Introduction
The global burden of CKD is increasing and it is estimated up to one in ten of the adult population has CKD, totaling around 850 million people worldwide (1). The major causes of CKD leading to ESKD are diabetes mellitus (DM), glomerular diseases, and hypertension (2). The epidemic of DM contributes to the major burden of CKD because, in 2010, 285 million (6%) of the adult population worldwide had DM and this is projected to increase to 8% in 2030 (2).
When patients with CKD reach ESKD, they will require RRT. Currently, the most common modalities are hemodialysis (HD) and peritoneal dialysis (PD). Continuous ambulatory PD (CAPD) started in 1976 and has gradually gained worldwide recognition as an established form of high-quality, cost-effective RRT for patients with kidney failure (3). Despite this, the global utilization of PD has remained <15%. Currently, PD can be divided into CAPD and automated PD (APD). Both CAPD and APD are home therapies for patients on dialysis, with the ratio of APD to CAPD varying in different countries. In 2019, President Trump signed the Executive Order on Advancing American Kidney Health (AAKH) (4). One of the three goals of the initiative is ensuring that 80% of new patients with kidney failure receive either dialysis at home or a kidney transplant by 2025. Lessons learned from other countries where utilization of PD is higher is therefore relevant to the United States nephrology community.
PD-First Policy in Hong Kong
A “PD-First Policy” was first coined and introduced in Hong Kong (HK) in 1985 (5). This program was started by the Central Renal Committee of the Medical and Health Department and later by the Hospital Authority of HK Government in light of rising demand from the escalating number of patients with kidney failure requiring dialysis (6).
The policy stated that all patients with kidney failure would be treated with PD first unless they had a medical contraindication (5). Although absolute medical contraindications to PD do occur (Table 1), in our own clinical practice, very few patients cannot be put on PD as first-line therapy. At the start of the PD-First Policy in HK, all patients put on PD were on CAPD. APD was gradually introduced into the program, with both CAPD and APD providing high-quality dialysis for HK patients (7). In 2018, there were 6097 patients on dialysis in HK with 75% on PD and 25% on HD (Figure 1). The mild decrease in the PD population from 82% in 2009 to 75% in 2018 was due to a planned increase in HD provision by the Hospital Authority to support the proactive switch of patients on PD to HD before their PD finally failed. HK still practices a “CAPD-First Policy” but the number of patients put on APD has been increasing, with APD accounting for 19% of the total PD population in HK in 2018.
Table 1.
Possible medical contraindications for peritoneal dialysis
| Possible Contraindication |
| Abdominal problems |
| Extensive abdominal adhesions |
| Multiple abdominal surgeries |
| Colostomy |
| Uncorrected hernias |
| Inflammatory or ischemic bowel disease |
| Extensive diverticulitis |
| Massive polycystic kidneys |
| Morbid obesity |
| Severe pulmonary disease |
| Severe lumbar disc problem |
| History of severe noncompliance |
Figure 1.
