Abstract
Oropouche (ORO) virus is an emerging infectious agent that has caused numerous outbreaks of an acute febrile (dengue-like) illness among humans in Brazil, Peru, and Panama. Diagnosis of ORO virus infection is based mainly on serology. Two different antigens, hamster serum antigen (HSA) and Vero cell lysate antigen (VCLA), are currently used in enzyme immunoassays (EIAs) in Brazil and Peru, respectively, to investigate the epidemiology of ORO virus infection. Both antigens involve use of infectious virus, and for this reason their use is restricted. Consequently, the frequency and distribution of ORO virus infection are largely unexplored in other countries of South America. This report describes the use of a bacterially expressed recombinant nucleocapsid (rN) protein of ORO virus in EIAs for the diagnosis of ORO virus infection. The data revealed that the purified rN protein is comparable to the authentic viral N protein in its antigenic characteristics and is highly sensitive and specific in EIAs. Among 183 serum samples tested, a high degree of concordance was found between rN protein-based EIA and HSA- and VCLA-based EIAs for the detection of both ORO virus-specific immunoglobulin M (IgM) and IgG antibodies. The high sensitivity, specificity, and safety of the rN protein-based EIA make it a useful diagnostic technique that can be widely used to detect ORO virus infection in South America.
Oropouche (ORO) virus, a member of the Simbu serogroup of the genus Bunyavirus, family Bunyaviridae, is an emerging human pathogen that is transmitted mainly by the biting midge Culicoides paraensis. ORO virus is the etiologic agent of ORO fever, a dengue-like acute febrile illness that is a significant public health problem in tropical South America. Over the past 39 years there have been at least 30 reported outbreaks of ORO fever, involving more than half a million people in tropical South America (14). Seroepidemiological investigations of ORO fever in Brazil indicate that, while the prevalence of ORO antibodies is relatively low (0 to 2%) in areas where epidemic transmission has not been reported (13), during epidemics an incidence rate of 30% is common and may reach as high as 60% (10). The disease has also been reported in Panama and is endemic in humid tropical regions of Peru, with a fairly high (18 to 46%) seroprevalence rate (20). Due to the debilitating nature and duration (5 to 6 days) of symptoms and the high incidence rates during epidemics, ORO fever clearly has the potential for substantial social and economic impact.
The clinical diagnosis of ORO fever is difficult because of the nonspecific nature of symptoms; it is easily confused with dengue fever and a number of other arbovirus illnesses that are common in tropical South America. Laboratory diagnosis of the disease is usually based on serological methods including the plaque reduction neutralization test, complement fixation test, hemagglutination inhibition (HI) test, and enzyme immunoassay (EIA). However, all of these techniques require the handling of infectious virus at some part of the process; since ORO virus is a biosafety level 3 agent, this limits the laboratories that can work with it. Consequently, only a few laboratories in South America currently conduct serological testing for ORO fever, and the frequency and distribution of the disease are not well known.
To investigate the epidemiology of ORO fever, we have developed an EIA for the laboratory diagnosis of ORO virus infection. This assay is based on the bacterially expressed recombinant nucleocapsid (rN) protein, and its advantages include simplicity, safety of use, and the possibility of standardization, in addition to good sensitivity and specificity. Thus, our rN protein-based EIA can potentially be used to investigate the epidemiology of ORO virus infection and possibly to obtain a more accurate estimate of the impact of ORO fever on human health in tropical South America.
MATERIALS AND METHODS
Virus and cell culture.
Vero-E6 cells were cultivated in Eagle's minimal essential medium (EMEM) supplemented with 10% fetal bovine serum (FBS) and penicillin (100 U/ml)-streptomycin (100 μg/ml). ORO virus strain MD023 was used; this virus was originally isolated from the blood of a human with ORO fever in Peru and was propagated in Vero cells in EMEM supplemented with 2% FBS and antibiotics. When 75 to 80% of the cellular monolayer had cytopathic effect, the culture supernatant was collected and centrifuged at 2,000 × g for 5 to 10 min to remove cellular debris. The supernatant was aliquoted in 1-ml volumes and frozen at −70°C as stock virus.
Serum samples and immunological reagents.
Of the 183 human serum samples used in this study, 108 were obtained from individuals during ORO fever epidemiological investigations in Brazil and 75 were obtained from individuals as part of previous ORO surveillance studies in Iquitos and Santa Clara, Peru. The negative-control human sera were obtained from the World Arbovirus Reference Center at the University of Texas Medical Branch (Galveston, Tex.). These sera were collected from healthy individuals residing in the United States who were known to have never visited South America.
Hyperimmune mouse ascitic fluids (HIMAF) used in Western blotting and EIA were obtained from the World Arbovirus Reference Center. Mice received four weekly intraperitoneal injections of 10%-infected newborn mouse brain mixed with Freund's adjuvant, followed by sarcoma-180 cells, as described by Tikasingh et al. (18).
Anti-Xpress antibody, used in a Western blot assay to identify the full-length recombinant N protein containing a His tag leader peptide, was purchased from Invitrogen (Carlsbad, Calif.). It is a mouse monoclonal antibody that specifically reacts with a His tag leader peptide encoded by the pTrcHis expression vector used in these studies.
Amplification and cloning of nucleocapsid gene.
Viral RNA was extracted from 0.5 ml of stock virus, as described previously (12). To amplify the nucleocapsid gene, the RNA was subjected to reverse transcription-PCR using primers ORON5 (5′-AAAGAGGATCCAATAATGTCAGAGTTCATTT-3′) and ORON3 (5′-GTGAATTCCACTATATGCCAATTCCGAATT-3′), as described previously (15). Primers ORON5 and ORON3 were designed so as to generate BamHI and EcoRI sites, respectively. The amplicon was first cloned into the TA cloning vector (pCR2.1; Invitrogen), amplified in bacteria, and digested with BamHI and EcoRI restriction enzymes. The insert DNA was then recovered and cloned into the BamHI and EcoRI sites of pTrcHisB expression vector (Invitrogen). The recombinant expression vector was transformed into cells, which were then selected on Luria-Bertani (LB) agar containing ampicillin (100 μg/ml). Following overnight incubation in LB broth containing ampicillin, plasmid DNA was extracted and analyzed for the presence of insert DNA by digestion with BamHI and EcoRI restriction enzymes.
