Abstract
Objectives
The current COVID-19 pandemic has created a huge impact across the globe. Recent literature has reported the occurrence of varied oral lesions in COVID-19 patients in the form of sporadic case reports. This analytical cross-sectional study was carried out to gauge and understand the pattern of oral lesions in qualitative RT-PCR-confirmed COVID-19 patients.
Methods
A cross-sectional study involves a total of 500 qualitative RT-PCR confirmed, hospitalized COVID-19 patients who were meticulously scanned for any hard and soft tissue lesions developing concomitantly with the disease occurrence.
Results
This study included a total of 367 (73.4%) males and 133 (26.6%) female patients with a mean age of 53.46 ± 17.50 years. Almost 51.2% of patients presented with gustatory disturbance, 28% with xerostomia and 15.4% of patients were found to have oral findings like erythema, ulcers, depapillation of tongue. There was a statistically significant correlation between oral manifestations and disease severity (p ≤ 0.001).
Conclusion
COVID-19 is found to effect oral health with greater probability in patients with severe diseases (SARI) which may be due to disease itself, immune response and lack of motivation for personal hygiene measures.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12663-021-01679-x.
Keywords: COVID-19, Oral manifestations, Xerostomia, Taste alteration, Oral ulcers
Introduction
It has been more than a year since the first case of COVID-19 was first reported in the city of Wuhan, China, in December 2019 [1]. The collaborative efforts from all countries and World Health Organization (WHO) specifically with the advent of vaccines have given a hope of complete eradication; however, with the emerging new strain and classical phasic appearance of pandemics, there is a new need to actively monitor the current situation. SARS-CoV-2 belongs to the family of beta coronaviruses (CoV), which are positive-stranded RNA viruses, and is a close relative of SARS-CoV and MERS-CoV.
Human-to-human transmission of the virus via respiratory droplets has already been established. The median incubation time generally ranges from 4 to 5 days and can extend for maximum up to 14 days [2]. There are various symptoms associated with COVID-19 infection which includes influenza-like illness (ILI) and severe acute respiratory infection (SARI). These symptoms include fever, dry cough, sore throat and respiratory rate more than 30 cycles/min and SpO2 less than 90% [3]. As defined by World Health Organization (WHO), patients were categorized as ILI if they have an acute respiratory infection with measured fever of ≥ 38 degree centigrades and cough with onset within the last 10 days and were referred as SARI if they have ILI symptoms that requires hospitalization. According to the recent data, 80% of infections are mild or asymptomatic, 15% are severe infections requiring oxygen and 5% are critical infection requiring ventilation [4]. Also, approximately 70% of the patients are diagnosed with anosmia or hyposmia as well as taste alterations, and sometimes, these gustatory and olfactory dysfunctions may be the first presenting symptom of COVID-19 [5].
Involvement of oral cavity by COVID-19 may be suggested to have multifactorial aetiology with different compounding mechanisms. The oral cavity forms a gateway to the external environment and plays a major role in the spread of SARS-CoV-2. Extensive research has identified the role of a metallopeptidase enzyme, i.e. angiotensin-converting enzyme 2 (ACE-2) as a functional receptor for SARS-CoV-2 [6]. Apart from localisation in the other parts of the body, ACE-2 expression is found extensively in the basal layer of the non-keratinizing squamous epithelium of nasal and oral mucosa as well as nasopharynx [7]. This could also be responsible for impaired taste sensation as a temporary symptom. Another school of thought proposed is that change in immune response or the direct systemic response to the disease can be a reason for oral manifestations of COVID-19 [8]. Vieira et al. [9] suggested the role of persistent inflammation acting as an initiator for the coagulation cascade leading to further worsening of the existing untreated periodontitis in the COVID-19-positive patients. The psychological impact of COVID-19 leads to panic behaviour with feelings of hopelessness and negative outcomes, and there is a complete shift of focus from daily hygiene maintenance further worsening the oral health. Drugs therapy used in the treatment of the COVID-19 like remdesivir, hydroxychloroquine, protease inhibitors and interferon regimen was also postulated as reasons although they have reported minimal effect on oral health, except Lopinavir which may cause taste alteration [10].
In recent literature, there is emerging evidence on oral manifestations in COVID-19 patients which are basically beefed up case reports, few case series and scarce cross-sectional studies from across the globe14−26. Most of these studies are from western world with different socio-economic background, healthcare facilities and insurances availability. Representation from India which is fast paced developing, densely populated and second worst COVID-19 hit country of the world can open up an altogether new realm in understanding this. An analytical cross-sectional study was thus planned in the most affected country of Asian continent, i.e. India to evaluate the detailed oral condition of COVID-19 patients and correlating it with severity of the disease and patient demographics.
