Abstract
Background
Cocaine dependence is a major public health problem that is characterised by recidivism and a host of medical and psychosocial complications. Although effective pharmacotherapy is available for alcohol and heroin dependence, none is currently available for cocaine dependence, despite two decades of clinical trials primarily involving antidepressant, anticonvulsivant and dopaminergic medications. Extensive consideration has been given to optimal pharmacological approaches to the treatment of individuals with cocaine dependence, and both dopamine antagonists and agonists have been considered. Anticonvulsants have been candidates for use in the treatment of addiction based on the hypothesis that seizure kindling‐like mechanisms contribute to addiction.
Objectives
To evaluate the efficacy and safety of anticonvulsants for individuals with cocaine dependence.
Search methods
We searched the Cochrane Drugs and Alcohol Group Trials Register (June 2014), the Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 6), MEDLINE (1966 to June 2014), EMBASE (1988 to June 2014), the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982 to June 2014), Web of Science (1991 to June 2014) and the reference lists of eligible articles.
Selection criteria
All randomised controlled trials and controlled clinical trials that focus on the use of anticonvulsant medications to treat individuals with cocaine dependence.
Data collection and analysis
We used the standard methodological procedures expected by The Cochrane Collaboration.
Main results
We included a total of 20 studies with 2068 participants. We studied the anticonvulsant drugs carbamazepine, gabapentin, lamotrigine, phenytoin, tiagabine, topiramate and vigabatrin. All studies compared anticonvulsants versus placebo. Only one study had one arm by which the anticonvulsant was compared with the antidepressant desipramine. Upon comparison of anticonvulsant versus placebo, we found no significant differences for any of the efficacy and safety measures. Dropouts: risk ratio (RR) 0.95, 95% confidence interval (CI) 0.86 to 1.05, 17 studies, 20 arms, 1695 participants, moderate quality of evidence. Use of cocaine: RR 0.92, 95% CI 0.84 to 1.02, nine studies, 11 arms, 867 participants, moderate quality of evidence; side effects: RR 1.39, 95% CI 1.01 to 1.90, eight studies, 775 participants; craving: standardised mean difference (SMD) ‐0.25, 95% CI ‐0.59 to 0.09, seven studies, eight arms, 428 participants, low quality of evidence.
Authors' conclusions
Although caution is needed when results from a limited number of small clinical trials are assessed, no current evidence supports the clinical use of anticonvulsant medications in the treatment of patients with cocaine dependence. Although the findings of new trials will improve the quality of study results, especially in relation to specific medications, anticonvulsants as a category cannot be considered first‐, second‐ or third‐line treatment for cocaine dependence.
Keywords: Humans, Anticonvulsants, Anticonvulsants/therapeutic use, Cocaine‐Related Disorders, Cocaine‐Related Disorders/drug therapy, Randomized Controlled Trials as Topic
Plain language summary
Anticonvulsants for cocaine dependence
Background
Cocaine is an illicit drug available as a powder for intranasal or intravenous use or smoked as crack. Short‐ and long‐term use of this drug results in the spread of infectious diseases (for example, AIDS, hepatitis, tuberculosis), crime, violence and prenatal drug exposure. Cocaine dependence is associated with medical and psychosocial complications and is a major public health problem. No proven pharmacological treatment for cocaine dependence is known. Antidepressant, anticonvulsant and dopaminergic medications have all been studied. The present review looked at the efficacy and safety of anticonvulsant drugs for treating cocaine dependence, both as a class and individually.
Study characteristics
The review authors searched scientific databases and Internet resources to identify randomised controlled trials (in which participants were allocated at random to any anticonvulsant drug or placebo or another type of drug or non‐pharmacological intervention intended to reduce,the use of cocaine). We assessed also dropout from treatment and frequency of side effects .We included people of any gender, age or ethnicity.
Key results
The review authors identified 20 studies with 2068 participants, 77% male, with a mean age of 36 years. The mean duration of the trials was 11.8 weeks (range eight to 24 weeks). All but two of the trials were conducted in the USA, all with outpatients. The anticonvulsant drugs studied were carbamazepine, gabapentin, lamotrigine, phenytoin, tiagabine, topiramate and vigabatrin. All studies compared anticonvulsants versus placebo. No significant differences were found between placebo and any anticonvulsant in reducing the number of dropouts from treatment, use of cocaine, craving and severity of dependence, depression or anxiety. Side effects were slightly more frequent in the anticonvulsant groups. No current evidence supports the clinical use of anticonvulsant medications for the treatment of cocaine dependence.
Quality of the evidence
The quality of the evidence was moderate for the outcomes dropout and use of cocaine, and was low for the outcomes side effects and craving. The major limitation of the trials was incomplete reporting of the methods used to protect against selection bias, randomly allocate participants to groups and conceal allocation. The evidence is current to June 2014.
Summary of findings
Summary of findings for the main comparison. Any anticonvulsant versus placebo for cocaine dependence.
| Any anticonvulsant versus placebo for cocaine dependence | ||||||
| Patient or population: patients with cocaine dependence Settings: outpatients Intervention: any anticonvulsant versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Any anticonvulsant versus placebo | |||||
| Dropout Number of participants who did not complete the treatment Follow‐up: mean 11.8 weeks1 | 45 per 100 | 42 per 100 (38 to 47) | RR 0.95 (0.86 to 1.05) | 1695 (17 studies2) | ⊕⊕⊕⊝ Moderate3,4 | |
| Use of cocaine (self reported or objective) Number of participants who reported the use of cocaine during treatment, and/or number of participants with urine samples positive for cocaine Follow‐up: mean 11.8 weeks1 | 77 per 100 | 71 per 100 (65 to 79) | RR 0.92 (0.84 to 1.02) | 867 (9 studies5) | ⊕⊕⊕⊝ Moderate6,7 | |
| Side effect Number of participants reporting at least 1 side effect and types of side effects experienced during treatment Follow‐up: mean 11.8 weeks1 | 46 per 100 | 65 per 100 (47 to 88) | RR 1.39 (1.01 to 1.9) | 775 (8 studies) | ⊕⊕⊝⊝ Low8,9 | |
| Craving (BSCS) Measured by validated scales (e.g. Brief Substance Craving Scale (BSCS)) Follow‐up: mean 11.8 weeks1 | The mean craving (bscs) in the intervention groups was 0.25 standard deviations lower (0.59 lower to 0.09 higher) | 428 (7 studies11) | ⊕⊕⊝⊝ Low11,12,13 | |||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE (Grades of Recommendation, Assessment, Development and Evaluation) Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Range 8 ‐ 24 weeks 2 20 treatment arms 3 In the Cornish 1995, Halikas 1997 arm a, Halikas 1997 arm b;Nuijten 2014, Umbricht 2014 studies an adequate sequence generation method was described and judged at low risk of selection bias. In the other 17 studies the method was not reported (unclear risk of bias). In five studies (Brodie 2009, Cornish 1995, Gonzalez 2007 arm a, Gonzalez 2007 arm b, Kranzler 1995, Umbricht 2014) an adequate method for allocation concealment was judged at low risk of selection bias. In all the other studies the method for allocation concealment was not reported (unclear risk of bias). Campbell 1994 arm was judged at high risk of selective reporting bias because results for drop out were not reported. 4 All the seventeen included studies were conducted in the USA 5 11 treatment arm 6 In the Cornish 1995, Halikas 1997 arm a, Halikas 1997 arm b;Nuijten 2014 studies an adequate sequence generation method was described and judged at low risk of selection bias. In the other studies the method was not reported (unclear risk of bias). In three studies (Brodie 2009, Cornish 1995, Gonzalez 2007 arm a, Gonzalez 2007 arm b) an adequate method for allocation concealment was judged at low risk of selection bias. In all the other studies the method for allocation concealment was not reported (unclear risk of bias). Nuijten 2014 were judged at high risk of performance bias and at unclear risk of detection bias All the other studies were judged at low risk of performance and detection bias. Cornish 1995, Halikas 1997 arm a, Halikas 1997 arm b, Kranzler 1995, Nuijten 2014 were judged at high risk of attrition bias. 7 I‐squared 30% 8 In the Brown 2012, Cornish 1995 and Nuijten 2014 studies an adequate sequence generation method was described and judged at low risk of selection bias. In the other studies the method was not reported (unclear risk of bias).One study was judged at high of bias ( Brown 2012) for allocation concealment. All the studies were judged at low risk of performance and detection bias. Brown 2012, Cornish 1995, Crosby 1996, Kranzler 1995, Nuijten 2014) were judged at high risk of attrition bias. All the other studies performed the analysis on the intention to treat basis or did not have withdrawn from the study. 9 I‐squared 81% 10 Eight treatment arms 11In the Nuijten 2014 studies an adequate sequence generation method was described and judged at low risk of selection bias. In the other studies the method was not reported (unclear risk of bias). In all the studies the method for allocation concealment was not reported (unclear risk of bias). Berger 2005 arm a, Berger 2005 arm b and Nuijten 2014 were judged at high risk of performance bias and at unclear risk of detection bias. Winhusen 2005 was judged at high risk both for performance and detection bias. Crosby 1996 and Nuijten 2014 were judged at high risk of attrition bias. 12I‐squared 63% 13 All the seven included studies were conducted in the USA
Background
Description of the condition
Cocaine is an alkaloid derived from the leaf of coca, which is commonly available as powder for intranasal or intravenous use, or as crack, a free‐base form that is smoked. Cocaine is a powerful stimulant that when abused typically quickly leads to dependence. Cocaine dependence is characterised by continued use of cocaine despite significant substance‐related problems.
Cocaine dependence is a major public health problem that is characterised by recidivism and a host of medical and psychosocial complications (EMCDDA 2006).
Among regular users, a broad distinction can be made between socially integrated consumers, who may be using the drug in a recreational context, and more marginalised drug users, who use cocaine, along with opioids, as part of a chronic drug problem. Regular cocaine use has been associated with cardiovascular, neurological and mental health problems, and with elevated risk of accident and dependence. Cocaine injection and use of crack cocaine are associated with the highest health risks, including transmission of infectious diseases (EMCDDA 2014).
In addition to these serious implications, cocaine use has been found to have direct negative cognitive effects on the brain, affecting tasks related to inhibition, memory, concentration, problem solving, learning, planning, attention and discrimination (Harvey 2004).
Cocaine is also implicated in acute hospital admissions, suicides and deaths (Degenhardt 2012).
Cocaine is the most commonly used illicit stimulant drug in Europe, although most users are found in only a few countries. It is estimated that about 2.2 million young adults 15 to 34 years of age (1.7% of this age group) used cocaine in the past year (EMCDDA 2014). Illicit use of cocaine is still a persistent health problem worldwide. According to recent national population surveys, between 0.4% and 9% of the adult population report that they have tried cocaine at least once (i.e. lifetime prevalence), with Italy (4.2%), Spain (8.8%) and the United Kingdom (9.0%) at the upper end of this range. In general, recent cocaine use (past 12 months) is reported by less than 2% of adults (range, 0.2% to 3.6%). In Spain and the United Kingdom, recent prevalence rates are higher than 3% (EMCDDA 2014).
The 2011 National Survey on Drug Use and Health found that the number of US citizens 12 years of age or older who are current users of cocaine has dropped by 44% since 2006. The US government survey on cocaine use found that in 2011, an estimated 1.4 million US citizens used cocaine ‐ down from 2.4 million in 2006. The number of people who first tried cocaine over the previous year decreased from one million in 2002 to 670,000 in 2011. In addition, the number of people who abused or were dependent on cocaine dropped from 1.7 million in 2006 to 0.8 million in 2011.
The number of people who tested positive for cocaine in the workplace dropped by 65% from 2006 to 2012, and a 44% decrease in cocaine‐related overdose deaths was reported from 2006 to 2010 (NSDUH 2011).
Although cocaine use in many South American countries has decreased or remained stable, a substantial increase in Brazil is obvious enough to be reflected in the regional prevalence rate for 2011. Cocaine use in Australia increased over the four years leading up to 2012 (UNODC 2013).
In 2012, a decrease in cocaine use among addicts seeking treatment was observed , after a peak in 2008, in Denmark, Spain and the United Kingdom, all countries reporting relatively high prevalence rates (EMCDDA 2014).
Description of the intervention
It has been estimated that at least 1.3 million people received treatment for illicit drug use in Europe during 2012.
Most treatment is provided in outpatient settings such as specialised centres, general healthcare centres such as general practitioners’ offices and low‐threshold facilities (EMCDDA 2014).
Cocaine was cited as the primary drug among 14% of all reported individuals entering specialised drug treatment in 2012 (55,000) and in 18% of those entering treatment for the first time (26,000). Differences between countries have been noted, with around 90% of all cocaine users reported by only five countries (Germany, Spain, Italy, Netherlands and United Kingdom). Together, these five countries account for just over half of the EU population (EMCDDA 2014).
Currently, no medications have been approved by the Food and Drug Administration (FDA) for the treatment of cocaine dependence (Pani 2010).
No effective pharmacotherapy is currently available for cocaine dependence despite two decades of clinical trials involving primarily antidepressant, antipsychotic, anticonvulsant and dopaminergic medications.
Recent controlled clinical studies have highlighted some promising medications, especially glutamatergic (N‐acetylcysteine, modafinil, topiramate) and GABAergic (vigabatrin) agents, agonist replacement therapy (sustained‐release methylphenidate, d‐amphetamine) and indirect dopaminergic agents (disulfiram). Additionally, immunotherapy is a newly investigated approach (Karila 2011).
Several Cochrane systematic reviews have been published on the efficacy of antidepressants (Pani 2011), dopamine agonists (Amato 2011), psychostimulants (Castells 2010), disulfiram (Pani 2010) and antipsychotics (Amato 2007) for the treatment of cocaine dependence, but none of these provided support for the efficacy of these treatments. One published review on the efficacy of psychosocial treatment for psychostimulant dependence (Knapp 2007) showed that existing treatments have yielded modest outcomes at best, leading to the conclusion that different formats of existing treatment models should be developed and tested and new psychosocial interventions should be undertaken.
A recent study found that topiramate was more efficacious than placebo in increasing the mean weekly proportion of cocaine non‐use days and associated measures of clinical improvement among cocaine‐dependent individuals (Johnson 2013).
Nonetheless, cocaine dependence remains a disorder for which no pharmacological treatment with proved efficacy is known, although considerable advances in the neurobiology of this addiction could guide future development of medication.
How the intervention might work
The effect of cocaine seems to rely on its ability to increase the availability of monoamines (dopamine, serotonin and noradrenaline) in the brain. The dopamine increase in specific areas of the mesolimbic system with cocaine, which is shared with other drugs such as heroin, alcohol, cannabis and nicotine, has been involved in the rewarding effects of drugs and self‐administration behaviour in animals and humans (Di Chiara 1988; Drevets 1999; Drevets 2001; Volkow 2003). Anticonvulsants have been regarded as candidates for the treatment of cocaine addiction based on the hypothesis that seizure kindling‐like mechanisms contribute to addiction (Crosby 1991; Kranzler 1995). In addiction, anticonvulsants potentiate gamma‐aminobutyric acid (GABA)‐mediated inhibitory neurotransmission (Czapinski 2005; Landmark 2007). GABA neurons are part of the mesolimbic dopamine system, and activation of GABA receptors in the ventral tegmental area is known to dampen dopamine neuronal activity in the nucleus accumbens (Koob 1997). The inhibitory capacity of GABA may be effective in blocking cocaine‐induced increases in extracellular dopamine in the nucleus accumbens, which may lead to a decrease in cocaine reinforcement and reduced cocaine self administration (Campbell 1999; Kushner 1999). Some of the anticonvulsants more commonly studied for this purpose are carbamazepine, tiagabine, gabapentin, lamotrigine, topiramate, valproate, phenobarbital, phenytoin and vigabatrin.
Why it is important to do this review
In 2008, we published a Cochrane systematic review of randomised controlled trials (RCTs) evaluating several anticonvulsant drugs (Minozzi 2008), with the aim of updating and completing the pre‐existing review on carbamazepine for the treatment of cocaine dependence (Lima Reisser 2000).
We concluded that no current evidence supports the clinical use of anticonvulsant medications in the treatment of cocaine dependence, and that larger randomised investigations analysing relevant outcomes (dropout, use of cocaine measured as number of individuals abstinent at the end of treatment) would have been necessary.
Since 2008, new RCTs on this topic have been published, and for this reason, an update of the systematic review is mandatory.
Objectives
To evaluate the efficacy and safety of anticonvulsants for individuals with cocaine dependence.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs and controlled clinical trials (CCTs) that focus on the use of anticonvulsant medication for cocaine dependence.
Types of participants
Cocaine‐dependent patients as diagnosed by the Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV‐R) or by specialists. Trials including patients with additional diagnoses of substance dependence were eligible. People younger than 18 years of age and pregnant women were excluded for the substantially different approach to clinical management that is used for these people. People with co‐morbid mental health conditions were included and were considered in the subgroup analysis.
Types of interventions
Experimental intervention
Any anticonvulsant medication alone or in combination with any psychosocial intervention.
Control interventions
Placebo.
No intervention.
Other pharmacological interventions.
Any psychosocial interventions.
When we found trials that compared different anticonvulsant medications, we performed separate subgroup analyses.
Types of outcome measures
Primary outcomes
Dropouts from treatment as the number of participants who did not complete the study protocol.
Use of primary substance of abuse as the number of participants who reported use of cocaine during treatment and/or the number of participants with urine samples positive for cocaine.
Acceptability of treatment as the number of participants reporting at least one side effect and types of side effects experienced during treatment.
Secondary outcomes
Compliance as the number of participants who were adherent to the treatment protocol, or as mean and standard deviation (SD) of pills taken.
Craving as measured by validated scales (e.g. Brief Substance Craving Scale (BSCS), visual analogue scale (VAS)).
Severity of dependence as measured by validated scales (e.g. Addiction Severity Index (ASI), Clinical Global Impression Scale (CGI‐S), Clinical Global Impression ‐ Observer Scale (CGI‐O)).
Psychiatric symptoms/psychological distress diagnosed using standard criteria (e.g. Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria, measurement by validated scales (e.g. Hamilton Depression Scale, Profile of Mood States Scale (POMSS), Positive and Negative Syndrome Scale (PANSS)).
Search methods for identification of studies
Electronic searches
For the original review (Minozzi 2008), we searched the following electronic databases from the earliest available date to March 2007.
The Cochrane Central Register of Controlled Trials (CENTRAL) (most recent).
MEDLINE (from 1966 to March 2007).
EMBASE (from 1988 to March 2007).
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982 to March 2007).
For this update, we searched the following electronic databases (search date: 23 June 2014).
Cochrane Drugs and Alcohol Group (CDAG) Specialised Register* (searched June 2014).
CENTRAL (2014, Issue 6).
MEDLINE (PubMed) (March 2007 to June 2014).
EMBASE (Elsevier, EMBASE.com) (March 2007 to June 2014).
CINAHL (EBSCO Host) (March 2007 to June 2014).
Web of Science (Thomson Reuters) (March 2007 to June 2014).
Search strategies used for all databases are shown in Appendix 1, Appendix 2, Appendix 3, Appendix 4 and Appendix 5.
In addition, we searched for ongoing clinical trials and unpublished studies via Internet searches on the following sites.
www.clinicaltrials.gov (search date: 27 June 2014).
www.who.int/ictrp/en/ (World Health Organization International Clinical Trials Registry Platform) (search date: 27 June 2014).
Searching other resources
We also searched the following.
Reference lists of all relevant papers to identify further studies.
Conference proceedings likely to include trials relevant to the review.
We contacted investigators to request information about unpublished or incomplete trials.
All searches included non‐English language literature, and we assessed studies with English abstracts for inclusion. When considered likely to meet inclusion criteria, we had studies translated.
Data collection and analysis
Selection of studies
One review author (LA) inspected the search hits by reading titles and abstracts. Two review authors (LA and SM) obtained full‐text articles for all potentially relevant studies located in the search and independently assessed them for inclusion. All review authors resolved doubts by discussion. For the present update, two review authors (MC and SM) independently inspected the search hits by reading titles and abstracts. These two review authors (MC and SM) also independently inspected full‐text versions of potentially relevant studies.
Data extraction and management
Two review authors (LA and SM) independently extracted data. For the present update, two review authors (MC and SM) independently extracted data. All review authors discussed disagreements. Key findings were summarised narratively in the first instance and were assessed for meta‐analysis when possible.
Assessment of risk of bias in included studies
Two review authors (SM and MC) independently assessed risk of bias of the included studies. They performed risk of bias assessment for RCTs and CCTs in this review using the criteria provided by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The approach recommended for assessing risk of bias in studies included in a Cochrane review involves a two‐part tool used to address seven specific domains, namely, sequence generation and allocation concealment (selection bias), blinding of participants and providers (performance bias), blinding of outcome assessors (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias) and other sources of bias. The first part of the tool involves describing what was reported to have happened in the study. The second part of the tool involves assigning a judgement related to the risk of bias for that entry in terms of low, high or unclear risk. To make these judgements, we used the criteria provided by the Cochrane Handbook for Systematic Reviews of Interventions as adapted for the field of addiction. See Appendix 6 for details.
The domains of sequence generation and allocation concealment (avoidance of selection bias) were addressed in the tool by a single entry for each study.
Blinding of participants, personnel and outcome assessors (avoidance of performance bias and detection bias) was considered separately for objective outcomes (e.g. dropout, use of substance of abuse as measured by urinalysis, relapse at the end of follow‐up) and subjective outcomes (e.g. duration and severity of signs and symptoms of withdrawal, patient self‐reported use of substance, side effects, craving, psychiatric symptoms).
Incomplete outcome data (avoidance of attrition bias) were considered for all outcomes except for dropout from treatment, which very often is the primary outcome measured in trials on addiction.
Grading of evidence
We assessed the overall quality of evidence for the primary outcome using the GRADE system. The Grading of Recommendation, Assessment, Development and Evaluation Working Group (GRADE) developed a system for grading the quality of evidence (Grade 2004; Guyatt 2008; Guyatt 2011; Shunemann 2006), which takes into account issues related not only to internal validity but also to external validity, such as directness of results. The 'Summary of findings' tables present the main findings of a review in a transparent and simple tabular format. In particular, they provide key information concerning the quality of evidence, the magnitude of effect of the interventions examined and the sum of available data on the main outcomes.
The GRADE system uses the following criteria in assigning grades of evidence.
High: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low: Any estimate of effect is very uncertain.
Grading is decreased for the following reasons.
Serious (‐1) or very serious (‐2) limitation to study quality.
Important inconsistency (‐1).
Some (‐1) or major (‐2) uncertainty about directness.
Imprecise or sparse data (‐1).
High probability of reporting bias (‐1).
Grading is increased for the following reasons.
Strong evidence of association: significant relative risk > 2 (< 0.5) based on consistent evidence from two or more observational studies, with no plausible confounders (+1).
Very strong evidence of association: significant relative risk > 5 (< 0.2) based on direct evidence with no major threats to validity (+2).
Evidence of a dose response gradient (+1).
Effect reduced by all plausible confounders (+1).
Measures of treatment effect
We analysed dichotomous outcomes by calculating the risk ratio (RR) for each trial with uncertainty in each result expressed by 95% confidence intervals (CIs). We analysed continuous outcomes by calculating the mean difference (MD) with 95% CI when studies used the same instrument in assessing the outcome. We used the standardised mean difference (SMD) when studies used different instruments. For craving score, severity of dependence (Addiction Severity Index (Drug ASI), Clinical Global Impression ‐ Observer (CGI‐O)), depression (Hamilton Depression Scale (HAM‐D)) and anxiety (Hamilton Anxiety Scale (HAM‐A)), we compared the postintervention mean scores of experimental and control groups.
