Abstract
Background
This study aimed to compare the efficacy and safety of intra-articular hyaluronic acid (HA) injection with different molecular weights (MWs) for treating hip osteoarthritis (OA).
Methods
A systematic literature search for relevant studies was conducted in 3 electronic databases, including PubMed, BMJ Journals, and Cochrane Library, from inception to April 2020. Extracted outcomes included visual analogue scale (VAS) (1, 3, and 6 months), Lequesne index (3 and 6 months), and adverse effects. HAs were classified into low-molecular-weight (LMW), moderate-molecular-weight (MMW), high-molecular-weight (HMW), and ultra-high-molecular-weight (UHMW) groups. Meta-analysis was performed using Review Manager 5.3.
Results
A total of 15 studies with 614 patients were included. Our meta-analysis showed that the HMW HA group had the best improvement in VAS and Lequesne index compared with other HA groups for all the follow-up visits. Moreover, the HMW group demonstrated significantly better improvement than the other groups in VAS at 6-month follow-up and in Lequesne index at 3- and 6-month follow-ups. Analysis for adverse effects revealed low rates of systemic adverse effects (≤0.6%) in all groups and similar rate of local adverse effects (around 10%) among the groups except for UHMW HA group (37.5%).
Conclusion
Among different MWs of HA for treating hip OA, HMW HA injection demonstrated the best efficacy for up to 6 months after treatment without increased risk of adverse effects. Further studies with more comprehensive data and a higher level of evidence are required to prove our results.
Keywords: hip osteoarthritis, hyaluronic acid, intra-articular, molecular weight, viscosupplementation
Introduction
Osteoarthritis (OA) is recognized as a “serious” disease in that it causes progressive disability and increased risk of death. 1 With estimated 240 million people affected by OA across the world currently, its prevalence is expected to rise due to aging of the population and increased incidence of obesity.1,2 The surge of disease burden embodies in the years lived with disability for OA, which has increased by 31.4% from 2007 to 2017. 3 Despite more and more people suffering from symptomatic OA, there is still no known curable strategy to stop, prevent, or even reduce its progression. Current pharmacological treatments for OA are limited to symptomatic control, including acetaminophen, nonsteroidal anti-inflammatory drugs (NSAID), and intra-articular (IA) hyaluronic acid (HA) or steroid. 4 NSAIDs are commonly prescribed to alleviate joint pain and inflammation, but they are associated with an increased risk of gastrointestinal and cardiovascular adverse effects. 5 In contrast, IA-HA injection can relieve joint pain with few systemic side effects, it thus has been developed and widely used in the past 20 years. 6
Within affected joints, chronic inflammatory process gives rise to reduced molecular weight (MW) and concentration of HA, impairing the lubricating and protecting effects of synovial fluid. 7 Physically, viscosupplementation with IA-HA injection could directly restore the rheologic properties of the synovial fluid and decrease the joint friction to prevent cartilage degradation. 8 The MW is positively correlated to rheologic properties and residence time. 9 Correspondingly, efficacy of different HA products is found to vary by MW with accumulating evidence.10 -12
Besides supplementation for viscoelasticity of synovial fluid, IA-HA may act through additional cellular modifying mechanisms, including anti-oxidative, anti-inflammatory, and analgesic effects. 13 The biologic effects of HA also vary widely with its MW. 14 An in vitro study revealed the correlation between MW and macrophage activation: HA with MW less than 5 kDa induced phenotypic changes of macrophage facilitating pro-inflammatory response; while HA with MW more than 800 kDa enhanced changes leading to pro-resolving response. 15 HA with MW of 2000 to 4000 kDa was found to inhibit interleukin-6 (IL-6)-induced matrix metalloproteinases production from human chondrocytes, repressing proteoglycan degradation in articular cartilage. 16
Clinically, the impact of MW on the effects of HA treatment has been extensively explored for knee OA. A recent meta-analysis evaluating the efficacy and safety of currently used IA treatments (HAs, platelet-rich plasma, and extended-release or standard release corticosteroids) demonstrated that HA with higher MW had more comprehensive therapeutic effects on both pain and function, outperforming other IA treatments. 11 Another meta-analysis assessing the efficacy and safety of different HA products for knee OA suggested that HA with MW more than 3000 kDa leads to lower incidence of discontinuation due to treatment-related adverse effects (0.77%), compared with 2.20% for HA with MW lower than 1500 kDa. 12 On the basis of studies in knee OA, it seems that HAs of different MWs work with distinguishing features, and they should not be categorized as a single group.
Although hip joint is the second common affected site of OA, literature regarding the efficacy of viscosupplementation for treating hip OA is much lesser than that of knee OA, presumably because it is relatively difficult to access the hip joint. 17 Previous investigations indicated amelioration of pain and function after viscosupplementation treatment for hip OA, but the efficacy of different MWs of HA remains unclear. Due to limited analyses and inconclusive opinions on HA of different MWs for treating hip OA, we therefore conducted this meta-analysis to make clear the role of MW in clinical therapeutic effects on hip OA.
Method
Search Strategy
We conducted this systematic review and meta-analysis by following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Three electronic databases were adopted to screen relevant articles from inception to April 2020, including PubMed, BMJ Journals, and Cochrane Library. The following key terms with Boolean operators were used to search articles: (“hip” AND (“osteoarthritis” OR “arthritis” AND (“hyaluronic acid” OR “viscosupplementation”)). For the searching procedure, duplicates in the identified articles were removed. Then, titles and abstracts of the remaining articles were screened for potential studies. Finally, full-texts of the potential studies were further examined. The searching procedure was performed independently by 2 reviewers (Y.Z.W. and C.L.S.). Any discrepancy was resolved after discussion by the 2 reviewers until a consensus was reached. We also manually searched the reference lists of related reviews and the included articles to include additional relevant articles.
Inclusion and Exclusion Criteria
Studies that met the following criteria were included: (1) clinical trials using IA-HA injection to treat patients with hip OA, without restriction to prospective or retrospective design; (2) diagnosis of hip OA confirmed by clinical or radiographical assessment; (3) explicit description of the type of HA used, dosage, and treatment course; and (4) clinical outcomes recorded using visual analogue score (VAS) or Lequesne index. The exclusion criteria were as follows: (1) HA treatment in combination with manual therapy, (2) lack of description of the brand name or MW of HA, and (3) lack of quantitative data for analysis, such as sample size, mean, or standard deviation.
Type of Outcome
For evaluating the therapeutic effect on hip OA, several scales or indices were commonly used, including VAS, Lequesne index, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score, and Harris hip score. After collecting data from potential studies, we decided to adopt VAS and Lequesne index due to well-documented records for most included studies.
VAS is a tool for the measurement of pain level, which contains a line with a fixed length of 10 cm or 100 mm. The left end anchored with 0 cm represents “no pain,” and the right end anchored with 10 cm for “the worst pain.” It is a continuous scale, and any point on the line between the ends can be selected. To standardize VAS among included studies, we used the unit of cm. We retrieved VAS data at follow-up time of 1, 3, and 6 months after initial injection, and we adopted the VAS while walking or in activity.
Lequesne index consists of 3 components, including evaluating discomforts, maximal walking distance and ability for daily activity. Scoring of each part ranges from 0 to 8, and the maximum total score is 24. Data of Lequesne index were retrieved at 3- and 6-month follow-ups after initial injection.
Data Extraction
The main characteristics of included studies were extracted, including the first author’s name, publication year, study design, treatment implementation (type of HA used, dosage, and course of injection), demographics of enrolled patients (sample size, male-to-female ratio, mean age, and OA radiographic grade), type of outcome, follow-up time, and adverse effects.
