Abstract
Background
Cancer immunotherapy shows insufficient efficacy for low immunogenic tumors. Furthermore, tumors often downregulate antigen and major histocompatibility complex expression to escape recognition by T cells, resulting in insufficient T cell receptor (TCR) stimulation in the tumor microenvironment. Thus, augmenting TCR-mediated recognition of tumor antigens is a useful strategy to improve the efficacy of cancer immunotherapy.
Methods
We screened 310 small molecules from our library and identified PQDN, a small molecule that activates CD8 T cells after TCR engagement, even when antigen stimulation is too weak for their activation. We used inhibitors of mitochondrial functions and Seahorse Flux Analyzer to investigate the mechanism underlying the effect of PQDN on T cells. Effect of PQDN on tumor-infiltrating CD8 T cells was examined using flow cytometry and TCR repertoire analysis.
Results
PQDN increased mitochondrial reciprocal capacity through enhancement of electron transport chains (ETCs) and facilitated glycolysis via mTOR/AKT signaling, resulting in augmented CD8 T cell activation, even when antigen stimulation is extremely weak. Intratumoral administration of this compound into tumor-bearing mice tunes inactivated T cell with tumor antigen recognition potent and expanded functional T cell receptor diversity of tumor-infiltrating T cells, augmenting antitumor immune responses and retarding tumor growth. Furthermore, PQDN has a synergistic potent with T cell dependent immunotherapy, such as checkpoint inhibitory therapy or adoptive cell therapy, even in a low immunogenic tumor. We also demonstrated that this compound enhances the activation of human CD8 T cells.
Conclusions
These data suggest that tuning the T cell activation threshold by chemical activation of mitochondrial ETC is a new strategy for improving therapeutic efficacy through the activation of low-avidity tumor-specific T cells.
Keywords: CD8-positive T-lymphocytes, lymphocytes, tumor-infiltrating, metabolic networks and pathways
Background
Cancer immunotherapy using tumor-specific T cells has led to great improvements since the discovery of tumor antigens. T cell-based immunotherapies, including checkpoint blockade therapies and adoptive cell therapy, have been developed, and some of these have been approved by the Food and Drug Administration and Pharmaceuticals and Medical Devices Agency and used for treating patients with cancer practically.1–3 In these therapies, antigen recognition on the major histocompatibility complex (MHC) by the T cell receptor (TCR) or cell surface targeted molecule recognition by CAR is a very important mechanism to induce antitumor effects. However, low immunogenic cancer cells or cancer cells that decrease MHC or antigen expression evade immune control due to the lack of tumor recognition by CD8 T cells.4–6 Therefore, a solution to increase TCR recognition ability is needed to improve the efficacy of cancer immunotherapy.
Strategies to enhance antigen recognition by T cells, such as modification of TCR genes in adoptive cell therapy or increased costimulatory signaling, have been developed to improve the efficacy of cancer immunotherapy, but these have not achieved sufficient antitumor activity.7–9 One of the important issues regarding these failures is that strategies for enhancing antigen recognition only involve infusion of cells in adoptive cell therapy; thus, antigen recognition by endogenous T cells is not affected. Therefore, a strategy that activates infused T cells as well as spontaneously induced endogenous tumor-specific T cells in patients with cancer is required for the increased efficacy of cancer immunotherapy.
TCR stimulation induces phosphorylation of immunoreceptor tyrosine-based activation motifs by lymphocyte protein tyrosine kinase (LCK) and leads to the nuclear translocation of nuclear factor of activated T cells (NFAT) and NF-κB, resulting in the transcription of various molecules involved in CD8 T cell activation. In addition to these transcriptional changes, metabolic activation is necessary for full activation of T cells with proliferation and acquisition of effector function. Although naïve T cells use oxidative phosphorylation (OXPHOS) to produce adenosine triphosphate (ATP),10 11 antigen-stimulated T cells use glycolysis to synthesize ATP for meeting the rapidly increasing energy demands.10 12–15 This reprogramming is controlled by pyruvate dehydrogenase kinase 1 (PDHK1) or phosphoinositide 3-kinase (PI3K).16 17 PDHK1 represses the import of glucose into mitochondria and attenuates mitochondrial activation, resulting in enhanced glycolysis and full activation.16 PI3K signaling facilitates Akt phosphorylation and promotes glycolytic ATP synthesis via LDHA in TCR-stimulated T cells.17 In addition to glycolysis, OXPHOS is essential for CD8 T cell activation after TCR engagement. Inhibitors of mitochondrial complex I and III, and ATP synthesis inhibitors such as rotenone, antimycin A, and oligomycin, completely block the activation of CD8 T cells.10 11 Furthermore, early activated CD8 T cells exhibit simultaneous peak oxidative and glycolytic activity before differentiation from naïve to memory or effector cells.18 Thus, both OXPHOS and glycolysis, induced after metabolic reprogramming, are essential to fully activate T cells after antigen recognition.
Here, we identified a small molecule that enhances proliferation and cytokine production in CD8 T cells even under insufficient TCR stimulation and elucidated the mechanism underlying the effect of this compound on T cells in a mitochondria-dependent manner.
Materials and methods
Mice
Female BALB/c and BALB/cnu/nu mice were obtained from SLC Japan and used at 6–12 weeks of age. DUC18 mice, which are transgenic for TCRα/β-reactive with a Kd-restricted 9 m epitope, were established as reported previously.19 20 All mice were maintained at the Animal Center of Nagasaki University and were used under protocols approved by the Institutional Review Boards.
Tumors
CMS5a is a subclone derived from the CMS5 cell line that expresses the mutated ERK2 antigen containing the 9 m epitope. The colon epithelial tumor cell line CT26 was purchased from the American Type Culture Collection. CMS5a and CT26 cells were cultured in RPMI 1640 medium containing 10% FBS. Mice were inoculated subcutaneously in the hind flank with 1×106 cells of the respective tumor cell line. Tumor volume was calculated using the following formula: tumor volume=0.5 × length in mm × (width in mm).2
Extracellular flux analysis
The oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were measured using an XFe96 Extracellular Flux Analyzer (Seahorse Bioscience, North Billerica, Massachusetts, USA). For the Cell Mito Stress Test, the OCR and ECAR were measured under basal conditions and in response to oligomycin (1 µM), FCCP (trifluoromethoxy carbonylcyanide phenylhydrazone, carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone) (2 µM), or rotenone/antimycin A (0.5 µM each) (Agilent). All tests were performed in triplicate wells per condition, and all data were analyzed using Seahorse Wave Software V.2.6. Spare capacity was calculated according to the following formulas: spare respiratory capacity=maximal respiration − basal respiration. The maximum ECAR was measured as the stressed ECAR.
Glucose uptake assay
Cells were treated in glucose-free medium and incubated with 20 µg of 2-NBDG for 10 min. The mean fluorescence intensity of 2-NBDG was determined using flow cytometry.
NAD+/NADH measurement
NAD+/NADH levels were determined using the NAD/NADH assay kit wst-8 according to the manufacturer’s instructions (DOJIDO). Briefly, 1×106 DUC splenocytes, following PQDN treatment, were lysed, and half of the cell lysate were incuvated for 60 min at 60°C to decompose the NAD+. All lysate samples were subjected to enzyme reaction for 60 min at 37°C; the color was measured at 450 nm using a SpectraMax microplate reader (Molecular Devices, Sunnyvale, California, USA). NAD contents were calculated by subtracting NADH contents from the total NAD and NADH.
Statistics
Statistical analyses were performed using unpaired two-tailed Student’s t-tests and one-way or two-way analysis of variance for multiple comparison with post hoc Tukey-Kramer tests. Statistically significant results are indicated as ***p<0.001, **p<0.01, and *p<0.05. To compare multiple groups in RNA-sequencing (RNA-seq), FDR was calculated. A p value or FDR less than 0.05 was considered to indicate statistical significance. All error bars represent the mean±SD. The Kaplan-Meier method was used for the survival analysis, using IBM SPSS Statistics Advanced (V.28). Otherwise, all statistical analyses were performed using R (V.4.0.2).
