Highlights
-
•
Steroid diminished the antitumor effect of combination therapy with anti-PD-1 Ab and CDDP in an HNSCC mouse model by reducing the T cell proliferation and suppressing memory T cells.
-
•
In vitro assessment using antigen-specific T cells demonstrated that steroid induced apoptosis, decreased proliferation, and reduced tumor cytotoxicity.
-
•
IL-2 or IL-2 Cx restored steroid-induced immunosuppression of T cells by restoring the proliferation and function of T cells in vitro and in vivo.
Keywords: Steroid, Immune checkpoint inhibitor, IL-2, IL-2/anti-IL-2 complexes, Immunochemotherapy, Head and neck squamous cell carcinoma
Abstract
Background: A combination therapy with immune checkpoint inhibitors (ICIs) and platinum-based chemotherapy has become the first-line treatment for recurrent or metastatic head and neck squamous carcinoma (HNSCC). Although steroids are often used as anti-emetic medications during chemotherapy, their adverse effects on immune-combined chemotherapy are unclear in HNSCC.
Methods: The effects of dexamethasone on tumor growth and immune cell population were evaluated in a mouse HNSCC model treated with PD-1 blockade combined with cisplatin. The effect of various doses of dexamethasone on cell proliferation, survival, surface markers, IFN-γ production, and antitumor effects in antigen-specific T cells was examined in vitro. The recovery of T cell dysfunction by IL-2 was assessed in vitro and in vivo.
Results: In a mouse HNSCC model, dexamethasone showed limited antitumor effects on immunochemotherapy. Dexamethasone decreased the number of T cells and inhibited T cell differentiation into effector and central memory T cells. In the in vitro assessment, dexamethasone induced cell death, limited proliferation, and reduced the reactivity against HNSCC cell lines of antigen-specific T cells in a dose-dependent manner. The expression of inhibitory receptors on T cells was not affected by steroids. This inhibition was recovered by IL-2 and IL-2/anti-IL-2 complexes (IL-2 Cx) in vitro and in vivo, respectively.
Conclusion: Our preclinical data indicate that dexamethasone diminishes the antitumor effects of immunochemotherapy in patients with HNSCC. IL-2 Cx recovered the inhibition of antitumor immunity by steroids and might be a potent immune adjuvant for patients who require steroids during PD-1 blockade and chemotherapy.
Background
Head and neck squamous cell carcinoma (HNSCC), which includes cancer of theoral cavity, nasal/sinonasal cavity, pharynx, and larynx, is the sixth most common cancer type worldwide, with little improvement in prognosis over the last few decades [1]. The first-line therapies for patients with advanced HNSCC are surgery and platinum-based chemoradiotherapy, both of which cause severe toxicity [2]. Despite initial treatment, several patients with HNSCC succumb to the disease due to recurrence or metastasis [3]. Cancer immunotherapy, including immune checkpoint inhibitors (ICIs), is a promising strategy to treat otherwise untreatable cancers, including HNSCC [4,5]. By blocking the interaction of negative immune checkpoint molecules with their ligands, the immune checkpoint blockade potentiates antitumor immune cells, followed by substantial antitumor responses. However, only 20% of the patients could receive the clinical benefits from ICI monotherapy [6,7]. Several clinical studies have shown that combination therapy with ICIs and cytotoxic chemotherapy (immunochemotherapy) is promising for the treatment of advanced cancer [8,9]. The spread of tumor epitopes through cytotoxic chemotherapy can augment antitumor T-cell stimulation by ICIs. Recently, pembrolizumab plus platinum and 5-fluorouracil chemotherapy have shown high clinical efficiency with acceptable safety compared to standard treatments (e.g., cetuximab plus platinum-based chemotherapy) in HNSCC [10]. Accordingly, the combination of ICI and platinum-based chemotherapy has become the first-line treatment for recurrent or metastatic HNSCC.
ICIs can induce autoimmune diseases, such as immune-related adverse events (irAEs) by disrupting the immune homeostasis [11]. Steroids are typically administered to treat irAEs, which damage the lungs, liver, colon, pancreas, and skin [12,13]. However, immunosuppressive drugs, including steroids, could be disadvantageous for cancer immunotherapy. Several clinical studies have shown that steroid use impeded the antitumor effect of ICI treatment [14], [15], [16]. In some clinical trials, the patients treated with corticosteroids were excluded from the ICI treatment [17]. Since patients undergoing platinum-based chemotherapy, such as cisplatin (CDDP), a key drug for the treatment of HNSCC, often receive dexamethasone to prevent severe nausea, no consensus has been reached as to how steroids influence the antitumor activity of ICIs combined with CDDP. Therefore, preclinical examination is necessary to determine whether immunosuppression by corticosteroids affects the efficacy of immunochemotherapy. In addition, few studies have elucidated the recovery of steroid-induced immunosuppression. Herein, we aimed to analyze whether steroids reduce the antitumor effect of T cell-based immunotherapy on HNSCC in vivo and in vitro. Additionally, we showed that IL-2 restored the steroid-derived dysfunction of antigen-specific T cells in vitro, and IL-2/anti-IL-2 complexes (IL-2 Cx) recovered immune inhibition of steroids in an HNSCC mouse model treated with immunochemotherapy.
Materials and methods
Cell line and mice
HSC4 (tongue SCC) was supplied by the RIKEN BioResource Center (Tsukuba, Ibaraki, Japan). HPC-92Y (hypopharyngeal SCC) was kindly provided by Dr. Syunsuke Yanoma (Yokohama Tsurugamine Hospital, Yokohama, Japan). MOC1 (tongue SCC derived from C57BL/6 mice) was supplied by Kerafast Inc. (Boston, MA, USA). All cell lines were maintained by tissue culture with RPMI1640 (Nacalai tesque, Japan), 10% FBS (Sigma-Aldrich, Burlington, MA, USA), and Penicillin-Streptomycin (Gibco, Waltham, MA, USA). All cell lines were used within 10 passage after obtaining from the distributors. C57BL/6 mice (female, 8 to 10 weeks old) were purchased from Charles River Laboratories Japan, Inc. (Yokohama, Japan). All the mice were maintained in a specific pathogen-free facility at the Asahikawa Medical University. The experimental protocol was approved by the Institutional Animal Care and Use Committee of Asahikawa Medical University (#20001).
In vitro proliferation and survival assay of antigen-specific CD4+ T cells
The induction of EGFR875–889 -specific CD4+ helper T lymphocytes (HTLs) has been previously described in detail [18]. Briefly, purified HTLs were stimulated weekly with EGFR875–889 peptide, and peptide-specific T cells were selected by limiting dilution. The proliferation of T cells and HNSCC cell lines in response to dexamethasone was investigated using the MTS assay (CellTiter 96® AQueous One Solution Cell Proliferation Assay, Promega, Madison, WI). The tumor cells (HSC4 or HPC-92Y) and EGFR875–889 -specific HTLs (T8) [18] were seeded in a 96-well culture plate and treated with dexamethasone (0–300 µg/mL) for 24 h. After incubation with MTS solution for 1 h, the absorption was measured at 490 nm using a GloMax Discover Microplate Reader (Promega, Madison, WI).
The T cell survival was assessed by flow cytometry (CytoFLEX flow cytometer, Beckman Coulter) using 7-AAD viability staining solution (BioLegend, San Diego, CA). EGFR875–889 -specific HTLs (T8) were co-cultured with dexamethasone (0–300 µg/mL) for 24 h, followed by 7-AAD staining.
