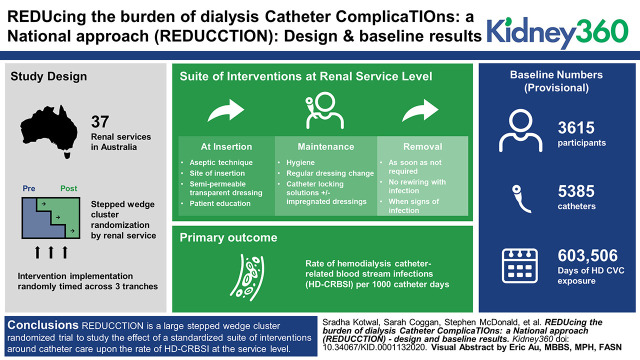
Visual Abstract
Keywords: dialysis, Australia, bacteremia, catheterization, central venous catheters, crossinfection, dialysis catheter, evidence-based medicine, health care costs, longitudinal studies, renal dialysis, vascular access
Abstract
Background
Patients with hemodialysis central venous catheters (HD CVCs) are susceptible to health care-associated infections, particularly hemodialysis catheter-related bloodstream infection (HD-CRBSI), which is associated with high mortality and health care costs. There have been few systematic attempts to reduce this burden and clinical practice remains highly variable. This manuscript will summarize the challenges in preventing HD-CRBSI and describe the methodology of the REDUcing the burden of dialysis Catheter ComplicaTIOns: a National approach (REDUCCTION) trial.
Methods
The REDUCCTION trial is a stepped-wedge cluster randomized trial of a suite of clinical interventions aimed at reducing HD-CRBSI across Australia. It clusters the intervention at the renal-service level with implementation randomly timed across three tranches. The primary outcome is the effect of this intervention upon the rate of HD-CRBSI. Patients who receive an HD CVC at a participating renal service are eligible for inclusion. A customized data collection tool allows near-to-real-time reporting of the number of active catheters, total exposure to catheters over time, and rates of HD-CRBSI in each service. The interventions are centered around the insertion, maintenance, and removal of HD CVC, informed by the most current evidence at the time of design (mid-2018).
Results
A total of 37 renal services are participating in the trial. Data collection is ongoing with results expected in the last quarter of 2020. The baseline phase of the study has collected provisional data on 5385 catheters in 3615 participants, representing 603,506 days of HD CVC exposure.
Conclusions
The REDUCCTION trial systematically measures the use of HD CVCs at a national level in Australia, accurately determines the rate of HD-CRBSI, and tests the effect of a multifaceted, evidence-based intervention upon the rate of HD-CRBSI. These results will have global relevance in nephrology and other specialties commonly using CVCs.
Introduction
Patients receiving hemodialysis (HD) are highly susceptible to health care-associated infections. Most prominent among these infections is HD catheter-related bloodstream infection (HD-CRBSI), which is associated with high mortality and health care costs (1). HD central venous catheters (HD CVCs) are ubiquitous in modern nephrology, used in up to 80% of incident maintenance HD patients, 20% of prevalent HD patients, and universally in patients requiring HD for AKI (2–5).
HD-CRBSI is associated with risks beyond the primary event, such as endocarditis, major organ abscesses, recurrent sepsis, and mortality. A 2013 meta-analysis concluded that the health care cost per episode of all CVC-related bacteremia, including HD-CRBSI, in the USA was $45,814 (95% confidence interval, $30,919–$65,245), constituting 18.9% of the total national cost of health care-associated infections or 1.85 billion dollars per annum (1). These costs exclude the effect on the patient’s quality of life from additional catheters, hospital admissions, and medical procedures.
There have been few systematic attempts to reduce this burden in dialysis services, and clinical practice remains highly variable (6). Many interventions have been studied with the aim of reducing HD-CRBSI, and the nature of this literature has likely contributed to practice variation (6–9). The recent Making Dialysis Safer for Patients Coalition initiative, led by the Centers for Disease Control and Prevention, is testament to the need for standardized approaches to infection prevention in dialysis services, as was the success of the Keystone Michigan Project (10,11). Herein, we review the research that formed the basis of the design and protocol of the REDUcing the burden of dialysis Catheter ComplicaTIOns: a National approach (REDUCCTION) trial, a stepped-wedge cluster randomized clinical trial of a suite of clinical interventions aimed at reducing the rate of HD-CRBSI across Australian renal services.
Challenges in Reducing HD-CRBSI
Reporting and Comparison of Rates of HD-CRBSI
A crucial requirement for the implementation and evaluation of strategies to reduce HD-CRBSI is the ability to accurately measure and report the disease burden. Whereas measurement has been done well in randomized controlled trials and prospective studies, sustainable, system-wide measurement in clinical practice can be difficult. Historically, HD-CRBSI rates have used different definitions and denominators, with the numerator usually being HD-CRBSI events and denominators using variable measures of catheter exposure, making comparisons difficult (12,13). Furthermore, the lack of clinical adjudication of BSI also adds uncertainty around the true rate of HD CVC-related HD-CRBSI. Our survey of Australia/New Zealand (ANZ) renal services found that 84% of services were collecting data on HD-CRBSI rates but only 51% could report a rate (6). The reported HD-CRBSI rates in the literature vary widely, with published rates of 1.1–5.5 episodes per 1000 days of catheter exposure at the time of design of the REDUCCTION trial (14–17). Subsequent studies from the USA and Canada have published much lower rates of between 0.19 and 0.84 per 1000 catheter days, making it difficult for clinicians to ascertain appropriate targets (13,18).
Complex Care
There are many components to catheter care, with breakdown in any element likely to influence the risk of HD-CRBSI. Published research has focused upon the use of prophylactic antimicrobial agents, either applied topically to the catheter exit site, or as solutions to “lock” the catheter lumen after insertion and between dialysis treatments, as well as nonmicrobial agents such as citrate taurolidine and chlorhexidine (8,19,20). Other interventions include catheter dressing types (21), catheter dressing frequency (22), catheters with subcutaneous tunnels (23) and, more recently, antimicrobial catheter lumen caps that form a physical and antimicrobial barrier at the closure of the dialysis catheter (24). A recent review highlighted the complexity of this literature, presenting challenges for clinicians in untangling the evidence to decide upon optimal catheter care (25). In addition, various hospital departments may play a role in managing any single HD CVC, complicating care and making standardization difficult (6).
