Abstract
Context: Continuous glucose monitoring (CGM) provides nuanced information on glucose patterns, but data in very old adults are scarce.
Objective: To evaluate CGM patterns in very old adults.
Design: Pilot study.
Setting: Participants recruited from one center during visit 7 (2019) of the community-based Atherosclerosis Risk in Communities (ARIC) Study.
Participants: We enrolled 27 adults (8 with type 2 diabetes and 19 without diabetes) who wore a CGM sensor (Abbott Libre Pro) for up to 14 days. Clinical and laboratory measures, including hemoglobin A1c (HbA1c), were obtained.
Main Outcomes: Mean CGM glucose, standard deviation (SD), coefficient of variation (CV), time-in-range (TIR) 70–180 mg/dL, and hypoglycemia.
Results: Mean age was 81 (range 77–91 years) and mean CGM wear time was 13.2 days. In persons without diabetes, there was a wide range of CGM parameters: range of mean glucose, 83.7–124.5 mg/dL, SD 12.2–27.3 mg/dL, CV 14.0%–26.7%, and TIR 71.1%–99.5%. In persons with diabetes, the range of mean CGM glucose was 105.5–223.0 mg/dL, SD, 22.3–86.6 mg/dL, CV 18.2%–38.8%, TIR 38.7%–98.3%. The Pearson's correlation of mean glucose with HbA1c was high overall (0.90); but, for some participants with similar HbA1c, glucose patterns differed substantially. There was a high prevalence of hypoglycemia (glucose <70 or <54 mg/dL) in both persons with and without diabetes.
Conclusions: There was high feasibility and acceptability of CGM in very old adults. Low readings on CGM are common, even in nondiabetic older adults; the clinical relevance of these low values is unclear. CGM may provide complementary information to HbA1c in some older adults.
Keywords: Very old adults, Elderly, Glycemic variability, Continuous glucose monitoring, Biomarkers, The ARIC study, HbA1c
Introduction
Continuous glucose monitoring (CGM) technology is the recommended approach to assessing biochemical hypoglycemia and glycemic variability.1 The accuracy of CGM systems has improved substantially over the past decade and their use in clinical practice is increasing, typically in populations with type 1 diabetes. The 2021 American Diabetes Association Standards of Medical Care for Diabetes now recommends CGM as useful for patients on multiple daily injections of insulin and other insulin therapy, regardless of diabetes type or age.2
The latest generation of CGM devices are easy to use and have revolutionized diabetes management for many patients. Nonetheless, CGM is underutilized as a research tool and has rarely been studied in persons without diabetes or in older adults. Indeed, there is no consensus on what constitutes “normal” glucose patterns in older adults as studies of glycemic variability have typically excluded older persons. There is a lack of data on the burden of low, high, and variable glucose patterns in older adults with and without diabetes.
Hemoglobin A1c (HbA1c) is the standard measure used to monitor glycemic control and guide diabetes management. Prior studies have established the robust association of HbA1c with CGM-defined mean glucose and other CGM parameters.1,3–5 There is growing interest in other laboratory measures of glycemic control, including fructosamine, glycated albumin, and 1,5-anhydroglucitol (1,5-AG). However, few studies have examined the comparative associations of these biomarkers with HbA1c, mean glucose, and other CGM parameters.
We undertook a pilot study to characterize glucose patterns and evaluate the acceptability and feasibility of conducting CGM in a community-based population of very old adults (ages 77–91 years) with and without diabetes. We also conducted laboratory measurements of HbA1c, fructosamine, glycated albumin, and 1,5-AG, and related them to CGM parameters in this pilot study.
Methods
Study population
The Atherosclerosis Risk in Communities (ARIC) Study is an ongoing community-based prospective epidemiology study of 15,792 participants (∼25% black) who were initially recruited in 1987–1989 when the participants were middle aged. For >30 years, ARIC investigators have conducted detailed assessments of clinical and subclinical cardiovascular disease and its risk factors, including diabetes. Participants are seen at four community-based field centers.
This study was a pilot study and was conducted during the final 2 months (October and November 2019) of ARIC visit 7 at the Washington County (Johns Hopkins) Field Center. At the time of this visit, all ARIC participants were aged ≥77 years. This CGM pilot study involved a separate consent and all participants who attended the last 2 months of visit 7 at the Washington County Field Center were invited to participate. Of these, 61% agreed. All CGM devices were returned, but one device did not record any data. We received valid CGM data (ranging from 7 to 14 days) from a total of 27 of participants, 8 with history of type 2 diabetes, and 19 without a history of diabetes.
