Abstract
Lipid nanoparticle (LNP)-formulated nucleoside-modified mRNA vaccines have proven to be very successful in the fight against the coronavirus disease 2019 (COVID-19) pandemic. They are effective, safe, and can be produced in large quantities. However, the long-term storage of mRNA-LNP vaccines without freezing is still a challenge. Here, we demonstrate that nucleoside-modified mRNA-LNPs can be lyophilized, and the physicochemical properties of the lyophilized material do not significantly change for 12 weeks after storage at room temperature and for at least 24 weeks after storage at 4°C. Importantly, we show in comparative mouse studies that lyophilized firefly luciferase-encoding mRNA-LNPs maintain their high expression, and no decrease in the immunogenicity of a lyophilized influenza virus hemagglutinin-encoding mRNA-LNP vaccine was observed after 12 weeks of storage at room temperature or for at least 24 weeks after storage at 4°C. Our studies offer a potential solution to overcome the long-term storage-related limitations of nucleoside-modified mRNA-LNP vaccines.
Keywords: mRNA, lipid nanoparticle, nucleoside modification, lyophilization, vaccine
Graphical abstract
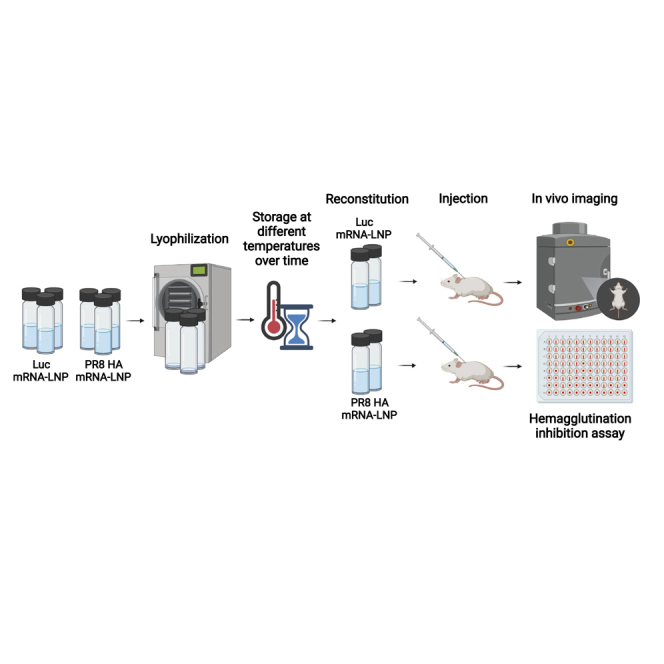
Long-term storage of mRNA-LNP vaccines requires freezing, posing a limitation for this successful, novel vaccine class. Pardi and colleagues demonstrate that lyophilization provides substantial physicochemical stability for mRNA-LNPs. Importantly, the authors observe no decrease in the immunogenicity of a lyophilized influenza mRNA-LNP vaccine after storage at 4°C for 24 weeks.
Introduction
Lipid nanoparticle (LNP)-formulated nucleoside-modified messenger RNA (mRNA) vaccines developed by Moderna and Pfizer-BioNTech demonstrated safety and very high (>90%) efficacy and are at the forefront of the battle against the coronavirus disease 2019 (COVID-19) pandemic.1, 2, 3 Currently, the most critical limitation of this novel vaccine platform is the requirement of a special cold-chain system for long-term storage. While most conventional vaccines can be stored at 2°C–8°C in a refrigerator for at least 6 months, mRNA-LNP vaccines need to be stored frozen, presenting a considerable obstacle to vaccine distribution in countries with poor infrastructure. Lyophilization (freeze-drying) is commonly used in the pharmaceutical industry to increase the stability and shelf life of various products by removing the water from drug formulations.4,5 In a freeze-dried form, mRNA-LNP vaccines could be conveniently shipped worldwide without the need for cooling or freezing.
However, lyophilization of LNPs is less than straightforward. While the process is readily applied to true solutions, LNPs are much more complex; carefully assembled using well-defined processes,6 these nanostructured particles are made from specific types of lipids at certain ratios.7 Physicochemical parameters, such as particle size, polydispersity, and proper payload encapsulation, are critical to biological performance and must be retained during the lyophilization process itself and subsequent storage. Careful selection of lyophilization buffers, cycle process parameters, and temperatures is of the utmost importance to ensure they are preserved.
Recent studies have shown that LNPs containing small interfering (si)RNA or mRNA can be successfully lyophilized.8, 9, 10 Tekmira Pharmaceuticals developed an LNP for treatment of Zaire Ebola virus (ZEBOV) infection containing siRNA targeting VP24, VP35, and L polymerase proteins.11,12 After demonstrating complete protection of non-human primates (NHPs) in an otherwise lethal model of ZEBOV, a reformulated, lyophilized version (TKM-100802) was assessed in a phase 1 clinical trial in 2014 (NCT02041715).13 While Tekmira reported equivalent efficacy with the wet and lyophilized formats of their siRNA-LNP, not all studies have had the same conclusion. Ball et al. found that siRNA-LNPs can be lyophilized, but they show significantly lower efficacy (gene silencing in cell culture) after reconstitution with water.8 Two recent studies demonstrated that the mRNA-LNP platform can also be lyophilized.9,10 Zhao et al. generated lyophilized firefly luciferase-encoding mRNA-LNPs and demonstrated that the reconstituted material maintains the mRNA expression efficiency in mice as observed with in vivo bioluminescence imaging studies.9 Hong et al. developed a lyophilized severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA-LNP vaccine formulation and showed that the reconstituted vaccine can induce strong immune responses in mice.10 Importantly, none of these publications provides information on the stability of lyophilized mRNA-LNP formulations over time.
Here, we describe a very efficient lyophilization procedure that can be used to produce nucleoside-modified mRNA-LNPs as freeze-dried cake. Lyophilized mRNA-LNPs were generated and stored at −80°C, −20°C, 4°C, 25°C (room temperature), and 42°C for 4, 12, or 24 weeks. We demonstrate that the physicochemical properties of mRNA-LNPs do not significantly change after storage at room temperature for 12 weeks, or at 4°C for at least 24 weeks, and then reconstitution with water. Using the same storage conditions, we show that firefly luciferase-encoding mRNA-LNPs do not lose their high translatability as measured by in vivo bioluminescence imaging studies in mice. Most importantly, we demonstrate in comparative mouse immunization studies that a nucleoside-modified mRNA-LNP influenza virus vaccine does not lose potency after 12 weeks of storage at room temperature or for at least 24 weeks at 4°C as a lyophilized product. We believe that this report represents a major advance in the field of mRNA-LNP vaccine development, as it offers a potential solution for the suboptimal long-term storage temperature requirements of these potent new-generation vaccines.
