Abstract
Currently, the world is facing a Coronavirus pandemic with a grave deficiency of specific therapy for Coronavirus Disease (COVID-19). Moreover, scientists attempt to discover the most refined approach to prevent this condition. Regarding COVID-19 infection, herbal medicines with immunomodulatory effects may offer patients a promising preventive treatment option. Several ayurvedic and Traditional Chinese Medicine (TCM) are effective during this worrisome Coronavirus pandemic i.e. Tinospora cordifolia (Willd.) Miers, Withania somnifera (L.) Dunal, Scutellaria baicalensis Georgi, Curcuma longa L. etc. TCM was shown to be utilized with over 90% efficacy when the COVID-19 pandemic broke out in early 2020. In addition to herbal treatments and nutraceutical drugs, dietary supplements such as vitamins and amino acid derivatives also play a significant part in COVID-19 management. Diet can assist in regulating inflammation, while nutraceuticals can aid in the prevention of viral invasion. Functional amino acids (e.g., arginine, cysteine, glutamate, glutamine, glycine, taurine, and tryptophan) and glutathione, which are all abundant in animal-sourced foodstuffs, are crucial for optimum immunity and health in humans and animals. The goal of this article is to thoroughly evaluate recent statistics on the effectiveness of herbal medicines in COVID-19, the antiviral activity of nutraceuticals, and the significance of these results in creating dietary supplements that would enhance innate immunity and contribute as preventive measures against severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2).
Keywords: COVID-19 management, Herbal drugs, Ayurvedic remedies, Nutraceutical drugs, Chinese herbs, Traditional Chinese Medicine, Dietary supplements
Graphical abstract
1. Introduction
The epidemic of a coronavirus has now become pandemic and spread across many countries (V.P. Chavda et al., 2021). The COVID-19 implications comprise fever, dry cough, and difficulties in breathing which may lead to severe respiratory complications (Nugraha et al., 2020). Along with various therapeutic agents established for the treatment of COVID-19, various herbal drugs and dietary supplements are also proven beneficial for the treatment. Herbal plants of various choices and their phytochemicals are used for treatment as well as in supportive therapy of this viral infection (Jalali et al., 2021). Various botanical drugs (like Boozari) have been recommended as adjuvant therapy for the treatment of COVID-19. Based on the mechanism of action, various natural extracts and TCM have been identified to be helpful, but their clinical studies have not yet been performed or proved (Brendler et al., 2021; Ren et al., 2020). The application of TCM was found as a result of in-silico screening methods with specific COVID targets like angiotensin-converting enzyme 2 (ACE-2) and SARS-COV-2 3Cl using the molecular docking studies (Gao et al., 2020). The natural antiviral components like baicalin, bilirubin, corosolic acid, glycyrrhizin, hesperidin, mulberroside A, rutin, saikosoponin A, and verbascoside were found to be effective in the treatment of covid infection evaluated by molecular docking (Janairo et al., 2021; Yan et al., 2020). Many patented and non-patented herbal drugs are found to relieve symptoms of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection like cough, fever, and fatigue. Furthermore, natural drugs have proven incidences of fewer side effects than synthetic ones, but some herbal medicines used in COVID-19 are found to have a few side effects (Wang et al., 2021). For instance, Xiyanping, a herbal drug formulation used in COVID treatment was restricted due to reports of adverse effects such as drowsiness, lightheadedness, vomit, and sometimes liver damage ( Yang, 2020). The traditional Indian herbal drugs are one of the oldest drugs used since humans' existence and play an important role in combating various disease conditions (Ravishankar et al., 2007). During COVID-19 pandemic, Minister of AYUSH (Ayurvedic, Yoga, and Naturopathy, Unani, Siddha, and Homeopathy) in India suggested drinking ‘Kadha’, Kadha is nothing but an extract prepared from various herbal drugs, and its benefits are proven that induces immunity in individuals (Khanna et al., 2021). Various Indian herbal drugs like Withania somnifera, Curcuma longa, Ocimum sanctum, and Phyllanthus emblica are found to be effective in COVID-19 related complications ( Singh et al., 2021; Brahmbhatt, 2020). Not only herbal medicines but also various dietary supplements were found to boost the immune system like Vitamin D, and C are recommended by various health experts during the pandemic as it is responsible for boosting immunity (Infusino et al., 2020). Generally, Vitamin D deficiency is a major issue among both sexes. This deficiency can further induce the risk of COVID-19 severity and even mortality. So, intake of Vitamin D supplements is proven beneficial in reducing the risk of covid infection (Grant et al., 2020).
2. Current Global Scenario of COVID-19
The COVID-19 outbreak started at the end of 2019, and it is currently causing havoc around the world. As per the Centers for Disease Control and Prevention (CDC), Coronavirus variations are categorized in great detail, and the mutation of this deadly virus is continuously underway (CDC, 2021). A mutation is a change in the sequence of genes. Nucleotide mutations arise in the fundamental chemicals that make up the large RNA and DNA molecules. A mutation in a viral genome can alter the encoded amino acid sequences, which can cause the virus to replicate. Mutations are classified into two types–deletion and substitution (Iengar, 2012). The rise in Coronavirus cases has been widely documented due to such constant mutational changes of SARS-CoV-2. According to the WHO estimation, approximately two hundred and thirty million confirmed cases were recorded in a single day. As of January 31, 2022, more than 8 billion vaccine doses have been delivered globally. Many drugs are repurposed, and vaccines are approved based on emergency use approval path to combat the pandemic (Chavda and Apostolopoulos, 2021; Chavda et al., 2021b; Chavda et al., 2021d; Chavda et al., 2021c; Chavda et al., 2021a). Even though there is a disparity in vaccination efforts in various nations, every effort is being made to manage and prevent this infection (World Health Organization, 2021). The major and unsolved question is how much contagious it will be and how long it will take to get out of lockdown. If it is substantially more contagious, people will fail to manage the spread, and perhaps some types of social distancing restrictions will very certainly be necessary for the future. If the infection rate increases exponentially, we are still likely to face severe sickness, increasing hospitalizations and significant strain on the national health system, including in those who have been vaccinated (Lai et al., 2020).
3. Immunopathology of COVID-19
3.1. Asymptomatic phase
SARS-CoV-2 enters the respiratory tract through the pulmonary route and binds to nasal epithelial cells. ACE-2 expression is high in adult nasal epithelial cells and during COVID-19; ACE-2 serves as a host receptor for viral entry. After entering the body, the virus replicates and multiplies locally, infecting ciliated cells in the conducting airways (Sims et al., 2005). This phase only persists for a few days, and the immunological response produced throughout this time is minimal. The viral load is minimal at this stage but is in the highly contagious form, causing widespread illness, and the virus has been identified by employing nose swab testing (Hoffmann et al., 2020b).
3.2. Virus entry to the host cell
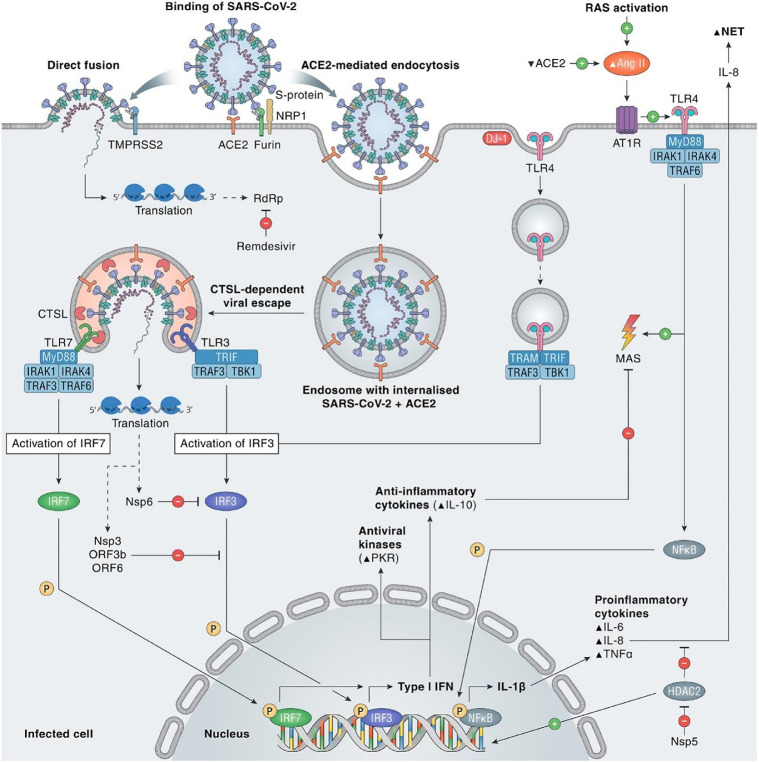
The SARS-CoV-2 enters the host cell through the droplets produced by the infected person while sneezing or coughing and when these droplets enter the nasal system of a person, the virus starts to replicate. SARS-CoV-2 has the spike(S) glycoprotein on its surface, which has S1 and S2 functional subunits (Li, 2016). The S1 subunit has 14–685 residues, whereas the S2 subunit has 686–1273 residues; the final two parts are crucial for receptor binding and membrane fusion. The S1 subunit's N-terminal domain (14–305 residues) and the S2 subunit's receptor-binding domain (RBD, 319–541 residues), as well as the fusion peptide (FP) (788–806 residues), the heptapeptide repeat sequence 1 (HR1) (912–984 residues), HR2 (1163–1213 residues) and the TM domain are all engaged in viral fusion and entrance (1213). The S2 subunit's viral fusion and entry functions are dependent on HR1 and HR2, which make up the six-helix bundle (Xia et al., 2020). As the virus binds to the ACE2 site, the virus enters the host cell by creating a furin cleavage site on the spike protein into the S1 and S2 subunits present on the virus by the host protease and begins its life cycle (Fig. 1 ) ( Wu et al., 2021).
Fig. 1.
Immunopathophysiology of COVID-19 (Adopted from (Eijk et al., 2021) under creative commons CC-BY-NC—ND).
Several proteases produced by the host cells then facilitate viral binding and enter into the body comprising cathepsins L and B, factor X, elastase, furin, transmembrane protease serine 2 (TMPRSS2), and trypsin (Hoffmann et al., 2020a). Trypsin is another host protease that cleaves the viral S Protein (Ou et al., 2020). TMPRSS2 is directly involved in the host interaction with viral spike protein (Mortaz et al., 2020). The virus penetrates the host cell and unfolds its genome, which is subsequently transcribed and translated.
3.3. Virus replication
The further step is the Coronavirus genome's replication and transcription by continuous and discontinuous RNA synthesis at cytoplasmic membranes. Coronavirus contains large RNA genomes flanked by 5′ and 3′ untranslated sections containing cis-acting secondary RNA structures, required for RNA amalgamation (Mousavizadeh and Ghasemi, 2021). The 5′ end of the genomic RNA comprises two enormous open reading frames (ORFs, ORF1a and ORF1b) that comprise two-thirds of the capped and polyadenylated genome. Genomic RNA is transformed into pp1a (protein phosphatase 1a) and pp1ab (protein phosphatase 1ab) that is processed into individual non-structural proteins (NSPS) when the virion releases RNA within the cell, producing the viral replication and transcription complex. According to NSPS expression, viral replication organelle synthesis occurs, which includes distinctive perinuclear double-membrane vesicles (DMVs), convoluted membranes (CMs), and tiny open double-membrane spherules (DMSs) (Astuti and Ysrafil, 2020). These elements help in creating a protective microenvironment for viral genomic RNA replication. The transcription of sub-genomic mRNAs contains the characteristic Coronavirus mRNAs. Structured proteins that have been translated translocate into the endoplasmic reticulum (ER) membranes and pass via the ER-Golgi intermediate compartment (ERGIC). The lumen of secretory vesicular compartments is formed later in the Golgi apparatus by interaction with N-encapsidate and freshly generated genomic RNA. Finally, virions are released from the infected cell through exocytosis and begin infesting the human body (V'kovski et al., 2021; Mousavizadeh and Ghasemi, 2021). It is documented that ACE2 of the host is the viral target, but it is not valid for all the ACE2 expressed in the human body (Zhang et al., 2021). Viral battle with alveolar macrophages of the host has been accounted for producing cytokines and chemokines. The S2 subunit is responsible for the host cell membrane, and viral membrane fusion during which the viral nucleocapsid reaches the cytoplasm of the host cell (Fig. 1) (Kuhn et al., 2004). A conformational change is seen in the S2 protein, which is irreversible and this eventually facilitates the entry of the virus (Matsuyama and Taguchi, 2002; Walls et al., 2020; Djomkam et al., 2020).
