Abstract
Host genetic factors can confer resistance against malaria1, raising the question of whether this has led to evolutionary adaptation of parasite populations. Here we searched for association between candidate host and parasite genetic variants in 3,346 Gambian and Kenyan children with severe malaria caused by Plasmodium falciparum. We identified a strong association between sickle haemoglobin (HbS) in the host and three regions of the parasite genome, which is not explained by population structure or other covariates, and which is replicated in additional samples. The HbS-associated alleles include nonsynonymous variants in the gene for the acyl-CoA synthetase family member2–4 PfACS8 on chromosome 2, in a second region of chromosome 2, and in a region containing structural variation on chromosome 11. The alleles are in strong linkage disequilibrium and have frequencies that covary with the frequency of HbS across populations, in particular being much more common in Africa than other parts of the world. The estimated protective effect of HbS against severe malaria, as determined by comparison of cases with population controls, varies greatly according to the parasite genotype at these three loci. These findings open up a new avenue of enquiry into the biological and epidemiological significance of the HbS-associated polymorphisms in the parasite genome and the evolutionary forces that have led to their high frequency and strong linkage disequilibrium in African P. falciparum populations.
Subject terms: Genetic association study, Evolutionary genetics, Malaria, Parasitic infection, Genetic interaction
A strong association has been found between three regions of the Plasmodium falciparum genome and sickle haemoglobin in children with severe malaria, suggesting parasites have adapted to overcome natural host immunity.
Main
Malaria can be viewed as an evolutionary arms race between the host and parasite populations. Human populations in Africa have acquired a high frequency of HbS and other erythrocyte polymorphisms that provide protection against the severe symptoms of P. falciparum infection1,5, while P. falciparum populations have evolved a complex repertoire of genetic variation to evade the human immune system and to resist antimalarial drugs6,7. This raises the basic question: are there genetic forms of P. falciparum that can overcome the human variants that confer resistance to this parasite?
To address this question, we analysed both host and parasite genome variation in samples from 5,096 children from Gambia and Kenya with severe malaria caused by P. falciparum (Extended Data Fig. 1, Supplementary Fig. 1, Methods). The samples were collected over the period 1995–2009 as part of a genome-wide association study (GWAS) of human resistance to severe malaria5,8,9. In brief, we sequenced the P. falciparum genome using the Illumina X Ten platform using two approaches based on sequencing whole DNA and selective whole-genome amplification10. We used an established pipeline11 to identify and call genotypes at more than two million single nucleotide polymorphisms (SNPs) and short insertion and deletion variants across the P. falciparum genome in these samples (Methods), although the majority of these occurred at low frequency. Our analysis is based on the 4,171 samples that had high quality data for both parasite and human genotypes, of which a subset of 3,346 had human genome-wide genotyping available and were used for discovery analysis. We focused on a set of 51,225 biallelic variants in the P. falciparum genome that passed all quality control filters and were observed in at least 25 infections in this subset (Methods). Our analyses exclude mixed-genotype calls that arise in malaria when a host is infected with multiple parasite lineages. Full details of our sequencing and data processing can be found in Supplementary Methods.
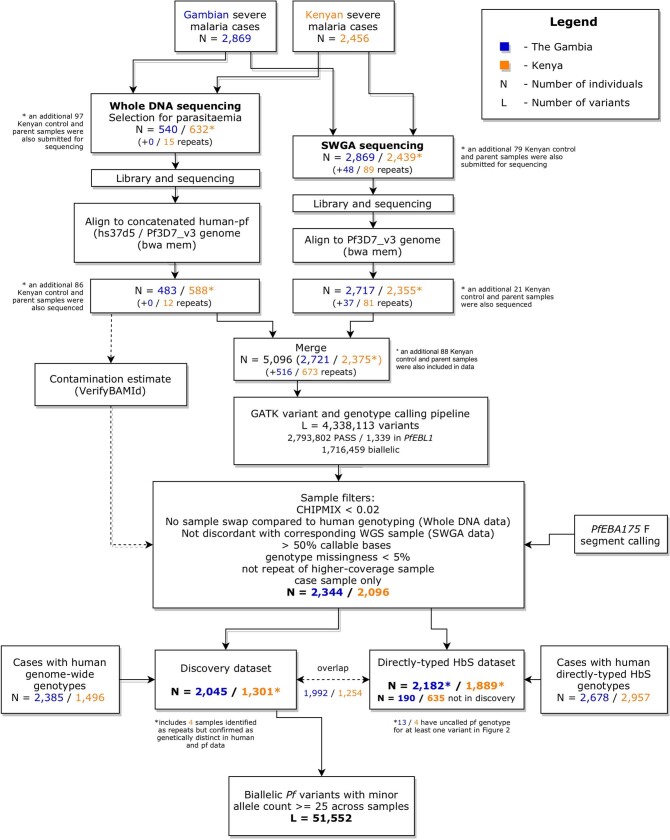
Extended Data Fig. 1. Flowchart showing generation and processing of P.falciparum (Pf) sequence data from 5,096 severe malaria cases.
Flowchart shows sample processing from initial selection for whole DNA and Selective Whole genome Amplification (SWGA) pipelines (top) to the curated analysis datasets (bottom). Numbers in each box show counts of severe cases in The Gambia (blue) and Kenya (orange) with the number of individuals sequenced multiple times indicated in brackets. Following curation and QC of data (large box), available Pf data was intersected with two existing human genotype datasets to for the analyses described in main text. The combined pf/human imputed dataset has 3,346 samples and the combined pf/human direct typing dataset contains 4,071 individuals. These two datasets have substantial overlap; 825 individuals were represented in the directly-typed data but not the imputed data and were used for replication.
We used a logistic-regression approach to test for pairwise association between these P. falciparum variants and four categories of human variants that are plausibly associated with malaria resistance: (1) known autosomal protective mutations, including HbS (in HBB), the common mutation that determines the O blood group (in ABO), regulatory variation associated with protection at ATP2B45,8,12 and the structural variant DUP4, which encodes the Dantu blood-group phenotype13; (2) variants that showed suggestive but not conclusive evidence for association with severe malaria in our previous GWAS8; (3) human leukocyte antigen (HLA) alleles and additional glycophorin structural variants that we previously imputed in these samples8,13; and (4) variants near genes that encode human blood-group antigens, which we tested against the subset of P. falciparum variants lying near genes that encode proteins important for the merozoite stage14,15, as these might conceivably interact during host cell invasion by the parasite. Although several factors could confound this analysis in principle—notably, if there was incidental association between human and parasite population structure—the distribution of test statistics suggested that our test was not affected by systematic confounding after including only an indicator of country as a covariate (Supplementary Fig. 2), and we used this approach for our main analysis. The full set of results is summarized in Fig. 1a, Supplementary Table 1.
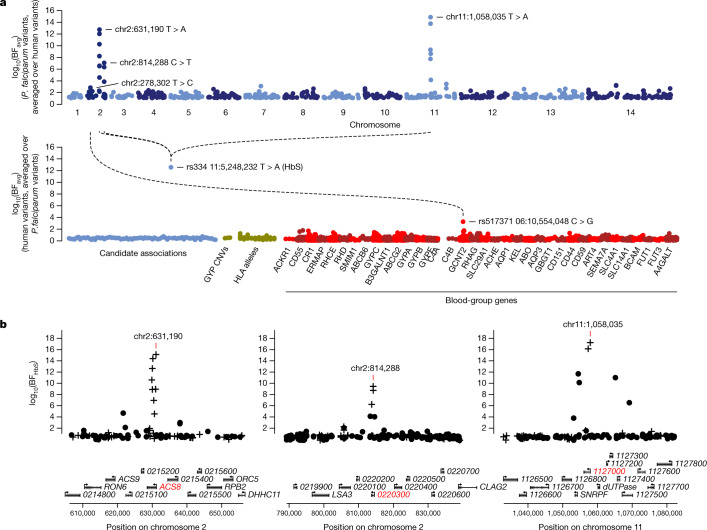
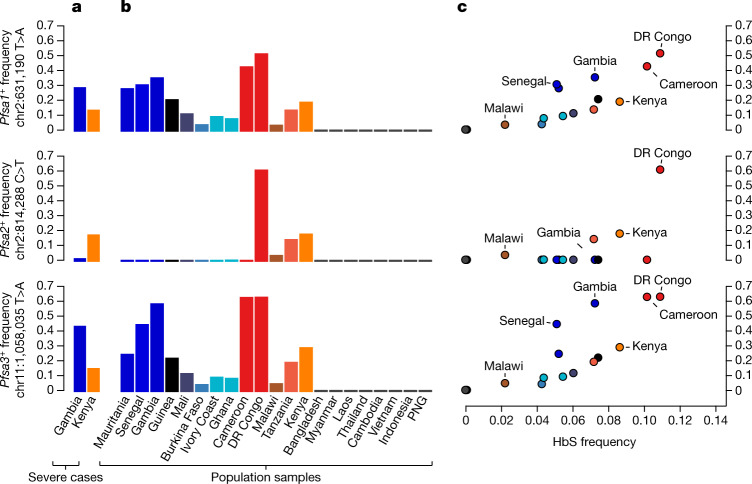
Fig. 1. Three regions of the P. falciparum genome are associated with HbS.
a, Points show the evidence for association between each P. falciparum variant and human genotypes (top row) or between each included human variant and P. falciparum genotypes (bottom row). Association evidence is summarized by averaging the evidence for pairwise association (Bayes factor (BF) for test in n = 3,346 samples) between each variant (points) and all variants in the other organism against which it was tested (log10 (BFavg)). P. falciparum variants are shown grouped by chromosome, and human variants are grouped by inclusion category as described in text and Methods. Dashed lines and variant annotations reflect pairwise tests with BF > 106; only the top signal in each association region pair is annotated (Methods). b, Detail of the association with HbS in the Pfsa1, Pfsa2 and Pfsa3 regions of the P. falciparum genome. Points show evidence for association with HbS (log10 (BFHbS)) for each regional variant. Variants that alter protein coding sequence are denoted by plus, and other variants are denoted by circles. Results are computed by logistic regression including an indicator of country as a covariate and assuming an additive model of association, with HbS genotypes based on imputation from genome-wide genotypes as previously described8. Mixed and missing P. falciparum genotype calls were excluded from the computation. Below, regional genes are annotated, with gene symbols given where the gene has an ascribed name in the PlasmoDB annotation (after removing ‘PF3D7_’ from the name where relevant); the three genes containing the most-associated variants are shown in red. A corresponding plot using directly typed HbS genotypes is presented in Extended Data Fig. 2.
Three P. falciparum loci are associated with HbS
The most prominent finding to arise from this joint analysis of host and parasite variation was a strong association between the sickle haemoglobin allele HbS and three separate regions in the P. falciparum genome (Fig. 1b). Additional associations with marginal levels of evidence were observed at a number of other loci, including a potential association between GCNT2 in the host and PfMSP4 in the parasite and associations involving HLA alleles (detailed in Supplementary Methods, Supplementary Table 1), but here we focus on the association with HbS.
The statistical evidence for association at the HbS-associated loci can be described as follows, focussing on the variant with the strongest association in each region and assuming an additive model of effect of the host allele on parasite genotype on the log-odds scale (Supplementary Table 1). The chr2: 631,190 T>A variant, which lies in PfACS8, was associated with HbS with a Bayes factor (BFHbS) of 1.1 × 1015 (computed under a log F(2,2) prior; Methods) and P value of 4.8 × 10−13 (computed using a Wald test; Supplementary Methods). At a second region on chromosome 2, the chr2: 814,288 C>T variant, which lies in Pf3D7_0220300, was associated with BFHbS = 2.4 × 109 and P = 1.6 × 10−10. At the chromosome 11 locus, the chr11: 1,058,035 T>A variant, which lies in Pf3D7_1127000, was associated with BFHbS = 1.5 × 1017 and P = 7.3 × 10−12. For brevity, we refer to these HbS-associated loci as Pfsa1, Pfsa2 and Pfsa3, respectively—for P. falciparum sickle-associated—and we use + and – signs to refer to alleles that are positively and negatively correlated with HbS, respectively. For example, Pfsa1+ denotes the allele that is positively correlated with HbS at the Pfsa1 locus. All three of the lead variants are nonsynonymous mutations of their respective genes, as are additional associated variants in these regions (Fig. 1, Supplementary Table 1).
