Abstract
The flavonoid silymarin extracted from the seeds of Sylibum marianum is a mixture of 6 flavolignan isomers. The 3 more important isomers are silybin (or silibinin), silydianin, and silychristin. Silybin is functionally the most active of these compounds. This group of flavonoids has been extensively studied and they have been used as hepato-protective substances for the mushroom Amanita phalloides intoxication and mainly chronic liver diseases such as alcoholic cirrhosis and nonalcoholic fatty liver. Hepatitis C progression is not, or slightly, modified by silymarin. Recently, it has also been proposed for SARS COVID-19 infection therapy. The biochemical and molecular mechanisms of action of these substances in cancer are subjects of ongoing research. Paradoxically, many of its identified actions such as antioxidant, promoter of ribosomal synthesis, and mitochondrial membrane stabilization, may seem protumoral at first sight, however, silymarin compounds have clear anticancer effects. Some of them are: decreasing migration through multiple targeting, decreasing hypoxia inducible factor-1α expression, inducing apoptosis in some malignant cells, and inhibiting promitotic signaling among others. Interestingly, the antitumoral activity of silymarin compounds is limited to malignant cells while the nonmalignant cells seem not to be affected. Furthermore, there is a long history of silymarin use in human diseases without toxicity after prolonged administration. The ample distribution and easy accessibility to milk thistle—the source of silymarin compounds, its over the counter availability, the fact that it is a weed, some controversial issues regarding bioavailability, and being a nutraceutical rather than a drug, has somehow led medical professionals to view its anticancer effects with skepticism. This is a fundamental reason why it never achieved bedside status in cancer treatment. However, in spite of all the antitumoral effects, silymarin actually has dual effects and in some cases such as pancreatic cancer it can promote stemness. This review deals with recent investigations to elucidate the molecular actions of this flavonoid in cancer, and to consider the possibility of repurposing it. Particular attention is dedicated to silymarin's dual role in cancer and to some controversies of its real effectiveness.
Keywords: antioxidant, cancer, invasion, migration, milk thistle, silybin, silymarin
Introduction
Research on plants and their possible curative properties is not new. It has been occurring since ancient times. In the last 200 years this search has become more scientifically oriented and led to discoveries such as curare, strychnine, atropine, salicylate, digitalis, and more recently taxanes, artemisinin, vitamins, and many others. These naturally originated molecules “have cellular targets similar to those of new drugs developed by pharmaceutical companies.” 1 Many of these natural products were so strikingly important for human health that they swiftly entered clinical practice. Sometimes, they were favorably modified by the pharmaceutical industry and then derivatives with enhanced benefits were born. While taxane compounds are one of the best examples of a success story in oncology, other compounds, not so blatantly effective as taxanes are on the waiting list. There is also a group of natural products that were, and are, used for known diseases other than cancer. In some cases, their antitumoral effects were slowly recognized and they were repurposed. Silymarin is one of this type of products, with some recognized antitumor effects however, repurposing has not yet occurred. Seeds of Silybum marianum, 2 popularly known as milk thistle, have been used since ancient times to treat diverse ailments, and more recently liver damage due to toxins, particularly Amanita phaloides poisoning (but including many others such as carbon tetrachloride, 3 metals, allylalcohol) and alcohol-induced damage, including hepatitis, cirrhosis, and jaundice.4–6 (From a technical point, what are commonly called seeds are actually fruits, but we shall call them seeds following other publication precedents). The last 15 years have witnessed a growing interest in silymarin and the plant it comes from: Silybum marianum (L.) Gaertn (also known as Carduus marianus and wild artichoke).
Although Silymarin is probably the most thoroughly studied nutraceutical, it is looked upon with skepticism by the medical profession for multiple reasons, such as:
ample distribution and easy accessibility to milk thistle;
over the counter availability;
the fact that it is a weed;
some controversial issues regarding bioavailability, and pharmacological actions;
its status as a nutraceutical rather than a drug according to FDA;
its vulgarization through many nonscientific Internet pages dedicated to silymarin compounds;
the enormous number of manufacturers, many of them scarcely known (Figure 1);
the direct consequence of this “popularization” is that it is available over the counter at the herbalist shop or through the Internet, rather than with a prescription in the pharmacy; 7
the lack of striking effects on the disease;
the fact that it is not usually considered in university-level pharmacology courses.
Figure 1.
A glimpse of the multiple brands and presentations of silymarin compounds in the US and European markets with the resultant “vulgarization.” There are many “silymarins” developed in well accredited pharmaceutical laboratories, but there are also many produced by scarcely known sources. Most can be acquired through the Internet.
Definition. Silymarin is the standardized extract obtained from the dried seeds of Silybum marianum (milk thistle) containing approximately 70% to 80% of the silymarin complex and an approximately 20% to 30% chemically undefined fraction, comprising mostly other polyphenolic compounds. The main component is silybin (silibinin). Silymarin and silybin are not synonyms. However, many older reports indistinctly use one or the other term, leading to some confusion. Silymarin extract and its components may frequently differ in their effects due to differences in solubility and bioavailability.
History. Silymarin has been used in Europe since the fourth century BCE by Theophrastus of Eresus, and reappears in the year 65 of current era in Pedanius Dioscorides’ De Materia Medica. Here he proposed milk thistle for the treatment of serpent venom bite and called it silybon. 8 It does not seem to be part of Traditional Chinese Medicine. 9 It was also used in Ancient Egypt, 10 however, we do not know exactly for what purpose. 11 During the Renaissance some of the therapeutic effects were discovered and published by herbalists and physicians such as Pietro Andrea Mattioli (1544) and Hieronymus Bock (1539), among others. In the seventeenth century, an English botanist, Nicholas Culpeper, suggested that milk thistle was useful for liver diseases.
Location and Habitat. This invasive annual plant was originally found in the Mediterranean basin, but now it is present in all the continents. It requires dry, warm soil and it is very competitive eliminating other plants. 12
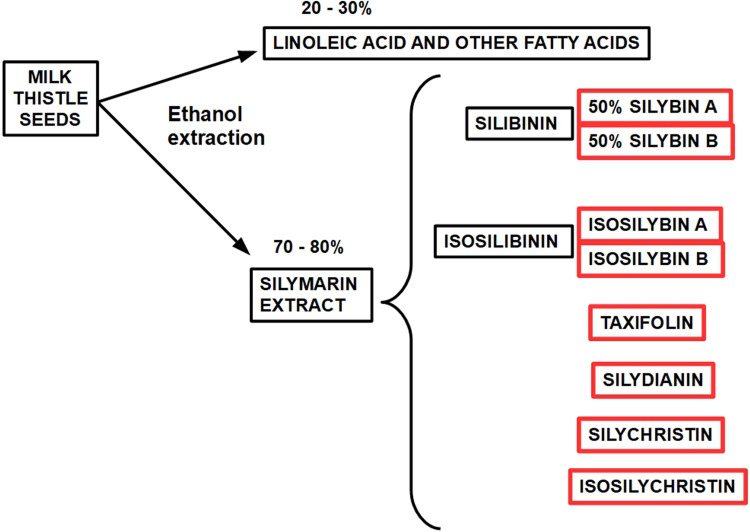
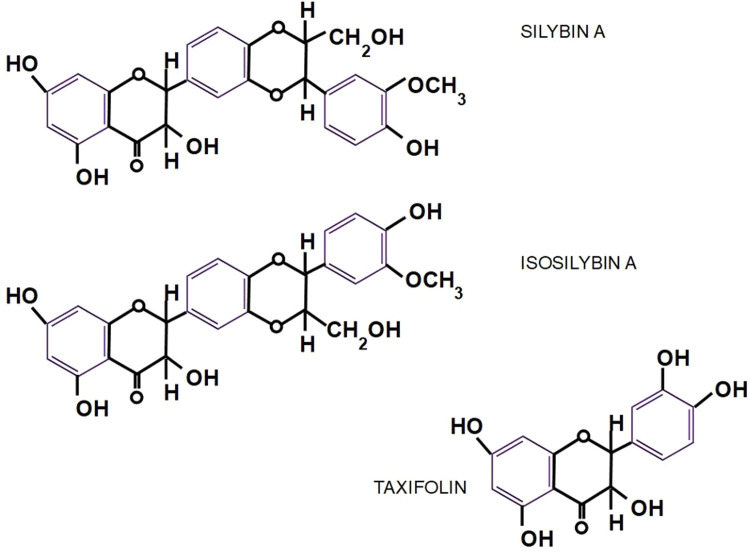
Chemistry. The standardized extract obtained from the seeds of Sylibum marianum is known as silymarin which contains between 70% and 80% of silymarin flavolignans. Sylibum marianum is a mixture of 8 flavolignan structurally related isomers: silybin (or silibinin), isosilibinin, silydianin, silychristin, isosilychristin, and taxifolin.13, 14 The main component of silymarin is Silibinin which is a compound consisting of equal amounts of silybin A and silybin B (CAS 22888-70-6).
BOX 1: Average composition of silymarin.
Silybin 60% to 70%
Silychristin 20%
Silydianin 10%
Isosilybin 5%
Taxifolin 1%
Small amounts of the flavonoids: quercetin, kaempferol, apigenin, naringin, eriodyctiol.
In 1959, Möschlin isolated silybin, 15 and then in 1968 silymarin chemistry was described in detail by Wagner et al,16, 17 and Pelter and Hansel. 18 Today, more than 50 years have elapsed since the initial hepatic antitoxic and protective function of the compound was discovered and now, its antitumor activity is under scrutiny (Figures 2 to 4). Silymarin, the active principal component of milk thistle, was originally thought to be one substance, until it was discovered that it is actually composed of a group of different flavolignans (Box 1).
Figure 2.
Milk thistle and the chemical structure of silybin (C25 H22 O10), with its proprietary numbering. Of note, is the similarity between silybin and steroid hormones. The lower panel shows silibinin's structural formula where 3 different chemical groups can be identified: a taxifolin and a coniferyl alcohol united by an oxirane ring. According to Biedermann et al, 22 “the 20-OH group was established to be the most active radical scavenging moiety and also the most important group responsible for the lipoperoxidation inhibitory activity.” Positions 2-3 also play a role in antioxidant activity because these positions can be oxidized to produce 2-3 dehydrosilybin (see Figure 3). Silybin has 5 hydroxyl groups in positions 3, 5, 7, 20, and 23 which are the targets to produce silybin derivatives. Positions 7 and 20 are usual sites of glucuronidation of silybin during its conjugation in human metabolism.
Figure 4.
Nomenclature diagram of the different components of milk thistle seed and silymarin. 24 Isosilybin B seems to be the most powerful anticancer fraction. 25 Silymarin and Silibinin are different concepts, however some older publications use both terms interchangeably. Silibinin is the more active form of the silymarin extract. Only silymarin extracts are available in pharmacies while researchers usually use silibinin for their experiments. Standard silymarin extracts usually contain 33.5% silybin, 13% silychristin, 8.35% isosilybin, 3.5% silydianin 26 (see Figure 5).
Figure 3.
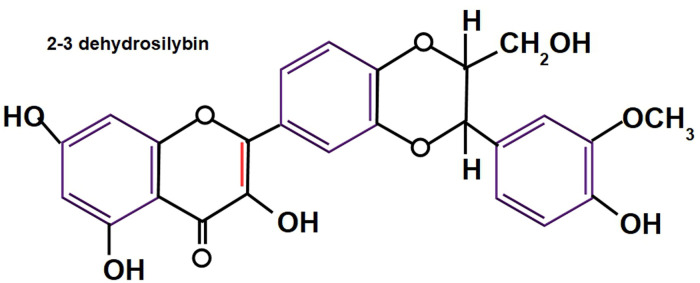
2-3 dehydrosilybin has 25-fold more antioxidant activity than silybin. 23 Taxifolin and quercetin have more antioxidant activity than 2-3 dehydrosilybin. Small amounts of 2-3 dehydrosilybin are found in silymarin.
Silybin is stable in acidic conditions but unstable under alkaline conditions. Alkaline media disrupt flavolignan's skeleton. This is important because the extracellular matrix of tumors has a low pH (approximate pH = 6.8), while intracellular tumor pH is alkaline (approximate pH = 7.5), but only slightly more alkaline than normal cell intracellular pH (approximately = 7.2). 19 Normal cells, on the other hand, have an alkaline extracellular milieu (approximate pH = 7.35). We presume, without evidence to sustain the presumption, that silybin can reach the malignant cell's acidic extracellular space without degradation. This singular feature, the acidic extracellular pH of tumors, 20 may explain why silybin effects differ in normal versus malignant cells. Silymarin may be able to better access the malignant cell compared with normal cells. This theory needs experimental confirmation.
Production. Silymarin extract is obtained by compressing the seeds which leads to a loss of lipids. Then, the active principal component is extracted with acetone, methanol, ethanol, or ethyl acetate. After a second lipid and impurities extraction, what is left is a mixture of flavolignans called silymarin. 21 Silybin is obtained from silymarin through methanolic extraction.
Biological activity. In 1975, Desplaces et al 27 showed that silymarin had a protective effect on hepatocytes against phalloidin, the toxin of Amanita phalloides, when it was administered before the poison. When it was given immediately after phalloidin, it still protected hepatocytes but when given 30 min later, this protective action was negligible. Phalloidin produces acute hemorrhagic necrosis of hepatocytes. When silymarin was administered before the poison there were no morphologic (electron microscopic level) or biochemical signs of hepatic lesions. 28 Silymarin was adopted as an “hepato-protector” by lay persons and the medical profession based on sometimes controversial evidence.
Hepato-protection. For example in:
Chronic hepatitis B and C: silymarin was able to lower transaminases but there was no change in viral load. 29 However, Fried et al did not find benefits in chronic hepatitis C virus infected patients with high doses of silymarin, and did not find effective lowering of transaminases. 30 No transaminase lowering was found with silymarin in hepatitis C virus infection in another study with very high doses of silymarin. 31 Other authors arrived to completely different results: silymarin had antiviral actions by blocking hepatitis C virus cellular entry and transmission. 32 As a first conclusion we may say that there is no clear evidence of silymarin's benefits in chronic hepatitis C.
Alcoholic hepatitis: Trinchet et al 33 found no significant favorable effects of silymarin in alcoholic hepatitis in a double blind randomized study.
Nonalcoholic fatty liver disease: In this case, silymarin has shown favorable and less controversial results.34–36
Reduction/inhibition of hepatic fibrosis: silymarin showed the ability to reduce hepatic fibrosis in the early stages of liver injuries. 37
Cirrhosis: a large population study showed that silymarin decreased mortality in patients with hepatic cirrhosis. 38
In spite of the evidence favoring its benefits in chronic liver disease, “the overall efficacy of silymarin remains unclear” according to Tighe et al. 39 However, there are many, and some potentially beneficial, known biochemical effects of silymarin and silybin. For example, free radical scavenging and antioxidative properties of silybin are well known and have been thoroughly investigated. 40 It is considered 10-fold more antioxidant than vitamin E. In 1977, Machicao and Sonnenbichler 41 showed that silybin increased RNA synthesis in rat liver cells and mainly increased the production of ribosomal RNA and polymerase A. Shriever et al 42 found that silymarin inhibited fatty acid synthesis in rat liver: fatty-acid-synthase and ATP-citrate-lyase, 2 of the main lipogenic enzymes, were diminished by about 50%. Fiebrich and Koch43,44 described silymarin as a blocker of prostaglandin production in vitro through inhibition of both prostaglandin synthetase and lipoxygenase. This reduction of lipoxygenation was confirmed on liver ribosomes and mitochondria as well and probably explains silymarin's hepatoprotective actions. 45
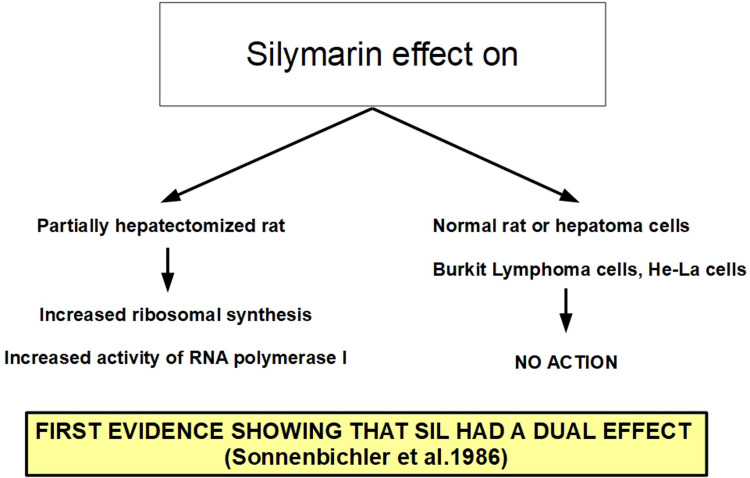
A few years later Sonnenbichler et al 46 presented the first evidence that silymarin acted in a different way in noncancerous hepatic tissue and malignant cells: in the first case it stimulated DNA synthesis, in the second it did not. Silymarin is also a potent blocker of cyclic AMP breakdown (in vitro) by a phosphodiesterase preparation, 47 an inhibitor of histamine release from human basophil leukocytes, 48 dose-dependent downregulator of in vitro lymphocyte blastogenesis 49 and alters the mitochondrial electron transport chain through mitochondrial calcium release, 50 in addition to its antioxidant properties. 51 Immunostimulatory effects of silymarin were also described in experimental models, but not in the context of cancer treatment.52,53
Silymarin and Other Diseases (Table 1)
Table 1.
Silymarin Research Beyond Hepatoprotection and Cancer: A Summary.
| Type of disease | Specific disease | References |
|---|---|---|
| Neurologic diseases | General | 62,63 |
| Parkinson's disease | 64 | |
| Alzheimer | 65–67 | |
| Multiple sclerosis | 68,69 | |
| Diabetic cognitive impairment | 70 | |
| Learning and memory deficits (in mice) | 71 | |
| Diabetes | Diabetic complications | 72–76 |
| Hypercholesterolemia | 77–81 | |
| Renal diseases | Cyclosporine nephrotoxicity | 82 |
| Diabetic nephropathy | 83,84 | |
| Ischemia/reperfusion | Damage prevention in general | 85 |
| In heart muscle | 86 | |
| In the central nervous system | 87,88 | |
| In the kidney | 89,90 | |
| In intestine and bowel | 91 | |
| In the stomach | 92 | |
| In the lungs | 93 | |
| In the liver | 94,95 | |
| Multivisceral | 96 | |
| Skin | Protection against UV radiation | 97–99 |
| Melasma | 100 | |
| Rosacea | 101 | |
| Immune system | Inhibition of UV-induced immune suppression | 102 |
| Infections: Viral | Covid infection | 103,104 |
| Anti-Mayaro virus | 105 | |
| Anti-Chikungunya virus | 106 | |
| Anti-Zika virus | 107 | |
| Infections: bacterial | Escherichia coli | 108 |
| Amiodarone | Improved effects on atrial flutter | 109 |
| Decreased Amiodarone side-effects | 110 | |
| Ulcerative colitis | Prolonged remission | 111 |
| Inflammatory bowel disease | 112 | |
| Irritable bowel syndrome | 113 | |
| Migraine | Reduced frequency of attacks | 114 |
| Endocrine | Hyperprolactinemia | 115 |
| Decreased hot flashes in menopause | 116 | |
| Polycystic ovarian syndrome | 117 |
Silymarin has been investigated and proposed for the treatment of many different diseases, from Alzheimer dementia 54 to SARS 2 Covid-19, including diabetes, 55 diabetic complications,56–58 hyperlipidemia, and hypercholesterolemia,50–61 among others. However, in the last 15 years, the main focus has been cancer.
Silymarin and Cancer
The first observation of silymarin's possible benefits in cancer is the 1991 publication by Mehta and Moon. 118 They showed that silymarin could act as a preventive (antipromoter) of cancer in mouse mammary glands treated with DMBA (dimethylbenzanthracene) and TPA (tetradecanoylphorbol acetate). The treatment protocol they employed made it possible to differentiate whether the chemoprevention worked at the initiation stage of carcinogenesis (DMBA phase) or during promotion (TPA phase). A 1991 review on the advances in pharmacological studies of silymarin by Rui, 119 did not mention anticancer activities. But in 1994, Agarwal et al 120 performed a study on skin treated with TPA confirming the protective effect of this flavonoid against tumor promotion. Silymarin protected against induction of ornithine decarboxylase by TPA. Ornithine decarboxylase inhibition protects against tumor promotion. A protective effect of silymarin was also found in colon and small intestine adenocarcinoma cells induced by 1,2-dimethylhydrazine. 121 Silymarin and its components also inhibit beta-glucuronidase. 122
Valenzuela and Garrido 123 proposed 3 levels for silymarin's action in experimental animals:
(a) as an antioxidant, by scavenging prooxidant free radicals and by increasing the intracellular concentration of the tripeptide glutathione;
(b) through a regulatory action of cell membrane permeability and increase in its stability against xenobiotic injury;
(c) through nuclear expression, by increasing ribosomal RNA synthesis, by stimulating DNA polymerase I, and by exerting a steroid-like regulatory effect on DNA transcription.
Silymarin also inhibits rat liver cytosolic glutathione S-transferase, 124 although this function does not clearly hint towards anticancer activity. On the other hand, silymarin scavenges reactive oxygen species as noted above, and inhibits arachidonic acid metabolism in human cells, 125 has antiinflammatory effects similar to those of indomethacin, 126 protects skin against carcinogenic agents127,128 and ultraviolet radiation.129–131 These publications strongly suggest a cancer-preventive activity and silymarin is slowly emerging as an anticancer drug. For example, Scambia et al 132 tested the antiproliferative activity of silymarin on human ovarian and breast cancer cell lines and found a growth-inhibiting effect on both. Silymarin also showed synergism with the commonly used anticancer compounds doxorubicin and cisplatin.
In DU145, prostate carcinoma cells, silymarin showed inhibition of Erb1 (eukaryotic ribosome biogenesis protein 1) signaling and G1 arrest. 133 In MDA-MB 486 breast cancer cells, G1 arrest was found due to increased p21 and decreased CDKs activity. 134 In advanced human prostate carcinoma cells, silymarin decreased ligand binding to Erb1 135 and NF-kB expression was strongly inhibited by silymarin in hepatoma cells, 136 as well as in histiocytic lymphoma, HeLa and Jurkat cells. 137
According to Zi and Agarwal, low doses of silymarin inhibited ERK1 and ERK2 Map kinases in a skin cancer cell line (human epidermoid carcinoma A431) and at higher doses activated MAPK/JNK1. This means that at lower doses the effect was antiproliferative and at higher doses proapoptotic. 138
Treating prostate carcinoma cells with silymarin the levels of PSA were significantly decreased and cell growth was inhibited through decreased CDK activity and induction of Cip1/p21 and Kip1/p27. 139
Silymarin has also been shown to have a variety of other protective effects in various cell types, such as anti-COX2 and anti-IL-1α activity, 140 antiangiogenic effects through inhibition of VEGF secretion, upregulation of Insulin like Growth Factor Binding Protein 3 (IGFBP3), 141 and inhibition of androgen receptors. 142 In leukemia HL-60 cells, silymarin inhibited proliferation and induced differentiation into monocytes in a dose-dependent manner. 143 Another important effect of silymarin in cancer is the downregulation of the STAT3 pathway which was seen in many cell models. STAT3 is active in many types of cancer and is associated with poor prognosis and resistance to treatments.144–146 Telomerase activity is another important factor in promoting carcinogenesis and evading senescense, thus inducing cancer cell immortality; silymarin has the ability to decrease telomerase activity in prostate cancer cells. 147
Silymarin and Apoptosis
The apoptotic mechanism silymarin employs on cancer cells is generally p53 dependent, and follows the usual steps: increased proapoptotic proteins; decreased antiapoptotic proteins; mitochondrial cytochrome C release-caspase activation. 148 Caspase inhibitors terminate silymarin apoptotic activity. Malignant p53 negative cells show only minimal apoptosis when treated with silymarin. Therefore, one conclusion is that silymarin may be useful in tumors with conserved p53.
Silymarin and Cancer Cell Migration
Enhanced cell migration is an important part of cancer progression. The antimigratory effects of silymarin in cancer cells are the result of mechanisms that 149 :
inhibit histone deacetylase activity; 149
increase histone acetyltransferase activity; 149
reduce expression of the transcription factor ZEB1; 149
increase expression of E-cadherin; 149
increase expression of miR-203; 149
reduce activation of sodium hydrogen isoform 1 exchanger (NHE1); 150
target β catenin and reduce the levels of MMP2 and MMP9; 151
reduce activation of prostaglandin E2; 152
suppress vimentin expression; 153
inhibit Wnt signaling; 154
modulate β1 integrin signaling. 155
Silymarin and Angiogenesis
Angiogenesis is important in cancer growth because solid tumors need a blood supply to grow. Silymarin inhibits angiogenesis. There are various postulated mechanisms:
Decreased migration of endothelial cells. 156
Flt1 (VEGFR1) upregulation. 157 (VEGFR1 upregulation may act as a negative regulator of VEGFA that is upheld by this receptor with low protein kinase activity and therefore VGEFA is unable to bind to KDR [VEGFR2] with much higher kinase activity). 158
VEGF downregulation. 40
Silymarin and Epithelial–Mesenchymal Transition (EMT)
EMT is involved in tumor progression and metastatic expansion. In a transcriptome study of nonsmall cell lung cancer (NSCLC) cells, Kaipa et al 159 found that silibinin had no effect on EMT. However, the opposite was found in other malignant tissues160–162 where it showed inhibitory effects.
Silymarin and TIMP1
High expression of the tissue inactivator of metalloproteases I, or TIMP1, in cancer is a marker of poor prognosis163,164 54 because it is involved in tumor progression, metastasis, and shorter overall patient survival. TIMP1 also promotes accumulation of tumor-associated fibroblasts. 165 Therefore, it may be considered a target in cancer treatment. Silymarin has the capacity to decrease TIMP1 expression166–168 in mice.
Silymarin and LPAR1
LPAR1 and 3 (lisophosphatidic receptors 1 and 3) are related to cancer invasiveness.169–172 Silymarin has the ability to downregulate LPAR1. 173
Silymarin and TGF β2
Silibinin reduces the expression of TGF β2 in different tumors such as triple negative breast, 174 prostate, and colorectal cancers. 175 TGF β2 downregulation impedes the TGF β2/Smad pathway reducing cellular motility and MMP2 and MMP9 (metalloproteases) reducing invasion. In the liver, TGF β2 downregulation results in an antifibrotic effect, preventing hepatic fibrosis induced by inflammatory liver diseases.166,176
Silymarin and Hypoxia Inducible Factor-1α (HIF-1α)
When cells are exposed to hypoxia, HIF-1α accumulates in the nucleus activating transcription of many genes and this plays an important role in tumor progression. Silymarin was found to decrease HIF-1α expression in rainbow trout brain 177 and in rat lung under hypoxic conditions. 93 In prostate cancer cells silibinin inhibited HIF-1α translation. 178
Silymarin and CD44 and EGFR
CD44, the transmembrane receptor for hyaluronan, is increased in breast cancer and many other tumors, due to EGF (epidermal growth factor) stimulation. Silibinin decreased CD44 expression and the activation of EGFR (epidermal growth factor receptor) by EGF. 179 In prostate cancer, silibinin decreased/inhibited CD44 expression as well. 180 CD44 binding with hyaluronan triggers important protumoral signaling from its intracellular segment, inducing cancer cell survival, angiogenesis, migration, and invasion. The CD44 antigen (synonym HCAM) is a glycoprotein acting as an adhesion molecule 181 on the cell surface. Cell adhesion molecules play an important role in cell migration. In fact, CD44 has been shown to be strongly correlated with invasion182,183 and metastasis.184–186
Silymarin Modulation of TNFα (Tumor Necrosis Factor Alpha)
Tyagi et al 187 showed that silibinin pretreatment of lung cancer cells inhibited TNFα induced “phosphorylation of STAT3, STAT1, and Erk1/2, NF-κB-DNA binding, and expression of COX2, iNOS, matrix metalloproteinases (MMP)2, and MMP9, which was mediated through impairment of STAT3 and STAT1 nuclear localization.”