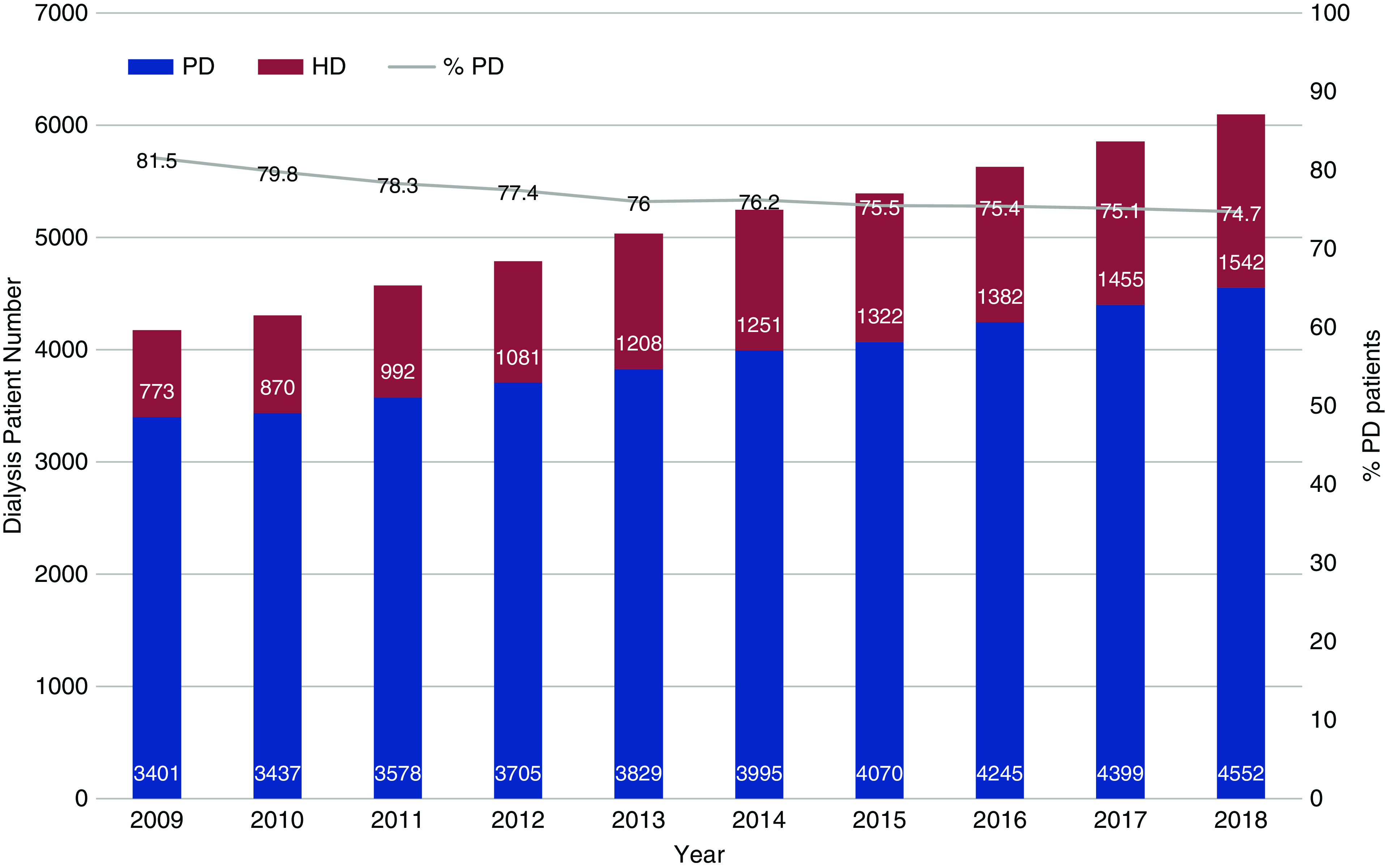
The total number of prevalent patients on peritoneal dialysis (PD) and hemodialysis (HD) from 2009 to 2018 in Hong Kong. PD accounts for over 75% of the dialysis population.
Why PD First?
PD First can be assessed based on clinical outcomes and cost issues. There have been multiple cohort studies and registry data suggesting that PD results in comparable survival compared with HD in both patients with and without diabetes, and PD may even result in a lower mortality with PD in the early years after initiation of dialysis (5,8). The United States Renal Data System’s 2018 data showed that the all-cause mortality, as reflected by deaths per 1000 patient years and adjusted for age, sex, race, ethnicity, primary diagnosis, and vintage, was higher for HD compared with PD starting in 2006 and persisting through 2016 (9). PD has always been found to have a more protective effect in preserving residual renal function compared with HD (5).
