Abstract
The investigation of per- and polyfluorinated alkyl substances (PFAS) in environmental and biological samples relies on both high- and low-resolution mass spectrometry (MS) techniques. While high-resolution MS (HRMS) can be used for identification and quantification of novel compounds, low-resolution MS is the more commonly used and affordable approach for studies examining previously identified PFAS. Of note, perfluorobutanoic acid (PFBA) is one of the smaller PFAS observed in biological and environmental samples and has only one major MS/MS transition, preventing the use of qualitative transitions for verification. Recently, our laboratories undertook a targeted investigation of PFAS in the human placenta from high-risk pregnancies utilizing low-resolution, targeted MS/MS. Examination of placental samples revealed a widespread (n = 93/122 (76%)) chemical interferent in the quantitative ion channel for PFBA (213 → 169). PFBA concentrations were influenced by up to ∼3 ng/g. Therefore, additional chromatographic and HRMS/MS instrumentation was utilized to investigate the suspect peak and putatively assign the identity of the interfering compound as the saturated oxo-fatty acid (SOFA) 3-oxo-dodecanoic acid.
Graphical Abstract
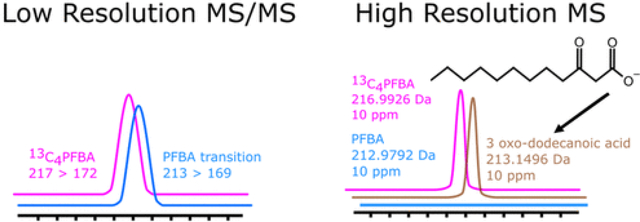
Introduction
Per- and polyfluoroalkyl acids (PFAS) are a class of thousands of unique chemicals containing carbon–fluorine bonds. (1) Production of the first PFAS began in the late 1940s, and since their genesis, usage has expanded to over 200 categories, from industrial applications to consumer products. (2) Because of their decades-long, widespread usage, PFAS are detectable in environmental media, wildlife, and humans across the globe. (3,4) The most investigated subclass of PFAS (often termed legacy PFAS) are the perfluoroalkyl acids (PFAAs). (5) Of the PFAAs, the two most studied to date are perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS); however, in recent decades, many applications of PFAS have transitioned to using shorter chain replacement PFAS, many of which have been quantified in both abiotic (e.g., drinking water) (6) and biotic matrices (e.g., plasma and tissue). (7)
The investigation of PFAS in environmental and biological samples relies on both high- and low-resolution mass spectrometry (MS) techniques. While high-resolution MS (HRMS) can be used for identification and quantification of novel compounds, low-resolution MS is the more widespread and affordable approach for studies examining previously identified PFAS. Low-resolution, targeted tandem mass spectrometry (MS/MS) quantification experiments are regarded as highly accurate and precise for chemical measurements but still possess the potential for unresolved chemical interference. Investigations of PFAS in biological matrices (e.g., blood, serum, muscle, brain) has identified several instances where compounds interfere with quantitation of PFAS at low resolution. For example, taurodeoxycholate (a common bile acid) has been observed to mimic the primary perfluorooctanesulfonic acid (PFOS) MS/MS transition (499 → 80), (8) while endogenous steroid sulfates have been observed to mimic two of the perfluorohexanesulfonic acid (PFHxS) MS/MS transitions (399 → 80 and 399 → 99). (9) In the case where such interference is anticipated, qualifying ion MS/MS transitions, along with enhanced chemical separation, can be used to ensure chemical specificity. As a result, the use of qualifying ion MS/MS transitions are suggested to verify the chemical identity for PFOS and PFHxS; however, the degree of historical over-reporting of PFOS and PFHxS from biological matrices in the literature as a result of these interferences is not fully known.
Recently, studies undertaken by the FDA and CDC have also reported matrix interferences in food and blood for perfluorobutanoic acid (PFBA) and perfluoropentanoic acid (PFPeA), (10,11) suggesting uncharacterized analytical interferences do exist for other PFAS when using low resolution MS/MS instrumentation. In 2020, our laboratories undertook an investigation into PFAS in the placenta from high-risk pregnancies. The study utilized a HPLC method similar to many HPLC methods in the literature paired with low-resolution MS/MS instrumentation. (12) Numerous PFAAs were quantified and reported in that study; however, because of some uncertainty around PFBA in the initial study, values for PFBA were not published in the original report. (12) More specifically, examination of placental samples revealed a widespread (n = 93/122) chemical interferent in the quantitative ion channel for PFBA (213 → 169), and as a result of the interferent, PFBA concentrations were influenced up to ∼3 ng/g. Unfortunately, the small size of PFBA results in limited fragmentation of the molecule, yielding one MS/MS transition for quantification and none for verification, increasing the likelihood of interference. A second parameter often utilized for identification is matched retention time (RT) with an isotope labeled PFBA standard. However, because of the short RT for PFBA and the RT allowance window for many programs that automatically integrate peaks during high throughput analysis, misidentification is still possible. Therefore, the current study was undertaken to identify the PFBA interferent present in the placental samples through HRMS and putatively assign a structure.
