Objectives:
To evaluate safety and immunogenicity of V114 [15-valent pneumococcal conjugate vaccine (PCV) containing serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F], followed by 23-valent pneumococcal polysaccharide vaccine (PPSV23) 8 weeks later, in adults living with HIV.
Design:
In this phase 3 study (V114-018; NCT03480802), pneumococcal vaccine-naive adults with HIV (CD4+ cell count ≥50 cells/μl, plasma HIV RNA <50 000 copies/ml, receiving antiretroviral therapy) were randomized 1 : 1 to receive one dose of V114 or licensed 13-valent PCV (PCV13) on day 1; participants received PPSV23 at week 8.
Methods:
Adverse events and serotype-specific opsonophagocytic activity (OPA) and immunoglobulin G (IgG) antibodies were evaluated after each vaccination.
Results:
Of 302 participants enrolled, 292 (96.7%) completed the study. Proportions of participants experiencing at least one adverse event were 73.0 and 62.7% in the V114 and PCV13 groups following PCV and 60.7 and 71.6% following PPSV23. Most solicited adverse events were of mild or moderate severity and short duration. OPA geometric mean titers (GMTs) and IgG geometric mean concentrations (GMCs) were generally comparable between groups for shared serotypes at day 30 and maintained at week 12. OPA and IgG responses for additional serotypes in V114 (22F, 33F) were higher following V114 than PCV13 at day 30 but comparable at week 12, 30 days post-PPSV23.
Conclusion:
In pneumococcal vaccine-naive adults living with HIV, V114 was well tolerated and induced immune responses for all 15 pneumococcal serotypes. V114 can be followed by PPSV23 8 weeks later to broaden serotype coverage.
Keywords: adult, clinical trial, HIV, pneumococcal infections, pneumococcal vaccines
Introduction
People with HIV are at an increased risk of pneumococcal disease, with an incidence of invasive pneumococcal disease (IPD) 30 times higher than uninfected individuals (300 vs. 10 per 100 000 person-years) [1,2]. Risk factors for increased incidence of IPD in individuals living with HIV include CD4+ cell counts less than 500 cells/μl and HIV RNA loads greater than 50 000 copies/ml [2].
Pneumococcal conjugate vaccines (PCVs) protect against recurrent IPD in adults living with HIV [3] and are recommended as sequential vaccination followed at least 8 weeks later by PPSV23 [4,5]. This strategy induces a T-cell-dependent response [6] and provides broad protection against pneumococcal disease caused by serotypes included in the two vaccines.
Since the introduction of PCVs into national infant immunization programs, pneumococcal disease caused by serotypes not included in licensed vaccines has become a public health concern [7,8]. Of serotypes not included in currently licensed PCVs, serotype 22F is among the most common cause of IPD in the United States, Europe, and Canada in recent years [7–11], and serotype 33F, which is associated with multidrug antibiotic resistance, has also been frequently associated with IPD in young children in the United States and Canada. V114 is a 15-valent vaccine containing the 13 serotypes included in the licensed 13-valent PCV (PCV13) as well as serotypes 22F and 33F; it is being developed to provide broader serotype coverage while maintaining strong immune responses to serotypes included in licensed PCVs. This study evaluated immunogenicity and safety of V114 followed by PPSV23 8 weeks later in pneumococcal vaccine-naive adults living with HIV.
Methods
Study design
This was a phase 3, multicenter, randomized, double-blind, active comparator-controlled, descriptive study to evaluate safety, tolerability, and immunogenicity of V114 or PCV13, followed by PPSV23 8 weeks later, in pneumococcal vaccine-naive adults living with HIV (Protocol V114-018; NCT03480802; EudraCT 2017-001909-32). The study was conducted from July 2018 to January 2020 at 13 sites worldwide (Supplementary Table 16).
The study used central randomization and assignment to vaccination group that was implemented using an interactive response technology system. Vaccines were dispensed and administered by unblinded study personnel who were not involved in any subsequent participant assessments. V114 and PCV13 were administered in a blinded fashion, and PPSV23 was administered open label. The study was conducted in accordance with the principles of Good Clinical Practice and was approved by appropriate institutional review boards. Written informed consent was obtained from each participant prior to any study procedure.
Participants were randomized in a 1 : 1 ratio to receive a single dose of either V114 or PCV13 on day 1 followed by PPSV23 at week 8. PCV13 was included to serve as a reference for the expected safety and immunogenicity profile of PCVs in this population. Randomization was stratified by CD4+ cell count (≥50 to <200, ≥200 to <500, and ≥500 cells/μl), with at least 50% of participants to be enrolled into the at least 200 to less than 500 cells/μl stratum. Blood samples were obtained for immunogenicity assays and/or CD4+ cell count and HIV RNA testing prevaccination on day 1, on day 30, and at week 12.
Participants
Pneumococcal vaccine-naive adults at least 18 years of age living with HIV, with CD4+ cell count at least 50 cells/μl and plasma HIV RNA less than 50 000 copies/ml at screening, were eligible for the study. All participants were receiving stable combination antiretroviral treatment (ART) for at least 6 weeks prior to enrollment. Key exclusion criteria included: history of IPD or other culture-positive pneumococcal disease within the previous 3 years, opportunistic infections within the previous 12 months, noninfectious AIDS-related illness and other immunocompromising conditions or therapies.
