Abstract
The abuse of oral formulations of prescription opioids has precipitated the current opioid epidemic. We developed an oral oxycodone consumption model consisting of a limited access (4h) two-bottle choice drinking in the dark (TBC-DID) paradigm and quantified dependence with naloxone challenge using mice of both sexes. We also assessed neurobiological correlates of withdrawal and dependence elicited via oral oxycodone consumption using immunohistochemistry for DeltaFosB (ΔFosB), a transcription factor described as a molecular marker for drug addiction. Neither sex developed a preference for the oxycodone bottle, irrespective of oxycodone concentration, bottle position or prior water restriction. Mice that volitionally consumed oxycodone exhibited hyperlocomotion in an open field test and supra-spinal but not spinally-mediated antinociception. Both sexes also developed robust, dose-dependent levels of opioid withdrawal that was precipitated by the opioid antagonist naloxone. Oral oxycodone consumption followed by naloxone challenge led to mesocorticolimbic region-specific increases in the number of ΔFosB expressing cells. Naloxone-precipitated withdrawal jumps, but not the oxycodone bottle % preference, was positively correlated with the number of ΔFosB expressing cells specifically in the nucleus accumbens shell. Thus, limited access oral consumption of oxycodone produced physical dependence and increased ΔFosB expression despite the absence of opioid preference. Our TBC-DID paradigm allows for the study of oral opioid consumption in a simple, high-throughput manner and elucidates the underlying neurobiological substrates that accompany opioid-induced physical dependence.
Keywords: dependence, mouse, oral self-administration, oxycodone, prescription opioid, withdrawal
Graphical Abstract
1. Introduction
The non-medical use and abuse of prescription opioids has reached epidemic levels and are a major public health problem globally (Ahmad et al., 2021; Cicero and Ellis, 2017). Oxycodone is one of the most commonly abused prescription opioids, accounting for a sizable proportion of drug overdose related deaths (Jalal et al., 2018; Mattson et al., 2021; Wilson et al., 2020). Despite recent changes in prescribing guidelines, oxycodone continues to be heavily prescribed in patients with high abuse risk (Scherrer et al., 2020). Clinical reports also suggest that the initial use of prescription opioids results in physical dependence that subsequently causes concurrent or replacement use of less expensive but more accessible and lethal opioids such as fentanyl (Cicero and Ellis, 2018).
Oxycodone (6-deoxy-7,8-dehydro-14-hydroxy-3-O-methyl-6-oxomorphine) is a semisynthetic opioid analgesic that is a derivative of the opioid alkaloid thebaine (Kalso, 2005). It is a μ-opioid receptor agonist that is approximately twice as potent as the prototypical μ-opioid agonist morphine (Benziger et al., 1997). Prolonged use of oxycodone leads to many unwanted side-effects such as addiction, tolerance, and physical dependence (Compton et al., 2015; Jimenez et al., 2017; Paulozzi et al., 2006). Among these side-effects, physical dependence manifests as the urge to continue drug consumption to avoid withdrawal symptoms, which occur following discontinuation of opioid use or following exposure to an opioid antagonist, such as naloxone (Kosten and George, 2002). Withdrawal symptoms such as increased heart rate, nausea, abdominal cramps, muscle spasms, anxiety, insomnia etc. (Webster et al., 2006) can lead to compulsive drug intake and short-term relapses, thereby increasing the potential for opioid addiction (Koob, 2000; Koob et al., 1989). Further, withdrawal also results in a negative affective state which is thought to contribute to the exacerbation of a drug relapse (Edwards and Koob, 2010; Koob, 2009; Koob and Volkow, 2010).
Oral ingestion is a prevalent route of administration of oxycodone abuse and the adverse health consequences that follow (Back et al., 2011; Gasior et al., 2016; Kirsh et al., 2012; Surratt et al., 2011; Young et al., 2010). Oral ingestion of oxycodone leads to slower pharmacokinetics compared to intravenous injections (Leow et al., 1992), but still elicits dose-dependent hedonic feelings and is a preferred route of administration among individuals suffering from substance use disorder (Kirsh et al., 2012; McCabe et al., 2007; Surratt et al., 2011; Walsh et al., 2008). Moreover, newly developed abuse-deterrent formulations of oxycodone have led to increases in abuse via oral consumption (Cicero and Ellis, 2015; Gasior et al., 2016). Therefore, the development of pre-clinical models of volitional oral oxycodone consumption that capture the salient aspects of physical dependence and the subsequent withdrawal are vitally important.
Both “two-bottle choice” (TBC) (Richter and Campbell, 1940) and “drinking in the dark” (DID) (Thiele et al., 2014) are paradigms commonly used to model ethanol consumption in rodents. An oral oxycodone TBC has been recently implemented in rats (Zanni et al., 2020) and mice (Reeves et al., 2020), but the physical dependence that follows short-term limited access to oral oxycodone remains uncharacterized. Here, we combined the TBC and DID procedures, which enabled mice to voluntarily consume oxycodone during their active dark cycle for a limited time period and permitted study of volitional oral oxycodone consumption in a simple and high-throughput manner. The use of an oral route of administration mimics a prevalent medical and non-medical route of administration and enhances the translational relevance of investigations into the neurobiological mechanisms of oxycodone abuse.
We used our limited access oral oxycodone TBC- DID paradigm to test the effects of water deprivation, fixed and escalating forced choice and sex differences on the development of preference for and dependence to oxycodone in mice. Further, we quantified the neurobiological alterations caused by such oxycodone intake using DeltaFosB (ΔFosB), a transcription factor implicated in drug addiction, as a marker for neuronal activation induced by oxycodone. In our studies, following limited volitional access in post-prandial conditions, mice that orally consumed oxycodone exhibited dose-dependent levels of naloxone-precipitated opioid withdrawal, despite lacking preference for the oxycodone containing bottle. A subsequent naloxone challenge increased levels of ΔFosB in mesocorticolimbic brain regions. Withdrawal jumps, but not % preference, correlated with ΔFosB expression levels in the nucleus accumbens shell, a key component of the mesocorticolimbic reward pathway. Our results show that preference is not an obligate condition for the development of physical dependence to oral oxycodone consumption. Furthermore, naloxone-precipitated withdrawal leads to increases in a biomarker linked to opioid addiction in mesocorticolimbic brain regions that are heavily implicated in opioid-mediated reward and physical dependence.
2. Materials and Methods
2.1. Subjects
One hundred and thirty-two adult male and female mice on a C57BL/6J background were purchased from The Jackson Laboratory (Bar Harbor, ME) and used in these studies. All mice weighed 25-30 g and were ~12-24 weeks old when used in this study. Mice were single housed several days before initiating the first TBC-DID session. All mice were maintained on a 12 h reverse light/dark cycle (lights on from 8 pm to 8 am) in a temperature and humidity-controlled facility. Access to food and water varied depending on the experimental paradigm being implemented. All experiments were approved by the Indiana University Bloomington Animal Care and Use Committee (Protocol 19-037) and followed the International Association for the Study of Pain (IASP) Guidelines for the Use of Animals in Research (Zimmermann, 1983).
2.2. Drugs and chemicals
Oxycodone hydrochloride was purchased from Sigma Aldrich (St. Louis, MO) and dissolved in the relevant concentrations in standard drinking water that was treated using reverse osmosis, deionized, and irradiated with a bactericidal UV lamp. Naloxone (Sigma Aldrich) was dissolved in saline and injected via the intraperitoneal (i.p.) route at a dose of 10 mg/kg in a volume of 10 ml/kg.
2.3. Bottle and cage construction
To measure daily liquid intake and preference, bottles were constructed from 10 ml serological pipettes, lixit sipper tubes and rubber stoppers. The bottom of the serological pipettes were trimmed, and the lixit sipper tubes were attached to the bottom end such that 5 cm of the sipper tube protruded from the end. Rubber tubing was wrapped around the junction of the tube and sipper to minimize leakage. Prior to their use in experiments, each bottle was filled with 3-4 ml of water and placed in food hoppers of empty cages to assess baseline leakage. Any bottles which leaked more than 0.5 ml in a 24 h period were discarded. To place the tubes securely in the cages, regular food hoppers of the mice cages were modified to include slots for the two tubes separated by 2 cm in the place of the single water bottle slot. The slots for the tubes were also designed to prevent the mice from moving the entire tube and thereby affecting the monitoring of fluid intake. The food section of the hopper was left unmodified. Around 3 cm of the sipper tube protruded into the cage to allow mice access while preventing them from gnawing on the bottle itself.
2.4. Oxycodone two bottle choice (General Procedure)
At the start of each TBC-DID paradigm, single-housed mice were provided with two bottles filled with 3-8 ml fluid each. Fluid consumption from each bottle was assessed at the end of the TBC-DID session each day after 4 h. The initial left/right positions of the test and control bottles were distributed evenly among subjects for each experiment, and the position was varied depending on the experimental paradigm. Initially, both bottles were filled with water, and the mice were acclimated to this set-up for 3-4 days as a baseline measure. Next, mice were assigned an “untreated’ bottle containing drinking water and a “treated’ bottle containing various concentrations of oxycodone depending on the experimental paradigm. The experimental timeline, oxycodone concentrations and number of forced and TBC-DID sessions for all experimental paradigms are presented in the figure schematics. All schematics were created using Biorender.com. The difference between each drinking tube’s initial and final volume after each TBC-DID test was measured as the volume consumed for either the treated or untreated bottle. Mice were weighed every other day to allow for calculation of a daily oral dose in mg/kg for each mouse. Percent preference for the treated bottle was calculated as: %preference = treated bottle volume / (treated + untreated volume) *100. The impact of oral oxycodone consumption on body weight (g) was calculated as: % difference = (body weight ~30 min following the last oxycodone TBC-DID session - corresponding body weight following the last water baseline session)* 100.
2.5. Water deprivation TBC-DID paradigm
Effects of water deprivation on the development of oxycodone preference were evaluated using a modification of our 6-day TBC-DID session where each TBC-DID session was preceded by 3h of water deprivation (removal of regular water bottle) in the home cage of the mice. Separate groups of mice receiving 0, 0.5 or 1.0 mg/ml of oxycodone in their treated bottle were subject to this experiment.
2.6. Tail-Flick Antinociception
The hot water tail-immersion test was used to assess the impact of oral oxycodone consumption on spinally mediated antinociception. In brief, the distal 2 cm of the tail of the mouse was immersed in the water bath at 53-54°C and the latency to elicit a ‘flick’ response was measured as described previously (Slivicki et al., 2020). A 15 s cut-off was applied to avoid tissue damage. Prior to oxycodone exposure, a baseline latency measure was carried out for all mice at the end of day 4 of the water TBC-DID sessions. Subsequently, both oxycodone and water drinking mice were tested on day 2 and day 6 of the escalating dose forced choice sessions in Experiment 3 to test the effects of oral oxycodone consumption.
2.7. Hot plate Antinociception
The hot plate test was used to assess the impact of oral oxycodone consumption on supra-spinally mediated antinociception. Mice were placed on a 56° C hot plate until jumping, paw shaking, or paw licking behaviors were observed or until the maximum cut-off latency time of 30 s was reached as described previously (Iyer et al., 2020). Prior to oxycodone exposure, a baseline latency measure was carried out for all mice at the end of day 4 of the water TBC-DID sessions. Subsequently, both oxycodone and water drinking mice were tested on day 6 of the fixed dose forced choice sessions in Experiment 4 to test the effects of oral oxycodone consumption.
2.8. Locomotor activity
An open field activity meter was used to assess the impact of volitional oral oxycodone consumption on locomotor activity. Mice were handled by the experimenter prior to exposure to the arena and were allowed to acclimate in the testing room (illuminated with red light bulbs and equipped with a white noise generator to create a steady sound level of 62-63 dB) for 15 min prior to testing at the end of the TBC-DID session. Mice were placed in Omnitech Superflex Node activity meters (Dimensions: 42 x 42 x 30 cm) and their locomotor activity was recorded using the Fusion 6.5 software (Omnitech Electronics, Columbus, OH). After a 5 min recording window mice were returned to their home cages and the chambers were cleaned with 70% ethanol prior to reuse. The distance travelled (cm) by each mouse during the observation window was recorded. The same mice used in the hot plate antinociception test were also tested in the open field test on day 1 of the TBC-DID sessions in Experiment 4 to test the effects of oral oxycodone consumption. No baseline measure was carried out to avoid any habituation effects.
