Abstract
This narrative review represents an output from the International Association for the Study of Pain’s global task force on the use of cannabis, cannabinoids, and cannabis-based medicines (CBM) for pain management, informed by our companion systematic review and meta-analysis of preclinical studies in this area. Our aims in this review are: 1) to describe the value of studying cannabinoids and endogenous cannabinoid (endocannabinoid) system modulators in preclinical/animal models of pain; 2) to discuss both pain-related efficacy and additional pain-relevant effects (adverse and beneficial) of cannabinoids and endocannabinoid system modulators as they pertain to animal models of pathological or injury-related persistent pain; and 3) to identify important directions for future research. In service of these goals, this review a) provides an overview of the endocannabinoid system and the pharmacology of cannabinoids and endocannabinoid system modulators, with specific relevance to animal models of pathological or injury-related persistent pain; b) describes pharmacokinetics of cannabinoids in rodents and humans; and c) highlights differences and discrepancies between preclinical and clinical studies in this area. Preclinical (rodent) models have advanced our understanding of the underlying sites and mechanisms of action of cannabinoids and the endocannabinoid system in suppressing nociceptive signaling and behaviors. We conclude that substantial evidence from animal models supports the contention that cannabinoids and endocannabinoid system modulators hold considerable promise for analgesic drug development, although the challenge of translating this knowledge into clinically useful medicines is not to be underestimated.
Keywords: Cannabinoid1 (CB1) receptor, Cannabinoid2 (CB2) receptor, Endocannabinoid, Chronic Pain, Neuropathic Pain, Inflammatory Pain, Nociception, rats, mice, behavior
Introduction
Cannabis, cannabis extracts or oils, individual cannabinoid substances, and modulators of the endogenous cannabinoid (endocannabinoid) system have all been suggested as therapeutic agents for pain management75,94,193. The primary drivers for interest in these agents include: 1) major scientific advances in our understanding of the biology of the endocannabinoid system and pharmacology of cannabinoids; 2) development and regulatory approval of cannabis-based (or cannabis-derived) medicines (e.g. Nabiximols/Sativex® and Epidiolex®); and 3) regulatory changes that permit use of cannabis for medical purposes, including pain management, following advocacy by patients and public support. However, the latter development has proven controversial amongst scientists, patients and healthcare professionals, with uncertainty over whether the current evidence base for efficacy and safety justifies change in legislation of cannabis for medical use, including pain management. In 2018, the International Association for Study of Pain (IASP) established a Presidential Task Force on Cannabis and Cannabinoids Analgesia. The present narrative review represents an output from Work Package (WP) 1 of the IASP task force which was focused on basic science, including definition of terminology, overview of the endogenous cannabinoid (endocannabinoid) system, compound classification, pharmacology and assessment of pain-related efficacy and additional pain-relevant effects (adverse and beneficial) in preclinical laboratory animal studies.
The aims of this review are:
To provide commentary on the efficacy and side effects of cannabinoids and endocannabinoid system modulators as they pertain to animal models of pathological paina, informed by our companion systematic review and meta-analysis of preclinical studies in this area215.
To discuss the value of studying cannabinoids and endocannabinoid system modulators in preclinical/animal models of pain, as well as discrepancies between preclinical and clinical studies in this area, and
Provide suggestions for future research directions.
In service of the above objectives, we:
Clearly define terminology used in this field.
Provide an overview of the endocannabinoid system.
Review the classification, chemistry, and pharmacology of cannabinoids, CBM and endocannabinoid system modulators, including structure-activity relationships and pharmacokinetics in rodents and humans.
Terminology and Definitions
One factor that has hampered public debate (and sometimes scientific/medical debate) on the topic of cannabis and cannabinoids for pain management is the inappropriate, inconsistent or unclear use of terminology. For example the terms ‘cannabis’, ‘cannabinoids’ and ‘cannabis-based medicines (CBM)’ are often used interchangeably or conflated, both within public discourse and the media, and within the scientific literature (e.g. pooling of data relating to cannabis and individual cannabinoids in meta-analyses and systematic reviews of clinical efficacy). Cannabis refers to the whole plant, or to its parts, and must be clearly distinguished from cannabinoid ligands (cannabinoids) that are either plant-derived natural (phytocannabinoids), synthetic or semi-synthetic, but always chemically defined, single entity compounds usually having affinity for and activity at cannabinoid receptors. Thus, cannabis, single entity cannabinoid compounds, and modulators of the endocannabinoid system should not be used synonymously or conflated within the same preclinical or clinical investigation for either efficacy or side effects. Furthermore, when considering or debating the merits, or otherwise, of cannabis, due regard must be given to the fact that cannabis is highly heterogeneous with many different chemical constituents and that a multitude of different strains of the plant exist, all containing different amounts of phytocannabinoids12,103, most particularly Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). THC, first isolated in the 1960s, is the primary psychoactive constituent of Cannabis sativa83,162. THC has psychoactive properties, and its pharmacological effects are attributable to agonist activity at both cannabinoid type 1 (CB1) and type 2 (CB2) receptors44,61,148,234. CBD, in contrast, does not have appreciable agonist activity at CB1 receptors (but may be a negative allosteric modulator of CB1), and lacks the psychoactivity profile of THC22,68,222. In addition to phytocannabinoids, the cannabis plant also contains a large number of terpenes, flavonoids and other compounds, which, themselves, may be pharmacologically active but remain understudied.
CBMs are registered medicinal cannabis extracts, approved by regulatory authorities, with well-defined, standardized THC/CBD content, and with no, or only trace levels of, terpenes, flavonoids and other compounds. Examples of CBMs are nabiximols (Sativex®), approved in some countries for adjunctive treatment of spasticity (and in some jurisdictions neuropathic pain) in multiple sclerosis, and cannabidiol extract (Epidiolex®), indicated for treatment of childhood epilepsy, that has only minor or trace levels of other phytocannabinoids. These medicinal products with regulatory approval should be distinguished from so-called cannabis or CBD oils or extracts which are numerous, typically sold in healthfood stores, pharmacies, cannabis dispensaries or over the internet, but often have uncertain and/or unverified THC/CBD content. Synthetic THC (e.g. dronabinol), and the synthetic THC analogue nabilone are also approved for indications such as anorexia and weight loss in AIDS, and chemotherapy-induced nausea and vomiting, and available on special prescription in many countries. Table 1 provides a glossary of terminology and definitions.
Table 1.
Terminology and Definitions (Adapted from Soliman et al., 2019, after modification from Hauser et al. 2018).
| Term | Definition | Examples/typical products |
|---|---|---|
| (Herbal) Cannabis | The whole plant or parts or material from the plant (e.g. flowers, buds, resin, leaves) | Cannabis sativa, hashish |
| Medical or medicinal cannabis | The term ‘medical/medicinal cannabis’ (or ‘medical/medicinal marijuana’) is used for cannabis plants, plant material, or full plant extracts used for medical purposes. | Bedrocan®, Bedrobinol®, Tilray 10THC/10CBD® |
| Cannabis-based (or cannabis-derived) medicines | Medicinal cannabis extracts with regulatory approval for marketing as a therapeutic with defined and standardized THC and/or CBD content. | Nabiximols (Sativex®), dronabinol (Marinol®), Epidiolex® |
| Cannabinoids | Cannabinoids are biologically active constituents of cannabis, or synthetic compounds, usually having affinity for and activity at cannabinoid receptors. | THC, CBD, CP55,940, WIN55,212-2, HU210, nabilone |
| Phytocannabinoid | A cannabinoid found in cannabis plants or purified/extracted from plant material | THC, CBD |
| Endocannabinoid | An endogenous ligand found in the body of humans and other animals and which has affinity for, and activity at, cannabinoid receptors | Anandamide, 2-AG |
| Modulators that decrease endocannabinoid system activity | Directly block cannabinoid receptors or reduce signalling indirectly via impeding action of endogenous ligand through actions at a distinct site | Cannabinoid receptor antagonists (rimonabant [SR141716A], AM251, SR144528, AM630), negative allosteric modulators (PSNCBAM-1), DAGL inhibitors (RHC80267) |
| Modulators that increase or enhance endocannabinoid system activity | In addition to individual phytocannabinoids, cannabis-derived or cannabis-based medicines, and cannabis extracts, other pharmacological approaches under development for manipulation of the endocannabinoid system include selective synthetic cannabinoid receptor agonists, inhibitors of the catabolism (e.g. fatty acid amide hydrolase [FAAH] inhibitors), transport (e.g. fatty acid binding protein [FABP] inhibitors) or reuptake of endocannabinoids, or positive allosteric modulators of cannabinoid receptor signalling. | FAAH inhibitors (PF-04457845, URB597, URB937), Anandamide transport inhibitors (AM404, VDM11), MGL inhibitors (URB602, JZL184, MJN110), Positive allosteric modulators of the CB1 receptor (ZCZ011, GAT211) |
CBD: cannabidiol; DAGL: Diacylglycerol lipase; FABP: fatty acid binding protein; THC: Δ9-tetrahydrocannabinol; 2-AG: 2-arachidonoyl glycerol; MGL: monoacylglycerol lipase.
The Endocannabinoid System
The discovery of THC in the 1960s as the psychoactive constituent of Cannabis inspired research into its pharmacology and mechanism of action. However, it was not until the late 1980s/early 1990s that the cannabinoid receptors were discovered (Figure 1). The first cannabinoid receptor to be discovered, cloned and characterised was the CB1 receptor, initially in rat brain57,159 and subsequently localized in human brain88. In 1993, a second cannabinoid receptor, CB2, was cloned and characterized in a human promyelocytic leukaemic cell line and in rat spleen167. Both CB1 and CB2 belong to the super-family of seven-transmembrane domain, G protein-coupled receptors (Gi/o coupled). The existence of cannabinoid receptors, highly conserved across species, implied the existence of endogenous cannabis-like molecules (endocannabinoids) that bind to and modulate cannabinoid receptors.
Figure 1.
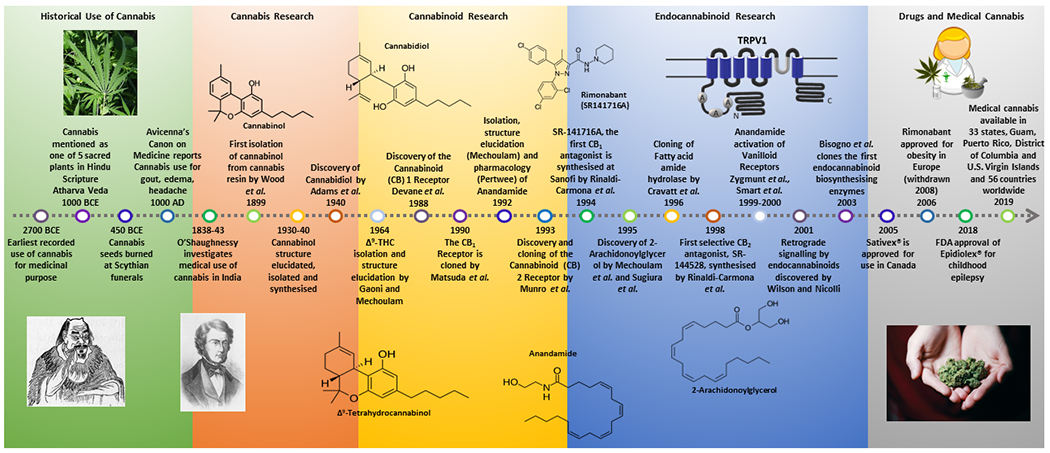
A historical timeline of key milestones in cannabis and cannabinoid research.
Research efforts sought to identify the endogenous ligands that bind to and modulate mammalian cannabinoid receptors. Two endocannabinoids, N-arachidonoylethanolamide (anandamide, AEA)58 and 2-arachidonoyl glycerol (2-AG)161,220, were discovered. The endocannabinoid system is an important physiological system, comprised of CB1 and CB2 receptors, their endogenous ligands, AEA and 2-AG, and the enzymes responsible for the synthesis and degradation of the endocannabinoids. AEA and 2-AG are the best characterised endocannabinoids. However, there are several other endogenous ligands with affinity and activity at cannabinoid receptors, including 2-AG ether (noladin ether), virodhamine, N-arachidonoyl dopamine (NADA) and others10,59,63,191,192.
CB1 is the most highly expressed G protein-coupled receptor subtype in the central nervous system (CNS)88,104,191. Within the brain, CB1 is found in high density in the basal ganglia, as well as in key components of the descending pain pathway and the stress/fear/anxiety circuitry. CB1 is localized to most other tissues and organs of the body. Of relevance to pain, in addition to their supraspinal localisation, CB1 receptors are expressed in the dorsal horn of the spinal cord, synthesized in dorsal root ganglion cells and transported in peripheral nerves21,74,108–111,178. Immunohistochemical studies have localized CB1 to dorsal root ganglia and identified CB1 receptors on primary afferent neurons21,110,111. CB2 receptors are mainly expressed in the periphery, with particularly high density on cells and tissues of the immune system11,167. Although localisation of CB2 to otherwise naïve CNS remains controversial, CB2 can be induced in the CNS in response to injury or pathophysiological states (for review see 99,134,205,240). Being Gi/o protein-coupled receptors, CB1 and CB2 are negatively coupled to adenylyl cyclase116–118, and positively coupled to mitogen-activated protein kinase18. Within neurons, CB1 activation results in inhibition of N- and P/Q-type voltage-activated Ca2+ channels, and induction of inwardly rectifying K+ currents, with consequent inhibition of neurotransmitter release55. Cannabinoids, including endocannabinoids, phytocannabinoids, and synthetic cannabinoids may potentially also act at other non-CB1/non-CB2 receptors, including the transient receptor potential cation channel subfamily V member 1 (TRPV1; also known as the capsaicin or vanilloid receptor VR1), peroxisome proliferator-activated receptors (PPARs), and G protein-coupled receptors such as GPR55 and GPR1196,23,179.
Mechanisms underlying endocannabinoid biosynthesis, signaling and degradation are quite well understood. Biosynthesis of AEA involves its formation from the precursor N-arachidonoylphosphatidylethanolamine (NAPE), catalysed by the hydrolytic activity of the phospholipase D enzyme known as NAPE-PLD (for review see13,30,31,62). 2-AG is synthesized by conversion of 1,2-diacylglycerol to 2-AG by diacylglycerol lipases (DAGL) (for review see59,135,219,227). AEA is primarily degraded to arachidonic acid and ethanolamine by the enzyme fatty acid amide hydrolase (FAAH), located in the endoplasmic reticulum of postsynaptic neuron46,59for review see86,182. FAAH also catabolizes other N-acylethanolamines including N-palmitoylethanolamide (PEA) and N-oleoylethanolamide (OEA) which themselves do not have appreciable activity at CB1 or CB2 receptors but may elevate levels of AEA through substrate competition at FAAH133. 2-AG is primarily metabolized to arachidonic acid and glycerol by the presynaptic enzyme monoacylglycerol lipase (MGL)65,227, with other enzymes including FAAH, ABHD6 and ABHD12 accounting for a modest (i.e. <10%) degree of 2-AG catabolism15,89. Within the nervous system, newly synthesized endocannabinoids leave the post-synaptic neuron to exert their effects on CB1 receptors expressed on pre-synaptic nerve terminals in a signaling process known as retrograde neurotransmission. Further work is required to better understand the mechanisms by which endocannabinoids are transported within cells and across cell membranes.
As a lipid signalling system whose components are expressed widely across the body, the endocannabinoid system plays a key role in the regulation of a wide array of physiological processes including metabolism, mood, motor function, appetite, cardiovascular control, stress response, gastrointestinal tract function, developmental biology, cell fate, immune and inflammatory response, endocrine function, neurotransmission, and pain (for review see 60,183,184). In the context of nociception and pain, key components of the endocannabinoid system are expressed throughout nociceptive pathways (Figure 2): in the periphery on primary afferent neurons, in the dorsal horn of the spinal cord and in multiple supraspinal regions of the brain associated with pain perception and modulation (for review see 96,99,196,207,217,240). As a result, targeting the endocannabinoid system via enhancement of the levels of endogenous cannabinoids (e.g. with FAAH or MGL inhibitors) or exogenous cannabinoid ligands (e.g. CB1 or CB2 receptor agonists) can reduce nociceptive transmission at all three of these neuroanatomical levels. Glial cells, which express components of the endocannabinoid system, represent another substrate though which cannabinoids or endocannabinoid system modulators may regulate pain through neuro-immune interactions29,157for review see233,238,242. Preclinical research indicates that endocannabinoids are synthesized on-demand in postsynaptic neurons in response to stress or pain and produce short-term antinociceptive effects via presynaptic inhibitory CB1 receptors92,113,144,181,218,239. Endocannabinoids are implicated in control of pain initiation32,38, and play an important role in the resolution of tonic pain and in stress-induced and fear-conditioned analgesia in rodents27,28,77,92,113. In animal models of pathological pain, the endocannabinoid system exhibits adaptive changes or plasticity (e.g. altered cannabinoid receptor expression/functionality and endocannabinoid levels) depending on the model and anatomical site under investigation100,200,206,240,241. These findings support the contention that the endocannabinoid system may represent a viable therapeutic target for chronic pain. This view is supported by numerous pharmacological studies demonstrating efficacy of cannabinoids or modulators of the endocannabinoid system in animal models of pathological or injury-related pain. These latter studies have been reviewed and analysed in our systematic review and meta-analysis of the preclinical literature published in this special issue of PAIN215. Herein, we include a summary of the key findings of this systematic review, and extended commentary on some elements not discussed in detail within the systematic review itself.
Figure 2.
