Abstract
Both cognitive impairment and cardiovascular diseases have a high incidence in the elderly population, increasing the burden of care and reducing the quality of life. Studies have suggested that cognitive impairment interacts with cardiovascular diseases such as coronary heart disease, abnormal blood pressure, heart failure, and arrhythmia. On one hand, cognitive impairment in the elderly influences the progression and self-management of cardiovascular diseases and increases the risk of cardiovascular-related adverse events. On the other hand, coronary heart disease, heart failure, higher blood pressure variability, orthostatic hypotension, and atrial fibrillation may aggravate cognitive impairment. The role of blood pressure levels on cognition remains controversial. Several shared biological pathways have been proposed as the underlying mechanism for the association. Cardiovascular diseases may lead to cognitive decline even dementia through cerebral perfusion damage, brain structural changes, inflammation, β-amyloid deposition, and neuroendocrine disorders. It is of great significance to study the interaction and put forward effective interventions in an overall perspective to reduce care burden and improve the quality of life of the elderly patients.
Keywords: aging, cardiovascular diseases, cognitive impairment, pathogenesis
Introduction
Cognitive impairment (CI) is defined as clear decline from a previous level of functioning in one or more of the key cognitive domains by Diagnostic and Statistical Manual of Mental Disorders (5th edition, DSM-5). The key domains include perceptual–motor function, language, executive function, learning and memory, complex attention and social cognition. 1 With aging, the function of nervous system and other organs is declining, and a series of changes appear, such as impaired automatic regulation of cerebral blood flow, 2 impaired ability of brain microvascular endothelial cells to form capillary like structures, 3 and increased oxidative stress. 4 Aging has been an important risk factor of CI. With the development of population aging, CI, as one of the common geriatric syndromes, has become an increasingly serious health problem worldwide.
CI endangers the health of the elderly. First, CI increases the risk of geriatric syndromes such as malnutrition, fall, and frailty,5–7 leading to increased vulnerability, fracture, and other adverse consequences. 8 Studies have demonstrated that physical frailty and cognitive frailty share biological mechanism and behavioral changes in some ways, such as gait disturbances. 9 As early as 2012, the studies released at Alzheimer’s Association Information Conference (AAIC) showed that gait disturbances indicate a decline in cognitive function. Processing speed is related to stride length and velocity. Executive function is associated with stride length, width, and variability. 10 Second, CI often co-exists with a variety of chronic diseases in aging population, 11 such as cardiovascular disease (CVD), chronic obstructive pulmonary disease, diabetes, chronic kidney disease, or sleep apnea.12–15 The pathological process and clinical manifestations influence each other, which increases the difficulty of diagnosis and treatment. Third, the decline in executive function and memory may impair clinical communication and compliance, and affect clinical decision-making.16,17 The decline in language, memory, and social cognitive function tends to lead to social isolation, anxiety, and psychological loneliness of the elderly. 18 In short, CI, which diminishes quality of life, shortens life expectancy, and exerts a substantial drain on healthcare resources, 19 has become a main medical problem of healthy aging.
Decades ago, the term ‘cardiogenic dementia’ which was proposed by Lancet opened research on the relationship between CVD with its risk factors and cognitive function. 20 Nowadays, there have been many studies which show that the link between CVD and CI is certainly present and probably strong (Figure 1) but is both complex and not fully understood. We sorted out recent studies on the relationship between CI and common CVDs in the elderly population, to explore the specific linkages between them, especially the shared pathogenesis that common CVDs affect cognitive function in the elderly. This review integrates the viewpoints with sufficient evidence, analyzes the controversies with possible reasonable explanations, and points out the limitations of current research, so as to provide reference for future research.
Figure 1.
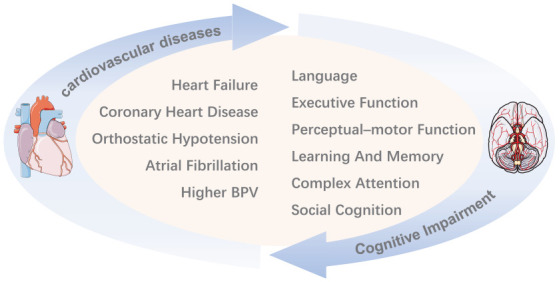
The vicious cycle of cardiovascular diseases and cognitive impairment. It is generally believed that above CVDs accelerate the decline of cognitive function. In turn, the impairment of above cognitive domains increases the risk of CVDs or aggravates the prognosis of them.
BPV, blood pressure variability.
The influence of CI on the development of CVDs in the elderly
It has been confirmed that CI in the elderly has a negative impact on the occurrence and development of CVDs. Leng et al. 21 found that older people with lower cognition or faster decline in overall cognitive function at baseline had a higher risk of occurrence of CVDs and a higher risk of death from it. Salzwedel et al. 22 observed that CI make secondary prevention, behavior change and self-management of patients with coronary heart disease (CHD) more complex. Medication adherence is critical to prevent recurrence of CVD. However, elderly people with CI are more likely to develop poor medication compliance or confusion over drugs. 23
CI in the elderly is also associated with an increased risk of adverse cardiovascular events. Tomioka et al. 24 reported that elderly patients who received percutaneous coronary intervention, the group with CI had higher rates of cardiac mortality and cardiac adverse events during 3-year follow-up than those without CI. Kewcharoe et al. 25 found that CI significantly increased the risk of 30-day readmission in patients with heart failure. Gu et al. 26 studied elderly patients with non ST-elevation myocardial infarction who underwent coronary angiography and found that the group with CI at baseline were more likely to have major adverse events (death, non-fatal myocardial infarction, emergency unplanned repeated vascular reperfusion, stroke, or significant bleeding) 1 year later than the group without CI.