Characterization of antigen-specific CD4+ T cells
EGFR875–889 -specific HTLs (T8) were treated with dexamethasone (0, 0.3, and 3 µg/mL) for 24 h, and stained with APC-conjugated anti-PD-1 (EH12.2H7) monoclonal antibody (mAb), PerCP-conjugated anti-LAG-3 (11C3C65) mAb, APC-conjugated anti-TIM3 (F38–2E2) mAb, APC/Cy7-conjugated anti-CD62L (DREG-56), PE-conjugated anti-CD44 (BJ18) mAb, and isotype monoclonal mAb. All the antibodies used for flow cytometric analysis were obtained from BioLegend. All the samples were analyzed using a CytoFLEX flow cytometer and CytExpert (Beckman Coulter).
In vitro antigen recognition and anti-tumor effect of CD4+ T cells
EGFR875–889 -specific HTLs (T8, 1 × 105) were co-cultured in 96-well culture plate with EGFR875–889 peptide-loaded antigen-presenting cells (APCs: γ-irradiated autologous PBMCs, 1 × 105) or HLA-DR-matched tumor cell lines (3 × 104) in the presence of dexamethasone (0–30 µg/mL) for 24 h. The tumor cell lines used in this study expressed EGFR, as described previously [18]. The supernatants were collected and evaluated for IFN-γ by ELISA (BD Pharmingen, San Diego, CA, USA) according to the manufacturer's instructions. To examine the timing of steroid treatment, T8 cells were pretreated with dexamethasone (Pre-Dex) for 48 h before the co-culture, as indicated in the figure. In the killing assay, the tumor cell lines were labeled using the CellTraceTM CFSE Cell Proliferation Kit (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) 6 h before co-culturing with several effector/target cell (E: T) ratios of T cells. The dead cells were assessed by flow cytometry (CytoFLEX flow cytometer, Beckman Coulter) using 7-AAD viability staining solution (BioLegend).
In some experiments, the T8 cells were cultured without dexamethasone for 5–10 days or co-cultured with IL-2 (100 U/ml) after treatment with dexamethasone (0.3 or 3 µg/mL); additionally, the peptide reactivity was evaluated using IFN-γ ELISA.
In vivo steroid treatment and tumor assessment
C57BL/6 mice were intradermally injected with MOC1 (1 × 106). The mice were intraperitoneally administered CDDP (6 mg/kg) on days 18, 25, and 32 after tumor inoculation. In the indicated group, anti-PD-1 Ab (200 μg/mouse) and dexamethasone (1 mg/kg) were intraperitoneally administered three times per week and three sequential days with CDDP administration, respectively. To assess the effect of IL-2 on steroids, IL-2 Cx was intraperitoneally administered three times every two days for one cycle from day 18. IL-2 Cx was prepared by mixing IL-2 (BioLegend) and IL-2 mAb (JES6–5H4, BioXcell), which has a high affinity for IL-2 receptor β chain (CD122), and incubating the complexes overnight at 4 °C. Tumor growth was monitored every 2–3 days. The results are presented as the mean tumor size (mm2) with SD.
The surface markers of immune cells in the spleen, tumor-draining lymph nodes (dLNs), and tumor-infiltrating lymphocytes (TILs) were assessed on day 45. The TILs were disaggregated from tumor tissues using collagenase (1 mg/ml) and gentlMACS (Miltenyi Biotec, Berguch, Germany) according to the manufacturer's instructions. The immune cells were stained with APC-conjugated anti-I-A-I-E (M5/114.15.2) mAb, PerCP-conjugated anti-CD4 (GK1.5) mAb, APC/Cy7-conjugated anti-CD8a (53–6.7) mAb, FITC-conjugated anti-CD62L (MEL-14) mAb, PC-7-conjugated anti-CD44 (IM7) mAb, PE-conjugated anti-PD-1 (29F.1A12) mAb, PC5.5-conjugated anti-NK-1.1 (PK136) mAb, APC/A750-conjugated anti-GR-1 (RB6–8C5) mAb, and the isotype monoclonal mAb. All the antibodies used for flow cytometric analysis were obtained from BioLegend. All samples were analyzed using a CytoFLEX flow cytometer and software (Beckman Coulter).
Statistical analysis
The statistical differences between groups were determined using the Student's t-test and one-way ANOVA with Tukey's method (GraphPad Prism 8). The statistical significance was set at p < 0.05.
Results
Steroid inhibits the effect of PD-1 blockade with chemotherapy in vivo
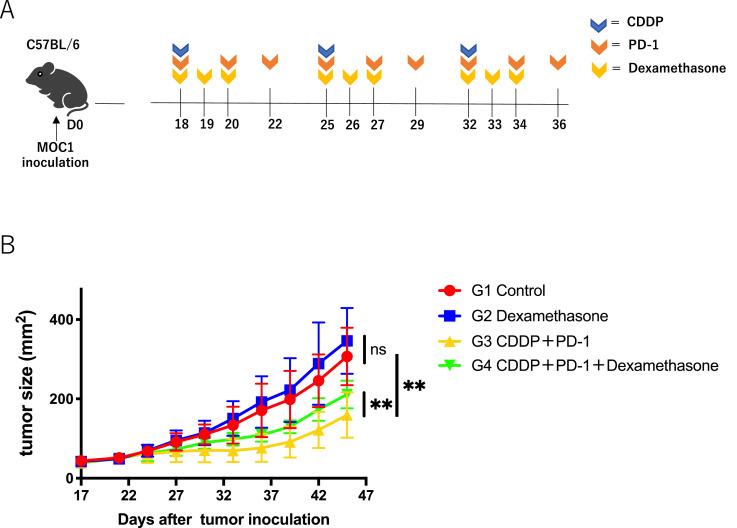
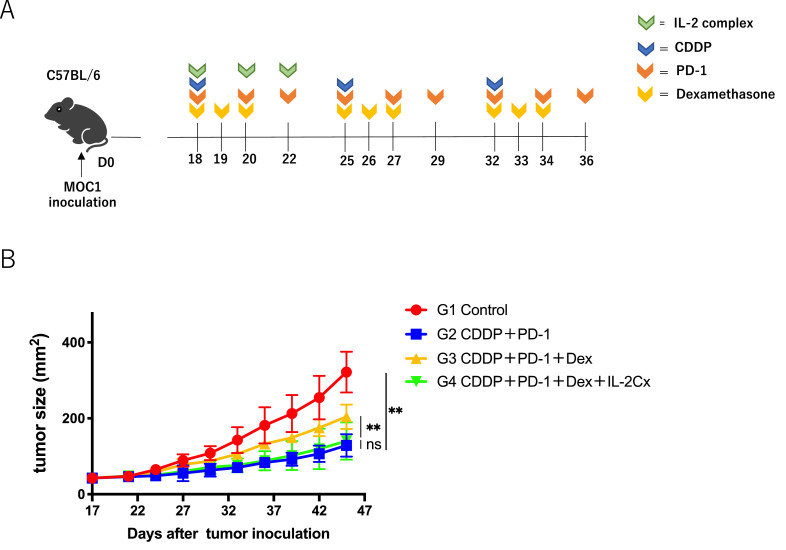
Since steroids are frequently used in clinics to relieve nausea with high-risk emetic chemotherapy including CDDP-combined PD-1 blockade, we evaluated whether immunosuppression by dexamethasone inhibits the anti-tumor effects of immunochemotherapy in a mouse model. The combination therapy consisted of CDDP and anti PD-1 with or without dexamethasone (Fig. 1A). As shown in Fig. 1B, dexamethasone significantly reduced the antitumor effects of the combination therapy. Dexamethasone alone had no effect on the tumor growth.
Fig. 1.
Effect of dexamethasone on anti-tumor activity of CDDP combined with anti-PD-1 Ab in HNSCC mouse model;(A) Experimental schema. C57BL/6 mice were intradermally injected with MOC1(1 × 106). On days 18, 25, and 32 after tumor inoculation, the mice were intraperitoneally administered CDDP (6 mg/kg). Anti-PD-1 Ab (200 μg/mice) was administered three times every other day, and dexamethasone (1 mg/kg) was administered for 3 sequential days from the day of CDDP administration.(B) Tumor growth curves. Control PBS (red), dexamethasone monotherapy (blue), combination therapy with CDDP and anti-PD-1 Ab (yellow), and tri-combination therapy with CDDP, anti-PD-1 Ab, and dexamethasone (Green) (n = 4 or 5 /group). Bars and error bars indicate the mean and SD, respectively (**p<0.01, one-way ANOVA).
