Abstract
Introduced less than two decades ago, glucagon‐like peptide‐1 receptor agonists rapidly reshaped the field of Type 2 diabetes mellitus (T2DM) care by providing glycaemic control in tandem with weight loss. However, FDA‐approved GLP‐1 receptor agonists are often accompanied by nausea and emesis and, in some lean T2DM patients, by undesired anorexia. Importantly, the hypophagic and emetic effects of GLP‐1 receptor agonists are caused by activation of central GLP‐1 receptors. This review summarizes two different approaches to mitigate the incidence and severity of nausea and emesis related to GLP‐1 receptor agonists: conjugation with vitamin B12, or related corrin ring‐containing compounds (‘corrination’), and development of dual agonists of GLP‐1 receptors with glucose‐dependent insulinotropic polypeptide (GIP). Such approaches could lead to the generation of GLP‐1 receptor agonists with improved therapeutic efficacy, thus decreasing treatment attrition, increasing patient compliance and extending treatment to a broader population of T2DM patients. The data reviewed show that it is possible to pharmacologically separate the emetic effects of GLP‐1 receptor agonists from their glucoregulatory action.
LINKED ARTICLES
This article is part of a themed issue on GLP1 receptor ligands (BJP 75th Anniversary). To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v179.4/issuetoc
Keywords: area postrema, cobinamide, corrination, diabetes mellitus, emesis, GIPR/GLP‐1R dual agonists, glucose‐dependent insulinotropic polypeptide, hindbrain, side effects, tirzepatide, vitamin B12
Abbreviations
- AP
area postrema
- B12
vitamin B12
- Cbi
cobinamide
- CDT
1,1‐dicarbonyl‐di‐(1,2,4‐triazole)
- CeA
central nucleus of the amygdala
- DMV
dorsal motor nucleus of the vagus
- DPP‐4
dipeptidyl peptidase‐4
- GI
gastrointestinal
- GIP
glucose‐dependent insulinotropic polypeptide
- GLP‐1
glucagon‐like peptide‐1
- HC
haptocorrin
- IF
intrinsic factor
- NTS
nucleus tractus solitarius
- PBN
parabrachial nucleus
- PPG
preproglucagon
- T2DM
type 2 diabetes mellitus
- TC
transcobalamin
1. INTRODUCTION
1.1. Type 2 diabetes mellitus is a global health problem, and pharmacological intervention requires refinement to increase tolerability for patient quality of life in disease management
Diabetes is one of the most common chronic diseases in the world. According to the International Diabetes Federation (IDF), the incidence of diabetes has tripled over the last 20 years, reaching a prevalence of nearly 10% among adults today (www.idf.org). Of the different forms of diabetes, Type 2 diabetes mellitus (T2DM) is the most prevalent form and accounts for the vast majority (~90%) of the total diabetic population (Saeedi, Petersohn et al., 2019). T2DM is a progressive metabolic disorder characterized by hyperglycaemia and deterioration in beta‐cell function, paradoxically accompanied by defective insulin action due to the development of insulin resistance (Nauck & Meier, 2016). The global prevalence of the disease is increasing rapidly, largely due to concomitant increases in obesity (Leitner et al., 2017), as clear underlying mechanistic co‐morbidities exist between these two pathologies. Unfortunately, lifestyle interventions alone (i.e., diet and exercise‐based interventions) are largely ineffective, in part due to poor adherence/compliance but also due to evolutionary metabolic mechanisms that intrinsically prevent sustained weight loss (Grill, 2020) and obfuscate T2DM management tools.
In the last few decades, many families of hypoglycaemic drugs have been developed for the treatment of T2DM such as synthetic insulin, metformin, dipeptidyl peptidase‐4 (DPP‐4) inhibitors, sodium/glucose cotransporter‐2 inhibitors and glucagon‐like peptide‐1 (GLP‐1) analogues (American Diabetes Association, 2020; Buse et al., 2020; Lovshin & Drucker, 2009). Despite cases of remarkable success, all these drugs have been characterized by pitfalls and side effects that limit efficacy and narrow therapeutic windows, requiring intensified research efforts to fully manage T2DM (McGovern et al., 2018; Thrasher, 2017). Second‐generation GLP‐1 receptor agonists have now been approved by the Food and Drug Administration (FDA) for the treatment of T2DM. The development and marketing of albiglutide, dulaglutide and semaglutide have been extensively discussed and reviewed elsewhere (Avgerinos et al., 2020; Grill, 2020; Jendle et al., 2016; Madsbad, 2016; Rendell, 2018; Sharma et al., 2018). In this review, we highlight recent advances in the development of promising GLP‐1 receptor agonist‐based approaches that have not yet received FDA approval and will describe the underlying mechanisms identified in preclinical studies, discussing potential translational relevance of these novel pharmacotherapies with special emphasis on ‘in‐build’ strategies to mitigate the most prominent side effects of the GLP‐1 receptor agonists, such as nausea and vomiting. Specifically, we discuss the advantages of GLP‐1 agonism in conjunction with glucose‐dependent insulinotropic polypeptide (GIP), and GLP‐1 conjugation with vitamin B12 (B12) as potential future strategies to mitigate or eliminate the side effects characteristic of current GLP‐1 receptor agonists.
1.2. The GLP‐1 system and the history of GLP‐1 analogues: Remarkable control of glycaemia, if tolerated
The search for insulin‐stimulating factors (the incretins) began over 100 years ago in the hopes of uncovering endogenous peptides with potential hypoglycaemic utility (Kim & Egan, 2008). GLP‐1 is a multifaceted hormone with broad pharmacological potential (Drucker, 2018). First isolated in 1986 from the gut, GLP‐1 has since been found to both stimulate insulin secretion and inhibit glucagon release (Drucker et al., 2017). Together with GIP, these two proteins are the only two known endogenous incretins (Holst, 2019). Glucose‐stimulated insulin secretion by GLP‐1 is only one of the beneficial effects for T2DM management. Other known GLP‐1 effects on metabolism that are relevant to T2DM include the slowing of gastric emptying, inhibition of food intake and modulation of beta‐cell proliferation (Holst, 2007; Muller et al., 2019; Rowlands et al., 2018).
GLP‐1 is a post‐translational product of the peptide preproglucagon (PPG) expressed in the enteroendocrine L cells of the small intestine and in pancreatic alpha‐cells (Eissele et al., 1992; Kauth & Metz, 1987). Within minutes of the ingestion of nutrients, GLP‐1 is released by enteroendocrine L cells (Jorsal et al., 2018). However, due to rapid degradation by the enzyme DPP‐4, only ~10% of endogenously released GLP‐1 reaches the systemic circulation (Muller et al., 2019). PPG is also expressed centrally, primarily in the nucleus tractus solitarius (NTS) (Merchenthaler et al., 1999) of the caudal brainstem, an integrative relay nucleus for satiety and emetic signals controlling ingestive and malaise behaviour (Grill & Hayes, 2012). GLP‐1‐expressing NTS neurons project to various GLP‐1 receptor‐expressing nuclei throughout the CNS (Llewellyn‐Smith et al., 2011) implicated in the control of food intake and energy balance (Dunphy et al., 1998; Merchenthaler et al., 1999). Central administration of GLP‐1 or GLP‐1 receptor agonists reduce food intake and body weight, reproducing the effects of systemically delivered GLP‐1 receptor agonists (Alhadeff et al., 2016; Barrera et al., 2011; Hayes et al., 2010; Turton et al., 1996) and highlighting a predominant role of the central GLP‐1 system in the regulation of feeding.
As a treatment for the management of metabolic diseases, native GLP‐1 has proven largely ineffective as a pharmacotherapy due to the necessity for repeated, large doses to overcome the extremely short half‐life, ~2 min, due to degradation by DPP‐4 (Muller et al., 2019). Nearly 30 years ago, it was discovered that a compound extracted from the salivary gland secretions of the Gila monster has similar properties to human GLP‐1, which led to the development of the peptide exendin‐4 and its synthetic form, exenatide (Eng et al., 1992). Exendin‐4 exhibits a modified amino acid sequence, relative to human GLP‐1, thereby allowing the compound to be remarkably resistant to enzymic degradation by DPP‐4 and extending its half‐life to ~2.5 h (Copley et al., 2006; Kieffer et al., 1995). In 2005, exenatide became the first FDA‐approved GLP‐1 analogue for the treatment of T2DM. Shortly after, in 2008, exendin‐4 was approved the by European Medicines Agency (EMA) for the treatment of obesity (Davidson et al., 2005). The entry of exendin‐4 into the T2DM landscape was followed in 2009 by liraglutide, an acylated (i.e., lipidated) and DPP‐4‐resistant GLP‐1 analogue derived from human GLP‐1 (Nauck & Meier, 2019), which was approved by the FDA for the treatment of obesity in 2014 (Muller et al., 2019). In doing so, exendin‐4 and liraglutide became the first glucose‐lowering medication options for reducing body weight, according to FDA/EMA standards, thus providing additional health benefits for the overweight T2DM patient population. With advances in biotherapeutic engineering, studies focused on exendin‐4 and liraglutide inspired drug discovery campaigns over the subsequent two decades to deliver ‘second‐generation’ GLP‐1 receptor agonists for treating T2DM, most notably albiglutide, dulaglutide and semaglutide (Williams et al., 2020). These developments yielded substantial overall metabolic improvements in patients compared with the first‐generation GLP‐1 receptor agonists that include superior and longer lasting hypoglycaemic actions and greater body weight loss. However, these compounds were still plagued by a high incidence of side effects, mostly of gastrointestinal (GI) nature, such as nausea and vomiting (Ahren et al., 2018; Bettge et al., 2017; Pratley et al., 2018).
1.3. Nausea and emesis are the most common side effects of all existing GLP‐1‐based therapies and limit T2DM management
The 13th US Surgeon General, C. Everett Koop, famously stated, ‘Drugs don't work in patients who don't take them’, encapsulating an important segment of T2DM patients and challenges to treatment adherence over time. Current GLP‐1 receptor agonists are extraordinary medicines but importantly are not immune to pitfalls characterized by all‐too‐common side effects such as GI distress, nausea, vomiting and diarrhoea (Bettge et al., 2017; Filippatos et al., 2014). These side effects occur in a dose‐dependent manner, which generally limits the use of higher doses to drive satisfactory glycaemic control and proper disease management. Evidence‐based medical reports are now clear that nausea and emesis are the principal reported side effects of existing GLP‐1‐based therapeutic agents (Bettge et al., 2017). A recent report from GlaxoSmithKline concluded that ‘Patients reported that GI‐related issues “Made me feel sick” (64.4%) and “Made me throw up” (45.4%) as their top reasons for discontinuation’ (Sikirica et al., 2017). The same report showed ‘disparities between patient experiences and physician perceptions’ and suggested major ‘gaps in physician–patient communication’ regarding GLP‐1‐therapeutic agents and incidence of illness. Indeed, it has been reported for decades in the cancer literature that physicians and nurses vastly underestimate incidence of nausea and emesis in outpatient settings (Aapro, 2018). Additionally, the documentation of these side effects in clinical trials is often via self‐reporting rather than validated, structured and homogenized questionnaires, which further complicates assessments and between‐study comparisons. Report after report for every GLP‐1 based therapeutic agent reads with incidence of nausea and emesis warranting strategies for improvement (see Bergenstal et al., 2010; Bettge et al., 2017; Buse et al., 2004; John et al., 2007; Jones et al., 2018; Kendall et al., 2005; Lean et al., 2014; Nauck et al., 2016; Ratner et al., 2010). These effects are not transient nor insignificant as they lead to discontinuation of treatment in ~6–10% and reduced dose tolerance in another ~15% of T2DM patients (Bergenstal et al., 2010; Buse et al., 2004; Capehorn et al., 2020; John et al., 2007; Kendall et al., 2005; O'Neil et al., 2018; Trujillo, 2020). To put this major issue into a different set of numbers demonstrating the magnitude of this problem, approximately one in four T2DM patients (~6.5 million US citizens) are prevented from the full benefit of current FDA‐approved GLP‐1‐based therapeutic agents, and up to 50% of patients (~13 million Americans) that may be prescribed an existing GLP‐1‐based therapeutic agent will experience nausea and emesis. These statistics together support the notion that future GLP‐1 receptor agonists designed to eliminate nausea and emesis have a high therapeutic potential for T2DM patients.