Influence of common CVDs on cognitive function in the elderly
CHD and CI
Studies have suggested that CHD is associated with accelerated cognitive decline in old age. A systematic review by Xia et al. 27 suggested that increased severity of coronary calcification was associated with the risk of occurrence of dementia and patients with coronary artery disease had a 50% increased risk of occurrence of mild cognitive impairment (MCI) and dementia. Xie et al. 28 reported that older adults with CHD events had a faster decline in cognitive function than those without CHD events during follow-up. At the genetic level, Elman et al. 29 found that the polygenic risk of CHD significantly changed the relationship between the genetic risk of Alzheimer’s disease (AD) and MCI, and those with higher polygenic risk of CHD were more likely to develop MCI. The diagnosis and intervention of CHD may also influence the cognitive function of the elderly. Coronary angiography may aggravate CI in patients. 30 Greaves et al. 17 found that among the patients who underwent coronary artery bypass grafting for the first time (mean age = 64.86 years), the percent of CI increased from 19% before operation sharply to about 43% after operation.
It is still controversial whether the CI in the elderly exists in the early stage or long term after the onset of CHD. Xie et al. 28 found that no short-term cognitive function decline was observed after the occurrence of CHD events. But in the following years after CHD events, cognitive function declined significantly faster than before the events. However, Pottle et al. 31 found that CI is common both in early and long-term rehabilitation among patients with acute coronary syndrome. The exact prevalence of CI at different stages of rehabilitation of patients with CHD is not known yet.
Abnormal blood pressure and cognitive function
Blood pressure levels
Blood pressure levels are associated with cognitive function in older adults, but the influence of higher or lower blood pressure on cognitive function is unclear. Despite some studies of high quality, no consistent conclusions have been reached. On one hand, studies demonstrated that hypertension has a protective influence on cognitive function. Corrada et al. 32 found that people with hypertension between the ages of 80 and 89 had a lower risk of dementia than those without hypertension, and those aged 90 and above had the lowest risk of dementia. Irimata et al. 33 analyzed the data of The National Alzheimer’s Coordinating Center (NACC) and found that recent or active hypertension was associated with a slower decline in cognitive function in patients with AD. A recent systematic review from JAMA, which covered SPRINT MIND study, concluded that blood pressure lowering with antihypertensive drugs was significantly associated with a lower risk of incident dementia or CI.34,35 On the other hand, hypertension has been considered to be a risk factor for CI. Framingham Heart Study showed an inverse relationship between blood pressure levels and cognitive function. 36 A prospective cohort study of 12,281 elderly people in China by Yuan et al. 37 observed that hypertension was associated with a significantly increased risk of CI in the elderly.
Since the criteria of hypertension includes the levels of systolic blood pressure (SBP) and/or diastolic blood pressure (DBP), and hypertension is divided into different grades and types. Their pathophysiological processes are different. Therefore, it is controversial to judge the relationship between hypertension and cognitive function only by the holistic concept of ‘hypertension’. First, cognitive function is associated with the duration and grades of hypertension. Wang et al. 38 found that compared with elderly patients without hypertension, patients with short-term (<10 years) and mild hypertension (grade 1) had a lower risk of MCI, while patients with grades 2–3 hypertension or duration ⩾10 years had a higher risk of MCI. It may be due to the fact that atherosclerotic plaques partially hinder the blood vessels of the elderly, so mild hypertension is beneficial to the maintenance of cerebral blood circulation. However, long-term hypertension can lead to vascular remodeling, microinfarcts, sparse cerebral microvessels, and decreased cerebral blood flow, even cerebral ischemia which aggravates the impairment of cognitive function. 39 Second, the difference also exists in different genders. Hestad et al. 40 found that higher SBP was associated with better cognitive function in elderly men, while the opposite was true in elderly women. Third, Taylor et al. 41 followed up 1484 middle-aged and elderly participants (aged 40–67 years) for 20 years and found that CI had a significant U-shaped relationship with DBP and mean arterial pressure at baseline, that is, too low and too high DBP and mean arterial pressure were related to CI. Therefore, more stratified studies on different blood pressure grade, duration or gender are needed to confirm the relationship between blood pressure levels and cognitive function.
Blood pressure variability
The degree of blood pressure fluctuation in a certain period of time has been defined as blood pressure variability (BPV). The standard deviation and coefficient of variation of ambulatory blood pressure are usually used to indicate the degree of blood pressure fluctuation over time, or the degree of overall change in blood pressure over a period of time. At present, it is generally believed that the increase of BPV in the elderly is related to the decline of cognitive function. Godai et al. 42 found that the cognitive function of elderly patients, with increased variability of systolic and DBP decreased significantly. And no matter how baseline blood pressure was, higher systolic BPV, diastolic BPV, and mean arterial pressure variability were associated with poorer cognition and greater dementia risk. 43 Zhou et al. 44 found that SBP and diastolic BPV have different influences on various cognitive domains. First, the larger DBP variability was associated with lower information processing speed and executive function, and significantly correlated with lower memory function. Second, the larger SBP variability was only significantly correlated with lower memory function.
Orthostatic hypotension
Classic orthostatic hypotension (COH) is defined as a drop in SBP of at least 20 mmHg or DBP of at least 10 mmHg within 3 minutes of standing from a supine position. 45 It is generally believed that the COH of the elderly is related to the decline of cognitive function. Zimmermann et al. 46 found that the executive and memory functions of elderly participants with COH deteriorated faster than those without COH. Cremer et al. followed 7425 participants (aged 65 or above) for 12 years and observed a significant correlation between the presence of COH at baseline and the occurrence of dementia. Regardless of the threshold of COH and the statistical methods used, the risk of dementia increased by at least 25%. 47
As a variant of COH, initial orthostatic hypotension (IOH) is defined as a transient reduction ⩾40 mmHg in SBP or ⩾20 mmHg in DBP within 15 seconds of standing. 45 Saedon et al. 45 practiced a study on 1245 participants (age ⩾ 55 years) and found that participants with IOH revealed better cognitive performance compared to those without OH. While it also demonstrated that COH group (those with a ⩾20/10 mmHg BP drop beyond 15 seconds and within 3 minutes) was also associated with better cognitive performance, which is contrary to the findings mentioned above. Therefore, a large amount of population-based data are still needed to provide information on the relationship between OH and cognitive function.
