Abstract
INTRODUCTION:
To examine the independent association of BMI in early adulthood with dementia incidence among men and women.
Methods:
We studied 5,104 older adults from the Cardiovascular Health Study and the Health, Aging, and Body Composition study. We imputed early adulthood and midlife BMI using a pooled parent cohort with complete adult lifespan coverage and previously established methods. Dementia was ascertained using criteria such as neuropsychological test battery, medical records, and dementia-related drug use. Pooled logistic regression models were used.
Results:
Compared to women with normal BMI in early adulthood, the odds of dementia were higher among both overweight (OR=1.8; 95%CI=1.31–2.54) and obese (OR=2.45; 95%CI=1.47–4.06) women, independent of mid and late life BMI. Similar relationship was observed in men.
Conclusions:
With the growing obesity epidemic among US adults, efforts aimed at reducing dementia may need to begin obesity prevention and treatment early in the life course.
Keywords: Body mass index, Cohort, Dementia, Life course
INTRODUCTION
Over the past few years, research has demonstrated that vascular factors are major contributors to late life cognitive decline and dementia.1–22 Despite this wealth of data, the timing and influence of key cardiovascular risk factors, including body mass index (BMI), remains controversial. For example, studies have shown that while high midlife BMI is associated with increased risk of dementia, high late life BMI may be unrelated or appear protective.14–16, 23–25 These associations may be subject to unmeasured confounding by earlier exposures, as well as other biases. For example, the unexpected null or inverse association of dementia with higher BMI in late life may be due to reverse causation, reflecting the long preclinical phase of dementia.23–25 Such inconsistencies support the need for a life course approach in which BMI is measured before any preclinical neurodegenerative changes, such as in early adulthood.
The association of BMI in early adulthood with dementia has not been thoroughly studied.26, 27 Several mechanisms have been hypothesized to link BMI to dementia, including vascular28–31, inflammation32, 33 and a direct effect of adiposity and fat-related hormones34–36 (e.g. leptin) on the brain. These pathways have also been suggested to vary by sex34 as has the incidence of dementia37–39. Understanding such relationship is particularly important in light of the persisting increases in obesity among US adults.40 Yet, such research has been mostly hampered by the lack of feasibility and difficulty of following participants from early adulthood to late life, when dementia almost exclusively occurs. With the absence of large and racially diverse single US cohorts with complete adult lifespan coverage, the only way to address the gap in our knowledge, on whether early adulthood BMI is a risk factor for dementia, is by leveraging lifecourse pooled cohorts. In this study, using BMI data from a pooled cohort spanning the lifecourse, we imputed adult life BMI for older adults, then examined its association with dementia incidence in late life.
METHODS
Parent pooled cohort
We previously pooled four prospective cohorts that span the adult life course, including a total of 15,001 white and black adults aged 18 to 95 years old at enrollment. Cohorts included 4,632 participants from the Coronary Artery Risk Development in Young Adults study (CARDIA) of young to middle-aged adults, 4,238 participants from the Multi Ethnic Study of Atherosclerosis (MESA) of middle to older-aged adults, 3,936 participants from the Cardiovascular Health Study (CHS), and 2,195 participants from the Health, Aging and Body Composition study (Health ABC) of older adults. Since, by design, some of the individual cohorts included only White and Black adults, as did our pooled cohort. Furthermore, each of the four cohorts is a prospective cohort study. Measures of BMI and other cardiovascular risk factors were harmonized across cohorts. Details of the parent pooled cohort have been published elsewhere.41 All participants provided written informed consent. The inclusion of the cohorts in our study was approved by local institutional review boards (IRBs) as well as the IRBs at Columbia University and the University of California San Francisco, and the present analysis was approved by the Publications & Presentations committee of each study.
Analytical cohort
Given that dementia incidence is our outcome of interest, for this analysis, we focused on the older adults of the parent pooled cohort, i.e. CHS and Health ABC cohorts. Our pooled sample included a total of 5,104 white and black participants aged 69 to 78 at enrollment, for whom BMI and dementia data were available, including 2,909 from CHS and 2,195 from Health ABC. Below are brief details of each study.
CHS is a prospective study of community-dwelling adults recruited from four U.S. communities: Washington County in Maryland, Forsyth County in North Carolina, Sacramento County in California, and Allegheny County in Pennsylvania. Participants were 65 or older at baseline in 1989–90 and included 57% women and 12% blacks. Participants were followed annually for up to 11 years.
Health ABC is a prospective cohort study of well-functioning community-dwelling adults aged 70–79 years at baseline in 1997. Participants were a random sample of Medicare-eligible older adults living in Memphis and Pittsburgh including 52% women and 42% blacks. Participants were followed annually or semi-annually for up to 11 years.
Measurement of early adulthood and midlife BMI for CHS/Health ABC older adults
In both CHS and Health ABC, BMI was calculated from measured height and weight, which were available at almost all study visits. Because BMI was only collected beginning in late life in the CHS and Health ABC cohorts, adult and midlife BMI exposures were imputed using the pooled parent cohort, which in addition to CHS and Health ABC, also included young and middle-age adults from CARDIA and MESA. In particular, using repeated measures of BMI, we estimated person-specific trajectories of BMI beginning from age 20 and forward using best linear unbiased predictions based on linear mixed models (LMMs) that included sex, race and smoking status. In brief, these borrow information across cohorts to extrapolate trends in observed BMI values in CHS and Health ABC, allowing for between-cohort differences in level, and accounting for birth year, on the assumption that covariate-specific age trends are common across the four cohorts. Details of our imputation procedure and accompanying simulation studies have been described elsewhere41 as well as in the Supplement. We then calculated time-weighted averages (TWAs), which are averages over a time interval, to summarize BMI in adult life (ages 20–49), midlife (ages 50–69) and late life (ages 70–89). These age cut-points were chosen based on prior work41–43. In sensitivity analyses, we considered alternative age cut-points. Period-specific BMI TWAs were then categorized at established cut-points into normal (<25 kg/m2), overweight (25–30 kg/m2) or obese (>30 kg/m2).
