Abstract
The worldwide incidence of obesity and its sequelae, such as type 2 diabetes mellitus, have reached pandemic levels. Central to the development of these metabolic disorders is adipose tissue. White adipose tissue stores excess energy, whereas brown adipose tissue (BAT) and beige (also known as brite) adipose tissue dissipate energy to generate heat in a process known as thermogenesis. Strategies that activate and expand BAT and beige adipose tissue increase energy expenditure in animal models and offer therapeutic promise to treat obesity. A better understanding of the molecular mechanisms underlying the development of BAT and beige adipose tissue and the activation of thermogenic function is the key to creating practical therapeutic interventions for obesity and metabolic disorders. In this Review, we discuss the regulation of the tissue microenvironment (the adipose niche) and inter-organ communication between BAT and other tissues. We also cover the activation of BAT and beige adipose tissue in response to physiological cues (such as cold exposure, exercise and diet). We highlight advances in harnessing the therapeutic potential of BAT and beige adipose tissue by genetic, pharmacological and cell-based approaches in obesity and metabolic disorders.
Adipose tissue has a major role in the regulation of whole-body energy homeostasis and impairments in adipose tissue function directly link to the aetiology of type 2 diabetes mellitus and cardiovascular diseases. White adipose tissue (WAT) depots, which contain lipid-filled white adipocytes, are distributed throughout the body and are major energy storage sites. In addition, two other functionally distinct types of adipocytes exist that are energy-burning (that is, thermogenic). These are brown adipocytes, which are present in brown adipose tissue (BAT), and related beige or brite adipocytes (hereafter referred to as beige adipocytes), which appear in certain WAT depots in response to cold acclimation, exercise training or pharmacological activation of β-adrenergic receptors1.
Adipose thermogenesis is mainly ascribed to a high density of mitochondria and uncoupling protein 1 (UCP1) expression in brown and beige adipocytes. UCP1 is located on the inner mitochondrial membrane and shuttles protons from the mitochondrial intermembrane space back to the mitochondrial matrix without generating ATP. This process uncouples the metabolism of glucose and fatty acids from ATP generation and results in energy dissipation as heat2. Stemming from their high energy expenditure, brown and beige adipocytes have a remarkable capacity to take up and utilize fuels, and therefore function as a metabolic sink for glucose and free fatty acids3. Furthermore, BAT and beige adipose tissues play major parts in the regulation of whole-body metabolism through their secretory function, releasing different endocrine signalling molecules, including proteins, lipids and microRNAs, into the circulation that exert regulatory effects on the target tissues or organs4,5.
In humans, UCP1-positive adipose tissue has been found in several depots, including the cervical–supraclavicular, perirenal–adrenal and paravertebral regions, and around the major arteries6–9. The activity of BAT in humans negatively correlates with BMI6,8–10, which suggests that BAT is an attractive target for anti-obesity therapies. Additionally, studies in humans and mice have shown that the amount of active BAT positively correlates with insulin sensitivity11,12. Therefore, any strategy that increases the amount and activity of BAT can potentially be applied to the treatment of obesity and its comorbidities.
In this Review, we provide a comprehensive discussion of the ontogeny of thermogenic adipocytes and we integrate the existing literature on the role of niche factors and intercellular communications in the regulation of BAT and beige adipose tissue function and remodelling. Furthermore, we focus on the endocrine functions of BAT and beige adipose tissue and discuss their contributions to whole-body metabolism via long-range inter-organ crosstalk. Finally, we review the translational implications of these findings and propose strategies to optimize these processes towards the development of novel therapies for obesity and metabolic diseases.
Origin of thermogenic adipocytes
Lineage tracing studies have revealed the heterogeneity of adipocyte lineages between and within adipose depots. Early histological examination of mouse embryonic development identified the mesoderm layer to be the primary origin of most adipocytes13. However, the cephalic adipocytes can be traced with the neural crest marker Sox10-Cre and are therefore thought to have a neural crest origin14.
Origin of brown adipocytes
Early lineage tracing studies in mice using En1-CreER, which marks somites and somite-derived tissues, revealed that the majority of adipocytes in the interscapular BAT are derived from progenitors in dermomyotome15. Later studies showed that brown adipocytes in the interscapular and perirenal BAT arise from Pax3+Pax7+Myf5+ progenitors, but that white adipocytes in the gluteal, inguinal and epididymal WAT depots do not arise from these cells16–18. These findings led to the assumption that brown and white adipocytes arise from completely distinct embryonic progenitors, and that functional and phenotypical distinctions between these two types of adipocytes can therefore be attributed to their ontogenetic differences18–20. However, further examination of other adipose depots using the Myf5-Cre lineage tracing system revealed that the Myf5-expressing progenitors not only give rise to the brown adipocytes present in the BAT depots originated from the somitic mesoderm (that is, interscapular, subscapular and cervical BAT), but they also contribute to adipocytes in some minor WAT depots, such as the anterior WAT (interscapular and axillary) and the retroperitoneal WAT21.
Somites.
Segmental axial structures located on either side of the neural tube in the developing vertebrate embryo that contain the precursor populations of cells, which give rise to vertebral column, ribs, skeletal and smooth muscles, dermis, tendons, ligaments, cartilage and adipose tissue.
Lineage tracing studies using multiple Cre drivers that label the progenitors in different mesodermal subcompartments have shed light on the exact regions in the somites that contribute to each adipose depot22. Tracing the Meox1-expressing progenitors in the somite revealed that both brown and white adipocytes of the dorsoaxial adipose depots (including retroperitoneal WAT, and interscapular BAT and WAT) arise from somitic mesoderm. Interestingly, more than half of perigonadal white adipocytes in male mice, but not female mice, are also derived from Meox1-expressing progenitors22. However, contrary to the previous observation that indicated Pax7-expressing progenitors in the central dermomyotome as the source of all brown adipocytes in the interscapular BAT17, the 2018 Pax7-Cre:mTmG lineage tracing study suggested that the majority of brown adipocytes originate from Pax7-negative progenitors in the epaxial dermomyotome22. The discrepancy between the results of two Pax7 lineage tracings might have been due to differences in the targeting strategies (for example, Pax7 heterozygote knockout versus Cre knock-in without disruption of the endogenous gene) and the use of different reporter mouse strains (for example, LacZ versus Rosa26-mTmG) in these studies. Of note, disruption of one copy of Pax7 locus in the earlier work by Lepper and Fan17 could potentially affect lineage specification; thus, it seems more likely that the Pax7-negative progenitors in the epaxial dermomyotome are the origin of most adipocytes in the interscapular BAT.
In rodents, chronic cold exposure increases BAT mass and activity through both de novo recruitment of brown adipocytes and enhancing the thermogenic capacity of pre-existing brown adipocytes23. Genetic lineage tracing using platelet-derived growth factor receptor-α reporter mice (Pdgfra-CreERT2/tdTomato mice) combined with BrdU labelling revealed the contribution of Pdgfra-expressing adipocyte progenitors to brown adipocytes; Pdgfra-expressing adipocyte progenitors are recruited mostly to the dorsal edges of BAT in the first week of cold acclimation24. Additionally, a single-cell RNA sequencing analysis of mouse BAT published in 2021 identified the transient receptor potential cation channel subfamily V member 1 (Trpv1)-expressing vascular smooth muscle-derived adipocyte progenitors as the origin of cold-induced brown adipogenesis. Cold exposure in mice induced the proliferation of Trpv1-expressing progenitors, which was followed by their differentiation to brown adipocytes25.
Origin of beige adipocytes
In adult humans, gene expression analysis of BAT from the supraclavicular region revealed the expression of markers of both classic brown and beige adipocytes, indicating that human BAT is a heterogeneous pool of brown and beige adipocytes26. Numerous studies in rodents have demonstrated the beneficial metabolic effects of WAT browning, therefore substantiating the contribution of beige adipocytes to whole-body metabolism. Importantly, some of the beneficial effects of these adipocytes are mediated through their secretory function and might be independent of thermogenic activity.
WAT browning.
The formation of thermogenic beige adipocytes within the white adipose tissue depots.
Two possible models of beige adipocyte recruitment.
The origin of beige adipocytes remains somewhat controversial. Two possible models for beige adipocyte recruitment have been proposed. First, beige adipocytes can form via reprogramming of white adipocytes: white to beige trans-differentiation. Second, beige adipocytes arise through de novo differentiation from tissue-resident adipocyte progenitors. The first model was originally supported by electron microscopy (EM) analysis of adipocytes in WAT of mice exposed to cold. One study found the presence of two types of UCP1-expressing cells: paucilocular adipocytes, which have a central large lipid droplet and several small lipid droplets in the periphery of the cytoplasm; and multilocular adipocytes, which have the typical morphology of the classic brown adipocytes with several small lipid droplets in the cytoplasm27. EM analysis of UCP1-expressing paucilocular adipocytes showed that they have a mixture of ‘brown’ mitochondria (large with numerous transverse cristae) and elongated ‘white’ mitochondria27, consistent with the presence of intermediate steps in the process of direct trans-differentiation of white into beige adipocytes. Consistently, genetic labelling of white adipocytes in mice with adiponectin-CreERT2 and tracing their outcome upon 7 days of cold exposure has revealed that all of the UCP1-expressing multilocular beige adipocytes are derived from pre-existing white adipocytes24. This interconversion process of beige and white adipocytes seems to be reversible. For example, transfer of animals from a cold environment to a warmer one results in the conversion of beige adipocytes into cells with the morphology and gene expression pattern of white adipocytes28.
By contrast, another study showed that the majority of beige adipocytes formed in mouse inguinal WAT (ingWAT) upon 3 days of cold exposure are the result of de novo adipogenesis from adipocyte progenitors. In that study, ‘AdipoChaser’ mice were used to enable doxycycline-inducible permanent labelling of adiponectin-expressing adipocytes, which were tracked upon cold exposure or treatment with β3-adrenergic receptor agonist CL316,243 (REF.29). Another study using the AdipoChaser mice combined with the Rosa26-mTmG reporter (a dual fluorescent reporter mouse strain) showed the contribution of both the trans-differentiation and de novo adipogenesis to beige adipocyte recruitment in ingWAT upon cold exposure in mice30.
The opposing findings of the above-mentioned studies could have been the result of differences in the lineage-tracing strategy used (tamoxifen versus doxycycline induction or LacZ reporter versus membrane tagged fluorescent proteins), as well as key differences in the experimental design of each study. Notably, tamoxifen was shown to be retained in adipose tissue for a long period following initial injection, essentially extending the ‘Pulse’ experimental period into the ‘Chase’ period. Another confounding variable is the housing temperature in which mice are raised before the experiments. A 2019 study31 examined the effects of housing temperatures early in life on beige adipogenesis. Using AdipoChaser mice, the researchers demonstrated that the majority of beige adipocytes formed upon transferring the mice from thermoneutrality (30 °C) to cold (6 °C) are the result of de novo adipogenesis. However, if the mice are raised at room temperature (22 °C) and then transferred to cold, only half of the beige adipocytes are formed through de novo adipogenesis and the rest originate from the pre-existing adipocytes. These findings indicate that beige adipocytes are predominantly derived from adipocyte progenitors during the first exposure of mice to cold. These beige adipocytes are converted to inactive ‘dormant’ thermogenic adipocytes, which are indistinguishable from white adipocytes, when the animals are returned to warm temperatures. Upon future exposures to cold, the dormant adipocytes can be activated to form the beige adipocytes. Of note, room temperature presents a mild cold exposure in mice and results in the appearance of the first wave of beige adipocytes observed in mice born and raised at room temperature.
One study also addressed the effect of the type of stimulus (cold versus β3-adrenergic receptor agonist CL316,243) on beige adipogenesis32. Compared with cold exposure, the administration of CL316,243 activated the conversion of dormant beige adipocytes more potently. This phenomenon probably occurs because adipocytes express the β3-adrenergic receptor; however, their progenitors do not. In humans, dormant BAT is found throughout the perirenal depot, especially in the region most distant to the adrenal gland32.
A role for mural progenitors.
Several studies have demonstrated the presence of white and beige adipocyte progenitors in the vessel-associated mural component of WAT25,33–37. Lineage tracing studies using Cre drivers that mark the vascular smooth muscle lineage (Pdgfrb, Acta2, Tagln, Cspg4, Myh11 and Trpv1) have shown the contribution of smooth muscle lineage to the white and beige adipocyte pool. Although different studies have shown varying extents to which vascular smooth muscles contribute to cold-induced beige adipogenesis, together they provide convincing evidence that progenitors derived from mural lineage are the key contributors to the browning of WAT depots. Impairing the adipogenic differentiation of these mural progenitors through the deletion of the key adipogenic transcription factor, Pparg, impedes browning of WAT in adult mice36,37.
Heterogeneity in adipocyte progenitors.
A remaining question is whether beige and white adipocytes derive from distinct types of adipocyte progenitors present in WAT, or if cold exposure stimulates the thermogenic differentiation of the common beige–white progenitors. Clonal analysis of adipocyte progenitors in ingWAT of mice identified a group of adipocyte progenitors with the potential to differentiate into beige adipocytes in vitro38. Such heterogeneity in adipocyte progenitors has also been observed in human neck adipose tissue39. One study identified Ebf2+PDGFRA+ progenitors present in mouse BAT and adult mouse ingWAT as beige adipocyte progenitors and showed that cold exposure increases the number of Ebf2+PDGFRA+ progenitors40. Furthermore, genetic deletion of Ebf2 in mice results in severe loss of thermogenic gene expression in BAT without any alteration in the expression of pro-adipogenic genes41.
Single-cell RNA sequencing was used to identify a population of Lin−Sca1+CD142+Abcg1+ adipocyte progenitor in mouse ingWAT that was refractory to adipogenesis in vitro. Of note, Lin−Sca1+CD142+Abcg1+ adipocyte progenitors inhibited adipogenesis of other adipocyte progenitors in a paracrine manner during co-culture, therefore suggesting a regulatory function of these cells in reducing adipogenesis in the whole adipose depot42. Another study43 identified three subpopulations of Sca1+PDGFRA+ adipocyte progenitors and used computational trajectory analysis to determine the hierarchical relationship between the cells in these populations. Such analysis combined with in vitro and in vivo characterization demonstrated that cells expressing dipeptidyl peptidase 4 are highly proliferative, multipotent progenitors that give rise to both committed pre-adipocytes (Icam1+) and CD142+ adipocyte progenitors. Contrary to the observation reported by Schwalie et al.42, the CD142+ cells were shown to be adipogenic in vitro and in vivo42,43. Another study used single-cell RNA sequencing to examine the effect of the β3-adrenergic agonist CL316,243 on lineage-negative cells from mouse ingWAT44. The analysis revealed that administration of CL316,243 for 3 days does not result in proliferation or differentiation of adipocyte progenitors to beige adipocytes. This finding supports the notion that the CL316,243-induced recruitment of beige adipo cytes in ingWAT mostly results from white to beige adipocyte conversion. By contrast, CL316,243 treatment stimulates the proliferation of PDGFRA+ adipocyte progenitors in perigonadal WAT (pgWAT), followed by induction of the adipogenic gene programme44. These studies provide strong evidence for the presence of depot-specific pathways involved in WAT browning induced by β3-adrenergic agonists or cold exposure.