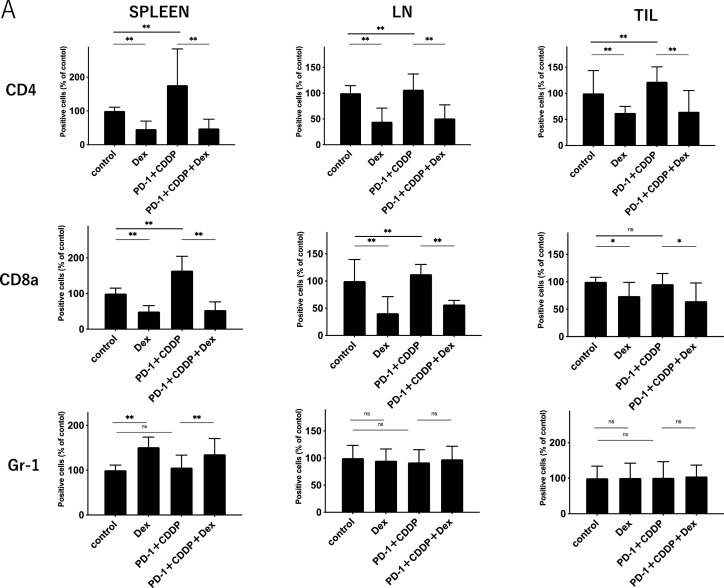
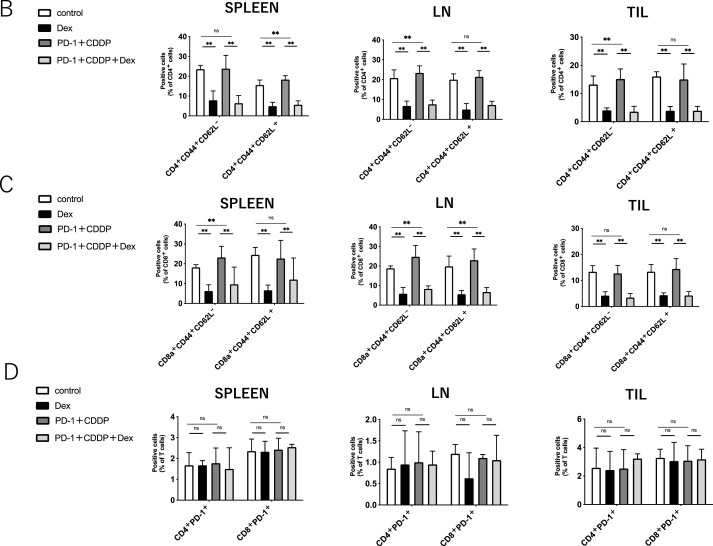
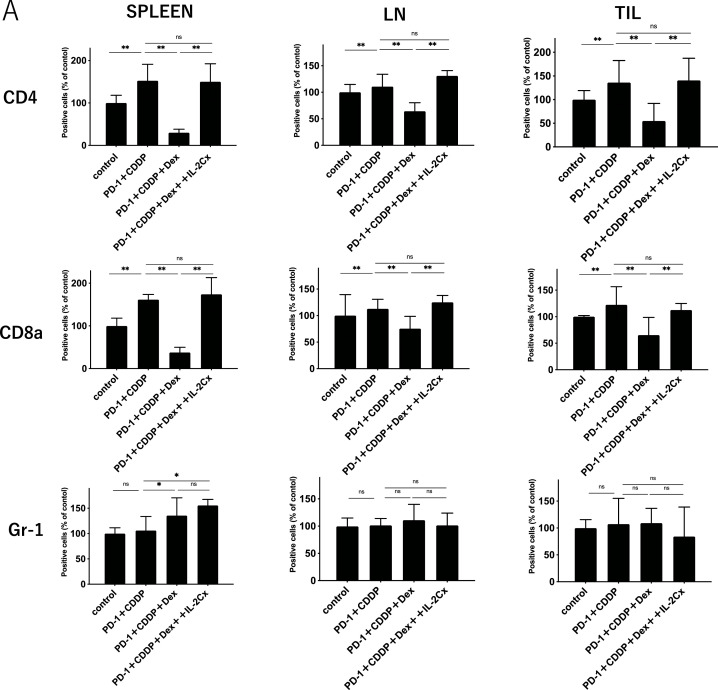
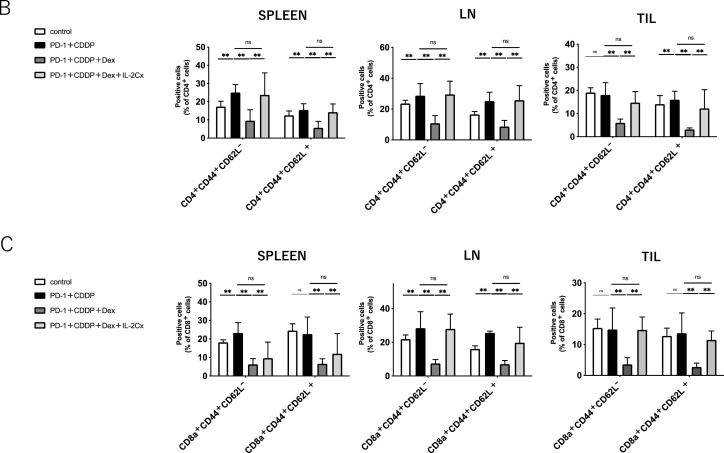
To elucidate the effect of dexamethasone on the immune cells in vivo, the immune cell profiles of spleens, dLNs, and TILs after 3 cycles of treatment (Day 45) were investigated using flow cytometric analysis (Supplementary Fig. 1). As shown in Fig. 2A, dexamethasone significantly reduced the percentage of CD4+ T cells and CD8+ T cells in spleens, dLNs, and TILs, irrespective of immunochemotherapy. In addition, NK cells also decreased following steroid treatment (Supplementary Fig. 2). In contrast, the percentage of Gr-1+ myeloid cells increased with steroid treatment in the spleen. Notably, the percentage of both CD44+ CD62L− (effector memory) and CD44+ CD62L+ (central memory) subsets in CD4+ and CD8+ T cells was reduced by dexamethasone in all the samples (Fig. 2B and C). The reduction of both effector and memory cells in PBMCs was found one week after treatment (Supplemental Fig. 3), indicating that steroids inhibit immune cells within the early period of treatment. The percentage of PD-1+ T cells was not affected by the steroids (Fig. 2D). These results suggest that steroids might inhibit antitumor immunity by reducing central memory and effector memory subsets, in addition to total CD4+ T cells and CD8+ T cells.
Fig. 2.
Immune cell population analysis of spleens, tumor-draining lymph nodes, and tumor infiltrating lymphocytes.Immune cells from the spleen, tumor-draining lymph nodes (dLNs), and tumor-infiltrating lymphocytes (TILs) were harvested on day 45 after MOC1 inoculation. Each group was treated as shown in Fig. 1.(A) The percentage of CD4+T cells, CD8+ T cells, and Gr-1+ cells in spleens, dLNs, and TILs.(B) The percentage of CD44+ CD62L+or CD44+ CD62L− in CD4+ T cells in spleens, dLNs, and TILs.(C) The percentage of CD44+ CD62L+or CD44+ CD62L− in CD8+ T cells in spleens, dLNs, and TILs.(D) The percentage of PD-1+in CD4+ or CD8+T cells in spleens, dLNs, and TILs. Bars and error bars indicate the mean and SD, respectively (*p<0.05, **p<0.01, Student's t-test).