Study protocols were approved by the institutional review board at the Johns Hopkins Bloomberg School of Public Health, and all participants provided written informed consent.
Continuous glucose monitoring
We used the FreeStyle Libre Pro (Abbott Diabetes Care) CGM system to measure glucose in up to 14 days in all participants who consented to participate in the pilot study protocol. The Pro system is factory calibrated (no finger stick), records interstitial glucose every 15 min, and stores the 14 days of data (participants are masked to the glucose readings). The devices were placed by a technician on the upper arm on participants during the clinic visit. Participants were provided with a prepaid mailer to return the sensor. They also had the option of returning to the clinic for removal.
Other measurements
All other measurements were obtained using standardized protocols as part of the main ARIC Study. Participants provided fasting blood samples and laboratory measurements of HbA1c, fasting glucose, glycated albumin, fructosamine, and 1,5-AG were obtained.
Glucose was measured in serum using the hexokinase method (Roche Diagnostics, Indianapolis, IN). HbA1c was measured in EDTA whole blood on the Tosoh G7 HPLC Glycohemoglobin Analyzer (Tosoh Medics, Inc., San Francisco CA). Fructosamine (Roche Diagnostics), glycated albumin (Asahi Kasei Pharma Corp, Tokyo, Japan), and 1,5-AG (GlycoMark, Inc., New York, NY) were measured in serum on the Roche Cobas 6000.
Statistical analyses
We evaluated characteristics of the pilot study participants, laboratory biomarkers of hyperglycemia, and CGM parameters according to a diagnosed diabetes status.
From the CGM data, we calculated the mean glucose (average of all available glucose measurements) and the corresponding standard deviation (SD). We also calculated the coefficient of variation (CV) and the interquartile range (75th percentile minus the 25th percentile). The percent time in the range of 70–180 mg/dL was calculated for each participant along with the percent time above or below the prespecified thresholds of 54, 70, 180, and 200 mg/dL. We calculated mean amplitude of glycemic excursions (MAGE), the mean of upward and downward glucose excursions exceeding the SD for the individual during the wear period, and the mean of daily difference (MODD).1,3,6
We also evaluated definitions of biochemical hypoglycemia, defined as glucose concentrations <70 mg/dL (Level 1) or <54 mg/dL (Level 2) for >15 min (i.e., at least two consecutive readings).1,7 We also conducted sensitivity analyses excluding values during the first 24 h of the CGM sensor wear period, the warm-up time.
For each participant, we generated a 14-day profile of CGM glucose plotted by time to visualize differences in glucose patterns. We also generated 24-h “Ambulatory Glucose Profiles” based on standard methods (aggregated data for each person over a 24-h window with Tukey smoothing and plotting of the 5th, 25th, 50th, 75th, and 95th percentiles). We generated scatterplots with corresponding regression lines (overall and by diabetes status), root mean squared errors (RMSE), and Pearson's correlations of CGM mean glucose and HbA1c, fasting glucose, fructosamine, glycated albumin, and 1,5-AG.
Results
Our study population was 100% white, 30% (n = 8) had a history of type 2 diabetes, and the mean age was 81 years (range 77–91 years). Participants with diagnosed diabetes had higher body mass index and a higher burden of chronic disease and mild cognitive impairment (Table 1). Prediabetes was highly prevalent among the older adults without a history of diabetes when defined by fasting glucose 100–125 mg/dL (36.8%, n = 7) or HbA1c 5.7%–6.4% (26.3%, n = 5).
Table 1.
