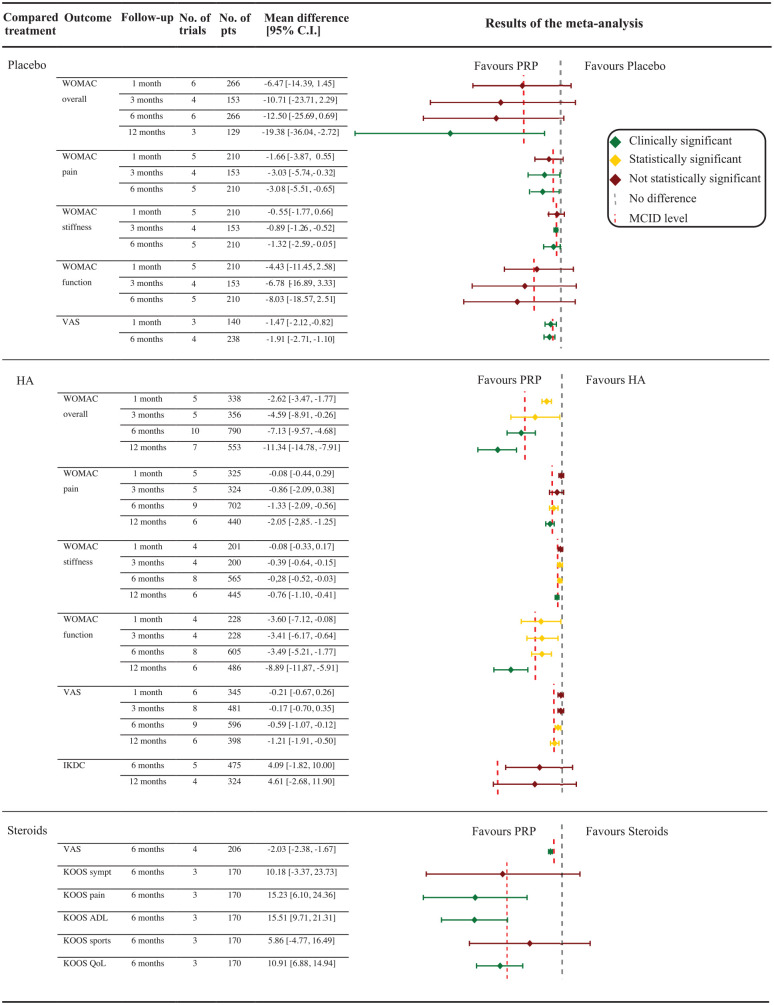
The overall WOMAC score at 6- and 12-month follow-ups, which was the primary outcome of this meta-analysis, showed that PRP improvement was not statistically higher than with placebo at 6-month follow-up, whereas a statistically as well as clinically significant difference favoring PRP was reported at 12-month follow-up (P = 0.02).
The analysis of the WOMAC score at earlier follow-ups showed a nonstatistically significant difference at 1 and 3 months. Considering the WOMAC subscores, in terms of WOMAC-pain, the benefit of PRP was statistically and clinically significant at 6-month follow-up (P = 0.02; P = 0.04) and nonstatistically significant at 1-month and 3-month follow-ups. With regard to WOMAC-stiffness, a statistically and clinically significant difference was documented at 3-month and 6-month follow-ups (P < 0.001; P = 0.04), whereas no significant difference was documented at all other follow-ups. No significant differences between treatments were documented in terms of WOMAC-function at any follow-up. A statistically and clinically significant difference favoring PRP against placebo was also documented in terms of VAS pain at 1-month and 6-month follow-ups (P < 0.001; P < 0.001). The meta-analysis on adverse events found no differences between PRP and placebo injections. Detailed results of this meta-analysis are reported in Figure 2 , whereas the forest plots of the different analyses are available in the supplementary data.
Figure 2.
Results of the meta-analysis for the different study outcomes.
PRP versus HA
The overall WOMAC score at 6-month and 12-month visits favors PRP with a statistically and clinically significant difference that improves over time (P < 0.001).
A statistically but not clinically significant difference favoring PRP was also documented for the overall WOMAC score at 1-month (P < 0.001) and 3-month follow-ups (P = 0.04). Similar results were reported in the different WOMAC subscores. Regarding the WOMAC-pain subscore, there was no difference at 1- and 3-month follow-ups, while a statistically significant difference was found at 6-month follow-up (P < 0.001) and a statistically and clinically significant difference was found after 12-month follow-up (P < 0.001). In terms of WOMAC-stiffness, no significant difference was documented after 1 month, a statistically significant difference was documented after 3 (P = 0.002) and 6 months (P = 0.03), and a difference that was both statistically and clinically significant was reported after 12 months (P < 0.001). For the WOMAC-function subscore, a statistically significant difference was documented at all follow-ups (P = 0.05; P = 0.02; P < 0.001; P < 0.001), but the benefit of PRP can be considered clinically relevant only at the 12-month visit. An improvement over time of the benefit of PRP over HA was also confirmed in terms of VAS pain with no difference at 1-month and 3-month follow-ups; however, a statistically but not clinically significant difference at 6-month follow-up (P = 0.01) and a statistically significant as well as clinically relevant benefit of PRP at 12-month follow-up (P < 0.001) were observed. A meta-analysis plotting the results for the IKDC score was possible only at 6-month and 12-month follow-ups and showed no statistically significant differences at both time points. The analysis of the responder rates was possible only for the 6-month follow-up and showed a responder rate of 60.8% (124/204) in the PRP group and 44.0% (84/191) in the HA group, without reaching a statistically significant difference between treatment groups. The analysis on adverse events reported no differences between PRP and HA injections, either overall, nor when specific adverse events, such as knee pain or knee swelling, where considered separately.
