Abstract
The ability to generate episodic details while recollecting autobiographical events is believed to depend on a collection of brain regions that form a posterior medial network (PMN). How age-related differences in episodic detail generation relate to the PMN, however, remains unclear. The present study sought to examine individual differences, and the role of age, in PMN resting state functional connectivity (rsFC) associations with episodic detail generation. Late middle-aged and older adults (N = 41, ages 52–81), and young adults (N = 21, ages 19–35) were asked to describe recent personal events, and these memory narratives were coded for episodic, semantic and ‘miscellaneous’ details. Independent components analysis and regions-of-interest analyses were used to assess rsFC within the PMN separately for anterior connections (hippocampal and medial prefrontal) and posterior connections (hippocampal, parahippocampal and parieto-occipital), as these connections purportedly serve different functional roles in episodic detail generation. Compared to younger adults, older adults produced memory narratives with lower episodic specificity (ratio of episodic:total details) and a greater amount of semantic detail. Among the older adults, episodic detail amounts and episodic specificity were reduced with increasing age. There were no significant age differences in PMN rsFC. Stronger anterior PMN rsFC was related to lower episodic detail in the older adult group, but not in the young. Among the older adults, increasing age brought on an association between increased anterior PMN rsFC and reduced episodic specificity. In contrast, increasing age brought on an association between increased posterior PMN rsFC and increased semantic detail. The present study provides evidence that functional connectivity within the PMN, particularly anterior PMN, tracks individual differences in the amount of episodic details retrieved by older adults. Furthermore, these brain-behavior relationships appear to be age-specific, indicating that some process within aging alters the nature of how anterior PMN rsFC and episodic detail relate to each other. Whether this process entails an age-related loss of integrity to the PMN, or an age-related shift toward semantic retrieval, remains to be determined.
Keywords: autobiographical memory, episodic memory, fMRI, functional connectivity, aging
1. Introduction
A key function of the autobiographical memory system is the retrieval of specific past personal events. When a specific autobiographical event is recalled, a variety of information can be brought to mind, including general semantics (i.e., knowledge about the world), personal semantics (i.e., knowledge about one’s self) and episodic details (i.e., perceptual, emotional and other elements specific to a spatio-temporally defined event) (Conway, 2009). Episodic details provide fine-grain information about what transpired in past events, and they may relate to phenomenological aspects (e.g. memory vividness) of recollection (Moscovitch et al., 2005). Episodic detail generation may also contribute towards various other cognitive functions related to episodic memory processes, such as decision-making (Lempert et al., 2020), imagining novel events (Addis, Wong & Schacter, 2008), and problem solving (Madore & Schacter, 2015; Peters, Fan & Sheldon, 2019).
In the present study, we build on recent work showing that the amount of episodic information an individual is generally capable of accessing during autobiographical recollection can be mapped to differences in the integrity of brain regions that support episodic detail generation (Ahmed et al., 2018; Irish et al., 2014; Esopenko & Levine, 2017; Palombo et al., 2018; Hodgetts et al., 2017; Memel et al., 2020; Sheldon et al., 2016). In particular, we assess the relationship between individual differences in episodic detail generation and resting state functional connectivity in a brain network that has emerged as critical for autobiographical memory (Ranganath and Ritchey, 2012).
1.1. Relationship between episodic detail generation and an MTL-neocortical network
A network of medial temporal lobe (MTL) and neocortical regions are believed to support the generation of episodic details during the recollection of an autobiographical event. According to several theories, the hippocampus is at the center of this network (Ranganath and Ritchey, 2012; Sekeres, Winocur, and Moscovitch, 2018; Sheldon & Levine, 2016; Addis, 2020), and is supplied input via extra-hippocampal regions of the MTL, including the perirhinal and parahippocampal cortices (Ranganath and Ritchey, 2012; Burke et al., 2018). Case studies of patients with hippocampal and broader MTL damage have demonstrated that the recollection of rich episodic details from autobiographical memories is compromised in the absence of normal hippocampal function (Rosenbaum et al., 2008). Converging evidence can be found in task-based functional magnetic resonance imaging (fMRI) studies, in which episodic autobiographical retrieval has been associated with greater hippocampal activation (for meta-analyses, see Martinelli, Sperduti, & Piolino, 2013 and Svoboda, McKinnon & Levine, 2006).
In addition to the hippocampus, a variety of neocortical regions have been implicated in the retrieval of richly detailed episodic memories (Ranganath & Ritchey, 2012; Moscovitch et al., 2016), including the posterior cingulate, precuneus, lateral parietal cortex, and medial prefrontal cortex (mPFC). Task-based fMRI studies have provided evidence that activation in these neocortical regions is associated with episodic autobiographical retrieval (for meta-analyses, see Svoboda, McKinnon & Levine, 2006, Benoit & Schacter, 2015, and Martinelli, Sperduti & Piolino, 2013), and have furthermore shown that the hippocampus interacts with neocortical regions during autobiographical retrieval for past events (St. Jacques, Kragel & Rubin, 2011; McCormick et al., 2015), as well as simulation of future events (Campbell et al., 2018). Additionally, episodic detail is reduced in cases of lesion damage to the mPFC (Bertossi et al., 2016), medial parietal cortex (Berryhill et al., 2007), and lateral parietal cortex (Berryhill et al., 2007; Davidson et al., 2008). Among healthy adults, transcranial magnetic stimulation of a lateral parietal region, the angular gyrus, induces a decrease in episodic detail generation (Bonnici et al., 2018; Thakral, Madore & Schacter, 2017).
Ranganath and Ritchey (2012) propose that interactions across the MTL, mPFC, and parieto-occipital regions (e.g. posterior cingulate, precuneus, angular gyrus) comprise a network that supports episodic recollection – known as the ‘posterior-medial network’ (PMN). The parieto-occipital components of the PMN and the mPFC may serve distinct roles during the process of episodic recollection. The parieto-occipital regions of this network may help generate scene imagery (McCormick et al., 2018; Barry et al., 2019) and represent the contextual information that characterizes episodic recollection, including perceptual and spatial detail (Gurguryan & Sheldon, 2019; Sekeres, Winocur & Moscovitch, 2018). The mPFC, in contrast, has been suggested to have a number of possible roles that could influence episodic detail generation. Several theories suggest that the mPFC promotes episodic detail generation by mediating access to event features (McCormick et al., 2018; Barry et al., 2019) and sustaining their elaboration during recollection (Ritchey & Cooper, 2020), or by aiding the hippocampus in integrating contextual and item memory (Ritchey, Libby & Ranganath, 2015). Other theories suggest that mPFC involvement can skew episodic recollection towards more schematic representations or schema-guided retrieval of details, when sufficient to fulfill the goals of the recollection situation (Sekeres, Winocur & Moscovitch, 2018). This may enhance the generation of semantic details, possibly with the added effect of dampening episodic detail generation. In summary, engagement of the posterior, parieto-occipital components of the PMN is hypothesized to support episodic detail generation during autobiographical recollection, while there are competing hypotheses as to whether engagement of the mPFC would result in increased or decreased episodic detail generation.
1.2. Evidence for individual differences using structural and functional MRI
A handful of studies have provided evidence that individual differences in episodic detail generation can be mapped to variability in the structural integrity of the PMN. Studies have shown that a greater amount of episodic details generated during autobiographical memory narration is associated with larger volumes in hippocampal (Irish et al., 2014), precuneus (Ahmed et al., 2018), posterior cingulate (Esopenko & Levine, 2017; Irish et al., 2014), and mPFC regions (Irish et al., 2014; Esopenko & Levine, 2017) in clinical populations (e.g. dementia and traumatic brain injury), as well as larger hippocampal subfield volumes in healthy young adults (Palombo et al., 2018). Studies using diffusion tensor imaging have similarly found that increased episodic detail generation is associated with greater white matter integrity, as measured via fractional anisotropy, for tracts connecting hippocampal, mPFC and posterior parietal regions including the cingulum (Irish et al., 2014; Memel et al., 2020), the fornix (Hodgetts et al., 2017; Memel et al., 2020; Williams et al., 2020) and the genu (Irish et al., 2014). Variability in the integrity of PMN regions and its connections, therefore, may partially account for why some individuals describe autobiographical events in richer episodic detail compared to others.
In addition to structural properties, examining functional characteristics of the PMN is important for understanding how this network contributes toward individual differences in the ability to generate episodic details. Current theories propose that information processing within the PMN relies on a complex system of both direct and indirect connections between network nodes (Cooper & Ritchey, 2019; Raganath & Ritchey, 2012; Ramanan, Piguet, & Irish, 2018; Reagh & Ranganath, 2018). Resting state fMRI enables the identification of regions that show synchronized fluctuations in the BOLD signal, capturing ‘intrinsic’, well-established patterns of functional interactions that do not necessarily have direct monosynaptic axonal connections (Greicius et al., 2009). Thus, resting state fMRI can provide us with a means of exploring how individual variability in PMN functional connections might impact the amount of episodic details provided during autobiographical narration tasks that occur outside the scanner. In support of this viewpoint, in a seed based resting state fMRI study of healthy young adults, Sheldon et al. (2016) observed that a greater self-reported tendency to remember events in rich episodic detail was associated with higher resting state functional connectivity (rsFC) between MTL seeds (posterior hippocampi and parahippocampal gyri) and medial parieto-occipital regions such as the posterior cingulate. No such relationship with the episodic detail measure, however, was noted for rsFC between the MTL seeds and the mPFC.
1.3. Understanding normal age-related changes in episodic detail generation
It is well-established that cognitively normal older adults tend to provide fewer episodic details, and more non-episodic details, when recounting autobiographical events relative to young adults (Acevedo-Molina, Matijevic, & Grilli, 2020; Robin & Moscovitch, 2017; Gaesser et al., 2011; St Jacques, & Levine, 2007; Addis, Wong & Schacter, 2008; Levine et al., 2002). There is also evidence to suggest that, among older adults, increasing age is associated with reduced episodic detail (Wank et al., 2020). Alterations to the PMN could contribute toward the age-related decreases in episodic detail generation given the prior evidence indicating that PMN integrity accounts for variability in episodic detail richness across various adult populations, including older adults. For example, Memel et al. (2020) noted, within a sample of older adults, that episodic detail generation was positively associated with fractional anisotropy of tracts connecting regions of the PMN, namely the fornix and cingulum. The potential moderating impact of age on these brain-behavior relationships was not directly tested, however, and thus it is still an open question as to whether age differences in episodic detail generation are related to age-associated modifications to PMN structural or functional integrity.
Exploring how age impacts the relationship between PMN functional connectivity and episodic detail generation could be particularly illuminating for our understanding of age-related episodic detail generation reductions, given the possible complexities of PMN rsFC alterations within aging. The PMN is identified through resting state fMRI as a subcomponent of the broader default network (Andrews-Hanna et al., 2010; Ritchey & Cooper, 2020), which commonly shows reduced rsFC among network regions in older adults (Andrews-Hanna et al., 2007; Damoiseaux et al., 2008; Betzel et al., 2014). However, in a more recent longitudinal study, Staffaroni et al. (2018) noted a non-linear trajectory of rsFC changes within the default network characterized by a trend toward rsFC increases from age 50 – 66 that shifts after age 74 to a significant rsFC decline. There is also evidence of rsFC decreases and increases co-occurring within the default network in aging, with more pronounced rsFC increases in the anterior portion of the default network and decreases in the posterior default network (Jones et al., 2011).
The behavioral consequences of age-related rsFC weakening or strengthening within the default network, and by extension PMN, could be distinct and emerge from different mechanisms (Andrews-Hanna, Smallwood & Spreng, 2014). According to the integrity hypothesis of default network functioning (Andrews-Hanna, Smallwood & Spreng, 2014; Andrews-Hanna, Grilli, & Irish, 2019), weakened rsFC can reflect structural and functional age-related alterations that compromise supported cognitive functions, which may include episodic detail generation during autobiographical narration. Decreased rsFC within the default network, and specifically PMN, would thus correlate with fewer episodic details among older adults. However, Hillary et al. (2015) argues that a compensatory increase in rsFC is the brain’s initial response to neural damage, such as in conditions like traumatic brain injury or mild cognitive impairment. A decline in rsFC is theorized to begin only once a critical loss of neural resources occurs, such as the severe atrophy in Alzheimer’s disease, and compensatory hyperconnectivity can no longer be maintained. With regards to aging, overuse of the default network and PMN “at rest” could thus signal poorer network efficiency, and increased rsFC would correlate to decreased episodic detail generation.
Rather than reflecting a loss of PMN integrity, episodic detail reductions in aging could also result from changes in how individuals engage with autobiographical memory recollection (Andrews-Hanna, Grilli, & Irish, 2019). Older adults may take on a recollection mode that favors schema-based or ‘semanticized’ representations of events over the retrieval of fine grain details, in order to best serve socio-motivational goals that focus on meaning making and emotional regulation (Carstensen, Isaacowitz, & Charles, 1999) and capitalize on a wealth of accumulated schemas and semantic knowledge (Spreng et al., 2018). If this process is occurring within aging, it may be supported by interactions between the hippocampus and mPFC, which are theorized to facilitate schema-driven memory retrieval (Sekeres, Winocur & Moscovitch, 2018; Guo & Yang, 2020). Thus, increased rsFC between the hippocampus and mPFC could relate to decreased episodic detail generation among older adults, but also increased semantic detail generation.
