Key Points
Question
Does an electronic patient portal message primer increase the completion rate of colorectal cancer (CRC) screening in a mailed fecal immunochemical test (FIT) program?
Findings
In this randomized clinical quality improvement trial of 2339 patients receiving a mailed FIT, the rate of screening completion was significantly higher in the group that received the electronic message than in the control group (38% vs 32%).
Meaning
Including an electronic patient portal message primer prior to a mailed FIT is an effective method to increase CRC screening rates in a managed care patient population.
Abstract
Importance
Colorectal cancer (CRC) screening reduces CRC mortality; however, screening rates remain well below the national benchmark of 80%.
Objective
To determine whether an electronic primer message delivered through the patient portal increases the completion rate of CRC screening in a mailed fecal immunochemical test (FIT) outreach program.
Design, Setting, and Participants
In this randomized clinical quality improvement trial at the University of California, Los Angeles Health of 2339 patients enrolled in a FIT mailing program from August 28, 2019, to September 20, 2020, patients were randomly assigned to either the control or intervention group, and the screening completion rate was measured at 6 months. Participants were average-risk managed care patients aged 50 to 75 years, with a valid mailing address, no mailed CRC outreach in the previous 6 months, and an active electronic health record (EHR) patient portal who were due for CRC screening. Data were analyzed on an intention-to-treat basis.
Interventions
Eligible patients were randomly assigned to receive either (1) the standard FIT mailed outreach (control group) or (2) the standard FIT mailed outreach plus an automated primer to notify patients of the upcoming mailed FIT sent through the electronic patient portal (intervention group).
Main Outcomes and Measures
The primary outcome was the screening completion rate (ie, returning the FIT). Secondary outcomes were (1) were the time to CRC screening from the FIT mailing date, (2) screening modality completed, and (3) the effect of opening the electronic primer on screening completion rate.
Results
The study included 2339 patients (1346 women [57.5%]; mean [SD] age, 58.9 [7.5] years). The screening completion rate was higher in the intervention group than in the control group (37.6% [445 of 1182] vs 32.1% [371 of 1157]; P = .005). The time to screening was shorter in the intervention group than in the control group (adjusted hazard ratio, 1.24; 95% CI, 1.08-1.42; P = .003). The proportion of each screening test modality completed was similar in both groups. In a subanalysis of the 900 of 1182 patients (76.1%) in the intervention group who opened the patient portal primer message, there was a 7.3–percentage point (95% CI, 2.3-12.4 percentage points) increase in CRC screening (local mean treatment effect; P = .004).
Conclusions and Relevance
Implementation of an electronic patient portal primer message in a mailed FIT outreach program led to a significant increase in CRC screening and improvement in the time to screening completion. The findings provide an evidence base for additional refinements to mailed FIT outreach quality improvement programs in large health systems.
Trial Registration
ClinicalTrials.gov Identifier: NCT05115916
This randomized clinical trial examines whether an electronic primer message delivered through the patient portal increased colorectal cancer (CRC) screening in a mailed fecal immunochemical test outreach program.
Introduction
Colorectal cancer (CRC) is the third most common cancer and the third leading cause of cancer-related deaths in the United States.1,2 Owing to the relatively slow growth of precancerous colon and rectal lesions, screening is effective for both prevention and early detection of disease and has been demonstrated to reduce mortality.3,4 The US Preventive Services Task Force recommends screening for all individuals from 50 to 75 years of age (grade A), and in May 2021, they updated their recommend screening guidelines to include individuals 45 to 49 years of age (grade B).5,6,7 Nonetheless, the US screening rate remains well below the national benchmark of 80% as established by the National Colorectal Cancer Roundtable.1,8
The fecal immunochemical test (FIT) is an effective screening modality for CRC.9,10,11,12,13 Although the US Preventive Services Task Force does indicate individuals complete screening via FIT, fecal occult blood test, stool DNA test, colonoscopy, computed tomography colonography, and flexible sigmoidoscopy, it does not recommend any one of the screening modality over another.7 The US Multi-Society Task Force, however, recommended FIT and colonoscopy as first-line screening modalities for CRC in 2017.14 This guidance has practical implications because FIT can be mailed to patients and completed at home, reaching patients who do not have access to colonoscopy or who have reservations about more invasive screening modalities. In light of decreasing CRC screening rates and patient hesitancy for in-person health visits during the COVID-19 pandemic, multiple medical and professional societies have emphasized the use of a mailed FIT outreach program.15,16,17