PRP versus Steroids
Only 2 studies comparing PRP versus steroid reported results in terms of WOMAC score at 6-month follow-up; thus, a meta-analysis was not possible. However, in both studies, a significant benefit of PRP was documented.26,27 Similarly, only one study reported the WOMAC score at 12-month follow-up with a clinically significant difference favoring PRP. 26
A statistically and clinically significant difference favoring PRP against steroids in terms of VAS-pain was documented at 6-month follow-up (P < 0.001). The comparison between PRP and steroids was also reported in terms of KOOS sub-scales at 6-month follow-up: statistically and clinically significant differences favoring PRP were documented for pain (P = 0.001), function in daily living (P < 0.001), and quality of life subscores (P < 0.001), whereas only a statistically significant difference was reported for function in sports, and no significant differences were reported for KOOS symptoms. The meta-analysis on adverse events was not possible due to lack of data.
PRP versus Other Intraarticular Options
The paucity of data hindered the possibility of performing a meta-analysis on the comparison between PRP and ozone and dextrose injections. However, in the studies comparing PRP and ozone injections,22,28 as well as PRP and dextrose injections, 29 a statistically and clinically significant difference favoring PRP was documented for all the outcomes.
Sensitivity Analyses, Risk of Bias, and Quality of Evidence
The sensitivity analyses including only results of double-blind RCTs confirmed most of the results of the overall analysis (Suppl. Table S2).
The risk of bias was low in 7 studies, whereas there were some concerns regarding 27 studies (Suppl. Table S1). The main reasons for these concerns are an unclear method to guarantee allocation concealment and the impossibility of retrieving a published protocol aimed at preventing the risk of selective reporting bias.
The level of evidence was low in almost all the outcomes, with only 5 outcomes with a very low level of evidence (Suppl. Table S2). The main reasons to downgrade the level of evidence were inconsistency due to the high heterogeneity of results and imprecision due to the limited numbers of patients included in these comparisons. The risk of bias of the included studies was judged not to significantly influence results when they were confirmed by double-blind RCTs. No points were lost for indirectness, and no evidence of publication bias was present.
Discussion
The main finding of this meta-analysis is that platelet concentrates offer a benefit that, although not significant at the earlier follow-ups, exceeds over time both the placebo effect and the improvement offered by other intraarticular options at 12 months, without an increased risk of adverse events. This finding is supported not only by the primary outcome but also by secondary evaluations. In fact, scales focused on function, pain, or other symptoms converge into one common trend: while no difference was observed in the first months after the injection, PRP benefit became evident starting from 6 months and increased up to the 12-month follow-up being statistically and clinically significant. This is an important finding, as patient-perceived benefit is a key aspect when choosing a treatment strategy.
Intraarticular treatments are a common approach for the management of knee degeneration, especially in early/moderate OA. Various substances have been proposed to obtain clinical improvement and possibly a disease-modifying effect; among these, corticosteroids and HA are routinely applied in clinical practice.10,30 However, not one of these substances has shown the characteristics of an ideal treatment. This explains the search for a new solution to improve joint status, 31 with platelet concentrates being proposed as intraarticular treatment to address joint degeneration.10,32 In this landscape, this meta-analysis allows us to draw important conclusions. First, an overall superiority versus saline was demonstrated, although it was mainly driven by pain-related measures more than by functional scales. Important placebo effects have been observed in almost every knee injection study and are even greater in biologic trials where patients perceive they are getting a “regenerative medicine.”33,34 However, while placebo plays an important role in PRP results, as demonstrated by the similar outcome compared to saline up to 6 months, the PRP benefit exceeds the mere placebo effect. Second, the comparison with other intraarticular treatments showed that the superiority of platelet concentrates should not be regarded as greater improvements but rather as longer lasting effects, with differences becoming apparent over time when the effects of placebo or other intraarticular treatments wear out. Third, the documented improvement is significant, not only statistically but also clinically, and thus the benefit is substantial and perceived by patients at 12 months.