Characteristics of Atherosclerosis Risk in Communities-Continuous Glucose Monitoring (CGM) Pilot Study Participants and CGM Parameters According to Diagnosed Diabetes Status, 2017
| No diabetes (n = 19) | Diagnosed diabetes (n = 8) | |
|---|---|---|
| Age in years, mean (SD) | 80.9 (4.3) | 82.0 (4.0) |
| Female | 58% | 63% |
| Body mass index, kg/m2, mean (SD) | 25.8 (4.3) | 32.4 (4.0) |
| Prevalent cardiovascular disease | 26% | 38% |
| Prevalent chronic kidney disease | 47% | 63% |
| Cognitive status | ||
| Normal | 94% | 88% |
| Mild cognitive impairment | 11% | 13% |
| Fasting glucose, mg/dL, mean (SD) | 96.7 (7.1) | 121.6 (11.8) |
| HbA1c, %-points, mean (SD) | 5.6 (0.3) | 7.0 (0.8) |
| Fructosamine, μmol/L, mean (SD) | 235.5 (15.6) | 275.5 (21.1) |
| Glycated albumin, %-points, mean (SD) | 13.4 (1.3) | 16.5 (1.8) |
| 1,5-AG, μg/mL, mean (SD) | 13.8 (6.3) | 12.9 (8.0) |
| Duration of diabetes in years, median (p25, p75) | — | 6.9 (4.4, 8.1) |
| Glucose lowering medication use | — | |
| None | — | 33% |
| Sulfonylurea or any insulin | — | 50% |
| Other oral medication | — | 17% |
| CGM parameters | ||
| Mean CGM wear time, days (min, max) | 13.2 (7, 14) | 13.3 (8, 14) |
| Glucose, mg/dL, mean (SD) | 99.4 (10.0) | 145.4 (36.9) |
| SD, mg/dL, mean (p25, p75) | 20.0 (18.0, 22.4) | 37.8 (27.9, 53.3) |
| CV, %, mean (SD) | 20 (3.3) | 29 (7.6) |
| IQR, mg/dL, median (p25, p75) | 25.0 (21.0, 28.0) | 54.0 (36.0, 71.5) |
| % of time glucose ≥200 mg/dL, median (p25, p75) | 0 | 5.7 (1.9, 20.7) |
| % of time glucose ≥180 mg/dL, median (p25, p75) | 0.2 (0.0, 0.5) | 13.6 (5.1, 30.9) |
| % of time glucose ≥140 mg/dL, median (p25, p75) | 4.7 (2.1, 8.1) | 45.6 (25.2, 60.8) |
| % of time glucose 70–180 mg/dL, median (p25, p75) | 96.8 (94.5, 99.0) | 84.0 (67.3, 92.4) |
| % of time glucose 70–140 mg/dL, median (p25, p75) | 92.3 (87.7, 95.4) | 52.5 (38.7, 72.7) |
| % of time glucose <70 mg/dL, median (p25, p75) | 2.2 (0.6, 5.1) | 1.1 (0.1, 3.2) |
| % of time glucose <54 mg/dL, median (p25, p75) | 0.2 (0.0, 0.8) | 0.1 (0.0, 0.9) |
| MAGE, mg/dL, median (p25, p75) | 33.3 (25.6, 37.3) | 71.2 (51.0, 86.8) |
| MODD, mg/dL, median (p25, p75) | 15.7 (13.9, 18.4) | 34.7 (23.7, 49.3) |
| Hypoglycemic eventsa | ||
| No. of participants with hypoglycemia events, n (%) | ||
| Level 1: glucose <70 mg/dL | 17 (89) | 6 (75) |
| Level 2: glucose <54 mg/dL | 10 (53) | 4 (50) |
| No. of hypoglycemic events, median (min, max) | ||
| Level 1: glucose <70 mg/dL | 4.0 (0, 42) | 3.0 (0, 13) |
| Level 2: glucose <54 mg/dL | 1.0 (0, 10) | 0.5 (0, 5) |
Hypoglycemia level 1: glucose <70 mg/dL for >15 min (at least two consecutive readings). Hypoglycemia level 2: glucose <54 mg/dL for >15 min (at least two consecutive readings).
1,5-AG, 1,5-anhydroglucitol; CGM, continuous glucose monitoring; CV, coefficient of variation; HbA1c, hemoglobin A1c; IQR, interquartile range; MAGE, mean amplitude of glycemic excursion; MODD, mean of daily difference; SD, standard deviation.
