Abstract
BACKGROUND
In COVID-19 pneumonia, cases of severe hypoxemia in the early stage and cases of sudden deterioration in respiratory status due to silent hypoxia leading to death, have been reported.
CASE SUMMARY
A 70-year-old Japanese man with essential hypertension, dyslipidemia, chronic kidney disease and emphysema was hospitalized with the novel coronavirus disease. He had hypoxemia that was disproportionate to the severity of pneumonia indicated by computed tomography (CT), along with coagulation abnormalities. We speculated that there was a high possibility that he had developed ventilation and blood flow imbalance due to pulmonary intravascular coagulopathy (PIC) or hypoxic pulmonary vasoconstriction (HPV). In this case, early, short-term combination therapy with remdesivir, nafamostat mesylate and low-dose dexamethasone (Dex) was successful.
CONCLUSION
In COVID-19 patients with multiple comorbidities who have hypoxemia and coagulation abnormalities that are disproportionate to the severity of pneumonia on CT, it is important to commence antiviral and anticoagulant therapy as soon as possible, followed by use of a low dose of Dex.
Keywords: COVID-19, hypoxic pulmonary vasoconstriction, pulmonary intravascular coagulopathy, remdesivir, nafamostat mesylate, dexamethasone
Background
At the end of 2019, an outbreak of atypical pneumonia was reported in Wuhan City, Hubei Province, China. This pneumonia was shown to be caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and was named coronavirus disease 2019 (COVID-19). To date, the number of infected people globally is about 242 million, and the number of deaths is about 5 million. It is known that the disease is more likely to become severe in the elderly than in the young, and the mortality rate of people aged 80 years and over is extremely high, at 14.8 to 20.2%.1,2 It has also been reported that there is a difference in mortality depending on the presence of underlying comorbidities, with reported mortality rates of 10.5% in patients with cardiovascular disease, 7.3% in diabetics, 6.3% in patients with chronic respiratory disease, and 6.0% in hypertensive patients. 3
SARS-CoV-2 has a positive-sense, single-stranded RNA genome, and binds to angiotensin-converting enzyme 2 (ACE2) receptors present on the cell surface with a coronal spike protein on its surface envelope. By doing so, it invades the cell by endocytosis due to membrane fusion, leading to viral amplification. 4 ACE2 receptors are known to be widely expressed in nerve cells, olfactory nerve epithelium, the tongue, intestinal epithelial cells, vascular endothelial cells, etc, in addition to alveolar type II epithelial cells.5,6 Therefore, SARS-CoV-2 causes not only severe pneumonia and fatal acute respiratory distress syndrome, but also multifaceted disorders in many cells, tissues, and organs. 7
In COVID-19 pneumonia, cases of severe hypoxemia in the early stage 8 and cases of sudden deterioration in respiratory status due to silent hypoxia leading to death, 9 have been reported. It is speculated that major changes in one of the following factors: (1) thrombosis due to impaired blood coagulation, (2) disordered hypoxic pulmonary vasoconstriction (HPV),10,11 and (3) imbalance between ventilation and blood flow in healthy lungs, or relatively small changes in these factors occurring at the same time 11 are responsible for the disease severity in such cases.
Here, we report a case of COVID-19 who showed progressive silent hypoxemia that was presumed to be due to an imbalance between ventilation and blood flow, but with only mild pneumonia visualized on computed tomography (CT), in whom early multidrug therapy was effective.
Case Report
The patient was a 70-year-old Japanese man (172.0 cm tall, weighing 68.0 kg, body mass index 23.0 kg/m2) who had smoked 20 cigarettes a day for 47 years until 5 years ago. He was under treatment and follow-up for essential hypertension, dyslipidemia and chronic kidney disease, and his blood pressure and lipid levels were well controlled with administration of 40 mg of olmesartan medoxomil (Olm) and 5 mg of atorvastatin calcium hydrate. His renal function was stable. The patient presented with nasal discharge, malaise and a mild cough, and tested positive for SARS-CoV-2 by the polymerase chain reaction (PCR) test three days after coming in extended contact with a colleague who was also diagnosed with SARS-CoV-2. Since he had many risk factors for disease aggravation, he was urgently admitted to our hospital (Day 1) the day after symptom onset and confirmation of the diagnosis.
