Abstract
Objective:
Co-prescription of opioids and benzodiazepines (BDZ) is associated with adverse outcomes, including greater healthcare utilization and overdose risk. This study aims to examine opioid and BDZ co-prescription, dosing, and mortality among patients with and without opioid use disorder (OUD) in a large healthcare system.
Methods:
Using data from the California state Prescription Drug Monitoring Program during 2010–2014 linked with a large healthcare system electronic health record database and mortality records from the Centers for Disease Control National Death Index, this study examined 5,202 patients (1,978 with OUD, 3,224 controls). Multiple logistic regression analyses were conducted to examine relationships between most recent BDZ and opioid prescription, and their interaction with respect to mortality.
Results:
10.5% of the sample died on or before December 31, 2014. 17.7% were prescribed BDZ during the final month of observation. Individuals with OUD were prescribed higher average BDZ and opioid doses than those without OUD. After adjusting for covariates, increased prescribed doses of BDZ (odds ratio [OR]=1.34, 95%CI: 1.15–1.55 per 10 mg/day increment) and opioids (OR=1.04, 95%CI: 1.02–1.05 per 10 mg/day increment) were positively associated with mortality. Non-OUD patients who received both BDZ and opioid prescriptions had a higher mortality than those who received only BDZ or opioids (The ratio of odds ratio (ROR) =3.83, 95%CI: 1.78–8.21).
Conclusions:
Study findings highlight significant mortality associated with the co-prescription of opioids and BDZ in a general healthcare setting. Further research is needed to elucidate factors associated with mortality among non-OUD patients who are co-prescribed opioids and BDZ.
Keywords: benzodiazepines prescription, PDMP, mortality, opioid use disorder, general healthcare system
Introduction
A significant proportion of opioid overdose deaths occur in the presence of benzodiazepines (BDZ), sedative medications commonly prescribed for anxiety and insomnia1–3. Benzodiazepines increase the risk of fatal overdose from respiratory suppression when taken together with other central nervous system depressants such as opioids or alcohol1,2. Though recent clinical guidelines caution against co-prescription of BDZ and opioids4, BDZ are commonly co-prescribed with opioids2,5,6, and rates of co-prescription in outpatient settings have risen in recent decades7. The number of BDZ prescriptions filled in the United States rose by two-thirds between 1996 and 20136, and in 2015 nearly a quarter of individuals who died from opioid overdose also tested positive for BDZ8. Among individuals prescribed opioids in the U.S., the proportion with co-prescribed BDZ rose from approximately 2001 to 20145,9. Studies during a similar period suggest increased BDZ quantity and dosage among those filling prescriptions6. Among individuals with non-cancer diagnoses prescribed opioids, BDZ co-prescription is associated with a higher prescribed opioid dose and longer duration of prescription10. Co-prescription is associated with emergency department visits and inpatient admissions9,11, and elevated rates of medical, mental and substance use comorbidities12.
Prior work has suggested associations between BDZ use and all-cause mortality, even for short durations of use13,14, as well as associations with specific causes of death including cardiovascular disease and cancer15,16, though findings have been mixed and inconclusive17. Studies investigating specific harms of BDZ and opioid co-prescription utilizing electronic health record (EHR) data linked with prescription drug monitoring databases (PDMP), and those focusing on at-risk populations, including those with OUD, are relatively lacking. Extant studies that have utilized EHRs of single healthcare systems or Medicaid claims18,19 do not capture a broader, comprehensive range of populations filling prescriptions from multiple providers and pharmacies, including simultaneous prescriptions.
We previously demonstrated that escalating prescribed opioid dose was associated with all-cause mortality in a large health system20. Linking individuals’ medical, death, and PDMP records, the present study expands our previous efforts to investigate BDZ and opioid co-prescribing and dosage among patients with OUD and matched patients without OUD in relation to their mortality. We hypothesized that patients with OUD would be more likely to be prescribed BDZ than non-OUD patients; average daily dose of prescribed BDZ would be higher in OUD patients than non-OUD patients; higher BDZ dose would be associated with greater mortality in both OUD and non-OUD patients; and that co-prescription of BDZ and opioids would be associated with greater mortality than either alone in both OUD and non-OUD patients.
Methods
Study participants
The sample is derived from 7,728 patients treated in an academic health center in Los Angeles, consisting of 2,576 patients with OUD diagnosis (International Classification of Diseases [ICD]-9th Edition-CM codes 304.0x, 304.7x, or 305.5x) in the EHR aged 18 to 64 years at their first OUD diagnosis and 5,152 control patients, matched by sex, date of birth (within three years), first encounter (within one year), and the Elixhauser Comorbidity Index20. Data from 2006 to 2014 were collected from an electronic health record (EHR) system utilizing Epic software. Among the 7,728 individuals, 5,202 individuals (1,978 cases and 3,224 controls) during 2010–2014 were matched in PDMP and were included in the study. This study was approved by the Institutional Review Boards at UCLA and the State of California.