Evidentiary Limitations
The difficulties posed by the literature examining HD-CRBSI are perhaps best illustrated by the differing and often outdated recommendations from guideline groups. The ANZ guideline, published in 2012, made a single recommendation based upon high-grade (level 1) evidence, and the Canadian guidelines from 2006 made two recommendations with high-level evidence (26,27). The US guidelines published in April 2020 make multiple recommendations around the use, insertion, management, and removal of HD-CVCs, most of which remain on the basis of expert opinion or at best a moderate level of evidence (28,29). The limited recommendations, although reflecting the available literature at the time of guideline production, are in notable contrast to the myriad elements of catheter insertion, management, and care. The guidelines also highlight the gaps in the literature, most notably the paucity of head-to-head studies comparing differing treatments and approaches, such that their relative benefits remain unclear (23).
In contrast, the Centers for Disease Control and Prevention guidelines for the prevention of intravascular catheter-related infections updated in 2017 cover 20 subtopic areas, illustrating the complexity of what most clinicians would consider a relatively routine part of clinical nephrology care (30). The final recommendation, that of using collaborative performance improvement initiatives and multifaceted strategies to improve practice, has been central to the development of the REDUCCTION trial.
Many other elements such as insertion site, showering/bathing, use of sterile techniques at insertion and when accessing the catheter, frequency of dressing changes, and patient factors (such as socioeconomic status, comorbidities, residence characteristics, and patients’ understanding of catheter care) could also influence HD-CRBSI, but are not readily studied. Furthermore, much of the current evidence arises from small studies, many of low quality, testing differing interventions with variable outcome reporting. These characteristics make the distillation of findings into a coherent and implementable practice change at a service level difficult and allow variability in clinical practice to flourish (25,31–33).
Practice Variation
A survey of ANZ renal services demonstrated wide variation in the processes around catheter care, such as prophylactic antibiotic use, exit-site dressings, and catheter insertion personnel (6,34). This showed that prophylactic antibiotics were used at 21% of renal services and eight different combinations of exit-site dressing were in use, with an antibiotic patch being most common (35%) (6).
Reducing such variation has resulted in some success in reducing CVC-related bacteremia. The Keystone intensive care unit project used a suite of interventions to address health care-associated infections, most notably CVC-associated bacteremia, and showed significant reductions in this outcome (11).
The REDUCCTION trial aims to systematically measure the use of HD CVCs for dialysis access at a national level in Australia, to accurately determine the rate of HD-CRBSI and test the effect of a multifaceted, evidence-based intervention upon the rate of HD-CRBSI.
Materials and Methods
Design
REDUCCTION uses a stepped-wedge cluster design (Figure 1) (35), clustering the intervention at the renal-service level, ensuring that all participating services receive the intervention and serve as their own control. The application of such an intervention at the individual-patient level was impractical due to the requirement of having two (or more) systems of HD CVC management within each service and the risk of “contamination” of the intervention practices into the control group. This approach implements the entire suite of interventions at a service level, minimizing contamination, uses the benefits of randomization in the timing of the application of the intervention, and accommodates changes in practice that occur with time. The trial was registered on the Australian New Zealand Clinical Trials Registry on the June 23, 2016 (ACTRN12616000830493).
Figure 1.
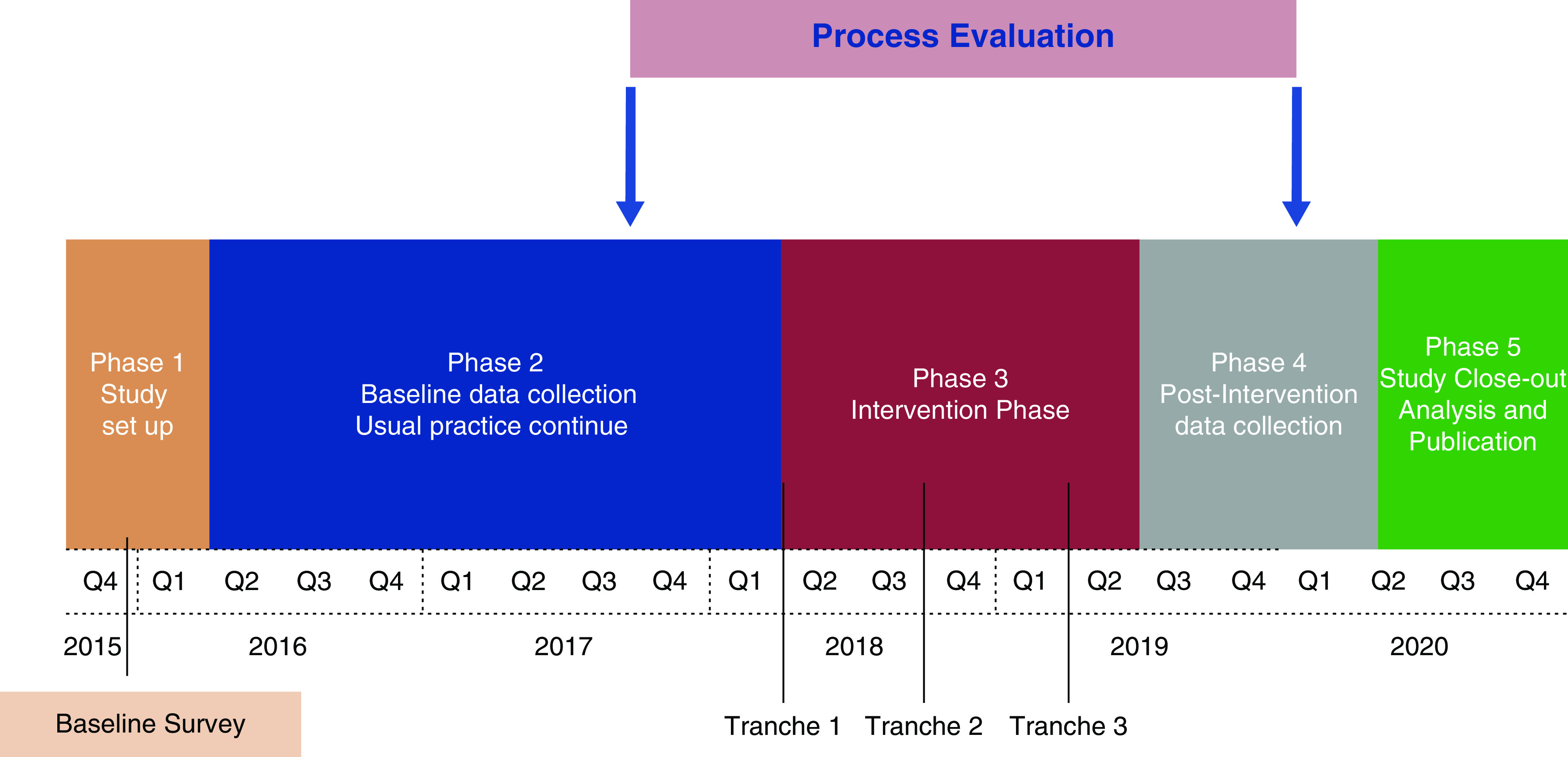
Trial timelines.