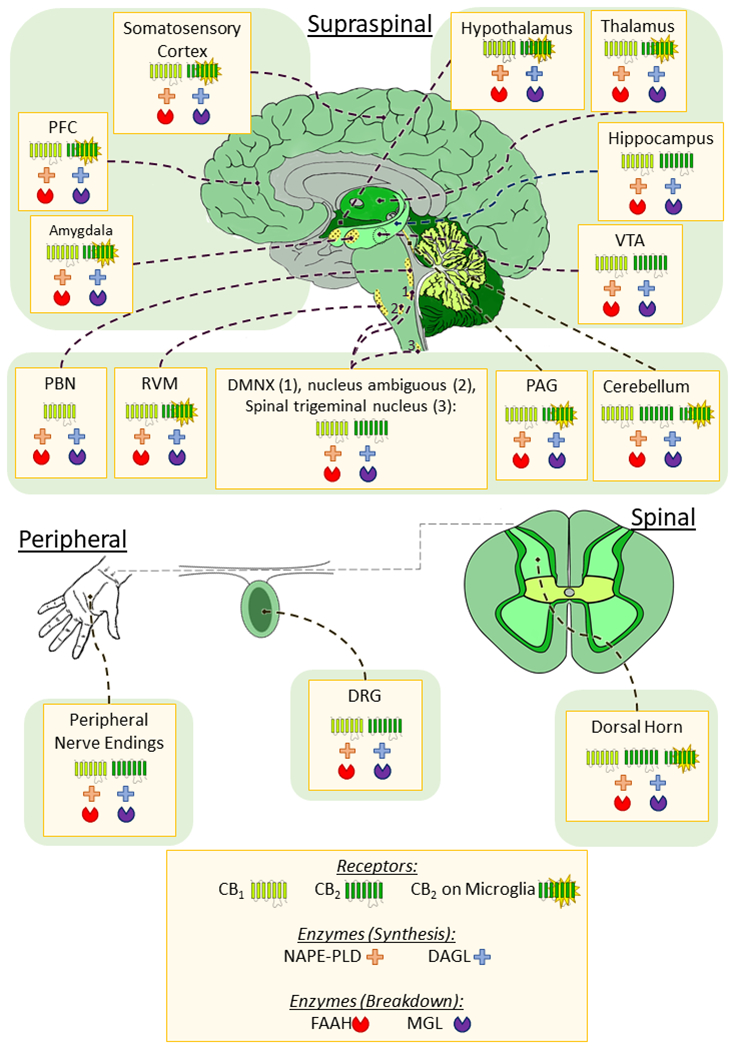
Distribution of the cannabinoid receptors and enzymes associated with endocannabinoid synthesis and degradation in pain pathways. The endcannabinoid system is widely distributed throughout regions associated with pain processing and modulation in the brain, spinal cord and periphery; Most particularly the CB1 receptor and the enzymes responsible for endocannabinoid synthesis (NAPE-PLD, DAGL) and degradation (FAAH, MGL). CB2 receptors are less abundant in the brain and are primarily located on microglia, however studies have shown expression of CB2 on neurons in the VTA, and several discrete nuclei of the brainstem. CB1 is also expressed on microglia at a lower level than CB2, and effects of CB1 on microglia may be mediated by neuronal CB1. Therefore, this was not depicted above. In the DRG and periphery both CB1 and CB2 receptors can be found on neurons (and glia in the dorsal horn), as well as the endocannabinoid enzymes. PFC – prefrontal cortex; VTA – Ventral Tegmental Area; PAG – periaqueductal grey; RVM – rostral ventromedial medulla; PBN – parabrachial nucleus; DMNX - dorsal motor nucleus of the vagus nerve; DRG – dorsal root ganglion; CB – cannabinoid receptor; NAPE-PLD – N-acylphosphatidylethanolamine-hydrolyzing phospholipase D; DAGL – diacylglycerol lipase; FAAH – fatty acid amide hydrolase; MGL – monoacylglycerol lipase.
Classification of cannabinoids and endocannabinoid system modulators of relevance to pain
Common pharmacological tools employed to manipulate the endocannabinoid system are summarized in Table 2.
Table 2.
Cannabinoid Ligands/Preparations and Pharmacological Tools
| Natural Cannabinoid Ligands/Synthetic Analogues | CB1-selective Agonists |
| *Δ9-THC (Dronabinol) | ACEA |
| *CBD | Met-F-AEA |
| * Cannabis extract, Δ9-THC:CBD (2.5:1.25 mg) | AZ11713908 (peripherally restricted with CB2 inverse agonist properties) |
| Mixed CB1/CB2 Agonists | |
| *Cannabis | BAY59-3074 |
| *eCBD | CP55,940 |
| *Nabilone (Δ9-THC analogue) | *CT-3 (Ajulemic Acid) |
| *Nabiximols (oral-mucosal spray, Δ9-THC:CBD, 2.7:2.5 mg) | HU-210 |
| Endocannabinoids | WIN55,212-2 |
| AEA | CB2-selective Agonists |
| 2-AG | AM1241 |
| Endocannabinoid Modulators | AM1714 |
| CB1 Positive Allosteric Modulators | AM1710 |
| GAT211 | A-796260 |
| GAT229 | A-836339 |
| ZCZ011 | *GW842166X |
| Uptake Inhibitors | *HU308 |
| AM404 | JWH015 |
| LY2318912 | JWH133 |
| VDM11 | LY2828360 |
| OMDM132 | MDA7 |
| UCM-707 | MDA19 |
| FAAH Inhibitors | CB1 Antagonists |
| OL135 | AM251 |
| *PF-00457845 | AM281 |
| URB597 | AM6545 (peripherally restricted) |
| URB937 (peripherally restricted) | SR141716 |
| MGL Inhibitors | CB2 Antagonists |
| JZL184 | AM630 |
| URB602 (local only) | SR144528 |
| MJN110 | Fatty Acids that do not bind CBRs |
| Dual FAAH-MGL Inhibitors | NaGly |
| JZL195 | PEA |
| SA57 | L-29 |
| FABP5 Inhibitors | |
| SBF126 |
Abbreviations: AEA, anandamide; 2-AG, 2-arachydonoylglycerol; CBD, cannabidiol; eCBD, high CBD cannabis; Δ9-THC, Δ9-tetrahydrocannabinol; FAAH, fatty-acid amide hydrolase; FABP, fatty-acid binding protein; FLAT, FAAH-like anandamide transporter; MGL, monoacylglycerol lipase; N-arachidonoyl glycine, NaGly; PEA, palmitoylethanolamine;
Denotes compounds used clinically; Adapted and updated from Hohmann and Rice (2013) Textbook of Pain 6th Edition and Rahn and Hohmann (2009) Neurotherapeutics 6: 713-3
Antinociceptive efficacy of cannabinoids in animal models of pathological or injury-related persistent pain
The antinociceptive efficacy of cannabinoids and endocannabinoid system modulators has been reviewed extensively99,196,206,217,240,241. Thus, herein, we instead provide a concise commentary on the efficacy of cannabinoids and endocannabinoid system modulators in animal models of pathological or injury-related persistent pain that is uniquely informed by our companion systematic review and meta-analysis of preclinical studies in this area215. We summarise the key findings and elaborate further on some of their implications for the field.
A systematic review of laboratory animal studies that employed models/conditions associated with persistent pain and reported a pain-relevant outcome measure, identified 473 published reports of which 374 reported data that could be included in a meta-analysis. Data from 6479 rats and 6876 mice respectively were included, reflecting 864 and 677 experimental comparisons, respectively. This is a very large data set by preclinical standards. No studies in other species were found. Ninety-nine studies (~20 %) were excluded from the meta-analysis because the methods and/or results were not reported in sufficient detail to permit extractable data to be included in a meta-analysis.
Overall, the data support the hypothesis of cannabinoid-mediated analgesia. The overall effect size (Hedge’s G Standardised Mean Difference, SMD) was 1.32 [Q = 4101.26, d.f. 1543, p <0.0001, I2 = 61.58 %] (Figure 3). The models used reflect a range of conventional and diverse inflammatory and neuropathy paradigms, particularly surgically-induced nerve injury for the latter. With regard to outcome measures, in common with other pre-clinical behavioral pain studies, limb withdrawal measures evoked by sensory stimuli were by far the most frequently reported outcome measure, with very few reports of complex behavioral assessments. Lack of consensus on predictive validity of such measures limits the degree to which the presence of pain, and thus pain relief from the intervention, can be inferred190,202. This contrasts with the clinical trial literature where patient reported pain intensity is the predominant metric. The formalin test also features strongly in the data; this model of tonic inflammatory pain entails measuring several spontaneous or non-evoked nociceptive behaviors e.g. licking, lifting, flinching, rearing, and guarding to generate a ‘combined or composite pain score’ which thereby affords a greater degree of confidence regarding the impact of pain on the animal’s behavior1,69,224.
Figure 3.
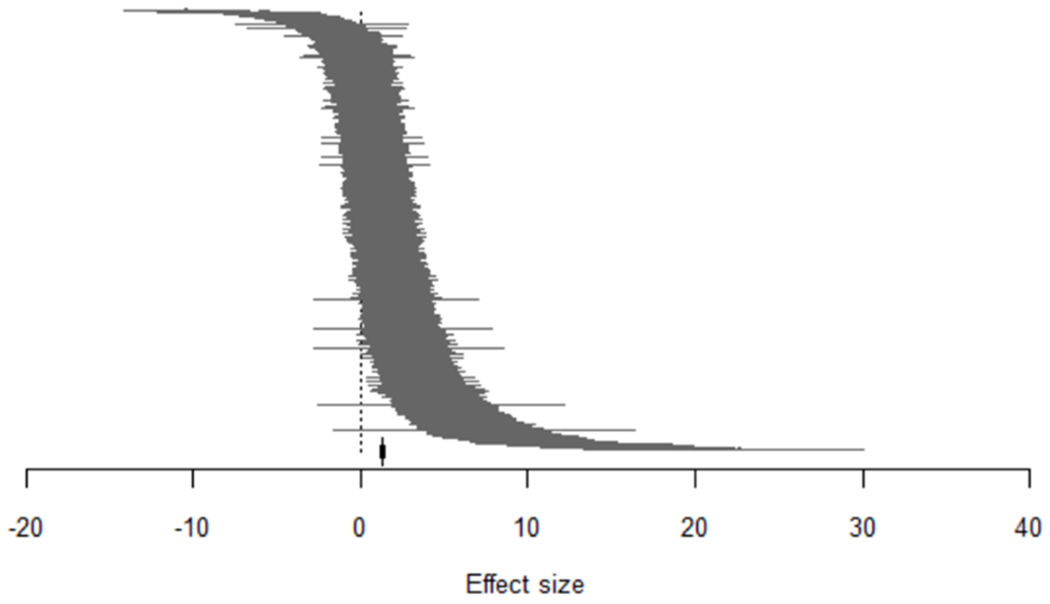
The caterpillar plot of 1544 nested comparisons extracted from 374 studies included in the meta-analysis. A Hedge’s G standardised mean difference effect size was calculated for each comparison. Overall effect size = 1.32215
Small molecule CB1 and CB2 receptor agonists and non-selective cannabinoid receptor agonists (including THC) were the most frequently assessed interventions. FAAH inhibitors and PPAR-α agonists (in particular palmitoylethanolamide; PEA) were also frequently evaluated. In general, studies demonstrated antinociceptive efficacy, as measured predominantly by attenuation of injury/inflammation-associated hypersensitivity in evoked limb withdrawal (Figure 4).
Figure 4.
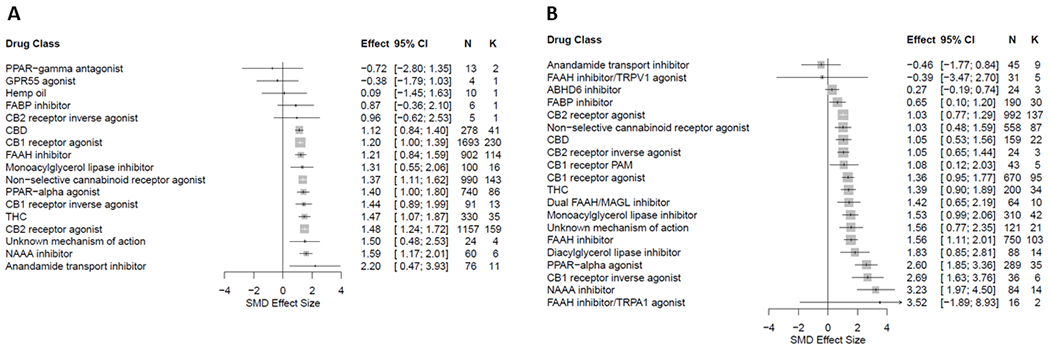
Forest plot of drug classes assessed for antinociceptive efficacy in rat (A) and mouse (B) models of injury-related or pathological persistent pain. The size of the squares represents the weight (%) and its influence on the pooled result. N denotes the number of animals and K the number of comparisons of each sub-group215.
The differences between the effect sizes of the different interventions may be inherent to the intervention, e.g., mechanism of action, route of administration and dosing regimens but are also likely to be influenced by other study design characteristics e.g. the model, choice of species, strain, sex and behavioral outcome measure.
In rodent inflammatory pain models (e.g. formalin, Complete Freund’s Adjuvant [CFA], carrageenan and osteoarthritis), CB1, CB2 receptor agonists and PEA consistently attenuated pain-related behaviors across a range of inflammatory pain models (Table 3). The only exception was for carrageenan-induced inflammation in rats, for which CB1 receptor agonists did not significantly attenuate pain-related behaviours. The efficacy of FAAH inhibitors was mixed; pain-related behaviors were significantly attenuated in formalin and CFA but not in osteoarthritis rodent models. In carrageenan models, a species difference was detected in which FAAH inhibitors significantly attenuated pain-related behaviors in mice but not rats. THC also significantly attenuated pain-related behaviors in formalin, CFA and carrageenan models. Like FAAH inhibitors, the efficacy of CBD was mixed; pain-related behaviors were significantly attenuated in formalin and CFA but not in carrageenan and osteoarthritis rodent models.
Table 3.
Summary of the effects of cannabinoids and endocannabinoid system modulators on pain-related behaviour in the most frequently used rodent models of inflammatory pain. Adapted from215.
| Formalin | Complete Freund’s Adjuvant | Carrageenan | Osteoarthritis | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug Class | K | N | Effect size | 95 % CI | K | N | Effect size | 95 % CI | K | N | Effect size | 95 % CI | K | N | Effect size | 95 % CI |
| Anandamide transport inhibitor | 10 | 64 | 1.97 | 0.608 - 3.342 | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT |
| CB1 receptor agonist | 59 | 496 | 1.12 | 0.731 - 1.502 | 21 | 144 | 0.74 | 0.378 - 1.107 | ns | ns | ns | ns | 3 | 16 | 1.22 | 0.378 - 1.107 |
| CB1 receptor inverse agonist | 1 | 7 | 1.71 | 0.427 - 3.002 | NT | NT | NT | NT | 5 | 30 | 0.44 | 0.055 - 0.822 | NT | NT | NT | NT |
| CB1 receptor PAM | NT | NT | NT | NT | NT | NT | NT | NT | 1 | 8 | 2.45 | 1.067 - 3.837 | NT | NT | NT | NT |
| CB2 receptor agonist | 33 | 265 | 0.81 | 0.316 - 1.307 | 44 | 338 | 1.10 | 0.829 - 1.366 | 10 | 74 | 1.39 | 0.885 - 1.896 | 24 | 173 | 1.42 | 0.829 - 1.366 |
| CB2 receptor inverse agonist | 3 | 22 | 1.05 | 0.653 - 1.444 | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT |
| CBD | 2 | 16 | 1.86 | 1.548 - 2.169 | 5 | 20 | 1.59 | 0.71 - 2.469 | ns | ns | ns | ns | ns | ns | ns | ns |
| Dual FAAH/MAGL inhibitor | NT | NT | NT | NT | ns | ns | ns | ns | 4 | 36 | 1.42 | 0.25 - 2.6 | NT | NT | NT | NT |
| FAAH inhibitor | 23 | 184 | 1.50 | 1.134 - 1.862 | 24 | 144 | 1.24 | 0.702 - 1.781 | ns | ns | ns | ns | ns | ns | ns | ns |
| FABP inhibitor | ns | ns | ns | ns | 15 | 98 | 0.78 | 0.194 - 1.373 | 6 | 36 | 1.42 | 0.947 - 1.895 | NT | NT | NT | NT |
| Monoacylglycerol lipase inhibitor | 8 | 69 | 2.28 | 1.382 - 3.175 | ns | ns | ns | ns | 8 | 60 | 2.67 | 0.967 - 4.378 | ns | ns | ns | ns |
| NAAA inhibitor | NT | NT | NT | NT | 3 | 30 | 1.63 | 1.235 - 2.025 | 12 | 72 | 1.91 | 0.923 - 2.897 | NT | NT | NT | NT |
| Non-selective cannabinoid receptor agonist | 38 | 253 | 1.56 | 1.019 - 2.11 | ns | ns | ns | ns | 45 | 311 | 0.74 | 0.202 - 1.268 | ns | ns | ns | ns |
| PPAR-alpha agonist | 21 | 143 | 1.73 | 0.701 - 2.761 | 2 | 16 | 2.53 | 0.851 - 4.205 | 26 | 173 | 3.26 | 1.51 - 5.006 | 9 | 75 | 0.98 | 0.851 - 4.205 |
| THC | 20 | 118 | 1.16 | 0.679 - 1.643 | 16 | 109 | 2.34 | 1.687 - 2.986 | 5 | 37 | 2.13 | 0.138 - 4.12 | NT | NT | NT | NT |
K denotes the number of comparisons and N denotes the number of animals within each sub-group. All entries with an effect size value were statistically significant. NT, not tested, ns, not significant.
In rodent neuropathic pain models e.g. nerve injury, chemotherapy-induced peripheral neuropathy and diabetes, CB1 and CB2 receptor agonists, FAAH inhibitors and CBD consistently demonstrated antinociceptive efficacy (Table 4). PEA also significantly attenuated pain-related behaviors in nerve injury and chemotherapy models. THC significantly attenuated pain-related behaviors in nerve injury models.
Table 4.
Summary of the effects of cannabinoids and endocannabinoid system modulators on pain-related behaviour in the most frequently used rodent models of neuropathic pain. Adapted from215.
| Nerve injury | Chemotherapy | Diabetes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug Class | K | N | Effect size | 95 % CI | K | N | Effect size | 95 % CI | K | N | Effect size | 95 % CI |
| Anandamide transport inhibitor | 1 | 5 | 7.95 | 3.26 - 12.643 | NT | NT | NT | NT | NT | NT | NT | NT |
| CB1 receptor agonist | 102 | 756 | 1.17 | 0.842 - 1.5 | 31 | 173 | 1.47 | 1.007 - 1.937 | 30 | 183 | 1.74 | 1.234 - 2.248 |
| CB1 receptor inverse agonist | 4 | 36 | 1.71 | 1.171 - 2.248 | NT | NT | NT | NT | 2 | 12 | 4.53 | 1.932 - 7.127 |
| CB2 receptor agonist | 87 | 639 | 1.15 | 0.812 - 1.497 | 27 | 192 | 1.549995 | 1.117 - 1.983 | 10 | 88 | 1.29 | 0.772 - 1.818 |
| CBD | 9 | 72 | 1.86 | 1.307 - 2.406 | 10 | 76 | 1.31 | 0.594 - 2.021 | 7 | 49 | 1.08 | 0.257 - 1.896 |
| Diacylglycerol lipase inhibitor | 1 | 6 | 3.69 | 1.235 - 6.148 | ns | ns | ns | ns | NT | NT | NT | NT |
| Dual FAAH/MAGL inhibitor | 2 | 12 | 2.08 | 1.348 - 2.808 | NT | NT | NT | NT | NT | NT | NT | NT |
| FAAH inhibitor | 59 | 439 | 1.75 | 1.212 - 2.281 | 19 | 148 | 1.96 | 1.048 - 2.868 | 17 | 180 | 2.53 | 1.455 - 3.604 |
| Monoacylglycerol lipase inhibitor | 16 | 119 | 1.31 | 0.774 - 1.84 | 12 | 80 | 1.42 | 0.179 - 2.653 | NT | NT | NT | NT |
| NAAA inhibitor | 5 | 34 | 4.54 | 2.594 - 6.496 | NT | NT | NT | NT | NT | NT | NT | NT |
| Non-selective cannabinoid receptor agonist | 75 | 504 | 1.18 | 0.825 - 1.536 | ns | ns | ns | ns | 8 | 48 | 1.89 | 0.335 - 3.437 |
| PPAR-alpha agonist | 26 | 229 | 1.14 | 0.493 - 1.786 | 11 | 74 | 2.54 | 1.341 - 3.746 | NT | NT | NT | NT |
| THC | 9 | 86 | 1.26 | 0.838 - 1.677 | ns | ns | ns | ns | NT | NT | NT | NT |
K denotes the number of comparisons and N denotes the number of animals within each sub-group. All entries with an effect size value were statistically significant. NT, not tested, ns, not significant.