Quality Assessment
We evaluated the quality of all the included studies using Downs and Black checklist for its applicability to randomized and nonrandomized studies. 18 There are 27 items with 1 point for each item, but there is an exceptional item: Up to 2 points can be given for the question regarding distributions of principal confounders in the reporting section. After scoring, the quality of articles can be classified as poor (score: 0-14), fair (score: 15-19), and good (score: 20-28).19,20
Data Synthesis and Analysis
For comparing the efficacy among different MWs of HA, HAs were grouped into low-molecular-weight (LMW), moderate-molecular-weight (MMW), high-molecular-weight (HMW), and ultra-high-molecular-weight (UHMW) with reference to previous studies through minor adjustments.11,12,21 The detailed classifications based on MWs and brand are shown in Table 1 .
Table 1.
Hyaluronic Acid Classified by Molecular Weight.
| Classification | Grouping Standard (kDa) | Bank of HA | Molecular Weight (kDa) | Crosslinking |
|---|---|---|---|---|
| LMW HA | <1,200 | Hyalgan | 500-730 | |
| Adant | 600-1,200 | |||
| MMW HA | 1,200-3,600 | Ostenil | 1,200-1,400 | |
| Hyalubrix | 1,500-3,200 | |||
| Hyalubrix 60 | 1,300-3,600 | |||
| Synocrom | Averaging 1,600 | |||
| HMW HA | 3,600-10,000 | Hylan G-F 20 | Averaging 6,000-7,000 | v |
| UHMW HA | > 10,000 | Durolane | Averaging >10,000 | v |
| Fermathron S | Not quantifiable | v |
HA = hyaluronic acid; LMW = low-molecular-weight; MMW = moderate-molecular-weight; HMW = high-molecular-weight; UHMW = ultra-high-molecular-weight.
All statistical analyses were conducted by Review Manager (version 5.3). Data were subgrouped based on MW classifications and follow-up time. VAS and Lequesne index were both continuous variables and were evaluated using a mean difference (MD) with 95% confidence intervals (CIs). Tau, chi-square test and I2 statistics were used to assess the heterogeneity. Noticeable heterogeneity was considered if any of the following criteria was met: Tau square test >0.1, P value of chi-square test <0.05, or I2 > 50%. Due to varied treatment courses among the included studies, we decided to adopt a random-effects model for all analyses, regardless of heterogeneity. A P value <0.05 was considered statistically significant for all analyses.
Results
Study Selection
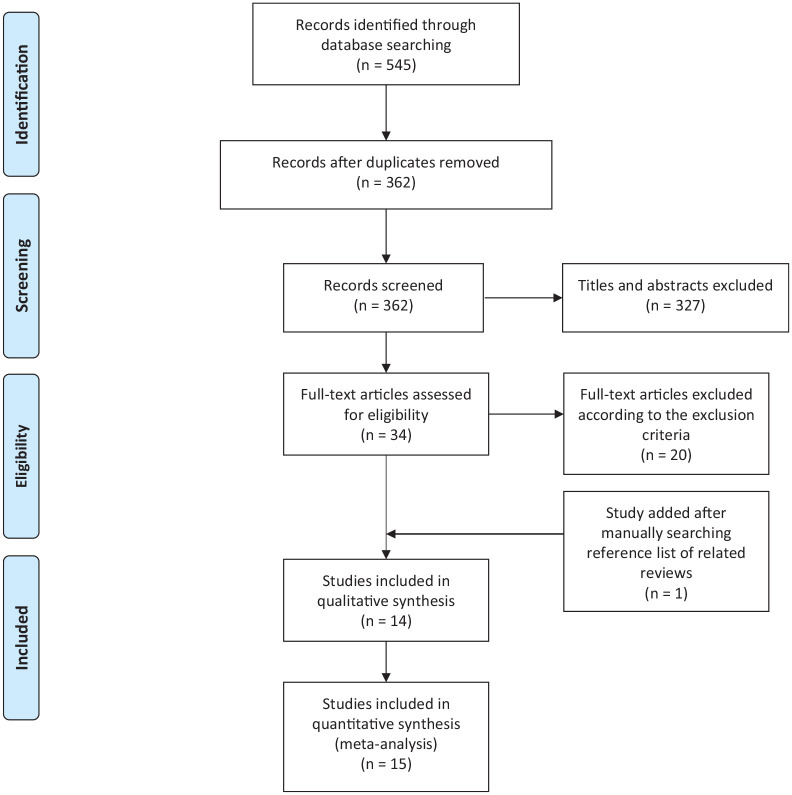
The flow diagram of the selecting process is shown in Figure 1 . Initially, 545 relevant articles were identified from the electronic databases. After removing duplicates, titles and abstracts of 362 articles were screened, and 34 of them were considered as eligible. We further reviewed the full texts of the remaining studies, and 14 studies met our inclusion criteria. Otherwise, an additional study was included by manually searching the reference lists of related reviews. Finally, a total of 15 studies were included for meta-analysis.
Figure 1.
Flow diagram of study selecting process.
Study Characteristics
The main characteristics of the included studies are shown in Table 2 . A total of 614 patients with 633 symptomatic hips were included in this meta-analysis, and 61% of the patients were female. Mean age of the participants ranged from 54.7 to 73.6 years. The severity of hip OA was assessed by Kellgren-Lawrence (KL) grade, and patients with grade II to III OA accounted for 68% to 100% of participants. The treatment course was scheduled according to the features of HA products, with additional injections performed at follow-ups if clinically necessary. For available clinical outcomes, we extracted VAS at follow-up time of 1, 3, and 6 months, as well as Lequesne index at 3 and 6 months. All of these studies reported VAS, and 9 of them reported Lequesne index.
Table 2.
Main Characteristics of the Included Studies.
| Study/Reference | Study Design | Treatment Type | Technique | Treatment Course | Sample Size a | Male:Female | Mean Age (Years) | OA Severity (Radiological Grade) | Follow-Up Time | Extracted Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Brocq et al. (2002) 22 | Prospective | HMW-HA (Hylan G-F 20) |
Fluoroscopy-assisted | 2 mL HA injection, once at baseline, and a second injection at 30th, 60th, or 90th day if clinically necessary | 22 | 9:13 | 54.7 | I: 2 (9.1%) II: 11 (50%) III: 9 (40.9%) |
1 month 3 months 6 months |
VAS Lequesne index |
| Conrozier et al. (2003) 23 | Prospective | HMW-HA (Hylan G-F 20) |
Fluoroscopy-assisted | 2 mL HA injection, once at baseline, and a second injection at 30th, 60th, or 90th day if clinically necessary | 56 | 23:33 | 59.8 | II: 25 (44.6%) III: 31 (55.4%) |
3 months | VAS |
| Caglar-Yagci et al. (2005) 24 | Not mentioned | HMW-HA (Hylan G-F 20) |
Ultrasound-guided | 2 mL HA injections, once a week for 3 injections | 14 | 4:10 | 65.35 | I: 1 (7.1%) II: 10 (71.4%) III: 3 (21.4%) |
