Abstract
Cyclic nucleotide phosphodiesterases (PDEs) are superfamily of enzymes that regulate the spatial and temporal relationship of second messenger signaling in the cellular system. Among the 11 different families of PDEs, phosphodiesterase 1 (PDE1) sub-family of enzymes hydrolyze both 3’,5’-cyclic adenosine monophosphate (cAMP) and 3’,5’-cyclic guanosine monophosphate (cGMP) in a mutually competitive manner. The catalytic activity of PDE1 is stimulated by their binding to Ca2+/calmodulin (CaM), resulting in the integration of Ca2+ and cyclic nucleotide-mediated signaling in various diseases. The PDE1 family includes three subtypes, PDE1A, PDE1B and PDE1C, which differ for their relative affinities for cAMP and cGMP. These isoforms are differentially expressed throughout the body, including the cardiovascular, central nervous system and other organs. Thus, PDE1 enzymes play a critical role in the pathophysiology of diseases through the fundamental regulation of cAMP and cGMP signaling. This comprehensive review provides the current research on PDE1 and its potential utility as a therapeutic target in diseases including the cardiovascular, pulmonary, metabolic, neurocognitive, renal, cancers and possibly others.
Keywords: Phosphodiesterase, cAMP, cGMP, Pulmonary arterial hypertension, Cardiac hypertrophy, Metabolic syndrome
1. Introduction
Phosphodiesterases (PDEs) belong to a class of phosphohydrolytic enzymes that modulate the intensity of intracellular second messenger signaling by catalyzing the degradation of 3’,5’-cyclic adenosine monophosphate (cAMP) and 3’,5’-cyclic guanosine monophosphate (cGMP) molecules to their inactive 5’ AMP and 5’ GMP forms, respectively. PDEs are divided into 11 different families, which include 21 different genes and comprises more than 100 enzyme variants that are generated from multiple promoters and products of alternative splicing (Baillie et al., 2019; Maurice et al., 2014). The PDE isoforms are widely distributed in various organs and possess unique functional properties. The characterization and functional analysis of PDE family members revealed their versatile role in multiple areas, including the cardiovascular biology, neurology, oncology and the endocrine system. Activation of PDEs may abolish the cellular effects of cAMP and cGMP second messengers signaling, whereas inhibition of PDEs would increase cAMP and/or cGMP signaling. This is exemplified by the therapeutic importance of phosphodiesterase 5 (PDE5) inhibitors in perpetuating nitric oxide (NO)-cGMP mediated smooth muscle cell relaxation in the cardiovascular system. The clinical impact of PDE inhibition was widely appreciated after the landmark discovery of the vasodilatory property of NO in the cardiovascular system (Arnold et al., 1977). This landmark observation highlighted the importance of PDE5 inhibition in regulating the cGMP levels and vasodilation for a sustained period. PDE5 also has prominent roles in the regulation of vascular tone through maintenance of intracellular cGMP and calcium levels, particularly in the lung and penis (Bender and Beavo, 2006). Widely used PDE inhibitors such as sildenafil (PDE5)(Guazzi et al., 2011; Kim et al., 2015; Lewis et al., 2007), milrinone (Baim et al., 1983) (PDE3), roflumilast (PDE4) are considered prominent cardiovascular drugs along with non-PDE drugs like angiotensin converting enzyme (ACE) inhibitors (enalapril) (McMurray, 2010), calcium channel blocker (Amlodipine) (Wang, 2009) and β-adrenergic agonist (Isoprenaline) (Wallukat, 2002).
PDE1 is a dual substrate (cAMP and cGMP) diesterase and a promising target for the treatment of heart disease, cancer, neurological disorder and other endocrine abnormalities. Several factors such as their unique tissue distribution and species dependent expression of isoforms, activation by cofactors and spatial localization need to be evaluated to establish the possible role of PDE1 inhibition as a treatment option in various diseases.
2. Structure, Gene Variants, and Properties of Phosphodiesterase 1
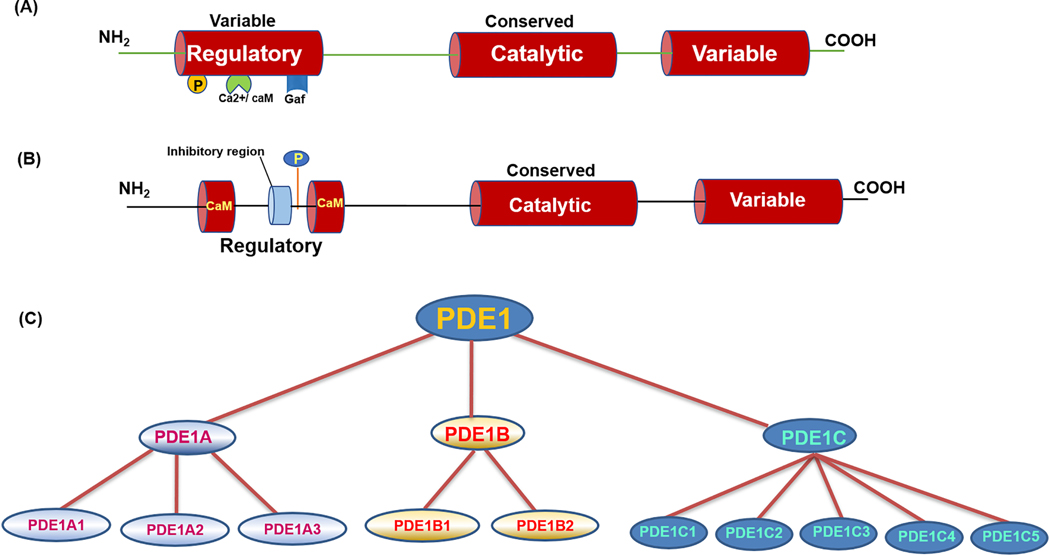
The basic structure of PDE consists of a variable amino terminal regulatory domain, a conserved catalytic domain of approximately 300 amino acids (20–25% identity with other isoforms) and a variable carboxyl terminal region (Fig. 1A). The catalytic domain contains the substrate binding pocket for cAMP and cGMP molecule and also encompasses the classic HD domain [(H(X)3H(X)25–35(D/E) and amino acid residues, histidine (H), aspartate (D) and glutamic acid (E)], which is a unique characteristic domain for metal-dependent phosphohydrolases enzymes (Aravind and Koonin, 1998). The presence of a unique allosteric binding domain confers each PDE their regulatory properties and their exclusive cellular function (Corbin et al., 2009; Pandit et al., 2009). The amino-terminal regulatory region of PDE encompasses several protein domains that are responsible for enzymatic activity, subcellular localization and their pharmacological properties (Kenan et al., 2000). The N-terminal end also contains essential sites for phosphorylation by cAMP-dependent protein kinase (PKA), protein kinase C (PKC) and cGMP-dependent protein kinase (PKG) as well as amino acid residues for ubiquitination (Wan et al., 2019). Apart from the conserved catalytic domain and HD domain, several PDEs have their distinct landmarks that defines their cAMP and cGMP hydrolyzing efficiency. For example, PDE5 contains two GAF domains (GAF-A and GAF-B) that increases their affinity towards cGMP molecule and acts as allosteric binding sites for cGMP (Heikaus et al., 2008). PDE1 is characterized by the presence of a Ca2+/calmodulin (CaM) binding site and is activated by the increase in cellular concentration of Ca2+/CaM (Fukunaga et al., 1984). The typical domain structure and the regulatory regions of the PDE1 subtype are presented in Fig.1B.
Figure 1.
(A). General domain organization of phosphodiesterase (PDE). PDE consists of variable amino terminal and carboxy end sequences responsible for gene splicing, isoform variation and a conserved catalytic domain with binding site specific for substrates and a metal binding motif. The regulatory amino terminal region containing amino acid residues correspond to phosphorylation, calcium calmodulin activation, allosteric GAF binding domain for catalytic activity. (B) Basic domain arrangement of PDE1 subtype. PDE1 family of isoforms contain unique calcium calmodulin binding domain (Ca2+/CaM) that leads to the activation of enzyme and other amino acid residues for phosphorylation and inhibition properties. (C) The family tree of PDE1 gene. There are multiple isoforms of enzyme due to alternative splicing and multiple promoter activity.
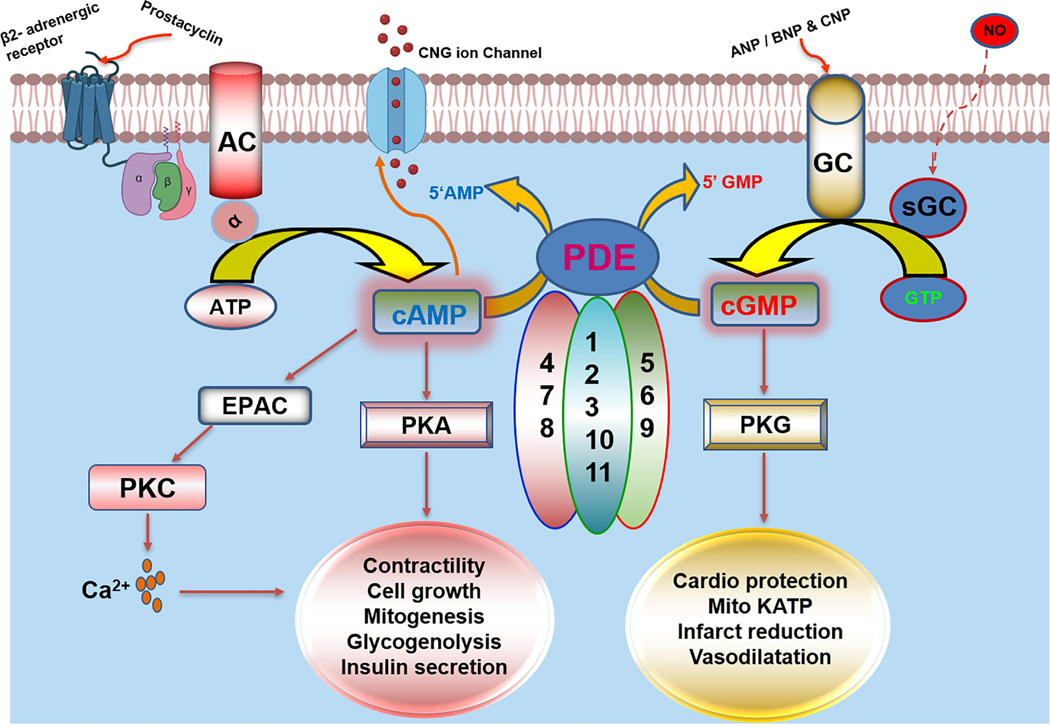
PDEs exhibit unique substrate specificity and hydrolyze either cAMP/cGMP or both. PDE isoenzymes 4, 7 and 8 are specific for cAMP degradation, whereas PDEs 5, 6 and 9 isoforms exclusively breakdown cGMP. PDE subtypes 1–3,10 and 11 hydrolyze both cAMP as well as cGMP (Fig. 2). These PDEs vary in their primary amino acid sequence as well as their kinetic and regulatory properties (Conti and Beavo, 2007). The cellular distribution, distinct compartmentalization, activation and inhibition by various effector molecules make PDE an attractive target for drug intervention in pathophysiological conditions. PDE1 is one of the foremost isoforms identified as a dual-substrate (hydrolyze both cAMP and cGMP) esterases and is the only PDE stimulated by Ca2+/CaM (Cheung, 1970; Sonnenburg et al., 1993).
Figure 2. Schematic overview of fundamental functions and regulations of phosphodiesterase (PDE) family of enzymes.
PDE enzymes are activated by multiple external stimuli through ligand binding to G-protein coupled receptor (GPCR) and synthesis of 3’,5’-cyclic adenosine monophosphate (cAMP) by adenylate cyclase or activation of guanylyl cyclase by ANP/BNP/CNP and nitric oxide produce cyclic guanosine monophosphate (cGMP). cAMP regulates its downstream target largely through activation of protein kinase A (PKA), EPAC or protein kinase C (PKC), while cGMP synthesis results in activation of protein kinase G (PKG) and controls various biological processes. PDE enzymes are substrate specific and gene families such as PDE 4,7 and 8 solely hydrolyze cAMP molecules while PDE subtypes 5,6 and 9 are unique for degradation of cGMP substrates. PDE 1,2,3,10 and 11 are family of enzymes that are dual substrate specific and can hydrolyze both cAMP and cGMP second messenger molecule.
PDE1 is encoded by three genes, namely PDE1A, 1B and 1C, which have several splice variants and show distinct distributions in multiple species, including humans, mice and rats (Francis et al., 2011; Kakkar et al., 1999; Lugnier, 2006). Fig. 1C shows the family tree of PDE1 gene and its subtypes identified from different mammalian tissues and species. PDE1A has three isoforms PDE1A1, PDE1A2 and PDE1A3 (Fidock et al., 2002; Yan et al., 1996). PDE1A1 is a 59 kDa protein isolated from bovine brain (Novack et al., 1991; Sonnenburg et al., 1995). PDE1A2 is a 61 kDa protein also obtained from bovine brain tissue (Charbonneau et al., 1991; Sonnenburg et al., 1993). PDE1A3 cDNA was isolated from human tissue using bovine cDNA fragment (61-kDa PDE1A2) as probe and has an additional 42-nucleotide insertion sequence than PDE1A2 (Loughney et al., 1996). All PDE1A isoforms are characterized by the presence of common Ca2+/CaM binding site. However, these isoforms possess different affinity towards Ca2+/CaM due to the variations in the amino acid sequence at the regulatory N-terminal region (Florio et al., 1994). PDE1B gene consists of two isoforms, PDE1B1 (encoding 63 kDa protein with 536 amino acid residues) and PDE1B2 (encoding 59 kDa protein containing 516 amino acid residues), which were identified using human cDNA library and lymphoblastoid B-cell line, respectively (Bentley et al., 1992; Jiang et al., 1996; Yu et al., 1997). PDE1B1 and PDE1B2 differ in their amino acid content in the N-terminal sequence due to the existence of alternate first exon. PDE1B1 contains a unique 38-amino acid residues in its N-terminal end, whereas PDE1B2 consists of 18 amino acid residues in its N-terminus (Bentley et al., 1992; Repaske et al., 1992). PDE1C gene contains multiple isoforms and responds differently to various cell-specific stimuli. So far, five different subtypes have been reported ranging from PDE1C1 to 1C5 and each isoform has their unique function in various disease conditions (Yan et al., 1996; Yan et al., 1995). PDE1C subtypes have a longer carboxy region than PDE1A and PDE1B and therefore result in large molecular weight protein transcripts that range from 72 to 87 kDa.
The km for all three PDE1 enzymes for cGMP is in the range of 1 to 5 μmol/L, among them, PDE1A and 1B have high affinity towards cGMP substrate, whereas PDE1C hydrolyses both cAMP and cGMP with equal efficacy. PDE1A affinity towards cAMP is lower (Km=50 to 100 μmol/L) than cGMP (Km=1 to 5 μmol/L). Compared to PDE1C4, PDE1A2 has an approximately 30-fold greater Km for cAMP. PDE1B has a slightly higher affinity for cAMP (Km=7 to 24 μmol/L) compared to PDE1A (Loughney et al., 1996). The cAMP hydrolysing capacity of PDE1A and B can be hindered by the level of cGMP in the system (Yan et al., 1996) with the exception of PDE1C, which can hydrolyze both cAMP and cGMP with equal efficacy. These enzyme kinetics become a crucial factor during the substrate availability and the preferential activation of PDE1C isoforms under certain cell stimuli. Table 1 shows the Km and affinity of each PDE1 isoforms towards cAMP and cGMP and its half-maximum inhibitor concentration for Ca2+/CaM binding. The varying enzyme kinetics among the PDE1 isoforms highlights a critical function in the cross talk between cAMP and cGMP signaling. Similarly, the role of these genes varies from species to species: for example PDE1C regulates the proliferative phenotype of smooth muscle cells (SMCs) in human (Rybalkin et al., 2002) whereas in rodent PDE1A maintains the quiescent phenotype of SMCs, while PDE1C expression remains unchanged or have a minor impact. Even though the dual substrate specificity of PDE1 gives the advantage to hydrolyze both cAMP as well as cGMP, the availability and proximity of these nucleotide second messenger to PDE1 enzyme is crucial and needs further investigation. Moreover, the lack of isoform-specific PDE1 inhibitor impedes the current research progress on dissecting the exact role of each PDE1 isoforms.
Table 1.