Intercellular crosstalk in the adipose niche
Although the developmental origin of adipocytes could partially explain the functional differences between adipocytes, the depot-specific microenvironment also plays a key part in directing adipocyte development and function. Adipose tissue is composed of mature adipocytes, adipocyte progenitors, immune cells, endothelial cells, smooth muscle cells, pericytes, neurons and Schwann cells. Adipocytes have a major role in maintaining energy balance, but other cell types form the adipocyte niche and regulate adipose tissue function through extensive cellular crosstalk. Such communication axes are essential for the regulation of adipose tissue turnover, expansion and remodelling, in response to external stimuli such as ambient temperature and diet. Numerous studies have demonstrated that healthy remodelling and expansion of adipose tissue is essential for adipose tissue function and metabolic health45. Impairment in the capacity of adipose tissue to remodel is a hallmark of obesity and its sequelae.
Cold exposure is a robust stimulus of BAT activity2. This stimulus increases BAT cellularity by recruitment of new brown adipocytes, as well as via expansion and remodelling of other cell types in BAT. Under conditions of increased thermogenic demand, a coordinated expansion of brown adipocyte progenitors, endothelial cells and nerve terminals occurs, as well as changes in the composition of BAT-resident immune cells, to enable maximal thermogenic activity46,47.
Here we focus on cellular crosstalk within adipose tissue mediated through ligand–receptor interactions. We summarize our current understanding of the role of paracrine niche factors in regulating the thermogenic function of brown and beige adipocytes and how their coordinated functions contribute to the adaptation of adipose depots to environmental stimuli (BOX 1).
Box 1 |. Different mechanisms for intercellular communication in the adipose tissue microenvironment.
Multicellular organisms have developed three main ways to establish intercellular communications over short or long distances. These include direct physical cell–cell contact between adjacent cells, ligand–receptor interactions, and extracellular vesicle (EV)-mediated cellular crosstalk. All three of these communication modes have been shown to mediate intercellular crosstalk in the adipose tissue microenvironment by integrating internal and external stimuli, which maintains tissue function and homeostasis. Direct cell–cell contacts between beige adipocytes are mediated by connexin 43 gap junction channels and are essential for propagation of sympathetic nerve signals in the sparsely innervated white adipose tissue215. Multiple ligand–receptor interactions between adipose-resident cells are discussed in this Review. EVs such as exosomes and microvesicles transport various cargoes, including proteins, lipids, DNA, RNA and other signalling molecules, between cells and tissues. For example, endothelial-derived EVs are taken up by adipocytes and are used to inform adipocytes about the systemic nutritional status216. Adipocyte-derived EVs have also been identified as a lipase-independent pathway for lipid transport to adipose tissue macrophages217. A 2021 study showed that mitochondria are transferred from adipocytes to macrophages in a heparan sulfate-dependent manner218. Although the mechanism involved in the transfer of mitochondria from adipocytes to macrophages is unclear, previous studies have identified EVs as a potential vehicle for intercellular transfer of mitochondria219. Thus, it is possible that adipocyte-derived EVs contain intact or fragmented mitochondria that are taken up by macrophages or other cells.
Neurons
BAT is innervated by an extensive network of sympathetic nerve projections that transmit signals from the central nervous system (CNS) to BAT, as well as afferent sensory neurons that convey inputs from BAT to the brain48. The sympathetic stimulation has a crucial role in BAT thermogenesis and energy expenditure through modulating noradrenaline production and secretion from sympathetic nerve terminals. In humans, the level of BAT innervation correlates with BAT activity49. In animals, cold exposure increases sympathetic activity in BAT and WAT by elevating the rate of noradrenaline turnover and increasing the density of sympathetic arborizations50,51. Dynamic communication between nerve fibres, brown adipocytes and other cell types in BAT enables the appropriate growth of neurites to provide the necessary network for noradrenaline-mediated activation of brown adipocytes and to transmit afferent signals from BAT to the CNS (FIG. 1).
Fig. 1 |. Cellular crosstalk between neurons and other adipose-resident cells.

Extensive communication between the neurons, thermogenic adipocytes and other cell types in adipose tissue is essential for brown adipose tissue (BAT) thermogenesis and cold-induced browning of white adipose tissue (WAT). The central nervous system (CNS) stimulates the release of neurotransmitters (pale blue), including noradrenaline (NE), neuropeptide Y (NPY) and ATP, from the sympathetic nerve terminals in BAT and WAT upon exposure to cold temperatures. Thermogenic adipocytes (here shown in beige and brown) release lipids and neurotrophic factors (pale yellow) that affect the growth, survival and function of sympathetic nerves in the adipose niche. These factors, whose production and release are stimulated by sympathetic nerve activity, promote the coordinated expansion of sympathetic innervation that is essential for maintaining tissue responsiveness to CNS signals. Both axon growth and angiogenic sprouting are regulated through a common array of attractive and repulsive cues. Nerve terminals release signalling molecules to guide and promote angiogenesis. Blood vessels also secrete factors (red) that attract and direct axons to innervate the vasculature. Sympathetic nerves regulate the development and function of immune cells by releasing neurotransmitters, such as NE and NPY. Several cytokines (grey) produced by adipose-resident immune cells also positively or negatively impact the survival and growth of sensory and sympathetic nerves. BMP, bone morphogenetic protein; NGF, nerve growth factor; NRG4, neuregulin 4; S100b, S100 calcium binding protein B; TGFβ1, transforming growth factor-β 1; VEGFA, vascular endothelial growth A factor.
Crosstalk between sympathetic nerves and adipocytes.
Sympathetic nerve terminals release factors such as noradrenaline, neuropeptide Y (NPY) and ATP to regulate adipocyte function48. Noradrenaline stimulates adipocytes mainly through activation of the β1–3-adrenergic receptors to promote lipolysis, increase the expression of thermogenic genes and facilitate adipose tissue remodelling52. Conversely, the secretion of NPY from sympathetic nerves antagonizes the function of noradrenaline and promotes adipogenesis and lipid accumulation53. Noradrenaline also increases the proliferation of adipocyte progenitors in BAT and pgWAT depots. This proliferative response seems to be considerably impaired in the absence of β1-adrenergic receptors24,54. However, the underlying molecular pathway leading to noradrenaline-induced proliferative responses has remained undefined.
Adipocytes produce fatty acids and other bioactive lipids, such as endocannabinoids, with potential effects on sympathetic nerves. Evidence from mouse models suggests that the endocannabinoid system has negative regulatory effects on adipose sympathetic innervation and thereby inhibits BAT thermogenesis and promotes WAT accumulation55. Whether these effects are mediated via direct action of endocannabinoids on sympathetic nerve activity in adipose tissue or through central mechanisms needs to be investigated.
Studies in mouse models have shown that, in addition to lipids, adipocytes secrete several neurotrophic factors including neuregulin 4 (REF.56), nerve growth factor57,58 and S-100 protein β-chain59 that promote neurite outgrowth. Furthermore, BMP8b secreted from adipocytes was shown to improve sympathetic innervation by upregulating neuregulin 4 expression in BAT and WAT in mice56. Cold exposure increases the expression of these neurotrophic factors in brown and beige adipocytes. Furthermore, loss or reduction in the expression of these factors is associated with impairment in BAT thermogenic capacity in mice, which results from reduced sympathetic innervation and activity56,58,59.
Crosstalk between sympathetic nerves and adipose vasculature.
The close anatomical and functional relationship between the vasculature and peripheral nerve fibres ensures the interrelated growth and remodelling of the neurovascular network in several tissues. Both axon growth and angiogenic sprouting are regulated through a common array of attractive and repulsive cues and a substantial overlap exists between the factors that direct these processes. Blood vessels secrete factors that attract and direct axons to innervate the vasculature. Conversely, nerves also release signalling molecules to guide and promote angiogenesis60.
Sympathetic activation of BAT in mice results in the rapid upregulation of vascular endothelial growth factor A (VEGFA) expression in brown and beige adipocytes61. Additionally, vascular cells also secrete VEGFA, which acts on the VEGFR2 receptor expressed on sympathetic nerves and promotes axon growth62. Transient overexpression of VEGFA in mouse WAT increases sympathetic innervation and promotes lipolysis, leading to WAT browning63.
Crosstalk between sympathetic nerves and immune cells.
Pro-inflammatory and anti-inflammatory cytokines produced by adipose-resident macrophages influence the survival and growth of sensory and sympathetic nerves. In conditions of chronic tissue inflammation, the accumulation of inflammatory cytokines might lead to the repulsion of sympathetic fibres and could even result in nerve damage64. Other evidence supporting the direct role of BAT-resident macrophages on sympathetic nerve activity emerged from a mouse model of macrophage-specific mutation in Mecp2, a gene mutated in the rare neurological disorder Rett syndrome. Mice lacking Mecp2 in CX3CR1-expressing macrophages gain more weight than their wild-type littermates on chow or high-fat diets. The macrophage-specific Mecp2-knockout mice show reduced BAT innervation and impaired thermogenesis65.
Sympathetic neurotransmitters such as noradrenaline and NPY have been shown to directly affect human immune cells66. Immune cells such as macrophages and T cells express the adrenergic receptor, suggesting the potential for direct communication axes between sympathetic nerves and immune cells67.
Vascular cells
BAT is one of the most vascularized tissues in the body68. Several external stimuli including cold, nutritional status, diet and exercise promote angiogenesis and vascular remodelling in adipose tissue. The reciprocal interactions between adipocytes and vascular cells are crucial for providing the optimal supply of oxygen, nutrients, hormones and other bioactive molecules for adipocytes.
In WAT, induction of angiogenesis is essential for the healthy expansion of adipose tissue. In individuals with obesity, the excessive and rapid expansion of WAT mass is usually not coordinated with the expansion of vasculature, resulting in tissue hypoxia and eventually leading to inflammation, fibrosis and insulin resistance. In mouse BAT, the triggering of angiogenic processes elevates thermogenesis and improves whole-body metabolism69.
Here we discuss the interactions between adipocytes, endothelial cells and other cell types in the adipose niche that have been shown to contribute to adipose function (FIG. 2).
Fig. 2 |. Cellular crosstalk between vasculature and other adipose-resident cells.

Accumulation of pro-angiogenic factors combined with reduced levels of angiogenic inhibitors in the adipose niche promotes the expansion and remodelling of the vascular network in response to thermogenic stimuli. Thermogenic adipocytes (shown in beige and brown) and their progenitors secrete several angiogenic factors (pale yellow) to guide vascular cells to expand, regress or remodel depending on the requirements of the adipose tissue microenvironment. Reciprocally, endothelial cells release factors (red), such as endothelin1 (EDN1) and nitric oxide (NO), that promote the thermogenic function of brown and beige adipocytes. EDN1 and platelet-derived growth factor C (PDGF-C) also regulate adipogenesis in progenitors. Pro-angiogenic and anti-angiogenic cytokines (grey) and growth factors secreted by immune cells regulate vasculature function and remodelling. ANGPT1, angiopoietin 1; FGF, fibroblast growth factor; HGF, hepatocyte growth factor; NPY, neuropeptide Y; TGFβ1, transforming growth factor-β 1; TNF, tumour necrosis factor; VEGF, vascular endothelial growth factor.
Cellular crosstalk between adipocytes and vascular cells.
Adipocytes secrete angiogenic factors including members of the VEGF family, angiopoietins (ANGPT1, ANGPT2) and hepatocyte growth factor (HGF)70.
In mice, VEGFA is the key factor responsible for the angiogenic response of BAT to cold exposure or β3-adrenergic stimulation71. VEGFA is a highly specific and potent angiogenic factor that promotes proliferation, migration and survival of vascular endothelial cells72. Specific overexpression of VEGFA in brown adipocytes enhances mitochondrial respiration and thermogenesis. This effect is associated with an increase in energy expenditure that protects mice against diet-induced obesity and improves their glucose and lipid metabolism69. Another member of the VEGF family, VEGFB, also has a role in adipose tissue vascular remodelling. VEGFB also acts on endothelial cells to enhance proliferation and fatty acid utilization73. Members of the VEGF family bind to two tyrosine kinase receptors, VEGFR1 and VEGFR2 (REF.72), and VEGF-induced angiogenesis is mediated through VEGFR2. By contrast, evidence from in vivo mouse studies suggests that VEGFR1 acts as a decoy receptor and limits the binding of VEGF ligands to VEGFR2 (REF.74). Together, these findings provide strong support for the contribution of the VEGF–VEGFR axis in the regulation of adipose tissue angiogenesis and thermogenic function. However, future studies are required to provide the mechanistic link between increased angiogenesis and the activation of the thermogenic programme in WAT.
Endothelial cells regulate adipocyte function through the secretion of endothelin 1 (EDN1) and nitric oxide. EDN1 inhibits differentiation of human and mouse adipocyte progenitors75. In mature adipocytes, EDN1 stimulates lipolysis through binding to endothelin receptor type A, but not endothelin receptor type B76. Nitric oxide triggers the relaxation of vascular smooth muscle to promote vasodilation77, which enables nutrients to influx into BAT, a process that is required for enhanced thermogenesis. Additionally, nitric oxide directly acts on brown and beige adipocytes to induce mitochondrial biogenesis and the thermogenesis process78.
Cellular crosstalk between adipocyte progenitors and vascular cells.
Adipocyte progenitors can also secrete several angiogenic factors, including VEGFA, HGF, fibroblast growth factor 1 (FGF1), FGF2, transforming growth factor-β1 (TGFβ1) and PDGFs70, to guide vascular cells to expand, regress or remodel depending on the requirements of the adipose tissue microenvironment. FGFs are suggested to indirectly modulate neovascularization and angiogenesis by inducing the production of other pro-angiogenic factors such as VEGFs and HGF79. PDGFs are ligands that bind to and signal through their cognate tyrosine kinase receptors (PDGFRA and PDGFRB)80. In addition to their role in the regulation of tissue vasculature, one study demonstrated the role of PDGFs in thermogenic adipocyte formation. Genetic deletion or pharmacological inhibition of PDGF-C in mice statistically significantly abrogated CL316,243-induced beige adipocyte formation in WAT, a phenotype that was rescued by overexpression of PDGF-C. Furthermore, PDGF-C treatment of both undifferentiated and differentiated PDGFRA-expressing progenitors induced thermogenic gene programme81. However, since the level of Pdgfra expression in adipocytes is much lower than in progenitors, in vivo PDGF-C probably acts on adipocyte progenitors to direct their differentiation towards beige adipocytes.
Cellular crosstalk between adipose immune cells and vasculature.