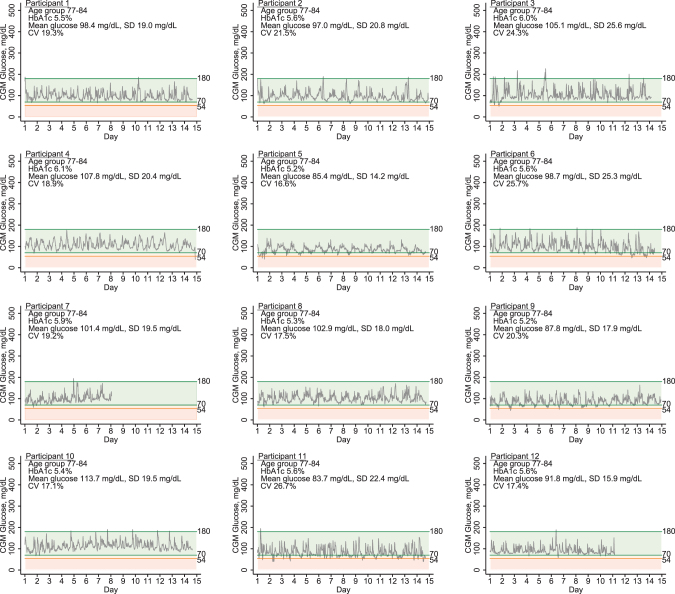
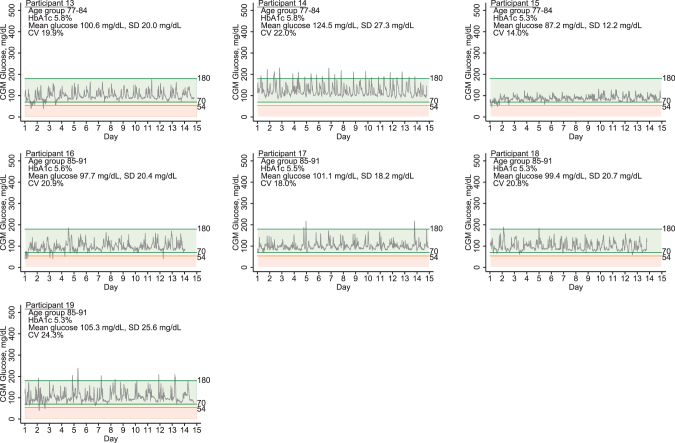
The mean wear time was 13.2 days (range 7–14) and was similar in persons with (13.3 [range 8–14] days) vs. without (13.2 [range 7–14] days) diabetes. There were no skin reactions or other adverse reactions related to placement or removal of the sensor. CGM parameters differed substantially between these older adult participants with and without diabetes (Table 1). Participants with diabetes had substantially higher mean glucose and more glucose variability. However, even among persons without diabetes, there was a range of CGM glucose parameters: mean CGM glucose was 99.4 mg/dL and ranged from 83.7 to 124.5 mg/dL; the mean CV was 20% and ranged from 14.0% to 26.7% (Fig. 1).
FIG. 1.
Daily glucose patterns of pilot study participants without diabetes.
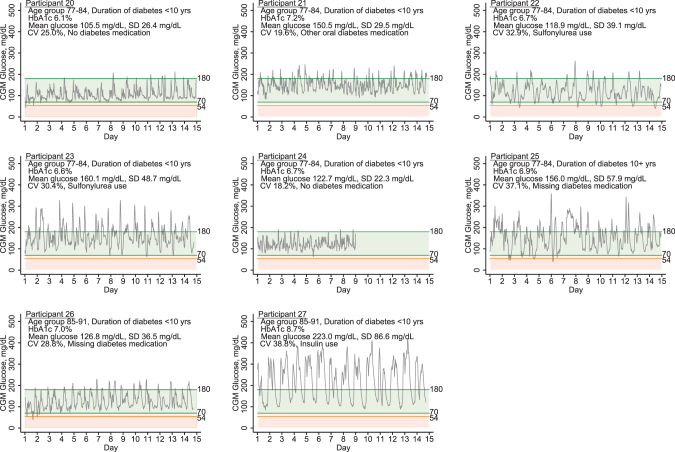
Among the eight study participants with diabetes, the mean CGM glucose was 145.4 mg/dL and ranged from individual means of 105.5–223.0 mg/dL (Fig. 2). The mean CV was 29% and ranged from 18.2% to 38.8% in these older adults with diabetes. The highest mean CGM glucose, CV, and HbA1c were observed in an 88-year-old participant who was currently taking insulin and oral diabetes medications (duration of diabetes = 8 years), mean CGM glucose 223.0 mg/dL, CV of 38.8%, and HbA1c of 8.7% (Participant 27, Fig. 2). Corresponding Ambulatory Glucose Profiles are provided in the Supplementary Figs. S1 and S2.