The meta-analysis suggests that the most frequently assessed cannabinoids, CB1 and CB2 receptor agonists and non-selective agonists, consistently attenuated pain-associated behaviors in a broad range of inflammatory and neuropathic pain models. Although tested in fewer model types, this was similarly evident for THC. CBD and FAAH inhibitors were not as effective in inflammatory pain models but may be a viable candidate for the treatment of neuropathic pain. However, this analysis does not take into account potential side effects e.g. motor impairment, hypothermia or anxiolysis, that could influence the behavioral outcomes. Prospective preclinical trials are required to better ascertain what factors are influencing the differences in observed efficacy between the different drug classes and model types.
The systematic review highlights several differences between the preclinical and clinical assessment of candidate treatments. The number and profile of the drugs assessed preclinically (171 different interventions) differs markedly from those investigated in clinical trials (11 different interventions in79) where the predominantly evaluated clinical interventions are pharmacologically complex cannabis-based medical extracts, THC or THC analogues. Indeed, an unusual feature of the cannabinoid field is that the initiation of clinical trials has not generally followed (or has been very slow to follow) the publication of preclinical data providing evidence of benefit for small molecule drugs, whereas this is the case for many other drug classes. Moreover, certain indications assessed in clinical trials (e.g. pain due to third molar extraction) are not represented in the preclinical literature.
The routes of administration also differ, and accompanying pharmacokinetic investigation was only evident in 7% of the studies. However, 69 % of studies did confirm CB1 or CB2 receptor involvement through the use of antagonists, transgenic mice or radioligand binding.
The systematic review also highlights several weaknesses in face and construct validity of the animal models and there are several study characteristics that can impact pain-associated behavioral outcome measures and the assessment of novel antinociceptive efficacy. The animal cohorts are genetically very similar, converse to the widely heterogeneous patient population. In rats, most studies employed either Sprague-Dawley or Wistar strains, although larger effect sizes were evident in the small number of studies reported with Lewis rats. There was a major bias to the use of male rats (91%). Similar findings were found in mice with 81% of studies reporting the use of males across 29 strains. This does not reflect the clinical situation where women are overrepresented among patients with chronic pain 164. In clinical trials male and female patients are more equally represented e.g. a recent systematic review of 36 randomised controlled trials assessing cannabinoids, cannabis, and CBMs, female patients outnumbered (n=3691) male patients (n=3613) 79.
More generally, the animal models do not effectively simulate multidimensional clinical pain conditions including the psychological component. Disease or injury is frequently induced in young, otherwise healthy animals contrasting with the clinical situation in which disease or injury predominantly occurs in older patients with co-morbidities. The duration of animal studies is usually brief (up to a few weeks) which results in candidate treatments being tested in the early stages of disease onset which does not adequately reproduce the impact of prolonged clinical pain nor address the clinical need for treatment in the later stages of disease. There is an over reliance on evoked limb withdrawal, a measure of hypersensitivity, which is not appropriate for pain that is characterized by sensory loss or spontaneous pain. Careful consideration should be given to the choice of species, strain, sex and age in relation to the clinical condition being modelled. To limit threats to external validity, researchers should balance the sexes39. A broader range of outcome measures that are of clinical relevance and include more complex, ethologically relevant behaviors is required202. Multicenter testing will increase environmental heterogeneity and study samples thereby improving the generalizability of preclinical findings231.
As with the vast majority of current preclinical neuroscience and pharmacological research employing animal models, the risk of bias is uncertain, due to a generic poverty of reporting sufficient details of the experimental design, conduct and analysis factors which govern the veracity of experimental internal validity. There is evidence to suggest that low prevalence of the reporting of measures to mitigate bias tend to give higher estimates of treatment effects48,105. The meta-analysis did not show a consistent relationship between the reporting of methodological quality criteria and smaller effect sizes, however, larger effect sizes were reported for studies that did not report allocation concealment and sample size calculations. In relation, the methods by which biases were mitigated were also infrequently reported. On the rare occasions where they were, the methods were often invalid230 e.g. randomization by ‘picking animals randomly from a cage’ rather than computer generated random sequence, or determining sample size based upon reported sample size norms rather than a power calculation. Thus, there is a critical need for transparency of reporting all experimental details so that the quality of research can be assessed, and the validity of the outcomes inferred.
There was also evidence of publication bias overestimating the effect size reported in the literature because of the well-known propensity to report studies which support the hypothesis, and historic difficulty of publishing data that show no effect. It is also to be expected that animal studies will yield larger effect sizes in comparison to clinical studies due to the more homogenous nature of the study population, and better opportunity to control experimental variables, reducing the observed variance and making direct comparison of effect sizes impossible.
Our accompanying systematic review and meta-analysis therefore highlights the need for improvements in experimental design and, perhaps even more importantly, the reporting of experimental design and analysis features in sufficient detail such that primary research can be reproduced and meta-analysed. Nevertheless, notwithstanding the caveats relating to animal studies of analgesia, substantial evidence from animal model experiments supports the hypothesis of cannabinoid-induced analgesia in inflammatory and neuropathy conditions.
Side effects relevant to preclinical pain studies
An important consideration in the evaluation of any pain medication is whether analgesic efficacy is accompanied by adverse side effects that limit therapeutic potential, or additional beneficial effects that might enhance therapeutic potential. The present evaluation of on-target “side effects” is restricted to effects of cannabinoids/endocannabinoid system modulators administered to rodents in adulthood. Additional preclinical research is needed to specifically assess potential harms in vulnerable populations (e.g. older adults, and the developing fetal and adolescent brain40,41,91). In this section, we consider both potential harms and benefits of pharmacological strategies assessed specifically in animal pain models, particularly as they may emerge with chronic dosing. We also consider pharmacological effects (e.g. motor impairment, hypothermia or anxiolysis) that could influence interpretation of pain-related behavior. Animal models have been used to investigate an array of mechanisms and sites of analgesic action using a much broader array of mechanistically distinct compounds compared to those evaluated in the clinical literature. Most therapeutic interventions that show promise in preclinical studies, nonetheless, fail in clinical trials, albeit for different reasons (i.e. efficacy, side effect profile, lack of adequate target engagement, therapeutic indication, variability in clinical populations, clinical primary outcome measure); this failure rate is not unique to cannabinoids. Moreover, animal models may not adequately capture adverse side effects that may be problematic in humans, and adverse side effects are often not systematically studied in animal experiments. Nevertheless, preclinical studies have provided evidence of both beneficial (i.e. antinociception, anti-emetic, anti-spasticity, anti-stress, anti-anxiety-like effects) and adverse (i.e. reward or aversion, dependence, tolerance, motor and memory impairment) effects of cannabinoids and endocannabinoid system modulators in otherwise normal animals (for review see183,184). Here, we primarily consider “on-target” pharmacological effects of cannabinoids in pain models only, including assessments of cannabimimetic effects (i.e. in the classic cannabinoid tetrad), tolerance, physical dependence, reward/reinforcement, opioid sparing effects, antinociceptive synergy and effects on stress-, anxiety- and depression-related behavior in animal models of pain.
Cardinal signs of CB1 receptor activation.
The classic cannabinoid tetrad assesses cardinal signs of CB1 activation (i.e. hypoactivity, reduction in body temperature, catalepsy, and tail-flick antinociception)156,214 in rodents and is produced by all CNS-penetrant CB1 agonists, including THC. These cannabimimetic effects are consistent with localisation of CB1 to motor and limbic regions in rodent brain104. Drug-induced motor impairment can mask detection of antinociception in rodents. Consequently, preclinical studies must show that antinociceptive effects of cannabinoids observed in behavioral studies are not artifacts of motor impairment. Whereas motor effects complicate behavioral evaluation of antinociceptive effects of cannabinoids, they do not preclude the existence of antinociceptive mechanisms. Our accompanying systematic review revealed that a minority (33%) of studies assessed the effects of the drugs on motor activity215. Direct CB1 agonists and non-selective cannabinoid receptor agonists that penetrate the CNS have potential to produce undesirable CB1-mediated pharmacological effects in humans (e.g. psychoactivity, motor and memory impairment) (for review see183,184). Electrophysiological measures of activity of nociceptive neurons and/or neurochemical measures of noxious stimulus-evoked neuronal activation provide independent lines of evidence that non-selective cannabinoid receptor agonists, CB1 agonists, FAAH inhibitors, and CB2 agonists suppress nociceptive processing in rodents (for review see98,99,217,240). Non-selective cannabinoid receptor agonists (e.g. WIN55,212-2) suppress electrophysiological112,114,115,158 and neurochemical markers of pain-evoked neuronal activation226 as well as pain behavior at doses that do not alter body temperature or produce immobility186,199. Drug-induced reductions in body temperature cannot explain electrophysiological or behavioral indicators of antinociceptive efficacy158,170,228. WIN55,212-2-induced reduction of evoked hypersensitivity in the chronic constriction injury (CCI) model of neuropathic pain, and side effects (motor incoordination, catalepsy and sedation), can occur at similar ED50s in mice3, however, antinociceptive effects of WIN55,212-2 correlate with suppression of firing in nociceptive neurons and is reported to outlast these motor effects158.
Drug development efforts have focused on elucidating therapeutic potential of small molecules that engage targets within the endocannabinoid system that lack unwanted cannabimimetic effects associated with direct CB1 activation (for review see50,113,139). CB2 agonists, FAAH inhibitors, MGL inhibitors (at appropriate doses), CB1 positive allosteric modulators (PAMs), peripherally-restricted non-selective cannabinoid receptor agonists (PrNM1; 166) have all been shown to suppress pain behavior without unwanted motor effects of CB1 agonists (for review see98,99,217,240). High dose MGL inhibitors can elicit tetrad effects whereas a dual FAAH/MGL inhibitor JZL195 suppressed neuropathic nociception in the mouse CCI model with an ED50 four times lower than that which produced side effects (motor incoordination, catalepsy, sedation)3, although brain permeant and impermeant FAAH inhibitors lack such effects5,73,197,223. Dual FAAH/MGL inhibitors represent a pharmacological strategy to elevate both AEA and 2-AG and produce antinociceptive efficacy without producing unwanted cannabimimetic effects in the tetrad66. CB1 knock-out (KO) mice have also been used to assess the impact of CB2 activation without the confound of CB1-mediated side-effects56,66,126,208. Such studies show that mixed cannabinoid agonists and CB2 agonists can engage CB2 receptors independently of CB1 to alleviate neuropathic pain-related behavior induced by paclitaxel administration or spinal nerve liagtion56,126.
The abundance of CB1 in brain regions controlling motor activity and memory accounts for adverse side effects of mixed and CB1-preferring strategies104. Very few studies evaluate possible memory impairment induced by pharmacological treatments in laboratory animal pain models. However, studies that involve learning approaches document that rodents in pathological pain states can show conditioned place preferences50,84 or perform operant responses102,166 to chambers/tasks associated with pain relief; memory impairment would preclude demonstrations of efficacy in such studies. Peripherally-restricted CB1 agonists, CB2 agonists, MGL inhibitors and CBD represent cannabinoid modulators that have shown efficacy in such assays in animal pain models50,84,166.
Tolerance
Tolerance, the loss of therapeutic efficacy with repeated administration, is undesirable in an analgesic and can lead to dose escalation and potential for misuse and abuse. In animal models of pathological pain, tolerance to therapeutic efficacy develops to direct acting CB1 agonists, non-selective cannabinoid receptor agonists, as well as high, but not low, doses of MGL inhibitors56,142,212, presumably via downregulation and desensitization of CB1 receptors. In a mouse model of chemotherapy-induced neuropathic pain induced by paclitaxel, tolerance developed to the antinociceptive effects of THC and other non-selective cannabinoid receptor agonists (CP55,940, WIN55.212-2), as well as to other classical cannabimimetic effects56,212. Low dose chronic infusion of WIN55,212-2 and AM1241 (CB2 agonist) were associated with sustained antinociceptive efficacy in the paclitaxel model without motor impairment199. Tolerance develops more quickly to high compared to low doses of the centrally acting CB1-preferring ligands56,212. By contrast, antinociceptive tolerance is typically absent in neuropathic as well as inflammatory pain models following repeated administration of CB2 agonists56,199,212, brain permeant and impermeant FAAH inhibitors38,212,213, low dose (but not high dose) MGL inhibitors142, CB1 PAMs128,212 and a peripherally restricted CB1 agonist166. The FAAH/MGL dual inhibitor JZL195 exhibited greater efficacy than FAAH or MGL inhibitor alone and did not produce tolerance, exhibiting an improved therapeutic window compared to direct cannabinoid CB1 agonists3. Further preclinical studies are necessary to determine whether different therapeutic strategies (i.e. biased CB1 agonism, peripherally restricted non-selective cannabinoid receptor agonists) can separate therapeutic efficacy from unwanted side effects.
Physical dependence
Receptor antagonists have been used to precipitate a withdrawal syndrome, a sign of physical dependence, in mice subjected to pathological pain states. In mice rendered neuropathic with paclitaxel, the CB1 antagonist rimonabant precipitates signs of physical dependence (i.e. paw tremors) in mice treated chronically with orthosteric cannabinoid agonists (i.e. THC, CP55940, WIN55,212-2); severity of withdrawal symptoms was dose-related56,212. In the same neuropathic pain model, both tolerance and rimonabant-precipitated withdrawal signs were produced by the MGL inhibitor JZL184 but not by a CB2 agonist56,212, brain permeant or impermeant inhibitors of FAAH213 or a CB1 PAM212. Where present, physical dependence was induced by challenge with CB1, but not CB2, antagonists and in animals receiving direct acting agonists (or high dose MGL inhibitor) that penetrated the CNS. By contrast, neither CB1 PAMs nor brain permeant or impermeant inhibitors of FAAH were associated with signs of physical dependence following 3 weeks of once daily administration in paclitaxel-treated mice212,213. Notably, CB1 antagonist-precipitated withdrawal syndromes (i.e. paw tremors) lacked the more striking somatic (i.e. jumping) and autonomic (i.e. diarrhea) signs associated with naloxone-precipitated opioid withdrawal9,212.
Reward/Reinforcement
THC can produce both rewarding and aversive effects in laboratory animals35,151,216. Rewarding properties of cannabinoids, have, historically, been demonstrated in otherwise healthy, young, male rodents which may not necessarily mimic the situation in people with chronic pain. In pathological pain states, interpretation of positive reinforcing effects of cannabinoids (i.e. reward in the typical drug abuse sense) should be differentiated from negative reinforcing effects (i.e. removal of an aversive pain state). Preclinical research involving rodent pain models has investigated therapeutic strategies targeting the endocannabinoid system that hold promise for suppressing pain without producing abuse liability. Using a classic drug self-administration approach, rats rendered neuropathic by a spared nerve injury self-administered a CB2 agonist in a CB2-dependent manner; naïve animals did not reliably self-administer the drug102. Moreover, CB2 agonists did not produce conditioned place preference (CPP)93,130. These observations suggest that the CB2 agonists were not inherently reinforcing in the absence of the pathological pain state. In the absence of pathological pain, CB1 PAMs ZCZ011 and GAT211 do not produce CPP when administered alone129,212. Moreover, ZCZ011 did not substitute for CB1 agonists CP55940 or AEA in a drug discrimination assay 129 suggesting it, does not produce cannabimimetic side effects. Inhibition of MGL with MINI110 also reversed a negative affective state associated with paclitaxel treatment using a CPP approach50. Similarly, a non-rewarding dose of CBD produced reward-related effects in the presence of pain due to incisional injury84. Thus, in the absence of pathological pain state, CB1 PAMs, and endocannabinoid deactivation inhibitors did not produce rewarding or aversive effects when administered alone in rodents. These studies raise the possibility that components of the endocannabinoid signaling system may be targeted for therapeutic benefit without the rewarding properties of direct CB1 activation in the CNS.