Setting
The trial is being conducted at 37 renal services across Australia that, collectively, manage 75% of the Australian prevalent dialysis population (Table 1). Many services oversee multiple dialysis facilities, such that a service is defined as a site, or sites (usually hospital-based in Australia), under the same clinical governance as it pertains to clinical decision making around HD CVC management. Australia has a universal health insurance system, covering the costs of inpatient public hospital care and varying proportions of outpatient health services. Dialysis is largely provided in public hospitals and is covered by the national insurance scheme, and private facilities manage a small proportion of dialysis patients.
Table 1.
Renal services participating in the trial
| Service Name | State | Number of Satellite Services | Urban/Regionala | Consent Model | Hemodialysis Patient Numbers from ANZDATA as on 31/12/2016 |
| Alice Springs | NT | 2 | Regional | Opt-out | 358 |
| Armadale Health Service | WA | 0 | Regional | Opt-out | 53 |
| Austin Health | VIC | 4 | Urban | Waiver | 243 |
| Cairns Hospital | QLD | 5 | Regional | Waiver | 266 |
| Concord Hospital | NSW | 2 | Urban | Waiver | 126 |
| Eastern Health | VIC | 5 | Urban | Opt-out | 179 |
| Fiona Stanley Hospital | WA | 7 | Urban | Opt-out | 469 |
| Flinders Medical Centre | SA | 4 | Urban | Waiver | 218 |
| Geelong Hospital (Barwon Health) | VIC | 4 | Urban | Waiver | 156 |
| Gold Coast Hospital and Health Service | QLD | 2 | Urban | Waiver | 151 |
| John Hunter Hospital | NSW | 5 | Urban | Waiver | 222 |
| Liverpool Hospital | NSW | 5 | Urban | Waiver | 424 |
| Mackay Hospital | QLD | 1 | Regional | Waiver | 55 |
| Mater Hospital, Brisbane | QLD | 2 | Urban | Waiver | 30 |
| Monash Health | VIC | 5 | Urban | Waiver | 507 |
| Sunshine Coast University Hospital and Health Service | QLD | 3 | Urban | Waiver | 128 |
| Nepean Hospital | NSW | 1 | Urban | Waiver | 119 |
| Prince of Wales Hospital | NSW | 1 | Urban | Waiver | 80 |
| Metro South Hospital and Health Service (including Princess Alexandria Hospital, Redlands and Logan Hospital) | QLD | 3 | Urban | Waiver | 328 |
| Rockhampton | QLD | 3 | Regional | Waiver | 89 |
| Royal Adelaide Hospital | SA | 17 | Urban | Waiver | 591 |
| Royal Brisbane Hospital | QLD | 3 | Urban | Waiver | 229 |
| Royal Darwin Hospital | NT | 4 | Urban | Waiver | 287 |
| Royal Hobart Hospital | TAS | 1 | Urban | Waiver | 87 |
| Royal Melbourne Hospital | VIC | 25 | Urban | Waiver | 483 |
| Royal North Shore Hospital | NSW | 2 | Urban | Opt-out | 225 |
| Royal Prince Alfred Hospital | NSW | 1 | Urban | Waiver | 223 |
| Sir Charles Gairdner Hospital | WA | 6 | Urban | Opt-out | 362 |
| St George Hospital | NSW | 1 | Urban | Waiver | 213 |
| St Vincent’s Hospital, Melbourne | VIC | 6 | Urban | Waiver | 225 |
| Tamworth Hospital | NSW | 3 | Rural | Waiver | 74 |
| The Alfred Hospital | VIC | 2 | Urban | Opt-out | 237 |
| The Canberra Hospital | ACT | 8 | Urban | Waiver | 259 |
| Toowoomba Hospital | QLD | 3 | Regional | Waiver | 81 |
| Western Health | VIC | 3 | Urban | Waiver | 231 |
| Western Sydney Local Health District (including Westmead, Auburn and Blacktown Hospitals) | NSW | 0 | Urban | Opt-out | 320 |
| Wollongong Hospital | NSW | 4 | Regional | Waiver | 170 |
ACT, Australian Capital Territory; ANZDATA, NSW, New South Wales; NT, Northern Territory; QLD, Queensland; SA, South Australia; TAS, Tasmania; VIC, Victoria; WA, Western Australia.
Urban was defined as being located in an area with a population of ≥100,000. All other services were categorized as regional/rural.