2.9. Precipitated Withdrawal
Mice of both sexes in Experiment 3 consuming either water or oxycodone were subject to a naloxone challenge on the last day of the TBC-DID sessions prior to euthanasia and tissue collection. A separate cohort of mice of both sexes in Experiment 4 consuming either water or oxycodone using a similar paradigm were not subject to naloxone challenge prior to euthanasia for tissue collection. In mice undergoing the naloxone challenge, following the end of the last TBC-DID session, mice were placed in individual plexiglass observation cylinders to allow for acclimation. Then, 30 min later mice in all groups were challenged with the opioid antagonist naloxone (10 mg/kg i.p.) to precipitate a μ-opioid receptor-dependent withdrawal syndrome as described previously (Iyer et al., 2020; Slivicki et al., 2020; Thomaz et al., 2021). Immediately following the naloxone injection, mice were again placed in the plexiglass observation cylinders. Mice were video recorded continually throughout the observation interval and the number of withdrawal jumps observed over 30 min following the naloxone challenge were subsequently scored by a treatment-blinded rater using the BORIS quantification software (Friard and Gamba, 2016). The impact of precipitated withdrawal on body weight (g) was calculated as a % difference: (body weight ~30 min following naloxone challenge - corresponding body weight prior to the naloxone injection)* 100.
2.10. ΔFosB immunohistochemistry
Approximately 2h after naloxone challenge, mice in Experiment 3 and 4 were deeply anesthetized with isoflurane, and transcardially perfused with 0.1% heparinized 0.1 M phosphate-buffered saline (PBS) followed by ice cold 4% paraformaldehyde (PFA). Brains were extracted post-mortem and were post-fixed in PFA (24 h) followed by cryoprotection in 30% sucrose. Brains were sectioned (coronal, 40 μm), and alternate floating sections were collected in PBS using neuroanatomical criteria according to the Paxinos and Watson Mouse Brain atlas (Keith B. J. Franklin and Paxinos, 2008) and based on the following regions of interest; two subregions within the nucleus accumbens (i.e. the nucleus accumbens shell and the nucleus accumbens core), two subregions within the amygdala (i.e. central amygdalar nucleus capsular part, basolateral amygdalar nucleus), and the ventral tegmental area. Free-floating sections were immersed in PBS containing 0.3 % hydrogen peroxide and non-specific binding was removed by incubation (1 h) with 5% goat serum diluted in PBS. Next, sections were incubated at 4 °C with a rabbit anti-ΔfosB antibody (1:10000, D3S8R, Cell Signaling Technology) in 0.4% Triton PBS for 48 h. The tissue was incubated in the presence of biotinylated goat anti-rabbit IgG followed by Vectastain elite ABC reagent (1:600, #PK6101, Vector Laboratories, Burlingame, USA). ΔFosB immunoreactive cells were visualized with the avidin-biotin peroxidase method using diaminobenzidine as a chromogen. Sections were washed with double-distilled water, slide mounted, air-dried, dehydrated, and cover-slipped with Neo-Mount® (Sigma Aldrich, St. Louis, USA).
2.11. Quantification of ΔFosB immunoreactive cells
Images were captured from slide-mounted section using a Leica (Wetzlar, Germany) DM6B microscope and a Leica DFC9000GT digital camera. The specificity of the immunostaining was verified by omission of the primary antibody from the immunostaining protocols. ΔFosB-expressing nuclei were counted at 20x magnification bilaterally using a computer-assisted image analysis system (LAS AF 2D, Leica). A square field (400 μm) (Supplementary Figure 1A, B, C) was superimposed upon the captured image to serve as a reference area and the number of cells were quantified by a scorer blinded to the experimental status of the mouse.
2.12. Statistical Analysis
Two-way repeated measures ANOVA followed by Bonferroni post hoc tests were used to compare all parameters of fluid intake in the Experiment 1 featuring male mice only. Two or three-way repeated measures ANOVA followed by Bonferroni post hoc tests were used to compare all parameters in Experiments 2-4 where mice of both sexes were used, as appropriate. One-way or two-way ANOVAs followed by Bonferroni post hoc tests were used to compare withdrawal behaviors induced following a naloxone challenge, the impact of oxycodone consumption and naloxone challenge on the number of ΔFosB expressing cells and the impact of oxycodone consumption on body weight loss across groups, as appropriate. Unpaired t-tests were used to analyze differences in number of ΔFosB positive cells between the oxycodone and water drinking mice. Linear regression analyses were used to assess the relationship between ΔFosB positive cell counts and the number of naloxone precipitated jumps or the oxycodone bottle % preference. All data was analyzed using GraphPad Prism version 7.05 or version 9 (GraphPad Software Inc., La Jolla, CA, USA). p < 0.05 was considered statistically significant.
3. Results
3.1. A limited access oxycodone TBC-DID paradigm leads to dose dependent levels of naloxone-precipitated withdrawal without the development of oxycodone preference
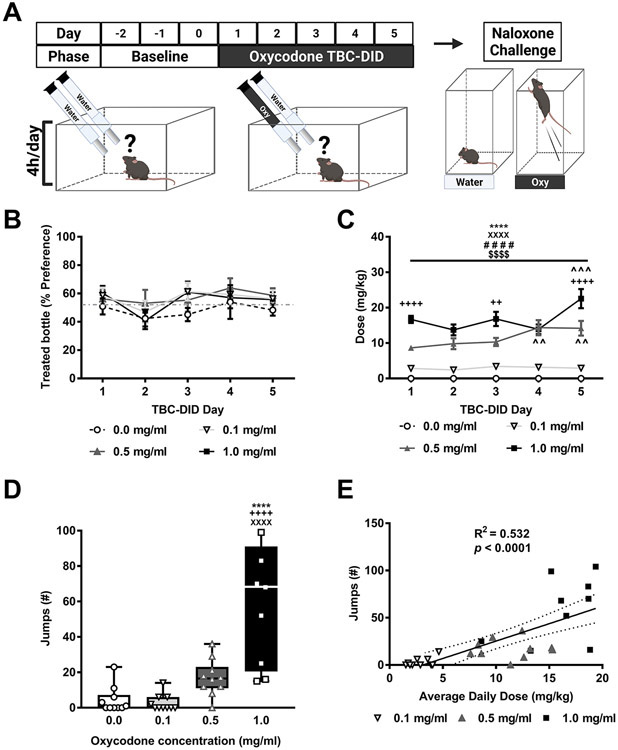
In Experiment 1, separate groups of male mice were given access to 0, 0.1, 0.5 or 1.0 mg/ml of oxycodone in their treated bottle in a 5-day limited access (4h/day) TBC-DID paradigm (Fig. 1A). The % preference did not differ between the different oxycodone groups overall, changed across TBC-DID sessions irrespective of composition of the treated bottle and the interaction was not significant (Oxycodone concentration: F3,36=1.074, p = 0.3723; Session: F4,144=2.641, p = 0.0362; Interaction: F12,144= 0.3686, p = 0.9725; Fig. 1B). Post hoc analyses failed to reveal any differences in % preference across sessions.
Fig. 1. A limited access oxycodone TBC-DID paradigm produces naloxone-precipitated withdrawal without the development preference.
A) The schematic shows the experimental timeline. B) An increasing oxycodone concentration did not impact the % preference for the treated bottle in mice subject to a six-day TBC-DID paradigm. C) The daily oxycodone dose (mg/kg) consumed increased as a function of oxycodone in a session-dependent manner. D) An increasing oxycodone concentration caused a dose-dependent increase in the number of naloxone-precipitated jumps. E) The number of naloxone-precipitated withdrawal jumps was positively correlated with the average daily dose (mg/kg) of oxycodone consumed during the TBC-DID sessions. Data are expressed as mean ± S.E.M. (n = 10 per group). “*” indicates high (1 mg/ml) concentration group vs. water (0 mg/ml) group where****p < 0.0001, **p < 0.01, *p < 0.05, “+”indicates high (1 mg/ml) vs. middle (0.5 mg/ml) concentration group, “X” indicates high (1 mg/ml) vs. low (0.1 mg/ml) concentration group, “#” indicates middle (0.5 mg/ml) concentration group vs. water (0 mg/ml) group, “$” indicates middle (0.5 mg/ml) vs. low (0.1 mg/ml) concentration group, and “^’indicates vs. Session 1 withing the same concentration group with the same symbol indications.
The daily orally consumed oxycodone dose (mg/kg) differed between the different oxycodone groups overall, changed across the five TBC-DID sessions and the interaction was significant (Oxycodone concentration: F3,36=102.7, p < 0.0001; Session: F4,144=5.67, p = 0.0003; Interaction: F12,144= 3.646, p < 0.0001; Fig. 1C). Post hoc analyses revealed that oxycodone dose was higher in the middle (0.5 mg/ml) and the high (1 mg/ml) oxycodone concentration groups across all sessions compared to the low (0.1 mg/ml) concentration and water TBC-DID groups (p < 0.0001 for each session 1-5). Effects of the low (0.1 mg/ml) oxycodone concentration group did not differ in any session compared to water TBC-DID groups (p > 0.05). Further, mice exposed to the high (1 mg/ml) oxycodone concentration consumed more oxycodone during session 1 (p < 0.0001), 3 (p < 0.01) and 5 (p < 0.0001) compared to the middle (0.5 mg/ml) concentration group. Post hoc analyses also revealed that oxycodone dose was higher in the middle (0.5 mg/ml) concentration group during session 4 (p = 0.0013) and 5 (p = 0.0019) compared to session 1 of TBC-DID whereas oxycodone dose in the high (1 mg/ml) concentration group was higher during session 5 compared to session 1 (p = 0.0009). Thus, oxycodone dose consumed increased over time for both middle and high concentrations.
Naloxone-precipitated withdrawal jumps differed between the different oxycodone groups overall (F3,35= 20.36, p < 0.0001; Fig. 1D). Post hoc analyses revealed that withdrawal jumps were higher in the high (1 mg/ml) concentration group compared to water, the low (0.1 mg/ml) and the middle (0.5 mg/ml) oxycodone concentration groups (p < 0.0001 vs. each group). One mouse from the high (1 mg/ml) concentration group died during the course of the withdrawal procedure and was excluded from the withdrawal analyses. No other fatalities were observed in any of the other TBC-DID paradigms.
A positive correlation was observed between the number of naloxone-precipitated withdrawal jumps and the average daily dose (mg/kg) of oxycodone consumed during the TBC sessions in all the oxycodone consuming groups (F1,37= 42.76, p < 0.0001; R2 = 0.532; Fig. 1E).
3.2. Water deprivation prior to oxycodone TBC-DID session does not impact the development of dose dependent levels of naloxone-precipitated withdrawal without oxycodone preference
Drinking and withdrawal parameters did not differ as function of water deprivation and were similar in levels to that observed in all other experiments (Data not shown).
3.3. Male and female mice show similar levels of oxycodone preference, dose and precipitated withdrawal following an oxycodone TBC-DID paradigm
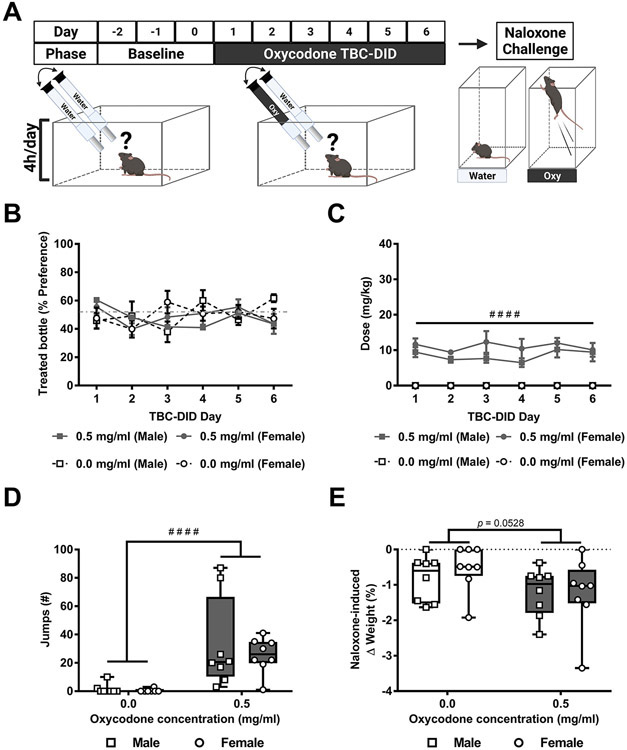
In Experiment 2, mice of both sexes were given access to either 0 or 0.5 mg/ml oxycodone in their treated bottle and the bottle positions were swapped daily in a 6-day limited access (4h/day) TBC-DID paradigm (Fig. 2A). The % preference did not differ as a function of the treated bottle, or across sessions or sexes and the interactions between any two or all three of the factors was not significant (Treated bottle : F1,28= 0.3085, p = 0.5830; Session: F3.832, 107.3= 0.8472, p = 0.4943, Sex: F1,28= 0.01091, p = 0.9176; Session x Treated bottle: F5,140= 1.586, p = 0.1677; Session x Sex: F5,140= 1.562, p = 0.1748, Treated bottle x Sex: F1,28= 0.1481, p = 0.7033; Session x Concentration x Sex: F5,140= 0.8960, p = 0.4857; Fig. 2B).
