ABSTRACT
Microalgae have been recognized as one of the most efficient microorganisms to remediate industrial effluents. Among microalgae diatoms are silica shelled unicellular eukaryotes, found in all types of water bodies and flourish very well even in wastewater. They have their silica cell wall made up of nano arrayed pores arranged in a uniform fashion. Therefore, they act as smart nanocontainers to adsorb various trace metals, dyes, polymers, and drugs which are hazardous to human as well to aquatic life. The beautiful nanoarchitecture in diatoms allows them to easily bind to ligands of choice to form a nanocomposite structure with the pollutants which can be a chemical or biological component. Such naturally available diatom nanomaterials are economical and highly sensitive compared to manmade artificial silica nanomaterials to help in facile removal of the toxic pollutants from wastewater. This review is thus focused on employing diatoms to remediate various pollutants such as heavy metals, dyes, hydrocarbons detected in the wastewater. It also includes different microalgae as biosensors for determination of pollutants in effluents and the perspectives for nanotechnological applications in the field of remediating pollutants through microalgae. The review also discusses in length the hurdles and perspectives of employing microalgae in wastewater remediation.
KEYWORDS: Biosensors, Diatoms, Microalgae, Nanomaterials, Pollutants, Wastewater
1. Introduction
Recent years, application of microalgae in the treatment of wastewater has increased remarkably. This is due to the fact that wastewater contain nutrients required for the growth of microalgae [1,2]. These nutrients mainly include nitrates (N), phosphates (P), and trace elements, essential for the growth of microalgae [3]. Since most of the microalgae are photosynthetic in nature, they do not need external organic compound for their growth and replication, whereas some strains could avail organic compounds in the absence of light too. Therefore, microalgal bioremediation could be combined with traditional and single biological treatment methods for efficiently treating wastewater [4,5]. Among microalgae both Chlorella and Micractinium showed good response in removing nitrates and phosphates from wastewater [6,7]. They were cultivated in wastewater in order to check the kinetics of nutrient removed and their growth [8]. About 95% of the soluble phosphorus (P) was removed, and soluble nitrogen (N) was reduced up to 95.7% and 93.9% for Chlorella and Micractinium, respectively. Soluble nitrogen decreased by 50.6% for Chlorella and 45.7% for Micractinium in primary effluent mixtures. Higher concentration of N and unbalanced N/P ratios in the mixed wastewater resulted in higher removal rates of N and potassium (K) accompanied by rapid uptake, simultaneously resulting into algae growing in the environment rich in extracellular proteins [8]. In a study of four different types of wastewater samples viz; before primary settling (type 1); after primary settling (type 2); wastewater after activated sludge tank (type 3); and centrate (type 4) were tested to observe the growth of green algae Chlorella sp. It was done to check how the Chlorella sp. removed N, P, chemical oxygen demand (COD), and trace metals from these four different samples of wastewater [9].
In case of wastewater after activated sludge tank (type 3), the major inorganic nitrogen (NO3–N) formed was 62.5% of which 6.3-fold NO2-N was removed. The average specific growth rates in the different wastewater type 1, 2, 3, and 4 at exponential period was 0.412, 0.429, 0.343, and 0.948, day−1 respectively. Algal growth increased in the centrate (type 4) because of higher levels of N, P, and COD compared to that in other three wastewater types, while the treatment of wastewater types 1, i.e. before and type 2, i.e. after primary settling was more systematic in nutrient depletion than that of pollutants. Metal ions like Fe, Ca, Mg, Mn, and Al in type 4, i.e. centrate were removed efficiently by algae growing in it [9]. In addition, it has been seen that among bacteria genetically engineered cells of Deinococcus radiodurans nonspecific for nitrogen phosphatase removes 70% of uranium from 1 g uranium/g dry weight cells [10]. Table 1 shows removal of different heavy metals by different microalgae at different efficiencies which vary obviously on the type of wastewater and type of microalgae strain.
Table 1.
Microalgae for metal removal from wastewater
| Metals | Microalgal strain | Removal efficiency (%) | References |
|---|---|---|---|
| Copper, zinc, cadmium, and mercury | Cladophora fracta; live algae | Cu2+, Zn2+, Cd2+, and Hg2+ were 99%, 85%, 97%, and 98%, respectively | [11] |
| Cadmium |
Scenedesmus sp. Chlorella sp. Chlorella vulgaris |
73% 33–41% 66% |
[12] |
| Chromium |
Chlorella vulgaris Scenedesmus sp. Spirulina sp. |
50.7–80.3% 92.89% 82.67% |
[13–15] |
| Copper |
Spirulina maxima Chlorella vulgaris Scenedesmus obliquus |
94.9% 96.3% 72.4–91.7% |
[16,30] |
| Lead |
Chlorella vulgaris Chlorella sp. Pseudochlorococcum typicum |
89.26% 66.3% 70% |
[17,18,32] |
| Mercury |
Chlorella vulgaris Chlorella vulgaris Pseudochlorococcum typicum |
79–86% 34.21–93% 97% |
[18–20] |
| Nickel |
Scenedesmus sp. Chlorella vulgaris Chlorella miniate |
97% 33–41% 60-73% |
[12,21] |
| Zinc |
Chlorella sp Synechocystis sp Scenedesmus sp |
60–70% 40% 98% |
[21,32] |
| Cadmium, Copper, Lead, and Zinc | Haslea ostrearia, Phaeodactylum tricornutum, Skeletonema costatum, and Tetraselmis suecica; live algae | Reduction in Cu and Cd | [22] |
| Chromium | Chlorella miniata; dried algae; cultivated in domestic wastewater | 75% and 100% removal for Cr (III) and Cr (VI), respectively | [23] |
| Lead, Cadmium, Copper, and Arsenic | Cyanophyta (Oscillatoria princeps 92%, Oscillatoria subbrevis 2%, and Oscillatoria formosa 1%) and Chlorophyta (Spirogyra aequinoctialis 3%, Mougeta sp. 1%, and others 1%); dried algae | Metals were removed | [24] |
In yet similar study microalgae Chlorella was grown in only bold basal medium (BBM), wastewater, and wastewater enriched with BBM elements [25]. It was observed that wastewater enriched with BBM elements showed highest growth in Chlorella cell density; however, due to rapid growth there was decrease in lipid accumulation. On the offset, wastewater showed maximum lipid accumulation of about 16.2 mg L−1 day−1, with removal of NO3-N, NH3-N, and TP, to an extent of 74.2, 83.3, and 78.0%, respectively. However, BBM media showed additional recovery of monounsaturated fatty acids without any nutrient recovery. Hence proving that microalgae grown in wastewater were very economical way of bio cycling important nutrients from wastewater while simultaneously producing lipids and biofuel [25,26]. On the other hand, phycoremediation of tannery wastewater (TWW) with Scenedesmus sp showed significant results. In this study, Scenedesmus sp. was grown in TWW under controlled laboratory environment which showed significant results on the 12th day of harvesting. The results showed that, during growth period the accumulated algal biomass decreased percentage amounts of heavy metals (Cu-73.2–98%, Cr-81.2–96%, Zn-65-98%, and Pb-75-98%) and nutrients (PO4 > 95% and NO3 > 44.3%). Thus showing that Scenedesmus sp. has potential for biomass production and also ability to phycoremediate the toxic pollutants found in TWW [27,28]. Another microalgae Coelastrum sp. has great future to produce large amounts of biomass while simultaneously removing nutrients from wastewater [29]. In this study outcome of different Soluble Chemical Oxygen Demand (sCOD) concentrations and light intensity was done on the total Kjeldahl nitrogen (TKN), nitrate and total phosphorus (TP) removal, microalgae growth, and CO2 utilization rate. It was observed that maximum growth of about 2.71 g L−1 day-1and CO2 removal of 53.12 mg L−1 day−1, took place at light intensity of 6900 Lux with wastewater having initial sCOD of 750 mg L−1. However, on 4th day Coelastrum sp. showed the removal efficiency of sCOD 53.45%, 91.18% TKN, 87.51% nitrate and 100% of TP. In addition, when Coelastrum sp. was cultivated under semi-batch conditions with continuous gas flow, the biomass productivity was seen to be 0.281 g L−1 day−1. In addition, under the same condition, the average removal rate of TKN, TP, N, and sCOD was 83.51%, 100%, 80.91%, and 41.4%, respectively, by this microalgae. These results suggest that Coelastrum sp. is a befitting microalgae for carbon dioxide bio fixation, and wastewater bioremediation [29]. Furthermore, a study on treating toxicity of metals like Cu is done by sulfate reducing bacteria (SRB) eg. Desulfovibrio sp. using novel sulfur reduction bacteria (SRB) microalgal spheres employing four microalgae viz; Scenedesmus obliquus, Selenastrum capricornutum, Chlorella vulgaris and Anabaena spiroides [30]. These microalgae were used as source of carbon to treat Cu (II) effluent wastewater at a concentration of 60 mg L−1 and 600 mg L−1 sulfate. The microalgae are broken down into fatty acids by sulfate reducing bacteria, which serve as carbon source for sulfur reduction bacteria (SRB) so as to lower the COD. Furthermore, it has been observed that immobilized SRB nutrient beads showed efficient remediation than free cells, because the beads not only secured SRB against toxicity by heavy metals but also increased the COD/SO42− ratios. Results showed that after 45 days, the Cu (II) elimination rates increased from 91.7 to 98.2% and sulfate removal rates increased from 72.4 to 74.4%. The outstanding efficiency and strength of this immobilized SRB microalgae bead provides an encouraging strategy to deal with pollution caused by heavy metals instead of using free microalgal cells [30]. Chlorella sp. regulates high removal of Zn2+ and has shown its 60–70% removal from culture medium containing from 5 to 20 mgL−1 Zn2+ hence reducing it to 42% at 50 mgL−1. However, a continuous fall in cell number from 538 × 105 to 8 × 105 cells mL–1 suggested Zn toxicity in Chlorella. The growth retardation in microalgae cells is dependent not only upon the amount of heavy metal ions immobilized on its surfaces but also on its amount present intracellularly [31] while in case of Zn, retardation in growth may be due to Zn ions present extracellularly. In the algal stabilization ponds, Zn2+ and Pb2+ were removed by 72% and 73%, respectively. These results display a good treatment capacity of algae stabilization pond system by Chlorella sp. along with remediation of toxic heavy metals [32]. It has been observed that the cosmopolitan microalgae Chlorella sp. assembles and remediates toxic heavy metals from the wastewater [33]. This is because heavy metals such as Cu, Zn, Fe, Co, Mo, and Mn are required as essential nutrients for its growth and multiplication. Chlorella shows adsorption of heavy metals found as pollutants in tannery industries [34]. The reduction of different metal ions was Pb 12.54 mg L−1; Co 7.37 mg L−1; Cr 10.92 mg L−1; Ni 9.15 mg L−1; Cd 8.48 mg L−1; Cu 10.71 mg L−1 Zn 11.56 mg L−1 at 20th day of the treatment [35]. The maximum bioadsorption capacity of Chlorella to remove heavy metals was found to be 83.43, 82.15, 63.29, 58.92, 70.53, 64.83, and 81.36, µg L−1 for Zn, Ni, Cd, Co, Pb, Cu, and Cr respectively. This was followed by optimal yield of value added products as well as cleaning of wastewater effectively yet economically especially if microalgal value added has high industrial demand and gives high yield in the presence of high light and other components which are freely available. For instance astaxanthin in green-red microalgae Haematococcus pluvialis and fucoxanthin in diatoms [36,37]. On the other hand, various studies showed that than other microalgae are also used widely in wastewater treatment such as Chlorella, Arthrospira [38], Scenedesmus [39], Botryococcus [40], Chlamydomonas [41], and Phormidium [42]. Brown microalgae has great potential in treating wastewater alone or in combination with bacteria [43]. Among the commonly existing microalgae used for wastewater treatment, optimal yield of value added products as well as cleaning of wastewater effectively yet economically is very important. Furthermore, if microalgae has high value added for instance astaxanthin in green-red microalgae Haematococcus pluvialis and fucoxanthin in diatoms. Among these two microalgae diatoms are robust microalgae due to their silica structure which not only remediate wastewater rich in micronutrients and macronutrients required for its growth but also acts as smart nanocontainers. Consequently, oxygen produced by diatoms during photosynthesis encourages heterotrophic bacterial growth which leads to enhanced degradation of organic matter including heavy metals by bacteria. They thus play an important role in biosensing pollutants in untreated sewage sites rich in pharmaceutical drugs, NH4+ and PO43- [44]. On the offset diatom bio-monitor wastewater rich in heavy metals like Cr, Pb, Cd, Cu by not only showing morphological changes in their silica frustule but also show significant physiological changes. They also effectively bio remediate not only heavy metals but also wastewater from different sources like dairy waste [45], dyes waste [46–48], and piggery waste [49] while simultaneously yielding value added products [49] and recycling the wastewater. In this article light has been thrown on the ability of diatom to bioremediate wastewater and biosense pollutants in it owing to its magnificent silica nanostructure which act as a nanocontainer for wastewater pollutants. The use of diatoms as nanomaterials alone or in conjunction with conjugate linkers has been elaborated. The teratological deformation of diatoms morphological outlines due to heavy metals has shown lipid stress and enhancement in lipid bodies has been explained. Besides this the diatoms as microfluidic devices to biosense the wastewater pollutants based on biosensor technology along with the hurdles and future research has been discussed in length.