The conclusions of this meta-analysis strengthen some findings of previous attempts to quantify PRP results while questioning others. Most of the previous meta-analyses focused on a smaller number of trials or included lower level trials, which weakened their findings supporting PRP benefit over placebo or HA. More recent attempts also presented methodological limitations, such as the one of Hohmann et al., who found no advantages of PRP over HA for clinical outcomes at both 6 and 12 months. However, they pooled all the different clinical outcomes together (such as VAS, WOMAC, KOOS, and IKDC) not accounting for the intrinsic differences of these scores. 35 Performing separate analyses for the different score and subscores, the present meta-analysis adds new insights to the field, taking further advantage of newly published high-level trials to provide enough data and separately address the different aspects related to patient symptomatology, thus deriving important points that other less updated meta-analyses failed to clarify.36-39 Moreover, previous literature analyses were focused on the comparison between PRP and HA, 39 thus missing the chance to address the questions on the placebo effect frequently raised in the field of intraarticular injections. The inclusion of a higher number of RCTs and of a broader range of comparator gave the possibility to better understand the role of PRP in the field of injectables for knee OA, with further insights also by including a comparative evaluation of the improvement in terms of MCID, which was proven significant versus placebo, as well as versus corticosteroids and HA.
This is an interesting finding considering that preclinical literature suggests a homeostatic improvement of the articular environment rather than a long-lasting regenerative effect. 1 However, the homeostatic effect can affect the imbalance between pro- and anti-inflammatory cytokines that leads to the perpetuation of inflammatory pathways. 40 As inflammation is a key OA feature associated with both joint symptoms and disease progression, anti-inflammatory approaches could counteract this key mechanism of disease progression. Other mechanisms can play an important role in the effects of blood derivatives.3,41 PRP might influence the entire joint environment, and synoviocytes are affected by platelet releasate as well as by meniscal cells and mesenchymal stem cells. Moreover, the chemo-attractant activity of PRP may contribute to the recruitment of other cells that might participate to the overall effect. 42 This milieu of actions could lead to anabolic effects, to an overall down-modulation of the joint inflammation, and might positively influence chondrocyte apoptosis, which could explain the clinical benefit beyond the earliest follow-ups. 43 In this light, PRP might not lead to hyaline cartilage regeneration, but it still might offer a clinical benefit with symptomatic and functional improvement and possibly a slowdown of the degenerative processes.
This meta-analysis pooled the overall effects reported for PRP. This leads to an oversimplification that, while offering interesting insights on the potential of this biological approach, fails to address the complexity of this field. Depending on the method of preparation, blood derivatives have been defined differently and include PRP, leukocyte-rich PRP, platelet-rich fibrin, PRP rich in GFs, platelet-rich fibrin matrix, autologous concentrated plasma, pure PRP, platelet releasate, platelet gel, autologous conditioned plasma, autologous protein solution, and so on. All these platelet products have varying concentrations of blood cells, plasma, or fibrinogen and, therefore, varying concentrations of GFs and bioactive molecules. It would not be surprising if the biological properties of those products could vary accordingly and impact their efficacy in knee OA.44,45 In particular, leukocytes are currently the most debated aspect, as in vitro experiments have revealed the stimulation of catabolic and pro-inflammatory molecule release.46,47 Nonetheless, a recent clinical study did not confirm an increase in the concentration of inflammatory molecules in the synovial fluid. 6 Moreover, the only available comparative trial revealed similar results when using leukocyte-rich and leukocyte-poor formulations, and recently good results have also been reported by exploiting the pleiotropic effects of leukocyte-rich concentrates, leaving the question regarding the in vivo role of leukocytes still open. 48 In this light, it is important to underline that, while the advantages of leukocyte-poor preparations have been previously suggested, 49 those claims are based on indirect comparisons with an inherently high risk of bias. Furthermore, the exact composition of PRP is not properly reported in many of the available studies, and the definitions themselves are often heterogeneous, grouping PRPs with normal leukocyte values with those with complete leukocyte depletion. PRP products should be better characterized in future trials to understand the role of leukocytes. Overall, the evidence on this issue is still weak, and new high-level studies, directly comparing different PRP formulations, are needed to perform reliable analysis and draw clear conclusions on this topic. To this purpose, such RCTs should provide overall similar conditions while differing only for the presence or not of leukocyte, as many different aspects could otherwise contribute to the observed results.
