Abstract
Pancreatic intraepithelial neoplasia (PanIN) is a microscopic precursor lesion to pancreatic ductal adenocarcinoma (PDAC), however there are few biomarkers that segregate high-grade PanIN/PDAC from low-grade PanIN lesions. mAb Das-1 is a monoclonal antibody against a colonic epithelial antigen that is reactive to premalignant conditions of the upper gastrointestinal tract including Barrett’s esophagus, incomplete-type gastric intestinal metaplasia, and intraductal papillary mucinous neoplasm (IPMN) of the pancreas at high risk of malignancy. We sought to examine a role for Das-1 expression in differentiating high-grade PanIN/PDAC from low-grade PanIN lesions. We examined surgical specimens from 86 patients and 2 autopsied pancreata (74 with and 14 without PDAC) with 107 distinct PanIN lesions, 74 PDAC, and 32 associated lymph node metastases with internal controls of normal pancreatic ducts observed in 56 cases. All of the normal pancreatic duct controls (0/56) and low-grade PanIN (0/95) lesions were non-reactive to Das-1. Das-1 expression amongst high-grade PanIN (7/12, 58%), PDAC (55/74, 74%), and lymph node metastasis (21/32, 66%) was significantly higher (p<0.0001). Clinicopathologically, Das-1 reactivity was significantly correlated to nodal metastasis (p=0.021). Overall, the sensitivity, specificity, and accuracy of Das-1 in segregating high-grade PanIN/PDAC from low-grade PanIN lesions and normal ducts were 72%, 100%, and 90% respectively. Thus, mAb Das-1 reacts with high specificity to high-grade PanIN and PDAC and may help in preoperative diagnosis and/or clinical risk stratification.
Keywords: Pancreatic intraepithelial neoplasia (PanIN), mAb Das-1, Pancreatic Adenocarcinoma, Biomarker, CEP
1. INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is projected to become the second leading cause of cancer death in the United States by 2030.(1) Unfortunately, the vast majority of patients present with locally advanced or metastatic disease, and only if the patient’s cancer is small and confined to the pancreas, or ideally preinvasive, will post-surgical overall survival be meaningfully improved.(2) Thus, biomarkers to identify PDAC, preferably in its preinvasive stage, are of utmost clinical need.
Pancreatic intraepithelial neoplasia (PanIN) is a precursor lesion to PDAC that is confined to pancreatic ducts and is 5mm or less in diameter. PanIN typically exhibits a gastric epithelial morphology and is currently classified into two grades: low-grade PanIN (PanIN-1 and PanIN-2) and high-grade PanIN (PanIN-3). The latter demonstrates pronounced cytologic and architectural atypia, equivalent to in situ carcinoma.(3–5) Morphological progression from low-grade to high-grade PanIN lesions is marked by successive accumulation of alterations in cancer-associated genes including KRAS, p16/CDKN2A, TP53 and SMAD4/DPC4.(3) Clinically, the presence of high-grade PanIN may impact the decision for pancreatic resection and if found in the context of a patient with hereditary pancreatitis for example, is an indication for total pancreatectomy.(6) Unfortunately, there is significant inter-observer variability in morphologic grading of PanIN(7, 8) creating the need for additional approaches for molecular grading. Indeed, given the high prevalence of low-grade PanIN lesions that are unlikely to progress to clinically important neoplasm in the general population,(4, 7, 9) markers are needed not only to help differentiate neoplastic from non-neoplastic pancreatic lesions, but also to identify high-grade PanIN that often accompanies invasive carcinoma or might indicate a future pancreatic cancer risk.
While genetic and/or epigenetic alterations of several genes such as KRAS, TP53 and DPC/SMAD4, and expression of several biomarkers such as COX2, S100A4, and Survivin have been identified and correlated to outcomes in PDAC, studies have not been able to show that their expression reliably identifies cancer at an early or preinvasive stage with high sensitivity or specificity.(10) Many markers are confounded by their expression in chronic pancreatitis (CP), from which PDAC may emerge. Even critical markers such as KRAS, which is found in the vast majority of PDAC, are ubiquitous in low-grade PanIN as well as in individuals who smoke, those with chronic pancreatitis, and in non-neoplastic ductal hyperplasia.(11–13) Available screening modalities may have difficulty in distinguishing mass-forming CP from PDAC, not only because PDAC may arise in the background of CP, but also because clinical signs and symptoms of the conditions can be quite similar.
Using a colon epithelial protein (CEP), we have previously developed a novel murine monoclonal antibody, mAb Das-1 (formerly known as 7E12H12, IgM isotype), that reacts specifically with normal colonic epithelium.(14) Both via immunoperoxidase and immunofluorescence assays, we and others, have independently demonstrated that mAb Das-1 specifically reacts with both non-goblet and goblet cell colonic epithelium, but not with normal small intestinal enterocytes from the duodenum, jejunum, or ileum.(14, 15) While mAb Das-1 reactivity is similarly absent from normal pancreatic, gastric, and esophageal mucosa, it is strongly expressed in pre-neoplastic and intestinal-phenotypic changes in these organs. Indeed, it has been shown that mAb Das-1 is both highly sensitive and specific for the detection of Barrett’s esophagus, incomplete type gastric intestinal metaplasia, and cancer developed therefrom.(16–19) We have reported that mAb Das-1 expression serves as a sensitive and highly specific marker segregating high-risk lesions of intraductal papillary mucinous neoplasm of the pancreas (IPMN) from low-risk lesions. While normal pancreatic ducts and low-grade (low- and intermediate-grade) gastric-type IPMN were minimally reactive, mAb Das-1 was significantly more reactive in high-risk/malignant lesions including: intestinal-type IPMN, high-grade dysplasia of any epithelial subtype, and IPMN associated carcinoma.(20) In a large, multi-center validation study with cyst fluid from 169 patients with resected pancreatic cystic lesions, ELISA for Das-1 identified high-risk lesions (those lesions warranting surgical resection) with 88% sensitivity, 99% specificity, and 95% accuracy which was significantly more accurate than any available clinical guideline.(21)
In the present study, we explore the ability of mAb Das-1 to distinguish non-neoplastic pancreatic ducts and low-grade PanIN from high-grade lesions. To that end we examined reactivity of the mAb by immunohistochemistry against tissue with all grades of PanIN, associated PDAC, and matched lymph node metastasis.