Vaccines and administration
V114 (VAXNEUVANCE; Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, USA) is a 15-valent PCV containing capsular polysaccharide from serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 23F, 22F, and 33F adjuvanted with aluminium phosphate [12–14]. PCV13 (Prevnar 13; Wyeth LLC, marketed by Pfizer, New York, New York, USA) is a 13-valent PCV containing capsular polysaccharide from serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F adjuvanted with aluminium phosphate [15]. PPSV23 (PNEUMOVAX23; Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, USA) is an unadjuvanted 23-valent pneumococcal polysaccharide vaccine containing capsular polysaccharide from serotypes 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, and 33F [16].
All vaccines were supplied in prefilled syringes and stored at 2–8 °C. They were all administered intramuscularly as 0.5 ml doses.
Safety assessments
Participants recorded postvaccination complaints using an electronic Vaccination Report Card (eVRC), which were subsequently assessed by study investigators to determine if they met protocol-defined adverse event criteria. Injection-site reactions (injection-site pain, injection-site swelling, and injection-site erythema) occurring from days 1–5 postvaccination and systemic adverse events (muscle pain/myalgia, joint pain/arthralgia, headache, and fatigue) occurring from days 1 to 14 postvaccination were solicited.
Daily body temperature was measured on days 1–5 postvaccination. Participants were also followed for nonsolicited adverse events from days 1 to 14 postvaccination. Serious adverse events (SAEs) and deaths, regardless of whether the events were considered to be vaccine-related by study investigators, were collected from time of signed consent through to end of study (approximately 6 months after the first vaccination).
All solicited and nonsolicited events were assessed by investigators for severity using adapted US Food and Drug Administration guidance on toxicity grading for vaccine trials [17].
All injection-site adverse events were considered to be vaccine-related. For systemic adverse events, relatedness to study vaccine was assessed by study investigators. The Medical Dictionary for Regulatory Activities version 22.1 was used in the reporting of this study.
Immunogenicity assessments
Serum samples were drawn prevaccination with PCV on day 1, 30 days postvaccination with PCV (day 30), and 30 days following vaccination with PPSV23 (week 12) to assess immune responses. Functional antibodies were measured using serotype-specific opsonophagocytic killing activity using a validated microcolony multiplexed opsonophagocytic assay (mMOPA) [18]. Serotype-specific pneumococcal capsular polysaccharide immunoglobulin G (IgG) antibodies were evaluated using a validated electrochemiluminescence (ECL) assay [19].
Study endpoints and statistical methods
Determination of study sample size
This descriptive study aimed to randomize approximately 300 participants overall, with 150 per vaccination group. The sample size was selected to achieve a reasonably sized safety database in this population of pneumococcal vaccine-naive adults living with HIV. It was assumed that 135 participants per vaccination group would be evaluable for immunogenicity analyses at day 30 (based on a 90% evaluability rate).
Analysis populations
Safety analyses were conducted on the All-Participants-as-Treated (APaT) population, consisting of all randomized participants who received relevant study vaccine for timepoint of interest.
The per-protocol population was the primary population used for analysis of immunogenicity data, consisting of all randomized participants without protocol deviations that could substantially affect results of immunogenicity endpoints.
Primary and secondary safety endpoints and statistical methods
Primary safety endpoints were assessed following administration of V114 or PCV13, and secondary safety endpoints were assessed following administration of PPSV23. Safety endpoints included proportions of participants with: solicited injection-site adverse events from days 1 to 5 postvaccination, solicited systemic adverse events from days 1 to 14 postvaccination, and any adverse event or SAE, including vaccine-related SAEs. Confidence intervals (CIs) were provided for each treatment group for proportions of participants with adverse events using the method proposed by Clopper and Pearson [20], without multiplicity adjustments.
Primary and secondary immunogenicity endpoints and statistical methods
Primary immunogenicity objective was to evaluate the serotype-specific opsonophagocytic activity (OPA) geometric mean titers (GMTs) for all 15 serotypes included in V114 at day 30 within each vaccination group separately. Secondary immunogenicity endpoints included observed serotype-specific IgG geometric mean concentrations (GMCs), OPA GMTs, and IgG GMCs at week 12, as well as geometric mean fold rises (GMFRs), and proportions of participants with at least a four-fold rise in OPA and IgG responses between day 1 and day 30 (prevaccination and postvaccination with PCV) and between day 1 and week 12 (prior to any vaccination and 30 days postvaccination with PPSV23) within each vaccination group separately. Within-group CIs were computed without multiplicity adjustment based on t distribution and the method proposed by Clopper and Pearson [20] for continuous and dichotomous endpoints, respectively. For comparison of OPA GMTs and IgG GMCs between vaccination groups, OPA GMTs/IgG GMCs and OPA GMT ratios/IgG GMC ratios (with corresponding 95% CIs) were estimated using serotype-specific constrained longitudinal data analysis models [21].
All analyses were performed using SAS© software, version 9.4 (SAS Institute Inc., Cary, North Carolina, USA).