Measures
The primary outcome was all-cause mortality status at the end of follow-up, which was December 31, 2014, for the alive patients or date of death for the deceased patients. Mortality records, available through December 31, 2014, were obtained from the Centers for Disease Control and Prevention (CDC) National Death Index (NDI).
The California state Prescription Drug Monitoring Program (PDMP), also known as CURES is a database of prescription records of Schedule II, III, and IV controlled substance prescriptions dispensed in California. The description of PDMP data and conversion of opioid medications to morphine milligram equivalent (MME) have been described previously20. All opioid prescriptions except buprenorphine were included in the analysis of opioid use (as its primary use is for treating OUD). We defined most recent opioid use as at least one opioid prescription in the last 30 days of observation (30 days prior to death or 12/31/2014). The daily opioid dose was calculated by averaging MME of all opioid prescriptions in the last month of observation over 30 days.
BDZ medications were converted to diazepam milligram equivalents (DME) for comparison purposes21,22. We defined most recent BDZ use as at least one BDZ prescription in the last 30 days of observation. The daily BDZ dose was calculated by averaging DME of all BDZ prescriptions in the last month of observation over 30 days.
Covariates were obtained from the medical records. Sociodemographic variables included age, sex, race, and insurance status. Clinical variables were defined from the ICD-9 codes. Included comorbidities were physical conditions (diabetes, sexually transmitted disease (STD), cancer, heart disease, respiratory disease, liver disease, sleep disorder, chronic pain, human immunodeficiency virus (HIV), and Hepatitis C (HCV); psychiatric conditions (depression, anxiety, bipolar disorder, psychotic disorder, and other); and substance use disorders (alcohol, amphetamine, cannabis, cocaine, hallucinogen, and tobacco).
Data Analysis
We conducted t-tests for continuous variables and chi-square tests for categorical variables to examine differences in demographics, comorbidities, prescription patterns, and mortality status between OUD and non-OUD patients. For multivariate analyses, a logistic regression model was used to examine the association between the prescription of BDZ together with opioids and mortality. In addition to the primary independent variable (OUD diagnosis in the EHR, and BDZ and opioid prescriptions in the last month of observation), other significant covariates (e.g. age, sex, race, insurance status, alcohol use disorders, heart disease) from univariate analyses were included to build additional models. Covariates considered as potential confounders were identified based on a combination of clinical significance and significant p value. Akaike Information Criterion (AIC) and deviance were calculated. Also, after stratifying by OUD diagnosis, an interaction term of BDZ and opioid use in the last month of observation was included in the models with same covariates as the third model to examine the moderating effect of co-prescription on mortality. All statistical tests were based on a significance level of α ≤ .05. Analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC).
Results
Patient demographics and comorbidities
We compared sample characteristics of demographics and comorbidities by OUD diagnosis (Table 1). Compared to the non-OUD patients, OUD patients had a higher proportion of white and self-pay individuals. In terms of substance use disorders, alcohol, amphetamine, cannabis, cocaine, hallucinogen, and tobacco use disorders were more prevalent (all p<0.001) among the OUD compared to non-OUD patients. Also, the OUD patients had more physical conditions (chronic pain (66.5% vs. 56.4%), HIV (2.3% vs. 1.4%, p<0.05), HCV (22.2% vs 10.7%); all p<0.001 unless noted otherwise) and psychiatric comorbidities (e.g. anxiety (35.9% vs. 31.4%), bipolar disorder (13.4% vs. 7.4%), psychotic disorder (10.9% vs. 8.2%, p<0.01); all p<0.001 unless noted otherwise)) relative to non-OUD patients.
Table 1.
