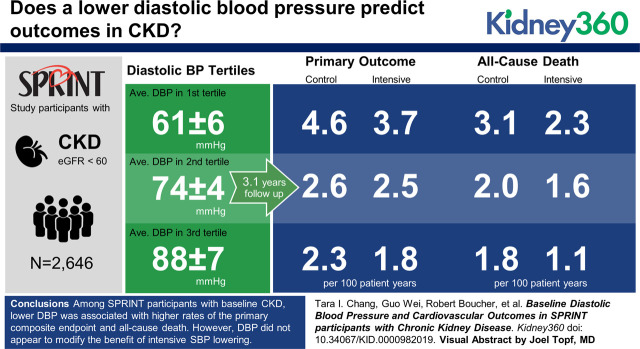
Visual Abstract
Keywords: Hypertension, Blood Pressure, Cause of Death, Hypertension, Randomized Controlled Trials, Renal Insufficiency, Chronic, SPRINT
Abstract
Background
We sought to determine whether intensive systolic BP (SBP) lowering was harmful in Systolic Blood Pressure Intervention Trial (SPRINT) participants with CKD (eGFR<60 ml/min per 1.73 m2) and lower baseline diastolic BP (DBP).
Methods
We related baseline DBP with the SPRINT primary composite end point (myocardial infarction, acute coronary syndrome, stroke, acute decompensated heart failure, or cardiovascular death) and all-cause death. We examined the effect of intensive SBP lowering on these outcomes across the range of baseline DBPs using Cox regression with treatment by baseline DBP interaction terms.
Results
Among 2646 SPRINT participants with CKD, lower baseline DBP was associated with a higher adjusted hazard of the primary composite end point and all-cause death. For example, participants with baseline DBP of 61 mm Hg (mean baseline DBP in the lowest tertile) experienced a 37% (95% CI, 7% to 75%) higher hazard of the primary outcome relative to participants with baseline DBP of 75 mm Hg (mean baseline DBP for overall). The benefit of intensive SBP lowering was consistent across a range of baseline DBPs on rates of the primary composite end point (linear interaction P value =0.56) and all-cause death (linear interaction P value =0.20).
Conclusions
Among SPRINT participants with baseline CKD, lower DBP was associated with higher rates of the primary composite end point and all-cause death. However, DBP did not seem to modify the benefit of intensive SBP lowering on the primary composite end point or all-cause death. Our results suggest that lower DBP should not necessarily impede more intensive SBP lowering in patients with mild to moderate CKD.
Introduction
Coronary perfusion primarily occurs during diastole, and intensive systolic BP (SBP) lowering often leads to diastolic BP (DBP) lowering. In observational on-treatment analyses, lower DBP is associated with an increased risk of cardiovascular disease events and death in patients at higher risk for cardiovascular events (1–4). Concern related to DBP lowering may be of particular relevance among patients with CKD given the higher prevalence of elevated SBP and lower DBP in that population due in part to known associations of CKD with premature vascular aging and vascular stiffness (5,6).
A recent analysis of data from the Systolic Blood Pressure Intervention Trial (SPRINT) demonstrated a U-curve relationship between baseline DBP and the primary outcome and all-cause death, in which participants with the lowest baseline DBP had the higher risk of adverse clinical events (7). However, comparison of randomized groups demonstrated that intensive SBP treatment reduced the risk of the primary outcome and all-cause death at every level of baseline DBP, including among participants with the lowest levels of baseline DBP. We sought to determine whether the effects of intensive SBP lowering in participants with CKD differed by baseline DBP and specifically, whether intensive SBP lowering was harmful in participants with CKD and lower baseline DBP.
Materials and Methods
The SPRINT was a multicenter, randomized outcome trial sponsored by the National Institutes of Health comparing the effects of intensive versus standard SBP lowering on cardiovascular and other outcomes, including all-cause death, kidney disease, dementia, and cognitive impairment (8–10).
Study Participants
Briefly, between November 2010 and March 2013, participants 50 years of age or older with treated or untreated SBP of 130–180 mm Hg and at least one of the following indicators of increased cardiovascular risk were enrolled: evidence of clinical or subclinical cardiovascular disease, CKD defined as an eGFR of 20 to <60 ml/min per 1.73 m2 using the four-variable Modification of Diet in Renal Disease equation, a 10-year Framingham risk score for cardiovascular events ≥15%, or age 75 years old or older. Persons with a history of stroke, diabetes mellitus, polycystic kidney disease, dementia, heart failure, nonadherence to medication, eGFR<20 ml/min per 1.73 m2, or ≥1 g of proteinuria per day (or the equivalent) were not eligible for participation. Participants enrolled were randomized to an intensive office SBP target of <120 mm Hg or a standard office SBP target of <140 mm Hg. This analysis includes the 2646 participants with baseline CKD defined as eGFR<60 ml/min per 1.73 m2.
All participants provided written informed consent for participation in the trial. The study was adherent to the Declaration of Helsinki. The trial was approved by an institutional review board at each site, and it was registered in ClinicalTrials.gov (NCT01206062).
BP Measurement
BP was estimated as the mean of three readings obtained at 1-minute intervals using standardized measurement techniques. Each BP reading was obtained using an automated machine (Model 907; Omron Healthcare) after the patient had been seated quietly for 5 minutes during a study office visit. During a 5-minute rest period prior to the BP measurements and during the measurements, study participants were either alone or with a member of the study team. Similar achieved BP levels and cardiovascular risk reduction were observed regardless of whether BP was measured with or without a staff member present (11).
