Abstract
Background
Lumbar spinal stenosis (LSS) affects mainly elderly patients. To this day, it is unclear whether comorbidities influence treatment success. The aim of this systematic review and meta-analysis was to assess the impact of comorbidities on the treatment effectiveness in symptomatic LSS.
Methods
We conducted a systematic review and meta-analysis and reviewed prospective or retrospective studies from Medline, Embase, Cochrane Library and CINAHL from inception to May 2020, including adult patients with LSS undergoing surgical or conservative treatment. Main outcomes were satisfaction, functional and symptoms improvement, and adverse events (AE). Proportions of outcomes within two subgroups of a comorbidity were compared with risk ratio (RR) as summary measure. Availability of ≥3 studies for the same subgroup and outcome was required for meta-analysis.
Results
Of 72 publications, 51 studies, mostly assessing surgery, there was no evidence reported that patients with comorbidities were less satisfied compared to patients without comorbidities (RR 1.06, 95% confidence interval (CI) 0.77 to 1.45, 94%), but they had an increased risk for AE (RR 1.46, 95% CI 1.06 to 2.01, 72%). A limited number of studies found no influence of comorbidities on functional and symptoms improvement. Older age did not affect satisfaction, symptoms and functional improvement, and AE (age >80 years RR 1.22, 95% CI 0.98 to 1.52, 60%). Diabetes was associated with more AE (RR 1.72, 95% CI 1.19 to 2.47, 58%).
Conclusion
In patients with LSS and comorbidities (in particular diabetes), a higher risk for AE should be considered in the treatment decision. Older age alone was not associated with an increased risk for AE, less functional and symptoms improvement, and less treatment satisfaction.
Keywords: Lumbar spinal stenosis, Comorbidities, Chronic disease, Systematic review, Meta-analysis, Treatment outcome, Adverse events, Diabetes, Elderly, Spine surgery
Introduction
In lumbar spinal stenosis (LSS) degenerative processes lead to a narrowing of the lumbar spine resulting in a compression of neurovascular structures [1], [2], [3]. Typical symptoms include neurogenic claudication or radiculopathy [1], [2], [3]. In symptomatic patients, LSS results in disability, limited mobility [4], which affects the physical, psychological and social health [5], [6], [7]. Degenerative LSS is the most common reason for spinal surgery in the elderly population [1,2,[7], [8], [9]]. Treatment options include physical therapy, pain medications [1,2,10] and in selected cases epidural injections [1,2,11], and surgery to improve function and relief of pain [1,2,10,12,13].
In the ageing population multimorbidity, defined as the presence of two or more chronic diseases, is common [14] and may affect treatment outcome in patients with LSS. Multimorbidity was associated with less favorable functional outcome after surgery [15,16] and with an increased risk for perioperative complications and mortality [9,17,18]. However, results from various studies were conflicting. Whereas some studies showed an increased risk for complications in elderly patients after surgery [8,[17], [18], [19]], others found no influence of age on the risk for complications [20], [21], [22], [23], [24], [25], [26]. Cardiovascular disease was associated with worse post-surgical outcomes in one study [27], but not in another study [21]. Conflicting results were also found for diabetes [21,22], psychiatric [21,22,28], and musculoskeletal diseases [21,29].
To date, the evidence of the influence of comorbidities on the treatment outcome in patients with LSS undergoing surgical or non-surgical treatments has not been systematically reviewed. Therefore, the aim was to summarize the evidence of the influence of comorbidities on the treatment outcome of patients undergoing treatment for LSS.
Methods
Study design
Systematic review and meta-analysis. We followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA) [30]. The study protocol has been described previously [7].
Literature search
We systematically searched on May 2, 2020: Medline (Ovid), Embase, the Cochrane Library, and CINAHL. All references from the inception of the database until the search date were considered. Search terms included MeSH terms (Medical Subject Headings) and keywords related to “lumbar spinal stenosis” and “comorbidities” (Appendix 1). We also searched bibliographies of studies, guidelines, and review articles and contacted authors of studies with insufficient details.