2. Diatoms silica shells as robust nanomaterials to treat heavy metals in waste water
Diatoms are golden brown algae, responsible for fixing about 25% of atmospheric CO2 [50]. They exist in different shapes and sizes ranging from 2 to 200 µm which are identified both morphologically and at molecular level [51]. There are about 20,000 species of diatoms with pores arranged in a beautiful and rigid walled nanoarchitecture [52–54]. These pores range in size from 3 to 50 nm in diameter [55]. Besides being found in all types of water bodies they are photosynthetic, convert atmospheric CO2 to carbohydrates, thus forming different biomolecules like proteins, lipids, carotenoids, etc. [56]. They are high source of naturally occurring value added products besides having immense applications in nanotechnology and sensing water quality of organic pollutants, metals, dyes, pesticides, hydrocarbons, and drugs etc. in aquatic ecosystems [50,57,58]. They are thus widely studied for their role as indicators of different kinds of water pollution, and their phytoremediation in the initial phase [59]. Since rapid industrialization and urbanization are the main reason for groundwater contamination andwater deficit [60,61]. There are various technologies to treat wastewater, however, each has its stumbling block and asset [62]. Therefore, it’s important to choose the one that could purify the wastewater and recover the resources from it. Since the disposal of wastewater into the water bodies results in the release of nutrients, this causes eutrophication in water bodies [63]. Furtheromre, heavy metals like Cu, Cd, Tl, Hg, As, Zn, Ni, Cr, and Pb in wastewater are harmful pollutants even in traces. The destructive nature of heavy metals is because of their bio toxicity in biotic systems [64]. Not to limit heavy metals, even dyes are the most common pollutants present in wastewater which is very important to remove or treat for clean environmental [65]. Therefore, it is is essential to not only utilize the nutrients present in wastewater but also remediate the heavy metals in it.
2.1. Diatoms as nanoabsorbents to remediate heavy metals
Recent study showed that the nanomaterials acts as sorbents for removing toxic heavy metals from wastewater due to their high surface to volume ratio, porous nature, and reactivity thus acting as suitable nanoadsorbents [66]. The selectivity and adsorption capacity of nanomaterial effectively eliminate heavy metals from water, even in trace concentrations [67]. The use of natural adsorbents to remove hazardous metals is both cost effective and environmentally beneficial. The treated clay minerals remove more metal ions under the same circumstances as untreated clay minerals due to increased surface area [68]. The immobilization of cement treated clay at different concentrations was tested and found that concentration of 6% and 9% was efficient in removing Pb+2 metal ions. The removal of Pb+2 increased with the increase in amount of cement in the clay. Furthermore, for the removal of heavy metals from wastewater, several remediation methods have been developed, the most common of which are filtration, oxidation and reduction, sedimentation, media filtration, and membrane filtration. Furthermore, heavy metals are unable to dissolve in flowing water and overcome variations in pH, conductivity, or microbiology that impact water quality. However, most of the techniques applied for removing heavy metals from wastewater are costly, thus adding financial burden [69]. Furthermore, owing to the specific properties of mesoporous silica materials they can be used as propitious adsorbents and catalytic support for treating wastewater [70,71]. Since manufacturing silica is arduous and costly and include toxic materials, there is a high demand to substitute these synthetic materials with steady and natural surrogates. On the offset, nature has treasured three-dimensional (3-D) porous silica structures known as diatoms, the single-celled photosynthetic microalgae [72] which are the rich source of natural porous silica [73].
The diatoms nano shells in conjugation with the ligands can be seen to adsorb toxic metals more in salinity-dependent marine diatoms as the cells at low salinity develop stronger affinity for adsorption of Cd++ [74]. It is observed that diatom cells thriving at lower salinity had a lower surface potential, higher specific surface area, and more sulfur-containing groups in the cell wall, followed by stronger Cd binding capacity in the cells. Hence, when the salinity decreases, more Si is found as poly-silicic acid. Thus change of Si content and speciation in the cell wall are considered as a key factor for variations of adsorption of Cd on its surface [75].
2.2. Teratological changes in living diatoms found in wastewater
Diatoms have capability to phycoremediate different kinds of wastewater due to their implicit cellular mechanisms. Furthermore, diatoms aid to rejuvenate the past climatic conditions by indicating water quality of water bodies. Silicified diatomaceous cell walls deform rapidly and characteristically in presence of chemical contamination, such as alterations in cell size, outline of frustules, patterns of raphe, and stria [76,77].
On the offset, diatoms flourish well in wastewaters by utilizing several macronutrients such as carbon, nitrogen, phosphorus, silica, and micronutrients such as iron, magnesium, calcium, potassium, manganese, copper, and cobalt required for their growth and photosynthesis to form biomass [78,79]. They not only utilize metals as nutrients but also remediate dyes, drugs, etc. Furthermore, exopolysaccharides secreted by microalgae prevents and slows down the leaching and chemical breakdown of plastics into micro and nanoplastics thus serving as biofoulant [80–82].
Additionally, the diatoms have a large impact on the biogeochemical cycles of carbon, phosphate, nitrate, and silica [83,84]. As a result, this mineral’s absorption is critical. The species-specific patterning of the silicon cell wall is the most distinguishing characteristic of a diatom [85]. When subjected to various types of stress throughout the reproductive process, the diatom cell outline and their frustule pattern can alter in a variety of ways, resulting in teratological forms. These changes might be minor, making it difficult to distinguish between normal and teratological cells. They can be so pronounced that it is impossible to determine if an unfamiliar form is teratological or belongs to a new species or variation. Teratological forms emerge as an unintended consequence of environmental stressors, which can be both physical and chemical in nature. Morphological variations in diatoms are often adaptive or nonadaptive in response to different environmental conditions [76]. Teratological forms are nonadaptive phenotypical abnormalities that typically have an effect on the contour of the valves or their stripe pattern. The rise in temperature can also affect the volume of the diatoms and reduce the size of the cells, although it is not the only relevant parameter [86]. This characteristic has no clear and universal influence on the carbon and nitrogen uptake of larger diatoms [87]. On the other hand, the conductivity influences the diatoms to a considerable extent. Diatoms can be attacked by metal contamination such as Ge, Cu, and Zn, which intervene silicate pathway in the diatoms [88]. For example, it was seen that Gomphonema pseudoagur found in Saraswati River Kurukshetra showed teratological deformation due to heavy metals [89]. The inductively coupled plasma mass spectrometry (ICP-MS) analysis of the site showed contamination by heavy metals, mainly Pb and Se. The deformity was further associated with high lipid content. Hence, the heavy metal pollutants result into valve or teratological deformation in diatom frustule. The morphological deformations not only make identification difficult but puts the diatom under metabolic stress by increasing the lipid bioaccumulation. Table 2 and Figure 1 shows how different heavy metals which are pollutants found in wastewater alter the morphology of diatoms by bringing undulating changes in frustule, stria, raphe and valve in diatoms and increasing the bioaccumulation of lipids.
Table 2.
Deformed diatoms on exposure to metals
| Diatoms | Teratology | Pollutant | References |
|---|---|---|---|
| Nitzschia fonticola | Deformed raphe channel | Zinc and copper | [90] |
| Achnanthidium pyrenaicum | Abnormal outline | Fluoranthene (pesticides) | [91] |
| Amphora pediculus | Distorted outline | Cadmium | [92] |
| Fragilaria capitellata | Deformed valve outline | Pesticides | [93] |
| Nitzschia amphibia | Distorted outline | Cadmium, arsenic, lead, mercury, copper, zinc | [94] |
| Fragilaria nanoides | Deformed central area | Copper | [95] |
| Eunotia sp. | Distorted outline (twist in middle of the valve) | Copper | [95] |
| Fragilaria recapitellata | Distorted valve outline | Zinc and copper | [96] |
| Reimeria uniseriata | Deformed valve outline | Cadmium, arsenic, lead, mercury, copper, zinc | [90] |
| Discostella pseudostelligera | Deformed striation pattern in the side of the valve | Cyanide and heavy metals | [97] |
| Eunotia spp. | Bent or dilate, absence of symmetry | Copper, iron, zinc, nickel | [98] |
| Brachysira microcephala | Distorted outline | Cadmium, zinc, lead | [77] |
| Phaeodactylum tricornutum | Abnormal boundaries or wrinkled shaped | Copper and zinc | [99] |
| Gomphonema pseudoaugur | Dilated in the middle of the valve | Lead and selenium | [89] |
Figure 1.