Opioid sparing effects and antinociceptive synergy
Opioids remain a mainstay of pain management but also produce tolerance, physical dependence, reward, constipation, and respiratory depression, among other effects. Efficacy of adjunctive therapies for suppressing pathological pain and producing opioid sparing effects has, consequently, been evaluated. THC produced synergistic antinociceptive effects with morphine in both arthritic (Freund’s adjuvant-induced) and naïve rats45; additive, rather than synergistic, interactions were reported for unwanted side effects (e.g. tetrad, motor ataxia)45. WIN55,212-2 produced synergistic antinociceptive interactions with morphine in mice with CCI of the sciatic nerve but only additive effects on motor coordination3. Brain permeant and impermeant inhibitors of FAAH212, MGL inhibitors237, dual FAAH-MGL inhibitor66, CB2 agonists93,130 and CB1 PAMs211 produce synergistic antinociceptive effects with morphine in neuropathic pain models. The CB2 agonist JWH133 produced additive antinociceptive effects with morphine in the formalin test245. Notably, in a mouse neuropathic pain model, brain permeant (URB597) and impermeant (URB937) FAAH inhibitors213, a CB1 PAM (GAT211;211) and CB2 agonists (AM1710, LY2828360;9,153,212) suppressed development of morphine tolerance without enhancing naloxone-precipitated opioid withdrawal. Multiple CB2 agonists attenuated opioid tolerance and naloxone-precipitated opioid withdrawal in neuropathic mice9,130,153,155,212. CB2 agonists produce synergistic antinociceptive effects with opioids and also attenuate opioid-induced respiratory depression236,246. Neither FAAH inhibitors (URB597, URB937) nor a CB1 PAM (GAT211) enhanced naloxone-precipitated opioid withdrawal in paclitaxel-treated mice212,213. Evaluations of other opioid side effects in the same studies (i.e. slowing of GI motility, reward), have typically employed normal animals not subjected to pathological pain states. Nonetheless, lowering opioid doses required to elicit therapeutic effects could enhance therapeutic ratios. Most studies have combined opioids with endocannabinoid modulators that themselves lack observable cannabimimetic side effects (JWH015, MJN110, URB597, URB937); consequently, additivity of adverse side effects would not be expected. Interestingly, CB2 agonists (JWH015; LY2828360) produced synergistic anti-allodynic effects with morphine in models of inflammatory (formalin), post-operative (paw incision) and neuropathic (SNI) nociception but did not produce synergy for nociceptive pain93,130. Synergy between CBD and morphine has also been reported in the acetic acid-induced writhing model176 and coadministration of THC and morphine reduced the second phase of formalin-evoked nociceptive behaviour in rats to a greater extent than either drug alone78. More work is necessary to examine whether cannabinoids alter other unwanted side effects of opioids (e.g. slowing of GI motility) in pain models, and evaluate the clinical relevance of these findings
Synergism of antinociceptive efficacy: Non-opioid analgesics
THC has been shown to produce synergistic antinociceptive interactions with gabapentin in the mouse CCI model of neuropathic pain9. Synergy between CBD and THC, and between PEA and gabapentin is also reported in chemotherapy-induced neuropathic pain models66,67,141, between CBD and THC in the mouse CCI model of neuropathic pain 34, and between PEA and acetaminophen (paracetamol) in the rat streptozotocin (STZ)-induced model of diabetic neuropathy53. The COX-2 inhibitor celecoxib increased the antihypersensitivity activity of the CB1 agonist Met-F-AEA and the CB2 agonist AM1241 in the STZ model26. Low doses of the MGL inhibitor JZL184 and the non-selective COX inhibitor diclofenac synergistically attenuated mechanical allodynia and additively reduced cold allodynia in the mouse CCI model of neuropathic pain49. Furthermore, co-administration of the FAAH inhibitor URB597 and diclofenac yielded synergistic antinociceptive effects in the acetic acid-induced abdominal stretching model of visceral nociception in mice174. In other work, anandamide co-administered with either ibuprofen (non-selective COX inhibitor) or rofecoxib (selective COX-2 inhibitor) resulted in synergistic antinociceptive effects in the rat formalin test97,101. Mechanistically, it is important to note that there are a number of interactions between COX/NSAIDs/acetaminophen and cannabinoids/endocannabinoid system, including inhibitory effects of NSAIDs or an acetaminophen metabolite on endocannabinoid catabolism or transport (for review see189). Thus, adjunctive therapies could enhance therapeutic ratios of existing treatments for both inflammatory and neuropathic pain. CB1 PAMs enhance antinociceptive and unwanted side effects of orthosteric CB1 agonists128,212 and produce synergistic anti-allodynic effects with FAAH and MGL inhibitors without enhancing other tetrad parameters212.
Beneficial on-target pharmacological effects
Beneficial on-target pharmacological effects (i.e. in suppressing stress, anxiety, nausea and producing improvements in sleep) of cannabinoids (for review see183,184) could contribute to perceived therapeutic benefits in relevant pathological pain states. However, only small numbers of studies have evaluated such features specifically in animal pain models. Indeed, our accompanying systematic review revealed that only 3% of studies assessed the anxiolytic or anti-depressant-like effects of the drugs in the animal models of injury-related or pathological persistent pain215. Systematic preclinical studies are necessary to determine whether, rather than purely modulating sensory thresholds, cannabinoid-based modulation of affective behavior could contribute to therapeutic efficacy of analgesics by attenuating symptoms that exacerbate pain.
Stress, anxiety, depression and pain-depressed behavior
The therapeutic potential of cannabinoids/endocannabinoid system modulators has been assessed in animal models of anxiety, stress, and depression (for review see76,90,165,183,184,187). However, fewer studies have evaluated such therapeutic effects within the context of pathological pain states (for review see42,80,217,240). The CB2 agonist GW405833 reduced immobility (i.e. a measure of depression-like behavior) in the forced swim test and allodynia (although mediation by CB2 was not assessed), whereas the anti-depressant desipramine primarily attenuated immobility time120. In the mouse monosodium iodoacetate (MIA) model of osteoarthritis pain, the CB1 agonist ACEA and the CB2 agonist JWH133 ameliorated nociceptive and affective alterations, and ACEA also improved associated memory impairment147. In rats with CCI, the brain permeant FAAH inhibitor URB597 but not the brain impermeant FAAH inhibitor URB937 suppressed immobility in the forced swim test, and attenuated novelty-induced suppression of feeding and CCI-induced reductions in hippocampal neurogenesis, all indicative of anti-depressant-like effects, whereas both URB597 and URB937 suppressed allodynia132. Thus, the CNS penetrant FAAH inhibitor produced beneficial effects on both evoked pain and pain-induced depression-like behavior. Rats with spared nerve injury exhibited an anxiety-like phenotype relative to shams; repeated CBD prevented anxiety-like behavior in the open field test, elevated plus maze and novelty-induced suppression of feeding test, in addition to suppressing allodynia via a 5-HT1A receptor mechanism52. Thus, effects of CBD revealed in that study are likely to be independent of cannabinoid receptor activation.
In addition to evaluations of responses to evoked pain (pain-stimulated behaviors), an emerging preclinical literature has evaluated pain-depressed behavior (e.g. marble burying, nestlet shredding, intracranial self-stimulation thresholds). While the extent to which assays of pain-depressed behavior assess stress-, anxiety-, or depression-related behavior is uncertain, they represent an attempt to more completely model the multifaceted complexities associated with pathological pain states177. Assessing the effects of drugs on pain-depressed behavior may also help to distinguish between antinociception and motoric side-effects of putative analgesics. THC and CP55,940 produced antinociception for pain-stimulated responding in an acid-induced writhing assay but exacerbated pain-depressed behavior (i.e. noxious stimulus-induced suppression of intracranial self-stimulation thresholds)146,150. By contrast, the FAAH inhibitor URB597 suppressed both pain-stimulated as well as pain-depressed behavior145. A MGL inhibitor (MJN110), CB2 agonist (LEI101), non-selective cannabinoid receptor agonist (CP55940), and reference analgesics (morphine, gabapentin, valdecoxib) all reversed pain-stimulated behavior (i.e. mechanical hypersensitivity) as well as pain-depressed behavior (i.e. deficits in marble burying) in mice with CCI50. By contrast, the benzodiazepine diazepam reversed neither dependent measure whereas a kappa-opioid receptor agonist (U69593) and a FAAH inhibitor (PF3845) reversed deficits in mechanical hypersensitivity only50. Deficits in marble burying resolved within a week of surgery50 and not all neuropathic pain models are associated with deficits in marble burying/nestlet shredding212. Thus, the translational relevance of these behaviors to pain and/or anxiety, consequently, remain unclear but may they provide insights of some relevance to general health/quality of life.
Sleep
Effects of cannabinoids on sleep have not been assessed in animal pain models. Clinical data on the FAAH inhibitor PF-04457845 in cannabis use disorder have suggested improvements in both withdrawal and sleep51. More work is necessary to ascertain presence of sleep disruptions in laboratory animal models of pathological pain states and ascertain whether endocannabinoid modulators could restore such deficits.
Pharmacokinetics of cannabinoids
In addition to the mechanisms of drug action that affect the endocannabinoid system, and cannabinoid structure-activity relationships (i.e. pharmacodynamics), the assessment of drug pharmacokinetics is of critical importance both for better understanding the preclinical pharmacology, and for improving animal-to-human translation235. The pharmacokinetic processes of drug absorption to the systemic circulation, its distribution to tissues of interest, as well as its metabolism and excretion will determine how much drug, after a particular dose delivered via a particular route, will reach its site of action. In addition, drug pharmacokinetics will determine the duration of a drug’s effect, and additional parameters related to drug-drug interactions and dose adjustments required in cases when organs such as liver or kidneys do not function properly.
Animal models provide advantages for investigating drug pharmacology because endogenous systems often cannot be manipulated safely in humans. However, since drug pharmacokinetics can be investigated directly in humans, this section of the manuscript will also discuss clinically-relevant pharmacokinetic data, where possible. Pharmacokinetic properties of cannabinoids, particularly their absorption, demonstrate substantial variability as a function of both route of administration, and the specific formulation in which the cannabinoids are delivered. Human studies repeatedly demonstrate large inter-subject variability in the pharmacokinetics of identical cannabinoid products, the basis of which is currently not well understood. Therefore, comparisons of non-human and human pharmacokinetics can have important translational relevance.
Absorption
Cannabinoid absorption is generally faster via the inhalational route than oral route, resulting in a more rapid onset of pharmacological effects, and shorter time to peak effect. In addition, cannabinoid delivery via the inhalational route circumvents the variability in oral absorption processes that are due to first pass metabolism. The limitations of inhalational routes include the variability in inter-individual efficiency that is caused by differences in inhalation techniques, respiratory tract irritation during inhalation, and the inconvenience or lack of adherence associated with smoked, vaporised, or nebulised cannabinoid products. While most preclinical experiments are performed with injected or oral/gastric administration of cannabinoids, most clinical therapeutic studies have used either inhaled products, oromucosal products such as nabiximols/Sativex®, or oral THC and its analogues.
The reported oral bioavailability of cannabinoids, and THC in particular, varies as a function of drug vehicle, and the co-ingested food. For example, orally ingested cannabis-containing cookies demonstrate only 6% THC bioavailability, while THC dissolved in sesame oil delivered in soft gelatin capsules is ~20% bioavailable (for review see122). A similar pattern of improved oral bioavailability approaching 30%, when dissolved in sesame oil, has been reported in rat studies with both THC and CBD131.
The bioavailability of THC delivered via smoked cannabis products reportedly varies between 18 to 50%180. In a small study comparing absorption of a physiologically compatible, nebulised inhalation solution of THC with that of intravenous THC in eight healthy volunteers, the average bioavailability of THC delivered via the inhalational route was approximately 28%172, somewhat comparable to average bioavailability with smoking. However, the measured area under the THC concentration time curve (AUC) among study participants varied more than 5-fold after intravenous administration, and more than 15-fold after inhalation. Adverse effects, as expected, were more prominent with intravenous dosing172.
After oral THC administration, the time to reach maximum plasma concentration (Tmax) has been reported between 30 and 120 min (but up to 6 hours in some studies) and is comparable between young and older adults4. Similarly, a wide-range of Tmax values, between 1-8 hours, has been reported with rat studies of THC, depending on the fasted/fed status, and the formulation in which THC is administered131,198. However, peak plasma concentrations (Cmax) of THC appear to be highly variable, with 4-7 fold differences among individuals, particularly in older adults4. With single oral doses of THC, the average Cmax ranged between 1.5-3 ng/mL after 3-6.5 mg doses4, and approximately 7ng/mL after 20mg oral THC (dronabinol) administration171. With inhaled doses, 0.053 mg/kg nebulised THC (mean dose 3.5mg)172 resulted in average Cmax of 20ng/mL,and with smoked cannabis, THC doses of 0.25 mg/kg body weight (14-22mg per cigarette) have resulted in mean Cmax of 48ng/mL. The plasma concentration profiles of key THC metabolites, namely 11-COOH-THC-, and 11-OH-THC, also vary substantially among people. With such variability in absorption and plasma concentrations of some cannabinoids, it is not surprising that clinical trial results are inconsistent79.
CBD, considered by many as devoid of the psychotropic side effects of other cannabinoids, has garnered attention in recent years. After oral administration, CBD follows close-to-linear absorption (e.g. Cmax of 530 ng/mL with 3000mg/day, and 780ng/mL with 6000mg/day oral dosing), but high-fat meals can increase CBD plasma exposure and peak plasma concentrations by 4-5 fold82,221. Following doses of 10 mg CBD with 10 mg THC either in an oral capsule or oromucosal nabiximols spray, mean reported Cmax was 2.5-3 ng/mL for CBD and 6.1-6.4 ng/mL for THC138.
Cannabinoids are highly lipophilic molecules with low aqueous solubility, and are susceptible to degradation and oxidation, especially in a solution. Drug formulation can thus play a crucial role in increasing the solubility and physicochemical stability of cannabinoid drugs, thus improving their pharmacokinetic properties25. Commonly used strategies in marketed products include pH adjustment, use of co-solvents, the use of micelles and nanoemulsions, complexation with cyclodextrins, or encapsulation in lipid-based formulations such as liposomes and nanoparticles. Even simple approaches such as administration of THC and CBD in lipid-rich oral formulations can increase plasma bioavailability of cannabinoids 2-3 fold, compared to lipid-free formulations248.
Formulations that are based on self-(nano)emulsifying drug delivery technology (SEDDS) have been proposed as a means of improving the oral bioavailability of drugs that show poor aqueous solubility, including cannabinoids. This approach results in a consistent increase in oral bioavailability of both THC (9-fold) and CBD (6-fold) in rats37, and a 1.5-fold and 2.2-fold increase respectively, in humans36.
Despite structural similarities, oral bioavailability differs between different cannabinoids. Nabilone, a synthetic cannabinoid derivative, is only slightly structurally different from THC (and dronabinol); however, it has substantially higher bioavailability than dronabinol (95% vs. 10–20%). One proposed difference relates to relative affinity to chylomicrons, and therefore the extent of lymphatic absorption of a particular cannabinoid85. Drug formulations containing long-chain triglycerides (LCT) may substantially improve the lymphatic absorption of selected cannabinoids85. Cannabinoids have demonstrated immunomodulatory effects in-vitro; however, plasma concentrations achieved with clinically-relevant dosing are well below those shown to affect lymphocyte function. By choosing the appropriate lipid-based formulation, lymphatic absorption of orally administered cannabinoids can be improved to achieve 100-fold and 250-fold higher lymph (vs plasma) concentrations of THC and CBD, respectively, which could help in the targeting of conditions where neuro-immunological effects of cannabinoids are of interest247,248.
While there is high variability with the oral routes, consideration of variability related to smoked or vaporised delivery is also important. In pulmonary delivery routes, the depth of inhalation, how long breath is held for, and vaporiser temperature all affect cannabinoid absorption25. Inhalational devices that are temperature-regulated to control the inhaled doses may allow less variable pharmacokinetic profile of inhaled cannabinoids70.
The transdermal route of administration provides an alternative approach for systemic delivery of lipophilic compounds with highly variable oral absorption, but more research is required to optimise these delivery systems and assess their efficacy and safety profiles188,229.
Distribution
Most naturally occurring cannabinoid receptor ligands are lipophilic, and readily penetrate the blood-brain barrier, and permeate well to lipid-rich tissues such as the brain and peripheral fat. Cannabinoids display slower elimination rates from these tissues compared to plasma24. THC achieves higher concentration in rodent brain than in plasma, and CBD achieves approximately a 1:1 ratio106. Many of the synthetic ligands behave similarly. For example, WIN 55,212-2 and MK-9470, cannabinoid receptor ligands commonly used in preclinical studies, demonstrate similarly high brain:plasma penetration ratio in rodents163,194.
In rhesus monkeys exposed to either intermittent (days) or long-term (years) cannabis consumption, the pharmacokinetics of single dose subcutaneous THC were not substantially different87. However, physiological responses of change in core temperature, or behavioral response time were different, and less profound, in monkeys with chronic exposure, likely suggesting physiological/behavioral tolerance to THC. Although blood THC levels were highest at 30-min post-injection, maximal effects on temperature and response rate did not occur until 120-min after injection, after blood THC levels had substantially decreased, suggesting a non-linear relationship between blood concentration of THC and its pharmacodynamic effects.
The existence of peripheral antinociceptive mechanisms32,81for review see107,152,169,196,204,240,250 prompted development of peripherally-restricted cannabinoid receptor ligands. However, developing such compounds that are not lipophilic but have high binding affinity to CB receptors has been challenging. Several compounds meeting the desired criteria of limited brain penetration with high affinity to CB1 and CB2 receptors have been synthesized2. Some achieve 0.16-0.18 brain:plasma ratio and produce antinociceptive effects in a rat model of neuropathy without producing catalepsy, but are yet to be evaluated clinically. Brain impermeant inhibitors of endocannabinoid metabolism have also been developed and show promise in preclinical studies of inflammatory and neuropathic pain38,100,212,213.
There may be sex-specific differences in cannabinoid pharmacokinetics. Plasma levels of THC after intraperitoneal injection are comparable between male and female rats but levels of THC metabolites in brain tissue, including 11-OH-THC, the major active metabolite, were higher in female than male rats. When behavioral effects such as response to heat nociception and catalepsy were tested, SKF525A, a cytochrome P450 inhibitor, attenuated THC-induced antinociception and catalepsy in female, but not in male rats. Greater levels of active THC metabolites produced by females could potentially contribute to greater behavioral effects of THC in female compared to male rats225. Human studies have found somewhat similar tendencies, with 11-OH-THC levels higher in female after THC administration, along with higher subjective ratings of drug effects210.
Metabolism and Excretion
Cannabinoids undergo a variety of metabolic processes in the gut, the liver, and various other tissues. Many metabolites have been identified, but the relative activity and toxicity of each is unknown. Once absorbed, THC is primarily oxidized by the cytochrome P450 hepatic mixed-function oxidase system to equipotent 11-hydroxy-THC (11-OH-THC), and further metabolized to inactive 11-COOH-THC. Most studies assessing the metabolic profile of THC have measured the plasma concentrations of these two metabolites, 11-OH-THC and 11-COOH-THC. The area under the concentration-time curve (AUC) of the psychoactive metabolite 11-OH-THC is 4-6 times lower compared to the AUC of THC itself after inhalational or IV administration, but overall, 11-OH-THC has a similar pharmacokinetic profile and elimination half-life171,172.
11-COOH-THC, the main cannabinoid metabolite detected upon urine drug screens, has average terminal elimination half-life of 3-5 days. However, due to high inter-patient variability, both in adults and adolescents, 11-COOH-THC can be detected in plasma or urine a month or more after biochemically-verified abstinence209.
CBD undergoes multiphasic elimination. Its effective half-life estimates ranged from 10 to 17 hours in humans, and the terminal elimination half-life is approximately 2-3 days221. The effective half-life of some of its metabolites, including 7-carboxy-cannabidiol (COOH-CBD), is around 24 hours, with plasma concentrations in hundreds of ng/mL detectable many days after single dose administration. In rats and mice, the half-life of CBD has been reported in the range of 1-4 hours, with some unexplained variability among administration by different routes and in different formulations20,54,137,244.