Methodology
This observational cross-sectional study was conducted in a DCH (Dedicated COVID Hospital) according to Ministry of Health & Family Welfare, Government of India. Study was commenced after Institutional Ethics Committee clearance and obtaining patient informed consent. This study has been done by abiding with the Declaration of Helsinki on medical protocol and ethics and according to STROBE guidelines. All the hospitalized patients of age 16 and above who were treated in the institute from 18/10/2020 to 07/11/2020 were included in the study. Paediatric population and patients with any other systemic conditions affecting oral mucosa were excluded from the study.
Thorough oral examination was performed under direct illumination by sterilized mouth mirror and probe under strict aseptic precautions by two post-graduate residents wearing complete Personal Protective Equipment (PPE) comprising of complete body suits, sterile gloves, N95 masks, goggles and face shields. All the oral findings were recorded using an elaborative proforma (Electronic Supplementary Material of Appendix I) with detailed soft tissue and hard tissue examination. Patient’s perception of xerostomia/dry mouth was asked, followed by soft tissue examination including examination of labial, buccal and gingival mucosa, tongue, floor of mouth and palate for presence of any inflammation, spontaneous bleeding, erythema, petechiae, ulcers, desquamation, macules and papules. Hard tissue examination included presence of any exposed or any necrosed bone, presence of any pus discharge and inadvertent tooth mobility (localized tooth mobility that cannot be explained by local periodontal factors). Thorough clinical examination was preceded by strict history confirming that only new lesions that appeared in prodromal and disease phase of COVID-19 patients were included. All symptom-positive patients were further cross-checked, and patients with previous history of oral lesions were strictly excluded. The positive findings were noted in the proforma with no further attempt to categorize the finding.
All numerical data were calculated as percentages. Spearman’s correlation coefficient was applied to find correlation between two components, and p value was generated. Data were considered significant if p value ≤ 0.05.
Results
A total of 500 (367 males and 133 females) RT-PCR-confirmed COVID-19 patients were included in the present cross-sectional study. The mean age of the patients was 53.46 ± 17.50 years. Seventeen percent of the study participants were asymptomatic, 64.6% had symptoms of ILI, and 18.4% suffered symptoms of SARI. A majority of the examined patients had no deleterious habits of tobacco consumption (59.4%), while the remaining patients had habits of consuming smokeless tobacco (22.8%) in the various regional forms of pan and supari (6.4%), gutka chewing (1.4%) and bidi/cigarette smoking (10.4%). Oral examination was performed at an average of 3.2 days (range 0–22 days) from the date of being tested positive for COVID-19. The demographic data of the patients are represented in Table 1.
Table 1.
Demographic details of study participants
| Parameter | No. of participants (%) | |
|---|---|---|
| Age (in years) (Mean ± SD) | 53.46 ± 17.50 | |
| Gender | Male | 367 (73.4%) |
| Female | 133 (26.6%) | |
| Habits | No habits | 297 (59.4%) |
| Tobacco | 114 (22.8%) | |
| Pan + Supari | 32 (6.4%) | |
| Gutka | 7 (1.4%) | |
| Alcohol | 5 (1%) | |
| Bidi/Cigarette | 52 (10.4%) | |
| Tobacco + Alcohol | 2 (0.4%) | |
| Tobacco + Pan | 1 (0.2%) | |
| Symptoms | Asymptomatic | 85 (17%) |
| ILI | 323 (64.6%) | |
| SARI | 92 (18.4%) | |
ILI influenza-like illness, SARI severe acute respiratory infection
The most common oral finding encountered was alteration in taste sensation seen in 51.2% where the patient had complete ageusia followed by xerostomia seen in 28% of the patients. The various oral lesions consisting of both mucosal (soft tissue) and bony or teeth (hard tissue) changes were seen only in 15.4% of all the COVID-19 patients. Of the observed soft tissue lesions, erythematous macules (7.2%) being the most common were followed by non-specific solitary ulcers (3%), depapillation of tongue (atrophic glossitis) (4.6%) to candida-like lesions (1%) as shown in Figs. 1 and 2. The observed incidence and site of lesions are tabulated in Table 2.
Fig. 1.