Eligibility criteria
Eligible were prospective or retrospective studies with adult patients with degenerative LSS undergoing surgical or conservative treatment. As subgroup analyses require a sufficient sample size to be robust, we included studies with at least 100 patients. All studies were considered in which we had sufficient language proficiency (i.e. English, French, German, Spanish, and Italian). Excluded were studies in patients aged <18 years or less than 100 patients, cross-sectional and case-control studies.
Study selection and data extraction
Two reviewers (AS, AB) independently screened all titles and abstracts, and reviewed all potentially relevant references in full text. Disagreement between the reviewers was discussed and resolved in consensus or by third party arbitration (MW). If there were several publications for the same study, we included publication(s) reporting findings relevant for the research question.
Data collection and data item
One reviewer (AS) extracted information, using a predefined and piloted extraction form. A second reviewer (AB) confirmed the accuracy of extracted data. All data included in the meta-analysis were confirmed by the third reviewer (MW). We extracted information on study characteristics, patients’ characteristics, comorbidities and comorbidity measures, treatments, and outcomes.
Outcomes of interest
The main outcomes of interest were treatment satisfaction, functional and symptoms improvement, and adverse events. Additional outcomes included mortality. All outcome variables were extracted as reported in the original studies and operationalized.
Comorbidities
We extracted information on comorbidity measures (Appendix 2) and comorbidities: disease specific (previous spine surgery, symptom duration), cardiovascular risk factors (age, obesity, smoking), chronic diseases (e.g. cardiovascular, lung, neurologic, or rheumatologic), and psychologic disease. Subgroup definitions were standardized into the most often reported categories: e.g. diabetes/no diabetes, obesity (body mass index (BMI) ≥30kg/m2 versus (vs.) <30kg/m2), high comorbidity burden (i.e. American Society of Anesthesiology score (ASA) >3 vs. ≤3, Charlson >1 vs. ≤1, comorbidity score >3 vs. ≤3, presence of diseases/comorbidities vs. no diseases/comorbidities).
Study quality
Two reviewers (AB, AS) independently assessed study quality using Scottish Intercollegiate Guidelines Networks (SIGN) checklists for randomized controlled trials (RCTs) and cohort studies [31]. For each study, internal validity was assessed (yes/no/can't say/doesn't apply) and a global quality assessment assigned according to pre-defined criteria into high, acceptable, or low (Appendix 3). Disagreements were discussed and resolved by consensus or third-party arbitration (MW).
Data synthesis and statistical analysis
We provided a descriptive synthesis of evidence by categorizing findings into strong, weak, or conflicting evidence for or against an influence of a comorbidity. We summarized continuous and categorical variables with number/percentage, mean/standard deviation or median/interquartile range. We reported regression factors with coefficients, 95% confidence intervals (CI) and p-values.
In the meta-analysis, associations of comorbidities with treatment outcomes were analyzed by restricting subsets with the same treatment outcome for surgical or non-surgical treatment. The proportions of the two subgroups were compared with risk ratio (RR) as summary measure. We explored potential publication bias by using funnel plots. Funnel plots were exploratory, as a study could have multiple study arms, thus the study dots in the funnel plot were not independent. We performed meta-analyses in subsets with the same treatment and with specific comorbidity subgroups only, if at least three studies were available. We used random-effects models for pooling RRs due to expected large heterogeneity.
Studies were weighted by the standard error of their estimates, i.e. by sample size. Heterogeneity measures and were quantified. Results in RRs were visualized in forest plots including the study-specific estimates and their 95% confidence intervals (CI). The statistical analysis was performed in the R programming language [32] using base and analysis-specific packages: Amelia, biostatUZH, dplyr, ggpubr, meta, metaviz, readxl, tableone, xtable.