Silymarin Inhibition of the Wnt/β-Catenin Signaling
The Wnt/β-catenin pathway is critical in cell proliferation, migration, and differentiation. It is a powerful regulator of embryonic development and tumorigenesis. Lu et al 188 showed that silibinin inhibited the Wnt/β-catenin pathway in both prostate and breast cancer cells.
Silymarin Potentiation of TRAIL-Induced Apoptosis
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is part of the TNF superfamily. It is known to selectively induce apoptosis in cancer cells without having significant toxicity toward normal cells. Kauntz et al189,190 found silibinin potentiated TRAIL-induced apoptosis in human colon adenocarcinoma cells. Furthermore, this potentiation was also found in TRAIL-resistant cells. Silibinin upregulated Death Receptors 4 and 5, thus increasing the number of receptors for TRAIL binding. Silibinin had the ability to induce not only the extrinsic apoptotic pathway, but also the intrinsic pathway. TRAIL sensitization by silymarin was also found in glioblastoma cells 191 and in hepatocarcinoma. 192
Silymarin and Phospholipase A2
Secreted phospholipase A2 participates in inflammation and carcinogenesis. Silibinin downregulates secreted phospholipase A2 in cancer cells. 193
Silymarin and Platelet-Derived Growth Factor (PDGF)
PDGF and its receptor are required for fibroblast proliferation and differentiation. It was found that silibinin had the ability to downregulate PDFG in fibroblasts, thus decreasing proliferation. 194
Silymarin Decreases the Levels of Interleukin 8 (IL-8)
Interleukin 8 has been identified as a protumoral cytokine195–198 and there is evidence showing that inhibition of IL-8 reduces tumorigenesis. 199 Flavonoids, in general, reduce levels of IL-8. Curcumin, 200 apigenin, 201 and silybin showed the ability to decrease IL-8 levels.150,202,203
Silymarin Inhibits the Signal Transducer and Activator of Transcription 3 (STAT3) Pathway
STAT3 exists in the cytosol of cells and is a focal point of multiple oncogenic pathways. Silymarin inhibited STAT3 phosphorylation and decreased the expression of intranuclear sterol regulatory element binding protein 1 (SREBP1), decreasing lipid synthesis. The final consequences of these inhibitions were growth arrest and apoptosis. 204
Silibinin Acts as a Mitochondrial “Poison” in Malignant Cells
Si et al, 205 experimenting with 2 human breast cancer cell lines, MCF7 and MDA-MB-231, found that silibinin produced morphological and functional changes in mitochondria: decreased mitochondrial mass, condensed crests, reduced membrane potential and ATP content, and decreased mitochondrial biogenesis.
Silibinin and Metalloproteases
MMP2 and MMP9 play an important role in extracellular matrix remodeling and their levels correlate with progression of neuroblastoma tumors. 206 Yousefi et al 207 found that silibinin decreased MMP2, MMP9, and urokinase plasminogen activator receptor level (uPAR) in neuroblastoma cells. uPAR is also a marker of cell invasion.
Silymarin and COX2
COX-2 expression in cancer can stimulate angiogenesis and is associated with tumor growth, invasion, and metastasis. 208 Silymarin decreased the expression of COX2 in a model of chemically induced hepatocarcinoma in rats. 209
Silibinin and Programmed Death-Ligand 1 (PD-L1)
The programmed cell death protein and its ligand (PD-L1) complex play a key role in tumor progression being involved in growth regulation disturbance. This results in a defect in programmed cell death, apoptosis. 210 Silibinin inhibits PD-L1 by impeding STAT5 binding in NSCLC. 211 This hints at the possible usefulness of silymarin as a complement to immune checkpoint inhibitors. A similar effect was found in nasopharyngeal carcinoma. 212 In renal carcinoma cells, silibinin decreased PD-L1 in murine renal cancer cells in vitro and in vivo. 213
Silybin and Notch Signaling
The Notch signaling pathway is highly conserved, regulating development and is involved in angiogenesis and metastasis. 214 Silybin inhibited Notch signaling in hepatocellular carcinoma cells showing antitumoral effects. 215 However, dibenzazepine is much more powerful in this respect. 216 Notch was also downregulated by silibinin in breast cancer cells impeding notch-1/ERK/Akt signaling 217 and inducing apoptosis.
Silymarin and SIRT1
SIRT1 can deacetylate histones and other substrates and may act in a dual manner: as tumor suppressor or tumor promoter.218,219 Silymarin has the ability to increase hepatic SIRT1 expression. 220 Silymarin can also increase SIRT1 expression in other tissues, such as hippocampus, 221 articular chondrocytes, 222 and heart muscle. 223 Silymarin seems to act differently in tumors: in lung cancer cells SIRT downregulated SIRT1 and exerted multiple antitumor effects such as reduced adhesion and migration and increased apoptosis. When SIRT1 was independently downregulated with siRNA the silymarin's antitumoral effects were increased. 224
Silymarin and VEGF/VEGFR
The angiogenic cytokine vascular endothelial growth factor (VEGF) and its receptor (VEGFR) play critical roles in vasculogenesis and angiogenesis. Jiang et al 225 found that adding silymarin to prostate and breast cancer cells swiftly reduced the secretion of VEGF to the medium in a dose-dependent manner. Silymarin also prevented VEGF expression in myocardial cells exposed to doxorubicin toxicity 226 as well as other manifestations of cardiotoxicity. A similar decrease in VEGF and VEGFR levels was found with silymarin in preeclamptic placenta, however the effect was very modest. 227 There is evidence showing that silymarin reduces VEGF expression at the transcription level. 228
Silymarin and Myc
C-Myc is a multifunctional master regulator transcription factor; it is activated by oncogenic pathways, drives many functions for rapid cell division, and inhibits antiproliferative pathways. 229 There is direct and indirect evidence that silymarin interacts with c-Myc, 230 in some cases increasing its expression in liver cells as a response to hepatic chemical injuries 231 or decreasing it in malignancies. 232 Rajamanickam et al233,234 found that silymarin could prevent spontaneous tumorigenesis in an APCmin/+ mouse model (prone to develop intestinal tumors) by decreasing β-catenin, cyclin D1, c-Myc, phospho-glycogen synthase kinase-3β expression, phospho-Akt, and cyclooxygenase-2 in polyps. This report confirms silymarin's multitargeting effects in tumors and its different behavior in nonmalignant cells.
Silymarin and Carbonic Anhydrases
Carbonic anhydrases (CAs) play an important role in cancer progression, particularly those associated with the cell membrane (membrane CAs), namely isoforms CA9 (CAIX) and CA12 (CAXII). These CAs intervene in acidifying the extracellular substance and, working in tandem with sodium bicarbonate cotransporters, increase the intracellular pH. Downregulating or inhibiting membrane CAs has become a valid target for cancer therapy. Silymarin has the ability to inhibit CA isoforms CA I and CA II.235,236 However, we could not find any publications specifically addressing silymarin's role as a possible inhibitor of membrane CAs.
Silymarin and Mitochondria
This is a controversial relationship. On one side, silymarin showed ability to reduce oxygen consumption in mitochondria NAD-dependent substrates, while on the other hand stimulating respiration in mitochondria oxidizing succinate. 50 Silymarin increases mitochondrial release of Ca++ and lowers mitochondrial membrane potential in cancer cells 237 and increases the transmembrane potential in toxic aggressions. 238 Regarding mitochondria we may presume that silymarin has context-dependent effects.
Antimetastatic Potential
Many of the features discussed above hint towards silymarin's antimetastatic potential. In a TRAMP (Transgenic Adenocarcinoma of the Mouse Prostate) model of prostate carcinoma, when mice were fed with silibinin invasion and metastasis were reduced. 239 The antimetastatic effect was due to less invasion, less EMT, less collagen I-cancer cell adhesion, and less expression of CD44. 240 In a randomized clinical study with patients harboring solid tumors, silymarin was added to standard chemotherapy. Although silymarin failed to improve the results, there was a a slight—not significant—trend towards reduced metastasis. 241 We think that this study had some flaws which included a small sample size (15 patients with silymarin and 15 with placebo), tumors being present in different organs, and very low dosage (420 mg/day). In spite of these flaws, the trend towards a decrease in metastasis is still interesting and further study with a larger sample population was suggested by the authors.
Silymarin: Decreasing Side Effects and Toxicity of Chemotherapeutic Drugs
Silymarin coadministered with chemotherapeutic drugs has the ability to reduce toxicity in normal organs242,243:
It protects against liver and kidney toxicities induced by methotrexate in children and adults treated for leukemia.244,245
Silibinin decreased cisplatin's nephrotoxicity without affecting its antitumoral effectiveness.246,247
There is also evidence that it protects the heart from doxorubicin toxicity, however, it is less potent than quercetin in this effect. 248
Silymarin reduced docetaxel central and peripheral neurotoxicity. 249
Silymarin was able to decrease diarrhea produced by irinotecan treatment. 250
Silymarin reduced hepatotoxicity in patients with nonmetastatic breast cancer receiving doxorubicin /cyclophosphamide-paclitaxel. 251
Silymarin and Resistance to Treatment
Rho et al 252 found that adding silymarin to epidermal growth factor receptor tyrosine kinase inhibitors could overcome resistance produced by the T790M mutation in NSCLC xenografts. The mechanism of action seems to be impeding EGFR dimerization. It was found that bladder cancer cell lines resistant to cisplatin could be resensitized with silymarin. 253 A similar result was obtained with ovarian cancer cells resistant to paclitaxel. 254 The mechanism involved in resensitization to chemotherapeutic drugs is not fully known, however possible factors are: inhibition of NF-kB nuclear migration, 136 inhibition of survivin protein levels, downregulation of Pgp (MDR 1),255–258 and multidrug resistance-associated protein 1 (MRP 1).258,259 Silychristin (a component of silymarin) and silychristin derivatives have shown the particular ability to inhibit Pgp activity in a concentration-dependent manner 260 (see Tables 2 to 12).
Table 2.
Melanoma.
| Year, Ref. | Findings |
|---|---|
| 2006 285 | Silymarin proposed for chemoprevention of melanoma. |
| 2007 286 | Silymarin increased proapoptotic effects of anti-Fas agonistic antibody CH11 in melanoma cells. |
| 2013 287 | Silymarin for prevention for melanoma invasion. |
| 2013 288 X | Silymarin decreased the growth of melanoma xenografts and locked MEK1/2-ERK1/2-RSK signaling that led to a reduction of NF-kB activator protein1 and STAT3, which resulted in cell cycle arrest and inhibited tumor growth in vitro and in vivo |
| 2015 289 X | Silymarin targeted cell cycle regulators, angiogenesis, and induced apoptosis in vitro and in vivo. |
| 2016 290 | Silymarin as a modulator of the wnt/β catenin pathway. |
Table 12.
Miscellaneous.
| Year, Ref. | Organ/tumor | Findings |
|---|---|---|
| 2013 361 | Pharynx | Silymarin increased PTEN, reduced Akt phosphorylation, and induced apoptosis in pharynx squamous cell carcinoma cells. |
| 2015 362 | Glioma | Silibinin induced apoptosis in glioma cells through downregulation of PI3K pathway and decreased FoxM1 transcription factor expression. |
| 2018 363 X | Oral cancer | In vivo, silymarin reduced tumor growth and volume; in vitro it produced apoptosis through induction of the DR5 (death receptor 5)-caspase 8-truncated Bid pathway. |
| 2017 364 | Salivary glands | Silymarin increased proapoptotic Bim protein in mucoepidermoid carcinoma cells, inducing apoptosis. |
| 2012 365 | Cervical | Silymarin induced apoptosis in cervical cancer cells through increase of PTEN, inhibition of Akt phosphorylation, and decreased expression of MMP9. |
| 2007 366 | Osteosarcoma | Silibinin decreased human osteosarcoma cell invasion through Erk inhibition of a FAK/ERK/uPA/MMP2 pathway. |
| 2017 367 | Rabdoid tumor | Silibinin inhibited rabdoid tumor cell migration and invasion through inhibition of the PI3K/Akt pathway. |
| 2019 368 | Human gastric cancer cells | Silymarin downregulated the MAP kinase pathway inhibiting growth and inducing apoptosis. |
Table 4.
Lung NSCLC: Nonsmall Cell Lung Cancer//SCLC: Small Cell Lung Cancer.
| Year, Ref. | Findings |
|---|---|
| 2004 306 | Silibinin impeded invasion by inhibiting expression of uPA and MMP2 and enhancing expression of TIMP2. |
| 2013 307 | Silibinin meglumine impeded the epithelial–mesenchymal transition in EGFR mutant NSCLC cells. |
| 2010 308 | Silibinin decreased NSCLC cell growth through cell cycle arrest and decreased cell cycle modulators. |
| 2011 309 | Silymarin produced apoptosis in a highly metastatic lung cancer cell line through the mitochondrial caspase pathway. |
| 2003 310 | Silibinin inhibited growth and apoptosis in NSCLC and SCLC line cells in a dose-dependent manner. |
| 2012 311 | HDAC inhibitors in combination with silibinin showed enhanced antitumor activity in NSCLC cells. There was also increased transcription of p21 through higher acetylation of its promoter. The augmented p21 was responsible for proteasomal destruction of cyclin B1. |
| 2013 312 | EGFR-mutated lung cancer cells, resistant to erlotinib and overexpressing ALDH, were resensitized by silibinin. |
| 2009 313 X | Treating a xenograft lung cancer mouse model with silibinin resulted in a decreased tumor size through decreased angiogenesis. HIF-1α was also decreased by silibinin. |
| 2016 314 | Through downregulation of STAT3, silibinin reinstated sensitivity to crizotinib therapy in ALK rearranged lung cancer. |
Table 7.
Breast.
| Year, Ref. | Findings |
|---|---|
| 2004 325 | Silymarin, as part of a mixture of flavonoids, downregulated the Breast Cancer Resistance Protein (BCRP). The authors propose a “flavonoid cocktail” for this purpose. |
| 2004 326 | Silibinin synergized with conventional chemotherapeutic drugs in anticancer effects on breast cancer cells. |
| 2009 327 | Silibinin decreased MMP9 and VEGF expression induced by TPA through downregulation of the Raf/Mek/Erk pathway. |
| 2013 328 | Silymarin showed synergy with doxorubicin in producing MCF7 cell apoptosis |
| 2014 329 | Silymarin showed much higher proapoptotic gene induction in a lung cancer cell line than in a breast cancer cell line. |
| 2014 330 X | Silibinin inhibited the accumulation of myeloid derived suppressor cells (MDSC) in murine breast cancer and increased overall survival. Silibinin decreased tumor volume. |
| 2015 331 | Silibinin induced autophagic death in breast cancer cells. Silibinin treatment decreased ATP levels and altered mitochondrial electric potential with increased ROS accumulation. |
| 2015 332 | Silibinin induced apoptosis in breast cancer cells. (Comment: the concentrations used were too high and are not achievable in human use). |
| 2015 333 | ERα inhibition was a key factor in silibinin-induced autophagy and apoptosis. Using ERα inhibitors with silibinin, both apoptosis and autophagia were further increased. |
| 2016 334 | Silibinin decreased BCL2 proteins in breast cancer cells and normal breast cells and ununiformly increased PTEN in different cancer cell lines. |
| 2017 335 | Silibinin sensitized breast cancer cells to doxorubicin treatment. (Comment: The concentrations used were excessively high and difficult to achieve in the clinical setting). |
| 2017 336 | Silymarin-loaded iron nanoparticles produced cell cycle arrest in triple negative breast cancer cells. |
| 2017 337 | Silymarin's anticancer effects were due to inhibition of Akt and MAPK pathway. |
| 2021 338 X | Silymarin decreased proliferation and viability of MDA-MB-231 and MCF-7 cells in a concentration-dependent manner, inducing apoptosis. These results were obtained in vitro and in vivo. |
Table 8.
Colon.
| Year, Ref. | Findings |
|---|---|
| 2002 339 X | Silymarin inhibited chemically induced carcinogenesis of the colon in mice. |
| 2013 340 X | Silibinin blocked TNFα-induced NF-kB activation in vitro and in vivo. Tumor growth and progression were concomitantly Inhibited. Bcl2, COX2, VEGF, and MMPs levels were also diminished by silibinin feeding of xenotransplanted mice. |
| 2015 341 | Silymarin induced proteasomal degradation of cyclin D1 and inhibited growth of colon cancer cells. |
| 2016 342 | Treatment with silymarin increased the efficacy of ionizing radiation on colon cancer cells causing increased cell death. |
| 2017 343 | Silibinin inhibited proliferation and increased apoptosis in colon cancer cells. |
| 2017 344 X | The combination of regorafenib and silybin had synergistic antiproliferative and proapoptotic effect. This combination was tested in 22 patients with metastatic colon cancer. No control group was available. |
| 2020 345 | Sylimarin, associated with other nutraceuticals, reduced intestinal polyp growth in an animal model. |
Table 9.
Liver.
| Year, Ref. | Findings |
|---|---|
| 2005 346 | Silibinin strongly inhibited growth of hepatocellular cancer cells. It also increased apoptosis with inhibition of CDK2, CDK4, and CDC2 kinases. |
| 2006 347 X | Silymarin inhibited hepatocarcinogenesis induced by nitrosodiethylamine. |
| 2008 348 X | Silymarin decreased the expression of MMP2 and MMP9 and decreased recruitment of mast cells in vivo in a rat liver carcinogenesis model. |
| 2008 349 | Silibinin decreased cell proliferation and migration of human hepatocellular cancer cells by inhibiting the Erk 1/2 cascade. |
| 2009 350 | Silymarin decreased growth of hepatocellular carcinoma (HCC) cells and induced apoptosis. |
| 2009 351 X | In a xenograft mouse model of HCC, silibinin reduced growth and proliferation through reduction of Akt/Erk signaling and increased histone acetylation. |
| 2015 352 | Silibinin increased growth inhibition of hepatocarcinoma cells by either sorafenib or gefitinib. |
| 2020 353 | Silymarin showed antimetastatic and proapoptotic effects on HepG2 cells through the Slit-2/Robo-1 pathway. |
Table 10.
Ovary.
| Year, Ref. | Findings |
|---|---|
| 2014 354 | Silymarin suppressed cell growth and induced caspase-dependent apoptosis with increased p53, p21, and p27, and decreased CDK2. |
| 2003 355 X | A silybin-phosphatidylcholine complex decreased tumor growth in xenografted mice (tumor weight inhibition of 78%). |
| 2013 356 X | Silibinin decreased tumor growth in vitro and in vivo through downregulation of Erk and Akt signaling. |
Table 11.
Hematologic.
| Year, Ref. | Disease | Findings |
|---|---|---|
| 2001 143 | Promyelocytic leukemia | Silymarin inhibited proliferation and induces differentiation into monocytes. It showed synergy with vitamin D3. |
| 2010 357 | Acute myeloid leukemia | Silibinin induced differentiation of acute myeloid leukemia cells ex vivo (only in cases in which there were no chromosome aberrations). |
| 2016 358 | Lymphoma | Silibinin induced apoptosis in Alk-positive anaplastic large cell lymphoma by suppressing the phosphorylation of NPM/ALK. |
| 2020 359 | Lymphoma | Epstein-Barr positive lymphoma cell proliferation was inhibited and apoptosis induced through NF-kB inhibition by silymarin. |
| 2016 360 | Multiple myeloma | Silybin suppressed myeloma cell proliferation and induced apoptosis by inhibiting the PI3K/Akt/mTOR pathway. |
Silymarin's Cancer Chemopreventive Actions
Table 5 summarizes the findings by Vinh et al that show that silymarin was able to significantly decrease the incidence of bladder neoplasms in male rats receiving the carcinogenic substance N-butyl-.N-(4-hydroxybutyl) nitrosamine. Interestingly, these results were achieved by oral administration of silymarin and were found in those animals that received silymarin not only at the initiation of carcinogenesis, but also in those of the postcarcinogenic period (for more examples on chemoprevention, see Tables 2 to 12).
Table 5.
Bladder.
| Year, Ref. | Findings |
|---|---|
| 2017 253 | Silibinin reversed chemotherapeutic resistance in bladder cancer cells in a NF-kB signal-dependent and independent manner. |
| 2017 315 | Silibinin is an antiproliferative compound whose mechanism of action depended on p53 status (WT or mutated). |
| 2002 316 X | In an induced bladder carcinogenesis mouse model, silymarin reduced the incidence of bladder lesions and cell proliferation. Silymarin acted as a preventive compound. |
| 2017 317 | The anticancer mechanism of silibinin in bladder cancer was through downregulation of the actin cytoskeleton, the PI3K/Akt pathway and KRAS. |
| 2011 318 X | Oral silibinin prevented carcinogenesis, decreased proliferation, and increased apoptosis in vivo in a mouse model. Intravesical silibinin had a similar effect. |
| 2013 319 X | Silibinin decreased bladder cancer metastasis and prolonged animal survival through downregulation of the GSK3β/β catenin pathway and Zeb1 expression. Silibinin also suppressed EMT and stemness. |
Silymarin and Hormonal Receptors
Silymarin is a selective estrogen β receptor (ER-β) agonist. 261 However, it also has some estrogenic effects through ER-α. 262 Silymarin has strong binding affinity to ER-β and a mild affinity for ER-α. 263 Silymarin's estrogenic actions should be seriously considered as a problem in female hormone-dependent tumors. Furthermore, silymarin's estrogenic effects are confirmed by the observation that it produces benefits in menopausal women with hot flashes. 116 Contrario sensu, it may be advantageous in benign prostate hyperplasia264,265 and prostate cancer. However, in an experiment carried out in albino rats, silymarin increased testosterone and LH. 266 It also increased spermatogenesis in rats. 267 In spite of these 2 findings, the evidence for silymarin benefits in prostate cancer is abundant (see Table 3). Our conclusion is that the possible benefits found in prostate cancer are independent of silymarin's hormonal effects.
Table 3.
Prostate.
| Year, Ref. | Findings |
|---|---|
| 2008 291 | Silibinin had inhibitory effects on survival, motility and adhesion of highly metastatic prostate cancer cells. |
| 2005 292 | Isosylibin B was the most potent fraction of silymarin against proliferation in different prostate cancer cell lines (LNCaP, DU145, and PC3). Silymarin used in this paper contained 5% isosylibin B. Isosilybin was a suppressor of the promoter of the topoisomerase IIα gene in DU145 cells. |
| 2001 142 | Silymarin inhibited the androgen receptor (AR) by reducing its localization in the nucleus without modifying AR expression or binding ability. This action was probably related to silymarin's downregulation of FKBP51 that is a probable transporter of AR to the nucleus. |
| 2020 293 | Silymarin inhibited DU 145 cell proliferation through two mechanisms: activation of the SLIT 2 protein and inhibition of CXCR4. |
| 1999 294 | Silibinin inhibited growth by G1 cell cycle arrest in hormone-refractory human prostate carcinoma cells without apoptosis. It decreased CDKs and PSA and increased p21 and p27. |
| 2006 295 | Silymarin and silibinin produced G1 and G2-M cell cycle arrest with a decrease in CDKs and Cdc2 kinase activity, and an increase in CDK inhibitors. |
| 2002 296 | Silibinin treatment caused growth inhibition, apoptosis, and decreased viability in different prostate cancer cell lines. |
| 2007 297 | Isosylibin A and Isosylibin B caused growth arrest and apoptosis in human prostate cancer cell lines. |
| 2004 298 | Silibinin inhibited mitogenic signaling in prostate cancer cells. |
| 2007 299 | Silibinin had a synergistic effect on Mitoxantrone inhibition of cell growth arrest and apoptosis of prostate cancer cells. |
| 2005 300 X | Dietary supplementation of silymarin in rats decreased the incidence of induced rat prostate carcinoma. |
| 2010 301 | Silibinin reversed epithelial mesenchymal transition, induced upregulation of cytokeratin-18, and downregulation of vimentin, MMP2, NF-kB nuclear translocation, and transcription factors ZEB1 and SLUG. |
| 2010 302 | Isosylibin A induced apoptosis in prostate cancer cells through phosphoAkt, NF-kB, and AR downregulation. |
| 2003 303 X | In nude mice with xenografted prostate carcinomas, silibinin feeding increased apoptosis and reduced growth and angiogenesis. |
| 2002 304 X | In nude mice xenografted with human prostate carcinoma cells silibinin feeding decreased tumor growth by 35% to 58%. |
| 2002 305 | In human androgen-dependent prostate cancer silibinin inhibited Rb phosphorylation and increased Rb-E2F. |
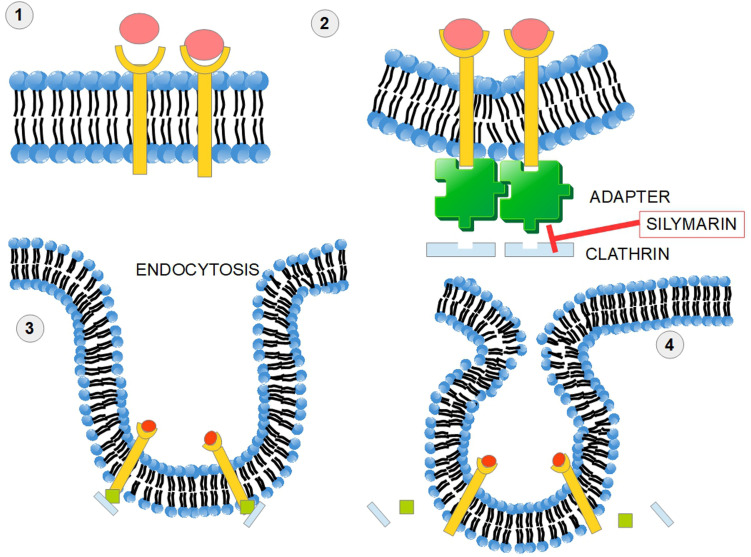
Silymarin Inhibits Clathrin-Dependent Trafficking
Endocytosis is an important mechanism of cell intercommunication which acquires major relevance in cancer. This process is initiated by the invagination of the plasma membrane. The protein clathrin provides A coat to this invagination (Figure 5). The clathrin coated vesicle has the ability to select for the adequate cellular receptor. 268 There is also endocytosis without clathrin coating. Silymarin has the ability to inhibit clathrin-dependent trafficking at least in the case of certain viruses such as Hepatitis C virus, reovirus, influenza virus,269,270 and Hepatitis B virus. 271 The mechanism behind this inhibition is through interference with the clathrin endocytic pathway. Actually, silymarin interferes with all the clathrin-dependent endocytic processes. Taxifolin, a close relative of silybin, was also found to inhibit receptor-mediated endocytosis of β-hexosaminidase in normal fibroblast culture. There were similar findings with other flavonoids. 272
Figure 5.