Purification of ORO virus rN protein.
Purification of the recombinant protein was carried out using the pTrcHis Xpress kit (Invitrogen) according to the manufacturer's instructions. Briefly, bacteria containing the recombinant expression vector were grown in LB broth supplemented with ampicillin. When the optical density at 600 nm (OD600) of the culture reached 0.6, protein expression was induced by the addition of 5 mM isopropyl-β-d-thiogalactopyranoside (IPTG). After 5 to 6 h of induction, bacteria were pelleted and resuspended in buffer (50 mM sodium phosphate and 300 mM NaCl, pH 7.8) containing lysozyme (100 μg/ml) and incubated on ice for 30 min. Thereafter, cells were sonicated and centrifuged at 10,000 × g for 15 min. The supernatant was loaded on a ProBond histidine-binding column, preequilibrated with buffer containing 50 mM sodium phosphate and 300 mM NaCl, pH 7.8. Subsequent to rinsing with the washing buffer (50 mM sodium phosphate and 300 mM NaCl, pH 6.0), the recombinant protein was eluted with a concentration gradient (0 to 1.0 M) of imidazole. Each of the eluted fractions was analyzed by electrophoresis on a sodium dodecyl sulfate (SDS)–12% polyacrylamide gel. The identity of the expressed protein was confirmed by Western blot analysis using ORO virus-specific HIMAF and human serum from an ORO virus-infected individual.
Preparation of hamster serum antigen (HSA).
One hundred microliters of brain homogenate of newborn mice infected with ORO virus (strain BeAn 19991) was inoculated intraperitoneally into 4- to 5-week-old female Syrian golden hamsters (Mesocricetus auratus). When the hamsters first showed signs of illness (36 to 48 h postinoculation), blood was collected by cardiac puncture and allowed to clot at 4°C. Subsequently, the serum was removed and diluted 10-fold with isotonic saline solution. The diluted serum was expressed into 20 volumes of dry, chilled acetone with an 18-gauge needle and extracted for 5 min with intermittent shaking. The sample was then centrifuged at 500 × g for 5 min at 4°C; the supernatant was discarded, and the sediment was resuspended in 20 volumes of chilled acetone by vigorous shaking. After incubation for 1 h at 4°C, the sample was centrifuged at 500 × g for 10 to 15 min and the sediment was dried under vacuum at room temperature for 1 h. Finally, the dried sediment was resuspended in a sufficient volume of borate-saline solution (0.12 M NaCl, 0.05 M H3BO3, 0.024 N NaOH, pH 9.0) to make a 1:10 dilution based on the original volume of serum and stored at −70°C in 1- to 2-ml aliquots.
The use of animals in this study was in accordance with a University of Texas Medical Branch protocol for the use of animals in biomedical research.
Preparation of VCLA.
Vero cell lysate antigen (VCLA) was prepared essentially as described by Beaty et al. (1). Briefly, Vero cells were infected with ORO virus (strain MD023). At the time when cytopathic effects began to appear (approximately 20 to 25% cell death), cells were harvested, centrifuged at 10,000 × g for 10 min at 4°C, and washed once with 0.1 M borate-saline solution (pH 9.0). Thereafter, the cells were resuspended in borate-saline containing 1% Triton X-100, sonicated, and centrifuged at 10,000 × g for 5 min at 4°C. The supernatant was collected, aliquoted, and stored at 4°C.
EIA. (i)IgG EIA.
Wells of microtiter plates were coated with antigen (purified ORO virus rN protein or HSA or VCLA) and diluted in carbonate-bicarbonate buffer, pH 9.6, and the plates were incubated at 4°C. Subsequently, the plates were washed five times with phosphate-buffered saline (PBS), pH 7.4 (Gibco-BRL), containing 0.05% Tween 20 (Sigma Chemical Co., St. Louis, Mo.) followed by the addition of 250 μl of blocking buffer (4% bovine serum albumin in PBS) to each well. After incubation for 15 to 20 min at 37°C, the blocking buffer was aspirated and 100-μl portions of serum samples (diluted 1:400 in blocking buffer) were added to the wells and the plates were incubated at 37°C for 1 h. Thereafter, the plates were washed five times as described above, and 100 μl of peroxidase-conjugated goat anti-human immunoglobulin G (IgG) (Kirkegaard & Perry Laboratories, Gaithersburg, Md.), diluted 1:2,000 in blocking buffer, was added to each well, followed by incubation for 1 h at 37°C. The plates were then washed as described above and 100 μl of peroxidase substrate, 2.2′-azino-di[3-ethyl-benzthiazoline sulfonate (6)] (Kirkegaard & Perry Laboratories), was added to each well. Plates were then incubated at room temperature for 15 to 30 min, and OD405 was measured in a microplate reader. At least one positive-control serum and three negative-control sera were included in each assay. The cutoff was the mean OD405 of negative samples plus three standard deviations. Test sera having an OD405 greater than the cutoff were considered positive.
(ii)IgM EIA.
An IgM capture method was used as follows. Wells of microtiter plates were coated with 100 μl of anti-human IgM (Kirkegaard & Perry Laboratories) diluted 1:200 in carbonate-bicarbonate buffer, pH 9.6, and the plates were incubated at 4°C for at least 16 h. Subsequently, the plates were washed five times with wash buffer (PBS–0.05% Tween 20), and 250 μl of blocking buffer (4% bovine serum albumin in PBS) was added to each well. After incubation for 15 to 20 min, the blocking buffer was aspirated and 50 μl of test sera (diluted 1:400 in blocking buffer) was added to wells and the plates were incubated at 37°C for 1 h. The plates were then washed as described above, and 50 μl of appropriately diluted antigen (rN protein or HSA) was added to each well, followed by incubation for 1 h at 37°C. The plates were again washed, and 50 μl of ORO virus-specific HIMAF, diluted 1:200 in blocking buffer, was added to each well. After a 1-h incubation at 37°C, the plates were washed, 50 μl of peroxidase-conjugated sheep anti-mouse Ig (diluted 1:2,000; Amersham) was added to each well, and the plates were incubated at 37°C for 1 h. Finally, after the washing, 100 μl of peroxidase substrate was added and the plates were incubated at room temperature for 15 to 30 min. OD was measured in a microplate reader at 405 nm. The cutoff was calculated as described above.
