Key Points
Question
For male androgenetic alopecia, what is the relative efficacy of monotherapy with orally and topically administered minoxidil, dutasteride, and finasteride?
Findings
In this network meta-analysis of 23 trials, the rank of efficacy across treatments and the relative efficacy for every possible pairwise comparison of treatments were determined for 4 end points (ie, change in total and terminal hair count after 24 and 48 weeks). The results of this meta-analysis indicate that 0.5 mg/d of oral dutasteride has the highest probability of being the most efficacious treatment, followed by these agents in decreasing order of efficacy: 5 mg/d of oral finasteride, 5 mg/d of oral minoxidil, 1 mg/d of oral finasteride, 5% topical minoxidil, 2% topical minoxidil, and 0.25 mg/d of oral minoxidil.
Meaning
The findings of this meta-analysis contribute to the comparative effectiveness literature for AGA therapies with regard to the compared interventions.
Abstract
Importance
There are knowledge gaps regarding the relative efficacy of 3 commonly used drugs for androgenetic alopecia (AGA), namely, minoxidil and the two 5-α reductase inhibitors dutasteride and finasteride.
Objective
To examine the relative efficacy of any dose and administration route of minoxidil, dutasteride, and finasteride for the treatment of male AGA.
Data Sources
Systematic searches were performed in PubMed on March 5, 2021, without date restrictions.
Study Selection
Eligible studies included those that investigated monotherapy with any dose and administration route of minoxidil, dutasteride, and finasteride.
Data Extraction and Synthesis
Data on the mean (SD) difference and sample size were used for the bayesian network meta-analyses. League tables and surface under the cumulative ranking curve values were used to examine the relative efficacy of the interventions.
Main Outcomes and Measures
Study end points were change in total and terminal hair count after 24 and 48 weeks of therapy. The 4 end points were quantified in hairs per square centimeters.
Results
The PubMed search yielded 848 records; after the 2 stages of screening, 23 studies were eligible for quantitative analyses. Mean (SD) age of patients ranged from 22.8 (3.3) years to 41.8 (12.3) years. The greatest increase in total hair count at 24 weeks (ie, first end point) was with 0.5 mg/d of dutasteride, which was significantly more efficacious than 1 mg/d of finasteride (mean difference, 7.1 hairs/cm2; 95% CI, 5.1-9.3 hairs/cm2) and minoxidil (0.25 mg/d [mean difference, 23.7 hairs/cm2; 95% CI, 9.5-38.0 hairs/cm2], 5 mg/d [mean difference, 15.0 hairs/cm2; 95% CI, 3.9-26.1 hairs/cm2], and 2% solution [mean difference, 8.5 hairs/cm2; 95% CI, 4.8-12.3 hairs/cm2]). The greatest increase in terminal hair count at 24 weeks (ie, second end point) was with 5 mg/d of minoxidil, which was significantly more efficacious than the 0.25-mg/d dose (mean difference, 43.6 hairs/cm2; 95% CI, 29.7-57.7 hairs/cm2) and its topical forms (in 2% [mean difference, 29.3 hairs/cm2; 95% CI, 21.1-37.5 hairs/cm2] and 5% [mean difference, 29.8 hairs/cm2; 95% CI, 19.7-39.8 hairs/cm2]); 5 mg/d of minoxidil was significantly more efficacious than 1 mg/d of finasteride (mean difference, 10.4 hairs/cm2; 95% CI, 2.2-18.6 hairs/cm2). The greatest increase in total hair count at 48 weeks (ie, third end point) was with 5 mg/d of finasteride, which was significantly more efficacious than 2% topical minoxidil (mean difference, 20.7 hairs/cm2; 95% CI, 9.5-31.9 hairs/cm2). The greatest increase in terminal hair count at 48 weeks (ie, fourth end point) was with 1 mg/d of finasteride, which was significantly more effective than topical minoxidil (in 2% [mean difference, 32.1 hairs/cm2; 95% CI, 23.9-40.3 hairs/cm2] and 5% [mean difference, 26.2 hairs/cm2; 95% CI, 16.2-36.2 hairs/cm2]).
Conclusions and Relevance
As efficacy data from head-to-head trials accumulate, there could be a better sense of the relative efficacy of the different doses of the 5-α reductase inhibitors and minoxidil. The findings of this meta-analysis contribute to the comparative effectiveness literature for AGA therapies with regard to the compared interventions.
This network meta-analysis examines whether minoxidil, dutasteride, or finasteride monotherapy is the most efficacious for the treatment of male androgenetic alopecia.
Introduction
Androgenetic alopecia (AGA) is a common occurrence in men and women1,2,3; the etiologic factors in this condition involve the enzyme 5-α reductase. Finasteride and dutasteride are 5-α reductase inhibitors (5-ARIs).4 Minoxidil is another drug for AGA; however, its mechanism of action is not fully understood.5 Numerous studies6,7 have investigated the efficacy of minoxidil and the 5-ARIs for the treatment of male AGA; however, evidence on their relative efficacy is scant. For example, there are no controlled trials on the efficacy of oral minoxidil for male AGA.8
Recently, the relative efficacy of oral and topical minoxidil was examined using network meta-analyses (NMAs),9 but to our knowledge no study has determined the comparative effectiveness of these 2 agents with that of local and systemic dutasteride and finasteride. Therefore, our objective was to examine the relative efficacy of monotherapy with the 5-ARIs and minoxidil—in any dose and administration route—for the treatment of male AGA.
Methods
The protocol for our work was registered with the International Platform of Registered Systematic Review and Meta-analysis Protocols.10 Our study did not have direct involvement with humans; therefore, the conduct of our work did not require approval from an institutional review board. The study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline for NMAs.
Identification of Eligible Studies
Per our objective, we had 4 outcomes of interest: change in total and terminal hair count after 24 and 48 weeks of therapy. A study was eligible for quantitative analyses if it investigated the therapeutic outcomes associated with monotherapy with minoxidil, dutasteride, and finasteride for male AGA. The 3 agents could be of any dose and administration route. We excluded evidence in languages other than English. A brief description for terminal and total hair count is provided in eAppendix 1 in the Supplement.11,12
We systematically searched PubMed on March 5, 2021, without date restrictions, with the following keywords: topical finasteride AND hair loss; oral finasteride AND hair loss; topical minoxidil AND hair loss; oral minoxidil AND hair loss; and dutasteride AND hair loss. We screened titles and abstract, reviewed full-texts, and extracted data from eligible studies. The searches, 2 stages of screening, and data extraction were performed independently (M.V. and M.A.B.); discrepancies were resolved in discussion with a third author (A.K.G.). Extracted data were organized into spreadsheets.
We obtained values for the mean (SD) and sample size from each arm. When the SD was not provided, we estimated it, wherever possible, using approaches supported by the literature.13,14
Network Plots
For each outcome, the network of interventions was depicted through a network plot, a diagram with nodes and edges. A node corresponds to a given intervention; an edge is the line between 2 interventions that were compared in a head-to-head trial.
Inclusion of Randomized and Observational Evidence
Our NMAs included data from both observational and randomized studies, a form of evidence synthesis commonly used.15,16 Per the Declaration of Helsinki,17 providing placebo (or vehicle) to participants in control arms of clinical trials can raise ethical concerns if therapeutic alternatives exist; given that the treatment options for any condition expand over time, newer trials can be ethically constrained to randomize participants to placebo or vehicle. In a network of interventions, absence of placebo or vehicle arms can disconnect recent drugs from former ones. Hence, use of observational data has been suggested15,16 as an approach to unite otherwise disconnected networks. Moreover, health technology assessment agencies have acknowledged the amalgamation of randomized and observational data as a pragmatic approach to evidence synthesis.
Single-arm trials were incorporated into a network of randomized studies by using a framework supported by the literature: we matched each single-arm study to a randomized trial whose participants had the closest distribution of disease severity; then, the matched single-arm trial was treated as an additional arm of the randomized trial to which it was matched.15,16 Randomized and observational data were combined through naive pooling, an approach in which study design is not differentiated. Naive pooling is the simplest approach for combining randomized and observational evidence and is commonly used.16
Network Meta-analyses
For each outcome, an NMA was conducted under a bayesian random-effects model with 200 000 iterations for each Markov chain Monte Carlo chain; uniform priors were used. In each of the 4 NMAs, studies were weighted according to inverse of variance—whose value is directly proportional to sample size.18 For each end point, we produced league tables and estimated treatments’ surface under the cumulative ranking curve (SUCRA). League tables present the relative efficacy for every possible pairwise combination. The point estimate for our league tables was the mean difference (with 95% credible intervals). A drug’s SUCRA corresponds to their overall rank for efficacy; the highest value corresponds to the most efficacious intervention. It is important to note that inferences for relative efficacy should not be based solely on SUCRA because this ranking metric does not incorporate statistical evidence (eg, league tables) or quality of evidence. Brief description for SUCRA estimation is provided in eAppendix 2 in the Supplement.