When oral CBD (Epidiolex®) was administered in people with variable extent of liver impairment, the total exposure (AUC) to CBD was increased by 50% in subjects with mild hepatic impairment, 2.5-fold in subjects with moderate, and 5-fold in severe hepatic impairment221.
There is debate in the literature on whether CBD potentiates or antagonises analgesic effects produced by THC. One hypothesis is that CBD is an inhibitor of CYP450 enzyme systems, and may affect the pharmacokinetic profile of THC. CBD does inhibit microsomal CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4 enzymes, but appears to do so at much higher concentrations than typically achieved in plasma with clinically-relevant doses138. In addition, in a human study of oromucosal THC and CBD administration, no major differences in THC pharmacokinetics were observed in the presence or absence of co-administered CBD138.
In summary, cannabinoids demonstrate substantial inter-individual variability in pharmacokinetics, particularly in absorption processes, whether via oral or inhaled routes. This can result in differences in drug concentrations at the desired sites of action, leading to inconsistent clinical effects. Only a minority of preclinical studies of cannabinoids and endocannabinoid system modulators in animal pain models have assessed drug pharmacokinetics. Particular attention is required in the future studies to understand the pharmacokinetic-pharmacodynamic relationships of drugs that interact with the endocannabinoid system, to improve translational success.
Discussion, conclusions and future perspectives
Preclinical laboratory animal models provide an opportunity to investigate molecular and cellular changes associated with cannabinoid-based interventions. Pharmacological, electrophysiological, genetic and optogenetic methodologies can facilitate understanding of the involvement of the endocannabinoid system in neural circuits, and the opportunities to modulate the system to interfere with nociception and pain behaviour.
Key differences exist between preclinical and clinical evaluations of cannabinoids, CBMs and endocannabinoid system modulators. Substantial evidence from the preclinical literature supports the hypothesis of cannabinoid-induced analgesia at multiple levels of analysis215, while there is less evidence of efficacy in human patients with pain and evidence is identified to be of low or very low-quality79. Foremost amongst these differences is that the clinical and preclinical literatures generally evaluate different compounds. For example, preclinical animal studies rarely test cannabis itself, while most human studies use whole cannabis or cannabis extracts containing THC and CBD in various ratios or doses and a limited number of other cannabinoids (e.g. THC or synthetic THC). Indeed, very few preclinical animal studies have evaluated vaporised/inhaled THC; none of the preclinical studies included in our meta-analysis assessed the effects of THC or cannabis administered in vaporised/inhaled form, and only 38 preclinical studies have administered cannabis extracts via other routes215. A recent preclinical brain imaging study showed that inhaled vaporised cannabis plant enriched in THC (10.3% THC; 0.,05% CBD) uncoupled brain resting state functional connectivity in the raphe nuclei in paclitaxel-treated rats, normalizing paclitaxel-induced hyperconnectivity to levels observed in vehicle-treated rats, and also produced antinociception in the cold plate test7. The paucity of such studies may reflect difficulty in administering cannabis plant material to rats and mice in a manner where the dose of THC and other phytocannabinoids administered would be precise. Preclinical animal studies have performed mechanistic evaluations using individual cannabinoids and endocannabinoid system modulators (or sometimes combinations of two or more of these), at very well-defined doses and via routes of administration (mostly parenteral) that yield predictable pharmacokinetics.
Second, a much wider variety of cannabinoids and endocannabinoid system modulators have been tested in animal models of pain than have been assessed in human pain patients. This point is underscored by comparing our IASP Task Force clinical and preclinical systemic reviews, which reviewed 1179 and 171215 pharmacological interventions, respectively. Ethical, safety and methodological standpoints require that novel/experimental drugs are administered in rodent models before human subjects, and where mechanism of action can also be identified. Significant barriers to performing clinical research with cannabis and cannabinoids also remain. There are, collectively, over 150 preclinical studies assessing the efficacy of CB2 receptor agonists, endocannabinoid re-uptake inhibitors, MGL inhibitors and FAAH inhibitors in animal models of pain, but not a single randomised controlled clinical trial (RCT) of the first 3 drug classes, and only 3 RCTs assessing the efficacy of FAAH inhibitors in pain patients19,123,232. This phenomenon, wherein the clinical studies lag significantly behind the preclinical studies, is, of course, to be expected, and is certainly not unique to the cannabinoid field. However, cannabis and cannabinoid use in humans has bypassed the usual preclinical and clinical studies typical of conventional drug development because of the widespread illicit, or more recently licit, use of cannabis by the public. The disparity that exists between the variety of cannabinoids and endocannabinoid system modulators that has been tested in animal models of pain versus what has been assessed in human pain patients should be kept in mind when comparing or contrasting efficacy of specific cannabinoids in animal models versus cannabis in human patients (in addition to translational relevance of the animal model and appropriateness of the clinical indication).
Another key difference between preclinical and clinical studies is that the latter very often administer the cannabinoid or cannabis as adjunctive/add-on treatment, alongside other analgesics that the patient is using. In contrast, preclinical studies in animals almost always investigate the cannabinoid drug against a ‘clean’ background, in the absence of any other analgesics (unless drug-drug interactions are specifically investigated). When administered adjunctively, the ‘window’ or opportunity to see therapeutic efficacy of the cannabinoid/cannabis may well be smaller than if it were investigated against a ‘clean’ background; if the other analgesics are already having partial effects in lowering pain, it may be more difficult for an add-on cannabinoid/cannabis to induce a further measurable reduction superimposed upon the pre-existing reduction.
Our accompanying systematic review and meta-analysis provides a comprehensive summary of studies in which cannabinoids, cannabis-based medicines and endocannabinoid system modulators were assessed for anti-nociceptive efficacy in animal models of injury-related or pathological persistent pain215. The overall behavioural data effect sizes are significant and it is clear that there is a spectrum of efficacy dependent on drug and pain model type, and also on species, strain and other experimental factors.
The risk of internal and external validity related biases confounding these data and exaggerating effect sizes is uncertain, but not insignificant. This could be better ascertained if future pre-clinical studies were reported with sufficient transparency to ascertain the methods that were used to mitigate against such biases8,121,140,143,201,203. There is a need for preclinical living systematic reviews that incorporate new evidence as it becomes available71, and closer, multi-disciplinary, cross sector collaboration to ensure that laboratory animal studies have sufficient rigor to identify promising candidate drugs and more accurately inform clinical trial design.
Several intervention strategies offer the potential for analgesia while also circumventing adverse side effects of direct acting CB1 agonists. These include cannabinoid CB2 agonists, peripherally restricted CB1 agonists, inhibitors of endocannabinoid deactivation (especially FAAH inhibitors), synergism between cannabinoids and other analgesics, mode of drug delivery (e.g. local, targeted delivery), biased agonism, CB1 PAMs and CBD. FAAH represents a promising target whose engagement is likely to show a reduced pattern of unwanted CB1-mediated effects, produce anxiolytic effects and opioid-sparing effects without being inherently reinforcing. Moreover, the recent case report of a woman with a mutation in the FAAH gene who does not experience pain or anxiety provides further support that FAAH inhibitors may not only exhibit reduced adverse side effects compared to conventional analgesic but may also exhibit additional desirable therapeutic properties (i.e. reduction of stress, fear and anxiety)64,160. With regards to reduction of abuse liability, CB2 receptor agonists deserve especial mention for their potential to produce anti-addiction effects9,17,130,175,212,249. CBD represents another promising therapeutic agent which does not produce THC-like effects in humans or animals95,127,173. The mechanism underlying therapeutic effects of CBD remains incompletely understood52,95. Preclinical studies nonetheless, suggest that CBD may attenuate opioid addiction124,125, THC withdrawal168, reduce nausea154,185, suppress inflammation and nociceptive behavior9,17,43,195,243 and attenuate stress responding14,16,33,47,72,119,136,149. Further preclinical studies of underlying mechanisms as well as clinical trials of CBD are warranted to better ascertain the therapeutic effects that may be exploited as adjunct or unitary therapy. There is also a pressing requirement to better understand the pharmacology and therapeutic potential of the other phytocannabinoids in the cannabis plant beyond THC and CBD, and whether/how these may interact with THC and CBD in the context of analgesia. Given the substantial inter-individual differences in cannabinoid pharmacokinetics among humans, future research is needed to improve the consistency of drug delivery methods, for better translational of preclinical developments to human studies. Preclinical laboratory animal models are evolving to capture aspects associated with pathological pain states observed clinically e.g. spontaneous pain, anxiety, stress and depression comorbidities, conditions under which dynamic changes in endocannabinoid tone, enzyme activity or receptors may be observed (for review see42,76,80,90,165,187). Consequently, future preclinical studies in laboratory animals, that evaluate novel cannabinoid interventions, reference analgesics, as well as drug development candidates that previously failed for efficacy, are required to validate treatments, understand mechanisms of action, and enhance clinical translation.
Acknowledgements.
This work is part of the effort of the IASP Presidential Taskforce on cannabis and cannabinoid analgesia. We would like to thank Dr. Emer Power and Darragh Mattimoe for assistance with preparation of figures, and Dr. Christopher Eccleston and Dr. Emma Fisher for comments on the manuscript.
Funding:
The International Association for the Study of Pain commissioned this work in the form of a Presidential Task Force and funded attendance for the authors at a working meeting in Washington DC, November 2019.
Conflicts of Interest
David P. Finn – Dr. Finn reports an Industry-Academia research grant from Alkermes Inc. and Science Foundation Ireland outside of the submitted work. He also reports research grants in the area of cannabinoids or the endocannabinoid system from Shionogi Ltd (Shionogi Science Programme), from B. Braun Ltd jointly with Science Foundation Ireland, and from the Irish Research Council, CNPq Brazil and EU INTERREG Programmes.
Simon Haroutounian – Dr. Haroutounian reports grants from Disarm Therapeutics, and advisory board/consultancy fees from Vertex Pharmaceuticals, Medoc Ltd and Rafa laboratories, outside the submitted work.
Andrea G Hohmann – Dr. Hohmann reports grants from the National Institutes of Health (National Institute on Drug Abuse, National Cancer Institute, National Institute of Neurological Diseases and Stroke), Indiana Addiction Grand Challenge and is a co-inventor on a provisional patent related to CB2-opioid interactions.
Elliot Krane – Dr. Krane has nothing to declare.
Nadia Soliman – Ms. Soliman has nothing to declare.
Andrew SC Rice – Dr. Rice is a Council Member of IASP and Chair of the Presidential Task Force of the IASP, He undertook consultancy and advisory board work for Imperial College Consultants-in the last 24 months this has included personally remunerated work outside of the submitted work for: Abide, Confo, Vertex, Pharmanovo, Lateral, Novartis, Mundipharma, Orion, Asahi Kasei, Toray & Theranexis. He was the owner of share options in Spinifex Pharmaceuticals from which personal benefit accrued between 2015 and 2019 upon the acquisition of Spinifex by Novartis. Prof Rice is a named inventor on the patents – Rice A.S.C, Vandevoorde S. and Lambert D. M. Methods using N-(2propenyl)hexadecanamide and related amides to relieve pain. WO2005/079771 pending, and Okuse K. et al Methods of treating pain by inhibition of vgf activity EP13702262.0/WO2013110945. During the conduct of the study Imperial College received grants funding to support Prof Rice’s programme of research from Biotechnology and Biological Sciences Research Council (BBSRC), Medical Research Council (MRC), Wellcome Trust, Alan and Sheila Diamond Charitable Trust, British Pain Society, Royal British Legion and the European Commission (IMI2 (EQIPD); FP7 (Neuropain) and H2020 (Dolorisk)).
Footnotes
The term ‘animal model of pain’ is not a universally agreed descriptor, but given that it is common usage we will use it herein as shorthand. It is also worth noting that there is a distinction between ‘model’ which reflects the underlying disease or injury and the pain-associated outcome measures used in evaluating such models.
References
- 1.Abbott FV, Franklin KB, Westbrook RF. The formalin test: scoring properties of the first and second phases of the pain response in rats. Pain. 60:91–102, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Adam JM, Clark JK, Davies K, Everett K, Fields R, Francis S, Jeremiah F, Kiyoi T, Maidment M, Morrison A, Ratcliffe P, Prosser A, Schulz J, Wishart G, Baker J, Boyce S, Campbell R, Cottney JE, Deehan M, Martin I. Low brain penetrant CB1 receptor agonists for the treatment of neuropathic pain. Bioorg Med Chem Lett. 22:2932–2937, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Adamson Barnes NS, Mitchell VA, Kazantzis NP, Vaughan CW. Actions of the dual FAAH/MAGL inhibitor JZL195 in a murine neuropathic pain model. Br J Pharmacol. 173:77–87, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed AI, van den Elsen GA, Colbers A, van der Marck MA, Burger DM, Feuth TB, Rikkert MG, Kramers C. Safety and pharmacokinetics of oral delta-9-tetrahydrocannabinol in healthy older subjects: a randomized controlled trial. Eur Neuropsychopharmacol. 24:1475–1482, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Ahn K, Smith SE, Liimatta MB, Beidler D, Sadagopan N, Dudley DT, Young T, Wren P, Zhang Y, Swaney S, Van Becelaere K, Blankman JL, Nomura DK, Bhattachar SN, Stiff C, Nomanbhoy TK, Weerapana E, Johnson DS, Cravatt BF. Mechanistic and pharmacological characterization of PF-04457845: a highly potent and selective fatty acid amide hydrolase inhibitor that reduces inflammatory and noninflammatory pain. J Pharmacol Exp Ther. 338:114–124, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexander SP, Kendall DA. The complications of promiscuity: endocannabinoid action and metabolism. Br J Pharmacol. 152:602–623, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alkislar I, Miller AR, Hohmann AG, Sadaka A, Cai X, Kulkarni P, Ferris CF Inhaled cannabis suppresses chemotherapy-induced neuropathic nociception by decoupling the raphe nucleus: a functional imaging study in rats. Biological Psychiatry, In Press. 10.1016/j.bpsc.2020.11.015, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrews NA, Latremoliere A, Basbaum AI, Mogil JS, Porreca F, Rice AS, Woolf CJ, Currie GL, Dworkin RH, Eisenach JC, Evans S, Gewandter JS, Gover TD, Handwerker H, Huang W, Iyengar S, Jensen MP, Kennedy JD, Lee N, Levine J, Lidster K, Machin I, McDermott MP, McMahon SB, Price TJ, Ross SE, Scherrer G, Seal RP, Sena ES, Silva E, Stone L, Svensson CI, Turk DC, Whiteside G. Ensuring transparency and minimization of methodologic bias in preclinical pain research: PPRECISE considerations. Pain. 157:901–909, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atwal N, Casey SL, Mitchell VA, Vaughan CW. THC and gabapentin interactions in a mouse neuropathic pain model. Neuropharmacology. 144:115–121, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Battista N, Di Tommaso M, Bari M, Maccarrone M. The endocannabinoid system: an overview. Front Behav Neurosci. 6:9, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berdyshev EV. Cannabinoid receptors and the regulation of immune response. Chem Phys Lipids. 108:169–190., 2000 [DOI] [PubMed] [Google Scholar]