Any strategy addressing emesis and emetic behaviour improvements must consider that the vomiting response is largely controlled by an emetic centre located in the brainstem (Babic & Browning, 2014; Baker et al., 2005; Hesketh, 2008; Horn, 2014; Miller & Leslie, 1994). This anatomical emetic ‘hub’ is composed of three distinct nuclei—the NTS, the adjacent dorsal motor nucleus of the vagus (DMV) and the area postrema (AP). Physiological and pathological modulators of energy balance share many common neural substrates and anatomical nodes within the brain including the AP/NTS (Babic & Browning, 2014; Hesketh, 2008; Miller & Leslie, 1994). The AP/NTS is the primary target of vagal afferent projections originating from the gut. Additionally, the presence of fenestrated capillaries allows neurons in the AP/NTS to be easily reached by circulating emetic/anorectic agents that cannot readily cross the blood–brain barrier (Miller & Leslie, 1994; Price et al., 2008). Increased neuronal activity in the AP/NTS is associated with emesis and nausea (Baker et al., 2005). Importantly, virtually, all existing approved GLP‐1 analogues, as well as other emetic stimuli, such as the highly emetogenic chemotherapeutic agent cisplatin, activate neurons in the AP/NTS and upstream CNS targets implicated in the regulation of not only feeding behaviour but also in the development of aversion and illness behaviours, such as the parabrachial nucleus (PBN) and the central nucleus of the amygdala (CeA) (Alhadeff et al., 2017; Baraboi et al., 2011; De Jonghe & Horn, 2009; Gabery et al., 2020; Salinas et al., 2018). Direct activation of the GLP‐1 receptors expressed in various nuclei of the CNS reproduces the body weight and food intake suppressive effects, as well as the behavioural signs of malaise observed following systemic administration of GLP‐1 receptor agonists (Kanoski et al., 2011; Kanoski et al., 2012; Mietlicki‐Baase et al., 2018; Secher et al., 2014; Sisley et al., 2014).
Despite the enhanced half‐life (i.e., sustained agonism) and the use of titration schemes to slowly increase drug levels as a means to reduce some side effects (Nauck & Meier, 2019), significant attrition due to intolerance to GLP‐1 pharmacotherapy has and continues to occur mainly due to reported GI side effects. Thus, considering the dose‐limiting side effects, there is a clear clinical need for safer and more effective GLP‐1‐based analogues. In this review, we discuss the advantages of GLP‐1 agonism in conjunction with GIP, as well as GLP‐1 conjugation with corrin ring‐containing molecules such as vitamin B12 (B12) or cobinamide (Cbi).
2. CONJUGATION WITH B12 AND Cbi: ALTERING PHARMACODYNAMICS TO PREVENT GLP‐1 RECEPTOR‐INDUCED EMESIS AND NAUSEA
2.1. General vitamin B12 physiology
Coined ‘nature's most beautiful cofactor’ due to its deep red colour, B12 is the largest and most complex of all vitamins and one of nature's rare organometallic compounds. B12 is a water‐soluble molecule that is critical for red blood cell formation, proper neurological function, and DNA and protein synthesis (Nielsen et al., 2012). Because B12 can only be synthesized by bacteria, mammals (including humans and rats) must ingest the vitamin for survival as an essential nutrient. A highly efficient system for the absorption and cellular uptake of B12 exists (see Figure 1 and Green et al., 2017). Once B12 is released in the gastric lumen, the glycoprotein haptocorrin (HC) binds it, protects it from the stomach's low pH and carries it to the duodenum (Morkbak et al., 2007), where HC is then degraded and B12 is free to bind another glycoprotein, the intrinsic factor (IF) (Allen et al., 1978; Gordon et al., 1991). This IF transports B12 through the intestine into the terminal ileum, where the IF‐B12 complex undergoes receptor‐mediated endocytosis into the ileal enterocyte (Birn et al., 1997; Fyfe et al., 2004; Kozyraki et al., 1998). In the cytoplasm of the ileal absorptive cells, B12 becomes bound to transcobalamin (TC), which mediates its secretion into the blood plasma and carries it to cells that require B12 (Russell‐Jones & Alpers, 1999).
FIGURE 1.
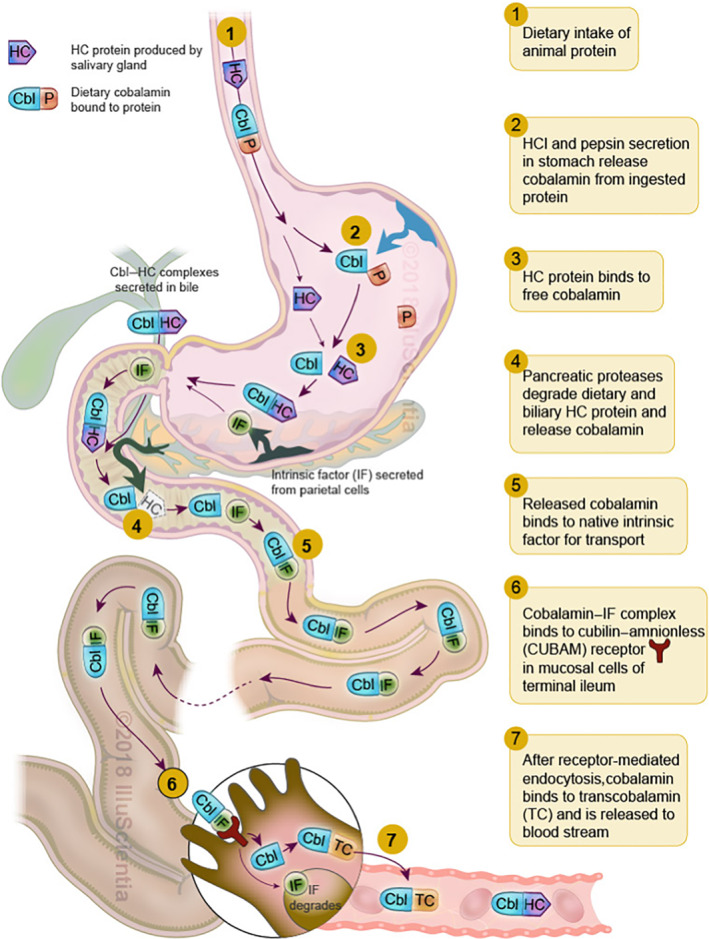
Schematic representation of the dietary uptake pathway of vitamin B12 in humans. Cbl, cobalamin/B12; HC, haptocorrin; IF, intrinsic factor; P, protein
2.2. The B12 corrination technology
The structural heart of B12 is a corrin ring: a 19‐carbon, asymmetric, mono‐protonated, tetrapyrrole ring system with a centrally coordinated cobalt atom (Co3+). This structure allows for multiple areas that can be chemically modified and/or conjugated to other moieties. However, maintaining recognition of B12 by its key transport proteins is critical but can be achieved through select modification of, and/or conjugation to, B12. Several modifications of B12 have been published, including those at the amide side chains, the phosphate group, the cobalt centre or the ribose secondary hydroxyl group (Figure 2) (ó Proinsias et al., 2013). Our team has successfully generated, validated and characterized several peptide conjugates, such as those with insulin (Petrus et al., 2007), PYY3–36 (Fazen et al., 2011) and GLP‐1 (Clardy‐James et al., 2013) at the 5′‐OH position (see Table 1). This site was previously shown to be reactive in a coupling reaction with 1,1‐dicarbonyl‐di‐(1,2,4‐triazole) (CDT) coupling agent, and such conjugates retained binding with IF and TC (Fedosov et al., 2006).
FIGURE 2.
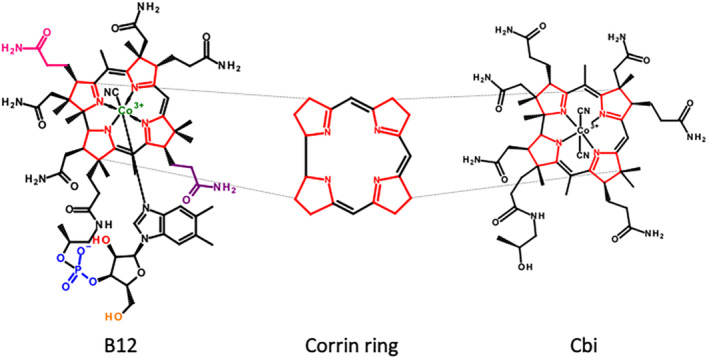
Schematic representation of vitamin B12 (B12), corrin ring (shown deprotonated) and dicyanocobinamide (Cbi). ‘Corrination’ is derived from the use of corrin ring‐containing molecules such as Cbi or B12
TABLE 1.
List of corrinated peptides tested in the context of energy balance and diabetes preclinical models
| Corrination moiety | Peptide/protein | In vivo model | Mode of administration | Key outcomes | Reference |
|---|---|---|---|---|---|
| B12 | Insulin | Rat | Oral | Prolonged hypoglycaemic activity and increased half‐life | (Petrus et al., 2007) |
| B12 | Ex4 |
Rat Mouse Shrew |
s.c., i.p. and i.c.v. | Maintained glucoregulation, no effect on food intake and body weight, and near absence of emetic events | (Borner, Shaulson, et al., 2020; Mietlicki‐Baase et al., 2018) |
| B12 | PYY3–36 | Rat | s.c. | Augmented hypophagia and weight loss | (Henry et al., 2015) |
| IF‐B12 | Ex4 | Not tested | Not applicable | Increased gut and kidney protease resistance | (Bonaccorso et al., 2015) |
| Cbi | Ex4 |
Rat Shrew |
i.p. | Improved glycaemic response in IPGTT and reduction of emetic events | (Borner, Workinger, et al., 2020) |
Abbreviations: B12, vitamin B12; Cbi, cobinamide; Ex4, exendin‐4; IF, intrinsic factor; IPGTT, intraperitoneal glucose tolerance test.
Successful conjugation strategies utilized for the improvement of GLP‐1 have focused on the use of lipidation and conjugation to antibody fragments. Dulaglutide, developed by Eli Lilly, successfully conjugated GLP‐1 (7–37) to an Fc fragment of IgG4. The use of fatty acid GLP‐1 conjugates is highlighted by liraglutide and semaglutide—compounds developed by Novo Nordisk. An additional conjugation strategy, although less explored, is corrination; the use of B12 or any of its biosynthetic corrin‐containing precursors (e.g., dicyanocobinamide [CN2Cbi]) (Figure 2), with the aim of altering pharmacology. Although exendin‐4 readily penetrates the CNS (Kastin & Akerstrom, 2003; Mietlicki‐Baase et al., 2018), less is known about the penetrance of B12 into the adult brain (Kanazawa & Herbert, 1983). Uptake of B12 into the brain is likely to be a receptor‐mediated process with megalin, a receptor capable of TC–B12 uptake, being expressed in the choroid plexus (Carro et al., 2005). Additional evidence points to the importance of the CD320 receptor, as well as the transmembrane protein, amnionless (Luder et al., 2008). Collectively, this information points to a receptor‐mediated process of B12 blood–brain barrier penetrance. It is, however, clear that in the adult brain with limited neurogenesis, uptake of B12 by the CNS is very low and considerably lower than in other organs, especially the liver and kidney (Ikotun et al., 2014; Sah et al., 2014). A recent study using radioactively labelled B12 (B12‐89Zr) reveals less than 0.1% injected dose per gram (ID·g−1) of B12 in brain in mouse models with over 5% ID·g−1 observed in pancreas (Kuda‐Wedagedara et al., 2017). Importantly, evidence collected post‐mortem from human brain and liver clearly demonstrates negligible amounts of B12 (11.3 pmol·g−1) and a ~10‐fold lower relative concentration of corrinoid‐type analogues (1.3 pmol·g−1; including Cbi) in the brain, with the liver being the main site of concentration for both B12 and corrinoid analogues (total >600 pmol·g−1) (Kanazawa & Herbert, 1983).
Overall, from a therapeutic perspective aimed at normalizing the chronic hyperglycaemia of T2DM patients, designing a GLP‐1 receptor agonist that does not penetrate readily into the CNS, but retains enhanced pharmacological action on beta cells, would theoretically provide an improved tool for glycaemic control without eliciting unwanted nausea/malaise and/or anorexia. Corrination has been utilized to deliver several bioactive and/or imaging molecules, ranging from 99mTc to insulin (see Table 1). The conjugation of peptides to corrins offers a unique delivery system of peptides through the B12 uptake pathway. Unlike other conjugation strategies, corrination increases the hydrophilicity of the peptide and thus increasing solubility, a common limitation to peptide therapeutic agents. The use of corrins for conjugation to peptides and other low MW compounds has been well explored, and their synthesis is simple and direct. As an additional benefit, corrination has also been shown to be successful at limiting proteolytic degradation of exendin‐4 (Bonaccorso et al., 2015).
2.3. In vitro and in vivo characterization of B12–exendin‐4: Do conjugates with reduced brain penetrance reduce GLP‐1‐induced nausea and vomiting?