Covariates
Trained study personnel ascertained information about participant baseline sociodemographic data, comorbid conditions, and antihypertensive medications during the screening or randomization visit. Fasting blood and urine samples were also collected. All assays were performed in a single central laboratory. Serum creatinine and urine creatinine were measured using an enzymatic procedure (Roche, Indianapolis, IN). Urine albumin was measured using a nephelometric method (Siemens, Tarrytown, NY).
Outcomes
The primary composite end point in SPRINT and in this analysis was the first occurrence of a myocardial infarction, acute coronary syndrome not resulting in myocardial infarction, stroke, acute decompensated heart failure, or a cardiovascular death. We also examined all-cause death. The primary composite end point and death from any cause were adjudicated by a committee blinded to the treatment group assignments.
Adverse events were monitored throughout the trial (9). In this analysis, we examined any serious adverse event and serious adverse events associated with hypotension, syncope, electrolyte abnormalities, and AKI or acute kidney failure.
Statistical Analyses
We computed the mean follow-up BP for each study participant by averaging the SBP or DBP measurements from month 3 to the last available reading. We used boxplots to display the mean follow-up SBP and DBP within the intensive and standard groups by tertile of baseline DBP. A total of 75 participants (2.8%; 28 in the intensive group and 47 in the standard group) had missing BP measurements after month 2 and were not included in this descriptive portion of the analysis.
Our analysis is on the basis of information provided in the SPRINT public access Biological Specimen and Data Repository Information Coordinating Center database. It includes events that occurred on or before the trial was stopped on August 20, 2015 and were recognized with the use of a data freeze date of October 14, 2015.
Associations of Baseline DBP with Outcomes.
We calculated the incidence per 100 person-years of the primary composite end point, all-cause death, and adverse events by tertile of baseline DBP for descriptive purposes. We related baseline DBP to these outcomes by fitting separate Cox regression models with baseline DBP as a cubic spline with knot points at the 25th, 50th, and 75th percentiles, with adjustment for the randomized SBP intervention, age, sex, and race as covariates. In sensitivity analyses, we repeated the above models with additional covariate adjustment for history of cardiovascular disease, Framingham 10-year cardiovascular disease risk score ≥15%, smoking history, and baseline eGFR.
Effect Modification by Baseline DBP of Intensive SBP Lowering on Outcomes.
We examined the effects of intensive SBP lowering on the primary composite end point and all-cause death across the range of baseline DBP in separate Cox regression models modeling baseline DBP as natural cubic spline terms with knot points at the 25th, 50th, and 75th percentiles. We included the cubic spline terms both as main effects and in multiplicative interactions with the randomized intensive SBP treatment group to display the results graphically. The linear interaction between baseline DBP and the randomized SBP intervention was tested by using a likelihood ratio test. To assist with the interpretation and graphic representation of our results, we conducted a companion analysis that evaluated the effect of the randomized SBP intervention on the primary outcome and all-cause death by tertile of baseline DBP. We compared the hazard ratios among the DBP tertiles to calculate a P value for interaction by using a likelihood ratio test. In sensitivity analyses, we included adjustments for age, sex, race, history of cardiovascular disease, Framingham 10-year cardiovascular disease risk score ≥15%, smoking history, and baseline eGFR in the models.
We performed all analyses using STATA version MP 14.0 or SAS version 9.4, and we used two-sided α=0.05 for hypothesis testing without adjustment for multiple comparisons. We compared numeric baseline characteristics between baseline DBP tertiles using one-way ANOVA and categorical variables using chi-squared tests.
Results
Among the 2646 SPRINT participants with baseline CKD, mean baseline DBP overall was 75±12 mm Hg, and mean baseline DBPs in the lowest, middle, and highest tertiles were 61±6, 74±3, and 88±7 mm Hg, respectively. Participants in the lowest tertile of baseline DBP were generally older, less often of black race, had a higher pulse pressure, had a higher prevalence of cardiovascular disease, and had a lower mean eGFR (Table 1).
Table 1.
Baseline characteristics by tertile of baseline diastolic BP among the Systolic Blood Pressure Intervention Trial participants with baseline CKD (N=2646)
| Baseline Characteristic | Lowest tertile <69 mm Hg, N=823 | Middle tertile =69–79 mm Hg, N=893 | Highest tertile ≥80 mm Hg, N=930 |
| Diastolic BP, mm Hg | 61±6 | 74±3 | 88±7 |
| Age, yr | 76.1±8.0 | 72.4±8.4 | 67.7±9.2 |
| Women, % | 42.0 | 40.8 | 37.4 |
| Black race, % | 19.9 | 21.7 | 30.8 |
| Cardiovascular disease, % | 30.7 | 26.5 | 16.6 |
| Framingham 10-yr cardiovascular disease risk score ≥15%, % | 63.5 | 64.6 | 65.4 |
| Never smoked, % | 44.2 | 45.4 | 47.1 |
| Antihypertensive agents, no. per patient | 2.3±0.9 | 2.1±1.0 | 1.9±1.0 |
| Systolic BP, mm Hg | 133±16 | 137±14 | 147±15 |
| Pulse pressure, mm Hg | 71±16 | 63±13 | 59±14 |
| Body mass index, kg/m2 | 28.5±5.6 | 29.3±5.6 | 30.4±5.9 |
| eGFR, ml/min per 1.73 m2 | 47±10 | 48±9 | 49±9 |
| CKD stage 3b or higher (eGFR<45 ml/min per 1.73 m2), % | 39.2 | 32.8 | 29.5 |
| Urine albumin-creatinine ratio, mg/g | 13.3 (6.8–38.8) | 11.6 (5.8–36.4) | 15.1 (6.7–52.8) |
Results are presented as percentage for binary variables and as mean ± SDs for continuous variables other than albumin-creatinine ratio, which is presented as median (interquartile range). For comparison of differences between the tertiles for numeric variables by using one-way ANOVA and categorical variables by using chi-squared tests, all P values were < 0.001, except for women (P=0.12), Framingham 10-year cardiovascular disease risk score ≥15% (P=0.72), and never smoked (P=0.48).