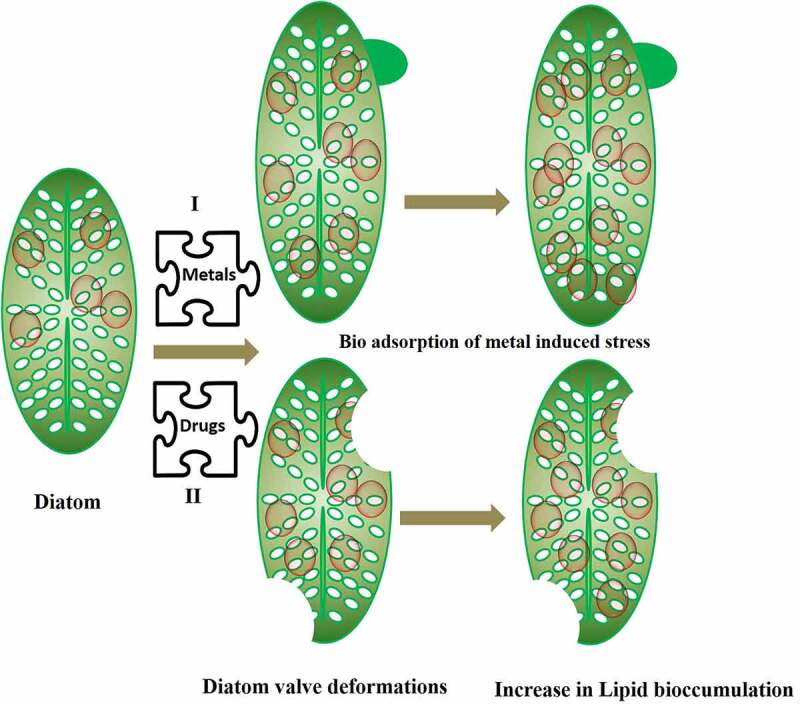
Diatoms undergoing valve deformation and increase lipid accumulation under stress conditions during remediation of pollutants
2.3. Effect on lipid bodies
Diatom showed large amount of lipid body (LB) accumulation under metal stress compared to the diatom living under standard conditions. It had been observed that fatty acid and sterol content reducedin the marine diatom Asterionella glacialis under Hg and Cd stress [77]. Although, the specific process of LB production in diatoms is unknown, research has shown that metal stress disrupts the diatom cell’s cytoplasmic homeostasis, which may enhance their sinking rate. Even though it’s not the case with all diatoms, as diatom Diadesmis confervacea has been discovered with oil oozing property in a clean fresh water lake of Haryana, India, without any heavy metal contamination [100]. Thus, increasing lipid content inside diatoms via LB production enhances buoyancy and decreases diatom sinking [101]. In a study by [102], it was shown that parameters like motility, LB, size reduction, and deformation of diatoms serves as biological monitoring tool for heavy metal detection [102]. In a study, it has been shown that there is a correlation of change in diatom morphology and lipid bodies (LB) as a function of metal concentration in the Khetri and Zawar regions in Rajasthan, India containing Cu and Zn [103]. All the heavily metal polluted sites showed increase in LB in about 8 diatom species viz; Craticula cuspidata, F. capucina, Navicula subminusula, Nitzschia amphibia, Nitzschia gracilis, Nitzschia linearis, Nitzschia sigmoidea, and P. conica found in these regions. As per available literature LB in diatoms increases under nutrient stress and its accumulation in metal stress is not clear. On the offset, deformities in about 20 diatom species were observed in Khetri and Zawar regions rich in Cu and Zn. The deformation in diatom raphe was seen in Cu polluted sites compared to Zn rich sites. Further these sites showed absence of centric diatoms and its species for, e.g. Acanthedium minnutissium showed its abundance in Zawar region than at Khetri. This is well proved by the fact that A. minutissimum is tolerant to metals like Cu, Fe, Pb, and Zn. Apart to this, reduction in diatom size was observed which was independent of the type of diatom and its morphology.
Pearson’s correlation analysis showed that there was a positive and statistically strong correlation between the induction of LB and the deformation by heavy metals (Pb and Se). Finally, based on this study, it was concluded that high levels of metal stress led to increase in LB and valve deformation in Gomphonema pseudoaugur. The deformation of diatoms, which can be used as an important indicator of biological monitoring and important parameter for the production of biofuels [89]. These deformed frustules can be effective indicators of water quality of wastewaters. Antoni et al. [104] found big LB in diatom cells treated with Zn compared to the control ones, and found that AgNP enhanced LB in Thalassiosira sp [105]. On the other hand [106], they observed no increase in LB in diatoms treated with Pb. [107]. They did a detailed study of teratological forms of diatoms under different environmental stresses making them a highly sensitive biosensor for all types of environmental pollutants [108]. This could be helpful in economical harvest of lipid/biofuel from diatoms also known as DiafuelTM [109], unlike the difficulties faced by researchers in optimizing the economical yet viable way of harvesting biofuel from diatoms using centrifugation, pulse electric field and resonance energies [110]. Besides teratological changes in diatoms there was decrease in chlorophyll pigments on exposure to trace metals [106,111,112], although it increased in some cases [113] whereas no changes were observed in many other cases [114]. Not limiting to this there is an alteration in the physiology as well organ structure of diatom on exposure to heavy metals. Diatom motility, nucleus anomalies have been observed in diatoms exposed to heavy metals. It has been observed in Fallacia pygmaea and Navicula novaesiberica there was damage in the nuclear membrane as well as abnormal nucleus location on exposure to Cr (IV) for 7 days [115].
3. Diatom as SiO2-nanoshells in conjunction with ligands to remediate wastewater
It is quite evident that the silica shells of the diatoms protect them from adverse environmental stresses. These encapsulated nano shells can be functionalized or modified without changing their abiotic properties by incorporating or doping of some elements and metal alkoxides via numerous processes of silicification [116,117]. Functionalization of natural silica shell is effortlessly attained by coupling of the surfaces silanol with suitable ligands on to their surface which is rich in -OH, -NH2, -COOH, -Si and other functional groups [118,119]. Moreover, several studies support that this rigid silica encapsulates biomolecules such as enzymes, lipids, protein, and other cell components, providing thermal stability and protection of the diatom cells from any aggressors viz; surfactant agent, cell lysis enzymes, pathogens, etc. [120]. This encapsulation concept can easily facilitate the silica nanoparticles on the cell surface which is extensible to any subjective nanoparticle to form the ligand chemistries [121]. The diatoms, silica cell wall owing to its porous structure permits the immobilization of target molecules onto its frustule surface as well as inside the pores, thus allowing the doping of sensing molecules, with high sensitivity and immediate responses [109,122]. In recent studies, various biosensors with ultrahigh sensitivity using diatom bio-silica, peptides and nanoparticles have been used in proteins self-assembly on Ag and Au NP on pores in diatom surface which under the laser beam undergo heating and thus release of bioenergy [123,124]. Its uniform porous architecture not only give it a porous sponge with wide surface area but is also photoluminescent which is an analog of crystalline silicon. Researchers have developed photoluminescence-based diatom biosensors for sensing the nitroaromatic compounds like 2, 4, 6-trinitrotoluene (TNT) [125]. Thus, adsorbents with essential parameters such as quick adsorption, easy separation, high adsorption capacity, equilibrium time, and with high stability are generally used for treatment of wastewater [126].
3.1. Heavy metals
Since diatoms synthesize antioxidant compounds such as polyunsaturated fatty acids (PUFA), terpenes, pigments and carotenoids like fucoxanthin to control uptake, detoxification, transport, and accumulation of heavy metals [37,127]. Photosynthetic pigments like chlorophyll a, b, and c are influenced by heavy metal toxicity, but pigment from diatoms enhance the detoxification of heavy metals. According to the study by Corcoll et al [128], a fast bioaccumulation of Zn after few hours of exposure in Osor stream, Spain produced different damaging effects on the biofilm assessed via diatom indices. This was associated with reduced photosynthetic efficiency effecting the photosynthetic pigments which depends on cell surface adsorption, intracellular, and extracellular accumulation of the heavy metals. Cell death generally takes place when the metals reach intracellular cavities of cell. After longer time of exposure there were changes in Zn and Fe bioaccumulation and concentration of Zn in water sites which were related to diatom community changes; decline in diatom cell biovolume and chlorophyll pigment studies [128]. Another common hazardous heavy metal is Pb2+, which is commonly found in our environment and results in serious health problems like bone, kidney damages, brain, and liver disorders. Therefore, it is very necessary to reduce the levels of these toxic contaminants from our wastewaters before releasing into the environment [129,130]. To overcome such issues a synthetic approach is usually taken to prepare conjugate hybrid complex structures by combining metal organic frameworks (MOFs) and diatoms. By using Metal-organic framework [MIL-100(Fe)] new hybrid MIL100(Fe)-diatomaceous earth a MOF was prepared for the very first time having a high surface area to remove Pb2+. Thus, the MOF of MIL-100(Fe)-diatomaceous earth showed removal efficiency of Pb2+ by 96.45% at adsorption rate of 120 min while simultaneously possessing high adsorption capacity of 155 mg/g [131]. So it is noteworthy that diatoms are capable for adsorbing heavy metal ions and trace metals from wastewater [132]. They have the capability to adsorb and desorb metals by metal binding functional groups on the siliceous cell surface forming the intracellular ligands with trace metals [133]. One such laboratory marine diatom which is potential candidate for laboratory experiments is Phaeodactylum tricornutum which shows the ability to adsorb the Cd metal ions and form the metal ligand [134]. Hernández-Á vila in 2017 reported absorption of Pb, Cd, and Cu by antioxidant enzymes and phytochelations [135]. Nitzschia was reported to adsorb the Zn, Cu, Co, and Mn chiefly by hydrogen bonding and van der Walls interactions. It demonstrates the adsorption of Cd by linking the shell surface [136–138]. Planothidium lanceolatum forms the ligands with metals like Cd, Cu, and Zn by adsorption and absorption of metals via π−π interactions [137]. Absorption of Cd in Thalassiosira weissflogii and Thalasssiosira oceanica by metallothione in transport system via chelation effect mechanisms and physical interactions and chemical bonding, respectively, was observed. While Thalassiosira pseudonana demonstrates the chemical adsorption of Zn, Cd, and Cu metal ions [134,139], reported that Chaetoceros costatum has high affinity for hydrogen bonds thus absorb heavy metals like Hg [140].