Dexamethasone regulates survival and proliferation of antigen-specific T cells in vitro
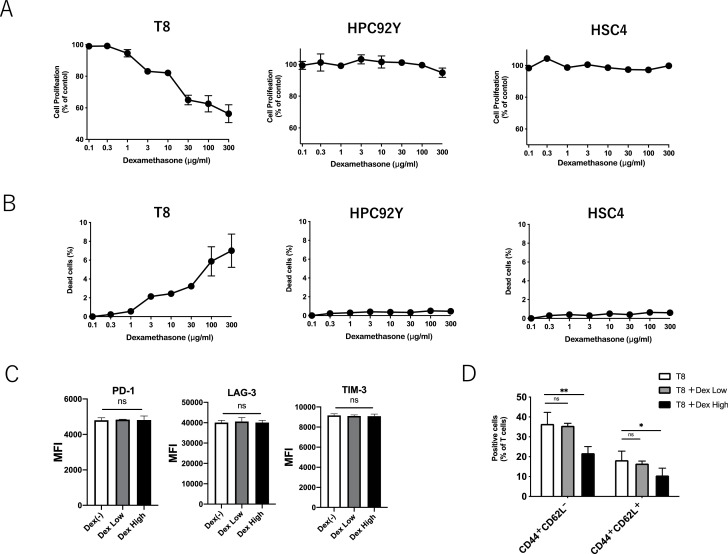
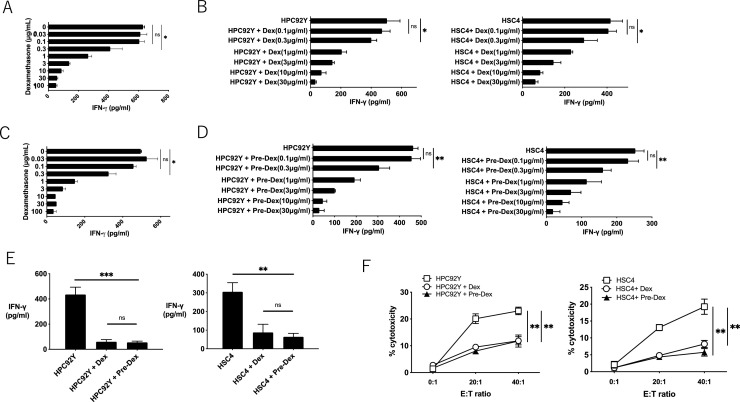
Several studies have shown that steroids lead to the apoptosis of T cells [19]. Because T cell epitopes have not been identified in the mouse HNSCC model, we used EGFR-specific CD4+ helper T lymphocyte (HTLs) clones from human PBMCs [18] to further elucidate the effects of steroid in tumor antigen-specific T cells. Being widely expressed in HNSCC, EGFR is selected as a model tumor antigen. HNSCC cell lines (HPC-92Y and HSC4) expressed EGFR, which remained stable with dexamethasone (Supplementary Fig. 4). After co-culturing with EGFR875–889-reactive HTLs (T8), dexamethasone reduced the proliferation of EGFR875–889-reactive HTLs in a dose-dependent manner (Fig. 3A). The proliferation of tumor cell lines (HPC-92Y and HSC4) was not affected by dexamethasone. Similar to the proliferation, the survival of T cells was directly impeded by dexamethasone in a dose-dependent manner, whereas the tumor cells were not affected (Fig. 3B). PD-1, LAG-3, and TIM-3 are negative immune checkpoints expressed on exhausted T cells. In addition to the in vivo model (Fig. 2D), the expression of these surface markers on EGFR875–889-reactive HTLs remained stable with high- (3 μg/ml) or low-dose (0.3 μg/ml) dexamethasone (Fig. 3C). Similar to the in vivo experiment (Fig. 2C), both the CD44+ CD62L− (effector memory) and CD44+ CD62L+ (central memory) cells in EGFR875–889-reactive HTLs were also decreased by dexamethasone (Fig. 3D). Altogether, steroid-induced apoptosis, the limited proliferation of antigen-specific CD4+ T cells, and reduced memory T cell proportion without upregulating inhibitory checkpoints.
Fig. 3.
Effects of dexamethasone on cell proliferation, survival, and surface markers of T cells and tumor cells;(A) The proliferation of EGFR875–889-reactive HTLs (T8) and tumor cell line (HPC-92Y and HSC4) co-cultured with various concentration of dexamethasone was evaluated by MTS assay.(B) The survival of T8 and tumor cell line (HPC-92Y and HSC4) co-cultured with various concentration of dexamethasone were evaluated by flow cytometry using 7-AAD viability staining solution.(C) Expression levels of PD-1, LAG-3, and TIM3 on T8 with high-dose (3 μg/ml) or low-dose (0.3 μg/ml) dexamethasone were evaluated by flow cytometry. The panels show averages values of mean fluorescence intensity (MFI). (ns: not significant, Student's t-test). The error bars indicate standard deviations of triplicates.(D) The percentage of CD44+ CD62L+or CD44+ CD62L− in T8 with high-dose (3 μg/ml) or low-dose (0.3 μg/ml) dexamethasone was assessed by flow cytometry.Bars and error bars indicate the mean and SD, respectively (*p<0.05, **p<0.01, Student's t-test).
Dexamethasone inhibits anti-tumor effect of antigen-specific T cells in vitro
To evaluate the functional regulation of HTLs by steroids, EGFR875–889-reactive HTLs (T8) were co-cultured with peptide-pulsed autologous PBMCs or EGFR-expressing HLA-DR-matched HNSCC cell lines in the presence of dexamethasone. As shown in Fig. 4A, IFN-γ production in T8 cells co-cultured with EGFR875–889 peptide-pulsed γ-irradiated autologous PBMCs was attenuated by dexamethasone in a dose-dependent manner. The IFN-γ production in T8 cells co-cultured with tumor cell lines was also reduced by dexamethasone (Fig. 4B). To examine whether the reduction of IFN-γ production from T cells requires concurrent culture with steroids and APCs, T8 cells were pre-treated with dexamethasone (0–30 µg/mL) for 48 h and washed with PBS. As shown in Fig. 4C and D, pretreatment with dexamethasone reduced the production of IFN-γ from T8 against γ-irradiated autologous PBMCs or tumor cell lines. The IFN-γ production was equally suppressed in T8 cells treated with concurrent or pre-dexamethasone (Fig. 4E). Similarly, the tumor-killing activity of T8 also decreased with or pre-treatment with dexamethasone (Fig. 4F). These results suggest that steroids can directly diminish the antigen reactivity and cytotoxicity of tumor-reactive HTLs, irrespective of the presence of APCs.
Fig. 4.
Direct recognition and anti-tumor effect by EGFR875–889-reactive HTLs with dexamethasone;(A) T8 were evaluated for IFN-γ production with EGFR875–889 peptide (3 µg/ml) and various concentration of dexamethasone in the context of autologous PBMCs as APCs.(B) Direct tumor recognition by T8 with various concentration of dexamethasone was evaluated by co-culturing T cells and tumor cell lines (HPC-92Y and HSC4). IFN-γ production was used as an output.(C, D) T8 were co-cultured with various concentration of dexamethasone for 48 h and washed with PBS. Subsequently, IFN-γ production of steroid-pretreated T8 in the context of (C) peptide-pulsed γ-irradiated autologous PBMCs or (D) tumor cell lines (HPC-92Y and HSC4) was evaluated.(E) T8 were concurrently (Dex) or pre-treated for 48 h (Pre-Dex) with dexamethasone (3 μg/mL), and evaluated for IFN-γ production with tumor cell lines.(F) Tumoricidal ability of T8 with concurrently (Dex) or pre-treated for 48 h (Pre-Dex) with dexamethasone (3 μg/mL). T8 was co-cultured with CSFE-labeled tumor cell lines (HPC-92Y and HSC4) for 6 h with several E: T (Effector: Target cells) ratio, and evaluated the percentages of dead tumor cells (CFSE+ 7-AAD+ cells) with flow cytometry. Symbols and error bars indicate the mean and SD, respectively. Experiments were performed in triplicate. (*p<0.05, **p<0.01, ***<0.001, Student's t-test).