Infection such as peritonitis may be an area of concern for not putting patients on PD. However, with the promulgation of guidelines for preventing and treating peritonitis by the International Society for PD (ISPD), and the implementation and adherence to these guidelines by PD centers globally, the overall peritonitis rate around the world can achieve the target rate of <0.5 episodes per year (10). Many high-performing centers can actually achieve rates significantly <0.5 episodes per year. Infectious complications in incident HD and PD patients are similar, with patients on PD being more likely to have peritonitis and patients on HD being more likely to have septicemia and pneumonia (5). An additional benefit of PD as home therapy in the current era of coronavirus disease 2019 (COVID-19) is to eliminate the exposure of patients on PD to those patients on HD who are potentially infected with severe acute respiratory syndrome coronavirus 2 at HD centers.
The economic benefits of using PD over HD have been consistently noted. A recent lifetime cost-effectiveness analysis in HK under the PD-First Policy showed that the hospital-based HD group had a higher annual cost of US$142,389 but lower effectiveness of 6.58 quality-adjusted life years compared with the PD group that had a lower annual cost of US$76,915 and higher effectiveness of 7.13 quality-adjusted life years (11). In most countries, the actual costs of PD are lower than those of in-center HD, and are commonly in the range of 10%–30% less expensive. Reasons for these cost differences are lower overheads, decreased staff/patient ratio, and the nature of home-based dialysis therapy itself (5). However, in some developing countries, the cost of PD may be higher than HD, mainly because of the high imported costs of PD fluids.
How To Make PD First Successful
A successful PD-First program is dependent on several factors as described below and summarized in Table 2.
Table 2.
Factors to facilitate a successful PD-First Policy
| Factors for Success |
| Patient selection |
| Appropriate choice of patients with physical and visual ability to perform PD |
| Family, social, and medical support for PD and assisted PD as required |
| Training and education |
| Patient and family should be educated about PD as a dialysis option |
| High-quality PD training program for patient and caregiver |
| Undergraduate and postgraduate education and training programs for nephrologists and renal nurses on PD management and the benefits of PD for patients with kidney failure |
| Center effects |
| Infrastructure for building and maintaining a PD unit |
| Reasonable patient number and center size for accumulation of PD experience and expertise |
| PD catheter insertion strategies and training programs for committed nephrologists and surgeons |
| Continuous quality improvement program to achieve good technique and patient outcomes on PD |
| Reimbursement policy |
| No financial disincentives for patients on PD |
| Financial incentives for hospitals, PD units, and nephrologists for putting patients on PD |
| Lower cost for PD fluids |
| Government policy |
| Implementation of PD-first or home therapy–first policy |
| Setting targets for achieving percentage of dialysis population on PD/home therapy |
PD, peritoneal dialysis.
Patient Selection
In HK, there are very few medical conditions that pose as an absolute contraindication to PD (Table 1) (12). We have patients with nonmajor abdominal operations and moderate-sized polycystic kidneys on PD with success. A smaller body size may allow for a lower dialytic dose. For CAPD in HK, we can achieve good dialysis adequacy by routinely starting patients with three 2-L exchanges a day, taking advantage of the patients’ residual renal function. The preservation of residual renal function in PD can be further improved with angiotensin-converting enzyme inhibitors (13). The lower dialytic dose and thus the number of PD exchanges have the benefits of savings in cost and time and can lead to better compliance. There is also a theoretically reduced risk of dialysis-related complications such as hyperglycemia, obesity, and peritonitis. The gradual incremental dialysis dose is adjusted based on the clinical condition of patients. The use of incremental PD is supported by a recent ISPD guideline in which less than the standard “full-dose” PD is prescribed to people initiating PD so that the combination of residual renal and peritoneal clearance achieved is sufficient to achieve individualized clearance goals (14).