- 12.Berman P, Futoran K, Lewitus GM, Mukha D, Benami M, Shlomi T, Meiri D. A new ESI-LC/MS approach for comprehensive metabolic profiling of phytocannabinoids in Cannabis. Sci Rep. 8:14280, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bisogno T, Ligresti A, Di Marzo V. The endocannabinoid signalling system: biochemical aspects. Pharmacol Biochem Behav. 81:224–238, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Bitencourt RM, Takahashi RN. Cannabidiol as a Therapeutic Alternative for Post-traumatic Stress Disorder: From Bench Research to Confirmation in Human Trials. Front Neurosci. 12:502, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 14:1347–1356, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blessing EM, Steenkamp MM, Manzanares J, Marmar CR. Cannabidiol as a Potential Treatment for Anxiety Disorders. Neurotherapeutics. 12:825–836, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Booz GW. Cannabidiol as an emergent therapeutic strategy for lessening the impact of inflammation on oxidative stress. Free Radic Biol Med. 51:1054–1061, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouaboula M, Poinot-Chazel C, Bourrie B, Canat X, Calandra B, Rinaldi-Carmona M, Le Fur G, Casellas P. Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1. Biochem J. 312:637–641., 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bradford D, Stirling A, Ernault E, Liosatos M, Tracy K, Moseley J, Blahunka P, Smith MD. The MOBILE Study-A Phase IIa Enriched Enrollment Randomized Withdrawal Trial to Assess the Analgesic Efficacy and Safety of ASP8477, a Fatty Acid Amide Hydrolase Inhibitor, in Patients with Peripheral Neuropathic Pain. Pain Med. 18:2388–2400, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brenneman DE, Petkanas D, Kinney WA. Pharmacological Comparisons Between Cannabidiol and KLS-13019. J Mol Neurosci. 66:121–134, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bridges D, Rice AS, Egertova M, Elphick MR, Winter J, Michael GJ. Localisation of cannabinoid receptor 1 in rat dorsal root ganglion using in situ hybridisation and immunohistochemistry. Neuroscience. 119:803–812, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Britch SC, Babalonis S, Walsh SL. Cannabidiol: pharmacology and therapeutic targets. Psychopharmacology (Berl). 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown AJ. Novel cannabinoid receptors. Br J Pharmacol. 152:567–575, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brunet B, Doucet C, Venisse N, Hauet T, Hébrard W, Papet Y, Mauco G, Mura P. Validation of Large White Pig as an animal model for the study of cannabinoids metabolism: application to the study of THC distribution in tissues. Forensic Sci Int. 161:169–174, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Bruni N, Della Pepa C, Oliaro-Bosso S, Pessione E, Gastaldi D, Dosio F. Cannabinoid Delivery Systems for Pain and Inflammation Treatment. Molecules. 23, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bujalska-Zadrozny M, de Corde A, Pawlik K. Influence of nitric oxide synthase or cyclooxygenase inhibitors on cannabinoids activity in streptozotocin-induced neuropathy. Pharmacol Rep. 67:209–216, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Butler RK, Finn DP. Stress-induced analgesia. Prog Neurobiol. 88:184–202, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Butler RK, Rea K, Lang Y, Gavin AM, Finn DP. Endocannabinoid-mediated enhancement of fear-conditioned analgesia in rats: Opioid receptor dependency and molecular correlates. Pain. 140:491–500, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Cabanero D, Ramirez-Lopez A, Drews E, Schmole A, Otte DM, Wawrzczak-Bargiela A, Huerga Encabo H, Kummer S, Ferrer-Montiel A, Przewlocki R, Zimmer A, Maldonado R. Protective role of neuronal and lymphoid cannabinoid CB2 receptors in neuropathic pain. Elife. 9, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cadas H, di Tomaso E, Piomelli D. Occurrence and biosynthesis of endogenous cannabinoid precursor, N-arachidonoyl phosphatidylethanolamine, in rat brain. J Neurosci. 17:1226–1242, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cadas H, Gaillet S, Beltramo M, Venance L, Piomelli D. Biosynthesis of an endogenous cannabinoid precursor in neurons and its control by calcium and cAMP. J Neurosci. 16:3934–3942, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calignano A, La Rana G, Giuffrida A, Piomelli D. Control of pain initiation by endogenous cannabinoids. Nature. 394:277–281, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Campos AC, Ferreira FR, Guimaraes FS. Cannabidiol blocks long-lasting behavioral consequences of predator threat stress: possible involvement of 5HT1A receptors. J Psychiatr Res. 46:1501–1510, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Casey SL, Atwal N, Vaughan CW. Cannabis constituent synergy in a mouse neuropathic pain model. Pain. 158:2452–2460, 2017 [DOI] [PubMed] [Google Scholar]
- 35.Chen JP, Paredes W, Li J, Smith D, Lowinson J, Gardner EL. Delta 9-tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology (Berl). 102:156–162, 1990 [DOI] [PubMed] [Google Scholar]
- 36.Cherniakov I, Izgelov D, Barasch D, Davidson E, Domb AJ, Hoffman A. Piperine-pro-nanolipospheres as a novel oral delivery system of cannabinoids: Pharmacokinetic evaluation in healthy volunteers in comparison to buccal spray administration. J Control Release. 266:1–7, 2017 [DOI] [PubMed] [Google Scholar]
- 37.Cherniakov I, Izgelov D, Domb AJ, Hoffman A. The effect of Pro NanoLipospheres (PNL) formulation containing natural absorption enhancers on the oral bioavailability of delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) in a rat model. Eur J Pharm Sci. 109:21–30, 2017 [DOI] [PubMed] [Google Scholar]
- 38.Clapper JR, Moreno-Sanz G, Russo R, Guijarro A, Vacondio F, Duranti A, Tontini A, Sanchini S, Sciolino NR, Spradley JM, Hohmann AG, Calignano A, Mor M, Tarzia G, Piomelli D. Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism. Nat Neurosci. 13:1265–1270, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 509:282–283, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conner SN, Bedell V, Lipsey K, Macones GA, Cahill AG, Tuuli MG. Maternal Marijuana Use and Adverse Neonatal Outcomes: A Systematic Review and Meta-analysis. Obstet Gynecol. 128:713–723, 2016 [DOI] [PubMed] [Google Scholar]
- 41.Conner SN, Carter EB, Tuuli MG, Macones GA, Cahill AG. Maternal marijuana use and neonatal morbidity. Am J Obstet Gynecol. 213:422 e421–424, 2015 [DOI] [PubMed] [Google Scholar]
- 42.Corcoran L, Roche M, Finn DP. The Role of the Brain’s Endocannabinoid System in Pain and Its Modulation by Stress. Int Rev Neurobiol. 125:203–255, 2015 [DOI] [PubMed] [Google Scholar]
- 43.Costa B, Trovato AE, Comelli F, Giagnoni G, Colleoni M. The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur J Pharmacol. 556:75–83, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Cox ML, Haller VL, Welch SP. The antinociceptive effect of Delta9-tetrahydrocannabinol in the arthritic rat involves the CB(2) cannabinoid receptor. Eur J Pharmacol. 570:50–56, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Cox ML, Haller VL, Welch SP. Synergy between delta9-tetrahydrocannabinol and morphine in the arthritic rat. Eur J Pharmacol. 567:125–130, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 384:83–87, 1996 [DOI] [PubMed] [Google Scholar]
- 47.Crippa JA, Guimaraes FS, Campos AC, Zuardi AW. Translational Investigation of the Therapeutic Potential of Cannabidiol (CBD): Toward a New Age. Front Immunol. 9:2009, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crossley NA, Sena E, Goehler J, Horn J, van der Worp B, Bath PM, Macleod M, Dirnagl U. Empirical evidence of bias in the design of experimental stroke studies: a metaepidemiologic approach. Stroke. 39:929–934, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Crowe MS, Leishman E, Banks ML, Gujjar R, Mahadevan A, Bradshaw HB, Kinsey SG. Combined inhibition of monoacylglycerol lipase and cyclooxygenases synergistically reduces neuropathic pain in mice. Br J Pharmacol. 172:1700–1712, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Curry ZA, Wilkerson JL, Bagdas D, Kyte SL, Patel N, Donvito G, Mustafa MA, Poklis JL, Niphakis MJ, Hsu KL, Cravatt BF, Gewirtz DA, Damaj MI, Lichtman AH. Monoacylglycerol Lipase Inhibitors Reverse Paclitaxel-Induced Nociceptive Behavior and Proinflammatory Markers in a Mouse Model of Chemotherapy-Induced Neuropathy. J Pharmacol Exp Ther. 366:169–183, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.D’Souza DC, Cortes-Briones J, Creatura G, Bluez G, Thurnauer H, Deaso E, Bielen K, Surti T, Radhakrishnan R, Gupta A, Gupta S, Cahill J, Sherif MA, Makriyannis A, Morgan PT, Ranganathan M, Skosnik PD. Efficacy and safety of a fatty acid amide hydrolase inhibitor (PF-04457845) in the treatment of cannabis withdrawal and dependence in men: a double-blind, placebo-controlled, parallel group, phase 2a single-site randomised controlled trial. Lancet Psychiatry. 6:35–45, 2019 [DOI] [PubMed] [Google Scholar]
- 52.De Gregorio D, McLaughlin RJ, Posa L, Ochoa-Sanchez R, Enns J, Lopez-Canul M, Aboud M, Maione S, Comai S, Gobbi G. Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain. 160:136–150, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deciga-Campos M, Ortiz-Andrade R. Enhancement of Antihyperalgesia by the Coadministration of N-palmitoylethanolamide and Acetaminophen in Diabetic Rats. Drug Dev Res. 76:228–234, 2015 [DOI] [PubMed] [Google Scholar]
- 54.Deiana S, Watanabe A, Yamasaki Y, Amada N, Arthur M, Fleming S, Woodcock H, Dorward P, Pigliacampo B, Close S, Platt B, Riedel G. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Delta(9)-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive behaviour. Psychopharmacology (Berl). 219:859–873, 2012 [DOI] [PubMed] [Google Scholar]
- 55.Demuth DG, Molleman A. Cannabinoid signalling. Life Sci. 78:549–563, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Deng L, Cornett BL, Mackie K, Hohmann AG. CB1 Knockout Mice Unveil Sustained CB2-Mediated Antiallodynic Effects of the Mixed CB1/CB2 Agonist CP55,940 in a Mouse Model of Paclitaxel-Induced Neuropathic Pain. Mol Pharmacol. 88:64–74, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Devane WA, Dysarz FA 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 34:605–613, 1988 [PubMed] [Google Scholar]
- 58.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor [see comments]. Science. 258:1946–1949, 1992 [DOI] [PubMed] [Google Scholar]
- 59.Di Marzo V Endocannabinoids: synthesis and degradation. Reviews of physiology, biochemistry and pharmacology. 160:1–24, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Di Marzo V Targeting the endocannabinoid system: to enhance or reduce? Nat Rev Drug Discov. 7:438–455, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Di Marzo V, Breivogel CS, Tao Q, Bridgen DT, Razdan RK, Zimmer AM, Zimmer A, Martin BR. Levels, metabolism, and pharmacological activity of anandamide in CB(1) cannabinoid receptor knockout mice: evidence for non-CB(1), non-CB(2) receptor-mediated actions of anandamide in mouse brain. J Neurochem. 75:2434–2444., 2000 [DOI] [PubMed] [Google Scholar]
- 62.Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 372:686–691, 1994 [DOI] [PubMed] [Google Scholar]
- 63.Di Marzo V, Stella N, Zimmer A. Endocannabinoid signalling and the deteriorating brain. Nat Rev Neurosci. 16:30–42, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dincheva I, Drysdale AT, Hartley CA, Johnson DC, Jing D, King EC, Ra S, Gray JM, Yang R, DeGruccio AM, Huang C, Cravatt BF, Glatt CE, Hill MN, Casey BJ, Lee FS. FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nat Commun. 6:6395, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 99:10819–10824, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Donvito G, Nass SR, Wilkerson JL, Curry ZA, Schurman LD, Kinsey SG, Lichtman AH. The Endogenous Cannabinoid System: A Budding Source of Targets for Treating Inflammatory and Neuropathic Pain. Neuropsychopharmacology. 43:52–79, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Donvito G, Wilkerson JL, Damaj MI, Lichtman AH. Palmitoylethanolamide Reverses Paclitaxel-Induced Allodynia in Mice. J Pharmacol Exp Ther. 359:310–318, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dos Santos RG, Hallak JEC, Crippa JAS. Neuropharmacological Effects of the Main Phytocannabinoids: A Narrative Review. Adv Exp Med Biol. 1264:29–45, 2021 [DOI] [PubMed] [Google Scholar]
- 69.Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 4:161–174, 1977 [DOI] [PubMed] [Google Scholar]
- 70.Eisenberg E, Ogintz M, Almog S. The pharmacokinetics, efficacy, safety, and ease of use of a novel portable metered-dose cannabis inhaler in patients with chronic neuropathic pain: a phase 1a study. J Pain Palliat Care Pharmacother. 28:216–225, 2014 [DOI] [PubMed] [Google Scholar]
- 71.Elliott JH, Synnot A, Turner T, Simmonds M, Akl EA, McDonald S, Salanti G, Meerpohl J, MacLehose H, Hilton J, Tovey D, Shemilt I, Thomas J, Living Systematic Review N. Living systematic review: 1. Introduction-the why, what, when, and how. J Clin Epidemiol. 91:23–30, 2017 [DOI] [PubMed] [Google Scholar]
- 72.Elms L, Shannon S, Hughes S, Lewis N. Cannabidiol in the Treatment of Post-Traumatic Stress Disorder: A Case Series. J Altern Complement Med. 25:392–397, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Endo T, Takeuchi T, Maehara S. Pharmacological characterization of a novel, potent, selective, and orally active fatty acid amide hydrolase inhibitor, PKM-833 [(R)-N-(pyridazin-3-yl)-4-(7-(trifluoromethyl)chroman-4-yl)piperazine-1-carboxami de] in rats: Potential for the treatment of inflammatory pain. Pharmacol Res Perspect. 8:e00569, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Farquhar-Smith WP, Egertova M, Bradbury EJ, McMahon SB, Rice AS, Elphick MR. Cannabinoid CB(1) receptor expression in rat spinal cord. Mol Cell Neurosci. 15:510–521., 2000 [DOI] [PubMed] [Google Scholar]
- 75.Fine PG, Rosenfeld MJ. The endocannabinoid system, cannabinoids, and pain. Rambam Maimonides Med J. 4:e0022, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Finn DP. Endocannabinoid-mediated modulation of stress responses: physiological and pathophysiological significance. Immunobiology. 215:629–646, 2010 [DOI] [PubMed] [Google Scholar]
- 77.Finn DP, Beckett SR, Richardson D, Kendall DA, Marsden CA, Chapman V. Evidence for differential modulation of conditioned aversion and fear-conditioned analgesia by CB1 receptors. Eur J Neurosci. 20:848–852, 2004 [DOI] [PubMed] [Google Scholar]
- 78.Finn DP, Beckett SR, Roe CH, Madjd A, Fone KC, Kendall DA, Marsden CA, Chapman V. Effects of coadministration of cannabinoids and morphine on nociceptive behaviour, brain monoamines and HPA axis activity in a rat model of persistent pain. Eur J Neurosci. 19:678–686, 2004 [DOI] [PubMed] [Google Scholar]
- 79.Fisher E, Moore RA, Fogarty AE, Finn DP, Finnerup NB, Gilron I, Haroutounian S, Krane E, Rice ASC, Rowbotham M, Wallace M, Eccleston C. Cannabinoids, cannabis, and cannabis-based medicine for pain management: a systematic review of randomised controlled trials. Pain, In Press. doi: 10.1097/j.pain.0000000000001929, 2020 [DOI] [PubMed] [Google Scholar]
- 80.Fitzgibbon M, Finn DP, Roche M. High Times for Painful Blues: The Endocannabinoid System in Pain-Depression Comorbidity. Int J Neuropsychopharmacol. 19:pyv095, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fox A, Kesingland A, Gentry C, McNair K, Patel S, Urban L, James I. The role of central and peripheral Cannabinoid1 receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic pain. Pain. 92:91–100, 2001 [DOI] [PubMed] [Google Scholar]
- 82.Franco V, Perucca E. Pharmacological and Therapeutic Properties of Cannabidiol for Epilepsy. Drugs. 79:1435–1454, 2019 [DOI] [PubMed] [Google Scholar]
- 83.Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. Journal of American Chemical Society. 86:1646–1647, 1964 [Google Scholar]
- 84.Genaro K, Fabris D, Arantes ALF, Zuardi AW, Crippa JAS, Prado WA. Cannabidiol Is a Potential Therapeutic for the Affective-Motivational Dimension of Incision Pain in Rats. Front Pharmacol. 8:391, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gershkovich P, Qadri B, Yacovan A, Amselem S, Hoffman A. Different impacts of intestinal lymphatic transport on the oral bioavailability of structurally similar synthetic lipophilic cannabinoids: dexanabinol and PRS-211,220. Eur J Pharm Sci. 31:298–305, 2007 [DOI] [PubMed] [Google Scholar]
- 86.Giang DK, Cravatt BF. Molecular characterization of human and mouse fatty acid amide hydrolases. Proc Natl Acad Sci U S A. 94:2238–2242, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ginsburg BC, Hruba L, Zaki A, Javors MA, McMahon LR. Blood levels do not predict behavioral or physiological effects of Δ9-tetrahydrocannabinol in rhesus monkeys with different patterns of exposure. Drug Alcohol Depend. 139:1–8, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Glass M, Dragunow M, Faull RLM. Cannabinoid receptors in the human brain: A detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 77:299–318, 1997 [DOI] [PubMed] [Google Scholar]
- 89.Goparaju SK, Ueda N, Yamaguchi H, Yamamoto S. Anandamide amidohydrolase reacting with 2-arachidonoylglycerol, another cannabinoid receptor ligand. FEBS Lett. 422:69–73, 1998 [DOI] [PubMed] [Google Scholar]
- 90.Gorzalka BB, Hill MN. Putative role of endocannabinoid signaling in the etiology of depression and actions of antidepressants. Prog Neuropsychopharmacol Biol Psychiatry. 35:1575–1585, 2011 [DOI] [PubMed] [Google Scholar]
- 91.Grant KS, Petroff R, Isoherranen N, Stella N, Burbacher TM. Cannabis use during pregnancy: Pharmacokinetics and effects on child development. Pharmacol Ther. 182:133–151, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gregg LC, Jung KM, Spradley JM, Nyilas R, Suplita RL 2nd, Zimmer A, Watanabe M, Mackie K, Katona I, Piomelli D, Hohmann AG. Activation of type 5 metabotropic glutamate receptors and diacylglycerol lipase-alpha initiates 2-arachidonoylglycerol formation and endocannabinoid-mediated analgesia. J Neurosci. 32:9457–9468, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grenald SA, Young MA, Wang Y, Ossipov MH, Ibrahim MM, Largent-Milnes TM, Vanderah TW. Synergistic attenuation of chronic pain using mu opioid and cannabinoid receptor 2 agonists. Neuropharmacology. 116:59–70, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grotenhermen F, Muller-Vahl K. The therapeutic potential of cannabis and cannabinoids. Dtsch Arztebl Int. 109:495–501, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grotenhermen F, Russo E, Zuardi AW. Even High Doses of Oral Cannabidol Do Not Cause THC-Like Effects in Humans: Comment on Merrick et al. Cannabis and Cannabinoid Research 2016; 1(1):102–112; DOI: 10.1089/can.2015.0004. Cannabis Cannabinoid Res. 2:1-4, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guindon J, Beaulieu P. The role of the endogenous cannabinoid system in peripheral analgesia. Curr Mol Pharmacol. 2:134–139, 2009 [DOI] [PubMed] [Google Scholar]