Materials and Methods
Initial, targeted HPLC-MS/MS analysis of placental samples was completed as detailed in Bangma et al. 2020. (12) (12) More details on the Bangma et al. MS/MS parameters can be found in the Supporting Information.
Following identification of an interference in low-resolution MS/MS, additional HRMS analysis of the samples was conducted on a separate system that employed an Agilent 1200 UHPLC coupled to an Agilent 6546 quadrupole time-of-flight mass spectrometer with electrospray ionization in negative mode. Chromatographic separation on the second system utilized a Waters BEH C18 column (50 mm × 30 mm, 1.7 μm). As before, 5 μL of extract was injected for each sample. Similar to the previous method, each sample run involved a ramping LC solvent gradient with methanol and deionized water both containing 2.5 mmol/L ammonium acetate. In brief, the LC gradient began at 25% organic and was held steady for the first minute, then ramped to achieve 55% organic at 2 min. A second ramp from 2 to 6 min was undertaken to achieve 65% organic. From there, the gradient was ramped to 100% organic at 8 min and held for 2 min to flush the column. System chromatographic pressure maintained around 700 bar (max system pressure 1300 bar). Under the new conditions, the unknown interferent was not observed to coelute with 13C4-PFBA internal standard. Therefore, to identify the interfering compound, several placenta samples from the previous study were selected, divided into groups with the highest levels of interferent (∼3 ng/g based on previous PFBA quantitation methods), midlevel of interferent (>1 ng/g to <3 ng/g), and no observable interferent (less than 0.5 ng/g). All samples contained a mass labeled PFBA internal standard (13C4PFBA), and samples were run alongside a method blank (previously processed for the original study) and solvent blanks on the QTOF. Method reporting limits (MRLs) for the HRMS were set based on the lowest point calibration standard with replicate precision <20% RSD and resulted in an MRL of 0.3 ng/g. Data was collected in 10 GHz high dynamic range mode at resolution ∼20 000 and scan rate of 2 Hz. Additional parameters for the HRMS can be found in Supporting Information, Table S1.
Results and Discussion
The presence of PFBA in placental samples previously analyzed using low-resolution MS was examined utilizing additional chromatographic and HRMS instrumentation. On the HRMS, PFBA presence or absence was investigated by extracting the deprotonated monoisotopic masses of PFBA ([M – H]−, 212.9792 amu) and 13C4–PFBA internal standard ([M – H]−, 216.9926 amu) with a 10 ppm window (Figure 1B) and compared to previous data collected from the low-resolution instrument (Figure 1A). Samples previously observed to contain chemical interferent in the PFBA ion channel (213 → 169) were found to contain no PFBA based on the absence of a detectable ion peak for the PFBA exact precursor mass across the entire chromatographic window.
Figure 1.