Results
Identification of a small molecule enhancing CD8 T cell activation even with weak TCR stimulation
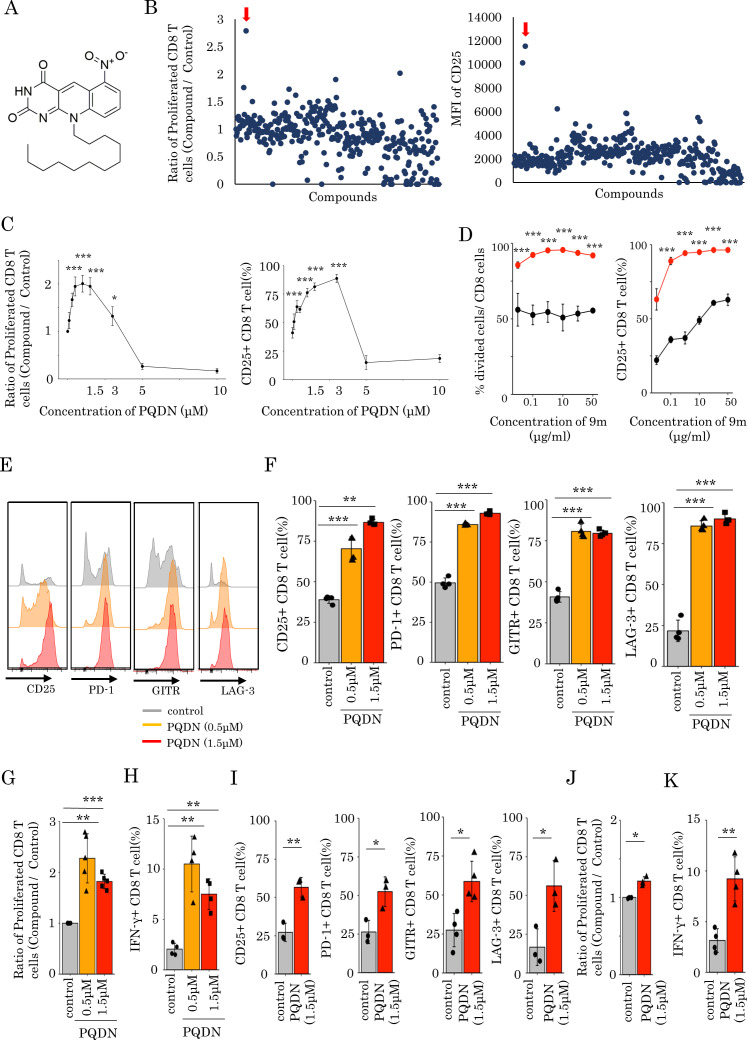
We screened 310 small molecules from the in-house chemical library of the University of Shizuoka to identify novel compounds that enhance CD8 T cell activation despite weak antigen stimulation. We cultured splenocytes from DUC18 transgenic mice, genetically engineered to express a 9m-specific TCR,19 20 in culture medium containing the low immunogenic antigen 9 m peptide and each small molecule for 72 hours and then evaluated CD25 expression and the proliferative activity of CD8 T cells. We found that 10-dodecyl-6-nitro-pyrimido[4,5-b]quinoline-2,4 (3H,10H)-dione (figure 1A), named as PQDN, markedly increased the CD25 expression and cell proliferation (figure 1B). We also observed that PQDN increased CD25 expression and cell proliferation up to 3 µM in a concentration-dependent manner, whereas these were decreased with further increasing concentrations due to toxicity (figure 1C). Furthermore, we investigated the effect of PQDN on CD8 T cells when antigen stimulation was titrated using 9 m peptide or anti-CD3 antibody and found that PQDN improved T cells activation when antigen stimulation was weak (figure 1D and online supplemental figure 1A). Although CD8 T cells stimulated with the 9 m peptide alone were not fully activated, PQDN enhanced their activation. These data show that PQDN improve the T cell activation threshold. Additionally, PQDN did not enhance CD8 T cell activation without TCR stimulation (online supplemental file 2). Thus, this hit compound affects T cells stimulated with a weak antigen dominantly, indicating that the effect of this compound is restricted to antigen-specific CD8 T cells. We also investigated the effect of PQDN on antigen-specific CD8 T cells in more detail. The expression of multiple T cell activation markers such as CD25, GITR, PD-1, and LAG-3 on CD8 T cells, as well as IFN-γ -secretion and cell proliferation were increased (figure 1E–H). Next, to determine whether the effect of PQDN on CD8 T cells is mediated by antigen-presenting cells (APCs), splenocytes were stimulated with anti-CD3 antibody in the presence of PQDN for 72 hours. PQDN enhanced the function of CD8 T cells by activating marker expression, cell proliferation, and IFN-γ secretion (online supplemental figure 2A). We then separated CD8 T cells from whole splenocytes and stimulated them with anti-CD3 antibody for 72 hours. Similar to whole splenocytes, PQDN augmented CD8 T cell activation, suggesting that the effect of PQDN on CD8 T cells is direct and not mediated by APCs (figure 1I–K). Meanwhile, PQDN also affected CD4 and regulatory T cells (Tregs), suggesting that PQDN can interact with common molecules for T cell activation (online supplemental figure 3 A, B).
Figure 1.
(A) Chemical structure of PQDN. (B) Approximately 310 compounds were screened to identify compounds enhancing the activation of CD8 T cells despite weak antigen stimulation. Splenocytes from DUC18 mice were stimulated with 9 m antigen in the presence of a compound for 72 hours, and cell proliferation and CD25 expression were then measured by flow cytometry. Red arrow indicates PQDN. (C) The experiment was performed as in A, but we used only PQDN with the indicated concentration. (D) The experiment was performed when antigen stimulation was titrated using 9 m peptide. (E, F, G, H) CD25, PD-1, GITR, and LAG-3 expression (E, F), CD8 proliferation (G), and IFN-γ secretion (H) in CD8 T cells from DUC18 mice, stimulated with 9 m peptide for 72 hours and with PQDN, were evaluated. Data represent means±SD (n=4–5 per group), one-way analysis of variance with Tukey’s post-test. (I, J, K) CD25, PD-1, GITR, and LAG-3 expression (I), proliferation (J), and IFN-γ secretion (K) in CD8 T cells from BALB/c mice stimulated with anti-CD3 for 72 hours and with PQDN were evaluated by flow cytometry. Data represent the means±SD. (n=3–4 per group), unpaired Student’s t-test. All experiments were repeated three or more times with similar results.