Ascertainment of dementia endpoints
In CHS, a neuropsychological test battery was administered to selected participants at high risk for dementia, defined by one of the following: Modified Mini-Mental State Examination (3MS)44 score<80, a decrease of 5 or more points on the 3MS, Informant Questionnaire on Cognitive Decline in the Elderly45 score > 3.6, incident stroke, medical record with dementia diagnosis, or nursing home residence. Participants who failed the memory test or in at least two cognitive domains underwent a detailed neurological examination by a neurologist. An expert committee of neurologists and psychiatrists classified participants as either normal, mild cognitive impairment (MCI), or dementia.46 In cases, when neuropsychological testing was not feasible, dementia classification was made based on collected neurological examination throughout the study period, medical records, and physician questionnaires.
In Health ABC, dementia diagnosis was determined according to any of the following criteria: 1) dementia-related hospitalization based on ICD codes for admission and discharge (assessed every 6 months); 2) use of prescribed dementia-related drugs determined using a drug inventory administered at annual visits; 3) decline of at least 1.5 race-specific standard deviations on the 3MS between baseline and last visit.47
Statistical Analysis
We first tabulated participant characteristics including period-specific BMI by sex and final dementia status. We then plotted the trajectory of average imputed BMI, also by sex and final dementia status. We used Poisson regression models, with person-years of exposure accrued from first to last study visit or dementia diagnosis, whichever is earlier, to estimate dementia incidence rates within period-specific BMI categories, first unadjusted, then adjusting for age, sex, race/ethnicity, education, and cohort; adjusted incidence rates were obtained using regression standardization. These models used log person-years at risk as an offset, and robust standard errors to allow for over-dispersion. To examine the associations between period-specific BMI and dementia incidence, we used pooled logistic regression (PLR) models48, with age as the time scale, and adjusting for sex, race/ethnicity, years of education, and cohort. Like Cox proportional hazards regression, PLR is a convenient alternative that also accommodates left truncation by age at study entry, censoring, and time-varying covariates, in particular, our late life BMI TWAs (i.e. for ages 70–89). Specifically, each individual in the analytic sample contributes a record to the PLR analysis for each year of age from our CHS-Health ABC cohort entry (range 69–78) until onset of dementia or censoring. Thus the BMI TWAs in early adulthood (ages 20–49) and in midlife (ages 50–69) are fixed covariates as they were accrued prior to cohort entry. However, the BMI TWAs in late life (ages 70–89) are time-varying. For example, for a CHS-Health ABC participant who enrolled at age 70 and contributed records (i.e. was followed) till age 75, their late life BMI TWAs for ages 70–89 are averages over fitted BMI values at ages 70–71, 70–72, 70–73, 70–75, and 70–75 respectively (i.e. time-varying or age-updated) (Supplemental Figure 1). All analyses are stratified by sex since interactions were significant at a p-value 0.05, and also based on known and well established sex differences in BMI patterns over the lifecourse as well as dementia. STATA version 16, including the margins command for regression standardization, was used for the analyses.
We also conducted the following sensitivity analyses: We examined the same relationships of BMI and dementia (1) adjusting for vascular risk factors that we hypothesize as mediators, (2) using different age-cutoffs for summarizing BMI exposure, (3) accounting for survival bias, (4) and separately for CHS and Health ABC to account for differences in dementia diagnoses.
RESULTS
The CHS/Health ABC sample included a total of 5,104 participants, of whom 493 (17.4%) of 2,835 women and 416 (18.3%) of 2,269 men were found to have dementia at some point during follow up (Table 1). Average follow-up was 7.9 years (range 0–11). Among both women and men, the number of dementia cases was higher among blacks and among subjects with fewer years of education, but did not vary by cohort. Among both women and men who eventually developed dementia, early adulthood BMI was higher but later life BMI was lower.
Table 1.