Results
Production of lyophilized mRNA-LNP formulations
LNPs comprising the ionizable lipid (6Z,16Z)-12-((Z)-dec-4-en-1-yl)docosa-6,16-dien-11-yl 5-(dimethylamino)pentanoate, 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), cholesterol, and PEG2000-C-DMA at a molar ratio of 50:10:38.5:1.5 were formulated using a modified version of our proprietary T-mixer manufacturing process [US 9,005,654]. This involves mixing lipids dissolved in ethanol with a low-pH, aqueous solution of nucleic acid in a T-shaped mixing chamber. LNPs form spontaneously as the ethanol concentration drops below the level required to support lipid solubility. Particles are then rapidly stabilized by further dilution with an aqueous buffer in the collection reservoir. The process is robust, highly scalable, and has been used to encapsulate a variety of nucleic acids, including siRNA and mRNA.6,12,14 Here, we separately encapsulated two different mRNAs encoding either firefly luciferase (Luc) or hemagglutinin from the A/Puerto Rico/8/1934 influenza virus strain (PR8 HA) in LNPs, subsequently exchanging the carrier buffer to 5 mM Tris pH 8, containing 10% sucrose and 10% maltose (w/v). LNP formulations were then freeze-dried using a VirTis Genesis Pilot Lyophilizer. The lyophilization cycle consisted of a freezing step at −45°C, a primary drying step at −25°C and 20 mTorr, and a secondary drying step at 30°C and 20 mTorr. At the completion of the cycle, samples were brought to atmospheric pressure, backfilled with ultrapure nitrogen gas, stoppered, and then transferred to various storage temperatures (−80°C, −20°C, 4°C, 25°C, 42°C) for stability monitoring. All samples were noted to have a dense, white, freeze-dried cake structure. Aliquots from the same batch of mRNA-LNP were stored frozen at −80°C and served as a benchmark control in this evaluation.
Physicochemical characterization of lyophilized mRNA-LNPs
At set time points (0, 4, 12, and 24 weeks after production), lyophilized mRNA-LNPs were removed from storage. The lyophilized vials were inspected for cake appearance, which can be indicative of physicochemical changes that may impact product quality and biological performance.15 All lyophilized vials contained a uniform and elegant cake (Figure S1), showing no signs of cake collapse, shrinkage, or cracked texture. This cake was quickly reconstituted by the addition of nuclease-free water to a target concentration of 0.5 mg/mL total mRNA. After the addition of water, vials were gently inverted several times and quickly acquired a clear, opalescent appearance with no visible solids (Figure S2).
We previously assessed the stability of this LNP composition in a frozen format. These studies found no change in key quality attributes when stored for 1 year at −80°C (Table S1). To generate a similar dataset with this set of lyophilized products, we first analyzed the properties of the wet Luc and PR8 HA mRNA-LNPs before and after freeze-thaw and post-reconstitution of the lyophilized samples at release, marking the week 0 time point (Table S2). Hereafter, the frozen and reconstituted samples in the time course study were analyzed using stability-indicating assays for total RNA content, mRNA purity, percentage of RNA encapsulation, lipid identity, lipid content, mean particle size, and polydispersity.
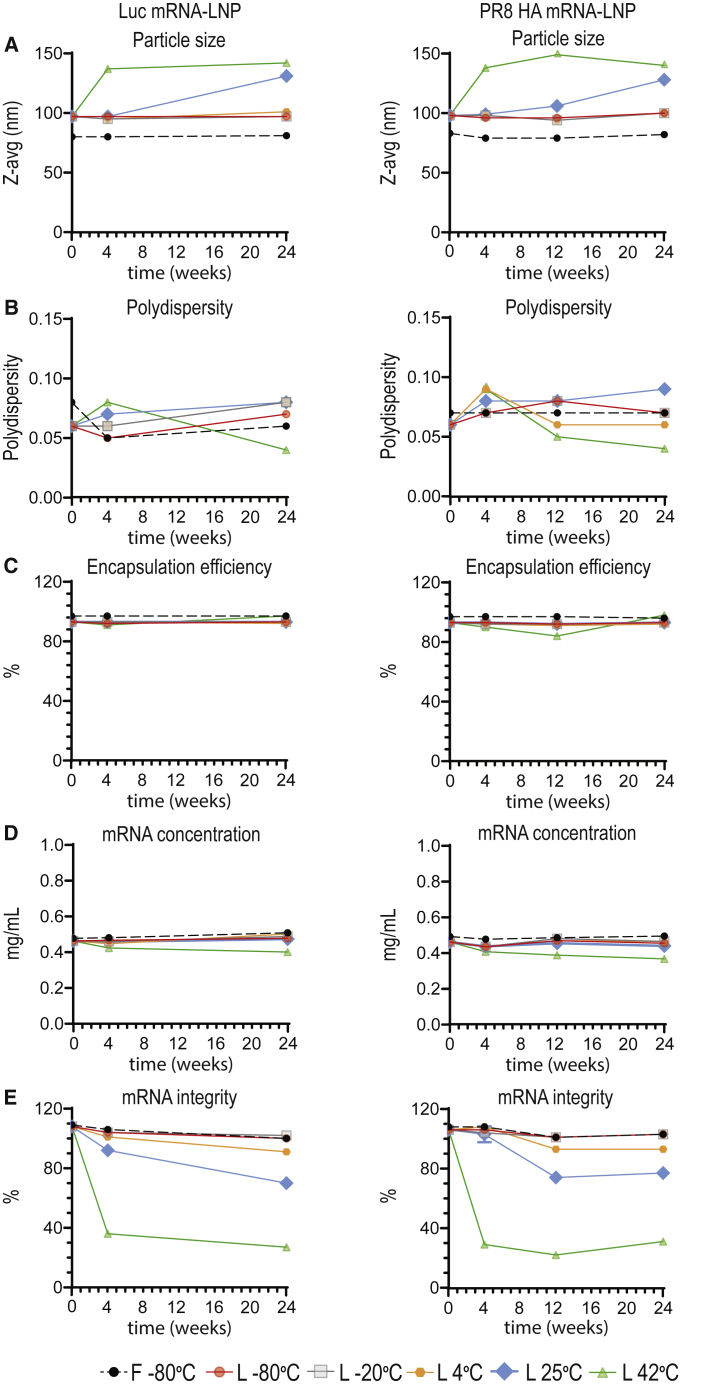
We utilized dynamic light scattering (DLS) to characterize particle size and polydispersity (size distribution). Frozen LNPs stored at −80°C showed no particle size change over time (Figure 1A). Similarly, lyophilized LNPs stored at 4°C and below also maintained particle-size integrity for at least 24 weeks after production. In contrast, an increase in z-average diameter was measured in lyophilized samples stored at elevated temperatures. Interestingly, despite exhibiting size growth, these samples maintained a narrow size distribution, where the polydispersity index was <0.10 (Figure 1B). We also noted that particle-size increase reached a plateau following 4-week storage of lyophilized LNPs at 42°C.
