Abstract
Pyroptosis, a lytic form of programmed cell death, both stimulates effective immune responses and causes tissue damage. Gasdermin (GSDM) proteins are a family of pore-forming executors of pyroptosis. While the most-studied member, GSDMD, exerts critical functions in inflammasome biology, emerging evidence demonstrates potential broad relevance for GSDM-mediated pyroptosis across diverse pathologies. In this review, we describe GSDM biology, outline conditions where inflammasomes and GSDM-mediated pyroptosis represent rational therapeutic targets, and delineate strategies to manipulate these central immunologic processes for the treatment of human disease.
Keywords: Inflammasome, Inflammasomopathy, Immunogenic cell death, Anti-tumor immunity, Pore-forming protein
Introduction
Inflammation acts as a double-edged sword: on one edge it is essential to launch protective responses against foreign pathogens and to promote tissue repair while on the other it drives tissue damage and malignant transformation [1]. Modern medicine has developed a robust armamentarium to inhibit inflammation, initially in the form of non-specific anti-inflammatory agents and more recently via the development of novel small molecules and biologics that target pathogenic inflammatory pathways and mediators [2]. In parallel, our understanding of inflammatory mechanisms has grown immensely. The past two decades of research in the field have uncovered a set of cellular responders to a multitude of infectious agents and markers of cellular dysfunction that initiate inflammatory cascades via so-called inflammasomes. Activation of inflammasomes often, but not always, results in a programmed form of cell death with necrotic morphology known as pyroptosis. This immunostimulatory form of cell death can alert the immune system to an invading pathogen or damaged tissue in order to coordinate an appropriate response to restore organismal homeostasis. However, when aberrantly activated inflammasomes instigate pathology of a diverse complement of diseases, ranging from sepsis to atherosclerosis to cancer. The gasdermin (GSDM) family of pore-forming proteins has emerged as a critical facilitator of inflammasome biology and pro-inflammatory cell death. In fact, many now define pyroptosis as GSDM-mediated lytic cell death [3]. The seminal contribution of GSDMD to the inflammatory pathways that produce such immense morbidity and mortality has made it a natural focus for pharmaceutical targeting. Moreover, study of related family members has identified additional diseases where GSDM targeting may harbor therapeutic potential. In this review, we will outline pathways to GSDM activation, their known roles in human pathology and strategies– pharmaceutical and otherwise– under development to modulate GSDM biology for the treatment of disease.
Gasdermin family members
The GSDM family consists of six members in humans: GSDMA, GSDMB, GSDMC, GSDMD, GSDME (DFNA5), and pejvakin (PJVK, DFNB59). Mice have three paralogs of GSDMA and four paralogs of GSDMC, but lack a detectable ortholog of GSDMB. Structurally, GSDMA-E consist of a two domain architecture, with an N-terminal (NT) pore forming domain connected to a C-terminal (CT) auto-inhibitory domain through a flexible linker region [4,5]. PJVK contains a truncated CT relative to the other GSDMs (Figure 1). Within the family, the GSDMNT display high sequence similarity with more variability in GSDMCT. All GSDMNT except for PJVK have been demonstrated to harbor cytolytic pore-forming activity. Tissue expression profiles differ among GSDM family members (summarized in several recent reviews [6–8]). While disease relevance for individual GSDMs likely coincides with the organs/cell types in which they are expressed, specific reported disease associations will be presented below.
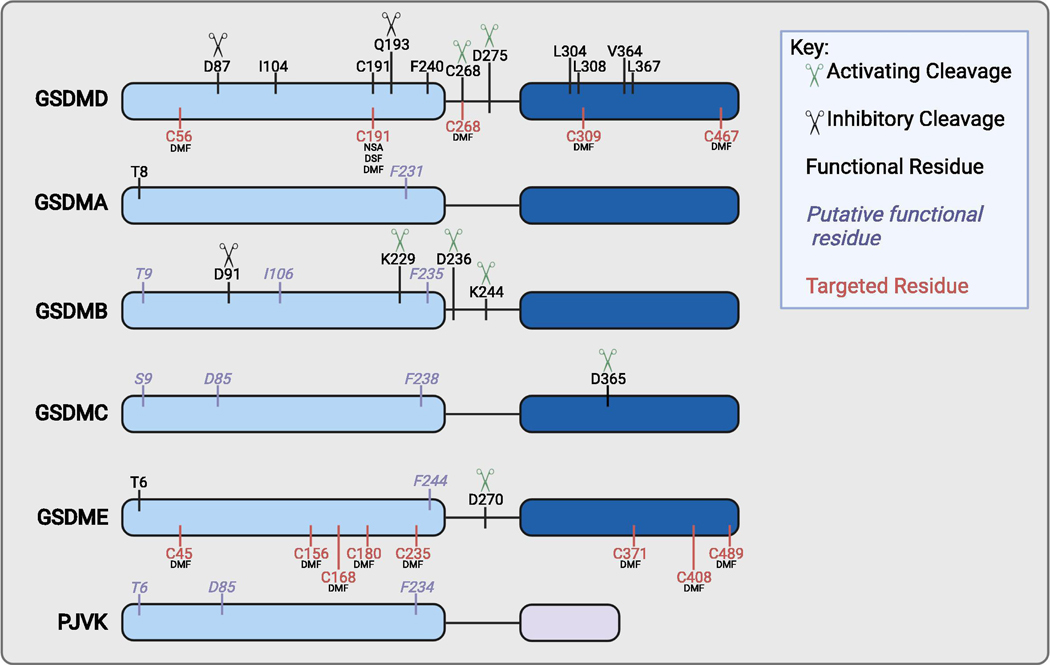
Figure 1: Schematic overview of critical residues and targetable sites of the gasdermins.
GSDMA-E contain an NT pore forming domain (light blue) and a CT inhibitory domain (dark blue). PJVK contains a putative pore forming domain (light blue) and a truncated CT domain (light purple). Activating (green) and inhibitory (black) cleavage sites are indicated by scissors. Residues annotated in black indicate those with known function. Residues annotated in purple italics indicate those with putative function due to conservation with other GSDM family members. Residues in red indicate sites modified by current GSDMD targeting drugs.
Human GSDMD is activated by caspases 1,4,5,8 at D275 and by neutrophil elastase (ELANE) at C268 [16,41,42,49,52–54]. GSDMD is inactivated by caspase-3 at D87 and by enterovirus 71 protease 3C at Q193 [113,121]. Among the other gasdermin family members, the D87 residue is conserved in GSDMB (D91), GSDMC (D85), and PJVK (D85), although proteolytic cleavage has not been shown at this site for GSDMC or PJVK. Important residues for GSDMD pore formation include C191 (oligomerization) and F240 (dimerization) [43–45]. The F240 residue is conserved in all GSDM family members, whereas C191 is not. Mutation of I104N results in a hypomorphic gasdermin D allele, and this site is conserved in GSDMB [49]. Four residues in GSDMDCT (L304, V308, V364, L367) are responsible for GSDMD-inflammatory caspase exosite interactions [50,51]. GSDMA and GSDME harbor an inhibitory phosphorylation site (T8, T6, respectively) [17]. A conserved residue is found in GSDMB, GSDMC and PJVK. There are no described proteases for GSDMA or PJVK. GSDMB is cleaved at K229 and K244 by granzyme A; some splice variants may undergo caspase-1 cleavage at D236 [28,32]. GSDMC is cleaved at D365 by caspase-8 [35]. GSDME is cleaved at D270 by caspase-3 or granzyme B [62,63,119,120]. Direct GSDMD inhibitors all modify C191 [44,45,57]. DMF additionally succinates C56, C268, C309, and C467. DMF additionally modifies GSDME on C45, C156, C168, C180, C235, C371, C408, and C489. See text for further details. Created with BioRender.com.
GSDMA
Discovery of the first identified GSDM homolog, GSDMA, occurred through positional cloning of a gene causing abnormal hair and skin phenotype in mice. The name comes from its localization to the gastrointestinal tract and skin [9,10]. In mice, mutations in GSDMA3CT cause excoriation, alopecia, and keratosis that are believed to arise due to disrupted auto-inhibition of GSDMA3NT [11–14]. In support of this view, no phenotype has been observed in a knockout mouse model [5,15]. At this time, it is not known whether GSDMA requires cleavage for activation or, if so, the identity of its activating protease. However, expression of GSDMANT is sufficient for plasma membrane permeabilization and lytic cell death [16] and this may be inhibited by phosphorylation at Thr8 by an unknown kinase [17].
In humans, polymorphisms in GSDMA have been linked to inflammatory bowel disease (IBD), systemic sclerosis and asthma susceptibility [18–21]. Furthermore, its expression is increased in asthmatic patients [22]. Conversely, GSDMA is silenced in gastric cancer. Early work demonstrated that restoration of GSDMA in these tumor lines increases susceptibility to TGF-β, suggesting a tumor suppressive role for the protein [9,23]. Thus, therapeutic modulation of GSDMA activity is likely to require disease specific strategies – it may be advantageous to inhibit GSDMA pore formation in some inflammatory diseases whereas reactivation may hold therapeutic potential in some cancers.
GSDMB
Human GSDMB clusters in the same genetic locus as GSDMA and is thought to have arisen due to a gene duplication event [6,18,20,24]. Similar to GSDMA, genome-wide association studies have revealed two human polymorphisms that segregate together with an increased risk of IBD and asthma. These polymorphisms, p.G299S and p.P306S, are suggested to alter the structure of GSDMB but the biochemical and physiological effects of these alterations have not been defined [18,21,25–27]. A separate variant may alter caspase processing of GSDMB [28].
In contrast to GDSMA, the data on GSDMB in cancer are more variable. Increased expression of GSDMB has been linked to cancer proliferation and metastasis, with ectopic expression enhancing in vitro growth of cervical cancer cells and increasing cell motility and invasion of breast cancer cell lines [29–31]. Additional data showed GSDMB to promote metastases in xenograft models and identified an association of high GSDMB expression with poor prognosis in HER2-positive breast cancer. However, GSDMB shows variable expression changes in tumor relative to normal tissues across different cancer types [32].
A recent study discovered a novel role by which GSDMB converts cytotoxic lymphocyte (CTL)-mediated cell killing from apoptotic to pyroptotic in nature. Granzyme A from CTLs cleaves GSDMB, predominantly at K244, to induce target cell pyroptosis and promote efficacy of anti-PD-1 immunotherapy [32]. This work also showed that interferons and TNF-α variably induce GSDMB, highlighting a potential mechanism to augment anti-tumor immunity in some cancers.
More studies on the role of GSDMB in inflammatory disease and cancer immunology are necessary to inform future therapeutic targeting of this GSDM. Similarly to GSDMA, its inhibition may be beneficial in certain inflammatory disorders. However, the therapeutic efficacy of GSDMB activation or inhibition may be stage- and/or tissue-specific in cancer.
GSDMC
GSDMC was first identified as a marker for melanoma progression and given the name melanoma-derived leucine zipper-containing extranuclear factor (MLZE) [33]. As one of the least well-studied GSDM family members, the physiological role of GSDMC remains uncertain. Early work characterized GSDMC as an oncogene that accelerates proliferation of colorectal cancer cells [34]. High expression additionally correlates with poor prognosis in breast and lung cancers [35,36]. Inducers of GSDMC expression include ultraviolet light, NFATc1 and STAT3-PD-L1 signaling [35,37,38]. Under hypoxic conditions, the STAT3-PD-L1 pathway switches TNF-α-induced apoptosis to pyroptosis via caspase-8 cleavage of GSDMC to drive tumor necrosis [35]. Although this study showed that GSDMC-driven necrosis promoted tumor progression, the authors also showed enhanced tumor cell pyroptosis in response to certain chemotherapeutics known to cause immunogenic cell death (ICD) [39]. Together these data suggest that although GSDMC may contribute to carcinogenesis and cancer aggressiveness, its expression may be exploited as a therapeutic target to drive immunostimulatory cancer cell pyroptosis.
GSDMD
GSDMD was originally identified by homology to other family members and recognized as the most efficient substrate of inflammatory caspases [40]. It was not until 2015, however, that GSDMD was identified as the conduit for IL-1β and IL-18 release and the primary executioner of pyroptosis downstream of both multiple inflammasomes [16,41,42]. Since its initial characterization, it has rapidly become the best understood GSDM family member. Unlike its homologs, no polymorphisms have been described linking GSDMD expression to human disease, though some demonstrate functional consequences [43]. Deficiency or inhibition of Gsdmd has been demonstrated to be protective in numerous mouse models of inflammatory disease, including sepsis, experimental autoimmune encephalitis (EAE), familial Mediterranean fever (FMF), and neonatal-onset multisystem inflammatory disorder (NOMID; see below), highlighting the allure of GSDMD as a drug target for treatment of diverse inflammatory conditions [42,44–48].
The first proteases identified for GSDMD were the inflammatory caspases-1/4/5, which cleave GSDMD after D275 (caspase-1/11 after D276 in mice)[16,40–42,49]. Emerging data have identified a critical inflammatory caspase exosite interaction with GSDMDCT [50,51]. This novel caspase-GSDM interface reveals a function for GSDMDCT outside of its traditional view as solely an autoinhibitory domain. Other proteases, including caspase-8, neutrophil elastase (ELANE) and cathepsin G, cleave GSDMD in the same interdomain linker region as caspase-1 [52–55]. The diversity of upstream signals that activate GSDMD further supports its therapeutic potential as a common bottleneck for multiple pyroptotic stimuli.
In addition to its activation (and inhibition; see below) by protease cleavage, several post-translational modifications (PTMs) alter GSDMD function. Itaconate causes inhibitory covalent modification at C77 (present in mice but not humans) while fumarate and its derivatives block GSDMD through modification of C191 and other residues [56,57]. The former change prevents GSDMD cleavage whereas the latter inhibits its oligomerization and is the target of several GSDMD inhibitors (see below). In contrast, oxidation of GSDMD at multiple cysteine residues reportedly augments its cleavage by caspase-1 [58]. These PTMs highlight the potential influence of cellular metabolic state on GSDMD activation. Surprisingly little is known about other PTMs, such as phosphorylation or ubiquitination, though prediction tools suggest that both may occur and a single study found that some sites for PTM have little effect on GSDMD function [43]. Future studies may further clarify the roles played by PTMs in tuning the threshold for GSDMD pyroptosis.
Specific downstream consequences of GSDMD activation are cell type-dependent. Among the possible outcomes are inflammatory cytokine release, neutrophil extracellular trap (NET) extrusion, and/or pyroptotic cell death. As the primary GSDM downstream of inflammasomes, GSDMD has become a major focus of pharmaceutical development across divers inflammatory diseases, a topic addressed more thoroughly below [44,45,57,59].
GSDME
GSDME is the most ancient family member [60] and gain-of-function mutations were originally described as a cause of non-syndromic hearing loss in humans [61]. Two groups recently characterized GSDME as a pore-forming protein that triggers pyroptosis upon cleavage by apoptotic caspase-3 after D270. The generated GSDMENT fragment in essence converts cells committed to apoptosis instead to a pyroptotic phenotype [62,63]. This cell fate switch appears to require a critical GSDME expression threshold, a phenomenon that may hold true for other GSDMs [64].
Recent studies of GSDME activation have fallen into three broad camps: caspase-9-driven intrinsic apoptotic activation of caspase-3 following chemotherapy regimens, caspase-8-driven activation of caspase-3 occurring via either inflammasomes or other signaling complexes, and cell extrinsic activation by granzyme B, all described below.
Targetable aspects of GSDME remain scant. One study provided evidence of inhibitory phosphorylation at the conserved T6 [17], and dozens of high-throughput studies have identified S252 and Y254 (www.phosphosite.org), both in the interdomain linker region, as potential phosphorylated sites conserved in mice but not zebrafish [65]. Further, the key modifiable C191 in human GSDMD is not present in GSDME. Whether a caspase exosite like in GSDMD exists for GSDME remains to be determined [50,51].
Pejvakin
PJVK, as previously mentioned, is the only member of the GSDM family without a complete CT domain. Further distinguishing it from other family members, loss of Pjvk in mice results in a distinct phenotype characterized primarily by hearing loss whereas other GSDM knock-out models lack a baseline phenotype [52,63,66–68]. While PJVK plays a role in protecting auditory hair cells from free radical-induced damage [69,70], no demonstration of pore-forming ability has been reported. Such data will be important to better understand its physiologic function.
Key residues involved in the regulation of individual human GSDMs are presented in Figure 1.
Pathways to GSDM Activation: Inflammasomes
The first recognized and most extensively studied pathways to GSDM cleavage and pyroptosis involve assembly of inflammasome complexes. Pathogen-associated molecular patterns (PAMPs) resulting from microbial and viral infections or damage-associated molecular patterns (DAMPs) released from host cells are detected by sensor proteins, including nucleotide-binding domain (NBD), leucine-rich repeat (LRR)-containing (NLR) proteins, pyrin and absent in melanoma 2 (AIM-2). Resultant activation of these proteins generally triggers recruitment of ASC [apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (CARD)] and subsequent association and proximity-induced auto-cleavage of pro-caspase-1. Once activated, caspase-1 cleaves the inflammatory cytokines pro-IL-1β and pro-IL-18, as well as GSDMD.
The discovery that NLRP1 forms a caspase-1 activating platform in complex with ASC served as the first description of an inflammasome [71]. This general structural framework whereby a sensor protein (here NLRP1) enables supramolecular complex formation to facilitate caspase-1 activation holds true as a model for canonical inflammasomes. The NLRP1 protein contains an NT pyrin domain (PYD), an NBD, an LRR domain, a ‘function to find’ domain (FIIND) and a CT CARD [71]. NLRP1 auto-proteolysis within the FIIND is requisite for its activation [72,73]. Upon stimulus-triggered degradation of NLRP1NT, the liberated CT CARD interacts with the ASC to promote its oligomerization into a single ASC focus, also called a speck, where pro-caspase-1 undergoes proximity-induced auto-activation [74,75].
