Abstract
Extramammary Paget’s disease (EMPD) is a rare adnexal neoplasm commonly seen in the genital areas among the senior population. The prognosis of advanced EMPD is not favorable; thus, the development of potential treatments has long been sought. Cyclin‐dependent kinase (CDK) 4/6 inhibitors such as abemaciclib and palbociclib have been proven effective against metastatic breast cancer; however, no studies have addressed CDK4/6 inhibitors as an EMPD treatment. We herein examine the efficacy of CDK4/6 inhibitors against an EMPD patient‐derived xenograft (PDX) model. Abemaciclib (50 mg/kg/day) or palbociclib (120 mg/kg/day) was given orally to tumor‐bearing NOD/Scid mice over a 3‐week period. We also investigated the protein expression levels of CDK4/6 and cyclin D1 through immunohistochemical staining using EMPD clinical samples. Treatment with abemaciclib or palbociclib as a single agent was found to significantly suppress tumor growth in EMPD‐PDX. The Ki‐67‐positive ratio of the treated EMPD‐PDX tumors was significantly lower than that of the nontreated tumors. Clinically, the expression levels of CDK4 and cyclin D1 were significantly higher in the EMPD tumor cells than in the normal epidermis. Our results suggest that CDK4/6 inhibitors could be novel and potent therapeutics for the treatment of EMPD.
Keywords: cancer, cyclin, cyclin‐dependent kinase, extramammary Paget’s disease, patient‐derived xenograft
The therapeutic efficacy of cyclin‐dependent kinase 4/6 inhibitors was assessed using extramammary Paget’s disease patient‐derived xenografts (EMPD‐PDX).Treatment with abemaciclib or palbociclib as a single agent was found to significantly suppress tumor growth in EMPD‐PDX.
![]()
Abbreviations
- CDK
cyclin‐dependent kinase
- EMPD
extramammary Paget’s disease
- HER2
human epidermal growth factor receptor 2
- PDX
patient‐derived xenograft
1. INTRODUCTION
Extramammary Paget’s disease (EMPD) is a rare cutaneous malignancy that occurs within the genital epithelium. 1 The incidence and mortality have been increasing over the last several decades. 2 A retrospective study reported that the 5‐year overall survival rate for EMPD patients with distant metastasis was only 7%. 3 Some cytotoxic agents, such as docetaxel, cisplatin, and 5‐fluorouracil, have been used to treat advanced EMPD; however, their efficacies are limited. 4 , 5 No standard therapies for advanced EMPD have been established; thus, efforts are needed to develop potential treatment options.
The significance of PDX has been proven in the development of treatments for some cancers. 6 Patient‐derived xenograft models can be established with the transplantation of cancerous cells or tissues from patients’ tumors into immunodeficient mice. Patient‐derived xenograft models can reproduce the conditions of human cancers better than conventional mouse models using cancer cell lines. In 2020, we established an EMPD‐PDX model (EMPD‐PDX‐H1) harboring a pathogenic ERBB2 S310F mutation for which anti‐HER2 therapies are effective. 7 To the best of our knowledge, this is the only EMPD‐PDX model that is currently available in the world.
Cyclin‐dependent kinases, a family of serine‐threonine kinases, play crucial roles in the cell cycle, neural development, and spermatogenesis. 8 Cyclin‐dependent kinase 4, and the closely related CDK6, are important in mammalian cell proliferation, where they help to lead the progression of cells into the S phase. 9 , 10 Inhibitors of CDK4/6, such as abemaciclib and palbociclib, have been proven effective as therapeutics against breast cancers. 11 These inhibitors are currently available for hormone receptor‐positive, HER2‐negative metastatic breast cancer treatment. 11 Whole‐exome sequencing revealed that some EMPD tumors share common genetic alterations with breast cancers 12 , 13 , 14 , 15 ; thus, we hypothesize that these drugs could be effective against EMPD tumor cells. No clinical or experimental studies have investigated CDK4/6 inhibitors against EMPD. We herein examine the efficacy of CDK4/6 inhibitors against an EMPD‐PDX model, and we investigate the protein expression of CDK4/6 and cyclin D1 using EMPD clinical samples.