The above results are based on HbS genotypes imputed from surrounding haplotype variation8, but we focus below on the larger set of 4,071 cases in which we have previously directly assayed HbS genotypes5 (Extended Data Fig. 1). This includes the majority of samples used in our discovery analysis. The Pfsa1 and Pfsa3 associations were clearly supported in both populations in this dataset, whereas Pfsa2+ appears rare in Gambia (Supplementary Tables 2, 3). We also observed convincing replication of the associations in the additional 825 samples that were not part of our discovery phase, with nominal replication of Pfsa3 in the Gambia (one-tailed P = 0.026, N = 163) and replication of all three loci in the larger sample from Kenya (P < 0.001, N > 540) (Supplementary Table 2). Across the full dataset there is thus very strong evidence of association with HbS at all three loci (BFHbS = 2.0 × 1021 for Pfsa1, 3.7 × 1012 for Pfsa2, and 1.4 × 1024 for Pfsa3; Extended Data Fig. 2) with corresponding large effect size estimates (estimated odds ratio (OR) = 12.8 for Pfsa1+, 7.5 for Pfsa2+ and 21.7 for Pfsa3+). As described above, these estimates assume an additive relationship between HbS and the P. falciparum genotype at each locus, but we also noted that there is greatest evidence for a dominance effect (Supplementary Tables 2, 3).
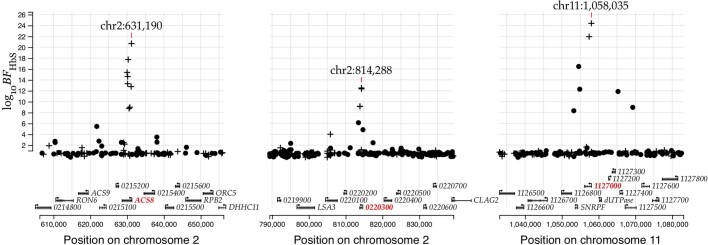
Extended Data Fig. 2. Evidence for association with HbS in three regions of the Pf genome using directly-typed HbS genotypes.
Points show evidence for association with HbS (log10 Bayes Factor for test in N = 4,071 samples, y axis) based on direct typing of HbS for variants in the Pfsa1, Pfsa2 and Pfsa3 regions of the Pf genome (panels). Variants which alter protein coding sequence are denoted by plusses, while other variants are denoted by circles. Results are computed by logistic regression including an indicator of country as a covariate and assuming an additive model of association; missing and mixed Pf genotype calls were excluded. A corresponding plot using imputed HbS genotypes can be found in Fig. 1. The variant with the strongest association in each region is annotated and the panels show regions of length 50kb centred at this variant. Below, regional genes are annotated, with gene symbols given where the gene has an ascribed name in the PlasmoDB annotation (after removing 'PF3D7_' from the name where relevant); the three genes containing the most-associated variants are shown in red.
We further examined the effect of adjusting for covariates in our data, including human and parasite principal components reflecting population structure, year of sampling, clinical type of severe malaria and technical features related to sequencing (Extended Data Fig. 3). Inclusion of these covariates did not substantially affect results with one exception: we found that parasite principal components computed across the whole P. falciparum genome included components that correlated with the Pfsa loci, and including these principal components reduced the association signal, particularly in Kenya. Altering the principal components by removing the Pfsa regions restored the association, indicating that this is not caused by a general population structure effect that is reflected in genotypes across the parasite genome, and we further discuss the reasons for this finding below. Finally, we analysed available data from a set of 32 uncomplicated infections of Malian children ascertained based on HbS genotypes16 (Methods); this provided further replication of the associations with Pfsa1 and Pfsa3 (Supplementary Table 2). Together, these data indicate that there are genuine differences in the distribution of parasite genotypes between infections of HbS and non-HbS genotype individuals.
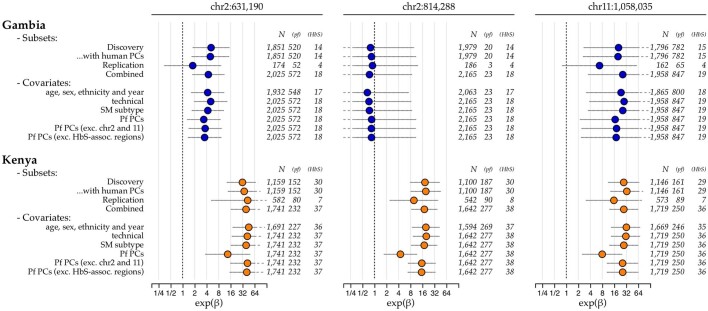
Extended Data Fig. 3. Odds ratios for association of HbS with the Pfsa variants in severe malaria cases.
Plot shows parameter estimates (points) and 95% posterior credible intervals (horizontal line segments) for the association of HbS with Pf genotype at each of the three Pfsa lead variants (columns), using several combinations of sample subsets and covariates (rows) in The Gambia and Kenya. Estimates are computed separately for each SNP using logistic regression with the given covariates included as fixed-effect terms, and are based on directly-typed HbS genotypes assuming a dominance model of HbS on Pf genotype. Samples with mixed Pf genotype calls are excluded from the regression. All estimates are made using a weakly-informative log-F(2,2) prior (Supplementary Methods) on the genetic effect; a diffuse log-F(0.08,0.08) prior is also applied to covariate effects. Row names are as follows: "Discovery": samples with human genome-wide imputed data that were included in our initial scan (Fig. 1); "Replication": the 825 additional samples that are not closely related to discovery samples (as determined previously8); "Combined": all samples with direct typing (as in Fig. 2 and Extended Data Fig. 2); "technical": indicators of sequencing performance including indicator of SWGA or whole DNA sequencing method for the sample, sequence read depth, insert size, and proportion of mixed genotype calls; "SM subtype": indicator of clinical presentation (cerebral malaria, severe malarial anaemia or other severe malaria) the individual was ascertained with; "Pf PCs": principal components (PCs) computed using all called biallelic SNPs having minor allele frequency at least 1% in each population and thinned to exclude variants closer than 1kb; additional rows are shown for PCs computed after excluding SNPs in chromosomes 2 and 11, or from the three regions of association shown in Fig. 1 plus a 25kb margin. Numbers to the right of each estimate show the total regression sample size, the number of samples having the non-reference allele at the given Pf SNP, and the number heterozygous or homozygous for HbS.
HbS protection varies with parasite type
The level of protection afforded by HbS against severe malaria can be estimated by comparing its frequency between cases and population controls. As shown in Fig. 2, the vast majority of children with HbS genotype in our data were infected with parasites that carry Pfsa+ alleles. Corresponding to this, our data show little evidence of a protective effect of HbS against severe malaria with parasites of Pfsa1+, Pfsa2+ and Pfsa3+ genotype (estimated relative risk (RR) = 0.83, 95% confidence interval = 0.53–1.30). By contrast, HbS is strongly associated with reduced risk of disease caused by parasites of Pfsa1−, Pfsa2− and Pfsa3− genotype (RR = 0.01, 95% confidence interval = 0.007–0.03). These estimates should be interpreted with caution because they are based on just 49 cases of severe malaria that had an HbS genotype, because many of these samples were included in the initial discovery dataset, and because there is some variation evident between populations. However, it can be concluded that the protective effect of HbS is dependent on parasite genotype at the Pfsa loci.
Fig. 2. The estimated relative risk for HbS varies by Pfsa genotype.
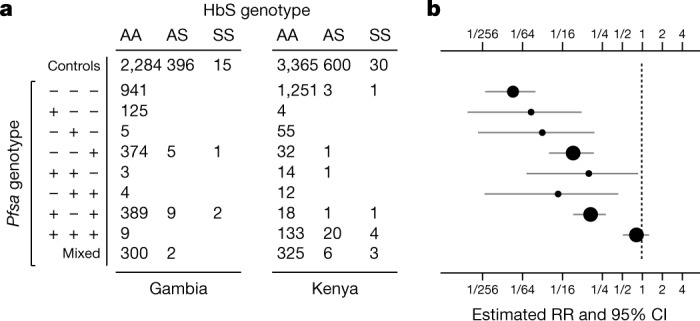
a, Numbers of cases of severe malaria from the Gambia and Kenya with indicated HbS genotype (columns) and carrying the indicated alleles at the Pfsa1, Pfsa2 and Pfsa3 loci (rows; using n = 4,054 samples with directly typed HbS genotype and non-missing genotype at the three P. falciparum loci). Pfsa alleles positively associated with HbS are denoted + and those negatively associated with HbS are denoted − for the respective loci. Samples with mixed P. falciparum genotype calls for at least one of the loci are shown in the bottom row and further detailed in Extended Data Fig. 4. The first row indicates counts of HbS genotypes in population control samples from the same populations8. b, The estimated relative risk of HbS for severe malaria with Pfsa genotypes (rows) as indicated in a. Relative risks were estimated using a multinomial logistic regression model with controls as the baseline outcome and assuming complete dominance (that is, that HbAS and HbSS genotypes have the same association with parasite genotype) as described in Supplementary Methods; an indicator of country was included as a covariate. Circles reflect posterior mean estimates and horizontal lines reflect the corresponding 95% credible intervals (CI). Estimates based on less than 5 individuals with HbAS or HbSS genotypes are represented by smaller circles. To reduce overfitting we used Stan46 to fit the model assuming a mild regularising Gaussian prior with mean zero and standard deviation of 2 on the log-odds scale (that is, with 95% of mass between 1/50 and 50 on the relative risk scale) for each parameter, and between-parameter correlations set to 0.5.
Population genetics of the Pfsa loci
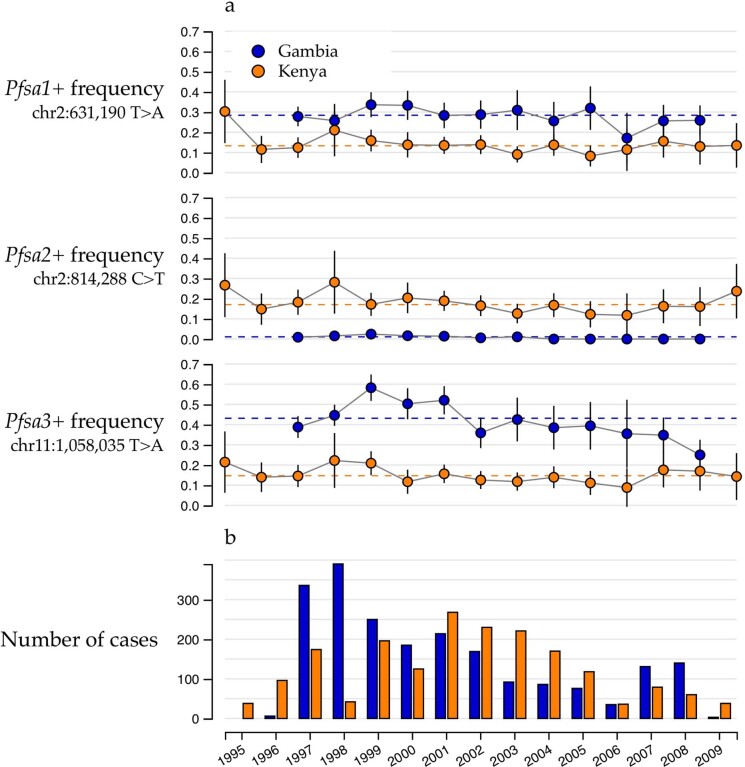
The Pfsa1+, Pfsa2+ and Pfsa3+ alleles had similar frequencies in Kenya (approximately 10–20%) whereas in Gambia Pfsa2+ had a much lower allele frequency than Pfsa1+ or Pfsa3+ (below 3% in all years studied, versus 25–60% for the Pfsa1+ or Pfsa3+ alleles; Fig. 3a). To explore the population genetic features of these loci in more detail, we analysed the MalariaGEN Pf6 open resource, which provides P. falciparum genome variation data for 7,000 worldwide samples11 (Fig. 3b). This showed considerable variation in the frequency of these alleles across Africa, the maximum observed value being 61% for Pfsa3+ in the Democratic Republic of Congo, and indicated that these alleles are rare outside Africa. Moreover, we found that within Africa, population frequencies of the Pfsa+ alleles are strongly correlated with the frequency of HbS (Fig. 3c; estimated using data from the Malaria Atlas Project17).