1.4. Present study
In the present study, we explore the questions raised here regarding the association between episodic detail generation and PMN rsFC in relation to normal cognitive aging. We assessed individual differences in autobiographical memory retrieval and rsFC in a group of 41 cognitively normal middle-aged and older adults (ages 52 – 81) and compared the older adult group to 21 young adults (ages 19 – 35). Measures of rsFC for an anterior division of PMN connections (mPFC and hippocampus) and a posterior division of PMN connections (parieto-occipital regions, parahippocampal cortex and hippocampus) were examined for associations with the amount of episodic detail participants generated while recalling autobiographical events in a separate behavioral session, using the Autobiographical Interview (Levine et al., 2002) scoring protocol.
We hypothesized that greater age would be associated with decreased episodic detail and elevated non-episodic (semantic and ‘miscellaneous’) details, both when comparing young and older adults and when analyzing age as a continuous variable within the older adult group. Based on the existing literature, we expected young adults to show greater PMN rsFC compared to the older adults; within the older adult group, we accounted for the possibility that age could have a non-linear effect on PMN rsFC. We considered three possible hypotheses as to brain-behavior relationships within the older adult group: 1) decreased PMN rsFC would be associated with lower episodic detail, resulting from a loss of PMN integrity, 2) increased PMN rsFC would be associated with lower episodic detail, reflecting functional compensation in response to a loss of PMN integrity, and 3) increased rsFC, specifically within mPFC to hippocampus connections, would be associated with lower episodic detail and greater semantic detail, resulting from the ‘semanticization’ of autobiographical recollection. To ascertain whether any of these outcomes within our older adult group were actually related to alterations with age, and not simply a reflection of general age-invariant relationships between PMN rsFC and episodic detail generation, we tested whether age moderated the effects of rsFC on behavior. Specifically, we compared brain-behavior relationships between younger and older adults and tested for an interaction between years of age and rsFC within the older adult group.
2. Methods
2.1. Participants
The sample consisted of 41 cognitively normal, community dwelling older adults (ages 52 – 81) recruited from Tucson, Arizona. Data from 29 of these participants were included in Memel et al. (2020). Additionally, a group of 21 young adults (ages 19 – 35) were recruited from the University of Arizona and Tucson area. Young and older adults did not differ in terms of education or sex (see Table 1). Participants were screened and excluded for neurological disorders, drug/alcohol abuse, psychiatric disorders, traumatic brain injury, and MRI contraindications. All participants scored within normal limits on the Mini Mental Status Exam (Folstein, Folstein & McHugh, 1975). Written, informed consent was obtained in accordance with the guidelines set by the University of Arizona’s institutional review board.
Table 1.
Demographics of the study sample are presented, split by age group.
| Younger adults (n=21) | Older adults (n=41) | |
|---|---|---|
| Age | ||
| Mean (SD) | 26.0 (4.20) | 68.6 (6.99) |
| Min, Max | 19.0, 35.0 | 52.0, 81.0 |
| Years of Education | ||
| Mean (SD) | 17.0 (1.72) | 16.9 (2.28) |
| Sex | ||
| Female | 16 (76.2%) | 29 (70.7%) |
| Male | 5 (23.8%) | 12 (29.3%) |
2.2. Autobiographical Interview
A detailed description of the Autobiographical Interview testing protocol used in this study can be found in Memel et al. (2020), adapted from Levine et al. (2002). Briefly, participants in the older adult group were asked to narrate in detail three memories of specific events from the recent past. An event was retrieved from each of the following time periods: 5 years to 1 year prior to the experiment, 1 year to 1 week prior to the experiment, and the week prior to the experiment (excluding the day of the experiment). A subset of participants (n = 20) also described three remote events, but these data were not included in the present study. Participants were given a limit of five minutes to describe each memory.
The instructions given to the younger adult participants partially differed in terms of the time periods they were asked to retrieve events from. Like the older adults, younger adults retrieved an event from 1 year to 1 week prior to the experiment, and an event from the week prior to the experiment. As an event 5 years to 1 year prior to the experiment would qualify as a childhood memory for some younger adults, the younger adult participants were simply asked to retrieve an event from childhood. All other instructions were equivalent to those received by the older adults. To more closely match the older adult data, details for only the two recent event memories were analyzed for the younger adults.
Memory narratives were recorded, transcribed, and scored for the types of details provided. In accordance with the original Autobiographical Interview scoring guidelines (Levine et al., 2002), we coded for ‘internal’ details that occurred during or characterized the specific event recalled. We subsequently refer to these ‘internal’ details as episodic details. The episodic detail category consisted of five sub-categories – event details (i.e. what occurred during the event), time details, place details, perceptual details, and thought/emotion details. We also coded for semantic details, which included autobiographical knowledge and general world knowledge. Repeated information, details pertaining to other events, and metacommentary were grouped together into a ‘miscellaneous’ category. The memories were scored by one trained rater who achieved excellent reliability with a secondary rater who scored 20 of the participants, as well as expert scorers from the Autobiographical Interview training materials, for the three detail types (i.e., episodic, semantic, and miscellaneous) and each of the sub-types (e.g., event details, time details) (Cronbach alpha’s ≥ .90). For each participant, the number of details generated across the three memories (two, for the young adults) were averaged by type. Episodic specificity was calculated as the ratio of episodic details to total details generated, which accounts for differences in narrative length.
2.3. MRI Acquisition and Pre-processing
MRI data were acquired with a 3.0 T Siemens Skyra scanner equipped with a 32 channel head coil. High-resolution, 3D T1-weighted (T1w) images was collected using an MPRAGE sequence with the following parameters: TR = 2300 ms, TE = 2.95 ms, FOV = 270 mm, slice thickness = 1 mm, in-plane resolution = 1.1 × 1.1 mm. Participants were asked to keep their eyes open and fixated on a white crosshair during a 6 minute resting state BOLD fMRI scan, collected with a 2D EPI sequence (45 axial slices, 121 volumes, TR = 3000 ms, TE = 26 ms, FOV = 224 mm, slice thickness = 3 mm, in-plane resolution = 3 × 3 mm).
T1w images underwent manual visual inspection to ensure quality and absence of pathological brain abnormalities. The resting state BOLD time-series were run through MRIQC’s automated quality assessment workflows to derive image quality metrics (Esteban et al., 2017). We excluded data from 2 young and 2 older participants (not included within the demographic reports) due to motion-related quality issues, determined on the basis of MRIQC’s quality metrics and visual inspection. Participants were excluded for exceeding a motion threshold of 0.8 mean framewise displacement, or for re-positioning during the scan such that the FOV coverage did not encompass the entire cerebrum. Mean framewise displacement was significantly greater in the older adult group compared to the young adults (t = −3.17, p = 0.002). Because in-scanner head motion can confound the results of rsFC analyses (Power et al., 2012; Kato et al., 2020), we decided to employ ICA-based Automatic Removal Of Motion Artifacts (ICA-AROMA, Pruim et al., 2015) as part of our denoising procedure (described in full later). ICA-AROMA’s ‘non-aggressive’ denoising strategy detects signal components related to motion and subtracts the signal variance associated with these components from the data, which has been shown to lessen correlations between rsFC and motion measures (Dipasquale et al., 2017).
Anatomical and functional data were pre-processed utilizing fMRIPrep 1.4.0 (Esteban et al., 2019; RRID:SCR_016216), a Nipype 1.2.0 based tool (Gorgolewski et al., 2011; RRID:SCR_002502). FMRIPrep is an automated pre-processing pipeline that flexibly employs tools from a variety of neuroimaging software packages, in order to optimize each pre-processing step. Many internal operations of fMRIPrep use Nilearn (Abraham et al., 2014, RRID:SCR_001362), mostly within the functional processing workflow. For more details, see https://fmriprep.org/en/1.4.0/workflows.html.
Utilizing tools from Advanced Normalization Tools (ANTs 2.2.0), T1w images were corrected for intensity non-uniformity using the N4BiasFieldCorrection algorithm (Tustison et al., 2010) and skull-stripped via a template-based brain extraction procedure (using the OASIS30ANTS template). Spatial normalization of T1w images to MNI space was performed through nonlinear registration with ANTs, using brain-extracted versions of both the T1w images and the ICBM 152 Nonlinear Asymmetrical template version 2009c (Fonov et al., 2009, RRID:SCR_008796). Brain tissue segmentation of cerebrospinal fluid (CSF), white matter and gray matter was performed on the brain-extracted T1w images using FSL’s FAST (FSL 5.0.9, RRID:SCR_002823, Zhang, Brady, & Smith, 2001).
Each participant’s functional data was slice time corrected with AFNI’s 3dTshift (Cox and Hyde, 1997, RRID:SCR_005927) and motion corrected with FSL’s MCFLIRT (Jenkinson et al., 2002). This was followed by co-registration to the participant’s corresponding T1w image using boundary-based registration (Greve & Fischl, 2009) with nine degrees of freedom, via FSL’s FLIRT (Jenkinson and Smith, 2001). Motion correcting transformations, the BOLD-to-T1 transformation and T1-to-template (MNI) warp were concatenated and applied in a single step using antsApplyTransforms (ANTs) with Lanczos interpolation, resulting in the functional data normalized to MNI space.
From the MNI normalized functional data, mean signals were calculated from voxels within the CSF and white matter separately for use as nuisance regressors. Spatial smoothing was applied using an isotropic Gaussian kernel of 6mm FWHM. Following smoothing, ICA-AROMA was performed to remove motion-related noise from the data. Tools from the CONN toolbox 18.a (www.nitrc.org/projects/conn, RRID:SCR_009550; Whitfield-Gabrieli & Nieto-Castanon, 2012) were used to further denoise the MNI normalized, smoothed and ICA-AROMA denoised outputs from fMRIPrep. A band pass filter (0.008 – 0.09 Hz) was applied to the functional data, preceded by linear detrending and nuisance regression of subject-specific measures: mean white matter signal, mean CSF signal and functional volumes that had been identified as non-steady state outliers by fMRIPrep.
2.4. Resting State fMRI ICA and ROI analyses
ICA and region of interest (ROI) correlations were performed using the CONN toolbox. A group ICA was performed on all participant functional data (from both young and older adult groups), with 20 unique components specified as the number of components to generate. From the group ICA output, we identified a component whose spatial map corresponded to the canonical default network and thresholded this map at z-score > 2. Voxel clusters within the following regions were identified as positively loading onto the thresholded default network component (MNI coordinates in brackets): anterior mPFC/ventral mPFC/anterior cingulate (0, 42, 3), right hippocampus (25, −20, −16), left hippocampus (−23, −21, −15), left parahippocampal cortex (−27, −35, −13), right lateral parietal cortex (44, −61, 36), left lateral parietal cortex (−39, −65, 38), posterior cingulate/precuneus (0, −51, 30), left thalamus (−4, −19, 8), left frontal pole (−23, 62, 10), and left superior/middle frontal gyri (−22, 29, 43). Masks were created from the voxel clusters in order to extract, from each participant, the average BOLD timeseries within ROIs (see Figure 1), namely those that corresponded to the PMN (anterior mPFC/ventral mPFC/anterior cingulate, posterior cingulate/precuneus, left and right lateral parietal cortices, left and right hippocampi, and left parahippocampal cortex).
Figure 1.