Mailed FIT outreach is effective at increasing CRC screening rates in health systems.18,19,20,21,22,23,24 The Community Preventive Services Task Force, in their 2012 systematic review, indicated with strong evidence that sending “client reminders or recalls” (ie, electronic or postal letters or telephone messages) increased screening completion rates via fecal occult blood test.25 Furthermore, a systematic review by Issaka et al23 demonstrated increased screening completion rates when mailed or telephone FIT primers were provided to patients prior to mailed FIT outreach. Primers preempt a future event (ie, arrival of a mailed FIT kit), with the goal to stimulate a positive behavioral response (ie, completion and return of the FIT kit), and are effective in improving health behaviors.26,27,28,29,30 Studies in behavioral economics and psychology indicate that how information or choice is framed affects behavior in predictable ways, which has applications in health and medicine, including the design of CRC screening strategies.19,31,32,33,34 However, while prior studies have demonstrated the positive effect of traditional mail or telephone primers by a median of 4.1%,23 there are no studies to date, to our knowledge that evaluate whether electronic primers can achieve the same gains. Leveraging electronic health record (EHR) patient portals to communicate with patients is a low-cost alternative to what has previously been demonstrated to increase CRC screening.
Thus, we developed an electronic primer within the EHR patient portal to alert patients due for CRC screening before arrival of a mailed FIT kit.35,36,37 As a part of our institution’s ongoing quality improvement efforts to increase CRC screening rates, we randomized implementation of the primer at the patient level to determine whether the electronic primer increased the CRC screening completion rate among patients enrolled in our mailed FIT program.
Methods
Setting and Population
This randomized clinical quality improvement trial was conducted from August 28, 2019, to September 20, 2020 (trial protocol in Supplement 1). University of California, Los Angeles (UCLA) Health is a large, integrated academic health system, with more than 390 000 primary care patients and 180 ambulatory primary care clinics across Southern California. Approximately 68 000 of these primary care patients form the managed care population, an insured group of patients enrolled in commercial health maintenance organization contracts covering services delivered only via UCLA Medical Group. In 2015, we implemented a biannual mailed FIT outreach program for managed care primary care patients who were overdue for average-risk CRC screening.20 All individuals between the ages of 50 and 75 years in this population who were overdue for CRC screening, based on their EHR information and the National Committee for Quality Assurance Healthcare Effectiveness Data and Information Set criteria, received a mailed FIT.20,38 The UCLA institutional review board deemed our work exempt because it is systems improvement consistent with ongoing hospital quality improvement efforts; patient consent was also waived because the study pertains to ongoing hospital quality improvement efforts. Our institution is implementing randomization as a best practice for quality improvement projects. As such, in reporting these findings to the public, we adhere to the standards set forth by the scientific community when randomizing participants to treatment. In addition, for this investigation, we followed and adhered to the Standards for Quality Improvement Reporting Excellence (SQUIRE) 2.0 reporting guideline for quality improvement projects and the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline for randomized clinical trials.39,40
The FIT outreach mailing includes an informational letter, which uses principles of behavioral science, to offer CRC screening (eAppendix 1 in Supplement 2), a preaddressed OC-Auto FIT CHEK Kit (Polymedco Inc) with instructions, and a 1-page form to report non-UCLA screening results.19 The letter accompanying the FIT kit provided the option to schedule a colonoscopy if preferred. All FIT kit mailers contained a postal label for return to the UCLA clinical laboratory and were processed per manufacturer protocol. For positive results, a population health patient coordinator notified the patient’s primary care physician of the result, and the primary care physician was responsible for generating a referral for a diagnostic colonoscopy.
Patient Selection and Randomization
With assistance from the UCLA Office of Population Health and Accountable Care, we used EHR data, billing data, and claims data to identify managed care patients, aged 50 to 75 years, at average risk for CRC and due for screening in August 2019 and again in March 2020. Due for screening was defined by a lack of FIT within the past year, flexible sigmoidoscopy within the past 5 years, colonoscopy within the past 10 years, computed tomography colonography within the past 5 years, or FIT-DNA test within the past 3 years. We excluded patients who had an invalid mailing address, had an inactive EHR patient portal (UCLA Health uses Epic MyChart), or who were considered high risk for CRC (personal history of CRC, adenomatous polyps, inflammatory bowel disease, familial polyposis syndrome, or a family history of CRC). We also excluded patients from inclusion to the March 2020 mailed outreach program who received a FIT mailer within the past 6 months.