Assessing Quality of Evidence Within Studies and Across Networks
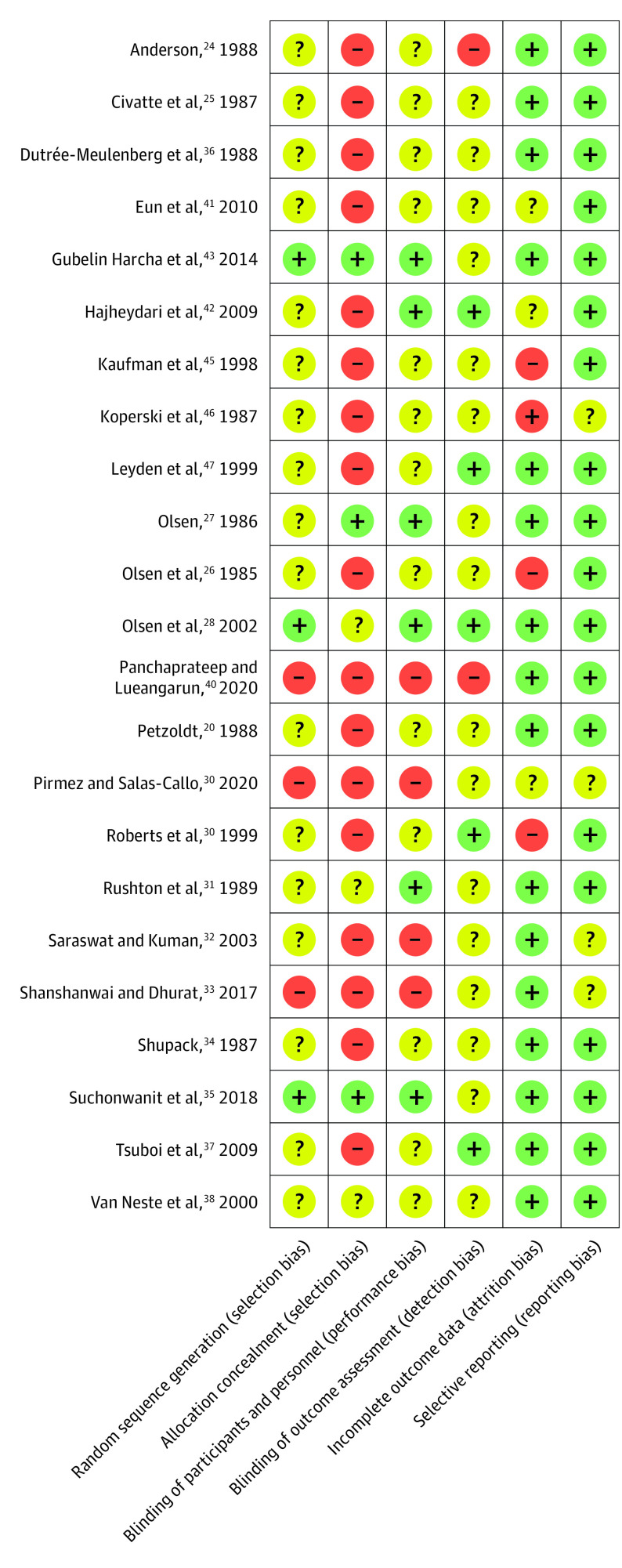
Each study that was eligible for quantitative analyses was evaluated for risk of bias using the Cochrane Collaboration risk of bias assessment tool wherein qualitative judgment is provided for 6 domains: randomization, allocation concealment, blinding of study participants and research personnel, blinding of the outcome assessor(s), attrition bias, and selective reporting.19 Attrition bias occurs when no outcome data are available—at follow up—for participants who were randomized to a group. Each domain could be judged as having low, unclear, or high risk of bias.19
For each outcome, quality of evidence across a network was assessed using the Confidence in Network Meta-Analysis (CINeMA) framework that is based on the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach. Under the CINeMA framework, qualitative judgments are made across 6 domains: within-study risk of bias, reporting bias, indirectness, imprecision, heterogeneity, and incoherence.20 Indirectness pertains to how relevant a study’s end point is to a given outcome of interest. Imprecision pertains to the 95% CI (ie, not the credible interval because CINeMA uses a frequentist framework); heterogeneity refers to the consistency—in terms of direction and magnitude of treatment effects. Incoherence pertains to congruence between direct and indirect evidence.20 In the CINeMA framework, a confidence rating is an overall qualitative assessment that summarizes judgment across the 6 domains that assess quality of evidence; just like the GRADE framework, confidence rating can be high, moderate, low, or very low.20,21
Kilim Plots
Given that we had more than 1 outcome of interest, the use of Kilim plots was appropriate for the presentation of our findings. A Kilim plot is a visual tool developed by Seo et al22 to aid researchers in the nontrivial task of intuitively—and efficiently—presenting results for multiple outcomes in NMAs. Kilim plots present estimates of absolute treatment effects, which are deemed clinically relevant. Furthermore, each treatment effect is color-coded to represent strength of statistical evidence. We modified the source code provided by Seo et al22 to produce Kilim plots for continuous point estimates and SUCRA ranks from a bayesian framework. We used R studio software (R Foundation for Statistical Computing) to conduct all our analyses23; a significance level of α = .05 was set for all analyses.
Results
Our search yielded 848 records (Figure 1); after the 2 stages of screening, 23 studies24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47 were eligible for quantitative analyses (Figure 1). Details from the eligible studies are presented in the Table; 2 were single-arm (ie, observational) studies, whereas the remaining 21 were randomized trials. The network plots for change in total hair count after 24 weeks of therapy, change in terminal hair count after 24 weeks of therapy, change in total hair count after 48 weeks of therapy, and change in terminal hair count after 48 weeks of therapy are presented in eFigures 1 to 4 in the Supplement. Within-study risk of bias assessments are presented in Figure 2; across-study evaluation of evidence quality is presented in eTables 1 to 4 in the Supplement. League tables for our first, second, third, and fourth outcomes are presented in eFigures 5 to 8 with further detail in eFigures 16 to 27 in the Supplement.
Figure 1. Search Process.
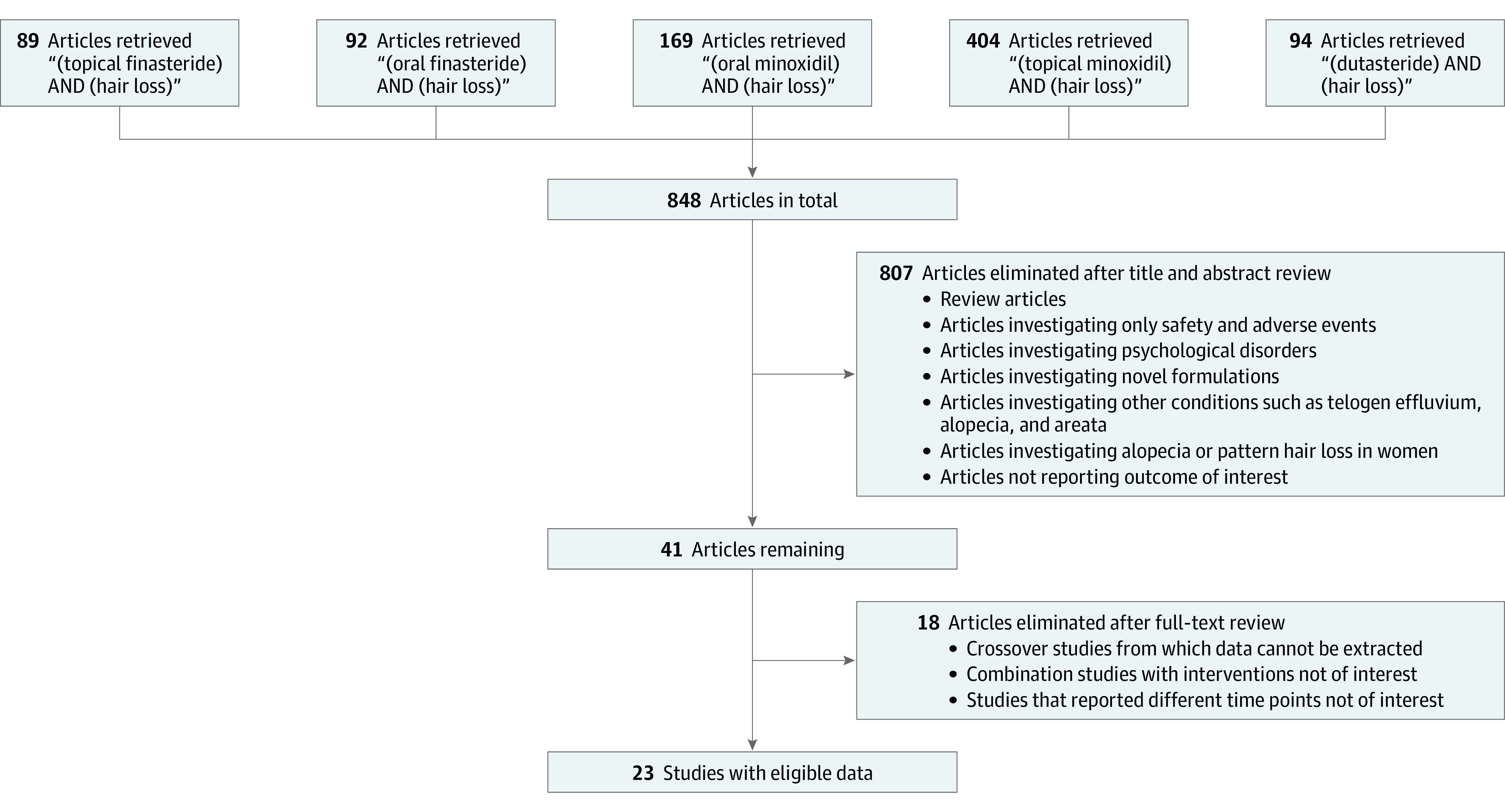
Schematic for identification of studies that were eligible for quantitative analyses.