Sociodemographic characteristics, other substance use disorders, physical and psychiatric comorbidity
| Characteristic | OUD (n=1,978) | Non-OUD (n=3,224) | Total (N=5,202) |
|---|---|---|---|
| Age at the end of follow-up (Death or 12/31/2014) | |||
| <30 | 324 (16.4) | 465 (14.4) | 789 (15.2) |
| 30–49 | 787 (39.8) | 1,248 (38.7) | 2035 (39.1) |
| 50–69 | 859 (43.4) | 1491 (46.2) | 2350 (45.2) |
| ≥70 | 8 (0.4) | 20 (0.6) | 28 (0.5) |
| Female | 815 (41.2) | 1,334 (41.4) | 2,149 (41.3) |
| Race/Ethnicity*** | |||
| Asian | 37 (1.9) | 156 (4.8) | 193 (3.7) |
| Black | 152 (7.7) | 279 (8.7) | 431 (8.3) |
| Hispanic | 64 (3.2) | 150 (4.7) | 214 (4.1) |
| Other | 174 (8.8) | 574 (17.8) | 748 (14.4) |
| White | 1,551 (78.4) | 2,065 (64.1) | 3,616 (69.5) |
| Insurance*** | |||
| Medicaid | 233 (11.8) | 190 (5.9) | 423 (8.1) |
| Medicare | 293 (14.8) | 424 (13.2) | 717 (32.6) |
| Private | 455 (23.0) | 1,239 (38.4) | 1,694 (32.6) |
| Self-pay | 997 (50.4) | 1,371 (42.5) | 2,368 (45.5) |
| Substance use disorder (ever diagnosed) | |||
| Alcohol*** | 503 (25.4) | 680 (21.1) | 1,183 (22.7) |
| Amphetamine*** | 238 (12.0) | 71 (2.2) | 309 (5.9) |
| Cannabis*** | 224 (11.3) | 151 (4.7) | 375 (7.2) |
| Cocaine*** | 259 (13.1) | 113 (3.5) | 372 (7.2) |
| Hallucinogen*** | 62 (3.1) | 15 (0.5) | 77 (1.5) |
| Tobacco*** | 402 (20.3) | 273 (8.5) | 675 (13.0) |
| Physical comorbidity (ever diagnosed) | |||
| Diabetes | 276 (14.0) | 444 (13.8) | 720 (13.8) |
| STD | 24 (1.2) | 40 (1.2) | 64 (1.2) |
| Cancer | 286 (14.5) | 468 (14.5) | 754 (14.5) |
| Heart disease | 1,104 (55.9) | 1,806 (56.0) | 2,910 (55.9) |
| Respiratory disease | 1,014 (51.3) | 1,725 (53.5) | 2,739 (52.7) |
| Liver disease | 404 (20.4) | 622 (19.3) | 1,026 (19.7) |
| Sleep disorder | 371 (18.8) | 666 (20.7) | 1,037 (19.9) |
| Chronic pain*** | 1,316 (66.5) | 1,818 (56.4) | 3,134 (60.3) |
| HIV* | 45 (2.3) | 45 (1.4) | 90 (1.7) |
| HCV*** | 439 (22.2) | 346 (10.7) | 785 (15.1) |
| Psychiatric comorbidity (ever diagnosed) | |||
| Any mental disorder*** | 1,360 (68.8) | 1,957 (60.7) | 3,317 (63.8) |
| Depression | 938 (47.4) | 1,510 (46.8) | 2,448 (47.1) |
| Anxiety*** | 710 (35.9) | 1,011 (31.4) | 1,721 (33.1) |
| Bipolar disorder*** | 265 (13.4) | 237 (7.4) | 502 (9.7) |
| Psychotic disorder** | 216 (10.9) | 265 (8.2) | 481 (9.3) |
| Other mental conditions*** | 713 (36.1) | 844 (26.2) | 1,557 (29.9) |
T-test for continuous variables and chi-square test for categorical variables;
p<.05,
p<.01,
p<.001
Association between opioid and BDZ prescription, OUD diagnosis, and mortality
In total, 10.5% of the sample (n=547) died at the end of follow-up (Table 2). The proportion of death was higher among OUD relative to non-OUD patients (13.7 % vs. 8.6%, p<0.001). BDZ and opioid prescriptions were calculated for the last month of follow-up. 17.7% of the sample (n=920) were prescribed BDZ during the last month of follow-up. More individuals with OUD were prescribed BDZ in the final month of follow-up than those without OUD (25.2% vs. 13.1%, p<0.001), and at higher average BDZ doses (DME, Mean: 1.9mg (SD 7.8) vs. 0.6mg (SD 3.1), p<0.001). OUD patients were prescribed higher average opioid doses (MME, Mean: 19.2mg (SD 105.9) vs. 4.0mg (SD 29.9), p<0.001). The proportion of co-prescription of BDZ and opioids in the final month of follow-up was higher among OUD compared with non-OUD patients (18.3% vs. 7.4%). Among those who received BDZ prescription in the last month of follow-up, the average opioid dose was higher among OUD relative to non-OUD patients (MME, Mean: 49.1mg (SD 165.7) vs. 19.2mg (SD 68.2), p<0.001,) in Supplementary Table 1.
Table 2.