Initial in vitro screenings demonstrated that covalent conjugation of the GLP‐1 receptor agonist exendin‐4 to B12 between the vitamin 5‐OH group and the K12 position of exendin‐4 retains potent agonism (68 pM) of the GLP‐1 receptor, either as the free conjugate or bound to the critical transport protein for B12 absorption IF (126 pM), confirming the ability of B12–exendin‐4 to interact with both systems. Results of in vivo evaluation of the effects of systemic B12–exendin‐4 and unconjugated exendin‐4 on food intake and body weight, glucose tolerance and in the development of taste avoidance (CTA, an assay of illness behaviour) in rodents showed that the B12–exendin‐4 conjugate improved glucose tolerance but did not elicit CTA and anorexia produced by unconjugated native exendin‐4 (Mietlicki‐Baase et al., 2018). Follow‐up studies also showed that the B12–exendin‐4 enhanced glucose clearance, relative to exendin‐4 and similar to liraglutide in Goto–Kakizaki (GK) rats, a lean, polygenetic model of T2DM (Goto et al., 1976; Ostenson & Efendic, 2007), but did not produce the CNS‐dependent outcomes of anorexia and body weight loss characteristic of these two compounds (Borner, Shaulson, et al., 2020). Rats treated with native exendin‐4 exhibit an acute hyperglycaemic response, a phenomenon tied to the high CNS penetrance of exendin‐4 and commonly observed across different rat models (Gao & Jusko, 2011; Perez‐Tilve et al., 2010). Conversely, B12–exendin‐4 did not produce a transient stress‐induced hyperglycaemic in lean healthy and GK diabetic rats (Borner, Shaulson, et al., 2020; Mietlicki‐Baase et al., 2018). To further evaluate whether differences in the effect profiles of B12–exendin‐4 and unconjugated exendin‐4 are the result of altered CNS penetrance, rats received systemic injections of fluorescein– exendin‐4 (Flex), Cy5–B12 or Cy5–B12–exendin‐4, and brain penetrance was evaluated using confocal microscopy. Although Flex robustly penetrates the brain (dorsal vagal complex and paraventricular nucleus of the hypothalamus), Cy5–B12 and Cy5–B12–exendin‐4 fluorescence were not observed centrally, supporting a lack of CNS penetrance into these regions of the brain, in line with observed reduction in CNS‐associated side effects of exendin‐4. However, Cy5–B12–exendin‐4 colocalized with insulin in the pancreas, suggesting direct pancreatic action as a potential mechanism underlying the hypoglycaemic effects of B12–exendin‐4 (Mietlicki‐Baase et al., 2018).
To definitively address the question of whether B12 conjugation would prevent emesis induced by exendin‐4, the musk shrew (Suncus murinus) was used to evaluate the in vivo efficacy and tolerability of B12–exendin‐4. In contrast to rodents, shrews are capable of emesis (Ueno et al., 1987) and are believed to have a nearly identical B12 binding profile in blood as humans (71% amino acid sequence identity to human HC in Tupaia chinensis [Chinese tree shrew], Accession No. XP_006147468.1). Importantly, in the context of modelling the GLP‐1 system, previous studies conducted by John Rudd and his research team amply demonstrated the ability of existing GLP‐1 receptor agonists to induce hypoglycaemia, anorexia and emesis in this model (Chan et al., 2011, 2013). In line with the rodent data, all exendin‐4 doses tested induced anorexia and body weight loss in shrews, but no such effects occurred after B12–exendin‐4, even at suprapharmacological doses (Borner, Shaulson, et al., 2020). Importantly, B12–exendin‐4 administration at ~10× the effective dose necessary for glucoregulation did not cause emesis compared with the potent emetic effects of exendin‐4 that were observed in all animals tested (Borner, Shaulson, et al., 2020). Additional evidence showing that B12–exendin‐4 induced profound emesis when administered centrally (i.e., direct CNS stimulation) further validates the thesis of an altered pharmacodynamic profile of the corrinated peptide.
2.4. The B12 precursor dicyanocobinamide (Cbi) as an alternative to B12
Cbi, a corrinated precursor of B12, has been identified in humans (Hardlei & Nexo, 2009) but has no known influence on normal B12 homeostasis because it is not recognized by the B12 blood‐transporting protein TC, critical for blood–brain barrier penetrance and cell entry via the CD320 receptor (Green et al., 2017; Luder et al., 2008). Instead, Cbi is recognized in the blood only by the B12‐binding protein HC. The function of circulating HC is unknown, and no known specific receptor for the Cbi–HC complex has been identified (Furger et al., 2012). Congenital defects in plasma HC are asymptomatic, suggesting that HC and Cbi are not physiologically relevant in humans (Rosenblatt et al., 2001). The theoretical major advantage of the Cbi–exendin‐4 construct over the B12–exendin‐4 is that by using Cbi as a pharmacodynamic/pharmacokinetic modifier of a target peptide pharmaceutical agent, one can generate an ‘inert’ carrier that does not alter B12 homeostasis. This is especially relevant for possible future applications in humans over chronic use (as it would be the case for T2DM patients), as there is a very limited, albeit possible, outcome where B12–exendin‐4 could interfere with the normal B12 physiology, potentially causing pernicious anaemia. This risk is completely avoidable by using Cbi, as it is only recognized by HC, and not by TC. A library of corrinated constructs of exendin‐4 were synthetized by introducing two specific conjugation sites (at position K12 and K40) into the exendin‐4 amino acid sequence and using various linkers with diverse chemical properties including hydrophobicity, amphiphilicity and rigidity (Borner, Workinger, et al., 2020; Tinsley et al., 2021). Two optimized conjugates were subsequently assayed ex vivo for GLP‐1 receptor‐binding in HEK‐239 cells stability transfected with hGlp1r and for insulin secretion in rat islet cells (Borner, Workinger, et al., 2020; Tinsley et al., 2021). Functional agonism screening revealed EC50 values between ~10 and 200 pM for the Cbi–exendin‐4 conjugates, thus effectively proving the successful generation of compounds with agonist activity comparable to that of than native exendin‐4. Using radio‐57Co–B12 binding assays and serum B12 protein isolation, the presence of both HC and TC in shrew blood was confirmed (Borner, Workinger, et al., 2020). Subsequent elisa‐based pharmacokinetic studies in shrews showed that Cbi– exendin‐4 exhibited reduced plasma clearance relative to native exendin‐4, most likely due to improved stability of the conjugate to proteolytic activity, as previously demonstrated for B12 conjugates (Bonaccorso et al., 2015), and/or reduced renal clearance, both key factors in determining the half‐life of exendin‐4 in vivo (Copley et al., 2006; Simonsen et al., 2006). Similar to what was performed for the B12– exendin‐4 conjugate, as a first validation in vivo, it was tested in shrews whether Cbi– exendin‐4 retained ability to reduce blood glucose following an intraperitoneal glucose tolerance test (IPGTT), across a wide dose range. At all doses tested, similar enhanced glucose clearance actions of Cbi–exendin‐4 and exendin‐4, compared with vehicle, were observed, indicative of a comparable glucoregulatory potency (Borner, Workinger, et al., 2020). It is likely that Cbi– exendin‐4 may also retain pharmacological actions for a longer duration compared with the native peptide as Cbi– exendin‐4, but not native exendin‐4, effectively suppressed plasma glucose concentrations following an IPGTT 6 h after drug administration. Extensive dose–response studies were conducted analysing the effects of Cbi– exendin‐4 on various aspects of feeding behaviour (food intake and body weight emesis) in shrews. Overall, the outcome of these studies was similar to results with B12– exendin‐4 (Borner, Workinger, et al., 2020), highlighting that Cbi can be leveraged for the generation of active corrinated compounds as shown previously for B12 constructs, which can improve glycaemic control without producing anorexia and drug treatment‐induced malaise but without the risk of causing alteration in the physiological B12 system.
By exploiting a B12 or its precursor Cbi, it is possible to generate potent and metabolically stable GLP‐1 receptor agonists via ‘corrination’, with enhanced and prolonged peripheral glucoregulatory action minimal effects on feeding and without emesis and nausea (see Figure 3). The striking difference in occurrence and severity of nausea and emesis described here is likely to be attributable to reduced penetrance of the corrinated constructs due to the presence of the highly polar nature of Cbi or B12 and the lack of an active transport mechanism into the adult brain. Consequently, this translates into a reduced activation of central GLP‐1 receptors, including those located in the AP/NTS, the activation of which is key for the induction of emesis/nausea following GLP‐1 receptor agonist treatment. Although longitudinal animal studies, as well as clinical trials, are needed to evaluate the long‐term efficacy and tolerability of the corrinated exendin‐4s, these preclinical data highlight the translational potential of the corrination technology in the context of GLP‐1 receptor agonists.
FIGURE 3.
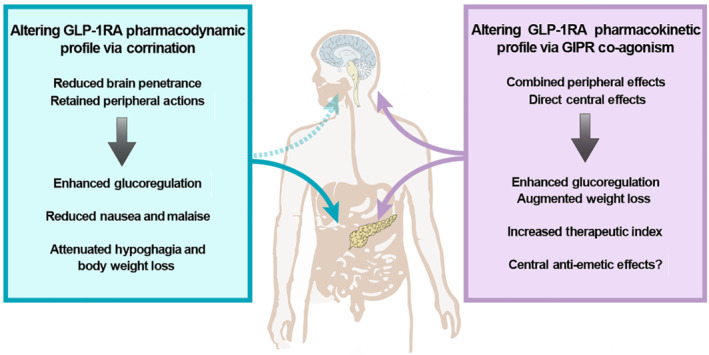
Main effects and mechanisms of vitamin B12 (B12)/cobinamide (Cbi)–GLP‐1 receptor agonist (GLP‐1RA) and GIP receptor (GIPR)/GLP‐1 receptor (GLP‐1R) strategies. Corrination reduces brain penetrance, radically changing the pharmacodynamic profile of GLP‐1 receptor agonists. Corrinated GLP‐1 receptor agonists retain a peripheral site of action when systemically administered, providing a pancreatic mechanism for GLP‐1 receptor‐mediated glycaemic control, without producing any centrally mediated illness‐like behaviours (i.e., nausea and emesis). GIPR/ GLP‐1 receptor dual agonism enhances peripheral effects on glucose handling, while simultaneously decreasing feeding and body weight via direct central actions. Such a combinatorial approach offers valuable opportunity to increase the therapeutic window/index via dose modifications. Moreover, GIP receptor agonism may also antagonize GLP‐1 receptor emetic signal(s) by engaging a yet unknown central mechanism(s) that could reduce the incidence and severity of nausea and emesis characteristic of current GLP‐1 receptor‐based approaches
3. DUAL GLP‐1 RECEPTOR AGONISTS
Dual agonism via a peptide–peptide conjugate or chimeric co‐agonism has several advantages often translating to improved clinical utility compared with the administration of individual single active peptides in combination, including a singular pharmacokinetic and pharmacodynamic profile (e.g., affecting rates of absorption, distribution and half‐life). In recent years, inspired by the early successful outcomes of GLP‐1/glucagon (Gcg) co‐treatment as a means to increase weight loss in preclinical models (Pocai et al., 2009), several peptide hybrids quickly populated the pharmacological landscape including dual receptor agonists of GLP‐1/Gcg (Ambery et al., 2018; Boland et al., 2020), GLP‐1/GLP‐2 (Wismann et al., 2018), GIP/GLP‐1 (Coskun et al., 2018; Finan et al., 2013; Frias et al., 2017, 2018, 2020) and GLP‐1 /NPY‐Y2 receptors (Chepurny et al., 2018; Milliken et al., 2021; Ostergaard et al., 2021). These dual agonists have been extensively reviewed elsewhere (Baggio & Drucker, 2020; Tschop et al., 2016). Their utility is currently being evaluated in the clinical setting, with the latter class being the most promising so far. Unfortunately, most of these studies did not include an exhaustive description of nausea and emesis side effects in humans and lack a proper in‐depth investigation of nausea and emesis in preclinical models. Additionally, groups such as a ‘classical’ GLP‐1 receptor agonist at doses matched for efficacy in body weight reduction or glucoregulatory effects were not included in many of the clinical trials. Therefore, interpretations regarding their ability to reduce nausea and emesis in humans and/or preclinical models, when matched for effect size in glucose regulation or body weight loss, are challenging.
3.1. GIP/GLP‐1 dual agonists, greater than the sum of their parts
GIP is a gut hormone released from the enteroendocrine cells in the duodenum and jejunum shortly after a meal (Baggio & Drucker, 2007). Together with GLP‐1, GIP plays an important role in orchestrating the body's response to the increase of postprandial glucose levels by augmenting insulin secretion (Dupre et al., 1973; Fehmann et al., 1995; Muller et al., 2019). GIP binds to its receptor (GIP receptor), which is widely expressed throughout the body including in several areas of the brain involved in regulating energy balance, such as the hypothalamus and the caudal hindbrain (Adriaenssens et al., 2019; Kaneko et al., 2019). Although GLP‐1 receptor agonists have been developed and employed with success for the treatment of T2DM and obesity, preclinical and clinical data regarding the potential use of GIP analogues are limited and controversial (seeFinan et al., 2016; Samms et al., 2020). Compounds targeting the GIP receptor alone were initially abandoned due to an overall weak biological effect, in part because of early findings suggesting GIP resistance in diabetes (Nauck et al., 1993) alongside incongruent results on its hypophagic and body weight‐lowering effects (Boylan et al., 2015; Finan et al., 2016; Killion et al., 2018; McClean et al., 2007; Miyawaki et al., 2002; Mroz et al., 2019). There is convincing evidence that the increase in glucose‐stimulated insulin secretion following administration of exogenously applied GIP is mediated by direct activation of GIP receptors expressed on pancreatic beta cells (Baggio & Drucker, 2007; Khan et al., 2020), but the role of GIP receptors expressed within the CNS is less clear and only a few studies have investigated the central actions of GIP ligands on feeding behaviours (Ambati et al., 2011; Kaneko et al., 2019; NamKoong et al., 2017; Zhang, Delessa, et al., 2021). Recent studies using long‐acting GLP‐1/GIP receptor dual agonists, however, have yielded promising results in preclinical models and clinical trials, providing greater body weight loss and better glycaemic control than GLP‐1 receptor agonists alone (Coskun et al., 2018; Frias et al., 2017; Frias et al., 2018; Killion et al., 2018; Norregaard et al., 2018).