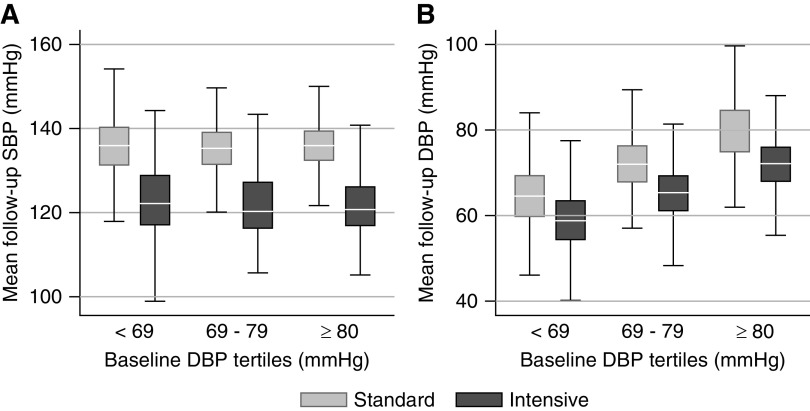
The achieved mean follow-up SBP was similar in the lowest and highest tertiles of baseline DBP in the intensive SBP intervention (123±10 versus 123±9 mm Hg, P=0.17) and standard SBP intervention (136±8 versus 136±7 mm Hg, P=0.85) (Figure 1A) groups. In contrast, the achieved mean follow-up DBP was significantly lower among participants in the lowest tertile compared with the highest tertile of DBP within the intensive SBP intervention (mean DBP of 59±7 versus 72±7 mm Hg, P<0.001) and standard SBP intervention (mean DBP of 65±8 versus 80±8 mm Hg, P<0.001) (Figure 1B) groups.
Figure 1.
Achieved mean SBP was similar across tertiles of baseline DBP, while achieved mean DBP was lower among participants in the lowest tertile of baseline DBP comred with the highest tertile. Follow-up minimum, median, 25th and 75th percentiles and maximum of patients' mean follow-up systolic BP (SBP; A) and diastoic BP (DBP; B) by randized SBP intervention and tertile of baseline DBP. Note that 75 of 2646 subjects (2.8%; 47 in the standard group and 28 in the intensive group) had missing BP measurements after month 2 and were excluded.
Associations of Baseline DBP with the Primary Composite End Point and All-Cause Death
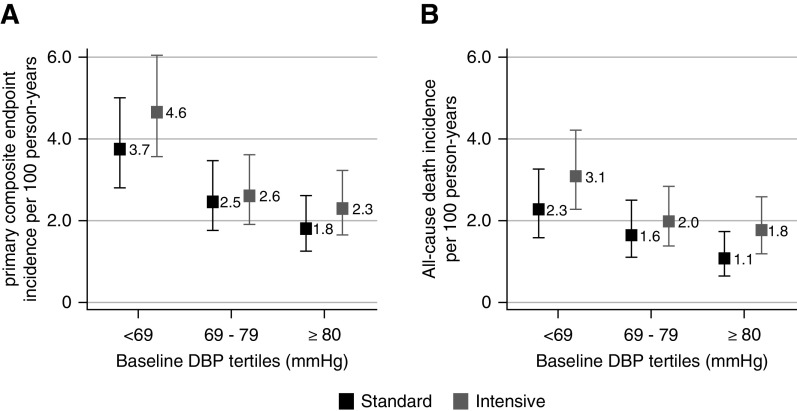
There were 234 primary outcome events during 8211 person-years of follow-up. The incidence rate of the primary outcome was highest in the lowest DBP tertiles (Figure 2A). In cubic spline regression models with adjustments for the randomized SBP intervention, age, sex, and race, baseline DBP had a curvilinear association with the primary composite end point, wherein lower baseline DBP was associated with higher adjusted hazard of the primary composite end point (Figure 3A). For example, baseline DBP of 61 mm Hg, which was the mean baseline DBP in the lowest baseline DBP tertile, was associated with a 37% (95% confidence interval, 7% to 75%) higher hazard of the primary composite end point compared with baseline DBP of 75 mm Hg (mean baseline DBP for the overall cohort).
Figure 2.
The incidence rate per 100 person-years of the primary outcome and all-cause death was highest in the lowest DBP tertiles in the Systolic Blood Pressure Intervention Trial participants with baseline CKD randomized to the intensive and standard treatment groups. (A) Primary composite end point. (B) All-cause death.
Figure 3.