At admission, his body temperature was 36.4 °C, blood pressure was 185/72 mm Hg, pulse was 86 beats/min, respiratory rate was 20 beats/min and peripheral oxygen saturation on pulse oximetry (SpO2) was 97.0%. Laboratory findings at this time showed lymphocyte depletion and coagulation abnormalities [lymphocytes 8.5%, fibrinogen 518 mg/dL, fibrinogen degradation products 5.26 μg/mL and D-dimer (D-D) 1.68 μg/mL] (Table 1). In addition, iron deficiency anemia was observed (he was scheduled for close examination of the gastrointestinal tract after COVID-19 was cured), and hence, it was difficult to evaluate the clinical implication of the observed low ferritin level (Table 1). CT showed emphysematous changes in the lung field. Ground glass opacities with neither a crazy-paving pattern nor consolidation was found below the dorsal pleura of the upper right lobe, and the total CT score was 1/25 points (upper right lobe 1/5 points, middle lobe 0/5 points, lower right lobe 0/5 points, upper left lobe 0/5 points and lower left lobe 0/5 points), based on the scoring system described by Pan et al. 12 In addition, fibrosis, subpleural lines, the reversed “halo sign”, pleural effusion and lymphadenopathy were not observed. It was considered to be a typical findings of early mild COVID-19 pneumonia (Figure 1a).
Table 1.
Laboratory findings on admission.
| Peripheral blood | WBC | 3270 | /μL | |
| NEUT (Neut) | 5.94 (80.5) | × 103/μL (%) | ||
| LYMPH (Lymph) | 0.63 (8.5) | × 103/μL (%) | [1.00 to 4.00 (18.0 to 50.0)] | |
| RBC | 504 | × 104/μL | ||
| Hb | 11.5 | g/dL | [13.7 to 16.8] | |
| MCV | 73.2 | fL | [83.6 to 98.2] | |
| MCH | 23 | pg | [27.5 to 33.2] | |
| MCHC | 31.4 | g/dL | [31.7 to 35.3] | |
| HCT | 36.9 | % | [40.7 to 50.1] | |
| PLT | 43.9 | × 104/μL | [15.8 to 34.8] | |
| Coagulation | PT | 90.4 | % | |
| PT-INR | 1.05 | |||
| APTT | 29.5 | sec | ||
| Fib | 518 | mg/dL | [200 to 400] | |
| FDP | 5.26 | μg/mL | [<5] | |
| D-D | 1.68 | μg/mL | [<1] | |
| AT-III | 107.4 | % | ||
| Biochemistry | AST | 14 | U/L | |
| ALT | 8 | U/L | ||
| LDH | 202 | U/L | ||
| ALP | 248 | U/L | ||
| γ-GTP | 19 | U/L | ||
| T. Bil | 0.53 | mg/dL | ||
| D. Bil | 0.22 | mg/dL | ||
| CK | 59 | U/L | ||
| TP | 7.15 | g/dL | ||
| Alb | 4.16 | g/dL | ||
| BUN | 12.4 | mg/dL | ||
| CRE | 1.4 | mg/dL | [0.65 to 1.07] | |
| eGFR | 39.7 | mL/min/1.73 m2 | ||
| PPG | 94 | mg/dL | ||
| CRP | 0.26 | mg/dL | [0.00 to 0.14] | |
| Ferritin | 7.37 | ng/mL | [25.80 to 280.50] | |
| Presepsin | 313 | pg/mL | ||
| Fe | 21 | μg/dL | [40 to 188] | |
| Urinalysis | Protein | ( + ) | ||
| Glucose | (-) | |||
| Occult blood | (-) | |||
| Others | Influenza A Ag | (-) | ||
| Influenza B Ag | (-) |
The reference ranges of data showing abnormal values are shown in brackets. WBC, leukocytes; NEUT, neutrophils (absolute value); Neut, neutrophils (percentage); LYMPH, lymphocytes (absolute value); Lymph, lymphocytes (percentage); Mono, monocytes (percentage); Eosin, eosinophils (percentage); Baso, basophils (percentage); RBC, red blood cells; Hb, hemoglobin; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; HCT, hematocrit; PLT, platelets; PT, prothrombin time; PT-INR, prothrombin time-international normalized ratio; APTT, activated partial thromboplastin time; Fib, fibrinogen; FDP, fibrin/fibrinogen degradation products; D-D, D-dimer; AT-III, antithrombin III; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; ALP, alkaline phosphatase; γ-GTP, γ-glutamyltransferase; ChE, cholinesterase; T. Bil, total bilirubin; D. Bil, direct bilirubin; CK, creatine kinase; TP, total protein; Alb, albumin; Na, sodium; K, potassium; Cl, chloride; BUN, blood urea nitrogen; CRE, creatinine; UA, uric acid; eGFR, estimated glomerular filtration rate; PPG, postprandial blood glucose; CRP, C-reactive protein; Fe, iron; Ag, antigen.