Fig. 2. Both male and female mice show similar levels of oral oxycodone preference, consumption and precipitated withdrawal.
A) The schematic shows the experimental timeline. B) No significant alterations in the % preference for oxycodone-treated bottle were seen in a sex or concentration or session-dependent manner. C) Both male and female oxycodone consuming mice consumed a higher dose compared to water consuming mice overall. D) Both male and female oxycodone consuming mice exhibited more naloxone-precipitated withdrawal jumps compared to water consuming mice overall. E) Naloxone-induced body weight loss trended to differ as a function of the treated bottle but was not altered by sex and the interaction was not significant. Data are expressed as mean ± S.E.M. (n = 8 per group). “#” indicates middle (0.5 mg/ml) concentration oxycodone group vs. water group where # # # #p < 0.0001.
The daily orally consumed oxycodone dose (mg/kg) differed as a function of the treated bottle but was not altered across sessions or sexes and the interactions between any two or all three of the factors was not significant (Treated bottle: F1,28= 113.5, p < 0.0001; Session: F3.187, 89.25= 1.019, p = 0.3913, Sex: F1,28= 1.983, p = 0.1700; Session x Treated bottle: F5,140= 1.019, p = 0.4089; Session x Sex: F5,140= 0.4648, p = 0.8020, Treated bottle x Sex: F1,28= 1.983, p = 0.1700; Session x Concentration x Sex: F5,140= 0.4648, p = 0.8020; Fig. 2C). Post hoc analyses revealed that a higher dose of oxycodone was consumed by the oxycodone (0.5 mg/ml) group compared to the water TBC-DID group overall (p < 0.0001).
Naloxone-precipitated withdrawal jumps differed as a function of the treated bottle but did not differ by sex and the interaction was not significant (Treated bottle: F1,28= 21.06, p < 0.0001, Sex: F1,28= 0.4648, p = 0.5010; Interaction: F1,28= 0.2486, p = 0.6220; Fig. 2D). Post hoc analysis revealed that, across groups, oxycodone consuming mice exhibited more withdrawal jumps compared to water consuming mice overall (p < 0.0001).
Naloxone-induced body weight changes trended to differ as a function of the treated bottle but was not altered by sex and the interaction was not significant (Treated bottle: F1,28= 4.089, p = 0.0528, Sex: F1,28= 0.2766, p = 0.6030; Interaction: F1,28= 0.3385, p = 0.5653; Fig. 2E).
3.4. Lack of preference following forced choice oxycodone access to escalating oxycodone concentrations prior to oxycodone TBC-DID
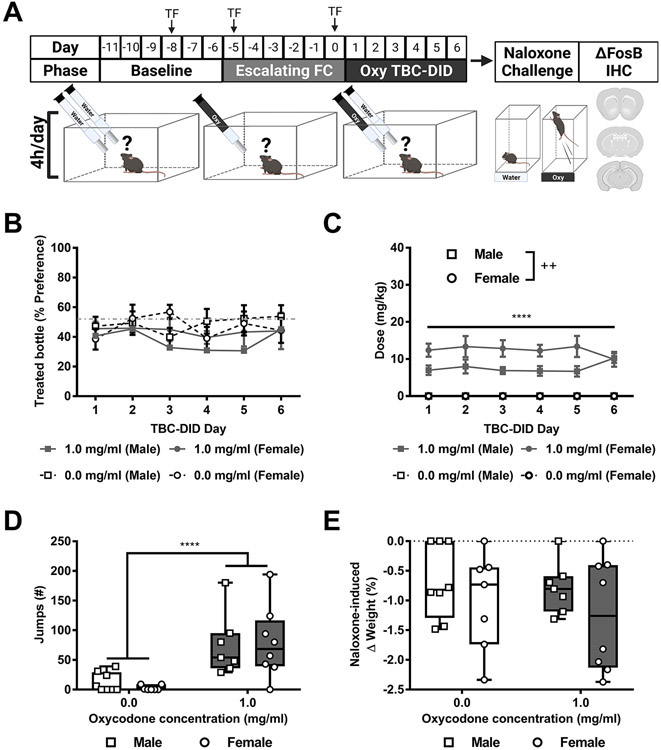
In Experiment 3, mice of both sexes were first given access to a six-day, escalating dose, limited access (4h/day), forced choice oxycodone regimen (2 days each of a single oxycodone bottle at concentrations of 0.5 mg/ml, 0.75 mg/ml, and 1 mg/ml). Then mice were given access to either 0 or 1 mg/ml oxycodone in their treated bottle in a 6-day limited access (4h/day) TBC-DID paradigm (Fig. 3A). The % preference did not differ as a function of the treated bottle, or across sessions or sexes and the interactions between any two or all three of the factors was not significant (Treated bottle: F1,26= 1.679, p = 0.2065; Session: F5, 130= 1.025, p = 0.4059, Sex: F1,26= 0.1427, p = 0.7086; Session x Treated bottle: F5,130= 0.7194, p = 0.6100; Session x Sex: F5,130= 1.475, p = 0.2025, Treated bottle x Sex: F1,26= 0.6125, p = 0.4409; Session x Concentration x Sex: F5,130= 0.7672, p = 0.5751; Fig. 3B).
Fig. 3. Escalating dose forced choice oxycodone prior to oxycodone TBC-DID does not lead to oxycodone bottle preference.
A) The schematic shows the experimental timeline with “TF” indicating days when mice were tested on the tail-flick test. B) The % preference for oxycodone-treated bottle did not differ as a function of sex, treated bottle or session. C) While across groups, oxycodone consuming mice consumed a higher dose compared to water consuming mice overall, across sexes, male mice consumed a lower dosage compared to female mice overall. D) Oxycodone consuming mice exhibited more naloxone-precipitated withdrawal jumps compared to water consuming mice overall. E) Naloxone-induced body weight loss did not differ as a function of the treated bottle or sex and the interaction was not significant. Data are expressed as mean ± S.E.M. (n = 7-8 per group). “*” indicates high (1 mg/ml) concentration oxycodone group vs. water group where ****p < 0.0001 “+” indicates male mice vs. female mice.
The daily orally consumed oxycodone dose (mg/kg) differed as a function of the treated bottle and sex but was not altered across sessions and the interaction was significant (Treated bottle: F1,26= 143.7, p < 0.0001; Session: F2.579, 67.05= 0.1120, p = 0.9342, Sex: F1,26= 8.220, p = 0.0081; Session x Treated bottle: F5,130= 0.1120, p = 0.9895; Session x Sex: F5,130= 1.124, p = 0.3511, Treated bottle x Sex: F1,26= 8.220, p = 0.0081; Session x Concentration x Sex: F5,130= 1.124, p = 0.3511; Fig. 3C). Post hoc analyses revealed that, across groups, oxycodone consuming mice consumed a higher dose compared to water consuming mice overall (p < 0.0001). Further, across sexes, male mice consumed a lower dosage compared to female mice overall (p = 0.0081).
Naloxone-precipitated withdrawal jumps differed as a function of the treated bottle but was not altered by sex and the interaction was not significant (Treated bottle: F1,26= 21.34, p < 0.0001, Sex: F1,26= 0. 03517, p = 0.8527; Interaction: F1,26= 0.2181, p = 0.6444; Fig. 3D). Post hoc analysis revealed that, across groups, oxycodone consuming mice exhibited more withdrawal jumps compared to water consuming mice overall (p < 0.0001).
Naloxone-induced body weight changes did not differ as a function of the treated bottle or sex and the interaction was not significant (Treated bottle: F1,26= 0.4043, p = 0.5304, Sex: F1,26= 2.064, p = 0.1628; Interaction: F1,26= 0.05147, p = 0.8223; Fig. 3E).
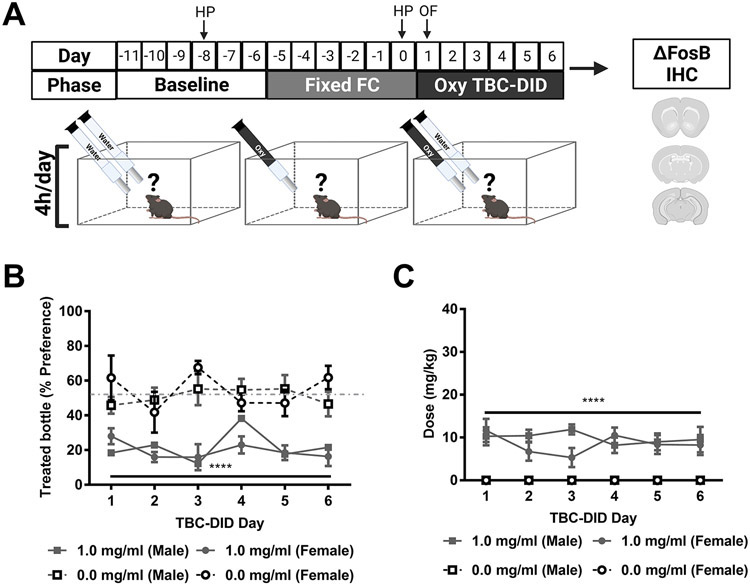
3.5. Forced choice oxycodone of fixed dosage prior to oxycodone TBC-DID leads to the development of treated bottle aversion
In Experiment 4, mice of both sexes were first given access to a six-day, fixed dose (1 mg/ml), limited access (4h/day), forced choice oxycodone regimen followed by access to either 0 or 1 mg/ml oxycodone in their treated bottle in a 6-day limited access (4h/day) TBC-DID paradigm (Fig. 4A). The % preference differed as a function of the treated bottle but was not altered across sessions or sexes and the interactions between any two or all three of the factors was not significant (Treated bottle: F1,16= 84.40, p < 0.0001; Session: F4.037, 64.60= 0.8609, p = 0.4931, Sex: F1,16= 0.03333, p = 0.8574; Session x Treated bottle: F5,80= 2.104, p = 0.0734; Session x Sex: F5,80= 2.187, p = 0.0637, Treated bottle x Sex: F1,16= 0.6576, p = 0.4293; Session x Concentration x Sex: F5,80= 0.6024, p = 0.6982; Fig. 4B). Post hoc analyses revealed that, across groups, oxycodone consuming mice had a lower % preference compared to water consuming mice overall (p < 0.0001).
Fig. 4. Fixed dose forced choice oxycodone prior to oxycodone TBC-DID leads to oxycodone bottle aversion.
A) The schematic shows the experimental timeline with “HP” and “OF” indicating days when mice were tested on the hot plate and open field activity meter test respectively. B) Oxycodone consuming mice had a lower % preference compared to water consuming mice overall. C) Oxycodone consuming mice consumed a higher dose compared to water consuming mice overall. Data are expressed as mean ± S.E.M. (n = 4-6 per group). “*” indicates high (1 mg/ml) concentration oxycodone group vs. water group where ****p < 0.0001.
The daily orally consumed oxycodone dose (mg/kg) differed as a function of the treated bottle but was not altered across sessions and sexes and the interactions between any two or all three of the factors was not significant (Treated bottle: F1,16= 186.2, p < 0.0001; Session: F2.800,44.81= 0.2872, p = 0.8212, Sex: F1,16= 1.103, p = 0.3092; Session x Treated bottle: F5,80= 0.2872, p = 0.9188; Session x Sex: F5,80= 0.8986, p = 0.4863, Treated bottle x Sex: F1,16= 1.103, p = 0.3092; Session x Concentration x Sex: F5,80= 0.8986, p = 0.4863; Fig. 4C). Post hoc analyses revealed that, across groups, oxycodone consuming mice consumed a higher dose compared to water consuming mice overall (p < 0.0001).