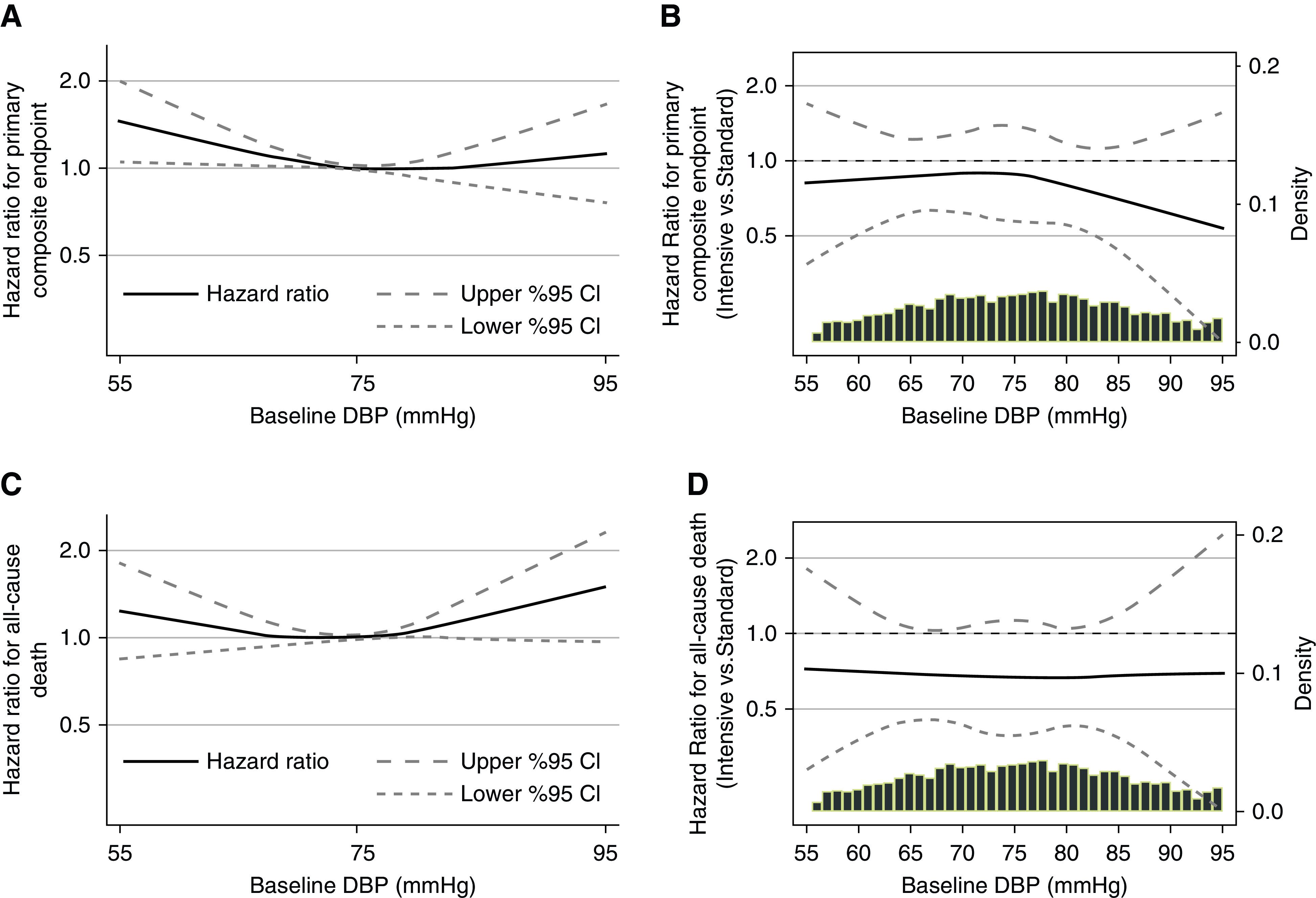
In cubic spline regression models, baseline DBP had a curvilinear association with the primary composite end point and all-cause death, but intensive SBP lowering did not increase the risk of these end points in participants with lower baseline DBP. (A and C) Cubic spline regression models showing hazard ratios with pointwise 95% confidence intervals (95% CIs) for the association of baseline DBP as a continuous variable with the primary composite end point (A) and all-cause death (C) in the Systolic Blood Pressure Intervention Trial participants with baseline CKD. Models include the randomized SBP intervention, age, sex, and race. (B and D) Cubic spline regression models showing hazard ratios with pointwise 95% CIs for the effect of the randomized SBP intervention across a range of baseline DBP on the primary composite end point (B) and all-cause death (D) in the Systolic Blood Pressure Intervention Trial participants with baseline CKD. Models are unadjusted. Likelihood ratio tests for the linear interaction item of the baseline DBP and the SBP intervention were nonsignificant (primary composite end point interaction P=0.56; all-cause death interaction P=0.20).
There were 165 deaths during 8576 person-years of follow-up. The incidence rate was higher for all-cause death in the lowest DBP tertiles (Figure 2B), and we saw a similar curvilinear association of DBP with all-cause death in cubic spline models (Figure 3C).
Results were not materially changed in sensitivity analyses that additionally adjusted for history of cardiovascular disease, Framingham 10-year cardiovascular disease risk score ≥15%, smoking history, and baseline eGFR (Supplemental Figure 1, A and C).