1 month 3 months |
VAS Lequesne index |
| Migliore et al. (2005) 25 | Prospective | HMW-HA (Hylan G-F 20) |
Ultrasound-guided | 2 mL HA injection, once at baseline, with up to 2 additional injections 1 month and/or 2 months later if clinically necessary | 30 | 7:23 | 70 | I: 2 (6.7%) II: 16 (53.3%) III: 11 (36.7%) |
6 months | VAS Lequesne index |
| Migliore et al. (2005) 26 | Not mentioned | LMW-HA (Hyalgan) |
Ultrasound-guided | 2 mL HA injection, with subsequent injections at an interval of 3-6 months if necessary | 19 | 3:11 | 71.64 | II: 6 (31.6%) III: 9 (47.4%) IV: 4 (21.1%) |
3 months 6 months |
VAS Lequesne index |
| Tikiz et al. (2005) 27 | Prospective | MMW-HA (Ostenil) |
Fluoroscopy-assisted | 2 mL HA injection, 3 times with interval of 1 week | 32 | 5:20 | 58.8 | I: 2 (6%) II: 10 (32%) III: 20 (62%) |
1 month 3 months 6 months |
VAS Lequesne index |
| HMW-HA (Hylan G-F 20) |
24 | 4:14 | 60.4 | I: 1 (4%) II: 7 (29%) III: 16 (67%) |
||||||
| van den Bekerom et al. (2008) 28 | Prospective | LMW-HA (Adant) |
Fluoroscopy-assisted | 1 injection at baseline, with a 2nd and 3rd injection or THA if condition worsened | 91 | 35:65 | 61.8 | NA | 1.5 months | VAS |
| MMW-HA (Synocrom) | 20 | 7:13 | 62.1 | |||||||
| HMW-HA (Hylan G-F 20) |
15 | 7:8 | 61.9 | |||||||
| Conrozier et al. (2009) 29 | Prospective | UHMW-HA (Durolane) | Fluoroscopy-assisted | 3 mL HA injection once at baseline | 34 | 17:23 | 63.1 | I: 4 (10%) II: 16 (40%) III: 20 (50%) |
6 months | VAS Lequesne index |
| Migliore et al. (2009) 30 | Prospective | MMW-HA (Hyalubrix) | Ultrasound-guided | 4 mL HA injections, once a month for 2 injections | 17 | 12:10 | 68 | I: 1 (4.5%) II: 21 (95.5%) III: 0 (0%) |
3 months 6 months |
VAS Lequesne index |
| Richette et al. (2009) 31 | Prospective | LMW-HA (Adant) |
Fluoroscopy-assisted | 2.5 mL HA injection once at baseline | 42 | 15:27 | 60.8 | II: 7 (16.7%) III: 35 (83.3%) |
3 months | VAS |
| Battaglia et al. (2013) 32 | Prospective | MMW-HA (Hyalubrix) | Ultrasound-guided | 2 mL HA injections, once every 2 weeks for 3 consecutive injections | 50 | 33:17 | 56 | NA | 1 month 3 months 6 months |
VAS |
| Di Sante et al. (2016) 33 | Prospective | MMW-HA (1000-2900 kDa) |
Ultrasound-guided | 2 mL HA injections, once a week for 3 injections | 22 | 9:13 | 73.62 | II: 7 (31.8%) III: 15 (68.2%) |
1 month | VAS |
| Doria et al. (2017) 34 | Prospective | MMW-HA (Hyalubrix) | Ultrasound-guided | 3 weekly injections, without mention of dosing | 40 | NA | 68 | grade 0-II | 6 months | VAS |
| Abate and Salini (2017) 35 | Retrospective | LMW-HA (hybrid HA with 1100-1400 kDa HA + 80-100 kDa HA) | Ultrasound-guided | 2 mL HA injection (both kinds of HA 32 mg), at baseline and after 40 days | 20 | NA | 63.3 | Grade II-IV | 3 months 6 months |
VAS Lequesne index |
| LMW-HA (800 - 1200 kDa) |
2.5 mL HA injection (50 mg), at baseline and after 40 days | 20 | NA | 63.6 | ||||||
| Clementi et al. (2018) 36 | Prospective | MMW-HA (Hyalubrix 60) | Ultrasound-guided | 4 mL HA injection, twice, at baseline and 3-4 weeks later | 27 | 11:16 | 67.4 | All patients were grade III | 3 months 6 months |
VAS Lequesne index |
| UHMW-HA (Fermathron S) | 3 mL HA injection once at baseline | 23 | 8:15 | 65.9 |
OA = osteoarthritis; HA = hyaluronic acid; LMW = low-molecular-weight; MMW = moderate-molecular-weight; HMW = high-molecular-weight; UHMW = ultra-high-molecular-weight; NA, not available; VAS = visual analogue scale.
Sample size is the number of hips included in the study, so it may not be equal to the number of patients.
Quality Assessment
The detailed quality assessment for each study is shown in Table 3 . In the reporting section, most of the included studies briefly reported adverse effects, and all the studies did not provide a complete list of possible adverse effects. Only 1 study reported statistical power calculation. Overall, the scores of the included studies ranged from 16 to 25, which were considered as fair to good quality.
Table 3.
Quality of the Included Studies Assessed by Using Downs and Black Checklist.
| Study | Study Design | Downs and Black Checklist | Quality | |||||
|---|---|---|---|---|---|---|---|---|
| Reporting (11) | External Validity (3) | Bias (7) | Confounding (6) | Power (1) | Total (28) | |||
| Brocq et al. (2002) 22 | Prospective | 9 | 3 | 6 | 3 | 0 | 21 | Good |
| Conrozier et al. (2003) 23 | Prospective | 10 | 2 | 6 | 3 | 0 | 21 | Good |
| Caglar-Yagci et al. (2005) 24 | Not mentioned | 9 | 3 | 6 | 3 | 0 | 21 | Good |
| Migliore et al. (2005) 25 | Prospective | 10 | 3 | 6 | 3 | 0 | 22 | Good |
| Migliore et al. (2005) 26 | Not mentioned | 9 | 2 | 6 | 3 | 0 | 20 | Good |
| Tikiz et al. (2005) 27 | Prospective | 10 | 3 | 7 | 4 | 0 | 24 | Good |