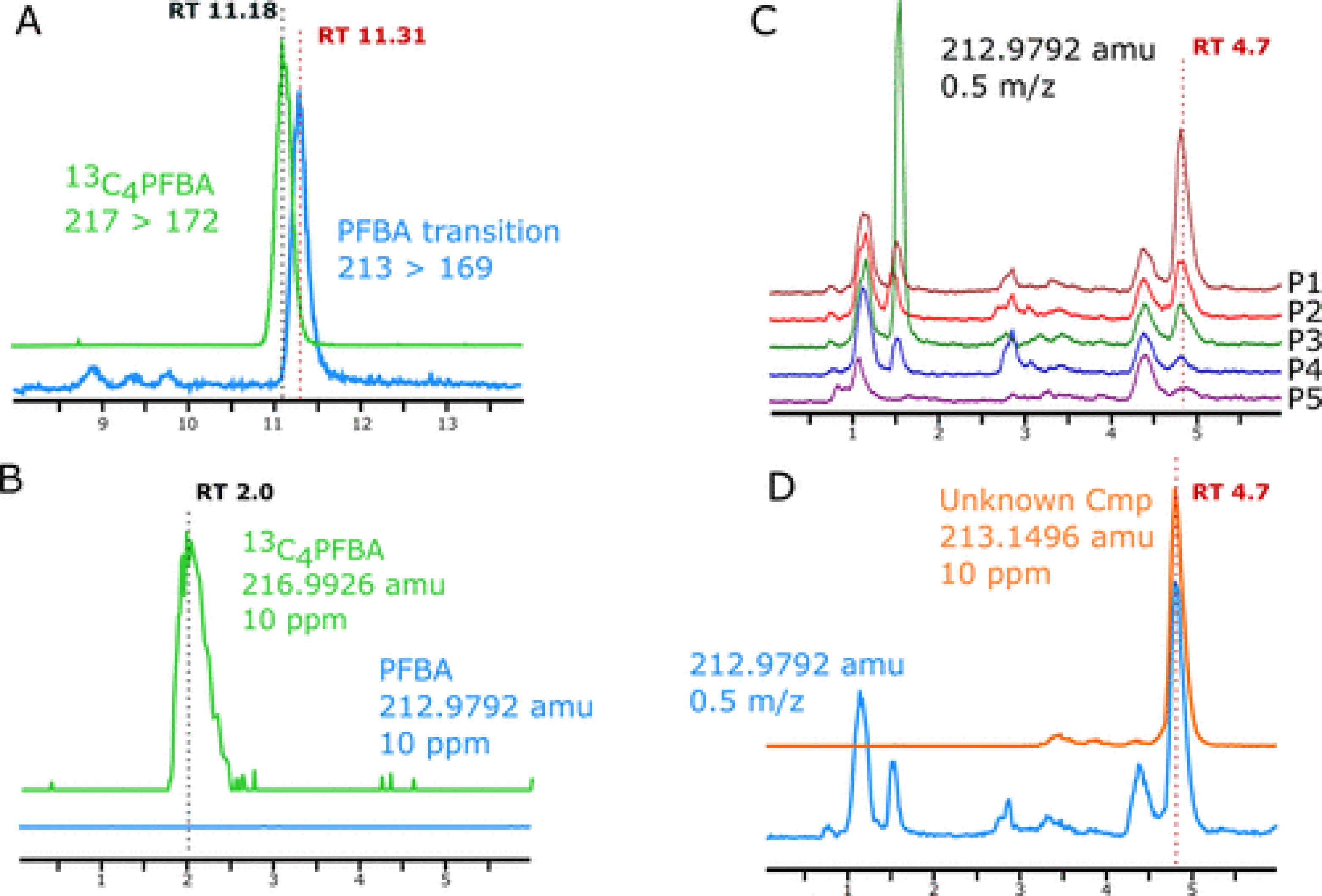
(A) Low-resolution MS/MS analysis of a placental sample for PFBA internal standard (13C4–PFBA) and PFBA mass transition ion channels (precursor > product ion). (B) High-resolution MS analysis of a placental sample for 13C4PFBA precursor ion and native PFBA precursor ion. (C) On the high-resolution MS, a simulated low-resolution mass window (±0.5 m/z) was set around the mass of the PFBA precursor ion in placentas with high level of chemical interferent (P1), midlevel of chemical interferent (P2 and P3), and levels below detection for the low-resolution method (P4 and P5). (D) Identified interfering compound at mass 213.1496 m/z. All extracted ion chromatograms in each window are normalized to the largest peak to maintain scale across grouped traces.
Because of altered chromatographic conditions (column and system pressure capabilities) on the HRMS system, the interfering ion no longer eluted in time with 13C4–PFBA internal standard. Therefore, to identify the previously coeluting ion, we recreated the low-resolution scan data by extracting ion chromatograms of PFBA with a ±0.5 m/z window, allowing us to replicate the chromatographic traces observed in the low-resolution instrument. We were then able to pinpoint the interfering ion through the observation of the increasing peak size of an unknown ion corresponding to the previously estimated quantity of PFBA interferent in the various placental samples (P1–P5, Figure 1C). Investigation of the mass spectrum of the identified interferent peak at retention time (RT) = 4.7 min identified an ion with monoisotopic mass of 213.1496 m/z (Figure 1D), capable of causing interference in the ∼1 Da precursor isolation window of a typical quadrupole. We hypothesize the identified ion undergoes an equivalent neutral loss of 44 amu (i.e., decarboxylation) to interfere with the MS/MS transition (213 → 169) in low resolution instrumentation.