While humans have one NLRP1 protein, mice express three paralogs, NLRP1a, NLRP1b, and NLRP1c, which all lack a PYD [76]. Mouse NLRP1b can be activated by the lethal toxin of Bacillus anthracis, whereas human NLRP1 is unaffected [76]. The NLRP1 inflammasome also responds to Toxoplasma gondii infection in mice and possibly humans [77–79]. Several recent studies report that multiple viral proteases cleave the auto-inhibitory NT fragment of NLRP1, releasing the active CT fragment to initiate inflammasome activation [80,81]. New evidence additionally describes NLRP1 as a double-stranded (ds)RNA sensor [82]. These studies expand the potential disease relevance of this earliest recognized inflammasome sensor.
Among the few proteins harboring both a FIIND and CARD, CARD8 shares a parallel activation mechanism with NLRP1 [72]. However, unlike NLRP1, human CARD8 reportedly forms an inflammasome independently of ASC [83]. Instead, direct CARD homotypic interactions between CARD8 and pro-caspase-1 enable proteolytic activity of the latter. Recent work has identified distinct features of the CARD of NLRP1 compared to that of CARD8 that explain the different composition of these two inflammasomes [84,85]. Of note, the ASC-independent inflammasomes appear to be inefficient at IL-1β and potentially GSDMD processing [86,87].
Perhaps the most studied inflammasome sensor, NLRP3 can form inflammasome complexes in response to a wide range of PAMPs and DAMPs, including bacteria, viruses, fungi, and protozoans, as well as monosodium urate and silica crystals, asbestos, extracellular ATP, DNA-RNA hybrids, and heme [88–93]. Given the diversity of activating stimuli, it has been proposed that NLRP3 responds to a common cellular perturbation. Proximal activating stimuli generated by NLRP3 agonists include reactive oxygen species, mitochondrial dysfunction, lysosomal damage, and ion flux; however, a common activation mechanism remains under investigation [94–100]. Multiple post-translational mechanisms also contribute to NLRP3 regulation (reviewed in [101]). Like GSDMD, the role of NLRP3 as a convergence point for diverse inflammatory stimuli increases its attractiveness as a therapeutic target.
In addition to canonical inflammasome activation, non-canonical activation occurs upon intracellular LPS binding to inflammatory caspase-4/5 in humans or caspase-11 in mice. This process activates GSDMD-dependent pyroptosis but these caspases lack activity toward pro-inflammatory cytokines pro-IL-1β and pro-IL-18 [102,103]. However, secondary NLRP3 activation can facilitate subsequent cytokine release, likely in response to potassium efflux through newly formed GSDMD pores [104,105].
Among other inflammasome sensors, the NLRC4 inflammasome is assembled in response to bacterial type 3 secretion systems in humans and mice, as well as flagellin in mice [106,107]. Recognition of cytosolic dsDNA activates the AIM2 inflammasome. Separately, the pyrin inflammasome responds to a number of bacterial toxins, including those of Clostridium difficile and Clostridium botulinum. However, rather than sensing them directly, these toxins inhibit RhoA GTPase activity [108], causing pyrin dephosphorylation and downstream inflammasome activation [109]. The reader is referred to recent reviews for more information about inflammasomes [110,111].
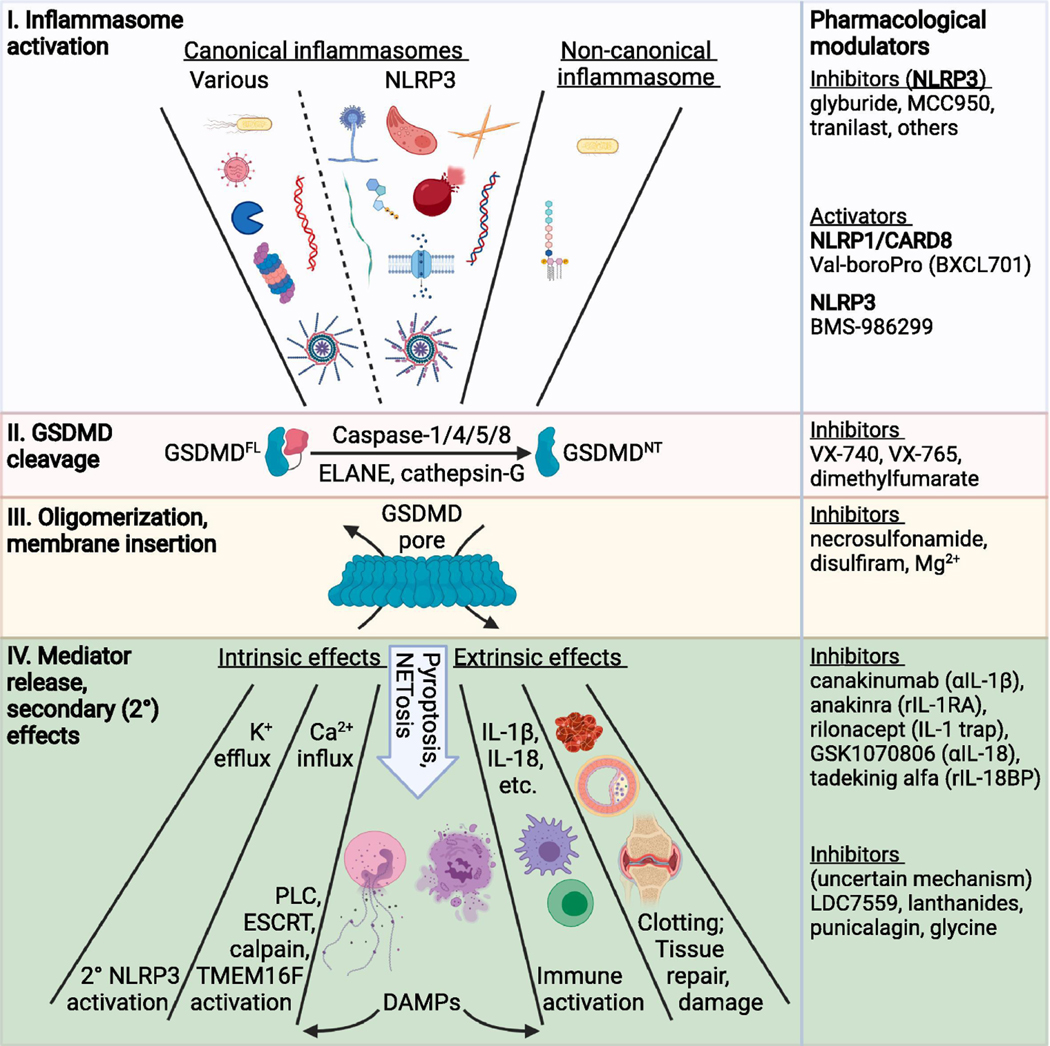
The central position of GSDMD as a bottleneck for inflammasome biology is depicted in Figure 2.
Figure 2: Understanding GSDMD as a bottleneck for inflammasome-driven effects.
Inflammasome-mediated GSDMD activation and the resulting downstream effects are divided into four general steps: (I) inflammasome activation, (II) GSDMD cleavage, (III) GSDMD oligomerization and membrane insertion and (IV) downstream processes/outcomes. (I) Numerous inflammasome activating stimuli are sensed by either a canonical or non-canonical inflammasome sensor protein. Depicted triggers for various canonical inflammasomes include bacterial components (type III secretion system, flagellin), dsRNA, viral proteases, and other stimuli that facilitate proteasomal activation of NLRP1 and CARD8. Depicted stimuli for NLRP3 canonical inflammasome activation include aspergillus, toxoplasma, monosodium urate (and other) crystals, amyloid β, ATP, heme released from lysed red blood cells, DNA:RNA hybrids, and K+ efflux. The non-canonical inflammasome is activated by LPS from gram-negative bacteria. (II) GSDMDFL (full-length) can be cleaved by multiple caspases and other proteases to release the GSDMDNT fragment. (III) The liberated GSDMDNT associates with membranes, oligomerizes, and inserts into the membrane as a higher-order multimeric pore (~27mer) upon conformational change. (IV) Assembled GSDMD pores cause both cell intrinsic and extrinsic effects. Cell intrinsic effects include ion fluxes (both efflux and influx), NETosis, and pyroptosis. Some DAMPs additionally produce cell intrinsic effects. Other DAMPs and factors released via GSDMD pores or following GSDMD-mediated NETosis or pyroptosis cause cell extrinsic effects, including immune cell activation, clotting, and tissue repair or damage. To the right are agents that function at each step. Of note, GSDMD can be activated outside of the context of inflammasomes and a variety of stimuli trigger cleavage of other GSDMs to produce similar downstream effects (see text for details). PLC = phospholipase C. Created with BioRender.com.
Other GSDM Regulatory Mechanisms: Beyond Inflammatory Caspases
While the inflammasome pathways described above converge on inflammatory caspase activation to cleave GSDMD and additional relevant targets (pro-IL-1β, pro-IL-18, etc.), subsequent research has revealed greater diversity of GSDM proteolysis mechanisms. The implicated proteases target both GSDMD and other family members and include both activating and inhibitory cleavage events. Furthermore, GSDM proteolysis can be broadly divided into caspase-dependent and caspase-independent events.
In contrast to the roles of inflammatory caspases in pyroptosis, separate homologs exert important functions during apoptosis either as initiators (caspases-2,8,9,10) or executioners (caspases-3,6,7). Classically, apoptosis is thought to be a non-lytic, non-inflammatory form of programmed cell death. However, shortly after the recognition of GSDMD cleavage and pore formation by inflammatory caspases, GSDME was shown to be cleaved at D270 by caspase-3 to produce secondary pyroptosis in response to apoptotic stimuli; activation by caspases-6/7 is currently undetermined [62,63]. This and subsequent findings[112,113] have blurred the lines between pyroptosis and apoptosis as discrete forms of cell death.
Cytotoxic chemotherapeutics are among many stimuli that activate intrinsic apoptosis through the caspase-9/apoptosome pathway [63]. Another apoptotic caspase, caspase-8, is a component of many complexes and thus has multiple triggers for activation. Both caspase-8 and caspase-9 converge on caspase-3 activation during apoptosis. Of note, caspase-8 is also recruited by ASC CARD to inflammasomes where it mediates downstream caspase-3 activation [114,115]. Caspase-1 can similarly perform this function, especially in the absence of effective GSDMD-dependent pyroptosis [116]. Any of these pathways can therefore lead to GSDME-dependent pyroptosis. In addition to its role in activating caspase-3, caspase-8 also directly liberates pore-competent GSDMCNT and GSDMDNT through proteolysis, providing additional routes to secondary pyroptosis [35,54]. The necessary complexes and recognition sites for these various cleavage events are yet to be fully elucidated.
On the other hand, caspase-3 can also cleave at D87 within GSDMD to generate an inactive 43 kDa fragment [113]. This cleavage can be concurrent with inflammatory pathway cleavage at D275, where it retains its inhibitory function [117]. A conserved site, D91, in GSDMB is likewise targeted by caspase-3 (and caspases-6/7), resulting in inhibitory cleavage [27,118]. Thus, caspase-3 can both promote and inhibit GSDM-pyroptosis in a context-dependent manner.
Recent data demonstrate that granzyme B targets the same activation site in GSDME as caspase-3 [119,120] with similar consequences as granzyme A activity toward GSDMB. These compelling studies demonstrate critical functions for these GSDMs in the development of anti-tumor immunity, to be discussed more below. Thus, although CTL-mediated killing was long held to be immunologically “silent” like apoptosis, GSDMB or GSDME expression can convert this process to an immune-stimulatory pyroptotic cell death.
Adding to the long list of GSDM-cleaving enzymes, both ELANE and cathepsin G activate GSDMD via proteolysis at linker-region sites close to the canonical D275 (C268 and L273, respectively). The only reported caspase-independent GSDM inactivating cleavage is performed by enteroviral protease 3C on GSDMD at Q193 [121]. As this protease was recently reported to activate NLRP1 [80], the overall consequences of its proteolytic activity require further investigation. In time, additional GSDM proteases may be uncovered.
The variety of GSDM activating and inhibitory stimuli underscores the potentially broad set of conditions where targeting GSDM pyroptosis may harbor therapeutic potential. However, there is no standardized approach to these diseases since GSDMs have variably been demonstrated to both ameliorate and worsen infectious and inflammatory conditions. Moreover, in oncology settings GSDM activation can both promote immunogenic cancer cell death and cause normal tissue toxicity and uncontrolled inflammatory responses. Below we will briefly outline a number of diseases where GSDM pyroptosis has been implicated and where GSDM-targeted therapies are likely to be tested in the future.
Disease Relevance
Monogenic Inflammatory Diseases
Genetic mutations in inflammasome components produce rare, heritable auto-inflammatory diseases (Table 1). These pathogenic mutations drive spontaneous inflammasome activation, resulting in sterile inflammation driven by IL-1β, IL-18 and other factors. The most prevalent of these diseases is FMF, a disease attributed to likely gain-of-function mutations in the MEFV gene that encodes the pyrin protein [122,123]. FMF is a highly heterogeneous disease that is characterized by recurring attacks of inflammation with fevers, peritonitis, pleuritic, polyarthritis, and an erysipelas-like skin disease. The mainstay of treatment for FMF is the microtubule targeting agent colchicine, which is effective for many patients though treatment resistance does occur. Patients have elevated serum levels of inflammatory cytokines such as IL-1β, IL-6, and IL-18 [124]. Newer treatments with biologics targeting individual cytokines such as canakinumab (anti-IL-1β mAb) and tocilizumab (anti-IL-6R mAb) have shown success in patients resistant to colchicine [125].
Table 1.
Monogenic diseases associated with inflammasome genes
| Gene | Disease | Current Treatments |
|---|---|---|
| MEFV | Familial Mediterranean Fever (FMF) | Colchicine, canakinumab |
| NLRC4 | Autoinflammation with infantile enterocolitis (AIFEC) | Under investigation |
| NLRP3 | Cryopyrin-associated Periodic Syndrome (CAPS) | Corticosteroids, canakinumab, anakinra, rilonacept |
| Familial Cold Autoinflammatory Syndrome (FCAS) | ||
| Neonatal-onset Multisystem Inflammatory Disease (NOMID) | ||
| Muckle-Wells Syndrome (MWS) | ||
| NLRP1 | Multiple Self-healing Palmoplantar Carcinoma (MSPC) | Retinoids, surgical removal of carcinomas |
| Juvenile Recurrent Respiratory Papillomatosis (JRRP) | Surgical removal of papillomas | |
| Familial Keratosis Lichenoides Chronica (FKLC) | Retinoids, corticosteroids, phototherapy |
Other inflammasome sensors have also been implicated in sterile auto-inflammatory diseases. Over 200 gain-of-function mutations in exon 3 of NLRP3 have been identified resulting in a spectrum of diseases that together are referred to as cryopyrin-associated periodic syndrome (CAPS). These syndromes range from the mildest presentation as familial cold auto-inflammatory syndrome (FCAS) to the most severe NOMID [126]. The most common treatments for CAPS are canakinumab or anakinra (IL-1R antagonist protein) [127]. Similar to NLRP3 mutations, NLRC4 inflammasomopathies also present with auto-inflammation, arthralgia, and rash. The observation that NLRC4 mutations can lead to FCAS and NOMID like diseases highlights the phenotypic convergence of aberrant inflammasome pathway activation [128–130].
As NLRP1 is predominantly expressed in the skin, mutations in this receptor lead to a clinical diseases such as multiple self-healing palmoplantar carcinoma (MSPC), juvenile recurrent respiratory papillomatosis (JRRP) and familial keratosis lichenoides chronica (FKLC) [131–133]. Some mutations lead to severe disease that closely mimics systemic inflammasome activation and presents as auto-inflammation with arthritis and dyskeratosis (AIADK) [132,133]. Since dipeptidyl peptidase 9 (DPP9) is an endogenous inhibitor of NLRP1, it not surprising that a recent preprint details a family with pathogenic DPP9 variants that allow NLRP1 activation and produce similar disease phenotypes [134]. Because DPP9 also restrains CARD8 activity, some phenotypes associated with DPP9 dysfunction may be due to pathologic CARD8 activation.
Despite the genetic diversity of the above inflammasomopathies, the affected pathways all converge on inflammasome-mediated GSDMD cytokine release and pyroptosis. Thus, the inflammasome-GSDMD axis represents a rational set of targets to modulate these diseases. While biologic drugs targeting disease-associated cytokines have proven to be beneficial, upstream targeting of inflammasomes or GSDMD may be more efficacious by simultaneously blocking the release of multiple inflammatory mediators. In mice, many of these diseases are rescued by knockout of ASC, inflammatory caspases, or GSDMD, supporting the viability of this approach [46–48].
Monogenic disorders, although rare, often serve as testing platforms for novel pathway modulating agents, benefitted by their orphan disease status and uniform pathophysiology. As such, these inflammasomopathies may become a primary point-of-entry into human use for novel inflammasome and GSDMD targeting therapies. The established efficacy of therapeutically blocking downstream mediators further enforces the potential for this approach. Identification of suitable orally bioavailable small molecules would also benefit patients who currently rely on regular injection of biologics. Demonstration of safety and efficacy of inflammasome-directed therapies in these rare genetic diseases will additionally pave the way for expanded use in diverse inflammatory diseases where inflammasomes and GSDM-mediated pyroptosis contribute to morbidity and mortality.
Diseases with Aberrant Inflammasome Activation
While the initial entry of inflammasome- and GSDM-targeted therapeutics into the clinical realm may be for the treatment of the above rare genetic disorders, the long-term impact of such therapeutics is expected to be greatest for complex diseases with an inflammasome-driven component to their pathophysiology.