| van den Bekerom et al. (2008) 28 | Prospective | 7 | 3 | 6 | 4 | 0 | 20 | Good |
| Conrozier et al. (2009) 29 | Prospective | 10 | 3 | 6 | 3 | 0 | 22 | Good |
| Migliore et al. (2009) 30 | Prospective | 9 | 3 | 7 | 5 | 0 | 24 | Good |
| Richette et al. (2009) 31 | Prospective | 10 | 2 | 7 | 6 | 0 | 25 | Good |
| Battaglia et al. (2013) 32 | Prospective | 8 | 3 | 7 | 5 | 1 | 24 | Good |
| Di Sante et al. (2016) 33 | Prospective | 10 | 2 | 6 | 4 | 0 | 22 | Good |
| Doria et al. (2017) 34 | Prospective | 10 | 3 | 7 | 5 | 0 | 25 | Good |
| Abate and Salini (2017) 35 | Retrospective | 8 | 1 | 6 | 1 | 0 | 16 | Fair |
| Clementi et al. (2018) 36 | Prospective | 10 | 3 | 6 | 5 | 0 | 24 | Good |
Meta-Analysis
Visual Analogue Scale
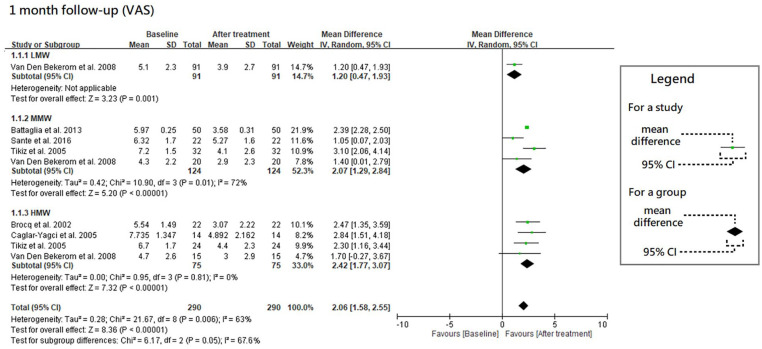
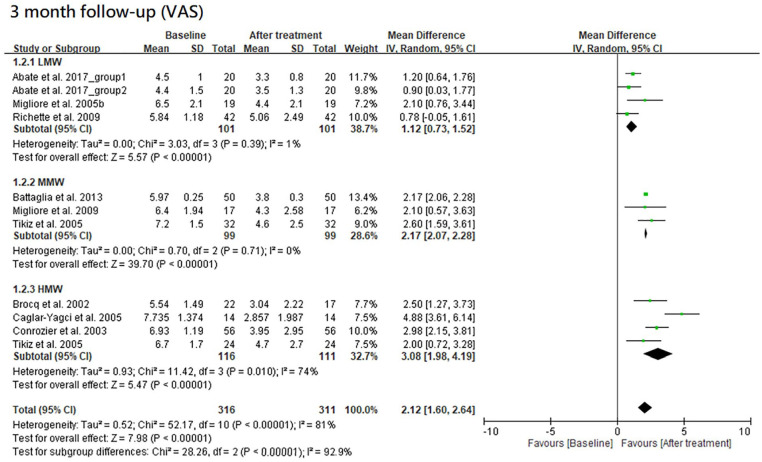
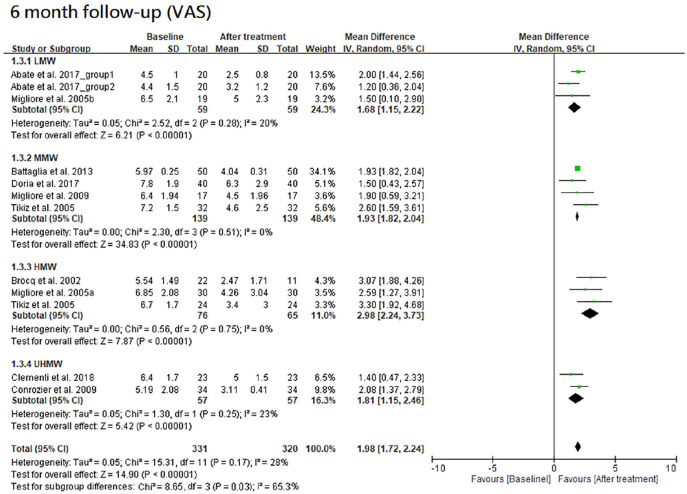
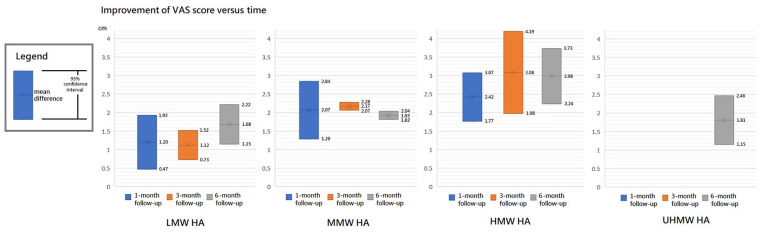
The results of VAS at 1-, 3- and 6-month follow-ups are shown in Figures 2 , 3 , and 4 , respectively. Moreover, the results of VAS at all 3 follow-ups are shown together in Figure 5 according to the classification by MW.
Figure 2.
Forest plots of meta-analysis in comparison of VAS score improvement before and after treatment at the 1-month follow-up for LMW, MMW, and HMW groups. VAS, visual analogue scale; LMW, low-molecular-weight; MMW, moderate-molecular-weight; HMW, high-molecular-weight.
Figure 3.
Forest plots of meta-analysis in comparison of VAS score improvement before and after treatment at the 3-month follow-up for LMW, MMW, and HMW groups. VAS, visual analogue scale; LMW, low-molecular-weight; MMW, moderate-molecular-weight; HMW, high-molecular-weight.
Figure 4.
Forest plots of meta-analysis in comparison of VAS score improvement before and after treatment at the 6-month follow-up for LMW, MMW, HMW, and UHMW groups. VAS, visual analogue scale; LMW, low-molecular-weight; MMW, moderate-molecular-weight; HMW, high-molecular-weight; UHMW, ultra-high-molecular-weight.
Figure 5.
Improvement of visual analogue scale (VAS) score versus time.
All the 3 HA groups had a significant improvement in VAS at the 1-month follow-up when compared with the baseline level (trial = 1, MD = 1.20, 95% CI = 0.47-1.93, P = 0.001 for LMW group; trial = 4, I2 = 72%, MD = 2.07, 95% CI = 1.29-2.84, P < 0.00001 for MMW group; trial = 4, I2 = 0%, MD = 2.42, 95% CI = 1.77-3.07, P < 0.00001 for HMW group). Although there were no significant differences in improvement among these 3 HA groups (P = 0.05), they had a possible trend in variance for efficacy, in which HMW HA was the best, MMW the second, and LMW the worst.
All the 3 HA groups had a significant improvement in VAS at the 3-month follow-up when compared with the baseline level (trial = 4, I2 = 1%, MD = 1.12, 95% CI = 0.73-1.52, P < 0.00001 for LMW group; trial = 3, I2 = 0%, MD = 2.17, 95% CI = 2.07-2.28, P < 0.00001 for MMW group; trial = 4, I2 = 74%, MD = 3.08, 95% CI = 1.98-4.19, P < 0.00001 for HMW group). There was a significant difference among these 3 groups (P < 0.00001). The HMW or MMW group had a significantly better improvement than LMW group (HMW vs. LMW, P = 0.001; MMW vs. LMW, P < 0.00001). There was no significant difference between the HMW and MMW groups (P = 0.11). These groups had a possible trend in variance for efficacy, in which HMW was the best, MMW the second, and LMW the worst.
All the 4 HA groups had a significant improvement in VAS at the 6-month follow-up when compared with the baseline level (trial = 3, I2 = 20%, MD = 1.68, 95% CI = 1.15-2.22, P < 0.00001 for LMW group; trial = 4, I2 = 0%, MD = 1.93, 95% CI = 1.82-2.04, P < 0.00001 for MMW group; trial = 3, I2 = 0%, MD = 2.98, 95% CI = 2.24-3.73, P < 0.00001 for HMW group; trial = 2, I2 = 23%, MD = 1.81, 95% CI = 1.15-2.46, P < 0.00001 for UHMW group). There was a significant difference among these 4 groups (P = 0.03). The efficacy of HMW group was significantly better than the LMW (P = 0.005), MMW (P = 0.006), or UHMW group (P = 0.02). However, there were no significant differences among the LMW, MMW, and UHMW groups (P = 0.63).
Overall, HMW group had the best improvement in VAS at all the 3 follow-ups, and it had a significantly better performance than other groups at 6-month follow-up. Moreover, the improvement of VAS score reached its peak between 1 and 6 months in both MMW and HMW groups, whereas no peaking was found in that of LMW group within 6 months after initial injection.
Lequesne Index
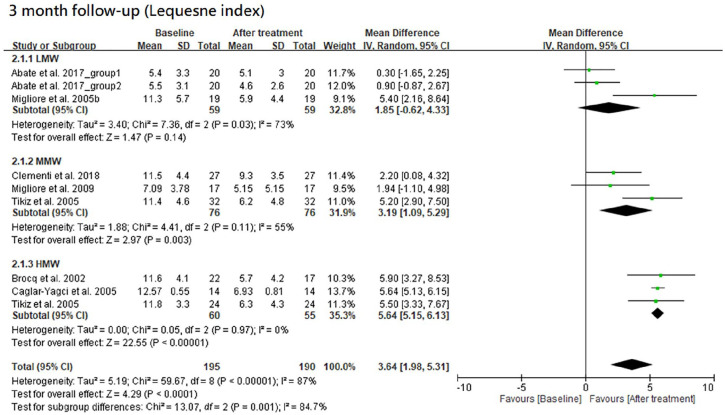
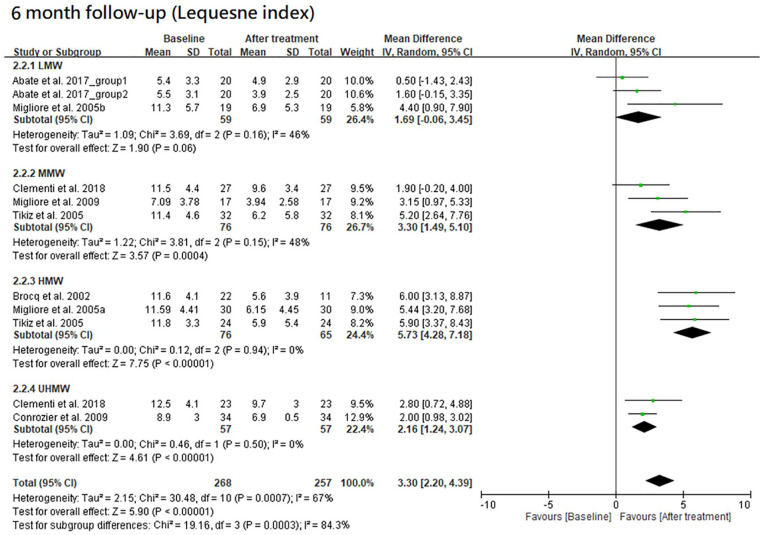
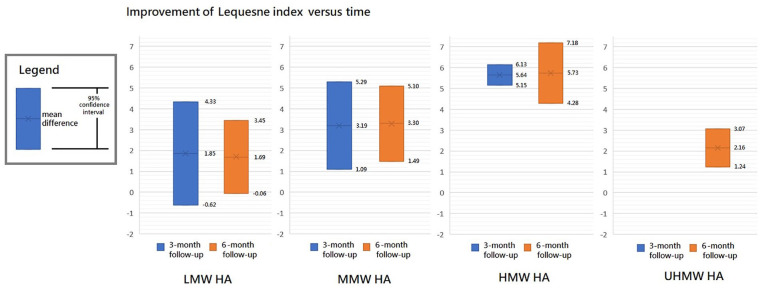
The results of Lequesne index at 3- and 6-month follow-ups are shown in Figures 6 and 7, respectively. Furthermore, the results of Lequesne index at both follow-ups are shown together in Figure 8 according to the classification by MW.