Neutralization assay.
Serum samples, diluted 10-fold in PBS, were heat-inactivated at 56°C for 30 min, mixed with an equal volume of ORO virus strain MD023 (diluted in PBS to give 40 to 50 PFU/0.1 ml), and incubated at 37°C for 1 h. Subsequently, 0.2 ml of virus-serum mixture was inoculated into confluent monolayer cultures of Vero cells grown in six-well tissue culture plates. After incubation for 30 min at room temperature, each well was overlaid with 5 ml of 1% agar (Sigma Chemical Co.) containing EMEM supplemented with 2% FBS. Thereafter, plates were incubated at 37°C. After 4 to 5 days, monolayers were stained with neutral red and the plaques in each well were counted. At least three negative-control serum samples were included. Serum samples that showed an 80% or greater reduction in number of plaques compared to negative-control sera were considered neutralizing, and those that showed less than 80% reduction at 1:20 serum dilution were considered to be negative.
HI assays.
The HI tests were performed according to the methods of Clarke and Casals (3) using modifications for microtiter plates (17). For these assays, the HSA described above was used as the antigen. Prior to the HI assay, the test sera were extracted with acetone and adsorbed with goose red blood cells as described by Beaty et al. (1).
SDS-polyacrylamide gel electrophoresis (PAGE) and Western blotting.
Protein samples were resolved on an SDS–12% polyacrylamide gel by electrophoresis, using the method of Laemmli (9). The resolved samples were then transferred to a polyvinylidene difluoride membrane (Bio-Rad) by electroblotting in a buffer containing Tris-HCl (25 mM), glycine (192 mM), and methanol (20% [vol/vol]). Subsequently, the membrane was incubated with 5% nonfat milk (in PBS) for 1 h at room temperature to block nonspecific protein binding sites. Thereafter, the membrane was incubated with the primary antibody (ORO virus-specific HIMAF diluted 1:200 or ORO virus-specific human serum diluted 1:50 or anti-Xpress diluted 1:5,000 in 5% nonfat milk) for 1 h at room temperature, followed by washing with PBS and incubation with a secondary antibody (alkaline phosphatase-conjugated goat anti-mouse or anti-human IgG; Sigma Chemical Co.). After 1 h at room temperature, the membrane was washed and the antigen-antibody complex on the membrane was detected by the addition of a small volume of BCIP (5-bromo-4-chloro-3-indolylphosphate)-nitroblue tetrazolium (Sigma Chemical Co.), the substrate for alkaline phosphatase.
RESULTS
Amplification and cloning of the ORO virus N cDNA.
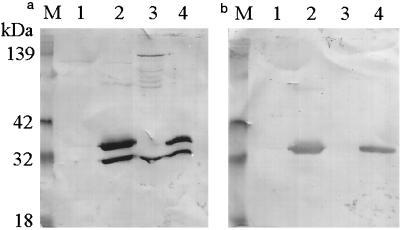
The genomic RNA of ORO virus (strain MD023) was extracted and subjected to reverse transcription-PCR using primers described above to amplify the N gene. The nucleotide sequence of the amplicon was confirmed by sequencing as described elsewhere (15). Subsequently, the amplified cDNA was cloned into the pTrcHisB expression vector (Invitrogen) between the BamHI and EcoRI sites in frame with the start codon of the His tag leader peptide. The recombinant construct was transformed into competent Escherichia coli cells, and plasmid DNA extraction and digestion with BamHI and EcoRI restriction enzymes confirmed the presence of the construct in transformed bacteria (data not shown). A single colony of the transformed bacteria was grown in LB broth and treated with IPTG to induce the expression of rN protein, which was then analyzed by PAGE and Western blotting, using HIMAF raised against ORO virus. The results (Fig. 1a) show the presence of two anti-ORO virus antibody-reactive bands in extracts of bacteria transformed with the recombinant plasmid (lane 2) but not in extracts of bacteria transformed with vector alone (lane 1). This suggested that the ORO virus rN protein was expressed in bacteria; however there were two forms of the protein. From the molecular weight estimates, the slower-migrating band corresponded to the full-length recombinant fusion protein, while the faster-migrating band represented the truncated version. SDS-PAGE revealed that the faster-migrating band corresponded in size to the authentic viral N protein expressed in ORO virus-infected Vero cells (Fig. 1a; compare lanes 2 and 3), suggesting that the truncated version consists of the entire N protein lacking the His tag leader peptide. This was confirmed by Western blot analysis using antibodies against the His tag fusion peptide (Invitrogen). The results show that, while both forms reacted with anti-ORO virus antibodies (Fig. 1a, lane 2), only the slower-migrating form was reactive to the anti-His tag antibodies (Fig. 1b, lane 2).
FIG. 1.
Reactivity of recombinant and virally expressed N proteins of ORO virus with anti-ORO virus and anti-His tag antibodies. Protein samples were resolved on an SDS-polyacrylamide gel and transferred to polyvinylidene difluoride membranes by electroblotting. The membranes were then probed with ORO virus-specific HIMAF (1:200) (a) or anti-Xpess (anti-His tag leader peptide; 1:5,000; Invitrogen) (b) antibody. Lane M, kaleidoscope molecular weight marker (Bio-Rad); lane 1, lysate from IPTG-induced control bacteria (bacteria transformed with vector alone); lane 2, lysate from IPTG-induced bacteria transformed with a recombinant plasmid (pTrcHisB-ORO18N); lane 3, lysate from ORO virus-infected Vero cells; lane 4, nickel chelate affinity-purified rN protein.
Purification of ORO virus rN protein.