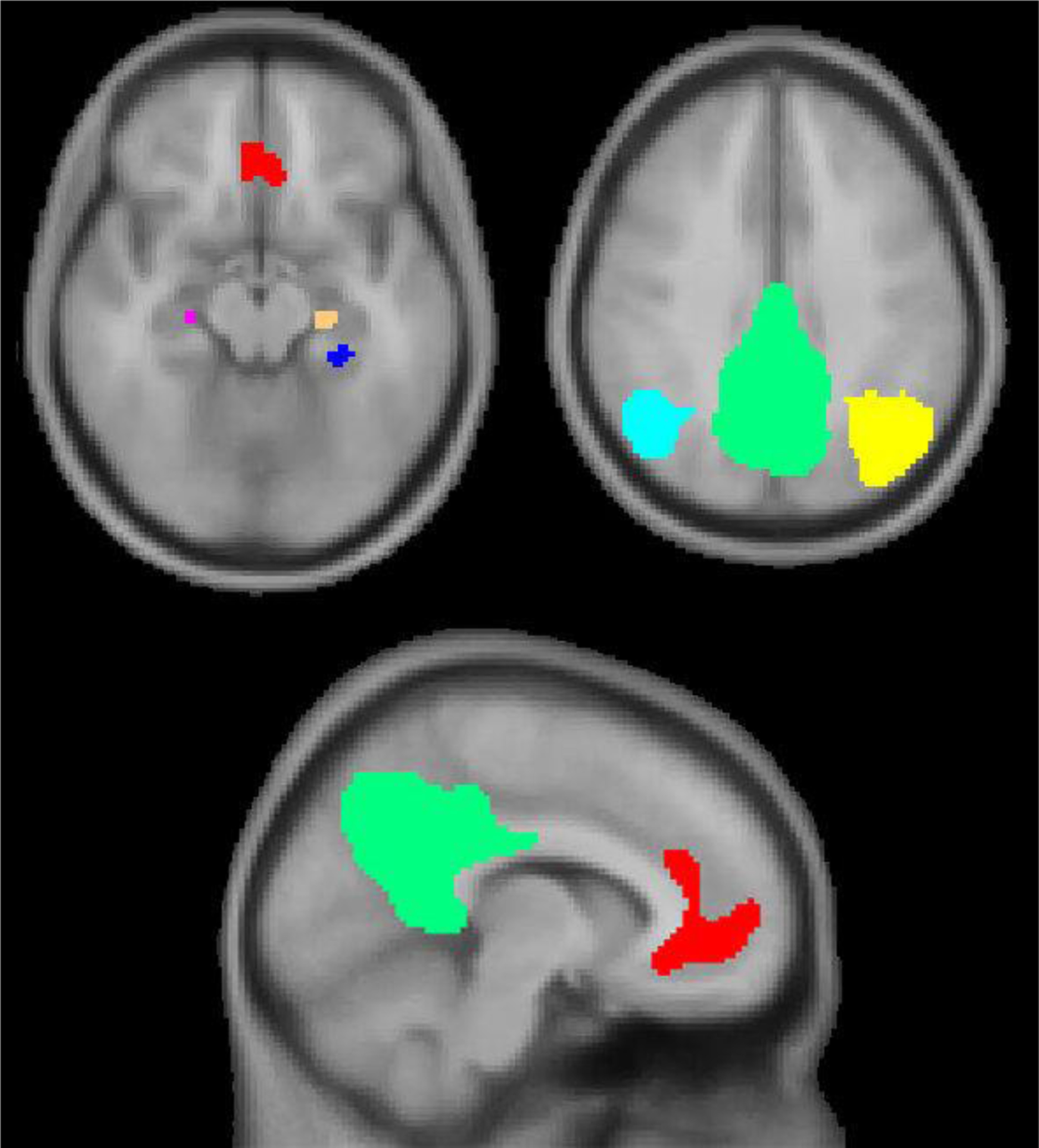
The PMN regions identified via ICA are overlaid on an MNI152 template. (L hippocampus = tan, R hippocampus = pink, L parahippocampal cortex = blue, anterior mPFC/ventral mPFC/anterior cingulate = red, posterior cingulate/precuneus = green, L lateral parietal cortex = yellow, R lateral parietal cortex = light blue).
For each participant, bivariate Pearson correlations were calculated for the average BOLD timeseries between pairs of ROIs (see Supplemental Tables 1 & 2 for average correlation values per age group). A Fischer z-transformation was applied to all correlation coefficients. Averaging these Fischer transformed correlation coefficients, we computed composite measures of anterior PMN and posterior PMN rsFC strength for use in our analyses. The anterior PMN measure was the average of correlations between the anterior cingulate/anterior mPFC/ventral mPFC and hippocampal ROIs, while the posterior PMN measure was the average of correlations between and among hippocampal, parahippocampal and parieto-occipital ROIs (see Figure 2). We were motivated to divide PMN connections into anterior and posterior components based on previous evidence suggesting variation in how age impacts anterior and posterior portions of the default network (Jones et al., 2011), and because we raised a hypothesis specific to anterior PMN (mPFC – hippocampal) connectivity.
Figure 2.
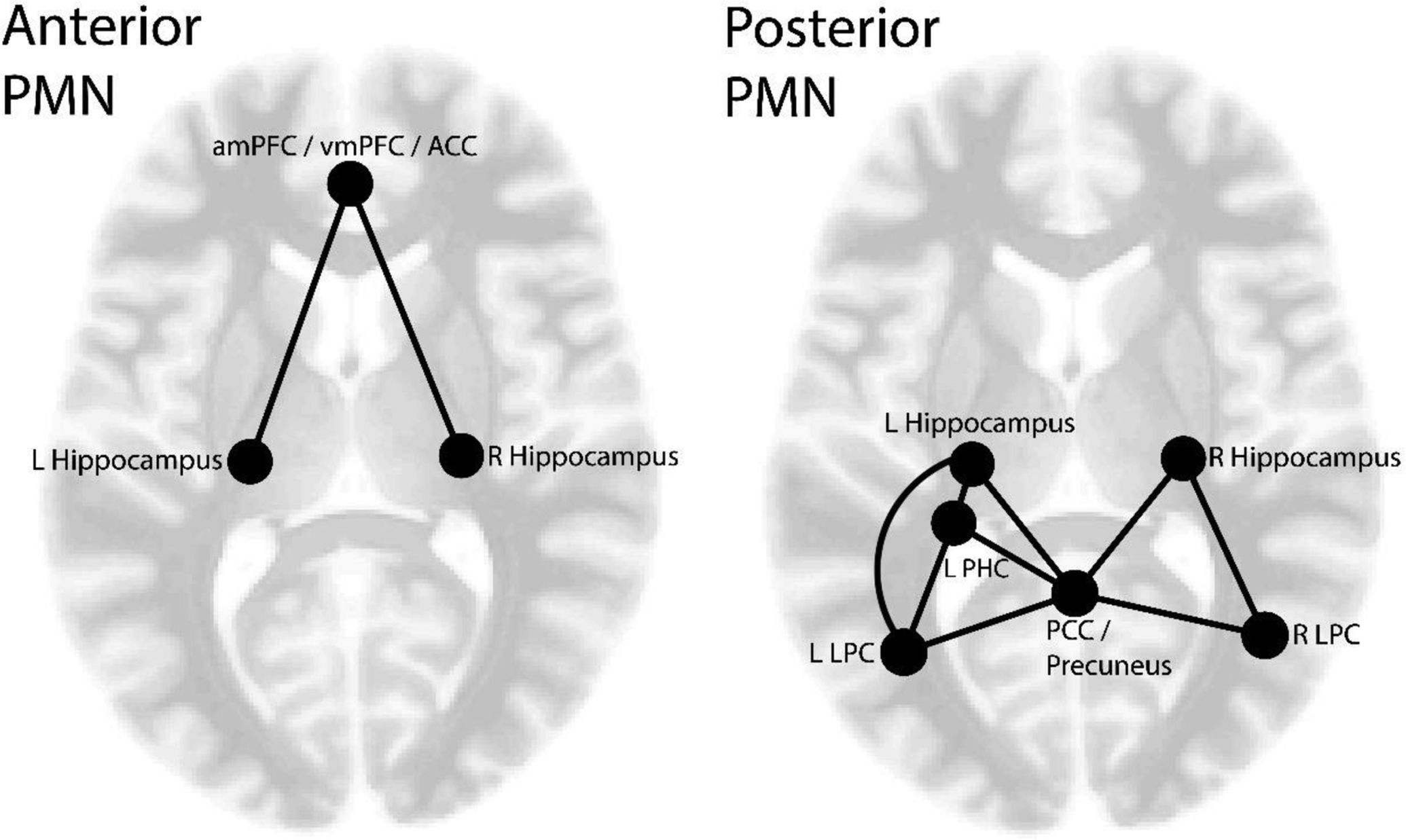
A depiction of the ROI-ROI correlations that are included within the anterior and posterior PMN rsFC measures. (amPFC = anterior mPFC, vmPFC = ventral mPFC, ACC = anterior cingulate cortex, PCC = posterior cingulate cortex, LPC = lateral parietal cortex, PHC = parahippocampal cortex).
2.5. Statistical analyses
Our first set of analyses assessed the impact of age on episodic detail amounts and episodic specificity, as well as semantic and miscellaneous detail amounts. We compared the behavioral measures between younger and older adult groups using Welch’s t-test, which is more robust to unequal variances stemming from group size imbalance. Within the older adult group, we computed Pearson bivariate correlations between years of age and the behavioral measures.
Second, we explored whether age influenced rsFC strength between age groups and within the older adult group for the anterior and posterior PMN measures. We compared rsFC strength between younger and older adult groups using Welch’s t-tests. Motivated by prior findings of non-linearity in default network rsFC alterations with age, we tested for both linear and quadratic effects of age on rsFC using polynomial regression models.
Next, we examined relationships between episodic detail generation and PMN rsFC within the older adult group and explored the impact of age on these relationships. First, linear regressions assessed the effects of rsFC strength on episodic detail and specificity within the older adult group. We tested whether significant brain-behavior relationships observed in the older adult group were also present for the younger adults, by modeling age group (younger adults, older adults), rsFC, and age group * rsFC interaction as predictors of behavior in regressions using the full sample. To determine whether age as a continuous variable influenced brain-behavior relationships among older adults, we performed regressions within the older adult group with years of age, rsFC and age * rsFC interaction modeled as predictors of behavior. Age and the rsFC measures were all mean centered prior to inclusion within these regression models
Post-hoc analyses included an examination of episodic detail sub-types and their associations with the two rsFC measures among the older adults. Also within the older adult group, we more explicitly tested the ‘semanticization’ hypothesis by assessing the impact of age, rsFC, and age * rsFC interaction on semantic detail generation. Age and the rsFC measures were mean centered.
3. Results
3.1. Behavioral Age Differences
Comparing Younger Adults vs. Older Adults
The event narratives from the younger adults showed greater episodic specificity than the narratives produced by older adults (Welch’s t(55.42) = 7.01, p < 0.001, younger mean = 0.83, older mean = 0.64). Interestingly, the amount of episodic detail was not significantly lower for the older adult group relative to the younger adult group (Welch’s t(33.44) = 1.55, p = 0.130, younger mean = 43.74, older mean = 35.91; see Figure 3). Older adults provided greater amounts of semantic detail (Welch’s t(57.42) = −6.18, p < 0.001, younger mean = 4.24, older mean = 13.76), but not miscellaneous detail (Welch’s t(38.31) = −1.01, p = 0.317, younger mean = 5.14, older mean = 6.28) compared to the younger adults.
Figure 3.
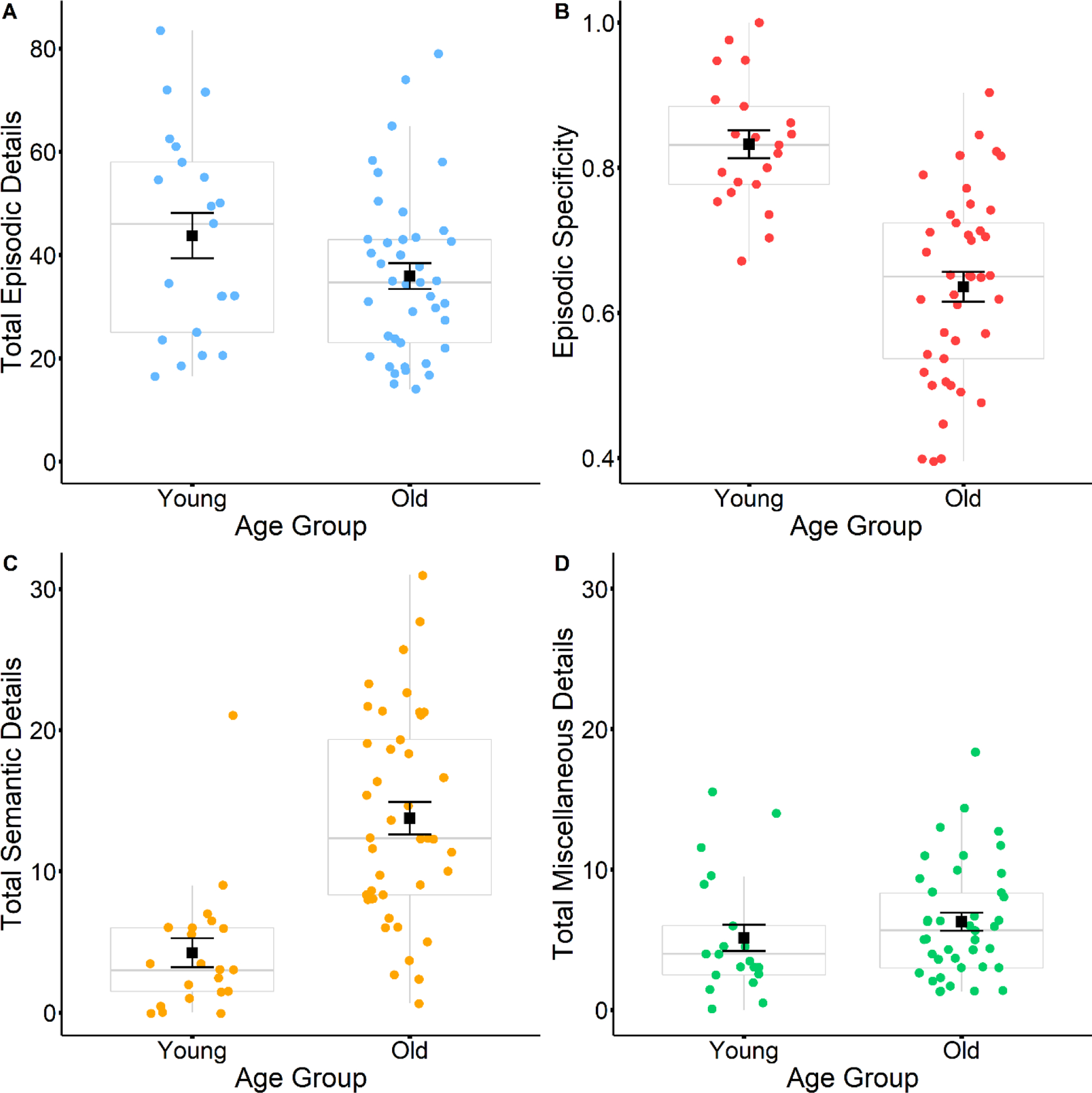
Boxplots display differences in total episodic details (A), episodic specificity (B), total semantic details (C) and total miscellaneous details (D) between young and older adult narratives. Black squares represent mean values, and black error bars represent the standard error.