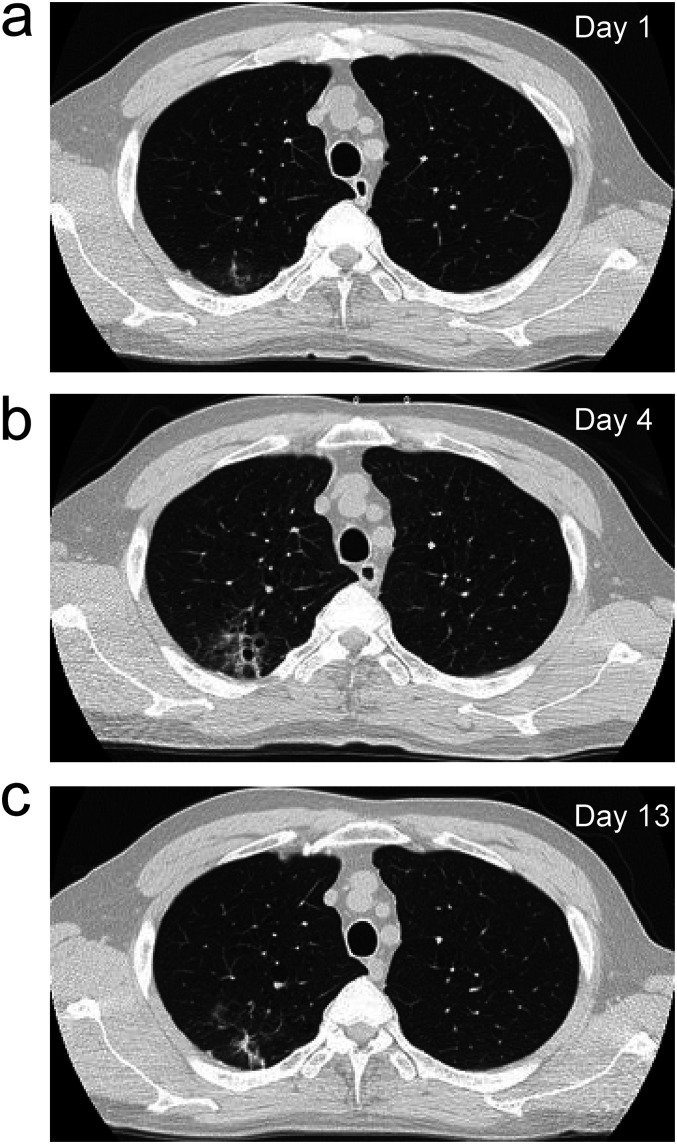
Figure 1.
Chest computed tomography images. (a) Image taken on Day 1 showing emphysematous changes in the lung field and ground-glass opacities with unclear borders below the dorsal pleura of the upper right lobe (b) Image taken on Day 7 showing slight exacerbation of the ground-glass opacities. (c) Image taken on Day 13 showing improvement in the ground-glass opacities.