In fact, other than the leukocyte content, protocols differ in terms of blood volume harvested, use of anticoagulant, number and speed of centrifugations, final volume obtained, overall number of platelets, their integrity and activation method, in addition to the possibility of cryopreserving them or using fresh products, all factors that could influence the properties of the releasate.44,50 Moreover, different application modalities have been reported, with single injections or injection cycles and different volumes and concentrations finally leading to the administration of heterogeneous doses of releasate. This could be an important aspect since preclinical and clinical studies suggested the importance of GF dosage.51,52 Beyond the variation in product content and its applicative modalities, also the selection of the patient to be treated may play a key role. Although the reported data were not suitable for a subanalysis based on the type of patient in this meta-analysis, literature reports suggested that patients with a more advanced OA tend to have less benefit.1,53,54 This situation may be due not only to the more compromised conditions and less responsiveness of joint tissues in advanced OA but also to their lower biological potential. In fact, factors that may suppress chondrocyte matrix synthesis and promote macrophage inflammation in vitro have been shown in platelet concentrates of older individuals with OA. 55
All the aforementioned factors increase heterogeneity in the field of PRP injections 56 for knee OA. There is an urgent need for more standardized procedures 5 and for the identification of patient subgroups that could benefit more from this treatment. 44 The lack of standardization is a limitation of the current literature and is reflected by this meta-analysis, which could not give further indications besides an overall positive effect. Further analyses are hindered by the lack of key data, the heterogeneous reports on the available data, and the lack of high level trials to investigate specific aspects in terms of PRP formulations and more suitable targets. All these aspects should be addressed to avoid the risk of drawing misleading conclusions. Moreover, also within the available RCTs, as reported in the Results section, the level of evidence for some of the outcomes is still low since they were documented in a small number of trials, which were often underpowered. Only 20 out of 33 studies were double blinded: given the relevance of the placebo effect in the field of knee injections, 34 this factor could have influenced the results, although the overall results were in line with those from the sensitivity analysis of double-blind trials. Finally, the MCID is primarily intended as a measure of clinically significant improvement in a patient undergoing a specific intervention, and the high variability in the MCIDs reported in the literature 57 suggests some caution when considering it in regard to the mean change in a heterogeneous population. Nonetheless, the MCID is increasingly used to interpret the relevance of the difference documented in a quantitative synthesis. 58
This meta-analysis showed that platelet concentrates offer an overall significant clinical improvement, which overcomes the mere placebo effect and results obtained with other intraarticular options 12 months after treatment. More controversial is the comparison at earlier follow-ups, for which further double-blind, high-level RCTs are needed. In this line, the benefit of platelet concentrates versus other options should not be regarded as a greater improvement but rather as a longer lasting effect. This benefit is, on one hand, statistically and clinically significant and therefore substantial and perceived by patients, but on the other hand, it is still just a partial improvement, far from reaching the full scores foreseen by symptoms and functional scales. This fact should be considered by both patients and physicians in order for them to have realistic expectations when considering this biological approach as intraarticular treatment for knee OA. Nonetheless, the effect of platelet concentrates goes beyond mere placebo effect, and PRP injections provide better results than other injective options. This benefit increases over time, as it is not significant at the earliest follow-up while it becomes clinically significant at 12 months. However, although substantial, the improvement remains partial and supported by low-quality evidence, urging further research to confirm benefits and to identify the best formulation and indications for PRP injections in knee OA.
Supplemental Material
Supplemental material, Supplementary_File for PRP Injections for the Treatment of Knee Osteoarthritis: A Meta-Analysis of Randomized Controlled Trials by Giuseppe Filardo, Davide Previtali, Francesca Napoli, Christian Candrian, Stefano Zaffagnini and Alberto Grassi in CARTILAGE
Footnotes
Supplementary material for this article is available on the Cartilage website at https://journals.sagepub.com/home/cara.
Authors’ Note: The study was performed at the Ospedale Regionale di Lugano, EOC, Lugano, Switzerland, and the IRCCS Istituto Ortopedico Rizzoli, Bologna, Italy. Christian Candrian is also affiliated with USI - Università della Svizzera Italiana, Facoltà di scienze biomediche, Lugano, Switzerland.
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article. All authors declare no support from any organization for the submitted work. CC has received institutional support outside the present work from Medacta International SA, Johnson & Johnson, Lima Corporate, Zimmer Biomet, and Oped AG. SZ received personal fees from I+ SRL, research grants outside the submitted work from Fidia Farmaceutici SPA, CartiHeal Ltd, IGEA Clinical Biophysics, BIOMET, and Kensey Nash, and has a Springer patent with royalties paid.
Ethical Approval: Ethical approval was not sought for the present study because it is a systematic review with meta-analysis and does not directly involve patients.
Informed Consent: Informed consent was not sought for the present study because it is a systematic review with meta-analysis and does not directly involve patients.
Trial Registration: CRD42019145409 PROSPERO.