Results
Study population
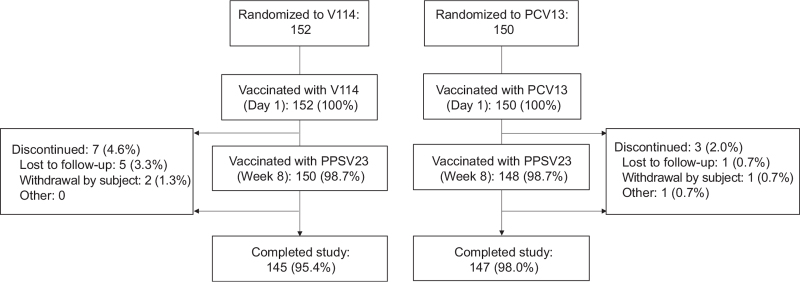
Of 302 randomized participants, all received either V114 or PCV13 and were included in the APaT population (Fig. 1). In total, 298 (98.7%) received PPSV23 and 292 (96.7%) completed the study. The number of study discontinuations and reasons for study discontinuation were similar across vaccination groups.
Fig. 1.
Participant disposition.
PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine; V114, 15-valent pneumococcal conjugate vaccine.
Demographic and baseline characteristics were generally comparable between vaccination groups (Table 1). The majority of participants (78.8%) were men. Approximately half of participants (50.3%) had a CD4+ cell count at least 200 to less than 500 cells/μl and only two participants in each vaccine group had a CD4+ cell count less than 200 cells/μl. The majority (>75%) of study participants had undetectable blood HIV RNA (<20 copies/ml).
Table 1.
Participant demographics and baseline characteristics.
| Characteristic | V114 (n = 152) | PCV13 (n = 150) |
| Age, mean (range) (years) | 42.4 (23–74) | 41.3 (21–69) |
| Sex [n (%)] | ||
| Male | 120 (78.9) | 118 (78.7) |
| Female | 32 (21.1) | 32 (21.3) |
| Race [n (%)] | ||
| Black or African American | 51 (33.6) | 43 (28.7) |
| White | 41 (27.0) | 48 (32.0) |
| Multiple | 36 (23.7) | 26 (17.3) |
| Asian | 24 (15.8) | 30 (20.0) |
| Native Hawaiian or other Pacific Islander | 0 (0.0) | 2 (1.3) |
| American Indian or Alaska native | 0 (0.0) | 1 (0.7) |
| Ethnicity [n (%)] | ||
| Not Hispanic or Latino | 102 (67.1) | 104 (69.3) |
| Hispanic or Latino | 49 (32.2) | 45 (30.0) |
| Not reported | 1 (0.7) | 1 (0.7) |
| Antiretroviral therapy [n (%)] | 152 (100) | 150 (100) |
| CD4+ T-cell count [n (%)] | ||
| ≥500 cells/μl | 74 (48.7) | 72 (48.0) |
| ≥200 to <500 cells/μl | 76 (50.0) | 76 (50.7) |
| ≥50 to <200 cells/μl | 2 (1.3) | 2 (1.3) |
| HIV RNA [n (%)] | ||
| <20 copies/ml | 123 (80.9) | 114 (76.0) |
| ≥20 copies/mla | 29 (19.1) | 36 (24.0) |
CD, cluster of differentiation; PCV13, 13-valent pneumococcal conjugate vaccine; RNA, ribonucleic acid; V114, 15-valent pneumococcal conjugate vaccine.
Maximum less than 50 000 copies/ml, per protocol.
Safety following vaccination with V114 or PCV13
Both V114 and PCV13 were generally well tolerated. Overall, 73 and 62.7% of participants in the V114 and PCV13 groups, respectively, experienced at least one adverse event. The proportion of participants experiencing solicited adverse events was generally higher in the V114 group than the PCV13 group, although no statistical comparisons were made (Table 2). Overall, 66.4% in the V114 group and 58.7% in the PCV13 group experienced an adverse event deemed to be related to study vaccine (Table 2).
Table 2.
Adverse events after vaccination.
| Adverse events after vaccination with V114 or PCV13 | Adverse events after vaccination with PPSV23 | |||||||
| V114 (n = 152) | PCV13 (n = 150) | V114 (n = 150) | PCV13 (n = 148) | |||||
| n (%) | 95% CI | n (%) | 95% CI | n (%) | 95% CI | n (%) | 95% CI | |
| Any AE | 111 (73.0) | 65.2–79.9 | 94 (62.7) | 54.4–70.4 | 91 (60.7) | 52.4–68.5 | 106 (71.6) | 63.6–78.7 |
| Injection site | 97 (63.8) | 82 (54.7) | 83 (55.3) | 96 (64.9) | ||||
| Systemic | 65 (42.8) | 54 (36.0) | 49 (32.7) | 51 (34.5) | ||||
| Any vaccine-related AE | 101 (66.4) | 58.3–73.9 | 88 (58.7) | 50.3–66.6 | 87 (58.0) | 49.7–66.0 | 99 (66.9) | 58.7–74.4 |
| Injection site | 97 (63.8) | 82 (54.7) | 83 (55.3) | 97 (65.5) | ||||
| Systemic | 40 (26.3) | 36 (24.0) | 34 (22.7) | 36 (24.3) | ||||
| Any SAE | 3 (2.0) | 0.4–5.7 | 0 (0.0) | 0.0–2.4 | 2 (1.3) | 0.2–4.7 | 6 (4.1) | 1.5–8.6 |
| Any vaccine-related SAE | 0 (0.0) | 0.0–2.4 | 0 (0.0) | 0.0–2.4 | 0 (0.0) | 0.0–2.4 | 0 (0.0) | 0.0–2.5 |