2. MATERIALS AND METHODS
2.1. Case Selection
The Massachusetts General Hospital institutional review board approved this study. The surgical pathology file of Massachusetts General Hospital was queued for pancreatic resections for PDAC between 2005 and 2007. After exclusion of post neoadjuvant therapy resections and those with insufficient materials, the remaining 74 cases as well as 10 pancreatic resections for mass-forming chronic pancreatitis, 2 pancreatic resections for pancreatic duct stricture with associated chronic pancreatitis, and 2 autopsied pancreata with PanIN lesions formed our study cohort (n=88). Of those, representative sections with various grades of PanIN, invasive adenocarcinoma, and/or associated lymph node (LN) metastasis were evaluated with Das-1 immunohistochemistry. All blocks were formalin fixed, paraffin embedded, and routine serial 5 μm sections were made. Hematoxylin-eosin (H&E) stained sections were reviewed to ensure the presence of representative lesions in the same block.
For the purpose of this study, PanIN lesions were classified into low-grade PanIN (PanIN-1 and PanIN-2), and high-grade PanIN (PanIN-3) using previously accepted criteria.(4) In brief, PanIN-1 is defined as either a flat (PanIN-1A) or papillary/micropapillary (PanIN-1B) epithelial lesion composed of columnar cells with basally located nuclei, abundant cytoplasmic mucin, and essentially no cytologic atypia. PanIN-2 is predominantly papillary and exhibits mild-to-moderate nuclear atypia, nuclear crowding, hyperchromatism, and pseudostratification. In contrast, PanIN-3 has pronounced architectural and cytologic atypia equivalent to carcinoma in situ that is usually papillary or micropapillary and demonstrates a loss of polarity with prominent nucleoli and mitoses.
2.2. Immunohistochemistry using mAb Das-1
Tissue sections from each lesion were examined with mAb Das-1 using a sensitive immunoperoxidase assay, as described previously.(14, 16, 17, 20) Each experiment included at least two slides of normal colon and duodenal tissue sections as positive and negative controls, respectively (Figure 1 A&B). Reactivity to mAb Das-1 was considered positive if a dark green to crisp golden-brown staining was present and were graded based on the percentage of affected cells that were positive: Grade 0 (negative) - < 5%, Grade 1 – 5–25%, Grade 2 – 25–50%, and Grade 3 - >50%. Two of the authors (KKD and MM-K) who were blinded to the clinical data evaluated and scored each section. There was agreement among the investigators in the vast majority of cases, and in the cases with discordant scores, the final score was determined after discussion.
Figure 1:
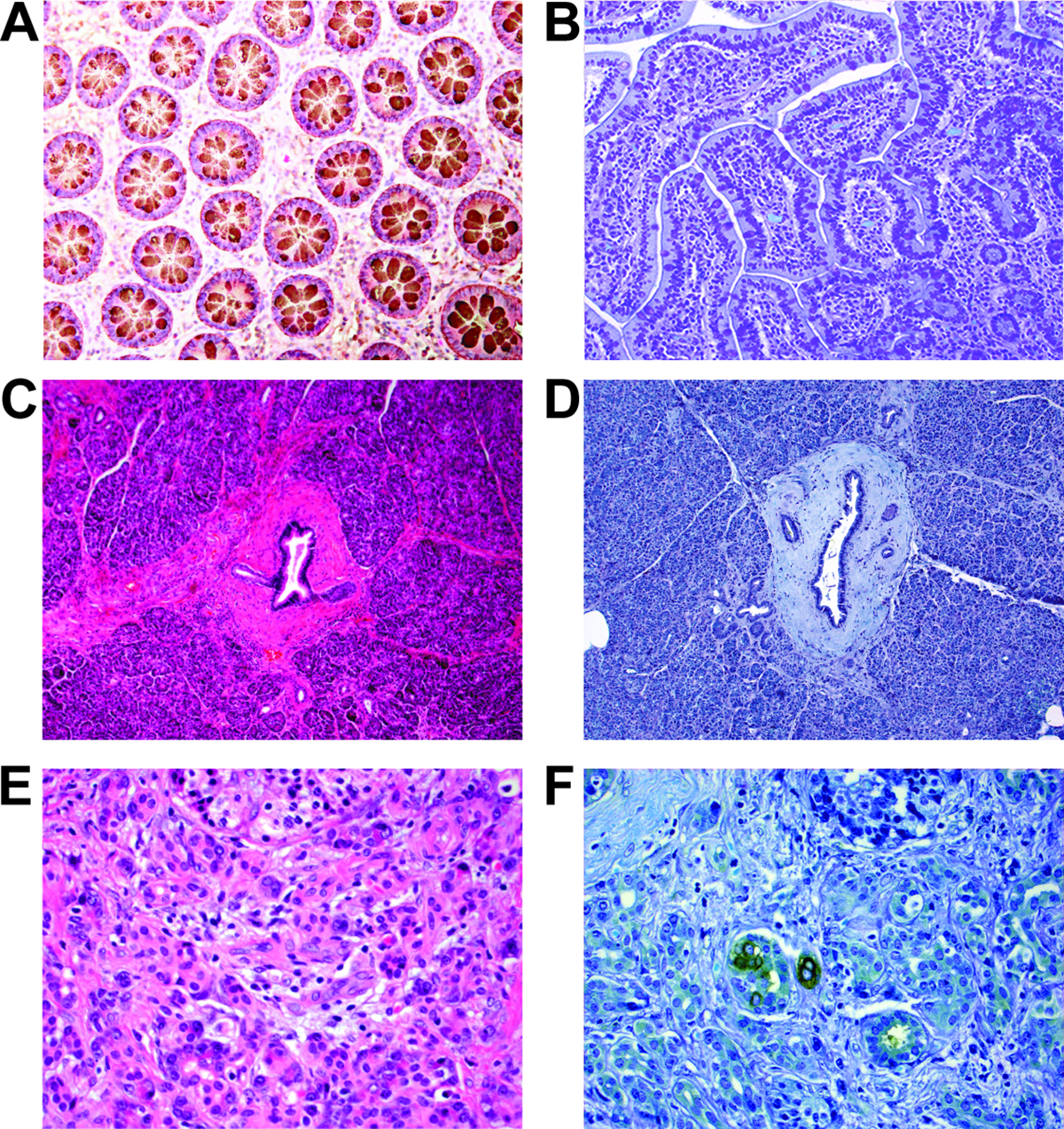
Normal colonic mucosa exhibits cytoplasmic expression of Das-1 (A), while normal small intestinal mucosa is negative for Das-1 (B). Similarly, normal pancreatic parenchyma including pancreatic ducts are non-reactive to Das-1 (C: H&E stain, D: Das-1 immunohistochemistry). Interestingly, rare small foci with acinar to ductal metaplasia in the setting of chronic pancreatitis secondary to pancreatic ductal adenocarcinoma shows strong membranous and/or cytoplasmic Das-1 expression (E: H&E stain, F: Das-1 immunohistochemistry).