Unit of analysis issues
If all arms in a multi‐arm trial were to be included in the meta‐analysis, and one treatment arm was to be included in more than one of the treatment comparisons, we divided the number of events and the number of participants in that arm by the number of treatment comparisons made. This method avoids the multiple use of participants in the pooled estimate of treatment effect while retaining information from each arm of the trial. It slightly compromises the precision of the pooled estimate.
Assessment of heterogeneity
We analysed heterogeneity by using the I2 statistic and the Chi2 test. Cut‐off points included an I2 value greater than 50% and a P value for the Chi2 test less than 0.1.
Assessment of reporting biases
A funnel plot (plot of the effect estimate from each study against the sample size or the effect standard error) was not used to assess the potential for bias related to the size of the trials, which could indicate possible publication bias, because all included studies had a small sample size and yielded results that were not statistically significant.
Data synthesis
Outcomes from the individual trials were combined through meta‐analysis when possible (comparability of interventions and outcomes between trials) using a random‐effects model because some degree of heterogeneity was expected among trials.
Subgroup analysis and investigation of heterogeneity
We first compared any anticonvulsant versus placebo. We then performed subgroup analyses for single types of anticonvulsants.
Sensitivity analysis
To incorporate our assessment of risk of bias into the review process, we first plotted the intervention effect estimates stratified for risk of bias for each relevant domain. If differences in results were noted among studies at different risks of bias, we performed sensitivity analysis by excluding from the analysis studies at high risk of bias.
Results
Description of studies
Results of the search
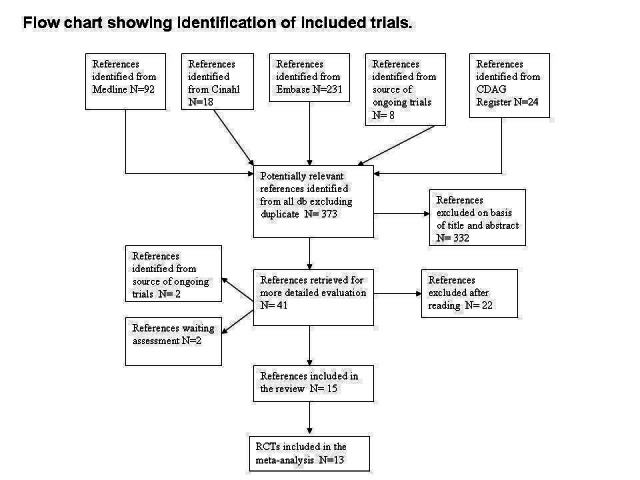
This is an update of a Cochrane review first published in 2008. In the first edition of this review, through bibliographic searches we identified 373 reports after removing duplicates; we excluded 332 studies on the basis of title and abstract; we retrieved 41 articles in full text for more detailed evaluation, 22 of which we excluded after reading the full text; of the remaining 19 studies, two were ongoing trials and two were unpublished studies. Therefore we excluded 22 studies and found that 15 satisfied all criteria required for inclusion in the review. See Figure 1.
1.
In the present update, through bibliographic searches we identified 322 records after removing duplicates; we excluded 298 studies on the basis of title and article; we retrieved 24 articles in full text for more detailed evaluation. We excluded eight articles related to five studies after reading the full text. We determined that 13 articles related to eight studies satisfied the inclusion criteria. Three were conference abstracts for which we were unable to retrieve the full publication, so we classified these as awaiting classification. We included no unpublished studies. See Figure 2.
2.
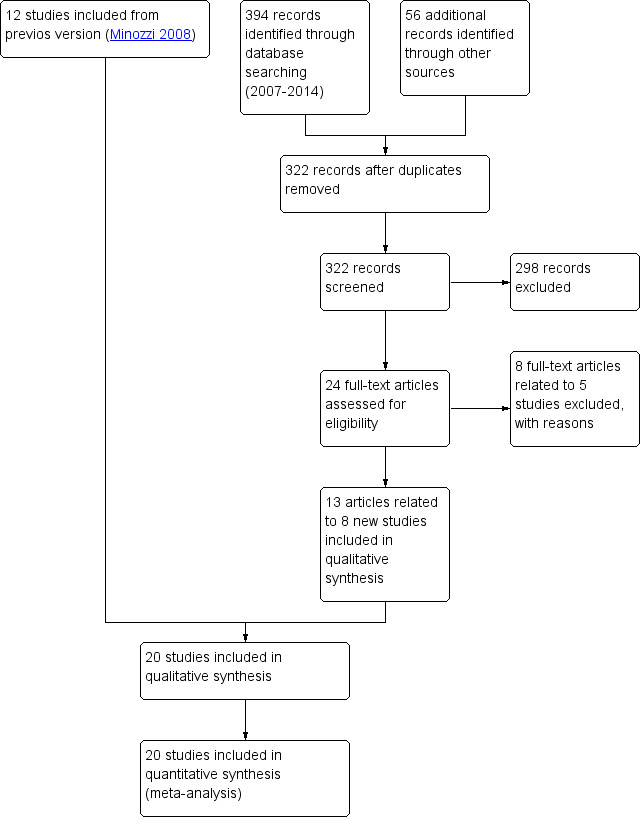
Study flow diagram of the updated version.
For substantive descriptions of studies, see Characteristics of included studies and Characteristics of excluded studies tables.
Included studies
Fifteen studies with 1066 participants met the inclusion criteria for this review in the first edition. In the update, 13 additional articles related to eight studies were further included. Moreover, for the updated version, we decided to exclude three studies that had been included in the first version: two (Reid 2005; Sofuoglu 1999) because they did not fulfil the inclusion criteria, and one (Gonzalez 2003) because it was an interim analysis of already included studies (Gonzalez 2007 arm a; Gonzalez 2007 arm b), and the same participants in two of three arms were counted in both studies. Finally, we included 20 studies with 2068 participants.
Duration of trials
The mean duration of the trials was 11.8 weeks (range, eight to 24 weeks).
Treatment regimens
The anticonvulsants utilised in the included studies were as follows.
Carbamazepine: six studies, nine arms (Campbell 1994 arm a; Campbell 1994 arm b; Campbell 2003 arm a; Campbell 2003 arm b; Cornish 1995; Halikas 1997 arm a; Halikas 1997 arm b; Kranzler 1995; Montoya 1994); mean dose 375 mg/d.
Tiagabine: three studies (Gonzalez 2007 arm a; Winhusen 2005, Winhusen 2007); mean dose 21 mg/d.
Gabapentin: three studies (Berger 2005 arm b; Bisaga 2006; Gonzalez 2007 arm b); mean dose 1933 mg/d.
Phenytoin: one study (Crosby 1996); doses of 100 mg/d.
Lamotrigine: two studies (Berger 2005 arm a; Brown 2012); dose max 150 mg/d in one study and not reported in the other.
Topiramate: five studies (Johnson 2013; Kampman 2004; Kampman 2013; Nuijten 2014; Umbricht 2014); dose max 200 mg/d in two studies, 300 mg/d in three studies.
Vigabatrin: two studies (Brodie 2009; Somoza 2013); 250 and 300 mL/d, respectively.
Setting
One study was conducted in Mexico, and one in The Netherlands; all others were conducted in the USA.
All studies were conducted in an outpatient setting.
Participants
A total of 2068 cocaine addicts according to Diagnostic and Statistical Manual of Mental Disorders Fourth Edition Revised (DSM‐IV‐R) criteria. A total of 77.4% were male; mean age was 36.2 years. All participants were actively using cocaine. Routes of administration of cocaine included 84.5% smoked crack cocaine, 10.6% intranasal and 6.6% intravenous in the 13 studies that reported this information.
Rating instruments used in these studies
Brief Substance Craving Scale (BSCS) (Somoza 1995): four studies, five arms (Berger 2005 arm a; Berger 2005 arm b; Somoza 2013; Winhusen 2005; Winhusen 2007).
Minnesota Cocaine Craving Scale (Halikas 1991): one study (Kampman 2013).
Adapted version of Obsessive Compulsive Dinking Scale for Craving (Anton 1996): one study (Nuijten 2014).
Halikas‐Crosby Drug Impairment Rating Scale for Craving (Hal‐DIRS): one study (Campbell 2003 arm a).
CSSA for Craving (Mulvaney 1999): one study (Umbricht 2014).
Addiction Severity Index (ASI) (McLellan 1992): six studies, seven arms (Berger 2005 arm a; Berger 2005 arm b; Kampman 2013; Kranzler 1995; Nuijten 2014; Winhusen 2005; Winhusen 2007).
Clinical Global Impression Scale (CGI‐O) (Guy 1976): six studies, seven arms (Berger 2005 arm a; Berger 2005 arm b; Brodie 2009; Kranzler 1995; Somoza 2013; Winhusen 2005; Winhusen 2007).
Hamilton Anxiety Rating Scale (Hamilton 1959): four studies, five arms (Berger 2005 arm a; Berger 2005 arm b; Brodie 2009; Brown 2012; Winhusen 2005).
Hamilton Depression Rating Scale (Hamilton 1967): three studies, four arms (Berger 2005 arm a; Berger 2005 arm b; Brodie 2009; Winhusen 2005).
Beck Depression Inventory (Beck 1961): two studies (Kranzler 1995; Umbricht 2014).
State Anxiety Inventory (Spielberg 1983): two studies (Kranzler 1995; Umbricht 2014).
Excluded studies
A total of 31 studies did not meet the criteria for inclusion in this review. Grounds for exclusion included study design not in the inclusion criteria: 15 studies (Ahmadi 2006, Brown 2003; Campbell 2001; Cornish 1995 b; Elkashef 2005; Halikas 1989; Johnoson 2005; Kampman 2005; Khun 1989; Leiderman 2005; Llopis Llacer 2008; Reis 2008; Salloum 2007; Vocci 2005; Zullino 2004); objectives not in the inclusion criteria: six studies (Haney 2005; Hart 2004; Hart 2007; Reid 2009; Sofuoglu 2005; Winter 2000); no useable outcome measures: three studies (Brady 2002; Halikas 1991; Hatsukami 1991); types of interventions not in the inclusion criteria: four studies (five articles) (Gorelick 1994; Mancino 2014; Mariani 2012; Reid 2005); and types of participants not in the inclusion criteria: two studies (Kemp 2009; Sofuoglu 1999). An interim analysis of already included studies was performed by one study (Gonzalez 2003).
Risk of bias in included studies
Allocation
Random sequence generation
Six studies; eight arms (Brown 2012; Campbell 1994 arm a; Campbell 1994 arm b; Cornish 1995; Halikas 1997 arm a; Halikas 1997 arm b; Nuijten 2014; Umbricht 2014) were judged at low risk of selection bias because they used an adequate sequence generation method. In all of the other studies, the method was not reported.
Allocation concealment
Six studies, seven arms (Bisaga 2006; Brodie 2009; Cornish 1995; Gonzalez 2007 arm a; Gonzalez 2007 arm b; Kranzler 1995; Umbricht 2014) were judged at low risk of selection bias because researchers used an adequate method for allocation concealment. One study was judged at high of bias (Brown 2012). In all of the other studies, investigators did not report the method used for allocation concealment.
Blinding
All but three studies (four arms) (Berger 2005 arm a; Berger 2005 arm b; Nuijten 2014; Winhusen 2005) were double‐blind controlled trials.
Objective outcomes
All studies were judged at low risk of bias.
Subjective outcomes
Performance bias
Three studies (Berger 2005 arm a; Berger 2005 arm b; Nuijten 2014 and Winhusen 2005) were judged at high risk. All other studies were judged at low risk of performance and detection bias.
Detection bias
One study (Winhusen 2005) was judged at high risk; 12 studies were judged at unclear risk of detection bias, and the remaining studies were at low risk.
Incomplete outcome data
Eight studies, nine arms (Brown 2012; Cornish 1995; Crosby 1996; Halikas 1997 arm a; Halikas 1997 arm b; Kranzler 1995; Montoya 1994; Nuijten 2014; Winhusen 2007) were judged at high risk of attrition bias. Investigators in all of the other studies performed the analysis on an intention‐to‐treat basis or did not report withdrawal from the study.
Selective reporting
Brodie 2009 was judged at high risk of selective reporting bias because results for cocaine craving, HAM‐A and HAM‐D scores or CGI severity and CGI were not reported. Study authors stated only that they observed no differences. Campbell 1994 arm a and Campbell 1994 arm b were judged at high risk of selective reporting bias because results for dropout were not reported. Study authors stated only that they observed no differences. Brown 2012 was judged at high risk of reporting bias because retention in treatment, which is one of the most relevant outcomes in the field of addiction, was not reported. For the other studies, the study protocol was not available but published reports included all expected outcomes, including those that were prespecified in the Methods section, so they were judged at low risk of reporting bias.
3.
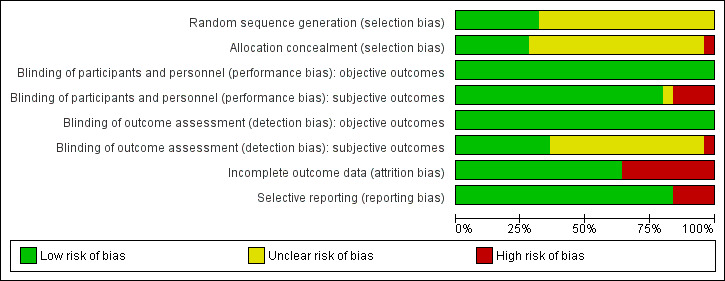
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.
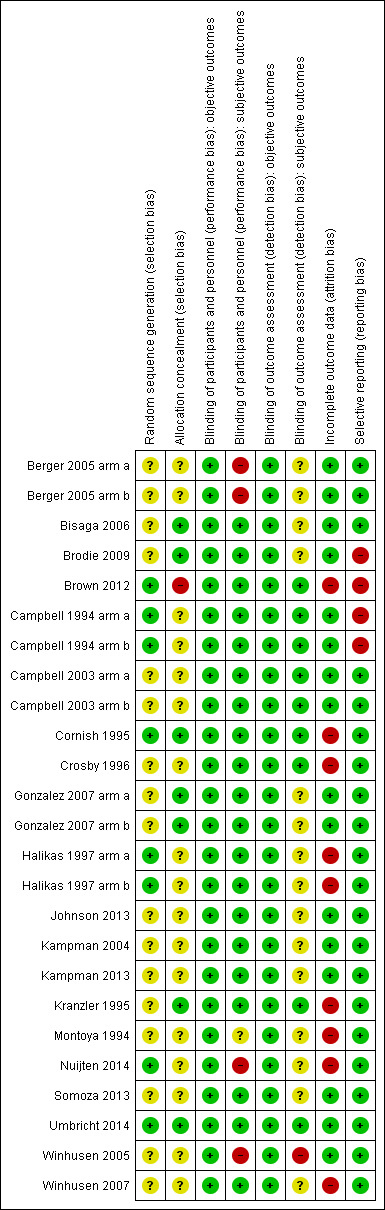
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1
Comparisons
Any anticonvulsant versus placebo: 21 studies, 25 arms.
-
Single anticonvulsant versus placebo.
Subcategory 2.1: carbamazepine versus placebo: six studies, seven arms.
Subcategory 2.2: tiagabine versus placebo: three studies.
Subcategory 2.3: gabapentin versus placebo: three studies.
Subcategory 2.4: phenytoin versus placebo: one study.
Subcategory 2.5: lamotrigine versus placebo: two studies.
Subcategory 2.6: topiramate versus placebo: five studies.
Subcategory 2.7: vigabatrin versus placebo: two studies.
Anticonvulsant versus antidepressive (desipramine): two studies.
Two studies (Berger 2005 and Gonzalez 2007) included three arms, each comparing lamotrigine (Berger 2005 arm a) and gabapentin (Berger 2005 arm b) versus placebo and tiagabine (Gonzalez 2007 arm a) and gabapentin (Gonzalez 2007 arm b) versus placebo; in order to do not doublecounting the participants, we divided the number participants and events in the placebo group for comparison 2 and 1. In cases where only one event occurred in the placebo group, this could not be divided, so events and participants have been counted twice.
Two studies (Campbell 1994 and Campbell 2003) had three arms, each comparing carbamazepine versus placebo (Campbell 1994 arm a; Campbell 2003 arm a) and desipramine (Campbell 1994 arm b; Campbell 2003 arm b). The study of Halikas 1997 had three arms comparing carbamazepine 400 mg (Halikas 1997 arm a) and carbamazepine 800 mg (Halikas 1997 arm b) versus placebo; in order to do not doublecounting the participants, we divided the number participants and events in the placebo group for comparison 2 and 1.
Primary outcomes
Dropouts from treatment as number of participants who did not complete treatment
(1) Any anticonvulsants versus placebo (see Table 1) 17 studies, 20 arms (Berger 2005 arm a; Berger 2005 arm b; Brodie 2009; Campbell 2003 arm a; Cornish 1995; Crosby 1996; Gonzalez 2007 arm a; Gonzalez 2007 arm b; Halikas 1997 arm a; Halikas 1997 arm b; Johnson 2013; Kampman 2004; Kampman 2013; Kranzler 1995; Montoya 1994; Nuijten 2014; Somoza 2013; Umbricht 2014; Winhusen 2005; Winhusen 2007), 1695 participants, RR 0.95, 95% CI 0.86 to 1.05; no significant difference between anticonvulsant and placebo (see Analysis 1.1).
1.1. Analysis.
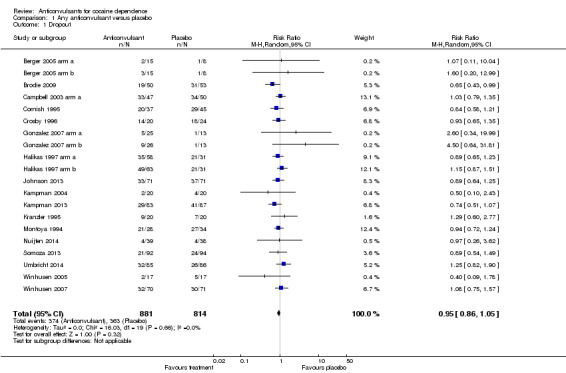
Comparison 1 Any anticonvulsant versus placebo, Outcome 1 Dropout.
(2) Single anticonvulsants versus placebo Subcategory 2.1: carbamazepine versus placebo, five studies, six arms (Campbell 2003 arm a; Cornish 1995; Halikas 1997 arm a; Halikas 1997 arm b; Kranzler 1995; Montoya 1994), 464 participants, RR 0.99, 95% CI 0.87 to 1.13; no significant difference (see Analysis 2.1). Subcategory 2.2: tiagabine versus placebo, three studies (Gonzalez 2007 arm a; Winhusen 2005; Winhusen 2007), 213 participants, RR 1.01, 95% CI 0.56 to 1.82; no significant difference (see Analysis 2.1). Subcategory 2.3: gabapentin versus placebo, two studies (Berger 2005 arm b; Gonzalez 2007 arm b), 62 participants, RR 2.78, 95% CI 0.67 to 11.61; the result is statistically significant in favour of placebo (see Analysis 2.1). Subcategory 2.4: phenytoin versus placebo, one study (Crosby 1996), 44 participants, RR 0.93, 95% CI 0.65 to 1.35; no significant difference (see Analysis 2.1). Subcategory 2.5: lamotrigine versus placebo, one study (Berger 2005 arm a), 23 participants, RR 1.07, 95% CI 0.11 to 10.04; no significant difference (see Analysis 2.1). Subcategory 2.6: topiramate versus placebo, four studies (Johnson 2013; Kampman 2004; Nuijten 2014; Umbricht 2014), 557 participants, RR 0.92, 95% CI 0.73 to 1.16; no significant difference (see Analysis 2.1).
2.1. Analysis.
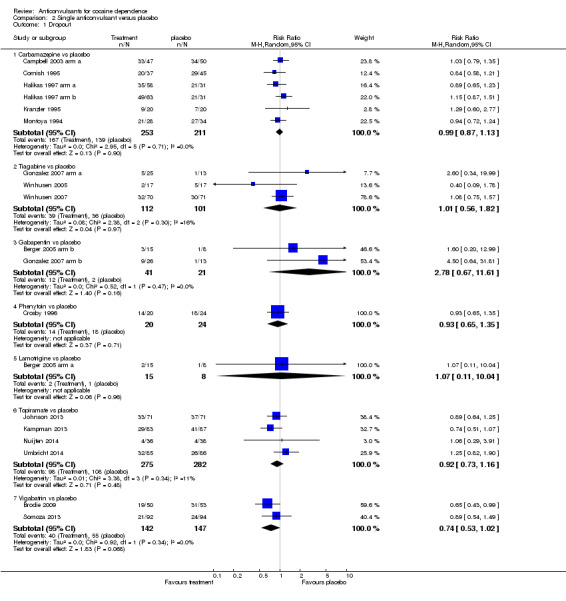
Comparison 2 Single anticonvulsant versus placebo, Outcome 1 Dropout.
Subcategory 2.7: vigabatrin versus placebo, two studies (Brodie 2009; Somoza 2013), 289 participants, RR 0.74, 95% CI 0.53 to 1.02; a trend favours vigabatrin.
(3) Carbamazepine versus desipramine One study (Campbell 2003 arm b), 96 participants, RR 1.15, 95% CI 0.86 to 1.53; no significant difference.
Use of cocaine (urinalysis or self reported)
(1) Any anticonvulsants versus placebo (see Table 1) Nine studies, 11 arms (Bisaga 2006; Brodie 2009; Cornish 1995; Crosby 1996; Gonzalez 2007 arm a; Gonzalez 2007 arm b; Halikas 1997 arm a; Halikas 1997 arm b; Kampman 2004; Kampman 2013; Somoza 2013), 867 participants (see Analysis 1.2), RR 0.92, 95% CI 0.84 to 1.02; no significant difference.
1.2. Analysis.
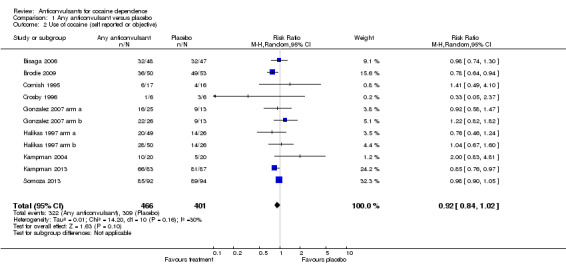
Comparison 1 Any anticonvulsant versus placebo, Outcome 2 Use of cocaine (self reported or objective).
(2) Single anticonvulsants versus placebo Subcategory 2.1: carbamazepine versus placebo, three studies, four arms (Campbell 2003 arm a; Cornish 1995; Halikas 1997 arm a; Halikas 1997 arm b), 214 participants (Analysis 2.2), RR 0.95, 95% CI 0.70 to 1.28; no significant difference. Subcategory 2.2: tiagabine versus placebo, one study (Gonzalez 2007 arm a), 50 participants, RR 0.89, 95% CI 0.61 to 1.30; no significant difference (see Analysis 2.2). Subcategory 2.3: gabapentin versus placebo, two studies (Bisaga 2006; Gonzalez 2007 arm b), 146 participants (see Analysis 2.2), RR 1.07, 95% CI 0.87 to 1.31; no significant difference (see Analysis 2.2). Subcategory 2.4: phenytoin versus placebo, one study (Crosby 1996), 12 participants, RR 0.33, 95% CI 0.05 to 2.37; no significant difference (see Analysis 2.2). Subcategory 2.6: topiramate versus placebo, two studies (Kampman 2004; Kampman 2013), 210 participants, RR 1.19, 95% CI 0.48 to 2.98; no significant difference.