Individual Differences Among Older Adults
Within the older adult group, increasing age was associated with a tendency to narrate events with fewer episodic details (r(39) = −0.42, p = 0.006) and lower episodic specificity (r(39) = −0.42, p = 0.006; see Figure 4). Age was not correlated with either semantic details (r(39) = 0.03, p = 0.830) or miscellaneous details (r(39) = 0.13, p = 0.426).
Figure 4.
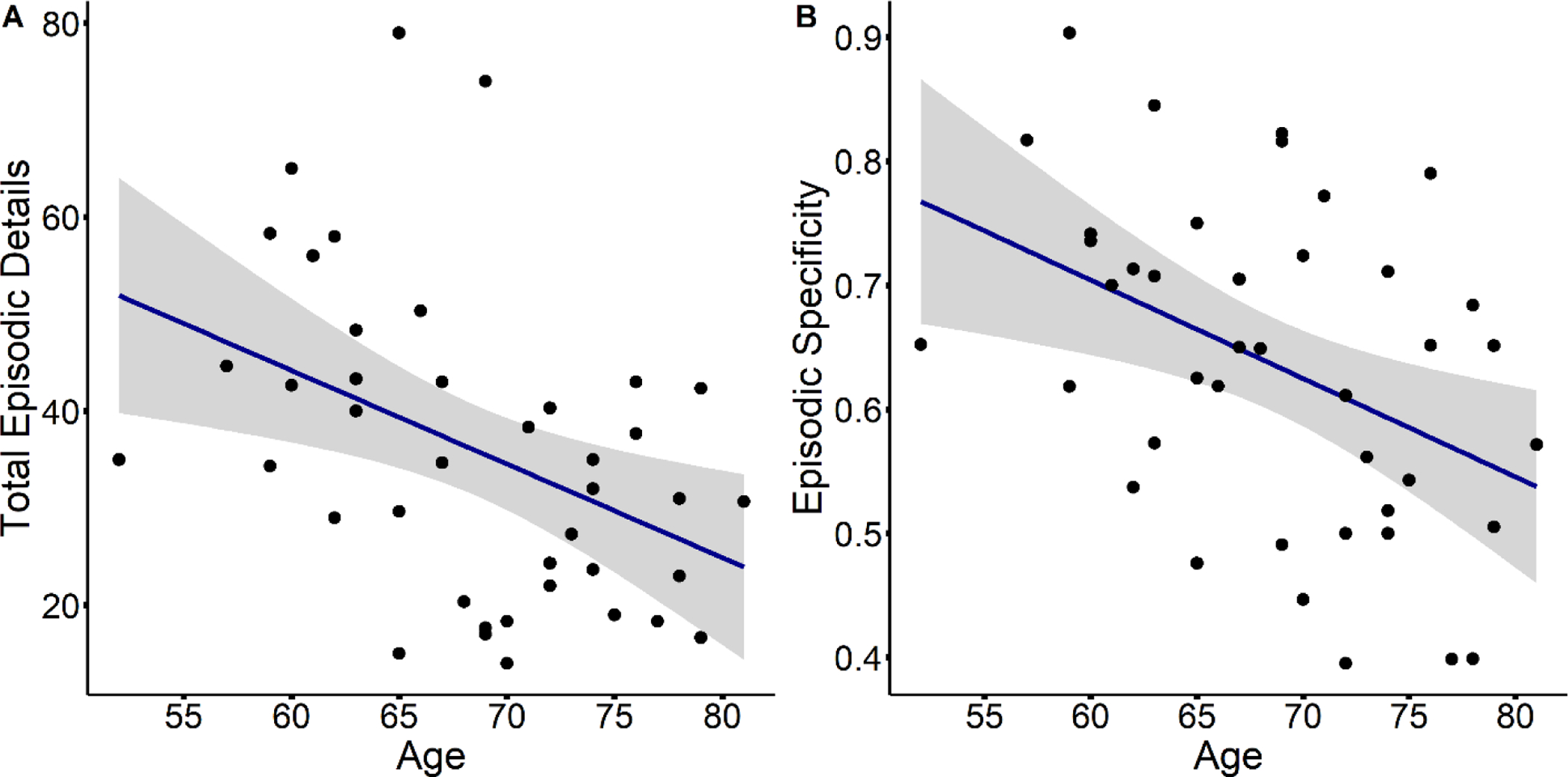
Scatterplots display age differences in total episodic detail (A) and episodic specificity (B), within the older adult group.
3.2. rsFC Age Differences
Despite age-associated behavioral differences, younger and older adult groups did not show significant differences in rsFC strength for the anterior PMN (Welch’s t(42.14) = 0.90, p = 0.375) or posterior PMN (Welch’s t(51.87) = −0.37, p = 0.714), rsFC measures. Similarly, rsFC strength did not vary across age within the older adult group for the anterior PMN (linear: β = 0.28, p = 0.073, quadratic: β = −0.18, p = 0.249, model R2 adj. = 0.06) or posterior PMN (linear: β = 0.20, p = 0.227, quadratic: β = 0.02, p = 0.890, model R2 adj. = −0.01) rsFC measures.
3.3. Effects of rsFC on Episodic Detail Generation Among Older Adults & Differences with Age
Individual Differences Among Older Adults
Within a simple linear regression, anterior PMN rsFC significantly predicted episodic detail (β = −0.46, p = 0.003, R2 = 0.21). This relationship indicates that stronger anterior PMN rsFC is associated with fewer episodic details (see Figure 5). There was no significant effect of posterior PMN rsFC on episodic detail (β = −0.29, p = 0.066, R2 = 0.08). The episodic specificity measure was not significantly predicted by anterior PMN rsFC (β = −0.29, p = 0.063, R2 = 0.09), or posterior PMN rsFC (β = −0.20, p = 0.212, R2 = 0.04).
Figure 5.
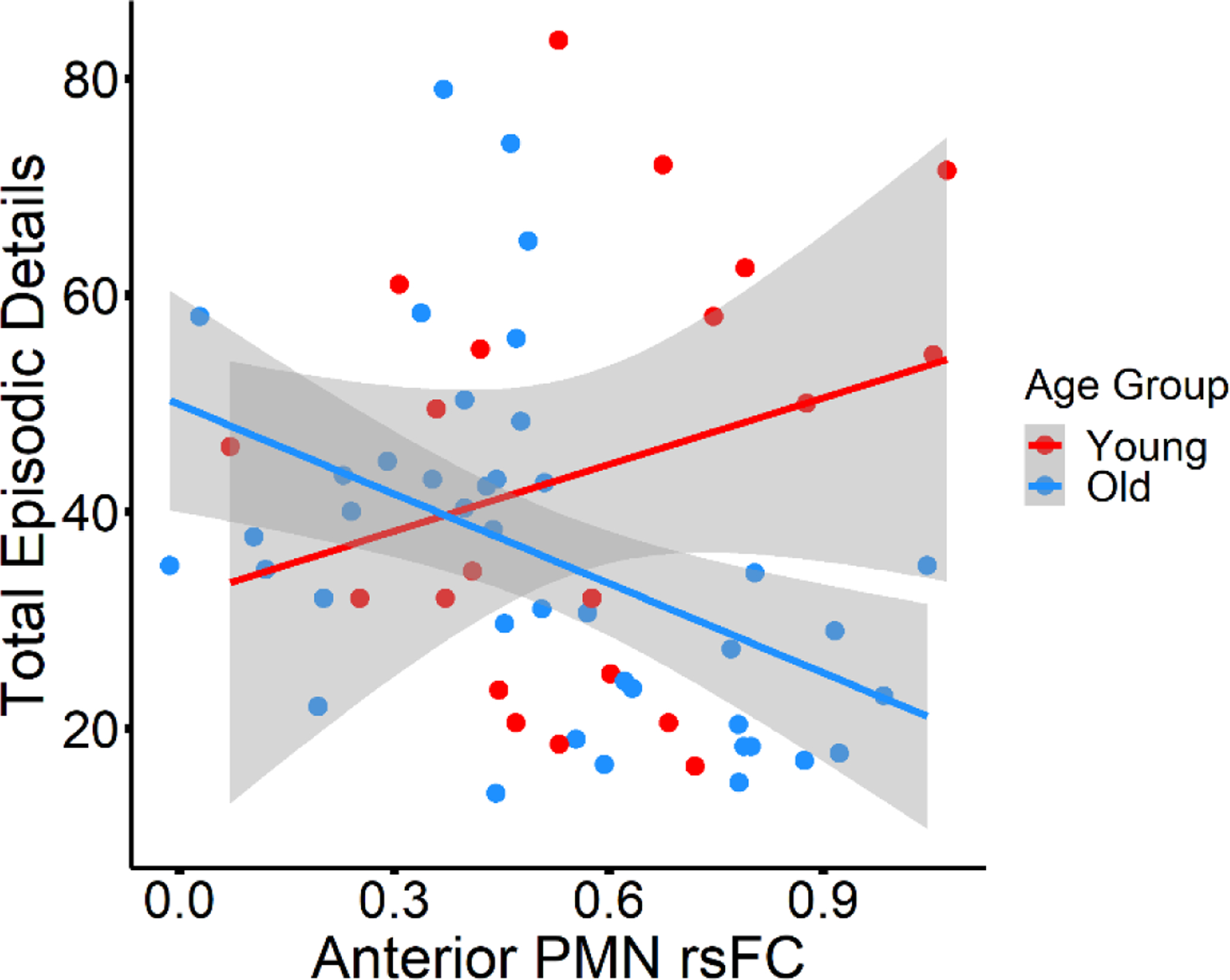
Young vs. older adult group difference in the relationship between anterior PMN rsFC and episodic detail.
Comparing Younger Adults and Older Adults
Because we observed that stronger anterior PMN rsFC predicted decreased episodic detail within the older adults, we examined this association within the full sample and tested for an interaction between age group and rsFC in order to determine if the relationship was unique to older adults. A significant age group * rsFC interaction (β = 0.35, p = 0.008, model R2 adjusted = 0.14) indicated an age group difference in the relationship between anterior PMN rsFC and episodic detail generation. Stronger anterior PMN rsFC was associated with fewer episodic details in the older adult group (r(39) = −0.46, p = 0.003), as we previously noted, but there was no such significant relationship between rsFC and episodic detail among the younger adults (r(19) = 0.26, p = 0.254; see Figure 5).
Age Differences in Brain-behavior Relationships Among Older Adults
We tested whether years of age interacted with rsFC to influence episodic detail and episodic specificity within the older adult group, in order to determine if the effects of PMN rsFC on behavior varied with age. The addition of age and age * rsFC interaction terms into each regression model left main effects of rsFC on episodic detail among older adults essentially unchanged (see Supplemental Table 3). We did not find evidence that age significantly moderated the relationships between episodic detail and rsFC strength for the anterior PMN (β = −0.08, p = 0.569, model R2 adj. = 0.25) or posterior PMN (β = 0.12, p = 0.427, model R2 adj. = 0.17). Likewise, the interaction between age and posterior PMN rsFC was also non-significant for episodic specificity (β = −0.22, p = 0.136, model R2 adj. = 0.18). However, age significantly moderated the relationship between episodic specificity and anterior PMN rsFC (β = −0.34, p = 0.021, model R2 adj. = 0.26). To explore the significant interaction, we computed simple slopes for the association between anterior PMN rsFC and episodic specificity, testing effects of holding age constant at the mean age (68.59 years), one SD below the mean age (61.6 years), and one SD above the mean age (75.57 years). The slope significantly differs from zero when age is held at 75.57 years (slope = −0.26, p = 0.012), but not at 68.59 years (slope = −0.10, p = 0.144) or at 61.6 years (slope = 0.05, p = 0.579). Among older adults, an association between stronger anterior network rsFC and lower episodic specificity appears as age increases (see Figure 6).