2.3. Statistical Analysis
Das-1 expression in high-grade PanIN, PDAC, and Lymph Node metastases were compared against low-grade PanIN and normal pancreatic ducts using Fisher’s Exact test. Das-1 reactivity was also correlated with the other clinicopathological parameters by Fisher’s Exact test. P<0.05 was considered statistically significant.
3. RESULTS
3.1. mAb Das-1 Expression in Non-neoplastic Pancreatic Parenchyma
Non-neoplastic pancreatic ducts and pancreatic parenchyma including chronic pancreatitis were essentially non-reactive in all the samples examined (Figures 1C&D). Notably, tumor associated chronic pancreatitis and stroma are essentially negative except in areas with nests of tumor cells or immediately adjacent, which may indicate localized extravasation. Das-1 expression was observed in rare, small foci with acinar to ductal metasplasia (ADM) in chronic pancreatitis secondary to PDAC (Figures1E&F).
3.2. mAb Das-1 is Specific for High-grade PanIN Lesions and PDAC
In total, the PanIN lesions consisted of 95 low-grade PanIN (58 PanIN-1 and 37 PanIN-2) and 12 high-grade PanIN lesions. The former included 16 low-grade PanIN (10 PanIN-1 and 6 PanIN-2) lesions from pancreatic resections for mass-forming chronic pancreatitis and the latter, 4 high-grade PanIN lesions in the pancreata that did not show invasive carcinoma. Of the low-grade PanIN, no lesions were reactive to Das-1 (Figures 2A&B) (Table 2). Compared to non-neoplastic pancreatic ducts (present in 56 individual sections) and low-grade PanIN lesions, Das-1 expression amongst high-grade PanIN was significantly higher (P<0.0001) with 7/12 (58%) staining positive (Grade 1: n=5, Grade 2: n=0; Grade 3: n=2). Of note, 2 (50%) of 4 high-grade PanIN lesions identified in the pancreata without PDAC were immunoreactive to Das-1. When reactive to mAb Das-1, the lesional epithelium exhibited intense staining in cytoplasmic and/or membranous patterns (Figures 2C&D). Within samples demonstrating various PanIN grades, mAb Das-1 preferentially reacted with high-grade PanIN and was absent in low-grade lesions.
Figure 2:
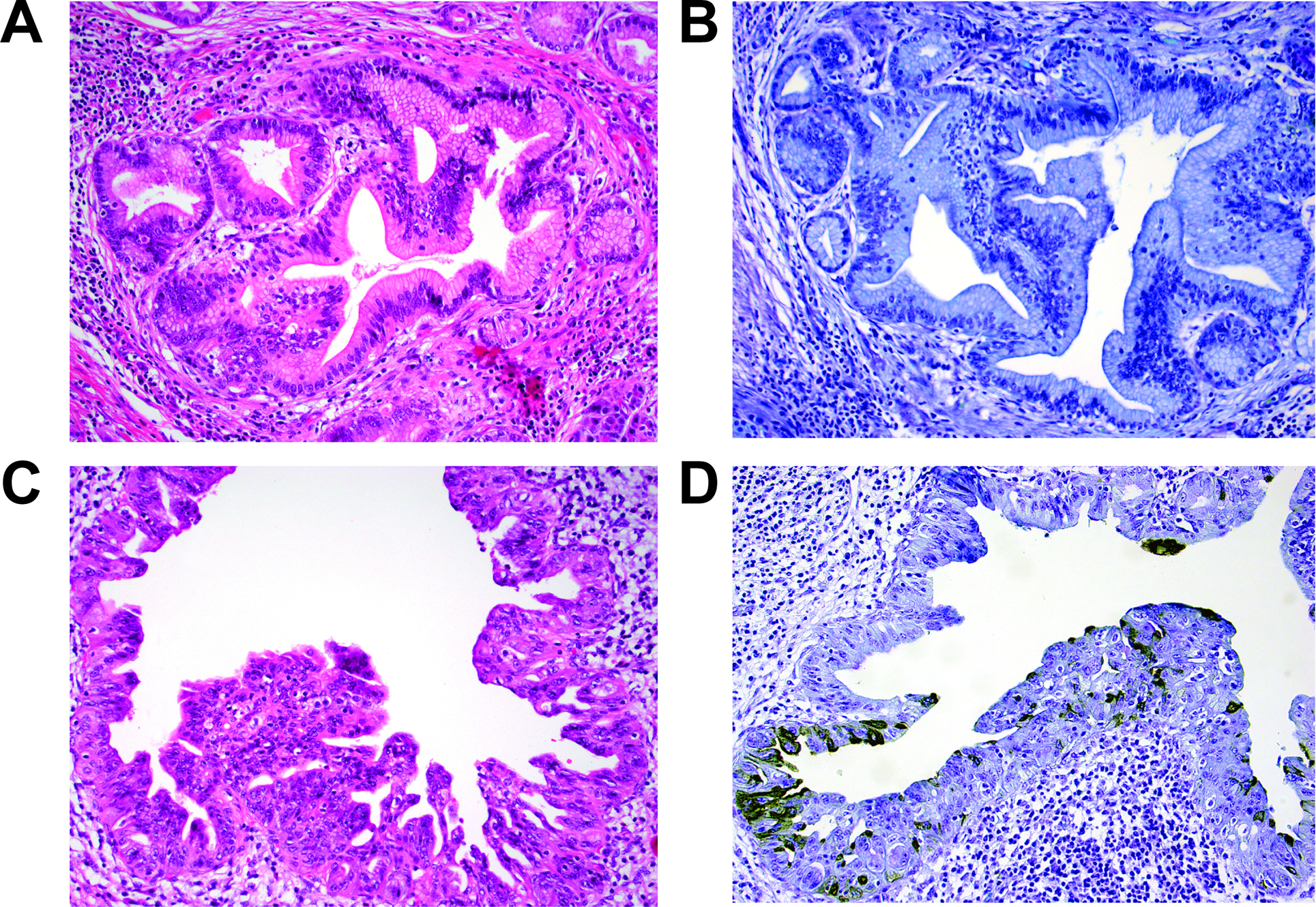
Low-grade PanIN (PanIN-1 and PanIN-2) are non-reactive to Das-1 (A: H&E stain, B: Das-1 immunohistochemistry), while cytoplasmic and/or membranous patterns of Das-1 expression is seen in the majority of high-grade PanIN (PanIN-3) lesions (C: H&E stain, D: Das-1 immunohistochemistry).