IL-2 recovers antigen reactivity of steroid-induced anergic T cells
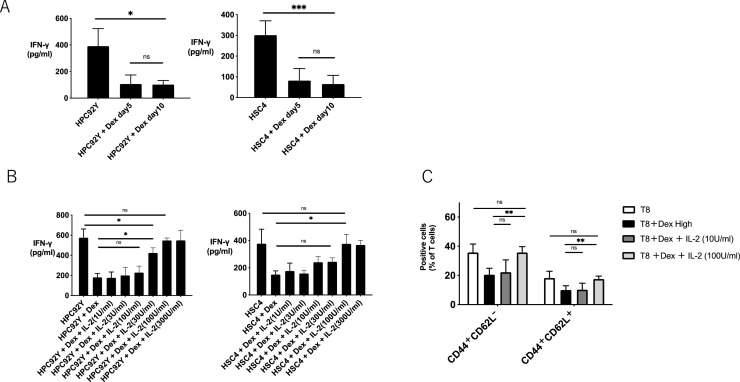
Subsequently, we investigated whether the reduced antigen reactivity of HTLs by dexamethasone was recovered by resting or adding cytokines. As shown in Fig. 5A, IFN-γ production from HTLs was not recovered by resting for 5 to 10 days after co-culturing with dexamethasone. In contrast, steroid-induced anergic HTLs recovered IFN-γ production in tumor cells with IL-2 supplementation (Fig. 5B). The proportion of memory T cells reduced by steroids was also recovered by 100 U/ml IL-2 (Fig. 5C). These results indicate that the steroid-induced suppression of antitumor T cells can be recovered by IL-2.
Fig. 5.
IL-2 recovers the function of EGFR-reactive HTLs impaired by dexamethasone;EGFR875–889 -specific HTLs (T8) were co-cultured with dexamethasone (3 μg/mL) for 48 h and washed with PBS. (A) Pre-treated T8 cells were rested for 5 or 10 days, and IFN-γ production in tumor cells was evaluated by ELISA. (B) Pre-treated T8 cells were co-cultured with various concentrations of IL-2 for 48 h after washing, and IFN-γ production in tumor cells was evaluated by ELISA. Bars and error bars indicate the mean and SD, respectively. The experiments were performed in triplicate (*p<0.05, Student's t-test). (C) Pre-treated T8 cells were co-cultured with IL-2 (10 U/ml or 100 U/ml) for 48 h after washing, and the percentage of CD44+ CD62L+or CD44+ CD62L− in T8 cells was evaluated by flow cytometry.
IL-2 complex recovers antitumor effect of immunochemotherapy impaired by steroid in HNSCC mouse model
To assess whether IL-2 restores the immunosuppression of antitumor T cells by steroids in vivo, IL-2 Cx was added to the immunochemotherapy/steroid mouse model (Fig. 6A). As shown in Fig. 6B, IL-2 Cx significantly diminished the negative effects of dexamethasone in the immunochemotherapy model. The percentage of CD4+ and CD8+ T cells decreased by dexamethasone in the spleen, dLNs, and TILs was restored by IL-2 Cx. The percentage of Gr-1+ myeloid cells was not affected by the IL-2 Cx treatment. Moreover, the percentages of both CD44+ CD62L− (effector memory) and CD44+ CD62L+ (central memory) subsets in CD4+ and CD8+ T cells were also recovered to the extent of the non-steroid group by IL-2 Cx (Fig. 7B and C). The recovery of T cells from steroids was observed during the early period of treatment (Supplementary Fig. 5). In addition, the number of NK cells, which was downregulated by steroids, was also recovered with IL-2 Cx (Supplementary Fig. 6). Collectively, these data demonstrate that IL-2 Cx recovered the antitumor effect of immunochemotherapy suppressed by dexamethasone by restoring the number of total CD4+ T cells, CD8+ T cells, memory T cells, and NK cells. IL-2 is a potent immune adjuvant that activates antitumor T cells during immunochemotherapy with steroids.
Fig. 6.
IL-2 complex recovers antitumor effect of immunochemotherapy impaired by steroids in HNSCC mouse model.(A) Experimental schema. C57BL/6 mice intradermally injected with MOC1 (1 × 106) were administered intraperitoneally with IL-2 Cx 3 times every other day for 1 cycle in addition to CDDP, anti-PD-1 Ab, and dexamethasone as indicated in Fig. 1.(B) Tumor growth curves. Control PBS (Red), combination therapy with CDDP and anti-PD-1 Ab (Blue), tri-combination therapy with CDDP, anti-PD-1 Ab and dexamethasone (Yellow), and quad-combination therapy with CDDP, anti-PD-1 Ab, dexamethasone, and IL-2 complex (Green) (n = 4 or 5 /group). Bars and error bars indicate the mean and SD, respectively (**p<0.01, one-way ANOVA).
Fig. 7.
IL-2 complex recovers immune cells reduced by steroid in HNSCC mouse model.Immune cells from the spleen, tumor-draining lymph nodes (dLNs), and tumor-infiltrating lymphocytes (TILs) were harvested on day 45 after MOC1 inoculation. Each group was treated as shown in Fig. 6.(A) The percentage of CD4+T cells, CD8+ T cells, and Gr-1+ cells in spleens, dLNs, and TILs. (B) The percentage of CD44+ CD62L+or CD44+ CD62L− in CD4+ T cells in spleens, dLNs, and TILs.(C) The percentage of CD44+ CD62L+or CD44+ CD62L− in CD8+ T cells in spleens, dLNs, and TILs.
Discussion
To the best of our knowledge, there have been no reports regarding the effects of steroids on ICIs combined with chemotherapy in clinical or preclinical models of HNSCC. In this study, we showed that dexamethasone caused immunosuppression and tumor growth inhibition in an HNSCC mouse model treated with PD-1 blockade and CDDP. In other types of cancer, several reports indicate that steroids have adverse effects on patients undergoing ICIs. Corticosteroids have been associated with poor prognosis in non-small-cell lung cancer patients treated with anti-PD-(L)1 antibody (Ab) [14,20]. In patients with glioblastoma receiving anti-PD-(L)1 Ab therapy, the baseline use of dexamethasone attenuated the overall survival [21]. Tallon de Lara et al. showed that the addition of dexamethasone to ICIs with gemcitabine resulted in a synergistic clinical response [22].The induction of IFN-γ responses to personalized neoantigen-targeting vaccines was also inhibited by dexamethasone [23]. Conversely, some studies have suggested that steroids have no significant impact on the efficacy of ICIs. In hepatocellular carcinoma, corticosteroid therapy did not influence the response rate or overall survival following ICIs [24]. Jeffrey et al. showed that corticosteroid use did not affect the overall response rate in advanced melanoma with nivolumab monotherapy [25]. However, these patients received corticosteroids due to irAEs, which is associated with improved survival in the patients treated with ICIs [26]. Thus, steroids are likely to have adverse effects on cancer immunotherapy; however, the direct association between steroids and treatment responses to ICIs remains to be elucidated in the clinic.