ORCID iD: Davide Previtali  https://orcid.org/0000-0002-0284-4368
https://orcid.org/0000-0002-0284-4368
References
- 1. Filardo G, Kon E, Roffi A, Di Matteo B, Merli ML, Marcacci M. Platelet-rich plasma: why intra-articular? A systematic review of preclinical studies and clinical evidence on PRP for joint degeneration. Knee Surg Sports Traumatol Arthrosc. 2015;23(9):2459-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gilbertie JM, Long JM, Schubert AG, Berglund AK, Schaer TP, Schnabel LV. Pooled platelet-rich plasma lysate therapy increases synoviocyte proliferation and hyaluronic acid production while protecting chondrocytes from synoviocyte-derived inflammatory mediators. Front Vet Sci. 2018;5:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sundman EA, Cole BJ, Karas V, Della Valle C, Tetreault MW, Mohammed HO, et al. The anti-inflammatory and matrix restorative mechanisms of platelet-rich plasma in osteoarthritis. Am J Sports Med. 2014;42(1_suppl):35-41. [DOI] [PubMed] [Google Scholar]
- 4. Chen WH, Lin CM, Huang CF, Hsu WC, Lee CH, Ou KL, et al. Functional recovery in osteoarthritic chondrocytes through hyaluronic acid and platelet-rich plasma-inhibited infrapatellar fat pad adipocytes. Am J Sports Med. 2016;44(10):2696-705. [DOI] [PubMed] [Google Scholar]
- 5. Gato-Calvo L, Hermida-Gomez T, Romero CR, Burguera EF, Blanco FJ. Anti-inflammatory effects of novel standardized platelet rich plasma releasates on knee osteoarthritic chondrocytes and cartilage in vitro. Curr Pharm Biotechnol. 2019;20:920-33. [DOI] [PubMed] [Google Scholar]
- 6. Mariani E, Canella V, Cattini L, Kon E, Marcacci M, Di Matteo B, et al. Leukocyte-rich platelet-rich plasma injections do not up-modulate intra-articular pro-inflammatory cytokines in the osteoarthritic knee. PLoS One. 2016;11(6):e0156137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mariani E, Canella V, Berlingeri A, Bielli A, Cattini L, Landini MP, et al. Leukocyte presence does not increase microbicidal activity of platelet-rich plasma in vitro. BMC Microbiol. 2015;15:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37(11):2259-72. [DOI] [PubMed] [Google Scholar]
- 9. Kon E, Filardo G, Di Martino A, Marcacci M. Platelet-rich plasma (PRP) to treat sports injuries: evidence to support its use. Knee Surg Sports Traumatol Arthrosc. 2011;19(4):516-27. [DOI] [PubMed] [Google Scholar]
- 10. Filardo G, Kon E, Longo UG, Madry H, Marchettini P, Marmotti A, et al. Non-surgical treatments for the management of early osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1775-85. [DOI] [PubMed] [Google Scholar]
- 11. Kwon DR, Park GY, Lee SU. The effects of intra-articular platelet-rich plasma injection according to the severity of collagenase-induced knee osteoarthritis in a rabbit model. Ann Rehabil Med. 2012;36(4):458-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khatab S, van Buul GM, Kops N, Bastiaansen-Jenniskens YM, Bos PK, Verhaar JA, et al. Intra-articular injections of platelet-rich plasma releasate reduce pain and synovial inflammation in a mouse model of osteoarthritis. Am J Sports Med. 2018;46(4):977-86. [DOI] [PubMed] [Google Scholar]
- 13. Di Martino A, Di Matteo B, Papio T, Tentoni F, Selleri F, Cenacchi A, et al. Platelet-rich plasma versus hyaluronic acid injections for the treatment of knee osteoarthritis: results at 5 years of a double-blind, randomized controlled trial. Am J Sports Med. 2019;47(2):347-54. [DOI] [PubMed] [Google Scholar]
- 14. Redelmeier DA, Lorig K. Assessing the clinical importance of symptomatic improvements: an illustration in rheumatology. JAMA Intern Med. 1993;153(11):1337-42. [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-9. [DOI] [PubMed] [Google Scholar]
- 16. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 17. Ryan R, Hill S. How to GRADE the quality of the evidence. Accessed May 15, 2020. https://colorectal.cochrane.org/sites/colorectal.cochrane.org/files/public/uploads/how_to_grade.pdf
- 18. Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45(4):384-91. [DOI] [PubMed] [Google Scholar]
- 19. Saltzman BM, Leroux T, Meyer MA, Basques BA, Chahal J, Bach BR, Jr, et al. The therapeutic effect of intra-articular normal saline injections for knee osteoarthritis: a meta-analysis of evidence level 1 studies. Am J Sports Med. 2017;45(11):2647-53. [DOI] [PubMed] [Google Scholar]
- 20. Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1(1_suppl):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Irrgang JJ, Anderson AF, Boland AL, Harner CD, Neyret P, Richmond JC, et al. ; International Knee Documentation Committee. Responsiveness of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med. 2006;34(10):1567-73. [DOI] [PubMed] [Google Scholar]
- 22. Duymus TM, Mutlu S, Dernek B, Komur B, Aydogmus S, Kesiktas FN. Choice of intra-articular injection in treatment of knee osteoarthritis: platelet-rich plasma, hyaluronic acid or ozone options. Knee Surg Sports Traumatol Arthrosc. 2017;25(2):485-92. [DOI] [PubMed] [Google Scholar]
