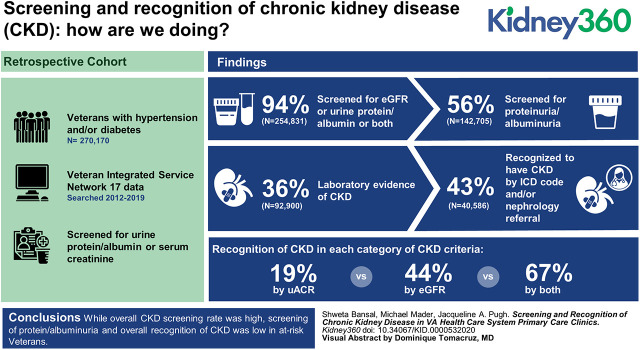
Visual Abstract
Keywords: chronic kidney disease, awareness, diuretics, glomerular filtration rate, hypertension, International Classification of Diseases, kidney function tests, renal insufficiency, chronic, retrospective studies, screening, urinalysis, proteinuria, albuminuria
Abstract
Background
The successful implementation of interventions targeted to improve kidney health requires early identification of CKD which involves screening at-risk populations as well as recognizing CKD. We aim to determine CKD screening and recognition rates, factors associated with these rates, and evaluate the effect of CKD awareness on delivery of care.
Methods
A retrospective cohort study of veterans enrolled with Veterans Integrated Service Network 17 who had hypertension (HTN) and/or diabetes (DM) and were seen at least twice in primary care clinics within 18 months. The final cohort of 270,170 patients (52% HTN, 5% DM, and 44% both) was examined for serum creatinine/eGFR, urine protein/albumin, International Classification of Diseases (ICD) codes for CKD, and nephrology referral. CKD was defined as eGFR <60 ml/min per 1.73 m2 and/or urine albumin-creatinine ratio (uACR) >30 mg/g at least twice 90 days apart. Clinical covariates, HTN control, and prescription rates of renal prudent medications and nonsteroidal anti-inflammatory drugs (NSAIDs) were assessed.
Results
Overall, 254,831 (94%) patients had either eGFR, urine protein/albumin, or both. However, screening for protein/albuminuria was low (56%), particularly in patients with isolated HTN (35%). Of 254,831 patients, 92,900 (36%) had laboratory evidence of CKD and, of these, 40,586 (44%) were recognized to have CKD by ICD code and/or nephrology referral. CKD due to presence of uACR criteria alone had the lowest recognition (19%) as compared with CKD due to eGFR criteria (44%) or both (67%). Frequency of emergency room visits, hospitalization, and cardiac and endovascular procedures requiring contrast had the highest odds and races other than white had the lower odds of screening. In contrast, CKD recognition was high in races other than white and increased with worsening eGFR and increasing uACR. In screened and recognized CKD, prescription was higher for angiotensin inhibitors, statins, and diuretics, and was lower for NSAIDs.
Conclusions
Although overall CKD screening rate was high, screening of protein/albuminuria in isolated HTN and overall recognition of CKD was low in at-risk veterans. Increased recognition was associated with a favorable prescription rate for renal prudent medications.
Introduction
CKD is a significant public health problem that affects approximately 14% of the adult United States population and results in considerable morbidities and health care costs (1). The recent Presidential Executive Orders reflect this issue and emphasize prevention of CKD progression rather than end stage treatments (2). A series of interventions have been suggested, among which are optimization of blood pressure (BP) control, angiotensin converting enzyme inhibitor/angiotensin receptor blocker (ACEI/ARB) in persons with diabetes (DM) and proteinuria, intensive management of cardiovascular disease (CVD) risk, and avoidance of nephrotoxins, to improve CKD outcomes; however, these interventions are successful when implemented early in CKD (3,4). Identification of early stage CKD involves screening high-risk populations as well as recognizing CKD. CKD screening has been recommended by Kidney Disease Improving Global Outcomes (KDIGO), Department of Veterans Affairs (VA), and Department of Defense (DOD) clinical practice guidelines in high-risk populations including patients with DM, hypertension (HTN), CVD, systemic illnesses such as HIV or lupus, history of AKI, and racial/ethnic groups with increased risk, e.g., those who are black, Hispanic, and Native American (5). In early CKD, the above mentioned interventions significantly reduce risk of ESKD, stroke, and all-cause mortality (3,4).
The Veterans Health Administration (VHA) is uniquely situated to address these objectives with a number of effective approaches already in use for prevention and chronic-illness care such as a robust electronic medical record system, clinical practice guidelines, performance measures (such as screening for albuminuria in persons with DM), and primary care population management. Of all patients with CKD, 95% are in the early stages and are cared for in primary care clinics (5). Data has been emerging on screening rates in primary care; however, it is limited to either populations with DM or HTN, or includes serum creatinine or albuminuria alone (6). In addition to screening, recognition of CKD by health care providers is also essential for optimal delivery of interventions to improve outcomes in CKD. Recently, cross-sectional observational studies have highlighted the low prevalence of recognition of CKD in patients with laboratory evidence of CKD (by eGFR) in primary care clinics (7,8). Similar to screening, previous studies have not taken albuminuria into account as a marker for CKD or were restricted to a diabetic population while evaluating recognition (9–11).
In brief, guidelines have recommended early identification of CKD in high-risk patients which involves both screening and recognition of CKD by providers for optimal delivery of care; however, it is largely unknown what the actual practice is in large health care organizations such as the VA health care system. We analyzed Veteran Integrated Service Network 17 (VISN 17) data to evaluate the CKD screening and recognition rate in at-risk veterans (those with HTN, DM, or both) enrolled in VA health care system primary care clinics. We also evaluated factors associated with screening and recognition, and whether screening and recognition of CKD have any effect on HTN control; prescription of ACEI/ARB, diuretics, and statins; and avoidance of nephrotoxins. The data from this analysis will inform the barriers to delivery of optimal CKD care and illuminate whether recognition changes behavior and thus may help develop actionable interventions.
Materials and Methods
In a retrospective cohort design, we queried the VHA Corporate Data Warehouse (CDW) to develop the study population, covariates, and patient outcomes. We examined CKD screening and recognition rates along with factors associated with them. The study was approved as exempt by the institutional review board.
Study Population
The initial sample consisted of 752,613 patients who visited one of the outpatient clinics in VHA VISN 17 at least twice, 90 days apart, and within 18 months between October 1, 2012 and September 30, 2019. Our goal was to derive a study cohort who is seen regularly in primary care clinics. Figure 1 outlines the data extraction steps. Because the presence of DM and HTN are the two major risk factors for CKD and have unambiguous diagnostic codes in patient charts, we used these two conditions to define the at-risk population. Patients were counted if the diagnosis of HTN or DM by International Classification of Diseases, Ninth Revision (ICD-9) or ICD-10 codes (Supplemental Table 1) appeared on the Problem List as of 2018 (i.e., at least 1 year before the end of the study period), or if the diagnosis was assigned to at least two visits that occurred within VISN 17 during October 1, 2012 to September 30, 2019 or in the three previous years (October 1, 2009 to September 30, 2012). The additional 3 years were included because it was presumed that a large percentage of the target population would have been diagnosed before the study period. Each qualifying member was assigned a cohort entry date, the later of (1) the earliest primary care visit within VISN 17, or (2) the earliest date of diagnosis with either HTN or DM. If both of those dates were before September 30, 2012, then the qualifying member was considered an “existing patient” and assigned a cohort entry date of October 1, 2012.
Figure 1.