Results
Study selection
We screened title and abstract of 3244 references and read 157 potentially relevant full texts (Figure 1). In total, 72 publications based on 51 studies (the Spine Patient Outcomes Research Trial (SPORT) study was counted as two studies with a randomized and an observational study arm) were included and analyzed. Main reasons for exclusion were insufficient sample size (n=47), other study population/research question (n=27), study protocol/conference proceedings (n=7), and no language proficiency (n=4, Chinese, Japanese, and Czech).
Fig. 1.
Study flow.
Baseline characteristics
Two studies were RCTs, 14 prospective observational, and 32 retrospective studies (Table 1). Three studies used mixed methods (retrospective chart review and cross-sectional follow-up assessments). Retrospective studies were chart reviews (n=14), databases (n=3, e.g. hospital databases), insurances claims data (n=7), and registries (n=8). The studies were performed in the USA (n=19), Europe (n=18), Asia (n=14), and in various countries (n=3) [23,33,34].
Table 1.
Baseline characteristics.
| Author, year, study number | Design | Setting | Inclusion criteria | Exclusion criteria | Treatment | Follow-up, months | Number of patients(% female) | Age: mean, years (SD) |
|---|---|---|---|---|---|---|---|---|
| Turner J et al, 2015, [51] 1.1 | Randomized controlled trial (RCT) | Lumbar Epidural Steroid Injections for Spinal Stenosis trial (LESS), multicenter study at 16 clinical sites, United States of America (USA). Follow-up data assessed by telephone interview, in-person interview or mailed questionnaires | Age ≥50 years, confirmed lumbar spinal stenosis (LSS) on computer tomography (CT)/magnetic resonance imaging (MRI), undergoing conservative treatment; average low back/buttock/leg pain while standing/walking/spinal extension in the past week of number rating scale (NRS) ≥5 (0-10); buttock/leg pain worse than back pain; Roland Morris Disability Questionnaire (RMDQ) physical disability score ≥7 | Epidural steroid injection (ESI) ≤6 months, previous lumbar spine surgery; cognitive impairment; fibromyalgia; chronic widespread pain; lower extremity amputation; Parkinson's disease; head injury; stroke, other neurologic conditions; severe vascular, pulmonary or coronary artery disease; spinal instability; osteoporosis, metastatic cancer, excessive alcohol consumption/drug use; pregnancy; pain with internal rotation of hip; active infection; allergy to local anesthetic, steroid or contrast | Double blind epidural steroid + lidocaine or lidocaine injections | 1.5 | 400 (45) | Median 68 |
| Friedly J et al, 2014, [67] 1.2 | Subgroup analysis with central canal stenosis | No central canal stenosis; spondylolisthesis requiring surgery | 1.5 | 386 (57) | 68.1 (10) | |||
| Friedly J et al, 2018, [68] 1.3 | Subgroup analysis: degree of cortisol suppression and risk factors | 0.75 | 307 (n.r.) |
N.r. | ||||
| Lurie JD et al, 2015, [69] 2.1 | Secondary analysis of a RCT and cohort study | Spine Patient Outcomes Research Trial (SPORT): RCT and cohort study; patient enrollment in 13 centers across 11 US-States 2000-2004, USA | Adults (age ≥18 years), LSS group: neurogenic claudication and/or radicular leg symptoms; LSS confirmed on imaging (≥1 level(s)); ongoing symptoms ≥12 weeks without sufficient improvement after non-surgical interventions | Degenerative spondylolisthesis, cauda equina syndrome, progressive symptoms with urgent surgery, overall health that makes spine surgery too life threatening; dramatic improvement with non-surgical care; pregnancy; active malignancy; current fracture, infection, significant deformity of the spine; previous lumbar spine surgery; unable to complete questionnaires or follow-up | RCT-group: surgery (posterior decompression laminectomy) or conservative treatment (physical therapy (PT), education, non-steroidal anti-inflammatory drugs (NSAID), ESI, spinal manipulation). Cohort study: surgery or conservative treatment | 96 | 306 (37) | 61.1 (10.4) |