TABLE 2:
Das-1 expression in pancreatic ducts, PanIN and pancreatic adenocarcinomas
| Non-neoplastic pancreatic duct | Low-grade PanIN a | High-grade PanIN a | PDAC b | LN c | |
|---|---|---|---|---|---|
| Total | 56 | 95 | 12 | 74 | 32 |
| Das-1 + | 0 | 0 | 7 | 55 | 21 |
| 0% | 0% | 58% | 74% | 66% | |
| Grade 1(1) | 0 | 0 | 5 | 10 | 6 |
| Grade 2(2) | 0 | 0 | 0 | 14 | 2 |
| Grade 3(3) | 0 | 0 | 2 | 31 | 13 |
| p-value * | NS | NS | <0.0001 | <0.0001 | <0.0001 |
PanIN - Pancreatic Intraepithelial Neoplasia
PDAC - Pancreatic Ductal Adenocarcinoma
LN – Lymph Node Metastasis
Grade 1: Das-1 expression was seen in 5–25% of tumor cells
Grade 2: Das-1 was positive in 25–50% of tumor cells
Grade 3: Das-1 was expressed in > 50% of tumor cells.
Each category was compared to low-grade PanIN lesions and non-neoplastic pancreatic duct controls.
Performance for High-grade PanIN/PDAC: Sensitivity 72% | Specificity 100%
3.3. mAb Das-1 Expression in Pancreatic Ductal Adenocarcinoma
In total, 55/74 (74%) invasive carcinomas were considered positive (Tables 1 & 2). Das-1 staining was often diffuse (Grade 1: n=10; Grade 2: n=14; Grade 3: n=34), and mostly cytoplasmic in the invasive ducts (Figure 3), and also highlighted perineural lymphatic, and venous invasion, and cancerization of the duct (intraductal spread of carcinoma) (Figures 4A,B&C, Table 2). Overall, the sensitivity and specificity of Das-1 in segregating high-grade PanIN/PDAC from non-neoplastic ducts and low-grade PanIN lesions were 72% (95% CI: 0.62–0.81) and 100% (95% CI: 0.98–1.00), respectively, with an accuracy of 90%.
Table 1:
Clinicopathological features of patients with pancreatic ductal adenocarcinoma stratified by Das-1 expression
| Clinical or pathologic feature | Total N |
Das-1 expression | P value | |
|---|---|---|---|---|
| Negative | Positive | |||
| All cases | 74 | 19 | 55 | |
| Gender | 0.785 | |||
| Male | 28 | 8 (42%) | 20 (36%) | |
| Female | 46 | 11 (58%) | 35 (64%) | |
| Age (years) | 0.100 | |||