Participant Inclusion Criteria
All Australian renal services were approached to participate in the trial in 2016, with 37 proceeding to enrollment. Adult patients who have a HD CVC inserted after the start of the trial at a participating renal service are eligible for inclusion in the data collection. Prevalent dialysis catheters inserted before the start of the trial are excluded. Data are collected from the time of HD CVC insertion or the time the HD CVC comes under the care of the renal service, until the HD CVC is removed, the patient is no longer under the care of the renal service, or the end of the trial.
Informed Consent
Ethics approval was obtained according to institutional processes across eight states and territories (Supplemental Table 1). The trial uses two approaches to consent: an “opt-out” approach or a “waiver-of-consent” approach, as decided by local research governance (Table 1). Renal services using the opt-out approach allow patients to opt out of the data collection after provision of a trial information sheet, but the renal service continues to treat such patients as per the service’s participation in the trial. Services with approval to use the “waiver-of-consent” approach are not required to perform any study-related consent activities. Any participant is able to decline participation in the data collection at any point in the trial.
Data Collection Methods
A web-based data collection tool was custom designed for the study and data are entered by each participating service. Renal services can see, in real time, all dialysis catheters currently in use and the total exposure of patients to dialysis catheters over various time periods. Services are able to see their own rates of HD-CRBSI during the baseline phase and, during the trial intervention phase, can also see the HD-CRBSI rate across the entire trial.
The data collection includes baseline patient and catheter characteristics, and classifies catheters by their reason for insertion: initiation of maintenance HD, AKI requiring HD (HD CVC is managed by the renal service), interim HD due to failure of an existing dialysis access, e.g., failure of peritoneal dialysis, or thrombosis of an existing arteriovenous fistula. Data on interventions upon the dialysis catheter (e.g., rewiring or resuturing), surgery for creation or revision of permanent dialysis access, the reasons for catheter removal, and the results of any blood or catheter-tip cultures are also collected. (Supplemental Table 3) In addition, service-level data on existing practices around catheter care and dialysis activity within each renal service at trial initiation, and before implementation, are collected. Services were discouraged from making changes to the processes of care for HD CVCs during the baseline phase of the trial.
Reporting of HD-CRBSI uses a standardized definition (Table 2) and a central blinded adjudication process. All possible HD-CRBSI events are reviewed by two adjudicators (clinicians who are independent from the research team). Disagreement between the two adjudicators requires review from the third adjudicator. If agreement is not reached by the third adjudication, the Trial Management Committee reviews the deidentified event.
Table 2.
Trial outcomes
| Outcome | Definition |
| Primary outcome | |
| Dialysis catheter-related bacteremia per 1000 catheter d | Clinical suspicion of HD-CRBSI and any one of the following: culture of the same organism from both the catheter tip and at least one percutaneous blood culture; culture of the same organism from at least two blood samples (one from a catheter hub and the other from a peripheral vein); or bacteremia in the absence of another source |
| Secondary outcomes | |
| Suspected or possible catheter-related bacteremia | Catheter removal for the reason of suspected infection with negative blood cultures |
| Total suspected and possible bacteremia | Catheter removal for the reason of suspected infection with positive or negative blood cultures |
Data linkage to state-specific hospitalization and death data sets will complete at the end of the intervention phase, after all trial data collection has ceased, allowing analyses of long-term patient outcomes and economic effects of catheter complications.
Interventions and Implementation
The suite of evidence-based interventions, designed by a subcommittee of the REDUCCTION Steering Committee in consultation with the Australasian renal guidelines group (36) and the Australian consumer body (Kidney Health Australia), was endorsed by the Trial Management Committee. The interventions were informed by the most current evidence at the time of design, including the Keystone Project in Michigan intensive care units (15,16) and expert opinion across ANZ, and were finalized in January 2018. The components of the intervention were separated into three time points in catheter use—insertion, maintenance, and removal—and were deemed to be guiding rather than prescriptive (Figure 2).
Figure 2.
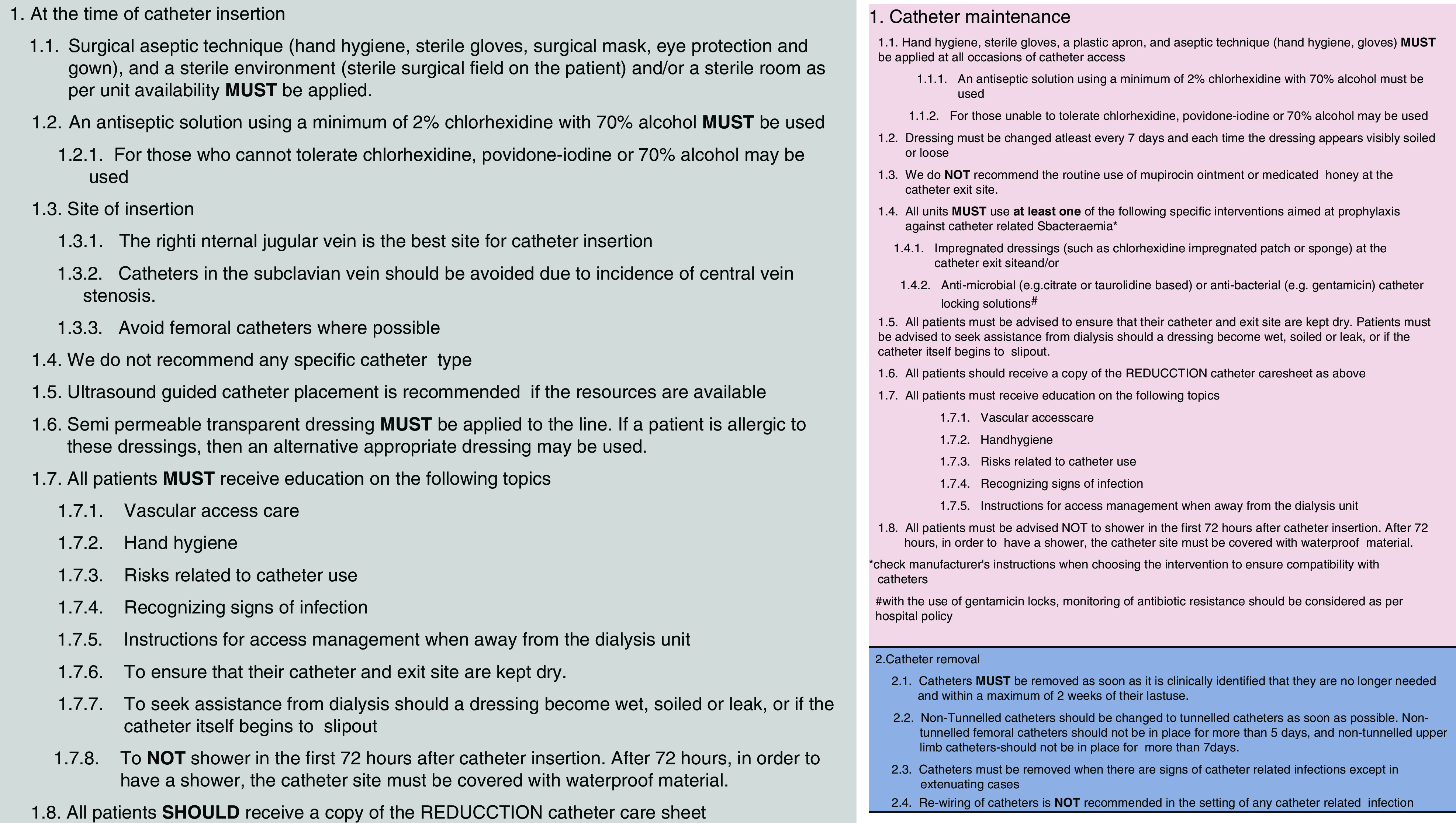
Interventions information sheet. REDUCCTION, REDUcing the burden of dialysis Catheter ComplicaTIOns: a National approach.