The first characterization of a dual GIP/GLP‐1 receptor agonist was reported in 2013 via landmark publications in multiple preclinical models and human subjects (Finan et al., 2013; Frias et al., 2017). Using an approach that leveraged the high degree of homology between GIP and GLP‐1 to generate hybridized GLP‐1/GIP mono‐molecules, amino acids from native GLP‐1 and GIP were introduced into the native glucagon sequence and the resulting peptides were screened for activity at the GLP‐1 and GIP receptors (Finan et al., 2013). The final, unimolecular balanced dual agonist (NNC0090‐2746/RG7697) was further modified to prevent DPP‐4 degradation and to extend solubility (Finan et al., 2013). In two mouse models for T2DM (i.e., diet‐induced obese and leptin‐deficient mice), daily administration of this dual agonist reduced body weight, hyperglycaemia and dyslipidaemia (Finan et al., 2013). In cynomolgus monkeys, GIP/GLP‐1 co‐agonism was shown to be superior in reducing blood glucose levels and increasing plasma insulin, compared with equimolar doses of liraglutide (Finan et al., 2013). The dual agonist also decreased plasma insulin and decreased blood glucose during a glucose infusion challenge, more effectively than liraglutide, in healthy, nondiabetic human subjects. Collectively, these data highlight the translational potential of this class of drugs. Importantly, there was a reported reduction in the incidence of gastric‐related adverse events for the co‐agonist as compared with GLP‐1 mono‐agonist treatment (Finan et al., 2013). In subsequent clinical trials conducted by the same research team in healthy and T2DM patients, the same peptide and further optimized versions demonstrated high tolerability, dose‐dependent reductions in fasting and postprandial plasma glucose, and reduced body weight, total cholesterol and leptin levels, relative to placebo controls (Frias et al., 2017). Together, these data clearly support the beneficial effects of targeting both incretin systems to provide enhanced effects, as well as to offer valuable opportunity of increasing the therapeutic window or index, via dose modifications (Finan et al., 2016; Samms et al., 2020) (see Figure 3).
3.2. Central GIP receptor activation: Anti‐emetic counteraction of GLP‐1‐induced emesis?
Activation of GIP receptors may have surprising anti‐emetic effects, as recently described in a patent application filed by the pharmaceutical company Takeda (Asami et al., 2018). In these studies, GIP receptor agonists were capable of reducing CTA and emetic responses that usually occur following administration of the gut peptide PYY and cisplatin, in ferrets and beagles (Asami et al., 2018).
One can therefore speculate that GIP could not only potentiate the peripheral effects of GLP‐1 receptor agonists on glucose handling but also antagonize GLP‐1 receptor emetic signal(s) by engaging a yet unknown central mechanism(s) that ultimately reduces the incidence and severity of nausea and emesis characteristic of current GLP‐1 receptor agonist‐based approaches. The mechanism(s) and the circuitry engaged by GIP, in particular those underlying its anti‐emetic actions, are lacking, despite recent work describing the phenotype of GIP receptor‐expressing neurons in the various CNS nuclei involved in the control of energy homeostasis, including the AP and NTS (Adriaenssens et al., 2019; Ludwig et al., 2021; Zhang, Kaye, et al., 2021). In one of these studies, Zhang, Kaye, et al. (2021) generated the full transcriptome profile of each individual AP neuron via single‐nuclei RNA sequencing of murine AP tissue. Intriguingly, a significant portion of the neurons expressing Gad1‐ and Gad2, genes that encode for enzymes responsible for GABA synthesis, also expressed Gipr, whereas only a few neurons expressed Glp1r and Gipr (Zhang, Kaye, et al., 2021). These data suggest the presence of two unique and very distinct neuronal circuitries within the AP and highlight the hypothesis of a local inhibitory network within the caudal hindbrain that could be exploited via activation of GIP receptors to reduce nausea and emesis mediated by hindbrain GLP‐1 receptors. More exhaustive studies are required to further investigate the applications of this promising, yet understudied system.
Several GIP/GLP‐1 receptor dual agonists have been or are currently being investigated in the clinical setting (see Bastin & Andreelli, 2019, for review) with none currently approved by the FDA. Tirzepatide (originally named LY3298176), a dual ‘sequence‐mixed’ GIP and GLP‐1 receptor agonist under development by Eli Lilly, Inc. for the treatment of T2DM, obesity and non‐alcoholic steatohepatitis was designed to increase the half‐life and resist enzymatic degradation to allow for once‐weekly administration (Frias, 2020; Hartman et al., 2020). In addition to an Arg to Lys substitution to protect from DPP‐4 degradation, its 39‐amino acid structure contains a C20 unsaturated di‐acid acyl side chain for enhanced albumin binding, further extending the peptide half‐life to approximately 5 days (Coskun et al., 2018). Preclinical evidence showed glucose‐dependent insulin secretion and improved glucose tolerance by acting on both GIP and GLP‐1 receptors in mice (Coskun et al., 2018). Chronic tirzepatide treatment in a mouse model of diet‐induced obesity, potently suppressed feeding, body weight and food intake with significantly greater effect than the GLP‐1 receptor agonist dulaglutide (Coskun et al., 2018). Early phase trials in T2DM patients indicate that tirzepatide improves clinical outcomes beyond those achieved by a selective GLP‐1 receptor agonist (Coskun et al., 2018). Phase II clinical investigations of tirzepatide actions in T2DM patients showed high efficacy for glucose handling and body weight reduction with approximately one third of patients receiving the 15‐mg dose attaining normoglycaemia (defined as HbA1C < 5.7%) and one fourth of the subjects losing ≥15% of their initial body weight after only 6 months (Frias et al., 2018). In addition, tirzepatide treatment reduced fasting glucose, insulin and triglyceride concentrations and improved insulin sensitivity. Importantly, tirzepatide showed significantly greater efficacy with regard to glucose control and weight loss than did monotherapy with a GLP‐1 receptor agonist (i.e., dulaglutide), with an acceptable safety and tolerability profile (Coskun et al., 2018;Frias et al., 2018, 2020). Several large‐scale Phase 3 clinical trials in T2DM (SURPASS) and obese (SURMOUNT) patients are currently ongoing (Frias, 2020; Min & Bain, 2021), with interim positive/promising results in line with the outcome of previous studies. This general metabolic improvement further underlines the beneficial contributions of GIPR activation to GLP‐1 agonist therapy.
Nausea and emesis were still present at a relatively high rate in tirzepatide‐treated T2DM patients (Frias et al., 2018, 2020), despite initial Phase 1 studies suggesting a potential improved tolerance and a greater therapeutic index compared with classical GLP‐1 receptor‐based monotherapies (Coskun et al., 2018). The incidence of GI events was dose‐related with most events being transient and categorized as mild to moderate in intensity. Nevertheless, it is surprising and contra‐intuitive to the ‘GIP anti‐emetic hypothesis’, as well as being in contrast with preclinical GIPR studies, in the context of emesis and malaise (Asami et al., 2018). Despite having an acceptable safety and tolerability profile, comparable with what is seen with GLP‐1 agonist monotherapies such as dulaglutide, GI events (e.g., nausea and vomiting) were the most common adverse events related to tirzepatide treatment. One possible explanation could be that the dose range and/or the administration regime employed were suboptimal. Another possible explanation could lie in the pharmacodynamic profile of tirzepatide compared with the individual profile of the two single separate components. A recent study demonstrated that tirzepatide possesses an imbalanced agonism favouring GIP receptors over GLP‐1 receptors (Willard et al., 2020). Additionally, at the GLP‐1 receptor, its action is biased in favour of cAMP generation over β‐arrestin recruitment, coincident with a weaker ability to drive GLP‐1 receptor internalization, compared with native GLP‐1 (Willard et al., 2020). Such experiments were conducted ex vivo in primary islets. Whether similar effects also occur in the CNS and whether they contribute to an enhanced ‘emetic’ signalling via GLP‐1 receptor activation or a reduction in the GIP receptor ‘anti‐emetic’ activity, thus cancelling the anti‐emetic effects of GIP receptor signalling in the hindbrain, still need to be elucidated.
4. GENERAL CONCLUSIONS AND OUTLOOK
After their introduction less than two decades ago, GLP‐1 receptor agonists reshaped the field of diabetes care. Pharmacological side effects such as nausea and emesis have often been downplayed or dismissed, left in the shadow of the overriding goal of glucoregulation and weight loss. In recent years, differently designed strategies were employed that led, to some extent, an improved tolerability of GLP‐1 receptor agonists. The data summarized here show high translational potential of new GLP‐1‐based approaches that will hopefully further reduce the incidence and severity of nausea and emesis, thus increasing the therapeutic efficacy, decreasing treatment attrition and extending their use to a broader population of T2DM patients (see Figure 3). In conclusion, these emerging findings demonstrate that it is possible and technically achievable to both physiologically and pharmacologically separate the emetic effects of GLP‐1 receptor agonists from their glucoregulatory actions.
The use of corrination combined with peptides that offer polypharmacy offers great scope for development. Combining B12 or Cbi with dual agonists of GLP‐1/GIP receptors or GLP‐1/NPY‐Y2 receptors is currently ongoing. In addition, the use of corrination to solubilize and/or stabilize challenging peptides such as glucagon or dual agonists integrating glucagon with GLP‐1 receptor agonists might solve issues with clinical development related to such problems and thus allowing the exploitation of exciting pharmacodynamic outcomes. Extensive preclinical and clinical studies investigating the effects of ‘corrinated’ GLP‐1 analogues, as well as GLP‐1/GIP dual agonist(s), are required to fully evaluate the effects of these drugs in these medical conditions, and we will soon have insights into how to broaden the pharmacopoeia of GLP‐1 receptor‐based therapies.
4.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in the IUPHAR/BPS Guide to PHARMACOLOGY (http://www.guidetopharmacology.org) and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Christopoulos, et al., 2019; Alexander, Fabbro et al., 2019; Alexander, Kelly, et al., 2019).
CONFLICT OF INTEREST
T.B. and I.C.T. have no conflicts to declare. R.P.D. is an inventor of patents related to the B12/Cbi–Ex4 technology. B.C.D.J. receives research funding from Eli Lilly and Company and Pfizer Inc. and provided remunerated consultancy services for Pfizer Inc. not supporting these studies. R.P.D. is a scientific advisory board member and received funds from Xeragenx LLC (St. Louis, NY) and Balchem, New Hampton, New York, that were not used in support of these studies. M.R.H. receives research funding from Eli Lilly and Company and Boehringer Ingelheim that was not used in support of these studies.
5.
ACKNOWLEDGEMENTS
This work was supported by the Swiss National Science Foundation (Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung; Grant SNF P400PB 186728) (T.B.) and by the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases; Grants DK097675 [R.P.D.], DK112812 [B.C.D.J.], DK097675 [M.R.H.], DK115762 [M.R.H.] and DK128443 [M.R.H., R.P.D. and B.C.D.J.]).
Borner, T. , Tinsley, I. C. , Doyle, R. P. , Hayes, M. R. , & De Jonghe, B. C. (2022). Glucagon‐like peptide‐1 in diabetes care: Can glycaemic control be achieved without nausea and vomiting? British Journal of Pharmacology, 179(4), 542–556. 10.1111/bph.15647
Funding information National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Numbers: DK097675, DK112812, DK115762, DK128443; Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung, Grant/Award Number: P400PB 186728
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article because no new data were created or analysed in this study.