| Mean | 65.8 | 62.5 | 67.0 | |
| SD | 10.2 | 9.7 | 10.3 | |
| Tumor size (cm) | 0.446 | |||
| Mean | 3.1 | 2.9 | 3.1 | |
| SD | 0.9 | 0.9 | 0.9 | |
| Tumor stage | 0.671 | |||
| pT1 | 1 | 0 (0%) | 1 (1.8%) | |
| pT2 | 7 | 1 (5.3%) | 6 (11%) | |
| pT3 | 65 | 18 (95%) | 47 (85%) | |
| pT4 | 1 | 0 (0%) | 1 (1.8%) | |
| Nodal stage | 0.021 | |||
| pN0 | 16 | 8 (42%) | 8 (11%) | |
| pN1 | 58 | 11 (58%) | 47 (89%) | |
| Stage | 1.000 | |||
| IA | 1 | 0 (0%) | 1 (1.8%) | |
| IB | 1 | 0 (0%) | 1 (1.8%) | |
| IIA | 14 | 8 (42%) | 6 (11%) | |
| IIB | 57 | 11 (58%) | 46 (84%) | |
| III | 1 | 0 (0%) | 1 (1.8%) | |
| Histological grade** | ||||
| Low | 35 | 10 (53%) | 25 (45%) | 0.606 |
| High | 39 | 9 (47%) | 30 (55%) | |
| Lymphatic invasion | ||||
| (−) | 25 | 8 (42%) | 17 (31%) | 0.408 |
| (+) | 49 | 11 (58%) | 38 (69%) | |
| Vascular invasion | ||||
| (−) | 26 | 9 (47%) | 17 (31%) | 0.266 |
| (+) | 48 | 10 (53%) | 38 (69%) | |
| Perineural invasion | ||||
| (−) | 6 | 1 (5.3%) | 5 (9.1%) | 1.000 |
| (+) | 68 | 18 (95%) | 50 (91%) | |
| Resection margin status | ||||
| Negative | 26 | 11 (58%) | 15 (27%) | 0.259 |
| Positive | 48 | 8 (42%) | 40 (73%) | |
low grade: well and moderately differentiated, high grade: poorly differentiated and undifferentiated
Figure 3:
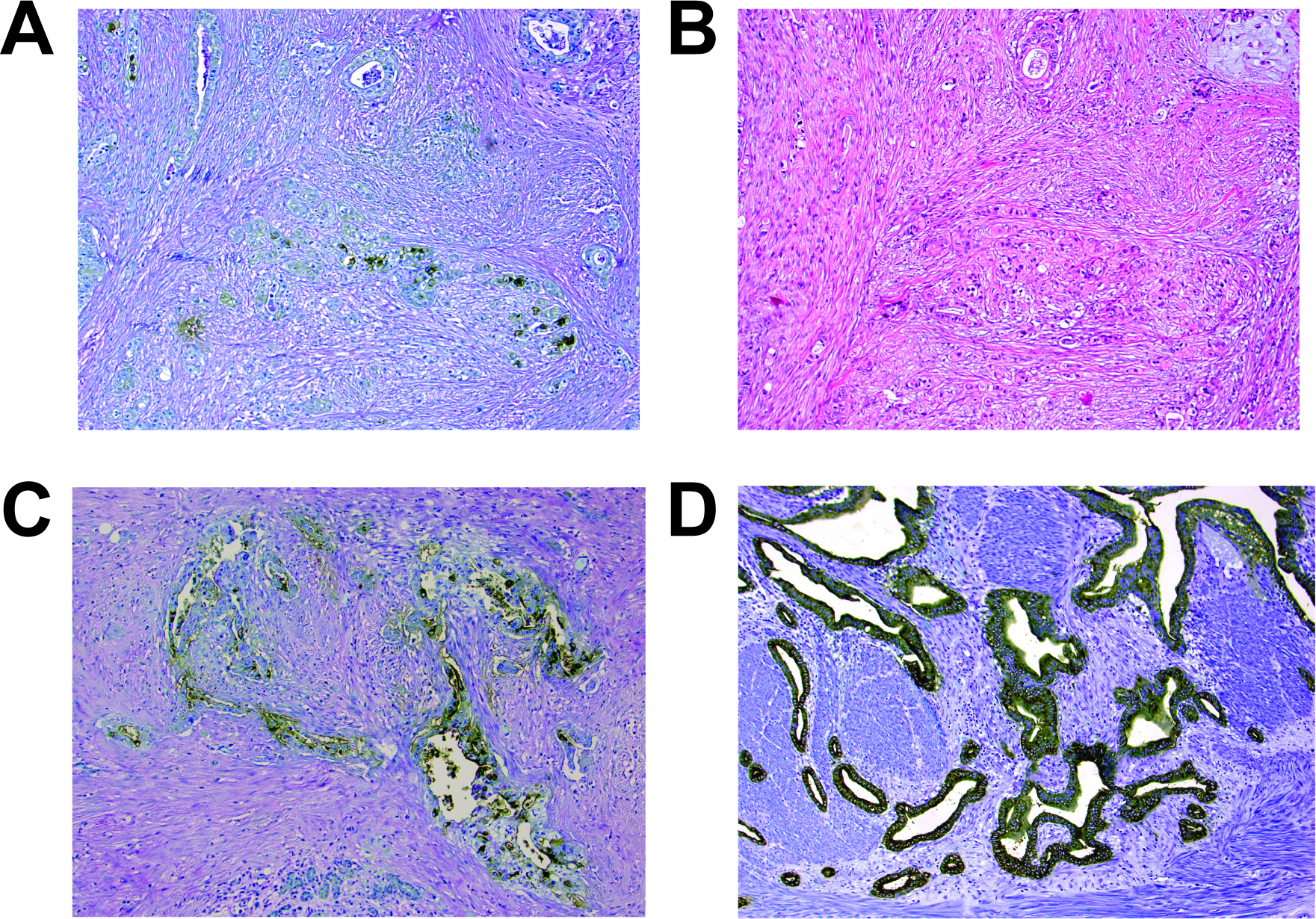
Examples of pancreatic ductal adenocarcinomas with grade 1 (A & B), grade 2 (C) and grade 3 (D) Das-1 expression (A, C & D: Das-1 immunohistochemistry, B: H&E stain).
Figure 4:
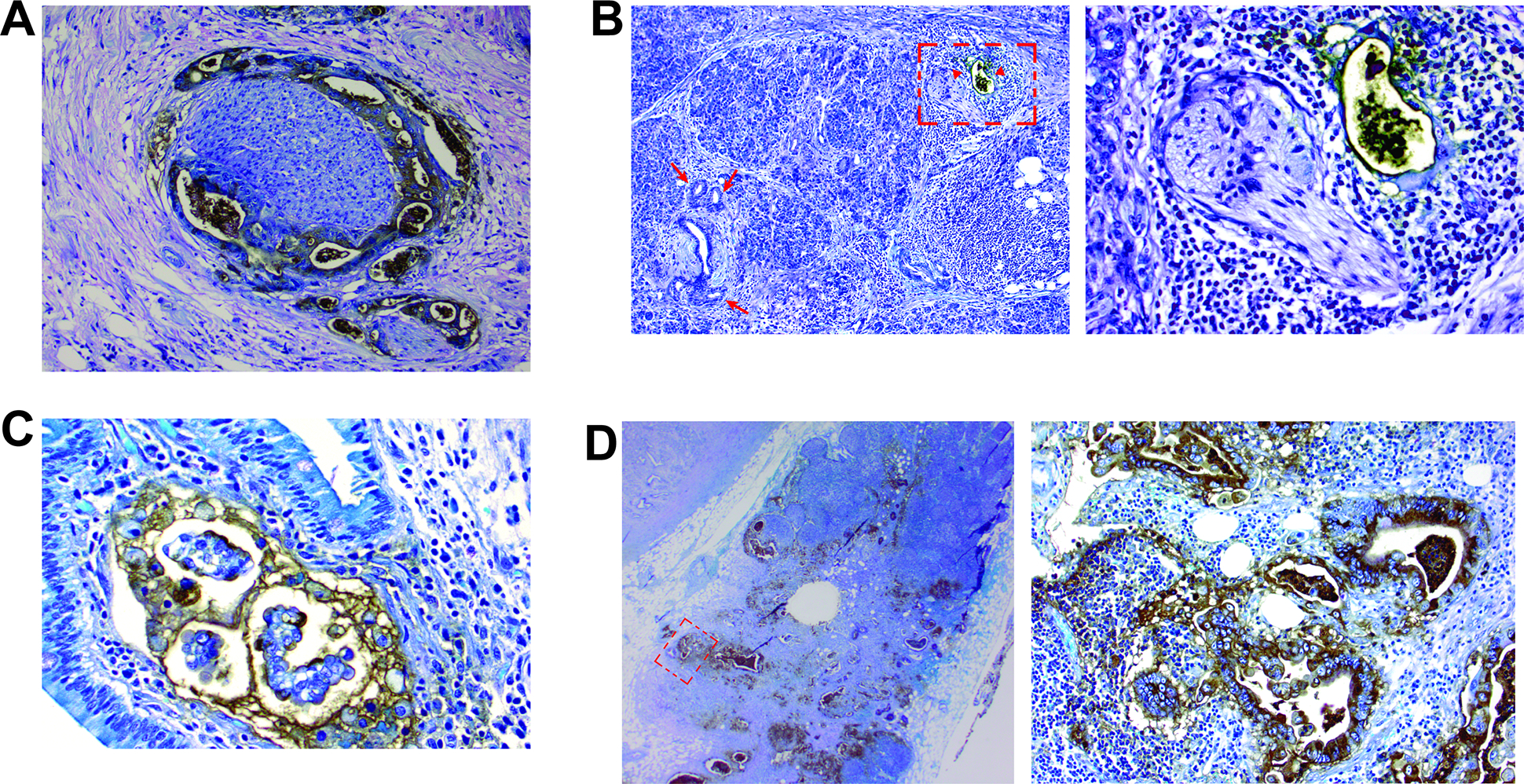
A) Das-1 staining highlighting perineural invasion. B) A malignant duct in the vicinity of neural tissue is positive for Das-1 (arrow heads; an area marked by a rectangle is magnified in the right-side image), while normal interlobular duct and low-grade PanIN lesions are non-reactive to Das-1 (arrows). C) Lymphatic invasion with Das-1 expression. D) An example of lymph node metastasis with grade 3 Das-1 expression (an area marked by a rectangle is magnified in the right-side image).