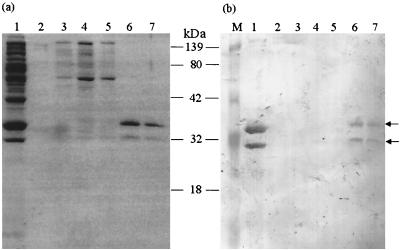
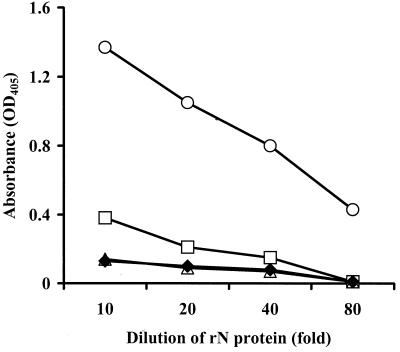
The ORO virus rN protein, expressed in E. coli, was purified by affinity chromatography. The recombinant protein was eluted with a gradient of 0 to 1.0 M imidazole. Lysate from bacteria transformed with vector alone was used as a negative control. Each fraction was analyzed by SDS-PAGE and immunoblotting. The results show that only two bands, representing the two forms of the rN protein, were present in fractions eluted with 0.5 M imidazole (Fig. 2a). Both bands were absent in corresponding fractions eluted from control bacterial lysate (data not shown). Western blot analyses revealed that both bands were reactive to ORO virus-specific mouse immune ascitic fluid (Fig. 2b) and human serum from an ORO virus-infected individual (data not shown). These data, therefore, attest that the rN protein was purified from the bacterial lysate to homogeneity. Use of the eluted fraction, containing both bands, in EIA using HIMAF raised against ORO, dengue-2, Mayaro, and Melao viruses (all of them prevalent in South America) showed that the rN protein reacted specifically to HIMAF raised against ORO virus and that there was no cross-reactivity to HIMAF raised against the other viruses examined (Fig. 3). These data, therefore, suggest that, despite the presence of two forms, the purified rN protein is highly specific in EIAs for detecting antibodies to ORO virus.
FIG. 2.
Purification of ORO virus rN protein. Following IPTG induction bacteria containing the recombinant plasmid were lysed and the rN protein was purified by metal chelate affinity chromatography. The bound protein was eluted from the column by a concentration gradient (0 to 0.5 M) of imidazole. The eluted fractions were analyzed by SDS-PAGE followed by Coomassie blue staining of the gel (a) and immunoblotting using ORO virus-specific HIMAF (1:200) (b). Lane M, kaleidoscope molecular weight marker (Bio-Rad); lane 1, bacterial lysate before purification; lane 2, fraction from 0.05 M imidazole elution; lane 3, fraction from 0.2 M imidazole elution; lanes 4 and 5, fractions from 0.35 M imidazole elution; lanes 6 and 7, fractions from 0.5 M imidazole elution. Arrows indicate the positions of two ORO antibody-reactive bands.
FIG. 3.
Specificity of purified rN protein in IgG EIA. Wells of a microtiter plate were coated with purified rN protein, diluted in carbonate-bicarbonate buffer, and incubated for 16 to 18 h at 4°C, followed by washing and addition of 100 μl of HIMAF (1:500) raised against ORO (○), dengue-2 (□), Melao (▵), and Mayaro (♦) viruses in separate wells. After 1 h at room temperature, the plate was washed and 100 μl of peroxidase-conjugated goat anti-mouse IgG (1:3,000) was added to each well for 1 h. Subsequently, the plate was washed and 100 μl of substrate was added to each well. After 15 to 20 min at room temperature absorbance was measured at a wavelength of 405 nm.
Antigenic characterization of the rN protein.
Serological tests have indicated that there is extensive cross-reactivity among the N proteins of many Simbu serogroup viruses (7). To determine if the antigenic epitopes were conserved on the rN protein, HIMAF raised against several Simbu serogroup viruses (Table 1) were obtained from World Arbovirus Reference Center and used in Western blot analyses to compare the antigenic reactivity of the rN protein with that of the authentic viral N protein expressed in ORO virus-infected Vero cells. The results (Table 1) revealed that the pattern of reactivity of the purified rN protein to the HIMAF used was exactly the same as that of the authentic viral N protein, except in one case, where antibodies to Sathuperi virus reacted to the viral N protein but not to the rN protein. Thus, these data suggest that most, if not all, of the antigenic epitopes on the rN protein are conserved.
TABLE 1.
Comparison of the reactivities of authentic viral and rN proteins by Western blotting with HIMAF raised against different Simbu serogroup bunyaviruses
| Bunyavirus HIMAF | Reactivitya of:
|
|
|---|---|---|
| Viral N | rN | |
| Para | + | + |
| Shamonda | − | − |
| Thimiri | + | + |
| Manzanilla | − | − |
| Ingwavuma | − | − |
| Utinga | + | + |
| Buttonwillow | − | − |
| Simbu | + | + |
| Sango | − | − |
| Sabo | − | − |
| Shuni | + | + |
| Sathuperi | + | − |
| Mermet | − | − |
| Peaton | − | − |
| Akabane | + | + |
+, positive reaction to the HIMAF; −, no reaction to the HIMAF.
Efficacy of rN protein in EIAs using human sera.
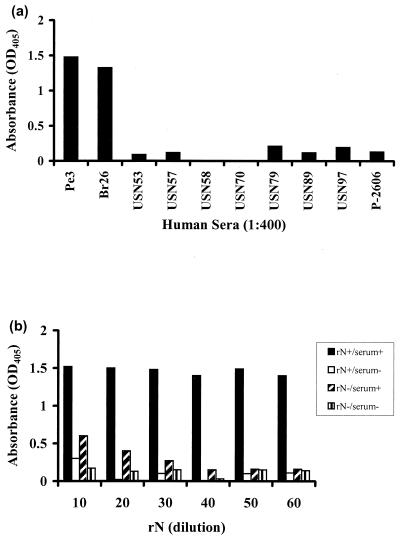
To evaluate the efficiency of the rN protein, human serum samples known to be positive or negative for ORO virus-specific antibodies were tested both in IgG-based and IgM capture EIAs. For IgG assays two positive samples (Br26 and Pe3) and eight negative samples (USN53, -57, -58, -70, -79, and -97 and P-2606) were used. Sample Br26 was obtained from an ORO virus-infected individual in Brazil, while sample Pe3 was obtained from an ORO virus-infected individual in Peru. All the negative serum samples were obtained from healthy individuals residing in the United States who were known to have never been to South America. The results, presented in Fig. 4a, demonstrate that the OD values obtained for both positive samples were significantly higher than that obtained for each of the eight negative-control samples. The mean OD value for the two positive samples was 1.4, while that for the eight negative-control samples was approximately 0.11 ± 0.08. This amounts to a difference of approximately 13-fold between the average OD values for positive- and negative-control samples, suggesting that the rN protein specifically reacts to ORO virus-specific IgG antibodies in human sera with no or very little nonspecific reaction. Similarly, in the IgM capture EIA, the OD value obtained for the positive-control sample was significantly higher than that for the negative-control sample (Fig. 4b), suggesting a highly specific reaction of the rN protein to ORO virus-specific IgM antibodies. Thus, these data suggest that the rN protein is quite efficient at specifically detecting both IgG and IgM antibodies to ORO virus in human sera.