2.2. Analysis.
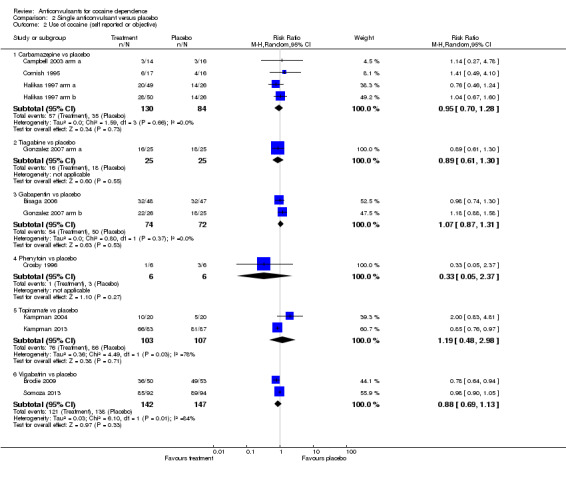
Comparison 2 Single anticonvulsant versus placebo, Outcome 2 Use of cocaine (self reported or objective).
Subcategory 2.7: vigabatrin versus placebo, two studies (Brodie 2009; Somoza 2013), 289 participants, RR 0.88, 95% CI 0.69 to 1.13; no significant difference (see Analysis 2.2).
Any side effects
(1) Any anticonvulsants versus placebo (see Table 1) Eight studies (Bisaga 2006; Brown 2012; Cornish 1995; Crosby 1996; Johnson 2013; Kranzler 1995; Nuijten 2014; Somoza 2013), 775 participants (see Analysis 1.3), RR 1.39, 95% CI 1.01 to 1.90. Heterogeneity in the results was very high (P value < 0.00001; I2 81%). Results favoured placebo.
1.3. Analysis.
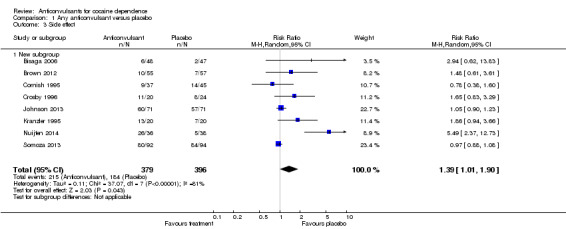
Comparison 1 Any anticonvulsant versus placebo, Outcome 3 Side effect.
Serious adverse events (SAEs): Seven studies reported data about SAEs. Berger 2005 arm a and Berger 2005 arm b reported three SAEs, none of which were related to the study medication. The first involved a placebo participant who accidentally shot himself in the eye with a nail gun, which required surgery, while he was in the follow‐up phase of the study. The other two SAEs occurred in participants who were taking gabapentin. Bisaga 2006 reported five SAEs, four occurring in participants randomly assigned to gabapentin. Three of the SAEs in the gabapentin group were mild and resolved without hospitalisation (chest pain, bloody stools and calf pain), and one warranted removal from the study (depression with suicidal tendencies). None of the SAEs were judged to be related to the gabapentin. In Cornish 1995, the only unexpected, serious adverse medical event that occurred was the death of one participant who was randomly assigned to carbamazepine. Montoya 1994 and Nuijten 2014 reported that no SAEs occurred. In Somoza 2013, 11 participants collectively experienced a total of 14 SAEs. The three SAEs involving placebo group participants (manic episode, hip replacement and insomnia) were determined to be unrelated to the study medication. Of 11 SAEs experienced by eight vigabatrin group participants, eight (experienced by five participants) were deemed to be unrelated or unlikely to be related to the study medication. Winhusen 2005 reported a total of three SAEs, none of which were related to the study medications. In Winhusen 2007, a total of 10 SAEs were reported for randomly assigned participants, with two participants experiencing two SAEs. Serious adverse events in the tiagabine group included hospitalisation due to suicidal ideation and hospitalisation for detoxification from alcohol and cocaine. An additional three tiagabine participants were hospitalised as the result of chest pains. Finally, one participant in the tiagabine group experienced two hospitalisations ‐ one for gallstones and one for being incoherent and agitated following use of a large amount of cocaine. One of the placebo participants was hospitalised as the result of experiencing visual hallucinations and agitation, and a second placebo participant experienced two hospitalisations, both due to chest pains, which he attributed to panic attacks. All SAEs were rated as unrelated to the study medication or with only a remote possibility of being related to the study medication.
(2) Single anticonvulsants versus placebo Subcategory 2.1: carbamazepine versus placebo, two studies (Cornish 1995; Kranzler 1995), 122 participants, RR 1.21, 95% CI 0.52 to 2.86; no significant difference (see Analysis 2.3). Subcategory 2.3: gabapentin versus placebo, one study (Bisaga 2006), 95 participants, RR 2.94, 95% CI 0.62 to 13.83; the result is statistically significant in favour of placebo (see Analysis 2.3). Subcategory 2.4: phenytoin versus placebo, one study (Crosby 1996), 44 participants, RR 1.65, 95% CI 0.83 to 3.29; no significant difference (see Analysis 2.3). Subcategory 2.6: topiramate versus placebo, two studies (Johnson 2013; Nuijten 2014), 216 participants, RR 2.42, 95% CI 0.27 to 21.87; no significant difference (see Analysis 2.3).
2.3. Analysis.
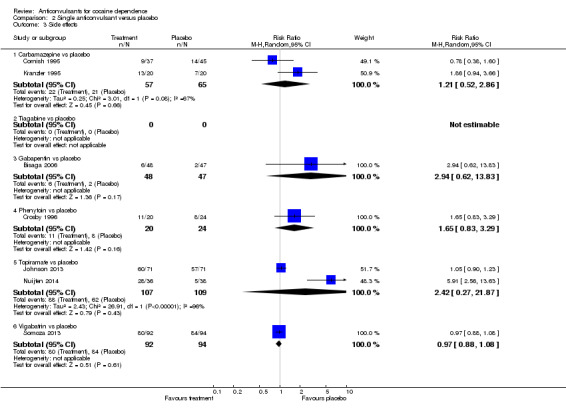
Comparison 2 Single anticonvulsant versus placebo, Outcome 3 Side effects.
Subcategory 2.7: vigabatrin versus placebo, one study (Somoza 2013), 186 participants, RR 0.97, 95% CI 0.88 to 1.08; no significant difference (see Analysis 2.3).
Sensitivity analysis
For comparison 1, we plotted the intervention effect estimates stratified for risk of bias for random sequence generation and allocation concealment. We found no difference in the results, so sensitivity analysis excluding studies with high risk of bias was not performed.
Secondary outcomes
Compliance
(1) Any anticonvulsants versus placebo
Four studies, six arms measured compliance as the number of participants fully compliant on the basis of pill counts (Berger 2005 arm a; Berger 2005 arm b; Brown 2012; Halikas 1997 arm a; Halikas 1997 arm b; Winhusen 2005), 343 participants, RR 1.01, 95% CI 0.93 to 1.08; no significant difference (see Analysis 1.9).
1.9. Analysis.
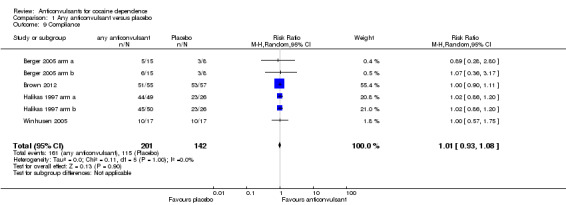
Comparison 1 Any anticonvulsant versus placebo, Outcome 9 Compliance.
Five studies measured compliance as a mean percentage of pills taken by each group (Crosby 1996; Johnson 2013; Kampman 2004; Kampman 2013; Winhusen 2007), 426 participants, MD 1.42, 95% CI ‐4.80 to 7.64; no significant difference (see Analysis 1.10).
1.10. Analysis.
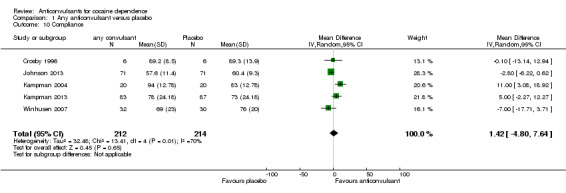
Comparison 1 Any anticonvulsant versus placebo, Outcome 10 Compliance.
Five studies, seven arms reported information on compliance in different and often incomplete modalities.
Bisaga 2006, Campbell 2003 arm a and Campbell 2003 arm b measured compliance by plasma level and reported only the percentage of participants compliant in the anticonvulsant group as 83%, 71% and 63%, respectively .
Gonzalez 2007 arm a and Gonzalez 2007 arm a reported “over 95% compliance with no significant difference between groups”.
Nuijten 2014 reported, “Topiramate titration (3weeks) was completed by 28 participants (77.6%). Of these, 27 participants were prescribed the maximum dose of 200mg/day and one patient received 150 mg/day due to adverse events. Twenty‐two patients (61.1%) received Topiramate treatment for at least 6 weeks, nine patients (25.0%) for at least 9 weeks and five patients (13.9%) completed at least 11 weeks. The mean dose of Topiramate in the 28 ‘titration completers’ was 189 mg/day (sd=32.1)”. Information on adherence was not reported for the placebo group.
Somoza 2013 reported, “Based on pill counts, 55.4% of participants were more than 90% compliant, and 66.2% of participants were more than 70% compliant, with no statistically significant difference between the treatment groups”.
The remaining studies did not assess this outcome.
Craving
(1) Any anticonvulsants versus placebo (see Table 1) Seven studies, eight arms (Berger 2005 arm a; Berger 2005 arm b; Campbell 2003 arm a; Crosby 1996; Nuijten 2014; Somoza 2013; Winhusen 2005; Winhusen 2007), 428 participants, SMD ‐0.25, 95% CI ‐0.59 to 0.09; no significant difference (see Analysis 1.4).
1.4. Analysis.
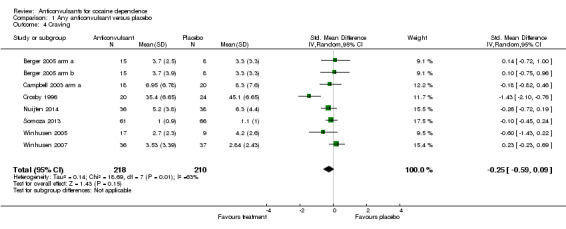
Comparison 1 Any anticonvulsant versus placebo, Outcome 4 Craving.
Severity of dependence (Addiction Severity Index)
(1) Any anticonvulsants versus placebo
Five studies, six arms (Berger 2005 arm a; Berger 2005 arm b; Kranzler 1995; Somoza 2013; Winhusen 2005, Winhusen 2007), 290 participants, MD 0.03, 95% CI ‐0.02 to 0.08; no significant difference (see Analysis 1.5).
1.5. Analysis.
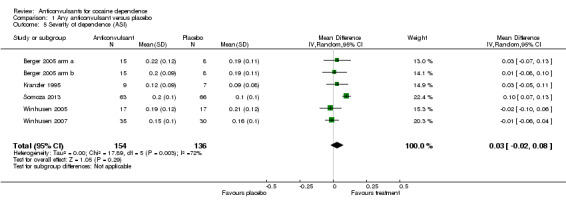
Comparison 1 Any anticonvulsant versus placebo, Outcome 5 Severity of dependence (ASI).
Severity of dependence (Clinical Global Impression Scale ‐ Observer)
(1) Any anticonvulsants versus placebo Four studies, five arms (Berger 2005 arm a; Berger 2005 arm b; Somoza 2013; Winhusen 2005; Winhusen 2007), 277 participants, MD ‐0.11, 95% CI ‐0.42 to 0.20; no significant difference (see Analysis 1.6).
1.6. Analysis.
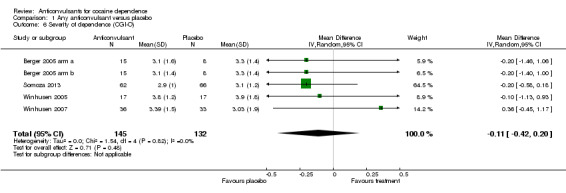
Comparison 1 Any anticonvulsant versus placebo, Outcome 6 Severity of dependence (CGI‐O).
Depression (Hamilton Depression Rating Scale)
(1) Any anticonvulsants versus placebo Two studies, three arms (Berger 2005 arm a; Berger 2005 arm b; Winhusen 2005), 80 participants, MD 1.80, 95% CI ‐0.59 to 4.19; no significant difference (see Analysis 1.7).
1.7. Analysis.
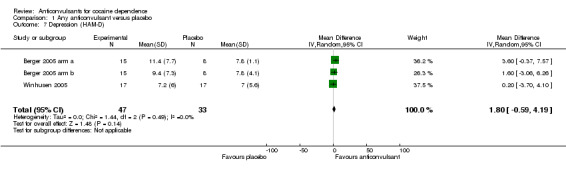
Comparison 1 Any anticonvulsant versus placebo, Outcome 7 Depression (HAM‐D).
Anxiety (Hamilton Anxiety Rating Scale)
(1) Any anticonvulsants versus placebo Two studies, three arms (Berger 2005 arm a; Berger 2005 arm b; Winhusen 2005), 78 participants, MD 1.79, 95% CI ‐1.02 to 4.60; no significant difference (see Analysis 1.8).
1.8. Analysis.
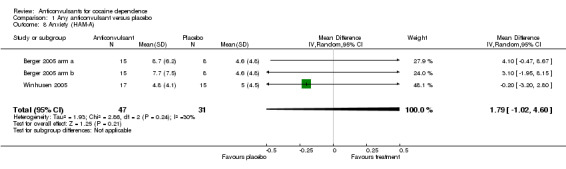
Comparison 1 Any anticonvulsant versus placebo, Outcome 8 Anxiety (HAM‐A).
Discussion
Summary of main results
We found 20 studies including 2068 participants comparing anticonvulsants versus placebo for the treatment of cocaine dependence. The anticonvulsants assessed were carbamazepine, gabapentin, lamotrigine, phenytoin, tiagabine, topiramate and vigabatrin.
Overall, no significant differences were found for any of the primary and secondary outcomes when any anticonvulsants were compared with placebo. Also in the subgroup analyses comparing a single anticonvulsant versus placebo, no differences were found in any of the primary or secondary outcomes.
Results on side effects are few because only 8/21 (38%) studies reported data on side effects in a useable way. Moreover heterogeneity is high between studies in the frequencies of any side effects; this suggests that side effects could have been defined differently within studies, and that probably studies for which the frequency of side effects was very low reported only the most significant or important ones, whereas studies with high frequency reported any and low relevant side effects.
Overall completeness and applicability of evidence
All but two of the 21 included studies were conducted in the USA. This could limit the generalisability of the results because health effects of various substances of abuse seem to be strongly dependent on social context, and the location at which studies were conducted could act as an effect modifier in the estimation of efficacy of treatment. Moreover among the included participants, 84.5% smoked crack cocaine, and only 10.6% used the intranasal route in the 13 studies that reported this information. These frequencies do not reflect the real prevalence of the different formulations of cocaine and route of administration: In European countries, crack cocaine represents around 13% of individuals seeking treatment (ranging from 1% in Italy and 36% in England) (EMCDDA 2012). This further limits on the generalisability of the results.
Quality of the evidence
Only 7/21 (30%) studies have been judged as having low risk of selection bias; all other studies were judged as having unclear risk of selection bias because the information was not reported. All but three studies (86%) were double‐blind. A total of 8/21 (38%) studies were judged as having high risk of attrition bias. All other studies performed the analysis on an intention‐to‐treat basis or reported no withdrawals from the study. Two studies were judged to be at high risk of selective reporting because they did not report results for dropout, and because they did not report raw data for craving or psychiatric outcomes but only stated that no differences were noted.
However, with subgroup analysis, as in the case of single classes of anticonvulsants, single types of medications and confounder/moderator evaluation, as well as with comparisons versus other medications, findings of the review were limited by the small number of studies included in the meta‐analysis of study outcomes. Therefore the precision of the calculated effects is low. Finally, the great heterogeneity of the scales used in the primary studies and the way in which results were reported often made a cumulative analysis impossible.
Potential biases in the review process
We found no unpublished studies despite efforts to contact all first authors of the included studies and to perform a search of conference proceedings. We did not use funnel plots to assess the possibility of publication bias because in this review, only small negative studies have been included, and in this situation, this method is not sensitive.
Agreements and disagreements with other studies or reviews
Both preclinical and clinical studies have investigated the potential involvement of this class of medication in the treatment of substance use disorders; clinical trials specifically designed for evaluation of anticonvulsants and their efficacy and safety in cocaine dependence have been performed. Among the reviews published after our previous Cochrane review on the topic, two (Cohen 2014; Shinn 2010) were exclusively interested in the efficacy of topiramate. They adopted a narrative approach and reported results of two clinical trials and one clinical trial, respectively. A third review (Alvarez 2010) was characterised by a meta‐analytical approach (Alvarez 2010). It included 15 randomised, double‐blind, placebo‐controlled clinical trials involving 1236 participants and evaluated seven anticonvulsant drugs. According to this review, treatments do not show improvement in subject retention compared with placebo, and the number of cocaine‐positive urine samples was close to reaching statistical significance (95% CI 0.85 to 1.06) compared with placebo.
Our review, besides applying Cochrane methodology, includes 20 studies with 2068 participants, and it extends evaluations to a wider range of primary and secondary outcomes. On the whole, the results that we obtained show no evidence of differences between anticonvulsants and placebo. However, it has to be considered that anticonvulsants constitute a really heterogeneous group. Besides their anticonvulsant action, they have different pharmacological profiles and indications. This would suggest the need for a more detailed evaluation of singular medications. Unfortunately, for most outcomes, meta‐analyses carried out on specific anticonvulsants included only one or two studies and few participants.
Authors' conclusions
Implications for practice.
Although caution is needed when results from a limited number of clinical trials are assessed, at present no current evidence supports the clinical use of anticonvulsants, as a category, in the treatment of cocaine dependence. In terms of specific medications, the insufficiency of evidence may leave to clinicians the alternative of balancing possible benefits against potential adverse effects of treatment.
Implications for research.
To answer the urgent demand of clinicians, patients, families and the community as a whole for adequate treatment for cocaine dependence, we must improve primary research in the field of addiction to make the best possible use of a single study. Researchers must design larger randomised investigations to analyse relevant outcomes (dropout, use of cocaine measured as number of participants abstinent at the end of treatment). The fact that this review has found that the anticonvulsants investigated are not efficacious for cocaine dependence should not discourage researchers from carrying out new clinical trials on anticonvulsants with different pharmacological characteristics. Some of these studies are ongoing and will be added to this review as soon as their results become available. Besides increasing the numbers of trials and participants, these studies (five on topiramate, two on vigabatrin, two on tiagabine, one on levetiracetam), when available, will contribute to evaluation of the efficacy of specific medications.
What's new
| Date | Event | Description |
|---|---|---|
| 8 June 2015 | Amended | Reference correction |
History
Protocol first published: Issue 4, 2007 Review first published: Issue 2, 2008
| Date | Event | Description |
|---|---|---|
| 11 March 2015 | New citation required but conclusions have not changed | The previous version of this review has been withdrawn because of conflicts of interest of one review author; the review team has been changed, and 5 new studies added |
| 11 March 2015 | New search has been performed | A new search was conducted |
| 6 September 2011 | Amended | Plain language summary was amended |
| 21 March 2008 | Amended | Review was converted to new review format |
| 7 February 2008 | New citation required and conclusions have changed | Substantive amendments were made |
Acknowledgements
We would like to thank Zuzana Mitrova for helping with search strategies and for facilitating the editorial process, and Professor Robert Ali, who is the contact editor for this review.