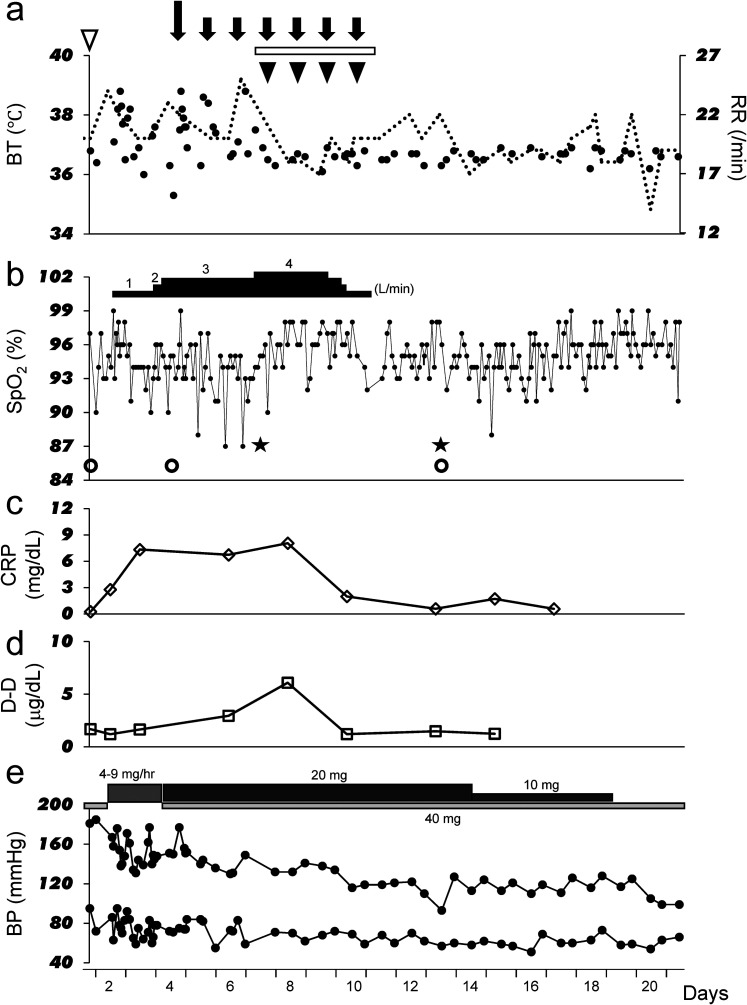
Although no decrease in SpO2 was observed, administration of favipiravir (Fav) 3600 mg daily was started from the evening of the same day, due to the presence of mild pneumonia as seen on CT and the presence of multiple comorbidities. In addition, since SpO2 decreased a little the following day, 1 L/min oxygen administration via a nasal cannula was also started. Weakness and a decreased level of consciousness (tendency to somnolence) appeared from the early morning of Day 2, but a neurologist ruled out stroke. Since somnolence and weakness were considered as side effects of Fav, the drug was discontinued on the evening of Day 2, and his symptoms improved by the morning of Day 3. Thereafter, since his temperature increased again, the frequency of administration of acetaminophen 500 mg was increased from 0 to 3 times a day. In addition, his CRP level increased and SpO2 decreased, requiring an increase in oxygen flow rate from 1 to 3 L/min by nasal cannula. CT performed on Day 4 showed slight deterioration in the pneumonia. The total CT score was 2/25 points (ground-glass opacities only below the dorsal pleura of the upper right lobe) (Figure 1b). Therefore, 200 mg of remdesivir (Rem) was administered on Day 4, with 100 mg daily being administered from Day 5 until Day 10, for a total of seven days (Figure 2a). On Day 7, blood gas data under administration of 4 L/min oxygen by nasal cannula (Figure 2b) showed that the alveolar-arterial oxygen difference (A-aDO2) had increased to 122.1 mm Hg (Table 2), indicating an imbalance between ventilation and blood flow. However, no pulmonary hypertension was observed on electrocardiogram or echocardiography. Furthermore, since a tendency of increasing D-D levels was also observed (Figure 2d), the combination of nafamostat mesylate (Naf) 100 mg daily by continuous intravenous infusion and dexamethasone (Dex) 6 mg daily was administered for four days from Day 7 (Figure 2a). The treatment was remarkably effective, resulting in fever reduction and a decrease in CRP and D-D levels (Figure 2a, c and d). On Day 10, oxygen administration could be discontinued, and at the same time (Figure 2b), his blood pressure control improved (Figure 2e). By Day 13, A-aDO2 had also significantly improved to 33.2 mm Hg without supplementary oxygen (Table 2), and CT showed a tendency for improvement in pneumonia. The total CT score at this time was still 2/25 points (ground-glass opacities only below the dorsal pleura of the upper right lobe) (Figure 1c).
Figure 2.