2. MATERIALS AND METHODS
2.1. Reagents and Abs
The following items were purchased from the indicated sources: Abs against CDK4 (11026‐1‐AP; Proteintech), CDK6 (ab124821; Abcam), and cyclin D1 (SP1; Invitrogen). Palbociclib was purchased from Sigma (PZ0383), and abemaciclib (LY2835219) was purchased from Adooq BioScience (A12989). These drugs were dissolved in DMSO (Sigma).
2.2. Patient‐derived xenografts from EMPD
We established PDXs from EMPD using Matrigel (BD Biosciences; EMPD‐PDX‐H1). 7 Animal use procedures were approved by the institutional committee of Hokkaido University (approval nos. 19‐0015 and 19‐0093). NOD/Scid mice were purchased from Clea Japan. All animals used for this study were maintained under pathogen‐free conditions. The tumor‐transplanted NOD/Scid mice were observed twice a week. The tumors were measured once a week by caliper. Tumor volume was calculated according to the following formula: (long axis × short axis2)/2. 8 Once the tumor volume reached 500–1000 mm3, the EMPD‐PDX‐H1 tumors were transplanted into the next generation of NOD/Scid mice. Treatment experiments in the present study were undertaken using the fourth to sixth generations. Targeted gene mutation analysis using a comprehensive cancer panel (Qiagen) revealed neither apparent mutations nor copy number alterations in the CDK4 gene of the EMPD‐PDX. 7
2.3. Treatment experiments using CDK4/6 inhibitors
Tumor growth curves for all of the EMPD‐PDX‐H1 tumors were generated by using the kinetic measurement of tumor volume. The tumor volume range of 50–100 mm3 in the tumor‐bearing NOD/Scid mice was randomized, and treatment experiments were started. All treatment experiments were carried out with a minimum of three mice per condition. Control mice were given 100 μL PBS once a day orally (n = 3). In the CDK4/6‐targeted treatments, abemaciclib (50 mg/kg/day) or palbociclib (120 mg/kg/day) was given orally once a day for 3 weeks in accordance with previous studies (Figure 1). 16 , 17
FIGURE 1.
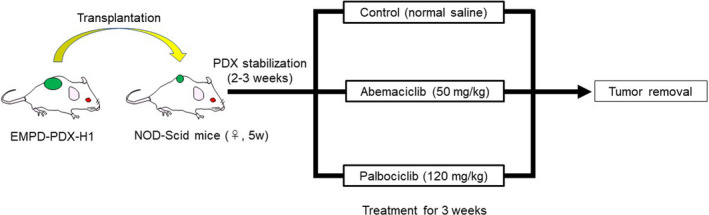
Schematic of treatment experiments with cyclin‐dependent kinase (CDK)4/6 inhibitors using extramammary Paget’s disease patient‐derived xenograft (EMPD‐PDX) model mice. Tumor‐bearing NOD/Scid mice were randomized. In the CDK4/6‐targeted treatments, abemaciclib (50 mg/kg/day) or palbociclib (120 mg/kg/day) was given orally once a day (n = 3)
2.4. Histopathological analyses
Immunohistochemical analysis was carried out on 4‐μm‐thick formalin‐fixed, paraffin‐embedded sections. 18 Immunostaining was evaluated by the same observer; the total immunostaining score for CDK4 and cyclin D1 was calculated as the sum of proportion scores (0, absent; 1, 0%–25%; 2, 25%–50%; 3, 50%–75%; and 4, 75%–100%) and intensity scores (0, absent; 1, faint; 2, moderate; and 3, strong) in accordance with our previous study. 19 The proportion of immunoreactive cells was determined by counting 100 cells in three randomly chosen fields.