Fig. 3. The relationship between Pfsa and HbS allele frequencies across populations.
a, Bars show the estimated frequency of each Pfsa+ allele in severe cases of malaria from each country. Details of allele frequencies and sample counts across years are presented in Extended Data Fig. 5. b, Estimated frequency of each Pfsa+ allele in worldwide populations from the MalariaGEN Pf6 resource11, which contains samples collected during the period 2008–2015. Only countries with at least 50 samples are shown (this excludes Columbia, Peru, Benin, Nigeria, Ethiopia, Madagascar and Uganda). c, Estimated population-level Pfsa+ allele frequency (as in a, b) against HbS allele frequency in populations from MalariaGEN Pf6 (coloured as in b; selected populations are also labelled). Pfsa+ allele frequencies were computed from the relevant genotypes, after excluding mixed or missing genotype calls. HbS allele frequencies were computed from frequency estimates previously published by the Malaria Atlas Project17 for each country, by averaging over the locations of MalariaGEN Pf6 sampling sites weighted by the sample size. DR, Democratic Republic; PNG, Papua New Guinea.
This analysis also revealed a further feature of the Pfsa+ alleles: although Pfsa1 and Pfsa2 are separated by 180 kb, and the Pfsa3 locus is on a different chromosome, they are in strong linkage disequilibrium (LD). This can be seen from the co-occurrence of these alleles in severe cases (Fig. 2), and from the fact that they covary over time in our sample (Extended Data Fig. 5) and geographically across populations (Fig. 3b). We computed LD metrics between the Pfsa+ alleles in each population (Supplementary Table 4) after excluding HbS-carrying individuals to avoid confounding with the association outlined above. Pfsa1+ and Pfsa2+ were strongly correlated in Kenyan severe cases (r = 0.75) and Pfsa1+ and Pfsa3+ were strongly correlated in both populations (r = 0.80 in Kenya; and r = 0.43 in severe cases from the Gambia). This high LD was not explained by population structure or other covariates in our data (Methods), and was also observed in multiple populations in MalariaGEN Pf6 (for example, r = 0.20 between Pfsa1+ and Pfsa3+ in the Gambia; r = 0.71 in Kenya; and r > 0.5 in all other African populations surveyed; Supplementary Table 4), showing that the LD is not purely an artefact of our sample of severe malaria cases.
Extended Data Fig. 5. Pfsa+ allele frequency and sample size by year of ascertainment.
a) points show the sample allele frequency (y axis) for each Pfsa variant (rows) in severe malaria cases by year of ascertainment (x axis) and country (colour). Vertical line segments show the 95% confidence interval corresponding to each estimate. Horizontal dashed lines show the overall estimate across all years in our data, as in Fig. 3a. b) Bars show the total number of severe case samples in our dataset (y axis) in each country (colour) by year of ascertainment (x axis).
This observation of strong correlation between alleles at distant loci is unexpected, because the P. falciparum genome undergoes recombination in the mosquito vector and typically shows very low levels of LD in malaria-endemic regions11,18,19. To confirm that this is unusual, we compared LD between the Pfsa loci with the distribution computed from all common biallelic variants on different chromosomes (Fig. 4). In Kenyan samples, the Pfsa loci have the highest between-chromosome LD of any pair of variants in the genome. In Gambia, between-chromosome LD at these SNPs is also extreme, but another pair of extensive regions on chromosomes 6 and 7 also show strong LD. These regions contain the chloroquine resistance-linked genes PfCRT and PfAAT120,21 and contain long stretches of DNA sharing identical by descent, consistent with positive selection of antimalarial-resistant haplotypes22. Moreover, we noted that these signals are among a larger set of HbS-associated and drug-resistance loci that appear to have increased between-chromosome LD in these data (Supplementary Table 4).
Fig. 4. HbS-associated variants show extreme between-chromosome correlation in severe P. falciparum infections.
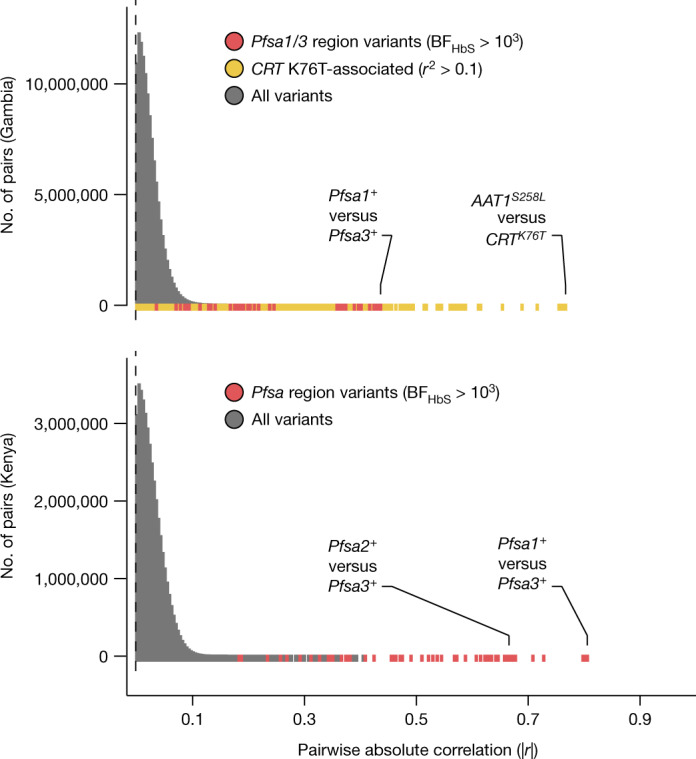
Empirical distribution of absolute genotype correlation (|r|) between pairs of variants on different P. falciparum chromosomes in the Gambia (top) and Kenya (bottom). To avoid capturing direct effects of the HbS association, correlation values are computed after excluding HbS-carrying individuals. All pairs of biallelic variants with estimated minor allele frequency at least 5% and at least 75% of samples having non-missing and non-mixed genotype call are shown (totalling 16,487 variants in the Gambia and 13,766 variants in Kenya). Colours indicate the subset of comparisons between HbS-associated variants in Pfsa regions relevant for the population (red) and between variants in LD with the CRT K76T mutation. Labelled points denote the variant pairs showing the highest and second-highest pairwise correlation in each population after grouping correlated variants into regions; for this purpose regions were defined to include all nearby pairs of correlated variants with minor allele frequency ≥5% and r2 > 0.05, such that no other such pair of variants within 10 kb of the given region boundaries is present (Methods). A longer list of regions showing increased between-chromosome LD is presented in Supplementary Table 5.
Combining these new findings with other population genetic evidence from multiple locations across Africa, including observations of frequency differentiation within and across P. falciparum populations11,23,24 and other metrics at these loci indicative of selection22,25,26, it appears likely that the allele frequencies and strong LD between Pfsa1, Pfsa2 and Pfsa3 are maintained by some form of natural selection. However, the mechanism for this is unclear. Given our findings, an obvious hypothesis is that the Pfsa1+, Pfsa2+ and Pfsa3+ alleles are positively selected in hosts with HbS, but since the frequency of HbS carriers5,17 is typically <20% it is not clear whether this alone is a sufficient explanation to account for the high population frequencies or the strong LD observed in non-HbS carriers. Equally, since the Pfsa+ alleles have not reached fixation (Fig. 3) and do not appear to be rapidly increasing in frequency (Extended Data Fig. 5), an opposing force may also be operating to maintain their frequency. However, the above data do not suggest strong fitness costs for Pfsa+-carrying parasites in HbAA individuals (Fig. 2), and the Pfsa2+ allele also appears to be present only in east Africa, further complicating these observations. It thus remains entirely possible that additional selective factors are involved, such as epistatic interactions between these loci, or further effects on fitness in the host or vector in addition to those observed here in relation to HbS.
The genomic context of the Pfsa variants
The biological function of these parasite loci is an area of considerable interest for future investigation. At the Pfsa1 locus, the signal of association includes non-synonymous changes in the PfACS8 gene, which encodes an acyl-CoA synthetase3 that belongs to a gene family that has expanded in the Laverania relative to other Plasmodium species4 and lies close to a paralogue PfACS9 on chromosome 2. PfACS8 has been predicted to localize to the apicoplast27, but it also contains a Plasmodium export element (PEXEL)-like motif28–30, which may instead indicate export to the host cytosol where other acyl-CoA synthetase family members have been observed31. The functions of the proteins encoded by PF3D7_0220300 (an exported protein, at the Pfsa2 locus) and PF3D7_1127000 (a putative tyrosine phosphatase, at Pfsa3) are not known; however, the protein encoded by PF3D7_0220300 has been observed to localise to the host membrane and to colocalise with host stomatin32, whereas the protein encoded by PF3D7_1127000 has been observed in the food vacuole33. All three genes appear to be expressed at multiple parasite lifecycle stages (Supplementary Text) in 3D7 parasites and are not essential for in vitro growth34.
We noted two further features that may point to the functional role of the Pfsa+ alleles themselves. The associated variants at Pfsa2 and Pfsa3 each include SNPs immediately downstream of a PEXEL motif (detailed in Supplementary Information), which mediates export through a pathway that involves protein cleavage at the motif35. This process leaves the downstream amino acids at the N terminus of the mature protein, and it is therefore possible that these variants influence successful export36,37. However, another possibility is that the Pfsa+ alleles affect levels of transcription of the relevant genes. In this context, we noted a recent study16 that found that PF3D7_1127000 is among the most differentially over-expressed genes in trophozoite-stage infections of children with HbAS compared with those with HbAA (more than 32-fold increase in transcripts per million (TPM) at the trophozoite stage; n = 12; unadjusted P = 5.6 × 10−22). We reanalysed these data in light of genotypes at the Pfsa loci (Supplementary Table 6), and found that the Pfsa3+ mutations plausibly explain this increased expression. In particular, read ratios at the second-most-associated Pfsa3 SNP (chr11:1,057,437 T > C) (Supplementary Table 1) appear especially strongly correlated with increased expression at trophozoite stage (Extended Data Fig. 6). Further support for this observation comes from an in vitro time-course experiment conducted in the same study16, in which the increased expression was also observed in HbAA erythrocytes infected with a Pfsa+-carrying isolate (Extended Data Figs. 7, 8, Methods). The mechanism of upregulation is not known, but a further relevant observation is that the Pfsa3+ alleles appear to be linked to a neighbouring copy number variant that includes duplication of the 5ʹ end of the small nuclear ribonucleoprotein gene SNRPF, upstream of PF3D7_1127000 (based on analysis of available genome assemblies of P. falciparum isolates38; Extended Data Fig. 9, Supplementary Fig. 3). We caution that these findings are tentative, and the manner in which Pfsa alleles affect genome function is a subject for future research. Understanding this functional role could provide important clues into how HbS protects against malaria and help to distinguish between the various proposed mechanisms, which include enhanced macrophage clearance of infected erythrocytes39, inhibition of intraerythrocytic growth dependent on oxygen levels40, altered cytoadherence of infected erythrocytes41 due to cytoskeleton remodelling42, and immune-mediated mechanisms43.