Table. Details of the 23 Studies Eligible for Quantitative Analyses.
| Study | Comparators and dosage | Administration route |
|---|---|---|
| Olsen et al,26 1985 | 2% Topical minoxidil; 3% topical minoxidil; placebo or vehicle for 4 mo and crossed over to 3% topical minoxidil | 1 mL twice daily for 12 mo; vehicle consisted of a mixture of propylene glycol, 95% alcohol, and water |
| Olsen et al,27 1986 | 0.01% Topical minoxidil; 0.1% topical minoxidil; 1% topical minoxidil; 2% topical minoxidil; placebo or vehicle | 1 mL twice daily for 6 mo |
| Civatte et al,25 1987 | 2% Topical minoxidil in the excipient placebo; placebo (composition: solution of alcohol, propylene glycol, and the remainder water) | 1 mL twice daily for 24 weeks; 1 mL twice daily for 24 weeks; from week 24 to week 48, all patients received active treatment |
| Koperski et al,46 1987 | 2% Topical minoxidil for 12 mo followed by 3% topical minoxidil for the remainder of the study; 3% topical minoxidil for the whole duration of the study; placebo for 4 mo followed by 3% topical minoxidil for the remainder of the study | 1 mL twice daily for 30 mo; placebo composed of 10% propylene glycol, 20% water, and 70% ethyl alcohol |
| Shupack et al,34 1987 | 2% Topical minoxidil; 1% topical minoxidil; 0.1% topical minoxidil; placebo | 1 mL twice daily for 6 mo |
| Anderson,24 1988 | 2% Topical minoxidil in the excipient placebo; placebo (composition: solution of alcohol [63%], propylene glycol [20%], and the remainder water) | 1 mL twice daily (morning and evening with an approximately 12-h gap) for 24 wk; 1 mL twice daily (morning and evening with an approximately 12-h gap) for 24 wk |
| Dutrée-Meulenberg et al,36 1988 | 2% Topical minoxidil (in the excipient placebo; placebo (composition: solution of alcohol, propylene glycol, and the remainder water) | 1 mL twice daily (morning and evening with an approximately 12-h gap) for 24 wk |
| Petzoldt,29 1988 | 2% Topical minoxidil; placebo excipient (propylene glycol, alcohol, and water) | 1 mL twice daily (morning and evening at approximately 12-h intervals) for 24 wk |
| Rushton et al,31 1989 | 2% Topical minoxidil; placebo | 2.0 cm3 applied twice daily for 6 mo |
| Kaufman et al,45 1998 | 1 mg of oral finasteride; placebo | After screening, patients entered a 2-week single-blind placebo run-in; then patients received a study shampoo for standardization of shampoo used and for prophylaxis of seborrheic dermatitis; patients were then randomized to once-daily finasteride or placebo for 1 y; extension study for another year (different randomization) |
| Leyden et al,47 1999 | 1 mg of oral finasteride; placebo | Once daily for 12 mo |
| Roberts et al,30 1999 | 5 mg of oral finasteride; 1 mg of oral finasteride; 0.2 mg of oral finasteride; 0.01 mg of oral finasteride; placebo | First clinical study evaluated finasteride; 5 mg/d vs placebo for 12 mo; second clinical trial evaluated the effects of 1, 0.2, and 0.01 mg/d of finasteride vs placebo for 6 mo; men completing the second trial were eligible to enter extension study without placebo for another 6 mo; patients receiving placebo were crossed over to the other 3 groups |
| Van Neste et al,38 2000 | 1 mg of oral finasteride; placebo | Patients completed a 2-week, single-blind, placebo run-in period; after randomization, patients received daily for 48 wk |
| Olsen et al,28 2002 | 5% Topical minoxidil; 2% topical minoxidil; placebo (vehicle for 5% solution) | 1 mL twice daily at approximately 12-h intervals for 48 wk |
| Saraswat and Kumar,32 2003 | 1 mg of oral finasteride; 2% topical minoxidil | Daily for 12 mo; some continued treatment for another year; 1 mL twice daily for 12 mo; some continued treatment for another year |
| Hajheydari et al,42 2009 | 1% Topical finasteride gel and placebo tablet; 1 mg of oral finasteride tablets and gel base placebo | Gel was to be used twice daily for 6 mo; tablet was once daily for 6 mo |
| Tsuboi et al,37 2009 | 5% Topical minoxidil; 1% topical minoxidil | 1 mL twice daily for 24 wk |
| Eun et al,41 2010 | 0.5 mg of oral dutasteride; placebo | Daily for 6 mo |
| Gubelin Harcha et al,43 2014 | 0.02 mg of oral dutasteride; 0.1 mg of oral dutasteride; 0.5 mg of oral dutasteride; 1 mg of oral finasteride; placebo | Daily for 24 wk |
| Shanshawal et al,33 2017 | 0.5 mg of oral dutasteride; 1 mg of oral finasteride | Daily for 24 wk |
| Suchonwanit et al,35 2018 | Finasteride (topical) 0.25% plus 3% topical minoxidil (propylene glycol, ethanol, and water base); 3% topical minoxidil | 1 mL twice daily for 24 wk |
| Panchaprateep and Lueangarun,40 2020 | 5 mg of oral minoxidil | Daily every morning for 24 wk |
| Pirmez and Salas-Callo,39 2020 | 0.25 mg of oral minoxidil | Daily for 24 wk |
Figure 2. Risk of Bias Summary.
Risk of bias across 6 domains for the 23 studies that were eligible for quantitative analyses is shown. Each domain received 1 of 3 judgments, namely, low risk of bias (denoted by green or the plus sign), unclear risk of bias (denoted by yellow or the question mark), and high risk of bias (denoted by red or the minus sign).
On the basis of the available information, we computed the proportion of patients whose severity of AGA was mild, moderate, and severe, which corresponded to the Hamilton-Norwood scores of I to II, III to IV, and V or above, respectively. Participants’ mean (SD) age ranged from 22.8 (3.3) years to 41.8 (12.3) years. Summary of patient demographic data are provided in eTable 5 in the Supplement.
Across the 23 studies, 15 regimens were identified: (1) 0.02 mg/d of oral dutasteride, (2) 0.1 mg/d of oral dutasteride, (3) 0.5 mg/d of oral dutasteride, (4) 0.2 mg/d of oral finasteride, (5) 1 mg/d of oral finasteride, (6) 5 mg/d or oral finasteride, (7) 1% topical finasteride, (8) 0.25 mg/d of oral minoxidil, (9) 5 mg/d or oral minoxidil, (10) 0.1% topical minoxidil, (11) 1% topical minoxidil, (12) 2% topical minoxidil, (13) 3% topical minoxidil, (14) 5% topical minoxidil, and (15) placebo or vehicle. For our analyses, placebo and vehicle were amalgamated into 1 group (ie, the control group).