Appendices
Appendix 1. CENTRAL search
MeSH descriptor: [Cocaine‐Related Disorders] explode all trees
(cocaine* or crack):ti,ab,kw (Word variations have been searched)
#1 or #2
(anticonvulsant* or carbamazepine or clorazepate or clobazam or clonazepam or chlordiazepoxide or divalproex or ethosuximide or ethosuximide or ethotoin or felbamate or fosphenytoin or gabapentin or lignocaine or lamotrigine or levetiracetam or lidocaine or hydantoins or levetiracetam or methsuximide or oxcarbazepine or paraldehyde or phenacemide or phenytoin or pregabalin or primidone or succinimide or tiagabine or topiramate or valproate or vigabatrin or zonisamide):ti,ab,kw (Word variations have been searched)
ACTH:ti,ab
#4 or #5
#3 and #6
Appendix 2. MEDLINE search strategy
Cocaine‐Related Disorders [Mesh]
((cocaine*[tiab]) AND (abuse*[tiab] OR addict*[tiab] OR dependen*[tiab]))
#1 OR #2
"Anticonvulsants"[Mesh]
anticonvulsant* [tiab]
ACTH[tiab]
carbamazepine OR clorazepate OR clobazam OR clonazepam OR chlordiazepoxide OR divalproex OR ethosuximide OR ethosuximide OR ethotoin OR felbamate OR fosphenytoin OR gabapentin OR lignocaine OR lamotrigine OR levetiracetam OR lidocaine OR hydantoins OR levetiracetam OR methsuximide OR oxcarbazepine OR paraldehyde OR phenacemide OR phenytoin OR pregabalin OR primidone OR succinimide OR tiagabine OR topiramate OR valproate OR vigabatrin OR zonisamide
#4 OR #5 OR #6 OR #7
randomized controlled trial [pt])
controlled clinical trial [pt])
randomized [tiab])
drug therapy [sh])
randomly [tiab])
trial [tiab])
groups [tiab])
placebo [tiab]
#9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16
animals [mh] NOT humans [mh]
#17 NOT #18
#3 AND #8 AND #19
Appendix 3. EMBASE search strategy
'cocaine dependence'/exp
(cocaine:ab,ti AND (abus*:ab,ti OR dependen*:ab,ti OR disorder*:ab,ti OR addict*:ab,ti))
'cocaine'/exp OR 'cocaine derivative'/exp AND
#1 OR #2 OR #3
'anticonvulsive agent'/exp OR
acth:ab,ti OR anticonvulsant*:ab,ti OR carbamazepine:ab,ti OR clorazepate:ab,ti OR clobazam:ab,ti OR clonazepam:ab,ti OR chlordiazepoxide:ab,ti OR divalproex:ab,ti OR ethosuximide:ab,ti OR ethotoin:ab,ti OR felbamate:ab,ti OR fosphenytoin:ab,ti OR gabapentin:ab,ti OR lignocaine:ab,ti OR lamotrigine:ab,ti OR lidocaine:ab,ti OR hydantoins:ab,ti OR levetiracetam:ab,ti OR methsuximide:ab,ti OR oxcarbazepine:ab,ti OR paraldehyde:ab,ti OR phenacemide:ab,ti OR phenytoin:ab,ti OR pregabalin:ab,ti OR primidone:ab,ti OR succinimide:ab,ti OR tiagabine:ab,ti OR topiramate:ab,ti OR valproate:ab,ti OR vigabatrin:ab,ti OR zonisamide:ab,ti AND
#5 OR #6
'randomized controlled trial'/exp
'crossover procedure'/exp
'double blind procedure'/exp
'single blind procedure'/exp
'controlled clinical trial'/exp
'clinical trial'/exp
placebo:ab,ti OR 'double blind':ab,ti OR 'single blind':ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti OR random*:ab,ti OR factorial*:ab,ti OR crossover:ab,ti OR (cross:ab,ti AND over:ab,ti)
#8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14
#4 AND #7 AND #15
Appendix 4. CINAHL search strategy
(MH "Substance Use Disorders+")
TX((cocaine) AND (abuse* OR dependen* OR addict* OR disorder*))
TI cocaine* OR AB cocaine* OR MH cocaine
S1 OR S2 OR S3
(MH "Anticonvulsants+")
TI (carbamazepine OR clorazepate OR clobazam OR clonazepam OR chlordiazepoxide OR divalproex OR ethosuximide OR ethosuximide OR ethotoin OR felbamate OR fosphenytoin OR gabapentin OR lignocaine OR lamotrigine OR levetiracetam OR lidocaine OR hydantoins OR levetiracetam OR methsuximide OR oxcarbazepine OR paraldehyde OR phenacemide OR phenytoin OR pregabalin OR primidone OR succinimide OR tiagabine OR topiramate OR valproate OR vigabatrin OR zonisamide)
AB (carbamazepine OR clorazepate OR clobazam OR clonazepam OR chlordiazepoxide OR divalproex OR ethosuximide OR ethosuximide OR ethotoin OR felbamate OR fosphenytoin OR gabapentin OR lignocaine OR lamotrigine OR levetiracetam OR lidocaine OR hydantoins OR levetiracetam OR methsuximide OR oxcarbazepine OR paraldehyde OR phenacemide OR phenytoin OR pregabalin OR primidone OR succinimide OR tiagabine OR topiramate OR valproate OR vigabatrin OR zonisamide)
TI anticonvulsant* OR AB anticonvulsant*
TI ACTH OR AB ACTH
S5 OR S6 OR S7 OR S8 OR S9
MH "Clinical Trials+"
PT Clinical trial
TI clinic* N1 trial* or AB clinic* N1 trial*
TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* )
AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* )
TI randomi?ed control* trial* or AB randomi?ed control* trial*
MH "Random Assignment"
TI random* allocat* or AB random* allocat*
MH "Placebos"
TI placebo* or AB placebo*
MH "Quantitative Studies"
S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21
S4 AND S10 AND S22
Appendix 5. Web of Science search strategy
TS=((( cocaine* OR crack) AND (abuse* OR depend* OR addict* OR disorder* OR detox* OR withdraw* OR abstinen* OR abstain*)))
TS=(anticonvulsant* OR carbamazepine OR clorazepate OR clobazam OR clonazepam OR chlordiazepoxide OR divalproex OR ethosuximide OR ethosuximide OR ethotoin OR felbamate OR fosphenytoin OR gabapentin OR lignocaine OR lamotrigine OR levetiracetam OR lidocaine OR hydantoins OR levetiracetam OR methsuximide OR oxcarbazepine OR paraldehyde OR phenacemide OR phenytoin OR pregabalin OR primidone OR succinimide OR tiagabine OR topiramate OR valproate OR vigabatrin OR zonisamide)
TS= clinical trial* OR TS=research design OR TS=comparative stud* OR TS=evaluation stud* OR TS=controlled trial* OR TS=follow‐up stud* OR TS=prospective stud* OR TS=random* OR TS=placebo* OR TS=(single blind*) OR TS=(double blind*)
#1 AND #2 AND #3
Indexes=SCI‐EXPANDED, SSCI, A&HCI Timespan=All years
Appendix 6. Criteria for risk of bias assessment
| Item | Judgment | Description |
| 1. Random sequence generation (selection bias) | Low risk | Investigators describe a random component in the sequence generation process such as random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation |
| High risk | Investigators describe a non‐random component in the sequence generation process such as odd or even date of birth; date (or day) of admission; hospital or clinic record number; alternation; judgement of the clinician; results of a laboratory test or a series of tests; availability of the intervention | |
| Unclear risk | Insufficient information about the sequence generation process to permit judgement of low or high risk | |
| 2. Allocation concealment (selection bias) | Low risk | Investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled, randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes |
| High risk | Investigators enrolling participants could possibly foresee assignments because one of the following methods was used: open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or were not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure | |
| Unclear risk | Insufficient information to permit judgement of low or high risk. This is usually the case if the method of concealment is not described or is not described in sufficient detail to allow a definitive judgement | |
| 3. Blinding of participants and providers (performance bias); objective outcomes |
Low risk | No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding Blinding of participants and key study personnel ensured, and unlikely that blinding could have been broken |
| High risk | No blinding or incomplete blinding, and outcome is likely to be influenced by lack of blinding Blinding of key study participants and personnel attempted, but likely that blinding could have been broken, and the outcome is likely to be influenced by lack of blinding |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk | |
| 4. Blinding of participants and providers (performance bias); subjective outcomes |
Low risk | Blinding of participants and providers and unlikely that blinding could have been broken |
| High risk | No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding Blinding of key study participants and personnel attempted, but likely that blinding could have been broken, and the outcome is likely to be influenced by lack of blinding |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk | |
| 5. Blinding of outcome assessor (detection bias); objective outcomes |
Low risk | No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding Blinding of outcome assessment ensured, and unlikely that blinding could have been broken |
| High risk | No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding Blinding of outcome assessment, but likely that blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk | |
| 6.Blinding of outcome assessor (detection bias); subjective outcomes |
Low risk | Blinding of outcome assessment ensured, and unlikely that blinding could have been broken |
| High risk | No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding Blinding of outcome assessment, but likely that blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk | |
| 7. Incomplete outcome data (attrition bias); for all outcomes except retention in treatment or dropout |
Low risk | No missing outcome data Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias) Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size Missing data have been imputed using appropriate methods All randomly assigned participants are reported/analysed in the group to which they were allocatedby randomisation, irrespective of non‐compliance and co‐interventions (intention to treat) |
| High risk | Reason for missing outcome data likely to be related to true outcome, with imbalance in numbers or reasons for missing data across intervention groups For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size ‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk (e.g. number randomised not stated, no reasons for missing data provided; number of dropouts not reported for each group) | |
| 8. Selective reporting (reporting bias) | Low risk | Study protocol is available, and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way Study protocol is not available, but it is clear that published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon) |
| High risk | Not all of the study’s prespecified primary outcomes have been reported One or more primary outcomes are reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not prespecified One or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect) One or more outcomes of interest in the review are reported incompletely, so that they cannot be entered in a meta‐analysis The study report fails to include results for a key outcome that would be expected to have been reported for such a study |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk |
Data and analyses
Comparison 1. Any anticonvulsant versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dropout | 20 | 1695 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.86, 1.05] |
| 2 Use of cocaine (self reported or objective) | 11 | 867 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.84, 1.02] |
| 3 Side effect | 8 | 775 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [1.01, 1.90] |
| 3.1 New subgroup | 8 | 775 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [1.01, 1.90] |
| 4 Craving | 8 | 428 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.59, 0.09] |
| 5 Severity of dependence (ASI) | 6 | 290 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.02, 0.08] |
| 6 Severity of dependence (CGI‐O) | 5 | 277 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.42, 0.20] |
| 7 Depression (HAM‐D) | 3 | 80 | Mean Difference (IV, Random, 95% CI) | 1.80 [‐0.59, 4.19] |
| 8 Anxiety (HAM‐A) | 3 | 78 | Mean Difference (IV, Random, 95% CI) | 1.79 [‐1.02, 4.60] |
| 9 Compliance | 6 | 343 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.93, 1.08] |
| 10 Compliance | 5 | 426 | Mean Difference (IV, Random, 95% CI) | 1.42 [‐4.80, 7.64] |
Comparison 2. Single anticonvulsant versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dropout | 19 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Carbamazepine vs placebo | 6 | 464 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.87, 1.13] |
| 1.2 Tiagabine vs placebo | 3 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.56, 1.82] |
| 1.3 Gabapentin vs placebo | 2 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 2.78 [0.67, 11.61] |
| 1.4 Phenytoin vs placebo | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.65, 1.35] |
| 1.5 Lamotrigine vs placebo | 1 | 23 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.11, 10.04] |
| 1.6 Topiramate vs placebo | 4 | 557 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.73, 1.16] |
| 1.7 Vigabatrin vs placebo | 2 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.53, 1.02] |
| 2 Use of cocaine (self reported or objective) | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Carbamazepine vs placebo | 4 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.70, 1.28] |
| 2.2 Tiagabine vs placebo | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.61, 1.30] |
| 2.3 Gabapentin vs placebo | 2 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.87, 1.31] |
| 2.4 Phenytoin vs placebo | 1 | 12 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.05, 2.37] |
| 2.5 Topiramate vs placebo | 2 | 210 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.48, 2.98] |
| 2.6 Vigabatrin vs placebo | 2 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.69, 1.13] |
| 3 Side effects | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Carbamazepine vs placebo | 2 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.52, 2.86] |
| 3.2 Tiagabine vs placebo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Gabapentin vs placebo | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 2.94 [0.62, 13.83] |
| 3.4 Phenytoin vs placebo | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.83, 3.29] |
| 3.5 Topiramate vs placebo | 2 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 2.42 [0.27, 21.87] |
| 3.6 Vigabatrin vs placebo | 1 | 186 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.88, 1.08] |
Comparison 3. Anticonvulsant (carbamazepine) vs antidepressant (desipramine).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dropout | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.86, 1.53] |
3.1. Analysis.
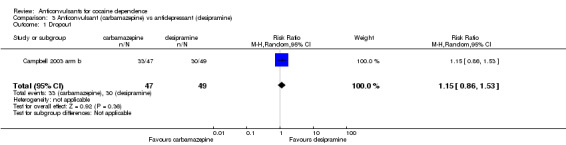
Comparison 3 Anticonvulsant (carbamazepine) vs antidepressant (desipramine), Outcome 1 Dropout.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Berger 2005 arm a.
| Methods | Randomised placebo‐controlled trial | |
| Participants | Participants: 60, mean age 39.1 years; male 70%; African American 92%, Caucasian 8%; married 23%, separated/divorced 31%, never married 45% Education: median 12.3 years Employment: full time 55%, part time 28%; student 8%, unemployed 8% Reporting cocaine use: 100% Use of cocaine in the past 30 days: 18.5 days Route of administration: smoked 93%, intravenous 5%, intranasal 2% Inclusion criteria: fulfilling DSM‐IV criteria for cocaine dependence Exclusion criteria: criteria utilised in CRES trial |
|
| Interventions | (1) lamotrigine 150 mg/d tapered, 15 participants; (2) placebo, 15 participants
For all adjunct cognitive‐behavioural therapy
Outpatient Duration: 10 weeks (2 of screening and 8 of intervention) Country of origin: USA |
|
| Outcomes | Dropout; Use of cocaine; Severity of dependence; Craving; Side effect; Depression; Anxiety; Compliance | |
| Notes | Funded by the National Institute on Drug Abuse (NIDA) under Interagency Agreement Y 01 DA
50038–00 Urinalyses were funded by NIDA contract N01DA‐7–8074 Conflict of interested: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were assigned randomly to a medication or placebo arm of the study at the end of the baseline period" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Participants were assigned randomly to a medication or placebo arm of the study at the end of the baseline period" |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Quote: "The medications used in this study were not blinded in terms of appearance; thus, the unblinded study pharmacist handled all medication manipulations, including weekly dispensing and pill counts" Comment: objective outcomes unlikely to be biased by lack of blinding |
| Blinding of participants and personnel (performance bias) subjective outcomes | High risk | Quote: "The medications used in this study were not blinded in terms of appearance; thus, the unblinded study pharmacist handled all medication manipulations, including weekly dispensing and pill counts" |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Information not reported Comment: objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | Information not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout: lamotrigine 20%; placebo 7%; P value ns Quote: "each participant’s end‐point is the last observation obtained regardless of the time‐point at which it was obtained. Thus, for a participant who dropped out after visit 1, his or her end‐point would be visit 1 and, thus, equivalent to the baseline observation. For a participant who completed the trial, his or her end‐point would be from study week 8" |
| Selective reporting (reporting bias) | Low risk | Published reports include all expected outcomes, including those that were prespecified |
Berger 2005 arm b.
| Methods | Randomised placebo‐controlled trial | |
| Participants | Participants: 60, mean age 39.1 years; male 70%; African American 92%, Caucasian 8%; married 23%, separated/divorced 31%, never married 45% Education: median 12.3 years Employment: full time 55%, part time 28%; student 8%, unemployed 8% Reporting cocaine use: 100% Use of cocaine in the past 30 days: 18.5 days Route of administration: smoked 93%, intravenous 5%, intranasal 2% Inclusion criteria: fulfilling DSM‐IV criteria for cocaine dependence Exclusion criteria: criteria utilised in CRES trial |
|
| Interventions | (1) gabapentin 1800 mg tapered, 15 participants; (2) placebo, 15 participants
For all adjunct cognitive‐behavioural therapy
Outpatient Duration: 10 weeks (2 of screening and 8 of intervention) Country of origin: USA |
|
| Outcomes | Dropout; Use of cocaine; Severity of dependence; Craving; Side effect; Depression; Anxiety; Compliance | |
| Notes | Funded by the National Institute on Drug Abuse (NIDA) under Interagency Agreement Y 01 DA
50038–00 Urinalyses were funded by NIDA contract N01DA‐7–8074 Conflict of interested: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were assigned randomly to a medication or placebo arm of the study at the end of the baseline period" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Participants were assigned randomly to a medication or placebo arm of the study at the end of the baseline period" |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Quote: "The medications used in this study were not blinded in terms of appearance; thus, the unblinded study pharmacist handled all medication manipulations, including weekly dispensing and pill counts Comment: objective outcomes unlikely to be biased by lack of blinding |
| Blinding of participants and personnel (performance bias) subjective outcomes | High risk | Quote: "The medications used in this study were not blinded in terms of appearance; thus, the unblinded study pharmacist handled all medication manipulations, including weekly dispensing and pill counts" |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Information not reported Comment: objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | Information not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout: gabapentin 13%; placebo 7%; P value ns Quote: "each participant’s end‐point is the last observation obtained regardless of the time‐point at which it was obtained. Thus, for a participant who dropped out after visit 1, his or her end‐point would be visit 1 and, thus, equivalent to the baseline observation. For a participant who completed the trial, his or her end‐point would be from study week 8" |
| Selective reporting (reporting bias) | Low risk | Published reports include all expected outcomes, including those that were prespecified |
Bisaga 2006.
| Methods | Randomised placebo‐controlled trial | |
| Participants | Participants: 99; mean age 39 years; male 88%; African American 46%, Caucasian 26%, Hispanic 20%; married/co‐habitant 30% Education: high school 26%, college 53% Employed: 87% Use of cocaine in the past 30 days: 14 days, average dollar amount per week spent on cocaine US$287 Reporting cocaine use: 100% Route of administration not reported Inclusion criteria: 18 to 60 years old, fulfilling DSM‐IV criteria for current cocaine dependence, used cocaine at least 4 days in the previous month Exclusion criteria: major affective or psychotic disorder, ADHD; physical dependence on opiates, sedative‐hypnotics or alcohol, or if the principal drug of dependence was not cocaine; ongoing treatment with psychotropic agents or other substance use treatment; unstable physical disorder, which might make participation hazardous; pregnancy or lactation |
|
| Interventions | (1) gabapentin 1600 mg maximum then tapered, 48 participants; (2) placebo, 47 participants
Outpatient Duration: 12 weeks of treatment followed by 2 weeks of placebo Country of origin: USA |
|
| Outcomes | Retention measured as mean week in treatment; Use of cocaine; Craving; Side effect; Compliance | |
| Notes | Supported by NIDA Center Grant DA09236 and Grants K23 DA00429 (Dr Bisaga), K23 DA16743 (Dr
Aharonovich), K02 DA00288 (Dr Nunes) and K02 DA00465 (Dr Levin) Conflict of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "A research pharmacist who was independent of the investigative team conducted randomization" |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Study declared as double‐blind Quote: "Gabapentin 400 mg and matching placebo capsules were provided by Parke‐Davis Pharmaceuticals Ltd. A matching capsule containing 50 mg of riboflavin (added as urine marker to assess compliance; see below) and lactose filler was manufactured by the research pharmacy of the New York State Psychiatric Institute" |
| Blinding of participants and personnel (performance bias) subjective outcomes | Low risk | Study declared as double‐blind Quote: "Gabapentin 400 mg and matching placebo capsules were provided by Parke‐Davis Pharmaceuticals Ltd. A matching capsule containing 50 mg of riboflavin (added as urine marker to assess compliance; see below) and lactose filler was manufactured by the research pharmacy of the New York State Psychiatric Institute" |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Information not reported Comment: objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | Information not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All analyses were performed on the intent‐to‐treat population" |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available, but published reports include all expected outcomes, including those that were prespecified in the Methods section |
Brodie 2009.
| Methods | Randomised placebo‐controlled trial | |
| Participants | Participants: 103 parolees of a Mexico City prison were recruited at parole centres and were active with cocaine and had a history of cocaine dependence. Mean age 29.1 years; male 95.5% Duration dependent on cocaine (years): 8.9; cocaine self reported use within the past 30 days: 100% Participants were reimbursed $7 (US dollars) per treatment visit plus an incentive payment of $25 (US dollars) upon treatment phase completion and inclusion in a drawing for 3 monetary prizes ($100, $50 or $25 (US dollars) Route of administration not reported Inclusion criteria: parolees; individuals 18 to 55 years old who were capable of giving informed consent, had DSM‐IV cocaine dependence and were urine positive for cocaine and negative for heroin and methamphetamine at screening Exclusion criteria: dependence on substances other than cocaine, alcohol, nicotine or marijuana; alcohol dependence requiring detoxification; prior cocaine use treatment; significant cocaine abstinence within 6 months; current court‐mandated cocaine use treatment; intravenous drug use within 2 months; recent medical study participation; or history of major medical, neurological or psychiatric disorders. Participants were also excluded for visual field defects or predisposing factors, including glaucoma, severe myopia, retinal disorder, cataracts, diabetes or uncontrolled hypertension. Participants were also excluded for a violent crime conviction or pending reincarceration or relocation |
|
| Interventions | (1) daily vigabatrin 250 mL, 50 participants; (2) placebo, 53 participants. For all participants, weekly individual cognitive‐behaviour therapy focused on supporting abstinence in accordance with routine clinic practice Outpatients Duration: 9‐week double‐blind trial and 4‐week follow‐up assessment Country of origin: Mexico |
|
| Outcomes | Cocaine abstinence, defined as twice‐weekly urine toxicology tests negative for cocaine (clean) during the last 3 weeks of the trial; self‐reported cocaine dose; cocaine craving; CGI Severity scores; CGI improvement scores at weeks 5 and 9; HAM‐A and HAM‐D scores; mood, anxiety and somatic symptoms; adverse events | |
| Notes | Supported in part by the Biochemical Psychiatry Fund of the New York University School of Medicine, an unrestricted grant from Catalyst Pharmaceutical Partners, Inc., the US Department of Energy Office of Biological Research (contract grant DE‐AC02‐98CH10886) and the National Institute on Drug Abuse Conflict of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "A research pharmacist dissolved each daily vigabatrin dose in 250 ml of orange juice according to a fixed titration. Placebo consisted of identical bottles of juice. The pharmacist maintained subject treatment assignments in a locked file that was inaccessible to study personnel" |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Study declared as double‐blind Quote: "Staff, blind to assignments, directly observed consumption of the dose for a particular day and distributed bottles for use until the next visit" |
| Blinding of participants and personnel (performance bias) subjective outcomes | Low risk | Study declared as double‐blind Quote: "Staff, blind to assignments, directly observed consumption of the dose for a particular day and distributed bottles for use until the next visit" |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Information not reported Comment: objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | Information not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis performed on the intention‐to‐treat principle |
| Selective reporting (reporting bias) | High risk | Results for cocaine craving, HAM‐A and HAM‐D scores, or CGI severity and CGI not reported. Study authors stated only that no differences were noted |
Brown 2012.
| Methods | Randomised placebo‐controlled trial | |
| Participants | Participants: 120 adult outpatients (results reported for only 112) with bipolar I, II NOS or cyclothymic disorders and current cocaine dependence; mean age 44 years; male 59.8%; Caucasian 30%, African American 35%, Hispanic 3% Education: 13.5 mean years Reporting cocaine use: 100% Route of administration: smoked 70%, intravenous 25.5%, intranasal 4.5% Inclusion criteria: men or women aged 18 to 70 years, diagnosis of bipolar I, II or NOS disorder, currently depressed or mixed mood as determined by SCID‐CV, current cocaine dependence with self reported cocaine use within 14 days before randomisation, English or Spanish speaking, baseline Hamilton Rating Scale for Depression 17‐item version (HRSD17) score ×10 Exclusion criteria: currently taking an enzyme‐inducing or ‐inhibiting anticonvulsant (e.g. valproic acid, carbamazepine), currently experiencing severe psychotic features (e.g. daily auditory hallucinations, fixed delusions, severely disorganised thought processes) that require antipsychotic therapy, and that do not appear to be secondary to cocaine use; active suicidal ideation (plan and intent) or ×2 attempts in past 12 months or any attempt in the past month, highly unstable medical condition, change in concomitant psychiatric medications (e.g. initiated antipsychotic) or in other substance abuse treatment (e.g. began intensive outpatient treatment) within 7 days before study entry, vulnerable populations (e.g. pregnant or nursing women, incarcerated or cognitively impaired individuals) |
|
| Interventions | (1) lamotrigine, dose not reported, patients: 55; (2) placebo, patients: 57 Outpatients Duration: 10 weeks. Country of origin: USA |
|
| Outcomes | Use of cocaine craving, amount spent on cocaine, mood symptoms (Hamilton, 1960), depressive symptoms‐SR (QIDS‐SR) (Rush et al, 2003), Young mania rating scale (YMRS) (Young et al, 1978) The psychobiology of recovery in depression IIIFsomatic symptom scale (PRD‐III), adverse events; compliance |
|
| Notes | Funded by the Stanley Medical Research Institute, grant number 05T‐704. Study drug was provided by GlaxoSmithKline. Neither organisation participated in the design, conduct or interpretation of the study Conflict of interest: Dr Brown would like to disclose funding from the following organisations: Stanley Medical Research Institute, Sunovion Pharmaceuticals, Forest Research Institute, GlaxoSmithKline and AstraZeneca Dr Sunderajan would like to disclose funding from the following organisations: Bristol‐Myers Squibb, Lilly USA, LLC and Takeda Pharmaceuticals North America Dr Carmody would like to disclose the following organisations from which he received consulting fees: Cyberonics and the Institute for Chronic Illness Ms Hu and Ms Sowell declare no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was conducted by the study statistician (TJC) through a computerized randomization process, which was downloaded to a spread‐sheet used by unblinded clinic staff to allocate medication" |
| Allocation concealment (selection bias) | High risk | Quote: "Randomization was conducted by the study statistician (TJC) through a computerized randomization process, which was downloaded to a spread‐sheet used by unblinded clinic staff to allocate medication" |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Study declared as double‐blind Quote: "All direct care staff (i.e. study physicians and raters) were blinded" |
| Blinding of participants and personnel (performance bias) subjective outcomes | Low risk | Study declared as double‐blind Quote: "All direct care staff (i.e. study physicians and raters) were blinded" |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Study declared as double‐blind Quote: "All direct care staff (i.e. study physicians and raters) were blinded" |
| Blinding of outcome assessment (detection bias) subjective outcomes | Low risk | Study declared as double‐blind Quote: "All direct care staff (i.e. study physicians and raters) were blinded" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "The number of subjects available for analysis was 112 (those with at least one post baseline assessment)" Comment: 6% lost at follow‐up; not specified from which groups; reason not given |
| Selective reporting (reporting bias) | High risk | Retention in treatment, which is one of the most relevant outcomes in the field of addiction, not reported |
Campbell 1994 arm a.