FIG. 4.
Efficacy of rN protein in EIAs using human sera. (a) IgG EIA. Wells of a microtiter plate were coated with rN protein overnight, rinsed, and incubated with human serum samples. After 1 h, the wells were rinsed, and a secondary antibody (peroxidase-conjugated goat anti-human IgG) was added to each well for 1 h. The plate was then washed, and peroxidase substrate was added to each well. After 15 min at room temperature the intensity of color in each well was recorded by a microtiter plate reader at a wavelength of 405 nm. (b) IgM capture EIA. Wells of a microtiter well plate were coated with anti-human IgM and incubated for 16 to 18 h at 4°C, followed by washing and addition of human serum samples for 1 h. The plate was subsequently washed, and rN protein was added to each well and incubated for 1 h, followed by washing and addition of ORO virus-specific HIMAF. After 1 h, the plate was washed, and peroxidase-conjugated sheep anti-mouse Ig was added to each well. Finally, the plate was washed and peroxidase substrate was added to each well, and after 15 min absorbance was measured at a wavelength of 405 nm. serum+, ORO virus-specific IgM-positive serum; serum−, negative-control serum; rN+, rN protein; rN−, negative-control antigen.
Diagnostic potential of purified rN protein by EIA.
Sensitivity and specificity are important issues for a diagnostic reagent. Statistically, sensitivity refers to the probability that the diagnostic reagent gives a positive result when the individual tested actually has the disease, while specificity is the probability that the diagnostic reagent gives a negative result when the individual tested does not have the disease. In other words, the sensitivity of a diagnostic reagent is high if it has no, or a very low frequency of, false negatives. Similarly, the specificity is high when the frequency of false positives is zero or very low. To evaluate the sensitivity and specificity of the rN protein in a diagnostic EIA, the purified-rN protein-based EIA was compared with VCLA-based and HSA-based EIAs using human serum samples.
Comparison with HSA for the detection of IgG antibodies.
Initially, 108 human serum samples from Brazil were tested for IgG by rN protein- and HSA-based EIAs. Of the 108 serum samples tested, 71 were positive and 25 were negative for ORO virus-specific IgG with both antigens (Table 2). This corresponds to a concordance of approximately 89% between the results obtained using the two antigens. Seven samples which tested positive in the rN protein-based EIA were negative in the HSA-based EIA, while five samples which tested positive in the HSA-based EIA were negative in the rN protein-based EIA. To investigate the discordance in more detail, neutralization and HI assays were performed on discordant samples plus control samples of positive and negative concordant samples. ORO virus strain MD023 was used for neutralization assays. The results (Table 3) show that six (Br21, Br44, Br69, Br85, Br89, and Br90) of the seven discordant samples that tested positive by rN protein-based EIA but negative by HSA-based EIA were positive for anti-ORO virus antibodies, both by neutralization (>80% plaque inhibition at 1:20 dilution) and HI (each with HI titer of ≥160) tests. The remaining sample (Br 40) tested negative both by neutralization (≤80% plaque inhibition at 1:20 dilution) and HI (titer of ≤20) tests. Similarly, four (Br30, Br32, Br36, and Br71) of the five discordant sera that tested positive by HSA-based EIA but negative by rN protein-based EIA tested positive by neutralization (>80% plaque inhibition at 1:20 dilution) and HI tests (HI titer of ≥80), while one sample (Br12) tested negative by the two assays (≤80% plaque inhibition at 1:20 dilution and HI titer of ≤20) (Table 3). Together, these data correspond to four false-negative results and one false-positive result by rN protein-based EIA compared to six false-negative results and one false-positive result by HSA-based EIA. Thus, based on 108 Brazilian samples tested, the error frequency for the rN protein is equivalent to, if not lower than, that for HSA, and therefore the rN protein was comparable to HSA in its sensitivity and specificity for detecting ORO virus-specific IgG antibodies in human sera.
TABLE 2.
Comparison of rN protein-based EIA with HSA-based EIA to detect ORO virus-specific IgG and IgM and VCLA-based EIA to detect ORO virus-specific IgG antibodies in human seraa
| HSA- or VCLA-based EIA result | No. of samples with indicated result in rN protein-based EIA
|
Concordance (%) | |
|---|---|---|---|
| Positive | Negative | ||
| HSA | |||
| IgG EIA | 88.9 | ||
| Positive | 71 | 5 | |
| Negative | 7 | 25 | |
| IgM EIA | 93.7 | ||
| Positive | 25 | 8 | |
| Negative | 0 | 94 | |
| VCLA | |||
| IgG EIA | 86.7 | ||
| Positive | 36 | 9 | |
| Negative | 1 | 29 | |
Positive and Negative, absorbance values above and below the cutoff, respectively. The cutoff was equal to the mean plus three standard deviations of the absorbance values obtained with three negative-control sera run alongside in each experiment.
TABLE 3.
Reactivities of Brazilian sera discordant in rN protein- and HSA-based EIAs for the detection of IgM antibodies
| Serum sample | Resulta for indicated antibody by:
|
Neutralization assay resultb | HI assay resultc | |||
|---|---|---|---|---|---|---|
| rN EIA
|
HSA EIA
|
|||||
| IgG | IgM | IgG | IgM | |||
| Br12 | Neg | C | Pos | C | Neg | Neg |
| Br21 | Pos | C | Neg | C | Pos | Pos |
| Br30 | Neg | Neg | Pos | Pos | Pos | Pos |
| Br32 | Neg | C | Pos | C | Pos | Pos |
| Br36 | Neg | C | Pos | C | Pos | Pos |
| Br40 | Pos | C | Neg | C | Neg | Neg |
| Br44 | Pos | Neg | Neg | Pos | Pos | Pos |
| Br69 | Pos | NT | Neg | NT | Pos | Pos |
| Br71 | Neg | NT | Pos | NT | Pos | Pos |
| Br85 | Pos | NT | Neg | NT | Pos | Pos |
| Br89 | Pos | NT | Neg | NT | Pos | Pos |
| Br90 | Pos | NT | Neg | NT | Pos | Pos |
Pos, positive reaction; Neg, negative reaction; C, concordance between results for rN and HSA antigens; NT, not tested.