Summary of enzyme kinetics, tissue expression, inhibitors and function of phosphodiesterase 1 superfamily.
| PDE1 type | Km cAMP (μm) | Km cGMP (μm) | Vmax (μm) cAMP/cGMP | IC50 (μm) for (Ca2+) | Inhibitor | Tissue Expression | Function |
|---|---|---|---|---|---|---|---|
| PDE1A/A1 (Novack et al., 1991; Sonnenburg et al., 1995) | 110–115 | 5 | 2–3 | 0.3 | Trifluoperazine, Vinpocetine, 8-MMX | Brain, cerebral cortex and hippocampus, smooth muscle cell, heart, testis, kidney, liver, and pancreas, B16F10 melanoma cells | Proliferation (Jeon et al., 2010; Miller et al., 2011), activates p27Kip1 and regulates cell cycle (Jeon et al., 2010), vascular contraction of mesenteric arteries (Giachini et al., 2011), sperm function (Lefievre et al., 2002), nitrate tolerance (Kim et al., 2001), myocardial alpha-crystallin B chain (Zhang et al., 2019), autosomal dominant polycystic kidney disease (ADPKD) (Ong, Devuyst, Knebelmann, & Walz, 2015). |
| PDE1A2 (Charbonneau et al., 1991; Sonnenburg et al., 1993) | 110–114 | 5 | 2–3 | 2.0 | 8-MMX, ITI-214 | Brain and neuronal tissue | Dopaminergic stimulation (Polli & Kincaid, 1992), Parkinson’s Disease(Nature Reviews. Drug Discovery, 2011). |
| PDE1A3 (Loughney et al., 1996) | Lu AF41228 and Lu AF58027 | Multiple human tissues | Hypertension (Laursen et al., 2017). | ||||
| PDE1B1 (Bentley et al., 1992; Jiang et al., 1996; Yu et al., 1997) | 24.3 | 3 | 0.9 | 1.3 | Vinpocetine, ITI-214 | Human brain, striatum, neurons, lymphocytes, smooth muscle, heart, skeletal muscle, leukemic cells | Dopaminergic function and neuronal learning (Reed, Repaske, Snyder, Greengard, & Vorhees, 2002), induces apoptosis in leukemia cells (Jiang et al., 1996), Alzheimer disease (Dyck et al., 2017; Pekcec et al., 2018; Snyder et al., 2016). |
| PDE1B2 (Fidock et al., 2002) | N/A | Macrophages, lymphocytes and lymphoblastoid B-cell | Monocyte-macrophage differentiation (Bender et al., 2004; Bender et al., 2005). | ||||
| PDE1C/C1 (Loughney et al., 1996; Yan et al., 1995) | 3.5 | 2.2 | 1.3 | 3.0 | 8-MMX, IC86340, 8-MM-IBMX Vinpocetine | Brain, cardiovascular system, cardiomyocyte, smooth muscle cell, pancreatic islets, osteosarcoma MG-63 cells, human glioblastoma SNB75 CNS system | Hypertrophy, fibrosis, vascular remodeling, (Schermuly et al., 2007), cardiomyocyte apoptosis (Zhang et al., 2018), vascular remodeling (Cai et al., 2011), pulmonary arterial hypertension, insulin secretion, (Pratt et al., 2019; Pyne & Furman, 2003), proliferation of migration of glioblastoma (Rowther et al., 2016),age related cAMP regulation (Kelly et al., 2014). |
| PDE1C2 (Yan et al., 1996) | 1.2 | 1.1 | 1.2 | 0.8 | Zaprinast and Vinpocetine | Human brain, heart, lung and kidney, olfactory epithelium | Odorant stimulation (Yan et al., 1995). |
| PDE1C3 (Loughney et al., 1996) | 0.3 | 0.6 | 1 | N/A | N/A | Newborn and adult aortas | Unknown |
| PDE1C4 (Yan et al., 1996) | 1.1 | 1.0 | 1.0 | 2.4 | N/A | Testis | Unknown |
Note: PDE1A and 1B have higher affinity towards cGMP substrate, while PDE1C hydrolyzes both cAMP and cGMP with equal efficacy. PDE1, kinetics data are adapted from Sonnenburg, W et al. 1998. Abbreviations: 8-MMX - 8-methoxymethyl-3-isobutyl-1-methylxanthine, IBMX- 3-isobutyl-l-methyl-xanthine, N/A- not available/reported.
3. cAMP and cGMP Signaling and their Cross-Talk
Second messengers cAMP and cGMP regulate a myriad of functions in the cardiovascular system, such as vasodilation, ventricular contraction, cardiac hypertrophy, apoptosis and cell metabolism. The intracellular levels of cAMP and cGMP molecules are maintained through a delicate balance between their synthesis by adenylate cyclases (AC) and guanylate cyclases (GC) and their degradation by the PDEs. cAMP exerts its function mainly via protein kinase A (PKA), the exchange protein directly activated by cAMP (EPAC) (de Rooij et al., 1998), and cyclic nucleotide-gated (CNG) ion channels, whereas cGMP largely functions through its downstream effector,PKG (Park et al., 2018). PKA mediates various cellular functions by direct phosphorylation of its protein targets and regulates events such as cell movement, migration, hormone secretion, cellular metabolism, and gene transcription (Francis et al., 2011). In the cardiovascular system, PKA controls the vascular tone through regulation of potassium (K+) channels (Johnson et al., 2009), modulation of Ca2+ sensitivity of the contractile apparatus in SMCs (Nishimura et al., 2001) and vascular permeability through phosphorylation of myosin light chain in endothelial cells (Moy et al., 1993). PKA also controls the catecholaminergic dependent force and frequency of cardiac contraction (Xiang and Kobilka, 2003) through L-type Ca2+ channels (LTCCs), ryanodine receptors (RyRs), and phosphorylation of phospholamban (PLB) in cardiomyocytes (Bers, 2008). Moreover, protein-protein interaction between A-Kinase–anchoring proteins (AKAPs) and PKA plays a crucial role in the uptake of Ca2+ by the sarcoplasmic reticulum (Lygren et al., 2007). PKA also participates in the regulated release of fatty acids and lipids for cardiac energy metabolism (Pollak et al., 2015). Thus, given the importance of cAMP dependent regulation of cardiac function, PDEs are good candidates for therapeutic intervention in cardiac disease.
The role of NO-cGMP pathway in cardiac disease has long been appreciated and continues to evolve to new paradigm (Michel and Loscalzo, 2015). The identification of the vasodilatory property of NO established the importance of cGMP molecule in cardiac physiology. Furthermore, treatment options targeted to increase the cGMP levels by using nitrate donors and natriuretic peptides are often hindered by the upregulation of PDEs. Therefore, inhibition of cGMP specific PDEs is an attractive approach to exploit the beneficial effects of sustained cGMP signaling in the cardiovascular system. NO-cGMP–PKG signaling is also implicated in the cardioprotection against ischemia/reperfusion (I/R) injury (Das et al., 2015; Kukreja et al., 2005). Activation of PKG via cGMP elevation has been shown to regulate the opening of the mitochondrial KATP channel in heart and cardiomyocyte (Das et al., 2009; Das et al., 2005; Kodani et al., 2002; Ockaili et al., 2002; Oldenburg et al., 2004; Salloum et al., 2009; Salloum et al., 2006). Studies in adult rat cardiomyocytes also provided evidence for cGMP-PKG role in attenuating I/R-induced apoptosis and necrosis (Das et al., 2006; Das et al., 2008; Kukreja, 2012; 2013; Kukreja et al., 2012).
Monitoring Ca2+ oscillation in the heart and real-time localization of cAMP in cells offered greater understanding on the temporal and spatial compartmentalization of PDEs (Tian et al., 2011). The complexity involved in the maintenance of cAMP and cGMP nucleotide levels and their relevance to the pathophysiology of diseases is further amplified by the possibility of the cross-talk between cAMP and cGMP signaling mediated by PDEs and their cellular localization. (Pavlaki and Nikolaev, 2018; Zaccolo and Movsesian, 2007). The mere presence of multiple PDE isoforms solely dedicated for the degradation of cAMP and cGMP and the existence of several gene products of AC, which catalyze the formation of cAMP, suggest the complex web of signaling network among these proteins. For instance, the cAMP-hydrolyzing activity of several families of PDEs found in the heart is differentially regulated by the concentration of cGMP level (Zaccolo and Movsesian, 2007). Binding of cGMP molecule to the allosteric site of PDE2 stimulates the hydrolysis of cAMP, whereas excessive accumulation of cGMP in the cell acts as a competitive inhibitor of cAMP hydrolysis by PDE3 (Hambleton et al., 2005; Stangherlin et al., 2011; Weber et al., 2017). Several cGMP-mediated responses in cardiac cells, including potentiation of Ca2+ currents and diminished responsiveness to β-adrenergic receptor agonists, are in part due to cGMP level dependent effect on the hydrolysis of cAMP (Zaccolo and Movsesian, 2007). Negative feedback mechanisms are also involved in the maintenance of cAMP and cGMP homeostasis. A classic example is the inhibition of PDE3 (which hydrolyzes both cAMP and cGMP) by elevated cGMP levels. This interplay has a huge effect in terms of in vivo translational studies, where it has been shown that PDE5 inhibition also reaps the beneficial effects of PDE3 blockade (Afzal et al., 2011).
The competitive substrate preference also plays a role in the regulation of PDE1, where hydrolysis of cAMP is compromised because cGMP is the preferred substrate of PDE1A and 1B, which have more affinity towards cGMP than cAMP (Bender and Beavo, 2006; Bender et al., 2005; Loughney et al., 1996). Such an effect is also evident with PDE2 isoforms, activated by cGMP (Mongillo et al., 2006). The feedback activation of PDE2 by β-adrenergic stimulation through NO-dependent increase in cGMP leads to the hydrolysis of cAMP levels in the cell (Mongillo et al., 2006). This, in turn, prevents the excessive activation of β-adrenergic complex at the resting level and interference of normal function. The discussion about the cAMP and cGMP crosstalk brings an interesting topic of compartmentalization and the presence of isolated pools of cAMP and cGMP in cardiac vasculature. Fluorescence Resonance Energy Transfer (FRET) based analysis showed confined packets of cAMP and cGMP molecules in rat cardiomyocytes (Zaccolo et al., 2005; Zaccolo and Pozzan, 2002). Also, the unique external stimuli that leads to the synthesis of cAMP and cGMP plays an important role in the activation of the specific isoform(s) of PDE. For example, the synthesis of cAMP mediated by the activation of β1 adrenergic receptor agonists was exclusively hydrolyzed by PDE4, whereas cAMP generated by the β2 adrenergic receptor agonists was degraded by other PDE isoforms (Nikolaev et al., 2006).
4. PDE1 in Cardiovascular System
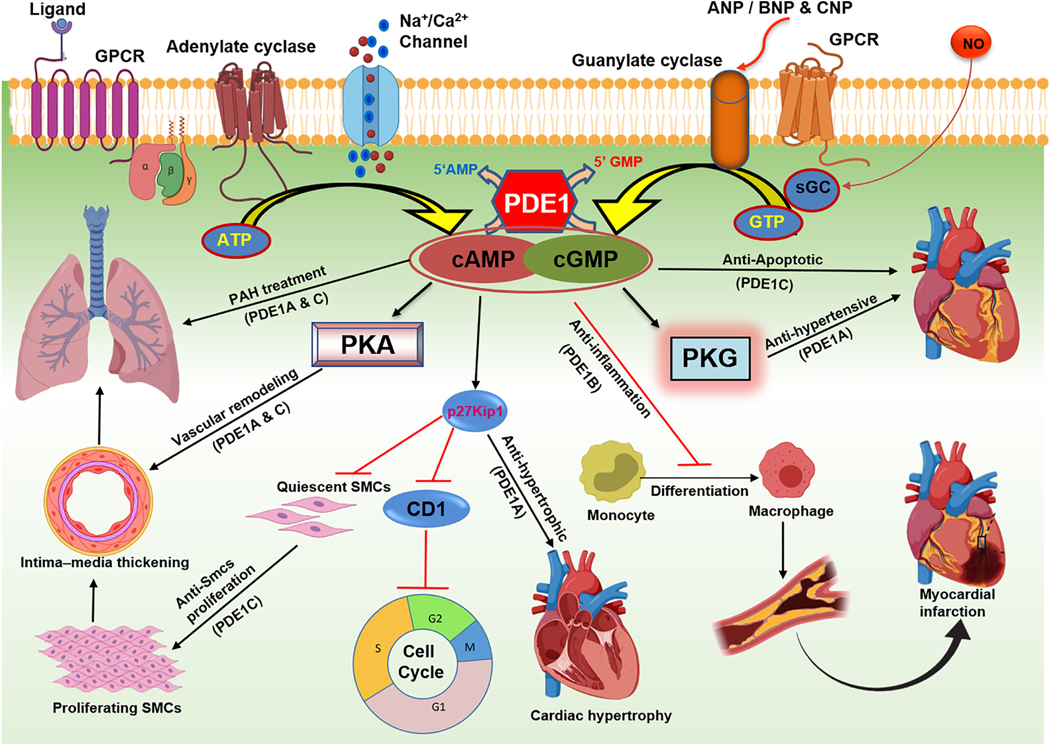
Seven different PDEs are expressed in the heart, PDE1–5, 8, and 9, which play important roles in regulating cardiac metabolism and function (Kostic et al., 1997; Loughney et al., 1996; Meacci et al., 1992; Onody et al., 2003; Senzaki et al., 2001; Soderling et al., 1998). Among these, PDE1 is present in abundant level in the heart and recent studies have confirmed its greater functional role in cardiac physiology and pathophysiology than previously realized (Dey et al., 2020; Hashimoto et al., 2018) PDE1 plays an important role in cardiac muscle physiology owing to its activation by intracellular calcium concentration and regulates cell metabolism as well as hypertrophy (Miller et al., 2011). PDE1A is mostly concentrated in the smooth muscle layer of muscular arteries (Sonnenburg et al., 1998) Since heart is an active organ that undergoes repeated cycles of contraction and relaxation, there is an associated dynamic oscillation of intracellular concentration of calcium. A detailed diagram representing the function and mechanism of PDE1 in various biological processes including the role in cardiovascular system is presented in Fig. 3.
Figure 3. Diagrammatic illustration of phosphodiesterase 1 (PDE1) enzyme functions in cardiovascular system.
Inhibition of PDE1A isoform results in vascular remodeling and inhibition of smooth muscle cells (SMCs) proliferation in lung vasculature. PDE1A inhibitors are used to treat pulmonary arterial hypertension (PAH) through blocking neo-intimal thickening. Inhibition of PDE1C attenuates apoptosis and results in the survival of cardiomyocytes. PDE1A inactivation increases the level of cGMP and reduces systemic blood pressure through PKG dependent mechanism. Abnormal upregulation of PDE1 isoforms leads to cardiac hypertrophy and its inhibition increases p27Kip1 level while simultaneously decreases cyclin D1 (CD1) through cAMP accumulation. Consequently cell cycle arrest results in anti-hypertrophic activity in heart. Activation of PDE1B promotes monocyte to macrophage differentiation and its inhibition reduces inflammation through upregulation of cGMP.
All three PDE1 isoforms have been associated with diverse cellular processes. PDE1 expression and activity were detected in the heart and cardiomyocytes from various mammalian species, including human, mouse, rat, bovine, and canine (Clapham and Wilderspin, 2001; Loughney et al., 1996; Miller et al., 2011; Vandeput et al., 2007), whereas the functional role for each of the PDE1 isoforms appears to be mainly species dependent. For example, PDE1C is the dominant isoform in humans that regulates smooth muscle cells (SMCs) proliferation (Rybalkin et al., 2002; Rybalkin et al., 2003), whereas PDE1A is the predominant isoform, which controls vascular SMCs proliferation in rodents (Johnson et al., 2012). PDE1A increases vascular contraction of mesentric arteries upon angiotensin II (Ang II) infusion in rats (Giachini et al., 2011) and also regulates cGMP hydrolysis in cardiomyocyte and vascular SMCs (Miller et al., 2011), which in turn controls smooth muscle tone (Nagel et al., 2006). Cell-based experiments uncovered distinct function of cytosolic and nuclear PDE1A. Cytosolic PDE1A was responsible for contractile phenotype of cardiomyocytes via phosphorylation of myosin light chain, whereas nuclear PDE1A contributed to proliferative phenotype by preventing cell cycle arrest at G1 stage (Jeon et al., 2010). Thus, blocking PDE1A reduced proliferation of vascular SMCs by increasing p27Kip1 and simultaneously downregulating cyclin D1. PDE1A stabilized nuclear β-catenin protein via the association with PP2A-GSKβ interaction (Jeon et al., 2010). In addition, the shuttling of PDE1A between cytosol and nucleus adds another dimension to PDE1A role in the maintenance of vascular tone. In the normal heart, PDE1A is localized mainly in the cytosol and translocates to the nucleus upon SMCs proliferation stimuli (Nagel et al., 2006). Nuclear PDE1A is critical for the synthesis of SMCs growth and survival (Nagel et al., 2006). Ang II or TGF-β mediated activation of cardiac myofibroblasts resulted in the induction of PDE1A, which caused fibrotic remodeling.Inhibition of PDE1A through either short hairpin RNA (shRNA) or pharmacological inhibitor IC86340, attenuated adverse cardiac remodeling through EPAC and PKG (Miller et al., 2011).
A possible link between PDE1 activity and development of nitrate tolerance where a 2.3-fold increase in PDE1A1 protein expression was observed in the nitroglycerin-tolerant rat aortas (Kim et al., 2001). A feedback activation of PDE1A1 by NO-cGMP signaling pathway in vascular SMCs was attributed as a major factor for nitrate tolerance in patients (Kim et al., 2001). In addition, pharmacological inhibition of PDE1 partially restored the sensitivity of the tolerant vasculature to subsequent nitroglycerin treatment. Therefore, the induction of PDE1A1 in nitrate-tolerant blood vessels is likely a key mechanism by which NO/cGMP-mediated vasodilation is desensitized and Ca2+ mediated vasoconstriction is hyper-sensitized. The beneficial effects of vasodilators such as nitroglycerin are limited by induction of PDE1A1. Thus, the inhibition of PDE1A1 may be a novel therapeutic approach to limit nitrate tolerance.
The role of PDE1B in the heart remains elusive. It has been suggested that PDE1B contributes to the pathological vascular remodeling through enhancing the differentiation of monocytes to macrophages. PDE1B increased macrophage maturation via cGMP hydrolysis in HL-60, a macrophage derived cell line (Bender et al., 2004; Bender et al., 2005). PDE1B may promote tissue inflammation during myocardial infarction (MI) by facilitating abnormal infiltration of macrophage and other immune cells into the myocardium (Hu et al., 2010; Moriwaki et al., 2004), which may eventually lead to cardiac tissue injury and fibrosis (Schulze and Lee, 2004). Thus, the recruitment of PDE1B into the myocardium could be deadly in pathologic conditions like atherosclerosis and diabetes.
PDE1C is abundantly expressed in the myocardium and serves as a mediator of SMCs proliferation in humans. PDE1C is expressed in primary cultures of SMCs derived from explants of human newborn and adult aortas, and in SMCs cultured from severe atherosclerotic lesions (Rybalkin et al., 1997). In fact, PDE1C is the primary isoform found in human cardiac tissues, localized along the Z and M bands of cardiomyocytes (Vandeput et al., 2007). The global RNA sequencing identified enhanced expression of PDE1C in failing heart tissues of the patients with ischemic heart disease and dilated cardiomyopathy (Vandeput et al., 2007). In dogs and rabbits, cardiac expression of PDE1C is greater than PDE1A, similar to humans, whereas abundant expression of PDE1A over PDE1C is found in mice and rats (Hashimoto et al., 2018) On the contrary, even though the expression of PDE1C mRNA was detected in mice, it did not translate to detectable protein level (Miller et al., 2011). Preconditioning with 7-oxo-prostacyclin upregulated PDE1C protein levels (Kostic et al., 1997) in rat hearts, suggesting a possible feedback desensitization of beneficial effects of 7-oxo-prostacyclin similar to nitroglycerin treatment.