In August 2019 and March 2020, we used simple randomization using a random number generator to assign eligible patients to receive either (1) the standard mailed FIT kit (control group) or (2) the standard mailed FIT kit plus an automated electronic primer sent through the personal health portal, alerting them that a FIT kit would be arriving to their home (intervention group).
Intervention Description
Only individuals with activated EHR patient portals were included in the investigation. Both patient groups received identical informational letters and FIT kits in the outreach (eAppendix 1 in Supplement 2). In addition to this mailed outreach, patients in the intervention group received an electronic message through their personal health portal (eAppendix 2 in Supplement 2). Patients received the primer approximately 1 to 2 weeks prior to arrival of the FIT kit, which informed patients about the incoming FIT kit and instructed patients to complete and return the kit promptly.
Outcomes and Variables
Our primary outcome was the CRC screening completion rate from FIT mailing date, over a 6-month follow-up period. We considered patients as screened for CRC if any screening modality (FIT, flexible sigmoidoscopy, colonoscopy, computed tomography colonography, or FIT-DNA) was completed between August 28, 2019, and February 28, 2020, for the August 2019 mailing and between March 20 and September 20, 2020, for the March 2020 mailing. We had 3 secondary outcomes: (1) time to screening completion in each group; (2) screening modality completion in each group at 6 months; and (3) receipt of the primer in the intervention group, as measured by opened primer message in the patient portal. We also included patient-level demographic variables, including age at time of randomization, sex, and race and ethnicity. The outcomes for this investigation were registered retrospectively.
Statistical Analysis
For all analyses, we excluded patients who died during the study period. We also excluded patients from the August 2019 cohort from analysis who received a mailed FIT kit in the previous 6 months, eliminating potential confounding among patients who received a FIT kit twice during the study period. We used frequencies and mean and median values to summarize demographic data for each study group. We first compared screening completion rates in the 2 study groups using an intention-to-treat analysis and the t test. We then used logistic regression and Cox proportional hazards regression model to compare time to screening in the 2 study groups, controlling for age, sex, and race and ethnicity; results were presented with odds ratios or hazards ratios and 95% CIs. After this, we used the Fisher exact test to compare the completion rates of individual screening modalities in the 2 study groups. Last, we performed a secondary analysis to determine the effect of opening the portal message on screening, using randomization group as an instrumental variable. In this analysis, we compared the subset of patients in the intervention group who opened the portal primer message with the control group. All P values were from 2-sided tests and results were deemed statistically significant at P < .05. All analyses were performed using R, version 3.6.2 (R Group for Statistical Computing).41 All analyses performed, despite formal registration occurring during manuscript development, were developed and conducted a priori.
Results
Characteristics of the Study Population
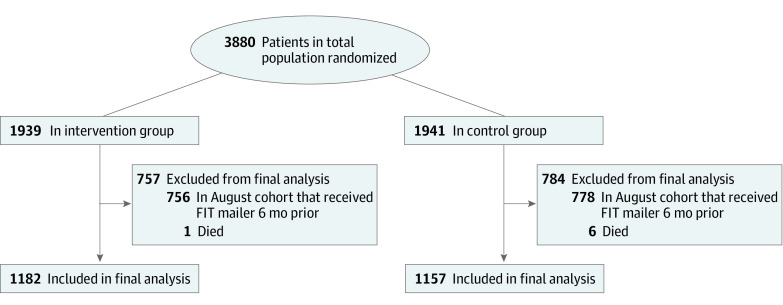
There were 1157 patients (666 women [57.6%]; mean [SD] age, 58.7 [7.5] years) in the control group and 1182 patients (680 women [57.5%]; mean [SD] age, 59.2 [7.5] years) in the intervention group (Figure 1 and Table 1). The sample included 346 Hispanic individuals (14.8%), 1350 White individuals (57.7%), and 196 Black individuals (8.4%). These baseline patient characteristics were similar in both groups.
Figure 1. Patient Flow Diagram.
FIT indicates fecal immunohistochemical test.