- 97.Guindon J, De Lean A, Beaulieu P. Local interactions between anandamide, an endocannabinoid, and ibuprofen, a nonsteroidal anti-inflammatory drug, in acute and inflammatory pain. Pain. 121:85–93, 2006 [DOI] [PubMed] [Google Scholar]
- 98.Guindon J, Hohmann AG. Cannabinoid CB2 receptors: a therapeutic target for the treatment of inflammatory and neuropathic pain. Br J Pharmacol. 153:319–334, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guindon J, Hohmann AG. The endocannabinoid system and pain. CNS Neurol Disord Drug Targets. 8:403–421, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guindon J, Lai Y, Takacs SM, Bradshaw HB, Hohmann AG. Alterations in endocannabinoid tone following chemotherapy-induced peripheral neuropathy: Effects of endocannabinoid deactivation inhibitors targeting fatty-acid amide hydrolase and monoacylglycerol lipase in comparison to reference analgesics following cisplatin treatment. Pharmacol Res. 67:94–109, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guindon J, LoVerme J, De Lean A, Piomelli D, Beaulieu P. Synergistic antinociceptive effects of anandamide, an endocannabinoid, and nonsteroidal anti-inflammatory drugs in peripheral tissue: a role for endogenous fatty-acid ethanolamides? Eur J Pharmacol. 550:68–77, 2006 [DOI] [PubMed] [Google Scholar]
- 102.Gutierrez T, Crystal JD, Zvonok AM, Makriyannis A, Hohmann AG. Self-medication of a cannabinoid CB2 agonist in an animal model of neuropathic pain. Pain. 152:1976–1987, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hanus LO, Meyer SM, Munoz E, Taglialatela-Scafati O, Appendino G. Phytocannabinoids: a unified critical inventory. Nat Prod Rep. 33:1357–1392, 2016 [DOI] [PubMed] [Google Scholar]
- 104.Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 11:563–583, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hirst JA, Howick J, Aronson JK, Roberts N, Perera R, Koshiaris C, Heneghan C. The need for randomization in animal trials: an overview of systematic reviews. PLoS One. 9:e98856, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hložek T, Uttl L, Kadeřábek L, Balíková M, Lhotková E, Horsley RR, Nováková P, Šíchová K, Štefková K, Tylš F, Kuchař M, Páleníček T. Pharmacokinetic and behavioural profile of THC, CBD, and THC+CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. Eur Neuropsychopharmacol. 27:1223–1237, 2017 [DOI] [PubMed] [Google Scholar]
- 107.Hohmann AG. Spinal and peripheral mechanisms of cannabinoid antinociception: behavioral, neurophysiological and neuroanatomical perspectives. Chemistry and Physics of Lipids. 121:173–190, 2002 [DOI] [PubMed] [Google Scholar]
- 108.Hohmann AG, Briley EM, Herkenham M. Pre- and postsynaptic distribution of cannabinoid and mu opioid receptors in rat spinal cord. Brain Res. 822:17–25., 1999 [DOI] [PubMed] [Google Scholar]
- 109.Hohmann AG, Herkenham M. Regulation of cannabinoid and mu opioid receptors in rat lumbar spinal cord following neonatal capsaicin treatment. Neurosci Lett. 252:13–16., 1998 [DOI] [PubMed] [Google Scholar]
- 110.Hohmann AG, Herkenham M. Cannabinoid receptors undergo axonal flow in sensory nerves. Neuroscience. 92:1171–1175, 1999 [DOI] [PubMed] [Google Scholar]
- 111.Hohmann AG, Herkenham M. Localization of central cannabinoid CB1 receptor messenger RNA in neuronal subpopulations of rat dorsal root ganglia: a double-label in situ hybridization study. Neuroscience. 90:923–931., 1999 [DOI] [PubMed] [Google Scholar]
- 112.Hohmann AG, Martin WJ, Tsou K, Walker JM. Inhibition of noxious stimulus-evoked activity of spinal cord dorsal horn neurons by the cannabinoid WIN 55,212-2. Life Sci. 56:2111–2118, 1995 [DOI] [PubMed] [Google Scholar]
- 113.Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes PV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. An endocannabinoid mechanism for stress-induced analgesia. Nature. 435:1108–1112, 2005 [DOI] [PubMed] [Google Scholar]
- 114.Hohmann AG, Tsou K, Walker JM. Cannabinoid modulation of wide dynamic range neurons in the lumbar dorsal horn of the rat by spinally administered WIN55,212-2. Neurosci Lett. 257:119–122, 1998 [DOI] [PubMed] [Google Scholar]
- 115.Hohmann AG, Tsou K, Walker JM. Intrathecal cannabinoid administration suppresses noxious stimulus- evoked Fos protein-like immunoreactivity in rat spinal cord: Comparison with morphine. Acta Pharmacol. Sin 20:1132–1136, 1999 [PubMed] [Google Scholar]
- 116.Howlett AC. Inhibition of neuroblastoma adenylate cyclase by cannabinoid and nantradol compounds. Life Sci. 35:1803–1810., 1984 [DOI] [PubMed] [Google Scholar]
- 117.Howlett AC, Fleming RM. Cannabinoid inhibition of adenylate cyclase. Pharmacology of the response in neuroblastoma cell membranes. Mol Pharmacol. 26:532–538., 1984 [PubMed] [Google Scholar]
- 118.Howlett AC, Mukhopadhyay S, Shim JY, Welsh WJ. Signal transduction of eicosanoid CB1 receptor ligands. Life Sci. 65:617–625, 1999 [DOI] [PubMed] [Google Scholar]
- 119.Hsiao YT, Yi PL, Li CL, Chang FC. Effect of cannabidiol on sleep disruption induced by the repeated combination tests consisting of open field and elevated plus-maze in rats. Neuropharmacology. 62:373–384, 2012 [DOI] [PubMed] [Google Scholar]
- 120.Hu B, Doods H, Treede RD, Ceci A. Depression-like behaviour in rats with mononeuropathy is reduced by the CB2-selective agonist GW405833. Pain. 143:206–212, 2009 [DOI] [PubMed] [Google Scholar]
- 121.Huang W, Percie du Sert N, Vollert J, Rice ASC. General Principles of Preclinical Study Design. Handb Exp Pharmacol. 257:55–69, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huestis M: Pharmacokinetics of THC in Inhaled and Oral Preparations. In: Marihuana and Medicine. (Nahas GG, Sutin KM, Harvey DJ, Agurell S, Ed.), Humana Press, 1999, pp. 105–116. [Google Scholar]
- 123.Huggins JP, Smart TS, Langman S, Taylor L, Young T. An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. Pain. 153:1837–1846, 2012 [DOI] [PubMed] [Google Scholar]
- 124.Hurd YL, Spriggs S, Alishayev J, Winkel G, Gurgov K, Kudrich C, Oprescu AM, Salsitz E. Cannabidiol for the Reduction of Cue-Induced Craving and Anxiety in Drug-Abstinent Individuals With Heroin Use Disorder: A Double-Blind Randomized Placebo-Controlled Trial. Am J Psychiatry. 176:911–922, 2019 [DOI] [PubMed] [Google Scholar]
- 125.Hurd YL, Yoon M, Manini AF, Hernandez S, Olmedo R, Ostman M, Jutras-Aswad D. Early Phase in the Development of Cannabidiol as a Treatment for Addiction: Opioid Relapse Takes Initial Center Stage. Neurotherapeutics. 12:807–815, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ibrahim MM, Deng H, Zvonok A, Cockayne DA, Kwan J, Mata HP, Vanderah TW, Lai J, Porreca F, Makriyannis A, Malan TP Jr. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci U S A. 100:10529–10533, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Iffland K, Grotenhermen F. An Update on Safety and Side Effects of Cannabidiol: A Review of Clinical Data and Relevant Animal Studies. Cannabis Cannabinoid Res. 2:139–154, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ignatowska-Jankowska B, Wilkerson JL, Mustafa M, Abdullah R, Niphakis M, Wiley JL, Cravatt BF, Lichtman AH. Selective monoacylglycerol lipase inhibitors: antinociceptive versus cannabimimetic effects in mice. J Pharmacol Exp Ther. 353:424–432, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ignatowska-Jankowska BM, Baillie GL, Kinsey S, Crowe M, Ghosh S, Owens RA, Damaj IM, Poklis J, Wiley JL, Zanda M, Zanato C, Greig IR, Lichtman AH, Ross RA. A Cannabinoid CB1 Receptor-Positive Allosteric Modulator Reduces Neuropathic Pain in the Mouse with No Psychoactive Effects. Neuropsychopharmacology. 40:2948–2959, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Iyer V, Slivicki RA, Thomaz AC, Crystal JD, Mackie K, Hohmann AG. The cannabinoid CB2 receptor agonist LY2828360 synergizes with morphine to suppress neuropathic nociception and attenuates morphine reward and physical dependence. Eur J Pharmacol. 886:173544, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Izgelov D, Regev A, Domb AJ, Hoffman A. Using the Absorption Cocktail Approach to Assess Differential Absorption Kinetics of Cannabidiol Administered in Lipid-Based Vehicles in Rats. Mol Pharm. 17:1979–1986, 2020 [DOI] [PubMed] [Google Scholar]
- 132.Jiang HX, Ke BW, Liu J, Ma G, Hai KR, Gong DY, Yang Z, Zhou C. Inhibition of Fatty Acid Amide Hydrolase Improves Depressive-Like Behaviors Independent of Its Peripheral Antinociceptive Effects in a Rat Model of Neuropathic Pain. Anesth Analg. 129:587–597, 2019 [DOI] [PubMed] [Google Scholar]
- 133.Jonsson KO, Vandevoorde S, Lambert DM, Tiger G, Fowler CJ. Effects of homologues and analogues of palmitoylethanolamide upon the inactivation of the endocannabinoid anandamide. Br J Pharmacol. 133:1263–1275, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jordan CJ, Xi ZX. Progress in brain cannabinoid CB2 receptor research: From genes to behavior. Neurosci Biobehav Rev. 98:208–220, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jung KM, Astarita G, Zhu C, Wallace M, Mackie K, Piomelli D. A key role for diacylglycerol lipase-alpha in metabotropic glutamate receptor-dependent endocannabinoid mobilization. Mol Pharmacol. 72:612–621, 2007 [DOI] [PubMed] [Google Scholar]
- 136.Jurkus R, Day HL, Guimaraes FS, Lee JL, Bertoglio LJ, Stevenson CW. Cannabidiol Regulation of Learned Fear: Implications for Treating Anxiety-Related Disorders. Front Pharmacol. 7:454, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Karler R, Sangdee P, Turkanis SA, Borys HK. The pharmacokinetic fate of cannabidiol and its relationship to barbiturate sleep time. Biochem Pharmacol. 28:777–784, 1979 [DOI] [PubMed] [Google Scholar]
- 138.Karschner EL, Darwin WD, Goodwin RS, Wright S, Huestis MA. Plasma cannabinoid pharmacokinetics following controlled oral delta9-tetrahydrocannabinol and oromucosal cannabis extract administration. Clin Chem. 57:66–75, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Khurana L, Mackie K, Piomelli D, Kendall DA. Modulation of CB1 cannabinoid receptor by allosteric ligands: Pharmacology and therapeutic opportunities. Neuropharmacology. 124:3–12, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG, Group NCRRGW. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 160:1577–1579, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.King KM, Myers AM, Soroka-Monzo AJ, Tuma RF, Tallarida RJ, Walker EA, Ward SJ. Single and combined effects of Delta(9)-tetrahydrocannabinol and cannabidiol in a mouse model of chemotherapy-induced neuropathic pain. Br J Pharmacol. 174:2832–2841, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kinsey SG, Wise LE, Ramesh D, Abdullah R, Selley DE, Cravatt BF, Lichtman AH. Repeated low-dose administration of the monoacylglycerol lipase inhibitor JZL184 retains cannabinoid receptor type 1-mediated antinociceptive and gastroprotective effects. J Pharmacol Exp Ther. 345:492–501, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Knopp KL, Stenfors C, Baastrup C, Bannon AW, Calvo M, Caspani O, Currie G, Finnerup NB, Huang W, Kennedy JD, Lefevre I, Machin I, Macleod M, Rees H, Rice ASC, Rutten K, Segerdahl M, Serra J, Wodarski R, Berge OG, Treede RD. Experimental design and reporting standards for improving the internal validity of pre-clinical studies in the field of pain: Consensus of the IMI-Europain consortium. Scand J Pain. 7:58–70, 2015 [DOI] [PubMed] [Google Scholar]
- 144.Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 29:717–727, 2001 [DOI] [PubMed] [Google Scholar]
- 145.Kwilasz AJ, Abdullah RA, Poklis JL, Lichtman AH, Negus SS. Effects of the fatty acid amide hydrolase inhibitor URB597 on pain-stimulated and pain-depressed behavior in rats. Behav Pharmacol. 25:119–129, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kwilasz AJ, Negus SS. Dissociable effects of the cannabinoid receptor agonists Delta9-tetrahydrocannabinol and CP55940 on pain-stimulated versus pain-depressed behavior in rats. J Pharmacol Exp Ther. 343:389–400, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.La Porta C, Bura SA, Llorente-Onaindia J, Pastor A, Navarrete F, Garcia-Gutierrez MS, De la Torre R, Manzanares J, Monfort J, Maldonado R. Role of the endocannabinoid system in the emotional manifestations of osteoarthritis pain. Pain. 156:2001–2012, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Bohme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 283:401–404, 1999 [DOI] [PubMed] [Google Scholar]
- 149.Lee JLC, Bertoglio LJ, Guimaraes FS, Stevenson CW. Cannabidiol regulation of emotion and emotional memory processing: relevance for treating anxiety-related and substance abuse disorders. Br J Pharmacol. 174:3242–3256, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Leitl MD, Negus SS. Pharmacological modulation of neuropathic pain-related depression of behavior: effects of morphine, ketoprofen, bupropion and [INCREMENT]9-tetrahydrocannabinol on formalin-induced depression of intracranial self-stimulation in rats. Behav Pharmacol. 27:364–376, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lepore M, Vorel SR, Lowinson J, Gardner EL. Conditioned place preference induced by delta 9-tetrahydrocannabinol: comparison with cocaine, morphine, and food reward. Life Sci. 56:2073–2080, 1995 [DOI] [PubMed] [Google Scholar]
- 152.Lever IJ, Pheby TM, Rice AS. Continuous infusion of the cannabinoid WIN 55,212-2 to the site of a peripheral nerve injury reduces mechanical and cold hypersensitivity. Br J Pharmacol. 151:292–302, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li AL, Lin X, Dhopeshwarkar AS, Thomaz AC, Carey LM, Liu Y, Nikas SP, Makriyannis A, Mackie K, Hohmann AG. Cannabinoid CB2 Agonist AM1710 Differentially Suppresses Distinct Pathological Pain States and Attenuates Morphine Tolerance and Withdrawal. Mol Pharmacol. 95:155–168, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Limebeer CL, Rock EM, Sharkey KA, Parker LA. Nausea-Induced 5-HT Release in the Interoceptive Insular Cortex and Regulation by Monoacylglycerol Lipase (MAGL) Inhibition and Cannabidiol. eNeuro. 5, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lin X, Dhopeshwarkar AS, Huibregtse M, Mackie K, Hohmann AG. Slowly Signaling G Protein-Biased CB2 Cannabinoid Receptor Agonist LY2828360 Suppresses Neuropathic Pain with Sustained Efficacy and Attenuates Morphine Tolerance and Dependence. Mol Pharmacol. 93:49–62, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Little PJ, Compton DR, Johnson MR, Melvin LS, Martin BR. Pharmacology and stereoselectivity of structurally novel cannabinoids in mice. J Pharmacol Exp Ther. 247:1046–1051., 1988 [PubMed] [Google Scholar]
- 157.Luongo L, Maione S, Di Marzo V. Endocannabinoids and neuropathic pain: focus on neuron-glia and endocannabinoid-neurotrophin interactions. Eur J Neurosci. 39:401–408, 2014 [DOI] [PubMed] [Google Scholar]
- 158.Martin WJ, Hohmann AG, Walker JM. Suppression of noxious stimulus-evoked activity in the ventral posterolateral nucleus of the thalamus by a cannabinoid agonist: correlation between electrophysiological and antinociceptive effects. J Neurosci. 16:6601–6611, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 346:561–564., 1990 [DOI] [PubMed] [Google Scholar]
- 160.Mayo LM, Asratian A, Linde J, Morena M, Haataja R, Hammar V, Augier G, Hill MN, Heilig M. Elevated Anandamide, Enhanced Recall of Fear Extinction, and Attenuated Stress Responses Following Inhibition of Fatty Acid Amide Hydrolase: A Randomized, Controlled Experimental Medicine Trial. Biol Psychiatry. 2019 [DOI] [PubMed] [Google Scholar]
- 161.Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 50:83–90, 1995 [DOI] [PubMed] [Google Scholar]
- 162.Mechoulam R, Gaoni Y. Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc. 86:1646–1647, 1967 [Google Scholar]
- 163.Miederer I, Buchholz HG, Kronfeld A, Maus S, Weyer-Elberich V, Mildenberger P, Lutz B, Schreckenberger M. Pharmacokinetics of the cannabinoid receptor ligand [UR - https://www.ncbi.nlm.nih.gov/pubmed/29244904. 45:725–734, 2018 [DOI] [PubMed] [Google Scholar]
- 164.Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 13:859–866, 2012 [DOI] [PubMed] [Google Scholar]
- 165.Morena M, Patel S, Bains JS, Hill MN. Neurobiological Interactions Between Stress and the Endocannabinoid System. Neuropsychopharmacology. 41:80–102, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mulpuri Y, Marty VN, Munier JJ, Mackie K, Schmidt BL, Seltzman HH, Spigelman I. Synthetic peripherally-restricted cannabinoid suppresses chemotherapy-induced peripheral neuropathy pain symptoms by CB1 receptor activation. Neuropharmacology. 139:85–97, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 365:61–65., 1993 [DOI] [PubMed] [Google Scholar]
- 168.Myers AM, Siegele PB, Foss JD, Tuma RF, Ward SJ. Single and combined effects of plant-derived and synthetic cannabinoids on cognition and cannabinoid-associated withdrawal signs in mice. Br J Pharmacol. 176:1552–1567, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nackley AG, Suplita RL 2nd, Hohmann AG. A peripheral cannabinoid mechanism suppresses spinal fos protein expression and pain behavior in a rat model of inflammation. Neuroscience. 117:659–670, 2003 [DOI] [PubMed] [Google Scholar]
- 170.Nackley AG, Zvonok AM, Makriyannis A, Hohmann AG. Activation of cannabinoid CB2 receptors suppresses C-fiber responses and windup in spinal wide dynamic range neurons in the absence and presence of inflammation. J Neurophysiol. 92:3562–3574, 2004 [DOI] [PubMed] [Google Scholar]
- 171.Naef M, Curatolo M, Petersen-Felix S, Arendt-Nielsen L, Zbinden A, Brenneisen R. The analgesic effect of oral delta-9-tetrahydrocannabinol (THC), morphine, and a THC-morphine combination in healthy subjects under experimental pain conditions. Pain. 105:79–88, 2003 [DOI] [PubMed] [Google Scholar]
- 172.Naef M, Russmann S, Petersen-Felix S, Brenneisen R. Development and pharmacokinetic characterization of pulmonal and intravenous delta-9-tetrahydrocannabinol (THC) in humans. J Pharm Sci. 93:1176–1184, 2004 [DOI] [PubMed] [Google Scholar]