2.5. Patient selection
Sixty‐one skin samples from EMPD patients were immunohistochemically assessed. All of the patients had been treated at the Department of Dermatology, Hokkaido University Hospital. The EMPD samples were obtained from patients whose ages ranged from 53 to 94 years (average, 75.0 years, male : female ratio, 36:25). This study was approved by the institutional review board of Hokkaido University Hospital (017‐0392). Initial clinical stages and observed time were retrieved from clinical data. The clinical information is summarized in Table S1. The clinical stages of EMPD were assessed in accordance with our previous study. 3 , 19
2.6. Statistical analysis
Quantitative data are shown as mean ± SD. All statistical analyses were calculated using Excel 2016 (Microsoft Corporation). To evaluate the statistical significance of the treatment experiments, Student’s t tests were used to compare tumor volumes between the treatment groups and the control group. At least three independent experiments were carried out for statistical comparison. We used the Steel‐Dwass test to assess pairwise comparisons among groups (immunohistochemistry). All analyses were carried out with a P < .05 level of significance unless otherwise indicated. The correlation between CDK4/cyclin D1 expression levels and clinical information was assessed using Spearman’s correlation coefficient by rank test, as in our previous study. 19
3. RESULTS
3.1. Cyclin‐dependent kinase 4/6 inhibitors suppress tumor growth in EMPD‐PDX
First, we investigated the protein expression of CDK4 and CDK6 in EMPD‐PDX using anti‐CDK4 and CDK6 Abs. The tumor cells in EMPD‐PDX were positive for CDK4 but negative for CDK6, which is consistent with the original metastatic tumor (Figure 2). Based on these results, we undertook therapeutic intervention using anti‐CDK4/6 inhibitors to investigate whether EMPD‐PDX‐H1 responds to such therapies. The CDK4/6 inhibitors, abemaciclib and palbociclib, were found to suppress tumor progression (Figure 3). We assessed the extracted tumors by H&E and Ki‐67 staining. In the Ki‐67 staining, the positive ratio was significantly lower for the treated EMPD‐PDX‐H1 tumors than for the nontreated tumors (Figure S1). There were no noticeable side‐effects, including fatigue, diarrhea, or body weight loss, during the observation period. These results suggest that CDK4/6 inhibitors arrest the cell cycle, leading to the suppression of tumor growth.
FIGURE 2.
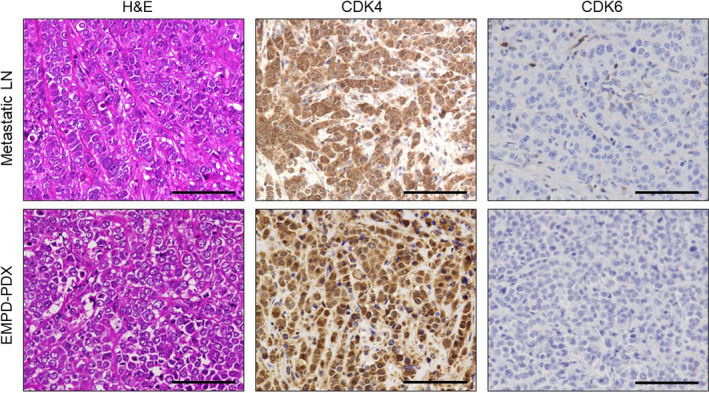
Histopathological and immunochemical manifestations in extramammary Paget’s disease patient‐derived xenograft (EMPD‐PDX) and the original metastatic tumor (lymph node [LN]). Hematoxylin and eosin staining and immunohistochemistry of cyclin‐dependent kinase (CDK)4 and CDK6 were carried out. Scale bars, 100 µm
FIGURE 3.