Clinical course of the patient. (a) The black dots indicate body temperature and dotted lines indicate respiratory rate. The white inverted triangle indicates 3600 mg favipiravir administration. The long downward-pointing black arrow indicates administration of 200 mg remdesivir, and the short downward-pointing black arrows indicate administration of 100 mg remdesivir. The horizontal white bars indicate continuous intravenous infusion of nafamostat mesylate 0.07 mg/kg/h. The black inverted triangles indicate administration of 6 mg dexamethasone. (b) Transition of SpO2. The horizontal black bars indicate the flow rate of oxygen administered via a nasal cannula. The white circles indicate the day when CT was performed, and the black stars indicate the day when confirmatory blood gas analyses were performed. (c) Transition of C-reactive protein (CRP) levels. (d) Transition of D-dimer (D-D) levels. (e) Transition of systolic blood pressure at the top and diastolic blood pressure at the bottom part of the graph. The horizontal light-gray bar indicates administration of olmesartan, the moderately-dark gray bar indicates continuous intravenous infusion of pernidipine, and the dark gray bar indicates administration of nifedipine.
BP, blood pressure; BT, body temperature; CRP, C-reactive protein; D-D, D-dimer; RR, respiratory rate; SpO2, peripheral oxygen saturation on pulse oximetry.
Table 2.
Blood gas analysis.
| DAY 7 | DAY 13 | |||
|---|---|---|---|---|
| O2 NASAL (L/MIN) | 4 | 0 | ||
| pH | 7.398 | 7.462 | μg/L | [7.36 to 7.44] |
| PCO2 | 33.1 | 32.4 | mm Hg | [35 to 45] |
| PO2 | 96.2 | 76.4 | mm Hg | [85 to 105] |
| HCO3- | 20 | 22.6 | mmol/L | [21 to 27] |
| BE | −4.1 | −0.5 | mmol/L | [−2.0 to 3.0] |
| A-aDO2 | 122.1 | 33.2 | mm Hg | [<10] |
| Lac | 2.2 | 1.7 | mmol/L | [0.5 to 1.6] |
The reference ranges of data are shown in brackets. pH, potential of hydrogen; PCO2, partial pressure of carbon dioxide; PO2, partial pressure of oxygen; HCO3−, actual bicarbonate; BE, actual base excess; A-aDO2, alveolar-arterial oxygen difference; Lac, lactate. A-aDO2 (Day 7) = [{ (760 − 47) × 0.21}-PCO2/0.8] − PO2 A-aDO2 (Day 13) = [{ (760 − 47) × 0.364} − PCO2/0.8] − PO2
For blood pressure control during the course of his hospitalization, continuous intravenous infusion of pernidipine (4-9 mg/h) was performed for two days when oral intake was difficult due to the decreased consciousness level, and from day 4, Olm 40 mg and nifedipine 20 mg were administered in combination. With the treatment of COVID-19, his blood pressure gradually stabilized, and the dose of nifedipine was reduced from Day 14 to 10 mg, and nifedipine was discontinued from Day 19 (Figure 2).
It took some more time for SpO2 to stabilize, but it eventually improved and the patient was discharged on Day 21 (Figure 2).
Discussion
Cases of COVID-19 often develop ischemic stroke, ischemic heart disease and venous thromboembolism. Guan et al. reported that 46.4% of COVID-19 patients had elevated D-D levels. 13 It has been reported that elevated D-D is a predictor of COVID-19 mortality,14,15 and that thrombi are frequently observed in pulmonary microarteries in autopsy cases.16,17 Carsana et al. stated that the D-D value reached more than 10 times the upper limit of normal in all autopsy cases with COVID-19 for which D-D could be examined. 17 On the other hand, Asakura classified the type of disseminated intravascular coagulation (DIC) and reported “DIC with suppressed fibrinolysis” as a more serious condition. In their report, the increase in D-D remained relatively mild even in a serious case leading to death. 18 Furthermore, in an observational study of 183 COVID-19 patients in China, the mean D-D values in survivor and deceased groups were reportedly 0.61 μg/mL and 2.12 μg/mL, respectively. 19 In our case, the highest D-D level during his clinical course was 2.95 μg/mL, which was only a slight increase above the reference range. This suggests that in COVID-19 cases, the absolute value of D-D does not necessarily correlate with the severity of coagulation/fibrinolytic abnormalities, and hence, it is important to evaluate the pathological condition by confirming the transition of biochemical and fibrinolytic markers together.