3.4. Body response to virus
In response to the invasion, the human immune system is activated against the virus. SARS-CoV-2 and the related immune responses are summarized in the coming sections (Yang et al., 2020a). Immune cells search for foreign bodies, recognize the virus and take action against the virus by secreting the antibodies. In SARS-CoV-2 virus infection, lymphocytes count is suddenly decreased and leads to lymphopenia (Tavakolpour et al., 2020). Lymphocytes include T-cell, B-cell, and Natural killer cells (NK cells). Among them, helper T-cells activate the immune system, and release signaling molecules called cytokines and toxic T-cell releases toxic chemicals to kill the virus (Fig. 1) (Catanzaro et al., 2020). B-cells’ primary function is to release antibodies while the NK-cells kill the cells that the virus has already infected. It is reported that the occurrence of lymphopenia can be indicated as a prognosis of COVID-19 (Huang et al., 2020).
3.5. Lymphocyte activation and dysfunction
As the virus enters into the host, subsequent activation of the T-cell in the body will lead to the production of certain antiviral compounds like cytokines, chemokines, and cytotoxic molecules (perforin and granzyme-B) (Yang et al., 2020b). INF-α directly inhibits viral replication and enhances antigen presentation. Chemokines enroll more natural and adaptive cells to control the virus. Cytotoxic molecules help in killing and removing infected epithelial cells and pathogens. The COVID-19 patients show marked leukopenia with severe lymphopenia, which involves the sudden loss of CD4+ and CD8+ T-cells (Croft et al., 2009). The marked higher expression of OX40 and 4–1BB has been observed in severely ill patients with COVID-19 ( Zhou et al., 2020b).
3.6. Granulocyte and monocyte irregularities
There is a reduction in various cell counts of the patients suffering from COVID-19. The NLR ratio of neutrophile to lymphocyte is considered an indicator of the intensity of the COVID-19. Also, reduced count of monocytes, eosinophils, and basophils are reported for the patients whose situation is critical due to COVID-19 (Lippi and Plebani, 2021).
3.7. Increased production of cytokines
In critically ill COVID-19 patients, “Cytokine storm” has played the role of the marker. Fig. 1 summarizes the cytokines which are activated in the patient (Huang et al., 2020a). Out of the cytokines released, IL-1β, IL-6, and IL-10 are majorly reported and diagnosed in severe cases (Wan et al., 2020). It is reported that in critical and severe COVID-19 patients, the levels of IL-10 and IL-6 are suddenly upregulated (Yang et al., 2020a).
3.8. Increased antibodies
The diagnosis of the COVID-19 is based on detecting the presence of various antibodies like IgM and IgE. Studies have reported the correlation of IgE with the severity of the illness, and hence it can be used for segregating the cases that are severe and non-severe (Zhang et al., 2020a).
4. Natural Compounds and Their Potential Targets against SARS-CoV-2 Infection
Several natural substances and their derivatives are beneficial against Coronavirus infection (Şimşek Yavuz and Ünal, 2020; Islam et al., 2020; Chojnacka et al., 2020; Orhan et al., 2020). Attempts are being taken to combat the disease indefinitely via preclinical and clinical studies even though many antiviral drugs used against SARS-CoV have already proven literature as the treatment measure. Nearly 10,000 molecules, including natural ingredients, were tested for antiviral activity against SARS-CoV-2 using a cell-based assay in Vero E6 cells using immunofluorescence (IFA), western blot, and flow cytometry analytical techniques (Wu et al., 2004; Orhan et al., 2020). Preliminary screening of phytoconstituents for bioactivity is an ideal exploratory tactic for obtaining their biological compounds in the end. Collaborative efforts among researchers, clinicians, governments, and ethnomedicinal professionals in the search and advancement of safe and effective therapies derived from plant sources for the treatment of COVID-19 could be a viable option (Omokhua-Uyi and Staden, 2021). According to recent in -silico analysis, various natural products have been discovered to be highly efficient in preventing enzymatic activity and cell wall receptors of human Coronavirus. Milder dosages of such bioactive molecules may inhibit or at least slow the spread of SARS-CoV-2 (Huang et al., 2020b). Furthermore, the development of COVID-19 is characterized by an unregulated inflammatory response, such as cytokine release syndrome, so anti-inflammatory herbs could be an effective instrument in suppressing such a catastrophic symptom (Huang et al., 2020b).
4.1. Spike glycoprotein
Several herb extracts belonging to the Polygonaceae family have been documented to inhibit the interaction of viral spike protein with host ACE-2 (Zhou et al., 2020a); one such example is emodin (Zhang et al., 2020b). Furthermore, the anthraquinone emodin, a component of the genus Rheum and Polygonum, hindered the SARS-CoV–ACE2 engagement in a concentration-dependent manner, and the infectivity of SARS-CoV spike-pseudotyped retrovirus to Vero cells.
4.2. Helicase
Helicase, also referred to as NTPase, is involved in viral genomic RNA replication, as well as transcription and translation. SARS CoV helicase is an SF1 family enzyme that hydrolyzes all NTPs and is used as substrates of ATP, dATP, and d-CTP (Karpe and Lole, 2010). The action of two naturally occurring flavonoids, particularly myricetin and scutellarein, is validated as possible inhibitors against SARS Cov helicase nsP133 (Squeglia et al., 2020).
4.3. Human-centered goals
4.3.1. ACE2 receptor
The human receptor for SARS-CoV and SARS-CoV-2 is the ACE-2 receptor. Such cell surface receptors are often present and seldom circulate in the soluble form (Zhou et al., 2020a). Many flavonoids are proved to be effective in COVID-19 by preventing viral attachment to host ACE-2. A successful clinical open-labeled randomized study was conducted in 447 patients with quercetin treatment (NCT04377789). Furthermore, the anthraquinone emodin, a component of the genus Rheum and Polygonum hindered the SARS-CoV–ACE2 engagement in a concentration-dependent manner, as well as the infectivity of SARS-CoV spike-pseudotyped retrovirus to Vero cells (Ho et al., 2007).
4.3.2. SARS-CoV chymotrypsin-like protease (3CLpro)
It is known that natural plant-derived compounds demonstrate SARS-CoV antiviral efficacy against SARS-CoV 3CL protease. Rhizoma cibotii rhizomes, dried Cibotium barometz rhizomes, Dioscoreae rhizoma rhizomes, Dioscorea batatas tubers showed a substantial decrease in SARS-CoV 3CL protease activity. Flavonoids are secondary metabolites of polyphenolic plants found in various fruits and vegetables. The Anti-SARS-CoV 3CLpro activity was demonstrated by flavonoids such as herbaceous, rhoifolin, and pectolinarin. Another amentoflavone flavonoid is also the most powerful inhibitor of SARS-CoV 3CLpro (Jo et al., 2019). Also, betulinic acid and triterpenes have been reported to have anti-3CLpro activity (Koulgi et al., 2020).
4.3.3. Papain-like cysteine protease (PLpro)
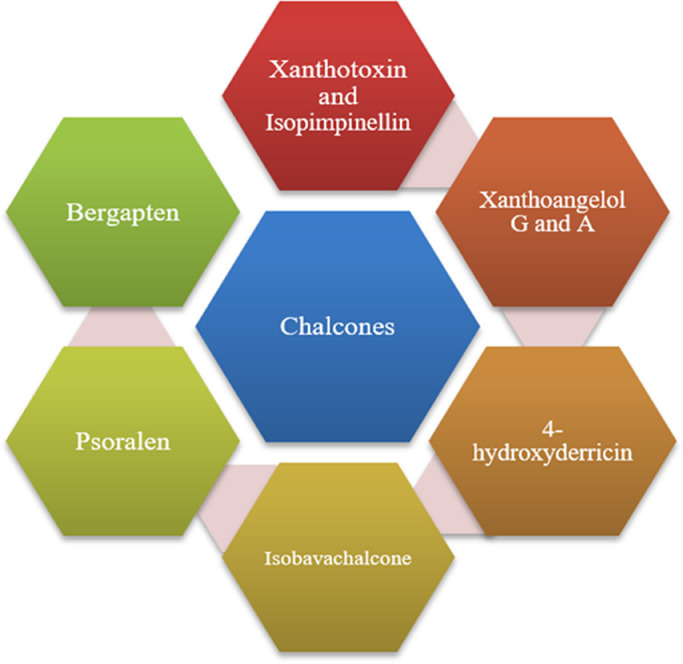
PLpro is associated with the replication of SARS-CoV viral genomic RNA (Fig. 2 ). PLpro might be a significant pharmacological target in the development of Anti-SARS medicines (Hilgenfeld, 2014).
Fig. 2.
Chalcones isolated from Angelica keiskei with Anti-SARS CoV activity targeting PLpro.
4.3.4. RNA-dependent RNA polymerase (RdRp)
The SARS-CoV RdRp is an important enzyme that is useful for viral RNA synthesis. Ganoderma lucidum extracts are effective against COVID-19 by targeting viral RdRpp (Yang et al., 2020c; Zhu et al., 2020).
Knowing and understanding the immunopathology of SARS-CoV-2, experts concluded that several phytochemicals can be proven extremely beneficial in preventing and curing the ongoing outbreak.
5. Methodology
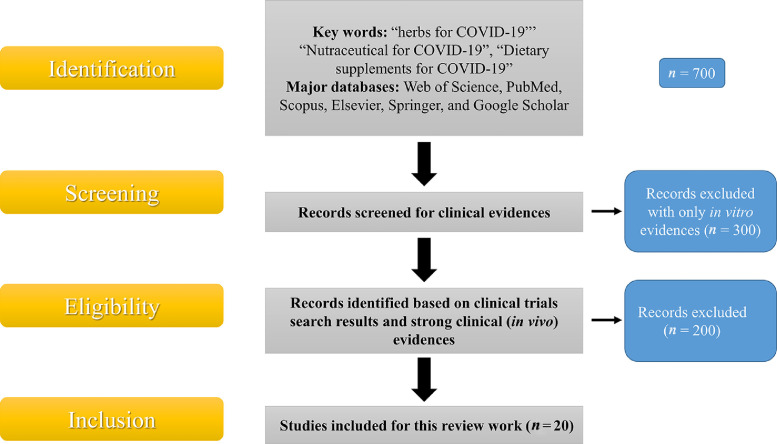
This review article tried to thoroughly evaluate recent statistics on the effectiveness of herbal medicines in COVID-19, the antiviral activity of nutraceuticals, and the significance of these results in creating dietary supplements that would enhance innate immunity contribute as preventive measures against SARS-CoV-2. The methodology used for this review work is summarized in Fig. 3 . We have started searching through major databases such as Web of Science, PubMed, Scopus, Elsevier, Springer, and Google Scholar with keywords like “herbs for COVID-19”, “Nutraceutical for COVID-19”, “Dietary supplements for COVID-19”, and then we started with a specific search.
Fig. 3.
Data base search methodology with inclusion/exclusion criteria.
From the identified components, first, we have omitted the components for which no strong literature support was available. From the remaining components, we have considered the compounds based on the in vivo data evaluation and searched for the specific research around COVID-19. Later on, from the highly researched components against SARS-CoV-2, the clinical trial database was evaluated. We have incorporated clinical trial outcomes for the majority of the components if not then a cogent discussion on in vivo studies was incorporated in certain cases. Here we have also considered the current use of such components in outpatient management as well as for hospitalized patients especially in the case of dietary supplements and nutraceuticals.
6. Herbal Remedies
The phytochemicals that are effective against SARS-CoV-2 are summarized in Table 1 . A few of them which have been used more frequently and widely are discussed below.
Table 1.