- 173.Nahler G, Grotenhermen F, Zuardi AW, Crippa JAS. A Conversion of Oral Cannabidiol to Delta9-Tetrahydrocannabinol Seems Not to Occur in Humans. Cannabis Cannabinoid Res. 2:81–86, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Naidu PS, Booker L, Cravatt BF, Lichtman AH. Synergy between enzyme inhibitors of fatty acid amide hydrolase and cyclooxygenase in visceral nociception. J Pharmacol Exp Ther. 329:48–56, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Navarrete F, Rodriguez-Arias M, Martin-Garcia E, Navarro D, Garcia-Gutierrez MS, Aguilar MA, Aracil-Fernandez A, Berbel P, Minarro J, Maldonado R, Manzanares J. Role of CB2 cannabinoid receptors in the rewarding, reinforcing, and physical effects of nicotine. Neuropsychopharmacology. 38:2515–2524, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Neelakantan H, Tallarida RJ, Reichenbach ZW, Tuma RF, Ward SJ, Walker EA. Distinct interactions of cannabidiol and morphine in three nociceptive behavioral models in mice. Behav Pharmacol. 26:304–314, 2015 [DOI] [PubMed] [Google Scholar]
- 177.Negus SS, Bilsky EJ, Do Carmo GP, Stevenson GW. Rationale and methods for assessment of pain-depressed behavior in preclinical assays of pain and analgesia. Methods Mol Biol. 617:79–91, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nyilas R, Gregg LC, Mackie K, Watanabe M, Zimmer A, Hohmann AG, Katona I. Molecular architecture of endocannabinoid signaling at nociceptive synapses mediating analgesia. Eur J Neurosci. 29:1964–1978, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.O’Sullivan SE. Cannabinoids go nuclear: evidence for activation of peroxisome proliferator-activated receptors. Br J Pharmacol. 152:576–582, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ohlsson A, Lindgren JE, Wahlen A, Agurell S, Hollister LE, Gillespie HK. Plasma delta-9 tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clin Pharmacol Ther. 28:409–416., 1980 [DOI] [PubMed] [Google Scholar]
- 181.Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 29:729–738, 2001 [DOI] [PubMed] [Google Scholar]
- 182.Otrubova K, Ezzili C, Boger DL. The discovery and development of inhibitors of fatty acid amide hydrolase (FAAH). Bioorg Med Chem Lett. 21:4674–4685, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 58:389–462, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pacher P, Kunos G. Modulating the endocannabinoid system in human health and disease--successes and failures. FEBS J. 280:1918–1943, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Parker LA, Mechoulam R, Schlievert C. Cannabidiol, a non-psychoactive component of cannabis and its synthetic dimethylheptyl homolog suppress nausea in an experimental model with rats. Neuroreport. 13:567–570, 2002 [DOI] [PubMed] [Google Scholar]
- 186.Pascual D, Goicoechea C, Suardiaz M, Martin MI. A cannabinoid agonist, WIN 55,212-2, reduces neuropathic nociception induced by paclitaxel in rats. Pain. 118:23–34, 2005 [DOI] [PubMed] [Google Scholar]
- 187.Patel S, Hill MN, Cheer JF, Wotjak CT, Holmes A. The endocannabinoid system as a target for novel anxiolytic drugs. Neurosci Biobehav Rev. 76:56–66, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Paudel KS, Hammell DC, Agu RU, Valiveti S, Stinchcomb AL. Cannabidiol bioavailability after nasal and transdermal application: effect of permeation enhancers. Drug Dev Ind Pharm. 36:1088–1097, 2010 [DOI] [PubMed] [Google Scholar]
- 189.Paunescu H, Coman OA, Coman L, Ghita I, Georgescu SR, Draghia F, Fulga I. Cannabinoid system and cyclooxygenases inhibitors. J Med Life. 4:11–20, 2011 [PMC free article] [PubMed] [Google Scholar]
- 190.Percie du Sert N, Rice AS. Improving the translation of analgesic drugs to the clinic: animal models of neuropathic pain. Br J Pharmacol. 171:2951–2963, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther 74:129–180, 1997 [DOI] [PubMed] [Google Scholar]
- 192.Pertwee RG. Cannabinoid receptors and pain. Prog Neurobiol. 63:569–611., 2001 [DOI] [PubMed] [Google Scholar]
- 193.Pertwee RG: Hanbook of Cannabis, Oxford University Press, 2016. [Google Scholar]
- 194.Petitet F, Jeantaud B, Bertrand P, Imperato A. Cannabinoid penetration into mouse brain as determined by ex vivo binding. Eur J Pharmacol. 374:417–421, 1999 [DOI] [PubMed] [Google Scholar]
- 195.Philpott HT, O’Brien M, McDougall JJ. Attenuation of early phase inflammation by cannabidiol prevents pain and nerve damage in rat osteoarthritis. Pain. 158:2442–2451, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Piomelli D, Hohmann AG, Seybold V, Hammock BD. A lipid gate for the peripheral control of pain. J Neurosci. 34:15184–15191, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Piomelli D, Tarzia G, Duranti A, Tontini A, Mor M, Compton TR, Dasse O, Monaghan EP, Parrott JA, Putman D. Pharmacological profile of the selective FAAH inhibitor KDS-4103 (URB597). CNS Drug Rev. 12:21–38, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Pryor GT, Husain S, Mitoma C. Influence of fasting on the absorption and effects of delta9-tetrahydrocannabinol after oral administration in sesame oil. Pharmacol Biochem Behav. 6:331–341, 1977 [DOI] [PubMed] [Google Scholar]
- 199.Rahn EJ, Deng L, Thakur GA, Vemuri K, Zvonok AM, Lai YY, Makriyannis A, Hohmann AG. Prophylactic cannabinoid administration blocks the development of paclitaxel-induced neuropathic nociception during analgesic treatment and following cessation of drug delivery. Mol Pain. 10:27, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Rani Sagar D, Burston JJ, Woodhams SG, Chapman V. Dynamic changes to the endocannabinoid system in models of chronic pain. Philos Trans R Soc Lond B Biol Sci. 367:3300–3311, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rice AS, Cimino-Brown D, Eisenach JC, Kontinen VK, Lacroix-Fralish ML, Machin I, Preclinical Pain C, Mogil JS, Stohr T. Animal models and the prediction of efficacy in clinical trials of analgesic drugs: a critical appraisal and call for uniform reporting standards. Pain. 139:243–247, 2008 [DOI] [PubMed] [Google Scholar]
- 202.Rice ASC, Finnerup NB, Kemp HI, Currie GL, Baron R. Sensory profiling in animal models of neuropathic pain: a call for back-translation. Pain. 159:819–824, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Rice ASC, Morland R, Huang W, Currie GL, Sena ES, Macleod MR. Transparency in the reporting of in vivo pre-clinical pain research: The relevance and implications of the ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines. Scand J Pain. 4:58–62, 2013 [DOI] [PubMed] [Google Scholar]
- 204.Richardson JD, Kilo S, Hargreaves KM. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. 75:111–119, 1998 [DOI] [PubMed] [Google Scholar]
- 205.Roche M, Finn DP. Brain CB(2) Receptors: Implications for Neuropsychiatric Disorders. Pharmaceuticals (Basel). 3:2517–2553, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sagar DR, Gaw AG, Okine BN, Woodhams SG, Wong A, Kendall DA, Chapman V. Dynamic regulation of the endocannabinoid system: implications for analgesia. Mol Pain. 5:59, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sagar DR, Jhaveri MD, Richardson D, Gray RA, de Lago E, Fernandez-Ruiz J, Barrett DA, Kendall DA, Chapman V. Endocannabinoid regulation of spinal nociceptive processing in a model of neuropathic pain. Eur J Neurosci. 31:1414–1422, 2010 [DOI] [PubMed] [Google Scholar]
- 208.Sain NM, Liang A, Kane SA, Urban MO. Antinociceptive effects of the non-selective cannabinoid receptor agonist CP 55,940 are absent in CB1(−/−) and not CB2(−/−) mice in models of acute and persistent pain. Neuropharmacology. 57:235–241, 2009 [DOI] [PubMed] [Google Scholar]
- 209.Schuster RM, Potter K, Vandrey R, Hareli M, Gilman J, Schoenfeld D, Evins AE. Urinary 11-nor-9-carboxy-tetrahydrocannabinol elimination in adolescent and young adult cannabis users during one month of sustained and biochemically-verified abstinence. J Psychopharmacol. 34:197–210, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sholler DJ, Strickland JC, Spindle TR, Weerts EM, Vandrey R. Sex differences in the acute effects of oral and vaporized cannabis among healthy adults. Addict Biol. e12968, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Slivicki RA, Iyer V, Mali SS, Garai S, Thakur GA, Crystal JD, Hohmann AG. Positive Allosteric Modulation of CB1 Cannabinoid Receptor Signaling Enhances Morphine Antinociception and Attenuates Morphine Tolerance Without Enhancing Morphine- Induced Dependence or Reward. Front Mol Neurosci. 13:54, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Slivicki RA, Saberi SA, Iyer V, Vemuri VK, Makriyannis A, Hohmann AG. Brain-Permeant and - Impermeant Inhibitors of Fatty Acid Amide Hydrolase Synergize with the Opioid Analgesic Morphine to Suppress Chemotherapy-Induced Neuropathic Nociception Without Enhancing Effects of Morphine on Gastrointestinal Transit. J Pharmacol Exp Ther. 367:551–563, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Slivicki RA, Xu Z, Mali SS, Hohmann AG. Brain permeant and impermeant inhibitors of fatty-acid amide hydrolase suppress the development and maintenance of paclitaxel-induced neuropathic pain without producing tolerance or physical dependence in vivo and synergize with paclitaxel to reduce tumor cell line viability in vitro. Pharmacol Res. 142:267–282, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Smith PB, Compton DR, Welch SP, Razdan RK, Mechoulam R, Martin BR. The pharmacological activity of anandamide, a putative endogenous cannabinoid, in mice. J Pharmacol Exp Ther. 270:219–227, 1994 [PubMed] [Google Scholar]
- 215.Soliman N, Vollert J, Sena C, Liao J, Macleod M, Thomas J,, Hohmann AG, Haroutounian S, Krane E, Alaverdyan H, Barakat A,, Barthlow T, Harris Bozer AL, Davidson A, DiazdelCastillo M, , Dolgorukova A, Ferdousi M, Healy C, Hong N, Hopkins M, James A,, Leake HB, Malewicz NM, Mansfield M, Mardon AK, , Mattimoe D, McLoone DP, Noes-Holt G, Pogatzki-Zahn EM, Power E, , Pradier B, Romanos-Sirakis E, Segelcke A, Segelcke D, Vinagre R, Yanes JA, Zhang J, Zhang XY, Finn DP, Rice ASC A Systematic Review and Meta-analysis of cannabis-based medicines, cannabinoids and endocannabinoid system modulators tested for antinociceptive effects in animal models of injury-related or pathological persistent pain. PAIN. Under Review, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Spencer S, Neuhofer D, Chioma VC, Garcia-Keller C, Schwartz DJ, Allen N, Scofield MD, Ortiz-Ithier T, Kalivas PW. A Model of Delta(9)-Tetrahydrocannabinol Self-administration and Reinstatement That Alters Synaptic Plasticity in Nucleus Accumbens. Biol Psychiatry. 84:601–610, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Starowicz K, Finn DP. Cannabinoids and Pain: Sites and Mechanisms of Action. Adv Pharmacol. 80:437–475, 2017 [DOI] [PubMed] [Google Scholar]
- 218.Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 388:773–778, 1997 [DOI] [PubMed] [Google Scholar]
- 219.Sugiura T, Kishimoto S, Oka S, Gokoh M. Biochemistry, pharmacology and physiology of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Prog Lipid Res. 45:405–446, 2006 [DOI] [PubMed] [Google Scholar]
- 220.Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 215:89–97, 1995 [DOI] [PubMed] [Google Scholar]
- 221.Taylor L, Crockett J, Tayo B, Morrison G. A Phase 1, Open-Label, Parallel-Group, Single-Dose Trial of the Pharmacokinetics and Safety of Cannabidiol (CBD) in Subjects With Mild to Severe Hepatic Impairment. J Clin Pharmacol. 59:1110–1119, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Tham M, Yilmaz O, Alaverdashvili M, Kelly MEM, Denovan-Wright EM, Laprairie RB. Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type 2 cannabinoid receptors. Br J Pharmacol. 176:1455–1469, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Thors L, Burston JJ, Alter BJ, McKinney MK, Cravatt BF, Ross RA, Pertwee RG, Gereau RWt, Wiley JL, Fowler CJ. Biochanin A, a naturally occurring inhibitor of fatty acid amide hydrolase. Br J Pharmacol. 160:549–560, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 51:5–17, 1992 [DOI] [PubMed] [Google Scholar]
- 225.Tseng AH, Harding JW, Craft RM. Pharmacokinetic factors in sex differences in Delta 9-tetrahydrocannabinol-induced behavioral effects in rats. Behav Brain Res. 154:77–83, 2004 [DOI] [PubMed] [Google Scholar]
- 226.Tsou K, Lowitz KA, Hohmann AG, Martin WJ, Hathaway CB, Bereiter DA, Walker JM. Suppression of noxious stimulus-evoked expression of Fos protein-like immunoreactivity in rat spinal cord by a selective cannabinoid agonist. Neuroscience. 70:791–798, 1996 [DOI] [PubMed] [Google Scholar]
- 227.Ueda N, Tsuboi K, Uyama T, Ohnishi T. Biosynthesis and degradation of the endocannabinoid 2-arachidonoylglycerol. Biofactors. 37:1–7, 2011 [DOI] [PubMed] [Google Scholar]
- 228.Uhelski ML, Khasabova IA, Simone DA. Inhibition of anandamide hydrolysis attenuates nociceptor sensitization in a murine model of chemotherapy-induced peripheral neuropathy. J Neurophysiol. 113:1501–1510, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Valiveti S, Hammell DC, Earles DC, Stinchcomb AL. Transdermal delivery of the synthetic cannabinoid WIN 55,212-2: in vitro/in vivo correlation. Pharm Res. 21:1137–1145, 2004 [DOI] [PubMed] [Google Scholar]
- 230.van der Worp HB, Howells DW, Sena ES, Porritt MJ, Rewell S, O’Collins V, Macleod MR. Can animal models of disease reliably inform human studies? PLoS Med. 7:e1000245, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Voelkl B, Vogt L, Sena ES, Wurbel H. Reproducibility of preclinical animal research improves with heterogeneity of study samples. PLoS Biol. 16:e2003693, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wagenlehner FME, van Till JWO, Houbiers JGA, Martina RV, Cerneus DP, Melis J, Majek A, Vjaters E, Urban M, Ramonas H, Shoskes DA, Nickel JC. Fatty Acid Amide Hydrolase Inhibitor Treatment in Men With Chronic Prostatitis/Chronic Pelvic Pain Syndrome: An Adaptive Double-blind, Randomized Controlled Trial. Urology. 103:191–197, 2017 [DOI] [PubMed] [Google Scholar]
- 233.Wang J Glial endocannabinoid system in pain modulation. Int J Neurosci. 129:94–100, 2019 [DOI] [PubMed] [Google Scholar]
- 234.Welch SP, Huffman JW, Lowe J. Differential blockade of the antinociceptive effects of centrally administered cannabinoids by SR141716A. Journal of Pharmacology & Experimental Therapeutics. 287:1301–1308, 1998 [PubMed] [Google Scholar]
- 235.Whiteside GT, Adedoyin A, Leventhal L. Predictive validity of animal pain models? A comparison of the pharmacokinetic-pharmacodynamic relationship for pain drugs in rats and humans. Neuropharmacology. 54:767–775, 2008 [DOI] [PubMed] [Google Scholar]
- 236.Wiese BM, Liktor-Busa E, Levine A, Couture SA, Nikas SP, Ji L, Liu Y, Mackie K, Makriyannis A, Largent-Milnes TM, and Vanderah TW Cannabinoid-2 Agonism with AM2301 Mitigates Morphine-Induced Respiratory Depression. Cannabis and Cannabinoid Research, ahead of print. 10.1089/can.2020.0076, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wilkerson JL, Ghosh S, Mustafa M, Abdullah RA, Niphakis MJ, Cabrera R, Maldonado RL, Cravatt BF, Lichtman AH. The endocannabinoid hydrolysis inhibitor SA-57: Intrinsic antinociceptive effects, augmented morphine-induced antinociception, and attenuated heroin seeking behavior in mice. Neuropharmacology. 114:156–167, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wilkerson JL, Milligan ED. The Central Role of Glia in Pathological Pain and the Potential of Targeting the Cannabinoid 2 Receptor for Pain Relief. ISRN Anesthesiol. 2011, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 410:588–592, 2001 [DOI] [PubMed] [Google Scholar]
- 240.Woodhams SG, Chapman V, Finn DP, Hohmann AG, Neugebauer V. The cannabinoid system and pain. Neuropharmacology. 124:105–120, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Woodhams SG, Sagar DR, Burston JJ, Chapman V. The role of the endocannabinoid system in pain. Handbook of experimental pharmacology. 227:119–143, 2015 [DOI] [PubMed] [Google Scholar]
- 242.Wu J, Hocevar M, Bie B, Foss JF, Naguib M. Cannabinoid Type 2 Receptor System Modulates Paclitaxel-Induced Microglial Dysregulation and Central Sensitization in Rats. J Pain. 20:501–514, 2019 [DOI] [PubMed] [Google Scholar]
- 243.Xiong W, Cui T, Cheng K, Yang F, Chen SR, Willenbring D, Guan Y, Pan HL, Ren K, Xu Y, Zhang L. Cannabinoids suppress inflammatory and neuropathic pain by targeting alpha3 glycine receptors. J Exp Med. 209:1121–1134, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Xu C, Chang T, Du Y, Yu C, Tan X, Li X. Pharmacokinetics of oral and intravenous cannabidiol and its antidepressant-like effects in chronic mild stress mouse model. Environ Toxicol Pharmacol. 70:103202, 2019 [DOI] [PubMed] [Google Scholar]
- 245.Yuill MB, Hale DE, Guindon J, Morgan DJ. Anti-nociceptive interactions between opioids and a cannabinoid receptor 2 agonist in inflammatory pain. Mol Pain. 13:1744806917728227, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zavala CA, Thomaz AC, Iyer V, Mackie K, and Hohmann AG Cannabinoid CB2 Receptor Activation Attenuates Fentanyl-Induced Respiratory Depression. Cannabis and Cannabinoid Research, ahead of print. 10.1089/can.2020.0059, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zgair A, Lee JB, Wong JCM, Taha DA, Aram J, Di Virgilio D, McArthur JW, Cheng YK, Hennig IM, Barrett DA, Fischer PM, Constantinescu CS, Gershkovich P. Oral administration of cannabis with lipids leads to high levels of cannabinoids in the intestinal lymphatic system and prominent immunomodulation. Sci Rep. 7:14542, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zgair A, Wong JC, Lee JB, Mistry J, Sivak O, Wasan KM, Hennig IM, Barrett DA, Constantinescu CS, Fischer PM, Gershkovich P. Dietary fats and pharmaceutical lipid excipients increase systemic exposure to orally administered cannabis and cannabis-based medicines. Am J Transl Res. 8:3448–3459, 2016 [PMC free article] [PubMed] [Google Scholar]
- 249.Zhang HY, Bi GH, Li X, Li J, Qu H, Zhang SJ, Li CY, Onaivi ES, Gardner EL, Xi ZX, Liu QR. Species differences in cannabinoid receptor 2 and receptor responses to cocaine self-administration in mice and rats. Neuropsychopharmacology. 40:1037–1051, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zhu CZ, Mikusa JP, Fan Y, Hollingsworth PR, Pai M, Chandran P, Daza AV, Yao BB, Dart MJ, Meyer MD, Decker MW, Hsieh GC, Honore P. Peripheral and central sites of action for the non-selective cannabinoid agonist WIN 55,212-2 in a rat model of post-operative pain. Br J Pharmacol. 157:645–655, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]