McGonagle et al. distinguished macrothrombosis and widespread microthrombosis in the lung from DIC and called it pulmonary intravascular coagulopathy (PIC). 20 Causes of the blood coagulation disorders in COVID-19 include hyperformation of neutrophil extracellular traps due to inappropriate release of nuclear chromatin from activated neutrophils that migrated and accumulated at the infected site, and subsequent platelet aggregation by their supplementation/activation.21,22 Furthermore, it is known that elevation of Von Willebrand factor (vWF), which is derived from macrophages and monospheres or is activated by inflammatory cytokines (Interleukin-6, Tumor necrosis factor-α, etc), coagulation-inducing factors such as factors VII and VIII, and plasminogen activator inhibitor-I, which suppresses urokinase-type or tissue plasminogen activator,19,21 are involved in the pathogenesis. On the other hand, it has been reported that vWF and P-selectin are released into the blood due to activation and damage of vascular endothelial cells, or that the loss of thrombomodulin, which is the center of the fibrinolytic system, causes microvascular thrombosis. 23 Downregulation of ACE2 by SARS-CoV-2 binding inhibits the conversion of angiotensin II (AII) to angiotensin 1 to 7 (AT1−7), resulting in an imbalance in the renin-angiotensin system. Reportedly, this results in further damage to vascular endothelial cells by AII, inflammation, promotion of oxidation, vasoconstriction, and coagulopathy mediated by an increase in plasminogen activator inhibitor-I.24–26 In this case, the patient's blood pressure, which was largely stable at the time of admission, was subsequently elevated. It has been suggested that Olm increases ACE2 and AT1−7, which binds to the Mas receptor to lower blood pressure and protect organs. 27 Due to the fact that blood pressure control was stabilized by the treatment of COVID-19 (Figure 2e), we speculated that the ACE2 consumption by SARS-CoV-2 might have made achievement of the antihypertensive effect of Olm via the Mas receptor difficult. On the other hand, the fact that blood pressure control was stabilized by the treatment of COVID-19 might suggest that direct vascular endothelial damage due to SARS-CoV-2 was progressing. In addition to the above-mentioned coagulopathy, the patient developed HPV disorder,9,10 which, we speculated, together with emphysematous changes in the lungs, rapidly caused imbalance between ventilation and blood flow.
In this case, CT suggested pneumonia, which had multiple aggravating factors. Therefore, administration of Fav was started immediately after admission (Figure 2a). Fav selectively inhibits RNA-dependent RNA polymerase (RdRp) by its metabolism and conversion to the active form favipiravir-ribofuranosyl triphosphate by an intracellular enzyme, which is recognized as a substrate for RdRp by RNA viruses. 28 After the commencement of Fav treatment, the patient exhibited weakness and decrease in consciousness (tendency to somnolence), which improved after discontinuation of Fav. Chen et al. reported that 4.31% of patients receiving Fav had a psychiatric reaction, 29 suggesting that these symptoms in our patient were likely to have been side effects of Fav. Subsequently, since fever persisted and the patient's SpO2 progressively decreased (Figure 2b), Rem was started on Day 4 (Figure 2a). Reportedly, Rem is phosphorylated in cells to a nucleic acid analog (triphosphate type remdesivir), which, like Fav, binds to RdRp and selectively inhibits it, thereby suppressing the growth of RNA viruses.28,30 Its effectiveness against SARS-CoV-2 by this mechanism has also been reported.30–32 However, although CT suggested only slight exacerbation of pneumonia, our patient's fever persisted, SpO2 decreased, and CRP and D-D levels tended to increase even after the start of Rem (Figure 2a to d). Therefore, Naf 100 mg and Dex 6 mg were also used from Day 8 (Figure 2a). Naf suppresses transmembrane protease serine 2 (TMPRSS2), an enzyme that cleaves and activates the spike protein, and blocks the invasion of SARS-CoV-2 into host cells.28,33 It is used for the treatment of DIC and acute pancreatitis because it binds to the active center (serine) of enzymes such as thrombin, XIIa, Xa, VIIa, kallikrein, plasmin and trypsin, and suppresses the coagulation/fibrinolytic system. Doi et al. have reported the efficacy of Naf 0.2 mg/kg/h in combination with Fav for COVID-19 when administered as a continuous intravenous infusion for an average of 14 days, 34 but the optimal administration method has not yet been established. We were able to confirm a decrease in D-D levels after 4 days of administration of Naf with Rem at approximately the smallest dose (0.07 mg/kg/h) used in DIC (Figure 2d). Preliminary results from a large randomized controlled trial conducted in the United Kingdom showed that once-daily administration of 6 mg Dex for up to 10 days resulted in a 35% reduction in mortality in patients with COVID-19 receiving invasive mechanical ventilation. 