Mortality status and prescription patterns in the final month of follow-up
| Characteristic | OUD (n=1,978) | Non-OUD (n=3,224) | Total (n=5,202) |
|---|---|---|---|
| Death status at the end of follow-up, n(%)*** | 270 (13.7) | 277 (8.6) | 547 (10.5) |
| Prescribed BDZ in the last 30 days, n(%)*** | 499 (25.2) | 421 (13.1) | 920 (17.7) |
| BDZ dose in the last 30 days (diazepam milligram equivalent per day), mean (SD)*** | 1.9 (7.8) | 0.6 (3.1) | 1.1 (5.4) |
| Prescribed opioid in the last 30 days, n(%) | 1083 (54.8) | 1692 (52.5) | 2775 (53.3) |
| Opioid dose in the last 30 days (morphine milligram equivalent per day), mean (SD)*** | 19.2 (105.9) | 4.0 (29.9) | 9.8 (69.8) |
| Co-prescription of BDZ and opioids, n(%)*** | 362 (18.3) | 238 (7.4) | 600 (11.5) |
T-test for continuous variables and chi-square test for categorical variables;
p<.05,
p<.01,
p<.001
Association between co-prescription of BDZ and opioids and mortality
Association between mortality and most recent medication use was examined using logistic regression models (Table 3). After adjusting for covariates including social-demographic factors, substance use disorders, and comorbidity in Model 3, increased average daily dose of BDZ (odds ratio [OR]=1.34, 95%CI: 1.15–1.55 per 10 mg/day increment) and opioid prescriptions (OR=1.04, 95%CI: 1.02–1.05 per 10 mg/day increment) was associated with elevated mortality. Results of multiple regression analyses showed that specific demographic and comorbidity variables were associated with mortality. Older age (OR=1.02, 95%CI: 1.01, 1.03) or self-pay for health care (OR=8.61, 95%CI: 6.72, 11.02) was associated with increased mortality. For substance use disorders, having a diagnosis of opioid (OR=1.42, 95%CI: 1.13, 1.77) or alcohol use disorder (OR=1.47, 95%CI: 1.16, 1.86) was associated with increased mortality. In terms of physical comorbidities, heart disease, liver disease, cancer, and HCV were statistically significant. In stratified analyses, addition of interaction terms did not change any model 3 findings. However, the multiplicative interaction between BDZ and opioid prescription on mortality was significant among non-OUD patients (The ratio of odds ratio (ROR) =3.83, 95%CI: 1.78, 8.21) meaning that non-OUD patients who received both BDZ and opioid prescriptions had higher mortality than those who received only BDZ or opioids. However, the multiplicative interaction was not significant among OUD patients (ROR=1.29, 95%CI: 0.63, 2.62),
Table 3.
Logistic regression model for mortality at the end of follow-up
| OR (95% CI) | |||
|---|---|---|---|
| Parameter | Model 1 | Model 2 | Model 3 |
| OUD (vs. non-OUD) | 1.44 (1.20–1.73)*** | 1.49 (1.22–1.81)*** | 1.42 (1.13–1.77)** |
| BDZ prescription in the last month (vs. none) | 2.58 (2.11–3.14)*** | 2.80 (2.26–3.48)*** | |
| Opioid prescription in the last month (vs. none) | 1.73 (1.43–2.10)*** | 1.68 (1.37–2.06)*** | |
| 10 DME per day in the last month | 1.34 (1.15–1.55)*** | ||
| 10 MME per day in the last month | 1.04 (1.02–1.05)*** | ||
| Socio-demographics | |||
| Age at the end of follow-up (years) | 1.05 (1.04–1.06)*** | 1.02 (1.01–1.03)*** | |
| Female (vs. male) | 0.66 (0.54–0.81)*** | 0.85 (0.67–1.06) | |
| White (vs. non-white) | 1.00 (0.81–1.24) | 0.92 (0.73–1.15) | |
| Self-pay/other (vs. public and private insurance) | 6.71 (5.35–8.41)*** | 8.61 (6.72–11.02)*** | |
| Substance use disorders | |||
| Alcohol | 1.47 (1.16–1.86)** | ||
| Amphetamine | 0.65 (0.42–1.01) | ||
| Cocaine | 0.72 (0.49–1.06) | ||
| Marijuana | 1.26 (0.88–1.80) | ||
| Tobacco | 0.96 (0.72–1.28) | ||
| Comorbidity | |||
| Heart | 2.69 (2.07–3.49)*** | ||
| Liver | 3.07 (2.38–3.95)*** | ||
| Cancer | 2.07 (1.60–2.67)*** | ||
| Chronic pain | 1.08 (0.85–1.36) | ||
| HIV | 1.44 (0.70–2.96) | ||
| HCV | 1.55 (1.18–2.04)** | ||
| Any mental disorders | 1.15 (0.92–1.45) | ||
| AIC | 3345.06 | 2883.19 | 2598.18 |
| −2 Log-likelihood | 3337.06 | 2867.19 | 2558.18 |
Note: OUD, opioid use disorder; BDZ, benzodiazepine; DME, diazepam milligram equivalents; MME, morphine milligram equivalent.