Compared to Das-1 negative tumors, those with Das-1 expression were more likely to have nodal metastasis (58% vs. 89%, p=0.021), but there was no association between Das-1 expression and other clinicopathological features including age, gender, pathologic T stage and AJCC stage, histologic grade of the tumor, lymphovascular and perineural invasion and resection margin status (Table 1). There was no difference in overall survival between the two groups as well. Two-year overall survival rate was 56% for those with negative Das-1 expression and 47% for those with positive Das-1 expression (p=0.313).
3.4. mAb Das-1 Reactivity in Nodal Metastases
mAb Das-1 was also examined in 32 lymph nodes with metastatic deposits, distant from the primary PDAC in 30 cases. Reactivity was evident in 21/32 (66%) of the lymph nodes (Grade 1: n=6, Grade 2: n=2; Grade 3: n=13) in these cases (Table 2). Staining was specific for metastatic adenocarcinoma tissue within the lymph node (Figure 4D). Among the primary tumors in these cases, 25/30 (83%) were positive for Das-1 and in no case was a lymph node reactive when a primary tumor was not. Only in 4 cases was Das-1 expression present in the primary tumor but absent in the nodal metastasis. Interestingly, in one case, a primary tumor exhibiting Das-1 expression had one lymph node that was focally positive for Das-1 and another that was negative.
4. DISCUSSION
In this study, we show that mAb Das-1is a potential novel biomarker to specifically identify high-grade PanIN and PDAC, but not non-neoplastic pancreatic ducts and low-grade PanIN lesions. Indeed, in our cohort, mAb Das-1 was able to distinguish high-grade PanIN/PDAC with 72% sensitivity, 100% specificity, and 90% accuracy. While mAb Das-1 was completely nonreactive with low-grade PanIN, expression among high-grade PanIN was significantly higher at 58% (7/12). With regards to the performance of the immunohistochemistry assay, 55% of positive samples stained at a grade 3 level (>50% of affected glands staining positive) and the vast majority (75%) of samples stained at a grade 2 level or higher (at least 25% of affected glands stained positive).
While low-grade PanIN lesions are commonly identified in benign pancreata and associated with few genetic alterations,(9, 22, 23) high-grade PanIN lesions are usually confined to the immediate vicinity of an infiltrating carcinoma with a genetic profile similar to that of PDAC.(24–27) In addition, we have recently demonstrated in a large autopsy study of 173 patients who did not die with any evidence of PDAC or IPMN, a frequency of PanIN-1 of 77% and PanIN 2 of 28% in comparison to PanIN-3 of 4%.(9) Given the ubiquity of these asymptomatic, low grade PanIN lesions, the ability to make the distinction between PanIN-2 and PanIN-3 lesions is clinically crucial. However, as with many features in surgical pathology that one attempts to stratify into ordinal categories, the features that separate PanIN-3 from PanIN-2 lie on a continuum, resulting in ambiguity in delineating PanIN-2 and 3 lesions.(4) Indeed, even expert pancreatic pathologists disagree in diagnosing high-grade dysplasia versus lower-grade dysplasia in 16% of cases.(8) Thus the development of biomarkers that can specifically delineate high-grade PanIN, but not low-grade PanIN may help to confidently grade lesions and optimally manage early PDAC.
Mass forming chronic pancreatitis including autoimmune pancreatitis can clinically mimic PDAC. Initial assessment of pancreatic masses frequently involves endoscopic ultrasound-guided, trans-duodenal/gastric fine needle aspiration. As such, pathologic evaluation of cytologic materials is often contaminated with gastric or duodenal glandular epithelium. Further, chronic pancreatitis frequently exhibits low-grade PanIN lesions which may be difficult to distinguish from well-differentiated PDAC. As mAb Das-1 is non-reactive in normal gastric, duodenal, and pancreatic epithelium, as well as in pancreatitis and low-grade PanIN lesions, its reactivity may be helpful in these initial evaluations. A few immunohistochemistry markers including SMAD4 (loss of expression), p53 (aberrant expression) and IPM3 have been evaluated for the differentiation of PDAC and/or high-grade PanIN from non-neoplastic pancreatic tissue and low-grade PanIN.(28, 29) Of those, IPM3 is expressed in 96% of PDAC, but its expression in high-grade PanIN lesions appears to be limited (<60%).(28) Thus, a panel of immunohistochemical stains including mAb Das-1 and IPM3 for example, may allow for more confident discrimination between well-differentiated PDAC and/or high-grade PanIN from gastric or duodenal epithelium and/or low-grade PanIN lesions.
Evidence in mouse models has suggested that ADM is a likely antecedent to the development of PanIN and subsequently PDAC.(30) ADM also may be a site of increased epithelial to mesenchymal transformation (EMT) in models of pancreatic cancer.(31) In our study, we found a few instances of ADM with strong membranous and cytoplasmic expression of mAb Das-1. Further evaluation in lineage labeled models of EMT may elucidate at what stage mAb Das-1 expression becomes apparent as well as whether gastric and/or intestinal programming is activated concurrently.
Previous studies of mAb Das-1 reactivity in fetal tissues by Badve et al. demonstrated expression of the Das-1 antigen in organs arising from the primitive gut, ureteric bud, and several other sites of ectodermal, mesodermal, and endodermal origin.(32) Subsequently it has been shown that while Das-1 is expressed in the fetal esophagus, stomach, and small intestine, it is lost in the respective adult organs, and reappears in precancerous and cancerous conditions like Barrett’s Esophagus/esophageal adenocarcinoma,(16, 33) incomplete-type gastric intestinal metaplasia/gastric adenocarcinoma,(17, 19) and small intestinal adenomas/small intestinal adenocarcinoma.(34) These findings suggest that mAb Das-1 may be a novel oncofetal marker. While expression of Das-1 is present in fetal pancreatic tissue and absent in adult pancreata,(32) we now demonstrate that expression returns in high-grade PanIN and PDAC. Furthermore, the reactivity remains upon metastasis into lymph nodes in the vast majority of PDACs.