All services implemented the entire suite of interventions at their randomly assigned time point, overseen by local physician and nursing leaders. The delivery of the intervention training was in the form of three short videos (one for each component of the intervention) delivered to the service 6 weeks before the implementation of the interventions as well as a preintervention teleconference. Upon intervention implementation, the data collection tool was modified to allow each service to compare the primary outcome, HD-CRBSI, at their service with the study-wide rate, and additional data prompts and fields were added to remind service staff of the intervention components, and allow monitoring. (Supplemental Table 4)
Blinding
Blinding of the trial intervention is not possible, but the timings and natures of the trial interventions were kept confidential and revealed before implementation at each service. Services were asked to keep the intervention confidential until all services had implemented the intervention.
Outcomes
The primary outcome of the trial is the comparative rate of HD-CRBSI per 1000 catheter days of exposure, between the baseline and intervention trial periods (Table 2). A modified version from the Infectious Diseases Society of America definition was used to define HD-CRBSI, as detailed in Table 2 (37). The secondary outcomes are suspected or possible HD-CRBSI; total suspected and possible bacteremia (Table 2).
Statistical Analyses
Covariate-based constrained randomization determined the division of participating services into three balanced tranches and the commencement of the intervention over the trial period (35,38). The allocation sequence was informed by the total number of catheters inserted in the baseline phase of the trial (December 2016–January 2018). One hundred thousand random allocation sequences were generated, and those that achieved a degree of balance of no more than a 10% different from the average number of dialysis catheters in the trial (between 73.2 and 89.8 average catheters per arm) were retained. Of the original sequences generated, 6865 met our balance criteria, and the final randomization allocation was chosen randomly from this subset.
Pilot data collected from a Sydney service (unpublished) allowed us to estimate that an average of 100 dialysis catheter insertions (100 observations) occurred per year at a medium-sized Australian renal service (defined on the basis of number of dialysis patients as per Australia and New Zealand Dialysis and Transplant Registry), with each catheter lasting for a mean of 46 days. The expected rate of baseline HD-CRBSI was 2.5 per 1000 catheter days, derived from a combination of pilot data and the published literature at the time (17). Further considerations included the intercluster correlation coefficient, which we estimated at 0.07 when the rate seen in a large US study from 2014 was 0.03 (9), and the number of intervention points (number of steps) planned was three. The powering was based upon the intervention resulting in a 50% reduction in the risk of catheter-related bacteremia, which was a conservative estimate on the basis of the Key Michigan project outcomes (11,39).
On the basis of these figures, the trial was estimated to have a power of >0.9 to detect a 50% reduction in the bacteremia rate using a proposed sample of 30 renal services after 100 patients per “step” in the stepped-wedge design. This is likely a conservative figure as the intercluster correlation coefficient in other studies has been substantially lower, the number of patients per step is likely to be >100 as the duration of follow-up postintervention is longer for some services, and the trial has included 37 renal services to ensure that a number of 100 patients per step during implementation is met. A formal statistical analysis plan has been derived for the trial and will be published on a preprint server (https://osf.io/preprints/).
Additional Analyses
There are two subgroup analyses of the primary outcome planned: the first examining differences in the primary outcome on the basis of renal service size, and the second on the basis of whether renal services were using either an impregnated dressing or an antimicrobial catheter lock, as per the Kidney Health Australia-Caring for Australasians with Renal Impairment guidelines (27), at baseline. A prospective process evaluation is being conducted during the baseline and intervention phases of the trial to better understand the factors that influence the implementation of evidence-based practices around dialysis catheter management (40).
In addition, this uniquely large prospective cohort of patients receiving dialysis catheters will allow future exploration of the effects of a variety of factors, including renal-service, patient, clinical, and HD CVC characteristics upon important patient outcomes such as HD-CRBSI, hospitalization, and mortality. These analyses will use trial data and linkage to administrative (hospitalization and death) data sets, allowing assessments of the health care costs of catheter practice.
