Keywords: calcium, disease, MCU, mitochondria, NCLX
Abstract
The uptake of calcium into and extrusion of calcium from the mitochondrial matrix is a fundamental biological process that has critical effects on cellular metabolism, signaling, and survival. Disruption of mitochondrial calcium (mCa2+) cycling is implicated in numerous acquired diseases such as heart failure, stroke, neurodegeneration, diabetes, and cancer and is genetically linked to several inherited neuromuscular disorders. Understanding the mechanisms responsible for mCa2+ exchange therefore holds great promise for the treatment of these diseases. The past decade has seen the genetic identification of many of the key proteins that mediate mitochondrial calcium uptake and efflux. Here, we present an overview of the phenomenon of mCa2+ transport and a comprehensive examination of the molecular machinery that mediates calcium flux across the inner mitochondrial membrane: the mitochondrial uniporter complex (consisting of MCU, EMRE, MICU1, MICU2, MICU3, MCUB, and MCUR1), NCLX, LETM1, the mitochondrial ryanodine receptor, and the mitochondrial permeability transition pore. We then consider the physiological implications of mCa2+ flux and evaluate how alterations in mCa2+ homeostasis contribute to human disease. This review concludes by highlighting opportunities and challenges for therapeutic intervention in pathologies characterized by aberrant mCa2+ handling and by summarizing critical unanswered questions regarding the biology of mCa2+ flux.
CLINICAL HIGHLIGHTS
Mitochondrial Calcium Homeostasis Is Perturbed in Human Disease
Primary mutations in genes encoding mitochondrial calcium-handling proteins including MICU1, MICU2, and LETM1 cause neuromuscular disorders.
Altered expression of mitochondrial calcium-handling genes and changes in the balance between mitochondrial calcium uptake and efflux are noted in acquired conditions such as heart failure, stroke, neurodegenerative disease, diabetes, and cancer.
Excessive mitochondrial calcium uptake is clearly detrimental in ischemia-reperfusion injury following myocardial infarction or stroke, and contributes to cell death and subsequent organ-level dysfunction.
Diminished mitochondrial calcium content can impair cellular bioenergetics and may contribute to organ-level dysfunction in chronic diseases such as heart failure.
Chronic neurodegenerative conditions such as Alzheimer’s disease and Parkinson’s disease are characterized by mitochondrial calcium overload.
Therapeutic Applications
Blocking mitochondrial calcium uptake through the mitochondrial calcium uniporter complex or enhancing mitochondrial calcium efflux through NCLX is protective in mouse models of ischemia-reperfusion injury.
NCLX has emerged as a promising target for neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease.
There is growing interest in manipulating either mitochondrial calcium uptake or mitochondrial calcium efflux for the treatment of cancer. However, more research is needed in this area, because increasing net mitochondrial calcium accumulation has variably been shown to promote either prosurvival signaling or apoptosis in tumor cells.
Current Challenges to Targeting Mitochondrial Calcium Exchange in Human Disease
Development of compounds that can specifically activate or inhibit the mitochondrial calcium uniporter complex or NCLX.
Ensuring tissue-specific drug delivery to avoid detrimental effects of altering mitochondrial calcium homeostasis in tissues that are not affected in a given disease.
Need for greater understanding of the temporal changes in mitochondrial calcium handling throughout the progression of chronic illness, to match the appropriate therapeutic strategy to each stage of the disease.
1. INTRODUCTION
The earliest observations of calcium (Ca2+) uptake by isolated mitochondria were reported by Slater and Cleland in the early 1950s (1) in studies examining the instability of cardiac mitochondrial or “sarcosome” preparations used to investigate oxidative phosphorylation. Slater and Cleland found that, after isolation, the mitochondria contained the vast majority of all Ca2+ originally distributed throughout the cardiac tissue, and further demonstrated in vitro that mitochondria isolated in Ca2+-free conditions, with the Ca2+ chelator ethylenediaminetetraacetic acid (EDTA), were capable of taking up nearly all the Ca2+ from a bath solution containing 1 µM CaCl2 (1). These early investigations found Ca2+ chelation with EDTA to have a stabilizing effect on oxidative phosphorylation (OXPHOS), as it prevented the gradual decline in the activity of OXPHOS reactions over the course of several hours of experimentation. Although this finding suggested the potential for mitochondrial Ca2+ (mCa2+) uptake to inhibit OXPHOS, likely due to deleterious mCa2+ overload, it stood in stark contrast to previous reports of Ca2+-dependent stimulation of OXPHOS (2). Slater and Cleland had the insight that mCa2+ may play dual roles, both to stimulate OXPHOS and, in some cases, to destroy or disrupt the OXPHOS machinery (1). Thus, this early work hinted at the biphasic effects of mCa2+ calcium and foreshadowed research into the roles of mCa2+ in metabolism and mitochondrial permeability transition (PT) and cell death that has continued ever since.
Subsequent in vitro studies by DeLuca and Engstrom (3) and by Vasington and Murphy (4) revealed rapid uptake of radiolabeled 45Ca2+ by isolated rat kidney mitochondria. These experiments demonstrated the requirement for an oxidizable carbon substrate such as succinate, α-ketoglutarate, glutamate, or malate; ATP or ADP + Pi; and magnesium (Mg2+) to permit mCa2+ uptake. They also revealed that electron transport chain (ETC) inhibition (with Antimycin A, dicumarol, or 2,3-dimercaptopropanol) attenuated mCa2+ uptake, but uncoupling or inhibition of ATP synthesis with dinitrophenol or oligomycin A did not (3). Vasington and Murphy (4) concluded that the activity of the ETC is required to drive mCa2+ uptake and could drive the import of as much as 2 µmol of Ca2+ per milligram of mitochondrial protein. They proposed a stoichiometric relationship between Ca2+ uptake and electron transport, and indeed, this robust capacity for mCa2+ uptake was later shown to depend on the establishment of a highly electronegative potential (ΔΨm, approximately −180 mV) across the inner mitochondrial membrane (IMM). As Peter Mitchell’s elegant work on chemiosmotic theory established, this mitochondrial membrane potential is generated as protons (H+) are pumped out of the mitochondrial matrix by the ETC (5, 6). Both DeLuca and Engstrom and Vasington and Murphy noted the eventual loss of Ca2+ from mitochondria upon prolonged time after isolation, removal of ADP, or addition of ETC inhibitors or uncouplers. These observations strengthened the notion that a functional ETC that maintains ΔΨm is required for mCa2+ sequestration and indicated that there are also controlled pathways for mCa2+ efflux.
The 1970s brought a growing appreciation that the release of Ca2+ from the mitochondrial matrix could occur in a regulated manner. In experiments investigating the mechanisms of excitation-contraction coupling in cardiac muscle, Carafoli and colleagues (7, 8) discovered that incubation with NaCl or LiCl, but not KCl, could stimulate the release of Ca2+ from rat heart mitochondria. This stimulated Ca2+ release occurred independent of respiratory function, suggesting the presence of an exchanger protein capable of extruding matrix Ca2+ in exchange for sodium (Na+) or lithium (Li+). Subsequent studies by Pozzan et al. (9) supported the idea that mCa2+ efflux could be coupled directly or indirectly to H+ leak into the matrix. This phenomenon, when coupled to cooperative activation of mCa2+ influx (10–12), would minimize mCa2+ accumulation under steady-state conditions despite the highly electronegative ΔΨm favoring mCa2+ influx. Thus, the net mitochondrial Ca2+ content would tend to increase only under conditions of increased cytosolic Ca2+ concentration. This pattern of mCa2+ accumulation could allow the mitochondria to buffer changes in cytosolic Ca2+ and in doing so act as a signal to couple changes in cellular function to coordinated responses in the mitochondria.
Direct proof of the principle that changes in cytosolic Ca2+ concentration drive changes in mitochondrial Ca2+ within intact cells was provided in 1992 in groundbreaking work by Pozzan’s group. In this study, Rizzuto et al. fused the Ca2+-sensitive photoprotein aequorin to the mitochondrial targeting sequence of subunit VIII of human cytochrome-c oxidase, to mediate mitochondrial import of the Ca2+ reporter to the inner mitochondrial membrane (IMM) (13). This approach allowed the first simultaneous live-cell measurements of both mitochondrial Ca2+ and cytosolic Ca2+, which was measured by the Ca2+-sensitive dye Fura-2. In the 30 years since, a wide array of reagents have been developed for the measurement of mitochondrial Ca2+ flux in intact cells. For an assessment of the tools currently available for assessing mitochondrial Ca2+ exchange in isolated mitochondria or cellular systems, and the strengths and limitations of these approaches, the reader is directed to two recent articles (14, 15). Advances in molecular biology have revealed conservation of mCa2+ flux across all tissues and taxa. The mitochondrial research community has also begun to identify the specific proteins that transport Ca2+ into and out of the mitochondria and that regulate this Ca2+ exchange. This review begins by summarizing the pathways responsible for raising and lowering cytosolic Ca2+ concentration. We then focus on the current understanding of the identity, function, and molecular regulation of the proteins that transport Ca2+ across the outer and inner mitochondrial membranes, and summarize the cellular processes directly impacted by mitochondrial Ca2+ signaling as well as the emerging functions of the mitochondrial Ca2+ microdomain. We conclude with an examination of the role of altered mCa2+ exchange in human disease (TABLE 1), a discussion of potential therapeutic strategies based on manipulation of mCa2+ handling, and a summary of the major gaps in the current understanding of mCa2+ exchange.
Table 1.
Human genetic diseases linked to mCa2+ handling proteins
| Reference | Genetic Defect | Disease/Major Phenotype(s) |
|---|---|---|
| Logan et al. (199) | Loss of function mutations in MICU1 | Excessive mCa2+ uptake, proximal myopathy, learning defects, and a progressive extrapyramidal movement disorder |
| Lewis-Smith et al. (200) | Homozygous, 2,755-bp deletion in MICU1 | Lethargy, fatigue, and muscle weakness presenting in childhood |
| O’Grady et al. (201) | Loss of function mutation in MICU1 | Congenital muscular dystrophy |
| Musa et al. (202) | Homozygous c.553C>T (p.Q185*) mutation in MICU1; predicted to cause complete loss of protein function | Muscle weakness, fatigue; in some instances accompanied by extrapyramidal signs, learning disability, nystagmus, and cataracts |
| Mojbafan et al. (203) | C1295delA mutation in exon 13 of MICU1, causing a frameshift and protein truncation | Myopathy with extrapyramidal signs |
| Wilton et al. (573) | Two distinct heterozygous MICU1 mutations in the same patient, one on the maternal allele and one on the paternal allele: • c.161 + 1G>A in MICU1, likely disrupting splicing and gene function • c.386G>C in MICU1, resulting in R129P missense mutation |
Myopathy with extrapyramidal signs, also acute encephalopathy and developmental brain abnormalities |
| Shamseldin et al. (233) | Homozygous c.42G>A:P.W14* mutation in MICU2, causing an early stop codon and protein truncation | Neurodevelopmental disorder with severe cognitive impairment, spasticity, and white matter involvement. Patient fibroblasts have excessive mCa2+ uptake. |
| Endele et al. (341) | Deletion of regions of the short arm of chromosome 4 (4p16.3), including LETM1 | Wolf–Hirschhorn syndrome: impaired growth, developmental delay, microcephaly, and mental defects. Sometimes involves impaired muscle tone, seizures, and congenital heart defects. |
| South et al. (371) | Microdeletion in 4p16.3, including LETM1 | Developmental delays and delayed growth, unique facial features distinct from those of Wolf–Hirschhorn syndrome; variable involvement of seizures |
| Cyr et al. (375) | Microduplication of 4p16.3, including LETM1 | Macrocephaly, normal growth, irregular iris pigmentation, delayed development, dysmorphic features, and seizures |
| Roselló et al. (376) | Submicroscopic duplication in 4p16.2, including LETM1 | Phenotype intermediate between Wolf–Hirschhorn syndrome and 4p trisomy; facial dysmorphism, delayed gross motor development |
See glossary for abbreviations.
2. INTRACELLULAR Ca2+ CYCLING
The fundamental stimulus for mitochondrial Ca2+ uptake is an increase in the local cytosolic Ca2+ concentration. Although a full accounting of the mechanisms controlling cytosolic Ca2+ flux is beyond the scope of this article, the key points are highlighted below and the reader is directed to the excellent reviews cited throughout this section for further details.
2.1. Ca2+ Transport Across the Plasma Membrane
Resting cytosolic calcium Ca2+ concentration in eukaryotic cells is typically held around 100 nM, ∼10,000–20,000 times lower than the extracellular Ca2+ concentration of ∼1 mM (16, 17). This large Ca2+ gradient represents a massive thermodynamic driving force for Ca2+ entry across the plasma membrane and is maintained by the action of the plasma membrane calcium ATPase (PMCA), which uses the energy of ATP hydrolysis to pump Ca2+ out of the cell (16). The ATP consumed to pump Ca2+ “uphill” against its electrochemical gradient represents an energetic investment by the cell to establish a strong driving force for subsequent, regulated Ca2+ entry into the cell. The electrical potential across the plasma membrane (ΔΨ, approximately −70 mV inside the cell relative to outside) is established by the electrogenic Na+-potassium (K+)-ATPase pumping 3 Na+ ions out of the cell in exchange for the import of 2 K+ ions, contributing to the net driving force favoring influx of positively charged Ca2+ across the plasma membrane (18). The plasma membrane potential and the Na+ gradient [∼140 mM Na+ extracellular, ∼8–12 mM Na+ cytosolic (19–21)] also drive Na+/Ca2+ exchange through the electrogenic plasma membrane Na+/Ca2+ exchanger, NCX. NCX exports 1 Ca2+ ion out of the cell in exchange for the entry of 3 Na+ ions, which helps to maintain the Ca2+ gradient across the plasma membrane at the expense of the net entry of 1 positive charge into the cell (16). Similarly, electrogenic plasma membrane Na+/Ca2+-K+ exchangers (NCKXs) use the driving force for Na+ entry established by the Na+-K+-ATPase to export 1 Ca2+ ion and 1 K+ ion in exchange for the entry of 4 Na+ ions (17). Ca2+ is also sequestered in intracellular stores, such as the endoplasmic reticulum (ER)/sarcoplasmic reticulum (SR), which contributes to the low resting cytosolic Ca2+ concentration. The accumulation of Ca2+ in intracellular stores is achieved via the action of the sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA), which pumps Ca2+ against its concentration gradient into the ER/SR (17).
When plasma membrane Ca2+ channels open, the potential energy stored across the plasma membrane allows Ca2+ to rapidly enter the cell, flowing down its electrochemical gradient. The resultant elevation of intracellular Ca2+ (iCa2+) is an early event in many intracellular signaling cascades including cAMP-response element binding protein (CREB)-dependent transcriptional responses, calcium/calmodulin-dependent signaling, calcineurin-NFAT signaling, synaptic vesicle release, and calcium-induced Ca2+ release that triggers myofilament cross-bridge cycling (reviewed in Refs. 22–26). Another fundamental consequence of elevated cytosolic Ca2+ concentration, and a major focus of this review, is that it drives mitochondrial Ca2+ uptake, which has numerous implications for cellular metabolism and viability. In this way, the cell can use the energy invested in the extracellular-intracellular Ca2+ gradient to effect rapid and reliable intracellular signaling.
The specific proteins that mediate Ca2+ influx across the plasma membrane vary across cell types but in general include voltage-gated Ca2+-permeant channels, including the L-type and T-type Ca2+ channels and transient receptor potential channels, which open in response to plasma membrane depolarization (for instance, during an action potential), and ligand-gated Ca2+-permeant channels including certain purinergic receptors, nicotinic receptors, and N-methyl-d-aspartate (NMDA) receptors, which open upon binding to extracellular signaling molecules such as ATP and neurotransmitters (17, 23, 24).
2.2. Release of Ca2+ From Intracellular Stores
A key feature of cellular Ca2+ signaling is that the activation of plasma membrane receptors can initiate intracellular signaling cascades that ultimately cause release of Ca2+ from the ER/SR. These pathways serve as another mechanism to raise iCa2+ concentration and can allow for localized release of Ca2+ at more discrete regions within the interior of the cell, a concept that is addressed in further detail in sect. 5. Gq-coupled receptors at the plasma membrane are activated by extracellular ligands such as histamine, 5-hydroxytryptamine, ATP, and angiotensin II. They then activate phospholipase Cβ, which hydrolyzes phosphatidylinositol-4,5-bisphosphate to generate diacylglycerol and inositol-(1,4,5)-trisphosphate (IP3) (27) (also reviewed in Refs. 28–32). Several additional phospholipase C isoforms are activated via other signaling mechanisms and likewise generate IP3 (31). IP3 then binds and activates the IP3 receptor (IP3R), a Ca2+ release channel on the ER membrane, allowing Ca2+ to flow from the ER lumen (∼0.5 mM Ca2+ concentration) to the cytosol (∼100 nM resting Ca2+ concentration) (33, 34).
In excitable cell types such as muscle and neurons, influx of extracellular Ca2+ through voltage-gated Ca2+ channels at the plasma membrane in response to an action potential also triggers release of Ca2+ from the SR/ER by activating the ryanodine receptor (RyR), in a process known as Ca2+-induced Ca2+ release (23, 24, 35, 36). It should be noted that in skeletal muscle intracellular Ca2+ release through the RyR does not strictly require the influx of extracellular Ca2+, as the predominant isoform expressed in this cell type (RyR1) can be activated in response to plasma membrane depolarization through its physical interaction with the voltage-sensitive dihydropyridine receptor that is located on the t-tubule membrane (36). Ca2+-induced Ca2+ release from the ER/SR can amplify the cytosolic Ca2+ signal in response to a relatively smaller influx of Ca2+ across the plasma membrane and give rise to global cytosolic Ca2+ transients such as those responsible for muscle contraction.
Another important point is that the ER/SR often closely associates with the mitochondria, allowing for precise positioning of intracellular Ca2+ release in close proximity to the mitochondria, at sites termed “mitochondria-associated membranes” (MAMs) (34). This organization creates local, spatially constrained cytosolic microdomains in which Ca2+ released from the ER/SR can accumulate at concentrations high enough to activate mCa2+ uptake, without requiring robust cell-wide increases in Ca2+ levels. The functional implications of this special arrangement for ER/SR-mitochondrial Ca2+ transfer and cellular function are explored in greater depth in sects. 3 and 5.
3. THE MOLECULAR MACHINERY CONTROLLING mCa2+ FLUX
Upon stimulation, cytosolic Ca2+ can rise because of the influx of extracellular Ca2+ and/or the release of Ca2+ from the ER/SR via the mechanisms introduced above (reviewed in Refs. 16, 17, 31, 37–39). As local concentrations of Ca2+ rise above ∼400 nM, as likely occurs at sites of ER-mitochondria contact, the mitochondria begin to rapidly take up Ca2+ from the cytosol. The highly electronegative inner mitochondrial membrane potential (ΔΨm, approximately −180 mV) provides the thermodynamic driving force for the flow of Ca2+ into the matrix, where the resting free Ca2+ concentration is kept low, ∼100 nM, due to buffering of Ca2+ ions as calcium phosphate (21, 40). The high buffering capacity of the mitochondrial matrix serves as a “sink” for cellular Ca2+ storage and favors net mCa2+ uptake when cytosolic Ca2+ concentration rises. Early studies noted that the kinetics of mCa2+ uptake follow a sigmoidal curve, with the rate of mCa2+ uptake accelerating rapidly as extramitochondrial Ca2+ concentration increases, until plateauing at a cytosolic or bath Ca2+ concentration around 200 µM (10–12). This behavior reflects robust Ca2+ regulation of the mCa2+ uptake machinery, which is a consequence of the numerous proteins that modulate the activity of the mCa2+ uptake channels.
3.1. Ca2+ Transport Across the Outer Mitochondrial Membrane
The outer mitochondrial membrane (OMM) is generally thought to impose minimal to no resistance to Ca2+ transport, with Ca2+ moving from the cytosol to the mitochondrial intermembrane space (IMS) through the 30- to 35-kDa voltage-dependent anion channel (VDAC), also referred to in the older literature as “porin” because of its similarity with pore-forming proteins in the membranes of bacteria (41). VDAC proteins are present in all metazoans, with a high degree of conservation among eukaryotes. Three related VDAC isoforms (VDAC1–3) are present in chordates (42), and VDAC1 is the most abundant isoform (43). The reader is referred to an excellent review by Shoshan-Barmatz et al. (42) for a comprehensive discussion of the genetic and structural diversity of VDAC variants across taxa and the phylogenetic relationships between VDAC isoforms.
The VDAC channel is made up of a 19-stranded β-barrel comprising the channel pore and a NH2-terminal α-helix that lies within the pore (44–46). VDAC is readily permeable to ions and small molecules < 5 kDa such as phosphate, chloride, and the adenine nucleotides, with a greater conductance for anions than for cations of similar size (47, 48). VDAC displays voltage sensitivity, in that it switches to “closed” states of diminished conductance as transmembrane voltage increases beyond ±30–40 mV (47, 48). In this way, VDAC behavior at the OMM may be influenced by the electrochemical gradient of H+ in the IMS that is established by the activity of the ETC at the inner membrane, which in turn influences the potential across the OMM (negative on the cytosolic side; positive on the IMS side) (47, 49, 50). Interestingly, the “substate” conformations of VDAC exhibit selectivity to cation transport (50, 51). VDAC contains Ca2+ binding sites and when constituted into lipid bilayers or liposomes is permeable to Ca2+ (52–54). Experiments in liposomes indicate that Ca2+ itself regulates VDAC conductance, with increased Ca2+ concentration favoring increased conductance of the closed or subconductance states of the channel (55). Ca2+ may also increase ATP transport across the OMM, indicating that Ca2+ can control the overall small molecule permeability of VDAC as well as its ionic conductance (55). The Ca2+ permeability of specific VDAC isoforms is further regulated by interaction with other proteins such as the antiapoptotic protein Bcl-xL (56). A number of posttranslational modifications of VDAC have been identified by mass spectrometry, including phosphorylation of multiple residues and lysine acetylation of VDAC1–3 (reviewed in Ref. 57). However, the consequences of such modifications for overall VDAC permeability or Ca2+ transport remain to be determined.
Some models propose that VDAC specifically regulates OMM Ca2+ transport at discrete spatial domains where the OMM is in proximity with Ca2+ release sites on the ER (discussed in greater detail in sect. 5). For instance, overexpression of rat VDAC1 in HeLa cells potentiates mCa2+ uptake in response to treatment with agonists that stimulate ER Ca2+ release (58). However, VDAC1 overexpression does not alter the extent or kinetics of mCa2+ uptake when permeabilized cells are exposed to increasing concentrations of bath Ca2+ (58). This finding indicates that VDAC does not increase OMM permeability to Ca2+ in a general, global fashion but instead predominantly increases OMM Ca2+ permeability at sites of ER-mitochondria contact. Fitting with this notion, silencing of VDAC1, 2, or 3 in HeLa cells attenuates and overexpression of VDAC1, 2, or 3 increases mCa2+ uptake that occurs after histamine-induced release of ER Ca2+ (59). These effects of VDAC on mCa2+ uptake occur independent of any alteration in agonist-induced ER Ca2+ release (59). Intriguing work suggests that the IP3R and VDAC interact in a physical complex that spans the ER and OMM, and that physical interaction between VDAC and the IP3R stimulates Ca2+ uptake through VDAC (60). This can explain why VDAC overexpression specifically potentiates mCa2+ uptake after agonist-induced ER Ca2+ release rather than after a global elevation of cytosolic or bath Ca2+, because VDAC activity may only increase at sites of agonist-induced ER Ca2+ release, where it is subject to positive regulation by the IP3R. However, given that Ca2+ permeation even occurs when VDAC is in the “closed” confirmation, it is unlikely that VDAC is a real-time regulator of mCa2+ flux; rather, it is permissive for Ca2+ transport across the OMM.
In addition to its roles in Ca2+ transport across the OMM, VDAC has also been implicated in cell death. VDAC can associate with the IMM adenine nucleotide translocator and form a large, nonspecific channel that spans both the outer and inner mitochondrial membranes and is believed to represent a component of the mitochondrial permeability transition pore (mPTP) (reviewed extensively in Refs. 42, 61 and discussed further below). However, the strict requirement for VDAC in mPTP opening and necrotic cell death is called into question by experiments in which genetic disruption of the three mammalian VDAC isoforms either alone or in combination fails to prevent mitochondrial permeability transition and cell death (62, 63).
A small number of case studies describe human patients with genetic deletion of VDAC1. The first was reported by Huizing et al. in a study of patients with mitochondrial myopathy in which no defect in mitochondrial metabolic enzymes could be identified, despite signs of impaired substrate oxidation and ATP production in muscle tissue (64). The affected patient exhibited psychomotor retardation and increased blood lactate in response to an intravenous glucose loading test, pointing to a mitochondrial defect (65). VDAC content was reduced 10-fold in skeletal muscle but was normal in fibroblasts from this patient, pointing to potential tissue-specific expression or modification of this protein (65). De Pinto and colleagues also observed reduced VDAC content in a muscle biopsy from a patient who likely suffered from Pearson’s disease due to a large mitochondrial DNA deletion, although it is possible that the reduction in VDAC expression was simply a consequence of gross mitochondrial dysfunction in this individual (42, 66).
Animal models with disruption of the various VDAC isoforms support a role for the channel in shaping mitochondrial metabolism and suggest a fair amount of functional redundancy among the three isoforms. Mouse embryonic stem cells with deletion of VDAC 1, VDAC2, or VDAC3 are viable but have diminished oxygen consumption, consistent with a role for VDAC in delivering metabolites such as phosphate and ADP needed for OXPHOS to the mitochondria (67). This study did not differentiate any potential effects of metabolite delivery versus Ca2+ transport to the mitochondria as potential causes for these deficits, but as this impaired metabolic phenotype is observed under basal conditions, rather than in response to any Ca2+-mobilizing stimulation, the authors’ interpretation that the phenotype is largely driven by reduced mitochondrial metabolite availability is likely correct.
Constitutive knockout (KO) of either Vdac1 or Vdac3 in mice yields viable animals (68, 69), but deletion of Vdac2 results in embryonic lethality (70). Vdac1−/− striated muscle has no change in basal respiration, despite altered mitochondrial sensitivity for ADP [Km(ADP)] in heart and skeletal muscle (68). Additionally, Vdac1 deletion causes ultrastructural changes including altered cristae structure in the mitochondria of the gastrocnemius and soleus muscles and, to a lesser extent, the heart (68). Vdac3−/− mice are healthy, but the males are infertile because of reduced sperm motility attributed to axoneme instability (69). Vdac3−/− animals also have enlarged mitochondria and diminished respiratory chain activity in skeletal muscle (69), consistent with a requirement for VDAC3 in normal skeletal muscle metabolism. Finally, mice lacking VDAC1 or VDAC3 alone or in combination exhibit disrupted fear conditioning and spatial learning, which are associated with defects in synaptic plasticity in the hippocampus (71). mPTP inhibition with cyclosporine A recapitulates the impaired synaptic plasticity observed in VDAC-deficient mice, suggesting that this defect is related to loss of VDAC-dependent mPTP opening and potential alterations to mCa2+ homeostasis in hippocampal neurons (71). Notably, treatment of Vdac1−/−/Vdac3−/− mouse embryonic fibroblasts (MEFs) with siRNA against Vdac2 in order to eliminate all cellular VDAC expression is insufficient to prevent mitochondrial permeability transition and cell death upon stimulation with H2O2, ionomycin, staurosporine, or TNF-α (62). Although this study did not specifically investigate the metabolic phenotype of these VDAC-deficient MEFs, the fact that these cells are viable suggests that alternative pathways are sufficient, at least under basal conditions, to supply adequate metabolites and Ca2+ to the mitochondria to support homeostatic metabolic function. It should also be noted that there was no difference in the ability of VDAC1/2/3-null cells to take up mCa2+, arguing against its requirement for OMM Ca2+ permeation (62). Functional redundancy and compensation among the three mammalian VDAC genes themselves and with other Ca2+- and metabolite-transporting pathways may also explain why pathological disruption of VDAC has so rarely been detected in the clinical setting. For example, the presence of VDAC2 appears sufficient to mostly compensate for the absence of VDAC1 and VDAC3 (68–71); at the very least, the loss of VDAC1 and/or VDAC3 is better tolerated than the loss of VDAC2 in terms of overall viability. These studies also relied on constitutive deletion of the various VDAC isoforms, so it is possible that there are robust compensatory mechanisms during development that minimize the detrimental effects of VDAC1 and/or VDAC3 deletion and result in relatively mild phenotypes.
3.2. Ca2+ Uptake Across the Inner Mitochondrial Membrane
After crossing the outer mitochondrial membrane via VDAC, Ca2+ must be transported from the intermembrane space across the inner mitochondrial membrane to enter the mitochondrial matrix. Two Ca2+ uptake mechanisms with distinct kinetics have been described for this process, the Ca2+ uniporter and the rapid mode of Ca2+ uptake (RaM). However, it has not yet been determined whether these two Ca2+ uptake modes are mediated by the same or different molecular machinery (72).
The more familiar mCa2+ uptake activity, described above and in Refs. 10–12, in which Ca2+ influx is driven by ΔΨm (approximately −180 mV on the matrix side of the IMM relative to the IMS; favoring influx of positively charged Ca2+), occurs without direct coupling to the transport of another ion and shows sigmoidal kinetics as a function of cytosolic Ca2+ concentration. This mode of mCa2+ uptake has been referred to as the “calcium uniporter” since the 1970s and 1980s (72–75). mCa2+ uptake via the uniporter can be inhibited by the hexavalent cation ruthenium red (76) and by its derivative Ru360 (77). Conversely, uniporter-mediated mCa2+ uptake can be activated by taurine (78); by several plant flavonoids including kaempferol, quercetin, genistein, and genistin (79); and, at low cytosolic Ca2+ concentrations, by spermine and spermidine (80).
The alternative, rapid mode of Ca2+ uptake (RaM) was first described in liver mitochondria by Sparagna et al. (81) as a mCa2+ uptake mechanism that functions at the beginning of a Ca2+ pulse and so allows mitochondria to accumulate Ca2+ even if the duration of the cytosolic Ca2+ pulse is insufficient for activation of the uniporter. The RaM functions at cytosolic Ca2+ concentrations below 200 nM that are too low for activation of the uniporter (81) but begins to inactivate as the surrounding Ca2+ concentration increases beyond 100–150 nM (72). Mallilankaraman et al. (82) hypothesize that this effect could arise from changes in MICU-dependent regulation of the uniporter as local cytosolic Ca2+ concentration changes. That is, at low Ca2+ concentrations the RaM may be mediated by an “ungated” form of the uniporter channel that is not subject to regulation by the Ca2+-binding protein MICU1, a concept that is discussed in detail below. As local Ca2+ concentration rises beyond 100–150 nM, increased binding of Ca2+ to MICU1 may enable MICU1 to “gate” the uniporter, thus terminating the RaM current, despite persistence of the thermodynamic driving force for mCa2+ uptake.
After inactivation, the RaM can be reset in response to a brief decrease in bath Ca2+ concentration (81). This behavior effectively allows for substantial mCa2+ sequestration via the RaM in response to a series of Ca2+ pulses. Thus, the RaM may provide a mechanism for mCa2+ accumulation in response to continuous, low-amplitude cytosolic Ca2+ oscillations that may occur physiologically. Sparagna et al. (81) interpret this rapid activation/inactivation feature of the RaM as a potential mechanism to tune mCa2+ accumulation based on the frequency of cytosolic Ca2+ oscillations. Subsequent studies detected the RaM in heart mitochondria and fast, high-capacity mCa2+ uptake consistent with the RaM in rat chromaffin cells (83), motor neurons (84), and mast cells (85). However, the properties of RaM activation and inhibition in the heart differ from the RaM observed in liver mitochondria. The time required for cytosolic Ca2+ to remain low in order to reset the RaM after its inactivation is longer in the heart than in the liver (86), and the relative sensitivity of the RaM to activation or inhibition by adenine nucleotides differs between the two tissues (86). Buntinas et al. (86) propose that these tissue-specific differences in the RaM may be matched to differences in patterns of cytosolic Ca2+ oscillations in liver (triggered by an external stimulus like a hormone; infrequent but of long duration), versus heart (continuous; frequent but of short duration), and may permit sufficient mCa2+ uptake to stimulate mitochondrial metabolism while at the same time preventing detrimental mCa2+ overload and permeability transition.
The pharmacological sensitivity of the RaM generally resembles that of the mCa2+ uniporter. The RaM can be blocked by ruthenium red, although this requires more than an order of magnitude greater concentration of ruthenium red than is required to inhibit the uniporter (81). Just as spermine activates the uniporter, spermine activates the RaM in liver mitochondria and, to a lesser extent, in heart mitochondria as well (81, 86). However, one point of distinction is that slow mCa2+ uptake (i.e., uniporter activity) is inhibited by cytosolic Mg2+ but rapid mCa2+ uptake is not (87).
3.3. Composition of the Mitochondrial Calcium Uniporter Complex
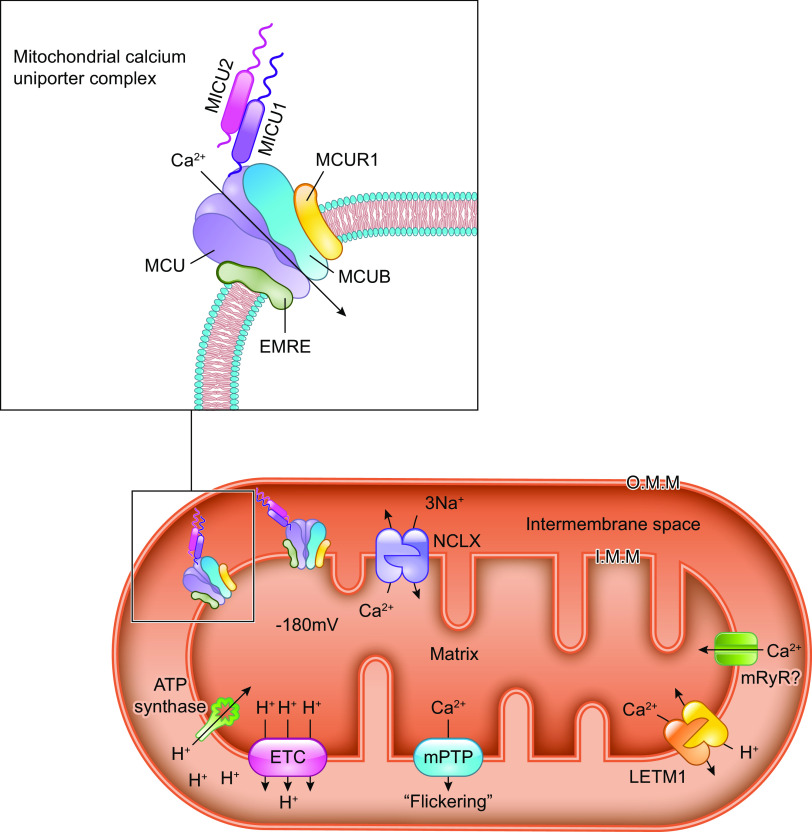
The molecular composition of the mitochondrial calcium uniporter channel began to be pieced together in the 2010s, as a number of research groups used evolutionary genetics and bioinformatic approaches to identify the mitochondrial inner membrane proteins required for or capable of modulating mitochondrial Ca2+ uptake. The multiprotein channel responsible for the uniporter activity described above has been termed the “mitochondrial uniporter channel” or “mtCU” (FIGURE 1).
FIGURE 1.
Ca2+ influx and efflux pathways of the inner mitochondrial membrane. The mitochondrial calcium uniporter channel (mtCU) exists as a channel composed of a total of 4 MCU subunits and up to 4 EMRE subunits. For simplicity, only a single EMRE subunit is shown here. In some cases, the dominant-negative MCU paralog, MCUB, replaces MCU subunits within the complex. The channel is gated by dimers of MICU1/2 or, in some tissues, dimers of MICU1/3. The accessory subunit MCUR1 also binds to and regulates the channel. Two uniporter channels can dimerize, and the function of these dimers is regulated by interactions between the MICU2 subunit associated with each channel. The driving force for Ca2+ entry through the mtCU is the highly negative (approximately −180 mV) potential established across the inner mitochondrial membrane (IMM) by the electron transport chain. Other proposed routes of mitochondrial Ca2+ (mCa2+) uptake may include the mitochondrial ryanodine receptor (mRyR) or reverse-mode operation of NCLX and LETM1. The Na+/Ca2+ exchanger NCLX is a major route for mCa2+ efflux, and the Ca2+/H+ exchanger LETM1 may contribute to mCa2+ efflux in some tissues. Transient opening of the mitochondrial permeability transition pore (mPTP), termed “flickering,” is proposed as another mechanism by which Ca2+ leaves the mitochondrial matrix under physiological conditions. See glossary for abbreviations.
This channel is composed of the channel-forming IMM protein, mitochondrial calcium uniporter (MCU); the integral scaffolding protein, essential mitochondrial response element (EMRE); and the regulatory proteins mitochondrial calcium uptake 1, 2, and 3 (MICU1/2/3) that project into the intermembrane space. The MCU paralog, MCUB, also contributes to the mtCU pore under certain conditions. Additional regulatory proteins including the IMM protein mitochondrial calcium uniporter regulator 1 (MCUR1) also form part of the mtCU and are reported to alter channel activity. These mtCU constituents, and their spatial arrangement within the uniporter complex, are discussed in detail below. The reader is directed to several articles (88–96) discussed throughout this section for excellent illustrations of the evolutionary relationships between mtCU components. The specific identity of the protein(s) that mediates the RaM remains unknown but may include one or more of the components of the mtCU.
3.3.1. MCU.
In the early 2000s, the Clapham group patch-clamped mitoplasts derived from the inner mitochondrial membrane of COS-7 cells and ascribed mitochondrial calcium uniporter activity to a single Ca2+-selective, inwardly rectifying ion channel with high Ca2+ affinity (97). This work provided evidence that the uniporter is a Ca2+ channel rather than a carrier protein and demonstrated that the properties of this channel, such as sensitivity to ruthenium red, match those of the activity of the theoretical uniporter postulated from historical studies of Ca2+ uptake in intact mitochondria (10–12, 72–76).
The genetic identity of the core pore-forming subunit of the mitochondrial calcium uniporter channel was determined in 2011 in a series of elegant papers from the Mootha and Rizzuto laboratories. These studies used a combination of phylogenetic profiling, RNA expression analysis, and mitochondrial protein expression analysis to identify genes coexpressed and functionally related to MICU1, the first uniporter complex component to be identified (98–100). These analyses identified a ubiquitously expressed transmembrane protein, CCDC109A, as a potential MICU1-interacting mitochondrial protein consisting of two transmembrane regions connected by a loop enriched with acidic residues (98, 99). CCDC109A coimmunoprecipitates with MICU1 and is localized to the inner mitochondrial membrane (98, 99). Knockdown of CCDC109A in HeLa cells or mouse liver attenuates rapid mCa2+ uptake, and mutational analysis of this protein further demonstrated the requirement of a conserved region that links the protein’s two transmembrane domains, termed the DIME motif, for Ca2+ permeation of the channel (98, 99). Mutation of a serine residue adjacent to the DIME motif diminishes the protein’s sensitivity to inhibition by Ru360, a ruthenium red derivative, providing further evidence that this protein forms part of the mitochondrial calcium uniporter channel (99). Recombinant CCDC109A exhibits ruthenium red-sensitive Ca2+ conductance when reconstituted into lipid bilayers, confirming its function as an inner membrane Ca2+ channel (98). As such, CCDC109A was renamed “MCU” for “mitochondrial calcium uniporter.” Additional experiments by the Mootha laboratory assessed the function of MCU in a more natural setting by using voltage clamp of whole mitoplasts. These studies confirmed MCU’s identity as the pore-forming subunit of the mitochondrial inner membrane Ca2+ channel that is responsible for the classically defined uniporter current (101).
3.3.1.1. mcu phylogenetic conservation.
MCU and its homologs are present in all branches of eukaryotes (88). MCU homologs appear in virtually all plants and metazoa, although these genes have been lost from some protozoan and fungal lineages, notably yeast fungi such as Saccharomyces cerevisiae (88). Most species have at least one or two MCU homologs, such as MCUB (discussed below), whereas some have as many as three or four homologs (88). Trypanosome parasites such as Trypanosoma cruzi and T. brucei have four MCU homologs (TcMCU as well as TcMCUb–d), all of which can diminish or enhance mCa2+ uptake when knocked out or overexpressed, respectively (102–105). Putative homologs for MCU have been detected in several species of bacteria (Prevotella oris, Chlorobium phaeobacteroides, and Cytophaga hutchinsonii), raising the possibility that the uniporter predates the emergence of Eukarya (88).
3.3.1.2. mcu structure.
MCU has a predicted molecular mass of ∼40 kDa but is processed to ∼34 kDa upon cleavage of an amino-terminal mitochondrial targeting sequence before incorporation into the inner mitochondrial membrane (99). MCU consists of a large NH2-terminal domain followed by a coiled-coil domain, a transmembrane domain, a short linker region, a second transmembrane domain, and a second coiled-coil domain (106). The NH2 and COOH termini of MCU face the mitochondrial matrix, whereas the loop that links the two transmembrane α-helices projects into the intermembrane space (99, 107). MCU can be detected as part of a large complex that migrates at ∼450 kDa when subjected to electrophoresis under nondenaturing conditions, and MCU is capable of oligomerizing with itself (99). Cryo-electron microscopy (cryo-EM) and X-ray crystallography of fungal MCUs support a model in which individual MCU proteins come together as a symmetric homotetramer consisting of a “dimer of dimers,” with fourfold symmetry of the transmembrane domains and twofold symmetry of the NH2-terminal domains (106, 108, 109) (FIGURE 2).
FIGURE 2.
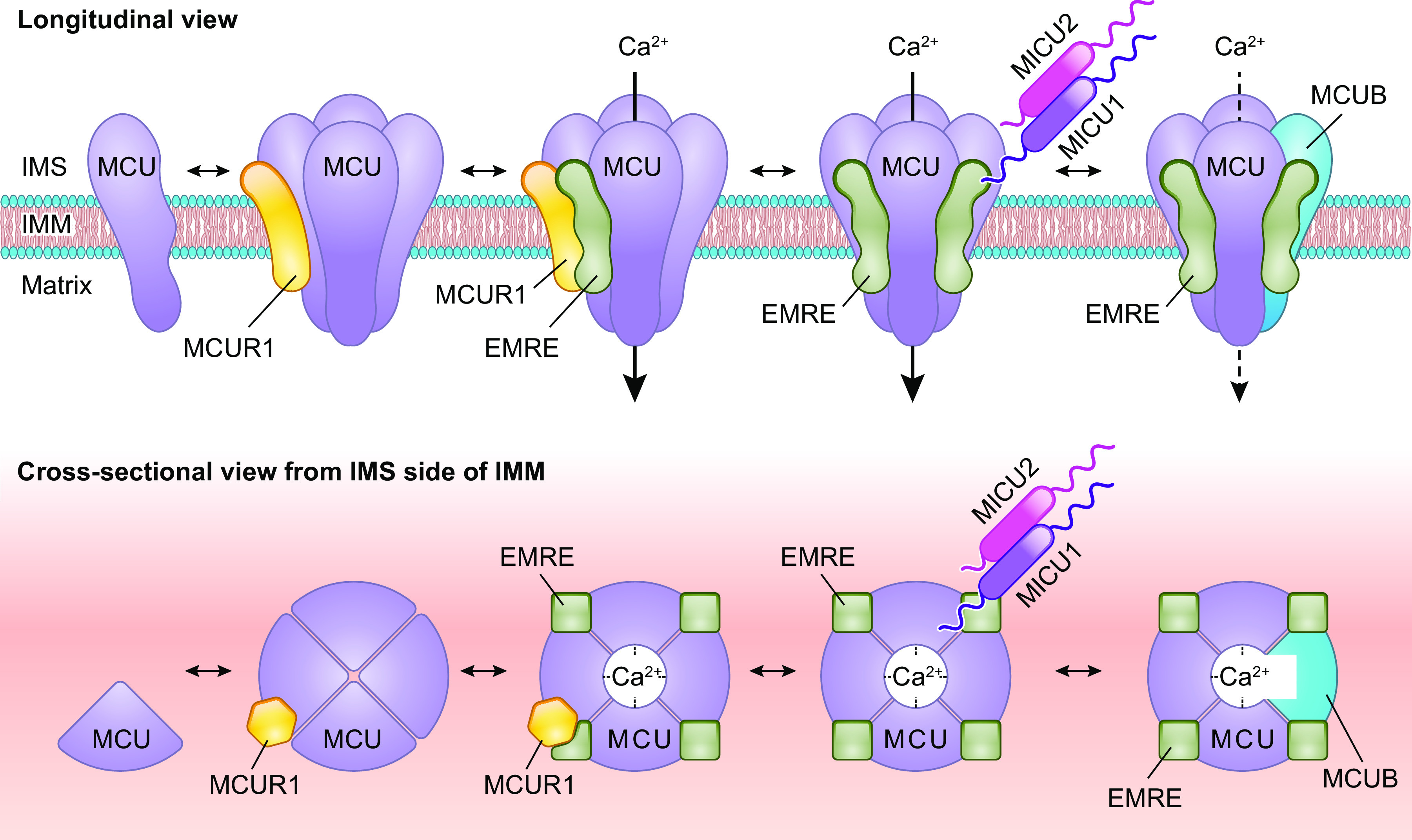
Assembly and stoichiometry of the mitochondrial calcium uniporter channel. Single MCU subunits assemble into tetramers with the assistance of the accessory mtCU subunit, MCUR1. In the absence of EMRE, these MCU tetramers are not capable of conducting Ca2+ across the inner mitochondrial membrane (IMM). EMRE associates with MCU at a likely 1-to-1 ratio in functional Ca2+ channels. The regulatory protein MICU1 binds to MCU and EMRE within the intermembrane space (IMS). In conjunction with its binding partners MICU2 or MICU3, MICU1 acts as a gatekeeper for the mtCU at low cytosolic Ca2+ concentrations and facilitates cooperative activation of the mtCU as cytosolic Ca2+ concentration rises. Current models suggest a stoichiometry of 1 MICU dimer (MICU1/1, MICU1/2, or MICU1/3):4 MCU:up to 4 EMRE. Binding of MICU1 to the channel is proposed to displace the scaffolding factor MCUR1. MCUB, a dominant-negative paralog of MCU, can replace MCU subunits within the channel pore. Incorporation of MCUB into the mtCU impairs Ca2+ conductance through the channel by disrupting the structure of channel pore and by displacing MICU dimers from the channel. See glossary for abbreviations.
Nuclear magnetic resonance and electron microscopy investigations of Caenorhabditis elegans MCU indicate that this protein also forms homooligomers in higher organisms (110). Recent data suggest that in zebrafish MCU also organizes into tetramers, although in these zebrafish tetramers the MCU NH2-terminal domains are arranged in an asymmetric fashion (111). The current structural models of MCU indicate that the conserved DIME motif (W-D-X-X-E-P-V-T-Y, where X indicates hydrophobic residues; corresponding to residues 260–268 of human MCU) that lies within the linker region forms part of the pore entrance and selectivity filter of the channel (106, 108–111). This model is strengthened by the observation that mutation of the DIME motif abrogates MCU-dependent mCa2+ uptake in vitro (99).
Although not part of the transmembrane MCU pore, the large NH2-terminal domain is thought to regulate MCU activity. Truncated human MCU lacking the NH2-terminal domain is incapable of restoring mitochondrial Ca2+ uptake when expressed in MCU-knockdown HeLa cells, despite correct assembly into complexes containing MICU1 and MICU2 (112). NH2-terminal deleted MCU also exerts dominant-negative effects on the function of wild-type (WT) MCU, suggesting that the NH2-terminal domain is critical for appropriate permeation of the channel. The crystal structure of the NH2-terminal domain of human MCU reveals the presence of a β-grasp-like fold in residues 72–189, which is capable of homooligomerization (113). Several acidic residues (D131, D142, D147, D148, and D166) within this region form a negatively charged patch that can bind divalent cations including Ca2+ and Mg2+. Such cation binding, or mutation of the acidic residues to eliminate their negative charge, disrupts the oligomerization of the β-grasp-like fold and inhibits MCU activity (113). The association of independent MCU proteins into functional multimeric channels is likewise disrupted by Ca2+ and Mg2+ (113). Furthermore, either loading of the mitochondria with Mg2+ or inhibition of mCa2+ efflux to increase mCa2+ concentration suppresses the rate of acute mCa2+ uptake, independent of changes in ΔΨm (113). These findings indicate that the NH2-terminal domain of MCU is important for oligomerization of MCU into functional mtCU channels and provide a potential mechanism by which matrix Mg2+ and possibly matrix Ca2+ inhibit MCU activity. Though hypothesized in the literature (114), the effect of certain concentrations of matrix Ca2+ to inhibit the uniporter remains controversial. It has been investigated further in recent years by the Foskett laboratory and may involve complex interactions between MCU (in particular, Ca2+ binding at the acidic residues D131 and D147 of MCU’s NH2-terminal domain), EMRE, and MICU1/2 (115, 116).
3.3.1.3. properties and function of mcu.
Patch-clamp experiments exploring the biophysical properties of mtCU complexes were performed by Kirichok’s laboratory following the genetic identification of MCU. Patch-clamp measurements of whole mitoplasts derived from mouse heart, skeletal muscle, liver, kidney, or brown adipose tissue revealed distinct differences in MCU current densities among these tissues, ranging from 58 pA/pF in skeletal muscle down to 2.1 pA/pF in the heart (117). Further analysis of the electrophysiological properties of the MCU current in heart and skeletal muscle mitoplasts confirmed that the same molecule transports this current in both tissues but that the overall activity of heart MCU is reduced compared with skeletal muscle MCU (117). The measured differences in MCU current across tissues likely also reflect tissue-specific differences in the incorporation of regulatory components of the mtCU into the intact uniporter channel. The observation that MCU activity is approximately fivefold higher in neonatal versus adult mouse heart mitoplasts (117) suggests that overall mtCU activity is regulated in a developmental manner as well.
3.3.1.4. genetic diseases associated with mcu.
In contrast to other mtCU components (TABLE 1), no primary mutations in MCU have yet been causatively linked to human disease. However, a recent report of increased MCU transcription and increased mCa2+ uptake occurring downstream of mutations in leucine-rich repeat kinase 2 (LRRK2), which is linked to late-onset familial Parkinson’s disease, supports a role for a secondary increase in mCa2+ uptake in the neurodegeneration observed in this disease (118). Such secondary alterations in MCU expression or function may likewise contribute to the development or progression of a wide variety of genetic diseases, but no causal relationships have yet been established.
3.3.1.5. animal models of mcu and roles of mcu in physiology.
3.3.1.5.1. Germline MCU deletion.
As the pore-forming subunit of the mitochondrial calcium uniporter complex, MCU is absolutely critical for the rapid mCa2+ uptake traditionally described as “uniporter” activity. This fact is supported by the phenotypes of numerous animal models in which MCU is knocked out or disrupted (TABLE 2). The first genetic mouse model for MCU disruption was published in 2013 by the Finkel and Murphy laboratories and employed a gene trap strategy to constitutively delete MCU from all tissues (119) (also reviewed in Refs. 120, 121). Somewhat surprisingly, these animals are viable on a hybrid background and show no obvious defects in mitochondrial structure or number. However, rapid Ca2+ uptake over a range of 0.5–5.7 mM bath Ca2+ is ablated in isolated MCU−/− skeletal muscle mitochondria and cardiac mitochondria. The same lack of rapid mCa2+ uptake is noted in intact mouse embryonic fibroblasts (MEFs) prepared from MCU−/− embryos and in isolated MCU−/− adult mouse cardiomyocytes. Despite lowering resting mCa2+ concentration, constitutive loss of MCU has little effect on basal metabolic function of MEFs or purified mitochondria (119). Rather, the main metabolic consequence of constitutive MCU ablation is loss of the ability to increase matrix Ca2+ concentration to stimulate mitochondrial respiration (119).
Table 2.
Animal models for mCa2+ handling proteins of the inner mitochondrial membrane
| Reference | Model | Major Phenotype(s) |
|---|---|---|
| Pan et al. (119) | Mcu gene trap mouse (Mcu-null) | Ablation of rapid mCa2+ uptake; reduced skeletal muscle PDH activity; impaired capacity for treadmill running; reduced grip strength. Loss of Ca2+-induced PT, but cells are not protected from death with other stimuli. No protection from cardiac I/R injury. |
| Holmstrom et al. (122) | Mcu gene trap mouse (Mcu-null) | Impaired Ca2+-induced respiration but no cardiac functional defects basally; no change in heart’s response to chronic pressure overload or acute isoproterenol stress |
| Wu et al. (124) | αMHC-DN-MCU mice [constitutive expression of dominant-negative mutant (DIME→QIMQ) MCU in cardiomyocytes] | Impaired chronotropic response to isoproterenol; aberrant cellular Ca2+ handling related to impaired ATP production |
| Rasmussen et al. (125) | αMHC-DN-MCU mice [constitutive expression of dominant-negative mutant (DIME→QIMQ) MCU in cardiomyocytes] | Increased basal oxygen consumption, impaired inotropic and lusitropic contractile responses to increased cardiac pacing frequency; no protection against cardiac I/R injury |
| Luongo et al. (126) | Mcuflfl × αMHC-MCM mice; tamoxifen-inducible deletion of Mcu in adult cardiomyocytes | Reduced mPTP activation upon acute Ca2+; stress protection from cardiac I/R injury; diminished contractile response to acute β-adrenergic stimulation |
| Kwong et al. (127) | Mcuflfl × αMHC-MCM mice; tamoxifen-inducible deletion of Mcu in adult cardiomyocytes | No basal cardiac phenotype out to 1 yr of age, diminished NCLX expression and activity; reduced mPTP activation upon acute Ca2+, stress protection from cardiac I/R injury; diminished contractile response to acute β-adrenergic stimulation; impaired treadmill running that could be overcome with an exercise warm-up period; no protection from chronic pressure overload |
| Mammucari et al. (128) | 8-wk AAV-mediated MCU overexpression in mouse hindlimb skeletal muscle | Increased skeletal muscle fiber size; increased mitochondrial content; increased PGC-1α4 and IGF1-Akt/PKB signaling |
| Mammucari et al. (128) | 8-wk AAV-mediated MCU knockdown in mouse hindlimb skeletal muscle | Decreased skeletal muscle fiber size; decreased mitochondrial content; attenuated PGC-1α4 and IGF1-Akt/PKB signaling |
| Altamimi et al. (130) | Mcuflfl × αMHC-MCM mice; tamoxifen-inducible deletion of Mcu in adult cardiomyocytes | Increased fatty acid oxidation supporting increased contractility at baseline and after isoproterenol |
| Kwong et al. (129) | Mcuflfl × MyoD-Cre mice; constitutive deletion of Mcu in skeletal muscle | Impaired mCa2+ uptake an attenuation of Ca2+-stimulated mitochondrial respiration; impaired treadmill running capacity that could be overcome with a warm-up period; impaired glucose oxidation and impaired entry of carbon substrates into the TCA cycle; increased capacity for fatty acid metabolism. |
| Kwong et al. (129) | Mcuflfl × skeletal muscle α actin-MCM mice; tamoxifen-inducible deletion of MCU in adult skeletal muscle | Impaired mCa2+ uptake but no effect on skeletal muscle growth |
| Flicker et al. (132) | Mcuflfl × UCP1-Cre mice; constitutive deletion of Mcu in brown adipose tissue | Little effect on cold tolerance, diet-induced obesity, or transcriptional responses to cold exposure, despite effective ablation of acute mCa2+ uptake |
| Drago and Davis (134) | Drosophila with RNAi-mediated silencing of MCU homolog (GC18769) in mushroom body neurons | Defects in memory, but not learning, in adult flies. This effect was caused by silencing of MCU specifically during pupal development, which led to altered structure of mushroom body neurons. |
| Drago and Davis (134) | Drosophila with RNAi-mediated global silencing of MCU homolog (GC18769) | Developmentally lethal |
| Choi et al. (133) | Drosophila with loss-of-function mutant of MCU homolog (GC18769) | No gross defects, but flies are protected from oxidative stress. impaired mCa2+ uptake in response to caffeine. |
| Hutto et al. (237) | Zebrafish with cone photoreceptor-specific MCU overexpression | Increased mCa2+ uptake and mitochondrial swelling, but this is tolerated through late adulthood. Compensatory downregulation of MICU3. |
| Choi et al. (133) | Drosophila with muscle-specific MCU overexpression | Lethal, but lethality could be blocked by simultaneous knockdown of IP3R; lethality not blocked by simultaneous knockdown of IP3R and Sod1 |
| Choi et al. (133) | Drosophila with eye-specific overexpression of EMRE | No defective phenotype unless combined with overexpression of MCU; combined overexpression of MCU and EMRE is lethal. |
| Choi et al. (133) | Drosophila with knockdown of EMRE | Impaired mCa2+ uptake; phenocopies deletion of MCU. |
| Liu et al. (164) | Mice with constitutive deletion of Emre | Homozygous Emre−/− mice are born less frequently than expected and are small but viable. Resistance to Ca2+-induced permeability transition but no protection from cardiac I/R injury. |
| Liu et al. (163) | Heterozygous Emre deletion rescues homozygous deletion of Micu1. | |
| Antony et al. (206) | Mice with constitutive deletion of Micu1 (Micu1flfl × germline Cre-eIIa) | Perinatal lethality, trend toward reduced neuron density in nucleus ambiguus and nucleus facialis, possible defect in respiration. MEFs show excessive mCa2+ at low bath Ca2+ concentration. |
| Antony et al. (206) | Mice with inducible deletion of Micu1 in the liver (Micu1flfl injected with AAV8-Cre under a hepatocyte-specific thyroxine-binding globulin promoter) | Tolerated at baseline, but increased susceptibility to liver injury and impaired liver regeneration following experimental stress with partial hepatectomy |
| Liu et al. (163) | Mice with CRISPR/Cas9 mediated constitutive deletion of Micu1 | Perinatal lethality, small body weight and developmental delay, brain abnormalities, ataxia, and muscle weakness. Gradual compensatory downregulation of EMRE with age is associated with partial normalization of body mass and brain histology. |
| Debattisti et al. (207) | Mice with constitutive deletion of Micu1 in skeletal muscle (Micu1flfl × Creatine kinase-Cre) | Impaired mCa2+ uptake during twitch and tetanic muscle contraction, and sarcolemmal repair defect leading to muscle weakness and wasting |
| Drago and Davis (134) | Drosophila with RNAi-mediated silencing of MICU1 homolog (CG4495) in mushroom body neurons | Defects in memory, but not learning, in adult flies |
| Drago and Davis (134) | Drosophila with RNAi-mediated global silencing of MICU1 homolog (CG4495) | Developmentally lethal |
| M’Angale and Staveley (179) | Drosophila with inducible RNAi-mediated silencing of MICU1 homolog (GC4495) in neurons | Reduced survival and early loss of locomotor function |
| Xue et al. (209) | siRNA knockdown of Micu1 via lentiviral injection in the mouse heart | Exacerbated mCa2+ overload and myocardial injury, with exacerbated cardiac dysfunction, upon myocardial I/R |
| Tufi et al. (195) | Drosophila with homozygous null mutation of MICU1 homolog | Lethal; lethality is not rescued by simultaneous knockout of MCU or EMRE, or by ubiquitous overexpression of MICU3. |
| Bick et al. (234) | Micu2 gene trap mice with constitutive, global knockout of Micu2 | Little baseline phenotype, but diastolic dysfunction leads to left atrial enlargement by 16–18 mo of age. Accelerated cardiac decompensation upon chronic angiotensin II infusion; increased susceptibility to abdominal aortic aneurysm with chronic angiotensin II infusion |
| Tufi et al. (195) | Drosophila with CRISPR/Cas9-induced disruption of MICU3 homolog | Viable, but have a modest reduction in life span, climbing defect (interpreted as neurological defect) in both young and older flies. No effect on basal mitochondrial respiration in fly head tissue. |
| Puente et al. (236) | Mice with germline Micu3 deletion | No basal cardiac phenotype but protection from chronic isoproterenol-induced mCa2+ overload, contractile dysfunction, and left ventricular dilation; protection from ex vivo I/R injury |
| Lambert et al. (242) | CAG-CAT-MCUB transgenic × αMHC-MerCreMer mice (inducible adult cardiomyocyte-specific MCUB overexpression): | |
| • 5-day cardiac MCUB overexpression | Acute 5-day overexpression: diminished acute mCa2+ uptake; diminished baseline cardiac contractility and diminished contractile response to acute β-adrenergic stimulation; reduced mitochondrial metabolism; increased mortality during cardiac ischemia | |
| • 1-mo cardiac MCUB overexpression: | Chronic 1-mo overexpression: diminished acute mCa2+ uptake; normal cardiac contractile response to acute β-adrenergic stimulation, normal mitochondrial metabolism; reduced myocardial infarct size upon I/R | |
| Huo et al. (241) | Tetracycline-off model of cardiomyocyte MCUB overexpression (αMHC-tTA × TRE-MCUB mice, constitutively kept off DOX to allow constitutive MCUB overexpression | No detrimental effect on baseline cardiac function; protection of heart from I/R injury and mPTP activation |
| Huo et al. (241) | Mice with Mcub knockout first allele, for constitutive whole body disruption of Mcub | No detrimental effect on mCa2+ uptake, oxygen consumption, or cardiac function at baseline; exacerbated myocardial injury, cardiac remodeling, and contractile function following cardiac I/R |
| Tomar et al. (253) | Mice with constitutive, endothelial cell-specific deletion of Mcur1 (Mcur1flfl × VE-Cad-Cre) | Impaired endothelial cell mCa2+ uptake and reduced basal and agonist-induced increase in ATP; diminished cell proliferation and migration but increased autophagy. Mice are normal but have increased body heat dissipation associated with increased UCP2 expression in Mcur1-KO endothelial cells. |
| Tomar et al. (253) | Mice with constitutive, cardiomyocyte-specific deletion of Mcur1 (Mcur1flfl × αMHC-Cre) | Born at expected ratios but are small and die within 3 wk of birth; decreased cardiomyocyte mCa2+ uptake and mtCU currents, but normal cardiomyocyte mCa2+ content; increased autophagy in Mcur1-KO cardiomyocytes |
| Beutner et al. (275) | Mice with constitutive RyR1 knockout (RyR1−/−) | Lethal birth defect; animals die immediately after birth; trend toward elevated basal oxygen consumption in neonatal RyR1−/− heart homogenates; loss of Ca2+-stimulated increase in mitochondrial respiration |
| Luongo et al. (166) | Mice with tamoxifen-inducible, cardiomyocyte-specific deletion of NCLX (Slc8b1fl/fl × αMHC-MerCreMer) | Reduced cardiomyocyte mCa2+ efflux, cardiomyocyte mCa2+ overload, increased ROS production, and cardiomyocyte necrosis; left ventricular dilation and impaired contractility, cardiac hypertrophy and fibrosis; 87% lethality within 2 wk of cardiomyocyte NCLX deletion; rescued by simultaneous deletion of the mPTP component Cyclophilin D |
| Luongo et al. (166) | Mice with constitutive, cardiomyocyte-specific NCLX deletion (Slc8b1flfl × αMHC-Cre) | Normal viability but reduced mCa2+ efflux; compensatory reduction in mCa2+ uptake; normal cytosolic Ca2+ handling |
| Luongo et al. (166) | Mice with cardiomyocyte-specific, doxycycline-controlled transgenic NCLX overexpression (TRE-NCLX × αMCH-tTA) | Increased cardiomyocyte mCa2+ efflux; increased resistance to permeability transition, protection against cardiac I/R injury; protection from LV dilation, contractile dysfunction, hypertrophy, fibrosis, and inflammation after myocardial infarction |
| Jadiya et al. (323) | Neuronal-specific, constitutive deletion of NCLX in 3xTg-AD Alzheimer’s disease mouse model (3xTg-AD × Slc8b1fl/fl × Camk2a-Cre) | Accelerated impairment in spatial working memory, contextual recall, and cued recall; increased amyloid burden and tau pathology |
| Jadiya et al. (323) | Neuronal-specific, doxycycline-controlled overexpression of NCLX in 3xTg-AD Alzheimer’s disease mouse model (3xTg-AD × TRE-NCLX × Camk2a-tTA) | Protection against age-related cognitive decline, reduced amyloid plaque burden and tau pathology; reduced susceptibility to permeability transition; reduced Alzheimer’s disease-associated increase in brain superoxide production and lipid peroxidation |
| Pathak et al. (325) | Mice with constitutive, global CRISPR/Cas9-meditated disruption of NCLX (Slc8b1−/−) | Viable and develop fewer and smaller tumors when subjected to the colitis-associated colorectal cancer model |
| Sharma et al. (326) | C. elegans with null mutation in the NCLX-like gene ncx-9 | Developmental defects in the left/right projection patterning of the GABAergic motor neuron circuit |
| Hasegawa and van der Bliek (343) | C. elegans with null mutation in letm-1 | Homozygous animals arrest in L3 larval stage and are small and infertile. Heterozygous animals are normal. |
| Hasegawa and van der Bliek (343) | C. elegans with RNAi-mediated knockdown of letm-1 | Delayed development and small body size at adulthood; swollen and disorganized mitochondria |
| Hasegawa and van der Bliek (343) | C. elegans with transgenic expression of LETM-1 under the control of the myo-3 promoter | Crimping of the mitochondrial matrix, swelling of outer mitochondrial membrane, occasional detachment of outer membrane from the inner mitochondrial membrane and matrix |
| McQuibban et al. (353) | Drosophila with ubiquitous LETM1 (CG4589) knockdown using the tub-GAL4 driver, the da-GAL4 driver, or the act-GAL4 driver | Lethal during development |
| McQuibban et al. (353) | Drosophila with muscle-specific LETM1 (CG4589) knockdown using the mef2-GAL4 driver | Larvae are small and have reduced physical activity; most flies arrest growth in the pupal stage. Flies that progress to adulthood are small, weak, and unable to fly. |
| McQuibban et al. (353) | Drosophila with eye-specific LETM1 (CG4589) knockdown using the ey-GAL4 driver | Small eye facets surrounded by scar tissue; ommatidia exhibit swollen mitochondria. |
| McQuibban et al. (353) | Drosophila with nervous system-specific LETM1 (CG4589) knockdown using the elav-GAL4 driver or s-nyb-GAL4 driver | Reduced speed of locomotion and increased time spent immobile; reduced neurotransmitter release following nerve stimulation |
| Jiang et al. (372) | Mice with homozygous deletion of Letm1 via gene trap | Lethal by day 6.5 of embryogenesis |
| Jiang et al. (372) | Mice with heterozygous deletion of Letm1 via gene trap | ∼50% die by day E13.5. Surviving mice are relatively normal and have normal mitochondrial morphology, but have impaired brain ATP concentration and reduced PDH activity. Have increased seizures in response to kainic acid. |
| Zhang et al. (377) | Rats with lentiviral knockdown of LETM1 in the hippocampus and dentate gyrus | Mitochondrial swelling and reduced mitochondrial gene expression; increased susceptibility to seizures in a pilocarpine-induced epilepsy model. Increased seizure susceptibility is not corrected by treatment with the K+/H+ ionophore nigericin. |
| Elrod et al. (414) | Mice with constitutive, global ablation of Cyclophilin D (Ppif−/−) | Loss of mPTP activity; impaired cardiac mCa2+ efflux; increased cardiac TCA cycle flux and shift toward glucose rather than fatty acid metabolism; impaired cardiac contractility in response to acute isoproterenol infusion; exaggerated pressure overload-induced cardiac dysfunction and hypertrophy; exaggerated cardiac dysfunction and death upon chronic exercise |
See glossary for abbreviations.
Pan et al. (119) report that germline MCU-null animals have reduced grip strength, forelimb muscle strength, and maximal work capacity when running on an inclined treadmill, despite no changes in skeletal muscle fiber type composition. These findings indicate that rapid mCa2+ uptake through the mtCU may be required for acute increases in muscle force production and/or power output, likely by supporting increased mitochondrial ATP production required to fuel increased muscle contraction (FIGURE 3).
FIGURE 3.
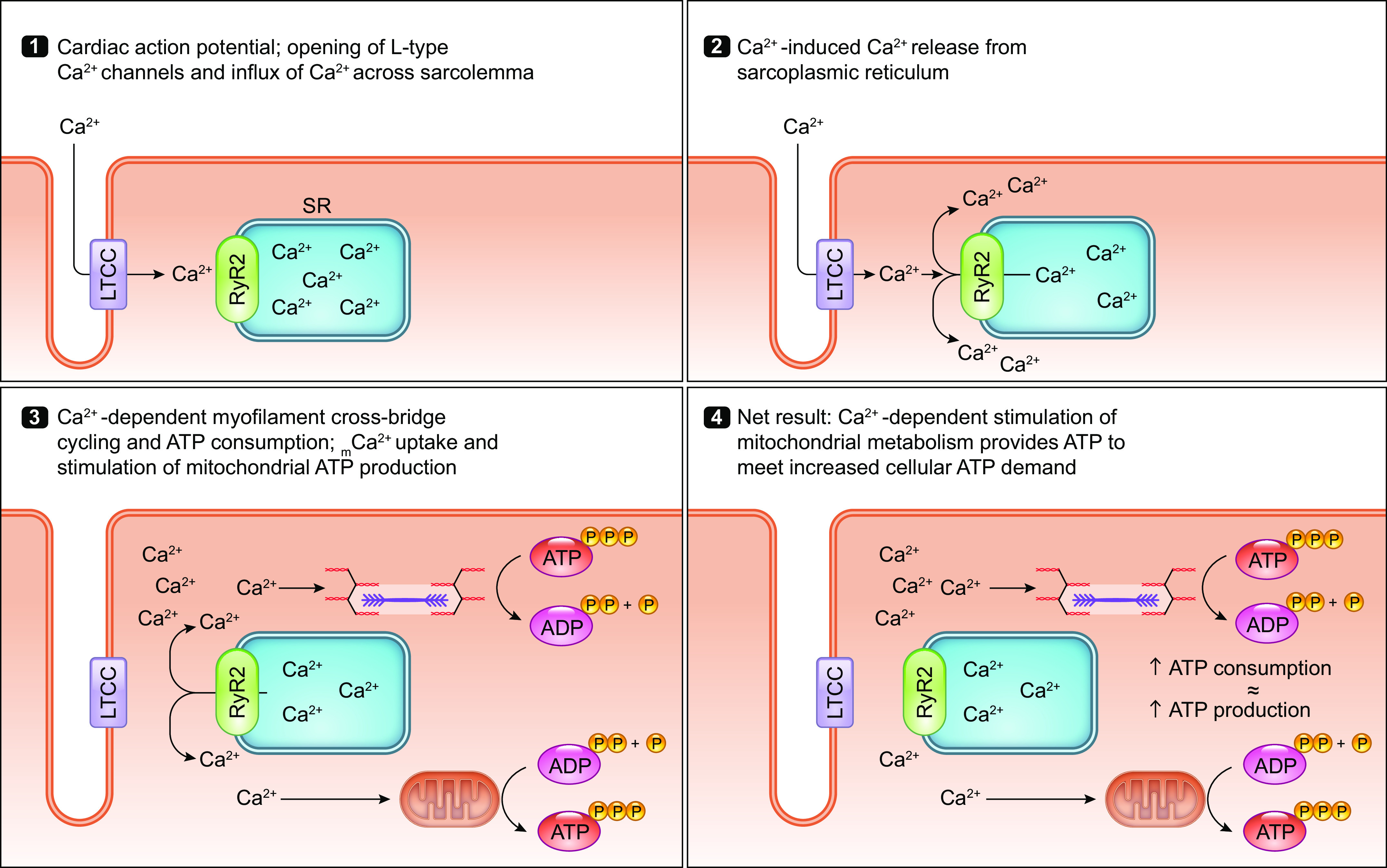
Ca2+ signaling in the parallel activation of ATP-consuming processes and ATP production. Ca2+ serves as a second messenger that coordinates the activation of both ATP-consuming and ATP-generating processes within the cell. This process is particularly important for tissues with a high energy demand such as the heart. The parallel activation of ATP consumption and ATP generation within cardiomyocytes is illustrated stepwise as follows: 1) Extracellular Ca2+ enters the cardiomyocyte through the L-type calcium channel (LTCC) that opens with each action potential. 2) Ca2+ that entered the cardiomyocyte through the LTCC activates the ryanodine receptor (RyR2), triggering calcium-induced calcium release from the sarcoplasmic reticulum (SR). 3) Increased cytosolic Ca2+ stimulates the ATP-consuming process of myofilament cross-bridge cycling and cardiac contraction. At the same time, increased cytosolic Ca2+ concentration drives increased mitochondrial Ca2+ uptake that stimulates mitochondrial ATP production. 4) This mechanism of Ca2+-dependent parallel activation allows the heart to respond to an increased workload with a sustained increase in mitochondrial ATP production that matches the increase in ATP consumption by the contractile apparatus. See glossary for abbreviations.