Acute pathologies that fit this mold include sepsis, disseminated intravascular coagulation (DIC) and cytokine release syndrome (CRS). Multiple studies have shown protection from sepsis in Gsdmd-deficient mice [42,44,45,135]. The importance of GSDMD for releasing NETs and activating tissue factor support its importance for DIC [136–139]. Finally, GSDME has been shown to cause CRS in models of chimeric antigen receptor (CAR) T cell therapy [119]. Whether other GSDMs play roles in acute inflammatory diseases remains to be seen. Still, the preponderance of data show that GSDM activation can contribute to an overwhelming inflammatory response. Given the profound disease burden attributed to these and similar life-threatening conditions, GSDM inhibitors may deliver groundbreaking therapeutic advances in these contexts.
In terms of chronic diseases with demonstrated inflammasome- and GSDM-mediated pathology, the list includes atherosclerosis, liver disease, diabetes, cardiovascular disease, ischemic stroke, graft-versus-host-disease, sickle cell anemia, neurodegenerative diseases and multiple sclerosis (MS), among others (reviewed in [7]). This laundry list of conditions (Table 2) includes some of the biggest killers and most costly diseases in medicine. The implication of inflammasomes and GSDMD in the pathogenesis of complications across such a prevalent and burdensome set of disease raises the potential impact of successfully targeting GSDM-mediated pyroptosis to revolutionary in its scope. Interested readers are referred to other reviews in this issue for more specifics regarding some of these disease topics.
Table 2.
Acute and chronic diseases associated with inflammasome activation
| Disease | References | |
|---|---|---|
| Acute | Sepsis | [44,45,135,210] |
| Disseminated Intravascular Coagulation (DIC) | [136–139] | |
| Cytokine Release Syndrome (CRS) | [119] | |
| Tumor Lysis Syndrome | [211] | |
| Chronic | Multiple Sclerosis | [212,213] |
| Atherosclerosis | [214,215] | |
| Diabetes | [216–218] | |
| Gout (and other crystal/particulate disorders) | [90,91,96,187,215,219,220] | |
| Alzheimer’s Disease | [221,222] | |
| Asthma | [20,25,26,80,223–226] | |
| Inflammatory Bowel Disease | [193,227,228] | |
| Non-alcoholic Fatty Liver Disease | [229] | |
| Ischemic Stroke | [230] | |
| Sickle Cell Anemia | [231–233] | |
| Rheumatoid Arthritis | [234,235] |
The data on GSDMs, especially GSDMD, in infectious diseases reveals a mixed picture. While GSDMD contributes to defense against some microorganisms [140–142], Gsdmd-deficient mice show better outcomes in other infection models [52,143]. Likely, the balance between GSDMD-mediated eradication of replicative environments and constructive immune stimulation versus inflammatory tissue pathology will determine whether therapeutic benefit can be derived from targeting GSDMD in distinct infectious diseases. Such information will need to be generated on a case-by-case basis.
Anti-Tumor Immunity
The extent to which GSDMs contribute to the immunogenicity of cancer cell death is incompletely understood, yet emerging evidence supports the sufficiency of multiple GSDMs to produce ICD [32,120]. The strongest data supporting the function of GSDMs in anti-tumor immunity come for GSDMB and GSDME, which are cleaved by granzyme A and B, respectively, from CTLs. Expression of GSDMB by cancer cells promoted efficacy of anti-PD-1 therapy in immune competent tumor-bearing mice [32]. For GSDME, two separate papers have now shown its role in driving ICD, as GSDME expressing tumors in immunocompetent mice fail to regrow tumors after either tumor re-challenge [120] or therapy cessation [144]. Furthermore, while low-level GSDMC cleavage may be responsible for tolerizing tumor necrosis in some tumors, activation of caspase-8-mediated cleavage by certain chemotherapeutics promotes cancer cell pyroptosis [35]. Likewise, secondary GSDMD activation can occur downstream of apoptotic stimuli [112]. These data highlight the potential for GSDMs to activate anti-tumor immunity in response to CTLs or chemotherapy, though much remains to be learned about the full extent to which individual GSDMs contribute to ICD in different malignancies. Approaches to therapeutically control GSDM activity to direct anti-tumor immunity will be discussed below.
Hematopoietic Disorders
Cytopenias (decreased blood cell counts) arise as part of many inflammatory and autoimmune disorders. Mechanistically, chronic inflammation drives hematopoietic impairment that involves inflammasome activation. For example, anemia of chronic disease shows contribution from caspase-1 activation and prolonged IL-1β exposure drives hematopoietic stem cell exhaustion in model systems [145,146]. Yet, the contributions of GSDMs to these effects remain largely unknown. This area deserves further investigation both to prevent bone marrow failure caused by chronic inflammation and to understand potential hematological consequences of therapeutically modulating the inflammasome-GSDMD axis.
Additionally, inflammasomes have emerging functions in age-related clonal hematopoiesis, also known as clonal hematopoiesis of indeterminate potential (CHIP). These terms describe the age-dependent acquisition of neoplasia-associated mutations without overt hematopoietic neoplasm. In addition to being a precursor to malignancies such as myelodysplastic syndromes (MDS) or acute myeloid leukemia (AML), CHIP is a risk factor for cardiovascular morbidity and mortality [147–149]. Atherosclerosis and heart failure are exacerbated in a CHIP mouse model through an NLRP3-dependent mechanism [150,151]. Inflammasome activation may, in fact, be a general feature of CHIP that provides a selective advantage to these clones [152–154]. These data suggest that CHIP-associated inflammasome activation may contribute to a variety of diseases of aging.
The exact functions exerted by inflammasomes and GSDMs in myeloid leukemogenesis should be the focus of future research. Additionally, whether they can be inhibited to limit myeloid leukemogenesis or selectively activated to treat myeloid leukemia remain open questions. Such efforts may turn CHIP from a risk factor to an actionable biomarker to prevent inflammatory pathology and forestall the development of MDS and AML.
Strategies to Target GSDM-Mediated Pyroptosis
Having laid the foundation to understand GSDM biology and disease associations, we now turn to approaches to modulate GSDM function therapeutically. Conceptually, chemical modulation of GSDM-mediated pyroptosis can be divided into three general categories of targets: the upstream pathways, the GSDMs themselves and the downstream effectors (Table 3).
Table 3.
Current pharmacological strategies for modulation of pyroptosis
| Target | Drug | Phase | References/ Clinical Trials |
|---|---|---|---|
| Inhibitors of Pyroptosis | |||
| NLRP3 | MCC950 | Failed Phase 2 trial in rheumatoid arthritis patients due to hepatotoxicity | [164] |
| Inzomelid | Completed Phase 1 | NCT04015076 | |
| IZD334 | Completed Phase 1 | NCT04086602 | |
| Tranilast | Approved outside the US for asthma, keloids, and conjunctivitis; Phase 2 trial ongoing in CAPS patients | NCT03923140 | |
| DFV890 (IFM-2427) | Phase 2 trials for knee osteoarthritis, FACS, and COVID-19 | NCT04868968; NCT04886258; NCT04382053 | |
| Dapansutrile | Phase 2 trial completed for gout and in progress for CRS | [168]; NCT04540120 | |
| RRx-001 | Phase 3 for oncology applications | [167] | |
| CY-09 | Pre-clinical | [169] | |
| Oridonin | Pre-clinical | [166] | |
| INF39 | Pre-clinical | [170] | |
| Caspase-1 | Pralnacasan (VX-740) | Failed Phase 2 trial in rheumatoid arthritis patients due to hepatotoxicity | [175] |
| Belnacasan (VX-765) | Failed to meet primary endpoints in Phase 2 trial in epilepsy patients | [175,178] | |
| GSDMD | Necrosulfonamide | Pre-clinical | [44] |
| Disulfiram | Approved for alcohol dependence; Phase 1–3 for oncology applications | [45,171] | |
| Dimethyl fumarate | Approved in the US for multiple sclerosis; Approved in the EU for plaque psoriasis | [172] | |
| Unknown | Punicalagin | Pre-clinical | [200] |
| LDC7559 | Pre-clinical | [138] | |
| Activators of Pyroptosis | |||
| NLRP1/ CARD8 | Val-boroPro (talabostat, BXCL701) | Phase 1–3 for oncology applications | NCT03910660; NCT03910660; NCT04171219 |
| NLRP3 | BMS-986299 | Phase 1 | NCT03444753 |
| GSDMA | Nanoparticle-conjugated delivery of GSDMA3 | Pre-clinical | [199] |
| GSDMD | AAV delivery of GSDMDNT | Pre-clinical | [198] |
AAV = adeno-associated virus; EU = European Union
Targeting upstream pathways
The most advanced therapeutic strategies directed at upstream drivers of GSDM-mediated pyroptosis target the NLRP3 inflammasome, for reasons related to its broad relevance to inflammatory diseases as mentioned above. Selective pharmacologic inhibition of the NLRP3 inflammasome was first demonstrated with the sulfonylurea glyburide in 2009 [155]. A more potent diarylsulfonylurea analog, MCC950, also selectively inhibits the NLRP3 inflammasome by binding to the NBD and blocking ATP hydrolysis [156–158]. While MCC950 has shown therapeutic benefit in mouse models of myocardial infarction, atherosclerosis, Parkinson’s disease, Alzheimer’s disease, EAE and colitis, it is unable to inhibit some CAPS-associated NLRP3 mutants [157,159–163]. Unfortunately, development of MCC950 stalled due to hepatotoxicity in a phase 2 clinical trial for rheumatoid arthritis [164]. Two analogs of MCC950, inzomelid and IZD334, have demonstrated safety for healthy adults in phase 1 clinical trials and offer the latest hope for this class of inflammasome inhibitors (https://clinicaltrials.gov/ct2/show/NCT04015076, https://clinicaltrials.gov/ct2/show/NCT04086602).
Tranilast, a tryptophan derivative, also binds within the NBD of NLRP3 but inhibits inflammasome activation by blocking oligomerization [165]. Tranilast is approved for use outside of the US in asthma, allergic conjunctivitis, and keloids and has shown efficacy in mouse models of gout and CAPS. A phase 2 clinical trial for its use in CAPS patients is ongoing (https://clinicaltrials.gov/ct2/show/NCT03923140). Additional NLRP3 inhibitors are in various stages of development ([166–172] and see recent reviews on the topic [110,164,173]).
Currently, specific inhibitors of other inflammasomes are not available. Proteasome inhibitors can block NLRP1 and CARD8 activation but have much broader cellular activity [174]. Specific inhibition of other inflammasomes may prove efficacious in diseases where that inflammasome drives detrimental pathology rather than playing a prominent role in disease resolution or pathogen eradication. However, such strategies may simply compromise immunity to specific infectious agents.
Another upstream approach to targeting GSDM-mediated pyroptosis is inhibition of caspase-1. Tetrapeptide inhibitors targeting the catalytic cysteine within the caspase-1 active site have failed clinically due to unfavorable pharmacology and toxicity profiles [175]. Synthetic peptidomimetic prodrugs were developed to overcome these issues; these drugs include pralnacasan (VX-740) and belnacasan (VX-765). The metabolic products of both of these drugs are reversible inhibitors of caspase-1/4/5 [176,177]. A phase 2 clinical trial of VX-740 in rheumatoid arthritis patients was stopped due to hepatotoxicity, while a phase 2 clinical trial of VX-765 in partial epilepsy patients was stopped after it failed to reach its primary endpoints [175,178]. At this time, the future of this therapeutic strategy remains uncertain.
While intense pharmaceutical efforts are directed toward inhibiting pyroptosis, it is also possible to induce inflammasome activation using the anticancer drug Val-boroPro (Talabostat, BXCL701) and similar agents. Val-boroPro is a nonselective inhibitor of post-proline cleaving serine proteases, including DPP8 and DPP9. While it was initially proposed that Val-boroPro treatment promoted caspase-1 activation directly, it has more recently been shown that inhibition of DPP8/9 activates the NLRP1 and CARD8 inflammasomes, leading to downstream caspase-1 activation and pyroptosis [86,174,179,180]. The mechanism for DPP8/9 regulation of inflammasome formation is still incompletely understood, with data supporting roles for both binding and enzymatic activity to repress CARD8 and NLRP1 [180,181]. In line with CARD8 being ASC-independent in humans, Val-boroPro appears to cause low-level caspase-1 and GSDMD cleavage. The therapeutic implications of this inefficient cleavage remain unclear. Yet, Val-boroPro shows efficacy in mouse models of AML. Earlier studies demonstrated immune activation against cancers in multiple mouse models [182,183]. However, this agent also kills normal B and T lymphocytes and CD34-positive hematopoietic progenitor cells in vitro [86,184]. Likewise, NLRP1 activation kills hematopoietic stem cells [185], raising questions about the therapeutic window for this strategy. Val-boroPro and a distinct inflammasome activator are in early clinical trials in solid tumors (https://clinicaltrials.gov/ct2/show/NCT03910660, https://clinicaltrials.gov/ct2/show/NCT03444753) [186]. Continued research may identify therapeutically tractable approaches to inflammasome activation that will break immune tolerance in solid tumors or directly treat cancers via induction of pyroptosis.
Targeting GSDMs: GSDMD Inhibitors
As a convergence point for upstream inflammasome pathways and as a conduit for Il-1 family cytokine and DAMP release, GSDMD represents a logical drug target for disease with inflammasome-mediated pathology. Based on the protective effects of GSDMD knockout across numerous murine inflammatory disease models, therapeutic inhibition of GSDMD may become an efficacious strategy to prevent inflammasome-mediated pathology.
Several inhibitors of GSDMD-mediated pyroptosis have been identified over the past few years. The first direct inhibitor of GSDMD, the cysteine-reactive alkylating agent necrosulfonamide(NSA), was found to bind directly to GSDMD at C191 and consequently to inhibit oligomerization and pore formation by GSDMDNT [44]. Other groups have since shown that NSA may also inhibit upstream steps on the way to GSDMD pyroptosis, such as caspase-1 activation and GSDMD cleavage [45,187]. While NSA shows efficacy in mouse models of sepsis[44], it lacks specificity for GSDMD, as it was originally identified as an inhibitor of human (but not murine) mixed-lineage kinase domain-like (MLKL) protein [188].
Identification of other small molecules that target C191 underscores the importance of this residue for GSDMD function and its potential as a site for rational drug development. Among them, disulfiram (DSF), a drug used to treat alcohol dependence, was identified through a liposome-leakage based screen as an inhibitor of GSDMD. Similar to NSA, it was found to bind directly to C191 on GSDMD and prevent IL-1β release as well as lytic cell death. Both DSF and NSA decreased serum cytokine levels and extended survival of wild-type mice after a lethal dose of LPS [44,45]. Likewise for both compounds, mutation of C191 in GSDMD reduces their ability to inhibit pyroptosis. However, both compounds additionally have upstream inhibitory effects. While GSDME lacks a residue corresponding to C191, DSF apparently inhibits GSDME processing by an uncertain mechanism [189].
A third inhibitor of GSDMD driven pyroptosis is the MS drug dimethyl fumarate (DMF). Endogenous fumarate, which accumulates in activated macrophages, causes GSDMD succination at C191 and other sites, which impairs inflammatory caspase binding [57]. The fumarate analog DMF functions similarly. Murine treatment with DMF protected mice against a lethal dose of LPS and FMF activating mutations in MEVF, as well as showing efficacy in an EAE model. Mass spectrometric analysis identified 5 cysteines in humans and 7 in mice that are modified by DMF. Furthermore, it was found to similarly modify GSDME at C45 and other sites, resulting in GSDME blockade. While this result again highlights the lack of specificity of GSDMD inhibitors, this dual inhibition could be beneficial given the compensatory role for GSDME in IL-1β release [57,64].
The non-cysteine-reactive molecule LDC7559 was identified through screening to block both PMA and cholesterol crystal-induced NET formation in neutrophils and silica-induced IL-1β release in human monocytes [138]. While it associates with GSDMD via affinity chromatography, direct binding was not verified and the compound did not appear to inhibit GSDMDNT activity in a liposome leakage assay [45]. Lanthanides, trivalent cations with broad channel blocking activity, also inhibit inflammasome-driven propidium dye influx and pyroptosis [190]. The recent recognition of important acidic residues within the GSDMD channel interior may help to explain these effects [191]. However, lanthanides did not significantly impair IL-1β release. Further studies are needed to validate the precise mechanism of action for these agents.
It is important to remember that the efficacy of GSDMD inhibition may be disease specific. For example, GSDMD exerts anti-inflammatory properties in some contexts and is critical for clearance of certain pathogens. Contradictory effects of GSDMD deletion have been seen in models of IBD [192,193]. Moreover, our recent work demonstrates that GSDME can compensate for GSDMD to release IL-1β [64]. Furthermore, other studies have shown that GSDMD loss does not prevent IL-1β release or cell death in some inflammasome-driven pathologies, suggesting that not all conditions resulting in inflammasome activation will benefit from therapeutic targeting of GSDMD alone [187]. The extensive ongoing efforts toward understanding GSDMD biology are likely to determine diseases where GSDMD inhibition will be of benefit.
Currently, only molecules targeting GSDMD (some with additional activities as above) have been reported. However, advances in the biology of other GSDMs are likely to reveal novel agents that inhibit other GSDM family members. Efforts will likely include pharmacological attempts to specifically target the C191 site in GDSMD and homologs. The recent identification of a caspase-1-GSDMDCT exosite binding interface may represent an additional site for rational drug design [51]. Other GSDMs may have similar or unique protease interaction sites that could serves as scaffolds for structure-based inhibitor development. Emerging data show that electrostatic forces additionally control mature IL-1β release via GSDMD pores, demonstrating an additional potential targetable feature [191]. Finally, pathway targeting agents that alter GSDM PTMs may exhibit therapeutic effects in GSDM-mediated pathologies.