Adipose tissue immune cells secrete several cytokines and growth factors with pro-angiogenic and anti-angiogenic potential82. Macrophages can regulate angiogenic processes and this regulation plays a key part in wound healing and tissue repair83,84. Macrophage infiltration in adipose tissue has been shown to promote angiogenesis in humans and animal models through secretion of factors such as tumour necrosis factor, IL-8, WNT and PDGF85–87. Adipose tissue macrophages were also shown to be a major source of PDGF, which is at least partially responsible for hypoxia-induced angiogenesis observed in adipose tissues of obese mice86.
Adipose immune cells
Adipose tissue depots house a wide array of immune cells including macrophages, dendritic cells, lymphocytes, neutrophils, eosinophils and mast cells. These resident immune cells have crucial roles in the maintenance of adipose tissue homeostasis. Emerging evidence demonstrates that multiple adipose-resident immune cell types are dysregulated in obesity and are associated with its progression and related metabolic complications. Obesity-induced adipose inflammation, which occurs as a result of chronic nutritional overload, mediates insulin resistance in type 2 diabetes mellitus88.
Over the past decade, studies have found unexpected roles for multiple immune cells in the development and function of BAT and beige adipose tissue. Although recruitment of M1 macrophages and other inflammatory immune cells is associated with suppression of thermogenesis89, a type 2 immune response is shown to promote BAT activation and WAT browning in mice90–95.
M1 macrophages.
Macrophages that secrete pro-inflammatory cytokines and chemokines and mediate host defence against pathogens.
Type 2 immune response.
An immune response characterized by infiltration of alternatively activated (or M2) macrophages, eosinophils and innate lymphoid type 2 cells.
Here we focus on reviewing the role of different immune cells in regulating thermogenic adipocytes (FIG. 3).
Fig. 3 |. Cellular crosstalk between immune cells and other adipose-resident cells.

Adipose tissue-resident immune cells are major contributors to the adipose niche by playing key roles in both the function of brown adipose tissue (BAT) and white adipose tissue (WAT) and homeostasis through extensive cellular crosstalk with other cell types. M1 macrophages (dark blue) secrete pro-inflammatory cytokines (pale red) that impair insulin signalling and suppress thermogenesis in adipocytes. Conversely, a type 2 immune response, which is mediated by M2 macrophages (yellow), eosinophils (red), innate lymphoid type 2 cells (ILC2, grey) and invariant natural killer T-cells (iNKTs, green), enhances BAT activation and browning of WAT (factors involved in the type 2 responses are shown in pale blue). Thermogenic adipocytes (shown in beige and brown) regulate the type 2 immune response by releasing ADIPOQ, CXCL14, GDF15, METRNL and CCL11 (adipocyte-released factors are shown in pale yellow). A multicellular communication axis between sympathetic nerves, adipocytes, eosinophils, adipocyte progenitors and macrophages also orchestrates the adaptive response of WAT to cold. Methionine-enkephalin (MET-ENK) are IL2C-derived secreted peptides that induce uncoupling protein 1 expression in white adipocytes. Sympathetic neuron-associated macrophages (SAMs) mediate the clearance of extracellular noradrenaline (NE, grey) and thereby negatively regulate NE availability and the thermogenic activity of BAT and beige adipose tissue. ADIPOQ, adiponectin; CCL11, chemokine (C-C motif) ligand 11; CXCL14, C-X-C motif chemokine ligand 14; FGF, fibroblast growth factor; GDF15, growth differentiation factor 15; METRNL, meteorin-like glial cell differentiation regulator; PDGF, platelet-derived growth factor; TNF, tumour necrosis factor.
Crosstalk between immune cells, adipocytes and adipocyte progenitors.
At the onset of obesity, the release of pro-inflammatory cytokines from adipocytes combined with the presence of other stressors favours polarization of macrophages in WAT to a M1-like phenotype. The recruitment of these activated M1-like macrophages facilitates the infiltration of other inflammatory immune cells into the adipose depot, which further exacerbates chronic inflammation and impairs insulin-regulated adipocyte metabolism in obesity96. In mice, obesity is associated with increased expression of pro-inflammatory cytokines in BAT and the recruitment of several immune cell types, albeit with less intensity than in WAT97. Similar to the processes occurring in WAT, the pro-inflammatory environment in BAT in rodents and individuals with obesity disturbs glucose metabolism and causes insulin resistance in brown adipocytes98. Additionally, pro-inflammatory cytokines can directly suppress thermogenic gene expression and hamper thermogenic function in vitro and in vivo89,99. These findings led to the conclusion that obesity produces a self-sustained inflammatory response in adipose tissue that suppresses beige adipogenesis100.
Although M2 macrophages have been reported to contribute to sustaining adaptive thermogenesis, the specific mechanism remains to be elucidated. Loss of IL-4 and IL-13 cytokine signalling, which is required for alternative activation of M2 macrophages, impairs cold-induced BAT thermogenesis and WAT lipolysis in mice90,91. Myeloid cell-specific deletion of tyrosine hydroxylase, which is the rate-limiting enzyme of noradrenaline biosynthesis, decreased noradrenaline content in ingWAT of cold-acclimated mice, suggesting that alternatively activated M2 macrophages are a source of catecholamine in WAT91. However, these findings were not reproduced by another study using a mouse model of inducible adult-onset loss of tyrosine hydroxylase in myeloid lineage101. This study detected no tyrosine hydroxylase expression in the macrophage populations isolated from BAT or ingWAT either at room temperature or following cold exposure101. Although the reasons for the striking discrepancies between these studies remain unclear, the use of different animal models (congenital versus adult-onset) might partially explain the differences.
A 2017 study identified a population of sympathetic neuron-associated macrophages that mediate the clearance of extracellular noradrenaline and thereby negatively regulate noradrenaline availability and thermogenic activity of BAT and beige adipose tissue102. Consistent with this finding, another group observed a higher frequency of sympathetic neuron-associated macrophages in two mouse models of obesity, indicating the role of these macrophages in regulating adipose tissue function and energy balance102.
Several adipocyte-derived factors have been shown to contribute to promoting the M2 macrophage phenotype in adipose tissue. For example, CXCL14 is a chemokine secreted from brown adipocytes that induces macrophages to favour M2 polarization103. Cold exposure increases both expression and release of CXCL14 protein from BAT. CXCL14-null mice display a substantial reduction of the number of M2 macrophages in interscapular BAT and ingWAT, accompanied by reduced thermogenesis and impaired glucose tolerance. Furthermore, systemic administration of recombinant CXCL14 in these mice promotes recruitment of M2 macrophages to WAT and induces WAT browning103. Adiponectin is another adipokine that was found to promote the proliferation of M2 macrophages in ingWAT of mice upon cold exposure93. Additionally, in vitro treatment of RAW 264.7 macrophages with the conditioned medium from brown adipocytes repressed the expression of inflammatory genes, and knocking down GDF15 in brown adipocytes reduces the anti-inflammatory effects of the brown adipocyte secretome. These findings suggest that GDF15 is a potential anti-inflammatory brown adipokine104.
Eosinophils seem to be the major source of IL-4 in ingWAT of mice91,105. Eosinophil-derived IL-4 acts through two key mechanisms to promote beige adipocyte formation. First, IL-4 induces alternative activation of macrophages through the secretion of type 2 cytokines within the adipose niche. For example, meteorin-like (METRNL) is a hormone that was found to induce the thermogenic programme in ingWAT through a mechanism that involves eosinophil-derived IL-4 and alternative activation of macrophages. Blocking IL4–IL13 signalling in mice impaired METRNL-induced thermogenesis and β-oxidation gene programmes106. Future investigations are required to identify the physiologically relevant METRNL-producing cell type(s) inside or outside adipose tissue.
The second mechanism by which eosinophils and IL-4 signalling regulate adipose thermogenesis is through the induction of proliferation and commitment of adipocyte precursors into beige adipocytes92,94. Genetic mouse models lacking eosinophils, IL-4 and IL-13, or IL-4Rα show a considerable reduction in both number and proliferation rate of PDGFRA+ adipocyte progenitors in ingWAT. Intriguingly, IL4Rα-mediated signalling in PDGFRA+ adipocyte progenitors, and not in myeloid cells, is responsible for the effects of IL-4 on the expansion of adipocyte progenitor pool and beige adipogenesis92. Consistent with these findings, adipocyte-derived FGF21 was shown to induce WAT browning through CCL11-mediated recruitment of eosinophils, which in turn secrete IL-4 and promote proliferation and adipogenic commitment of PDGFRA+ adipocyte progenitors94.
Innate lymphoid type 2 cells (ILC2s) are another group of adipose-resident immune cells that are the major source of IL-5 and IL-13, which promote the recruitment and accumulation of eosinophils and alternatively activated M2 macrophages107. Upon activation with cytokines IL-33, IL-25 and thymic stromal lymphopoietin, ILC2s produce IL-5, IL-13 and IL-9, and initiate a type 2 immune response108. Studies have revealed that ILC2s also directly regulate beige adipocyte formation by regulating adipocyte precursor numbers and fate92,95. In a study in which IL-33 was administered to activate ILC2s in mice housed under thermoneutral conditions, ILC2-derived IL-13 and eosinophil-derived IL-4 were observed to stimulate the proliferation of adipocyte progenitors in ingWAT and increased the expression of beige markers CD137 and TMEM26 (REF.38). The effects of IL-4 and IL-13 on the expansion of beige adipocyte progenitors required IL-4Ra expression on PDGFRA+ progenitors, indicating that these cytokines act directly on adipocyte progenitors to increase WAT browning92. Future studies should address the cellular source and pathways involved in endogenous IL-33 production in adipose tissue, which could potentially be targeted as a therapeutic strategy to induce beige adipose tissue expansion to counteract obesity. ILC2s also produce methionine–enkephalin peptides that enhance UCP1 expression by acting directly on adipocytes and inducing browning of ingWAT in vivo95.
Another population of lymphocytes implicated in a 2020 study of the regulation of BAT thermogenesis in mice are γδ T cells109. The pro-thermogenic function of γδ T cells is mediated by IL-17F release from γδ T cells and interaction with the receptor IL-17RC on brown adipocytes. Perturbing this signalling axis causes defects in BAT innervation and impaired adaptive thermogenesis. TGFβ1 has been identified as the secreted factor downstream of the IL-17F–IL-17RC pathway that communicates with sympathetic nerves to promote neurite growth and expand BAT innervation109 (FIG. 1). Finally, B220+ B cells, CD4+ αβ T cells and M2 macrophages in ingWAT in mice have been shown to produce acetylcholine, which acts through the CHRNA2 (cholinergic receptor nicotinic α2-subunit) on beige adipocytes to induce the thermogenic response110.
Autocrine and paracrine factors
In addition to the interactions between adipocytes and other cell types within adipose tissue, adipocytes release multiple factors (proteins111–122, lipids123,124 and metabolites125) into their niche that act in an autocrine and/or paracrine manner to regulate the thermogenic function of brown and/or beige adipocytes (FIG. 4). The thermogenic adipocyte-derived secreted factors include members of the BMP–TGFβ superfamily122, FGF families of growth factors113,120, the secreted SLIT2 fragments118, bradykinin121, the secreted enzyme peptidase M20 domain containing 1 (PM20D1)117, ependymin-related protein 1 (EPDR1)119, adenosine126, and lipokines 12,13-diHOME123,127 and 12-HEPE124, as well as others. Some of them also mediate inter-organ communications. Using single-nucleus RNA sequencing and functional studies, a population of adipocytes were identified that express CYP2E1 and ALDH1A1 that can secrete acetate to repress the thermogenic activity of neighbouring adipose cells125. The role of these autocrine and/or paracrine factors in adipose tissue has been extensively reviewed elsewhere128,129. Thus, we only highlight the key factors regulating adipocyte thermogenesis in FIG. 4.
Fig. 4 |. Autocrine and paracrine factors regulating brown and beige adipocytes.
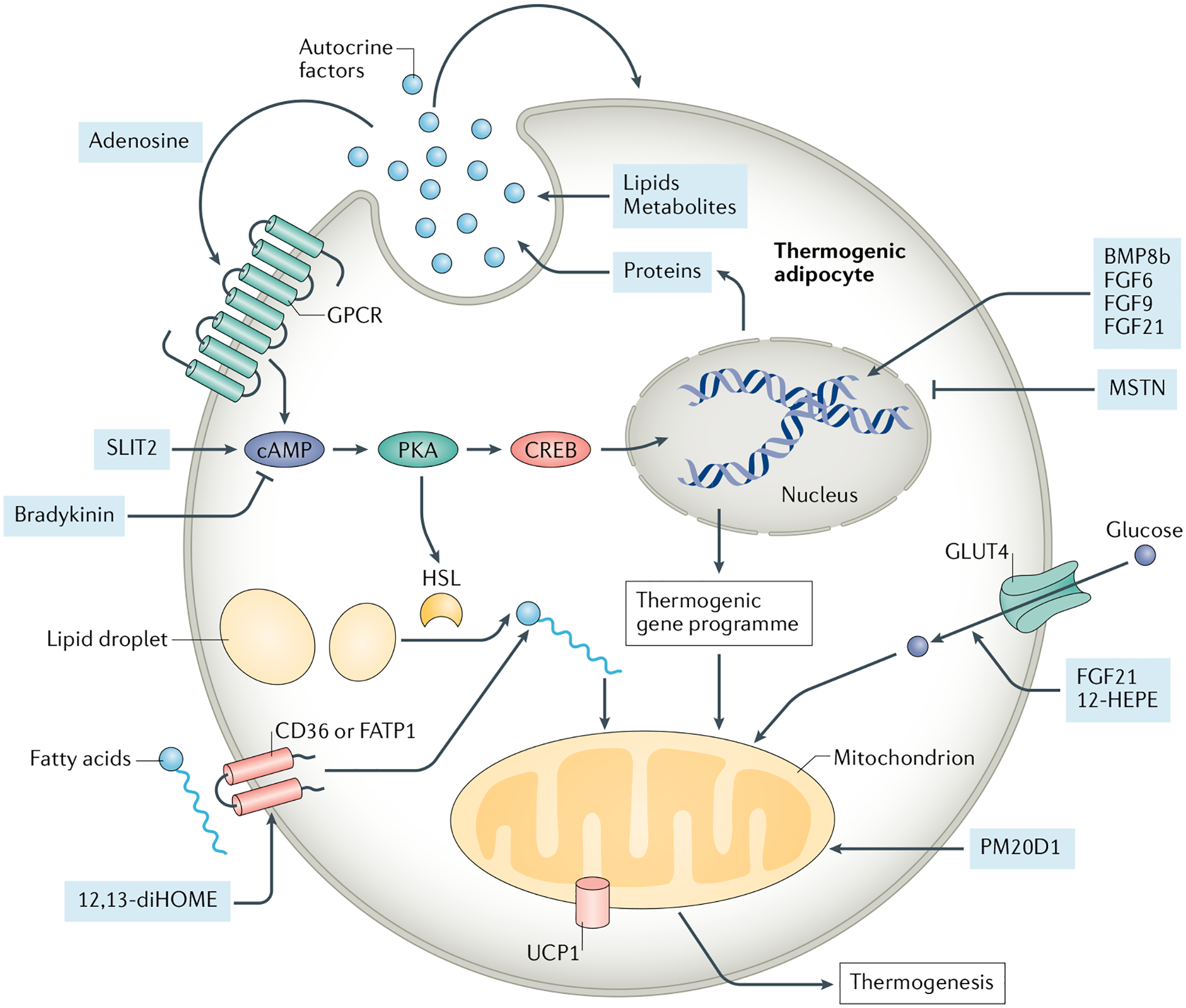
Several factors released by brown and beige adipocytes function in an autocrine and/or paracrine manner to regulate nutrient utilization and thermogenic gene expression in adipocytes. The secreted factors (pale blue circles and boxes) include members of bone morphogenetic protein (BMP) and transforming growth factor-β (TGFβ), such as BMP8b, myostatin (MSTN), fibroblast growth factor (FGF) families of growth factors (for example, FGF6, FGF9 and FGF21), bradykinin, SLIT2, PM20D1, adenosine and others. Additionally, thermogenic adipocyte-derived lipokines, such as 12,13-diHOME and 12-HEPE, promote fatty acid and glucose uptake to adipocytes, thereby controlling key aspects of fuel utilization and energy metabolism in brown adipose tissue and white adipose tissue. CREB, cAMP responsive element binding protein; FATP1, fatty acid transport protein 1; GPCR, G protein-coupled receptor; HSL, hormone-sensitive lipase; PKA, protein kinase A; PM20D1, the secreted enzyme peptidase M20 domain containing 1. UCP1, uncoupling protein 1.