| Methods | Randomised placebo‐controlled trial | |
| Participants | Participants: 65, cocaine dependents (DSM‐III‐R); mean age 32 years (range 20 to 60); 63% male; 90% black; 16 also had current diagnosis of alcohol dependence, 11 major depression, 2 generalized anxiety disorder and 16 antisocial personality disorder Reporting cocaine use: 100% Route of administration not reported Inclusion criteria: cocaine dependence according to DSM‐III criteria Exclusion criteria: patients with psychosis or major medical disorders |
|
| Interventions | (1) carbamazepine (n = 19), dose unknown;
(2) placebo (n = 25)
Outpatient Duration: 6 months Country of origin: USA |
|
| Outcomes | Dropout; Use of cocaine | |
| Notes | Source of funding and conflict of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "subjects were randomly assigned to desipramine, carbamazepine or placebo according to a computer generated list of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Information not reported |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Study declared as double‐blind Quote: "the study physician was blind to medication status" |
| Blinding of participants and personnel (performance bias) subjective outcomes | Low risk | Study declared as double‐blind Quote: "the study physician was blind to medication status" |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Study declared as double‐blind Quote: "the study physician was blind to medication status" |
| Blinding of outcome assessment (detection bias) subjective outcomes | Low risk | Study declared as double‐blind Quote: "the study physician was blind to medication status" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals from the study |
| Selective reporting (reporting bias) | High risk | Study protocol is not available. Study report declares no significant differences in retention in treatment but does not report data |
Campbell 1994 arm b.
| Methods | Randomised placebo‐controlled trial | |
| Participants | Participants: 65, cocaine dependents (DSM‐III‐R); mean age 32 years (range 20 to 60); 63% male; 90% black; 16 participants also had current diagnosis of alcohol dependence, 11 major depression, 2 generalised anxiety disorder and 16 antisocial personality disorder Reporting cocaine use: 100% Route of administration not reported Inclusion criteria: cocaine dependence according to DSM‐III criteria Exclusion criteria: patients with psychosis or major medical disorders |
|
| Interventions | (1) carbamazepine (n = 19), dose unknown;
(2) desipramine (n = 21), dose unknown
Outpatient Duration: 6 months Country of origin: USA |
|
| Outcomes | Dropout | |
| Notes | Source of funding and conflict of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "subjects were randomly assigned to desipramine, carbamazepine or placebo according to a computer generated list of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Information not reported |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Study declared as double‐blind Quote: "the study physician was blind to medication status" |
| Blinding of participants and personnel (performance bias) subjective outcomes | Low risk | Study declared as double‐blind Quote: "the study physician was blind to medication status" |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Study declared as double‐blind Quote: "the study physician was blind to medication status" |
| Blinding of outcome assessment (detection bias) subjective outcomes | Low risk | Study declared as double‐blind Quote: "the study physician was blind to medication status" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals from the study |
| Selective reporting (reporting bias) | High risk | Study protocol is not available. Study report declares no significant differences in retention in treatment but does not report data |
Campbell 2003 arm a.
| Methods | Randomised placebo‐controlled trial Allocation concealment: unclear Double‐blind Blinding of outcome assessor: unclear | |
| Participants | Participants: 146; mean age 33.4 years, male 69,6%, 16,33% white, 82,3% black; probation/parole 51.3%. Education: < 11: 36%, high school 39%, > 13: 21.3% Patients with depression 30%, antisocial PD 39%, anxiety disorders 6.3% Reporting cocaine use: 100% Route of administration not reported Inclusion criteria: not reported Exclusion criteria: psychosis, organic brain syndromes, suicidal or homicidal ideation, unstable medical disorders |
|
| Interventions | (1) carbamazepine: 47 participants; 200 mg up to 800 mg;
(2) placebo: 50 participants
Outpatients Duration of follow‐up: 8 weeks Country of origin: USA |
|
| Outcomes | Dropout; Use of cocaine; Side effect; Compliance | |
| Notes | Supported by grant R18DA‐06954 from the National Institute on Drug Abuse, Bethesda, Maryland (Dr Campbell) Ciba‐Geigy (now Novartis) and Marion Merrill Dow (now Aventis) pharmaceutical companies provided medication and matching placebo capsules | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not reported |
| Allocation concealment (selection bias) | Unclear risk | Information not reported |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Study declared as double‐blind Quote: "Serum concentration of medication was monitored by an unblinded physician who had no contact with subjects. If plasma concentration of desipramine was >300 ng/ml or carbamazepine was >10mcg/ml, the study physician was instructed to change the dose for both an active medication subject and a placebo subject in order to maintain the blind" |
| Blinding of participants and personnel (performance bias) subjective outcomes | Low risk | Study declared as double‐blind Quote: "Serum concentration of medication was monitored by an unblinded physician who had no contact with subjects. If plasma concentration of desipramine was >300 ng/ml or carbamazepine was >10mcg/ml, the study physician was instructed to change the dose for both an active medication subject and a placebo subject in order to maintain the blind" |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Study declared as double‐blind Quote: "patient ratings, urine drug screen, and blood samples were obtained and medication side effects were evaluated by the study physician, who was blind to treatment condition" |
| Blinding of outcome assessment (detection bias) subjective outcomes | Low risk | Study declared as double‐blind Quote: "patient ratings, urine drug screen, and blood samples were obtained and medication side effects were evaluated by the study physician, who was blind to treatment condition" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "For each outcome, two stages of analyses were conducted: one with groups defined by randomisation (the intent to treat group), and a secondary analysis with groups based on treatment as received" |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified in the Methods section |
Campbell 2003 arm b.
| Methods | Randomised placebo‐controlled trial | |
| Participants | Participants: 146; mean age 33.4 years; male 69.6%; 16.33% white, 82.3% black; probation/parole: 51.3% Education: < 11: 36%, 3, high school: 39%, > 13: 21.3% Patients with depression 30%, antisocial PD 39%, anxiety disorders 6.3% Reporting cocaine use: 100% Route of administration not reported Inclusion criteria: not reported Exclusion criteria: psychosis, organic brain syndromes, suicidal or homicidal ideation, unstable medical disorders |
|
| Interventions | (1) desipramine: 49 participants; 50 mg up to 200 mg;
(2) carbamazepine: 47 participants; 200 mg tapered up to 800 mg
Outpatients Duration of follow‐up: 8 weeks Country of origin: USA |
|
| Outcomes | Dropout; Use of cocaine; Side effect; Compliance | |
| Notes | Supported by grant R18DA‐06954 from the National Institute on Drug Abuse, Bethesda, Maryland (Dr Campbell) Ciba‐Geigy (now Novartis) and Marion Merrill Dow (now Aventis) pharmaceutical companies provided medication and matching placebo capsules | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not reported |
| Allocation concealment (selection bias) | Unclear risk | Information not reported |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Study declared as double‐blind Quote: "Serum concentration of medication was monitored by an unblinded physician who had no contact with subjects. If plasma concentration of desipramine was >300 ng/ml or carbamazepine was >10mcg/ml, the study physician was instructed to change the dose for both an active medication subject and a placebo subject in order to maintain the blind" |
| Blinding of participants and personnel (performance bias) subjective outcomes | Low risk | Study declared as double‐blind Quote: "Serum concentration of medication was monitored by an unblinded physician who had no contact with subjects. If plasma concentration of desipramine was >300 ng/ml or carbamazepine was >10mcg/ml, the study physician was instructed to change the dose for both an active medication subject and a placebo subject in order to maintain the blind" |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Study declared as double‐blind Patient ratings, urine drug screen and blood samples were obtained and medication side effects were evaluated by the study physician, who was blind to treatment condition |
| Blinding of outcome assessment (detection bias) subjective outcomes | Low risk | Study declared as double‐blind Quote: "patient ratings, urine drug screen, and blood samples were obtained and medication side effects were evaluated by the study physician, who was blind to treatment condition" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "For each outcome, two stages of analyses were conducted: one with groups defined by randomization (the intent to treat group), and a secondary analysis with groups based on treatment as received" |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified in the Methods section |
Cornish 1995.
| Methods | Randomised placebo‐controlled trial | |
| Participants | Participants: 95, cocaine dependents (DSM‐III‐R); age range 21 to 51 years; 98% male; 98% black Reporting cocaine use: 100% Route of administration not reported Inclusion criteria: between 21 and 51 years of age, diagnosis of cocaine dependence according to DSM‐III‐R Participants may have had a concurrent diagnosis of alcohol dependence (DSM‐III‐R) as long as they had been detoxified from alcohol and were alcohol‐free for a period of 7 days before the study Exclusion criteria: medical or psychiatric condition (such as anaemia, bipolar disorder, schizophrenia) existed that would contraindicate the administration of carbamazepine or result in confounding data |
|
| Interventions | (1) carbamazepine (n = 37); dose started at 200 mg/d, increased to reach serium levels of 4 to 12 μg/mL; (2) placebo (n = 45) Setting: outpatient Follow‐up: 10 weeks Country of origin: USA |
|
| Outcomes | Dropout; Use of cocaine; Craving; Side effect | |
| Notes | Supported by NIDA Grant DA 00144 and DA 05186, and the DVA Medical Research Service Ciba‐Geigy Corporation (Summit, NJ 07901) provided carbamazepine and matching placebo as study medication Conflict of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information not reported |
| Allocation concealment (selection bias) | Low risk | Information not reported |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Study declared as double‐blind Quote: "Subjects were randomly assigned to receive either carbamazepine (CBZ), 200 mg, or ‘identically appearing' placebo tablets throughout the study. One of the authors (Iraj Maany) was an unblinded physician who reviewed carbamazepine serum levels to ensure that subjects were maintained within a constant therapeutic range of 4‐12 ml. He also adjusted placebo medication (using a method of yoked controls) in such a manner that blinded co‐investigators would not be able to identify subjects receiving active medication based upon dosage adjustments" |
| Blinding of participants and personnel (performance bias) subjective outcomes | Low risk | Study declared as double‐blind Quote: "Subjects were randomly assigned to receive either carbamazepine (CBZ), 200 mg, or ‘identically appearing' placebo tablets throughout the study. One of the authors (Iraj Maany) was an unblinded physician who reviewed carbamazepine serum levels to ensure that subjects were maintained within a constant therapeutic range of 4‐12 ml. He also adjusted placebo medication (using a method of yoked controls) in such a manner that blinded co‐investigators would not be able to identify subjects receiving active medication based upon dosage adjustments" |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Study declared as double‐blind Quote: "Subjects were randomly assigned to receive either carbamazepine (CBZ), 200 mg, or identically appearing' placebo tablets throughout the study. One of the authors (Iraj Maany) was an unblinded physician who reviewed carbamazepine serum levels to ensure that subjects were maintained within a constant therapeutic range of 4‐12 ml. He also adjusted placebo medication (using a method of yoked controls) in such a manner that blinded co‐investigators would not be able to identify subjects receiving active medication based upon dosage adjustments" |
| Blinding of outcome assessment (detection bias) subjective outcomes | Low risk | Study declared as double‐blind Quote: "Subjects were randomly assigned to receive either carbamazepine (CBZ), 200 mg, or ‘identically appearing' placebo tablets throughout the study. One of the authors (Iraj Maany) was an unblinded physician who reviewed carbamazepine serum levels to ensure that subjects were maintained within a constant therapeutic range of 4‐12 ml. He also adjusted placebo medication (using a method of yoked controls) in such a manner that blinded co‐investigators would not be able to identify subjects receiving active medication based upon dosage adjustments" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Forty‐six percent of carbamazepine‐treated and 33% of placebo‐treated subjects remained in treatment through day 70 of the protocol" Comment: high percentage of dropout unbalanced between groups |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified in the Methods section |
Crosby 1996.
| Methods | Randomised placebo‐controlled trial | |
| Participants | Participants: 44; mean age 34 years; male 79.5%; African American 57%; married 34% Education: less than high school 14%, high school 70%, college 15%: current employed 59% Reporting cocaine use: 100% Route of cocaine ingestion: intranasal 4.5%, smoking 93%, intravenous 2%; cocaine use in the past 30 days mean 11.7 days; number of previous treatments mean 1.5; lifetime cocaine abuse mean 90 months; alcohol use to intoxication in the past 30 days: 3.0 days Inclusion criteria: 18 years old, fulfilling DSM‐IV criteria for current cocaine abuse/dependence Exclusion criteria: current clinical unstable medical illness or history of seizure disorder, history of head trauma resulting in brain injury or history of heart block or other cardiac disorder; lifetime psychiatric diagnosis of mental retardation precluding the ability to read, understand and complete written tests; organic brain syndrome with cognitive impairment; schizophrenia, bipolar affective disorder, schizoaffective disorder, suicidal risk, pregnancy, use of any psychiatric agent or mood‐altering medication in the previous 6 weeks |
|
| Interventions | (1) phenytoin 100 mg/d, 20 participants; (2) placebo, 24 participants
Outpatient Duration: 12 weeks Country of origin: USA |
|
| Outcomes | Dropout; Use of cocaine; Craving; Side effect; Compliance | |
| Notes | Source of funding and conflict of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not reported |
| Allocation concealment (selection bias) | Unclear risk | Information not reported |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Study declared as double‐blind Quote: "subjects were seen weekly by a trained psychotherapist blinded to treatment condition" |
| Blinding of participants and personnel (performance bias) subjective outcomes | Low risk | Study declared as double‐blind Quote: "subjects were seen weekly by a trained psychotherapist blinded to treatment condition" |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Study declared as double‐blind Quote: "all clinical rating were made by trained chemical dependency counsellors who were blinded to treatment condition" |
| Blinding of outcome assessment (detection bias) subjective outcomes | Low risk | Study declared as double‐blind Quote: "all clinical rating were made by trained chemical dependency counsellors who were blinded to treatment condition" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "16 subjects, 9 receiving phenytoin (31%) and 7 receiving placebo (23%) failed to return to visit 1 and were eliminated from the analysis. 12 subjects completed the twelve weeks protocol, 6 receiving phenytoin (20.7%) and 6 (19.4%) receiving placebo" |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified in the Methods section |
Gonzalez 2007 arm a.
| Methods | Randomised placebo‐controlled trial | |
| Participants | Participants: 76; mean age 37 years; male 76%; Caucasian 70%; unemployed 54% Reporting cocaine use: 100% Route of administration: smoked 73% Mean days of cocaine use during the past 30 days: 16 Inclusion criteria: fulfilling DSM‐IV criteria for current opioid dependence and weekly use of cocaine within a month before study entry Exclusion criteria: major cardiovascular, renal, endocrine or hepatic disorder; history of psychotic disorder or schizophrenia, or current suicidal ideation; pregnant or breast feeding women |
|
| Interventions | (1) tiagabine 24 mg/d then tapered, 25 participants; (2) placebo, 25 participants
Outpatient Duration: 10 weeks Country of origin: USA |
|
| Outcomes | Dropout; Use of cocaine; Compliance | |
| Notes | Supported by the National Institute on Drug Abuse grants K23DA14331 (GG), K05DA00454 (TRK), R01‐DA05626, P50‐DA12762 and the Veterans Administration Mental Illness Research, Education and Clinical Center (MIRECC) Conflict of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "Only the data manager who did not have direct contact with research subjects during their assessments and the pharmacist preparing the study medication were aware of the allocation, and the principal investigator kept the medication assignment code in a sealed envelope for access in case of medical emergency" |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Study declared as double‐blind Quote: "Tiagabine (Gabitril), gabapentin (Neurontin) and placebo were placed in identical capsules" |
| Blinding of participants and personnel (performance bias) subjective outcomes | Low risk | Study declared as double‐blind Quote: "Tiagabine (Gabitril), gabapentin (Neurontin) and placebo were placed in identical capsules" |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Information not reported Comment: objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | Information not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "We conducted the primary analyses on the total intent‐to‐treat sample (N = 76)" |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified in the Methods section |
Gonzalez 2007 arm b.
| Methods | Randomised placebo‐controlled trial | |
| Participants | Participants: 76; mean age 37 years; male 76%; Caucasian 70%; unemployed 54% Reporting cocaine use: 100% Route of administration: smoked 73% Mean days of cocaine use during the past 30 days: 16 Inclusion criteria: fulfilling DSM‐IV criteria for current opioid dependence and weekly use of cocaine within a month before study entry Exclusion criteria: major cardiovascular, renal, endocrine or hepatic disorder; history of psychotic disorder or schizophrenia, or current suicidal ideation; pregnant or breast feeding women |
|
| Interventions | (1) gabapentin 2400 mg/d then tapered, 26 participants; (2) placebo, 25 participants
Outpatient Duration: 10 weeks Country of origin: USA |
|
| Outcomes | Dropout; Use of cocaine; Compliance | |
| Notes | Supported by the National Institute on Drug Abuse grants K23DA14331 (GG), K05DA00454 (TRK), R01‐DA05626, P50‐DA12762 and the Veterans Administration Mental Illness Research, Education and Clinical Center (MIRECC) Conflict of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "Only the data manager who did not have direct contact with research subjects during their assessments and the pharmacist preparing the study medication were aware of the allocation, and the principal investigator kept the medication assignment code in a sealed envelope for access in case of medical emergency" |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Study declared as double‐blind Quote: "Tiagabine (Gabitril), gabapentin (Neurontin) and placebo were placed in identical capsules" |
| Blinding of participants and personnel (performance bias) subjective outcomes | Low risk | Study declared as double‐blind Quote: "Tiagabine (Gabitril), gabapentin (Neurontin) and placebo were placed in identical capsules" |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Information not reported Comment: objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | Information not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "We conducted the primary analyses on the total intent‐to‐treat sample (N = 76)" |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified in the Methods section |
Halikas 1997 arm a.
| Methods | Randomised placebo‐controlled trial | |
| Participants | Participants: 183, cocaine dependents (DSM‐III‐R); mean age 32.5 years; 71% male; 66.1% white; participants had at least an eighth grade education Reporting cocaine use: 100% Route of administration not reported Inclusion criteria: at least 18 years old and eighth grade education, met DSM‐III‐R criteria for cocaine dependence, reported cocaine use for at least 10 of the previous 25 days Exclusion criteria: suicidal ideation, clinically unstable clinical condition, narrow angle glaucoma, seizure disorder, head trauma, blood dyscrasia, heart block, organic brain syndrome, bipolar affective disorder, schizoaffective illness, lactating or pregnant women, patients taking any psychotropic medications |
|
| Interventions | (1) carbamazepine 400 mg (n = 62); (2) placebo (n = 63) A range of psychosocial interventions was offered to participants including group educational, cognitive, behavioural and supportive approaches Setting: outpatient Duration: 12 weeks Country of origin: USA | |
| Outcomes | Dropout; Use of cocaine; Craving; Side effect; Compliance | |
| Notes | Source of funding and conflict of interest: not reported; placebo tablets were provided by Ciba Geigy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "subjects were assigned to one of the three groups by means of randomized block design" |
| Allocation concealment (selection bias) | Unclear risk | Information not reported |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Study declared as double‐blind Quote: "carbamazepine tablets were used; identical placebo tablets were provided by Ciba Geigy; double blind medications were packaged by the unblinded pharmacist into ten days blister packs" |
| Blinding of participants and personnel (performance bias) subjective outcomes | Low risk | Study declared as double‐blind Quote: "carbamazepine tablets were used; identical placebo tablets were provided by Ciba Geigy; double blind medications were packaged by the unblinded pharmacist into ten days blister packs" |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Information not reported Comment: objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | Information not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 18% of participants failed to return at visit 1: 11 from the placebo group, 9 from the 400‐mg condition, 13 from the 800‐mg condition Evaluable participants: 150 out of 183 randomly assigned |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified in the Methods section |
Halikas 1997 arm b.
| Methods | Randomised placebo‐controlled trial | |
| Participants | Participants: 183, cocaine dependents (DSM‐III‐R); mean age 32.5 years; 71% male; 66.1% white; participants had at least an eighth grade education Reporting cocaine use: 100% Route of administration not reported Inclusion criteria: at least 18 years old and eighth grade education, met DSM‐III‐R criteria for cocaine dependence, reported cocaine use for at least 10 of the previous 25 days Exclusion criteria: suicidal ideation, clinically unstable clinical condition, narrow angle glaucoma, seizure disorder, head trauma, blood dyscrasia, heart block, organic brain syndrome, bipolar affective disorder, schizoaffective illness, lactating or pregnant women, patients taking any psychotropic medications |
|
| Interventions | (1) carbamazepine 800 mg (n = 58) (2) placebo (n = 63) Range of psychosocial interventions was offered to participants including group educational, cognitive, behavioural and supportive approaches Setting: outpatient Duration: 12 weeks Country of origin: USA | |
| Outcomes | Dropout; Use of cocaine; Craving; Side effect; Compliance | |
| Notes | Source of funding and conflict of interest: not reported; placebo tablets were provided by Ciba Geigy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "subjects were assigned to one of the three groups by means of randomized block design" |
| Allocation concealment (selection bias) | Unclear risk | Information not reported |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Study declared as double‐blind Quote: "carbamazepine tablets were used; identical placebo tablets were provided by Ciba Geigy; double blind medications were packaged by the unblinded pharmacist into ten days blister packs" |
| Blinding of participants and personnel (performance bias) subjective outcomes | Low risk | Study declared as double‐blind Quote: "carbamazepine tablets were used; identical placebo tablets were provided by Ciba Geigy; double blind medications were packaged by the unblinded pharmacist into ten days blister packs" |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Information not reported Comment: objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | Information not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 18% of participants failed to return at visit 1: 11 from the placebo group, 9 from the 400‐mg condition, 13 from the 800‐mg condition Evaluable participants: 150 out of 183 randomly assigned |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified in the Methods section |
Johnson 2013.
| Methods | Randomised placebo‐controlled trial | |
| Participants | Participants:142 cocaine‐dependent individuals diagnosed according to DSM‐IV; mean age 43.7 years; male 72.5%; white 28.9%, black 70%; full‐time employment: 60.6%; married 54.9% Reporting cocaine use: 100% Route of administration: smoking 86%, nasal 14.8%, oral 1.4%, injection 0 Inclusion criteria: recent history of cocaine use (1 or more, cocaine‐positive urine specimens (> 300 ng/mL) during screening and 4 or more urine specimens during the 2‐week baseline screening period. Eligibility criteria based on diagnosis and health checks. Alcohol‐dependent individuals were included Exclusion criteria: significant withdrawal symptoms that required medical detoxification |
|
| Interventions | (1) topiramate from 50 mg/d and escalated during the first 6 weeks until the ceiling dose of 300 mg/d or the participant’s maximum tolerated dose was achieved. During weeks 6 to 12, the maximum achieved dose of topiramate or matching placebo was maintained. If, however, a participant was intolerant of adverse events, the investigator could reduce the daily dose to obtain a minimum topiramate or matching placebo maintenance dose of 200 mg/d. Participants: 71 (2) placebo; participants: 71 All participants received, as an adjunct to the medication, weekly cognitive‐behavioural treatment, a manual‐driven, psychosocial treatment Duration: 12 weeks Settings: outpatient Country of origin: USA |
|
| Outcomes | Dropout; Cocaine use; Compliance | |
| Notes | Supported by grant 5 R01 DA017296‐04 from the National Institute on Drug Abuse (Dr Johnson), grant 5 RC1 AA019274‐02 from the National Institute on Alcohol Abuse and Alcoholism and grant 7 R01 HS020263‐02 from the Agency for Healthcare Research and Quality (Dr Liu) Conflict of interest: Dr Johnson reported serving as a consultant for Johnson & Johnson (Ortho‐McNeil Janssen Scientific Affairs, LLC) from 2003‐2008, Transcept Pharmaceuticals, Inc from 2006‐2009, Eli Lilly and Company from 2009‐2010 and Organon from 2007‐2010; he currently consults for D&A Pharma, ADial Pharmaceuticals, LLC (with which he also serves as chairman) and Psychological Education Publishing Company (PEPCo), LLC. Dr Liu reported serving as a consultant for Celladon Corporation. No other disclosures were reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Study medication was randomized in a 1:1 ratio of daily oral topiramate or matched placebo. Randomization was stratified to balance participants between groups on age, sex, and frequency of cocaine use (>18 vs 18 daysof use in the past 30 days according to self‐report, urine sample, or both) before randomization" |
| Allocation concealment (selection bias) | Unclear risk | Information not reported |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Study declared as double‐blind Quote: "After randomization, double blind treatment medication was provided twice daily (ie, morning and night) for 12 weeks (ie, weeks 1‐12) using a double‐dummy procedure that ensured that placebo and topiramate recipients received the same number of capsules" |
| Blinding of participants and personnel (performance bias) subjective outcomes | Low risk | Study declared as double‐blind Quote: "After randomization, double blind treatment medication was provided twice daily (ie, morning and night) for 12 weeks (ie, weeks 1‐12) using a double‐dummy procedure that ensured that placebo and topiramate recipients received the same number of capsules" |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Information not reported Comment: objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | Information not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis performed on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified in the Methods section |
Kampman 2004.