Pos, >80% plaque inhibition at 1:20 serum dilution; Neg, <80% plaque inhibition at 1:20 dilution.
Pos, HI titer, >160; Neg, HI titer, <20.
Comparison with HSA for the detection IgM antibodies.
The rN protein and HSA were compared using a total of 127 serum samples (52 from Brazil and 75 from Peru) in IgM capture EIAs. Of the 52 Brazilian samples tested, 25 were positive and 19 were negative, while all of the Peruvian samples were negative for ORO virus-specific IgM antibodies with both antigens (Table 2). This corresponds to a concordance of approximately 94% between the results obtained by rN protein- and HSA-based EIAs. The eight discordant samples (Br8, -11, -17, -19, -30, -41, -43, and -44) represented those that tested positive by HSA-based EIA but negative by rN protein-based EIA. All of the discordant samples were positive in neutralization tests (>80% plaque inhibition at 1:20 dilution; Table 4), suggesting that the sensitivity of the rN protein for detecting ORO virus-specific IgM antibodies may be slightly lower than that of HSA. However, in the IgG EIAs, six of the discordant sera (Br 8, -11, -17, -19, -41, and -43) were positive and concordant in rN protein- and HSA-based EIAs, while one sample (Br44) was positive by rN protein-based EIA and negative by HSA-based EIA (Table 3), suggesting that the overall sensitivities of the two antigens are comparable.
TABLE 4.
Reactivities of Brazilian sera discordant in rN protein- and HSA-based EIAs for the detection of IgM antibodies
| Serum sample | Resulta for indicated antibody by:
|
Neutralization assay resultb | |||
|---|---|---|---|---|---|
| rN EIA
|
HSA EIA
|
||||
| IgG | IgM | IgG | IgM | ||
| Br8 | C | Neg | C | Pos | Pos |
| Br11 | C | Neg | C | Pos | Pos |
| Br17 | C | Neg | C | Pos | Pos |
| Br19 | C | Neg | C | Pos | Pos |
| Br30 | Neg | Neg | Pos | Pos | Pos |
| Br41 | C | Neg | C | Pos | Pos |
| Br43 | C | Neg | C | Pos | Pos |
| Br44 | Pos | Neg | Neg | Pos | Pos |
Pos, positive reaction; Neg, negative reaction; C, concordance between results for rN and HSA antigens.
Pos, >80% plaque inhibition at 1:20 serum dilution.
Comparison with VCLA for the detection of IgG antibodies.
The rN protein was compared with VCLA for the detection of ORO virus-specific IgG antibodies in human sera (Table 2). For this purpose, 75 serum samples from Peru were tested using rN protein-based EIA and the results were compared to those obtained by VCLA-based EIA. Of the 75 samples, 36 were positive and 29 were negative with both antigens, corresponding to a concordance of approximately 87% (Table 2). Of the 10 discordant samples, one (Pe70) tested positive by rN protein-based EIA but negative by VCLA-based EIA. The remaining nine discordant samples were positive in VCLA-based EIA but negative in rN protein-based EIA. To examine these results further, discordant samples were tested by neutralization assays, using a Peruvian strain (MD023) of ORO virus. Of the nine discordant samples that were positive in VCLA-based EIA but negative in rN protein-based EIA, four were negative (≤80% plaque inhibition at 1:20 dilution) and five were positive (>80% plaque inhibition at 1:20 dilution) in neutralization assays (Table 5). The sample (Pe70) that was positive by rN protein-based EIA but negative by VCLA-based EIA was positive in the neutralization test (Table 5). These data correspond to five false-negative and no false-positive results in rN protein-based EIA compared to four false-positive results and one false-negative result in VCLA-based EIA. Together, the results of EIA and neutralization assays revealed that the overall sensitivity of rN protein-based EIA is 93%, which is comparable to that of VCLA-based EIA; however, the VCLA has a higher error frequency in terms of false-positive results.
TABLE 5.
Neutralization activity of Peruvian sera that were discordant in rN protein- and HSA-based EIAs for the detection of IgG antibodies
| Serum sample | Resulta by:
|
Neutralization assay resultb | |||
|---|---|---|---|---|---|
| IgG EIA
|
IgM EIA
|
||||
| rN | VCLA | rN | HSA | ||
| Pe15 | Neg | Pos | Neg | Neg | Neg |
| Pe30 | Neg | Pos | Neg | Neg | Neg |
| Pe35 | Neg | Pos | NT | NT | Pos |
| Pe48 | Neg | Pos | Neg | Neg | Pos |
| Pe50 | Neg | Pos | Neg | Neg | Pos |
| Pe53 | Neg | Pos | Neg | Neg | Pos |
| Pe55 | Neg | Pos | Neg | Neg | Pos |
| Pe58 | Neg | Pos | Neg | Neg | Neg |
| Pe61 | Neg | Pos | Neg | Neg | Neg |
| Pe70 | Pos | Neg | Neg | Neg | Pos |
Pos, positive reaction; Neg, negative reaction; NT, not tested.
Pos, >80% plaque inhibition at 1:20 serum dilution; Neg, <80% plaque inhibition at 1:20 serum dilution.
DISCUSSION
Recombinant antigens offer several advantages over conventional antigens as diagnostic reagents. They are generally less expensive, safer, fairly specific, and can be produced in large quantity with relatively little effort. Use of recombinant antigens has proved useful in the serodiagnosis of several viral infections, such as herpes simplex (5) and Japanese encephalitis (8). The N protein of members of Bunyaviridae is a major virus antigen. Schwarz et al. (16) showed that in Toscana virus infection the N protein is the immunodominant antigen recognized by the humoral immune response and that both IgM and IgG antibodies are produced against this antigen. In recent years, the rN protein has been used for the serodiagnosis of several members of the family Bunyaviridae, including Hantaan (21), Puumala (6), Sin Nombre (4), Crimean Congo hemorrhagic fever (11), and Toscana (19, 2) viruses. This paper describes the expression of the ORO virus N gene in E. coli, and the use of the purified rN protein in diagnostic EIAs.