Effect Modification by Baseline DBP of Intensive SBP Lowering on the Primary Composite End Point and All-Cause Death
Compared with standard SBP lowering, intensive SBP lowering had a lower hazard of the primary composite end point, which did not reach statistical significance in this CKD cohort (hazard ratio, 0.84; 95% confidence interval, 0.65 to 1.09). When we used cubic spline regression models to examine a range of baseline DBPs, we found no evidence that intensive SBP lowering increased the risk of the primary composite end point in participants with lower baseline DBP (Figure 3B) (linear interaction P value =0.56). In companion analyses where we analyzed the effect of intensive SBP lowering on outcomes by tertile of baseline DBP, hazard ratios for the SBP intervention for the primary composite end point were similar (Figure 4A) (interaction P value =0.85).
Figure 4.
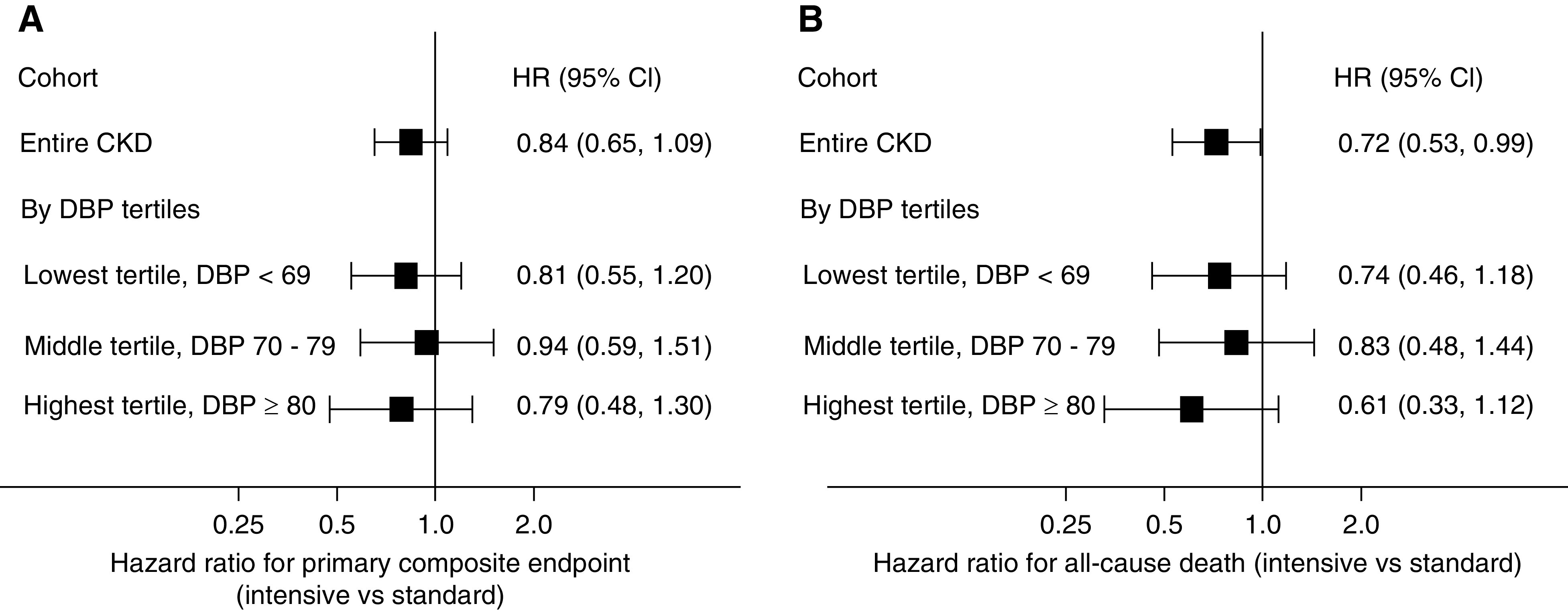
The effect of intensive SBP lowering on outcomes by tertile of baseline DBP, hazard ratios for the SBP intervention for the primary composite end point and all-cause death were similar. Forest plots showing hazard ratios (HRs) and 95% CIs for the effect of the randomized SBP intervention on the primary composite end point (A) and all-cause death (B) for the entire CKD cohort and by baseline DBP tertile. Likelihood ratio tests comparing HR for the SBP intervention among baseline DBP tertiles were nonsignificant (primary composite end point interaction P=0.85; all-cause death interaction P=0.75). Models are unadjusted.
Compared with standard SBP lowering, intensive SBP lowering had a lower hazard for all-cause death (hazard ratio, 0.72; 95% confidence interval, 0.53 to 0.99). Again, there was no evidence that intensive SBP lowering increased mortality in participants with lower baseline DBP in cubic spline regression models (Figure 3D) (linear interaction P value =0.20) or in analyses stratified by baseline DBP tertiles (Figure 4B) (interaction P value =0.75).
Results were not materially changed in sensitivity analyses that adjusted for age, sex, race, history of cardiovascular disease, Framingham 10-year cardiovascular disease risk score ≥15%, smoking history, and baseline eGFR (Supplemental Figures 1, B and D and 2).
Adverse Events
The incidence of any serious adverse event was higher in the lowest tertiles of baseline DBP in both the intensive and standard SBP lowering arms (Table 2). The effect of intensive versus standard SBP lowering on adverse events did not differ by tertiles of baseline DBP.
Table 2.