Inter-organ communication
Although the beneficial anti-obesity effects of BAT have been traditionally attributed to its high metabolic rate and fuel utilization capacity, in the past 10 years studies have highlighted the secretory role of BAT. Some of these adipocyte-released factors act locally and others can target distant organs that express the corresponding receptors for the ligands. Notably, certain factors can target both BAT and other organs. Some of these interactions could trigger positive or negative feedback loops that further modulate BAT-mediated thermogenesis. Here we review the current understanding of the endocrine BAT and/or beige adipose tissue-derived factors (batokines) and their roles in systemic metabolism. We also discuss factors secreted from other organs that modulate functions of thermogenic adipocytes (FIG. 5).
Fig. 5 |. Inter-organ communication of thermogenic adipose tissue and other tissues.

Thermogenic adipose tissue (shown here as beige and brown adipocytes) is recognized as an endocrine organ, which can secrete several factors (pale yellow) to regulate the gene expression or functions in distant organs, such as the heart, liver, muscle and white adipose tissue. These organs, as well as the brain and gut, can also communicate to thermogenic adipose tissue through the secretion of endocrine factors (pale blue) in response to different stimulations. This inter-organ communication highlights the importance of thermogenic adipose tissue in whole-body metabolism. ACTH, adrenocorticotropic hormone; BAIBA, β-aminoisobutyric acid; EPDR1, ependymin-related protein 1; EVs, extracellular vesicles; FGF, fibroblast growth factor; GCs, glucocorticoids; GLP1, glucagon-like peptide 1; METRNL, meteorin-like glial cell differentiation regulator; NE, noradrenaline; NPs, natriuretic peptides; NRG4, neuregulin 4; PM20D1, peptidase M20 domain containing 1; SLIT2, slit guidance ligand 2; TGFβ2, transforming growth factor-β2; TSH, thyroid-stimulating hormone.
BAT–WAT communication
Fatty acids are key contributors to the thermogenesis process in BAT and beige adipose tissue by serving both as fuel for thermogenesis and stimulators of UCP1 function. Studies have demonstrated that in the absence of food or BAT lipolysis, fatty acids released from WAT are indispensable for BAT thermogenesis. Blocking lipolysis in BAT and beige adipose tissue in mice by genetic ablation of Atgl (that encodes an enzyme that catalyses the initial step in triglyceride hydrolysis)130 or CGI-58 (that encodes an activator of ATGL)131 in UCP1-expressing cells did not impair cold-induced thermogenesis. A 2019 study showed that TGFβ2 protein is upregulated and secreted from subcutaneous WAT of mice after exercise training. Transplantation of WAT from trained mice to untrained mice promotes glucose uptake in many tissues, including endogenous BAT, through secretion of TGFβ2 (REF.132).
BAT–brain communication
Within adipose tissue, BAT-secreted factors can target sympathetic and sensory nerves133 to promote neurite projection and activity. In addition to these local effects, the BAT–brain communication axis regulates systemic energy balance and food intake by informing the CNS of the energetic status of the body.
Besides the well-known sympathetic signalling pathway, several central actions mediated by hormones also contribute to BAT thermogenesis. For example, cold exposure stimulates the hypothalamus to release TSH-releasing hormone, which stimulates the pituitary to release TSH that acts on the thyroid to induce thyroid hormone secretion; thyroid hormone, T3 and/or T4, then directly drives BAT thermogenesis by induction of UCP1 (REF.134). T3 can also act on the hypothalamus to regulate WAT browning, lipid oxidation in BAT and hepatic lipogenesis135. Mechanistically, T3 suppress AMPK signalling in the ventromedial nucleus of the hypothalamus to modulate peripheral metabolism via activation of the parasympathetic nervous system and sympathetic nervous system (SNS).
BMP8b122 and oestrogens also contribute to energy expenditure via this hypothalamus–SNS–BAT axis. The central action of oestradiol decreases ceramide-induced lipotoxicity and oestrogen receptor stress in mice136. Moreover, the neurons in the arcuate nucleus of the hypothalamus contribute to WAT browning. Coordination of leptin and insulin signalling in pro-opiomelanocortin (POMC)-expressing neurons, the anorexigenic (appetite-suppressing) neurons, promote WAT browning in mice137; however, activation of O-GlcNAc signalling in AgRP orexigenic (appetite-inducing) neurons upon fasting in mice suppresses WAT browning to conserve energy138.
Glucocorticoids are a type of corticosteroid that are synthesized in the adrenal cortex. In rodents, glucocorticoids have been demonstrated to suppress the expression of UCP1 in brown adipocytes139,140; however, a 2019 study demonstrated that glucocorticoid-induced obesity is independent of the decrease in UCP1-mediated thermogenesis in mice141. Interestingly, glucocorticoids seem to exert opposite effects in humans. For example, acute administration of the synthetic glucocorticoid predniso-lone promoted glucose uptake and increases skin temperatures in the supraclavicular BAT region of human volunteers during mild cold exposure (16–17 °C)142. Similarly, in human brown adipocytes, glucocorticoids increased isoprenaline-induced UCP1 expression and mitochondrial respiration142. Further studies are needed to investigate the mechanisms underlying the observed differences between humans and mouse models.
BAT–heart communication
Increasing evidence indicates an association between reduced BAT activity and cardiovascular abnormalities in humans and mice143–145. For example, a mouse model with loss of Ucp1-expressing cells is prone to developing obesity on a normal chow diet but also displays deleterious cardiovascular phenotypes such as cardiomyopathy, fibrosis and hypertension143. Genetic ablation of Ucp1 in mice also results in cardiac hypertrophy and adverse echocardiographic function in response to stimulation. Consistently, transplantation of normal BAT into Ucp1−/− mice provides cardioprotective effects144. In 2021, a large retrospective study demonstrated that the presence of BAT is associated with a low prevalence of cardiometabolic diseases and improved blood glucose and lipid content in humans, including individuals with obesity145. The authors of this study proposed that BAT mediates cardiovascular health, at least partially, through the release of some potential cardiovascular regulators.
The direct causal relationship between BAT-secreted FGF21 and cardiovascular function was established in a study in hypertensive mice146. Activation of adenosine receptor A2aR signalling in hypertensive rats ameliorated hypertension-related cardiac hypertrophy126. Notably, in a chemical-induced hypertension mouse model, A2aR activation in BAT promoted FGF21 release and improved heart performance. Specific blockage of FGF21 secretion from BAT attenuates the recovery from heart injury in the mice, indicating crosstalk between BAT and the heart through FGF21 (REF.146).
Factors produced by the heart could regulate BAT activity. Cold exposure increases secretion of B-type natriuretic peptides from the heart into the circulation147. Natriuretic peptide treatment in mouse and human adipocytes promotes mitochondrial biogenesis, thermogenic gene expression and induces lipolysis in brown and beige adipocytes via the cGMP–PKG–p38 signalling pathway147.
BAT–liver communication
Enhanced fatty acid utilization by activated BAT results in fatty acid clearance from the circulation, which potentially improves the ectopic deposition of lipids in the liver148,149. Emerging evidence also indicates the presence of direct crosstalk between brown and/or beige adipocytes and hepatocytes through batokines (such as NRG4 (REF.148), adiponectin149 and FGF21 (REF.150)). MicroRNAs (miRNAs) are packaged into extracellular vesicles (EVs), thereby providing a potent and targeted way for miRNA shuttling between organs, as EVs can maintain miRNA stability and enable specific delivery to certain tissues via surface ligands or receptors151. One study in rodents152 demonstrated that BAT serves as a major source of circulating miRNA in EVs. Furthermore, miRNAs in BAT-derived EVs regulate FGF21 gene expression and secretion in the liver. Of note, the levels of circulating EVs are considerably reduced in mice with severe lipodystrophy, and BAT transplantation from wild-type mice rescues the levels of most miRNAs in EVs152.
FGF21 mediates a bidirectional communication axis between BAT and the liver. Liver-derived FGF21 has a role in the activation of thermogenesis in mouse neonatal BAT153. Moreover, cold exposure in mice was found to stimulate the liver to produce acylcarnitines, which can be delivered to BAT to act as substrates to facilitate UCP1-dependent uncoupling respiration and heat production154. The action of CPT1B in generating acylcarnitines from free fatty acids is usually inhibited by elevated levels of malonyl-CoA in brown adipocytes upon cold exposure154. Thus, the rate-limiting step of fatty acid oxidation is bypassed by using liver-derived acylcarnitines as fuel, which is more efficient for thermogenesis.
Beyond their role in lipid absorption of the intestine, bile acids also serve as signalling molecules to regulate lipid metabolism and energy expenditure in BAT. Bile acids bind to the G protein-coupled receptor (GPCR) TGR5 in brown adipocytes, and induce cAMP-dependent activation of type 2 iodothyronine deiodinase and thermogenesis155. Genetic deletion of hepatic Na+ taurocholate co-transporting polypeptide in mice inhibits the clearance of bile acids from plasma, which increases the circulating levels of bile acids and protects the mice against diet-induced obesity by promoting BAT function156. Similarly, the bile acid–TGR5 axis is also involved in subcutaneous WAT browning in mice after cold exposure or high-fat diet feeding157. In humans, oral administration of the bile acid chenodeoxycholic acid elevates whole-body energy expenditure and glucose uptake in BAT158.
BAT–skeletal muscle communication
Increasing evidence shows that exercise triggers major adaptations in BAT and WAT, including BAT activation, remodelling of WAT depots and subcutaneous WAT browning159. Other than changes in energetic demands, these adaptations are thought to result from altered secretion of muscle-derived factors (myokines) that affect adipose tissue function. Additionally, in 2018, two studies identified the cold or exercise-induced batokines that can influence skeletal muscle function127,160.
12,13-diHOME is a cold-induced and exercise-induced batokine in mice and humans123,127. In addition to enhancing fatty acid utilization in BAT123, 12,13-diHOME can also promote fatty acid uptake and oxidation in myocytes127. Moreover, a BAT–muscle crosstalk axis was identified that is mediated by myostatin secretion from BAT160. Loss of the transcription factor IRF4 in BAT of mice induces myostatin expression and secretion in BAT, resulting in reduced exercise capacity, impaired mitochondrial function and several other abnormalities in skeletal muscles. Furthermore, the authors of this study observed a similar increase in myostatin expression in BAT and circulating levels of myostatin when housing mice at thermoneutrality as compared with room temperature, which also led to decreased exercise capacity160.
Accumulating evidence suggests that the exercise-induced myokine IL-6 is required for exercise-induced BAT activity and WAT browning, based on studies using an IL-6-deficient mouse model161,162. Moreover, daily injection of IL-6 in mice for a week stimulated UCP1 induction in BAT and beige adipose tissue162. Of note, IL-6 is also a batokine161,163. For example, acute psychological stress in rodents was demonstrated to induce IL-6 secretion from BAT via β3-adrenergic signalling. This effect anticipates adaptation of fight or flight responses by promoting hepatic gluconeogenesis, but also reducing tolerance to inflammation163. In addition, exercise-induced increases in circulating METRNL were found to improve glucose tolerance and energy expenditure in mice via the promotion of BAT and/or beige adipose tissue activity and the induction of anti-inflammatory cytokines106. Conversely, blocking METRNL actions through neutralizing antibodies attenuates the exercise-induced thermogenesis response and M2 macrophage activation upon exercising in mice106. Other exercise-induced myokines (including irisin164, lactate132 and β-aminoisobutyric acid165) have also been found to promote the activity of BAT and beige adipose tissue. These findings indicate that mutual communication between BAT and skeletal muscle maintains the balance between energy utilization and storage depending on the physiological demands.
BAT–gut communication
The gastrointestinal tract (gut) has been recognized for its role in diet-induced thermogenesis through secreted factors from intestinal cells that trigger the gut–brain–BAT axis or directly activate the gut–BAT axis. Moreover, an increasing number of studies have demonstrated the roles of gut microbiota in whole-body metabolism of the host via the pleiotropic effects of microbial metabolites.
Glucagon-like peptide 1 (GLP1) is a peptide hormone that is secreted from intestinal enteroendocrine L cells. GLP1 not only enhances glucose-stimulated insulin secretion in β-cells but also activates BAT thermogenesis. Meal-induced thermogenesis is generally believed to be induced through GLP1-mediated regulation of efferent sympathetic innervation in BAT by modulating AMPK activation in the hypothalamus in rodent models166. A 2018 study showed a novel gut–BAT–brain axis involving secretin, which is secreted by the duodenum. Prandial increases in the release of secretin result in its direct binding to the secretin receptor in BAT, which leads to the activation of lipolysis and thermogenesis. BAT, in turn, relays unknown signals to the brain to suppress food intake167. In humans, the level of circulating secretin after a meal is correlated with energy expenditure and fatty acid uptake167. Administration of secretin substantially promotes glucose uptake in human neck BAT167,168.
The gut microbiota produces metabolites, nutrients and vitamins in a dynamic manner169 and has been linked with the activities of BAT and WAT. Germ-free mice or mice with microbiota depletion display increased lipolysis in BAT170 and browning of subcutaneous and visceral WAT depots171. By contrast, antibiotic-induced microbiota depletion in mice impaired the thermogenic function of BAT and reduced WAT browning172. These conflicting observations might result from the differences in the compositions of the antibiotic cocktail and the duration of treatment used in these studies. Of note, the composition of gut microbiota substantially changes upon cold exposure. Transplantation of the microbiome from cold-induced mice improved BAT function173 and WAT browning174 in recipient mice, leading to improvements in insulin sensitivity and cold tolerance. Furthermore, intermittent fasting in mice alters the composition of the microbiota, which activates the function of BAT, thereby contributing in part to the anti-obesity effects of intermittent fasting175.