3.6. Oral oxycodone consumption leads to hyperlocomotion and preferentially enhances supra-spinally mediated hot plate antinociception compared to spinally mediated tail-flick antinociception
In Experiment 3, tail-flick latencies did not differ between groups at baseline. After oxycodone treatment, tail-flick latencies showed a modest but reliable alteration as a function of the treated bottle and differed by sex, but did not differ across sessions and the interaction was not significant (Treated bottle: F1,26= 15.68, p = 0.0005; Session: F1,26 = 0.04545, p = 0.8328, Sex: F1,26= 7.170, p = 0.0127; Session x Treated bottle: F1,26 = 0.4994, p = 0.4860; Session x Sex: F1,26= 0.02645, p = 0.8721, Treated bottle x Sex: F1,26= 1.078, p = 0.3088; Session x Concentration x Sex: F1,26= 0.4197, p = 0.5228; Fig. 5A). Post hoc analyses revealed that, across groups, oxycodone consuming mice exhibited slightly longer tail-flick latencies compared to water consuming mice overall (p < 0.0005). Further, male mice showed slighly longer tail-flick latencies compared to female mice overall (p = 0.0127).
Figure 5: Oral oxycodone at behaviorally active doses produces hyperlocomotion and preferentially produces hot plate antinociception compared to tail-flick antinociception.
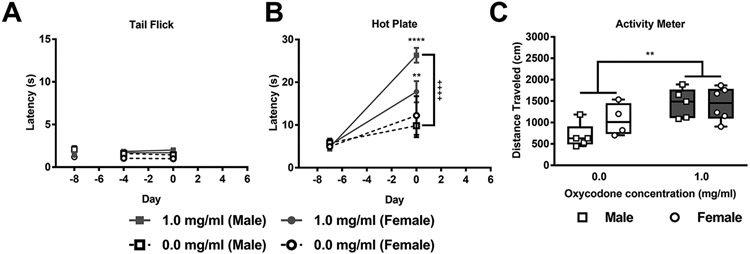
A) Hot water tail-flick latencies showed modest but reliable alterations as a function of the treated bottle and differed by sex. B) Hot plate latencies differed as a function of the treated bottle and session and the interaction between treated bottle and session and sex was significant. Both male and female mice showed longer withdrawal latencies in the hot plate test post-oxycodone compared to their water baseline levels. In male mice, oxycodone consumption increased response latencies in the hot plate test post-oxycodone compared to water consumption. C) Oxycodone consuming mice travelled a greater distance compared to water consuming mice overall in the open field activity meter test. Data are expressed as mean ± S.E.M. (n = 4-6 per group). “*” indicates high (1 mg/ml) concentration oxycodone group vs. water group where ****p < 0.0001 and **p < 0.01, “+” indicates oxycodone consuming male mice vs. water consuming male mice with the same symbol indications.
In Experiment 4, hot plate latencies did not differ between groups at baseline. After oxycodone treatment, the hot plate latency differed as a function of the treated bottle and session (pre vs. post-treatment) but not sex; the interactions between the treated bottle and session and between the treated bottle and sex and session were significant (Treated bottle: F1,16= 11.72, p = 0.0035; Session: F1,16= 68.78, p < 0.0001, Sex: F1,16= 1.086, p = 0.3129; Session x Treated bottle: F1,16= 17.26, p = 0.0007; Session x Sex: F1,16= 1.124, p = 0.3048, Treated bottle x Sex: F1,16= 2.300, p = 0.1489; Session x Concentration x Sex: F1,16= 5.055, p = 0.0390; Fig. 5B). Post hoc analyses revealed that, across sessions, both male (p < 0.0001) and female (p = 0.0033) mice showed longer withdrawal latencies in the hot plate test post-oxycodone compared to their water baseline levels. Further, oxycodone consuming male mice had exhibited longer latencies in the hot plate test post-oxycodone compared to water consuming male mice (p < 0.0001).
In Experiment 4, locomotor activity differed as a function of the treated bottle but not sex and the interaction was not significant (Treated bottle: F1,16= 12.52, p = 0.0027, Sex: F1,16= 1.305, p = 0.2701; Interaction: F1,16= 1.522, p = 0.2352; Fig. 5C). Post hoc analyses revealed that oxycodone consuming mice travelled a greater distance compared to water consuming mice overall (p = 0.0027).
3.7. Oxycodone consuming mice subjected to naloxone challenge show mesocorticolimbic region-specific increases in ΔFosB-expressing cells compared to water consuming controls
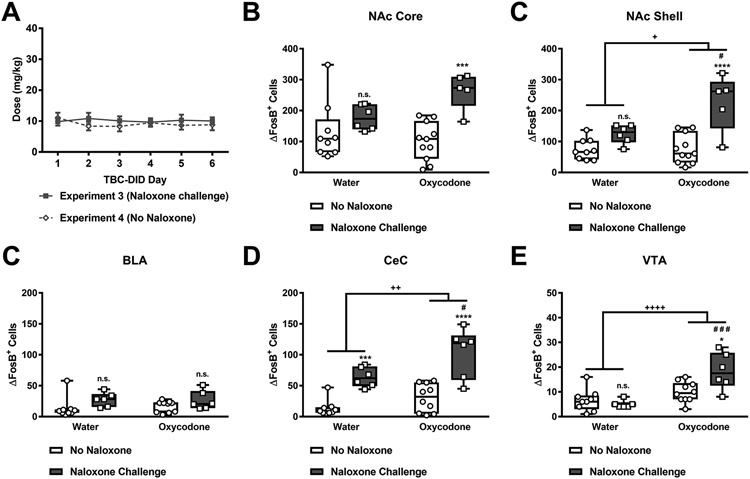
In Experiment 3, both male and female oxycodone consuming mice subjected to naloxone challenge (Fig. 6B, D, F) exhibited greater numbers of ΔFosB-expressing cells compared to water consuming mice subjected to naloxone challenge (Fig. 6A, C, E). Greater numbers of ΔFosB expressing cells were observed in the nucleus accumbens core (t9= 2.893, p = 0.0178; Fig. 7A), nucleus accumbens shell (t9= 2.609, p = 0.0283; Fig. 7B), central amygdalar nucleus capsular part (t10= 2.246, p = 0.0485; Fig. 7E), and ventral tegmental area (t10= 4.293, p = 0.0016; Fig. 7F). The number of ΔFosB expressing cells did not differ between oxycodone and water consuming mice in the basolateral amygdala (p > 0.05; Fig. 7D). A positive correlation was observed between the number of naloxone-precipitated jumps (R2= 0.403, p = 0.0359; Fig. 7C; left panel) but not the % preference for oxycodone-treated bottle (Fig. 7C; right panel) and the number of ΔFosB-expressing cells in the nucleus accumbens shell. No significant correlations were observed with the number of naloxone-precipitated jumps or % preference for oxycodone-treated bottle in any of the other brain regions evaluated (Data not shown).
Fig. 6. Representative photomicrographs show impact of oxycodone consumption and naloxone challenge on ΔFosB-expressing cells in the NAc, amygdala and VTA.
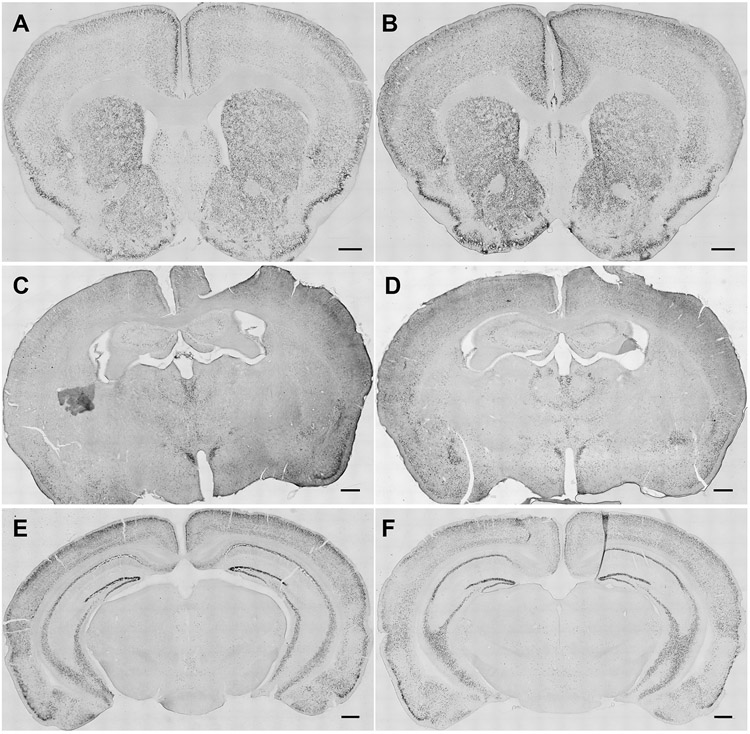
Representative images show distribution of ΔFosB staining in coronal sections at the level of the NAc, amygdala and VTA derived from A, C, E) water- and B, D, F) oxycodone-consuming mice respectively. The scale bar equals 500 μm. (Nucleus accumbens – NAc, Basolateral amygdalar nucleus - BLA, Central amygdalar nucleus capsular part – CeC, Ventral tegmental area - VTA)
Fig. 7. Naloxone challenge following oral oxycodone consumption leads to greater numbers of ΔFosB expressing cells in the NAc core, shell, CeC, and VTA.
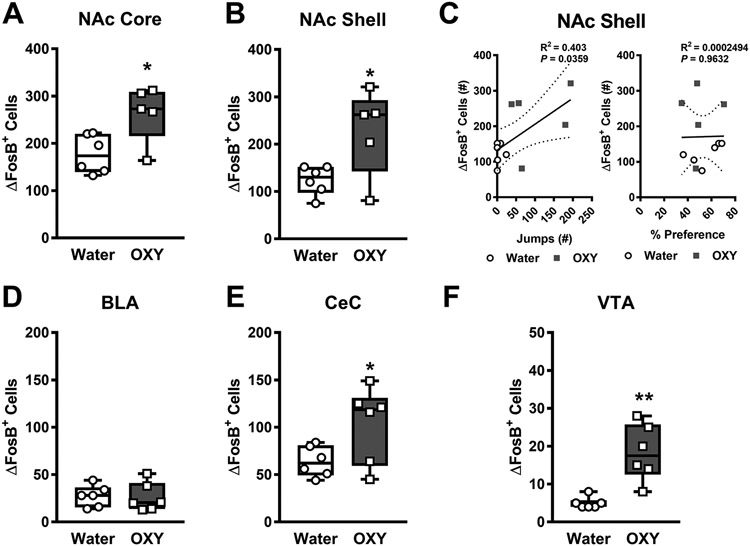
Following naloxone challenge, oxycodone consuming mice showed higher numbers of ΔFosB expressing cells in the A) NAc core, B) NAc shell, E) CeC, and F) VTA but not the D) BLA when compared to water consuming mice. C) The number of naloxone-precipitated withdrawal jumps was positively correlated with the number of ΔFosB-expressing cells in the NAc shell (left panel). In contrast, no significant correlations was observed between the % preference and the number of ΔFosB-expressing cells in the NAc shell (right panel). (Nucleus accumbens – NAc, Basolateral amygdalar nucleus - BLA, Central amygdalar nucleus capsular part – CeC, Ventral tegmental area - VTA) Data are expressed as mean ± S.E.M. (n = 5-6 per group). *p < 0.05 vs. water consuming mice.
3.8. Oral oxycodone consuming mice not subject to naloxone challenge do not show differences in ΔFosB cell counts compared to water consuming controls
Oxycodone consuming mice of both sexes that were not challenged with naloxone (Supplementary Fig. 2B, D, F) did not show reliable differences in the number of ΔFosB expressing cells in the nucleus accumbens core (Fig. 8B), nucleus accumbens shell (Fig. 8C), basolateral amygdala (Fig. 8D), central amygdalar nucleus capsular part (Fig. 8E), or ventral tegmental area (Fig. 8F) compared to water consuming mice (Supplementary Fig. 2A, C, E) (p > 0.05 for all brain regions). No significant correlations were observed with the % preference for oxycodone-treated bottle in any of the other brain regions evaluated (Data not shown).
Fig. 8. Naloxone challenge increases the number of ΔFosB-expressing cells preferentially in oxycodone consuming but not water consuming mice.
A) Male and female mice subjected to either the escalating (Fig. 3) or fixed (Fig. 4) forced choice regimen followed by a six-day oxycodone TBC-DID sessions consumed similar doses of oxycodone. Oxycodone consumption followed by naloxone challenge led to higher numbers of ΔFosB expressing cells in the B) NAc core, C) NAc shell, E) CeC, and F) VTA but not the D) BLA compared to oxycodone consumption without naloxone challenge. In contrast, water consumption followed by naloxone challenge led to higher numbers of ΔFosB expressing cells in the E) CeC alone. (Nucleus accumbens – NAc, Basolateral amygdalar nucleus - BLA, Central amygdalar nucleus capsular part – CeC, Ventral tegmental area - VTA) Data are expressed as mean ± S.E.M. (n = 5-11 per group). “*” indicates vs. corresponding no naloxone group where ****p < 0.0001, ***p < 0.001, and *p < 0.05, “#” indicates vs. corresponding naloxone challenge group, and “+” indicates main effect of oxycodone consumption with the same symbol indications. n.s. - non-significant.