Heart failure and CI
There is a consensus that heart failure increases the risk of CI in the elderly. Even mild cardiac dysfunction or lower-than-normal cardiac output is associated with accelerated brain aging and CI. 48 Data from the Framingham Heart Study suggested that reduced cardiac index and left ventricular ejection fraction are associated with CI. 49 Jefferson et al. found that the relative risk of dementia and AD increased with each decrease in cardiac index of one standard deviation unit in a study of 1039 Framingham offspring cohort participants (age = 69 ± 6 years) who had no clinical stroke, transient ischemic attack, or dementia. Participants with lower cardiac index had more than twice the risk of developing dementia compared with those with normal cardiac index. When participants with clinically prevalent CVD and atrial fibrillation were excluded, participants with a clinically low cardiac index had nearly three times the risk of the control group. 50 In a 15-year study of 5,414 elderly people, Witt et al. found that heart failure was associated with an increased risk of MCI and dementia. Patients with heart failure showed greater cognitive decline than those without. 51 The cognitive domains most commonly impaired in patients with heart failure are complex attention, executive function, learning, and memory. 52
Arrhythmias and CI
Atrial fibrillation is one of the most common arrhythmias in the elderly. It was demonstrated by several researches that atrial fibrillation may increase the risk of CI through multiple mechanisms.53–56 Manolis et al. 54 also suggested that the correlation between persistent/permanent atrial fibrillation and CI was stronger than that between paroxysmal atrial fibrillation and CI.
There are few studies on the relationship between CI and arrhythmia except atrial fibrillation. Even so, most of them demonstrated that arrhythmia can affect cognitive function through mechanisms such as reduced cerebral blood flow. The study by Mateen et al. 57 reported that long-term survivors of cardiac arrest due to ventricular fibrillation outside the hospital may have impaired long-term memory. Barbe et al. 58 found that patients with bradycardia may show mental decline in intelligence, and that implantation of pacemakers may improve cognitive function in elderly patients with bradycardia.
Possible pathogenesis of CVDs on cognitive function
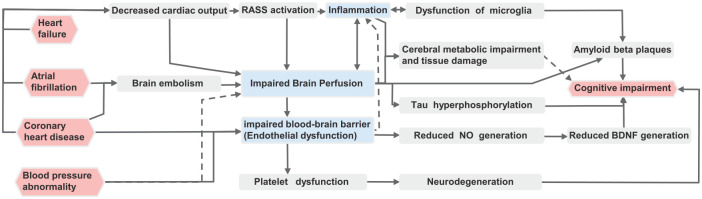
More and more theories and hypotheses attempt to elucidate the interaction between cardiovascular system and brain. The concept of neurovascular units was proposed many years ago. 59 Havakuk et al. 60 combined various factors that lead to CI in patients with heart failure into ‘cardio-brain syndrome’. Abete et al. 61 proposed the heart-brain continuum hypothesis. In 2018, the European Society of Cardiology advanced a new system to describe heart–brain interactions. 62 We have brought together classical and up-to-date possible mechanisms to study the networks and common pathways of CI caused by various CVDs (Figure 2).
Figure 2.
Systematic overview on pathogenesis of cognitive impairment caused by cardiovascular diseases in the elderly.
BDNF, brain-derived neurotrophic factor; NO, nitric oxide; RAAS, renin-angiotensin-aldosterone system.
Vascular pathogenesis
Hemodynamic abnormalities and vascular integrity damage are the first step in CI caused by CVDs, including decreased cardiac output, abnormal blood pressure, impaired blood–brain barrier (BBB), platelet dysfunction, and microthrombosis.
Many CVDs such as CHD, heart failure, and atrial fibrillation in the elderly, can lead to cognitive decline through the decrease of cardiac output. 48 On one hand, the decrease of cardiac output can directly lead to impaired brain perfusion, so as to reduce the energy and oxygen supply of brain cells, which causes injury or death of brain cells. 63 Cerebral hypoperfusion can also lead to hyperphosphorylation of tau protein and formation of neurofibrillary tangles, which is one of the pathological characteristics of AD. 64 On the other hand, decreased cardiac output can directly lead to decreased renal blood flow, so as to activate the renin-angiotensin-aldosterone system (RAAS), and then cause impaired brain perfusion, or lead to brain tissue damage through systemic inflammation, ultimately leading to cognitive decline. 65
In addition, cerebral embolism caused by atrial fibrillation, 66 increased variability in daily blood pressure, 67 and orthostatic hypotension, 47 may also lead to cerebral hypoperfusion. Pantoni 68 found that persistent hypertension caused cerebral small vessel lesions (loss of smooth muscle cells in small vessels, deposition of fibrous hyaline substances, lumen stenosis, and vascular wall thickening), leading to ischemia or hemorrhage in the corresponding brain regions and parenchymal lesions, and finally manifested as CI. Coronary angiography or percutaneous coronary intervention may remove atherosclerotic plaques from the aortic wall, allowing microemboli to enter the cerebral circulation, leading to CI.30,69
Beta-amyloid (Aβ) deposition is one of the pathological features of AD. Aβ is normally cleared by endocytosis of the BBB and is transported into the blood vessels. 70 When the BBB is damaged, this mechanism fails, leading to deposition of Aβ in the brain. The BBB is composed of continuous capillary endothelium of the brain, and the close connections between its cells, complete basement membrane, pericytes and the foot plate of astrocytes, and the endothelial is the main structure of BBB. Both CHD and hypertension can lead to endothelial dysfunction. 71 Fujiyoshi et al. 72 found that endothelial dysfunction was significantly associated with CI in elderly patients with CVD. Saleem et al. 73 observed patients with CHDs and found that the improvement in endothelial function was significantly associated with improvement in overall cognition function at 3 months compared to baseline. In addition, endothelial dysfunction may lead to vascular stress and reduced production of nitric oxide (NO). 74 NO is involved in the production of brain-derived neurotrophic factor (BDNF), which plays an important role in cognitive function, especially in learning and memory. 75
Platelets may also play a role in CVDs and CI. According to the heart–brain continuity hypothesis proposed by Abete, hypertension, CHD, atrial fibrillation, and heart failure cause neurodegeneration through platelet activation, and then lead to CI and even dementia. 61 Stellos et al. 76 found that the expression of platelet activation biomarkers (activated glycoprotein IIb–IIIa complex and P-selectin) in AD patients with faster cognitive decline at baseline was significantly higher than that with slower cognitive decline.