Training and Education
Nephrologists and PD nurses with more training and experience with PD are more likely to effectively manage peritoneal access creation, volume status, infectious complications, and cardiovascular disease (5). Greater PD exposure and training throughout the medical education continuum can achieve better outcomes and are emphasized in our program. Tenckhoff catheter insertion can be performed by nephrologists, using techniques such as the open dissection technique, either with the use of a laparoscope or peritoneoscope, inserted percutaneously after blind (or with a modified Seldinger technique) puncture of the abdomen or with fluoroscopy assistance. The survival rates of PD catheters inserted by nephrologists, in many reported studies, have been excellent (5,12). Timely and effective catheter insertion avoids unduly long waiting times and helps provide dialysis to patients who need semi-urgent PD initiation. This also helps to shorten the waiting time, during which potential PD candidates may lose interest in this dialysis modality. PD nurse education is crucial and there are excellent PD training curricula published by ISPD for patients and caregivers.
Center Effects
Many studies have shown that center size with >20 patients on PD, or centers with >500 cumulative patients on PD have significantly improved patient and technique survivals (5,12). This is because of the gathering of medical expertise in PD, dedicated PD staff, well designed patient training programs, and integrated backup facilities for PD care (5,12). PD centers should have a multidisciplinary predialysis clinic. Early referral of patients to such centers will allow for predialysis education for patients, their relatives, and caretakers. This also allows for timely insertion of Tenckhoff catheters and avoidance of acute HD and HD catheter insertion.
Reimbursement Policy
The reimbursement policy of the health system will definitely help to improve the outcome of PD. The provision of financial incentives, or at least a reduction in disincentives, to patients and healthcare providers facilitate the implementation of a PD-First Policy as shown in HK and many parts of the world. An assisted PD program with a paid nurse or caregiver who goes from home to home to assist in placing patients on PD and then disconnecting them has been shown to be effective in supporting functionally limited patients on PD and yields acceptable clinical outcomes as shown in countries like Canada, Denmark, and France. The program is less expensive than transfer to HD or long-term care, thereby minimizing costs for patients with failing self-care PD.
PD-First Policy or PD-Favored Policy in Other Countries and Regions
Following HK, Thailand started a PD-First Policy to provide more comprehensive dialysis services for their citizens in 2008 (15). Canada, China, Guatemala, India, Mexico, Spain, Taiwan, and the United States have PD-favored policies; Australia, Finland, and New Zealand have home dialysis–first policies (15). Interestingly, the success of a given country’s PD-favored policy was inversely associated with the extent of HD infrastructure (15).
Implications for the Executive Order on AAKH
In the United States, the costs of treatment for kidney failure are covered by the national Medicare health insurance program no matter the age of the patient. Approximately 100,000 patients begin dialysis each year, with the majority (87%) starting with in-center HD (9). The treatment modality of prevalent patients with kidney failure is 63% HD (with 98% of these patients on in-center HD), 7% PD, and 30% with functioning kidney transplants. Many more patients with kidney failure would be eligible for home dialysis and, when studied, 25%–40% of patients would choose home dialysis if given the option (16).
Committing the US Government to create a system that “pays for kidney health, rather than kidney sickness,” US President Trump signed the AAKH Executive Order on July 10, 2019 (4). The nation’s first kidney health strategy aims to reduce the number of Americans with kidney failure by 25% over the next decade; to double the number of kidneys available for transplant; and to provide a greater emphasis on transplantation, home dialysis, and new therapies, such as the artificial kidney. Expanding the number of patients on home dialysis is now a priority in the United States, with the initial goal of having 80% of incident patients with ESKD treated with either home dialysis or kidney transplantation by 2025.
The emphasis on home dialysis and the development of innovative alternative therapies, including the artificial kidney, seeks to provide patients with access to more patient-centered treatment options and allows for improved education around these options. AAKH will pilot new payment and care options that engage nephrologists earlier in the disease course and that incentivize the movement to greater use of home dialysis.