Figure 1.
Physicochemical characterization of frozen and lyophilized mRNA-LNPs
Analytical testing post-thawing of frozen mRNA-LNPs stored at −80°C and post-reconstitution of lyophilized mRNA-LNPs stored at −80°C, −20°C, 4°C, 25°C, or 42°C for 0, 4, or 24 (Luc) or 0, 4, 12, or 24 (PR8 HA) weeks. (A–E) Particle size (A) and polydispersity (B) were measured by DLS, mRNA encapsulation (C) and mRNA concentration (D) were determined by RiboGreen assay, and mRNA integrity (E) was assessed by capillary electrophoresis. For (A) and (B), each data point represents one sample, and each data point is an aggregate of at least 10 readings on the particle sizer, resulting in the mean z-average and polydispersity values displayed on the graphs. Analyses in (C)–(E) were done in triplicate. Error bars are SEM. See also Figures S1–S5 and Tables S1–S3.
Encapsulation efficiency was measured by the RiboGreen assay, which relies on a dye that fluoresces upon binding to single-stranded mRNA. Dye accessibility is low with intact LNPs, so only unencapsulated mRNA is detected. To determine the total mRNA concentration, entrapped mRNA is released by addition of a detergent (Triton X-100) to lyse the LNPs. The ratio of fluorescence intensity before and after addition of Triton allows for the calculation of the proportion of encapsulated mRNA payload, typically > 90% in stable formulations. There was no significant change in encapsulation efficiency of mRNA-LNPs stored under most conditions, including lyophilized mRNA-LNPs stored for 24 weeks at room temperature (Figure 1C). Only at 42°C storage, the lyophilized PR8 HA mRNA-LNP product showed a steady decline in encapsulation efficiency after the first 12 weeks, followed by an increase between 12 and 24 weeks. While there is no binding interference of RiboGreen with unencapsulated single nucleotides, the increased encapsulation observed at 24 weeks may be due to binding of the dye to degraded segments of the mRNA payload. This hypothesis correlates well with the measured total mRNA concentration. A steady decline in total mRNA content over time, including the 24-week time point, was reported for the lyophilized product stored at 42°C. For all other storage formats and conditions, no significant change in total mRNA concentration was reported over this time period (Figure 1D).
The integrity of nucleoside-modified mRNA-LNPs was assessed by capillary electrophoresis. No notable changes in mRNA integrity were observed for the −80°C frozen product, as well as the lyophilized product stored at either −20°C or −80°C, for at least 24 weeks (Figures 1E and S3). Above subzero temperatures, mRNA chemical degradation was observed in a temperature-dependent manner. For lyophilized mRNA-LNPs stored at 4°C for 24 weeks, there was an approximate 10%–15% decrease in RNA integrity. Meanwhile, for samples stored at 25°C, approximately 30% reduction in mRNA integrity was reported. It is important to note that no further mRNA degradation was observed for the PR8 HA mRNA-LNPs between 12 and 24 weeks for both 4°C and 25°C storage conditions. The greatest loss of mRNA integrity (approximately 70%) was reported for the lyophilized samples stored at 42°C.
All four lipid components were analyzed by ultra high-performance liquid chromatography (UHPLC) and are stable for at least 24 weeks, regardless of the LNP format and storage temperature (Figures S4 and S5). Importantly, the lipid composition maintained the target molar ratio of 1.5:50:38.5:10 (polyethylene glycol [PEG] lipid: ionizable lipid: cholesterol: distearoylphosphatidylcholine [DSPC]).
To further demonstrate the benefits of lyophilization, we conducted a comparative study of non-lyophilized and lyophilized samples stored at different temperatures for 4 weeks. Since the datasets for Luc and PR8 HA mRNA-LNPs were very comparable in the main stability arm, we conducted this direct comparison with Luc mRNA-LNPs only. In this short-term stability study, non-lyophilized LNPs were stored as a wet formulation at −80°C, −20°C, 4°C, 25°C, and 42°C for 4 weeks. In comparison with the lyophilized LNPs, more changes in particle characteristics were reported for the non-lyophilized counterpart by 4 weeks. Particle size of non-lyophilized samples increased at all storage temperatures, which was not observed with lyophilized samples stored below 42°C. At 42°C, lower mRNA integrity was reported for the non-lyophilized sample than for the lyophilized sample. Lower RNA encapsulation was also reported for the non-lyophilized sample stored at −20°C. Overall, these results demonstrate that lyophilized samples provide improved stability over non-lyophilized samples (Table S3).
Additionally, an in-use stability study was performed at room temperature with the Luc mRNA-LNPs. Both frozen and lyophilized samples were removed from −80°C storage, thawed, and reconstituted (if applicable) and then held at 25°C for up to 24 h. All attributes were comparable to those of the samples at time 0 (Table 1). These results support in-use stability of both −80°C frozen and lyophilized/reconstituted samples for at least 24 h at 25°C, which exceeds the current in-use stability instructions of approved mRNA-based COVID-19 vaccines.16
Table 1.
Luc mRNA-LNP in-use stability at room temperature
| Times (h) | −80°C frozen Luc mRNA-LNPs |
Lyophilized and reconstituted Luc mRNA-LNPs |
||||||
|---|---|---|---|---|---|---|---|---|
| Z-avg (nm) | PDI | [mRNA] mg/mL | % EE | z-avg (nm) | PDI | [mRNA] mg/mL | % EE | |
| 0 | 81 ± 0.6 | 0.07 ± 0.00 | 0.53 ± 0.00 | 97 ± 0.2 | 99 ± 0.6 | 0.07 ± 0.02 | 0.50 ± 0.00 | 95 ± 2.0 |
| 1 | 81 ± 0.9 | 0.06 ± 0.02 | 0.51 ± 0.00 | 97 ± 0.1 | 98 ± 0.3 | 0.07 ± 0.00 | 0.49 ± 0.00 | 93 ± 0.2 |
| 2 | 80 ± 0.0 | 0.09 ± 0.01 | 0.51 ± 0.01 | 97 ± 0.1 | 98 ± 0.3 | 0.05 ± 0.01 | 0.49 ± 0.00 | 93 ± 0.2 |
| 4 | 79 ± 0.3 | 0.09 ± 0.01 | 0.51 ± 0.01 | 97 ± 0.4 | 96 ± 0.6 | 0.08 ± 0.01 | 0.48 ± 0.01 | 93 ± 0.3 |
| 6 | 79 ± 0.7 | 0.08 ± 0.02 | 0.53 ± 0.00 | 97 ± 0.2 | 96 ± 0.7 | 0.07 ± 0.01 | 0.49 ± 0.01 | 92 ± 0.0 |
| 24 | 81 ± 0.3 | 0.08 ± 0.01 | 0.51 ± 0.00 | 97 ± 0.1 | 98 ± 0.3 | 0.09 ± 0.01 | 0.50 ± 0.01 | 92 ± 0.3 |
Particle size, polydispersity, mRNA concentration, and encapsulation efficiency of frozen and reconstituted lyophilized Luc mRNA-LNPs were measured at room temperature (25°C) at set time points for 24 h. PDI, polydispersity; EE, encapsulation efficiency.