Therapeutic applications
Given the role of BAT and beige adipose tissue in energy expenditure and modulation of glucose and lipid metabolism, and its crosstalk to other tissues, targeting thermogenic adipose tissue provides a promising therapeutic approach to combatting obesity and metabolic dis orders. Several studies have demonstrated that increasing the amount and activity of BAT improves metabolism in mouse models of obesity or diabetes mellitus176. However, rodent and human thermogenic adipose tissue depots are different in their anatomical locations and amounts relative to total body weight, as well as the molecular basis and developmental origin of the tissue. It is generally believed that merely increasing the level of UCP1 expression or the amount of thermogenic adipose tissue might not be sufficient to drive thermogenesis; constant activation signals are also required. From the translational point of view, we focus here on the therapeutic potential of activating thermogenic adipose tissue in humans and/or converting human white adipocytes into thermogenic adipocytes through pharmacological, gene-based or cell-based therapies (FIG. 6).
Fig. 6 |. Cell-based, gene-based and pharmacological therapies for activation and conversion of thermogenic adipose tissue.
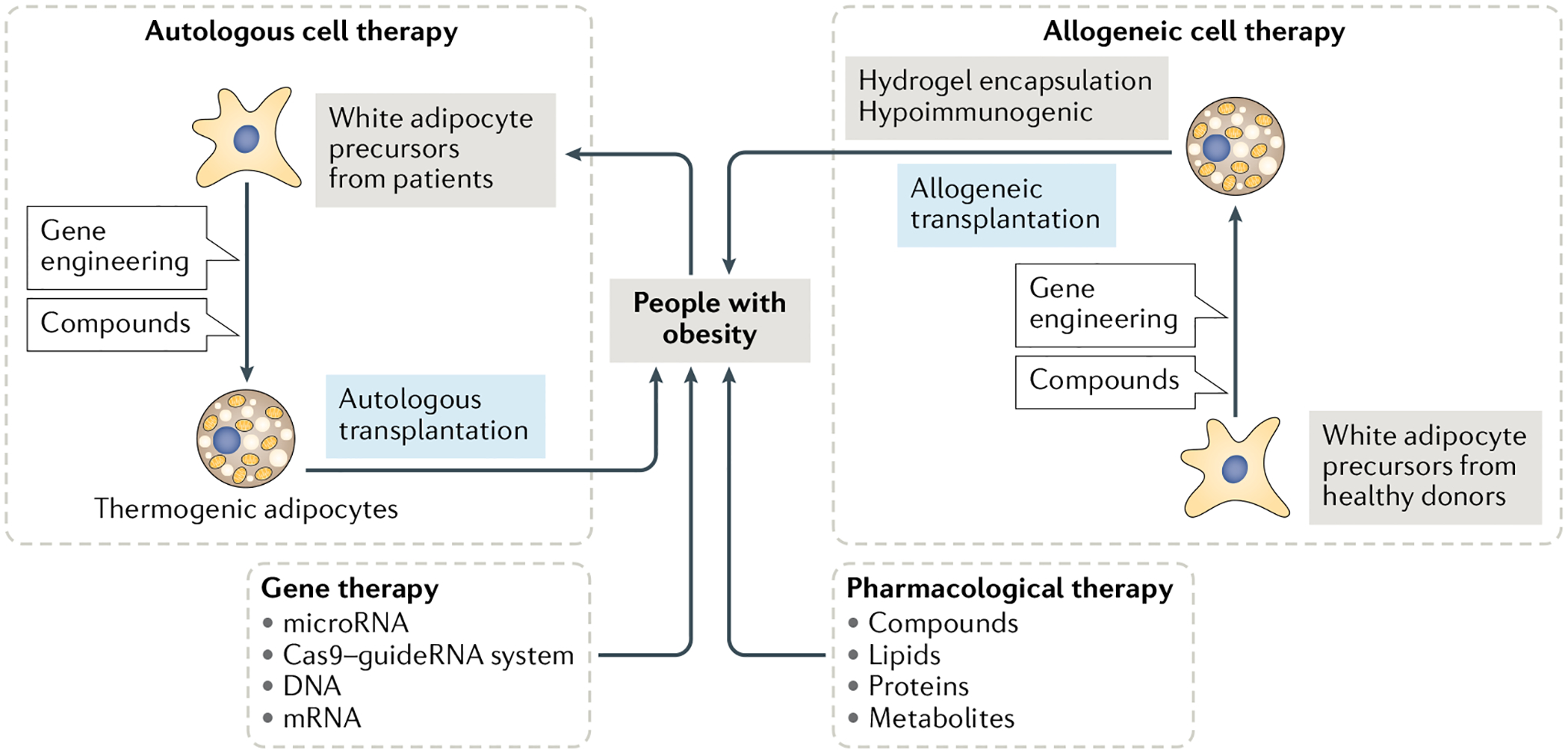
In pharmacological therapies, several compounds, proteins, lipids or metabolites have been applied for treating obesity in human clinical trials through the regulation of thermogenic adipose tissue activity and energy expenditure. In gene therapy, the delivery of DNA (transgene), mRNA, microRNA or Cas9–guideRNA systems is used for increasing the expression of genes involved in the thermogenic pathway. Targeted delivery into thermogenic adipose tissue or white adipose tissue increases efficiency and specificity and minimizes the adverse effects on other cell types. Cell-based therapies include autologous and allogeneic cell therapy according to the source of the transplanted cells. In autologous cell therapy, precursor cells isolated from an individual with obesity can be engineered and differentiated to thermogenic adipose tissue, followed by transplanting back to the same individual. Allogeneic cell therapy uses precursor cells from healthy donors. Although this strategy overcomes the burden of cell source, preventing transplanted cells from immune rejection needs to be considered using hydrogel encapsulation or reducing immunogenicity before allogeneic cell transplantation into the recipients. Although gene-based and cell-based therapy have not yet been applied for treating obesity in humans, these might be potential strategies.
Pharmacological approaches
Exposure to cold is a potent stimulus activating BAT function and inducing the conversion of WAT to beige adipose tissue through stimulating β-adrenergic signalling in both humans and mice. However, cold exposure is inconvenient, uncomfortable and ultimately an unrealistic intervention in many cases. Besides, cold exposure might induce other unwanted effects such as an increase in blood pressure177. Therefore, much effort has been invested in identifying more specific, potent pharmacological products with minimized adverse consequences (TABLE 1).
Table 1 |.
Pharmacological therapies targeting activation of thermogenic adipose tissue in humans
| Type of therapy | Compound | Manipulation | Study participants | Effects | Refs |
|---|---|---|---|---|---|
| β3-Adrenergic agonist | Mirabegron | Oral administration: 200 mg, one dose | Healthy lean men | Higher BAT activity than in placebo- treated individuals; increased energy expenditure | (NCT01950520)178,179 |
| Oral administration: 50 mg or 200 mg, one dose | Healthy lean men | Compared with pretreatment: no change in BAT activity with 50 mg dose; higher BAT activity with 200 mg dose | (NCT02811289)180 | ||
| Oral administration: 100 mg per day for 4 weeks | Healthy lean women | Higher BAT activity than pretreatment; increased energy expenditure | (NCT03049462)181 | ||
| Oral administration: 50 mg per day for 12 weeks | People with obesity | Activation of WAT beiging; improved insulin sensitivity; improved β-cell function | (NCT02919176)182 | ||
| Capsaicinoids | Capsinoids | Oral administration: 10 mg/kg per day for 4 weeks | People with obesity | Increased energy expenditure | 184 |
| Oral administration: 6 mg per day for 12 weeks | People with obesity | Increased fat oxidation; no change in energy expenditure | 185 | ||
| Oral administration: 9 mg, one dose | Healthy lean men | Higher BAT activity than in placebo- treated individuals; increased energy expenditure | 186 | ||
| Thyroid hormones | Levothyroxine | Oral administration: 137.75 μg per day for 24 weeks | Patients with thyroidectomy | Higher BAT activity than pretreatment; increased energy expenditure | (NCT02499471)190 |
| Liothyronine | Oral administration: 570 ng/kg per day for 2 weeks | Patients with insulin receptor mutation | Increased glucose disposal | (NCT02457897)191 | |
| GLP1 | GLP1 | Intravenous infusion: 50 pmol/kg per h for 4 h | Healthy lean men | Decreased energy expenditure | 195 |
| Exenatide | Subcutaneous injection: 10 μg twice per day for 24 weeks | People with obesity but without diabetes mellitus | Decreased body weight and food intake; no change in energy expenditure | (NCT00856609)196 |
BAT, brown adipose tissue; GLP1, glucagon-like peptide 1; WAT, white adipose tissue.
Mirabegron.
Mirabegron is a β3-adrenergic receptor agonist approved by the FDA for treating overactive bladder by relaxing the bladder muscles and ameliorating the symptoms. Acute, single-dose mirabegron treatment (200 mg) increased BAT glucose uptake and energy expenditure in healthy humans178,179. Unfortunately, this dosage was associated with the adverse effect of elevated blood pressure, which explains why dosages higher than 50 mg per day are prohibited for clinical use. A 2020 study in humans found that 200 mg of mirabegron activates BAT, yet 50 mg cannot, although both doses increase energy expenditure in humans180. In two 2020 studies, healthy human volunteers and individuals with obesity were treated with lower doses of mirabegron, 100 mg for 4 weeks181 and 50 mg for 12 weeks182, respectively. This dosage leads to activation of thermogenic adipose tissue, reduced inflammation in adipose tissues and skeletal muscle, improvements in glucose tolerance, insulin sensitivity and pancreatic β-cell function, with few adverse effects on cardiovascular function181,182.
Capsaicinoids.
Capsaicinoids are a group of phenolic alkaloids and the active components in chili peppers183. Interest in using capsaicinoids is growing due to their potential anti-obesity effects. Compared with control individuals who received placebo, oral supplementation of capsaicinoids in individuals with overweight or obesity induced adipose tissue loss by increasing fatty acid oxidation and elevating resting energy expenditure184,185 that was correlated with enhanced glucose uptake in human BAT186. Capsaicin acts on TRPV1, which is primarily expressed in afferent sensory neurons and arterial smooth muscle within skeletal muscle, heart and adipose tissues187 and its activation leads to elevation of intracellular calcium levels and downstream signalling. In mice, TRPV1 deletion completely blocks the anti-obesity effects mediated by capsaicin188. In addition, in a 2016 study, the combination of capsaicinoid treatment and mild cold exposure (17 °C) synergistically promoted beige adipocyte development and weight loss in mice189. This finding provides a potential therapeutic regimen combining dietary and environmental modifications in mammals.
Thyroid hormone analogues.
As discussed above, thyroid hormones, T3 and/or T4, activate thermogenic functioning in the BAT of mice. In a longitudinal study in patients with thyroid carcinoma after thyroidectomy, substitutional treatment with levothyroxine (a T4 analogue) was associated with increased basal metabolic rate and increased glucose uptake by BAT (NCT02499471)190. In addition, patients with severe insulin resistance caused by mutations of the insulin receptor showed improved glucose uptake in WAT and muscle after administration of liothyronine (a T3 analogue) for 6 months. However, glucose uptake by BAT was not quantified in that study (NCT02457897)191. Notably, two independent studies reported in 2019 found that BAT thermogenesis is dispensable for thyroid hormone-induced energy expenditure192,193, to which the main contributor might be skeletal muscle194.
GLP1 agonists.
As discussed above, GLP1 activates BAT thermogenesis via the brain in mice166, whereas it seems to have an opposite effect in humans. GLP1 infusion in healthy men has been reported to decrease diet-induced thermogenesis due to a reduction in food absorption and by limiting the nutrients supplied by food195. Another study demonstrated that subcutaneous injection of exenatide, a GLP1 agonist, considerably decreased energy intake with no change in the resting energy expenditure in people with obesity (NCT00856609)196. Considering the profound effects on the gut, and other systemic effects of GLP1, an ongoing clinical trial aims to investigate the specific effects of SAR425899, a novel GLP1 agonist, through direct injection into subcutaneous WAT of people with obesity (NCT03376802).
Gene-based therapy
Gene-based therapy is a technique in which genetic material is introduced into the target cells or tissues to permanently or transiently manipulate gene expression for the correction of mutations or treatment of diseases. Selective genes have been targeted in vitro and in animal models to activate BAT function and promote WAT browning. For example, the activation of UCP1-mediated thermogenesis provides an efficient way to dissipate excess energy and consume fuels to provide metabolic health benefits197.
Mice that ectopically express UCP1 in adipose tissue198 display increased energy expenditure and are protected from diet-induced obesity. Several studies have demonstrated that overexpression of UCP1 in non-thermogenic human cells enables uncoupling respiration from mitochondrial biogenesis199,200. However, the potential leakage of the mitochondrial membrane that results from unsuitable incorporation of extremely high levels of UCP1 should be taken into consideration. Introduction of upstream transcriptional regulators such as PRDM16 or PPARGC1A induces UCP1 transcription and drives the conversion of white adipocytes to beige adipocytes in mice176 and in human cells in vitro201. Furthermore, introducing CEBPB and PRDM16 transgenes into human inducible pluripotent stem cells (iPSCs) or CEBPB and MYC into human dermal fibroblasts, induces the formation of lipid-laden brown adipocytes202. Of note, engineering fibroblasts to become human brown adipocytes seems to provide a more convenient and potent system than using iPSCs, the use of which also raises a safety issue due to the use of an oncogene such as MYC. A 2020 study showed that a long intergenic non-coding RNA that is induced in adipocytes by adrenergic signalling stimulates transcription of mitochondrial oxidative metabolism genes, lipolysis and respiration in human adipocytes203. This regulatory process might provide another strategy for gene-based BAT activation.
Several new tools have been developed based on CRISPR–Cas9 that enable targeted inhibition or activation of gene expression, or the insertion of a transgene into genomic DNA. One study takes advantage of the CRISPR–Cas9 method to replace a truncated UCP1 gene with a UCP1 transgene driven using an adiponectin promoter into endogenous loci in pigs. Pigs with the reconstituted UCP1 gene became leaner and displayed improved cold tolerance204. Of note, the expression and activity of UCP1 must be physiologically regulated, and not constitutively activated, which would exhaust energy. A 2018 study showed the development of CRISPR delivery particles (CriPs), which are Cas9–single guide RNA ribonucleoprotein (RNP) complexes coated with amphipathic peptides, enabling more efficient gene editing in primary mouse white pre-adipocytes205. CriP-mediated deletion of nuclear receptor-interacting protein 1 (NRIP1) successfully promoted browning of white adipocytes in vitro. NRIP1 is a co-repressor known to negatively regulate UCP1 expression. However, further in vivo investigation is required to reveal the therapeutic potential of targeting NRIP1. In summary, gene therapy performed in human adipocytes or stem cells is a potential therapeutic approach in humans, after appropriate safety evaluations.