Additional experiments by Pan et al. argue for a role for MCU-dependent mCa2+ uptake in mitochondrial permeability transition. Both liver and heart MCU−/− mitochondria are remarkably resistant to Ca2+-induced mPTP opening (119). Somewhat unexpectedly, this protection against Ca2+-induced mPTP opening does not translate to any measurable protection of constitutive MCU-null hearts from ex vivo, global ischemia-reperfusion (I/R) injury (119). The reasons for this discrepancy are incompletely understood but may involve roles for elevated cytosolic, rather than mitochondrial, Ca2+ in cell death signaling during I/R injury or upregulation of mPTP-independent cell death pathways such as necroptosis in Mcu−/− mice (119, 120).
A later study by the same group used the MCU gene trap mouse model to examine the specific consequences of germline MCU deletion on cardiac physiology. Mcu−/− cardiac mitochondria exhibit reduced resting matrix Ca2+ content and an impaired respiratory response to Ca2+ (122). EMRE levels are also reduced in Mcu−/− hearts. However, constitutive loss of MCU has no detectable effect on baseline cardiac function out to nearly 2 yr of age, on cardiac structural or functional phenotype after 8 wk of transverse aortic constriction (TAC), or on contractile responses to acute in vivo isoproterenol stimulation (122). The lack of a significant defect in acute functional responses to β-adrenergic stimulation in MCU-null hearts, despite a loss of mitochondrial Ca2+ uptake (119), cast some doubt on the link between acute mCa2+ signaling and the increase in mitochondrial ATP synthesis required to fuel an increase in cardiac contractility. However, in light of findings in conditional MCU models discussed below, this negative result may simply be the result of the constitutive germline deletion strategy allowing for significant compensatory mechanisms to be activated during both development and aging, thereby masking any role for MCU in these processes (121, 123).
3.3.1.5.2. Conditional MCU models.
A series of conditional MCU mutant mouse models published in 2015 offer further insight into the role of MCU in cardiac physiology and disease. Mark Anderson’s group (124) developed transgenic mice in which the cardiac-specific α-myosin heavy chain (α-MHC) promoter drives expression of a dominant-negative MCU (DN-MCU) shortly after birth. In this construct, the aspartic acid and glutamic acid residues of the critical DIME motive are mutated to glutamine (i.e., DIME→QIMQ), resulting in ablation of MCU activity, presumably due to the displacement of endogenous MCU from the uniporter channel. DN-MCU mice have a normal basal heart rate but have a blunted increase in heart rate upon in vivo isoproterenol administration or increased physical activity, which correlates with diminished atrial ATP content. Administration of exogenous ATP in vitro to isolated cardiac sinoatrial node cells expressing DN-MCU rescues the isoproterenol-induced increase in action potential frequency, suggesting a link between mCa2+ uptake and cellular ATP production required for chronotropic responsiveness (124). DN-MCU sinoatrial cells also exhibit diminished SR Ca2+ content and reduced diastolic Ca2+ release. Wu et al. (124) hypothesize that this effect results from impaired ATP-dependent SERCA activity required for Ca2+ reuptake into the SR. They propose that MCU is specifically required for the increase in sinoatrial node activity and heart rate in response to stress, because mCa2+ uptake through MCU enhances OXPHOS activity to increase ATP levels sufficiently to power ionic homeostasis and cellular Ca2+ cycling.
DN-MCU hearts also have higher rates of oxygen consumption than controls across multiple frequencies of electrical pacing, despite a diminishment in left ventricular inotropic and lusitropic responses to electrical pacing (125). This effect on oxygen consumption rate (OCR) is not observed under low-Ca2+ conditions in isolated cardiomyocytes or mitochondria, suggesting that MCU inhibition causes extramitochondrial adaptations leading to increased OCR during Ca2+ stress. Such adaptations could arise as a result of the increased energetic demand needed to maintain cytosolic Ca2+ homeostasis in the absence of MCU-dependent mCa2+ uptake. Similar to the MCU gene trap hearts (122), DN-MCU hearts are not protected from I/R-induced cell death (125). This observation reinforces the notion that chronic disruption of MCU activity may drive compensatory adaptations to maintain critical cellular processes (cell death signaling, etc.) in the absence of rapid mCa2+ uptake. Indeed, this study reports increased expression of the cell death gene Bax in DN-MCU hearts, which may explain why DN-MCU hearts are not protected from I/R injury despite abolished rapid mCa2+ uptake (125).
The question of the acute role for MCU in cardiac stress physiology, separable from compensatory effects allowing adaptation to chronic knockout or inhibition of MCU, was clarified by the generation of MCU floxed mice by our group (126) and the Molkentin laboratory (127), allowing for tissue-specific and temporally controlled MCU ablation when crossed to inducible Cre drivers. The major findings from these studies are presented here; for a more thorough examination and a discussion of how we can reconcile conflicting results from different genetic MCU models, the reader is directed to recent reviews (121, 123). Rapid mCa2+ uptake is ablated in cardiomyocytes isolated from tamoxifen-treated Mcufl/fl × αMHC-MerCreMer (MCM) mice. However, no differences in basal cardiomyocyte mCa2+ are observed between controls and Mcufl/fl × αMHC-MCM mice (126, 127). This finding is consistent with the Molkentin laboratory’s observation of no baseline phenotype in MCU conditional knockout hearts at up to 1 yr of age (127). Decreased expression and activity of the mitochondrial sodium/calcium exchanger, NCLX, may help to maintain relatively normal basal matrix Ca2+ content in these hearts (127).
Acute cardiomyocyte Mcu deletion blunts the increase in cardiac contractility upon adrenergic stimulation; this is associated with an impaired increase in mCa2+ content, OCR, and NADH production (126, 127). Kwong et al. (127) also note a diminished maximal treadmill running capacity in mice with acute cardiomyocyte Mcu deletion, presumably related to impaired matching of cardiac energetic demand and cardiac energy production. However, this exercise defect can be overcome if these mice are allowed a prolonged exercise warm-up period before the running test (127). Furthermore, when subjected to prolonged isoproterenol stimulation, the OCR, cardiac contractility, and mCa2+ content of MCU-knockout cardiomyocytes or hearts eventually attain values matching those of control mice (127). These results strengthen the idea that MCU is specifically required for acute increases in mitochondrial Ca2+, to mediate acute adaptations to the bioenergetic stress of increased contractile demand (FIGURE 3). Given sufficient time to take effect, though, additional MCU-independent pathways can increase mCa2+ content and enable mitochondrial and/or extramitochondrial energetic pathways to provide cardiomyocytes with sufficient ATP to support contractility.
In contrast to findings in mice with germline MCU deletion (119) or constitutive cardiomyocyte expression of DN-MCU (125), acute loss of MCU from adult cardiomyocytes in Mcufl/fl × αMHC-MCM mice protects against myocardial I/R injury and preserves contractile function after reperfusion (126, 127). Acute MCU deletion also reduces mitochondrial swelling and the frequency of mPTP opening in response to Ca2+ stress, indicating that the mtCU is responsible for acute mCa2+ overload that triggers permeability transition and cardiomyocyte death upon intracellular Ca2+ overload during I/R injury (126, 127). Finally, a small study testing the consequences of adult cardiomyocyte-specific MCU knockout on the heart’s response to chronic hemodynamic stress resulting from 8 wk of transverse aortic constriction (TAC) showed no effect of MCU loss on pathological cardiac remodeling or contractile function (127). These data would suggest that MCU is dispensable for the cardiomyocytes’ adaptations to sustained hemodynamic or energetic stress. However, the length of time (18 wk) between the start of tamoxifen administration and the end point of the TAC study warrants caution in this interpretation, as this may have been sufficient time for alternative pathways to compensate for the lack of MCU and mask any beneficial or detrimental effects that might have been observed with more acute Mcu deletion.
Despite the discrepancies in the above models of constitutive versus conditional MCU disruption, several consistent themes regarding the physiological role of MCU emerge from these animal models: 1) MCU is required for rapid mCa2+ uptake, which may be most physiologically relevant in situations requiring acute increases in cellular energetic demand (i.e., during exercise or sympathetic stimulation) rather than required for basal cellular function. 2) Additional, slower routes of mCa2+ entry independent of MCU and/or the mtCU complex may exist to support residual matrix Ca2+ levels in MCU-deficient mitochondria, with the caveat that accurate measurement of basal matrix Ca2+ levels is tricky business (126) and so any additional routes of mCa2+ entry may be moot in these experiments. 3) Excessive Ca2+ uptake via the mtCU complex contributes to mCa2+-dependent mPTP opening. 4) Over time, cells may remodel to compensate for the loss of MCU to sustain critical processes such as energy metabolism and cell death versus survival signaling via alternative, MCU-independent mechanisms. Such adaptive mechanisms may help to explain why disruption of MCU in adult animals has little apparent effect on the basal, unstressed phenotype of tissues such as the heart.
Additional animal models of MCU manipulation have yielded further insights into the physiological roles of MCU in tissues beyond the heart. Adeno-associated virus (AAV)-mediated MCU overexpression in mouse skeletal muscle increases fiber size in both slow and fast muscles, whereas MCU knockdown decreases fiber size in both muscle types (128). Skeletal muscle MCU overexpression is associated with increased mitochondrial content, increased expression of PGC-1α4 (a regulator of mitochondrial biogenesis), and increased downstream signaling by prohypertrophic insulin, IGF1-Akt/PKB, and mTOR pathways (128). In contrast, MCU knockdown attenuates these pathways (128). Mammucari et al. (128) demonstrate that MCU overexpression is sufficient to attenuate muscle atrophy following denervation caused by sciatic nerve section, suggesting that MCU-dependent mCa2+ uptake can counteract pathological atrophy as well as stimulate hypertrophy. These authors propose that MCU overexpression triggers muscle growth by altering the anabolic versus catabolic balance, rather than exclusively via a metabolic effect, since aerobic metabolism is not altered by MCU levels and hypertrophic effects of MCU overexpression are observed in both oxidative and glycolytic muscles.
Constitutive deletion of MCU in skeletal muscle with a MyoD-Cre driver in floxed MCU mice does not affect muscle size or AKT signaling (129). In contrast to the findings by Mammucari et al. (128), genetic deletion of MCU in adult skeletal muscle likewise has no effect on muscle weight or pathology (129). However, the disparity in these results may reflect differences in MCU knockout versus knockdown strategy or the sex or genetic background of the mice used [CD1 males (128) vs. C57BL/6 males and females (129)]. MCU-knockout skeletal muscle exhibits impaired glucose oxidation, consistent with limited mCa2+-dependent stimulation of pyruvate dehydrogenase (PDH) activity, but an increased capacity for fatty acid metabolism (129). A similar phenotype of increased cardiac fatty acid metabolism is observed in mice with adult cardiomyocyte-specific loss of MCU when examined 3 mo after MCU deletion (130). In this study, ex vivo working hearts with MCU deletion (Mcufl/fl × αMHC-MCM mice) unexpectedly show enhanced contractile function compared with Mcufl/fl controls, both at baseline and after acute isoproterenol challenge. Hearts of both genotypes increase glucose oxidation in response to isoproterenol, but only hearts with Mcu deletion increase fatty acid oxidation after isoproterenol administration. Thus, MCU-null striated muscle may adapt to impaired mCa2+-regulated carbohydrate oxidation by upregulating pathways for fatty acid metabolism as an alternative mechanism to access energy reserves during periods of stress. Altamimi et al. (130) propose that such increased capacity for fatty acid utilization explains the enhanced contractile function observed in MCU-deleted hearts. Unfortunately, αMHC-MCM controls were not included in this study (130), thus precluding any discrimination of any specific effects of MCU loss alone on cardiac function and metabolism, as opposed to effects of cardiac Cre expression, which is noted to generate significant phenotypes (131). Despite these limitations, the studies discussed above support the hypothesis that acute mCa2+ uptake through MCU supports the use of carbohydrates by striated muscle tissue, with particularly important acute effects on muscle energetics and work capacity, but that with sufficient time, muscle can functionally compensate for the loss of MCU by upregulating fatty acid oxidation. That is, MCU-dependent Ca2+ uptake is likely not a direct regulator of fatty acid oxidation, but rather increased fatty acid oxidation is a downstream compensatory adaptation to diminished mCa2+ uptake.
Elucidation of the role of MCU in the physiology of nonmuscle tissues is ongoing. A third Mcufl/fl mouse on the C57BL/6 background was independently generated by Vamsi Mootha’s group and crossed to mice expressing Cre under the control of the uncoupling protein 1 (UCP1) promoter, allowing for constitutive deletion of Mcu in brown adipose tissue (BAT) (132). Despite evidence for large mtCU currents in BAT (117), ablation of acute mCa2+ uptake via MCU knockout has little effect on cold tolerance, diet-induced obesity, or cold-induced transcriptional responses (132). Thus, MCU appears largely dispensable for bioenergetics in BAT (132), in contrast to the effects described above for striated muscle. These findings may reflect diversity in the physiological relevance of MCU-mediated mCa2+ uptake across different tissue types. It is important to note, however, that in this constitutive MCU-knockout model compensatory adaptations akin to those discussed for the original MCU gene trap mice (119–121, 123) may have obscured any physiological roles for MCU within BAT.
Research in Drosophila offers additional evidence for the importance of this protein to cellular function. Deletion of the Drosophila MCU homolog, GC18769, effectively ablates rapid mCa2+ uptake but has no effect on viability or metabolic phenotype (133). MCU-mutant flies exhibit increased resistance to oxidative stress (H2O2) compared with wild-type flies, as indicated by increased survival, reduced TUNEL staining, and reduced expression of proapoptotic markers. This phenotype is attributed to a reduction in mCa2+ uptake otherwise triggered by oxidative stress. Drosophila have also been used to investigate MCU in the brain. Drago and Davis (134) describe flies with knockdown of CG18769 via RNA interference (RNAi) in different neuronal populations of the olfactory system and show that silencing of MCU in mushroom body neurons impairs memory without affecting learning. Mutation of the MCU DIME motif in mushroom body neurons yields the same effect, indicating that functional MCU within this cell population is required for memory. Remarkably, memory seems to rely predominantly on the appropriate expression of MCU during development. Silencing of MCU specifically during the pupal stage affects memory in adult flies, whereas silencing of MCU earlier in development or in adult flies produces no memory defect (134). The loss of MCU during pupal development reduces synaptic vesicle number and/or size in the horizontal lobes of the mushroom body neurons and increases the length and field volume of the axons of αβ mushroom body neurons. Thus, MCU function is required during neuronal development to establish the appropriate neural circuitry or neuronal structure and function that underlies adult memory (134).
3.3.1.6. regulation of mcu.
The mouse models of MCU deletion discussed above suggest that MCU may be an attractive therapeutic target for pathologies involving cellular death caused by acute mCa2+ overload, such as ischemia-reperfusion injury following treatment for a myocardial infarction. The molecular mechanisms controlling MCU expression and activity are thus a topic of active research. In vitro experiments in chicken DT40 B lymphocytes and HeLa cells reveal that the Ca2+-regulated transcription factor cyclic adenosine monophosphate response element-binding protein (CREB) binds directly to the MCU promoter and stimulates transcription of MCU (135). This mechanism is thought to increase MCU expression in order to increase mCa2+ uptake in response to cytosolic Ca2+ signaling, perhaps as a way for the cell to buffer excess cytosolic Ca2+ and shape Ca2+-regulated signaling within the cell. A recent study in rats indicates that increased phospho-CREB-dependent MCU expression in the spinal cord dorsal horn contributes to the development of morphine tolerance (136). This work also detected binding of cytoplasmic polyadenylation element binding protein 1 (CPEB1) to the 3′ untranslated region of MCU mRNA, and showed that knockdown of CPEB1 decreases MCU protein expression, suggesting that CPEB1 facilitates translation of MCU mRNA into protein (136). Although CREB-dependent MCU transcription may occur in some cell types, one caveat to this hypothesis, which may preclude it from representing a robust mode of physiological regulation, is that the proposed signaling would put the cell in peril of mitochondrial Ca2+ overload and cell death.
MCU expression is also regulated by microRNAs (miRs). Zaglia et al. (137) find that miR-1 inhibits the translation of MCU mRNA and is inversely correlated with increasing MCU levels observed in both mouse and human hearts during physiological and pathological cardiac hypertrophy. These authors propose that chronic β-adrenergic receptor activation in pathological hypertrophy represses miR-1 expression and allows for increased cardiac MCU content. Fitting with this model, pharmacological β-adrenergic receptor blockade prevents the decline in miR-1 levels and associated increase in MCU protein expression observed in the hearts of mice subjected to transverse aortic constriction (137). miR-25 likewise downregulates MCU expression, which may protect cardiomyocytes against the damaging effects of oxidative stress by preventing mCa2+ overload (138). miR-25 is also linked to downregulation of MCU in colon cancer and cancer cell lines, possibly functioning as a mechanism that prevents apoptosis and promotes the survival of cancer cells (139). In line with these findings, enhanced proliferation, migration, and apoptosis resistance of pulmonary artery smooth muscle cells in pulmonary arterial hypertension are associated with increased miR-138 expression and a trend toward increased expression of miR-25. These miRs together downregulate MCU expression, reduce mCa2+ levels, and increase cytosolic Ca2+ concentration (140). However, a conflicting report suggests that, in breast cancer, increased MCU expression instead promotes cell migration, invasion, and metastasis by increasing glycolysis and the Warburg effect (141). In this study, increased expression of miR-340, another negative regulator of MCU expression, reduces the metastatic potential of breast cancer cell lines injected into nude mice. Considered together, these results indicate that numerous miRs can negatively regulate MCU expression, although the specific consequences of altered cellular MCU protein levels for cellular survival, growth, and proliferation can vary across different cell types and across distinct pathologies. However, it should be noted that a full review of the literature suggests minimal to no changes in MCU expression in response to physiological or pathological stimuli. Indeed, transcriptional modulation of the mtCU appears much greater for the mtCU regulators MICU1, EMRE, and MCUB.
MCU protein is subjected to a variety of posttranslational modifications that alter overall MCU activity. Acute regulation of MCU by a kinase, CaMKII, was first reported by Mark Anderson’s laboratory in 2012 (142). Genetic inhibition of CaMKII activity attenuates MCU current and protects the heart against pathologies associated with mCa2+ overload, including I/R injury, myocardial infarction, and injury due to acute isoprenaline stimulation (142). This study identified serines 57 and 92 of MCU as potential CaMKII phosphorylation sites and demonstrated that phospho-null mutation of these sites to alanine is sufficient to block a CaMKII-dependent increase in MCU current in vitro in mitoplasts prepared from human embryonic kidney (HEK) cells. It should be noted, though, that concrete evidence of CaMKII at the IMM remains elusive. The idea that CaMKII signaling acutely regulates MCU current in the heart is challenged by Kirichok’s group, who question the identity of the large currents that Joiner et al. (142) identified as IMCU, on the basis of the currents being two orders of magnitude greater than MCU currents measured in the Kirichok laboratory; discrepancies in Ru360 sensitivity of the measured current; and failure to replicate Joiner et al.’s finding of increased mitoplast MCU current upon addition of constitutively active CaMKII in vitro (143). However, other data indicate that S92A mutation of MCU impairs MCU activity in vitro (112), strengthening the idea that phosphorylation or other modification of this residue can modulate mtCU function and mCa2+ uptake. The possible functional consequences of MCU S92 phosphorylation by CaMKII or other kinases remains a topic of active research. Conflicting studies variously indicate a role for CaMKII in phosphorylating S92 to promote MCU activity, mitochondrial mobility, and the migration of vascular smooth muscle cells (144) or, instead, little consequence of CaMKII deletion on mCa2+ uptake, mitochondrial redox state, respiration, or reactive oxygen species (ROS) production in cardiac mitochondria (145). A possible explanation for these disparate findings is that the role of CaMKII in MCU regulation at S92 or other sites is highly dependent on the particular tissue of study. The identification of AMP-activated protein kinase (AMPK) as another potential regulator of MCU S57 phosphorylation and uniporter activity (146) emphasizes that multiple signaling cascades likely converge to affect multiple regulatory residues on MCU, with the ultimate functional consequences for MCU activity depending on the particular combination of posttranslational modifications present on the protein at any given time.
MCU is also phosphorylated at as yet undefined tyrosine residue(s) by the Ca2+-sensitive protein tyrosine kinase, proline-rich tyrosine kinase 2 (PYK2), leading to an increase in mCa2+ uptake capacity (147). Pyk2-dependent tyrosine phosphorylation and stimulation of MCU may underlie the increase in mCa2+ uptake observed upon treatment of H9c2 cardiac myoblasts and neonatal rat cardiomyocytes with α-adrenergic agonists such as phenylephrine. Interestingly, Pyk2-dependent MCU phosphorylation is associated with increased oligomerization of MCU, suggesting that tyrosine phosphorylation of MCU may promote mCa2+ uptake by facilitating the organization of individual MCU monomers into functional multisubunit channels (147).
Additional forms of posttranslational modification beyond phosphorylation have been detected for MCU as well. The Madesh laboratory identified a conserved residue, C97, as a key residue of human MCU that is subject to oxidative modification (148). Upon oxidative stress, C97 undergoes S-glutathionylation, which enhances mCa2+ uptake via a mechanism that likely involves structural changes in the MCU NH2-terminal domain that allow for increased oligomerization of MCU into high-molecular weight, functional uniporter complexes. Thus, Dong et al. (148) propose that cellular oxidative stress promotes mCa2+ uptake through MCU, which may stimulate further production of ROS by the mitochondria and set up a positive feedback loop of increasing mCa2+ uptake and ROS production that could predispose cells to death. Similar to the feedback look reported for transcriptional regulation of MCU, this mechanism may contribute to pathophysiology but is likely not a physiological regulator of the mtCU.
3.3.1.7. pharmacological modulation of mcu.
The mtCU can be inhibited by ruthenium red and its derivatives, such as Ru360. Early mutagenesis studies revealed that Ru360 blocks MCU activity by binding at or near the MCU DIME motif (99). Later studies suggest that Ru360 specifically binds the DIME motif at the carboxylate ring of D240, which is exposed to the intermembrane space at the entrance of the MCU selectivity filter (149). Despite the value of ruthenium red and Ru360 as experimental tools to study mCa2+ uptake and its biological consequences in vitro, their potential therapeutic utility is limited by their poor cell permeability and off-target effects (150) (also reviewed in Ref. 151). Woods et al. (152) developed a Ru360 analog, termed Ru265, that exhibits improved cell permeability over Ru360 and that inhibits MCU in a manner that is not affected by MCU S259A mutation, indicating that its site of action is distinct from that of Ru360. Instead, Ru265 inhibits MCU by binding to the NH2-terminal domain and favoring MCU aggregation in a configuration that is distinct from typical MCU oligomerization and inhibits MCU Ca2+ conductance. Recent small-molecule screens have also identified a number of additional MCU inhibitors that may be amenable to clinical use. The chemotherapeutic drug mitoxantrone inhibits MCU by interacting with acidic residues of the selectivity filter (153). The cell-permeant compound DS16570511 also inhibits mCa2+ uptake in HEK293A cells and in isolated perfused rat hearts (154). The exact site(s) of DS16570511 action within MCU or the uniporter complex has yet to be identified, although its inhibition of mCa2+ uptake may involve effects on MCU and/or MICU1 (154).
A number of chemical MCU activators have been described as well. MCU is reversibly activated by several plant flavonoids, the most potent of which is kaempferol, which triggers a nearly twofold increase in mCa2+ uptake rate in HeLa cells at as little as 1 µM concentration (79). Proof of concept for the therapeutic application of MCU activation by kaempferol was demonstrated in 2017 as Schweitzer et al. (155) showed that treatment of a mouse model of catecholaminergic polymorphic ventricular tachycardia with kaempferol attenuates isoproterenol-induced arrhythmogenic cytosolic Ca2+ waves in cardiomyocytes in vitro, and attenuates caffeine + epinephrine-induced ventricular tachycardia in mice in vivo. These findings suggest that pharmacological manipulation of MCU activity may be a feasible approach not only for preventing mCa2+ overload but also for correcting pathogenic defects in cytosolic Ca2+ handling.
3.3.2. EMRE.
The transmembrane protein EMRE (Essential MCU Regulator; gene name: Single-pass membrane protein with aspartate-rich tail 1, mitochondrial, SMDT1) was identified as an mtCU component in a proteomics screen in HEK293T cells performed by the Mootha laboratory (89). Knockdown of EMRE with RNA interference (RNAi) in HEK293T or HeLa cells phenocopies MCU knockdown and abrogates rapid mCa2+ uptake, indicating that EMRE is required for MCU function in human cells. Furthermore, rapid mCa2+ uptake is not rescued by overexpression of MCU in EMRE-deficient cells (89), indicating that such Ca2+ flux requires the simultaneous expression of both MCU and EMRE (FIGURE 2). Subsequent analysis revealed that EMRE is broadly expressed across mammalian tissues, with both EMRE RNA (89) and protein (156) detected in all 14 tissues examined by Mootha’s group. This ubiquitous expression of mammalian EMRE likely reflects its essential role in mtCU function in higher organisms.
3.3.2.1. emre phylogenetic conservation.
In contrast to MCU, which is found in nearly all eukaryotes, EMRE arose more recently in evolutionary history. EMRE is found only in metazoans and not in plants, protozoa, or fungi (89). EMRE is required for Ca2+-transporting activity of the mtCU in animal lineages from Drosophila to human (133). However, the absence of EMRE in lower taxa such as the Amoebozoa slime mold Dictyostelium discoideum (157) and the plant Arabidopsis thaliana (158) does not disrupt uniporter activity, suggesting that evolutionary changes in MCU or overall uniporter complex structure and function in animals necessitated the coordinated development of EMRE to maintain MCU in a functional state. Indeed, the expression of MCU from species that lack endogenous EMRE is sufficient to restore rapid, Ru360-sensitive mCa2+ uptake in mammalian MCU/EMRE double-knockout cells, whereas expression of MCU from other metazoans such as C. elegans or Drosophila is not (158).
3.3.2.2. emre structure.
EMRE is a single-pass, transmembrane protein ∼10 kDa in size, with a predicted mitochondrial targeting sequence [amino acid (AA) 1–47], a transmembrane region (AA 65–84), and a conserved, aspartate-rich COOH terminus (AA 85–107) (89, 159). The membrane topology of EMRE remained a matter of debate for some time after its identification, with Vais et al. (115) initially asserting that the COOH terminus of EMRE faces the mitochondrial matrix based on patterns of proteinase digestion assays. However, this study tested only truncated NH2- or COOH-terminal EMRE constructs or COOH-terminal FLAG-tagged EMRE, which may not have appropriately incorporated into the inner mitochondrial membrane or may have displayed alternative secondary structure. The current consensus, based on multiple antibody binding and proteinase protection assays comparing NH2- or COOH-terminal FLAG-tagged EMRE, is that the NH2 terminus of EMRE faces the mitochondrial matrix, whereas the aspartate-rich COOH terminus extends into the IMS (158, 159). This orientation is supported by the finding by Tsai et al. (158) that only cysteines engineered into the COOH terminus, and not the NH2 terminus, of EMRE can be labeled by addition of polyethylene glycol maleimide to the IMS side of isolated mitoplasts. More recently, cryo-EM resolution of EMRE structure has confirmed that its NH2 terminus is located in the matrix (160). Fitting with this model for EMRE orientation is the finding that the COOH terminus of EMRE binds to MICU1, which associates with the mtCU in the IMS. This interaction occurs via an interaction between a polybasic sequence in MICU1 (KKKR) that likely binds to the polyaspartate tail of EMRE (EDDDDDD) (158).
P60 and the region encompassing S85 to A90 are critical for EMRE function. Mutation of these residues of EMRE disrupts the MCU-EMRE interaction and abrogates mtCU Ca2+ uptake activity when EMRE is coexpressed with MCU in yeast (159). Deletion of EMRE residues 48–53 or 54–57 partially reduces MCU-dependent Ca2+ uptake, indicating that these residues may also alter the MCU-EMRE interaction (159). Tsai et al. further support the notion that the transmembrane helix of EMRE, rather than its NH2 or COOH terminus, is critical for mtCU activity. They show that the GxxxS motif spanning residues G81–S85 within EMRE’s transmembrane helix is crucial for MCU-EMRE binding and mtCU-dependent Ca2+ uptake (158, 161). They propose that this region of EMRE’s transmembrane helix interacts with a region of MCU’s first transmembrane helix (158).
3.3.2.3. properties and function of emre.
The initial characterization of EMRE by Mootha’s laboratory indicated that MCU activity is lost in human HEK293T cells with shRNA knockdown or TALEN-mediated deletion of EMRE (89). This finding supports the conclusion that EMRE is required for mtCU channel activity. However, interpretation of the precise role or strict requirement for EMRE in mtCU function is complicated by the prior observation by De Stefani et al. (98) that His-tagged mouse MCU expressed in Escherichia coli or a cell-free transcription/translation system conducts calcium Ca2+ when reconstituted in vitro in lipid bilayers. Possible explanations for this discrepancy are structural changes in MCU induced by addition of the His tag or the absence of additional endogenous inhibitory binding partners or normal inhibitory posttranslational modifications on MCU that would not have been added to the construct during expression in bacteria or in vitro. Recent studies concur with the early assertion that EMRE is required for metazoan MCU to conduct Ca2+ (157–159, 161, 162). Furthermore, expression of mouse EMRE alone in yeast, in the absence of MCU, does not confer yeast mitochondria with uniporter activity, demonstrating that EMRE itself is not a Ca2+ transporter (159).
Examination of uniporter function across taxa indicates that EMRE arose evolutionarily in metazoan lineages in concert with structural changes to MCU that rendered MCU incapable of conducting Ca2+ on its own. When incorporated into yeast, human MCU requires coexpression of EMRE to conduct Ca2+, whereas MCU from the slime mold D. discoideum (DdMCU) is competent to transport Ca2+ in the absence of EMRE (157). Furthermore, DdMCU is sufficient to restore uniporter activity in human HEK293T MCU- or EMRE-knockout (KO) cells (157). Wu et al. report similar distinctions concerning the requirement for EMRE between taxa. When reconstituted in lipid bilayers, fungal MCU from Pyronema omphalodes (pMCU) conducts Ca2+ by itself, but human MCU requires coexpression of EMRE to transport Ca2+ (162). The structural features of MCU that may determine the differences in its dependence on EMRE in metazoans versus other groups are discussed below.
3.3.2.4. interaction of emre with the metazoan mtcu complex.
EMRE protein is stabilized by association with MCU and possibly other components of the mtCU complex. Loss of MCU in HEK293T cells causes a coordinate decrease in EMRE protein level despite normal levels of EMRE mRNA (89). Selective loss of EMRE does not affect the protein expression or stability of other uniporter components, although it does shift the molecular mass of immunoprecipitated, MCU-containing complexes from ∼480 kDa to ∼300 kDa, similar to the shift in molecular mass caused by loss of MICU1 (89). Indeed, the presence of EMRE is required for the interaction of MICU1/2 with the uniporter complex, although MICU1/2 and MCUB are not required for EMRE to interact with MCU (89). Thus, Sancak et al. propose a model in which EMRE binds to MICU1/2 in the intermembrane space and with MCU in the inner mitochondrial membrane. These authors speculate that these interactions allow EMRE to couple the Ca2+-sensing abilities of MICU1/2 to MCU’s ability to open and allow mCa2+ influx in response to an increase in extramitochondrial Ca2+ concentration (89).
Kevin Foskett’s laboratory additionally proposed that EMRE functions as a sensor of matrix Ca2+ content and helps to inhibit MCU in response to rising matrix Ca2+ levels as a mechanism to prevent mCa2+ overload (115, 116). In this model, the mtCU complex has Ca2+-sensing regulatory functions on both the matrix side (dependent on MCU’s NH2 terminus) and the IMS side (conferred by MICU1/2). EMRE functionally couples these two Ca2+-sensing functions via its interactions with MCU and MICU1, resulting in maximal matrix-dependent inhibition of the mtCU at a matrix Ca2+ concentration of ∼400 nM. EMRE binds MICU1 via its polyaspartate COOH-terminal tail (158), and EMRE-KO cells rescued with EMRE COOH-terminal mutants display normal mCa2+ uptake capacity but fail to shut off mCa2+ uptake upon a drop in cytosolic or bath Ca2+ concentration (115). This behavior resembles the phenotype of MICU1-null cells (described below) and suggests that the COOH-terminal tail of EMRE is required for the functional interaction of MICU1/2 and EMRE, which transmits the sensing of IMS Ca2+ concentration by MICU1/2 into functional changes in the structure of MCU.
Fitting with a model in which the COOH terminus of EMRE mediates EMRE/MICU1 binding, Tsai et al. (158) report that COOH-terminal deletion of EMRE results in increased mCa2+ uptake at low bath Ca2+ concentrations, likely because of disruption of MICU1-EMRE interaction and the loss of MICU1/2 from the uniporter complex. This study also identified the first transmembrane helix of MCU as the region that interacts with the transmembrane helix of EMRE (158). Thus, the authors proposed that EMRE is critical for the normal function of the mtCU via two main effects: first, EMRE activates MCU or is permissive for its Ca2+-transporting activity, and second, EMRE localizes MICU1 in proximity to MCU in order to allow Ca2+-sensitive regulation of MCU activity.
A major advance in understanding the mechanism by which EMRE controls MCU function was the recent report of a 3.6-Å-resolution cryo-EM structure of human MCU and EMRE complexed together (160). This study confirmed a tetrameric assembly of human MCU and also defined a 1:1 stoichiometry of MCU and EMRE subunits within the mtCU complex (FIGURE 2). Each EMRE subunit is located at the periphery of the MCU transmembrane pore and interacts with two neighboring MCU subunits at a total of three points of contact. First, the central portion of the EMRE transmembrane helix sits at a 45° angle against the first transmembrane helix of one MCU subunit, consistent with previous reports of these two regions interacting (158, 159, 161). Second, the NH2-terminal portion of the EMRE transmembrane helix contacts the COOH terminus of the second transmembrane helix of the neighboring MCU subunit. Third, the NH2 terminus of EMRE forms a β-hairpin that nestles into a groove between coiled-coil domain helices (CC2a and CC2b) of the neighboring MCU subunit. Wang et al. (160) report that a six-residue juxtamembrane loop (JML) that connects CC2a and the second transmembrane helix of each MCU subunit is highly ordered in human MCU and sits parallel to the lipid bilayer and blocks the matrix side of the MCU transmembrane domain. In fungal MCU, the structure of the JML is much less ordered. The JML of human MCU is linked to EMRE via hydrogen bonds between R297 in CC2a of MCU, T285 in the JML of MCU, and the carbonyl group of EMRE V61 (160). They propose that the MCU JML forms a luminal gate, specifically in metazoan MCUs, that may require EMRE for an open conformation and Ca2+ permeation. In a series of elegant experiments, Wang et al. demonstrate that substitution of the JML of human MCU with the JML from either D. discoideum or the fungus Neosartorya fischeri, but not the JML from Drosophila, allows the modified human MCU to conduct Ca2+ in the absence of EMRE. Thus, it appears that the JML of MCU in metazoans versus lower organisms dictates the uniporter’s functional dependence on EMRE. In the absence of EMRE, the coiled-coil domains of MCU lie closer to the central axis of the pore, potentially bringing the JMLs of neighboring MCU subunits into close proximity to block Ca2+ flow through the channel. In the Wang et al. model, the NH2 terminus of EMRE pulls the MCU CC2a domain away from the central axis of the channel, along with coiled-coil domain helix1, CC2b, and the JML, to maintain the MCU luminal gate in an open conformation for Ca2+ permeation (160). In the absence of EMRE, the MCU NH2-terminal domains undergo a conformational change that disrupts MCU dimerization, indicating that EMRE also promotes MCU oligomerization (160).
3.3.2.5. genetic diseases associated with emre.
As for MCU, no mutations in EMRE have yet been reported to cause human disease.
3.3.2.6. animal models of emre.
The first EMRE-knockout mouse model was generated by Toren Finkel’s and Elizabeth Murphy’s laboratories in 2016 (163) (TABLE 2). This was prompted by the observation that a decrease in EMRE expression tends to correlate with a less severe phenotype of MICU1-null mice that survive the perinatal period (discussed in greater detail below). Heterozygous deletion of EMRE rescues the perinatal lethality and excessive mCa2+ uptake, and corrects defects in motor performance and skeletal muscle strength in Micu1−/− mice. These data suggest that the reduction in EMRE levels reduces overall uniporter activity to compensate for the loss of MICU1’s gatekeeping function, which normally minimizes mCa2+ uptake at low Ca2+ concentrations. Interestingly, mice with homozygous deletion of EMRE alone are viable on a mixed genetic background and have no obvious neurological or skeletal muscle defects under basal conditions, similar to previous findings for a gene-trap MCU-knockout model on a mixed genetic background (119, 163). Although mice with constitutive Emre deletion have impaired mCa2+ uptake and resistance to Ca2+-induced permeability transition, they are not protected from cardiac I/R injury (164). This lack of obvious phenotypic consequence for constitutive EMRE deletion, despite a strict requirement of EMRE for mtCU function in animals, may reflect the initiation of pathways that compensate for the lack of mCa2+ uptake throughout development and/or may reflect the biological robustness of the mixed genetic background.
In Drosophila, knockdown of EMRE via RNA interference (RNAi) impairs mCa2+ uptake, as expected (133). Overexpression of EMRE, specifically in the Drosophila eye, has no obvious consequences, possibly because the addition of EMRE alone has little functional effect in the absence of concurrent MCU overexpression. Consistent with this idea, simultaneous overexpression of MCU and EMRE is lethal (133). Further studies of EMRE-null animal models under stressed conditions, and the development of mice with conditional rather than constitutive, global deletion of EMRE, will further clarify the in vivo requirements for EMRE in mCa2+ handling and associated biological functions.
3.3.2.7. regulation of emre.
The predominant endogenous mode of regulation of EMRE is the control of its protein level via proteolytic degradation by mitochondrial proteases. MCU−/− HEK293T cells have reduced levels of EMRE protein expression, despite normal levels of EMRE mRNA (89), a phenomenon confirmed with acute deletion of Mcu in MEFs (165). Moreover, acute deletion of NCLX in adult cardiomyocytes results in a rapid reduction in EMRE protein, suggesting that proteolysis of EMRE may be a mechanism to downregulate mtCU function in the context of mCa2+ overload (166). A proteomic screen of the neuronal interactome of mitochondrial m-AAA proteases consisting of homooligomers of AFG3L2 or heterooligomers of AFG3L2 and SPG7 revealed that the m-AAA protease binds to the matrix protein C2ORF47, which was renamed m-AAA protease interacting protein 1 (MIAP1) (167). Knockdown of either MAIP1 or m-AAA protease subunits in cells reduces the accumulation of an 11-kDa form of EMRE, without affecting protein levels of other mtCU components or mCa2+-handling proteins (167). The 11-kDa form corresponds to full-length EMRE, before cleavage of the NH2-terminal mitochondrion-targeting sequence (MTS); postprocessed EMRE = 7 kDa. Protease protection assays indicated that preprocessed EMRE is localized to the intermembrane space, whereas the MTS-cleaved EMRE is fully incorporated into the IMM. Additional experiments suggest that MAIP1 binds to nascent EMRE in the intermembrane space and protects it from degradation by the i-AAA protease, YME1L (167).
Once incorporated into the IMM, EMRE participates in the assembly of MCU and MICU1/2 into the mtCU complex as described above. Loss of the m-AAA protease promotes mtCU assembly into high-molecular weight complexes by stabilizing EMRE expression (167). Later work corroborated the role of SPG7 or AFG3L2 in EMRE degradation (168). EMRE in low-molecular weight complexes, smaller than the mtCU, is especially susceptible to degradation by the m-AAA protease, leading Konig et al. (167) to conclude that the protease preferentially degrades EMRE that is not already incorporated into the mtCU, and so may limit the assembly of additional functional mtCU complexes. Indeed, the expression of mutant forms of EMRE or MCU that abolish the EMRE-MCU interaction accelerate the turnover of EMRE, supporting the notion that binding to MCU protects EMRE from proteolytic degradation (168). The above-mentioned data obtained with acute deletion of NCLX (166) suggest that disaggregase activity may precede EMRE proteolysis as a mechanism to downregulate functional, high-molecular weight mtCU activity. The relevance of the m-AAA protease for EMRE stability, mtCU formation, and mitochondrial Ca2+ homeostasis was validated in vivo in mice with neuronal-specific knockout of the AFG3L2 subunit of the protease (167). Loss of the protease results in an accumulation of MCU-EMRE complexes ∼400 kDa in size, which lack MICU1/3 regulatory subunits that are normally part of the mtCU in the brain. Consistent with a loss of mtCU gatekeeping function, these neurons exhibit increased mCa2+ uptake and increased sensitivity to mPTP opening. m-AAA protease-deficient neurons also display increased levels of an ∼100-kDa complex likely containing MICU1, MICU3, and EMRE, which might represent an intermediate step in mtCU assembly. Konig et al. (167) propose that the MICU subunits titrate EMRE away from MCU during uniporter assembly and that the m-AAA protease degrades any noncomplexed EMRE. According to this model, the presence of the m-AAA protease protects the cell from mCa2+ overload by preventing the formation of “ungated” EMRE-MCU uniporter channels (i.e., channels lacking MICU1/2/3) that might mediate unchecked mCa2+ uptake. One challenge to this idea is a recent report that expression of a protease-resistant EMRE mutant (P76A) does not cause excess mCa2+ uptake unless MICU1 is simultaneously depleted by ∼35% (168). This result suggests that cells normally have sufficient MICU1 to bind to and gate all EMRE-MCU complexes and that there is little accumulation of ungated EMRE-MCU complexes. The discrepancy between these two studies may arise from different extents of EMRE stabilization and accumulation in the two model systems (point mutant of EMRE vs. m-AAA protease depletion), or differences in the availability of endogenous MICU1 between the different cell types used for the experiments (neurons and HeLa cells vs. HEK293 cells). Despite these inconsistencies, the studies agree that accumulation of EMRE has the potential to increase the number of ungated EMRE-MCU complexes, which may result in excess mCa2+ uptake at low cytosolic Ca2+ levels, and both highlight the importance of EMRE expression and stability in mtCU regulation.
3.3.3. MICU proteins: the gatekeepers of the mtCU.
A distinct property of the mtCU is that channel activation by cytosolic or IMS Ca2+ differs across distinct tissues and can change during development or with disease. This sensitivity of mtCU activation to changes in Ca2+ concentration is largely determined by the interactions of three regulatory mtCU components, the MICU proteins. Current models suggest that MICU1 binds directly to the core of the mtCU (MCU + EMRE) and has dual functions to regulate the channel: MICU1 functions as the gatekeeper that prevents Ca2+ flux through the channel when cytosolic/IMS Ca2+ concentration is low, but also facilitates the cooperative activation of the mtCU as cytosolic/IMS Ca2+ concentration rises. MICU2 and MICU3 do not bind to the core mtCU (MCU + EMRE) directly, but rather interact with the channel complex via dimerization with MICU1. The dimerization of MICU1 with MICU2 versus MICU3, or versus another molecule of MICU1, modulates the Ca2+ responsiveness of mtCU gatekeeping and cooperative activation. The molecular interactions between the MICU proteins that allow for this complex regulation of mtCU function are examined in the following sections.
3.3.3.1. micu1.
The peripheral membrane protein MICU1 [Mitochondrial Calcium Uptake Protein 1; gene name: MICU1 (or CBARA1, calcium-binding atopy-related autoantigen 1)] was the first mtCU component to be identified, using an integrative screen designed to identify genes involved in mCa2+ uptake on the basis of comparative physiology, evolutionary genomics, and mitochondrial proteomics (100). In this study, Perocchi et al. (100) showed that knockdown of MICU1 in HeLa cells greatly reduces mCa2+ uptake in response to histamine and that MICU1 is required for the coupling of increased cytosolic Ca2+ content to an increase in mitochondrial bioenergetics. The observation that silencing of MICU1 reduces rather than completely ablates rapid mCa2+ uptake suggested that MICU1 is a regulator of the mitochondrial calcium uniporter complex rather than a necessary component of its Ca2+-conducting pore. This was known by the Mootha group at the time of publication, as their identification of MCU, the pore-forming component, was published only a few months later. MICU1 contains two canonical, Ca2+-binding EF-hand domains that are critical for mCa2+ uptake, suggesting a role for MICU1 in Ca2+-dependent regulation of the uniporter (100). MICU1 is broadly expressed and is present in the mitochondria of at least 11 different mammalian tissues (156). Within the mitochondrion, MICU1 is located within the IMS, where it is associates with the inner mitochondrial membrane (IMM) (100, 158, 169, 170). Tagging of MICU1 with the peroxidase reporter APEX2 and examination of MICU1-APEX2 localization via electron microscopy indicated that MICU1 is present both within the intercristae IMM, as well as within the cristae themselves (170). This was later confirmed in another study examining MICU1 submitochondrial localization (171).
MICU1 has distinct effects on uniporter function depending on the concentration of cytosolic or IMS Ca2+ to which it is exposed. MICU1 limits or inhibits mCa2+ uptake under basal conditions, despite the strong driving force for mCa2+ entry created by the highly electronegative mitochondrial membrane potential (82). Knockdown of MICU1 in HeLa cells elevates resting mCa2+ concentration, although in contrast to initial work on MICU1 (100), Mallilankaraman et al. (82) observe no effect of MICU1 knockdown on mCa2+ uptake in response to an agonist-induced increase in cytosolic Ca2+. Permeabilized MICU1 knockdown cells readily take up Ca2+ even at low bath Ca2+ concentrations (0.5–1 µM Ca2+) that are insufficient to trigger rapid mCa2+ uptake in control cells. This finding suggests that MICU1 acts as a gatekeeper to prevent mCa2+ uptake through the uniporter when cytosolic and IMS Ca2+ concentration is low (82). This “gatekeeping” function is lost upon mutation of the EF-hands of MICU1, indicating that a Ca2+-sensing function of MICU1 is necessary for its inhibition of the channel under resting, low-cytosolic Ca2+ conditions (82), although the requirement of MICU1 EF-hands in this function has since been challenged, as EF-hand mutants are capable of restoring gating (172, 173). Similar effects of MICU1 knockdown on uniporter function are found when Ca2+ currents of HeLa mitoplasts are examined by patch clamp (174). Experiments in primary mouse hepatocytes and HeLa cells verified that MICU1 acts as a gatekeeper to set the threshold for Ca2+ activation of uniporter mCa2+ uptake, with MICU1 knockdown increasing mCa2+ uptake at extramitochondrial Ca2+ concentrations over the range of ∼0.3–2 µM (169). The slope of the linear fit of the double logarithmic plot of initial mCa2+ uptake rate versus cytosolic Ca2+ concentration is reduced in MICU1-knockdown cells, leading Csordás et al. (169) to propose that MICU1 also contributes to cooperative activation of MCU-dependent mCa2+ uptake (i.e., greater rates of mCa2+ uptake when the mitochondria are exposed to higher cytosolic or IMS Ca2+ concentration). However, a caveat of this interpretation is that it is mostly based on the greater rate of uptake observed in MICU1-knockdown cells at low cytosolic Ca2+, as the uptake rates for WT and MICU1-knockdown cells at high cytosolic Ca2+ in this study appear similar in magnitude (169). Current models propose that MICU1 modulates uptake in a biphasic fashion by both inhibiting the mtCU at low cytosolic/IMS Ca2+ concentration (i.e., the gatekeeping function that “sets the Ca2+ threshold” for mtCU activation) as well as facilitating cooperative activation of the channel as Ca2+ concentration rises (175) (FIGURE 4).
FIGURE 4.
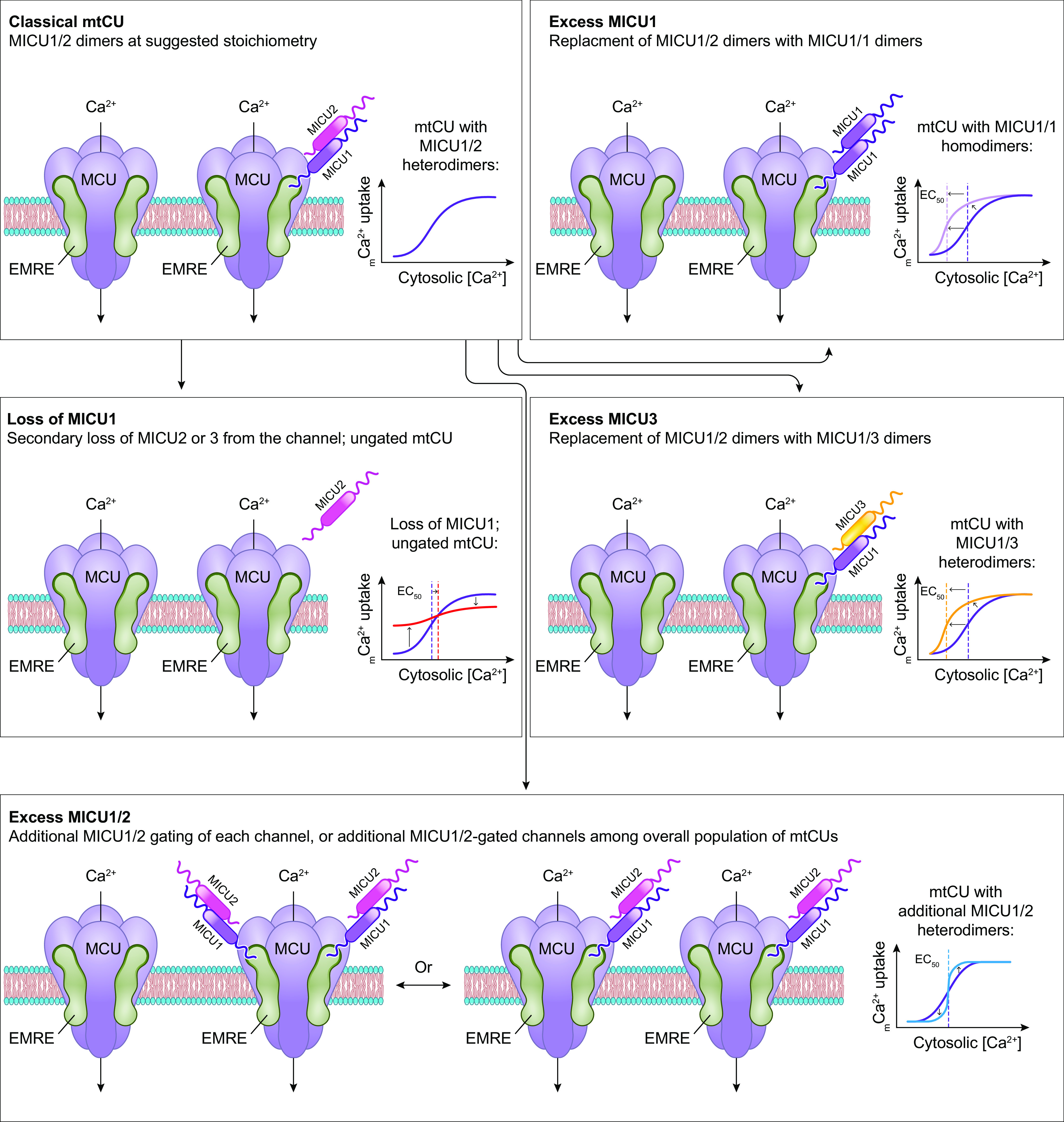
Gating of the mitochondrial calcium uniporter channel and consequences of changes in MICU composition. Clockwise from top left: Classical mtCU: MICU1/2 dimers bind to the mtCU and regulate its activation by Ca2+ within the intermembrane space (IMS). The stoichiometry suggested by structural studies of the mtCU is 4 MCU:3 or 4 EMRE:1 MICU1:1 MICU2, although the proportion of the total mtCUs that are regulated by MICUs is determined by the relative MICU expression within each tissue. At low cytosolic/IMS Ca2+ concentration ([Ca2+]), the MICU1/2 dimer acts as a gatekeeper to keep the channel impermeable to Ca2+. As cytosolic/IMS [Ca2+] rises, the MICU1/2 dimer facilitates the cooperative activation of the channel, resulting in a sigmoidal relationship between mtCU-dependent mCa2+ uptake and cytosolic/IMS [Ca2+]. Excess MICU1: Increased expression of MICU1 may displace MICU2 from existing MICU1/2 dimers, resulting in MICU1 homodimers. Displacement of MICU2 results in loss of its ability to tune MICU1-dependent mtCU gating and cooperative activation, shifting the relationship between mtCU-dependent mCa2+ uptake and cytosolic/IMS Ca2+ concentration to the left. Excess MICU3: Increased expression of MICU3 may displace MICU2 from existing MICU1/2 dimers, resulting in MICU1/3 dimers. The properties of MICU3-dependent regulation of MICU1 differ somewhat from those of MICU2 (see text for details); thus, replacement of MICU2 with MICU3 also shifts the relationship between mtCU-dependent mCa2+ uptake and cytosolic/IMS Ca2+ concentration to the left. Excess MICU1/2: Increased expression of MICU1/2 results in more stringent regulation of Ca2+ flux through the mtCU, with enhanced channel gatekeeping at low cytosolic/IMS [Ca2+], but greater activation of the channel at higher [Ca2+]. This behavior may result from a greater number of MICU1/2 dimers binding to each mtCU, or from an increase in the number of mtCUs within the tissue being gated by MICU1/2. Loss of MICU1: MICU2 and MICU3 are incapable of binding to and regulating the mtCU in the absence of MICU1. Loss of MICU1 therefore results in loss of all MICU-dependent mtCU regulation. The absence of MICU-dependent channel gatekeeping results in increased mCa2+ uptake at low cytosolic/IMS [Ca2+], while the absence of MICU-dependent cooperative activation makes further mtCU activation less responsive to increases in cytosolic/IMS [Ca2+]. See glossary for abbreviations.
These properties allow MICU1, when forming heterodimers with MICU2, to act as Ca2+-sensitive “on-off switch” for the mitochondrial calcium uniporter channel (173, 176). As noted above, the ability for MICU1 to inhibit the uniporter within the range of typical resting cytosolic Ca2+ levels (∼100–150 nM) may also contribute to inactivation of the RaM current over this range of Ca2+ concentrations (82). The consequences of the multifaceted functions of MICU1—both preventing and promoting mCa2+ uptake—are discussed in further detail below.
3.3.3.1.1. MICU1 phylogenetic conservation.
Most species of Eukarya that express MCU also express MICU1, and the few species that lack MCU typically also lack MICU1 (88). A notable exception is that fungi that contain MCU generally lack MICU1 and its homologs, indicating that these lineages may rely on alternative, MICU1-independent mechanisms for regulation of the uniporter channel (88). In fungal species that do express MICU1 homologs, it appears that these homologs are not involved in mtCU regulation. For example, the fungus Aspergillis fumigatus contains a putative MCU homolog named McuA, which is required for acute mCa2+ uptake (177). A. fumigatus also contains a putative MICU1 homolog named AgcA, which contains two EF-hands and thus is predicted to bind Ca2+. Although AgcA deletion improves resistance to azole and oxidative stress, it does not modulate acute mCa2+ uptake, suggesting that the predominant function of AgcA is independent of the mtCU (177). An additional layer of complexity is seen in trypanosomatids, where the mtCU-gatekeeping and mtCU-cooperative activation functions of MICU1 are separable. MICU1 does not prevent mCa2+ uptake at low, basal Ca2+ concentrations in the parasite T. cruzi as it does in mammalian cells, suggesting that MICU1 does not function in channel gatekeeping in this species (94). Rather, disruption of MICU1 in T. cruzi ablates mCa2+ uptake at both low (1.5 µM) and high (up to 50 µM) bath or cytosolic Ca2+ concentration (94). This result is consistent with the purported role of MICU1 in facilitating the cooperative activation of the mtCU.
In vertebrate species, the MICU1 and MCU genes are located next to each other on the same chromosome and share a potential bidirectional promoter (88). Bick et al. (88) propose that this arrangement allows for highly coordinated expression of MICU1 and MCU proteins within these taxa. However, this genetic organization does not preclude the possibility that MICU1 protein could have both mtCU-dependent and mtCU-independent functions within these species. Despite the apparent diversity of mtCU-dependent and/or mtCU-independent MICU1 functions across evolution, the Ca2+-coordinating residues within its two EF-hands remain highly conserved from protozoa to humans (100). This degree of conservation indicates that a fundamental function of MICU1 across species is to serve as a mitochondrial Ca2+ sensor.
3.3.3.1.2. MICU1 structure.
The human MICU1 gene encodes a 476-amino acid protein of ∼54 kDa comprised mostly of α-helices (178). The NH2 terminus consists of a presequence (residues 1–33) followed by a predicted transmembrane domain (residues 34–52; numbers throughout this paragraph correspond to residues within 476-amino acid human MICU1 isoform 2) and a mitochondrial targeting sequence with a cleavage site at residue 115 (100, 179). A second transmembrane domain is predicted around residues 194–216 (179). The predominant feature of MICU1 is a pair of two highly conserved, classical Ca2+-binding EF-hands (“EF1” and “EF2”) in the COOH-terminal half of the protein. The EF-hands are located at residues 218–253 and 408–443 and are separated by a long α-helical spacer (100, 178, 179). A conserved cysteine residue (C463) allows MICU1 to form disulfide bridges with other MICU proteins, resulting in MICU1 homodimers or MICU1/2 or MICU1/3 heterodimers (96, 180).
3.3.3.1.3. Alternative splice/transcript variants of MICU1.
Several transcript variants for human MICU1 are reported in the NCBI Gene database, including the canonical transcript encoding the classical 476-AA MICU1 protein (MICU1 isoform 2). Another encodes a 482-AA protein (MICU1 isoform 4) due to inclusion of an additional alternative exon, which introduces four amino acids (DFWQ) after residue 179.
Six protein-coding splice variants for mouse Micu1 are annotated on ENSEMBL. The expression and function of these various MICU1 protein isoforms remain largely unknown except for the mouse isoform that corresponds to human MICU1 isoform 4. This variant (named “MICU1.1”) is characterized by the insertion of four residues (EFWQ) after residue 181 of mouse MICU1, a location that is relatively far from the two EF-hand domains (181). MICU1.1 heterodimerizes with MICU2, and upon Ca2+ binding MICU1.1/2 heterodimers facilitate more net mCa2+ uptake than MICU1/2 heterodimers. Such MICU1.1/2 dimers still act as a gatekeeper for the mtCU at low cytosolic Ca2+ concentrations but shift the threshold for channel activation to a lower Ca2+ concentration compared with MICU1/2 dimers (181). Despite its distance from the EF-hand domains, the 4-AA insertion increases the Ca2+-binding affinity of the MICU1.1 isoform (181). This change likely accounts for the shift in the Ca2+ threshold for activation of uniporter channels containing MICU1.1. Micu1.1 mRNA expression is mostly restricted to skeletal muscle, where the Micu1.1-to-Micu1 ratio is 8:1 (181). Micu1.1 mRNA is also detected in the brain, at a 1-to-1 ratio with Micu1 (181). Vecellio Reane et al. (181) propose that MICU1.1 allows skeletal muscle to be highly sensitive to changes in cytosolic Ca2+, such as occur during muscle stimulation, and thus allows sufficient mCa2+ uptake through the mtCU to increase ATP production for use in muscle contraction.
3.3.3.1.4. Properties and function of MICU1.
Early work in HEK293 cells confirmed the initial findings (82, 169) that MICU1 inhibits mCa2+ uptake at low cytosolic or IMS Ca2+ concentrations < 2 µM, but promotes mCa2+ uptake as extramitochondrial Ca2+ concentration rises past ∼4 µM (114, 158). MICU1 modulation of mCa2+ uptake is sigmoidal, with the rate plateauing at higher extramitochondrial Ca2+ concentrations ∼30 µM, at which point mCa2+ uptake in MICU1-knockdown cells is similar to that in wild-type cells (158). Initial structural studies indicated that when extramitochondrial Ca2+ concentration is low MICU1 forms hexamers that may inhibit MCU. As the local Ca2+ concentration rises, MICU1 binds Ca2+ with high affinity (∼15–20 µM) and undergoes conformational changes, particularly at the EF-hands, and forms multiple lower-order oligomers to allow Ca2+ permeation of MCU (182). Such MICU1 rearrangement is specifically controlled by changes in cytosolic or IMS Ca2+ concentration, rather than changes in mCa2+ content, and occurs regardless of the presence of EMRE and MCU (183). Although the hexameric structure of MICU1 was determined in the absence of MCU (182), MICU1 expressed in eukaryotic cells can be detected on blue native-PAGE gels at molecular weights corresponding to MICU1/2 hexamers, suggesting that higher-order MICU oligomers are capable of forming in intact systems (181). However, subsequent studies of the mtCU holocomplex, discussed below, indicate that it is MICU dimers, rather than hexamers, that are functionally relevant for regulating individual uniporter channels (184–186).
The relative ratio MICU1:MCU within a cell confers an additional layer of regulation on mtCU function because it controls how much of the uniporter channel population is subject to gatekeeping and regulation, and how responsive the tissue’s mitochondria will be to changes in cytosolic Ca2+ (FIGURE 4). For example, liver exhibits a high MICU1-to-MCU ratio, and therefore is effectively gated, but also has enhanced cooperative activation of the channel (187). This means that liver has low Ca2+ uptake at low cytosolic Ca2+ levels, because of a high threshold for Ca2+ activation set by the presence of MICU dimers on the mtCU, but also has profound mCa2+ uptake at high cytosolic Ca2+ levels. Tissues such as the heart and skeletal muscle that have a low ratio of MICU1:MCU have a greater portion of “ungated” mtCUs and exhibit a lower Ca2+ threshold (i.e., greater mCa2+ uptake at low Ca2+ levels), but relatively lower mCa2+ uptake at high cytosolic Ca2+ concentrations due to fewer channels exhibiting cooperative activation (187) (FIGURE 4). Paillard et al. (187) hypothesize that regulation of the MICU1-to-MCU ratio allows tissues to tune how responsive the uniporter is to cytosolic Ca2+ concentration, as a mechanism to modulate the Ca2+ sensitivity of oxidative metabolism. In support of this view, Payne et al. (188) propose an average stoichiometry of two MICU1/2 dimers associated with two or three EMRE subunits per MCU tetramer in HEK293T cells and demonstrate that an increase in the relative MICU1-to-MCU ratio enhances gatekeeping at low cytosolic Ca2+ concentrations (i.e., increases the threshold cytosolic Ca2+ concentration required to relieve channel inhibition). The existence of tissue-specific regulation of the Ca2+ sensitivity of mitochondrial metabolism is not surprising. Different tissues are exposed to very different frequencies and amplitudes of cytosolic Ca2+ transients or spikes (31). For instance, the heart is constantly exposed to cyclic changes in cytosolic Ca2+ concentration and thus has more background “noise” to filter out before modifying steady-state mCa2+ content, compared with more quiescent tissues that may only undergo Ca2+ transients in response to discrete, infrequent physiological signals.
MICU1 also appears to facilitate mCa2+ uptake by overriding the eventual inhibition of mtCU activity that otherwise occurs as matrix Ca2+ content rises upon rapid mCa2+ uptake (114). MCU current is partially inhibited at a matrix Ca2+ concentration of ∼400 nM, with greater MCU current observed at higher or lower mCa2+ concentrations (115). The Foskett group proposes that an inhibitory matrix Ca2+ sensor within the MCU NH2-terminal domain (including residues D131 and D147) allows the channel to close as matrix Ca2+ concentration rises up to this level, even despite persistent Ca2+ binding by MICU1 and MICU2 in the IMS (116). Such matrix Ca2+-dependent inhibition of the mtCU is lost upon mutation of EMRE’s COOH terminus (115), the portion of EMRE that normally helps anchor MICU1 to the mtCU (158), or in the absence of MICU1 (115). These results suggest that the inhibitory mechanism is responsive to Ca2+ concentration within the IMS, in addition to the matrix, or at least somehow is sensed by MICU1 (115). Vais et al. (116) therefore reason that the mechanisms of Ca2+ sensing and changes in protein function that control Ca2+ flux through the mtCU depend on both MICU1/2 and MCU, and are coupled to each other across the IMM. With continued Ca2+ influx, mtCU inhibition is relieved as mCa2+ concentration rises past ∼400 nM (115). This behavior may help account for the phenomenon of cooperative activation of the uniporter and may enable appropriate loading of the mitochondria with Ca2+ in response to prolonged, moderate increases in cytosolic Ca2+ concentration. The finding that MICU1 and MICU2 mediate the gradual activation of rapid mCa2+ uptake that occurs upon small but sustained increases in cytosolic Ca2+ concentration (189) supports the possibility that MICU1/2 are directly involved in the relief of matrix Ca2+-dependent MCU inhibition. This behavior, specifically the relief of partial MCU inhibition as matrix Ca2+ rises past 400 nM, also enables continued mCa2+ uptake to the point of depolarization, as observed in Ca2+ retention capacity experiments or mitochondrial swelling assays.
Finally, MICU1 lets the mtCU distinguish between Ca2+ and manganese (Mn2+) and allows the channel to favor the transport of Ca2+ over other ions (190). Kamer et al. (190) attribute this discriminatory function to the fact that Ca2+ elicits a conformational change in MICU1 but Mn2+ does not. These findings agree with the current structural models (discussed below) in which MICU1 physically blocks Ca2+ permeation through the mtCU pore under basal conditions and in which this inhibition is relieved only upon a Ca2+-induced conformational shift in MICU1 that alters its interaction with the channel pore. Loss of MICU1 therefore makes human cells more susceptible to Mn2+ toxicity (190), because it no longer blocks the channel pore to prevent Mn2+ permeation. Conversely, expression of MICU1 is sufficient to protect against mtCU-dependent Mn2+ toxicity (191). This protection likely results from MICU1’s physical blocking of the channel pore in low-Ca2+ conditions rather than any direct action of MICU1 to bind Ca2+ or Mn2+, because it is preserved even with mutation of MICU1’s EF-hands (191).
3.3.3.1.5. Interaction of MICU1 with the metazoan mtCU complex.
MICU1 association with the mtCU requires an interaction with either MCU or EMRE: MICU1 binds directly to MCU independent of EMRE but also binds directly to EMRE independent of MCU (158) (FIGURE 2). MICU1 binds to MCU via an NH2-terminal polybasic domain (residues 99–110 of MICU1) that contains a series of positively charged lysine residues (174). These residues interact with the two coiled-coil domains of MCU and anchor MICU1 to the mtCU, independent of changes in local Ca2+ concentration (174). The same polybasic sequence of MICU1 (KKKKR) mediates its electrostatic interaction with the COOH-terminal polyaspartate tail of EMRE. This MICU1-EMRE interaction is required to maintain MICU1 near the uniporter pore, where it can function in channel gatekeeping (158). Two positively charged arginine residues of MICU1 (R440 and R442, comprising a “DIME interacting domain”) also bind directly to negatively charged residues within the MCU DIME motif (D261 and E264), allowing MICU1 to block the pore of the uniporter by competing for the same site on MCU where the pharmacological inhibitor Ru360 binds (192).
MICU1 dimerizes with MICU2 (180) or MICU3 (96) and can also form homodimers or higher-order hexamers (trimers of dimers) (174, 176, 182). Binding of MICU2 or MICU3 to the uniporter is dependent upon association with MICU1, i.e., neither protein directly tethers to MCU or EMRE independent of MICU1 (96, 172, 180). Structural studies using cryo-EM reveal how the MICU1/2 heterodimer gates the mtCU. Under conditions of no cytosolic/IMS Ca2+, the uniporter complex exists predominantly in a “uniplex” form with individual uniporter channels with a stoichiometry of 4 MCU:4 EMRE:1 MICU1:1 MICU2 (184) (FIGURE 2). In this conformation, the polybasic sequence of a single MICU1 molecule interacts directly with a ring of D261 residues formed by the four MCU subunits to seal the entrance of the channel pore. A single molecule of MICU2 binds to MICU1, without directly interacting with the MCU subunits (184).
Wang et al. (185) propose an alternative low-Ca2+ (<1 nM) stoichiometry of 4 MCU:3 EMRE:1 MICU1:1 MICU2, where the MICU1/2 heterodimer displaces one EMRE subunit from the complex. However, these authors agree that, at low Ca2+, MICU1 blocks the IMS entrance of the uniporter pore via its interaction with the D ring created by the MCU subunits (185). At high Ca2+ concentrations (∼2 mM Ca2+), a greater proportion of the uniporter channels exist as V-shaped dimers of the individual uniplex channels formed by MCU + EMRE, with two MICU1-MICU2 heterodimers bridging the two uniplexes, although it should be noted that dimerization is not strictly required for mtCU channel function (184, 186). A portion of the dimers lose MICU1/2 (184), but the functional consequence of MICU1/2 loss specifically from the dimerized uniplex has not yet been tested. Channel dimerization is mediated by MCU’s NH2-terminal domain (160, 184). Rearrangement of the uniporter under high-Ca2+ conditions does not appreciably alter the structure of the channel pore, indicating that the mechanism for channel gating occurs elsewhere within the complex (184). In the high-Ca2+ channel dimer conformation, the MICU1/2 dimers have bound Ca2+ and the MICU1 subunits have rotated such that the uniporter interaction domains no longer cover the pores of their respective channels, potentially allowing for Ca2+ permeation (184–186). MICU1 remains connected to the channel in this high-Ca2+ state via its contacts with the COOH terminus of EMRE (184).