In vivo activity of frozen and reconstituted lyophilized Luc mRNA-LNPs
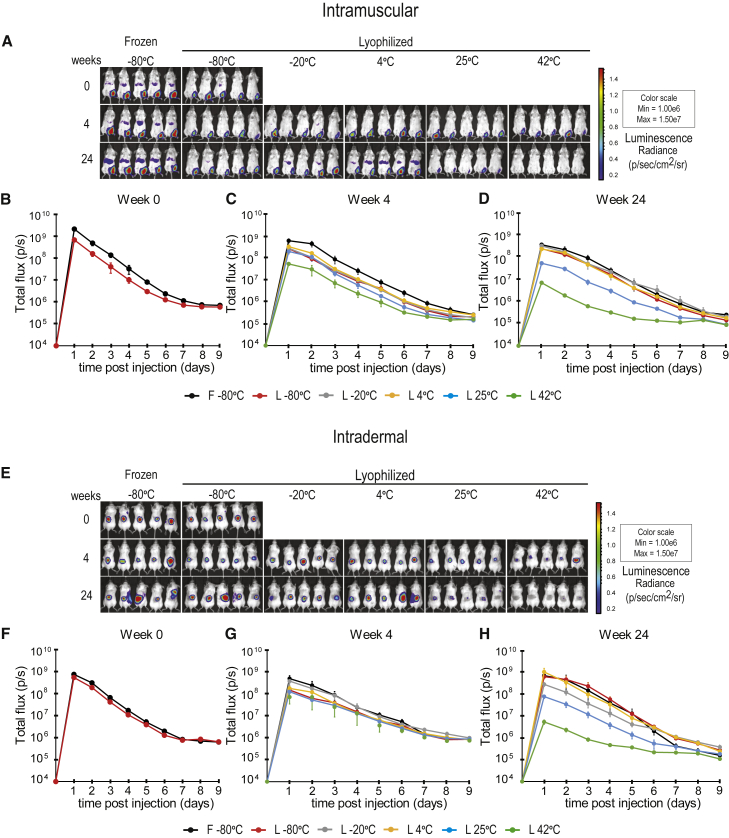
The translatability of Luc mRNA-LNPs was evaluated in mice by in vivo imaging studies. As most vaccines are given intramuscularly (IM) or intradermally (ID), mRNA-LNPs were tested after IM and ID injections (Figures 2, S6;, and S7). Animals were injected IM with Luc mRNA-LNPs, and protein production from the frozen and reconstituted lyophilized products stored for 0, 4, and 24 weeks was examined (Figures 2A and S6). Lyophilization results in some decrease in activity of Luc mRNA-LNPs compared with the frozen formulations (Figures 2A, 2B, and S6A). Lyophilized mRNA-LNPs stored at room temperature (or lower temperatures) displayed a high level of protein production at week 4 (Figures 2A, 2C, and S6B). Storing mRNA-LNPs at 42°C results in a substantial drop in activity compared with storage in other conditions by week 4 (Figures 2A, 2C, and S6B). Impressively, lyophilized mRNA-LNPs stored at 4°C remain stable for at least 24 weeks (Figures 2A, 2D, and S6C). A decrease in protein production from Luc mRNA-LNPs was found after storage at room temperature for 24 weeks (Figures 2A, 2D, and S6C). Storing mRNA-LNPs at 42°C for 24 weeks results in a substantial further drop in activity compared with week 4 (Figures 2A, 2D, S6B, and S6C). We obtained very similar results after evaluating protein production from ID-administered Luc mRNA-LNPs (Figures 2E–2H and S7). In summary, lyophilized Luc mRNA-LNPs remained stable at room temperature for at least 4 weeks and at 4°C for at least 24 weeks.
Figure 2.
In vivo imaging studies with Luc mRNA-LNPs
Frozen Luc mRNA-LNPs were stored at −80°C throughout the study, and lyophilized Luc mRNA-LNPs were stored at −80°C, −20°C, 4°C, 25°C, or 42°C for 0, 4, or 24 weeks prior to reconstitution. (A–H) Mice were (A–D) IM- or (E–H) ID-injected with 3 μg Luc mRNA-LNPs (frozen or reconstituted lyophilized products), and bioluminescence was monitored for 9 days. (A) Representative IVIS images taken 2 days post-IM Luc mRNA-LNP injection. (B–D) Quantification of the bioluminescent signal obtained after IM injection of frozen and reconstituted lyophilized Luc mRNA-LNPs after (B) 0, (C) 4- or (D) 24-week storage. (E) Representative IVIS images taken 2 days post-ID Luc mRNA-LNP injection. (F–H) Quantification of the bioluminescent signal obtained after the ID injection of frozen and reconstituted lyophilized Luc mRNA-LNPs after (F) 0, (G) 4-, or (H) 24-week storage. n = 5 mice per group. Error bars are SEM. See also Figures S6 and S7.