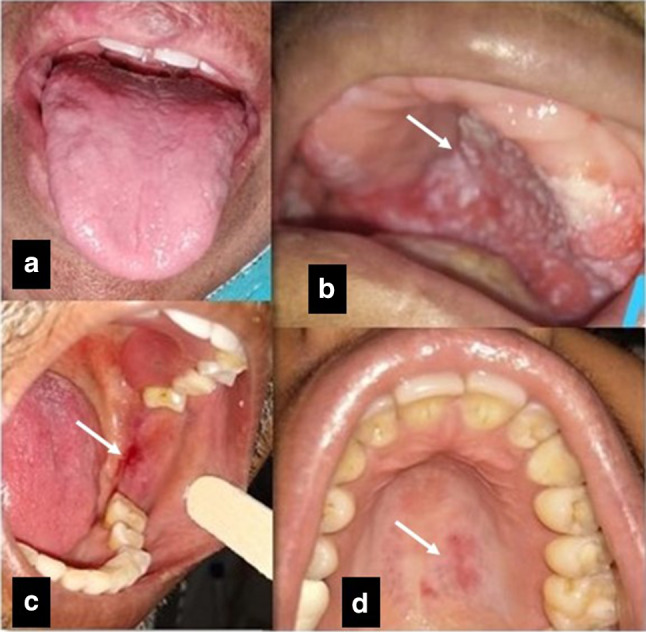
a Depapillation of tongue. b White scrapable lesion, most likely oral candidiasis on hard palate. c, d Erythematous patch over left buccal mucosa and palate
Fig. 2.
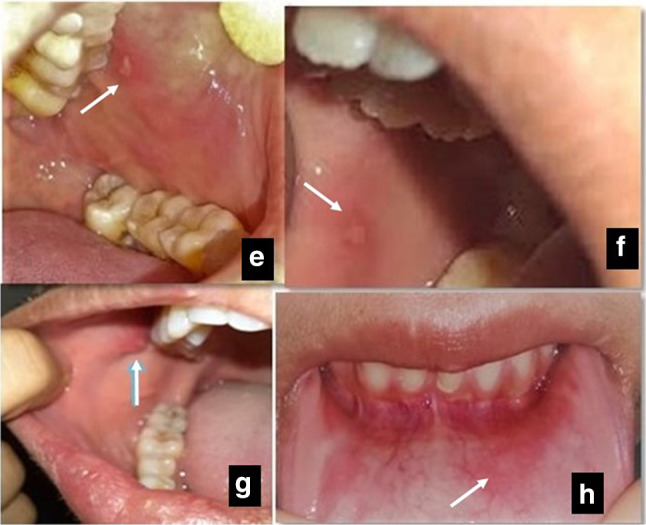
e, f Solitary aphthous-like ulcer in buccal mucosa. g Inflamed Stensen’s ductal orifice. h Diffuse erythema over labial mucosa
Table 2.
Oral Manifestations in COVID-19
| S. no | Oral findings | Number of patients (%) | Description | |
|---|---|---|---|---|
| I | Taste alteration | 256 (51.2%) | Complete absence of taste sensation | |
| II | Xerostomia | 140 (28%) | Thick, ropy saliva with dryness in mouth | |
| III | Excessive salivation | 0 | ||
| IV | Oral lesions present | 77 (15.4%) | Ulcers, erythema, white patch, depapillation of tongue/atrophic glossitis, candida-like lesions, ductal inflammation and tooth mobility | |
| IV A | Hard tissue lesions | Inadvertent mobility | 2 (0.4%) | |
| Pus discharge | None | |||
| Necrotic bone | None | |||
| IV B | Soft tissue lesions | Erythema (7.2%) |
Labial mucosa − 8 (1.6%) Buccal mucosa − 18 (3.6%) Palate − 8 (1.6%) Gingiva – 2 (0.4%) |
Diffuse Erythematous macules with irregular margins associated with burning sensation |
| Depapillation of tongue/Atrophic glossitis | 23 (4.6%) |
Complete or partial depapillation Associated with inflammation in 3 cases (0.6%) |
||
| Non-specific ulcers (3%) |
Labial mucosa − 5 (1%) Buccal mucosa − 8 (1.6%) Palate − 2 (0.4%) |
Solitary ulcer with well-defined margins, measuring approximately 5 mm x 5 mm with erythematous halo Surrounding mucosa appears normal |
||
| White patch (1%) | Buccal mucosa – 5 (1%) |
Diffuse white patch with ill-defined borders associated with an ulcer in 0.4% of cases Surrounding mucosa pigmented or normal in appearance |
||
| Candida-like lesion | 6 (1%) | White coating over dorsum tongue with associated depapillation/white scrapable lesion in palate was seen in 0.2% of cases | ||
| Ductal orifice | 4 (0.4%) | Inflamed ductal orifice |
The occurrence of various oral manifestations in patients according to the severity of COVID-19 is depicted in Table 3. As depicted in Table 4, there was no significant correlation found between age, gender or habit history with the oral manifestations of COVID-19. However, there was a significant correlation between severity of the disease and oral findings (value < 0.001) as per Spearman’s correlation coefficient.