Phytochemicals that are beneficial as adjunct agents in COVID-19 management.
| Plant name | Biological source | Family | Plant part used | Phytochemical useful in COVID-19 | Mechanism of action |
|---|---|---|---|---|---|
| Ashwagandha (Indian ginseng) | Withania somnifera (L.) Dunal | Solanaceae | Roots | Withaferin A, withanolide A, withanolide D, sitoindosides, 12-deoxywithastramonolide, and withanoside V. |
Improves cell-mediated immunity. Controls alpha-2 macroglobulin synthesis during inflammation and decreases oxidative stress (Shree et al., 2020). |
| Baicalein (Huang Qin) |
Scutellaria baicalensis Georgi | Lamiaceae | Roots | Baicalin, wogonin, norwogonin, and oroxylin A. | Inhibition of replication of SARS-CoV-2 virus (Zhao et al., 2019; Song et al., 2021). |
| China Root (Poria cocos) |
Wolfiporia extensa | Polyporaceae | Sclerotia root | Pachymic acid | Mpro is the protease enzyme of the SARS CoV virus which helps in replication. Pachymic acid binds to this Mpro enzyme and arrests the further growth of an organism (Du et al., 2021). |
| Ginger (Soonth) | Zingiber officinale Roscoe | Zingiberaceae | Rhizomes | Gingerol, gingerone, gingiberol, and shagaol. | Provides symptomatic relief from the sore throat, which in turn can be utilized as a possible marker in SARS-CoV-2 infection (Haridas et al., 2021). |
| Guduchi (Giloy) |
Tinospora cordifolia (Willd.) Hook. f. & Thomson | Menispermaceae | Whole plant | Berberine, palmatine, tinocordiside, tinocrodifolioside A, cordioside, cordofoliside A, B, C, D, and E, tinospora, tinosporides, jateorine, and columbine. | Immunomodulatory, anti-inflammatory, and antioxidant effects (Balkrishna et al., 2021) . |
| Holy Basil (Tulsi) |
Ocimum tenuiflorum L. Ocimum caryophyllinum F.Muell. |
Lamiaceae | Leaves | Vicenin, Isorientin, 4′-O- Glucoside 2″-O-p-Hydroxybenzoagte, Ursolic Acid, Tulsinol A,B,C,D,E,F and G |
Potential inhibitor of Papain-like-protease and SARS CoV19 Main Protease(Mpro) thus hindering binding of the virus to ACE2 host receptors (Mody et al., 2021). |
| Japanese Honeysuckle | Lonicera japonica Thunb. | Caprifoliaceae | Seeds, fruits, and leaves | Hydroxycinnamic acid, isoflavone, and flavanones. | Reduction of toll-like receptor 3 and tank bound kinase 1 caused by respiratory syncytial viral infection (Tsai et al., 2021). |
| Liquorice (Mulethi) | Glycyrrhiza glabra L. | Leguminosae | Roots and rootlets | Glycyrrhizin | Glycyrrhizin prevents the release of HMGB1 nuclear protein (High Motility Group Box Protein 1) from infected cells which leads to failure in activation of macrophages responsible for the release of pro-inflammatory cytokines. Thus, preventing the ‘Cytokine Storm’ and its detrimental consequences (Pastorino et al., 2018; Gomaa and Abdel-Wadood, 2021). |
| Mongolian milkvetch (Huáng qí) | Astragalus mongholicus Bunge | Leguminosae | Root | Astragalosides, kumatakenin, and calycosin | The polysaccharide of Astragalus regulates the expression of TNFα, IL-1β, and NFATc4 and thus exerts an immunomodulating effect (Dashdorj et al., 2021). |
| Turmeric (Haldi) | Curcuma longa L. | Zingiberaceae | Dried roots and rhizomes | Curcumin | Probable inhibition of 3CL (3 Chymotrypsin) like protease in viral cells and thus preventing replication in the host. Inhibition of pro-inflammatory mediators like IL-6, IL-1β TNF α, bradykinin, cyclooxygenase (COX), caspase 3 (Cas 3), and NF-kB (Rattis et al., 2021). |
| Vasaka (Ardusi) |
Adhatoda vasica Justicia adhatoda L. |
Acanthaceae | Leaves | Vascicine, vascinone, vascinolone, and adhatodine. | Antitussive and bronchodilatory actions relieving pneumonia-like symptoms (Kotecha, 2015; Gheware et al., 2021). |
6.1. Giloy (Guduchi)
It consists of the entire plant of Tinospora cordifolia and belongs to the family Menispermaceae. Berberine and palmatine are important alkaloids; glycosides that are beneficial comprise tinocordiside, tinocrodifolioside A, cordioside, cordofoliside A, B, C, D, and E, and diterpenoids such as tinosporon, tinosporides, jateorine, and columbine are useful chemical constituents present (Arunachalam et al., 2022; Reddi and Tetali, 2019).
The powdered giloy plant is thoroughly used during the pre-covid as well as post-covid times due to its immunomodulatory properties. Moreover, the giloy plant is also useful as an antioxidant and therefore decreases the overall oxidative stress in the body (Chowdhury, 2021). This further prevents the oxidative breakdown of essential biomolecules in the body. During chronic COVID-19 infection, giloy exhibits anti-inflammatory actions, which are of great use in corona virus-induced pneumonia and inflammation of the lungs. The oxygen saturation level in the blood falls below the normal range, inducing hypoxia, fatigue, and even death (Dhama et al., 2017; Kumar et al., 2020a). Hence, a clinical trial was conducted on the ayurvedic formulation, Guduchi Ghan Vati-extract of Giloy, to evaluate its efficacy in COVID-19 confirmed asymptomatic to mild symptomatic patients. Retrospectively, 91 patients between the ages of 18 to 75 years old were studied, from them, 11.7% of the control group developed mild symptoms after an average of 1.8 days, and none in the Ayurveda group reported any other symptoms. The researchers suggested from the study that the Guduchi Ghan Vati can act as a preventive and treatment agent in the case of asymptomatic COVID-19 patients (NCT04480398).
6.2. Turmeric (Haldi)
It consists of entire dried and powdered roots and rhizomes of Curcuma longa belonging to the family Zingiberaceae. Turmeric is an extensively used ayurvedic remedy for its antimicrobial activities and as a spice in Indian culture and other parts of the world. The active principle of turmeric, curcumin, acts against COVID-19 infection by exhibiting anti-bacterial, antiviral, and anti-inflammatory effects (Benzie and Wachtel-Galor, 2011). When applied topically or ingested orally, it shows a significant reduction in inflammation, which can act as a countering characteristic in respiratory inflammation (Brahmbhatt, 2020). It is also responsible for the prevention of ‘cytokine storm’ induced at once due to excessive secretion of pro-inflammatory mediators and factors like interleukin IL-1β, IL-6, IL-8 and IL-10, tumor necrosis factor-alpha, cytokines, cyclooxygenase enzymes, and bradykinin. Furthermore, studies have shown that curcumin may block the 3-chymotrypsin-like protease enzyme in Vero cells, therefore halting cellular reproduction, an important mechanism for virus cellular replication, and thus gradually over time reducing the viral load in the body (US20140369938). Moreover, to evaluate the efficacy of turmeric in COVID-19, a double-blind, randomized, controlled clinical trial of its constituent, curcumin coadministered with piperine delivered orally, was analyzed on symptomatic COVID-19 patients. Patients with mild, moderate, and severe symptoms who received curcumin/piperine treatment showed early symptomatic recovery (fever, cough, sore throat, and breathlessness), better ability to maintain oxygen saturation above 94% on room air and better clinical outcomes compared to the patients of the control group. The researchers suggested using this combination tablet as symptomatic adjuvant therapy in COVID-19 treatment with substantially reduced morbidity and mortality and safe use of curcumin in post-covid thromboembolic events (CTRI/2020/05/025,482).
6.3. Liquorice (Mulethi)
It comprises of roots and rootlets of Glycyrrhiza glabra from the family Leguminosae and its active chemical constituent is glycyrrhizin. Licorice is an ayurvedic medicine known for its peripheral as well as centrally acting anti-tussive properties. Studies demonstrated that glycyrrhizin exhibits cough suppressing properties, which subdues the coughing, and the associated shortness of breath; the primary symptoms of COVID-19 infestation (Isbrucker et al., 2006). It has been proven to prevent the release of HMGB1 nuclear protein (high motility group box protein 1) from the cells infected by SARS (Mamedov et al., 2019). This fails macrophages, which play an active role in activating immunity and are responsible for producing pro-inflammatory cytokines. As a result, the start of the ‘cytokine storm' and its adverse health effects are avoided (Mamedov et al., 2019). Furthermore, a single-center, open-label, randomized, clinical trial with a parallel-group design was conducted in Iran to evaluate the efficacy of anti-inflammatory action of licorice on 60 COVID-19 patients whose age was ≥ 18 years old (weight ≥ 35 kg) (IRCT20200506047323N2).
6.4. China root (Poria cocos)
It consists of sclerotia (fungus) obtained from the roots of Wolfiporia extensa belonging to the family Polyporaceae with the active constituent pachymic acid. Protease enzymes are in charge of an organism's replication in the host cell. One such protease enzyme found in Coronaviruses is the Mpro enzyme ( Lee et al., 2001). Mpro is the key enzyme of SARS-CoV-2 involved in mediating viral replication and transcription. Pachymic acid, the main active constituent of Poria cocos, binds to the Mpro enzyme and inhibits it, thus arresting an organism's further replication and growth in the host cells (Lee et al., 2001). In COVID-19 severe cases, few patients may develop pneumonia complications that are even higher in people who are 65 or older. In such conditions, the anti-inflammatory property of pachymic acid can be accounted for providing relief. In one study, the rat lungs with pneumonia were treated with pachymic acid, and results showed an inhibition in the expression of inflammatory cytokines through the NF-κB pathway (Gui et al., 2021).
6.5. Mongolian milkvetch
It consists of roots, stems, and leaves of Astragalus membranaceus belonging to the family Leguminosae. The active phytochemicals are astragalosides, kumatakenin, and calycosin. Astragaloside, the polysaccharide found in Astragalus roots, stems, and leaves, regulates the expression of pro-inflammatory factors like cytokines, TNFα, IL-1β, and NFATc4 and thus exerts an immunomodulating effect. It has been found useful in averting the condition of the cytokine storm. Moreover, kumatakenin is also, up to a certain extent, successful in inhibiting the Mpro enzyme (Gonzalez, 2021). As an immune regulator, Mongolian milkvetch can be administered in the case of COVID-19 to boost immunity as a preventive measure. To evaluate this, a metabonomic study of mice plasma was conducted. The results demonstrated that simultaneous ingestion of Mongolian milkvetch with ginseng strengthened the immune system by enhancing the spleen and thymus index, proliferation of splenic lymphocytes, and cytotoxic activity of NK cells (Liu et al., 2021).
7. Dietary Supplements and Nutraceuticals
It is already known that COVID-19 is responsible for displaying flu-like symptoms, so one way to combat it can be by incorporating several dietary supplements like zinc, Vit. C, D, B, and probiotics as immunity boosters (Louca et al., 2021). Dietary supplements are the nutrients intended to be taken in a capsule, tablet, powder, or liquid extracted from natural food sources for boosting one's immunity against various diseases like viral infection, inflammation, and respiratory complications. They compromise vitamins, minerals, botanicals, enzymes, fatty acids, proteins, and probiotics. In most cases, they are used as adjuvant therapy or additives along with the main pharmacological drug (Das et al., 2012). The broader term than dietary supplements dealing with whole food instead of extracting nutrients as an enhancer of health is called nutraceuticals, which are equally important when dealing with Coronavirus. It is derived from nutrition and pharmaceutical, meaning “food as a medicine”. They provide physiological benefits to an individual's health and prevent various diseases (Savant et al., 2021). Most diseases like diabetes, hypertension, obesity, respiratory, cardiovascular, and inflammatory bowel diseases are manifested because of complicated and improper lifestyle and food habits (Lordan et al., 2021b). Therefore, the primary level of treatment can be achieved by just some lifestyle modifications and the incorporation of nutraceuticals.
In respect to the current scenario of the COVID-19 pandemic, dietary supplements are the only most important section of nutraceuticals that exhibit good immunity-boosting abilities (Subedi et al., 2021; Savant et al., 2021). The innate immune system acts as the first-line defense mechanism against pathogens responsible for manipulating the illness, and the consecutive events are part of adaptive reactions (Brendler et al., 2021). In Table 2 , we discussed various types of dietary supplements obtained from the active constituents extracted from animals and plants utilized against COVID-19 and selected based on the research evidence against COVID-19 or under investigation for the same. Hospitalized patients also have a higher rate of Vitamin D deficiency or inadequacies related to disease severity. As a result, more study is needed to find how dietary supplements and nutraceuticals can aid in the cure of critically ill and recovering patients who frequently rely on treatment (Huang et al., 2020b). People should be aware of misleading information, lies, and deceit underlying certain supplements that might be subject to strict government intervention. However, more studies are needed to determine whether dietary supplements and nutraceuticals have prophylactic and therapeutic significance against SARS-CoV-2 infection and COVID-19 illness (Ronan et al., 2021).