Our results indicate that the ORO virus N protein was expressed in E. coli and purified to homogeneity (Fig. 2). However, the protein was expressed in two forms: high- and low-molecular-weight forms. Through immunoblotting, using ORO virus-specific HIMAF and anti-His tag leader peptide antibodies, it was shown that the high-molecular-weight form represents the full-length fusion protein containing the His tag leader peptide fused to the amino terminus of the ORO virus rN protein, while the low-molecular-weight form represents the rN protein without the leader peptide (Fig. 1). The reason why the protein is expressed in two forms may relate to AUG initiation codons. Since the complete coding region of the ORO virus N gene, including start and stop codons, was cloned into the expression vector downstream of, and in frame with, the His tag leader sequences, it is possible that the translation of the mRNA transcript is initiated at two AUG codons, one provided in the vector at the beginning of the leader sequence and the other present in the N cDNA, thereby giving two forms of the recombinant protein. Nevertheless, the purified bacterially expressed ORO virus rN protein was comparable to the authentic viral N protein in its antigenic characteristics (Table 1). In EIAs, the purified rN protein was found to be able to detect specifically both IgG and IgM antibodies to ORO virus with high efficiency and no or very little background (Fig. 4). The specificity and sensitivity of the rN protein-based EIA were compared with those of HSA- and VCLA-based EIAs, the two assays currently used for the serodiagnosis of ORO virus infection in Brazil and Peru, respectively. The rN protein was found to be highly sensitive and specific in detecting IgG and IgM antibodies to ORO virus in human sera. A total of 183 human serum samples (108 from Brazil and 75 from Peru) were tested for the presence of ORO virus-specific IgG antibodies, and 127 serum samples (52 from Brazil and 75 from Peru) were tested for the presence of ORO virus-specific IgM antibodies. A high degree of concordance was found between the results obtained with the rN protein and with either of the other two antigens (89% with HSA and 87% with VCLA) for the detection of ORO virus-specific IgG (Table 2). Examination of the discordant samples by neutralization and/or HI assays revealed that, out of 183 serum samples tested by rN protein-based EIA, only 1 (0.5%) was a false positive and 9 (4.9%) were false negatives, which raises the overall sensitivity and specificity of the rN protein-based EIA to approximately 95 and 99.5%, respectively. In contrast, of the 108 serum samples tested by HSA-based EIA, one (0.9%) was a false positive and six (5.5%) were false negatives, which equates to an overall sensitivity of approximately 93% and specificity of 99% for HSA-based EIA. Similarly, of the 75 samples tested by VCLA-based EIA, four (5.3%) were false positives and one (1.3%) was a false negative, which equates to an overall sensitivity of 98% and specificity of approximately 95% for VCLA-based EIA. Thus, these data suggest that the sensitivity of the rN protein for the detection of ORO virus-specific IgG antibodies in human sera is comparable to those of the other two antigens currently used for the diagnosis of ORO virus infection; however, in terms of specificity the rN protein is better than VCLA. In IgM capture assays, the frequency of false-negative samples was relatively higher for the rN protein (approximately 6%) than for HSA, suggesting that HSA has a relatively higher sensitivity than the rN protein for the detection of ORO virus-specific IgM antibodies. However, the overall concordance between the two antigens was approximately 95% (Table 2).
Because of its high sensitivity (95%) and specificity (99.5%), the rN protein may prove very useful in obtaining a more accurate estimate of the seroprevalence of ORO virus infection in tropical regions of South America; it may also help in obtaining an estimate of the prevalence of infection by other ORO-like viruses and reassortants. Although the percentage of false-negative results for IgG antibodies detected by the rN protein-based EIA (4.9%) is comparable to that of the HSA-based EIA (5. 5%), it is still higher than that of the VCLA-based EIA (1.3%). There may be several explanations for this. First, it is possible that the samples that gave false-negative results may contain IgG antibodies to only the viral surface glycoproteins. Presumably, during ORO virus infection IgG antibodies to viral surface glycoproteins appear earlier than the IgG antibodies to the N protein. Thus, if a serum is collected at a stage when IgG antibodies to the N protein are undetectable or below detectable levels, the rN protein-based EIA would not score that serum as positive, while HSA and VCLA, being whole-virus antigens, would be able to detect IgG antibodies to viral surface glycoproteins. Similarly, being directed towards surface glycoproteins, neutralization and HI assays would also score such a serum as positive. An alternate explanation may be infection by a reassortant virus having the medium (M) RNA segment of ORO virus and the small (S) RNA segment of a different bunyavirus. In such a situation, neutralization and HI assays and VCLA-based and HSA-based EIAs, since they are able to detect viral glycoproteins (products of the M RNA segment), would score the serum sample as positive. On the other hand the rN protein-based EIA, which detects antibodies to the N protein (a product of the S RNA segment), would score the serum sample as negative. This issue needs to be further investigated, especially in view of the need for more-accurate epidemiological investigations, to define the distribution and prevalence of ORO infection in South America.
In conclusion, the rN protein-based EIA is a potentially good assay for the detection of both IgG and IgM antibodies to ORO virus with high sensitivity and specificity. Thus, the rN protein-based EIA appears to be a simple and readily accessible assay for diagnosing ORO virus infection. These characteristics make the rN protein a useful diagnostic reagent for diagnostic and epidemiological purposes in tropical South America.
ACKNOWLEDGMENTS
We are thankful to David Beasley of our laboratory for help with graphics and to Stuart Nichol, Mike Bowen, Pierre Rollin, and C. J. Peters of the National Centers for Disease Control and Prevention, Atlanta, Ga., for helpful discussions.
This work was supported in part by NIH grants AI 43336 and AI 10984. Mohammad Saeed was supported in part by the James W. McLaughlin fellowship fund.