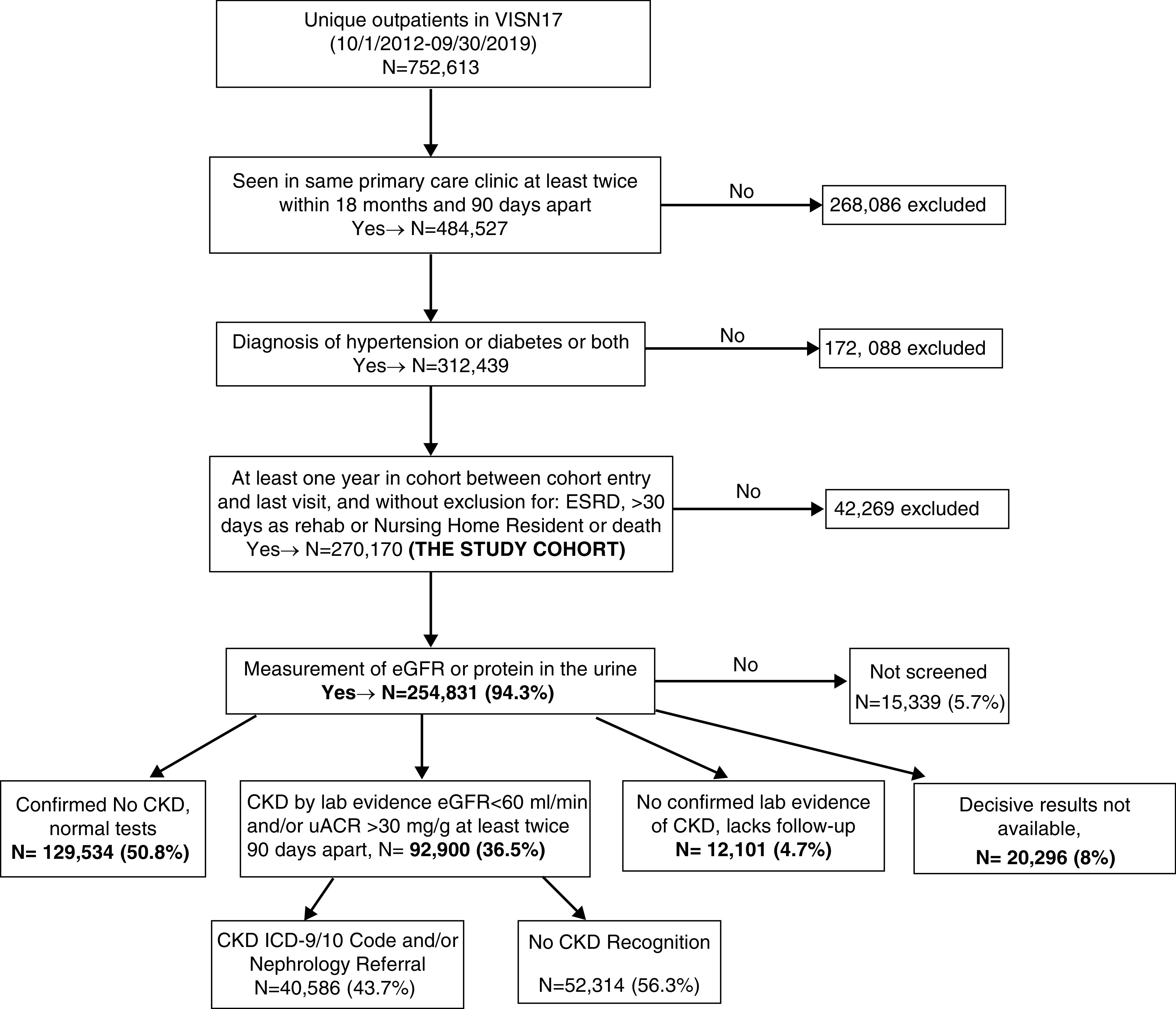
The flow of the study population from identification of the study cohort to status of screening tests done, to who had laboratory evidence of CKD, to who were recognized to have CKD. ICD-9/10, International Classification of Diseases, Ninth and Tenth Revision; lab, laboratory; uACR, urinary albumin-creatinine ratio; VISN 17, Veteran Integrated Service Network 17.
Assessment of eGFR, Urine Protein/Albumin, CKD, and Other Covariates
CKD Screening
We examined the CDW for either (1) at least one measurement of serum creatinine, whether or not converted to an eGFR; or (2) at least one measurement of protein in urine, reported as urinary albumin-creatinine ratio (uACR), protein-creatinine ratio, or 24-hour urinalysis for protein. Laboratory results considered were those from 1 year before cohort entry date until the end of the study period, on the assumption that the provider would consider a year’s worth of previous tests in deciding whether or not to screen the patient for CKD. Samples collected during an inpatient stay or in association with an emergency room (ER) visit were excluded to avoid possible confounding with AKI. If no laboratory screening tests were found, the patient was categorized as “no CKD screening.”
CKD Recognition
If at least two consecutive eGFR or uACR values over a 90 day period (or longer) were <60 ml/min per 1.73 m2 or >30 mg/g, respectively, the patient was counted as positive for CKD by serum creatinine/eGFR or uACR, respectively. Patients were then categorized as “positive by both tests,” “positive by eGFR only,” “positive by uACR only,” “negative by all available tests,” “follow-up lacking” (if a positive value did not have a follow-up laboratory test to confirm/refute), or “decisive test results not available” (if no serum creatinine was converted to eGFR and if none of the available urine tests included uACR). In a parallel process, the CDW was queried for assigned ICD-9 or ICD-10 codes that indicated CKD diagnosis including hypertensive CKD and diabetic CKD (Supplemental Table 1). If at least one of the codes was listed in the Problem List, or at least two outpatient visits had one of the codes between October 1, 2009 and September 30, 2019, the patient was considered to be recognized with CKD. Referral to a nephrologist within VISN 17 (either in a VA setting or in a community setting) was also considered recognition of CKD.
Demographics
Age, sex, and ethnicity/race were obtained for each patient. Age was calculated based on the date of cohort entry. The value of ethnicity/race was determined in accordance with current recommendations of the National Veterans Health Equity Report (12).
Assessment of Predictive Factors
The CDW was queried to create variables that might be associated with ordering of screening tests and recognition of CKD in those with laboratory evidence of CKD.
Comorbidities
The comorbidities of CVD, peripheral arterial disease (PAD), stroke, and tumor (either malignant or benign, because the initial work up of any mass/tumor would require serum chemistry) were determined by identifying ICD-9 and -10 codes (Supplemental Table 1). Patients were counted if a diagnosis was assigned to at least two visits during the years of interest or appeared on the Problem List no later than September 30, 2019.
Clinic Visits
The number of primary care visits, specialty care visits (cardiology, endocrinology, rheumatology, or general surgery clinics), and ER visits plus hospitalizations that occurred between the cohort entry date and the last outpatient visit date were identified through the use of “clinic stop codes” which are used to identify specific functions of the visit. To limit counting of repeat primary care visits (such as BP checks or tuberculosis tests), only one visit with a primary care stop code within a 7-day period was counted.
Elective Procedures
The numbers of elective procedures requiring contrast administration were identified by Current Procedural Terminology (CPT) codes (Supplemental Table 1) and were counted if they occurred between the years of interest. These procedures were categorized into radiologic (computed tomography scans and interventional procedures), cardiac (heart catheterization and percutaneous coronary intervention), and endovascular (peripheral angiography, venography, and percutaneous interventions) procedures. The rates of clinic visits or elective procedures were calculated by dividing the total number of visits or procedures by the number of years between cohort entry and last visit date.
HTN Control
All BPs for patients with HTN were collected beginning 1 year after the HTN diagnosis date (or from the cohort entry date, whichever was later), through February 29, 2020, except for BP measured during an inpatient stay or an ER visit. If there were more than one BP measurements on a given day, the lowest value of systolic and diastolic pressures on that day were selected. The median values of all collected systolic and diastolic pressures were used to assess control. HTN control was categorized into three groups: <130/80 mm Hg, 130–140/80–90 mm Hg, and >140/90 mm Hg.