jitc-2021-003958supp002.pdf (14.1MB, pdf)
PQDN enhances mitochondrial function and glycolysis via mitochondrial complex I and III
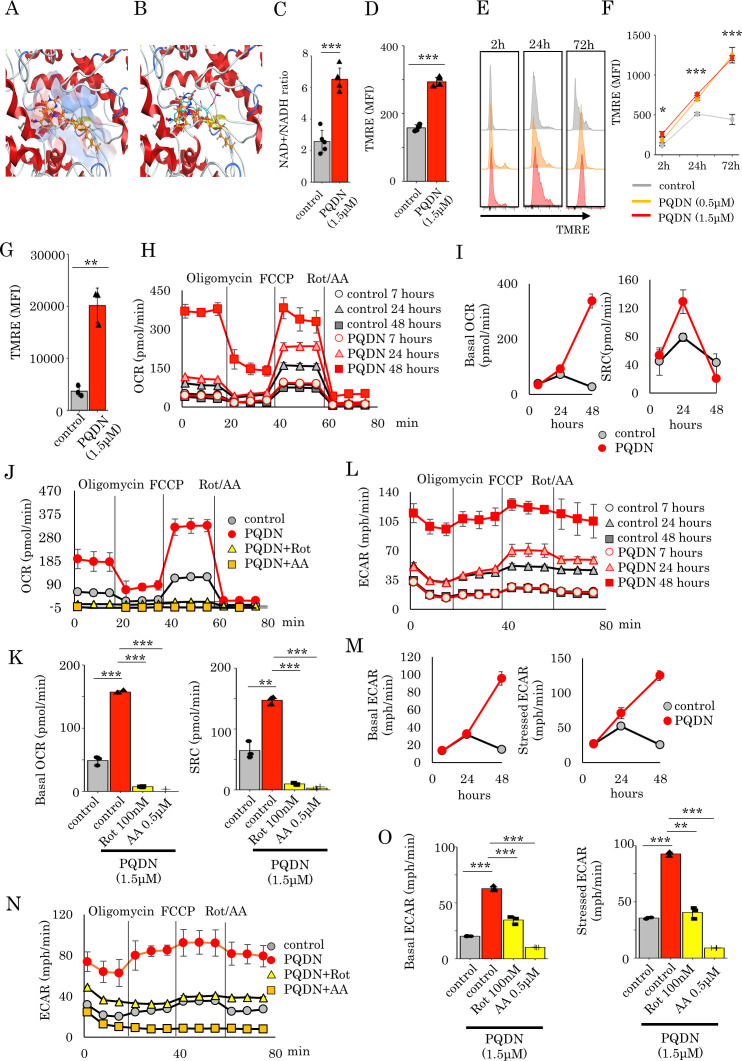
To elucidate the mechanism underlying the effect of PQDN on T cells, we analyzed the whole gene expression in splenocytes cells treated with antigen and PQDN for 24 hours using RNA-Seq. Gene set enrichment analysis revealed that PQDN upregulated mitochondria-related genes, especially for mitochondrial membrane potential to transport proteins such as TIMM9 and TOMM22 (online supplemental figure 4A, B).21 22 Pyrimido[4,5-b]quinoline-2,4 (3H,10H)-dione, a substructure of PQDN, is a substitution of CH for nitrogen at the N5 position of flavin and is similar to that of flavin mononucleotide (FMN). Since FMN acts as a coenzyme for NADH dehydrogenase in mitochondria, we performed a molecular docking of PQDN on the FMN-binding site of mouse mitochondrial complex I. The tricyclic ring of PQDN could effectively fit into the hydrophobic pocket in a similar orientation to that of FMN (figure 2A, B). Hydrogen bond formation between the nitro group and pyrimidine moiety of PQDN with side chains such as Tyr184 and Asp98 were also observed. The docking score of the redocked FMN was –9.12 kcal/mol, while that of the PQDN was similar at approximately –8.72 kcal/mol (online supplemental figure 5A, B). Additionally, we evaluated NAD+/NADH following PQDN treatment to determine whether PQDN can activate NADH as FMN and found that PQDN promotes the conversion of NADH to NAD+ (figure 2C). These results suggest that PQDN may contribute to the electron transport chains (ETCs) in mitochondria. Next, we assessed the membrane potential of mitochondria using flow cytometry with tetramethylrhodamine methyl ester (TMRE) as a fluorescent probe to determine whether PQDN induced mitochondrial activation. The mitochondrial membrane potential is increased 30 min after PQDN treatment in naive T cells (figure 2D), and similar results were obtained in antigen-stimulated T cells; this elevation continued for at least 72 hours after stimulation (figure 2E, F). Similar results were also observed among CD8 tumor-infiltrating lymphocytes (TILs) in a CMS5a murine model (figure 2G). As PQDN elevated mitochondrial membrane potential, we investigated whether it affects mitochondrial respiration. OXPHOS profiles of CD8 T cells were also determined using extracellular flux analysis for the OCR at 7, 24, and 48 hours after PQDN treatment (figure 2H). Basal respiration was increased in both T cells with or without PQDN treatment at 24 hours after antigen stimulation, and this increment was continued in PQDN treated T cells; however, in peptide-stimulated T cells without PQDN, this increment was not observed even at 48 hours (figure 2I). Spare respiratory capacity (SRC) was also increased in both T cells with or without PQDN at 24 hours, and PQDN treatment significantly elevated SRC compared with the vehicle control (figure 2I). This significant increase in SRC by PQDN was temporary and was not observed at 48 hours. Mitochondrial respiration complexes comprise five complexes (complexes I–V). Of these, proton pumping using ubiquinone and ETC mainly occur in mitochondrial complexes I and III, which are required for mitochondrial activation.23 Therefore, to verify whether the effect of PQDN on mitochondrial respiration is mediated by mitochondrial complexes I and III, we cultured DUC18 lymphocytes in the presence of PQDN for 24 hours with a mitochondrial complex I inhibitor (rotenone (Rot)) or complex III inhibitor (antimycin A (AA)); the elevation of basal OCR or SRC by PQDN treatment was diminished by both Rot and AA (figure 2J, K). Considering that OXPHOS as well as glycolysis is necessary for full activation of T cells after antigen recognition,18 we next evaluated the ECAR. Both basal and stressed ECAR were significantly increased by PQDN treatment, and this increase was diminished by both Rot and AA (figure 2L–O).
Figure 2.
(A) Docking model of PQDN to an NADH dehydrogenase FMN-binding site of mouse mitochondrial complex I using MOE. PQDN is indicated in orange in ball-stick representation. (B) Overlay of the original FMN and PQDN generated using MOE. FMN is indicated in cyan in line representation. (C) NADH activation induced by PQDN was measured using an NAD+/NADH assay kit (n=4 per group). (D) Flow cytometric analysis of the mean fluorescence intensity of TMRE in CD8 T cells treated with PQDN. (n=3 per group). (E, F) Kinetics of the TMRE dependent on stimulation time. (G) Flow cytometric analysis of the TMRE in CD8 TILs from CMS5a tumor-bearing mice (n=3 per group). (H and I) The oxygen consumption rate (OCR) of CD8 T cells treated with PQDN. The basal OCR or SRC are shown in figure 2I. (J and K) The OCR of CD8 T cells treated with PQDN for 24 hours using Rot or AA. The basal OCR or SRC are shown in figure 2K (n=3 per group). (L and M) The extracellular acidification rate (ECAR) of CD8 T cells treated with PQDN were measured. The basal and stressed ECAR are shown in figure 2M. (N and O) The ECAR of CD8 T cells treated with PQDN for 24 hours using Rot or AA. Basal and stressed ECAR are shown in figure 2O (n=3 per group). Data are presented as mean±SD. P values were determined using unpaired Student’s t-test or one-way analysis of variance with Tukey’s post-test. *P<0.05. The experiments were repeated at least two to three times. AA, antimycin A; FMN, flavin mononucleotide; Rot, rotenone; SRC, spare respiratory capacity; TMRE, tetramethylrhodamine methyl ester.
Glycolysis induced by PQDN through mitochondrial respiration is essential for CD8 T cell activation
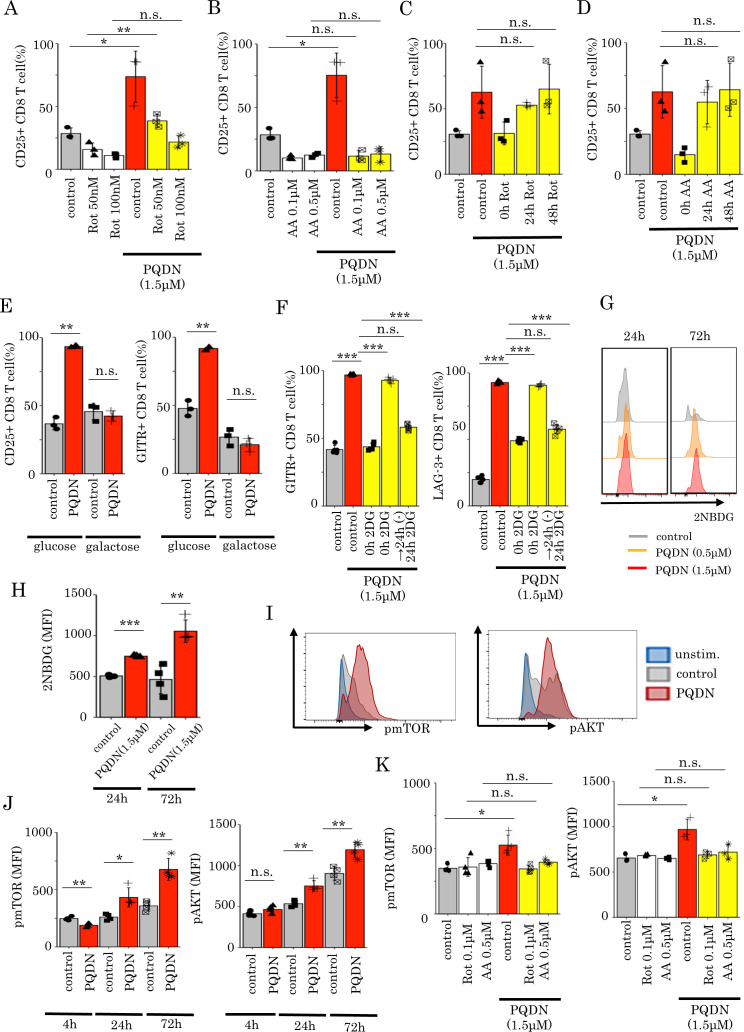