Brain structural changes
Evidences of neuroimaging studies suggest that CVDs and the risk factors are associated with brain structural abnormalities. Aljondi et al. 77 found that the higher Framingham Cardiovascular Risk Profile (FCRP) score in middle age was associated with higher white matter hyperintensity (WMH) volume in old age, and the relationship between FCRP score in middle age and executive function in old age was mediated by WMH volume. Guevarra et al. 78 found that hypertension was particularly associated with WMH in the 60–69 age group.
CVDs are associated with damages to multiple brain regions. Vogels et al. 79 found significant increases in WMH, lacunar infarction, and temporal lobe atrophy in patients with heart failure compared with healthy controls. Rosano et al. 80 confirmed that higher mean SBP was related to lower white matter integrity in uncinate and superior lateral fasciculi bilaterally. Debette et al. 81 found that cardiovascular risk factors are associated with accelerated progression of vascular brain injury, global brain and hippocampal atrophy, and decline in executive function. Gonzales et al. 82 found that hypertension was associated with poor learning, memory, and executive function, as well as lower cortical thickness of right occipital lobe. Tarasova et al. 83 studied the electroencephalography (EEG) and cognitive performance in patients with CHD and found that higher θ/α ratio, θ1 rhythm power of closed eyes in left frontal and occipital regions and α2 rhythm power of opening eyes in right frontal region were associated with an increased risk of MCI. Current research on areas of brain damage caused by CVD and its risk factors is less consistent, and the relationship needs to be further clarified by angiography and neuroimaging of the brain in larger populations.
Inflammation and immunomodulation
Whether it is the lack of tissue energy supply, oxygen supply or vascular damage caused by CVD, it can lead to inflammation. Inflammation aggravates existing CVDs but also damages the nervous system, leading to CI and even dementia. 84 Previous studies have confirmed the existence of systemic inflammatory network after ischemic heart disease. 85 Thackeray et al. 86 found that acute myocardial infarction leads to early inflammatory response, left ventricular remodeling, and activation of brain microglia. Hypertension can destroy BBB through angiotensin II and hypoxia, increase its permeability resulting in plasma extravasation and perivascular inflammation. The interaction between pro-inflammatory mediator and BBB on the lumen side further destroys the BBB. 71 RAAS is activated, which in turn activates the sympathetic nervous system, releasing reactive oxygen species that enhances oxidative stress and lead to a self-perpetuating inflammatory state. 65
As the main immune cells of the central nervous system, microglia plays a key role in regulating neuroinflammation and antibody deposition. Through the comprehensive analysis of gene regulatory networks, Zhang et al. 87 emphasized that the immune/microglia module is the molecular system most closely related to the pathophysiological mechanism of late-onset Alzheimer’s disease. Over-activation of microglia leads to over-release of pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin (IL)-1, and increases the expression of amyloid precursor protein and Aβ. 88 In addition to endocytosis of BBB, Aβ can also be cleared by the phagocytosis of microglia. 70 When activated microglia cannot phagocytize excessive Aβ, Aβ deposits form amyloid plaques. Cerebral perfusion also reduced the clearance rate of Aβ.
Endocrine and metabolic disorders
Neuroendocrine disorders influences hemodynamics and inflammation, and then influences cognitive functions, such as the activation of the RAAS system. Apolipoprotein (Apo) and other components also have an impact on the occurrence and development of CVD and CI. Lutski et al. 89 found that patients with blood Apo-B ⩾ 105 mg/dL and low-density lipopropetin cholesterol (LDL-C) ⩾ 160 mg/d in middle age had more significant decline in comprehensive cognitive function in old age. ApoE regulates cholesterol homeostasis by mediating low-density lipoprotein uptake and metabolism. ApoE2 and ApoE4 increase the risk of heart disease. ApoE4 also increases the risk of neurodegenerative diseases and reduces the age of onset. 90 The ApoE4 allele has been considered as a genetic risk factor for Alzheimer’s disease for many years. 91 Lipnicki et al. 92 found that the cognitive function of most ApoE4 genotype carriers decreased faster than that of non-ApoE4 carriers, and the difference in processing speed was the largest.
Limitations and future directions
There are still some limitations in current studies, and further studies may be needed. First, most of the current studies are cross-sectional studies, and more large, multicenter prospective cohort studies are needed to detemine the causal relationship between these variables. Second, patients with CI are rarely allowed to participate in randomized cardiovascular clinical trials, most of which are observational studies, and evidence for procedural interventions in elderly patients with CI and CVD is weak. Third, there is no unified standard or consensus on the tools used in cognitive function assessment. Different assessment tools and standards may have an impact on the consistency of the research results, and even some studies only use some areas of cognitive function to represent the overall cognition. Therefore, it is necessary to establish a sensitive and standardized clinical assessment tools to diagnose and predict CI in future research. In addition to rating scales, machine examination techniques also deserve attention, just like blood oxygenation level-dependent functional magnetic resonance imaging (BOLD fMRI). 93 Fourth, due to the lack of understanding of CI among the elderly and even clinicians, the accelerated decline of cognitive function may be attributed to normal aging. It is recommended that institutions should conduct routine cognitive function assessment for elderly patients with CVD when conditions are available.