3.9. Oxycodone consuming mice subjected to naloxone challenge show greater numbers of ΔFosB-expressing cells compared to mice consuming similar doses of oxycodone that were not challenged with naloxone
Mice of both sexes in Experiment 3 or 4, subject to either the escalating (Fig. 3) or the fixed (Fig. 4) forced choice regimens did not differ in the oxycodone dose (mg/kg) consumed during the TBC-DID sessions. The oxycodone dose (mg/kg) did not differ as a function of experimental paradigm or session and the interaction between was not significant (Experimental Paradigm: F1,24=0.6148, p = 0.4406; Session: F5,120=0.1994, p = 0.9622; Interaction: F5,120= 0.454, p = 0.8097; Fig. 8A).
In the nucleus accumbens core, naloxone challenge preferentially increased ΔFosB expression levels in oxycodone compared to water drinking mice, and the number of ΔFosB expressing cells increased as a function of naloxone challenge, but not oxycodone consumption, overall (Interaction: F1,27=5.178, p = 0.0310; Oxycodone consumption: F1,27=1.129, p = 0.2974; Naloxone challenge: F1,27=15.93, p = 0.0005; Fig. 8B). Post hoc analyses revealed that, across groups, oxycodone (p = 0.0010) but not water consuming mice (p > 0.05) exhibited greater numbers of ΔFosB expressing cells in response to naloxone challenge compared to mice with similar oral consumption histories that were not challenged with naloxone.
In the nucleus accumbens shell, naloxone challenge preferentially increased ΔFosB expression levels in oxycodone compared to water drinking mice, and the number of ΔFosB expressing cells was higher in oxycodone consuming mice, and following naloxone challenge, overall (Interaction: F1,27=6.964, p = 0.0136; Oxycodone consumption: F1,27=7.589, p = 0.0104; Naloxone challenge: F1,27=27.81, p < 0.0001; Fig. 8C). Post hoc analyses revealed that oxycodone (p < 0.0001) but not water consuming mice (p > 0.05) exhibited greater numbers of ΔFosB expressing cells in response to naloxone challenge compared to their counterparts that were not challenged with naloxone. Further, oxycodone consuming mice exhibited greater numbers of ΔFosB-expressing cells compared to water consuming mice subjected to naloxone challenge (p = 0.0141).
In the basolateral amygdala, the number of ΔFosB positive cells increased as a function of naloxone challenge, but not oxycodone consumption, overall and the interaction between oxycodone consumption and naloxone challenge was not significant (Interaction: F1,27=0.1662, p = 0.6867; Oxycodone consumption: F1,27= 0.02931, p = 0.8653; Naloxone challenge: F1,27=5.798, p = 0.0231; Fig. 8C). However, post hoc analyses failed to reveal any differences in ΔFosB expressing cells across groups.
In the central amygdalar nucleus capsular part, both oxycodone consumption and naloxone challenge overall increased the number of ΔFosB expressing cells and the interaction between naloxone challenge and oxycodone consumption was not significant (Oxycodone consumption: F1,27=10.33, p = 0.0034; Naloxone challenge: F1,27=49.27, p < 0.0001; Interaction: F1,27= 1.647, p = 0.2103; Fig. 8D). Post hoc analyses revealed that, across groups, both oxycodone (p < 0.0001) and water consuming mice (p = 0.0008) exhibited greater numbers of ΔFosB expressing cells in response to naloxone challenge compared to their counterparts that were not challenged with naloxone. Further, oxycodone consuming mice exhibited greater numbers of ΔFosB-expressing cells compared to water consuming mice subjected to naloxone challenge (p = 0.0156).
In the ventral tegmental area, naloxone challenge preferentially increased ΔFosB expression levels in oxycodone compared to water drinking mice, and the number of ΔFosB expressing cells increased as function of oxycodone consumption, but not naloxone challenge, overall (Interaction: F1,27=8.296, p = 0.0077; Oxycodone consumption: F1,27=23.13, p < 0.0001; Naloxone challenge: F1,27=3.933, p = 0.0576; Fig. 8E). Post hoc analyses revealed that, across groups, oxycodone (p = 0.0105) but not water consuming mice (p > 0.05) exhibited greater numbers of ΔFosB expressing cells in response to naloxone challenge compared to mice not challenged with naloxone. Further, oxycodone consuming mice exhibited greater numbers of ΔFosB-expressing cells compared to water consuming mice subjected to naloxone challenge (p = 0.0002).
3.10. Oral oxycodone consumption impacts body weight change.
In Experiment 1, oxycodone produced concentration-dependent changes in body weight overall (F3,36= 25.01, p < 0.0001; Supplementary Fig. 3A). Post hoc analyses revealed that, across groups, the low (0.1 mg/ml), middle (0.5 mg/ml) and high (1 mg/ml) oxycodone concentration groups all showed a greater reduction in body weight compared to the water group (p < 0.0001 vs. each group).
In Experiment 2, change in body weight, relative to baseline, did not differ as a function of oxycodone concentration, and changed in a sex-dependent manner although the interaction was not significant (Oxycodone concentration: F1,28=0.01383, p = 0.9072; Sex: F1,28=9.144, p = 0.0053; Interaction: F1,28= 0.6234, p = 0.4364; Supplementary Fig. 3B). Post hoc analyses revealed that male mice had a higher loss in body weight compared to female mice overall (p = 0.0053).
In Experiment 3, change in body weight, relative to baseline, differed as a function of oxycodone concentration and sex and the interaction was significant (Oxycodone concentration: F1,26= 5.21, p = 0.0309, Sex: F1,26= 7.412, p = 0.0114; Interaction: F1,26= 6.047, p = 0.0209; Supplementary Fig. 3C). Post hoc analyses revealed that oxycodone consuming male mice showed a greater reduction in body weight compared to oxycodone consuming female mice (p = 0.0067). Further, oxycodone consuming male mice also showed greater reduction in body weight compared to the water consuming male mice (p = 0.0148) while no such differences in body weight were found corresponding groups of female mice (p > 0.05).
In Experiment 4, body weight changes, relative to baseline, did not differ as a function of oxycodone concentration or sex and the interaction was not significant (Oxycodone concentration: F1,16= 0.8724, p = 0.3642, Sex: F1,16= 0.5028, p = 0.4885; Interaction: F1,16= 1.662, p = 0.2157; Supplementary Fig. 3D).
4. Discussion
Prescription opioids are important therapeutic agents in the treatment of pain and remain a mainstay of chronic pain management (Minhas and Leri, 2018). Despite changes to the CDC Guideline for Prescribing Opioids for Chronic Pain, modest increases in new oxycodone prescriptions have been reported (Tucker et al., 2021), potentially contributing to iatrogenic opioid dependence (Theisen et al., 2018; Waljee et al., 2017). Abuse liability and overdose deaths attributable to oxycodone have exceeded those of other semi-synthetic opioids such as hydrocodone (Control and Prevention, 2009; Wightman et al., 2012). There is, therefore, an urgent need to better understand the neurobiological changes that follow the oxycodone intake.
Both continuous drug delivery (Mori et al., 2013; Raehal and Bohn, 2011) and chronic s.c. dosing (Bhalla et al., 2015) of oxycodone are shown to produce physical dependence. While volitional consumption of oral oxycodone has recently been modeled in rodents (Enga et al., 2016; Zanni et al., 2020), the behavioral and neurobiological characteristics underlying oral oxycodone-induced physical dependence remain poorly understood. In the present study, mice subjected to a limited access oral intermittent oxycodone paradigm developed robust, dose-dependent physical dependence without the concurrent development of escalating oxycodone intake or preference. Strikingly, when challenged with naloxone, these mice showed greater numbers of ΔFosB-expressing cells in several brain regions important in drug abuse and reward. Limited access to oral oxycodone also affected appetitive behavior as evidenced by a decrease in total fluid consumption across our experimental parameters (Data not shown) and produced behavioral alterations including hyperlocomotion and supra-spinally-mediated hot plate antinociception.
Rodents are unlikely to show a preference for oral oxycodone when using a limited access TBC-DID paradigm. Mice subjected to a 24 h TBC oral oxycodone paradigm did not develop a preference for the oxycodone bottle compared to the water bottle (Reeves et al., 2020). Similarly, rats do not develop a preference or escalate their intake of morphine following a limited access oral intake in a morphine TBC paradigm (Badawy et al., 1982; Gellert and Holtzman, 1978). However, following initial forced exposure in a long-term, chronic and continuous access oral oxycodone TBC lasting 22 weeks, both dose escalation and preference for the oxycodone-treated bottle was observed in rats (Zanni et al., 2020). Several factors could account for the lack of development of preference or dose escalation in our TBC-DID paradigms. Our studies did not include forced exposure to oxycodone where the only source of fluid for an extended period was the oxycodone-treated bottle, a procedure which is likely to precipitate development of dependence. All opioids including morphine, fentanyl and oxycodone are reported to have a bitter taste at certain concentrations, and this may also account for the lack of preference seen in our paradigm due to a bitter taste-aversion (Belknap, 1990; Carlson, 1989; Jimenez et al., 2017). The extensive first-pass metabolism of oral oxycodone via N-demethylation to noroxycodone also leads to low bioavailability and a longer latency to induce oxycodone reward (Chan et al., 2008) which may affect the development of preference following short-term limited access. Finally, test duration influences the sensitivity of the TBC-DID test (Tordoff and Bachmanov, 2002) and a longer duration of access to the treated bottle either as an increase in the number of sessions and/or increase in session duration could be needed for the development of preference for oral oxycodone.
Prescription opioid abuse liability may be impacted by sex differences (see reviews in (Becker and Koob, 2016; Bobzean et al., 2014; Lynch et al., 2002; Serdarevic et al., 2017)). Sex-dependent differences in the pharmacokinetic, metabolic and analgesic effects of oxycodone are observed in rodents (Chan et al., 2008; Holtman and Wala, 2006; Neelakantan et al., 2015). Female mice had higher levels of oxycodone self-administration, but equivalent reinstatement levels compared to male mice in a study modeling an operant contingency based oral oxycodone consumption (Phillips et al., 2019). However, such sex differences in oxycodone self-administration behavior may not be due to differences in oxycodone pharmacokinetics (Mavrikaki et al., 2017). In our study, male and female mice did not differ in oxycodone preference, oxycodone dose consumed, and oxycodone-induced weight loss. Since female mice had a lower body weight on average compared to male mice, they consumed higher oxycodone doses compared to male mice though these differences did not reach levels of statistical significance.
Few preclinical studies have characterized the effects of oxycodone-induced physical dependence in rodent models. Naloxone-precipitated body-weight loss, forepaw tremors, rearing and diarrhea were observed in mice subjected to subcutaneous slow-release oxycodone emulsion at levels similar to those produced by morphine (Mori et al., 2013). Removal of an osmotic minipump delivering oxycodone in rats produced weight loss characteristic of physical dependence (Hutchinson et al., 2009). Somatic signs of naloxone-precipitated withdrawal were also observed in mice subjected to a 9-day subcutaneous oxycodone b.i.d. dosing schedule (Enga et al., 2016). However, to our knowledge, no prior study has evaluated the development of physical dependence following the volitional oral consumption of oxycodone or its impact on ΔFosB expression. We show that both male and female mice exhibited robust dose-dependent levels of naloxone precipitated withdrawal jumps following oral oxycodone consumption which was indicative of the development of physical dependence. Further, this method does not involve surgical procedures (i.e. those required for osmotic pump or subcutaneous pellet implantation) and did not produce any mortality attributable to opioid consumption. Our TBC-DID paradigm followed by naloxone precipitated withdrawal therefore provides a novel, easy, and high-throughput method to accurately measure physical dependence produced by volitional consumption of oral oxycodone.