Dementia status according to participant characteristics and period-specific BMI, pooled CHS and Health ABC
| Women | Men | |||
|---|---|---|---|---|
| Dementia status | Dementia status | |||
| No N = 2,342 | Yes N = 493 | No N = 1,853 | Yes N = 416 | |
| Age (years) | 80.0 (77.0 – 83.0) | 81.0 (77.0 – 84.0) | 81.0 (78.0 – 84.0) | 81.0 (78.0 – 84.0) |
| Race/ethnicity | ||||
| White | 1,916 (84.4%) | 355 (15.6%) | 1,568 (82.9%) | 324 (17.1%) |
| Black | 426 (75.5%) | 138 (24.5%) | 285 (75.6%) | 92 (24.4%) |
| Education | ||||
| <HS | 483 (74.9%) | 162 (25.1%) | 394 (75.0%) | 131 (25.0%) |
| Completed HS | 824 (83.1%) | 167 (16.9%) | 458 (83.3%) | 92 (16.7%) |
| >HS | 1,031 (86.3%) | 163 (13.7%) | 1,000 (83.8%) | 193 (16.2%) |
| Cohort | ||||
| CHS | 1,391 (83.2%) | 280 (16.8%) | 1,010 (81.6%) | 228 (18.4%) |
| HABC | 951 (81.7%) | 213 (18.3%) | 843 (81.8%) | 188 (18.2%) |
| Early Adulthood BMI TWA (ages 20–49) | ||||
| <25 | 1,731 (84.0%) | 329 (16.0%) | 1,357 (83.1%) | 276 (16.9%) |
| 25–30 | 477 (80.6%) | 115 (19.4%) | 457 (80.0%) | 114 (20.0%) |
| >30 | 134 (73.2%) | 49 (26.8%) | 39 (60.0%) | 26 (40.0%) |
|
Midlife BMI TWA
(ages 50–69) |
||||
| <25 | 919 (81.0%) | 215 (19.0%) | 625 (82.9%) | 129 (17.1%) |
| 25–30 | 898 (85.1%) | 157 (14.9%) | 936 (82.1%) | 204 (17.9%) |
| >30 | 525 (81.3%) | 121 (18.7%) | 292 (77.9%) | 83 (22.1%) |
|
Late life BMI TWA
(ages 70–89) |
||||
| <25 | 888 (78.8%) | 239 (21.2%) | 602 (78.3%) | 167 (21.7%) |
| 25–30 | 898 (84.6%) | 163 (15.4%) | 936 (83.1%) | 191 (16.9%) |
| >30 | 556 (85.9%) | 91 (14.1%) | 315 (84.5%) | 58 (15.5%) |
BMI indicates Body Mass Index, TWA indicates Time Weighted Averages.
Data shown as median (IQR) and n (row %).
Measures of period-specific BMI at the last observed age of each participant.
Numbers of dementia cases, person-years of follow-up, and unadjusted dementia incidence rates are shown in Table 2. Among women, dementia incidence increased across early adulthood BMI categories, from 15.9/1000PY (95% CI 14.1, 17.8) among women with BMI <25 kg/m2, to 29.7/1000 PY (95% CI 22.0, 37.2) and 46/1000 PY (95% CI 27.7, 64.3) among those with BMI 25–30 kg/m2 and >30 kg/m2 respectively. In contrast, dementia incidence among women decreased across mid and late life BMI TWA categories. Among men as among women, dementia incidence increased across early adulthood BMI categories and decreased across late life BMI categories, but in contrast to women increased across midlife BMI categories. These patterns were unchanged after adjustment for age, race/ethnicity, education, and study cohort (Figure 1).
Table 2.
Incidence of dementia events across categories of period-specific BMI, by sex, pooled CHS and Health ABC.
| Women | Men | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of Events | Person-years | IR* | 95% CI | Number of Events | Person-years | IR* | 95% CI | ||
| Overall | 493 | 25581 | 416 | 20097 | |||||
| Early Adulthood BMI TWA (ages 20–49) | |||||||||
| <25 | 329 | 18377 | 15.9 | 14.1, 17.8 | 276 | 14298 | 19.0 | 16.6, 21.4 | |
| 25–30 | 115 | 5469 | 29.7 | 22.0, 37.2 | 114 | 5190 | 22.8 | 18.0, 27.7 | |
| >30 | 49 | 1735 | 46.0 | 27.7, 64.3 | 26 | 609 | 44.6 | 26.0, 63.2 | |
| Midlife BMI TWA (ages 50–69) | |||||||||
| <25 | 215 | 9947 | 24.6 | 19.3, 29.9 | 129 | 6551 | 16.8 | 13.2, 20.4 | |
| 25–30 | 157 | 9611 | 17.8 | 14.7, 20.9 | 204 | 10070 | 22.0 | 18.8, 25.3 | |
| >30 | 121 | 6023 | 15.1 | 10.6, 19.5 | 83 | 3476 | 26.2 | 17.4, 34.9 | |
| Late life BMI TWA (ages 70–89) | |||||||||
| <25 | 239 | 10895 | 22.0 | 18.8, 25.3 | 167 | 7006 | 29.4 | 24.0, 34.7 | |
| 25–30 | 163 | 9144 | 19.5 | 16.3, 22.6 | 191 | 9813 | 19.4 | 16.5, 22.2 | |
| >30 | 91 | 5542 | 14.3 | 10.7, 17.9 | 58 | 3278 | 12.8 | 8.9, 16.6 | |
IR: numbers of events per 1000 person-years at risk
Figure 1. Incidence rates* of dementia (per 1000 person-years) across categories of period-specific BMI, by sex, pooled CHS and Health ABC.