Table 2.
Dietary supplements against COVID-19.
| Dietary supplements | Biological sources | Active constituents |
|---|---|---|
| Echinacea (NCT04981314) | Group of purple coneflower obtained from the genus Echinacea, majorly extracted from E. purpurea, E. angustifolia, and E. pallid (Gurley et al., 2012; Nagoor Meeran et al., 2021) | Polysaccharides Polyacetylenes Volatile-terpenes Caffeic acid esters Phenolic compounds Alkamides Glycoproteins |
| Elderberry (NCT03410862) | Fruit of Sambucus nigra (Porter and Bode, 2017; Wieland et al., 2021) | Anthocyanins Flavonols Phenolic acids |
| Ginseng (NCT01478009) |
Ginseng is obtained from the genus Panax most commonly from Panax quinquefolius (American variety) and Panax ginseng(Asian variety) (Mancuso and Santangelo, 2017; Fang et al., 2020; Lee and Rhee, 2021). |
Ginsenosides Triterpene glycosides |
| N-acetylcysteine (NCT04419025) |
A derivative of the cysteine amino acid (Bauer et al., 2020; Rahimi et al., 2021) | N-acetylcysteine |
| Omega-3 fatty acid (NCT04836052) | It is found mostly in flaxseeds and fatty fish, both including fish oil (Hathaway et al., 2020). | Derived as Eicosapentaenoic acid (EPA) Alpha-linolenic acid (ALA) Docosahexaenoic acid (DHA) |
| Probiotics (NCT04390477) | Obtained from bacteria like Lactobacillus acidophilus, Lactobacillus rhamnosus, and Bifidobacterium longum, and yeasts like Saccharomyces boulardii ( Kumar et al., 2020b; Singh and Rao, 2021) | Lactic acid |
| Vitamin C (NCT04682574) |
Derived from fruits and vegetables of the citrus family, and also includes tomatoes, potatoes, peppers, kiwifruit, broccoli, strawberries, and cantaloupe (Hemilä and Man, 2021). | Ascorbic acid Citric acid |
| Vitamin D (NCT04536298) | The primary ingredient is found in fatty fish (such as tuna fish and salmon fish) and their liver oils. It is also obtained from cheese, cow liver oil, and egg yolks in the form of Vitamin D1, D2, D3, D4, and D5 (Wallace et al., 2013; Butler-Laporte et al., 2021) | Ergocalciferol Cholecalciferol 22-dihydroergocalciferol Sitocalciferol Ergocalciferol with lumisterol |
| Zinc (NCT04558424) |
Zinc is mainly found in the foods like crab, whole grains, crab, beef, beans, nuts, pork, lobster, oysters, and dairy products ( Kumar et al., 2020b). | Zinc |
The diet helps in modulating inflammation, and nutraceuticals can inhibit viral entry. Hence, we have collated literature on antiviral nutraceuticals effective against other similar Coronaviruses. The objective of this study is to review available information on the antiviral activity of nutraceuticals comprehensively and to discuss the implications of these findings in designing a diet that would boost innate immunity and act as preventive care against COVID-19. The most widely used dietary supplements and nutraceuticals are discussed below.
7.1. Echinacea
Volatile terpenes, polyacetylenes, alkamides, phenolic compounds, polysaccharides, caffeic acid esters, and glycoproteins are the major components of Echinacea (Gurley et al., 2012). Its chemical constituents mainly exhibit anti-oxidant and anti-inflammatory activities, they also stimulate monocytes and natural killer cells that might have a role in reducing the binding of the virus to human host cells. It also reduces inflammation by inhibiting inflammatory mediators like inflammatory cytokines (Dimmito et al., 2021; Gurley et al., 2012). There are some reviews by investigators suggesting that it has an immunostimulatory effect that worsens the cytokine storm developed in novel Coronavirus patients. Preferentially, it has a long history of usage in wound healing, and there is very little evidence that it can help with common colds and upper respiratory infections (Sharifi-Rad et al., 2018). Therefore, some researchers believed that it might have similar effects on the COVID-19. In a double-blind study, 230 individuals with moderate COVID-19 were administered echinacea or placebo. The efficacy of therapy vs. placebo on clinical symptoms, fever, and overall illness duration and proportion of return to the emergency department and/or hospitalization was studied during 4 weeks (NCT04981314). It is considered safe and has very few adverse effects, including gastrointestinal tract upset and skin rashes. Also, if it is contaminated or the product separation is observed, it might cause allergic reactions with elevated liver enzymes, leading to liver injuries (Jawad et al., 2012).
7.2. Elderberry
Elderberry is a fruit of Sambucus nigra, a small deciduous tree, and it mainly contains anthocyanins, flavonols, and phenolic acids as principle constituents (Ulbricht et al., 2014). These components have antioxidant, anti-inflammatory, antiviral, antibacterial, and immune-stimulant properties. According to several experts, elderberry as an adjuvant treatment might benefit the present pandemic crisis. According to early laboratory and animal studies, the active components of elderberry described before may aid in the prevention or treatment of upper respiratory tract infections by blocking viral binding to the host cell and activating the immune system (Hawkins et al., 2019). A randomized, double-blinded, placebo-controlled clinical trial with a parallel-group design of 40 patients was studied to evaluate the effects of elderberry extract syrup against COVID-19 symptoms in outpatients and home quarantined patients (IRCT20200406046965N1). However, no exact results were revealed on it. Although the flowers and ripe fruits have seemed to be safe to eat, and extracts are made from the ripe fruits, the bark, leaves, seeds, and raw or unripe fruit of Sambucus nigra contain a cyanogenic glycoside that has the potential to cause nausea, vomiting, diarrhea, dehydration due to diuresis, and cyanide poisoning if consumed in excess or improperly (Appenteng et al., 2021; Ulbricht et al., 2014).
7.3. Ginseng
According to laboratory and animal research, ginseng promotes B-lymphocyte proliferation and enhances the synthesis of inflammatory mediators, including interferon-gamma and interleukins, which affect immunological activation and regulation. It also has anti-inflammatory properties and inhibits viral multiplication (Antonelli et al., 2020). However, it is unclear if ginseng has a therapeutic impact on immunological function. Many experts believe that ginseng can help cure upper respiratory infections and reduce the usual cold and flu, yet there is no firm data to back up this claim in the COVID pandemic (Silveira et al., 2020; Mancuso and Santangelo, 2017). Though many types of studies are being conducted with COVID-19 patients to determine the relationship between the effect of ginseng on upper respiratory tract infection and anti-inflammatory activity, there have been no conclusive findings on the influence of ginseng on COVID-19 (Coon et al., 2002; Lee and Rhee, 2021). Currently, phase 2 and 3 clinical trials on traditional Chinese medicine, Shen Cao Gan Jiang Tang including Gan Cao Gan Jiang Tang with the addition of Ginseng for strengthening immunity system against SARS-CoV-2 virus is continuing. The results are yet to be awaited (NCT05055427).
7.4. N-acetylcysteine (NAC)
NAC is an amino acid derivative of cysteine. It is an antioxidant that primarily boosts the body's glutathione levels. It has mucolytic action, which aids in the removal of mucus from the lungs (Bauer et al., 2020). In addition, some laboratory studies show that it may strengthen the immune system and inhibit viral replication, both of which are beneficial in the COVID scenario. It is also helpful in decreasing the inflammatory mediator level, interleukin-6. However, there are no recommendations to use NAC in the COVID-19 treatment, but investigators believe its anti-inflammatory and anti-oxidant properties are useful (Flora et al., 2020). In one N-acetylcysteine clinical trial, 42 persons with COVID-19 were given 600, 1200, and 1800 mg NAC thrice a day for about three months (NCT04545008). Another investigator was looking to see if taking NAC with glycine for two weeks in 64 COVID-19-positive hospitalized patients aged 55 to 85 would improve their conditions (NCT04703036). In addition, studies have revealed that its oral dose has some effect in bronchopulmonary disease, and due to its mucolytic activity, it might have an action in cardiac obstructive pulmonary disease (COPD) too. NAC's safety profile has been examined because it is an FDA-approved medication. Nausea, vomiting, stomach pain, diarrhea, indigestion, and epigastric discomfort have all been described as typical adverse effects of oral NAC (Fowdar et al., 2017).
7.5. Omega-3 fatty acid
Omega-3 is a type of phospholipid found in the cell membrane, and hence it is critical for the body's cardiovascular, pulmonary, immunological, and endocrine systems to function properly (Lordan et al., 2021a). They are polyunsaturated fatty acids derived from eicosanoids and are more powerful mediators of inflammation, vasoconstriction, and platelet aggregation than omega-6 fatty acids. As a result, higher omega-3 eicosanoid concentrations balance less inflammatory activity than omega-6 eicosanoids (Simopoulos, 2016). Moreover, higher levels of eicosapentaenoic acid and docosahexaenoic acid from which omega-3 is derived in blood vessels are linked to lower levels of inflammatory cytokines. It influences immune function by modulating the activity of macrophages, neutrophils, T cells, B cells, natural killer cells, and other immune system cells (Hathaway et al., 2020). In the case of COVID-19, higher doses of omega-3s may reduce the patients’ risk or severity, which is yet to be proven; but experts suggest its advantages in the COVID-19 pandemic because of its proven and accepted ability as an anti-inflammatory and immune-stimulating agent (Hathaway et al., 2020). Moreover, according to an analysis of red blood cells containing active ingredients of omega-3 s, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) among hospitalized patients with a range of ages from 33 to 72 years, those who took supplements had a greater survival rate by one month than those who did not. Also, supplementing with omega-3s enhanced numerous respiratory and hepatic functions, including blood urea nitrogen, atrial blood pH, and creatinine levels, without affecting oxygen saturation or WBC count (Gutiérrez et al., 2019). The recommended dose of omega-3 approved by the FDA includes not exceeding 3 g/day EPA and DHA combined, including up to 2 g/day from dietary supplements (n.d.). In Norway, 80,000 healthy people aged 18 to 75 years were being studied to see if taking a daily cod liver oil supplement containing 1200 mg of long-chain omega-3 s, namely EPA and DHA, for 6 months decreases the chance of developing COVID-19 and the severity of illness or not (NCT04609423). Moreover, in Jordan, over 100 healthy people aged 30 to 66 years were studied to evaluate daily intake of 300 mg of omega-3 s for two months on changes in levels of interleukin-1 beta, interleukin-6, and tumor necrosis factor; which are implicated in the cytokine storm in case of COVID-19 (NCT04483271).
7.6. Probiotics
Probiotics might cause the improvement of the immune system in several ways, and their main desirable acting site is the gastrointestinal tract (Brendler et al., 2021). However, their method of improving COVID-19 is still vague, but a few mechanisms like improving gut barrier function, boosting immunoglobulin synthesis, blocking virus multiplication, and raising white blood cell phagocytic activity can be considered beneficial (Lehtoranta et al., 2014; Darbandi et al., 2021). Many pieces of evidence are present stating that probiotics are effective against several viral infections by improving the immunity or producing anti-inflammatory activity in the host who is taking them as a supplement. Nevertheless, the method by which probiotics impact these numerous diseases differs between strains, with each strain having its significant impact and dependent on its particular diverse set. Researchers also stated that one probiotic strain cannot be extrapolated to another (Hao et al., 2015; Olaimat et al., 2020). Many researchers believe that probiotics are useful in the COVID-19 situation as an adjuvant therapy or supplements that may be given along with the potent pharmaceutical ingredients. For checking these possibilities, a country like Italy performed a clinical trial with 70 hospitalized patients (mean age of 59 years), in which they gave hydroxychloroquine, tocilizumab, and other antibiotics to 70 patients in combination or alone (Das et al., 2012). In addition, 28 out of 70 received probiotics comprising a mixture of Streptococcus, Lactobacillus, and Bifidobacterium three times a day, daily up to 14 days. After 7 days, symptoms like fever, diarrhea, headaches, myalgia, and dyspnea were significantly reduced compared to patients who did not receive probiotics as adjuvant therapy (Akour, 2020). Probiotics like Lactobacillus, Bifidobacterium, and Propionibacterium are usually considered safe nutrients as they display side effects rarely, and if they occur, they are observed after prolonged use. However, partial toxic effects such as fungemia (fungi in the blood), and bacteremia (bacteria in the blood) can be observed, but they are rare (Lu et al., 2021).