Similarities and differences among silymarin's components.
Although there is no experimental evidence in this sense, we may presume that silymarin decreases endocytic trafficking in cancer cells too. Additionally, clathrin has protumoral effects beyond endocytosis: it switches TGF-β into a procancer role. 273
Figure 6 shows a simplified overview of the clathrin-dependent endocytosis.
Figure 6.
Silymarin impedes clathrin-dependent endocytosis. In receptor-mediated endocytosis, or clathrin-mediated endocytosis, cells incorporate hormones, proteins, and sometimes viruses in a selective manner that depends on a ligand-receptor interaction. (1) The ligand receptor interaction takes place on the cell surface. (2) An adapter protein and clathrin “coat” the internal surface of the receptors and a process of membrane invagination starts. (3) Advanced invagination process. (4) The adaptor protein and clathrin are released. Silymarin impedes this mechanism of endocytosis. Clathrin-dependent endocytosis of surface receptors participates in cellular signaling pathways. Clathrin seems to be a valid target in cancer therapy. 274
Silymarin and Renal Carcinoma
When targeting renal carcinoma cells with silymarin, migration and invasion were significantly decreased by inhibition of the EGFR/MMP-9 pathway: silymarin blocked phosphorylation of EGFR and ERK1/ERK2 and reduced expression of MMP-9. 275 This was confirmed by Liang et al 276 and by Chang et al in vivo 277 and in our unpublished observations with 2 patients with grade IV clear cell renal carcinoma, who experienced no new metastasis after they started on silymarin.
Silymarin and Pancreatic Cancer
Silymarin has not been extensively tested in pancreatic ductal adenocarcinoma (PDAC) (see Table 6). However, it does have an important antifibrotic effect. One of the major problems in PDAC is the intense stromal reaction with abundant production of stromal collagen fibers. 278 These impede delivery of the chemotherapeutic drug to the tumor mass and create interstitial hypertension through the strongly hydrophilic hyluronan. Therefore, silymarin's antifibrotic effects may provide an interesting complement to standard treatment. 279
Table 6.
Pancreas.
| Year, Ref. | Findings |
|---|---|
| 2011 320 | Silibinin induced cell cycle arrest and apoptosis in certain pancreatic cancer cell lines. |
| 2015 321 | The combination of an HDAC inhibitor and silibinin had additive effects on growth inhibition and apoptosis of pancreatic cancer cells. |
| 2015 322 X | In an orthotopic model of pancreatic cancer, silibinin reduced glycolytic activity of cancer cells, proliferation, and cachexia. |
| 2013 323 X | Dose-dependent cell growth inhibition was produced by silibinin concentrations between 25 and 100 μM. In xenograft in nude mice, tumor weight was significantly decreased by dietary silibinin. |
| 2018 324 | SW1990 pancreatic cancer cells showed G1 arrest with decreased cyclins and CDKs and apoptosis with silibinin. |
Desmoplastic tumors are the consequence of the intense activity of cancer-associated fibroblasts (CAFs) producing collagen fibers. PDAC and the liver have specialized CAFs known as stellate cells considered the producers of the desmoplastic reaction. Silymarin has been found to inhibit/decrease the desmoplastic reaction through 2 mechanisms:
it inhibits TGF β2 that induces the desmoplastic phenotype of naïve fibroblasts; 280
it increases E-cadherin expression281,282 decreasing the invasive nature of the desmoplastic reaction.
Silymarin decreased fibrosis not only in 2 models of induced liver fibrosis37,283 but also in lung fibrosis induced by cigarette smoke. 284 In this last case, this occurred by downregulation of the TGF-β1/Smad 2/3 pathway signaling.
Although we could not find any publication showing that silymarin could reduce the desmoplastic reaction in pancreatic cancer, we may assume that it has the potential to do so, because the mechanisms behind this are similar to those found in liver and lung cancers. Long et al suggested this possibility, however they did not incorporate any evidence in their review. 279
Tables 2 to 12: Evidence of silymarin's anticancer effects. In vivo experiments are marked with an X in column 1.
In spite of the long list of publications mentioned in Tables 2 to 12, 5 cautionary notes should be added:
In a mouse model of induced mammary carcinogenesis, the administration of silymarin, slightly increased mammary tumor incidence. 369 This may be due to silymarin's estrogenic effects,115,261,370 however, the issue remains controversial because silymarin increases ERβ and decreases ERα expression. 264
In a model of mouse hepatic carcinogenesis (with diethylnitrosamine), silymarin showed no effects at all. 371
In a mouse model of alcohol-dependent hepatocarcinoma, silibinin increased tumor progression if chronic alcohol intake continued. 372
Many of the in vitro experiments described in Table 2 to 12 were performed at very high concentrations that are difficult or impossible to achieve in vivo. On the other hand, in vivo experiments (marked with an X in Tables 2 to 12) were mainly conducted with oral administration of silymarin or silibinin, so those results should have a more significant impact on future clinical research.
Most of the published literature on silymarin and cancer does not mention the p53 status of the cells and this information is of capital importance (silymarin shows apoptotic effects on p53 positive cells but not on mutated p53).
Pharmacokinetics
Flavolignans (silymarin is a mixture of flavolignans) generally have poor bioavailability. This is the consequence of:
their strongly hydrophobic nature that does permit dilution to more than 50 μg/mL in water. Some organic solvents have a much better performance for this purpose. For example, ethanol shows a solubility of 225 mg/mL; 373
the fact that they are quickly metabolized;
the fact they are poorly absorbed in the intestine.
The pharmacokinetic considerations we shall make refer to the standardized form of silymarin with known amounts of silybin.
Absorption. silymarin is not soluble in water and oral administration shows poor absorption in the alimentary tract (approximately 1% in rats, 374 however, other authors mention a higher absorption around 30%). In spite of this low absorption, according to Janiak et al, a plasma level of 500 mg/L (500 μg/mL) is achievable 90 min after oral administration of 200 mg/kg of silymarin in mice. 375
Excretion. Silymarin is mainly excreted in the bile and half-life is 6 h.
Toxicity. Toxicity is almost absent 376 and therefore high oral doses can be administered with negligible side effects.
Dose/absorption studies in humans. A number of other studies have administered various doses and studied the plasma concentration. For example, with oral administration of 240 mg of silybin to 6 healthy volunteers the following results were obtained 377 :
and time to maximum plasma concentration 1.32 ± 0.45 h. Absorption half life 0.17 ± 0.09 h, elimination half life 6.32 ± 3.94 h. 377 Beckmann-Knopp et al 378 also found: “Mean maximum plasma concentration after an oral dose of 700 mg silymarin, containing 254 mg of silibinin, is 317 ng/ml or 0.6 mM. Accumulation in plasma during three daily medications is negligible. Plasma protein binding is reported to reach about 90–95%.” After feeding volunteers with a smaller dose of 80 mg of a lipophilic silybin-phospatidylcholine complex (silipide) Gatti et al 379 found that free unconjugated silybin reached a maximum concentration of 141 ng/mL after 2.4 h. The level of conjugated silybin peaked after 3.8 h reaching 255 ng/mL. Another study on 6 healthy volunteers used a larger dose of 560 mg of silymarin and attained concentrations starting at 0.18 and going as high as 0.64 μg/mL. 377 These results are quite different and to some extent controversial.
Absorption studies in animals. Administration of silybin to animals also showed divergent results. In dogs, 380 the silybin-phosphatidylcholine complex (SPC) showed increased concentrations when compared with silymarin extract, however, the results showed a low level in general: SPC: 1.310 ± 880 ng/ml; silymarin: 383 ± 472 ng/ml. While Morazzoni et al 381 found higher peak levels of silybin in the form of silipide when administered to rats: “After oral silipide, silybin reached peak plasma levels within 2 h, with a Cmax of 9.0 ± 3.0 μg/ml for unconjugated drug and 93.4 ± 16.7 μg/ml for total (free + unconjugated drug).”
Pharmacodynamic conclusions: The above studies show that the achievable concentration in humans (with a low dose) is far lower than what was found in rodents (with a high dose). The important issue is that most of the experiments found in the literature at cellular level used a concentration around 100 μg/mL. Even in the study by Morazzoni et al, 381 the level of 100 μg/mL was not achieved and in any case it is a peak level that cannot be sustained. Therefore, is the experimental level of 100 μg/mL achievable at the bedside?
We think that there is no evidence that it can be. Oral administration of silymarin in humans achieves nanogram, but not microgram levels. Furthermore, we should not extrapolate Morazzoni's findings in rats to humans as their pharmacokinetics may differ.
Therefore, the evidence based on these high concentration experiments should be viewed with caution. On the other hand, experiments with xenograft models are more reliable (Tables 2 to 12, xenograft results are marked with an X).
Tissue concentration. For cancer treatment purposes the important data to know are the concentrations achievable in tissues. Zhao and Agarwal 382 found the following results in mice 30 min after administration:
Liver: 8.8 μg per gram of tissue
Lung: 4.3 μg per gram of tissue
Stomach: 123 μg per gram of tissue
Pancreas: 5.8 μg per gram of tissue
Prostate: 2.5 μg per gram of tissue after 1 h.
After an oral intake, silipide (the lipophilic SPC), achieved a maximum concentration of silybin in bile within 4 h and then declined with a mean time of approximately 10 h. 383 Silibinin complexed with the amino-sugar meglumine is water soluble and can reach a tissue concentration high enough to show clear antigrowth effects in NSCLC xenografts. 307 The distribution in different tissues also varies widely according to the type of tissue considered. It is higher in the liver and dimishes in lungs, pancreas, and prostate. 382 A relatively high concentration is achievable in colorectal muscosa (20-141 nmol/g of tissue). 384
The tissue levels obtainable compare unfavorably with those used in cell studies. To achieve apoptosis in cell studies, a concentration of more than 20 μM was necessary, 385 and this concentration does not seem easy to achieve by oral intake of standard preparations. It was also necessary to use a concentration of 100 μg/mL to induce apoptosis in Ramos cells (B lymphocytes). 386 Kamrani et al 387 used concentrations between 50 and 100 μg/mL to induce apoptosis in colon cancer cells. Therefore, while only a nanomolar concentration can be attained in tissues, micromolar concentrations were needed to induce apoptosis in these studies (the molecular weight of silybin is 482, 100 μg/ml = 207 μM).
In spite of this difficulty, Sing and Agarwal 298 found an important decrease in tumor volume in xenografted mice with human prostate carcinoma cells when the mice were orally fed with silymarin.
There are also different requirements for effects on cell migration versus proliferation. For endothelial cells, it was necessary to use a concentration of 48.1 μg/mL of silymarin to achieve a 20% reduction in proliferation and 16.1 μg/mL to achieve the same reduction in proliferation of LoVo colon cancer cells 156 to achieve a reduction of migration of 50%, it is necessary a concentration of 1.15 μg/mL on endothelial cells (with silibinin instead of silymarin 0.66 μg/mL were enough to achieve the same). 156 Our conclusion is that, from a bioavailability standpoint, it is much easier to achieve migration inhibition, than proliferative reduction.
In Europe one of the most used brands of silymarin is Legalon® L (silybin 3,23-O-bis-hemisuccinate) that comes in capsules of 150 mg. It also comes in vials containing 350 mg of silibinin for intravenous use. In the United States, silymarin is considered a nutritional supplement. 388 The intake of 5 of these capsules in 6 human volunteers, showed no adverse events. The concentration in plasma correlated with the dose and only 10% of it was unconjugated silymarin. A half life of 6 h was estimated. 389
In experimental conditions, many researchers dilute silybin in DMSO, a polar solvent in which silymarin is highly soluble. Unfortunately, this is not possible at the bedside.
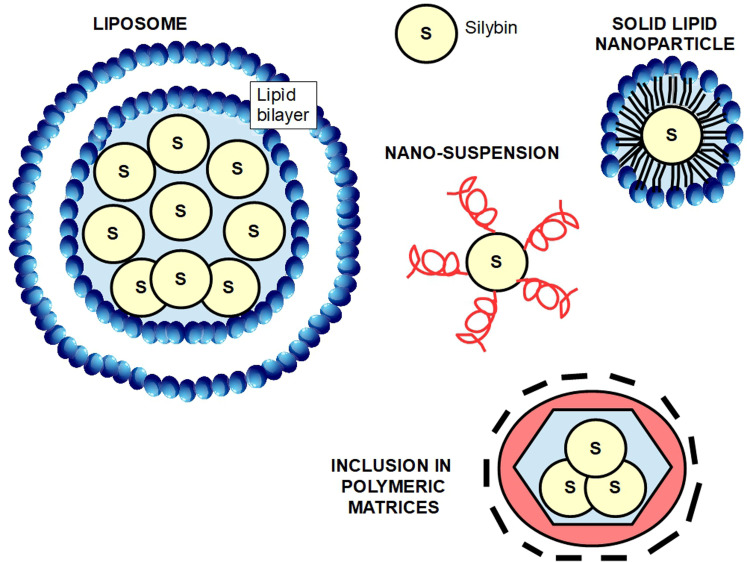
Pharmaceutical Methods to Increase Bioavailability
Silymarin's low solubility, rapid metabolism, and quick excretion, led researchers and pharmaceutical industry to develop methods that could solve these very important drawbacks. Therefore, many compounds have been formulated mainly using nanotechnology. These compounds include nanosuspensions, solid dispersions, complexes with cyclodextrins and phospholipids, microemulsions, nanoemulsions, liposomes, polymer nanocarriers, solid-lipid nanoparticles and nanostructured lipid carriers, and polymer-based nanocarriers. 390 We shall discuss only a few of them.
► Combination with succinate: is available on the market under the trade mark Legalon® (bis hemisuccinate silybin).
► Combination with phosphatidylcholine: this was the first system developed for a better bioavailability: it consists of the combination of 2 molecules of phosphatidylcholine with one of sylibin. It has been registered under the name Siliphos®, but is also known as Idb1016, silipide, or phytosome.391–394 This method increased bioavailability 10-fold. 395
► Silybin-cyclodextrin complex: adding cyclodextrin considerably enhances silymarin's water solubility.
► Other combinations with: meglumine, 23-O-phosphate.
The problem with silybin combinations is that although they increase water solubility at the same time, they may reduce other effects such as antioxidant properties. 23
► Nanosuspensions: are colloidal dispersions of drug particles with surfactants on the surface or other kind of synthetic stabilizers. This method improves dissolution and prolongs drug half life. 396
► Polymeric micelles: are nano-sized particles in which a hydrophobic substance is fully covered by a hydrophilic external layer. Wu et al 397 developed a silybin core included in amphiphilic chitosan micelles.
► Self micro-emulsifying drug delivery systems (SMEDDS): are mixtures of oil and surfactants. Liu et al 398 developed a silybin SMEDD that significantly increased its bioavailability.
► Liposomes: are lipid bilayer structures with a silybin core. This composition substantially improves bioavailability. 399
► Inclusion in polymeric matrices that carry and protect the drug. 400
There are many other mechanisms based on nano-particles that increase absorption, prolong half life, and improve water solubility of silym arin, that escape the scope of this article. For a review of the issue, see Di Costanzo et al 401 and Piazzini et al 402 (Figure 7).
Figure 7.
Methods to increase silymarin's bioavailability.
Dosage and side Effects
A phase I study of silymarin in prostate cancer patients showed that 13 g daily per os divided into 3 doses was well tolerated. The most frequent adverse event was asymptomatic liver toxicity. 403 Side effects, although rare, were mainly related to the gastrointestinal tract, such as diarrhea, bloating, and nausea. 404 Abenavoli et al 405 found that daily doses beyond 1500 mg had laxative effects and increased bile flow. The usual dose of 400 or 800 mg a day is probably insufficient to achieve anticancer effects. It may be necessary to administer 800 mg 4 times a day because the half-life is short. However, the dose of silymarin for cancer treatment remains controversial. In one study, a high dose of silybinin was administered to patients prior to prostatectomy (13 g daily). They achieved high plasma concentrations, but nevertheless, low levels of silibinin were found in prostate tissue. 406 In an attempt to circumvent some of these problems one group used a silymarin-phosphatidylcholine compound administered orally as a daily dose of 2.8 g for 4 weeks prior to surgery. They achieved high levels in human breast cancer tissue. 407 This high bioavailability in this breast cancer study is an encouraging signal for a phase II clinical trial. It should also be noted that silymarin constituents have different anticancer abilities, 22 therefore a formulation of the strongest combination would represent a fundamental step in order to incorporate this flavonoid into standard treatments.
The Main Problems with Silymarin
Problem 1: Bioavailability. The evidence gathered in Tables 2 to 12 clearly shows that silymarin should have a place in cancer treatment. The main problem is its bioavailability. Many of the in vitro investigations have used concentrations that are very difficult to achieve at the bedside. The combination of silymarin with phosphatidylcholine (silipide) has a better bioavailability, however this combination is not available for clinical use.
Problem 2: Dual nature of silymarin's effects. Silymarin has protumoral and antitumoral effects. For example, in pancreatic cancer it promotes growth arrest and apoptosis (see Table 6) and decreases CD44 signaling. However, Lee et al 408 found that in addition to the antitumoral actions, silymarin also upregulated cancer stemness-related genes, namely TWIST1, Snail, and c-Jun. At the same time, it decreased p53 wild type and increased Ki-67 (a marker of proliferation). This is a powerful call for caution. On the other hand, in bladder cancer, silymarin seems to decrease stemness through inhibition of the β-cathenin/ZEB1 signaling (Wu 178). In pancreatic tumors (PANC1), it was also found that silymarin targeted stem cells decreasing proliferation and increasing apoptosis, 409 and had similar effects in breast cancer cells.410,411 These controversies on silymarin prostemness or antistemness effects may be due to context or tumor dependency. The question remains unsolved.
Problem 3: DNA intercalation. In 2020, Pawar and Jaldappagari 412 reported that flavolignans had the ability to intercalate into the DNA double helix with moderate binding affinity. Other authors have vehemently contradicted this finding. 413 However, if this silymarin effect on DNA is confirmed, it may have unthought consequences which are favorable (modulating gene activities against cancer) or undesirable (genotoxicity and or mutations). The issue is important enough to encourage further basic research in this area.
Clinical Trials
The United States Clinical Trials web-page lists the following trials for silymarin in cancer:
-
NCT03130634: The Efficacy of Silymarin as Adjuvant Therapy on Colorectal Cancer patients Undergoing FOLFIRI Treatment.
State: recruiting since 2017.
Kaohsiung Medical University Chung-Ho Memorial Hospital. Taiwan.
Study Design: This is an open-label, randomized, comparative, double arm, single center study to assess efficacy of Silymarin (150 mg 3 times a day) as adjuvant therapy on metastatic colorectal cancer patients undergoing FOLFIRI chemotherapy in Taiwan.
-
NCT00487721: The Effect of High-dose Silybin-phytosome in Men With Prostate Cancer. (A Pilot Biomarker Study of Oral Silybin-Phytosome Followed by Prostatectomy in Patients With Localized prostate cancer).
State: completed 2014
University of Colorado. Denver
Subjects will take Silibin-Phytosome for 2 to 10 weeks. The dose of Silibin-Phytosome is 13 g daily, in 3 divided doses.
Outcome: To determine if measurable silibinin tissue levels are detectable in the prostate glands of men treated with Silybin-Phytosome administered according to the protocol.
Results: low concentration of silymarin in prostatic tissue. 117
-
NCT01829178: Evaluation of Effects of Silymarin on Cisplatin Induced Nephrotoxicity in Upper Gastrointestinal Adenocarcinoma.
State: completed 2015
University of Tehran
This study looked for possible protective effects of silymarin on kidney injury in patients receiving cisplatin.
No results posted.
-
NCT00055718: Silymarin (Milk Thistle Extract) in Treating Patients with Acute Lymphoblastic Leukemia Who Are Receiving Chemotherapy.
State: completed 2013
Miami Children's Hospital, Winthrop University Hospital, Mount Sinai Medical School.
This study looked for hepatoprotective effects of silymarin in patients receiving chemotherapy.
No results posted.
-
NCT02146118: A Phase II Study to Assess Efficacy of Combined Treatment with Erlotinib (Tarceva) and Silybin-phytosome (Siliphos) in Patients With EGFR Mutant Lung Adenocarcinoma.
State: unknown
Gosin University. Busan, Korea.
No results.
-
NCT01402648: Estrogen Receptor Beta Agonists (Eviendep) and Polyp Recurrence
State: completed 2011
Ospedale Policlinico Consorziale—Gastroenterology Unit. Bari, Italy
No results.
The conclusion we reach regarding clinical trials is:
There were only 2 clinical trials (4 and 5) to determine therapeutic possibilities of silymarin against cancer. Neither have published results.
The dose used in the clinical trials showed differences of up to 1000% which clearly means that there is no standard dose.
Schröder et al 414 conducted a randomized double-blind, cross-over placebo-controlled trial (with 2 periods of 10 weeks with a wash out period in the middle) with 49 patients that showed rising PSA levels after radical prostatectomy (34) or radiotherapy (15). They received a supplement containing soy, different isoflavones, silymarin, vitamins, minerals, and antioxidants. While receiving the treatment the doubling time for PSA was 1150 days compared with 445 days with the placebo. The fact that the supplement contained many other components besides the silymarin makes it impossible to draw conclusions about this compound. But it is evident that the supplement modified the biochemical evolution of the disease, delaying PSA progression.
Four Clinical Cases
Four clinical case reports are available, which though they cannot in themselves constitute a proof for the efficacy of silymarin, are nonetheless interesting and suggest a need for further studies. Hsu et al 415 describe the case of a 66-year-old Taiwanese patient with a regression of an 11 cm diameter hepatocellular carcinoma. The patient was receiving 450 mg of silymarin daily, and no other medication. Even if we cannot consider this regression as a consequence of silymarin treatment, the fact that spontaneous regression of hepatomas is quite infrequent, makes us think of some intervention of silymarin in this unusual event. Moroni and Zanlorenzi 416 published another case of complete regression of an advanced unresectable hepatocellular carcinoma treated with sorafenib and silymarin. Additionally, Bosch-Barrera et al 417 presented 2 cases of brain metastases from lung cancer in which the treatment with silymarin decreased edema and the size of metastases, without improvement of the primary tumor.
Discussion
The concept of a tumor as a consequence of the mutation of one gene, and with one driver signaling or metabolic pathway, is flawed in most cases with the exception of cases such as chronic myeloid leukemia. Usually many genes and pathways are involved. The approach of attacking only one of the many hallmarks of cancer is also flawed. 418 Recent evidence suggests that multiple genes are usually involved along with many signaling pathways, all interconnected, and interdependent and generating an extraordinary ability of tumor cells to survive and resist internal and external threats. 419 This is one of the reasons why treatments made up of many different drugs are implemented in most treatment protocols.
Silymarin and its derivatives, through its multipronged attacks, allow one drug to reach many targets at the same time. Of course, we cannot expect silymarin to “cure” cancer all by itself, and it cannot replace any conventional chemotherapeutic treatment, but it is rather a privileged companion to therapeutic schemes in which it may develop useful complementary activity. This activity entails 3 concepts:
cancer prevention;
synergy with some treatment protocols;
decrease of collateral damage induced by chemotherapeutic drugs.
Silymarin's clinically achievable concentration in serum and at the tumor site, with the possible exception of the liver, seems insufficient for inducing apoptosis. However, xenograft model experiments showed that even with this low bioavailability drawback, silymarin could stop tumor growth.288,351,356
The first studies on silymarin activity in cancer were performed in hepatic cells showing some characteristics that cannot be really considered antitumoral such as increased ribosomal synthesis and RNA polymerase I activation. This did not happen in hepatoma cells or in other malignant cells (Figure 8).
Figure 8.
Different effects of silymarin according to context.
Antiproliferative activity was found against almost all types of tumors, whether solid or nonsolid (Tables 2 to 12). These findings were confirmed not only at cellular level but also in vivo .
Silymarin has many other antitumor effects that can complement mainstream treatment protocols, such as:
reduction of cell motility and invasion through TGF-β2 inhibition;
inhibition of HIF-1α translation;
decreased TIMP1 expression, thus decreasing metalloproteases activation;
inhibition of the EGFR-MMP9 pathway;
decreasing the accumulation of MDSCs in the tumor;
inhibition of ERK and AKT signaling;
protection against off-target toxicity of chemotherapeutic drugs;
synergistic or added effects with some chemotherapeutics;
reduction of extracellular fibronectin production.
To this short list we must add that there is evidence sustaining clear benefits in clinical cases such as hepatocarcinoma and clear cell renal carcinoma.
However, silymarin also has some effects that work against classical chemotherapy. For example, its ability as an antioxidant reduces ROS production. Many of the drugs currently used against cancer are precisely based on the creation of an oxidative stress with increased ROS that induces apoptosis of malignant cells.
Therefore, we must ask: why is silymarin a useful complement to chemotherapy?
Evidence indicates that there may be 2 possible answers:
silymarin has context-dependent effects: its behavior is different in normal and malignant cells as can be seen in BOX 2.
Its anticancer effects overwhelm those that seem favorable for cancer cell survival.
Box 2.
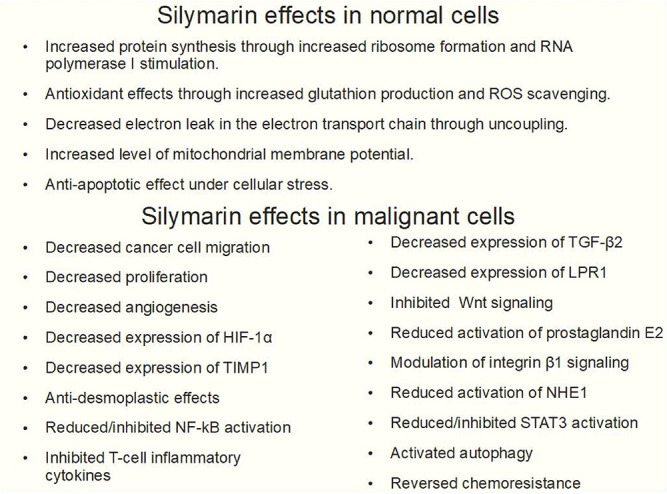
The dual effects of silymarin can be clearly seen in an experiment by Su et al. 424 Here nasopharyngeal carcinoma cells were exposed to low concentrations of silymarin and this showed protumoral effects: “Silymarin increased the expressions of superoxide dismutase 1, catalase, and glutathione peroxidase. Consequently, the cell apoptosis was reduced markedly. An increase of Bcl-2 expression and a decrease of activated caspase-3….” Additionally, there is the problem of increased stemness induced by silymarin in pancreatic adenocarcinoma cells.