Key Limitations
A limitation of the trial and its design is that the completeness of data collection is not known, and there is a risk that this could change between the baseline and intervention phases. Potentially, the risk of the primary outcome across services may also have varied between the two study phases, but the absence of tools to guide clinicians in such patient selection makes this unlikely. Most importantly, the Hawthorne effect may affect the trial outcomes: the phenomenon whereby individuals change their behavior as a result of their knowledge of being observed (41). To mitigate this, services are discouraged from changing to their clinical practices around catheter care during the trial, beyond those changes arising from the trial intervention. The risk of under-reporting of HD-CRBSI remains, but is mitigated by the requirement to centrally report all possible infectious events and the central adjudication of these events.
Results
Participant Characteristics
Data collection commenced on the December 20, 2016 and is ongoing. A provisional extract from the trial data set (prior to data lock), encompassing the intervention phase at all services, includes data on 5385 HD CVCs in 3615 participants, representing 603,506 days of HD CVC exposure.
The average age of the participants was 63.0 years [interquartile range (IQR) 50–73.0 years], 60.1% (n=2174) were men, and 43.7% (n=1578) had diabetes (Table 3). The majority of HD CVCs inserted were tunneled catheters with a median catheter duration of 90.0 days (IQR 28–207 days). Nontunneled HD CVCs had a median catheter duration of 6 days (IQR 3–8 days).
Table 3.
Participant characteristics of all participants with data entered during the baseline phase
| Patient Characteristics | n=3615 (%) |
| Men | 2174 (60.1) |
| Age, yr, median (IQR) | 63.0 (50–73) |
| Ethnicity/ancestry | |
| Asian (including Chinese, Malay, Filipino, Vietnamese, and Indonesian) | 292 (8.1) |
| Indigenous Australian (including Aboriginal and Torres Strait Islander) | 350 (9.7) |
| White | 2331 (64.5) |
| Māori | 31 (0.9) |
| Pacific Islander (including Tongan, Samoan and Cook Islander) | 91 (2.5) |
| Other | 112 (3.1) |
| Medical records did not indicate ethnicity/ancestry | 408 (11.3) |
| State of enrolment | |
| ACT | 96 (2.7) |
| NSW | 1232 (34.1) |
| QLD | 877 (24.3) |
| NT | 194 (5.4) |
| SA | 245 (6.8) |
| VIC | 758 (21.1) |
| WA | 165 (4.6) |
| TAS | 48 (1.3) |
| Location of enrolling renal service | |
| Urban | 3111 (86.1) |
| Regional/rural | 504 (13.9) |
| Diabetes mellitus | |
| Yes, diet controlled | 309 (8.6%) |
| Yes, medication controlled | 1268 (35.1%) |
| Immunosuppressant use | 477 (13.2%) |
ACT, Australian Capital Territory; NSW, New South Wales; NT, Northern Territory; QLD, Queensland; SA, South Australia; TAS, Tasmania; VIC, Victoria; WA, Western Australia.
The major indications for HD CVC insertion were for AKI (n=1896, 35.0%) or for initiation of maintenance HD without permanent access (n=1703, 31.6%) (Table 4). The majority of tunneled catheters were inserted by interventional radiology services (n=2699; 67%), whereas the nontunneled catheters were inserted predominantly by intensive care units (n=753; 55.8%). The internal jugular vein was the most popular site for HD CVC insertion [total 4619; tunneled=3780 (81.8%); nontunneled=838 (18.1%)], with femoral being the second most popular site [total 577; tunneled=83 (14.4%); nontunneled=494 (85.6%)]. REDUCCTION is a large stepped-wedge cluster randomized trial, which systematically measures the use of dialysis catheters across 37 Australian renal services in real time. It will study the effect of a standardized suite of evidence-based interventions around catheter care upon the rate of HD-CRBSI at the service level and, by virtue of its scale and robust design, will provide novel insights into dialysis catheter care and its effects upon patients.
Table 4.
Reasons for catheter insertion
| Reason for CVC Insertion | n=5385 (%) |
| AKI | 1896 (35.0%) |
| Initiation of maintenance dialysis with no functioning access | 1703 (31.6%) |
| Transfer from peritoneal dialysis | 632 (11.7%) |
| AVF/AVG problems | 706 (13.1%) |
| Other | 446 (8.3%) |
AVF, arteriovenous fistula; AVG, arteriovenous graft.
Disclosures
N. Gray reports personal fees from Baxter Healthcare and nonfinancial support from Amgen Australia outside the submitted work. M. Gallagher reports grants from Australian National Health and Medical Research Council, nonfinancial support from Multiple partner hospitals, grants from Victorian Department of Health, and grants from Queensland Department of Health during the conduct of the study; the George Institute and its affiliated entities work with numerous health and pharmaceutical companies in the design, implementation, and analyses of clinical research and clinical trials. It is possible that some of these companies have products relevant to the clinical space covered in this analysis, but Dr. Gallagher is not aware of any possible conflicts arising from this work. All remaining authors have nothing to disclose.
Funding
The REDUcing the burden of dialysis Catheter ComplicaTIOns: a National approach (REDUCCTION) trial is supported by National Health and Medical Research Council (partnership grant APP1103241), Department of Health Victoria, and Queensland Health. The Australia and New Zealand Dialysis and Transplant Registry, Kidney Health Australia, Kidney Health Australia–Caring for Australasians with Renal Impairment, Alice Springs Hospital, Royal Darwin Hospital, Royal Adelaide Hospital, Austin Hospital, Alfred Hospital, Eastern health, Monash Medical Centre, Royal Melbourne Hospital, Western Health, Royal Hobart Hospital, Cairns Hospital, Princess Alexandria Hospital, Royal Brisbane Hospital, Sunshine Coast Hospital and Health Service, Toowoomba Hospital, Australian Capital Territory/New South Wales Southern Highlands Local District Renal Network, Concord Repatriation General Hospital, Royal Prince Alfred Hospital, Liverpool Hospital, Nepean Hospital, and the Western Sydney Local Health District provided in-kind support. S. Kotwal is supported by the Department of Health, Australian Government via Medical Research Future Fund Next-Generation Translating Research into Practice Fellowship (MRF1150335).