- 23. Filardo G, Di Matteo B, Di Martino A, Merli ML, Cenacchi A, Fornasari P, et al. Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med. 2015;43(7):1575-82. [DOI] [PubMed] [Google Scholar]
- 24. Lin KY, Yang CC, Hsu CJ, Yeh ML, Renn JH. Intra-articular injection of platelet-rich plasma is superior to hyaluronic acid or saline solution in the treatment of mild to moderate knee osteoarthritis: a randomized, double-blind, triple-parallel, placebo-controlled clinical trial. Arthroscopy. 2019;35(1_suppl):106-17. [DOI] [PubMed] [Google Scholar]
- 25. Raeissadat SA, Rayegani SM, Hassanabadi H, Fathi M, Ghorbani E, Babaee M, et al. Knee osteoarthritis injection choices: platelet-rich plasma (PRP) versus hyaluronic acid (a one-year randomized clinical trial). Clin Med Insights Arthritis Musculoskelet Disord. 2015;8:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang Y, Liu X, Xu X, Liu J. Intra-articular injections of platelet-rich plasma, hyaluronic acid or corticosteroids for knee osteoarthritis. Orthopäde. 2019;48(3):239-47. [DOI] [PubMed] [Google Scholar]
- 27. Güvendi EU, Aşkin A, Güvendi G, Koçyiğit H. Comparison of efficiency between corticosteroid and platelet rich plasma injection therapies in patients with knee osteoarthritis. Arch Rheumatol. 2018;33(3):273-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gaballa NM, Mohammed YA, Kamel LM, Mahgoub HM. Therapeutic efficacy of intra-articular injection of platelet–rich plasma and ozone therapy in patients with primary knee osteoarthritis. Egyptian Rheumatol. 2019;41(3):183-7. [Google Scholar]
- 29. Rahimzadeh P, Imani F, Faiz SHR, Entezary SR, Zamanabadi MN, Alebouyeh MR. The effects of injecting intra-articular platelet-rich plasma or prolotherapy on pain score and function in knee osteoarthritis. Clin Interv Aging. 2018;13:73-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bannuru RR, Schmid CH, Kent DM, Vaysbrot EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1_suppl):46-54. [DOI] [PubMed] [Google Scholar]
- 31. Pas HI, Winters M, Haisma HJ, Koenis MJ, Tol JL, Moen MH. Stem cell injections in knee osteoarthritis: a systematic review of the literature. Br J Sports Med. 2017;51(15):1125-33. [DOI] [PubMed] [Google Scholar]
- 32. Dai WL, Zhou AG, Zhang H, Zhang J. Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Arthroscopy. 2017;33(3):659-70. [DOI] [PubMed] [Google Scholar]
- 33. Kon E, Engebretsen L, Verdonk P, Nehrer S, Filardo G. Clinical outcomes of knee osteoarthritis treated with an autologous protein solution injection: a 1-year pilot double-blinded randomized controlled trial. Am J Sports Med. 2018;46(1_suppl):171-80. [DOI] [PubMed] [Google Scholar]
- 34. Previtali D, Merli G, Di Laura Frattura G, Candrian C, Zaffagnini S, Filardo G. The long-lasting effects of “placebo injections” in knee osteoarthritis: a meta-analysis. Cartilage. Epub Mar 18, 2020. doi: 10.1177/1947603520906597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hohmann E, Tetsworth K, Glatt V. Is platelet-rich plasma effective for the treatment of knee osteoarthritis? A systematic review and meta-analysis of level 1 and 2 randomized controlled trials. Eur J Orthop Surg Traumatol. Epub Feb 14, 2020. doi: 10.1007/s00590-020-02623-4 [DOI] [PubMed] [Google Scholar]
- 36. Phillips M, Vannabouathong C, Devji T, Patel R, Gomes Z, Patel A, et al. Differentiating factors of intra-articular injectables have a meaningful impact on knee osteoarthritis outcomes: a network meta-analysis. Knee Surg Sports Traumatol Arthrosc. Epub Jan 3, 2020. doi: 10.1007/s00167-019-05763-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen P, Huang L, Ma Y, Zhang D, Zhang X, Zhou J, et al. Intra-articular platelet-rich plasma injection for knee osteoarthritis: a summary of meta-analyses. J Orthop Surg Res. 2019;14(1_suppl):385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wu Q, Luo X, Xiong Y, Liu G, Wang J, Chen X, et al. Platelet-rich plasma versus hyaluronic acid in knee osteoarthritis: a meta-analysis with the consistent ratio of injection. J Orthop Surg (Hong Kong). 2020;28(1_suppl):2309499019887660. [DOI] [PubMed] [Google Scholar]
- 39. Han Y, Huang H, Pan J, Lin J, Zeng L, Liang G, et al. Meta-analysis comparing platelet-rich plasma vs hyaluronic acid injection in patients with knee osteoarthritis. Pain Med. 2019;20(7):1418-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lieberthal J, Sambamurthy N, Scanzello CR. Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2015;23(11):1825-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee HR, Park KM, Joung YK, Park KD, Do SH. Platelet-rich plasma loaded hydrogel scaffold enhances chondrogenic differentiation and maturation with up-regulation of CB1 and CB2. J Control Release. 2012;159(3):332-7. [DOI] [PubMed] [Google Scholar]
- 42. Kruger JP, Hondke S, Endres M, Pruss A, Siclari A, Kaps C. Human platelet-rich plasma stimulates migration and chondrogenic differentiation of human subchondral progenitor cells. J Orthop Res. 2012;30(6):845-52. [DOI] [PubMed] [Google Scholar]