FIG. 2.
Daily glucose patterns of pilot study participants with diagnosed type 2 diabetes.
There was a high prevalence of CGM-defined hypoglycemia in both participants with and those without diabetes. Among participants without diabetes, there were 17 participants (89%) who experienced at least one biochemical hypoglycemic event (two consecutive CGM glucose values <70 mg/dL, Level 1) (Table 1) and 10 (53%) who experienced at least one episode of two or more consecutive glucose values <54 mg/dL (Level 2). Among the participants with diabetes, six persons (75%) had a hypoglycemic event meeting Level 1 criteria and four participants (50%) had events meeting Level 2 criteria. Nonetheless, the numbers of hypoglycemic events ranged substantially among persons without diabetes: median of 4.0 events (range 0–42) for Level 1 and median of 1.0 event (range 0–10) for Level 2. Among persons with diabetes, the median number of events for Level 1 was 3.0 (range 0–13) and 0.5 (range 0–5) for Level 2. The numbers of hypoglycemic events decreased somewhat after excluding measurements obtained during the first 24 h of wear (Supplementary Table S1).
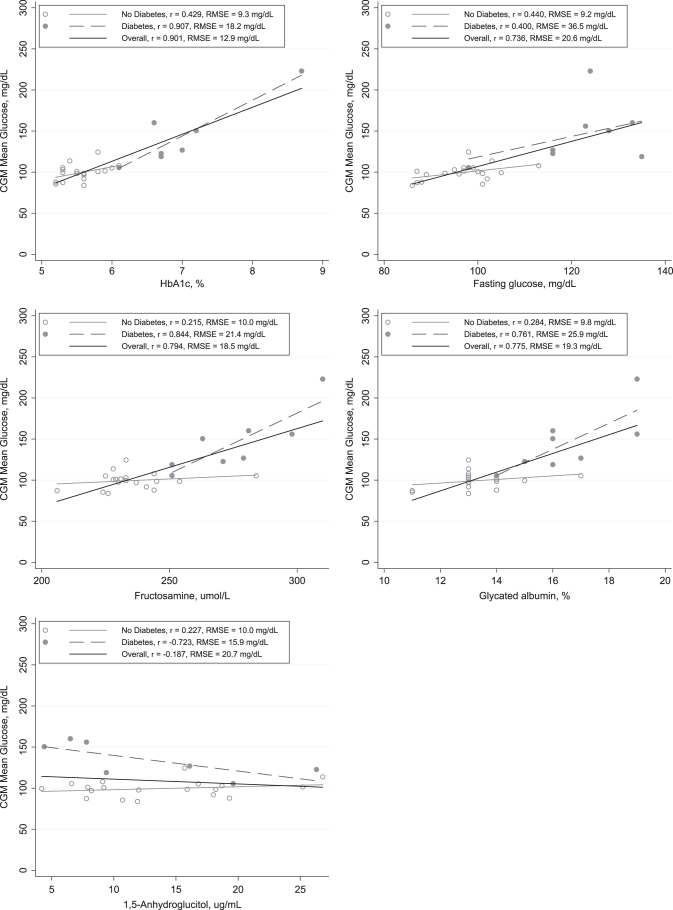
There was a strong positive correlation between mean CGM glucose and HbA1c (Pearson's correlation, 0.90), although this was predominately driven by persons with diabetes (Person's correlation, 0.91) (Table 2 and Fig. 3). Mean CGM glucose was also strongly positively associated with fructosamine and glycated albumin, with more moderate correlations observed for laboratory fasting glucose. 1,5-AG was inversely correlated with CGM mean glucose in persons with diabetes (Pearson's correlation, −0.72) but not in those without diabetes. The RSMEs also demonstrate the higher variability of mean CGM glucose for any given laboratory value (fasting glucose, HbA1c, fructosamine, glycated albumin, or 1,5-AG) in persons with diabetes.
Table 2.
Correlations of Mean Glucose with Hemoglobin A1c, Fasting Glucose, Fructosamine, Glycated Albumin and 1,5-Anhydroglucitol, Overall and by Diabetes Status
| Overall (n = 27a) | No diabetes (n = 19) | Diagnosed diabetes (n = 8b) | |
|---|---|---|---|
| HbA1c, % | 0.90 | 0.43 | 0.91 |
| Fasting glucose, mg/dL | 0.74 | 0.44 | 0.40 |
| Fructosamine, μmol/L | 0.79 | 0.22 | 0.84 |
| Glycated albumin, % | 0.78 | 0.28 | 0.76 |
| 1,5-AG, μg/mL | −0.19 | 0.23 | −0.72 |
n = 26 for HbA1c and 1,5-AG.
n = 7 for HbA1c and 1,5-AG.