| Methods | Randomised placebo‐controlled trial | |
| Participants | Participants: 40; mean age 40 years; male 97.5%; African American 90%, Caucasian 10%; mean years of education 12 years; use of cocaine in the previous month: mean 7 days Reporting cocaine use: 100% Route of cocaine ingestion: intranasal 12.5%, smoked 87.5% Inclusion criteria: age 18 to 60 years, fulfilling DSM‐IV criteria for cocaine dependence Exclusion criteria: current dependence (DSM‐IV) on any additional drug except nicotine; psychosis, dementia, use of psychotropic medication; unstable medical illness, use of antiepileptic medication, history of nephrolithiasis, history of glaucoma, hypersensitivity to topiramate |
|
| Interventions | (1) topiramate starting dose 25 mg/d increased to 200 mg/d then tapered, 20 participants; (2) placebo, 20 participants Outpatient Duration: 13 weeks Country of origin: USA |
|
| Outcomes | Dropout; Use of cocaine; Severity of dependence; Craving; Side effect; Compliance | |
| Notes | Study funded by NIDA grant DA12756 Conflict of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not reported |
| Allocation concealment (selection bias) | Unclear risk | Information not reported |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Study declared as double‐blind Quote: "Study medication was prepared by the Research Pharmacist at the Hospital of the University of Pennsylvania by over‐encapsulating topiramate 25 and 100 mg tablets, purchased from a commercial pharmacy, and producing identical‐appearing lactose‐containing placebo capsules, Study medication was placed in blister packs with each day’s dose clearly marked. Medications were dispensed by the study physician each week and the previous week’s blister pack was collected" |
| Blinding of participants and personnel (performance bias) subjective outcomes | Low risk | Study declared as double‐blind Quote: "Study medication was prepared by the Research Pharmacist at the Hospital of the University of Pennsylvania by over‐encapsulating topiramate 25 and 100 mg tablets, purchased from a commercial pharmacy, and producing identical‐appearing lactose‐containing placebo capsules, Study medication was placed in blister packs with each day’s dose clearly marked. Medications were dispensed by the study physician each week and the previous week’s blister pack was collected" |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Information not reported Comment: objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | Information not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis performed on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified in the Methods section |
Kampman 2013.
| Methods | Randomised placebo‐controlled trial | |
| Participants | Participants: 170 DSM‐IV cocaine dependent; mean age 44 years; male 79%. African American 83%, Caucasian 17% Education: 12.7 years (mean) Reporting cocaine use: 100% Route of cocaine used: smoked 78%, intranasal 21%: years of cocaine use: 14 (mean) Inclusion criteria: In the 30 days before study entry, participants used no less than $200‐worth of cocaine and met the following drinking criteria as measured by the Timeline Followback (TLFB; Sobelland Sobell, 1995): (1) drank within 30 days of intake day, (2) reported a minimum of 48 standard alcoholic drinks (avg 12 drinks/wk) for women and 60 standard drinks (15 drinks/wk) for men in a consecutive 30‐day period over the 90‐day period before starting intake, and (3) had 2 or more days of heavy drinking (defined as 5 or more drinks/d in males and 4 or more drinks/d in females) in this same pretreatment period Exclusion criteria: Patients with current dependence (DSM‐IV criteria) on any additional drug except nicotine and cannabis were excluded. Psychiatric exclusion criteria included psychosis, dementia and use of other psychotropic medications. Medical exclusion criteria included unstable medical illness, impaired renal function and a history of hypersensitivity to topiramate. Patients with a history of kidney stones and those taking carbonic anhydrase inhibitors or any other antiepileptic drugs were excluded from the study |
|
| Interventions | (1) topiramate titrated to 300 mg daily; participants 83; (2) placebo, participants 87 In addition to medication or placebo, participants received weekly individual cognitive‐behavioural relapse prevention therapy utilising a Cognitive‐Behavioural Coping Skills Therapy (CBT) manual Setting: outpatient Follow‐up: 13 weeks Country of origin: USA |
|
| Outcomes | Alcohol and cocaine use; Treatment retention; Severity of addiction‐related problems measured by the Addiction Severity Index (ASI); Minnesota Cocaine Craving Scale (MCCS) for cocaine craving intensity (MCCS‐I), cocaine craving frequency (MCCS‐F) and cocaine craving duration (MCCS‐D); Adverse events; Compliance | |
| Notes | The National Institute on Drug Abuse (NIDA) provided funding for this trial through the following grants: P60‐DA‐05186‐17, P50DA012756 and T32 MH065218. NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper Conflict of interest: All study authors declare they have no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not reported |
| Allocation concealment (selection bias) | Unclear risk | Information not reported |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Study declared double‐blind Quote: "The Investigational Drug Service of the University of Pennsylvania prepared study medication by over encapsulating topiramate tablets and preparing identical appearing placebo capsules" |
| Blinding of participants and personnel (performance bias) subjective outcomes | Low risk | Study declared double‐blind Quote: "The Investigational Drug Service of the University of Pennsylvania prepared study medication by over encapsulating topiramate tablets and preparing identical appearing placebo capsules" |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Information not reported Comment: objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | Information not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis performed on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all but 1 expected outcomes, including those that were prespecified in the Methods section. Results for cocaine craving not reported. Study authors only stated that they noted no differences |
Kranzler 1995.
| Methods | Randomised placebo‐controlled trial | |
| Participants | Participants: 40, cocaine dependents (DSM‐III‐R); age range 18 to 45 years; 100% male; 32% black Reporting cocaine use: 100% Use of cocaine: at least 4 g of cocaine during the preceding month Route of administration: smoked 75%, intranasal 25% Inclusion criteria: men, aged 18 to 45 years, reading comprehension skills adequate for providing written informed consent and for completing study questionnaires, met DSM‐III‐R criteria for current cocaine dependence, having used at least 4 g of cocaine during the preceding month Exclusion criteria: met DSM‐III‐R criteria for current dependence on any drug other than cocaine and nicotine, major medical (hematological, neurological, renal, cardiovascular or hepatic) disorder, had an unstable psychiatric condition (e.g. schizophrenic, acutely suicidal). Recent use of prescription or over‐the‐counter psychoactive medications other than cocaine, a history of seizures (including drug‐related seizures) or a history of serious head injury (i.e. resulting in loss of consciousness for longer than 30 minutes), absence of a stable living situation |
|
| Interventions | (1) carbamazepine (20 participants): 200 mg/d up to 600 mg/d
(2) placebo (20 participants)
Setting: outpatient Follow‐up: 12 weeks of treatment; 3 months after treatment Country of origin: USA |
|
| Outcomes | Retention; Use of cocaine; Use of other substances; Side effect | |
| Notes | Study supported by grants PSO‐DA04060 from the National Institute on Drug Abuse and K20‐
AA00143 (to Henry R. Kranzler) from the National Institute on Alcohol Abuse and Alcoholism. Carbamazepine
and matching placebo were generously donated by Ciba‐Geigy Pharmaceuticals Conflict of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "Subjects were randomly assigned to treatment condition by a research pharmacist, who also was not involved in the clinical care of the subjects" |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Study declared as double‐blind Quote: "Medication was contained in identical opaque capsules containing carbamazepine 100 mg or matching placebo" |
| Blinding of participants and personnel (performance bias) subjective outcomes | Low risk | Study declared as double‐blind Quote: "Medication was contained in identical opaque capsules containing carbamazepine 100 mg or matching placebo" |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Study declared as double‐blind Quote: "All assessments were conducted by a research evaluator who was blind to the treatment condition and had no other contact with the subjects. A psychiatrist monitored plasma levels of carbamazepine and clinical laboratory results to protect against adverse effects. This psychiatrist had no direct contact with subjects once they were randomized" |
| Blinding of outcome assessment (detection bias) subjective outcomes | Low risk | Study declared as double‐blind Quote: "All assessments were conducted by a research evaluator who was blind to the treatment condition and had no other contact with the subjects. A psychiatrist monitored plasma levels of carbamazepine and clinical laboratory results to protect against adverse effects. This psychiatrist had no direct contact with subjects once they were randomized" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported how many participants' results were included. Use of cocaine and of other substances was computed |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified in the Methods section |
Montoya 1994.
| Methods | Randomised placebo‐controlled trial | |
| Participants | Participants: 72 randomly assigned; 62 included in the analysis; mean age 33.2 years; 79% male; 68% black. Use of cocaine: at least 14 g of self reported cocaine use in the prior 3 months Route of administration: not reported Inclusion criteria: diagnosis: cocaine dependence (DSM‐lll‐R) Exclusion criteria: concurrent dependence on other drugs, concurrent institutional residence, illiteracy, history of seizure disorders, glaucoma, renal failure, asthma, bone marrow suppression, liver disease, lupus, other severe or uncontrolled psychiatric or medical disorders |
|
| Interventions | (1) carbamazepine (28 participants): starting dose 200 mg up to 800 mg then gradually reduced by up to 200 mg;
(2) placebo (34 participants)
Setting: outpatient Follow‐up: 8 weeks Country of origin: USA |
|
| Outcomes | Use of cocaine; Craving | |
| Notes | Study supported through NIH‐NIDA intramural research funds Conflict of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not reported |
| Allocation concealment (selection bias) | Unclear risk | Information not reported |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Study declared as double‐blind; no further information provided |
| Blinding of participants and personnel (performance bias) subjective outcomes | Unclear risk | Study declared as double‐blind; no further information provided |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Information not reported Comment: objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | Information not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10 participants (13.8%) started treatment but discontinued participation before completing 1 week of treatment. Not reported from which group they dropped out |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified in the Methods section |
Nuijten 2014.
| Methods | Randomised controlled feasibility trial | |
| Participants | Participants: 82 crack cocaine dependent; 8 refused informed consent from the topiramate group: analysed 74; mean age 42 years; male 81.6% Education: 11.2 years (mean); years of regular cocaine use: 13 (mean) Reporting cocaine use: 100% Route of administration: smoked 100% Inclusion criteria: Eligible patients had to (1) be at least 18 years old, (2) be cocaine dependent according to the Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM‐IV), (3) regularly use cocaine (8 days in the previous month), (4) administer cocaine primarily by means of basing, (5) be able and willing to participate in the study treatment and associated assessments and (6) provide written informed consent Exclusion criteria: Patients were excluded in case of (1) severe medical (e.g. renal insufficiency; cardiovascular problems) or psychiatric problems (e.g. acute psychosis, suicidality), (2) pregnancy or breastfeeding, (3) pharmacotherapy with a potentially effective medication for cocaine dependence (i.e. naltrexone, disulfiram, acamprosate, methylphenidate, modafinil, dexamphetamine or baclofen), (4) indication for residential treatment, (5) insufficient command of the Dutch language and (6) current participation in another addiction treatment trial |
|
| Interventions | (1) CBT plus topiramate (200 mg/d), participants 44; (2) CBT only, participants 38 Setting: outpatient Follow‐up: 12 weeks Country of origin: The Netherlands |
|
| Outcomes | Retention; Safety; Cocaine use (ASI); Cocaine craving (OCDS); Use of other substances; Physical and mental health; Social functioning; Participant satisfaction; Compliance | |
| Notes | Funding by The Netherlands Organization for Health Research and Development (ZonMw), project number 31160012; ZonMw had no further role in the study design; in the collection,analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication Conflict of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was computer‐generated and stratified by gender,cultural background (European/non‐European) and participation in methadone maintenance treatment (MMT)" |
| Allocation concealment (selection bias) | Unclear risk | Information not reported |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Open‐label Quote: "a pre‐randomization, double‐consent design (Zelen, 1979) was used. Prior to randomization, all participants were asked to provide informed consent about participating in a study evaluating the effectiveness of CBT. Following randomization, a second informed consent, pertaining to the treatment with topiramate, was obtained only in those participants randomized to the experimental group. Hence, participants were only informed about the assigned treatment and not about the condition they were compared with" Comment: objective outcomes unlikely to be biased by lack of blinding |
| Blinding of participants and personnel (performance bias) subjective outcomes | High risk | Open‐label Quote: "a pre‐randomization,double‐consent design (Zelen, 1979) was used. Prior to randomization, all participants were asked to provide informed consent about participating in a study evaluating the effectiveness of CBT. Following randomization, a second informed consent, pertaining to the treatment with topiramate, was obtained only in those participants randomized to the experimental group. Hence, participants were only informed about the assigned treatment and not about the condition they were compared with" |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Information not reported Comment: objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | Information not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Analysis performed on an intention‐to‐treat basis after the second informed consent: 8 in the topiramate group did not given informed consent and were excluded from the analysis |
| Selective reporting (reporting bias) | Low risk | Study protocol is available, and published reports include all expected outcomes, including those that were prespecified in the protocol and in the Methods section |
Somoza 2013.
| Methods | Multi‐site randomised placebo‐controlled trial | |
| Participants | Participants: 186 treatment‐seeking with cocaine dependence; mean age 45 years; male 66.5%; African American 60%, white 31% Reporting cocaine use: 100% Route of cocaine administration: smoked or intravenous 85%, nasal 15% Frequency of cocaine use during the last 30 days before screening < 18 days: 67.5% Inclusion criteria: at least 18 years old, normal visual fields as measured by a Humphrey field analyser, in good physical health as determined by the results of a medical history, physical examination, electrocardiogram and standard laboratory tests; met DSM‐IV criteria for cocaine dependence and hd at least 1 positive (benzoylecgonine [BE] level 300 ng/mL) urine drug screen during the 14‐day baseline Exclusion criteria: individuals who required detoxification from alcohol, who had been court‐ordered to seek cocaine‐dependence treatment or who met DSM‐IV criteria for dependence for any substance other than cocaine, alcohol, nicotine or marijuana. Pregnant and lactating women and women unwilling to use an adequate method of birth control; patients who had ever taken vigabatrin, had received electroconvulsive therapy within 3 months of randomisation or had been enrolled in an opioid substitution programme in the past 2 months. Patients who had taken a drug with known major organ toxic effects, including retinotoxic effects, within 30 days of randomisation or who had clinically significant ophthalmological disease |
|
| Interventions | (1) twice‐daily doses of vigabatrin (total dosage 3.0 g/d), participants 92; (2) placebo, participants 94 All participants received weekly computerised cognitive‐behavioural therapy at 21 plus biweekly half‐hour individual sessions with a counselor Setting: outpatient Follow‐up: duration of the trial: 12 weeks; follow‐up: 24 weeks Country of origin: USA |
|
| Outcomes | Cocaine abstinence, Cocaine use, Craving (as assessed by BSCS), Addiction Severity and Substance Clinical Global Impression (SCGI) scores; Adverse events; Compliance | |
| Notes | Funding and support for this study were provided by Catalyst Pharmaceutical Partners, Inc. The study medication and matching placebo were provided by the company at no cost. The sponsor provided funding to a clinical research organisation, Health Decisions (Durham, NC), which provided day‐to‐day data collection management and analysis and initial interpretation of data Conflict of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "those meeting study criteria were randomized in a 1:1 ratio to vigabatrin or placebo, stratified by sex, primary route of cocaine administration (ie, smoked or intravenous vs nasal), and frequency of cocaine use during the last 30 days before screening (18 vs18 days)" |
| Allocation concealment (selection bias) | Unclear risk | Information not reported |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Study declared as double‐blind Quote: "Vigabatrin and its matching placebo were supplied as white, film‐coated, capsule‐shaped 500‐mg tablets with a bisect on one side. They were custom manufactured for Catalyst Pharmaceutical Partners, Inc" |
| Blinding of participants and personnel (performance bias) subjective outcomes | Low risk | Study declared as double‐blind Quote: "Vigabatrin and its matching placebo were supplied as white, film‐coated, capsule‐shaped 500‐mg tablets with a bisect on one side. They were custom manufactured for Catalyst Pharmaceutical Partners, Inc" |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Information not reported Comment: objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | Information not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis performed on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified in the Methods section |
Umbricht 2014.
| Methods | Randomised controlled trial with a 2×2 factorial design | |
| Participants | Participants: 171 cocaine‐dependent methadone maintenance patients; mean age 42 years; male 52% Education < 12 years: 42% Reporting cocaine use: 100%; mean past 30 days cocaine use: 20.8 Route of administration not reported Inclusion criteria: (1) cocaine and opioid dependent and seeking treatment; (2) between 18 and 55 years old; (3) eligible for methadone maintenance; and (4) able to comply with study requirements Exclusion criteria: (1) sulfonamide or topiramate allergy; (2) diabetes, respiratory insufficiency or other chronic risk factor for acidosis; (3) prior kidney stones, or unexplained blood in the urine; (4) current participation in highly active antiretroviral therapy; (5) glaucoma, family history of glaucoma, intraocular hypertension or 1‐sided blindness; (6) seizure disorder or use of antiepileptic medications; (7) current benzodiazepine dependence; (8) serious psychiatric illness; and (9) pregnancy, lactation or sexual activity without effective contraception |
|
| Interventions | (1) topiramate 300 mg + contingency management; participants 40; (2) topiramate 300 mg non‐contingency management; participants 45; (3) placebo + contingency management; participants 39; (4) placebo non‐contingency management; participants 47. All participants received methadone maintenance Setting: outpatients Follow‐up: 18 weeks Country of origin: USA |
|
| Outcomes | Cocaine abstinence; Retention in treatment; Cocaine craving (CSSA); Depression (BDI); Anxiety (State Trait Anxiety Inventory); Pain symptoms | |
| Notes | Study was supported by grants from the National Institute on Drug Abuse (DA021808) with additional funds from grant T32DA07209 and grant K24DA023186 Conflict of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computerized stratified randomization with a 1:1:1:1 allocation ratio was implemented by staff members with no participant contact" |
| Allocation concealment (selection bias) | Low risk | Quote: "Computerized stratified randomization with a 1:1:1:1 allocation ratio was implemented by staff members with no participant contact" |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Quote: "Participants and staff were blind to time of randomization and changes in medication doses. All study capsules were prepared at the on‐site research pharmacy from bulk topiramate and lactose monohydrate powder as filler. Lactose was premixed with 5PPM denatonimbenzoate to give a similar bitter taste to all capsules" |
| Blinding of participants and personnel (performance bias) subjective outcomes | Low risk | Quote: "Participants and staff were blind to time of randomization and changes in medication doses. All study capsules were prepared at the on‐site research pharmacy from bulk topiramate and lactose monohydrate powder as filler. Lactose was premixed with 5PPM denatonimbenzoate to give a similar bitter taste to all capsules" |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Quote: "Participants and staff were blind to time of randomization and changes in medication doses. All study capsules were prepared at the on‐site research pharmacy from bulk topiramate and lactose monohydrate powder as filler. Lactose was premixed with 5PPM denatonimbenzoate to give a similar bitter taste to all capsules" |
| Blinding of outcome assessment (detection bias) subjective outcomes | Low risk | Quote: "Participants and staff were blind to time of randomization and changes in medication doses. All study capsules were prepared at the on‐site research pharmacy from bulk topiramate and lactose monohydrate powder as filler. Lactose was premixed with 5PPM denatonimbenzoate to give a similar bitter taste to all capsules" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All analyses were intent‐to‐treat" |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified in the Methods section |
Winhusen 2005.
| Methods | Randomised placebo‐controlled trial | |
| Participants | Participants: 34; male 82%; mean age 40 years; all African American; years of education 12.8; married 12%, separated 9%, divorced 35%, never married 44%; employment: full time 53%, part time 44%, unemployed 3%
Reporting cocaine use: 100% Route of cocaine administration: smoked 97%, intranasal 3%; use of cocaine in the past 30 days, 20.0 days Inclusion criteria: CREST criteria Exclusion criteria: CREST criteria plus history of rashes or other sensitivity reactions to tiagabine |
|
| Interventions | (1) tiagabine, 20 mg/d, 17 participants; (2) placebo, 17 participants
For all cognitive‐behavioural therapy: 1‐hour individual weekly
Outpatient Duration: 10 weeks Country of origin: USA |
|
| Outcomes | Dropout; Use of cocaine; Side effect; Depression; Anxiety; Compliance | |
| Notes | Funded by the National Institute on Drug Abuse (NIDA) under Interagency Agreement Y 01 DA 50038–00. Urinalyses were funded by NIDA contract N01DA‐7‐8074 Conflict of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not reported |
| Allocation concealment (selection bias) | Unclear risk | Information not reported |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Quote: "The medications used in this study were not blinded in terms of appearance; thus, the unblinded study pharmacist handled all medication manipulations, including weekly dispensing and pill counts" Comment: objective outcomes unlikely to be biased by lack of blinding |
| Blinding of participants and personnel (performance bias) subjective outcomes | High risk | Quote: "The medications used in this study were not blinded in terms of appearance; thus, the unblinded study pharmacist handled all medication manipulations, including weekly dispensing and pill counts" |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Quote: "The medications used in this study were not blinded in terms of appearance; thus, the unblinded study pharmacist handled all medication manipulations, including weekly dispensing and pill counts" Comment: objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | High risk | Quote: "The medications used in this study were not blinded in terms of appearance; thus, the unblinded study pharmacist handled all medication manipulations, including weekly dispensing and pill counts" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis performed on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified in the Methods section |
Winhusen 2007.