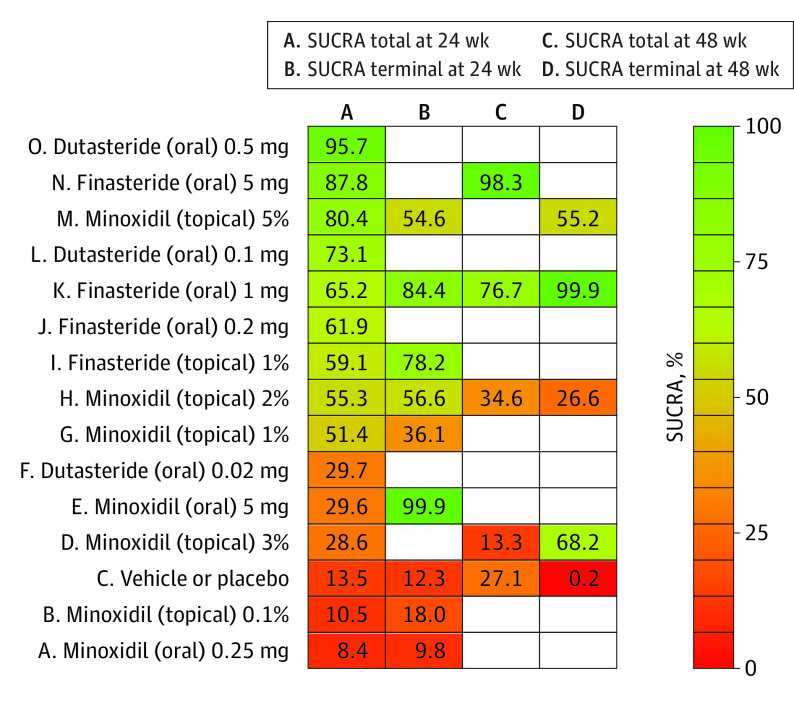
Figure 3 presents a modified Kilim plot for the SUCRA values of the interventions in all 4 networks. Figure 4 presents a Kilim plot for each estimated treatment effect relative to the reference group (ie, the placebo or vehicle arm). In each network, the magnitude of each treatment’s effect—relative to the reference—followed an order like that of their SUCRA; for example, in the first network, we found a mean increase of 18.7 hairs/cm2 for 0.5 mg of oral dutasteride (SUCRA = 96%), 16.4 hairs/cm2 for 5 mg of oral finasteride (SUCRA = 88%), and 15.3 hairs/cm2 for 5% topical minoxidil (SUCRA = 80%) (Figures 3 and 4).
Figure 3. Presentation of Treatments’ Surface Under the Cumulative Ranking Curve (SUCRA) via a Modified Kilim Plot.
Names were prefixed with uppercase letters solely for the purpose of ordering. A treatment’s SUCRA corresponds to its overall rank for efficacy (higher values corresponding to greater efficacy). The vertical and horizontal axes correspond to the treatments and the end points, respectively. For example, SUCRA total at 24 weeks corresponds to change in total hair count (per square centimeters) after 24 weeks of therapy (ie, outcome 1). A color chart for SUCRA values is shown; red and green (which are arbitrarily chosen colors) correspond to lowest and highest SUCRA values, which, in turn, correspond to the least and the most effective treatment per this ranking metric, respectively.
Figure 4. Kilim Plot for the Comparison of 15 Interventions and 4 End Points.
Names were prefixed with uppercase letters solely for the purpose of ordering. Each cell represents the mean difference between the change in hair count after the respective duration in the treatment group and the change in hair count after the respective duration in the reference comparator. Our reference comparator (ie, control) was the arm for vehicle (placebo). Hence, the treatment effect for the control (ie, the reference) is 0; so, the nonzero values represented in the blue cells are pooled estimates from 1-group meta-analyses. P values close to green or red represent mean differences that are significantly more or less effective than the control, respectively; yellow corresponds to nonsignificant mean differences.
Change in Total Hair Count After 24 Weeks of Therapy
For the first NMA, 0.5 mg of oral dutasteride was ranked the most efficacious (SUCRA = 96%) relative to 5 mg of oral finasteride (SUCRA = 88%), 5% topical minoxidil (SUCRA = 80%), 1 mg of oral finasteride (SUCRA = 65%), 2% topical minoxidil (SUCRA = 55%), 5 mg of oral minoxidil (SUCRA = 30%), and 0.25 mg of oral minoxidil (SUCRA = 8%) (Figure 3). However, our league table showed that the efficacy of the highest-ranked intervention, 0.5 mg of dutasteride, did not differ from that of 5% topical minoxidil or 5 mg of oral finasteride; the quality of evidence for these 2 comparisons was low. In addition, 0.5 mg of oral dutasteride was significantly more efficacious than 1 mg of oral finasteride (mean difference, 7.1 hairs/cm2; 95% CI, 5.1-9.3 hairs/cm2) and 2% topical minoxidil (mean difference, 8.5 hairs/cm2; 95% CI, 4.8-12.3 hairs/cm2); the quality of evidence for these 2 comparisons was low (eTable 1 and eFigure 5 in the Supplement).
Our results show that 5 mg of oral minoxidil was significantly less efficacious than 0.5 mg of oral dutasteride (mean difference, −15.0 hairs/cm2; 95% CI, −26.1 to −3.9 hairs/cm2) and 5 mg of oral finasteride (mean difference, −12.7 hairs/cm2; 95% CI, −24.9 to −0.5 hairs/cm2); the quality of evidence for both comparisons was low. However, the efficacy of 5 mg of oral minoxidil did not significantly differ from that of 0.25 mg of oral minoxidil (mean difference, 8.7 hairs/cm2; 95% CI, −9.2 to 26.5 hairs/cm2), 1 mg of oral finasteride (mean difference, −7.9 hairs/cm2; 95% CI, −18.9 to 3.1 hairs/cm2), and the 2% (mean difference, −6.5 hairs/cm2; 95% CI, −16.9 to 3.9 hairs/cm2) and 5% (mean difference, −11.7 hairs/cm2; 95% CI, −27.8 to 4.4 hairs/cm2) concentrations of topical minoxidil; evidence quality for these pairwise comparisons ranged from low to very low (eFigure 5 and eTable 1 in the Supplement). We found that 0.25 mg of oral minoxidil was less efficacious than 1 mg of oral finasteride (moderate evidence quality) and 5 mg of finasteride (low evidence quality), less efficacious than the 2% and 5% concentrations of topical minoxidil (low evidence quality), and less efficacious than 0.5 mg of oral dutasteride (moderate evidence quality) (eFigure 5 and eTable 1 in the Supplement).
Change in Terminal Hair Count After 24 Weeks of Therapy
For our second NMA, 5 mg of oral minoxidil had the highest SUCRA (100%) compared with 1 mg of oral finasteride (SUCRA = 84%), 2% topical minoxidil (SUCRA = 57%), 5% topical minoxidil (SUCRA = 55%), and 0.25 mg of oral minoxidil (SUCRA = 10%) (Figure 3). The 5-mg oral dose of minoxidil was significantly more efficacious than 1 mg of oral finasteride (mean difference, 10.4 hairs/cm2; 95% CI, 2.2 − 18.6 hairs/cm2), and the evidence quality for this comparison was low. The 1-mg oral dose of finasteride was significantly more efficacious than 0.25 mg of oral minoxidil (mean difference, 33.2 hairs/cm2; 95% CI, 19.1-47.3 hairs/cm2) (moderate evidence quality), 5% topical minoxidil (mean difference, 19.4 hairs/cm2; 95% CI, 9.2-29.5 hairs/cm2) (moderate evidence quality), and 2% topical minoxidil (mean difference, 18.8 hairs/cm2; 95% CI, 10.7-27.0 hairs/cm2) (low evidence quality). Furthermore, we found that 5 mg of oral minoxidil was significantly more efficacious than 0.25 mg of oral minoxidil (mean difference, 43.6 hairs/cm2; 95% CI, 29.7-57.7 hairs/cm2) (moderate evidence quality), 5% topical minoxidil (mean difference, 29.8 hairs/cm2; 95% CI, 19.7-39.8 hairs/cm2) (high evidence quality), and 2% topical minoxidil (mean difference, 29.3 hairs/cm2; 95% CI, 21.1-37.5 hairs/cm2) (low evidence quality) (eFigure 6 and eTable 2 in the Supplement).