The final mandatory and voluntary models that will provide the framework for accomplishing the goals of the executive order have not been finalized. These models will affect the success of increasing the number of patients treated with home dialysis therapy. The current COVID-2019 pandemic has had significant short-term consequences on advancing these policy changes and on the tolerance for nephrologists and their health systems to take on any financial risk that the proposed models would involve. The many lessons learned from the disruptions caused by COVID-19 have involved the importance of being able to deliver care by telehealth and the potential benefits of therapies that can keep patients at home (17). These lessons will undoubtedly have a lasting effect that could increase the willingness of patients to be on home PD and enhance the skill of nephrologists to manage patients at remote locations.
Policy change can affect modality choice in the United States. For example, in 2011 the ESRD Prospective Payment System was implemented that bundled payment for dialysis services into a single payment and provided financial incentives for providing care for patients on PD. The ESRD Prospective Payment System resulted in a modest increase in PD as a dialysis modality, including HD to PD switches (18).
As nephrologists, other kidney health professionals, and dialysis facilities work to achieve the bold goals outlined in AAKH, lessons can be learned from HK and other countries where a greater emphasis on home therapy and PD have been in place. As outlined above, these lessons include liberalization of patient selection for PD and home HD and, in the future, the use of machine learning and artificial intelligence to help guide clinical decision making and modality choice. Training and education of kidney health professionals must evolve to develop greater expertise and confidence in prioritizing home therapy, with a greater emphasis on home dialysis during nephrology training and for practicing nephrologists. A component of this training could include the insertion of PD catheters by a larger number of nephrologists. Involving nephrologists earlier in the care of people with kidney diseases will also facilitate a more educated choice of dialysis modality selection. Infrastructure for PD and home therapy needs to be expanded, either in specialized home dialysis centers or more broadly in a greater number of dialysis facilities. Developments in home monitoring and telemedicine will also facilitate this transition. Reimbursement policy, as proposed in the AAKH mandatory, voluntary care, and payment models, will also be an important driver of change (19).
A large variation in dialysis treatment modality for patients with kidney failure exists across the world. Countries and regions such as HK have successfully implemented a PD-first approach to dialysis. Lessons learned from HK and other countries and regions can help inform change in the United States as kidney health professionals and dialysis facilities prioritize home dialysis to achieve the bold goals outlined in AAKH.
Disclosures
P. Li is a former president of the ISPD and the Asian Pacific Society of Nephrology (APSN). The views expressed are his own and do not represent those of ISPD or APSN. He reports honorarium from Fibrogen. M. Rosenberg is a former president of the American Society of Nephrology (ASN). The views expressed are his own and do not represent those of ASN. He reports honorarium support from Wolters Kluwer.
Funding
None.
Acknowledgments
The authors thank Central Renal Committee, Renal Registry of Hospital Authority of HK and Dr. C. B. Leung for providing the data for Figure 1.
Author Contributions
P. Li and M. Rosenberg conceptualized the study and wrote the original draft; and P. Li was responsible for data curation.