In future studies, it should also be noted that some factors in pathological processes may be as predictors of cognitive decline in elderly patients with CVD. For example, reactive hyperemia index, used to measure endothelial function, may be a predictive tool for cognitive decline in patients with CHD. 73 Apo-B, LDL-C, and APO-B/LDL-C ratios are potential predictors of accelerated cognitive decline in later life in male patients with CHD. 89 Serum dihigh-gamma linolenic acid may be a useful marker for identifying early cognitive decline in elderly patients with CHD. 94 Higher preoperative red blood cell distribution width may be an independent predictor of CI after coronary artery bypass grafting. 95
Ultimately, developing novel tools and interventions to improve cardiovascular health and cognition is a pressing need. The above studies to clarify these pathophysiological mechanisms have provided some directions for follow-up research. In clinical research, improving treatment compliance and lifestyle may be critical. CI independently increased the risk of non-adherence to antithrombotic therapy in the elderly with atrial fibrillation. 23 In secondary medication prevention after acute myocardial infarction, patients with well adherence to medications have a significantly reduced risk of all-cause mortality and major adverse cardiovascular events. 16 Comprehensive cardiac rehabilitation programs which combined exercise, dietary therapy, and disease management might help improve cognitive function. 96 Meanwhile, it is also important to remove risk factors of CVDs, such as somking, lack of exercise and poor blood glucose control. Interventions against major cardiovascular risk factors at midlife might be effective to reduce the development of WMH lesions and thus late life cognitive decline. 77
Pre-clinical studies are mainly focused on Alzheimer’s disease and vascular CI. Some experimental and new therapeutic avenues can potentially rescue cerebromicrovascular endothelial function and neurovascular coupling responses to prevent or delay CI.93,97 Interventions in nutrition and dietary habits, such as time-restricted feeding (TRF), are basal and effective measures. 98 Nicotinamide mononucleotide has also been proved such a role.3,99 The effect of abnormal tau proteins can be blocked by targeted immunotherapy. 100 Peroxisome proliferator-activated receptor-γ co-activator-1α (PGC-1α) is also a potential therapeutic target for CI because it suppresses the excessive reactive oxygen species and neuroinflammation caused by chronic cerebral hypoperfusion. 101
Conclusion
There is sufficient evidence that common CVDs interacts with cognition in the elderly. On one hand, CI in the elderly has a negative impact on the prognosis of CVDs and increases the risk of cardiovascular-related adverse events through behavioral changes, self-management difficulties and decreased drug compliance. On the other hand, CHD, higher BPV, orthostatic hypotension, and atrial fibrillation may aggravate CI. The influence of blood pressure level and duration on cognitive function in the elderly is still controversial. CVDs may lead to cognitive decline even dementia through cerebral perfusion damage, brain structural changes, inflammation, β-amyloid deposition, and neuroendocrine disorders. More prospective cohort studies of the elderly and more unified cognitive function assessment tool are needed to provide sufficient evidence for intervention for CI and CVD, which is of great significance to reduce care burden and improve the quality of life.
Footnotes
Author contributions: Wenhang Zuo: Conceptualization; Resources; Visualization; Writing – original draft; Writing – review & editing.
Jinhui Wu: Conceptualization; Project administration; Formal analysis; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by China National Key R&D Program (grant no.2018YFC2002100 and 2018YFC2002103).
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs: Wenhang Zuo  https://orcid.org/0000-0003-1119-558X
https://orcid.org/0000-0003-1119-558X
Jinhui Wu  https://orcid.org/0000-0003-0763-7728
https://orcid.org/0000-0003-0763-7728
Contributor Information
Wenhang Zuo, National Clinical Research Center for Geriatrics, Department of Gerontology and Geriatrics, West China Hospital, Sichuan University, Chengdu, People’s Republic of China.
Jinhui Wu, National Clinical Research Center for Geriatrics, Department of Gerontology and Geriatrics, West China Hospital, Sichuan University, Chengdu 610041, Sichuan, People’s Republic of China.
References
- 1. Sachdev PS, Blacker D, Blazer DG, et al. Classifying neurocognitive disorders: the DSM-5 approach. Nat Rev Neurol 2014; 10: 634–642. [DOI] [PubMed] [Google Scholar]
- 2. Wang S, Lv W, Zhang H, et al. Aging exacerbates impairments of cerebral blood flow autoregulation and cognition in diabetic rats. Geroscience 2020; 42: 1387–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kiss T, Balasubramanian P, Valcarcel-Ares MN, et al. Nicotinamide mononucleotide (NMN) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: a potential mechanism for the prevention of vascular cognitive impairment. Geroscience 2019; 41: 619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, et al. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell 2018; 17: e12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kiiti Borges M, Oiring de Castro Cezar N, Silva Santos Siqueira A, et al. The relationship between physical frailty and mild cognitive impairment in the elderly: a systematic review. J Frailty Aging 2019; 8: 192–197. [DOI] [PubMed] [Google Scholar]
- 6. Chatindiara I, Allen J, Popman A, et al. Dysphagia risk, low muscle strength and poor cognition predict malnutrition risk in older adults athospital admission. BMC Geriatr 2018; 18: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Muir SW, Gopaul K, Montero Odasso MM. The role of cognitive impairment in fall risk among older adults: a systematic review and meta-analysis. Age Ageing 2012; 41: 299–308. [DOI] [PubMed] [Google Scholar]
- 8. Tsutsumimoto K, Doi T, Makizako H, et al. Cognitive frailty is associated with fall-related fracture among older people. J Nutr Health Aging 2018; 22: 1216–1220. [DOI] [PubMed] [Google Scholar]
- 9. Waters DL, Vlietstra L, Qualls C, et al. Sex-specific muscle and metabolic biomarkers associated with gait speed and cognitive transitions in older adults: a 9-year follow-up. Geroscience 2020; 42: 585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ikram M, Verlinden V, Hofman A, et al. O1-08-02: cognition and gait reveal distinct patterns of association in an aging population. Alzheimers Dement 2012; 8: P99. [Google Scholar]