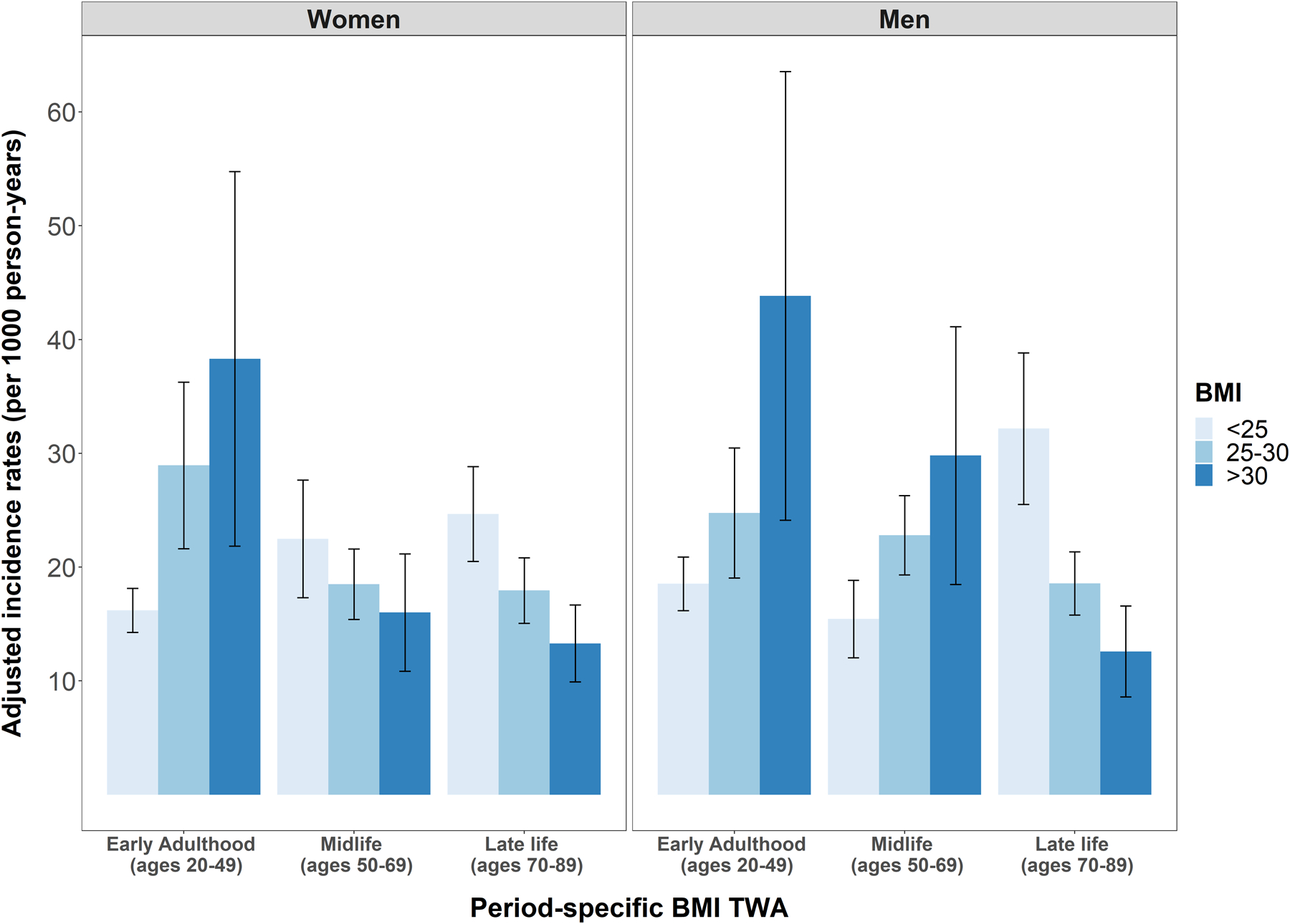
TWA indicates Time weighted averages.
*Adjusted for sex and race/ethnicity, education and cohort
Early Adulthood BMI exposure and dementia (Table 3):
Table 3.
Associations of period-specific BMI with incident dementia from pooled logistic regression by sex, pooled CHS and Health ABC
| Women | Men | ||||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | ||
| Early Adulthood BMI TWA (ages 20–49) | |||||||
| <25 | (ref) | (ref) | |||||
| 25–30 | 1.82 | 1.31, 2.54 | <0.001 | 1.35 | 0.99, 1.82 | 0.06 | |
| >30 | 2.45 | 1.47, 4.06 | <0.001 | 2.47 | 1.46, 4.19 | <0.001 | |
| Midlife BMI TWA (ages 50–69) | |||||||
| <25 | (ref) | (ref) | |||||
| 25–30 | 0.82 | 0.62, 1.09 | 0.17 | 1.51 | 1.11, 2.05 | 0.009 | |
| >30 | 0.70 | 0.42, 1.19 | 0.19 | 2.00 | 1.16, 3.42 | 0.012 | |
| Late life BMI TWA (ages 70–89) | |||||||
| <25 | (ref) | (ref) | |||||
| 25–30 | 0.72 | 0.55, 0.93 | 0.012 | 0.56 | 0.42, 0.75 | <0.001 | |
| >30 | 0.52 | 0.36, 0.76 | <0.001 | 0.37 | 0.24, 0.59 | <0.001 | |
BMI indicates Body Mass Index, TWA indicates Time Weighted Averages.
Adjusted for age, sex and race/ethnicity, years of education, cohort (CHS, Health ABC), and BMI TWAs from all three time periods.
Using PLR models to adjust for race/ethnicity, education, cohort, and BMI exposure in mid and late life, the adjusted odds of incident dementia increased across early adulthood BMI categories among both women and men. In particular, compared to women with an early adulthood BMI<25 kg/m2, the adjusted odds of dementia were 1.8 times higher among overweight women (aOR 1.82; 95% CI 1.31, 2.54) and 2.5 times higher among obese women (aOR 2.45; 95% CI 1.47, 4.06). Similarly, compared to men with early adulthood BMI TWA<25 kg/m2, the odds of incident dementia were 1.35 times higher among overweight men (aOR 1.35; 95% CI 0.99, 1.82) and 2.47 times higher among obese men (aOR 2.47; 95% CI 1.46, 4.19).