Prescriptions
CDW was queried to determine the prescription status of ACEI/ARBs, diuretics, statin, and nonsteroidal anti-inflammatory drugs (NSAIDs) for each of the patients in the study groups. Patients were counted if there was a record of having been prescribed these medications at least once between the time of cohort entry and the last visit date.
Statistical Analyses
Means with SDs and percentages were used to summarize continuous and categoric measures, respectively. Tests of significance were run to detect differences in the variables across the CKD screening and recognition status, using a level of significance of 0.001. However, it is acknowledged that with such a large data set even clinically insignificant differences can achieve statistical significance, therefore, we emphasized the differences where these were clinically meaningful. For univariate analysis, the chi-squared test for homogeneity was used to detect statistical differences in proportions for categoric variables and the t test was used to detect statistical differences in means for continuous variables.
Logistic regression was used to assess the multivariate association of variables with the CKD screening and recognition status. The logistic regression models were set up to predict a status of “screened” using all members of the cohort, and a status of “recognized” using all members with laboratory evidence of CKD. Reference values used for categoric variables were a white, male patient, with both DM and HTN but without any of the other comorbidities, with negative results for eGFR and uACR, and no prescription for any of the medications. The reference value for age was 18 years, and for clinic visits and elective procedures was “zero.” The odds ratios (ORs) for age are for a unit change of 1 year, and are a unit change of 1 per year for visits or procedures. A level of significance of 0.01 was used to create 99% confidence intervals for ORs. All statistical analysis was conducted using the SAS/STAT program version 9.4 (SAS Institute, Cary, NC).
Results
Overall Study Cohort
We identified 270,170 unique patients who were seen regularly in primary care clinics and were at risk of developing CKD either due to presence of HTN (n=140,266; 52%) or DM (n=12,295; 5%), or both (n= 117,609; 44%). Figure 1 describes the flow of the study population from identification of the study cohort to status of screening tests done, to who had laboratory evidence of CKD, to who were recognized to have CKD. Table 1 demonstrates the characteristics of these different stages of the population. The pertinent findings are described in this section under the respective headings.
Table 1.
Characteristics of total population and by CKD screening, evidence, and recognition status
| Characteristics | Total (N=270,170) | Screened for CKD (N=254,831; 94%) | Laboratory Evidence of CKD (N=92,900; 37%) | Recognized as CKD (N=40,586; 44%) |
| Existing patients on October 1, 2012, n (%) | 171,089 (63%) | 160,987 (94%) | 70,478 (44%) | 31,616 (45%) |
| Entered cohort between October 2012 and September 2014, n (%) | 37,249 (14%) | 35,359 (95%) | 10,121 (29%) | 4006 (40%) |
| Entered cohort between October 2014 and September 2016, n (%) | 33,886 (13%) | 31,977 (94%) | 7453 (23%) | 2984 (40%) |
| Entered cohort between October 2016 and September 2018, n (%) | 27,946 (10%) | 26,508 (95%) | 4848 (18%) | 1980 (41%) |
| Age, mean±SD | 62.1±13.3 | 62.1±13.2 | 67.5±11.3 | 68.3±11.0 |
| Male, n (%) | 253,094 (94%) | 238,582 (94%) | 88,514 (95%) | 39,270 (97%) |
| Race, n (%) | ||||
| Non-Hispanic white | 145,620 (54%) | 140,998 (97%) | 54,302 (39%) | 2,2961 (42%) |
| Non-Hispanic black | 47,414 (18%) | 46,013 (97%) | 16,823 (37%) | 7395 (44%) |
| Hispanic | 44,362 (16%) | 39,137 (88%) | 11,206 (29%) | 5564 (50%) |
| Asian | 1121 (0.4%) | 1032 (92%) | 211 (20%) | 99 (47%) |
| American Indian/Pacific Islander | 4095 (2%) | 3552 (87%) | 1278 (36%) | 609 (48%) |
| Multirace | 1859 (0.7%) | 1811 (97%) | 648 (36%) | 294 (45%) |
| Unknown | 25,699 (10%) | 22,288 (87%) | 8432 (38%) | 3664 (44%) |
| BMI, mean±SD | 30.7±6.1 | 30.7±6.1 | 30.5±6.1 | 30.6±6.1 |
| eGFR, n (%) | ||||
| Not available | 47,993 (18%) | 32,654 (68%) | 1757 (5%)a | 233 (13%)a |
| >60 ml/min | 123,640 (46%) | 123,640 (100%) | 8321 (7%)a | 1547(19%)a |
| <60 ml/min but not confirmed | 17,292 (6%) | 17,292 (100%) | 1577 (9%)a | 387 (25%)a |
| 45–60 ml/min | 44,055 (16%) | 44,055 (100%) | 44,055 (100%) | 11,603 (26%) |
| 30–45 ml/min | 26,297 (10%) | 26,297 (100%) | 26,297 (100%) | 17,184 (65%) |
| <30 ml/min | 10,893 (4%) | 10,893 (100%) | 10,893 (100%) | 9632 (88%) |
| uACR, n (%) | ||||
| Not available | 144,514 (54%) | 129,175 (89%) | 36,808 (50%) | 14,841 (40%) |
| <30 mg/g | 84,054 (31%) | 84,054 (100%) | 23,418 (28%) | 10,152 (43%) |
| >30 mg/g, but not confirmed | 16,601 (6%) | 16,601 (100%) | 7673 (46%) | 4543 (59%) |
| 30–300 mg/g | 20,415 (8%) | 20,415 (100%) | 20,415 (100%) | 8068 (40%) |
| >300 mg/g | 4586 (2%) | 4586 (100%) | 4586 (100%) | 2982 (65%) |
| Comorbidities, n (%) | ||||
| Diabetes w/o hypertension | 12,295 (5%) | 11,329 (92%) | 2109 (19%) | 595 (28%) |
| Hypertension w/o diabetes | 140,266 (52%) | 132,040 (94%) | 37,774 (29%) | 14,541 (39%) |
| Both diabetes and hypertension | 117,609 (44%) | 111,462 (95%) | 53,017 (48%) | 25,450 (48%) |
| Cardiovascular disease | 122,293 (45%) | 116,361 (95%) | 56,042 (48%) | 27,047 (48%) |
| Peripheral arterial disease | 26,701 (10%) | 25,232 (95%) | 13,927 (55%) | 7803 (56%) |
| Stroke | 34,192 (13%) | 32,739 (96%) | 17,192 (53%) | 8747 (51%) |
| Tumor | 118,718 (44%) | 113,267 (95%) | 48,499 (43%) | 22,192 (46%) |
| Primary care visits per year, mean±SD | 4.09±2.62 | 4.09±2.64 | 4.46±2.80 | 4.80±2.98 |
| Specialty care visits per year, mean±SD | 0.57±1.39 | 0.58±1.42 | 0.81±1.76 | 0.99±2.05 |
| ER visit and hospitalization per year, mean±SD | 0.53±1.40 | 0.56±1.43 | 0.71±1.60 | 0.87±1.83 |
| Elective procedures, n/yr, mean±SD | ||||
| Radiologic | 0.58±1.74 | 0.61±1.78 | 0.717±2.06 | 0.57±1.76 |
| Endovascular | 0.051±0.43 | 0.054±0.44 | 0.091±0.58 | 0.120±0.71 |
| Cardiac | 0.056±0.36 | 0.059±0.37 | 0.098±0.51 | 0.123±0.61 |
| Median BP value, n (%) | ||||
| Not diagnosed with HTN | 12,295 (5%) | 11,329 (92%) | 2109 (19%) | 595 (28%) |
| Not available | 2720 (1%) | 2291 (84%) | 348 (15%) | 135 (38%) |
| <130/80 mm Hg | 77,947 (29%) | 73,528 (94%) | 29,340 (40%) | 12,678 (43%) |
| 130–140/80–90 mm Hg | 142,746 (53%) | 135,050 (95%) | 48,044 (36%) | 20,661 (43%) |
| >140/90 mmHg | 34,462 (13%) | 32,633 (95%) | 13,059 (40%) | 6517 (50%) |
| Medications, n (%) | ||||
| ACEI/ARB | 171,813 (64%) | 162,498 (95%) | 66,266 (41%) | 30,028 (45%) |
| Diuretics | 129,377 (48%) | 122,629 (95%) | 54,591 (45%) | 26,029 (48%) |
| Statin | 183,984 (68%) | 174,210 (95%) | 71,467 (41%) | 32,359 (45%) |
| NSAIDs | 138,333 (51%) | 129,710 (94%) | 40,681 (31%) | 15,433 (38%) |
The percentages are calculated with the denominator from the preceding column in this row, e.g., the “Total” column is the denominator for “Screened for CKD.” However, for the column labeled "Total", the denominator is the cohort total (270,170), and for sex percentage in each column, the denominator is total of that column in the top row. BMI, body mass index; uACR, urinary albumin-creatinine ratio; w/o, without; ER, emergency room; HTN, hypertension; ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; NSAIDs, nonsteroidal anti-inflammatory drugs.