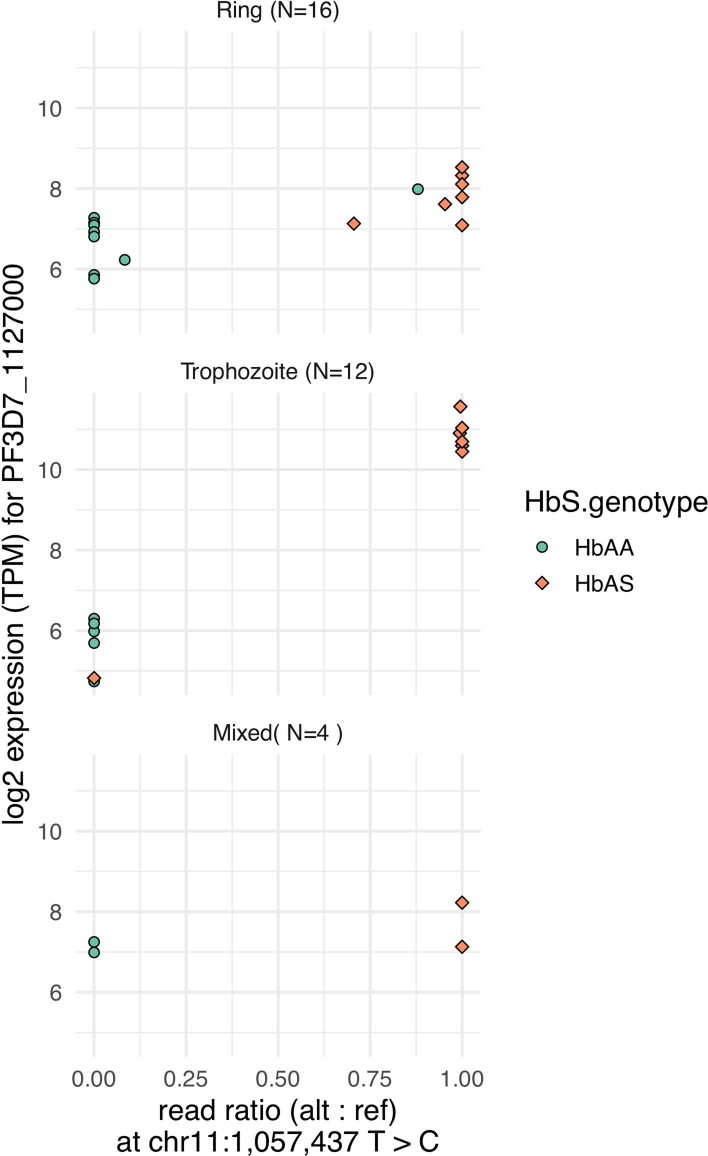
Extended Data Fig. 6. Pfsa3+ genotypes are correlated with PF3D7_1127000 transcript levels in trophozoite-stage infections.
Plot shows the ratio of RNA-seq reads carrying the non-reference allele at the chr11:1,057,437 T > C mutation (x axis) against the estimated transcript abundance of PF3D7_1127000 (log2 TPM, y axis), for 32 children from Mali ascertained with HbAA or HbAS genotype (colours, as detailed in the legend). Underlying data is as published by Saelens et al16 and is further detailed in Supplementary Table 6. The plot is separated by parasite stage (panel labels) as previously inferred16. Among trophozoite-stage infections, we noted one infection of an HbAS individual (AS08) that has low expression of PF3D7_1127000 (TPM = 28.3); this sample appears to have Pfsa3- genotype although we caution that only two reads with reasonable mapping quality were observed. Conversely, one ring-stage infection of an HbAA genotype individual (AA01) has relatively high expression (TPM = 253.2) of PF3D7_1127000; this sample is likely mixed as it appears to express gene copies with both Pfsa3- and Pfsa3+ genotypes, with Pfsa3+ predominant.
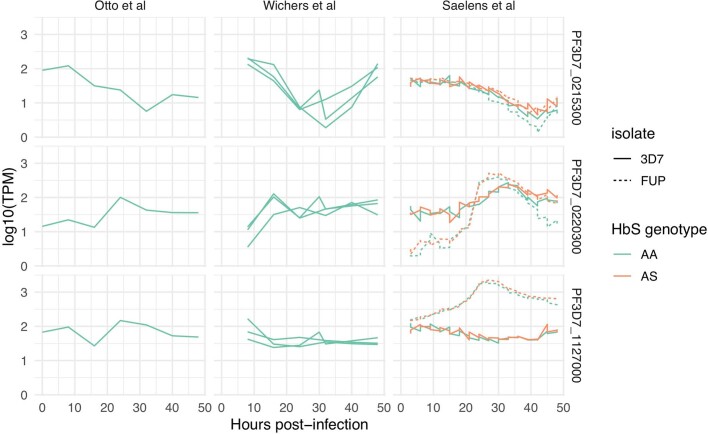
Extended Data Fig. 7. Estimated abundance of Pfsa region gene transcripts from in vitro intraerythrocytic time course experiments.
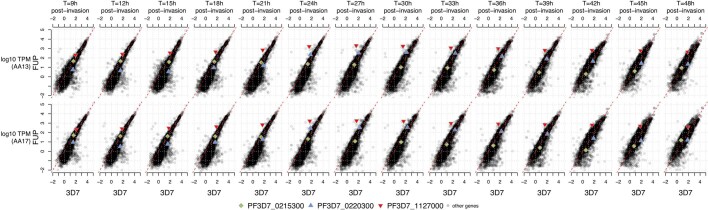
Plot shows the estimated relative transcript abundance (log10 TPM, y axis) against hours post-infection of erythrocytes (x axis) for the three Pfsa region genes containing nonsynonymous sickle-associated polymorphisms (Supplementary Table 1). Data is from three studies which analysed the 3D7 isolate (Otto et al58, Wichers et al59 and Saelens et al16) as indicated by columns; the Saelens et al study also analysed the FUP/H isolate (dashed lines). Time points can be roughly interpreted58 as: ring stage (~0-16h post-invasion); trophozoite stage (16-40h post-invasion); schizont stage (40-48h post-invasion). Replicate experiments are indicated by multiple lines in each panel; colours indicate the HbS genotype of the erythrocytes used as noted in the legend. TPM values are estimated based on reads aligning to the 3D7 reference genome; for the Saelens et al study these were used as reported previously while for the other studies we recomputed TPM as described in Methods.
Extended Data Fig. 8. Estimated abundance of all transcripts in 3D7 and FUP/H parasites across the intraerythrocytic time course.
Plot shows the estimated relative transcript abundance (TPM) of P.falciparum genes measured in 3D7 (x axis) and in FUP/H (Uganda Palo Alto, y axis) parasites, using the data reported by Saelens et al16. Transcript abundance is measured in vitro using erythrocytes from two HbAA genotype individuals (rows), and at multiple time points post-infection (columns). TPM is measured by alignment to the 3D7 genome followed transcript quantification as described by Saelens et al16. The genes PF3D7_0215300 (PfACS8, Pfsa1 locus), PF3D7_0220300 (Pfsa2 locus), and PF3D7_1127000 (Pfsa3 locus) are denoted by coloured points as shown in the legend. Both PF3D7_1127000 and to a lesser extent PF3D7_0220300 show an increase in expression at trophozoite stage in FUP/H parasites. We determined the genotypes of FUP/H. We determined the Pfsa genotypes of FUP/H as + + + (using the notation of Fig. 2) by aligning available short-read sequencing reads (SRA accessions SRR530503, SRR629055, and SRR629078; Broad Institute 2014).
Extended Data Fig. 9. Structural variation at the Pfsa3 locus.
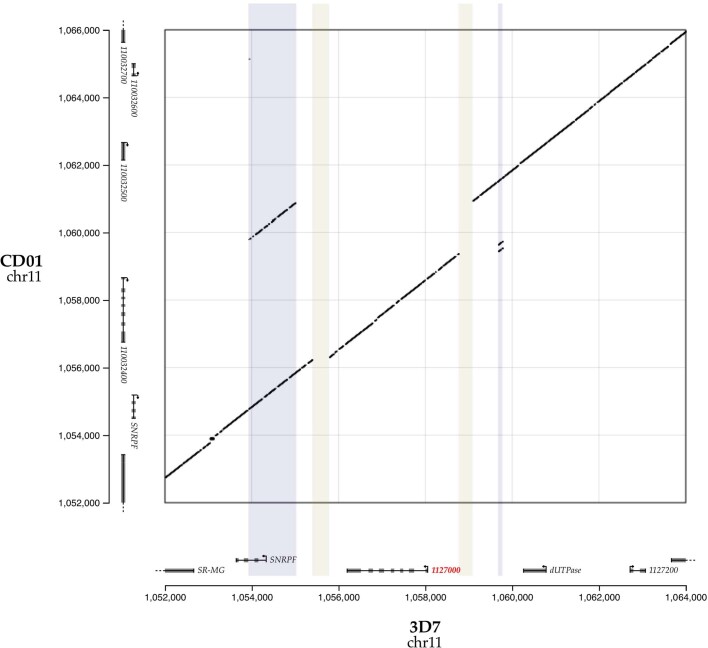
Plot shows all DNA segments of length 50 (50-mers) that are shared identically between the 3D7 genome assembly (x axis) and CD01 genome assembly38 (y axis) in the Pfsa3 region. Points near the diagonal indicate similar structure, while sequences of off-diagonal points indicate structural differences between genomes. Coloured regions indicate approximate regions of 3D7 that contain increased copy number (light blue) or deletions (light yellow) in CD01 relative to 3D7. Segment endpoints are determined by inspection of shared kmer locations and are: 1,053,925 - 1055024 (duplication); 1,055,395-1,055,784 (deletion); 1,058,765-1,059,087 (deletion); 1,059,675-1,059,777 (triplication). The CD01 assembly carries Pfsa1+, Pfsa2+ and Pfsa3+ alleles. Comparisons of 3D7 to other available assembled Pf genomes in Pfsa regions can be found in Supplementary Fig. 3.
Discussion
A fundamental question in the biology of host–pathogen interactions is whether the genetic makeup of infections is determined by the genotype of the host. While there is some previous evidence of this in malaria—for example, allelic variants of the PfCSP gene have been associated with HLA type44 and HbS has itself previously been associated with MSP-1 alleles45 (described further in Supplementary Information)—our findings provide clear evidence of an interaction between genetic variants in the parasite and the host. Our central discovery is that among African children with severe malaria there is a strong association between HbS in the host and three loci in different regions of the parasite genome. Based on estimation of relative risk, HbS has no apparent protective effect against severe malaria in the presence of the Pfsa1+, Pfsa2+ and Pfsa3+ alleles. These alleles, which are much more common in Africa than elsewhere, are positively correlated with HbS allele frequencies across populations. However, they are also found in substantial numbers of individuals without HbS, reaching up to 60% allele frequency in some populations. The Pfsa1, Pfsa2 and Pfsa3 loci also show remarkably high levels of long-range between-locus LD relative to other loci in the P. falciparum genome, which is equally difficult to explain without postulating ongoing evolutionary selection. Although it seems clear that HbS has a key role in this selective process, there is a need for further population surveys (that include asymptomatic and uncomplicated cases of malaria) to gain a more detailed understanding of the genetic interactions between HbS and these parasite loci, and how they affect the overall protective effect of HbS against severe malaria.