Figure 6.
Forest plots of meta-analysis in comparison of Lequesne index improvement before and after treatment at the 3-month follow-up for LMW, MMW, and HMW groups. LMW, low-molecular-weight; MMW, moderate-molecular-weight; HMW, high-molecular-weight.
Figure 7.
Forest plots of meta-analysis in comparison of Lequesne index improvement before and after treatment at the 6-month follow-up for LMW, MMW, HMW, and UHMW groups. LMW, low-molecular-weight; MMW, moderate-molecular-weight; HMW, high-molecular-weight; UHMW, ultra-high-molecular-weight.
Figure 8.
Improvement of Lequesne index versus time.
Among the LMW, MMW, and HMW groups, the MMW and HMW groups had a significant improvement in Lequesne index at the 3-month follow-up when compared with the baseline level (trial = 3, I2 = 73%, MD = 1.85, 95% CI = −0.62 to 4.33, P = 0.14 for LMW group; trial = 3, I2 = 55%, MD = 3.19, 95% CI = 1.09-5.29, P = 0.003 for MMW group; trial = 3, I2 = 0%, MD = 5.64, 95%CI = 5.15-6.13, P < 0.00001 for HMW group). There was a significant difference in improvement among the 4 groups (P = 0.001). The efficacy of HMW group was significantly better than the LMW (P = 0.003) or MMW group (P = 0.03). However, there was no significant difference between the LMW and MMW groups (P = 0.42). These groups had a possible trend in variance for efficacy, in which HMW was the best, MMW the second, and LMW the worst.
There were 4 HA groups at the 6-month follow-up, including LMW, MMW, HMW, and UHMW groups. They had a significant improvement in Lequesne index when compared with the baseline level except for the LMW group (trial = 3, I2 = 46%, MD = 1.69, 95% CI = −0.06 to 3.45, P = 0.06 for LMW group; trial = 3, I2 = 48%, MD = 3.30, 95% CI = 1.49-5.10, P = 0.0004 for MMW group; trial = 3, I2 = 0%, MD = 5.73, 95% CI = 4.28-7.18, P < 0.00001 for HMW group; trial = 2, I2 = 0%, MD = 2.16, 95% CI = 1.24-3.07, P < 0.00001 for UHMW group). There was a significant difference among these groups (P = 0.0003). The efficacy of HMW group was significantly better than LMW (P = 0.0005), MMW (P = 0.04), or UHMW group (P < 0.0001). There was a possible trend in variance for efficacy, in which higher MW of HA had better efficacy except for the UHMW group.
As a whole, HMW group had significantly better improvement in Lequesne index at both follow-ups. Moreover, the mean improvement of Lequesne index remained stationary from 3- to 6-month follow-ups in LMW, MMW, and HMW groups.
Adverse Effects
All the included studies reported adverse effects of HA treatment, and their results are summarized in Table 4. Of the 614 patients included, only 2 cases were reported having systemic adverse effects after injection. One patient suffered from aseptic arthritis with fever after injection of HMW HA (Hylan G-F 20), and the symptoms resolved within 2 days under treatment of NSAIDs alone; the other had pruritus after injection of LMW HA (Adant), but no further description of the involved area or treatment course was recorded. The incidence of local adverse effects was relatively higher, ranging from 5% to 37.5%. Most of the local adverse effects were transient local pain or hematoma, which usually resolved within hours or days. UHMW HA group had a relatively high rate of local adverse effects (37.5%) when compared with the other groups (8.2%-12.2%).
Table 4.
Adverse Effects of the Included Studies Stratified by Molecular Weight Group.
| Classification | Study | HA Type | Sample Size | Systemic Adverse Effects | Local Adverse Effects | ||
|---|---|---|---|---|---|---|---|
| Rate | Event | Rate | Event | ||||
| LMW group | Migliore et al. (2005) 26 | Hyalgan | 19 | 0% (0/19) | None | NA | NA |
| van den Bekerom et al. (2008) 28 | Adant | 91 | 0% (0/91) | None | NA | NA | |
| Richette et al. (2009) 31 | Adant | 42 | 2.3% (1/42) | Pruritis | 9.5% (4/42) | Local hematoma: 1 Pain flares: 3 |
|
| Abate and Salini (2017) 35 | Hybrid HA (1100-1400 kDa HA and 80-100 kDa HA) | 20 | 0% (0/20) | None | NA | Only slight discomfort | |
| HA (800-1200 kDa) | 20 | 0% (0/20) | None | NA | |||
| Subtotal | 192 | 0.5% (1/192) | 9.5% (4/42) | ||||
| MMW group | Tikiz et al. (2005) 27 | Ostenil | 32 | 0% (0/32) | none | 9.3% (3/32) | Pain or swelling |
| van den Bekerom et al. (2008) 28 | Synocrom | 20 | 0% (0/20) | NA | NA | ||
| Migliore et al. (2009) 30 | Hyalubrix | 17 | 0% (0/17) | 5.9% (1/17) | Moderate hip pain | ||
| Battaglia et al. (2013) 32 | Hyalubrix | 50 | 0% (0/50) | NA | Peri- or posttreatment pain Superficial hematoma: 1 |
||
| Di Sante et al. (2016) 33 | HA (1000-2900 kDa) | 22 | 0% (0/22) | NA | NA | ||
| Doria et al. (2017) 34 | Hyalubrix | 40 | 0% (0/40) | NA | Self-limited postinjection Pain reaction |
||
| Clementi et al. (2018) 36 | Hyalubrix 60 | 27 | 0% (0/27) | NA | NA | ||
| Subtotal | 208 | 0% (0/208) | 8.2% (4/49) | ||||
| HMW group | Brocq et al. (2002) 22 | Hylan G-F 20 | 22 | 4.5% (1/22) | Aseptic arthritis with fever up to 38.5 °C | 9.1% (2/22) | Transient local pain |
| Conrozier et al. (2003) 23 | Hylan G-F 20 | 56 | 0% (0/56) | None | NA | 1. Transient hip pain: 10.1% of injections 2. Mild synovial fluid aseptic effusions: 2 |
|
| Caglar-Yagci et al. (2005) 24 | Hylan G-F 20 | 14 | 0% (0/14) | None | 21.4% (3/14) | Transient local pain | |
| Migliore et al. (2005) 25 | Hylan G-F 20 | 30 | 0% (0/30) | None | 10.0% (3/30) | Transient local heaviness | |
| Tikiz et al. (2005) 27 | Hylan G-F 20 | 24 | 0% (0/24) | None | 12.5% (3/24) | Pain or swelling | |
| van den Bekerom et al. (2008) 28 | Hylan G-F 20 | 15 | 0% (0/15) | None | NA | NA | |
| Subtotal | 161 | 0.6% (1/161) | 12.2% (11/90) | ||||
| UHMW group | Conrozier et al. (2009) 23 | Durolane | 40 | 0% (0/40) | None | 37.5% (15/40) | Local pain Mild: 10/40 Moderate: 3/40 Severe: 2/40 |
| Clementi et al. (2018) 36 | Fermathron S | 23 | 0% (0/23) | None | NA | NA | |
| Subtotal | 63 | 0% (0/63) | 37.5% (15/40) | ||||
HA = hyaluronic acid; LMW = low-molecular-weight; MMW = moderate-molecular-weight; HMW = high-molecular-weight; UHMW = ultra-high-molecular-weight; NA = not available.