The Fos family of transcription factors act as neuronal activation markers in response to several drugs of abuse (Nestler et al., 2001). FosB are members of this Fos family, and are implicated in drug addiction mediated neural plasticity (Kaplan et al., 2011). ΔFosB is a highly stable and truncated splice variant of the full-length FosB (Marttila et al., 2006). Unlike full-length FosB which is expressed in a rapid and transient manner, ΔFosB gradually accumulates over a relatively prolonged period in response to chronic stimuli due to its very long half-life and stability (McClung et al., 2004; Nestler et al., 2001). ΔFosB expression is induced in the brain in response to chronic administration of drugs of abuse including morphine (Muller and Unterwald, 2005; Nye and Nestler, 1996; Wang et al., 2005; Zhang et al., 2016a), cocaine (Hope et al., 1994; Moratalla et al., 1996; Nye et al., 1995), and nicotine (Pich et al., 1997). Due to its long half-life, ΔFosB levels gradually accumulate with repeated drug exposure (Chen et al., 1997) and may represent a mechanism by which drugs of abuse produce lasting changes in gene expression patterns (Chao and Nestler, 2004). Such increasing levels of ΔFosB may also lead to an increased sensitivity to drugs of abuse and manifest as addictive behaviors (Kaplan et al., 2011; McClung et al., 2005; Nye and Nestler, 1996). Spontaneous withdrawal following chronic morphine treatment has been linked to increased ΔFosB levels in the nucleus accumbens core, nucleus accumbens shell, central amygdalar nucleus capsular part, ventral tegmental area, and cingulate cortex in rats (McDaid et al., 2006; Zhang et al., 2016a). ΔFosB expression is also increased in the nucleus accumbens following naloxone- (Nunez et al., 2010; Wang et al., 2005) or naltrexone-precipitated (Nye and Nestler, 1996) withdrawal in non-contingent morphine-dependent rats. Using a bi-transgenic mouse line to overexpress ΔFosB in the nucleus accumbens in morphine-dependent mice, ΔFosB overexpression was associated with exacerbated morphine sensitivity, tolerance and naloxone-precipitated withdrawal while reducing morphine analgesia (Zachariou et al., 2006). These findings underscore the essential role of ΔFosB in opioid-mediated mechanisms.
Our results show, for the first time, that oxycodone consuming mice exhibited greater numbers of ΔFosB expressing cells in the nucleus accumbens core, nucleus accumbens shell, central amygdalar nucleus capsular part, and ventral tegmental area following naloxone challenge compared to all other groups. Increased ΔFosB expression was higher in oxycodone consuming animals challenged with naloxone compared to either water consuming controls or oxycodone consuming mice that were not challenged with naloxone. Moreover, ΔFosB expression was preferentially impacted by naloxone in oxycodone consuming mice alone as water consuming mice did not show alterations in ΔFosB expression in response to naloxone challenge in any region studied, with the exception of the CeC. In naloxone-treated mice, the number of naloxone-precipitated withdrawal jumps, but not the % preference for oxycodone-treated bottle, was positively correlated with ΔFosB in the nucleus accumbens shell only. ΔFosB expression did not correlate with the either the number withdrawal jumps or the % preference for oxycodone-treated bottle in any other region evaluated. A potential caveat in interpreting our data is that we did not identify the phenotypes of neurons expressing ΔFosB. Moreover, the timepoints post-perfusion were matched in the oxycodone consumption conditions in which naloxone challenge was either present or absent; thus, our no naloxone condition is likely to reflect a condition of oxycodone dependence rather than spontaneous withdrawal. Finally, it must be noted that our studies were powered to evaluate differences in ΔFosB expression levels in oxycodone versus water-consuming controls and were not powered to assess correlations between ΔFosB expression levels in specific brain regions and withdrawal jumps. Further studies are required to examine the regional specificity of this phenomenon more extensively.
μ-opioid receptor agonists dose-dependently increase locomotor activity (Collins et al., 2016; Niikura et al., 2013) and produce antinociception in mouse models of neuropathic or inflammatory pain (Narita et al., 2008; Nozaki et al., 2005). Oxycodone (10 mg/kg p.o.) also increases tail-flick antinociception in mice; this effect was blocked by β-funaltrexamine, a selective μ-opioid receptor antagonist (Nozaki et al., 2006). However, evidence for sex differences in pharmacological effects of oxycodone are conflicting and depend on the model being tested (Chan et al., 2008; Collins et al., 2016). While the mice in our study did not show the development of preference or escalation in oxycodone intake, orally consumed oxycodone was behaviorally active in producing μ-opioid receptor mediated effects including the Straub tail (Aceto et al., 1969; Hecht and Schiorring, 1979), hyperlocomotion in an open field and supra-spinally-mediated hot plate antinociception. By contrast, a very modest but reliable spinally-mediated tail-flick antinociception was observed following oral consumption of low doses of oxycodone in our study, suggesting that the hot plate test was more sensitive than the tail-flick test to antinociceptive effects of oral oxycodone consumption at the timepoint evaluated. While the antinociceptive effects of systemic, intrathecal and intracerebroventricularly injected oxycodone on the tail-flick (Beardsley et al., 2004; Cleary et al., 1994; Ma et al., 2001; Narita et al., 2008; Nielsen et al., 2000; Ross et al., 2000; Yang et al., 2016) and hot plate (Austin Zamarripa et al., 2018; Beardsley et al., 2004; Carter, 1991; Poyhia and Kalso, 1992; Zhang et al., 2016b) assays are well documented, to our knowledge, no prior study has evaluated these effects following voluntary oral oxycodone consumption. Differences in opioid receptor affinities, lipo-solubility, and metabolism may account for the disparity between spinal and supraspinal antinociception produced by oral oxycodone consumption in our study (Lemberg et al., 2006; Narita et al., 2008; Poyhia and Kalso, 1992). Further, while our TBC-DID apparatus permits quantification of overall oxycodone drinking, it did not permit analysis of the timeline of this drinking. Future studies investigating the microstructure of such drinking could determine whether higher oral doses are needed to elicit spinal antinociception or whether the pharmacological effects of oral oxycodone are distinct, with tail-flick antinociception dissipating faster than the hot plate antinociception.
Some potential caveats should be considered in interpreting our oxycodone drinking data. The initial baseline TBC-DID sessions use water alone in both bottles and all the experimental paradigms took place under post-prandial conditions. While water deprivation initially increased the total fluid consumption, this tapered down during the course of the experiment back to levels similar to non-deprived controls. Therefore, water deprivation did not significantly alter the dependence or preference endpoints in our paradigm. We did not test effects of food deprivation on oral oxycodone consumption in any of our experimental paradigms. However, food- restriction strongly influences opioid- seeking behavior (Carroll and Meisch, 1981; Meisch and Kliner, 1979; Shalev, 2012). While our volitional exposure paradigm allows mice to self-regulate their consumption of oxycodone and produces concentration-dependent signs of physical dependence, more work is necessary to compare these effects with other non-contingent forms of oxycodone or other opioid administration (e.g. using pellets or repeated injections). Finally, we did not analyze the impact of any taste adulterants in our oral oxycodone model. Both positive and negative taste adulterants such as saccharine (Belknap, 1990; Horowitz et al., 1977) and quinine (Forgie et al., 1988; Grim et al., 2018) impact the oral consumption of opioids; future studies could potentially explore their role in the development of oral oxycodone preference.
The oral route of prescription opioid administration has been heavily linked to substance use disorder (Back et al., 2011; Gasior et al., 2016; Kirsh et al., 2012; Surratt et al., 2011). Further, the neuroadaptive effects produced by volitional versus forced (experimenter-administered) opioids markedly differ (Jacobs et al., 2003). The limited access volitional oral oxycodone paradigm presented here gives insight into the mechanisms underlying oxycodone-induced physical dependence and its neurobiological substrates, thereby providing a model that could be leveraged to evaluate pharmacotherapeutics that attenuate oxycodone withdrawal.
Supplementary Material
Supplementary Fig. 1. Schematic showing representative brain sections used for quantification of ΔFosB expressing cells. Mouse brain atlas images with superimposed square fields (400 μm) show regions used for quantification of ΔFosB cell counts in representative sections of the A) NAc (core (1) and shell (2)), B) amygdala (CeC (3) and BLA (4)), and C) VTA (5). Black numbered square areas indicate regions where a square field was superimposed by a blinded experimenter and ΔfosB-expressing nuclei were counted at 20x magnification using a computer-assisted image analysis system (LAS AF 2D, Leica). Images also show distance of each coronal plane of section from bregma in mm. (Nucleus accumbens – NAc, Basolateral amygdalar nucleus - BLA, Central amygdalar nucleus capsular part – CeC, Ventral tegmental area - VTA). Schematics of coronal sections were adapted from (Keith B. J. Franklin and Paxinos, 2008).
Supplementary Fig. 2. Representative photomicrographs show impact of oxycodone consumption without naloxone challenge on ΔFosB-expressing cells in the NAc, amygdala and VTA. Representative images show distribution of ΔFosB staining in coronal sections at the level of the NAc, amygdala and VTA derived from A, C, E) water- and B, D, F) oxycodone-consuming mice not subject to naloxone challenge respectively. The scale bar equals 500 μm. (Nucleus accumbens – NAc, Basolateral amygdalar nucleus - BLA, Central amygdalar nucleus capsular part – CeC, Ventral tegmental area - VTA)
Supplementary Fig. 3. Oral oxycodone consumption impacts body weight change compared to water consumption. A) In Experiment 1, across groups, the low (0.1 mg/ml), middle (0.5 mg/ml) and high (1 mg/ml) oxycodone concentration groups all showed a greater reduction in body weight compared to the water group. B) In Experiment 2, male mice had a higher loss in body weight compared to female mice overall irrespective of treatment. C) In Experiment 3, male mice consuming oxycodone showed a greater reduction in body weight compared to female mice consuming oxycodone and compared to male mice consuming water. D) In Experiment 4, body weight changes, relative to baseline, did not differ as a function of oxycodone concentration or sex and the interaction was not significant. Data are expressed as mean ± S.E.M. (n = 4-10 per group). “*” indicates high (1 mg/ml) concentration oxycodone group vs. water group where ****p < 0.0001 ,**p < 0.01 and, *p < 0.05, “#” indicates middle (0.5 mg/ml) concentration group vs. water (0 mg/ml) group, “&” indicates low (0.1 mg/ml) concentration group vs. water (0 mg/ml) group, “+” indicates oxycodone consuming male mice vs. female mice with the same symbol indications.
Highlights.
Naloxone induces withdrawal following limited access oral oxycodone administration
Dose-dependent withdrawal occurs without concurrent preference or dose escalation
Preference was absent irrespective of sex, bottle position or water restriction
Volitional oral oxycodone produced hyperlocomotion and hot plate antinociception
Naloxone challenge post-oxycodone increased ΔFosB in mesocorticolimbic regions
Acknowledgements:
The authors wish to thank Allen Cody, Jesse Goode and Cameron Walker from the Indiana University Department of Psychological and Brain Sciences Technical Support Group for their expert help in creating the mice cage racks and drinking tubes.
Support:
This work is supported by the National Institutes of Health National Institute on Drug Abuse (NIDA) [Grants DA047858, DA041229 and DA042584] (A.G.H.), the Indiana Addiction Grand Challenge Grant (A.G.H.), the Gill Graduate Student Fellowship and the Harlan Scholars Research Program (V.I.) and a T32 NIDA Predoctoral training grant DA024628 (T.J.W.).
Footnotes
Conflict of Interest: None of the authors report any conflicts of interest.