3.2. Dyes
Dyes are toxic, mutagenic, carcinogenic in nature and are also non-biodegradable due to their aromatic structure. Synthetic dyes are widely used in the leather, cosmetic, paper, plastics, and textile industries [141,142]. Bio removal of these dyes such as azo dyes, acid dyes, cationic dyes, and others has gained more consideration due to their highly tenacious and xenobiotic properties [143]. When these chemicals are disposed in water stream leading to an upsurge in water pollution and change in aquatic life. The research showed that SiO2 in diatom frustule, which is a naturally available low cost bio-silica material, could be used for eriochrome black T (EBT) dye removal. To increase its efficiency, its surface is modified or engineered with epichlorohydrin followed by NH4OH so as to introduce amine functionality on the silanol groups on diatom surface. This could help in adsorbing the EBT dye selectively even in the presence of other interfering dyes. Henceforth, this was found to be an efficient adsorbent for the removal of EBT dye from the effluents of dye-based and textile industries [65]. Figure 2 shows surface modification of diatom and their role in removal of dyes and metal ions.
Figure 2.
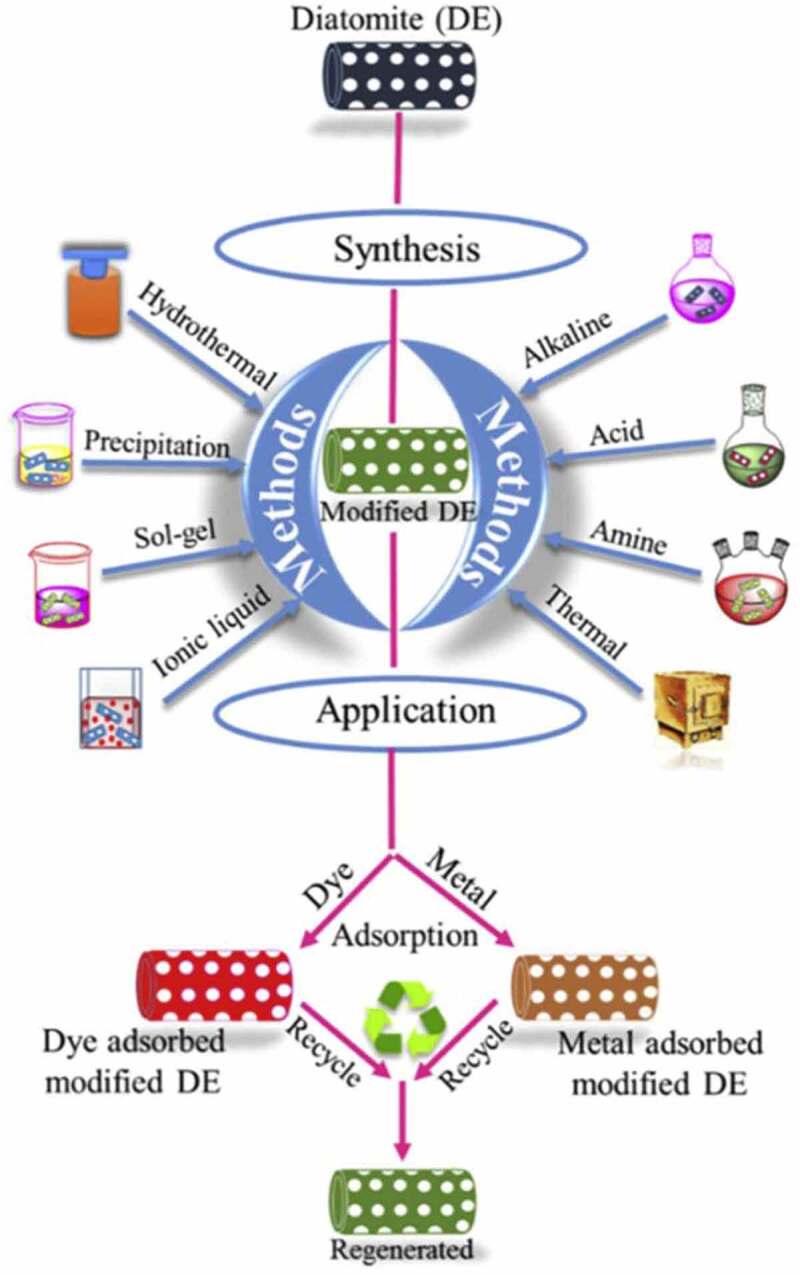
Schematic representation of surface modification and treatment of DE, and their application in the removal of dyes and metal ions along with regeneration. Reproduced with permission from [145]
In yet another study, diatoms frustules or diatomaceous earth were modified into bio-inspired polydopamine (PDA), DE-PDA and decorated with silver nanoparticles (AgNPs) in-situ to be used as nanocatalyst for the catalytic application. This designed nanocatalyst has been used for the degradation of cationic methylene blue (MB) and anionic Congo red (CR) dyes showing their fast degradation [144].
Similarly nano structure of MnO2-coated diatomite (MOCD) was used to remediate the methylene blue and methyl orange dyes, which showed biocatalytic activity and fast degradation within 30 minute or less [144]. Thus, they have potential applications in the field of wastewater treatment, and pollution degradation. Furthermore, removal of reactive blue 160 dye was demonstrated by Sriram et al [145], using diatomaceous earth at pH 2 with high adsorption capacity of up to 7.96 mg g-1 for treatment of wastewater from textile industries.
Thus, there is high requirement of cost-effective techniques, sustainable using hybrid diatom materials for the removal of toxic heavy metal ions and dyes.
3.3. Hydrocarbons
Polycyclic Aromatic Hydrocarbons (PAHs) are environmental contaminants and is a vast global concern [146,147]. PAHs have a wide range of hydrocarbons with over 100 compounds identified, each containing at least two aromatic rings in their assembly [48,148]. Owing to its hydrophobic nature, PAHs tend to accumulate in the aquatic sediments, results in its bioaccumulation and increased concentrations over time [149–151].
Diatoms are described to uptake polychlorinated biphenyls congener 2, 2′, 6, 6′-tetrachlorobiphenyl under standard nutrient availability conditions [143,152]. Due to the severe toxicity by polyaromatic hydrocarbons (PAHs), bioremediation has very limited success rate; however, marine diatoms Skeletonema costratum and Nitzschia sp. have revealed the quick biodegradation of hydrocarbons like phenanthrone (PHE) and fluoranthene (PLA), found commonly from marine sediments [153]. Furthermore, diatoms generate oxygen that can be utilized for the degradation of poly aromatic hydrocarbons, phenolic groups, and organic groups from the environments.
Research has shown the effects of three PAHs, viz; fluoranthene, pyrene, and benzo pyrene, on diatom Thalassiosira pseudonana, another common laboratory candidate diatom that is generally found globally in ocean and freshwaters. Modification at gene expression level by real-time PCR can be further investigated as the result of four chosen concentrations obtained from growth inhibition curves. According to the results, two out of eight genes were strongly influenced by the PAHs treatments. These were lacsA (involving the fatty acid metabolism) and sil3 (involving the formation of the silica shell). The observations showed that, it can be assumed that PAHs impaired the fatty acid metabolism and silica shell formation [154].
155 Li et al grew diatom Stephanodiscus hantzschii in titanium (IV) bis (ammonium lactato) dihydroxide solution in a continuous flow system. This integrated 7.2 ± 1.4% (mass fraction) of titanium in the diatom cell wall and thus forming silica-titania frustules and henceforth reported the photodegradation of three different types of pharmaceuticals and personal care products viz; triclosan, BPW and N, N-Diethylmeta-toluamide. The silica-titania frustules with hierarchical nano/microstructures which served as a prefiltration unit by selectively allowing pharmaceuticals and personal care products to pass through their nanopores and are therefore fit for the applications in nanotechnology and in environmental remediation [155].
4. Diatoms in microfluidics to treat wastewater
Among microalgae, diatoms due to nanoporous silica wall are suitable material to be used in fabricating micro and nanosized devices. They acting as a photobioreactor for production of value added products mainly biofuel and carotenoids and simultaneously adsorbing the toxic metals, dyes and other pollutants present in the wastewater added as nutrient supply for their growth [156]. In one such study, it was found that a microfluidic diatomite analytical device (μDADs) was fabricated to remove illicit drugs [122]. This has been inspired from microfluidic paper devices which are capable to remove heavy metals and other analytes like glucose effectively and economically [157]. This led to fabrication of μDADs which were constructed on a simple glass slide with diatomaceous earth spin coated in a mixture of 0.4% of carboxymethyl cellulose at an area of 400 × 30 μm2. It has been used as lab on chip chromatography in conjunction with surface enhanced Raman spectroscopy (SERS) sensing protocol to separate cocaine (C17H21NO4). The lab on chip (LOC) device can not only detect toxic drugs but also mixture of drugs in any aqueous solution and even wastewater via capillary forces operating in μDADs. Besides this other toxic compound like PAH for eg. pyrene, 4-mercaptobenzoic acid (MBA) are nuisance in our environment which can also be removed by μDADs, which is a novel way for economic route to use microfluidics in the field of nanotechnology and nanosciences for remediation of toxic compounds from wastewater environments. Furthermore, fabricating a lab on chip diatom electromachine (LOC-DEM) model on a glass wafer may direct a hydrodynamic flow of fluorophore doped algal cells kept under pressure in an intracellular spectral recomposition of light as seen in Figure 3. The lipophilic fluorophore further enhances the photosynthesis and thus rate of synthesis of petrochemical and bioactive components. The separation of these metabolites and bacterial contaminants further takes place via pores of size as small as 5–10 nm. This will not only separate the algal concentrate which can be re-fed in fresh media for manufacture of more bioactive and petrochemical products but the different fluorophores sensitive to reactive oxygen species serve as pollutant indicators too. This type of microfluidic system helps in overcoming dewatering the microalgae a crucial step during algal biomass processing, sensing the pollutants like herbicides, phenols, metals, and biopolymers and harvesting enhanced valuable biometabolites under the influence of intracellular spectral recompositing of light.
Figure 3.