Cell-based therapy
In clinical applications, the unlimited resource provided by cells renders stem cell therapy a more feasible strategy than tissue transplantation. Considering the limited amount of BAT and brown adipocyte progenitor cells that are present in adult humans, the approaches of using white adipocyte progenitors or even fewer committed stem cells seem more applicable (BOX 2).
Box 2 |. Cell-based therapies.
Cell-based therapies offer treatment strategies for patients and diseases that existing pharmaceuticals cannot adequately address. One potential benefit of a cell-based approach, compared to strategies based around single molecules, is that the cells serve as a ‘living factory’ to provide a more comprehensive and persistent therapeutic effect.
Autologous cell therapy
Cells are taken from an individual and administered back into the same individual to minimize the possibility of immune rejection. Autologous cell-based therapy is a preferred therapeutic intervention and has been an active area of research. This approach is moving towards successful commercial development and patient access partially due to the advances in the delivery systems and genome engineering methods220,221 in the past 10 years.
Allogeneic cell therapy
In older people (aged ≥65 years) or patients with lipodystrophy, the limited number of adipocytes does not allow autologous cell therapy. In these cases, progenitors derived from healthy individuals provide an alternative for allogeneic cell therapy. Strategies to minimize immune rejection should be considered in individuals undergoing allogeneic transplantation. Hydrogel-mediated encapsulation has been applied to minimize the immune response and maintain the transplant–host communication for transplanted cells222. Moreover, engineering iPSCs in order to generate hypoimmunogenic iPSCs, followed by differentiation, can enable immune escape in immunocompetent allogeneic recipients223.
Overexpression of PRDM16 and CEBPB, two important factors involved in thermogenic differentiation, stimulated fibroblasts to differentiate into adipocytes and form an ectopic adipose pad with BAT-like characteristics after transplantation into mice. These differentiated brown-like adipocytes have been shown to be functional and able to take up glucose206. Alternatively, other groups have used pharmacological treatments to induce thermogenic differentiation in stem cells. For example, one method was established to differentiate human PSCs to brown adipocytes using a haemopoietin cocktail, which was composed of KIT ligand, Fms-related tyrosine kinase 3 ligand, IL-6, VEGF and BMP7 (REF.207). Upon subcutaneous transplantation into mice, these brown-like adipocytes were stimulated with β-adrenergic receptor agonists and behaved similarly to endogenous classic brown adipocytes. Alongside the contribution to energy expenditure by transplants themselves, batokines secreted from transplants might add to the regulation of whole-body metabolism161,208,209.
Another group isolated adipocyte progenitors residing in the capillary network of human WAT and defined an in vitro protocol using forskolin to differentiate them into beige adipocytes. After implanting them into the subcutaneous dorsal region of immuno-deficient NOD-SCID IL2rg-null (NSG) mice, human beige adipocytes can enhance glucose utilization and improve glucose tolerance when the mice are fed a high-fat diet208. More recently, in 2020, a CRISPR synergistic activator was used to establish human brown-like (HUMBLE) adipocytes via overexpressing UCP1 in human white adipocytes. HUMBLE cells acquired brown adipocyte-like features and provided long-term metabolic benefits in nude mice fed a high-fat diet after subcutaneous transplantation209,210.
Of note, different transplantation studies have identified distinct secreted factors mediating the activation of endogenous thermogenic adipose and/or other tissues and the modulation of systemic metabolism. The differences can be attributed to the use of different species (mice or humans), different transplants (whole tissues, beige cells or brown-like cells), and different transplantation sites (visceral cavity or subcutaneous). Each study examined a unique condition that generates certain signals contributing to the wide range of effects and phenotypes reported. Collectively, these investigations enabled us to gain a comprehensive understanding of the potential of cell-based therapy approaches to tackle obesity and metabolic diseases.
Conclusions
Brown and beige adipocytes arise from distinct origins during embryonic development; however, they share similar effects in thermoregulation and nutrient utilization. The thermogenic adipose niche has an essential role in shaping adipose tissue function and homeostasis. Secreted factors from brown and/or beige adipocytes regulate adipocytes and other cell types within the adipose niche and control systemic metabolism through long-range communications with other organs. Scientific advances have shed light on both intercellular interactions within adipose tissue and inter-organ communications at the systemic level. The knowledge learned, when combined with genetic, pharmacological and cell-based tools, as well as biocompatible delivery systems, promises to lead to effective therapeutics to meet the ever-growing demand for preventing and treating obesity, type 2 diabetes mellitus and related metabolic disorders.
Although multiple therapeutic strategies targeting BAT and beige adipose tissue have been shown to work well in rodent models, most of these tools are not applicable to humans. A notable knowledge gap exists in the translational application from mice to humans, especially considering the differences in BAT between the two species. For example, a 2020 study showed that thermogenesis in human BAT is driven by the β2-adrenergic receptor, not by the β3-adrenergic receptor, which is the dominant isoform in adipose tissue of mice180; however, β3-adrenergic receptor agonists can activate BAT in humans as noted above. One group also claimed that the β1-adrenergic receptor is the predominant adrenergic receptor and contributes to the function of human BAT211. In addition, to avoid unwanted adverse effects of pharmacological therapy on other tissues, targeted delivery of drugs to adipose tissues would offer a promising solution (BOX 3).
Box 3 |. Targeted delivery.
In development of therapeutics using either genetic or pharmacological approaches, tissue-specific and cell type-specific targeting strategies are greatly desired. Improving targeting specificity would enable reductions in effective dosages and off-target effects and would improve cost effectiveness and safety of treatment. Customization of gene expression with the cell-specific or inducible promoters would allow selective genetic manipulation in a spatiotemporal manner. Another strategy would be to engineer the designated surface receptors or ligands on nanoparticles, carriers or Cas9 protein to be selectively delivered to certain cells via specific binding. To achieve targeted delivery of white adipose tissue, two adipose vasculature targeting peptides, iRGD and P3, have been conjugated to the membrane of lipid nanoparticles and have delivered either a PPARγ activator or a prostaglandin E2 analogue to induce WAT browning224.
To mimic human conditions in mice, studies were performed in middle-aged mice housed under thermoneutral conditions (30 °C) and fed with a diet containing 45% fat. These studies concluded that classic BAT obtained from mice subjected to this humanized physiological condition is similar to human BAT in terms of cellular, molecular and morphological characteristics212. The concept of using environmental and dietary cues in mouse models, instead of inserting human genes to establish humanized mice, provides a system mimicking the current obesogenic human lifestyle for metabolic studies, especially for BAT metabolism, which is highly regulated by temperature and diet. Although this manipulation aimed to create a ‘humanized’ condition in mice, issues related to the heterogeneity of human BAT, and the origin and identity of thermogenic adipose tissue, distinguish humanized mouse models and humans213,214. In addition, considering the complexity and crosstalk of different cell types within BAT and beige adipose tissue, using human adipose organ-oids as platforms to develop a therapeutic strategy might shorten the gaps of translational medicine.
Regarding therapeutic approaches that aim to increase the amount or activity of thermogenic adipose tissue, besides conventional pharmacological interventions, cell-based and gene therapies also offer feasible therapeutic options. Autologous cell therapy is considered a safer and minimally invasive approach compared with conventional treatments because it reduces the risk of rejection and provides longer lasting effects after a single administration. Gene therapy using the viral delivery system has been applied in many non-metabolic diseases due to its high efficacy. However, unintended genome integration, high immunogenicity and safety issues associated with gene delivery need to be addressed. Other non-insertional genetic approaches, such as microRNA-based or mRNA-based medicine, which are associated with a low risk of permanent genomic alteration, might be more applicable in humans. Nevertheless, future research on the compatibility of such approaches to target adipose tissue is warranted. In conclusion, the current advances in fundamental knowledge and new technologies hold promise for beginning to fully harness the therapeutic potential of thermogenic adipose tissue to combat metabolic diseases.
Key points.
Brown adipocytes and white adipocytes in different depots originate from distinct progenitor pools in the embryonic mesoderm.
Beige adipocytes are formed in white adipose tissue in response to cold acclimation, exercise training or pharmacological activation of β-adrenergic receptors through reprogramming of white adipocytes or de novo differentiation from adipocyte progenitors.
Adipose tissue plasticity enables the rapid adaptation of the organism to fluctuations in nutritional load and metabolic demand and is a hallmark of metabolic health.
Adipose-resident cell types establish an extensive network of cellular crosstalk to coordinate depot-specific functions and remodelling.
Communication between thermogenic adipose tissue and distant organs enables the regulation of systemic metabolism beyond thermogenesis.
Targeting thermogenic adipose tissue offers a potential therapeutic strategy to combat obesity and metabolic disorders, such as type 2 diabetes mellitus and cardiovascular diseases.
Acknowledgements
The authors acknowledge the support of NIH grants R01DK077097, R01DK102898 and R01DK122808 (to Y.-H.T.), and P30DK036836 (to Joslin Diabetes Center’s Diabetes Research Center, DRC) from the National Institute of Diabetes and Digestive and Kidney Diseases, and of US Army Medical Research grant W81XWH-17-1-0428 (to Y.-H.T.). F.S. acknowledges the support of an American Diabetes Association Postdoctoral Fellowship #1-18-PDF-169 and NIH K01DK125608. C.-H.W. acknowledges the support of a Postdoctoral Research Abroad Program (106-2917-I-564-069) and a grant (MOST 110-2320-B-039-063-MY3) from the Ministry of Science and Technology, Taiwan.
Footnotes
Competing interests
Y.-H.T. is an inventor on US Patent 7,576,052 related to BMP7 and US patent applications related to 12,13-diHOME and FGF6/9. The other authors declare no competing interests.
References
- 1.Peres Valgas da Silva C, Hernandez-Saavedra D, White JD & Stanford KI Cold and exercise: therapeutic tools to activate brown adipose tissue and combat obesity. Biology 8, 9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cannon B & Nedergaard J Brown adipose tissue: function and physiological significance. Physiol. Rev 84, 277–359 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Bartelt A et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med 17, 200–205 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Scheja L & Heeren J The endocrine function of adipose tissues in health and cardiometabolic disease. Nat. Rev. Endocrinol 15, 507–524 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Villarroya F, Cereijo R, Villarroya J & Giralt M Brown adipose tissue as a secretory organ. Nat. Rev. Endocrinol 13, 26–35 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Cypess AM et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med 360, 1509–1517 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leitner BP et al. Mapping of human brown adipose tissue in lean and obese young men. Proc. Natl Acad. Sci. USA 114, 8649–8654 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Marken Lichtenbelt WD et al. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med 360, 1500–1508 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Virtanen KA et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med 360, 1518–1525 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Saito M et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58, 1526–1531 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poher AL, Altirriba J, Veyrat-Durebex C & Rohner-Jeanrenaud F Brown adipose tissue activity as a target for the treatment of obesity/insulin resistance. Front. Physiol 6, 4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peirce V & Vidal-Puig A Regulation of glucose homoeostasis by brown adipose tissue. Lancet Diabetes Endocrinol 1, 353–360 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Flemming W Ueber Bildung und Rückbildung der Fettzelle im Bindegewebe, und Bemerkungen über die Structur des Letzteren. Arch. Mikrosk. Anat 7, 32–80 (1871). [Google Scholar]
- 14.Billon N et al. The generation of adipocytes by the neural crest. Development 134, 2283–2292 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sgaier SK et al. Morphogenetic and cellular movements that shape the mouse cerebellum; insights from genetic fate mapping. Neuron 45, 27–40 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Lang D et al. Pax3 functions at a nodal point in melanocyte stem cell differentiation. Nature 433, 884–887 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Lepper C & Fan CM Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis 48, 424–436 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seale P et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 454, 961–967 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Timmons JA et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc. Natl Acad. Sci. USA 104, 4401–4406 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosen ED & Spiegelman BM What we talk about when we talk about fat. Cell 156, 20–44 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez-Gurmaches J et al. PTEN loss in the Myf5 lineage redistributes body fat and reveals subsets of white adipocytes that arise from Myf5 precursors. Cell Metab 16, 348–362 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sebo ZL, Jeffery E, Holtrup B & Rodeheffer MS A mesodermal fate map for adipose tissue. Development 145, dev166801 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cameron IL & Smith RE Cytological responses of brown fat tissue in cold-exposed rats. J. Cell Biol 23, 89–100 (1964). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee YH, Petkova AP, Konkar AA & Granneman JG Cellular origins of cold-induced brown adipocytes in adult mice. FASEB J 29, 286–299 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shamsi F et al. Vascular smooth muscle-derived Trpv1+ progenitors are a source of cold-induced thermogenic adipocytes. Nat. Metab 3, 485–495 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jespersen NZ et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab 17, 798–805 (2013). [DOI] [PubMed] [Google Scholar]
- 27.Barbatelli G et al. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am. J. Physiol. Endocrinol. Metab 298, E1244–E1253 (2010). [DOI] [PubMed] [Google Scholar]