| Gerling M et al, 2016, [70] 2.2 | Subgroup analysis: risk factors for reoperation in patients treated surgically for LSS | Fixed or unstable lumbar spondylolisthesis or spondylolysis | Surgery (posterior decompression laminectomy) | 96 | 417 (39) |
63.3 (11.35) | ||
| Freedman M et al, 2011, [59] 2.3 | Subgroup analysis: influence of diabetes on the outcome after treatment for LSS | Surgical or conservative treatment | 48 | 627 (40) |
65.75 (10.65) | |||
| McGuire K et al, 2014, [41] 2.4 | Subgroup analysis: influence of extreme obesity (BMI ≥35 kg/m2) on outcomes of treatment for LSS | Spondylolysis and isthmic spondylolisthesis | Surgical or conservative treatment | 48 | 634 (39) | 63.47 (10.87) | ||
| Rihn J et al, 2015, [71] 2.5 | Subgroup analysis in old age (<80 years compared to ≥80) | Surgical or conservative treatment | 48 | 1235 (54) |
73.35 (6.4) | |||
| Rihn J et al, 2012, [72] 2.6 | Subgroup analysis: obesity (body mass index (BMI) >30 kg/m2) compared with non-obese (BMI ≤30) | 48 | 634 (39) |
64.25 (11.35) | ||||
| Radcliff K et al, 2011, [73] 2.7 | Subgroup analysis: symptom duration | 48 | 634 (39) |
64.65 (11.75) | ||||
| Atlas SJ et al, 2005, [74] 3 | Multicenter cohort study | Maine Lumbar Spine Study, community-based practices in Maine, USA; recruitment 1990-1992, interviews (baseline), mailed questionnaires (follow-up) | Patients with a diagnosis of LSS based on physician assessment of appropriate symptoms, examination, and radiographic findings undergoing operative or non-operative treatment | Previous lumbar surgery; cauda equina syndrome; developmental spine deformities; vertebral fractures; spine infection or tumor; inflammatory spondyloarthropathy; pregnancy; severe comorbid conditions | Surgery (laminectomy, no fusion) or conservative treatment (exercises, bedrest, PT, spinal manipulation, opioid, ESI) | 120 | 97 (60) |
65.6 (11.55) |
| Katz JN et al, 1999, [27] 4.1 | Multicenter prospective observational study | Four referral centers, Brigham and Women's Hospital, and Beth Israel Hospital in Boston, University of Vermont and University of Iowa Hospitals and Clinics, USA. Baseline/follow-up questionnaires by mail and medical records | Age ≥50 years, surgery for degenerative LSS confirmed by imaging studies (compression of cauda equina on CT/myelography followed by contrast enhanced CT or MRI); presence of back/buttock and/or lower extremity pain; opinion of the attending surgeon that patients had clinically significant degenerative LSS | Previous surgery for LSS; limitations to complete questionnaires; patients who had non-surgical treatment | Surgery (decompression with/without fusion) | 24 | 199 (n.r.) |
69 (range 50-92) |
| Katz JN et al, 1995, [38] 4.2 | First results on treatment satisfaction at 6 months follow-up | 6 | 194 (60) |
68.5 (8.6) | ||||
| Herron L et al, 1991, [75] 5 | Single center prospective observational study | Central Coast Spine Institute San Luis Obispo, USA. Baseline/follow-up clinical examinations | N.r. | N.r. | Surgery (decompression) | Mean 42 | 140 (50) |
Mean 63 (range 30-87) |
| Ilyas H et al, 2019, [54] 6 | Single center retrospective chart review | Cleveland Clinic, Cleveland, USA. Baseline and follow-up data from medical records | All patients with diagnosis of LSS with claudication undergoing surgery between 01/2014 and 12/2015 | Age <18 years, spinal tumor or infection, anterior lumbar surgery, planned elective readmission or reoperation | Posterior lumbar decompression (with/without fusion) | 3 | 1592 (45.5) | 67.4 (10.1) |