Immunogenicity of frozen and reconstituted lyophilized PR8 HA mRNA-LNP influenza vaccines
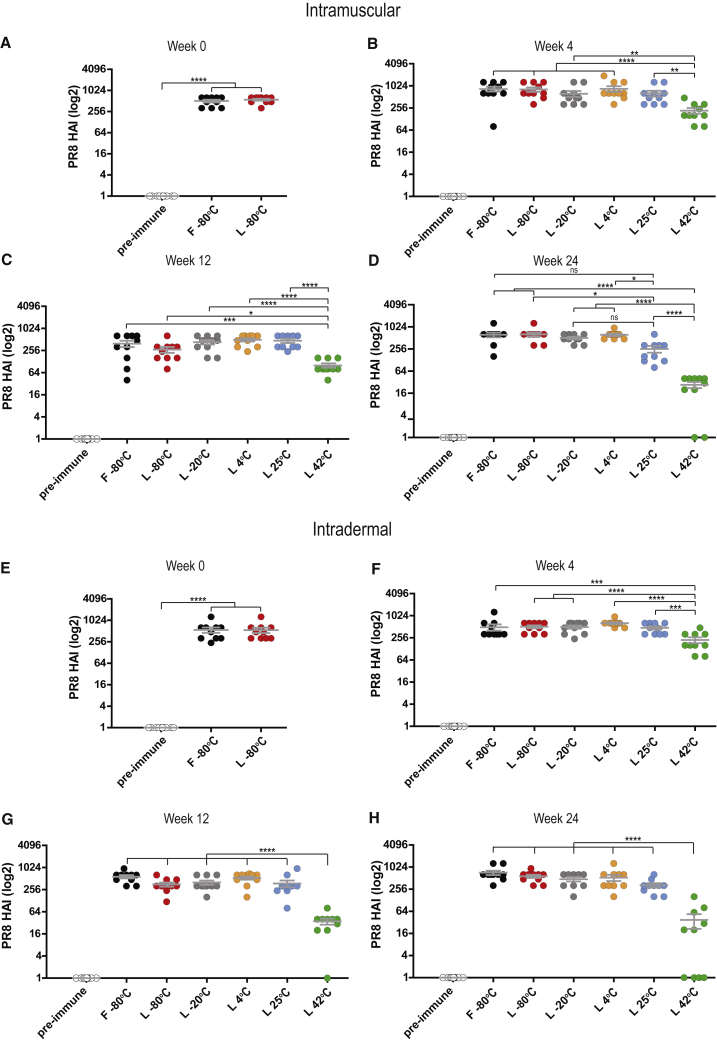
To investigate the performance of the reconstituted lyophilized PR8 HA mRNA-LNP vaccines stored at various temperatures, mice were immunized IM or ID with a single dose of vaccine, and HA inhibition (HAI) titers in sera of these vaccinated mice were determined 4 weeks later (Figure 3). HAI titers are a simple readout and clearly reflect the quality and magnitude of immune responses induced by PR8 HA mRNA-LNP vaccines. As immunogenicity is a critical parameter, an additional time point (week 12) was added to the PR8 HA study protocol to provide more detailed information. As in previous experiments, frozen mRNA-LNPs served as a benchmark control in this evaluation. When administered IM, no significant difference between the activity of frozen and freshly reconstituted lyophilized formulations was found (Figure 3A). Despite some decrease in mRNA integrity (Figure 1D), the immunogenicity of the lyophilized vaccines did not decrease after 12 weeks of storage at room temperature (Figures 3B and 3C). Impressively, no drop in PR8 HAI activity and only a modest decrease in immunogenicity were found after 24 weeks of storage at 4°C and at room temperature, respectively (Figure 3D). In accordance with previous measurements, storing PR8 HA mRNA-LNPs at 42°C resulted in a significant drop in activity by week 4 (Figure 3B), a substantial further decrease by week 12 (Figure 3C), and very low to no activity by week 24 (Figure 3D). Similar results were obtained after ID immunizations with PR8 HA mRNA-LNP vaccines (Figures 3E–3H). In summary, lyophilized PR8 mRNA-LNP vaccines did not lose activity after storage at room temperature for 12 weeks and lost some activity by 24 weeks. Storing the lyophilized vaccines at 4°C for at least 24 weeks did not result in any loss of immunogenicity.
Figure 3.
Immunogenicity of PR8 HA mRNA-LNP vaccines
Frozen PR8 HA mRNA-LNPs were stored at −80°C throughout the study, and lyophilized PR8 HA mRNA-LNPs were stored at −80°C, −20°C, 4°C, 25°C, or 42°C for 0, 4, 12, or 24 weeks prior to reconstitution. (A–H) mice were (A–D) IM- or (E–H) ID-injected with 10 μg PR8 HA mRNA-LNPs (frozen or reconstituted lyophilized products), serum was collected 4 weeks post-immunization, and PR8 HAI titers were determined. n = 10 mice per group. Error bars are SEM. Each symbol represents one animal. HAI titers below the limit of detection are shown equal to 1 on the graph. Statistical analysis: one-way ANOVA with Bonferroni's multiple comparisons test on log-transformed data was performed; ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, ∗∗∗∗p ≤ 0.0001.
Discussion
The emergence of SARS-CoV-2 has motivated a global effort to develop a protective vaccine. mRNA is an attractive vaccine modality, owing to its flexibility in antigen design and the speed of development and production. However, effective mRNA vaccines require both efficient mRNA delivery to cells and a high level of antigen expression to induce robust immune responses coupled with durable protective immunity. Specialized delivery technologies such as LNPs are required to realize their full potential. Despite real-world evidence demonstrating the advantages of this promising novel platform, the instability of mRNA-LNP vaccines and the need for frozen storage remain major limitations. Here, we investigated lyophilization to achieve long-term pharmaceutical stability in these formulations.
Lyophilization, or freeze-drying, is one of the most common methods used for long-term preservation of drug products, including colloidal nanoparticle suspensions.4,5 Physical instability can be characterized as aggregation or fusion of the nanoparticles, manifesting as an increase in particle size or polydispersity. Chemical instability is most often observed as degradation of the mRNA payload and/or lipid components. Either form of instability would pose challenges for storage in an aqueous buffer as a wet formulation. Extrinsic parameters, such as storage-buffer pH and temperature, may further impact stability. As a result, lyophilization buffers, cycle times, and temperature are important parameters for preserving the physicochemical parameters of LNPs.
Nucleoside-modified mRNA-LNPs were produced as a freeze-dried cake through lyophilization. For a quick assessment of the success of this process, macroscopic analysis was performed by visually inspecting the lyophilized product for cake appearance. Observations such as cake collapse, shrinkage, or cracked texture may indicate potential changes in LNP characteristics. The lyophilized vials in this study presented a uniform and elegant cake that was rapidly restored to its original state after resuspension in nuclease-free water. The reconstituted samples acquired a clear, opalescent appearance with no visible solids.
Particle size is an important stability parameter and can influence pharmacokinetics, distribution, safety, and efficacy. For frozen and lyophilized LNPs stored at 4°C (and lower temperatures), particle size stability was reported for at least 24 weeks. Room-temperature storage of the lyophilized vials provided at least 4 to 12 weeks of particle size stability. Under the most accelerated storage condition of 42oC, the lyophilized product exhibited a large increase in particle size between 0 and 4 weeks, but remained stable for at least 24 weeks. Under these conditions, the maximum particle diameter was less than 150 nm. Importantly, this particle size is still within the range where LNPs have been reported to elicit robust immune responses in animals, including NHPs.17
As mRNA is an active drug substance, contributing to the immunogenicity and efficacy of the successful mRNA-LNP vaccines, it is important to monitor its encapsulation efficiency, integrity, and content. With intact and stable LNPs, the encapsulation efficiency of an mRNA payload is typically >90%. Here, we found no change in this parameter under most storage conditions, including lyophilized mRNA-LNPs stored for 24 weeks at room temperature. Moreover, no change was reported in total mRNA concentration in lyophilized LNPs stored at room temperature and below during the course of this study. While currently there are no published criteria on the acceptable limits of RNA integrity and its threshold in respect to vaccine efficacy, it is critical to prevent mRNA degradation to ensure biological performance. RNA integrity represents the most temperature-sensitive, stability-limiting parameter. Remarkably, no notable changes in Luc and PR8 HA mRNA integrity were observed for LNP products stored at subzero temperatures for at least 24 weeks. mRNA chemical degradation was observed in a temperature-dependent pattern, resulting in an approximately 10%–15% decrease in RNA integrity for lyophilized mRNA-LNPs stored for 24 weeks at 4°C and an approximately 30% decrease in RNA integrity for lyophilized mRNA-LNPs stored for 24 weeks at room temperature. Impressively, the 4°C lyophilized samples maintained the same in vivo potency as the frozen samples. Meanwhile, only slight decreases in immunogenicity were observed with lyophilized PR8 HA mRNA-LNP samples stored at room temperature for 24 weeks. It is important to note that no further mRNA degradation was observed for the PR8 HA mRNA-LNP samples between 12 and 24 weeks for both 4°C and room temperature storage conditions. The greatest loss of mRNA integrity (approximately 70%) was reported for the lyophilized samples stored at 42°C; however, this degree of degradation did not completely abrogate in vivo potency.