Table 1. Sample Characteristicsa.
| Characteristic | Patients, No. (%) | |
|---|---|---|
| Intervention (n = 1182) | Control (n = 1157) | |
| Age, mean (SD), y | 59.2 (7.5) | 58.7 (7.5) |
| Median (IQR), y | 58 (52-65) | 57 (52-65) |
| Range, y | 50-75 | 50-75 |
| Sex | ||
| Female | 680 (57.5) | 666 (57.6) |
| Male | 502 (42.5) | 491 (42.4) |
| Ethnicity | ||
| Hispanic | 182 (15.4) | 164 (14.2) |
| Non-Hispanic | 896 (75.8) | 901 (77.9) |
| Unknown | 104 (8.8) | 92 (8.0) |
| Race | ||
| American Indian or Alaska Native | 1 (0.08) | 0 |
| Asian | 133 (11.3) | 146 (12.6) |
| Black | 98 (8.3) | 98 (8.5) |
| White | 681 (57.6) | 669 (57.8) |
| Otherb | 172 (14.6) | 172 (14.9) |
| Unknown | 92 (7.8) | 69 (6.0) |
This information was obtained from the electronic health records.
All other races that a person can identify as outside of American Indian or Alaska Native, Asian, Black, White, or unknown. These categories were determined at the system level.
CRC Screening Completion Rates
At 6 months, the screening completion rate was 32.1% (n = 371) in the control group and 37.6% (n = 445) in the intervention group (P = .005) (Table 2). After adjusting for patient demographic characteristics, we found that the intervention group had significantly increased odds of completing CRC screening compared with the control group (odds ratio, 1.29; 95% CI, 1.08-1.53; P = .004) (eTable 1 in Supplement 2).
Table 2. Colorectal Cancer Screening Completion Rate at 6 Months After Intervention.
| Study group | Patients, No. (%) | P valuea | |
|---|---|---|---|
| Not up to date | Up to date | ||
| Intervention (n = 1182) | 737 (62.4) | 445 (37.6) | .005 |
| Control (n = 1157) | 786 (67.9) | 371 (32.1) | |
Significance set at P < .05.
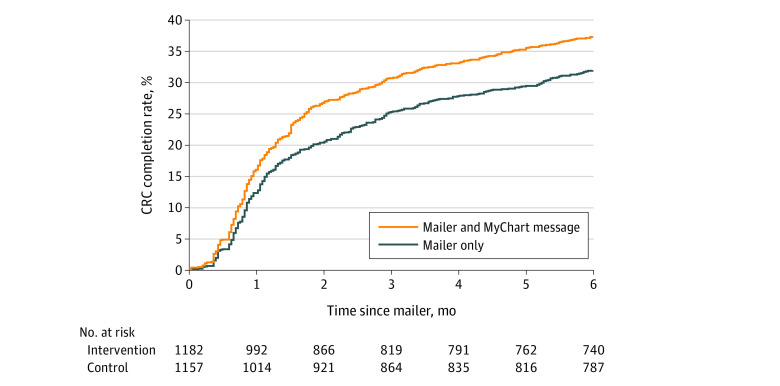
Furthermore, Figure 2 displays the estimated cumulative incidence of CRC screening for each group, showing that the intervention group diverged from the control group in the first 2 months of the intervention, after which it maintained consistently higher screening rates. The median time to CRC screening completion after receipt of the mailer among those who received the FIT kit within the 6-month follow-up was 35 days (IQR, 22-72 days) for the intervention group and 38 days (IQR, 24-84 days) for the control group. The Cox proportional hazards regression model demonstrated that the time to CRC screening in the intervention group was significantly shorter than the time to screening in the control group (hazards ratio, 1.24; 95% CI, 1.08-1.42; P = .003) (Table 3).
Figure 2. Cumulative Risk Curve, Stratified by Study Group.
CRC indicates colorectal cancer.
Table 3. Cox Proportional Hazards Regression Model.
| Effect | Adjusted HR (95% CI) | P valuea |
|---|---|---|
| Intervention vs control | 1.24 (1.08 to 1.42) | .003 |
| Age (per 1-y increment) | 1.02 (1.01 to 1.03) | <.001 |
| Male vs female | 1.14 (0.99 to 1.31) | .06 |
| Ethnicity | ||
| Hispanic | 0.88 (0.70 to 1.10) | .25 |
| Non-Hispanic | 1 [Reference] | NA |
| Unknown | 1.48 (1.08 to 2.02) | .02 |
| Race | ||
| American Indian or Alaska Native | <0.01 (<0.01 to >0.99) | .99 |
| Asian | 1.25 (1.02 to 1.54) | .03 |
| Black | 1.01 (0.78 to 1.30) | .96 |
| White | 1 [Reference] | NA |
| Other | 1.02 (0.82 to 1.26) | .89 |
| Unknown | 0.62 (0.42 to 0.91) | .02 |
Abbreviations: HR, hazard ratio; NA, not applicable.