REFERENCES
- Aapro, M. (2018). CINV: Still troubling patients after all these years. Support Care Cancer, 26, 5–9. 10.1007/s00520-018-4131-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adriaenssens, A. E. , Biggs, E. K. , Darwish, T. , Tadross, J. , Sukthankar, T. , Girish, M. , Polex‐Wolf, J. , Lam, B. Y. , Zvetkova, I. , Pan, W. , & Chiarugi, D. (2019). Glucose‐dependent insulinotropic polypeptide receptor‐expressing cells in the hypothalamus regulate food intake. Cell Metabolism, 30(987–996), e986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahren, B. , Atkin, S. L. , Charpentier, G. , Warren, M. L. , Wilding, J. P. H. , Birch, S. , Holst, A. G. , & Leiter, L. A. (2018). Semaglutide induces weight loss in subjects with type 2 diabetes regardless of baseline BMI or gastrointestinal adverse events in the SUSTAIN 1 to 5 trials. Diabetes, Obesity & Metabolism, 20, 2210–2219. 10.1111/dom.13353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , & Pawson, A. J. (2019). The Concise Guide to PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176(Suppl 1), S21–S141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Sharman, J. L. , Southan, C. , Davies, J. A. , CGTP Collaborators , Beuve, A. , Boison, D. , Brouckaert, P. , Burnett, J. C. , Burns, K. , … Watts, V. (2019). The Concise Guide to PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176, S297–S396. 10.1111/bph.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Sharman, J. L. , Southan, C. , Davies, J. A. , CGTP Collaborators , Anderson, C. M. H. , Bröer, S. , Dawson, P. , Hagenbuch, B. , Hammond, J. R. , Hancox, J. , … Verri, T. (2019). The Concise Guide to PHARMACOLOGY 2019/20: Transporters. British Journal of Pharmacology, 176, S397–S493. 10.1111/bph.14753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhadeff, A. L. , Holland, R. A. , Zheng, H. , Rinaman, L. , Grill, H. J. , & De Jonghe, B. C. (2017). Excitatory hindbrain–forebrain communication is required for cisplatin‐induced anorexia and weight loss. The Journal of Neuroscience, 37, 362–370. 10.1523/JNEUROSCI.2714-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhadeff, A. L. , Mergler, B. D. , Zimmer, D. J. , Turner, C. A. , Reiner, D. J. , Schmidt, H. D. , Grill, H. J. , & Hayes, M. R. (2016). Endogenous glucagon‐like peptide‐1 receptor signaling in the nucleus tractus solitarius is required for food intake control. Neuropsychopharmacology, 42(7), 1471–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, R. H. , Seetharam, B. , Podell, E. , & Alpers, D. H. (1978). Effect of proteolytic enzymes on the binding of cobalamin to R protein and intrinsic factor: In vitro evidence that a failure to partially degrader protein is responsible for cobalamin malabsorption in pancreatic insufficiency. Journal of Clinical Investigation, 61, 47–54. 10.1172/JCI108924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambati, S. , Duan, J. , Hartzell, D. L. , Choi, Y. H. , Della‐Fera, M. A. , & Baile, C. A. (2011). GIP‐dependent expression of hypothalamic genes. Physiological Research, 60, 941–950. 10.33549/physiolres.932151 [DOI] [PubMed] [Google Scholar]
- Ambery, P. , Parker, V. E. , Stumvoll, M. , Posch, M. G. , Heise, T. , Plum‐Moerschel, L. , Tsai, L. F. , Robertson, D. , Jain, M. , Petrone, M. , Rondinone, C. , Hirshberg, B. , & Jermutus, L. (2018). MEDI0382, a GLP‐1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: A randomised, controlled, double‐blind, ascending dose and phase 2a study. Lancet, 391, 2607–2618. 10.1016/S0140-6736(18)30726-8 [DOI] [PubMed] [Google Scholar]
- American Diabetes Association . (2020). 9. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes‐2020. Diabetes Care, 43, S98–S110. 10.2337/dc20-S009 [DOI] [PubMed] [Google Scholar]
- Asami, T. , Nishizawa, N. , Niida, A. , Kanematsu, Y. , Adachi, M. , Takekawa, S. , & Morimoto, T. (2018). Gip receptor activating peptide (WO2018181864A1). Takeda Pharmaceutical Company Limited. [Google Scholar]
- Avgerinos, I. , Michailidis, T. , Liakos, A. , Karagiannis, T. , Matthews, D. R. , Tsapas, A. , & Bekiari, E. (2020). Oral semaglutide for type 2 diabetes: A systematic review and meta‐analysis. Diabetes, Obesity & Metabolism, 22, 335–345. 10.1111/dom.13899 [DOI] [PubMed] [Google Scholar]
- Babic, T. , & Browning, K. N. (2014). The role of vagal neurocircuits in the regulation of nausea and vomiting. European Journal of Pharmacology, 722, 38–47. 10.1016/j.ejphar.2013.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio, L. L. , & Drucker, D. J. (2007). Biology of incretins: GLP‐1 and GIP. Gastroenterology, 132, 2131–2157. 10.1053/j.gastro.2007.03.054 [DOI] [PubMed] [Google Scholar]
- Baggio, L. L. , & Drucker, D. J. (2020). Glucagon‐like peptide‐1 receptor co‐agonists for the treatment of metabolic disease. Molecular Metabolism, 46, 101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, P. D. , Morzorati, S. L. , & Ellett, M. L. (2005). The pathophysiology of chemotherapy‐induced nausea and vomiting. Gastroenterology Nursing, 28, 469–480. 10.1097/00001610-200511000-00003 [DOI] [PubMed] [Google Scholar]
- Baraboi, E. D. , St‐Pierre, D. H. , Shooner, J. , Timofeeva, E. , & Richard, D. (2011). Brain activation following peripheral administration of the GLP‐1 receptor agonist exendin‐4. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 301, R1011–R1024. 10.1152/ajpregu.00424.2010 [DOI] [PubMed] [Google Scholar]
- Barrera, J. G. , Jones, K. R. , Herman, J. P. , D'Alessio, D. A. , Woods, S. C. , & Seeley, R. J. (2011). Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon‐like peptide‐1 loss of function. The Journal of Neuroscience, 31, 3904–3913. 10.1523/JNEUROSCI.2212-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastin, M. , & Andreelli, F. (2019). Dual GIP–GLP1‐receptor agonists in the treatment of type 2 diabetes: A short review on emerging data and therapeutic potential. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy, 12, 1973–1985. 10.2147/DMSO.S191438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergenstal, R. M. , Wysham, C. , Macconell, L. , Malloy, J. , Walsh, B. , Yan, P. , Wilhelm, K. , Malone, J. , Porter, L. E. , & DURATION‐2 Study Group . (2010). Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION‐2): A randomised trial. Lancet, 376, 431–439. 10.1016/S0140-6736(10)60590-9 [DOI] [PubMed] [Google Scholar]
- Bettge, K. , Kahle, M. , Abd El Aziz, M. S. , Meier, J. J. , & Nauck, M. A. (2017). Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon‐like peptide‐1 receptor agonists: A systematic analysis of published clinical trials. Diabetes, Obesity & Metabolism, 19, 336–347. 10.1111/dom.12824 [DOI] [PubMed] [Google Scholar]
- Birn, H. , Verroust, P. J. , Nexø, E. , Hager, H. , Jacobsen, C. , Christensen, E. I. , & Moestrup, S. K. (1997). Characterization of an epithelial ∼460‐kDa protein that facilitates endocytosis of intrinsic factor‐vitamin B12 and binds receptor‐associated protein. Journal of Biological Chemistry, 272, 26497–26504. 10.1074/jbc.272.42.26497 [DOI] [PubMed] [Google Scholar]
- Boland, M. L. , Laker, R. C. , Mather, K. , Nawrocki, A. , Oldham, S. , Boland, B. B. , Lewis, H. , Conway, J. , Naylor, J. , Guionaud, S. , Feigh, M. , Veidal, S. S. , Lantier, L. , McGuinness, O. P. , Grimsby, J. , Rondinone, C. M. , Jermutus, L. , Larsen, M. R. , Trevaskis, J. L. , & Rhodes, C. J. (2020). Resolution of NASH and hepatic fibrosis by the GLP‐1R and GcgR dual‐agonist cotadutide via modulating mitochondrial function and lipogenesis. Nature Metabolism, 2, 413–431. 10.1038/s42255-020-0209-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaccorso, R. L. , Chepurny, O. G. , Becker‐Pauly, C. , Holz, G. G. , & Doyle, R. P. (2015). Enhanced peptide stability against protease digestion induced by intrinsic factor binding of a vitamin B12 conjugate of exendin‐4. Molecular Pharmaceutics, 12, 3502–3506. 10.1021/acs.molpharmaceut.5b00390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner, T. , Shaulson, E. D. , Tinsley, I. C. , Stein, L. M. , Horn, C. C. , Hayes, M. R. , Doyle, R. P. , & De Jonghe, B. C. (2020). A second‐generation glucagon‐like peptide‐1 receptor agonist mitigates vomiting and anorexia while retaining glucoregulatory potency in lean diabetic and emetic mammalian models. Diabetes, Obesity & Metabolism, 22, 1729–1741. 10.1111/dom.14089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner, T. , Workinger, J. L. , Tinsley, I. C. , Fortin, S. M. , Stein, L. M. , Chepurny, O. G. , Holz, G. G. , Wierzba, A. J. , Gryko, D. , Nexø, E. , Shaulson, E. D. , Bamezai, A. , da Silva, V. A. R. , De Jonghe, B. C. , Hayes, M. R. , & Doyle, R. P. (2020). Corrination of a GLP‐1 receptor agonist for glycemic control without emesis. Cell Reports, 31, 107768. 10.1016/j.celrep.2020.107768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan, M. O. , Glazebrook, P. A. , Tatalovic, M. , & Wolfe, M. M. (2015). Gastric inhibitory polypeptide immunoneutralization attenuates development of obesity in mice. American Journal of Physiology. Endocrinology and Metabolism, 309, E1008–E1018. 10.1152/ajpendo.00345.2015 [DOI] [PubMed] [Google Scholar]
- Buse, J. B. , Henry, R. R. , Han, J. , Kim, D. D. , Fineman, M. S. , & Baron, A. D. (2004). Effects of exenatide (exendin‐4) on glycemic control over 30 weeks in sulfonylurea‐treated patients with type 2 diabetes. Diabetes Care, 27, 2628–2635. 10.2337/diacare.27.11.2628 [DOI] [PubMed] [Google Scholar]
- Buse, J. B. , Wexler, D. J. , Tsapas, A. , Rossing, P. , Mingrone, G. , Mathieu, C. , D'Alessio, D. A. , & Davies, M. J. (2020). 2019 update to: Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care, 43, 487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capehorn, M. S. , Catarig, A. M. , Furberg, J. K. , Janez, A. , Price, H. C. , Tadayon, S. , Vergès, B. , & Marre, M. (2020). Efficacy and safety of once‐weekly semaglutide 1.0 mg vs once‐daily liraglutide 1.2 mg as add‐on to 1–3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes & Metabolism, 46, 100–109. 10.1016/j.diabet.2019.101117 [DOI] [PubMed] [Google Scholar]
- Carro, E. , Spuch, C. , Trejo, J. L. , Antequera, D. , & Torres‐Aleman, I. (2005). Choroid plexus megalin is involved in neuroprotection by serum insulin‐like growth factor I. The Journal of Neuroscience, 25, 10884–10893. 10.1523/JNEUROSCI.2909-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, S. W. , Lin, G. , Yew, D. T. , & Rudd, J. A. (2011). A physiological role of glucagon‐like peptide‐1 receptors in the central nervous system of Suncus murinus (house musk shrew). European Journal of Pharmacology, 668, 340–346. 10.1016/j.ejphar.2011.06.036 [DOI] [PubMed] [Google Scholar]
- Chan, S. W. , Lin, G. , Yew, D. T. , Yeung, C. K. , & Rudd, J. A. (2013). Separation of emetic and anorexic responses of exendin‐4, a GLP‐1 receptor agonist in Suncus murinus (house musk shrew). Neuropharmacology, 70, 141–147. 10.1016/j.neuropharm.2013.01.013 [DOI] [PubMed] [Google Scholar]
- Chepurny, O. G. , Bonaccorso, R. L. , Leech, C. A. , Wollert, T. , Langford, G. M. , Schwede, F. , Roth, C. L. , Doyle, R. P. , & Holz, G. G. (2018). Chimeric peptide EP45 as a dual agonist at GLP‐1 and NPY2R receptors. Scientific Reports, 8, 3749. 10.1038/s41598-018-22106-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clardy‐James, S. , Chepurny, O. G. , Leech, C. A. , Holz, G. G. , & Doyle, R. P. (2013). Synthesis, characterization and pharmacodynamics of vitamin‐B12‐conjugated glucagon‐like peptide‐1. ChemMedChem, 8, 582–586. 10.1002/cmdc.201200461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copley, K. , McCowen, K. , Hiles, R. , Nielsen, L. L. , Young, A. , & Parkes, D. G. (2006). Investigation of exenatide elimination and its in vivo and in vitro degradation. Current Drug Metabolism, 7, 367–374. 10.2174/138920006776873490 [DOI] [PubMed] [Google Scholar]