As each lipid component in the LNP has specific functions during particle formation, stabilization, and biological performance, it is critical to maintain the stability of lipid components to ensure a pharmacologically active drug product. The amine group of the ionizable lipid is positively charged at acidic pH, promoting encapsulation of the negatively charged mRNA payload during particle formation. Following cellular uptake of the LNP, it further drives endosomal fusion and cytoplasmic release of payload. The PEG-conjugated lipid controls particle size during formation and prevents particle aggregation by sterically stabilizing the LNP. DSPC and cholesterol are often referred to as structural lipids with concentrations chosen to optimize particle size, encapsulation, and stability. In aggregate, the LNP serves to protect the delicate RNA molecule from serum nucleases during transition to target cells and promotes uptake and delivery. All four lipids maintained their integrity under all storage conditions tested in this study. These trends are impressive, as some ionizable lipids and DSPC are susceptible to temperature and pH-dependent hydrolysis, which was not observed here.18 All frozen and lyophilized LNPs maintained the target molar ratio of 1.5:50:38.5:10 (PEG lipid:ionizable lipid:cholesterol:DSPC).
Overall, these results are very encouraging, as other groups that evaluated the stability of lipid-based nanoparticles encapsulating Luc mRNA reported a significantly lower bioluminescence signal in vivo after storing the lyophilized product for 1 week at −80°C.9 Although the presence of a cryoprotectant stabilized particle size, they speculated that the nanostructure of these mRNA formulations was altered during the lyophilization and reconstitution process, thereby affecting their delivery efficiency in vivo. In our studies, we were able to maintain key physicochemical attributes of our lyophilized mRNA-LNP product and demonstrate high in vivo translation.
A preliminary shelf life of at least 24 weeks at 4°C offers increased flexibility over the current options. Both authorized COVID-19 mRNA vaccines require frozen storage in the presence of sucrose.18 SpikeVax is stable for up to 6 months at −15°C to −20°C, whereas Comirnaty is stable at −60°C to −80°C for up to 6 months or −15°C to −25°C for 2 weeks.19 Moreover, in-use stability assessment of our mRNA-LNPs reported no change in physicochemical characteristics at room temperature for at least 24 h. This provides a significant advantage, as it maximizes the use of drug product in a single vial and enables efficient administration over this 24-h period. Currently, SpikeVax is reported to have up to 12 h of 25°C stability, whereas Comirnaty has up to 2 h stability at 25°C or 6 h stability after dilution with 0.9% saline for injection.16
The urgency of the COVID-19 pandemic demanded the rapid identification and development of a protective vaccine. Although Moderna and Pfizer/BioNTech quickly developed very effective nucleoside-modified mRNA-LNP vaccines,1, 2, 3 some critical aspects of vaccine stability have yet to be addressed. In the few reports published on lyophilized mRNA-LNPs, there has been no discussion of the key quality attributes of these products after long-term storage and the biological impact of long-term storage. We believe that this report represents an important advancement in the field of mRNA-LNP vaccine research, as our dataset provides a better understanding of the physicochemical characteristics and in vivo activity (translatability and immunogenicity) of this new-generation platform. The lyophilization approach represents a compelling opportunity for improving thermostability of mRNA-LNP vaccines and will be critical in facilitating rapid global distribution of these vaccines in the future.
Materials and methods
Ethics statement
The investigators faithfully adhered to the “Guide for the Care and Use of Laboratory Animals” by the Committee on Care of Laboratory Animal Resources Commission on Life Sciences, National Research Council. Mouse studies were conducted under protocols approved by the Institutional Animal Care and Use Committees (IACUC) of the University of Pennsylvania. All animals were housed and cared for according to local, state, and federal policies in an Association for Assessment and Accreditation of Laboratory Animal Care International- (AAALAC)-accredited facility.
Production of mRNA-LNPs
mRNAs were produced from linearized plasmids encoding codon-optimized firefly Luc or HA of A/Puerto Rico/8/1934 influenza virus as described.20 Briefly, mRNAs were transcribed to contain 101-nt-long poly(A) tails. m1Ψ-5′-triphosphate (TriLink) instead of uridine 5'-triphosphate (UTP) was used to generate modified nucleoside-containing mRNA. Capping of the in-vitro-transcribed mRNAs was performed co-transcriptionally using the trinucleotide cap1 analog CleanCap (TriLink). mRNA was purified by cellulose purification, as described.21 All mRNAs were analyzed by agarose gel electrophoresis and were stored frozen at −20°C.
Cellulose-purified m1Ψ-containing mRNA was encapsulated in LNPs using a controlled mixing process (US 9,005,654) in which an aqueous solution of mRNA at pH 5 was combined with an ethanolic solution of lipids, containing the ionizable lipid (6Z,16Z)-12-((Z)-dec-4-en-1-yl)docosa-6,16-dien-11-yl 5-(dimethylamino)pentanoate, DSPC, cholesterol, and PEG2000-C-DMA22 at a molar ratio of 50:10:38.5:1.5. Ethanol was removed by tangential flow ultrafiltration, followed by buffer exchange and concentration. The formulation was adjusted to 0.5 mg/mL, and sterile was filtered through a 0.2 μm polyethersulfone membrane. Aliquots were subsequently stored frozen at −80°C (0.25 mL fill) or lyophilized (0.5 mL fill) in 5-mM Tris buffer, pH 8, with 10% sucrose and 10% maltose.