Figure 6.
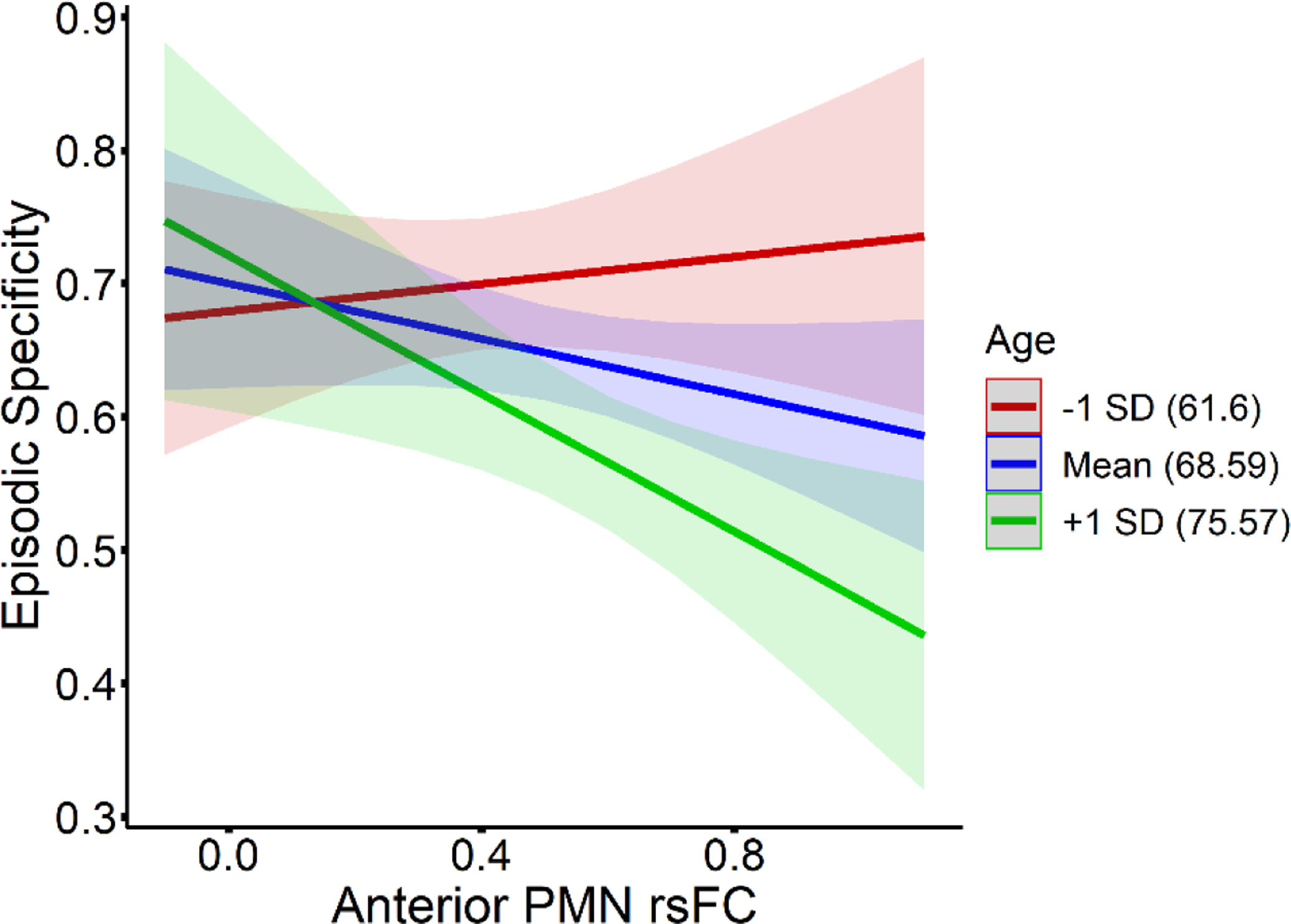
Simple slopes illustrating the interaction between age and anterior PMN rsFC for effects on episodic specificity, among the older adults. Slopes for episodic specificity and rsFC relationships at 1 SD below the mean age (61.6 years) are plotted in red, slopes at the mean age (68.59 years) are plotted in blue, and slopes at 1 SD above the mean age (75.57 years) are plotted in green, as denoted in the figure legend.
3.4. Post-Hoc Analyses
Associations Between rsFC and Episodic Detail Sub-types
Because the effect of posterior PMN rsFC on episodic detail approached significance, and because Ahmed et al. (2018) demonstrated a specific relationship between perceptual details and precuneus volume, we speculated that posterior PMN rsFC could have a more targeted effect on specific episodic detail sub-types that would not show through in our analyses of total episodic detail. Specifically, we hypothesized that posterior PMN rsFC might be more closely related to details relating to place, time and perceptual information than other details. To explore this possibility, we performed post hoc correlations between the episodic detail sub-types and the two rsFC measures in our older adult group.
The results of these analyses are shown in Table 2. Anterior PMN rsFC was significantly associated with event details (r = −0.45, p = 0.003, FDR corrected p = 0.017) and thought/emotion details (r = −0.45, p = 0.003, FDR corrected p = 0.017). There was a significant correlation between posterior PMN rsFC and event details that did not survive FDR correction (r = −0.31, p = 0.048, FDR corrected p = 0.159). Across all these associations, stronger rsFC was related to fewer details.
Table 2.
Correlations between episodic detail sub-types and rsFC measures, within the older adult group. Significant p-values are shown in italics and bold.
| Detail type | rsFC measure | Pearson r | Original p-value | FDR corrected p-value |
|---|---|---|---|---|
| Event | Anterior PMN | −0.446 | 0.003 | 0.017 |
| Place | Anterior PMN | −0.241 | 0.13 | 0.187 |
| Time | Anterior PMN | −0.257 | 0.104 | 0.187 |
| Thought/Emotion | Anterior PMN | −0.452 | 0.003 | 0.017 |
| Perceptual | Anterior PMN | −0.035 | 0.83 | 0.848 |
| Event | Posterior PMN | −0.311 | 0.048 | 0.159 |
| Place | Posterior PMN | −0.254 | 0.108 | 0.187 |
| Time | Posterior PMN | −0.240 | 0.131 | 0.187 |
| Thought/Emotion | Posterior PMN | −0.054 | 0.738 | 0.848 |
| Perceptual | Posterior PMN | 0.031 | 0.848 | 0.848 |
Association of rsFC to Semantic Detail Generation Among Older Adults & Moderation by Age
We hypothesized that interactions between the mPFC and hippocampus could contribute toward an age-related ‘semanticization’ of autobiographical recollection, or increased reliance on semantic over episodic retrieval, such that greater mPFC – hippocampal connectivity (captured within our anterior PMN rsFC measure) would relate to decreased episodic detail and increased semantic detail generation. The results of our rsFC and episodic detail generation analyses provide evidence consistent with this hypothesis, particularly the finding that decreased episodic specificity, the ratio of episodic to total combined details, becomes associated with heightened anterior PMN rsFC as age increases among older adults. Although the episodic specificity measure provides insight into the relationship between episodic and semantic detail, it is not an exact test of this relationship per se, as miscellaneous details also factor into its calculation. Therefore, as a follow-up analysis, we sought to more explicitly test the ‘semanticization’ hypothesis, by modeling age, rsFC, and age by rsFC interaction as predictors of semantic details and a semantic density score within the older adult group. The semantic density score was calculated as the ratio of semantic details to the sum of episodic and semantic details to more precisely capture the relationship between episodic and semantic details within the memory narratives. We expected stronger rsFC to predict greater semantic detail and semantic density, especially with increasing age, for the anterior PMN but not the posterior PMN.
There were no main effects of rsFC on semantic detail for the anterior PMN (β = 0.04, p = 0.832, model R2 adjusted = −0.03) or posterior PMN (β = 0.13, p = 0.383, model R2 adjusted = 0.14) among the older adults. Likewise, there were no main effects of rsFC on the semantic density score for either the anterior PMN (β = 0.21, p = 0.169, model R2 adjusted = 0.15) or posterior PMN (β = 0.19, p = 0.211, model R2 adjusted = 0.18). However, an age by posterior PMN rsFC interaction was significant for both semantic detail (β = 0.45, p = 0.004, model R2 adjusted = 0.14) and semantic density (β = 0.32, p = 0.031, model R2 adjusted = 0.18). In contrast, there was no significant age by anterior PMN rsFC interaction for either semantic detail (β = 0.21, p = 0.211, model R2 adjusted = −0.03) or semantic density (β = 0.23, p = 0.134, model R2 adjusted = 0.15).
Simple slope analyses of the two age by posterior PMN rsFC interactions revealed a similar pattern of results (see Figure 7). For the association between posterior PMN rsFC and semantic detail, simple slopes were non-significant at the mean age of 68.59 (slope = 5.21, p = 0.383) and at 61.6 years of age, one SD below the mean age (slope = −12.90, p = 0.100). The slope was significant at 75.57 years of age, one SD above the mean age (slope = 23.33, p = 0.014). For the association between posterior PMN rsFC and semantic density, simple slopes were again non-significant at age 68.59 (slope = 0.13, p = 0.211) and age 61.6 (slope = −0.10, p = 0.448), but significant at age 75.57 (slope = 0.35, p = 0.027). These results indicate that stronger posterior PMN rsFC becomes associated with increased semantic detail and higher semantic density as age increases.
Figure 7.
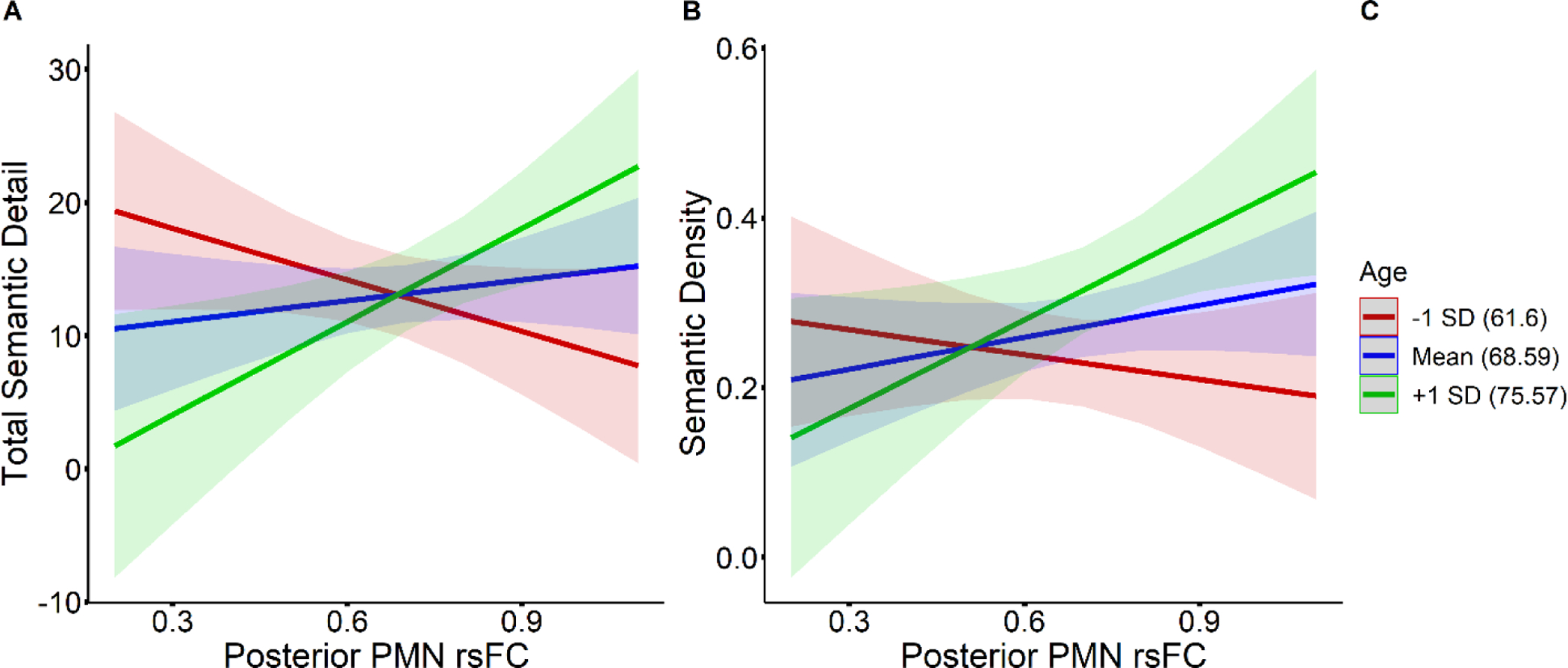
Simple slopes illustrating the interaction between age and posterior PMN rsFC for effects on semantic detail (A) and semantic density (B), among older adults. Slopes at 1 SD below the mean age (61.6 years) are plotted in red, slopes at the mean age (68.59 years) are plotted in blue, and slopes at 1 SD above the mean age (75.57 years) are plotted in green, as denoted in the figure legend (C).