p<0.05,
p<0.01,
p<0.001
Discussion
Using the state’s PDMP records linked with EHR and CDC NDI data, this study explored the co-prescription of BDZ and opioids and its association with all-cause mortality among patients in a large healthcare system. We found that 1) OUD patients had higher rates of other substance use disorders, mental and physical comorbidities relative to non-OUD; 2) OUD patients were prescribed both BDZ and opioids in significantly higher doses compared to non-OUD patients; 3) most recent average daily BDZ or opioid prescription dose was significantly associated with increased all-cause mortality after adjusting for covariates, and 4) there is a significant interaction between BDZ and opioid co-prescription on mortality among non-OUD patients. Though this study analyzed records prior to 2015, national efforts and guidelines have been implemented since that time to help combat problems associated with co-prescription of BDZ and opioids. Findings from this study support recommendations to minimize co-prescription of BDZ and opioids whenever possible4, and to use lowest effective doses when prescribing BDZ or opioids.
OUD vs. non-OUD patients
Our study extends prior literature by examining a general patient population from a large health care system with a larger proportion of privately insured individuals and focusing on a population at risk given OUD diagnosis history. Compared to non-OUD patients, we found higher rates of all-cause mortality and higher average doses of BDZ prescribed among OUD patients. Prior research has demonstrated an increased likelihood of BDZ prescription in persons with chronic noncancer pain prescribed higher doses of opioids12,23. We also observed elevated rates of physical and mental health comorbidities in OUD patients relative to non-OUD patients. Accumulating evidence has supported the positive association between opioid use and physical or psychiatric comorbidities, which presents considerable management challenges in clinical practice and contributes to mortality risk24,25.
Opioid and BDZ prescriptions and mortality
The findings in this study suggest that use of either opioids or BDZ is significantly associated with all-cause mortality, which is consistent with our previous findings20 and prior studies26. Furthermore, our finding of the association between opioid dosage and mortality was in accordance with prior studies demonstrating mortality risk associated with opioid prescribing in a dose-dependent manner27,28. The CDC guideline recommends risk review and mitigation for opioid dosages greater than 90 MME per day4; dosages as low as 50 MME per day can increase the risk for drug-related adverse events for patients with chronic pain29. For BDZ, we found 36.3% (98/270) of deaths among OUD patients and 34.7% (96/277) among non-OUD patients were BDZ involved. We also found that increased daily BDZ dosage was associated with mortality risk after adjusting for potential confounders, which is consistent with prior literature focused on overdose risk6,30 as well as all-cause mortality13,15 associated with BDZ. Prior studies have reported BDZ dose relationships with mortality as quantified by cumulative prescribed doses over observation periods14,31; this study extends prior work to include average prescribed dose in the final month of observation. This finding of increased mortality associated with BDZ dose may be related to management of underlying medical conditions closer to time of death, medication interaction effects, or other factors.
The association between co-prescription of opioids and BDZ and overdose risk or mortality has been reported among Medicare32 and Medicaid33,34 enrollees and veterans30. Findings from this study extend to a primarily privately insured population. In our study, it is worth noting that there was a significant interaction between BDZ and opioid co-prescription on all-cause mortality among patients without OUD diagnosis, but not in those with a diagnosis of OUD, despite elevated rates of physical and psychiatric comorbidities in those with OUD. Although our observational study cannot determine the etiology of this differential finding, a contributing factor might be higher opioid tolerance to the effect of respiratory suppression among OUD patients compared with non-OUD patients. As reported, higher doses of opioids and BDZ were prescribed in patients with OUD. Clinicians should be cautious about prescribing BDZ to patients using opioids, whether or not they have a diagnosis of OUD, and should be aware of the high rates of comorbidity in this population. More studies are warranted to help elucidate the potential medication interaction of opioid and BDZ.
Other factors associated with mortality
The results of this study revealed several covariates associated with increased risk for all-cause mortality in addition to opioid and BDZ use. We found that self-pay for health care was a strong risk factor associated with mortality compared with public and private insurance; this could be related to health care access or utilization in this population or other factors and warrants further study. Not surprisingly, older age, alcohol use disorder comorbidity, and some physical health comorbidities, such as heart, liver, cancer, and HCV, were significantly associated with all-cause mortality.
Limitations
This study has several limitations. First, findings based on EHR data are limited by provider diagnostic coding and documentation choices, which are influenced by clinical experience and billing requirements. This may affect the accuracy of the data as it was not collected specifically for research purposes; diagnoses may be over- or under-captured. Like other records-based research, diagnoses and prescriptions are limited to this healthcare system and PDMP records; those obtained outside of the system were not captured, and thus dosages may not accurately reflect the total amount consumed. It is possible that patients used remaining BDZ or opioids from prescriptions prior to the last 30 days of the follow-up or from other sources such as friends or illicit purchases. Therefore, our findings are likely a conservative estimate. Second, the number of overdose deaths is relatively low in this study, and overdose deaths may be misclassified, so we decided to use all-cause mortality instead. Participants were predominantly white, insured individuals living in the Los Angeles area, limiting the generalizability of findings. Lastly, residual confounding is still possible, even after adjusting for many demographic and comorbidity covariates.