Significance set at P < .05.
Furthermore, for both our adjusted logistic regression and Cox proportional hazards regression analysis, age and Asian race were significantly associated with CRC screening (age: hazard ratio, 1.02; 95% CI, 1.10-1.03; P < .001; Asian race: hazard ratio, 1.25; 95% CI, 1.02-1.54; P = .03) (eTable 1 in Supplement 2; Table 3). In addition, unknown race and ethnicity were also significantly associated with CRC screening (hazard ratio, 0.62; 95% CI, 0.42-0.91; P = .02); however, these variables need to be interpreted with caution (eTable 1 in Supplement 2; Table 3).
Screening Modality Used
Screening modalities did not differ between the study groups (P = .65) (eTable 2 in Supplement 2). For the 371 patients in the control group who completed screening, 337 (90.8%) used FIT and 34 (9.2%) underwent colonoscopy; no patients underwent flexible sigmoidoscopy. For the 445 patients in the intervention group who completed screening, 409 (91.9%) used FIT, 35 (7.9%) underwent colonoscopy, and 1 (0.2%) underwent flexible sigmoidoscopy.
Receipt of Patient Portal Primer
A total of 900 patients (76.1%) in the intervention group opened the patient portal primer message. We found that those who opened the message had a 7.3–percentage point increase (95% CI, 2.3-12.4 percentage points) in CRC completion (local mean treatment effect; P = .004).
Discussion
In our study, we found that the addition of an electronic primer message sent through patients’ EHR portal significantly increased the CRC screening completion rate by 5.5% for patients in our mailed FIT outreach program. The rate of screening completion was statistically significant among patients who opened the primer message. The effect of the electronic primer was most prominent during the first month after mailing and was maintained throughout the 6-month study period despite an attenuation in screening participation over time. Screening test choice did not vary between the study groups. We believe that our findings highlight the growing importance of early detection in CRC prevention and treatment, as well as the growing need for effective and scalable screening programs.
This investigation has been a part of a series of quality improvement projects at UCLA Health focused on increasing CRC screening rates through a mailed FIT outreach program.19,20 Our intervention group had higher screening rates (37.6%) than previously published studies in the same managed care population (24.1% in the study by Bakr et al19 and 33.2% in the study by Yu et al20). We believe the increased screening completion rates in our study is due to improvement in care processes that have occurred in the years since the FIT outreach program began, the primer implementation, and the health care system’s emphasis on increasing CRC rates for this population. The absolute effect size (5.5%-7.3%) of our intervention on CRC screening completion rates was similar to other studies that have evaluated the effect of primers on FIT outreach.42,43,44,45 In an analysis of a mailed FIT outreach program in a large health maintenance organization in Colorado, use of a telephone primer increased the screening completion rate by 4.5%.45 In a separate population-based Dutch randomized clinical trial that used a similar mailed letter primer design as ours, researchers saw a 3.3% increase in the screening completion rate.42
Our findings are also consistent with behavioral science literature that demonstrates that successful implementation of priming messages influences behavior. Primers motivate individual behavior by providing a stimulus that will influence action on a subsequent process, using subconscious memory association.26,27,46 Although there is debate about the robustness of primers, primer messages have been used successfully in psychology to influence task completion, biases, and health behaviors.27,28,30,47,48 Specifically, primers have been shown to be effective at improving health behaviors, such as increasing physical activity, vaccination uptake, reducing unhealthy snack purchase, and increasing hand hygiene in clinical situations.27,28,36,37,49
Strengths and Limitations
Our study has some strengths. First, the randomization successfully resulted in balanced study groups, which is the optimal way to control for potential unmeasured confounders. Second, our patient population is racially and ethnically diverse, which enhances the generalizability of our findings to other study populations. Third, although existing studies have demonstrated the benefits of primers delivered via telephone, this is the first study, to our knowledge, to show the benefit of an electronic primer in conjunction with a mailed FIT outreach program. Fourth, our intervention is low cost and does not require additional mailings or personnel, so it can be easily adapted for any health care system that integrates patient communication electronically.