CRediT author statement
Vishakh Iyer: Conceptualization, Methodology, Formal analysis, Investigation, Writing - Original Draft, Writing - Review & Editing, Visualization
Taylor Woodward: Conceptualization, Methodology, Formal analysis, Investigation, Writing - Review & Editing, Visualization
Romario Pacheco: Conceptualization, Methodology, Investigation, Writing - Review & Editing, Visualization
Andrea G. Hohmann: Conceptualization, Methodology, Formal analysis, Resources, Writing - Review & Editing, Supervision, Project administration, Funding acquisition
References
- Aceto MD, McKean DB, Pearl J, 1969. Effects of opiates and opiate antagonists on the Straub tail reaction in mice. Br J Pharmacol 36, 225–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad F, Rossen L, Sutton P, 2021. Provisional drug overdose death counts National Center for Health Statistics. 2021. Statistics, Centers for Disease Control and Prevention (CDC). [Google Scholar]
- Austin Zamarripa C, Edwards SR, Qureshi HN, Yi JN, Blough BE, Freeman KB, 2018. The G-protein biased mu-opioid agonist, TRV130, produces reinforcing and antinociceptive effects that are comparable to oxycodone in rats. Drug Alcohol Depend 192, 158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Lawson KM, Singleton LM, Brady KT, 2011. Characteristics and correlates of men and women with prescription opioid dependence. Addict Behav 36, 829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy AA, Evans CM, Evans M, 1982. Production of tolerance and physical dependence in the rat by simple administration of morphine in drinking water. Br J Pharmacol 75, 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Aceto MD, Cook CD, Bowman ER, Newman JL, Harris LS, 2004. Discriminative stimulus, reinforcing, physical dependence, and antinociceptive effects of oxycodone in mice, rats, and rhesus monkeys. Exp Clin Psychopharmacol 12, 163–172. [DOI] [PubMed] [Google Scholar]
- Becker JB, Koob GF, 2016. Sex Differences in Animal Models: Focus on Addiction. Pharmacol Rev 68, 242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap JK, 1990. Physical dependence induced by the voluntary consumption of morphine in inbred mice. Pharmacol Biochem Behav 35, 311–315. [DOI] [PubMed] [Google Scholar]
- Benziger DP, Miotto J, Grandy RP, Thomas GB, Swanton RE, Fitzmartin RD, 1997. A pharmacokinetic/pharmacodynamic study of controlled-release oxycodone. J Pain Symptom Manage 13, 75–82. [DOI] [PubMed] [Google Scholar]
- Bhalla S, Pais G, Tapia M, Gulati A, 2015. Endothelin ETA receptor antagonist reverses naloxone-precipitated opioid withdrawal in mice. Can J Physiol Pharmacol 93, 935–944. [DOI] [PubMed] [Google Scholar]
- Bobzean SA, DeNobrega AK, Perrotti LI, 2014. Sex differences in the neurobiology of drug addiction. Exp Neurol 259, 64–74. [DOI] [PubMed] [Google Scholar]
- Carlson KR, 1989. Taste vs. CNS effects in voluntary oral opiate intake: studies with a novel device and technique. Pharmacol Biochem Behav 34, 419–423. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Meisch RA, 1981. Determinants of increased drug self-administration due to food deprivation. Psychopharmacology (Berl) 74, 197–200. [DOI] [PubMed] [Google Scholar]
- Carter RB, 1991. Differentiating analgesic and non-analgesic drug activities on rat hot plate: effect of behavioral endpoint. Pain 47, 211–220. [DOI] [PubMed] [Google Scholar]
- Chan S, Edwards SR, Wyse BD, Smith MT, 2008. Sex differences in the pharmacokinetics, oxidative metabolism and oral bioavailability of oxycodone in the Sprague-Dawley rat. Clin Exp Pharmacol Physiol 35, 295–302. [DOI] [PubMed] [Google Scholar]
- Chao J, Nestler EJ, 2004. Molecular neurobiology of drug addiction. Annu Rev Med 55, 113–132. [DOI] [PubMed] [Google Scholar]
- Chen J, Kelz MB, Hope BT, Nakabeppu Y, Nestler EJ, 1997. Chronic Fos-related antigens: stable variants of deltaFosB induced in brain by chronic treatments. J Neurosci 17, 4933–4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, 2015. Abuse-Deterrent Formulations and the Prescription Opioid Abuse Epidemic in the United States: Lessons Learned From OxyContin. JAMA Psychiatry 72, 424–430. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, 2017. The prescription opioid epidemic: a review of qualitative studies on the progression from initial use to abuse. Dialogues Clin Neurosci 19, 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, 2018. Oral and non-oral routes of administration among prescription opioid users: Pathways, decision-making and directionality. Addict Behav 86, 11–16. [DOI] [PubMed] [Google Scholar]
- Cleary J, Mikus G, Somogyi A, Bochner F, 1994. The influence of pharmacogenetics on opioid analgesia: studies with codeine and oxycodone in the Sprague-Dawley/Dark Agouti rat model. J Pharmacol Exp Ther 271, 1528–1534. [PubMed] [Google Scholar]
- Collins D, Reed B, Zhang Y, Kreek MJ, 2016. Sex differences in responsiveness to the prescription opioid oxycodone in mice. Pharmacol Biochem Behav 148, 99–105. [DOI] [PubMed] [Google Scholar]
- Compton WM, Boyle M, Wargo E, 2015. Prescription opioid abuse: Problems and responses. Prev Med 80, 5–9. [DOI] [PubMed] [Google Scholar]
- Control, C. f. D., Prevention, 2009. Overdose deaths involving prescription opioids among Medicaid enrollees-Washington, 2004-2007. MMWR: Morbidity and mortality weekly report 58, 1171–1175. [PubMed] [Google Scholar]
- Edwards S, Koob GF, 2010. Neurobiology of dysregulated motivational systems in drug addiction. Future Neurol 5, 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enga RM, Jackson A, Damaj MI, Beardsley PM, 2016. Oxycodone physical dependence and its oral self-administration in C57BL/6J mice. Eur J Pharmacol 789, 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgie ML, Beyerstein BL, Alexander BK, 1988. Contributions of taste factors and gender to opioid preference in C57BL and DBA mice. Psychopharmacology (Berl) 95, 237–244. [DOI] [PubMed] [Google Scholar]
- Friard O, Gamba M, 2016. BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods in Ecology and Evolution 7, 1325–1330. [Google Scholar]
- Gasior M, Bond M, Malamut R, 2016. Routes of abuse of prescription opioid analgesics: a review and assessment of the potential impact of abuse-deterrent formulations. Postgrad Med 128, 85–96. [DOI] [PubMed] [Google Scholar]
- Gellert VF, Holtzman SG, 1978. Development and maintenance of morphine tolerance and dependence in the rat by scheduled access to morphine drinking solutions. J Pharmacol Exp Ther 205, 536–546. [PubMed] [Google Scholar]
- Grim TW, Park SJ, Schmid CL, Laprairie RB, Cameron M, Bohn LM, 2018. The effect of quinine in two bottle choice procedures in C57BL6 mice: Opioid preference, somatic withdrawal, and pharmacokinetic outcomes. Drug Alcohol Depend 191, 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht A, Schiorring E, 1979. Behavioral effects of low and high acute doses of morphine in solitary mice. Psychopharmacology (Berl) 64, 73–79. [DOI] [PubMed] [Google Scholar]
- Holtman JR Jr., Wala EP, 2006. Characterization of the antinociceptive effect of oxycodone in male and female rats. Pharmacol Biochem Behav 83, 100–108. [DOI] [PubMed] [Google Scholar]
- Hope BT, Nye HE, Kelz MB, Self DW, Iadarola MJ, Nakabeppu Y, Duman RS, Nestler EJ, 1994. Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron 13, 1235–1244. [DOI] [PubMed] [Google Scholar]
- Horowitz GP, Whitney G, Smith JC, Stephan FK, 1977. Morphine ingestion: genetic control in mice. Psychopharmacology (Berl) 52, 119–122. [DOI] [PubMed] [Google Scholar]
- Hutchinson MR, Lewis SS, Coats BD, Skyba DA, Crysdale NY, Berkelhammer DL, Brzeski A, Northcutt A, Vietz CM, Judd CM, Maier SF, Watkins LR, Johnson KW, 2009. Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast). Brain Behav Immun 23, 240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer V, Slivicki RA, Thomaz AC, Crystal JD, Mackie K, Hohmann AG, 2020. The cannabinoid CB2 receptor agonist LY2828360 synergizes with morphine to suppress neuropathic nociception and attenuates morphine reward and physical dependence. Eur J Pharmacol 886, 173544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EH, Smit AB, de Vries TJ, Schoffelmeer AN, 2003. Neuroadaptive effects of active versus passive drug administration in addiction research. Trends Pharmacol Sci 24, 566–573. [DOI] [PubMed] [Google Scholar]
- Jalal H, Buchanich JM, Roberts MS, Balmert LC, Zhang K, Burke DS, 2018. Changing dynamics of the drug overdose epidemic in the United States from 1979 through 2016. Science 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez SM, Healy AF, Coelho MA, Brown CN, Kippin TE, Szumlinski KK, 2017. Variability in prescription opioid intake and reinforcement amongst 129 substrains. Genes Brain Behav 16, 709–724. [DOI] [PubMed] [Google Scholar]
- Kalso E, 2005. Oxycodone. J Pain Symptom Manage 29, S47–56. [DOI] [PubMed] [Google Scholar]
- Kaplan GB, Leite-Morris KA, Fan W, Young AJ, Guy MD, 2011. Opiate sensitization induces FosB/DeltaFosB expression in prefrontal cortical, striatal and amygdala brain regions. PLoS One 6, e23574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith BJ Franklin M, Paxinos G, 2008. The Mouse Brain in Stereotaxic Coordinates, Compact: The Coronal Plates and Diagrams. Elsevier Science. [Google Scholar]
- Kirsh K, Peppin J, Coleman J, 2012. Characterization of prescription opioid abuse in the United States: focus on route of administration. J Pain Palliat Care Pharmacother 26, 348–361. [DOI] [PubMed] [Google Scholar]
- Koob GF, 2000. Neurobiology of addiction. Toward the development of new therapies. Ann N Y Acad Sci 909, 170–185. [DOI] [PubMed] [Google Scholar]
- Koob GF, 2009. Dynamics of neuronal circuits in addiction: reward, antireward, and emotional memory. Pharmacopsychiatry 42 Suppl 1, S32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Stinus L, Le Moal M, Bloom FE, 1989. Opponent process theory of motivation: neurobiological evidence from studies of opiate dependence. Neurosci Biobehav Rev 13, 135–140. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2010. Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, George TP, 2002. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect 1, 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemberg KK, Kontinen VK, Siiskonen AO, Viljakka KM, Yli-Kauhaluoma JT, Korpi ER, Kalso EA, 2006. Antinociception by spinal and systemic oxycodone: why does the route make a difference? In vitro and in vivo studies in rats. Anesthesiology 105, 801–812. [DOI] [PubMed] [Google Scholar]
- Leow KP, Smith MT, Watt JA, Williams BE, Cramond T, 1992. Comparative oxycodone pharmacokinetics in humans after intravenous, oral, and rectal administration. Ther Drug Monit 14, 479–484. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME, 2002. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 164, 121–137. [DOI] [PubMed] [Google Scholar]
- Ma HC, Dohi S, Wang YF, Ishizawa Y, Yanagidate F, 2001. The antinociceptive and sedative effects of carbachol and oxycodone administered into brainstem pontine reticular formation and spinal subarachnoid space in rats. Anesth Analg 92, 1307–1315. [DOI] [PubMed] [Google Scholar]
- Marttila K, Raattamaa H, Ahtee L, 2006. Effects of chronic nicotine administration and its withdrawal on striatal FosB/DeltaFosB and c-Fos expression in rats and mice. Neuropharmacology 51, 44–51. [DOI] [PubMed] [Google Scholar]
- Mattson CL, Tanz LJ, Quinn K, Kariisa M, Patel P, Davis NL, 2021. Trends and geographic patterns in drug and synthetic opioid overdose deaths—United States, 2013–2019. Morbidity and Mortality Weekly Report 70, 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrikaki M, Pravetoni M, Page S, Potter D, Chartoff E, 2017. Oxycodone self-administration in male and female rats. Psychopharmacology (Berl) 234, 977–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, Cranford JA, Boyd CJ, Teter CJ, 2007. Motives, diversion and routes of administration associated with nonmedical use of prescription opioids. Addict Behav 32, 562–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ, Zachariou V, 2005. Regulation of gene expression by chronic morphine and morphine withdrawal in the locus ceruleus and ventral tegmental area. J Neurosci 25, 6005–6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ, 2004. DeltaFosB: a molecular switch for long-term adaptation in the brain. Brain Res Mol Brain Res 132, 146–154. [DOI] [PubMed] [Google Scholar]
- McDaid J, Dallimore JE, Mackie AR, Napier TC, 2006. Changes in accumbal and pallidal pCREB and deltaFosB in morphine-sensitized rats: correlations with receptor-evoked electrophysiological measures in the ventral pallidum. Neuropsychopharmacology 31, 1212–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisch RA, Kliner DJ, 1979. Etonitazene as a reinforcer for rats: increased etonitazene-reinforced behavior due to food deprivation. Psychopharmacology (Berl) 63, 97–98. [DOI] [PubMed] [Google Scholar]