- 28.Rosenwald M, Perdikari A, Rulicke T & Wolfrum C Bi-directional interconversion of brite and white adipocytes. Nat. Cell Biol 15, 659–667 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Wang QA, Tao C, Gupta RK & Scherer PE Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med 19, 1338–1344 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shao M et al. Zfp423 maintains white adipocyte identity through suppression of the beige cell thermogenic gene program. Cell Metab 23, 1167–1184 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shao M et al. Cellular origins of beige fat cells revisited. Diabetes 68, 1874–1885 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jespersen NZ et al. Heterogeneity in the perirenal region of humans suggests presence of dormant brown adipose tissue that contains brown fat precursor cells. Mol. Metab 24, 30–43 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vishvanath L et al. Pdgfrβ+ mural preadipocytes contribute to adipocyte hyperplasia induced by high-fat-diet feeding and prolonged cold exposure in adult mice. Cell Metab 23, 350–359 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang W et al. White fat progenitor cells reside in the adipose vasculature. Science 322, 583–586 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Long JZ et al. A smooth muscle-like origin for beige adipocytes. Cell Metab 19, 810–820 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang Y, Berry DC, Tang W & Graff JM Independent stem cell lineages regulate adipose organogenesis and adipose homeostasis. Cell Rep 9, 1007–1022 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berry DC, Jiang Y & Graff JM Mouse strains to study cold-inducible beige progenitors and beige adipocyte formation and function. Nat. Commun 7, 10184 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu J et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xue R et al. Clonal analyses and gene profiling identify genetic biomarkers of the thermogenic potential of human brown and white preadipocytes. Nat. Med 21, 760–768 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang W et al. Ebf2 is a selective marker of brown and beige adipogenic precursor cells. Proc. Natl Acad. Sci. USA 111, 14466–14471 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rajakumari S et al. EBF2 determines and maintains brown adipocyte identity. Cell Metab 17, 562–574 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwalie PC et al. A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature 559, 103–108 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Merrick D et al. Identification of a mesenchymal progenitor cell hierarchy in adipose tissue. Science 364, eaav2501 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burl RB et al. Deconstructing adipogenesis induced by β3-adrenergic receptor activation with single-cell expression profiling. Cell Metab 28, 300–309.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun K, Kusminski CM & Scherer PE Adipose tissue remodeling and obesity. J. Clin. Invest 121, 2094–2101 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geloen A, Collet AJ, Guay G & Bukowiecki LJ Beta-adrenergic stimulation of brown adipocyte proliferation. Am. J. Physiol 254, C175–C182 (1988). [DOI] [PubMed] [Google Scholar]
- 47.Bukowiecki LJ, Geloen A & Collet AJ Proliferation and differentiation of brown adipocytes from interstitial cells during cold acclimation. Am. J. Physiol 250, C880–C887 (1986). [DOI] [PubMed] [Google Scholar]
- 48.Guilherme A, Henriques F, Bedard AH & Czech MP Molecular pathways linking adipose innervation to insulin action in obesity and diabetes mellitus. Nat. Rev. Endocrinol 15, 207–225 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muzik O, Mangner TJ, Leonard WR, Kumar A & Granneman JG Sympathetic innervation of cold-activated brown and white fat in lean young adults. J. Nucl. Med 58, 799–806 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang H, Ding X, Cao Y, Wang H & Zeng W Dense intra-adipose sympathetic arborizations are essential for cold-induced beiging of mouse white adipose tissue. Cell Metab 26, 686–692.e3 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Murano I, Barbatelli G, Giordano A & Cinti S Noradrenergic parenchymal nerve fiber branching after cold acclimatisation correlates with brown adipocyte density in mouse adipose organ. J. Anat 214, 171–178 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Collins S β-adrenoceptor signaling networks in adipocytes for recruiting stored fat and energy expenditure. Front. Endocrinol 2, 102 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang W, Cline MA & Gilbert ER Hypothalamus-adipose tissue crosstalk: neuropeptide Y and the regulation of energy metabolism. Nutr. Metab 11, 27 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bronnikov G, Houstek J & Nedergaard J Beta-adrenergic, cAMP-mediated stimulation of proliferation of brown fat cells in primary culture. Mediation via beta 1 but not via beta 3 adrenoceptors. J. Biol. Chem 267, 2006–2013 (1992). [PubMed] [Google Scholar]
- 55.Quarta C et al. CB(1) signaling in forebrain and sympathetic neurons is a key determinant of endocannabinoid actions on energy balance. Cell Metab 11, 273–285 (2010). [DOI] [PubMed] [Google Scholar]
- 56.Pellegrinelli V et al. Adipocyte-secreted BMP8b mediates adrenergic-induced remodeling of the neurovascular network in adipose tissue. Nat. Commun 9, 4974 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nechad M, Ruka E & Thibault J Production of nerve growth factor by brown fat in culture: relation with the in vivo developmental stage of the tissue. Comp. Biochem. Physiol. Comp. Physiol 107, 381–388 (1994). [DOI] [PubMed] [Google Scholar]
- 58.Nisoli E, Tonello C, Benarese M, Liberini P & Carruba MO Expression of nerve growth factor in brown adipose tissue: implications for thermogenesis and obesity. Endocrinology 137, 495–503 (1996). [DOI] [PubMed] [Google Scholar]
- 59.Zeng X et al. Innervation of thermogenic adipose tissue via a calsyntenin 3β-S100b axis. Nature 569, 229–235 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carmeliet P & Tessier-Lavigne M Common mechanisms of nerve and blood vessel wiring. Nature 436, 193–200 (2005). [DOI] [PubMed] [Google Scholar]
- 61.Asano A, Morimatsu M, Nikami H, Yoshida T & Saito M Adrenergic activation of vascular endothelial growth factor mRNA expression in rat brown adipose tissue: implication in cold-induced angiogenesis. Biochem. J 328, 179–183 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marko SB & Damon DH VEGF promotes vascular sympathetic innervation. Am. J. Physiol. Heart Circ. Physiol 294, H2646–H2652 (2008). [DOI] [PubMed] [Google Scholar]
- 63.Zhao Y et al. Transient overexpression of vascular endothelial growth factor A in adipose tissue promotes energy expenditure via activation of the sympathetic nervous system. Mol. Cell Biol 38, e00242–18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pongratz G & Straub RH The sympathetic nervous response in inflammation. Arthritis Res. Ther 16, 504 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wolf Y et al. Brown-adipose-tissue macrophages control tissue innervation and homeostatic energy expenditure. Nat. Immunol 18, 665–674 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sung CP, Arleth AJ & Feuerstein GZ Neuropeptide Y upregulates the adhesiveness of human endothelial cells for leukocytes. Circ. Res 68, 314–318 (1991). [DOI] [PubMed] [Google Scholar]
- 67.Rached MT et al. Deletion of myeloid IRS2 enhances adipose tissue sympathetic nerve function and limits obesity. Mol. Metab 20, 38–50 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brakenhielm E & Cao Y Angiogenesis in adipose tissue. Methods Mol. Biol 456, 65–81 (2008). [DOI] [PubMed] [Google Scholar]
- 69.Sun K et al. Brown adipose tissue derived VEGF-A modulates cold tolerance and energy expenditure. Mol. Metab 3, 474–483 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cao Y Angiogenesis and vascular functions in modulation of obesity, adipose metabolism, and insulin sensitivity. Cell Metab 18, 478–489 (2013). [DOI] [PubMed] [Google Scholar]
- 71.Xue Y et al. Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell Metab 9, 99–109 (2009). [DOI] [PubMed] [Google Scholar]
- 72.Neufeld G, Cohen T, Gengrinovitch S & Poltorak Z Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 13, 9–22 (1999). [PubMed] [Google Scholar]
- 73.Hagberg CE et al. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature 464, 917–921 (2010). [DOI] [PubMed] [Google Scholar]
- 74.Koch S, Tugues S, Li X, Gualandi L & Claesson-Welsh L Signal transduction by vascular endothelial growth factor receptors. Biochem. J 437, 169–183 (2011). [DOI] [PubMed] [Google Scholar]
- 75.Bhattacharya I & Ullrich A Endothelin-1 inhibits adipogenesis: role of phosphorylation of Akt and ERK1/2. FEBS Lett 580, 5765–5771 (2006). [DOI] [PubMed] [Google Scholar]
- 76.van Harmelen V et al. Vascular peptide endothelin-1 links fat accumulation with alterations of visceral adipocyte lipolysis. Diabetes 57, 378–386 (2008). [DOI] [PubMed] [Google Scholar]
- 77.Rapoport RM, Draznin MB & Murad F Endothelium-dependent relaxation in rat aorta may be mediated through cyclic GMP-dependent protein phosphorylation. Nature 306, 174–176 (1983). [DOI] [PubMed] [Google Scholar]
- 78.Bossy-Wetzel E & Lipton SA Nitric oxide signaling regulates mitochondrial number and function. Cell Death Differ 10, 757–760 (2003). [DOI] [PubMed] [Google Scholar]
- 79.Murakami M & Simons M Fibroblast growth factor regulation of neovascularization. Curr. Opin. Hematol 15, 215–220 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fredriksson L, Li H & Eriksson U The PDGF family: four gene products form five dimeric isoforms. Cytokine Growth Factor. Rev 15, 197–204 (2004). [DOI] [PubMed] [Google Scholar]
- 81.Seki T et al. Endothelial PDGF-CC regulates angiogenesis-dependent thermogenesis in beige fat. Nat. Commun 7, 12152 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aharonov O, Maftzir G & Benezra D The role of cytokines in angiogenesis. Ocul. Immunol. Inflamm 1, 135–142 (1993). [DOI] [PubMed] [Google Scholar]
- 83.Lucas T et al. Differential roles of macrophages in diverse phases of skin repair. J. Immunol 184, 3964–3977 (2010). [DOI] [PubMed] [Google Scholar]
- 84.Brancato SK & Albina JE Wound macrophages as key regulators of repair: origin, phenotype, and function. Am. J. Pathol 178, 19–25 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Newman AC & Hughes CC Macrophages and angiogenesis: a role for Wnt signaling. Vasc. Cell 4, 13 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pang C et al. Macrophage infiltration into adipose tissue may promote angiogenesis for adipose tissue remodeling in obesity. Am. J. Physiol. Endocrinol. Metab 295, E313–E322 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stefater JA III et al. Macrophage Wnt-calcineurin-Flt1 signaling regulates mouse wound angiogenesis and repair. Blood 121, 2574–2578 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu J, Zhao J, Meng H & Zhang X Adipose tissue-resident immune cells in obesity and type 2 diabetes. Front. Immunol 10, 1173 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sakamoto T et al. Macrophage infiltration into obese adipose tissues suppresses the induction of UCP1 level in mice. Am. J. Physiol. Endocrinol. Metab 310, E676–E687 (2016). [DOI] [PubMed] [Google Scholar]
- 90.Nguyen KD et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 480, 104–108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Qiu Y et al. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 157, 1292–1308 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee MW et al. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell 160, 74–87 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hui X et al. Adiponectin enhances cold-induced browning of subcutaneous adipose tissue via promoting M2 macrophage proliferation. Cell Metab 22, 279–290 (2015). [DOI] [PubMed] [Google Scholar]
- 94.Huang Z et al. The FGF21-CCL11 axis mediates beiging of white adipose tissues by coupling sympathetic nervous system to type 2 immunity. Cell Metab 26, 493–508.e4 (2017). [DOI] [PubMed] [Google Scholar]
- 95.Brestoff JR et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 519, 242–246 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu R & Nikolajczyk BS Tissue immune cells fuel obesity-associated inflammation in adipose tissue and beyond. Front. Immunol 10, 1587 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Villarroya F, Cereijo R, Gavalda-Navarro A, Villarroya J & Giralt M Inflammation of brown/beige adipose tissues in obesity and metabolic disease. J. Intern. Med 284, 492–504 (2018). [DOI] [PubMed] [Google Scholar]
- 98.Lorenzo M et al. Insulin resistance induced by tumor necrosis factor-α in myocytes and brown adipocytes. J. Anim. Sci 86, E94–E104 (2008). [DOI] [PubMed] [Google Scholar]
- 99.Goto T et al. Proinflammatory cytokine interleukin-1β suppresses cold-induced thermogenesis in adipocytes. Cytokine 77, 107–114 (2016). [DOI] [PubMed] [Google Scholar]
- 100.Chung KJ et al. A self-sustained loop of inflammation-driven inhibition of beige adipogenesis in obesity. Nat. Immunol 18, 654–664 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fischer K et al. Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nat. Med 23, 623–630 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pirzgalska RM et al. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat. Med 23, 1309–1318 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cereijo R et al. CXCL14, a brown adipokine that mediates brown-fat-to-macrophage communication in thermogenic adaptation. Cell Metab 28, 750–763.e6 (2018). [DOI] [PubMed] [Google Scholar]
- 104.Campderros L et al. Brown adipocytes secrete GDF15 in response to thermogenic activation. Obesity 27, 1606–1616 (2019). [DOI] [PubMed] [Google Scholar]
- 105.Wu D et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332, 243–247 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rao RR et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 157, 1279–1291 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Molofsky AB et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J. Exp. Med 210, 535–549 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Herbert DR, Douglas B & Zullo K Group 2 innate lymphoid cells (ILC2): type 2 immunity and helminth immunity. Int. J. Mol. Sci 20, 2276 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hu B et al. ɣδ T cells and adipocyte IL-17RC control fat innervation and thermogenesis. Nature 578, 610–614 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jun H et al. An immune-beige adipocyte communication via nicotinic acetylcholine receptor signaling. Nat. Med 24, 814–822 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zamani N & Brown CW Emerging roles for the transforming growth factor-β superfamily in regulating adiposity and energy expenditure. Endocr. Rev 32, 387–403 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tseng YH et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 454, 1000–1004 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fisher FM et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes. Dev 26, 271–281 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Keipert S et al. Genetic disruption of uncoupling protein 1 in mice renders brown adipose tissue a significant source of FGF21 secretion. Mol. Metab 4, 537–542 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lebrasseur NK Building muscle, browning fat and preventing obesity by inhibiting myostatin. Diabetologia 55, 13–17 (2012). [DOI] [PubMed] [Google Scholar]
- 116.Braga M et al. Follistatin promotes adipocyte differentiation, browning, and energy metabolism. J. Lipid Res 55, 375–384 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Long JZ et al. Ablation of PM20D1 reveals N-acyl amino acid control of metabolism and nociception. Proc. Natl Acad. Sci. USA 115, E6937–E6945 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Svensson KJ et al. A Secreted Slit2 fragment regulates adipose tissue thermogenesis and metabolic function. Cell Metab 23, 454–466 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Deshmukh AS et al. Proteomics-based comparative mapping of the secretomes of human brown and white adipocytes reveals EPDR1 as a novel batokine. Cell Metab 30, 963–975.e7 (2019). [DOI] [PubMed] [Google Scholar]
- 120.Shamsi F et al. FGF6 and FGF9 regulate UCP1 expression independent of brown adipogenesis. Nat. Commun 11, 1421 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Peyrou M et al. The kallikrein-kinin pathway as a mechanism for auto-control of brown adipose tissue activity. Nat. Commun 11, 2132 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Whittle AJ et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell 149, 871–885 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lynes MD et al. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat. Med 23, 631–637 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Leiria LO et al. 12-Lipoxygenase regulates cold adaptation and glucose metabolism by producing the omega-3 lipid 12-HEPE from brown fat. Cell Metab 30, 768–783.e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sun W et al. snRNA-seq reveals a subpopulation of adipocytes that regulates thermogenesis. Nature 587, 98–102 (2020). [DOI] [PubMed] [Google Scholar]
- 126.da Silva JS et al. Adenosine A2A receptor agonist prevents cardiac remodeling and dysfunction in spontaneously hypertensive male rats after myocardial infarction. Drug Des. Devel Ther 11, 553–562 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stanford KI et al. 12,13-diHOME: an exercise-induced lipokine that increases skeletal muscle fatty acid uptake. Cell Metab 27, 1357 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Scheele C & Wolfrum C Brown adipose crosstalk in tissue plasticity and human metabolism. Endocr. Rev 41, 53–65 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Villarroya J et al. New insights into the secretory functions of brown adipose tissue. J. Endocrinol 243, R19–R27 (2019). [DOI] [PubMed] [Google Scholar]