Our study also has some limitations. First, our investigation was conducted in a single academic health system, which may limit the generalizability of the findings. Our health system is large, however, and the patient population is diverse. Second, we included and randomized only patients with active patient EHR portal accounts, which introduces the possibility of selection bias. Our findings may be more relevant to patient populations more engaged in their health or who are more technologically savvy. In our health care system, approximately 84% of patients have an activated portal, so this population is quite robust. Third, the population under investigation is routinely included in CRC screening efforts by the health care system. Although our randomization was balanced, a potential limitation of our analysis is that we did not adjust for whether patients received or completed prior CRC screening. Fourth, by focusing only on individuals who had not received FIT in the prior 6 months, we omitted individuals who may be particularly difficult to screen. Omitting these patients was necessary to create comparable populations and pool the August 2019 and March 2020 mailed outreach cohorts.
Conclusions
Our randomized clinical quality improvement study in a large academic health care system demonstrated that a simple electronic patient portal primer sent prior to mailed FIT outreach may significantly increase CRC screening rates. Our future work will focus on understanding populations that do not participate in screening despite our current efforts and the design of interventions to reach these populations. Increasing suboptimal CRC screening rates in the US is essential to reducing the burden of CRC, and using simple, low-cost, and effective population health interventions, grounded in proven behavioral principles, will allow us to detect disease earlier and improve the quality of life of our patients.
Trial Protocol
eAppendix 1. FIT Kit Mailer Letter
eAppendix 2. MyChart Electronic Message Example
eTable 1. Logistic Regression Model of CRC Screening by Study Group
eTable 2. Screening Modality by Study Group
Data Sharing Statement
References
- 1.American Cancer Society . Colorectal cancer facts & figures 2017-2019. Accessed November 29, 2020. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2017-2019.pdf
- 2.American Cancer Society . Cancer facts & figures 2020. Accessed December 2, 2020. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2020.html [DOI] [PMC free article] [PubMed]
- 3.National Cancer Institute . Colon cancer treatment (PDQ)—health professional version. Accessed March 24, 2021. https://www.cancer.gov/types/colorectal/hp/colon-treatment-pdq
- 4.National Cancer Institute . Colorectal cancer screening (PDQ)—health professional version. Accessed December 2, 2020. https://www.cancer.gov/types/colorectal/hp/colorectal-screening-pdq
- 5.Davidson KW, Barry MJ, Mangione CM, et al. ; US Preventive Services Task Force . Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi: 10.1001/jama.2021.6238 [DOI] [PubMed] [Google Scholar]
- 6.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. ; US Preventive Services Task Force . Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;315(23):2564-2575. doi: 10.1001/jama.2016.5989 [DOI] [PubMed] [Google Scholar]
- 7.US Preventive Services Task Force . Colorectal cancer: screening. Published 2021. Accessed March 24, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening
- 8.National Colorectal Cancer Roundtable . 80% By 2018: working toward the shared goal of 80% screened for colorectal cancer by 2018. Accessed March 24, 2021. https://nccrt.org/what-we-do/80-percent-by-2018/
- 9.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood: Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328(19):1365-1371. doi: 10.1056/NEJM199305133281901 [DOI] [PubMed] [Google Scholar]
- 10.Scholefield JH, Moss S, Sufi F, Mangham CM, Hardcastle JD. Effect of faecal occult blood screening on mortality from colorectal cancer: results from a randomised controlled trial. Gut. 2002;50(6):840-844. doi: 10.1136/gut.50.6.840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, Wilschut J, van Ballegooijen M, Kuntz KM. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149(9):659-669. doi: 10.7326/0003-4819-149-9-200811040-00244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med. 2014;160(3):171. doi: 10.7326/M13-1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robertson DJ, Lee JK, Boland CR, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task Force on colorectal cancer. Gastrointest Endosc. 2017;85(1):2-21. doi: 10.1016/j.gie.2016.09.025 [DOI] [PubMed] [Google Scholar]