REFERENCES
- 1.Beaty B J, Calisher C H, Shope R E. Arboviruses. In: Lennette E H, Lennette D A, Lennette E T, editors. Diagnostic procedures for viral, rickettsial and chlamydial infections. Washington, D.C.: American Public Health Association; 1995. pp. 189–212. [Google Scholar]
- 2.Ciufolini M G, Fiorentini C, Di Bonito P, Mochi S, Giorgi C. Detection of Toscana virus-specific immunoglobulins G and M by an enzyme-linked immunosorbent assay based on recombinant viral nucleoprotein. J Clin Microbiol. 1999;37:2010–2012. doi: 10.1128/jcm.37.6.2010-2012.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke D H, Casals J. Techniques for haemagglutination and haemagglutination inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958;7:561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- 4.Feldmann H, Sanchez A, Morzunov S, Spiropoulou C F, Rollin P E, Ksiazek T G, Peters C J, Nichols S T. Utilization of autopsy RNA for the synthesis of the nucleocapsid antigen of a newly recognized virus associated with hantavirus pulmonary syndrome. Virus Res. 1993;30:351–367. doi: 10.1016/0168-1702(93)90101-r. [DOI] [PubMed] [Google Scholar]
- 5.Kakkanas A, Papadogeorgaki H, Manservigi R, Miriagou V, Georgopoulou U, Mavromara P. Escherichia coli expressed herpes simplex virus gG1 and gG2 proteins in ELISA and immunoblotting assays. Intervirology. 1995;38:346–351. doi: 10.1159/000150462. [DOI] [PubMed] [Google Scholar]
- 6.Kallio-Kokko H, Vapalahit O, Lundkvist A, Vaheri A. Evaluation of Puumala virus IgG and IgM enzyme immunoassays based on recombinant baculovirus-expressed nucleocapsid protein for early nephropathia epidemica diagnosis. Clin Diagn Virol. 1998;10:83–90. doi: 10.1016/s0928-0197(97)10019-8. [DOI] [PubMed] [Google Scholar]
- 7.Kinney R M, Calisher C H. Antigenic relationship among Simbu serogroup (Bunyaviridae) viruses. Am J Trop Med Hyg. 1981;30:1307–1318. doi: 10.4269/ajtmh.1981.30.1307. [DOI] [PubMed] [Google Scholar]
- 8.Konishi E, Mason P W, Shope R E. Enzyme-linked immunosorbent assay using recombinant antigens for serodiagnosis of Japanese encephalitis. J Med Virol. 1996;48:76–79. doi: 10.1002/(SICI)1096-9071(199601)48:1<76::AID-JMV12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 10.Leduc J W, Pinheiro F P. Oropouche fever. In: Monath T P, editor. The arboviruses: epidemiology and ecology. Vol. 4. Boca Raton, Fla: CRC Press; 1989. pp. 1–15. [Google Scholar]
- 11.Marriot A C, Polyzoni T, Antoniadis A, Nuttall P A. Detection of human antibodies to Crimean-Congo hemorrhagic fever virus using expressed viral nucleocapsid protein. J Gen Virol. 1994;75:2157–2161. doi: 10.1099/0022-1317-75-9-2157. [DOI] [PubMed] [Google Scholar]
- 12.Ni H, Barrett A D T. Nucleotide and amino acid differences of the structural protein genes of Japanese encephalitis virus from different geographical locations. J Gen Virol. 1995;76:401–407. doi: 10.1099/0022-1317-76-2-401. [DOI] [PubMed] [Google Scholar]
- 13.Pinheiro F P, Travassos da Rosa A P A, Ishak R, Freitas R B, Gomez M L, LeDuc J W, Olivia O F. Oropouche virus: a review of clinical, epidemiological and ecological findings. Am J Trop Med Hyg. 1981;30:149–160. [PubMed] [Google Scholar]
- 14.Pinheiro F P, Travassos da Rosa A P A, Vasconcelos P F C. An overview of Oropouche fever epidemics in Brazil and neighbouring countries. In: Travassos da Rosa A P A, Vasconcelos P F C, Travassos da Rosa J F S, editors. An overview of arbovirology in Brazil and neighbouring countries. Belem, Brazil: The Evandro Chagas Institute; 1998. pp. 186–192. [Google Scholar]
- 15.Saeed M F, Wang H, Nunes M, Vasconcelos P, Weaver S C, Shope R E, Watts D M, Tesh R B, Barrett A D T. Nucleotide sequences and phylogeny of the nucleocapsid gene of Oropouche virus. J Gen Virol. 2000;81:743–748. doi: 10.1099/0022-1317-81-3-743. [DOI] [PubMed] [Google Scholar]
- 16.Schwarz T F, Gilch S, Pauli C, Jager G. Immunoblot detection of antibodies to Toscana virus. J Med Virol. 1996;49:83–86. doi: 10.1002/(SICI)1096-9071(199606)49:2<83::AID-JMV2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 17.Sever J H, Ley A C, Walman F, Caplan B M, Crockett P W, Turner H C. Utilization of disposable plastic plates with a serologic microtechnique. Am J Clin Pathol. 1964;41:167–170. doi: 10.1093/ajcp/41.2.167. [DOI] [PubMed] [Google Scholar]
- 18.Tikasingh E S, Spence L, Downs W G. The use of adjuvants and sarcoma 180 cells in the production of mouse hyperimmune ascitic fluids to arboviruses. Am J Trop Med Hyg. 1966;15:219–226. doi: 10.4269/ajtmh.1966.15.219. [DOI] [PubMed] [Google Scholar]
- 19.Valassina M, Soldateschi D, Maria dal Maso G, Santini L, Bianchi S, Valensin P E, Cusi M G. Diagnostic potential of Toscana virus N protein expressed in Escherichia coli. J Clin Microbiol. 1998;36:3170–3172. doi: 10.1128/jcm.36.11.3170-3172.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watts D M, Phillips I, Callahan J D, Greibenow W, Hyams K C, Hayes C G. Oropouche virus transmission in Amazon river basin of Peru. Am J Trop Med Hyg. 1997;56:148–152. doi: 10.4269/ajtmh.1997.56.148. [DOI] [PubMed] [Google Scholar]
- 21.Zoller L, Yang S, Gott P, Bautz E K, Darai G. Use recombinant nucleocapsid proteins of the Hantaan and nephropathia epidemica serotypes of Hantaviruses as immunodiagnostic antigens. J Med Virol. 1993;39:200–207. doi: 10.1002/jmv.1890390305. [DOI] [PubMed] [Google Scholar]