In general, steroids attenuate T cell activation, differentiation, migration, and cytokine production with increasing regulatory T cells [19,27]. Our results show that steroids suppress effector memory T cells and central memory CD4+ T cells and CD8+ T cells both of which play essential roles in ICIs [28] [29]. The impediment of CD28-mediated cell cycle entry and CTLA-4 induction by steroids might inhibit the differentiation of naïve T cells into these two essential subsets [30]. Since steroid inhibits the migration of T cells, the number of periphery T cells was inhibited in addition to lymph tissues and tumor-infiltrating cells. Thus, lymphocyte migration might be indispensable for the inhibition of antitumor T cell responses by dexamethasone in the anti-PD1 therapy model. Moreover, we showed that dexamethasone decreased the NK cells, which can kill tumor cells through antibody-dependent cytotoxic activity [31]. Steroids not only suppress T cells and NK cells, but also stimulate M2 macrophage skewing [32], and inhibit dendric cells proliferation and maturation [33].
In this study, the function of T cells remained anergic after dexamethasone removal. Giles et al. have shown that dexamethasone upregulates CTLA-4 molecules on the T cells and blocks CD28-mediated cell cycle entry and differentiation [30] suggesting that the absence of CD28/CD80 or CD28/CD86 costimulatory signaling induces an anergic state in T cells. Moreover, the T cell receptor complex is disrupted after binding the glucocorticoid to the corresponding receptor [34]. Since Tokunaga et al. have shown that early corticosteroids in malignant melanoma patients treated with CTLA-4 inhibitors shortened the overall survival [35], the CTLA-4 blockade alone is not sufficient to overcome steroid-mediated immunosuppression. As Wayne et al. have reported [36], the T cell exhaustion markers (PD-1, LAG3, and TIM3) did not change with steroid treatment in this study, indicating that PD-1 blockade cannot overcome steroid-induced immunosuppression. In addition to the lack of costimulatory signaling, the corticosteroids cause T cell dysfunction by suppressing IL-2 production [19]. Because IL-2 is an essential cytokine that activates the antitumor activity of cytotoxic T cells and NK cells [37], we used IL-2 as an adjuvant to overcome steroid-induced immunosuppression. The supplementation with IL-2 was efficient in recovering the inhibited antitumor T cells by steroids in vitro and in vivo. Regardless of steroids, IL-2 is considered as a promising immunoadjuvant in various types of immunotherapy, including peptide vaccines [38,39]. Although the safety of IL-2 administration in humans has been confirmed in clinical practice for treating patients with melanoma and renal cell carcinoma, IL-2 is rapidly degraded in vivo. To increase the half-life of IL-2, IL-2 can be combined with an anti-IL-2 antibody to produce IL-2 Cx that augments tumor-reactive T cell responses [38,39] and efficiency of ICIs [40,41]. Since we have shown that steroids inhibit the stimulation of T cells through the MHC-peptide-T cell receptor complex, further studies are required to elucidate whether the pre-activated T cells are suppressed by steroids, and the appropriate dose or timing to spare the negative effects of corticosteroids on ICIs in a larger cohort of patients. The maximum recommended dose of dexamethasone is 20 mg for antiemetic as well as in the treatment for immune-related adverse effects or auto-immune diseases. The maximum observed concentration of 20 mg dexamethasone is 0.26 ng/ml [42], which is almost similar to the dose sufficient to inhibit antitumor T cell responses in this study (0.3 ng/ml, Fig. 4). Thus, we believe that the dexamethasone dose in our in vitro experiments is relevant to the clinical setting. The dose used in our mouse models (1 mg/kg) was relatively higher than the dose used for antiemetic in human. However, it would be difficult to directly compare the intravenously administered dose in human with the intraperitoneally administered dose in mice since the bioavailability of drug is different between intravenous and intraperitoneal route due to the first pass metabolism [43]. Further studies to examine the different doses of steroid with PD-1 blockade are required. As dexamethasone is more potent and long-acting than other corticosteroids, immunosuppression among the types of steroids should also be examined. A limitation of the mouse model in study is that the T cell epitope from mouse HNSCC has not been identified. To study the influence of steroid in antigen-specific T cell responses, we used human EGFR-reactive HTLs. Fortunately, steroid decreased T cells in both mouse and human models. The establishment of a tumor antigen-specific T cell model in mouse HNSCC is required to further examine the antitumor T cell responses in the future.
Conclusions
Dexamethasone decreased the antitumor effect of combination therapy with anti-PD-1 Ab and CDDP in an HNSCC mouse model by reducing the T cell proliferation and suppressing memory T cells. In vitro assessment using antigen-specific T cells showed that dexamethasone induced apoptosis, decreased proliferation, and reduced tumor cytotoxicity. Notably, IL-2 or IL-2 Cx restored steroid-induced immunosuppression of T cells by restoring the proliferation and function of T cells in vitro and in vivo. These results suggest that the use of steroids should be avoided unless necessary during immunochemotherapy in HNSCC patients, and IL-2 Cx might be a promising adjuvant to recover immunosuppression with steroids.
CRediT authorship contribution statement
Michihisa Kono: Funding acquisition, Formal analysis, Data curation, Conceptualization, Visualization, Supervision, Writing – original draft. Hidekiyo Yamaki: Funding acquisition, Formal analysis, Data curation. Hiroki Komatsuda: Funding acquisition, Formal analysis, Data curation. Takumi Kumai: Funding acquisition, Formal analysis, Data curation, Methodology, Conceptualization, Visualization, Supervision, Writing – original draft, Writing – review & editing. Ryusuke Hayashi: Funding acquisition, Formal analysis, Data curation. Risa Wakisaka: Funding acquisition, Formal analysis, Data curation. Ryosuke Sato: Formal analysis. Kenzo Ohara: . Kan Kishibe: Resources. Miki Takahara: Formal analysis. Akihiro Katada: Formal analysis. Tatsuya Hayashi: Resources, Methodology. Yasuaki Harabuchi: Methodology, Writing – review & editing.
Declaration of Competing Interest
None declared.
Acknowledgments
Funding
This work was supported by JSPS KAKENHI Grant Number 20K09724.
Availability of data and material
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics approval and consent to participate, Consent for publication
All experiments were approved by the institutional ethics committee on the Asahikawa Medical University (#16217). The study was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Acknowledgments
The authors also thank Dr. Hajime Kamada (Hokuto Social Medical Corporation) for his excellent suggestions for the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2022.101358.