Methods
Ethics and consent
Sample collection and design of our case-control study8 was approved by Oxford University Tropical Research Ethics committee (OXTREC), Oxford, United Kingdom (OXTREC 020-006). Informed consent was obtained from parents or guardians of patients with malaria, and from mothers for population controls. Local approving bodies were the MRC/Gambia Government Ethics Committee (SCC 1029v2 and SCC670/630) and the KEMRI Research Ethics Committee (SCC1192).
Building a combined dataset of human and P. falciparum genotypes for severe cases
We used Illumina sequencing to generate two datasets jointly reflecting human and P. falciparum genetic variation, using a sample of severe malaria cases from the Gambia and Kenya for which human genotypes have previously been reported5,8. A full description of our sequencing and data processing is given in Supplementary Methods and summarized in Extended Data Fig. 1. In brief, following a process of sequence data quality control and merging across platforms, we generated (1) a dataset of microarray and imputed human genotypes, and genome-wide P. falciparum genotypes, in 3,346 individuals previously identified as without close relationships8; and (2) a dataset of HbS genotypes directly typed on the Sequenom iPLEX Mass-Array platform (Agena Biosciences)5, and genome-wide P. falciparum genotypes, in 4,071 individuals without close relationships8. Parasite DNA was sequenced from whole DNA in samples with high parasitaemia, and using selective whole-genome amplification (SWGA) to amplify P. falciparum DNA in all samples. P. falciparum genotypes were called using an established pipeline11 based on GATK, which calls single nucleotide polymorphisms and short insertion–deletion variants relative to the Pf3D7 reference sequence. This pipeline deals with mixed infections by calling parasite variants as if the samples were diploid; in practice this means that variants with substantial numbers of reads covering reference and alternate alleles are called as heterozygous genotypes.
For the analyses presented in main text, we used the 3,346 samples with imputed human genotypes for our initial discovery analysis, and the 4,071 individuals with directly typed HbS genotypes for all other analysis. The individuals in these two datasets substantially overlap (Extended Data Fig. 1), but a subset of 825 individuals have directly typed for HbS but were not in the discovery data and we used these for replication.
Inference of genetic interaction from severe malaria cases
To describe our approach, we first consider a simplified model of infection in which parasites have a single definite (measurable) genotype, acquired at time of biting, that is relevant to disease outcome—that is, we neglect any effects of within-host mutation, co- and super-infection at the relevant genetic variants. We consider a population of individualswho are susceptible to being been bitten by an infected mosquito. A subset of infections go on to cause severe disease. Among individuals who are bitten and infected with a particular parasite type I = x, the association of a human allele E = e with disease outcome can be measured by the relative risk,
| 1 |
where we have used E = 0 to denote a chosen baseline human genotype against which risks are measured. If the strength of association further varies between parasite types (say between and a chosen infection type ) then these relative risks will vary, and thus the ratio of relative risks (RRR) will differ from 1. If the host genotype e confers protection against severe malaria, the ratio of relative risks will therefore capture variation in the level of protection compared between different parasite types.
Although phrased above in terms of a relative risk for human genotypes, the RRR can be equivalently expressed as a ratio of relative risks for a given parasite genotype compared between two human genotypes (Supplementary Methods). It is thus conceptually symmetric with respect to human and parasite alleles, and would equally well capture variation in the level of pathogenicity conferred by a particular parasite type compared between different human genotypes.
The OR for specific human and parasite alleles computed in severe malaria cases is formally similar to the ratio of relative risks but with the roles of the genotypes and disease status interchanged. We show in Supplementary Methods that in fact
| 2 |
where ORbiting is a term that reflects possible association of human and parasite genotypes at the time of mosquito biting. Thus, under this model and in the absence of confounding factors, implies either that host and parasite genotypes are not independent at time of biting, or that there is an interaction between host and parasite genotypes in determining disease risk. The former possibility may be considered less plausible because it would seem to imply that relevant host and parasite genotypes can be detected by mosquitos prior to or during biting, but we stress that this cannot be tested formally without data on mosquito-borne parasites. A further discussion of these assumptions can be found in Supplementary Methods.
Testing for genome-to-genome correlation
We developed a C++ program (HPTEST) to efficiently estimate the odds ratio (equation (2)) across multiple human and parasite variants, similar in principle to approaches that have been developed for human-viral and human-bacterial GWAS47–49. HPTEST implements a logistic regression model in which genotypes from one file are included as the outcome variable and genotypes from a second file on the same samples are included as predictors. Measured covariates may also be included, and the model accounts for uncertainty in imputed predictor genotypes using the approach from SNPTEST50. The model is fit using a modified Newton-Raphson with line search method. For our main analysis we applied HPTEST with the parasite genotype as outcome and the host genotype as predictor, assuming an additive effect of the host genotype on the log-odds scale, and treating parasite genotype as a binary outcome (after excluding mixed and missing genotype calls.).
To mitigate effects of finite sample bias, we implemented regression regularised by a weakly informative log F(2,2) prior distribution51 on the effect of the host allele (similar to a Gaussian distribution with standard deviation 1.87; Supplementary Methods). Covariate effects were assigned a log F(0.08,0.08) prior, which has similar 95% coverage interval to a gaussian with zero mean and standard deviation of 40. We summarised the strength of evidence using a Bayes factor against the null model that the effect of the host allele is zero. A P-value can also be computed under an asymptotic approximation by comparing the maximum posterior estimate of effect size to its expected distribution under the null model (Supplementary Methods). For our main results we included only one covariate, an indicator of the country from which the case was ascertained (Gambia or Kenya); additional exploration of covariates is described below.
Choice of genetic variants for testing
For our initial discovery analysis we concentrated on a set of 51,552 P. falciparum variants that were observed in at least 25 individuals in our discovery set, after excluding any mixed or missing genotype calls. These comprised: 51,453 variants that were called as biallelic and passed quality filters (detailed in Supplementary Methods; including the requirement to lie in the core genome52); an additional 98 biallelic variants in the region of PfEBL1 (which lies outside the core genome but otherwise appeared reliably callable); and an indicator of the PfEBA175 ‘F’ segment, which we called based on sequence coverage as described in Supplementary Methods and Supplementary Fig. 6. We included PfEBL1 and PfEBA175 variation because these genes encode known or putative receptors for P. falciparum during invasion of erythrocytes15.
We concentrated on a set of human variants chosen as follows: we included the 94 autosomal variants from our previously reported list of variants with the most evidence for association with severe malaria8, which includes confirmed associations at HBB, ABO, ATP2B4 and the glycophorin locus. We also included three glycophorin structural variants13, and 132 HLA alleles (62 at 2-digit and 70 at 4-digit resolution) that were imputed with reasonable accuracy (determined as having minor allele frequency > 5% and IMPUTE info at least 0.8 in at least one of the two populations in our dataset). We tested these variants against all 51,552 P. falciparum variants described above. We also included all common, well-imputed human variants within 2 kb of a gene determining a blood-group antigen (defined as variants within 2 kb of a gene in the HUGO blood-group antigen family53 and having a minor allele frequency of 5% and an IMPUTE info score of at least 0.8 in at least one of the two populations in our dataset; this includes 39 autosomal genes and 4,613 variants in total). We tested these against all variants lying within 2 kb of P. falciparum genes previously identified as associated or involved in erythrocyte invasion14,15 (60 genes, 1740 variants in total). In total we tested 19,830,288 distinct human-parasite variant pairs in the discovery dataset (Fig. 1a).
Definition of regions of pairwise association
We grouped all associated variant pairs (defined as pairs (v,w) having BF(v,w) > 100, where BF(v,w) is the association test Bayes factor for the variant pair) into regions using an iterative algorithm as follows. For each associated pair (v,w), we found the smallest enclosing regions (Rv, Rw) such that any other associated pair either lay with (Rv, Rw) or lay further than 10 kb from (Rv, Rw) in the host or parasite genomes, repeating until all associated pairs were assigned to regions. For each association region pair, we then recorded the region boundaries and the lead variants (defined as the regional variant pair with the highest Bayes factor), and we identified genes intersecting the region and the gene nearest to the lead variants using the NCBI refGene54 and PlasmoDB v4455 gene annotations. Due to our testing a selected list of variant pairs as described above, in some cases these regions contain a single human or parasite variant. Supplementary Table 1 summarises these regions for variant pairs with BF > 1,000.
Interpretation of association test results
We compared association test P-values to the expectation under the null model of no association using a quantile-quantile plot, both before and after removing comparisons with HbS (Supplementary Fig. 2; HbS is encoded by the ‘A’ allele at rs334, chr11:5,248,232 T -> A). A simple way to interpret individual points on the QQ-plot is to compare each P-value to its expected distribution under the relevant order statistic (depicted by the grey area in Supplementary Fig. 2); for the lowest P-value this is similar to considering a Bonferroni correction. However, we caution that thresholds determined by this approach are dependent on the set of tests carried out. A more defensible approach is to ask what P-value threshold is needed to generate confidence that a particular pair of variants is genuinely associated. This depends on both the prior probability of association and on the statistical power, with the relationship.
| 3 |
for any P-value threshold T (Supplementary Methods)50. The term on the left is the odds of true association given observation of a P-value below the given threshold; the corresponding probability is therefore equal to one minus the positive false discovery rate56. Interpretation of (3) requires knowledge of both a relevant prior odds of association, and the power, which in turn depends on the true effect size distribution and the underlying frequencies of the variants.
A similar approach conditional on the observed data (Supplementary Methods) leads to an analogous formula involving the Bayes factor instead of the power term and T. If the distribution of truly associated variant effect sizes is similar to the log F(2,2) distribution we have used to calculate Bayes factors, and if variant pairs have approximately similar prior probability of association, then a fixed threshold on the Bayes factor would provide an approximately constant posterior probability of association.
We illustrate a possible computation as follows. The 51,552 P. falciparum variants represent around 20,000 1 kb regions of the P. falciparum genome, which might be thought of as approximately independent given LD decay rates11; similarly the human genome may be thought of as consisting of around 2 million approximately independent regions. If we take the view that a small number—say up to ten—of pairs of regions might be associated, this dictates prior odds on the order of 1 in 4 billion. A Bayes factor around 1010 would therefore be needed to generate substantial posterior odds of association, while a Bayes factor an order of magnitude higher would provide compelling evidence (posterior probability > 95%). In Supplementary Fig. 4 and Supplementary Methods we detail the analogous calculation applied to P-values. For large effect sizes on the order of OR ≈ 4, this suggests that P-values on the order of 1 × 10−10 to 1 × 10−12 might provide compelling evidence for association, depending on the allele frequencies, but weaker effects would require lower thresholds and would be less easily detectable.
It might be considered that the human variants and genes that we have considered here are among those with the highest prior plausibility for association with parasites, and thus the above choice of prior may be considered somewhat conservative. However, even under stronger prior odds on the order of 1 in 2 million (for example, assuming 10 associations among the variant pairs tested in our study), our results do not identify any associations additional to the HbS–Pfsa associations with very strong evidence. Particular variants may however be of further interest due to specific prior plausibility; in Supplementary Methods we give further details on putative associations with BF > 105 and those involving known malaria-protective mutations in the human genome.
Summarizing evidence for each variant
For each human variant v, we further summarised the evidence that v is associated with variation in the parasite genome using the mean Bayes factor , computed as the average of the Bayes factor BF(v,w) across all the parasite variants w tested against v. Under the restrictive assumption that at most one parasite variant is associated with w, can be interpreted as a model-averaged Bayes factor reflecting the evidence for association of v with parasite variants; more generally provides a pragmatic way to combines evidence across all tested variants. We similarly define for each parasite variant w averaged over all human variants tested against v. BFavg is plotted for human and parasite variants in Fig. 1a.