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0001132020/-/DCSupplemental.
List of REDUCCTION Partnership Project contributors. Download Supplemental Appendix, PDF file, 238 KB (154.4KB, pdf) Supplemental Appendix, PDF file, 155 KB (347KB, pdf)
Ethics committee and services covered. Download Supplemental Table 1, PDF file, 347 KB (347KB, pdf)
Allocation schedule. Download Supplemental Table 2, PDF file, 347 KB (347KB, pdf)
Data collected in the trial/case report form. Download Supplemental Table 3, PDF file, 347 KB (347KB, pdf)
New questions added in the intervention phase. Download Supplemental Table 4, PDF file, 347 KB (347KB, pdf)
Author Contributions
M. Gallagher, N. Gray, S. Kotwal, S. McDonald, K. Polkinghorne, and G. Talaulikar conceptualized the study; S. Kotwal was responsible for formal analysis, validation, and drafting the manuscript; M. Gallagher, S. Jan, S. Kotwal, S. McDonald, K. Polkinghorne, and G. Talaulikar were responsible for methodology; S. Coggan, M. Gallagher, and S. Kotwal were responsible for project administration; A. Cass, M. Gallagher, N. Gray, S. Jan, S. McDonald, K. Polkinghorne, and G. Talaulikar were responsible for funding acquisition; M. Gallagher was responses for resources and supervision; and all authors reviewed and edited the manuscript.
References
- 1.Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, Keohane C, Denham CR, Bates DW: Health care-associated infections: A meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med 173: 2039–2046, 2013 [DOI] [PubMed] [Google Scholar]
- 2.United States Renal Data System : 2018 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2018 [Google Scholar]
- 3.Byrne CCF, Castledine C, Davenport A, Dawnay A, Fraser S, Maxwell H, Medcalf JF, Wilkie M, Williams AJ: UK Renal Registry - 20th Annual Report of the Renal Association, Bristol, UK, UK Renal Registry, 2017 [Google Scholar]
- 4.Robinson BM, Akizawa T, Jager KJ, Kerr PG, Saran R, Pisoni RL: Factors affecting outcomes in patients reaching end-stage kidney disease worldwide: Differences in access to renal replacement therapy, modality use, and haemodialysis practices. Lancet 388: 294–306, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Registry ANZDATA: 41st Report, Haemodialysis. Australia and New Zealand Dialysis and Transplant Registry. 2018, Haemodialysis. Australia and New Zealand Dialysis and Transplant Registry, 2018. Available at: http://www.anzdata.org.au. Accessed May 10, 2020 [Google Scholar]
- 6.Smyth B, Kotwal S, Gallagher M, Gray NA, Polkinghorne K; REDUCCTION Partnership Project : Dialysis catheter management practices in Australia and New Zealand. Nephrology (Carlton) 24: 827–834, 2019 [DOI] [PubMed] [Google Scholar]
- 7.Aslam S, Vaida F, Ritter M, Mehta RL: Systematic review and meta-analysis on management of hemodialysis catheter-related bacteremia. J Am Soc Nephrol 25: 2927–2941, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaffer Y, Selby NM, Taal MW, Fluck RJ, McIntyre CW: A meta-analysis of hemodialysis catheter locking solutions in the prevention of catheter-related infection. Am J Kidney Dis 51: 233–241, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Rosenblum A, Wang W, Ball LK, Latham C, Maddux FW, Lacson E Jr.: Hemodialysis catheter care strategies: A cluster-randomized quality improvement initiative. Am J Kidney Dis 63: 259–267, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Patel PR, Brinsley-Rainisch K: The making dialysis safer for patients coalition: A new partnership to prevent hemodialysis-related infections. Clin J Am Soc Nephrol 13: 175–181, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, Sexton B, Hyzy R, Welsh R, Roth G, Bander J, Kepros J, Goeschel C: An intervention to decrease catheter-related bloodstream infections in the ICU [published correction appears in N Engl J Med 356: 2660, 2007]. N Engl J Med 355: 2725–2732, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Klevens RM, Edwards JR, Andrus ML, Peterson KD, Dudeck MA, Horan TC; NHSN Participants in Outpatient Dialysis Surveillance : Dialysis surveillance report: National healthcare safety Network (NHSN)-data summary for 2006. Semin Dial 21: 24–28, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Thompson S, Wiebe N, Klarenbach S, Pelletier R, Hemmelgarn BR, Gill JS, Manns BJ, Tonelli M; Alberta Kidney Disease Network : Catheter-related blood stream infections in hemodialysis patients: A prospective cohort study. BMC Nephrol 18: 357, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen DB, Shugart A, Lines C, Shah AB, Edwards J, Pollock D, Sievert D, Patel PR: National healthcare safety Network (NHSN) dialysis event surveillance report for 2014. Clin J Am Soc Nephrol 12: 1139–1146, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aitken E, Thomson P, Bainbridge L, Kasthuri R, Mohr B, Kingsmore D: A randomized controlled trial and cost-effectiveness analysis of early cannulation arteriovenous grafts versus tunneled central venous catheters in patients requiring urgent vascular access for hemodialysis. J Vasc Surg 65: 766–774, 2017 [DOI] [PubMed] [Google Scholar]