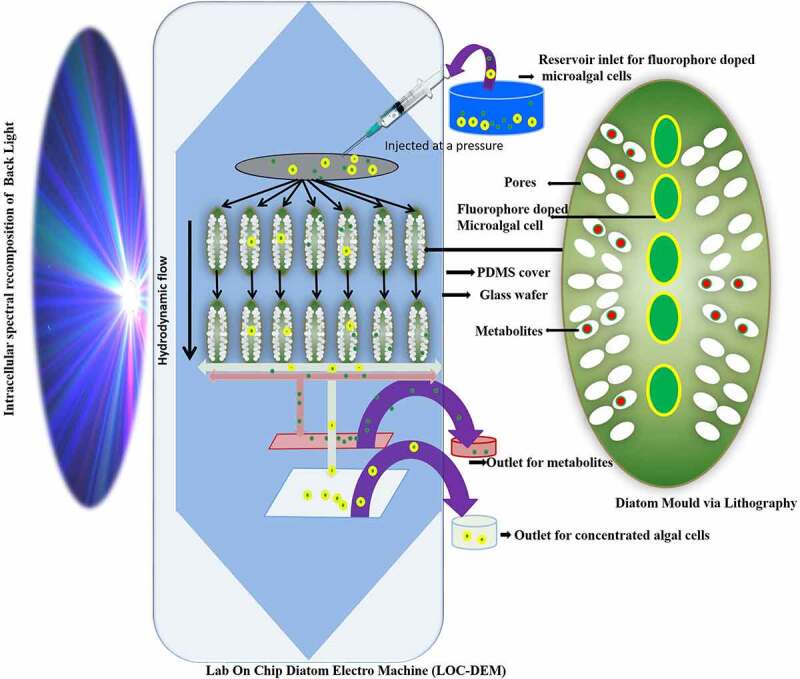
Lab on Chip Diatom Electro Machine (LOC-DEM) to remove wastewater pollutants while clean separating the algal concentrate flowing at hydrodynamic flow under the influence of intracellular spectral recomposition of light
5. Drawbacks and future perspectives
Drawbacks of usual methods for wastewater treatment make it difficult to develop a continuous treatment for wastewater disposal and recycling. So, it is very essential to find an alternative that would be high on resource recycling which take less energy and encourage economical circular bio-refinery concepts. Thus, microalgae can be a good surrogate for wastewater treatment [158].
Even though, microalgae utilize the nutrients from wastewater and not only recycle the wastewater but also help in remediating the toxic pollutants from it. This makes the wastewater reusable for agricultural and washing purpose. This is deficiently economical compared to the expensive water treatment methods in which industries dump their waste into oceans, rivers and lakes deteriorating the healthy flora and fauna. On the other hand, the use of microalgae has significant applications if they are used in economical microbial fuel cells (MFC) to not only clean the wastewater [159], but also produce electricity besides producing value added products like biofuel, carotenoids, proteins, carbohydrates, and polyhydroxyalkanoates [160,161]. Furthermore, diatoms along with other existing microalgae are responsible for synthesizing nanoparticles and synthesize various value added products while remediating wastewater employing various techniques including microbial fuel cells [162,163]. They thus behave differentially to different pollutants as seen in a case where diatom Nitzschia palea was exposed to seven different pollutants viz; paint, β propranolol drugs, sewage water, metasilicates, Eichhornia crassipes, detergents, hydrocarbons like petrol. It was found that diatom grew best in sewage water at a dilution of 5 mg and 10 mg/L, silica (60–120 µl/L), and petrol (5–15 µl/L) with moderate increase in lipid percentage [164]. Hydrocarbons at higher concentration than this are detrimental to microorganism growth and have different set of bacterium in it to valorize the soil petroleum sludge [27].
Although many different diatom based fuel cells besides MFC like diatom dye sensitized solar cells have been made to generate electricity by metabolically engineering diatoms with TiO2 nanoparticles using electrolyte and photosensitive dye [123,165]. Such fuel cells besides yielding biofuel need to be up scaled utilizing wastewater instead of electrolyte or even cost-effective nutrient media. Hence it is still challenging to researchers to find an economic and effective wastewater treatment methods that can be utilized and accepted globally [161].
6. Conclusions
Diatoms can be considered as sea jewels due to their rigid silica cell wall, which resists decomposition and are rich in value-added products. They besides cleaning, decrease 25% of our atmospheric CO2, thus reducing global warming. The diatoms frustule is made up of porous structure that has large surface area due to uniform nanoarray pattern and thus serves as nanoadsorbent for toxic metals and dyes. Even though live diatom cells equally adsorb the toxic pollutants but the live cells show deformities and abnormalities in their morphology and many times the cell undergo lipid stress thus resulting into high lipid bioaccumulation. Furthermore the nanoarchitecture of dead diatoms makes them an excellent choice to be used in microfluidic devices to sieve drugs and metals of varied sizes and shapes. Thus, biosensing the pollutants by diatoms living or dead at nanoscale level makes them a smart nanocontainers to biosense the wastewater rich metal and other pollutants.
Acknowledgments
MJK is grateful to Department of Science and Technology Nanomission Government of India for the Postdoc fellowship. AA is thankful to CEFIPRA Indo French project for Pre doctoral fellowships. VV would like to thank Department of Science and Technology Nanomission Government of India research project number (SR/NM/NT-1090/2014(G) and Indo-French Centre for the Promotion of Advanced Research (IFCPAR/CEFIPRA) project number (PPMB-7133/2020) Indo France project sanctioned to VV for the financial aids.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Nagarajan D, Varjani S, Lee DJ, et al. Sustainable aquaculture and animal feed from microalgae - nutritive value and techno-functional components. Renewable Sustainable Energy Rev. 2021;150:111549. [Google Scholar]
- [2].Nguyen -T-T, Bui X-T, Ngo HH, et al. Nutrient recovery and microalgae biomass production from urine by membrane photobioreactor at low biomass retention times. SciTotal Environ. 2021;785:147423. [Google Scholar]
- [3].Nguyen TTD, Bui XT, Nguyen TT, et al. Co-culture of microalgae-activated sludge in sequencing batch photobioreactor systems: effects of natural and artificial lighting on wastewater treatment. Bioresour Technol. 2022;343:126091. [DOI] [PubMed] [Google Scholar]
- [4].Devda V, Chaudhary K, Varjani S, et al. Recovery of resources from industrial wastewater employing electrochemical technologies: status, advancements and perspectives. Bioengineered. 2021;12(1):4697–4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lage S, Toffolo A, Gentili FG.. Microalgal growth, nitrogen uptake and storage, and dissolved oxygen production in a polyculture based-open pond fed with municipal wastewater in northern Sweden. Chemosphere. 2021;276:130122. [DOI] [PubMed] [Google Scholar]
- [6].Oyebamiji OO, Boeing WJ, Holguin FO, et al. Green microalgae cultured in textile wastewater for biomass generation and biodetoxification of heavy metals and chromogenic substances. Bioresour Technol Rep. 2019;7:100247. [Google Scholar]
- [7].Shen Q-H, Gong Y-P, Fang W-Z, et al. Saline wastewater treatment by Chlorella vulgaris with simultaneous algal lipid accumulation triggered by nitrate deficiency. Bioresour Technol. 2015;193:68–75. [DOI] [PubMed] [Google Scholar]
- [8].Wang C-H, Lee Y-H, Kuo H-T, et al. Dielectrophoretically-assisted electroporation using light-activated virtual microelectrodes for multiple DNA transfection. Lab Chip. 2014;14(3):592–601. [DOI] [PubMed] [Google Scholar]
- [9].Wang L, Min M, Li Y, et al. Cultivation of green algae Chlorella sp. in different wastewaters from municipal wastewater treatment plant. Appl Biochem Biotechnol. 2010;162(4):1174–1186. [DOI] [PubMed] [Google Scholar]
- [10].Misra CS, Appukuttan D, Kantamreddi VSS, et al. Recombinant D. radiodurans cells for bioremediation of heavy metals from acidic/neutral aqueous wastes. Bioengineered. 2012;3(1):44–48. [DOI] [PubMed] [Google Scholar]
- [11].Ji L, Xie S, Feng J, et al. Heavy metal uptake capacities by the common freshwater green algae Cladophora fracta. J Appl Phycol. 2012;24(4):979–983. [Google Scholar]
- [12].Wong J, Wong Y, Tam N.. Nickel biosorption by two chlorella species, C. Vulgaris (a commercial species) and C. Miniata (a local isolate). Bioresour Technol. 2000;73(2):133–137. [Google Scholar]
- [13].Pradhan D, Sukla LB, Mishra BB, et al. Biosorption for removal of hexavalent chromium using microalgae Scenedesmus sp. J Clean Prod. 2019;209:617–629. [Google Scholar]
- [14].Rezaei H. Biosorption of chromium by using Spirulina sp. Arabian J Chem. 2016;9(6):846–853. [Google Scholar]
- [15].Sibi G. Biosorption of chromium from electroplating and galvanizing industrial effluents under extreme conditions using Chlorella vulgaris. Green Energy Environ. 2016;1(2):172–177. [Google Scholar]
- [16].Chan A, Salsali H, McBean E. Heavy metal removal (copper and zinc) in secondary effluent from wastewater treatment plants by microalgae. ACS Sustain Chem Eng. 2014;2(2):130–137. [Google Scholar]
- [17].Malakootian M, Yousefi Z, Limoni ZK. Removal of lead from battery industry wastewater by Chlorella vulgaris as green micro-algae (case study: Kerman, Iran). Desalin Water Treat. 2019;141:248–255. [Google Scholar]
- [18].Shanab S, Essa A, Shalaby E. Bioremoval capacity of three heavy metals by some microalgae species (Egyptian Isolates). Plant Signal Behav. 2012;7(3):392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fard GH, Mehrnia MR. Investigation of mercury removal by Micro-Algae dynamic membrane bioreactor from simulated dental waste water. J Environ Chem Eng. 2017;5(1):366–372. [Google Scholar]
- [20].Kumar M, Singh AK, Sikandar M. Biosorption of Hg (II) from aqueous solution using algal biomass: kinetics and isotherm studies. Heliyon. 2020;6(1):e03321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chong A, Wong Y, Tam N. Performance of different microalgal species in removing nickel and zinc from industrial wastewater. Chemosphere. 2000;41(1–2):251–257. [DOI] [PubMed] [Google Scholar]
- [22].Gagneux-Moreaux S, Cosson RP, Bustamante P, et al. Growth and metal uptake of microalgae produced using salt groundwaters from the Bay of Bourgneuf. Aquat Living Resour. 2006;19(3):247–255. [Google Scholar]