Change in Total Hair Count After 48 Weeks of Therapy
For our third NMA, we found that 5 mg of oral finasteride had the highest SUCRA (98%), followed by 1 mg of oral finasteride (SUCRA = 77%) and 2% topical minoxidil (SUCRA = 35%) (Figure 3). For this outcome, efficacy data were not available for oral minoxidil or dutasteride. We found that the efficacy of this highest-ranked intervention (ie, 5 mg of oral finasteride) did not differ from that of 1 mg of oral finasteride (low evidence quality); however, our results showed that 5 mg of oral finasteride was significantly more efficacious than 2% topical minoxidil (mean difference, 20.7 hairs/cm2; 95% CI, 9.5-31.9 hairs/cm2) (low evidence quality) (eFigure 7 and eTable 3 in the Supplement).
Change in Terminal Hair Count After 48 Weeks of Therapy
For our fourth NMA, 1 mg of oral finasteride had the highest SUCRA (100%), followed by 5% topical minoxidil (SUCRA = 55%) and 2% topical minoxidil (SUCRA = 27%) (Figure 3). This top ranked treatment (1 mg of oral finasteride) was significantly more efficacious than the 2% (mean difference, 32.1 hairs/cm2; 95% CI, 23.9-40.3 hairs/cm2) and 5% (mean difference, 26.2 hairs/cm2; 95% CI, 16.2-36.2 hairs/cm2) concentrations of topical minoxidil; evidence quality for these 2 comparisons was low (eFigure 8 and eTable 4 in the Supplement).
Discussion
The focus of our discussion pertains to our results on the relative efficacy of topical minoxidil (2% and 5%), 0.5 mg of oral dutasteride, and 1 and 5 mg of oral finasteride because these regimens are regularly used for treating male AGA.7,48,49,50 We also expound on our findings on the relative efficacy of oral minoxidil. One possible ranking of efficacies—in decreasing order—is 0.5 mg/d of oral dutasteride, 5 mg/d of oral finasteride, 5 mg/d of oral minoxidil, 1 mg/d of oral finasteride, 5% topical minoxidil, 2% topical minoxidil, and 0.25 mg/d of oral minoxidil; the robustness of this order could be confirmed by results from future randomized clinical trials.
Dutasteride
We found that 0.5 mg/d of dutasteride, which is used off-label for male AGA in North America, is more efficacious than 1 mg/d of finasteride (which is approved by the US Food and Drug Administration [FDA] for male AGA). The higher potency can be ascribed to the fact that dutasteride, compared with finasteride, is 100 times more potent in inhibiting the type I isoenzyme of 5-α reductase and 3 times more potent in inhibiting the type II isoenzyme.51 Furthermore, finasteride inhibits only the type II isoenzyme.52 Dutasteride is not approved by the FDA for AGA possibly because its half-life is much longer than that of finasteride (5 weeks vs 6 hours).53,54 Nonetheless, 0.5 mg/d of dutasteride has been approved for the treatment of male AGA in Japan and South Korea.4 The treatment effect of dutasteride is deemed superior to that of finasteride,4 and our results (eFigure 5 in the Supplement) support this. Data on dutasteride’s relative efficacy after 48 weeks of therapy were unavailable for change in total and terminal hair count. Hence, future randomized trials for male AGA could investigate the efficacy of dutasteride—per change in hair count—at longer time points.
Finasteride
Although 0.5 mg/d of dutasteride was more efficacious than 1 mg/d of finasteride (change in total hair count at 24 weeks), it was not different from 5 mg/d of finasteride. For 5 mg/d of finasteride, data were available only for change in total hair count at 24 and 48 weeks; at both time points, the efficacy of the daily 5-mg and 1-mg doses of finasteride were not different from each other. For change in total hair count at 24 weeks, the efficacy of the daily 0.2-mg dose of finasteride is similar to that of the daily 1-mg and 5-mg doses; however, it is not clear whether the safety profiles of these 3 doses vary. The efficacy of 1% topical finasteride was similar to 1 mg/d of finasteride (eFigures 5 and 7 in the Supplement); albeit, the safety profiles of the 2 may differ.
Minoxidil
Oral minoxidil has never been approved as a hair loss treatment in any country48; despite this, many physicians prescribe oral minoxidil for male AGA.48,50 The 2% and 5% concentrations of topical minoxidil are approved by the FDA for the treatment of male AGA. In the literature for male AGA, there is a paucity—if not complete absence—of randomized evidence for the efficacy of oral minoxidil.48
The 5-mg/d dose of minoxidil was more efficacious than the 0.25-mg/d dose for terminal hair count, and 5 mg/d of minoxidil was more efficacious than the 5% and 2% topical concentrations for terminal hair count. Per our outcomes, the 5% concentration of topical minoxidil was more efficacious than its 2% counterpart for change in terminal hairs after 48 weeks of therapy; after 24 weeks, the efficacy of the 5% and 2% concentrations—per total and terminal hair count—are not different from each other (eFigures 5 to 8 in the Supplement). It has been suggested that the oral preparation may result in better adherence.
The relative efficacy of 3 or more therapies may arguably be determined more efficiently with NMAs than through head-to-head trials; furthermore, this statistical tool permits researchers to produce evidence on comparative effectiveness without ethical constraints. However, unlike actual randomized trials, meta-analytic procedures cannot determine causality.55
Nonetheless, our conclusions remained virtually unchanged when we re-ran all our analyses with only randomized data. Only the first and second outcomes (ie, change in total and terminal hair count after 24 weeks) incorporated observational data; an updated Kilim plot of SUCRA values—and updated league tables—are provided in eFigures 9 to 11 in the Supplement. For both outcomes (ie, change in total and terminal hair count after 24 weeks), the rank order of SUCRAs were unaltered in the randomized set; furthermore, the SUCRA values of the treatments (in the randomized set) were highly similar (eFigure 9 in the Supplement). For both outcomes, pairwise comparisons in league tables for the randomized set were similar—in magnitude, direction, and statistical significance—to those of the randomized and observational set (eFigures 10 and 11 in the Supplement). Having unaltered conclusions after conducting sensitivity analyses that excluded observational data add to the robustness of our findings; moreover, the evidence for our base analyses was majorly randomized.
We conducted additional analyses; through network meta-regressions, we determined the relative efficacy for mild, moderate, and severe AGA. For each outcome, we produced SUCRA ranks and values and league tables for each severity level. These additional analyses had to exclude a few trials because some studies (ie, 4 of the 23) did not provide severity information (eTable 5 in the Supplement). For each outcome, we found that the SUCRA ranks and values were, for the most part, similar across mild, moderate, and severe AGA (eFigures 12 to 15 in the Supplement). Regarding pairwise associations presented in league tables, a general conclusion is that the direction, magnitude and statistical significance of effect were—more often than not—similar across the mild, moderate, severe, and combined severity levels sets. We did not stratify according to age because approximately 70% of the included studies reported a mean age between 30 and 40 years (eTable 5 in the Supplement); therefore, our findings may not be generalizable to the senior (ie, at least 65 years of age) and juvenile (ie, <18 years of age) populations.
Limitations
This study has limitations. The use of hair count as an end point may pose limits. First, the included studies did not necessarily quantify hair count from the same scalp region; given that hair count tends to be highest at the vertex,56 our findings could be subject to selection bias that arises from measurement at different scalp regions. Second, the trials included in our analyses had no exclusion criteria for participant race and ethnicity, so no assumptions can be made about the generalizability of the findings with regard to race and ethnicity. We did not explore end points such as global photographic assessment because we deemed such end points to be less objective than hair count.