Discussion
With growing disease burden of hip OA, it is imperative to develop appropriate options for conservative treatment. The use of IA-HA to treat hip OA gets more and more common, but the efficacy of HA with different MWs remains unclear. Our study aimed to figure out how the MW of HA determines its therapeutic effects. The meta-analysis for efficacy revealed a clear trend that was consistently observed in both VAS and Lequesne index: improvement of the outcomes increased from LMW, MMW to HMW groups, then decreased from HMW to UHMW groups, regardless of the follow-up time within 6 months after initial injection. Moreover, analysis for safety of HA showed rare systemic adverse effects (≤0.6%) in all groups and similar rate of local adverse effects (LMW 4/42, 9.5%; MMW 4/49, 8.2%; HMW 11/90, 12.2%) among the groups except for UHMW group (15/40, 37.5%).
Although the effective mechanisms of IA-HA injection in hip OA were still not fully understood, we tried to interpret our findings on the basis of current knowledge of HA, in which relevant researches in knee OA served as particularly useful references. The efficacy of HA would be discussed below in time order since the injected HAs were so short-lived within the joints that the mechanisms of action probably varied with time. It was reported that Hyalgan (LMW HA) had a half-life of 17 hours in the joints, Hylan G-F 20 (HMW HA) 8.8 days, and Durolane (UHMW HA) around 1 month.37,38 Therefore, the physical properties of the injected HA might just partially affect the outcomes at 1-month follow-up, whereas the outcomes at 3- and 6-month follow-ups were solely the results of its subsequent reactions.
Before considerable degradation of the injected HA, it directly changed the rheologic properties of the synovial fluid, and its physiological concentration and MW were the main determinants. 39 For concentration, it was expected that higher HA concentration led to better viscoelasticity, but the clinical effects could not be proved in our analysis due to lack of relevant measurement in our included studies. On the other hand, from the aspect of the MW, our meta-analysis showed that the improvement in VAS at 1-month follow-up rose from LMW, MMW, to HMW groups. However, we did not place a high value on the direct effect by MW itself of the injected HAs for the following 2 reasons. One is that the residence time of HAs in the joints was not long enough to provide a sustained physical change. The other is that the HMW HA group had the peaked improvement in VAS and Lequesne index after 1 month-follow up, suggesting that the change caused by physical properties of the injected HA was not the most important mechanism of action. Besides, 2 randomized control trials researching into the efficacy of Durolane (UHMW HA) in knee OA also showed an improvement in outcome from 2 to 6 weeks, further confirming our point of view.40,41
After most of the injected HA was degraded, we could see from our analysis that the superior efficacy in HMW HA group remained at 6-month follow-up. It was thus reasonable to take into account the possible subsequent biological reactions that HMW HA brought about, such as endogenous HA synthesis by synovial fibroblast and immunomodulary effects that impeded the progression of early-stage OA.42 -44 To better understand the different biological effects of HA with different MWs, further studies were needed to shed light on the mechanisms contributing to long-term effects of HMW HA.
Regarding the relevant experience in knee OA as valuable reference, we compared the results of our analysis with those already studied in knee OA. Similar results were reported by Rutjes et al. 21 and Altman et al. 12 They both showed an increasing pooled effect size in pain improvement from lower MW (<1500 kDa) to higher MW HAs (>3000 or 6000 kDa) over placebo, indicating that IA-HA with larger MW was more effective than placebo.12,21 Although a recent meta-analysis with level I evidence revealed that there were no significant differences between cross-linked hylan (HMW HA) and linear hyaluronic acid (LMW or MMW HA) in the improvement of VAS and Lequesne index, the results of the study may be blurred by the variety of LMW and MMW HAs. 45 Compared with the aforementioned 3 studies for knee OA with each including at least 3,000 patients, our study for hip OA included only 614 patients; thus, the power of our study could not match them. However, our study used a clearly defined MW classification of HA. Furthermore, we reported the clinical efficacy of IA-HA for OA at 1, 3, and 6 months, demonstrating the effects of HA over time within the first 6 months instead of evaluating a chosen timepoint as the previous studies did.12,21,45 That is, our results provided stronger reasoning for the relationship between varied efficacy of HAs and their MWs.
Besides the efficacy of HA, adverse effects caused by HA were also reviewed carefully. Systemic adverse effects were rare (≤0.6%), but the rate of local adverse reactions ranged from 5% to 37.5% in our included studies. Compared with the incidence of local adverse events in knee OA (0.6%, 31/5241 in Rutjes et al. 21 ; flare-up at the injection site 9.5%, 459/4846 in Altman et al. 12 ), it seemed to be slightly higher in hip OA, which may be related to difficulty in approaching the joint space of hip.12,17,21,46 Our study further showed that the rates of local adverse events were comparable (8.2%-12.2%) among the groups with different MWs except for UHMW HA group (37.5%). In terms of the few available data for adverse effects in our included studies, it was noteworthy that the results might not be representative of the population. Despite the fact that the linker (1,4-butanediol diglycidyl ether) used for Durolane and Fermathron S (UHMW HAs) was a concern for adverse effects in cosmetic use due to its causing higher resistance to enzyme degradation, Durolane was not found to induce more tissue responses or antibodies specific to bacterial products than Hylan G-F 20 for viscosupplementation in a mice model, and it was reported having acceptable adverse effects (12%-22%) in the previous studies treating knee OA.47-49 Therefore, we thought that the low rate of systemic adverse effects among all the groups with different MWs was the mainstay of their safety, but more data were needed to get a clear idea of the difference in rate of local adverse effects among them.
Considering the efficacy and adverse effects clinically, the aim of our study was to point out an optimal IA-HA injection with high and long-lasting efficacy so that ideal therapeutic effect and reduced injection times could be simultaneously achieved, decreasing the times of approaching the hip joint while injection and the risk of adverse events. The results of our study showed that the HMW HA group had the best performance in the improvement of outcomes without obvious increased risk of adverse events, HMW HA was therefore considered the most appropriate choice of HA to treat hip OA.
There are several limitations in our analysis. First, because our goal was to find studies that compared the assessment under the posttreatment to baseline conditions, we did not restrict the study design to randomized controlled trials, and patient’s preference and/or bias for treatment thus could not be avoided. Second, there was some variance in patients’ OA severity and treatment course (e.g., injection times) among the studies, which may be important factors affecting the results. Third, there were a few subgroups that included no more than 2 trials, and the sample size of some trials was no more than 20, making our conclusions less convincing. Fourth, we analyzed the effects of HA in hip OA up to 6 months, but the effects in the long run could not be learned. Finally, HA concentrations could affect the efficacy of HA treatment for hip OA. However, this was not considered in our meta-analysis and may generate bias in our results.