In spite of these isolated discouraging report, 424 the overwhelming sum of experimental evidence shows that silymarin has clear anticancer effect, and at the same time protects normal cells from the collateral damage of chemotherapy. There is reasonable doubt that the concentration that can be reached in vivo is enough to induce apoptosis. However, xenografted tumors were reduced in size suggesting that somehow silymarin induces tumor apoptosis. In a small cohort prospective study there were no benefits found when silymarin was added to standard chemotherapy. On the other hand, a slight tendency to lower metastatic behavior was seen in the cohort receiving silymarin. 425
As already mentioned, silymarin is not a stand-alone compound against cancer and it should be used alongside other medications. On this point, it is important to note that silymarin is an inhibitor of the Cytochrome P450 system, particularly silychristin. Therefore, caution should be exercised in this respect. 426
Silybin could be used as a scaffold or structure that can be modified improving its antitumoral effects. For example, Manivannan et al 427 have synthesized silybin analogues with increased anticancer capacity. One of these compounds named “15k,” was very potent and selective for ovarian cancer cells, where it bound to tubulin with high affinity. Subsequent experiments found that 15k induced growth arrest and apoptosis of ovarian cancer cells at a much lower concentration than silymarin. Furthermore, it showed no toxicity in animals. 428
Finally, it is important to note that many of silymarin's multipronged antitumoral actions are equally, or sometimes even better conveyed by other flavonoids such as genistein and epigallocatechin gallate.429,430
What Remains to be Done?
In the first place, some of silymarin's protumoral effects demand further research with the objective of ascertaining if they need to be counteracted. Then, the precise silymarin concentrations required for the different antitumoral effects need to be established. And finally, the tumor concentration achievable with the different pharmaceutical preparations has to be determined. Once these 3 pieces of information are combined silymarin will be ready for serious clinical trials as a complement to classical chemotherapeutic schedules.
Conclusions
Silymarin compounds have considerable antitumoral effects. Well-planned clinical trials should be necessary to finally asses its bedside indications. Its dual, antitumoral and protumoral, effects merit further research.
Silymarin should be used at very high doses because low concentrations may induce protumoral effects. There is no toxicity even with very high doses. Silymarin's low absorption and bioavailability make it preferable to use modified pharmaceutical forms, such as nanoparticles or conjugated with compounds that increase its water solubility. These combinations already exist even if they have not been marketed as yet. The abundant existing evidence shows that silymarin has a definite place in cancer treatment. It has the ability to interfere with the expression of proteins related to cell cycle regulation, apoptosis, angiogenesis, and multidrug resistance. These characteristics define an anticancer drug. On the other hand, its strong antioxidant activity makes it a useful drug in cancer prevention.
Silymarin's lack of toxicity, even at very high doses, and the lack of effects on normal cells are important reasons for its further development.
Is there any other drug, that with no toxicity at all that can:
Inhibit EGFR signaling
Upregulate CDK inhibitors such as p21 and p27
Downregulate CDKs
Induce growth arrest by interfering MAPkinases cascade
Induce apoptosis
Inhibit TGF-alpha
Reduce the expression of VEGF and VEGFR
Inhibit the AKT axis
Decrease tumoral fibrosis
and have many other antitumoral effects?
Probably no other drug can achieve all these results without adverse events or high toxicity. We cannot expect that such a nontoxic pharmaceutical work as a stand-alone drug against cancer. But it can be an important factor in a multidrug anticancer schedule. Having mentioned this, the reports of increased stemness problem remain an unsolved issue which needs further investigation.
The inability to patent the compound is no doubt a drawback for the pharmaceutical industry and will restrict investment in these types of compounds. In its bioavailable formulations, silymarin deserves to be tested on clinical grounds, not as a stand-alone pharmaceutical, but as part of a treatment schedule.
Finally, the low concentrations that can be achieved with silymarin extracts at the bedside (in the order of ng/mL) hints to a serious bias in much of the past and present research at the cellular level where the average concentration range used was between 50 and 100 μg/mL. As a precondition for repurposing silymarin, newer pharmaceutical formulations should be screened in order to establish whether they can reach the necessary therapeutic concentrations.
Acknowledgments
Ms. Julia Hanna Weiss cooperated in the revision and correction of the article.
Abbreviations
- AR
androgen receptor
- BCRP
Breast cancer resistance protein.
- CDK
cyclin dependent kinase
- CA
carbonic anhydrase
- CAF
cancer associated fibroblast
- COX2
cyclooxygenase 2
- CXCR4
C-X-C chemokine receptor type 4
- DMBA
dimethylbenzanthracene
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- EMT
epithelial–mesenchymal transition
- ER
estrogen receptor.
- ERB1
eukaryotic ribosome biogenesis protein 1
- ERK
extracellular signal-regulated kinases
- FDA
Food and Drug Administration (USA)
- FKBP5
FK506 binding protein 5
- HCC
hepatocellular carcinoma
- HIF-1 alpha
hypoxia inducible factor 1 alpha
- HDAC
histone deacetylase
- IGFBP3
insulin like growth factor binding protein 3
- IL
interleukin
- MDSC
myeloid derived suppressor cell
- MAPK
mitogen-activated protein kinase
- MMP
metallopreoteases
- MDR
multidrug resistance protein
- MRP1
multidrug resistance-associated protein 1
- NF-kB
nuclear factor-kappa B
- NSCLC
nonsmall cell lung cancer
- PDAC
pancreatic ductal adenocarcinoma
- PDGF
platelet derived growth factor
- PD-L1
programmed death ligand 1
- PSA
prostate specific antigen
- Rb
retinoblastoma protein
- ROS
reactive oxygen species
- SCLC
small cell lung cancer
- SIRT1
NAD-dependent deacetylase sirtuin-1
- SLIT2
slit homolog 2 protein
- SMEDDS
self micro-emulsifying drug delivery systems
- SPC
silybin phosphatidyl coline
- SREBP1
sterol regulatory element binding protein 1
- STAT3
signal transducer and activator of transcription 3
- TGF
transforming growth factor
- TPA
tetradecanoylphorbol acetate
- TRAIL
tumor necrosis factor (TNF)-related apoptosis-inducing ligand
- TRAMP
transgenic adenocarcinoma of the mouse prostate
- UPAR
urokinase plasminogen activator receptor
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
- ZEB1
zinc finger E-box-binding homeobox 1
Footnotes
Author Contributions: Both authors equally contributed in the writing of the article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: Not applicable, because this article does not contain any studies with human or animal subjects
ORCID iD: Tomas Koltai https://orcid.org/0000-0002-7398-5096
Trial Registration: Not applicable, because this article does not contain any clinical trials.
Plagiarism: All the figures, tables, and boxes are original and were developed by the authors.
References
- 1.Delmas D. Silymarin and derivatives: from biosynthesis to health benefits. Molecules. 2020;25(10):2415. 10.3390/molecules25102415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young JA, Evans RA, Hawkes RB. Milk thistle (Silybum marianum) seed germination. Weed Sci. 1978;26(4):395-398. 10.1017/S0043174500050189 [DOI] [Google Scholar]
- 3.Letteron P, Labbe G, Degott Cet al. et al. Mechanism for the protective effects of silymarin against carbon tetrachloride-induced lipid peroxidation and hepatotoxicity in mice. Biochem. Pharmacol. 1990;39:2027-2034. 10.1016/0006-2952(90)90625-U [DOI] [PubMed] [Google Scholar]
- 4.Křen V, Walterova D. Silybin and silymarin–new effects and applications. Biomed Papers. 2005;149(1):29-41. 10.5507/bp.2005.002 [DOI] [PubMed] [Google Scholar]
- 5.Saller R, Meier R, Brignoli R. The use of silymarin in the treatment of liver diseases. Drugs. 2001;61(14):2035-2063. 10.2165/00003495-200161140-00003 [DOI] [PubMed] [Google Scholar]
- 6.Vargas-Mendoza N, Madrigal-Santillán E, Morales-González Áet al. Hepatoprotective effect of silymarin. World J Hepatol. 2014;6(3):144. 10.4254/wjh.v6.i3.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flora K, Hahn M, Rosen H, Benner K. Milk thistle (Silybum marianum) for the therapy of liver disease. Am J Gastroenterol. 1998;93(2):139-143. 10.1016/S0002-9270(97)00082-8 [DOI] [PubMed] [Google Scholar]
- 8.Theodosiou E, Purchartová K, Stamatis H, Kolisis F, Křen V. Bioavailability of silymarin flavonolignans: drug formulations and biotransformation. Phytochem Rev. 2014;13(1):1-18. 10.1007/s11101-013-9285-5 [DOI] [Google Scholar]
- 9.Polyak SJ, Ferenci P, Pawlotsky JM. Hepatoprotective and antiviral functions of silymarin components in hepatitis C virus infection. Hepatology. 2013;57(3):1262-1271. 10.1002/hep.26179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Federico A, Dallio M, Loguercio C. Silymarin/silybin and chronic liver disease: a marriage of many years. Molecules. 2017;22(2):191. 10.3390/molecules22020191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schadewaldt H. The history of silymarin. Contribution to the history of liver therapy. Med Welt. 1969;20(15):902-914. PMID: 5784380. [PubMed] [Google Scholar]
- 12.Andrzejewska J, Sadowska K, Mielcarek S. Effect of sowing date and rate on the yield and flavonolignan content of the fruits of milk thistle (Silybum marianum L. Gaertn.) grown on light soil in a moderate climate. Ind Crops Prod. 2011;33(2):462-468. 10.1016/j.indcrop.2010.10.027 [DOI] [Google Scholar]
- 13.Takemoto T, Ikegawa S, Nomoto K. Studies on constituents of Silybum marianum (L.) gaertn. I. New flavonolignans named 2,3–dehydrosilymarin and 2,3-dehydrosilychristin. AGRIS (FAO of the UN). 1975; 95(8):1017 PMID: 123760. 10.1248/yakushi1947 [DOI] [PubMed] [Google Scholar]
- 14.Kim NC, Graf TN, Sparacino CM, Wani MC, Wall ME. Complete isolation and characterization of silybins and isosilybins from milk thistle (Silybum marianum). Org Biomol Chem. 2003;1:1684-1689. 10.1039/B300099K [DOI] [PubMed] [Google Scholar]
- 15.Möschlin G. Phd thesis, Karlsruhe University, 1959, mentioned in Biedermann, D., Vavříková, E., Cvak, L., & Křen, V. Chemistry of silybin. Natural Prod Rep. 2014;31(9):1138-1157. 10.1039/C3NP70122K [DOI] [PubMed] [Google Scholar]
- 16.Wagner H, Hörhammer L, Seitz M. Chemical evaluation of a silymarin-containing flavonoid concentrate from Silybum marianum (L.) Gaertn. Arzneimittelforschung. 1968;8(6):696-698. PMID: 5755806. [PubMed] [Google Scholar]
- 17.Wagner H, Hörhammer L, Münster R. On the chemistry of silymarin (silybin), the active principle of the fruits from Silybum marianum (L.) Gaertn. (Carduus marianus L.). Arzneimittelforschung. 1968;18(6):688-696. PMID: 5755805. [PubMed] [Google Scholar]
- 18.Pelter A, Hansel R. The structure of silybin (silybum substance E6), the first flavonolignan. Tetrahedron Lett. 1968;9(25):2911-2916. 10.1016/S0040-4039(00)89610-0 [DOI] [Google Scholar]
- 19.Cardone RA, Alfarouk KO, Elliott RLet al. et al. The role of sodium hydrogen exchanger 1 in dysregulation of proton dynamics and reprogramming of cancer metabolism as a sequela. Int J Mol Sci. 2019;20(15):3694. 10.3390/ijms20153694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer. 2011;11(9):671-677. 10.1038/nrc3110 [DOI] [PubMed] [Google Scholar]
- 21.Simánek V, Kren V, Ulrichová J, Vicar J, Cvak L. Silymarin: what is in the name…? An appeal for a change of editorial policy. Hepatology. 2000;32(2):442-444. 10.1053/jhep.2000.9770 [DOI] [PubMed] [Google Scholar]
- 22.Biedermann D, Vavříková E, Cvak L, Křen V. Chemistry of silybin. Nat Prod Rep. 2014;31(9):1138-1157. 10.1039/C3NP70122K [DOI] [PubMed] [Google Scholar]
- 23.Gažák R, Svobodová A, Psotová Jet al. et al. Oxidised derivatives of silybin and their antiradical and antioxidant activity. Bioorg Med Chem. 2004;12(21):5677-5687. 10.1016/j.bmc.2004.07.064 [DOI] [PubMed] [Google Scholar]
- 24.Kroll DJ, Shaw HS, Oberlies NH. Milk thistle nomenclature: why it matters in cancer research and pharmacokinetic studies. Integr Cancer Ther. 2007;6(2):110-119. 10.1177/1534735407301825 [DOI] [PubMed] [Google Scholar]
- 25.Deep G, Oberlies NH, Kroll DJ, Agarwal R. Identifying the differential effects of silymarin constituents on cell growth and cell cycle regulatory molecules in human prostate cancer cells. Int J Cancer. 2008;123(1):41-50. 10.1002/ijc.23485 [DOI] [PubMed] [Google Scholar]
- 26.Ding TM, Tian SJ, Zhang ZXet al. et al. Determination of active component in silymarin by RP-LC and LC/MS. J Pharm Biomed Anal. 2001;26(1):155-161. 10.1016/S0731-7085(01)00364-8 [DOI] [PubMed] [Google Scholar]
- 27.Desplaces A, Choppin J, Vogel G, Trost W. The effects of silymarin on experimental phalloidine poisoning. Arzneim-Forsch. 1975;25(1):89-96. PMID: 125090. [PubMed] [Google Scholar]
- 28.Tuchweber B, Sieck R, Trost W. Prevention by silybin of phalloidin-induced acute hepatoxicity. Toxicol Appl Pharmacol. 1979;51(2):265-275. 10.1016/0041-008X(79)90469-1 [DOI] [PubMed] [Google Scholar]
- 29.Mayer KE, Myers RP, Lee SS. Silymarin treatment of viral hepatitis: a systematic review. J Viral Hepat. 2005;12(6):559-567. 10.1111/j.1365-2893.2005.00636.x [DOI] [PubMed] [Google Scholar]
- 30.Fried MW, Navarro VJ, Afdhal Net al. et al. Effect of silymarin (milk thistle) on liver disease in patients with chronic hepatitis C unsuccessfully treated with interferon therapy: a randomized controlled trial. Jama. 2012;308(3):274-282. 10.1001/jama.2012.8265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hawke RL, Schrieber SJ, Soule TA, Wen Z, Smith PC, Reddy KR, SyNCH Trial Group. Silymarin ascending multiple oral dosing phase I study in noncirrhotic patients with chronic hepatitis C. J Clin Pharmacol. 2010;50(4):434-449. 10.1177/0091270009347475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagoner J, Negash A, Kane OJet al. et al. Multiple effects of silymarin on the hepatitis C virus lifecycle. Hepatology. 2010;51(6):1912-1921. doi : 10.1002/hep.23587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trinchet JC, Coste T, Levy VGet al. et al. Treatment of alcoholic hepatitis with silymarin. A double-blind comparative study in 116 patients. Gastroenterol Clin Biol. 1989;13(2):120-124. PMID: 2707520. [PubMed] [Google Scholar]
- 34.Soliman NA, El-Dardiry SA. Taraxacum officinale and Silybum marianum alone or combined orchestrate experimentally induced hepatic steatosis through lipogenecity, glucose tolerance and oxidant/antioxidant status. Int J Biol Chem Sci. 2015;9(4):1918-1928. 10.4314/ijbcs.v9i4.17 [DOI] [Google Scholar]
- 35.Ni X, Wang H. Silymarin attenuated hepatic steatosis through regulation of lipid metabolism and oxidative stress in a mouse model of nonalcoholic fatty liver disease (NAFLD). Am J Transl Res. 2016;8(2):1073. PMID: 27158393. [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu SY, Jiang N, Yang Jet al. et al. Silybum marianum oil attenuates hepatic steatosis and oxidative stress in high fat diet-fed mice. Biomed Pharmacother. 2018;100:191-197. 10.1016/j.biopha.2018.01.144 [DOI] [PubMed] [Google Scholar]
- 37.Clichici S, Olteanu D, Nagy AL, Oros A, Filip A, Mircea PA. Silymarin inhibits the progression of fibrosis in the early stages of liver injury in CCl4-treated rats. J Med Food. 2015;18(3):290-298. 10.1089/jmf.2013.0179 [DOI] [PubMed] [Google Scholar]
- 38.Ferenci P, Dragosics B, Dittrich Het al. et al. Randomized controlled trial of silymarin treatment in patients with cirrhosis of the liver. J. Hepatol. 1989;9(1):105-113. 10.1016/0168-8278(89)90083-4 [DOI] [PubMed] [Google Scholar]
- 39.Tighe SP, Akhtar D, Iqbal U, Ahmed A. Chronic liver disease and silymarin: a biochemical and clinical review. J Clin Transl Hepatol. 2020;8(4):454. 10.14218/JCTH.2020.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Basaga H, Poli G, Tekkaya C, Aras I. Free radical scavenging and antioxidative properties of ‘silibin’ complexes on microsomal lipid peroxidation. Cell Biochem Funct. 1997;15(1):27-33. [DOI] [PubMed] [Google Scholar]
- 41.Machicao F, Sonnenbichler J. Mechanism of the stimulation of RNA synthesis in rat liver nuclei by silybin. Hoppe Seylers Z Physiol Chem. 1977;358(2):141-147. 10.1515/bchm2.1977.358.1.141 [DOI] [PubMed] [Google Scholar]
- 42.Shriewer H, Krämer U, Rukowski G, Borjis KJ. Influence of silybin-dihemisuccinate on fatty acid synthesis in rat liver. Arzneimittelforschung. 1979;29(3):524-526. PMID: 39576. [PubMed] [Google Scholar]
- 43.Fiebrich F, Koch H. Silymarin an inhibitor of prostaglandin synthetase. Experientia. 1979;35(12):1550-1552. 10.1007/BF01953185 [DOI] [PubMed] [Google Scholar]
- 44.Fiebrich F, Koch H. Silymarin an inhibitor of lipooxygenase. Experientia. 1979;35(12):1548-1560. 10.1007/BF01953184 [DOI] [PubMed] [Google Scholar]
- 45.Bindoli A, Cavallini L, Siliprandi N. Inhibitory action of silymarin of lipid peroxide formation in rat liver mitochondria and microsomes. Biochem Pharmacol. 1977;26(24):2405-2409. 10.1016/0006-2952(77)90449-X [DOI] [PubMed] [Google Scholar]
- 46.Sonnenbichler J, Goldberg M, Hane L, Madubunyi I, Vogl S, Zetl I. Stimulatory effect of silibinin on the DNA synthesis in partially hepatectomized rat livers: non-response in hepatoma and other malign cell lines. Biochem Pharmacol. 1986;35(3):538-541. 10.1016/0006-2952(86)90233-9 [DOI] [PubMed] [Google Scholar]
- 47.Koch HP, Bachner J, Löffler E. Silymarin: potent inhibitor of cyclic AMP phosphodiesterase. Methods Find Exp Clin Pharmacol. 1985;7(8):409-413. PMID: 3001454. [PubMed] [Google Scholar]
- 48.Miadonna A, Tedeschi A, Leggieri E, Lorini M, Froldi M, Zanussi C. Effects of silybin on histamine release from human basophil leucocytes. Br J Clin Pharmacol. 1987;24(6):747-752. 10.1111/j.1365-2125.1987.tb03241.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moroni PL, Barcellini W, Borghi M. Sylibinin inhibition of human T lymphocyte. Activation. Int J Tissue React. 1988;10(3):177-181. PMID: 3265704. [PubMed] [Google Scholar]
- 50.Chávez E, Bravo C. Silymarin-induced mitochondrial Ca2 + release. Life Sci. 1988;43(12):975-981. 10.1016/0024-3205(88)90542-5 [DOI] [PubMed] [Google Scholar]
- 51.Valenzuela A, Guerra R, Videla LA. Antioxidant properties of the flavonoids silybin and ( + )- cyanidanol-3: comparison with butylated hydroxyanisole and butylated hydroxytoluene. Planta Me. 1986;52(6):438-440. 10.1055/s-2007-969247 [DOI] [PubMed] [Google Scholar]
- 52.Wilasrusmee C, Kittur S, Shah Get al. et al. Immunostimulatory effect of Silybum Marianum (milk thistle) extract. Med Sci Monit. 2002;8(11):BR439-BR443. PMID: 12444368. [PubMed] [Google Scholar]
- 53.Esmaeil N, Anaraki SB, Gharagozloo M, Moayedi B. Silymarin impacts on immune system as an immunomodulator: one key for many locks. Int Immunopharmacol. 2017;50:194-201. 10.1016/j.intimp.2017.06.030 [DOI] [PubMed] [Google Scholar]
- 54.Wu ZS, Wu Q, Yang JHet al. et al. Prognostic significance of MMP-9 and TIMP-1 serum and tissue expression in breast cancer. Int J Cancer. 2008;122(9):2050-2056. 10.1002/ijc.23337 [DOI] [PubMed] [Google Scholar]
- 55.Stolf AM, Cardoso CC, Acco A. Effects of silymarin on diabetes mellitus complications: a review. Phytother Res. 2017;31(3):366-374. 10.1002/ptr.5768 [DOI] [PubMed] [Google Scholar]
- 56.Tuorkey MJ, El-Desouki NI, Kamel RA. Cytoprotective effect of silymarin against diabetes-induced cardiomyocyte apoptosis in diabetic rats. Biomed Environ Sci. 2015;28(1):36-43. 10.3967/bes2015.004 [DOI] [PubMed] [Google Scholar]
- 57.Rafieian-Kopaie M, Nasri H. Silymarin and diabetic nephropathy. J Renal Inj Prev. 2012;1(1):3. 10.12861/jrip.2012.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.García-Ramírez M, Turch M, Simó-Servat O, Hernández C, Simó R. Silymarin prevents diabetes-induced hyperpermeability in human retinal endothelial cells. Endocrinologia, diabetes y nutricion. 2018;65(4):200-205. 10.1016/j.endinu.2017.12.004 [DOI] [PubMed] [Google Scholar]
- 59.Krečman V, Škottová N, Walterová D, Ulrichová J, Šimánek V. Silymarin inhibits the development of diet-induced hypercholesterolemia in rats. Planta Med. 1998;64(02):138-142. 10.1055/s-2006-957391 [DOI] [PubMed] [Google Scholar]
- 60.Milić N, Milošević N, Suvajdžić L, Žarkov M, Abenavoli L. New therapeutic potentials of milk thistle (Silybum marianum). Nat Prod Commun. 2013;8(12). 10.1177/1934578X1300801236 [DOI] [PubMed] [Google Scholar]
- 61.Skottova N, Krecman V, Walterova D, Ulrichová J, Simanek V. Effects of silymarin and silybin on lipoprotein cholesterol levels and oxidizability of low density lipoproteins in rats. Atherosclerosis. 1997;134(1–2):134-134. eLIBRARY ID: 279046. ISSN0021-9150. [Google Scholar]
- 62.Haddadi R, Shahidi Z, Eyvari-Brooshghalan S. Silymarin and neurodegenerative diseases: therapeutic potential and basic molecular mechanisms. Phytomedicine. 2020;79:153320. 10.1016/j.phymed.2020.153320 [DOI] [PubMed] [Google Scholar]
- 63.Borah A, Paul R, Choudhury Set al. et al. Neuroprotective potential of silymarin against CNS disorders: insight into the pathways and molecular mechanisms of action. CNS Neurosci Ther. 2013;19(11):847-853. 10.1111/cns.12175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang MJ, Lin WW, Chen HLet al. et al. Silymarin protects dopaminergic neurons against lipopolysaccharide-induced neurotoxicity by inhibiting microglia activation. Eur J Neurosci. 2002;16(11):2103-2112. 10.1046/j.1460-9568.2002.02290.x [DOI] [PubMed] [Google Scholar]
- 65.Murata N, Murakami K, Ozawa Yet al. et al. Silymarin attenuated the amyloid β plaque burden and improved behavioral abnormalities in an Alzheimer's disease mouse model. Biosci, Biotechnol, Biochem. 2010;74(11):2299-2306. 10.1271/bbb.100524 [DOI] [PubMed] [Google Scholar]
- 66.Yaghmaei P, Azarfar K, Dezfulian M, Ebrahim-Habibi A. Silymarin effect on amyloid-β plaque accumulation and gene expression of APP in an Alzheimer's disease rat model. DARU J Pharmaceut Sci. 2014;22(1):1-7. 10.1186/2008-2231-22-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sato M, Murakami K, Uno Met al. et al. Structure–activity relationship for ( + )-taxifolin isolated from silymarin as an inhibitor of amyloid β aggregation. Biosci, Biotechnol, Biochem. 2013;77(5):1100-1103. 10.1271/bbb.120925 [DOI] [PubMed] [Google Scholar]
- 68.Shariati M, Shaygannejad V, Abbasirad Fet al. et al. Silymarin restores regulatory T cells (tregs) function in multiple sclerosis (MS) patients in vitro. Inflammation. 2019;42(4):1203-1214. 10.1007/s10753-019-00980-9 [DOI] [PubMed] [Google Scholar]
- 69.Navabi F, Shaygannejad V, Abbasirad Fet al. et al. Immunoregulatory effects of silymarin on proliferation and activation of Th1 cells isolated from newly diagnosed and IFN-β 1b-treated MS patients. Inflammation. 2019;42(1):54-63. 10.1007/s10753-018-0872-x [DOI] [PubMed] [Google Scholar]