Conclusions
As efficacy data from head-to-head trials accumulate, there could be a better sense of the relative efficacy of the different doses of the 5-ARIs and minoxidil. The findings of this meta-analysis contribute to the comparative effectiveness literature for AGA therapies vis-à-vis the compared interventions.
eAppendix 1. Terminal and Vellus Hair (Basic Description)
eAppendix 2. Estimation of Surface Under the Cumulative Ranking Curve (SUCRA)
eFigure 1. Network Plot for Outcome 1, Change in Total Hair Count (per cm2) After 24 Weeks of Therapy
eFigure 2. Network Plot for Outcome 2, Change in Terminal Hair Count (per cm2) After 24 Weeks of Therapy
eFigure 3. Network Plot for Outcome 3, Change in Total Hair Count (per cm2) After 48 Weeks of Therapy
eFigure 4. Network Plot for Outcome 4, Change in Terminal Hair Count (per cm2) After 48 Weeks of Therapy
eTable 1. Quality of Evidence Assessment for the Network of Outcome 1, Change in Total Hair Count (per cm2) After 24 Weeks of Therapy for Male Pattern Hair Loss
eTable 2. Quality of Evidence Assessment for the Network of Outcome 2, Change in Terminal Hair Count (per cm2) After 24 Weeks of Therapy for Male Androgenetic Alopecia
eTable 3. Quality of Evidence Assessment for the Network of Outcome 3, Change in Total Hair Count (per cm2) After 48 Weeks of Therapy for Male Androgenetic Alopecia
eTable 4. Quality of Evidence Assessment for the Network of Outcome 4, Change in Terminal Hair Count (per cm2) After 48 Weeks of Therapy for Male Androgenetic Alopecia
eTable 5. Demographics of Patients From Studies Included in Quantitative Analyses
eFigure 5. League Table for Our First Endpoint (i.e., ‘Outcome 1’)
eFigure 6. League Table for Our Second Endpoint (i.e., ‘Outcome 2’)
eFigure 7. League Table for Our Third Endpoint (i.e., ‘Outcome 3’)
eFigure 8. League Table for Our Fourth Endpoint (i.e., ‘Outcome 4’)
eFigure 9. Presentation of Treatments’ Surface Under the Cumulative Ranking Curve (SUCRA) Via A Modified Kilim Plot for Data From Only Randomized Trials and Both Randomized and Observational Studies.
eFigure 10. League Table for Our First Endpoint (i.e., ‘Outcome 1’) Based on Solely on Data From Randomized Studies
eFigure 11. League Table for Our Second Endpoint (i.e., ‘Outcome 2’) Based on Solely on Data From Randomized Studies
eFigure 12. Presentation of Treatments’ Surface Under the Cumulative Ranking Curve (SUCRA) Via A Modified Kilim Plot for ‘Outcome 1’ in Patients With: (1) any Level of Severity, (2) Mild Form of the Condition, (3) Moderate Form of the Condition, and (4) Severe Form of the Condition
eFigure 13. Presentation of Treatments’ Surface Under the Cumulative Ranking Curve (SUCRA) Via A Modified Kilim Plot for ‘Outcome 2’ in Patients With: (1) any Level of Severity, (2) Mild Form of the Condition, (3) Moderate Form of the Condition, and (4) Severe Form of the Condition
eFigure 14. Presentation of Treatments’ Surface Under the Cumulative Ranking Curve (SUCRA) Via A Modified Kilim Plot for ‘Outcome 3’ in Patients With: (1) any Level of Severity, (2) Mild Form of the Condition, (3) Moderate Form of the Condition, and (4) Severe Form of the Condition
eFigure 15. Presentation of Treatments’ Surface Under the Cumulative Ranking Curve (SUCRA) Via A Modified Kilim Plot for ‘Outcome 4’ in Patients With: (1) any Level of Severity, (2) Mild Form of the Condition, (3) Moderate Form of the Condition, and (4) Severe Form of the Condition
eFigure 16. League Table for Our First Endpoint (i.e., ‘Outcome 1’)(for Androgenetic Alopecia That Is MILD)
eFigure 17. League Table for Our First Endpoint (i.e., ‘Outcome 1’) (for Androgenetic Alopecia That Is MODERATE)
eFigure 18. League Table for Our First Endpoint (i.e., ‘Outcome 1’) (for Androgenetic Alopecia That Is SEVERE)
eFigure 19. League Table for Our Second Endpoint (i.e., ‘Outcome 2’) (for Androgenetic Alopecia That Is MILD)
eFigure 20. League Table for Our Second Endpoint (i.e., ‘Outcome 2’) (for Androgenetic Alopecia That Is MODERATE)
eFigure 21. League Table for Our Second Endpoint (i.e., ‘Outcome 2’) (for Androgenetic Alopecia That Is SEVERE)
eFigure 22. League Table for Our Third Endpoint (i.e., ‘Outcome 3’) (for Androgenetic Alopecia That Is MILD)
eFigure 23. League Table for Our Third Endpoint (i.e., ‘Outcome 3’) (for Androgenetic Alopecia That Is MODERATE)
eFigure 24. League Table for Our Third Endpoint (i.e., ‘Outcome 3’) (for Androgenetic Alopecia That Is SEVERE)
eFigure 25. League Table for Our Fourth Endpoint (i.e., ‘Outcome 4’) (for Androgenetic Alopecia That Is MILD)
eFigure 26. League Table for Our Fourth Endpoint (i.e., ‘Outcome 4’) (for Androgenetic Alopecia That Is MODERATE)
eFigure 27. League Table for Our Fourth Endpoint (i.e., ‘Outcome 4’) (for Androgenetic Alopecia That Is SEVERE)
eReferences
References
- 1.Chanprapaph K, Sutharaphan T, Suchonwanit P. Scalp biophysical characteristics in males with androgenetic alopecia: a comparative study with healthy controls. Clin Interv Aging. 2021;16:781-787. doi: 10.2147/CIA.S310178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoedemaker C, van Egmond S, Sinclair R. Treatment of female pattern hair loss with a combination of spironolactone and minoxidil. Australas J Dermatol. 2007;48(1):43-45. doi: 10.1111/j.1440-0960.2007.00332.x [DOI] [PubMed] [Google Scholar]
- 3.Sadick N, Arruda S. Understanding causes of hair loss in women. Dermatol Clin. 2021;39(3):371-374. doi: 10.1016/j.det.2021.03.002 [DOI] [PubMed] [Google Scholar]
- 4.Dhurat R, Sharma A, Rudnicka L, et al. 5-Alpha reductase inhibitors in androgenetic alopecia: Shifting paradigms, current concepts, comparative efficacy, and safety. Dermatol Ther. 2020;33(3):e13379. doi: 10.1111/dth.13379 [DOI] [PubMed] [Google Scholar]
- 5.Villani A, Fabbrocini G, Ocampo-Candiani J, Ruggiero A, Ocampo-Garza SS. Review of oral minoxidil as treatment of hair disorders: in search of the perfect dose. J Eur Acad Dermatol Venereol. 2021;35(7):1485-1492. doi: 10.1111/jdv.17216 [DOI] [PubMed] [Google Scholar]
- 6.Blumeyer A, Tosti A, Messenger A, et al. ; European Dermatology Forum (EDF) . Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men. J Dtsch Dermatol Ges. 2011;9(suppl 6):S1-S57. doi: 10.1111/j.1610-0379.2011.07802.x [DOI] [PubMed] [Google Scholar]
- 7.Kanti V, Messenger A, Dobos G, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men - short version. J Eur Acad Dermatol Venereol. 2018;32(1):11-22. doi: 10.1111/jdv.14624 [DOI] [PubMed] [Google Scholar]
- 8.Gomolin A, Litvinov IV, Netchiporouk E. Oral minoxidil: a possible new therapy for androgenetic alopecia. J Cutan Med Surg. 2020;24(1):88-89. doi: 10.1177/1203475419879887 [DOI] [PubMed] [Google Scholar]
- 9.Gupta AK, Talukder M, Venkataraman M, Bamimore MA. Minoxidil: a comprehensive review. J Dermatolog Treat. 2021;•••:1-11. [DOI] [PubMed] [Google Scholar]
- 10.Gupta A, Venkataraman M, Talukder M, Bamimore M. Relative efficacy of minoxidil and 5-alpha-reductase inhibitors in the treatment of male androgenetic alopecia: protocol for a network meta-analysis study. Inplasy protocol 202150083. Accessed December 21, 2021. https://inplasy.com/inplasy-2021-5-0083/
- 11.Van Neste D. Placebo-controlled dose-effect studies with topical minoxidil 2% or 5% in male-patterned hair loss treated with oral finasteride employing an analytical and exhaustive study protocol. Skin Res Technol. 2020;26(4):542-557. doi: 10.1111/srt.12827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hugh Rushton D, Norris MJ, Van Neste D. Hair regrowth in male and female pattern hair loss does not involve the conversion of vellus hair to terminal hair. Exp Dermatol. 2016;25(6):482-484. doi: 10.1111/exd.12945 [DOI] [PubMed] [Google Scholar]