Conclusion
Overall, our study showed that HMW HA was a potentially optimal choice for pain relief and functional improvement in the first 6 months of IA-HA treatment for hip OA, without additional risk of adverse effects. Our finding suggested that HAs could not be classified as a single group, and it was necessary for guideline makers to consider this point. The American Academy of Orthopaedic Surgeons currently does not support the use of IA-HA in symptomatic hip OA because no better performance over placebo in improving symptoms was proved. 50 We hoped that our study would be helpful for recognizing the difference among HAs with different MWs, and HMW HA could be viewed as a potential candidate for recommended conservative treatment. In terms of several limitations in our study, further studies with more comprehensive data and a higher level of evidence for this topic are needed.
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported in part by the Kaohsiung Medical University Hospital (KMUH109-9R35). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs: Yen-Zung Wu  https://orcid.org/0000-0002-0559-785X
https://orcid.org/0000-0002-0559-785X
Chung-Hwan Chen  https://orcid.org/0000-0001-8941-4792
https://orcid.org/0000-0001-8941-4792
References
- 1. Osteoarthritis Research Society International. Osteoarthritis: a serious disease, submitted to the US Food and Drug Administration. Available from: https://oarsi.org/sites/default/files/docs/2016/oarsi_white_paper_oa_serious_disease_121416_1.pdf
- 2. Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35. doi: 10.1002/art.23176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1859-922. doi: 10.1016/s0140-6736(18)32335-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wieland HA, Michaelis M, Kirschbaum BJ, Rudolphi KA. Osteoarthritis—an untreatable disease? Nat Rev Drug Discov. 2005;4:331-44. doi: 10.1038/nrd1693 [DOI] [PubMed] [Google Scholar]
- 5. Scarpignato C, Lanas A, Blandizzi C, Lems WF, Hermann M, Hunt RH, et al. Safe prescribing of non-steroidal anti-inflammatory drugs in patients with osteoarthritis—an expert consensus addressing benefits as well as gastrointestinal and cardiovascular risks. BMC Med. 2015;13:55. doi: 10.1186/s12916-015-0285-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Honvo G, Reginster JY, Rannou F, Rygaert X, Geerinck A, Rabenda V, et al. Safety of intra-articular hyaluronic acid injections in osteoarthritis: outcomes of a systematic review and meta-analysis. Drugs Aging. 2019;36(Suppl 1):101-27. doi: 10.1007/s40266-019-00657-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hyaluronan or hylans for knee osteoarthritis? Drug Ther Bull. 1999;37:71-2. doi: 10.1136/dtb.1999.37971 [DOI] [PubMed] [Google Scholar]
- 8. Waller KA, Zhang LX, Fleming BC, Jay GD. Preventing friction-induced chondrocyte apoptosis: comparison of human synovial fluid and hylan G-F 20. J Rheumatol. 2012;39:1473-80. doi: 10.3899/jrheum.111427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Agerup B, Berg P, Akermark C. Non-animal stabilized hyaluronic acid: a new formulation for the treatment of osteoarthritis. BioDrugs. 2005;19:23-30. doi: 10.2165/00063030-200519010-00003 [DOI] [PubMed] [Google Scholar]
- 10. Band PA, Heeter J, Wisniewski HG, Liublinska V, Pattanayak CW, Karia RJ, et al. Hyaluronan molecular weight distribution is associated with the risk of knee osteoarthritis progression. Osteoarthritis Cartilage. 2015;23:70-6. doi: 10.1016/j.joca.2014.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Phillips M, Vannabouathong C, Devji T, Patel R, Gomes Z, Patel A, et al. Differentiating factors of intra-articular injectables have a meaningful impact on knee osteoarthritis outcomes: a network meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2020;28:3031-9. doi: 10.1007/s00167-019-05763-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Altman RD, Bedi A, Karlsson J, Sancheti P, Schemitsch E. Product differences in intra-articular hyaluronic acids for osteoarthritis of the knee. Am J Sports Med. 2016;44:2158-65. doi: 10.1177/0363546515609599 [DOI] [PubMed] [Google Scholar]
- 13. Gupta RC, Lall R, Srivastava A, Sinha A. Hyaluronic acid: molecular mechanisms and therapeutic trajectory. Front Vet Sci. 2019;6:192. doi: 10.3389/fvets.2019.00192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garantziotis S, Savani RC. Hyaluronan biology: a complex balancing act of structure, function, location and context. Matrix Biol. 2019;78-79:1-10. doi: 10.1016/j.matbio.2019.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rayahin JE, Buhrman JS, Zhang Y, Koh TJ, Gemeinhart RA. High and low molecular weight hyaluronic acid differentially influence macrophage activation. ACS Biomater Sci Eng. 2015;1:481-93. doi: 10.1021/acsbiomaterials.5b00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hashizume M, Mihara M. High molecular weight hyaluronic acid inhibits IL-6-induced MMP production from human chondrocytes by up-regulating the ERK inhibitor, MKP-1. Biochem Biophys Res Commun. 2010;403:184-9. doi: 10.1016/j.bbrc.2010.10.135 [DOI] [PubMed] [Google Scholar]
- 17. Singh J, Khan WS, Marwah S, Wells G, Tannous DK, Sharma HK. Do we need radiological guidance for intra-articular hip injections? Open Orthop J. 2014;8:114-7. doi: 10.2174/1874325001408010114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377-84. doi: 10.1136/jech.52.6.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu Y, Cheng J, Gao Y, Qin L, Min X, Zhang M. Efficacy of switching therapy to aflibercept for patients with persistent diabetic macular edema: a systematic review and meta-analysis. Ann Transl Med. 2020;8:382. doi: 10.21037/atm.2020.02.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hooper P, Jutai JW, Strong G, Russell-Minda E. Age-related macular degeneration and low-vision rehabilitation: a systematic review. Can J Ophthalmol. 2008;43:180-7. doi: 10.3129/i08-001 [DOI] [PubMed] [Google Scholar]
- 21. Rutjes AW, Jüni P, da Costa BR, Trelle S, Nüesch E, Reichenbach S. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med. 2012;157:180-91. doi: 10.7326/0003-4819-157-3-201208070-00473 [DOI] [PubMed] [Google Scholar]
- 22. Brocq O, Tran G, Breuil V, Grisot C, Flory P, Euller-Ziegler L. Hip osteoarthritis: short-term efficacy and safety of viscosupplementation by hylan G-F 20. An open-label study in 22 patients. Joint Bone Spine. 2002;69:388-91. doi: 10.1016/s1297-319x(02)00416-5 [DOI] [PubMed] [Google Scholar]
- 23. Conrozier T, Bertin P, Mathieu P, Charlot J, Bailleul F, Treves R, et al. Intra-articular injections of hylan G-F 20 in patients with symptomatic hip osteoarthritis: an open-label, multicentre, pilot study. Clin Exp Rheumatol. 2003;21:605-10. [PubMed] [Google Scholar]
- 24. Caglar-Yagci H, Unsal S, Yagci I, Dulgeroglu D, Ozel S. Safety and efficacy of ultrasound-guided intra-articular hylan G-F 20 injection in osteoarthritis of the hip: a pilot study. Rheumatol Int. 2005;25:341-4. doi: 10.1007/s00296-004-0441-5 [DOI] [PubMed] [Google Scholar]
- 25. Migliore A, Tormenta S, Valente C, Massafra U, Martin LS, Carmenini E, et al. Intra-articulaar treatment with Hylan G-F 20 under ultrasound guidance in hip osteoarthritis. Clinical results after 12 months follow-up [in Italian]. Reumatismo. 2005;57:36-43. [DOI] [PubMed] [Google Scholar]