- 70.Yön B, Belviranlı M, Okudan N. The effect of silymarin supplementation on cognitive impairment induced by diabetes in rats. J Basic Clin Physiol Pharmacol. 2019;30(4). 10.1515/jbcpp-2018-0109 [DOI] [PubMed] [Google Scholar]
- 71.Jin G, Bai D, Yin Set al. et al. Silibinin rescues learning and memory deficits by attenuating microglia activation and preventing neuroinflammatory reactions in SAMP8 mice. Neurosci Lett. 2016;629:256-261. 10.1016/j.neulet.2016.06.008 [DOI] [PubMed] [Google Scholar]
- 72.Soto CP, Perez BL, Favari LP, Reyes JL. Prevention of alloxan-induced diabetes mellitus in the rat by silymarin. Comparative Biochemistry and Physiology Part C: pharmacology. Toxicol Endocrinol. 1998;119(2):125-129. 10.1016/S0742-8413(97)00198-9 [DOI] [PubMed] [Google Scholar]
- 73.Matsuda T, Ferreri K, Todorov Iet al. et al. Silymarin protects pancreatic β-cells against cytokine-mediated toxicity: implication of c-Jun NH2-terminal kinase and janus kinase/signal transducer and activator of transcription pathways. Endocrinology. 2005;146(1):175-185. 10.1210/en.2004-0850 [DOI] [PubMed] [Google Scholar]
- 74.Huseini HF, Larijani B, Heshmat Ret al. et al. The efficacy of Silybum marianum (L.) gaertn.(silymarin) in the treatment of type II diabetes: a randomized, double-blind, placebo-controlled, clinical trial. Phytother Res, Int J Devoted Pharmacol Toxicol Eval Nat Prod Derivat. 2006;20(12):1036-1039. 10.1002/ptr.1988 [DOI] [PubMed] [Google Scholar]
- 75.Hussain SAR. Silymarin as an adjunct to glibenclamide therapy improves long-term and postprandial glycemic control and body mass index in type 2 diabetes. J Med Food. 2007;10(3):543-547. 10.1089/jmf.2006.089 [DOI] [PubMed] [Google Scholar]
- 76.Voroneanu L, Nistor I, Dumea R, Apetrii M, Covic A. Silymarin in type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. J Diabetes Res. 2016; 20(12) Volume 2016. Article ID 5147468 10.1155/2016/5147468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sobolová L, Škottová N, Večeřa R, Urbánek K. Effect of silymarin and its polyphenolic fraction on cholesterol absorption in rats. Pharmacol Res. 2006;53(2):104-112. 10.1016/j.phrs.2005.09.004 [DOI] [PubMed] [Google Scholar]
- 78.Škottová N, Večeřa R, Urbánek K, Váňa P, Walterová D, Cvak L. Effects of polyphenolic fraction of silymarin on lipoprotein profile in rats fed cholesterol-rich diets. Pharmacol Res. 2003;47(1):17-26. 10.1016/S1043-6618(02)00252-9 [DOI] [PubMed] [Google Scholar]
- 79.Škottová N, Krečman V. Silymarin as a potential hypocholesterolaemic drug. Physiol Res. 1998;47(1):1-7. PMID: 9708694. [PubMed] [Google Scholar]
- 80.Nassuato G, Iemmolo RM, Strazzabosco M. Effect of silibinin on biliary lipid composition experimental and clinical study. J Hepatol. 1992;12:290-295. 10.1016/0168-8278(91)90829-Z [DOI] [PubMed] [Google Scholar]
- 81.Somogyi A, Ecsedi GG, Blazovics A, Miskolczi K, Gergely P, Feher J. Short term treatment of type II hyperlipoproteinaemia with silymarin. Acta Med Hung. 1989;46:289-295. PMID: 2699920. [PubMed] [Google Scholar]
- 82.Zima T, Kamenikova L, Janebova M, Buchar E, Crkovska J, Tesar V. The effect of silibinin on experimental cyclosporine nephrotoxicity. Renal Fail. 1998;20(3):471-479. 10.3109/08860229809045136 [DOI] [PubMed] [Google Scholar]
- 83.Soto C, Pérez J, García V, Uría E, Vadillo M, Raya L. Effect of silymarin on kidneys of rats suffering from alloxan-induced diabetes mellitus. Phytomedicine. 2010;17(14):1090-1094. 10.1016/j.phymed.2010.04.011 [DOI] [PubMed] [Google Scholar]
- 84.Fallahzadeh MK, Dormanesh B, Sagheb MMet al. et al. Effect of addition of silymarin to renin-angiotensin system inhibitors on proteinuria in type 2 diabetic patients with overt nephropathy: a randomized, double-blind, placebo-controlled trial. Am J Kidney Dis. 2012;60(6):896-903. 10.1053/j.ajkd.2012.06.005 [DOI] [PubMed] [Google Scholar]
- 85.Akbari-Kordkheyli V, Abbaszadeh-Goudarzi K, Nejati-Laskokalayeh M, Zarpou S, Khonakdar-Tarsi A. The protective effects of silymarin on ischemia-reperfusion injuries: a mechanistic review. Iran J Basic Med Sci. 2019;22(9):968. 10.22038/ijbms.2019.34284.8147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rao PR, Viswanath RK. Cardioprotective activity of silymarin in ischemia-reperfusion-induced myocardial infarction in albino rats. Exper Clin Cardiol. 2007;12(4):179. PMID: 18651002. [PMC free article] [PubMed] [Google Scholar]
- 87.Hou YC, Liou KT, Chern CMet al. et al. Preventive effect of silymarin in cerebral ischemia–reperfusion-induced brain injury in rats possibly through impairing NF-κB and STAT-1 activation. Phytomedicine. 2010;17(12):963-973. 10.1016/j.phymed.2010.03.012 [DOI] [PubMed] [Google Scholar]
- 88.Moghaddam AH, Sangdehi SRM, Ranjbar M, Hasantabar V. Preventive effect of silymarin-loaded chitosan nanoparticles against global cerebral ischemia/reperfusion injury in rats. Eur J Pharmacol. 2020;877:173066. 10.1016/j.ejphar.2020.173066 [DOI] [PubMed] [Google Scholar]
- 89.Senturk H, Kabay S, Bayramoglu Get al. et al. Silymarin attenuates the renal ischemia/reperfusion injury-induced morphological changes in the rat kidney. World J Urol. 2008;26(4):401-407. 10.1007/s00345-008-0256-1 [DOI] [PubMed] [Google Scholar]
- 90.Turgut F, Bayrak O, Catal Fet al. et al. Antioxidant and protective effects of silymarin on ischemia and reperfusion injury in the kidney tissues of rats. Int Urol Nephrol. 2008;40(2):453-460. 10.1007/s11255-008-9365-4 [DOI] [PubMed] [Google Scholar]
- 91.Demir M, Amanvermez R, Polat AKet al. et al. The effect of silymarin on mesenteric ischemia-reperfusion injury. Med Princ Pract. 2014;23(2):140-144. 10.1159/000356860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.de la Lastra CA, Martin MJ, Motilva V, Jimenez M, La Casa C, Lopez A. Gastroprotection induced by silymarin, the hepatoprotective principle of Silybum marianum in ischemia-reperfusion mucosal injury: role of neutrophils. Planta Med. 1995;61(02):116-119. 10.1055/s-2006-958028 [DOI] [PubMed] [Google Scholar]
- 93.Jin Y, Zhao X, Zhang H, Li Q, Lu G, Zhao X. Modulatory effect of silymarin on pulmonary vascular dysfunction through HIF-1α-iNOS following rat lung ischemia-reperfusion injury. Exp Ther Med. 2016;12(2):1135-1140. 10.3892/etm.2016.3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Younis NN, Shaheen MA, Mahmoud MF. Silymarin preconditioning protected insulin resistant rats from liver ischemia-reperfusion injury: role of endogenous H2S. J Surg Res. 2016;204(2):398-409. 10.1016/j.jss.2016.04.069 [DOI] [PubMed] [Google Scholar]
- 95.Rolo AP, Oliveira PJ, Moreno AJ, Palmeira CM. Protection against post-ischemic mitochondrial injury in rat liver by silymarin or TUDC. Hepatol Res. 2003;26(3):217-224. 10.1016/S1386-6346(03)00108-6 [DOI] [PubMed] [Google Scholar]
- 96.Koçarslan A, Koçarslan S, Aydin MSet al. et al. Intraperitoneal administration of silymarin protects end organs from multivisceral ischemia/reperfusion injury in a rat model. Brazilian J Cardiovasc Surg. 2016;31:434-439. 10.5935/1678-9741.20160072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Svobodová A, Zdařilová A, Mališková J, Mikulková H, Walterová D, Vostalová J. Attenuation of UVA-induced damage to human keratinocytes by silymarin. J Dermatol Sci. 2007;46(1):21-30. 10.1016/j.jdermsci.2006.12.009 [DOI] [PubMed] [Google Scholar]
- 98.Katiyar SK, Mantena SK, Meeran SM. Silymarin protects epidermal keratinocytes from ultraviolet radiation-induced apoptosis and DNA damage by nucleotide excision repair mechanism. PLoS ONE. 2011;6(6):e21410. 10.1371/journal.pone.0021410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vostálová J, Tinková E, Biedermann D, Kosina P, Ulrichová J, Rajnochová Svobodová A. Skin protective activity of silymarin and its flavonolignans. Molecules. 2019;24(6):1022. 10.3390/molecules24061022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Altaei T. The treatment of melasma by silymarin cream. BMC Dermatol. 2012;12(1):1-6. 10.1186/1471-5945-12-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Berardesca E, Cameli N, Cavallotti C, Levy JL, Piérard GE, de Paoli Ambrosi G. Combined effects of silymarin and methylsulfonylmethane in the management of rosacea: clinical and instrumental evaluation. J Cosmet Dermatol. 2008;7(1):8-14. 10.1111/j.1473-2165.2008.00355.x [DOI] [PubMed] [Google Scholar]
- 102.Meeran SM, Katiyar S, Elmets CA, Katiyar SK. Silymarin inhibits UV radiation-induced immunosuppression through augmentation of interleukin-12 in mice. Mol Cancer Ther. 2006;5(7):1660-1668. 10.1158/1535-7163.MCT-06-0095 [DOI] [PubMed] [Google Scholar]
- 103.Palit P, Mukhopadhyay A, Chattopadhyay D. Phyto-pharmacological perspective of silymarin: a potential prophylactic or therapeutic agent for COVID-19, based on its promising immunomodulatory, anti-coagulant and anti-viral property. Phytother Res. 2021;2021:1-12. 10.1002/ptr.7084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gorla US, Rao K, Kulandaivelu US, Alavala RR, Panda SP. Lead finding from selected flavonoids with antiviral (SARS-CoV-2) potentials against COVID-19: an in-silico evaluation. Comb Chem High Throughput Screening. 2021;24(6):879-890. 10.2174/1386207323999200818162706 [DOI] [PubMed] [Google Scholar]
- 105.Camini FC, da Silva TF, da Silva Caetano CCet al. Antiviral activity of silymarin against mayaro virus and protective effect in virus-induced oxidative stress. Antiviral Res. 2018;158:8-12. 10.1016/j.antiviral.2018.07.023 [DOI] [PubMed] [Google Scholar]
- 106.Lani R, Hassandarvish P, Chiam CWet al. et al. Antiviral activity of silymarin against chikungunya virus. Sci Rep. 2015;5(1):1-10. 10.1038/srep11421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.da Silva TF, Ferraz AC, Almeida LTet al. Antiviral effect of silymarin against Zika virus in vitro. Acta Trop. 2020;211:105613. 10.1016/j.actatropica.2020.105613 [DOI] [PubMed] [Google Scholar]
- 108.Rakelly de Oliveira D, Relison Tintino S, Morais Braga MFBet al. In vitro antimicrobial and modulatory activity of the natural products silymarin and silibinin. BioMed Res Int. 2015;2015. 10.1155/2015/292797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vereckei AS, Besch HR, Zipes DP. Combined amiodarone and silymarin treatment, but not amiodarone alone, prevents sustained atrial flutter in dogs. J. Cardiovasc Electrophysiol. 2003;14:861-867. 10.1046/j.1540-8167.2003.02446.x [DOI] [PubMed] [Google Scholar]
- 110.Ágoston M, Örsi F, Free Eet al. et al. Silymarin and vitamin E reduce amiodarone-induced lysosomal phospholipidosis in rats. Toxicology. 2003;190:231-241. 10.1016/S0300-483X(03)00188-4 [DOI] [PubMed] [Google Scholar]
- 111.Rastegarpanah M, Malekzadeh R, Vahedi Het al. et al. A randomized, double blinded, placebo-controlled clinical trial of silymarin in ulcerative colitis. Chin J Integr Med. 2015;21(12):902-906. 10.1007/s11655-012-1026-x [DOI] [PubMed] [Google Scholar]
- 112.Nguyen THT, Trinh NT, Tran HNet al. et al. Improving silymarin oral bioavailability using silica-installed redox nanoparticle to suppress inflammatory bowel disease. J Controlled Release. 2021;331:515-524. 10.1016/j.jconrel.2020.10.042. [DOI] [PubMed] [Google Scholar]
- 113.Hagan M, Hayee BH, Rodriguez-Mateos A. (Poly) phenols in inflammatory bowel disease and irritable bowel syndrome: a review. Molecules. 2021;26(7):1843. 10.3390/molecules26071843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Monfared ME, Jadidi A, Hosseini SM, Ashtiani AR. Investigating the effectiveness of silymarin in treatment of migraine patients referred to medical centers affiliated to arak university of medical sciences. J Biochem Tech. 2018;9(2):98-101. [Google Scholar]
- 115.Capasso R, Aviello G, Capasso Fet al. et al. Silymarin BIO-C®, an extract from Silybum marianum fruits, induces hyperprolactinemia in intact female rats. Phytomedicine. 2009;16(9):839-844. 10.1016/j.phymed.2009.02.007 [DOI] [PubMed] [Google Scholar]
- 116.Saberi Z, Gorji N, Memariani Z, Moeini R, Shirafkan H, Amiri M. Evaluation of the effect of Silybum marianum extract on menopausal symptoms: a randomized, double-blind placebo-controlled trial. Phytother Res. 2020;34(12):3359-3366. 10.1002/ptr.6789 [DOI] [PubMed] [Google Scholar]
- 117.Kayedpoor P, Mohamadi S, Karimzadeh-Bardei L, Nabiuni M. Anti-inflammatory effect of silymarin on ovarian immunohistochemical localization of TNF-α associated with systemic inflammation in polycystic ovarian syndrome. Int J Morphol. 2017;35(2):723-732. 10.4067/S0717-95022017000200054 [DOI] [Google Scholar]
- 118.Mehta RG, Moon RC. Characterization of effective chemopreventive agents in mammary gland in vitro using an initiation-promotion protocol. Anticancer Res. 1991;11(2):593-596. PMID: 1905902. [PubMed] [Google Scholar]
- 119.Ruy YC. Advances in pharmacological studies of silymarin. Mem Inst Oswaldo Cruz. 1991;86, Suppl 2, 79–85. https://doi.org/10.1590/s0074-02761991000600020. [DOI] [PubMed] [Google Scholar]
- 120.Agarwal R, Katiyar SK, Lundgren DW, Mukhtar H. Inhibitory effect of silymarin, an antihepatotoxic flavonoid, on 12-O-tetradecanoylphorbol-13-acetate-induced epidermal ornithine decarboxylase activity and mRNA in SENCAR mice. Carcinogenesis. 1994;15(6):1099-1103. 10.1093/carcin/15.6.1099 [DOI] [PubMed] [Google Scholar]
- 121.Gershbein LL. Action of dietary trypsin, pressed coffee oil, silymarin and iron salt on 1,2- dimethylhydrazine tumorigenesis by gavage. Anticancer Res. 1994;14(3A):1113-1116. PMID: 8074460. [PubMed] [Google Scholar]
- 122.Kim DH, Jin YH, Park JB, Kobashi K. Silymarin and its components are inhibitors of betaglucuronidase. Biol Pharm Bull. 1994;17(3):443-445. 10.1248/bpb.17.443 [DOI] [PubMed] [Google Scholar]
- 123.Valenzuela A, Garrido A. Biochemical bases of the pharmacological action of the flavonoid silymarin and of its structural isomer silibinin. Biol Res. 1994;27(2):105-112. PMID: 8640239. [PubMed] [Google Scholar]
- 124.Bartholomaeus AR, Bolton R, Ahokas JT. Inhibition of rat liver cytosolic glutathione S transferase by silybin. Xenobiotica. 1994;24(1):17-24. 10.3109/00498259409043217 [DOI] [PubMed] [Google Scholar]
- 125.Dehmlow C, Murawski N, de Groot H. Scavenging of reactive oxygen species and inhibition of arachidonic acid metabolism by silibinin in human cells. Life Sci. 1996;58(18):1591-1600. 10.1016/0024-3205(96)00134-8 [DOI] [PubMed] [Google Scholar]
- 126.De la Puerta R, Martinez E, Bravo L, Ahumada MC. Effect of silymarin on different acute inflammatory models and leukocyte migration. J Pharm Pharmacol. 1996;48(9):968-970. 10.1111/j.2042-7158.1996.tb06014.x [DOI] [PubMed] [Google Scholar]
- 127.Zi X, Mukhtar H, Agarwal R. Novel cancer chemopreventive effects of a flavonoid antioxidant silymarin: inhibition of mRNA expression of an endogenous tumor promoter TNF alpha. Biochem Biophys Res Commun. 1997;239(1):334-339. 10.1006/bbrc.1997.7375 [DOI] [PubMed] [Google Scholar]
- 128.Ahmad N, Gali H, Javed S, Agarwal R. Skin cancer chemopreventive effects of a flavonoid antioxidant silymarin are mediated via impairment of receptor tyrosine kinase signaling and perturbation in cell cycle progression. Biochem Biophys Res Commun. 1998;247(2):294-301. 10.1006/bbrc.1998.8748 [DOI] [PubMed] [Google Scholar]
- 129.Katiyar SK, Korman NJ, Mukhtar H, Agarwal R. Protective effects of silymarin against photocarcinogenesis in a mouse skin model. J Natl Cancer Inst. 1997;89(8):556-566. 10.1093/jnci/89.8.556 [DOI] [PubMed] [Google Scholar]
- 130.Saliou C, Kitazawa M, McLaughlin Let al. et al. Antioxidants modulate acute solar ultraviolet radiation-induced NF-kappa-B activation in a human keratinocyte cell line. Free Radical Biology and Medicine. 1999;26(1–2):174-183. 10.1016/S0891-5849(98)00212-3 [DOI] [PubMed] [Google Scholar]
- 131.Lahiri-Chatterjee M, Katiyar SK, Mohan RR, Agarwal R. A flavonoid antioxidant, silymarin, affords exceptionally high protection against tumor promotion in the SENCAR mouse skin tumorigenesis model. Cancer Res. 1999;59(3):622-632. PMID: 9973210. [PubMed] [Google Scholar]
- 132.Scambia G, De Vincenzo R, Ranelletti POet al. et al. Antiproliferative effect of silybin on gynaecological malignancies: synergism with cisplatin and doxorubicin. Eur J Cancer. 1996;32(5):877-882. 10.1016/0959-8049(96)00011-1 [DOI] [PubMed] [Google Scholar]
- 133.Zi X, Grasso AW, Kung HJ, Agarwal R. A flavonoid antioxidant, silymarin, inhibits activation of erbB1 signaling and induces cyclin-dependent kinase inhibitors, G1 arrest, and anticarcinogenic effects in human prostate carcinoma DU145 cells. Cancer Res. 1998;58(9):1920-1929. PMID: 9581834. [PubMed] [Google Scholar]
- 134.Zi X, Feyes DK, Agarwal R. Anticarcinogenic effect of a flavonoid antioxidant, silymarin, in human breast cancer cells MDA-MB 468: induction of G1 arrest through an increase in Cip1/p21 concomitant with a decrease in kinase activity of cyclin-dependent kinases and associated cyclins. Clin Cancer Res. 1998;4(4):1055-1064. PMID: 9563902. [PubMed] [Google Scholar]
- 135.Sharma Y, Agarwal C, Singh AK, Agarwa lR. Inhibitory effect of silibinin on ligand binding to erbB1 and associated mitogenic signaling, growth, and DNA synthesis in advanced human prostate carcinoma cells. Mol Carcinog. 2001;30(4):224-236. 10.1002/mc.1032 [DOI] [PubMed] [Google Scholar]
- 136.Saliou C, Rihn B, Cillard J, Okamaoto T, Packer L. Selective inhibition of NF-kB activation by the flavonoid hepatoprotector silymarin in HepG2. Evidence for different activating pathways. FEBS Lett. 1998;440:8-12. 10.1016/S0014-5793(98)01409-4 [DOI] [PubMed] [Google Scholar]
- 137.Manna SK, Mukhopadhyay A, Van NT, Aggarwal BB. Silymarin suppresses TNF-induced activation of NF-kappa B, c-Jun N-terminal kinase, and apoptosis. J Immunol. 1999;163(12):6800-6809. PMID: 10586080. [PubMed] [Google Scholar]
- 138.Zi X, Agarwal R. Modulation of mitogen-activated protein kinase activation and cell cycle regulators by the potent skin cancer preventive agent silymarin. Biochem Biophys Res Commun. 1999;263(2):528-536. 10.1006/bbrc.1999.1398 [DOI] [PubMed] [Google Scholar]
- 139.Agarwal R, Agarwal C, Ichikawa H, Singh RP, Aggarwal BB. Anticancer potential of silymarin: from bench to bed side. Anticancer Res. 2006;26(6B):4457-4498. PMID: 17201169. [PubMed] [Google Scholar]
- 140.Zhao J, Sharma Y, Agarwal R. Significant inhibition by the flavonoid antioxidant silymarin against 12–O-tetradecanoylphorbol 13-acetate-caused modulation of antioxidant and inflammatory enzymes, and cyclooxygenase 2 and interleukin-1alpha expression in SENCAR mouse epidermis: implications in the prevention of stage I tumor promotion. Mol Carcinog. 1999;26(4):321-333. [DOI] [PubMed] [Google Scholar]
- 141.Zi X, Zhang J, Agarwal R, Pollak M. Silibinin up-regulates insulin-like growth factor-binding protein 3 expression and inhibits proliferation of androgen-independent prostate cancer cells. Cancer Res. 2000;60(20):5617-5620. PMID: 11059749. [PubMed] [Google Scholar]
- 142.Zhu W, Zhang JS, Young CY. Silymarin inhibits function of the androgen receptor by reducing nuclear localization of the receptor in the human prostate cancer cell line LNCaP. Carcinogenesis. 2001;22(9):1399-1403. 10.1093/carcin/22.9.1399 [DOI] [PubMed] [Google Scholar]
- 143.Kang SN, Lee MH, Kim KM, Cho D, Kim TS. Induction of human promyelocytic leukemia HL-60 cell differentiation into monocytes by silibinin: involvement of protein kinase C. Biochem Pharmacol. 2001;61(12):1487-1495. 10.1016/S0006-2952(01)00626-8 [DOI] [PubMed] [Google Scholar]
- 144.Verdura S, Cuyàs E, Llorach-Parés Let al. et al. Silibinin is a direct inhibitor of STAT3. Food Chem Toxicol. 2018;116:161-172. 10.1016/j.fct.2018.04.028 [DOI] [PubMed] [Google Scholar]
- 145.Bosch-Barrera J, Queralt B, Menendez JA. Targeting STAT3 with silibinin to improve cancer therapeutics. Cancer Treat Rev. 2017;58:61-69. 10.1016/j.ctrv.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 146.Mao J, Yang H, Cui Tet al. et al. Combined treatment with sorafenib and silibinin synergistically targets both HCC cells and cancer stem cells by enhanced inhibition of the phosphorylation of STAT3/ERK/AKT. Eur J Pharmacol. 2018;832:39-49. 10.1016/j.ejphar.2018.05.027 [DOI] [PubMed] [Google Scholar]
- 147.Thelen P, Wuttke W, Jarry H, Grzmil M, Ringert RH. Inhibition of telomerase activity and secretion of prostate specific antigen by silibinin in prostate cancer cells. J Urol. 2004;171(5):1934-1938. 10.1097/01.ju.0000121329.37206.1b [DOI] [PubMed] [Google Scholar]
- 148.Katiyar SK, Roy AM, Baliga MS. Silymarin induces apoptosis primarily through a p53-dependent pathway involving Bcl-2/Bax, cytochrome c release, and caspase activation. Mol Cancer Ther. 2005;4(2):207-216. PMID: 15713892. [PubMed] [Google Scholar]
- 149.Singh T, Prasad R, Katiyar SK. Therapeutic intervention of silymarin on the migration of non-small cell lung cancer cells is associated with the axis of multiple molecular targets including class 1 HDACs, ZEB1 expression, and restoration of miR-203 and E-cadherin expression. Am J Cancer Res. 2016;6(6):1287. PMID: 27429844. [PMC free article] [PubMed] [Google Scholar]
- 150.Trappoliere M, Caligiuri A, Schmid Met al. et al. Silybin, a component of sylimarin, exerts anti-inflammatory and anti-fibrogenic effects on human hepatic stellate cells. J Hepatol. 2009;50(6):1102-1111. 10.1016/j.jhep.2009.02.023 [DOI] [PubMed] [Google Scholar]
- 151.Vaid M, Prasad R, Sun Q, Katiyar SK. Silymarin targets β-catenin signaling in blocking migration/invasion of human melanoma cells. PLoS ONE. 2011;6(7):e23000. 10.1371/journal.pone.0023000 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 152.Woo SM, Min KJ, Chae IG, Chun KS, Kwon TK. Silymarin suppresses the PGE2-induced cell migration through inhibition of EP2 activation; G protein-dependent PKA-CREB and G protein-independent Src-STAT3 signal pathways. Mol Carcinog. 2015;54(3):216-228. 10.1002/mc.22092 [DOI] [PubMed] [Google Scholar]
- 153.Wu KJ, Zeng J, Zhu GDet al. et al. Silibinin inhibits prostate cancer invasion, motility and migration by suppressing vimentin and MMP-2 expression. Acta Pharmacol Sin. 2009;30(8):1162-1168. 10.1038/aps.2009.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Eo HJ, Park GH, Jeong JB. Inhibition of wnt signaling by silymarin in human colorectal cancer cells. Biomol Ther. 2016;24(4):380. 10.4062/biomolther.2015.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dastpeyman M, Motamed N, Azadmanesh Ket al. et al. Inhibition of silibinin on migration and adhesion capacity of human highly metastatic breast cancer cell line, MDA-MB-231, by evaluation of β1-integrin and downstream molecules, Cdc42, Raf-1 and D4GDI. Med Oncol. 2012;29(4):2512-2518. 10.1007/s12032-011-0113-8 [DOI] [PubMed] [Google Scholar]
- 156.Yang SH, Lin JK, Chen WS, Chiu JH. Anti-angiogenic effect of silymarin on colon cancer LoVo cell line. J Surg Res. 2003;113(1):133-138. 10.1016/S0022-4804(03)00229-4 [DOI] [PubMed] [Google Scholar]
- 157.Yang SH, Lin JK, Huang CJ, Chen WS, Li SY, Chiu JH. Silibinin inhibits angiogenesis via Flt-1, but not KDR, receptor up-regulation. J Surg Res. 2005;128(1):140-146. 10.1016/j.jss.2005.04.042 [DOI] [PubMed] [Google Scholar]
- 158. http://www.uniprot.org/uniprot/P17948 Accessed 10/20/2020.