| Methods | Multi‐centre randomised controlled trial | |
| Participants | Participants: 141 cocaine‐dependent patients; mean age 42.5 years; male 67%; African American 67%; full‐time employment 29%; married 18.5% Reporting cocaine use: 100% Route of cocaine administration: smoked 95%, intranasal 4%, intravenous 0.5%; oral 0.5% Cocaine use past 30 days: mean 17.2 (SD 9.4) Inclusion criteria: at least 28 years old, good physical health, at least 1 positive urine toxicology screen for cocaine metabolites, met DSM‐IV criteria for cocaine dependence Exclusion criteria: requirement of detoxification for alcohol, met DSM‐IV criteria for dependence on other substances than cocaine, alcohol, nicotine and marijuana; serious psychological disorders, enrolment in opiate substitution programme, current suicidal ideation, currently taking tiagabine or having medical condition exacerbated by tiagabine; pregnant or unwilling to use an adequate method of birth control (women) |
|
| Interventions | (1) tiagabine 20 mg/d, 70 participants; (2) placebo 71 participants All participants received 1 h of manualised individual cognitive‐behavioural therapy on a weekly basis Outpatients Duration: 12 weeks Country of origin: USA |
|
| Outcomes | Cocaine non‐use days (self report confirmed or disproved by urine BE levels) expressed as weekly proportion of non‐use days to total number of use and non‐use study days that week; Craving (BSCS); Addiction Severity (ASI); Clinical Global Impression scores (CGI‐O); Compliance | |
| Notes | Supported by the National Institutes of Health, National Institute on Drug Abuse through contract N01‐DA‐9‐
8095 (E. Somoza). The study medication and matching placebo were provided by Cephalon at no cost as a consultant for Alkermes, Astra Zeneca, Bristol Myers Squibb, Cephalon, Johnson & Johnson, Ortho‐McNeil and UCB Pharma |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Stratified randomization, balancing for gender and self‐report of cocaine use (<18 or ≥18 days of use in the last 30 days), was used to assign eligible participants to tiagabine or placebo within each study site" |
| Allocation concealment (selection bias) | Unclear risk | Information not reported |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Study declared as double‐blind Quote: "Participants assigned to placebo took tablets that looked identical to the tiagabine tablets and were scheduled to take the same number of tablets as those in the tiagabine condition" |
| Blinding of participants and personnel (performance bias) subjective outcomes | Low risk | Study declared as double‐blind Quote: "Participants assigned to placebo took tablets that looked identical to the tiagabine tablets and were scheduled to take the same number of tablets as those in the tiagabine condition" |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Information not reported Comment: objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | Information not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 56% of participants completed the study; study completion rates did not differ significantly
between tiagabine (54%) and placebo (58%) treatment groups (Chi2 = 0.17, df = 1, P value > 0.05) Reason for discontinuation reported and not significantly different between groups Comment: high dropout rate; per‐protocol analysis |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified in the Methods section |
ADHD: attention deficit hyperactivity disorder. CRES: Cocaine Rapid Efficacy Screening Trial. DSM: Diagnostic and Statistical Manual of Mental Disorders. MMT: methadone maintenance treatment.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahmadi 2006 | Excluded, as study design and objective were not found in the inclusion criteria: non‐RCT study designed to identify outcome predictors in cocaine dependence treatment trials |
| Brady 2002 | Excluded, as no useable outcome measures were included |
| Brown 2003 | Excluded, as study design was not found in the inclusion criteria: non‐RCT |
| Campbell 2001 | Excluded, as the study design was not found in the inclusion criteria: non‐RCT |
| Cornish 1995 b | Excluded, as the study design was not found in the inclusion criteria: non‐RCT |
| Elkashef 2005 | Excluded, as the study design was not found in the inclusion criteria: non‐RCT |
| Gonzalez 2003 | Excluded, as interim analysis was performed on already included studies |
| Gorelick 1994 | Excluded, as the type of intervention was not found in the inclusion criteria: laboratory study |
| Halikas 1989 | Excluded, as the study design was not found in the inclusion criteria: non‐RCT |
| Halikas 1991 | Excluded, as no useable outcome measures were included |
| Haney 2005 | Excluded, as the objective of the study and the outcomes were not found in the inclusion criteria: Drug and cocaine were given simultaneously by the researcher to assess their effects on the cardiovascular, nervous system and subjective response to cocaine |
| Hart 2004 | Excluded, as the objective of the study and the outcomes were not found in the inclusion criteria: Drug and cocaine were given simultaneously by the researcher to assess their effects on the cardiovascular, nervous system and subjective response to cocaine |
| Hart 2007 | Excluded, as the objective of the study and the outcomes were not found in the inclusion criteria: Drug and cocaine were given simultaneously by the researcher to assess their effects on the cardiovascular, nervous system and subjective response to cocaine |
| Hatsukami 1991 | Excluded, as no useable outcome measures were included |
| Johnoson 2005 | Excluded, as the study design was not found in the inclusion criteria: non‐RCT |
| Kampman 2005 | Excluded, as the study design was not found in the inclusion criteria: non‐RCT |
| Kemp 2009 | Excluded, as patients were not found in the inclusion criteria: Patients with bipolar disorders and concurrent alcohol, cannabis or cocaine abuse within the past 3 months, or dependence within the past 6 months. Only 9 participants with cocaine “disorders” were included; it was not specified whether they were dependent or abusing |
| Khun 1989 | Excluded, as the study design was not found in the inclusion criteria: non‐RCT |
| Leiderman 2005 | Excluded, as the study design was not found in the inclusion criteria: non‐RCT |
| Llopis Llacer 2008 | Excluded, as the study design was not found in the inclusion criteria: non‐RCT |
| Mancino 2014 | Excluded, as the type of intervention was not found in the inclusion criteria: sertraline plus gabapentin vs placebo; not possible to ascertain the effect of an anticonvulsant alone |
| Mariani 2012 | Excluded, as the type of intervention was not found in the inclusion criteria: combination of mixed amphetamine salts extended‐release (MAS‐ER) and topiramate; not possible to ascertain the effect of an anticonvulsant alone |
| Reid 2005 | Excluded, as the experimental intervention was not found in the inclusion criteria: Celecoxib has anticonvulsant properties not yet proved in clinical trials |
| Reid 2009 | Excluded, as the objective was not found in the inclusion criteria. Laboratory study performed to assess the effect of cue‐induced cocaine craving |
| Reis 2008 | Excluded, as the study design was not found in the inclusion criteria: non‐RCT |
| Salloum 2007 | Excluded, as the study design was not found in the inclusion criteria: non‐RCT |
| Sofuoglu 1999 | Excluded, as participants were not included in the inclusion criteria: no cocaine‐dependent patients, according to DSM criteria |
| Sofuoglu 2005 | Excluded, as the objective of the study and the outcomes were not found in the inclusion criteria: Drug and cocaine were given simultaneously by the researcher to assess their effects on the cardiovascular, nervous system and subjective response to cocaine |
| Vocci 2005 | Excluded, as the study design was not found in the inclusion criteria: non‐RCT |
| Winter 2000 | Excluded, as the objective of the study and the outcomes were not found in the inclusion criteria: Drug and cocaine were given simultaneously by the researcher to assess their effects on the cardiovascular, nervous system and subjective response to cocaine |
| Zullino 2004 | Excluded, as the study design was not found in the inclusion criteria: non‐RCT |
Characteristics of studies awaiting assessment [ordered by study ID]
Gonzalez 2009.
| Methods | 12‐Week randomised double‐blind placebo‐controlled trial |
| Participants | 8 cases of individuals who reported abnormal movements were identified among cocaine‐ and opiate‐dependent participants of a 12‐week randomised double‐blind placebo‐controlled trial |
| Interventions | (1) tiagabine 32 mg; (2) placebo |
| Outcomes | Any abnormal movement or changes in mental status |
| Notes | Conference proceeding. Not able to retrieve the full publication |
Llorens 2007.
| Methods | Randomised controlled trial |
| Participants | 43 individuals diagnosed as cocaine abusers or dependents (sniffed) |
| Interventions | (1) topiramate, (2) exposition and (3) topiramate exposure |
| Outcomes | Craving; Cocaine self regulation; Impulsiveness; Relapse; Abstinence |
| Notes | Conference proceeding. Not able to retrieve the full publication |
Sherwood Brown 2011.
| Methods | 10‐Week, randomised, double‐blind, placebo‐controlled trial |
| Participants | 120 adult outpatients with bipolar disorder, depressed or mixed mood state and cocaine dependence |
| Interventions | (1) lamotrigine (titrated up to 400 mg/d); (2) placebo |
| Outcomes | Cocaine use; Depression; Mania; Side effects; Cocaine craving |
| Notes | Conference proceeding. Not able to retrieve the full publication |
Characteristics of ongoing studies [ordered by study ID]
Jenkins‐Mendoza 2005.
| Trial name or title | Effectiveness of topiramate in treating cocaine‐dependent individuals |
| Methods | Randomised, double‐blind, placebo‐controlled trial |
| Participants | 180 |
| Interventions | Topiramate or placebo |
| Outcomes | Cocaine use at 2 weeks and at 1, 2 and 3 months following completion of treatment |
| Starting date | October 2005 |
| Contact information | Eva Jenkins‐Mendoza, UVA Care, Charlottesville, VA 22911 USA Phone: 434‐243‐0562 emj9c@virginia.edu |
| Notes |
NCT00086255.
| Trial name or title | Tiagabine for the treatment of cocaine dependence |
| Methods | Randomised, double‐blind, placebo‐controlled trial |
| Participants | 140 |
| Interventions | Tiagabine or placebo |
| Outcomes | Days of cocaine use as assessed by self report confirmed with urine assays for benzoylecgonine (BE) |
| Starting date | October 2002 |
| Contact information | Eugene Somoza, MD, PhD, Cincinnati VA Medical Center |
| Notes |
NCT00448825.
| Trial name or title | Novel pharmacotherapy for dual dependence |
| Methods | Randomised, double‐blind, placebo‐controlled trial |
| Participants | 180 |
| Interventions | Topiramate + Cognitive‐behavioural therapy or placebo + Cognitive‐behavioural therapy |
| Outcomes | Primary outcome measures: Weekly mean proportion of cocaine‐free days; Self‐reported drinking and craving for cocaine and alcohol, as assessed by self report of use; Biochemical markers (GGT, CDT); Urine assay Secondary outcome measures: Cocaine‐free weeks; Psychosocial functioning; Quality of life |
| Starting date | March 2007 |
| Contact information | Mindy Borszich 888‐882‐2345 mcb3x@virginia.edu; Eva Jenkins‐Mendoza 434‐243‐0562 emj9c@virginia.edu; University of Virginia Center for Addiction Research and Education, Charlottesville, VA 22911 USA |
| Notes |
NCT00577005.
| Trial name or title | Effectiveness of tiagabine for cocaine dependence in methadone maintenance individuals |
| Methods | Randomised, double‐blind, placebo‐controlled trial |
| Participants | 120 |
| Interventions | Tiagabine or placebo, while concurrently receiving methadone treatment |
| Outcomes | Urine toxicology for cocaine; Self report and other drug use; Craving |
| Starting date | December 2004 |
| Contact information | Gerardo Gonzalez, Department of Psychiatry, Yale University, New Haven, CT 06511 USA; gerardo.gonzalez‐haddad@yale.edu |
| Notes |
NCT00593125.
| Trial name or title | Efficacy of levetiracetam in cocaine‐abusing methadone maintained patients (Keppra‐DB) |
| Methods | Randomised, double‐blind, placebo‐controlled trial |
| Participants | 40 cocaine‐dependent opioid‐dependent patients |
| Interventions | Levetiracetam or placebo |
| Outcomes | Primary outcome measures: Thrice‐weekly urine toxicology Secondary outcome measures: Weekly self report use of cocaine and opiate; Treatment retention; Cocaine craving; Anxiety symptoms; Opioid withdrawal symptoms; Adverse events |
| Starting date | July 2007 |
| Contact information | Gerardo Gonzalez, Department of Psychiatry, Yale University, New Haven, CT 06511 USA; gerardo.gonzalez‐haddad@yale.edu |
| Notes |
NCT01281202.
| Trial name or title | Vigabatrin for the treatment of cocaine dependency |
| Methods | Randomised, double‐blind, placebo‐controlled trial |
| Participants | 200 |
| Interventions | Vigabatrin or placebo |
| Outcomes | Primary outcome measures: Proportion of participants in each treatment group who are cocaine abstinent during the past 2 weeks of the treatment phase (weeks 8 and 9) Secondary outcome measures: Weekly fraction of cocaine use days; Percent of clean urines (BE < 300 ng/mL) collected during treatment phase; Time to exit from abstinence state up to weeks 13 and 24 among participants who were cocaine free in weeks 8 and 9 |
| Starting date | January 2011 |
| Contact information | Kathleen Brady, MD, PhD, Medical University of South Carolina |
| Notes |
NCT01335867.
| Trial name or title | A phase II, double‐blind, placebo‐controlled, pilot trial of vigabatrin for the treatment of cocaine and alcohol dependence |
| Methods | Randomised, double‐blind, placebo‐controlled trial |
| Participants | 38 |
| Interventions | Vigabatrin or placebo |
| Outcomes | Primary outcomes: Reduction in cocaine use (number of benzoylecgonine (BE)‐negative urine samples); Alcohol abstinent days and heavy drinking days, recorded using the Timeline Followback method Secondary outcomes: Measures of cocaine and alcohol craving, measured using the Minnesota Cocaine Craving Scale and the Penn Alcohol Craving Scale; Addiction severity by the Addiction Severity Index (ASI); Disease severity and improvement, including the Clinical Global Impression Scale; Alcohol and cocaine withdrawal severity, including the Clinical Institutes Withdrawal Scale for Alcohol and Cocaine Selective Severity Assessment; Depression and anxiety, using the Hamilton Depression Rating Scale and the Hamilton Anxiety Rating Scale |
| Starting date | April 2011 |
| Contact information | Treatment Research Center, Philadelphia, PA 19104 USA |
| Notes |
NCT01811940.
| Trial name or title | Multi‐centre trial of combined pharmacotherapy to treat cocaine dependence (TACT2) |
| Methods | Randomised, double‐blind, placebo‐controlled trial |
| Participants | 176 |
| Interventions | Topiramate and Adderal or placebo |
| Outcomes | Primary outcome measures: Proportion of participants in each study arm achieving sustained cocaine abstinence for 3 consecutive weeks at the end of the study. This will be measured by self reported cocaine use on the daily Time line Follow Back (TLFB) and corroborated by the urine toxicology samples collected 3 times per week Secondary outcome measures: Proportion of urine samples negative for cocaine metabolites over the course of the 14 weeks of the study, Length of study participation |
| Starting date | |
| Contact information | |
| Notes |
NTR2576.
| Trial name or title | New pharmacotherapeutic treatment options for crack cocaine‐dependent people in the Netherlands. ‐ CATCH‐study |
| Methods | Randomised open |
| Participants | |
| Interventions | Topiramate + psychosocial treatment or psychosocial treatment alone |
| Outcomes | Primary outcome: Treatment retention Secondary outcomes: Safety, Illicit cocaine use, Cocaine craving, Use of other substances, Physical and mental health, Social functioning (including criminality), Patient satisfaction |
| Starting date | April 2010 |
| Contact information | Vincent Hendriks Parnassia Addiction Research Centre (PARC) Monsterseweg 83 2553, Parnassia Addiction Research Centre (PARC) Monsterseweg 83 2553 RJ Den, Haag, The Netherlands; vincent.hendriks@brijder.nl |
| Notes |
RBR‐3vwfjs.
| Trial name or title | The use of topiramate in the crack addiction |
| Methods | Randomised, double‐blind, placebo‐controlled trial |
| Participants | 40 |
| Interventions | Topiramate or placebo |
| Outcomes | Primary outcome: Urine tests free from cocaine Secondary outcomes: Average number of drugs consumed per week; Average number of days per week that the patient remains without the drug; Weekly side effects |
| Starting date | February 2013 |
| Contact information | Leonardo Baldaçara, Universidade Federal do Tocantins, Av. NS 15, ALCNO 14 77000‐000 Palma Brazil. +55(63)3228‐1807; leonardobaldassara@gmail.com |
| Notes |
Contributions of authors
Amato wrote the background, Pani and Minozzi helped with suggestions and comments and Marina Davoli supervised. Amato inspected the search hits by reading titles and abstracts. Each potentially relevant study identified in the search was obtained in full text and assessed for inclusion independently by two review authors (Minozzi, Amato). Doubts were resolved by discussion between all review authors. Minozzi assessed the quality of the included studies, and Amato and Minozzi extracted data. The other review authors are the former authors of a review of carbamazepine for cocaine dependence, which now is joined with this review.
For the update, Minozzi and Cinquini inspected the search hits by reading titles and abstracts. Each potentially relevant study identified in the search was obtained in full text and assessed for inclusion independently by two review authors (Minozzi, Cinquinii). Pani supervised. Minozzi and Cinquini assessed risk of bias and extracted data. Pani and Amato supervised and contributed to the discussion and the conclusions.
Sources of support
Internal sources
Department of Epidemiology, ASL RM E, Italy.
External sources
No sources of support supplied
Declarations of interest
None.
Edited (no change to conclusions)
References
References to studies included in this review
Berger 2005 arm a {published data only}
- Berger PS, Winhusen TM, Somoza EC, Harrer JM, Mezinskis JP, Leiderman DB, et al. A medication screening trial evaluation of reserpine, gabapentin and lamotrigine pharmacotherapy of cocaine dependence. Addiction 2005;100(Suppl 1):58‐67. [DOI] [PubMed] [Google Scholar]
Berger 2005 arm b {published data only}
- Berger PS, Winhusen TM, Somoza EC, Harrer JM, Mezinskis JP, Leiderman DB, et al. A medication screening trial evaluation of reserpine, gabapentin and lamotrigine pharmacotherapy of cocaine dependence. Addiction 2005;100(Suppl 1):58‐67. [DOI] [PubMed] [Google Scholar]
Bisaga 2006 {published data only}
- Bisaga A, Aharonovich E, Garawi F, Levin FR, Rubin E, Raby WN, et al. A randomised placebo‐controlled trial of gabapentin for cocaine dependence. Drug and Alcohol Dependence 2006;81(3):267‐74. [DOI] [PubMed] [Google Scholar]
Brodie 2009 {published data only}
- Brodie JD, Case BG, Figueroa E, Dewey SL, Robinson JA, Wanderling JA, et al. Randomized, double‐blind, placebo‐controlled trial of vigabatrin for the treatment of cocaine dependence in Mexican parolees. American Journal of Psychiatry 2009;166(11):1269‐77. [DOI] [PubMed] [Google Scholar]
Brown 2012 {published data only}
- Brown ES. Comparative medication strategies in treatment trials for bipolar disorder and substance use disorders. Bipolar Disorder 2013;15:33. [Google Scholar]
- Brown ES, Sunderajan P, Hu LT, Sowell SM, Carmody TJ. A randomized, double‐blind, placebo‐controlled, trial of lamotrigine therapy in bipolar disorder, depressed or mixed phase and cocaine dependence. Neuropsychopharmacology 2012;37(11):2347‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 1994 arm a {published data only}
- Campbell JL, Thomas HM, Gabrielli W, Liskow BI, Powell BJ. Impact of Desipramine or Carbamazepine on patient retention in outpatient cocaine treatment: preliminary findings. Journal of Addictive Diseases 1994;13(4):191‐9. [DOI] [PubMed] [Google Scholar]
Campbell 1994 arm b {published data only}
- Campbell JL, Thomas HM, Gabrielli W, Liskow BI, Powell BJ. Impact of Desipramine or Carbamazepine on patient retention in outpatient cocaine treatment: preliminary findings. Journal of Addictive Diseases 1994;13(49:191‐9. [DOI] [PubMed] [Google Scholar]
Campbell 2003 arm a {published data only}
- Campbell J, Nickel EJ, Penick EC, Wallace D, Gabrrelli WF, Rowe C, et al. Comparison of desipramine or carbamazepine to placebo for crack cocaine dependent patients. American Journal of Addiction 2003;12:122‐36. [PubMed] [Google Scholar]
- Campbell J, Nickel EJ, Penlick EC, Liskow B, Powell BJ. Gender differences in pharmacologic treatment for cocaine dependence. NIDA Research Monograph 1999:54. [Google Scholar]
- Campbell L, Thomas HM, Gabrielli WF, Samuelson SD. Efficacy and compliance with carbamazepine and desipramine versus placebo for cocaine abusers. NIDA Research Monograph 1996;162:147. [Google Scholar]
Campbell 2003 arm b {published data only}
- Campbell J, Nickel EJ, Penick EC, Wallace D, Gabrrelli WF, Rowe C, et al. Comparison of desipramine or carbamazepine to placebo for crack cocaine dependent patients. American Journal of Addiction 2003;12:122‐36. [PubMed] [Google Scholar]
Cornish 1995 {published data only}
- Cornish JW, Manny I, Fudala PJ, Neal S, Poole SA, Volpicelli P, et al. Carbamazepine treatment for cocaine dependence. Drug and Alcohol Dependence 1995;38(3):221‐7. [DOI] [PubMed] [Google Scholar]
Crosby 1996 {published data only}
- Crosby RD, Pearson VL, Eller C, Winegarden T, Graves NL. Phenytoin in the treatment of cocaine abuse: a double blind study. Clinical Pharmacology & Therapeutics 1996;59(4):458‐68. [DOI] [PubMed] [Google Scholar]
Gonzalez 2007 arm a {published data only}
- Gonzalez G, Desai R, Sofuoglu M, Poling J, Oliveto A, Gonsai K, et al. Clinical efficacy of gabapentin versus tiagabine for reducing cocaine use among cocaine dependent methadone‐treated patients. Drug and Alcohol Dependence 2007;87(1):1‐9. [DOI] [PubMed] [Google Scholar]
- Gonzalez G, Sevarino K, Sofuoglu M, Poling J, Oliveto A, Gonsai K, et al. Tiagabine increases cocaine‐free urine in cocaine‐dependent methadone‐treated patients: results of a randomised pilot study. Addiction 2003;98(11):1625‐32. [DOI] [PubMed] [Google Scholar]
Gonzalez 2007 arm b {published data only}
- Gonzalez G, Desai R, Sofuoglu M, Poling J, Oliveto A, Gonsai K, et al. Clinical efficacy of gabapentin versus tiagabine for reducing cocaine use among cocaine dependent methadone‐treated patients. Drug and Alcohol Dependence 2007;87(1):1‐9. [DOI] [PubMed] [Google Scholar]
Halikas 1997 arm a {published data only}
- Halikas JA, Crosby RD, Koop LP, Crea F, Nugent SM, Carlson GA. Daily monitored cardiovascular effects of Carbamazepine in chronic crack cocaine use. Psychopharmacological Bullettin 1991;27(3):345‐51. [PubMed] [Google Scholar]
- Halikas JA, Crosby RD, Pearson VL, Graves NM. A randomised double‐blind study of carbamazepine in the treatment of cocaine abuse. Clinical Pharmacology and Therapeutics 1997;62(1):89‐105. [DOI] [PubMed] [Google Scholar]
Halikas 1997 arm b {published data only}
- Halikas JA, Crosby RD, Koop LP, Crea F, Nugent SM, Carlson GA. Daily monitored cardiovascular effects of Carbamazepine in chronic crack cocaine use. Psychopharmacological Bullettin 1991;27(3):345‐51. [PubMed] [Google Scholar]
- Halikas JA, Crosby RD, Pearson VL, Graves NM. A randomised double‐blind study of carbamazepine in the treatment of cocaine abuse. Clinical Pharmacology and Therapeutics 1997;62(1):89‐105. [DOI] [PubMed] [Google Scholar]
Johnson 2013 {published data only}
- Johnson BA, Ait‐Daoud N, Wang XQ, Penberthy K, Javors MA, Seneviratne C, Liu L. Topiramate for the treatment of cocaine addiction: a randomised clinical trial. JAMA Psychiatry 2013;70(12):1388‐46. [DOI] [PubMed] [Google Scholar]
Kampman 2004 {published data only}
- Kampan KM, Pettinati H, Lynch KG, Dackis C, Sparkman T, Weigley C, et al. A pilot trial of topiramate for the treatment of cocaine dependence. Drug and Alcohol Dependence 2004;75(3):233‐40. [DOI] [PubMed] [Google Scholar]
Kampman 2013 {published data only}
- Kampman K, Pettinati H, Lynch K, Jones A, Varillo K, O'Brian C. A double‐blind, placebo controlled trial of topiramate for the treatment of comorbid alcohol and cocaine dependence. Neuropsychopharmacology. 2010; Vol. 35(Suppl 1):S207.
- Kampman KM, Pettinati HM, Lynch KG, et al. Topiramate for the treatment of comorbid alcohol and cocaine dependence. Proceedings of the 73rd Annual Scientific Meeting of the College on Problems of Drug Dependence;. Hollywood, Florida, 2011; Vol. 84:Abstract no: 334.