It is not immediately clear whether the disruption of the MICU-pore interaction occurs independent of channel dimerization, due to conformational changes within MICU1/2 themselves, or rather depends on dimer formation and the association between the bridge of MICU1/2 heterodimers, which may pull MICU1 away from the pore. Wang et al. (186) suggest that the two MICU1/2 heterodimers that bridge the dimerized uniplexes become more compact in structure upon binding Ca2+. This conformational shift in the MICU1/2 heterodimer likely involves a transition from interactions between E242 in MICU1 and R352 in MICU2 in the Ca2+-free state to an interaction dependent on MICU1 F383 and MICU2 E196, I333, and M337 in the Ca2+-bound state (193). The MICU1/2 heterodimer then changes position relative to the channel pore, rotating out of a state in which each MICU1/2 dimer faces the center of its corresponding uniplex channel and blocks the pore, and into a state where the MICU1/2 dimer is pointed toward the periphery of the uniplex channel and can contact the MICU1/2 dimer from the adjacent uniplex channel (186). Consistent with this view, the interaction between MICU1 and MCU is favored in the absence of Ca2+, whereas Ca2+ binding to the MICU1/2 heterodimer favors the interaction between MICU1 and EMRE (194). A shift in MICU1/2 positioning from the center to the periphery of each uniplex channel upon Ca2+ binding agrees with the finding that Ca2+ induces a conformational change in the MICU1 bound to a single mtCU from a more condensed arrangement to a more spatially distributed arrangement (183).
Fan et al. (184) report that the bridge structure composed of two MICU1/2 heterodimers is formed via back-to-back contacts between the two MICU2 subunits and that this bridge structure may allow for cooperative activation of the two dimerized channels. According to this model, Ca2+ would be required to bind to both of the MCU1/2 heterodimers in order to elicit reciprocal conformational changes in both of the dimerized uniplex channels and fully open both pores (184). It is also possible that the bridge structure formed by the two MICU1/2 heterodimers facilitates mCa2+ uptake simply by stabilizing the open-pore conformation of the mtCU.
These elegant structural studies present an attractive model for how MICU1/2 control mtCU activity. MICU1 gates the channel by acting like a rotating lid that either covers or uncovers the pore depending on Ca2+ binding to MICU1/2, thus prohibiting or permitting Ca2+ permeation, respectively. In turn, contacts between MICU2 subunits of adjacent, dimerized uniplex channels help to hold each channel’s “lid” in the open conformation, away from the channel pore.
3.3.3.1.6. mtCU-independent functions of MICU1.
Genetic experiments in Drosophila indicate that MICU1 has important mtCU-independent functions distinct from its classical role in regulating mCa2+ uptake. Deletion of MICU1 is lethal to flies and is associated with deleterious mCa2+ overload, whereas deletion of MCU or EMRE abolishes mCa2+ uptake but has little impact on viability (195). Surprisingly, simultaneous loss of MICU1 with either MCU or EMRE in order to ablate mCa2+ uptake through the mtCU is insufficient to rescue lethality (195). These results suggest that loss of MICU1 has additional harmful consequences besides mCa2+ overload. The putative mtCU-independent functions of MICU1 are the subject of active investigation. CBARA1/MICU1 was detected in a genome-wide screen for genetic adaptation to high altitude (196) and may be involved in hypoxia-inducible factor-1 (HIF-1)-dependent regulation of mitochondrial metabolism in low-oxygen environments (197). Whether such a role for MICU1 is related to its regulation of the mtCU or to some other cellular function remains to be determined. A recent study using structured illumination microscopy detected MICU1 at the inner boundary membrane of the IMM, where it localizes to mitochondrial cristae junctions (171, 198). In contrast, MCU and EMRE are distributed all throughout the IMM under basal conditions and become associated with MICU1 at the inner boundary membrane only upon an increase in IMS Ca2+ concentration (171). This intriguing result suggests a novel mechanism by which changes in cytosolic or IMS Ca2+ concentration may regulate mtCU complex assembly and mCa2+ uptake.
3.3.3.1.7. Genetic diseases associated with MICU1.
MICU1 is unique among the other mtCU components in that there is substantial evidence that genetic mutation of MICU1 is linked to human disease (TABLE 1). MICU1 mutations associated with excessive mCa2+ uptake are found in patients exhibiting proximal myopathy, learning deficits, and a progressive extrapyramidal movement disorder (involuntary/uncontrolled movement or tremors) (199). Homozygous deletions in exon 1 of MICU1 resulting in loss of protein impair mCa2+ uptake and are associated with fatigue, lethargy, and weakness, although patients have normal muscle biopsies (200). Disruption of either the gatekeeping or cooperative activation functions of MICU1 thus appears sufficient to cause human neuromuscular disease. Additional reports of MICU1 mutations in patients with congenital muscular dystrophy (201) and syndromes characterized by developmental delays and neuromuscular impairments (202, 203) support this conclusion. The mechanisms by which various mutations in MICU1 lead to neuromuscular disease probably vary depending on whether the particular mutation favors or instead diminishes mCa2+ uptake. For instance, loss of MICU1’s gatekeeping function may lead to futile Ca2+ cycling that ultimately runs down the mitochondrial membrane potential (ΔΨm) via secondary effects through the mitochondrial sodium/calcium exchanger (NCLX) and the mitochondrial sodium/hydrogen exchanger, and/or via mitochondrial Ca2+ overload inducing mitochondrial dysfunction and permeability transition. Reduction of ΔΨm would limit mitochondrial ATP production and thus place an energetic stress on the cell (204). On the other hand, mutations that impair mCa2+ uptake could impair energy production by limiting Ca2+-dependent stimulation of the tricarboxylic acid (TCA) cycle and respiratory complexes. Excess mCa2+ uptake upon loss of MICU1 gatekeeping also sensitizes tissues to cell death (82) and could explain neural as well as skeletal muscle defects in MICU1-mutant patients. Finally, since MICU1 regulates regrowth following axon injury in adult neurons (205), defective neuronal homeostasis may also contribute to neuromuscular defects in these individuals.
3.3.3.1.8. Animal models of MICU1.
Several different genetic knockout strategies have been employed to examine the function of MICU1 in vivo (TABLE 2). The Hajnóczky laboratory crossed Micu1loxP/loxP mice to germline-expressing E2a-Cre animals to generate a constitutive Micu1-knockout model (206). Heterozygous Micu1+/− mice are viable and fertile, but homozygous Micu1−/− mice die within several hours of birth (206). Loss of MICU1 is hypothesized to cause perinatal lethality because it interferes with brain stem control of respiratory function (206). Micu1−/− MEFs exhibit excessive mCa2+ uptake at low cytoplasmic Ca2+ concentrations and diminished mCa2+ uptake at high cytosolic Ca2+ concentrations, as expected for loss of gatekeeping and cooperative activation of the mtCU (206). Acute Micu1 deletion alone has little effect on the adult mouse liver but renders it more susceptible to necrosis and microvesicular steatosis following partial hepatectomy and impairs hepatocyte proliferation and liver regeneration after injury (206). The regenerative capacity of Micu1−/− livers is rescued by the mPTP inhibitor NIM811, suggesting that the tissue damage and impaired proliferation are caused by mCa2+ overload-induced mitochondrial permeability transition and cell death (206).
Constitutive deletion of Micu1 via CRISPR/Cas9 also yields high rates of perinatal mortality in Micu1−/− pups (163). Micu1−/− pups that survive the perinatal period are ∼50% smaller than WT littermates at 1 wk of age (163). By 1 mo of age, surviving Micu1−/− mice show signs of ataxia and have impaired performance on a balance beam and impaired skeletal muscle strength and coordination, much like human patients with MICU1 mutations (163). These neuromuscular defects are associated with abnormal brain development and reduced skeletal muscle fiber size (163). Micu1−/− animals also have reduced numbers of splenic B cells, indicating that MICU1 exerts important functions in the immune system (163). Liu et al. (163) observe that, despite these defects in early life, the overall appearance, body weight, and elevated resting mCa2+ levels in the brains of surviving Micu1−/− mice improve as the animals age. These improvements are attributed to a gradual downregulation of EMRE expression that normalizes mCa2+ uptake at low cytosolic Ca2+ concentrations and thus minimizes mCa2+ overload (163). These findings highlight the plasticity of mtCU composition and the existence of counterregulatory mechanisms that can normalize mCa2+ handling despite the disruption of a given mtCU component.
Constitutive deletion of Micu1 specifically in skeletal muscle (Creatine kinase-Cre × Micu1fl/fl) reduces the Ca2+ threshold for mCa2+ uptake and results in proximal muscle weakness and atrophy (207). Skeletal muscle knockout (skmKO) of Micu1 does not alter cytosolic Ca2+ transients but reduces mCa2+ accumulation during single-fiber twitches and during tetanic contraction (207). Resting mCa2+ content is elevated in Micu1-skmKO fibers (207), and this may contribute to the smaller overall amplitude of mCa2+ transients during muscle contraction, as opposed to a direct effect of Micu1 deletion to reduce net mCa2+ uptake. Interestingly, though, Micu1-skmKO muscles have impaired sarcolemmal repair after acute muscle injury (207), and efficient muscle fiber repair depends on acute mCa2+ uptake (208). These findings suggest that loss of MICU1 impairs skeletal muscle function by two mechanisms: 1) reducing mCa2+ uptake during muscle contraction, leading to impaired force generation, and 2) diminishing the efficiency of sarcolemmal repair after injury to a muscle fiber, thus increasing the likelihood of muscle cell necrosis and progressive loss of muscle mass and strength (207).
These studies in mice with genetic Micu1 disruption demonstrate that the regulation of acute mCa2+ uptake—not simply whether or not acute mCa2+ is able to occur—is sufficient to affect a number of cellular processes that rely on Ca2+ handling and that impact physiological function at the level of the whole organism. This conclusion is further supported by in vivo research in Drosophila, where global silencing of the MICU1 homolog CG4495 via RNAi is lethal, and where silencing of CG4495 specifically in mushroom body neurons causes memory deficits that recapitulate the effects of silencing of the MCU homolog CG18769 (134). Likewise, inducible knockdown of GC4495 in Drosophila neurons reduces survival and is associated with early loss of locomotor ability (179). These effects are rescued by overexpression of the antiapoptotic Drosophila Bcl-2 homolog, Buffy, implicating aberrant neuronal death in the phenotype of flies with neuronal GC4495 knockdown (179). The recent observation that acute knockdown of Micu1 in the adult mouse heart aggravates mCa2+ overload, infarct size, cardiac dysfunction, and apoptosis following myocardial ischemia-reperfusion injury (209) provides further evidence that appropriate modulation of uniporter activity by MICU1 is critical for cell survival during stress and has substantial impacts on whole organism physiology and disease.
This interesting result indicates that MICU1 activity is relevant for the regulation of mCa2+ handling within the heart, at least under stressed conditions where cytosolic Ca2+ concentration may be elevated beyond normal ranges. That is, MICU1 appears to oppose net mCa2+ accumulation at high cytosolic Ca2+ concentrations within the cardiomyocyte. How it may do so is puzzling. Whether MICU1 is still functioning as a mtCU gatekeeper in this context or is exerting additional functions at sites distinct from the mtCU remains to be determined. Recent work by the Lederer group suggests that cardiomyocytes exhibit no Ca2+ threshold for mtCU gatekeeping, at least over the range of ∼400 nM to 12 µM extramitochondrial Ca2+ (15, 210). It is possible that these experiments simply missed the range of high extramitochondrial Ca2+ concentrations where MICU1-dependent mtCU regulation becomes appreciable within the heart. Cytosolic Ca2+ can reach ∼2 µM during the cardiomyocyte Ca2+ transient (21), and recall that this concentration may be 5–20 times higher in microdomains between the ER/SR and mitochondria. Ca2+ concentration in this microdomain may be elevated even more in pathological settings. If the net ratio of MICU1:MCU in the heart is indeed very low compared with other tissues (187) and the majority of mtCUs in the cardiomyocyte are ungated, this could result in the appearance of minimal gatekeeping activity and minimal extramitochondrial/IMS Ca2+-dependent mtCU activation over the range of 400 nM to 12 µM Ca2+. This is particularly true for experiments considering the overall behavior of a population of mtCUs (188) (FIGURE 4), whether measured within a batch of isolated cardiomyocytes or even within a patch-clamped cardiomyocyte mitoplast.
Further complicating the question of whether MICU1 has an appreciable effect on mtCU function in the heart is the possibility that the susceptibility of MICU1-knockdown hearts to I/R injury reflects the effects of altered mtCU activity and/or the loss of critical mtCU-independent MICU1 functions. To this point, it is interesting to consider how the baseline phenotypes of animal models of MICU1 knockout or knockdown differ from baseline phenotypes of animal models of MCU knockout. In contrast to the effects of MICU1 disruption discussed above, global loss of MCU is well tolerated under basal, unstressed conditions in flies (195) and in mice on a mixed genetic background (119). At least two factors may account for these differences. First, MICU1 disruption generally results in cellular phenotypes associated with mCa2+ overload (tissue damage, permeability transition, necrosis, death of specific cell populations), consistent with a loss of MICU1-dependent mtCU gatekeeping activity. That mPTP inhibition (206) or downregulation of EMRE to limit mCa2+ uptake (163) corrects some of these defects supports this notion. This mCa2+-overload phenotype of MICU1-deficient animals is the opposite of that seen with loss of MCU function, which ablates all measurable rapid mCa2+ uptake and so minimizes the risk of pathogenic mCa2+ overload, which is most relevant during acute cellular Ca2+ stress. MCU seems to be less critical for baseline, unstressed function; thus, MCU-knockout animals display a mild baseline/homeostatic phenotype. A second, intriguing hypothesis to explain the discrepancies between animals with loss of MCU versus loss of MICU1 is the possibility that MICU1 exerts functions independent of the mtCU, for instance at cristae junctions (171, 198). Thus, loss of MICU1 may disrupt other Ca2+-sensitive mitochondrial processes that are independent of changes in mtCU activity and that cause additional functional defects that cannot be recapitulated with disruption of MCU alone. The generation of vertebrate models in which the effects of MICU1 knockout or overexpression are examined independent of the mtCU (i.e., in a MCU- or EMRE-knockout background) will help to elucidate possible additional functions of MICU1 and their impact on physiology.
3.3.3.1.9. Regulation of MICU1.
MICU1 gene expression is controlled by a number of transcription factors and translational repressors. MICU1 mRNA and protein levels are reduced during hypoxia because of increased expression of the transcription factor Foxd1, which binds to the MICU1 promoter and blocks MICU1 gene expression (211). MICU1 is particularly responsive to profibrotic stimuli such as transforming growth factor (TGF)-β and angiotensin II that acutely increase its gene expression (165), although the specific transcription factors responsible for this pathway have yet to be elucidated. The transcription factor early growth response 1 (EGR1) increases MICU1 expression under conditions of nutrient deficiency and limited TCA cycle substrate availability (212). Nemani et al. (212) demonstrate that such upregulation of MICU1 limits mCa2+ uptake and hypothesize that nutrient-sensitive transcriptional control of MICU1 is a mechanism to prevent mCa2+ overload and cell death during starvation conditions such as ischemia. Several caveats to this work exist, though, limiting confidence in this model of MICU1 regulation (213).
The translation of MICU1 transcripts is repressed by microRNAs (miRs) including miR-195, which is reduced in ovarian cancer, leading to elevated MICU1 expression (214). miR-181c is upregulated in obesity and also indirectly affects MICU1 expression. Increased expression of miR-181c promotes cellular ROS production, causing oxidation and downregulation of the MICU1 transcription factor Sp1 (215), leading to net downregulation of MICU1 (216). This effect of miR-181c is likely due to its direct repression of the mitochondrial gene mt-COX1, which encodes a subunit of complex IV. Downregulation of mt-COX1 and subsequent disruption of complex IV activity increases mitochondrial ROS (217). MICU1 expression is thus regulated by retrograde mitochondria-to-nucleus signaling involving mitochondrial ROS production, which in turn is influenced by changes in mCa2+ concentration (218). These observations suggest the intriguing possibility of a feedback loop involving mitochondrial ROS production that modulates MICU1 expression in response to perturbations in mCa2+ homeostasis. Studies in C. elegans indicate that MICU1 protein translation is also repressed by the mRNA decay factor CAR-1, an ortholog of LSM14 that is downregulated during axon regrowth in conjunction with an increase in MICU1 expression (205).
The import of MICU1 protein into the mitochondria depends on the mitochondrial import complex component Tom70 (209). Experiments in the mouse heart reveal that decreased mitochondrial Tom70 abundance after I/R limits mitochondrial MICU1 content and thereby contributes to mCa2+ overload and myocardial I/R injury (209). MICU1 protein is subject to proteasomal degradation, and its half-life is substantially shorter than that of MCU and MICU2 (219). Matteucci et al. (219) attribute MICU1’s degradation to an interaction between MICU1 and the E3 ubiquitin ligase Parkin. Parkin’s Ubl domain, but not its E3 ubiquitin ligase activity, is required for proteasomal degradation of MICU1, raising the possibility that Parkin acts as a scaffold for the interaction of MICU1 with other ubiquitin ligases rather than ubiquitinating MICU1 itself (219). Valosin-containing protein (VCP) promotes the degradation of ubiquitinated MICU1 in the mouse heart and so inhibits mCa2+ uptake at high cytosolic Ca2+ concentrations (220). The specific proteins involved in MICU1 degradation downstream of its interaction with Parkin or VCP remain to be determined.
MICU1 protein function is regulated by a number of posttranslational modifications, the most prominent being redox modification of the cysteine residue that forms disulfide bridges connecting MICU1 homodimers or MICU1/2 or MICU1/3 heterodimers. C55 of the intermembrane space oxidoreductase Mia40/CHCHD4 forms a disulfide bond with MICU1 C465 (C463 according to the numbering scheme noted above) after MICU1 is imported across the OMM (221). Formation of this bond primes MICU1 for subsequent disulfide bond formation and heterodimerization with MICU2 (221). Methylation of R455 by protein arginine methyltransferase 1 (PRMT1) renders MICU1 less sensitive to Ca2+ and consequently impairs mCa2+ uptake (222). Preferential binding of uncoupling protein 2 (UCP2) to methylated MICU1 resensitizes MICU1 to Ca2+ and restores mCa2+ uptake (222). Uncoupling protein-dependent sensitization of MICU1 is proposed to maintain appropriate mCa2+ cycling in settings of increased cellular PRMT activity such as cancer (222). Finally, S124 within the NH2 terminus of MICU1 can be phosphorylated by serine/threonine kinase 1 (AKT). Phosphorylation of S124 disrupts MICU1 proteolytic processing and destabilizes MICU1, ultimately causing loss of gatekeeper function, increased basal mCa2+ levels, and increased ROS production (223). This study reports that activated AKT can be detected within isolated mitochondria and localizes to the IMS, where it would have access to MICU1. Multiple investigations support that AKT can translocate from the cytosol to the mitochondrial IMS or matrix (224, 225), which may occur via mechanisms involving the chaperone heat shock protein 90 (226) and/or redox signaling (227). Phospho-null mutation of MICU1 S124 is sufficient to suppress tumor growth in vivo even in the presence of active AKT, indicating that AKT-dependent MICU1 phosphorylation is relevant to cancer biology (223).
3.3.3.1.10. Pharmacological modulation of MICU1.
A recent high-throughput screen designed to identify small molecules capable of modifying mtCU function identified two compounds, named MCU-i4 and MCU-i11, that inhibit mCa2+ uptake (228). Both of these compounds bind within a specific cleft that separates the N lobe and C lobe of MICU1 and fail to inhibit mCa2+ uptake when residues in this cleft (Q302, Q306, and L443) are mutated, suggesting that their inhibitory effect is mediated specifically by MICU1 (228). Both MCU-i4 and MCU-i11 inhibit mCa2+ uptake in skeletal muscle fibers following caffeine-induced SR Ca2+ release and are sufficient to attenuate myotube growth in vitro (228). These findings indicate that MCU-i4 and MCU-i11 interfere with cooperative activation rather than the gatekeeping function of MICU1 and therefore are promising tools for the prevention of mCa2+ uptake and mCa2+ overload. An intriguing question for further research is whether these compounds may also be used to regulate mtCU-independent functions of MICU1.
3.3.3.2. micu2.
MICU2 (Mitochondrial Calcium Uptake Protein 2; gene name = MICU2, previously annotated as EFHA1) was identified via bioinformatics as a paralog of MICU1 that arose via gene duplication (93). MICU2 localizes to the mitochondrial IMS and is anchored to the uniporter complex via a physical interaction with MICU1 (FIGURE 2). The interaction between MICU1 and MICU2 is preserved in both high-Ca2+ and low-Ca2+ conditions (93). siRNA-mediated knockdown of MICU2 or MICU1, either alone or in combination, does not affect basal respiration or oxidative phosphorylation in mitochondria isolated from mouse liver. However, single knockdown of either MICU1 or MICU2 slows the rate of mCa2+ uptake in response to a single large (50 µM) Ca2+ bolus and limits the ability of mitochondria to buffer extramitochondrial Ca2+ (93). These inhibitory effects on mCa2+ uptake are additive upon simultaneous knockdown of MICU1 + MICU2. However, interpretation of the direct consequences of MICU1 or MICU2 depletion is confounded by the finding that, in certain cell types, knockdown of either of these proteins reduces net MCU protein expression, and is also hindered by the finding that MICU2 knockdown reduces MICU1 protein levels by about half (93). Based on these findings, Plovanich et al. (93) suggest that a major role of the MICU1/2 interaction is for intraprotein stability. Knockdown of MICU1 alone, or in combination with MICU2 knockdown, decreases the overall molecular weight of the mtCU (93), consistent with complete loss of MICU1/2 heterodimers and/or loss of MICU1 homodimers from the uniporter complex. In contrast, knockdown of MICU2 alone shifts only a portion of the existing mtCU complexes to a lower molecular weight (93). This result is consistent with the replacement of MICU1/2 heterodimers by MICU1 homodimers, which have a similar molecular mass, thus preserving the overall mass of a subset of mtCU complexes (∼480 kDa). The portion of mtCUs that do shift to a lower molecular weight upon MICU2 knockdown likely results from a secondary reduction in MICU1 protein abundance, and therefore complete loss of MICU dimers from those particular complexes. Such complex effects of MICU1 and MICU2 on each other’s stability and capacity to regulate the mtCU emphasize the technical challenges in the design and evaluation of experiments aimed at elucidating the role of a single MICU on mCa2+ uptake. This also has important implications for interpretation of experiments using genetic knockout as opposed to partial knockdown of the various MICU proteins (172).
MICU2 contains a pair of highly conserved Ca2+-binding EF-hand domains, just like MICU1, that allow it to respond to local changes in Ca2+ concentration (93). However, distinct tissue distributions suggest that MICUs have unique roles in cellular physiology. Whereas MICU1 is expressed in the mitochondria of most mammalian tissues, MICU2 expression is less ubiquitous and was detected in mitochondria of only 7 of 14 mouse tissues examined by mass spectrometry (93, 156). Both MICU2 and MICU1 show particularly strong mRNA expression in organs such as the stomach and intestines, but MICU2 expression is enriched over MICU1 in tissues including the heart and prostate (93). Within the mitochondria, MICU2 is closely associated with the outer leaflet of the IMM because of obligate heterodimerization with MICU1 (158, 180).
Early work from Rizzuto’s laboratory suggested that MICU2 inhibits the activity of purified MCU reconstituted in lipid bilayers, leading the group to propose that MICU2 functions as a bona fide negative regulator or “gatekeeper” for the mtCU (180). How the addition of MICU2 could produce this effect on purified MCU when MICU2 is not capable of binding directly to MCU (180) is unclear. Patron et al. (180) show that either MICU1 silencing or MICU1 overexpression in HeLa cells increases mCa2+ uptake, consistent with loss of mtCU gatekeeping. In contrast, they report that knockdown of MICU2 increases whereas overexpression of MICU2 decreases mCa2+ uptake, in broad agreement with their results in lipid bilayers. It is important to note that these experiments leading to the initial view of a MICU2 as a “MCU inhibitor” were not performed over a wide range of experimentally controlled extramitochondrial Ca2+ concentrations. Rather, this study examined net mCa2+ transients in response to histamine-induced Ca2+ transients in intact cells or mCa2+ uptake in permeabilized cells in response to increasing the bath Ca2+ concentration from 0 to the relatively low concentration of 400 nM (180).
Additional studies conducted over a wider range of physiological Ca2+ concentrations have refined the field’s understanding of MICU2’s influence on MICU1 behavior and overall mtCU regulation. The Mootha group reports that MICU1 and MICU2 have nonredundant functions but nevertheless work together to prevent mCa2+ uptake through the mtCU when cytosolic Ca2+ concentration is low, and to facilitate mCa2+ uptake when cytosolic Ca2+ concentration rises (172). Genetic knockout of MICU2 in HEK293T cells increases mCa2+ uptake in response to a pulse of moderate-concentration (1 µM) Ca2+, in agreement with Patron et al. (180) and suggesting that MICU2, like MICU1, contributes to overall gatekeeping of the mtCU (172). In this model system, knockout of MICU2 does not reduce MICU1 protein levels, so any effects of MICU2 loss can be specifically attributed to loss of MICU2 function rather than destabilization of MICU1. Mutation of MICU2’s EF-hand domains prevents mitochondria from taking up Ca2+ at both moderate (1 µM) and high (40 µM) concentrations, indicating that when MICU2 binds Ca2+ it contributes to activation of the mtCU (172). Thus, Kamer and Mootha (172) suggest that MICU1 and MICU2 together gate the mtCU when cytosolic Ca2+ concentration is low but then bind Ca2+ and alleviate this inhibition as cytosolic Ca2+ concentration rises. Loss of MICU2 results in a shift in the Ca2+ threshold for this Ca2+-dependent activation of mCa2+ uptake (172) (FIGURE 4). Furthermore, since disruption of either MICU1’s or MICU2’s EF-hands ablates mCa2+ uptake, the relief of mtCU channel gatekeeping by rising cytosolic Ca2+ concentrations depends upon Ca2+ binding to both MICU components of a MICU1/2 heterodimer. Mutation of the MICU2 EF-hands has no effect on mCa2+ uptake in MICU1-knockout cells, indicating that MICU2-dependent channel regulation depends on the presence of MICU1 (172). Indeed, since MICU2 cannot bind to MCU, MICU2 must mediate its effects on mtCU function via its physical interaction with MICU1 (172, 180).
Studies by the Foskett laboratory further clarify MICU2’s role in mtCU regulation. Payne et al. (173) show that genetic knockout of MICU2 in HEK293T cells has no effect on MICU1 expression or MICU1 binding to MCU, and does not alter mtCU gatekeeping at low (100–300 nM) cytosolic Ca2+ concentrations or affect basal mCa2+ concentration. That is, MICU2 is not strictly required for mtCU gatekeeping over this Ca2+ range. The authors suggest that MICU1, possibly functioning as MICU1 homodimers, is sufficient to gate the mtCU and prevent mCa2+ uptake at low, resting extramitochondrial Ca2+ levels. It should be noted, though, that any inferences about specific MICU1 homodimer function in MICU2-knockout cells, where the presence or absence of MICU3 has not been confirmed, remain speculative because such function could instead reflect the consequences of an increase in the relative cellular population of MICU1/3 homodimers in the absence of MICU2.
In contrast to the lipid bilayer results cited above (180), overexpression of MICU2 in MICU1-knockout cells cannot rescue channel gatekeeping and so does not prevent excessive mCa2+ uptake at low cytosolic Ca2+ concentrations (173). Again, this fits with the observation that MICU2 does not bind to the mtCU in the absence of MICU1. At higher cytosolic Ca2+ concentrations (500 nM to 10 µM), mCa2+ uptake is more sensitive to an increase in Ca2+ concentrations in MICU2-knockout cells, and the relationship between mCa2+ uptake rate and cytosolic Ca2+ concentration is shifted to the left (173) (FIGURE 4). Thus, Payne et al. propose that MICU2 does not act as an inhibitor of the mtCU but instead modulates the threshold Ca2+ concentration at which MICU1’s mtCU gatekeeping activity is relieved. They further suggest that MICU2 reduces the gain of mtCU activation, by opposing MICU1-dependent channel activation at higher Ca2+ concentrations via a mechanism that reduces the Ca2+ affinity of this activation (173). Thus, the presence of MICU2 shifts the Ca2+ dependence of cooperative activation of the channel to the right, making it less responsive to low cytosolic Ca2+ concentrations. However, the cooperative nature of mtCU activation by Ca2+ is otherwise much the same regardless of whether is MICU2 is present (173). Interestingly, disruption of either of MICU2’s EF-hands disrupts cooperative activation of the mtCU, indicating that MICU2’s ability to bind Ca2+ is required both for high Ca2+ concentrations to relieve mtCU gating and for cooperative activation of the channel (172, 173).
Considered together, these reports support a model in which the MICU1/2 dimer functions as an “on-off switch” for the uniporter (176) and in which MICU2 specifically modulates the threshold and gain of MICU1’s gatekeeping and cooperative activation functions (173). At low, resting cytosolic Ca2+ concentrations, MICU1 gates the mtCU and prevents mCa2+ uptake through the channels; as cytosolic Ca2+ concentration rises, MICU1’s gatekeeping function is relieved. Whether the channel is gated by MICU1/1 homodimers or MICU1/2 heterodimers determines the cytosolic/IMS Ca2+ concentration that is required to relieve gatekeeping and allow cooperative activation of the channel to occur (FIGURE 4). In the absence of MICU2, the Ca2+ sensitivity of mtCU activation is increased, shifting this relationship to the left. This allows for greater mCa2+ uptake at low and moderate Ca2+ concentrations, consistent with the initial observations of the effects of MICU2 knockdown (180) and MICU2 knockout (172). When MICU2 is present, it reduces the Ca2+ sensitivity of mtCU activation, pushing the curve to the right, and with MICU1 participates in the cooperative activation of the channel at Ca2+ concentrations above the new activation threshold. Thus, the Ca2+-dependent regulation of the mtCU is mediated by the net effects of MICU1 and MICU2 and by the relative availability of each within the cell.
3.3.3.2.1. MICU2 phylogenetic conservation.
MICU2, along with MICU1, is conserved among vertebrate species, but only a single ancestral MICU isoform is present in plants and protozoa. Sequence analysis suggests that MICU2 arose via gene duplication of the ancestral MICU1-like isoform (93). A MICU2 ortholog is also present in trypanosomatids and C. elegans (93–95), and suggests that the initial gene duplication occurred before divergence of these lineages. MICU2 was subsequently lost from several genera including Leptomona, Crithidia, and Leischmania (94). The observation that the parasite T. cruzi can survive genetic loss of MICU1 or MICU2 but that simultaneous loss of MICU1 and MICU2 is lethal (94) supports the idea that these proteins have nonredundant roles in lower organisms. In contrast to results in HeLa cells (180), MICU2 overexpression in T. cruzi surprisingly does not affect mCa2+ uptake (94). This finding suggests some divergence of MICU2 protein function in parasites versus humans. Another possibility is that T. cruzi already have a high MICU2-to-MICU1 ratio and that the introduction of additional exogenous MICU2 does little to alter mtCU activity. MICU2 knockout in T. cruzi phenocopies MICU1 knockout and limits acute mCa2+ uptake over a range of 1.5–50 µM extramitochondrial Ca2+ (94). However, it is not clear whether this finding represents a function of MICU2 that is unique to trypanosomatids or whether impaired mCa2+ uptake upon MICU2 deletion is due to destabilization of MICU1 protein and loss of MICU1-dependent cooperative activation of the mtCU, as occurs with MICU2 knockdown in some mammalian cells.
3.3.3.2.2. MICU2 structure.
MICU2 shares 20–25% protein sequence identity and ∼40% sequence similarity with MICU1, depending on species (93, 94, 180). MICU2 has a molecular mass of 45 kDa (versus ∼50 kDa for MICU1) (180). Much of MICU2’s overall domain architecture is conserved with that of MICU1 and is characterized by two highly conserved Ca2+-binding EF-hands separated by a long linker region composed of α-helices (93). The first EF-hand is critical for MICU2 to undergo conformational changes needed to form dimers following binding of Ca2+ (229). Like MICU1, MICU2’s NH2 terminus contains a mitochondrial targeting sequence (93). However, several differences in the NH2- and COOH-terminal regions of MICU2 and MICU1 contribute to their distinct functional properties. MICU2 has a longer COOH-terminal helix, which is more rigid in structure than the corresponding COOH terminus of MICU1 (230). This COOH-terminal helix is not needed for dimerization between MICU1 and MICU2, but it is required for MICU2-dependent gatekeeping function within cells (230). MICU2’s COOH-terminal helix is stabilized by a short, NH2-terminal β-strand that is also specific to MICU2 (230).
Dimerization between MICU1 and MICU2 is mediated by disulfide bond formation between C465 of MICU1 and C410 of MICU2. Both of these cysteine residues are critical for heterodimer formation, as mutant C465A MICU1 and C410A MICU2 are incapable of heterodimerization with wild-type MICU2 or MICU1, respectively (180). MICU1/2 heterodimers are further stabilized by the formation of a salt bridge between residues R221 in MICU1 and D330 in MICU2 (229), although this interaction itself is not sufficient to mediate dimerization in the absence of disulfide bond formation. A second salt bridge forms between D231 of MICU1 and R352 of MICU2 (231). Finally, the MICU1/2 heterodimer is stabilized by hydrophobic interactions between M229 of MICU1 and M337 of MICU2 (231). Park et al. (231) suggest that these salt bridges and hydrophobic interactions make the intermolecular association of MICU1/2 heterodimers stronger than the intermolecular association between either MICU1 homodimers or MICU2 homodimers.
As noted above, dimerization of MICU2 with MICU1 is critical for anchoring of MICU2 to the mtCU and for MICU2-dependent regulation of the uniporter channel (172, 180). The three-dimensional structure of the MICU1/2 heterodimer is also responsive to Ca2+ binding. In the Ca2+-free state, the MICU1/2 heterodimer is characterized by electrostatic interactions between E242 of MICU1 and R352 of MICU2 (193). Upon Ca2+ binding, the MICU1/2 heterodimer instead depends on F383 of MICU1 and E196 of MICU2, and is more compact in structure (193). The shift to the compact form of the heterodimer involves the establishment of hydrophobic interactions between F383 of MICU1 and I333 and M337 of MICU2 that are exposed as the EF-hands of MICU1/2 rotate outward after they bind Ca2+ (193). The Ca2+-induced conformational shift in the MICU1/2 heterodimer is likely responsible for Ca2+-dependent changes in mtCU gating and activation. Xing et al. (232) also note that MICU2 forms “back-to-back” homodimers involving residues R107, K121, and D154 that are critical for mtCU gatekeeping and that dissociate in response to Ca2+. Thus, contact between multiple MICU2 subunits, possibly involving multiple MICU1/2 dimers present at a given mtCU uniplex, may contribute to MICU2’s regulation of the channel. This model is discussed in further detail below.
3.3.3.2.3. Properties and function of MICU2.
Research since the initial characterization of MICU2 has enhanced our understanding of how MICU2 cooperates with MICU1 to regulate mtCU activity. Matesanz-Isabel et al. (175) agree with the original Rizzuto model and suggest that MICU2 functions as a pure inhibitor of the uniporter, but acknowledge that this apparent inhibitory effect is minimized as cytosolic/IMS Ca2+ levels rise past 7 µM. However, this same phenomenon can be explained by a MICU2-dependent decrease in the Ca2+ sensitivity of mtCU activation, which reduces channel function at low cytosolic Ca2+ concentrations. This function of MICU2 agrees with data indicating that the formation of MICU2 back-to-back dimers is required to suppress mCa2+ uptake at moderate (1 µM) cytosolic Ca2+ concentrations and that these dimers dissociate when local Ca2+ concentration rises (232). Whether such MICU2 dimer structures within a given uniplex channel influence MICU2’s role in the integration of matrix and IMS Ca2+ signals (115, 116) or other functions is undetermined. An interesting concept, though, is the possibility for distinct MICU2 molecules to functionally interact, perhaps influencing the collective behavior of populations of MICU1/2 heterodimers, conferring additional, “emergent” levels of channel regulation beyond that provided by MICU1/1 homodimers alone.
Interesting studies by the Pozzan and Riemer laboratories demonstrate that Ca2+-dependent dissociation of the MICU1/2 heterodimer from MCU mediates the gradual activation of the mtCU that occurs in response to sustained, moderate increases in cytosolic Ca2+, such as in ischemia-reperfusion (189, 221). Paillard et al. (187) suggest that tissue-specific expression of MICU1 is the limiting factor for gating of the mtCU by MICU1-2/3 heterodimers or MICU1 homodimers, because MICU2 and MICU3 cannot bind to the complex on their own. Thus, even under physiological conditions there is a balance between “ungated” mtCUs that lack MICU dimers and “gated” mtCUs that are regulated by the MICUs, depending on the availability of MICU1 within the tissue (187). In addition to the presence versus absence of MICU dimers on the mtCU (MICU-to-MCU ratio), another key parameter that shapes uniporter regulation is the relative ratio of MICU1:MICU2 at the uniporter. Deletion of MICU2, leaving only the possibility of MICU1 homodimers (or MICU1/3 heterodimers, depending on cell type) increases the ratio of MICU1:MICU2 and shifts the uniporter’s Ca2+ activation threshold from ∼600–800 nM down to ∼350 nM (173, 176) (FIGURE 4). The relative ratio of MICU1:MICU2 is particularly low in tissues such as the heart and prostate (93). Assuming that MICU1 abundance is not limiting, a high level of MICU2 expression and corresponding low ratio of MICU1:MICU2 is predicted to replace some MICU1 homodimers with MICU1/2 heterodimers, and so would reduce the Ca2+ sensitivity of mtCU activation, thus requiring higher cytosolic Ca2+ levels to permit mCa2+ uptake through the channel. Therefore, the relative expression of MICU2 versus MICU1 is another determinant of tissue-specific properties of mCa2+ uptake.
3.3.3.2.4. Interaction of MICU2 with the metazoan mtCU complex.
Binding of MICU2 to the mtCU is mediated by heterodimerization of MICU2 with MICU1 (180), which then interacts with MCU and EMRE and tethers the heterodimer to the complex (158). MICU2 is not capable of binding to MCU directly, in the absence of MICU2 dimerization with MICU1 (172, 180). MICU2 also differs from MICU1 in that it does not interact stably with EMRE (158, 172). The current structural models for the mtCU suggest a “dimer of uniplex channels” configuration (introduced above in sect. 3.3.3.1). This highlights how MICU2 and the MICU1/2 heterodimer cooperate to keep the channel open or closed depending on local Ca2+ concentration. Within a single “uniplex” channel (4 MCU:3 or 4 EMRE:1 MICU1:1 MICU2), MICU2 binds to MICU1, which blocks the uniporter pore, but MICU2 does not interact directly with MCU (184, 185). The regions of MICU1’s uniporter interaction domain that directly contact MCU (the α1-helix, P124 in the bend between the α1- and α2-helices, and amino acids that interact with MCU’s D ring) are not conserved in MICU2 (185). So, MICU2 is not capable of blocking the mtCU pore on its own, in the absence of MICU1. Xing et al. (232) propose that contacts between the MICU2 proteins of multiple MICU1/2 heterodimers present on each uniplex channel act as an inhibitory latch that keeps the heterodimers in a position where MICU1 can block the channel pore under low-Ca2+ conditions. This is an intriguing idea, although it is at odds with structural models in which each uniplex channel has only a single MICU1/2 heterodimer (184, 185).
As Ca2+ concentration increases, favoring dimerization of the individual uniplex channels, the MICU1/2 heterodimers of each uniplex form a back-to-back bridge spanning the distance between the two channels (184, 186). Although the channel activity of the mtCU does not require such dimerization of the uniplex channels (that is, monomeric uniplex channels are fully functional and subject to regulation by the MICU proteins), the pore of each mtCU uniplex does become unblocked in the dimer conformation (184–186). This conformational shift is associated with a change in MICU1 from binding MCU to favoring an interaction with EMRE (194), and so may alter MICU-dependent channel regulation. These observations fit with the finding that the MICU1/2 heterodimer is bound to MCU at low Ca2+ concentrations but dissociates from MCU as Ca2+ levels rise (221). Both MICU1 and MICU2 can associate with cardiolipin in the IMM (176). It is tempting to speculate that cardiolipin binding stabilizes the Ca2+-bound MICU1/2 heterodimer in the “bridging” state where MICU1 releases MCU in favor of EMRE and the heterodimer is pulled away from the mtCU pore. Indeed, structures of the Ca2+-bound MICU1/2 heterodimers either in single uniplex channels or in the “bridge” between dimerized uniplex channels place MICU2 in proximity with the outer leaflet of the IMM (185, 186). Thus, cardiolipin binding by MICU2 may stabilize the MICU1/2 heterodimer in the “open,” bridging state.
The particular composition of MICU dimers present on the mtCU uniplex channels will influence how the channels are gated in these structural models. Kamer et al. propose that, in the low-Ca2+ state, the relatively inflexible COOH-terminal helix of MICU2 is stabilized by a segment of the MICU2 NH2 terminus and points toward the center of the mtCU pore, contributing to an “inhibitory latch” for the channel as hypothesized by Xing et al. (230, 232). The COOH-terminal helix of MICU1, in contrast, is much less rigid, which may make it less effective at forming this latch on its own, even though it is predicted to interact with the COOH-terminal helix of MICU2 (230). The unique “inhibitory latch” gating function conferred by MICU2 should then be absent from mtCU channels gated by MICU1/1 homodimers, potentially explaining the increased sensitivity of the mtCU to activation by Ca2+ that is observed upon genetic MICU2 disruption. Thus, interactions between the MICU2 constituents of a pair of MICU1/2 dimers present on a single uniplex mtCU may be one mechanism by which the presence of MICU2 decreases the Ca2+ sensitivity of mtCU activation.
Another mechanism differentiating the gating behavior of MICU1 homodimers versus MICU1/2 heterodimers is the difference in their affinity for Ca2+. MICU1 and MICU2 both bind Ca2+ with high affinity, but the Ca2+-binding affinity of MICU2 is lower than that of MICU1 (i.e., MICU2 is less sensitive to Ca2+) (176). MICU1/2 heterodimers thus have a lower overall Ca2+ affinity (∼600 nM, similar to the mtCU gatekeeping threshold observed in wild-type cells) than MICU1 homodimers (172, 176, 231). Park et al. (231) hypothesize that the greater binding affinity of MICU1-MICU2 interactions within heterodimers, versus the binding affinity of MICU1-MICU1 interactions within homodimers, makes it more difficult for MICU1/2 heterodimers to undergo conformational changes required for Ca2+ binding. Since Ca2+ binds to MICU1 and MICU2 cooperatively and requires conformational changes in both proteins, the net result is that the greater structural stability of the Ca2+-free MICU1/2 heterodimer reduces its affinity for Ca2+ and makes the mtCU less sensitive to activation at low Ca2+ than the MICU1 homodimer does (231). Therefore, a higher cytosolic/IMS Ca2+ concentration is required to open mtCU channels gated by MICU1/2 heterodimers than those gated by MICU1 homodimers.
3.3.3.2.5. Genetic diseases associated with MICU2.
Homozygous truncation of MICU2 was recently found in patients with a neurodevelopmental disorder characterized by severe cognitive impairment, spasticity, and white matter involvement (233) (TABLE 1). The mutation (c.42G>A) generates an early stop codon that results in truncation of MICU2 after residue 13 and expression of a shortened, <10-kDa MICU2 protein. Patient skin fibroblasts with this mutation exhibit elevated resting mCa2+ content, as well as diminished cytosolic Ca2+ transients and prolonged mCa2+ influx upon stimulation with bradykinin (233). These observations are consistent with the purported role of MICU2 to modulate the Ca2+ sensitivity of mtCU gatekeeping and cooperative activation. MICU1 protein levels are normal in MICU2-mutant fibroblasts (233), suggesting that this aberrant Ca2+-handling behavior is specifically attributable to loss of MICU2 function, and likely replacement of MICU1/2 heterodimers with MICU1/1 homodimers (173), rather than any secondary effect on MICU1 protein stability. The altered mCa2+ handling in MICU2-mutant cells is associated with a greater sensitivity to oxidative stress in vitro (233). It remains to be determined how increased susceptibility to oxidative stress, and any subsequent neuronal dysfunction or death, may contribute to the neurological phenotypes of individuals with homozygous MICU2 truncation.
3.3.3.2.6. Animal models of MICU2.
Only one genetic animal model to date has been developed to investigate MICU2 function in vivo (TABLE 2). Bick et al. disrupted Micu2 via gene trap to generate mice with constitutive, germline loss of MICU2. Micu2−/− mice are born at normal Mendelian ratios and have a normal life span (234). That loss of MICU2 is developmentally tolerated much better than loss of MICU1 (163) recapitulates observations in Drosophila, where loss of MICU1 is lethal but loss of MICU3, the only other MICU1 ortholog present in flies, has no impact on viability (195). Bick et al. (234) report that MICU1 and MCU protein levels are reduced in the liver of Micu2−/− mice but do not comment on other tissues. Loss of MICU2 protein results in more rapid mCa2+ uptake at low Ca2+ concentrations, as expected upon loss of MICU2-dependent mtCU regulation (234). However, mCa2+ uptake is slower in response to high Ca2+, which the authors attribute to the secondary reduction in MCU expression.
Mitochondria in Micu2−/− hearts are slightly smaller and more eccentric compared with WT hearts, although cristae structure is normal (234). Micu2−/− cardiomyocytes exhibit slower relaxation rates due to slowed cytosolic Ca2+ clearance, indicative of diastolic dysfunction. As a consequence of this, Micu2−/− mice develop left atrial enlargement at advanced ages of 16–18 mo (234). Loss of MICU2 does not affect cardiac hypertrophy in response to chronic angiotensin II infusion, but it accelerates angiotensin II-induced decompensation of contractile function (234). MICU2 deletion also increases the animals’ susceptibility to abdominal aortic aneurysm during chronic angiotensin II infusion. This defect is associated with increased expression of inflammatory and extracellular matrix genes, decreased expression of cell-junction genes, and markers of increased ROS generation. Single-cell RNA sequencing revealed that such transcriptional changes occurred in both aortic smooth muscle cells and aortic fibroblasts (234). Collectively, these experiments confirm that MICU2’s modulation of MICU1 function and overall mtCU channel behavior is functionally relevant in vivo and that its expression is particularly important in the adaption of multiple different cell types to stress. An interesting question for future studies will be to determine whether acute and/or tissue-specific Micu2 deletion reveals any further phenotypes that may have been masked by gradual compensation to constitutive deletion of Micu2 in gene trap mice.
3.3.3.2.7. Regulation of MICU2.
Little is known about how MICU2 is regulated at the transcriptional or posttranslational level. As noted above, MICU2 protein is destabilized in the absence of MICU1 (93). This finding suggests the presence of unidentified quality control mechanisms that degrade MICU2 protein that is not appropriately dimerized with MICU1 and anchored to the uniporter complex. Matteucci et al. (219) find that overexpression of the E3 ubiquitin ligase Parkin reduces the stability of MICU2 protein. However, this effect is likely an indirect consequence of Parkin-dependent degradation of MICU1, since, unlike MICU1, mature MICU2 protein within the mitochondria is not rapidly degraded by the ubiquitin proteasome system and persists even with Parkin overexpression (219).
3.3.3.3. micu3.
MICU3 (Mitochondrial Calcium Uptake Protein 3; gene name = MICU3 or EFHA2) was identified as a second paralog of MICU1 at the time of MICU2 discovery (93). Just like MICU1 and MICU2, MICU3 contains two conserved EF-hands that bind Ca2+ and allow it to serve as a Ca2+ sensor (93, 96). MICU3 protein is detected in the mitochondria of 6 out of 14 mouse tissues with high expression in excitable tissues including the brain, spinal cord, and skeletal muscle (93, 156). The relative mRNA expression of MICU3 tends to be high in tissues where MICU2 expression is low (such as neural tissue and skeletal muscle), and MICU3 expression is low in most other tissues where MICU2 expression is high (93). This pattern suggests a reciprocal relationship between MICU2 and MICU3 expression within each cell type. Like MICU2, MICU3 heterodimerizes with MICU1 (96) and therefore competes with MICU2 for binding to MICU1 (FIGURE 4). MICU2 and MICU3 do not appear to heterodimerize with each other (96). Although MICU3 subcellular localization has not yet been experimentally demonstrated, binding to MICU1 would place MICU3 within the mitochondrial IMS.
MICU3 strongly potentiates histamine-induced mCa2+ transients and increases mCa2+ accumulation at low (400 nM) cytosolic Ca2+ concentration (96). These effects are dependent upon simultaneous expression of MICU1 (96), suggesting that, just like MICU2, MICU3 requires MICU1 to regulate mtCU function. MICU3 is less effective than MICU2 at increasing mtCU gatekeeping and minimizing basal mCa2+ uptake when expressed in cells that also overexpress MCU and MICU1 (96). Therefore, Patron et al. (96) conclude that any gatekeeping functions of MICU3 are less potent than gatekeeping functions of MICU2. Indeed, they suggest that MICU3 disrupts normal mtCU gatekeeping function because it displaces MICU2 from MICU1, thereby causing loss of the more robust (less Ca2+ sensitive) MICU2-dependent gatekeeping (96) (FIGURE 4). Accordingly, increasing MICU3 expression in a cell that normally expresses MICU2 would replace MICU1/2 dimers with MICU1/3 dimers, resulting in the shift to greater channel activity at lower cytosolic Ca2+ concentrations, as observed by Patron et al. (FIGURE 4).
MICU3 silencing downregulates the amplitude of mCa2+ transients in primary cortical neurons (96). Thus, a fundamental role of MICU3 is to augment Ca2+ uptake through the mtCU. In theory, this might occur via a mechanism similar to that described for MICU2, where Ca2+ binding to both MICU1 and its partner MICU2 (or MICU3) is required for MICU1’s cooperative activation of the channel (172, 173). The notion that MICU3 augments mCa2+ uptake is challenged, though, by experiments in HeLa cells, where MICU3 overexpression prevents increased mCa2+ uptake in MICU1-knockdown cells or MICU2-knockdown cells treated with 1 µM Ca2+ (232). This result suggests that MICU3 may attenuate mCa2+ uptake rather than augmenting it. However, this study did not examine the consequences of MICU3 overexpression alone on a wild-type background, so it is difficult to dissect the specific contributions of MICU3 versus potential secondary effects due to stabilization of other mtCU subunits on mCa2+ uptake.
One reported behavior unique to MICU3 is that its overexpression in wild-type cells shortens the time between an agonist-induced increase in cytosolic Ca2+ concentration and the initiation of subsequent mCa2+ uptake (96). How this phenomenon arises is unclear, but one possibility is that it results from a lower binding affinity between the constituent subunits of MICU1/3 heterodimers versus MICU1/2 heterodimers. As introduced above, a lower binding affinity between the members of a MICU dimer makes it easier for each subunit to undergo the conformational changes needed to bind and respond to Ca2+. If the requisite Ca2+-induced conformational changes occur more readily in MICU1/3 heterodimers than in MICU1/2 heterodimers, it would shorten the time between a rise in cytosolic Ca2+ and the subsequent activation of mCa2+ uptake in cells that have a greater relative population of MICU1/3 heterodimers than MICU1/2 heterodimers. Indeed, this concept agrees with the idea that MICU1/3 heterodimers have a greater Ca2+ affinity than MICU1/2 heterodimers and so do not shift the Ca2+ sensitivity of mtCU regulation as far to the right (relative to MICU1/1 homodimers) as MICU1/2 heterodimers.
3.3.3.3.1. MICU3 phylogenetic conservation.
MICU3 arose from an ancestral MICU1-like gene via gene duplication, similar to MICU2. MICU3 is not present in plants or protozoa but is conserved among vertebrate species (93). Drosophila contain MICU1 and a second MICU isoform, which Tufi et al. (195) identify as a paralog of MICU3. The presence of MICU3 but not MICU2 in flies suggests that the gene duplication that gave rise to MICU3 occurred prior in evolutionary history to a subsequent duplication that gave rise to MICU2. The evolution of MICU3 and MICU2 conferred species with an extra level of mtCU regulation—modulation of the function of the ancestral MICU1—and an enhanced ability to control mtCU activity in a tissue-specific manner based on the relative expression of the three MICU isoforms (96).
3.3.3.3.2. MICU3 structure.
MICU3 protein shares ∼25% sequence identity and 34% sequence similarity with MICU1, whereas MICU3 shares ∼47% sequence similarity with MICU2 (93, 96). MICU3 contains an NH2-terminal mitochondrial targeting sequence and two Ca2+-binding EF-hands (93, 96). Mutation of MICU3’s EF-hands diminishes agonist-induced mCa2+ uptake but does not completely abolish it, indicating that the EF-hands contribute to positive regulation of the mtCU but are not strictly required for it (96). A unique feature of MICU3, versus MICU1 and MICU2, is the inclusion of an extra 50-amino acid domain in the middle of the protein. This additional sequence makes MICU3 slightly larger than the other MICU isoforms, with a molecular mass of ∼55 kDa (96).
MICU3 dimerizes with MICU1 via a disulfide bond formed between MICU3 C515 and MICU1 C465. Both of these cysteine residues must be present for MICU1/3 dimer formation (96). Wu et al. (193) predict that E256, M445, and R460 of MICU3 also contribute to MICU1/3 heterodimerization, based on conservation with corresponding residues in MICU2 that promote MICU1/2 heterodimerization.
3.3.3.3.3. Interaction of MICU3 with the metazoan mtCU complex.
MICU3 binds to the mtCU via its heterodimerization with MICU1 (96). The structural features of MICU1’s uniporter interaction domain that allow it to bind to MCU are not conserved in MICU3 (185). MICU3, like MICU2, therefore strictly depends upon dimerization with MICU1 to bind to and regulate the uniporter. Accordingly, MICU3 does not directly contact MCU or directly block the uniporter pore. In the structural model for the mtCU as a dimer of uniplex channels discussed above in sects. 3.3.3.1 and 3.3.3.2, MICU3 is expected to replace MICU2 in tissues like the brain where MICU3 is highly expressed. This would result in each uniplex mtCU containing 4 MCU:3 or 4 EMRE:1 MICU1:1 MICU3 (184, 185).
Structural studies indicate some degree of functional redundancy between MICU3 and MICU2 in regulating mtCU activity. In Ca2+-free conditions, MICU3 can form back-to-back homodimers, just like MICU2 (232). The ability to form back-to-back dimers is likely a consequence of the structure of the MICU3 NH2 domain, which is very similar in structure to the MICU2 NH2 domain (232). Xing et al. (232) hypothesize that back-to-back MICU3 dimers act in the same way as back-to-back MICU2 dimers to create an inhibitory latch that positions MICU1 appropriately to keep the mtCU closed. However, this idea has not yet been experimentally tested and at first consideration conflicts with the current model of MICU3 increasing the Ca2+ affinity of MICU1-dependent mtCU regulation relative to MICU2. These conflicting views may be reconciled by a model in which back-to-back interactions between the MICU3 subunits of two MICU1/3 heterodimers occur and do contribute to overall channel gatekeeping, but are simply less stable and therefore less effective at inhibiting channel activity as cytosolic Ca2+ concentrations rise than back-to-back interactions between the MICU2 subunits of two MICU1/2 heterodimers. This interpretation is supported by the finding that MICU3 is not as effective as MICU2 at limiting basal mCa2+ uptake in cells that overexpress MCU + MICU1 (96).
Ca2+ binding induces structural rearrangements within the first EF-hand of MICU3, but the second EF-hand does not change conformation upon binding Ca2+ (232). Thus, the overall three-dimensional structure of MICU3 does not appreciably differ between the Ca2+-free and Ca2+-bound states. MICU3 behavior resembles MICU1 in this respect (182) and stands in contrast to MICU2, which changes its overall conformation upon binding Ca2+ (232). Ca2+-bound MICU3 is capable of forming “face-to-face” dimers via its EF-hands, much like the face-to-face MICU1 dimers that form in the presence of Ca2+ (232). Thus, Xing et al. (232) suggest that Ca2+ binding to MICU1/2/3 triggers a shift in the distribution of different mtCU forms present within a cell. At low cytosolic Ca2+ concentration, MICU1/3 heterodimers form back-to-back dimers via the association of their constituent MICU3 subunits with the corresponding MICU3 subunit of a second MICU1/3 heterodimer. This organization of the MICU proteins generates the “inhibitory latch” that Xing et al. (232) propose helps keep the mtCU closed. MICU1 does not form back-to-back dimers itself (232), so it is less effective at channel inhibition on its own than when interacting with MICU2 or MICU3. At higher Ca2+ concentrations, a greater proportion of the individual MICU1/2 and MICU1/3 dimers adopt a face-to-face conformation that allows for mtCU activation as the inhibitory latch disassembles (232). Based on this proposed model, one can speculate that the apparent inhibitory function of MICU3 reported by Xing et al. (232) is not an intrinsic property of an individual MICU3 dimerizing with MICU1, which should lower the Ca2+ threshold for activation of the channel compared with a single MICU1/2 dimer. Rather, the inhibitory function of MICU3 may instead be an emergent property predicted to arise from the interaction between multiple MICU1/3 heterodimers acting within the same uniporter channel. Thus, when participating in back-to-back dimers, either MICU2 or MICU3 may contribute to channel gatekeeping or inhibition, albeit with different degrees of efficacy. Direct experimental evidence for or against this hypothesized function of MICU3 is needed to settle whether MICU3 indeed forms back-to-back dimers in situ and the effect they may have on mtCU function.
A relevant question for future studies is how the subunit-subunit binding affinity compares among MICU1 homodimers, MICU1/2 heterodimers, and MICU1/3 heterodimers. As noted above, a weaker binding affinity between the two constituent subunits of a MICU dimer is correlated with a higher Ca2+-binding affinity for the dimer, because it is easier for the individual subunits to undergo conformational changes related to Ca2+ binding (231). A higher Ca2+-binding affinity makes the MICU dimer more sensitive to increases in cytosolic Ca2+ concentration, and so reduces the gatekeeping threshold for the mtCU and lets the channel open at a lower cytosolic Ca2+ concentration. If MICU3 truly allows the mtCU to be activated at lower Ca2+ thresholds than when regulated by MICU2, then this function should be associated with a higher degree of subunit mobility (i.e., weaker subunit-subunit binding affinity) and a corresponding increase in the Ca2+-binding affinity of MICU1/3 heterodimers compared with MICU1/2 heterodimers. A MICU1-MICU3 subunit binding affinity that is weaker than the affinity between MICU1 and MICU2 subunits aligns well with the observation that MICU3-regulated mtCU gatekeeping is relieved at lower Ca2+ concentrations than MICU2-regulated gatekeeping (96) (FIGURE 4).
3.3.3.3.4. Special role of MICU3 in physiology.
Examination of MICU3’s particular role within neurons highlights how the distinct functional properties of the various mtCU regulatory subunits tune mtCU activity to match tissue-specific needs. The unique features of MICU3 and its interaction with MICU1 and the mtCU confer neural tissue with specific mCa2+ handling properties that support its specialized physiology. Neurons express MICU1/3 and MICU1/2 heterodimers at similar levels (96), and this will result in a higher proportion of neuronal mtCU channels gated by MICU1/3 than in other cell types. Since MICU1/3 dimers have a lower Ca2+ activation threshold than MICU1/2 dimers, this makes neuronal mitochondria able to take up Ca2+ at lower cytosolic Ca2+ concentrations than mitochondria in other tissues. As a result, neuronal mitochondria are more sensitive to changes in cellular activity that elevates cytosolic Ca2+ than mitochondria in other cell types (235). The notion that, relative to MICU2, MICU3 increases the sensitivity of mCa2+ uptake to small increases in cytosolic Ca2+ concentration agrees with the finding that MICU3 knockdown in primary cortical neurons decreases the amplitude of mCa2+ transients caused by cytosolic Ca2+ oscillations (96).
The enhanced Ca2+ sensitivity conferred by MICU3 may be critical for presynaptic neurons to ramp up local mitochondrial metabolism when needed, such as during neurotransmission. After an action potential reaches the presynaptic terminal, the subsequent increase in cytosolic Ca2+ concentration is transmitted to the mitochondrial matrix, where Ca2+ stimulates ATP production needed to fuel the synaptic vesicle recycling that is required for sustained neurotransmitter release. The Ryan laboratory proposes that the MICU3-dependent reduction in the mtCU Ca2+ threshold allows for “feedforward regulation of ATP production” within the nerve terminals, such that the very stimulus (local elevation in cytosolic Ca2+) that triggers an energy-consuming process (neurotransmitter release and vesicle recycling) is the same signal that stimulates energy production needed to sustain this process (235). This is the same concept as the parallel stimulation of mitochondrial oxidative metabolism and ATP-consuming myofilament cross-bridge cycling during sympathetic stimulation of the heart, introduced above in sect. 3.3.1.
3.3.3.3.5. Genetic diseases associated with MICU3.
MICU3 has not yet been reported to cause genetic disease in humans. Also, very little is known about whether MICU3 is involved in the pathological mechanisms by which mutations in other genes cause disease. Given the importance of MICU3 in neurons, future studies should explore its potential involvement as a driver and/or modifier of neurological and neurodegenerative disease. Conditional knockout models are needed to begin to dissect the role of MICU3 in pathophysiology.
3.3.3.3.6. Animal models of MICU3.
Tufi et al. (195) recently described a Drosophila model with CRISPR/Cas9-induced genetic disruption of MICU3 (CG4662 in the fly) (TABLE 2). Homozygous MICU3-mutant flies are viable but have a 7% reduction in life span, in contrast to MICU1 disruption, which is lethal (195). MICU3-mutant flies exhibit a climbing defect, even at young ages, which the authors interpret as a neurological defect (195). However, MICU3 disruption has no significant effect on basal mitochondrial respiration in fly head tissue (195). This suggests that loss of MICU3 has little effect on basal mCa2+ uptake and respiration, even though increased expression of MICU3 is reported to make mCa2+ uptake in mouse neurons more sensitive to small increases in cytosolic Ca2+ concentration (235). These results imply that the most relevant effects of MICU3-dependent modulation of MICU1 function in neurons may occur at higher cytosolic Ca2+ concentrations, where MICU3 would be more important for enhancing mtCU activation than in gatekeeping. The interpretation is also consistent with the observation that although MICU1 overexpression prevents the deleterious effects of overexpression of MCU + EMRE in the Drosophila eye (i.e., mCa2+ overload and cell death), overexpression of MICU3 with MCU + EMRE is not capable of preventing these effects (195). The finding that MICU3 + MCU overexpression gives a stronger deleterious eye phenotype than MICU1 + MCU overexpression likewise suggests that MICU3 is most relevant for enhancing rather than attenuating mCa2+ uptake through the mtCU within neural tissue (195). Finally, Tufi et al. (195) report that genetic disruption of MICU1 is lethal to flies and that ubiquitous overexpression of MICU3 in MICU1-mutant flies is not able to rescue this lethality. This result reinforces the views that MICU1 and MICU3 are not functionally redundant and play unique roles within the cell, and that MICU3 depends on the presence of MICU1 to exert its effects on Ca2+ uptake through the mtCU.
A recent study from the Murphy laboratory notes that the ratio of MICU3:MCU protein in the heart is about threefold higher than in the liver, suggesting that MICU3 may be particularly relevant for cardiac physiology (236). Germline deletion of Micu3 in mice produces no effect on baseline cardiac function, but prevents left ventricular dilation and the corresponding decline in cardiac contractility upon chronic isoproterenol infusion. This protection is associated with increased inhibitory phosphorylation of PDH, suggesting that loss of MICU3 prevents mCa2+ overload. Finally, loss of MICU3 preserves contractile function and reduces infarct size in hearts subjected to ex vivo I/R (236). Puente et al. (236) conclude that loss of MICU3 prevents pathological mCa2+ overload in the heart. Conversely, then, the presence of MICU3 in the heart contributes to mCa2+ overload during cellular Ca2+ stress, just as it appears to in the nervous system. Interestingly, Puente et al. note that constitutive disruption of Micu3 does not induce the same kind of compensatory adaptations in the heart as observed in earlier models of constitutive Mcu or Emre deletion (119, 164), but why this is so remains to be determined.
3.3.3.3.7. Regulation of MICU3.
The field has only recently begun to examine the function of MICU3 in mCa2+ uptake, and little is known about how MICU3 expression and protein function are regulated. Recent work in the zebrafish retina suggests that MICU3 expression is responsive to changes in MCU expression. MCU expression is low in cone photoreceptors, presumably to limit mCa2+ uptake and prevent mCa2+ overload in this highly active, excitable cell type (237). Experimental overexpression of MCU in zebrafish cone photoreceptors increases mCa2+ concentration and causes some mitochondria to swell and lose cristae (237). MICU3 transcripts are downregulated over time in retinas with MCU overexpression, perhaps as a compensatory mechanism that favors the presence of MICU1/2 heterodimers at the mtCU and thereby reduces the Ca2+ sensitivity of channel activation. This may be an adaptive response to limit pathogenic mCa2+ uptake (237). Whether this transcriptional downregulation of MICU3 is a direct response to increased mCa2+ content, other cell stress pathways secondary to mCa2+ overload, altered mitochondrial energetics, or some other mechanism is yet to be determined.
3.3.3.4. mcub.
MCUB (Mitochondrial Calcium Uniporter Dominant-Negative Beta Subunit; gene name = MCUB or CCDC109B) was identified via its sequence similarity with MCU (90). MCUB protein shares ∼50% homology with MCU and like MCU contains two transmembrane domains (90). MCUB is located in the inner mitochondrial membrane, and MCUB immunofluorescence overlaps with that of MCU (89, 90). However, MCUB exhibits a distinct expression profile from MCU that likely reflects tissue-specific regulation of mtCU function. MCUB expression is particularly high in the lung, heart, and brain, which have relatively low MCU expression (MCU:MCUB mRNA ratio of ∼3:1 in heart and lung). In contrast, MCUB expression is low in tissues with high MCU expression, such as skeletal muscle (MCU:MCUB of ∼40:1) (90). This pattern suggests a reciprocal relationship between MCUB and MCU expression and is consistent with a model in which MCU and MCUB proteins compete for incorporation into the same uniporter complexes (FIGURE 2). MCUB expression is also enriched in hematopoietic and immune cells (90), suggesting that MCUB is highly relevant in the immune system. MCUB protein is detected only in mitochondria from the placenta and not in mitochondria from any of the 13 other mouse tissues examined in the MitoCarta database (156, 238).
MCUB acts as a dominant-negative inhibitor of mtCU function. MCUB protein alone is not capable of conducting Ca2+ when inserted into lipid bilayers (90). Furthermore, coexpression of MCUB along with MCU decreases the probability of observing MCU channel activity in lipid bilayers, consistent with an inhibitory effect of MCUB on uniporter function (90) (FIGURE 5).
FIGURE 5.
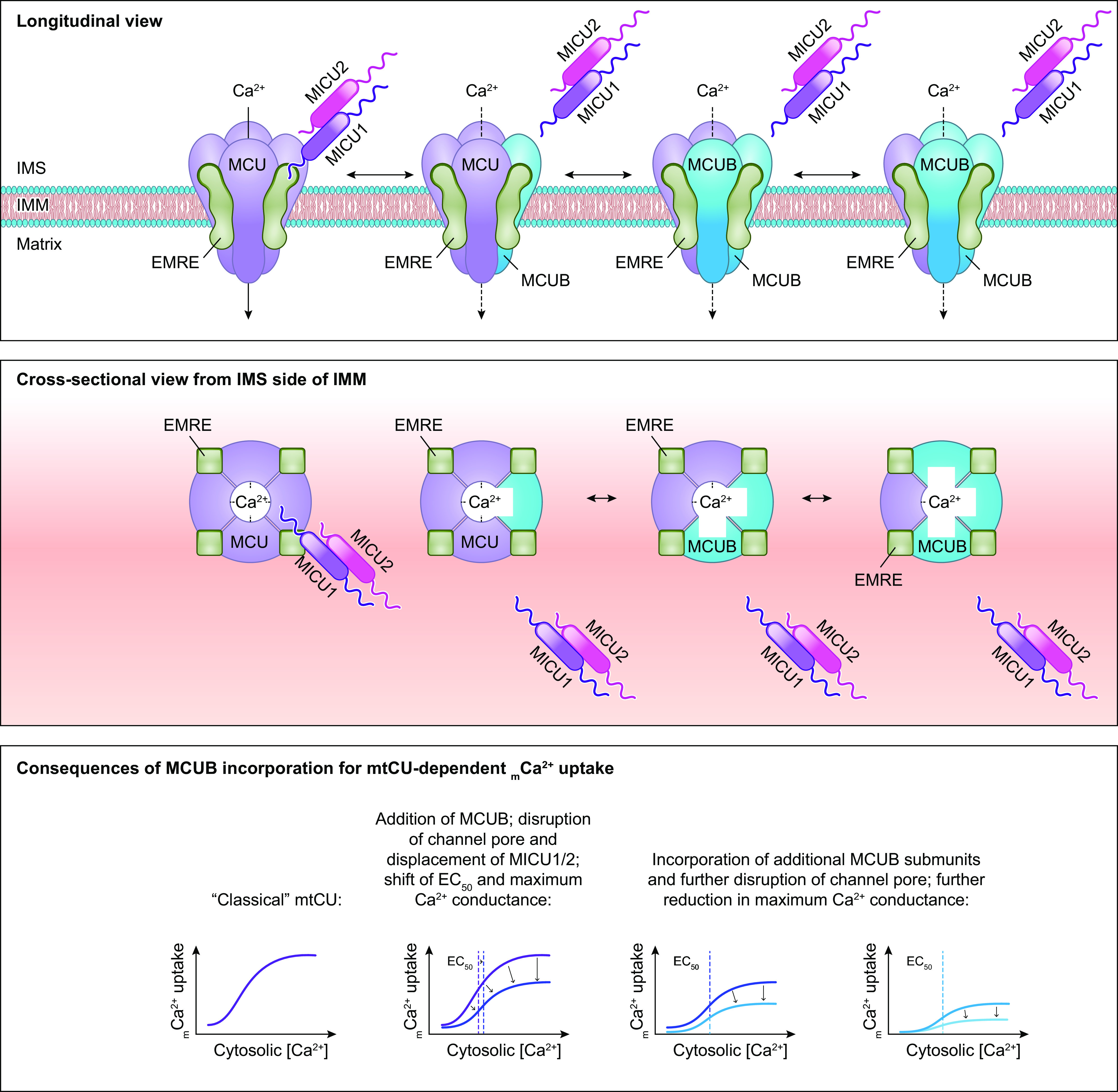
Consequences of MCUB incorporation on mitochondrial calcium uniporter channel function. Replacement of MCU with its dominant-negative paralog, MCUB, impairs Ca2+ uptake through the mtCU. At least 2 mechanisms are responsible for this phenomenon. Replacement of MCU with MCUB can disrupt binding between MICU1 and the mtCU, resulting in loss of MICU-dependent regulation of the channel. Here, cooperative activation of the mtCU is impaired, shifting the relationship between mtCU-dependent mCa2+ uptake and cytosolic/IMS Ca2+ concentration to the right. However, the consequences of the loss of MICU-dependent gatekeeping function at low cytosolic/IMS Ca2+ concentration are mitigated by MCUB’s additional effect to disrupt Ca2+ flux through the channel. As a result, mCa2+ uptake through the channel is reduced compared with the classical mtCU. Replacement of additional MCU subunits with MCUB further attenuates Ca2+ flux through the channel. Structural modeling suggests that this effect results from a widening of the channel pore in the presence of MCUB, which may disrupt Ca2+ coordination within the channel. See glossary for abbreviations.