- 159.Kaipa JM, Starkuviene V, Erfle H, Eils R, Gladilin E. Transcriptome profiling reveals silibinin dose-dependent response network in non-small lung cancer cells. PeerJ. 2020;8:e10373. 10.7717/peerj.10373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Adhikari M, Kaushik N, Ghimire Bet al. et al. Cold atmospheric plasma and silymarin nanoemulsion synergistically inhibits human melanoma tumorigenesis via targeting HGF/c-MET downstream pathway. Cell Commun Signal. 2019;17(1):1-14. 10.1186/s12964-019-0360-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kim DH, Park SJ, Lee SY, Yoon HS, Park CM. Silymarin attenuates invasion and migration through the regulation of epithelial-mesenchymal transition in Huh7 cells. Korean J Clin Lab Sci. 2018;50(3):337-344. 10.15324/kjcls.2018.50.3.337 [DOI] [Google Scholar]
- 162.Li J, Hu L, Zhou Tet al. et al. Taxifolin inhibits breast cancer cells proliferation, migration and invasion by promoting mesenchymal to epithelial transition via β-catenin signaling. Life Sci. 2019;232:116617. 10.1016/j.lfs.2019.116617 [DOI] [PubMed] [Google Scholar]
- 163.McCarthy K, Maguire T, McGreal G, McDermott E, O’Higgins N, Duffy MJ. High levels of tissue inhibitor of metalloproteinase-1 predict poor outcome in patients with breast cancer. Int J Cancer. 1999;84(1):44-48. [DOI] [PubMed] [Google Scholar]
- 164.Ylisirniö S, Höyhtyä M, Turpeenniemi-Hujanen T. Serum matrix metalloproteinases-2,-9 and tissue inhibitors of metalloproteinases-1,-2 in lung cancer--TIMP-1 as a prognostic marker. Anticancer Res. 2000;20(2B):1311-1316. PMID: 10810441. [PubMed] [Google Scholar]
- 165.Gong Y, Scott E, Lu R, Xu Y, Oh WK, Yu Q. TIMP-1 Promotes accumulation of cancer associated fibroblasts and cancer progression. Kyprianou N, ed. PLoS ONE. 2013;8(10):e77366. 10.1371/journal.pone.0077366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jia JD, Bauer M, Cho JJet al. et al. Antifibrotic effect of silymarin in rat secondary biliary fibrosis is mediated by downregulation of procollagen α1 (I) and TIMP-1. J Hepatol. 2001;35(3):392-398. 10.1016/S0168-8278(01)00148-9 [DOI] [PubMed] [Google Scholar]
- 167.Chen IS, Chen YC, Chou CH, Chuang RF, Sheen LY, Chiu CH. Hepatoprotection of silymarin against thioacetamide-induced chronic liver fibrosis. J Sci Food Agric. 2012;92(7):1441-1447. 10.1002/jsfa.4723 [DOI] [PubMed] [Google Scholar]
- 168.Di Sario A, Bendia E, Taffetani Set al. et al. Hepatoprotective and antifibrotic effect of a new silybin–phosphatidylcholine–vitamin E complex in rats. Dig Liver Dis. 2005;37(11):869-876. 10.1016/j.dld.2005.05.011 [DOI] [PubMed] [Google Scholar]
- 169.Ward Y, Lake R, Yin JJet al. et al. LPA Receptor heterodimerizes with CD97 to amplify LPA-initiated RHO-dependent signaling and invasion in prostate cancer cells. Cancer Res. 2011;71(23):7301-7311. 10.1158/0008-5472.CAN-11-2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kato K, Yoshikawa K, Tanabe Eet al. et al. Opposite roles of LPA 1 and LPA 3 on cell motile and invasive activities of pancreatic cancer cells. Tumor Biol. 2012;33(5):1739-1744. 10.1007/s13277-012-0433-0 [DOI] [PubMed] [Google Scholar]
- 171.Wei JS, Johansson P, Chen Let al. et al. Massively parallel sequencing reveals an accumulation of de novo mutations and an activating mutation of LPAR1 in a patient with metastatic neuroblastoma. PLoS ONE. 2013;8(10):e77731. 10.1371/journal.pone.0077731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sahay D, Leblanc R, Grunewald TGet al. et al. The LPA1/ZEB1/miR-21-activation pathway regulates metastasis in basal breast cancer. Oncotarget. 2015;6(24):20604. 10.18632/oncotarget.3774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Eraky SM, El-Mesery M, El-Karef A, Eissa LA, El-Gayar AM. Silymarin and caffeine combination ameliorates experimentally-induced hepatic fibrosis through down-regulation of LPAR1 expression. Biomed Pharmacother. 2018;101:49-57. 10.1016/j.biopha.2018.02.064 [DOI] [PubMed] [Google Scholar]
- 174.Kim S, Han J, Jeon Met al. et al. Silibinin inhibits triple negative breast cancer cell motility by suppressing TGF-β2 expression. Tumor Biol. 2016;37(8):11397-11407. 10.1007/s13277-016-5000-7 [DOI] [PubMed] [Google Scholar]
- 175.Zare Z, Dizaj TN, Lohrasbi Aet al. et al. Silibinin inhibits TGF-β-induced MMP-2 and MMP-9 through smad signaling pathway in colorectal cancer HT-29 cells. Basic Clin Cancer Res. 2020;12(2):81-90. [Google Scholar]
- 176.El-Lakkany NM, Hammam OA, El-Maadawy WH, Badawy AA, Ain-Shoka AA, Ebeid FA. Anti-inflammatory/anti-fibrotic effects of the hepatoprotective silymarin and the schistosomicide praziquantel against schistosoma mansoni-induced liver fibrosis. Parasit Vectors. 2012;5(1):1-14. 10.1186/1756-3305-5-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Malekinejad H, Taheri-Brujerdi M, Janbaz-Acyabar H, Amniattalab A. Silymarin regulates HIF-1α and iNOS expression in the brain and gills of hypoxic-reoxygenated rainbow trout oncorhynchus mykiss. Aquatic Biology. 2012;15(3):261-273. 10.3354/ab00427 [DOI] [Google Scholar]
- 178.Jung HJ, Park JW, Lee JSet al. et al. Silibinin inhibits expression of HIF-1α through suppression of protein translation in prostate cancer cells. Biochem Biophys Res Commun. 2009;390(1):71-76. 10.1016/j.bbrc.2009.09.068 [DOI] [PubMed] [Google Scholar]
- 179.Kim S, Han J, Kim JSet al. et al. Silibinin suppresses EGFR ligand-induced CD44 expression through inhibition of EGFR activity in breast cancer cells. Anticancer Res. 2011;31(11):3767-3773. PMID: 22110198. [PubMed] [Google Scholar]
- 180.Handorean AM, Yang K, Robbins EW, Flaig TW, Iczkowski KA. Silibinin suppresses CD44 expression in prostate cancer cells. Am J Transl Res. 2009;1(1):80. PMID: 19966941. [PMC free article] [PubMed] [Google Scholar]
- 181.Wang B, He G, Xu G, Wen J, Yu X. miRNA-34a inhibits cell adhesion by targeting CD44 in human renal epithelial cells: implications for renal stone disease. Urolithiasis. 2019;48:1-8. 10.1007/s00240-019-01155-9 [DOI] [PubMed] [Google Scholar]
- 182.Merzak A, Koocheckpour S, Pilkington GJ. CD44 Mediates human glioma cell adhesion and invasion in vitro. Cancer Res. 1994;54(15):3988-3992. PMID: 7518347. [PubMed] [Google Scholar]
- 183.Dohadwala M, Luo J, Zhu Let al. et al. Non-small cell lung cancer cyclooxygenase-2-dependent invasion is mediated by CD44. J Biol Chem. 2001;276(24):20809-20812. 10.1074/jbc.C100140200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jothy S. CD44 And its partners in metastasis. Clin Exp Metastasis. 2003;20(3):195-201. 10.1023/A:1022931016285 [DOI] [PubMed] [Google Scholar]
- 185.Rutnam ZJ, Yang BB. The non-coding 3′ UTR of CD44 induces metastasis by regulating extracellular matrix functions. J Cell Sci. 2012;125(8):2075-2085. 10.1242/jcs.100818 [DOI] [PubMed] [Google Scholar]
- 186.Troness B, Spartz A, Sharma Uet al. et al. CD44 Facilitates metastasis by promoting co-clustering of breast cancer cells and cancer associated fibroblasts. Cancer Res. 2019;79(13 Supplement):2044. 10.1158/1538-7445.AM2019-2044 [DOI] [Google Scholar]
- 187.Tyagi A, Agarwal C, Dwyer-Nield LD, Singh RP, Malkinson AM, Agarwal R. Silibinin modulates TNF-α and IFN-γ mediated signaling to regulate COX2 and iNOS expression in tumorigenic mouse lung epithelial LM2 cells. Mol Carcinog. 2012;51(10):832-842. 10.1002/mc.20851 [DOI] [PubMed] [Google Scholar]
- 188.Lu W, Lin C, King TD, Chen H, Reynolds RC, Li Y. Silibinin inhibits Wnt/β-catenin signaling by suppressing Wnt co-receptor LRP6 expression in human prostate and breast cancer cells. Cell Signal. 2012;24(12):2291-2296. 10.1016/j.cellsig.2012.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kauntz H, Bousserouel S, Gossé F, Raul F. The flavonolignan silibinin potentiates TRAIL-induced apoptosis in human colon adenocarcinoma and in derived TRAIL-resistant metastatic cells. Apoptosis. 2012;17(8):797-809. 10.1007/s10495-012-0731-4 [DOI] [PubMed] [Google Scholar]
- 190.Kauntz H, Bousserouel S, Gossé F, Raul F. Silibinin triggers apoptotic signaling pathways and autophagic survival response in human colon adenocarcinoma cells and their derived metastatic cells. Apoptosis. 2011;16(10):1042. 10.1007/s10495-011-0631-z [DOI] [PubMed] [Google Scholar]
- 191.Son YG, Kim EH, Kim JYet al. et al. Silibinin sensitizes human glioma cells to TRAIL-mediated apoptosis via DR5 up-regulation and down-regulation of c-FLIP and survivin. Cancer Res. 2007;67(17):8274-8284. 10.1158/0008-5472.CAN-07-0407 [DOI] [PubMed] [Google Scholar]
- 192.Bousserouel S, Bour G, Kauntz H, Gosse F, Marescaux J, Raul F. Silibinin inhibits tumor growth in a murine orthotopic hepatocarcinoma model and activates the TRAIL apoptotic signaling pathway. Anticancer Res. 2012;32(7):2455-2462. PMID: 22753701. [PubMed] [Google Scholar]
- 193.Hagelgans A, Nacke B, Zamaraeva M, Siegert G, Menschikowski M. Silibinin down-regulates expression of secreted phospholipase A2 enzymes in cancer cells. Anticancer Res. 2014;34(4):1723-1729. PMID: 24692702. [PubMed] [Google Scholar]
- 194.Chen YH, Chen CL, Lu DW, Liang CM, Tai MC, Chen JT. Silibinin inhibits platelet-derived growth factor-driven cell proliferation via downregulation of N-glycosylation in human Tenon's Fibroblasts in a proteasome-dependent manner. PLoS ONE. 2016;11(12):e0168765. 10.1371/journal.pone.0168765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Inoue K, Slaton JW, Eve BYet al. et al. Interleukin 8 expression regulates tumorigenicity and metastases in androgen-independent prostate cancer. Clin Cancer Res. 2000;6(5):2104-2119. PMID: 10815938. [PubMed] [Google Scholar]
- 196.Mizukami Y, Jo WS, Duerr EMet al. et al. Induction of interleukin-8 preserves the angiogenic response in HIF-1α–deficient colon cancer cells. Nat Med. 2005;11(9):992. 10.1038/nm1294 [DOI] [PubMed] [Google Scholar]
- 197.Kozłowski L, Zakrzewska I, Tokajuk P, Wojtukiewicz MZ. Concentration of interleukin-6 (IL-6), interleukin-8 (IL-8) and interleukin-10 (IL-10) in blood serum of breast cancer patients. Roczniki Akademii Medycznej w Bialymstoku (1995). 2003;48:82-84. PMID: 14737948. [PubMed] [Google Scholar]
- 198.Inoue K, Slaton JW, Kim SJet al. et al. Interleukin 8 expression regulates tumorigenicity and metastasis in human bladder cancer. Cancer Res. 2000;60(8):2290-2299. PMID: 10786697. [PubMed] [Google Scholar]
- 199.Arenberg DA, Kunkel SL, Polverini PJ, Glass M, Burdick MD, Strieter RM. Inhibition of interleukin-8 reduces tumorigenesis of human non-small cell lung cancer in SCID mice. J Clin Invest. 1996;97(12):2792-2802. 10.1172/JCI118734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Biswas SK, McClure D, Jimenez LA, Megson IL, Rahman I. Curcumin induces glutathione biosynthesis and inhibits NF-κB activation and interleukin-8 release in alveolar epithelial cells: mechanism of free radical scavenging activity. Antioxid Redox Signaling. 2005;7(1–2):32-41. 10.1089/ars.2005.7.32 [DOI] [PubMed] [Google Scholar]
- 201.Gerritsen ME, Carley WW, Ranges GEet al. et al. Flavonoids inhibit cytokine-induced endothelial cell adhesion protein gene expression. Am J Pathol. 1995;147(2):278. PMID: 7543732. [PMC free article] [PubMed] [Google Scholar]
- 202.Hussain SA, Jassim NA, Numan IT, Al-Khalifa II, Abdullah TA. Anti-inflammatory activity of silymarin in patients with knee osteoarthritis. Saudi Med J. 2009;30(1):98-103. PMID: 19139781. [PubMed] [Google Scholar]
- 203.Au AY, Hasenwinkel JM, Frondoza CG. Silybin inhibits interleukin-1β-induced production of pro-inflammatory mediators in canine hepatocyte cultures. J Vet Pharmacol Ther. 2011;34(2):120-129. 10.1111/j.1365-2885.2010.01200.x [DOI] [PubMed] [Google Scholar]
- 204.Shi Z, Zhou Q, Gao Set al. et al. Silibinin inhibits endometrial carcinoma via blocking pathways of STAT3 activation and SREBP1-mediated lipid accumulation. Life Sci. 2019;217:70-80. 10.1016/j.lfs.2018.11.037 [DOI] [PubMed] [Google Scholar]
- 205.Si L, Liu W, Hayashi Tet al. et al. Silibinin-induced apoptosis of breast cancer cells involves mitochondrial impairment. Arch Biochem Biophys. 2019;671:42-51. 10.1016/j.abb.2019.05.009 [DOI] [PubMed] [Google Scholar]
- 206.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases structure, function, and biochemistry. Circ Res. 2003;92(8):827-839. 10.1161/01.RES.0000070112.80711.3D [DOI] [PubMed] [Google Scholar]
- 207.Yousefi M, Ghaffari SH, Soltani BMet al. et al. Therapeutic efficacy of silibinin on human neuroblastoma cells: akt and NF-κB expressions may play an important role in silibinin-induced response. Neurochem Res. 2012;37(9):2053-2063. [DOI] [PubMed] [Google Scholar]
- 208.Tang TC, Poon RT, Lau CP, Xie D, Fan ST. Tumor cyclooxygenase-2 levels correlate with tumor invasiveness in human hepatocellular carcinoma. World J Gastroenterol. 2005;11(13):1896-1902. 10.3748/wjg.v11.i13.1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ramakrishnan G, Elinos-Báez CM, Jagan Set al. et al. Silymarin downregulates COX-2 expression and attenuates hyperlipidemia during NDEA-induced rat hepatocellular carcinoma. Mol Cell Biochem. 2008;313(1):53-61. 10.1007/s11010-008-9741-5 [DOI] [PubMed] [Google Scholar]
- 210.Wyllie AH. Apoptosis and carcinogenesis. Eur. J. Cell Biol. 1997;73(3):189-197. [PubMed] [Google Scholar]
- 211.Rugamba A, Kang DY, Sp Net al. et al. Silibinin regulates tumor progression and tumorsphere formation by suppressing PD-L1 expression in Non-small cell lung cancer (NSCLC) cells. Cells. 2021;10(7):1632. 10.3390/cells10071632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sellam LS, Zappasodi R, Chettibi Fet al. et al. Silibinin down-regulates PD-L1 expression in nasopharyngeal carcinoma by interfering with tumor cell glycolytic metabolism. Arch Biochem Biophys. 2020;690:108479. 10.1016/j.abb.2020.108479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yang C, Li F, Ma Jet al. et al. Evaluation of anti-cancer potency of silibinin on murine renal carcinoma RenCa cells in an animal model with an intact immune system. Anti-Cancer Drugs. 2020;31(8):785-791. 10.1097/CAD.0000000000000931 [DOI] [PubMed] [Google Scholar]
- 214.Capaccione KM, Pine SR. The notch signaling pathway as a mediator of tumor survival. Carcinogenesis. 2013;34(7):1420-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhang S, Yang Y, Liang Zet al. et al. Silybin-mediated inhibition of notch signaling exerts antitumor activity in human hepatocellular carcinoma cells. PLoS ONE. 2013;8(12):e83699. 10.1371/journal.pone.0083699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ahmed LA, Abd El-Rhman RH, Gad AM, Hassaneen SK, El-Yamany MF. Dibenzazepine combats acute liver injury in rats via amendments of notch signaling and activation of autophagy. Naunyn-Schmiedeberg's Archives of Pharmacology. 2021;394(2):337-348. 10.1007/s00210-020-01977-0 [DOI] [PubMed] [Google Scholar]
- 217.Kim TH, Woo JS, Kim YK, Kim KH. Silibinin induces cell death through reactive oxygen species–dependent downregulation of notch-1/ERK/Akt signaling in human breast cancer cells. J Pharmacol Exp Ther. 2014;349(2):268-278. 10.1124/jpet.113.207563 [DOI] [PubMed] [Google Scholar]
- 218.Lin Z, Fang D. The roles of SIRT1 in cancer. Genes Cancer. 2013;4(3–4):97-104. 10.1177/1947601912475079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yang H, Bi Y, Xue Let al. et al. Multifaceted modulation of SIRT1 in cancer and inflammation. Critical Reviews™ in Oncogenesis. 2015;20:(1–2). 10.1615/CritRevOncog.2014012374 [DOI] [PubMed] [Google Scholar]
- 220.Feng B, Huang B, Jing Yet al. et al. Silymarin ameliorates the disordered glucose metabolism of mice with diet-induced obesity by activating the hepatic SIRT1 pathway. Cell Signal. 2021;84:110023. 10.1016/j.cellsig.2021.110023 [DOI] [PubMed] [Google Scholar]
- 221.Sarubbo F, Ramis MR, Kienzer Cet al. et al. Chronic silymarin, quercetin and naringenin treatments increase monoamines synthesis and hippocampal Sirt1 levels improving cognition in aged rats. J Neuroimmune Pharmacol. 2018;13(1):24-38. 10.1007/s11481-017-9759-0 [DOI] [PubMed] [Google Scholar]
- 222.Wu WT, Chen YR, Lu DH, Senatov FS, Yang KC, Wang CC. Silymarin modulates catabolic cytokine expression through Sirt1 and SOX9 in human articular chondrocytes. J Orthop Surg Res. 2021;16(1):1-9. 10.1186/s13018-021-02305-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zhou B, Wu LJ, Li LHet al. et al. Silibinin protects against isoproterenol-induced rat cardiac myocyte injury through mitochondrial pathway after up-regulation of SIRT1. J Pharmacol Sci. 2006;102(4):387-395. 10.1254/jphs.FPJ06005X [DOI] [PubMed] [Google Scholar]
- 224.Liang Z, Yang Y, Wang Het al. et al. Inhibition of SIRT1 signaling sensitizes the antitumor activity of silybin against human lung adenocarcinoma cells in vitro and in vivo. Mol Cancer Ther. 2014;13(7):1860-1872. 10.1158/1535-7163.MCT-13-0942 [DOI] [PubMed] [Google Scholar]
- 225.Jiang C, Agarwal R, Lü J. Anti-angiogenic potential of a cancer chemopreventive flavonoid antioxidant, silymarin: inhibition of key attributes of vascular endothelial cells and angiogenic cytokine secretion by cancer epithelial cells. Biochem Biophys Res Commun. 2000;276(1):371-378. 10.1006/bbrc.2000.3474 [DOI] [PubMed] [Google Scholar]
- 226.Attia GM, Elmansy RA, Algaidi SA. Silymarin decreases the expression of VEGF-A, iNOS and caspase-3 and preserves the ultrastructure of cardiac cells in doxorubicin induced cardiotoxicity in rats: a possible protective role. Int J Clin Exp Med. 2017;10(2):4158-4173. www.ijcem.com /ISSN:1940-5901/IJCEM0043306. [Google Scholar]
- 227.Kaya MD, Başer E, Kaya Set al. et al. The effect of silymarin on VEGF, VEGFR-1 and IL-1α levels in placental cultures of severe preeclamptic women. J Turk Ger Gynecol Assoc. 2014;15(1):30. 10.5152/jtgga.2014.81592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zhang J, Ma H, Yang L. Silymarin inhibits invasion and migration of hepatoma cell line MHCC97. Basic & Clinical Medicine. 2019;39(12):1741. http://journal11.magtechjournal.com/Jwk_jcyxylc. [Google Scholar]
- 229.Miller DM, Thomas SD, Islam A, Muench D, Sedoris K. c-Myc and cancer metabolism. Clin Cancer Res. 2012;18(20):5546-5553. 10.1158/1078-0432.CCR-12-0977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Rainone F. Milk thistle. Am Fam Physician. 2005;72(7):1285-1288. PMID: 16225032. [PubMed] [Google Scholar]
- 231.He Q, Osuchowski MF, Johnson VJ, Sharma RP. Physiological responses to a natural antioxidant flavonoid mixture, silymarin, in BALB/c mice: i. Induction of transforming growth factor beta1 and c-myc in liver with marginal effects on other genes. Planta Med. 2002;68(01):676-679. 10.1080/713611034 [DOI] [PubMed] [Google Scholar]
- 232.Mastron JK, Siveen KS, Sethi G, Bishayee A. Silymarin and hepatocellular carcinoma: a systematic, comprehensive, and critical review. Anti-Cancer Drugs. 2015;26(5):475-486. 10.1097/CAD.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 233.Rajamanickam S, Kaur M, Velmurugan B, Singh RP, Agarwal R. Silibinin suppresses spontaneous tumorigenesis in APC min/ + mouse model by modulating beta-catenin pathway. Pharm Res. 2009;26(12):2558-2567. 10.1007/s11095-009-9968-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Rajamanickam S, Velmurugan B, Kaur M, Singh RP, Agarwal R. Chemoprevention of intestinal tumorigenesis in APCmin/ + mice by silibinin. Cancer Res. 2010;70(6):2368-2378. 10.1158/0008-5472.CAN-09-3249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Şentürk M, Gülçin İ, Beydemir Ş, Küfrevioğlu Öİ, Supuran CT. In vitro inhibition of human carbonic anhydrase I and II isozymes with natural phenolic compounds. Chem Biol Drug Des. 2011;77(6):494-499. 10.1111/j.1747-0285.2011.01104.x [DOI] [PubMed] [Google Scholar]
- 236.Koz Ö, Ekinci D, Perrone Aet al. et al. Analysis of saponins and phenolic compounds as inhibitors of α-carbonic anhydrase isoenzymes. J Enzyme Inhib Med Chem. 2013;28(2):412-417. 10.3109/14756366.2011.651464 [DOI] [PubMed] [Google Scholar]
- 237.Jaggi AS, Singh N. Silymarin and its role in chronic diseases. Drug discovery from mother nature. 2016; 929:25–44. https://doi.org/10.1007/978-3-319-41342-6_2. [DOI] [PubMed] [Google Scholar]
- 238.Feng B, Meng R, Huang B, Bi Y, Shen S, Zhu D. Silymarin protects against renal injury through normalization of lipid metabolism and mitochondrial biogenesis in high fat-fed mice. Free Radical Biology and Medicine. 2017;110:240-249. 10.1016/j.freeradbiomed.2017.06.009 [DOI] [PubMed] [Google Scholar]
- 239.Singh RP, Raina K, Sharma G, Agarwal R. Silibinin inhibits established prostate tumor growth, progression, invasion, and metastasis and suppresses tumor angiogenesis and epithelial-mesenchymal transition in transgenic adenocarcinoma of the mouse prostate model mice. Clin Cancer Res. 2008;14(23):7773-7780. 10.1158/1078-0432.CCR-08-1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Deep G, Agarwal R. Antimetastatic efficacy of silibinin: molecular mechanisms and therapeutic potential against cancer. Cancer Metastasis Rev. 2010;29(3):447-463. 10.1007/s10555-010-9237-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Sadighi S, Dashti-Khavidaki S, Shahbazi Fet al. et al. The effects of concomitant use of silymarin and chemotherapy on solid tumors: a pilot randomized controlled trial. Basic & Clinical Cancer Research. 2017;9(2):13-19. [Google Scholar]
- 242.Fanoudi S, Alavi MS, Karimi G, Hosseinzadeh H. Milk thistle (Silybum Marianum) as an antidote or a protective agent against natural or chemical toxicities: a review. Drug Chem Toxicol. 2020;43(3):240-254. 10.1080/01480545.2018.1485687 [DOI] [PubMed] [Google Scholar]
- 243.Comelli MC, Mengs U, Schneider C, Prosdocimi M. Toward the definition of the mechanism of action of silymarin: activities related to cellular protection from toxic damage induced by chemotherapy. Integr Cancer Ther. 2007;6(2):120-129. 10.1177/1534735407302349 [DOI] [PubMed] [Google Scholar]
- 244.Hagag AA, Elgamsy MA, El-Asy HM, Mabrouk MM. Protective role of silymarin on hepatic and renal toxicity induced by MTX based chemotherapy in children with acute lymphoblastic leukemia. Mediterr J Hematol Infect Dis. 2016;8:(1): e2016043. 10.4084/MJHID.2016.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Invernizzi R, Bernuzzi S, Ciani D, Ascari E. Silymarine during maintenance therapy of acute promyelocytic leukemia. Haematologica. 1993;78(5):340-341. PMID: 8314167. [PubMed] [Google Scholar]
- 246.Bokemeyer C, Fels LM, Dunn Tet al. et al. Silibinin protects against cisplatin-induced nephrotoxicity without compromising cisplatin or ifosfamide anti-tumour activity. Br J Cancer. 1996;74(12):2036-2041. 10.1038/bjc.1996.673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Gaedeke J, Fels LM, Bokemeyer C, Mengs U, Stolte H, Lentzen H. Cisplatin nephrotoxicity and protection by silibinin. Nephrol Dial Transplant. 1996;11(1):55-62. 10.1093/ndt/11.1.55 [DOI] [PubMed] [Google Scholar]
- 248.Psotová J, Chlopčíková Š, Grambal F, Šimánek V, Ulrichová J. Influence of silymarin and its flavonolignans on doxorubicin-iron induced lipid peroxidation in rat heart microsomes and mitochondria in comparison with quercetin. Phytother Res. 2002;16(S1):63-67. 10.1002/ptr.811 [DOI] [PubMed] [Google Scholar]
- 249.Yardım A, Kucukler S, Özdemir Set al. et al. Silymarin alleviates docetaxel-induced central and peripheral neurotoxicity by reducing oxidative stress, inflammation and apoptosis in rats. Gene. 2021;769:145239. 10.1016/j.gene.2020.145239 [DOI] [PubMed] [Google Scholar]