PDE1C is also involved in vascular remodeling, especially SMCs proliferation and collagen deposition (Knight et al., 2016), which is supported by the correlation between collagen deposition and increased PDE1C expression and attenuation of vascular remodeling by the PDE1 inhibitor IC86340 (Cai et al., 2011). Though the expression of PDE1C is less abundant in rodents (Hashimoto et al., 2018), genetic deletion of PDE1C in mice attenuated transaortic constriction (TAC)-induced cardiac structural remodeling. In human saphenous vein explants, induction of PDE1C expression was associated with collagen deposition and PDE1 inhibition with IC86340 attenuated vascular remodeling (Cai et al., 2011). A recent study described unique mechanism underlying PDE1C dependent protection against stress-induced apoptosis in cardiomyocytes (Zhang et al., 2018b). A multi-protein complex comprising adenosine A2 receptor (A2R), PDE1C, and transient receptor potential channel 3 (TRPC3) was identified in which PDE1C was activated by TRPC3-derived Ca2+, thereby antagonizing A2R-cAMP signaling and promoting cardiomyocyte death/apoptosis. Likewise, isolated adult mouse cardiomyocytes treated with doxorubicin and/or a quinazoline-based PDE1 inhibitor for 24 hours showed significant reduction of necrosis as well as apoptosis and preservation of mitochondrial membrane potential. These results suggested that PDE1 inhibition could potentially be an efficient therapeutic strategy to reduce doxorubicin-induced cardiotoxic effects (Vigneshwar et al., 2019). Treatment with the selective PDE1 inhibitor ITI-214 induced positive inotropic, lusitropic, chronotropic, and vasodilatory effects in conscious dogs and rabbits under normal or tachycardia pacing-induced heart failure (Hashimoto et al., 2018). Similarly, PDE1 inhibition with non-specific inhibitor, BTTQ reduced peripheral vascular resistance to a maximum of 24 mmHG in spontaneously hypertensive Dahl salt-sensitive rats (Dey et al., 2020). However, BTTQ significantly increased heart rate, and symptoms of tachycardia-like effects were also reported. In a rat model of hypertension, novel selective PDE1 inhibitors (Lu AF41228 and Lu AF58027) promoted vasodilatation and lowered blood pressure via suppression of PDE1A, PDE1B and PDE1C enzyme activities (Laursen et al., 2017).
PDE1 inhibitors were also beneficial in alleviating vascular aging (Bautista Nino et al., 2015). Senescent human vascular SMCs have elevated PDE1A, PDE1C, and PDE5 mRNA levels, which are associated with the markers of cellular senescence. PDE1 inhibition lowered the expression of these markers, suggesting its important role in the aging-related loss of vasodilator function. Thus PDE1 inhibitors could be potentially explored to improve healthy cardiovascular aging in the general population (Bautista Nino et al., 2015).
Vinpocetine, a derivative of the alkaloid vincamine, is a PDE1 inhibitor, which raises cAMP and cGMP levels has been approved in European countries for the treatment of dementia and stroke for over 30 years, and it is available in the US as a dietary supplement (e.g., Cavinton or Intelectol, Richter Gedeon; Cognitex, Life Extension). Vinpocetin partially restores the vasodilation by increasing cGMP level and decreasing Ca2+ concentration in rat aortic vasculature (Giachini et al., 2011). Pharmacologic preconditioning with vinpocetine was recently shown to reduce the oxidative stress levels via enhancement of antioxidant enzyme activity (Ristic et al., 2020). The effect of myocardial preconditioning was studied by treatment with vinpocetine (10 mg/kg/day) alone or in combination with a non-pharmacological preconditioning maneuver (i.e. exercise training). Several serological markers of oxidative stress including reactive oxygen species (ROS; H2O2, superoxide anion) and thiobarbituric acid reactive substances were measured. Treatment with Vinpocetine alone or in combination with exercise, significantly reduced ROS levels and increased the antioxidant enzyme activities such as catalase (Ristic et al., 2020). PDE1 inhibition also alleviated cytotoxic stress in heart failure with preserved ejection fraction (HFpEF), eliminated the misfolded myocardial alpha-crystallin B chain protein through increased levels of PKA as well as PKG and improved the survival of mice (Zhang et al., 2019).
5. PDE1 in Pulmonary Arterial Hypertension
The possible use of PDE1 inhibitor as a protective agent is also extended to a few other types of cardiovascular pathologies, especially the pulmonary arterial hypertension (PAH). PAH is a severe and poorly understood disease that affects the lungs and right ventricle (RV) of the heart. PAH is defined as elevated resting mean pulmonary arterial pressure above 20 mmHg in the presence of a pulmonary capillary wedge pressure less than or equal to 15 mmHg (Gaine and Rubin, 1998). It can occur as a primary idiopathic disease with a sporadic/familial mutation, specifically bone morphogenetic protein receptor II mutations, or can manifest secondary to left heart failure, congenital heart disease, chronic obstructive pulmonary disease, or sustained hypoxia (Murray et al., 2007). The disease process begins with prolonged pulmonary vasoconstriction and structural remodeling of small pulmonary arteries leading to increased pulmonary vascular resistance which raises right ventricular afterload ultimately leading to RV failure and death (Humbert et al., 2004). While a rare disease, increasing mortality rates in the past decade indicate a need for improved treatment options. In the pulmonary system, endothelial nitric oxide synthase (eNOS) produces NO in response to hypoxia, inflammation and oxidative stress. NO activates soluble guanylate cyclase in SMCs (Napoli and Loscalzo, 2004; Xue and Johns, 1995), which in turn, catalyzes the formation of cGMP from GTP. In PAH, lung tissue exhibits decreased eNOS expression and patients are reported to have low levels of exhaled NO resulting in low cGMP, decreased vasodilation and increased vascular resistance (Girgis et al., 2005). Patients with severe PAH are manifested by low NO production and enhanced activation of PDE5 enzyme, which results in overall decrease of cGMP levels. Treatment with PDE5 inhibitors reduce pulmonary artery pressure by increasing cGMP levels, prolonging vasodilation, and in turn, decreasing vascular resistance. The current treatment options for PAH include prostanoid replacement as well as endothelin receptor antagonists, all of which directly or indirectly target cGMP or cAMP signaling (O’Callaghan et al., 2011). PDE5 inhibitor sildenafil, was approved for the treatment of PAH by the Food and Drug Administration (FDA) in 2005 and was clinically proven to extend the life expectancy of PAH patients (Archer and Michelakis, 2009). However, a sustained high levels of cGMP in the system increases intracellular calcium and activates cascade of events that leads to cell proliferation (Wang et al., 2008).
Endothelial prostacyclin produced by prostaglandin H2 drives cAMP production via prostacyclin synthase. Levels of prostacyclin synthase in pulmonary arteries and urinary metabolites of prostacyclin are significantly reduced in PAH patients resulting in decreased cAMP vasodilation (Christman et al., 1992; Tuder et al., 1999). The importance of cAMP is demonstrated by the successful use of prostacyclin analogs, such as Epoprostenol, to treat PAH. Several studies have shown the importance of both cAMP and cGMP signaling in PAH development (Johnson et al., 2012). cAMP elevating agents, such as iloprost or epoprostenol, in conjunction with cGMP elevating PDE inhibitors have synergistic effect in the treatment of PAH (Ghofrani et al., 2002; Simonneau, 2008).
Current research on the role of PDE1 in PAH is in infancy, with majority of the work being performed in animal models. The role of PDE1 in pulmonary disease is detailed in Fig 3. The PDE1 isoforms are activated by Ca2+/CaM and are abundantly expressed in pulmonary SMCs. With their cAMP and cGMP co-specificity, PDE1 isoforms could be a promising new target for therapeutic intervention for PAH. Levels of cAMP and cGMP are significantly decreased in a chronic hypoxia rat model of PAH. This decrease in cyclic nucleotides corresponded with a profound increase in the PDE1 activity in main pulmonary arteries (Maclean et al., 1997). Rat model of monocrotaline (MCT)-induced PAH showed that PDE1A expression was significantly and specifically upregulated in pulmonary arterial SMCs of MCT injected rats, with no expression changes found in aortic tissue. Treatment with the PDE1 inhibitor, 8-methoxymethyl-isobutyl-1-methylxanthine (8MM-IBMX), significantly improved hemodynamics, right heart hypertrophy and vascular remodeling (Schermuly et al., 2007). Moreover, 8MM-IBMX reversed the acute hypoxia-induced vasoconstriction in isolated mouse lungs and improved RV hypertrophy, vascular remodeling and hemodynamics in chronically hypoxic mice (Schermuly et al., 2007).
The effect of PDE1 inhibitors on vascular tone was also demonstrated in an ovine model of acute thromboxane-induced PAH. Vinpocetine augmented the pulmonary vasodilator response and transpulmonary cGMP release after inhalation (Evgenov et al., 2006). These promising results in animal models have been carried over to human tissue studies. Human pulmonary artery lung tissue of patients with PAH demonstrated strong immunoreactivity of PDE1C with specific changes in the medial layer of the pulmonary arteries. At the same time, only weak expression of PDE1C was detected in healthy donor pulmonary arteries. Furthermore, PDE1 inhibitor, PI79, dose dependently inhibited DNA synthesis of human pulmonary artery SMCs (Schermuly et al., 2007). Interestingly, the porcine coronary artery subjected to hypoxic vasoconstriction demonstrated that PDE1 inhibition with 8-MM-IBMX or PDE5 inactivation using zaprinast increased cGMP level and augmented hypoxic constriction without interfering with the relaxation response (Nan et al., 2020). Furthermore, administration of BTTQ alone was reported to reduce systemic blood pressure by up to 24 mmHg in Dahl salt-sensitive rats and an additional 22 mmHg with a supplemental ACE inhibitor lisinopril (Dey et al., 2020). Similarly, inhibition of all PDE1 isoforms with Lu AF41228 and Lu AF58027 reduced mean arterial blood pressure of about 10–15 mmHg in both conscious and anesthetized phenylephrine-induced rat model of hypertension (Laursen et al., 2017).
PDE1A and PDE1C expression was enhanced in pulmonary artery SMCs of both idiopathic PAH (IPAH) and secondary PAH (SPAH) patients when compared to control patients (Schermuly et al., 2007). In addition, cAMP levels were significantly reduced in IPAH and SPH pulmonary artery SMCs (PASMCs). Increased PDE1 activity accounted for a large portion of the reduced cAMP levels and increased proliferation of IPAH and SPH PASMCs. Furthermore, treatment with PDE1C-targeted small interference RNA enhanced cAMP accumulation and inhibited cellular proliferation to a greater extent in PAH PASMCs than in the control group. Taken together, PDE1 seems to play a major role in the development of both idiopathic and SPAH and its use as a novel therapeutic strategy may lead to exciting advances in the treatment of this severe and debilitating disease.
6. PDE1 and Cancer
Intracellular cAMP/cGMP signaling is tightly woven into the development of tumors by regulating cellular proliferation, growth, and apoptosis (Das et al., 2010; Peng et al., 2018). Several studies reported that overexpression of individual PDE isozymes in various tumor tissues play a critical role in tumor development by negatively regulating the intracellular cAMP and/or cGMP levels (Marko et al., 1998; Zhang et al., 2008). Stimulation of intracellular cAMP/cGMP signaling with specific PDE inhibitors could provide efficient antitumor therapy by inhibiting disease progression with reduced adverse effects as compared with other chemotherapeutic agents (Abusnina et al., 2011a; Abusnina et al., 2011b; Rowther et al., 2016). Specifically, several studies have described that restoration of intracellular cGMP levels prevents hypoxia-induced resistance to chemotherapeutic agents and cancer progression (Bell et al., 2007; Frederiksen et al., 2007; Siemens et al., 2009; Yasuda et al., 2006). PDEs therefore show promise as potential diagnostic markers or therapeutic targets in multiple cancers.
Non-selective PDE inhibitors and selective inhibitors of cGMP-specific PDE enzymes have demonstrated antitumorigenic potency through induction of apoptosis in malignant cells and indirectly by enhancing the efficacy of other chemotherapeutic drugs in various cancers (Abdollahi et al., 2003; Bender and Beavo, 2006; Black et al., 2008; Booth et al., 2014; Maxwell et al., 2004; Sarfati et al., 2003; Zhu et al., 2005). We and others demonstrated that PDE5 inhibitors (sildenafil or zaprinast) improved chemotherapeutic efficacy of anticancer drugs in prostate and other tumor models by inducing intracellular cGMP levels (Black et al., 2008; Das et al., 2016; Das et al., 2010; Hamilton et al., 2013; Hu et al., 2010; Muniyan et al., 2020; Roberts et al., 2014; Sarfati et al., 2003). Increased cGMP levels activate PKG and its downstream effectors, which are key in enhancing antitumor efficacy of chemotherapeutic agent by promoting apoptosis and curbing inflammation (Das et al., 2015; Levy et al., 2011).
Alterations in cAMP signaling components have been identified in various cancers, which contribute to the prognosis of cancer treatment (James et al., 2009; Merkle and Hoffmann, 2011; Miller, 2002). For example, EPAC, PKA, ERK, and ion channels are important downstream targets of cAMP that mediate cellular homeostasis by regulating cell proliferation, migration, differentiation, and apoptosis (Levy et al., 2011; Stork and Schmitt, 2002). EPAC is an integrin-mediated cell adhesion and cell-cell junction formation regulator, pertinent to migration/metastasis of malignant cells (Wehbe et al., 2020). Accordingly, EPAC has been extensively explored as the potential target for multiple cancer therapies (Wehbe et al., 2020).
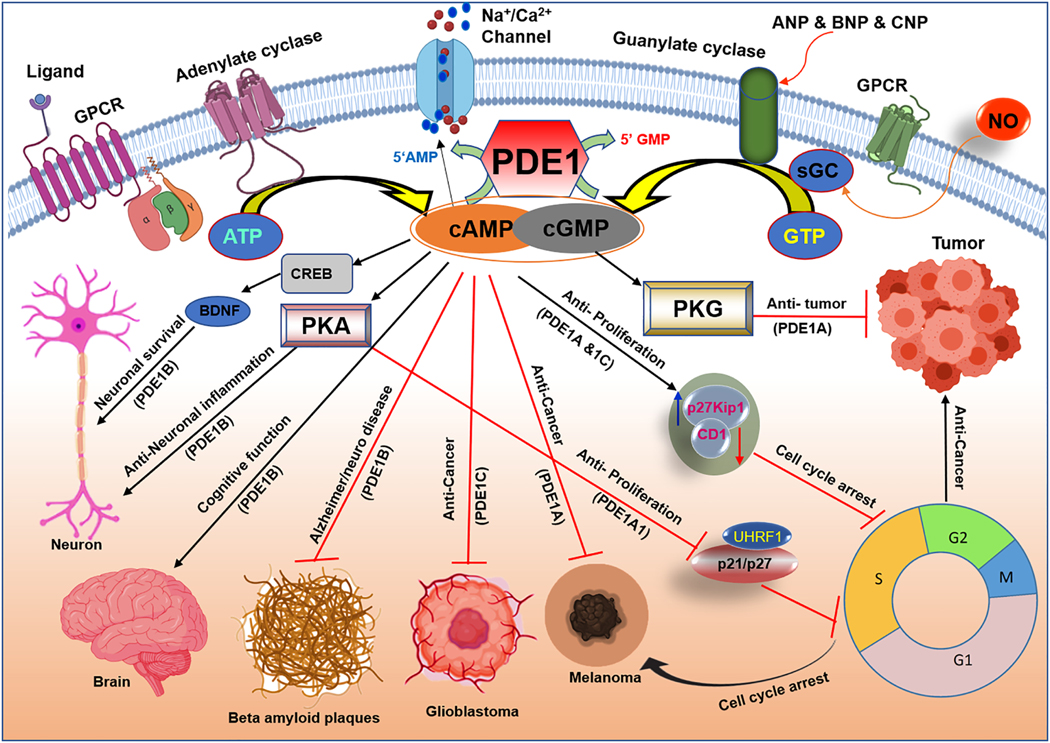
Different PDE isoforms are predominantly expressed in several cancer cell lines: PDE1A in B16F10 melanoma cells (Abusnina et al., 2011b), PDE1B in lymphoblastoid B-cell RPMI-8392 cells (Jiang et al., 1996), PDE1A and PDE1B in retinoblastoma Y79 cells (White et al., 2004), PDE1A and PDE1C in osteosarcoma MG-63 cells (Ahlstrom et al., 2005), PDE1C in human glioblastoma SNB75 and SF295 cells (Vatter et al., 2005) and melanoma MAA cells (Fajardo et al., 2014; Shimizu et al., 2009). The type of PDE1 isoforms and its function in various cancers is depicted in Fig. 4. The increased presence of PDE1 in these cancer cells suggests its potential involvement in carcinogenesis. Although studies on the specific role of PDE1 in cancer are limited, some of the recent developments targeting PDEs in cancer therapy are encouraging (Rowther et al., 2016; Shimizu et al., 2004). For example, a potent CaM (Ca2+-dependent regulatory protein) antagonist, tehranolide, significantly reduced cell proliferation of human erythroleukemic cell line (K562) through inducing cAMP/PKA signaling pathways via CaM and PDE1 inhibition (Noori and Hassan, 2014). Treatment of MDA-MB-157 breast cancer cell line with the non-selective PDE inhibitors/cAMP modulators (1-isobutyl-3-methylxanthine (IBMX) or lovastatin) inhibited cell growth through induction of p21/p27 (Rao et al., 1999). These reports validate the known ability of cancer cells to aberrantly alter cyclic nucleotide, protein kinase, and downstream effector levels, which shift the cellular processes away from these regulatory activities toward cell growth and metastasis (Savai et al., 2010). A recent study revealed that the cationic amphiphilic drugs (CAD) enhance the efficacy of chemotherapy by inducing lysosome-dependent cancer cell death (Anand et al., 2019). Increase of cellular cAMP levels sensitized cancer cells to CAD-induced lysosome-dependent cell death either by activating cAMP-inducing G-protein–coupled receptors (GPCR) or by inhibiting cAMP-PDEs. Further investigations are needed to develop a potent combination of cancer therapy with CAD and cAMP-inducing drugs.
Figure 4. Phosphodiesterase 1 (PDE1) regulated cAMP and cGMP signaling pathway in cancer and neurological disorders.