These individuals’ CKD diagnosis is based on proteinuria alone.
CKD Screening
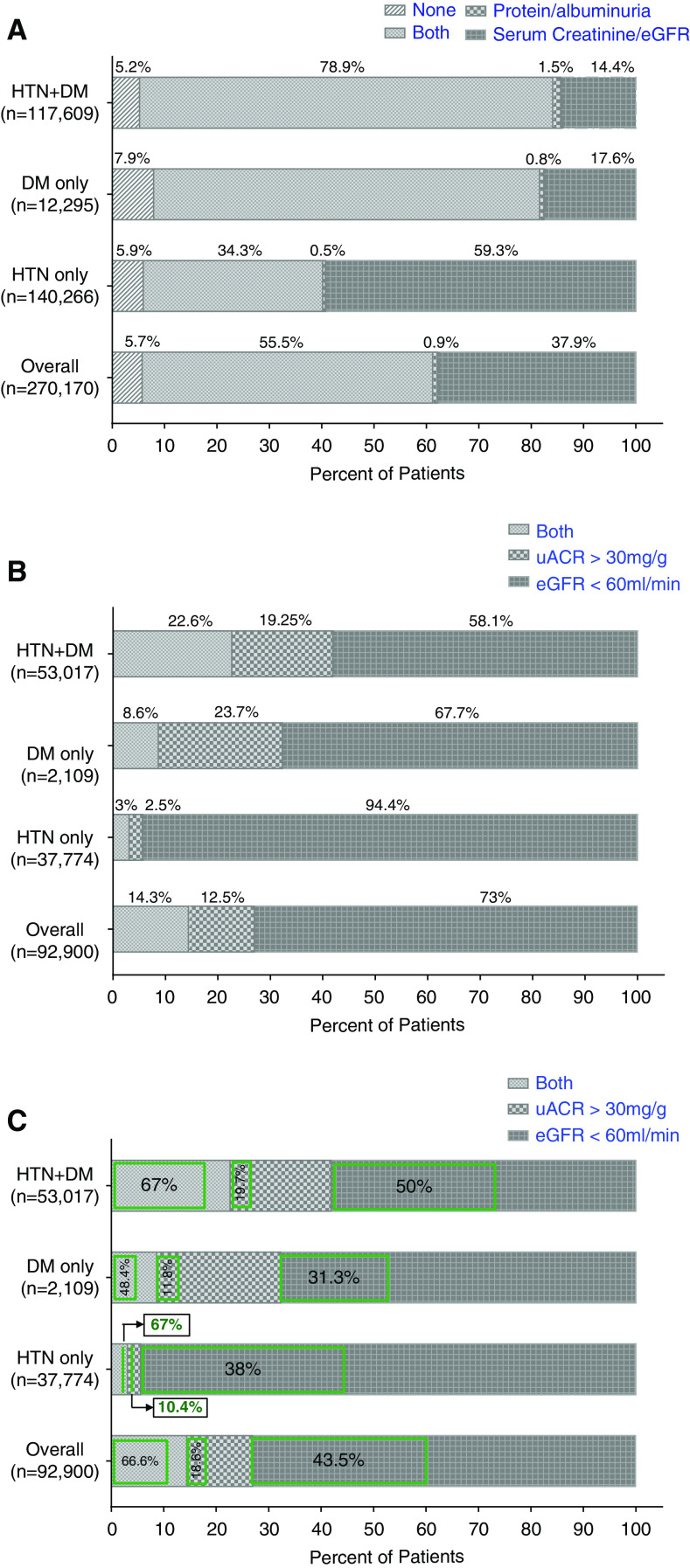
As shown in Figure 1, 254,831 (94%) patients had CKD screening tests (eGFR, urine protein/albumin, or both) in the chart. On examination of data from the different time periods, the frequency of screening tests did not change over time (Table 1). Figure 2A shows the individual CKD screening tests in these patients by DM, HTN, or both. Overall, 149,903 (56%) patients had both serum and urine screening tests. Urine protein measurements were present in 56% of patients’ charts. This rate was worse in isolated HTN (35%) compared with DM (75%) or both conditions (80%). Table 2 describes the characteristics of the patients by the presence or absence of screening tests. Patients who were Hispanic, American Indian/Pacific Islander, Asian, and those with unknown race were less likely to be screened. Patients who had screening tests were more likely to have comorbidities such as stroke, CVD, and tumors, and had a higher frequency of specialist visits, ER visits, hospitalization, and elective procedures.
Figure 2.
The screening for protein/albuminuria was low, particularly in patients with HTN only and recognition for CKD was very low in patients with CKD due to uACR criteria irrespective of presence of DM or HTN or both. (A) Percentage of patients with diabetes (DM), hypertension (HTN), or both who had screening tests in the chart. (B) Percentage of patients with DM, HTN, or both who had laboratory evidence of CKD by either eGFR, uACR, or both criteria. (C) Within each criterion of CKD, green box represents the percentage of patients who were recognized to have CKD.
Table 2.