Table 3.
Distribution of oral manifestations and severity of disease
| Oral manifestation | Alteration in taste | Xerostomia | Ulcers | Erythema | White patch | Depapillation/atrophic glossitis | White coating | Inflammation | Inflammation + Depapillation | Inflamed ductal orifice |
|---|---|---|---|---|---|---|---|---|---|---|
| Asymptomatic (N = 85) | 42 (49.4%) | 30 (35.3%) | 1 (1.2%) | 4 (4.7%) | 0 | 1 (1.2%) | 0 | 0 | 0 | 0 |
| ILI (N = 323) | 168 (52%) | 92 (28.5%) | 9 (2.8%) | 22 (6.8%) | 4 (1.2%) | 15 (4.6%) | 0 | 5 (1.5%) | 2 (0.6%) | 3 (0.9%) |
| SARI (N = 92) | 46 (50%) | 18 (19.6%) | 5 (5.4%) | 10 (10.9%) | 1 (1.9%) | 8 (8.7%) | 5 (5.4%) | 1 (1.9%) | 1 (1.9%) | 1 (1.9%) |
ILI influenza-like illness, SARI severe acute respiratory infection
Table 4.
Correlation of oral findings with patient factors
| Correlation of oral findings | P value | ρ value (Spearman’s coefficient) | Correlation |
|---|---|---|---|
| Age | 0.323* | 0.044 | No |
| Gender | 0.329* | NA | No |
| Habit history | 0.478* | 0.032 | No |
| Severity of disease | < 0.001** | NA | Yes |
*Not significant; **Highly significant; NA not applicable
The odds of having oral manifestations in asymptomatic patients were 0.29 and 0.12 times than ILI and SARI patients, respectively. Similarly, the odds of having oral manifestations in ILI patients were 0.43 times than SARI patients as shown in Table 5.
Table 5.
Odds ratio between disease severity and oral manifestations
| Oral findings | Odds ratio (OR) | |||
|---|---|---|---|---|
| Present | Absent | |||
| Asymptomatic | 4 | 81 | 0.29* | 0.12** |
| ILI | 47 | 276 | 0.43*** | |
| SARI | 26 | 66 | ||
*OR calculated between Asymptomatic and ILI
**OR calculated between Asymptomatic and SARI
***OR calculated between ILI and SARI
Discussion
The current COVID-19 pandemic has affected all the realm of human existence. A systematic review by Santos J et al [11] published on oral manifestations in COVID-19 patients included 7 case reports and 33 cross-sectional studies. Interestingly in all these cross-sectional studies, alteration in taste was the only oral finding assessed. A rigorous search of complete COVID literature in MEDSCAPE carried out on oral findings in COVID-19 patients with last date of search in on 30 November reveals a total of 13 case reports with findings tabulated in Table 6.
Table 6.
Available literature regarding Oral manifestations of COVID-19
| Study | Study design and sample size | Oral signs and symptoms | Location on oral mucosa | History of appearance | Duration and recovery | Reported diagnosis |
|---|---|---|---|---|---|---|
| Amorim dos Santos [16] (2020), Brazil | Case Report, 01 patient | (1) White plaque. (2) Multiple yellowish aphthae. (3) Nodule. (4) Severe geographic tongue + fissured tongue. (5) Extremely viscous saliva | (1) Dorsum of tongue. (2) Lower lip (3) Tongue dorsum | Persistent white plaque and associated yellowish aphthae on the 24th day of hospitalization. Severe geographic tongue was observed after 2 weeks | Lesions on tongue dorsum were resolved at almost 14d after the first oral examination. Severe geographic tongue was also resolved to moderate degree within approximately 17 days after its appearance | (1) Fungus infection. (2) Herpetic recurrent oral lesion. (3) Fibroma. (4) Geographic tongue |
| Ansari [17] (2020), Iran | Case Report, 02 patients | Several painful aphthae having irregular margins and varying sizes with the background mainly red and non-haemorrhagic | Case 1: Hard palate. Case 2: Anterior region of the tongue | Case 1: 5 days after the onset of symptoms. Case 2: 1 week after hospitalization | For complete recovery approximately 07 days of duration | Diffuse oedema with desquamation, granulation, and ulceration under the mucosa, with invasion of mononuclear and neutrophilic cells, indicating a secondary bacterial infection. Negative serologic tests for herpes simplex virus type 1 and 2 |
| Cebeci Kahraman [18] (2020), Turkey | Case Report, 01 patient | (1) Largely erythematous surface. (2) Few petechiae. (3) Numerous pustular enanthema (1 to 3 mm in diameter) | (1) Oropharynx and hard palate. (2) Palate midline. (3) Near soft palate border, more prominent on the left side | 10 days duration after the onset symptoms | After a few days of therapy | Diffuse oropharyngeal erythema, petechia and pustule formation |