- 16.Al-Solaiman Y, Estrada E, Allon M: The spectrum of infections in catheter-dependent hemodialysis patients. Clin J Am Soc Nephrol 6: 2247–2252, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller LM, Clark E, Dipchand C, Hiremath S, Kappel J, Kiaii M, Lok C, Luscombe R, Moist L, Oliver M, MacRae J; Canadian Society of Nephrology Vascular Access Work Group : Hemodialysis tunneled catheter-related infections. Can J Kidney Health Dis 3: 1–11, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang HH, Cortés-Penfield NW, Mandayam S, Niu J, Atmar RL, Wu E, Chen D, Zamani R, Shah MK: Dialysis catheter-related bloodstream infections in patients receiving hemodialysis on an emergency-only basis: A retrospective cohort analysis. Clin Infect Dis 68: 1011–1016, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landry DL, Braden GL, Gobeille SL, Haessler SD, Vaidya CK, Sweet SJ: Emergence of gentamicin-resistant bacteremia in hemodialysis patients receiving gentamicin lock catheter prophylaxis. Clin J Am Soc Nephrol 5: 1799–1804, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arechabala MC, Catoni MI, Claro JC, Rojas NP, Rubio ME, Calvo MA, Letelier LM: Antimicrobial lock solutions for preventing catheter-related infections in haemodialysis. Cochrane Database Syst Rev 4: CD010597, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCann M, Moore ZE: Interventions for preventing infectious complications in haemodialysis patients with central venous catheters. Cochrane Database Syst Rev 1: CD006894, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Kosa SD, Lok CE: The economics of hemodialysis catheter-related infection prophylaxis. Semin Dial 26: 482–493, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Atapour A, Shahidi S, Sairafian S: Does tunneling the temporary vascular access extend its lifetime? J Res Med Sci 11: 41–47, 2006 [Google Scholar]
- 24.Brunelli SM, Van Wyck DB, Njord L, Ziebol RJ, Lynch LE, Killion DP: Cluster-randomized trial of devices to prevent catheter-related bloodstream infection. J Am Soc Nephrol 29: 1336–1343, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher M, Golestaneh L, Allon M, Abreo K, Mokrzycki MH: Prevention of bloodstream infections in patients undergoing hemodialysis. Clin J Am Soc Nephrol 15: 132–151, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jindal K, Chan CT, Deziel C, Hirsch D, Soroka SD, Tonelli M, Culleton BF: CHAPTER 4: Vascular access. J Am Soc Nephrol 17: S16–S23, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Polkinghorne KR, Chin GK, MacGinley RJ, Owen AR, Russell C, Talaulikar GS, Vale E, Lopez-Vargas PA: KHA-CARI guideline: Vascular access - central venous catheters, arteriovenous fistulae and arteriovenous grafts. Nephrology (Carlton) 18: 701–705, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Lok CE, Huber TS, Lee T, Shenoy S, Yevzlin AS, Abreo K, Allon M, Asif A, Astor BC, Glickman MH, Graham J, Moist LM, Rajan DK, Roberts C, Vachharajani TJ, Valentini RP; National Kidney Foundation : KDOQI clinical practice guideline for vascular access: 2019 update. Am J Kidney Dis 75[Suppl 2]: S1–S164, 2020 [DOI] [PubMed] [Google Scholar]
- 29.Nkf KDOQI: Clinical practice guidelines for vascular adequacy, update. 2006. Available at: http://kidneyfoundation.cachefly.net/professionals/KDOQI/guideline_upHD_PD_VA/index.htm. Accessed January 30, 2020
- 30.O’Grady NP, Alexander M, Burns L, Dellinger P, Garland J, Heard S, Lipsett P, Masur H, Mermel L, Pearson M, Raad I, Randolph A, Rupp M, Saint S; and the Healthcare Infection Control Practices Advisory Committee : Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis 52: e162–e193, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lok CE, Mokrzycki MH: Prevention and management of catheter-related infection in hemodialysis patients. Kidney Int 79: 587–598, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Allon M: Vascular access for hemodialysis patients: New data should guide decision making. Clin J Am Soc Nephrol 14: 954–961, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niyyar VD: Catheter dysfunction and lock solutions: Are we there yet? Nephrol Dial Transplant 34: 1626–1628, 2019 [DOI] [PubMed] [Google Scholar]
- 34.Smyth B, Kotwal S, Gallagher M, Gray NA, Polkinghorne KR: Arteriovenous access practices in Australian and New Zealand dialysis units. J Vasc Access 20: 740–745, 2019 [DOI] [PubMed] [Google Scholar]
- 35.Hussey MA, Hughes JP: Design and analysis of stepped wedge cluster randomized trials. Contemp Clin Trials 28: 182–191, 2007 [DOI] [PubMed] [Google Scholar]
- 36.KHA-CARI : Kidney Health Australia-Caring for Australasians with renal impairment. 2020. Available at: http://www.cari.org.au/. Accessed February 14, 2020
- 37.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK: Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America [published correction appears in Clin Infect Dis 50: 1079, 2010]. Clin Infect Dis 49: 1–45, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moulton LH: Covariate-based constrained randomization of group-randomized trials. Clin Trials 1: 297–305, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Nuckols TK, Keeler E, Morton SC, Anderson L, Doyle B, Booth M, Shanman R, Grein J, Shekelle P: Economic evaluation of quality improvement interventions for bloodstream infections related to central catheters: A systematic review [published correction appears in JAMA Intern Med 176: 1884, 2016]. JAMA Intern Med 176: 1843–1854, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Craswell A, Massey D, Wallis M, Sriram D, Gray NA, Kotwal S; REDUCCTION Investigators : Current practice in dialysis central venous catheter management: Multi-disciplinary renal team perspectives. Nephrology (Carlton) 25: 406–412, 2020 [DOI] [PubMed] [Google Scholar]
- 41.Landsberger H: HAWTHORNE REVISITED, Ithaca, New York, The New York State School of Industrial and Labor Relations, 1958 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of REDUCCTION Partnership Project contributors. Download Supplemental Appendix, PDF file, 238 KB (154.4KB, pdf) Supplemental Appendix, PDF file, 155 KB (347KB, pdf)
Ethics committee and services covered. Download Supplemental Table 1, PDF file, 347 KB (347KB, pdf)
Allocation schedule. Download Supplemental Table 2, PDF file, 347 KB (347KB, pdf)
Data collected in the trial/case report form. Download Supplemental Table 3, PDF file, 347 KB (347KB, pdf)
New questions added in the intervention phase. Download Supplemental Table 4, PDF file, 347 KB (347KB, pdf)