In intact cells, MCUB overexpression suppresses histamine-induced mCa2+ uptake. In contrast, MCUB silencing increases agonist-induced mCa2+ transients (90). These studies suggest that control of MCUB expression is yet another mechanism that can regulate overall mtCU function.
3.3.3.4.1. MCUB phylogenetic conservation.
MCUB is conserved in vertebrates but is lacking in lower organisms that do contain an MCU homolog, including plants, kinetoplastids, Amoebozoa, nematodes, and arthropods (90, 157). Trypanosomatids are a peculiar example of organisms that contain not only MCUB but additional unique MCU paralogs as well. The parasites T. cruzi and T. brucei each have a total of four MCU homologs: MCU, MCUb, MCUc, and MCUd. A critical distinction between these trypanosome MCU paralogs and mammalian MCUB is that knockout of any of the four trypanosome genes (MCU and MCUb–d) diminishes mCa2+ uptake, whereas their overexpression enhances mCa2+ uptake (102–105). Functional studies further support the idea that trypanosome MCUb–d contribute to rather than inhibit mCa2+ uptake. In T. cruzi, knockout of TcMCU has little phenotypic consequence, but knockout of TcMCUb, TcMCUc, or TcMCUd reduces the parasite’s growth, respiration, and infectivity (102, 105). These findings suggest that replication of the MCU gene in trypanosomatids has allowed for functional divergence of the various MCU isoforms, such that MCUb and the other variants (MCUc and MCUd) are most important for acute mCa2+ uptake, whereas trypanosome MCU is relatively less important for mCa2+ uptake than in higher species. T. brucei likewise contains MCUb–d, all of which promote rather than inhibit mCa2+ uptake (104). T. brucei MCUb, MCUc, and MCUd all interact with MCU, and each of these four paralogs can immunoprecipitate all of the other three paralogs as well (104). Thus, T. brucei MCU, MCUb, MCUc, and MCUd can all exist within a given mtCU complex and are capable of interchanging with each other to form a functional complex.
Recent work provides insight into why mammalian MCUB functions as a dominant-negative inhibitor of the mtCU but trypanosomatid MCUB–D do not. All four T. cruzi MCU isoforms share the DIME (WDXXEPXTY) motif’s critical glutamic acid (E226 in TcMCU, corresponding to E264 in human MCU and E249 in human MCUB) (102). All four T. cruzi MCU isoforms also have at least one additional negatively charged residue between the linker domain and the DIME motif (102), which may provide sufficient negative charge near the pore of the uniporter to allow Ca2+ permeation. The region surrounding the DIME motif is largely conserved between human MCUB and T. cruzi MCUb, suggesting that differences in the ability of these two proteins to conduct Ca2+ may arise from structural difference elsewhere in the primary sequence of the proteins or possibly from species-specific differences in the interaction of MCUB with other mtCU components such as EMRE, which is not conserved in trypanosomes (89, 95).
3.3.3.4.2. MCUB structure.
Human MCUB protein is 336 residues long and contains two transmembrane domains that are connected by a short linker (90). MCUB has several critical amino acid substitutions in the pore region compared with MCU (90). A conserved arginine residue (R251 in mouse, R252 in human) in MCU’s first transmembrane domain is replaced by tryptophan in MCUB (W246 in mouse, W237 in human). Additionally, a conserved glutamic acid (E256 in mouse, E257 in human MCU) in MCU is replaced by a valine in MCUB (V251 in mouse, V242 in human MCUB), resulting in loss of a negative charge from the loop connecting the two transmembrane domains. As a result, the surface of MCUB is less negatively charged than the surface of MCU, which may interfere with Ca2+ permeation of the channel (90). Combined mutation of MCU R251W and E256V to make the protein resemble MCUB reduces histamine-induced mCa2+ uptake, suggesting that these residues contribute to the differences in Ca2+ conduction between MCU and MCUB (90).
Computational modeling of predicted human MCU and MCUB protein reveals overall structural similarity between both monomeric and tetrameric MCU and MCUB, with the greatest structural divergence occurring at their NH2 and COOH termini (239). In addition to the differences in the pore region noted above (90), several amino acid differences in the transmembrane domains of MCU and MCUB are predicted to affect their function. The first transmembrane domain of MCU contains several hydrophobic residues (L240, A244, F247, A251, and W255) that interact with other hydrophobic and neutral amino acids (F269, Y272, M276, and Y279) in the second transmembrane domain of the neighboring MCU subunit. Two of these residues, Y272 and Y279, are replaced by phenylalanines F257 and F264 in MCUB. What’s more, the hydrophobic alanine and phenylalanine residues present in MCU’s first transmembrane domain (A244 and F247) are replaced by neutral serine and glycine residues in MCUB (S229 and G332). In silico modeling predicts that these amino acid substitutions in MCUB together widen the opening of the uniporter pore (239), which may be sufficient to disrupt Ca2+ coordination and Ca2+ permeation through the channel (FIGURE 5). The current model therefore suggests that the primary mechanism by which MCUB inhibits uniporter function is by disrupting the three-dimensional structure and function of the channel pore (90). However, effects of MCUB to disrupt the negatively charged surface of the uniporter raise the possibility that MCUB also interferes with MICU1 binding to the pore, and therefore limits cooperative activation of the uniporter as well.
3.3.3.4.3. Properties and function of MCUB.
The current consensus is that MCUB is not capable of forming a Ca2+-permeable channel on its own (90, 240). Coexpression of MCU and MCUB at a 1-to-3 ratio, corresponding to tetrameric channels consisting of a single MCU subunit and three MCUB subunits, produces channels that are capable of conducting Ca2+. However, the incorporation of MCUB into the uniporter reduces the probability of recording channel activity from 89% in channels composed of MCU alone to 13% in channels composed of 1 MCU:3 MCUB (240). These data are consistent with MCUB exerting a net inhibitory effect on uniporter Ca2+ conductance. It is also interesting to note that MCUB alone does form a channel capable of conducting sodium (90). This observation suggests that the major functional difference between MCU and MCUB is in their ion selectivity, with both proteins capable of forming functional ion channels but only MCU being sufficient to transport Ca2+. A relevant question is whether EMRE may be required for MCUB to function as a Ca2+ channel, given that EMRE is required for the function of metazoan MCU (89, 157–159, 161, 162). Furthermore, does “functional” MCUB, when coupled to the appropriate binding partners (such as EMRE), conduct Ca2+ at all, albeit perhaps with a lower conductance than channels composed of MCU? This seems a distinct possibility, given that the addition of EMRE confers on MCU the ability to conduct Ca2+, whereas MCU alone is incapable of Ca2+ transport but, like MCUB, can conduct Na+ in the absence of EMRE (90, 180). One important caveat, though, is that these conclusions regarding the competence of MCUB and MCU for ion transport in the absence of EMRE (90, 180) were based on experiments conducted in lipid bilayers with reconstituted channels, which did not include all the relevant mtCU components and therefore may not fully or accurately recapitulate the in situ behavior of MCU or MCUB, as would be observed by using patch clamp of mitoplasts.
Intriguing data from genetic mouse models suggest a downregulation of EMRE protein upon MCUB overexpression (241). Thus, displacement of EMRE from the mtCU may be one way in which MCUB disrupts uniporter function. Alternatively, downregulation of EMRE with increased MCUB expression may indicate that EMRE is not required for the fundamental function(s) of MCUB. Using a variety of methodologies including reductionist systems to answer fundamental questions such as these will clarify the molecular mechanism(s) by which increased mitochondrial MCUB content diminishes acute mCa2+ uptake. It will also help to determine whether MCUB may function as an alternative, MCU-independent mCa2+ uptake pathway.
Recent research supports the view that, in cells that also express MCU, the net effect of MCUB is to disrupt mtCU-dependent Ca2+ transport. Deletion of MCUB in HeLa cells increases the amplitude of mCa2+ transients while decreasing the amplitude of cytosolic Ca2+ transients, suggesting that when MCUB is expressed it limits the ability of mitochondria to buffer changes in cytosolic Ca2+ (242). Loss of MCUB from HeLa cells shifts the relationship of mCa2+ uptake rate versus bath Ca2+ concentration to the left, with MCUB-deleted cells exhibiting an enhanced rate of mCa2+ uptake at both high and low bath Ca2+ concentrations (242). This effect is particularly strong at bath Ca2+ concentrations above 3 µM and in some ways resembles the effects of MICU1 deletion to disrupt gatekeeping and cooperative activation of the uniporter (169). MCUB deletion likewise increases net mtCU current density in HeLa mitoplasts, consistent with an overall increase in uniporter function in the absence of MCUB (242). These findings led Lambert et al. (242) to conclude that loss of MCUB enhances net mtCU function and/or increases cooperative activation and Ca2+ uptake rate of the uniporter, especially in conditions of high cytosolic/IMS Ca2+. The physiological function of MCUB, then, may be to limit mCa2+ uptake during periods of cytosolic Ca2+ stress. This hypothesis is supported by experiments performed in genetic animal models of MCUB deletion and overexpression.
The relative ratio of MCU:MCUB tracks closely with the net mtCU activity observed across different tissues. For example, skeletal muscle has an exceptionally high mtCU current density. Skeletal muscle also has a very high ratio of MCU:MCUB, with 4 times higher MCU expression and 3 times lower MCUB expression compared with the heart (90). The relative MCUB content within a tissue may therefore modify the mitochondria’s ability to take up Ca2+ in response to changes in cytosolic/IMS Ca2+ and so serve as a control point to regulate the mitochondria’s sensitivity to signals that are transduced by an increase in cytosolic Ca2+ concentration (90). This mode of regulation is analogous to the proposed mechanism in which the relative ratio of MICU1:MCU controls net mtCU activity in a tissue-specific manner (187).
3.3.3.4.4. Interaction of MCUB with the metazoan mtCU complex.
MCUB incorporates into the endogenous mtCU complex of HEK293T and HeLa cells and coimmunoprecipitates with MCU (89, 90). Huang et al. (104) propose that the interactions between MCU and MCUB are mediated by their transmembrane domains, just like the interactions between MCU subunits. MCUB is also capable of self-oligomerizing into complexes of ∼170 kDa in cells in which MCU is silenced, demonstrating that MCUB does not need MCU to form a tetrameric channel (90). The population of mtCUs present within a cell may therefore be a heterogeneous mix of mtCUs composed of MCU-MCUB heterotetrameric complexes, MCU homotetrameric complexes, and MCUB homotetrameric complexes (90). A shift in the relative distribution of these different types of uniporter channels is yet another way that a tissue may modulate its mCa2+ handling.
Differences in the residues of the pore regions of MCU and MCUB and in their interactions with other mtCU subunits give clues as to how MCUB can inhibit Ca2+ uptake through the mtCU. Like MCU, MCUB binds to EMRE (242). However, EMRE and MCUB subunits appear to compete for inclusion in the mtCU, because MCUB deletion from HeLa cells or the mouse heart increases EMRE expression, and MCUB overexpression in cardiomyocytes decreases EMRE expression (241, 242). Since MCU depends on EMRE to conduct Ca2+, it is possible that MCUB’s specific displacement of EMRE from the mtCU is a key mechanism that disrupts the function of the remaining MCU subunits.
Overexpression of MCUB in the mouse heart also reduces the total molecular weight of the mtCU and reduces the total amount of MICU1 and MICU2 present in high-molecular mass (∼500–800 kDa) uniporter complexes (242). Immunoprecipitation experiments show that MCUB does not interact with MICU1 or MICU2 (242). These results indicate that when MCUB replaces MCU within a mtCU channel, it causes displacement of MICU1/2 from the channel as well because MCUB is not able to bind to the MICU dimer (FIGURE 5). Fitting with this view, deletion of MCUB from HeLa cells (allowing for greater MCU + MICU1/2 incorporation into the complex) increases MCU, EMRE, MICU1, and MICU2 protein expression and increases the overall molecular weight of the mtCU (242). At least two mechanisms may account for the difference in MCU’s and MCUB’s ability to bind the MICU1/2 dimer. First, the replacement of E257 (residue numbers in this paragraph refer to the human proteins) in MCU’s loop region with V242 in MCUB renders the IMS-facing surface of MCUB less negatively charged than the corresponding surface of MCU (90). Besides a possible direct effect to disrupt Ca2+ permeation through the channel, the loss of negative charge from the MCUB surface may also make MCUB less capable of binding MICU1 compared with MCU. A direct MCU-MICU1 interaction occurs between positively charged arginine residues (R440 and R442) on MICU1 and the negatively charged DIME motif residues D261 and E264 of MCU, and prevents Ca2+ permeation of the channel when cytosolic Ca2+ concentration is low (192). Given its proximity to the DIME motif, it is possible that the replacement of negatively charged E257 in MCU with neutral V242 in MCUB also affects the electrostatic interaction between MICU1 and the region around the channel pore. Second, EMRE helps to anchor MICU1 to the mtCU (158). MCUB’s displacement of EMRE, from the mtCU could therefore have an additional, indirect effect to dislodge the MICU1/2 dimer from the channel.
The overall consequence of increased MCUB incorporation on mtCU function, then, is to inhibit the channel by disrupting Ca2+ permeation directly and/or via displacement of EMRE, and by disrupting the channel gatekeeping and cooperative activation functions of the MICUs. Increased MCUB expression would result in a greater proportion of the mtCU population existing in an ungated, but still inhibited, state, resulting in less net mCa2+ uptake over a range of cytosolic Ca2+ concentrations. Conversely, decreasing cellular MCUB content would allow more mtCUs to be gated by MICU dimers. The net effect here would be to increase the cooperative activation of the channel, particularly at higher Ca2+ concentrations. Basal mCa2+ uptake at low (<3 µM) cytosolic Ca2+ does not appear to be affected as strongly by loss of MCUB (242). This phenomenon can be explained by the increased expression of MICU1/2 in observed MCUB−/− cells (242). The addition of the MICU1/2 gatekeeping activity at each uniporter would functionally compensate for loss of MCUB to keep the channel inhibited at low Ca2+ concentrations. The relative ratio of MCUB:MCU in a tissue therefore may exert more stringent control over its net mtCU activity than the MICU1-to-MCU ratio, because MCUB content would affect both the channel pore and, via secondary displacement of MICU1/2, its gating properties.
3.3.3.4.5. Genetic diseases associated with MCUB.
No primary mutations in MCUB have yet been reported to cause human disease. However, changes in MCUB expression are noted as secondary features of a number of human pathologies including diabetes (243), cancer (244), atopic dermatitis (245), and muscular dystrophy (246, 247).
3.3.3.4.6. Animal models of MCUB.
Evidence from genetic mouse models implicates MCUB in tissues’ responses to pathological stress (TABLE 2). Our laboratory and others note that MCUB is not normally expressed in the mouse heart but is upregulated at both the mRNA and protein levels and incorporated into the mtCU after myocardial infarction or myocardial ischemia-reperfusion injury (241, 242). MCUB expression thus appears to be induced by pathological signals such as hypoxia. The first genetic mouse model of MCUB employed a flox-stop MCUB transgene crossed to cardiomyocyte-specific, tamoxifen-inducible αMHC-MCM to enable inducible overexpression of MCUB in the adult mouse heart (242). Acute ∼5-day cardiomyocyte MCUB overexpression largely replicates the phenotype of cardiac MCU deletion (119, 122, 126, 127) in that it diminishes the heart’s contractile responses to acute β-adrenergic stimulation (242). Acute MCUB overexpression also reduces the maximum oxygen consumption rate and reserve capacity of adult cardiomyocytes, although it does not affect basal cardiomyocyte respiration in vitro (242). Consistent with reduced mCa2+ content, acute MCUB overexpression is associated with greater inhibitory phosphorylation of pyruvate dehydrogenase both at rest and after acute isoproterenol stimulation (242). When mice are stressed with 40 min of cardiac ischemia + 24 h of reperfusion, animals with acute MCUB overexpression have substantially increased mortality, with 12 of 13 MCUB overexpressor mice dying several minutes after the onset of ischemia. Lambert et al. (242) hypothesize that this increase in mortality is related to an inability of the MCUB overexpressor heart to quickly adapt to the energetic stress of the ischemia. Another prominent distinction between earlier models of cardiomyocyte MCU deletion and short-term cardiomyocyte MCUB overexpression is that acute MCUB overexpression causes substantial baseline contractile dysfunction (242).
In contrast, after ∼1 mo of cardiac MCUB overexpression, the predominant effects of increased cardiomyocyte MCUB content are protective. Acute mCa2+ uptake is still inhibited in MCUB overexpressor cardiomyocytes, but there is no longer any detrimental effect of MCUB overexpression on baseline heart function or on survival during cardiac I/R (242). These improvements are associated with a normalization of PDH phosphorylation and cellular bioenergetics. Such gradual metabolic compensation also normalizes the MCUB overexpressor heart’s contractile responses to acute isoproterenol stimulation (242). These findings suggest that the metabolic deficits that impair β-adrenergic responsiveness in mice with 1-wk MCUB overexpression and that predispose them to death during I/R are largely overcome via undefined compensatory mechanisms that take effect over the course of several weeks. Since acute mCa2+ uptake is still inhibited after 1 mo of MCUB overexpression, the compensatory response likely involves a shift toward mCa2+-independent regulation of cellular energy production (242). Fitting with maintained inhibition of acute mCa2+ uptake, 1-mo MCUB overexpression before I/R reduces myocardial infarct size. This indicates that MCUB overexpression is sufficient to protect the cardiomyocytes against mCa2+ overload and necrosis when challenged with cellular Ca2+ stress during I/R (242).
Many of the observations in mice with chronic 1-mo MCUB overexpression were recently recapitulated by Huo et al. (241) using mice in which MCUB is overexpressed in the cardiomyocytes because of expression of an α-myosin heavy chain-driven tetracycline-transactivator transgene and a mouse MCUB cDNA under the control of tetracycline operator sequences. Here, chronic MCUB overexpression within the cardiomyocytes has no detrimental effect on baseline cardiac function but protects the heart from I/R injury and mPTP opening (241). These results agree that with sufficient time for metabolic compensation, the beneficial effects of MCUB overexpression to limit deleterious mCa2+ uptake under stress outweigh any detrimental effects. Huo et al. (241) also generated a knockout first mouse line resulting in constitutive disruption of the Mcub gene (Mcub−/−). Cardiomyocytes from MCUB−/− mice do not exhibit any alteration in basal mCa2+ uptake, oxygen consumption, or cardiac function, as expected from the observation that the unstressed heart expresses very little MCUB (241). However, constitutive MCUB deletion exacerbates myocardial ischemic injury, subsequent cardiac remodeling, and contractile dysfunction (241). These detrimental effects of MCUB deletion are associated with increased mCa2+ uptake at 7 days post-I/R, consistent with loss of the MCUB upregulation that normally occurs as an adaptive response to ischemia (241). Huo et al. (241) additionally report that hindlimb remote ischemic preconditioning is sufficient to induce MCUB expression and diminish mCa2+ uptake in the mouse heart. This suggests the existence of regulatory mechanisms that communicate the presence of stress in one part of the body in order to modulate mCa2+ handing in other tissues and protect them from subsequent Ca2+ stress. Upregulation of MCUB appears to be just one component of this protective response, since remote ischemic preconditioning is still capable of eliciting a partial reduction in acute mCa2+ uptake in Mcub−/− cardiac mitochondria (241).
Considered together, these mouse models suggest that MCUB has a beneficial role in protecting tissues from or adapting them to pathologically high cytosolic Ca2+ levels that would otherwise cause mCa2+ overload, permeability transition, and cell death. They also indicate that increased MCUB expression, and potentially any other alteration in that inhibits mtCU function, is sufficient to perturb mitochondrial energetics and cardiac contractility. Given sufficient time, though, the heart can adapt and rewire its metabolic regulation such that impaired mCa2+ uptake no longer disrupts bioenergetics or cardiac pump function. The reasons for the discrepancies between findings in mice with constitutive MCU deletion, inducible but chronic MCU deletion, and acute versus chronic inducible MCUB overexpression likely relate to the time available for the activation of alternative cell death pathways and compensatory changes to cellular metabolism. This topic has recently been reviewed in depth elsewhere (121).
3.3.3.4.7. Regulation of MCUB.
Much less is known about the regulation of MCUB than about MCU. MCUB mRNA expression is responsive to changes in cellular Ca2+ handling in lymphocytes, and in silico analysis suggests that MCUB is transcriptionally controlled by NFAT (135). These data agree with the observation that ischemia, which increases cellular Ca2+ content via a shift to glycolysis and activation of plasma membrane Na+/H+ and Na+/Ca2+ exchange, upregulates MCUB (241, 242). Despite conserving the cysteine residue that is oxidized in MCU (C97 in MCU/C82 in MCUB), MCUB is not as readily oxidized as MCU (148). MCUB therefore may differ from MCU with regard to its regulation by cellular redox signaling.
Many questions remain about what controls MCUB versus MCU incorporation into the uniporter complex. Given that MCU protein has a relatively long half-life in vivo (126, 127), how is MCUB able to quickly incorporate into the mtCU upon its induction within ∼1 day of I/R? Does the relative incorporation of MCU and MCUB into the intact mtCU align directly with their relative protein expression? Or do active mechanisms regulate which of the available subunits is selected for incorporation into and retention within a uniporter complex? Answering such questions will provide useful insight into how the relative composition of the core mtCU can be modulated as a therapy for human disease.
3.3.3.5. mcur1.
MCUR1 (Mitochondrial Calcium Uniporter Regulator 1, gene name = MCUR1, previously annotated as CCDC90A) was identified in an RNAi screen for proteins that affect mCa2+ uptake in HEK293T cells (248). MCUR1 is an integral membrane protein that localizes to the IMM. It is expressed ubiquitously and has particularly high expression in skeletal muscle, kidney, and the large intestine (248). MCUR1 is not sufficient to conduct Ca2+ when reconstituted in yeast expression systems, indicating that it does not form a channel on its own (159). Mallilankaraman et al. (248) suggest that MCUR1 physically interacts with MCU in COS7 cells, although this notion is contested by findings from Sancak et al. (89) that MCUR1 does not copurify with MCU isolated from HEK293T cells. Nevertheless, knockdown of MCUR1 in HEK293T and HeLa cells reduces acute mCa2+ uptake (248, 249). MCUR1 overexpression enhances agonist-induced mCa2+ uptake, and this effect depends upon the expression of MCU (248, 249). These findings support the view that MCUR1 regulates uniporter function and that both MCUR1 and MCU are required for full mtCU activity (248, 249).
3.3.3.5.1. MCUR1 phylogenetic conservation.
MCUR1 exhibits a unique pattern of conservation across species. MCUR1 is conserved among vertebrates but is not found in Drosophila or C. elegans (91, 250). MCUR1 homologs are present in yeast as well as species of fungi that do not express homologs of MCU (91, 251). These data are consistent with MCUR1 having cellular functions that are independent of the mtCU. For example, the yeast MCUR1 homologs Put6 (Fmp32) and Put7 (Ylr283w) localize to the IMM and form large heterooligomeric complexes that are involved in proline metabolism (91). Thus, a generalizable feature of MCUR1 across species is that it participates in large complexes of mitochondrial proteins.
3.3.3.5.2. MCUR1 structure.
Human MCUR1 consists of 359 amino acids and has a molecular mass of ∼40 kDa. The first 60 residues form an NH2-terminal domain that is followed by two transmembrane helices and a short COOH-terminal domain. Both the NH2 and COOH termini of MCUR1 extend into the IMS, whereas the bulk of the protein extends into the mitochondrial matrix (248). The conserved three-dimensional structure of MCUR1 and related proteins is described as a head-neck-stalk-anchor, where a coiled-coil stalk is anchored in a lipid membrane via a COOH-terminal transmembrane region and a β-layer neck connects the NH2-terminal head to the other end of the stalk (251). The NH2 terminus of MCUR1 contains a mitochondrial signal peptide that is followed by a disordered segment of 160 residues (251). In vitro, MCUR1 constructs lacking the first 160 residues and the COOH-terminal anchor form trimers, suggesting that the intact MCUR1 protein can oligomerize (251). The head domain has small patches of negative charge due to residues E176 and E177 (251). Immunoprecipitation of MCUR1 deletion constructs in HEK293T cells show that MCUR1 interacts with MCU and that MCUR1’s head domain is required for MCUR1-MCU binding (251). The MCUR1 coiled-coil stalk domain is also critical for the interaction with MCU (251). The head domain of MCUR1 is capable of binding Ca2+ (251). This raises the possibility that Ca2+ regulates MCUR1 binding interactions, with potential implications for MCUR1-dependent regulation of uniporter function.
3.3.3.5.3. Properties and function of MCUR1.
The function of MCUR1 remains more controversial than that of other mtCU components. MCUR1 is necessary for full MCU activity, and knockdown of MCUR1 disrupts oxidative phosphorylation and ATP production (248). These effects are very similar to the effects of MCU knockdown and support the hypothesis that MCUR1 promotes mCa2+ uptake through MCU. Consistent with this idea, MCUR1 knockdown in HeLa cells decreases basal oxygen consumption and reduces resting mCa2+ content (248).
Conflicting data indicate that the effects of MCUR1 on mCa2+ uptake may be indirect and attributable to alteration of mitochondrial membrane potential (ΔΨm) rather than a direct influence on MCU. MCUR1 is required for appropriate assembly of cytochrome-c oxidase (COX; respiratory complex IV), with knockdown of MCUR1 in human fibroblasts causing an increase in the protein turnover of COXI and COXII (252). MCUR1 appears to facilitate the maturation of COXII or its incorporation into the cytochrome-c oxidase complex rather than residing in mature complex IV (252). This study agrees with Mallilankaraman et al. (248) that loss of MCUR1 impairs mCa2+ uptake, but claims that impaired mCa2+ uptake is a consequence of depleted membrane potential (the driving force for mCa2+ uptake) secondary to respiratory impairment (252). Likewise, Paupe et al. (252) reason that the respiratory defect observed by Mallilankaraman et al. (248) is not a secondary effect of diminished mCa2+ uptake but rather is a primary consequence of the failure to assemble COX and maintain ΔΨm in MCUR1-deficient cells. Fitting with the view that MCUR1 affects ΔΨm, which subsequently impacts mCa2+ uptake (as opposed to disrupted mCa2+ uptake compromising basal metabolism and ΔΨm), Paupe et al. (252) show that silencing of MCU alone is not sufficient to disrupt ΔΨm. On the other hand, Mallilankaraman et al. (248) report that knockdown of MCUR1 does not change ΔΨm. Mitoplast patch-clamp experiments in which membrane potential is controlled show that MCUR1 knockdown in HEK293 cells reduces mtCU Ca2+ currents, independent of changes in membrane potential (249). Thus, although MCUR1 may influence multiple protein complexes within mitochondria, it does appear to act as a direct regulator of the mtCU. MCUR1 directly promotes mtCU activity by acting as a scaffolding factor to help assemble the intact uniporter complex (FIGURE 2). MCUR1 binds to both of the core uniporter components, MCU and EMRE, and coexpression of MCU and MCUR1 increases the proportion of MCU in high-molecular weight protein fractions (253). Loss of MCUR1 decreases the higher-order oligomerization of MCU, suggesting that MCUR1 is necessary for proper formation of the intact, heterooligomeric uniporter complex (253).
A number of cellular functions are ascribed to MCUR1, although how each of these functions specifically relates to MCUR1’s effect on mtCU-dependent mCa2+ uptake versus MCUR1’s effects on other protein complexes is still under investigation. MCUR1-dependent uniporter function is required for maintenance of mitochondrial bioenergetics. Without MCUR1, cells become energy starved and upregulate alternative metabolic pathways such as autophagy in order to survive (253). MCUR1 has also been implicated in mitochondrial permeability transition. Expression of MCUR1 in Drosophila cells (which normally lack MCUR1) renders the mPTP sensitive to mCa2+ overload (250). Conversely, siRNA knockdown of MCUR1 in HeLa cells renders the mPTP less sensitive to permeability transition in response to an increase in mCa2+ content (250). Chaudhuri et al. (250) report that MCUR1 interacts with the mPTP component cyclophilin D (CypD) and hypothesize that MCUR1 brings the mPTP into proximity with MCU, so that the mPTP is exposed to a local domain of high mCa2+ concentration as Ca2+ flows through the mtCU into the mitochondria. However, these data conflict with a report that HeLa cells with stable MCUR1 overexpression take up more Ca2+ pulses into the mitochondria before permeability transition, which suggests that MCUR1 instead diminishes mCa2+-sensitivity of the mPTP (248).
Since MCUR1 binds divalent cations and has a particularly high affinity for Ca2+ (10 times higher than its affinity for Mg2+) (251), another interpretation that may reconcile the existing data is that MCUR1 also functions as a Ca2+ sensor to mediate cellular responses—including susceptibility or resistance to mitochondrial permeability transition (PT)—upon changes in mCa2+ content. Indeed, the head domain of MCUR1 is destabilized in the presence of Ca2+, and chronic exposure to elevated Ca2+ levels causes purified MCUR1 to form amyloid fibrils (251). The functional consequences of these Ca2+-dependent changes in MCUR1 have not yet been demonstrated experimentally, but Ca2+-dependent regulation of MCUR1 structure is consistent with a model in which Ca2+ binding to MCUR1 can act as a switch to regulate other proteins or cellular processes. In this model, the apparent effects of MCUR1 expression on susceptibility to Ca2+-induced mitochondrial PT would arise from a complex interaction between the separate effects of MCUR1’s ability to sense Ca2+, MCUR1’s promotion of mtCU activity, and MCUR1’s physical interaction with the mPTP modulator CypD, which may or may not be in competition with MCUR1’s interaction with the uniporter. These multiple functions of MCUR1 would allow it to contribute to both physiological and pathological responses to elevated mCa2+.
In addition to its regulatory effects on the mtCU and possible responses to changes in mCa2+, MCUR1 also has mtCU-independent functions that are specifically responsive to other intramitochondrial signals. For instance, protein expression of the yeast homologs of MCUR1, Put6 and Put7, is positively regulated by proline concentration (91). Deletion of Put6 and Put7 reveals that they are necessary for proline metabolism in yeast, serving as negative regulators of mitochondrial proline uptake, and are needed to maintain cellular redox balance (91). Further investigation into the multiple effects of MCUR1 will shed greater light on its involvement in such additional processes and how they are related to mCa2+.
3.3.3.5.4. Interaction of MCUR1 with the metazoan mtCU complex.
MCUR1 interacts with the uniporter complex via direct binding to MCU as well as EMRE (248, 253) (FIGURE 2). The interaction between MCU and MCUR1 is mediated by the MCUR1 head domain and additionally depends on the coiled-coil stalk, which may position the MCUR1 head in the appropriate position to interact with MCU (251). The NH2-terminal domain and coiled-coil domain of MCU are also critical for MCU-MCUR1 binding (253). The MCU-MCUR1 interaction occurs independent of Ca2+ concentration, although since Ca2+ destabilizes the MCUR1 head domain (251) changes in Ca2+ concentration near the uniporter may change the nature of the MCU-MCUR1 interaction. MCUR1 thus represents another potential control point at which the local Ca2+ microdomain may regulate mtCU function. MCUR1 interacts with EMRE (253) but, unlike MCU and EMRE, does not directly bind to MICU1 (248, 253). MICU1 coimmunoprecipitates MCU but not MCUR1, whereas MCU coimmunoprecipitates both MCUR1 and MICU1, and MCUR1 coimmunoprecipitates only MCU and not MICU1 (248). MCUR1 and MICU1 therefore do not appear to exist within the same uniporter complex, which suggests the presence of multiple forms of uniporter channels with distinct compositions, one containing MCU-MICU1 and another containing MCU-MCUR1 (248). A possible explanation for these data is that MCUR1 associates with the nascent core uniporter composed of MCU + EMRE to ensure appropriate assembly of the channel pore, but MCUR1 is displaced by MICU1 in the mature, fully gated uniporter (FIGURE 2).
Knockdown of MCUR1 results in specific upregulation of MCU but does not affect the expression of other mtCU components (248, 249). These data could be interpreted as MCUR1 and MCU being in competition for incorporation into mtCU channels, analogous to the competition between MCU and MCUB for inclusion in the mtCU. Alternatively, upregulation of MCU in MCUR1-knockdown cells could simply be a compensatory response to normalize the cell’s net mCa2+ uptake capacity in the face of diminished mCa2+ uptake through each MCUR1-deficient uniporter channel. MCUR1 was not included in the structural studies of the uniporter complex discussed above in section 3.3.3.1 (184, 185), so the typical stoichiometry of MCUR1 within the mtCU remains unknown.
3.3.3.5.5. Genetic diseases associated with MCUR1.
Primary mutations in MCUR1 have not yet been reported to cause human genetic disease, although MCUR1 is implicated in the pathogenesis of hepatocellular carcinoma (254).
3.3.3.5.6. Animal models of MCUR1.
C57BL/6 mice with deletion of Mcur1 from cardiomyocytes (Mcur1fl/fl × αMHC-Cre) or from endothelial cells (Mcur1fl/fl × VE-Cad-Cre) are viable and born at expected Mendelian ratios (253) (TABLE 2). However, cardiac Mcur1-knockout animals are smaller than expected and die within 3 wk of birth (253). Mcur1-null cardiomyocytes exhibit impaired mCa2+ uptake and diminished mtCU currents, but have normal basal mCa2+ content (253). In contrast, basal mCa2+ content, mCa2+ uptake, basal ATP levels, and agonist-induced increases in ATP concentration are all reduced in MCUR1-deficient endothelial cells. Proliferation and migration are attenuated in MCUR1-deficient endothelial cells, similar to observations in MCU-deficient endothelial cells (253). Mcur1 endothelial cell knockout mice have normal metabolic profiles and normal serum chemistry, but have increased heat dissipation that may be related to higher expression of uncoupling protein 2 and a greater degree of mitochondrial uncoupling within the MCUR1-deficient cells (253). Autophagy is increased in both Mcur1-KO endothelial cells and Mcur1-KO cardiomyocytes (253). This finding is consistent with a requirement for MCUR1 in normal mtCU-dependent mCa2+ uptake that stimulates mitochondrial metabolism: without MCUR1, cells upregulate autophagic pathways as an alternative fuel source in order to survive the bioenergetic stress imposed by limiting mtCU function (253).
3.3.3.5.7. Regulation of MCUR1.
MCUR1 protein is subject to several posttranslational modifications. Binding of Ca2+ to the MCUR1 head region destabilizes its three-dimensional structure (251). A large portion of the MCUR1 expressed in HEK293T cells is proteolytically cleaved, resulting in loss of the first 140 residues from the NH2 terminus of the protein (251). This cleavage removes most of the disordered region that precedes the MCUR1 head domain (251). Both full-length and cleaved MCUR are capable of binding MCU (251, 253), but it is unknown whether these forms differ in their ability to regulate uniporter function.
MCUR1 is also sensitive to metabolic stress. MCUR1 protein is downregulated in human pulmonary microvascular endothelial cells subjected to oxygen and glucose deprivation (255). MCUR1 downregulation is associated with increased autophagic flux in microvascular endothelial cells, in agreement with earlier findings by Tomar et al. (253) (255).The finding that MCUR1 is the only mtCU component significantly affected by oxygen and glucose deprivation in this cell type (255) suggests the presence of specific regulatory pathways that act through MCUR1 to impact mtCU function. However, it should be acknowledged that such regulatory pathways could preferentially affect MCUR1 protein that participates in other mitochondrial protein complexes besides the mtCU, such as respiratory complex IV. Any impact of MCUR1 downregulation on mCa2+ handling in the context of oxygen and nutrient deprivation remains to be determined. Finally, experiments in mtNOD mice with polymorphisms in the mitochondrial COX3 gene indicate that MCUR1 protein expression is also responsive to changes in the Ca2+ microdomain surrounding the mitochondria. Aged mtNOD mice exhibit a hyperfused and elongated mitochondrial network, in contrast to a more fragmented mitochondrial network in aged control animals (256). Niemann et al. (256) observe that MCUR1 gene expression is diminished in aged mtNOD hepatocytes and propose that this change relates to changes in mitochondrial structure. In normal aged tissue, the fragmented mitochondrial network requires a high mCa2+ uptake capacity (corresponding to higher MCUR1 expression), because some mitochondria will be located far from ER Ca2+ release sites and will not be exposed to as high of a cytosolic/IMS Ca2+ concentration as those mitochondria located closer to the ER (256). These fragmented mitochondria would require robust pathways for mCa2+ uptake to ensure that they still import sufficient Ca2+ to support mitochondrial metabolism. However, this problem is not encountered in aged mtNOD cells, as the existence of hyperfused mitochondria means that each mitochondrion is more likely to have a portion of its structure in proximity to ER Ca2+ release sites. Each mitochondrion will consequently be exposed to a locally high Ca2+ microdomain upon ER Ca2+ release, which can trigger sufficient mCa2+ uptake for cellular needs, independent of positive mtCU regulation by MCUR1 (256). mtNOD cells would thus be able to effectively couple changes in cytosolic Ca2+ concentration or ER Ca2+ release to mitochondrial responses, while requiring a lower level of MCUR1 expression than that needed in wild-type cells.
3.4. Structure and Stoichiometry of the Proteins Within the mtCU
Several features of the regulation of mCa2+ uptake emerge only from consideration of the intact uniporter complex and are not readily apparent from studies of its individual protein constituents. The net mass of native, intact protein complexes that contain MCU varies from ∼450 kDa up to ∼800 kDa (99, 242, 253). The lower end of this range aligns closely with the 4 MCU:4 EMRE:1 MICU1:1 MICU2 channel stoichiometry proposed by Fan et al. (184) and is consistent with the incorporation of additional components such as MCUR1 into the complex (FIGURE 2). The higher end of the range is consistent with the formation of dimers of mtCU uniplex channels as observed in structural studies of the uniporter (184, 186). That the higher end of the range of reported mtCU weights is not double the molecular mass of the individual uniporter (∼450 kDa) perhaps reflects the loss of MICU1/2 from some dimerized channels (184) or the displacement of accessory subunits as the uniporter forms mature dimers. Unfortunately, cryo-EM studies of uniporter structure did not include accessory subunits like MCUR1 and MCUB, so the precise stoichiometry of their incorporation into mature mtCU uniplex channels and dimers remains unresolved.
How the appropriate uniporter stoichiometry is maintained requires further investigation. Several questions regarding this matter are particularly relevant to our understanding of uniporter regulation. For example, does the MICU1-to-MCU ratio at a given uniporter channel correspond directly to the relative gene expression of MICU1 and MCU, or do additional mechanisms control whether or not MICU1 will be selected for inclusion in a complex composed of MCU + EMRE? Are there additional physiological mechanisms besides replacement of MCU with MCUB that dictate whether or not the uniporter channel binds to and is gated by the MICU proteins?
What is clear is that alteration of the normal mtCU stoichiometry is a fundamental mechanism by which cells modulate mCa2+ uptake. Transcriptional control of relative MCU, MICU1, and MICU2/3 content allows for tissue specificity of mCa2+ handling and the sensitivity of the mitochondria to changes in cytosolic Ca2+ concentration (187). Uniporter current density varies widely across tissues and tends to be low in highly metabolically active tissues like the heart, possibly to prevent excess mitochondrial buffering of cytosolic Ca2+ signals or to prevent mCa2+ overload in the face of repeated bouts of high cytosolic Ca2+ (117). Temporal changes in mtCU composition within a given tissue can also mediate changes in mCa2+ handling over time as part of normal biological processes. For example, the placenta and myometrium exhibit distinct changes in mtCU component expression late in pregnancy. MCU, MICU1, MICU2, and EMRE mRNA and MCU and MICU1 protein expression increase in the placenta throughout gestation, along with an increase in the MICU1-to-MCU protein ratio (257). MICU2 protein expression in the placenta then decreases in the later stages of pregnancy. This response is consistent with a greater membrane depolarization observed in response to bolus Ca2+ addition (257), suggestive of increased mCa2+ uptake. At the same time, protein expression of MCU and MCUB increases while MICU1 protein expression decreases in the myometrium through the late stages of pregnancy (257). How these specific changes may be important for uterine or placental function is still under investigation, but they highlight that coordinated changes in the expression of uniporter components occur in response to physiological stimuli.
Changes in uniporter component expression also allow the mitochondrion to modulate its Ca2+ uptake in order to withstand pathological cellular stress, as is seen with upregulation of MCUB in the heart in response to I/R injury (242). The plasticity of uniporter composition is also evident from studies in which experimental manipulation of a given subunit results in a reflex up- or downregulation of another homologous subunit to maintain overall uniporter structure. For example, knockout of MCUB increases MCU and EMRE protein expression (242), and MCUB overexpression downregulates EMRE (241). Likewise, overexpression of a uniporter subunit can increase the expression of its binding partners: MCU overexpression increases the expression of MICU1 and 2, and MICU1 overexpression results in increased MICU2 expression (93). These findings reflect the existence of homeostatic control mechanisms that maintain tissue-appropriate uniporter function and may minimize the abundance of inappropriately regulated channels.
Since certain subunits like MICU1 and MICU2/3 depend on the presence of others (i.e., MCU rather than MCUB) for uniporter complex binding and regulation, changes in the composition of the core uniporter channel (MCU vs. MCUB) also have secondary effects on the gating and regulation of each uniplex mtCU. Additionally, since back-to-back MICU2 dimers impact the function of dimerized mtCUs (232), changes in the relative MCU versus MCUB content and subsequent binding versus displacement of MICU1/2 from each individual uniplex channel may have further effects on the specific behavior of dimerized channels. For example, incorporation of increasing amounts of MCUB within the each uniplex channel would limit the amount of bound MICU2 available to participate in the back-to-back dimers proposed to act as the inhibitory latch within a set of dimerized uniplex channels.
3.5. Regulators of the mtCU
Several IMM proteins that are not considered part of the mtCU nevertheless have direct impacts on uniporter function.
3.5.1. SLC25A23.
The mitochondrial ATP-magnesium/phosphate carrier SLC25A23 (also called SCaMC-3) is required for Ca2+-dependent accumulation of adenine nucleotides and the subsequent increase in OXPHOS in liver mitochondria (258). This activity may depend on SLC25A23’s EF-hands, which lie in the IMS and sense an increase in IMS Ca2+ (258, 259). Interestingly, the Ca2+-induced increase in SLC25A23 activity is also associated with increased mitochondrial Ca2+ retention capacity (258). Amigo et al. (258) suggest that this effect on Ca2+ retention is attributable to SLC25A23’s transport of adenine nucleotides into the mitochondria, which may facilitate the formation of Ca2+-phosphate precipitates. SLC25A23 also has direct effects to promote mCa2+ uptake. Knockdown of SLC25A23 reduces mCa2+ uptake rate and cytosolic Ca2+ clearance in response to histamine stimulation (260). Likewise, mutation of the SLC25A23 EF-hands reduces mCa2+ uptake (260). These observations suggest that SLC25A23 promotes mtCU activity in response to increased Ca2+ concentration in the cytosol or IMS. SLC25A23’s effect on mCa2+ uptake may be mediated by a direct protein-protein interaction with the uniporter complex, as SLC25A23 binds both MCU and MICU1 when overexpressed in COS7 cells (260). Consistent with a role for SLC25A23 in promoting mCa2+ uptake, knockdown of SLC25A23 reduces mitochondrial ROS production and protects against oxidant-induced cell death, both of which are phenomena related to increased mCa2+ content (260).
3.5.2. Uncoupling proteins 2 and 3.
Uncoupling proteins 2 and 3 (UCP2 and UCP3) are implicated in the regulation of mCa2+ uptake. Overexpression of UCP2 or UCP3 increases and knockdown of these proteins reduces agonist-induced mCa2+ uptake (261). This activity of UCP2 and UCP3 is in contrast to UCP1, which is the major player in nonshivering thermogenesis but has no impact on mCa2+ uptake (261). In UCP2−/− liver mitochondria, all ruthenium red-sensitive mCa2+ uptake is ablated, indicating that UCP2 is absolutely required for uniporter function in this tissue (261). Expression of UCP2 or UCP3 alone is insufficient to confer ruthenium red-sensitive mCa2+ uptake in yeast, suggesting that UCP2/3 modulate mtCU function in mammalian cells rather than conducting Ca2+ themselves (261). Trenker et al. (261) demonstrate that mutation of the second intermembrane loop of either UCP2 or UCP3 has a dominant-negative effect to limit mCa2+ uptake, and propose that wild-type UCP2 and UCP3 form homo- and heteromultimers that normally promote mtCU function. Uniporter channels containing MCU + EMRE mediate several distinct ruthenium red-sensitive Ca2+ currents that are observed in electrophysiological experiments, including the extra-large mitochondrial/mitoplast Ca2+ current (xl-MCC) and the intermediate mitochondrial/mitoplast Ca2+ current (i-MCC) (262–265). Overexpression of UCP2 increases and knockdown of UCP2 decreases the open probability of xl-MCC (265). Thus, even though UCP2 is not a core uniporter component, it stimulates the form of the uniporter responsible for conducting xl-MCC. The specific molecular composition of the distinct forms of mtCU that conduct xl-MCC and i-MCC, and how these forms may be differentially regulated by proteins besides UCP2, largely remains to be determined. However, one report suggests that UCP2/3 affect the Ca2+ sensitivity of MICU1, and thereby regulate the activity of MICU1-gated forms of the mtCU (222).
3.6. Additional Mitochondrial Ca2+ Influx Pathways Distinct From the mtCU
Apart from the mtCU, several additional channels and exchangers contribute to Ca2+ uptake across the inner mitochondrial membrane. These proteins are candidates for the pathways that allow for more gradual mCa2+ uptake compared with the mtCU and may help normalize homeostatic mCa2+ content as observed in some tissues with chronic genetic mtCU disruption (126, 127). They may also contribute to the eventual Ca2+ loading of MCU-deficient cardiac mitochondria upon prolonged dobutamine stimulation (127). Thus, these proteins may participate in pathological mCa2+ loading, particularly in chronic stress or disease states. They therefore represent attractive, alternative targets to the mtCU for therapeutic strategies that aim to prevent mCa2+ overload in pathologies characterized by sustained elevations in cytosolic Ca2+ levels.
3.6.1. Mitochondrial ryanodine receptor.
The Sheu laboratory identified a ryanodine receptor, referred to as the “mitochondrial ryanodine receptor (mRyR),” in the IMM of cardiac mitochondria (266, 267). Cardiac mitochondria bind both the RyR ligand ryanodine and ruthenium red in a competitive manner, and their net mCa2+ uptake is sensitive to inhibition by ruthenium red, ryanodine, and the type 1 ryanodine receptor (RyR1) inhibitor dantrolene (266). Both ryanodine and dantrolene inhibit mitochondrial swelling upon exposure to high cytosolic Ca2+ concentration, suggesting that, like the mtCU, the mRyR mediates mCa2+ uptake and contributes to pathogenic mCa2+ overload (266) (FIGURE 1). mCa2+ uptake through the mRyR is driven by the same thermodynamic forces as Ca2+ flux through the mtCU: the membrane potential across the IMM (ΔΨm, approximately −180 mV on the matrix side of the IMM relative to the IMS; favoring influx of positively charged Ca2+) and the concentration gradient of free Ca2+ ions [∼100 nM at rest in the matrix (40, 268, 269), up to micromolar concentrations in nearby cytosolic microdomains/the IMS during cellular Ca2+ release events (83, 270–274)]. Subsequent genetic studies confirmed the identify of cardiac mRyR as RyR1, the isoform that is typically found in the sarcoplasmic reticulum of skeletal muscle (275). Electrophysiological characterization of purified mitochondrial membranes fused with lipid bilayers further revealed that the Ca2+-dependent activation of mRyR more closely resembles that of RyR1 than that of the cardiac type 2 ryanodine receptor, RyR2 (276).
The function of a mRyR as an alternative route of mCa2+ uptake may be important for certain cell types, particularly in excitable tissues. Since its initial characterization in the heart, mRyR activity has also been measured in neurons, and the RyR has been detected via immunofluorescence in neuronal mitochondria (277). Acute mCa2+ uptake may only be partially inhibited in MCU-null neurons (278), consistent with a role for MCU-independent mCa2+ uptake pathways such as the mRyR. Similar observations of MCU-independent, albeit gradual, mCa2+ uptake are reported in MCU-null MEFs (126). Whether the mRyR may be relevant to mCa2+ uptake in other tissues is less clear. The original studies investigating the role of MCU in mCa2+ uptake in tissue culture cell lines relied on MCU knockdown, rather than complete genetic MCU knockout, and overexpression of nonfunctional MCU mutants (98, 99, 101). These studies reported reduced but not complete loss of acute mCa2+ uptake, but the relative contribution of any residual MCU expressed in these cells, as opposed to alternative pathways such as the mRyR, to the residual mCa2+ uptake is indiscernible. Relevant questions for future study are whether the extent of MCU-independent mCa2+ uptake observed in different tissues is correlated with the relative expression and mitochondrial localization of mRyR and whether deletion of mRyR on MCU-null backgrounds is sufficient to ablate residual mCa2+ uptake in cell types such as cardiomyocytes and neurons.
To date, studies of the function and physiological effects of the putative mRyR have mostly been carried out in cardiac mitochondria or cardiomyocytes by a single laboratory. Whereas mCa2+ uptake following exposure of cardiac mitochondria to 10 µM Ca2+ is sensitive to both ryanodine and dantrolene, mCa2+ uptake is less sensitive to these inhibitors at higher (50 µM) extramitochondrial Ca2+ concentration. This result suggests that mRyR may be most relevant for mCa2+ uptake at lower cytosolic Ca2+ concentrations and less important at higher cytosolic Ca2+ concentrations, where its contribution to net mCa2+ uptake may be masked by a greater relative contribution of mCa2+ uptake through the mtCU (275). The observation that IP3 receptor-dependent Ca2+ release from the ER/SR, but not global cytosolic Ca2+ elevation under adrenergic stimulation, elicits dantrolene-sensitive, mRyR-dependent mCa2+ uptake suggests that mRyR is particularly responsive to changes in Ca2+ concentration in local microdomains adjacent to cellular Ca2+ release sites (279).
Like the mtCU, mRyR contributes to Ca2+-dependent regulation of the TCA cycle and oxidative phosphorylation. Acute mRyR inhibition with ryanodine attenuates the increase in oxygen consumption induced by incubating isolated cardiac mitochondria or whole heart lysates with 10 µM bath Ca2+, although it does not affect basal oxygen consumption (275). The view of these channels’ roles in mCa2+ uptake and metabolic regulation is more complicated, though, when mRyR versus mtCU function is studied within their native environment. Experiments comparing local IP3 receptor-dependent Ca2+ release from the ER/SR to global cytosolic Ca2+ elevation with β-adrenergic stimulation in intact cardiomyocytes reveal differences in mCa2+ uptake under these two conditions. IP3 receptor activation by endothelin 1 stimulates ER/SR Ca2+ release, mCa2+ uptake through the mRyR, and an increase in ATP production in both resting and electrically paced cardiomyocytes (279). In contrast, global cytosolic Ca2+ elevation with isoproterenol treatment stimulates mCa2+ uptake through the mtCU, and this increases ATP production only in electrically paced and not quiescent cells (279). These results may reflect particular enrichment of mRyR versus the mtCU at distinct mitochondrial subdomains (i.e., at regions closer to or farther from ER Ca2+ release sites) or additional regulatory signals that differentially modulate the mtCU and mRyR in response to Gs- versus Gq-coupled receptor signaling. They also suggest that mRyR is relatively more important than the mtCU in maintaining basal mitochondrial ATP production, whereas the mtCU contributes more to enhancing mitochondrial ATP production when cellular workload and ATP consumption are increased. A role for mRyR in maintaining basal mitochondrial ATP production in unstressed cells could help to explain why the hearts of animals with adult cardiomyocyte-specific MCU deletion do not have any baseline defects in metabolism or cardiac contractility (126, 127, 279). In this model, mRyR would help to maintain homeostatic mCa2+ uptake and mitochondrial metabolism, while the mtCU is specifically activated during periods of increased energetic demand. Mathematical modeling further suggests that the relative balance of cytosolic Ca2+ and Mg2+ concentrations may shift the balance between mtCU- and mRyR-dependent mCa2+ uptake. It also reinforces the notion that mRyR-dependent uptake is favored under basal, physiological conditions (280).
Heart homogenates from neonatal RyR1-deficient mice exhibit elevated basal oxygen consumption, but their oxygen consumption is not increased further in response to an elevation in extramitochondrial Ca2+ (275) (TABLE 2). Overexpression of mRyR (RyR1) but not RyR2 or MCU in cardiac H9c2 myoblasts yields the opposite effect, enhancing mCa2+ transients and augmenting mitochondrial ATP production triggered by an increase in cytosolic Ca2+ concentration (281). These observations suggest that mRyR has some physiological redundancy with the mtCU and, like the uniporter, contributes to Ca2+-dependent parallel activation of oxidative metabolism and myofilament cross-bridge cycling. They also highlight mRyR-dependent mCa2+ uptake as a mechanism that may partially compensate for loss of uniporter function in order to maintain basal metabolism and mCa2+ content in animal models with mtCU disruption, as well as to gradually increase mitochondrial Ca2+ concentration and oxygen consumption in response to prolonged elevations in cytosolic Ca2+ concentration, even in the absence of MCU. It will be informative for future studies to examine how disruption of mtCU function specifically affects the expression and activity of proteins such as mRyR/RyR1 that mediate alternative routes of mCa2+ entry.
Two ion exchangers of the IMM that typically function in mCa2+ efflux, NCLX and LETM1, exhibit reversible activity and so can mediate mCa2+ uptake under appropriate cellular conditions.
3.6.2. NCLX in mCa2+ uptake.
NCLX is the mitochondrial Na+/Ca2+ exchanger that is primarily responsible for Na+-linked mCa2+ efflux (282). This forward-mode NCLX activity is discussed below in sect. 3.7. Mitochondrial Na+/Ca2+ exchange can occur in the reverse direction under nonrespiring conditions or perhaps pathological states where mitochondrial membrane potential is depolarized (283), leading to mCa2+ uptake. Here, if cytosolic Ca2+ concentration becomes elevated relative to free matrix Ca2+ [∼100 nM at rest (40, 268, 269)], reverse-mode NCLX activity can allow the influx of 1 Ca2+ ion across the IMM in exchange for the efflux of 3 Na+ ions. Note that the resting concentration gradient for Na+ is relatively small [∼8 mM in cytosol and ∼6 mM in the mitochondrial matrix (21)] and so may offer minimal opposition to reverse-mode flux through NCLX compared with the driving force for this flux that could be provided by a large cytosolic-mitochondria Ca2+ gradient. Such reverse-mode flux through NCLX is electrogenic, resulting in the net loss of 1 positive charge from the matrix. Thus, under normal conditions where ΔΨm is maintained at approximately −180 mV, this potential tends to oppose reverse-mode NCLX activity.
Fitting with this model, when rat cardiomyocytes are subjected to metabolic inhibition mimicking hypoxia, reverse-mode Na+/Ca2+ exchange mediates influx of Ca2+ into the matrix (284). Reverse-mode mitochondrial Na+/Ca2+ exchange also occurs in metabolically inhibited kidney epithelial cells (285). The NCLX inhibitor CGP-37157 attenuates this reverse Na+/Ca2+ exchange, supporting the notion that it occurs via NCLX (286) Although forward-mode NCLX exchange would be favored under physiological conditions where ΔΨm is intact, reverse-mode NCLX exchange may have pathological consequences, such as contributing to mCa2+ overload that occurs in conjunction with impaired mitochondrial metabolism during hypoxia and ischemia (287). However, a limitation of these studies of reverse-mode mitochondrial Na+/Ca2+ exchange is that they typically rely on the direct manipulation of ion gradients or the use of pharmacological inhibitors such as CGP-37157, which is not strictly specific to NCLX. Further validation of the purported role of NCLX in reverse-mode mitochondrial Na+/Ca2+ exchange via approaches that genetically target NCLX is therefore needed. Indeed, knockdown of NCLX is associated with a slight increase in mCa2+ uptake rather than any appreciable decrease, arguing against NCLX operating in reverse mode (166).
3.6.3. LETM1 in mCa2+ uptake.
LETM1 is a reversible IMM Ca2+/H+ antiporter (288). LETM1’s function in mCa2+ efflux is discussed in detail below; here we focus on its role in mCa2+ uptake. LETM1 mediates mCa2+ uptake and mitochondrial H+ extrusion at cytosolic Ca2+ concentrations < 1 µM, where mtCU-dependent Ca2+ uptake is minimal (288, 289). At cytosolic Ca2+ concentrations > 1 µM, LETM1-independent mCa2+ uptake predominates and is not coupled to mitochondrial H+ extrusion, consistent with mCa2+ uptake through the mtCU (288). LETM1 has a specific role in mCa2+ uptake following influx of Ca2+ across the plasma membrane rather than in response to Ca2+ release from intracellular stores (289, 290). This contrast with mtCU-mediated mCa2+ uptake reflects LETM1’s high affinity for Ca2+, which makes mCa2+ uptake through LETM1 less reliant on high-Ca2+ microdomains than mCa2+ uptake through the mtCU (287). The existence of high- and low-affinity modes of mCa2+ uptake enables mitochondria to differentiate slow, sustained increases in cytosolic Ca2+, which trigger mCa2+ uptake through high-affinity pathways like LETM1 and mRyR/RyR1, from high-amplitude cytosolic Ca2+ oscillations, which trigger mCa2+ uptake through lower-affinity pathways such as the mtCU (287).
Jiang et al. (288) propose an electrogenic 1 Ca2+:1 H+ stoichiometry for Ca2+/H+ exchange through LETM1. Consistent with this model, LETM1-dependent mCa2+ uptake is driven by ΔΨm and favored by the Ca2+ concentration gradient [∼100 nM free resting matrix Ca2+, up to micromolar cytosolic Ca2+ (40, 83, 268–274)], but it is limited by the proton gradient [matrix pH ∼7.7–8.2; cytosolic/IMS pH ∼7-7.2 (21, 291–294)] across the IMM (288). The highly negative ΔΨm (approximately −180 mV) that exists under physiological conditions favors mCa2+ uptake, although, assuming electrogenic uptake of Ca2+ through LETM1, equilibrium would be reached once matrix Ca2+ concentration reaches ∼10 µM (287). As a result, mCa2+ uptake through an electrogenic LETM1 should not be able to cause pathological mCa2+ overload (287). This suggests that whereas the mtCU contributes to both physiological mCa2+ uptake and pathological mCa2+ overload, LETM1 is most relevant in physiological mCa2+ uptake.
3.7. Mitochondrial Ca2+ Efflux Pathways
Evidence for regulated, physiological efflux of Ca2+ from the mitochondria dates back to the 1970s and 1980s. Carafoli et al. (7) first reported that Na+ addition stimulates the release of Ca2+ from isolated heart mitochondria. Lithium also stimulates some mCa2+ efflux, although other cations such as potassium, rubidium, cesium, and magnesium do not (7). Na+-induced mCa2+ efflux persists in the presence of ruthenium red, so it does not occur through the mtCU and is not coupled to transport of H+ across the IMM (8). Crompton et al. (8) propose a mechanism for mCa2+ cycling in which Ca2+ enters the matrix through a ruthenium red-sensitive influx pathway (the mtCU) and then is recycled via a Na+-dependent efflux pathway. Na+-coupled mCa2+ efflux does not strictly require the mitochondria to be energized, although it occurs at a greater rate in energized mitochondria. Crompton et al. (8) therefore postulated the existence of a mitochondrial sodium/lithium-calcium antiporter and suggested that it operates with an electrogenic 3 Na+:1 Ca2+ stoichiometry. Bernardi and Azzone and colleagues (295–297) further suggested the presence of an electrogenic mitochondrial H+/Ca2+ exchanger that drives mCa2+ efflux and is regulated by ΔΨm. Together, these mechanisms of regulated mCa2+ efflux reflect the activities of at least two IMM cation exchangers: the Na+,Li+/Ca2+ exchanger, NCLX, and the H+/Ca2+ exchanger, LETM1 (FIGURE 1).
3.7.1. NCLX in mCa2+ efflux.
NCLX (mitochondrial Na+/Ca2+,Li+ exchanger; gene name = SLC8B1, previously annotated as NCKX6 or NCLX) was first isolated as a 110-kDa mitochondrial protein that catalyzes Na+/Ca2+ exchange when reconstituted into proteoliposomes (298). Palty et al. (299) later identified the human gene encoding this particular exchanger and showed that the protein it encodes mediates K+-independent Na+/Ca2+ exchange. NCLX is unique in that it is the only member of the Na+/Ca2+ exchanger (NCX) family that also mediates Li+/Ca2+ exchange (299). NCLX was later detected specifically within the cristae of mitochondria (282). Its identity as the principal mitochondrial Na+/Ca2+ exchanger was confirmed by findings that NCLX overexpression enhances mitochondrial Na+-dependent Ca2+ efflux and that NCLX knockdown diminishes this activity (282). Moreover, Ca2+ transport through NCLX is activated by Li+ and is inhibited by the benzothiazepine CGP-37157, an accepted inhibitor of mitochondrial Na+/Ca2+ exchange (282). These results support NCLX as the primary mitochondrial Na+/Ca2+ exchanger. Fitting with such a fundamental function, NCLX is widely expressed across tissues, with particularly high expression in the pancreas, skeletal muscle, and stomach (298–300). In cardiomyocytes, the rate of mCa2+ efflux is much slower than the rate of mCa2+ uptake through the mtCU. mCa2+ efflux is faster and roughly equal to mCa2+ uptake rate in skeletal muscle, which has greater NCLX expression than cardiomyocytes. NCLX thus represents the rate-limiting component of mCa2+ exchange (282), meaning that changes in NCLX expression and activity exert a major influence over mCa2+ homeostasis.
3.7.1.1. nclx phylogenetic conservation.
Human and mouse NCLX share 83% amino acid identity (300). NCLX diverged early in the evolutionary history of mammalian sodium/calcium exchanger genes and forms its own subfamily, separate from the NCX1–3 and NCKX1–4 subfamilies (illustrated in Ref. 300). Phylogenetic analysis reveals the presence of cation/Ca2+ exchanger proteins related to NCLX in protozoa, fungi, plants, invertebrates, and mammals (301).
3.7.1.2. nclx structure.
Full-length NCLX protein is 584 residues long in humans and 585 residues long in the mouse, with a predicted mass of 64 kDa (300). NCLX contains 13 transmembrane regions, a NH2-terminal sequence variably identified as a mitochondrial targeting sequence or signal peptide, and two consensus glycosylation sites on the extracellular loop between transmembrane regions M0 and M1 (300). NCLX contains a large regulatory loop region that contains two potential PKA phosphorylation sites and lies between transmembrane regions M5 and M6 (300). Whether NCLX is oriented so that this loop lies within the matrix or within the IMS has not yet been definitively demonstrated.
The structural features of NCLX resemble the structure of plasma membrane Na+/Ca2+ exchangers and plasma membrane Na+/Ca2+,K+ exchangers (301). NCLX also shares the two α-repeat domains (designated α1 and α2) that are typical of NCX proteins. These repeats are located in the clusters of hydrophobic transmembrane domains located on either side of the loop region, the first consisting of transmembrane regions M2 and M3 and the other consisting of transmembrane regions M9 and M10 (300). These α-repeats are hypothesized to form the ion binding sites required for ion exchange (301).
NCLX is detected as ∼70-kDa and ∼55-kDa proteins upon Western blotting, and Palty et al. (299) ascribe these bands to full-length NCLX and a shorter splice variant, s-NCLX. Others suggest that the 55-kDa form of NCLX represents the full-length variant, but that its protein is subject to protranslational cleavage that reduces its molecular mass (300). NCLX can also appear as a ∼100-kDa band, which likely represents dimers of the ∼50-kDa NCLX monomers noted above (282, 299). A technical challenge facing studies of NCLX structure and function, which is augmented by the apparent diversity of NCLX proteoforms, is that the research community at large suffers from a lack of specific, genetically validated, and reliably available antibodies for NCLX. Alternative methodologies for detecting, isolating, and manipulating NCLX protein are greatly needed. Care must also be taken in assessing the reliability of any reported literature that depends solely on the performance of NCLX-directed antibodies or pharmacological targeting of NCLX.
3.7.1.3. alternative splice/transcript variants of nclx.
A ∼60-kDa mouse NCLX variant results from the skipping of exons 13 and 14, which removes 149 nucleotides, causing a frame shift. The resultant protein is 552 amino acids long and has a unique COOH terminus. This short form exhibits K+-dependent Na+/Ca2+ exchange, whereas full-length NCLX can mediate Na+/Ca2+ exchange independent of K+ (300). The 58-kDa NCLX variant identified in humans results from the exclusion of exon 7. This results in an in-frame deletion and disruption of the 3rd and 4th transmembrane regions (M3 and M4), but has no impact on NCLX ion exchanger activity (299).
3.7.1.4. properties and function of nclx.
NCLX is proposed to operate with 3 Na+:1 Ca2+ stoichiometry and like other Na+/Ca2+ exchangers mediate electrogenic ion exchange (302–305). Physiological conditions [ΔΨm approximately −180 mV, free matrix and cytosolic Ca2+ ∼100 nM, matrix Na+ ∼6 mM, and cytosolic Na+ ∼8 mM (16, 21, 37, 40, 268, 269)] will favor forward-mode operation of NCLX, with mCa2+ efflux and mitochondrial Na+ influx resulting in the net flow of 1 positive charge into the matrix. This ion exchange occurs via a ping-pong mechanism, in which Na+ and Ca2+ compete for binding to 4 potential ion-binding sites within the exchanger and are transported in separate steps. The ion binding sites are alternately exposed to opposite sides of the IMM, with the ion of highest local abundance outcompeting the other for access to the binding sites and subsequent transport across the IMM (306, 307). This model is consistent with the observed electrogenicity and voltage dependence of mitochondrial Na+/Ca2+ exchange (286). NCLX also exhibits sensitivity to pH and is inhibited at basic extramitochondrial pH, which increases the Km of NCLX for Ca2+ (308). Although K+ is not required for NCLX activity (299), K+ can stimulate mitochondrial Na+/Ca2+ exchange because it reduces the exchanger’s Km for Ca2+ (308).
Both α-repeats are not required within the same molecule of NCLX in order for it to catalyze ion exchange. Instead, NCLX may function as a dimer or trimer, in which the contribution of a single α-repeat from each subunit is sufficient for ion transport (309). This model agrees with the detection of ∼100-kDa dimers in Western blots for NCLX (282). That NCLX functions as an oligomer is further supported by the finding that expression of a nonfunctional mutant NCLX exerts a dominant-negative effect to inhibit the activity of single α-repeat forms of NCLX (309). These findings imply that short NCLX splice variants with disruption of either the α1 or α2 domain should still be capable of Na+/Ca2+ exchange when dimerized with an additional copy of NCLX.
NCLX catalyzes Na+/Ca2+ exchange and Li+/Ca2+ exchange at similar rates (299). NCLX S468 is highly conserved among NCX and NCKX exchangers and is critical for mCa2+ transport by NCLX (282, 309). N149, P152, D153, and G176 within the NCLX α1 domain and N467, S468, G494, and N498 within the α2 domain are required for full mCa2+ exchange activity (306). N149A, P152A, D153A, N467Q, S468T, and G494S mutations do not affect Li+/Ca2+ exchange but disrupt Na+/Ca2+ exchange, suggesting that these sites are critical specifically for Na+-mediated Ca2+ transport (306). In contrast, D471 is critical for Li+/Ca2+ exchange but is dispensable for Na+/Ca2+ exchange (306). Simultaneous disruption of four critical Ca2+-binding residues (N149A, D153A, N467A, D471A) is required to fully disrupt all Ca2+ exchange by NCLX (306). Nine out of 12 ion coordinating residues differ between the four ion binding sites of NCLX and archaeal NCX (307, 310). Replacement of these nine residues in NCX with the corresponding residues from NCLX (N159, G150, D153, A177, V181, N467, D471, G494, N498) allows the mutant NCX to catalyze Li+/Ca2+ exchange, suggesting that some of these sites confer the Li+-transporting activity of NCLX (307). Refaeli et al. (307) propose that this difference in Li+ binding in NCLX versus NCX is also related to the fact that all four of the residues that coordinate Ca2+ at the SCa ion binding site (N149, D153, D471, and N467 in NCLX) differ between these two proteins. This particular structural difference may induce structural rearrangements in the other three ion binding sites that ultimately affect the proteins’ ability to bind Na+ versus Li+ (307). Since intracellular Li+ concentration is low (0.6–0.8 mM) and far below NCLX’s Kd for Li+, whether NCLX actually transports Li+ under physiological conditions (306) and what functional consequences this Li+ transport may have for the cell are pertinent unanswered questions.
3.7.1.5. special roles of nclx in physiology.
NCLX’s role in mCa2+ efflux allows it to shape cellular processes that depend on mitochondrial or cytosolic Ca2+ signals. mCa2+ efflux through NCLX can limit mCa2+ accumulation and so limit TCA cycle flux, NADH production, and the rate of oxidative phosphorylation (311). This means that fluctuations in cytosolic Na+ can indirectly impact metabolism and influence the mitochondria’s ability to buffer cytosolic Ca2+. This effect is apparent in tetanus-induced synaptic potentiation, where increased cytosolic Na+ drives mCa2+ release, and this Ca2+ stimulates neurotransmitter release at neuromuscular synapses (312). In a similar fashion, NCLX contributes to cytosolic Ca2+ signals that cause pancreatic β-cells to secrete insulin after stimulation with glucose (313). NCLX-dependent mCa2+ efflux is also essential for maintaining Ca2+ recycling from the mitochondria to the SR in HL-1 cardiomyocytes, and disruption of this process diminishes cardiomyocyte automaticity (314).
NCLX-dependent changes in mCa2+ content affect mitochondrial ROS production, which itself affects cellular Ca2+ homoeostasis. During store-operated Ca2+ entry (SOCE), elevated cytosolic Ca2+ is transmitted to the mitochondria, causing a spike in ROS production. Without NCLX to extrude mCa2+, excessive mitochondrial ROS causes oxidation and inactivation of Orai1. This prematurely terminates SOCE and limits the filling of intracellular Ca2+ stores (315). NCLX is likewise implicated in control of Ca2+-dependent signals relevant for diverse processes including nociceptive signaling (316) and B lymphocyte chemotaxis (317). Finally, recent work demonstrates that NCLX is necessary to limit mCa2+ accumulation that would otherwise lead to permeability transition and cell death during processes involving increased cytosolic Ca2+ signaling, such as during the adrenergic stimulation of brown fat that triggers nonshivering thermogenesis (318).