- 13.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(135):135. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Cochrane Collaboration . Obtaining Standard Deviations from Standard Errors and Confidence Intervals for Group Means. In: Higgins J, Green S, eds. The Cochrane Handbook. Cochrane Collaboration; 2011. Accessed December 21, 2021. https://handbook-5-1.cochrane.org/chapter_7/7_7_3_2_obtaining_standard_deviations_from_standard_errors_and.htm
- 15.Leahy J, Thom H, Jansen JP, et al. Incorporating single-arm evidence into a network meta-analysis using aggregate level matching: Assessing the impact. Stat Med. 2019;38(14):2505-2523. doi: 10.1002/sim.8139 [DOI] [PubMed] [Google Scholar]
- 16.Cameron C, Fireman B, Hutton B, et al. Network meta-analysis incorporating randomized controlled trials and non-randomized comparative cohort studies for assessing the safety and effectiveness of medical treatments: challenges and opportunities. Syst Rev. 2015;4:147. doi: 10.1186/s13643-015-0133-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 18.Borenstein M, Hedges L, Higgins J, Rothstein H. Introduction to Meta-Analysis. Wiley; 2009. doi: 10.1002/9780470743386 [DOI] [Google Scholar]
- 19.Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikolakopoulou A, Higgins JPT, Papakonstantinou T, et al. CINeMA: An approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020;17(4):e1003082. doi: 10.1371/journal.pmed.1003082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papakonstantinou T, Nikolakopoulou A, Higgins JPT, Egger M, Salanti G. CINeMA: software for semiautomated assessment of the confidence in the results of network meta-analysis. Campbell Syst Rev. 2020;16(1):e1080. doi: 10.1002/cl2.1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seo M, Furukawa TA, Veroniki AA, et al. The Kilim plot: A tool for visualizing network meta-analysis results for multiple outcomes. Res Synth Methods. 2021;12(1):86-95. doi: 10.1002/jrsm.1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.RStudio Team . RStudio: Integrated Development Environment for R. Published online 2020. Accessed December 21, 2021. https://www.rstudio.com/
- 24.Anderson CD. Topical minoxidil in androgenetic alopecia Scandinavian and Middle East experience. Int J Dermatol. 1988;27:447-451. doi: 10.1111/j.1365-4362.1988.tb00006.x [DOI] [Google Scholar]
- 25.Civatte J, Laux B, Simpson NB, Vickers CFH. 2% topical minoxidil solution in male-pattern baldness: preliminary European results. Dermatologica. 1987;175(suppl 2):42-49. doi: 10.1159/000248902 [DOI] [PubMed] [Google Scholar]
- 26.Olsen EA, Weiner MS, Delong ER, Pinnell SR. Topical minoxidil in early male pattern baldness. J Am Acad Dermatol. 1985;13(2 Pt 1):185-192. doi: 10.1016/S0190-9622(85)70157-0 [DOI] [PubMed] [Google Scholar]
- 27.Olsen EA, DeLong ER, Weiner MS. Dose-response study of topical minoxidil in male pattern baldness. J Am Acad Dermatol. 1986;15(1):30-37. doi: 10.1016/S0190-9622(86)70138-2 [DOI] [PubMed] [Google Scholar]
- 28.Olsen EA, Dunlap FE, Funicella T, et al. A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. J Am Acad Dermatol. 2002;47(3):377-385. doi: 10.1067/mjd.2002.124088 [DOI] [PubMed] [Google Scholar]
- 29.Petzoldt D. The German double-blind placebo-controlled evaluation of topical minoxidil solution in the treatment of early male pattern baldness. Int J Dermatol. 1988;27:430-434. doi: 10.1111/j.1365-4362.1988.tb00003.x [DOI] [Google Scholar]
- 30.Roberts JL, Fiedler V, Imperato-McGinley J, et al. Clinical dose ranging studies with finasteride, a type 2 5α-reductase inhibitor, in men with male pattern hair loss. J Am Acad Dermatol. 1999;41(4):555-563. [PubMed] [Google Scholar]
- 31.Rushton DH, Unger WP, Cotterill PC, Kingsley P, James KC. Quantitative assessment of 2% topical minoxidil in the treatment of male pattern baldness. Clin Exp Dermatol. 1989;14(1):40-46. doi: 10.1111/j.1365-2230.1989.tb00881.x [DOI] [PubMed] [Google Scholar]
- 32.Saraswat A, Kumar B. Minoxidil vs finasteride in the treatment of men with androgenetic alopecia. Arch Dermatol. 2003;139(9):1219-1221. doi: 10.1001/archderm.139.9.1219-b [DOI] [PubMed] [Google Scholar]
- 33.Shanshanwal SJS, Dhurat RS. Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: A randomized controlled open-label, evaluator-blinded study. Indian J Dermatol Venereol Leprol. 2017;83(1):47-54. doi: 10.4103/0378-6323.188652 [DOI] [PubMed] [Google Scholar]
- 34.Shupack JL, Kassimir JJ, Thirumoorthy T, Reed ML, Jondreau L. Dose-response study of topical minoxidil in male pattern alopecia. J Am Acad Dermatol. 1987;16(3 Pt 2):673-676. doi: 10.1016/S0190-9622(87)70086-3 [DOI] [PubMed] [Google Scholar]
- 35.Suchonwanit P, Srisuwanwattana P, Chalermroj N, Khunkhet S. A randomized, double-blind controlled study of the efficacy and safety of topical solution of 0.25% finasteride admixed with 3% minoxidil vs. 3% minoxidil solution in the treatment of male androgenetic alopecia. J Eur Acad Dermatol Venereol. 2018;32(12):2257-2263. doi: 10.1111/jdv.15171 [DOI] [PubMed] [Google Scholar]
- 36.Dutrée-Meulenberg ROGM, Nieboer C, Koedijk FHJ, Stolz E. Treatment of male pattern alopecia using topical minoxidil in the Netherlands. Int J Dermatol. 1988;27(suppl 6):435-440. doi: 10.1111/j.1365-4362.1988.tb00004.x [DOI] [Google Scholar]
- 37.Tsuboi R, Arano O, Nishikawa T, Yamada H, Katsuoka K. Randomized clinical trial comparing 5% and 1% topical minoxidil for the treatment of androgenetic alopecia in Japanese men. J Dermatol. 2009;36(8):437-446. doi: 10.1111/j.1346-8138.2009.00673.x [DOI] [PubMed] [Google Scholar]
- 38.Van Neste D, Fuh V, Sanchez-Pedreno P, et al. Finasteride increases anagen hair in men with androgenetic alopecia. Br J Dermatol. 2000;143(4):804-810. doi: 10.1046/j.1365-2133.2000.03780.x [DOI] [PubMed] [Google Scholar]
- 39.Pirmez R, Salas-Callo C-I. Very-low-dose oral minoxidil in male androgenetic alopecia: A study with quantitative trichoscopic documentation. J Am Acad Dermatol. 2020;82(1):e21-e22. doi: 10.1016/j.jaad.2019.08.084 [DOI] [PubMed] [Google Scholar]
- 40.Panchaprateep R, Lueangarun S. Efficacy and safety of oral minoxidil 5 mg once daily in the treatment of male patients with androgenetic alopecia: an open-label and global photographic assessment. Dermatol Ther (Heidelb). 2020;10(6):1345-1357. doi: 10.1007/s13555-020-00448-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eun HC, Kwon OS, Yeon JH, et al. Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male pattern hair loss: a randomized, double-blind, placebo-controlled, phase III study. J Am Acad Dermatol. 2010;63(2):252-258. doi: 10.1016/j.jaad.2009.09.018 [DOI] [PubMed] [Google Scholar]
- 42.Hajheydari Z, Akbari J, Saeedi M, Shokoohi L. Comparing the therapeutic effects of finasteride gel and tablet in treatment of the androgenetic alopecia. Indian J Dermatol Venereol Leprol. 2009;75(1):47-51. doi: 10.4103/0378-6323.45220 [DOI] [PubMed] [Google Scholar]
- 43.Gubelin Harcha W, Barboza Martínez J, Tsai T-F, et al. A randomized, active- and placebo-controlled study of the efficacy and safety of different doses of dutasteride versus placebo and finasteride in the treatment of male subjects with androgenetic alopecia. J Am Acad Dermatol. 2014;70(3):489-498.e3. doi: 10.1016/j.jaad.2013.10.049 [DOI] [PubMed] [Google Scholar]