Targeting GSDMs: Therapeutic Activation
Given the transformational nature of recent therapeutic advances harnessing the immune system to treat cancer [194], the excitement surrounding GSDM functions in anti-tumor immunity is justified. This burgeoning area of GSDM biology includes several approaches to activate GSDMs for cancer therapy. For example, GSDME is hypermethylated in many cancers and its expression can be induced by hypomethylating agents such as decitabine [63,195,196]. As mentioned earlier, research into other GSDMs has identified positive regulatory factors for other GSDMs that may present additional means to enhance GSDM expression by cancer cells. These inducing factors include interferons and TNF-α for GSDMB [32], STAT3 among other stimuli for GSDMC [35], and IRF2 for GSDMD [197]. Agents to enhance GSDM expression in cancers may work alone or in combination with therapeutics to activate appropriate GSDM-cleaving proteases.
Additionally, enforced expression or selective delivery of GSDMs can induce ICD. Established experimental techniques to this end include adenoviral expression of GSDMDNT under a tumor specific promoter and delivery of nanoparticles conjugated to a modified active GSDMA3 molecule that co-accumulates in tumors with its releasing agent [198,199]. Of note, one of these studies showed that pyroptosis of fewer than 15% of tumor cells was sufficient to promote complete tumor regression. If appropriate specificity can be achieved to prevent off-target pyroptosis, this finding suggests that similar approaches may be able to activate an anti-tumor immune response without causing tumor lysis syndrome or CRS.
Beyond target specificity, one great hurdle for GSDM activation as a cancer therapeutic will be the avoidance of normal tissue damage and systemic inflammatory complications. The initial description of GSDME pyroptosis showed that it contributed to the tissue toxicity of chemotherapy [63]. Subsequent work showed that GSDME contributed to CRS upon CAR T cell therapy [119]. Likewise, while DPP8/9 inhibitors can induce pyroptosis in AML blasts, they also show on-target toxicity toward normal hematopoietic cells [86]. Thus, whether such strategies can be safely applied will be an important area of research moving forward. Of note, loss-of-function mutations in GSDMs are seen in cancer [120]. Such mutations would be predicted to impair anti-tumor immunity.
Targeting Downstream Processes and Effectors
Billed as a GSDMD inhibitor, punicalagin, a complex polyphenol found in pomegranate extract, was found to prevent ATP-induced IL-1β secretion and pyroptosis. The mechanism by which it inhibits cell death is unknown. However, it was also shown to protect against detergent lysis and may inhibit GSMD membrane insertion via lipid membrane stabilization [200]. Glycine, a cytoprotective agent, has long been known to prevent pyroptosis by an uncertain mechanism that likewise occurs downstream of GSDMD [190,201]. Further studies are needed to clarify the targets of these agents.
Cellular mechanisms in place to repair membrane integrity can be engaged upon insertion of GSDMD pores. The endosomal sorting complex required for transport (ESCRT) machinery is activated by GSDMD-dependent Ca2+ influx to excise GSDMD pore-containing membrane vesicles. However, it seems that pyroptosis proceeds when a critical threshold of GSDMD pores accumulate [202]. At this time the involvement of other membrane repair pathways and the contribution of the ESCRT pathway to forestalling pyroptosis downstream of other GSDMs remain to be determined [203]. Additionally, a recent report showed that the small transmembrane protein NINJ1 facilitates a common pathway for plasma membrane rupture downstream of both pyroptotic and apoptotic stimuli [204]. The therapeutic potential of these downstream pathways is uncertain but merits further investigation. Inhibiting ESCRT may prevent replication of bacteria through rapid pyroptosis or cause a dramatic systemic inflammatory response syndrome. Inducing ESCRT pathway activity will allow prolonged inflammatory cytokine egress through GSDMD pores prior to repair which may also result in a hyperactive state [205]. Therefore, the effects of ESCRT modulation on systemic immune responses need to be addressed in appropriate models.
Other Ca2+ mediated events downstream of GSDMD pore formation include activation of calpains (whose targets include vimentin and IL-1α), TMEM16F (which can accelerate coagulation via tissue factor-phosphatidylserine interaction) and phospholipases [135,206–208]. Oligomerization of GDMD appears to require Ca2+ and this step can be blocked by supraphysiological levels of Mg2+ [209]. The diversity of processes engaged downstream of GSDMD pores further highlights its critical placement as a limiting step in inflammasome-driven mediator release and pyroptosis. The utility of targeting these downstream events individually is not known.
As mentioned above, several monoclonal antibody therapies targeting inflammatory cytokines released upon inflammasome activation have become clinically available for treatment of inflammatory diseases. These agents come with their own drawbacks, including infection risk, route of administration by injection, lack of central nervous system penetration and specificity for a single downstream mediator. Still, the burden of proof will fall on the field to demonstrate acceptable safety and efficacy of inflammasome- and GSDM-targeted therapies before such agents can overtake non-specific anti-inflammatory drugs and these biologic agents as mainstays of treatment for inflammatory diseases.
Future directions
The discovery of pore-forming ability of GSDMD in 2015 answered an important question in the field of inflammasome biology. It simultaneously opened new avenues of inquiry and created opportunity for the development of novel therapeutics for a wide variety of diseases. A robust body of research has now determined regulatory mechanisms and highlighted potential translational relevance for many of the GSDMs. Critical next steps for inflammatory diseases will include the development of specific GSDM inhibitors, the determination of appropriate disease contexts and the evaluation of safety and efficacy of targeting at each step outlined in this review. For cancer, the contribution of GSDMs to ICD and anti-tumor immunity should be determined on a global scale. These data may lead to personalized approaches to anti-tumor immunity involving combinatorial therapies targeting GSDM-expression in tumors. Less than a decade since the initial discovery of GSDMD pore formation, the broad potential of therapeutic modulation of GSDM-mediated pyroptosis is just beginning to be realized. Much work remains to be done, yet the promise of these strategies is undeniable.
Highlights.
Gasdermins have emerged as critical effectors of inflammatory mediator release and pyroptosis
The central position of gasdermins as a bottleneck between diverse upstream activating pathways and release of myriad effectors makes them a prime target for therapeutic intervention
This review will discuss the known function and regulation of gasdermins in human disease and describe emerging strategies to target gasdermin-mediated pyroptosis therapeutically
Abbreviations
- AML
Acute myeloid leukemia
- ASC
Apoptosis-associated speck-like protein containing a CARD
- CAPS
Cryopyrin-associated periodic syndrome
- CAR
Chimeric antigen receptor
- CARD
Caspase activation and recruitment domain
- CHIP
Clonal hematopoiesis of indeterminate potential
- CRS
Cytokine release syndrome
- CT
C-terminal/C=terminus
- CTL
Cytotoxic T lymphocyte
- DAMP
Danger-associated molecular pattern
- DIC
Disseminated intravascular coagulation
- DMF
Dimethylfumarate
- DPP9
Dipeptidyl peptidase 9
- Ds
Double-stranded
- DSF
Disulfiram
- EAE
Experimental autoimmune encephalitis
- ELANE
Neutrophil elastase
- ESCRT
Endosomal sorting complex required for transport
- FCAS
Familial cold auto-inflammatory syndrome
- FIIND
Function to find’ domain
- FKLC
Familial keratosis lichenoides chronica
- FMF
Familial Mediterranean fever
- GSDM
Gasdermin
- IBD
Inflammatory bowel disease
- ICD
Immunogenic cell death
- JRRP
Juvenile recurrent respiratory papillomatosis
- MDS
Myelodysplastic syndrome
- MLKL
Mixed-lineage kinase domain-like
- MS
Multiple sclerosis
- MSPC
Multiple self-healing palmoplantar carcinoma
- NET
Neutrophil extracellular trap
- NLR
Nucleotide-binding domain (NBD), leucine-rich repeat (LRR)-containing
- NOMID
Neonatal-onset multisystem inflammatory disorder
- NSA
Necrosulfonamide
- NT
N-terminal/N-terminus
- PLC
Phospholipase C
- PTM
Post-translational modification
- PYD
Pyrin domain
Footnotes
CRedit Author Statement
Christopher Ryder: Wrote portions of manuscript, edited entire manuscript, Figure and Table preparation
Hannah Kondolf: Wrote portions of manuscript, Figure and Table preparation
Meghan O’Keefe: Wrote portions of manuscript. Table preparation
Bowen Zhou: Wrote portions of manuscript
Derek Abbott: Edited manuscript, contributed to Figure and Table preparation, oversaw assembly and vision.
Declaration of interests
☐ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Shalapour S, Karin M, Immunity, inflammation, and cancer: An eternal fight between good and evil, J. Clin. Invest. 125 (2015) 3347–3355. 10.1172/JCI80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rainsford KD, Anti-inflammatory drugs in the 21st century, Subcell. Biochem. 42 (2007) 3–27. 10.1007/1-4020-5688-5_1. [DOI] [PubMed] [Google Scholar]
- [3].Shi J, Gao W, Shao F, Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death, Trends Biochem. Sci. 42 (2017) 245–254. 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- [4].Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, Sun H, Wang DC, Shao F, Pore-forming activity and structural autoinhibition of the gasdermin family, Nature. 535 (2016) 111–116. 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- [5].Shi P, Tang A, Xian L, Hou S, Zou D, Lv Y, Huang Z, Wang Q, Song A, Lin Z, Gao X, Loss of conserved Gsdma3 self-regulation causes autophagy and cell death, Biochem. J. 468 (2015) 325–336. 10.1042/BJ20150204. [DOI] [PubMed] [Google Scholar]
- [6].De Schutter E, Roelandt R, Riquet FB, Van Camp G, Wullaert A, Vandenabeele P, Punching Holes in Cellular Membranes: Biology and Evolution of Gasdermins, Trends Cell Biol. 31 (2021). 10.1016/j.tcb.2021.03.004. [DOI] [PubMed] [Google Scholar]
- [7].Liu X, Xia S, Zhang Z, Wu H, Lieberman J, Channelling inflammation: gasdermins in physiology and disease, Nat. Rev. Drug Discov. 20 (2021) 384–405. 10.1038/s41573-021-00154-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Broz P, Pelegrín P, Shao F, The gasdermins, a protein family executing cell death and inflammation, Nat. Rev. Immunol. 20 (2020) 143–157. 10.1038/s41577-019-0228-2. [DOI] [PubMed] [Google Scholar]
- [9].Saeki N, Kuwahara Y, Sasaki H, Satoh H, Shiroishi T, Gasdermin (Gsdm) localizing to mouse chromosome 11 is predominantly expressed in upper gastroiatestinal tract but significantly suppressed in human gastric cancer cells, Mamm. Genome. 11 (2000) 718–724. 10.1007/s003350010138. [DOI] [PubMed] [Google Scholar]
- [10].Sato H, Koide T, Masuya H, Wakana S, Sagai T, Umezawa A, Ishiguro S, Tama M, A new mutation Rim3 resembling Rede” is mapped close to retinoic acid receptor alpha (Rara) gene on mouse Chromosome 11, 25 (1998) 20–25. [DOI] [PubMed] [Google Scholar]
- [11].Runkel F, Marquardt A, Stoeger C, Kochmann E, Simon D, Kohnke B, Korthaus D, Wattler F, Fuchs H, Hrabé De Angelis M, Stumm G, Nehls M, Wattler S, Franz T, Augustin M, The dominant alopecia phenotypes Bareskin, Rex-denuded, and Reduced Coat 2 are caused by mutations in gasdermin 3, Genomics. 84 (2004) 824–835. 10.1016/j.ygeno.2004.07.003. [DOI] [PubMed] [Google Scholar]
- [12].Tanaka S, Tamura M, Aoki A, Fujii T, Komiyama H, Sagai T, Shiroishi T, A new Gsdma3 mutation affecting anagen phase of first hair cycle, Biochem. Biophys. Res. Commun. 359 (2007) 902–907. 10.1016/j.bbrc.2007.05.209. [DOI] [PubMed] [Google Scholar]
- [13].Li J, Zhou Y, Yang T, Wang N, Lian X, Yang L, Gsdma3 is required for hair follicle differentiation in mice, Biochem. Biophys. Res. Commun. 403 (2010) 18–23. 10.1016/j.bbrc.2010.10.094. [DOI] [PubMed] [Google Scholar]
- [14].Kumar S, Rathkolb B, Budde BS, Nürnberg P, de Angelis MH, Aigner B, Schneider MR, Gsdma3I359N is a novel ENU-induced mutant mouse line for studying the function of Gasdermin A3 in the hair follicle and epidermis, J. Dermatol. Sci. 67 (2012) 190–192. 10.1016/j.jdermsci.2012.05.001. [DOI] [PubMed] [Google Scholar]
- [15].Tanaka S, Mizushina Y, Kato Y, Tamura M, Shiroishi T, Functional Conservation of Gsdma Cluster Genes Specifically Duplicated in the Mouse Genome, G3 Genes, Genomes, Genet. 3 (2013) 1843–1850. 10.1534/g3.113.007393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F, Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death, Nature. 526 (2015) 660–665. 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- [17].Rogers C, Erkes DA, Nardone A, Aplin AE, Fernandes-Alnemri T, Alnemri ES, Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation, Nat. Commun. 10 (2019) 1689. 10.1038/s41467-019-09397-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Söderman J, Berglind L, Almer S, Gene expression-genotype analysis implicates GSDMA, GSDMB, and LRRC3C as contributors to inflammatory bowel disease susceptibility, Biomed Res. Int. 2015 (2015). 10.1155/2015/834805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Terao C, Kawaguchi T, Dieude P, Varga J, Kuwana M, Hudson M, Kawaguchi Y, Matucci-Cerinic M, Ohmura K, Riemekasten G, Kawasaki A, Airo P, Horita T, Oka A, Hachulla E, Yoshifuji H, Caramaschi P, Hunzelmann N, Baron M, Atsumi T, Hassoun P, Torii T, Takahashi M, Tabara Y, Shimizu M, Tochimoto A, Ayuzawa N, Yanagida H, Furukawa H, Tohma S, Hasegawa M, Fujimoto M, Ishikawa O, Yamamoto T, Goto D, Asano Y, Jinnin M, Endo H, Takahashi H, Takehara K, Sato S, Ihn H, Raychaudhuri S, Liao K, Gregersen P, Tsuchiya N, Riccieri V, Melchers I, Valentini G, Cauvet A, Martinez M, Mimori T, Matsuda F, Allanore Y, Transethnic meta-Analysis identifies GSDMA and PRDM1 as susceptibility genes to systemic sclerosis, Ann. Rheum. Dis. 76 (2017) 1150–1158. 10.1136/annrheumdis-2016-210645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yu J, Kang MJ, Kim BJ, Kwon JW, Song YH, Choi WA, Shin YJ, Hong SJ, Polymorphisms in GSDMA and GSDMB are associated with asthma susceptibility, atopy and BHR, Pediatr. Pulmonol. 46 (2011) 701–708. 10.1002/ppul.21424. [DOI] [PubMed] [Google Scholar]
- [21].Zihlif M, Obeidat NM, Zihlif N, Mahafza T, Froukh T, Ghanim MT, Beano H, AL-Akhras FM, Naffa R, Association Between Gasdermin A and Gasdermin B Polymorphisms and Susceptibility to Adult and Childhood Asthma Among Jordanians, Genet. Test. Mol. Biomarkers. 20 (2016) 143–148. 10.1089/gtmb.2015.0174. [DOI] [PubMed] [Google Scholar]
- [22].Moussette S, Al Tuwaijri A, Kohan-Ghadr HR, Elzein S, Farias R, Bérubé J, Ho B, Laprise C, Goodyer CG, Rousseau S, Naumova AK, Role of DNA methylation in expression control of the IKZF3-GSDMA region in human epithelial cells, PLoS One. 12 (2017) 1–13. 10.1371/journal.pone.0172707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Saeki N, Kim DH, Usui T, Aoyagi K, Tatsuta T, Aoki K, Yanagihara K, Tamura M, Mizushima H, Sakamoto H, Ogawa K, Ohki M, Shiroishi T, Yoshida T, Sasaki H, GASDERMIN, suppressed frequently in gastric cancer, is a target of LMO1 in TGF-β-dependent apoptotic signalling, Oncogene. 26 (2007) 6488–6498. 10.1038/sj.onc.1210475. [DOI] [PubMed] [Google Scholar]