Midlife BMI exposure and dementia (Table 3):
Based on the same adjusted PLR models, we found no statistically significant association between midlife BMI and dementia among women, after adjusting for earlier and later BMI exposures. In contrast, higher midlife BMI was associated with higher odds of incident dementia among men. In particular, compared to men with midlife BMI≤25 kg/m2, the adjusted odds of incident dementia were 1.51 times higher among overweight men (aOR 1.51; 95% CI 1.11, 2.05) and 2.00 times higher among obese men (aOR 2.00; 95% CI 1.16, 3.42).
Late life BMI exposure and dementia (Table 3):
For women and men, higher late life BMI (ages 70–89) was associated with lower adjusted odds of incident dementia, after adjusting for early adulthood and midlife BMI exposures. In particular, compared to women with late life BMI≤25 kg/m2, the adjusted odds of incident dementia were 28% lower among overweight women (aOR 0.72; 95% CI 0.55, 0.93) and 48% lower among obese women (OR 0.52; 95% CI 0.36, 0.76). Similarly, compared to men with late life BMI≤25 kg/m2, the adjusted odds of incident dementia were 44% lower among overweight men (aOR 0.56; 95% CI 0.42, 0.75) and 63% lower among obese men (aOR 0.37; 95% CI 0.24, 0.59).
Interactions with BMI exposure:
The interaction with sex was statistically significant (P=0.02) for the midlife BMI TWAs but not the early adulthood (P=0.32) or late life (P=0.53) BMI TWAs. However, we did not find statistically significant interactions with a four-level categorization with both sex and race (P=0.19, 0.35 and 0.90 for the three BMI TWA age ranges).
Sensitivity analyses.
Our findings were unchanged in models adjusted for systolic blood pressure, low density lipoprotein and glucose (Supplementary Table 1), which we hypothesized as potential mediators that are downstream from BMI. Our findings were similar in sensitivity analyses summarizing BMI exposures over alternate age ranges (Supplementary Table 2). Our findings were also unchanged in models using inverse probability of inclusion weights to mitigate possible survival bias (data not shown). Finally, to address differences in dementia diagnoses between CHS and Health ABC, we repeated the analyses examining whether BMI in early adulthood is a risk factor for dementia, separately for CHS and Health ABC, and results remained overall similar (Supplementary Tables 3 and 4).
DISCUSSION
In this study, we found that higher early adulthood BMI was independently associated with higher dementia incidence among both women and men. Higher midlife BMI was associated with higher dementia incidence among men, but not among women. In both groups, higher late life BMI was independently associated with lower dementia incidence. By using a pooled cohort spanning the life course, we were able to impute early adulthood BMI exposure, then estimate its association with incident dementia, which occurs almost exclusively in late life; this would not have been possible using data for CHS and Health ABC alone. Our findings support the hypothesis that dementia may be influenced by cardiovascular risk factors, such as high BMI, developing many decades before dementia is typically diagnosed.
Our study contributes to a sparse literature examining the influence of BMI early in the lifecourse on dementia. To our knowledge, only one previous US study, the Harvard Alumni Health Study26, examined such relationship, and in contrast to our findings, they reported no association between BMI measured in early adulthood and dementia-related deaths. In the Harvard alumni study, early adulthood BMI was measured only once, at an average age of 18 years old; in contrast, we imputed early adulthood BMI from age 20 through 49, a time in life when average BMI increases substantially. Another recent, non-US study, examined the relationship between BMI across the lifespan and dementia risk among subjects from the Swedish Twin Registry and found a relationship between higher BMI in early adulthood and higher dementia risk.27 While we do not study the exact pathways linking BMI early in the lifecourse to dementia in late life, exposure at an early age could mean a longer duration of exposure to higher BMI as well as to a cascade of behavioral and biological processes28–33 that result in vascular damage and leads to the neuropathological changes accompanying dementia. In light of the growing obesity epidemic among US adults,40 with recent figures suggesting about 40% of US adults ages 20 years or older are obese, our findings suggest that interventions aimed at modifying trends in obesity early in the lifecourse may reduce the risk of dementia by potentially modifying the course of its preclinical phase.
Compared to the sparse evidence on early adulthood BMI and dementia, higher BMI in midlife is suggested as a well-established risk factor for dementia.14–16, 49, 50 According to a meta-analysis, being overweight or obese in midlife is associated with greater risk for Alzheimer’s, vascular, and all cause dementia.49 Studies among patients from the Northern California Kaiser Permanente health care system have found associations between being overweight or obese in midlife (ages 40 to 45 years old) and increased odds of being diagnosed with Alzheimer’s and vascular dementia, and shown that these associations were stronger in women than in men.14, 50 Somewhat unexpectedly, we found no association with midlife BMI among women, above and beyond early adulthood BMI. Our finding is however consistent with a recent study among 7,029 participants from the Health and Retirement Study (HRS) in which midlife BMI, measured at an average age of 58, was not associated with memory loss.25 While we do not examine underlying pathways, several mechanisms have been hypothesized to link BMI to dementia, and some of which have been suggested to vary by sex. For example, it has been previously reported that among older adults, higher levels of adiposity measured by imaging were associated with worsening cognitive function in men, but not in women.34 Overall, further studies are needed to better understand the lifecourse nature of the associations of BMI and other CVD risk factors with dementia risk across sex, as well as the role of underlying mechanisms. This is especially relevant in light of the mixed evidence regarding sex differences in dementia incidence37, 39, 51 (in our pooled cohort, 17.4% among women vs. 18.3% among men).