| Death | 0 (0.0) | 0.0–2.4 | 0 (0.0) | 0.0–2.4 | 0 (0.0) | 0.0–2.4 | 0 (0.0) | 0.0–2.5 |
| Solicited injection-site AEs (days 1–5) | ||||||||
| Injection-site pain | 87 (57.2) | 49.0–65.2 | 77 (51.3) | 43.0–59.6 | 80 (53.3) | 45.0–61.5 | 91 (61.5) | 53.1–69.4 |
| Injection-site swelling | 18 (11.8) | 7.2–18.1 | 6 (4.0) | 1.5–8.5 | 30 (20.0) | 13.9–27.3 | 43 (29.1) | 21.9–37.1 |
| Injection-site erythema | 7 (4.6) | 1.9–9.3 | 5 (3.3) | 1.1–7.6 | 15 (10.0) | 5.7–16.0 | 18 (12.2) | 7.4–18.5 |
| Solicited systemic AEs (days 1–14) | ||||||||
| Fatigue | 31 (20.4) | 14.3–27.7 | 20 (13.3) | 8.3–19.8 | 19 (12.7) | 7.8–19.1 | 16 (10.8) | 6.3–17.0 |
| Headache | 20 (13.2) | 8.2–19.6 | 14 (9.3) | 5.2–15.2 | 17 (11.3) | 6.7–17.5 | 18 (12.2) | 7.4–18.5 |
| Myalgia | 19 (12.5) | 7.7–18.8 | 14 (9.3) | 5.2–15.2 | 13 (8.7) | 4.7–14.4 | 13 (8.8) | 4.8–14.6 |
| Arthralgia | 5 (3.3) | 1.1–7.5 | 6 (4.0) | 1.5–8.5 | 4 (2.7) | 0.7–6.7 | 2 (1.4) | 0.2–4.8 |
AE, adverse event; CI, confidence interval; PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine; SAE, serious AE; V114, 15-valent pneumococcal conjugate vaccine.
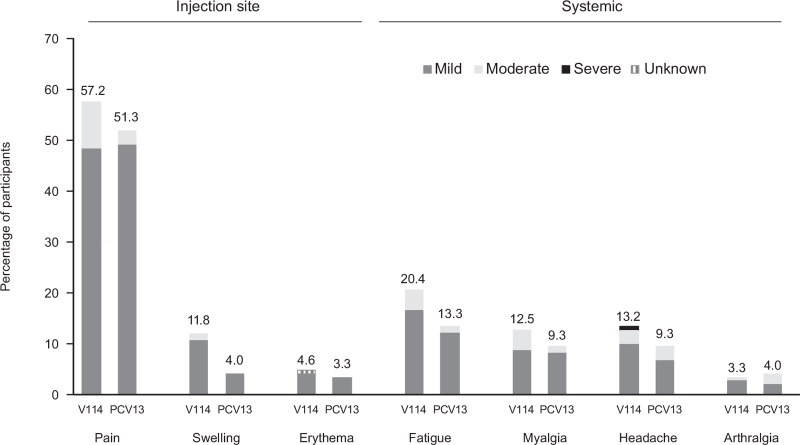
The most commonly reported solicited adverse event in both groups was injection-site pain, and the most commonly reported solicited systemic adverse event was fatigue (Fig. 2). The majority of solicited adverse events lasted 3 days or less (data not shown). Only three participants (two following V114 and one following PCV13) had elevated body temperature of at least 38 °C (100.4 °F), but less than 39 °C (102.2 °F).
Fig. 2.
Proportions of participants with solicited adverse events after vaccination with V114 or PCV13 by severity.
Solicited adverse events collected postvaccination (days 1–5 for injection-site events and days 1–14 for systemic events) are shown with severity grades (V114: n = 152; PCV13: n = 150). The height of the stacked bar represents the total percentage of participants reporting the adverse event. The severity grades (mild, moderate, or severe) within the bar indicate the proportion of the total attributed to each respective category. PCV13, 13-valent pneumococcal conjugate vaccine; V114, 15-valent pneumococcal conjugate vaccine.
The proportion of participants who experienced SAEs following either V114 or PCV13 was low (≤2%) (Table 2) and none were considered by study investigators to be related to study vaccine. No participant discontinued the study because of an adverse event following PCV vaccination.
V114 was generally well tolerated across subgroups based on sex, age, CD4+ cell count, or HIV viral load (Supplementary Tables 1–3).
Safety following vaccination with PPSV23
Following vaccination with PPSV23, 60.7% of participants in the V114 group and 71.6% in the PCV13 group experienced at least one adverse event. In contrast to observations following V114/PCV13 vaccination, after vaccination with PPSV23, the proportion of participants experiencing adverse events was generally higher in the PCV13 group, although no statistical comparisons were made (Table 2).
Adverse events considered to be related to PPSV23 were reported by 58.0 and 66.9% of participants in the V114 and PCV13 groups, respectively (data not shown).
Consistent with observations following V114/PCV13 vaccination, the majority of solicited adverse events after PPSV23 were of short duration (≤3 days), and less than 3% of participants in either vaccination group reported solicited adverse events that were graded as severe (data not shown). The proportion of participants who experienced SAEs was low (<5%) in both intervention groups (Table 2) and none were considered by study investigators to be related to study vaccine.