3.7.1.6. genetic diseases associated with nclx.
No primary mutations in NCLX are yet reported to cause human disease, but altered NCLX function has been implicated in the pathogenesis of several neurological and neuromuscular disorders. Loss of frataxin in Friedreich’s ataxia causes a secondary increase in cytosolic Ca2+ concentration in neurons (319) and cardiomyocytes (320). Elevated Ca2+ activates calpains that degrade NCLX and thereby reduce mCa2+ efflux activity (321, 322). As a result, mitochondria overload with Ca2+ and become susceptible to permeability transition, leading to cell death. Downregulation of NCLX is also implicated in Alzheimer’s disease and Parkinson’s disease (323, 324).
3.7.1.7. animal models of nclx.
The first NCLX loss-of-function model, developed by our laboratory, is a mouse in which exons 5–7 of Slc8b1 are floxed (TABLE 2). Tamoxifen-inducible deletion of Nclx in the adult cardiomyocytes of Slc8b1fl/fl × αMHC-MerCreMer mice reduces mCa2+ efflux and results in mCa2+ overload, leading to excessive ROS production, necrotic cell death, and rapidly progressing heart failure (166). Eighty-seven percent of these Nclx conditional-knockout animals die within 2 wk of tamoxifen administration, reflecting the critical requirement for NCLX within the heart. Cardiac mitochondria from these animals exhibit increased permeability transition (PT) and swelling following a Ca2+ challenge, and already tend to be swollen at baseline, suggesting that they are already overloaded with Ca2+. EMRE protein expression decreases somewhat in Nclx-conditional knockout hearts, possibly as a compensatory response to reduce mCa2+ uptake and limit mCa2+ overload (166). Deletion of the mPTP component cyclophilin D rescues lethality, contractile function, cardiac hypertrophy, and mitochondrial structural defects in mice with acute cardiomyocyte Nclx deletion (166). This confirms that these defects result in large part from mCa2+ overload, PT, and cardiomyocyte death. Despite the critical role of NCLX in maintaining cardiomyocyte mCa2+ homeostasis and survival, Slc8b1fl/fl mice crossed to the αMHC-Cre driver to enable constitutive deletion of Nclx in the cardiomyocytes are viable because of undefined compensatory responses (166). Cardiomyocytes from these animals have a profound reduction in mCa2+ efflux rate but no change in cytosolic Ca2+ handling. mCa2+ uptake is also somewhat reduced in these cells, even though the expression of mtCU components is not changed, suggesting that some aspect of their posttranslational regulation is altered in the absence of NCLX. Our laboratory also developed mice with cardiac-specific, doxycycline-controlled NCLX overexpression (TRE-NCLX × αMHC-tTA mice) that exhibit enhanced mCa2+ efflux and increased resistance to PT (166). Cardiomyocyte NCLX overexpression in these animals reduces infarct size and cell death following cardiac ischemia-reperfusion injury, and protects contractile function, reduces cardiomyocyte superoxide production, and minimizes pathological cardiac remodeling following permanent myocardial infarction (166). Together, these models indicate that NCLX is the predominant route of mCa2+ efflux within the heart and is required for normal mitochondrial function and cell survival. They also suggest that mitochondrial Ca2+ cycling occurs continuously within the heart, such that disruption of mCa2+ efflux quickly results in pathological mCa2+ overload.
Our laboratory recently used these genetic models of NCLX to examine its contribution to the progression of Alzheimer’s disease (AD). NCLXfl/fl mice were crossed to mice with neuronal-restricted Camk2a-Cre to delete NCLX from the forebrain and then crossed to the 3xTg-AD mouse model of AD (323). Loss of NCLX accelerates impairments in spatial working memory, contextual recall, and cued recall in 3xTg-AD mice. These defects are associated with increased amyloid plaque burden and tau hyperphosphorylation (323). These findings suggest that the reduced NCLX expression observed in AD patients contributes to AD progression. Rescue experiments crossed the 3xTg-AD line to mice with neuronal-specific, doxycycline-controlled NCLX overexpression (3xTg-AD × TRE-NCLX × Camk2a-tTA) to investigate the impact of increased neuronal NCLX activity on AD. Here, increased NCLX expression protects against age-related cognitive decline and reduces amyloid plaque burden and tau pathology in AD mice (323). NCLX overexpression also reduces the susceptibility to mitochondrial PT and limits superoxide formation and lipid peroxidation in 3xTg-AD brains (323). These animal studies and reports of altered NCLX expression or activity in neurodegeneration and heart failure suggest that NCLX could be a powerful therapeutic target for human diseases driven by mCa2+ overload.
Recently, the Trebak laboratory studied a Slc8b1-knockout mouse model generated via CRISPR/Cas9 to produce constitutive, global disruption of NCLX (325). Interestingly, these animals are viable, pointing to compensatory mechanisms during development that minimize the deleterious effects of NCLX ablation, although it should be noted that loss of NCLX protein expression is not demonstrated in these animals. Pathak et al. (325) used this model to investigate the link between colorectal cancer and the downregulation of NCLX expression observed in human colorectal tumors. NCLX-knockout mice subjected to the colitis-induced colorectal cancer model develop fewer and smaller colorectal tumors than wild-type control mice. Likewise, NOD-SCID mice xenografted with NCLX-knockout human HCT116 colorectal cancer cells exhibit less tumor growth than animals receiving control HCT116 colorectal cancer cells (325). Surprisingly, though, mice receiving NCLX-knockout cells have increased metastasis and reduced overall survival, suggesting that NCLX has distinct roles in tumor cell proliferation versus tumor cell migration or invasion in vivo. Further in vitro work suggests that loss of NCLX in colorectal cancer cells depolarizes mitochondria, disrupts mitochondrial morphology, and increases ROS production. These changes are associated with prometastatic transcriptional reprogramming, chemoresistance, and stabilization of HIF-1α, which results in increased glycolysis that drives enhanced cellular migration (325). These findings suggest that targeting NCLX as a therapy for cancer is a complicated objective, as manipulating NCLX activity in this context could have both desirable and deleterious outcomes.
Null mutation of the C. elegans NCLX-type gene, ncx-9, causes defects in axon guidance within the GABAergic motor neuron circuit, leading Sharma et al. (326) to conclude that NCLX is critical for neural circuit patterning. Beyond its critical function in adult organisms, then, NCLX also appears to have important roles during development, even if it is not strictly required for viability. It will be interesting for future studies to determine whether any such developmental defects also occur within the brain or other tissues in mice with constitutive loss of NCLX. Continued research into NCLX will likely uncover more roles for this protein in normal physiology and additional consequences of altered NCLX function in organs other than the heart and brain.
3.7.1.8. regulation of nclx.
Na+/Ca2+ exchange through NCLX is electrogenic and therefore sensitive to the mitochondrial membrane potential (ΔΨm). Furthermore, NCLX protein is itself allosterically regulated by membrane potential, with small changes in ΔΨm that are insufficient to affect mCa2+ uptake instead altering mCa2+ efflux. Mild mitochondrial depolarization inhibits mCa2+ efflux through NCLX (327). This effect is dependent on a cluster of positively charged arginine and lysine residues (R253, R255, R256, and K325) located near the interface between the regulatory loop of NCLX and the membrane. Kostic et al. (327) propose that these charged residues respond to changes in membrane potential by changing the interaction of NCLX’s regulatory loop with the rest of the exchanger. They suggest that such allosteric inhibition of NCLX allows the mitochondria to respond to metabolic stress (partial depolarization of ΔΨm) by increasing mCa2+ content, which in turn would stimulate the TCA cycle and oxidative phosphorylation. As mitochondrial metabolism rises to meet cellular demand, membrane potential recovers, relieving the inhibition of NCLX and allowing mCa2+ efflux to resume (327). Phosphorylation of NCLX S258, which lies near the positively charged residues involved in this mechanism [see Kostic et al. (327) for an illustration], overrides the ability of membrane depolarization to inhibit NCLX, possibly because it changes the net charge in this region of the protein. Alternatively, S258 phosphorylation may change the nature of the interactions between this residue and R253, R255, R256, and K325, leading to secondary conformational changes in the catalytic α-domains of NCLX that favor ion exchange (327).
Phosphorylation of NCLX S258 or phosphomimetic S258D mutation rescues impaired NCLX exchanger activity in PINK1-deficient neurons, a model for Parkinson’s disease (328). Pharmacological activation of PKA is also sufficient to stimulate NCLX activity in this context, and S258 of NCLX lies within a putative PKA phosphorylation site. Furthermore, purified NCLX is phosphorylated at S258 when incubated with the PKA catalytic subunit, suggesting that PKA is capable of directly phosphorylating NCLX at this site to stimulate its activity (328). Knockout or inhibition of the leucine-rich repeat kinase 2 (LRRK2) impairs mCa2+ efflux through NCLX (324), although whether LRRK2 directly regulates NCLX activity is unclear. Phosphorylation of NCLX at the putative PKA-regulated site, S258, recovers NCLX activity in LRRK2-deficient cells (324), but whether PKA-dependent phosphorylation of NCLX represents a downstream step in LRKK2-dependent signaling or rather is an independent regulatory event remains to be determined.
How NCLX may be regulated by protein-protein interactions is another area of active investigation. The activity of NCX family proteins is regulated by other membrane and membrane-associated proteins such as CRMP2 and 14-3-3 proteins (329, 330). 14-3-3 proteins bind to the cytoplasmic loops of NCX proteins and inhibit their activity (330). Since NCLX contains an analogous regulatory loop, this may be a site at which binding partners can modulate NCLX function. The antiapoptotic protein Bcl-2 inhibits mitochondrial Na+/Ca2+ exchange and thereby enhances mCa2+ content and oxidative phosphorylation (331). Bcl-2 thus is a putative candidate that may regulate NCLX activity via a direct physical interaction. As noted above, NCLX appears to be degraded by calpains in Friedreich’s ataxia (321, 322). Recent work suggests that calpains may also be responsible for downregulation of NCLX activity after traumatic brain injury (332). Thus, proteolytic cleavage of NCLX is yet another mechanism that can control net cellular NCLX activity.
3.7.1.9. pharmacological modulation of nclx.
A number of compounds including tetraphenylphosphonium (TPP+) and benzodiazepines inhibit mitochondrial Na+/Ca2+ exchange and may act directly on NCLX (333, 334). Several Ca2+ antagonists such as diltiazem, prenylamine, fendiline, nifedipine, and verapamil also inhibit mitochondrial Na+/Ca2+ exchange (335), although their lack of specificity toward NCLX limits their utility as NCLX modulators. Likewise, KB-R7942 shows partial inhibition of NCLX but also affects Na+/Ca2+ exchangers (299). The benzothiazepine CGP-37157 inhibits Na+/Ca2+ exchange in cardiac mitochondria without affecting the L-type calcium channel, plasma membrane NCX proteins, or the Na+-K+-ATPase (336). CGP-37157 directly inhibits NCLX (282) and so has become a widely accepted experimental tool to control NCLX activity. However, some reports indicate that CGP-37157 also inhibits other proteins involved in cellular Ca2+ handling including plasma membrane Ca2+ channels (337, 338), SERCA (339), LETM1 (288), and even, in contrast to other reports, the L-type calcium channel (340). Experiments using intact or permeabilized cells should consider potential effects of CGP-37157 on these other targets. CGP-37157 also causes Ca2+ leak through the RyR in both cardiac and skeletal muscle, further complicating interpretation of experiments using this drug (339). Possible effects of CGP-37157 on the RyR in cell types where the presence of a mitochondrial RyR is suspected (see above) should also be taken into account when using this drug. There is therefore a great need for the development of more specific pharmacological NCLX inhibitors. No pharmacological activators of NCLX are currently known. Given the attractiveness of enhancing NCLX activity as a strategy to treat human disease, the development of potent and specific NCLX activators is another high-priority goal for the mCa2+ field.
3.7.2. LETM1 in mCa2+ efflux.
LETM1 (Leucine Zipper and EF-Hand Containing Transmembrane Protein 1; gene name = LETM1 or SLC55A1) was initially identified as a gene that is deleted in the majority of patients with the developmental disorder Wolf–Hirschhorn syndrome (WHS) (341). The presence of two EF-hands in LETM1 hints at its potential role in intracellular Ca2+ signaling (341), and its localization to the mitochondria suggests that some neuromuscular features of WHS are related to mitochondrial defects (342). LETM1 is broadly expressed, with transcripts detected in the heart, brain, placenta, lung, liver, skeletal muscle, kidney, and pancreas (341). Within the mitochondria, LETM1 is specifically localized to the IMM (288, 343, 344). LETM1 is also detected in the endoplasmic reticulum (ER), and at least one report suggests that it may contribute to ER Ca2+ exchange (345).
Early studies proposed LETM1 as a mitochondrial K+/H+ exchanger (346). The Drosophila homolog of LETM1, CG4589, was identified specifically as a mitochondrial Ca2+/H+ exchanger in an RNA interference screen for genes affecting mCa2+ transport (288). When permeabilized Drosophila cells incubated in Na+-free solution to limit mitochondrial Na+/Ca2+ and Na+/H+ exchange are exposed to low (<1 µM) Ca2+, mitochondria take up Ca2+ in exchange for extrusion of H+ from the matrix (288). This activity is inhibited by knockdown of LETM1, suggesting that LETM1 mediates mCa2+ uptake under low-cytosolic Ca2+ conditions (288). Such mCa2+ uptake/H+ extrusion through LETM1 is normally limited by the pH gradient across the IMM [pH ∼7.7–8.2 in the matrix; pH ∼7-7.2 in the cytosol/IMS (21, 291–294)], thus preventing free mCa2+ concentration from reaching equilibrium under physiological conditions (288). Fitting with this model, reduction of cytosolic Ca2+ concentration or acidification of the cytosol reverses LETM1 transport, causing mCa2+ extrusion coupled to mitochondrial H+ (mH+) influx (288). Human cell lines overexpressing LETM1 exhibit augmented pH-driven mCa2+ uptake and mH+ extrusion. Conversely, LETM1 knockdown in HeLa cells ablates both early mCa2+ uptake/mH+ extrusion and subsequent mCa2+ efflux/mH+ uptake that are triggered by histamine stimulation (288). Loss of this reversible LETM1 activity, in particular the second phase of mCa2+ efflux/mH+ uptake, causes eventual mCa2+ overload (288). The immediate effects of LETM1 knockdown on mitochondrial Ca2+/H+ exchange are independent of any impairment in electron transport chain function, as basal ΔΨm is slightly increased in LETM1-knockdown cells (288).
Further support for LETM1 as a Ca2+/H+ exchanger comes from experiments on purified His-tagged LETM1. LETM1-containing liposomes rapidly take up Ca2+, and this flux is blocked by ruthenium red and Ru360 and is partially inhibited by GCP-37157 (288). H+ efflux from liposomes is triggered by addition of Ca2+ to or alkalinization of the external buffer solution. Likewise, acidification of the external buffer solution drives Ca2+ efflux from the liposomes, whereas alkalinization of the external buffer stimulates Ca2+ uptake (288). Although LETM1 transports a number of cations, its affinity for Ca2+ is much greater than its affinity for other cations, including K+ (347). LETM1 thus has a fundamental function as a reversible Ca2+/H+ exchanger that will mediate either mCa2+ uptake or mCa2+ efflux depending on the given conditions within a cell.
3.7.2.1. letm1 phylogenetic conservation.
LETM1 is conserved throughout Eukarya, including in protozoa such as Toxoplasma gondii (342, 348). Evolutionary relationships among LETM1 homologs are illustrated by Austin and Nowikovsky (349). Mouse and human LETM1 proteins share 83.3% sequence identity at the amino acid level, and LETM1 homologs in species from yeast to C. elegans share ∼25–43% homology with human LETM1 (341). The central region of LETM1 containing the transmembrane domain is the most conserved region of LETM1 among such divergent species, whereas the rest of the protein varies more, indicating that LETM1 homologs may have distinct functions in different taxa (341). Of note, PKC and casein kinase phosphorylation sites located NH2-terminal to the transmembrane domain are conserved, suggesting that LETM1 regulation is consistent across distant species (341). Several additional features are conserved among some species, including an α-helical domain predicted to form coiled coils, the leucine zipper domain, and a SAP-like region of unknown function that is located 138–145 residues after the transmembrane domain (342). A COOH-terminal, Ca2+-binding EF-hand domain is present in plants and animals but absent in yeast; and the leucine zipper is conserved only among animals (350).
Studies of LETM1 homologs in various species support conserved, Ca2+-independent functions of this protein related to its role in K+/H+ exchange. Knockout of the yeast LETM1 homolog, MDM38/YOL027C, causes mitochondrial swelling, defective mitochondrial K+/H+ exchange, increased mitochondrial K+ content, and a diminished ΔΨm (346, 351). Thus, a fundamental function of LETM1 is to extrude K+ from the matrix, thereby lowering matrix osmolarity in order to control mitochondrial volume (343, 346). This function of LETM1 in mitochondrial K+ handling and volume control is shared in T. gondii, T. brucei, C. elegans, Drosophila, and humans (343, 348, 352, 353). The potential conservation of LETM1’s role in mCa2+ exchange has not been studied in as great detail as its role in K+ flux, although the finding that the Ca2+-sensing EF-hand of LETM1 is not as widely conserved indicates that LETM1’s role in mCa2+ handling may not be as consistent across taxa. LETM1 and its homologs have been implicated in a number of processes, including mitochondrial translation and protein homeostasis via interactions with the mitochondrial ribosome (350, 354). Some reports indicate that these effects are specifically related to and downstream of LETM1’s role in mitochondrial K+ exchange (352, 355). In many cases, though, any potential contribution of LETM1-dependent mCa2+ transport to these processes has not been thoroughly investigated.
3.7.2.2. letm1 structure.
Human LETM1 encodes an 83.4-kDa, 739-amino acid transmembrane protein (341). LETM1 is detected in high-molecular mass (∼550 kDa) protein complexes (343, 356). LETM1 can homooligomerize, and the ∼550-kDa molecular mass of LETM1-containing complexes corresponds to the oligomerization of up to six or seven LETM1 subunits (343). LETM1 also forms 140-kDa dimers and 210-kDa trimers, and blue native gel electrophoresis further reveals that LETM1 participates in at least three distinct high-molecular mass complexes, of 250 kDa, 500 kDa, and 650 kDa. Since these high-mass complexes persist regardless of LETM1 knockdown or overexpression, they likely contain other proteins in addition to LETM1 (356).
The protein structure of LETM1 begins with an NH2-terminal mitochondrial targeting sequence within the first 167 residues (342), followed by a transmembrane region. The transmembrane region is followed by a region of α-helices that may form coiled coils, a leucine zipper motif, and two EF-hand motifs (341). The first EF-hand domain (residues 569–597 of human LETM1) is located within the leucine zipper, and divergence of its sequence from the consensus EF-hand divalent cation binding sequence suggests that it has lost the ability to bind Ca2+ (341). The second EF-hand (residues 667–695 of human LETM1) conforms to the EF-hand consensus sequence and so is predicted to bind Ca2+ (341). Expression of LETM1 either with the second EF-hand deleted or with point mutation of two critical residues within this EF-hand (D676A and D688K) impairs mitochondrial Ca2+ flux in HeLa cells (357). LETM1 also contains several predicted phosphorylation sites for protein kinase C, casein kinase, and tyrosine kinase, as well as a potential glycosylation site (341).
The transmembrane domain of LETM1 anchors it in the IMM. Protease protection assays indicate that the majority of the protein, which lies COOH-terminal to the transmembrane domain, extends into the matrix and only a small and/or protease-resistant region extends into the IMS (344, 356). Since a single-pass transmembrane protein should not be capable of ion exchange, homooligomerization of LETM1 or its heterooligomerization with other proteins may be required for LETM1 to form a functional K+/H+ or Ca2+/H+ exchanger (297). Recent studies labeling the matrix and IMS sides of the IMM via genetically targeted peroxidases challenge this structural model and suggest that both the NH2 and COOH termini of LETM1 reside in the mitochondrial matrix (358). According to this model, LETM1 has two relevant transmembrane domains (residues 204–232 and 413–421), and only a small linker region between them is exposed in the IMS (358). Further investigation into the structure of LETM1 and whether or not oligomerization is in fact required for LETM1-mediated cation exchange is warranted.
3.7.2.3. properties and function of letm1.
This section focuses on the ion transport properties of LETM1 specifically as they pertain to Ca2+/H+ exchange. For a discussion of the properties of LETM1-mediated K+/H+ exchange, the reader is referred to recent reviews on this topic (297, 351, 359).
Several recent papers reinforce LETM1’s role as a Ca2+/H+ exchanger and indicate that, under normal conditions, it is particularly important for mCa2+ efflux. LETM1 knockdown increases net mCa2+ content (357). In HeLa cells, LETM1 knockdown increases mCa2+ transients in response to histamine stimulation and overexpression of wild-type LETM1 reduces mCa2+ transients (360). Similar effects are observed in pancreatic β-cells, where silencing of LETM1 causes mitochondria to accumulate more Ca2+ and take up less H+ in response to a rise in extramitochondrial Ca2+ (361). Mutation of E221 diminishes the ability of LETM1 to reduce mCa2+ transients, suggesting that E221 is critical for its Ca2+ exchange activity (360). These studies collectively support a critical role for LETM1 in mCa2+ efflux, although the relative contribution of LETM1 versus NCLX to mCa2+ efflux is the matter of some debate. De Marchi et al. (362) find that LETM1 overexpression in HeLa cells has no effect on mCa2+ efflux following agonist stimulation, whereas NCLX overexpression clearly increases mCa2+ efflux. Heterozygous deletion of NCLX in chicken DT40 B lymphocytes or siRNA-mediated NCLX knockdown in mouse A20 B lymphocytes causes a marked reduction in, but not complete loss of, mCa2+ efflux, and the residual efflux occurs even in the absence of Na+ (363). This suggests that additional, Na+-independent exchangers such as LETM1 contribute to mCa2+ efflux in immune cells. An interesting question is whether either of these two exchange systems may be able to compensate for loss or dysfunction of the other in order to maintain mCa2+ homeostasis. For example, does a compensatory increase in LETM1 function account for the viability of NCLX-constitutive knockout mice (325)? Much more work is clearly required to elucidate the relative importance of LETM1 and NCLX to mCa2+ efflux, and to understand how the relative contributions of these proteins vary across distinct tissues.
How LETM1 mediates ion exchange while it may have only a single transmembrane domain is an area of active investigation. LETM1’s function as a Ca2+ exchanger is independent of the function of other proteins involved in mCa2+ uptake, as knockdown of LETM1 does not affect MCU current or MCU, MCUR1, or MICU1 protein level (357). Likewise, in fibroblasts of patients with genetic disruption of LETM1, the loss of LETM1 protein has no effect on the expression of these mtCU components (357). LETM1 does not physically interact with NCLX, indicating that LETM1 has a direct role in mCa2+ efflux rather than simply binding to NCLX to modulate its activity (357). Shao et al. report that reconstituted LETM1 forms hexamers with a pore of 10.5 Å at pH 8.0, which is predicted to allow ion flux (360). This pore becomes blocked by a “plungerlike” structure at pH 6.5, which presumably inhibits ion transport (360). LETM1 therefore oligomerizes to a channel that appears competent for both ion exchange and pH-dependent regulation, independent of any requirement for other proteins.
Initial studies proposed LETM1 to operate with a stoichiometry of 1 Ca2+:1 H+ and mediate electrogenic cation exchange (288). Such electrogenic exchange means that the transport of Ca2+ and H+ by LETM1 is influenced by membrane voltage (287) and so could respond to changes in ΔΨm. Other studies of purified LETM1 indicate that it instead mediates electroneutral cation exchange and operates with a stoichiometry of 1 Ca2+:2 H+ (347). However, the observation that cation exchange through LETM1 is indeed sensitive to changes in membrane voltage (288) favors the electrogenic 1 Ca2+:1 H+ model of LETM1 stoichiometry. Furthermore, the observation that LETM1 mediates mCa2+ uptake at low cytosolic Ca2+ concentrations (<1 µM) (288, 289) is inconsistent with electroneutral Ca2+/H+ exchange, which would require cytosolic Ca2+ to rise to a >100-fold higher concentration than the matrix Ca2+ concentration in order to drive mCa2+ uptake through LETM1 (297). So, assuming an electrogenic 1:1 stoichiometry of Ca2+/H+ exchange, LETM1 should mediate mCa2+ efflux under conditions where mitochondrial Ca2+ is sufficiently high relative to cytosolic Ca2+ or where cytosolic pH is sufficiently low (acidic, high H+ concentration) relative to the matrix, and/or ΔΨm is sufficiently depolarized to reverse LETM1 directionality. Indeed, this prediction agrees with the observation that, in response to agonist-induced elevation of cytosolic Ca2+, LETM1 first contributes to mCa2+ uptake but then subsequently contributes to mCa2+ efflux as the cytosolic Ca2+ transient subsides (288). Furthermore, Na+-independent mCa2+ efflux, likely via LETM1, is sensitive to total matrix Ca2+ content. In experiments conducted in the absence of Na+ so as to eliminate Ca2+ transport by NCLX, increased mCa2+ load increases the rate of mCa2+ efflux via Ca2+/H+ exchange (364). LETM1 thus may serve as a mechanism to protect mitochondria from mCa2+ overload, because it favors mCa2+ efflux as mCa2+ concentration rises (364). Such a protective ability is apparently of limited efficacy in some tissues, though, as it is not sufficient to compensate for loss of Na+/Ca2+ exchange and prevent basal mCa2+ overload when NCLX is deleted in the adult heart (166). A limitation of existing studies of LETM1 ion transport is that few have been conducted in the presence of physiological concentrations of K+ and Mg2+ (287, 297). Thus, understanding the truly “physiological” function of LETM1 as it operates in intact cells requires further investigation.
3.7.2.4. special roles of letm1 in physiology.
LETM1 is implicated in a number of cellular processes, some of which have obvious associations with mCa2+ handling. Knockdown of LETM1 impairs cellular bioenergetics and oxygen consumption and is associated with defects in complex IV (357), suggesting a critical role for LETM1 in oxidative phosphorylation. One possible mechanism linking LETM1-dependent changes in mCa2+ homeostasis to disrupted bioenergetics is increased mitochondrial ROS production. Consistent with this notion, overexpression of antioxidant genes in LETM1-knockdown cells corrects cellular energetics (357). Other effects of LETM1 disruption, such as altered mitochondrial fusion, apoptosis, and mitophagy, may instead reflect LETM1’s functions in K+ homeostasis and mitochondrial volume regulation (355, 365). LETM1 and its homologs also have pleiotropic effects on mitochondrial DNA metabolism and pyruvate dehydrogenase activity (366); mitochondrial mRNA translation and respiratory chain biogenesis (367); and mitochondrial morphology (342) and cristae organization (368). LETM1 is also required for the maturation of preadipocytes and the differentiation of beige adipocytes (369). Upregulation of LETM1 causes upregulation of YAP and the stimulation of proliferative pathways (370), suggesting an additional role for LETM1 in cell proliferation. Which of these phenomena are specifically related to LETM1-dependent mCa2+ exchange versus LETM1-dependent mitochondrial K+ homeostasis or binding and regulation of other mitochondrial proteins has not yet been fully elucidated. Any interpretation of the putative impact of LETM1’s Ca2+ exchanger activity on cellular and whole animal phenotypes should therefore take into consideration the possible contribution of these Ca2+-independent effects of LETM1 on cell function.
3.7.2.5. genetic diseases associated with letm1.
LETM1 is deleted in almost all patients who have Wolf–Hirschhorn syndrome (WHS), a developmental disorder caused by partial deletion of the short arm of chromosome 4 (341) (TABLE 1). WHS is characterized by impaired growth, developmental delay, microcephaly, and mental defects. It often presents with impaired muscle tone and seizures, and congenital heart defects are sometimes observed as well (341, 342). WHS patient fibroblasts have reduced LETM1 mRNA and protein content (356), suggesting that loss of LETM1 contributes to these phenotypes. Consistent with this idea, disruption of LETM1 is implicated in the seizure phenotype of WHS (371), and LETM1 haploinsufficiency disrupts normal glucose metabolism, reduces brain ATP levels, and increases the occurrence of seizures in mice (372). LETM1 haploinsufficiency also alters mitochondrial function in cells derived from WHS patients, which display diminished Ca2+ sensitivity of the mPTP, hyperpolarization of ΔΨm, and elevated mitochondrial superoxide production (373). WHS patient fibroblasts surprisingly do not exhibit the same mitochondrial fragmentation observed with acute LETM1 silencing, suggesting that some compensatory mechanisms exist to maintain mitochondrial network structure in the face of chronic LETM1 disruption, despite persistent disruption of mitochondrial ion homoeostasis (356).
Although LETM1 deletion is necessary for WHS, LETM1 deletion alone is not sufficient to cause the full spectrum of phenotypes that are associated with this disease (374). Rather, the deletion of additional genes adjacent to LETM1 contributes to WHS as well (374). Further complicating our understanding is the observation that duplication of genetic regions containing LETM1 can also cause several features of WHS such as microcephaly, seizures, and delayed development (375), as well as syndromes distinct from but related to WHS (376). These findings indicate that either increased or decreased LETM1 expression can be detrimental, and that the specific dosage of LETM1 is critical for maintaining normal cellular function. How each of the individual phenotypic features of WHS specifically relates to altered mCa2+ homeostasis versus other consequences of altered LETM1 expression remains to be determined.
Altered LETM1 expression is observed in a number of conditions including temporal lobe epilepsy (377), obesity (378), and diabetes (379). LETM1 is also upregulated in various types of cancer, where it may inhibit mitochondrial function and secondarily enhance glycolysis and cell survival signaling (380, 381). Multiple studies have proposed LETM1 as a prognostic cancer biomarker, as LETM1 expression typically correlates with poorer outcomes (382–391). This has raised interest in targeting LETM1 as a cancer therapeutic (392–394), although several cases suggest that LETM1 instead is protective and inhibits tumor growth in liver and lung cancer (395, 396). How these potential detrimental versus protective effects of LETM1 may be related to its specific role in mCa2+ exchange is an open question.
3.7.2.6. animal models of letm1.
Null mutation of the LETM1 homolog (F58G11.1 or letm-1) in C. elegans causes mitochondrial swelling (TABLE 2). C. elegans homozygous for this mutation arrest in the L3 larval stage and are small and infertile, although they can live longer than wild-type worms (343). Animals that are heterozygous for this mutation appear no different from wild type and, in contrast to human WHS patients, have no signs of seizures (343). Wild-type worms subjected to RNAi to knock down letm-1 after hatching mature more slowly and remain smaller than control worms upon reaching adulthood (343). These phenotypes are associated with swollen and highly disorganized mitochondria, consistent with accepted roles for LETM1 in mitochondrial volume regulation. Conversely, transgenic overexpression of LETM-1 in C. elegans results in the opposite effect, causing “crimping” of the mitochondrial matrix and in some cases detachment of the mitochondrial outer membrane from the IMM and matrix (343). These findings indicate that LETM1 is inversely related with mitochondrial matrix volume and support the notion that mitochondrial defects in LETM1-mutant animals have broad effects to limit organismal growth and development.
In higher animals, loss of LETM1 is lethal during development. In vivo RNAi to ubiquitously knock down LETM1 in Drosophila results in lethality in the early larval stage or the pupal stage (353). Specific knockdown of LETM1 in muscle cells reduces the size of larvae, reduces their physical behavior, and arrests the growth of most flies in the pupal stage (353). The few animals that develop to adulthood are smaller than normal, weak, and unable to fly (353). Knockdown of LETM1 in the fly eye results in smaller than normal eyes surrounded by scar tissue, with many ommatidia exhibiting swollen mitochondria (353). Finally, downregulation of LETM1 in the nervous system reduces the speed of locomotion and increases the time that flies remain immobile, suggestive of neuromuscular impairments (353). This locomotor defect is associated with a reduction in neurotransmitter release following nerve stimulation (353). McQuibban et al. (353) conclude that loss of LETM1 has direct effects on neurotransmission, with subsequent impairments of neuromuscular function. This particular study did not distinguish whether these effects are related to disrupted mitochondrial K+ transport, Ca2+ transport, or both.
In mice, homozygous deletion of Letm1 via gene trap is lethal by day 6.5 of embryogenesis (day E6.5) (372). About half of the mice with heterozygous Letm1 deletion die by day E13.5. Once past this critical developmental stage, surviving heterozygous embryos are largely normal, although their fibroblasts have reduced mCa2+ uptake rates, low homeostatic mCa2+ concentration, and lower matrix pH. Letm1 heterozygous mice that survive to birth do not exhibit craniofacial or midline developmental defects as seen in WHS patients and have normal body weight up to at least 6 wk of age (372). The mitochondrial morphology of adult Letm1+/− mice is relatively normal, much like in human WHS patients (356), suggesting that over time the mechanisms regulating mitochondrial structure compensate for loss of LETM1. Interestingly, the brains of Letm1+/− mice have an impaired ability to maintain ATP concentration and reduced PDH activity, suggesting that LETM1 is required for glucose metabolism and pyruvate oxidation (372). Letm1+/− mice exhibit increased seizure activity when challenged with kainic acid, which increases intracellular Ca2+ concentration (372). This study did not specifically examine whether altered mCa2+ handling specifically contributes to increased seizure susceptibility. However, observations in rats with lentivirus-mediated knockdown of LETM1 in the hippocampus and dentate gyrus suggest that disrupted mitochondrial K+ homeostasis is not the key factor causing neuronal dysfunction upon loss of LETM1. Knockdown of LETM1 in these brain regions results in mitochondrial swelling and reduces mitochondrial gene expression, and increases the susceptibility to and duration of seizures in experimental pilocarpine-induced epilepsy (377). Although the K+/H+ ionophore nigericin corrects mitochondrial swelling in other models of LETM1 deficiency (353, 355), nigericin treatment fails to reduce this seizure susceptibility in rats with LETM1 knockdown (377). This suggests that other consequences of LETM1 disruption, like altered mCa2+ homeostasis, contribute to seizures in LETM1-deficient subjects.
3.7.2.7. regulation of letm1.
PTEN-induced kinase 1 (PINK1) phosphorylates LETM1 at T192 to stimulate LETM1-mediated mCa2+ uptake and efflux (397). Phosphorylation of LETM1 at this site protects neurons against MPP+-induced permeability transition and cell death, indicating that PINK1-dependent activation of LETM1 protects cells against mCa2+ overload (397). Several binding partners also regulate LETM1 function. The mitochondrial AAA-ATPase BCS1L binds directly to LETM1 via an interaction that depends on R155 of BCS1L but is independent of BCS1L ATP hydrolysis activity (344). LETM1 incorporates into high-molecular mass complexes of ∼300 kDa and ∼500 kDa, and BCS1L specifically regulates the incorporation of LETM1 into the larger ∼500-kDa complex (344). As LETM1 forms homooligomers in vitro (343), Tamai et al. (344) propose that BCS1L facilitates this oligomerization process. LETM1 and BCS1L also interact with the mitochondrial peptidase neurolysin, which like BCS1L is required for LETM1 complex formation (398). Thus, cleavage of LETM1 via neurolysin, or some other protease, may be a necessary step for its incorporation into intact high-mass complexes. The carboxyl-terminal modulator protein (CTMP), a tumor suppressor-like protein that negatively regulates PKB/Akt, also associates with LETM1 (365), although any direct effect of CTMP on LETM1 function remains to be determined. Finally, studies of LETM1 activity in liposomes of defined phospholipid composition indicate that cardiolipin is required for full LETM1 Ca2+ exchanger activity (399). The authors hypothesize that this effect may arise from the ability of cardiolipin to facilitate LETM1 oligomerization into hexameric, functional transporters (399).
3.7.2.8. pharmacological modulation of letm1.
LETM1 activity in liposomes is inhibited by CGP-37157, ruthenium red, and Ru360 (288). However, these effects are not consistently observed (347). Furthermore, these compounds all inhibit other proteins involved in mitochondrial Ca2+ flux, notably the mtCU, which is inhibited by ruthenium red and Ru360, and NCLX, which is inhibited by CGP-37157. The development of LETM1-specific activators and inhibitors would thus be of great experimental and perhaps clinical utility.
3.7.3. The mitochondrial permeability transition pore.
The mitochondrial permeability transition pore is a high-conductance channel spanning the IMM and OMM. It is best appreciated for its classical role in stress-induced mitochondrial depolarization, swelling, rupture, and necrotic cell death. This function of the mPTP in cell death and the regulation of the mPTP by mCa2+ are reviewed extensively elsewhere (150, 400–402). The mPTP also has a physiological function in mCa2+ efflux, in which transient, low-conductance opening of the mPTP, termed “flickering,” selectively releases Ca2+ and so helps to limit mCa2+ accumulation (401, 403–406) (FIGURE 1). Indeed, such physiological “venting” of Ca2+ out of the mitochondria, while ΔΨm is still largely intact, would imply that such mCa2+ efflux is driven by a free mCa2+ concentration that is greater than the local cytosolic/IMS Ca2+ concentration. The structure of the mPTP is largely undefined, although numerous proteins are suggested to form or regulate the pore, including the adenine nucleotide translocator (ANT) (407), the F0/F1 ATP synthase (408), the voltage-dependent anion channel (409), cyclophilin D (CypD) (410, 411), and the mitochondrial phosphate carrier (PiC) (401, 402, 412).
The mPTP inhibitor cyclosporin attenuates physiological mCa2+ efflux in healthy, isolated rat cardiomyocytes (413). What’s more, ablation of CypD, the only genetically confirmed mPTP component, results in diminished cardiomyocyte mCa2+ efflux and increased mCa2+ content (414) (TABLE 2). Protective, transient mPTP openings that prevent mCa2+ overload are observed in neurons, and Ca2+ efflux via this mechanism is disrupted in the absence of CypD (415). CypD-null hearts exhibit increased TCA cycle flux and a shift in cardiac metabolism toward glucose rather than fatty acid utilization, all expected consequences of elevated mCa2+. Despite protection from permeability transition, CypD-null mice have exaggerated cardiac dysfunction, cardiac hypertrophy, and maladaptive cardiac remodeling following chronic pressure overload or chronic exercise (414). These phenotypes are consistent with purported roles for mCa2+ in cell growth and potentially deleterious mitochondrial ROS generation. These observations collectively reinforce the idea that the mPTP is a physiologically relevant route for mCa2+ efflux that helps to prevent detrimental mCa2+ overload.
4. BUFFERING OF MITOCHONDRIAL Ca2+
In addition to the proteins that mediate mCa2+ exchange, the ability of the mitochondria to buffer Ca2+ can influence cellular Ca2+ homeostasis. During acute mCa2+ uptake, free, ionic Ca2+ in the matrix increases from ∼0.1 to ∼5 µM. Beyond this point, additional influx of Ca2+ is buffered by inorganic phosphate (40, 268, 269). Inorganic phosphate exists as polyphosphate polymers within the matrix, and this conformation minimizes the precipitation of buffered Ca2+-phosphate complexes despite their high concentration (416). The Ca2+ buffering capacity of the mitochondria is exceptional, with the ratio of phosphate-bound Ca2+:free Ca2+ estimated to rise as high as 100,000:1 (268). The proportion of phosphate-bound to free Ca2+ is sensitive to matrix pH, with more acidic matrix conditions favoring dissociation of Ca2+-phosphate complexes and increased free matrix Ca2+ (268).
The high mitochondrial capacity for Ca2+ buffering can influence cytosolic Ca2+ dynamics, as the presence of inorganic phosphate in the matrix accelerates mCa2+ uptake and thus accelerates the clearance of cytosolic Ca2+ spikes (417). It should be noted, though, that the extent of mitochondrial buffering of cytosolic Ca2+ signals varies across tissues and with the amplitude and frequency of the Ca2+ signal (FIGURE 6).
FIGURE 6.
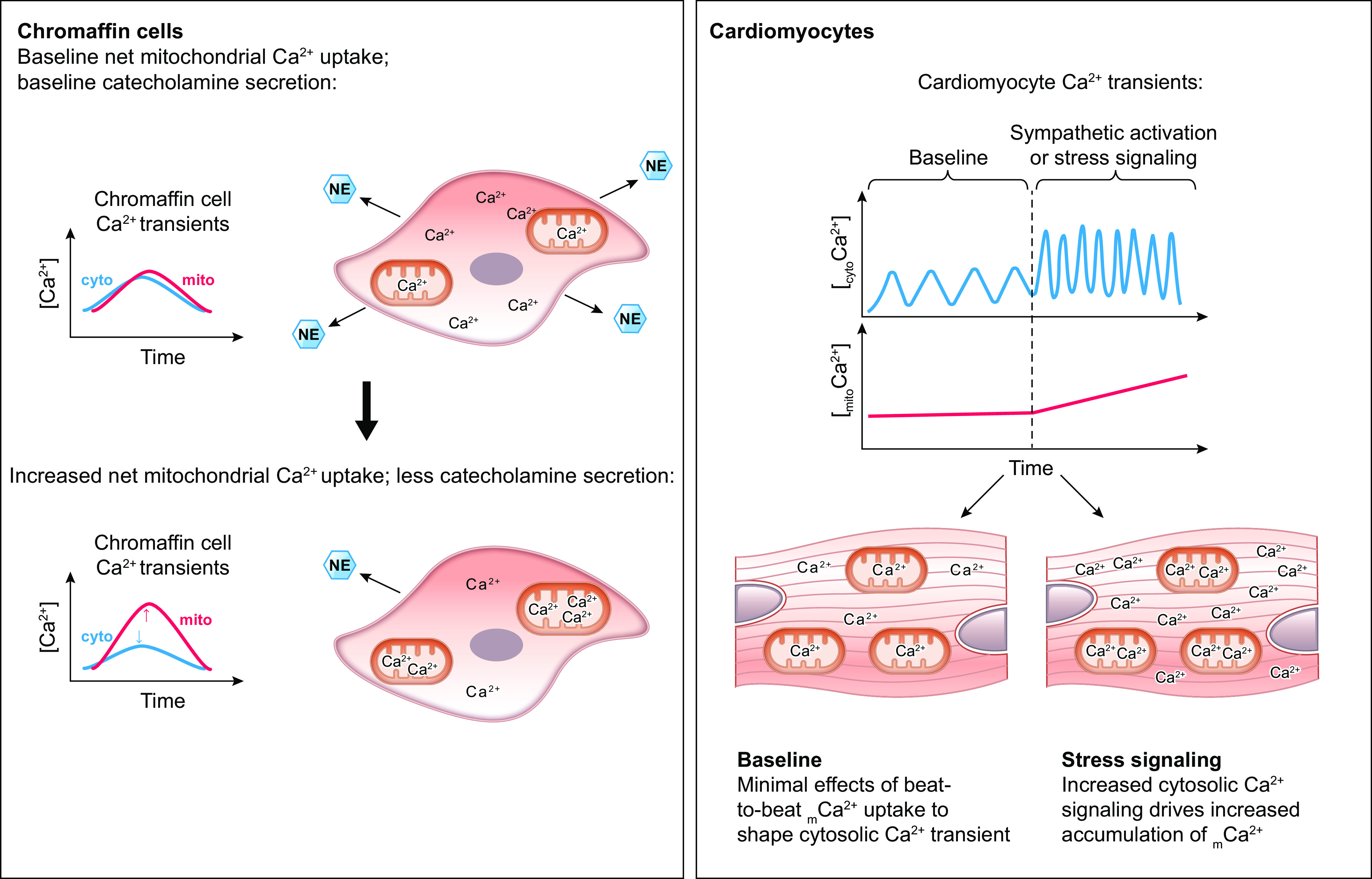
Cell-type dependent effects of mCa2+ uptake on cytosolic Ca2+ signals. Stimulation of secretory cells elicits cytosolic Ca2+ transients that are rapidly transmitted to the mitochondria, resulting in large mCa2+ transients. Modulation of net mCa2+ uptake affects the magnitude of both the cytosolic Ca2+ transient and the mitochondrial Ca2+ transient, and impacts physiologically relevant cellular functions. For example, increased net mCa2+ uptake in adrenal chromaffin cells can limit the magnitude of the cytosolic Ca2+ transient and thereby limit the amount of cytosolic Ca2+ available to stimulate secretion of catecholamines such as norepinephrine (NE). In other cell types, however, mitochondrial Ca2+ signals are not coupled as directly to each cytosolic Ca2+ transient. For example, cardiomyocytes do not generally appear to have beat-to-beat oscillations in mCa2+ content that mirror cytosolic Ca2+ transients. Rather, alterations in the amplitude and/or frequency of cytosolic Ca2+ transients drive integrated changes in mCa2+ concentration. For instance, sympathetic stimulation of cardiomyocytes increases the amplitude and frequency of cytosolic Ca2+ transients, causing a progressive increase in mCa2+ concentration over time. [Ca2+], Ca2+ concentration; [cytoCa2+], cytosolic [Ca2+]; [mitoCa2+], mitochondrial [Ca2+]; see glossary for other abbreviations.
For example, mCa2+ buffering has very little effect on the high-frequency, high-amplitude cytosolic Ca2+ transients that stimulate cardiomyocyte contraction (418). In contrast, mCa2+ uptake limits the increase in cytosolic Ca2+ concentration in presynaptic neurons that occurs during neurotransmission (84). The shaping of cytosolic Ca2+ signals via mitochondrial Ca2+ buffering regulates catecholamine secretion from chromaffin cells (419) and so has the potential to regulate physiologically relevant biological phenomena. Additional examples of how mCa2+ buffering shapes cellular function and can spatially regulate cytosolic Ca2+ signaling are discussed below.
5. SPATIAL ARRANGEMENT OF MITOCHONDRIA AND THE MITOCHONDRIAL Ca2+-HANDLING MACHINERY
Efficient mCa2+ uptake depends on the exposure of mitochondria to local concentrations of Ca2+ high enough to activate the Ca2+ uptake machinery. For acute uptake through the mtCU, this threshold is ∼400 nM Ca2+, roughly 4 times higher than the bulk resting cytosolic Ca2+ concentration (10–12, 16). The placement of mitochondria in close proximity to sites of intracellular Ca2+ release creates local microdomains where Ca2+ released from the store is concentrated in a small volume of cytosol. Ca2+ concentration in these microdomains can be 5- to 20-fold higher than Ca2+ concentration in the bulk cytosol and so is sufficient to activate low-affinity mCa2+ uptake through the mtCU (83, 270–274). Thus, the spatial organization of the mitochondria within the cell and with respect to other organelles is a major factor controlling mCa2+ flux and cellular Ca2+ homeostasis. Local Ca2+ release events can even reduce the motility of nearby mitochondria, thereby trapping them within the region of high Ca2+ concentration and further guaranteeing efficient Ca2+ transfer to the mitochondria (420). That mitochondrial localization is actively regulated by Ca2+ signals highlights just how critical the spatial organization of the mitochondria is for cellular Ca2+ handling.
5.1. Localization of Mitochondria With Respect to Cellular Domains and Other Organelles
5.1.1. Interactions with plasma membrane.
All mitochondria contact the ER, and a small portion are located in proximity to the plasma membrane. Even fewer of these peri-plasma membrane mitochondria actually contact the plasma membrane, and those that do seem to interact with the membrane via an ER stack (421). This population of mitochondria may be specifically involved in store-operated Ca2+ entry (421). As reviewed elsewhere, striated muscle contains distinct populations of subsarcolemmal and intermyofibrillar mitochondria with unique functional properties and features of Ca2+ handling. These distinctions likely relate to differences in proximity to the plasma membrane versus the SR and, respectively, exposure to Na+ and Ca2+ fluxes during the action potential versus exposure to large Ca2+ transients upon SR Ca2+ release (422, 423).
5.1.2. Interactions with the endoplasmic/sarcoplasmic reticulum.
Fluorescent proteins targeted specifically to the mitochondria or ER reveal the existence of numerous close contacts between these two compartments (273). The term “mitochondria-associated membranes (MAMs)” is used to describe regions of the ER that interact with the mitochondria. MAMs are critical sites of Ca2+ exchange between these organelles and generate local cytosolic microdomains where Ca2+ can accumulate to high enough concentrations to activate mCa2+ uptake through the mtCU. These sites of ER-mitochondria contact also have essential roles in lipid biosynthesis, regulation of mitochondrial morphology, autophagy, and apoptosis, which have been reviewed extensively elsewhere (424–427).
MAMs are maintained by protein-based tethers that hold the OMM and ER membrane at a distance of ∼10–25 nm. Changes in the spacing between these two surfaces modulates the dynamics of ER-to-mitochondria Ca2+ transfer. Narrowing of the ER-mitochondrial distance is observed under conditions that trigger apoptosis, and the expression of shortened artificial tethers to reduce this distance to 5 nm increases mCa2+ uptake and the susceptibility to permeability transition (428). These findings suggest that endogenous mechanisms can actively modify the structure of ER-mitochondrial contacts in order to perturb mCa2+ homeostasis. Mitofusin 2 is suggested as one ER-mitochondria tether based on its enrichment at ER-mitochondria junctions. Consistent with this model, knockout of mitofusin 2 in mouse embryonic fibroblasts reduces the area of interorganelle contact and interferes with propagation of Ca2+ released from the ER into the mitochondrial matrix (429). ER/SR-mitochondrial Ca2+ transfer is disrupted enough in mitofusin 2-knockout cardiomyocytes that it compromises cellular bioenergetics during electrical pacing (430). de Brito et al. (429) propose that ER-localized mitofusin 2 forms a tether by interacting with mitofusin 1 and mitofusin 2 on the OMM. This view is challenged, though, by experiments showing that mitofusin 2 knockout or knockdown increases rather than decreases juxtaposition of the ER and mitochondria and sensitizes cells to mCa2+ overload-induced cell death (431, 432). Filadi et al. (432) reason that mitofusin 2 antagonizes rather than promotes the apposition of the ER and mitochondria.
The search for alternative tethers is ongoing. Szabadkai et al. (60) report that the inositol-(1,4,5)-trisphosphate receptor (IP3R) on the ER membrane and VDAC on the IMM physically interact via the chaperone glucose-regulated protein 75 (grp75). Thus, the very proteins that mediate Ca2+ flux out of the ER and across the OMM help anchor these organelles together. Furthermore, this physical interaction can permit VDAC-dependent transport of mCa2+ across the OMM (60). Finally, the ER protein PDZD8 was recently described as another metazoan tethering protein. PDZD8 is orthologous to Mmm1, a component of the yeast ER-mitochondrial encounter structure (ERMES) (433). PDZD8 is required for the maintenance of ER-mitochondrial contacts, and PDZD8 knockout disrupts ER-to-mitochondria Ca2+ transfer (433). The tethering function of PDZD8 may have particular consequences for neuronal biology. Loss of PDZD8 in neurons and the subsequent reduction in ER-mitochondrial contacts results in loss of mitochondrial Ca2+ buffering and therefore allows greater cytosolic Ca2+ elevations in dendrites after synaptic stimulation and ER Ca2+ release (433). Thus, maintenance or disruption of the microdomain between the ER and mitochondria can have appreciable effects to shape cellular Ca2+ signaling.
5.1.3. Localization in neurons and effects at synaptic terminals.
Mitochondria are found at many presynaptic nerve terminals, where they can modulate local Ca2+ dynamics and ATP production (reviewed in Ref. 434). This population of mitochondria maintains neurotransmission during high-frequency stimulation because they provide the ATP needed to restore the readily releasable pool of synaptic vesicles (435, 436). Presynaptic mitochondria also buffer cytosolic Ca2+ and so limit the amount of cytosolic Ca2+ locally available to stimulate post-tetanic potentiation, a phenomenon in which synaptic transmission is enhanced for minutes after high-frequency stimulation (434).
5.1.4. Localization in exocrine gland acinar cells and segregation of Ca2+ signals.
A characteristic feature of the secretory cells found in exocrine glands, such as the acinar cells of the pancreas or the salivary glands, is that they are polarized, with distinct processes occurring at the apical versus basolateral domains (437). This includes compartmentalization of Ca2+ signaling at the apical domain, which allows the cell to separate Ca2+-dependent processes such as exocytosis from other processes such as transduction of extracellular signals that originate on the basolateral membrane (438). Mitochondria in pancreatic acinar cells help to segregate these cellular domains because they form a “belt” around the secretory granule area and buffer changes in cytosolic Ca2+. Consequently, Ca2+ signals that originate in the apical domain are restricted to that region, where they stimulate secretion, and do not propagate beyond the mitochondrial belt to the basolateral side of the cell (439–441).
5.2. Spatial Segregation of Ca2+ Influx and Efflux Machinery within the Mitochondria
The spatial arrangement of the proteins involved in Ca2+ transport across the OMM and IMM is another mechanism that ensures efficient mCa2+ exchange. In cardiomyocytes, contact points between the IMM and OMM align with the junctions that link the OMM and the SR (442). VDAC is enriched in these regions of the OMM and so acts as a direct conduit for Ca2+ released from the SR to gain access to the IMS and the mCa2+ uptake machinery of the IMM (442). This arrangement represents the most direct route for Ca2+ transfer from the SR to the mitochondrial matrix. In support of this model, within cardiac muscle MCU and EMRE are enriched at the regions of the IMM that contact the OMM (443). Interestingly, the proteins responsible for mCa2+ uptake and mCa2+ efflux are segregated to distinct regions of the IMM (FIGURE 7).
FIGURE 7.
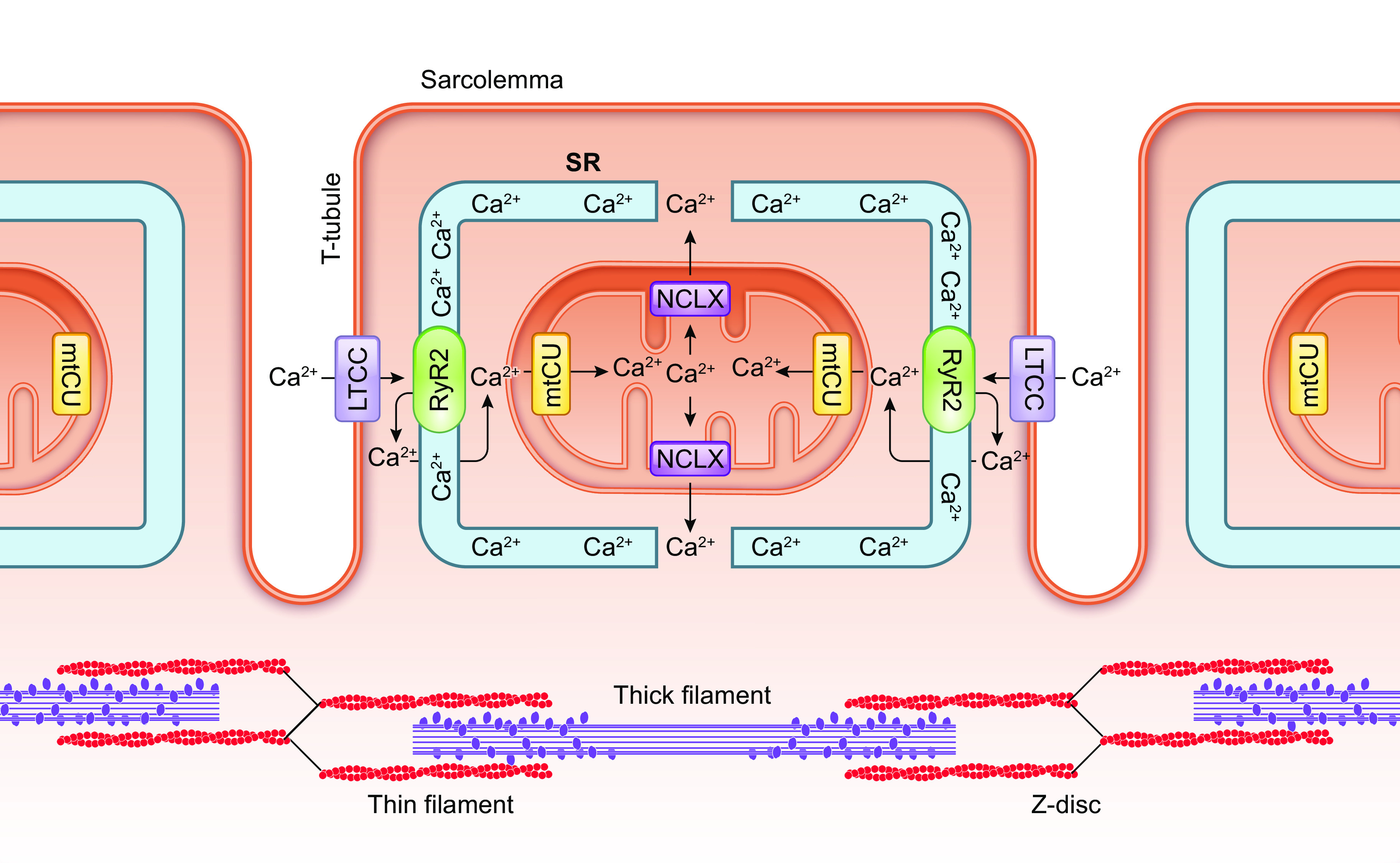
Spatial segregation of mitochondrial Ca2+ influx and efflux pathways in cardiomyocytes. In cardiac muscle, the mitochondrial calcium uniporter complex (mtCU) is found at regions of the inner mitochondrial membrane (IMM) near contacts between the outer mitochondrial membrane and Ca2+ release sites on the sarcoplasmic reticulum (SR). This arrangement enables Ca2+ released from the SR via the ryanodine receptor (RyR2) to be taken up efficiently into the mitochondrial matrix. NCLX, the principal route for mitochondrial Ca2+ efflux in cardiomyocytes, is instead distributed at regions of the IMM that are more distant from the sites of SR-mitochondria contact. This spatial separation of mitochondrial Ca2+ uptake and efflux is thought to minimize futile cycling of Ca2+ flux through the mtCU and NCLX that would otherwise depolarize mitochondrial membrane potential. See glossary for abbreviations.
Mitochondrial regions associated with the SR contain minimal NCLX and do not exhibit Na+-induced mCa2+ efflux; instead, NCLX localizes to regions of the mitochondria that are more distant from the SR (444). This organization is proposed to minimize futile cycling of mCa2+ uptake through the mtCU and immediate, subsequent mCa2+ efflux via Na+/Ca2+ exchange. Such futile Ca2+ cycling could occur if matrix Ca2+ diffuses slowly after uptake and so persists at comparatively high concentrations in the vicinity of the mtCU. If this assumption of slow diffusion of matrix Ca2+ is valid and the mtCU and NCLX were located in close proximity, futile cycles of mCa2+ uptake and efflux would ultimately run down ΔΨm because of the net charge influx through forward-mode operation of NCLX, without effectively increasing mCa2+ content (444). Recent data indicating that discrete cristae within the mitochondria may maintain distinct ΔΨm and operate independently from each other reveal the possibility that distinct regions of the IMM can be electrically insulated from other regions (445). These findings fit with the notion that mCa2+ uptake and stimulation of OXPHOS (thus contributing to ΔΨm at nearby cristae) could be spatially and functionally isolated from NCLX-dependent mCa2+ efflux, which would otherwise dissipate ΔΨm and potentially compromise ATP synthesis. Thus, the spatial segregation of mCa2+ uptake and extrusion is proposed to protect cellular bioenergetics (444). How precisely the distinct localization of the mtCU versus NCLX is maintained is an intriguing question with direct implications for cellular energy production.
6. PHYSIOLOGICAL CONSEQUENCES OF MITOCHONDRIAL Ca2+ FLUX
6.1. Influence on Metabolism
The best-appreciated physiological consequence of an acute increase in mCa2+ concentration is the rapid stimulation of mitochondrial metabolism. mCa2+ uptake is associated with an increase in respiration and ATP production (446), indicating that matrix Ca2+ stimulates oxidative phosphorylation (FIGURE 8).
FIGURE 8.
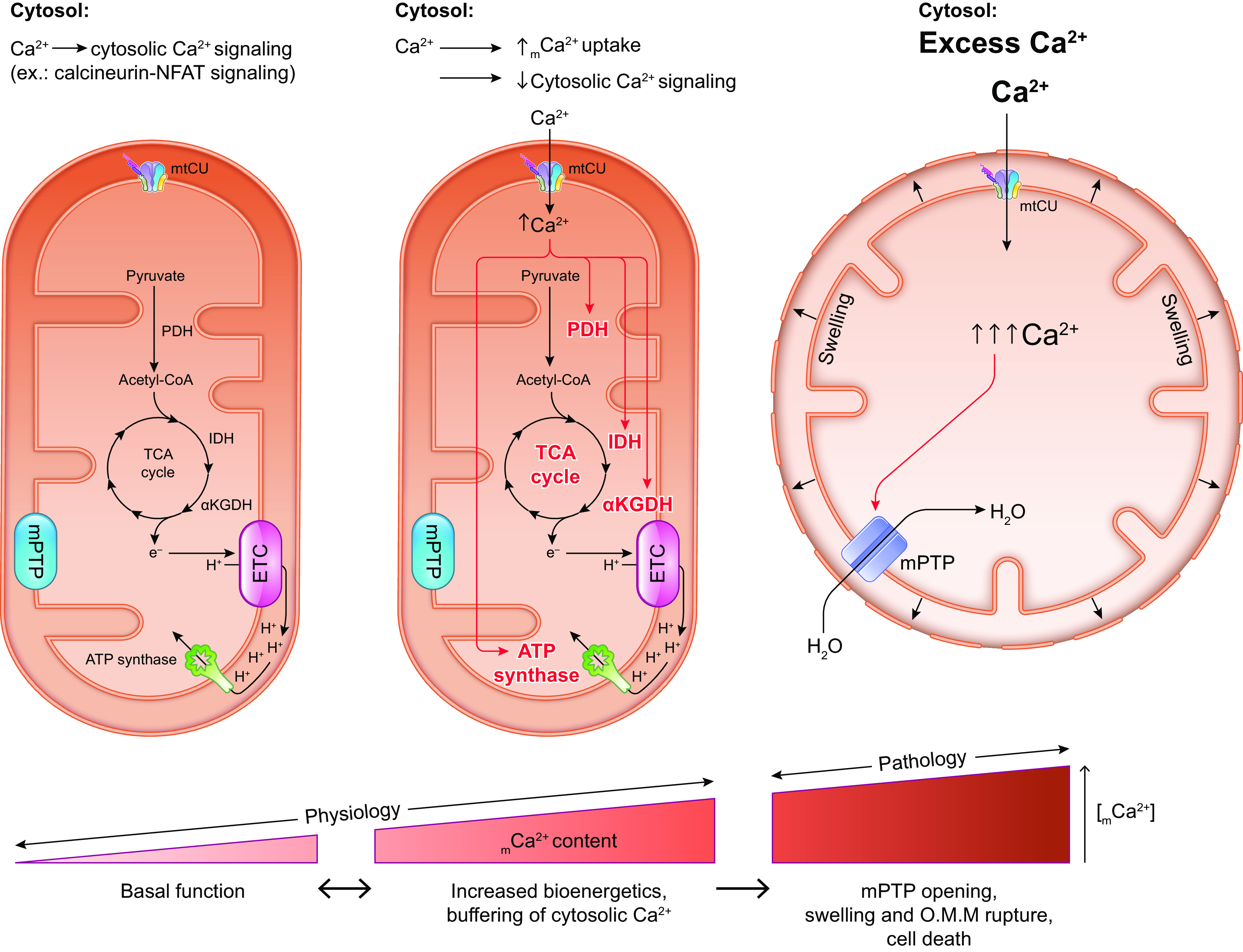
Physiological and pathological functions of acute mCa2+ uptake. Physiological cellular Ca2+ signaling simulates signal transduction pathways in the cytosol and, if cytosolic Ca2+ concentration reaches a high enough threshold, can trigger mCa2+ uptake through the mitochondrial calcium uniporter complex (mtCU). In some cell types, mitochondrial Ca2+ uptake can buffer further changes in cytosolic Ca2+ concentration and so affect Ca2+-dependent signaling throughout the cell. Increasing matrix Ca2+ concentration stimulates the activity of pyruvate dehydrogenase (PDH), isocitrate dehydrogenase (IDH), and α-ketoglutarate dehydrogenase (αKGDH) to increase TCA cycle flux and delivery of electrons to the electron transport chain to stimulate ATP production. Matrix Ca2+ may also stimulate ATP synthase directly, although this concept is controversial. Rapid mCa2+ uptake through the mtCU allows cells to couple Ca2+-dependent, energy-consuming processes in the cytosol, such as muscle contraction, with a coordinated increase in mitochondrial ATP production in order to supply the cell’s increased energetic demand. A pathological increase in cytosolic Ca2+ concentration, as occurs during ischemia-reperfusion, can drive excessive Ca2+ uptake through the mtCU, leading to activation of the mitochondrial permeability transition pore, collapse of mitochondrial membrane potential, influx of water and swelling of the matrix, and rupture of the outer mitochondrial membrane (OMM), culminating in cell death. See glossary for abbreviations.
Ca2+ exerts this effect via several distinct mechanisms: 1) Activation of pyruvate dehydrogenase through the stimulation of pyruvate dehydrogenase phosphatase, which removes inhibitory phosphorylation at S232, S293, and S300 of the PDH E1α regulatory subunit (447–449). Increased PDH activity increases pyruvate oxidation to generate acetyl-CoA, which enters the TCA cycle. This reaction also generates NADH, which can deliver electrons to the electron transfer chain (450). 2) Stimulation of the TCA cycle enzymes isocitrate dehydrogenase (IDH) and α-ketoglutarate dehydrogenase (αKGDH) (451–454). Ca2+ lowers the Km of these enzymes for their substrates and thereby enhances TCA cycle flux. Increased TCA cycle activity increases the production of reducing equivalents (NADH and FADH2) that deliver electrons to the ETC (311). 3) Stimulation of respiratory complexes I, III, and IV (455) to increase ETC activity. Whether the activity of complex V (ATP synthase) is also directly increased in response to elevated mCa2+ is debated (210, 456, 457). On an acute timescale, these effects of increased mCa2+ allow cells such as cardiomyocytes, neurons, and pancreatic β-cells to rapidly increase ATP production needed for energy-consuming processes like muscle contraction or vesicle trafficking for neurotransmission and insulin secretion (126, 127, 235, 290, 361, 458–461). mCa2+-dependent effects on mitochondrial metabolism can also persist and allow for a sustained increase in mitochondrial ATP production, thus supporting continued cellular energy consumption even after the initial rise in mCa2+ concentration subsides (462).
Since TCA cycle function is intricately linked to the metabolism of biosynthetic building blocks such as amino acids, nucleotides, and lipids, as well as signaling molecules such as ROS (reviewed in Refs. 463–465), Ca2+-dependent changes in mitochondrial metabolism can have wide-reaching effects on cellular function and growth. Consistent with this notion, ablation of mCa2+ uptake disrupts mitochondrial bioenergetics, alters pyruvate and proline metabolism, and impairs growth in T. brucei (103).
6.2. Buffering of Cytosolic Ca2+ and Modulation of Processes Regulated by It
Depending on the cell type, mCa2+ uptake can buffer changes in cytosolic Ca2+ concentration and thereby influence cellular functions and signaling cascades that respond to cytosolic Ca2+. For example, uptake of Ca2+ by mitochondria helps to maintain cellular Ca2+ signaling that is required for T-cell activation. Stimulation of T cells by antigens causes IP3-dependent Ca2+ release from the ER. Depletion of ER Ca2+ stores activates the store-operated Ca2+ release-activated Ca2+ current (ICRAC) to allow entry of Ca2+ across the plasma membrane. This replenishes the ER Ca2+ stores and allows for a sustained elevation in cytosolic Ca2+ that is required for T-cell activation and associated transcriptional responses (17, 466–468). If mitochondria are depolarized, diminishing the driving force for mCa2+ uptake, ICRAC becomes inactivated, which is likely due to a local elevation in cytosolic Ca2+ concentration in the absence of mCa2+ buffering (469). Inactivation of ICRAC impairs store-operated Ca2+ entry and consequently attenuates the broader cytosolic Ca2+ response required for the activation of the transcription factor NFAT (469). Thus, mCa2+ uptake is required to maintain sustained cytosolic Ca2+ signaling that ultimately drives T cell-mediated immune responses.
Another recent example is the control of AMPK signaling in hepatocytes. mCa2+ uptake through the mtCU minimizes cytosolic Ca2+ concentration, but, when mCa2+ uptake is disrupted, elevated cytosolic Ca2+ activates protein phosphatase-4, leading to dephosphorylation and inhibition of AMPK. Reduced AMPK signaling then impairs hepatocyte lipid metabolism (470). Thus, by altering cytosolic Ca2+, mCa2+ exchange may exert secondary effects on diverse, seemingly unrelated cellular processes.
6.3. Role in Cell Death
Excessive accumulation of Ca2+ in the mitochondrial matrix causes sustained activation of the mitochondrial permeability transition pore (mPTP), a process known as permeability transition (PT) (FIGURE 8). Opening of the mPTP results in collapse of the mitochondrial membrane potential, release of Ca2+ from the matrix, mitochondrial swelling, and rupture of the outer mitochondrial membrane. This leads to cell death via either apoptosis or necrosis, depending on the cellular ATP supply (reviewed in Refs. 150, 400–402). PT thus contributes to much of the tissue injury that occurs in pathologies marked by excessive mCa2+ uptake, such as ischemia-reperfusion and myocardial infarction. Although mCa2+ is a clear trigger for PT and mitochondrial swelling (471), whether mCa2+ directly activates the mPTP is less certain. Increased mCa2+ stimulates ROS production, which is itself a stimulus for PT (401). Increased ROS production can occur as a direct result of increased TCA cycle flux and electron transport stimulated by elevated mCa2+ (472, 473). Another idea proposes that, with continued mCa2+ uptake, excessive mCa2+ loading causes precipitation of Ca2+-phosphate complexes within the matrix, which interfere with the ETC by inhibiting complex I, disrupting electron transfer between NADH and complex I, and altering the structure of cristae where the ETC is located, ultimately increasing ROS production (474). Increased mCa2+ stimulates nitric oxide production, which inhibits complexes I and IV, thus blocking electron flow through the ETC and favoring ROS production (401). Increased ROS production due to increased mCa2+ interferes with cytochrome c’s association with cardiolipin and the IMM, further disrupting the ETC and driving even more ROS production (475). Increased mCa2+ also interacts with cardiolipin to dissociate complex II and generate a subcomplex that makes excessive ROS (476). Thus, excessive mCa2+ accumulation drives ROS production via multiple mechanisms that can contribute to PT. In turn, increased mitochondrial ROS production drives further mCa2+ accumulation by promoting leak of Ca2+ from intracellular stores such as the ER (477), setting up a vicious cycle that triggers PT via the combined effects of mCa2+ overload and oxidative stress. Breaking this Ca2+-ROS cycle via the administration of antioxidants is sufficient to prevent PT and cell death in the context of I/R (478). Likewise, VDAC1 knockdown, MCU knockdown, or Ru360 administration to minimize mCa2+ uptake prevents PT and apoptosis of cells subjected to oxidative stress (59, 479, 480). These findings underscore the relevance of the interplay between mCa2+ and ROS in permeability transition and cell death.