Incidence rates (per 100 person-years [N of event per follow-up year]) for intensive and standard treatment groups, hazard ratios, and 95% confidence intervals for the effect of the intensive versus standard systolic BP intervention on serious adverse events in the Systolic Blood Pressure Intervention Trial participants with CKD by tertile of baseline diastolic BP
| Adverse Event Type | Lowest tertile <69 mm Hg | Middle tertile =69–79 mm Hg | Highest tertile ≥80 mm Hg |
| Any serious adverse event a | |||
| Incidence rate | |||
| Intensive | 24.3 (223/919) | 21.6 (218/1010) | 15.0 (187/1246) |
| Standard | 26.4 (233/883) | 18.5 (206/1111) | 18.1 (202/1118) |
| HR (95% CI) intensive versus standard | 0.93 (0.78 to 1.12) | 1.11 (0.92 to 1.35) | 0.84 (0.69 to 1.03) |
| Hypotension | |||
| Incidence rate | |||
| Intensive | 1.0 (13/1263) | 1.3 (18/1338) | 1.1 (17/1525) |
| Standard | 0.9 (11/1238) | 0.8 (12/1423) | 0.8 (11/1424) |
| HR (95% CI) intensive versus standard | 1.14 (0.51 to 2.55) | 1.55 (0.75 to 3.23) | 1.37 (0.64 to 3.00) |
| Syncope | |||
| Incidence rate | |||
| Intensive | 1.3 (16/1264) | 1.1 (15/1349) | 0.7 (10/1538) |
| Standard | 0.9 (11/1244) | 0.8 (12/1426) | 0.5 (7/1431) |
| HR (95% CI) intensive versus standard | 1.38 (0.64 to 3.00) | 1.28 (0.60 to 2.74) | 1.25 (0.46 to 3.37) |
| Electrolyte abnormality | |||
| Incidence rate | |||
| Intensive | 1.8 (22/1245) | 2.0 (27/1324) | 0.9 (14/1532) |
| Standard | 1.4 (17/1228) | 0.8 (12/1425) | 1.1 (16/1408) |
| HR (95% CI) intensive versus standard | 1.37 (0.73 to 2.60) | 2.33 (1.18 to 4.61) | 0.73 (0.36 to 1.51) |
| AKI or acute kidney failure | |||
| Incidence rate | |||
| Intensive | 3.7 (45/1220) | 3.1 (41/1302) | 1.8 (27/1515) |
| Standard | 2.4 (29/1227) | 1.6 (23/1418) | 1.9 (27/1394) |
| HR (95% CI) intensive versus standard | 1.53 (0.95 to 2.44) | 1.81 (1.08 to 3.01) | 1.00 (0.58 to 1.72) |
HR, hazard ratio; 95% CI, 95% confidence interval.
Models adjusted for age, sex, race, cardiovascular disease, Framingham 10-year cardiovascular disease risk score ≥15%, smoking history, and baseline eGFR. Likelihood ratio tests comparing HRs for the systolic BP intervention among baseline diastolic BP tertiles were nonsignificant.
Discussion
On-treatment observational studies have shown associations among lower DBP and higher rates of adverse cardiac ischemic events, particularly in populations with established coronary artery disease (1–4,12). For example, a secondary observational reanalysis of 22,576 patients with hypertension and coronary artery disease enrolled in the International Verapamil-Trandolapril study showed that patients with an achieved mean DBP of <60 mm Hg had a higher incidence of cardiovascular events and death compared with their counterparts who had a higher mean achieved DBP (12). An observational reanalysis of the Treating to New Targets trial in which patients with clinically evident coronary artery disease were treated with higher or lower doses of atorvastatin again showed that an achieved DBP <80 mm Hg was associated with higher rates of fatal and nonfatal cardiovascular events (2).
Given the associations among lower DBP and cardiovascular events in these observational studies, concerns about DBP lowering could be even more pronounced in patients with CKD. Interventions aimed at lowering SBP will generally reduce DBP as well and could theoretically lead to underperfusion and ischemia of vital organs. Patients with CKD also have a high prevalence of hypertension and left ventricular hypertrophy, which impair coronary blood flow autoregulation, wherein more intensive BP lowering could lead to more marked declines in coronary perfusion pressures (13).
In this study of SPRINT participants with CKD, we found that an observational analysis yielded the expected association between lower baseline DBP and higher rates of the primary outcome and all-cause mortality. However, in randomized comparisons of the two treatment groups, we found no indication that the beneficial effect of the intensive SBP intervention in SPRINT participants with CKD on the primary composite end point and all-cause death differed by baseline level of DBP. Therefore, although lower baseline DBP may serve as a marker of poor vascular health and/or other conditions that signal higher risk of cardiovascular and other adverse events, more intensive BP treatment in participants with lower DBP and CKD still proved beneficial.
Our analysis has several strengths, including the inclusion of patients with CKD in the SPRINT as a prespecified subgroup and the randomized allocation to an intensive or standard SBP treatment target. Observational analyses that rely on examination of achieved DBP can lead to different inferences due at least in part to confounding of achieved BP by differences in disease severity and adherence (14). However, our analysis also has some limitations. First, although the SPRINT represents the largest BP intervention trial in CKD to date, it was not designed to detect interaction by baseline DBP in the CKD subgroup, and thus, we may have lacked the power to detect a statistically significant interaction. Second, although our cohort was diverse in terms of age, sex, race, and inclusion of patients with baseline heart disease, the study excluded patients with diabetes mellitus or history of stroke, which may limit the generalizability of our findings to those important patient populations. In addition, the SPRINT enrolled relatively few patients with stage 3b or higher CKD and excluded patients with >1 g of proteinuria per day. Because the efficacy and safety of intensive BP lowering may differ in patients with more advanced (stage 4/5) CKD, future BP intervention studies dedicated to enrolling this patient population specifically are needed.