Thus far, we showed that PQDN augments glycolysis following OXPHOS activation via mitochondrial complexes I and III. Next, we investigated whether mitochondrial activation is necessary for the effects of PQDN on CD8 T cells. Thus, DUC18 lymphocytes were cultured with PQDN and with mitochondrial complex inhibitors. Both Rot and AA abolished the effect of PQDN in terms of CD25 expression (figure 3A, B). In addition to mitochondrial complexes I and III, we also examined whether this compound affects T cells via reactive oxygen species (ROS), mitochondrial complex II, or mitochondrial pyruvate carrier (MPC), which are known to be involved in T cell activation.24–26 However, although PQDN produced higher ROS both in vitro and in vivo (online supplemental figure 6A), the antioxidant N-acetylcysteine did not inhibit the effect of PQDN (online supplemental figure 6E). The mitochondrial complex II inhibitor (dimethyl malonate, DMM) and MPC inhibitor (UK5099) also did not diminish the effect of PQDN (online supplemental figure 7A, B). These data suggest that activation of mitochondrial respiration directly through mitochondrial complexes I and III is crucial for CD8 T cell activation induced by PQDN. Interestingly, this cancelation of the effect of PQDN was not observed if Rot or AA was added 24 hours after TCR stimulation (figure 3C, D). From these data, we hypothesized that T cells treated with antigen and PQDN use glycolysis to synthesizes ATP for their activation after 24 hours later following stimulation. Therefore, to verify whether PQDN-induced glycolysis is required for CD8 T cell activation, we cultured DUC18 lymphocytes with PQDN in galactose medium. The effect of PQDN was decreased when CD8 T cells were cultured in glucose-free medium (figure 3E).12 27 Notably, though 2-deoxy-d-glucose (2DG), an inhibitor of d-glucose metabolism, canceled the effect of PQDN, the effect of PQDN was maintained when cultured with 2DG for only 24 hours since the beginning of stimulation and was canceled with 2DG addition at 24 hours after TCR stimulation (figure 3F). Furthermore, we also estimated glucose uptake ability by flow cytometry using a fluorescent d-glucose analog, 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]−2-deoxy-D-glucose (2NBDG). We observed that PQDN improved glucose uptake in CD8 T cells 24 hours and later after TCR stimulation, consistent with the time point of glycolysis acceleration (figure 3G, H). These results suggest that glycolysis induced by PQDN is essential for CD8 T cell activation, especially within the initial 24 hours following TCR stimulation. Next, we investigated how PQDN induces glycolysis via mitochondrial respiration. mTOR/AKT signaling pathways can induce glucose uptake in antigen-stimulated CD8 T cells,28 where glycolysis is regulated by mTOR signaling; this metabolic shift contributes to CD8 T cell differentiation.29 Therefore, we hypothesized that mTOR/AKT signaling may be involved in the activation of glycolysis by PQDN; hence, we investigated whether PQDN accelerates mTOR/AKT signaling in CD8 T cells and found that PQDN induced AKT and mTOR phosphorylation, 24 hours following TCR stimulation, but not at 4 hour (figure 3I, J). The effect of PQDN on the mTOR/AKT signaling was inhibited when CD8 T cells were cultured with mitochondrial inhibitors (Rot and AA) (figure 3K). These results suggest that PQDN accelerates mTOR/AKT signaling through mitochondrial activation and subsequently activates glycolytic pathway as well as mitochondrial respiration.
Figure 3.
(A and B) Flow cytometric analysis of CD25 expression in DUC18 CD8 T cells treated with PQDN using Rot (A) or AA (B) (n=3 per group) (C and D) Flow cytometric analysis of CD25 and CD69 expression in DUC18 CD8 T cells treated with PQDN and Rot (C) or AA (D) (n=3 per group). (E) CD25 and GITR expression in DUC18 CD8 T cells treated with PQDN in glucose or glucose-free medium (n=3 per group). (F) Flow cytometric analysis of GITR and LAG-3 expression in DUC18 CD8 T cells treated with PQDN with 2DG at 0 and 24 hours after stimulation, and subsequently removed 2DG 24 hours after stimulation (n=5 per group). (G and H) Glucose uptake ability in CD8 T cells after treatment with antigen and PQDN was evaluated using 2NBDG (n=4 per group). (I and J) Phosphorylation of mTOR and AKT in DUC18 CD8 T cells treated with antigen and PQDN was measured by flow cytometry at 24 hours (I) or at the indicated time points (J) (n=4 per group). (K) Phosphorylation of mTOR and AKT in CD8 T cells after treatment with antigen and PQDN for 72 hours using Rot or AA (n=3–4 per group). Data are presented as mean±SD. P values were determined using unpaired Student’s t-test or one-way analysis of variance with Tukey’s post-test. *P<0.05. The experiments were repeated at least two to three times. AA, antimycin A; Rot, rotenone.
PQDN tunes inactivated T cell with tumor antigen recognition potent and enhances antitumor immunity
Next to evaluate the in vivo effects of PQDN, we used the CT26 and CMS5a tumor-bearing mice model. PQDN has no toxicity effects on these cell lines at concentrations lower than 10 uM, which suggests that these cell lines show high resistance to the direct effect of PQDN, at such concentrations, and are suitable for evaluating the immune system-mediated effect of PQDN (online supplemental figure 8Supplementary Fig. 8 A, B). First, we examined the change in immune cells following PQDN treatment and found that PQDN increased the tumor-infiltrating CD8 T cells and Tregs and decreases CD4 T cells (online supplemental figure 9). Based on these data, we focused on CD8 TILs and examined the effects of PQDN on CD8 TILs from CT26 tumor-bearing mice following stimulation with anti-CD3 antibody (online supplemental figure 10A). Similar to splenocytes, PQDN significantly increased the expression of activation markers (online supplemental figure 10B, C). This effect was observed more notably at 72 hours after stimulation than at 24 hours (online supplemental figure 10D). Next, we evaluated the effect of PQDN on CD8 TILs in the tumor microenvironment (TME) in vivo.
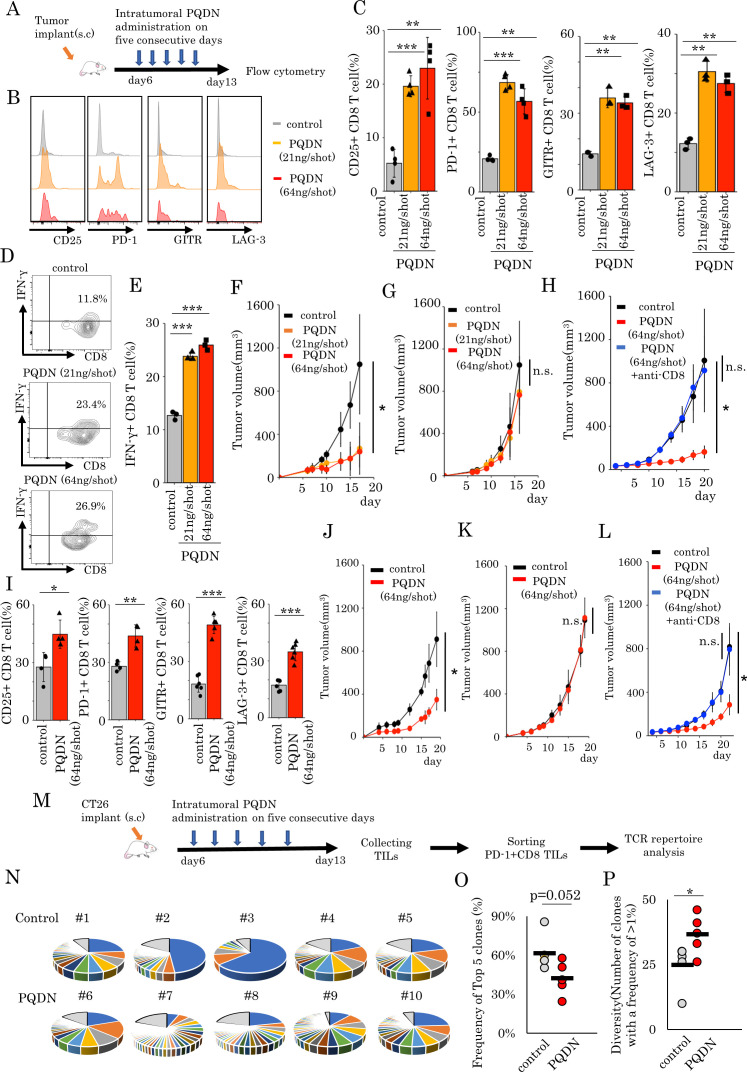
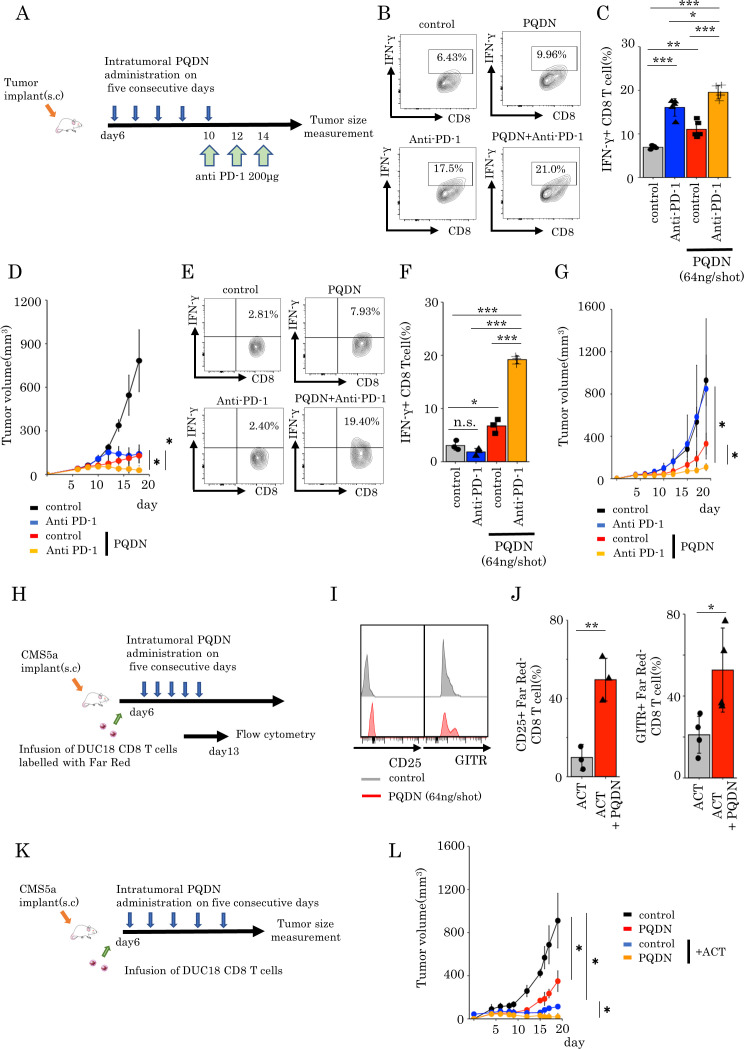