- 26. Migliore A, Tormenta S, Massafra U, Carloni E, Padalino C, Iannessi F, et al. Repeated ultrasound-guided intra-articular injections of 40 mg of Hyalgan may be useful in symptomatic relief of hip osteoarthritis. Osteoarthritis Cartilage. 2005;13:1126-7. doi: 10.1016/j.joca.2005.08.001 [DOI] [PubMed] [Google Scholar]
- 27. Tikiz C, Unlü Z, Sener A, Efe M, Tüzün C. Comparison of the efficacy of lower and higher molecular weight viscosupplementation in the treatment of hip osteoarthritis. Clin Rheumatol. 2005;24:244-50. doi: 10.1007/s10067-004-1013-5 [DOI] [PubMed] [Google Scholar]
- 28. van den Bekerom MP, Rys B, Mulier M. Viscosupplementation in the hip: evaluation of hyaluronic acid formulations. Arch Orthop Trauma Surg. 2008;128:275-80. doi: 10.1007/s00402-007-0374-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Conrozier T, Couris CM, Mathieu P, Merle-Vincent F, Piperno M, Coury F, et al. Safety, efficacy and predictive factors of efficacy of a single intra-articular injection of non-animal-stabilized-hyaluronic-acid in the hip joint: results of a standardized follow-up of patients treated for hip osteoarthritis in daily practice. Arch Orthop Trauma Surg. 2009;129:843-8. doi: 10.1007/s00402-008-0778-4 [DOI] [PubMed] [Google Scholar]
- 30. Migliore A, Massafra U, Bizzi E, Vacca F, Martin-Martin S, Granata M, et al. Comparative, double-blind, controlled study of intra-articular hyaluronic acid (Hyalubrix®) injections versus local anesthetic in osteoarthritis of the hip. Arthritis Res Ther. 2009;11:R183. doi: 10.1186/ar2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Richette P, Ravaud P, Conrozier T, Euller-Ziegler L, Mazières B, Maugars Y, et al. Effect of hyaluronic acid in symptomatic hip osteoarthritis: a multicenter, randomized, placebo-controlled trial. Arthritis Rheum. 2009;60:824-30. doi: 10.1002/art.24301 [DOI] [PubMed] [Google Scholar]
- 32. Bragantini A, Molinaroli F. A pilot clinical evaluation of the treatment of hip osteoarthritis with hyaluronic acid. Curr Ther Res. 1994;55:319-30. doi: 10.1016/S0011-393X(05)80175-4 [DOI] [Google Scholar]
- 33. Di Sante L, Villani C, Santilli V, Valeo M, Bologna E, Imparato L, et al. Intra-articular hyaluronic acid vs platelet-rich plasma in the treatment of hip osteoarthritis. Med Ultrason. 2016;18:463-8. doi: 10.11152/mu-874 [DOI] [PubMed] [Google Scholar]
- 34. Doria C, Mosele GR, Caggiari G, Puddu L, Ciurlia E. Treatment of early hip osteoarthritis: ultrasound-guided platelet rich plasma versus hyaluronic acid injections in a randomized clinical trial. Joints. 2017;5:152-5. doi: 10.1055/s-0037-1605584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Abate M, Salini V. Efficacy and safety study on a new compound associating low and high molecular weight hyaluronic acid in the treatment of hip osteoarthritis. Int J Immunopathol Pharmacol. 2017;30:89-93. doi: 10.1177/0394632016689275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Clementi D, D’Ambrosi R, Bertocco P, Bucci MS, Cardile C, Ragni P, et al. Efficacy of a single intra-articular injection of ultra-high molecular weight hyaluronic acid for hip osteoarthritis: a randomized controlled study. Eur J Orthop Surg Traumatol. 2018;28:915-22. doi: 10.1007/s00590-017-2083-9 [DOI] [PubMed] [Google Scholar]
- 37. Brandt KD, Smith GN, Jr, Simon LS. Intraarticular injection of hyaluronan as treatment for knee osteoarthritis: what is the evidence? Arthritis Rheum. 2000;43:1192-203. doi: [DOI] [PubMed] [Google Scholar]
- 38. Lindqvist U, Tolmachev V, Kairemo K, Aström G, Jonsson E, Lundqvist H. Elimination of stabilised hyaluronan from the knee joint in healthy men. Clin Pharmacokinet. 2002;41:603-13. doi: 10.2165/00003088-200241080-00004 [DOI] [PubMed] [Google Scholar]
- 39. Dahl LB, Dahl IM, Engström-Laurent A, Granath K. Concentration and molecular weight of sodium hyaluronate in synovial fluid from patients with rheumatoid arthritis and other arthropathies. Ann Rheum Dis. 1985;44:817-22. doi: 10.1136/ard.44.12.817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Arden NK, Åkermark C, Andersson M, Todman MG, Altman RD. A randomized saline-controlled trial of NASHA hyaluronic acid for knee osteoarthritis. Curr Med Res Opin. 2014;30:279-86. doi: 10.1185/03007995.2013.855631 [DOI] [PubMed] [Google Scholar]
- 41. Altman RD, Akermark C, Beaulieu AD, Schnitzer T; Durolane International Study Group. Efficacy and safety of a single intra-articular injection of non-animal stabilized hyaluronic acid (NASHA) in patients with osteoarthritis of the knee. Osteoarthritis Cartilage. 2004;12:642-9. doi: 10.1016/j.joca.2004.04.010 [DOI] [PubMed] [Google Scholar]
- 42. Smith MM, Ghosh P. The synthesis of hyaluronic acid by human synovial fibroblasts is influenced by the nature of the hyaluronate in the extracellular environment. Rheumatol Int. 1987;7:113-22. doi: 10.1007/bf00270463 [DOI] [PubMed] [Google Scholar]
- 43. Bagga H, Burkhardt D, Sambrook P, March L. Longterm effects of intraarticular hyaluronan on synovial fluid in osteoarthritis of the knee. J Rheumatol. 2006;33:946-50. [PubMed] [Google Scholar]
- 44. Hsieh YS, Yang SF, Lue KH, Chu SC, Lu KH. Effects of different molecular weight hyaluronan products on the expression of urokinase plasminogen activator and inhibitor and gelatinases during the early stage of osteoarthritis. J Orthop Res. 2008;26:475-84. doi: 10.1002/jor.20524 [DOI] [PubMed] [Google Scholar]
- 45. Dai WL, Lin ZM, Guo DH, Shi ZJ, Wang J. Efficacy and safety of hylan versus hyaluronic acid in the treatment of knee osteoarthritis. J Knee Surg. 2018;32:259-68. doi: 10.1055/s-0038-1641142 [DOI] [PubMed] [Google Scholar]
- 46. Adams ME, Lussier AJ, Peyron JG. A risk-benefit assessment of injections of hyaluronan and its derivatives in the treatment of osteoarthritis of the knee. Drug Saf. 2000;23:115-30. doi: 10.2165/00002018-200023020-00003 [DOI] [PubMed] [Google Scholar]
- 47. Keizers PHJ, Vanhee C, van den Elzen EMW, de Jong WH, Venhuis BJ, Hodemaekers HM, et al. A high crosslinking grade of hyaluronic acid found in a dermal filler causing adverse effects. J Pharm Biomed Anal. 2018;159:173-8. doi: 10.1016/j.jpba.2018.06.066 [DOI] [PubMed] [Google Scholar]
- 48. Wooley PH, Song Z, Harrison A. Hyaluronic acid viscosupplements from avian and non-mammalian sources exhibit biocompatibility profiles with unique, source-specific, antigenic profiles. J Biomed Mater Res B Appl Biomater. 2012;100:808-16. doi: 10.1002/jbm.b.32514 [DOI] [PubMed] [Google Scholar]
- 49. Leighton R, Fitzpatrick J, Smith H, Crandall D, Flannery CR, Conrozier T. Systematic clinical evidence review of NASHA (Durolane hyaluronic acid) for the treatment of knee osteoarthritis. Open Access Rheumatol. 2018;10:43-54. doi: 10.2147/oarrr.S162127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. American Academy of Orthopaedic Surgeons. Management of osteoarthritis of the hip: evidence-based clinical practice guideline. Available from: https://aaos.org/globalassets/quality-and-practice-resources/osteoarthritis-of-the-hip/management-of-osteoarthritis-of-the-hip-7-31-19.pdf