Comparisons of patient characteristics between CKD screening versus not and CKD recognition versus not
| Characteristics | Screening | Recognition | ||
| Screened (N=254,831; 94%) | Not Screened (N=15,339; 6%) | Recognized (N=40,586; 44%) | Not Recognized (N=52,314; 56%) | |
| Existing patients at start of October 2012, n (%)a | 160,987 (94%) | 10,102 (6%) | 31,616 (45%) | 38,862 (55%) |
| Entered cohort in October 2012 to September 2014, n (%)a | 35,359 (95%) | 1890 (5%) | 4006 (40%) | 6115 (60%) |
| Entered cohort in October 2014 to September 2016, n (%)a | 31,977 (94%) | 1909 (6%) | 2984 (40%) | 4469 (60%) |
| Entered cohort in October 2016 to September 2018, n (%)a | 26,508 (95%) | 1438 (5%) | 1980 (41%) | 2868 (59%) |
| Age, mean±SD | 62.1 (13.2) | 61.7 (14.0) | 68.3 (11.0) | 66.9 (11.4) |
| Male, n (%) | 238,582 (94%) | 14,511 (95%) | 39,270 (97%) | 49,244 (94%) |
| Race, n (%) | ||||
| Non-Hispanic white | 140,998 (55%) | 4622 (30%) | 22,961 (57%) | 31,341 (60%) |
| Non-Hispanic black | 46,013 (18%) | 1401 (9%) | 7395 (18%) | 9428 (18%) |
| Hispanic | 39,137 (15%) | 5225 (34%) | 5564 (14%) | 5642 (11%) |
| Asian | 1032 (0.4%) | 89 (0.6%) | 99 (0.2%) | 112 (0.2%) |
| American Indian/Pacific Islander | 3552 (1%) | 543 (4%) | 609 (2%) | 669 (1%) |
| Multirace | 1811 (0.7%) | 48 (0.3%) | 3664 (9%) | 4768 (9%) |
| Unknown | 22,288 (9%) | 3411 (22%) | 294 (0.7%) | 354 (0.7%) |
| BMI, mean (SD) | 30.7 (6.1) | 30.4 (5.7) | 30.6 (6.1) | 30.4 (6.0) |
| eGFR ml/min per 1.73 m2, median (IQR) | 39 (29.9–46.2) | 51 (45.0–55.0) | ||
| Not available, n (%) | 32,654 (13%) | 15,339 (100%) | 233 (0.6%) | 1524 (3%) |
| >60 ml/min, n (%) | 123,640 (49%) | 0 | 1547 (4%) | 6774 (13%) |
| <60 ml/min but not confirmed, n (%) | 17,292 (7%) | 0 | 387 (1%) | 1190 (2%) |
| 45–60 ml/min, n (%) | 44,055 (17%) | 0 | 11,603 (29%) | 32,452 (62%) |
| 30–45 ml/min, n (%) | 26,297 (10%) | 0 | 17,184 (42%) | 9113 (17%) |
| <30 ml/min, n (%) | 10,893 (4%) | 0 | 9632 (24%) | 1261 (2%) |
| uACR in mg/g, median (IQR) | 132 (65.7–326.8) | 78.5 (48–156.3) | ||
| Not available, n (%) | 129,175 (51%) | 15,339 (100%) | 14,841 (37%) | 21,967 (42%) |
| <30 mg/g, n (%) | 84,054 (33%) | 0 | 10,152 (25%) | 13,266 (25%) |
| >30 mg/g but not confirmed, n (%) | 16,601 (7%) | 0 | 4543 (11%) | 3130 (6%) |
| 30–300 mg/g, n (%) | 20,415 (8%) | 0 | 8068 (20%) | 12,347 (24%) |
| >300 mg/g, n (%) | 4586 (2%) | 0 | 2982 (7%) | 1604 (3%) |
| Comorbidities, n (%) | ||||
| Diabetes w/o hypertension | 11,329 (5%) | 966 (6%) | 595 (2%) | 1514 (3%) |
| Hypertension w/o diabetes | 132,040 (52%) | 8226 (54%) | 14,541 (36%) | 23,233 (44%) |
| Both diabetes and hypertension | 111,462 (44%) | 6147 (40%) | 25,450 (63%) | 27,567 (53%) |
| Cardiovascular disease | 116,361 (46%) | 5932 (39%) | 27,047 (67%) | 28,995 (52%) |
| Peripheral arterial disease | 25,232 (10%) | 1469 (10%) | 7803 (19%) | 6124 (12%) |
| Stroke | 32,739 (13%) | 1453 (10%) | 8747 (22%) | 8445 (16%) |
| Tumor | 113,267 (44%) | 5451 (36%) | 22,192 (55%) | 26,307 (50%) |
| Primary care visits per year, mean±SD | 4.09 (2.64) | 3.97 (2.33) | 4.80 (2.98) | 4.19 (2.62) |
| Specialty care visits per year, mean±SD | 0.58 (1.42) | 0.33 (0.85) | 0.99 (2.05) | 0.66 (1.48) |
| ER visit and hospitalization per year, mean±SD | 0.56 (1.43) | 0.015 (0.20) | 0.87 (1.83) | 0.59 (1.37) |
| Elective procedures, n/yr, mean±SD | ||||
| Radiologic | 0.61 (1.78) | 0.16 (0.55) | 0.57 (1.76) | 0.83 (2.26) |
| Endovascular | 0.054 (0.44) | 0.0004 (0.02) | 0.120 (0.71) | 0.069 (0.46) |
| Cardiac | 0.059 (0.37) | 0.0006 (0.02) | 0.123 (0.61) | 0.078 (0.41) |
| Median BP value, n (%) | ||||
| Not diagnosed with HTN | 11,329 (4%) | 966 (6%) | 595 (2%) | 1514 (3%) |
| Not available | 2291 (0.9%) | 429 (3%) | 135 (0.3%) | 213 (0.4%) |
| <130/80 mm Hg | 73,528 (29%) | 4419 (29%) | 12,678 (31%) | 16,662 (32%) |
| 130–140/80–90 mm Hg | 135050 (53%) | 7696 (50%) | 20,661 (51%) | 27,383 (52%) |
| >140/90 mm Hg | 32,633 (13%) | 1829 (12%) | 6517 (16%) | 6542 (13%) |
| Medications, n (%) | ||||
| ACEI/ARB | 162,498 (64%) | 9315 (61%) | 30,028 (74%) | 36,238 (69%) |
| Diuretics | 122,629 (48%) | 6748 (44%) | 26,029 (64%) | 28,562 (55%) |
| Statin | 174,210 (68%) | 9774 (64%) | 32,359 (80%) | 39,108 (75%) |
| NSAIDs | 129,710 (51%) | 8623 (56%) | 15,433 (38%) | 25,248 (48%) |
All the comparisons in this table have a P value <0.001, except PAD in screening group (P=0.19) and BMI in CKD recognition (P=0.013). BMI, body mass index; IQR, interquartile range; uACR, urinary albumin-creatinine ratio; w/o, without; ER, emergency room; HTN, hypertension; ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; NSAIDs, nonsteroidal anti-inflammatory drugs; PAD, peripheral artery disease.
The percentages for the cohort groups are based on the total for that group from Table 1. The rest of the percentages (rows without a) are calculated with N from the column as the denominator.
Laboratory Evidence of CKD
Of the 254,831 patients who had any screening procedures done, 92,900 (37%) had laboratory evidence of CKD either by low eGFR, high uACR, or both tests. Figure 2B shows that the majority of patients had CKD based on eGFR <60 ml/min per 1.73 m2 criteria (73%) as compared with uACR >30 mg/g (13%) or both (14%). In patients with isolated HTN, 94% had CKD based on eGFR <60 ml/min per 1.73 m2 criteria as compared with 68% with DM, reflecting a lower rate of uACR testing in isolated HTN. Supplemental Table 2 displays the comparisons between those who were diagnosed for CKD by uACR alone, eGFR alone, or both.
CKD Recognition
Of the patients with laboratory evidence of CKD, 40,586 (44%) were recognized as having CKD either by ICD codes or nephrology referral (Figure 1). Of these, 7341 patients had both ICD code and referral, 1710 had nephrology referral only, and the rest had ICD codes only. On examination of the different time periods, the rate of CKD recognition was slightly lower in later time periods (Table 1). Table 2 describes the characteristics of the patients who had laboratory evidence of CKD by recognition status. The median (interquartile range) eGFR was lower (39 [29.9–46.2] versus 51 [45.0–55.0] ml/min per 1.73 m2) and uACR was higher (132.1 [65.7–326.8] versus 78.5 [48–156.3] mg/g) in patients with recognized CKD compared with those with unrecognized CKD. Patients with advanced CKD (eGFR <45 ml/min, 66% versus 20%) and severe albuminuria (uACR >300 mg/g, 7% versus 3%) were more likely to have recognized CKD. Notably, 20% of patients who had advanced CKD (eGFR <45 ml/min) were not recognized.
When comparing CKD recognition in patients with CKD either due to eGFR <60 ml/min per 1.73 m2 or uACR >30 mg/g criteria, there was increased likelihood to be recognized if CKD was due to both eGFR and uACR criteria (67%) versus eGFR alone (44%) or uACR alone (19%) (Figure 2C). Supplemental Table 2 displays comparisons between those who were versus those who were not recognized by categories of CKD by eGFR only, uACR only, or both.