In summary, patients with mild to moderate CKD are at high risk for cardiovascular events and death. In SPRINT participants with CKD at baseline, lower DBP was associated with higher rates of the primary composite end point and all-cause death as well as higher rates of serious adverse events, including hypotension, syncope, electrolyte abnormalities, and AKI, in an observational on-treatment analysis. However, in randomized comparisons, lower baseline levels of DBP did not modify the beneficial effect of the intensive SBP intervention on the primary outcome and all-cause death. Our results suggest that lower DBP should not necessarily be a barrier to more intensive SBP lowering in patients with mild to moderate CKD. Careful treatment and follow-up are essential in all patients with CKD, especially patients with more advanced disease.
Disclosures
S. Beddhu reports grants from NHLBI and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), during the conduct of the study, grants from Bayer, AbbVie, and Boehringer Ingelheim, and consultation fee from Reata, outside the submitted work. T. I. Chang reports receiving consulting fees from Fresenius Medical Care Renal Therapies Group, LLC; Janssen Research and Development, LLC; Novo Nordisk; and Tricida Inc. and grant support from Satellite Health Care for work unrelated to this manuscript. R. Boucher, G. M. Chertow, A. K. Cheung, T. Greene, H. Kramer, G. Wei, and P. K. Whelton have nothing to disclose.
Funding
Statistical analyses and preparation of this manuscript were supported by NIDDK grants R21HL145494, R01DK115814, and R21DK106574, and the University of Utah Study Design and Biostatistics Center funded in part from National Center for Research Resources Public Health Services research grants UL1-RR025764 and C06-RR11234. The Systolic Blood Pressure Intervention Trial was funded with federal funds from the National Institutes of Health, including NHLBI; NIDDK; National Institute on Aging; and National Institute of Neurological Disorders and Stroke contracts HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, and HHSN268200900049C and interagency agreement A-HL-13-002-001. It was also supported in part with resources and use of facilities through the US Department of Veterans Affairs. We also acknowledge the support from the following Clinical and Translational Science Awards funded by National Center for Advancing Translational Sciences grants Case Western Reserve University: UL1TR000439; Ohio State University: UL1RR025755; University of Pennsylvania: UL1RR024134 and UL1TR000003; Boston: UL1RR025771; Stanford: UL1TR000093; Tufts: UL1RR025752, UL1TR000073, and UL1TR001064; University of Illinois: UL1TR000050; University of Pittsburgh: UL1TR000005; University of Texas Southwestern: 9U54TR000017-06; University of Utah: UL1TR000105-05; Vanderbilt University: UL1TR000445; George Washington University: UL1TR000075; University of California, Davis: UL1TR000002; University of Florida: UL1TR000064; University of Michigan: UL1TR000433; and Tulane University: P30GM103337 Center of Biomedical Research Excellence Award National Institute of General Medical Sciences.
Supplemental Material
This article contains supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0000982019/-/DCSupplemental.
Cubic spline regression models showing hazard ratios with pointwise 95% confidence intervals for the association of baseline diastolic BP as a continuous variable with the primary composite end point and all-cause death and for the effect of the randomized systolic BP intervention across a range of baseline diastolic BPs on the primary composite end point and all-cause death in the Systolic Blood Pressure Intervention Trial participants with baseline CKD. Download Supplemental Figure 1, PDF file, 260 KB (259.4KB, pdf)
Forest plots showing hazard ratios and 95% confidence intervals for the effect of the randomized systolic BP intervention on the primary composite end point and all-cause death for the entire CKD cohort and by baseline diastolic BP tertile. Download Supplemental Figure 2, PDF file, 260 KB (259.4KB, pdf)
Acknowledgments
The Systolic Blood Pressure Intervention Trial (SPRINT) investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals International, Inc.
All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing and revision were carried out by the coauthors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the US Department of Veterans Affairs, or the US Government. This manuscript was prepared using SPRINT Primary Outcome Paper Data (SPRINT-POP) Research Materials obtained from the National Heart, Lung, and Blood Institute (NHLBI) Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the SPRINT_POP or the NHLBI. All authors are SPRINT investigators. This manuscript was prepared using SPRINT data obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center. The views expressed in this paper are those of the authors and do not represent the official position of the National Institutes of Health, the Department of Veterans Affairs, the US Government, or the SPRINT Research Group. This paper was approved by the SPRINT Publications and Presentations Committee.
Author Contributions
S. Beddhu, R. Boucher, T. I. Chang, T. Greene, and G. Wei conceptualized the study; S. Beddhu and T. I. Chang were responsible for investigation; S. Beddhu, R. Boucher, T. I. Chang, and G. Wei were responsible for methodology; S. Beddhu, T. I. Chang, and T. Greene provided supervision; S. Beddhu and T. I. Chang wrote the original draft; R. Boucher and G. Wei were responsible for data curation; G. Wei was responsible for formal analysis; S. Beddhu and G. Wei were responsible for software; S. Beddhu, T. Greene, and G. Wei were responsible for validation; T.I. Chang, S. Beddhu, and T. Greene were responsible for project administration; S. Beddhu was responsible for funding acquisition and resources; and S. Beddhu, R. Boucher, T. I. Chang, G. M. Chertow, A. K. Cheung, T. Greene, H. Kramer, G. Wei, and P. K. Whelton reviewed and edited the manuscript.