6.4. Role in Autophagy and Mitophagy
Autophagy is a highly conserved process whereby cells envelope organelles and other cytosolic components in a double-membraned vesicle, which then fuses with lysosomes enabling degradation of the contents (481). This catabolic process provides cells with energy when nutrients are scarce. Mitophagy is a related process allowing cells to selectively degrade damaged mitochondria and thus functions in mitochondrial quality control (482, 483). Several studies suggest that these processes are influenced by mCa2+. In fibroblast lines from patients with distinct complex I mutations, those with mutations associated with reduced mCa2+ uptake have enhanced autophagic flux and increased mitophagy (484). This is due to increased AMPK activity, which stimulates the autophagic machinery. The same relationship between MCU downregulation and increased autophagy is seen in a dog model of electrical pacing-induced heart failure and cardiac resynchronization therapy (485). Autophagy and, specifically, mitophagy are noted to increase upon MCU inhibition and to provide cardioprotection in a mouse model of pressure overload-induced heart failure (486). A link between MCU-dependent mCa2+ uptake and autophagy is also observed in neuronal cell lines treated with MPP+, an in vitro model for Parkinson’s disease that involves autophagic cell death. Here, pharmacological MCU activation or MCU overexpression reduces MPP+-induced autophagic death, whereas downregulation of MCU activates AMPK and enhances autophagy (487). These studies did not distinguish whether AMPK activity increases as a result of metabolic stress due to impaired Ca2+-dependent stimulation of mitochondrial metabolism or rather as a result of increased cytosolic Ca2+ singling, which could activate AMPK through upstream kinases such as CaMKK. However, knockout or pharmacological inhibition of IP3 receptors to limit ER-to-mitochondria Ca2+ transfer triggers energetic stress, AMPK activation, and autophagy (488). This suggests that energetic starvation related to impaired mCa2+ uptake contributes to autophagy, independent of gross changes in cytosolic Ca2+ signaling.
mCa2+ uptake is also specifically involved in mitophagy. A key trigger for mitophagy is mitochondrial depolarization associated with permeability transition (489–491), which can occur as a consequence of mCa2+ overload. MacVicar et al. (492) further suggest that ER-to-mitochondria Ca2+ transfer “primes” mitochondria for mitophagy, because knockdown of MCU prevents mitophagy that otherwise follows pharmacological mitochondrial depolarization. Thus, changes in mCa2+ handling influence autophagy and mitophagy indirectly via effects on cellular energetics, and directly by affecting the susceptibility to PT as well as the mitochondria’s response to depolarization.
6.5. Influence on Mitochondrial Signaling to Other Cellular Compartments
An emerging concept is that mitochondria actively signal to other cellular compartments (FIGURE 9).
FIGURE 9.
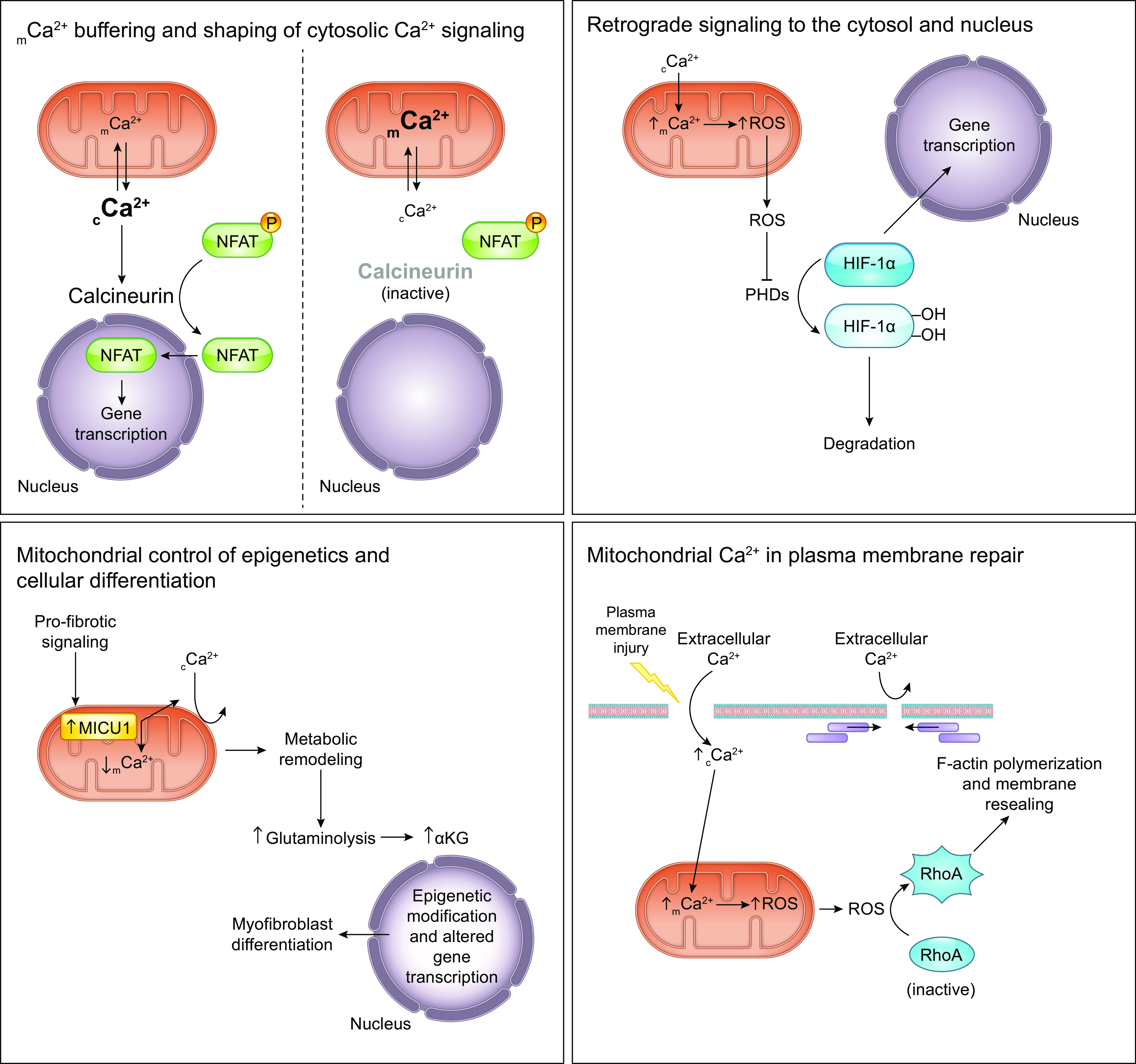
Mitochondrial Ca2+ handling as a master regulator of cellular signaling pathways. Clockwise from top left: mCa2+ buffering and shaping of cytosolic Ca2+ signaling: Net mCa2+ flux influences the amount of cytosolic Ca2+ available to participate in cytosolic signaling pathways. For instance, changes in net mCa2+ uptake can influence NFAT signaling and affect gene expression by influencing amount of cytosolic Ca2+ available to activate the Ca2+-sensitive phosphatase calcineurin. Retrograde signaling to the cytosol and nucleus: Prolyl hydroxylases (PHDs) hydroxylate the transcription factor hypoxia-inducible factor (HIF-1α) and target it for degradation. Increased net mCa2+ accumulation triggers the mitochondrial production of reactive oxygen species (ROS), which inhibit PHDs, thereby favoring HIF-1α accumulation and the transcription of its target genes. Mitochondrial Ca2+ in plasma membrane repair: Lesion of the plasma membrane allows extracellular Ca2+ to flow into the cell, elevating cytosolic Ca2+ concentration. Subsequent mCa2+ uptake drives increased mitochondrial production of ROS. ROS activates the GTPase RhoA, which then promotes F-actin polymerization to facilitate plasma membrane repair. Effective resealing of the plasma membrane completes this feedback loop by preventing further influx of extracellular Ca2+. Mitochondrial control of epigenetics and cellular differentiation: Profibrotic stimulation of fibroblasts upregulates the mtCU gatekeeper MICU1, thereby attenuating mCa2+ uptake. Altered mCa2+ handling remodels fibroblast metabolism and increases glutaminolysis to increase the cellular supply of α-ketoglutarate (αKG). αKG serves as a cofactor for histone demethylases and so modulates gene expression that ultimately causes differentiation of the fibroblasts into myofibroblasts. See glossary for abbreviations.
A classic example of retrograde signaling between the mitochondria and nucleus is that mitochondrial dysfunction triggers depolarization of ΔΨm, which favors release of mCa2+ to the cytosol. Increased cytosolic Ca2+ stimulates the phosphatase calcineurin and the kinases PKC, JNK, CamKIV, and MAPK, leading to activation of the transcription factors NFAT, NF-κB, ATF2, CEBP/δ, CREB, Egr-1, and CHOP (25). This allows changes in mitochondrial function to alter nuclear gene transcription. Another example of retrograde signaling that can be regulated by mCa2+ is mitochondrial ROS production, which activates the transcription factor hypoxia-inducible factor (HIF-1) (493) and can affect cell differentiation and proliferation (494). TCA cycle intermediates such as acetyl-CoA, succinate, fumarate, and α-ketoglutarate can function as signaling molecules by affecting the activity of proteins such as prolyl hydroxylases and Jumonji domain-containing histone demethylase (reviewed in Refs. 495–497). Notably, acetyl-CoA and succinyl-CoA also act as substrates for the posttranslational modification of lysine residues (498–500); a familiar example is histone acetylation. Mitochondrial metabolism influences levels of S-adenosyl methionine, which is required for DNA methylation (501, 502). By altering the availability of such compounds, changes in mitochondrial metabolism can thus affect epigenetic marks and patterns of gene expression. That altered mCa2+ homeostasis is sufficient to influence this kind of signaling is supported by the observation that MICU1 upregulation and diminished mCa2+ uptake drives metabolic reprogramming to control epigenetic signals critical for myofibroblast differentiation (165, 211).
Several additional examples illustrate how changes in mCa2+ handling affect signaling to other parts of the cell. In lung carcinoma cells, depletion of mitochondrial DNA leads to loss of mitochondrial membrane potential, diminishing the driving force for mCa2+ uptake and so elevating cytosolic Ca2+. This in turn increases the expression of genes involved in tumor invasion and increases invasive behavior, indicating that altered mCa2+ handling and retrograde signaling contribute to cancer metastasis (503). In plants, the upregulation of genes involved in adaptive responses to salt stress is blocked by MCU inhibition, suggesting that increased mCa2+ is a key step in this signaling pathway (504). Together these studies reveal that both increased and diminished mCa2+ uptake affect retrograde signaling to regulate gene expression. Finally, in C. elegans mCa2+ uptake through MCU stimulates superoxide production near skin wound sites; subsequent redox modulation of RHO-1 GTPase then facilitates actin-dependent wound closure (505). A similar phenomenon occurs in mammals to allow resealing of the sarcolemma in damaged skeletal muscle cells, although in this instance mCa2+-driven ROS production instead activates RhoA GTPase to promote F-actin accumulation near the site of the membrane lesion and facilitate membrane resealing (208). mCa2+ uptake following sarcolemmal injury is impaired in MICU1-deficient muscle cells, leading to impaired membrane repair (207). Thus, defective mCa2+-dependent signaling and plasma membrane sealing likely contribute to ongoing muscle damage and exaggerated muscle weakness in patients with MICU1 mutations (199).
7. MITOCHONDRIAL Ca2+ FLUX IN DISEASE
As noted above, several human genetic diseases are caused by primary disruption of mCa2+-handling proteins, such as myopathy (MICU1), a neurodevelopmental disorder (MICU2), and Wolf–Hirschhorn syndrome (LETM1) (TABLE 1). Secondary alterations in the expression of nearly all the proteins involved in mCa2+ exchange are reported in the context of at least one acquired human disease. Here, we discuss evidence for perturbations in mCa2+ homeostasis as a driving factor behind these pathologies.
7.1. Cardiac Ischemia-Reperfusion Injury and Heart Failure
Acute mitochondrial Ca2+ overload resulting in permeability transition is a major contributor to cardiomyocyte death that results from myocardial infarction and I/R injury (reviewed in Refs. 400, 401) (FIGURE 10).
FIGURE 10.
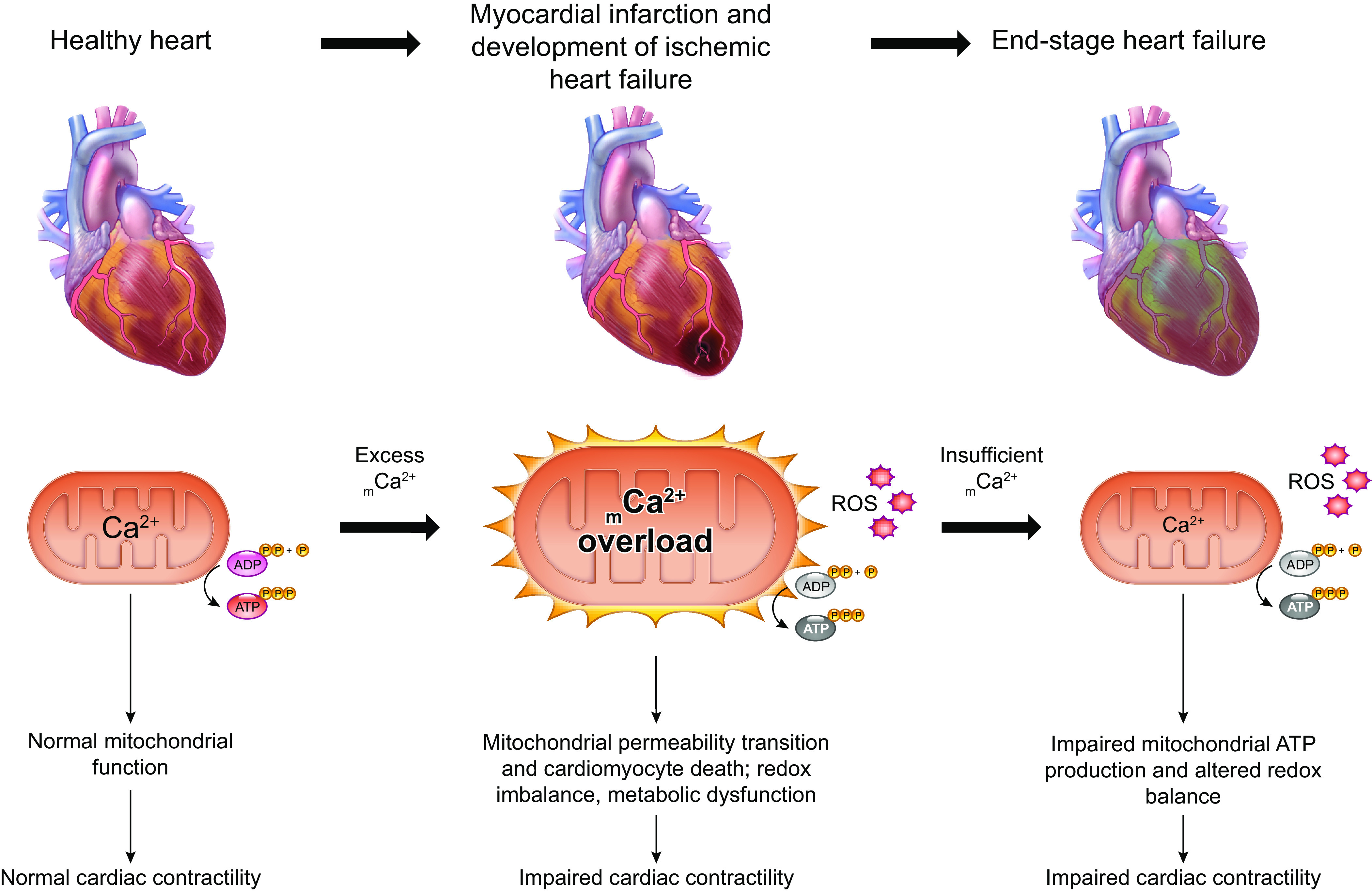
Either too much or too little mCa2+ can contribute to disease. mCa2+ has pleiotropic roles in diseases such as cancer, neurodegeneration, and heart failure that relate to its fundamental functions in supporting mitochondrial bioenergetics, influencing mitochondrial signaling, and cell death. For example, acute mCa2+ overload during cardiac ischemia-reperfusion injury causes excess reactive oxygen species (ROS) production and elicits mitochondrial permeability transition, which compromises mitochondrial ATP production and triggers cardiomyocyte death. These immediate effects of mCa2+ overload impair the heart’s mechanical pump function and over time cause the heart to fail. In end-stage heart failure, however, mCa2+ content may be reduced below normal levels because of excessive mitochondrial Na+/Ca2+ exchange. Here, diminished mCa2+-dependent stimulation of TCA cycle flux can disrupt mitochondrial redox balance, favor ROS production, and impair mitochondrial ATP production. The diminished ATP supply and excessive oxidative stress drive further impairment of the heart’s contractile function. See glossary for abbreviations.
Research in mouse models shows that acute deletion of MCU from adult cardiomyocytes protects the heart from I/R injury and cell death (126, 127). In a similar fashion, overexpression of NCLX to enhance cardiomyocyte mCa2+ efflux, or upregulation of MCUB to attenuate mCa2+ uptake, can also protect against I/R injury and, in the case of NCLX upregulation, is sufficient to preserve contractile function even after permanent myocardial infarction (166, 242). The heart has some endogenous capacity to upregulate such adaptive proteins in order to minimize I/R injury, as both NCLX and MCUB gene expression are increased after ischemic insult to the heart (166, 242). Interestingly, MICU1 transcripts are also elevated in end-stage ischemic heart failure (166). In the short term (up to 24 h of reperfusion), MICU1’s gating of the mtCU has a protective role in I/R, as knockdown of MICU1 exaggerates I/R-induced mCa2+ overload, increases infarct size and apoptosis, and worsens contractile function (209). However, whether MICU1 upregulation in end-stage failure is an ongoing mechanism to minimize mCa2+ overload, or rather is an adaptation to facilitate cooperative activation of mCa2+ uptake in support of cardiac energetics, is less clear.
To this point, beyond its initial role in triggering cardiomyocyte death during I/R, excessive mCa2+ uptake through the mtCU has additional consequences that lead to further deterioration of contractile function over time after I/R injury. Increased mitochondrial ROS production after myocardial infarction oxidizes critical residues on the cardiac RyR, leading to SR Ca2+ leak that further exacerbates mCa2+ loading and ROS production (506). This interorganelle cross talk can set up a cycle of positive feedback favoring continued SR Ca2+ leak, mitochondrial dysfunction, and further ROS production that contributes to the progressive decline in contractile function in the weeks following infarction (506). Thus, therapeutic strategies that limit excessive, pathological mCa2+ uptake should be beneficial both during I/R and during the course of cardiac decompensation that occurs afterward.
Current understanding of the role of excessive mCa2+ uptake in chronic, nonischemic heart failure that develops in response to hemodynamic stresses such as hypertension or aortic stenosis is more complicated. During I/R, cardiomyocytes are subjected to acute elevations in cytosolic Ca2+ and subsequent mCa2+ overload. In contrast, in conditions of chronic hemodynamic stress, cardiomyocytes are subjected to a sustained increase in cytosolic Ca2+ signals that may be of lower magnitude than the massive cytosolic Ca2+ stress experienced in I/R. Thus, blockade of acute mCa2+ uptake through the mtCU may be insufficient to prevent an increase in mCa2+ content in this context, because there may be ongoing, increased mCa2+ entry via alternative Ca2+ transporters that can be activated at lower Ca2+ thresholds than the mtCU. This topic has recently been reviewed elsewhere (121).
Finally, whereas excessive mCa2+ loading drives cardiac dysfunction in the early stages of ischemic heart failure, an interesting hypothesis is that the end-stage failing heart is instead characterized by insufficient mCa2+ content that is inadequate to meet bioenergetic needs and maintain mitochondrial redox balance (FIGURE 10). In the failing heart, cytosolic Na+ concentration is increased by ∼3–6 mM over its normal concentration of ∼7–8 mM (21, 507). This increases the driving force for Na+ entry into the mitochondria (matrix Na+ ∼6 mM) and so stimulates Na+/Ca2+ exchange through NCLX, which limits the accumulation of mCa2+ (21, 508, 509). This effect is sufficient to impair TCA cycle NADH production, particularly under conditions of increased cardiac workload (508, 509). By limiting NADH production, enhanced mCa2+ extrusion may have secondary effects to limit ETC flux and mitochondrial ATP production, further limiting contractility of the failing heart. In agreement with this concept, compensatory changes in mCa2+-handling proteins are observed as an adaptive response in a mouse model of mitochondrial cardiomyopathy. Mice with cardiac-specific ablation of the mitochondrial transcription factor TFAM develop dilated cardiomyopathy. In these animals, increased MCU expression enhances mCa2+ uptake and decreased NCLX expression minimizes mCa2+ efflux in order to increase the sensitivity of mitochondrial metabolism to increases in cytosolic Ca2+ concentration, ultimately helping to maintain cardiac bioenergetics (510). The recent report that increasing cardiac MCU expression in a guinea pig model of severe heart failure protects contractile function strengthens this notion (511, 512). Together, these findings indicate that promoting mCa2+ accumulation, rather than inhibiting it, may be an appropriate therapeutic goal in a heart that is already failing.
7.2. Stroke
I/R injury constitutes a fundamental mechanism that damages the brain upon recanalization of an occluded artery following ischemic stroke (513). Just like in cardiac I/R injury, excessive uptake of Ca2+ through the mtCU is a critical factor in the ensuing damage to brain tissue. In rats subjected to middle cerebral artery occlusion and reperfusion, I/R causes progressive inhibition of the ETC, impairment of ATP production, dissipation of ΔΨm, and increased ROS production, leading to neuronal damage and apoptosis (514). Pharmacological MCU inhibition prior to ischemia attenuates these changes and reduces brain infarct volume, whereas pharmacological MCU activation exacerbates brain injury (514). Conditional knockout of MCU in adult neurons similarly protects mice from hypoxic/ischemic brain injury and minimizes neuron loss and mitochondrial damage. Neuronal MCU ablation also attenuates sensorimotor deficits associated with hypoxic/ischemic injury (515), suggesting that this therapeutic strategy improves functional outcomes at the level of the intact organism. These findings together provide therapeutic rationale for inhibiting mCa2+ uptake in stroke. Inhibition of mCa2+ uptake also attenuates brain damage following acute traumatic brain injury, which like stroke is characterized by excessive ROS production, impaired mitochondrial energetics, and apoptosis (516).
7.3. Neurodegenerative Disease
Altered mitochondrial function leading to impaired oxidative phosphorylation, oxidative stress, and apoptosis are common features of neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), and Huntington’s disease (HD) (517, 518). mCa2+ overload is a common feature of neurodegenerative diseases and may impair cellular energetics by inducing oxidative stress and by triggering mitochondrial permeability transition, leading to collapse of ΔΨm that compromises ATP production (519, 520). In some cases, though, diminished mCa2+ accumulation is implicated in neurodegeneration.
7.3.1. Alzheimer’s disease.
Alzheimer’s disease (AD) is a form of dementia characterized by neuronal dysfunction and cell death, and has hallmark pathological features including the deposition of amyloid-β plaques and neurofibrillary tangles comprised of tau protein (521). Increased connection of the ER and mitochondria is noted in cell models of AD, raising the possibility of increased communication between these organelles (522). Fitting with this view, overexpression of presenilin 2 mutants linked to familial AD increases Ca2+ transfer from the ER to the mitochondria (523). Several reports demonstrate that this phenomenon results from effects of mutant presenilin to increase ER Ca2+ release through the IP3R, thereby enhancing mCa2+ uptake that in turn increases the likelihood of mCa2+ overload and permeability transition (524–527). That disruption of a single Itpr1 allele and subsequent reduction of IP3R1 expression by ∼50% protects against AD pathology and ameliorates memory impairments in the 3xTg mouse model of AD (528) supports a causative role for elevated cytosolic Ca2+ signaling in this disease. Increased phosphorylation and altered oxidative modification of RyR2 in AD neurons similarly favor excessive ER Ca2+ leak, and such altered RyR2 activity has been implicated in cognitive dysfunction (529). Amyloid-β oligomers can also stimulate ER Ca2+ release, leading to mCa2+ overload and neuronal death (530, 531). These findings are consistent with the observation that senile plaques impair neuronal Ca2+ homeostasis in the APP mouse model of AD (532) and that the presence of neurofibrillary tangles is associated with elevated iCa2+ concentration in neurons (533, 534). These and numerous other observations beyond the scope of this discussion have collectively led to the formulation of a “Ca2+ hypothesis of AD,” which posits that dysregulated cellular Ca2+ handling drives AD pathogenesis (535). The reader is directed to several comprehensive reviews (535–537) for an in-depth examination of data in support of this hypothesis.
Altered mCa2+ homeostasis resulting directly from either aberrant cytosolic Ca2+ signaling or altered expression or function of mCa2+ handling proteins is emerging as a central mechanism behind neuronal dysfunction in AD (reviewed in Ref. 538). For example, the presence of misfolded or mutated tau protein is sufficient to inhibit NCLX activity in neurons and astrocytes, and thereby increases their susceptibility to Ca2+-induced death (539). Our laboratory’s findings of reduced NCLX expression in AD brains, and the ability for NCLX overexpression to rescue numerous AD phenotypes both in vitro and in vivo, further support the notion that increased mCa2+ load drives AD progression (323). However, one recent report suggests that, in aged neurons, amyloid-β oligomers still increase ER-mitochondria contacts but surprisingly inhibit ER-to mitochondria Ca2+ transfer. Impaired mCa2+ uptake has direct consequences to limit TCA cycle flux and thus can alter mitochondrial redox balance, contributing to excessive ROS accumulation and apoptosis (540). This work complicates our understanding of the role of mCa2+ in AD and suggests that treatments aimed at limiting versus increasing mCa2+ content may have to be matched to the particular stage of the disease.
7.3.2. Parkinson’s disease.
Parkinson’s disease (PD) is caused by death of dopaminergic neurons in the substantia nigra pars compacta and is characterized by motor symptoms including bradykinesia, tremor, muscle rigidity, and gait impairments (541). In models of PD caused by mutant α-synuclein, excessive mCa2+ uptake causes mitochondrial ROS production, collapse of ΔΨm, and impaired ATP production (542, 543). Loss of function mutations in PTEN-induced kinase 1 (PINK1) also cause PD and are associated with mCa2+ overload. PINK1 deficiency impairs NCLX activity and elicits mCa2+ overload in dopaminergic neurons (328, 544). PKA-dependent phosphorylation of NCLX is able to restore mCa2+ extrusion in these cells, suggesting a therapeutic avenue to prevent mCa2+ overload in this setting (328). Likewise, genetic disruption or pharmacological inactivation of MCU improves mitochondrial bioenergetics and prevents the death of dopaminergic neurons in pink1-mutant zebrafish (545). The E3 ubiquitin ligase Parkin promotes the degradation of MICU1 protein, and Parkin mutations cause familial PD. Thus, Matteucci et al. (219) propose that altered proteostasis of MICU1 contributes to altered mCa2+ handing in PD. Finally, expression of mutant forms of leucine-rich repeat kinase 2 (LRKK2) associated with PD increase the transcription of MCU and MICU2 and increase mCa2+ uptake in mouse cortical neurons and PD patient fibroblasts, making them susceptible to injury and neurite and dendrite shortening (118). Impaired NCLX activity also contributes to mCa2+ overload in LRKK2-deficient neurons (324). mCa2+ overload and associated deleterious effects are attenuated by pharmacological MCU inhibition, knockdown of MCU, expression of the constitutively active phosphomimetic S258D NCLX mutant, or pharmacological activation of PKA to promote stimulatory NCLX phosphorylation (118, 324). These results support a causative role for both increased mCa2+ uptake and diminished mCa2+ efflux in PD-associated neurodegeneration.
7.3.3. Amyotrophic lateral sclerosis.
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disorder characterized by muscle weakness, muscle atrophy, and eventual paralysis due to the degeneration of motor neurons in the brain, brain stem, and spinal cord (546). Impaired neuronal mCa2+ uptake is noted in cell and mouse models of ALS and is associated with impaired complex I activity, depolarized ΔΨm, and impaired ATP synthesis (547–549). Likewise, induced pluripotent stem cell-derived motor neurons from ALS patients with C9ORF72 or TARDBP mutations exhibit impaired mCa2+ buffering. As a result, cytosolic Ca2+ concentration becomes abnormally elevated during glutamate stimulation, causing excitotoxity (550). These studies suggest that impaired mCa2+ uptake contributes to motor neuron death in ALS. In agreement with this notion, MCU is downregulated in motor neurons from presymptomatic and symptomatic ALS model mice (551). However, motor neurons that still remain at the end stage of ALS show upregulation of MCU, MICU1, and LETM1, suggesting that neurons with the highest capacity for mCa2+ uptake or overall mCa2+ buffering may be the most resistant to death (552). This implies that upregulation of mCa2+ levels could be protective in ALS. Fitting with this view, inhibition of mitochondrial Na+/Ca2+ exchange with the NCLX inhibitor GCP-37157 prevents kainate-induced excitotoxicity in a mouse motor neuron model of ALS (553). Conflicting reports, though, indicate instead that, in embryonic motor neurons from ALS mice, MCU is upregulated and pharmacological suppression of MCU is protective against excitotoxicity (551). Thus, like in AD, properties of mCa2+ exchange may change throughout the progression of ALS. Therapeutic goals centering on control of mCa2+ homeostasis may therefore need to be tailored to distinct stages of the disease.
7.3.4. Huntington’s disease.
Huntington’s disease (HD) results from the expansion of a poly-glutamine tract in the huntingtin gene (517). Acute mCa2+ uptake is impaired in HD (554); however, the susceptibility to Ca2+-induced depolarization and permeability transition is increased in HD mitochondria (555, 556). These changes in Ca2+ handling precede the development of pathology and behavioral abnormalities in HD mutant mice (554), but whether they indeed contribute to HD progression remains to be tested.
7.4. Diabetes and Obesity
Mitochondrial Ca2+ exchange has direct roles to regulate pancreatic insulin secretion by supporting mitochondrial ATP production and shaping cytosolic Ca2+ signals required for this process (290, 313, 361, 458, 460, 461). Therefore, alterations in mCa2+ uptake and efflux have the potential to influence whole body glucose homeostasis. Mutations in mCa2+ handling genes have not been causatively linked to either type 1 or type 2 diabetes mellitus. However, some reports suggest that diabetes and obesity are associated with changes in mCa2+ handling proteins that can contribute to the characteristic sequelae of these conditions.
Chronically increased circulating glucose in uncontrolled diabetes impairs pancreatic β-cell insulin secretion (557), further disrupting glucose homeostasis. Chronic exposure of human pancreatic islets to high glucose in vitro upregulates EMRE, downregulates MICU3, and upregulates NCLX (558), indicating that impaired insulin secretion under chronic glucose stress may result from remodeling of mCa2+ exchange. Likewise, changes in mCa2+ handling proteins are observed in multiple tissues that show dysfunction in diabetes. MCU protein expression and mCa2+ content are reduced in cardiomyocytes exposed to high glucose (559). Similarly, MCU and EMRE expression are decreased and MCUB expression is increased in the hearts of mice with streptozotocin-induced type 1 diabetes, resulting in impaired mCa2+ uptake, compromised oxidative metabolism, and impaired cardiomyocyte contractility (560). Viral overexpression of MCU corrects these defects. At the same time, impaired mCa2+ uptake due to downregulation of MICU1 is implicated in contractile dysfunction, cardiac hypertrophy, and fibrosis in the db/db model of type 2 diabetes (215). This study indicates that diminished mCa2+ content causes myocardial apoptosis because it limits NAD(P)H production, thereby limiting mitochondrial antioxidant potential and increasing mitochondrial ROS signaling. These findings implicate impaired mCa2+ uptake in diabetic cardiomyopathy. Downregulation of MICU1 is also reported in a mouse model of high-fat diet-induced obesity, but in this instance it instead leads to increased mCa2+ accumulation and associated pathological cardiac remodeling (216).The discrepancies between these studies of diabetes versus obesity on the effect of MICU1 downregulation on mCa2+ content likely reflect the biphasic nature of MICU1’s regulation of the mtCU.
Finally, hyperglycemia upregulates MCU and MCUR1 and increases mCa2+ content in endothelial cells. These changes contribute to increased ROS production and apoptosis, suggesting that increased mCa2+ uptake plays a role in endothelial dysfunction in diabetes (561). mCa2+ uptake increases in the liver of rats with streptozotocin-induced type 1 diabetes, although in this case enhanced mCa2+ results from downregulation of MCUB (243). Collectively, these studies support the notion that modulation of mtCU composition in response to metabolic dysregulation plays a role in pathological changes that commonly present alongside diabetes and obesity.
7.5. Cancer
Cancer is characterized by metabolic reprogramming including a shift to aerobic glycolysis (the “Warburg effect”) and associated phenotypic changes that allow unrestricted cell proliferation, resistance to cell death, and the spread of cancerous cells throughout the body. Given the central role of mitochondria in providing molecular building blocks for anabolism, modulating cellular Ca2+ homeostasis and cell death, and coordinating changes in nuclear gene transcription, increasing research is directed at understanding the role of mitochondrial biology in cancer (reviewed in Refs. 562, 563). Alterations in Ca2+ flux can affect all of these mitochondrial functions, and, indeed, growing evidence indicates that the mitochondrial Ca2+-handling machinery and mCa2+ homeostasis are altered in diverse forms of cancer.
MCU expression is increased in some breast cancers and positively correlates with tumor size and infiltration (564, 565). This may result from downregulation of miR-340, which normally inhibits MCU expression (141). Upregulation of MCU promotes metabolic adaptations that favor prosurvival signaling (564) and metastasis (141). For example, increased mCa2+ uptake through MCU increases mitochondrial ROS production, leading to induction of HIF-1α (565). Induction of this same pathway occurs in colorectal cancer as a result of NCLX downregulation and mCa2+ overload (325). RIPK1 is overexpressed in colon cancer, binds to MCU, and stimulates mCa2+ uptake to promote cancer growth and oncogenesis (566). Thus, procancer signaling also promotes mCa2+ uptake, independent of changes in MCU expression. Finally, silencing of MCU in colon cancer due to increased expression of miR-25 is associated with increased resistance to apoptosis (139). This effect on MCU still favors tumor survival, albeit by a distinct mechanism that prevents lethal mCa2+ overload.
In pancreatic cancer, downregulation of histidine triad nucleotide-binding protein (HINT2), a protein that sensitizes cells to apoptosis, is correlated with poor prognosis. Restoration of HINT2 expression increases expression of EMRE and downregulates the mtCU gatekeepers MICU1 and MICU2, and resensitizes cells to apoptosis by favoring mCa2+ overload (567). These data suggest that modulation of regulatory mtCU components to limit lethal mCa2+ accumulation is one mechanism that cancers cells employ to avoid apoptosis. Fitting with this view, EMRE expression is decreased in pancreatic adenocarcinoma, and expression of EMRE is positively correlated with better prognosis in this type of cancer. This protective effect of EMRE is related to increased cell death signaling, which likely results from a higher propensity for mCa2+ overload (568). Increased MICU1 expression in ovarian cancer, resulting from reduced expression of miR-195, can limit mCa2+ uptake and so prevent apoptosis to enable tumor cell survival. Increased MICU1 expression in this setting also stimulates aerobic glycolysis and associated metabolic reprogramming that drives cell growth, migration, and invasion (214, 569). Likewise, increased expression of the ribosomal protein S3 in melanoma and enhancer of zeste homolog 2 in squamous cell carcinoma can drive MICU1 upregulation, and so increase resistance to cell death (570, 571). In contrast, other studies indicate that increased expression of mtCU regulators that promote rather than oppose mCa2+ uptake favor cancer progression. Upregulation of MCUR1 in hepatocellular carcinoma promotes mCa2+ uptake and drives mitochondrial ROS signaling that leads to degradation of p53 and disruption of apoptotic pathways (572), and to Notch signaling that stimulates epithelial-mesenchymal transition and metastasis (254).
These diverse pro- and anticancer effects of the expression of mCa2+-handling genes reflect the wide range of consequences of mCa2+ uptake, from the more physiological range of mCa2+ concentrations that regulate metabolism and signaling up to toxic levels of mCa2+ that elicit permeability transition and cell death (FIGURE 8). The conclusion that altering mCa2+ flux may therefore promote or inhibit cancer cell growth and survival, depending on the context, highlights the challenges in targeting mCa2+ exchange as a generalizable therapeutic approach for cancer.
8. CONCLUSIONS AND FUTURE DIRECTIONS
8.1. The Case for Modulating Mitochondrial Ca2+ Flux as a Therapy for Disease
Mitochondrial Ca2+ exchange has emerged as a fundamental cellular process that, when perturbed, can initiate disease or help to drive disease progression. Since 2010, the genetic identification of many of the proteins that mediate mCa2+ uptake and mCa2+ efflux has greatly advanced our understanding of how mitochondrial Ca2+ exchange operates on a molecular level. It has also enabled the generation of genetic animal models that have been used to understand the cellular and molecular mechanisms by which an imbalance between mCa2+ uptake and efflux impairs cellular, tissue, and whole organism function. Increasing evidence suggests that mCa2+ homeostasis is indeed disrupted both in response to acute pathological stress such as ischemia-reperfusion and as a secondary consequence of chronic acquired diseases such as neurodegeneration and cancer. The next challenge is to understand 1) whether therapeutic manipulation of mCa2+ exchange is sufficient to alter the course of these diseases and 2) whether the appropriate goal in each case should be to increase or decrease net mCa2+ accumulation. Research into cardiac I/R injury in genetic animal models with knockout or overexpression of mCa2+ exchangers offers powerful proof-of-concept evidence that modifying mCa2+ flux is indeed an effective strategy to protect the heart from ischemic damage. Recent studies in mouse models of Alzheimer’s disease likewise argue for the utility of this approach in slowing the progression of neurodegeneration. More examples of how modifying mCa2+ exchange in vivo improves or exacerbates disease are sure to follow. Future research should focus on the development of therapies that can modulate mCa2+ exchange specifically in those tissues affected by a given disease. More research is also needed to understand how mCa2+ handling may change and influence pathology via distinct mechanisms throughout different stages of chronic disease. This would provide needed insight into how therapeutic strategies for a given patient may need to be altered over time. Fulfilling these two goals will bring us closer to translating our fundamental understanding of mCa2+ exchange into clinically useful approaches for the treatment of human disease.
8.2. Unanswered Questions and Future Directions
Several major unanswered questions regarding the basic molecular biology of mCa2+ exchange remain and are directly relevant to the development of therapeutic approaches targeting mCa2+ flux. First, have we identified all of the relevant players in mCa2+ exchange? Our incomplete understanding of the molecular composition of the mPTP is one example of this problem, with relevance for both physiological mCa2+ efflux and cellular responses to mCa2+ overload. Likewise, do any additional proteins besides those discussed above contribute to MCU-independent mCa2+ uptake? Second, do we fully understand the function of the mCa2+-handling proteins we have already identified? For example, might MCUB, when paired with the appropriate regulatory subunits, have some capacity to conduct Ca2+? And how do we appropriately distinguish the mCa2+-dependent versus mCa2+-independent effects of a protein like MICU1, which has additional cellular functions independent of the mtCU? Third, how are the composition and stoichiometry of the mitochondrial calcium uniporter complex maintained? Is mtCU protein composition a direct reflection of gene expression of the various mtCU components, or do active mechanisms select particular subunits such as MCUB for incorporation into or exclusion from the complex? Fourth, how is the molecular function of each protein involved in mCa2+ exchange regulated? This is a particularly relevant translational question for NCLX, since increasing NCLX activity is protective in several mouse models of human disease but no reliable pharmacological activators for NCLX yet exist. And finally, what are the compensatory mechanisms that eventually enable tissues to metabolically adapt to the loss of mtCU function? How might we exploit these pathways therapeutically to prevent deleterious mCa2+ overload, while at the same time preserving mitochondrial bioenergetics? Resolving these fundamental questions regarding mCa2+ exchange will help us to attain the therapeutic objectives outlined above.
GLOSSARY
- AA
Amino acid
- AD
Alzheimer’s disease
- ALS
Amyotrophic lateral sclerosis
- Ca2+
Calcium
- COX
Cytochrome-c oxidase
- CypD
Cyclophilin D
- EMRE
Essential MCU regulator
- ER
Endoplasmic reticulum
- ETC
Electron transport chain
- H+
Proton
- HD
Huntington’s disease
- iCa2+
Intracellular calcium
- IDH
Isocitrate dehydrogenase
- IMM
Inner mitochondrial membrane
- IMS
Intermembrane space
- IP3
Inositol-(1,4,5)-trisphosphate
- IP3R
Inositol-(1,4,5)-trisphosphate receptor
- I/R
Ischemia-reperfusion
- K+
Potassium
- KO
Knockout
- LETM1
Leucine zipper and EF-hand containing transmembrane protein 1
- Li+
Lithium
- mCa2+
Mitochondrial Ca2+
- MCU
Mitochondrial calcium uniporter
- MCUB
Mitochondrial calcium uniporter dominant-negative beta subunit
- MCUR1
Mitochondrial calcium uniporter regulator 1
- MEF
Mouse embryonic fibroblast
- Mg2+
Magnesium
- mH+
Mitochondrial H+
- MICU1
Mitochondrial calcium uptake protein 1
- MICU2
Mitochondrial calcium uptake protein 2
- MICU3
Mitochondrial calcium uptake protein 3
- Mn2+
Manganese
- mPTP
Mitochondrial permeability transition pore
- mRyR
Mitochondrial ryanodine receptor
- mtCU:
Mitochondrial calcium uniporter channel
- Na+
Sodium
- NCLX
Mitochondrial Na+/Ca2+,Li+ exchanger
- OCR
Oxygen consumption rate
- OMM
Outer mitochondrial membrane
- OXPHOS
Oxidative phosphorylation
- PD
Parkinson’s disease
- PDH
Pyruvate dehydrogenase
- PT
Permeability transition
- RaM
Rapid mode of Ca2+ uptake
- ROS
Reactive oxygen species
- RyR
Ryanodine receptor
- SERCA
Sarco(endo)plasmic reticulum Ca2+-ATPase
- SLC25A23
Mitochondrial ATP-magnesium/phosphate carrier
- SR
Sarcoplasmic reticulum
- TCA
Tricarboxylic acid
- VDAC
Voltage-dependent anion channel
- WHS
Wolf–Hirschhorn syndrome
- WT
Wild type
- αKGDH
α-Ketoglutarate dehydrogenase
- ΔΨm
Mitochondrial membrane potential
GRANTS
This work was supported by National Institutes of Health (T32HL091804 to J.F.G.; F32HL151146 to J.F.G.; P01HL147841, R01HL142271, R01HL136954, P01HL134608, and R01NS121379 to J.W.E.) and the American Heart Association (20EIA35320226 to J.W.E.).
DISCLOSURES
J.W.E. is a paid consultant for Mitobridge and Janssen. J.F.G. has no conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
J.F.G. prepared figures; J.F.G. and J.W.E. drafted manuscript; J.F.G. and J.W.E. edited and revised manuscript; J.F.G. and J.W.E. approved final version of manuscript.
REFERENCES
- 1.Slater EC, Cleland KW. The effect of calcium on the respiratory and phosphorylative activities of heart-muscle sarcosomes. Biochem J 55: 566–590, 1953. doi: 10.1042/bj0550566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lehninger AL. Enzymes and Enzyme Systems: Their State in Nature. Cambridge, MA: Harvard University Press, 1951. [Google Scholar]
- 3.DeLuca HF, Engstrom GW. Calcium uptake by rat kidney mitochondria. Proc Natl Acad Sci USA 47: 1744–1750, 1961. doi: 10.1073/pnas.47.11.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vasington FD, Murphy JV. Ca ion uptake by rat kidney mitochondria and its dependence on respiration and phosphorylation. J Biol Chem 237: 2670–2677, 1962. doi: 10.1016/S0021-9258(19)73805-8. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191: 144–148, 1961. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc 41: 445–502, 1966. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- 7.Carafoli E, Tiozzo R, Lugli G, Crovetti F, Kratzing C. The release of calcium from heart mitochondria by sodium. J Mol Cell Cardiol 6: 361–371, 1974. doi: 10.1016/0022-2828(74)90077-7. [DOI] [PubMed] [Google Scholar]
- 8.Crompton M, Capano M, Carafoli E. Sodium-induced efflux of calcium from heart mitochondria—possible mechanism for regulation of mitochondrial calcium. Eur J Biochem 69: 453–462, 1976. doi: 10.1111/j.1432-1033.1976.tb10930.x. [DOI] [Google Scholar]
- 9.Pozzan T, Bragadin M, Azzone GF. Disequilibrium between steady-state Ca2+ accumulation ratio and membrane potential in mitochondria. Pathway and role of Ca2+ efflux. Biochemistry 16: 5618–5625, 1977. doi: 10.1021/bi00644a036. [DOI] [PubMed] [Google Scholar]
- 10.Bygrave FL, Reed KC, Spencer T. cooperative interactions in energy-dependent accumulation of Ca2+ by isolated rat liver mitochondria. Nat New Biol 230: 89, 1971. doi: 10.1038/newbio230089a0. [DOI] [PubMed] [Google Scholar]
- 11.Vinogradov A, Scarpa A. Initial velocities of calcium uptake by rat-liver mitochondria. J Biol Chem 248: 5527–5531, 1973. doi: 10.1016/S0021-9258(19)43634-X. [DOI] [PubMed] [Google Scholar]
- 12.Scarpa A, Graziotti P. Mechanisms for intracellular calcium regulation in heart. 1. stopped-flow measurements of Ca++ uptake by cardiac mitochondria. J Gen Physiol 62: 756–772, 1973. doi: 10.1085/jgp.62.6.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rizzuto R, Simpson AW, Brini M, Pozzan T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature 358: 325–327, 1992. doi: 10.1038/358325a0. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-Sanz C, De la Fuente S, Sheu SS. Mitochondrial Ca2+ concentrations in live cells: quantification methods and discrepancies. FEBS Lett 593: 1528–1541, 2019. doi: 10.1002/1873-3468.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyman L, Greiser M, Lederer WJ. Calcium influx through the mitochondrial calcium uniporter holocomplex, MCUcx. J Mol Cell Cardiol 151: 145–154, 2021. doi: 10.1016/j.yjmcc.2020.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rizzuto R, Marchi S, Bonora M, Aguiari P, Bononi A, De Stefani D, Giorgi C, Leo S, Rimessi A, Siviero R, Zecchini E, Pinton P. Ca2+ transfer from the ER to mitochondria: when, how and why. Biochim Biophys Acta 1787: 1342–1351, 2009. doi: 10.1016/j.bbabio.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clapham DE. Calcium signaling. Cell 131: 1047–1058, 2007. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan JH. Biochemistry of Na,K-ATPase. Annu Rev Biochem 71: 511–535, 2002. doi: 10.1146/annurev.biochem.71.102201.141218. [DOI] [PubMed] [Google Scholar]
- 19.Boron WF, Boulpaep EL. Medical Physiology: a Cellular and Molecular Approach. Philadelphia, PA: Saunders/Elsevier, 2009. [Google Scholar]
- 20.Guyton AC, Hall JE. Textbook of Medical Physiology. Philadelphia, PA: Elsevier Saunders, 2006. [Google Scholar]
- 21.Murphy E, Eisner DA. Regulation of intracellular and mitochondrial sodium in health and disease. Circ Res 104: 292–303, 2009. doi: 10.1161/CIRCRESAHA.108.189050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, Takasu MA, Tao X, Greenberg ME. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci USA 98: 11024–11031, 2001. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bers DM. Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 24.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol 70: 23–49, 2008. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 25.da Cunha FM, Torelli NQ, Kowaltowski AJ. Mitochondrial retrograde signaling: triggers, pathways, and outcomes. Oxid Med Cell Longev 2015: 482582, 2015. doi: 10.1155/2015/482582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Südhof TC. Calcium control of neurotransmitter release. Cold Spring Harb Perspect Biol 4: a011353, 2012. doi: 10.1101/cshperspect.a011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee CH, Park D, Wu D, Rhee SG, Simon MI. Members of the Gq alpha subunit gene family activate phospholipase C beta isozymes. J Biol Chem 267: 16044–16047, 1992. doi: 10.1016/S0021-9258(18)41962-X. [DOI] [PubMed] [Google Scholar]
- 28.Harden TK, Waldo GL, Hicks SN, Sondek J. Mechanism of activation and inactivation of Gq/phospholipase C-beta signaling nodes. Chem Rev 111: 6120–6129, 2011. doi: 10.1021/cr200209p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berridge MJ, Irvine RF. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature 312: 315–321, 1984. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- 30.Berridge MJ. Inositol trisphosphate and calcium signalling mechanisms. Biochim Biophys Acta 1793: 933–940, 2009. doi: 10.1016/j.bbamcr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4: 517–529, 2003. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 32.Pfleger J, Gresham K, Koch WJ. G protein-coupled receptor kinases as therapeutic targets in the heart. Nat Rev Cardiol 16: 612–622, 2019. doi: 10.1038/s41569-019-0220-3. [DOI] [PubMed] [Google Scholar]
- 33.Berridge MJ. The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol Rev 96: 261–1296, 2016. doi: 10.1152/physrev.00006.2016. [DOI] [PubMed] [Google Scholar]
- 34.Raturi A, Simmen T. Where the endoplasmic reticulum and the mitochondrion tie the knot: the mitochondria-associated membrane (MAM). Biochim Biophys Acta 1833: 213–224, 2013. doi: 10.1016/j.bbamcr.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 35.Berridge MJ. Smooth muscle cell calcium activation mechanisms. J Physiol 586: 5047–5061, 2008. doi: 10.1113/jphysiol.2008.160440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Endo M. Calcium-induced calcium release in skeletal muscle. Physiol Rev 89: 1153–1176, 2009. doi: 10.1152/physrev.00040.2008. [DOI] [PubMed] [Google Scholar]
- 37.Belosludtsev KN, Dubinin MV, Belosludtseva NV, Mironova GD. Mitochondrial Ca2+ transport: mechanisms, molecular structures, and role in cells. Biochemistry (Mosc) 84: 593–607, 2019. doi: 10.1134/S0006297919060026. [DOI] [PubMed] [Google Scholar]
- 38.Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev 86: 369–408, 2006. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- 39.Pozzan T, Rizzuto R, Volpe P, Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev 74: 595–636, 1994. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- 40.Finkel T, Menazza S, Holmström KM, Parks RJ, Liu J, Sun J, Liu J, Pan X, Murphy E. The ins and outs of mitochondrial calcium. Circ Res 116: 1810–1819, 2015. doi: 10.1161/CIRCRESAHA.116.305484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benz R. Porin from bacterial and mitochondrial outer membranes. CRC Crit Rev Biochem 19: 145–190, 1985. doi: 10.3109/10409238509082542. [DOI] [PubMed] [Google Scholar]
- 42.Shoshan-Barmatz V, De Pinto V, Zweckstetter M, Raviv Z, Keinan N, Arbel N. VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol Aspects Med 31: 227–285, 2010. doi: 10.1016/j.mam.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto T, Yamada A, Watanabe M, Yoshimura Y, Yamazaki N, Yoshimura Y, Yamauchi T, Kataoka M, Nagata T, Terada H, Shinohara Y. VDAC1, having a shorter N-terminus than VDAC2 but showing the same migration in an SDS polyacrylamide gel, is the predominant form expressed in mitochondria of various tissues. J Proteome Res 5: 3336–3344, 2006. doi: 10.1021/pr060291w. [DOI] [PubMed] [Google Scholar]
- 44.Bayrhuber M, Meins T, Habeck M, Becker S, Giller K, Villinger S, Vonrhein C, Griesinger C, Zweckstetter M, Zeth K. Structure of the human voltage-dependent anion channel. Proc Natl Acad Sci USA 105: 15370–15375, 2008. doi: 10.1073/pnas.0808115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hiller S, Garces RG, Malia TJ, Orekhov VY, Colombini M, Wagner G. Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science 321: 1206–1210, 2008. doi: 10.1126/science.1161302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ujwal R, Cascio D, Colletier JP, Faham S, Zhang J, Toro L, Ping P, Abramson J. The crystal structure of mouse VDAC1 at 2.3 A resolution reveals mechanistic insights into metabolite gating. Proc Natl Acad Sci USA 105: 17742–17747, 2008. doi: 10.1073/pnas.0809634105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colombini M. A candidate for the permeability pathway of the outer mitochondrial membrane. Nature 279: 643–645, 1979. doi: 10.1038/279643a0. [DOI] [PubMed] [Google Scholar]
- 48.Schein SJ, Colombini M, Finkelstein A. Reconstitution in planar lipid bilayers of a voltage-dependent anion-selective channel obtained from paramecium mitochondria. J Membr Biol 30: 99–120, 1976. doi: 10.1007/BF01869662. [DOI] [PubMed] [Google Scholar]
- 49.Roos N, Benz R, Brdiczka D. Identification and characterization of the pore-forming protein in the outer membrane of rat liver mitochondria. Biochim Biophys Acta 686: 204–214, 1982. doi: 10.1016/0005-2736(82)90114-6. [DOI] [PubMed] [Google Scholar]
- 50.Benz R, Brdiczka D. The cation-selective substate of the mitochondrial outer membrane pore—single-channel conductance and influence on intermembrane and peripheral kinases. J Bioenerg Biomembr 24: 33–39, 1992. doi: 10.1007/BF00769528. [DOI] [PubMed] [Google Scholar]
- 51.Benz R, Kottke M, Brdiczka D. The cationically selective state of the mitochondrial outer membrane pore: a study with intact mitochondria and reconstituted mitochondrial porin. Biochim Biophys Acta 1022: 311–318, 1990. doi: 10.1016/0005-2736(90)90279-W. [DOI] [PubMed] [Google Scholar]
- 52.Gincel D, Zaid H, Shoshan-Barmatz V. Calcium binding and translocation by the voltage-dependent anion channel: a possible regulatory mechanism in mitochondrial function. Biochem J 358: 147–155, 2001. doi: 10.1042/bj3580147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deniaud A, Rossi C, Berquand A, Homand J, Campagna S, Knoll W, Brenner C, Chopineau J. Voltage-dependent anion channel transports calcium ions through biomimetic membranes. Langmuir 23: 3898–3905, 2007. doi: 10.1021/la063105+. [DOI] [PubMed] [Google Scholar]
- 54.Tan W, Colombini M. VDAC closure increases calcium ion flux. Biochim Biophys Acta 1768: 2510–2515, 2007. doi: 10.1016/j.bbamem.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Báthori G, Csordás G, Garcia-Perez C, Davies E, Hajnóczky G. Ca2+-dependent control of the permeability properties of the mitochondrial outer membrane and voltage-dependent anion-selective channel (VDAC). J Biol Chem 281: 17347–17358, 2006. doi: 10.1074/jbc.M600906200. [DOI] [PubMed] [Google Scholar]
- 56.Huang H, Hu X, Eno CO, Zhao G, Li C, White C. An interaction between Bcl-xL and the voltage-dependent anion channel (VDAC) promotes mitochondrial Ca2+ uptake. J Biol Chem 288: 19870–19881, 2013. doi: 10.1074/jbc.M112.448290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kerner J, Lee K, Tandler B, Hoppel CL. VDAC proteomics: post-translation modifications. Biochim Biophys Acta 1818: 1520–1525, 2012. doi: 10.1016/j.bbamem.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rapizzi E, Pinton P, Szabadkai G, Wieckowski MR, Vandecasteele G, Baird G, Tuft RA, Fogarty KE, Rizzuto R. Recombinant expression of the voltage-dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. J Cell Biol 159: 613–624, 2002. doi: 10.1083/jcb.200205091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Stefani D, Bononi A, Romagnoli A, Messina A, De Pinto V, Pinton P, Rizzuto R. VDAC1 selectively transfers apoptotic Ca2+ signals to mitochondria. Cell Death Differ 19: 267–273, 2012. doi: 10.1038/cdd.2011.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Szabadkai G, Bianchi K, Várnai P, De Stefani D, Wieckowski MR, Cavagna D, Nagy AI, Balla T, Rizzuto R. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol 175: 901–911, 2006. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shoshan-Barmatz V, Gincel D. The voltage-dependent anion channel: characterization, modulation, and role in mitochondrial function in cell life and death. Cell Biochem Biophys 39: 279–292, 2003. doi: 10.1385/CBB:39:3:279. [DOI] [PubMed] [Google Scholar]
- 62.Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol 9: 550–555, 2007. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krauskopf A, Eriksson O, Craigen WJ, Forte MA, Bernardi P. Properties of the permeability transition in VDAC1-/- mitochondria. Biochim Biophys Acta 1757: 590–595, 2006. doi: 10.1016/j.bbabio.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 64.Huizing M, Ruitenbeek W, Thinnes FP, DePinto V. Lack of voltage-dependent anion channel in human mitochondrial myopathies. Lancet 344: 762, 1994. doi: 10.1016/S0140-6736(94)92257-8. [DOI] [PubMed] [Google Scholar]
- 65.Huizing M, Ruitenbeek W, Thinnes FP, DePinto V, Wendel U, Trijbels FJ, Smit LM, ter Laak HJ, van den Heuvel LP. Deficiency of the voltage-dependent anion channel: a novel cause of mitochondriopathy. Pediatr Res 39: 760–765, 1996. doi: 10.1203/00006450-199605000-00003. [DOI] [PubMed] [Google Scholar]
- 66.De Pinto V, Messina A, Schmid A, Simonetti S, Carnevale F, Benz R. Characterization of channel-forming activity in muscle biopsy from a porin-deficient human patient. J Bioenerg Biomembr 32: 585–593, 2000. doi: 10.1023/A:1005622611410. [DOI] [PubMed] [Google Scholar]
- 67.Wu S, Sampson MJ, Decker WK, Craigen WJ. Each mammalian mitochondrial outer membrane porin protein is dispensable: effects on cellular respiration. Biochim Biophys Acta 1452: 68–78, 1999. doi: 10.1016/S0167-4889(99)00120-2. [DOI] [PubMed] [Google Scholar]
- 68.Anflous K, Armstrong DD, Craigen WJ. Altered mitochondrial sensitivity for ADP and maintenance of creatine-stimulated respiration in oxidative striated muscles from VDAC1-deficient mice. J Biol Chem 276: 1954–1960, 2001. doi: 10.1074/jbc.M006587200. [DOI] [PubMed] [Google Scholar]
- 69.Sampson MJ, Decker WK, Beaudet AL, Ruitenbeek W, Armstrong D, Hicks MJ, Craigen WJ. Immotile sperm and infertility in mice lacking mitochondrial voltage-dependent anion channel type 3. J Biol Chem 276: 39206–39212, 2001. doi: 10.1074/jbc.M104724200. [DOI] [PubMed] [Google Scholar]
- 70.Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science 301: 513–517, 2003. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- 71.Weeber EJ, Levy M, Sampson MJ, Anflous K, Armstrong DL, Brown SE, Sweatt JD, Craigen WJ. The role of mitochondrial porins and the permeability transition pore in learning and synaptic plasticity. J Biol Chem 277: 18891–18897, 2002. doi: 10.1074/jbc.M201649200. [DOI] [PubMed] [Google Scholar]
- 72.Gunter TE, Gunter KK. Uptake of calcium by mitochondria: transport and possible function. IUBMB Life 52: 197–204, 2001. doi: 10.1080/15216540152846000. [DOI] [PubMed] [Google Scholar]
- 73.Panfili E, Crompton M, Sottocasa GL. Immunochemical evidence of the independence of the Ca2+/Na2+ antiporter and electrophoretic Ca2+ uniporter in heart mitochondria. FEBS Lett 123: 30–32, 1981. doi: 10.1016/0014-5793(81)80012-9. [DOI] [PubMed] [Google Scholar]
- 74.Selwyn MJ, Dawson AP, Dunnett SJ. Calcium transport in mitochondria. FEBS Lett 10: 1–5, 1970. doi: 10.1016/0014-5793(70)80402-1. [DOI] [PubMed] [Google Scholar]
- 75.Nicholls DG, Scott ID. The regulation of brain mitochondrial calcium-ion transport. The role of ATP in the discrimination between kinetic and membrane-potential-dependent calcium-ion efflux mechanisms. Biochem J 186: 833–839, 1980. doi: 10.1042/bj1860833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rossi CS, Vasington FD, Carafoli E. The effect of ruthenium red on the uptake and release of Ca2+ by mitochondria. Biochem Biophys Res Commun 50: 846–852, 1973. doi: 10.1016/0006-291X(73)91322-3. [DOI] [PubMed] [Google Scholar]
- 77.Zazueta C, Sosa-Torres ME, Correa F, Garza-Ortiz A. Inhibitory properties of ruthenium amine complexes on mitochondrial calcium uptake. J Bioenerg Biomembr 31: 551–557, 1999. doi: 10.1023/A:1005464927366. [DOI] [PubMed] [Google Scholar]
- 78.Palmi M, Youmbi GT, Fusi F, Sgaragli GP, Dixon HB, Frosini M, Tipton KF. Potentiation of mitochondrial Ca2+ sequestration by taurine. Biochem Pharmacol 58: 1123–1131, 1999. doi: 10.1016/S0006-2952(99)00183-5. [DOI] [PubMed] [Google Scholar]
- 79.Montero M, Lobatón CD, Hernández-Sanmiguel E, Santodomingo J, Vay L, Moreno A, Alvarez J. Direct activation of the mitochondrial calcium uniporter by natural plant flavonoids. Biochem J 384: 19–24, 2004. doi: 10.1042/BJ20040990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nicchitta CV, Williamson JR. Spermine. A regulator of mitochondrial calcium cycling. J Biol Chem 259: 12978–12983, 1984. doi: 10.1016/S0021-9258(18)90643-5. [DOI] [PubMed] [Google Scholar]
- 81.Sparagna GC, Gunter KK, Sheu SS, Gunter TE. Mitochondrial calcium uptake from physiological-type pulses of calcium. A description of the rapid uptake mode. J Biol Chem 270: 27510–27515, 1995. doi: 10.1074/jbc.270.46.27510. [DOI] [PubMed] [Google Scholar]
- 82.Mallilankaraman K, Doonan P, Cárdenas C, Chandramoorthy HC, Müller M, Miller R, Hoffman NE, Gandhirajan RK, Molgó J, Birnbaum MJ, Rothberg BS, Mak DO, Foskett JK, Madesh M. MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca2+ uptake that regulates cell survival. Cell 151: 630–644, 2012. doi: 10.1016/j.cell.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Babcock DF, Herrington J, Goodwin PC, Park YB, Hille B. Mitochondrial participation in the intracellular Ca2+ network. J Cell Biol 136: 833–844, 1997. doi: 10.1083/jcb.136.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.David G, Barrett JN, Barrett EF. Evidence that mitochondria buffer physiological Ca2+ loads in lizard motor nerve terminals. J Physiol 509: 59–65, 1998. doi: 10.1111/j.1469-7793.1998.059bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Csordás G, Hajnóczky G. Sorting of calcium signals at the junctions of endoplasmic reticulum and mitochondria. Cell Calcium 29: 249–262, 2001. doi: 10.1054/ceca.2000.0191. [DOI] [PubMed] [Google Scholar]
- 86.Buntinas L, Gunter KK, Sparagna GC, Gunter TE. The rapid mode of calcium uptake into heart mitochondria (RaM): comparison to RaM in liver mitochondria. Biochim Biophys Acta 1504: 248–261, 2001. doi: 10.1016/S0005-2728(00)00254-1. [DOI] [PubMed] [Google Scholar]
- 87.Blomeyer CA, Bazil JN, Stowe DF, Dash RK, Camara AK. Mg2+ differentially regulates two modes of mitochondrial Ca2+ uptake in isolated cardiac mitochondria: implications for mitochondrial Ca2+ sequestration. J Bioenerg Biomembr 48: 175–188, 2016. doi: 10.1007/s10863-016-9644-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bick AG, Calvo SE, Mootha VK. Evolutionary diversity of the mitochondrial calcium uniporter. Science 336: 886, 2012. doi: 10.1126/science.1214977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sancak Y, Markhard AL, Kitami T, Kovács-Bogdán E, Kamer KJ, Udeshi ND, Carr SA, Chaudhuri D, Clapham DE, Li AA, Calvo SE, Goldberger O, Mootha VK. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science 342: 1379–1382, 2013. doi: 10.1126/science.1242993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Raffaello A, De Stefani D, Sabbadin D, Teardo E, Merli G, Picard A, Checchetto V, Moro S, Szabó I, Rizzuto R. The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. EMBO J 32: 2362–2376, 2013. doi: 10.1038/emboj.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zulkifli M, Neff JK, Timbalia SA, Garza NM, Chen Y, Watrous JD, Murgia M, Trivedi PP, Anderson SK, Tomar D, Nilsson R, Madesh M, Jain M, Gohil VM. Yeast homologs of human MCUR1 regulate mitochondrial proline metabolism. Nat Commun 11: 4866, 2020. doi: 10.1038/s41467-020-18704-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pittis AA, Goh V, Cebrian-Serrano A, Wettmarshausen J, Perocchi F, Gabaldón T. Discovery of EMRE in fungi resolves the true evolutionary history of the mitochondrial calcium uniporter. Nat Commun 11: 4031, 2020. doi: 10.1038/s41467-020-17705-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Plovanich M, Bogorad RL, Sancak Y, Kamer KJ, Strittmatter L, Li AA, Girgis HS, Kuchimanchi S, De Groot J, Speciner L, Taneja N, Oshea J, Koteliansky V, Mootha VK. MICU2, a paralog of MICU1, resides within the mitochondrial uniporter complex to regulate calcium handling. PLoS One 8: e55785, 2013. doi: 10.1371/journal.pone.0055785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bertolini MS, Chiurillo MA, Lander N, Vercesi AE, Docampo R. MICU1 and MICU2 play an essential role in mitochondrial Ca2+ uptake, growth, and infectivity of the human pathogen Trypanosoma cruzi. mBio 10: e00348-19, 2019. doi: 10.1128/mBio.00348-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Docampo R, Vercesi AE, Huang G. Mitochondrial calcium transport in trypanosomes. Mol Biochem Parasitol 196: 108–116, 2014. doi: 10.1016/j.molbiopara.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Patron M, Granatiero V, Espino J, Rizzuto R, De Stefani D. MICU3 is a tissue-specific enhancer of mitochondrial calcium uptake. Cell Death Differ 26: 179–195, 2019. doi: 10.1038/s41418-018-0113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427: 360–364, 2004. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 98.De Stefani D, Raffaello A, Teardo E, Szabò I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476: 336–340, 2011. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, Mootha VK. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476: 341–345, 2011. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, Mootha VK. MICU1 encodes a mitochondrial EF hand protein required for Ca2+ uptake. Nature 467: 291–296, 2010. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chaudhuri D, Sancak Y, Mootha VK, Clapham DE. MCU encodes the pore conducting mitochondrial calcium currents. Elife 2: e00704, 2013. doi: 10.7554/eLife.00704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chiurillo MA, Lander N, Bertolini MS, Vercesi AE, Docampo R. Functional analysis and importance for host cell infection of the Ca2+-conducting subunits of the mitochondrial calcium uniporter of Trypanosoma cruzi. Mol Biol Cell 30: 1676–1690, 2019. doi: 10.1091/mbc.E19-03-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang G, Vercesi AE, Docampo R. Essential regulation of cell bioenergetics in Trypanosoma brucei by the mitochondrial calcium uniporter. Nat Commun 4: 2865, 2013. doi: 10.1038/ncomms3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang G, Docampo R. The mitochondrial Ca2+ uniporter complex (MCUC) of Trypanosoma brucei is a hetero-oligomer that contains novel subunits essential for Ca2+ uptake. mBio 9: e01700-18, 2018. doi: 10.1128/mBio.01700-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chiurillo MA, Lander N, Bertolini MS, Storey M, Vercesi AE, Docampo R. Different roles of mitochondrial calcium uniporter complex subunits in growth and infectivity of Trypanosoma cruzi. mBio 8: e00574-17, 2017. doi: 10.1128/mBio.00574-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yoo J, Wu M, Yin Y, Herzik MA Jr, Lander GC, Lee SY. Cryo-EM structure of a mitochondrial calcium uniporter. Science 361: 506–511, 2018. doi: 10.1126/science.aar4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Martell JD, Deerinck TJ, Sancak Y, Poulos TL, Mootha VK, Sosinsky GE, Ellisman MH, Ting AY. Engineered ascorbate peroxidase as a genetically encoded reporter for electron microscopy. Nat Biotechnol 30: 1143–1148, 2012. doi: 10.1038/nbt.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nguyen NX, Armache JP, Lee C, Yang Y, Zeng W, Mootha VK, Cheng Y, Bai XC, Jiang Y. Cryo-EM structure of a fungal mitochondrial calcium uniporter. Nature 559: 570–574, 2018. doi: 10.1038/s41586-018-0333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fan C, Fan MR, Orlando BJ, Fastman NM, Zhang JR, Xu Y, Chambers MG, Xu XF, Perry K, Liao MF, Feng L. X-ray and cryo-EM structures of the mitochondrial calcium uniporter. Nature 559: 575–579, 2018. doi: 10.1038/s41586-018-0330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Oxenoid K, Dong Y, Cao C, Cui T, Sancak Y, Markhard AL, Grabarek Z, Kong L, Liu Z, Ouyang B, Cong Y, Mootha VK, Chou JJ. Architecture of the mitochondrial calcium uniporter. Nature 533: 269–273, 2016. doi: 10.1038/nature17656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Baradaran R, Wang CY, Siliciano AF, Long SB. Cryo-EM structures of fungal and metazoan mitochondrial calcium uniporters. Nature 559: 580–584, 2018. doi: 10.1038/s41586-018-0331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee Y, Min CK, Kim TG, Song HK, Lim Y, Kim D, Shin K, Kang M, Kang JY, Youn HS, Lee JG, An JY, Park KR, Lim JJ, Kim JH, Kim JH, Park ZY, Kim YS, Wang J, Kim DH, Eom SH. Structure and function of the N-terminal domain of the human mitochondrial calcium uniporter. EMBO Rep 16: 1318–1333, 2015. doi: 10.15252/embr.201540436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee SK, Shanmughapriya S, Mok MC, Dong Z, Tomar D, Carvalho E, Rajan S, Junop MS, Madesh M, Stathopulos PB. structural insights into mitochondrial calcium uniporter regulation by divalent cations. Cell Chem Biol 23: 1157–1169, 2016. doi: 10.1016/j.chembiol.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.de la Fuente S, Matesanz-Isabel J, Fonteriz RI, Montero M, Alvarez J. Dynamics of mitochondrial Ca2+ uptake in MICU1-knockdown cells. Biochem J 458: 33–40, 2014. doi: 10.1042/BJ20131025. [DOI] [PubMed] [Google Scholar]