PDE1A1 activation leads to upregulation of ubiquitin-like protein, containing PHD and RING finger domains 1 (UHRF1) in melanoma cells and in lymphoblastic leukemia cancer. Targeted inhibition of PDE1A and PDE1C increases p27Kip1 and downregulates cyclin D1 (CD1) through cGMP-PKG dependent mechanism and results in cell cycle arrest at G1 stage. PDE1B inhibition activates cAMP response element-binding protein (CREB) through cAMP accumulation and regulates brain-derived neurotrophic factor (BDNF) which results in neuronal survival and reduction of beta amyloid plaques in Alzheimer and Parkinson disease. Inhibition of PDE1B may improve cognitive function and memory impairment.
PDE1C is identified as a potential mediator of many of the aggressive characteristics of glioblastoma multiforme cells (GBM) (Rowther et al., 2016). Treatment with vinpocetine, attenuated proliferation of GBM cells with no effect on invasion/migration (Rowther et al., 2016). PDE1C transcript depletion showed reduced proliferation, invasion, and migration of glioblastoma cells (Rowther et al., 2016). This was associated with changes in expression of genes involved in the cell cycle, cell adhesion, and those maintaining the carcinogenic phenotype. Thus PDE1C may play a more specific role in metastatic activity; proliferation may depend on a broad set of PDE-dependent pathways (Rowther et al., 2016).
In the malignant melanoma MAA cell line, treatment with vinpocetine inhibited cell growth (Shimizu et al., 2009). Curcumin, a polyphenol found in turmeric, is recognized as a non-specific PDE inhibitor with actions mediated mainly through PDE1A. In melanoma cell line, curcumin treatment paused the cell cycle at the S- and G2/M phases through decreased ubiquitin-like containing PHD and Ring Finger domains 1 (UHRF1), DNA methyltransferase 1 (DNMT1) p21, and p27 (Abusnina et al., 2011a; Abusnina et al., 2011b; Alhosin et al., 2010).
PDE1A1 has also been examined in an acute lymphoblastic leukemia model (Jurkat cells). Thymoquinone, a major bioactive constituent of black seed oil, induced apoptosis in the Jurkat cells by increasing p72 and decreasing PDE1A and UHRF1 expression. Similar results obtained by RNA-interference of PDE1A expression provided further support for this mechanism (Abusnina et al., 2011b). Leukemia cells were also used to reveal the actions of the anti-proliferative mammalian protein, differentiation-inducing factor-1 (DIF-1). DIF-1 acts as a competitive inhibitor of PDE1 which, increases cAMP levels and inhibits cell growth (Shimizu et al., 2004). Activity against a different PDE1 isoform, PDE1B1, in the setting of cancer was explored in a different cell line, lymphoblastoid B-cells (RPMI-8392). Induction of PDE1B1 gene expression occurred in response to mitogenic stimulation. Moreover, antisense and pharmacological inhibition of PDE1B1 not only reduced cell growth, but also induced apoptosis (Jiang et al., 1996). Similar results were obtained in HeLa cells using Diethyl flavon-7-yl phosphate, a Ca2+-CaM dependent PDE1 inhibitor. PDE1 inhibition leads to elevated cAMP levels which then acted through a p21 and cleaved caspase-3/ PARP-1 mechanism to eventually cause cell cycle arrest, growth inhibition, and apoptosis (Zhang et al., 2012).
Overall, these studies provide evidence for the potential role of PDE1 in carcinogenesis. Based on the abnormal expression of different PDE1 isoforms in various cancer cell lines and the benefits of PDE1 inhibition impeding the regulatory growth/proliferation factors, it appears that PDE1 may be a potential therapeutic target in the treatment of cancer. Additionally, the upregulation of PDE expression in malignant cells may provide a therapeutic window to specifically target these diseased cells.
7. PDE1 in Neurocognitive Disorders
The role of PDE1 in various neurological and cognitive pathologies has gained significant attention in recent times. PDE1 subtypes are widely expressed in different regions of the brain and are associated with neurodevelopmental and neurodegeneration diseases (Fig.4). PDE1C is ubiquitously expressed, whereas PDE1A is predominantly expressed in the cortex and the neostriatum. PDE1B is also expressed in the neostriatum and prefrontal cortex, but is additionally found in the hippocampus and olfactory tubercle (Polli and Kincaid, 1992). Specific localization of PDE1 isoforms in important functional areas of the brain suggest a potential role in basal neuronal activity including phasic neurotransmitter signaling by dopamine, acetylcholine, glutamate, and certain peptidergic systems (Polli and Kincaid, 1992). PDE1B has been shown to co-localize with dopamine D1 receptors (D1R) where it triggers signal transduction through the generation of cAMP. Both PDE1B and D1R are abundantly expressed in the prefrontal cortex which controls working memory function (Lange et al., 1988; Polli and Kincaid, 1992). Inadequate D1R-mediated signaling in the prefrontal cortex contributed to impaired cognitive function in both preclinical and clinical studies (Castner et al., 2000). Inhibition of PDE1 can amplify dopaminergic neurotransmission downstream of D1 receptors, which signal through modulation of AC with subsequent production of cAMP. The cAMP response element binding protein (CREB), which is abundantly expressed in the hippocampus, is regulated by cAMP/cGMP and promotes the neuronal survival through mechanisms associated with the synaptic stimulation of strengthening and memory formation (Teich et al., 2015). Memory consolidation and performance are positively regulated by CREB through upregulating the brain derived neurotrophic factor (BDNF) (Ran et al., 2012; Suzuki et al., 2011).
Due to the broad spectrum of PDE1 activity and its contribution to the disease processes, several studies have focused on developing drugs targeting PDE1. Early studies proposed thienopyrimidine/pyridopyrimidine fused quinazolinone derivatives as likely anti-inflammatory and anti-Parkinsonian medications (Laddha and Bhatnagar, 2009). The knockout studies revealed an even stronger rationale for the clinical application of PDE1 inhibition. The phenotype of PDE1B knockout mice is characterized by strong striatal and dopaminergic activity. This is a desirable effect in low striatal and dopaminergic disease states, such as Parkinson’s disease and attention deficit hyperactivity disorder (ADHD). The PDE1B knockout model provided evidence for PDE1B inhibition in the treatment of cognitive conditions (Siuciak et al., 2007). PDE1 inhibition also impacts Alzheimer’s disease (AD), a progressive neurodegenerative disorder affecting millions of people worldwide. In AD, intracellular amyloid plaques consisting of amyloid beta (Aβ) aggregates and extracellular neurofibrillary tangles are formed by hyperphosphorylated tau fibrils which appear to interfere with the normal neuronal functioning (Maccioni et al., 2001). Currently used drugs, including acetylcholinesterase (AChE) inhibitors or N-methyl-d-aspartate (NMDA) antagonists have limited efficacy. Consequently, there is a dire need for novel treatment strategies for AD. Interestingly PDE1 inhibitor, vinpocetine was shown to enhance learning and memory in the animal model (Deshmukh et al., 2009). The intracerebroventricular infusion of streptozotocin in rats impaired learning as well as memory and increased oxidative–nitritive stress which manifested clinical features of the sporadic dementia of Alzheimer. Also, chronic treatment with vinpocetine significantly blunted the enhanced activity of acetylcholinesterase. Findings in this rat model of Alzheimer’s sporadic dementia provided a strong backing for PDE1 as a therapeutic target. Age related induction of PDE1C1 protein was responsible for the decrease in the cAMP levels in the hippocampus region of the brain in older population (Kelly et al., 2014). Interestingly, this study also reported a difference in comparmental localization of PDE1C in young versus older subjects (Kelly et al., 2014). Pharmacological inhibition of PDE1 in intracerebroventricular streptozotocin mouse model resulted in significant improvement in learning and memory function after 21 days. In addition, PDE1 inhibition mitigated oxidative damage and normalized acetylcholine levels (Deshmukh et al., 2009). Similar beneficial results including the anti-oxidative action and acetylcholine regulation were shown in Huntington’s disease rat model (Gupta and Sharma, 2014). PDE1-inhibition with vinpocetine was also protective against cognitive deficits in the preclinical AD model through signaling pathways which inhibit apoptosis (Zhang et al., 2018a), inflammation and arrest of the cell cycle in the G1 phase by downregulating cyclin D1 and upregulating p27(Kip1) (Zhang et al., 2018b). Despite these promising preclinical findings, there is very little evidence about vinpocetine improving AD symptoms in humans. ITI-214 is a broad-spectrum PDE1 inhibitor, with IC50 in the range of picomolar concentration for PDE1A, PDE1B and PDE1C and greater than 1000-fold selectivity compared to the cAMP-specific PDE4 (Li et al., 2016; Snyder et al., 2016). Inhibition of PDE1 with ITI-214 at a dose range of 0.1–10.0 mg/kg was reported to improve memory performance in animal models of novel object recognition (Pekcec et al., 2018; Snyder et al., 2016). The specific isoform of PDE1 mediating the cognition-enhancing effects of ITI-214 remains undetermined, although PDE1B could be the most likely candidate because of its localization closer to D1-expressing neurons (Pekcec et al., 2018). Moreover, similar cognition-enhancing effects were observed with the PDE1B inhibition (Dyck et al., 2017).
Neuroinflammation, an adverse pathological process caused by brain’s innate immune system in response to injury, infection or due to aging contributes to many neurological and neurodegenerative diseases. Activation of brain resident immune cells, microglia have been suggested to participate in the inflammatory processes in diseases of the central nervous system. Treatment with ITI-214 had anti-inflammatory action in lipopolysaccharide-challenged BV2 cells, downregulating some of the inflammatory cytokines and gene expression related to chemotaxis (O’Brien et al., 2020). Thus, the ability of PDE1 inhibitors to prevent or dampen excessive inflammatory responses of microglia was suggested to have therapeutic utility in the treatment of neurodegenerative diseases such as Parkinson’s disease and AD. In fact, Intra Cellular Therapies completed a randomized, placebo-controlled, double-blind study of safety, tolerability, pharmacokinetics and pharmacodynamics of multiple doses of ITI-214 in patients with idiopathic Parkinson’s Disease (NCT03257046).
Building on the theme of improved cognitive function with PDE1 inhibition, fetal alcohol spectrum disorder (FASD) entered the discussion. Fetal alcohol spectrum disorders is a general term used to describe the range of disabilities caused by prenatal exposure to alcohol. Attention-deficit/hyperactivity disorder (ADHD) is possibly the most common behavioral problem in children with FASD (Bhatara et al., 2006; Burd et al., 2003; Doig et al., 2008) and devising novel strategies that can ameliorate this condition has great clinical relevance (Nunes et al., 2011). Studies in rodent models of ADHD and FASD suggest that impairments in the cAMP signaling cascade contributed to the hyperactivity phenotype (Paine et al., 2009). During development, ethanol exposure can alter several key factors in the cAMP/PKA signaling pathway with long-lasting effects (Kumada et al., 2010). Rodents exposed to alcohol during the third trimester, equivalent to human gestation showed an increased spatial learning and memory deficits (Nunes et al., 2011). Since PDE1 is significantly expressed in neurons of the hippocampus and cortex (Lugnier, 2006), this enzyme may control cAMP levels in areas impacted by ethanol exposure during the brain growth spurt (Gil-Mohapel et al., 2010; Olney et al., 2002). Vinpocetine was able to restore ocular dominance plasticity (ODP) in the visual cortex of ferrets exposed to alcohol (Medina et al., 2006). ODP shares similar mechanisms with learning and memory, such as the dependence on the CREB function (Mower et al., 2002).
8. PDE1 in Metabolic Syndrome and Diabetes
Obesity and its common pathological outcome type 2 diabetes have emerged as primary risk factors for the increased morbidity and mortality in recent decades (Ginter and Simko, 2012; Nichols et al., 2013). The cellular cAMP and cGMP signaling and its regulator PDEs have been implicated in the complex pathogenic process of obesity and diabetes, from adipocyte lipolysis (Snyder et al., 2005) to insulin secretion and glycemic control (Laychock, 1983). In addition, autonomic control of ventricular function occurs through regulation of PKA. The diabetic heart has suppressed β-adrenergic responsiveness which is attributable to both β-AR and PKA signaling changes (Bockus and Humphries, 2015). Diabetic mice also displayed impaired inotropic and lusitropic responses, mediated by reduced PKA activity as well as catalytic subunit content in the cytoplasm and myofilaments (Bockus and Humphries, 2015). Since cAMP and cGMP are the key mediators in the regulation of lipolysis, glycogenolysis, gluconeogenesis and pancreatic β cell insulin secretion, PDEs play a vital role in metabolic syndromes. Research findings from our laboratory and others have demonstrated remarkable efficacy of the inhibitors of PDE3 (Degerman et al., 2011) and PDE5 (Koka et al., 2014; Koka et al., 2012; Varma et al., 2012) in alleviating obesity and type 2 diabetes. Our recent work in a mouse model of metabolic syndrome also showed that chronic PDE5 inhibition with tadalafil significantly improved left ventricular diastolic function, lipid profile and reduced infarct size following ischemia-reperfusion injury, via enhanced NO production (Koka et al., 2020), a known activator of cGMP signaling.
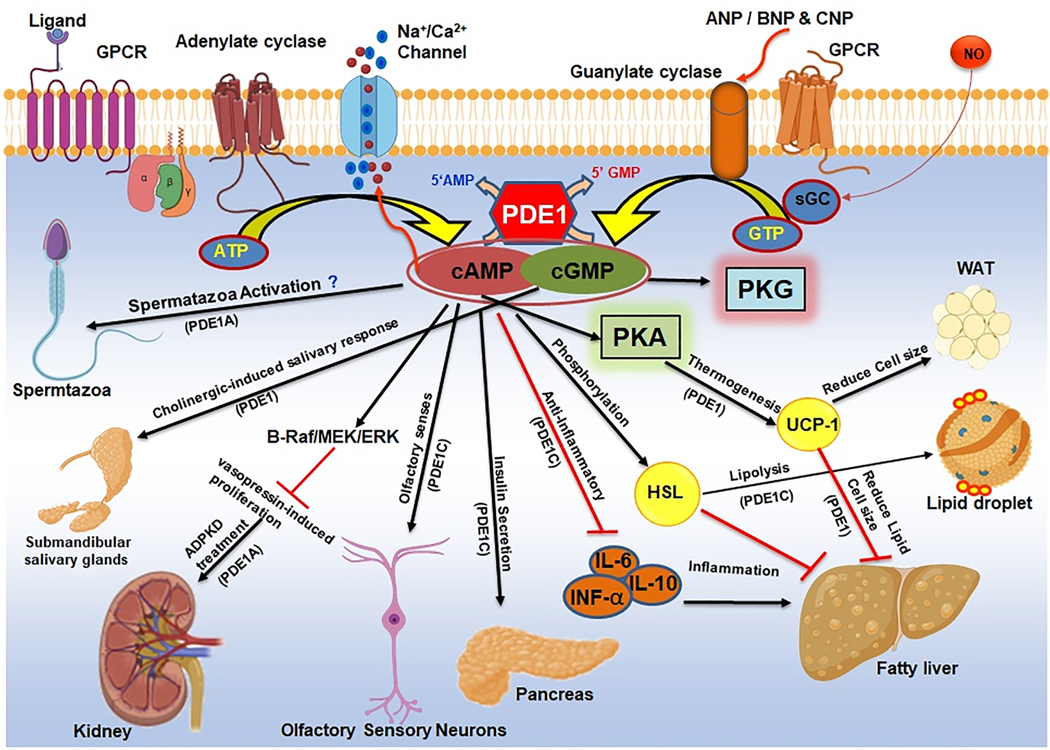
The presence of PDE1 in adipose tissue has sparked interest in exploring the possible role of this enzyme in the pathogenesis of metabolic syndrome and associated co-morbidities (Lugnier, 2011). Fig. 5 shows the regulation of several metabolic and endocrine function by PDE1 isoforms. Interestingly, despite its abundant expression in adipose tissue, outcomes of experiments with PDE1 inhibitor - IC224 ruled out any significant role of PDE1 in lipolysis (Baragli et al., 2011). In contrast, it was recently demonstrated that vinpocetine effectively suppressed adipogenesis and lipid accumulation (Kim et al., 2019). In these studies, vinpocetine decreased adipogenic cell signaling, and phosphorylation of ERK, AKT, JAK2, and STAT3, and adipokine secretion. Vinpocetine also reduced IL-6, IL-10, and IFN-α levels. PDE1 inhibition also increased phosphorylation of hormone-sensitive lipase suggesting the induction of the lipolysis pathway (Kim et al., 2019). Moreover, vinpocetine increased uncoupling protein 1 (UCP1) expression via increasing cAMP and PKA phosphorylation (Kim et al., 2019). Further, treatment of high fat diet fed mice with vinpocetine prevented weight gain, and drastically decreased fat weight, adipocyte cell size in gonadal and inguinal white adipose tissues as well as in the liver. Vinpocetine also decreased serum triacylglycerol and glucose levels (Kim et al., 2019). This study provided a compelling rationale to further explore the beneficial effects of vinpocetine in the treatment of obesity and diabetes and inhibition of adipogenesis.
Figure 5. Role of phosphodiesterase 1 (PDE1) in kidney disease, metabolic syndrome and endocrine system and other diseases.
PDE1 isoforms regulate calcium induced-insulin secretion in pancreatic β-cells upon activation by glucose and enhances lipolysis through phosphorylation of hormone sensitive lipase (HSL) which reduces lipid size and lowers risk of obesity and diabetes. Elevation of cAMP levels through inhibition of PDE1 activates uncoupling protein 1 (UCP-1) and increases lipolysis and inhibits lipid accumulation. Blocking PDE1C, decreases pro-inflammatory adipokines IL-6, IL-10 and INF-α levels and influences obesity-related insulin resistance. Inhibition of PDE1B alleviates vasopressin induced proliferation in renal glomerulus and tubule epithelium which could be the potential treatment option for autosomal dominant polycystic kidney disease (ADPKD). Elevation of cGMP levels through PDE1 inhibition leads to activation of submandibular salivary glands in response to cholinergic stimuli.
The coordinated oscillation of AMP and calcium-induced insulin secretion from pancreatic β-cells upon activation by glucose suggests a potential relationship between PDE1 activity and insulin secretion (Tian et al., 2012). Although PDE3B and PDE4 play a predominant role in insulin regulation, PDE1 is responsible for 30–37% of total PDE activity in pancreatic islets (Heimann et al., 2010; Tian et al., 2012). PDE1C is involved in the glucose-dependent feedback mechanism of insulin secretion (Han et al., 1999). Despite the role of PDE1 in regulation of insulin secretion, the effects of PDE1 on cAMP oscillation remains debatable. PDE1 inhibition disrupts the physiological insulin pulsatile secretion pattern (Pratt et al., 2019; Pyne and Furman, 2003), and the insulin secretion was greatly increased (Waddleton et al., 2008). To provide a clear picture, further investigations are needed to show the effects of PDE1 inhibition on a glucose-induced cAMP response in β-cells.