Appendix. Supplementary materials
References
- 1.Moy J.D., Moskovitz J.M., Ferris R.L. Biological mechanisms of immune escape and implications for immunotherapy in head and neck squamous cell carcinoma. Eur. J. Cancer. 2017;76:152–166. doi: 10.1016/j.ejca.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernier J., Domenge C., Ozsahin M., Matuszewska K., Lefebvre J.L., Greiner R.H., Giralt J., Maingon P., Rolland F., Bolla M., Cognetti F., Bourhis J., Kirkpatrick A., van Glabbeke M., European Organization for Research and Treatment of Cancer Trail 22931 Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N. Engl. J. Med. 2004;350:1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 3.Cooper J.S., Pajak T.F., Forastiere A.A., Jacobs J., Campbell B.H., Saxman S.B., Kish J.A., Kim H.E., Cmelak A.J., Rotman M., Machtay M., Ensley J.F., Chao K.S., Schultz C.J., Lee N., Fu K.K., Radiation Therapy Oncology Group 9501/Intergroup Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 4.Ferris R.L., Blumenschein G., Fayette J., Guigay J., Colevas A.D., Licitra L., Harrington K., Kasper S., Vokes E.E., Even C., Worden F., Saba N.F., Iglesias Docampo L.C., Haddad R., Rordorf T., Kiyota N., Tahara M., Monga M., Lynch M., Geese W.J., Kopit J., Shaw J.W., Gillison M.L. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seiwert T.Y., Burtness B., Mehra R., Weiss J., Berger R., Eder J.P., Heath K., McClanahan T., Lunceford J., Gause C., Cheng J.D., Chow L.Q. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17:956–965. doi: 10.1016/S1470-2045(16)30066-3. [DOI] [PubMed] [Google Scholar]
- 6.Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C., McDermott D.F., Powderly J.D., Carvajal R.D., Sosman J.A., Atkins M.B., Leming P.D., Spigel D.R., Antonia S.J., Horn L., Drake C.G., Pardoll D.M., Chen L., Sharfman W.H., Anders R.A., Taube J.M., McMiller T.L., Xu H., Korman A.J., Jure-Kunkel M., Agrawal S., McDonald D., Kollia G.D., Gupta A., Wigginton J.M., Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamid O., Robert C., Daud A., Hodi F.S., Hwu W.J., Kefford R., Wolchok J.D., Hersey P., Joseph R.W., Weber J.S., Dronca R., Gangadhar T.C., Patnaik A., Zarour H., Joshua A.M., Gergich K., Elassaiss-Schaap J., Algazi A., Mateus C., Boasberg P., Tumeh P.C., Chmielowski B., Ebbinghaus S.W., Li X.N., Kang S.P., Ribas A. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandhi L., Rodriguez-Abreu D., Gadgeel S., Esteban E., Felip E., De Angelis F., Domine M., Clingan P., Hochmair M.J., Powell S.F., Cheng S.Y., Bischoff H.G., Peled N., Grossi F., Jennens R.R., Reck M., Hui R., Garon E.B., Boyer M., Rubio-Viqueira B., Novello S., Kurata T., Gray J.E., Vida J., Wei Z., Yang J., Raftopoulos H., Pietanza M.C., Garassino M.C., Investigators K.-. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 9.Paz-Ares L., Luft A., Vicente D., Tafreshi A., Gumus M., Mazieres J., Hermes B., Cay Senler F., Csoszi T., Fulop A., Rodriguez-Cid J., Wilson J., Sugawara S., Kato T., Lee K.H., Cheng Y., Novello S., Halmos B., Li X., Lubiniecki G.M., Piperdi B., Kowalski D.M., Investigators K. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N. Engl. J. Med. 2018;379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 10.Burtness B., Harrington K.J., Greil R., Soulieres D., Tahara M., de Castro G., Psyrri A., Baste N., Neupane P., Bratland A., Fuereder T., Hughes B.G.M., Mesia R., Ngamphaiboon N., Rordorf T., Wan Ishak W.Z., Hong R.L., Gonzalez Mendoza R., Roy A., Zhang Y., Gumuscu B., Cheng J.D., Jin F., Rischin D., Investigators K. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 11.June C.H., Warshauer J.T., Bluestone J.A. Is autoimmunity the Achilles' heel of cancer immunotherapy? Nat. Med. 2017;23:540–547. doi: 10.1038/nm.4321. [DOI] [PubMed] [Google Scholar]
- 12.Hofmann L., Forschner A., Loquai C., Goldinger S.M., Zimmer L., Ugurel S., Schmidgen M.I., Gutzmer R., Utikal J.S., Goppner D., Hassel J.C., Meier F., Tietze J.K., Thomas I., Weishaupt C., Leverkus M., Wahl R., Dietrich U., Garbe C., Kirchberger M.C., Eigentler T., Berking C., Gesierich A., Krackhardt A.M., Schadendorf D., Schuler G., Dummer R., Heinzerling L.M. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur. J. Cancer. 2016;60:190–209. doi: 10.1016/j.ejca.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 13.Friedman C.F., Proverbs-Singh T.A., Postow M.A. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2:1346–1353. doi: 10.1001/jamaoncol.2016.1051. [DOI] [PubMed] [Google Scholar]
- 14.Arbour K.C., Mezquita L., Long N., Rizvi H., Auclin E., Ni A., Martinez-Bernal G., Ferrara R., Lai W.V., Hendriks L.E.L., Sabari J.K., Caramella C., Plodkowski A.J., Halpenny D., Chaft J.E., Planchard D., Riely G.J., Besse B., Hellmann M.D. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J. Clin. Oncol. 2018;36:2872–2878. doi: 10.1200/JCO.2018.79.0006. [DOI] [PubMed] [Google Scholar]
- 15.Della Corte C.M., Morgillo F. Early use of steroids affects immune cells and impairs immunotherapy efficacy. ESMO Open. 2019;4 doi: 10.1136/esmoopen-2018-000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuca G., Galli G., Poggi M., Russo G.Lo, Proto C., Imbimbo M., Ferrara R., Zilembo N., Ganzinelli M., Sica A., Torri V., Colombo M.P., Vernieri C., Balsari A., de Braud F., Garassino M.C., Signorelli D. Modulation of peripheral blood immune cells by early use of steroids and its association with clinical outcomes in patients with metastatic non-small cell lung cancer treated with immune checkpoint inhibitors. ESMO Open. 2019;4 doi: 10.1136/esmoopen-2018-000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Souza A., Hari P., Pasquini M., Braun T., Johnson B., Lundy S., Couriel D., Hamadani M., Magenau J., Dhakal B., Shah N.N., Riwes M., Parkin B., Reddy P., Pawarode A. A Phase 2 study of pembrolizumab during lymphodepletion after autologous hematopoietic cell transplantation for multiple myeloma. Biol. Blood Marrow Transplant. 2019;25:1492–1497. doi: 10.1016/j.bbmt.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Kumai T., Matsuda Y., Oikawa K., Aoki N., Kimura S., Harabuchi Y., Celis E., Kobayashi H. EGFR inhibitors augment antitumour helper T-cell responses of HER family-specific immunotherapy. Br. J. Cancer. 2013;109:2155–2166. doi: 10.1038/bjc.2013.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Libert C., Dejager L. How steroids steer T cells. Cell Rep. 2014;7:938–939. doi: 10.1016/j.celrep.2014.04.041. [DOI] [PubMed] [Google Scholar]
- 20.Ricciuti B., Dahlberg S.E., Adeni A., Sholl L.M., Nishino M., Awad M.M. Immune checkpoint inhibitor outcomes for patients with non-small-cell lung cancer receiving baseline corticosteroids for palliative versus nonpalliative indications. J. Clin. Oncol. 2019;37:1927–1934. doi: 10.1200/JCO.19.00189. [DOI] [PubMed] [Google Scholar]
- 21.Iorgulescu J.B., Gokhale P.C., Speranza M.C., Eschle B.K., Poitras M.J., Wilkens M.K., Soroko K.M., Chhoeu C., Knott A., Gao Y., Lim-Fat M.J., Baker G.J., Bonal D.M., Nguyen Q.D., Grant G.R.L., Ligon K.L., Sorger P.K., Chiocca E.A., Anderson A.C., Kirschmeier P.T., Sharpe A.H., Freeman G.J., Reardon D.A. Concurrent dexamethasone limits the clinical benefit of immune checkpoint blockade in glioblastoma. Clin. Cancer Res. 2021;27:276–287. doi: 10.1158/1078-0432.CCR-20-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tallon de Lara P., Cecconi V., Hiltbrunner S., Yagita H., Friess M., Bode B., Opitz I., Vrugt B., Weder W., Stolzmann P., Felley-Bosco E., Stahel R.A., Tischler V., Britschgi C., Soldini D., van den Broek M., Curioni-Fontecedro A. Gemcitabine synergizes with immune checkpoint inhibitors and overcomes resistance in a preclinical model and mesothelioma patients. Clin. Cancer Res. 2018;24:6345–6354. doi: 10.1158/1078-0432.CCR-18-1231. [DOI] [PubMed] [Google Scholar]