- 115.Vais H, Mallilankaraman K, Mak DD, Hoff H, Payne R, Tanis JE, Foskett JK. EMRE is a matrix Ca2+ sensor that governs gatekeeping of the mitochondrial Ca2+ uniporter. Cell Rep 14: 403–410, 2016. doi: 10.1016/j.celrep.2015.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vais H, Payne R, Paudel U, Li C, Foskett JK. Coupled transmembrane mechanisms control MCU-mediated mitochondrial Ca2+ uptake. Proc Natl Acad Sci USA 117: 21731–21739, 2020. doi: 10.1073/pnas.2005976117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fieni F, Lee SB, Jan YN, Kirichok Y. Activity of the mitochondrial calcium uniporter varies greatly between tissues. Nat Commun 3: 1317, 2012. doi: 10.1038/ncomms2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Verma M, Callio J, Otero PA, Sekler I, Wills ZP, Chu CT. Mitochondrial calcium dysregulation contributes to dendrite degeneration mediated by PD/LBD-associated LRRK2 mutants. J Neurosci 37: 11151–11165, 2017. doi: 10.1523/JNEUROSCI.3791-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pan X, Liu J, Nguyen T, Liu C, Sun J, Teng Y, Fergusson MM, Rovira II, Allen M, Springer DA, Aponte AM, Gucek M, Balaban RS, Murphy E, Finkel T. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat Cell Biol 15: 1464–1472, 2013. doi: 10.1038/ncb2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Murphy E, Pan X, Nguyen T, Liu J, Holmström KM, Finkel T. Unresolved questions from the analysis of mice lacking MCU expression. Biochem Biophys Res Commun 449: 384–385, 2014. doi: 10.1016/j.bbrc.2014.04.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Garbincius JF, Luongo TS, Elrod JW. The debate continues—what is the role of MCU and mitochondrial calcium uptake in the heart? J Mol Cell Cardiol 143: 163–174, 2020. doi: 10.1016/j.yjmcc.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Holmström KM, Pan X, Liu JC, Menazza S, Liu J, Nguyen TT, Pan H, Parks RJ, Anderson S, Noguchi A, Springer D, Murphy E, Finkel T. Assessment of cardiac function in mice lacking the mitochondrial calcium uniporter. J Mol Cell Cardiol 85: 178–182, 2015. doi: 10.1016/j.yjmcc.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu JC. Is MCU dispensable for normal heart function? J Mol Cell Cardiol 143: 175–183, 2020. doi: 10.1016/j.yjmcc.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu Y, Rasmussen TP, Koval OM, Joiner ML, Hall DD, Chen B, Luczak ED, Wang Q, Rokita AG, Wehrens XH, Song LS, Anderson ME. The mitochondrial uniporter controls fight or flight heart rate increases. Nat Commun 6: 6081, 2015. doi: 10.1038/ncomms7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rasmussen TP, Wu Y, Joiner ML, Koval OM, Wilson NR, Luczak ED, Wang Q, Chen B, Gao Z, Zhu Z, Wagner BA, Soto J, McCormick ML, Kutschke W, Weiss RM, Yu L, Boudreau RL, Abel ED, Zhan F, Spitz DR, Buettner GR, Song LS, Zingman LV, Anderson ME. Inhibition of MCU forces extramitochondrial adaptations governing physiological and pathological stress responses in heart. Proc Natl Acad Sci USA 112: 9129–9134, 2015. doi: 10.1073/pnas.1504705112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Luongo TS, Lambert JP, Yuan A, Zhang X, Gross P, Song J, Shanmughapriya S, Gao E, Jain M, Houser SR, Koch WJ, Cheung JY, Madesh M, Elrod JW. The mitochondrial calcium uniporter matches energetic supply with cardiac workload during stress and modulates permeability transition. Cell Rep 12: 23–34, 2015. doi: 10.1016/j.celrep.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kwong JQ, Lu X, Correll RN, Schwanekamp JA, Vagnozzi RJ, Sargent MA, York AJ, Zhang J, Bers DM, Molkentin JD. The mitochondrial calcium uniporter selectively matches metabolic output to acute contractile stress in the heart. Cell Rep 12: 15–22, 2015. doi: 10.1016/j.celrep.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mammucari C, Gherardi G, Zamparo I, Raffaello A, Boncompagni S, Chemello F, Cagnin S, Braga A, Zanin S, Pallafacchina G, Zentilin L, Sandri M, De Stefani D, Protasi F, Lanfranchi G, Rizzuto R. The mitochondrial calcium uniporter controls skeletal muscle trophism in vivo. Cell Rep 10: 1269–1279, 2015. doi: 10.1016/j.celrep.2015.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kwong JQ, Huo J, Bround MJ, Boyer JG, Schwanekamp JA, Ghazal N, Maxwell JT, Jang YC, Khuchua Z, Shi K, Bers DM, Davis J, Molkentin JD. The mitochondrial calcium uniporter underlies metabolic fuel preference in skeletal muscle. JCI Insight 3: e121689, 2018. doi: 10.1172/jci.insight.121689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Altamimi TR, Karwi QG, Uddin GM, Fukushima A, Kwong JQ, Molkentin JD, Lopaschuk GD. Cardiac-specific deficiency of the mitochondrial calcium uniporter augments fatty acid oxidation and functional reserve. J Mol Cell Cardiol 127: 223–231, 2019. doi: 10.1016/j.yjmcc.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 131.Doetschman T, Azhar M. Cardiac-specific inducible and conditional gene targeting in mice. Circ Res 110: 1498–1512, 2012. doi: 10.1161/CIRCRESAHA.112.265066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Flicker D, Sancak Y, Mick E, Goldberger O, Mootha VK. Exploring the in vivo role of the mitochondrial calcium uniporter in brown fat bioenergetics. Cell Rep 27: 1364–1375.e5, 2019. doi: 10.1016/j.celrep.2019.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Choi S, Quan X, Bang S, Yoo H, Kim J, Park J, Park KS, Chung J. Mitochondrial calcium uniporter in Drosophila transfers calcium between the endoplasmic reticulum and mitochondria in oxidative stress-induced cell death. J Biol Chem 292: 14473–14485, 2017. doi: 10.1074/jbc.M116.765578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Drago I, Davis RL. Inhibiting the mitochondrial calcium uniporter during development impairs memory in adult Drosophila. Cell Rep 16: 2763–2776, 2016. doi: 10.1016/j.celrep.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shanmughapriya S, Rajan S, Hoffman NE, Zhang X, Guo S, Kolesar JE, Hines KJ, Ragheb J, Jog NR, Caricchio R, Baba Y, Zhou Y, Kaufman BA, Cheung JY, Kurosaki T, Gill DL, Madesh M. Ca2+ signals regulate mitochondrial metabolism by stimulating CREB-mediated expression of the mitochondrial Ca2+ uniporter gene MCU. Sci Signal 8: ra23, 2015. doi: 10.1126/scisignal.2005673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Takahashi K, Yi H, Gu J, Ikegami D, Liu S, Iida T, Kashiwagi Y, Dong C, Kunisawa T, Hao S. The mitochondrial calcium uniporter contributes to morphine tolerance through pCREB and CPEB1 in rat spinal cord dorsal horn. Br J Anaesth 123: e226–e238, 2019. doi: 10.1016/j.bja.2019.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zaglia T, Ceriotti P, Campo A, Borile G, Armani A, Carullo P, Prando V, Coppini R, Vida V, Stolen TO, Ulrik W, Cerbai E, Stellin G, Faggian G, De Stefani D, Sandri M, Rizzuto R, Di Lisa F, Pozzan T, Catalucci D, Mongillo M. Content of mitochondrial calcium uniporter (MCU) in cardiomyocytes is regulated by microRNA-1 in physiologic and pathologic hypertrophy. Proc Natl Acad Sci USA 114: E9006–E9015, 2017. doi: 10.1073/pnas.1708772114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pan L, Huang BJ, Ma XE, Wang SY, Feng J, Lv F, Liu Y, Liu Y, Li CM, Liang DD, Li J, Xu L, Chen YH. MiR-25 protects cardiomyocytes against oxidative damage by targeting the mitochondrial calcium uniporter. Int J Mol Sci 16: 5420–5433, 2015. doi: 10.3390/ijms16035420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Marchi S, Lupini L, Patergnani S, Rimessi A, Missiroli S, Bonora M, Bononi A, Corrà F, Giorgi C, De Marchi E, Poletti F, Gafà R, Lanza G, Negrini M, Rizzuto R, Pinton P. Downregulation of the mitochondrial calcium uniporter by cancer-related miR-25. Curr Biol 23: 58–63, 2013. doi: 10.1016/j.cub.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hong Z, Chen KH, DasGupta A, Potus F, Dunham-Snary K, Bonnet S, Tian L, Fu J, Breuils-Bonnet S, Provencher S, Wu D, Mewburn J, Ormiston ML, Archer SL. MicroRNA-138 and microRNA-25 down-regulate mitochondrial calcium uniporter, causing the pulmonary arterial hypertension cancer phenotype. Am J Respir Crit Care Med 195: 515–529, 2017. doi: 10.1164/rccm.201604-0814OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yu C, Wang Y, Peng J, Shen Q, Chen M, Tang W, Li X, Cai C, Wang B, Cai S, Meng X, Zou F. Mitochondrial calcium uniporter as a target of microRNA-340 and promoter of metastasis via enhancing the Warburg effect. Oncotarget 8: 83831–83844, 2017. doi: 10.18632/oncotarget.19747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Joiner ML, Koval OM, Li J, He BJ, Allamargot C, Gao Z, Luczak ED, Hall DD, Fink BD, Chen B, Yang J, Moore SA, Scholz TD, Strack S, Mohler PJ, Sivitz WI, Song LS, Anderson ME. CaMKII determines mitochondrial stress responses in heart. Nature 491: 269–273, 2012. doi: 10.1038/nature11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fieni F, Johnson DE, Hudmon A, Kirichok Y. Mitochondrial Ca2+ uniporter and CaMKII in heart. Nature 513: E1–E2, 2014. doi: 10.1038/nature13626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nguyen EK, Koval OM, Noble P, Broadhurst K, Allamargot C, Wu M, Strack S, Thiel WH, Grumbach IM. CaMKII (Ca2+/calmodulin-dependent kinase II) in mitochondria of smooth muscle cells controls mitochondrial mobility, migration, and neointima formation. Arterioscler Thromb Vasc Biol 38: 1333–1345, 2018. doi: 10.1161/ATVBAHA.118.310951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nickel AG, Kohlhaas M, Bertero E, Wilhelm D, Wagner M, Sequeira V, Kreusser MM, Dewenter M, Kappl R, Hoth M, Dudek J, Backs J, Maack C. CaMKII does not control mitochondrial Ca2+ uptake in cardiac myocytes. J Physiol 598: 1361–1376, 2020. doi: 10.1113/JP276766. [DOI] [PubMed] [Google Scholar]
- 146.Zhao H, Li T, Wang K, Zhao F, Chen J, Xu G, Zhao J, Li T, Chen L, Li L, Xia Q, Zhou T, Li HY, Li AL, Finkel T, Zhang XM, Pan X. AMPK-mediated activation of MCU stimulates mitochondrial Ca2+ entry to promote mitotic progression. Nat Cell Biol 21: 476–486, 2019. doi: 10.1038/s41556-019-0296-3. [DOI] [PubMed] [Google Scholar]
- 147.O-Uchi J, Jhun BS, Xu S, Hurst S, Raffaello A, Liu X, Yi B, Zhang H, Gross P, Mishra J, Ainbinder A, Kettlewell S, Smith GL, Dirksen RT, Wang W, Rizzuto R, Sheu SS. Adrenergic signaling regulates mitochondrial Ca2+ uptake through Pyk2-dependent tyrosine phosphorylation of the mitochondrial Ca2+ uniporter. Antioxid Redox Signal 21: 863–879, 2014. doi: 10.1089/ars.2013.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dong Z, Shanmughapriya S, Tomar D, Siddiqui N, Lynch S, Nemani N, Breves SL, Zhang X, Tripathi A, Palaniappan P, Riitano MF, Worth AM, Seelam A, Carvalho E, Subbiah R, Jaña F, Soboloff J, Peng Y, Cheung JY, Joseph SK, Caplan J, Rajan S, Stathopulos PB, Madesh M. Mitochondrial Ca2+ uniporter is a mitochondrial luminal redox sensor that augments MCU channel activity. Mol Cell 65: 1014–1028.e7, 2017. doi: 10.1016/j.molcel.2017.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cao C, Wang S, Cui T, Su XC, Chou JJ. Ion and inhibitor binding of the double-ring ion selectivity filter of the mitochondrial calcium uniporter. Proc Natl Acad Sci USA 114: E2846–E2851, 2017. doi: 10.1073/pnas.1620316114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hajnóczky G, Csordás G, Das S, Garcia-Perez C, Saotome M, Sinha Roy S, Yi M. Mitochondrial calcium signalling and cell death: approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium 40: 553–560, 2006. doi: 10.1016/j.ceca.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Arduino DM, Perocchi F. Pharmacological modulation of mitochondrial calcium homeostasis. J Physiol 596: 2717–2733, 2018. doi: 10.1113/JP274959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Woods JJ, Nemani N, Shanmughapriya S, Kumar A, Zhang M, Nathan SR, Thomas M, Carvalho E, Ramachandran K, Srikantan S, Stathopulos PB, Wilson JJ, Madesh M. A selective and cell-permeable mitochondrial calcium uniporter (MCU) inhibitor preserves mitochondrial bioenergetics after hypoxia/reoxygenation injury. ACS Cent Sci 5: 153–166, 2019. doi: 10.1021/acscentsci.8b00773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Arduino DM, Wettmarshausen J, Vais H, Navas-Navarro P, Cheng Y, Leimpek A, Ma Z, Delrio-Lorenzo A, Giordano A, Garcia-Perez C, Médard G, Kuster B, García-Sancho J, Mokranjac D, Foskett JK, Alonso MT, Perocchi F. Systematic identification of MCU modulators by orthogonal interspecies chemical screening. Mol Cell 67: 711–723.e7, 2017. doi: 10.1016/j.molcel.2017.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kon N, Murakoshi M, Isobe A, Kagechika K, Miyoshi N, Nagayama T. DS16570511 is a small-molecule inhibitor of the mitochondrial calcium uniporter. Cell Death Discov 3: 17045, 2017. doi: 10.1038/cddiscovery.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schweitzer MK, Wilting F, Sedej S, Dreizehnter L, Dupper NJ, Tian Q, Moretti A, My I, Kwon O, Priori SG, Laugwitz KL, Storch U, Lipp P, Breit A, Mederos Y Schnitzler M, Gudermann T, Schredelseker J. Suppression of arrhythmia by enhancing mitochondrial Ca2+ uptake in catecholaminergic ventricular tachycardia models. JACC Basic Transl Sci 2: 737–747, 2017. doi: 10.1016/j.jacbts.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong SE, Walford GA, Sugiana C, Boneh A, Chen WK, Hill DE, Vidal M, Evans JG, Thorburn DR, Carr SA, Mootha VK. A mitochondrial protein compendium elucidates complex I disease biology. Cell 134: 112–123, 2008. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kovacs-Bogdan E, Sancak Y, Kamer KJ, Plovanich M, Jambhekar A, Huber RJ, Myre MA, Blower MD, Mootha VK. Reconstitution of the mitochondrial calcium uniporter in yeast. Proc Natl Acad Sci USA 111: 8985–8990, 2014. doi: 10.1073/pnas.1400514111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tsai MF, Phillips CB, Ranaghan M, Tsai CW, Wu Y, Williams C, Miller C. Dual functions of a small regulatory subunit in the mitochondrial calcium uniporter complex. Elife 5: e15545, 2016. doi: 10.7554/eLife.15545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yamamoto T, Yamagoshi R, Harada K, Kawano M, Minami N, Ido Y, Kuwahara K, Fujita A, Ozono M, Watanabe A, Yamada A, Terada H, Shinohara Y. Analysis of the structure and function of EMRE in a yeast expression system. Biochim Biophys Acta 1857: 831–839, 2016. doi: 10.1016/j.bbabio.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 160.Wang Y, Nguyen NX, She J, Zeng W, Yang Y, Bai XC, Jiang Y. Structural mechanism of EMRE-dependent gating of the human mitochondrial calcium uniporter. Cell 177: 1252–1261.e13, 2019. doi: 10.1016/j.cell.2019.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tsai CW, Tsai MF. Electrical recordings of the mitochondrial calcium uniporter in Xenopus oocytes. J Gen Physiol 150: 1035–1043, 2018. doi: 10.1085/jgp.201812015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wu G, Li S, Zong G, Liu X, Fei S, Shen L, Guan X, Yang X, Shen Y. Single channel recording of a mitochondrial calcium uniporter. Biochem Biophys Res Commun 496: 127–132, 2018. doi: 10.1016/j.bbrc.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 163.Liu JC, Liu J, Holmström KM, Menazza S, Parks RJ, Fergusson MM, Yu ZX, Springer DA, Halsey C, Liu C, Murphy E, Finkel T. MICU1 serves as a molecular gatekeeper to prevent in vivo mitochondrial calcium overload. Cell Rep 16: 1561–1573, 2016. doi: 10.1016/j.celrep.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liu JC, Syder NC, Ghorashi NS, Willingham TB, Parks RJ, Sun J, Fergusson MM, Liu J, Holmstrom KM, Menazza S, Springer DA, Liu C, Glancy B, Finkel T, Murphy E. EMRE is essential for mitochondrial calcium uniporter activity in a mouse model. JCI Insight 5: e134063, 2020. doi: 10.1172/jci.insight.134063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lombardi AA, Gibb AA, Arif E, Kolmetzky DW, Tomar D, Luongo TS, Jadiya P, Murray EK, Lorkiewicz PK, Hajnóczky G, Murphy E, Arany ZP, Kelly DP, Margulies KB, Hill BG, Elrod JW. Mitochondrial calcium exchange links metabolism with the epigenome to control cellular differentiation. Nat Commun 10: 4509, 2019. doi: 10.1038/s41467-019-12103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Luongo TS, Lambert JP, Gross P, Nwokedi M, Lombardi AA, Shanmughapriya S, Carpenter AC, Kolmetzky D, Gao E, van Berlo JH, Tsai EJ, Molkentin JD, Chen X, Madesh M, Houser SR, Elrod JW. The mitochondrial Na+/Ca2+ exchanger is essential for Ca2+ homeostasis and viability. Nature 545: 93–97, 2017. doi: 10.1038/nature22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.König T, Tröder SE, Bakka K, Korwitz A, Richter-Dennerlein R, Lampe PA, Patron M, Mühlmeister M, Guerrero-Castillo S, Brandt U, Decker T, Lauria I, Paggio A, Rizzuto R, Rugarli EI, De Stefani D, Langer T. The m-AAA protease associated with neurodegeneration limits MCU activity in mitochondria. Mol Cell 64: 148–162, 2016. doi: 10.1016/j.molcel.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 168.Tsai CW, Wu Y, Pao PC, Phillips CB, Williams C, Miller C, Ranaghan M, Tsai MF. Proteolytic control of the mitochondrial calcium uniporter complex. Proc Natl Acad Sci USA 114: 4388–4393, 2017. doi: 10.1073/pnas.1702938114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Csordás G, Golenár T, Seifert EL, Kamer KJ, Sancak Y, Perocchi F, Moffat C, Weaver D, Fuente Perez SF, Bogorad R, Koteliansky V, Adijanto J, Mootha VK, Hajnóczky G. MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca2+ uniporter. Cell Metab 17: 976–987, 2013. doi: 10.1016/j.cmet.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lam SS, Martell JD, Kamer KJ, Deerinck TJ, Ellisman MH, Mootha VK, Ting AY. Directed evolution of APEX2 for electron microscopy and proximity labeling. Nat Methods 12: 51–54, 2015. doi: 10.1038/nmeth.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gottschalk B, Klec C, Leitinger G, Bernhart E, Rost R, Bischof H, Madreiter-Sokolowski CT, Radulović S, Eroglu E, Sattler W, Waldeck-Weiermair M, Malli R, Graier WF. MICU1 controls cristae junction and spatially anchors mitochondrial Ca2+ uniporter complex. Nat Commun 10: 3732, 2019. doi: 10.1038/s41467-019-11692-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kamer KJ, Mootha VK. MICU1 and MICU2 play nonredundant roles in the regulation of the mitochondrial calcium uniporter. EMBO Rep 15: 299–307, 2014. doi: 10.1002/embr.201337946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Payne R, Hoff H, Roskowski A, Foskett JK. MICU2 restricts spatial crosstalk between InsP3R and MCU channels by regulating threshold and gain of MICU1-mediated inhibition and activation of MCU. Cell Rep 21: 3141–3154, 2017. doi: 10.1016/j.celrep.2017.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hoffman NE, Chandramoorthy HC, Shamugapriya S, Zhang X, Rajan S, Mallilankaraman K, Gandhirajan RK, Vagnozzi RJ, Ferrer LM, Sreekrishnanilayam K, Natarajaseenivasan K, Vallem S, Force T, Choi ET, Cheung JY, Madesh M. MICU1 motifs define mitochondrial calcium uniporter binding and activity. Cell Rep 5: 1576–1588, 2013. doi: 10.1016/j.celrep.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Matesanz-Isabel J, Arias-del-Val J, Alvarez-Illera P, Fonteriz RI, Montero M, Alvarez J. Functional roles of MICU1 and MICU2 in mitochondrial Ca2+ uptake. Biochim Biophys Acta 1858: 1110–1117, 2016. doi: 10.1016/j.bbamem.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 176.Kamer KJ, Grabarek Z, Mootha VK. High-affinity cooperative Ca2+ binding by MICU1-MICU2 serves as an on-off switch for the uniporter. EMBO Rep 18: 1397–1411, 2017. doi: 10.15252/embr.201643748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Song J, Liu X, Zhai P, Huang J, Lu L. A putative mitochondrial calcium uniporter in A. fumigatus contributes to mitochondrial Ca2+ homeostasis and stress responses. Fungal Genet Biol 94: 15–22, 2016. doi: 10.1016/j.fgb.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 178.Aichberger KJ, Mittermann I, Reininger R, Seiberler S, Swoboda I, Spitzauer S, Kopp T, Stingl G, Sperr WR, Valent P, Repa A, Bohle B, Kraft D, Valenta R. Hom s 4, an IgE-reactive autoantigen belonging to a new subfamily of calcium-binding proteins, can induce Th cell type 1-mediated autoreactivity. J Immunol 175: 1286–1294, 2005. doi: 10.4049/jimmunol.175.2.1286. [DOI] [PubMed] [Google Scholar]
- 179.M’Angale PG, Staveley BE. Inhibition of mitochondrial calcium uptake 1 in Drosophila neurons. Genet Mol Res 16: gmr16019436, 2017. doi: 10.4238/gmr16019436. [DOI] [PubMed] [Google Scholar]
- 180.Patron M, Checchetto V, Raffaello A, Teardo E, Vecellio Reane D, Mantoan M, Granatiero V, Szabò I, De Stefani D, Rizzuto R. MICU1 and MICU2 finely tune the mitochondrial Ca2+ uniporter by exerting opposite effects on MCU activity. Mol Cell 53: 726–737, 2014. doi: 10.1016/j.molcel.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Vecellio Reane D, Vallese F, Checchetto V, Acquasaliente L, Butera G, De Filippis V, Szabò I, Zanotti G, Rizzuto R, Raffaello A. A MICU1 splice variant confers high sensitivity to the mitochondrial Ca2+ uptake machinery of skeletal muscle. Mol Cell 64: 760–773, 2016. doi: 10.1016/j.molcel.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 182.Wang L, Yang X, Li S, Wang Z, Liu Y, Feng J, Zhu Y, Shen Y. Structural and mechanistic insights into MICU1 regulation of mitochondrial calcium uptake. EMBO J 33: 594–604, 2014. doi: 10.1002/embj.201386523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Waldeck-Weiermair M, Malli R, Parichatikanond W, Gottschalk B, Madreiter-Sokolowski CT, Klec C, Rost R, Graier WF. Rearrangement of MICU1 multimers for activation of MCU is solely controlled by cytosolic Ca2+. Sci Rep 5: 15602, 2015. doi: 10.1038/srep15602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Fan M, Zhang J, Tsai CW, Orlando BJ, Rodriguez M, Xu Y, Liao M, Tsai MF, Feng L. Structure and mechanism of the mitochondrial Ca2+ uniporter holocomplex. Nature 582: 129–133, 2020. doi: 10.1038/s41586-020-2309-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wang C, Jacewicz A, Delgado BD, Baradaran R, Long SB. Structures reveal gatekeeping of the mitochondrial Ca2+ uniporter by MICU1-MICU2. Elife 9: e59991, 2020. doi: 10.7554/eLife.59991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang Y, Han Y, She J, Nguyen NX, Mootha VK, Bai XC, Jiang Y. Structural insights into the Ca2+-dependent gating of the human mitochondrial calcium uniporter. Elife 9: e60513, 2020. doi: 10.7554/eLife.60513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Paillard M, Csordás G, Szanda G, Golenár T, Debattisti V, Bartok A, Wang N, Moffat C, Seifert EL, Spät A, Hajnóczky G. Tissue-specific mitochondrial decoding of cytoplasmic Ca2+ signals is controlled by the stoichiometry of MICU1/2 and MCU. Cell Rep 18: 2291–2300, 2017. doi: 10.1016/j.celrep.2017.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Payne R, Li C, Foskett JK. Variable assembly of EMRE and MCU creates functional channels with distinct gatekeeping profiles. iScience 23: 101037, 2020. doi: 10.1016/j.isci.2020.101037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Basso E, Rigotto G, Zucchetti AE, Pozzan T. Slow activation of fast mitochondrial Ca2+ uptake by cytosolic Ca2+. J Biol Chem 293: 17081–17094, 2018. doi: 10.1074/jbc.RA118.002332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kamer KJ, Sancak Y, Fomina Y, Meisel JD, Chaudhuri D, Grabarek Z, Mootha VK. MICU1 imparts the mitochondrial uniporter with the ability to discriminate between Ca2+ and Mn2+. Proc Natl Acad Sci USA 115: E7960–E7969, 2018. doi: 10.1073/pnas.1807811115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wettmarshausen J, Goh V, Huang KT, Arduino DM, Tripathi U, Leimpek A, Cheng Y, Pittis AA, Gabaldón T, Mokranjac D, Hajnóczky G, Perocchi F. MICU1 confers protection from MCU-dependent manganese toxicity. Cell Rep 25: 1425–1435.e7, 2018. doi: 10.1016/j.celrep.2018.10.037. [DOI] [PubMed] [Google Scholar]
- 192.Paillard M, Csordás G, Huang KT, Várnai P, Joseph SK, Hajnóczky G. MICU1 interacts with the D-ring of the MCU pore to control its Ca2+ flux and sensitivity to Ru360. Mol Cell 72: 778–785.e3, 2018. doi: 10.1016/j.molcel.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wu W, Shen Q, Lei Z, Qiu Z, Li D, Pei H, Zheng J, Jia Z. The crystal structure of MICU2 provides insight into Ca2+ binding and MICU1-MICU2 heterodimer formation. EMBO Rep 20: e47488, 2019. doi: 10.15252/embr.201847488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wu W, Shen Q, Zhang R, Qiu Z, Wang Y, Zheng J, Jia Z. The structure of the MICU1-MICU2 complex unveils the regulation of the mitochondrial calcium uniporter. EMBO J 39: e104285, 2020. doi: 10.15252/embj.2019104285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tufi R, Gleeson TP, von Stockum S, Hewitt VL, Lee JJ, Terriente-Felix A, Sanchez-Martinez A, Ziviani E, Whitworth AJ. Comprehensive genetic characterization of mitochondrial Ca2+ uniporter components reveals their different physiological requirements in vivo. Cell Rep 27: 1541–1550.e5, 2019. doi: 10.1016/j.celrep.2019.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Scheinfeldt LB, Soi S, Thompson S, Ranciaro A, Woldemeskel D, Beggs W, Lambert C, Jarvis JP, Abate D, Belay G, Tishkoff SA. Genetic adaptation to high altitude in the Ethiopian highlands. Genome Biol 13: R1, 2012. doi: 10.1186/gb-2012-13-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Semenza GL. Hypoxia-inducible factor 1: regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochim Biophys Acta 1813: 1263–1268, 2011. doi: 10.1016/j.bbamcr.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tomar D, Thomas M, Garbincius JF, Kolmetzky DW, Salik O, Jadiya P, Carpenter AC, Elrod JW. MICU1 regulates mitochondrial cristae structure and function independent of the mitochondrial calcium uniporter channel (Preprint). bioRxiv 803213, 2019. doi: 10.1101/803213. [DOI] [PMC free article] [PubMed]
- 199.Logan CV, Szabadkai G, Sharpe JA, Parry DA, Torelli S, Childs AM, et al. Loss-of-function mutations in MICU1 cause a brain and muscle disorder linked to primary alterations in mitochondrial calcium signaling. Nat Genet 46: 188–193, 2014. doi: 10.1038/ng.2851. [DOI] [PubMed] [Google Scholar]
- 200.Lewis-Smith D, Kamer KJ, Griffin H, Childs AM, Pysden K, Titov D, Duff J, Pyle A, Taylor RW, Yu-Wai-Man P, Ramesh V, Horvath R, Mootha VK, Chinnery PF. Homozygous deletion in MICU1 presenting with fatigue and lethargy in childhood. Neurol Genet 2: e59, 2016. doi: 10.1212/NXG.0000000000000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.O’Grady GL, Lek M, Lamande SR, Waddell L, Oates EC, Punetha J, Ghaoui R, Sandaradura SA, Best H, Kaur S, Davis M, Laing NG, Muntoni F, Hoffman E, MacArthur DG, Clarke NF, Cooper S, North K. Diagnosis and etiology of congenital muscular dystrophy: we are halfway there. Ann Neurol 80: 101–111, 2016. doi: 10.1002/ana.24687. [DOI] [PubMed] [Google Scholar]
- 202.Musa S, Eyaid W, Kamer K, Ali R, Al-Mureikhi M, Shahbeck N, Al Mesaifri F, Makhseed N, Mohamed Z, AlShehhi WA, Mootha VK, Juusola J, Ben-Omran T. A Middle Eastern founder mutation expands the genotypic and phenotypic spectrum of mitochondrial MICU1 deficiency: a report of 13 patients. JIMD Rep 43: 79–83, 2019. doi: 10.1007/8904_2018_107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mojbafan M, Nojehdeh ST, Rahiminejad F, Nilipour Y, Tonekaboni SH, Zeinali S. Reporting a rare form of myopathy, myopathy with extrapyramidal signs, in an Iranian family using next generation sequencing: a case report. BMC Med Genet 21: 77, 2020. doi: 10.1186/s12881-020-01016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bhosale G, Sharpe JA, Koh A, Kouli A, Szabadkai G, Duchen MR. Pathological consequences of MICU1 mutations on mitochondrial calcium signalling and bioenergetics. Biochim Biophys Acta Mol Cell Res 1864: 1009–1017, 2017. doi: 10.1016/j.bbamcr.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tang NH, Kim KW, Xu S, Blazie SM, Yee BA, Yeo GW, Jin Y, Chisholm AD. The mRNA decay factor CAR-1/LSM14 regulates axon regeneration via mitochondrial calcium dynamics. Curr Biol 30: 865–876.e7, 2020. doi: 10.1016/j.cub.2019.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Antony AN, Paillard M, Moffat C, Juskeviciute E, Correnti J, Bolon B, Rubin E, Csordás G, Seifert EL, Hoek JB, Hajnóczky G. MICU1 regulation of mitochondrial Ca2+ uptake dictates survival and tissue regeneration. Nat Commun 7: 10955, 2016. doi: 10.1038/ncomms10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Debattisti V, Horn A, Singh R, Seifert EL, Hogarth MW, Mazala DA, Huang KT, Horvath R, Jaiswal JK, Hajnóczky G. Dysregulation of mitochondrial Ca2+ uptake and sarcolemma repair underlie muscle weakness and wasting in patients and mice lacking MICU1. Cell Rep 29: 1274–1286.e6, 2019. doi: 10.1016/j.celrep.2019.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Horn A, Van der Meulen JH, Defour A, Hogarth M, Sreetama SC, Reed A, Scheffer L, Chandel NS, Jaiswal JK. Mitochondrial redox signaling enables repair of injured skeletal muscle cells. Sci Signal 10: eeaj1978, 2017. doi: 10.1126/scisignal.aaj1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Xue Q, Pei H, Liu Q, Zhao M, Sun J, Gao E, Ma X, Tao L. MICU1 protects against myocardial ischemia/reperfusion injury and its control by the importer receptor Tom70. Cell Death Dis 8: e2923, 2017. doi: 10.1038/cddis.2017.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wescott AP, Kao JP, Lederer WJ, Boyman L. Voltage-energized calcium-sensitive ATP production by mitochondria. Nat Metab 1: 975–984, 2019. doi: 10.1038/s42255-019-0126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Shanmughapriya S, Tomar D, Dong Z, Slovik KJ, Nemani N, Natarajaseenivasan K, Carvalho E, Lu C, Corrigan K, Garikipati VN, Ibetti J, Rajan S, Barrero C, Chuprun K, Kishore R, Merali S, Tian Y, Yang W, Madesh M. FOXD1-dependent MICU1 expression regulates mitochondrial activity and cell differentiation. Nat Commun 9: 3449, 2018. doi: 10.1038/s41467-018-05856-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Nemani N, Dong Z, Daw CC, Madaris TR, Ramachandran K, Enslow BT, Rubannelsonkumar CS, Shanmughapriya S, Mallireddigari V, Maity S, SinghMalla P, Natarajanseenivasan K, Hooper R, Shannon CE, Tourtellotte WG, Singh BB, Reeves WB, Sharma K, Norton L, Srikantan S, Soboloff J, Madesh M. Mitochondrial pyruvate and fatty acid flux modulate MICU1-dependent control of MCU activity. Sci Signal 13: eaaz6206, 2020. doi: 10.1126/scisignal.aaz6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tomar D, Elrod JW. Metabolite regulation of the mitochondrial calcium uniporter channel. Cell Calcium 92: 102288, 2020. doi: 10.1016/j.ceca.2020.102288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Rao G, Dwivedi SK, Zhang Y, Dey A, Shameer K, Karthik R, Srikantan S, Hossen MN, Wren JD, Madesh M, Dudley JT, Bhattacharya R, Mukherjee P. MicroRNA-195 controls MICU1 expression and tumor growth in ovarian cancer. EMBO Rep 21: e48483, 2020. doi: 10.15252/embr.201948483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ji L, Liu F, Jing Z, Huang Q, Zhao Y, Cao H, Li J, Yin C, Xing J, Li F. MICU1 alleviates diabetic cardiomyopathy through mitochondrial Ca2+-dependent antioxidant response. Diabetes 66: 1586–1600, 2017. doi: 10.2337/db16-1237. [DOI] [PubMed] [Google Scholar]
- 216.Roman B, Kaur P, Ashok D, Kohr M, Biswas R, O’Rourke B, Steenbergen C, Das S. Nuclear-mitochondrial communication involving miR-181c plays an important role in cardiac dysfunction during obesity. J Mol Cell Cardiol 144: 87–96, 2020. doi: 10.1016/j.yjmcc.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Banavath HN, Roman B, Mackowski N, Biswas D, Afzal J, Nomura Y, Solhjoo S, O’Rourke B, Kohr M, Murphy E, Steenbergen C, Das S. miR-181c activates mitochondrial calcium uptake by regulating MICU1 in the heart. J Am Heart Assoc 8: e012919, 2019. doi: 10.1161/JAHA.119.012919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Feissner RF, Skalska J, Gaum WE, Sheu SS. Crosstalk signaling between mitochondrial Ca2+ and ROS. Front Biosci (Landmark Ed) 14: 1197–1218, 2009. doi: 10.2741/3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Matteucci A, Patron M, Vecellio Reane D, Gastaldello S, Amoroso S, Rizzuto R, Brini M, Raffaello A, Calì T. Parkin-dependent regulation of the MCU complex component MICU1. Sci Rep 8: 14199, 2018. doi: 10.1038/s41598-018-32551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Stoll S, Xi J, Ma B, Leimena C, Behringer EJ, Qin G, Qiu H. The valosin-containing protein protects the heart against pathological Ca2+ overload by modulating Ca2+ uptake proteins. Toxicol Sci 171: 473–484, 2019. doi: 10.1093/toxsci/kfz164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Petrungaro C, Zimmermann KM, Küttner V, Fischer M, Dengjel J, Bogeski I, Riemer J. The Ca2+-dependent release of the Mia40-induced MICU1-MICU2 dimer from MCU regulates mitochondrial Ca2+ uptake. Cell Metab 22: 721–733, 2015. doi: 10.1016/j.cmet.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 222.Madreiter-Sokolowski CT, Klec C, Parichatikanond W, Stryeck S, Gottschalk B, Pulido S, Rost R, Eroglu E, Hofmann NA, Bondarenko AI, Madl T, Waldeck-Weiermair M, Malli R, Graier WF. PRMT1-mediated methylation of MICU1 determines the UCP2/3 dependency of mitochondrial Ca2+ uptake in immortalized cells. Nat Commun 7: 12897, 2016. doi: 10.1038/ncomms12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Marchi S, Corricelli M, Branchini A, Vitto VA, Missiroli S, Morciano G, Perrone M, Ferrarese M, Giorgi C, Pinotti M, Galluzzi L, Kroemer G, Pinton P. Akt-mediated phosphorylation of MICU1 regulates mitochondrial Ca2+ levels and tumor growth. EMBO J 38: e99435, 2019. doi: 10.15252/embj.201899435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Bijur GN, Jope RS. Rapid accumulation of Akt in mitochondria following phosphatidylinositol 3-kinase activation. J Neurochem 87: 1427–1435, 2003. doi: 10.1046/j.1471-4159.2003.02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Yang JY, Deng W, Chen Y, Fan W, Baldwin KM, Jope RS, Wallace DC, Wang PH. Impaired translocation and activation of mitochondrial Akt1 mitigated mitochondrial oxidative phosphorylation complex V activity in diabetic myocardium. J Mol Cell Cardiol 59: 167–175, 2013. doi: 10.1016/j.yjmcc.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Barksdale KA, Bijur GN. The basal flux of Akt in the mitochondria is mediated by heat shock protein 90. J Neurochem 108: 1289–1299, 2009. doi: 10.1111/j.1471-4159.2009.05878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Antico Arciuch VG, Galli S, Franco MC, Lam PY, Cadenas E, Carreras MC, Poderoso JJ. Akt1 intramitochondrial cycling is a crucial step in the redox modulation of cell cycle progression. PLoS One 4: e7523, 2009. doi: 10.1371/journal.pone.0007523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Di Marco G, Vallese F, Jourde B, Bergsdorf C, Sturlese M, De Mario A, Techer-Etienne V, Haasen D, Oberhauser B, Schleeger S, Minetti G, Moro S, Rizzuto R, De Stefani D, Fornaro M, Mammucari C. A high-throughput screening identifies MICU1 targeting compounds. Cell Rep 30: 2321–2331.6, 2020. doi: 10.1016/j.celrep.2020.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Li D, Wu W, Pei H, Wei Q, Yang Q, Zheng J, Jia Z. Expression and preliminary characterization of human MICU2. Biol Open 5: 962–969, 2016. doi: 10.1242/bio.018572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kamer KJ, Jiang W, Kaushik VK, Mootha VK, Grabarek Z. Crystal structure of MICU2 and comparison with MICU1 reveal insights into the uniporter gating mechanism. Proc Natl Acad Sci USA 116: 3546–3555, 2019. doi: 10.1073/pnas.1817759116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Park J, Lee Y, Park T, Kang JY, Mun SA, Jin M, Yang J, Eom SH. Structure of the MICU1-MICU2 heterodimer provides insights into the gatekeeping threshold shift. IUCrJ 7: 355–365, 2020. doi: 10.1107/S2052252520001840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Xing Y, Wang M, Wang J, Nie Z, Wu G, Yang X, Shen Y. Dimerization of MICU proteins controls Ca2+ influx through the mitochondrial Ca2+ uniporter. Cell Rep 26: 1203–1212.e4, 2019. doi: 10.1016/j.celrep.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 233.Shamseldin HE, Alasmari A, Salih MA, Samman MM, Mian SA, Alshidi T, Ibrahim N, Hashem M, Faqeih E, Al-Mohanna F, Alkuraya FS. A null mutation in MICU2 causes abnormal mitochondrial calcium homeostasis and a severe neurodevelopmental disorder. Brain 140: 2806–2813, 2017. doi: 10.1093/brain/awx237. [DOI] [PubMed] [Google Scholar]
- 234.Bick AG, Wakimoto H, Kamer KJ, Sancak Y, Goldberger O, Axelsson A, DeLaughter DM, Gorham JM, Mootha VK, Seidman JG, Seidman CE. Cardiovascular homeostasis dependence on MICU2, a regulatory subunit of the mitochondrial calcium uniporter. Proc Natl Acad Sci USA 114: E9096–E9104, 2017. doi: 10.1073/pnas.1711303114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ashrafi G, de Juan-Sanz J, Farrell RJ, Ryan TA. Molecular tuning of the axonal mitochondrial Ca2+ uniporter ensures metabolic flexibility of neurotransmission. Neuron 105: 678–687.e5, 2019. doi: 10.1016/j.neuron.2019.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Puente BN, Sun J, Parks RJ, Fergusson MM, Liu C, Springer DA, Aponte AM, Liu JC, Murphy E. MICU3 plays an important role in cardiovascular function. Circ Res 127: 1571–1573, 2020. doi: 10.1161/CIRCRESAHA.120.317177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Hutto RA, Bisbach CM, Abbas F, Brock DC, Cleghorn WM, Parker ED, Bauer BH, Ge W, Vinberg F, Hurley JB, Brockerhoff SE. Increasing Ca2+ in photoreceptor mitochondria alters metabolites, accelerates photoresponse recovery, and reveals adaptations to mitochondrial stress. Cell Death Differ 27: 1067–1085, 2020. doi: 10.1038/s41418-019-0398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Calvo SE, Clauser KR, Mootha VK. MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res 44: D1251–D1257, 2016. doi: 10.1093/nar/gkv1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Lambert JP, Murray EK, Elrod JW. MCUB and mitochondrial calcium uptake—modeling, function, and therapeutic potential. Expert Opin Ther Targets 24: 163–169, 2020. doi: 10.1080/14728222.2020.1732926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Checchetto V, Szabò I. MCU regulation in lipid bilayer and electrophysiological recording. Methods Mol Biol 1925: 59–63, 2019. doi: 10.1007/978-1-4939-9018-4_5. [DOI] [PubMed] [Google Scholar]
- 241.Huo J, Lu S, Kwong JQ, Bround MJ, Grimes KM, Sargent MA, Brown ME, Davis ME, Bers DM, Molkentin JD. MCUb Induction Protects the Heart From Postischemic Remodeling. Circ Res 127: 379–390, 2020. doi: 10.1161/CIRCRESAHA.119.316369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Lambert JP, Luongo TS, Tomar D, Jadiya P, Gao E, Zhang X, Lucchese AM, Kolmetzky DW, Shah NS, Elrod JW. MCUB Regulates the molecular composition of the mitochondrial calcium uniporter channel to limit mitochondrial calcium overload during stress. Circulation 140: 1720–1733, 2019. doi: 10.1161/CIRCULATIONAHA.118.037968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Belosludtsev KN, Talanov EY, Starinets VS, Agafonov AV, Dubinin MV, Belosludtseva NV. Transport of Ca2+ and Ca2+-dependent permeability transition in rat liver mitochondria under the streptozotocin-induced type I diabetes. Cells 8: 1014, 2019. doi: 10.3390/cells8091014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Xu R, Han M, Xu Y, Zhang X, Zhang C, Zhang D, Ji J, Wei Y, Wang S, Huang B, Chen A, Zhang Q, Li W, Sun T, Wang F, Li X, Wang J. Coiled-coil domain containing 109B is a HIF1alpha-regulated gene critical for progression of human gliomas. J Transl Med 15: 165, 2017. doi: 10.1186/s12967-017-1266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Rebane A, Zimmermann M, Aab A, Baurecht H, Koreck A, Karelson M, Abram K, Metsalu T, Pihlap M, Meyer N, Fölster-Holst R, Nagy N, Kemeny L, Kingo K, Vilo J, Illig T, Akdis M, Franke A, Novak N, Weidinger S, Akdis CA. Mechanisms of IFN-gamma-induced apoptosis of human skin keratinocytes in patients with atopic dermatitis. J Allergy Clin Immunol 129: 1297–1306, 2012. doi: 10.1016/j.jaci.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 246.Dubinin MV, Talanov EY, Tenkov KS, Starinets VS, Mikheeva IB, Sharapov MG, Belosludtsev KN. Duchenne muscular dystrophy is associated with the inhibition of calcium uniport in mitochondria and an increased sensitivity of the organelles to the calcium-induced permeability transition. Biochim Biophys Acta Mol Basis Dis 1866: 165674, 2020. doi: 10.1016/j.bbadis.2020.165674. [DOI] [PubMed] [Google Scholar]
- 247.Dubinin MV, Talanov EY, Tenkov KS, Starinets VS, Mikheeva IB, Belosludtsev KN. Transport of Ca2+ and Ca2+-dependent permeability transition in heart mitochondria in the early stages of Duchenne muscular dystrophy. Biochim Biophys Acta Bioenerg 1861: 148250, 2020. doi: 10.1016/j.bbabio.2020.148250. [DOI] [PubMed] [Google Scholar]
- 248.Mallilankaraman K, Cárdenas C, Doonan PJ, Chandramoorthy HC, Irrinki KM, Golenár T, Csordás G, Madireddi P, Yang J, Müller M, Miller R, Kolesar JE, Molgó J, Kaufman B, Hajnóczky G, Foskett JK, Madesh M. MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat Cell Biol 14: 1336–1343, 2012. doi: 10.1038/ncb2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Vais H, Tanis JE, Müller M, Payne R, Mallilankaraman K, Foskett JK. MCUR1, CCDC90A, Is a Regulator of the Mitochondrial Calcium Uniporter. Cell Metab 22: 533–535, 2015. doi: 10.1016/j.cmet.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Chaudhuri D, Artiga DJ, Abiria SA, Clapham DE. Mitochondrial calcium uniporter regulator 1 (MCUR1) regulates the calcium threshold for the mitochondrial permeability transition. Proc Natl Acad Sci USA 113: E1872–E1880, 2016. doi: 10.1073/pnas.1602264113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Adlakha J, Karamichali I, Sangwallek J, Deiss S, Bär K, Coles M, Hartmann MD, Lupas AN, Hernandez Alvarez B. Characterization of MCU-binding proteins MCUR1 and CCDC90B—representatives of a protein family conserved in prokaryotes and eukaryotic organelles. Structure 27: 464–475.e6, 2019. doi: 10.1016/j.str.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 252.Paupe V, Prudent J, Dassa EP, Rendon OZ, Shoubridge EA. CCDC90A (MCUR1) is a cytochrome c oxidase assembly factor and not a regulator of the mitochondrial calcium uniporter. Cell Metab 21: 109–116, 2015. doi: 10.1016/j.cmet.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 253.Tomar D, Dong Z, Shanmughapriya S, Koch DA, Thomas T, Hoffman NE, et al. MCUR1 Is a Scaffold Factor for the MCU Complex Function and Promotes Mitochondrial Bioenergetics. Cell Rep 15: 1673–1685, 2016. doi: 10.1016/j.celrep.2016.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Jin M, Wang J, Ji X, Cao H, Zhu J, Chen Y, Yang J, Zhao Z, Ren T, Xing J. MCUR1 facilitates epithelial-mesenchymal transition and metastasis via the mitochondrial calcium dependent ROS/Nrf2/Notch pathway in hepatocellular carcinoma. J Exp Clin Cancer Res 38: 136, 2019. doi: 10.1186/s13046-019-1135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Natarajan V, Mah T, Peishi C, Tan SY, Chawla R, Arumugam TV, Ramasamy A, Mallilankaraman K. Oxygen glucose deprivation induced prosurvival autophagy is insufficient to rescue endothelial function. Front Physiol 11: 533683, 2020. doi: 10.3389/fphys.2020.533683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Niemann J, Zehm C, Waterstradt R, Tiedge M, Baltrusch S. Cytosolic and mitochondrial Ca2+ concentrations in primary hepatocytes change with ageing and in consequence of an mtDNA mutation. Cell Calcium 82: 102055, 2019. doi: 10.1016/j.ceca.2019.102055. [DOI] [PubMed] [Google Scholar]
- 257.Vishnyakova PA, Tarasova NV, Volodina MA, Tsvirkun DV, Sukhanova IA, Kurchakova TA, Kan NE, Medzidova MK, Sukhikh GT, Vysokikh MY. Gestation age-associated dynamics of mitochondrial calcium uniporter subunits expression in feto-maternal complex at term and preterm delivery. Sci Rep 9: 5501, 2019. doi: 10.1038/s41598-019-41996-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Amigo I, Traba J, González-Barroso MC, Rueda CB, Fernández M, Rial E, Sánchez A, Satrústegui J, Del Arco A. Glucagon regulation of oxidative phosphorylation requires an increase in matrix adenine nucleotide content through Ca2+ activation of the mitochondrial ATP-Mg/Pi carrier SCaMC-3. J Biol Chem 288: 7791–7802, 2013. doi: 10.1074/jbc.M112.409144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Satrústegui J, Pardo B, Del Arco A. Mitochondrial transporters as novel targets for intracellular calcium signaling. Physiol Rev 87: 29–67, 2007. doi: 10.1152/physrev.00005.2006. [DOI] [PubMed] [Google Scholar]
- 260.Hoffman NE, Chandramoorthy HC, Shanmughapriya S, Zhang XQ, Vallem S, Doonan PJ, Malliankaraman K, Guo S, Rajan S, Elrod JW, Koch WJ, Cheung JY, Madesh M. SLC25A23 augments mitochondrial Ca2+ uptake, interacts with MCU, and induces oxidative stress-mediated cell death. Mol Biol Cell 25: 936–947, 2014. doi: 10.1091/mbc.e13-08-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Trenker M, Malli R, Fertschai I, Levak-Frank S, Graier WF. Uncoupling proteins 2 and 3 are fundamental for mitochondrial Ca2+ uniport. Nat Cell Biol 9: 445–452, 2007. doi: 10.1038/ncb1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Jean-Quartier C, Bondarenko AI, Alam MR, Trenker M, Waldeck-Weiermair M, Malli R, Graier WF. Studying mitochondrial Ca2+ uptake—a revisit. Mol Cell Endocrinol 353: 114–127, 2012. doi: 10.1016/j.mce.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Bondarenko AI, Jean-Quartier C, Malli R, Graier WF. Characterization of distinct single-channel properties of Ca2+ inward currents in mitochondria. Pflugers Arch 465: 997–1010, 2013. doi: 10.1007/s00424-013-1224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Bondarenko AI, Jean-Quartier C, Parichatikanond W, Alam MR, Waldeck-Weiermair M, Malli R, Graier WF. Mitochondrial Ca2+ uniporter (MCU)-dependent and MCU-independent Ca2+ channels coexist in the inner mitochondrial membrane. Pflugers Arch 466: 1411–1420, 2014. doi: 10.1007/s00424-013-1383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Bondarenko AI, Parichatikanond W, Madreiter CT, Rost R, Waldeck-Weiermair M, Malli R, Graier WF. UCP2 modulates single-channel properties of a MCU-dependent Ca2+ inward current in mitochondria. Pflugers Arch 467: 2509–2518, 2015. doi: 10.1007/s00424-015-1727-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Beutner G, Sharma VK, Giovannucci DR, Yule DI, Sheu SS. Identification of a ryanodine receptor in rat heart mitochondria. J Biol Chem 276: 21482–21488, 2001. doi: 10.1074/jbc.M101486200. [DOI] [PubMed] [Google Scholar]
- 267.Ryu SY, Beutner G, Kinnally KW, Dirksen RT, Sheu SS. Single channel characterization of the mitochondrial ryanodine receptor in heart mitoplasts. J Biol Chem 286: 21324–21329, 2011. doi: 10.1074/jbc.C111.245597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Chalmers S, Nicholls DG. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J Biol Chem 278: 19062–19070, 2003. doi: 10.1074/jbc.M212661200. [DOI] [PubMed] [Google Scholar]
- 269.Wei AC, Liu T, Winslow RL, O’Rourke B. Dynamics of matrix-free Ca2+ in cardiac mitochondria: two components of Ca2+ uptake and role of phosphate buffering. J Gen Physiol 139: 465–478, 2012. doi: 10.1085/jgp.201210784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Giacomello M, Drago I, Bortolozzi M, Scorzeto M, Gianelle A, Pizzo P, Pozzan T. Ca2+ hot spots on the mitochondrial surface are generated by Ca2+ mobilization from stores, but not by activation of store-operated Ca2+ channels. Mol Cell 38: 280–290, 2010. doi: 10.1016/j.molcel.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 271.Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science 262: 744–747, 1993. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- 272.Hajnóczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell 82: 415–424, 1995. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- 273.Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280: 1763–1766, 1998. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 274.Csordás G, Thomas AP, Hajnóczky G. Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J 18: 96–108, 1999. doi: 10.1093/emboj/18.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Beutner G, Sharma VK, Lin L, Ryu SY, Dirksen RT, Sheu SS. Type 1 ryanodine receptor in cardiac mitochondria: transducer of excitation-metabolism coupling. Biochim Biophys Acta 1717: 1–10, 2005. doi: 10.1016/j.bbamem.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 276.Altschafl BA, Beutner G, Sharma VK, Sheu SS, Valdivia HH. The mitochondrial ryanodine receptor in rat heart: a pharmaco-kinetic profile. Biochim Biophys Acta 1768: 1784–1795, 2007. doi: 10.1016/j.bbamem.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 277.Jakob R, Beutner G, Sharma VK, Duan Y, Gross RA, Hurst S, Jhun BS, O-Uchi J, Sheu SS. Molecular and functional identification of a mitochondrial ryanodine receptor in neurons. Neurosci Lett 575: 7–12, 2014. doi: 10.1016/j.neulet.2014.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Hamilton J, Brustovetsky T, Rysted JE, Lin Z, Usachev YM, Brustovetsky N. Deletion of mitochondrial calcium uniporter incompletely inhibits calcium uptake and induction of the permeability transition pore in brain mitochondria. J Biol Chem 293: 15652–15663, 2018. doi: 10.1074/jbc.RA118.002926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Seidlmayer LK, Kuhn J, Berbner A, Arias-Loza PA, Williams T, Kaspar M, Czolbe M, Kwong JQ, Molkentin JD, Heinze KG, Dedkova EN, Ritter O. Inositol 1,4,5-trisphosphate-mediated sarcoplasmic reticulum-mitochondrial crosstalk influences adenosine triphosphate production via mitochondrial Ca2+ uptake through the mitochondrial ryanodine receptor in cardiac myocytes. Cardiovasc Res 112: 491–501, 2016. doi: 10.1093/cvr/cvw185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Tewari SG, Camara AK, Stowe DF, Dash RK. Computational analysis of Ca2+ dynamics in isolated cardiac mitochondria predicts two distinct modes of Ca2+ uptake. J Physiol 592: 1917–1930, 2014. doi: 10.1113/jphysiol.2013.268847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.O-Uchi J, Jhun BS, Hurst S, Bisetto S, Gross P, Chen M, Kettlewell S, Park J, Oyamada H, Smith GL, Murayama T, Sheu SS. Overexpression of ryanodine receptor type 1 enhances mitochondrial fragmentation and Ca2+-induced ATP production in cardiac H9c2 myoblasts. Am J Physiol Heart Circ Physiol 305: H1736–H1751, 2013. doi: 10.1152/ajpheart.00094.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Palty R, Silverman WF, Hershfinkel M, Caporale T, Sensi SL, Parnis J, Nolte C, Fishman D, Shoshan-Barmatz V, Herrmann S, Khananshvili D, Sekler I. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc Natl Acad Sci USA 107: 436–441, 2010. doi: 10.1073/pnas.0908099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Jung DW, Baysal K, Brierley GP. The sodium-calcium antiport of heart mitochondria is not electroneutral. J Biol Chem 270: 672–678, 1995. doi: 10.1074/jbc.270.2.672. [DOI] [PubMed] [Google Scholar]
- 284.Griffiths EJ. Reversal of mitochondrial Na/Ca exchange during metabolic inhibition in rat cardiomyocytes. FEBS Lett 453: 400–404, 1999. doi: 10.1016/S0014-5793(99)00726-7. [DOI] [PubMed] [Google Scholar]
- 285.Smets I, Caplanusi A, Despa S, Molnar Z, Radu M, VandeVen M, Ameloot M, Steels P. Ca2+ uptake in mitochondria occurs via the reverse action of the Na+/Ca2+ exchanger in metabolically inhibited MDCK cells. Am J Physiol Renal Physiol 286: F784–F794, 2004. doi: 10.1152/ajprenal.00284.2003. [DOI] [PubMed] [Google Scholar]
- 286.Kim B, Matsuoka S. Cytoplasmic Na+-dependent modulation of mitochondrial Ca2+ via electrogenic mitochondrial Na+-Ca2+ exchange. J Physiol 586: 1683–1697, 2008. doi: 10.1113/jphysiol.2007.148726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Santo-Domingo J, Demaurex N. Calcium uptake mechanisms of mitochondria. Biochim Biophys Acta 1797: 907–912, 2010. doi: 10.1016/j.bbabio.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 288.Jiang D, Zhao L, Clapham DE. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science 326: 144–147, 2009. doi: 10.1126/science.1175145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Waldeck-Weiermair M, Jean-Quartier C, Rost R, Khan MJ, Vishnu N, Bondarenko AI, Imamura H, Malli R, Graier WF. Leucine zipper EF hand-containing transmembrane protein 1 (Letm1) and uncoupling proteins 2 and 3 (UCP2/3) contribute to two distinct mitochondrial Ca2+ uptake pathways. J Biol Chem 286: 28444–28455, 2011. doi: 10.1074/jbc.M111.244517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Alam MR, Groschner LN, Parichatikanond W, Kuo L, Bondarenko AI, Rost R, Waldeck-Weiermair M, Malli R, Graier WF. Mitochondrial Ca2+ uptake 1 (MICU1) and mitochondrial Ca2+ uniporter (MCU) contribute to metabolism-secretion coupling in clonal pancreatic beta-cells. J Biol Chem 287: 34445–34454, 2012. doi: 10.1074/jbc.M112.392084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Akhmedov D, Braun M, Mataki C, Park KS, Pozzan T, Schoonjans K, Rorsman P, Wollheim CB, Wiederkehr A. Mitochondrial matrix pH controls oxidative phosphorylation and metabolism-secretion coupling in INS-1E clonal beta cells. FASEB J 24: 4613–4626, 2010. doi: 10.1096/fj.10-162222. [DOI] [PubMed] [Google Scholar]
- 292.Llopis J, McCaffery JM, Miyawaki A, Farquhar MG, Tsien RY. Measurement of cytosolic, mitochondrial, and Golgi pH in single living cells with green fluorescent proteins. Proc Natl Acad Sci USA 95: 6803–6808, 1998. doi: 10.1073/pnas.95.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Abad MF, Di Benedetto G, Magalhães PJ, Filippin L, Pozzan T. Mitochondrial pH monitored by a new engineered green fluorescent protein mutant. J Biol Chem 279: 11521–11529, 2004. doi: 10.1074/jbc.M306766200. [DOI] [PubMed] [Google Scholar]
- 294.Rossignol R, Gilkerson R, Aggeler R, Yamagata K, Remington SJ, Capaldi RA. Energy substrate modulates mitochondrial structure and oxidative capacity in cancer cells. Cancer Res 64: 985–993, 2004. doi: 10.1158/0008-5472.CAN-03-1101. [DOI] [PubMed] [Google Scholar]
- 295.Bernardi P, Azzone GF. A membrane potential-modulated pathway for Ca2+ efflux in rat liver mitochondria. FEBS Lett 139: 13–16, 1982. doi: 10.1016/0014-5793(82)80476-6. [DOI] [PubMed] [Google Scholar]
- 296.Bernardi P, Azzone GF. Regulation of Ca2+ efflux in rat liver mitochondria. Role of membrane potential. Eur J Biochem 134: 377–383, 1983. doi: 10.1111/j.1432-1033.1983.tb07578.x. [DOI] [PubMed] [Google Scholar]
- 297.Nowikovsky K, Pozzan T, Rizzuto R, Scorrano L, Bernardi P. Perspectives on: SGP symposium on mitochondrial physiology and medicine: the pathophysiology of LETM1. J Gen Physiol 139: 445–454, 2012. doi: 10.1085/jgp.201110757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Li W, Shariat-Madar Z, Powers M, Sun X, Lane RD, Garlid KD. Reconstitution, identification, purification, and immunological characterization of the 110-kDa Na+/Ca2+ antiporter from beef heart mitochondria. J Biol Chem 267: 17983–17989, 1992. doi: 10.1016/S0021-9258(19)37140-6. [DOI] [PubMed] [Google Scholar]
- 299.Palty R, Ohana E, Hershfinkel M, Volokita M, Elgazar V, Beharier O, Silverman WF, Argaman M, Sekler I. Lithium-calcium exchange is mediated by a distinct potassium-independent sodium-calcium exchanger. J Biol Chem 279: 25234–25240, 2004. doi: 10.1074/jbc.M401229200. [DOI] [PubMed] [Google Scholar]
- 300.Cai X, Lytton J. Molecular cloning of a sixth member of the K+-dependent Na+/Ca2+ exchanger gene family, NCKX6. J Biol Chem 279: 5867–5876, 2004. doi: 10.1074/jbc.M310908200. [DOI] [PubMed] [Google Scholar]
- 301.Cai X, Lytton J. The cation/Ca2+ exchanger superfamily: phylogenetic analysis and structural implications. Mol Biol Evol 21: 1692–1703, 2004. doi: 10.1093/molbev/msh177. [DOI] [PubMed] [Google Scholar]
- 302.Granatiero V, De Stefani D, Rizzuto R. Mitochondrial calcium handling in physiology and disease. Adv Exp Med Biol 982: 25–47, 2017. doi: 10.1007/978-3-319-55330-6_2. [DOI] [PubMed] [Google Scholar]
- 303.Liao J, Li H, Zeng W, Sauer DB, Belmares R, Jiang Y. Structural insight into the ion-exchange mechanism of the sodium/calcium exchanger. Science 335: 686–690, 2012. doi: 10.1126/science.1215759. [DOI] [PubMed] [Google Scholar]
- 304.Marinelli F, Almagor L, Hiller R, Giladi M, Khananshvili D, Faraldo-Gomez JD. Sodium recognition by the Na+/Ca2+ exchanger in the outward-facing conformation. Proc Natl Acad Sci USA 111: E5354–E5362, 2014. doi: 10.1073/pnas.1415751111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Dash RK, Beard DA. Analysis of cardiac mitochondrial Na+-Ca2+ exchanger kinetics with a biophysical model of mitochondrial Ca2+ handling suggests a 3:1 stoichiometry. J Physiol 586: 3267–3285, 2008. doi: 10.1113/jphysiol.2008.151977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Roy S, Dey K, Hershfinkel M, Ohana E, Sekler I. Identification of residues that control Li+ versus Na+ dependent Ca2+ exchange at the transport site of the mitochondrial NCLX. Biochim Biophys Acta 1864: 997–1008, 2017. doi: 10.1016/j.bbamcr.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 307.Refaeli B, Giladi M, Hiller R, Khananshvili D. Structure-based engineering of lithium-transport capacity in an archaeal sodium-calcium exchanger. Biochemistry 55: 1673–1676, 2016. doi: 10.1021/acs.biochem.6b00119. [DOI] [PubMed] [Google Scholar]
- 308.Paucek P, Jabůrek M. Kinetics and ion specificity of Na+/Ca2+ exchange mediated by the reconstituted beef heart mitochondrial Na+/Ca2+ antiporter. Biochim Biophys Acta 1659: 83–91, 2004. doi: 10.1016/j.bbabio.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 309.Palty R, Hershfinkel M, Yagev O, Saar D, Barkalifa R, Khananshvili D, Peretz A, Grossman Y, Sekler I. Single alpha-domain constructs of the Na+/Ca2+ exchanger, NCLX, oligomerize to form a functional exchanger. Biochemistry 45: 11856–11866, 2006. doi: 10.1021/bi060633b. [DOI] [PubMed] [Google Scholar]
- 310.Giladi M, Lee SY, Refaeli B, Hiller R, Chung KY, Khananshvili D. Structure-dynamic and functional relationships in a Li+-transporting sodium-calcium exchanger mutant. Biochim Biophys Acta Bioenerg 1860: 189–200, 2019. doi: 10.1016/j.bbabio.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 311.Cox DA, Matlib MA. A role for the mitochondrial Na+-Ca2+ exchanger in the regulation of oxidative phosphorylation in isolated heart mitochondria. J Biol Chem 268: 938–947, 1993. doi: 10.1016/S0021-9258(18)54024-2. [DOI] [PubMed] [Google Scholar]
- 312.Yang F, He XP, Russell J, Lu B. Ca2+ influx-independent synaptic potentiation mediated by mitochondrial Na+-Ca2+ exchanger and protein kinase C. J Cell Biol 163: 511–523, 2003. doi: 10.1083/jcb.200307027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Nita II, Hershfinkel M, Fishman D, Ozeri E, Rutter GA, Sensi SL, Khananshvili D, Lewis EC, Sekler I. The mitochondrial Na+/Ca2+ exchanger upregulates glucose dependent Ca2+ signalling linked to insulin secretion. PLoS One 7: e46649, 2012. doi: 10.1371/journal.pone.0046649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Takeuchi A, Kim B, Matsuoka S. The mitochondrial Na+-Ca2+ exchanger, NCLX, regulates automaticity of HL-1 cardiomyocytes. Sci Rep 3: 2766, 2013. doi: 10.1038/srep02766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Ben-Kasus Nissim T, Zhang X, Elazar A, Roy S, Stolwijk JA, Zhou Y, Motiani RK, Gueguinou M, Hempel N, Hershfinkel M, Gill DL, Trebak M, Sekler I. Mitochondria control store-operated Ca2+ entry through Na+ and redox signals. EMBO J 36: 797–815, 2017. doi: 10.15252/embj.201592481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Nita II, Caspi Y, Gudes S, Fishman D, Lev S, Hersfinkel M, Sekler I, Binshtok AM. Privileged crosstalk between TRPV1 channels and mitochondrial calcium shuttling machinery controls nociception. Biochim Biophys Acta 1863: 2868–2880, 2016. doi: 10.1016/j.bbamcr.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 317.Kim B, Takeuchi A, Hikida M, Matsuoka S. Roles of the mitochondrial Na+-Ca2+ exchanger, NCLX, in B lymphocyte chemotaxis. Sci Rep 6: 28378, 2016. doi: 10.1038/srep28378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Assali EA, Jones AE, Veliova M, Acin-Pérez R, Taha M, Miller N, Shum M, Oliveira MF, Las G, Liesa M, Sekler I, Shirihai OS. NCLX prevents cell death during adrenergic activation of the brown adipose tissue. Nat Commun 11: 3347, 2020. doi: 10.1038/s41467-020-16572-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Mincheva-Tasheva S, Obis E, Tamarit J, Ros J. Apoptotic cell death and altered calcium homeostasis caused by frataxin depletion in dorsal root ganglia neurons can be prevented by BH4 domain of Bcl-xL protein. Hum Mol Genet 23: 1829–1841, 2014. doi: 10.1093/hmg/ddt576. [DOI] [PubMed] [Google Scholar]
- 320.Obis È, Irazusta V, Sanchís D, Ros J, Tamarit J. Frataxin deficiency in neonatal rat ventricular myocytes targets mitochondria and lipid metabolism. Free Radic Biol Med 73: 21–33, 2014. doi: 10.1016/j.freeradbiomed.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 321.Purroy R, Britti E, Delaspre F, Tamarit J, Ros J. Mitochondrial pore opening and loss of Ca2+ exchanger NCLX levels occur after frataxin depletion. Biochim Biophys Acta Mol Basis Dis 1864: 618–631, 2018. doi: 10.1016/j.bbadis.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 322.Britti E, Delaspre F, Tamarit J, Ros J. Calpain-Inhibitors protect frataxin-deficient dorsal root ganglia neurons from loss of mitochondrial Na+/Ca2+ exchanger, NCLX, and apoptosis. Neurochem Res 46: 108–119, 2021. doi: 10.1007/s11064-020-03020-3. [DOI] [PubMed] [Google Scholar]
- 323.Jadiya P, Kolmetzky DW, Tomar D, Di Meco A, Lombardi AA, Lambert JP, Luongo TS, Ludtmann MH, Praticò D, Elrod JW. Impaired mitochondrial calcium efflux contributes to disease progression in models of Alzheimer’s disease. Nat Commun 10: 3885, 2019. doi: 10.1038/s41467-019-11813-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Ludtmann MH, Kostic M, Horne A, Gandhi S, Sekler I, Abramov AY. LRRK2 deficiency induced mitochondrial Ca2+ efflux inhibition can be rescued by Na+/Ca2+/Li+ exchanger upregulation. Cell Death Dis 10: 265, 2019. doi: 10.1038/s41419-019-1469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Pathak T, Gueguinou M, Walter V, Delierneux C, Johnson MT, Zhang X, Xin P, Yoast RE, Emrich SM, Yochum GS, Sekler I, Koltun WA, Gill DL, Hempel N, Trebak M. Dichotomous role of the human mitochondrial Na+/Ca2+/Li+ exchanger NCLX in colorectal cancer growth and metastasis. Elife 9: e59686, 2020. doi: 10.7554/eLife.59686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Sharma V, Roy S, Sekler I, O’Halloran DM. The NCLX-type Na+/Ca2+ exchanger NCX-9 is required for patterning of neural circuits in Caenorhabditis elegans. J Biol Chem 292: 5364–5377, 2017. doi: 10.1074/jbc.M116.758953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Kostic M, Katoshevski T, Sekler I. Allosteric regulation of NCLX by mitochondrial membrane potential links the metabolic state and Ca2+ signaling in mitochondria. Cell Rep 25: 3465–3475.e4, 2018. doi: 10.1016/j.celrep.2018.11.084. [DOI] [PubMed] [Google Scholar]
- 328.Kostic M, Ludtmann MH, Bading H, Hershfinkel M, Steer E, Chu CT, Abramov AY, Sekler I. PKA phosphorylation of NCLX reverses mitochondrial calcium overload and depolarization, promoting survival of PINK1-deficient dopaminergic neurons. Cell Rep 13: 376–386, 2015. doi: 10.1016/j.celrep.2015.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Brustovetsky T, Pellman JJ, Yang XF, Khanna R, Brustovetsky N. Collapsin response mediator protein 2 (CRMP2) interacts with N-methyl-D-aspartate (NMDA) receptor and Na+/Ca2+ exchanger and regulates their functional activity. J Biol Chem 289: 7470–7482, 2014. doi: 10.1074/jbc.M113.518472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Pulina MV, Rizzuto R, Brini M, Carafoli E. Inhibitory interaction of the plasma membrane Na+/Ca2+ exchangers with the 14-3-3 proteins. J Biol Chem 281: 19645–19654, 2006. doi: 10.1074/jbc.M602033200. [DOI] [PubMed] [Google Scholar]
- 331.Zhu L, Yu Y, Chua BH, Ho YS, Kuo TH. Regulation of sodium-calcium exchange and mitochondrial energetics by Bcl-2 in the heart of transgenic mice. J Mol Cell Cardiol 33: 2135–2144, 2001. doi: 10.1006/jmcc.2001.1476. [DOI] [PubMed] [Google Scholar]