9. PDE1 and Kidney Disease
The role of PDE1 subtypes in kidney function is shown in Fig.5. PDE1A is strongly expressed in the kidney and hydrolyzes cyclic nucleotides in the renal glomerulus and tubule epithelium, suggesting their potential role in renal pathologies. Diabetic nephropathy (DN) is a major complication and the most common cause of the end stage renal disease in diabetics (Takayanagi et al., 2011). DN is characterized by mesangial cell proliferation and matrix accumulation, renal hypertrophy and later glomerulosclerosis (Wu et al., 2012). Proteinuria is an important manifestation of glomerular injury and an independent mediator of progressive kidney damage in diabetic patients (Remuzzi et al., 2004). Treatment with vinpocetine had profound effect in reducing renal injury and proteinuria in diabetic rats through improvement of redox status and interfering of NF-κB signaling pathway in addition to its antidiabetic effect (Wadie and El-Tanbouly, 2017).
Autosomal dominant polycystic kidney disease (ADPKD) is another leading causes of end stage kidney disease which is caused by mutations in PKD1 or PKD2 genes encoding polycystin 1 and polycystin 2 proteins (Ong et al., 2015). Disruption of polycystin function results in dysregulation of intracellular calcium dynamics and upregulation of cAMP and PKA signaling (Torres and Harris, 2014). The reduction in intracellular calcium caused upregulation of cAMP and PKA through stimulation of calcium inhibitable adenylyl cyclase 6 (AC6) and inhibition of PDE1 thereby degrading cAMP in the distal nephron and collecting duct (Gattone et al., 2003). Collecting duct-specific AC6 knockout mice afforded protection in a polycystic kidney disease (PKD) model (Rees et al., 2014). Since cyclic nucleotides hydrolysis capacity of PDEs far exceeds the synthesis by ACs (Dousa, 1999), modulation of PDE activity, particularly PDE1 activity, may be crucial in PKD. PDE1 specifically affects the cAMP signal to the B-Raf/MEK/ERK pathway and regulates vasopressin-induced proliferation in ADPKD cells (Pinto et al., 2016). PDE1 inhibition may also generate cGMP through PDE3 inhibition which may cause downregulation of PDE1 indirectly thereby affecting PDE3-regulated specific pool of intracellular cAMP. The depletion of either, PDE1A or PDE3A using morpholinos caused pronephric cysts, body curvature, and hydrocephalus in zebrafish (Sussman et al., 2014). Moreover, the inhibition of PDE1 or PDE3 increased proliferation of ADPKD cells. The PDE1 inhibition triggered a mitogenic response to vasopressin in normal human kidney cells similar to the effect of restricting intracellular calcium (Cai et al., 2004; Cai et al., 2015; Pinto et al., 2016). Therefore, PDE1 may function as a link connecting changes in intracellular calcium and the activity of PDE3 pool controlling cell proliferation. Studies using PDE1A knockout mice (Wang et al., 2017) showed signs of mild renal cystic disease and a urine concentrating defect. This was associated with upregulation of PDE4 activity and a decrease in PKA dependent phosphorylation of the membrane water channel, aquaporin-2. PDE1A mutants had lower aortic blood pressure and increased LV ejection fraction without a change in LV mass index, consistent with the high aortic and low cardiac expression of PDE1A in wild-type mice. These results supported an important role of PDE1A in the renal pathogenesis of ADPKD and regulation of blood pressure.
10. PDE1 in Other Diseases
PDE1A and 1C regulate the response to corticotrophin-releasing factor (CRF) and arginine vasopressin, two stress hormones that increase cAMP in the adenohypophysis. Treatment with vinpocetine enhanced the response to combined CRF and arginine vasopressin, but not CRF alone (Ang and Antoni, 2002). These results suggest that PDE1 may act as a low-affinity regulator of CRF under extreme stress. PDE1 in the pituitary gland could induce several changes in Ca2+-activated K+ channel kinetics. Vinpocetine application in the rat pituitary GH3 lactotrophs increased Ca2+-activated K+ currents and lowered the threshold for voltage-dependent channel opening. These results suggested that PDE1 inhibition stimulated K+ currents by direct channel activation and raising intracellular Ca2+ through a cGMP-dependent mechanism (Wu et al., 2001).
The discovery of PDE1A expression in the head and flagellum of ejaculated human sperm suggests PDE1A may have a role in male fertility (Lefievre et al., 2002). Interestingly, PDE1A was unresponsive to Ca2+ and calmodulin and functions differently in flagellum compared to their role in other tissues (Fig.5). The PDE1A-calmodulin complex did not dissociate when perturbed by powerful detergents. These results indicate that spermatozoa activation is likely a result of increased AC activity as opposed to PDE1A inhibition. PDE1 has also been shown to present in the submandibular salivary glands, where it plays a role in cholinergic-induced salivary response (Michikawa et al., 2005). In addition, PDE1 makes an appearance in the odorant senses by playing a part in olfactory stimulus termination. It has been reported that PDE1C partially assists in stimulus-induced cAMP degradation (Cygnar and Zhao, 2009).
11. PDE1 Inhibitors in Clinical Trials
Given the broad spectrum of PDE1 activity and their involvement in diverse disease conditions, several studies have focused on developing drugs targeting PDE1. A summary of the enzyme kinetics of PDE1 isoforms, available inhibitors and clinical outcome is presented in Table 1. Previous studies proposed thienopyrimidine/pyridopyrimidine fused quinazolinone derivatives as likely anti-inflammatory and anti-Parkinsonian medications (Laddha and Bhatnagar, 2009). In 2011, Takeda and Intra-Cellular Therapies, Inc. launched ITI-002, a PDE1 inhibitor targeted to treat cognitive component of schizophrenia (2011) (Deal watch: Intra-cellular therapies and Takeda to develop PDE1 inhibitors for schizophrenia.2011). After completing phase I of the clinical trial, Intra-Cellular Therapies, Inc. reported a safety profile for ITI-002 (Intra-cellular therapies unveil positive phase I data for PDE1 inhibitor in CIAS.2013). The overall efficacy of this treatment has yet to be determined, but the recent update demonstrating early signs of improvement in motor symptoms for patients with PD. Most recently, a search for centrally-acting neuropsychiatric agents led to development of a new drug class, quinazoline PDE1 inhibitors (Humphrey et al., 2018). These developments are perhaps a sign that a new frontier of PDE1 therapeutic applications is on the horizon.
The positive outcomes associated with diverse applications of PDE1 inhibition relate back to a common mechanistic tie: reliance on cAMP-CREB protein activity (Filgueiras et al., 2010). Though these several neurological and cognitive actions are grounded in a common mechanism, an element of individualization remains. The clinical importance of PDE1 in the brain is further reinforced by its involvement in varying drug responses. PDE1B levels increase following treatment with the typical anti-psychotic medication, haloperidol. PDE1A polymorphisms are also associated with variable efficacies of anti-depressant therapies (Wong et al., 2006). These findings suggest that PDE1 may influence individual responses to different cognitive therapeutic agents (Dlaboga et al., 2008). Overall, the broad spectrum of PDE1 action in many disease states presents an opportunity for clinical manipulation but it is perhaps confounded by genetic variability.
12. Conclusion
PDE1 is a versatile enzyme and a therapeutic target, which plays a central functional role in many diseases and its inhibition using pharmaceutical drugs can attenuate disease progression and outcomes. Though, PDE1 is relatively a new target among the PDE family in the treatment of cardiovascular disease, cancer, and other neurological disorders, our understanding of PDE1 continues to grow. Recent evidences provide hope for a promising future for development of new inhibitors. Expression levels of specific PDE1 isoforms in the target tissues and tailored drug dosages for the individual disease models need future investigations.
Acknowledgment
PDE1 enzyme kinetics data are adapted from book chapter on “5-Cyclic Nucleotide Regulation by Calmodulin” by Sonnenburg, W et al., 1998, Pages 237-286; Diagrammatic illustrations are created with Biorender.com.
Sources of Funding
This work was supported by grants from the National Institutes of Health R01HL124366 (RCK & AD), R01HL118808 (RCK), R01CA221813, R01DK120866 (DKS & RCK), R01HL057244 (PLL), National Institutes of Health (UO1 CA185148 to SKB) and the Department of Defense Idea Award [W81XWH-18-1-0308 (PC170891) to SKB & RCK.
Abbreviations
- 8MM-IBMX
8-methoxymethyl-isobutyl-1-methylxanthine
- AC
Adenylate cyclases
- AD
Alzheimer’s disease
- ADHD
Attention-deficit/hyperactivity disorder
- ADPKD
Autosomal dominant polycystic kidney disease
- AKAPs
A-Kinase-anchoring proteins
- A2R
Adenosine A2 receptor
- CAD
Cationic amphiphilic drugs
- CaM
Calmodulin
- cAMP
3’,5’-cyclic adenosine monophosphate
- cGMP
3’,5’-Cyclic guanosine monophosphate
- CNG
Cyclic nucleotide-gated
- D1R
Dopamine D1 receptors
- eNOS
Endothelial nitric oxide synthase
- EPAC
The exchange protein directly activated by cAMP
- FASD
Fetal alcohol spectrum disorder
- FRET
Fluorescence resonance energy transfer
- GBM
Glioblastoma multiforme cells
- GC
Guanylate cyclases
- GPCR
G-protein-coupled receptors
- HFpEF
Heart failure with preserved ejection fraction
- I/R
Ischemia/reperfusion
- IBMX
1-isobutyl-3-methylxanthine
- IPAH
Idiopathic pulmonary arterial hypertension
- NMDA
N-methyl-d-aspartate
- NO
Nitric oxide
- ODP
Ocular dominance plasticity
- PAH
Pulmonary arterial hypertension
- PDE
Phosphodiesterases
- PKA
cAMP-dependent protein kinase
- PKC
Protein kinase C
- PKD
Polycystic kidney disease
- PKG
cGMP-dependent protein kinase
- RV
Right ventricular
- SMCs
Smooth muscle cells
- SPAH
Secondary pulmonary arterial hypertension
- TRPC3
Transient receptor potential channel 3
- UHRF1
Ring finger domains 1
Footnotes
Declaration of Competing Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (2011) Deal watch: Intra-Cellular Therapies and Takeda to develop PDE1 inhibitors for schizophrenia. Nat Rev Drug Discov 10:329. [DOI] [PubMed] [Google Scholar]
- Abdollahi M, Chan TS, Subrahmanyam V and O’Brien PJ (2003) Effects of phosphodiesterase 3,4,5 inhibitors on hepatocyte cAMP levels, glycogenolysis, gluconeogenesis and susceptibility to a mitochondrial toxin. Mol Cell Biochem 252:205–211. [DOI] [PubMed] [Google Scholar]
- Abusnina A, Alhosin M, Keravis T, Muller CD, Fuhrmann G, Bronner C and Lugnier C (2011a) Down-regulation of cyclic nucleotide phosphodiesterase PDE1A is the key event of p73 and UHRF1 deregulation in thymoquinone-induced acute lymphoblastic leukemia cell apoptosis. Cell Signal 23:152–160. [DOI] [PubMed] [Google Scholar]
- Abusnina A, Keravis T, Yougbare I, Bronner C and Lugnier C (2011b) Anti-proliferative effect of curcumin on melanoma cells is mediated by PDE1A inhibition that regulates the epigenetic integrator UHRF1. Mol Nutr Food Res 55:1677–1689. [DOI] [PubMed] [Google Scholar]
- Afzal F, Qvigstad E, Aronsen JM, Moltzau LR, Sjaastad I, Skomedal T, Osnes JB and Levy FO (2011) Agents increasing cyclic GMP amplify 5-HT4-elicited positive inotropic response in failing rat cardiac ventricle. Naunyn Schmiedebergs Arch Pharmacol 384:543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlstrom M, Pekkinen M, Huttunen M and Lamberg-Allardt C (2005) Cyclic nucleotide phosphodiesterases (PDEs) in human osteoblastic cells; the effect of PDE inhibition on cAMP accumulation. Cell Mol Biol Lett 10:305–319. [PubMed] [Google Scholar]
- Alhosin M, Abusnina A, Achour M, Sharif T, Muller C, Peluso J, Chataigneau T, Lugnier C, Schini-Kerth VB, Bronner C and Fuhrmann G (2010) Induction of apoptosis by thymoquinone in lymphoblastic leukemia Jurkat cells is mediated by a p73-dependent pathway which targets the epigenetic integrator UHRF1. Biochem Pharmacol 79:1251–1260. [DOI] [PubMed] [Google Scholar]
- Anand A, Liu B, Dicroce Giacobini J, Maeda K, Rohde M and Jaattela M (2019) Cell Death Induced by Cationic Amphiphilic Drugs Depends on Lysosomal Ca(2+) Release and Cyclic AMP. Mol Cancer Ther 18:1602–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang KL and Antoni FA (2002) Functional plasticity of cyclic AMP hydrolysis in rat adenohypophysial corticotroph cells. Cell Signal 14:445–452. [DOI] [PubMed] [Google Scholar]
- Aravind L and Koonin EV (1998) The HD domain defines a new superfamily of metal-dependent phosphohydrolases. Trends Biochem Sci 23:469–472. [DOI] [PubMed] [Google Scholar]
- Archer SL and Michelakis ED (2009) Phosphodiesterase type 5 inhibitors for pulmonary arterial hypertension. N Engl J Med 361:1864–1871. [DOI] [PubMed] [Google Scholar]
- Arnold WP, Mittal CK, Katsuki S and Murad F (1977) Nitric oxide activates guanylate cyclase and increases guanosine 3’:5’-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci U S A 74:3203–3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillie GS, Tejeda GS and Kelly MP (2019) Therapeutic targeting of 3’,5’-cyclic nucleotide phosphodiesterases: inhibition and beyond. Nat Rev Drug Discov 18:770–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baim DS, McDowell AV, Cherniles J, Monrad ES, Parker JA, Edelson J, Braunwald E and Grossman W (1983) Evaluation of a new bipyridine inotropic agent--milrinone--in patients with severe congestive heart failure. N Engl J Med 309:748–756. [DOI] [PubMed] [Google Scholar]
- Baragli A, Ghe C, Arnoletti E, Granata R, Ghigo E and Muccioli G (2011) Acylated and unacylated ghrelin attenuate isoproterenol-induced lipolysis in isolated rat visceral adipocytes through activation of phosphoinositide 3-kinase gamma and phosphodiesterase 3B. Biochim Biophys Acta 1811:386–396. [DOI] [PubMed] [Google Scholar]
- Bautista Nino PK, Durik M, Danser AH, de Vries R, Musterd-Bhaggoe UM, Meima ME, Kavousi M, Ghanbari M, Hoeijmakers JH, O’Donnell CJ, Franceschini N, Janssen GM, De Mey JG, Liu Y, Shanahan CM, Franco OH, Dehghan A and Roks AJ (2015) Phosphodiesterase 1 regulation is a key mechanism in vascular aging. Clin Sci (Lond) 129:1061–1075. [DOI] [PubMed] [Google Scholar]
- Bell EN, Tse MY, Frederiksen LJ, Gardhouse A, Pang SC, Graham CH and Siemens DR (2007) Atrial natriuretic peptide attenuates hypoxia induced chemoresistance in prostate cancer cells. J Urol 177:751–756. [DOI] [PubMed] [Google Scholar]
- Bender AT and Beavo JA (2006) Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 58:488–520. [DOI] [PubMed] [Google Scholar]
- Bender AT, Ostenson CL, Giordano D and Beavo JA (2004) Differentiation of human monocytes in vitro with granulocyte-macrophage colony-stimulating factor and macrophage colony-stimulating factor produces distinct changes in cGMP phosphodiesterase expression. Cell Signal 16:365–374. [DOI] [PubMed] [Google Scholar]
- Bender AT, Ostenson CL, Wang EH and Beavo JA (2005) Selective up-regulation of PDE1B2 upon monocyte-to-macrophage differentiation. Proc Natl Acad Sci U S A 102:497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley JK, Kadlecek A, Sherbert CH, Seger D, Sonnenburg WK, Charbonneau H, Novack JP and Beavo JA (1992) Molecular cloning of cDNA encoding a “63”-kDa calmodulin-stimulated phosphodiesterase from bovine brain. J Biol Chem 267:18676–18682. [PubMed] [Google Scholar]
- Bers DM (2008) Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol 70:23–49. [DOI] [PubMed] [Google Scholar]
- Bhatara V, Loudenberg R and Ellis R (2006) Association of attention deficit hyperactivity disorder and gestational alcohol exposure: an exploratory study. J Atten Disord 9:515–522. [DOI] [PubMed] [Google Scholar]
- Black KL, Yin D, Ong JM, Hu J, Konda BM, Wang X, Ko MK, Bayan JA, Sacapano MR, Espinoza A, Irvin DK and Shu Y (2008) PDE5 inhibitors enhance tumor permeability and efficacy of chemotherapy in a rat brain tumor model. Brain Res 1230:290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockus LB and Humphries KM (2015) cAMP-dependent Protein Kinase (PKA) Signaling Is Impaired in the Diabetic Heart. J Biol Chem 290:29250–29258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth L, Roberts JL, Cruickshanks N, Conley A, Durrant DE, Das A, Fisher PB, Kukreja RC, Grant S, Poklepovic A and Dent P (2014) Phosphodiesterase 5 inhibitors enhance chemotherapy killing in gastrointestinal/genitourinary cancer cells. Mol Pharmacol 85:408–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd L, Klug MG, Martsolf JT and Kerbeshian J (2003) Fetal alcohol syndrome: neuropsychiatric phenomics. Neurotoxicol Teratol 25:697–705. [DOI] [PubMed] [Google Scholar]