- Coskun, T. , Sloop, K. W. , Loghin, C. , Alsina‐Fernandez, J. , Urva, S. , Bokvist, K. B. , Cui, X. , Briere, D. A. , Cabrera, O. , Roell, W. C. , Kuchibhotla, U. , Moyers, J. S. , Benson, C. T. , Gimeno, R. E. , D'Alessio, D. A. , & Haupt, A. (2018). LY3298176, a novel dual GIP and GLP‐1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept. Molecular Metabolism, 18, 3–14. 10.1016/j.molmet.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, M. B. , Bate, G. , & Kirkpatrick, P. (2005). Exenatide. Nature Reviews. Drug Discovery, 4, 713–714. 10.1038/nrd1828 [DOI] [PubMed] [Google Scholar]
- De Jonghe, B. C. , & Horn, C. C. (2009). Chemotherapy agent cisplatin induces 48‐h Fos expression in the brain of a vomiting species, the house musk shrew (Suncus murinus). American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 296, R902–R911. 10.1152/ajpregu.90952.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker, D. J. (2018). Mechanisms of action and therapeutic application of glucagon‐like peptide‐1. Cell Metabolism, 27, 740–756. 10.1016/j.cmet.2018.03.001 [DOI] [PubMed] [Google Scholar]
- Drucker, D. J. , Habener, J. F. , & Holst, J. J. (2017). Discovery, characterization, and clinical development of the glucagon‐like peptides. The Journal of Clinical Investigation, 127, 4217–4227. 10.1172/JCI97233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy, J. L. , Taylor, R. G. , & Fuller, P. J. (1998). Tissue distribution of rat glucagon receptor and GLP‐1 receptor gene expression. Molecular and Cellular Endocrinology, 141, 179–186. 10.1016/S0303-7207(98)00096-3 [DOI] [PubMed] [Google Scholar]
- Dupre, J. , Ross, S. A. , Watson, D. , & Brown, J. C. (1973). Stimulation of insulin secretion by gastric inhibitory polypeptide in man. The Journal of Clinical Endocrinology and Metabolism, 37, 826–828. 10.1210/jcem-37-5-826 [DOI] [PubMed] [Google Scholar]
- Eissele, R. , Goke, R. , Willemer, S. , Harthus, H. P. , Vermeer, H. , Arnold, R. , & Göke, B. (1992). Glucagon‐like peptide‐1 cells in the gastrointestinal tract and pancreas of rat, pig and man. European Journal of Clinical Investigation, 22, 283–291. 10.1111/j.1365-2362.1992.tb01464.x [DOI] [PubMed] [Google Scholar]
- Eng, J. , Kleinman, W. A. , Singh, L. , Singh, G. , & Raufman, J. P. (1992). Isolation and characterization of exendin‐4, an exendin‐3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. The Journal of Biological Chemistry, 267, 7402–7405. [PubMed] [Google Scholar]
- Fazen, C. H. , Valentin, D. , Fairchild, T. J. , & Doyle, R. P. (2011). Oral delivery of the appetite suppressing peptide hPYY(3–36) through the vitamin B12 uptake pathway. Journal of Medicinal Chemistry, 54, 8707–8711. 10.1021/jm2012547 [DOI] [PubMed] [Google Scholar]
- Fedosov, S. N. , Grissom, C. B. , Fedosova, N. U. , Moestrup, S. K. , Nexo, E. , & Petersen, T. E. (2006). Application of a fluorescent cobalamin analogue for analysis of the binding kinetics. A study employing recombinant human transcobalamin and intrinsic factor. The FEBS Journal, 273, 4742–4753. 10.1111/j.1742-4658.2006.05478.x [DOI] [PubMed] [Google Scholar]
- Fehmann, H. C. , Goke, R. , & Goke, B. (1995). Cell and molecular biology of the incretin hormones glucagon‐like peptide‐I and glucose‐dependent insulin releasing polypeptide. Endocrine Reviews, 16, 390–410. 10.1210/edrv-16-3-390 [DOI] [PubMed] [Google Scholar]
- Filippatos, T. D. , Panagiotopoulou, T. V. , & Elisaf, M. S. (2014). Adverse effects of GLP‐1 receptor agonists. The Review of Diabetic Studies, 11, 202–230. 10.1900/RDS.2014.11.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan, B. , Ma, T. , Ottaway, N. , Muller, T. D. , Habegger, K. M. , Heppner, K. M. , Kirchner, H. , Holland, J. , Hembree, J. , Raver, C. , & Lockie, S. H. (2013). Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Science Translational Medicine, 5, 209ra151. [DOI] [PubMed] [Google Scholar]
- Finan, B. , Muller, T. D. , Clemmensen, C. , Perez‐Tilve, D. , DiMarchi, R. D. , & Tschop, M. H. (2016). Reappraisal of GIP pharmacology for metabolic diseases. Trends in Molecular Medicine, 22, 359–376. 10.1016/j.molmed.2016.03.005 [DOI] [PubMed] [Google Scholar]
- Frias, J. P. (2020). Tirzepatide: A glucose‐dependent insulinotropic polypeptide (GIP) and glucagon‐like peptide‐1 (GLP‐1) dual agonist in development for the treatment of type 2 diabetes. Expert Review of Endocrinology and Metabolism, 15, 379–394. 10.1080/17446651.2020.1830759 [DOI] [PubMed] [Google Scholar]
- Frias, J. P. , Bastyr, E. J. 3rd , Vignati, L. , Tschop, M. H. , Schmitt, C. , Owen, K. , Christensen, R. H. , & DiMarchi (2017). The sustained effects of a dual GIP/GLP‐1 receptor agonist, NNC0090‐2746, in patients with type 2 diabetes. Cell Metabolism, 26(343–352), e342. [DOI] [PubMed] [Google Scholar]
- Frias, J. P. , Nauck, M. A. , Van, J. , Benson, C. , Bray, R. , Cui, X. , Milicevic, Z. , Urva, S. , Haupt, A. , & Robins, D. A. (2020). Efficacy and tolerability of tirzepatide, a dual glucose‐dependent insulinotropic peptide and glucagon‐like peptide‐1 receptor agonist in patients with type 2 diabetes: A 12‐week, randomized, double‐blind, placebo‐controlled study to evaluate different dose‐escalation regimens. Diabetes, Obesity & Metabolism, 22, 938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias, J. P. , Nauck, M. A. , Van, J. , Kutner, M. E. , Cui, X. , Benson, C. , Urva, S. , Gimeno, R. E. , Milicevic, Z. , Robins, D. , & Haupt, A. (2018). Efficacy and safety of LY3298176, a novel dual GIP and GLP‐1 receptor agonist, in patients with type 2 diabetes: A randomised, placebo‐controlled and active comparator‐controlled phase 2 trial. Lancet, 392, 2180–2193. 10.1016/S0140-6736(18)32260-8 [DOI] [PubMed] [Google Scholar]
- Furger, E. , Fedosov, S. N. , Lildballe, D. L. , Waibel, R. , Schibli, R. , Nexo, E. , & Fischer, E. (2012). Comparison of recombinant human haptocorrin expressed in human embryonic kidney cells and native haptocorrin. PLoS ONE, 7, e37421. 10.1371/journal.pone.0037421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe, J. C. , Madsen, M. , Højrup, P. , Christensen, E. I. , Tanner, S. M. , de la Chapelle, A. , He, Q. , & Moestrup, S. K. (2004). The functional cobalamin (vitamin B12)–intrinsic factor receptor is a novel complex of cubilin and amnionless. Blood, 103, 1573–1579. [DOI] [PubMed] [Google Scholar]
- Gabery, S. , Salinas, C. G. , Paulsen, S. J. , Ahnfelt‐Ronne, J. , Alanentalo, T. , Baquero, A. F. , Buckley, S. T. , Farkas, E. , Fekete, C. , Frederiksen, K. S. , & Hogendorf, W. F. (2020). Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, W. , & Jusko, W. J. (2011). Pharmacokinetic and pharmacodynamic modeling of exendin‐4 in type 2 diabetic Goto‐Kakizaki rats. The Journal of Pharmacology and Experimental Therapeutics, 336, 881–890. 10.1124/jpet.110.175752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, M. M. , Hu, C. , Chokshi, H. , Hewitt, J. E. , & Alpers, D. H. (1991). Glycosylation is not required for ligand or receptor binding by expressed rat intrinsic factor. American Journal of Physiology. Gastrointestinal and Liver Physiology, 260, G736–G742. [DOI] [PubMed] [Google Scholar]
- Goto, Y. , Kakizaki, M. , & Masaki, N. (1976). Production of spontaneous diabetic rats by repetition of selective breeding. The Tohoku Journal of Experimental Medicine, 119, 85–90. 10.1620/tjem.119.85 [DOI] [PubMed] [Google Scholar]
- Green, R. , Allen, L. H. , Bjorke‐Monsen, A. L. , Brito, A. , Gueant, J. L. , Miller, J. W. , Molloy, A. M. , Nexo, E. , Stabler, S. , Toh, B. H. , & Ueland, P. M. (2017). Vitamin B12 deficiency. Nature Reviews. Disease Primers, 3, 17040. 10.1038/nrdp.2017.40 [DOI] [PubMed] [Google Scholar]
- Grill, H. J. (2020). A role for GLP‐1 in treating hyperphagia and obesity. Endocrinology, 161. 10.1210/endocr/bqaa093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill, H. J. , & Hayes, M. R. (2012). Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metabolism, 16, 296–309. 10.1016/j.cmet.2012.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardlei, T. F. , & Nexo, E. (2009). A new principle for measurement of cobalamin and corrinoids, used for studies of cobalamin analogs on serum haptocorrin. Clinical Chemistry, 55, 1002–1010. 10.1373/clinchem.2008.114132 [DOI] [PubMed] [Google Scholar]
- Hartman, M. L. , Sanyal, A. J. , Loomba, R. , Wilson, J. M. , Nikooienejad, A. , Bray, R. , Karanikas, C. A. , Duffin, K. L. , Robins, D. A. , & Haupt, A. (2020). Effects of novel dual GIP and GLP‐1 receptor agonist tirzepatide on biomarkers of nonalcoholic steatohepatitis in patients with type 2 diabetes. Diabetes Care, 43, 1352–1355. 10.2337/dc19-1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, M. R. , De Jonghe, B. C. , & Kanoski, S. E. (2010). Role of the glucagon‐like‐peptide‐1 receptor in the control of energy balance. Physiology & Behavior, 100, 503–510. 10.1016/j.physbeh.2010.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry, K. E. , Elfers, C. T. , Burke, R. M. , Chepurny, O. G. , Holz, G. G. , Blevins, J. E. , Roth, C. L. , & Doyle, R. P. (2015). Vitamin B12 conjugation of peptide‐YY3–36 decreases food intake compared to native peptide‐YY3–36 upon subcutaneous administration in male rats. Endocrinology, 156, 1739–1749. 10.1210/en.2014-1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesketh, P. J. (2008). Chemotherapy‐induced nausea and vomiting. The New England Journal of Medicine, 358, 2482–2494. 10.1056/NEJMra0706547 [DOI] [PubMed] [Google Scholar]
- Holst, J. J. (2007). The physiology of glucagon‐like peptide 1. Physiological Reviews, 87, 1409–1439. 10.1152/physrev.00034.2006 [DOI] [PubMed] [Google Scholar]
- Holst, J. J. (2019). From the incretin concept and the discovery of GLP‐1 to today's diabetes therapy. Frontiers in Endocrinology, 10, 260. 10.3389/fendo.2019.00260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn, C. C. (2014). Measuring the nausea‐to‐emesis continuum in non‐human animals: Refocusing on gastrointestinal vagal signaling. Experimental Brain Research, 232, 2471–2481. 10.1007/s00221-014-3985-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikotun, O. F. , Marquez, B. V. , Fazen, C. H. , Kahkoska, A. R. , Doyle, R. P. , & Lapi, S. E. (2014). Investigation of a vitamin B12 conjugate as a PET imaging probe. ChemMedChem, 9, 1244–1251. 10.1002/cmdc.201400048 [DOI] [PubMed] [Google Scholar]
- Jendle, J. , Grunberger, G. , Blevins, T. , Giorgino, F. , Hietpas, R. T. , & Botros, F. T. (2016). Efficacy and safety of dulaglutide in the treatment of type 2 diabetes: A comprehensive review of the dulaglutide clinical data focusing on the AWARD phase 3 clinical trial program. Diabetes/Metabolism Research and Reviews, 32, 776–790. 10.1002/dmrr.2810 [DOI] [PubMed] [Google Scholar]
- John, L. E. , Kane, M. P. , Busch, R. S. , & Hamilton, R. A. (2007). Expanded use of exenatide in the management of type 2 diabetes. Diabetes Spectrum: A Publication of the American Diabetes Association, 20, 59–63. 10.2337/diaspect.20.1.59 [DOI] [Google Scholar]