- 23.Keskin D.B., Anandappa A.J., Sun J., Tirosh I., Mathewson N.D., Li S., Oliveira G., Giobbie-Hurder A., Felt K., Gjini E., Shukla S.A., Hu Z., Li L., Le P.M., Allesoe R.L., Richman A.R., Kowalczyk M.S., Abdelrahman S., Geduldig J.E., Charbonneau S., Pelton K., Iorgulescu J.B., Elagina L., Zhang W., Olive O., McCluskey C., Olsen L.R., Stevens J., Lane W.J., Salazar A.M., Daley H., Wen P.Y., Chiocca E.A., Harden M., Lennon N.J., Gabriel S., Getz G., Lander E.S., Regev A., Ritz J., Neuberg D., Rodig S.J., Ligon K.L., Suva M.L., Wucherpfennig K.W., Hacohen N., Fritsch E.F., Livak K.J., Ott P.A., Wu C.J., Reardon D.A. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565:234–239. doi: 10.1038/s41586-018-0792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinato D.J., Kaseb A., Wang Y., Saeed A., Szafron D., Jun T., Dharmapuri S., Naqash A.R., Muzaffar M., Navaid M., Khan U., Lee C., Bulumulle A., Yu B., Paul S., Fessas P., Nimkar N., Bettinger D., Hildebrand H., Pressiani T., Abugabal Y.I., Personeni N., Huang Y.H., Lozano-Kuehne J., Rimassa L., Ang C., Marron T.U. Impact of corticosteroid therapy on the outcomes of hepatocellular carcinoma treated with immune checkpoint inhibitor therapy. J. Immunother. Cancer. 2020;8:e000726. doi: 10.1136/jitc-2020-000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber J.S., Hodi F.S., Wolchok J.D., Topalian S.L., Schadendorf D., Larkin J., Sznol M., Long G.V., Li H., Waxman I.M., Jiang J., Robert C. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J. Clin. Oncol. 2017;35:785–792. doi: 10.1200/JCO.2015.66.1389. [DOI] [PubMed] [Google Scholar]
- 26.Shankar B., Zhang J., Naqash A.R., Forde P.M., Feliciano J.L., Marrone K.A., Ettinger D.S., Hann C.L., Brahmer J.R., Ricciuti B., Owen D., Toi Y., Walker P., Otterson G.A., Patel S.H., Sugawara S., Naidoo J. Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non-small cell lung cancer. JAMA Oncol. 2020;6:1952–1956. doi: 10.1001/jamaoncol.2020.5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen X., Oppenheim J.J., Winkler-Pickett R.T., Ortaldo J.R., Howard O.M. Glucocorticoid amplifies IL-2-dependent expansion of functional FoxP3(+)CD4(+)CD25(+) T regulatory cells in vivo and enhances their capacity to suppress EAE. Eur. J. Immunol. 2006;36:2139–2149. doi: 10.1002/eji.200635873. [DOI] [PubMed] [Google Scholar]
- 28.Maxwell R., Luksik A.S., Garzon-Muvdi T., Hung A.L., Kim E.S., Wu A., Xia Y., Belcaid Z., Gorelick N., Choi J., Theodros D., Jackson C.M., Mathios D., Ye X., Tran P.T., Redmond K.J., Brem H., Pardoll D.M., Kleinberg L.R., Lim M. Contrasting impact of corticosteroids on anti-PD-1 immunotherapy efficacy for tumor histologies located within or outside the central nervous system. Oncoimmunology. 2018;7 doi: 10.1080/2162402X.2018.1500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wherry E.J., Teichgraber V., Becker T.C., Masopust D., Kaech S.M., Antia R., von Andrian U.H., Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 30.Giles A.J., Hutchinson M.N.D., Sonnemann H.M., Jung J., Fecci P.E., Ratnam N.M., Zhang W., Song H., Bailey R., Davis D., Reid C.M., Park D.M., Gilbert M.R. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J. Immunother. Cancer. 2018;6:51. doi: 10.1186/s40425-018-0371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumai T., Oikawa K., Aoki N., Kimura S., Harabuchi Y., Kobayashi H. Assessment of the change in cetuximab-induced antibody-dependent cellular cytotoxicity activity of natural killer cells by steroid. Head Neck. 2016;38:410–416. doi: 10.1002/hed.23906. [DOI] [PubMed] [Google Scholar]
- 32.Sica A., Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szatmari I., Nagy L. Nuclear receptor signalling in dendritic cells connects lipids, the genome and immune function. EMBO J. 2008;27:2353–2362. doi: 10.1038/emboj.2008.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowenberg M., Verhaar A.P., van den Brink G.R., Hommes D.W. Glucocorticoid signaling: a nongenomic mechanism for T-cell immunosuppression. Trends Mol. Med. 2007;13:158–163. doi: 10.1016/j.molmed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Tokunaga A., Sugiyama D., Maeda Y., Warner A.B., Panageas K.S., Ito S., Togashi Y., Sakai C., Wolchok J.D., Nishikawa H. Selective inhibition of low-affinity memory CD8(+) T cells by corticosteroids. J. Exp. Med. 2019;216:2701–2713. doi: 10.1084/jem.20190738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aston W.J., Hope D.E., Cook A.M., Boon L., Dick I., Nowak A.K., Lake R.A., Lesterhuis W.J. Dexamethasone differentially depletes tumour and peripheral blood lymphocytes and can impact the efficacy of chemotherapy/checkpoint blockade combination treatment. Oncoimmunology. 2019;8 doi: 10.1080/2162402X.2019.1641390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spolski R., Li P., Leonard W.J. Biology and regulation of IL-2: from molecular mechanisms to human therapy. Nat. Rev. Immunol. 2018;18:648–659. doi: 10.1038/s41577-018-0046-y. [DOI] [PubMed] [Google Scholar]
- 38.Sultan H., Kumai T., Fesenkova V.I., Fan A.E., Wu J., Cho H.I., Kobayashi H., Harabuchi Y., Celis E. Sustained persistence of IL2 signaling enhances the antitumor effect of peptide vaccines through T-cell expansion and preventing PD-1 inhibition. Cancer Immunol. Res. 2018;6:617–627. doi: 10.1158/2326-6066.CIR-17-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verdeil G., Marquardt K., Surh C.D., Sherman L.A. Adjuvants targeting innate and adaptive immunity synergize to enhance tumor immunotherapy. Proc. Natl. Acad. Sci. U. S. A. 2008;105:16683–16688. doi: 10.1073/pnas.0805054105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drerup J.M., Deng Y., Pandeswara S.L., Padron A.S., Reyes R.M., Zhang X., Mendez J., Liu A., Clark C.A., Chen W., Conejo-Garcia J.R., Hurez V., Gupta H., Curiel T.J. CD122-selective IL2 complexes reduce immunosuppression, promote treg fragility, and sensitize tumor response to PD-L1 blockade. Cancer Res. 2020;80:5063–5075. doi: 10.1158/0008-5472.CAN-20-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reyes R.M., Deng Y., Zhang D., Ji N., Mukherjee N., Wheeler K., Gupta H.B., Padron A.S., Kancharla A., Zhang C., Garcia M., Kornepati A.V.R., Boyman O., Conejo-Garcia J.R., Svatek R.S., Curiel T.J. CD122-directed interleukin-2 treatment mechanisms in bladder cancer differ from alphaPD-L1 and include tissue-selective gammadelta T cell activation. J. Immunother. Cancer. 2021;9:e002051. doi: 10.1136/jitc-2020-002051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bashir Q., Acosta M. Comparative safety, bioavailability, and pharmacokinetics of oral dexamethasone, 4-mg and 20-mg tablets, in healthy volunteers under fasting and fed conditions: a randomized open-label, 3-way crossover study. Clin. Lymphoma Myeloma Leuk. 2020;20:768–773. doi: 10.1016/j.clml.2020.06.022. [DOI] [PubMed] [Google Scholar]
- 43.Al Shoyaib A., Archie S.R., Karamyan V.T. Intraperitoneal route of drug administration: should it be used in experimental animal studies? Pharm. Res. 2019;37:12. doi: 10.1007/s11095-019-2745-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.