We have previously described the expression of mAb Das-1 in high-grade IPMN lesions including those of the gastric epithelial type, as well as associated invasive carcinoma.(20, 21) It is not certain why this colonic specific marker would be expressed in dysplastic lesions of the pancreas and PDAC. As the antigen of the Das-1 antibody, CEP, has not been fully characterized and its function not elucidated, further research is ongoing to assess the functional significance of the expression of this marker in these preinvasive and invasive conditions of the pancreas.
There are several limitations of our study. First, as our surgical specimens were entirely from a single tertiary referral center, there may be a referral or treatment access bias. In addition, our PDAC cases were chosen non-randomly on the basis of availability of tissue samples from sequentially resected PDACs. This selection should not bias our sample as all cases by definition contained a combination of pre-neoplastic and neoplastic lesions and analysis was completed on a per-lesional basis. Second, the numbers of PanIN lesions in individual histologic categories, particularly high-grade PanIN, are small. However, the differences in reactivity among low-grade PanIN and high-grade PanIN were still highly statistically significant (p<0.001). Third, additional biomarkers, and clinicopathologic parameters like biliary obstruction/jaundice and plasma Ca19–9 were not available to correlate to mAb Das-1 expression. Nevertheless, we have demonstrated that expression of mAb Das-1 segregates high-grade PanIN/PDAC from low-grade PanIN by immunohistochemical analysis.
In conclusion, mAb Das-1 is a sensitive and highly specific biomarker for high-grade PanIN and PDAC in the pancreas. The inclusion of the Das-1 marker into the analysis of tissue may aid in the pre-operative diagnosis and/or risk stratification of patients with a pancreatic mass lesion.
ACKNOWLEDGEMENTS
We would like to thank Kelly Walton for her technical advice for immunohistochemical staining.
FUNDING:
This work was supported by the American Society for Gastrointestinal Endoscopy (KKD), National Pancreas Foundation (KMD), National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) T32DK007130–41 and Digestive Diseases Research Core Centers Pilot & Feasibility Grant as part of P30 DK052574 (JWB). Development of mAb Das-1 was supported in part by research grants NIDDK, R01 DK47673 and R01 DK63618 to KMD.
Footnotes
DECLARATION OF INTEREST
A patent for the use of mAb Das-1 in the detection of precancerous lesions of the esophagus has been awarded (KMD) and a patent for its possible use in the detection of pancreatic cancer is currently pending (KKD, MMK, KMD). These patents have not been licensed and these authors hold no commercial interests at this time.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer research 2014; 74, 2913–2921. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011. CA: A Cancer Journal for Clinicians 2011; 61, 212–236. [DOI] [PubMed] [Google Scholar]
- 3.Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Cancer Res 2000; 6, 2969–2972. [PubMed] [Google Scholar]
- 4.Hruban RH, Takaori K, Klimstra DS, Adsay NV, Albores-Saavedra J, Biankin AV, Biankin SA, Compton C, Fukushima N, Furukawa T, Goggins M, Kato Y, Kloppel G, Longnecker DS, Luttges J, Maitra A, Offerhaus GJ, Shimizu M, Yonezawa S. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol 2004; 28, 977–987. [DOI] [PubMed] [Google Scholar]
- 5.Basturk O, Hong SM, Wood LD, Adsay NV, Albores-Saavedra J, Biankin AV, Brosens LA, Fukushima N, Goggins M, Hruban RH, Kato Y, Klimstra DS, Kloppel G, Krasinskas A, Longnecker DS, Matthaei H, Offerhaus GJ, Shimizu M, Takaori K, Terris B, Yachida S, Esposito I, Furukawa T, Baltimore Consensus M. A Revised Classification System and Recommendations From the Baltimore Consensus Meeting for Neoplastic Precursor Lesions in the Pancreas. The American journal of surgical pathology 2015; 39, 1730–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rebours V, Lévy P, Mosnier JF, Scoazec JY, Soubeyrand MS, Fléjou JF, Turlin B, Hammel P, Ruszniewski P, Bedossa P, Couvelard A. Pathology analysis reveals that dysplastic pancreatic ductal lesions are frequent in patients with hereditary pancreatitis. Clin Gastroenterol Hepatol 2010; 8, 206–212. [DOI] [PubMed] [Google Scholar]
- 7.Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, Kern SE, Klimstra DS, Kloppel G, Longnecker DS, Luttges J, Offerhaus GJ. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol 2001; 25, 579–586. [DOI] [PubMed] [Google Scholar]
- 8.Longnecker DS, Adsay NV, Fernandez-del Castillo C, Hruban RH, Kasugai T, Klimstra DS, Kloppel G, Luttges J, Memoli VA, Tosteson TD, Yanagisawa A, Wilentz R, Zamboni G. Histopathological diagnosis of pancreatic intraepithelial neoplasia and intraductal papillary-mucinous neoplasms: interobserver agreement. Pancreas 2005; 31, 344–349. [DOI] [PubMed] [Google Scholar]
- 9.Matsuda Y, Furukawa T, Yachida S, Nishimura M, Seki A, Nonaka K, Aida J, Takubo K, Ishiwata T, Kimura W, Arai T, Mino-Kenudson M. The Prevalence and Clinicopathological Characteristics of High-Grade Pancreatic Intraepithelial Neoplasia: Autopsy Study Evaluating the Entire Pancreatic Parenchyma. Pancreas 2017; 46, 658–664. [DOI] [PubMed] [Google Scholar]
- 10.Ansari D, Rosendahl A, Elebro J, Andersson R. Systematic review of immunohistochemical biomarkers to identify prognostic subgroups of patients with pancreatic cancer. British Journal of Surgery 2011; 98, 1041–1055. [DOI] [PubMed] [Google Scholar]
- 11.Kanda M, Matthaei H, Wu J, Hong SM, Yu J, Borges M, Hruban RH, Maitra A, Kinzler K, Vogelstein B, Goggins M. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology 2012; 142, 730–733 e739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luttges J, Diederichs A, Menke MA, Vogel I, Kremer B, Kloppel G. Ductal lesions in patients with chronic pancreatitis show K-ras mutations in a frequency similar to that in the normal pancreas and lack nuclear immunoreactivity for p53. Cancer 2000; 88, 2495–2504. [DOI] [PubMed] [Google Scholar]