Comparing Young vs. Middle-Old vs. Older-Old Adults
Motivated by the lack of clear age group differences for episodic detail and rsFC measures, we conducted the following post-hoc analyses. The older adult group was split by the median age (69 years) to create a middle-old group (N = 19, mean age = 62, ages 52 – 68) and an older-old group (N = 22, mean age = 74, ages 69 – 81). The two older adult groups did not differ in years of education. Using one-way ANOVAs to test for differences in the detail measures across the three age groups (young, middle-old, older-old), we found significant effects of age group on episodic detail (F(2,59) = 5.25, p = 0.008), episodic specificity (F(2,59) = 24.31, p < 0.001), and semantic detail (F(2,59) = 14.49, p < 0.001), but not miscellaneous detail (F(2,59) = 0.95, p = 0.39) (see Figure 8). The older-old group produced less episodic detail than the younger adults (Welch’s t(34.91) = 2.74, p = 0.01) and the middle-old group (Welch’s t(36) = 3.08, p = 0.004), but younger adults and the middle-old group did not differ (Welch’s t(37.21) = 0.04, p = 0.97). In contrast, younger adults provided less semantic detail than the middle-old group (Welch’s t(28.97) = −5.08, p < 0.001) and older-old group (Welch’s t(35.91) = −4.74, p < 0.001), while the middle-old and older-old groups did not differ (Welch’s t(37.38) = 0.67, p = 0.51). Episodic specificity was higher for younger adults compared to the middle-old group (Welch’s t(35.35) = 4.79, p < 0.001) and the older-old group (Welch’s t(35.82) = 6.79, p < 0.001), and also higher for the middle-old group compared to the older-old group (Welch’s t(38.39) = 2.40, p = 0.021).
Figure 8.
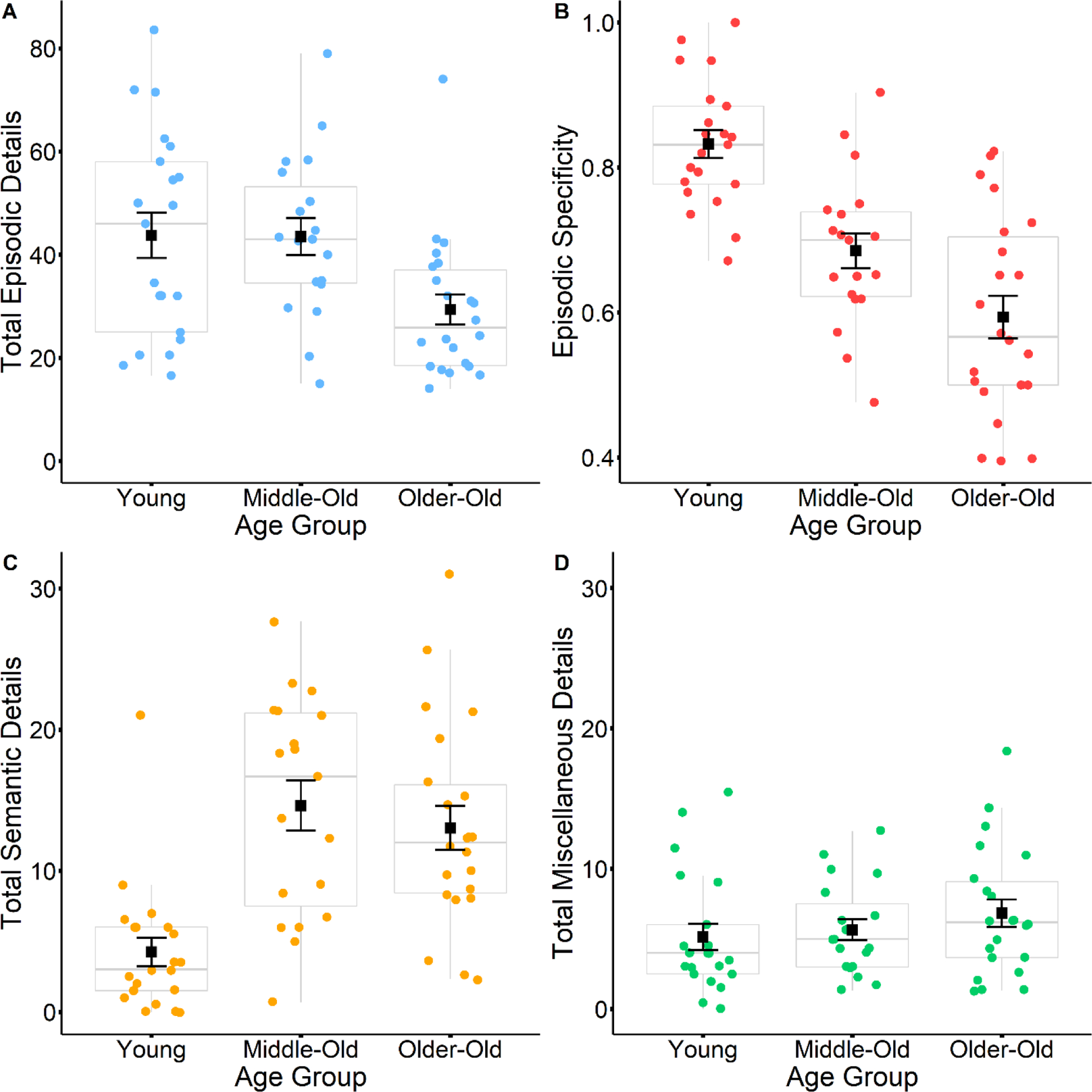
Boxplots display differences in total episodic details (A), episodic specificity (B), total semantic details (C) and total miscellaneous details (D) between young, middle-old, and older-old adult narratives. Black squares represent mean values, and black error bars represent the standard error.
Despite age differences in the brain-behavior relationships, there were no age differences in the rsFC measures. Using one-way ANOVAs to test for differences in the rsFC measures across the three age groups, there were no significant effects of age group on either anterior PMN rsFC (F(2,59) = 2.12, p = 0.13) or posterior PMN rsFC (F(2,59) = 1.70, p = 0.19).
4. Discussion
The present study sought to characterize the extent to which episodic detail generation is related to individual differences in rsFC within the PMN, and to determine whether these brain-behavior relationships are affected by aging. We demonstrated that the pattern of details generated during autobiographical memory recollection differs in complex ways between younger and older adults, and across older adults of varying ages. Our results also provided novel evidence that among older adults, the ability to construct autobiographical event narratives with rich episodic detail is associated with rsFC patterns within the PMN. Specifically, we demonstrated that stronger connectivity within the PMN related to reduced episodic detail generation, as well as increased semantic detail generation, among older adults. Age differences were found in these brain-behavior relationships, such that the association between anterior PMN rsFC and episodic detail within the older adult group was not present for younger adults, and also that age moderated certain brain-behavior relationships among the older adults. Further details of the results, and their implications, are discussed below.
Age differences in detail generation among younger and older adults
On average, episodic specificity was higher for event memory narratives produced by the younger adults compared to narratives produced by the older adults, even though the amount of episodic details within the narratives did not substantially differ between the two age groups. This divergence could be explained by the finding that older adults generated a greater amount of non-episodic content, particularly semantic details, relative to the younger adults, consistent with previous studies (Acevedo-Molina, Matijevic, & Grilli, 2020; Robin & Moscovitch, 2017; Gaesser et al., 2011; St Jacques, & Levine, 2007; Addis, Wong & Schacter, 2008; Levine et al., 2002). The lack of an age group difference in the amount of episodic details generated, though not entirely unprecedented (Wank, Andrews-Hanna & Grilli, 2021), was still somewhat surprising given the previous literature (Acevedo-Molina, Matijevic, & Grilli, 2020; Robin & Moscovitch, 2017; Gaesser et al., 2011; St Jacques, & Levine, 2007; Addis, Wong & Schacter, 2008; Levine et al., 2002). Importantly, however, within the older adult group, episodic detail and episodic specificity both decreased linearly with increasing age, suggesting that the inclusion of participants aged 52 to 65, within middle-age, could account for the lack of a clear group difference between younger and older adults. In support of this idea, the post-hoc analysis splitting older adults into two groups of individuals below and above 69 years of age showed that episodic detail amounts significantly differed between the younger adults and “older-old” group, but not between the younger adults and “middle-old” group. A similar pattern of results was seen when the age groups were compared only on the episodic details generated for the two event memories that younger and older adults retrieved from equivalent time periods (see Supplemental Results). Altogether, these results are consistent with the notion that episodic details decrease with age, but they also indicate that this decrease may not appear prominently until the 7th and 8th decades of life. In contrast, although semantic detail was increased for older adults relative to younger adults, it was not linearly related to increasing age, suggesting that the increase in semantic detail might actually precede the decrease in episodic detail with age. Episodic specificity also decreases with age, reflecting the combination of decreased episodic detail generation and steady generation of non-episodic information during autobiographical event narration by older adults.
The present findings raise the question of when in the adult lifespan are age differences in detail generation apparent? The answer to this question will likely have implications for theories on autobiographical memory and aging. For instance, if we interpret these findings as an indication that the age-related increase in semantic detail generation plateaus by the mid-50’s, while episodic detail generation steadily declines into the 7th and 8th decades, then it complicates arguments that increased inclusion of semantic detail is compensating for episodic reductions in the event narratives produced by older adults. These results point to the limitations of younger and older adult group comparisons that are most often employed in studies of memory and aging. In order to better understand the trajectory of age differences in autobiographical recollection, future research that includes participants across the full range of the adult lifespan is needed to clarify when these differences emerge.
PMN rsFC does not differ with age
We observed no age differences in PMN rsFC between the younger and older adult age groups, or an age-related decrease in PMN rsFC within the older adult group. The post-hoc analyses of older adults split into two groups likewise failed to reveal any age differences in PMN rsFC. Age-related reductions in rsFC within the default network have been demonstrated across several studies, both in group comparisons between younger and older adults (Damosieaux et al., 2008; Andrews-Hanna et al., 2007; Grady et al., 2016), in examinations of individual differences within old age (Andrews-Hanna et al., 2007; Ng et al., 2016), and across the adult lifespan (Betzel et al., 2014). However, rsFC measures have been shown to be exquisitely sensitive to head motion artifacts, and differences in the degree of head motion across individuals and groups may confound results (Power et al., 2012; Kato et al., 2020). This poses a significant problem for studies of aging, since increasing age is associated with greater within-scan head motion (Savalia et al., 2017). Van Dijk, Sabuncu, and Buckner (2012) demonstrated that head motion relates to rsFC reductions within the default network, and that controlling for motion can attenuate a significant association between age and rsFC between default network regions. The implications of this finding are that age differences in default network rsFC will be more subtle than reported in studies that lack careful suppression of motion-related noise. The absence of an association between age and rsFC in the present study could be explained by the use of ICA-AROMA, a robust motion mitigation strategy, within our processing pipeline. In addition, variation across studies in the methods used for defining the default network, such as anatomically-based versus ICA-based approaches, and for comparing functional connectivity between age groups could also contribute to the conflicting findings regarding default network alterations in aging. It may also be possible that the age range included in our sample of older adults may not represent the correct age distribution needed to capture a non-linear trajectory of rsFC shifts with aging, as Staffaroni et al. (2018) noted in a longitudinal study that rsFC within the default network only showed declines after age 70. A larger sample that included a greater proportion of ‘older’ old adults above age 80 perhaps could have enabled us to detect a non-linear pattern of change in rsFC within the older adult group.
PMN rsFC tracks with episodic detail generation among older adults
The number of episodic details generated by individuals within the older adult group was related to the strength of anterior PMN rsFC, with a similar trend also observed with posterior PMN rsFC. In a follow-up analysis, we noted selective associations between episodic detail sub-types and our PMN rsFC measures. For the anterior PMN, rsFC was correlated with event details and thought/emotion details. Posterior PMN rsFC was also related to event details, although this correlation was nonsignificant following multiple comparisons correction. The lack of an association between posterior PMN and place details in particular is interesting, given that a recent task-based fMRI study of the Autobiographical Interview showed that the generation of place details engages the parahippocampal cortex, angular gyrus and retrosplenial cortex (Gilmore et al., 2021). However, event details made up a majority of the episodic details that older adults provided (see Supplemental Figure 1), perhaps explaining the stronger relationship to PMN rsFC relative to other detail types, as well as the lack of an association between posterior PMN rsFC and place, time and perceptual details as hypothesized. Overall, the results of these brain-behavior analyses indicate that the strength of rsFC within the PMN is related to individual differences in episodic detail generation, among older adults.