- [24].Verlaan DJ, Berlivet S, Hunninghake GM, Madore AM, Larivière M, Moussette S, Grundberg E, Kwan T, Ouimet M, Ge B, Hoberman R, Swiatek M, Dias J, Lam KCL, Koka V, Harmsen E, Soto-Quiros M, Avila L, Celedón JC, Weiss ST, Dewar K, Sinnett D, Laprise C, Raby BA, Pastinen T, Naumova AK, Allele-Specific Chromatin Remodeling in the ZPBP2/GSDMB/ORMDL3 Locus Associated with the Risk of Asthma and Autoimmune Disease, Am. J. Hum. Genet. 85 (2009) 377–393. 10.1016/j.ajhg.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kang MJ, Yu HS, Seo JH, Kim HY, Jung YH, Kim YJ, Kim HJ, Lee SY, Hong SJ, GSDMB/ORMDL3 variants contribute to asthma susceptibility and eosinophil-mediated bronchial hyperresponsiveness, Hum. Immunol. 73 (2012) 954–959. 10.1016/j.humimm.2012.06.009. [DOI] [PubMed] [Google Scholar]
- [26].Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, von Mutius E, Farrall M, Lathrop M, Cookson WOCM, A Large-Scale, Consortium-Based Genomewide Association Study of Asthma, N. Engl. J. Med. 363 (2010) 1211–1221. 10.1056/nejmoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chao KL, Kulakova L, Herzberg O, Gene polymorphism linked to increased asthma and IBD risk alters gasdermin-B structure, a sulfatide and phosphoinositide binding protein, Proc. Natl. Acad. Sci. U. S. A. 114 (2017) E1128–E1137. 10.1073/pnas.1616783114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Panganiban RA, Sun M, Dahlin A, Park HR, Kan M, Himes BE, Mitchel JA, Iribarren C, Jorgenson E, Randell SH, Israel E, Tantisira K, Shore S, Park JA, Weiss ST, Wu AC, Lu Q, A functional splice variant associated with decreased asthma risk abolishes the ability of gasdermin B to induce epithelial cell pyroptosis, J. Allergy Clin. Immunol. 142 (2018) 1469–1478.e2. 10.1016/j.jaci.2017.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sun Q, Yang J, Xing G, Sun Q, Zhang L, He F, Expression of GSDML associates with tumor progression in uterine cervix cancer, Transl. Oncol. 1 (2008) 73–83. 10.1593/tlo.08112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hergueta-Redondo M, Sarrió D, Molina-Crespo Á, Megias D, Mota A, Rojo-Sebastian A, García-Sanz P, Morales S, Abril S, Cano A, Peinado H, Moreno-Bueno G, Gasdermin-B promotes invasion and metastasis in breast cancer cells, PLoS One. 9 (2014). 10.1371/journal.pone.0090099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hergueta-Redondo M, Sarrio D, Molina-Crespo Á, Vicario R, Bernadó-Morales C, Martínez L, Rojo-Sebastián A, Serra-Musach J, Mota A, Martínez-Ramírez Á, Castilla MÁ, González-Martin A, Pernas S, Cano A, Cortes J, Nuciforo PG, Peg V, Palacios J, Pujana MÁ, Arribas J, Moreno-Bueno G, Gasdermin B expression predicts poor clinical outcome in HER2-positive breast cancer, Oncotarget. 7 (2016) 56295–56308. 10.18632/oncotarget.10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhou Z, He H, Wang K, Shi X, Wang Y, Su Y, Wang Y, Li D, Liu W, Zhang Y, Shen L, Han W, Shen L, Ding J, Shao F, Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells, Science (80-. ). 182 (2020) eaaz7548. 10.1126/science.aaz7548. [DOI] [PubMed] [Google Scholar]
- [33].Watabe K, Ito A, Asada H, Endo Y, Kobayashi T, Nakamoto K, Itami S, Takao S, Shinomura Y, Aikou T, Yoshikawa K, Matsuzawa Y, Kitamura Y, Nojima H, Structure, expression and chromosome mapping of MLZE, a novel gene which is preferentially expressed in metastatic melanoma cells, Japanese J. Cancer Res. 92 (2001) 140–151. 10.1111/j.1349-7006.2001.tb01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Miguchi M, Hinoi T, Shimomura M, Adachi T, Saito Y, Niitsu H, Kochi M, Sada H, Sotomaru Y, Ikenoue T, Shigeyasu K, Tanakaya K, Kitadai Y, Sentani K, Oue N, Yasui W, Ohdan H, Gasdermin C Is Upregulated by Inactivation of Transforming Growth Factor β Receptor Type II in the Presence of Mutated Apc, Promoting Colorectal Cancer Proliferation, PLoS One. 11 (2016) e0166422. 10.1371/journal.pone.0166422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hou J, Zhao R, Xia W, Chang C-W, You Y, Hsu J-M, Nie L, Chen Y, Wang Y-C, Liu C, Wang W-J, Wu Y, Ke B, Hsu JL, Huang K, Ye Z, Yang Y, Xia X, Li Y, Li C-W, Shao B, Tainer JA, Hung M-C, PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis, Nat. Cell Biol. (2020). 10.1038/s41556-020-0575-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wei J, Xu Z, Chen X, Wang X, Zeng S, Qian L, Yang X, Ou C, Lin W, Gong Z, Yan Y, Overexpression of GSDMC is a prognostic factor for predicting a poor outcome in lung adenocarcinoma, Mol. Med. Rep. 21 (2020) 360–370. 10.3892/mmr.2019.10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kusumaningrum N, Lee DH, Yoon HS, Park CH, Chung JH, Ultraviolet light-induced Gasdermin C expression is mediated via TRPV1/calcium/calcineurin/NFATc1 signaling, Int. J. Mol. Med. 42 (2018) 2859–2866. 10.3892/ijmm.2018.3839. [DOI] [PubMed] [Google Scholar]
- [38].Kusumaningrum N, Lee DH, Yoon HS, Kim YK, Park CH, Chung JH, Gasdermin C is induced by ultraviolet light and contributes to MMP-1 expression via activation of ERK and JNK pathways, J. Dermatol. Sci. 90 (2018) 180–189. 10.1016/j.jdermsci.2018.01.015. [DOI] [PubMed] [Google Scholar]
- [39].Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G, Immunogenic cell death in cancer and infectious disease, Nat. Rev. Immunol. 17 (2017) 97–111. 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- [40].Agard NJ, Maltby D, Wells JA, Inflammatory stimuli regulate caspase substrate profiles, Mol. Cell. Proteomics. 9 (2010) 880–893. 10.1074/mcp.M900528-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].He W, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang Z-H, Zhong C-Q, Han J, Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion., Cell Res. 25 (2015) 1285–1298. 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, Dixit VM, Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling, Nature. 526 (2015) 666–671. 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- [43].Rathkey JK, Xiao TS, Abbott DW, Human polymorphisms in GSDMD alter the inflammatory response, J. Biol. Chem. 295 (2020) 3228–3238. 10.1074/jbc.RA119.010604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rathkey JK, Zhao J, Liu Z, Chen Y, Yang J, Kondolf HC, Benson BL, Chirieleison SM, Huang AY, Dubyak GR, Xiao TS, Li X, Abbott DW, Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis, Sci. Immunol. 3 (2018). 10.1126/sciimmunol.aat2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hu JJ, Liu X, Xia S, Zhang Z, Zhang Y, Zhao J, Ruan J, Luo X, Lou X, Bai Y, Wang J, Hollingsworth LR, Magupalli VG, Zhao L, Luo HR, Kim J, Lieberman J, Wu H, FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation, Nat. Immunol. 21 (2020) 736–745. 10.1038/s41590-020-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kanneganti A, Malireddi RKS, V Saavedra PH, Vande Walle L, Van Gorp H, Kambara H, Tillman H, Vogel P, Luo HR, Xavier RJ, Chi H, Lamkanfi M, GSDMD is critical for autoinflammatory pathology in a mouse model of Familial Mediterranean Fever, J. Exp. Med. 215 (2018) 1519–1529. 10.1084/jem.20172060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Li S, Wu Y, Yang D, Wu C, Ma C, Liu X, Moynagh PN, Wang B, Hu G, Yang S, Gasdermin D in peripheral myeloid cells drives neuroinflammation in experimental autoimmune encephalomyelitis, J. Exp. Med. 216 (2019) 2562–2581. 10.1084/jem.20190377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Xiao J, Wang C, Yao JC, Alippe Y, Xu C, Kress D, Civitelli R, Abu-Amer Y, Kanneganti TD, Link DC, Mbalaviele G, Gasdermin D mediates the pathogenesis of neonatal-onset multisystem inflammatory disease in mice, PLoS Biol. 16 (2018) 1–13. 10.1371/journal.pbio.3000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Aglietti RA, Estevez A, Gupta A, Ramirez MG, Liu PS, Kayagaki N, Ciferri C, Dixit VM, Dueber EC, GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes, Proc. Natl. Acad. Sci. (2016). 10.1073/pnas.1607769113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wang K, Sun Q, Zhong X, Zeng M, Zeng H, Shi X, Li Z, Wang Y, Zhao Q, Shao F, Ding J, Structural Mechanism for GSDMD Targeting by Autoprocessed Caspases in Pyroptosis, Cell. 180 (2020) 941–955.e20. 10.1016/j.cell.2020.02.002. [DOI] [PubMed] [Google Scholar]
- [51].Liu Z, Wang C, Yang J, Chen Y, Zhou B, Abbott DW, Xiao TS, Caspase-1 Engages Full-Length Gasdermin D through Two Distinct Interfaces That Mediate Caspase Recruitment and Substrate Cleavage, Immunity. 53 (2020) 106–114.e5. 10.1016/j.immuni.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kambara H, Liu F, Zhang X, Liu P, Bajrami B, Teng Y, Zhao L, Zhou S, Yu H, Zhou W, Silberstein LE, Cheng T, Han M, Xu Y, Luo HR, Gasdermin D Exerts Anti-inflammatory Effects by Promoting Neutrophil Death, Cell Rep. 22 (2018) 2924–2936. 10.1016/j.celrep.2018.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Burgener SS, Leborgne NGF, Snipas SJ, Salvesen GS, Bird PI, Benarafa C, Cathepsin G Inhibition by Serpinb1 and Serpinb6 Prevents Programmed Necrosis in Neutrophils and Monocytes and Reduces GSDMD-Driven Inflammation, Cell Rep. 27 (2019) 3646–3656.e5. 10.1016/j.celrep.2019.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sarhan J, Liu BC, Muendlein HI, Li P, Nilson R, Tang AY, Rongvaux A, Bunnell SC, Shao F, Green DR, Poltorak A, Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection, Proc. Natl. Acad. Sci. U. S. A. 115 (2018) E10888–E10897. 10.1073/pnas.1809548115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Orning P, Weng D, Starheim K, Ratner D, Best Z, Lee B, Brooks A, Xia S, Wu H, Kelliher MA, Berger SB, Gough PJ, Bertin J, Proulx MM, Goguen JD, Kayagaki N, Fitzgerald KA, Lien E, Pathogen blockade of TAK1 triggers caspase-8–dependent cleavage of gasdermin D and cell death, Science (80-. ). 362 (2018) 1064–1069. 10.1126/science.aau2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Bambouskova M, Potuckova L, Paulenda T, Kerndl M, Mogilenko DA, Lizotte K, Swain A, Hayes S, Sheldon RD, Kim H, Kapadnis U, Ellis AE, Isaguirre C, Burdess S, Laha A, Amarasinghe GK, Chubukov V, Roddy TP, Diamond MS, Jones RG, Simons DM, Artyomov MN, Itaconate confers tolerance to late NLRP3 inflammasome activation, Cell Rep. 34 (2021) 108756. 10.1016/j.celrep.2021.108756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Humphries F, Shmuel-Galia L, Ketelut-Carneiro N, Li S, Wang B, V Nemmara V, Wilson R, Jiang Z, Khalighinejad F, Muneeruddin K, Shaffer SA, Dutta R, Ionete C, Pesiridis S, Yang S, Thompson PR, Fitzgerald KA, Succination inactivates gasdermin D and blocks pyroptosis, Science (80-. ). 182 (2020) eabb9818. 10.1126/science.abb9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wang Y, Shi P, Chen Q, Huang Z, Zou D, Zhang J, Gao X, Lin Z, Mitochondrial ROS promote macrophage pyroptosis by inducing GSDMD oxidation, J. Mol. Cell Biol. 11 (2019) 1069–1082. 10.1093/jmcb/mjz020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kayagaki N, Dixit VM, Rescue from a fiery death: A therapeutic endeavor, Science (80-. ). 366 (2019) 688–689. 10.1126/science.aaw1177. [DOI] [PubMed] [Google Scholar]
- [60].Jiang S, Zhou Z, Sun Y, Zhang T, Sun L, Coral gasdermin triggers pyroptosis, Sci. Immunol. 5 (2020). 10.1126/SCIIMMUNOL.ABD2591. [DOI] [PubMed] [Google Scholar]
- [61].van Camp G, Coucke P, Balemans W, van Velzen D, van de Bilt C, van Laer L, Smith RJ, Fukushima K, Padberg GW, Frants RR, Localization of a gene for non-syndromic hearing loss (DFNA5) to chromosome 7p15., Hum. Mol. Genet. 4 (1995) 2159–2163. 10.1093/hmg/4.11.2159. [DOI] [PubMed] [Google Scholar]
- [62].Rogers C, Fernandes-Alnemri T, Mayes L, Alnemri D, Cingolani G, Alnemri ES, Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death, Nat. Commun. 8 (2017) 14128. 10.1038/ncomms14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wang Y, Gao W, Shi X, Ding J, Liu W, He H, Wang K, Shao F, Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin, Nature. 547 (2017) 99–103. 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- [64].Zhou B, Abbott DW, Gasdermin E permits interleukin-1 beta release in distinct sublytic and pyroptotic phases, Cell Rep. 35 (2021). 10.1016/j.celrep.2021.108998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Jiang S, Gu H, Zhao Y, Sun L, Teleost Gasdermin E Is Cleaved by Caspase 1, 3, and 7 and Induces Pyroptosis, J. Immunol. 203 (2019) 1369–1382. 10.4049/jimmunol.1900383. [DOI] [PubMed] [Google Scholar]
- [66].Harris SL, Kazmierczak M, Pangršič T, Shah P, Chuchvara N, Barrantes-Freer A, Moser T, Schwander M, Conditional deletion of pejvakin in adult outer hair cells causes progressive hearing loss in mice, Neuroscience. 344 (2017) 380–393. 10.1016/j.neuroscience.2016.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Schwander M, Sczaniecka A, Grillet N, Bailey JS, Avenarius M, Najmabadi H, Steffy BM, Federe GC, Lagler EA, Banan R, Hice R, Grabowski-Boase L, Keithley EM, Ryan AF, Housley GD, Wiltshire T, Smith RJH, Tarantino LM, Müller U, A forward genetics screen in mice identifies recessive deafness traits and reveals that pejvakin is essential for outer hair cell function, J. Neurosci. 27 (2007) 2163–2175. 10.1523/JNEUROSCI.4975-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Delmaghani S, Del Castillo FJ, Michel V, Leibovici M, Aghaie A, Ron U, Van Laer L, Ben-Tal N, Van Camp G, Weil D, Langa F, Lathrop M, Avan P, Petit C, Mutations in the gene encoding pejvakin, a newly identified protein of the afferent auditory pathway, cause DFNB59 auditory neuropathy, Nat. Genet. 38 (2006) 770–778. 10.1038/ng1829. [DOI] [PubMed] [Google Scholar]
- [69].Delmaghani S, Defourny J, Aghaie A, Beurg M, Dulon D, Thelen N, Perfettini I, Zelles T, Aller M, Meyer A, Emptoz A, Giraudet F, Leibovici M, Dartevelle S, Soubigou G, Thiry M, Vizi ES, Safieddine S, Hardelin JP, Avan P, Petit C, Hypervulnerability to Sound Exposure through Impaired Adaptive Proliferation of Peroxisomes, Cell. 163 (2015) 894–906. 10.1016/j.cell.2015.10.023. [DOI] [PubMed] [Google Scholar]
- [70].Defourny J, Aghaie A, Perfettini I, Avan P, Delmaghani S, Petit C, Pejvakin-mediated pexophagy protects auditory hair cells against noise-induced damage, Proc. Natl. Acad. Sci. U. S. A. 116 (2019) 8010–8017. 10.1073/pnas.1821844116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Martinon F, Burns K, Tschopp J, The Inflammasome: A Molecular Platform Triggering Activation of Inflammatory Caspases and Processing of proIL-β, Mol. Cell. 10 (2002) 417–426. 10.1016/S1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- [72].D’Osualdo A, Weichenberger CX, Wagner RN, Godzik A, Wooley J, Reed JC, CARD8 and NLRP1 Undergo Autoproteolytic Processing through a ZU5-Like Domain, PLoS One. 6 (2011). 10.1371/journal.pone.0027396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Finger JN, Lich JD, Dare LC, Cook MN, Brown KK, Duraiswamis C, Bertin JJ, Gough PJ, Autolytic proteolysis within the function to find domain (FIIND) is required for NLRP1 inflammasome activity, J. Biol. Chem. 287 (2012) 25030–25037. 10.1074/jbc.M112.378323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Franklin BS, Bossaller L, De Nardo D, Ratter JM, Stutz A, Engels G, Brenker C, Nordhoff M, Mirandola SR, Al-Amoudi A, Mangan MS, Zimmer S, Monks BG, Fricke M, Schmidt RE, Espevik T, Jones B, Jarnicki AG, Hansbro PM, Busto P, Marshak-Rothstein A, Hornemann S, Aguzzi A, Kastenmüller W, Latz E, The adaptor ASC has extracellular and “prionoid” activities that propagate inflammation, Nat. Immunol. 15 (2014) 727–737. 10.1038/ni.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Sandstrom A, Mitchell PS, Goers L, Mu EW, Lesser CF, Vance RE, Functional degradation: A mechanism of NLRP1 inflammasome activation by diverse pathogen enzymes, Science (80-. ). 364 (2019). 10.1126/science.aau1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Boyden ED, Dietrich WF, Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin, Nat. Genet. 38 (2006) 240–244. 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- [77].Ewald SE, Chavarria-Smith J, Boothroyd JC, NLRP1 Is an Inflammasome Sensor for Toxoplasma gondii, Infect. Immun. 82 (2014) 460–468. 10.1128/IAI.01170-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Witola WH, Mui E, Hargrave A, Liu S, Hypolite M, Montpetit A, Cavailles P, Bisanz C, Cesbron-Delauw M-F, Fournié GJ, McLeod R, NALP1 Influences Susceptibility to Human Congenital Toxoplasmosis, Proinflammatory Cytokine Response, and Fate of Toxoplasma gondii-Infected Monocytic Cells, Infect. Immun. 79 (2011) 756–766. 10.1128/IAI.00898-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Gov L, Karimzadeh A, Ueno N, Lodoen MB, Human Innate Immunity to Toxoplasma gondii Is Mediated by Host Caspase-1 and ASC and Parasite GRA15, MBio. 4 (2013). 10.1128/mBio.00255-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Robinson KS, Teo DET, Sen Tan K, Toh GA, Ong HH, Lim CK, Lay K, Au BV, Lew TS, Chu JJH, Chow VTK, Wang DY, Zhong FL, Reversade B, Enteroviral 3C protease activates the human NLRP1 inflammasome in airway epithelia, Science (80-. ). 370 (2020). 10.1126/science.aay2002. [DOI] [PubMed] [Google Scholar]