- 11. Morley JE. Cognition and chronic disease. J Am Med Dir Assoc 2017; 18: 369–371. [DOI] [PubMed] [Google Scholar]
- 12. Yeh YC, Kuo YT, Huang MF, et al. Association of brain white matter lesions and atrophy with cognitive function in chronic kidney disease. Int J Geriatr Psychiatry 2019; 34: 1826–1832. [DOI] [PubMed] [Google Scholar]
- 13. Gosselin N, Baril AA, Osorio RS, et al. Obstructive sleep apnea and the risk of cognitive decline in older adults. Am J Respir Crit Care Med 2019; 199: 142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cleutjens F, Spruit MA, Ponds R, et al. Cognitive impairment and clinical characteristics in patients with chronic obstructive pulmonary disease. Chron Respir Dis 2018; 15: 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol 2018; 14: 591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huber CA, Meyer MR, Steffel J, et al. Post-myocardial infarction (MI) care: medication adherence for secondary prevention after MI in a large real-world population. Clin Ther 2019; 41: 107–117. [DOI] [PubMed] [Google Scholar]
- 17. Greaves D, Psaltis PJ, Ross TJ, et al. Cognitive outcomes following coronary artery bypass grafting: a systematic review and meta-analysis of 91,829 patients. Int J Cardiol 2019; 289: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lara E, Caballero FF, Rico-Uribe LA, et al. Are loneliness and social isolation associated with cognitive decline? Int J Geriatr Psychiatry 2019; 34: 1613–1622. [DOI] [PubMed] [Google Scholar]
- 19. Jia J, Wei C, Chen S, et al. The cost of Alzheimer’s disease in China and re-estimation of costs worldwide. Alzheimers Dement 2018; 14: 483–491. [DOI] [PubMed] [Google Scholar]
- 20. Editorial. Cardiogenic dementia. Lancet 1977; 309: 27–28. [PubMed] [Google Scholar]
- 21. Leng X, Espeland MA, Manson JE, et al. Cognitive function and changes in cognitive function as predictors of incident cardiovascular disease: the Women’s Health Initiative Memory Study. J Gerontol A Biol Sci Med Sci 2018; 73: 779–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Salzwedel A, Heidler MD, Haubold K, et al. Prevalence of mild cognitive impairment in employable patients after acute coronary event in cardiac rehabilitation. Vasc Health Risk Manag 2017; 13: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seong HJ, Lee K, Kim BH, et al. Cognitive impairment is independently associated with non-adherence to antithrombotic therapy in older patients with atrial fibrillation. Int J Environ Res Public Health 2019; 16: 2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tomioka T, Takahashi R, Ikumi Y, et al. Influence of cognitive impairment on cardiac mortality after percutaneous coronary intervention in very elderly patients: a retrospective observational study. J Geriatr Cardiol 2019; 16: 733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kewcharoen J, Trongtorsak A, Kanitsoraphan C, et al. Cognitive impairment and 30-day rehospitalization rate in patients with acute heart failure: a systematic review and meta-analysis. Indian Heart J 2019; 71: 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gu SZ, Beska B, Chan D, et al. Cognitive decline in older patients with non-ST elevation acute coronary syndrome. J Am Heart Assoc 2019; 8: e011218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xia C, Vonder M, Sidorenkov G, et al. The relationship of coronary artery calcium and clinical coronary artery disease with cognitive function: a systematic review and meta-analysis. J Atheroscler Thromb 2020; 27: 934–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xie W, Zheng F, Yan L, et al. Cognitive decline before and after incident coronary events. J Am Coll Cardiol 2019; 73: 3041–3050. [DOI] [PubMed] [Google Scholar]
- 29. Elman JA, Panizzon MS, Logue MW, et al. Genetic risk for coronary heart disease alters the influence of Alzheimer’s genetic risk on mild cognitive impairment. Neurobiol Aging 2019; 84: 237.e5–237.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Devapalasundarum AN, Silbert BS, Evered LA, et al. Cognitive function in patients undergoing coronary angiography. Heart Asia 2010; 2: 75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pottle A. Prevalence and patterns of cognitive impairment in acute coronary syndrome patients: a systematic review. Eur J Prev Cardiol 2020; 27: 281–283. [DOI] [PubMed] [Google Scholar]
- 32. Corrada MM, Hayden KM, Paganini-Hill A, et al. Age of onset of hypertension and risk of dementia in the oldest-old: the 90+ study. Alzheimers Dement 2017; 13: 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Irimata KE, Dugger BN, Wilson JR. Impact of the presence of select cardiovascular risk factors on cognitive changes among dementia subtypes. Curr Alzheimer Res 2018; 15: 1032–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Williamson JD, Pajewski NM, Auchus AP, et al. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA 2019; 321: 553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hughes D, Judge C, Murphy R, et al. Association of blood pressure lowering with incident dementia or cognitive impairment: a systematic review and meta-analysis. JAMA 2020; 323: 1934–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wolf PA. Contributions of the Framingham Heart Study to stroke and dementia epidemiologic research at 60 years. Arch Neurol 2012; 69: 567–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yuan JQ, Lv YB, Chen HS, et al. Association between late-life blood pressure and the incidence of cognitive impairment: a community-based prospective cohort study. J Am Med Dir Assoc 2019; 20: 177–182.e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang F, Li D, Wang L, et al. Mild hypertension protects the elderly from cognitive impairment: a 7-year retrospective cohort study. Psychogeriatrics 2020; 20: 412–418. [DOI] [PubMed] [Google Scholar]
- 39. Rêgo ML, Cabral DA, Costa EC, et al. Physical exercise for individuals with hypertension: it is time to emphasize its benefits on the brain and cognition. Clin Med Insights Cardiol 2019; 13: 1179546819839411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hestad K, Engedal K, Schirmer H, et al. The effect of blood pressure on cognitive performance. An 8-year follow-up of the Tromsø study, comprising people aged 45-74 years. Front Psychol 2020; 11: 607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Taylor C, Tillin T, Chaturvedi N, et al. Midlife hypertensive status and cognitive function 20 years later: the Southall and Brent revisited study. J Am Geriatr Soc 2013; 61: 1489–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Godai K, Kabayama M, Gondo Y, et al. Day-to-day blood pressure variability is associated with lower cognitive performance among the Japanese community-dwelling oldest-old population: the SONIC study. Hypertens Res 2020; 43: 404–411. [DOI] [PubMed] [Google Scholar]