Lyophilization process
Lyophilization was performed in a glass chamber of a Pilot Freeze Dryer (SP Scientific VirTis Genesis 35L Pilot Lyophilizer). Samples were frozen at −45°C for 3 h, this being followed by a primary dry cycle at −25°C/20 mTorr for 84 h. During the secondary dry cycle, samples were warmed to 30°C/20 mTorr and held for 5 h. Vials were backfilled with nitrogen, capped, and transferred to various storage temperatures (−80°C, −20°C, 4°C, 25°C, 42°C) for stability assessments.
LNP characterization
Frozen and lyophilized vials were removed from storage at set time points (e.g., weeks 0, 4, 12, and 24) and equilibrated to room temperature. Lyophilized samples were reconstituted by quick addition of 500 μL nuclease-free water and gently mixed.
LNPs were diluted to 0.8–1.6 ng/μL total mRNA in phosphate buffered saline (PBS), pH 7.4, and transferred into a polystyrene cuvette to measure particle size and polydispersity by DLS (Malvern Nano ZS Zetasizer), using a refractive index (RI) of 1.590 and an absorption of 0.010 in PBS at 25°C and a viscosity of 0.9073 centipoise (cP) and RI of 1.332. Measurements were made with 10-s run durations with the number of runs automatically determined. Each measurement had a fixed position of 4.65 mm in the cuvette with an automatic attenuation selection. Diameters are reported as z-average.
RNA encapsulation efficiency and concentration were determined by the Quant-iT RiboGreen Assay (Life Technologies). Quantification of RNA in LNP formulation was conducted using a standard curve generated from a dilution series of the corresponding RNA stock (either Luc or PR8 mRNA). Both standards and samples were diluted with 1x Tris-EDTA (TE) buffer, pH 8.0. Samples were targeted to reach 0.1 ng/μL in the final sample in the polystyrene cuvette. Fluorescence was measured using a spectrofluorophotometer (Varian Cary Eclipse) set at 500-nm excitation and 525-nm emission. The standard curve was calculated by linear regression analysis of the fluorescence intensity plotted against the concentration of the standard. RNA encapsulation of LNP samples was determined by comparing the signal of the RNA-binding fluorescent dye RiboGreen in the absence and presence of a detergent (0.1% Triton X-100). In the absence of a detergent, the signal comes only from accessible (unencapsulated) RNA. In the presence of a detergent, the LNP is disrupted so that the measured signal comes from the total RNA (both encapsulated and non-encapsulated). The encapsulation percentage is calculated using the following equation: Encapsulation efficiency (%) = ([Fluorescence]total – [Fluorescence]unencapsulated)/(Fluorescence)total × 100%.
RNA integrity was measured by capillary electrophoresis on the Agilent 5200 Fragment Analyzer, using the Agilent HS RNA Kit (DNF-472-1000). At each time point, LNP samples were treated with Triton X-100 to disrupt the particles, diluted to 0.0025 mg/mL, mixed with the marker diluent, and then heat denatured at 70°C for 2 min. The unformulated RNA payloads were treated in exactly the same manner. The Fragment Analyzer injected the sample at 7 kV for 150 s, with separation at 8 kV for 45 min. Data from each run were analyzed using PROSize 3.0 software (Agilent Technologies). RNA integrity of the formulated mRNA-LNPs is represented as the percentage relative to the unformulated mRNA standard assayed within the same run.
Lipid content was determined by UHPLC using the Thermo-Scientific Vanquish UHPLC system with a CAD detector. The UHPLC method uses an Ace Ultracore superC18 column (100 × 2.1 mm: 2.5 μm) with 20-mM ammonium acetate in water (Solvent A) and 1:1 IPA:MeOH (Solvent B). The step gradient was 0–0.3 min 70% Solvent B; 0.3–1.8 min 90% Solvent B; 1.8–4.3 min 100% Solvent B; 4.3–4.8 min 100% Solvent B; 4.8–4.9 min 70% Solvent B; and 4.9–8 min 70% Solvent B. LNP samples were diluted to 1 mg/mL total lipid with ethanol (which also served to dissociate the LNP) and quantified against a 5-point calibration curve for each of the components of interest.
Administration of mRNA-LNPs to mice
Eight-week-old female BALB/c mice (Charles River Laboratories) were utilized for this study. Lyophilized mRNA-LNPs were reconstituted by the addition of nuclease-free water to a target concentration of 0.5 mg/mL total mRNA. Reconstituted mRNA-LNPs were filtered by using a 13-mm 0.2 μm syringe filter (Pall Acrodisc). mRNA-LNPs were diluted with sterile PBS (Corning) and administered via the IM or ID routes using a 3/10cc 29½G insulin syringe (Covidien) and 40- or 30-μL injection volumes, respectively.
Blood collection
Mice were isoflurane-anesthetized, and blood was collected through the retro-orbital route. Serum was separated from blood by centrifugation at 10,000 × g for 5 min. Separated serum was stored at −20°C until used.
Bioluminescence imaging studies
Bioluminescence imaging was performed with an In Vivo Imaging System (IVIS) Spectrum imaging system (Caliper Life Sciences). Mice were administered D-luciferin (Regis Technologies) at a dose of 150 mg/kg intraperitoneally. Mice were anesthetized after receiving D-luciferin in a chamber with 3% isoflurane (Piramal Healthcare Limited) and placed on the imaging platform while being maintained on 2% isoflurane via a nose cone. Mice were imaged at 5-min post-administration of D-luciferin using an exposure time of 5–60 s to ensure that the signal acquired was within effective detection range (above noise levels and below charge-coupled device [CCD] saturation limit). Bioluminescence values were quantified by measuring photon flux (photons/second) in the region of interest from where a bioluminescence signal emanated using the Living IMAGE Software provided by Caliper.
HAI assay
Sera were heat-inactivated (55°C) for 30 min, spun down at 11,000 RPM for 2 min, and diluted 1:20 in PBS, then serially diluted 1:2 in 50 μL in 96-well U-bottom plates (lowest concentration: 1:2,560) using a multichannel pipette. Then, four hemagglutinating doses of PR8 HA virus were added in the same volume as diluted sera. Finally, 12.5 μL of turkey erythrocyte solution (Lampire)—washed twice in PBS and diluted to a final concentration of 2% (v/v)—was added and gently mixed with the sera-virus solution (final volume of 125 μL). Samples were incubated for 45 min at room temperature, after which the plates were turned on their side for one minute, then scanned on a regular office scanner. HAI titers were determined as the highest dilution of the sample that inhibited four agglutinating doses of the influenza virus. Inhibition of agglutination was observed as the blood forming a “tear-drop” shape.
Statistical analysis
Data were collected and expressed as average ±standard error of the mean (SEM). Statistical analysis was conducted using the GraphPad Prism v.9.0.0 (GraphPad Software) software package.
Acknowledgments
N.P. was supported by the National Institute of Allergy and Infectious Diseases (R01AI146101 and R01AI153064) and the Katalin Karikó Fellowship Fund in Vaccine Development (University of Pennsylvania). Cs.B. was a post-doctoral fellow supported by the Biotechnological National Laboratory, Szeged, Hungary. The graphical abstract was created with BioRender.