Immunogenicity
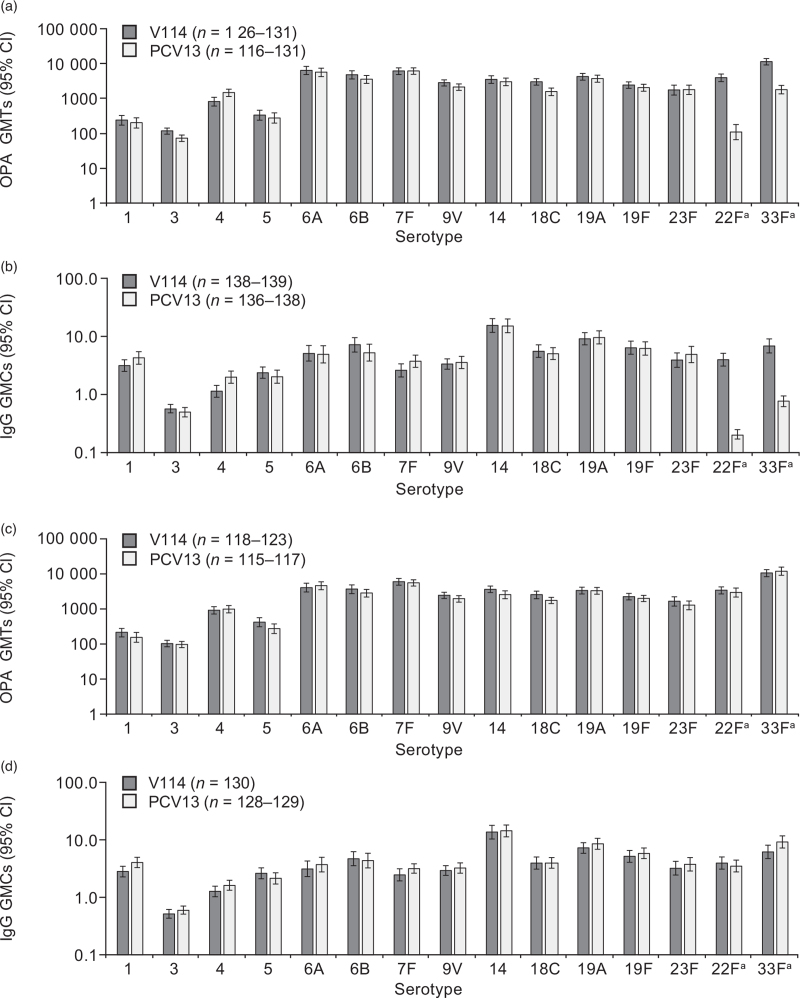
Both V114 and PCV13 were immunogenic, as assessed by OPA GMTs and IgG GMCs at 30 days postvaccination for all serotypes contained in each vaccine (Fig. 3a and b; Supplementary Tables 4 and 5). OPA GMTs and IgG GMCs for the two serotypes unique to V114 (22F and 33F), were higher in recipients of V114 than PCV13 at 30 days postvaccination. Although OPA GMTs and IgG GMCs were generally comparable between recipients of V114 and PCV13 for most shared serotypes, differences in mean antibody titers were observed and 95% CIs did not overlap across groups for three shared serotypes, favoring either PCV13 (serotype 4 IgG GMC and OPA GMT) or V114 (serotype 3 OPA GMT and serotype 18C OPA GMT). This study was not powered to show statistical differences between groups.
Fig. 3.
Serotype-specific immune responses at day 30 (opsonophagocytic activity geometric mean titers: panel a; IgG geometric mean concentrations: panel b) and week 12 (opsonophagocytic activity geometric mean titers: panel c; IgG geometric mean concentrations: panel d).
OPA GMTs and IgG GMCs are displayed with the corresponding 95% CIs. aSerotypes 22F and 33F are unique to V114. CI, confidence interval; GMC, geometric mean concentration (μg/ml); GMT, geometric mean titer (1/dil); IgG, immunoglobulin G; OPA, opsonophagocytic activity; PCV13, 13-valent pneumococcal conjugate vaccine; V114; 15-valent pneumococcal conjugate vaccine.
V114 and PCV13 were immunogenic across subgroups by age, sex, race ethnicity, viral load, and CD4+ cell count at 30 days postvaccination. A trend towards higher OPA GMTs and IgG GMCs at day 30 was observed in participants with a CD4+ cell count of at least 500 cells/μl and participants with plasma HIV RNA concentrations of less than 20 copies/ml, when compared with those with CD4+ cell count less than 500 cells/μl or HIV RNA at least 20 copies/ml, across both vaccination groups (Supplementary Tables 6–9).
At week 12 (30 days post-PPSV23), serotype-specific OPA and IgG responses were generally comparable to those observed at 30 days postvaccination with PCV for all 15 serotypes in the V114 group [which includes 14 shared serotypes between V114 and PPSV23 and one serotype unique to V114/PCV13 (serotype 6A)] and in the PCV13 group for all 13 serotypes in PCV13 (Fig. 3c and d, Supplementary Tables 10 and 11). PPSV23 elicited an immune response for serotypes 22F and 33F in the PCV13 group, as assessed at 30 days postvaccination with PPSV23, reaching levels comparable to those observed in the V114 group at both timepoints.