The precursor mass spectrum of the interferent was processed using Agilent MassHunter 10 to predict an empirical formula for the compound (elements and limits allowed included C,3–50; H, 0–120; C, l0–10; S, 0–3; F, 0–100; O, 0–10). The most plausible predicted formula was assigned as C12H22O3 with a score of 99.91 (composite scoring of monoisotopic mass, isotope cluster abundance, and isotope cluster spacing). The assigned formula of C12H22O3 was searched against the Environmental Protection Agency CompTox Chemicals Dashboard to yield 149 plausible chemical structures (Supporting Information). Results were rank-ordered by the number of associated data sources which have been used in the past to identify probable compound identity and examined based on exogenous compounds that mothers might have a potential for exposure. Two exogenous compounds (ethyl 2-acetyloctanoate, CASRN 29214–60-6 https://comptox.epa.gov/dashboard/dsstoxdb/results?search=DTXSID3051969; and tetrahydro-3-pentyl-2H-pyran-4-yl acetate, CASRN 18871–142, https://comptox.epa.gov/dashboard/dsstoxdb/results?search=DTXSID7044556) listed as cosmetic and/or food additives were selected, standards purchased, and determined to not match the spectra of the interferent in the placentas. We then investigated the possibility that the interferent was an endogenous compound and searched the Human Metabolome Database (HMDB) (13,14) to identify endogenous molecules with plausible ionization in negative electrospray ionization; product ion species were predicted using CFM-ID 3.0. (15) We assigned the most likely chemical class to be a saturated oxo-fatty acid (SOFA), which comprised seven of the 149 chemical structures identified in the CompTox Chemicals Dashboard search. SOFAs are involved in fatty acid biosynthesis pathways in all eukaryotes, contain a carboxylic acid group for negative mode ionization, and have been documented in bacteria, (16) yeast, (17) human breath, (18) human plasma, (19) rabbit plasma, (20) and cows and goats milk. (21)
Of the several potential dodecanoic SOFAs with the assigned formula C12H22O3, we hypothesize that 3-oxo-dodecanoic acid (Figure 2) is the major chemical interferent observed in the placenta samples, with the interfering 213 → 169 transition occurring as a minor, secondary fragment (Figure 2). CFM-ID predictions yielded a primary fragment at 169.1598 m/z, which matches the observed interference in the 213 → 169 ion channel in the low-resolution instrument, but empirical measurements of the small concentrations of interfering precursor mass on the HRMS observed 59.0134 amu as being the major ion fragment (Supporting Information, Figure S1). Fragmentation of 213.1496 amu at HCD 10, 20, and 40 all yielded the same resulting spectra, with the 59.0134 fragment being the major detected fragment on the HRMS. This is consistent with previous reports of this lipid in exhaled human breath, (18) with a primary 59 fragment and the observed 169 fragment appearing as a minor transition (∼1% relative intensity). We predict the 3-oxo fatty acid as the main SOFA present in placental samples due to the preferential generation of the 59-fragment resulting from the relative positioning of the ketone at the 3 position (Figure 2).
Figure 2.
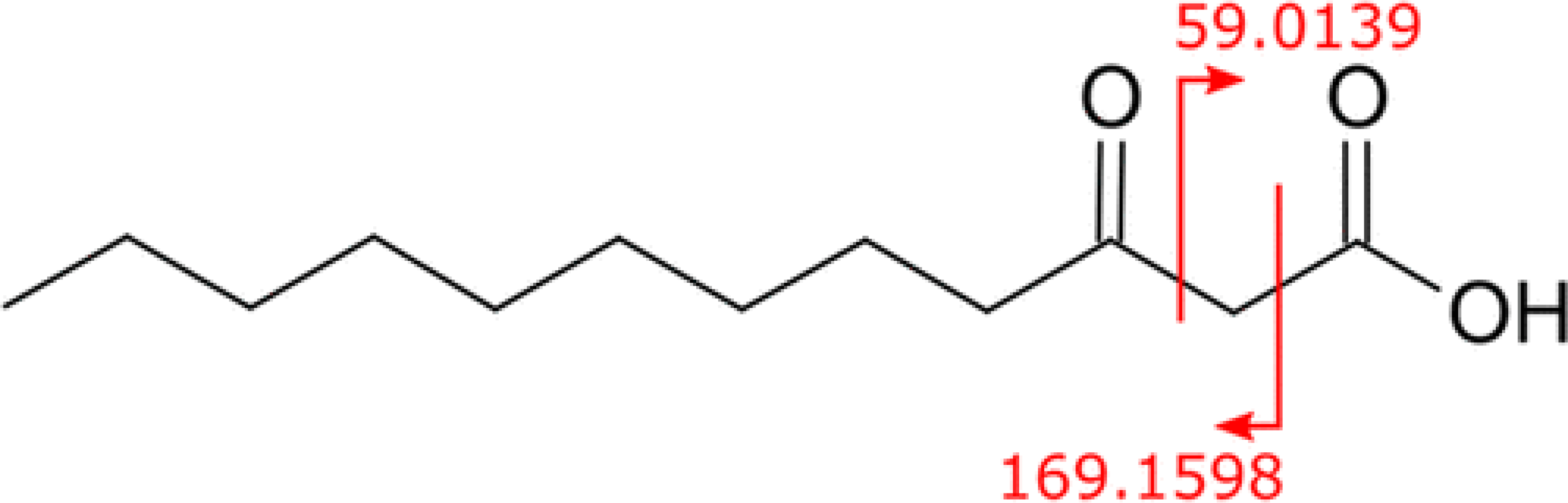
Structure of 3-oxo-dodecanoic acid with major (59.0139) and minor (169.1598) ion fragmentation annotated.