Increased connectivity within the PMN was associated with fewer episodic details generated. In other words, among older adults there was a negative relationship between rsFC within the PMN and episodic detail, which was consistent across regions and detail sub-types. Prior evidence from Sheldon et al. (2016) noted a different relationship among young adults, that is, stronger rsFC between MTL and parieto-occipital regions was related to an increase in the self-reported tendency to remember events with rich episodic detail. This discrepancy could be attributed to age differences in PMN rsFC and episodic detail relationships. Consistent with this notion, the negative association between anterior PMN rsFC and episodic detail within the older adult group was not present in the younger adults. We note that our younger adult group was relatively small, which was why individual differences within the group were only assessed to compare against significant findings in the older adult group. With a sample size more adequate for exploring individual differences, we perhaps could have seen a significant positive relationship between episodic detail generation and posterior PMN rsFC among the younger adults, consistent with Sheldon et al. (2016).
That stronger anterior PMN rsFC predicts decreased episodic detail among older adults, but not younger adults, suggests that strengthened connectivity within the anterior PMN is not always associated with adverse outcomes for episodic detail generation. Rather, this particular relationship appears specific to late adulthood. Interestingly, while there was no effect of age on the association between anterior PMN rsFC and episodic detail within the older adult group, we did observe such an interaction for episodic specificity. Using simple slope tests to evaluate the anterior PMN rsFC and episodic specificity relationship across three different ages within the older adult group, we found that stronger anterior PMN rsFC became associated with lower episodic specificity scores as age increased. This age-dependent effect provides additional support for the idea that the negative association between anterior PMN rsFC and episodic detail generation is unique to older adults, likely emerging as the result of changes within aging. Below, we discuss potential reasons as to why age might bring about this relationship.
Why does age modify the relationship between PMN rsFC and episodic detail generation?
In line with the idea that a loss of integrity in the default network will disrupt successful use of the cognitive functions it supports (Andrews-Hanna, Smallwood & Spreng, 2014; Andrews-Hanna, Grilli, & Irish, 2019), the age-dependent relationship between heightened anterior PMN rsFC and reduced episodic detail generation could be the consequence of damage or dysfunction within the anterior PMN. Increased rsFC within the anterior PMN might reflect the type of compensatory hyperconnectivity that Hillary et al. (2015) argue is a common response to neural damage. Therefore, reduced episodic detail generation would be related to strengthened anterior PMN rsFC among older adults, but not young adults, because network dysfunction is underlying the heightened connectivity among the older adults. However, as previously discussed, anterior PMN rsFC did not vary with age. If a compensatory increase in anterior PMN connectivity is occurring among the older adults, it is unclear how this age-associated rsFC increase would go undetected but still have an impact on behavior.
The age-dependent association between anterior PMN rsFC and episodic detail generation can also be viewed through the lens of the ‘semanticization’ hypothesis, the idea that older adults adopt a recollection mode that favors retrieval of semantic information over episodic details. Given the possible role of the mPFC and its interactions with the hippocampus in facilitating schema-guided retrieval, adoption of the ‘semanticized’ recollection mode by older adults may place a greater burden on mPFC and hippocampal connections, reflected at ‘rest’ as elevated connectivity. The association between heightened anterior PMN rsFC and reduced episodic detail generation might thus result from an increased reliance on anterior PMN interactions to accommodate age-related changes in autobiographical recollection strategies, rather than reflecting a loss of integrity within the PMN. However, the ‘semanticization’ hypothesis cannot account for the finding that stronger connectivity within the posterior PMN showed a similar, though subtle, relationship with decreased episodic detail generation among the older adults. Furthermore, anterior PMN rsFC did not seem to influence semantic detail generation among the older adults, as is expected by the ‘semanticization’ hypothesis. Anterior PMN rsFC was not related to either semantic detail or semantic density, a measure assessing the relationship between semantic and episodic detail generation. Surprisingly, we instead saw age-dependent effects of posterior PMN rsFC on both semantic detail and density among older adults. Stronger posterior PMN rsFC was associated with greater semantic detail and higher semantic density, although this association only appeared as age increased. This unexpected finding, along with the age-dependent effects of PMN rsFC on episodic detail generation, point toward a complex pattern of PMN interactions underlying alterations to autobiographical memory recollection in aging. Together, anterior and posterior connections of the PMN may jointly contribute toward, or at least reflect “at rest”, shifts in the balance of episodic and semantic detail generation with age.
The relationship demonstrated in the present study between heightened rsFC within the PMN and reduced episodic detail generation among older adults has a variety of interpretations as to its possible underlying mechanisms. While we cannot conclusively determine the most likely interpretation with the available data, we believe that future research efforts that adopt a multi-modality approach will be capable of disentangling these potential mechanisms. For example, resting state fMRI can be paired with task-based fMRI assessments of episodic and semantic autobiographical memory retrieval, in order to examine and compare PMN interactions during autobiographical recollection to rsFC patterns. This approach could provide insight as to whether the association between heightened PMN rsFC and poorer episodic detail generation among the elderly reflects either a diminishing ability to support episodic recollection or increasing involvement of semantic memory processes, or potentially both. Importantly, this approach could also elucidate the seemingly differential contributions of anterior and posterior connections to age-related alterations in detail generation. As well, research ought to be undertaken to discern whether age-related changes to the structural integrity of the PMN contribute to alterations in how PMN rsFC influences episodic detail generation. Further exploration of not just the PMN, but its interactions with other regions and networks implicated in memory, may also provide for a more comprehensive understanding of age differences in detail generation. One candidate is the lateral prefrontal cortex, as Spreng et al. (2018) previously noted that greater coupling between lateral prefrontal and default network regions was associated with increased generation of non-episodic details among older adults. Another candidate may be the anterior temporal network, as this network also involves the hippocampus and is theorized to contribute toward retrieval of conceptual, item-specific information from memory (Ritchey et al., 2015). Ideally, the results of the present study will generate a number of hypotheses for further exploration of the role of PMN interactions in supporting episodic detail generation, across the adult lifespan.
Supplementary Material
Age differentially impacts episodic and semantic detail generation.
MTL-neocortical resting state functional connectivity (rsFC) does not vary with age.
Stronger MTL-neocortical rsFC relates to lower episodic detail generation among older adults.
Associations between MTL-neocortical rsFC and episodic detail generation are age-dependent.
Acknowledgements
We thank our generous participants for taking part in this study. We also thank research assistants from the Cognition & Neuroimaging Laboratory for their aid in pre-processing the neuroimaging data, and research assistants from the Human Memory Laboratory for their help in memory narrative detail scoring. Research reported in this publication was supported by the Arizona Department of Health Services/Arizona Alzheimer’s Consortium (MDG & LR) and the National Institute on Aging of the National Institutes of Health under Award Number R03AG060271 (MDG). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. SM & AAW were supported by National Science Foundation Graduate Research Fellowship Grant DGE-1746060.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham A, Pedregosa F, Eickenberg M, Gervais P, Mueller A, Kossaifi J, Gramfort A, Thirion B, & Varoquaux G (2014). Machine learning for neuroimaging with scikit-learn. Frontiers in neuroinformatics, 8, 14. 10.3389/fninf.2014.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo-Molina MC, Matijevic S, & Grilli MD (2020). Beyond episodic remembering: elaborative retrieval of lifetime periods in young and older adults. Memory (Hove, England), 28(1), 83–93. 10.1080/09658211.2019.1686152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR (2020). Mental Time Travel? A Neurocognitive Model of Event Simulation. Review of Philosophy and Psychology, 11(2), 233–259. 10.1007/s13164-020-00470-0 [DOI] [Google Scholar]
- Addis DR, Wong AT, & Schacter DL (2008). Age-Related Changes in the Episodic Simulation of Future Events. Psychological Science, 19(1), 33–41. 10.1111/j.1467-9280.2008.02043.x [DOI] [PubMed] [Google Scholar]
- Ahmed S, Irish M, Loane C, Baker I, Husain M, Thompson S, … Butler C (2018). Association between precuneus volume and autobiographical memory impairment in posterior cortical atrophy: Beyond the visual syndrome. NeuroImage: Clinical, 18(March), 822–834. 10.1016/j.nicl.2018.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle MEE, & Buckner RL (2007). Disruption of Large-Scale Brain Systems in Advanced Aging. Neuron, 56(5), 924–935. 10.1016/j.neuron.2007.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, & Buckner RL (2010). Functional-Anatomic Fractionation of the Brain’s Default Network. Neuron, 65(4), 550–562. 10.1016/j.neuron.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood J, & Spreng RN (2014). The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences, 1316(1), 29–52. 10.1111/nyas.12360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Grilli MD, & Irish M (2019). A Review and Reappraisal of the Default Network in Normal Aging and Dementia. In Oxford Research Encyclopedia of Psychology. 10.1093/acrefore/9780190236557.013.384 [DOI] [Google Scholar]
- Barry DN, Barnes GR, Clark IA, & Maguire EA (2019). The Neural Dynamics of Novel Scene Imagery. Journal of Neuroscience, 39(22), 4375–4386. 10.1523/JNEUROSCI.2497-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit RG, & Schacter DL (2015). Specifying the core network supporting episodic simulation and episodic memory by activation likelihood estimation. Neuropsychologia, 75, 450–457. 10.1016/j.neuropsychologia.2015.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME, Phuong L, Picasso L, Cabeza R, & Olson IR (2007). Parietal lobe and episodic memory: Bilateral damage causes impaired free recall of autobiographical memory. Journal of Neuroscience, 27(52), 14415–14423. 10.1523/JNEUROSCI.4163-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertossi E, Tesini C, Cappelli A, & Ciaramelli E (2016). Neuropsychologia Ventromedial prefrontal damage causes a pervasive impairment of episodic memory and future thinking. Neuropsychologia, 90, 12–24. 10.1016/j.neuropsychologia.2016.01.034 [DOI] [PubMed] [Google Scholar]
- Betzel RF, Byrge L, He Y, Goñi J, Zuo XN, & Sporns O (2014). Changes in structural and functional connectivity among resting-state networks across the human lifespan. NeuroImage, 102(P2), 345–357. 10.1016/j.neuroimage.2014.07.067 [DOI] [PubMed] [Google Scholar]
- Bonnici HM, Cheke LG, Green DAE, FitzGerald THB, & Simons JS (2018). Specifying a causal role for angular gyrus in autobiographical memory. BioRxiv, 38(49), 10438–10443. 10.1101/323733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Gaynor LS, Barnes CA, Bauer RM, Bizon JL, Roberson ED, & Ryan L (2018). Shared Functions of Perirhinal and Parahippocampal Cortices: Implications for Cognitive Aging. Trends in Neurosciences, 41(6), 349–359. 10.1016/j.tins.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KL, Madore KP, Benoit RG, Thakral PP, & Schacter DL (2018). Increased hippocampus to ventromedial prefrontal connectivity during the construction of episodic future events. Hippocampus, 28(2), 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL, Isaacowitz DM, & Charles ST (1999). Taking time seriously. A theory of socioemotional selectivity. The American psychologist, 54(3), 165–181. 10.1037//0003-066x.54.3.165 [DOI] [PubMed] [Google Scholar]
- Conway MA (2009). Episodic memories. Neuropsychologia, 47(11), 2305–2313. 10.1016/j.neuropsychologia.2009.02.003 [DOI] [PubMed] [Google Scholar]
- Cooper RA, & Ritchey M (2019). Cortico-hippocampal network connections support the multidimensional quality of episodic memory. eLife, 8, e45591. 10.7554/eLife.45591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, & Hyde JS (1997). Software tools for analysis and visualization of fMRI data. NMR in Biomedicine, 10(4–5), 171–178. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, & Rombouts SA (2008). Reduced resting-state brain activity in the “default network” in normal aging. Cerebral cortex (New York, N.Y. : 1991), 18(8), 1856–1864. 10.1093/cercor/bhm207 [DOI] [PubMed] [Google Scholar]