A direct interpretation of these average Bayes factors can be carried in a similar way to the individual Bayes factors as described above. To illustrate, if as above we assume that around 10 of the 20,000 1 kb regions of the P. falciparum genome might be associated with human genetic variants among those tested, an average Bayes factor > 10,000 would be needed to indicate > 80% posterior odds of association; this is only achieved for the Pfsa variants in our data (Fig. 1a). This calculation can be adjusted as appropriate to take into account specific information about individual variants.
Investigation of additional associations
In addition to the HbS-Pfsa associations, we also observed moderate evidence for association at a number of other variant pairs. These include associations between variation in the human gene GCNT2 and PfMSP4 with BF = 2.8 × 106, and between HLA variation and multiple parasite variants with BF in the range 105–106 (Fig. 1a, Supplementary Table 1). A fuller description of the context of these SNPs can be found in Supplementary Methods. Our interpretation is that the statistical evidence for these associations is not sufficiently strong on its own to make these signals compelling without additional evidence.
Assessment of possible confounding factors
To assess whether the observed association between HbS and P. falciparum alleleles might be driven by confounding factors we conducted additional pairwise association tests as follows using HPTEST, based on directly typed HbS genotypes and working seperately in the two populations. Results are shown in Extended Data Fig. 3. First, we repeated the pairwise association test including only individuals overlapping the discovery dataset, and separately in the remaining set of 825 individuals. For discovery samples a set of population-specific principal components (PCs) reflecting human population structure were previously computed8 and we included these as covariates (including 20 PCs in total). Second, across all 4,071 individuals with directly typed HbS data, we repeated tests including measured covariates as additional predictors. Specifically, we considered: (1) the age of individual at time of ascertainment (measured in years; range 0–12; treated as a categorical covariate), sex, reported ethnic group, and year of admission (range 1995–2010, treated as a categorical covariate); (2) technical covariates including an indicator of method of sequencing (SWGA or whole DNA), mean depth of coverage of the P. falciparum genome, mean insert size computed from aligned reads, and percentage of mixed calls; and (3) an indicator of the clinical form of severe malaria with which the sample was ascertained (‘SM subtype’; either cerebral malaria, severe malarial anaemia, or other).
To assess the possibility that parasite population structure might impact results, we also included PCs computed in parasite populations as follows. Working in each population separately, we started with the subset of biallelic SNPs with minor allele frequency at least 1% from among the 51,552 analysed variants (50,547 SNPs in Gambia and 48,821 SNPs in Kenya respectively). We thinned variants by iteratively picking variants at random from this list and excluding all others closer than 1 kb (leaving 12,036 SNPs in Gambia and 11,902 SNPs in Kenya). We used QCTOOL to compute PCs using this list of SNPs. Several of the top PCs had elevated loadings from SNPs in specific genomic regions. This was especially noticeable in Kenya and included the widely reported extensive regions of LD around the AAT1 and CRT regions on chromosomes 6 and 7, and also the HbS-associated chromosome 2 and 11 loci. We therefore also considered separate sets of PCs computed after excluding SNPs in chromosomes 6 and 7 (leaving 9,933 and 9,812 SNPs respectively), after excluding chromosomes 2 and 11 (10,521 and 10,421 SNPs respectively) or after excluding 100 kb regions centred on the lead HbS-associated SNPs (11,866 and 11,732 SNPs respectively). For each set of PCs, we repeated association tests including 20 PCs as fixed covariates.
For each subset of individuals, each HbS-associated variant and each set of covariates described above, we plotted the estimated effect size and 95% posterior interval, annotated with the total number of samples, the number carrying the non-reference allele at the given variant, and the number carrying heterozygous or homozygous HbS genotypes (Extended Data Fig. 3). Corresponding genotype counts can be found in Supplementary Table 3. To assess mixed genotypes calls, we also plotted the ratio of reads with reference and nonreference alleles at each site; this can be found in Extended Data Fig. 4.
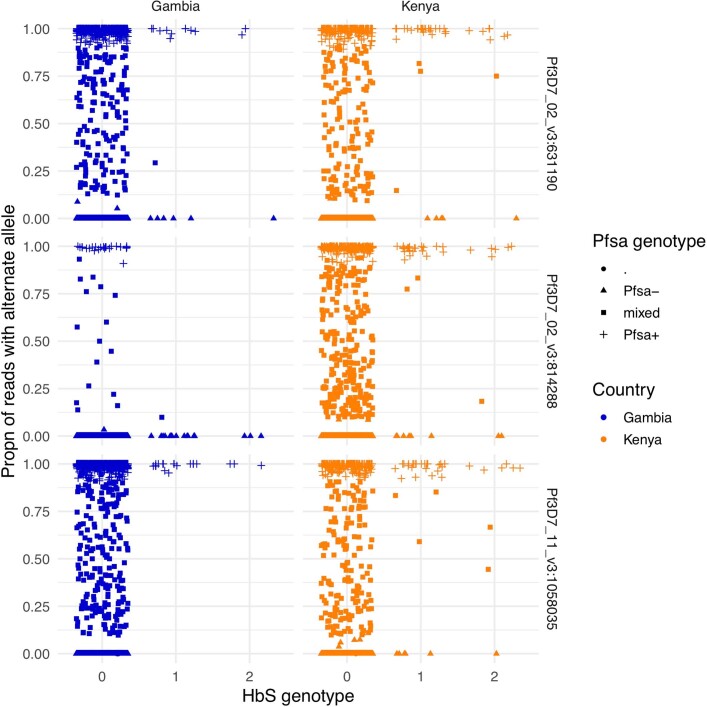
Extended Data Fig. 4. Allele read ratio versus HbS genotype at the three HbS-associated loci.
For each sample (points) and each of the three HbS-associated loci (rows), the figure shows the proportion of sequencing reads that carry the nonreference allele (y axis). Points are separated by country (columns) and HbS genotype (x axis); the x axis values are jittered to visually separate. The called Pf genotype of each sample is indicated by the shape, with mixed calls indicated by squares.
Interpretation in terms of causal relationships
Observing implies non-independence between host and parasite genotypes in individuals with severe disease, but does not determine the mechanism by which this could occur. Assuming , we show in Supplementary Methods that is equivalent to a multiplicative model in which host and parasite genotypes separately and multiplicatively affect disease risk (equations (S6) and (S7) in Supplementary Methods). In general deviation from this model could arise in several ways, including through within-host selection, interaction effects determining disease tolerance, as well as potential effects not specific to individual variants but relating to disease diagnosis (similar to Berkson’s paradox57). Our study provides only limited data to distinguish these possible mechanisms. For the HbS association described in main text, we note in Supplementary Methods that there is little evidence that the Pfsa+ variants are themselves associated with increased disease risk, and little evidence that the Pfsa+ variants associate with other host protective variants, suggesting that the observed interaction is specific to HbS. The appearance of the association in uncomplicated cases further suggests that the effect is not specifically related to infections manifesting as severe disease.
Genotype inference from uncomplicated cases from Mali
We called genotypes from previously published RNA-seq data from 32 children ascertained with uncomplicated malaria from Mali16 (NCBI BioProject PRJNA685106). We used BAM files of reads aligned to the 3D7 reference genome as generated previously16. All data were from 50 bp paired end RNA-seq of total RNA. For each Pfsa lead variant, we counted the number of reads aligning to the variant with the reference, alternate, or other allele, ignoring those with lower than 20 mapping quality. These read counts are presented in Supplementary Table 6 for the lead Pfsa mutations. We treated genotypes as mixed (and excluded these from association analysis in Supplementary Table 2) if > 10% of reads covering both alleles were observed; otherwise, we assigned a specific genotype. At Pfsa3, we also tabulated genotypes for the second-most associated SNP (chr11:1,057,437 T>C) because we noted one sample (AS15) that had different genotype between this and the chr11:1,085,035 T>A variant.
Comparison of severe cases to human population controls
Equation (3) represents the odds ratio in severe cases as a ratio of relative risks for a given human allele e conditional on the parasite genotype at infection time. A closely related interpretation involves the relative risk of severe malaria with the observed (disease-time) parasite type,
| 4 |
A calculation shows that the OR in severe cases is also equal to the ratio . The relative risk (equation (4)) can be computed by comparing the frequencies of host genotypes between disease cases with infection genotype y and in the whole population. In Supplementary Methods we extend this to show that can be estimated using multinomial logistic regression with population controls and cases stratified by parasite type as outcome levels, and with the human genotype and any covariates included as predictors. We apply this approach in Fig. 2 to estimate , where y ranges over combined genotypes at the three Pfsa loci, conditional on the country of sampling.
Assessing sequencing performance in HbS-associated regions
We assessed sequencing performance at the chr2:631,190, chr2:814,288 and chr11:1,058,035 loci by computing counts of reads aligning to each position (‘coverage’) and comparing this to the distribution of coverage across all biallelic sites in our dataset, treating each sample separately (Supplementary Fig. 5). In general coverage at the three sites was high; we noted especially high coverage at chr2:814,288 in sWGA sequencing data (for example, >90% of samples have coverage among the top 80% of that at biallelic variants genome-wide) but somewhat lower coverage in WGS samples at the chr11:1,058,035 locus. Variation in coverage between loci and samples is expected due to variation in DNA quantities, DNA amplification and sequencing processes, but we did not observe systematic differences in coverage between the different Pfsa genotypes at these loci. To further establish alignment accuracy, we also inspected alignment metrics. Across all analysis samples, over 99% of reads at each location carried either the reference or the identified non-reference allele, and over 99% of these reads had mapping quality at least 50 (representing confident read alignment). These results suggest sequence reads provide generally accurate genotype calls at these sites.
Assessing the distribution of between-chromosome LD
We developed a C++ program (LDBIRD) to efficiently compute LD between all pairs of P. falciparum variants. LDBIRD computes the frequency of each variant, and computes the correlation between genotypes at each pair of variants with sufficiently high frequency. It then generates a histogram of correlation values and reports pairs of variants with squared correlation above a specified level. We applied LDBIRD separately to P. falciparum data from Gambian and Kenyan severe malaria cases. We restricted attention to comparisons between biallelic variants that had frequency at least 5% in the given population and with at least 75% of samples having non-missing genotypes at both variants in the pair, after treating mixed genotype calls as missing, and output all pairs with r2 at least 0.01 for further consideration. To avoid confounding of LD by the HbS association signal, we also repeated this analysis after excluding individuals that carry the HbS allele (with the latter results presented in Fig. 4 and Supplementary Table 4).
To summarise between-chromosome LD results we grouped signals into regions as follows. First, we observed that most variant pairs have |r| < 0.15 and hence r2 > 0.05 is typically a substantially outlying degree of inter-chromosomal LD (Fig. 4). We therefore focussed on variant pairs (v1,v2) with r2 > 0.05. To each such pair (v1,v2) we assigned a pair of LD regions (R1,R2) with the property that R1 and R2 capture all other nearby variants with high r2. Specifically, R1 and R2 are defined as the smallest regions containing v1 and v2 respectively, such that no other variant pair within 10 kb of (R1,R2) has r2 > 0.05. To compute R1 and R2, we implemented an iterative algorithm that successively expands the initial pair until no additional nearby pairs with high r2 can be found. For each LD region pair we recorded the region boundaries and the most-correlated pair of variants. A full list of region pairs with r2 > 0.05 is given in Supplementary Table 5; the highest LD pairs for Pfsa regions and for PfCRT–PfAAT1 are shown in Fig. 4.