To determine how to inject PQDN into tumor-bearing mice, we examined the optimal dose and times of PQDN injection and decided to inject more than 21 ng PQDN for five consequent days to induce therapeutic effects (online supplemental figure 11A, B). Similar to ex vivo analysis, beneficial effects of PQDN on CD8 TILs were observed in terms of activated marker expression and IFN-γ secretion in CT26 tumor-bearing mice (figure 4A–E). Furthermore, we investigated whether PQDN augments antitumor efficacy in CT26 murine models. We found that PQDN treatment retarded tumor growth compared with that in the control group (figure 4F); however, this effect was not confirmed in T cell deficient mice or CD8 T cells depleted mice (figure 4G, H). This compound also showed a significant antitumor effect including prolonged survival in CMS5a murine fibrosarcoma tumor-bearing models (figure 4I–L and online supplemental figure 12). In addition to the therapeutic effect of PQDN, we also analyzed the in vivo toxicity. PQDN injection for five consequent days did not induce changes in any enzyme levels including aspartate aminotransferase and alanine aminotransferase (online supplemental figure 13A). Additionaly, weight loss related to PQDN treatment was not observed in tumor-bearing mice (online supplemental figure 13B). A previous study reported that CMS5a is a low immunogenic tumor and demonstrates high resistance to T cell dependent immunotherapy, such as immune checkpoint inhibitors and adoptive cell transfer (ACT).5 These data suggest that PQDN improves CD8 T cell function and promotes antitumor immunity in a T cell mediated manner, even in a low immunogenic tumor model. Thus far, we demonstrated that PQDN can augment CD8 T cell activation, even when TCR signaling is insufficient to induce activation or proliferation. Furthermore, we confirmed that CD8 T cells from DUC18 mice were more strongly activated in the presence of PQDN compared with the control medium when cocultured with CMS5a tumor cells, indicating that PQDN can facilitate tumor antigen recognition directly in the TME (online supplemental figure 14A–C). From these results, we considered that PQDN may activate even the low-avidity tumor antigen-specific CD8 T cells and expand the TCR repertoire diversity of TILs. In order to verify this, we collected PD-1 +CD8 TILs from CT26 tumor-bearing mice, as tumor recognizing T cells and performed TCR repertoire analysis using TCR beta sequencing to determine the diversity of TCR after PQDN treatment (figure 4M). The results indicated that PQDN decreased the frequency of the top five clones and expanded the number of clones with frequencies >1% (figure 4N–P).
Figure 4.
(A) The murine tumor cell line CT26 (B–H) or CMS5a (I–L) was subcutaneously inoculated into BALB/c mice. PQDN (n=8 mice per group) intratumorally administered on five consecutive days (days 6–10). (B–E) Flow cytometric analysis of CD25, PD-1, GITR, and LAG-3 expression (B, C, I) or IFN-γ secretion (D, E) in CD8 TILs (n=3–4 per group). (F and J) Tumor-bearing mice were treated with intratumoral PQDN. Tumor sizes are shown (n=5–6 per group). (G and K) The experiment was performed in nude mice as described in G or K. (H and L) The experiment was performed in CD8 depleted mice as described in H or L. (M–P) The murine tumor cell line CT26 was subcutaneously inoculated into BALB/c mice. PQDN (n=5 mice per group) was injected intratumorally on five consecutive days. Three days after the final administration, PD-1+CD8 TILs were isolated and analyzed for their TCR repertoire. (N) The circle graph represents the frequency of clones in PD-1+CD8 TILs from CT26 tumors (n=5 per group). (O and P) The frequency of the top five clones (O) and the number of clones with a frequency of >1% (P) in PD-1+CD8 TILs from CT26 tumors (n=5 per group) are shown. Data are presented as mean±SD. P values were determined using unpaired Student’s t-test or one-way analysis of variance with Tukey’s post-test. *P<0.05. The experiments were repeated at least two to three times. TILs, tumor-infiltrating lymphocytes.
PQDN has a synergistic potent with T cell dependent immunotherapy even in a low immunogenic tumor
As manipulation of mitochondrial quality or quantity or changing mitochondrial activity controls T cell function and improves the efficacy of cancer immunotherapies such as checkpoint inhibitory therapy or adoptive cell therapy,26 30–32 we hypothesized that PQDN can enhance tumors sensitivity to cancer immunotherapy, hence we investigated whether PQDN can enhance the antitumor effects of these therapies in tumor-bearing models. First, we examined whether PQDN improves the efficacy of PD-1 blockade in CT26 murine models (figure 5A). Combination therapy induces the production of higher IFN-γ levels (figure 5B, C) and significantly inhibited tumor growth compared with monotherapy (anti-PD-1 therapy or PQDN) in the CMS5a tumor model (figure 5D). Furthermore, these synergistic effects were also observed in CMS5a mouse models showing a resistance for immune checkpoint inhibitor therapy (figure 5E–G). Next, we determined whether PQDN can also improve the efficacy of adoptive cell therapy. Though adoptive cell therapy is effective, it is not sufficient. Because activation of endogenous tumor-specific immune responses were essential to induce the efficacy of this therapy.7–9 It has been reported that induction of endogenous immune responses against tumor through antigen spreading contributes to retardation of tumor growth after adoptive cell therapy. Thus, we examined whether intratumoral injection of this compound after adoptive T cell therapy activates the function of endogenous T cells at tumor after infusion of tumor-specific CD8 T cells. CD8 T cells derived from DUC18 mice labeled with Far Red were intravenously infused into CMS5a tumor-bearing mice 6 days after inoculation. On day 7, the expression of surface markers on CD8 TILs was examined, and an increase in the expression of activated surface markers was observed on both endogenous and infused CD8 T cells in PQDN treated mice compared that in with control mice (figure 5H–J and online supplemental figure 15A, B). Furthermore, intratumoral injection of PQDN remarkably increased the therapeutic efficacy of ACT with PQDN in mice bearing CMS5a (figure 5K, L). These results suggest that PQDN can improve the function of endogenous T cells and enhance immune checkpoint inhibitors and adoptive cell therapy, even in low immunogenic tumors.