The majority of participants achieved robust serotype-specific GMFRs and at least a four-fold rise in OPA GMT or IgG GMC from baseline to day 30 and week 12 for most serotypes in each vaccine (Supplementary Tables 12–15). Reverse cumulative distribution curves showed that distributions of OPA and IgG responses were generally comparable between the two groups (Supplementary Figure 1 and 2). Serotype-specific OPA GMTs and IgG GMCs were generally comparable across vaccination groups for the 13 shared serotypes, and higher in the V114 group than in the PCV13 group for serotypes 22F and 33F (Supplementary Figure 3 and 4).
Discussion
In this study of pneumococcal vaccine-naive adults living with HIV receiving ART, V114 was generally well tolerated and induced immune responses for all 15 pneumococcal serotypes included in the vaccine, as assessed by OPA and IgG responses at 30 days postvaccination. Responses were comparable to those observed in the PCV13 group for shared serotypes and greater for serotypes 22F and 33F. Sequential administration of PPSV23 8 weeks after V114 or PCV13 was well tolerated, with maintained immune responses to most serotypes shared with both PCVs and no clear evidence of hyporesponsiveness. Compared with PCV13, V114 therefore has the potential to broaden protection against pneumococcal disease caused by two important serotypes (22F and 33F). As part of a sequential regimen that includes PPSV23, V114 provides earlier coverage against serotypes 22F and 33F than PCV13. The findings of this study are consistent with HIV management guidelines recommending sequential administration of PCV13 and PPSV23 in many countries [4,5,22].
Reactogenicity was generally comparable for V114 and PCV13. Although there was a trend toward a higher proportion of participants experiencing adverse events with V114 than PCV13 after the initial vaccination, the reverse was observed after PPSV23 vaccination. These differences are unlikely to be of clinical significance, as the majority of adverse events reported were of mild-to-moderate severity and short duration. Although small differences in proportions of participants reporting adverse events were observed in some racial and ethnic subgroups, the number of participants in many subgroups was small.
For most shared serotypes, antibody responses were generally comparable across the two vaccine groups at 30 days after vaccination with V114 or PCV13. Although differences were observed in OPA GMTs and IgG GMCs between recipients of V114 and PCV13 for some shared serotypes (i.e. serotypes 3, 4, and 18C), the clinical significance of these differences in this descriptive study is unclear, given the number of endpoints examined and the absence of a defined correlate of protection in adults.
At week 12 (30 days after PPSV23 administration), antibody responses were generally comparable between the V114 and PCV13 groups for all 15 serotypes. Findings were consistent with a previous study evaluating sequential vaccination with PCV13 and PPSV23 in people with HIV, in which antibody levels were comparable or moderately increased after PPSV23 compared with post-PCV13 levels [23]. Although immune responses to serotypes 22F and 33F were greater in the V114 group than the PCV13 group at day 30, responses were generally comparable across groups at week 12 as they were maintained in the V114 group and induced in the PCV13 group to levels generally comparable to those observed in the V114 group at both timepoints. The lack of difference between groups at week 12 is likely because of the fact that participants in the V114 group had higher levels of antibodies to serotypes 22F and 33F than the PCV13 recipients pre-PPSV23 administration, resulting in a lower response to PPSV23 compared with those who were still vaccine-naive to these serotypes. This is supported by results from the aforementioned previous study in individuals living with HIV, in which antibody levels after three doses of PCV13 were similar to those observed after sequential administration of three doses of PCV13 followed by PPSV23 [23]. Notably, in this study, immune responses to serotypes 22F and 33F were similar at day 30 in the V114 group and week 12 in the PCV13 group, suggesting that PPSV23 elicits a similar response to PCVs in people previously naive to these vaccine serotypes, consistent with other studies in adults living with HIV [24]. However, as immune responses immediately prior to administration of PPSV23 were not measured in the current study, the magnitude of the ‘boost’ response to the shared serotypes is unknown.
A trend towards higher immune responses in participants with CD4+ cell counts at least 500 cells/μl or HIV RNA concentrations less than 20 copies/ml compared with those with CD4+ cell counts of less than 500 cells/μl or HIV RNA at least 20 copies/ml, respectively, was observed. Although these correlations have not been consistently observed in previous studies of pneumococcal vaccines in individuals living with HIV [25], several studies of other vaccines in people with HIV have reported similar observations [26–28].
Limitations to this study include the small sample size and the challenges experienced recruiting participants to the lowest CD4+ cell count stratum, which resulted in only four participants with a CD4+ cell count less than 200 cells/μl. Participants with low CD4+ cell counts were less likely to meet other inclusion criteria. This limits the generalizability of our findings to adults living with HIV with higher degrees of immunosuppression. Furthermore, immune responses to pneumococcal serotypes unique to PPSV23 were not measured, and immunogenicity was not assessed beyond 12 weeks following administration of V114. In addition, the descriptive nature of the trial did not allow for a formal statistical comparison between the safety and immunogenicity profiles of V114 and PCV13. A formal statistical comparison of these features between these two vaccines was evaluated in a larger study in adults ≥50 years of age (V114-019) [29]. Finally, our study did not evaluate the protective benefits of immune responses afforded by study vaccines as we did not evaluate vaccine effectiveness against pneumococcal disease.