- 43. Rouch L, Cestac P, Sallerin B, et al. Visit-to-visit blood pressure variability is associated with cognitive decline and incident dementia: the S.AGES cohort. Hypertension 2020; 76: 1280–1288. [DOI] [PubMed] [Google Scholar]
- 44. Zhou TL, Kroon AA, van Sloten TT, et al. Greater blood pressure variability is associated with lower cognitive performance. Hypertension 2019; 73: 803–811. [DOI] [PubMed] [Google Scholar]
- 45. Saedon NI, Frith J, Goh CH, et al. Orthostatic blood pressure changes and physical, functional and cognitive performance: the MELoR study. Clin Auton Res 2020; 30: 129–137. [DOI] [PubMed] [Google Scholar]
- 46. Zimmermann M, Wurster I, Lerche S, et al. Orthostatic hypotension as a risk factor for longitudinal deterioration of cognitive function in the elderly. Eur J Neurol 2020; 27: 160–167. [DOI] [PubMed] [Google Scholar]
- 47. Cremer A, Soumaré A, Berr C, et al. Orthostatic hypotension and risk of incident dementia: results from a 12-year follow-up of the three-city study cohort. Hypertension 2017; 70: 44–49. [DOI] [PubMed] [Google Scholar]
- 48. Jefferson AL. Cardiac output as a potential risk factor for abnormal brain aging. J Alzheimers Dis 2010; 20: 813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jefferson AL, Himali JJ, Au R, et al. Relation of left ventricular ejection fraction to cognitive aging (from the Framingham Heart Study). Am J Cardiol 2011; 108: 1346–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jefferson AL, Beiser AS, Himali JJ, et al. Low cardiac index is associated with incident dementia and Alzheimer disease: the Framingham Heart Study. Circulation 2015; 131: 1333–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Witt LS, Rotter J, Stearns SC, et al. Heart failure and cognitive impairment in the atherosclerosis risk in communities (ARIC) study. J Gen Intern Med 2018; 33: 1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gorodeski EZ, Hashmi AZ. Integrating assessment of cognitive status in elderly cardiovascular care. Clin Cardiol 2020; 43: 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sepehri Shamloo A, Dagres N, Müssigbrodt A, et al. Atrial fibrillation and cognitive impairment: new insights and future directions. Heart Lung Circ 2020; 29: 69–85. [DOI] [PubMed] [Google Scholar]
- 54. Manolis TA, Manolis AA, Apostolopoulos EJ, et al. Atrial fibrillation and cognitive impairment: an associated burden or burden by association? Angiology 2020; 71: 498–519. [DOI] [PubMed] [Google Scholar]
- 55. Diener HC, Hart RG, Koudstaal PJ, et al. Atrial fibrillation and cognitive function: JACC review topic of the week. J Am Coll Cardiol 2019; 73: 612–619. [DOI] [PubMed] [Google Scholar]
- 56. Stefanidis KB, Askew CD, Greaves K, et al. The effect of non-stroke cardiovascular disease states on risk for cognitive decline and dementia: a systematic and meta-analytic review. Neuropsychol Rev 2018; 28: 1–15. [DOI] [PubMed] [Google Scholar]
- 57. Mateen FJ, Josephs KA, Trenerry MR, et al. Long-term cognitive outcomes following out-of-hospital cardiac arrest: a population-based study. Neurology 2011; 77: 1438–1445. [DOI] [PubMed] [Google Scholar]
- 58. Barbe C, Puisieux F, Jansen I, et al. Improvement of cognitive function after pacemaker implantation in very old persons with bradycardia. J Am Geriatr Soc 2002; 50: 778–780. [DOI] [PubMed] [Google Scholar]
- 59. Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci 2004; 5: 347–360. [DOI] [PubMed] [Google Scholar]
- 60. Havakuk O, King KS, Grazette L, et al. Heart failure-induced brain injury. J Am Coll Cardiol 2017; 69: 1609–1616. [DOI] [PubMed] [Google Scholar]
- 61. Abete P, Della-Morte D, Gargiulo G, et al. Cognitive impairment and cardiovascular diseases in the elderly. A heart-brain continuum hypothesis. Ageing Res Rev 2014; 18: 41–52. [DOI] [PubMed] [Google Scholar]
- 62. Doehner W, Ural D, Haeusler KG, et al. Heart and brain interaction in patients with heart failure: overview and proposal for a taxonomy. A position paper from the Study Group on Heart and Brain Interaction of the Heart Failure Association. Eur J Heart Fail 2018; 20: 199–215. [DOI] [PubMed] [Google Scholar]
- 63. Fraser KS, Heckman GA, McKelvie RS, et al. Cerebral hypoperfusion is exaggerated with an upright posture in heart failure: impact of depressed cardiac output. JACC Heart Fail 2015; 3: 168–175. [DOI] [PubMed] [Google Scholar]
- 64. Zhang Q, Gao T, Luo Y, et al. Transient focal cerebral ischemia/reperfusion induces early and chronic axonal changes in rats: its importance for the risk of Alzheimer’s disease. PLoS ONE 2012; 7: e33722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mene-Afejuku TO, Pernia M, Ibebuogu UN, et al. Heart failure and cognitive impairment: clinical relevance and therapeutic considerations. Curr Cardiol Rev 2019; 15: 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Banerjee G, Chan E, Ambler G, et al. Effect of small-vessel disease on cognitive trajectory after atrial fibrillation-related ischaemic stroke or TIA. J Neurol 2019; 266: 1250–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Oishi E, Ohara T, Sakata S, et al. Day-to-day blood pressure variability and risk of dementia in a general Japanese elderly population: the Hisayama study. Circulation 2017; 136: 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010; 9: 689–701. [DOI] [PubMed] [Google Scholar]
- 69. Leclercq F, Kassnasrallah S, Cesari JB, et al. Transcranial Doppler detection of cerebral microemboli during left heart catheterization. Cerebrovasc Dis 2001; 12: 59–65. [DOI] [PubMed] [Google Scholar]
- 70. Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008; 57: 178–201. [DOI] [PubMed] [Google Scholar]
- 71. Iadecola C, Yaffe K, Biller J, et al. Impact of hypertension on cognitive function: a scientific statement from the American Heart Association. Hypertension 2016; 68: e67–e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fujiyoshi K, Yamaoka-Tojo M, Minami Y, et al. Endothelial dysfunction is associated with cognitive impairment of elderly cardiovascular disease patients. Int Heart J 2018; 59: 1034–1040. [DOI] [PubMed] [Google Scholar]