Our findings of an inverse association between higher BMI in late life and reduced dementia incidence, among both women and men, is consistent with most prior literature23, 24. In particular, our findings are consistent with a recent meta-analysis23 using data for 1.3 million adults showing that the relationship of BMI and dementia becomes protective as the follow-up time separating the two measurements gets shorter. Reverse causation is often hypothesized to explain this pattern. In particular, a recent HRS study25 showed that BMI loss preceding dementia occurred as early as late 50s. This is further mirrored by our finding that declines in BMI begin in midlife, and in our case, begin at an earlier age for women than men. In particular, we found that BMI trajectories for those with and without dementia begin to diverge as early as in mid-50s for women compared to the 70s for men. Altogether, these findings are indicative that reverse causation may begin earlier in the life course than originally thought.
Our findings support the hypothesis that dementia-related processes beginning decades prior to diagnosis may result in weight loss.52–55 Findings from the Alzheimer’s Disease Neuroimaging Initiative (ADNI)52 showed an association between biomarkers of increased AD burden and lower BMI, particularly among individual who were normal or with mild cognitive impairment. The latter suggests that the pathophysiological changes that accompany AD may result in weight loss and begin well before dementia diagnosis. While the exact pathways are unclear, several hypotheses have been suggested, including decreased appetite and food intake – maybe partially due to underlying cognitive changes56 or reduced smell and taste57 – and overall frailty and comorbidities58.
Our study has several limitations. Our imputations of early adulthood and midlife BMI are biased towards the overall averages41, attenuating the estimated associations of imputed early adulthood exposures with late life events. Yet, despite such attenuation, our findings still showed that exposure to high BMI in early adulthood is a significant risk factor for dementia in late life. Methods for diagnosing dementia differed between CHS and Health ABC. Yet, when we repeated the same analyses but separately for CHS and Health ABC, the results were overall similar. We also acknowledge that since the time of these two studies, dementia diagnosis has become more routed in biomarkers, as such it would be important to replicate our findings with more recent criteria. CHS and Health ABC participants had to survive until cohort entry in old age, and thus may be atypical of the younger adult populations they are taken to represent. Yet, our findings were unchanged in models using inverse probability of inclusion weights to mitigate possible survival bias. Concerning differential definition of early adulthood, midlife and late life across studies, our results were unchanged in sensitivity analyses using different age-cutoffs to define these time periods. It is also important to note that our methods of imputation do not allow us to distinguish life-course effects in a desirable level of detail beyond the age categories we have used. Information available for each subject in their 60s and beyond does not allow us to impute much more than a level and trend in early adulthood. Under those circumstances, we have little ability to distinguish the effects of BMI (or other exposures) in the 20s, 30s, and 40s, because we do not have independent information about those periods. That said, we do consider the TWAs we use to summarize the trajectories informatively.41 Finally, we acknowledge that while other factors such as traumatic brain injury and sleep disorders and other comorbidities may act as potential confounders, we do not have pooled and harmonized information on some factors and thus could not be accounted for. Despite those limitations, this study has several strengths. First, due to pooling multiple cohorts, our study was powered to make inferences among women and men separately. Second, to our knowledge, this is the first study to use these innovative methods to impute early adulthood and midlife BMI, and to examine their associations with dementia which occurs almost exclusively in late life. This would not have been possible using data for either cohort alone. Furthermore, the motivation for this study and the methods are not exclusive to BMI and will be important to other risk factors for which evidence has been inconsistent in midlife and late life, such as systolic blood pressure. Our methods and imputation framework have been previously described, including results of simulations that show their accuracy and usefulness in assessing the effects of early and midlife exposures on late life outcomes.41
In summary, our data show an independent association of dementia incidence with higher early adulthood BMI among both women and men. Accounting for early adulthood BMI exposure, we found no association between midlife BMI and dementia among women, but strong inverse associations between late life BMI and dementia incidence in both women and men. The latter is consistent with growing evidence that pre-clinical dementia may begin in midlife, earlier than originally thought. Taken together, our findings suggest that interventions aimed at reducing dementia, in part by modifying the course of its preclinical phase, may need to begin earlier in the life course with a focus on obesity prevention and treatment. Future studies, especially those with observed lifecourse BMI data, should replicate our findings as well as examine potential underlying pathways.
Supplementary Material
FUNDING
This work was supported by grants from the National Institutes of Health, National Institute on Aging (1RF1AG054443). CARDIA is supported by contracts HHSN268201800003I, HHSN268201800004I, HHSN268201800005I, HHSN268201800006I, and HHSN268201800007I from the National Heart, Lung, and Blood Institute (NHLBI).
MESA is supported by was supported by contracts HHSN268201500003I, N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-TR-001079 from NCRR.
CHS is supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org.
Health ABC study is supported by National Institute on Aging (NIA) Contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050, and NINR grant R01-NR012459. Health ABC was funded in part by the Intramural Research Program of the NIH, National Institute on Aging.
The funding organization or sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Drs. Zeki Al Hazzouri and Vittinghoff had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Conflict of Interest: none declared.