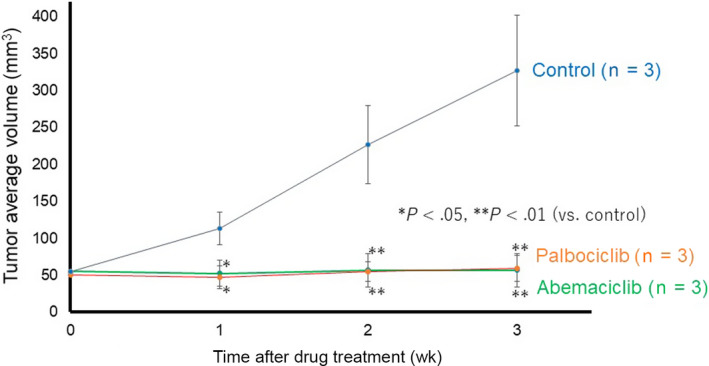
Cyclin‐dependent kinase 4/6 inhibitors suppress tumor growth in extramammary Paget’s disease patient‐derived xenograft. Tumor‐bearing NOD/Scid mice were randomized into no therapy (control, black line), abemaciclib (green line), and palbociclib (orange line). Tumor volumes were calculated according to the formula: (long axis × short axis2)/2. The results are presented as means, with error bars representing the SD from the mean. *P < .05, **P < .01 vs control
3.2. Expression levels of CDK4 and cyclin D1 are high in EMPD
The results of the therapeutic experiments using EMPD‐PDX prompted us to examine the CDK4 expression in clinical samples of EMPD. Immunohistochemically, the expression levels of CDK4 in EMPD tumor cells were higher than in normal epidermal keratinocytes or adnexal epithelium (hair follicles and sweat glands; Figure 4, *P < .01). The expression levels of CDK4 in lymph node metastatic tumor cells were also higher than in normal epidermis. These results are consistent with the previous study by Urata et al. 20 Next, we assessed the expression levels of cyclin D1 in EMPD tumors. The EMPD primary tumor cells showed higher cyclin D1 expression levels than normal epidermis (Figure S2). To examine the clinical significance of high expression levels of CDK4 and cyclin D1 in EMPD, we assessed the correlations between immunostaining results and clinical information. No correlation was observed between CDK4/cyclin D1 expression and clinical stage in EMPD (data not shown).
FIGURE 4.
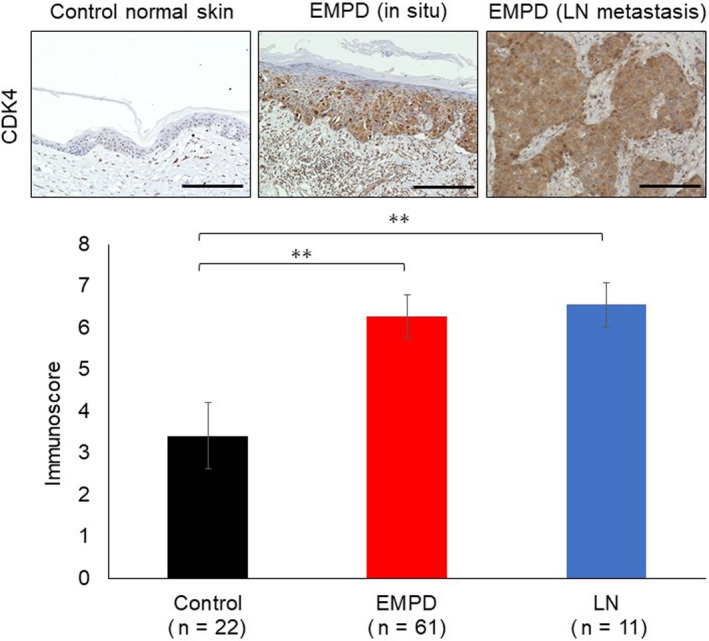
Cyclin‐dependent kinase 4 (CDK4) expression is elevated in extramammary Paget’s disease (EMPD). Upper panels: CDK4 immunostaining was carried out using specimens of normal skin (n = 22), primary tumor (n = 61), and lymph node (LN) metastasis (n = 11). Panels show representative images. Scale bars, 100 µm. Lower panel: The bar graph shows the CDK4 immunoscore. **P < .01
4. DISCUSSION
Cyclin‐dependent kinase 4/6 inhibitors are powerful therapeutics for cancer treatment, especially breast cancer. In preclinical models, CDK4/6 inhibitors have shown positive results against many kinds of tumors, including colon cancer, glioblastomas, prostate carcinomas, sarcomas, pancreatic adenocarcinomas, melanomas, and non‐small‐cell lung cancer. 11 Based on the preclinical evidence, clinical trials using CDK4/6 inhibitors (palbociclib and abemaciclib) as single agents have been undertaken as a treatment for solid tumors, including sarcomas, thymic epithelial tumors, gastrointestinal stromal tumors, brain tumors, teratomas, and head and neck squamous cell carcinomas. 11 In the dermatologic field, clinical investigations of the concurrent inhibition of MEK and CDK4/6 have been carried out using data from patients with NRAS‐mutant melanoma. 21 It is our hope that in several years the results of ongoing clinical trials will provide novel treatment options for many kinds of solid tumors.