- [23].Han X, Wong YS, Wong MH, et al. Feasibility of using microalgal biomass cultured in domestic wastewater for the removal of chromium pollutants. Water Environ Res. 2008;80(7):647–653. [PubMed] [Google Scholar]
- [24].Sulaymon AH, Mohammed AA, Al-Musawi TJ. Competitive biosorption of lead, cadmium, copper, and arsenic ions using algae. Environ Sci Pollut Res. 2013;20(5):3011–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Eladel H, Abomohra AE-F, Battah M, et al. Evaluation of Chlorella sorokiniana isolated from local municipal wastewater for dual application in nutrient removal and biodiesel production. Bioprocess Biosyst Eng. 2019;42(3):425–433. [DOI] [PubMed] [Google Scholar]
- [26].Cho HU, Kim YM, Park JM. Enhanced microalgal biomass and lipid production from a consortium of indigenous microalgae and bacteria present in municipal wastewater under gradually mixotrophic culture conditions. Bioresour Technol. 2017;228:290–297. [DOI] [PubMed] [Google Scholar]
- [27].Varjani S, Pandey A, Upasani VN. Petroleum sludge polluted soil remediation: integrated approach involving novel bacterial consortium and nutrient application. SciTotal Environ. 2021a;763:142934. [DOI] [PubMed] [Google Scholar]
- [28].Yan A, Wang Y, Tan SN, et al. Phytoremediation: a promising approach for revegetation of heavy metal-polluted Land. Front Plant Sci. 2020;11:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mousavi S, Najafpour GD, Mohammadi M, et al. Cultivation of newly isolated microalgae Coelastrum sp. in wastewater for simultaneous CO 2 fixation, lipid production and wastewater treatment. Bioprocess Biosyst Eng. 2018;41(4):519–530. [DOI] [PubMed] [Google Scholar]
- [30].Li Y, Yang X, Geng B. Preparation of immobilized sulfate-reducing bacteria-microalgae beads for effective bioremediation of copper-containing wastewater. Water Air Soil Pollut. 2018;229(3):1–13. [Google Scholar]
- [31].Franklin N, Adams M, Stauber J, et al. Development of an improved rapid enzyme inhibition bioassay with marine and freshwater microalgae using flow cytometry. Arch Environ Contam Toxicol. 2001;40(4):469–480. [DOI] [PubMed] [Google Scholar]
- [32].Kumar R, Goyal D. Waste water treatment and metal (Pb 2+, Zn 2+) removal by microalgal based stabilization pond system. Indian J Microbiol. 2010;50(S1):34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gaur VK, Sharma P, Gaur P, et al. Sustainable mitigation of heavy metals from effluents: toxicity and fate with recent technological advancements. Bioengineered. 2021;12(1):7297–7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Vo TDH, Bui XT, Dang BT, et al. Influence of organic loading rates on treatment performance of membrane bioreactor treating tannery wastewater. Environ Technol Innovation. 2021;24:101810. [Google Scholar]
- [35].Rajalakshmi A, Silambarasan T, Dhandapani R. Small scale photo bioreactor treatment of tannery wastewater, heavy metal biosorption and CO2 sequestration using microalgae Chlorella sp.: a biodegradation approach. Appl Water Sci. 2021;11(7):1–12. [Google Scholar]
- [36].Ahirwar A, Meignen G, Khan MJ, et al. Light modulates transcriptomic dynamics upregulating astaxanthin accumulation in Haematococcus: a review. Bioresour Technol. 2021b;340:125707. [DOI] [PubMed] [Google Scholar]
- [37].Gupta AK, Seth K, Maheshwari K, et al. Biosynthesis and extraction of high-value carotenoid from algae. Front Biosci (Landmark Ed). 2021;26(6):171–190. [DOI] [PubMed] [Google Scholar]
- [38].López-Pacheco IY, Carrillo-Nieves D, Salinas-Salazar C, et al. Combination of nejayote and swine wastewater as a medium for Arthrospira maxima and Chlorella vulgaris production and wastewater treatment. SciTotal Environ. 2019;676:356–367. [DOI] [PubMed] [Google Scholar]
- [39].Zambrano J, García-Encina PA, Hernández F, et al. Removal of a mixture of veterinary medicinal products by adsorption onto a Scenedesmus almeriensis microalgae-bacteria consortium. Journal of Water Process Engineering. 2021;43:102226. [Google Scholar]
- [40].Abirama V, Mohamed RMSR, Al-Gheethi A, et al. Meat processing wastewater Phycoremediation by Botryococcus sp.: a biokinetic study and a techno-economic analysis. Sep Sci Technol. 2021;56(3):577–591. [Google Scholar]
- [41].Harshkova D, Majewska M, Pokora W, et al. Diclofenac and atrazine restrict the growth of a synchronous Chlamydomonas reinhardtii population via various mechanisms. Aquatic Toxicol. 2021;230:105698. [DOI] [PubMed] [Google Scholar]
- [42].Abdel-Raouf N, Sholkamy EN, Bukhari N, et al. Bioremoval capacity of Co+ 2 using Phormidium tenue and Chlorella vulgaris as biosorbents. Environ Res. 2021;204:111630. [DOI] [PubMed] [Google Scholar]
- [43].Su C, Sun X, Mu Y, et al. Multilayer calcium alginate beads containing Diatom biosilica and bacillus subtilis as microecologics for sewage treatment. Carbohydr Polym. 2021;256:117603. [DOI] [PubMed] [Google Scholar]
- [44].Tornés E, Mor J-R, Mandaric L, et al. Diatom responses to sewage inputs and hydrological alteration in Mediterranean streams. Environ Pollut. 2018;238:369–378. [DOI] [PubMed] [Google Scholar]
- [45].Adesra A, Srivastava VK, Varjani S. Valorization of dairy wastes: integrative approaches for value added products. Indian J Microbiol. 2021;61(3):270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bharathiraja B, Selvakumari IAE, Iyyappan J, et al. Itaconic acid: an effective sorbent for removal of pollutants from dye industry effluents. Curr Opin Environ Sci Health. 2019;12:6–17. [Google Scholar]
- [47].Varjani S, Rakholiya P, Ng HY, et al. Microbial degradation of dyes: an overview. Bioresour Technol. 2020;314:123728. [DOI] [PubMed] [Google Scholar]
- [48].Varjani S, Rakholiya P, Shindhal T, et al. Trends in dye industry effluent treatment and recovery of value added products. Journal of Water Process Engineering. 2021b;39:101734. [Google Scholar]
- [49].Chen C-Y, Kuo E-W, Nagarajan D, et al. Semi-batch cultivation of Chlorella sorokiniana AK-1 with dual carriers for the effective treatment of full strength piggery wastewater treatment. Bioresour Technol. 2021;326:124773. [DOI] [PubMed] [Google Scholar]
- [50].Seth K, Kumar A, Rastogi RP, et al. Bioprospecting of fucoxanthin from diatoms—Challenges and perspectives. Algal Res. 2021;60:102475. [Google Scholar]
- [51].Vinayak V, Khan MJ, Jha AN. Photosystem I P700 chlorophyll a apoprotein A1 as PCR marker to identify diatoms and their associated lineage. J Eukaryotic Microbiol. 2021a;e12866. 10.1111/jeu.12866. [DOI] [PubMed] [Google Scholar]
- [52].Ahirwar A, Meignen G, Khan M, et al. Nanotechnological approaches to disrupt the rigid cell walled microalgae grown in wastewater for value-added biocompounds: commercial applications, challenges, and breakthrough. Biomass Convers Biorefin. 2021a. 10.1007/s13399-021-01965-1 [DOI] [Google Scholar]
- [53].Arif M, Bai Y, Usman M, et al. Highest accumulated microalgal lipids (polar and non-polar) for biodiesel production with advanced wastewater treatment: role of lipidomics. Bioresour Technol. 2020;298:122299. [DOI] [PubMed] [Google Scholar]
- [54].Bach LT, Riebesell U, Sett S, et al. An approach for particle sinking velocity measurements in the 3–400 μm size range and considerations on the effect of temperature on sinking rates. Mar Biol. 2012;159(8):1853–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Soleimani M, Rutten L, Maddala SP, et al. Modifying the thickness, pore size, and composition of diatom frustule in Pinnularia sp. with Al 3+ ions. Sci Rep. 2020;10(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Vinayak V, Manoylov KM, Gateau H, et al. Diatom milking: a review and new approaches. Mar Drugs. 2015;13(5):2629–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Rogato A, De Tommasi E. Physical, chemical, and genetic techniques for diatom frustule modification: applications in nanotechnology. Appl Sci. 2020;10(23):8738. [Google Scholar]
- [58].Zeb S, Ali N, Ali Z, et al. Silica-based nanomaterials as designer adsorbents to mitigate emerging organic contaminants from water matrices. Journal of Water Process Engineering. 2020;38:101675. [Google Scholar]
- [59].Kiran MT, Bhaskar MV, Tiwari A. Phycoremediation of eutrophic lakes using diatom algae. In: Lake sciences and climate change. IntechOpen, UK, 2016. p. 103–115. [Google Scholar]
- [60].Jayaswal K, Sahu V, Gurjar B. Water pollution, human health and remediation. In: Water Remediation. Springer, Singapore; 2018. p. 11–27. [Google Scholar]
- [61].Rashid H, Manzoor MM, Mukhtar S. Urbanization and its effects on water resources: an exploratory analysis. Asian J Water Environ Pollut. 2018;15(1):67–74 [Google Scholar]
- [62].Tramontano C, Chianese G, Terracciano M, et al. Nanostructured biosilica of diatoms: from water world to biomedical applications. Appl Sci. 2020;10(19):6811. [Google Scholar]
- [63].Li K, Liu Q, Fang F, et al. Microalgae-based wastewater treatment for nutrients recovery: a review. Bioresour Technol. 2019;291:121934. [DOI] [PubMed] [Google Scholar]