- 14.Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on colorectal cancer. Gastrointest Endosc. 2017;86(1):18-33. doi: 10.1016/j.gie.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 15.Gupta S, Lieberman D. Screening and surveillance colonoscopy and COVID-19: avoiding more casualties. Gastroenterology. 2020;159(4):1205-1208. doi: 10.1053/j.gastro.2020.06.091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel S, Issaka RB, Chen E, Somsouk M. Colorectal cancer screening and COVID-19. Am J Gastroenterol. 2021;116(2):433-434. doi: 10.14309/ajg.0000000000000970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myint A, Roh L, Yang L, Connolly L, Esrailian E, May FP. Noninvasive colorectal cancer screening tests help close screening gaps during coronavirus disease 2019 pandemic. Gastroenterology. 2021;161(2):712-714.e1. doi: 10.1053/j.gastro.2021.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta S, Halm EA, Rockey DC, et al. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the underserved: a randomized clinical trial. JAMA Intern Med. 2013;173(18):1725-1732. doi: 10.1001/jamainternmed.2013.9294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakr O, Afsar-Manesh N, Raja N, et al. Application of behavioral economics principles improves participation in mailed outreach for colorectal cancer screening. Clin Transl Gastroenterol. 2020;11(1):e00115. doi: 10.14309/ctg.0000000000000115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu C, Skootsky S, Grossman M, et al. A multi-level fit-based quality improvement initiative to improve colorectal cancer screening in a managed care population. Clin Transl Gastroenterol. 2018;9(8):177. doi: 10.1038/s41424-018-0046-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klabunde C, Blom J, Bulliard JL, et al. Participation rates for organized colorectal cancer screening programmes: an international comparison. J Med Screen. 2015;22(3):119-126. doi: 10.1177/0969141315584694 [DOI] [PubMed] [Google Scholar]
- 22.Levin TR. Colorectal cancer screening: 80% by 2018: colonoscopists simply cannot do it alone. Gastrointest Endosc. 2016;83(3):552-554. doi: 10.1016/j.gie.2015.08.023 [DOI] [PubMed] [Google Scholar]
- 23.Issaka RB, Avila P, Whitaker E, Bent S, Somsouk M. Population health interventions to improve colorectal cancer screening by fecal immunochemical tests: a systematic review. Prev Med. 2019;118(118):113-121. doi: 10.1016/j.ypmed.2018.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dougherty MK, Brenner AT, Crockett SD, et al. Evaluation of interventions intended to increase colorectal cancer screening rates in the United States: a systematic review and meta-analysis. JAMA Intern Med. 2018;178(12):1645-1658. doi: 10.1001/jamainternmed.2018.4637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabatino SA, Lawrence B, Elder R, et al. ; Community Preventive Services Task Force . Effectiveness of interventions to increase screening for breast, cervical, and colorectal cancers: nine updated systematic reviews for the guide to community preventive services. Am J Prev Med. 2012;43(1):97-118. doi: 10.1016/j.amepre.2012.04.009 [DOI] [PubMed] [Google Scholar]
- 26.The Decision Lab. Why do some ideas prompt other ideas later on without our conscious awareness? priming, explained. Accessed March 24, 2021. https://thedecisionlab.com/biases/priming/
- 27.Blumenthal-Barby JS, Burroughs H. Seeking better health care outcomes: the ethics of using the “nudge”. Am J Bioeth. 2012;12(2):1-10. doi: 10.1080/15265161.2011.634481 [DOI] [PubMed] [Google Scholar]
- 28.Wryobeck J, Chen Y. Using priming techniques to facilitate health behaviours. Clin Psychol. 2003;7(2):105-108. doi: 10.1080/13284200410001707553 [DOI] [Google Scholar]
- 29.Bargh JA, Gollwitzer PM, Lee-Chai A, Barndollar K, Trötschel R. The automated will: nonconscious activation and pursuit of behavioral goals. J Pers Soc Psychol. 2001;81(6):1014-1027. doi: 10.1037/0022-3514.81.6.1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chartrand TL, Bargh JA. Automatic activation of impression formation and memorization goals: nonconscious goal priming reproduces effects of explicit task instructions. J Pers Soc Psychol. 1996;71(3):464-478. doi: 10.1037/0022-3514.71.3.464 [DOI] [Google Scholar]