- Jones, B. , Buenaventura, T. , Kanda, N. , Chabosseau, P. , Owen, B. M. , Scott, R. , Goldin, R. , Angkathunyakul, N. , Corrêa Jr, I. R. , Bosco, D. , Johnson, P. R. , Piemonti, L. , Marchetti, P. , Shapiro, A. M. J. , Cochran, B. J. , Hanyaloglu, A. C. , Inoue, A. , Tan, T. , Rutter, G. A. , … Bloom, S. R. (2018). Targeting GLP‐1 receptor trafficking to improve agonist efficacy. Nature Communications, 9, 1602. 10.1038/s41467-018-03941-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorsal, T. , Rhee, N. A. , Pedersen, J. , Wahlgren, C. D. , Mortensen, B. , Jepsen, S. L. , Jelsing, J. , Dalbøge, L. S. , Vilmann, P. , Hassan, H. , Hendel, J. W. , Poulsen, S. S. , Holst, J. J. , Vilsbøll, T. , & Knop, F. K. (2018). Enteroendocrine K and L cells in healthy and type 2 diabetic individuals. Diabetologia, 61, 284–294. 10.1007/s00125-017-4450-9 [DOI] [PubMed] [Google Scholar]
- Kanazawa, S. , & Herbert, V. (1983). Noncobalamin vitamin B12 analogues in human red cells, liver, and brain. The American Journal of Clinical Nutrition, 37, 774–777. 10.1093/ajcn/37.5.774 [DOI] [PubMed] [Google Scholar]
- Kaneko, K. , Fu, Y. , Lin, H. Y. , Cordonier, E. L. , Mo, Q. , Gao, Y. , Yao, T. , Naylor, J. , Howard, V. , Saito, K. , Xu, P. , Chen, S. S. , Chen, M. H. , Xu, Y. , Williams, K. W. , Ravn, P. , & Fukuda, M. (2019). Gut‐derived GIP activates central Rap1 to impair neural leptin sensitivity during overnutrition. The Journal of Clinical Investigation, 129, 3786–3791. 10.1172/JCI126107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski, S. E. , Fortin, S. M. , Arnold, M. , Grill, H. J. , & Hayes, M. R. (2011). Peripheral and central GLP‐1 receptor populations mediate the anorectic effects of peripherally administered GLP‐1 receptor agonists, liraglutide and exendin‐4. Endocrinology, 152, 3103–3112. 10.1210/en.2011-0174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski, S. E. , Rupprecht, L. E. , Fortin, S. M. , De Jonghe, B. C. , & Hayes, M. R. (2012). The role of nausea in food intake and body weight suppression by peripheral GLP‐1 receptor agonists, exendin‐4 and liraglutide. Neuropharmacology, 62, 1916–1927. 10.1016/j.neuropharm.2011.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastin, A. J. , & Akerstrom, V. (2003). Entry of exendin‐4 into brain is rapid but may be limited at high doses. International Journal of Obesity and Related Metabolic Disorders, 27, 313–318. 10.1038/sj.ijo.0802206 [DOI] [PubMed] [Google Scholar]
- Kauth, T. , & Metz, J. (1987). Immunohistochemical localization of glucagon‐like peptide 1. Use of poly‐ and monoclonal antibodies. Histochemistry, 86, 509–515. 10.1007/BF00500625 [DOI] [PubMed] [Google Scholar]
- Kendall, D. M. , Riddle, M. C. , Rosenstock, J. , Zhuang, D. , Kim, D. D. , Fineman, M. S. , & Baron, A. D. (2005). Effects of exenatide (exendin‐4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care, 28, 1083–1091. 10.2337/diacare.28.5.1083 [DOI] [PubMed] [Google Scholar]
- Khan, R. , Tomas, A. , & Rutter, G. A. (2020). Effects on pancreatic beta and other islet cells of the glucose‐dependent insulinotropic polypeptide. Peptides, 125, 170201. 10.1016/j.peptides.2019.170201 [DOI] [PubMed] [Google Scholar]
- Kieffer, T. J. , McIntosh, C. H. , & Pederson, R. A. (1995). Degradation of glucose‐dependent insulinotropic polypeptide and truncated glucagon‐like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology, 136, 3585–3596. 10.1210/endo.136.8.7628397 [DOI] [PubMed] [Google Scholar]
- Killion, E. A. , Wang, J. , Yie, J. , Shi, S. D. , Bates, D. , Min, X. , Komorowski, R. , Hager, T. , Deng, L. , Atangan, L. , & Lu, S. C. (2018). Anti‐obesity effects of GIPR antagonists alone and in combination with GLP‐1R agonists in preclinical models. Science Translational Medicine, 10, eaat3392. 10.1126/scitranslmed.aat3392 [DOI] [PubMed] [Google Scholar]
- Kim, W. , & Egan, J. M. (2008). The role of incretins in glucose homeostasis and diabetes treatment. Pharmacological Reviews, 60, 470–512. 10.1124/pr.108.000604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozyraki, R. , Kristiansen, M. , Silahtaroglu, A. , Hansen, C. , Jacobsen, C. , Tommerup, N. , Verroust, P. J. , & Moestrup, S. K. (1998). The human intrinsic factor‐vitamin B12 receptor, cubilin: Molecular characterization and chromosomal mapping of the gene to 10p within the autosomal recessive megaloblastic anemia (MGA1) region. Blood, 91, 3593–3600. 10.1182/blood.V91.10.3593 [DOI] [PubMed] [Google Scholar]
- Kuda‐Wedagedara, A. N. W. , Workinger, J. L. , Nexo, E. , Doyle, R. P. , & Viola‐Villegas, N. (2017). 89Zr‐cobalamin PET tracer: Synthesis, cellular uptake, and use for tumor imaging. ACS Omega, 2, 6314–6320. 10.1021/acsomega.7b01180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lean, M. E. , Carraro, R. , Finer, N. , Hartvig, H. , Lindegaard, M. L. , Rossner, S. , Van Gaal, L. , & Astrup, A. (2014). Tolerability of nausea and vomiting and associations with weight loss in a randomized trial of liraglutide in obese, non‐diabetic adults. International Journal of Obesity, 38, 689–697. 10.1038/ijo.2013.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner, D. R. , Fruhbeck, G. , Yumuk, V. , Schindler, K. , Micic, D. , Woodward, E. , & Toplak, H. (2017). Obesity and type 2 diabetes: Two diseases with a need for combined treatment strategies—EASO can lead the way. Obesity Facts, 10, 483–492. 10.1159/000480525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn‐Smith, I. J. , Reimann, F. , Gribble, F. M. , & Trapp, S. (2011). Preproglucagon neurons project widely to autonomic control areas in the mouse brain. Neuroscience, 180, 111–121. 10.1016/j.neuroscience.2011.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovshin, J. A. , & Drucker, D. J. (2009). Incretin‐based therapies for type 2 diabetes mellitus. Nature Reviews. Endocrinology, 5, 262–269. 10.1038/nrendo.2009.48 [DOI] [PubMed] [Google Scholar]
- Luder, A. S. , Tanner, S. M. , de la Chapelle, A. , & Walter, J. H. (2008). Amnionless (AMN) mutations in Imerslund–Gräsbeck syndrome may be associated with disturbed vitamin B12 transport into the CNS. Journal of Inherited Metabolic Disease, 31(Suppl 3), 493–496. 10.1007/s10545-007-0760-2 [DOI] [PubMed] [Google Scholar]
- Ludwig, M. Q. , Cheng, W. , Gordian, D. , Lee, J. , Paulsen, S. J. , Hansen, S. N. , Egerod, K. L. , Barkholt, P. , Rhodes, C. J. , Secher, A. , Knudsen, L. B. , Pyke, C. , Myers, M. G. Jr. , & Pers, T. H. (2021). A genetic map of the mouse dorsal vagal complex and its role in obesity. Nature Metabolism, 3, 530–545. 10.1038/s42255-021-00363-1 [DOI] [PubMed] [Google Scholar]
- Madsbad, S. (2016). Review of head‐to‐head comparisons of glucagon‐like peptide‐1 receptor agonists. Diabetes, Obesity & Metabolism, 18, 317–332. 10.1111/dom.12596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClean, P. L. , Irwin, N. , Cassidy, R. S. , Holst, J. J. , Gault, V. A. , & Flatt, P. R. (2007). GIP receptor antagonism reverses obesity, insulin resistance, and associated metabolic disturbances induced in mice by prolonged consumption of high‐fat diet. American Journal of Physiology. Endocrinology and Metabolism, 293, E1746–E1755. 10.1152/ajpendo.00460.2007 [DOI] [PubMed] [Google Scholar]
- McGovern, A. , Tippu, Z. , Hinton, W. , Munro, N. , Whyte, M. , & de Lusignan, S. (2018). Comparison of medication adherence and persistence in type 2 diabetes: A systematic review and meta‐analysis. Diabetes, Obesity & Metabolism, 20, 1040–1043. 10.1111/dom.13160 [DOI] [PubMed] [Google Scholar]
- Merchenthaler, I. , Lane, M. , & Shughrue, P. (1999). Distribution of pre‐pro‐glucagon and glucagon‐like peptide‐1 receptor messenger RNAs in the rat central nervous system. The Journal of Comparative Neurology, 403, 261–280. [DOI] [PubMed] [Google Scholar]
- Mietlicki‐Baase, E. G. , Liberini, C. G. , Workinger, J. L. , Bonaccorso, R. L. , Borner, T. , Reiner, D. J. , Koch‐Laskowski, K. , McGrath, L. E. , Lhamo, R. , Stein, L. M. , De Jonghe, B. C. , Holz, G. G. , Roth, C. L. , Doyle, R. P. , & Hayes, M. R. (2018). A vitamin B12 conjugate of exendin‐4 improves glucose tolerance without associated nausea or hypophagia in rodents. Diabetes, Obesity & Metabolism, 20, 1223–1234. 10.1111/dom.13222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, A. D. , & Leslie, R. A. (1994). The area postrema and vomiting. Frontiers in Neuroendocrinology, 15, 301–320. 10.1006/frne.1994.1012 [DOI] [PubMed] [Google Scholar]
- Milliken, B. T. , Elfers, C. , Chepurny, O. G. , Chichura, K. S. , Sweet, I. R. , Borner, T. , Hayes, M. R. , De Jonghe, B. C. , Holz, G. G. , Roth, C. L. , & Doyle, R. P. (2021). Design and evaluation of peptide dual‐agonists of GLP‐1 and NPY2 receptors for glucoregulation and weight loss with mitigated nausea and emesis. Journal of Medicinal Chemistry, 64, 1127–1138. 10.1021/acs.jmedchem.0c01783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min, T. , & Bain, S. C. (2021). The role of tirzepatide, dual GIP and GLP‐1 receptor agonist, in the management of type 2 diabetes: The SURPASS clinical trials. Diabetes Therapy, 12, 143–157. 10.1007/s13300-020-00981-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki, K. , Yamada, Y. , Ban, N. , Ihara, Y. , Tsukiyama, K. , Zhou, H. , Fujimoto, S. , Oku, A. , Tsuda, K. , Toyokuni, S. , Hiai, H. , Mizunoya, W. , Fushiki, T. , Holst, J. J. , Makino, M. , Tashita, A. , Kobara, Y. , Tsubamoto, Y. , Jinnouchi, T. , … Seino, Y. (2002). Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nature Medicine, 8, 738–742. 10.1038/nm727 [DOI] [PubMed] [Google Scholar]
- Morkbak, A. L. , Poulsen, S. S. , & Nexo, E. (2007). Haptocorrin in humans. Clinical Chemistry and Laboratory Medicine, 45, 1751–1759. 10.1515/CCLM.2007.343 [DOI] [PubMed] [Google Scholar]
- Mroz, P. A. , Finan, B. , Gelfanov, V. , Yang, B. , Tschop, M. H. , DiMarchi, R. D. , & Perez‐Tilve, D. (2019). Optimized GIP analogs promote body weight lowering in mice through GIPR agonism not antagonism. Molecular Metabolism, 20, 51–62. 10.1016/j.molmet.2018.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, T. D. , Finan, B. , Bloom, S. R. , D'Alessio, D. , Drucker, D. J. , Flatt, P. R. , Fritsche, A. , Gribble, F. , Grill, H. J. , Habener, J. F. , & Holst, J. J. (2019). Glucagon‐like peptide 1 (GLP‐1). Molecular Metabolism, 30, 72–130. 10.1016/j.molmet.2019.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NamKoong, C. , Kim, M. S. , Jang, B. T. , Lee, Y. H. , Cho, Y. M. , & Choi, H. J. (2017). Central administration of GLP‐1 and GIP decreases feeding in mice. Biochemical and Biophysical Research Communications, 490, 247–252. 10.1016/j.bbrc.2017.06.031 [DOI] [PubMed] [Google Scholar]
- Nauck, M. A. , Heimesaat, M. M. , Orskov, C. , Holst, J. J. , Ebert, R. , & Creutzfeldt, W. (1993). Preserved incretin activity of glucagon‐like peptide 1 [7‐36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type‐2 diabetes mellitus. The Journal of Clinical Investigation, 91, 301–307. 10.1172/JCI116186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauck, M. A. , & Meier, J. J. (2016). The incretin effect in healthy individuals and those with type 2 diabetes: Physiology, pathophysiology, and response to therapeutic interventions. The Lancet Diabetes and Endocrinology, 4, 525–536. 10.1016/S2213-8587(15)00482-9 [DOI] [PubMed] [Google Scholar]