| Lubelski D et al, 2015, [52] 7 | Single center retrospective chart review | Cleveland Clinic Center for Spine Health, Cleveland, USA. Baseline chart review; outcome measures prospectively collected in a database | Age ≥18 years; diagnosis of LSS (gluteal and/or lower extremity pain and/or fatigue with/without back pain, symptoms aggravated by upright exercise or position-induced neurogenic claudication and relief with forward flexion, sitting or recumbency) undergoing conservative treatment | Previous spine surgery or treatment with a membrane stabilizing agent (MSA); spinal tumors or fracture; cauda equina syndrome; foot drop; epilepsy; renal failure; not participating in Quality of Life outcome data collection | Membrane stabilizing agents (MSA): gabapentin, pregabalin (treatment duration and drug dose not reported) | Mean 6 (range 2-12) | 1346 (49) |
66.3 (10.1) |
| Javalkar V et al, 2010, [76] 8 | Single center retrospective chart review | Department of Neurosurgery, Louisiana State University Health Sciences Center, Shreveport, Louisiana, USA; analysis of reoperation after surgery | Patients aged ≥18 years with symptomatic, confirmed LSS (MRI/x-rays) undergoing treatment for LSS after insufficient improvement during conservative treatment (epidural/facet/foraminal injections, PT) | N.r. | Surgery (decompression +/- fusion) | Undefined | 335 (50) |
Mean patients with re-operation: 60.8 (range 33-83) |
| Movassaghi K et al, 2019, [77] 9 | Single center retrospective chart review | Department of Orthopedic surgery, Rush University Medical Center, Chicago, USA | Lumbar decompression for LSS from 01/2008-12/2015, radiculopathy and/or neurogenic claudication, no motor deficit, failed conservative treatment (activity modification, anti-inflammatory medications, PT, injections for ≥3 months) | Age <18 years, previous lumbar surgery, herniated disc, follow-up <3 months | Decompressive laminectomy | Mean 24.1 range (3-78) | 210 (24.3) | 54.1 (16.3) |
| Ragab A et al, 2003, [78] 10 | Single center retrospective chart review | Spine Institute, Orthopedic surgery Department, Case Western Reserve University, Cleveland, USA. Medical charts review, follow-up questionnaire by mail | Age ≥70 years, follow-up ≥2 years (out of 1152 patients who underwent lumbar spinal surgery, 118 patients met these criteria) | <70 years of age; <2-year follow- up | Surgery (decompression +/- fusion) | Mean 84 (range 24-168) | 118 (56) |
74 (range 70-101) |
| Li G et al, 2008, [35] 11 | Database study | National Inpatient Sample (NIS) hospital discharge database (Agency for Healthcare Research and Quality), USA | All patients with primary diagnosis of LSS undergoing lumbar laminectomy without fusion from 1993 to 2002 | N.r. | Surgery (lumbar laminectomy) | Within the hospitalization time | 471215 (50) | 67 |
| Deyo R et al, 2010, [8] 12 | Insurance claims database analysis (Medicare) | Medicare claims data analysis (Medicare Provider Analysis and Review database (MedPAR)), 2002-2007, USA | Age ≥65 years; primary diagnosis of LSS or "spondylogenic compression of lumbar spinal cord"; all surgical procedure: patient identified by surgical procedure codes (international classification of disease 9th edition (ICD-9)) | Any diagnosis as cancer, vehicular accident, spinal infection, inflammatory spondyloarthropathy, vertebral fracture or dislocation or cervical or thoracic spine procedures | Surgery (decompression, simple fusion, complex fusion and any combination) | 1 | 32152 (54) |
75 |
| Drazin D et al, 2017, [55] 13 | Medicare claims data analysis, MedPAR data from 2005-2011 USA | Age ≥65 years; LSS diagnosis; patient identification by LSS diagnosis code (ICD-9) | Death during the index hospitalization; cancer <6 months prior to diagnosis; back surgery <1 year prior to the index hospitalization | Surgery (laminectomy or fusion) | Mean 40.4 (SD 23.5) | 12807 (58) |