7.7. Vitamin C
Vitamin C mainly demonstrates anti-oxidant, anti-inflammatory, and immunomodulatory effects. It is used in several viral infections. In addition, studies have revealed that it acts on both innate and adaptive immunity and enhances both of them. It helps in maintaining epithelial integrity, enhancement of phagocytic reaction, differentiation enhancement, normalization of cytokine production, decrease in histamine levels, and proliferation of B and T cells (Hemilä and Chalker, 2013). Its deficiency in the body leads to impairment of immune functions and increases the risk of viral infection as the immune functions are disturbed and susceptibility towards the particular viral infection increases (Arvinte et al., 2020). However, direct use of Vitamin C for the treatment of COVID-19 does not have any strong pieces of evidence and recommendations but, researchers suggest that it may act as an adjuvant supplement in the COVID-19 complications such as septic shock and ARDS (Acute respiratory distress syndrome) (Holford et al., 2020). Several studies are now being conducted to assess the efficacy of Vitamin C against COVID-19. A clinical retrospective cohort study was carried out on 78 participants to apply intravenous high dose Vitamin C to determine its effect on short-term mortality and length of stay in intensive care in critically ill COVID-19 patients (NCT04710329). Moreover, Vitamin C intravenous injection is classified as a medication by the FDA, and only oral dosages may be evaluated and used as dietary supplements in doses of 400 to 1800 mg per day for children (depending on age), and 2000 mg per day for adults is considered safe (Thomas et al., 2021).
7.8. Vitamin D
Vitamin D, which is physiologically inactive, undergoes double hydroxylation and transforms into an active form in the body when exposed to sunshine and food. The first hydroxylation reaction takes place in the liver, converting Vitamin D to 25-hydroxy Vitamin D [25(OH) D], and the second hydroxylation reaction takes place in the kidney, resulting in physiologically active 1, 25-dihydroxy Vitamin D [1, 25(OH) 2D](Lordan et al., 2021a). Blood Vitamin D concentration levels of 50 nmol·L−1 (20 ng·mL−1) or above are considered enough for bone and overall health in the majority of people (Wallace et al., 2013). It is essential for sustaining immunity in addition to its powerful impacts on calcium absorption and bone strength. It also slows down viral replication, decreases inflammatory activity, and increases the amount of T regulatory cells in the body (Iddir et al., 2020). It may also have effects such as suppressing the cytokine system, boosting the synthesis of antimicrobial peptides in the lungs to aid in respiratory infection prevention, and increasing ACE2 gene expression, which is suppressed by COVID-19. Vitamin D also alleviates complications such as infectious diseases, septic shock, and ARDS diseases (due to low levels of Vitamin D), which might aid in the prevention of novel Coronavirus as an adjuvant supplement (Zhou et al., 2019; Mitchell, 2020). Despite wide application, it does not exhibit strong recommendations for the use in the treatment or the prevention of COVID-19 (Grant et al., 2020). However, some experts believe that Vitamin D supplementation can assist patients with 25(OH) D levels less than 25 nmol·L−1 (10 ng·mL−1) to avoid respiratory infections. As a result, researchers are keen on investigating whether Vitamin D might help cure COVID-19 or not (Martineau et al., 2017). Apart from its advantageous features, it can cause some uncommon side effects like nausea, disorientation, hypercalcemia, stomach discomfort, polyuria, and dehydration. Moreover, its deficiency along with decalcification, and rickettsia increases the susceptibility towards the viral infection which is mainly associated with influenza, human immunodeficiency virus (HIV), and hepatitis (Aranow, 2011). Nevertheless, research is underway to determine the role of Vitamin D in COVID-19, with some claiming that individuals with low Vitamin D levels are more likely to develop COVID-19 and have a more severe illness. One study compared the levels of blood 25(OH)D in 335 patients with COVID-19 in China to 560 healthy volunteers and found that patients with COVID-19 had significantly lower 25(OH)D concentrations (median of 26.5 nmol·L−1 [10.6 ng·mL−1]) than healthy volunteers (median of 32.5 nmol·L−1 [13 ng·mL−1]) (Yisak et al., 2021). Furthermore, Vitamin D insufficiency [specifically defined as serum 25(OH) D less than 30 nmol·L−1 (12 ng·mL−1)] was shown to be more common in COVID-19 patients than in healthy volunteers. Daily Vitamin D doses of up to 25–100 mcg (1000–4000 IU) in foods and dietary supplements are acceptable for children (depending on their age) and up to 100mcg (4000 IU) are safe for adults, as per the institute of medicine's food and nutrition board in Washington (Wallace et al., 2013). Toxic effects like nausea, vomiting, muscular weakness, disorientation, lack of appetite, dehydration, increased urination, and thirst may occur if you consume more than the recommended quantity (Yisak et al., 2021).
7.9. Zinc
Zinc is the most prevalent and significant trace element, and it is involved in many facets of cellular metabolism and the catalytic activity of almost 100 enzymes (Kumar et al., 2020b). It stimulates both adaptive and inherent immunity, as well as antiviral and anti-inflammatory characteristics that aid in the maintenance of tissue barriers such as the respiratory epithelia (Wessels et al., 2017). Zinc deficiency impedes lymphocyte production, stimulation, and maturation, resulting in decreased immunological function. It also impacts immunological mediators, suppressing T cells, interleukin-2 synthesis, and inhibiting natural killer and cytotoxic T cell activity. Furthermore, its absence is linked to higher amounts of pro-inflammatory mediators, which may enhance vulnerability to disease and inflammatory disorders, especially those affecting the lungs (Prasad, 2020). Currently, the data and shreds of evidence are not that strong in favor of zinc as a drug for treating or preventing COVID disease, but its anti-inflammatory, anti-viral activity, and effect on the immune system might be helpful adjunctive therapy along with other drugs (Wessels et al., 2020). To evaluate the use of immune-based therapy consisting of zinc supplements with Vitamin D a 2×2 factorial randomized double-blind, placebo-controlled phase 3 clinical trial on 700 participants with COVID-19 in India is being carried out (NCT04641195). According to the Institute of Medicine's Food and Nutrition Board, Washington, zinc intakes of up to 4–34 mg/day in foods and dietary supplements for children (varies according to age) and up to 40 mg/day for adults are healthy. At larger doses, side effects such as nausea, vomiting, stomach cramps, lack of appetite, and diarrhea might occur (Wessels et al., 2020).
8. Conclusion
Researchers and the healthcare sectors have been trying to repurpose or invent innovative host-directed medicines to combat the COVID-19 outbreak triggered by the emergence of the SARS-CoV-2 in the last two years. This quick effort resulted in discovering potential pharmacological medicines for hospitalized patients, including remdesivir and dexamethasone. Moreover, the majority of cultures have undertaken non-pharmacological preventative measures, such as public health initiatives to reduce SARS-CoV-2 transmission. However, during this period, many people would seek extra defense by consuming different dietary supplements and nutraceuticals that felt to demonstrate positive effects. Our review indicates that numerous herbal remedies, dietary supplements, and nutraceutical products have higher safety tolerances than standard pharmaceutical drugs and gives certain amounts of proof to begin a clinical conversation regarding their possible use in the treatment of COVID-19. These herbal medications cannot prevent the virus but may enhance the patient's well-being. Pharmacokinetic studies on enticing bioactive components are rare and must be undertaken to acquire a pharmacokinetic profile, which includes absorption, distribution, metabolism, and excretion metrics. Furthermore, clinical studies (phase I to III) are needed to analyze anti-CoV safety and effectiveness in humans. Even with all of the possible advantages of nutraceutical and dietary supplement approaches presented, there is a shortage of clinical proof to substantiate their use for COVID-19 infection prevention and management. Nonetheless, by supporting normal immune system preservation, an individual's immune system can be primed to safeguard even against the effects of acute respiratory viral diseases. Undoubtedly, in light of such an overview, it would be prudent to abide by national guidelines for a healthy diet and lifestyle to maintain excellent immune health. Due to the sheer global appeal of dietary supplements and nutraceuticals, assessing the proof to support their use is critical.
Ethical Approval
Not Applicable.
Data Availability
Nil.
Declaration of Competing Interest
The authors claim that they have no conflicts of interest in respect to the authorship and publishing of this work.
CRediT authorship contribution statement
Vivek P Chavda: Conceptualization, Data curation, Methodology, Supervision, Writing – original draft, Writing – review & editing. Aayushi B. Patel: Writing – original draft. Disha Vihol: Writing – original draft. Darsh D. Vaghasiya: Writing – original draft. Khandu Muhammed Saad Bashir Ahmed: Writing – original draft. Kushal U. Trivedi: Writing – original draft. Divyang J Dave: Writing – review & editing.
Acknowledgments
Acknowledgments
V.P.C. wants to dedicate this work to L M College of pharmacy as a part of the 75th year celebration of the college.
Funding
Nil.
ORCID
Vivek P Chavda, https://orcid.org/0000-0002-7701-8597.
Supplementary Materials
Nil.