HTN, DM Control, and Renal Prudent Medications
Overall, in the total cohort, 29% had well controlled BP <130/80 mm Hg and 13% had BP >140/90 mm Hg (Table 1). The BP control rates were not different by CKD screening status; however, more patients with recognized CKD had BP >140/90 mm Hg (16% versus 13%) (Table 2). ACEI/ARBs, statin, and diuretics were prescribed in 64%, 68%, and 48% of the total cohort, respectively (Table 1). Patients with screening tests and recognized CKD were more likely to be on these medications as compared with unscreened and unrecognized patients with CKD (Table 2). NSAIDs were prescribed less frequently in screened (51% versus 56%) and recognized (38% versus 48%) CKD versus unscreened and unrecognized groups (Table 2). Supplemental Table 2 describes the prescription of ACEI/ARB, diuretics, statin, and NSAIDs in recognized patients versus those who were not, by categories of CKD by eGFR only, uACR only, or both.
Multivariate Association between Predictors and CKD Screening and Recognition
Table 3 describes the multivariable regression analysis. Higher frequency of ER visits and hospitalizations (OR, 222.4; 99% CI, 166.54 to 304.2), cardiology (OR, 9.48; 99% CI, 4.02 to 22.3) and endovascular (OR, 9.44; 99% CI, 3.36 to 26.53) procedures associated most strongly with higher screening. Hispanic, Asian, Native American, and unknown race; history of DM or HTN alone as compared to both; PAD; and diuretic prescription were associated with reduced screening. For CKD recognition, all races associated with higher recognition compared with white race. The odds of recognition increased with worsening eGFR and uACR. Similar to screening, patients with DM or HTN alone as compared with both were less likely to be recognized, whereas those with CVD, PAD, tumor, ACEI/ARB, and statin prescription were more likely to be recognized. Interestingly, frequency of ER visits and hospitalization had a very small association with recognition (OR, 1.03; 99% CI, 1.02 to 1.05; P<0.001) in contrast to screening. Surprisingly, NSAID prescription associated with lower odds for screening (OR, 0.64; 99% CI, 0.6 to 0.67) and recognition (OR, 0.77; 99% CI, 0.74 to 0.81).
Table 3.
Logistic regression analysis with CKD screening and recognition as the outcome variables
| Parameters | CKD Screening | CKD Recognition | ||
| Odds Ratio (99% CI) | P Value | Odds Ratio (99% CI) | P Value | |
| Age (ref: 18 yr)a | 1.00 (0.99 to 1.003) | 0.05 | 0.99 (0.99 to 0.99) | <0.001 |
| Sex, female versus male | 1.02 (0.92 to 1.13) | 0.66 | 0.52 (0.47 to 0.57) | <0.001 |
| Cohort entry (ref: before October 1, 2012) b | ||||
| October 1, 2012 to September 30, 2014 | 1.02 (0.95 to 1.09) | 0.5 | ||
| October 1, 2014 to September 30, 2016 | 1.10 (1.02 to 1.19) | 0.0008 | ||
| October 1, 2016 to September 30, 2018 | 1.17 (1.07 to 1.28) | <0.001 | ||
| Race | ||||
| Unknown versus white | 0.23 (0.22 to 0.25) | <0.001 | 1.12 (1.04 to 1.20) | <0.001 |
| Black versus white | 0.96 (0.89 to 1.05) | 0.25 | 1.13 (1.07 to 1.19) | <0.001 |
| Asian versus white | 0.45 (0.34 to 0.60) | <0.001 | 1.60 (1.06 to 2.40) | 0.003 |
| American Indian versus white | 0.21 (0.19 to 0.24) | <0.001 | 1.28 (1.08 to 1.52) | 0.0002 |
| Hispanic versus white | 0.28 (0.27 to 0.30) | <0.001 | 1.50 (1.41 to 1.60) | <0.001 |
| eGFR >60 ml/min (reference) | Not applicable | |||
| Not available | 0.75 (0.61 to 0.91) | 0.0001 | ||
| 45–60 ml/min | 2.90 (2.64 to 3.20) | <0.001 | ||
| 30–45 ml/min | 14.32 (12.98 to 15.8) | <0.001 | ||
| <30 ml/min | 49.99 (44.27 to 56.45) | <0.001 | ||
| uACR <30 mg/g (reference) | Not applicable | |||
| Not available | 0.79 (0.75 to 0.83) | <0.001 | ||
| 30–300 mg/g | 1.33 (1.24 to 1.43) | <0.001 | ||
| >300 mg/g | 2.19 (1.95 to 2.45) | <0.001 | ||
| Comorbidities | ||||
| DM (ref: both HTN and DM) | 0.87 (0.83 to 0.91) | <0.001 | 0.63 (0.54 to 0.73) | <0.001 |
| HTN (ref: both HTN and DM) | 0.78 (0.70 to 0.87) | <0.001 | 0.90 (0.86 to 0.95) | <0.001 |
| CVD (ref: no CVD) | 1.01 (0.96 to 1.06) | 0.58 | 1.09 (1.04 to 1.14) | <0.001 |
| PAD (ref: no PAD) | 0.78 (0.72 to 0.85) | <0.001 | 1.29 (1.21 to 1.37) | <0.001 |
| Stroke (ref: no stroke) | 1.06 (0.98 to 1.15) | 0.05 | 1.05 (0.99 to 1.11) | 0.011 |
| Tumor (ref: no tumor) | 1.06 (1.01 to 1.11) | 0.001 | 1.13 (1.08 to 1.18) | <0.001 |
| Visits (ref: no visit) c | ||||
| Primary care visits | 0.99 (0.98 to 0.99) | <0.001 | 1.04 (1.03 to 1.04) | <0.001 |
| Specialty care visits | 0.91 (0.89 to 0.94) | <0.001 | 1.00 (0.98 to 1.01) | 0.8 |
| ER visit and hospitalization | 222.37 (162.5 to 304.2) | <0.001 | 1.03 (1.02 to 1.05) | <0.001 |
| Elective procedures (ref: no procedure) a | ||||
| Radiologic | 1.18 (1.14 to 1.23) | <0.001 | 0.91 (0.90 to 0.93) | <0.001 |
| Endovascular | 9.44 (3.36 to 26.53) | <0.001 | 0.98 (0.94 to 1.02) | 0.1 |
| Cardiac | 9.48 (4.02 to 22.32) | <0.001 | 0.97 (0.93 to 1.01) | 0.07 |
| Medications (ref: no medication) | ||||
| ACEI/ARB | 1.01 (0.96 to 1.07) | 0.58 | 1.06 (1.01 to 1.12) | 0.002 |
| Diuretics | 0.91 (0.87 to 0.96) | <0.001 | 0.99 (0.95 to 1.04) | 0.72 |
| NSAIDs | 0.64 (0.6 to 0.67) | <0.001 | 0.77 (0.74 to 0.81) | <0.001 |
| Statin | 1.11 (1.06 to 1.17) | <0.001 | 1.1 (1.04 to 1.16) | <0.001 |
An odds ratio of <1 in this analysis means that the category, compared with the reference group, is less likely to be screened or recognized. An odds ratio of >1 means the category is more likely to get screened than the reference category, e.g., those not on diuretics are less likely to get screened than those on diuretics. Ref, reference; uACR, urinary albumin-creatinine ratio; DM, diabetes; HTN, hypertension; CVD, cardiovascular disease; PAD, peripheral artery disease; ER, emergency room; ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; NSAIDs, nonsteroidal anti-inflammatory drugs.
Odds ratio is for a unit change of 1 yr.