- 31.Matjasko JL, Cawley JH, Baker-Goering MM, Yokum DV. Applying behavioral economics to public health policy: illustrative examples and promising directions. Am J Prev Med. 2016;50(5)(suppl 1):S13-S19. doi: 10.1016/j.amepre.2016.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King D, Greaves F, Vlaev I, Darzi A. Approaches based on behavioral economics could help nudge patients and providers toward lower health spending growth. Health Aff (Millwood). 2013;32(4):661-668. doi: 10.1377/hlthaff.2012.1348 [DOI] [PubMed] [Google Scholar]
- 33.Voyer B. “Nudging” behaviours in healthcare: insights from behavioural economics. Br J Heal Care Manag. 2015;21(3):130-135. doi: 10.12968/bjhc.2015.21.3.130 [DOI] [Google Scholar]
- 34.Mehta SJ, Reitz C, Niewood T, Volpp KG, Asch DA. Effect of behavioral economic incentives for colorectal cancer screening in a randomized trial. Clin Gastroenterol Hepatol. 2021;19(8):1635-1641. doi: 10.1016/j.cgh.2020.06.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curtis JR, Downey L, Back AL, et al. Effect of a patient and clinician communication-priming intervention on patient-reported goals-of-care discussions between patients with serious illness and clinicians: a randomized clinical trial. JAMA Intern Med. 2018;178(7):930-940. doi: 10.1001/jamainternmed.2018.2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King D, Vlaev I, Everett-Thomas R, Fitzpatrick M, Darzi A, Birnbach DJ. “Priming” hand hygiene compliance in clinical environments. Health Psychol. 2016;35(1):96-101. doi: 10.1037/hea0000239 [DOI] [PubMed] [Google Scholar]
- 37.Papies EK, Potjes I, Keesman M, Schwinghammer S, van Koningsbruggen GM. Using health primes to reduce unhealthy snack purchases among overweight consumers in a grocery store. Int J Obes (Lond). 2014;38(4):597-602. doi: 10.1038/ijo.2013.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Committee for Quality Assurance . Colorectal cancer screening (COL). Accessed April 2, 2021. https://www.ncqa.org/hedis/measures/colorectal-cancer-screening/
- 39.Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi: 10.1136/bmjqs-2015-004411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. doi: 10.1136/bmj.c869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The R Project for Statistical Computing . Getting started. Accessed December 21, 2021. http://www.r-project.org/
- 42.van Roon AH, Hol L, Wilschut JA, et al. Advance notification letters increase adherence in colorectal cancer screening: a population-based randomized trial. Prev Med. 2011;52(6):448-451. doi: 10.1016/j.ypmed.2011.01.032 [DOI] [PubMed] [Google Scholar]
- 43.Cole SR, Smith A, Wilson C, Turnbull D, Esterman A, Young GP. An advance notification letter increases participation in colorectal cancer screening. J Med Screen. 2007;14(2):73-75. doi: 10.1258/096914107781261927 [DOI] [PubMed] [Google Scholar]
- 44.Senore C, Ederle A, DePretis G, et al. Invitation strategies for colorectal cancer screening programmes: the impact of an advance notification letter. Prev Med. 2015;73:106-111. doi: 10.1016/j.ypmed.2015.01.005 [DOI] [PubMed] [Google Scholar]
- 45.Kempe KL, Shetterly SM, France EK, Levin TR. Automated phone and mail population outreach to promote colorectal cancer screening. Am J Manag Care. 2012;18(7):370-378. [PubMed] [Google Scholar]
- 46.Molden DC. Understanding priming effects in social psychology: what is “social priming” and how does it occur? Soc Cogn. 2014;32(special issue):1-11. doi: 10.1521/soco.2014.32.supp.1 [DOI] [Google Scholar]
- 47.Bargh JA. The ecology of automaticity: toward establishing the conditions needed to produce automatic processing effects. Am J Psychol. 1992;105(2):181-199. doi: 10.2307/1423027 [DOI] [PubMed] [Google Scholar]
- 48.Chivers T. What’s next for psychology’s embattled field of social priming. Nature. 2019;576:200-202. doi: 10.1038/d41586-019-03755-2 [DOI] [PubMed] [Google Scholar]
- 49.Jacobson Vann JC, Jacobson RM, Coyne-Beasley T, Asafu-Adjei JK, Szilagyi PG. Patient reminder and recall interventions to improve immunization rates. Cochrane Database Syst Rev. 2018;1(1):CD003941. doi: 10.1002/14651858.CD003941.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix 1. FIT Kit Mailer Letter
eAppendix 2. MyChart Electronic Message Example
eTable 1. Logistic Regression Model of CRC Screening by Study Group
eTable 2. Screening Modality by Study Group
Data Sharing Statement