- Davidson PSR, Anaki D, Ciaramelli E, Cohn M, Kim ASN, Murphy KJ, … Levine B (2008). Does lateral parietal cortex support episodic memory ? Evidence from focal lesion patients. Neuropsychologia, 46(7), 1743–1755. 10.1016/j.neuropsychologia.2008.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipasquale O, Sethi A, Laganà MM, Baglio F, Baselli G, Kundu P, Harrison NA, & Cercignani M (2017). Comparing resting state fMRI de-noising approaches using multi- and single-echo acquisitions. PloS one, 12(3), e0173289. 10.1371/journal.pone.0173289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esopenko C, & Levine B (2017). Autobiographical memory and structural brain changes in chronic phase TBI. Cortex, 89, 1–10. 10.1016/j.cortex.2017.01.007 [DOI] [PubMed] [Google Scholar]
- Esteban O, Birman D, Schaer M, Koyejo OO, Poldrack A, & Gorgolewski KJ (2017). MRIQC: Advancing the automatic prediction of image quality in MRI from unseen sites. PLOS ONE 12(9): e0184661. 10.1371/journal.pone.0184661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, … Gorgolewski KJ (2019). fMRIPrep: a robust preprocessing pipeline for functional MRI. Nature Methods, 16, 111–116. 10.1038/s41592-018-0235-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research, 12(3), 189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Fonov V, Evans A, McKinstry R, Almli C, & Collins D (2009). Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. NeuroImage, 47, S102. 10.1016/s1053-8119(09)70884-5 [DOI] [Google Scholar]
- Gaesser B, Sacchetti DC, Addis DR, & Schacter DL (2011). Characterizing age-related changes in remembering the past and imagining the future. Psychology and aging, 26(1), 80–84. 10.1037/a0021054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AW, Quach A, Kalinowski SE, Gotts SJ, Schacter DL, & Martin A (2021). Dynamic Content Reactivation Supports Naturalistic Autobiographical Recall in Humans. The Journal of neuroscience : the official journal of the Society for Neuroscience, 41(1), 153–166. 10.1523/JNEUROSCI.1490-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski K, Burns CD, Madison C, Clark D, Halchenko YO, Waskom ML, & Ghosh SS (2011). Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Frontiers in neuroinformatics, 5, 13. 10.3389/fninf.2011.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady C, Sarraf S, Saverino C, & Campbell K (2016). Age differences in the functional interactions among the default, frontoparietal control, and dorsal attention networks. Neurobiology of Aging, 41, 159–172. 10.1016/j.neurobiolaging.2016.02.020 [DOI] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, & Dougherty RF (2009). Resting-state functional connectivity reflects structural connectivity in the default mode network. Cerebral cortex (New York, N.Y. : 1991), 19(1), 72–78. 10.1093/cercor/bhn059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve DN, & Fischl B (2009). Accurate and robust brain image alignment using boundary-based registration. NeuroImage, 48(1), 63–72. 10.1016/j.neuroimage.2009.06.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, & Yang J (2020). Interplay of the long axis of the hippocampus and ventromedial prefrontal cortex in schema-related memory retrieval. Hippocampus, 30, 263–277. 10.1002/hipo.23154 [DOI] [PubMed] [Google Scholar]
- Gurguryan L, & Sheldon S (2019). Retrieval orientation alters neural activity during autobiographical memory recollection. NeuroImage, 199, 534–544. 10.1016/j.neuroimage.2019.05.077 [DOI] [PubMed] [Google Scholar]
- Hillary FG, Roman CA, Venkatesan U, Rajtmajer SM, Bajo R, & Castellanos ND (2015). Hyperconnectivity is a Fundamental Response to Neurological Disruption Introduction : Disconnecting the Brain. Neuropsychology, 29(1), 59–75. [DOI] [PubMed] [Google Scholar]
- Hodgetts CJ, Postans M, Warne N, Varnava A, Lawrence AD, & Graham KS (2017). Distinct contributions of the fornix and inferior longitudinal fasciculus to episodic and semantic autobiographical memory. Cortex, 94, 1–14. 10.1016/j.cortex.2017.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish M, Hornberger M, El Wahsh S, Lam BYK, Lah S, Miller L, … Piguet O (2014). Grey and white matter correlates of recent and remote autobiographical memory retrieval -insights from the dementias. PLoS ONE, 9(11). 10.1371/journal.pone.0113081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, & Smith S (2001). A global optimisation method for robust affine registration of brain images. Medical Image Analysis, 5(2), 143–156. 10.1016/S1361-8415(01)00036-6 [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady JM & Smith SM (2002). Improved Optimisation for the Robust and Accurate Linear Registration and Motion Correction of Brain Images. NeuroImage, 17(2), 825–841. [DOI] [PubMed] [Google Scholar]
- Jones DT, MacHulda MM, Vemuri P, McDade EM, Zeng G, Senjem ML, … Jack CR (2011). Age-related changes in the default mode network are more advanced in Alzheimer disease. Neurology, 77(16), 1524–1531. 10.1212/WNL.0b013e318233b33d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Bagarinao E, Isoda H, Koyama S, Watanabe H, Maesawa S, … Sobue G (2020). Effects of Head Motion on the Evaluation of Age-related Brain Network Changes Using Resting State Functional MRI. Magnetic Resonance in Medical Sciences, 10.2463/mrms.mp.2020-0081. Advance online publication. 10.2463/mrms.mp.2020-0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lempert KM, MacNear KA, Wolk DA, & Kable JW (2020). Links between autobiographical memory richness and temporal discounting in older adults. Scientific Reports, 10(1), 1–13. 10.1038/s41598-020-63373-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, & Moscovitch M (2002). Aging and autobiographical memory: Dissociating episodic from semantic retrieval. Psychology and Aging, 17(4), 677–689. 10.1037//0882-7974.17.4.677 [DOI] [PubMed] [Google Scholar]
- Madore KP, & Schacter DL (2014). An episodic specificity induction enhances means-end problem solving in young and older adults. Psychology and Aging, 29(4), 913–924. 10.1037/a0038209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli P, Sperduti M, & Piolino P (2013). Neural substrates of the self-memory system: New insights from a meta-analysis. Human Brain Mapping, 34(7), 1515–1529. 10.1002/hbm.22008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick C, St-Laurent M, Ty A, Valiante TA, & McAndrews MP (2015). Functional and effective hippocampal-neocortical connectivity during construction and elaboration of autobiographical memory retrieval. Cerebral cortex (New York, N.Y. : 1991), 25(5), 1297–1305. 10.1093/cercor/bht324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccormick C, Ciaramelli E, Luca D, & Maguire EA (2018). Comparing and Contrasting the Cognitive Effects of Hippocampal and Ventromedial Prefrontal Cortex Damage : A Review of Human Lesion Studies. Neuroscience, 374, 295–318. 10.1016/j.neuroscience.2017.07.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memel M, Wank AA, Ryan L, & Grilli MD (2020). The relationship between episodic detail generation and anterotemporal, posteromedial, and hippocampal white matter tracts. Cortex, 123, 124–140. 10.1016/j.cortex.2019.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M, Cabeza R, Winocur G, & Nadel L (2016). Episodic Memory and Beyond: The Hippocampus and Neocortex in Transformation. Annual Review of Psychology, 67(1), 105–134. 10.1146/annurev-psych-113011-143733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M, Rosenbaum RS, Gilboa A, Addis DR, Westmacott R, Grady C, … Nadel L (2005). Functional neuroanatomy of remote episodic, semantic and spatial memory : a unified account based on multiple trace theory. J. Anat, 207, 35–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KK, Lo JC, Lim JKW, Chee MWL, & Zhou J (2016). Reduced functional segregation between the default mode network and the executive control network in healthy older adults: A longitudinal study. NeuroImage, 133, 321–330. 10.1016/j.neuroimage.2016.03.029 [DOI] [PubMed] [Google Scholar]
- Palombo DJ, Bacopulos A, Amaral RSC, Olsen RK, Todd RM, Anderson AK, & Levine B (2018). Episodic autobiographical memory is associated with variation in the size of hippocampal subregions. Hippocampus, 28(2), 69–75. 10.1002/hipo.22818 [DOI] [PubMed] [Google Scholar]
- Peters SL, Fan CL, & Sheldon S (2019). Episodic memory contributions to autobiographical memory and open-ended problem-solving specificity in younger and older adults. Memory and Cognition, 47(8), 1592–1605. 10.3758/s13421-019-00953-1 [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, & Petersen SE (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59(3), 2142–2154. 10.1016/j.neuroimage.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim RHR, Mennes M, van Rooij D, Llera A, Buitelaar JK, & Beckmann CF (2015). ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. NeuroImage, 112, 267–277. 10.1016/j.neuroimage.2015.02.064 [DOI] [PubMed] [Google Scholar]
- Ramanan S, Piguet O, & Irish M (2018). Rethinking the Role of the Angular Gyrus in Remembering the Past and Imagining the Future: The Contextual Integration Model. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry, 24(4), 342–352. 10.1177/1073858417735514 [DOI] [PubMed] [Google Scholar]
- Ranganath C, & Ritchey M (2012). Two cortical systems for memory-guided behaviour. Nature Reviews Neuroscience, 13(10), 713–726. 10.1038/nrn3338 [DOI] [PubMed] [Google Scholar]
- Reagh ZM, & Ranganath C (2018). What does the functional organization of cortico-hippocampal networks tell us about the functional organization of memory?. Neuroscience letters, 680, 69–76. 10.1016/j.neulet.2018.04.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M, & Cooper RA (2020). Deconstructing the Posterior Medial Episodic Network. Trends in Cognitive Sciences, 24(6), 451–465. 10.1016/j.tics.2020.03.006 [DOI] [PubMed] [Google Scholar]
- Ritchey M, Libby LA, & Ranganath C (2015). Cortico-hippocampal systems involved in memory and cognition: the PMAT framework. Progress in brain research, 219, 45–64. 10.1016/bs.pbr.2015.04.001 [DOI] [PubMed] [Google Scholar]
- Robin J, & Moscovitch M (2017). Familiar real-world spatial cues provide memory benefits in older and younger adults. Psychology and aging, 32(3), 210–219. 10.1037/pag0000162 [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Moscovitch M, Foster JK, Schnyer DM, Gao F, Kovacevic N, … Levine B (2008). Patterns of autobiographical memory loss in medial-temporal lobe amnesic patients. Journal of Cognitive Neuroscience, 20(8), 1490–1506. 10.1162/jocn.2008.20105 [DOI] [PubMed] [Google Scholar]
- Savalia NK, Agres PF, Chan MY, Feczko EJ, Kennedy KM, & Wig GS (2017). Motion-related artifacts in structural brain images revealed with independent estimates of in-scanner head motion. Human Brain Mapping, 38(1), 472–492. 10.1002/hbm.23397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekeres MJ, Winocur G, & Moscovitch M (2018). The hippocampus and related neocortical structures in memory transformation. Neuroscience Letters, 680, 39–53. 10.1016/j.neulet.2018.05.006 [DOI] [PubMed] [Google Scholar]
- Sheldon S, Farb N, Palombo DJ, & Levine B (2016). Intrinsic medial temporal lobe connectivity relates to individual differences in episodic autobiographical remembering. Cortex, 74, 206–216. 10.1016/j.cortex.2015.11.005 [DOI] [PubMed] [Google Scholar]
- Sheldon S, & Levine B (2016). The role of the hippocampus in memory and mental construction. Annals of the New York Academy of Sciences, 1369(1), 76–92. 10.1111/nyas.13006 [DOI] [PubMed] [Google Scholar]
- Spreng RN, Lockrow AW, DuPre E, Setton R, Spreng K, & Turner GR (2018). Semanticized autobiographical memory and the default - executive coupling hypothesis of aging. Neuropsychologia, 110, 37–43. 10.1016/j.neuropsychologia.2017.06.009 [DOI] [PubMed] [Google Scholar]
- Staffaroni XAM, Brown JA, Casaletto KB, Elahi FM, Neuhaus J, Cobigo Y, … Kramer JH (2018). The Longitudinal Trajectory of Default Mode Network Connectivity in Healthy Older Adults Varies As a Function of Processing Speed. The Journal of Neuroscience, 38(11), 2809–2817. 10.1523/JNEUROSCI.3067-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, & Levine B (2007). Ageing and autobiographical memory for emotional and neutral events. Memory (Hove, England), 15(2), 129–144. 10.1080/09658210601119762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, Kragel PA, & Rubin DC (2011). Dynamic neural networks supporting memory retrieval. NeuroImage, 57(2), 608–616. 10.1016/j.neuroimage.2011.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, & Levine B (2006). The functional neuroanatomy of autobiographical memory: A meta-analysis. Neuropsychologia, 44(12), 2189–2208. 10.1016/j.neuropsychologia.2006.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakral PP, Madore KP, & Schacter DL (2017). A Role for the Left Angular Gyrus in Episodic Simulation and Memory. The Journal of Neuroscience, 37(34), 8142–8149. 10.1523/JNEUROSCI.1319-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, & Gee JC (2010). N4ITK: Improved N3 Bias Correction. IEEE Transactions on Medical Imaging, 29(6), 1310–1320. 10.1109/TMI.2010.2046908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk KRA, Sabuncu MR, & Buckner RL (2012). The influence of head motion on intrinsic functional connectivity MRI. NeuroImage, 59(1), 431–438. 10.1016/j.neuroimage.2011.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wank AA, Andrews-Hanna JR, & Grilli MD (2021). Searching for the past: Exploring the dynamics of direct and generative autobiographical memory reconstruction among young and cognitively normal older adults. Memory & cognition, 49(3), 422–437. 10.3758/s13421-020-01098-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wank AA, Mehl MR, Andrews-Hanna JR, Polsinelli AJ, Moseley S, Glisky EL, & Grilli MD (2020). Eavesdropping on Autobiographical Memory: A Naturalistic Observation Study of Older Adults’ Memory Sharing in Daily Conversations. Frontiers in human neuroscience, 14, 238. 10.3389/fnhum.2020.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, & Nieto-Castanon A (2012). Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connectivity, 2(3), 125–141. 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- Williams AN, Ridgeway S, Postans M, Graham KS, Lawrence AD, & Hodgetts CJ (2020). The role of the pre-commissural fornix in episodic autobiographical memory and simulation. Neuropsychologia, 142, 107457. 10.1016/j.neuropsychologia.2020.107457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brady M, & Smith S (2001). Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging, 20(1), 45–57. 10.1109/42.906424 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