Assessing the influence of covariates on LD estimates
To investigate whether the observed between-locus LD might arise due to population structure effects or due to other artefacts captured by measured covariates in our data, we used HPTEST to fit a logistic regression model of association with the genotypes at one Pfsa locus as outcome and the genotypes at a second Pfsa locus as predictor, repeating for each pair of Pfsa regions, in each population separately. We fit the model including each of a set of covariates as follows: (1) no covariates; (2) 20 parasite PCs; (3) technical covariates including an indicator of the type of sequencing and sequence depth (as in Extended Data Fig. 3); (4) year of admission, or (5) all of the above combined. For each set of covariates we compared the estimated odds ratio indicating the strength of association to the unadjusted odds ratio. In Kenya, across covariate sets, the minimum and unadjusted estimates were 128.0 and 128.0 (Pfsa1+ vs Pfsa2+; minimum with no covariates), 218.0 vs 219.4 (Pfsa1+ vs Pfsa3+; minimum when including technical covariates) and 40.2 vs 47.2 (Pfsa2+ vs Pfsa3+; minimum when including parasite PCs). In Gambia the minimum and unadjusted estimates were 7.0 and 7.7 (Pfsa1+ and Pfsa3+, minimum when including parasite PCs). These results therefore suggest the observed LD is not substantially explained by population structure or other features of our sample that are captured by these covariates.
Genotype inference from the Uganda Palo Alto isolate
To determine the genotype of the Uganda Palo Alto (FUP/H) isolate (Extended Data Figures 7-8), we downloaded Illumina sequence read data from the Sequence Read Archive (accessions SRR530503, SRR629055 and SRR629078, generated by the Broad Institute). All reads were 101 base pair paired end. We aligned the reads to the Pf3D7_v3 genome using bwa mem and inspected read pileups to determine the genotypes at HbS-associated mutations. These data clearly indicate that FUP/H carries the alternate allele at the Pfsa1–3 lead SNPs as well as at chr:1,057,437 T>C (based on >98% of reads carrying the non-reference allele).
Analysis of transcript expression from time course experiments
We analysed data from three previously reported experiments that measured the transcription of genes in 3D7 at different time stages following invasion of erythrocytes (accessions PRJEB201558 and PRJEB3153559). Data were processed using a similar pipeline to that described previously16. In brief, reads were aligned to a concatenated human GRCh38 / Pf3D7 genome using STAR v2.7.3a, informed by the Gencode v38 human and the PlasmoDB v52 Pf3D7 gene annotations. Reads aligning to Pf3D7 were then extracted and transcript abundance (TPM) was estimated using Salmon v1.5.1. Estimated TPM values for genes in the Pfsa regions, along with previously computed TPM values from Saelens et al.16, are shown in Extended Data Fig. 7.
Assessing the structure of Pfsa regions in available genome assemblies
We extracted 101 bp and 1001 bp flanking sequence centred at the chr2:631,190, chr2:814,288 and chr11:1,058,035 loci from the Pf3D7 reference sequence. We then used minimap260 to align these sequences to a previously generated set of genome assemblies from P. falciparum isolates and laboratory strains38 (Supplementary Table 7), allowing for multiple possible mapping locations. Each flanking sequence aligned to a single location on the corresponding chromosome in all included genomes, with the exception that sequence flanking the chromosome 11 locus aligned to two locations in the ML01 sample. This sample was excluded from previous analysis38 as it represents a multiple infection; we comment further on this below.
To further inspect sequence identity, we used MAFFT to generate a multiple sequence alignment (MSA) corresponding to the 1001 bp sequence centred at each locus. Four isolates (GA01 from The Gabon, SN01 from Senegal, Congo CD01 and ML01 from Mali) carry the non-reference ‘A’ allele at the chr11:1,058,035 SNP; two of these (GA01 and CD01) also carry the non-reference allele at the chr2:631,190 SNP and one (CD01) carries the non-reference allele at all three SNPs. However, expansion of alignments to include a 10,001 bp segment indicated that these four samples also carry a structural rearrangement at the chr11 locus. Specifically, GA01, SN01, CD01 and ML01 genomes include a ~1 kb insertion present approximately 900 bp to the right of chr11:1,058,035, and also a ~400 bp deletion approximately 2,400 bp to the left of chr11:1,058,035. To investigate this, we generated k-mer sharing ‘dot’ plots for k = 50 across the region (Supplementary Fig. 3, Extended Data Fig. 9), revealing a complex rearrangement carrying both deleted and duplicated segments. The duplicated sequence includes a segment (approximate coordinates 1,054,000–1,055,000 in Pf3D7) that contains the gene SNRPF (‘small nuclear ribonucleoprotein F, putative’) in the Pf3D7 reference. Inspection of breakpoints did not reveal any other predicted gene copy number changes in this region, including for Pf3D7_1127000.
As noted above, the chromosome 11 region aligns to a second contig in ML01 (contig chr0_142, Supplementary Table 7). This contig appears to have a different tandem duplication of a ~4 kb segment lying to the right of the associated SNP (approximately corresponding to the range 11:1,060,100–1,064,000 in Pf3D7; Supplementary Fig. 3). This segment contains a number of genes including dUTPase, which has been under investigation as a potential drug target61. We interpret this second contig as arising due to the multiple infection in this sample38, and given challenges inherent in genome assembly of mixed samples it is unclear whether this duplication represents an assembly artefact or a second genuine regional structural variant.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this paper.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41586-021-04288-3.
Supplementary information
This file contains Supplementary text, Figs. 1–6, Methods and references.
Acknowledgements
We thank the patients and staff of Kilifi County Hospital and the KEMRI-Wellcome Trust Research Programme, Kilifi for their help with this study, members of the Human Genetics Group in Kilifi for help with sample collection and processing, and the patients and staff at the Paediatric Department of the Royal Victoria Hospital in Banjul, Gambia for their help with the study. The human genetic data from severe cases and controls used in this study have previously been reported by the Malaria Genomic Epidemiology Network, and we thank all our colleagues who contributed to this previous work as part of MalariaGEN Consortial Project 1. A full list of consortium members is provided at https://www.malariagen.net/projects/consortial-project-1/malariagen-consortium-members. The MalariaGEN Pf6 open resource11 was generated through the Malaria Genomic Epidemiology Network Plasmodium falciparum Community Project (https://www.malariagen.net/resource/26). The Malaria Genomic Epidemiology Network study of severe malaria was supported by Wellcome (https://wellcome.ac.uk/) (WT077383/Z/05/Z (MalariaGEN)) and the Bill & Melinda Gates Foundation (https://www.gatesfoundation.org/) through the Foundations of the National Institutes of Health (https://fnih.org/) (566 (MalariaGEN)) as part of the Grand Challenges in Global Health Initiative. The Resource Centre for Genomic Epidemiology of Malaria is supported by Wellcome (090770/Z/09/Z and 204911/Z/16/Z (MalariaGEN)). This research was supported by the Medical Research Council (https://mrc.ukri.org/) (G0600718, G0600230 and MR/M006212/1 (MalariaGEN)). Wellcome also provides core awards to the Wellcome Centre for Human Genetics (203141/Z/16/Z (WCHG)) and the Wellcome Sanger Institute (206194 (WSI)). Genome sequencing was carried out at the Wellcome Sanger Institute and we thank the staff of the Wellcome Sanger Institute Sample Logistics, Sequencing, and Informatics facilities for their contribution. T.N.W. is supported through a Senior Fellowship from Wellcome (202800/Z/16/Z). This paper is published with permission from the Director of the Kenya Medical Research Institute (KEMRI). Sequencing and initial analysis of the Mali uncomplicated malaria cases was funded by NIAID (R21AI125988 to S.M.T.). J.W.S. received support from the National Center for Advancing Translational Sciences (UL1TR002553). This research was funded in whole or in part by Wellcome as detailed above. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank D. Goldberg (Washington University in St.Louis) and M. Lee (Wellcome Sanger Institute) for their assistance with revisions.
Extended data figures and tables
Author contributions
Conceptualization: G.B., E.M.L., T.N.W., K.A.R. and D.P.K. Data curation: G.B., E.M.L., T.N., M.J., C.M.N., R.D.P., R.A. and K.A.R. Formal analysis: G.B., E.M.L. and K.A.R. Funding acquisition: D.P.K. Investigation: C.H., A.E.J., K.R., E.D. and K.A.R. Methodology: G.B., K.A.R. and D.P.K. Project administration: S.G., E.D., K.A.R. and D.P.K. Resources: S.G., E.D., J.S., C.V.A., R.A., R.D.P., M.J., F.S.-J., K.A.B., G.S., C.M.N., A.W.M., N.P., J.W.S., M.D., S.M.T., C.H., A.E.J., K.R., E.D. and K.A.R. Software and visualisation: G.B. Supervision: D.J.C., U.d.A., K.M., T.N.W., S.G., K.A.R. and D.P.K. Writing: G.B., E.M.L., S.M.T., D.J.C., T.N.W., K.A.R. and D.P.K. in collaboration with all authors.
Peer review information
Nature thanks Daniel Falush, John Lees and the other, anonymous reviewers for their contribution to the peer review of this work. Peer review reports are available.
Data availability
Sequence read data from whole DNA and SWGA sequencing of P. falciparum genomes (as detailed in Extended Data Fig. 1) are available from the European Nucleotide Archive (study accession ERP000190). A full list of relevant sample accessions can be found at http://www.malariagen.net/resource/32. Human genotype data used in this study have been described previously5,8 and are available under managed-access terms from the European Genome–Phenome Archive (study accession EGAS00001001311), as detailed at https://www.malariagen.net/resource/25. A dataset of the human and P. falciparum genotypes for 3,346 severe cases of malaria used in our discovery scan (Fig. 1), and HbS genotypes and P. falciparum genotypes in the larger set of 4,071 severe cases with direct HbS typing (Fig. 2), is available from Zenodo (10.5281/zenodo.4973476). Association test summary statistics from our discovery data (Fig. 1) are also available from Zenodo (10.5281/zenodo.5722497). A full list of data generated by this study and associated resources can be found at http://www.malariagen.net/resource/32.
Code availability
Source code for HPTEST and LDBIRD is available at https://code.enkre.net/qctool. A snapshot has also been deposited at Zenodo (10.5281/zenodo.5685581).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gavin Band, Email: gavin.band@well.ox.ac.uk.
Kirk A. Rockett, Email: kirk.rockett@well.ox.ac.uk
Dominic P. Kwiatkowski, Email: dominic@sanger.ac.uk
Extended data
is available for this paper at 10.1038/s41586-021-04288-3.
Supplementary information
The online version contains supplementary material available at 10.1038/s41586-021-04288-3.