There was no time trend for screening in univariate analysis, so not included in the regression analysis.
Odds ratio is for a unit change of 1/year.
Discussion
Our findings have important clinical implications that can provide a platform for designing interventions to improve the delivery of care to patients at risk for CKD. In VISN 17 primary care clinics, the overall CKD screening rate in patients with DM and HTN was high (94%); however, rate of screening for protein/albuminuria was low (56%), particularly in isolated HTN (35%). More strikingly, CKD recognition was low (44%). This rate increased with the severity of the CKD; however, substantial numbers of patients with advanced CKD were still unrecognized. The level of BP control was similar irrespective of CKD screening and recognition; however, ACEI/ARB, diuretics, and statin prescription rates were higher, and NSAID prescription was lower in patients with screened and recognized CKD as compared with the unscreened cohort and unrecognized CKD.
Previous studies have reported process measures in patients with CKD such as rate of ACEI/ARB prescription, BP control, or urine albumin or parathyroid hormone testing (13). However, optimal delivery of care first necessitates timely screening and early recognition of CKD. Lee et al. (6) reported uACR testing in 35% of patients with isolated HTN within a year of diagnosis in adult primary care patients. We found a similar rate but we describe CKD screening by both eGFR and uACR in patients with both HTN and DM. The screening rate for CKD by eGFR was high in VISN 17; however, the low rate of protein/albuminuria screening in isolated HTN was likely driven by the lack of VA performance measures for such screening. Of note, VA has performance measures and clinical reminders for screening for urine albumin in patients with DM. Moreover, although VA/DOD guidelines clearly recommend albuminuria testing in individuals with isolated HTN, the American College of Physicians’ recommendations for screening in at-risk populations are vague without particular mention of isolated HTN, resulting in uncertainty and confusion for this patient population. In addition, many primary care clinicians remain skeptical with the concept of early CKD and find this area of patient management of debatable benefit because adequate HTN and DM control is recommended irrespective of presence of CKD, and that CKD status does not change this management. However, we argue that diagnosis of CKD, particularly the presence of albuminuria, changes the treatment approach and prognosis. For example, KDIGO recommends a BP goal of <130/80 mm Hg in the presence of uACR >30 mg/g as compared with <140/90 mm Hg in patients with CKD who do not have albuminuria (14). Of note, the latest American College of Cardiology guidelines recommends goal BP <130/80 mm Hg for CKD irrespective of albuminuria (15). Albuminuria has consistently been shown to be the major predictor of uncontrolled HTN and worse cardiovascular outcomes in several study populations (16,17). In our study, we also observed higher rates of severe albuminuria (uACR >300 mg/g) in patients with uncontrolled HTN (data not shown). Much has been learned about the benefits of reduction of albuminuria on the outcome of CKD over the last two decades in patients with or without DM (3,4). In addition to HTN control, other albuminuria-reduction strategies include dietary salt reduction, use of ACEI/ARBs, and diuretics (14,18,19). However, for these interventions to be implemented, albuminuria needs to be identified in at-risk patients early (3,4). In our study, 65% of patients with HTN did not have testing for protein/albuminuria. Further studies are required to determine the reasons for this gap between guidelines and implementation.
A recent methodologic study reported that documentation of ICD diagnostic codes is a relatively good surrogate for primary care physician awareness (7). In a recent cross-sectional observational study, only 47% of patients with laboratory evidence of CKD in primary care had a CKD diagnosis in their chart (7). Several other publications have examined CKD awareness in primary care settings and reported rates ranging from 12% to 38% (8,9,11,20). Consistent with these prior studies in the primary care practices, we found low awareness (44%) of CKD in VISN 17. However, most of the prior studies considered only eGFR without including uACR as the criteria for CKD diagnosis, resulting in a lower denominator, and the study by Rothberg et al. (9) was limited to the elderly. Szczech et al. (10) examined CKD screening and diagnosis by both eGFR and uACR and recognition at the same time in primary care practices; however, they included patients with DM only and used only one-time eGFR and uACR values to define CKD instead of the recommended two values over 3 months, thus inflating the denominator. We are the first to present CKD screening and diagnosis with both eGFR and uACR and simultaneous recognition data in high-risk veterans with both DM and HTN. We found that many of those unrecognized patients had CKD based on moderately high albuminuria (>30 mg/gm) with eGFR >60 ml/min, suggesting either lack of knowledge of isolated albuminuria as a diagnosis of CKD or lag in practice change because the emphasis on albuminuria as a diagnostic and prognostic tool has been recent (21). However, lack of recognition of 20% patients with advanced CKD (eGFR <45 ml/min) is concerning.
For the first time, we report factors associated with screening and awareness of CKD. The most striking finding that patients with higher ER visits, hospitalization, and elective procedures were at the highest odds of having eGFR and uACR in the chart suggests that these tests were not performed with the intention of screening, but instead were secondary as a result of follow-up and the requirement to check renal function peri-procedure. This higher screening rate with the higher ER visits, hospitalization, and elective procedures did not convert into higher recognition rates, which again suggests these tests were not ordered as screening tests for CKD itself. Of note, in this analysis, we excluded laboratory results obtained during ER visit or hospital admissions. Another striking finding was the lower screening tests in races (except for black race) other than white; however, there was higher recognition in all of the races other than white. Higher recognition might reflect the knowledge of the providers of higher prevalence of CKD in races other than white or more advanced severity of CKD in these groups at time of recognition. In univariate analysis, the recognition rate was higher in the existent cohort compared with the later three cohorts, likely because of longer disease burden and perhaps more severe disease; therefore, in logistic regression analysis after adjustment for multiple other variates, particularly the worst eGFR and uACR, the patients in the later cohorts appeared to have higher instead of lower odds of recognition. Higher rates in patients with comorbidities and DM and HTN together versus DM or HTN alone suggests that more disease burden triggers CKD screening and recognition. Positive associations of ACEI/ARB and statin prescription with higher recognition likely suggests that providers using these medications are aware of the CKD recommendations. Lower screening in patients prescribed diuretics is counterintuitive because diuretic prescription should instigate serum chemistry checkup. Similarly, the finding of lower odds of screening and recognition in patients with NSAID prescription is perplexing. NSAID prescriptions imply patients who are suffering from pain and perhaps, during these types of interactions, providers may not have enough time to address preventive chronic health issues or may attribute decreased eGFR to NSAIDs rather than labeling it CKD. Data collection directly from providers and patients will be necessary to understand the prescription pattern in the face of CKD.
It is important to determine if CKD screening and recognition enhance the care process before the strained providers are tasked with an additional item. We found that screening and recognition of CKD was not associated with better BP control. Nonetheless, in accordance with prior studies, we found more patients with uncontrolled HTN had high albuminuria (data not shown). Presumably, as stated above, increased screening for albuminuria and recognition of its presence may offer opportunities to emphasize delivery of risk reduction strategies. ACEI/ARBs are one of the primary risk-reduction strategies for CKD with albuminuria and the prescription of these medications was 3% higher in screened and 5% higher in recognized patients with CKD. Studies are needed to determine whether increased recognition of albuminuria escalates risk-reduction strategies and improves HTN and CKD outcomes. Furthermore, screened and recognized patients with CKD had a 4% and 10% higher diuretic prescription rate, respectively. Diuretics are the mainstay for the difficult to control HTN in patients with CKD because the impaired natriuresis is the fundamental pathogenetic mechanism of HTN in CKD (14,22). In an uncontrolled HTN setting, recognition of CKD may increase the prescription of diuretics and help achieve target BP goals. In our study, overall statin prescription rates were high and were even higher in screened and recognized patients with CKD. Moreover, NSAID prescription rates were lower in patients that were screened and recognized with CKD by providers, suggesting that increased CKD screening and awareness may likely optimize the prescription of renal prudent medications.