- Cai Y, Anyatonwu G, Okuhara D, Lee KB, Yu Z, Onoe T, Mei CL, Qian Q, Geng L, Wiztgall R, Ehrlich BE and Somlo S (2004) Calcium dependence of polycystin-2 channel activity is modulated by phosphorylation at Ser812. J Biol Chem 279:19987–19995. [DOI] [PubMed] [Google Scholar]
- Cai Y, Miller CL, Nagel DJ, Jeon KI, Lim S, Gao P, Knight PA and Yan C (2011) Cyclic nucleotide phosphodiesterase 1 regulates lysosome-dependent type I collagen protein degradation in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 31:616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Nagel DJ, Zhou Q, Cygnar KD, Zhao H, Li F, Pi X, Knight PA and Yan C (2015) Role of cAMP-phosphodiesterase 1C signaling in regulating growth factor receptor stability, vascular smooth muscle cell growth, migration, and neointimal hyperplasia. Circ Res 116:1120–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castner SA, Williams GV and Goldman-Rakic PS (2000) Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science 287:2020–2022. [DOI] [PubMed] [Google Scholar]
- Charbonneau H, Kumar S, Novack JP, Blumenthal DK, Griffin PR, Shabanowitz J, Hunt DF, Beavo JA and Walsh KA (1991) Evidence for domain organization within the 61-kDa calmodulin-dependent cyclic nucleotide phosphodiesterase from bovine brain. Biochemistry 30:7931–7940. [DOI] [PubMed] [Google Scholar]
- Cheung WY (1970) Cyclic 3’,5’-nucleotide phosphodiesterase. Demonstration of an activator. Biochem Biophys Res Commun 38:533–538. [DOI] [PubMed] [Google Scholar]
- Christman BW, McPherson CD, Newman JH, King GA, Bernard GR, Groves BM and Loyd JE (1992) An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med 327:70–75. [DOI] [PubMed] [Google Scholar]
- Clapham JC and Wilderspin AF (2001) Cloning of dog heart PDE1A - a first detailed characterization at the molecular level in this species. Gene 268:165–171. [DOI] [PubMed] [Google Scholar]
- Conti M and Beavo J (2007) Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem 76:481–511. [DOI] [PubMed] [Google Scholar]
- Corbin JD, Zoraghi R and Francis SH (2009) Allosteric-site and catalytic-site ligand effects on PDE5 functions are associated with distinct changes in physical form of the enzyme. Cell Signal 21:1768–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cygnar KD and Zhao H (2009) Phosphodiesterase 1C is dispensable for rapid response termination of olfactory sensory neurons. Nat Neurosci 12:454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Durrant D, Mitchell C, Dent P, Batra SK and Kukreja RC (2016) Sildenafil (Viagra) sensitizes prostate cancer cells to doxorubicin-mediated apoptosis through CD95. Oncotarget 7:4399–4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Durrant D, Mitchell C, Mayton E, Hoke NN, Salloum FN, Park MA, Qureshi I, Lee R, Dent P and Kukreja RC (2010) Sildenafil increases chemotherapeutic efficacy of doxorubicin in prostate cancer and ameliorates cardiac dysfunction. Proc Natl Acad Sci U S A 107:18202–18207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Durrant D, Salloum FN, Xi L and Kukreja RC (2015) PDE5 inhibitors as therapeutics for heart disease, diabetes and cancer. Pharmacol Ther 147:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Salloum FN, Xi L, Rao YJ and Kukreja RC (2009) ERK phosphorylation mediates sildenafil-induced myocardial protection against ischemia-reperfusion injury in mice. Am J Physiol Heart Circ Physiol 296:H1236–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Smolenski A, Lohmann SM and Kukreja RC (2006) Cyclic GMP-dependent protein kinase Ialpha attenuates necrosis and apoptosis following ischemia/reoxygenation in adult cardiomyocyte. J Biol Chem 281:38644–38652. [DOI] [PubMed] [Google Scholar]
- Das A, Xi L and Kukreja RC (2005) Phosphodiesterase-5 inhibitor sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis. Essential role of nitric oxide signaling. J Biol Chem 280:12944–12955. [DOI] [PubMed] [Google Scholar]
- Das A, Xi L and Kukreja RC (2008) Protein kinase G-dependent cardioprotective mechanism of phosphodiesterase-5 inhibition involves phosphorylation of ERK and GSK3beta. J Biol Chem 283:29572–29585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A and Bos JL (1998) Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396:474–477. [DOI] [PubMed] [Google Scholar]
- Degerman E, Ahmad F, Chung YW, Guirguis E, Omar B, Stenson L and Manganiello V (2011) From PDE3B to the regulation of energy homeostasis. Curr Opin Pharmacol 11:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh R, Sharma V, Mehan S, Sharma N and Bedi KL (2009) Amelioration of intracerebroventricular streptozotocin induced cognitive dysfunction and oxidative stress by vinpocetine -- a PDE1 inhibitor. Eur J Pharmacol 620:49–56. [DOI] [PubMed] [Google Scholar]
- Dey AB, Khedr S, Bean J, Porras LL, Meredith TD, Willard FS, Hass JV, Zhou X, Terashvili M, Jesudason CD, Ruley KM, Wiley MR, Kowala M, Atkinson SJ, Staruschenko A and Rekhter MD (2020) Selective Phosphodiesterase 1 Inhibitor BTTQ Reduces Blood Pressure in Spontaneously Hypertensive and Dahl Salt Sensitive Rats: Role of Peripheral Vasodilation. Front Physiol 11:543727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlaboga D, Hajjhussein H and O’Donnell JM (2008) Chronic haloperidol and clozapine produce different patterns of effects on phosphodiesterase-1B, −4B, and −10A expression in rat striatum. Neuropharmacology 54:745–754. [DOI] [PubMed] [Google Scholar]
- Doig J, McLennan JD and Gibbard WB (2008) Medication effects on symptoms of attention-deficit/hyperactivity disorder in children with fetal alcohol spectrum disorder. J Child Adolesc Psychopharmacol 18:365–371. [DOI] [PubMed] [Google Scholar]
- Dousa TP (1999) Cyclic-3’,5’-nucleotide phosphodiesterase isozymes in cell biology and pathophysiology of the kidney. Kidney Int 55:29–62. [DOI] [PubMed] [Google Scholar]
- Dyck B, Branstetter B, Gharbaoui T, Hudson AR, Breitenbucher JG, Gomez L, Botrous I, Marrone T, Barido R, Allerston CK, Cedervall EP, Xu R, Sridhar V, Barker R, Aertgeerts K, Schmelzer K, Neul D, Lee D, Massari ME, Andersen CB, Sebring K, Zhou X, Petroski R, Limberis J, Augustin M, Chun LE, Edwards TE, Peters M and Tabatabaei A (2017) Discovery of Selective Phosphodiesterase 1 Inhibitors with Memory Enhancing Properties. J Med Chem 60:3472–3483. [DOI] [PubMed] [Google Scholar]
- Evgenov OV, Busch CJ, Evgenov NV, Liu R, Petersen B, Falkowski GE, Petho B, Vas A, Bloch KD, Zapol WM and Ichinose F (2006) Inhibition of phosphodiesterase 1 augments the pulmonary vasodilator response to inhaled nitric oxide in awake lambs with acute pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 290:L723–L729. [DOI] [PubMed] [Google Scholar]
- Fajardo AM, Piazza GA and Tinsley HN (2014) The role of cyclic nucleotide signaling pathways in cancer: targets for prevention and treatment. Cancers (Basel) 6:436–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidock M, Miller M and Lanfear J (2002) Isolation and differential tissue distribution of two human cDNAs encoding PDE1 splice variants. Cell Signal 14:53–60. [DOI] [PubMed] [Google Scholar]
- Filgueiras CC, Krahe TE and Medina AE (2010) Phosphodiesterase type 1 inhibition improves learning in rats exposed to alcohol during the third trimester equivalent of human gestation. Neurosci Lett 473:202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florio VA, Sonnenburg WK, Johnson R, Kwak KS, Jensen GS, Walsh KA and Beavo JA (1994) Phosphorylation of the 61-kDa calmodulin-stimulated cyclic nucleotide phosphodiesterase at serine 120 reduces its affinity for calmodulin. Biochemistry 33:8948–8954. [DOI] [PubMed] [Google Scholar]
- Francis SH, Blount MA and Corbin JD (2011) Mammalian cyclic nucleotide phosphodiesterases: molecular mechanisms and physiological functions. Physiol Rev 91:651–690. [DOI] [PubMed] [Google Scholar]
- Frederiksen LJ, Sullivan R, Maxwell LR, Macdonald-Goodfellow SK, Adams MA, Bennett BM, Siemens DR and Graham CH (2007) Chemosensitization of cancer in vitro and in vivo by nitric oxide signaling. Clin Cancer Res 13:2199–2206. [DOI] [PubMed] [Google Scholar]
- Fukunaga K, Yamamoto H, Tanaka E, Iwasa T and Miyamoto E (1984) Phosphorylation and activation of calmodulin-sensitive cyclic nucleotide phosphodiesterase by a brain Ca2+, calmodulin-dependent protein kinase. Life Sci 35:493–499. [DOI] [PubMed] [Google Scholar]
- Gaine SP and Rubin LJ (1998) Primary pulmonary hypertension. Lancet 352:719–725. [DOI] [PubMed] [Google Scholar]
- Gattone VH 2nd, Wang X, Harris PCand Torres VE(2003) Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 9:1323–1326. [DOI] [PubMed] [Google Scholar]
- Ghofrani HA, Wiedemann R, Rose F, Olschewski H, Schermuly RT, Weissmann N, Seeger W and Grimminger F (2002) Combination therapy with oral sildenafil and inhaled iloprost for severe pulmonary hypertension. Ann Intern Med 136:515–522. [DOI] [PubMed] [Google Scholar]
- Giachini FR, Lima VV, Carneiro FS, Tostes RC and Webb RC (2011) Decreased cGMP level contributes to increased contraction in arteries from hypertensive rats: role of phosphodiesterase 1. Hypertension 57:655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Mohapel J, Boehme F, Kainer L and Christie BR (2010) Hippocampal cell loss and neurogenesis after fetal alcohol exposure: insights from different rodent models. Brain Res Rev 64:283–303. [DOI] [PubMed] [Google Scholar]
- Ginter E and Simko V (2012) Type 2 diabetes mellitus, pandemic in 21st century. Adv Exp Med Biol 771:42–50. [DOI] [PubMed] [Google Scholar]
- Girgis RE, Champion HC, Diette GB, Johns RA, Permutt S and Sylvester JT (2005) Decreased exhaled nitric oxide in pulmonary arterial hypertension: response to bosentan therapy. Am J Respir Crit Care Med 172:352–357. [DOI] [PubMed] [Google Scholar]
- Guazzi M, Vicenzi M, Arena R and Guazzi MD (2011) PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1-year, prospective, randomized, placebo-controlled study. Circ Heart Fail 4:8–17. [DOI] [PubMed] [Google Scholar]
- Gupta S and Sharma B (2014) Protective effects of phosphodiesterase-1 (PDE1) and ATP sensitive potassium (KATP) channel modulators against 3-nitropropionic acid induced behavioral and biochemical toxicities in experimental Huntingtons disease. Eur J Pharmacol 732:111–122. [DOI] [PubMed] [Google Scholar]
- Hambleton R, Krall J, Tikishvili E, Honeggar M, Ahmad F, Manganiello VC and Movsesian MA (2005) Isoforms of cyclic nucleotide phosphodiesterase PDE3 and their contribution to cAMP hydrolytic activity in subcellular fractions of human myocardium. J Biol Chem 280:39168–39174. [DOI] [PubMed] [Google Scholar]
- Hamilton TK, Hu N, Kolomitro K, Bell EN, Maurice DH, Graham CH and Siemens DR (2013) Potential therapeutic applications of phosphodiesterase inhibition in prostate cancer. World J Urol 31:325–330. [DOI] [PubMed] [Google Scholar]
- Han P, Werber J, Surana M, Fleischer N and Michaeli T (1999) The calcium/calmodulin-dependent phosphodiesterase PDE1C down-regulates glucose-induced insulin secretion. J Biol Chem 274:22337–22344. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Kim GE, Tunin RS, Adesiyun T, Hsu S, Nakagawa R, Zhu G, O’Brien JJ, Hendrick JP, Davis RE, Yao W, Beard D, Hoxie HR, Wennogle LP, Lee DI and Kass DA (2018) Acute Enhancement of Cardiac Function by Phosphodiesterase Type 1 Inhibition. Circulation 138:1974–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikaus CC, Stout JR, Sekharan MR, Eakin CM, Rajagopal P, Brzovic PS, Beavo JA and Klevit RE (2008) Solution structure of the cGMP binding GAF domain from phosphodiesterase 5: insights into nucleotide specificity, dimerization, and cGMP-dependent conformational change. J Biol Chem 283:22749–22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimann E, Jones HA, Resjo S, Manganiello VC, Stenson L and Degerman E (2010) Expression and regulation of cyclic nucleotide phosphodiesterases in human and rat pancreatic islets. PLoS One 5:e14191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JH, Du L, Chu T, Otsuka G, Dronadula N, Jaffe M, Gill SE, Parks WC and Dichek DA (2010) Overexpression of urokinase by plaque macrophages causes histological features of plaque rupture and increases vascular matrix metalloproteinase activity in aged apolipoprotein e-null mice. Circulation 121:1637–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF and Rabinovitch M (2004) Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 43:13S–24S. [DOI] [PubMed] [Google Scholar]
- Humphrey JM, Movsesian M, Am Ende CW, Becker SL, Chappie TA, Jenkinson S, Liras JL, Liras S, Orozco C, Pandit J, Vajdos FF, Vandeput F, Yang E and Menniti FS (2018) Discovery of Potent and Selective Periphery-Restricted Quinazoline Inhibitors of the Cyclic Nucleotide Phosphodiesterase PDE1. J Med Chem 61:4635–4640. [DOI] [PubMed] [Google Scholar]
- James MA, Lu Y, Liu Y, Vikis HG and You M (2009) RGS17, an overexpressed gene in human lung and prostate cancer, induces tumor cell proliferation through the cyclic AMP-PKA-CREB pathway. Cancer Res 69:2108–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon KI, Jono H, Miller CL, Cai Y, Lim S, Liu X, Gao P, Abe J, Li JD and Yan C (2010) Ca2+/calmodulin-stimulated PDE1 regulates the beta-catenin/TCF signaling through PP2A B56 gamma subunit in proliferating vascular smooth muscle cells. FEBS J 277:5026–5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Li J, Paskind M and Epstein PM (1996) Inhibition of calmodulin-dependent phosphodiesterase induces apoptosis in human leukemic cells. Proc Natl Acad Sci U S A 93:11236–11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RP, El-Yazbi AF, Hughes MF, Schriemer DC, Walsh EJ, Walsh MP and Cole WC (2009) Identification and functional characterization of protein kinase A-catalyzed phosphorylation of potassium channel Kv1.2 at serine 449. J Biol Chem 284:16562–16574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WB, Katugampola S, Able S, Napier C and Harding SE (2012) Profiling of cAMP and cGMP phosphodiesterases in isolated ventricular cardiomyocytes from human hearts: comparison with rat and guinea pig. Life Sci 90:328–336. [DOI] [PubMed] [Google Scholar]
- Kakkar R, Raju RV and Sharma RK (1999) Calmodulin-dependent cyclic nucleotide phosphodiesterase (PDE1). Cell Mol Life Sci 55:1164–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MP, Adamowicz W, Bove S, Hartman AJ, Mariga A, Pathak G, Reinhart V, Romegialli A and Kleiman RJ (2014) Select 3’,5’-cyclic nucleotide phosphodiesterases exhibit altered expression in the aged rodent brain. Cell Signal 26:383–397. [DOI] [PubMed] [Google Scholar]
- Kenan Y, Murata T, Shakur Y, Degerman E and Manganiello VC (2000) Functions of the N-terminal region of cyclic nucleotide phosphodiesterase 3 (PDE 3) isoforms. J Biol Chem 275:12331–12338. [DOI] [PubMed] [Google Scholar]
- Kim D, Rybalkin SD, Pi X, Wang Y, Zhang C, Munzel T, Beavo JA, Berk BC and Yan C (2001) Upregulation of phosphodiesterase 1A1 expression is associated with the development of nitrate tolerance. Circulation 104:2338–2343. [DOI] [PubMed] [Google Scholar]
- Kim KH, Kim HK, Hwang IC, Cho HJ, Je N, Kwon OM, Choi SJ, Lee SP, Kim YJ and Sohn DW (2015) PDE 5 inhibition with udenafil improves left ventricular systolic/diastolic functions and exercise capacity in patients with chronic heart failure with reduced ejection fraction; A 12-week, randomized, double-blind, placebo-controlled trial. Am Heart J 169:813–822 e813. [DOI] [PubMed] [Google Scholar]
- Kim NJ, Baek JH, Lee J, Kim H, Song JK and Chun KH (2019) A PDE1 inhibitor reduces adipogenesis in mice via regulation of lipolysis and adipogenic cell signaling. Exp Mol Med 51:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight WE, Chen S, Zhang Y, Oikawa M, Wu M, Zhou Q, Miller CL, Cai Y, Mickelsen DM, Moravec C, Small EM, Abe J and Yan C (2016) PDE1C deficiency antagonizes pathological cardiac remodeling and dysfunction. Proc Natl Acad Sci U S A 113:E7116–E7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodani E, Xuan YT, Takano H, Shinmura K, Tang XL and Bolli R (2002) Role of cyclic guanosine monophosphate in late preconditioning in conscious rabbits. Circulation 105:3046–3052. [DOI] [PubMed] [Google Scholar]