Figure 5.
(A–G) CT26 (A–D) or CMS5a (E–G) cells were subcutaneously inoculated into BALB/c mice. PQDN was injected intratumorally into the same mice on five consequent days (6–10). Anti-PD-1 antibody was administrated intravenously on days 10, 12, and 14 into the same mice. (B, C) Flow cytometric analysis of IFN-γ secretion in CD8 TILs from CT26 tumors treated with the combination of intratumoral PQDN and anti-PD-1 antibody. Data are shown as mean±SD (n=5 per group) and analyzed using one-way analysis of variance (ANOVA) with Tukey’s post-test. (D) CT26-bearing mice were treated with a combination of intra tumorous PQDN and anti-PD-1 antibody. Tumor sizes are shown (n=4–5 per group) and analyzed using two-way ANOVA with Tukey’s post-test. (E, F) Flow cytometric analysis of IFN-γ secretion in CD8 TILs from CMS5a tumors treated with the combination of intratumoral PQDN and anti-PD-1 antibody. Data are shown as mean±SD (n=3 per group) and analyzed using one-way ANOVA with Tukey’s post-test. (G) CMS5a-bearing mice were treated with a combination of intratumoral PQDN and anti-PD-1 antibody. Tumor sizes are shown (n=3–4 per group) and analyzed using two-way ANOVA with Tukey’s post-test. (H–L) CMS5a cells were subcutaneously inoculated into BALB/c mice. CD8 cells isolated from DUC18 mice were infused on day 6. PQDN was injected intratumorally into the same mice for five consequent days (days 6 to 10). (I, J) CD25, GITR expression in endogenous CD8 T cells (Far Red – T cells) from CMS5a tumors. Data are shown as mean±SD (n=3–4 per group) and analyzed using unpaired Student’s t-test. (L) Tumor sizes are shown (n=3 per group) and analyzed using two-way ANOVA with Tukey’s post-test. TILs, tumor-infiltrating lymphocytes.
PQDN enhances the activation of human CD8 T cells
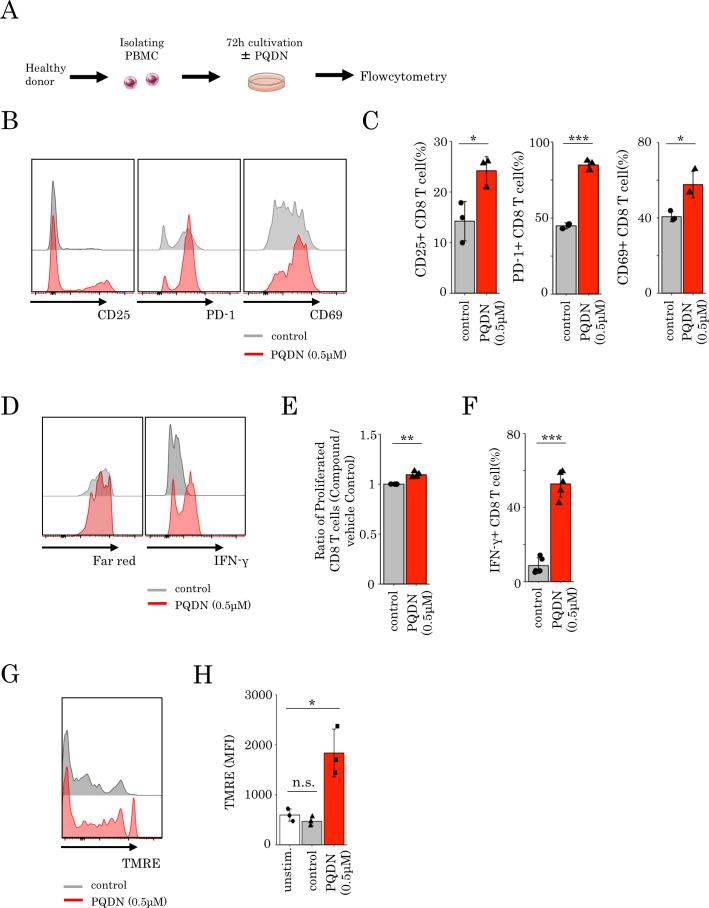
The results from our mouse studies prompted us to investigate whether PQDN might also augment CD8 T cell function in human PBMCs. Therefore, we examined the cell surface marker expression, cell proliferation, and IFN-γ secretion in CD8 T cells derived from healthy donor PBMCs stimulated with anti CD3 alone or in combination with PQDN (figure 6A). We found a significant increase in activation markers, cell proliferation, and IFN-γ secretion with PQDN treatment at 0.5 µM (figure 6B–F). Furthermore, to verify whether PQDN affects the mitochondrial membrane potential in human CD8 T cells, we assessed the change in TMRE in CD8 T cells and found that PQDN elevated TMRE at 2 hours after anti-CD3 stimulation (figure 6G, H). These results suggest that PQDN is also a promising compound to augment CD8 T cell function through mitochondrial activation in humans.
Figure 6.
(A–F) PBMCs isolated from healthy donors were stimulated with anti-CD3 antibody in the presence or absence of PQDN for 72 hours. CD25, PD-1, and CD69 expression (B, C), proliferation (D, E) and IFN-γ secretion (D, F) in CD8 T cells were measured by flow cytometry. Data represent means±SD (n=3–5 per group), unpaired Student’s t-test. (G, H) Mean fluorescence intensity of TMRE in CD8 T cells from healthy donor PBMCs stimulated with anti-CD3 for 2 hours. Data represent means±SD (n=3 per group), one-way analysis of variance with Tukey’s post-test.
Discussion
In this study, we identified PQDN as a small molecule that can augment CD8 T cell activation even with weak TCR stimulation, which is insufficient to activate these cells, indicating that this compound tunes T cell activation thresholds following antigen recognition. Mechanistically, PQDN enhances mitochondrial reciprocal capacity and subsequent glycolysis acceleration via the activating of ETC. Furthermore, intratumoral administration of PQDN increases the frequency of PD-1 +CD8 T cells and expands the TCR repertoire diversity of these cells at the tumor site. These results suggest that PQDN can activate inactive CD8 TILs that recognize the tumor antigen but do not respond to a tumor due to weak antigen stimulation. Previous studies have reported that diverse qualities of TCR are associated with the therapeutic efficacy of cancer immunotherapy, including checkpoint blockade therapy or cancer vaccines.33–35 Therefore, an increase in functional TCR diversity by PQDN may be useful for improving cancer immunotherapy. Furthermore, these findings suggest that tumor-specific T cells with low antigen recognition ability exist at the tumor site and that these T cells might be a target for developing novel cancer immunotherapies.
Modulating the metabolism of T cells at the tumor site improves these functions and augments antitumor immune responses.26 30–32 Attenuation of mitochondrial activity induces TIL dysfunction.26 31 32 36 37 Reduction of PPAR-gamma coactivator 1a (PGC1a) in TILs reduces mitochondrial function and mass and leads to metabolic exhaustion, resulting in T cell dysfunction. However, enforced expression of PGC1a in TILs or treatment with bezafibrate, a pan-PPAR agonist, bolsters mitochondrial biogenesis and improves antitumor T cell function.32 36 ROS generation is also a crucial mechanism for enhancing T cell activity following mitochondrial activation.38 ROS are generated at mitochondrial complexes I and III through the ETC39 and activate CD8 T cells by enhancing NFAT signaling and cytokine secretion.24 25 Trifluoromethoxy carbonylcyanide phenylhydrazone (FCCP), an ROS inducer, increases the functional potency of T cells by activating mitochondria.26 In this study, we observed that PQDN enhanced mitochondrial activation and ROS generation in antigen-stimulated T cells. However, ROS generation by PQDN was not related to CD8 T cell activation (online supplemental figure 6A–E). Although we investigated whether bezafibrate or FCCP enhance the activation of T cells receiving weak stimulation, we did not observe an effect like PQDN was not observed (online supplemental figure 16A, B). This difference might be caused by differences in the mode of mitochondrial activation by these compounds; for instance, bezafibrate or FCCP increases mitochondrial activity indirectly in a PPAR-mediated or ROS-mediated manner, whereas PQDN affects the mitochondria directly via ETC. Manipulating mitochondrial function directly might be critical for tuning the T cell activation threshold in low antigen-stimulated T cells. Guo et al reported that IL-10 directly enhances the expansion and effector function of TILs by promoting OXPHOS.31 This reactivation of OXPHOS in TILs is dependent on MPC, which plays a role in cytosolic pyruvate incorporation into the mitochondrial matrix.40 However, the effect of PQDN was not mediated by MPC. Altogether, although PQDN augments TIL activation by enhancing mitochondrial function, the mode of action of PQDN differs from that of various mitochondrial activators reported previously.