| Chaux-Bodard [19] (2020), France | Case Report, 01 patient | Irregular ulcer | Dorsal side of the tongue | First symptom: a painful inflammation of a tongue papilla. 24 h later: erythematous macula. After, the lesion turned to an irregular and asymptomatic ulcer | 10d of duration until complete recovery | COVID-19 is associated with inflammatory reactions, such as vascular inflammation. The ulcer observed after a macular erythematous lesion could be explained by vasculitis |
| Martín Carreras-Presas [20] (2020), Spain | Case Report, 03 patients | (1) Pain. (2) Small blisters. (3) Desquamative gingivitis | (1) Tongue. (2) Internal lip mucosa. (3) Gingiva | (1) With first symptoms. (2) and (3) 1 mo after first symptoms | 3d of duration and treatment until recovery | Suggestive of erythema multiforme |
| Putra [21] (2020), Indonesia | Case Report, 01 patient | Stomatitis aphthous | Not reported | 7 days after the first symptom (fever) | 3 days of duration until recovery | Stomatitis aphthous |
| Soares [22] (2020), Brazil | Case Report, 01 patient | (1) Painful ulceration. (2) Multiple reddish macules of different sizes | (1) Buccal mucosa. (2) Scattered along the hard palate, tongue, and lip | Not reported | 21 days of duration until complete recovery | Diffuse chronic inflammatory infiltrate with focal areas of necrosis and haemorrhage in the lamina propria. Intense lymphocytic infiltration in adjacent minor salivary glands. Negative IHC reactions against HHV-1, HHV-2, CMV, treponema pallidum, and EBV |
| Patel et al [23] 2020, London | Case Report, 01 patient | (1) Severe Halitosis. (2) Necrotic interdental papillae | Gingival tissue | 03 days after fever | Not reported | Not reported |
| Sakaida et al. [24] 2020, Inazawa (Japan) | Case Report, 01 patient | Erythematous lesions and erosions on lips and buccal mucosa | Lip and buccal mucosa | 2 days after dental drugs administered | Not reported | Not reported |
| Brandão et al. [25] 2020, Sao Paulo (Brazil) | Case Report, 07 patient | Multiple shallow aphthous-like painful lesions of varying sizes covered with mucopurulent membrane found in the upper and lower labial mucosa and in the anterior dorsal tongue | Labial mucosa and dorsal of tongue | Not reported | Not reported | Not reported |
| Rodríguez et al. [26] 2020, Madrid (Spain) | Case Report, 01 patient | Aphthous-like Stomatitis, burning tongue sensation and tongue depapillation | Buccal mucosa and tongue | Not reported | Not reported | Not reported |
| Dominguez‐Santas et al. [27] 2020, Madrid (Spain) | Case Report, 04 patients | Minor Aphthous ulcers | Non-keratinized mucosa | Not reported | Not reported | Not reported |
| Corchuelo et al. [28] 2020, Cali (Colombia) | Case Report, 01 patient | Aphthous lesion | Attached gingiva | Not reported | Not reported | Not reported |
Not even a single cross-sectional study has been done on complete oral findings in COVID-19 patients, thus making our study distinctive. This cross-sectional study includes a detailed analysis of 500 COVID-19 patients in a dedicated COVID hospital (DCH). A thorough complete soft and hard tissue examination was done in varying severity of COVID-19 patients ranging from asymptomatic to SARI in strict aseptic environment by two post-graduate students in complete PPE. A thorough history regarding habits and co-existing medical conditions were taken to understand all the possible confounding factors. Every effort was made to rule out any oral lesion that was present prior to infection so that inclusion of only the lesions occurring during the prodromal and disease phase of COVID-19 was considered in our study. Understanding the pattern of oral manifestations of COVID-19 infection is of utmost importance, as oral lesions can compromise the nutrition of the patients, their psychological well-being, quality of life and final outcome, and it may also work as a diagnostic-screening tool due to easy accessibility of the oral cavity to health care workers.