- [81].V Tsu B, Beierschmitt C, Ryan AP, Agarwal R, Mitchell PS, Daugherty MD, Diverse viral proteases activate the NLRP1 inflammasome., Elife. 10 (2021). 10.7554/eLife.60609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Bauernfried S, Scherr MJ, Pichlmair A, Duderstadt KE, Hornung V, Human NLRP1 is a sensor for double-stranded RNA., Science. 371 (2021). 10.1126/science.abd0811. [DOI] [PubMed] [Google Scholar]
- [83].Ball DP, Taabazuing CY, Griswold AR, Orth EL, Rao SD, Kotliar IB, Vostal LE, Johnson DC, Bachovchin DA, Caspase-1 interdomain linker cleavage is required for pyroptosis, Life Sci. Alliance. 3 (2020). 10.26508/lsa.202000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Gong Q, Robinson K, Xu C, Huynh PT, Chong KHC, Tan EYJ, Zhang J, Boo ZZ, Teo DET, Lay K, Zhang Y, Lim JSY, Goh WI, Wright G, Zhong FL, Reversade B, Wu B, Structural basis for distinct inflammasome complex assembly by human NLRP1 and CARD8, Nat. Commun. 12 (2021) 188. 10.1038/s41467-020-20319-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Robert Hollingsworth L, David L, Li Y, Griswold AR, Ruan J, Sharif H, Fontana P, Orth-He EL, Fu TM, Bachovchin DA, Wu H, Mechanism of filament formation in UPA-promoted CARD8 and NLRP1 inflammasomes, Nat. Commun. 12 (2021). 10.1038/s41467-020-20320-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Johnson DC, Taabazuing CY, Okondo MC, Chui AJ, Rao SD, Brown FC, Reed C, Peguero E, de Stanchina E, Kentsis A, Bachovchin DA, DPP8/DPP9 inhibitor-induced pyroptosis for treatment of acute myeloid leukemia, Nat. Med. 24 (2018) 1151–1156. 10.1038/s41591-018-0082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Broz P, Von Moltke J, Jones JW, Vance RE, Monack DM, Differential requirement for caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing, Cell Host Microbe. 8 (2010) 471–483. 10.1016/j.chom.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM, Cryopyrin activates the inflammasome in response to toxins and ATP, Nature. 440 (2006) 228–232. 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- [89].Sander LE, Davis MJ, V Boekschoten M, Amsen D, Dascher CC, Ryffel B, Swanson JA, Müller M, Blander JM, Detection of prokaryotic mRNA signifies microbial viability and promotes immunity, Nature. 474 (2011) 385–389. 10.1038/nature10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J, Gout-associated uric acid crystals activate the NALP3 inflammasome, Nature. 440 (2006) 237–241. 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- [91].Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J, Innate Immune Activation Through Nalp3 Inflammasome Sensing of Asbestos and Silica, Science (80-. ). 320 (2008) 674–677. 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Vanaja SK, Rathinam VAK, Atianand MK, Kalantari P, Skehan B, Fitzgerald KA, Leong JM, Bacterial RNA:DNA hybrids are activators of the NLRP3 inflammasome, Proc. Natl. Acad. Sci. U. S. A. 111 (2014) 7765–7770. 10.1073/pnas.1400075111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Dutra FF, Alves LS, Rodrigues D, Fernandez PL, De Oliveira RB, Golenbock DT, Zamboni DS, Bozza MT, Hemolysis-induced lethality involves inflammasome activation by heme, Proc. Natl. Acad. Sci. U. S. A. 111 (2014) E4110–E4118. 10.1073/pnas.1405023111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Zhou R, Yazdi AS, Menu P, Tschopp J, A role for mitochondria in NLRP3 inflammasome activation, Nature. 469 (2011) 221–225. 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- [95].Iyer SS, He Q, Janczy JR, Elliott EI, Zhong Z, Olivier AK, Sadler JJ, Knepper-Adrian V, Han R, Qiao L, Eisenbarth SC, Nauseef WM, Cassel SL, Sutterwala FS, Mitochondrial Cardiolipin Is Required for Nlrp3 Inflammasome Activation, Immunity. 39 (2013) 311–323. 10.1016/j.immuni.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E, Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization, Nat. Immunol. 9 (2008) 847–856. 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, Horng T, Critical role for calcium mobilization in activation of the NLRP3 inflammasome, Proc. Natl. Acad. Sci. 109 (2012) 11282–11287. 10.1073/pnas.1117765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Tang T, Lang X, Xu C, Wang X, Gong T, Yang Y, Cui J, Bai L, Wang J, Jiang W, Zhou R, CLICs-dependent chloride efflux is an essential and proximal upstream event for NLRP3 inflammasome activation, Nat. Commun. 8 (2017) 202. 10.1038/s41467-017-00227-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Muñoz-Planillo R, Kuffa P, Martínez-Colón G, Smith BL, Rajendiran TM, Núñez G, K+ Efflux Is the Common Trigger of NLRP3 Inflammasome Activation by Bacterial Toxins and Particulate Matter, Immunity. 38 (2013) 1142–1153. 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Chen J, Chen ZJ, PtdIns4P on dispersed trans-Golgi network mediates NLRP3 inflammasome activation, Nature. 564 (2018) 71–76. 10.1038/s41586-018-0761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Paik S, Kim JK, Silwal P, Sasakawa C, Jo EK, An update on the regulatory mechanisms of NLRP3 inflammasome activation, Cell. Mol. Immunol. 18 (2021) 1141–1160. 10.1038/s41423-021-00670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM, Non-canonical inflammasome activation targets caspase-11, Nature. 479 (2011) 117–121. 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- [103].Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F, Inflammatory caspases are innate immune receptors for intracellular LPS, Nature. 514 (2014) 187–192. 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- [104].Baker PJ, Boucher D, Bierschenk D, Tebartz C, Whitney PG, D’Silva DB, Tanzer MC, Monteleone M, Robertson AAB, Cooper MA, Alvarez-Diaz S, Herold MJ, Bedoui S, Schroder K, Masters SL, NLRP3 inflammasome activation downstream of cytoplasmic LPS recognition by both caspase-4 and caspase-5, Eur. J. Immunol. 45 (2015) 2918–2926. 10.1002/eji.201545655. [DOI] [PubMed] [Google Scholar]
- [105].Rühl S, Broz P, Caspase-11 activates a canonical NLRP3 inflammasome by promoting K+ efflux, Eur. J. Immunol. 45 (2015) 2927–2936. 10.1002/eji.201545772. [DOI] [PubMed] [Google Scholar]
- [106].Zhao Y, Yang J, Shi J, Gong Y-N, Lu Q, Xu H, Liu L, Shao F, The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus, Nature. 477 (2011) 596–600. 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- [107].Yang J, Zhao Y, Shi J, Shao F, Human NAIP and mouse NAIP1 recognize bacterial type III secretion needle protein for inflammasome activation, Proc. Natl. Acad. Sci. 110 (2013) 14408–14413. 10.1073/pnas.1306376110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Xu H, Yang J, Gao W, Li L, Li P, Zhang L, Gong Y-N, Peng X, Xi JJ, Chen S, Wang F, Shao F, Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome, Nature. 513 (2014) 237–241. 10.1038/nature13449. [DOI] [PubMed] [Google Scholar]
- [109].Gao W, Yang J, Liu W, Wang Y, Shao F, Site-specific phosphorylation and microtubule dynamics control Pyrin inflammasome activation, Proc. Natl. Acad. Sci. 113 (2016) E4857–E4866. 10.1073/pnas.1601700113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Chauhan D, Vande Walle L, Lamkanfi M, Therapeutic modulation of inflammasome pathways, Immunol. Rev. 297 (2020) 123–138. 10.1111/imr.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Broz P, Dixit VM, Inflammasomes: Mechanism of assembly, regulation and signalling, Nat. Rev. Immunol. 16 (2016) 407–420. 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- [112].Chen KW, Demarco B, Heilig R, Shkarina K, Boettcher A, Farady CJ, Pelczar P, Broz P, Extrinsic and intrinsic apoptosis activate pannexin-1 to drive NLRP 3 inflammasome assembly , EMBO J. 38 (2019). 10.15252/embj.2019101638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Taabazuing CY, Okondo MC, Bachovchin DA, Pyroptosis and Apoptosis Pathways Engage in Bidirectional Crosstalk in Monocytes and Macrophages, Cell Chem. Biol. 24 (2017) 507–514.e4. 10.1016/j.chembiol.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Roberts TL, Idris A, Dunn JA, Kelly GM, Burnton CM, Hodgson S, Hardy LL, Garceau V, Sweet MJ, Ross IL, Hume DA, Stacey KJ, HIN-200 Proteins Regulate Caspase Activation in Response to Foreign Cytoplasmic DNA, Science (80-. ). 323 (2009) 1057–1060. 10.1126/science.1169841. [DOI] [PubMed] [Google Scholar]
- [115].Sagulenko V, Thygesen SJ, Sester DP, Idris A, Cridland JA, Vajjhala PR, Roberts TL, Schroder K, Vince JE, Hill JM, Silke J, Stacey KJ, AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC, Cell Death Differ. 20 (2013) 1149–1160. 10.1038/cdd.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Tsuchiya K, Nakajima S, Hosojima S, Thi Nguyen D, Hattori T, Manh Le T, Hori O, Mahib MR, Yamaguchi Y, Miura M, Kinoshita T, Kushiyama H, Sakurai M, Shiroishi T, Suda T, Caspase-1 initiates apoptosis in the absence of gasdermin D, Nat. Commun. 10 (2019) 2091. 10.1038/s41467-019-09753-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Chen KW, Demarco B, Broz P, Pannexin-1 promotes NLRP3 activation during apoptosis but is dispensable for canonical or noncanonical inflammasome activation, Eur. J. Immunol. 50 (2020) 170–177. 10.1002/eji.201948254. [DOI] [PubMed] [Google Scholar]
- [118].Chen Q, Shi P, Wang Y, Zou D, Wu X, Wang D, Hu Q, Zou Y, Huang Z, Ren J, Lin Z, Gao X, GSDMB promotes non-canonical pyroptosis by enhancing caspase-4 activity, J. Mol. Cell Biol. 11 (2019) 496–508. 10.1093/jmcb/mjy056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Liu Y, Fang Y, Chen X, Wang Z, Liang X, Zhang T, Liu M, Zhou N, Lv J, Tang K, Xie J, Gao Y, Cheng F, Zhou Y, Zhang Z, Hu Y, Zhang X, Gao Q, Zhang Y, Huang B, Gasdermin E–mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome, Sci. Immunol. 5 (2020) eaax7969. 10.1126/sciimmunol.aax7969. [DOI] [PubMed] [Google Scholar]
- [120].Zhang Z, Zhang Y, Xia S, Kong Q, Li S, Liu X, Junqueira C, Meza-Sosa KF, Mok TMY, Ansara J, Sengupta S, Yao Y, Wu H, Lieberman J, Gasdermin E suppresses tumour growth by activating anti-tumour immunity, Nature. 579 (2020) 415–420. 10.1038/s41586-020-2071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Lei X, Zhang Z, Xiao X, Qi J, He B, Wang J, Enterovirus 71 Inhibits Pyroptosis through Cleavage of Gasdermin D, J. Virol. 91 (2017). 10.1128/jvi.01069-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Bernot A, Clepet C, Dasilva C, Devaud C, Petit JL, Caloustian C, Cruaud C, Samson D, Pulcini F, Weissenbach J, Heilig R, Notanicola C, Domingo C, Rozenbaum M, Benchetrit E, Topaloglu R, Dewalle M, Dross C, Hadjari P, Dupant M, Demaille J, Touitou I, Smaoui N, Nedelec B, Méry JP, Chaabouni H, Delpech M, Grateau G, A candidate gene for familial Mediterranean fever, Nat. Genet. 17 (1997) 25–31. 10.1038/ng0997-25. [DOI] [PubMed] [Google Scholar]
- [123].Park YH, Wood G, Kastner DL, Chae JJ, Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS, Nat. Immunol. 17 (2016) 914–921. 10.1038/ni.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Wada T, Toma T, Miyazawa H, Koizumi E, Shirahashi T, Matsuda Y, Yachie A, Longitudinal analysis of serum interleukin-18 in patients with familial Mediterranean fever carrying MEFV mutations in exon 10, Cytokine. 104 (2018) 143–146. 10.1016/j.cyto.2017.10.007. [DOI] [PubMed] [Google Scholar]
- [125].El Hasbani G, Jawad A, Uthman I, Update on the management of colchicine resistant Familial Mediterranean Fever (FMF), Orphanet J. Rare Dis. 14 (2019) 224. 10.1186/s13023-019-1201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Feldmann J, Prieur A-M, Quartier P, Berquin P, Certain S, Cortis E, Teillac-Hamel D, Fischer A, de Saint Basile G, Chronic Infantile Neurological Cutaneous and Articular Syndrome Is Caused by Mutations in CIAS1, a Gene Highly Expressed in Polymorphonuclear Cells and Chondrocytes, Am. J. Hum. Genet. 71 (2002) 198–203. 10.1086/341357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Kuemmerle-Deschner JB, Gautam R, George AT, Raza S, Lomax KG, Hur P, Systematic literature review of efficacy/effectiveness and safety of current therapies for the treatment of cryopyrin-associated periodic syndrome, hyperimmunoglobulin D syndrome and tumour necrosis factor receptor-associated periodic syndrome, RMD Open. 6 (2020) e001227. 10.1136/rmdopen-2020-001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Romberg N, Vogel TP, Canna SW, NLRC4 inflammasomopathies, Curr. Opin. Allergy Clin. Immunol. 17 (2017) 398–404. 10.1097/ACI.0000000000000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Kitamura A, Sasaki Y, Abe T, Kano H, Yasutomo K, An inherited mutation in NLRC4 causes autoinflammation in human and mice, J. Exp. Med. 211 (2014) 2385–2396. 10.1084/jem.20141091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Canna SW, De Jesus AA, Gouni S, Brooks SR, Marrero B, Liu Y, Dimattia MA, Zaal KJM, Sanchez GAM, Kim H, Chapelle D, Plass N, Huang Y, V Villarino A, Biancotto A, Fleisher TA, Duncan JA, O’Shea JJ, Benseler S, Grom A, Deng Z, Laxer RM, Goldbach-Mansky R, An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome, Nat. Genet. 46 (2014) 1140–1146. 10.1038/ng.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Drutman SB, Haerynck F, Zhong FL, Hum D, Hernandez NJ, Belkaya S, Rapaport F, de Jong SJ, Creytens D, Tavernier SJ, Bonte K, De Schepper S, van der Werff ten Bosch J, Lorenzo-Diaz L, Wullaert A, Bossuyt X, Orth G, Bonagura VR, Béziat V, Abel L, Jouanguy E, Reversade B, Laurent-Casanova J, Homozygous NLRP1 gain-of-function mutation in siblings with a syndromic form of recurrent respiratory papillomatosis, Proc. Natl. Acad. Sci. U. S. A. 116 (2019) 19055–19063. 10.1073/pnas.1906184116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Grandemange S, Sanchez E, Louis-Plence P, Tran Mau-Them F, Bessis D, Coubes C, Frouin E, Seyger M, Girard M, Puechberty J, Costes V, Rodière M, Carbasse A, Jeziorski E, Portales P, Sarrabay G, Mondain M, Jorgensen C, Apparailly F, Hoppenreijs E, Touitou I, Geneviève D, A new autoinflammatory and autoimmune syndrome associated with NLRP1 mutations: NAIAD (NLRP1- associated autoinflammation with arthritis and dyskeratosis), Ann. Rheum. Dis. 76 (2017) 1191–1198. 10.1136/annrheumdis-2016-210021. [DOI] [PubMed] [Google Scholar]