- 43. Huang G, Hua S, Yang T, Ma J, Yu W, Chen X. Platelet-rich plasma shows beneficial effects for patients with knee osteoarthritis by suppressing inflammatory factors. Exp Ther Med. 2018;15(3):3096-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Harrison P; Subcommittee on Platelet Physiology. The use of platelets in regenerative medicine and proposal for a new classification system: guidance from the SSC of the ISTH. J Thromb Haemost. 2018;16(9):1895-900. [DOI] [PubMed] [Google Scholar]
- 45. Mei-Dan O, Mann G, Maffulli N. Platelet-rich plasma: any substance into it? Br J Sports Med. 2010;44(9):618-9. [DOI] [PubMed] [Google Scholar]
- 46. Braun HJ, Kim HJ, Chu CR, Dragoo JL. The effect of platelet-rich plasma formulations and blood products on human synoviocytes: implications for intra-articular injury and therapy. Am J Sports Med. 2014;42(5):1204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39(10):2135-40. [DOI] [PubMed] [Google Scholar]
- 48. Filardo G, Kon E, Pereira Ruiz MT, Vaccaro F, Guitaldi R, Di Martino A, et al. Platelet-rich plasma intra-articular injections for cartilage degeneration and osteoarthritis: single- versus double-spinning approach. Knee Surg Sports Traumatol Arthrosc. 2012;20(10):2082-91. [DOI] [PubMed] [Google Scholar]
- 49. Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med. 2015;44:792-800. [DOI] [PubMed] [Google Scholar]
- 50. Roffi A, Filardo G, Assirelli E, Cavallo C, Cenacchi A, Facchini A, et al. Does platelet-rich plasma freeze-thawing influence growth factor release and their effects on chondrocytes and synoviocytes? Biomed Res Int. 2014;2014:692913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chouhan DK, Dhillon MS, Patel S, Bansal T, Bhatia A, Kanwat H. Multiple platelet-rich plasma injections versus single platelet-rich plasma injection in early osteoarthritis of the knee: an experimental study in a Guinea pig model of early knee osteoarthritis. Am J Sports Med. 2019;47(10):2300-7. [DOI] [PubMed] [Google Scholar]
- 52. Louis ML, Magalon J, Jouve E, Bornet CE, Mattei JC, Chagnaud C, et al. Growth factors levels determine efficacy of platelets rich plasma injection in knee osteoarthritis: a randomized double blind noninferiority trial compared with viscosupplementation. Arthroscopy. 2018;34(5):1530-40. [DOI] [PubMed] [Google Scholar]
- 53. Cole BJ, Karas V, Hussey K, Pilz K, Fortier LA. Hyaluronic acid versus platelet-rich plasma: a prospective, double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. 2017;45(2):339-46. [DOI] [PubMed] [Google Scholar]
- 54. Kon E, Mandelbaum B, Buda R, Filardo G, Delcogliano M, Timoncini A, et al. Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: from early degeneration to osteoarthritis. Arthroscopy. 2011;27(11):1490-501. [DOI] [PubMed] [Google Scholar]
- 55. O’Donnell C, Migliore E, Grandi FC, Koltsov J, Lingampalli N, Cisar C, et al. Platelet-rich plasma (PRP) from older males with knee osteoarthritis depresses chondrocyte metabolism and upregulates inflammation. J Orthop Res. 2019;37(8):1760-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bennell KL, Hunter DJ, Paterson KL. Platelet-rich plasma for the management of hip and knee osteoarthritis. Curr Rheumatol Rep. 2017;19(5):24. [DOI] [PubMed] [Google Scholar]
- 57. Olsen MF, Bjerre E, Hansen MD, Tendal B, Hilden J, Hróbjartsson A. Minimum clinically important differences in chronic pain vary considerably by baseline pain and methodological factors: systematic review of empirical studies. J Clin Epidemiol. 2018;101:87-106. [DOI] [PubMed] [Google Scholar]
- 58. Johnston BC, Thorlund K, da Costa BR, Furukawa TA, Guyatt GH. New methods can extend the use of minimal important difference units in meta-analyses of continuous outcome measures. J Clin Epidemiol. 2012;65:817-26. [DOI] [PubMed] [Google Scholar]
- 59. Ahmad HS, Farrag SE, Okasha AE, Kadry AO, Ata TB, Monir AA, et al. Clinical outcomes are associated with changes in ultrasonographic structural appearance after platelet-rich plasma treatment for knee osteoarthritis. Int J Rheum Dis. 2018;21(5):960-6. [DOI] [PubMed] [Google Scholar]
- 60. Buendía-López D, Medina-Quirós M, Fernández-Villacañas Marín MÁ. Clinical and radiographic comparison of a single LP-PRP injection, a single hyaluronic acid injection and daily NSAID administration with a 52-week follow-up: a randomized controlled trial. J Orthop Traumatol. 2018;19(1_suppl):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cerza F, Carnì S, Carcangiu A, Di Vavo I, Schiavilla V, Pecora A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40(12):2822-7. [DOI] [PubMed] [Google Scholar]
- 62. Elik H, Doğu B, Yılmaz F, Begoğlu FA, Kuran B. Platelet rich plasma in osteoarthritis the efficiency of platelet rich plasma treatment in patients with knee osteoarthritis. J Back Musculoskelet Rehabil. 2020;33:127-38. [DOI] [PubMed] [Google Scholar]
- 63. Forogh B, Mianehsaz E, Shoaee S, Ahadi T, Raissi GR, Sajadi S. Effect of single injection of platelet-rich plasma in comparison with corticosteroid on knee osteoarthritis: a double-blind randomized clinical trial. J Sports Med Phys Fitness. 2016;56(7-8):901-8. [PubMed] [Google Scholar]