75.4 (5.9) | |
| Ciol M et al, 1996, [17] 14 | Medicare and National Hospital Discharge Survey (NDHS, for all acute-care non-federal hospitals in the USA) data | Age ≥65 years with primary diagnosis for spinal stenosis (ICD-9 for spinal stenosis or "spondylogenic compression of the lumbar spine") undergoing surgery in 1985 or 1989 | Cervical/thoracic spine diagnosis; cancer; spinal infection; inflammatory spondylitis; fracture; vehicular trauma; other surgical procedure; living outside the US; Medicare eligibility based on end-stage renal disease or disability; <12 months Medicare eligibility | Surgery (decompression with or without fusion) | 36 (1989 cohort),84 (1985 cohort) | 28915 (59) |
73.35 (5.35) | |
| Lad S et al, 2013, [79] 15 | Insurance claims database analysis | Patient-level data from Medicaid and private insurance (Thomson Reuter's MarketScan), USA | Primary diagnosis of LSS; laminectomy or fusion between 01-2000 and 12-2009; patient identification using procedure codes (current procedural terminology 4th edition (CTP-4) and international classification of disease 9th edition, clinical modification (ICD-9-CM)) | Patients ≤18 or ≥65 years | Surgery (laminectomy or fusion) | 24 | 28462 (52) |
56 (8) |
| Sharma M et al, 2019, [37] 16 | Insurance claims database analysis | MarketScan database from Truven Health Analytics - IBM Watson Health. Claims data from private, Medicaid, Medicare supplemental insurances | Age ≥80 years and older with primary diagnosis of LSS and decompression between 2000-2016 | Age <80 years, no 12-months post-surgical insurance enrollment | Decompression +/- fusion (laminectomy, laminotomy, discectomy, vertebrectomy, corpectomy, foreign body removal or repair of vertebral fracture) | 12 | 5387 (48.3) | 83.1 (2.9) |
| Basques B et al, 2014, [80] 17 | Database study | American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database; >370 hospitals, USA | Postoperative diagnosis of LSS (ICD-9) with the primary Current Procedural Terminology code for lumbar laminectomy | Other spinal procedures including lumbar fusion; urgent/emergent surgery; previous evidence of infection | Surgery (decompression) | 1 | 2358 (40) |
66.4 (11.7) |
| Merrill R et al, 2018, [50] 18 | Database study | Surgical database of procedures 2015-2016 performed by 4 surgeons in 1 academic center, USA. Questionnaires collected during clinical visits | Symptomatic LSS (claudication or radiculopathy), age ≥18 years; surgery with lumbar laminectomy without fusion | Lumbar decompression with associated fusion; decompression performed for trauma or malignancy; incomplete follow-up; incomplete Patient-Reported Outcome Measurement Information System (PROMIS) questionnaires | Surgery (lumbar laminectomy) | 6 | 111 (51) |
60.0 (1.94) |
| Adogwa O et al, 2012, [81] 19 | Mixed methods (baseline chart review, prospective follow-up interview) | Clinic of Neurosurgery and Orthopedic Surgery and Rehabilitation, Vanderbilt University Medical Center, Nashville, USA | Revision lumbar decompression/instrumented fusion for symptomatic adjacent segment disease, pseudoarthrosis, or same-level restenosis; age 18-70 years; no improvement after ≥6 months conservative therapy | Extra-spinal cause of back pain; an active workman's compensation lawsuit; having no wish to take part to follow-up; fractured rods and screws without evidence of nonunion | Revision surgery | 24 | 150 (63) |
57 (22) |