- [64].Olteanu M, Baraitaru A, Panait A-M, et al. Advanced SiO 2 composite materials for heavy metal removal from wastewater. Water Air Soil Pollut. 2019;230(8):1–10. [Google Scholar]
- [65].Sriram G, Bhat Mp, Kigga M, et al. Amine activated diatom xerogel hybrid material for efficient removal of hazardous dye. Mater Chem Phys. 2019;235:121738. [Google Scholar]
- [66].Janani R, Baskar G, Sivakumar K, et al. Advancements in heavy metals removal from effluents employing nano-adsorbents: way towards cleaner production. Environ Res. 2021;203:111815. [DOI] [PubMed] [Google Scholar]
- [67].Gupta V, Tyagi I, Sadegh H, et al. Nanoparticles as adsorbent; a positive approach for removal of noxious metal ions: a review. Sci Techno Soc. 2015;34(3):195–214. [Google Scholar]
- [68].Teerawattanasuk C, Voottipruex P, Horpibulsuk S. Improved heavy metal immobilization of compacted clay by cement treatment. Heliyon. 2021;7(4):e06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Anjum A. Adsorption technology for removal of toxic pollutants. In: Sustainable heavy metal remediation. Springer, Cham; 2017. p. 25–80. [Google Scholar]
- [70].Hoffmann F, Cornelius M, Morell J, et al. Silica‐based mesoporous organic–inorganic hybrid materials. Angew Chem. 2006;45(20):3216–3251. [DOI] [PubMed] [Google Scholar]
- [71].Vinu A, Hossain KZ, Ariga K. Recent advances in functionalization of mesoporous silica. J Nanosci Nanotechnol. 2005;5(3):347–371. [DOI] [PubMed] [Google Scholar]
- [72].De Stefano L, De Stefano M, De Tommasi E, et al. A natural source of porous biosilica for nanotech applications: the diatoms microalgae. Phys Status Solidi C. 2011;8(6):1820–1825. [Google Scholar]
- [73].Terracciano M, De Stefano L, Rea I. Diatoms green nanotechnology for biosilica-based drug delivery systems. Pharmaceutics. 2018;10(4):242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Sbihi K, Cherifi O, Bertrand M, et al. Biosorption of metals (Cd, Cu and Zn) by the freshwater diatom Planothidium lanceolatum: a laboratory study. Diatom Res. 2014;29(1):55–63 [Google Scholar]
- [75].Ma J, Zhou B, Duan D, et al. Salinity-dependent nanostructures and composition of cell surface and its relation to Cd toxicity in an estuarine diatom. Chemosphere. 2019;215:807–814. [DOI] [PubMed] [Google Scholar]
- [76].Falasco E, Bona F, Ginepro M, et al. Morphological abnormalities of diatom silica walls in relation to heavy metal contamination and artificial growth conditions. Water Sa. 2009b;35(5):5. [Google Scholar]
- [77].Pandey LK, Kumar D, Yadav A, et al. Morphological abnormalities in periphytic diatoms as a tool for biomonitoring of heavy metal pollution in a river. Ecol Indic. 2014;36:272–279. [Google Scholar]
- [78].Olguín EJ. Dual purpose microalgae–bacteria-based systems that treat wastewater and produce biodiesel and chemical products within a Biorefinery. Biotechnol Adv. 2012;30(5):1031–1046. [DOI] [PubMed] [Google Scholar]
- [79].Rout PR, Shahid MK, Dash RR, et al. Nutrient removal from domestic wastewater: a comprehensive review on conventional and advanced technologies. J Environ Manage. 2021;296:113246. [DOI] [PubMed] [Google Scholar]
- [80].Feng S, Ngo HH, Guo W, et al. Roles and applications of enzymes for resistant pollutants removal in wastewater treatment. Bioresour Technol. 2021;335:125278. [DOI] [PubMed] [Google Scholar]
- [81].Khan MJ, Singh R, Shewani K, et al. Exopolysaccharides directed embellishment of diatoms triggered on plastics and other marine litter. Sci Rep. 2020;10(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Saravanan A, Kumar PS, Varjani S, et al. A review on algal-bacterial symbiotic system for effective treatment of wastewater. Chemosphere. 2021;271:129540. [DOI] [PubMed] [Google Scholar]
- [83].Khan MJ, Singh N, Mishra S, et al. Impact of light on Microalgal photosynthetic microbial fuel cells and removal of pollutants by nanoadsorbent biopolymers: updates, challenges and innovations. Chemosphere. 2022;132589, 10.1016/j.chemosphere.2021.132589. [DOI] [PubMed] [Google Scholar]
- [84].Launay H, Huang W, Maberly SC, et al. Regulation of carbon metabolism by environmental conditions: a perspective from diatoms and other chromalveolates. Front Plant Sci. 2020;11:1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Falasco E, Bona F, Badino G, et al. Diatom teratological forms and environmental alterations: a review. Hydrobiologia. 2009a;623(1):1–35. [Google Scholar]
- [86].Schiffman E, Ohrbach R, Truelove E, et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the International RDC/TMD consortium network and orofacial pain special interest group. J Oral Facial Pain Headache. 2014;28(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Montagnes DJ, Franklin M. Effect of temperature on diatom volume, growth rate, and carbon and nitrogen content: reconsidering some paradigms. Limnol Oceanography. 2001;46(8):2008–2018. [Google Scholar]
- [88].Pandey LK, Bergey EA. Exploring the status of motility, lipid bodies, deformities and size reduction in periphytic diatom community from chronically metal (Cu, Zn) polluted water bodies as a biomonitoring tool. SciTotal Environ. 2016;550:372–381. [DOI] [PubMed] [Google Scholar]
- [89].Gautam S, Pandey LK, Vinayak V, et al. Morphological and physiological alterations in the diatom Gomphonema pseudoaugur due to heavy metal stress. Ecol Indic. 2017;72:67–76. [Google Scholar]
- [90].Morin S, Coste M. 2006. Metal-induced shifts in the morphology of diatoms from the Riou mort and Riou viou streams (South West France). 91–106.
- [91].Rimet F, Ector L, Dohet A, et al. Impacts of fluoranthene on diatom assemblages and frustule morphology in indoor microcosms. Vie et Milieu. 2004;54:145–156. [Google Scholar]
- [92].Morin S, Coste M, Hamilton PB. SCANNING ELECTRON MICROSCOPY OBSERVATIONS OF DEFORMITIES IN SMALL PENNATE DIATOMS EXPOSED TO HIGH CADMIUM CONCENTRATIONS(1). J Phycol. 2008;44(6):1512–1518. [DOI] [PubMed] [Google Scholar]
- [93].Debenest T, Coste M, Delmas F, et al. Les frustules déformés de diatomées benthiques et les pesticides: le cas des pollutions agricoles dans les coteaux de Gascogne (Sud-Ouest de la France). Diatomania. 2006;10:62–65. [Google Scholar]
- [94].Woodard K, Neustupa J. Morphometric asymmetry of frustule outlines in the pennate diatom luticola poulickovae (Bacillariophyceae). Symmetry. 2016;8(12):150. [Google Scholar]
- [95].Cattaneo A, Couillard Y, Wunsam S, et al. Diatom taxonomic and morphological changes as indicators of metal pollution and recovery in Lac Dufault (Québec, Canada). J Paleolimnol. 2004;32(2):163–175 [Google Scholar]
- [96].Olenici A, Blanco S, Borrego-Ramos M, et al. Exploring the effects of acid mine drainage on diatom teratology using geometric morphometry. Ecotoxicology. 2017;26(8):1018–1030. [DOI] [PubMed] [Google Scholar]
- [97].Szabó K, Kiss KT, Taba G, et al. Epiphytic diatoms of the Tisza River, kisköre reservoir and some oxbows of the Tisza River after the cyanide and heavy metal pollution in 2000. Acta Bot Croat. 2005;64(1):1–46. [Google Scholar]
- [98].Sienkiewicz E, Gąsiorowski M. The evolution of a mining lake-From acidity to natural neutralization. SciTotal Environ. 2016;557-558:343–354. [DOI] [PubMed] [Google Scholar]
- [99].Renzi M, Roselli L, Giovani A, et al. Early warning tools for ecotoxicity assessment based on Phaeodactylum tricornutum. Ecotoxicology. 2014;23(6):1055–1072. [DOI] [PubMed] [Google Scholar]
- [100].Vinayak V, Gordon R, Gautam S, et al. Discovery of a diatom that oozes oil. Adv Sci Lett. 2014;20(7):1256–1267. [Google Scholar]
- [101].Khan MJ, Singh R, Joshi KB, et al. TiO 2 doped polydimethylsiloxane (PDMS) and Luffa cylindrica based photocatalytic nanosponge to absorb and desorb oil in diatom solar panels. RSC Adv. 2019;9(39):22410–22416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Cantonati M, Angeli N, Virtanen L, et al. Achnanthidium minutissimum (Bacillariophyta) valve deformities as indicators of metal enrichment in diverse widely-distributed freshwater habitats. SciTotal Environ. 2014;475:201–215. [DOI] [PubMed] [Google Scholar]
- [103].Pandey LK, Han T, Gaur J. Response of a phytoplanktonic assemblage to copper and zinc enrichment in microcosm. Ecotoxicology. 2015;24(3):573–582. [DOI] [PubMed] [Google Scholar]
- [104].Antoni JS, Daglio Y, Areco MM, et al. Zinc-induced stress on cells of Halamphora luciae (Bacillariophyceae). Eur J Phycol. 2021;56(1):37–50. [Google Scholar]
- [105].Pham T-L. Effect of silver nanoparticles on tropical freshwater and marine microalgae. J Chem. 2019;2019:1-7. [Google Scholar]
- [106].Park J, Lee H, Depuydt S, et al. Assessment of five live-cell characteristics in periphytic diatoms as a measure of copper stress. J Hazard Mater. 2020;400:123113. [DOI] [PubMed] [Google Scholar]
- [107].Falasco E, Ector L, Wetzel CE, et al. Looking back, looking forward: a review of the new literature on diatom teratological forms (2010–2020). Hydrobiologia. 2021;848:1–79.33424031 [Google Scholar]
- [108].Vinayak V, Gordon R, Joshi K, et al. 2018. Diafuel. Trademark application no 3778882; Trade Marks Journal No: 1846(Class 4.).