Conclusions
This study replicates and extends literature highlighting elevated mortality risk associated with BDZ and opioid co-prescription, particularly among individuals without OUD. Further research is needed to elucidate factors associated with mortality among patients who are co-prescribed opioids and BDZ.
Supplementary Material
Figure 1.
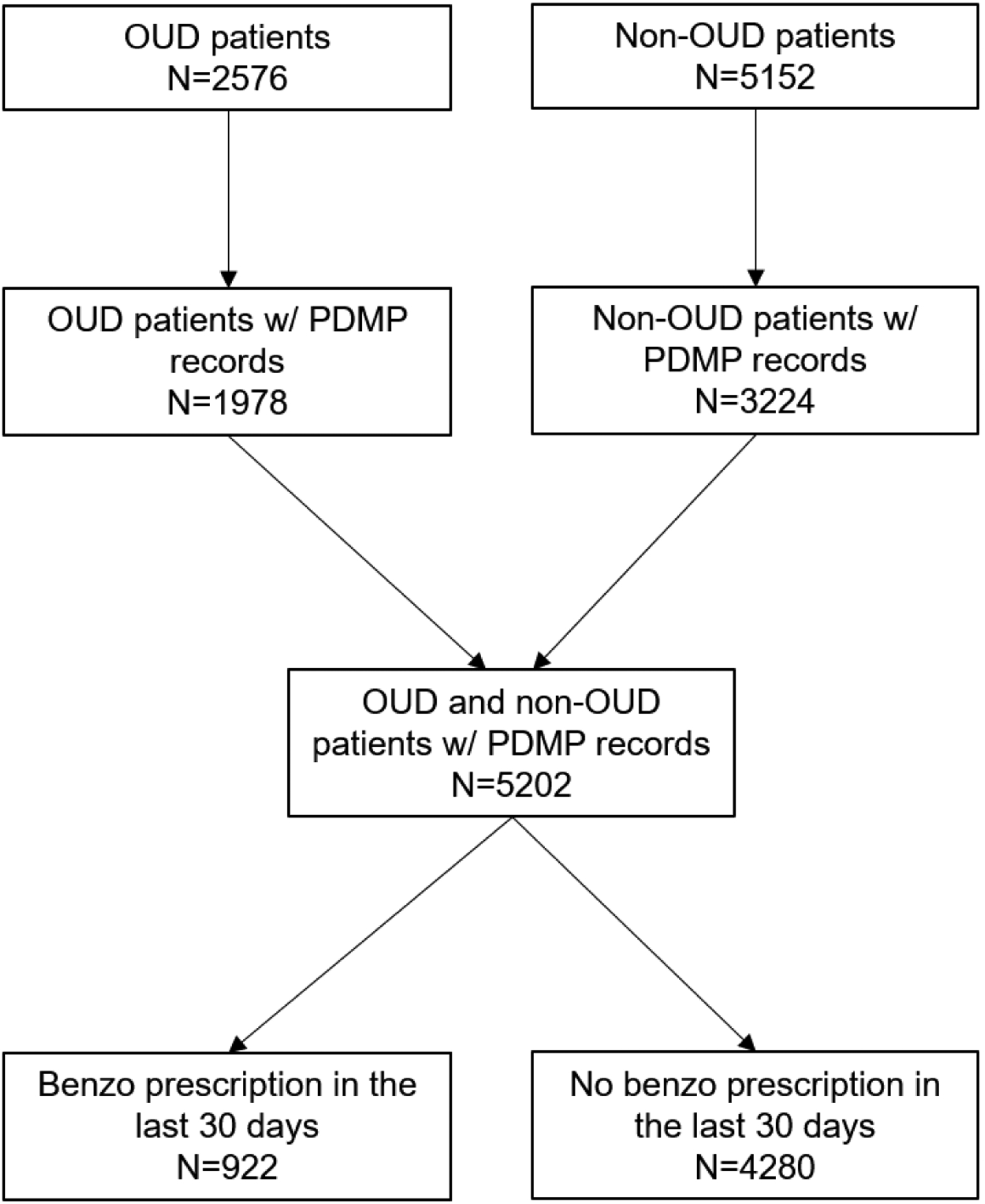
Sample flowchart
Table 4.