References
- 1.Kariuki SN, Williams TN. Human genetics and malaria resistance. Hum. Genet. 2020;139:801–811. doi: 10.1007/s00439-020-02142-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bethke LL, et al. Duplication, gene conversion, and genetic diversity in the species-specific acyl-CoA synthetase gene family of Plasmodium falciparum. Mol. Biochem. Parasitol. 2006;150:10–24. doi: 10.1016/j.molbiopara.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Matesanz F, Téllez MA-D-M, Alcina A. The Plasmodium falciparum fatty acyl-CoA synthetase family (PfACS) and differential stage-specific expression in infected erythrocytes. Mol. Biochem. Parasitol. 2003;126:109–112. doi: 10.1016/s0166-6851(02)00242-6. [DOI] [PubMed] [Google Scholar]
- 4.Otto TD, et al. Genomes of all known members of a Plasmodium subgenus reveal paths to virulent human malaria. Nat. Microbiol. 2018;3:687–697. doi: 10.1038/s41564-018-0162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malaria Genomic Epidemiology Network. Reappraisal of known malaria resistance loci in a large multicenter study. Nat. Genet. 2014;46:1197–1204. doi: 10.1038/ng.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cowell AN, Winzeler EA. The genomic architecture of antimalarial drug resistance. Brief. Funct. Genomics. 2019;18:314–328. doi: 10.1093/bfgp/elz008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomes PS, Bhardwaj J, Rivera-Correa J, Freire-De-Lima CG, Morrot A. Immune escape strategies of malaria parasites. Front. Microbiol. 2016;7:1617. doi: 10.3389/fmicb.2016.01617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Band G, et al. Insights into malaria susceptibility using genome-wide data on 17,000 individuals from Africa, Asia and Oceania. Nat. Commun. 2019;10:5732. doi: 10.1038/s41467-019-13480-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Band G, et al. A novel locus of resistance to severe malaria in a region of ancient balancing selection. Nature. 2015;526:253–257. doi: 10.1038/nature15390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oyola SO, et al. Whole genome sequencing of Plasmodium falciparum from dried blood spots using selective whole genome amplification. Malaria J. 2016;15:597. doi: 10.1186/s12936-016-1641-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahouidi A, et al. An open dataset of Plasmodium falciparum genome variation in 7,000 worldwide samples. Wellcome Open Research. 2021;6:42. doi: 10.12688/wellcomeopenres.16168.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Timmann C, et al. Genome-wide association study indicates two novel resistance loci for severe malaria. Nature. 2012;489:443–446. doi: 10.1038/nature11334. [DOI] [PubMed] [Google Scholar]
- 13.Leffler EM, et al. Resistance to malaria through structural variation of red blood cell invasion receptors. Science. 2017;356:aam6393. doi: 10.1126/science.aam6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowman AF, Berry D, Baum J. The cellular and molecular basis for malaria parasite invasion of the human red blood cell. J. Cell Biol. 2012;198:961–971. doi: 10.1083/jcb.201206112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cowman AF, Tonkin CJ, Tham WH, Duraisingh MT. The molecular basis of erythrocyte invasion by malaria parasites. Cell Host Microbe. 2017;22:232–245. doi: 10.1016/j.chom.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Saelens JW, et al. Impact of sickle cell trait hemoglobin on the intraerythrocytic transcriptional program of Plasmodium falciparum. mSphere. 2021;6:e0075521. doi: 10.1128/mSphere.00755-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piel FB, et al. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet. 2013;381:142–151. doi: 10.1016/S0140-6736(12)61229-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manske M, et al. Analysis of Plasmodium falciparum diversity in natural infections by deep sequencing. Nature. 2012;487:375–379. doi: 10.1038/nature11174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mzilahowa T, McCall PJ, Hastings IM. “Sexual” population structure and genetics of the malaria agent P. falciparum. PLoS ONE. 2007;2:e613–e613. doi: 10.1371/journal.pone.0000613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tindall SM, et al. Heterologous expression of a novel drug transporter from the malaria parasite alters resistance to quinoline antimalarials. Sci. Rep. 2018;8:2464. doi: 10.1038/s41598-018-20816-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, et al. Genome-wide association analysis identifies genetic loci associated with resistance to multiple antimalarials in Plasmodium falciparum from China–Myanmar border. Sci. Rep. 2016;6:33891. doi: 10.1038/srep33891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amambua-Ngwa A, et al. Major subpopulations of Plasmodium falciparum in sub-Saharan Africa. Science. 2019;365:813–816. doi: 10.1126/science.aav5427. [DOI] [PubMed] [Google Scholar]
- 23.Moser KA, et al. Describing the current status of Plasmodium falciparum population structure and drug resistance within mainland Tanzania using molecular inversion probes. Mol. Ecol. 2021;30:100–113. doi: 10.1111/mec.15706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verity R, et al. The impact of antimalarial resistance on the genetic structure of Plasmodium falciparum in the DRC. Nat. Commun. 2020;11:2107. doi: 10.1038/s41467-020-15779-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang H-H, et al. Genomic sequencing of Plasmodium falciparum malaria parasites from senegal reveals the demographic history of the population. Mol. Biol. Evol. 2012;29:3427–3439. doi: 10.1093/molbev/mss161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park DJ, et al. Sequence-based association and selection scans identify drug resistance loci in the Plasmodium falciparum malaria parasite. Proc. Natl Acad. Sci. USA. 2012;109:13052. doi: 10.1073/pnas.1210585109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ralph SA, et al. Metabolic maps and functions of the Plasmodium falciparum apicoplast. Nat. Rev. Microbiol. 2004;2:203–216. doi: 10.1038/nrmicro843. [DOI] [PubMed] [Google Scholar]
- 28.Hiller NL, et al. A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science. 2004;306:1934–1937. doi: 10.1126/science.1102737. [DOI] [PubMed] [Google Scholar]
- 29.Jonsdottir TK, Gabriela M, Crabb BS, F. de Koning-Ward T, Gilson PR. Defining the essential exportome of the malaria parasite. Trends Parasitol. 2021;37:664–675. doi: 10.1016/j.pt.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 30.Marti M, Good RT, Rug M, Knuepfer E, Cowman AF. Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science. 2004;306:1930–1933. doi: 10.1126/science.1102452. [DOI] [PubMed] [Google Scholar]
- 31.Matesanz F, Durán-Chica I, Alcina A. The cloning and expression of Pfacs1, a Plasmodium falciparum fatty acyl coenzyme A synthetase-1 targeted to the host erythrocyte cytoplasm11. J. Mol. Biol. 1999;291:59–70. doi: 10.1006/jmbi.1999.2964. [DOI] [PubMed] [Google Scholar]
- 32.Butler, T. K. An Exported Malaria Protein Regulates Glucose Uptake During Intraerythrocytic Infection. PhD thesis, Washington Univ. in St Louis (2014).
- 33.Lamarque M, et al. Food vacuole proteome of the malarial parasite Plasmodium falciparum. Proteomics Clin. Appl. 2008;2:1361–1374. doi: 10.1002/prca.200700112. [DOI] [PubMed] [Google Scholar]
- 34.Zhang M, et al. Uncovering the essential genes of the human malaria parasite Plasmodium falciparum by saturation mutagenesis. Science. 2018;360:eaap7847. doi: 10.1126/science.aap7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russo I, et al. Plasmepsin V licenses Plasmodium proteins for export into the host erythrocyte. Nature. 2010;463:632–636. doi: 10.1038/nature08726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boddey JA, et al. Role of plasmepsin V in export of diverse protein families from the Plasmodium falciparum exportome. Traffic. 2013;14:532–550. doi: 10.1111/tra.12053. [DOI] [PubMed] [Google Scholar]
- 37.Grüring C, et al. Uncovering common principles in protein export of malaria parasites. Cell Host Microbe. 2012;12:717–729. doi: 10.1016/j.chom.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 38.Otto TD, et al. Long read assemblies of geographically dispersed Plasmodium falciparum isolates reveal highly structured subtelomeres. Wellcome Open Res. 2018;3:52. doi: 10.12688/wellcomeopenres.14571.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luzzatto L. Sickle cell anaemia and malaria. Mediterr. J. Hematol. Infect. Dis. 2012;4:e2012065. doi: 10.4084/MJHID.2012.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Archer NM, et al. Resistance to Plasmodium falciparum in sickle cell trait erythrocytes is driven by oxygen-dependent growth inhibition. Proc. Natl Acad. Sci. USA. 2018;115:7350–7355. doi: 10.1073/pnas.1804388115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cholera R, et al. Impaired cytoadherence of Plasmodium falciparum-infected erythrocytes containing sickle hemoglobin. Proc. Natl Acad. Sci. USA. 2008;105:991–996. doi: 10.1073/pnas.0711401105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cyrklaff M, et al. Hemoglobins S and C interfere with actin remodeling in Plasmodium falciparum infected erythrocytes. Science. 2011;334:1283–1286. doi: 10.1126/science.1213775. [DOI] [PubMed] [Google Scholar]
- 43.Williams TN, et al. An immune basis for malaria protection by the sickle cell trait. PLoS Med. 2005;2:e128. doi: 10.1371/journal.pmed.0020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilbert SC, et al. Association of malaria parasite population structure, HLA, and immunological antagonism. Science. 1998;279:1173–1177. doi: 10.1126/science.279.5354.1173. [DOI] [PubMed] [Google Scholar]
- 45.Ntoumi F, et al. Imbalanced distribution of Plasmodium falciparum MSP-1 genotypes related to sickle-cell trait. Mol. Med. 1997;3:581–592. [PMC free article] [PubMed] [Google Scholar]
- 46.Stan Development Team. Stan Modeling Language Users Guide and Reference Manual. https://mc-stan.org (2021).
- 47.Ansari MA, et al. Genome-to-genome analysis highlights the effect of the human innate and adaptive immune systems on the hepatitis C virus. Nat. Genet. 2017;49:666–673. doi: 10.1038/ng.3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bartha I, et al. A genome-to-genome analysis of associations between human genetic variation, HIV-1 sequence diversity, and viral control. Elife. 2013;2:e01123. doi: 10.7554/eLife.01123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lees JA, et al. Joint sequencing of human and pathogen genomes reveals the genetics of pneumococcal meningitis. Nat. Commun. 2019;10:2176. doi: 10.1038/s41467-019-09976-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wellcome Trust Case Control Consortium Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greenland S, Mansournia MA. Penalization, bias reduction, and default priors in logistic and related categorical and survival regressions. Stat. Med. 2015;34:3133–3143. doi: 10.1002/sim.6537. [DOI] [PubMed] [Google Scholar]
- 52.Miles A, et al. Indels, structural variation, and recombination drive genomic diversity in Plasmodium falciparum. Genome Res. 2016;26:1288–1299. doi: 10.1101/gr.203711.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Braschi B, et al. Genenames.org: the HGNC and VGNC resources in 2019. Nucleic Acids Res. 2019;47:D786–D792. doi: 10.1093/nar/gky930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O'Leary NA, et al. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016;44:D733–D74. doi: 10.1093/nar/gkv1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aurrecoechea C, et al. PlasmoDB: a functional genomic database for malaria parasites. Nucleic Acids Res. 2009;37:D539–D543. doi: 10.1093/nar/gkn814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Storey JD. The positive false discovery rate: a Bayesian interpretation and the q-value. Ann. Stat. 2003;31:2013–2035. [Google Scholar]
- 57.Berkson J. Limitations of the application of fourfold table analysis to hospital data. Int. J. Epidemiol. 2014;43:511–515. doi: 10.1093/ije/dyu022. [DOI] [PubMed] [Google Scholar]
- 58.Otto TD, et al. New insights into the blood-stage transcriptome of Plasmodium falciparum using RNA-seq. Mol. Microbiol. 2010;76:12–24. doi: 10.1111/j.1365-2958.2009.07026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wichers JS, et al. Dissecting the gene expression, localization, membrane topology, and function of the Plasmodium falciparum STEVOR protein family. mBio. 2019;10:e01500–e01519. doi: 10.1128/mBio.01500-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics. 2018;34:3094–3100. doi: 10.1093/bioinformatics/bty191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pérez-Moreno G, et al. Validation of Plasmodium falciparum dUTPase as the target of 5′-tritylated deoxyuridine analogues with anti-malarial activity. Malar. J. 2019;18:392. doi: 10.1186/s12936-019-3025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains Supplementary text, Figs. 1–6, Methods and references.
Data Availability Statement
Sequence read data from whole DNA and SWGA sequencing of P. falciparum genomes (as detailed in Extended Data Fig. 1) are available from the European Nucleotide Archive (study accession ERP000190). A full list of relevant sample accessions can be found at http://www.malariagen.net/resource/32. Human genotype data used in this study have been described previously5,8 and are available under managed-access terms from the European Genome–Phenome Archive (study accession EGAS00001001311), as detailed at https://www.malariagen.net/resource/25. A dataset of the human and P. falciparum genotypes for 3,346 severe cases of malaria used in our discovery scan (Fig. 1), and HbS genotypes and P. falciparum genotypes in the larger set of 4,071 severe cases with direct HbS typing (Fig. 2), is available from Zenodo (10.5281/zenodo.4973476). Association test summary statistics from our discovery data (Fig. 1) are also available from Zenodo (10.5281/zenodo.5722497). A full list of data generated by this study and associated resources can be found at http://www.malariagen.net/resource/32.
Source code for HPTEST and LDBIRD is available at https://code.enkre.net/qctool. A snapshot has also been deposited at Zenodo (10.5281/zenodo.5685581).