As the efficacy of CDK4/6 inhibition is limited by resistance mechanisms, combined therapeutic strategies have been studied. Through in vitro and in vivo investigations, some of the molecular mechanisms associated with resistance to CDK4/6 inhibition have been elucidated. 22 In breast cancer, the HER2 pathway has been thought to be a resistance mechanism, as HER2 activation correlates with resistance to CDK4/6 inhibitors. 22 Additionally, activation of the CDK4 pathway contributes to resistance to HER2‐directed therapies. 23 It has been reported that CDK4/6 inhibitors and anti‐HER2 therapies have synergistic effects on model mice with breast cancer. 24 Furthermore, recent clinical studies found the combination therapy of CDK4/6 inhibitors and trastuzumab had promising results for the treatment of HER2‐positive breast cancers. 24 , 25 The expression levels of CDK4 and cyclin D1 are high in EMPD (Figure 4); furthermore, pathogenic ERBB2 mutations and/or higher expression levels of HER2 protein are detected in some EMPD cases. In light of the above, not only could CDK4/6‐targeted therapies be promising as single‐agent therapies against advanced EMPD with HER2 activation, but they could also be promising therapies in combination with anti‐HER2 Abs. Our study currently has the limitation that we have established only one EMPD‐PDX model, 7 and we used it to evaluate the efficacy of CDK4/6 inhibitors. We are now working on establishing other EMPD‐PDX models and will verify our results in the future.
In conclusion, the present study indicates that CDK4/6 inhibitors suppress tumor growth in EMPD‐PDX, suggesting that these drugs could be promising treatment options for advanced EMPD patients.
DISCLOSURE
The authors declare that they have no conflict of interest.
Supporting information
Fig S1‐S2
Table S1
Kitamura S, Yanagi T, Maeda T, Ujiie H. Cyclin‐dependent kinase 4/6 inhibitors suppress tumor growth in extramammary Paget’s disease. Cancer Sci.2022;113:802–807. doi: 10.1111/cas.15234
Funding information
This work was supported in part by JSPS KAKENHI, grant numbers JP18K08259 (to TY) and JP#21K1620101 (to SK)
REFERENCES
- 1. Fukuda K, Funakoshi T. Metastatic extramammary Paget's disease: pathogenesis and novel therapeutic approach. Front Oncol. 2018;8:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Herrel LA, Weiss AD, Goodman M, et al. Extramammary Paget's disease in males: survival outcomes in 495 patients. Ann Surg Oncol. 2015;22(5):1625‐1630. [DOI] [PubMed] [Google Scholar]
- 3. Ohara K, Fujisawa Y, Yoshino K, et al. A proposal for a TNM staging system for extramammary Paget disease: retrospective analysis of 301 patients with invasive primary tumors. J Dermatol Sci. 2016;83(3):234‐239. [DOI] [PubMed] [Google Scholar]
- 4. Kato M, Yoshino K, Maeda T, et al. Single‐agent taxane is useful in palliative chemotherapy for advanced extramammary Paget disease: a case series. Br J Dermatol. 2019;181(4):831‐832. [DOI] [PubMed] [Google Scholar]
- 5. Hirai I, Tanese K, Nakamura Y, Ishii M, Kawakami Y, Funakoshi T. Combination cisplatin‐epirubicin‐paclitaxel therapy for metastatic extramammary Paget's disease. Oncologist. 2019;24(6):e394‐e396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hidalgo M, Amant F, Biankin AV, et al. Patient‐derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4(9):998‐1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maeda T, Kitamura S, Nishihara H, Yanagi T. Extramammary Paget's disease patient‐derived xenografts harboring ERBB2 S310F mutation show sensitivity to HER2‐targeted therapies. Oncogene. 2020;39(36):5867‐5875. [DOI] [PubMed] [Google Scholar]