- Nauck, M. A. , & Meier, J. J. (2019). Management of endocrine disease: Are all GLP‐1 agonists equal in the treatment of type 2 diabetes? European Journal of Endocrinology, 181, R211–R234. 10.1530/EJE-19-0566 [DOI] [PubMed] [Google Scholar]
- Nauck, M. A. , Petrie, J. R. , Sesti, G. , Mannucci, E. , Courreges, J. P. , Lindegaard, M. L. , Jensen, C. B. , & Atkin, S. L. (2016). A phase 2, randomized, dose‐finding study of the novel once‐weekly human GLP‐1 analog, semaglutide, compared with placebo and open‐label liraglutide in patients with type 2 diabetes. Diabetes Care, 39, 231–241. 10.2337/dc15-0165 [DOI] [PubMed] [Google Scholar]
- Nielsen, M. J. , Rasmussen, M. R. , Andersen, C. B. , Nexo, E. , & Moestrup, S. K. (2012). Vitamin B12 transport from food to the body's cells—A sophisticated, multistep pathway. Nature Reviews Gastroenterology & Hepatology, 9, 345–354. 10.1038/nrgastro.2012.76 [DOI] [PubMed] [Google Scholar]
- Norregaard, P. K. , Deryabina, M. A. , Tofteng Shelton, P. , Fog, J. U. , Daugaard, J. R. , Eriksson, P. O. , Larsen, L. F. , & Jessen, L. (2018). A novel GIP analogue, ZP4165, enhances glucagon‐like peptide‐1‐induced body weight loss and improves glycaemic control in rodents. Diabetes, Obesity & Metabolism, 20, 60–68. 10.1111/dom.13034 [DOI] [PubMed] [Google Scholar]
- ó Proinsias, K. , Giedyk, M. , & Gryko, D. (2013). Vitamin B12: Chemical modifications. Chemical Society Reviews, 42, 6605–6619. 10.1039/c3cs60062a [DOI] [PubMed] [Google Scholar]
- O'Neil, P. M. , Birkenfeld, A. L. , McGowan, B. , Mosenzon, O. , Pedersen, S. D. , Wharton, S. , Carson, C. G. , Jepsen, C. H. , Kabisch, M. , & Wilding, J. P. H. (2018). Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: A randomised, double‐blind, placebo and active controlled, dose‐ranging, phase 2 trial. Lancet, 392, 637–649. 10.1016/S0140-6736(18)31773-2 [DOI] [PubMed] [Google Scholar]
- Ostenson, C. G. , & Efendic, S. (2007). Islet gene expression and function in type 2 diabetes; studies in the Goto‐Kakizaki rat and humans. Diabetes, Obesity & Metabolism, 9(Suppl 2), 180–186. 10.1111/j.1463-1326.2007.00787.x [DOI] [PubMed] [Google Scholar]
- Ostergaard, S. , Paulsson, J. F. , Kjaergaard Gerstenberg, M. , & Wulff, B. S. (2021). The design of a GLP‐1/PYY dual acting agonist. Angewandte Chemie (International Ed. in English), 133(15), 8349–8356. [DOI] [PubMed] [Google Scholar]
- Perez‐Tilve, D. , Gonzalez‐Matias, L. , Aulinger, B. A. , Alvarez‐Crespo, M. , Gil‐Lozano, M. , Alvarez, E. , Andrade‐Olivie, A. M. , Tschöp, M. H. , D'Alessio, D. A. , & Mallo, F. (2010). Exendin‐4 increases blood glucose levels acutely in rats by activation of the sympathetic nervous system. American Journal of Physiology. Endocrinology and Metabolism, 298, E1088–E1096. 10.1152/ajpendo.00464.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrus, A. K. , Vortherms, A. R. , Fairchild, T. J. , & Doyle, R. P. (2007). Vitamin B12 as a carrier for the oral delivery of insulin. ChemMedChem, 2, 1717–1721. 10.1002/cmdc.200700239 [DOI] [PubMed] [Google Scholar]
- Pocai, A. , Carrington, P. E. , Adams, J. R. , Wright, M. , Eiermann, G. , Zhu, L. , du, X. , Petrov, A. , Lassman, M. E. , Jiang, G. , Liu, F. , Miller, C. , Tota, L. M. , Zhou, G. , Zhang, X. , Sountis, M. M. , Santoprete, A. , Capito', E. , Chicchi, G. G. , … SinhaRoy, R. (2009). Glucagon‐like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes, 58, 2258–2266. 10.2337/db09-0278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratley, R. E. , Aroda, V. R. , Lingvay, I. , Ludemann, J. , Andreassen, C. , Navarria, A. , & Viljoen, A. (2018). Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): A randomised, open‐label, phase 3b trial. The Lancet Diabetes and Endocrinology, 6, 275–286. 10.1016/S2213-8587(18)30024-X [DOI] [PubMed] [Google Scholar]
- Price, C. J. , Hoyda, T. D. , & Ferguson, A. V. (2008). The area postrema: A brain monitor and integrator of systemic autonomic state. The Neuroscientist, 14, 182–194. 10.1177/1073858407311100 [DOI] [PubMed] [Google Scholar]
- Ratner, R. E. , Rosenstock, J. , Boka, G. , & Investigators, D. R. I. S. (2010). Dose‐dependent effects of the once‐daily GLP‐1 receptor agonist lixisenatide in patients with type 2 diabetes inadequately controlled with metformin: A randomized, double‐blind, placebo‐controlled trial. Diabetic Medicine, 27, 1024–1032. 10.1111/j.1464-5491.2010.03020.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendell, M. S. (2018). Albiglutide for the management of type 2 diabetes. Expert Review of Endocrinology and Metabolism, 13, 1–8. 10.1080/17446651.2018.1419061 [DOI] [PubMed] [Google Scholar]
- Rosenblatt, D. S. , Fenton, W. A. , Scriver, C. R. , Beaudet, A. L. , Valle, D. , & Sly, W. S. (2001). Inherited disorders of folate and cobalamin transport and metabolism. New York, USA: McGraw Hill. [Google Scholar]
- Rowlands, J. , Heng, J. , Newsholme, P. , & Carlessi, R. (2018). Pleiotropic effects of GLP‐1 and analogs on cell signaling, metabolism, and function. Frontiers in Endocrinology, 9, 672. 10.3389/fendo.2018.00672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell‐Jones, G. J. , & Alpers, D. H. (1999). Vitamin B12 transporters. Pharmaceutical Biotechnology, 12, 493–520. 10.1007/0-306-46812-3_17 [DOI] [PubMed] [Google Scholar]
- Saeedi, P. , Petersohn, I. , Salpea, P. , Malanda, B. , Karuranga, S. , Unwin, N. , Colagiuri, S. , Guariguata, L. , Motala, A. A. , Ogurtsova, K. , Shaw, J. E. , Bright, D. , Williams, R. , & IDF Diabetes Atlas Committee . (2019). Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Research and Clinical Practice, 157, 107843. 10.1016/j.diabres.2019.107843 [DOI] [PubMed] [Google Scholar]
- Sah, B. R. , Schibli, R. , Waibel, R. , von Boehmer, L. , Blauenstein, P. , Nexo, E. , Johayem, A. , Fischer, E. , Müller, E. , Soyka, J. D. , & Knuth, A. K. (2014). Tumor imaging in patients with advanced tumors using a new 99mTc‐radiolabeled vitamin B12 derivative. Journal of Nuclear Medicine, 55, 43–49. 10.2967/jnumed.113.122499 [DOI] [PubMed] [Google Scholar]
- Salinas, C. B. G. , Lu, T. T. , Gabery, S. , Marstal, K. , Alanentalo, T. , Mercer, A. J. , Cornea, A. , Conradsen, K. , Hecksher‐Sørensen, J. , Dahl, A. B. , & Knudsen, L. B. (2018). Integrated brain atlas for unbiased mapping of nervous system effects following liraglutide treatment. Scientific Reports, 8, 10310. 10.1038/s41598-018-28496-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samms, R. J. , Coghlan, M. P. , & Sloop, K. W. (2020). How may GIP enhance the therapeutic efficacy of GLP‐1? Trends in Endocrinology and Metabolism, 31, 410–421. 10.1016/j.tem.2020.02.006 [DOI] [PubMed] [Google Scholar]
- Secher, A. , Jelsing, J. , Baquero, A. F. , Hecksher‐Sorensen, J. , Cowley, M. A. , Dalboge, L. S. , Hansen, G. , Grove, K. L. , Pyke, C. , Raun, K. , & Schäffer, L. (2014). The arcuate nucleus mediates GLP‐1 receptor agonist liraglutide‐dependent weight loss. The Journal of Clinical Investigation, 124, 4473–4488. 10.1172/JCI75276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, D. , Verma, S. , Vaidya, S. , Kalia, K. , & Tiwari, V. (2018). Recent updates on GLP‐1 agonists: Current advancements & challenges. Biomedicine & Pharmacotherapy, 108, 952–962. 10.1016/j.biopha.2018.08.088 [DOI] [PubMed] [Google Scholar]
- Sikirica, M. V. , Martin, A. A. , Wood, R. , Leith, A. , Piercy, J. , & Higgins, V. (2017). Reasons for discontinuation of GLP1 receptor agonists: Data from a real‐world cross‐sectional survey of physicians and their patients with type 2 diabetes. Diabetes, Metabolic Syndrome and Obesity, 10, 403–412. 10.2147/DMSO.S141235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen, L. , Holst, J. J. , & Deacon, C. F. (2006). Exendin‐4, but not glucagon‐like peptide‐1, is cleared exclusively by glomerular filtration in anaesthetised pigs. Diabetologia, 49, 706–712. 10.1007/s00125-005-0128-9 [DOI] [PubMed] [Google Scholar]
- Sisley, S. , Gutierrez‐Aguilar, R. , Scott, M. , D'Alessio, D. A. , Sandoval, D. A. , & Seeley, R. J. (2014). Neuronal GLP1R mediates liraglutide's anorectic but not glucose‐lowering effect. The Journal of Clinical Investigation, 124, 2456–2463. 10.1172/JCI72434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrasher, J. (2017). Pharmacologic management of type 2 diabetes mellitus: Available therapies. The American Journal of Medicine, 130, S4–S17. 10.1016/j.amjmed.2017.04.004 [DOI] [PubMed] [Google Scholar]
- Tinsley, I. C. , Borner, T. , Swanson, M. L. , Chepurny, O. G. , Doebley, S. A. , Kamat, V. , Sweet, I. R. , Holz, G. G. , Hayes, M. R. , De Jonghe, B. C. , & Doyle, R. P. (2021). Synthesis, optimization, and biological evaluation of corrinated conjugates of the GLP‐1R agonist exendin‐4. Journal of Medicinal Chemistry, 64, 3479–3492. 10.1021/acs.jmedchem.1c00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo, J. (2020). Safety and tolerability of once‐weekly GLP‐1 receptor agonists in type 2 diabetes. Journal of Clinical Pharmacy and Therapeutics, 45(Suppl 1), 43–60. 10.1111/jcpt.13225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschop, M. H. , Finan, B. , Clemmensen, C. , Gelfanov, V. , Perez‐Tilve, D. , Muller, T. D. , & DiMarchi, R. D. (2016). Unimolecular polypharmacy for treatment of diabetes and obesity. Cell Metabolism, 24, 51–62. 10.1016/j.cmet.2016.06.021 [DOI] [PubMed] [Google Scholar]
- Turton, M. D. , O'Shea, D. , Gunn, I. , Beak, S. A. , Edwards, C. M. , Meeran, K. , Choi, S. J. , Taylor, G. M. , Heath, M. M. , Lambert, P. D. , & Wilding, J. P. (1996). A role for glucagon‐like peptide‐1 in the central regulation of feeding. Nature, 379, 69–72. 10.1038/379069a0 [DOI] [PubMed] [Google Scholar]
- Ueno, S. , Matsuki, N. , & Saito, H. (1987). Suncus murinus: A new experimental model in emesis research. Life Sciences, 41, 513–518. 10.1016/0024-3205(87)90229-3 [DOI] [PubMed] [Google Scholar]
- Willard, F. S. , Douros, J. D. , Gabe, M. B. , Showalter, A. D. , Wainscott, D. B. , Suter, T. M. , Capozzi, M. E. , van der Velden, W. J. , Stutsman, C. , Cardona, G. R. , & Urva, S. (2020). Tirzepatide is an imbalanced and biased dual GIP and GLP‐1 receptor agonist. JCI Insight, 5(17), e140532. 10.1172/jci.insight.140532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, D. M. , Nawaz, A. , & Evans, M. (2020). Drug therapy in obesity: A review of current and emerging treatments. Diabetes Therapy, 11, 1199–1216. 10.1007/s13300-020-00816-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wismann, P. , Pedersen, S. L. , Hansen, G. , Mannerstedt, K. , Pedersen, P. J. , Jeppesen, P. B. , Vrang, N. , Fosgerau, K. , & Jelsing, J. (2018). Novel GLP‐1/GLP‐2 co‐agonists display marked effects on gut volume and improves glycemic control in mice. Physiology & Behavior, 192, 72–81. 10.1016/j.physbeh.2018.03.004 [DOI] [PubMed] [Google Scholar]
- Zhang, C. , Kaye, J. A. , Cai, Z. , Wang, Y. , Prescott, S. L. , & Liberles, S. D. (2021). Area postrema cell types that mediate nausea‐associated behaviors. Neuron, 109(461–472), e465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q. , Delessa, C. T. , Augustin, R. , Bakhti, M. , Collden, G. , Drucker, D. J. , Feuchtinger, A. , Caceres, C. G. , Grandl, G. , Harger, A. , & Herzig, S. (2021). The glucose‐dependent insulinotropic polypeptide (GIP) regulates body weight and food intake via CNS‐GIPR signaling. Cell Metabolism, 33(4), 833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no new data were created or analysed in this study.