In the present study, prevalence of taste alteration (51.2%) was quite high as compared to other manifestations. All these patients presented with complete ageusia. Although the disorders of olfactory systems have been implicated as the cause in 95% of the cases with taste disorders, taste and smell disturbances are difficult to delineate for the patients [12]. Therefore, in such patients where both taste and olfactory disturbances are present, primary aetiology is considered to be an underlying olfactory disturbance. Taste alteration is considered in situations where either dysgeusia or ageusia is present. Matsuo et al. [13] in 2000 established the presence of gustatory receptors in the papillae present on the dorsum of the tongue which helps in perceiving taste. According to Xu et al. [14], the SAR-CoV-2 virus binds to angiotensin-converting enzyme 2 (ACE2) receptors which are highly expressed in the epithelial cells present on the tongue. Thus, the alteration in taste perception could be attributed to the inactivation of ACE2 receptors present on the taste buds.
Xerostomia was found in 28% of our patients which could be attributed to either poor hydration, prolonged antibiotic course or an existing candida infection. The occurrence of xerostomia showed old mild variation with disease severity, 35.3% in asymptomatic patients, 28% in ILI patients and 19.6% in SARI patients, thus indicating that the existing COVID-19 viral infection could be the cause of xerostomia.
We observed atypical erythematous lesions (7.2%) on the buccal mucosa adjacent to the molar region in addition to isolated, solitary ulcers (3%) resembling minor aphthae. Initially, these lesions are asymptomatic and begin as inconspicuous isolated erythematous areas, which then progresses to a diffuse erythematous area with irregular borders. The ulcers observed were less than 1 cm with a well-defined erythematous halo. However, 8% of such patients complained of burning sensation localized to the erythematous region. The burning sensation could be attributed to the persistent inflammatory processes or alterations in the immune response or frequent use of antibiotics. Although the recent literature which included mainly case reports mentioned about the COVID-19-infected patients with oral manifestations, none of the studies till date has supported or has sufficient evidence to correlate the exact cause of such manifestations. In the time of pandemic where the overburdened health care system is struggling to save lives, the very idea to give a definitive diagnosis of oral findings with no added therapeutic advantage to the patient seems to be far-fetched.
A various hypothesis with limited supporting evidence has been postulated. These lesions could be a result of direct viral replication process or could be because of increased probability of opportunistic injuries caused by products of systemic deterioration. According to Ciccarese et al. 2020, the presence of cutaneous or oral mucosal petechiae could be possibly related to SARS-CoV-2-induced thrombocytopenia [15].
For erythematous patches, ulcers and white patches buccal mucosa (6.2%) was afflicted most, followed by labial mucosa (2.6%) and palate (2%). Another frequent oral manifestation which was present in the population of our study was depapillation of tongue/atrophic glossitis (4.6%). As histopathological confirmation was not carried out, possible hypothesis could be an association with candida, which being an oral commensal may be the cause of opportunistic infection in these immunocompromised patients. As patients were put on antibiotics, this may be an additive factor for atrophic glossitis with candidal aetiology. Further, authors like Petrescu et al. (2020) stated high expression of ACE2 in epithelial cells of oral mucosa, confirming early susceptibility.
Ductal orifice inflammation (Stensen’s/Wharton’s) (0.4%) and in hard tissues inadvertent tooth mobility (0.4%) were also picked up in our study. None of the patients presented with any vesiculobullous lesions, pus discharge or any other frank signs of osteomyelitis.
Of all the patients with positive oral findings, 41.5% of patients had a positive history of either diabetes mellitus, hypertension, cerebrovascular accident, bronchial asthma or chronic kidney disease. Xerostomia and oral candidiasis are a common finding in diabetes mellitus and thus may be confounding factor in the study. Strict history to include the findings present only in the prodromal or disease phase of COVID was taken with an aim to balance these confounders. Another possible confounding factor could be various habit histories. However, in our study, all the patients except one confirmed the appearance of the lesions after the onset of COVID-19 infection. Also, the patients reported an associated sudden onset of burning sensation which further confirms the recent onset of the lesions and rules out the chronicity.
The site predilection for occurrence of oral lesions was found to be the maximum in the tongue (7.8%), followed by buccal mucosa (6.2%), labial mucosa (2.6%), palate (2%) and the least in gingiva (0.4%).
We further evaluated the relationship of oral findings with age, gender and severity of the disease, and we found a statistically significant correlation only with the disease severity. The odds of an asymptomatic COVID-19 patients presenting with oral manifestations are less than patients with ILI or SARI, and patients with ILI have lesser odds than SARI patients in developing oral lesions. Hence, it is evident from our study that patients with more severe disease had greater chances of presenting with oral findings. The comparison of the severity of the disease among the three groups suggested that the occurrence of alteration in taste, xerostomia and ulcers was comparable. However, we observed an increased incidence of candidal lesions on tongue (5.4%) and depapillation (8.7%) in patients with SARI. This could possibly be due to the fact that the patients presenting with SARI were either intubated or had a prolonged hospital course along with a compromised oral hygiene state.