In conclusion, in pneumococcal vaccine-naive adults living with HIV, V114 was generally well tolerated and induced immune responses for all 15 pneumococcal serotypes. V114 can be followed by PPSV23 at 8 weeks, as the immune response was maintained for shared serotypes and sequential administration was well tolerated.
Acknowledgements
The authors would like to thank each of the participants, study staff, and investigators in the V114-018 (PNEU-WAY) study group for their invaluable contributions to this study. A full list of investigators for this study can be found in Supplementary Table 16. Medical writing support, including assisting authors with the development of the initial draft and incorporation of comments was provided by Rachel Wright, PhD, and editorial support was provided by Ian Norton, PhD, all of Scion, London, according to Good Publication Practice guidelines (Link). This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, USA.
Funding: This study was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, USA.
Author contributions: L.M., Y.P., O.O., K.S., W.R., J.M. contributed to the enrolment of participants and data collection; review of the manuscript. J.S., U.K.B. contributed to the study concept and design; analysis and interpretation of data, preparation of the manuscript; review of the manuscript. G.T., T.S., Y.Z., A.P., Y.K., K.H. contributed to the analysis and interpretation of data; preparation of the manuscript; review of the manuscript. R.D., J.H., L.K.M. contributed to the study concept and design; analysis and interpretation of data; review of the manuscript.
Role of the funding source: this study was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, USA. The sponsor was involved in study design, collection, analysis, and interpretation of data, as well as data checking of information provided herein. However, ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors.
Data sharing: Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, USA's data sharing policy, including restrictions, is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the EngageZone site or via e-mail to dataaccess@merck.com.
Conflicts of interest
G.T., T.S., Y.Z., A.P., J.H., Y.K., K.H., L.M., and J.S. are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, USA, and may own stock and/or stock options in Merck & Co., Inc., Kenilworth, New Jersey, USA. U.K.B was an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, USA, at the time of the study and may own stock and/or stock options in Merck & Co., Inc., Kenilworth, New Jersey, USA. R.D. has received grants/research support from Pfizer, Merck Sharp & Dohme, and MedImmune, has been a scientific consultant for Pfizer, MeMed, Merck Sharp & Dohme, and Biondvax, has served on advisory boards of Pfizer, Merck Sharp & Dohme, and Biondvax, and has been a speaker for Pfizer. K.S. and Y.P.R. have received grants/research support from Merck Sharp & Dohme. L.M. has received grants from Merck Sharp & Dohme, GlaxoSmithKline - ViiV Healthcare, and Johnson & Johnson, and nonfinancial support from Kowa Pharmaceuticals America. J.M.M. has received grants from Gilead, and personal fees from Merck Sharp & Dohme, GlaxoSmithKline - ViiV Healthcare, Sanofi, and Gilead. O.O. has received personal fees from Merck Sharp & Dohme, GlaxoSmithKline – ViiV Healthcare, and Gilead.
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
References
- 1.van Aalst M, Lotsch F, Spijker R, van der Meer JTM, Langendam MW, Goorhuis A, et al. Incidence of invasive pneumococcal disease in immunocompromised patients: a systematic review and meta-analysis. Travel Med Infect Dis 2018; 24:89–100. [DOI] [PubMed] [Google Scholar]
- 2.Harboe ZB, Larsen MV, Ladelund S, Kronborg G, Konradsen HB, Gerstoft J, et al. Incidence and risk factors for invasive pneumococcal disease in HIV-infected and non-HIV-infected individuals before and after the introduction of combination antiretroviral therapy: persistent high risk among HIV-infected injecting drug users. Clin Infect Dis 2014; 59:1168–1176. [DOI] [PubMed] [Google Scholar]
- 3.French N, Gordon SB, Mwalukomo T, White SA, Mwafulirwa G, Longwe H, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Engl J Med 2010; 362:812–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012; 61:816–819. [PubMed] [Google Scholar]
- 5.Robert Koch-Institut. Empfehlungen der Ständigen Impfkommission (STIKO) am Robert Koch-Institut– 2017/2018. Available at: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2017/Ausgaben/34_17.pdf?__blob=publicationFile. [Accessed 19 March 2020]. [Google Scholar]
- 6.Avci FY, Li X, Tsuji M, Kasper DL. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat Med 2011; 17:1602–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. European Centre for Disease Prevention and Control. Invasive pneumococcal disease - annual epidemiological report for 2017. Available at: https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2017-invasive-pneumococcal-disease.pdf. [Accessed 4 October 2019] [Google Scholar]
- 8.Varghese J, Chochua S, Tran T, Walker H, Li Z, Snippes Vagnone PM, et al. Multistate population and whole genome sequence-based strain surveillance of invasive pneumococci recovered in the USA during 2017. Clin Microbiol Infect 2020; 26:512.e1–512.e10. [DOI] [PubMed] [Google Scholar]