The current study has several limitations. Many populations are at risk for CKD; however, we included only patients with DM and HTN because these two populations incorporate >80% of patients with CKD and have unambiguous diagnostic codes in patient charts. Second, we assessed screening rate based on the eGFR and urine reporting in the VA-CDW; some patients might have had these screening tests performed outside the VA health system. Nonetheless, we assume that including patients seen at least twice within 18 months excludes many of those patients. Third, the medication data captures only the VA prescriptions; however, we assume that our inclusion criteria might have excluded many of those patients who obtain prescriptions from outside VA. Fourth, we examined only the prescription rates of medications to gauge the providers’ engagement in patient care, not the prescription filling and adherence to treatment and thus this data cannot be used to ascertain association of medications with BP control or any other outcome. Last but not least, an electronic medical record–based, retrospective design precludes identification of other factors that are related to patients’ and providers’ knowledge and perception and would require additional primary data collections such as surveys, interviews, or direct observations.
For the first time, we report rate of CKD screening with both eGFR and uACR and simultaneous recognition of CKD in at-risk veterans, which are essential steps to guide the policies to advance American kidney health as mandated in recent Presidential Executive Orders. Screening for protein/albuminuria, particularly in isolated HTN, and CKD awareness was low. These results highlight a major gap in the understanding of CKD among primary care, particularly, albuminuria as the marker of CKD and the mediator of worse outcomes, which represents a missed opportunity. CKD screening and recognition was associated with favorable prescription of renal prudent medications. Our findings indicate that the dissemination of information regarding significance of CKD screening by both eGFR and uACR and need for early identification of CKD in primary care would be the next step to improve kidney health.
Disclosures
J. Pugh's salary is supported by the South Texas Veterans Health Care System and Michael Mader's by the South Texas Veterans Health Care System Research Service. All remaining authors have nothing to disclose.
Funding
None.
Author Contributions
S. Bansal was responsible for formal analysis and resources and wrote the original draft; S. Bansal and M. Mader were responsible for data curation, software, and visualization; S. Bansal, M. Mader, and J. Pugh reviewed and edited the manuscript and were responsible for methodology, project administration, and validation; S. Bansal and J. Pugh conceptualized the study, were responsible for investigation, and provided supervision.
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0000532020/-/DCSupplemental.
ICD and CPT codes used. Download Supplemental Table 1, PDF file, 66 KB (65.2KB, pdf)
Patient characteristics by CKD recognition status in patients with lab evidence of CKD by eGFR or uACR or both criteria. Download Supplemental Table 2, PDF file, 66 KB (65.2KB, pdf)
References
- 1.Murphy D, McCulloch CE, Lin F, Banerjee T, Bragg-Gresham JL, Eberhardt MS, Morgenstern H, Pavkov ME, Saran R, Powe NR, Hsu CY; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team : Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med 165: 473–481, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United States Executive Office of the President [Donald J. Trump] : Executive Order 13879: Advancing American Kidney Health, July 10, 2019. Fed Regist 84: 33817–33819, 2019 [Google Scholar]
- 3.Oellgaard J, Gæde P, Rossing P, Persson F, Parving HH, Pedersen O: Intensified multifactorial intervention in type 2 diabetics with microalbuminuria leads to long-term renal benefits [published correction appears in Kidney Int 91: 1257, 2017]. Kidney Int 91: 982–988, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Narva A: Population health for CKD and diabetes: Lessons From the Indian health Service. Am J Kidney Dis 71: 407–411, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Department of Veterans Affairs/Department of Defense : VA/DoD Clinical Practice Guideline for the Management of Chronic Kidney Disease in Primary Care, Washington, D.C., US Department of Veterans Affairs and Department of Defense, 2014 [Google Scholar]
- 6.Lee J, Chu C, Guzman D, Fontil V, Velasquez A, Powe NR, Tuot DS: Albuminuria testing by race and ethnicity among patients with hypertension with and without diabetes. Am J Nephrol 50: 48–54, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frigaard M, Rubinsky A, Lowell L, Malkina A, Karliner L, Kohn M, Peralta CA: Validating laboratory defined chronic kidney disease in the electronic health record for patients in primary care. BMC Nephrol 20: 3, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samal L, Linder JA, Bates DW, Wright A: Electronic problem list documentation of chronic kidney disease and quality of care. BMC Nephrol 15: 70, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothberg MB, Kehoe ED, Courtemanche AL, Kenosi T, Pekow PS, Brennan MJ, Mulhern JG, Braden GL: Recognition and management of chronic kidney disease in an elderly ambulatory population. J Gen Intern Med 23: 1125–1130, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szczech LA, Stewart RC, Su HL, DeLoskey RJ, Astor BC, Fox CH, McCullough PA, Vassalotti JA: Primary care detection of chronic kidney disease in adults with type-2 diabetes: The ADD-CKD study (awareness, detection and drug therapy in type 2 diabetes and chronic kidney disease). PLoS One 9: e110535, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guessous I, McClellan W, Vupputuri S, Wasse H: Low documentation of chronic kidney disease among high-risk patients in a managed care population: A retrospective cohort study. BMC Nephrol 10: 25, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.US Deparmtent of Veterans Affairs Office of Health Equity: National veteran health equity report FY2013. Washington, DC, US Department of Veterans Affairs, 2016. Available at: http://www.va.gov/healthequity/NVHER.asp. Accessed July 21, 2020 [Google Scholar]
- 13.Tiwari A, Tseng CL, Kern EF, Maney M, Miller DR, Pogach L: Facility variation in utilization of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in patients with diabetes mellitus and chronic kidney disease. Am J Manag Care 13: 73–79, 2007 [PubMed] [Google Scholar]
- 14.National Kidney Foundation: KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Available at https://kdigo.org/guidelines/blood-pressure-in-ckd/. Accessed July 21, 2020
- 15.Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee : Prevention, detection, evaluation, and management of high blood pressure in adults: Synopsis of the 2017 American College of cardiology/American heart association hypertension guideline. Ann Intern Med 168: 351–358, 2018 [DOI] [PubMed] [Google Scholar]
- 16.Muntner P, Anderson A, Charleston J, Chen Z, Ford V, Makos G, O’Connor A, Perumal K, Rahman M, Steigerwalt S, Teal V, Townsend R, Weir M, Wright JT Jr.; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : Hypertension awareness, treatment, and control in adults with CKD: Results from the chronic renal insufficiency cohort (CRIC) study. Am J Kidney Dis 55: 441–451, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plantinga LC, Miller ER 3rd, Stevens LA, Saran R, Messer K, Flowers N, Geiss L, Powe NR; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team : Blood pressure control among persons without and with chronic kidney disease: US trends and risk factors 1999-2006. Hypertension 54: 47–56, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buter H, Hemmelder MH, Navis G, de Jong PE, de Zeeuw D: The blunting of the antiproteinuric efficacy of ACE inhibition by high sodium intake can be restored by hydrochlorothiazide. Nephrol Dial Transplant 13: 1682–1685, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Vogt L, Waanders F, Boomsma F, de Zeeuw D, Navis G: Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J Am Soc Nephrol 19: 999–1007, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allen AS, Forman JP, Orav EJ, Bates DW, Denker BM, Sequist TD: Primary care management of chronic kidney disease. J Gen Intern Med 26: 386–392, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andrassy KM: Comments on ‘KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease’. Kidney Int 84: 622–623, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Toto RD: Treatment of hypertension in chronic kidney disease. Semin Nephrol 25: 435–439, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ICD and CPT codes used. Download Supplemental Table 1, PDF file, 66 KB (65.2KB, pdf)
Patient characteristics by CKD recognition status in patients with lab evidence of CKD by eGFR or uACR or both criteria. Download Supplemental Table 2, PDF file, 66 KB (65.2KB, pdf)