- [109].Vinayak V, Joshi KB, Gordon R, et al. Nanoengineering of diatom surfaces for emerging applications. In: Diatom Nanotechnology, RSC, United Kingdom. 2017. p. 55–78. [Google Scholar]
- [110].Khan MJ, Bawra N, Verma A, et al. Cultivation of diatom Pinnularia saprophila for lipid production: a comparison of methods for harvesting the lipid from the cells. Bioresour Technol. 2021a;319:124129. [DOI] [PubMed] [Google Scholar]
- [111].Pandey LK. In situ assessment of metal toxicity in riverine periphytic algae as a tool for biomonitoring of fluvial ecosystems. Environ Technol Innovation. 2020;18:100675. [Google Scholar]
- [112].Pandey LK, Bergey EA. Metal toxicity and recovery response of riverine periphytic algae. SciTotal Environ. 2018;642:1020–1031. [DOI] [PubMed] [Google Scholar]
- [113].Mu W, Jia K, Liu Y, et al. Response of the freshwater diatom Halamphora veneta (Kützing) Levkov to copper and mercury and its potential for bioassessment of heavy metal toxicity in aquatic habitats. Environ Sci Pollut Res. 2017;24(34):26375–26386. [DOI] [PubMed] [Google Scholar]
- [114].Mu W, Chen Y, Liu Y, et al. Toxicological effects of cadmium and lead on two freshwater diatoms. Environ Toxicol Pharmacol. 2018;59:152–162. [DOI] [PubMed] [Google Scholar]
- [115].Licursi M, Gómez N. Short-term toxicity of hexavalent-chromium to epipsammic diatoms of a microtidal estuary (Río de la Plata): responses from the individual cell to the community structure. Aquatic Toxicol. 2013;134-135:82–91. [DOI] [PubMed] [Google Scholar]
- [116].Lee J, Choi J, Park JH, et al. Cytoprotective silica coating of individual mammalian cells through bioinspired silicification. Angew Chem. 2014;53(31):8056–8059. [DOI] [PubMed] [Google Scholar]
- [117].Yang SH, Lee KB, Kong B, et al. Biomimetic encapsulation of individual cells with silica. Angew Chem. 2009;48(48):9160–9163. [DOI] [PubMed] [Google Scholar]
- [118].Kumar V, Kashyap M, Gautam S, et al. Fast Fourier infrared spectroscopy to characterize the biochemical composition in diatoms. J Biosci. 2018;43(4):717–729. [PubMed] [Google Scholar]
- [119].Yang SH, Ko EH, Jung YH, et al. Bioinspired functionalization of silica‐encapsulated yeast cells. Angew Chem. 2011;123(27):6239–6242. [DOI] [PubMed] [Google Scholar]
- [120].Lee H, Hong D, Cho H, et al. Turning diamagnetic microbes into multinary micro-magnets: magnetophoresis and spatio-temporal manipulation of individual living cells. Sci Rep. 2016;6(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Lei Q, Guo J, Kong F, et al. Bioinspired Cell Silicification: from Extracellular to Intracellular. J Am Chem Soc. 2021;143(17):6305–6322. [DOI] [PubMed] [Google Scholar]
- [122].Kong X, Chong X, Squire K, et al. Microfluidic diatomite analytical devices for illicit drug sensing with ppb-Level sensitivity. Sens Actuators B Chem. 2018;259:587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Gupta S, Kashyap M, Kumar V, et al. Peptide mediated facile fabrication of silver nanoparticles over living diatom surface and its application. J Mol Liq. 2018;249:600–608. [Google Scholar]
- [124].Leonardo S, Prieto-Simón B, Campàs M. Past, present and future of diatoms in biosensing. Trends Analyt Chem. 2016;79:276–285. [Google Scholar]
- [125].Zhen L, Ford N, Gale DK, et al. Photoluminescence detection of 2, 4, 6-trinitrotoluene (TNT) binding on diatom frustule biosilica functionalized with an anti-TNT monoclonal antibody fragment. Biosens Bioelectron. 2016;79:742–748. [DOI] [PubMed] [Google Scholar]
- [126].Lu M, Li L, Shen S, et al. Highly efficient removal of Pb 2+ by a sandwich structure of metal–organic framework/GO composite with enhanced stability. New J Chem. 2019;43(2):1032–1037. [Google Scholar]
- [127].Fu W, Wichuk K, Brynjólfsson S. Developing diatoms for value-added products: challenges and opportunities. N Biotechnol. 2015b;32(6):547–551. [DOI] [PubMed] [Google Scholar]
- [128].Corcoll N, Bonet B, Morin S, et al. The effect of metals on photosynthesis processes and diatom metrics of biofilm from a metal-contaminated river: a translocation experiment. Ecol Indic. 2012;18:620–631. [Google Scholar]
- [129].Bao S, Li K, Ning P, et al. Highly effective removal of mercury and lead ions from wastewater by mercaptoamine-functionalised silica-coated magnetic nano-adsorbents: behaviours and mechanisms. Appl Surf Sci. 2017;393:457–466. [Google Scholar]
- [130].Repo E, Warchoł JK, Bhatnagar A, et al. Heavy metals adsorption by novel EDTA-modified chitosan–silica hybrid materials. J Colloid Interface Sci. 2011;358(1):261–267. [DOI] [PubMed] [Google Scholar]
- [131].Uthappa U, Sriram G, Arvind O, et al. Engineering MIL-100 (Fe) on 3D porous natural diatoms as a versatile high performing platform for controlled isoniazid drug release, Fenton’s catalysis for malachite green dye degradation and environmental adsorbents for Pb2+ removal and dyes. Appl Surf Sci. 2020;528:146974. [Google Scholar]
- [132].Baeyens W, Gao Y, Davison W, et al. In situ measurements of micronutrient dynamics in open seawater show that complex dissociation rates may limit diatom growth. Sci Rep. 2018;8(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Fu L, Hamzeh M, Dodard S, et al. Effects of TiO2 nanoparticles on ROS production and growth inhibition using freshwater green algae pre-exposed to UV irradiation. Environ Toxicol Pharmacol. 2015a;39(3):1074–1080. [DOI] [PubMed] [Google Scholar]
- [134].Loix C, Huybrechts M, Vangronsveld J, et al. Reciprocal interactions between cadmium-induced cell wall responses and oxidative stress in plants. Front Plant Sci. 2017;8:1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Hernández-Ávila J, Salinas-Rodríguez E, Cerecedo-Sáenz E, et al. Diatoms and their capability for heavy metal removal by cationic exchange. Metals. 2017;7(5):169. [Google Scholar]
- [136].Jadoon S, Malik A. DNA damage by heavy metals in animals and human beings: an overview. Biochem Pharmacol. 2017;6(3):1–8. [Google Scholar]
- [137].Łukowski A, Dec D. Influence of Zn, Cd, and Cu fractions on enzymatic activity of arable soils. Environ Monit Assess. 2018;190(5):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Santos J, Almeida SF, Figueira E. Cadmium chelation by frustulins: a novel metal tolerance mechanism in Nitzschia palea (Kützing) W. Smith. Ecotoxicology. 2013;22(1):166–173. [DOI] [PubMed] [Google Scholar]
- [139].Lane ES, Jang K, Cullen JT, et al. The interaction between inorganic iron and cadmium uptake in the marine diatom Thalassiosira oceanica. Limnol Oceanography. 2008;53(5):1784–1789. [Google Scholar]
- [140].Volland S, Bayer E, Baumgartner V, et al. Rescue of heavy metal effects on cell physiology of the algal model system Micrasterias by divalent ions. J Plant Physiol. 2014;171(2):154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Guo Y, Wang X, Hu P, et al. ZIF-8 coated polyvinylidene fluoride (PVDF) hollow fiber for highly efficient separation of small dye molecules. Appl Mater Today. 2016;5:103–110. [Google Scholar]
- [142].Shah AV, Varjani S, Srivastava VK, et al. Zero liquid discharge as a sustainable technology - A review on assessment of technology, challenges and perspectives. Indian J Exp Biol. 2020;58(8):508–514. [Google Scholar]
- [143].Shindhal T, Rakholiya P, Varjani S, et al. A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered. 2021;12(1):70–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Uthappa U, Kigga M, Sriram G, et al. Facile green synthetic approach of bio inspired polydopamine coated diatoms as a drug vehicle for controlled drug release and active catalyst for dye degradation. Microporous Mesoporous Mater. 2019;288:109572. [Google Scholar]
- [145].Sriram G, Kigga M, Uthappa U, et al. Naturally available diatomite and their surface modification for the removal of hazardous dye and metal ions: a review. Adv Colloid Interface Sci. 2020;282:102198. [DOI] [PubMed] [Google Scholar]
- [146].Varjani SJ. Microbial degradation of petroleum hydrocarbons. Bioresour Technol. 2017;223:277–286. [DOI] [PubMed] [Google Scholar]
- [147].Varjani SJ, Gnansounou E, Pandey A. Comprehensive review on toxicity of persistent organic pollutants from petroleum refinery waste and their degradation by microorganisms. Chemosphere. 2017;188:280–291. [DOI] [PubMed] [Google Scholar]
- [148].Varjani SJ, Rana DP, Jain AK, et al. Synergistic ex-situ biodegradation of crude oil by halotolerant bacterial consortium of indigenous strains isolated from on shore sites of Gujarat, India. Int Biodeterior Biodegrad. 2015;103:116–124. [Google Scholar]
- [149].Behera BK, Das A, Sarkar DJ, et al. Polycyclic Aromatic Hydrocarbons (PAHs) in inland aquatic ecosystems: perils and remedies through biosensors and bioremediation. Environ Pollut. 2018;241:212–233. [DOI] [PubMed] [Google Scholar]
- [150].Varjani S, Joshi R, Srivastava VK, et al. Treatment of wastewater from petroleum industry: current practices and perspectives. Environ Sci Pollut Res. 2019;1-9. 10.1007/s11356-019-04725-x. [DOI] [PubMed] [Google Scholar]
- [151].Varjani SJ, Upasani VN. A new look on factors affecting microbial degradation of petroleum hydrocarbon pollutants. Int Biodeterior Biodegrad. 2017;120:71–83. [Google Scholar]
- [152].Lynn SG, Price DJ, Birge WJ, et al. Effect of nutrient availability on the uptake of PCB congener 2, 2′, 6, 6′-tetrachlorobiphenyl by a diatom (Stephanodiscus minutulus) and transfer to a zooplankton (Daphnia pulicaria). Aquatic Toxicol. 2007;83(1):24–32. [DOI] [PubMed] [Google Scholar]
- [153].Hong Y-W, Yuan D-X, Lin Q-M, et al. Accumulation and biodegradation of phenanthrene and fluoranthene by the algae enriched from a mangrove aquatic ecosystem. Mar Pollut Bull. 2008;56(8):1400–1405. [DOI] [PubMed] [Google Scholar]
- [154].Bopp SK, Lettieri T. Gene regulation in the marine diatom Thalassiosira pseudonana upon exposure to polycyclic aromatic hydrocarbons (PAHs). Gene. 2007;396(2):293–302. [DOI] [PubMed] [Google Scholar]
- [155].Li Y, Zhang C, Hu Z. Selective removal of pharmaceuticals and personal care products from water by titanium incorporated hierarchical diatoms in the presence of natural organic matter. Water Res. 2021;189:116628. [DOI] [PubMed] [Google Scholar]
- [156].Vinayak V, Kumar V, Kashyap M, et al. 2016. Fabrication of resonating microfluidic chamber for biofuel production in diatoms (Resonating device for biofuel production). 2016 3rd International Conference on Emerging Electronics (ICEE); IEEE, Mumbai, India. pp. 1–6. [Google Scholar]
- [157].Nie Z, Nijhuis CA, Gong J, et al. Electrochemical sensing in paper-based microfluidic devices. Lab Chip. 2010;10(4):477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Al-jabri H, Das P, Khan S, et al. Treatment of wastewaters by microalgae and the potential applications of the produced biomass—A review. Water. 2021;13(1):27. [Google Scholar]
- [159].Mishra B, Varjani S, Iragavarapu GP, et al. Microbial fingerprinting of potential biodegrading organisms. Curr Pollut Rep. 2019;5(4):181–197. [Google Scholar]
- [160].Do MH, Ngo HH, Guo W, et al. Microbial fuel cell-based biosensor for online monitoring wastewater quality: a critical review. SciTotal Environ. 2020;712:135612. [DOI] [PubMed] [Google Scholar]
- [161].Vinayak V, Khan MJ, Varjani S, et al. Microbial fuel cells for remediation of environmental pollutants and value addition: special focus on coupling diatom microbial fuel cells with photocatalytic and photoelectric fuel cells. J Biotechnol. 2021b;338:5–19. [DOI] [PubMed] [Google Scholar]
- [162].Khan MJ, Mangesh H, Ahirwar A, et al. Insights into diatom microalgal farming for treatment of wastewater and pretreatment of algal cells by ultrasonication for value creation. Environ Res. 2021b;201:111550. [DOI] [PubMed] [Google Scholar]
- [163].Koyande AK, Chew KW, Manickam S, et al. Emerging algal nanotechnology for high-value compounds: a direction to future food production. Trends Food SciTechnol. 2021;116:290–302. [Google Scholar]
- [164].Ahirwar A, Gupta S, Kashyap M, et al. Differential cell viability in Nitzschia palea on exposure to different organic and inorganic environmental effluents. Int J Environ Sci Technol. 2020;17(1):493–504. [Google Scholar]
- [165].Gautam S, Kashyap M, Gupta S, et al. Metabolic engineering of tio 2 nanoparticles in nitzschia palea to form diatom nanotubes: an ingredient for solar cells to produce electricity and biofuel. RSC Adv. 2016;6(99):97276–97284. [Google Scholar]