As a part of the strength of our study, to the best of our knowledge, till date no study has been conducted with such a large sample size of varying severity of COVID-19 patients. Moreover, most of the literature published is mainly case reports in nature. This study in addition to meticulously scanning all the oral findings further analysed the oral finding relationship with patient demographics and disease severity as a part of an analytical cross-sectional study. This study has an additional advantage of being conducted in Asian subcontinent where most of the countries are still developing. Health care facilities and medical services are different compared to the developed countries in terms of healthcare facility distribution, infrastructure availability, patient’s education, motivation and standard of living.
As a part of limitations of our study, appropriate history could not be obtained from intubated patients. We would like to highlight that the aim of the present cross-sectional study was to assess oral findings of COVID patients. No further attempt was made to diagnose the lesions histopathologically due to ethical reasons, cost factors and overburdened healthcare system of developing countries like India especially during COVID times. Swab from the lesion site and its evaluation with RT-PCR would have robustly confirmed the lesions as a part of SARS-CoV-2 infection, but this would have unnecessarily wasted the diagnostic resources, increasing the cost of the study without actually benefitting the patient. For a developing country like India, which is the second hit country, such a cost rise for the research purpose is not justifiable. Further, the virulence of COVID-19 virus is not evaluated in the saliva of the sample studied for the above reason only. Although in our study, samples from oral lesions were not tested, therefore we could not investigate the immune expression of ACE2 either. Lastly, the evolution of oral lesion and their course of resolution whether it occurred in parallel with COVID-19 infection resolution were not assessed as a part of the study design.
Conclusion
COVID-19 infection is definitely found to have a significant effect on the oral health of the patients with increased findings in severe form of the disease. As oral health care providers, it is important for us to understand the common presentations so that we can screen such patients and direct them for appropriate screening and testing centres. Further as a dentist, it is indispensable for us to identify such lesions and manage them adequately to enhance the recovery process and improve the quality of life of COVID-19 patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to express our sincere gratitude to Dr. Arun Kumar Patnana, Senior Resident, Department of Dentistry, All India Institute of Medical Sciences, Jodhpur, for his expertise with the statistical analysis. We thank our patients for their cooperation and wish them a speedy recovery.
Declarations
Conflict of interest
The authors declare that they have no competing interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Aparna Ganesan, Email: ganesan.aparna@gmail.com.
Shailendra Kumar, Email: kumar.shailendra0904@gmail.com.
Amanjot Kaur, Email: amanjotkaur1992@yahoo.com.
Kirti Chaudhry, Email: chaudhry_kirti@yahoo.com.
Pravin Kumar, Email: pravinkumar5@hotmail.com.
Naveen Dutt, Email: duttn@aiimsjodhpur.edu.in.
Vijaya Lakshmi Nag, Email: vijayalakshmi005@gmail.com.
M. K. Garg, Email: gargmk@aiimsjodhpur.edu.in
References
- 1.Huang C, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC, “Coronavirus Disease 2019 (COVID-19),” Centers for Disease Control and Prevention (2020) https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. Accessed 13 Dec 2020
- 3.Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R (2020) Features, evaluation, and treatment of coronavirus. In: StatPearls, Treasure Island (FL): StatPearls Publishing, 2020. Accessed: Dec. 13, 2020. [Online]. http://www.ncbi.nlm.nih.gov/books/NBK554776/ [PubMed]
- 4.“20200306-sitrep-46-covid-19.pdf.” Accessed 13 Dec 2020. [Online]. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200306-sitrep-46-covid-19.pdf?sfvrsn=96b04adf_4
- 5.Dell’Era V, Farri F, Garzaro G, Gatto M, Aluffi Valletti P, Garzaro M (2020) Smell and taste disorders during COVID‐19 outbreak: a cross‐sectional study on 355 patients. Head Neck. 10.1002/hed.26288 [DOI] [PMC free article] [PubMed]