- 9.Wijayasri S, Hillier K, Lim GH, Harris TM, Wilson SE, Deeks SL. The shifting epidemiology and serotype distribution of invasive pneumococcal disease in Ontario, Canada, 2007–2017. PLoS One 2019; 14:e0226353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adam HJ, Golden AR, Karlowsky JA, Baxter MR, Nichol KA, Martin I, et al. Canadian Antimicrobial Resistance Alliance (CARA). Analysis of multidrug resistance in the predominant Streptococcus pneumoniae serotypes in Canada: the SAVE study, 2011–15. J Antimicrob Chemother 2018; 73: (Suppl_7): vii12–vii19. [DOI] [PubMed] [Google Scholar]
- 11. Public Health Canada. National laboratory surveillance of invasive Streptococcal disease in Canada - annual summary 2017. Available at: https://www.canada.ca/en/public-health/services/publications/drugs-health-products/national-laboratory-surveillance-invasive-streptococcal-disease-annual-summary-2017.html. [Accessed 8 June 2020] [Google Scholar]
- 12.Stacey HL, Rosen J, Peterson JT, Williams-Diaz A, Gakhar V, Sterling TM, et al. Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine (PCV-15) compared to PCV-13 in healthy older adults. Hum Vaccin Immunother 2019; 15:530–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. The United States Food and Drug Administration. PNEUMOVAX 23 Prescribing Information. Available at: https://www.fda.gov/media/80547/download. [Accessed 16 August 2021] [Google Scholar]
- 14. The United States Food and Drug Administration. PREVNAR 13 Prescribing Information. Available at: https://www.fda.gov/files/vaccines%2C%20blood%20%26%20biologics/published/Package-Insert------Prevnar-13.pdf. [Accessed 11 June 2021] [Google Scholar]
- 15. Pfizer. PREVNAR 13 (Pneumococcal 13-valent Conjugate Vaccine [Diphtheria CRM197 Protein]). Available at: https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM574852.pdf. [Accessed 11 May 2020] [Google Scholar]
- 16. Food and Drug Administration. PNEUMOVAX® 23 (pneumococcal vaccine polyvalent). Available at: https://www.fda.gov/media/80547/download. [Accessed 25 March 2019] [Google Scholar]
- 17. Food and Drug Administration. Toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. Available at: https://www.fda.gov/media/73679/download. [Accessed 29 January 2021] [Google Scholar]
- 18.Nolan KM, Bonhomme ME, Schier CJ, Green T, Antonello JM, Murphy RD. Optimization and validation of a microcolony multiplexed opsonophagocytic killing assay for 15 pneumococcal serotypes. Bioanalysis 2020; 12:1003–1020. [DOI] [PubMed] [Google Scholar]
- 19.Nolan KM, Zhang Y, Antonello JM, Howlett AH, Bonhomme CJ, Greway R, et al. Enhanced antipneumococcal antibody electrochemiluminescence assay: validation and bridging to the WHO reference ELISA. Bioanalysis 2020; 12:1363–1375. [DOI] [PubMed] [Google Scholar]
- 20.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934; 26:404–413. [Google Scholar]
- 21.Liang K-Y, Zeger SL. Longitudinal data analysis of continuous and discrete responses for prepost designs. Sankhyā Indian J Stat 2000; 62:134–148. [Google Scholar]
- 22. National Institute for Health and Care Excellence. Pneumococcal vaccine. Available at: https://bnf.nice.org.uk/treatment-summary/pneumococcal-vaccine.html. [Accessed 28 April 2020] [Google Scholar]
- 23.Bhorat AE, Madhi SA, Laudat F, Sundaraiyer V, Gurtman A, Jansen KU, et al. Immunogenicity and safety of the 13-valent pneumococcal conjugate vaccine in HIV-infected individuals naive to pneumococcal vaccination. AIDS 2015; 29:1345–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garrido HMG, Schnyder JL, Tanck MWT, Vollaard A, Spijker R, Grobusch MP, et al. Immunogenicity of pneumococcal vaccination in HIV infected individuals: a systematic review and meta-analysis. EClinicalMedicine 2020; 29–30:100576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez-Barradas MC, Serpa JA, Munjal I, Mendoza D, Rueda AM, Mushtaq M, et al. Quantitative and qualitative antibody responses to immunization with the pneumococcal polysaccharide vaccine in HIV-infected patients after initiation of antiretroviral treatment: results from a randomized clinical trial. J Infect Dis 2015; 211:1703–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lesprit P, Pedrono G, Molina JM, Goujard C, Girard PM, Sarrazin N, et al. ANRS 114-Pneumovac Study Group. Immunological efficacy of a prime-boost pneumococcal vaccination in HIV-infected adults. AIDS 2007; 21:2425–2434. [DOI] [PubMed] [Google Scholar]
- 27.Kroon FP, van Dissel JT, de Jong JC, Zwinderman K, van Furth R. Antibody response after influenza vaccination in HIV-infected individuals: a consecutive 3-year study. Vaccine 2000; 18:3040–3049. [DOI] [PubMed] [Google Scholar]
- 28.Kroon FP, van Dissel JT, de Jong JC, van Furth R. Antibody response to influenza, tetanus and pneumococcal vaccines in HIV-seropositive individuals in relation to the number of CD4+ lymphocytes. AIDS 1994; 8:469–476. [DOI] [PubMed] [Google Scholar]
- 29.Platt HL, Cardona JF, Haranaka M, Schwartz HI, Narejos Perez S, Dowell A, et al. A phase 3 trial of safety, tolerability, and immunogenicity of V114, 15-valent pneumococcal conjugate vaccine, compared with 13-valent pneumococcal conjugate vaccine in adults 50 years of age and older (PNEU-AGE). Vaccine 2021; doi: 10.1016/j.vaccine.2021.08.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.