35 On the other hand, the mortality rate in patients treated with Dex who received only oxygen support without invasive ventilatory management reduced by 20%. 35 Corticosteroids (CS) are known to bind to cytoplasmic CS receptors and translocate to the nucleus, reducing the activity of pro-inflammatory transcription factors, such as nuclear factor of κB and activator protein-1, and to regulate transcription of anti-inflammatory genes. 36 As a result, they reduce a number of inflammatory mediators involved in excessive cytokine responses (cytokine storms), although their effect on COVID-19 remains controversial.37,38 Even in the large-scale randomized controlled trial mentioned above, no benefit of Dex administration to a group of patients with independent ventilation was recognized. 36 Since CS affects the function of many immune cells and suppresses both innate and acquired immunity, smaller doses and shorter treatment durations are recommended. 39 For this reason, we administered Dex 6 mg once daily for 4 days using a decrease in CRP as an indicator of response to therapy, which proved to be successful (Figure 2a). Asakura et al. have demonstrated the concept of antiviral, anticytokine and anticoagulant combination therapy according to the stage of COVID-19. 40 In our case, the combination therapy was extremely effective within a short period of time, confirming its importance in the treatment of COVID-19.
The limitations of our report are that we did not confirm the histopathology of the pulmonary pathology, and that since this was a case report, we cannot confirm that our therapy would be effective in all cases.
Conclusion
In COVID-19 patients with multiple comorbidities who have hypoxemia and coagulation abnormalities that are disproportionate to the severity of pneumonia on CT, there is a high possibility that ventilation and blood flow imbalance due to PIC or HPV disorder has occurred. We believe it is important to commence antiviral and anticoagulant therapy as soon as possible, followed by use of a low dose of Dex for a short period to control cytokine hyperreactivity.
Acknowledgements
We thank the patient for his permission to publish this manuscript. Furthermore, we thank Forte, Inc. for their medical editing services.
Footnotes
Author Contributions: N.K., AM, K.H., T.K., T.A., G.T., H.M., and M.O. attended to the patient; N.K. wrote the manuscript; M.O. supervised management of the case and contributed to writing and editing the manuscript. All authors have read and approved the final manuscript.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING: The author(s) received no financial support for the research, authorship and/or publication of this article.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
ORCID iD: Nobuyuki Koriyama https://orcid.org/0000-0002-4830-7154
REFERENCES
- 1.Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145‐151. [DOI] [PubMed] [Google Scholar]
- 2.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775‐1776. [DOI] [PubMed] [Google Scholar]
- 3.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239‐1241. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamming I, Timens W, Bulthuis ML, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lukiw WJ, Pogue A, Hill JM. SARS-CoV-2 infectivity and neurological targets in the brain. Cell Mol Neurobiol. 2020;25:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asselah T, Durantel D, Pasmant E, et al. COVID-19: discovery, diagnostics and drug development. J Hepatol. 2021;74(1):168‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatia P, Mohammed S. Severe hypoxemia in early COVID-19 pneumonia. Am J Respir Crit Care Med. 2020;202(4):621‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siswanto Gani M, Fauzi AR, et al. Possible silent hypoxemia in a COVID-19 patient: a case report. Ann Med Surg (Lond). 2020;60:583‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gattinoni L, Coppola S, Cressoni M, et al. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201(10):1299‐1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrmann J, Mori V, Bates JHT, et al. Modeling lung perfusion abnormalities to explain early COVID-19 hypoxemia. Nat Commun. 2020;11(1):4883. doi: 10.1038/s41467-020-18672-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan F, Ye T, Sun P, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19). Radiology. 2020;295(3):715‐721. doi: 10.1148/radiol.2020200370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shibata S, Arima H, Asayama K, et al. Hypertension and related diseases in the era of COVID-19: a report from the Japanese Society of Hypertension Task Force on COVID-19. Hypertens Res. 2020;43(10):1028‐1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao D, Zhou F, Luo L, et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. Lancet Haematol. 