1,5-AG, 1,5-anhydroglucitol; HbA1c, hemoglobin A1c.
FIG. 3.
Scatterplots of mean glucose with HbA1c, fasting glucose, fructosamine, glycated albumin, and 1,5-AG. Lines are from linear regression models in the overall study population (solid black line) and in persons with diabetes (dotted line) and without diabetes (solid gray line). 1,5-AG, 1,5-anhydroglucitol; HbA1c, hemoglobin A1c.
Discussion
Data on glucose patterns in older adults are scarce. Our study provides some of the first data documenting glucose patterns in much older adults (aged 77 years and older) in a community-based population, primarily adults without diabetes. There was a range of CGM-defined glucose patterns in this very old adult population, with a range of values even among those without diabetes. Mean CGM glucose was strongly correlated with HbA1c in the overall population, but this was primarily driven by participants with diabetes. Similar, although somewhat more modest correlations, were observed for CGM mean glucose with fructosamine and glycated albumin.
Time-in-range (TIR) is a measure of glycemic control with growing evidence for clinical utility in the setting of type 1 diabetes.3 In persons with type 2 diabetes, the value and interpretation of TIR is less clear.8 The CV is a primary measure of glycemic variability in diabetes care. In our study, there were two participants with diabetes (25%) who had CVs exceeding the clinically recommended threshold of 36%, typically considered the threshold for intervention.3 In persons without diabetes, CVs were lower but ranged from 14.0% to 26.7%. In adults with diabetes, there is controversy whether glycemic variability contributes to complications above and beyond measures of average glucose.9–11 Studies are needed to understand whether glycemic instability in older adults in the presence of—and possibly even in the absence of—diabetes is related to health outcomes.
In our study, the correlation of CGM mean glucose and HbA1c was high, although and partly driven by the high leverage of one participant with both very high mean CGM glucose and HbA1c values (Participant 27). This individual was on insulin and also had the highest observed measures of glucose variability (SD, 86.6 mg/dL; CV, 38.8%). Even in our small sample, it is interesting to observe the diversity of glucose patterns even at similar HbA1c values (e.g., Participants 22 and 23). Our results suggest that CGM in older adults with type 2 diabetes can provide complementary information to HbA1c.
The ability of the body to mount an adaptive response to hypoglycemia decreases with age,12 putting older adults at higher risk for hypoglycemic episodes. Symptomatic nondiabetic hypoglycemia is thought to be rare,13 but there is preliminary evidence that hypoglycemia in older adults is more common than previously recognized.7 A 2019 study by Shah et al. conducted in a population of 153 nondiabetic children and adults (aged 7–80 years) with up to 10 days of CGM data using an early generation Dexcom G6 CGM Pro system. The authors found that nondiabetic adults aged 60–80 years had significantly different glucose patterns compared with younger individuals, with higher mean CGM glucose and lower time spent in range. They reported a 31% prevalence of hypoglycemia (defined as sustained glucose <54 mg/dL for at least 15 min during the 10-day period) in the study participants aged 60–70 years of age.7 The accuracy of CGM devices is lower at low glucose concentrations.14,15 The FreeStyle Libre sensor has previously been shown to overestimate the degree of hypoglycemia compared with venous glucose.16,17 Our study demonstrates that low glucose values are likely to be observed when CGM systems are used in older adults without diabetes. The clinical relevance of these low glucose values is not clear. Our results extend these prior findings to a different population of nondiabetic adults aged 77–91 years and demonstrate high rates of CGM-detected hypoglycemic values using the Abbott sensor.
Recruitment for our study and acceptability of wearing the CGM device was high in this older population. Participants were recruited from a single field center during the last 2 months of visit 7 of the ARIC Study. Our study population was disproportionately made up of more reticent participants, including those who rescheduled, were difficult to schedule, and/or who delayed attending the clinic examination.