CD28 signaling or IL-2 signaling modulates the activation threshold and induces the biological response of T cells.41 42 Additionally, these signaling pathways induce glycolysis in antigen-stimulated T cells.43 Therefore, these reports suggest that glycolysis plays a crucial role in tuning the activation threshold. Considering this, modification of the activation threshold in PQDN-treated cells might be induced by accelerated glycolysis, which leads to T cell activation despite weak antigen stimulation.
Although PQDN activates mitochondrial respiration, mode of action of PQDN is unique. Several compounds that activate mitochondria have been reported including dichloroacetate (DCA)44 or triptolinamide (TLAM).45 DCA, an inhibitor of PDK, enhances the phosphorylation of PDH and increases the flux of pyruvate into mitochondria, resulting in OXPHOS activation.44 Furthermore, TLAM increases OXPHOS via the AMPK/fatty acid oxidation pathway by inhibiting phosphofructokinase-1.45 However, these compounds simultaneously reduce glycolysis, because PDK and PFK are important regulators of glycolysis,46 47 meaning that these compounds enhance mitochondrial function as a replacement for glycolysis. Unlike these compounds, PQDN enhanced mitochondrial function as well as glycolysis. The reason for this might be that OXPHOS was directly activated by PQDN and not by inhibiting the molecules involved in glycolysis. Antigen-stimulated T cells require a large amount of energy for activation and acquire energy by activating both OXPHOS and glycolysis.18 Thus, PQDN is an ideal compound that augments T cell activation through mitochondrial activation. Meanwhile, PQDN may also activate Tregs at tumor site. Since mitochondrial capacity is essential for the Tregs survival and function,48 ETC activation by PQDN may contribute to Tregs increment. However, although PQDN increased Tregs at tumor site, it can lead to tumor growth retardation, possibly due to the PQDN induction of antigen specific-CD8 T cells to overcome suppression by Tregs.
In this study, we indicated that PQDN may bind to NADH dehydrogenase in the mitochondrial respiratory complex I like FMN (figure 2A, B). PQDN was previously reported as a cell growth inhibitory compound.49 Shinkai et al50 reported that pyrimido[4,5-b]quinoline-2,4 (3H,10H)-dione structure, known as 5-deazaflavin, can promote electron bridge from NADH to FMN in an in vitro benzylamine oxidation reaction model. However, to date, there have been no reports of the biological effect of PQDN on mitochondrial function. Conversely, when FMN binds to NADH dehydrogenase as a prosthetic group, FMN receives hydrogen from NADH along with two electrons and transfers these electrons to iron-sulfur clusters. This electron transport induces leakage of protons into the mitochondrial intermembrane space (IMS); ATP is then synthesized from ADP when the protons transfer from IMS to the matrix through complex V. Altogether, based on the structure of PQDN, this compound is considered to promote ETC in mitochondria, resulting in increased mitochondrial activity. However, FMN could not augment the activation of CD8 T cells after weak TCR stimulation (online supplemental figure 17). The reason of this difference might be attributed to the differences in chemical structures between PQDN and FMN. FMN is water soluble while PQDN is lipophilic. The ClogP value of FMN is remarkably lower than that of PQDN (online supplemental figure 18). Thus, PQDN might enhance the ETC at mitochondria after penetrating cell membranes, which FMN might not be able to. It is unclear how activating mitochondrial function by PQDN accelerates glycolytic activity. Further studies will provide insight into the fundamental mechanisms of T cell activation and metabolic reprogramming.
In conclusion, we discovered a novel compound, PQDN, that increases mitochondrial respiration capacity and enhances glycolysis following mitochondrial activation. To our knowledge, no other compound that can simultaneously activate mitochondria and glycolysis has been reported; therefore, this compound is valuable for cancer immunotherapy. Furthermore, this compound augments antitumor immunity by expanding functional TCR diversity. Our findings indicate that PQDN can create an immune-responsive environment even in a low immunogenic tumor by modulating the activation threshold. However, further research is necessary to understand its antitumor effects in patients with cancer. Taken together, our results indicate that PQDN activates CD8 T cells in a previously unknown manner and is highly potent in overcoming immunotherapy resistance in tumors.
jitc-2021-003958supp001.pdf (14.1MB, pdf)
Acknowledgments
We would like to thank S Yamaguchi and H Masumoto for providing technical assistance.
Footnotes
YD and DM contributed equally.
Correction notice: This article has been corrected since it was first published. The author name Daisuke Muraoka was incorrectly spelt as Daisuk Muraoka.
Contributors: DM, HYam, HM and HI supervised the study. YD, DM, KY and HI conceived and designed the experiments. YD and DM performed the experiments. YD and DM performed data analysis. YS, NO, AA, and HYag contributed the reagents and materials. YD and DM wrote the manuscript. All authors reviewed and approved the final manuscript. DM is a guarantor for this study and publication.
Funding: This study was partially supported by the Japan Society for the Promotion of Science KAKENHI (Grant Number 17K07222 and 21H03005).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study does not involve human participants.
References
- 1.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28:3167–75. 10.1200/JCO.2009.26.7609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robbins PF, Kassim SH, Tran TLN, et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res 2015;21:1019–27. 10.1158/1078-0432.CCR-14-2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 2018;378:439–48. 10.1056/NEJMoa1709866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevanović S, Pasetto A, Helman SR, et al. Landscape of immunogenic tumor antigens in successful immunotherapy of virally induced epithelial cancer. Science 2017;356:200–5. 10.1126/science.aak9510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muraoka D, Seo N, Hayashi T, et al. Antigen delivery targeted to tumor-associated macrophages overcomes tumor immune resistance. J Clin Invest 2019;129:1278–94. 10.1172/JCI97642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt J, Smith AR, Magnin M, et al. Prediction of neo-epitope immunogenicity reveals TCR recognition determinants and provides insight into immunoediting. Cell Rep Med 2021;2:100194. 10.1016/j.xcrm.2021.100194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robbins PF, Li YF, El-Gamil M, et al. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J Immunol 2008;180:6116–31. 10.4049/jimmunol.180.9.6116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parkhurst MR, Joo J, Riley JP, et al. Characterization of genetically modified T-cell receptors that recognize the CEA:691-699 peptide in the context of HLA-A2.1 on human colorectal cancer cells. Clin Cancer Res 2009;15:169–80. 10.1158/1078-0432.CCR-08-1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaczanowska S, Joseph AM, Guo J, et al. A synthetic CD8α:MyD88 Coreceptor enhances CD8+ T-cell responses to weakly immunogenic and lowly expressed tumor antigens. Cancer Res 2017;77:7049–58. 10.1158/0008-5472.CAN-17-0653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phan AT, Doedens AL, Palazon A, et al. Constitutive glycolytic metabolism supports CD8+ T cell effector memory differentiation during viral infection. Immunity 2016;45:1024–37. 10.1016/j.immuni.2016.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ron-Harel N, Santos D, Ghergurovich JM, et al. Mitochondrial biogenesis and proteome remodeling promote one-carbon metabolism for T cell activation. Cell Metab 2016;24:104–17. 10.1016/j.cmet.2016.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang C-H, Curtis JD, Maggi LB, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 2013;153:1239–51. 10.1016/j.cell.2013.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang R, Dillon CP, Shi LZ, et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 2011;35:871–82. 10.1016/j.immuni.2011.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein-Hessling S, Muhammad K, Klein M, et al. NFATc1 controls the cytotoxicity of CD8+ T cells. Nat Commun 2017;8:511. 