- 130.Schreiber R et al. Cold-induced thermogenesis depends on ATGL-mediated lipolysis in cardiac muscle, but not brown adipose tissue. Cell Metab 26, 753–763.e7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shin H et al. Lipolysis in brown adipocytes is not essential for cold-induced thermogenesis in mice. Cell Metab 26, 764–777.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Takahashi H et al. TGF-β2 is an exercise-induced adipokine that regulates glucose and fatty acid metabolism. Nat. Metab 1, 291–303 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Henningsen JB & Scheele C Brown adipose tissue: a metabolic regulator in a hypothalamic cross talk? Annu. Rev. Physiol 83, 279–301 (2021). [DOI] [PubMed] [Google Scholar]
- 134.Lopez M, Alvarez CV, Nogueiras R & Dieguez C Energy balance regulation by thyroid hormones at central level. Trends Mol. Med 19, 418–427 (2013). [DOI] [PubMed] [Google Scholar]
- 135.Martinez-Sanchez N et al. Hypothalamic AMPK-ER Stress-JNK1 axis mediates the central actions of thyroid hormones on energy balance. Cell Metab 26, 212–229.e12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gonzalez-Garcia I et al. Estradiol regulates energy balance by ameliorating hypothalamic ceramide-induced ER stress. Cell Rep 25, 413–423.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dodd GT et al. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell 160, 88–104 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ruan HB et al. O-GlcNAc transferase enables AgRP neurons to suppress browning of white fat. Cell 159, 306–317 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Viengchareun S, Penfornis P, Zennaro MC & Lombes M Mineralocorticoid and glucocorticoid receptors inhibit UCP expression and function in brown adipocytes. Am. J. Physiol. Endocrinol. Metab 280, E640–E649 (2001). [DOI] [PubMed] [Google Scholar]
- 140.Soumano K et al. Glucocorticoids inhibit the transcriptional response of the uncoupling protein-1 gene to adrenergic stimulation in a brown adipose cell line. Mol. Cell Endocrinol 165, 7–15 (2000). [DOI] [PubMed] [Google Scholar]
- 141.Luijten IHN et al. Glucocorticoid-induced obesity develops independently of UCP1. Cell Rep 27, 1686–1698.e5 (2019). [DOI] [PubMed] [Google Scholar]
- 142.Ramage LE et al. Glucocorticoids acutely increase brown adipose tissue activity in humans, revealing species-specific differences in UCP-1 regulation. Cell Metab 24, 130–141 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cittadini A et al. Cardiovascular abnormalities in transgenic mice with reduced brown fat: an animal model of human obesity. Circulation 100, 2177–2183 (1999). [DOI] [PubMed] [Google Scholar]
- 144.Thoonen R et al. Functional brown adipose tissue limits cardiomyocyte injury and adverse remodeling in catecholamine-induced cardiomyopathy. J. Mol. Cell Cardiol 84, 202–211 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Becher T et al. Brown adipose tissue is associated with cardiometabolic health. Nat. Med 27, 58–65 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ruan CC et al. A2A receptor activation attenuates hypertensive cardiac remodeling via promoting brown adipose tissue-derived FGF21. Cell Metab 28, 476–489.e5 (2018). [DOI] [PubMed] [Google Scholar]
- 147.Bordicchia M et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J. Clin. Invest 122, 1022–1036 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang GX et al. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat. Med 20, 1436–1443 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shen H, Jiang L, Lin JD, Omary MB & Rui L Brown fat activation mitigates alcohol-induced liver steatosis and injury in mice. J. Clin. Invest 129, 2305–2317 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lee P et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab 19, 302–309 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gebert LFR & MacRae IJ Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol 20, 21–37 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Thomou T et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 542, 450–455 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hondares E et al. Hepatic FGF21 expression is induced at birth via PPARα in response to milk intake and contributes to thermogenic activation of neonatal brown fat. Cell Metab 11, 206–212 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Simcox J et al. Global analysis of plasma lipids identifies liver-derived acylcarnitines as a fuel source for brown fat thermogenesis. Cell Metab 26, 509–522.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Watanabe M et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439, 484–489 (2006). [DOI] [PubMed] [Google Scholar]
- 156.Donkers JM et al. NTCP deficiency in mice protects against obesity and hepatosteatosis. JCI Insight 5, e127197 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Velazquez-Villegas LA et al. TGR5 signalling promotes mitochondrial fission and beige remodelling of white adipose tissue. Nat. Commun 9, 245 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Broeders EP et al. The bile acid chenodeoxycholic acid increases human brown adipose tissue activity. Cell Metab 22, 418–426 (2015). [DOI] [PubMed] [Google Scholar]
- 159.Lehnig AC et al. Exercise training induces depot-specific adaptations to white and brown adipose tissue. iScience 11, 425–439 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kong X et al. Brown adipose tissue controls skeletal muscle function via the secretion of myostatin. Cell Metab 28, 631–643.e3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Stanford KI et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Invest 123, 215–223 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Knudsen JG et al. Role of IL-6 in exercise training- and cold-induced UCP1 expression in subcutaneous white adipose tissue. PLoS ONE 9, e84910 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Qing H et al. Origin and function of stress-induced IL-6 in murine models. Cell 182, 372–387.e14 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bostrom P et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481, 463–468 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Roberts LD et al. β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab 19, 96–108 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Beiroa D et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes 63, 3346–3358 (2014). [DOI] [PubMed] [Google Scholar]
- 167.Li Y et al. Secretin-activated brown fat mediates prandial thermogenesis to induce satiation. Cell 175, 1561–1574.e12 (2018). [DOI] [PubMed] [Google Scholar]
- 168.Laurila S et al. Secretin activates brown fat and induces satiation. Nat. Metab 3, 798–809 (2021). [DOI] [PubMed] [Google Scholar]
- 169.Dabke K, Hendrick G & Devkota S The gut microbiome and metabolic syndrome. J. Clin. Invest 129, 4050–4057 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mestdagh R et al. Gut microbiota modulate the metabolism of brown adipose tissue in mice. J. Proteome Res 11, 620–630 (2012). [DOI] [PubMed] [Google Scholar]
- 171.Suarez-Zamorano N et al. Microbiota depletion promotes browning of white adipose tissue and reduces obesity. Nat. Med 21, 1497–1501 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li B et al. Microbiota depletion impairs thermogenesis of brown adipose tissue and browning of white adipose tissue. Cell Rep 26, 2720–2737.e5 (2019). [DOI] [PubMed] [Google Scholar]
- 173.Chevalier C et al. Gut microbiota orchestrates energy homeostasis during cold. Cell 163, 1360–1374 (2015). [DOI] [PubMed] [Google Scholar]
- 174.Zietak M et al. Altered microbiota contributes to reduced diet-induced obesity upon cold exposure. Cell Metab 23, 1216–1223 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Li G et al. Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. Cell Metab 26, 672–685.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Harms M & Seale P Brown and beige fat: development, function and therapeutic potential. Nat. Med 19, 1252–1263 (2013). [DOI] [PubMed] [Google Scholar]
- 177.van der Lans AA et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J. Clin. Invest 123, 3395–3403 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cypess AM et al. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab 21, 33–38 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Baskin AS et al. Regulation of human adipose tissue activation, gallbladder size, and bile acid metabolism by a β3-adrenergic receptor agonist. Diabetes 67, 2113–2125 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Blondin DP et al. Human brown adipocyte thermogenesis is driven by β2-AR stimulation. Cell Metab 32, 287–300 e287 (2020). [DOI] [PubMed] [Google Scholar]
- 181.O’Mara AE et al. Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. J. Clin. Invest 130, 2209–2219 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Finlin BS et al. The β3-adrenergic receptor agonist mirabegron improves glucose homeostasis in obese humans. J. Clin. Invest 130, 2319–2331 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tremblay A, Arguin H & Panahi S Capsaicinoids: a spicy solution to the management of obesity? Int. J. Obes 40, 1198–1204 (2016). [DOI] [PubMed] [Google Scholar]
- 184.Inoue N, Matsunaga Y, Satoh H & Takahashi M Enhanced energy expenditure and fat oxidation in humans with high BMI scores by the ingestion of novel and non-pungent capsaicin analogues (capsinoids). Biosci. Biotechnol. Biochem 71, 380–389 (2007). [DOI] [PubMed] [Google Scholar]
- 185.Snitker S et al. Effects of novel capsinoid treatment on fatness and energy metabolism in humans: possible pharmacogenetic implications. Am. J. Clin. Nutr 89, 45–50 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yoneshiro T, Aita S, Kawai Y, Iwanaga T & Saito M Nonpungent capsaicin analogs (capsinoids) increase energy expenditure through the activation of brown adipose tissue in humans. Am. J. Clin. Nutr 95, 845–850 (2012). [DOI] [PubMed] [Google Scholar]
- 187.Phan TX et al. TRPV1 expressed throughout the arterial circulation regulates vasoconstriction and blood pressure. J. Physiol 598, 5639–5659 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Baskaran P, Krishnan V, Ren J & Thyagarajan B Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br. J. Pharmacol 173, 2369–2389 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ohyama K et al. A synergistic antiobesity effect by a combination of capsinoids and cold temperature through promoting beige adipocyte biogenesis. Diabetes 65, 1410–1423 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Broeders EP et al. Thyroid hormone activates brown adipose tissue and increases non-shivering thermogenesis – a cohort study in a group of thyroid carcinoma patients. PLoS ONE 11, e0145049 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kushchayeva YS et al. Thyroid hormone effects on glucose disposal in patients with insulin receptor mutations. J. Clin. Endocrinol. Metab 105, e158–e171 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Dittner C, Lindsund E, Cannon B & Nedergaard J At thermoneutrality, acute thyroxine-induced thermogenesis and pyrexia are independent of UCP1. Mol. Metab 25, 20–34 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Johann K et al. Thyroid-hormone-induced browning of white adipose tissue does not contribute to thermogenesis and glucose consumption. Cell Rep 27, 3385–3400.e3 (2019). [DOI] [PubMed] [Google Scholar]
- 194.Nicolaisen TS et al. Thyroid hormone receptor α in skeletal muscle is essential for T3-mediated increase in energy expenditure. FASEB J 34, 15480–15491 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Flint A, Raben A, Rehfeld JF, Holst JJ & Astrup A The effect of glucagon-like peptide-1 on energy expenditure and substrate metabolism in humans. Int. J. Obes. Relat. Metab. Disord 24, 288–298 (2000). [DOI] [PubMed] [Google Scholar]
- 196.Basolo A et al. Exenatide has a pronounced effect on energy intake but not energy expenditure in non-diabetic subjects with obesity: a randomized, double-blind, placebo-controlled trial. Metabolism 85, 116–125 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ost M, Keipert S & Klaus S Targeted mitochondrial uncoupling beyond UCP1 – The fine line between death and metabolic health. Biochimie 134, 77–85 (2017). [DOI] [PubMed] [Google Scholar]
- 198.Kopecky J, Clarke G, Enerback S, Spiegelman B & Kozak LP Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J. Clin. Invest 96, 2914–2923 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Casteilla L et al. Stable expression of functional mitochondrial uncoupling protein in Chinese hamster ovary cells. Proc. Natl Acad. Sci. USA 87, 5124–5128 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gonzalez-Muniesa P, Milagro FI, Campion J & Martinez JA Ectopic UCP1 gene expression in HepG2 cells affects ATP production. J. Physiol. Biochem 61, 389–393 (2005). [DOI] [PubMed] [Google Scholar]
- 201.Tiraby C et al. Acquirement of brown fat cell features by human white adipocytes. J. Biol. Chem 278, 33370–33376 (2003). [DOI] [PubMed] [Google Scholar]
- 202.Kishida T et al. Reprogrammed functional brown adipocytes ameliorate insulin resistance and dyslipidemia in diet-induced obesity and type 2 diabetes. Stem Cell Rep 5, 569–581 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Tran KV et al. Human thermogenic adipocyte regulation by the long noncoding RNA LINC00473. Nat. Metab 2, 397–412 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zheng Q et al. Reconstitution of UCP1 using CRISPR/Cas9 in the white adipose tissue of pigs decreases fat deposition and improves thermogenic capacity. Proc. Natl Acad. Sci. USA 114, E9474–E9482 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Shen Y et al. CRISPR-delivery particles targeting nuclear receptor-interacting protein 1 (Nrip1) in adipose cells to enhance energy expenditure. J. Biol. Chem 293, 17291–17305 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kajimura S et al. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-β transcriptional complex. Nature 460, 1154–1158 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Nishio M et al. Production of functional classical brown adipocytes from human pluripotent stem cells using specific hemopoietin cocktail without gene transfer. Cell Metab 16, 394–406 (2012). [DOI] [PubMed] [Google Scholar]
- 208.Min SY et al. Human ‘brite/beige’ adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nat. Med 22, 312–318 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wang CH et al. CRISPR-engineered human brown-like adipocytes prevent diet-induced obesity and ameliorate metabolic syndrome in mice. Sci. Transl. Med 12, eaaz8664 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Crunkhorn S CRISPR-engineered fat cells prevent obesity. Nat. Rev. Drug Discov 19, 672 (2020). [DOI] [PubMed] [Google Scholar]
- 211.Riis-Vestergaard MJ et al. Beta-1 and not beta-3 adrenergic receptors may be the primary regulator of human brown adipocyte metabolism. J. Clin. Endocrinol. Metab 105, e994–e1005 (2020). [DOI] [PubMed] [Google Scholar]
- 212.de Jong JMA et al. Human brown adipose tissue is phenocopied by classical brown adipose tissue in physiologically humanized mice. Nat. Metab 1, 830–843 (2019). [DOI] [PubMed] [Google Scholar]
- 213.Kajimura S & Spiegelman BM Confounding issues in the “humanized” BAT of mice. Nat. Metab 2, 303–304 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.de Jong JMA, Cannon B, Nedergaard J, Wolfrum C & Petrovic N Reply to ‘Confounding issues in the ‘humanized’ brown fat of mice’. Nat. Metab 2, 305–306 (2020). [DOI] [PubMed] [Google Scholar]
- 215.Zhu Y et al. Connexin 43 mediates white adipose tissue beiging by facilitating the propagation of sympathetic neuronal signals. Cell Metab 24, 420–433 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Crewe C et al. An endothelial-to-adipocyte extracellular vesicle axis governed by metabolic state. Cell 175, 695–708.e13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Flaherty SE III et al. A lipase-independent pathway of lipid release and immune modulation by adipocytes. Science 363, 989–993 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Brestoff JR et al. Intercellular mitochondria transfer to macrophages regulates white adipose tissue homeostasis and is impaired in obesity. Cell Metab 33, 270–282.e8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Torralba D, Baixauli F & Sanchez-Madrid F Mitochondria know no boundaries: mechanisms and functions of intercellular mitochondrial transfer. Front. Cell Dev. Biol 4, 107 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.De Luca M et al. Advances in stem cell research and therapeutic development. Nat. Cell Biol 21, 801–811 (2019). [DOI] [PubMed] [Google Scholar]
- 221.Yin H, Xue W & Anderson DG CRISPR-Cas: a tool for cancer research and therapeutics. Nat. Rev. Clin. Oncol 16, 281–295 (2019). [DOI] [PubMed] [Google Scholar]
- 222.Song W et al. Engraftment of human induced pluripotent stem cell-derived hepatocytes in immunocompetent mice via 3D co-aggregation and encapsulation. Sci. Rep 5, 16884 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Deuse T et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat. Biotechnol 37, 252–258 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Xue Y, Xu X, Zhang XQ, Farokhzad OC & Langer R Preventing diet-induced obesity in mice by adipose tissue transformation and angiogenesis using targeted nanoparticles. Proc. Natl Acad. Sci. USA 113, 5552–5557 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]