2020;7(9):e671‐e678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox SE, Akmatbekov A, Harbert JL, et al. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8(7):681‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carsana L, Sonzogni A, Nasr A, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020;20(10):1135‐1140. doi: 10.1016/S1473-3099(20)30434-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asakura H. Classifying types of disseminated intravascular coagulation: clinical and animal models. J Intensive Care. 2014;2(20):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGonagle D, O’Donnell JS, Sharif K, et al. Immune mechanisms of pulmonary intravascular coagulopathy (PIC) in COVID-19 pneumonia. Lancet Rheumatol. 2020;2(7):e437‐e445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee C, Choi WJ. Overview of COVID-19 inflammatory pathogenesis from the therapeutic perspective. Arch Pharm Res. 2021. 10.1007/s12272-020-01301-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiménez-Alcázar M, Rangaswamy C, Panda R, et al. Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science. 2017;358(6367):1202‐1206. [DOI] [PubMed] [Google Scholar]
- 23.O’Sullivan JM, McGonagle D, Ward SE, et al. Endothelial cells orchestrate COVID-19 coagulopathy. Lancet Haematol. 2020;7:e553‐e555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henry BM, Vikse J, Benoit S, et al. Hyperinflammation and derangement of renin-angiotensin-aldosterone system in COVID-19: a novel hypothesis for clinically suspected hypercoagulopathy and microvascular immunothrombosis. Clin Chim Acta. 2020;507:163‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gan R, Rosoman NP, Henshaw DJE, et al. COVID-19 as a viral functional ACE2 deficiency disorder with ACE2 related multi-organ disease. Med Hypotheses. 2020;144:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasal DA, De Lorenzo A, Tibiriçá E. COVID-19 and microvascular disease: pathophysiology of SARS-CoV-2 infection with focus on the renin-angiotensin system. Heart Lung Circ. 2020;29(11):1596‐1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furuhashi M, Moniwa N, Mita T, et al. Urinary angiotensin-converting enzyme 2 in hypertensive patients may be increased by olmesartan, an angiotensin II receptor blocker. Am J Hypertens. 2015;28(1):15‐21. [DOI] [PubMed] [Google Scholar]
- 28.Hosoki K, Chakraborty A, Sur S. Molecular mechanisms and epidemiology of COVID-19 from an allergist’s perspective. J Allergy Clin Immunol. 2020;146(2):285‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen C, Zhang Y, Huang J, et al. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. medRxiv. 2020. 10.1101/2020.03.17.20037432 [DOI] [Google Scholar]
- 30.Frediansyah A, Nainu F, Dhama K, et al. Remdesivir and its antiviral activity against COVID-19: a systematic review. Clin Epidemiol Glob Health. 2021;9:123‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh AK, Singh A, Singh R, et al. Remdesivir in COVID-19: a critical review of pharmacology, pre-clinical and clinical studies. Diabetes Metab Syndr. 2020;14(4):641‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hillaker E, Belfer J, Bondici A, et al. Delayed initiation of remdesivir in a COVID-19-positive patient. Pharmacotherapy. 2020. doi: 10.1002/phar.2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffmann M, Schroeder S, Kleine-Weber H, et al. Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for COVID-19. Antimicrob Agents Chemother. 2020;64(6):e00754‐20. doi: 10.1128/AAC.00754-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doi K, Ikeda M, Hayase N, et al. COVID-UTH Study group. Nafamostat mesylate treatment in combination with favipiravir for patients critically ill with covid-19: a case series. Crit Care. 2020;24(1):392. doi: 10.1186/s13054-020-03078-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with covid-19. N Engl J Med. 2021;384(8):693‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brattsand R, Linden M. Cytokine modulation by glucocorticoids: mechanisms and actions in cellular studies. Aliment Pharmacol Ther. 1996;10(Suppl. 2):81‐90. discussion 1-2. [DOI] [PubMed] [Google Scholar]
- 37.Shang L, Zhao J, Hu Y, et al. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395(10225):683‐684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Youssef J, Novosad SA, Winthrop KL. Infection risk and safety of corticosteroid use. Rheum Dis Clin N Am. 2016;42(1):157‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asakura H, Ogawa H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int J Hematol. 2021;113(1):45‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]