References
- 1.Yaffe K, Weston AL, Blackwell T, Krueger KA. The metabolic syndrome and development of cognitive impairment among older women. Archives of neurology 2009;66:324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young SE, Mainous AG 3rd, Carnemolla M. Hyperinsulinemia and cognitive decline in a middle-aged cohort. Diabetes care 2006;29:2688–2693. [DOI] [PubMed] [Google Scholar]
- 3.Kalmijn S, Foley D, White L, et al. Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men. The Honolulu-Asia aging study. Arteriosclerosis, thrombosis, and vascular biology 2000;20:2255–2260. [DOI] [PubMed] [Google Scholar]
- 4.Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. Jama 2004;292:1454–1461. [DOI] [PubMed] [Google Scholar]
- 5.Ravaglia G, Forti P, Lucicesare A, et al. Physical activity and dementia risk in the elderly: findings from a prospective Italian study. Neurology 2008;70:1786–1794. [DOI] [PubMed] [Google Scholar]
- 6.Middleton LE, Barnes DE, Lui LY, Yaffe K. Physical activity over the life course and its association with cognitive performance and impairment in old age. Journal of the American Geriatrics Society 2010;58:1322–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes DE, Santos-Modesitt W, Poelke G, et al. The Mental Activity and eXercise (MAX) trial: a randomized controlled trial to enhance cognitive function in older adults. JAMA internal medicine 2013;173:797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Middleton LE, Manini TM, Simonsick EM, et al. Activity energy expenditure and incident cognitive impairment in older adults. Archives of internal medicine 2011;171:1251–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anstey KJ, von Sanden C, Salim A, O’Kearney R. Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies. American journal of epidemiology 2007;166:367–378. [DOI] [PubMed] [Google Scholar]
- 10.Kalmijn S, van Boxtel MP, Verschuren MW, Jolles J, Launer LJ. Cigarette smoking and alcohol consumption in relation to cognitive performance in middle age. American journal of epidemiology 2002;156:936–944. [DOI] [PubMed] [Google Scholar]
- 11.Yaffe K Preclinical Alzheimer disease: Prevention Holy Grail or Pandora’s Box?: Comment on “Heavy smoking in midlife and long-term risk of Alzheimer disease and vascular dementia”. Archives of internal medicine 2011;171:339–340. [DOI] [PubMed] [Google Scholar]
- 12.Anstey KJ, von Sanden C, Salim A, O’Kearney R. Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies. Am J Epidemiol 2007;166:367–378. [DOI] [PubMed] [Google Scholar]
- 13.Kalmijn S, van Boxtel MP, Verschuren MW, Jolles J, Launer LJ. Cigarette smoking and alcohol consumption in relation to cognitive performance in middle age. Am J Epidemiol 2002;156:936–944. [DOI] [PubMed] [Google Scholar]
- 14.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP Jr., Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. Bmj 2005;330:1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrett-Connor E An introduction to obesity and dementia. Curr Alzheimer Res 2007;4:97–101. [DOI] [PubMed] [Google Scholar]
- 16.Fitzpatrick AL, Kuller LH, Lopez OL, et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Archives of neurology 2009;66:336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solomon A, Kareholt I, Ngandu T, et al. Serum total cholesterol, statins and cognition in non-demented elderly. Neurobiology of aging 2009;30:1006–1009. [DOI] [PubMed] [Google Scholar]
- 18.Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA. Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dementia and geriatric cognitive disorders 2009;28:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reitz C, Tang MX, Luchsinger J, Mayeux R. Relation of plasma lipids to Alzheimer disease and vascular dementia. Archives of neurology 2004;61:705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mielke MM, Zandi PP, Sjogren M, et al. High total cholesterol levels in late life associated with a reduced risk of dementia. Neurology 2005;64:1689–1695. [DOI] [PubMed] [Google Scholar]
- 21.Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiology of aging 2000;21:49–55. [DOI] [PubMed] [Google Scholar]
- 22.Birns J, Kalra L. Cognitive function and hypertension. Journal of human hypertension 2009;23:86–96. [DOI] [PubMed] [Google Scholar]
- 23.Kivimaki M, Luukkonen R, Batty GD, et al. Body mass index and risk of dementia: Analysis of individual-level data from 1.3 million individuals. Alzheimers Dement 2018;14:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh-Manoux A, Dugravot A, Shipley M, et al. Obesity trajectories and risk of dementia: 28 years of follow-up in the Whitehall II Study. Alzheimers Dement 2018;14:178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suemoto CK, Gilsanz P, Mayeda ER, Glymour MM. Body mass index and cognitive function: the potential for reverse causation. Int J Obes (Lond) 2015;39:1383–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russ TC, Lee IM, Sesso HD, Muniz-Terrera G, Batty GD. Five-decade trajectories in body mass index in relation to dementia death: follow-up of 33,083 male Harvard University alumni. Int J Obes (Lond) 2019;43:1822–1829. [DOI] [PubMed] [Google Scholar]