- 6.McLachlan CS (2020) The angiotensin-converting enzyme 2 (ACE2) receptor in the prevention and treatment of COVID-19 are distinctly different paradigms. Clin Hypertens 26. 10.1186/s40885-020-00147-x. [DOI] [PMC free article] [PubMed]
- 7.Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dziedzic A, Wojtyczka R. The impact of coronavirus infectious disease 19 (COVID-19) on oral health. Oral Dis. 2020 doi: 10.1111/odi.13359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vieira AR (2019) Oral manifestations in coronavirus disease 2019 (COVID-19). Oral Dis vol n/a, no n/a. 10.1111/odi.13463 [DOI] [PMC free article] [PubMed]
- 10.Schiffman SS, Zervakis J, Heffron S, Heald AE. Effect of protease inhibitors on the sense of taste. Nutrition. 1999;15(10):767–772. doi: 10.1016/s0899-9007(99)00152-5. [DOI] [PubMed] [Google Scholar]
- 11.Amorim Dos Santos J et al (2020) Oral manifestations in patients with COVID-19: a Living systematic review. J Dent Res 22034520957289. 10.1177/0022034520957289 [DOI] [PubMed]
- 12.Vinayachandran D, Balasubramanian S. Is gustatory impairment the first report of an oral manifestation in COVID-19? Oral Dis. 2020 doi: 10.1111/odi.13371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuo R. Role of saliva in the maintenance of taste sensitivity. Crit Rev Oral Biol Med. 2000;11(2):216–229. doi: 10.1177/10454411000110020501. [DOI] [PubMed] [Google Scholar]
- 14.Xu Z, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciccarese G, Drago F, Boatti M, Porro A, Muzic SI, Parodi A. Oral erosions and petechiae during SARS-CoV-2 infection. J Med Virol. 2020 doi: 10.1002/jmv.26221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amorim Dos Santos J, et al. Oral mucosal lesions in a COVID-19 patient: new signs or secondary manifestations? Int J Infect Dis. 2020;97:326–328. doi: 10.1016/j.ijid.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ansari R, Gheitani M, Heidari F, Heidari F. Oral cavity lesions as a manifestation of the novel virus (COVID-19) Oral Dis. 2020 doi: 10.1111/odi.13465. [DOI] [PubMed] [Google Scholar]
- 18.Cebeci Kahraman F, ÇaŞkurlu H (2020) Mucosal involvement in a COVID‐19‐positive patient: a case report. Dermatol Ther. 10.1111/dth.13797 [DOI] [PMC free article] [PubMed]
- 19.Chaux-Bodard A-G, Deneuve S, Desoutter A (2020) Oral manifestation of Covid-19 as an inaugural symptom? J Oral Med Oral Surg 26(2), Art. no. 2. 10.1051/mbcb/2020011
- 20.Martín Carreras-Presas C, Amaro Sánchez J, López-Sánchez AF, Jané-Salas E, Somacarrera Pérez ML (2020) Oral vesiculobullous lesions associated with SARS-CoV-2 infection. Oral Dis. 10.1111/odi.13382 [DOI] [PMC free article] [PubMed]
- 21.Eka Putra B, Adiarto S, Dewayanti S, Juzar D (2020) Viral Exanthem with ‘Pin and Needles Sensation’ on extremities of COVID-19 patient. Int J Infect Dis 96. 10.1016/j.ijid.2020.05.020 [DOI] [PMC free article] [PubMed]
- 22.Soares CD, de Carvalho RA, de Carvalho KA, de Carvalho MGF, de Almeida OP. Letter to editor: oral lesions in a patient with Covid-19. Med Oral Patol Oral Cir Bucal. 2020;25(4):e563–e564. doi: 10.4317/medoral.24044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel J, Woolley J Necrotizing periodontal disease: oral manifestation of COVID-19. Oral Dis vol n/a, no n/a. 10.1111/odi.13462 [DOI] [PMC free article] [PubMed]
- 24.Sakaida T, Tanimoto I, Matsubara A, Nakamura M, Morita A. Unique skin manifestations of COVID-19: Is drug eruption specific to COVID-19? J Dermatol Sci. 2020;99(1):62–64. doi: 10.1016/j.jdermsci.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brandão TB, et al. Oral lesions in patients with SARS-CoV-2 infection: Could the oral cavity be a target organ? Oral Surg Oral Med Oral Pathol Oral Radiol. 2020 doi: 10.1016/j.oooo.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Díaz Rodríguez M, Jimenez Romera A, Villarroel M (2020) Oral manifestations associated with COVID‐19. Oral Dis. 10.1111/odi.13555 [DOI] [PMC free article] [PubMed]
- 27.Dominguez-Santas M, Diaz-Guimaraens B, Fernandez-Nieto D, Jimenez-Cauhe J, Ortega-Quijano D, Suarez-Valle A. Minor aphthae associated with SARS-CoV-2 infection. Int J Dermatol. 2020 doi: 10.1111/ijd.15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corchuelo J, Ulloa FC. Oral manifestations in a patient with a history of asymptomatic COVID-19: Case report. Int J Infect Dis. 2020;100:154–157. doi: 10.1016/j.ijid.2020.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