- 250.Chang TK, Yin TC, Su WCet al. et al. Pilot study of silymarin as supplementation to reduce toxicities in metastatic colorectal cancer patients treated with first-line FOLFIRI Plus bevacizumab. Oncol Res Featuring Preclin Clin Cancer Ther. 2021; 7-8: 801-809. 10.3727/096504021X16218531628569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Moezian GSA, Javadinia SA, Sales SS, Fanipakdel A, Elyasi S, Karimi G. Oral silymarin formulation efficacy in management of AC-T protocol induced hepatotoxicity in breast cancer patients: a randomized, triple blind, placebo-controlled clinical trial. J Oncol Pharm Pract. 2021;10781552211006182. 10.1177/10781552211006182 [DOI] [PubMed] [Google Scholar]
- 252.Rho JK, Choi YJ, Jeon BSet al. et al. Combined treatment with silibinin and epidermal growth factor receptor tyrosine kinase inhibitors overcomes drug resistance caused by T790 M mutation. Mol Cancer Ther. 2010;9(12):3233-3243. 10.1158/1535-7163.MCT-10-0625 [DOI] [PubMed] [Google Scholar]
- 253.Sun Y, Guan Z, Zhao Wet al. et al. Silibinin suppresses bladder cancer cell malignancy and chemoresistance in an NF-κB signal-dependent and signal-independent manner. Int J Oncol. 2017;51(4):1219-1226. 10.3892/ijo.2017.4089 [DOI] [PubMed] [Google Scholar]
- 254.Zhou L, Liu P, Chen Bet al. et al. Silibinin restores paclitaxel sensitivity to paclitaxel-resistant human ovarian carcinoma cells. Anticancer Res. 2008;28(2A):1119-1127. PMID: 18507063. [PubMed] [Google Scholar]
- 255.Zhang S, Morris ME. Effects of the flavonoids biochanin A, morin, phloretin, and silymarin on P-glycoprotein-mediated transport. J Pharmacol Exp Ther. 2003;304(3):1258-1267. 10.1124/jpet.102.044412 [DOI] [PubMed] [Google Scholar]
- 256.Maitrejean M, Comte G, Barron D, El Kirat K, Conseil G, Di Pietro A. The flavanolignan silybin and its hemisynthetic derivatives, a novel series of potential modulators of P-glycoprotein. Bioorg Med Chem Lett. 2000;10(2):157-160. 10.1016/S0960-894X(99)00636-8 [DOI] [PubMed] [Google Scholar]
- 257.Džubák P, Hajdúch M, Gažák Ret al. et al. New derivatives of silybin and 2, 3–dehydrosilybin and their cytotoxic and P-glycoprotein modulatory activity. Bioorg Med Chem. 2006;14(11):3793-3810. 10.1016/j.bmc.2006.01.035 [DOI] [PubMed] [Google Scholar]
- 258.Leslie EM, Mao Q, Oleschuk CJ, Deeley RG, Cole SP. Modulation of multidrug resistance protein 1 (MRP1/ABCC1) transport and ATPase activities by interaction with dietary flavonoids. Mol Pharmacol. 2001;59(5):1171-1180. 10.1124/mol.59.5.1171 [DOI] [PubMed] [Google Scholar]
- 259.Nguyen H, Zhang SZ, Morris ME. Effect of flavonoids on MRP1-mediated transport in Panc-1 cells. J Pharm Sci. 2002;92:250-257. 10.1002/jps.10283 [DOI] [PubMed] [Google Scholar]
- 260.Viktorová J, Dobiasová S, Řehořová Ket al. et al. Antioxidant, anti-inflammatory, and multidrug resistance modulation activity of silychristin derivatives. Antioxidants. 2019;8(8):303. 10.3390/antiox8080303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Seidlova-Wuttke D, Becker T, Christoffel V, Jarry H, Wuttke W. Silymarin is a selective estrogen receptor β (ERβ) agonist and has estrogenic effects in the metaphysis of the femur but no or antiestrogenic effects in the uterus of ovariectomized (ovx) rats. J Steroid Biochem Mol Biol. 2003;86(2):179-188. 10.1016/S0960-0760(03)00270-X [DOI] [PubMed] [Google Scholar]
- 262.Plíšková M, Vondráček J, Křen Vet al. et al. Effects of silymarin flavonolignans and synthetic silybin derivatives on estrogen and aryl hydrocarbon receptor activation. Toxicology. 2005;215(1–2):80-89. 10.1016/j.tox.2005.06.020 [DOI] [PubMed] [Google Scholar]
- 263.El-Shitany NA, Hegazy S, El-Desoky K. Evidences for antiosteoporotic and selective estrogen receptor modulator activity of silymarin compared with ethinylestradiol in ovariectomized rats. Phytomedicine. 2010;17(2):116-125. 10.1016/j.phymed.2009.05.012 [DOI] [PubMed] [Google Scholar]
- 264.Atawia RT, Tadros MG, Khalifa AE, Mosli HA, Abdel-Naim AB. Role of the phytoestrogenic, pro-apoptotic and anti-oxidative properties of silymarin in inhibiting experimental benign prostatic hyperplasia in rats. Toxicol Lett. 2013;219(2):160-169. 10.1016/j.toxlet.2013.03.002 [DOI] [PubMed] [Google Scholar]
- 265.Vostalova J, Vidlar A, Ulrichova J, Vrbkova J, Simanek V, Student V. Use of selenium–silymarin mix reduces lower urinary tract symptoms and prostate specific antigen in men. Phytomedicine. 2013;21(1):75-81. 10.1016/j.phymed.2013.07.018 [DOI] [PubMed] [Google Scholar]
- 266.Khalil EA. Hormonal profile and histopathological study on the influence of silymarin on both female and male albino rats. Egyptian J Hosp Med. 2003;13(1):112-122. 10.21608/ejhm.2003.18236 [DOI] [Google Scholar]
- 267.Abedi H, Jahromi HK, Hashemi SMA, Jashni HK, Jahromi ZK, Pourahmadi M. The effect of silymarin on spermatogenesis process in rats. Int J Med Res Health Sci. 2016;5(6):146-150. [Google Scholar]
- 268.Mellman I, Yarden Y. Endocytosis and cancer. Cold Spring Harbor Perspect Biol. 2013;5(12):a016949. 10.1101/cshperspect.a016949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Blaising J, Lévy PL, Gondeau Cet al. et al. Silibinin inhibits hepatitis C virus entry into hepatocytes by hindering clathrin-dependent trafficking. Cell Microbiol. 2013;15(11):1866-1882. 10.1111/cmi.12155 [DOI] [PubMed] [Google Scholar]
- 270.Polyak SJ, Oberlies NH, Pécheur EI, Dahari H, Ferenci P, Pawlotsky JM. Silymarin for hepatitis C virus infection. Antiviral Ther. 2013;18(2):141. 10.3851/IMP2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Umetsu T, Inoue J, Kogure Tet al. et al. Inhibitory effect of silibinin on hepatitis B virus entry. Biochem Biophys Rep. 2018;14:20-25. 10.1016/j.bbrep.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Vladutiu GD, Middleton Jr E. Effects of flavonoids on enzyme secretion and endocytosis in normal and mucolipidosis II fibroblasts. Life Sci. 1986;39(8):717-726. 10.1016/0024-3205(86)90019-6 [DOI] [PubMed] [Google Scholar]
- 273.Caballero-Díaz D, Bertran E, Peñuelas-Haro Iet al. Clathrin switches transforming growth factor-β role to pro-tumorigenic in liver cancer. J Hepatol. 2020;72(1):125-134. 10.1016/j.jhep.2019.09.012 [DOI] [PubMed] [Google Scholar]
- 274.Smith CM, Haucke V, McCluskey A, Robinson PJ, Chircop M. Inhibition of clathrin by pitstop 2 activates the spindle assembly checkpoint and induces cell death in dividing HeLa cancer cells. Mol Cancer. 2013;12(1):4. 10.1186/1476-4598-12-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Li L, Gao Y, Zhang L, Zeng J, He D, Sun Y. Silibinin inhibits cell growth and induces apoptosis by caspase activation, down regulating survivin and blocking EGFR-ERK activation in renal cell carcinoma. Cancer Lett. 2008;272(1):61-69. 10.1016/j.canlet.2008.06.033 [DOI] [PubMed] [Google Scholar]
- 276.Liang L, Li L, Zeng Jet al. et al. Inhibitory effect of silibinin on EGFR signal-induced renal cell carcinoma progression via suppression of the EGFR/MMP-9 signaling pathway. Oncol Rep. 2012;28(3):999-1005. 10.3892/or.2012.1874 [DOI] [PubMed] [Google Scholar]
- 277.Chang HR, Chen PN, Yang SFet al. et al. Silibinin inhibits the invasion and migration of renal carcinoma 786-O cells in vitro, inhibits the growth of xenografts in vivo and enhances chemosensitivity to 5-fluorouracil and paclitaxel. Mol Carcinog. 2011;50(10):811-823. 10.1002/mc.20756 [DOI] [PubMed] [Google Scholar]
- 278.Koltai T, Reshkin SJ, Carvalho T, Cardone RA. Targeting the stromal pro-tumoral hyaluronan-CD44 pathway in pancreatic cancer. Int J Mol Sci. 2021;22(8):3953. 10.3390/ijms22083953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Long J, Zhang Y, Yu Xet al. et al. Overcoming drug resistance in pancreatic cancer. Expert Opin Ther Targets. 2011;15(7):817-828. 10.1517/14728222.2011.566216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Ting HJ, Deep G, Jain AKet al. et al. Silibinin prevents prostate cancer cell-mediated differentiation of naïve fibroblasts into cancer-associated fibroblast phenotype by targeting TGF β2. Mol Carcinog. 2015;54(9):730-741. 10.1002/mc.22135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Amawi H, Hussein NA, Karthikeyan Cet al. et al. HM015k, A novel silybin derivative, multi-targets metastatic ovarian cancer cells and is safe in zebrafish toxicity studies. Front Pharmacol. 2017;8:498. 10.3389/fphar.2017.00498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Song Y, Ye M, Zhou J, Wang ZW, Zhu X. Restoring E-cadherin expression by natural compounds for anticancer therapies in genital and urinary cancers. Mol Therapy-Oncol. 2019;14:130-138. 10.1016/j.omto.2019.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Lieber CS, Leo MA, Cao Q, Ren C, DeCarli LM. Silymarin retards the progression of alcohol-induced hepatic fibrosis in baboons. J Clin Gastroenterol. 2003;37(4):336-339. 10.1097/00004836-200310000-00013. [DOI] [PubMed] [Google Scholar]
- 284.Ko JW, Shin NR, Park SHet al. et al. Silibinin inhibits the fibrotic responses induced by cigarette smoke via suppression of TGF-β1/smad 2/3 signaling. Food Chem Toxicol. 2017;106:424-429. 10.1016/j.fct.2017.06.016 [DOI] [PubMed] [Google Scholar]
- 285.Lao CD, Demierre MF, Sondak VK. Targeting events in melanoma carcinogenesis for the prevention of melanoma. Expert Rev Anticancer Ther. 2006;6(11):1559-1568. 10.1586/14737140.6.11.1559 [DOI] [PubMed] [Google Scholar]
- 286.Li LH, Wu LJ, Jiang YYet al. et al. Silymarin enhanced cytotoxic effect of anti-Fas agonistic antibody CH11 on A375-S2 cells. J Asian Nat Prod Res. 2007;9(7):593-602. 10.1080/10286020600882502 [DOI] [PubMed] [Google Scholar]
- 287.Jones V, Katiyar SK. Emerging phytochemicals for prevention of melanoma invasion. Cancer Lett. 2013;335(2):251-258. 10.1016/j.canlet.2013.02.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Lee MH, Huang Z, Kim DJet al. et al. Direct targeting of MEK1/2 and RSK2 by silybin induces cell-cycle arrest and inhibits melanoma cell growth. Cancer Prev Res. 2013;6(5):455-465. 10.1158/1940-6207.CAPR-12-0425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Vaid M, Singh T, Prasad R, Katiyar SK. Silymarin inhibits melanoma cell growth both in vitro and in vivo by targeting cell cycle regulators, angiogenic biomarkers and induction of apoptosis. Mol Carcinog. 2015;54(11):1328-1339. 10.1002/mc.22208 [DOI] [PubMed] [Google Scholar]
- 290.Gajos-Michniewicz A, Czyz M. Modulation of WNT/β-catenin pathway in melanoma by biologically active components derived from plants. Fitoterapia. 2016;109:283-292. 10.1016/j.fitote.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 291.Mokhtari MJ, Motamed N, Shokrgozar MA. Evaluation of silibinin on the viability, migration and adhesion of the human prostate adenocarcinoma (PC-3) cell line. Cell Biol Int. 2008;32(8):888-892. 10.1016/j.cellbi.2008.03.019 [DOI] [PubMed] [Google Scholar]
- 292.Davis-Searles PR, Nakanishi Y, Kim NCet al. et al. Milk thistle and prostate cancer: differential effects of pure flavonolignans from Silybum marianum on antiproliferative end points in human prostate carcinoma cells. Cancer Res. 2005;65(10):4448-4457. 10.1158/0008-5472.CAN-04-4662 [DOI] [PubMed] [Google Scholar]
- 293.Kacar S, Aykanat NEB, Sahinturk V. Silymarin inhibited DU145 cells by activating SLIT2 protein and suppressing expression of CXCR4. Med Oncol. 2020;37(3):1-9. 10.1007/s12032-020-1343-4 [DOI] [PubMed] [Google Scholar]
- 294.Zi X, Agarwal R. Silibinin decreases prostate-specific antigen with cell growth inhibition via G1 arrest, leading to differentiation of prostate carcinoma cells: implications for prostate cancer intervention. Proc Natl Acad Sci USA. 1999;96:7490-7495. 10.1073/pnas.96.13.7490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Deep G, Singh RP, Agarwal C, Kroll DJ, Agarwal R. Silymarin and silibinin cause G1 and G2–M cell cycle arrest via distinct circuitries in human prostate cancer PC3 cells: a comparison of flavanone silibinin with flavanolignan mixture silymarin. Oncogene. 2006;25(7):1053. 10.1038/sj.onc.1209146 [DOI] [PubMed] [Google Scholar]
- 296.Tyagi A, Bhatia N, Condon MS, Bosland MC, Agarwal C, Agarwal R. Antiproliferative and apoptotic effects of silibinin in rat prostate cancer cells. Prostate. 2002;53(3):211-217. 10.1002/pros.10146 [DOI] [PubMed] [Google Scholar]
- 297.Deep G, Oberlies NH, Kroll DJ, Agarwal R. Isosilybin B and isosilybin A inhibit growth, induce G1 arrest and cause apoptosis in human prostate cancer LNCaP and 22Rv1 cells. Carcinogenesis. 2007;28(7):1533-1542. 10.1093/carcin/bgm069 [DOI] [PubMed] [Google Scholar]
- 298.Singh RP, Agarwal R. A cancer chemopreventive agent silibinin, targets mitogenic and survival signaling in prostate cancer. Mutation Res/Fund Mol Mech Mutagenesis. 2004;555(1):21-32. 10.1016/j.mrfmmm.2004.05.017 [DOI] [PubMed] [Google Scholar]
- 299.Flaig TW, Su LJ, Harrison G, Agarwal R, Glodé LM. Silibinin synergizes with mitoxantrone to inhibit cell growth and induce apoptosis in human prostate cancer cells. Int J Cancer. 2007;120(9):2028-2033. 10.1002/ijc.22465 [DOI] [PubMed] [Google Scholar]
- 300.Kohno H, Suzuki R, Sugie S, Tsuda H, Tanaka T. Dietary supplementation with silymarin inhibits 3, 2′-dimethyl-4-aminobiphenyl–induced prostate carcinogenesis in male F344 rats. Clin Cancer Res. 2005;11(13):4962-4967. 10.1158/1078-0432.CCR-05-0137 [DOI] [PubMed] [Google Scholar]
- 301.Wu K, Jin Z, Lei Let al. et al. Silibinin reverses epithelial-to-mesenchymal transition in metastatic prostate cancer cells by targeting transcription factors. Oncol Rep. 2010;23(6):1545-1552. 10.3892/or_00000794 [DOI] [PubMed] [Google Scholar]
- 302.Deep G, Gangar SC, Oberlies NH, Kroll DJ, Agarwal R. Isosilybin A induces apoptosis in human prostate cancer cells via targeting Akt, NF-κB, and androgen receptor signaling. Mol Carcinog. 2010;49(10):902-912. 10.1002/mc.20670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Singh RP, Sharma G, Dhanalakshmi S, Agarwal C, Agarwal R. Suppression of advanced human prostate tumor growth in athymic mice by silibinin feeding is associated with reduced cell proliferation, increased apoptosis, and inhibition of angiogenesis. Cancer Epidemiol Prev Biomarkers. 2003;12(9):933-939. PMID: 14504208. [PubMed] [Google Scholar]
- 304.Singh RP, Dhanalakshmi S, Tyagi AK, Chan DC, Agarwal C, Agarwal R. Dietary feeding of silibinin inhibits advance human prostate carcinoma growth in athymic nude mice and increases plasma insulin-like growth factor-binding protein-3 levels. Cancer Res. 2002;62:3063-3069. PMID: 12036915. [PubMed] [Google Scholar]
- 305.Tyagi A, Agarwal C, Agarwal R. Inhibition of retinoblastoma protein (Rb) phosphorylation at serine sites and an increase in Rb-E2F complex formation by silibinin in androgen-dependent human prostate carcinoma LNCaP cells: role in prostate cancer prevention. Mol Cancer Ther. 2002;1(7):525-532. PMID: 12479270. [PubMed] [Google Scholar]
- 306.Chu SC, Chiou HL, Chen PN, Yang SF, Hsieh YS. Silibinin inhibits the invasion of human lung cancer cells via decreased productions of urokinase-plasminogen activator and matrix metalloproteinase-2. Mol Carcinog. 2004;40(3):143-149. 10.1002/mc.20018 [DOI] [PubMed] [Google Scholar]
- 307.Cufí S, Bonavia R, Vazquez-Martin Aet al. Silibinin meglumine, a water-soluble form of milk thistle silymarin, is an orally active anti-cancer agent that impedes the epithelial-to-mesenchymal transition (EMT) in EGFR-mutant non-small-cell lung carcinoma cells. Food Chem Toxicol. 2013;60:360-368. 10.1016/j.fct.2013.07.063 [DOI] [PubMed] [Google Scholar]
- 308.Mateen S, Tyagi A, Agarwal C, Singh RP, Agarwal R. Silibinin inhibits human nonsmall cell lung cancer cell growth through cell-cycle arrest by modulating expression and function of key cell-cycle regulators. Mol Carcinog. 2010;49(3):247-258. 10.1002/mc.20595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Li W, Mu D, Song Let al. et al. Molecular mechanism of silymarin-induced apoptosis in a highly metastatic lung cancer cell line anip973. Cancer Biother Radiopharm. 2011;26(3):317-324. 10.1089/cbr.2010.0892 [DOI] [PubMed] [Google Scholar]
- 310.Sharma G, Singh RP, Chan DC, Agarwal R. Silibinin induces growth inhibition and apoptotic cell death in human lung carcinoma cells. Anticancer Res. 2003;23(3B):2649-2655. PMID: 12894553. [PubMed] [Google Scholar]
- 311.Mateen S, Raina K, Jain AK, Agarwal C, Chan D, Agarwal R. Epigenetic modifications and p21-cyclin B1 nexus in anticancer effect of histone deacetylase inhibitors in combination with silibinin on non-small cell lung cancer cells. Epigenetics. 2012;7(10):1161-1172. 10.4161/epi.22070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Corominas-Faja B, Oliveras-Ferraros C, Cuyas Eet al. Stem cell-like ALDHbright cellular states in EGFR-mutant non-small cell lung cancer: a novel mechanism of acquired resistance to erlotinib targetable with the natural polyphenol silibinin. Cell Cycle. 2013;12(21):3390-3404. 10.4161/cc.26417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Tyagi A, Singh RP, Ramasamy Ket al. et al. Growth inhibition and regression of lung tumors by silibinin: modulation of angiogenesis by macrophage-associated cytokines and nuclear factor-κB and signal transducers and activators of transcription 3. Cancer Prev Res. 2009;2(1):74-83. 10.1158/1940-6207.CAPR-08-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Cuyas E, Perez-Sanchez A, Micol V, Menendez JA, Bosch-Barrera J. STAT3-targeted Treatment with silibinin overcomes the acquired resistance to crizotinib in ALK-rearranged lung cancer. Cell Cycle. 2016;15:3413-3418. 10.1080/15384101.2016.1245249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.de Oliveira DT, Sávio ALV, de Castro Marcondes JPet al. et al. Cytotoxic and toxicogenomic effects of silibinin in bladder cancer cells with different TP53 status. J Biosci. 2017;42(1):91-101. 10.1007/s12038-016-9654-5 [DOI] [PubMed] [Google Scholar]
- 316.Vinh PQ, Sugie S, Tanaka Tet al. et al. Chemopreventive effects of a flavonoid antioxidant silymarin on N-butyl-N-(4-hydroxybutyl) nitrosamine-induced urinary bladder carcinogenesis in male ICR mice. Jpn J Cancer Res. 2002;93(1):42-49. 10.1111/j.1349-7006.2002.tb01199.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Imai-Sumida M, Chiyomaru T, Majid Set al. et al. Silibinin suppresses bladder cancer through down-regulation of actin cytoskeleton and PI3K/Akt signaling pathways. Oncotarget. 2017;8(54):92032. 10.18632/oncotarget.20734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Zeng J, Sun Y, Wu Ket al. et al. Chemopreventive and chemotherapeutic effects of intravesical silibinin against bladder cancer by acting on mitochondria. Mol Cancer Ther. 2011;10(1):104-116. 10.1158/1535-7163.MCT-10-0577 [DOI] [PubMed] [Google Scholar]
- 319.Wu K, Ning Z, Zeng Jet al. et al. Silibinin inhibits β-catenin/ZEB1 signaling and suppresses bladder cancer metastasis via dual-blocking epithelial–mesenchymal transition and stemness. Cell Signal. 2013;25(12):2625-2633. 10.1016/j.cellsig.2013.08.028 [DOI] [PubMed] [Google Scholar]
- 320.Ge Y, Zhang Y, Chen Yet al. et al. Silibinin causes apoptosis and cell cycle arrest in some human pancreatic cancer cells. Int J Mol Sci. 2011;12(8):4861-4871. 10.3390/ijms12084861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Feng W, Cai D, Zhang B, Lou G, Zou X. Combination of HDAC inhibitor TSA and silibinin induces cell cycle arrest and apoptosis by targeting survivin and cyclinB1/Cdk1 in pancreatic cancer cells. Biomed Pharmacother. 2015;74:257-264. 10.1016/j.biopha.2015.08.017 [DOI] [PubMed] [Google Scholar]
- 322.Shukla SK, Dasgupta A, Mehla Ket al. et al. Silibinin-mediated metabolic reprogramming attenuates pancreatic cancer-induced cachexia and tumor growth. Oncotarget. 2015;6(38):41146. 10.18632/oncotarget.5843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Nambiar D, Prajapati V, Agarwal R, Singh RP. In vitro and in vivo anticancer efficacy of silibinin against human pancreatic cancer BxPC-3 and PANC-1 cells. Cancer Lett. 2013;334(1):109-117. 10.1016/j.canlet.2012.09.004 [DOI] [PubMed] [Google Scholar]
- 324.Zhang X, Liu J, Zhang Pet al. et al. Silibinin induces G1 arrest, apoptosis and JNK/SAPK upregulation in SW1990 human pancreatic cancer cells. Oncol Lett. 2018;15(6):9868-9876. 10.3892/ol.2018.8541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Zhang S, Yang X, Morris ME. Combined effects of multiple flavonoids on breast cancer resistance protein (ABCG2)-mediated transport. Pharm Res. 2004;21(7):1263-1273. 10.1023/B:PHAM.0000033015.84146.4c [DOI] [PubMed] [Google Scholar]
- 326.Tyagi AK, Agarwal C, Chan DC, Agarwal R. Synergistic anti-cancer effects of silibinin with conventional cytotoxic agents doxorubicin, cisplatin and carboplatin against human breast carcinoma MCF-7 and MDA-MB468 cells. Oncol Rep. 2004;11(2):493-499. 10.3892/or.11.2.493 [DOI] [PubMed] [Google Scholar]
- 327.Kim S, Choi JH, Lim HIet al. et al. Silibinin prevents TPA-induced MMP-9 expression and VEGF secretion by inactivation of the Raf/MEK/ERK pathway in MCF-7 human breast cancer cells. Phytomedicine. 2009;16(6–7):573-580. 10.1016/j.phymed.2008.11.006 [DOI] [PubMed] [Google Scholar]
- 328.Rastegar H, Ashtiani HA, Anjarani S, Bokaee S, Khaki A, Javadi L. The role of milk thistle extract in breast carcinoma cell line (MCF-7) apoptosis with doxorubicin. Acta Med Iran. 2013:591-598. [PubMed] [Google Scholar]
- 329.Kalla PK, Chitti S, Aghamirzaei ST, Senthilkumar R, Arjunan S. Anti-cancer activity of silymarin on MCF-7 and NCIH-23 cell lines. Adv Biol Res. 2014;8(2):57-61. 10.5829/idosi.abr.2014.8.2.82286 [DOI] [Google Scholar]
- 330.Forghani P, Khorramizadeh MR, Waller EK. Silibinin inhibits accumulation of myeloid-derived suppressor cells and tumor growth of murine breast cancer. Cancer Med. 2014;3:215-224. 10.1002/cam4.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Jiang K, Wang W, Jin X, Wang Z, Ji Z, Meng G. Silibinin, a natural flavonoid, induces autophagy via ROS-dependent mitochondrial dysfunction and loss of ATP involving BNIP3 in human MCF7 breast cancer cells. Oncol Rep. 2015;33(6):2711-2718. 10.3892/or.2015.3915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Pirouzpanah MB, Sabzichi M, Pirouzpanah S, Chavoshi H, Samadi N. Silibilin-induces apoptosis in breast cancer cells by modulating p53, p21, Bak and Bcl-XL pathways. Asian Pac J Cancer Prev. 2015;16(5):2087-2092. 10.7314/apjcp.2015.16.5.2087. [DOI] [PubMed] [Google Scholar]
- 333.Zheng N, Zhang P, Huang Het al. et al. ERα down-regulation plays a key role in silibinin-induced autophagy and apoptosis in human breast cancer MCF-7 cells. J Pharmacol Sci. 2015;128(3):97-107. 10.1016/j.jphs.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 334.Jahanafrooz Z, Motameh N, Bakhshandeh B. Comparative evaluation of silibinin effects on cell cycling and apoptosis in human breast cancer MCF-7 and T47D cell lines. Asian Pac J Cancer Prev. 2016;17(5):2661-2665. PMID: 27268647. [PubMed] [Google Scholar]