- [133].Zhong FL, Mamaï O, Sborgi L, Boussofara L, Hopkins R, Robinson K, Szeverényi I, Takeichi T, Balaji R, Lau A, Tye H, Roy K, Bonnard C, Ahl PJ, Jones LA, Baker P, Lacina L, Otsuka A, Fournie PR, Malecaze F, Lane EB, Akiyama M, Kabashima K, Connolly JE, Masters SL, Soler VJ, Omar SS, McGrath JA, Nedelcu R, Gribaa M, Denguezli M, Saad A, Hiller S, Reversade B, Germline NLRP1 Mutations Cause Skin Inflammatory and Cancer Susceptibility Syndromes via Inflammasome Activation, Cell. 167 (2016) 187–202.e17. 10.1016/j.cell.2016.09.001. [DOI] [PubMed] [Google Scholar]
- [134].Harapas CR, Robinson KS, Lay K, Wong J, Bernstein JA, Zhong FL, Masters SL, Reversade B, DPP9 deficiency : an Inflammasomopathy which can be rescued by lowering NLRP1/IL-1 signaling, MedRxiv. 13 (2021) 14. 10.1101/2021.01.31.21250067. [DOI] [Google Scholar]
- [135].Kang R, Zeng L, Zhu S, Xie Y, Liu J, Wen Q, Cao L, Xie M, Ran Q, Kroemer G, Wang H, Billiar TR, Jiang J, Tang D, Lipid Peroxidation Drives Gasdermin D-Mediated Pyroptosis in Lethal Polymicrobial Sepsis, Cell Host Microbe. 24 (2018) 97–108.e4. 10.1016/j.chom.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Chen KW, Monteleone M, Boucher D, Sollberger G, Ramnath D, Condon ND, von Pein JB, Broz P, Sweet MJ, Schroder K, Noncanonical inflammasome signaling elicits gasdermin D–dependent neutrophil extracellular traps, Sci. Immunol. 3 (2018). 10.1126/sciimmunol.aar6676. [DOI] [PubMed] [Google Scholar]
- [137].Wu C, Lu W, Zhang Y, Zhang G, Shi X, Hisada Y, Grover SP, Zhang X, Li L, Xiang B, Shi J, Li XA, Daugherty A, Smyth SS, Kirchhofer D, Shiroishi T, Shao F, Mackman N, Wei Y, Li Z, Inflammasome Activation Triggers Blood Clotting and Host Death through Pyroptosis, Immunity. 50 (2019) 1401–1411.e4. 10.1016/j.immuni.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Sollberger G, Choidas A, Burn GL, Habenberger P, Di Lucrezia R, Kordes S, Menninger S, Eickhoff J, Nussbaumer P, Klebl B, Krüger R, Herzig A, Zychlinsky A, Gasdermin D plays a vital role in the generation of neutrophil extracellular traps, Sci. Immunol. 3 (2018). 10.1126/sciimmunol.aar6689. [DOI] [PubMed] [Google Scholar]
- [139].Yang X, Cheng X, Tang Y, Qiu X, Wang Y, Kang H, Wu J, Wang Z, Liu Y, Chen F, Xiao X, Mackman N, Billiar TR, Han J, Lu B, Bacterial Endotoxin Activates the Coagulation Cascade through Gasdermin D-Dependent Phosphatidylserine Exposure, Immunity. 51 (2019) 983–996.e6. 10.1016/j.immuni.2019.11.005. [DOI] [PubMed] [Google Scholar]
- [140].Cerqueira DM, Gomes MTR, Silva ALN, Rungue M, Assis NRG, Guimarães ES, Morais SB, Broz P, Zamboni DS, Oliveira SC, Guanylate-binding protein 5 licenses caspase-11 for Gasdermin-D mediated host resistance to Brucella abortus infection, PLoS Pathog. 14 (2018). 10.1371/journal.ppat.1007519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Zhu Q, Zheng M, Balakrishnan A, Karki R, Kanneganti T-D, Gasdermin D Promotes AIM2 Inflammasome Activation and Is Required for Host Protection against Francisella novicida , J. Immunol. 201 (2018) 3662–3668. 10.4049/jimmunol.1800788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Wang J, Deobald K, Re F, Gasdermin D Protects from Melioidosis through Pyroptosis and Direct Killing of Bacteria, J. Immunol. 202 (2019) 3468–3473. 10.4049/jimmunol.1900045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Dubois H, Sorgeloos F, Sarvestani ST, Martens L, Saeys Y, MacKenzie JM, Lamkanfi M, Van Loo G, Goodfellow I, Wullaert A, Nlrp3 inflammasome activation and Gasdermin D-driven pyroptosis are immunopathogenic upon gastrointestinal norovirus infection, PLoS Pathog. 15 (2019). 10.1371/journal.ppat.1007709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Erkes DA, Cai W, Sanchez IM, Purwin TJ, Rogers C, Field CO, Berger AC, Hartsough EJ, Rodeck U, Alnemri ES, Aplin AE, Mutant BRAF and MEK inhibitors regulate the tumor immune microenvironment via pyroptosis, Cancer Discov. 10 (2020) 255–269. 10.1158/2159-8290.CD-19-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Tyrkalska SD, Pérez-Oliva AB, Rodríguez-Ruiz L, Martínez-Morcillo FJ, Alcaraz-Pérez F, Martínez-Navarro FJ, Lachaud C, Ahmed N, Schroeder T, Pardo-Sánchez I, Candel S, López-Muñoz A, Choudhuri A, Rossmann MP, Zon LI, Cayuela ML, García-Moreno D, Mulero V, Inflammasome Regulates Hematopoiesis through Cleavage of the Master Erythroid Transcription Factor GATA1, Immunity. 51 (2019) 50–63.e5. 10.1016/j.immuni.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Pietras EM, Mirantes-Barbeito C, Fong S, Loeffler D, Kovtonyuk LV, Zhang S, Lakshminarasimhan R, Chin CP, Techner JM, Will B, Nerlov C, Steidl U, Manz MG, Schroeder T, Passegué E, Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal, Nat. Cell Biol. 18 (2016) 607–618. 10.1038/ncb3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, Baber U, Mehran R, Fuster V, Danesh J, Frossard P, Saleheen D, Melander O, Sukhova GK, Neuberg D, Libby P, Kathiresan S, Ebert BL, Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease, N. Engl. J. Med. 377 (2017) 111–121. 10.1056/nejmoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, Higgins JM, Moltchanov V, Kuo FC, Kluk MJ, Henderson B, Kinnunen L, Koistinen HA, Ladenvall C, Getz G, Correa A, Banahan BF, Gabriel S, Kathiresan S, Stringham HM, McCarthy MI, Boehnke M, Tuomilehto J, Haiman C, Groop L, Atzmon G, Wilson JG, Neuberg D, Altshuler D, Ebert BL, Age-Related Clonal Hematopoiesis Associated with Adverse Outcomes, N. Engl. J. Med. 371 (2014) 2488–2498. 10.1056/nejmoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Genovese G, Kähler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, Chambert K, Mick E, Neale BM, Fromer M, Purcell SM, Svantesson O, Landén M, Höglund M, Lehmann S, Gabriel SB, Moran JL, Lander ES, Sullivan PF, Sklar P, Grönberg H, Hultman CM, McCarroll SA, Clonal Hematopoiesis and Blood-Cancer Risk Inferred from Blood DNA Sequence, N. Engl. J. Med. 371 (2014) 2477–2487. 10.1056/nejmoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, Wu CL, Sano S, Muralidharan S, Rius C, Vuong J, Jacob S, Muralidhar V, Robertson AAB, Cooper MA, Andrés V, Hirschi KK, Martin KA, Walsh K, Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice, Science (80-. ). 355 (2017) 842–847. 10.1126/science.aag1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Sano S, Oshima K, Wang Y, MacLauchlan S, Katanasaka Y, Sano M, Zuriaga MA, Yoshiyama M, Goukassian D, Cooper MA, Fuster JJ, Walsh K, Tet2-Mediated Clonal Hematopoiesis Accelerates Heart Failure Through a Mechanism Involving the IL-1β/NLRP3 Inflammasome, J. Am. Coll. Cardiol. 71 (2018) 875–886. 10.1016/j.jacc.2017.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Hamarsheh S, Osswald L, Saller BS, Unger S, De Feo D, Vinnakota JM, Konantz M, Uhl FM, Becker H, Lübbert M, Shoumariyeh K, Schürch C, Andrieux G, Venhoff N, Schmitt-Graeff A, Duquesne S, Pfeifer D, Cooper MA, Lengerke C, Boerries M, Duyster J, Niemeyer CM, Erlacher M, Blazar BR, Becher B, Groß O, Brummer T, Zeiser R, Oncogenic KrasG12D causes myeloproliferation via NLRP3 inflammasome activation, Nat. Commun. 11 (2020). 10.1038/s41467-020-15497-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Höckendorf U, Yabal M, Herold T, Munkhbaatar E, Rott S, Jilg S, Kauschinger J, Magnani G, Reisinger F, Heuser M, Kreipe H, Sotlar K, Engleitner T, Rad R, Weichert W, Peschel C, Ruland J, Heikenwalder M, Spiekermann K, Slotta-Huspenina J, Groß O, Jost PJ, RIPK3 Restricts Myeloid Leukemogenesis by Promoting Cell Death and Differentiation of Leukemia Initiating Cells, Cancer Cell. 30 (2016) 75–91. 10.1016/j.ccell.2016.06.002. [DOI] [PubMed] [Google Scholar]
- [154].Basiorka AA, McGraw KL, Eksioglu EA, Chen X, Johnson J, Zhang L, Zhang Q, Irvine BA, Cluzeau T, Sallman DA, Padron E, Komrokji R, Sokol L, Coll RC, Robertson AAB, Cooper MA, Cleveland JL, O’Neill LA, Wei S, List AF, The NLRP3 inflammasome functions as a driver of the myelodysplastic syndrome phenotype, Blood. 128 (2016) 2960–2975. 10.1182/blood-2016-07-730556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, Deshayes K, Lee WP, Hoffman HM, Dixit VM, Glyburide inhibits the Cryopyrin/Nalp3 inflammasome, J. Cell Biol. 187 (2009) 61–70. 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Coll RC, Hill JR, Day CJ, Zamoshnikova A, Boucher D, Massey NL, Chitty JL, Fraser JA, Jennings MP, Robertson AAB, Schroder K, MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition, Nat. Chem. Biol. 15 (2019) 556–559. 10.1038/s41589-019-0277-7. [DOI] [PubMed] [Google Scholar]
- [157].Coll RC, Robertson AAB, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Núñez G, Latz E, Kastner DL, Mills KHG, Masters SL, Schroder K, Cooper MA, O’Neill LAJ, A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases, Nat. Med. 21 (2015) 248–255. 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Tapia-Abellán A, Angosto-Bazarra D, Martínez-Banaclocha H, de Torre-Minguela C, Cerón-Carrasco JP, Pérez-Sánchez H, Arostegui JI, Pelegrin P, MCC950 closes the active conformation of NLRP3 to an inactive state, Nat. Chem. Biol. 15 (2019) 560–564. 10.1038/s41589-019-0278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].van Hout GPJ, Bosch L, Ellenbroek GHJM, de Haan JJ, van Solinge WW, Cooper MA, Arslan F, de Jager SCA, Robertson AAB, Pasterkamp G, Hoefer IE, The selective NLRP3-inflammasome inhibitor MCC950 reduces infarct size and preserves cardiac function in a pig model of myocardial infarction, Eur. Heart J. 38 (2017) 828–836. 10.1093/eurheartj/ehw247. [DOI] [PubMed] [Google Scholar]
- [160].van der Heijden T, Kritikou E, Venema W, van Duijn J, van Santbrink P, Slutter B, Foks FA, Bot I, Kuiper J, NLRP3 Inflammasome Inhibition by MCC950 Reduces Atherosclerotic Lesion Development in Apolipoprotein E–Deficient Mice—Brief Report, Arterioscler. Thromb. Vasc. Biol. 37 (2017) 1457–1461. 10.1161/ATVBAHA.117.309575. [DOI] [PubMed] [Google Scholar]
- [161].Dempsey C, Rubio Araiz A, Bryson KJ, Finucane O, Larkin C, Mills EL, Robertson AAB, Cooper MA, O’Neill LAJ, Lynch MA, Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-β and cognitive function in APP/PS1 mice, Brain. Behav. Immun. 61 (2017) 306–316. 10.1016/j.bbi.2016.12.014. [DOI] [PubMed] [Google Scholar]
- [162].Perera AP, Fernando R, Shinde T, Gundamaraju R, Southam B, Sohal SS, Robertson AAB, Schroder K, Kunde D, Eri R, MCC950, a specific small molecule inhibitor of NLRP3 inflammasome attenuates colonic inflammation in spontaneous colitis mice, Sci. Rep. 8 (2018) 8618. 10.1038/s41598-018-26775-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Vande Walle L, Stowe IB, Šácha P, Lee BL, Demon D, Fossoul A, Van Hauwermeiren F, V Saavedra PH, Šimon P, Šubrt V, Kostka L, Stivala CE, Pham VC, Staben ST, Yamazoe S, Konvalinka J, Kayagaki N, Lamkanfi M, MCC950/CRID3 potently targets the NACHT domain of wild-type NLRP3 but not disease-associated mutants for inflammasome inhibition, PLOS Biol. 17 (2019) e3000354. 10.1371/journal.pbio.3000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E, Targeting the NLRP3 inflammasome in inflammatory diseases, Nat. Rev. Drug Discov. 17 (2018) 588–606. 10.1038/nrd.2018.97. [DOI] [PubMed] [Google Scholar]
- [165].Huang Y, Jiang H, Chen Y, Wang X, Yang Y, Tao J, Deng X, Liang G, Zhang H, Jiang W, Zhou R, Tranilast directly targets NLRP3 to treat inflammasome-driven diseases, EMBO Mol. Med. 10 (2018) e8689. 10.15252/emmm.201708689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].He H, Jiang H, Chen Y, Ye J, Wang A, Wang C, Liu Q, Liang G, Deng X, Jiang W, Zhou R, Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity, Nat. Commun. 9 (2018) 1–12. 10.1038/s41467-018-04947-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Chen Y, He H, Lin B, Chen Y, Deng X, Jiang W, Zhou R, RRx-001 ameliorates inflammatory diseases by acting as a potent covalent NLRP3 inhibitor, Cell. Mol. Immunol. 18 (2021) 1425–1436. 10.1038/s41423-021-00683-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Klück V, Jansen TLTA, Janssen M, Comarniceanu A, Efdé M, Tengesdal IW, Schraa K, Cleophas MCP, Scribner CL, Skouras DB, Marchetti C, Dinarello CA, Joosten LAB, Dapansutrile, an oral selective NLRP3 inflammasome inhibitor, for treatment of gout flares: an open-label, dose-adaptive, proof-of-concept, phase 2a trial, Lancet Rheumatol. 2 (2020) e270–e280. 10.1016/S2665-9913(20)30065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Jiang H, He H, Chen Y, Huang W, Cheng J, Ye J, Wang A, Tao J, Wang C, Liu Q, Jin T, Jiang W, Deng X, Zhou R, Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders, J. Exp. Med. 214 (2017) 3219–3238. 10.1084/jem.20171419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Cocco M, Pellegrini C, Martínez-Banaclocha H, Giorgis M, Marini E, Costale A, Miglio G, Fornai M, Antonioli L, López-Castejón G, Tapia-Abellán A, Angosto D, Hafner-Bratkovič I, Regazzoni L, Blandizzi C, Pelegrín P, Bertinaria M, Development of an Acrylate Derivative Targeting the NLRP3 Inflammasome for the Treatment of Inflammatory Bowel Disease, J. Med. Chem. 60 (2017) 3656–3671. 10.1021/acs.jmedchem.6b01624. [DOI] [PubMed] [Google Scholar]
- [171].Li H, Wang J, Wu C, Wang L, Chen ZS, Cui W, The combination of disulfiram and copper for cancer treatment, Drug Discov. Today. 25 (2020) 1099–1108. 10.1016/j.drudis.2020.04.003. [DOI] [PubMed] [Google Scholar]
- [172].Blair HA, Dimethyl Fumarate: A Review in Moderate to Severe Plaque Psoriasis, Drugs. 78 (2018) 123–130. 10.1007/s40265-017-0854-6. [DOI] [PubMed] [Google Scholar]
- [173].Zahid A, Li B, Kombe AJK, Jin T, Tao J, Pharmacological inhibitors of the nlrp3 inflammasome, Front. Immunol. 10 (2019). 10.3389/fimmu.2019.02538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Okondo MC, Rao SD, Taabazuing CY, Chui AJ, Poplawski SE, Johnson DC, Bachovchin DA, Inhibition of Dpp8/9 Activates the Nlrp1b Inflammasome, Cell Chem. Biol. 25 (2018) 262–267.e5. 10.1016/j.chembiol.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].MacKenzie SH, Schipper JL, Clark AC, The potential for caspases in drug discovery, Curr. Opin. Drug Discov. Devel. 13 (2010) 568–576. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3289102/. [PMC free article] [PubMed] [Google Scholar]
- [176].Rudolphi K, Gerwin N, Verzijl N, van der Kraan P, van den Berg W, Pralnacasan, an inhibitor of interleukin-1β converting enzyme, reduces joint damage in two murine models of osteoarthritis, Osteoarthr. Cartil. 11 (2003) 738–746. 10.1016/S1063-4584(03)00153-5. [DOI] [PubMed] [Google Scholar]
- [177].Wannamaker W, Davies R, Namchuk M, Pollard J, Ford P, Ku G, Decker C, Charifson P, Weber P, Germann UA, Kuida K, Randle JCR, (S)-1-((S)-2-{[1-(4-Amino-3-chloro-phenyl)-methanoyl]-amino}−3,3-dimethyl-butanoyl)-pyrrolidine-2-carboxylic acid ((2R,3S)-2-ethoxy-5-oxo-tetrahydro-furan-3-yl)-amide (VX-765), an Orally Available Selective Interleukin (IL)-Converting Enzyme/Caspase-1 Inh, J. Pharmacol. Exp. Ther. 321 (2007) 509–516. 10.1124/jpet.106.111344. [DOI] [PubMed] [Google Scholar]
- [178].Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS, Progress report on new antiepileptic drugs: A summary of the Eleventh Eilat Conference (EILAT XI), Epilepsy Res. 103 (2013) 2–30. 10.1016/j.eplepsyres.2012.10.001. [DOI] [PubMed] [Google Scholar]
- [179].Okondo MC, Johnson DC, Sridharan R, Bin Go E, Chui AJ, Wang MS, Poplawski SE, Wu W, Liu Y, Lai JH, Sanford DG, Arciprete MO, Golub TR, Bachovchin WW, Bachovchin DA, DPP8 and DPP9 inhibition induces pro-caspase-1-dependent monocyte and macrophage pyroptosis, Nat. Chem. Biol. 13 (2017) 46–53. 10.1038/nchembio.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Zhong FL, Robinson K, Teo DET, Tan K-Y, Lim C, Harapas CR, Yu C-H, Xie WH, Sobota RM, Au VB, Hopkins R, D’Osualdo A, Reed JC, Connolly JE, Masters SL, Reversade B, Human DPP9 represses NLRP1 inflammasome and protects against autoinflammatory diseases via both peptidase activity and FIIND domain binding, J. Biol. Chem. 293 (2018) 18864–18878. 10.1074/jbc.RA118.004350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Griswold AR, Ball DP, Bhattacharjee A, Chui AJ, Rao SD, Taabazuing CY, Bachovchin DA, DPP9’s Enzymatic Activity and Not Its Binding to CARD8 Inhibits Inflammasome Activation, ACS Chem. Biol. 14 (2019) 2424–2429. 10.1021/acschembio.9b00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Adams S, Miller GT, Jesson ML, Watanabe T, Jones B, Wallner BP, PT-100, a small molecule dipeptidyl peptidase inhibitor, has potent antitumor effects and augments antibody-mediated cytotoxicity via a novel immune mechanism, Cancer Res. 64 (2004) 5471–5480. 10.1158/0008-5472.CAN-04-0447. [DOI] [PubMed] [Google Scholar]