- Koka S, Aluri HS, Xi L, Lesnefsky EJ and Kukreja RC (2014) Chronic inhibition of phosphodiesterase 5 with tadalafil attenuates mitochondrial dysfunction in type 2 diabetic hearts: potential role of NO/SIRT1/PGC-1alpha signaling. Am J Physiol Heart Circ Physiol 306:H1558–H1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koka S, Xi L and Kukreja RC (2012) Chronic treatment with long acting phosphodiesterase-5 inhibitor tadalafil alters proteomic changes associated with cytoskeletal rearrangement and redox regulation in Type 2 diabetic hearts. Basic Res Cardiol 107:249. [DOI] [PubMed] [Google Scholar]
- Koka S, Xi L and Kukreja RC (2020) Chronic inhibition of phosphodiesterase 5 with tadalafil affords cardioprotection in a mouse model of metabolic syndrome: role of nitric oxide. Mol Cell Biochem 468:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic MM, Erdogan S, Rena G, Borchert G, Hoch B, Bartel S, Scotland G, Huston E, Houslay MD and Krause EG (1997) Altered expression of PDE1 and PDE4 cyclic nucleotide phosphodiesterase isoforms in 7-oxo-prostacyclin-preconditioned rat heart. J Mol Cell Cardiol 29:3135–3146. [DOI] [PubMed] [Google Scholar]
- Kukreja RC (2012) Phosphodiesterase-5 and retargeting of subcellular cGMP signaling during pathological hypertrophy. Circulation 126:916–919. [DOI] [PubMed] [Google Scholar]
- Kukreja RC (2013) Sildenafil and cardioprotection. Curr Pharm Des 19:6842–6847. [DOI] [PubMed] [Google Scholar]
- Kukreja RC, Salloum F, Das A, Ockaili R, Yin C, Bremer YA, Fisher PW, Wittkamp M, Hawkins J, Chou E, Kukreja AK, Wang X, Marwaha VR and Xi L (2005) Pharmacological preconditioning with sildenafil: Basic mechanisms and clinical implications. Vascul Pharmacol 42:219–232. [DOI] [PubMed] [Google Scholar]
- Kukreja RC, Salloum FN and Das A (2012) Cyclic guanosine monophosphate signaling and phosphodiesterase-5 inhibitors in cardioprotection. J Am Coll Cardiol 59:1921–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumada T, Komuro Y, Li Y, Hu T, Wang Z, Littner Y and Komuro H (2010) Inhibition of cerebellar granule cell turning by alcohol. Neuroscience 170:1328–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laddha SS and Bhatnagar SP (2009) A new therapeutic approach in Parkinson’s disease: some novel quinazoline derivatives as dual selective phosphodiesterase 1 inhibitors and anti-inflammatory agents. Bioorg Med Chem 17:6796–6802. [DOI] [PubMed] [Google Scholar]
- Lange H, Belz GG, Tschollar W and Wolf GK (1988) [Cilazapril in essential hypertension. A study of one year of continuous treatment]. Med Klin (Munich) 83:554–558. [PubMed] [Google Scholar]
- Laursen M, Beck L, Kehler J, Christoffersen CT, Bundgaard C, Mogensen S, Mow TJ, Pinilla E, Knudsen JS, Hedegaard ER, Grunnet M and Simonsen U (2017) Novel selective PDE type 1 inhibitors cause vasodilatation and lower blood pressure in rats. Br J Pharmacol 174:2563–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laychock SG (1983) Mediation of insulin release by cGMP and cAMP in a starved animal model. Mol Cell Endocrinol 32:157–170. [DOI] [PubMed] [Google Scholar]
- Lefievre L, de Lamirande E and Gagnon C (2002) Presence of cyclic nucleotide phosphodiesterases PDE1A, existing as a stable complex with calmodulin, and PDE3A in human spermatozoa. Biol Reprod 67:423–430. [DOI] [PubMed] [Google Scholar]
- Levy I, Horvath A, Azevedo M, de Alexandre RB and Stratakis CA (2011) Phosphodiesterase function and endocrine cells: links to human disease and roles in tumor development and treatment. Curr Opin Pharmacol 11:689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, Tawakol A, Gerszten RE, Systrom DM, Bloch KD and Semigran MJ (2007) Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation 116:1555–1562. [DOI] [PubMed] [Google Scholar]
- Li P, Zheng H, Zhao J, Zhang L, Yao W, Zhu H, Beard JD, Ida K, Lane W, Snell G, Sogabe S, Heyser CJ, Snyder GL, Hendrick JP, Vanover KE, Davis RE and Wennogle LP (2016) Discovery of Potent and Selective Inhibitors of Phosphodiesterase 1 for the Treatment of Cognitive Impairment Associated with Neurodegenerative and Neuropsychiatric Diseases. J Med Chem 59:1149–1164. [DOI] [PubMed] [Google Scholar]
- Loughney K, Martins TJ, Harris EA, Sadhu K, Hicks JB, Sonnenburg WK, Beavo JA and Ferguson K (1996) Isolation and characterization of cDNAs corresponding to two human calcium, calmodulin-regulated, 3’,5’-cyclic nucleotide phosphodiesterases. J Biol Chem 271:796–806. [DOI] [PubMed] [Google Scholar]
- Lugnier C (2006) Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther 109:366–398. [DOI] [PubMed] [Google Scholar]
- Lugnier C (2011) PDE inhibitors: a new approach to treat metabolic syndrome? Curr Opin Pharmacol 11:698–706. [DOI] [PubMed] [Google Scholar]
- Lygren B, Carlson CR, Santamaria K, Lissandron V, McSorley T, Litzenberg J, Lorenz D, Wiesner B, Rosenthal W, Zaccolo M, Tasken K and Klussmann E (2007) AKAP complex regulates Ca2+ re-uptake into heart sarcoplasmic reticulum. EMBO Rep 8:1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccioni RB, Munoz JP and Barbeito L (2001) The molecular bases of Alzheimer’s disease and other neurodegenerative disorders. Arch Med Res 32:367–381. [DOI] [PubMed] [Google Scholar]
- Maclean MR, Johnston ED, McCulloch KM, Pooley L, Houslay MD and Sweeney G (1997) Phosphodiesterase isoforms in the pulmonary arterial circulation of the rat: changes in pulmonary hypertension. J Pharmacol Exp Ther 283:619–624. [PubMed] [Google Scholar]
- Marko D, Romanakis K, Zankl H, Furstenberger G, Steinbauer B and Eisenbrand G (1998) Induction of apoptosis by an inhibitor of cAMP-specific PDE in malignant murine carcinoma cells overexpressing PDE activity in comparison to their nonmalignant counterparts. Cell Biochem Biophys 28:75–101. [DOI] [PubMed] [Google Scholar]
- Maurice DH, Ke H, Ahmad F, Wang Y, Chung J and Manganiello VC (2014) Advances in targeting cyclic nucleotide phosphodiesterases. Nat Rev Drug Discov 13:290–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell CR, Kanes SJ, Abel T and Siegel SJ (2004) Phosphodiesterase inhibitors: a novel mechanism for receptor-independent antipsychotic medications. Neuroscience 129:101–107. [DOI] [PubMed] [Google Scholar]
- McMurray JJ (2010) Clinical practice. Systolic heart failure. N Engl J Med 362:228–238. [DOI] [PubMed] [Google Scholar]
- Meacci E, Taira M, Moos M Jr., Smith CJ, Movsesian MA, Degerman E, Belfrage P and Manganiello V (1992) Molecular cloning and expression of human myocardial cGMP-inhibited cAMP phosphodiesterase. Proc Natl Acad Sci U S A 89:3721–3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina AE, Krahe TE and Ramoa AS (2006) Restoration of neuronal plasticity by a phosphodiesterase type 1 inhibitor in a model of fetal alcohol exposure. J Neurosci 26:1057–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle D and Hoffmann R (2011) Roles of cAMP and cAMP-dependent protein kinase in the progression of prostate cancer: cross-talk with the androgen receptor. Cell Signal 23:507–515. [DOI] [PubMed] [Google Scholar]
- Michel T and Loscalzo J (2015) Nitroglycerin and Nitric Oxide--A Rondo of Themes in Cardiovascular Therapeutics. N Engl J Med 373:1789. [DOI] [PubMed] [Google Scholar]
- Michikawa H, Sugiya H, Yoshigaki T, Fujita-Yoshigaki J and Furuyama S (2005) Phosphodiesterases 1 and 2 regulate cellular cGMP level in rabbit submandibular gland cells. Int J Biochem Cell Biol 37:876–886. [DOI] [PubMed] [Google Scholar]
- Miller CL, Cai Y, Oikawa M, Thomas T, Dostmann WR, Zaccolo M, Fujiwara K and Yan C (2011) Cyclic nucleotide phosphodiesterase 1A: a key regulator of cardiac fibroblast activation and extracellular matrix remodeling in the heart. Basic Res Cardiol 106:1023–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR (2002) Regulatory subunits of PKA and breast cancer. Ann N Y Acad Sci 968:37–48. [DOI] [PubMed] [Google Scholar]
- Mongillo M, Tocchetti CG, Terrin A, Lissandron V, Cheung YF, Dostmann WR, Pozzan T, Kass DA, Paolocci N, Houslay MD and Zaccolo M (2006) Compartmentalized phosphodiesterase-2 activity blunts beta-adrenergic cardiac inotropy via an NO/cGMP-dependent pathway. Circ Res 98:226–234. [DOI] [PubMed] [Google Scholar]
- Moriwaki H, Stempien-Otero A, Kremen M, Cozen AE and Dichek DA (2004) Overexpression of urokinase by macrophages or deficiency of plasminogen activator inhibitor type 1 causes cardiac fibrosis in mice. Circ Res 95:637–644. [DOI] [PubMed] [Google Scholar]
- Mower AF, Liao DS, Nestler EJ, Neve RL and Ramoa AS (2002) cAMP/Ca2+ response element-binding protein function is essential for ocular dominance plasticity. J Neurosci 22:2237–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy AB, Shasby SS, Scott BD and Shasby DM (1993) The effect of histamine and cyclic adenosine monophosphate on myosin light chain phosphorylation in human umbilical vein endothelial cells. J Clin Invest 92:1198–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniyan S, Rachagani S, Parte S, Halder S, Seshacharyulu P, Kshirsagar P, Siddiqui JA, Vengoji R, Rauth S, Islam R, Mallya K, Datta K, Xi L, Das A, Teply BA, Kukreja RC and Batra SK (2020) Sildenafil Potentiates the Therapeutic Efficacy of Docetaxel in Advanced Prostate Cancer by Stimulating NO-cGMP Signaling. Clin Cancer Res 26:5720–5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray F, Patel HH, Suda RY, Zhang S, Thistlethwaite PA, Yuan JX and Insel PA (2007) Expression and activity of cAMP phosphodiesterase isoforms in pulmonary artery smooth muscle cells from patients with pulmonary hypertension: role for PDE1. Am J Physiol Lung Cell Mol Physiol 292:L294–303. [DOI] [PubMed] [Google Scholar]
- Nagel DJ, Aizawa T, Jeon KI, Liu W, Mohan A, Wei H, Miano JM, Florio VA, Gao P, Korshunov VA, Berk BC and Yan C (2006) Role of nuclear Ca2+/calmodulin-stimulated phosphodiesterase 1A in vascular smooth muscle cell growth and survival. Circ Res 98:777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan Y, Zeng X, Jin Z, Li N, Chen Z, Chen J, Wang D, Wang Y, Lin Z and Ying L (2020) PDE1 or PDE5 inhibition augments NO-dependent hypoxic constriction of porcine coronary artery via elevating inosine 3’,5’-cyclic monophosphate level. J Cell Mol Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C and Loscalzo J (2004) Nitric oxide and other novel therapies for pulmonary hypertension. J Cardiovasc Pharmacol Ther 9:1–8. [DOI] [PubMed] [Google Scholar]
- Nichols GA, Joshua-Gotlib S and Parasuraman S (2013) Glycemic control and risk of cardiovascular disease hospitalization and all-cause mortality. J Am Coll Cardiol 62:121–127. [DOI] [PubMed] [Google Scholar]
- Nikolaev VO, Bunemann M, Schmitteckert E, Lohse MJ and Engelhardt S (2006) Cyclic AMP imaging in adult cardiac myocytes reveals far-reaching beta1-adrenergic but locally confined beta2-adrenergic receptor-mediated signaling. Circ Res 99:1084–1091. [DOI] [PubMed] [Google Scholar]
- Nishimura J, Setoguchi H and Kanaide H (2001) [Regulation of vascular tone by the modulation of Ca2+ sensitivity]. Clin Calcium 11:411–417. [PubMed] [Google Scholar]
- Noori S and Hassan ZM (2014) Tehranolide inhibits cell proliferation via calmodulin inhibition, PDE, and PKA activation. Tumour Biol 35:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novack JP, Charbonneau H, Bentley JK, Walsh KA and Beavo JA (1991) Sequence comparison of the 63-, 61-, and 59-kDa calmodulin-dependent cyclic nucleotide phosphodiesterases. Biochemistry 30:7940–7947. [DOI] [PubMed] [Google Scholar]
- Nunes F, Ferreira-Rosa K, Pereira Mdos S, Kubrusly RC, Manhaes AC, Abreu-Villaca Y and Filgueiras CC (2011) Acute administration of vinpocetine, a phosphodiesterase type 1 inhibitor, ameliorates hyperactivity in a mice model of fetal alcohol spectrum disorder. Drug Alcohol Depend 119:81–87. [DOI] [PubMed] [Google Scholar]
- O’Brien JJ, O’Callaghan JP, Miller DB, Chalgeri S, Wennogle LP, Davis RE, Snyder GL and Hendrick JP (2020) Inhibition of calcium-calmodulin-dependent phosphodiesterase (PDE1) suppresses inflammatory responses. Mol Cell Neurosci 102:103449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan DS, Savale L, Montani D, Jais X, Sitbon O, Simonneau G and Humbert M (2011) Treatment of pulmonary arterial hypertension with targeted therapies. Nat Rev Cardiol 8:526–538. [DOI] [PubMed] [Google Scholar]
- Ockaili R, Salloum F, Hawkins J and Kukreja RC (2002) Sildenafil (Viagra) induces powerful cardioprotective effect via opening of mitochondrial K(ATP) channels in rabbits. Am J Physiol Heart Circ Physiol 283:H1263–1269. [DOI] [PubMed] [Google Scholar]
- Oldenburg O, Qin Q, Krieg T, Yang XM, Philipp S, Critz SD, Cohen MV and Downey JM (2004) Bradykinin induces mitochondrial ROS generation via NO, cGMP, PKG, and mitoKATP channel opening and leads to cardioprotection. Am J Physiol Heart Circ Physiol 286:H468–476. [DOI] [PubMed] [Google Scholar]
- Olney JW, Tenkova T, Dikranian K, Qin YQ, Labruyere J and Ikonomidou C (2002) Ethanol-induced apoptotic neurodegeneration in the developing C57BL/6 mouse brain. Brain Res Dev Brain Res 133:115–126. [DOI] [PubMed] [Google Scholar]
- Ong AC, Devuyst O, Knebelmann B, Walz G and Diseases E-EWGfIK(2015) Autosomal dominant polycystic kidney disease: the changing face of clinical management. Lancet 385:1993–2002. [DOI] [PubMed] [Google Scholar]
- Onody A, Zvara A, Hackler L Jr., Vigh L, Ferdinandy P and Puskas LG(2003) Effect of classic preconditioning on the gene expression pattern of rat hearts: a DNA microarray study. FEBS Lett 536:35–40. [DOI] [PubMed] [Google Scholar]
- Paine TA, Neve RL and Carlezon WA Jr. (2009) Attention deficits and hyperactivity following inhibition of cAMP-dependent protein kinase within the medial prefrontal cortex of rats. Neuropsychopharmacology 34:2143–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit J, Forman MD, Fennell KF, Dillman KS and Menniti FS (2009) Mechanism for the allosteric regulation of phosphodiesterase 2A deduced from the X-ray structure of a near full-length construct. Proc Natl Acad Sci U S A 106:18225–18230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Sandner P and Krieg T (2018) cGMP at the centre of attention: emerging strategies for activating the cardioprotective PKG pathway. Basic Res Cardiol 113:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlaki N and Nikolaev VO (2018) Imaging of PDE2- and PDE3-Mediated cGMP-to-cAMP Cross-Talk in Cardiomyocytes. J Cardiovasc Dev Dis 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekcec A, Schulert N, Stierstorfer B, Deiana S, Dorner-Ciossek C and Rosenbrock H (2018) Targeting the dopamine D1 receptor or its downstream signalling by inhibiting phosphodiesterase-1 improves cognitive performance. Br J Pharmacol 175:3021–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng T, Gong J, Jin Y, Zhou Y, Tong R, Wei X, Bai L and Shi J (2018) Inhibitors of phosphodiesterase as cancer therapeutics. Eur J Med Chem 150:742–756. [DOI] [PubMed] [Google Scholar]
- Pinto CS, Raman A, Reif GA, Magenheimer BS, White C, Calvet JP and Wallace DP (2016) Phosphodiesterase Isoform Regulation of Cell Proliferation and Fluid Secretion in Autosomal Dominant Polycystic Kidney Disease. J Am Soc Nephrol 27:1124–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak NM, Jaeger D, Kolleritsch S, Zimmermann R, Zechner R, Lass A and Haemmerle G (2015) The interplay of protein kinase A and perilipin 5 regulates cardiac lipolysis. J Biol Chem 290:1295–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polli JW and Kincaid RL (1992) Molecular cloning of DNA encoding a calmodulin-dependent phosphodiesterase enriched in striatum. Proc Natl Acad Sci U S A 89:11079–11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt EPS, Harvey KE, Salyer AE and Hockerman GH (2019) Regulation of cAMP accumulation and activity by distinct phosphodiesterase subtypes in INS-1 cells and human pancreatic beta-cells. PLoS One 14:e0215188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne NJ and Furman BL (2003) Cyclic nucleotide phosphodiesterases in pancreatic islets. Diabetologia 46:1179–1189. [DOI] [PubMed] [Google Scholar]
- Ran I, Laplante I and Lacaille JC (2012) CREB-dependent transcriptional control and quantal changes in persistent long-term potentiation in hippocampal interneurons. J Neurosci 32:6335–6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S, Gray-Bablin J, Herliczek TW and Keyomarsi K (1999) The biphasic induction of p21 and p27 in breast cancer cells by modulators of cAMP is posttranscriptionally regulated and independent of the PKA pathway. Exp Cell Res 252:211–223. [DOI] [PubMed] [Google Scholar]
- Rees S, Kittikulsuth W, Roos K, Strait KA, Van Hoek A and Kohan DE (2014) Adenylyl cyclase 6 deficiency ameliorates polycystic kidney disease. J Am Soc Nephrol 25:232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remuzzi G, Chiurchiu C and Ruggenenti P (2004) Proteinuria predicting outcome in renal disease: nondiabetic nephropathies (REIN). Kidney Int Suppl:S 90–96. [DOI] [PubMed] [Google Scholar]