The interactions between opioid and benzodiazepine prescriptions in the last month of follow up on mortality, stratified by OUD diagnosis
| OUD | Non-OUD | ||||
|---|---|---|---|---|---|
| Opioid prescription | BDZ prescription | OR (95% CI) | ROR | OR (95% CI) | ROR |
| No | No | 1.00 | 1.29 (0.63, 2.62) | 1.00 | 3.83 (1.78, 8.21)*** |
| No | Yes | 2.00 (1.10–3.62)* | 1.64 (0.86, 3.14) | ||
| Yes | No | 1.20 (0.82–1.74) | 1.22 (0.86, 1.72) | ||
| Yes | Yes | 3.08 (2.02, 4.69)*** | 7.65 (4.94, 11.84)*** | ||
ROR: the ratio of odds ratio; Adjusted for all covariates in the Model 3 (
p<0.05,
p<0.01,
p<0.001)
Acknowledgments
Special appreciation to UCLA CareConnect, UCLA Clinical and Institutional Science Institute (CTSI), EMMES Corporation NIDA Data and Statistics Center (DSC), Center for Clinical Trials Network (CCTN), and National Institute on Drug Abuse. Thanks to Mike Small, Program Manager, Department of Justice, California Prescription Drug Monitoring Program.
Funding Source:
Support provided through the National Institute on Drug Abuse (UG1DA049435).
Footnotes
Declaration of Interest:
Larissa J. Mooney: received prior travel support and consultant fees from Alkermes, Inc.
All other authors report no financial or other possible conflicts of interest.
References
- 1.NIDA. Benzodiazepines and opioids. https://www.drugabuse.gov/drugs-abuse/opioids/benzodiazepines-opioids. Published 2018. Accessed August 5, 2019.
- 2.Schmitz A Benzodiazepine use, misuse, and abuse: A review. Ment Heal Clin. 2016;6(3):120–126. doi: 10.9740/mhc.2016.05.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darke S, Duflou J, Torok M. The comparative toxicology and major organ pathology of fatal methadone and heroin toxicity cases. Drug Alcohol Depend. 2010;106(1):1–6. doi: 10.1016/j.drugalcdep.2009.07.014 [DOI] [PubMed] [Google Scholar]
- 4.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624–1645. doi: 10.1001/jama.2016.1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hwang CS, Kang EM, Kornegay CJ, Staffa JA, Jones CM, McAninch JK. Trends in the concomitant prescribing of opioids and benzodiazepines, 2002−2014. Am J Prev Med. 2016;51(2):151–160. doi: 10.1016/J.AMEPRE.2016.02.014 [DOI] [PubMed] [Google Scholar]
- 6.Bachhuber MA, Hennessy S, Cunningham CO, Starrels JL. Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996–2013. Am J Public Health. 2016;106(4):686–688. doi: 10.2105/AJPH.2016.303061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirschtritt ME, Delucchi KL, Olfson M. Outpatient, combined use of opioid and benzodiazepine medications in the United States, 1993–2014. Prev Med Reports. 2018;9:49–54. doi: 10.1016/J.PMEDR.2017.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Multiple cause of death, 1999–2015. CDC WONDER Online Database. https://wonder.cdc.gov/mcd-icd10.html. Published 2017. Accessed March 12, 2019. [Google Scholar]
- 9.Sun EC, Dixit A, Humphreys K, Darnall BD, Baker LC, Mackey S. Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ. 2017;356:j760. doi: 10.1136/bmj.j760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paulozzi LJ, Zhang K, Jones CM, Mack KA. Risk of adverse health outcomes with increasing duration and regularity of opioid therapy. J Am Board Fam Med. 2014;27(3):329–338. doi: 10.3122/jabfm.2014.03.130290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones CM, McAninch JK. Emergency department visits and overdose deaths from combined use of opioids and benzodiazepines. Am J Prev Med. 2015;49(4):493–501. doi: 10.1016/j.amepre.2015.03.040 [DOI] [PubMed] [Google Scholar]
- 12.Nielsen S, Lintzeris N, Bruno R, et al. Benzodiazepine use among chronic pain patients prescribed opioids: associations with pain, physical and mental health, and health service utilization. Pain Med. 2015;16(2):356–366. doi: 10.1111/pme.12594 [DOI] [PubMed] [Google Scholar]
- 13.Kripke DF, Langer RD, Kline LE. Hypnotics’ association with mortality or cancer: a matched cohort study. BMJ Open. 2012;2(1):e000850. doi: 10.1136/bmjopen-2012-000850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weich S, Pearce HL, Croft P, et al. Effect of anxiolytic and hypnotic drug prescriptions on mortality hazards: retrospective cohort study. BMJ. 2014;348:g1996. doi: 10.1136/BMJ.G1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mallon L, Broman J-E, Hetta J. Is usage of hypnotics associated with mortality? Sleep Med. 2009;10(3):279–286. doi: 10.1016/J.SLEEP.2008.12.004 [DOI] [PubMed] [Google Scholar]