| Held U et al, 2019, [82] 20.1 | Multicenter cohort study | Lumbar stenosis outcome study (LSOS), Rheumatology/Spine surgery units at 8 hospitals, Switzerland. Baseline/follow-up questionnaires/interview. | Follow-up results 1 year: age ≥50 years, symptomatic LSS (neurogenic claudication) and verified degenerative LSS (MRI/CT) | Cancer, infection, or significant deformity; previous lumbar spine surgery; clinically relevant peripheral artery disease | Treatment according to patient/physician preferences: surgery; non-surgical treatment (analgesics, physiotherapy, +/- lumbar ESI) | 12 | 222 (55) |
74.2 (8.1) |
| Held U et al, 2018, [46] 20.2 | Subgroup analysis: patients undergoing surgical treatment; +/- previous spine surgery | Exclusion from analysis: patient did not undergo surgery <6 months after enrollment; not completed follow-up at 12 months | Surgery (open posterior lumbar laminotomy +/-fusion) | 12 | 300 (51) |
N.r. | ||
| Fekete T et al, 2015, [83] 20.3 | Subgroup analysis: ESI prior to surgical/non-surgical intervention | Surgery (first-time decompression without fusion) or conservative treatment (PT, oral analgesics) | 6 | 281 (52) |
75.0 (8.7) | |||
| Burgstaller J et al, 2016, [84] 20.4 | Subgroup analysis in patients undergoing surgery: influence of obesity on postoperative outcome | Diagnosis of diabetes mellitus | Surgery (open posterior lumbar laminectomy or laminotomy (no instrumentation)) | 12 | 166 (48) |
Median 74 (IQR 12) | ||
| Burgstaller J et al, 2017, [53] 20.5 | Subgroup analysis in patients undergoing surgery: influence of pre- and postoperative fear avoidance beliefs on post-surgical pain and disability | Surgery (first- decompression only) | 12 | 234 (51) | Median 75.0 (IQR 68, 80) | |||
| Ulrich NH et al, 2015, [20] 20.6 | Subgroup analysis in patients aged >80 years undergoing surgery (compared to <80 years) | Surgery (open posterior lumbar laminectomy or laminotomy (no instrumentation)) | 12 | 93 (39) | 78.0 (2.6) | |||
| Aalto T et al, 2012, [40] 21.1 Sinikallio S et al, 2007 [43] 21.2 |
Single center cohort study | Clinic of Orthopedics and Neurosurgery at Kuopio University Hospital, Kuopio, Finland. Baseline and follow-up questionnaires | Surgery for degenerative, symptomatic LSS; (back/buttock/lower extremity pain); radiographic evidence of cauda equina compression +/- exiting nerve roots; insufficient improvement after conservative treatment. Preoperative predictors for post-surgical outcome at 3 months [43] and 12 months [40] follow-up | Emergency spinal operation precluding recruitment and protocol investigations; failures in cooperation; MRI contraindications | Surgery (open or microscopic decompression) | 24 | 102 (58) |
N.r. |
| Tuomainen I et al, 2018, [48] 21.3 Pakarinen M et al, 2014, [85] 21.4 Sinikallio S et al, 2011 [86] 21.5 |
Analysis of influence of depression on the outcome at 2-year, [86] 5-year [85] and 10-year [48] follow-up | 120 | 72 (60) |
68.5 (10.9) | ||||
| Airaksinen O et al, 1997, [22] 22 | Mixed methods (chart review baseline, follow-up interview) | Department of Surgery, Kuopio University Hospital, Kuopio, Finland | Surgery for LSS between 1974 and 1987 | N.r. | Surgery (decompression) | Mean 52 | 438 (42) |
53 (9.5) |
| Jakola A et al, 2010, [24] 23 | Single center cohort study | Department of Neurosurgery, St. Olavs Hospital, Trondheim, Norway. Questionnaires at baseline/follow-up | Age ≥70 years, isolated LSS undergoing conventional decompression laminectomy | Radiological signs of instability (spondylolisthesis) considered for fusion procedure | Surgery (decompression) | 12 | 101 (50) |
75.3 |
| Guigui P et al, 2002, [87] 24 | Single center prospective observational study | Orthopedic/Surgery Unit, Beaujon Hospital, Clichy, France. Follow-up visits at 3, 6, 12 months | Patients undergoing surgery for LSS at hospital of Beaujon from 1998 to 2000 | Patients with a deviation of the spine (>20°) in the frontal or sagittal plane | Surgery (decompression, and/or fusion) | 12 | 306 (55) |