In addition to the main interfering SOFA, several additional SOFA isomers were observed in the placenta. These peaks are discernible as minor peaks eluting prior to 4.7 min in the HRMS spectra at 213.1496 m/z (Figure 1D). Other isomer forms of dodecanoic SOFAs with the ketone in a different position could yield similar interferents in a MS/MS spectrum, although the relative yield of 213 → 169 transitions (and potential for interference) will vary. With this information combined, we are confident in a level 3 identification on the Schymanski scale with a tentative candidate of 3-oxo-dodecanoic acid as the major SOFA present in the placenta samples in this study. (22) Identifying the exact isomeric structure of the major interfering SOFA with a chemical standard would be ideal and improve the Schymanski identification confidence level; however, several SOFAs appear to be present in the placenta in varying abundances and verifying with single a SOFA chemical standard not to mention several SOFA chemical standards is cost and time prohibitive.
Our study investigating PFAS in the placenta revealed that SOFAs are the suspected chemical interferents in the PFBA ion channel. Additionally, our study also revealed that depending on the LC conditions related to the column and system pressure capabilities, the major interfering SOFA can be separated chromatographically from PFBA, as observed in the differences between the original LC method (Figure 1A) and the follow-up UHPLC method utilized in tandem with HRMS (Figure 1B,D). In the future, we suggest caution when observing widespread PFBA measurements by low-resolution MS/MS in biological matrices. On the basis of the current literature, we hypothesize that the suspected interfering SOFA is likely only to be an interferent in biological matrices and not present in high enough quantities in environmental samples such as tap water and/or groundwater, although this cannot be ruled out. In addition, as a short chained PFAS, PFBA is rapidly eliminated from the body via urine in humans (23) and animals (24,25) and therefore is unlikely to be observed at a high frequency in biological samples unless there is a chronic point source exposure to the humans or wildlife in question. In fact, a recent study from Germany has questioned previous reports of PFBA in human tissues (26) after finding little to no PFBA in human lung and kidney biopsies from a separate cohort study that employed HRMS instrumentation. Our followup analysis in placenta is similar to Abraham and colleague’s study in that it seeks to verify or refute the presence of PFBA in biological samples previously thought to contain PFBA. It is with this in mind that we have built upon Abraham’s and colleagues’ findings to tentatively identify the PFBA interfering ion in the placenta as 3-oxo-dodecanoic acid. Additional research is warranted to verify 3-oxo-dodecanoic acid is in fact the SOFA of concern in the placenta. (27)
Armed with this knowledge, researchers can make more informed decisions as to whether it is necessary to confirm the presence or absence of PFBA in biological samples via secondary methods. Confirmation analysis can be undertaken with altered chromatographic conditions and or HRMS, analyzing only a handful of the samples (a few with suspected high PFBA and a few samples with no PFBA) to confirm the presence or absence of interfering SOFAs. Another avenue that may prove useful for investigators with limited access to additional chromatographic or HRMS instrumentation would be to scan for the 213 → 59 transition to indicate if an interfering SOFA elutes at the same retention time as PFBA. Authors reason that most if not all SOFA isomers with the molecular formula C12H22O3 should theoretically produce the 59 fragment (−COOH loss). However, authors are hesitant at this time to declare the 213 → 59 transition as an absolute guaranteed way to identify interfering SOFAs in a sample. While it is a valid method for the placental samples analyzed in this study, we reason that different isomeric SOFAs or changes in ionization conditions may lead to different major fragments besides the 59 fragment, and thus, depending on instrument settings and sensitivity, may lead to false negatives. More research on SOFA isomers is warranted to verify the 213 → 59 transition. Alternatively, sample preparation methods to eliminate interfering lipids (28) and/or enhanced chromatographic separations to eliminate coelution may reduce the potential for false-positives, but such sample preparation methods have not been investigated as a possibility for removing SOFAs from extracted tissues. Last, it is imperative that researcher closely monitor the retention time for PFBA and isotope labeled PFBA from investigations to confirm exact matching elution time as there is no confirmatory ion for ion ratio flagging.