References
- Akour A. Probiotics and COVID-19–Is there any link? Lett. Appl. Microbiol. 2020;71(3):229–234. doi: 10.1111/lam.13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonelli M., Donelli D., Firenzuoli F. Ginseng integrative supplementation for seasonal acute upper respiratory infections–A systematic review and meta-analysis. Complement. Ther. Med. 2020;52 doi: 10.1016/j.ctim.2020.102457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appenteng M.K., Krueger R., Johnson M.C., Ingold H., Bell R., Thomas A.L., Greenlief C.M. Cyanogenic glycoside analysis in American Elderberry. Molecules. 2021;26(5):1384. doi: 10.3390/molecules26051384. from https://pubmed.ncbi.nlm.nih.gov/33806603 March 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranow C. Vitamin D and the immune system. J. Investig. Med. 2011;59(6):881–886. doi: 10.2310/JIM.0B013E31821B8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunachalam K., Yang X., San T.T. Tinospora Cordifolia (Willd.) Miers–Protection mechanisms and strategies against oxidative stress-related diseases. J. Ethnopharmacol. 2022;283 doi: 10.1016/j.jep.2021.114540. [DOI] [PubMed] [Google Scholar]
- Arvinte C., Singh M., Marik P.E. Serum levels of Vitamin C and Vitamin D in a cohort of critically ill COVID-19 patients of a North American community hospital intensive care unit in May 2020–A pilot study. Med. Drug Discov. 2020;8 doi: 10.1016/J.MEDIDD.2020.100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astuti I., Ysrafil Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)–An overview of viral structure and host response. Diabetes Metab. Syndr. 2020;14(4):407–412. doi: 10.1016/j.dsx.2020.04.020. from https://pubmed.ncbi.nlm.nih.gov/32335367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkrishna A., Bhatt A.Ben, Singh P., Haldar S., Varshney A. Comparative retrospective open-label study of ayurvedic medicines and their combination with allopathic drugs on asymptomatic and mildly-symptomatic COVID-19 patients. J. Herb. Med. October 2021;29 doi: 10.1016/j.hermed.2021.100472. from https://pubmed.ncbi.nlm.nih.gov/34055580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S.R., Kapoor A., Rath M., Thomas S.A. What is the role of supplementation with ascorbic acid, zinc, Vitamin D, or N-acetylcysteine for prevention or treatment of COVID-19? Clevel. Clin. J. Med. 2020 doi: 10.3949/ccjm.87a.ccc046. [DOI] [PubMed] [Google Scholar]
- Benzie I.F.F., Wachtel-Galor S. Chapter 13–Turmeric, the golden spice. Herb. Med. 2011;II(2):1–31. [PubMed] [Google Scholar]
- Brahmbhatt R.V. Herbal medicines in management and prevention of COVID-19. Phytopathology. 2020;9(3):1221–1223. doi: 10.22271/PHYTO.2020.V9.I3T.11460. [DOI] [Google Scholar]
- Brendler T., Al-Harrasi A., Bauer R., Gafner S., Hardy M.L., Heinrich M., Hosseinzadeh H., et al. Botanical drugs and supplements affecting the immune response in the time of COVID-19–Implications for research and clinical practice. Phytother. Res. 2021;35(6):3013–3031. doi: 10.1002/ptr.7008. [DOI] [PubMed] [Google Scholar]
- Butler-Laporte G., Nakanishi T., Mooser V., Morrison D.R., Abdullah T., Adeleye O., Mamlouk N., et al. Vitamin D and COVID-19 susceptibility and severity in the COVID-19 host genetics initiative–A mendelian randomization study. PLoS Med. 2021;18(6) doi: 10.1371/journal.pmed.1003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catanzaro M., Fagiani F., Racchi M., Corsini E., Govoni S., Lanni C. Immune response in COVID-19–Addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct. Target. Ther. 2020;5(1):84. doi: 10.1038/s41392-020-0191-1. from 10.1038/s41392-020-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . 2021. SARS-CoV-2 Variant Classifications and Definitions. [Google Scholar]
- Chavda V.P., Apostolopoulos V. Mucormycosis – An opportunistic infection in the aged immunocompromized individual–A reason for concern in COVID-19. Maturitas. 2021 doi: 10.1016/j.maturitas.2021.07.009. from https://www.sciencedirect.com/science/article/pii/S0378512221001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavda V.P., Gajjar N., Shah N., Dave D.J., Ethanolate Darunavir. Repurposing an anti-HIV drug in COVID-19 treatment. Eur. J. Med. Chem. Rep. 2021;3 doi: 10.1016/j.ejmcr.2021.100013. from https://www.sciencedirect.com/science/article/pii/S2772417421000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavda V.P., Hossain M.K., Beladiya J., Apostolopoulos V. Nucleic acid vaccines for COVID-19–A paradigm shift in the vaccine development arena. Biologics. 2021 [Google Scholar]
- Chavda V.P., Pandya R., Apostolopoulos V. DNA vaccines for SARS-CoV-2–Towards third generation vaccination era. Expert Rev. Vaccines. 2021 doi: 10.1080/14760584.2021.1987223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavda V.P., Vora L.K., Pandya A.K., Patravale V.B. Intranasal vaccines for SARS-CoV-2–From challenges to potential in COVID-19 management. Drug Discov. Today. 2021;26(11):2619–2636. doi: 10.1016/j.drudis.2021.07.021. from https://www.sciencedirect.com/science/article/pii/S1359644621003317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavda V.P., Vora L.K., Vihol D.R. COVAX-19Ⓡ vaccine–Completely blocks virus transmission to non-immune individuals. Clin. Complement. Med. Pharmacol. 2021;1(1) doi: 10.1016/j.ccmp.2021.100004. from https://www.sciencedirect.com/science/article/pii/S2772371221000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chojnacka K., Witek-Krowiak A., Skrzypczak D., Mikula K., Młynarz P. Phytochemicals containing biologically active polyphenols as an effective agent against COVID-19-Inducing Coronavirus. J. Funct. Foods. 2020;73 doi: 10.1016/j.jff.2020.104146. from https://www.sciencedirect.com/science/article/pii/S1756464620303704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury P. Silico investigation of phytoconstituents from indian medicinal herb “Tinospora Cordifolia (Giloy)” against SARS-CoV-2 (COVID-19) by molecular dynamics approach. J. Biomol. Struct. Dyn. 2021;39(17):6792–6809. doi: 10.1080/07391102.2020.1803968. from https://pubmed.ncbi.nlm.nih.gov/32762511October. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coon J.T., Ernst E., Ginseng Panax. A systematic review of adverse effects and drug interactions. Drug Saf. 2002;25(5):323–344. doi: 10.2165/00002018-200225050-00003. [DOI] [PubMed] [Google Scholar]
- Croft M., So T., Duan W., Soroosh P. The significance of OX40 and OX40L to T-Cell biology and immune disease. Immunol. Rev. 2009;229(1):173–191. doi: 10.1111/j.1600-065X.2009.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbandi A., Asadi A., Ghanavati R., Afifirad R., Darb Emamie A., kakanj M., Talebi M. The effect of probiotics on respiratory tract infection with special emphasis on COVID-19–Systemic review 2010–20. Int. J. Infect. Dis. 2021;105:91–104. doi: 10.1016/j.ijid.2021.02.011. from https://www.sciencedirect.com/science/article/pii/S1201971221000977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashdorj N.J., Dashdorj N.D., Mishra M., Danzig L., Briese T., Lipkin W.I., Mishra N. Molecular and serological investigation of the 2021 COVID-19 case surge in mongolian vaccines. MedRxiv. 2021 doi: 10.1101/2021.08.11.21261915. 2021.08.11.21261915, from http://medrxiv.org/content/early/2021/08/13/2021.08.11.21261915.abstractJanuary 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Data, N.B., US20140369938 - Curcumin coated magnetite, p. 108270, 2021.
- Dhama K., Sachan S., Khandia R., Munjal A., Iqbal H.M.N., Latheef S.K., Karthik K., Samad H.A., Tiwari R., Dadar M. Medicinal and beneficial health applications of Tinospora Cordifolia (Guduchi)–A miraculous herb countering various diseases/disorders and its immunomodulatory effects. Recent Pat. Endocr. Metab. Immune Drug Discov. 2017;10(2):96–111. doi: 10.2174/1872214811666170301105101. [DOI] [PubMed] [Google Scholar]
- Dimmito M.P., Stefanucci A., Valle A.Della, Scioli G., Cichelli A., Mollica A. An overview on plants cannabinoids endorsed with cardiovascular effects. Biomed. Pharmacother. 2021;142 doi: 10.1016/j.biopha.2021.111963. from https://www.sciencedirect.com/science/article/pii/S0753332221007459. [DOI] [PubMed] [Google Scholar]
- Djomkam A.L.Z., Olwal C.O., Sala T.B., Paemka L. Commentary–SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Front. Oncol. 2020;10(5):1–5. doi: 10.3389/fonc.2020.01448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X., Shi L., Cao W., Zuo B., Zhou A. Add-on effect of chinese herbal medicine in the treatment of mild to moderate COVID-19–A systematic review and meta-analysis. PLoS One. 20, 2021;16(8) doi: 10.1371/journal.pone.0256429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijk L.E.van, Binkhorst M., Bourgonje A.R., Offringa A.K., Mulder D.J., Bos E.M., Kolundzic N., et al. COVID-19–Immunopathology, pathophysiological mechanisms, and treatment options. J. Pathol. 2021;254(4):307–331. doi: 10.1002/path.5642. from 10.1002/path.5642 July 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang B., Zhang W., Wu X., Huang T., Li H., Zheng Y., Che J., et al. Shenhuang granule in the treatment of severe Coronavirus Disease 2019 (COVID-19)–Study protocol for an open-label randomized controlled clinical trial. Trials. 2020;21(1):568. doi: 10.1186/s13063-020-04498-6. from 10.1186/s13063-020-04498-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora S.De, Balansky R., Maestra S.La. Rationale for the use of N-acetylcysteine in both prevention and adjuvant therapy of COVID-19. FASEB J. 2020;34(10):13185–13193. doi: 10.1096/fj.202001807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowdar K., Chen H., He Z., Zhang J., Zhong X., Zhang J., Li M., Bai J. The effect of N-acetylcysteine on exacerbations of chronic obstructive pulmonary disease–A meta-analysis and systematic review. Heart Lung. 2017;46(2):120–128. doi: 10.1016/j.hrtlng.2016.12.004. [DOI] [PubMed] [Google Scholar]
- Gao L.qin, Xu J., Chen S.dong. In silico screening of potential chinese herbal medicine against COVID-19 by targeting SARS-CoV-2 3CLpro and angiotensin converting enzyme II using molecular docking. Chin. J. Integr. Med. 2020;26(7):527–532. doi: 10.1007/s11655-020-3476-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheware A., Dholakia D., Kannan S., Panda L., Rani R., Pattnaik B.R., Jain V., et al. Adhatoda vasica attenuates inflammatory and hypoxic responses in preclinical mouse models–Potential for repurposing in COVID-19-like conditions. Respir. Res. 2021;22(1):99. doi: 10.1186/s12931-021-01698-9. from 10.1186/s12931-021-01698-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomaa A.A., Abdel-Wadood Y.A. The potential of glycyrrhizin and licorice extract in combating COVID-19 and associated conditions. Phytomed. Plus. 2021;1(3) doi: 10.1016/j.phyplu.2021.100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez, A., Mongolian milk vetch (Hu Ang z Hi), pp. 1–5, 2021.
- Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L., Bhattoa H.P. Evidence that Vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):1–19. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui Y., Sun L., Liu R., Luo J. Pachymic acid inhibits inflammation and cell apoptosis in lipopolysaccharide (LPS)-induced rat model with pneumonia by regulating NF-ΚB and MAPK pathways. Allergol. Immunopathol. 2021;49(5):87–93. doi: 10.15586/aei.v49i5.468. [DOI] [PubMed] [Google Scholar]
- Gurley B.J., Fifer E.K., Gardner Z. Pharmacokinetic herb-drug interactions (Part 2)–Drug interactions involving popular botanical dietary supplements and their clinical relevance. Planta Med. 2012;78(13):1490–1514. doi: 10.1055/s-0031-1298331. [DOI] [PubMed] [Google Scholar]
- Gutiérrez S., Svahn S.L., Johansson M.E. Effects of omega-3 fatty acids on immune cells. Int. J. Mol. Sci. 2019;20(20):5028. doi: 10.3390/ijms20205028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Q., Dong B.R., Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst. Rev. 2015;(2) doi: 10.1002/14651858.CD006895.pub3. [DOI] [PubMed] [Google Scholar]
- Haridas M., Sasidhar V., Nath P., Abhithaj J., Sabu A., Rammanohar P. Compounds of citrus medica and zingiber officinale for COVID-19 inhibition–In silico evidence for cues from ayurveda. Futur. J. Pharma. Sci. 2021;7(1):13. doi: 10.1186/s43094-020-00171-6. from https://pubmed.ncbi.nlm.nih.gov/33457429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway D., Pandav K., Patel M., Riva-Moscoso A., Singh B.M., Patel A., Min Z.C., et al. Omega 3 fatty acids and COVID-19–A comprehensive review. Infect. Chemother. 2020;52(4):478–495. doi: 10.3947/ic.2020.52.4.478. from https://pubmed.ncbi.nlm.nih.gov/33377319 December. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins J., Baker C., Cherry L., Dunne E. Black Elderberry (Sambucus Nigra) supplementation effectively treats upper respiratory symptoms–A meta-analysis of randomized, controlled clinical trials. Complement. Ther. Med. 2019;42:361–365. doi: 10.1016/j.ctim.2018.12.004. [DOI] [PubMed] [Google Scholar]