- 44.Hillmann K, Garcia Bartels N, Kottner J, Stroux A, Canfield D, Blume-Peytavi U. A single-centre, randomized, double-blind, placebo-controlled clinical trial to investigate the efficacy and safety of minoxidil topical foam in frontotemporal and vertex androgenetic alopecia in men. Skin Pharmacol Physiol. 2015;28(5):236-244. doi: 10.1159/000375320 [DOI] [PubMed] [Google Scholar]
- 45.Kaufman KD, Olsen EA, Whiting D, et al. ; Finasteride Male Pattern Hair Loss Study Group . Finasteride in the treatment of men with androgenetic alopecia. J Am Acad Dermatol. 1998;39(4 Pt 1):578-589. doi: 10.1016/S0190-9622(98)70007-6 [DOI] [PubMed] [Google Scholar]
- 46.Koperski JA, Orenberg EK, Wilkinson DI. Topical minoxidil therapy for androgenetic alopecia. A 30-month study. Arch Dermatol. 1987;123(11):1483-1487. doi: 10.1001/archderm.1987.01660350083018 [DOI] [PubMed] [Google Scholar]
- 47.Leyden J, Dunlap F, Miller B, et al. Finasteride in the treatment of men with frontal male pattern hair loss. J Am Acad Dermatol. 1999;40(6 Pt 1):930-937. doi: 10.1016/S0190-9622(99)70081-2 [DOI] [PubMed] [Google Scholar]
- 48.Manabe M, Tsuboi R, Itami S, et al. ; Drafting Committee for the Guidelines for the Diagnosis and Treatment of Male- and Female-Pattern Hair Loss . Guidelines for the diagnosis and treatment of male-pattern and female-pattern hair loss, 2017 version. J Dermatol. 2018;45(9):1031-1043. doi: 10.1111/1346-8138.14470 [DOI] [PubMed] [Google Scholar]
- 49.Alessandrini A, Starace M, D’''''Ovidio R, et al. Androgenetic alopecia in women and men: Italian guidelines adapted from European Dermatology Forum/European Academy of Dermatology and Venereology guidelines. G Ital Dermatol Venereol. 2020;155(5):622-631. doi: 10.23736/S0392-0488.19.06399-5 [DOI] [PubMed] [Google Scholar]
- 50.Pathomvanich D, Imagawa K. Hair Restoration Surgery in Asians. Springer; 2010. doi: 10.1007/978-4-431-99659-0 [DOI] [Google Scholar]
- 51.Hirshburg JM, Kelsey PA, Therrien CA, Gavino AC, Reichenberg JS. Adverse effects and safety of 5-alpha reductase inhibitors (finasteride, dutasteride): a systematic review. J Clin Aesthet Dermatol. 2016;9(7):56-62. [PMC free article] [PubMed] [Google Scholar]
- 52.Miller J, Tarter TH. Update on the use of dutasteride in the management of benign prostatic hypertrophy. Clin Interv Aging. 2007;2(1):99-104. doi: 10.2147/ciia.2007.2.1.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Disorders of micturition. In: Waller DG, Sampson A, eds. Medical Pharmacology and Therapeutics E-Book. 5th ed. Elsevier; 2018:219-229. [Google Scholar]
- 54.Katzung BG, ed. Basic & Clinical Pharmacology. 14th ed. McGraw Hill; 2018. [Google Scholar]
- 55.Greco T, Zangrillo A, Biondi-Zoccai G, Landoni G. Meta-analysis: pitfalls and hints. Heart Lung Vessel. 2013;5(4):219-225. [PMC free article] [PubMed] [Google Scholar]
- 56.Leerunyakul K, Suchonwanit P. Evaluation of hair density and hair diameter in the adult Thai population using quantitative trichoscopic analysis. Biomed Res Int. 2020;2020:2476890. doi: 10.1155/2020/2476890 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Terminal and Vellus Hair (Basic Description)
eAppendix 2. Estimation of Surface Under the Cumulative Ranking Curve (SUCRA)
eFigure 1. Network Plot for Outcome 1, Change in Total Hair Count (per cm2) After 24 Weeks of Therapy
eFigure 2. Network Plot for Outcome 2, Change in Terminal Hair Count (per cm2) After 24 Weeks of Therapy
eFigure 3. Network Plot for Outcome 3, Change in Total Hair Count (per cm2) After 48 Weeks of Therapy
eFigure 4. Network Plot for Outcome 4, Change in Terminal Hair Count (per cm2) After 48 Weeks of Therapy
eTable 1. Quality of Evidence Assessment for the Network of Outcome 1, Change in Total Hair Count (per cm2) After 24 Weeks of Therapy for Male Pattern Hair Loss
eTable 2. Quality of Evidence Assessment for the Network of Outcome 2, Change in Terminal Hair Count (per cm2) After 24 Weeks of Therapy for Male Androgenetic Alopecia
eTable 3. Quality of Evidence Assessment for the Network of Outcome 3, Change in Total Hair Count (per cm2) After 48 Weeks of Therapy for Male Androgenetic Alopecia
eTable 4. Quality of Evidence Assessment for the Network of Outcome 4, Change in Terminal Hair Count (per cm2) After 48 Weeks of Therapy for Male Androgenetic Alopecia
eTable 5. Demographics of Patients From Studies Included in Quantitative Analyses
eFigure 5. League Table for Our First Endpoint (i.e., ‘Outcome 1’)
eFigure 6. League Table for Our Second Endpoint (i.e., ‘Outcome 2’)
eFigure 7. League Table for Our Third Endpoint (i.e., ‘Outcome 3’)
eFigure 8. League Table for Our Fourth Endpoint (i.e., ‘Outcome 4’)
eFigure 9. Presentation of Treatments’ Surface Under the Cumulative Ranking Curve (SUCRA) Via A Modified Kilim Plot for Data From Only Randomized Trials and Both Randomized and Observational Studies.
eFigure 10. League Table for Our First Endpoint (i.e., ‘Outcome 1’) Based on Solely on Data From Randomized Studies
eFigure 11. League Table for Our Second Endpoint (i.e., ‘Outcome 2’) Based on Solely on Data From Randomized Studies
eFigure 12. Presentation of Treatments’ Surface Under the Cumulative Ranking Curve (SUCRA) Via A Modified Kilim Plot for ‘Outcome 1’ in Patients With: (1) any Level of Severity, (2) Mild Form of the Condition, (3) Moderate Form of the Condition, and (4) Severe Form of the Condition
eFigure 13. Presentation of Treatments’ Surface Under the Cumulative Ranking Curve (SUCRA) Via A Modified Kilim Plot for ‘Outcome 2’ in Patients With: (1) any Level of Severity, (2) Mild Form of the Condition, (3) Moderate Form of the Condition, and (4) Severe Form of the Condition
eFigure 14. Presentation of Treatments’ Surface Under the Cumulative Ranking Curve (SUCRA) Via A Modified Kilim Plot for ‘Outcome 3’ in Patients With: (1) any Level of Severity, (2) Mild Form of the Condition, (3) Moderate Form of the Condition, and (4) Severe Form of the Condition
eFigure 15. Presentation of Treatments’ Surface Under the Cumulative Ranking Curve (SUCRA) Via A Modified Kilim Plot for ‘Outcome 4’ in Patients With: (1) any Level of Severity, (2) Mild Form of the Condition, (3) Moderate Form of the Condition, and (4) Severe Form of the Condition
eFigure 16. League Table for Our First Endpoint (i.e., ‘Outcome 1’)(for Androgenetic Alopecia That Is MILD)
eFigure 17. League Table for Our First Endpoint (i.e., ‘Outcome 1’) (for Androgenetic Alopecia That Is MODERATE)
eFigure 18. League Table for Our First Endpoint (i.e., ‘Outcome 1’) (for Androgenetic Alopecia That Is SEVERE)
eFigure 19. League Table for Our Second Endpoint (i.e., ‘Outcome 2’) (for Androgenetic Alopecia That Is MILD)
eFigure 20. League Table for Our Second Endpoint (i.e., ‘Outcome 2’) (for Androgenetic Alopecia That Is MODERATE)
eFigure 21. League Table for Our Second Endpoint (i.e., ‘Outcome 2’) (for Androgenetic Alopecia That Is SEVERE)
eFigure 22. League Table for Our Third Endpoint (i.e., ‘Outcome 3’) (for Androgenetic Alopecia That Is MILD)
eFigure 23. League Table for Our Third Endpoint (i.e., ‘Outcome 3’) (for Androgenetic Alopecia That Is MODERATE)
eFigure 24. League Table for Our Third Endpoint (i.e., ‘Outcome 3’) (for Androgenetic Alopecia That Is SEVERE)
eFigure 25. League Table for Our Fourth Endpoint (i.e., ‘Outcome 4’) (for Androgenetic Alopecia That Is MILD)
eFigure 26. League Table for Our Fourth Endpoint (i.e., ‘Outcome 4’) (for Androgenetic Alopecia That Is MODERATE)
eFigure 27. League Table for Our Fourth Endpoint (i.e., ‘Outcome 4’) (for Androgenetic Alopecia That Is SEVERE)
eReferences