- 73. Saleem M, Herrmann N, Dinoff A, et al. Association between endothelial function and cognitive performance in patients with coronary artery disease during cardiac rehabilitation. Psychosom Med 2019; 81: 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bruno RM, Masi S, Taddei M, et al. Essential hypertension and functional microvascular ageing. High Blood Press Cardiovasc Prev 2018; 25: 35–40. [DOI] [PubMed] [Google Scholar]
- 75. Monnier A, Prigent-Tessier A, Quirié A, et al. Brain-derived neurotrophic factor of the cerebral microvasculature: a forgotten and nitric oxide-dependent contributor of brain-derived neurotrophic factor in the brain. Acta Physiol 2017; 219: 790–802. [DOI] [PubMed] [Google Scholar]
- 76. Stellos K, Panagiota V, Kogel A, et al. Predictive value of platelet activation for the rate of cognitive decline in Alzheimer’s disease patients. J Cereb Blood Flow Metab 2010; 30: 1817–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Aljondi R, Szoeke C, Steward C, et al. The effect of midlife cardiovascular risk factors on white matter hyperintensity volume and cognition two decades later in normal ageing women. Brain Imaging Behav 2020; 14: 51–61. [DOI] [PubMed] [Google Scholar]
- 78. Guevarra AC, Ng SC, Saffari SE, et al. Age moderates associations of hypertension, white matter hyperintensities, and cognition. J Alzheimers Dis 2020; 75: 1351–1360. [DOI] [PubMed] [Google Scholar]
- 79. Vogels RL, van der Flier WM, van Harten B, et al. Brain magnetic resonance imaging abnormalities in patients with heart failure. Eur J Heart Fail 2007; 9: 1003–1009. [DOI] [PubMed] [Google Scholar]
- 80. Rosano C, Abebe KZ, Aizenstein HJ, et al. Longitudinal systolic blood pressure characteristics and integrity of white matter tracts in a cohort of very old black and white adults. Am J Hypertens 2015; 28: 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Debette S, Seshadri S, Beiser A, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 2011; 77: 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gonzales MM, Ajilore O, Charlton RC, et al. Divergent influences of cardiovascular disease risk factor domains on cognition and gray and white matter morphology. Psychosom Med 2017; 79: 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tarasova IV, Trubnikova OA, Barbarash OL. EEG and clinical factors associated with mild cognitive impairment in coronary artery disease patients. Dement Geriatr Cogn Disord 2018; 46: 275–284. [DOI] [PubMed] [Google Scholar]
- 84. Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol 2015; 14: 388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Nahrendorf M, Frantz S, Swirski FK, et al. Imaging systemic inflammatory networks in ischemic heart disease. J Am Coll Cardiol 2015; 65: 1583–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Thackeray JT, Hupe HC, Wang Y, et al. Myocardial inflammation predicts remodeling and neuroinflammation after myocardial infarction. J Am Coll Cardiol 2018; 71: 263–275. [DOI] [PubMed] [Google Scholar]
- 87. Zhang B, Gaiteri C, Bodea LG, et al. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell 2013; 153: 707–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lee M, McGeer E, McGeer PL. Activated human microglia stimulate neuroblastoma cells to upregulate production of beta amyloid protein and tau: implications for Alzheimer’s disease pathogenesis. Neurobiol Aging 2015; 36: 42–52. [DOI] [PubMed] [Google Scholar]
- 89. Lutski M, Weinstein G, Goldbourt U, et al. Plasma lipids, apolipoproteins, and subsequent cognitive decline in men with coronary heart disease. J Alzheimers Dis 2019; 67: 827–837. [DOI] [PubMed] [Google Scholar]
- 90. Mahley RW. Apolipoprotein E: from cardiovascular disease to neurodegenerative disorders. J Mol Med 2016; 94: 739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Saunders AM, Schmader K, Breitner JC, et al. Apolipoprotein E epsilon 4 allele distributions in late-onset Alzheimer’s disease and in other amyloid-forming diseases. Lancet 1993; 342: 710–711. [DOI] [PubMed] [Google Scholar]
- 92. Lipnicki DM, Crawford JD, Dutta R, et al. Age-related cognitive decline and associations with sex, education and apolipoprotein E genotype across ethnocultural groups and geographic regions: a collaborative cohort study. PLoS Med 2017; 14: e1002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Yabluchanskiy A, Nyul-Toth A, Csiszar A, et al. Age-related alterations in the cerebrovasculature affect neurovascular coupling and BOLD fMRI responses: insights from animal models of aging. Psychophysiology 2021; 58: e13718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ishihara K, Izawa KP, Kitamura M, et al. Serum concentration of dihomo-γ-linolenic acid is associated with cognitive function and mild cognitive impairment in coronary artery disease patients. Prostaglandins Leukot Essent Fatty Acids 2019; 158: 102038. [DOI] [PubMed] [Google Scholar]
- 95. Wan J, Luo P, Du X, et al. Preoperative red cell distribution width predicts postoperative cognitive dysfunction after coronary artery bypass grafting. Biosci Rep 2020; 40: BSR20194448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Fujiyoshi K, Minami Y, Yamaoka-Tojo M, et al. Effect of cardiac rehabilitation on cognitive function in elderly patients with cardiovascular diseases. PLoS ONE 2020; 15: e0233688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yabluchanskiy A, Balasubramanian P, Tarantini S. Cerebrovascular rejuvenation: novel strategies for prevention of vascular cognitive impairment. Rejuvenation Res 2020; 23: 451–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Balasubramanian P, DelFavero J, Ungvari A, et al. Time-restricted feeding (TRF) for prevention of age-related vascular cognitive impairment and dementia. Ageing Res Rev 2020; 64: 101189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tarantini S, Valcarcel-Ares MN, Toth P, et al. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol 2019; 24: 101192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Qiu C, Albayram O, Kondo A, et al. Cis P-tau underlies vascular contribution to cognitive impairment and dementia and can be effectively targeted by immunotherapy in mice. Sci Transl Med 2021; 13: eaaz7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Han B, Jiang W, Liu H, et al. Upregulation of neuronal PGC-1α ameliorates cognitive impairment induced by chronic cerebral hypoperfusion. Theranostics 2020; 10: 2832–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]