- [183].Walsh MP, Duncan B, Larabee S, Krauss A, Davis JPE, Cui Y, Kim SY, Guimond M, Bachovchin W, Fry TJ, Val-BoroPro Accelerates T Cell Priming via Modulation of Dendritic Cell Trafficking Resulting in Complete Regression of Established Murine Tumors, PLoS One. 8 (2013). 10.1371/journal.pone.0058860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Linder A, Bauernfried S, Cheng Y, Albanese M, Jung C, Keppler OT, Hornung V, CARD8 inflammasome activation triggers pyroptosis in human T cells, EMBO J. 39 (2020). 10.15252/embj.2020105071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Masters SL, Gerlic M, Metcalf D, Preston S, Pellegrini M, O’Donnell JA, McArthur K, Baldwin TM, Chevrier S, Nowell CJ, Cengia LH, Henley KJ, Collinge JE, Kastner DL, Feigenbaum L, Hilton DJ, Alexander WS, Kile BT, Croker BA, NLRP1 Inflammasome Activation Induces Pyroptosis of Hematopoietic Progenitor Cells, Immunity. 37 (2012) 1009–1023. 10.1016/j.immuni.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Aggarwal RR, Costin D, O’Neill VJ, Corsi-Travali S, Adurthi S, Adedoyin A, Healey DI, De Bono JS, Monk P, Zhang J, Small EJ, Phase 1b study of BXCL701, a novel small molecule inhibitor of dipeptidyl peptidases (DPP), combined with pembrolizumab (pembro), in men with metastatic castration-resistant prostate cancer (mCRPC)., J. Clin. Oncol. 38 (2020) e17581–e17581. 10.1200/jco.2020.38.15_suppl.e17581. [DOI] [Google Scholar]
- [187].Rashidi M, Simpson DS, Hempel A, Frank D, Petrie E, Vince A, Feltham R, Murphy J, Chatfield SM, Salvesen GS, Murphy JM, Wicks IP, Vince JE, The Pyroptotic Cell Death Effector Gasdermin D Is Activated by Gout-Associated Uric Acid Crystals but Is Dispensable for Cell Death and IL-1β Release, J. Immunol. 203 (2019) 736–748. 10.4049/jimmunol.1900228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X, Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase, Cell. 148 (2012) 213–227. 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- [189].Wang C, Yang T, Xiao J, Xu C, Alippe Y, Sun K, Kanneganti T-D, Monahan JB, Abu-Amer Y, Lieberman J, Mbalaviele G, Activation of GSDME compensates for GSDMD deficiency in a mouse model of NLRP3 inflammasomopathy, BioRxiv. (2021) 2021.01.06.425634. 10.1101/2021.01.06.425634. [DOI] [Google Scholar]
- [190].Russo HM, Rathkey J, Boyd-Tressler A, Katsnelson MA, Abbott DW, Dubyak GR, Active Caspase-1 Induces Plasma Membrane Pores That Precede Pyroptotic Lysis and Are Blocked by Lanthanides, J. Immunol. 197 (2016) 1353–1367. 10.4049/jimmunol.1600699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Xia S, Zhang Z, Magupalli VG, Pablo JL, Dong Y, Vora SM, Wang L, Fu TM, Jacobson MP, Greka A, Lieberman J, Ruan J, Wu H, Gasdermin D pore structure reveals preferential release of mature interleukin-1, Nature. 593 (2021) 607–611. 10.1038/s41586-021-03478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Ma C, Yang D, Wang B, Wu C, Wu Y, Li S, Liu X, Lassen K, Dai L, Yang S, Gasdermin D in macrophages restrains colitis by controlling cGAS-mediated inflammation, Sci. Adv. 6 (2020) eaaz6717. 10.1126/sciadv.aaz6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Bulek K, Zhao J, Liao Y, Rana N, Corridoni D, Antanaviciute A, Chen X, Wang H, Qian W, Miller-Little WA, Swaidani S, Tang F, Willard BB, McCrae K, Kang Z, Dubyak GR, Cominelli F, Simmons A, Pizarro TT, Li X, Epithelial-derived gasdermin D mediates nonlytic IL-1β release during experimental colitis, J. Clin. Invest. 140 (2020) 4218–4234. 10.1172/JCI138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Waldman AD, Fritz JM, Lenardo MJ, A guide to cancer immunotherapy: from T cell basic science to clinical practice, Nat. Rev. Immunol. 20 (2020) 651–668. 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Kim MS, Chang X, Yamashita K, Nagpal JK, Baek JH, Wu G, Trink B, Ratovitski EA, Mori M, Sidransky D, Aberrant promoter methylation and tumor suppressive activity of the DFNA5 gene in colorectal carcinoma, Oncogene. 27 (2008) 3624–3634. 10.1038/sj.onc.1211021. [DOI] [PubMed] [Google Scholar]
- [196].Akino K, Toyota M, Suzuki H, Imai T, Maruyama R, Kusano M, Nishikawa N, Watanabe Y, Sasaki Y, Abe T, Yamamoto E, Tarasawa I, Sonoda T, Mori M, Imai K, Shinomura Y, Tokino T, Identification of DFNA5 as a target of epigenetic inactivation in gastric cancer, Cancer Sci. 98 (2007) 88–95. 10.1111/j.1349-7006.2006.00351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Kayagaki N, Lee BL, Stowe IB, Kornfeld OS, O’Rourke K, Mirrashidi KM, Haley B, Watanabe C, Roose-Girma M, Modrusan Z, Kummerfeld S, Reja R, Zhang Y, Cho V, Daniel Andrews T, Morris LX, Goodnow CC, Bertram EM, Dixit VM, IRF2 transcriptionally induces GSDMD expression for pyroptosis, Sci. Signal. 12 (2019). 10.1126/scisignal.aax4917. [DOI] [PubMed] [Google Scholar]
- [198].Ahmed SG, Abdelanabi A, Doha M, Brenner GJ, Schwannoma gene therapy by adeno-associated virus delivery of the pore-forming protein Gasdermin-D, Cancer Gene Ther. 26 (2019) 259–267. 10.1038/s41417-018-0077-3. [DOI] [PubMed] [Google Scholar]
- [199].Wang Q, Wang Y, Ding J, Wang C, Zhou X, Gao W, Huang H, Shao F, Liu Z, A bioorthogonal system reveals antitumour immune function of pyroptosis, Nature. 579 (2020) 421–426. 10.1038/s41586-020-2079-1. [DOI] [PubMed] [Google Scholar]
- [200].Martin-Sanchez F, Diamond C, Zeitler M, Gomez AI, Baroja-Mazo A, Bagnall J, Spiller D, White M, Daniels MJD, Mortellaro A, Penalver M, Paszek P, Steringer JP, Nickel W, Brough D, Pelegrin P, Inflammasome-dependent IL-1β release depends upon membrane permeabilisation, Cell Death Differ. 23 (2016) 1219–1231. 10.1038/cdd.2015.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Fink SL, Cookson BT, Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages, J. Immunol. 202 (2006) 1913–1926. 10.1111/J.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- [202].Rühl S, Shkarina K, Demarco B, Heilig R, Santos JC, Broz P, ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation, Science (80-. ). 362 (2018) 956–960. 10.1126/science.aar7607. [DOI] [PubMed] [Google Scholar]
- [203].Andrews NW, Corrotte M, Plasma membrane repair, Curr. Biol. 28 (2018) R392–R397. 10.1016/j.cub.2017.12.034. [DOI] [PubMed] [Google Scholar]
- [204].Kayagaki N, Kornfeld OS, Lee BL, Stowe IB, O’Rourke K, Li Q, Sandoval W, Yan D, Kang J, Xu M, Zhang J, Lee WP, McKenzie BS, Ulas G, Payandeh J, Roose-Girma M, Modrusan Z, Reja R, Sagolla M, Webster JD, Cho V, Andrews TD, Morris LX, Miosge LA, Goodnow CC, Bertram EM, Dixit VM, NINJ1 mediates plasma membrane rupture during lytic cell death, Nature. 591 (2021) 131–136. 10.1038/s41586-021-03218-7. [DOI] [PubMed] [Google Scholar]
- [205].Evavold CL, Ruan J, Tan Y, Xia S, Wu H, Kagan JC, The Pore-Forming Protein Gasdermin D Regulates Interleukin-1 Secretion from Living Macrophages, Immunity. 48 (2018) 35–44.e6. 10.1016/j.immuni.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Davis MA, Fairgrieve MR, Den Hartigh A, Yakovenko O, Duvvuri B, Lood C, Thomas WE, Fink SL, Gale M, Calpain drives pyroptotic vimentin cleavage, intermediate filament loss, and cell rupture that mediates immunostimulation, Proc. Natl. Acad. Sci. U. S. A. 116 (2019) 5061–5070. 10.1073/pnas.1818598116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Tsuchiya K, Hosojima S, Hara H, Kushiyama H, Mahib MR, Kinoshita T, Suda T, Gasdermin D mediates the maturation and release of IL-1α downstream of inflammasomes, Cell Rep. 34 (2021) 108887. 10.1016/j.celrep.2021.108887. [DOI] [PubMed] [Google Scholar]
- [208].Von Moltke J, Trinidad NJ, Moayeri M, Kintzer AF, Wang SB, Van Rooijen N, Brown CR, Krantz BA, Leppla SH, Gronert K, Vance RE, Rapid induction of inflammatory lipid mediators by the inflammasome in vivo, Nature. 490 (2012) 107–111. 10.1038/nature11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Wang D, Zheng J, Hu Q, Zhao C, Chen Q, Shi P, Chen Q, Zou Y, Zou D, Liu Q, Pei J, Wu X, Gao X, Ren J, Lin Z, Magnesium protects against sepsis by blocking gasdermin D N-terminal-induced pyroptosis, Cell Death Differ. 27 (2020) 466–481. 10.1038/s41418-019-0366-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Danielski LG, Della Giustina A, Bonfante S, Barichello T, Petronilho F, The NLRP3 Inflammasome and Its Role in Sepsis Development, Inflammation. 43 (2020) 24–31. 10.1007/s10753-019-01124-9. [DOI] [PubMed] [Google Scholar]
- [211].Kono H, Kimura Y, Latz E, Inflammasome activation in response to dead cells and their metabolites, Curr. Opin. Immunol. 30 (2014) 91–98. 10.1016/j.coi.2014.09.001. [DOI] [PubMed] [Google Scholar]
- [212].Keane RW, Dietrich WD, de Rivero Vaccari JP, Inflammasome Proteins As Biomarkers of Multiple Sclerosis, Front. Neurol. 9 (2018). 10.3389/fneur.2018.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Barclay W, Shinohara ML, Inflammasome activation in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE), Brain Pathol. 27 (2017) 213–219. 10.1111/bpa.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Hoseini Z, Sepahvand F, Rashidi B, Sahebkar A, Masoudifar A, Mirzaei H, NLRP3 inflammasome: Its regulation and involvement in atherosclerosis, J. Cell. Physiol. 233 (2018) 2116–2132. 10.1002/jcp.25930. [DOI] [PubMed] [Google Scholar]
- [215].Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nũez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E, NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals, Nature. 464 (2010) 1357–1361. 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Yu Z-W, Zhang J, Li X, Wang Y, Fu Y-H, Gao X-Y, A new research hot spot: The role of NLRP3 inflammasome activation, a key step in pyroptosis, in diabetes and diabetic complications, Life Sci. 240 (2020) 117138. 10.1016/j.lfs.2019.117138. [DOI] [PubMed] [Google Scholar]
- [217].Sun X, Pang H, Li J, Luo S, Huang G, Li X, Xie Z, Zhou Z, The NLRP3 Inflammasome and Its Role in T1DM, Front. Immunol. 11 (2020). 10.3389/fimmu.2020.01595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Stienstra R, Van Diepen JA, Tack CJ, Zaki MH, Van De Veerdonk FL, Perera D, Neale GA, Hooiveld GJ, Hijmans A, Vroegrijk I, Van Den Berg S, Romijn J, Rensen PCN, Joosten LAB, Netea MG, Kanneganti TD, Inflammasome is a central player in the induction of obesity and insulin resistance, Proc. Natl. Acad. Sci. U. S. A. 108 (2011) 15324–15329. 10.1073/pnas.1100255108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Szekanecz Z, Szamosi S, Kovács GE, Kocsis E, Benkő S, The NLRP3 inflammasome - interleukin 1 pathway as a therapeutic target in gout, Arch. Biochem. Biophys. 670 (2019) 82–93. 10.1016/j.abb.2019.01.031. [DOI] [PubMed] [Google Scholar]
- [220].Dempsey C, Rubio Araiz A, Bryson KJ, Finucane O, Larkin C, Mills EL, Robertson AAB, Cooper MA, O’Neill LAJ, Lynch MA, Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-β and cognitive function in APP/PS1 mice, Brain. Behav. Immun. 61 (2017) 306–316. 10.1016/j.bbi.2016.12.014. [DOI] [PubMed] [Google Scholar]
- [221].Yap JKY, Pickard BS, Chan EWL, Gan SY, The Role of Neuronal NLRP1 Inflammasome in Alzheimer’s Disease: Bringing Neurons into the Neuroinflammation Game, Mol. Neurobiol. 56 (2019) 7741–7753. 10.1007/s12035-019-1638-7. [DOI] [PubMed] [Google Scholar]
- [222].Severini C, Barbato C, Grazia Di Certo M, Gabanella F, Petrella P, Di Stadio A, de Vincentiis M, Polimeni A, Ralli M, Greco A, Alzheimer’s Disease: New Concepts on the Role of Autoimmunity and NLRP3 Inflammasome in the Pathogenesis of the Disease, Curr. Neuropharmacol. 19 (2021) 498–512. https://www.eurekaselect.com/183024/article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Kim RY, Pinkerton JW, Gibson PG, Cooper MA, Horvat JC, Hansbro PM, Inflammasomes in COPD and neutrophilic asthma, Thorax. 70 (2015) 1199–1201. 10.1136/thoraxjnl-2014-206736. [DOI] [PubMed] [Google Scholar]
- [224].Ather JL, Ckless K, Martin R, Foley KL, Suratt BT, Boyson JE, Fitzgerald KA, Flavell RA, Eisenbarth SC, Poynter ME, Serum Amyloid A Activates the NLRP3 Inflammasome and Promotes Th17 Allergic Asthma in Mice, J. Immunol. 187 (2011) 64–73. 10.4049/jimmunol.1100500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Das S, Miller M, Beppu AK, Mueller J, McGeough MD, Vuong C, Karta MR, Rosenthal P, Chouiali F, Doherty TA, Kurten RC, Hamid Q, Hoffman HM, Broide DH, GSDMB induces an asthma phenotype characterized by increased airway responsiveness and remodeling without lung inflammation, Proc. Natl. Acad. Sci. U. S. A. 113 (2016) 13132–13137. 10.1073/pnas.1610433113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Wu H, Romieu I, Sienra-Monge JJ, Li H, Del Rio-Navarro BE, London SJ, Genetic variation in ORM1-like 3 (ORMDL3) and gasdermin-like (GSDML) and childhood asthma, Allergy Eur. J. Allergy Clin. Immunol. 64 (2009) 629–635. 10.1111/j.1398-9995.2008.01912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Zhen Y, Zhang H, NLRP3 Inflammasome and Inflammatory Bowel Disease, Front. Immunol. 10 (2019). 10.3389/fimmu.2019.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Chunmei M, Yang D, Wang B, Wu C, Wu Y, Li S, Liu X, Lassen K, Dai L, Yang S, Gasdermin d in macrophages restrains colitis by controlling cgas-mediated inflammation, Sci. Adv. 6 (2020). 10.1126/sciadv.aaz6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Wu X, Dong L, Lin X, Li J, Relevance of the NLRP3 Inflammasome in the Pathogenesis of Chronic Liver Disease, Front. Immunol. 8 (2017). 10.3389/fimmu.2017.01728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Gao L, Dong Q, Song Z, Shen F, Shi J, Li Y, NLRP3 inflammasome: a promising target in ischemic stroke, Inflamm. Res. 66 (2017) 17–24. 10.1007/s00011-016-0981-7. [DOI] [PubMed] [Google Scholar]
- [231].Vogel S, Arora T, Wang X, Mendelsohn L, Nichols J, Allen D, Shet AS, Combs CA, Quezado ZMN, Thein SL, The platelet NLRP3 inflammasome is upregulated in sickle cell disease via HMGB1/TLR4 and Bruton tyrosine kinase, Blood Adv. 2 (2018) 2672–2680. 10.1182/bloodadvances.2018021709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Tumburu L, Ghosh-Choudhary S, Seifuddin F, Barbu EA, Yang S, Ahmad MM, Wilkins LHW, Tunc I, Sivakumar I, Nichols JS, Dagur PK, Yang S, Almeida LE, Quezado ZM, Combs C, Lindberg EJ, Bleck CKE, Zhu J, Shet AS, Chung JH, Pirooznia M, Thein SL, Circulating mitochondrial DNA is a pro-inflammatory DAMP in sickle cell disease, Blood. (2021). 10.1182/blood.2020009063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Chen G, Zhang D, Fuchs TA, Manwani D, Wagner DD, Frenette PS, Heme-induced neutrophil extracellular traps contribute to the pathogenesis of sickle cell disease, Blood. 123 (2014) 3818–3827. 10.1182/blood-2013-10-529982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Vande Walle L, Van Opdenbosch N, Jacques P, Fossoul A, Verheugen E, Vogel P, Beyaert R, Elewaut D, Kanneganti TD, Van Loo G, Lamkanfi M, Negative regulation of the NLRP3 inflammasome by A20 protects against arthritis, Nature. 512 (2014) 69–73. 10.1038/nature13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Zhao C, Gu Y, Zeng X, Wang J, NLRP3 inflammasome regulates Th17 differentiation in rheumatoid arthritis, Clin. Immunol. 197 (2018) 154–160. 10.1016/j.clim.2018.09.007. [DOI] [PubMed] [Google Scholar]