- 335.Molavi O, Narimani F, Asiaee Fet al. et al. Silibinin sensitizes chemo-resistant breast cancer cells to chemotherapy. Pharm Biol. 2017;55(1):729-739. 10.1080/13880209.2016.1270972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Paulpandi M. 78P In vivo activation of mitochondrial pathway and cell cycle arrest through silymarin loaded iron nanoparticles as proficient nanocomplex system for triple negative breast cancer therapy. Ann Oncol. 2017;28(suppl_10):mdx655–mdx020. 10.1093/annonc/mdx655.020 [DOI] [Google Scholar]
- 337.Sik CG, Seon YE, Jung KYet al. et al. Anticancer effect of silymarin on breast cancer cells through inhibition of Akt and MAPK pathway expression. 151–151. KALAS Int Symp. 2017;8:151-151. [Google Scholar]
- 338.Kim SH, Choo GS, Yoo ESet al. et al. Silymarin inhibits proliferation of human breast cancer cells via regulation of the MAPK signaling pathway and induction of apoptosis. Oncol Lett. 2021;21(6):1-10. 10.3892/ol.2021.12753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Kohno H, Tanaka T, Kawabata Ket al. et al. Silymarin, a naturally occurring polyphenolic antioxidant flavonoid, inhibits azoxymethane-induced colon carcinogenesis in male F344 rats. Int J Cancer. 2002;101(5):461-468. 10.1002/ijc.10625 [DOI] [PubMed] [Google Scholar]
- 340.Raina K, Agarwal C, Agarwal R. Effect of silibinin in human colorectal cancer cells: targeting the activation of NF-κB signaling. Mol Carcinog. 2013;52(3):195-206. 10.1002/mc.21843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Eo HJ, Park GH, Song HMet al. et al. Silymarin induces cyclin D1 proteasomal degradation via its phosphorylation of threonine-286 in human colorectal cancer cells. Int Immunopharmacol. 2015;24(1):1-6. 10.1016/j.intimp.2014.11.009 [DOI] [PubMed] [Google Scholar]
- 342.Lal M, Gupta D. Studies on radiation sensitization efficacy by silymarin in colon carcinoma cells. Discoveries. 2016;4(1):1. 10.15190/d.2016.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Horita MA, Ezekiel U. The effects of silibinin on colorectal cancer cell line. FASEB J. 2017;31(1 Supplement):766. 10.1096/fasebj.31.1_supplement.766.5 [DOI] [Google Scholar]
- 344.Belli V, Sforza V, Cardone Cet al. et al. Regorafenib in combination with silybin as a novel potential strategy for the treatment of metastatic colorectal cancer. Oncotarget. 2017;8(40):68305. 10.18632/oncotarget.20054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Girardi B, Pricci M, Giorgio Fet al. et al. Silymarin, boswellic acid and curcumin enriched dietetic formulation reduces the growth of inherited intestinal polyps in an animal model. World J Gastroenterol. 2020;26(14):1601. 10.3748/wjg.v26.i14.1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Varghese L, Agarwal C, Tyagi A, Singh RP, Agarwal R. Silibinin efficacy against human hepatocellular carcinoma. Clin Cancer Res. 2005;11(23):8441-8448. 10.1158/1078-0432.CCR-05-1646 [DOI] [PubMed] [Google Scholar]
- 347.Ramakrishnan G, Raghavendran HRB, Vinodhkumar R, Devaki T. Suppression of N-nitrosodiethylamine induced hepatocarcinogenesis by silymarin in rats. Chem-Biol Interact. 2006;161(2):104-114. 10.1016/j.cbi.2006.03.007 [DOI] [PubMed] [Google Scholar]
- 348.Ramakrishnan G, Jagan S, Kamaraj S, Anandakumar P, Devaki T. Silymarin attenuated mast cell recruitment thereby decreased the expressions of matrix metalloproteinases-2 and 9 in rat liver carcinogenesis. Invest New Drugs. 2009;27(3):233-240. 10.1007/s10637-008-9163-y [DOI] [PubMed] [Google Scholar]
- 349.Momeny M, Khorramizadeh MR, Ghaffari SHet al. et al. Effects of silibinin on cell growth and invasive properties of a human hepatocellular carcinoma cell line, HepG-2, through inhibition of extracellular signal-regulated kinase 1/2 phosphorylation. Eur J Pharmacol. 2008;591(1–3):13-20. 10.1016/j.ejphar.2008.06.011 [DOI] [PubMed] [Google Scholar]
- 350.Ramakrishnan G, Lo Muzio L, Elinos-Báez CMet al. et al. Silymarin inhibited proliferation and induced apoptosis in hepatic cancer cells. Cell Prolif. 2009;42(2):229-240. 10.1111/j.1365-2184.2008.00581.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Cui W, Gu F, Hu KQ. Effects and mechanisms of silibinin on human hepatocellular carcinoma xenografts in nude mice. World J Gastroenterol. 2009;15(16):1943. 10.3748/wjg.15.1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Gu HR, Park SC, Choi SJet al. et al. Combined treatment with silibinin and either sorafenib or gefitinib enhances their growth-inhibiting effects in hepatocellular carcinoma cells. Clin Mol Hepatol. 2015;21(1):49. 10.3350/cmh.2015.21.1.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Aykanat NEB, Kacar S, Karakaya S, Sahinturk V. Silymarin suppresses HepG2 hepatocarcinoma cell progression through downregulation of slit-2/robo-1 pathway. Pharmacol Rep. 2020;72(1):199-207. 10.1007/s43440-019-00040-x [DOI] [PubMed] [Google Scholar]
- 354.Fan L, Ma Y, Liu Y, Zheng D, Huang G. Silymarin induces cell cycle arrest and apoptosis in ovarian cancer cells. Eur J Pharmacol. 2014;743:79-88. 10.1016/j.ejphar.2014.09.019 [DOI] [PubMed] [Google Scholar]
- 355.Gallo D, Giacomelli S, Ferlini Cet al. et al. Antitumour activity of the silybin-phosphatidylcholine complex, IdB 1016, against human ovarian cancer. Eur J Cancer. 2003;39(16):2403-2410. 10.1016/S0959-8049(03)00624-5 [DOI] [PubMed] [Google Scholar]
- 356.Cho HJ, Suh DS, Moon SHet al. et al. Silibinin inhibits tumor growth through downregulation of extracellular signal-regulated kinase and Akt in vitro and in vivo in human ovarian cancer cells. J Agric Food Chem. 2013;61(17):4089-4096. 10.1021/jf400192v [DOI] [PubMed] [Google Scholar]
- 357.Zhang J, Harrison JS, Uskokovic M, Danilenko M, Studzinski GP. Silibinin can induce differentiation as well as enhance vitamin D3-induced differentiation of human AML cells ex vivo and regulates the levels of differentiation-related transcription factors. Hematol Oncol. 2010;28:124-132. 10.1002/hon.929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Molavi O, Samadi N, Wu C, Lavasanifar A, Lai R. Silibinin suppresses NPM-ALK, potently induces apoptosis and enhances chemosensitivity in ALK-positive anaplastic large cell lymphoma. Leuk Lymphoma. 2016;57(5):1154-1162. 10.3109/10428194.2015.1068306 [DOI] [PubMed] [Google Scholar]
- 359.Lu S, Zhang J, Wang Y. Silymarin inhibits proliferation and induces apoptosis in epstein-barr virus-positive lymphoma cells by suppressing nuclear factor-kappa B pathway. Curr Top Nutraceutical Res. 2020;18(4). [Google Scholar]
- 360.Feng N, Luo J, Guo X. Silybin suppresses cell proliferation and induces apoptosis of multiple myeloma cells via the PI3K/Akt/mTOR signaling pathway. Mol Med Rep. 2016;13:3243-3248. 10.3892/mmr.2016.4887 [DOI] [PubMed] [Google Scholar]
- 361.Su CH, Chen LJ, Liao JF, Cheng JT. Increase of phosphatase and tensin homolog by silymarin to inhibit human pharynx squamous cancer. J Med Food. 2013;16(9):778-784. 10.1089/jmf.2012.2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Zhang M, Liu Y, Gao Y, Li S. Silibinin-induced glioma cell apoptosis by PI3K-mediated but Akt-independent downregulation of FoxM1 expression. Eur J Pharmacol. 2015;765:346-354. 10.1016/j.ejphar.2015.08.057 [DOI] [PubMed] [Google Scholar]
- 363.Won DH, Kim LH, Jang Bet al. et al. In vitro and in vivo anti-cancer activity of silymarin on oral cancer. Tumor Biol. 2018;40(5), 1010428318776170. https://doi.org/10.1177/1010428318776170. [DOI] [PubMed] [Google Scholar]
- 364.Choi ES, Oh S, Jang Bet al. et al. Silymarin and its active component silibinin act as novel therapeutic alternatives for salivary gland cancer by targeting the ERK1/2-Bim signaling cascade. Cell Oncol. 2017;40(3):235-246. 10.1007/s13402-017-0318-8 [DOI] [PubMed] [Google Scholar]
- 365.Yu HC, Chen LJ, Cheng KC, Li YX, Yeh CH, Cheng JT. Silymarin inhibits cervical cancer cell through an increase of phosphatase and tensin homolog. Phytother Res. 2012;26(5):709-715. 10.1002/ptr.3618 [DOI] [PubMed] [Google Scholar]
- 366.Hsieh YS, Chu SC, Yang SF, Chen PN, Liu YC, Lu KH. Silibinin suppresses human osteosarcoma MG-63 cell invasion by inhibiting the ERK-dependent c-Jun/AP-1 induction of MMP-2. Carcinogenesis. 2007;28:977-987. 10.1093/carcin/bgl221 [DOI] [PubMed] [Google Scholar]
- 367.Li Y, Zhang C, Cai D, Chen C, Mu D. Silibinin inhibits migration and invasion of the rhabdoid tumor G401 cell line via inactivation of the PI3K/Akt signaling pathway. Oncol Lett. 2017;14(6):8035-8041. 10.3892/ol.2017.7246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Kim SH, Choo GS, Yoo ESet al. et al. Silymarin induces inhibition of growth and apoptosis through modulation of the MAPK signaling pathway in AGS human gastric cancer cells. Oncol Rep. 2019;42(5):1904-1914. 10.3892/or.2019.7295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Malewicz B, Wang Z, Jiang Cet al. et al. Enhancement of mammary carcinogenesis in two rodent models by silymarin dietary supplements. Carcinogenesis. 2006;27(9):1739-1747. 10.1093/carcin/bgl032 [DOI] [PubMed] [Google Scholar]
- 370.Kummer V, Maskova J, Canderle J, Zraly Z, Neca J, Machala M. Estrogenic effects of silymarin in ovariectomized rats. VETERINARNI MEDICINA-PRAHA. 2001;46(1):17-23. 10.17221/7846-VETMED [DOI] [Google Scholar]
- 371.Imamoto R, Okano JI, Sawada S, Fujise Y, Abe R, Murawaki Y. Null anticarcinogenic effect of silymarin on diethylnitrosamine-induced hepatocarcinogenesis in rats. Exp Ther Med. 2014;7(1):31-38. 10.3892/etm.2013.1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Brandon-Warner E, Eheim AL, Foureau DM, Walling TL, Schrum LW, McKillop IH. Silibinin (milk thistle) potentiates ethanol-dependent hepatocellular carcinoma progression in male mice. Cancer Lett. 2012;326(1):88-95. 10.1016/j.canlet.2012.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Javed S, Kohli K, Ali M. Reassessing bioavailability of silymarin. Altern. Med. Rev. 2011;16:239-249. PMID: 21951025. [PubMed] [Google Scholar]
- 374.Wu JW, Lin LC, Hung SC, Chi CW, Tsai TH. Analysis of silibinin in rat plasma and bile for hepatobiliary excretion and oral bioavailability application. J. Pharm. Biomed. Anal. 2007;45:635-641. 10.1016/j.jpba.2007.06.026 [DOI] [PubMed] [Google Scholar]
- 375.Janiak B, Kessler B, Kunz W, Schnieders B. Die wirkung von silymarin auf gehalt und function einiger durch einwirkung von tetrachlorkohlenstoff bzw. Halothan beeinflussten mikrosomalen leberenzyme. Arzneimittelforschung. 1973;23:1322-1326. PMID: 4801229. [PubMed] [Google Scholar]
- 376.Fraschini F, Demartini G, Esposti D. Pharmacology of silymarin. Clin Drug Investig. 2002;22(1):51-65. 10.2165/00044011-200222010-00007 [DOI] [Google Scholar]
- 377.Lorenz D, Lucker PW, Mennicke WH, Wetzelsberger N. Pharmacokinetic studies with silymarin in human serum and bile. Methods find. Exp Clin Pharmacol. 1984;6(10):655-661. PMID: 6513680. [PubMed] [Google Scholar]
- 378.Beckmann-Knopp S, Rietbrock S, Weyhenmeyer Ret al. et al. Inhibitory effects of silibinin on cytochrome P-450 enzymes in human liver microsomes. Pharmacol Toxicol. 2000;86(6):250-256. 10.1111/j.0901-9928.2000.860602.x [DOI] [PubMed] [Google Scholar]
- 379.Gatti G, Perucca E. Plasma concentrations of free and conjugated silybin after oral intake of a silybin-phosphatidylcholine complex (silipide) in healthy volunteers. Int J Clin Pharm Ther. 1994;32(11):614-617. PMID: 7874377. [PubMed] [Google Scholar]
- 380.Filburn CR, Kettenacker R, Griffin DW. Bioavailability of a silybin–phosphatidylcholine complex in dogs. J Vet Pharmacol Ther. 2007;30(2):132-138. 10.1111/j.1365-2885.2007.00834.x [DOI] [PubMed] [Google Scholar]
- 381.Morazzoni P, Montalbetti A, Malandrino S, Pifferi G. Comparative pharmacokinetics of silipide and silymarin in rats. Eur J Drug Metab Pharmacokinet. 1993;18(3):289-297. 10.1007/BF03188811 [DOI] [PubMed] [Google Scholar]
- 382.Zhao J, Agarwal R. Tissue distribution of silibinin, the major active constituent of silymarin, in mice and its association with enhancement of phase II enzymes: implications in cancer chemoprevention. Carcinogenesis. 1999;20(11):2101-2108. 10.1093/carcin/20.11.2101 [DOI] [PubMed] [Google Scholar]
- 383.Schandalik R, Gatti G, Perucca E. Pharmacokinetics of silybin in bile following administration of silipide and silymarin in cholecystectomy patients. Arzneimittelforschung. 1992;42:964-968. PMID: 1329780. [PubMed] [Google Scholar]
- 384.Hoh C, Boocock D, Marczylo Tet al. et al. Pilot study of oral silibinin, a putative chemopreventive agent, in colorectal cancer patients: silibinin levels in plasma, colorectum, and liver and their pharmacodynamic consequences. Clin Cancer Res. 2006;12(9):2944-2950. 10.1158/1078-0432.CCR-05-2724 [DOI] [PubMed] [Google Scholar]
- 385.Tyagi AK, Singh RP, Agarwal C, Chan DC, Agarwal R. Silibinin strongly synergizes human prostate carcinoma DU145 cells to doxorubicin-induced growth inhibition, G2–M arrest, and apoptosis. Clin Cancer Res. 2002;8(11):3512-3519. PMID: 12429642. [PubMed] [Google Scholar]
- 386.Ranjbar N, Saravani R, Faezizadeh Z. Silymarin inhibits toll-like receptor 8 gene expression and apoptosis in ramos cancer cell line. Avicenna J Phytomed. 2020;10(2):161. PMID: 32257888. [PMC free article] [PubMed] [Google Scholar]
- 387.Kamrani Z, Heshmati M, Babashah S. Evauation effects of silymarin on cytotoxicity and apoptosis on SW480 colon cancer cell line. Research in Karyotic Cell &Tissue. 2021;1(3). [Google Scholar]
- 388.Pradhan SC, Girish C. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J Med Res. 2013;124(5):491-504. PMID: 17213517. [PubMed] [Google Scholar]
- 389.Weyhenmeyer R, Mascher H, Birkmayer J. Study on dose-linearity of the pharmacokinetics of silibinin diastereomers using a new stereospecific assay. Int J Clin Pharmacol Ther Toxicol. 1992;30(4):134-138. PMID: 1572758. [PubMed] [Google Scholar]
- 390.Di Costanzo A, Angelico R. Formulation strategies for enhancing the bioavailability of silymarin: the state of the art. Molecules. 2019;24(11):2155. 10.3390/molecules24112155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Kidd P, Head K. A review of the bioavailability and clinical efficacy of milk thistle phytosome: a silybin-phosphatidylcholine complex (siliphos). Altern Med Rev. 2005;10:193-203. PMID: 16164374. [PubMed] [Google Scholar]
- 392.Comoglio A, Tomasi A, Malandrino S, Poli G, Albano E. Scavenging effect of silipide, a new silybin-phospholipid complex, on ethanol-derived free radicals. Biochem Pharmacol. 1995;50(8):1313-1316. 10.1016/0006-2952(95)02001-S [DOI] [PubMed] [Google Scholar]
- 393.Tvrdý V, Pourová J, Jirkovský E, Křen V, Valentová K, Mladěnka P. Systematic review of pharmacokinetics and potential pharmacokinetic interactions of flavonolignans from silymarin. Med Res Rev. 2021;41(4):2195-2246. 10.1002/med.21791 [DOI] [PubMed] [Google Scholar]
- 394.Pawar HA, Bhangale BD. Phytosome as a novel biomedicine: a microencapsulated drug delivery system. J Bioanal Biomed. 2015;7(1):6-12. 10.4172/1948-593X.1000116 [DOI] [Google Scholar]
- 395.Morazzoni P, Magistretti MJ, Giachetti C, Zanolo G. Comparative bioavailability of silipide, a new !avanolignan complex, in rats. Eur J Drug Metab Pharmacokinet. 1992;117:39-44. 10.1007/BF03189986 [DOI] [PubMed] [Google Scholar]
- 396.Zhang ZB, Shen ZG, Wang JXet al. et al. Micronization of silybin by the emulsion solvent diffusion method. Int J Pharm. 2009;376(1–2):116-122. 10.1016/j.ijpharm.2009.04.028 [DOI] [PubMed] [Google Scholar]
- 397.Wu W, Wang Y, Que L. Enhanced bioavailability of silymarin by selfmicroemulsifying drug delivery system. Eur J Pharm Biopharm. 2006;63(3):288-294. 10.1016/j.ejpb.2005.12.005 [DOI] [PubMed] [Google Scholar]
- 398.Liu L, Pang X, Zhang W, Wang S. Formulation design and in vitro evaluation of silymarin loaded self micro emulsifying drug delivery systems. Asian J Pharm Sci. 2007;2:150-160. [Google Scholar]
- 399.Maheshwari H, Aggarwal R, Patil C, Katare OP. Preparation and pharmacological evaluation of silibinin liposomes. Arzneimittelforschung. 2003;53(06):420-427. 10.1055/s-0031-1297130 [DOI] [PubMed] [Google Scholar]
- 400.Nguyen MH, Yu H, Dong B, Hadinoto K. A supersaturating delivery system of silibinin exhibiting high payload achieved by amorphous nano-complexation with chitosan. Eur J Pharm Sci. 2016;89:163-171. 10.1016/j.ejps.2016.04.036 [DOI] [PubMed] [Google Scholar]
- 401.Di Costanzo A, Angelico R. Formulation strategies for enhancing the bioavailability of silymarin: the state of the art. Molecules. 2019;24(11):2155. 10.3390/molecules24112155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Piazzini V, D’Ambrosio M, Luceri Cet al. et al. Formulation of nanomicelles to improve the solubility and the oral absorption of silymarin. Molecules. 2019;24(9):1688. 10.3390/molecules24091688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Flaig TW, Gustafson DL, Su LJet al. et al. A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Invest New Drugs. 2007;25(2):139-146. 10.1007/s10637-006-9019-2 [DOI] [PubMed] [Google Scholar]
- 404.Jacobs PB, Dennehy C, Ramirez G, Sapp J, Lawrence VA. Milk thistle for the treatment of liver disease: a systematic review and meta-analysis. Am J Med. 2002;113:506-515. 10.1016/S0002-9343(02)01244-5 [DOI] [PubMed] [Google Scholar]
- 405.Abenavoli L, Capasso R, Milic N, Capasso F. Milk thistle in liver diseases: past, present, future. Phytother Res. 2010;24(10):1423-1432. 10.1002/ptr.3207 [DOI] [PubMed] [Google Scholar]
- 406.Flaig TW, Glodé M, Gustafson Det al. et al. A study of high-dose oral silybin-phytosome followed by prostatectomy in patients with localized prostate cancer. Prostate. 2010;70(8):848-855. 10.1002/pros.21118 [DOI] [PubMed] [Google Scholar]
- 407.Lazzeroni M, Guerrieri-Gonzaga A, Gandini Set al. et al. A presurgical study of oral silybin-phosphatidylcholine in patients with early breast cancer. Cancer Prev Res. 2016;9(1):89-95. 10.1158/1940-6207.CAPR-15-0123 [DOI] [PubMed] [Google Scholar]
- 408.Lee SM, Lee GW, Park SYet al. et al. 2020. Dual Effects of Silibinin on Human Pancreatic Cancer Cells. Preprint downloaded from https://assets.researchsquare.com/files/rs-130714/v1/6f2d72c0-7e77-448d-85b5-b2bdeea220a0.pdf?c=1608755626 accessed 7/7/2021.
- 409.Tehrani FK, Ranji N, Kouhkan F, Hosseinzadeh S. PANC-1 cancer stem-like cell death with silybin encapsulated in polymersomes and deregulation of stemness-related miRNAs and their potential targets. Iran J Basic Med Sci. 2021;24(4):514. 10.22038/ijbms.2021.54001.12136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Abdollahi P, Ebrahimi M, Motamed N, Samani FS. Silibinin affects tumor cell growth because of reduction of stemness properties and induction of apoptosis in 2D and 3D models of MDA-MB-468. Anti-cancer Drugs. 2015;26(5):487-497. 10.1097/CAD.0000000000000205 [DOI] [PubMed] [Google Scholar]
- 411.Firouzi J, Ebrahimi M, Sotoodehnejadnematalahi F. Evaluation of inhibitory effect of silibinin on growth and stemness property of MCF-7 cell line derived mammospheres. SSU_Journals. 2017;25(2):111-122. http://jssu.ssu.ac.ir/article-1-3944-en.html. [Google Scholar]
- 412.Pawar SK, Jaldappagari S. Intercalation of a flavonoid, silibinin into DNA base pairs: experimental and theoretical approach. J Mol Recognit. 2020;33:e2812. 10.1002/jmr.2812 [DOI] [PubMed] [Google Scholar]
- 413.Biedermann D, Hurtová M, Biedermannová L, Valentová K, Křen V. Flavonolignans from silymarin do not intercalate into DNA: rebuttal of data published in the paper J. Mol. Recognit. e2812 (2019). J Mol Recognit. 2021;34(7):e2888. 10.1002/jmr.2888 [DOI] [PubMed] [Google Scholar]
- 414.Schröder FH, Roobol MJ, Boevé ERet al. Randomized, double-blind, placebo-controlled crossover study in men with prostate cancer and rising PSA: effectiveness of a dietary supplement. Eur Urol. 2005;48(6):922-931. 10.1016/j.eururo.2005.08.005 [DOI] [PubMed] [Google Scholar]
- 415.Hsu CY, Sun PL, Chang HC, Perng DS, Chen YS. Spontaneous regression of advanced hepatocellular carcinoma: a case report. Cases J. 2009;2(1):6251. 10.4076/1757-1626-2-6251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Moroni M, Zanlorenzi L. Complete regression following sorafenib in unresectable, locally advanced hepatocellular carcinoma. Future Oncol. 2013;9(8):1231-1237. 10.2217/fon.13.86 [DOI] [PubMed] [Google Scholar]
- 417.Bosch-Barrera J, Sais E, Cañete Net al. et al. Response of brain metastasis from lung cancer patients to an oral nutraceutical product containing silibinin. Oncotarget. 2016;7(22):32006. 10.18632/oncotarget.7900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-674. [DOI] [PubMed] [Google Scholar]
- 419.Gladilin E. Graph-theoretical model of global human interactome reveals enhanced long-range communicability in cancer networks. PLoS ONE. 2017;12(1):e0170953. 10.1371/journal.pone.0170953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Polyak SJ, Morishima C, Shuhart MC, Wang CC, Liu Y, Lee DYW. Inhibition of T-cell inflammatory cytokines, hepatocyte NF-κB signaling, and HCV infection by standardized silymarin. Gastroenterology. 2007;132(5):1925-1936. 10.1053/j.gastro.2007.02.038 [DOI] [PubMed] [Google Scholar]
- 421.Zhong X, Zhu Y, Lu Q, Zhang J, Ge Z, Zheng S. Silymarin causes caspases activation and apoptosis in K562 leukemia cells through inactivation of Akt pathway. Toxicology. 2006;227(3):211-216. 10.1016/j.tox.2006.07.021 [DOI] [PubMed] [Google Scholar]
- 422.Křen V, Walterová D. Silybin and silymarin-new effects and applications. Biomed Papers. 2005;149(1):29-41. PMID: 16170386. [DOI] [PubMed] [Google Scholar]
- 423.Saller R, Brignoli R, Melzer J, Meier R. An updated systematic review with meta-analysis for the clinical evidence of silymarin. Complement Med Res. 2008;15(1):9-20. 10.1159/000113648 [DOI] [PubMed] [Google Scholar]
- 424.Su CH, Chen LJ, Liao JF, Cheng JT. Dual effects of silymarin on nasopharyngeal carcinoma cells (NPC-TW01). Complement Med Res. 2013;20(4):261-266. 10.1159/000354594 [DOI] [PubMed] [Google Scholar]
- 425.Sadighi S, Dashti-Khavidaki S, Shahbazi Fet al. et al. The effects of concomitant use of silymarin and chemotherapy on solid tumors: a pilot randomized controlled trial. Basic Clin Cancer Res. 2017;9(2):13-19. [Google Scholar]
- 426.Faisal Z, Mohos V, Fliszár-Nyúl Eet al. et al. Interaction of silymarin components and their sulfate metabolites with human serum albumin and cytochrome P450 (2C9, 2C19, 2D6, and 3A4) enzymes. Biomed Pharmacother. 2021;138:111459. 10.1016/j.biopha.2021.111459 [DOI] [PubMed] [Google Scholar]
- 427.Manivannan E, Amawi H, Hussein N, et al. Design and discovery of silybin analogues as antiproliferative compounds using a ring disjunctive–based, natural product lead optimization approach. Eur J Med Chem. 2017;133:365-378. 10.1016/j.ejmech.2017.03.033 [DOI] [PubMed] [Google Scholar]
- 428.Amawi H, Hussein NA, Karthikeyan Cet al. et al. HM015k, A novel silybin derivative, multi-targets metastatic ovarian cancer cells and is safe in zebrafish toxicity studies. Front Pharmacol. 2017;8:498. 10.3389/fphar.2017.00498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Bhatia N, Agarwal R. Detrimental effect of cancer preventive phytochemicals silymarin, genistein and epigallocatechin 3-gallate on epigenetic events in human prostate carcinoma DU145 cells. Prostate. 2001;46(2):98-107. [DOI] [PubMed] [Google Scholar]
- 430.Fallah M., Davoodvandi A., Nikmanzar S., et al. (2021). Silymarin (milk thistle extract) as a therapeutic agent in gastrointestinal cancer. Biomed Pharmacother., 142, 112024. [DOI] [PMC free article] [PubMed] [Google Scholar]