- 13.Berger DH, Chang H, Wood M, Huang L, Heath CW, Lehman T, Ruggeri BA. Mutational activation of K-ras in nonneoplastic exocrine pancreatic lesions in relation to cigarette smoking status. Cancer 1999; 85, 326–332. [DOI] [PubMed] [Google Scholar]
- 14.Das KM, Sakamaki S, Vecchi M, Diamond B. The production and characterization of monoclonal antibodies to a human colonic antigen associated with ulcerative colitis: cellular localization of the antigen by using the monoclonal antibody. J Immunol 1987; 139, 77–84. [PubMed] [Google Scholar]
- 15.Halstensen TS, Das KM, Brandtzaeg P. Epithelial deposits of immunoglobulin G1 and activated complement colocalise with the M(r) 40 kD putative autoantigen in ulcerative colitis. Gut 1993; 34, 650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das KM, Prasad I, Garla S, Amenta PS. Detection of a shared colon epithelial epitope on Barrett epithelium by a novel monoclonal antibody. Ann Intern Med 1994; 120, 753–756. [DOI] [PubMed] [Google Scholar]
- 17.Mirza ZK, Das KK, Slate J, Mapitigama RN, Amenta PS, Griffel LH, Ramsundar L, Watari J, Yokota K, Tanabe H, Sato T, Kohgo Y, Das KM. Gastric intestinal metaplasia as detected by a monoclonal antibody is highly associated with gastric adenocarcinoma. Gut 2003; 52, 807–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piazuelo MB, Haque S, Delgado A, Du JX, Rodriguez F, Correa P. Phenotypic differences between esophageal and gastric intestinal metaplasia. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 2004; 17, 62–74. [DOI] [PubMed] [Google Scholar]
- 19.Watari J, Moriichi K, Tanabe H, Kashima S, Nomura Y, Fujiya M, Tomita T, Oshima T, Fukui H, Miwa H, Das KM, Kohgo Y. Biomarkers predicting development of metachronous gastric cancer after endoscopic resection: an analysis of molecular pathology of Helicobacter pylori eradication. Int J Cancer 2012; 130, 2349–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Das KK, Xiao H, Geng X, Fernandez-Del-Castillo C, Morales-Oyarvide V, Daglilar E, Forcione DG, Bounds BC, Brugge WR, Pitman MB, Mino-Kenudson M, Das KM. mAb Das-1 is specific for high-risk and malignant intraductal papillary mucinous neoplasm (IPMN). Gut 2014; 63, 1626–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Das KK, Geng X, Brown JW, Morales-Oyarvide V, Huynh T, Pergolini I, Pitman MB, Ferrone C, Al Efishat M, Haviland D, Thompson E, Wolfgang C, Lennon AM, Allen P, Lillemoe KD, Fields RC, Hawkins WG, Liu J, Castillo CF, Das KM, Mino-Kenudson M. Cross Validation of the Monoclonal Antibody Das-1 in Identification of High-Risk Mucinous Pancreatic Cystic Lesions. Gastroenterology 2019; 157, 720–730.e722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luttges J, Galehdari H, Brocker V, Schwarte-Waldhoff I, Henne-Bruns D, Kloppel G, Schmiegel W, Hahn SA. Allelic loss is often the first hit in the biallelic inactivation of the p53 and DPC4 genes during pancreatic carcinogenesis. Am J Pathol 2001; 158, 1677–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andea A, Sarkar F, Adsay VN. Clinicopathological correlates of pancreatic intraepithelial neoplasia: a comparative analysis of 82 cases with and 152 cases without pancreatic ductal adenocarcinoma. Mod Pathol 2003; 16, 996–1006. [DOI] [PubMed] [Google Scholar]
- 24.Kozuka S, Sassa R, Taki T, Masamoto K, Nagasawa S, Saga S, Hasegawa K, Takeuchi M. Relation of pancreatic duct hyperplasia to carcinoma. Cancer 1979; 43, 1418–1428. [DOI] [PubMed] [Google Scholar]
- 25.Hisa T, Suda K, Nobukawa B, Ohkubo H, Shiozawa S, Ishigame H, Yamao K, Yatabe Y. Distribution of intraductal lesions in small invasive ductal carcinoma of the pancreas. Pancreatology 2007; 7, 341–346. [DOI] [PubMed] [Google Scholar]
- 26.Wilentz RE, Iacobuzio-Donahue CA, Argani P, McCarthy DM, Parsons JL, Yeo CJ, Kern SE, Hruban RH. Loss of expression of Dpc4 in pancreatic intraepithelial neoplasia: evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res 2000; 60, 2002–2006. [PubMed] [Google Scholar]
- 27.Yamano M, Fujii H, Takagaki T, Kadowaki N, Watanabe H, Shirai T. Genetic progression and divergence in pancreatic carcinoma. Am J Pathol 2000; 156, 2123–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yantiss RK, Woda BA, Fanger GR, Kalos M, Whalen GF, Tada H, Andersen DK, Rock KL, Dresser K. KOC (K homology domain containing protein overexpressed in cancer): a novel molecular marker that distinguishes between benign and malignant lesions of the pancreas. The American journal of surgical pathology 2005; 29, 188–195. [DOI] [PubMed] [Google Scholar]
- 29.Maitra A, Adsay NV, Argani P, Iacobuzio-Donahue C, De Marzo A, Cameron JL, Yeo CJ, Hruban RH. Multicomponent analysis of the pancreatic adenocarcinoma progression model using a pancreatic intraepithelial neoplasia tissue microarray. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 2003; 16, 902–912. [DOI] [PubMed] [Google Scholar]
- 30.Reichert M, Rustgi AK. Pancreatic ductal cells in development, regeneration, and neoplasia. J Clin Invest 2011; 121, 4572–4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH, Leach SD, Stanger BZ. EMT and dissemination precede pancreatic tumor formation. Cell 2012; 148, 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Badve S, Logdberg L, Sokhi R, Sigal SH, Botros N, Chae S, Das KM, Gupta S. An antigen reacting with das-1 monoclonal antibody is ontogenically regulated in diverse organs including liver and indicates sharing of developmental mechanisms among cell lineages. Pathobiology 2000; 68, 76–86. [DOI] [PubMed] [Google Scholar]
- 33.Griffel LH, Amenta PS, Das KM. Use of a novel monoclonal antibody in diagnosis of Barrett’s esophagus. Dig Dis Sci 2000; 45, 40–48. [DOI] [PubMed] [Google Scholar]
- 34.Onuma EK, Amenta PS, Jukkola AF, Mohan V, Borra S, Das KM. A phenotypic change of small intestinal epithelium to colonocytes in small intestinal adenomas and adenocarcinomas. Am J Gastroenterol 2001; 96, 2480–2485. [DOI] [PubMed] [Google Scholar]