10.1038/s41467-017-00612-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toriyama K, Kuwahara M, Kondoh H, et al. T cell-specific deletion of Pgam1 reveals a critical role for glycolysis in T cell responses. Commun Biol 2020;3:394. 10.1038/s42003-020-01122-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menk AV, Scharping NE, Moreci RS, et al. Early TCR signaling induces rapid aerobic glycolysis enabling distinct acute T cell effector functions. Cell Rep 2018;22:1509–21. 10.1016/j.celrep.2018.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu K, Yin N, Peng M, et al. Glycolysis fuels phosphoinositide 3-kinase signaling to bolster T cell immunity. Science 2021;371:405–10. 10.1126/science.abb2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine LS, Hiam-Galvez KJ, Marquez DM, et al. Single-cell analysis by mass cytometry reveals metabolic states of early-activated CD8+ T cells during the primary immune response. Immunity 2021;54:e825:829–44. 10.1016/j.immuni.2021.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikeda H, Ohta N, Furukawa K, et al. Mutated mitogen-activated protein kinase: a tumor rejection antigen of mouse sarcoma. Proc Natl Acad Sci U S A 1997;94:6375–9. 10.1073/pnas.94.12.6375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanson HL, Donermeyer DL, Ikeda H, et al. Eradication of established tumors by CD8+ T cell adoptive immunotherapy. Immunity 2000;13:265–76. 10.1016/S1074-7613(00)00026-1 [DOI] [PubMed] [Google Scholar]
- 21.Qiu J, Wenz L-S, Zerbes RM, et al. Coupling of mitochondrial import and export translocases by receptor-mediated supercomplex formation. Cell 2013;154:596–608. 10.1016/j.cell.2013.06.033 [DOI] [PubMed] [Google Scholar]
- 22.Sotgia F, Whitaker-Menezes D, Martinez-Outschoorn UE, et al. Mitochondria “fuel” breast cancer metabolism: fifteen markers of mitochondrial biogenesis label epithelial cancer cells, but are excluded from adjacent stromal cells. Cell Cycle 2012;11:4390–401. 10.4161/cc.22777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cogliati S, Lorenzi I, Rigoni G, et al. Regulation of mitochondrial electron transport chain assembly. J Mol Biol 2018;430:4849–73. 10.1016/j.jmb.2018.09.016 [DOI] [PubMed] [Google Scholar]
- 24.Weinberg SE, Sena LA, Chandel NS. Mitochondria in the regulation of innate and adaptive immunity. Immunity 2015;42:406–17. 10.1016/j.immuni.2015.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinberg F, Hamanaka R, Wheaton WW, et al. Mitochondrial metabolism and ROS generation are essential for KRAS-mediated tumorigenicity. Proc Natl Acad Sci U S A 2010;107:8788–93. 10.1073/pnas.1003428107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chamoto K, Chowdhury PS, Kumar A, et al. Mitochondrial activation chemicals synergize with surface receptor PD-1 blockade for T cell-dependent antitumor activity. Proc Natl Acad Sci U S A 2017;114:E761–70. 10.1073/pnas.1620433114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cham CM, Driessens G, O'Keefe JP, et al. Glucose deprivation inhibits multiple key gene expression events and effector functions in CD8+ T cells. Eur J Immunol 2008;38:2438–50. 10.1002/eji.200838289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wofford JA, Wieman HL, Jacobs SR, et al. IL-7 promotes GLUT1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood 2008;111:2101–11. 10.1182/blood-2007-06-096297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buck MD, O'Sullivan D, Pearce EL. T cell metabolism drives immunity. J Exp Med 2015;212:1345–60. 10.1084/jem.20151159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eikawa S, Nishida M, Mizukami S, et al. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc Natl Acad Sci U S A 2015;112:1809–14. 10.1073/pnas.1417636112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo Y, Xie Y-Q, Gao M, et al. Metabolic reprogramming of terminally exhausted CD8+ T cells by IL-10 enhances anti-tumor immunity. Nat Immunol 2021;22:746–56. 10.1038/s41590-021-00940-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chowdhury PS, Chamoto K, Kumar A, et al. PPAR-induced fatty acid oxidation in T cells increases the number of tumor-reactive CD8+ T cells and facilitates anti-PD-1 therapy. Cancer Immunol Res 2018;6:1375–87. 10.1158/2326-6066.CIR-18-0095 [DOI] [PubMed] [Google Scholar]
- 33.Han J, Duan J, Bai H, et al. TCR repertoire diversity of peripheral PD-1+CD8+ T cells predicts clinical outcomes after immunotherapy in patients with non-small cell lung cancer. Cancer Immunol Res 2020;8:146–54. 10.1158/2326-6066.CIR-19-0398 [DOI] [PubMed] [Google Scholar]
- 34.Poran A, Scherer J, Bushway ME. Combined TCR repertoire profiles and blood cell phenotypes predict melanoma patient response to personalized neoantigen therapy plus anti-PD-1. Cell Rep Med 2020;1:100141. 10.1016/j.xcrm.2020.100141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hopkins AC, Yarchoan M, Durham JN, et al. T cell receptor repertoire features associated with survival in immunotherapy-treated pancreatic ductal adenocarcinoma. JCI Insight 2018;3 10.1172/jci.insight.122092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scharping NE, Menk AV, Moreci RS, et al. The tumor microenvironment represses T cell mitochondrial biogenesis to drive intratumoral T cell metabolic insufficiency and dysfunction. Immunity 2016;45:374–88. 10.1016/j.immuni.2016.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu Y-R, Imrichova H, Wang H, et al. Disturbed mitochondrial dynamics in CD8+ TILs reinforce T cell exhaustion. Nat Immunol 2020;21:1540–51. 10.1038/s41590-020-0793-3 [DOI] [PubMed] [Google Scholar]
- 38.Sena LA, Li S, Jairaman A, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 2013;38:225–36. 10.1016/j.immuni.2012.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol 2003;552:335–44. 10.1113/jphysiol.2003.049478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herzig S, Raemy E, Montessuit S, et al. Identification and functional expression of the mitochondrial pyruvate carrier. Science 2012;337:93–6. 10.1126/science.1218530 [DOI] [PubMed] [Google Scholar]
- 41.Acuto O, Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat Rev Immunol 2003;3:939–51. 10.1038/nri1248 [DOI] [PubMed] [Google Scholar]
- 42.Au-Yeung BB, Smith GA, Mueller JL, et al. IL-2 modulates the TCR signaling threshold for CD8 but not CD4 T cell proliferation on a single-cell level. J Immunol 2017;198:2445–56. 10.4049/jimmunol.1601453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frauwirth KA, Riley JL, Harris MH, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity 2002;16:769–77. 10.1016/S1074-7613(02)00323-0 [DOI] [PubMed] [Google Scholar]
- 44.Shen Y-C, Ou D-L, Hsu C, et al. Activating oxidative phosphorylation by a pyruvate dehydrogenase kinase inhibitor overcomes sorafenib resistance of hepatocellular carcinoma. Br J Cancer 2013;108:72–81. 10.1038/bjc.2012.559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kobayashi H, Hatakeyama H, Nishimura H, et al. Chemical reversal of abnormalities in cells carrying mitochondrial DNA mutations. Nat Chem Biol 2021;17:335–43. 10.1038/s41589-020-00676-4 [DOI] [PubMed] [Google Scholar]
- 46.Mor I, Cheung EC, Vousden KH. Control of glycolysis through regulation of PFK1: old friends and recent additions. Cold Spring Harb Symp Quant Biol 2011;76:211–6. 10.1101/sqb.2011.76.010868 [DOI] [PubMed] [Google Scholar]
- 47.Takubo K, Nagamatsu G, Kobayashi CI, et al. Regulation of glycolysis by PDK functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells. Cell Stem Cell 2013;12:49–61. 10.1016/j.stem.2012.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weinberg SE, Singer BD, Steinert EM, et al. Mitochondrial complex III is essential for suppressive function of regulatory T cells. Nature 2019;565:495–9. 10.1038/s41586-018-0846-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawamoto T, Ikeuchi Y, Hiraki J, et al. Synthesis and evaluation of nitro 5-deazaflavins as novel bioreductive antitumor agents. Bioorg Med Chem Lett 1995;5:2109–14. 10.1016/0960-894X(95)00353-U [DOI] [PubMed] [Google Scholar]
- 50.Shinkai S, Kuroda H, Manabe O, et al. Flavin plus 5-deazaflavin as a turnover oxidation system: model for the electron bridge from NAD+ to flavin. J Chem Soc Chem Commun 1981;8:391–2. 10.1039/c39810000391 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2021-003958supp002.pdf (14.1MB, pdf)
jitc-2021-003958supp001.pdf (14.1MB, pdf)
Data Availability Statement
Data are available on reasonable request.