- Hemilä H., Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst. Rev. 2013;2013(1) doi: 10.1002/14651858.CD000980.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemilä H., Man A.M.E. Vitamin C and COVID-19. Front. Med. 2021 doi: 10.3389/fmed.2020.559811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgenfeld R. From SARS to MERS –Crystallographic studies on coronaviral proteases enable antiviral drug design. FEBS J. 2014;281(1):4085–4096. doi: 10.1111/febs.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T.-.Y., Wu S.-.L., Chen J.-.C., Li C.-.C., Hsiang C.-.Y. Emodin blocks the SARS Coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antiviral Res. 2007;74(2):92–101. doi: 10.1016/j.antiviral.2006.04.014. from https://www.sciencedirect.com/science/article/pii/S0166354206001215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holford P., Carr A.C., Jovic T.H., Ali S.R., Whitaker I.S., Marik P.E., Smith A.D. Vitamin C-An adjunctive therapy for respiratory infection, sepsis and COVID-19. Nutrients. 2020;12(12) doi: 10.3390/nu12123760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao L., Li Zang, Fan G. Clinical features of patients infected with 2019 novel Coronavirus in Wuhan, China. Lancet N. Am. Ed. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Tao G., Liu J., Cai J., Huang Z., Chen J. Current prevention of COVID-19–Natural products and herbal medicine. Front. Pharmacol. 2020 doi: 10.3389/fphar.2020.588508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iddir M., Brito A., Dingeo G., Fernandez Del Campo S.S., Samouda H., Frano M.R.La, Bohn T. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition–Considerations during the COVID-19 crisis. Nutrients. May 2020;12(6) doi: 10.3390/nu12061562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iengar P. An analysis of substitution, deletion and insertion mutations in cancer genes. Nucleic Acids Res. 2012;40(14):6401–6413. doi: 10.1093/nar/gks290. from https://pubmed.ncbi.nlm.nih.gov/22492711August. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infusino F., Marazzato M., Mancone M., Fedele F., Mastroianni C.M., Severino P., Ceccarelli G., Santinelli L., Cavarretta E., Marullo A.G.M., Miraldi F., Carnevale R., Nocella C., Biondi-Zoccai G., et al. Diet supplementation, probiotics, and nutraceuticals in SARS-CoV-2 infection–A scoping review. Nutrients. 2020;12(6):1718. doi: 10.3390/nu12061718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbrucker R.A., Burdock G.A. Risk and safety assessment on the consumption of licorice root (Glycyrrhiza Sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regul. Toxicol. Pharmacol. 2006;46(3):167–192. doi: 10.1016/j.yrtph.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Islam M.T., Sarkar C., El-Kersh D.M., Jamaddar S., Uddin S.J., Shilpi J.A., Mubarak M.S. Natural products and their derivatives against Coronavirus–A review of the non-clinical and pre-clinical data. Phytother. Res. 2020;34(10):2471–2492. doi: 10.1002/ptr.6700. [DOI] [PubMed] [Google Scholar]
- Jalali A., Dabaghian F., Akbrialiabad H., Foroughinia F., Zarshenas M.M. A pharmacology-based comprehensive review on medicinal plants and phytoactive constituents possibly effective in the management of COVID-19. Phytother. Res. 2021;35(4):1925–1938. doi: 10.1002/ptr.6936. [DOI] [PubMed] [Google Scholar]
- Janairo G.I.B., Yu D.E.C., Janairo J.I.B. A machine learning regression model for the screening and design of potential SARS-CoV-2 protease inhibitors. Netw. Model. Anal. Health Inform. Bioinform. 2021;10(1):51. doi: 10.1007/s13721-021-00326-2. from https://pubmed.ncbi.nlm.nih.gov/34336544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawad M., Schoop R., Suter A., Klein P., Eccles R. Safety and efficacy profile of echinacea purpurea to prevent common cold episodes–A randomized, double-blind, placebo-controlled trial. Evid. Based Complement. Altern. Med. 2012;2012 doi: 10.1155/2012/841315. from 10.1155/2012/841315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo S., Kim S., Shin D.H., Kim M. Inhibition of SARS-CoV 3CL protease by flavonoids. J. Enzyme Inhib. Med. Chem. 2019;35(1):145–151. doi: 10.1080/14756366.2019.1690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpe Y.A., Lole K.S. NTPase and 5 Ј to 3 Ј RNA duplex-unwinding activities of the hepatitis E virus helicase domain. J. Virol. 2010;84(7):3595–3602. doi: 10.1128/JVI.02130-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna K., Kohli S.K., Kaur R., Bhardwaj A., Bhardwaj V., Ohri P., Sharma A., Ahmad A., Bhardwaj R., Ahmad P. Herbal immune-boosters–Substantial warriors of pandemic COVID-19 battle. Phytomed. 2021;85 doi: 10.1016/j.phymed.2020.153361. from https://pubmed.ncbi.nlm.nih.gov/33485605May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotecha V. Ayurveda research publications–A serious concern. AYU. 2015;36(1):1. doi: 10.4103/0974-8520.169004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulgi S., Jani V., Uppuladinne M., Sonavane U., Nath A.K. Drug repurposing studies targeting SARS-CoV-2 –An ensemble docking approach on drug target 3C-like protease (3CL Pro) J. Biomol. Struct. Dyn. 2020:1–21. doi: 10.1080/07391102.2020.1792344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn J.H., Li W., Choe H., Farzan M. Angiotensin-converting enzyme 2–A functional receptor for SARS Coronavirus. Cell. Mol. Life Sci. 2004;61(21):2738–2743. doi: 10.1007/s00018-004-4242-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar Abhimanyu, Prasad G., Srivastav S., Gautam V.K., Sharma N. A retrospective study on efficacy and safety of Guduchi Ghan Vati for COVID-19 asymptomatic patients. MedRxiv. 2020 doi: 10.1101/2020.07.23.20160424. p. 2020.07.23.20160424, from http://medrxiv.org/content/early/2020/07/29/2020.07.23.20160424.abstract January 1. [DOI] [Google Scholar]
- Kumar Amit, Kubota Y., Chernov M., Kasuya H. Potential role of zinc supplementation in prophylaxis and treatment of COVID-19. Med. Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.109848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Coronavirus Disease-2019 (COVID-19)–The epidemic and the challenges. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.E., Ju E.M., Kim J.H. Free radical scavenging and antioxidant enzyme fortifying activities of extracts from smilax China root. Exp. Mol. Med. 2001;33(4):263–268. doi: 10.1038/emm.2001.43. [DOI] [PubMed] [Google Scholar]
- Lee W.S., Rhee D.-.K. Corona-Cov-2 (COVID-19) and ginseng–Comparison of possible use in COVID-19 and influenza. J. Ginseng Res. 2021;45(4):535–537. doi: 10.1016/j.jgr.2020.12.005. from https://www.sciencedirect.com/science/article/pii/S1226845320301883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtoranta L., Pitkäranta A., Korpela R. Probiotics in respiratory virus infections. Eur. J. Clin. Microbiol. Infect. Dis. 2014;33(8):1289–1302. doi: 10.1007/s10096-014-2086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. Structure, function, and evolution of Coronavirus spike proteins. Annu. Rev. Virol. 2016;3:237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das Lipi, Eshani Bhaumik U.R., Chakraborty R. Role of nutraceuticals in human health. J. Food Sci. Technol. 2012;49(2):173–183. doi: 10.1007/s13197-011-0269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi, G. and Plebani, M., Our privacy with COVID-2019 laboratory abnormalities infection, vol. 58, no. Cclm, pp. 1–36, 2021. [DOI] [PubMed]
- Liu J., Nile S.H., Xu G., Wang Y., Kai G. Systematic exploration of astragalus membranaceus and panax ginseng as immune regulators–Insights from the comparative biological and computational analysis. Phytomedicine. 2021;86 doi: 10.1016/j.phymed.2019.153077. [DOI] [PubMed] [Google Scholar]
- Lordan R., Rando H.M., Greene C.S. Dietary supplements and nutraceuticals under investigation for COVID-19 prevention and treatment. MSystems. 2021;6(3) doi: 10.1128/msystems.00122-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lordan R., Rando H.M., Greene C.S. Dietary supplements and nutraceuticals under investigation for COVID-19 prevention and treatment. MSystems. 2021 doi: 10.1128/mSystems.00122-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louca P., Murray B., Klaser K., Graham M.S., Mazidi M., Leeming E.R., Thompson E., et al. Modest effects of dietary supplements during the COVID-19 pandemic–Insights from 445 850 users of the COVID-19 symptom study app. BMJ Nutr. Prev. Health. 2021;4(1) doi: 10.1136/BMJNPH-2021-000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Zhao W., Liu W.-.H., Sun T., Lou H., Wei T., Hung W.-.L., Chen Q. Safety evaluation of Bifidobacterium Lactis BL-99 and Lacticaseibacillus Paracasei K56 and ET-22 in vitro and in vivo. Front. Microbiol. 2021 doi: 10.3389/fmicb.2021.686541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamedov N.A., Egamberdieva D. Phytochemical constituents and pharmacological effects of licorice–A review. Plant Hum. Health. 2019;3:1–21. doi: 10.1007/978-3-030-04408-4_1. [DOI] [Google Scholar]
- Mancuso C., Santangelo R. Panax Ginseng and Panax Quinquefolius–From pharmacology to toxicology. Food Chem. Toxicol. 2017;107(Pt A):362–372. doi: 10.1016/j.fct.2017.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau A.R., Jolliffe D.A., Hooper R.L., Greenberg L., Aloia J.F., Bergman P., Dubnov-Raz G., et al. Vitamin D supplementation to prevent acute respiratory tract infections–Systematic review and meta-analysis of individual participant data. BMJ. 2017;356 doi: 10.1136/BMJ.I6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S., Taguchi F. Receptor-induced conformational changes of murine Coronavirus spike protein. J. Virol. 2002;76(23):11819–11826. doi: 10.1128/jvi.76.23.11819-11826.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell F. Vitamin-D and COVID-19–Do deficient risk a poorer outcome? Lancet Diabetes Endocrinol. 2020;8(7):570. doi: 10.1016/S2213-8587(20)30183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody V., Ho J., Wills S., Mawri A., Lawson L., Ebert M.C.C.J.C., Fortin G.M., Rayalam S., Taval S. Identification of 3-chymotrypsin like protease (3CLPro) inhibitors as potential Anti-SARS-CoV-2 agents. Commun. Biol. 2021;4(1):93. doi: 10.1038/s42003-020-01577-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortaz E., Tabarsi P., Varahram M., Folkerts G., Adcock I.M. The immune response and immunopathology of COVID-19. Front. Immunol. 2020;11(August):1–9. doi: 10.3389/fimmu.2020.02037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavizadeh L., Ghasemi S. Genotype and phenotype of COVID-19–Their roles in pathogenesis. J. Microbiol. Immunol. Infect. 2021;54(2):159–163. doi: 10.1016/j.jmii.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagoor Meeran M.F., Javed H., Sharma C., Goyal S.N., Kumar S., Jha N.K., Ojha S. Can echinacea be a potential candidate to target immunity, inflammation, and infection - The trinity of Coronavirus Disease 2019. Heliyon. 2021;7(2):e05990. doi: 10.1016/j.heliyon.2021.e05990. from https://www.sciencedirect.com/science/article/pii/S2405844021000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugraha R.V., Ridwansyah H., Ghozali M., Khairani A.F., Atik N. Traditional herbal medicine candidates as complementary treatments for COVID-19–A review of their mechanisms, pros and cons. Evid. Based Complement. Altern. Med. 2020;2020 doi: 10.1155/2020/2560645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaimat A.N., Aolymat I., Al-Holy M., Ayyash M., Abu Ghoush M., Al-Nabulsi A.A., Osaili T., Apostolopoulos V., Liu S.-.Q., Shah N.P. The potential application of probiotics and prebiotics for the prevention and treatment of COVID-19. NPJ Sci. Food. 2020;4:17. doi: 10.1038/s41538-020-00078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omokhua-Uyi A.G., Staden J.Van. Natural product remedies for COVID-19–A focus on safety. S. Afr. J. Bot. July 2021;139:386–398. doi: 10.1016/j.sajb.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orhan I.E., Senol Deniz F.S. Natural products as potential leads against Coronaviruses–Could they be encouraging structural models against SARS-CoV-2? Nat. Prod. Bioprospect. 2020;10(4):171–186. doi: 10.1007/s13659-020-00250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11(1):1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorino G., Cornara L., Soares S., Rodrigues F., Oliveira M.B.P.P. Liquorice (Glycyrrhiza Glabra)–A Phytochemical and pharmacological review. Phytother. Res. 2018;32(12):2323–2339. doi: 10.1002/ptr.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R.S., Bode R.F. A review of the antiviral properties of black elder (Sambucus Nigra L.) products. Phytother. Res. 2017;31(4):533–554. doi: 10.1002/ptr.5782. [DOI] [PubMed] [Google Scholar]