The major limitation of this study was the very small sample size; this was a pilot study designed to generate information on the feasibility and acceptability of the study protocols. Owing to the small sample size, our study is particularly sensitive to influential values. We also did not have information on symptomology or capillary blood glucose during the 2-week CGM wear period in our study, which could help with the interpretation of the low glucose values. Such information may be particularly important among individuals without diabetes, as it is unclear whether low CGM-based glucose values warrant intervention.
In conclusion, our results demonstrate the feasibility of conducting CGM in older adults with and without diabetes and is confirmatory of prior studies demonstrating robust associations of CGM mean glucose with HbA1c, fructosamine, and glycated albumin.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
Author Disclosure Statement
E.S. receives payments from Wolters Kluwer for chapters in UpToDate on measurements of glycemic control and screening tests for type 2 diabetes.
Funding Information
The ARIC study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under contract nos. HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, and HHSN268201700004I. E.S. was supported by NIH/NHLBI grant K24 HL152440 and NIH/NIDDK grant R01DK089174. J.B.E.T. was supported by NIH/NHLBI K23 HL153774. O.T. was supported by NIH/NIDDK grant F30 DK120160.
Supplementary Material
References
- 1. Danne T, Nimri R, Battelino T, et al. : International consensus on use of continuous glucose monitoring. Diabetes Care 2017;40:1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. 7. Diabetes technology: standards of medical care in diabetes—2021. Diabetes Care 2021;44:S85–S99. [DOI] [PubMed] [Google Scholar]
- 3. Battelino T, Danne T, Bergenstal RM, et al. : Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 2019:dci190028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vigersky RA, McMahon C: The relationship of hemoglobin a1c to time-in-range in patients with diabetes. Diabetes Technol Ther 2019;21:81–85. [DOI] [PubMed] [Google Scholar]
- 5. Beck RW, Bergenstal RM, Cheng P, et al. : The relationships between time in range, hyperglycemia metrics, and hba1c. J Diabetes Sci Technol 2019;13:614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rawlings RA, Shi H, Yuan L-H, et al. : Translating glucose variability metrics into the clinic via continuous glucose monitoring: a graphical user interface for diabetes evaluation (cgm-guide©). Diabetes Technol Ther 2011;13:1241–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shah VN, DuBose SN, Li Z, et al. : Continuous glucose monitoring profiles in healthy nondiabetic participants: a multicenter prospective study. J Clin Endocrinol Metab 2019;104:4356–4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Selvin E: The prognostic value of time in range in type 2 diabetes. Diabetes Care 2021;44:319–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ceriello A, Monnier L, Owens D: Glycaemic variability in diabetes: clinical and therapeutic implications. Lancet Diabetes Endocrinol 2019;7:221–230. [DOI] [PubMed] [Google Scholar]
- 10. Cavalot F: Do data in the literature indicate that glycaemic variability is a clinical problem? Glycaemic variability and vascular complications of diabetes. Diabetes Obes Metab 2013;15(Suppl. 2):3–8. [DOI] [PubMed] [Google Scholar]
- 11. Suh S, Kim JH: Glycemic variability: how do we measure it and why is it important? Diabetes Metab J 2015;39:273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pomatto LCD, Davies KJA: The role of declining adaptive homeostasis in ageing. J Physiol 2017;595:7275–7309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Desimone ME, Weinstock RS: Non-diabetic hypoglycemia. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc., 2000. [Google Scholar]
- 14. Freckmann G, Pleus S, Grady M, et al. : Measures of accuracy for continuous glucose monitoring and blood glucose monitoring devices. J Diabetes Sci Technol 2019;13:575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moser O, Pandis M, Aberer F, et al. : A head-to-head comparison of personal and professional continuous glucose monitoring systems in people with type 1 diabetes: hypoglycaemia remains the weak spot. Diabetes Obes Metab 2019;21:1043–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moser O, Eckstein ML, McCarthy O, et al. : Performance of the freestyle libre flash glucose monitoring (flash gm) system in individuals with type 1 diabetes: a secondary outcome analysis of a randomized crossover trial. Diabetes Obes Metab 2019;21:2505–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abbott Diabetes Care, Inc.: FDA summary of safety and effectiveness data—sensor, glucose, invasive, non-adjunctive, factory-calibrated, user-initiated, freestyle libre 14 day flash glucose monitoring system. https://www.accessdata.fda.gov/cdrh_docs/pdf16/P160030S017B.pdf (accessed July 23, 2018).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.