- 27.Karlsson IK, Lehto K, Gatz M, Reynolds CA, Dahl Aslan AK. Age-dependent effects of body mass index across the adult life span on the risk of dementia: a cohort study with a genetic approach. BMC Med 2020;18:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kivimaki M, Singh-Manoux A, Pentti J, et al. Physical inactivity, cardiometabolic disease, and risk of dementia: an individual-participant meta-analysis. Bmj 2019;365:l1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reis JP, Launer LJ, Terry JG, et al. Subclinical atherosclerotic calcification and cognitive functioning in middle-aged adults: the CARDIA study. Atherosclerosis 2013;231:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reis JP, Loria CM, Lewis CE, et al. Association between duration of overall and abdominal obesity beginning in young adulthood and coronary artery calcification in middle age. Jama 2013;310:280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeki Al Hazzouri A, Vittinghoff E, Sidney S, Reis JP, Jacobs DR Jr., Yaffe K. Intima-Media Thickness and Cognitive Function in Stroke-Free Middle-Aged Adults: Findings From the Coronary Artery Risk Development in Young Adults Study. Stroke 2015;46:2190–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fransson EI, Batty GD, Tabak AG, et al. Association between change in body composition and change in inflammatory markers: an 11-year follow-up in the Whitehall II Study. J Clin Endocrinol Metab 2010;95:5370–5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yaffe K, Kanaya A, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. Jama 2004;292:2237–2242. [DOI] [PubMed] [Google Scholar]
- 34.Kanaya AM, Lindquist K, Harris TB, et al. Total and regional adiposity and cognitive change in older adults: The Health, Aging and Body Composition (ABC) study. Archives of neurology 2009;66:329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeki Al Hazzouri A, Stone KL, Haan MN, Yaffe K. Leptin, mild cognitive impairment, and dementia among elderly women. J Gerontol A Biol Sci Med Sci 2013;68:175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeki Al Hazzouri A, Haan MN, Whitmer RA, Yaffe K, Neuhaus J. Central obesity, leptin and cognitive decline: the Sacramento Area Latino Study on Aging. Dementia and geriatric cognitive disorders 2012;33:400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barnes LL, Wilson RS, Schneider JA, Bienias JL, Evans DA, Bennett DA. Gender, cognitive decline, and risk of AD in older persons. Neurology 2003;60:1777–1781. [DOI] [PubMed] [Google Scholar]
- 38.Miech RA, Breitner JC, Zandi PP, Khachaturian AS, Anthony JC, Mayer L. Incidence of AD may decline in the early 90s for men, later for women: The Cache County study. Neurology 2002;58:209–218. [DOI] [PubMed] [Google Scholar]
- 39.Hebert LE, Scherr PA, McCann JJ, Beckett LA, Evans DA. Is the risk of developing Alzheimer’s disease greater for women than for men? American journal of epidemiology 2001;153:132–136. [DOI] [PubMed] [Google Scholar]
- 40.Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in Obesity and Severe Obesity Prevalence in US Youth and Adults by Sex and Age, 2007–2008 to 2015–2016. Jama 2018;319:1723–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeki Al Hazzouri A, Vittinghoff E, Zhang Y, et al. Use of a pooled cohort to impute cardiovascular disease risk factors across the adult life course. Int J Epidemiol 2019;48:1004–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Vittinghoff E, Pletcher MJ, et al. Associations of Blood Pressure and Cholesterol Levels During Young Adulthood With Later Cardiovascular Events. J Am Coll Cardiol 2019;74:330–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yaffe K, Vittinghoff E, Pletcher MJ, et al. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation 2014;129:1560–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bland RC, Newman SC. Mild dementia or cognitive impairment: the Modified Mini-Mental State examination (3MS) as a screen for dementia. Can J Psychiatry 2001;46:506–510. [DOI] [PubMed] [Google Scholar]
- 45.Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med 1989;19:1015–1022. [DOI] [PubMed] [Google Scholar]
- 46.Fitzpatrick AL, Kuller LH, Ives DG, et al. Incidence and prevalence of dementia in the Cardiovascular Health Study. Journal of the American Geriatrics Society 2004;52:195–204. [DOI] [PubMed] [Google Scholar]
- 47.Yaffe K, Falvey C, Harris TB, et al. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. Bmj 2013;347:f7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.D’Agostino RB, Lee ML, Belanger AJ, Cupples LA, Anderson K, Kannel WB. Relation of pooled logistic regression to time dependent Cox regression analysis: the Framingham Heart Study. Stat Med 1990;9:1501–1515. [DOI] [PubMed] [Google Scholar]
- 49.Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev 2011;12:e426–437. [DOI] [PubMed] [Google Scholar]
- 50.Whitmer RA, Gunderson EP, Quesenberry CP Jr., Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res 2007;4:103–109. [DOI] [PubMed] [Google Scholar]
- 51.Rocca WA, Cha RH, Waring SC, Kokmen E. Incidence of dementia and Alzheimer’s disease: a reanalysis of data from Rochester, Minnesota, 1975–1984. Am J Epidemiol 1998;148:51–62. [DOI] [PubMed] [Google Scholar]
- 52.Vidoni ED, Townley RA, Honea RA, Burns JM, Alzheimer’s Disease Neuroimaging I. Alzheimer disease biomarkers are associated with body mass index. Neurology 2011;77:1913–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burns JM, Johnson DK, Watts A, Swerdlow RH, Brooks WM. Reduced lean mass in early Alzheimer disease and its association with brain atrophy. Archives of neurology 2010;67:428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson DK, Wilkins CH, Morris JC. Accelerated weight loss may precede diagnosis in Alzheimer disease. Archives of neurology 2006;63:1312–1317. [DOI] [PubMed] [Google Scholar]
- 55.Barrett-Connor E, Edelstein SL, Corey-Bloom J, Wiederholt WC. Weight loss precedes dementia in community-dwelling older adults. Journal of the American Geriatrics Society 1996;44:1147–1152. [DOI] [PubMed] [Google Scholar]
- 56.Tamura BK, Bell CL, Masaki KH, Amella EJ. Factors associated with weight loss, low BMI, and malnutrition among nursing home patients: a systematic review of the literature. J Am Med Dir Assoc 2013;14:649–655. [DOI] [PubMed] [Google Scholar]
- 57.Yaffe K, Freimer D, Chen H, et al. Olfaction and risk of dementia in a biracial cohort of older adults. Neurology 2017;88:456–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fried LP, Kronmal RA, Newman AB, et al. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. Jama 1998;279:585–592. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