Author contributions
N.P., K.L., and J.H. conceptualized the study. N.P, K.L., and J.H. wrote the paper with help from co-authors. K.L. and Cs.B. graphed data and produced figures. H.M. produced all mRNAs and performed all mouse studies. P.S. encapsulated mRNAs into LNPs and generated lyophilized mRNA-LNPs. P.S. and A.M. performed the physicochemical characterization of all mRNA-LNP formulations. P.L. provided input on the lyophilization process. K.K. designed the untranslated region of the mRNA. Cs.B. and D.L. performed HAI assays.
Declaration of interests
N.P. is named on a patent describing the use of nucleoside-modified mRNA in LNPs as a vaccine platform. He has disclosed those interests fully to the University of Pennsylvania, and he has in place an approved plan for managing any potential conflicts arising from licensing of that patent. K.K. is an employee of BioNTech.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2022.02.001.
Supplemental information
References
- 1.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Perez Marc G., Moreira E.D., Zerbini C., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hogan M.J., Pardi N. mRNA vaccines in the COVID-19 pandemic and beyond. Annu. Rev. Med. 2022;73:17–39. doi: 10.1146/annurev-med-042420-112725. [DOI] [PubMed] [Google Scholar]
- 4.Chen C., Han D., Cai C., Tang X. An overview of liposome lyophilization and its future potential. J. Control. Release. 2010;142:299–311. doi: 10.1016/j.jconrel.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 5.Kasper J.C., Winter G., Friess W. Recent advances and further challenges in lyophilization. Eur. J. Pharm. Biopharm. 2013;85:162–169. doi: 10.1016/j.ejpb.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 6.Jeffs L.B., Palmer L.R., Ambegia E.G., Giesbrecht C., Ewanick S., MacLachlan I. A scalable, extrusion-free method for efficient liposomal encapsulation of plasmid DNA. Pharm. Res. 2005;22:362–372. doi: 10.1007/s11095-004-1873-z. [DOI] [PubMed] [Google Scholar]
- 7.Semple S.C., Akinc A., Chen J., Sandhu A.P., Mui B.L., Cho C.K., Sah D.W., Stebbing D., Crosley E.J., Yaworski E., et al. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 2010;28:172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 8.Ball R.L., Bajaj P., Whitehead K.A. Achieving long-term stability of lipid nanoparticles: examining the effect of pH, temperature, and lyophilization. Int. J. Nanomed. 2017;12:305–315. doi: 10.2147/IJN.S123062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao P., Hou X., Yan J., Du S., Xue Y., Li W., Xiang G., Dong Y. Long-term storage of lipid-like nanoparticles for mRNA delivery. Bioact. Mater. 2020;5:358–363. doi: 10.1016/j.bioactmat.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong C.H., Kim K.S., Park S.A., Chun M.J., Hong E.Y., Chung S.W., et al. An mRNA vaccine against SARS-CoV-2: lyophilized, liposome-based vaccine candidate EG-COVID induces high levels of virus neutralizing antibodies. Preprint at bioRxiv. 2021 doi: 10.1101/2021.03.22.436375. [DOI] [Google Scholar]
- 11.Geisbert T.W., Hensley L.E., Kagan E., Yu E.Y.Z., Geisbert J.B., Daddario-DiCaprio K., Fritz E.A., Jahrling P.B., McClintock K., Phelps J.R., et al. Postexposure protection of Guinea pigs against a lethal Ebola virus challenge is conferred by RNA interference. J. Infect. Dis. 2006;193:1650–1657. doi: 10.1086/504267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geisbert T.W., Lee A.C., Robbins M., Geisbert J.B., Honko A.N., Sood V., Johnson J.C., de Jong S., Tavakoli I., Judge A., et al. Postexposure protection of non-human primates against a lethal Ebola virus challenge with RNA interference: a proof-of-concept study. Lancet. 2010;375:1896–1905. doi: 10.1016/S0140-6736(10)60357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacLachlan I. Proceedings of the ASGCT 17th Annual Meeting. 2014. Progress in the development of lipid nanoparticle-RNA therapeutics. [Google Scholar]
- 14.Buschmann M.D., Carrasco M.J., Alishetty S., Paige M., Alameh M.G., Weissman D. Nanomaterial delivery systems for mRNA vaccines. Vaccines (Basel) 2021;9:65. doi: 10.3390/vaccines9010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel S.M., Nail S.L., Pikal M.J., Geidobler R., Winter G., Hawe A., Davagnino J., Rambhatla Gupta S. Lyophilized drug product cake appearance: what is acceptable? J. Pharm. Sci. 2017;106:1706–1721. doi: 10.1016/j.xphs.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Crommelin D.J.A., Anchordoquy T.J., Volkin D.B., Jiskoot W., Mastrobattista E. Addressing the cold reality of mRNA vaccine stability. J. Pharm. Sci-US. 2021;110:997–1001. doi: 10.1016/j.xphs.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hassett K.J., Higgins J., Woods A., Levy B., Xia Y., Hsiao C.J., Acosta E., Almarsson O., Moore M.J., Brito L.A. Impact of lipid nanoparticle size on mRNA vaccine immunogenicity. J. Control. Release. 2021;335:237–246. doi: 10.1016/j.jconrel.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 18.Schoenmaker L., Witzigmann D., Kulkarni J.A., Verbeke R., Kersten G., Jiskoot W., Crommelin D.J.A. mRNA-lipid nanoparticle COVID-19 vaccines: structure and stability. Int. J. Pharm. 2021;601:120586. doi: 10.1016/j.ijpharm.2021.120586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou X., Zaks T., Langer R., Dong Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021;6:1078–1094. doi: 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freyn A.W., Ramos da Silva J., Rosado V.C., Bliss C.M., Pine M., Mui B.L., Tam Y.K., Madden T.D., de Souza Ferreira L.C., Weissman D., et al. A multi-targeting, nucleoside-modified mRNA influenza virus vaccine provides broad protection in mice. Mol. Ther. 2020;28:1569–1584. doi: 10.1016/j.ymthe.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baiersdorfer M., Boros G., Muramatsu H., Mahiny A., Vlatkovic I., Sahin U., Kariko K. A facile method for the removal of dsRNA contaminant from in vitro-transcribed mRNA. Mol. Ther. Nucleic Acids. 2019;15:26–35. doi: 10.1016/j.omtn.2019.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heyes J., Hall K., Tailor V., Lenz R., MacLachlan I. Synthesis and characterization of novel poly(ethylene glycol)-lipid conjugates suitable for use in drug delivery. J. Control. Release. 2006;112:280–290. doi: 10.1016/j.jconrel.2006.02.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.