- 16.Belleville G Mortality hazard associated with anxiolytic and hypnotic drug use in the national population health survey. Can J Psychiatry. 2010;55(9):558–567. doi: 10.1177/070674371005500904 [DOI] [PubMed] [Google Scholar]
- 17.Patorno E, Glynn RJ, Levin R, Lee MP, Huybrechts KF. Benzodiazepines and risk of all cause mortality in adults: cohort study. BMJ. 2017;358:j2941. doi: 10.1136/bmj.j2941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell CI, Bahorik AL, VanVeldhuisen P, Weisner C, Rubinstein AL, Ray GT. Use of a prescription opioid registry to examine opioid misuse and overdose in an integrated health system. Prev Med (Baltim). 2018;110:31–37. doi: 10.1016/J.YPMED.2018.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali MM, Tehrani AB, Mutter R, et al. Potentially problematic opioid prescriptions among individuals with private insurance and medicaid. Psychiatr Serv. 2019;70(8):681–688. doi: 10.1176/appi.ps.201800555 [DOI] [PubMed] [Google Scholar]
- 20.Hser Y-I, Saxon AJ, Mooney LJ, et al. Escalating opioid dose is associated with mortality: A comparison of patients with and without opioid use disorder. J Addict Med. 2019;13(1):41–46. doi: 10.1097/ADM.0000000000000458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galanter M, Kleber HD, Brady KT. The American Psychiatric Publishing Textbook of Substance Abuse Treatment. 5th ed. American Psychiatric Publishing, Washington, DC; 2014. [Google Scholar]
- 22.Bope ET, Kellerman RD. Conn’s Current Therapy 2017. Philadelphia: Elsevier; 2016. [Google Scholar]
- 23.Saunders KW, Von Korff M, Campbell CI, et al. Concurrent use of alcohol and sedatives among persons prescribed chronic opioid therapy: prevalence and risk factors. J Pain. 2012;13(3):266–275. doi: 10.1016/J.JPAIN.2011.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slawek DE, Lu TY, Hayes B, Fox AD. Caring for patients with opioid use disorder: what clinicians should know about comorbid medical conditions. Psychiatr Res Clin Pract. 2019;1(1):16–26. doi: 10.1176/appi.prcp.20180005 [DOI] [Google Scholar]
- 25.Barry DT, Cutter CJ, Beitel M, Kerns RD, Liong C, Schottenfeld RS. Psychiatric disorders among patients seeking treatment for co-occurring chronic pain and opioid use disorder HHS Public Access. J Clin Psychiatry. 2016;77(10):1413–1419. doi: 10.4088/JCP.15m09963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King NB, Fraser V, Boikos C, Richardson R, Harper S. Determinants of increased opioid-related mortality in the United States and Canada, 1990–2013: a systematic review. Am J Public Health. 2014;104(8):e32–42. doi: 10.2105/AJPH.2014.301966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dasgupta N, Funk MJ, Proescholdbell S, Hirsch A, Ribisl KM, Marshall S. Cohort study of the impact of high-dose opioid analgesics on overdose mortality. Pain Med. 2016;17:85–98. doi: 10.1111/pme.12907 [DOI] [PubMed] [Google Scholar]
- 28.Adewumi AD, Hollingworth SA, Maravilla JC, Connor JP, Alati R. Prescribed dose of opioids and overdose: a systematic review and meta-analysis of unintentional prescription opioid overdose. CNS Drugs. 2018;32(2):101–116. doi: 10.1007/s40263-018-0499-3 [DOI] [PubMed] [Google Scholar]
- 29.Bohnert ASB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315. doi: 10.1001/jama.2011.370 [DOI] [PubMed] [Google Scholar]
- 30.Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert ASB. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350(jun10 9):h2698. doi: 10.1136/bmj.h2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kripke DF. Hypnotic drug risks of mortality, infection, depression, and cancer: But lack of benefit. F1000Research. 2016;5:918. doi: 10.12688/f1000research.8729.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hernandez I, He M, Brooks MM, Zhang Y. Exposure-response association between concurrent opioid and benzodiazepine use and risk of opioid-related overdose in medicare part D beneficiaries. JAMA Netw Open. 2018;1(2):e180919. doi: 10.1001/jamanetworkopen.2018.0919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cochran G, Lo-Ciganic W-H, Gellad WF, et al. Prescription opioid quality measures applied among Pennsylvania medicaid enrollees. J Manag Care Spec Pharm. 2018;24(9):875–885. doi: 10.18553/jmcp.2018.24.9.875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pham TT, Skrepnek GH, Bond C, Alfieri T, Cothran TJ, Keast SL. Overview of prescription opioid deaths in the Oklahoma state medicaid population, 2012–2016. Med Care. 2018;56(8):727–735. doi: 10.1097/MLR.0000000000000944 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