- 332.Carteri RB, Kopczynski A, Rodolphi MS, Strogulski NR, Sartor M, Feldmann M, De Bastiani MA, Duval Wannmacher CM, de Franceschi ID, Hansel G, Smith DH, Portela LV. Testosterone administration after traumatic brain injury reduces mitochondrial dysfunction and neurodegeneration. J Neurotrauma 36: 2246–2259, 2019. doi: 10.1089/neu.2018.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Karadjov JS, Kudzina LY, Zinchenko VP. TPP+ inhibits Na+-stimulated Ca2+ efflux from brain mitochondria. Cell Calcium 7: 115–119, 1986. doi: 10.1016/0143-4160(86)90014-X. [DOI] [PubMed] [Google Scholar]
- 334.Chiesi M, Schwaller R, Eichenberger K. Structural dependency of the inhibitory action of benzodiazepines and related compounds on the mitochondrial Na+-Ca2+ exchanger. Biochem Pharmacol 37: 4399–4403, 1988. doi: 10.1016/0006-2952(88)90623-5. [DOI] [PubMed] [Google Scholar]
- 335.Vághy PL, Johnson JD, Matlib MA, Wang T, Schwartz A. Selective inhibition of Na+-induced Ca2+ release from heart mitochondria by diltiazem and certain other Ca2+ antagonist drugs. J Biol Chem 257: 6000–6002, 1982. doi: 10.1016/S0021-9258(20)65094-3. [DOI] [PubMed] [Google Scholar]
- 336.Cox DA, Conforti L, Sperelakis N, Matlib MA. Selectivity of inhibition of Na+-Ca2+ exchange of heart mitochondria by benzothiazepine CGP-37157. J Cardiovasc Pharmacol 21: 595–599, 1993. doi: 10.1097/00005344-199304000-00013. [DOI] [PubMed] [Google Scholar]
- 337.Ruiz A, Alberdi E, Matute C. CGP37157, an inhibitor of the mitochondrial Na+/Ca2+ exchanger, protects neurons from excitotoxicity by blocking voltage-gated Ca2+ channels. Cell Death Dis 5: e1156, 2014. doi: 10.1038/cddis.2014.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Moreno-Ortega AJ, Martínez-Sanz FJ, Lajarín-Cuesta R, de Los Rios C, Cano-Abad MF. Benzothiazepine CGP37157 and its 2'-isopropyl analogue modulate Ca2+ entry through CALHM1. Neuropharmacology 95: 503–510, 2015. doi: 10.1016/j.neuropharm.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 339.Neumann JT, Diaz-Sylvester PL, Fleischer S, Copello JA. CGP-37157 inhibits the sarcoplasmic reticulum Ca2+ ATPase and activates ryanodine receptor channels in striated muscle. Mol Pharmacol 79: 141–147, 2011. doi: 10.1124/mol.110.067165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Thu le T, Ahn JR, Woo SH. Inhibition of L-type Ca2+ channel by mitochondrial Na+-Ca2+ exchange inhibitor CGP-37157 in rat atrial myocytes. Eur J Pharmacol 552: 15–19, 2006. doi: 10.1016/j.ejphar.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 341.Endele S, Fuhry M, Pak SJ, Zabel BU, Winterpacht A. LETM1, a novel gene encoding a putative EF-hand Ca2+-binding protein, flanks the Wolf-Hirschhorn syndrome (WHS) critical region and is deleted in most WHS patients. Genomics 60: 218–225, 1999. doi: 10.1006/geno.1999.5881. [DOI] [PubMed] [Google Scholar]
- 342.Schlickum S, Moghekar A, Simpson JC, Steglich C, O’Brien RJ, Winterpacht A, Endele SU. LETM1, a gene deleted in Wolf-Hirschhorn syndrome, encodes an evolutionarily conserved mitochondrial protein. Genomics 83: 254–261, 2004. doi: 10.1016/j.ygeno.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 343.Hasegawa A, van der Bliek AM. Inverse correlation between expression of the Wolfs Hirschhorn candidate gene Letm1 and mitochondrial volume in C. elegans and in mammalian cells. Hum Mol Genet 16: 2061–2071, 2007. doi: 10.1093/hmg/ddm154. [DOI] [PubMed] [Google Scholar]
- 344.Tamai S, Iida H, Yokota S, Sayano T, Kiguchiya S, Ishihara N, Hayashi J, Mihara K, Oka T. Characterization of the mitochondrial protein LETM1, which maintains the mitochondrial tubular shapes and interacts with the AAA-ATPase BCS1L. J Cell Sci 121: 2588–2600, 2008. doi: 10.1242/jcs.026625. [DOI] [PubMed] [Google Scholar]
- 345.Kuum M, Veksler V, Liiv J, Ventura-Clapier R, Kaasik A. Endoplasmic reticulum potassium-hydrogen exchanger and small conductance calcium-activated potassium channel activities are essential for ER calcium uptake in neurons and cardiomyocytes. J Cell Sci 125: 625–633, 2012. doi: 10.1242/jcs.090126. [DOI] [PubMed] [Google Scholar]
- 346.Nowikovsky K, Froschauer EM, Zsurka G, Samaj J, Reipert S, Kolisek M, Wiesenberger G, Schweyen RJ. The LETM1/YOL027 gene family encodes a factor of the mitochondrial K+ homeostasis with a potential role in the Wolf-Hirschhorn syndrome. J Biol Chem 279: 30307–30315, 2004. doi: 10.1074/jbc.M403607200. [DOI] [PubMed] [Google Scholar]
- 347.Tsai MF, Jiang D, Zhao L, Clapham D, Miller C. Functional reconstitution of the mitochondrial Ca2+/H+ antiporter Letm1. J Gen Physiol 143: 67–73, 2014. doi: 10.1085/jgp.201311096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Chang L, Zhang X, Gong P, Wang Y, Du B, Li J. Identification and characterization of Letm1 gene in Toxoplasma gondii. Acta Biochim Biophys Sin (Shanghai) 51: 78–87, 2019. doi: 10.1093/abbs/gmy138. [DOI] [PubMed] [Google Scholar]
- 349.Austin S, Nowikovsky K. LETM1: essential for mitochondrial biology and cation homeostasis? Trends Biochem Sci 44: 648–658, 2019. doi: 10.1016/j.tibs.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 350.Zhang B, Carrie C, Ivanova A, Narsai R, Murcha MW, Duncan O, Wang Y, Law SR, Albrecht V, Pogson B, Giraud E, Van Aken O, Whelan J. LETM proteins play a role in the accumulation of mitochondrially encoded proteins in Arabidopsis thaliana and AtLETM2 displays parent of origin effects. J Biol Chem 287: 41757–41773, 2012. doi: 10.1074/jbc.M112.383836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Froschauer E, Nowikovsky K, Schweyen RJ. Electroneutral K+/H+ exchange in mitochondrial membrane vesicles involves Yol027/Letm1 proteins. Biochim Biophys Acta 1711: 41–48, 2005. doi: 10.1016/j.bbamem.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 352.Hashimi H, McDonald L, Stribrná E, Lukeš J. Trypanosome Letm1 protein is essential for mitochondrial potassium homeostasis. J Biol Chem 288: 26914–26925, 2013. doi: 10.1074/jbc.M113.495119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.McQuibban AG, Joza N, Megighian A, Scorzeto M, Zanini D, Reipert S, Richter C, Schweyen RJ, Nowikovsky K. A Drosophila mutant of LETM1, a candidate gene for seizures in Wolf-Hirschhorn syndrome. Hum Mol Genet 19: 987–1000, 2010. doi: 10.1093/hmg/ddp563. [DOI] [PubMed] [Google Scholar]
- 354.Frazier AE, Taylor RD, Mick DU, Warscheid B, Stoepel N, Meyer HE, Ryan MT, Guiard B, Rehling P. Mdm38 interacts with ribosomes and is a component of the mitochondrial protein export machinery. J Cell Biol 172: 553–564, 2006. doi: 10.1083/jcb.200505060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Nowikovsky K, Reipert S, Devenish RJ, Schweyen RJ. Mdm38 protein depletion causes loss of mitochondrial K+/H+ exchange activity, osmotic swelling and mitophagy. Cell Death Differ 14: 1647–1656, 2007. doi: 10.1038/sj.cdd.4402167. [DOI] [PubMed] [Google Scholar]
- 356.Dimmer KS, Navoni F, Casarin A, Trevisson E, Endele S, Winterpacht A, Salviati L, Scorrano L. LETM1, deleted in Wolf-Hirschhorn syndrome is required for normal mitochondrial morphology and cellular viability. Hum Mol Genet 17: 201–214, 2008. doi: 10.1093/hmg/ddm297. [DOI] [PubMed] [Google Scholar]
- 357.Doonan PJ, Chandramoorthy HC, Hoffman NE, Zhang X, Cárdenas C, Shanmughapriya S, Rajan S, Vallem S, Chen X, Foskett JK, Cheung JY, Houser SR, Madesh M. LETM1-dependent mitochondrial Ca2+ flux modulates cellular bioenergetics and proliferation. FASEB J 28: 4936–4949, 2014. doi: 10.1096/fj.14-256453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Lee SY, Kang MG, Shin S, Kwak C, Kwon T, Seo JK, Kim JS, Rhee HW. Architecture mapping of the inner mitochondrial membrane proteome by chemical tools in live cells. J Am Chem Soc 139: 3651–3662, 2017. doi: 10.1021/jacs.6b10418. [DOI] [PubMed] [Google Scholar]
- 359.Nowikovsky K, Bernardi P. LETM1 in mitochondrial cation transport. Front Physiol 5: 83, 2014. doi: 10.3389/fphys.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Shao J, Fu Z, Ji Y, Guan X, Guo S, Ding Z, Yang X, Cong Y, Shen Y. Leucine zipper-EF-hand containing transmembrane protein 1 (LETM1) forms a Ca2+/H+ antiporter. Sci Rep 6: 34174, 2016. doi: 10.1038/srep34174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Quan X, Nguyen TT, Choi SK, Xu S, Das R, Cha SK, Kim N, Han J, Wiederkehr A, Wollheim CB, Park KS. Essential role of mitochondrial Ca2+ uniporter in the generation of mitochondrial pH gradient and metabolism-secretion coupling in insulin-releasing cells. J Biol Chem 290: 4086–4096, 2015. doi: 10.1074/jbc.M114.632547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.De Marchi U, Santo-Domingo J, Castelbou C, Sekler I, Wiederkehr A, Demaurex N. NCLX protein, but not LETM1, mediates mitochondrial Ca2+ extrusion, thereby limiting Ca2+-induced NAD(P)H production and modulating matrix redox state. J Biol Chem 289: 20377–20385, 2014. doi: 10.1074/jbc.M113.540898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Kim B, Takeuchi A, Koga O, Hikida M, Matsuoka S. Pivotal role of mitochondrial Na+-Ca2+ exchange in antigen receptor mediated Ca2+ signalling in DT40 and A20 B lymphocytes. J Physiol 590: 459–474, 2012. doi: 10.1113/jphysiol.2011.222927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Natarajan GK, Glait L, Mishra J, Stowe DF, Camara AK, Kwok WM. Total matrix Ca2+ modulates Ca2+ efflux via the Ca2+/H+ exchanger in cardiac mitochondria. Front Physiol 11: 510600, 2020. doi: 10.3389/fphys.2020.510600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Piao L, Li Y, Kim SJ, Sohn KC, Yang KJ, Park KA, Byun HS, Won M, Hong J, Hur GM, Seok JH, Shong M, Sack R, Brazil DP, Hemmings BA, Park J. Regulation of OPA1-mediated mitochondrial fusion by leucine zipper/EF-hand-containing transmembrane protein-1 plays a role in apoptosis. Cell Signal 21: 767–777, 2009. doi: 10.1016/j.cellsig.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 366.Durigon R, Mitchell AL, Jones AW, Manole A, Mennuni M, Hirst EM, Houlden H, Maragni G, Lattante S, Doronzio PN, Dalla Rosa I, Zollino M, Holt IJ, Spinazzola A. LETM1 couples mitochondrial DNA metabolism and nutrient preference. EMBO Mol Med 10: e8550, 2018. doi: 10.15252/emmm.201708550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Bauerschmitt H, Mick DU, Deckers M, Vollmer C, Funes S, Kehrein K, Ott M, Rehling P, Herrmann JM. Ribosome-binding proteins Mdm38 and Mba1 display overlapping functions for regulation of mitochondrial translation. Mol Biol Cell 21: 1937–1944, 2010. doi: 10.1091/mbc.e10-02-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Nakamura S, Matsui A, Akabane S, Tamura Y, Hatano A, Miyano Y, Omote H, Kajikawa M, Maenaka K, Moriyama Y, Endo T, Oka T. The mitochondrial inner membrane protein LETM1 modulates cristae organization through its LETM domain. Commun Biol 3: 99, 2020. doi: 10.1038/s42003-020-0832-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Xue H, Wang Z, Hua Y, Ke S, Wang Y, Zhang J, Pan YH, Huang W, Irwin DM, Zhang S. Molecular signatures and functional analysis of beige adipocytes induced from in vivo intra-abdominal adipocytes. Sci Adv 4: eaar5319, 2018. doi: 10.1126/sciadv.aar5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Lee J, Lee WK, Seol MY, Lee SG, Kim D, Kim H, Park J, Jung SG, Chung WY, Lee EJ, Jo YS. Coupling of LETM1 up-regulation with oxidative phosphorylation and platelet-derived growth factor receptor signaling via YAP1 transactivation. Oncotarget 7: 66728–66739, 2016. doi: 10.18632/oncotarget.11456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.South ST, Bleyl SB, Carey JC. Two unique patients with novel microdeletions in 4p16.3 that exclude the WHS critical regions: implications for critical region designation. Am J Med Genet A 143A: 2137–2142, 2007. doi: 10.1002/ajmg.a.31900. [DOI] [PubMed] [Google Scholar]
- 372.Jiang D, Zhao L, Clish CB, Clapham DE. Letm1, the mitochondrial Ca2+/H+ antiporter, is essential for normal glucose metabolism and alters brain function in Wolf-Hirschhorn syndrome. Proc Natl Acad Sci USA 110: E2249–E2254, 2013. doi: 10.1073/pnas.1308558110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Hart L, Rauch A, Carr AM, Vermeesch JR, O’Driscoll M. LETM1 haploinsufficiency causes mitochondrial defects in cells from humans with Wolf-Hirschhorn syndrome: implications for dissecting the underlying pathomechanisms in this condition. Dis Model Mech 7: 535–545, 2014. doi: 10.1242/dmm.014464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Andersen EF, Carey JC, Earl DL, Corzo D, Suttie M, Hammond P, South ST. Deletions involving genes WHSC1 and LETM1 may be necessary, but are not sufficient to cause Wolf-Hirschhorn syndrome. Eur J Hum Genet 22: 464–470, 2014. doi: 10.1038/ejhg.2013.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Cyr AB, Nimmakayalu M, Longmuir SQ, Patil SR, Keppler-Noreuil KM, Shchelochkov OA. A novel 4p16.3 microduplication distal to WHSC1 and WHSC2 characterized by oligonucleotide array with new phenotypic features. Am J Med Genet A 155A: 2224–2228, 2011. doi: 10.1002/ajmg.a.34120. [DOI] [PubMed] [Google Scholar]
- 376.Roselló M, Monfort S, Orellana C, Ferrer-Bolufer I, Quiroga R, Oltra S, Martínez F. Submicroscopic duplication of the Wolf-Hirschhorn critical region with a 4p terminal deletion. Cytogenet Genome Res 125: 103–108, 2009. doi: 10.1159/000227833. [DOI] [PubMed] [Google Scholar]
- 377.Zhang X, Chen G, Lu Y, Liu J, Fang M, Luo J, Cao Q, Wang X. Association of mitochondrial letm1 with epileptic seizures. Cereb Cortex 24: 2533–2540, 2014. doi: 10.1093/cercor/bht118. [DOI] [PubMed] [Google Scholar]
- 378.Lindinger PW, Christe M, Eberle AN, Kern B, Peterli R, Peters T, Jayawardene KJ, Fearnley IM, Walker JE. Important mitochondrial proteins in human omental adipose tissue show reduced expression in obesity. J Proteomics 124: 79–87, 2015. doi: 10.1016/j.jprot.2015.03.037. [DOI] [PubMed] [Google Scholar]
- 379.Park J, Li Y, Kim SH, Yang KJ, Kong G, Shrestha R, Tran Q, Park KA, Jeon J, Hur GM, Lee CH, Kim DH, Park J. New players in high fat diet-induced obesity: LETM1 and CTMP. Metabolism 63: 318–327, 2014. doi: 10.1016/j.metabol.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 380.Piao L, Li Y, Kim SJ, Byun HS, Huang SM, Hwang SK, Yang KJ, Park KA, Won M, Hong J, Hur GM, Seok JH, Shong M, Cho MH, Brazil DP, Hemmings BA, Park J. Association of LETM1 and MRPL36 contributes to the regulation of mitochondrial ATP production and necrotic cell death. Cancer Res 69: 3397–3404, 2009. doi: 10.1158/0008-5472.CAN-08-3235. [DOI] [PubMed] [Google Scholar]
- 381.Lee YY, McKinney KQ, Ghosh S, Iannitti DA, Martinie JB, Caballes FR, Russo MW, Ahrens WA, Lundgren DH, Han DK, Bonkovsky HL, Hwang SI. Subcellular tissue proteomics of hepatocellular carcinoma for molecular signature discovery. J Proteome Res 10: 5070–5083, 2011. doi: 10.1021/pr2005204. [DOI] [PubMed] [Google Scholar]
- 382.Chen L, Yang Y, Liu S, Piao L, Zhang Y, Lin Z, Li Z. High expression of leucine zipper-EF-hand containing transmembrane protein 1 predicts poor prognosis in head and neck squamous cell carcinoma. Biomed Res Int 2014: 850316, 2014. doi: 10.1155/2014/850316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Wang CA, Liu Q, Chen Y, Liu S, Xu J, Cui X, Zhang Y, Piao L. Clinical implication of leucine zipper/EF hand-containing transmembrane-1 overexpression in the prognosis of triple-negative breast cancer. Exp Mol Pathol 98: 254–259, 2015. doi: 10.1016/j.yexmp.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 384.Li N, Zheng Y, Xuan C, Lin Z, Piao L, Liu S. LETM1 overexpression is correlated with the clinical features and survival outcome of breast cancer. Int J Clin Exp Pathol 8: 12893–12900, 2015. [PMC free article] [PubMed] [Google Scholar]
- 385.Yang Z, Ni W, Cui C, Qi W, Piao L, Xuan Y. Identification of LETM1 as a marker of cancer stem-like cells and predictor of poor prognosis in esophageal squamous cell carcinoma. Hum Pathol 81: 148–156, 2018. doi: 10.1016/j.humpath.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 386.Piao L, Feng Y, Yang Z, Qi W, Li H, Han H, Xuan Y. LETM1 is a potential cancer stem-like cell marker and predicts poor prognosis in colorectal adenocarcinoma. Pathol Res Pract 215: 152437, 2019. doi: 10.1016/j.prp.2019.152437. [DOI] [PubMed] [Google Scholar]
- 387.Piao L, Yang Z, Feng Y, Zhang C, Cui C, Xuan Y. LETM1 is a potential biomarker of prognosis in lung non-small cell carcinoma. BMC Cancer 19: 898, 2019. doi: 10.1186/s12885-019-6128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Li H, Piao L, Xu D, Xuan Y. LETM1 is a potential biomarker that predicts poor prognosis in gastric adenocarcinoma. Exp Mol Pathol 112: 104333, 2020. doi: 10.1016/j.yexmp.2019.104333. [DOI] [PubMed] [Google Scholar]
- 389.Wang J, Ding W, Xu Y, Tao E, Mo M, Xu W, Cai X, Chen X, Yuan J, Wu X. Long non-coding RNA RHPN1-AS1 promotes tumorigenesis and metastasis of ovarian cancer by acting as a ceRNA against miR-596 and upregulating LETM1. Aging (Albany NY) 12: 4558–4572, 2020. doi: 10.18632/aging.102911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Piao L, Li H, Feng Y, Li X, Cui Y, Xuan Y. Leucine zipper-EF-hand containing transmembrane protein 1 is a potential prognostic biomarker and promotes cell progression in prostate cancer. Cancer Manag Res 12: 1649–1660, 2020. doi: 10.2147/CMAR.S236482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Zhang Y, Chen L, Cao Y, Chen S, Xu C, Xing J, Zhang K. LETM1 Promotes gastric cancer cell proliferation, migration, and invasion via the PI3K/Akt signaling pathway. J Gastric Cancer 20: 139–151, 2020. doi: 10.5230/jgc.2020.20.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Ji H, Hu NJ. MiR-613 blocked the progression of cervical cancer by targeting LETM1. Eur Rev Med Pharmacol Sci 24: 6576–6582, 2020. doi: 10.26355/eurrev_202006_21642. [DOI] [PubMed] [Google Scholar]
- 393.Xu J, Huang B, Li S, Zhang X, Xie T, Xu Y. Knockdown of LETM1 inhibits proliferation and metastasis of human renal cell carcinoma cells. Oncol Lett 16: 6377–6382, 2018. doi: 10.3892/ol.2018.9449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Huang B, Zhang J, Zhang X, Huang C, Hu G, Li S, Xie T, Liu M, Xu Y. Suppression of LETM1 by siRNA inhibits cell proliferation and invasion of bladder cancer cells. Oncol Rep 38: 2935–2940, 2017. doi: 10.3892/or.2017.5959. [DOI] [PubMed] [Google Scholar]
- 395.Shin JY, Chung YS, Kang B, Jiang HL, Yu DY, Han K, Chae C, Moon JH, Jang G, Cho MH. Co-delivery of LETM1 and CTMP synergistically inhibits tumor growth in H-ras12V liver cancer model mice. Cancer Gene Ther 20: 186–194, 2013. doi: 10.1038/cgt.2013.6. [DOI] [PubMed] [Google Scholar]
- 396.Hwang SK, Piao L, Lim HT, Minai-Tehrani A, Yu KN, Ha YC, Chae CH, Lee KH, Beck GR, Park J, Cho MH. Suppression of lung tumorigenesis by leucine zipper/EF hand-containing transmembrane-1. PLoS One 5: e12535, 2010. doi: 10.1371/journal.pone.0012535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Huang E, Qu D, Huang T, Rizzi N, Boonying W, Krolak D, Ciana P, Woulfe J, Klein C, Slack RS, Figeys D, Park DS. PINK1-mediated phosphorylation of LETM1 regulates mitochondrial calcium transport and protects neurons against mitochondrial stress. Nat Commun 8: 1399, 2017. doi: 10.1038/s41467-017-01435-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Mirali S, Botham A, Voisin V, Xu C, St-Germain J, Sharon D, Hoff FW, Qiu Y, Hurren R, Gronda M, Jitkova Y, Nachmias B, MacLean N, Wang X, Arruda A, Minden MD, Horton TM, Kornblau SM, Chan SM, Bader GD, Raught B, Schimmer AD. The mitochondrial peptidase, neurolysin, regulates respiratory chain supercomplex formation and is necessary for AML viability. Sci Transl Med 12: eaaz8264, 2020. doi: 10.1126/scitranslmed.aaz8264. [DOI] [PubMed] [Google Scholar]
- 399.Okamura K, Matsushita S, Kato Y, Watanabe H, Matsui A, Oka T, Matsuura T. In vitro synthesis of the human calcium transporter Letm1 within cell-sized liposomes and investigation of its lipid dependency. J Biosci Bioeng 127: 544–548, 2019. doi: 10.1016/j.jbiosc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 400.Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta 1787: 1395–1401, 2009. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Rasola A, Bernardi P. Mitochondrial permeability transition in Ca2+-dependent apoptosis and necrosis. Cell Calcium 50: 222–233, 2011. doi: 10.1016/j.ceca.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 402.Elrod JW, Molkentin JD. Physiologic functions of cyclophilin D and the mitochondrial permeability transition pore. Circ J 77: 1111–1122, 2013. doi: 10.1253/circj.CJ-13-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Bernardi P, Petronilli V. The permeability transition pore as a mitochondrial calcium release channel: a critical appraisal. J Bioenerg Biomembr 28: 131–138, 1996. doi: 10.1007/BF02110643. [DOI] [PubMed] [Google Scholar]
- 404.Ichas F, Jouaville LS, Mazat JP. Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell 89: 1145–1153, 1997. doi: 10.1016/S0092-8674(00)80301-3. [DOI] [PubMed] [Google Scholar]
- 405.Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev 79: doi: 10.1152/physrev.1999.79.4.1127. 1127–1155, 1999. [DOI] [PubMed] [Google Scholar]
- 406.Loupatatzis C, Seitz G, Schönfeld P, Lang F, Siemen D. Single-channel currents of the permeability transition pore from the inner mitochondrial membrane of rat liver and of a human hepatoma cell line. Cell Physiol Biochem 12: 269–278, 2002. doi: 10.1159/000067897. [DOI] [PubMed] [Google Scholar]
- 407.Halestrap AP, Davidson AM. Inhibition of Ca2(+)-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem J 268: 153–160, 1990. doi: 10.1042/bj2680153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Hunter DR, Haworth RA, Southard JH. Relationship between configuration, function, and permeability in calcium-treated mitochondria. J Biol Chem 251: 5069–5077, 1976. doi: 10.1016/S0021-9258(17)33220-9. [DOI] [PubMed] [Google Scholar]
- 409.Crompton M, Virji S, Ward JM. Cyclophilin-D binds strongly to complexes of the voltage-dependent anion channel and the adenine nucleotide translocase to form the permeability transition pore. Eur J Biochem 258: 729–735, 1998. doi: 10.1046/j.1432-1327.1998.2580729.x. [DOI] [PubMed] [Google Scholar]
- 410.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434: 658–662, 2005. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 411.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434: 652–658, 2005. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 412.Leung AW, Varanyuwatana P, Halestrap AP. The mitochondrial phosphate carrier interacts with cyclophilin D and may play a key role in the permeability transition. J Biol Chem 283: 26312–26323, 2008. doi: 10.1074/jbc.M805235200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Altschuld RA, Hohl CM, Castillo LC, Garleb AA, Starling RC, Brierley GP. Cyclosporin inhibits mitochondrial calcium efflux in isolated adult rat ventricular cardiomyocytes. Am J Physiol Heart Circ Physiol 262: H1699–H1704, 1992. doi: 10.1152/ajpheart.1992.262.6.H1699. [DOI] [PubMed] [Google Scholar]
- 414.Elrod JW, Wong R, Mishra S, Vagnozzi RJ, Sakthievel B, Goonasekera SA, Karch J, Gabel S, Farber J, Force T, Brown JH, Murphy E, Molkentin JD. Cyclophilin D controls mitochondrial pore-dependent Ca2+ exchange, metabolic flexibility, and propensity for heart failure in mice. J Clin Invest 120: 3680–3687, 2010. doi: 10.1172/JCI43171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Barsukova A, Komarov A, Hajnóczky G, Bernardi P, Bourdette D, Forte M. Activation of the mitochondrial permeability transition pore modulates Ca2+ responses to physiological stimuli in adult neurons. Eur J Neurosci 33: 831–842, 2011. doi: 10.1111/j.1460-9568.2010.07576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Solesio ME, Garcia Del Molino LC, Elustondo PA, Diao C, Chang JC, Pavlov EV. Inorganic polyphosphate is required for sustained free mitochondrial calcium elevation, following calcium uptake. Cell Calcium 86: 102127, 2020. doi: 10.1016/j.ceca.2019.102127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Wei AC, Liu T, O’Rourke B. Dual effect of phosphate transport on mitochondrial Ca2+ dynamics. J Biol Chem 290: 16088–16098, 2015. doi: 10.1074/jbc.M114.628446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Boyman L, Chikando AC, Williams GS, Khairallah RJ, Kettlewell S, Ward CW, Smith GL, Kao JP, Lederer WJ. Calcium movement in cardiac mitochondria. Biophys J 107: 1289–1301, 2014. doi: 10.1016/j.bpj.2014.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Montero M, Alonso MT, Carnicero E, Cuchillo-Ibáñez I, Albillos A, Gárcia AG, García-Sancho J, Alvarez J. Chromaffin-cell stimulation triggers fast millimolar mitochondrial Ca2+ transients that modulate secretion. Nat Cell Biol 2: 57–61, 2000. doi: 10.1038/35000001. [DOI] [PubMed] [Google Scholar]
- 420.Yi M, Weaver D, Hajnóczky G. Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J Cell Biol 167: 661–672, 2004. doi: 10.1083/jcb.200406038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Csordás G, Várnai P, Golenár T, Roy S, Purkins G, Schneider TG, Balla T, Hajnóczky G. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol Cell 39: 121–132, 2010. doi: 10.1016/j.molcel.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Hollander JM, Thapa D, Shepherd DL. Physiological and structural differences in spatially distinct subpopulations of cardiac mitochondria: influence of cardiac pathologies. Am J Physiol Heart Circ Physiol 307: H1–H14, 2014. doi: 10.1152/ajpheart.00747.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Díaz-Vegas A, Eisner V, Jaimovich E. Skeletal muscle excitation-metabolism coupling. Arch Biochem Biophys 664: 89–94, 2019. doi: 10.1016/j.abb.2019.01.037. [DOI] [PubMed] [Google Scholar]
- 424.Vance JE. MAM (mitochondria-associated membranes) in mammalian cells: lipids and beyond. Biochim Biophys Acta 1841: 595–609, 2014. doi: 10.1016/j.bbalip.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 425.Paillusson S, Stoica R, Gomez-Suaga P, Lau DH, Mueller S, Miller T, Miller CC. There’s something wrong with my MAM; the ER-mitochondria axis and neurodegenerative diseases. Trends Neurosci 39: 146–157, 2016. doi: 10.1016/j.tins.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Marchi S, Patergnani S, Missiroli S, Morciano G, Rimessi A, Wieckowski MR, Giorgi C, Pinton P. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium 69: 62–72, 2018. doi: 10.1016/j.ceca.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 427.Marchi S, Bittremieux M, Missiroli S, Morganti C, Patergnani S, Sbano L, Rimessi A, Kerkhofs M, Parys JB, Bultynck G, Giorgi C, Pinton P. Endoplasmic reticulum-mitochondria communication through Ca2+ signaling: the importance of mitochondria-associated membranes (MAMs). Adv Exp Med Biol 997: 49–67, 2017. doi: 10.1007/978-981-10-4567-7_4. [DOI] [PubMed] [Google Scholar]
- 428.Csordás GR, Renken C, Várnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnóczky GR, Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol 174: 915–921, 2006. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456: 605–610, 2008. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 430.Chen Y, Csordás G, Jowdy C, Schneider TG, Csordás N, Wang W, Liu Y, Kohlhaas M, Meiser M, Bergem S, Nerbonne JM, Dorn GW 2nd, Maack C. Mitofusin 2-containing mitochondrial-reticular microdomains direct rapid cardiomyocyte bioenergetic responses via interorganelle Ca2+ crosstalk. Circ Res 111: 863–875, 2012. doi: 10.1161/CIRCRESAHA.112.266585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Cosson P, Marchetti A, Ravazzola M, Orci L. Mitofusin-2 independent juxtaposition of endoplasmic reticulum and mitochondria: an ultrastructural study. PLoS One 7: e46293, 2012. doi: 10.1371/journal.pone.0046293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Filadi R, Greotti E, Turacchio G, Luini A, Pozzan T, Pizzo P. Mitofusin 2 ablation increases endoplasmic reticulum-mitochondria coupling. Proc Natl Acad Sci USA 112: E2174–E2181, 2015. doi: 10.1073/pnas.1504880112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Hirabayashi Y, Kwon SK, Paek H, Pernice WM, Paul MA, Lee J, Erfani P, Raczkowski A, Petrey DS, Pon LA, Polleux F. ER-mitochondria tethering by PDZD8 regulates Ca2+ dynamics in mammalian neurons. Science 358: 623–630, 2017. doi: 10.1126/science.aan6009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Devine MJ, Kittler JT. Mitochondria at the neuronal presynapse in health and disease. Nat Rev Neurosci 19: 63–80, 2018. doi: 10.1038/nrn.2017.170. [DOI] [PubMed] [Google Scholar]
- 435.Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron 47: 365–378, 2005. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 436.Shields LY, Kim H, Zhu L, Haddad D, Berthet A, Pathak D, Lam M, Ponnusamy R, Diaz-Ramirez LG, Gill TM, Sesaki H, Mucke L, Nakamura K. Dynamin-related protein 1 is required for normal mitochondrial bioenergetic and synaptic function in CA1 hippocampal neurons. Cell Death Dis 6: e1725, 2015. doi: 10.1038/cddis.2015.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Lombardi T, Montesano R, Wohlwend A, Amherdt M, Vassalli JD, Orci L. Evidence for polarization of plasma membrane domains in pancreatic endocrine cells. Nature 313: 694–696, 1985. doi: 10.1038/313694a0. [DOI] [PubMed] [Google Scholar]
- 438.Ambudkar IS. Polarization of calcium signaling and fluid secretion in salivary gland cells. Curr Med Chem 19: 5774–5781, 2012. doi: 10.2174/092986712804143321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Tinel H, Cancela JM, Mogami H, Gerasimenko JV, Gerasimenko OV, Tepikin AV, Petersen OH. Active mitochondria surrounding the pancreatic acinar granule region prevent spreading of inositol trisphosphate-evoked local cytosolic Ca2+ signals. EMBO J 18: 4999–5008, 1999. doi: 10.1093/emboj/18.18.4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Park MK, Ashby MC, Erdemli G, Petersen OH, Tepikin AV. Perinuclear, perigranular and sub-plasmalemmal mitochondria have distinct functions in the regulation of cellular calcium transport. EMBO J 20: 1863–1874, 2001. doi: 10.1093/emboj/20.8.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Petersen OH. Ca2+ signalling and Ca2+-activated ion channels in exocrine acinar cells. Cell Calcium 38: 171–200, 2005. doi: 10.1016/j.ceca.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 442.García-Pérez C, Schneider TG, Hajnóczky G, Csordás G. Alignment of sarcoplasmic reticulum-mitochondrial junctions with mitochondrial contact points. Am J Physiol Heart Circ Physiol 301: H1907–H1915, 2011. doi: 10.1152/ajpheart.00397.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.De La Fuente S, Fernandez-Sanz C, Vail C, Agra EJ, Holmstrom K, Sun J, Mishra J, Williams D, Finkel T, Murphy E, Joseph SK, Sheu SS, Csordás G. Strategic positioning and biased activity of the mitochondrial calcium uniporter in cardiac muscle. J Biol Chem 291: 23343–23362, 2016. doi: 10.1074/jbc.M116.755496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.De La Fuente S, Lambert JP, Nichtova Z, Fernandez Sanz C, Elrod JW, Sheu SS, Csordás G. Spatial separation of mitochondrial calcium uptake and extrusion for energy-efficient mitochondrial calcium signaling in the heart. Cell Rep 24: 3099–3107.e4, 2018. doi: 10.1016/j.celrep.2018.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Wolf DM, Segawa M, Kondadi AK, Anand R, Bailey ST, Reichert AS, van der Bliek AM, Shackelford DB, Liesa M, Shirihai OS. Individual cristae within the same mitochondrion display different membrane potentials and are functionally independent. EMBO J 38: e101056, 2019. doi: 10.15252/embj.2018101056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Rossi CS, Lehninger AL. Stoichiometry of respiratory stimulation, accumulation of Ca++ and phosphate, and oxidative phosphorylation in rat liver mitochondria. J Biol Chem 239: 3971–3980, 1964. doi: 10.1016/S0021-9258(18)91230-5. [DOI] [PubMed] [Google Scholar]
- 447.Denton RM, Randle PJ, Martin BR. Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. Biochem J 128: 161–163, 1972. doi: 10.1042/bj1280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Denton RM. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim Biophys Acta 1787: 1309–1316, 2009. doi: 10.1016/j.bbabio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 449.Rardin MJ, Wiley SE, Naviaux RK, Murphy AN, Dixon JE. Monitoring phosphorylation of the pyruvate dehydrogenase complex. Anal Biochem 389: 157–164, 2009. doi: 10.1016/j.ab.2009.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Gray LR, Tompkins SC, Taylor EB. Regulation of pyruvate metabolism and human disease. Cell Mol Life Sci 71: 2577–2604, 2014. doi: 10.1007/s00018-013-1539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Denton RM, Richards DA, Chin JG. Calcium ions and the regulation of NAD+-linked isocitrate dehydrogenase from the mitochondria of rat heart and other tissues. Biochem J 176: 899–906, 1978. doi: 10.1042/bj1760899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.McCormack JG, Denton RM. The effects of calcium ions and adenine nucleotides on the activity of pig heart 2-oxoglutarate dehydrogenase complex. Biochem J 180: 533–544, 1979. doi: 10.1042/bj1800533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Lawlis VB, Roche TE. Effect of micromolar Ca2+ on NADH inhibition of bovine kidney alpha-ketoglutarate dehydrogenase complex and possible role of Ca2+ in signal amplification. Mol Cell Biochem 32: 147–152, 1980. doi: 10.1007/BF00227441. [DOI] [PubMed] [Google Scholar]
- 454.Rutter GA, Denton RM. Regulation of NAD+-linked isocitrate dehydrogenase and 2-oxoglutarate dehydrogenase by Ca2+ ions within toluene-permeabilized rat heart mitochondria. Interactions with regulation by adenine nucleotides and NADH/NAD+ ratios. Biochem J 252: 181–189, 1988. doi: 10.1042/bj2520181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Glancy B, Willis WT, Chess DJ, Balaban RS. Effect of calcium on the oxidative phosphorylation cascade in skeletal muscle mitochondria. Biochemistry 52: 2793–2809, 2013. doi: 10.1021/bi3015983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Territo PR, Mootha VK, French SA, Balaban RS. Ca2+ activation of heart mitochondrial oxidative phosphorylation: role of the F0/F1-ATPase. Am J Physiol Cell Physiol 278: C423–C435, 2000. doi: 10.1152/ajpcell.2000.278.2.C423. [DOI] [PubMed] [Google Scholar]
- 457.Williams GS, Boyman L, Lederer WJ. Mitochondrial calcium and the regulation of metabolism in the heart. J Mol Cell Cardiol 78: 35–45, 2015. doi: 10.1016/j.yjmcc.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Lee B, Miles PD, Vargas L, Luan P, Glasco S, Kushnareva Y, Kornbrust ES, Grako KA, Wollheim CB, Maechler P, Olefsky JM, Anderson CM. Inhibition of mitochondrial Na+-Ca2+ exchanger increases mitochondrial metabolism and potentiates glucose-stimulated insulin secretion in rat pancreatic islets. Diabetes 52: 965–973, 2003. doi: 10.2337/diabetes.52.4.965. [DOI] [PubMed] [Google Scholar]
- 459.Wiederkehr A, Szanda G, Akhmedov D, Mataki C, Heizmann CW, Schoonjans K, Pozzan T, Spät A, Wollheim CB. Mitochondrial matrix calcium is an activating signal for hormone secretion. Cell Metab 13: 601–611, 2011. doi: 10.1016/j.cmet.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 460.Tarasov AI, Semplici F, Ravier MA, Bellomo EA, Pullen TJ, Gilon P, Sekler I, Rizzuto R, Rutter GA. The mitochondrial Ca2+ uniporter MCU is essential for glucose-induced ATP increases in pancreatic beta-cells. PLoS One 7: e39722, 2012. doi: 10.1371/journal.pone.0039722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Tarasov AI, Semplici F, Li D, Rizzuto R, Ravier MA, Gilon P, Rutter GA. Frequency-dependent mitochondrial Ca2+ accumulation regulates ATP synthesis in pancreatic beta cells. Pflugers Arch 465: 543–554, 2013. doi: 10.1007/s00424-012-1177-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci USA 96: 13807–13812, 1999. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Owen OE, Kalhan SC, Hanson RW. The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem 277: 30409–30412, 2002. doi: 10.1074/jbc.R200006200. [DOI] [PubMed] [Google Scholar]
- 464.Doenst T, Nguyen TD, Abel ED. Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res 113: 709–724, 2013. doi: 10.1161/CIRCRESAHA.113.300376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Martínez-Reyes I, Chandel NS. Mitochondrial TCA cycle metabolites control physiology and disease. Nat Commun 11: 102, 2020. doi: 10.1038/s41467-019-13668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Zweifach A, Lewis RS. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc Natl Acad Sci USA 90: 6295–6299, 1993. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Partiseti M, Le Deist F, Hivroz C, Fischer A, Korn H, Choquet D. The calcium current activated by T cell receptor and store depletion in human lymphocytes is absent in a primary immunodeficiency. J Biol Chem 269: 32327–32335, 1994. doi: 10.1016/S0021-9258(18)31639-9. [DOI] [PubMed] [Google Scholar]
- 468.Fanger CM, Hoth M, Crabtree GR, Lewis RS. Characterization of T cell mutants with defects in capacitative calcium entry: genetic evidence for the physiological roles of CRAC channels. J Cell Biol 131: 655–667, 1995. doi: 10.1083/jcb.131.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Hoth M, Button DC, Lewis RS. Mitochondrial control of calcium-channel gating: a mechanism for sustained signaling and transcriptional activation in T lymphocytes. Proc Natl Acad Sci USA 97: 10607–10612, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Tomar D, Jaña F, Dong Z, Quinn WJ, Jadiya P, Breves SL, et al. Blockade of MCU-mediated Ca2+ uptake perturbs lipid metabolism via PP4-dependent AMPK dephosphorylation. Cell Rep 26: 3709–3725.e7, 2019. doi: 10.1016/j.celrep.2019.02.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Greenawalt JW, Rossi CS, Lehninger AL. Effect of Active Accumulation of Calcium and Phosphate Ions on the Structure of Rat Liver Mitochondria. J Cell Biol 23: 21–38, 1964. doi: 10.1083/jcb.23.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol 287: C817–C833, 2004. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 473.Sohal RS, Allen RG. Relationship between metabolic rate, free radicals, differentiation and aging: a unified theory. Basic Life Sci 35: 75–104, 1985. [DOI] [PubMed] [Google Scholar]
- 474.Malyala S, Zhang Y, Strubbe JO, Bazil JN. Calcium phosphate precipitation inhibits mitochondrial energy metabolism. PLoS Comput Biol 15: e1006719, 2019. doi: 10.1371/journal.pcbi.1006719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Petrosillo G, Ruggiero FM, Pistolese M, Paradies G. Ca2+-induced reactive oxygen species production promotes cytochrome c release from rat liver mitochondria via mitochondrial permeability transition (MPT)-dependent and MPT-independent mechanisms: role of cardiolipin. J Biol Chem 279: 53103–53108, 2004. doi: 10.1074/jbc.M407500200. [DOI] [PubMed] [Google Scholar]
- 476.Hwang MS, Schwall CT, Pazarentzos E, Datler C, Alder NN, Grimm S. Mitochondrial Ca2+ influx targets cardiolipin to disintegrate respiratory chain complex II for cell death induction. Cell Death Differ 21: 1733–1745, 2014. doi: 10.1038/cdd.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Görlach A, Bertram K, Hudecova S, Krizanova O. Calcium and ROS: a mutual interplay. Redox Biol 6: 260–271, 2015. doi: 10.1016/j.redox.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Seidlmayer LK, Juettner VV, Kettlewell S, Pavlov EV, Blatter LA, Dedkova EN. Distinct mPTP activation mechanisms in ischaemia-reperfusion: contributions of Ca2+, ROS, pH, and inorganic polyphosphate. Cardiovasc Res 106: 237–248, 2015. doi: 10.1093/cvr/cvv097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Baumgartner HK, Gerasimenko JV, Thorne C, Ferdek P, Pozzan T, Tepikin AV, Petersen OH, Sutton R, Watson AJ, Gerasimenko OV. Calcium elevation in mitochondria is the main Ca2+ requirement for mitochondrial permeability transition pore (mPTP) opening. J Biol Chem 284: 20796–20803, 2009. doi: 10.1074/jbc.M109.025353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Liao Y, Hao Y, Chen H, He Q, Yuan Z, Cheng J. Mitochondrial calcium uniporter protein MCU is involved in oxidative stress-induced cell death. Protein Cell 6: 434–442, 2015. doi: 10.1007/s13238-015-0144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Galluzzi L, Pietrocola F, Levine B, Kroemer G. Metabolic control of autophagy. Cell 159: 1263–1276, 2014. doi: 10.1016/j.cell.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol 12: 9–14, 2011. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ 20: 31–42, 2013. doi: 10.1038/cdd.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Granatiero V, Giorgio V, Calì T, Patron M, Brini M, Bernardi P, Tiranti V, Zeviani M, Pallafacchina G, De Stefani D, Rizzuto R. Reduced mitochondrial Ca2+ transients stimulate autophagy in human fibroblasts carrying the 13514A>G mutation of the ND5 subunit of NADH dehydrogenase. Cell Death Differ 23: 231–241, 2016. doi: 10.1038/cdd.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Yu Z, Gong X, Yu Y, Li M, Liang Y, Qin S, Fulati Z, Zhou N, Shu X, Nie Z, Dai S, Chen X, Wang J, Chen R, Su Y, Ge J. The mechanical effects of CRT promoting autophagy via mitochondrial calcium uniporter down-regulation and mitochondrial dynamics alteration. J Cell Mol Med 23: 3833–3842, 2019. doi: 10.1111/jcmm.14227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Yu Z, Chen R, Li M, Yu Y, Liang Y, Han F, Qin S, Chen X, Su Y, Ge J. Mitochondrial calcium uniporter inhibition provides cardioprotection in pressure overload-induced heart failure through autophagy enhancement. Int J Cardiol 271: 161–168, 2018. doi: 10.1016/j.ijcard.2018.05.054. [DOI] [PubMed] [Google Scholar]
- 487.Zhao M, Chen J, Mao K, She H, Ren Y, Gui C, Wu X, Zou F, Li W. Mitochondrial calcium dysfunction contributes to autophagic cell death induced by MPP+ via AMPK pathway. Biochem Biophys Res Commun 509: 390–394, 2019. doi: 10.1016/j.bbrc.2018.12.148. [DOI] [PubMed] [Google Scholar]
- 488.Cárdenas C, Miller RA, Smith I, Bui T, Molgó J, Müller M, Vais H, Cheung KH, Yang J, Parker I, Thompson CB, Birnbaum MJ, Hallows KR, Foskett JK. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell 142: 270–283, 2010. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Elmore SP, Qian T, Grissom SF, Lemasters JJ. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. FASEB J 15: 2286–2287, 2001. doi: 10.1096/fj.01-0206fje. [DOI] [PubMed] [Google Scholar]
- 490.Rodriguez-Enriquez S, Kim I, Currin RT, Lemasters JJ. Tracker dyes to probe mitochondrial autophagy (mitophagy) in rat hepatocytes. Autophagy 2: 39–46, 2006. doi: 10.4161/auto.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys 462: 245–253, 2007. doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.MacVicar TD, Mannack LV, Lees RM, Lane JD. Targeted siRNA screens identify ER-to-mitochondrial calcium exchange in autophagy and mitophagy responses in RPE1 cells. Int J Mol Sci 16: 13356–13380, 2015. doi: 10.3390/ijms160613356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci USA 95: 11715–11720, 1998. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell 48: 158–167, 2012. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Metallo CM, Vander Heiden MG. Metabolism strikes back: metabolic flux regulates cell signaling. Genes Dev 24: 2717–2722, 2010. doi: 10.1101/gad.2010510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Kaelin WG Jr, McKnight SL. Influence of metabolism on epigenetics and disease. Cell 153: 56–69, 2013. doi: 10.1016/j.cell.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Weinberg SE, Sena LA, Chandel NS. Mitochondria in the regulation of innate and adaptive immunity. Immunity 42: 406–417, 2015. doi: 10.1016/j.immuni.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, Cheng T, Kho Y, Xiao H, Xiao L, Grishin NV, White M, Yang XJ, Zhao Y. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell 23: 607–618, 2006. doi: 10.1016/j.molcel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 499.Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325: 834–840, 2009. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 500.Weinert BT, Schölz C, Wagner SA, Iesmantavicius V, Su D, Daniel JA, Choudhary C. Lysine succinylation is a frequently occurring modification in prokaryotes and eukaryotes and extensively overlaps with acetylation. Cell Rep 4: 842–851, 2013. doi: 10.1016/j.celrep.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 501.Naviaux RK. Mitochondrial control of epigenetics. Cancer Biol Ther 7: 1191–1193, 2008. doi: 10.4161/cbt.7.8.6741. [DOI] [PubMed] [Google Scholar]
- 502.Wallace DC, Fan W. Energetics, epigenetics, mitochondrial genetics. Mitochondrion 10: 12–31, 2010. doi: 10.1016/j.mito.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Amuthan G, Biswas G, Ananadatheerthavarada HK, Vijayasarathy C, Shephard HM, Avadhani NG. Mitochondrial stress-induced calcium signaling, phenotypic changes and invasive behavior in human lung carcinoma A549 cells. Oncogene 21: 7839–7849, 2002. doi: 10.1038/sj.onc.1205983. [DOI] [PubMed] [Google Scholar]
- 504.Vanderauwera S, Vandenbroucke K, Inzé A, van de Cotte B, Mühlenbock P, De Rycke R, Naouar N, Van Gaever T, Van Montagu MC, Van Breusegem F. AtWRKY15 perturbation abolishes the mitochondrial stress response that steers osmotic stress tolerance in Arabidopsis. Proc Natl Acad Sci USA 109: 20113–20118, 2012. doi: 10.1073/pnas.1217516109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Xu S, Chisholm A. C. elegans epidermal wounding induces a mitochondrial ROS burst that promotes wound repair. Dev Cell 31: 48–60, 2014. doi: 10.1016/j.devcel.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Santulli G, Xie W, Reiken SR, Marks AR. Mitochondrial calcium overload is a key determinant in heart failure. Proc Natl Acad Sci USA 112: 11389–11394, 2015. doi: 10.1073/pnas.1513047112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Pogwizd SM, Sipido KR, Verdonck F, Bers DM. Intracellular Na in animal models of hypertrophy and heart failure: contractile function and arrhythmogenesis. Cardiovasc Res 57: 887–896, 2003. doi: 10.1016/S0008-6363(02)00735-6. [DOI] [PubMed] [Google Scholar]
- 508.Maack C, Cortassa S, Aon MA, Ganesan AN, Liu T, O’Rourke B. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ Res 99: 172–182, 2006. doi: 10.1161/01.RES.0000232546.92777.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Liu T, O’Rourke B. Enhancing mitochondrial Ca2+ uptake in myocytes from failing hearts restores energy supply and demand matching. Circ Res 103: 279–288, 2008. doi: 10.1161/CIRCRESAHA.108.175919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Sommakia S, Houlihan PR, Deane SS, Simcox JA, Torres NS, Jeong MY, Winge DR, Villanueva CJ, Chaudhuri D. Mitochondrial cardiomyopathies feature increased uptake and diminished efflux of mitochondrial calcium. J Mol Cell Cardiol 113: 22–32, 2017. doi: 10.1016/j.yjmcc.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Liu T, Yang N, Sidor A, O’Rourke B. MCU overexpression rescues inotropy and reverses heart failure by reducing SR Ca2+ leak. Circ Res 128: 1191–1204, 2021. doi: 10.1161/CIRCRESAHA.120.318562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Garbincius JF, Elrod JW. Is the failing heart starved of mitochondrial calcium? Circ Res 128: 1205–1207, 2021. doi: 10.1161/CIRCRESAHA.121.319030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Nour M, Scalzo F, Liebeskind DS. Ischemia-reperfusion injury in stroke. Interv Neurol 1: 185–199, 2013. doi: 10.1159/000353125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Zhao Q, Wang S, Li Y, Wang P, Li S, Guo Y, Yao R. The role of the mitochondrial calcium uniporter in cerebral ischemia/reperfusion injury in rats involves regulation of mitochondrial energy metabolism. Mol Med Rep 7: 1073–1080, 2013. doi: 10.3892/mmr.2013.1321. [DOI] [PubMed] [Google Scholar]
- 515.Nichols M, Pavlov EV, Robertson GS. Tamoxifen-induced knockdown of the mitochondrial calcium uniporter in Thy1-expressing neurons protects mice from hypoxic/ischemic brain injury. Cell Death Dis 9: 606, 2018. doi: 10.1038/s41419-018-0607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Zhang L, Wang H, Zhou X, Mao L, Ding K, Hu Z. Role of mitochondrial calcium uniporter-mediated Ca2+ and iron accumulation in traumatic brain injury. J Cell Mol Med 23: 2995–3009, 2019. doi: 10.1111/jcmm.14206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Zeviani M, Carelli V. Mitochondrial disorders. Curr Opin Neurol 20: 564–571, 2007. doi: 10.1097/WCO.0b013e3282ef58cd. [DOI] [PubMed] [Google Scholar]
- 518.Area-Gomez E, Guardia-Laguarta C, Schon EA, Przedborski S. Mitochondria, OxPhos, and neurodegeneration: cells are not just running out of gas. J Clin Invest 129: 34–45, 2019. doi: 10.1172/JCI120848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Jiang D, Sullivan PG, Sensi SL, Steward O, Weiss JH. Zn2+ induces permeability transition pore opening and release of pro-apoptotic peptides from neuronal mitochondria. J Biol Chem 276: 47524–47529, 2001. doi: 10.1074/jbc.M108834200. [DOI] [PubMed] [Google Scholar]
- 520.Calì T, Ottolini D, Brini M. Mitochondrial Ca2+ and neurodegeneration. Cell Calcium 52: 73–85, 2012. doi: 10.1016/j.ceca.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Hardy J, Duff K, Hardy KG, Perez-Tur J, Hutton M. Genetic dissection of Alzheimer’s disease and related dementias: amyloid and its relationship to tau. Nat Neurosci 1: 355–358, 1998. doi: 10.1038/1565. [DOI] [PubMed] [Google Scholar]
- 522.Area-Gomez E, Del Carmen Lara Castillo M, Tambini MD, Guardia-Laguarta C, de Groof AJ, Madra M, Ikenouchi J, Umeda M, Bird TD, Sturley SL, Schon EA. Upregulated function of mitochondria-associated ER membranes in Alzheimer disease. EMBO J 31: 4106–4123, 2012. doi: 10.1038/emboj.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Zampese E, Fasolato C, Kipanyula MJ, Bortolozzi M, Pozzan T, Pizzo P. Presenilin 2 modulates endoplasmic reticulum (ER)-mitochondria interactions and Ca2+ cross-talk. Proc Natl Acad Sci USA 108: 2777–2782, 2011. doi: 10.1073/pnas.1100735108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Stutzmann GE, Caccamo A, LaFerla FM, Parker I. Dysregulated IP3 signaling in cortical neurons of knock-in mice expressing an Alzheimer’s-linked mutation in presenilin1 results in exaggerated Ca2+ signals and altered membrane excitability. J Neurosci 24: 508–513, 2004. doi: 10.1523/JNEUROSCI.4386-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Cheung KH, Shineman D, Müller M, Cárdenas C, Mei L, Yang J, Tomita T, Iwatsubo T, Lee VM, Foskett JK. Mechanism of Ca2+ disruption in Alzheimer’s disease by presenilin regulation of InsP3 receptor channel gating. Neuron 58: 871–883, 2008. doi: 10.1016/j.neuron.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Cheung KH, Mei L, Mak DO, Hayashi I, Iwatsubo T, Kang DE, Foskett JK. Gain-of-function enhancement of IP3 receptor modal gating by familial Alzheimer’s disease-linked presenilin mutants in human cells and mouse neurons. Sci Signal 3: ra22, 2010. doi: 10.1126/scisignal.2000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Toglia P, Ullah G. The gain-of-function enhancement of IP3-receptor channel gating by familial Alzheimer’s disease-linked presenilin mutants increases the open probability of mitochondrial permeability transition pore. Cell Calcium 60: 13–24, 2016. doi: 10.1016/j.ceca.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 528.Shilling D, Müller M, Takano H, Mak DO, Abel T, Coulter DA, Foskett JK. Suppression of InsP3 receptor-mediated Ca2+ signaling alleviates mutant presenilin-linked familial Alzheimer’s disease pathogenesis. J Neurosci 34: 6910–6923, 2014. doi: 10.1523/JNEUROSCI.5441-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Lacampagne A, Liu X, Reiken S, Bussiere R, Meli AC, Lauritzen I, Teich AF, Zalk R, Saint N, Arancio O, Bauer C, Duprat F, Briggs CA, Chakroborty S, Stutzmann GE, Shelanski ML, Checler F, Chami M, Marks AR. Post-translational remodeling of ryanodine receptor induces calcium leak leading to Alzheimer’s disease-like pathologies and cognitive deficits. Acta Neuropathol 134: 749–767, 2017. doi: 10.1007/s00401-017-1733-7. [DOI] [PubMed] [Google Scholar]
- 530.Ferreiro E, Oliveira CR, Pereira CM. The release of calcium from the endoplasmic reticulum induced by amyloid-beta and prion peptides activates the mitochondrial apoptotic pathway. Neurobiol Dis 30: 331–342, 2008. doi: 10.1016/j.nbd.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 531.Sanz-Blasco S, Valero RA, Rodríguez-Crespo I, Villalobos C, Núñez L. Mitochondrial Ca2+ overload underlies Abeta oligomers neurotoxicity providing an unexpected mechanism of neuroprotection by NSAIDs. PLoS One 3: e2718, 2008. doi: 10.1371/journal.pone.0002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Kuchibhotla KV, Goldman ST, Lattarulo CR, Wu HY, Hyman BT, Bacskai BJ. Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron 59: 214–225, 2008. doi: 10.1016/j.neuron.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Murray FE, Landsberg JP, Williams RJ, Esiri MM, Watt F. Elemental analysis of neurofibrillary tangles in Alzheimer’s disease using proton-induced X-ray analysis. Ciba Found Symp 169: 201–210, 1992. doi: 10.1002/9780470514306.ch12. [DOI] [PubMed] [Google Scholar]
- 534.Mattson MP. Calcium and neurodegeneration. Aging Cell 6: 337–350, 2007. doi: 10.1111/j.1474-9726.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- 535.Alzheimer’s Association Calcium Hypothesis Workgroup. Calcium hypothesis of Alzheimer’s disease and brain aging: a framework for integrating new evidence into a comprehensive theory of pathogenesis. Alzheimers Dement 13: 178–182.e17, 2017. doi: 10.1016/j.jalz.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 536.Bezprozvanny I, Mattson MP. Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci 31: 454–463, 2008. doi: 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Popugaeva E, Pchitskaya E, Bezprozvanny I. Dysregulation of intracellular calcium signaling in Alzheimer’s disease. Antioxid Redox Signal 29: 1176–1188, 2018. doi: 10.1089/ars.2018.7506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Jadiya P, Garbincius JF, Elrod JW. Reappraisal of metabolic dysfunction in neurodegeneration: focus on mitochondrial function and calcium signaling. Acta Neuropathol Commun 9: 124, 2021. doi: 10.1186/s40478-021-01224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Britti E, Ros J, Esteras N, Abramov AY. Tau inhibits mitochondrial calcium efflux and makes neurons vulnerable to calcium-induced cell death. Cell Calcium 86: 102150, 2020. doi: 10.1016/j.ceca.2019.102150. [DOI] [PubMed] [Google Scholar]
- 540.Calvo-Rodriguez M, Hernando-Perez E, Nuñez L, Villalobos C. Amyloid beta oligomers increase ER-mitochondria Ca2+ cross talk in young hippocampal neurons and exacerbate aging-induced intracellular Ca2+ remodeling. Front Cell Neurosci 13: 22, 2019. doi: 10.3389/fncel.2019.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Kalia LV, Lang AE. Parkinson’s disease. Lancet 386: 896–912, 2015. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 542.Marongiu R, Spencer B, Crews L, Adame A, Patrick C, Trejo M, Dallapiccola B, Valente EM, Masliah E. Mutant Pink1 induces mitochondrial dysfunction in a neuronal cell model of Parkinson’s disease by disturbing calcium flux. J Neurochem 108: 1561–1574, 2009. doi: 10.1111/j.1471-4159.2009.05932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Parihar MS, Parihar A, Fujita M, Hashimoto M, Ghafourifar P. Alpha-synuclein overexpression and aggregation exacerbates impairment of mitochondrial functions by augmenting oxidative stress in human neuroblastoma cells. Int J Biochem Cell Biol 41: 2015–2024, 2009. doi: 10.1016/j.biocel.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 544.Gandhi S, Wood-Kaczmar A, Yao Z, Plun-Favreau H, Deas E, Klupsch K, Downward J, Latchman DS, Tabrizi SJ, Wood NW, Duchen MR, Abramov AY. PINK1-associated Parkinson’s disease is caused by neuronal vulnerability to calcium-induced cell death. Mol Cell 33: 627–638, 2009. doi: 10.1016/j.molcel.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Soman S, Keatinge M, Moein M, Da Costa M, Mortiboys H, Skupin A, Sugunan S, Bazala M, Kuznicki J, Bandmann O. Inhibition of the mitochondrial calcium uniporter rescues dopaminergic neurons in pink1-/- zebrafish. Eur J Neurosci 45: 528–535, 2017. doi: 10.1111/ejn.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Martin LJ. Mitochondrial pathobiology in ALS. J Bioenerg Biomembr 43: 569–579, 2011. doi: 10.1007/s10863-011-9395-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Coussee E, De Smet P, Bogaert E, Elens I, Van Damme P, Willems P, Koopman W, Van Den Bosch L, Callewaert G. G37R SOD1 mutant alters mitochondrial complex I activity, Ca2+ uptake and ATP production. Cell Calcium 49: 217–225, 2011. doi: 10.1016/j.ceca.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 548.Jaiswal MK, Keller BU. Cu/Zn superoxide dismutase typical for familial amyotrophic lateral sclerosis increases the vulnerability of mitochondria and perturbs Ca2+ homeostasis in SOD1G93A mice. Mol Pharmacol 75: 478–489, 2009. doi: 10.1124/mol.108.050831. [DOI] [PubMed] [Google Scholar]
- 549.Fuchs A, Kutterer S, Mühling T, Duda J, Schütz B, Liss B, Keller BU, Roeper J. Selective mitochondrial Ca2+ uptake deficit in disease endstage vulnerable motoneurons of the SOD1G93A mouse model of amyotrophic lateral sclerosis. J Physiol 591: 2723–2745, 2013. doi: 10.1113/jphysiol.2012.247981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Dafinca R, Barbagallo P, Farrimond L, Candalija A, Scaber J, Ababneh NA, Sathyaprakash C, Vowles J, Cowley SA, Talbot K. Impairment of mitochondrial calcium buffering links mutations in C9ORF72 and TARDBP in iPS-derived motor neurons from patients with ALS/FTD. Stem Cell Rep 14: 892–908, 2020. doi: 10.1016/j.stemcr.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Tadić V, Adam A, Goldhammer N, Lautenschlaeger J, Oberstadt M, Malci A, Le TT, Sengupta S, Stubendorff B, Keiner S, Witte OW, Grosskreutz J. Investigation of mitochondrial calcium uniporter role in embryonic and adult motor neurons from G93A(hSOD1) mice. Neurobiol Aging 75: 209–222, 2019. doi: 10.1016/j.neurobiolaging.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 552.Mühling T, Duda J, Weishaupt JH, Ludolph AC, Liss B. Elevated mRNA-levels of distinct mitochondrial and plasma membrane Ca2+ transporters in individual hypoglossal motor neurons of endstage SOD1 transgenic mice. Front Cell Neurosci 8: 353, 2014. doi: 10.3389/fncel.2014.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Lautenschläger J, Prell T, Ruhmer J, Weidemann L, Witte OW, Grosskreutz J. Overexpression of human mutated G93A SOD1 changes dynamics of the ER mitochondria calcium cycle specifically in mouse embryonic motor neurons. Exp Neurol 247: 91–100, 2013. doi: 10.1016/j.expneurol.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 554.Panov AV, Gutekunst CA, Leavitt BR, Hayden MR, Burke JR, Strittmatter WJ, Greenamyre JT. Early mitochondrial calcium defects in Huntington’s disease are a direct effect of polyglutamines. Nat Neurosci 5: 731–736, 2002. doi: 10.1038/nn884. [DOI] [PubMed] [Google Scholar]
- 555.Panov AV, Lund S, Greenamyre JT. Ca2+-induced permeability transition in human lymphoblastoid cell mitochondria from normal and Huntington’s disease individuals. Mol Cell Biochem 269: 143–152, 2005. doi: 10.1007/s11010-005-3454-9. [DOI] [PubMed] [Google Scholar]
- 556.Lim D, Fedrizzi L, Tartari M, Zuccato C, Cattaneo E, Brini M, Carafoli E. Calcium homeostasis and mitochondrial dysfunction in striatal neurons of Huntington disease. J Biol Chem 283: 5780–5789, 2008. doi: 10.1074/jbc.M704704200. [DOI] [PubMed] [Google Scholar]
- 557.Leahy JL, Cooper HE, Deal DA, Weir GC. Chronic hyperglycemia is associated with impaired glucose influence on insulin secretion. A study in normal rats using chronic in vivo glucose infusions. J Clin Invest 77: 908–915, 1986. doi: 10.1172/JCI112389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Jimenez-Sánchez C, Brun T, Maechler P. Mitochondrial carriers regulating insulin secretion profiled in human islets upon metabolic stress. Biomolecules 10: 1543, 2020. doi: 10.3390/biom10111543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Diaz-Juarez J, Suarez J, Cividini F, Scott BT, Diemer T, Dai A, Dillmann WH. Expression of the mitochondrial calcium uniporter in cardiac myocytes improves impaired mitochondrial calcium handling and metabolism in simulated hyperglycemia. Am J Physiol Cell Physiol 311: C1005–C1013, 2016. doi: 10.1152/ajpcell.00236.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Suarez J, Cividini F, Scott BT, Lehmann K, Diaz-Juarez J, Diemer T, Dai A, Suarez JA, Jain M, Dillmann WH. Restoring mitochondrial calcium uniporter expression in diabetic mouse heart improves mitochondrial calcium handling and cardiac function. J Biol Chem 293: 8182–8195, 2018. doi: 10.1074/jbc.RA118.002066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Chen W, Yang J, Chen S, Xiang H, Liu H, Lin D, Zhao S, Peng H, Chen P, Chen AF, Lu H. Importance of mitochondrial calcium uniporter in high glucose-induced endothelial cell dysfunction. Diab Vasc Dis Res 14: 494–501, 2017. doi: 10.1177/1479164117723270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Porporato PE, Filigheddu N, Pedro JM, Kroemer G, Galluzzi L. Mitochondrial metabolism and cancer. Cell Res 28: 265–280, 2018. doi: 10.1038/cr.2017.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Marchi S, Vitto VA, Danese A, Wieckowski MR, Giorgi C, Pinton P. Mitochondrial calcium uniporter complex modulation in cancerogenesis. Cell Cycle 18: 1068–1083, 2019. doi: 10.1080/15384101.2019.1612698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Curry MC, Peters AA, Kenny PA, Roberts-Thomson SJ, Monteith GR. Mitochondrial calcium uniporter silencing potentiates caspase-independent cell death in MDA-MB-231 breast cancer cells. Biochem Biophys Res Commun 434: 695–700, 2013. doi: 10.1016/j.bbrc.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 565.Tosatto A, Sommaggio R, Kummerow C, Bentham RB, Blacker TS, Berecz T, Duchen MR, Rosato A, Bogeski I, Szabadkai G, Rizzuto R, Mammucari C. The mitochondrial calcium uniporter regulates breast cancer progression via HIF-1alpha. EMBO Mol Med 8: 569–585, 2016. doi: 10.15252/emmm.201606255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Zeng F, Chen X, Cui W, Wen W, Lu F, Sun X, Ma D, Yuan Y, Li Z, Hou N, Zhao H, Bi X, Zhao J, Zhou J, Zhang Y, Xiao RP, Cai J, Zhang X. RIPK1 binds MCU to mediate induction of mitochondrial Ca2+ uptake and promotes colorectal oncogenesis. Cancer Res 78: 2876–2885, 2018. doi: 10.1158/0008-5472.CAN-17-3082. [DOI] [PubMed] [Google Scholar]
- 567.Chen L, Sun Q, Zhou D, Song W, Yang Q, Ju B, Zhang L, Xie H, Zhou L, Hu Z, Yao H, Zheng S, Wang W. HINT2 triggers mitochondrial Ca2+ influx by regulating the mitochondrial Ca2+ uniporter (MCU) complex and enhances gemcitabine apoptotic effect in pancreatic cancer. Cancer Lett 411: 106–116, 2017. doi: 10.1016/j.canlet.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 568.Xie KF, Guo DD, Luo XJ. SMDT1-driven change in mitochondrial dynamics mediate cell apoptosis in PDAC. Biochem Biophys Res Commun 511: 323–329, 2019. doi: 10.1016/j.bbrc.2019.02.043. [DOI] [PubMed] [Google Scholar]
- 569.Chakraborty PK, Mustafi SB, Xiong X, Dwivedi SK, Nesin V, Saha S, Zhang M, Dhanasekaran D, Jayaraman M, Mannel R, Moore K, McMeekin S, Yang D, Zuna R, Ding K, Tsiokas L, Bhattacharya R, Mukherjee P. MICU1 drives glycolysis and chemoresistance in ovarian cancer. Nat Commun 8: 14634, 2017. doi: 10.1038/ncomms14634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Tian Y, Qin L, Qiu H, Shi D, Sun R, Li W, Liu T, Wang J, Xu T, Guo W, Kang T, Huang W, Wang G, Deng W. RPS3 regulates melanoma cell growth and apoptosis by targeting Cyto C/Ca2+/MICU1 dependent mitochondrial signaling. Oncotarget 6: 29614–29625, 2015. doi: 10.18632/oncotarget.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Zhou X, Ren Y, Kong L, Cai G, Sun S, Song W, Wang Y, Jin R, Qi L, Mei M, Wang X, Kang C, Li M, Zhang L. Targeting EZH2 regulates tumor growth and apoptosis through modulating mitochondria dependent cell-death pathway in HNSCC. Oncotarget 6: 33720–33732, 2015. doi: 10.18632/oncotarget.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Ren T, Wang J, Zhang H, Yuan P, Zhu J, Wu Y, Huang Q, Guo X, Zhang J, Ji L, Li J, Zhang H, Yang H, Xing J. MCUR1-mediated mitochondrial calcium signaling facilitates cell survival of hepatocellular carcinoma via reactive oxygen species-dependent p53 degradation. Antioxid Redox Signal 28: 1120–1136, 2018. doi: 10.1089/ars.2017.6990. [DOI] [PubMed] [Google Scholar]
- 573.Wilton KM, Morales-Rosado JA, Selcen D, Muthusamy K, Ewing S, Agre K, Nickels K, Klee EW, Ho ML, Morava E. Developmental brain abnormalities and acute encephalopathy in a patient with myopathy with extrapyramidal signs secondary to pathogenic variants in MICU1. JIMD Rep 53: 22–28, 2020. doi: 10.1002/jmd2.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]