- Repaske DR, Swinnen JV, Jin SL, Van Wyk JJ and Conti M (1992) A polymerase chain reaction strategy to identify and clone cyclic nucleotide phosphodiesterase cDNAs. Molecular cloning of the cDNA encoding the 63-kDa calmodulin-dependent phosphodiesterase. J Biol Chem 267:18683–18688. [PubMed] [Google Scholar]
- Ristic J, Folic M, Radonjic K, Rosic MI, Bolevich S, Alisultanovich OI, Draginic N, Andjic M, Jeremic J, Milosavljevic I, Zivkovic V and Jakovljevic V (2020) Preconditioning with PDE1 Inhibitors and Moderate-Intensity Training Positively Affect Systemic Redox State of Rats. Oxid Med Cell Longev 2020:6361703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JL, Booth L, Conley A, Cruickshanks N, Malkin M, Kukreja RC, Grant S, Poklepovic A and Dent P (2014) PDE5 inhibitors enhance the lethality of standard of care chemotherapy in pediatric CNS tumor cells. Cancer Biol Ther 15:758–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowther FB, Wei W, Dawson TP, Ashton K, Singh A, Madiesse-Timchou MP, Thomas DG, Darling JL and Warr T (2016) Cyclic nucleotide phosphodiesterase-1C (PDE1C) drives cell proliferation, migration and invasion in glioblastoma multiforme cells in vitro. Mol Carcinog 55:268–279. [DOI] [PubMed] [Google Scholar]
- Rybalkin SD, Bornfeldt KE, Sonnenburg WK, Rybalkina IG, Kwak KS, Hanson K, Krebs EG and Beavo JA (1997) Calmodulin-stimulated cyclic nucleotide phosphodiesterase (PDE1C) is induced in human arterial smooth muscle cells of the synthetic, proliferative phenotype. J Clin Invest 100:2611–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybalkin SD, Rybalkina I, Beavo JA and Bornfeldt KE (2002) Cyclic nucleotide phosphodiesterase 1C promotes human arterial smooth muscle cell proliferation. Circ Res 90:151–157. [DOI] [PubMed] [Google Scholar]
- Rybalkin SD, Yan C, Bornfeldt KE and Beavo JA (2003) Cyclic GMP phosphodiesterases and regulation of smooth muscle function. Circ Res 93:280–291. [DOI] [PubMed] [Google Scholar]
- Salloum FN, Chau VQ, Hoke NN, Abbate A, Varma A, Ockaili RA, Toldo S and Kukreja RC (2009) Phosphodiesterase-5 inhibitor, tadalafil, protects against myocardial ischemia/reperfusion through protein-kinase g-dependent generation of hydrogen sulfide. Circulation 120:S31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloum FN, Ockaili RA, Wittkamp M, Marwaha VR and Kukreja RC (2006) Vardenafil: a novel type 5 phosphodiesterase inhibitor reduces myocardial infarct size following ischemia/reperfusion injury via opening of mitochondrial K(ATP) channels in rabbits. J Mol Cell Cardiol 40:405–411. [DOI] [PubMed] [Google Scholar]
- Sarfati M, Mateo V, Baudet S, Rubio M, Fernandez C, Davi F, Binet JL, Delic J and Merle-Beral H (2003) Sildenafil and vardenafil, types 5 and 6 phosphodiesterase inhibitors, induce caspase-dependent apoptosis of B-chronic lymphocytic leukemia cells. Blood 101:265–269. [DOI] [PubMed] [Google Scholar]
- Savai R, Pullamsetti SS, Banat GA, Weissmann N, Ghofrani HA, Grimminger F and Schermuly RT (2010) Targeting cancer with phosphodiesterase inhibitors. Expert Opin Investig Drugs 19:117–131. [DOI] [PubMed] [Google Scholar]
- Schermuly RT, Pullamsetti SS, Kwapiszewska G, Dumitrascu R, Tian X, Weissmann N, Ghofrani HA, Kaulen C, Dunkern T, Schudt C, Voswinckel R, Zhou J, Samidurai A, Klepetko W, Paddenberg R, Kummer W, Seeger W and Grimminger F (2007) Phosphodiesterase 1 upregulation in pulmonary arterial hypertension: target for reverse-remodeling therapy. Circulation 115:2331–2339. [DOI] [PubMed] [Google Scholar]
- Schulze PC and Lee RT (2004) Macrophage-mediated cardiac fibrosis. Circ Res 95:552–553. [DOI] [PubMed] [Google Scholar]
- Senzaki H, Smith CJ, Juang GJ, Isoda T, Mayer SP, Ohler A, Paolocci N, Tomaselli GF, Hare JM and Kass DA (2001) Cardiac phosphodiesterase 5 (cGMP-specific) modulates beta-adrenergic signaling in vivo and is down-regulated in heart failure. FASEB J 15:1718–1726. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Murata T, Tagawa T, Takahashi K, Ishikawa R, Abe Y, Hosaka K and Kubohara Y (2004) Calmodulin-dependent cyclic nucleotide phosphodiesterase (PDE1) is a pharmacological target of differentiation-inducing factor-1, an antitumor agent isolated from Dictyostelium. Cancer Res 64:2568–2571. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Murata T, Watanabe Y, Sato C, Morita H and Tagawa T (2009) Characterization of phosphodiesterase 1 in human malignant melanoma cell lines. Anticancer Res 29:1119–1122. [PubMed] [Google Scholar]
- Siemens DR, Heaton JP, Adams MA, Kawakami J and Graham CH (2009) Phase II study of nitric oxide donor for men with increasing prostate-specific antigen level after surgery or radiotherapy for prostate cancer. Urology 74:878–883. [DOI] [PubMed] [Google Scholar]
- Simonneau G (2008) [Pulmonary hypertension. Major therapeutic advances]. Rev Prat 58:1989–1990. [PubMed] [Google Scholar]
- Siuciak JA, McCarthy SA, Chapin DS, Reed TM, Vorhees CV and Repaske DR (2007) Behavioral and neurochemical characterization of mice deficient in the phosphodiesterase-1B (PDE1B) enzyme. Neuropharmacology 53:113–124. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Prickaerts J, Wadenberg ML, Zhang L, Zheng H, Yao W, Akkerman S, Zhu H, Hendrick JP, Vanover KE, Davis R, Li P, Mates S and Wennogle LP (2016) Preclinical profile of ITI-214, an inhibitor of phosphodiesterase 1, for enhancement of memory performance in rats. Psychopharmacology (Berl) 233:3113–3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder PB, Esselstyn JM, Loughney K, Wolda SL and Florio VA (2005) The role of cyclic nucleotide phosphodiesterases in the regulation of adipocyte lipolysis. J Lipid Res 46:494–503. [DOI] [PubMed] [Google Scholar]
- Soderling SH, Bayuga SJ and Beavo JA (1998) Cloning and characterization of a cAMP-specific cyclic nucleotide phosphodiesterase. Proc Natl Acad Sci U S A 95:8991–8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg WK, Rybalkin SD, Bornfeldt KE, Kwak KS, Rybalkina IG and Beavo JA (1998) Identification, quantitation, and cellular localization of PDE1 calmodulin-stimulated cyclic nucleotide phosphodiesterases. Methods 14:3–19. [DOI] [PubMed] [Google Scholar]
- Sonnenburg WK, Seger D and Beavo JA (1993) Molecular cloning of a cDNA encoding the “61-kDa” calmodulin-stimulated cyclic nucleotide phosphodiesterase. Tissue-specific expression of structurally related isoforms. J Biol Chem 268:645–652. [PubMed] [Google Scholar]
- Sonnenburg WK, Seger D, Kwak KS, Huang J, Charbonneau H and Beavo JA (1995) Identification of inhibitory and calmodulin-binding domains of the PDE1A1 and PDE1A2 calmodulin-stimulated cyclic nucleotide phosphodiesterases. J Biol Chem 270:30989–31000. [DOI] [PubMed] [Google Scholar]
- Stangherlin A, Gesellchen F, Zoccarato A, Terrin A, Fields LA, Berrera M, Surdo NC, Craig MA, Smith G, Hamilton G and Zaccolo M (2011) cGMP signals modulate cAMP levels in a compartment-specific manner to regulate catecholamine-dependent signaling in cardiac myocytes. Circ Res 108:929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork PJ and Schmitt JM (2002) Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol 12:258–266. [DOI] [PubMed] [Google Scholar]
- Sussman CR, Ward CJ, Leightner AC, Smith JL, Agarwal R, Harris PC and Torres VE (2014) Phosphodiesterase 1A modulates cystogenesis in zebrafish. J Am Soc Nephrol 25:2222–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Fukushima H, Mukawa T, Toyoda H, Wu LJ, Zhao MG, Xu H, Shang Y, Endoh K, Iwamoto T, Mamiya N, Okano E, Hasegawa S, Mercaldo V, Zhang Y, Maeda R, Ohta M, Josselyn SA, Zhuo M and Kida S (2011) Upregulation of CREB-mediated transcription enhances both short- and long-term memory. J Neurosci 31:8786–8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi R, Inoguchi T and Ohnaka K (2011) Clinical and experimental evidence for oxidative stress as an exacerbating factor of diabetes mellitus. J Clin Biochem Nutr 48:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teich AF, Nicholls RE, Puzzo D, Fiorito J, Purgatorio R, Fa M and Arancio O (2015) Synaptic therapy in Alzheimer’s disease: a CREB-centric approach. Neurotherapeutics 12:29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian G, Sagetorp J, Xu Y, Shuai H, Degerman E and Tengholm A (2012) Role of phosphodiesterases in the shaping of sub-plasma-membrane cAMP oscillations and pulsatile insulin secretion. J Cell Sci 125:5084–5095. [DOI] [PubMed] [Google Scholar]
- Tian G, Sandler S, Gylfe E and Tengholm A (2011) Glucose- and hormone-induced cAMP oscillations in alpha- and beta-cells within intact pancreatic islets. Diabetes 60:1535–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres VE and Harris PC (2014) Strategies targeting cAMP signaling in the treatment of polycystic kidney disease. J Am Soc Nephrol 25:18–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuder RM, Cool CD, Geraci MW, Wang J, Abman SH, Wright L, Badesch D and Voelkel NF (1999) Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med 159:1925–1932. [DOI] [PubMed] [Google Scholar]
- Vandeput F, Wolda SL, Krall J, Hambleton R, Uher L, McCaw KN, Radwanski PB, Florio V and Movsesian MA (2007) Cyclic nucleotide phosphodiesterase PDE1C1 in human cardiac myocytes. J Biol Chem 282:32749–32757. [DOI] [PubMed] [Google Scholar]
- Varma A, Das A, Hoke NN, Durrant DE, Salloum FN and Kukreja RC (2012) Anti-inflammatory and cardioprotective effects of tadalafil in diabetic mice. PLoS One 7:e45243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatter S, Pahlke G, Deitmer JW and Eisenbrand G (2005) Differential phosphodiesterase expression and cytosolic Ca2+ in human CNS tumour cells and in non-malignant and malignant cells of rat origin. J Neurochem 93:321–329. [DOI] [PubMed] [Google Scholar]
- Vigneshwar N, Samidurai A, Movsesian M, Das A and Kukreja RC (2019) PDE1 inhibition attenuates doxorubicin-induced toxicity in primary mouse cardiomyocytes. FASEB J 33: doi: 10.1096/fasebj.2019.33.1_supplement.817.12. (Abstract). [DOI] [Google Scholar]
- Waddleton D, Wu W, Feng Y, Thompson C, Wu M, Zhou YP, Howard A, Thornberry N, Li J and Mancini JA (2008) Phosphodiesterase 3 and 4 comprise the major cAMP metabolizing enzymes responsible for insulin secretion in INS-1 (832/13) cells and rat islets. Biochem Pharmacol 76:884–893. [DOI] [PubMed] [Google Scholar]
- Wadie W and El-Tanbouly DM (2017) Vinpocetine mitigates proteinuria and podocytes injury in a rat model of diabetic nephropathy. Eur J Pharmacol 814:187–195. [DOI] [PubMed] [Google Scholar]
- Wallukat G (2002) The beta-adrenergic receptors. Herz 27:683–690. [DOI] [PubMed] [Google Scholar]
- Wan M, Sulpizio AG, Akturk A, Beck WHJ, Lanz M, Faca VM, Smolka MB, Vogel JP and Mao Y (2019) Deubiquitination of phosphoribosyl-ubiquitin conjugates by phosphodiesterase-domain-containing Legionella effectors. Proc Natl Acad Sci U S A 116:23518–23526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Wang J, Zhao L, Wang Y, Liu J, Shi L, Xu M and Wang C (2008) Sildenafil inhibits human pulmonary artery smooth muscle cell proliferation by decreasing capacitative Ca2+ entry. J Pharmacol Sci 108:71–78. [DOI] [PubMed] [Google Scholar]
- Wang JG (2009) A combined role of calcium channel blockers and angiotensin receptor blockers in stroke prevention. Vasc Health Risk Manag 5:593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Yamada S, LaRiviere WB, Ye H, Bakeberg JL, Irazabal MV, Chebib FT, van Deursen J, Harris PC, Sussman CR, Behfar A, Ward CJ and Torres VE (2017) Generation and phenotypic characterization of Pde1a mutant mice. PLoS One 12:e0181087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber S, Zeller M, Guan K, Wunder F, Wagner M and El-Armouche A (2017) PDE2 at the crossway between cAMP and cGMP signalling in the heart. Cell Signal 38:76–84. [DOI] [PubMed] [Google Scholar]
- Wehbe N, Slika H, Mesmar J, Nasser SA, Pintus G, Baydoun S, Badran A, Kobeissy F, Eid AH and Baydoun E (2020) The Role of Epac in Cancer Progression. Int J Mol Sci 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JB, Thompson WJ and Pittler SJ (2004) Characterization of 3’,5’ cyclic nucleotide phosphodiesterase activity in Y79 retinoblastoma cells: absence of functional PDE6. Mol Vis 10:738–749. [PubMed] [Google Scholar]
- Wong ML, Whelan F, Deloukas P, Whittaker P, Delgado M, Cantor RM, McCann SM and Licinio J (2006) Phosphodiesterase genes are associated with susceptibility to major depression and antidepressant treatment response. Proc Natl Acad Sci U S A 103:15124–15129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Wen W, Qi CL, Zhao RX, Lu JH, Zhong CY and Chen YY (2012) Ameliorative effect of berberine on renal damage in rats with diabetes induced by high-fat diet and streptozotocin. Phytomedicine 19:712–718. [DOI] [PubMed] [Google Scholar]
- Wu SN, Li HF and Chiang HT (2001) Vinpocetine-induced stimulation of calcium-activated potassium currents in rat pituitary GH3 cells. Biochem Pharmacol 61:877–892. [DOI] [PubMed] [Google Scholar]
- Xiang Y and Kobilka BK (2003) Myocyte adrenoceptor signaling pathways. Science 300:1530–1532. [DOI] [PubMed] [Google Scholar]
- Xue C and Johns RA (1995) Endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med 333:1642–1644. [DOI] [PubMed] [Google Scholar]
- Yan C, Zhao AZ, Bentley JK and Beavo JA (1996) The calmodulin-dependent phosphodiesterase gene PDE1C encodes several functionally different splice variants in a tissue-specific manner. J Biol Chem 271:25699–25706. [DOI] [PubMed] [Google Scholar]
- Yan C, Zhao AZ, Bentley JK, Loughney K, Ferguson K and Beavo JA (1995) Molecular cloning and characterization of a calmodulin-dependent phosphodiesterase enriched in olfactory sensory neurons. Proc Natl Acad Sci U S A 92:9677–9681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda H, Nakayama K, Watanabe M, Suzuki S, Fuji H, Okinaga S, Kanda A, Zayasu K, Sasaki T, Asada M, Suzuki T, Yoshida M, Yamanda S, Inoue D, Kaneta T, Kondo T, Takai Y, Sasaki H, Yanagihara K and Yamaya M (2006) Nitroglycerin treatment may enhance chemosensitivity to docetaxel and carboplatin in patients with lung adenocarcinoma. Clin Cancer Res 12:6748–6757. [DOI] [PubMed] [Google Scholar]
- Yu J, Wolda SL, Frazier AL, Florio VA, Martins TJ, Snyder PB, Harris EA, McCaw KN, Farrell CA, Steiner B, Bentley JK, Beavo JA, Ferguson K and Gelinas R (1997) Identification and characterisation of a human calmodulin-stimulated phosphodiesterase PDE1B1. Cell Signal 9:519–529. [DOI] [PubMed] [Google Scholar]
- Zaccolo M, Cesetti T, Di Benedetto G, Mongillo M, Lissandron V, Terrin A and Zamparo I (2005) Imaging the cAMP-dependent signal transduction pathway. Biochem Soc Trans 33:1323–1326. [DOI] [PubMed] [Google Scholar]
- Zaccolo M and Movsesian MA (2007) cAMP and cGMP signaling cross-talk: role of phosphodiesterases and implications for cardiac pathophysiology. Circ Res 100:1569–1578. [DOI] [PubMed] [Google Scholar]
- Zaccolo M and Pozzan T (2002) Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science 295:1711–1715. [DOI] [PubMed] [Google Scholar]
- Zhang H, Pan B, Wu P, Parajuli N, Rekhter MD, Goldberg AL and Wang X (2019) PDE1 inhibition facilitates proteasomal degradation of misfolded proteins and protects against cardiac proteinopathy. Sci Adv 5:eaaw5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Murray F, Zahno A, Kanter JR, Chou D, Suda R, Fenlon M, Rassenti L, Cottam H, Kipps TJ and Insel PA (2008) Cyclic nucleotide phosphodiesterase profiling reveals increased expression of phosphodiesterase 7B in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A 105:19532–19537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Du J, Liu L, Chen X, Yang F and Jin Q (2012) Inhibitory effects and underlying mechanism of 7-hydroxyflavone phosphate ester in HeLa cells. PLoS One 7:e36652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Knight W, Chen S, Mohan A and Yan C (2018a) Multiprotein Complex With TRPC (Transient Receptor Potential-Canonical) Channel, PDE1C (Phosphodiesterase 1C), and A2R (Adenosine A2 Receptor) Plays a Critical Role in Regulating Cardiomyocyte cAMP and Survival. Circulation 138:1988–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YS, Li JD and Yan C (2018b) An update on vinpocetine: New discoveries and clinical implications. Eur J Pharmacol 819:30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B, Vemavarapu L, Thompson WJ and Strada SJ (2005) Suppression of cyclic GMP-specific phosphodiesterase 5 promotes apoptosis and inhibits growth in HT29 cells. J Cell Biochem 94:336–350. [DOI] [PubMed] [Google Scholar]