- 8. Yanagi T, Krajewska M, Matsuzawa S, Reed JC. PCTAIRE1 phosphorylates p27 and regulates mitosis in cancer cells. Cancer Res. 2014;74(20):5795‐5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sherr CJ, Beach D, Shapiro GI. Targeting CDK4 and CDK6: from discovery to therapy. Cancer Discov. 2016;6(4):353‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cersosimo RJ. Cyclin‐dependent kinase 4/6 inhibitors for the management of advanced or metastatic breast cancer in women. Am J Health Syst Pharm. 2019;76(16):1183‐1202. [DOI] [PubMed] [Google Scholar]
- 11. Schettini F, De Santo I, Rea CG, et al. CDK 4/6 inhibitors as single agent in advanced solid tumors. Front Oncol. 2018;8:608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang G, Zhou S, Zhong W, et al. Whole‐exome sequencing reveals frequent mutations in chromatin remodeling genes in mammary and extramammary Paget's diseases. J Invest Dermatol. 2019;139(4):789‐795. [DOI] [PubMed] [Google Scholar]
- 13. Kiniwa Y, Yasuda J, Saito S, et al. Identification of genetic alterations in extramammary Paget disease using whole exome analysis. J Dermatol Sci. 2019;94(1):229‐235. [DOI] [PubMed] [Google Scholar]
- 14. Ishida Y, Kakiuchi N, Yoshida K, et al. Unbiased detection of driver mutations in extramammary Paget disease. Clin Cancer Res. 2021;27(6):1756‐1765. [DOI] [PubMed] [Google Scholar]
- 15. Takeichi T, Okuno Y, Matsumoto T, et al. Frequent FOXA1‐activating mutations in extramammary Paget's disease. Cancers (Basel). 2020;12(4):820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dowless M, Lowery CD, Shackleford T, et al. Abemaciclib is active in preclinical models of ewing sarcoma via multipronged regulation of cell cycle, DNA methylation, and interferon pathway signaling. Clin Cancer Res. 2018;24(23):6028‐6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cook Sangar ML, Genovesi LA, Nakamoto MW, et al. Inhibition of CDK4/6 by palbociclib significantly extends survival in medulloblastoma patient‐derived xenograft mouse models. Clin Cancer Res. 2017;23(19):5802‐5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kitamura S, Yanagi T, Imafuku K, Hata H, Abe R, Shimizu H. Drp1 regulates mitochondrial morphology and cell proliferation in cutaneous squamous cell carcinoma. J Dermatol Sci. 2017;88(3):298‐307. [DOI] [PubMed] [Google Scholar]
- 19. Kitamura S, Yanagi T, Maeda T, Shimizu H. Drp1 expression levels correlate with clinical stage in extramammary Paget's disease. J Eur Acad Dermatol Venereol. 2020;34(9):e510‐e513. [DOI] [PubMed] [Google Scholar]
- 20. Urata K, Kajihara I, Myangat TM, et al. Overexpression of cyclin‐dependent kinase 4 protein in extramammary Paget's disease. J Dermatol. 2019;46(5):444‐448. [DOI] [PubMed] [Google Scholar]
- 21. Hayes TK, Luo F, Cohen O, et al. A functional landscape of resistance to MEK1/2 and CDK4/6 inhibition in NRAS‐mutant melanoma. Cancer Res. 2019;79(9):2352‐2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Álvarez‐Fernández M, Malumbres M. Mechanisms of sensitivity and resistance to CDK4/6 Inhibition. Cancer Cell. 2020;37(4):514‐529. [DOI] [PubMed] [Google Scholar]
- 23. Goel S, Wang Q, Watt AC, et al. Overcoming therapeutic resistance in HER2‐positive breast cancers with CDK4/6 inhibitors. Cancer Cell. 2016;29(3):255‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patnaik A, Rosen LS, Tolaney SM, et al. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non‐small cell lung cancer, and other solid tumors. Cancer Discov. 2016;6(7):740‐753. [DOI] [PubMed] [Google Scholar]
- 25. Goel S, Pernas S, Tan‐Wasielewski Z, et al. Ribociclib plus trastuzumab in advanced HER2‐positive breast cancer: results of a phase 1b/2 trial. Clin Breast Cancer. 2019;19(6):399‐404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S2
Table S1