- 64. de Menezes Freire MR, da Silva PMC, Azevedo AR, Silva DS, da Silva RBB, Cardoso JC. Efeito comparativo entre a infiltração de plasma rico em plaquetas e o uso de corticosteroides no tratamento de osteoartrite do joelho: estudo clínico prospectivo e randomizado. Rev Bras Ortop. 2018. doi: 10.1016/j.rbo.2018.01.001 [DOI] [Google Scholar]
- 65. Ghai B, Gupta V, Jain A, Goel N, Chouhan D, Batra YK. Effectiveness of platelet rich plasma in pain management of osteoarthritis knee: double blind, randomized comparative study [in Portuguese]. Rev Bras Anestesiol. 2019;69(5):439-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Görmeli G, Görmeli CA, Ataoglu B, Çolak C, Aslantürk O, Ertem K. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2017;25(3):958-65. [DOI] [PubMed] [Google Scholar]
- 67. Montañez-Heredia E, Irízar S, Huertas P, Otero E, del Valle M, Prat I, et al. Intra-articular injections of platelet-rich plasma versus hyaluronic acid in the treatment of osteoarthritic knee pain: a randomized clinical trial in the context of the Spanish National Health Care System. Int J Mol Sci. 2016;17(7):E1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Joshi Jubert N, Rodríguez L, Reverté-Vinaixa MM, Navarro A. Platelet-rich plasma injections for advanced knee osteoarthritis: a prospective, randomized, double-blinded clinical trial. Orthop J Sports Med. 2017;5(2):2325967116689386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lana JF, Weglein A, Sampson SE, Vicente EF, Huber SC, Souza CV, et al. Randomized controlled trial comparing hyaluronic acid, platelet-rich plasma and the combination of both in the treatment of mild and moderate osteoarthritis of the knee. J Stem Cells Regen Med. 2016;12(2):69-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lisi C, Perotti C, Scudeller L, Sammarchi L, Dametti F, Musella V, et al. Treatment of knee osteoarthritis: platelet-derived growth factors vs. hyaluronic acid. A randomized controlled trial. Clin Rehabil. 2018;32(3):330-9. [DOI] [PubMed] [Google Scholar]
- 71. Nabi BN, Sedighinejad A, Mardani-Kivi M, Haghighi M, Roushan ZA, Biazar G. Comparing the effectiveness of intra-articular platelet-rich plasma and corticosteroid injection under ultrasound guidance on pain control of knee osteoarthritis. Iran Red Crescent Med J. 2018;20(3):e62157. [Google Scholar]
- 72. Papalia R, Zampogna B, Russo F, Vasta S, Tirindelli M, Nobile C, et al. Comparing hybrid hyaluronic acid with PRP in end career athletes with degenerative cartilage lesions of the knee. J Biol Regul Homeost Agents. 2016;30(4 Suppl. 1):17-23. [PubMed] [Google Scholar]
- 73. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356-64. [DOI] [PubMed] [Google Scholar]
- 74. Paterson KL, Nicholls M, Bennell KL, Bates D. Intra-articular injection of photo-activated platelet-rich plasma in patients with knee osteoarthritis: a double-blind, randomized controlled pilot study. BMC Musculoskelet Disord. 2016;17(1_suppl):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Raeissadat SA, Rayegani SM, Ahangar AG, Abadi PH, Mojgani P, Ahangar OG. Efficacy of intra-articular injection of a newly developed plasma rich in growth factor (PRGF) versus hyaluronic acid on pain and function of patients with knee osteoarthritis: a single-blinded randomized clinical trial. Clin Med Insights Arthritis Musculoskelet Disord. 2017;10:1179544117733452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sánchez M, Fiz N, Azofra J, Usabiaga J, Recalde EA, Gutierrez AG, et al. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy. 2012;28(8):1070-8. [DOI] [PubMed] [Google Scholar]
- 77. Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee osteoarthritis: an FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44(4):884-91. [DOI] [PubMed] [Google Scholar]
- 78. Su K, Bai Y, Wang J, Zhang H, Liu H, Ma S. Comparison of hyaluronic acid and PRP intra-articular injection with combined intra-articular and intraosseous PRP injections to treat patients with knee osteoarthritis. Clin Rheumatol. 2018;37(5):1341-50. [DOI] [PubMed] [Google Scholar]
- 79. Vaquerizo V, Plasencia MÁ, Arribas I, Seijas R, Padilla S, Orive G, et al. Comparison of intra-articular injections of plasma rich in growth factors (PRGF-Endoret) versus durolane hyaluronic acid in the treatment of patients with symptomatic osteoarthritis: a randomized controlled trial. Arthroscopy. 2013;29(10):1635-43. [DOI] [PubMed] [Google Scholar]
- 80. Wu YT, Hsu KC, Li TY, Chang CK, Chen LC. Effects of platelet-rich plasma on pain and muscle strength in patients with knee osteoarthritis. Am J Phys Med Rehabil. 2018;97(4):248-54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_File for PRP Injections for the Treatment of Knee Osteoarthritis: A Meta-Analysis of Randomized Controlled Trials by Giuseppe Filardo, Davide Previtali, Francesca Napoli, Christian Candrian, Stefano Zaffagnini and Alberto Grassi in CARTILAGE