In spite of this apparent interferent, we acknowledge that PFBA is likely to be present in various collected environmental samples (29) and may pose a health risk to highly exposed individuals. (30) For example, recent analysis of groundwater in China using HRMS has observed PFBA as one of the dominantly detected PFAS. (6) Another study utilizing MS/MS and HRMS observed high frequency of PFBA in seabird livers. (7) We hope this information will help to guide PFAS researchers in how to best verify PFBA quantification in biological matrices in the future to reduce potential over reporting of PFBA in the literature.
Supplementary Material
Acknowledgments
We the Oak Ridge Institute for Science and Education fellowship funding for the successful completion of this work. We also thank internal EPA reviewers Theresa Cantu-Guillette and Kelsey Miller for their time and expertise.
Footnotes
The research presented was not performed or funded by EPA and was not subject to EPA’s quality system requirements. The views expressed in this article are those of the author(s) and do not necessarily represent the views or the policies of the U.S. Environmental Protection Agency. The authors declare no competing financial interest.
- Additional materials and methods, average fragmentation spectrum for 213.1496 → [52.0000–223.0000], and ESI source parameters for MS1 data collection (PDF)
- Plausible chemical structures for the assigned formula of C12H22O3 from the Environmental Protection Agency CompTox Chemicals Dashboard, (XLSX)
References
- 1.Toward a New Comprehensive Global Database of Per- and Polyfluoroalkyl Substances (PFASs): Summary Report on Updating the OECD 2007 List of Per- and Polyfluoroalkyl Substances (PFASs); Organisation for Economic Cooperation and Development (OECD)/UNEP, 2018.
- 2.Glüge J; Scheringer M; Cousins I; DeWitt JC; Goldenman G; Herzke D; Lohmann R; Ng C; Trier X; Wang Z An overview of the uses of per-and polyfluoroalkyl substances (PFAS). Environ. Sci.: Processes Impacts 2020, 22, 2345–2373, DOI: 10.1039/D0EM00291G [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houde M; De Silva AO; Muir DCG; Letcher RJ Monitoring of Perfluorinated Compounds in Aquatic Biota: An Updated Review. Environ. Sci. Technol. 2011, 45 (19), 7962–7973, DOI: 10.1021/es104326w [DOI] [PubMed] [Google Scholar]
- 4.Jian J-M; Chen D; Han F-J; Guo Y; Zeng L; Lu X; Wang F A short review on human exposure to and tissue distribution of per- and polyfluoroalkyl substances (PFASs). Sci. Total Environ. 2018, 636, 1058–1069, DOI: 10.1016/j.scitotenv.2018.04.380 [DOI] [PubMed] [Google Scholar]
- 5.Wang Z; DeWitt JC; Higgins CP; Cousins IT A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)? Environ. Sci. Technol. 2017, 51, 2508, DOI: 10.1021/acs.est.6b04806 [DOI] [PubMed] [Google Scholar]
- 6.Liu S; Junaid M; Zhong W; Zhu Y; Xu N A sensitive method for simultaneous determination of 12 classes of per- and polyfluoroalkyl substances (PFASs) in groundwater by ultrahigh performance liquid chromatography coupled with quadrupole orbitrap high resolution mass spectrometry. Chemosphere 2020, 251, 125327, DOI: 10.1016/j.chemosphere.2020.126327 [DOI] [PubMed] [Google Scholar]
- 7.Robuck A; Cantwell MG; McCord JP; Addison LM; Pfohl M; Strynar MJ; McKinney RA; Katz DR; Wiley DN; Lohmann R Legacy and Novel Per- and Polyfluoroalkyl Substances (PFAS) in Juvenile Seabirds from the US Atlantic Coast. Environ. Sci. Technol. 2020, 54, 12938, DOI: 10.1021/acs.est.0c01951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benskin JP; Bataineh M; Martin JW Simultaneous Characterization of Perfluoroalkyl Carboxylate, Sulfonate, and Sulfonamide Isomers by Liquid Chromatography–Tandem Mass Spectrometry. Anal. Chem. 2007, 79 (17), 6455–6464, DOI: 10.1021/ac070802d [DOI] [PubMed] [Google Scholar]