References
- 1.Vidal-Petiot E, Ford I, Greenlaw N, Ferrari R, Fox KM, Tardif JC, Tendera M, Tavazzi L, Bhatt DL, Steg PG; CLARIFY Investigators : Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: An international cohort study. Lancet 388: 2142–2152, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Bangalore S, Messerli FH, Wun CC, Zuckerman AL, DeMicco D, Kostis JB, LaRosa JC; Treating to New Targets Steering Committee and Investigators : J-curve revisited: An analysis of blood pressure and cardiovascular events in the Treating to New Targets (TNT) Trial. Eur Heart J 31: 2897–2908, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Protogerou AD, Safar ME, Iaria P, Safar H, Le Dudal K, Filipovsky J, Henry O, Ducimetière P, Blacher J: Diastolic blood pressure and mortality in the elderly with cardiovascular disease. Hypertension 50: 172–180, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Itoga NK, Tawfik DS, Lee CK, Maruyama S, Leeper NJ, Chang TI: Association of blood pressure measurements with peripheral artery disease events. Circulation 138: 1805–1814, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sedaghat S, Mattace-Raso FUS, Hoorn EJ, Uitterlinden AG, Hofman A, Ikram MA, Franco OH, Dehghan A: Arterial stiffness and decline in kidney function. Clin J Am Soc Nephrol 10: 2190–2197, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Covic A, Kanbay M, Voroneanu L, Turgut F, Serban DN, Serban IL, Goldsmith DJ: Vascular calcification in chronic kidney disease. Clin Sci (Lond) 119: 111–121, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Beddhu S, Chertow GM, Cheung AK, Cushman WC, Rahman M, Greene T, Wei G, Campbell RC, Conroy M, Freedman BI, Haley W, Horwitz E, Kitzman D, Lash J, Papademetriou V, Pisoni R, Riessen E, Rosendorff C, Watnick SG, Whittle J, Whelton PK; SPRINT Research Group : Influence of baseline diastolic blood pressure on effects of intensive compared with standard blood pressure control. Circulation 137: 134–143, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, Fine LJ, Goff DC Jr., Johnson KC, Killeen AA, Lewis CE, Oparil S, Reboussin DM, Rocco MV, Snyder JK, Williamson JD, Wright JT Jr., Whelton PK; SPRINT Study Research Group : The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: The systolic blood pressure intervention trial (SPRINT). Clin Trials 11: 532–546, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT; SPRINT Research Group: A randomized trial of intensive versus standard blood-pressure control [published correction appears in N Engl J Med 377: 2506, 2017]. N Engl J Med 373: 2103–2116, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, Cheung AK, Cleveland ML, Coker LH, Crowe MG, Cushman WC, Cutler JA, Davatzikos C, Desiderio L, Erus G, Fine LJ, Gaussoin SA, Harris D, Hsieh MK, Johnson KC, Kimmel PL, Tamura MK, Launer LJ, Lerner AJ, Lewis CE, Martindale-Adams J, Moy CS, Nasrallah IM, Nichols LO, Oparil S, Ogrocki PK, Rahman M, Rapp SR, Reboussin DM, Rocco MV, Sachs BC, Sink KM, Still CH, Supiano MA, Snyder JK, Wadley VG, Walker J, Weiner DE, Whelton PK, Wilson VM, Woolard N, Wright JT Jr., Wright CB; SPRINT MIND Investigators for the SPRINT Research Group : Effect of intensive vs standard blood pressure control on probable dementia: A randomized clinical trial. JAMA 321: 553–561, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson KC, Whelton PK, Cushman WC, Cutler JA, Evans GW, Snyder JK, Ambrosius WT, Beddhu S, Cheung AK, Fine LJ, Lewis CE, Rahman M, Reboussin DM, Rocco MV, Oparil S, Wright JT Jr.; SPRINT Research Group : Blood pressure measurement in sprint (systolic blood pressure intervention trial). Hypertension 71: 848–857, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Messerli FH, Mancia G, Conti CR, Hewkin AC, Kupfer S, Champion A, Kolloch R, Benetos A, Pepine CJ: Dogma disputed: Can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med 144: 884–893, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Polese A, De Cesare N, Montorsi P, Fabbiocchi F, Guazzi M, Loaldi A, Guazzi MD: Upward shift of the lower range of coronary flow autoregulation in hypertensive patients with hypertrophy of the left ventricle. Circulation 83: 845–853, 1991 [DOI] [PubMed] [Google Scholar]
- 14.Davis EM, Appel LJ, Wang X, Greene T, Astor BC, Rahman M, Toto R, Lipkowitz MS, Pogue VA, Wright JT Jr.; African American Study of Kidney Disease and Hypertension Research Collaborative Group : Limitations of analyses based on achieved blood pressure: Lessons from the African American study of kidney disease and hypertension trial. Hypertension 57: 1061–1068, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cubic spline regression models showing hazard ratios with pointwise 95% confidence intervals for the association of baseline diastolic BP as a continuous variable with the primary composite end point and all-cause death and for the effect of the randomized systolic BP intervention across a range of baseline diastolic BPs on the primary composite end point and all-cause death in the Systolic Blood Pressure Intervention Trial participants with baseline CKD. Download Supplemental Figure 1, PDF file, 260 KB (259.4KB, pdf)
Forest plots showing hazard ratios and 95% confidence intervals for the effect of the randomized systolic BP intervention on the primary composite end point and all-cause death for the entire CKD cohort and by baseline diastolic BP tertile. Download Supplemental Figure 2, PDF file, 260 KB (259.4KB, pdf)