- Prasad A.S. Lessons learned from experimental human model of zinc deficiency. J. Immunol. Res. 2020;2020 doi: 10.1155/2020/9207279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi A., Samimagham H.R., Azad M.H., Hooshyar D., Arabi M., KazemiJahromi M. The efficacy of N-acetylcysteine in severe COVID-19 patients–A structured summary of a study protocol for a randomized controlled trial. Trials. 2021;22(1):271. doi: 10.1186/s13063-021-05242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattis B.A.C., Ramos S.G., Celes M.R.N. Curcumin as a potential treatment for COVID-19. Front. Pharmacol. 2021 doi: 10.3389/fphar.2021.675287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravishankar B., Shukla V.J. Indian systems of medicine–A brief profile. Afr. J. Tradit. Complement. Altern. Med. 2007;4(3):319–337. doi: 10.4314/ajtcam.v4i3.31226. from https://pubmed.ncbi.nlm.nih.gov/20161896 February 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddi K.K., Tetali S.D. Dry leaf extracts of Tinospora Cordifolia (Willd.) Miers attenuate oxidative stress and inflammatory condition in human monocytic (THP-1) cells. Phytomed. 2019;61 doi: 10.1016/j.phymed.2019.152831. [DOI] [PubMed] [Google Scholar]
- Ren J.-.L., Zhang A.-.H., Wang X.-.J. Traditional Chinese medicine for COVID-19 treatment. Pharmacol. Res. 2020 doi: 10.1016/j.phrs.2020.104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronan L., null M.R.H., null S., G.C., A G.J. Dietary supplements and nutraceuticals under investigation for COVID-19 prevention and treatment. MSystems. 2021;6(3) doi: 10.1128/mSystems.00122-21. e00122-21from 10.1128/mSystems.00122-21 November 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savant S., Srinivasan S., Kruthiventi A.K. Potential nutraceuticals for COVID-19. Nutr. Diet. Suppl. 2021;13:25–51. doi: 10.2147/nds.s294231. [DOI] [Google Scholar]
- Sharifi-Rad M., Mnayer D., Morais-Braga M.F.B., Carneiro J.N.P., Bezerra C.F., Coutinho H.D.M., Salehi B., et al. Echinacea plants as antioxidant and antibacterial agents–From traditional medicine to biotechnological applications. Phytother. Res. 2018;32(9):1653–1663. doi: 10.1002/ptr.6101. [DOI] [PubMed] [Google Scholar]
- Shree P., Mishra P., Selvaraj C., Singh S.K., Chaube R., Garg N., Tripathi Y.B. Targeting COVID-19 (SARS-CoV-2) main protease through active phytochemicals of ayurvedic medicinal plants - Withania Somnifera (Ashwagandha), Tinospora Cordifolia (Giloy) and Ocimum Sanctum (Tulsi)–A molecular docking study. J. Biomol. Struct. Dyn. 2020:1–14. doi: 10.1080/07391102.2020.1810778. from https://pubmed.ncbi.nlm.nih.gov/32851919August 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira D., Prieto-Garcia J.M., Boylan F., Estrada O., Fonseca-Bazzo Y.M., Jamal C.M., Magalhães P.O., Pereira E.O., Tomczyk M., Heinrich M. COVID-19–Is there evidence for the use of herbal medicines as adjuvant symptomatic therapy? Front. Pharmacol. 2020 doi: 10.3389/fphar.2020.581840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simopoulos A.P. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients. 2016;8(3):128. doi: 10.3390/nu8030128. from https://pubmed.ncbi.nlm.nih.gov/26950145 March 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims A.C., Baric R.S., Yount B., Burkett S.E., Collins P.L., Pickles R.J. Severe acute respiratory syndrome Coronavirus infection of human ciliated airway epithelia–Role of ciliated cells in viral spread in the conducting airways of the lungs. J. Virol. 2005;79(24):15511–15524. doi: 10.1128/JVI.79.24.15511-15524.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Şimşek Yavuz S., Ünal S. Antiviral treatment of COVID-19. Turk. J. Med. Sci. 2020;50(SI–1):611–619. doi: 10.3906/sag-2004-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K., Rao A. Probiotics–A potential immunomodulator in COVID-19 infection management. Nutr. Res. 2021;87:1–12. doi: 10.1016/j.nutres.2020.12.014. from https://www.sciencedirect.com/science/article/pii/S0271531720305984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R.S., Singh A., Kaur H., Batra G., Sarma P., Kaur H., Bhattacharyya A., et al. Promising traditional indian medicinal plants for the management of novel Coronavirus Disease–A systematic review. Phytother. Res. 2021;35(8):4456–4484. doi: 10.1002/ptr.7150. from https://pubmed.ncbi.nlm.nih.gov/34132429August. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Zhang L., Xu Y., Yang D., Zhang L., Yang S., Zhang W., et al. The comprehensive study on the therapeutic effects of Baicalein for the treatment of COVID-19 in vivo and in vitro. Biochem. Pharmacol. 2021;183 doi: 10.1016/j.bcp.2020.114302. from https://www.sciencedirect.com/science/article/pii/S0006295220305384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia F., Romano M., Ruggiero A., Maga G., Berisio R. Host DDX helicases as possible SARS-CoV-2 proviral factors–A structural overview of their hijacking through multiple viral proteins. Front. Chem. 2020 doi: 10.3389/fchem.2020.602162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subedi L., Tchen S., Gaire B.P., Hu B., Hu K. Adjunctive nutraceutical therapies for COVID-19. Int. J. Mol. Sci. 2021;22(4) doi: 10.3390/ijms22041963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakolpour S., Rakhshandehroo T., Wei E.X., Rashidian M. Lymphopenia during the COVID-19 Infection–What it shows and what can be learned. Immunol. Lett. 2020;225:31–32. doi: 10.1016/j.imlet.2020.06.013. September. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S., Patel D., Bittel B., Wolski K., Wang Q., Kumar A., Il'Giovine Z.J., et al. Effect of high-dose zinc and ascorbic acid supplementation vs. usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection–The COVID A to Z randomized clinical trial. JAMA Netw. Open. 2021;4(2):e210369. doi: 10.1001/jamanetworkopen.2021.0369. from 10.1001/jamanetworkopen.2021.0369February 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai K.-.C., Huang Y.-.C., Liaw C.-.C., Tsai C.-.I., Chiou C.-.T., Lin C.-.J., Wei W.-.C., et al. A traditional Chinese medicine formula NRICM101 to target COVID-19 through multiple pathways–A bedside-to-bench study. Biomed. Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.111037. from https://www.sciencedirect.com/science/article/pii/S0753332220312294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbricht C., Basch E., Cheung L., Goldberg H., Hammerness P., Isaac R., Khalsa K.P.S., et al. An evidence-based systematic review of elderberry and elderflower (Sambucus Nigra) by the natural standard research collaboration. J. Diet. Suppl. 2014;11(1):80–120. doi: 10.3109/19390211.2013.859852. March. [DOI] [PubMed] [Google Scholar]
- V'kovski P., Kratzel A., Steiner S., Stalder H., Thiel V. Coronavirus biology and replication–Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021;19(3):155–170. doi: 10.1038/s41579-020-00468-6. from 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace T.C., Reider C., Fulgoni V.L. Calcium and Vitamin D disparities are related to gender, age, race, household income level, and weight classification but not vegetarian status in the United States–Analysis of the NHANES 2001-2008 data set. J. Am. Coll. Nutr. 2013;32(5):321–330. doi: 10.1080/07315724.2013.839905. October. [DOI] [PubMed] [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292. doi: 10.1016/j.cell.2020.02.058. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S., Yi Q., Fan S., Lv J., Zhang X., Guo L., Lang C., et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 Novel Coronavirus Pneumonia (NCP) MedRxiv. 2020:1–5. doi: 10.1101/2020.02.10.20021832. [DOI] [Google Scholar]
- Wang H., Xu B., Zhang Y., Duan Y., Gao R., He H., Li X., Li J. Efficacy and safety of traditional Chinese medicine in Coronavirus Disease 2019 (COVID-19)–A systematic review and meta-analysis. Front. Pharmacol. 2021 doi: 10.3389/fphar.2021.609213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels I., Maywald M., Rink L. Zinc as a gatekeeper of immune function. Nutrients. 2017;9(12) doi: 10.3390/nu9121286. November. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels I., Rolles B., Rink L. The potential impact of zinc supplementation on COVID-19 pathogenesis. Front. Immunol. 2020;11(July):1–11. doi: 10.3389/fimmu.2020.01712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland L.S., Piechotta V., Feinberg T., Ludeman E., Hutton B., Kanji S., Seely D., Garritty C. Elderberry for prevention and treatment of viral respiratory illnesses–A systematic review. BMC Complement. Med. Ther. 2021;21(1):112. doi: 10.1186/s12906-021-03283-5. April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; 2021. COVID-19 Weekly Epidemiological Update 22; pp. 1–3. [Google Scholar]
- Wu C.-.Y., Jan J.-.T., Ma S.-.H., Kuo C.-.J., Juan H.-.F., Cheng Y.-S.E., Hsu H.-.H., et al. Proceedings of the National Academy of Sciences of the United States of America. Vol. 101. 2004. Small molecules targeting severe acute respiratory syndrome human Coronavirus; pp. 10012–10017. July. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Li Z., Zhao Y., Huang Y., Jiang M., Luo H. Therapeutic targets and potential agents for the treatment of COVID-19. Med. Res. Rev. 2021;(December):1–23. doi: 10.1002/med.21776. [DOI] [PubMed] [Google Scholar]
- Xia S., Zhu Y., Liu M., Lan Q., Xu W., Wu Y., Ying T., et al. Fusion mechanism of 2019-NCoV and fusion inhibitors targeting HR1 domain in spike protein. Cell. Mol. Immunol. 2020;17(7):765–767. doi: 10.1038/s41423-020-0374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, Y.-.M., Shen, X., Cao, Y.-.K., Zhang, J.-.J., Wang, Y., and Cheng, Y.-.X., Discovery of Anti-2019-NCoV agents from Chinese patent drugs via docking screening<Strong> </Strong>, 2020.
- Yang L., Gou J., Gao J., Huang L., Zhu Z., Ji S., Liu H., Xing L., Yao M., Zhang Y. Immune characteristics of severe and critical COVID-19 patients. Signal Transduct. Target. Ther. 2020;5(1) doi: 10.1038/s41392-020-00296-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Liu S., Liu J., Zhang Z., Wan X., Huang B., Chen Y., Zhang Y. COVID-19–Immunopathogenesis and immunotherapeutics. Signal Transduct. Target. Ther. 2020;5(1):1–8. doi: 10.1038/s41392-020-00243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Yang, Islam M.S., Wang J., Li Y., Chen X. Traditional Chinese medicine in the treatment of patients infected with 2019-new Coronavirus (SARS-CoV-2)–A review and perspective. Int. J. Biol. Sci. 2020;16(10):1708–1717. doi: 10.7150/ijbs.45538. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Yichang. Use of herbal drugs to treat COVID-19 should be with caution. Lancet N. Am. Ed. 2020;395(10238):1689–1690. doi: 10.1016/S0140-6736(20)31143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yisak H., Ewunetei A., Kefale B., Mamuye M., Teshome F., Ambaw B., Yideg Yitbarek G. Effects of Vitamin D on COVID-19 infection and prognosis–A systematic review. Risk Manag. Healthc. Policy. 2021;14:31–38. doi: 10.2147/RMHP.S291584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Zhou X., Zhu C., Song Y., Feng F., Qiu Y., Feng J., et al. Immune phenotyping based on the Neutrophil-to-Lymphocyte ratio and IgG level predicts disease severity and outcome for patients with COVID-19. Front. Mol. Biosci. 2020;7(July):1–7. doi: 10.3389/fmolb.2020.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., M P.J., L Y., N Z., A S. Angiotensin ‑ Converting Enzyme 2 (ACE2) as a SARS-CoV-2 receptor–Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Xiang R., Huo S., Zhou Y., Jiang S., Wang Q., Yu F. Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy. Signal Transduct. Target. Ther. 2021;6(1):233. doi: 10.1038/s41392-021-00653-w. from 10.1038/s41392-021-00653-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Yang J., Cui M.Y., Liu J., Fang Y., Yan M., Qiu W., et al. The reference genome sequence of Scutellaria Baicalensis provides insights into the evolution of wogonin biosynthesis. Mol. Plant. 2019;12(7):935–950. doi: 10.1016/j.molp.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Zhou P., Yang X., Wang X., Hu B., Zhang L., Zhang W., Guo H., et al. A pneumonia outbreak associated with a new Coronavirus of probable bat origin. Nature. 2020;579(1):270–280. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Fu B., Zheng X., Wang D., Zhao C., Qi Y., Sun R., Tian Z., Xu X., Wei H. Pathogenic T-Cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl. Sci. Rev. 2020;7(6):998–1002. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.F., Luo B.A., Qin L.L., Shidoji Y. The association between Vitamin D deficiency and community-acquired pneumonia–A meta-analysis of observational studies. Medicine. 2019;98(38) doi: 10.1097/MD.0000000000017252. September. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W., Chen C.Z., Gorshkov K., Xu M., Lo D.C., Zheng W. RNA-Dependent RNA polymerase as a target for COVID-19 drug discovery. SLAS Discov. 2020;25(10):1141–1151. doi: 10.1177/2472555220942123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Nil.