- 9.Chan E; Sandhu M; Benskin JP; Ralitsch M; Thibault N; Birkholz D; Martin JW Endogenous high-performance liquid chromatography/tandem mass spectrometry interferences and the case of perfluorohexane sulfonate (PFHxS) in human serum; are we overestimating exposure? Rapid Commun. Mass Spectrom. 2009, 23 (10), 1405–1410, DOI: 10.1002/rcm.4012 [DOI] [PubMed] [Google Scholar]
- 10.Genualdi S; Beekman J; Carlos K; Fisher CM; Young W; DeJager L; Begley T, Analysis of per- and poly-fluoroalkyl substances (PFAS) in processed foods from FDA’s Total Diet Study. Anal. Bioanal. Chem. 2021. DOI: 10.1007/s00216-021-03610-2 [DOI] [PubMed] [Google Scholar]
- 11.Kato K; Kalathil AA; Patel AM; Ye X; Calafat AM Per- and polyfluoroalkyl substances and fluorinated alternatives in urine and serum by on-line solid phase extraction-liquid chromatography-tandem mass spectrometry. Chemosphere 2018, 209, 338–345, DOI: 10.1016/j.chemosphere.2018.06.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bangma J; Eaves LA; Oldenburg K; Reiner JL; Manuck T; Fry RC Identifying risk factors for levels of per-and polyfluoroalkyl substances (PFAS) in the placenta in a high-risk pregnancy cohort in North Carolina. Environ. Sci. Technol. 2020, 54 (13), 8158–8166, DOI: 10.1021/acs.est.9b07102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McEachran AD; Sobus JR; Williams AJ Identifying known unknowns using the US EPA’s CompTox Chemistry Dashboard. Anal. Bioanal. Chem. 2017, 409 (7), 1729–1735, DOI: 10.1007/s00216-016-0139-z [DOI] [PubMed] [Google Scholar]
- 14.Wishart DS; Feunang YD; Marcu A; Guo AC; Liang K; Vázquez-Fresno R; Sajed T; Johnson D; Li C; Karu N; Sayeeda Z; Lo E; Assempour N; Berjanskii M; Singhal S; Arndt D; Liang Y; Badran H; Grant J; Serra-Cayuela A; Liu Y; Mandal R; Neveu V; Pon A; Knox C; Wilson M; Manach C; Scalbert A HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608, DOI: 10.1093/nar/gkx1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Djoumbou-Feunang Y; Pon A; Karu N; Zheng J; Li C; Arndt D; Gautam M; Allen F; Wishart DS CFM-ID 3.0: Significantly Improved ESI-MS/MS Prediction and Compound Identification. Metabolites 2019, 9 (4), 72, DOI: 10.3390/metabo9040072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryu EH; Yang EJ; Woo ER; Chang HC Purification and characterization of antifungal compounds from Lactobacillus plantarum HD1 isolated from kimchi. Food Microbiol. 2014, 41, 19–26, DOI: 10.1016/j.fm.2014.01.011 [DOI] [PubMed] [Google Scholar]
- 17.Kim D; Cryle MJ; De Voss JJ; Ortiz de Montellano PR Functional expression and characterization of cytochrome P450 52A21 from Candida albicans. Arch. Biochem. Biophys. 2007, 464 (2), 213–20, DOI: 10.1016/j.abb.2007.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaugg MT; Bruderer T; Nowak N; Eiffert L; Martinez-Lozano Sinues P; Kohler M; Zenobi R Mass-Spectrometric Detection of Omega-Oxidation Products of Aliphatic Fatty Acids in Exhaled Breath. Anal. Chem. 2017, 89 (19), 10329–10334, DOI: 10.1021/acs.analchem.7b02092 [DOI] [PubMed] [Google Scholar]