- Minhas M, Leri F, 2018. A Multifaceted Analysis of Oxycodone Addiction. International Journal of Mental Health and Addiction 16, 1016–1032. [Google Scholar]
- Moratalla R, Elibol B, Vallejo M, Graybiel AM, 1996. Network-level changes in expression of inducible Fos-Jun proteins in the striatum during chronic cocaine treatment and withdrawal. Neuron 17, 147–156. [DOI] [PubMed] [Google Scholar]
- Mori T, Komiya S, Uzawa N, Inoue K, Itoh T, Aoki S, Shibasaki M, Suzuki T, 2013. Involvement of supraspinal and peripheral naloxonazine-insensitive opioid receptor sites in the expression of mu-opioid receptor agonist-induced physical dependence. Eur J Pharmacol 715, 238–245. [DOI] [PubMed] [Google Scholar]
- Muller DL, Unterwald EM, 2005. D1 dopamine receptors modulate deltaFosB induction in rat striatum after intermittent morphine administration. J Pharmacol Exp Ther 314, 148–154. [DOI] [PubMed] [Google Scholar]
- Narita M, Nakamura A, Ozaki M, Imai S, Miyoshi K, Suzuki M, Suzuki T, 2008. Comparative pharmacological profiles of morphine and oxycodone under a neuropathic pain-like state in mice: evidence for less sensitivity to morphine. Neuropsychopharmacology 33, 1097–1112. [DOI] [PubMed] [Google Scholar]
- Neelakantan H, Ward SJ, Walker EA, 2015. Discriminative stimulus effects of morphine and oxycodone in the absence and presence of acetic acid in male and female C57Bl/6 mice. Exp Clin Psychopharmacol 23, 217–227. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, Self DW, 2001. DeltaFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci U S A 98, 11042–11046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen CK, Ross FB, Smith MT, 2000. Incomplete, asymmetric, and route-dependent cross-tolerance between oxycodone and morphine in the Dark Agouti rat. J Pharmacol Exp Ther 295, 91–99. [PubMed] [Google Scholar]
- Niikura K, Ho A, Kreek MJ, Zhang Y, 2013. Oxycodone-induced conditioned place preference and sensitization of locomotor activity in adolescent and adult mice. Pharmacol Biochem Behav 110, 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki C, Saitoh A, Kamei J, 2006. Characterization of the antinociceptive effects of oxycodone in diabetic mice. Eur J Pharmacol 535, 145–151. [DOI] [PubMed] [Google Scholar]
- Nozaki C, Saitoh A, Tamura N, Kamei J, 2005. Antinociceptive effect of oxycodone in diabetic mice. Eur J Pharmacol 524, 75–79. [DOI] [PubMed] [Google Scholar]
- Nunez C, Martin F, Foldes A, Luisa Laorden M, Kovacs KJ, Victoria Milanes M, 2010. Induction of FosB/DeltaFosB in the brain stress system-related structures during morphine dependence and withdrawal. J Neurochem 114, 475–487. [DOI] [PubMed] [Google Scholar]
- Nye HE, Hope BT, Kelz MB, Iadarola M, Nestler EJ, 1995. Pharmacological studies of the regulation of chronic FOS-related antigen induction by cocaine in the striatum and nucleus accumbens. J Pharmacol Exp Ther 275, 1671–1680. [PubMed] [Google Scholar]
- Nye HE, Nestler EJ, 1996. Induction of chronic Fos-related antigens in rat brain by chronic morphine administration. Mol Pharmacol 49, 636–645. [PubMed] [Google Scholar]
- Paulozzi LJ, Budnitz DS, Xi Y, 2006. Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiol Drug Saf 15, 618–627. [DOI] [PubMed] [Google Scholar]
- Phillips AG, McGovern DJ, Lee S, Ro K, Huynh DT, Elvig SK, Fegan KN, Root DH, 2019. Oral prescription opioid-seeking behavior in male and female mice. Addict Biol, e12828. [DOI] [PubMed] [Google Scholar]
- Pich EM, Pagliusi SR, Tessari M, Talabot-Ayer D, Hooft van Huijsduijnen R, Chiamulera C, 1997. Common neural substrates for the addictive properties of nicotine and cocaine. Science 275, 83–86. [DOI] [PubMed] [Google Scholar]
- Poyhia R, Kalso EA, 1992. Antinociceptive effects and central nervous system depression caused by oxycodone and morphine in rats. Pharmacol Toxicol 70, 125–130. [DOI] [PubMed] [Google Scholar]
- Raehal KM, Bohn LM, 2011. The role of beta-arrestin2 in the severity of antinociceptive tolerance and physical dependence induced by different opioid pain therapeutics. Neuropharmacology 60, 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves KC, Kube MJ, Grecco GG, Fritz BM, Munoz B, Yin F, Gao Y, Haggerty DL, Hoffman HJ, Atwood BK, 2020. Mu opioid receptors on vGluT2-expressing glutamatergic neurons modulate opioid reward. Addict Biol, e12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter CP, Campbell KH, 1940. Alcohol Taste Thresholds and Concentrations of Solution Preferred by Rats. Science 91, 507–508. [DOI] [PubMed] [Google Scholar]
- Ross FB, Wallis SC, Smith MT, 2000. Co-administration of sub-antinociceptive doses of oxycodone and morphine produces marked antinociceptive synergy with reduced CNS side-effects in rats. Pain 84, 421–428. [DOI] [PubMed] [Google Scholar]
- Scherrer JF, Tucker J, Salas J, Zhang Z, Grucza R, 2020. Comparison of Opioids Prescribed for Patients at Risk for Opioid Misuse Before and After Publication of the Centers for Disease Control and Prevention's Opioid Prescribing Guidelines. JAMA Netw Open 3, e2027481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serdarevic M, Striley CW, Cottler LB, 2017. Sex differences in prescription opioid use. Curr Opin Psychiatry 30, 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, 2012. Chronic food restriction augments the reinstatement of extinguished heroin-seeking behavior in rats. Addict Biol 17, 691–693. [DOI] [PubMed] [Google Scholar]
- Slivicki RA, Iyer V, Mali SS, Garai S, Thakur GA, Crystal JD, Hohmann AG, 2020. Positive Allosteric Modulation of CB1 Cannabinoid Receptor Signaling Enhances Morphine Antinociception and Attenuates Morphine Tolerance Without Enhancing Morphine- Induced Dependence or Reward. Front Mol Neurosci 13, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surratt H, Kurtz SP, Cicero TJ, 2011. Alternate routes of administration and risk for HIV among prescription opioid abusers. J Addict Dis 30, 334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theisen K, Jacobs B, Macleod L, Davies B, 2018. The United States opioid epidemic: a review of the surgeon's contribution to it and health policy initiatives. BJU international 122, 754–759. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Crabbe JC, Boehm SL 2nd, 2014. "Drinking in the Dark" (DID): a simple mouse model of binge-like alcohol intake. Curr Protoc Neurosci 68, 9 49 41–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomaz AC, Iyer V, Woodward TJ, Hohmann AG, 2021. Fecal microbiota transplantation and antibiotic treatment attenuate naloxone-precipitated opioid withdrawal in morphine-dependent mice. Exp Neurol 343, 113787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff MG, Bachmanov AA, 2002. Influence of test duration on the sensitivity of the two-bottle choice test. Chem Senses 27, 759–768. [DOI] [PubMed] [Google Scholar]
- Tucker J, Salas J, Zhang Z, Grucza R, Scherrer JF, 2021. Provider specialty and odds of a new codeine, hydrocodone, oxycodone and tramadol prescription before and after the CDC opioid prescribing guideline publication. Preventive Medicine 146, 106466. [DOI] [PubMed] [Google Scholar]
- Waljee JF, Li L, Brummett CM, Englesbe MJ, 2017. Iatrogenic opioid dependence in the United States: are surgeons the gatekeepers? Annals of surgery 265, 728–730. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Nuzzo PA, Lofwall MR, Holtman JR Jr., 2008. The relative abuse liability of oral oxycodone, hydrocodone and hydromorphone assessed in prescription opioid abusers. Drug Alcohol Depend 98, 191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Xiang XH, Guo Y, Wu WR, Cao DY, Wang HS, Zhao Y, 2005. Ionotropic glutamatergic neurotransmission in the ventral tegmental area modulates DeltaFosB expression in the nucleus accumbens and abstinence syndrome in morphine withdrawal rats. Eur J Pharmacol 527, 94–104. [DOI] [PubMed] [Google Scholar]
- Webster LR, Butera PG, Moran LV, Wu N, Burns LH, Friedmann N, 2006. Oxytrex minimizes physical dependence while providing effective analgesia: a randomized controlled trial in low back pain. J Pain 7, 937–946. [DOI] [PubMed] [Google Scholar]
- Wightman R, Perrone J, Portelli I, Nelson L, 2012. Likeability and abuse liability of commonly prescribed opioids. Journal of Medical Toxicology 8, 335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson N, Kariisa M, Seth P, Smith H. t., Davis NL, 2020. Drug and Opioid-Involved Overdose Deaths - United States, 2017-2018. MMWR Morb Mortal Wkly Rep 69, 290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang PP, Yeh GC, Yeh TK, Xi J, Loh HH, Law PY, Tao PL, 2016. Activation of delta-opioid receptor contributes to the antinociceptive effect of oxycodone in mice. Pharmacol Res 111, 867–876. [DOI] [PubMed] [Google Scholar]
- Young AM, Havens JR, Leukefeld CG, 2010. Route of administration for illicit prescription opioids: a comparison of rural and urban drug users. Harm Reduct J 7, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariou V, Bolanos CA, Selley DE, Theobald D, Cassidy MP, Kelz MB, Shaw-Lutchman T, Berton O, Sim-Selley LJ, Dileone RJ, Kumar A, Nestler EJ, 2006. An essential role for DeltaFosB in the nucleus accumbens in morphine action. Nat Neurosci 9, 205–211. [DOI] [PubMed] [Google Scholar]
- Zanni G, DeSalle MJ, Deutsch HM, Barr GA, Eisch AJ, 2020. Female and male rats readily consume and prefer oxycodone to water in a chronic, continuous access, two-bottle oral voluntary paradigm. Neuropharmacology 167, 107978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Liu Q, Li T, Liu Y, Wang L, Zhang Z, Liu H, Hu M, Qiao Y, Niu H, 2016a. Expression and colocalization of NMDA receptor and FosB/DeltaFosB in sensitive brain regions in rats after chronic morphine exposure. Neurosci Lett 614, 70–76. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Windisch K, Altschuler J, Rahm S, Butelman ER, Kreek MJ, 2016b. Adolescent oxycodone self administration alters subsequent oxycodone-induced conditioned place preference and anti-nociceptive effect in C57BL/6J mice in adulthood. Neuropharmacology 111, 314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M, 1983. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16, 109–110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Schematic showing representative brain sections used for quantification of ΔFosB expressing cells. Mouse brain atlas images with superimposed square fields (400 μm) show regions used for quantification of ΔFosB cell counts in representative sections of the A) NAc (core (1) and shell (2)), B) amygdala (CeC (3) and BLA (4)), and C) VTA (5). Black numbered square areas indicate regions where a square field was superimposed by a blinded experimenter and ΔfosB-expressing nuclei were counted at 20x magnification using a computer-assisted image analysis system (LAS AF 2D, Leica). Images also show distance of each coronal plane of section from bregma in mm. (Nucleus accumbens – NAc, Basolateral amygdalar nucleus - BLA, Central amygdalar nucleus capsular part – CeC, Ventral tegmental area - VTA). Schematics of coronal sections were adapted from (Keith B. J. Franklin and Paxinos, 2008).
Supplementary Fig. 2. Representative photomicrographs show impact of oxycodone consumption without naloxone challenge on ΔFosB-expressing cells in the NAc, amygdala and VTA. Representative images show distribution of ΔFosB staining in coronal sections at the level of the NAc, amygdala and VTA derived from A, C, E) water- and B, D, F) oxycodone-consuming mice not subject to naloxone challenge respectively. The scale bar equals 500 μm. (Nucleus accumbens – NAc, Basolateral amygdalar nucleus - BLA, Central amygdalar nucleus capsular part – CeC, Ventral tegmental area - VTA)
Supplementary Fig. 3. Oral oxycodone consumption impacts body weight change compared to water consumption. A) In Experiment 1, across groups, the low (0.1 mg/ml), middle (0.5 mg/ml) and high (1 mg/ml) oxycodone concentration groups all showed a greater reduction in body weight compared to the water group. B) In Experiment 2, male mice had a higher loss in body weight compared to female mice overall irrespective of treatment. C) In Experiment 3, male mice consuming oxycodone showed a greater reduction in body weight compared to female mice consuming oxycodone and compared to male mice consuming water. D) In Experiment 4, body weight changes, relative to baseline, did not differ as a function of oxycodone concentration or sex and the interaction was not significant. Data are expressed as mean ± S.E.M. (n = 4-10 per group). “*” indicates high (1 mg/ml) concentration oxycodone group vs. water group where ****p < 0.0001 ,**p < 0.01 and, *p < 0.05, “#” indicates middle (0.5 mg/ml) concentration group vs. water (0 mg/ml) group, “&” indicates low (0.1 mg/ml) concentration group vs. water (0 mg/ml) group, “+” indicates oxycodone consuming male mice vs. female mice with the same symbol indications.