- Kampman KM, Pettinatia HM, Lyncha KG, Spratt BK, Wierzbickic MR, O'Briena CP. A double‐blind, placebo‐controlled trial of topiramate for the treatment of comorbid cocaine and alcohol dependence. Drug and Alcohol Dependence 2013;133:94‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kranzler 1995 {published data only}
- Hersh D, Bauer LO, Kranzler HR. Carbamazepine and cocaine‐cue reactivity. Drug and Alcohol Dependence 1995;39:213‐21. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Bauer LO, Hersh D, Klinghoffer V. Carbamazepine treatment of cocaine dependence: a placebo‐controlled trial. Drug and Alcohol Dependence 1995;38(3):203‐11. [DOI] [PubMed] [Google Scholar]
Montoya 1994 {published data only}
- Montoya ID, Levin FR, Fudala PJ, Gorelick DA. Double‐blind comparison of carbamazepine and placebo for treatment of cocaine dependence. Drug and Alcohol Dependence 1995;38(3):213‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nuijten 2014 {published data only}
- Nuijten M, Blanken P, Brink W, Hendriks V. Cocaine Addiction Treatments to improve Control and reduce Harm (CATCH): new pharmacological treatment options for crack‐cocaine dependence in the Netherlands. BMC Psychiatry 2011;11:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuijten M, Blanken P, Brink W, Hendriks V. Treatment of crack‐cocaine dependence with topiramate: a randomized controlled feasibility trial in The Netherlands. Drug and Alcohol Dependence 2014;138:177‐84. [DOI] [PubMed] [Google Scholar]
Somoza 2013 {published data only}
- Somoza EC, Winship D, Gorodetzky CW, Lewis D, Ciraulo DA, Galloway GP, et al. A multisite, double‐blind, placebo‐controlled clinical trial to evaluate the safety and efficacy of vigabatrin for treating cocaine dependence. JAMA Psychiatry 2013;70(6):630‐7. [DOI] [PubMed] [Google Scholar]
Umbricht 2014 {published data only}
- Umbricht A, DeFulio A, Tompkins DA. Topiramate and contingency management in the treatment of cocaine dependence: a randomized controlled trial. Proceedings of the 74th Annual Scientific Meeting of the College on Problems of Drug Dependence;. Palm Springs, CA, 2012; Vol. Abstract no: 681.
- Umbricht A, DeFulio A, Winstanley EL, Tompkins DA, Peirce J, Mintzer MZ, et al. Topiramate for cocaine dependence during methadone maintenance treatment: a randomized controlled trial. Drug and Alcohol Dependence 2014;140:92‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Winhusen 2005 {published data only}
- Winhusen TM, Somoza EC, Harrer JM, Mezinskis JP, Montgomery MA, Goldsmith RJ, et al. A placebo‐controlled screening trial of tiagabine, sertraline and donepezil as cocaine dependence treatments. Addiction 2005;100(Suppl 1):68‐77. [DOI] [PubMed] [Google Scholar]
Winhusen 2007 {published data only}
- Winhusen T, Somoza E, Ciraulo DA, Harrer JM, Goldsmith RJ, Grabowski J, et al. A double‐blind, placebo‐controlled trial of tiagabine for the treatment of cocaine dependence. Drug and Alcohol Dependence 2007;91(2‐3):141‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ahmadi 2006 {published data only}
- Ahmadi J, Kampman K, Dackis C. Outcome predictors in cocaine dependence treatment trials. The American Journal of Addiction Psychiatry 2006;15(6):434‐9. [DOI] [PubMed] [Google Scholar]
Brady 2002 {published data only}
- Brady KT, Sonne SC, Malcolm RJ, Randall CL. Carbamazepine in the teratment of cocaine dependence: subtyping by affective disorders. Experimental and Clinical Pharmacology 2002;10(3):276‐85. [DOI] [PubMed] [Google Scholar]
Brown 2003 {published data only}
- Brown ES, Nejtek VA, Perantie DC, Orsulak PJ, Bobadilla LB. Lamotrigine in patients with bipolar disorder and cocaine dependence. Journal of Clinical Psychiatry 2003;64(2):198‐201. [DOI] [PubMed] [Google Scholar]
Campbell 2001 {unpublished data only}
- Campbell J, Gorodetzky C, Nickel J, Pennick E. Does worse predict better? Characteristics and outcomes of volunteers for a medication trial or Psychosocial trial. Drud and Alcohol Dependence. Sixty‐Thrid Annual Scientific Meetind Scottsdale, Arizona. 2001; Vol. Vol. 63 supplement 1, S23.
Cornish 1995 b {unpublished data only}
- Cornish JW, Maany I, Fudala PJ, Poole SA, O'Brien CP. An analysis of factors influencing subject participation in a trial of carbamazepine for cocaine dependence treatment. NIDA Research Monograph 1995;135:312. [Google Scholar]
Elkashef 2005 {published data only}
- Elkashef A, Holmes TH, Bloch DA, Shoptaw S, Kampman K, Reid MS. Retrospective analyses of pooled data from CREST I and CREST II trials for treatment of cocaine dependence. Addiction 2005;100(Suppl 1):91‐101. [DOI] [PubMed] [Google Scholar]
Gonzalez 2003 {published data only}
- Gonzalez G, Sevarino K, Sofuoglu M, Poling J, Oliveto A, Gonsai K, et al. Tiagabine increases cocaine‐free urine in cocaine‐dependent methadone‐treated patients: results of a randomised pilot study. Addiction 2003;98(11):1625‐32. [DOI] [PubMed] [Google Scholar]
Gorelick 1994 {published data only (unpublished sought but not used)}
- Gorelick DA, Weinhold LL, Cone EJ, Henningfield JE. Carbamazepine (CBS) does not alter cocaine self‐administration in human cocaine addicts. NIDA Research Monograph 1994;141:137. [Google Scholar]
Halikas 1989 {published data only}
- Halikas J, Kemp K, Kuhn K, Carlson G, Crea F. Carbamazepine for cocaine addiction. The Lancet 1989;1(8638):623‐4. [DOI] [PubMed] [Google Scholar]
Halikas 1991 {published data only}
- Halikas JA, Crosby RD, Carlson GA, Crea F, Graves NM, et al. Cocaine reduction in unmotivated crack users using carbamazepine versus placebo in a short term, double blind cross over design. Clinical Pharmacology & Therapeutics 1991;50:81‐95. [DOI] [PubMed] [Google Scholar]
Haney 2005 {published data only}
- Haney M, Hart C, Collins ED, Foltin RW. Smoked cocaine discrimination in humans: effects of gabapentin. Drug and Alcohol Dependence 2005;80(1):53‐61. [DOI] [PubMed] [Google Scholar]
Hart 2004 {published data only}
- Hart CL, Waerd AS, Collins ED, Haney M, Foltin RW. Gabapentin maintenance decreases smoked cocaine‐related subjective effects, but not self‐administration by humans. Drug and Alcohol Dependence 2004;73(3):279‐87. [DOI] [PubMed] [Google Scholar]
Hart 2007 {published data only}
- Hart CL, Haney M, Collins ED, Rubin E, Foltin RW. Smoked cocaine self‐administration by humans is not reduced by large gabapentin maintenance doses. Drug and Alcohol Dependence 2007;86(2‐3):274‐7. [DOI] [PubMed] [Google Scholar]
Hatsukami 1991 {published data only}
- Hatsukami D, Keenan R, Halikas J, Pentel PR, Brauer LH. Effects of carbamazepine on acute responses to smoked cocaine‐base in human cocaine users. Psychopharmacology 1991;104:120‐4. [DOI] [PubMed] [Google Scholar]
Johnoson 2005 {published data only}
- Johnson BA. Recent advances in the development of treatments for alcohol and cocaine dependence. CNS Drugs 2005;19(10):873‐96. [DOI] [PubMed] [Google Scholar]
Kampman 2005 {published data only}
- Kampman KM, Leiderman D, Holmes T, LoCastro J, Bloch DA, Reid MS, et al. Cocaine rapid efficacy screening trials (CREST): lessons learned. Addiction 2005;100(Suppl 1):102‐10. [DOI] [PubMed] [Google Scholar]
Kemp 2009 {published data only}
- Calabrese JR, Kemp DE, Ganocy SJ, et al. A 6‐month, pilot, double‐blind, maintenance trial of lithium monotherapy versus the combination of lithium and divalproex for dual diagnosis rapid‐cycling bipolar disorder. International Journal of Psychiatry in Clinical Practice. 2007; Vol. 11(Suppl 1):15‐6.
- Kemp DE, Gao K, Ganocy SJ, et al. A 6‐month, double‐blind, maintenance trial of lithium monotherapy versus the combination of lithium and divalproex for rapid‐cycling bipolar disorder and co‐occurring substance abuse or dependence. Journal of Clinical Psychiatry 2 2009;70(1):113‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khun 1989 {published data only}
- Kuhn KL, Halikas JA, Kemp KD. Carbamazepine treatment of cocaine dependence in methadone maintenance patient with dual opiate‐cocaine addiction. NIDA Research Monograph 1989;95:316‐7. [PubMed] [Google Scholar]
Leiderman 2005 {published data only}
- Leiderman DB, Shoptaw S, Montgomery A, Bloch DA, Elkashef A, LoCastro JL, et al. Cocaine rapid efficacy screening trial (CREST): a paradigm for the controlled evaluation of candidate medications for cocaine dependence. Addiction 2005;100(Suppl 1):1‐11. [DOI] [PubMed] [Google Scholar]
Llopis Llacer 2008 {published data only}
- Llopis Llacer JJ, Aguilella AC. Efficacy of oxcarbazepine treatment in patients diagnosed with cocaine abuse/dependence. Adicciones 2008;20(3):263‐70. [PubMed] [Google Scholar]
Mancino 2014 {published data only}
- Mancino MJ, Feldman Z, Chopra M, Cargile CS, Oliveto A. Randomized placebo controlled trial of sertraline and sertraline plus gabapentin in depressed, recently abstinent cocaine‐dependent patients. Proceedings of the 71th Annual Scientific Meeting of the College on Problems of Drug Dependence. Nevada USA: Reno/Sparks, 2009; Vol. 94.
- Mancino MJ, McGaugh J, Chopra MP, et al. Clinical efficacy of sertraline alone and augmented with gabapentin in recently abstinent cocaine‐dependent patients with depressive symptoms. Journal of Clinical Psychopharmacology 2014;34(2):234‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mariani 2012 {published data only}
- Mariani JJ, Pavlicova M, Bisaga A, Nunes EV, Brooks DJ, Levin FR. Extended‐release mixed amphetamine salts and topiramate for cocaine dependence: a randomized controlled trial. Biological Psychiatry 2012;72(11):950‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reid 2005 {published data only}
- Reid MS, Casadonte P, Baker S, Sanfilippo M, Braunstein D, Hitzemann R, et al. A placebo‐controlled screening trial of olanzapine, valproate and coenzyme Q10/L‐carnitine for the treatment of cocaine dependence. Addiction 2005;100(Suppl 1):43‐57. [DOI] [PubMed] [Google Scholar]
Reid 2009 {published data only}
- Reid MS, Thakkar V. Valproate treatment and cocaine cue reactivity in cocaine dependent individuals. Drug and Alcohol Dependence 2009;102(1‐3):144‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reis 2008 {published data only}
- Reis AD, Castro LA, Faria R, Laranjeira R. Craving decrease with topiramate in outpatient treatment for cocaine dependence: an open label trial. Rev Bras Psiquiatr 2008;30(2):132‐5. [DOI] [PubMed] [Google Scholar]
Salloum 2007 {published data only}
- Salloum IM, Douaihy A, Cornelius JR, Kirisci L, Kelly TM, Hayes J. Divalproex utility in bipolar disorder with co‐occurring cocaine dependence: a pilot study. Addictive Behaviors 2007;32(2):410‐5. [DOI] [PubMed] [Google Scholar]
Sofuoglu 1999 {published data only}
- Sofuoglu M, Pentel PR, Bliss RL, Goldman AI, Hatsukami DK. Effects of phenytoin on cocaine self‐administration in humans. Drug and Alcohol Dependence 1999;53(3):273‐5. [DOI] [PubMed] [Google Scholar]
Sofuoglu 2005 {published data only}
- Sofuoglu M, Poling J, Mitchell E, Kosten TR. Tiagabine affects the subjective response to cocaine in humans. Pharmacology, Biochemistry and Behavior 2005;82(3):569‐73. [DOI] [PubMed] [Google Scholar]
Vocci 2005 {published data only}
- Vocci FJ, Elkashef A. Pharmacotherapy and other treatments for cocaine abuse and dependence. Current Opinion in Psychiatry 2005;18:235‐70. [DOI] [PubMed] [Google Scholar]
Winter 2000 {published data only}
- Winther LC, Saleem R, McCance‐Katz EF, Rosen MI, Hameedi FA, Pearsall HR, et al. Effects of lamotrigine on behavioral and cardiovascular responses to cocaine in human subjects. American Journal on Drug and Alcohol Abuse 2000;26(1):47‐59. [DOI] [PubMed] [Google Scholar]
Zullino 2004 {published data only}
- Zullino DF, Khazaal Y, Hattenschwiler J, Borgeat F, Besson J. Anticonvulsant drugs in the treatment of substance withdrawal. Drugs of Today 2004;40(7):603‐19. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Gonzalez 2009 {published data only}
- Gonzalez G, Romero‐Gonzalez M, Desai R, et al. Myoclonus cases associated with tiagabine treatment for cocaine and opioid dependent patients. American Journal on Addictions 2009;18(4):324. [Google Scholar]
Llorens 2007 {published data only}
- Llorens N, Perello MJ, Senchez A, et al. Exposuure techniques versus topiramate treatment on cocaine addiction. Proceedings of the 69th Annual Scientific Meeting of the College on Problems of Drug Dependence. Quebec City, Canada, 2007.
Sherwood Brown 2011 {published data only}
- Sherwood Brown E, Sunderajan P, Hu LT, Sowell S, Carmody T. Lamotrigine in bipolar disorder, depressed or mixed phase and cocaine dependence. Neuropsychopharmacology 2011;36:S425‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Jenkins‐Mendoza 2005 {unpublished data only}
- Effectiveness of topiramate in treating cocaine‐dependent individuals. Ongoing study October 2005.
NCT00086255 {unpublished data only}
- Tiagabine for the treatment of cocaine dependence. Ongoing study October 2002.
NCT00448825 {unpublished data only}
- Novel pharmacotherapy for dual dependence. Ongoing study March 2007.
NCT00577005 {unpublished data only}
- Effectiveness of tiagabine for cocaine dependence in methadone maintenance individuals. Ongoing study December 2004.
NCT00593125 {unpublished data only}
- Efficacy of levetiracetam in cocaine‐abusing methadone maintained patients (Keppra‐DB). Ongoing study July 2007.
NCT01281202 {unpublished data only}
- Vigabatrin for the treatment of cocaine dependency. Ongoing study January 2011.
NCT01335867 {unpublished data only}
- A phase II, double‐blind, placebo‐controlled, pilot trial of vigabatrin for the treatment of cocaine and alcohol dependence. Ongoing study April 2011.
NCT01811940 {unpublished data only}
- Multi‐centre trial of combined pharmacotherapy to treat cocaine dependence (TACT2). Ongoing study Starting date of trial not provided. Contact author for more information.
NTR2576 {unpublished data only}
- New pharmacotherapeutic treatment options for crack cocaine‐dependent people in the Netherlands. ‐ CATCH‐study. Ongoing study April 2010.
RBR‐3vwfjs {unpublished data only}
- The use of topiramate in the crack addiction. Ongoing study February 2013.
Additional references
Alvarez 2010
- Alvarez Y, Farré M, Fonseca F, Torrens M. Anticonvulsant drugs in cocaine dependence: a systematic review and meta‐analysis. Journal of Substance Abuse Treatment 2010;38(1):66‐73. [DOI] [PubMed] [Google Scholar]
Amato 2007
- Amato L, Minozzi S, Pani PP, Davoli M. Antipsychotic medication for cocaine dependence. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD006306.pub2] [DOI] [PubMed] [Google Scholar]
Amato 2011
- Amato L, Minozzi S, Pani PP, Solimini R, Vecchi S, Zuccaro P, et al. Dopamine agonists for the treatment of cocaine dependence. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD003352.pub3] [DOI] [PubMed] [Google Scholar]
Anton 1996
- Anton RF, Moak DH, Latham PK. The obsessive compulsive drinking scale:a new method of assessing outcome in alcoholism treatment studies. Archives of General Psychiatry 1996;53:225‐31. [DOI] [PubMed] [Google Scholar]
Beck 1961
- Beck AT, Word CH, Mendelson M, Mock J, Rebaugh J. An inventory for measuring depression. Archives of General Psichiatry 1961;4:461‐71. [DOI] [PubMed] [Google Scholar]
Campbell 1999
- Campbell UC, Lac ST, Carroll ME. Effects of baclofen on maintenance and reinstatement of intravenous cocaine self‐administration in rats. Psychopharmacology 1999;143(2):209‐14. [DOI] [PubMed] [Google Scholar]
Castells 2010
- Castells X, Casas M, Pérez‐Mañá C, Roncero C, Vidal X, Capellà D. Efficacy of psychostimulant drugs for cocaine dependence. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD007380.pub3] [DOI] [PubMed] [Google Scholar]
Cohen 2014
- Cohen J, Dervaux A, Laqueille X. Topiramate in substance‐related and addictive disorders. Presse Medicale 2014;43(9):892‐901. [DOI] [PubMed] [Google Scholar]
Crosby 1991
- Crosby RD, Halikas JA, Carlson G. Pharmacotherapeutic interventions for cocaine abuse: present practices and future directions. Journal of Addictive Diseases 1991;10(4):13‐30. [DOI] [PubMed] [Google Scholar]
Czapinski 2005
- Czapinski P, Blaszczyk B, Czuczwar SJ. Mechanisms of action of antiepileptic drugs. Current Topics in Medicinal Chemistry 2005;5(1):3‐14. [DOI] [PubMed] [Google Scholar]
Degenhardt 2012
- Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet 2012;379:55‐70. [DOI] [PubMed] [Google Scholar]
Di Chiara 1988
- Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America 1988;85(14):5274‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Drevets 1999
- Drevets WC, Price JC, Kupfer DJ, Kinahan PE, Lopresti B, Holt D, et al. PET measures of amphetamine‐induced dopamine release in ventral versus dorsal striatum. Neuropsychopharmacology 1999;21(6):694‐709. [DOI] [PubMed] [Google Scholar]
Drevets 2001
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, et al. Amphetamine‐induced dopamine release in human ventral striatum correlates with euphoria. Biological Psychiatry 2001;49(2):81‐96. [DOI] [PubMed] [Google Scholar]
DSM‐IV‐R
- American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington DC: American Psychiatric Association, 1994. [Google Scholar]
EMCDDA 2006
- EMCDDA. The state of the drugs problem in the European Union and Norway. The State of the Drug Problem in the European Union and Norway. Luxemburg: European Monitoring Centre for Drug and Drug Abuse, 2006. [Google Scholar]
EMCDDA 2012
- EMCDDA. The State of the Drug Problem in Europe. Annual Report. Luxemburg: European Monitoring Centre for Drug and Drug Abuse, 2012. [Google Scholar]
EMCDDA 2014
- EMCDDA. European Drug Report: Trends and Developments. European Drug Report: Trends and Developments. Luxemburg: European Monitoring Centre for Drugs and Drug Addiction, 2014. [Google Scholar]
Grade 2004
- The GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guy 1976
- Guy W. Assessment Manual for Psychopharmacology. Publication ADM 76‐338. Washington DC: US Department of Health Education and Welfare, 1976. [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64:383‐94. [DOI] [PubMed] [Google Scholar]
Halikas 1991
- Halikas JA, Kuhn KL, Crosby R, Carlson G, Crea F. The measurement of craving in cocaine patients using the Minnesota Cocaine Craving Scale. Comprehensive Psychiatry 1991;32:22‐7. [DOI] [PubMed] [Google Scholar]
Hamilton 1959
- Hamilton M. The assessment of anxiety states by rating. British Journal of Medical Psychology 1959;32(1):50‐5. [DOI] [PubMed] [Google Scholar]
Hamilton 1967
- Hamilton M. Development of a rating scale for primary depressive illness. British Journal of Social and Clinical Psychology 1967;6(4):278‐96. [DOI] [PubMed] [Google Scholar]
Harvey 2004
- Harvey JA. Cocaine effects on the developing brain: current status. Neuroscience & Biobehavioral Reviews 2004;27(8):751‐64. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. www.cochrane‐handbook.org, 2011. [Google Scholar]
Karila 2011
- Karila L, Reynaud M, Aubin HJ, Rolland B, Guardia D, Cottencin O, Benyamina A. Pharmacological treatments for cocaine dependence: is there something new?. Current Pharmaceutical Design 2011;17(14):1359‐68. [DOI] [PubMed] [Google Scholar]
Knapp 2007
- Knapp WP, Soares BGO, Farrel M, Lima MS. Psychosocial interventions for cocaine and psychostimulant amphetamines related disorders. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD006754.pub3.] [DOI] [PubMed] [Google Scholar]
Koob 1997
- Koob GF, Nestler EJ. The neurobiology of drug addiction. Journal of Neuropsychiatry and Clinical Neurosciences 1997;9(3):482‐97. [DOI] [PubMed] [Google Scholar]
Kranzler 1995
- Kranzler HR, Bauer LO, Hersh D, Klinghoffer V. Carbamazepine treatment of cocaine dependence: a placebo‐controlled trial. Drug and Alcohol Dependence 1995;38(3):203‐11. [DOI] [PubMed] [Google Scholar]
Kushner 1999
- Kushner SA, Dewey SL, Kornetsky C. The irreversible gamma‐aminobutyric acid (GABA) transaminase inhibitor gamma‐vinyl‐GABA blocks cocaine self‐administration in rats. Journal of Pharmacology and Experimental Therapeutics 1999;290(2):792‐802. [PubMed] [Google Scholar]
Landmark 2007
- Landmark CJ. Targets for antiepileptic drugs in the synapse. Medical Science Monitor 2007;13(1):RA1‐7. [PubMed] [Google Scholar]
Lima Reisser 2000
- Lima Reisser A, Lima MS, Soares BGO, Farrell M. Carbamazepine for cocaine dependence. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD002023.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
McLellan 1992
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The fifth edition of the Addiction Severity Index. Journal Substance Abuse Treatment 1992;9(3):199‐213. [DOI] [PubMed] [Google Scholar]
Minozzi 2008
- Minozzi S, Amato L, Davoli M, Farrell MF, Lima Reisser AARL, Pani PP, et al. Anticonvulsants for cocaine dependence. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD006754.pub3] [DOI] [PubMed] [Google Scholar]
Mulvaney 1999
- Mulvaney FD, Alterman AI, Boardman CR, Kampman K. Cocaine abstinence symptomatology and treatment attrition. Journal of Substance Abuse Treatment 1999;16:129‐35. [DOI] [PubMed] [Google Scholar]
NSDUH 2011
- Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration Center for Behavioral Health Statistics and Quality, 2011.
Pani 2010
- Pani PP, Trogu E, Vacca R, Amato L, Vecchi S, Davoli M. Disulfiram for the treatment of cocaine dependence. Cochrane Database of Systematic Reviews 2010, Issue 1. [10.1002/14651858.CD007024.pub2] [DOI] [PubMed] [Google Scholar]
Pani 2011
- Pani PP, Trogu E, Vecchi S, Amato L. Antidepressants for cocaine dependence and problematic cocaine use. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD002950.pub3] [DOI] [PubMed] [Google Scholar]
Shinn 2010
- Shinn AK, Greenfield SF. Topiramate in the treatment of substance‐related disorders: a critical review of the literature. Journal of Clinical Psychiatry 2010;71(5):634‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shunemann 2006
- Schünemann HJ, Jaeschke R, Cook D, Bria W, El‐Solh A, Ernst A, et al. An official ATS statement: grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. American Journal of Respiratory and Critical Care Medicine 2006;174:605‐14. [DOI] [PubMed] [Google Scholar]
Somoza 1995
- Somoza E, Dyrenforth S, Goldsmith J, Mezinskis J, Cohen M. In search of a universal drug craving scale. Annual Meeting of the American Psychiatric Association. 1995.
Spielberg 1983
- Spielberg DC. State‐Trate Anxiety Inventory. Palo Alto CA: Consulting Psychologist Press, 1983. [Google Scholar]
UNODC 2013
- UNODC, World Drug Report 2013 (United Nations publication, Sales No. E.13.XI.6).
Volkow 2003
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights for imaging studies. Journal of Clinical Investigation 2003;111(10):1444‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]