- 19.Batsika CS; Mantzourani C; Gkikas D; Kokotou MG; Mountanea OG; Kokotos CG; Politis PK; Kokotos G Saturated Oxo Fatty Acids (SOFAs): A Previously Unrecognized Class of Endogenous Bioactive Lipids Exhibiting a Cell Growth Inhibitory Activity. J. Med. Chem. 2021, 64, 5654, DOI: 10.1021/acs.jmedchem.0c02058 [DOI] [PubMed] [Google Scholar]
- 20.Shestakova K; Brito A; Mesonzhnik NV; Moskaleva NE; Kurynina KO; Grestskaya NM; Serkov IV; Lyubimov II; Bezuglov VV; Appolonova SA Rabbit plasma metabolomic analysis of Nitroproston®: a multi target natural prostaglandin based-drug. Metabolomics 2018, 14 (9), 112, DOI: 10.1007/s11306-018-1413-1 [DOI] [PubMed] [Google Scholar]
- 21.Kokotou MG; Batsika CS; Mantzourani C; Kokotos G Free Saturated Oxo Fatty Acids (SOFAs) and Ricinoleic Acid in Milk Determined by a Liquid Chromatography-High-Resolution Mass Spectrometry (LC-HRMS) Method. Metabolites 2021, 11 (1), 46, DOI: 10.3390/metabo11010046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schymanski EL; Jeon J; Gulde R; Fenner K; Ruff M; Singer HP; Hollender J Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48 (4), 2097–2098, DOI: 10.1021/es5002105 [DOI] [PubMed] [Google Scholar]
- 23.Peng L; Xu W; Zeng Q; Cheng Y; Zhang Y; Guo Y; Chen D; Jiang C; Wang F, Distribution characteristics of per- and polyfluoroalkyl substances (PFASs) in human urines of acrylic fiber plant and chemical plant. Environ. Sci. Pollut. Res 2021. DOI: 10.1007/s11356-021-15355-7 [DOI] [PubMed] [Google Scholar]
- 24.Crebelli R; Caiola S; Conti L; Cordelli E; De Luca G; Dellatte E; Eleuteri P; Iacovella N; Leopardi P; Marcon F; Sanchez M; Sestili P; Siniscalchi E; Villani P Can sustained exposure to PFAS trigger a genotoxic response? A comprehensive genotoxicity assessment in mice after subacute oral administration of PFOA and PFBA. Regul. Toxicol. Pharmacol. 2019, 106, 169–177, DOI: 10.1016/j.yrtph.2019.05.005 [DOI] [PubMed] [Google Scholar]
- 25.Chang SC; Das K; Ehresman DJ; Ellefson ME; Gorman GS; Hart JA; Noker PE; Tan YM; Lieder PH; Lau C; Olsen GW; Butenhoff JL Comparative pharmacokinetics of perfluorobutyrate in rats, mice, monkeys, and humans and relevance to human exposure via drinking water. Toxicol. Sci. 2008, 104 (1), 40–53, DOI: 10.1093/toxsci/kfn057 [DOI] [PubMed] [Google Scholar]
- 26.Pérez F; Nadal M; Navarro-Ortega A; Fàbrega F; Domingo JL; Barceló D; Farré M Accumulation of perfluoroalkyl substances in human tissues. Environ. Int. 2013, 59, 354–362, DOI: 10.1016/j.envint.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 27.Abraham K; El-Khatib AH; Schwerdtle T; Monien BH Perfluorobutanoic acid (PFBA): No high-level accumulation in human lung and kidney tissue. Int. J. Hyg. Environ. Health 2021, 237, 113830, DOI: 10.1016/j.ijheh.2021.113830 [DOI] [PubMed] [Google Scholar]
- 28.Gao Y; Zhang Q; Li X; Li X; Li H Simultaneous determination of legacy and emerging per- and polyfluoroalkyl substances in fish by QuEChERS coupled with ultrahigh performance liquid chromatography tandem mass spectrometry. Anal. Methods 2018, 10 (47), 5715–5722, DOI: 10.1039/C8AY01478G [DOI] [Google Scholar]
- 29.Sun M; Cui J; Guo J; Zhai Z; Zuo P; Zhang J Fluorochemicals biodegradation as a potential source of trifluoroacetic acid (TFA) to the environment. Chemosphere 2020, 254, 126894, DOI: 10.1016/j.chemosphere.2020.126894 [DOI] [PubMed] [Google Scholar]
- 30.Weatherly LM; Shane HL; Lukomska E; Baur R; Anderson SE Systemic toxicity induced by topical application of heptafluorobutyric acid (PFBA) in a murine model. Food Chem. Toxicol. 2021, 156, 112528, DOI: 10.1016/j.fct.2021.112528 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