Footnotes
References
- 1.Li PKT, Garcia-Garcia G, Lui SF, Andreoli S, Fung WWS, Hradsky A, Kumaraswami L, Liakopoulos V, Rakhimova Z, Saadi G, Strani L, Ulasi I, Kalantar-Zadeh K; World Kidney Day Steering Committee : Kidney health for everyone everywhere - from prevention to detection and equitable Access to care. Kidney Int 97: 226–232, 2020 [DOI] [PubMed] [Google Scholar]
- 2.Li PKT, Ma TK: Global impact of nephropathies. Nephrology (Carlton) 22[Suppl 4]: 9–13, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Li PKT, Chow KM, Van de Luijtgaarden MW, Johnson DW, Jager KJ, Mehrotra R, Naicker S, Pecoits-Filho R, Yu XQ, Lameire N: Changes in the worldwide epidemiology of peritoneal dialysis. Nat Rev Nephrol 13: 90–103, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Office of the Assistant Secretary for Planning and Evaluation: Advancing American Kidney Health, Washington, DC, US Department of Health and Human Services, 2019. Available at: https://aspe.hhs.gov/pdf-report/advancing-american-kidney-health. Accessed July 30, 2019 [Google Scholar]
- 5.Li PKT, Chow KM: Peritoneal dialysis-first policy made successful: Perspectives and actions. Am J Kidney Dis 62: 993–1005, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Leung CB, Cheung WL, Li PKT: Renal registry in Hong Kong-the first 20 years. Kidney Int Suppl (2011) 5: 33–38, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li PKT, Chung KY, Chow KM: Continuous ambulatory peritoneal dialysis is better than automated peritoneal dialysis as first-line treatment in renal replacement therapy. Perit Dial Int 27[Suppl 2]: S153–S157, 2007 [PubMed] [Google Scholar]
- 8.Couchoud C, Bolignano D, Nistor I, Jager KJ, Heaf J, Heimburger O, Van Biesen W; European Renal Best Practice (ERBP) Diabetes Guideline Development Group : Dialysis modality choice in diabetic patients with end-stage kidney disease: A systematic review of the available evidence. Nephrol Dial Transplant 30: 310–320, 2015 [DOI] [PubMed] [Google Scholar]
- 9.United States Renal Data System: 2018 USRDS annual data report: Epidemiology of kidney disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2018. Available at: https://www.usrds.org/2018/view/Default.aspx. Accessed March 9, 2020 [Google Scholar]
- 10.Li PKT, Szeto CC, Piraino B, de Arteaga J, Fan S, Figueiredo AE, Fish DN, Goffin E, Kim YL, Salzer W, Struijk DG, Teitelbaum I, Johnson DW: ISPD peritonitis recommendations: 2016 update on prevention and treatment [published correction appears in Perit Dial Int 38: 313, 2018]. Perit Dial Int 36: 481–508, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong CKH, Chen J, Fung SKS, Mok M, Cheng YL, Kong I, Lo WK, Lui SL, Chan TM, Lam CLK: Lifetime cost-effectiveness analysis of first-line dialysis modalities for patients with end-stage renal disease under peritoneal dialysis first policy. BMC Nephrol 21: 42, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li PKT, Chow KM: Peritoneal dialysis patient selection: Characteristics for success. Adv Chronic Kidney Dis 16: 160–168, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Li PKT, Chow KM, Wong TY, Leung CB, Szeto CC: Effects of an angiotensin-converting enzyme inhibitor on residual renal function in patients receiving peritoneal dialysis. A randomized, controlled study. Ann Intern Med 139: 105–112, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Blake PG, Dong J, Davies SJ: Incremental peritoneal dialysis. Perit Dial Int 40: 320–326, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Liu FX, Gao X, Inglese G, Chuengsaman P, Pecoits-Filho R, Yu A: A global overview of the impact of peritoneal dialysis first or favored policies: An opinion. Perit Dial Int 35: 406–420, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maaroufi A, Fafin C, Mougel S, Favre G, Seitz-Polski B, Jeribi A, Vido S, Dewisme C, Albano L, Esnault V, Moranne O: Patients’ preferences regarding choice of end-stage renal disease treatment options. Am J Nephrol 37: 359–369, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Agarwal R: The aftermath of coronavirus disease of 2019: devastation or a new dawn for nephrology? [published online ahead of print April 17, 2020] Nephrol Dial Transplant doi: 10.1093/ndt/gfaa094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sloan CE, Coffman CJ, Sanders LL, Maciejewski ML, Lee SD, Hirth RA, Wang V: Trends in peritoneal dialysis use in the United States after Medicare payment reform. Clin J Am Soc Nephrol 14: 1763–1772, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flanagin EP, Chivate Y, Weiner DE: Home dialysis in the United States: A roadmap for increasing peritoneal dialysis utilization. Am J Kidney Dis 75: 413–416, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]