60 (range 22-90) |
| Ferrero E et al, 2018, [88] 25 | Single center prospective observational study | Department of orthopedic surgery, Hôpital européen Georges-Pompidou, Paris, France. Questionnaires at follow-up | LSS diagnosis based on clinical and imaging studies (CT/MRI; ≥1 level(s) narrowing of the central spinal canal (area <100mm2), a foraminal diameter or lateral recess diameter <3mm); neurogenic claudication and/or signs of chronic neurogenic compression | Previous spinal surgery; coronal Cobb angle ≥10°; other disease causing polyneuropathy; LSS secondary to tumor or infection; language limitations | Unspecified surgery | 12 | 250 (57) |
65.6 (12) |
| Papavero L et al, 2009, [89] 26 | Single center prospective observational study | Spine Surgery Center, Eilbek Medical Center, Hamburg, Germany. Baseline/outcome data assessed by independent observer | Patients with LSS undergoing surgery; back/leg pain refractory to conservative treatment for ≥3 months; decreased walking capacity | Mobile vertebral slip; previous surgery at one of the stenotic levels | Surgery (microsurgical bilateral decompression using unilateral laminotomy) | 12 | 165 (50) |
69.27 |
| Costa F et al, 2007, [90] 27 | Single center retrospective chart review | Department of Neurosurgery, Milan, Italy. Chart review of medical records | Patients with confirmed single/multilevel LSS (CT/MRI) undergoing surgery; neurogenic claudication or radiculopathy; failure of conservative treatment with NSAID, corticosteroids, and physiotherapy for ≥3 months | Segmental instability | Surgery (unilateral laminotomy for bilateral micro-decompression) | 30.3 (range 16-53) | 374 (51) |
64.7 (9) |
| Rillardon L et al, 2003, [91] 28.1 | Single center retrospective chart review | Orthopedic Surgery Clinic, Hospital Beaujon, Clichy, France. Chart review. Additional in-person/phone questionnaire for follow-up | Surgery for symptomatic and confirmed LSS 1990-1992; symptoms: neurogenic claudication/compression of peripheral nerves; analysis of long-term outcome after surgery |
Previous spine surgery; scoliosis of ≥20° | Surgery (decompression +/- fusion) | Mean 120 | 105 (66) |
58 (11.3) |
| Lenoir T et al, 2008, [36] 28.2 | Analysis on the long-term risk of reoperation after initial surgery between 1989 and 1992 | 180 | 262 (56) |
61 (10.8) | ||||
| Aghayev E et al, 2019, [34] 29 | Spine Tango registry (Eurospine) | 38 centers, 10 countries. Pre-and postoperative questionnaires | Age 18-100 years; decompression surgery for LSS 2004-2017, known American Society of Anesthesiologists (ASA) classification, no other spinal pathology | Anterior dynamic stabilization, any previous spine surgery, no pre- or ≥1 postoperative Core Outcome Measure Index (COMI) between 3-30 months available | Surgery (decompression with at least laminotomy, hemi-/laminectomy, partial facet joint resection or interspinous spacer) | Mean 15.6 (10.8-24) | 4504 (46.6) |
67.1 (12) |
| Sobottke R et al, 2017, [23] 30 | 35 centers, 9 countries. | Age ≥20 years; surgery for LSS 2004-2015; no other spinal pathology; no anterior surgical procedure | No ASA classification available, no pre- or ≥1 postoperative COMI between 3-30 months available | Surgery (open decompression +/- rigid or dynamic stabilization +/-fusion | Mean 15.8 (8.5) | 4768 (47) |
67.4 (11.9) | |
| Kleinstück F et al, 2009, [33] 31 | Spine Center, Schulthess Klinik, Zürich, Switzerland | Degenerative LSS diagnosed by surgeons (clinical and radiological findings), decompression only (02-2004 to 03-2007); fluent in German/English; ≤3 segments affected | <1-year follow-up; disc herniation; previous surgery at the same level; fusion/stabilization | Surgery (decompression) without fusion | 12 | 221 (49) |
72.4 (9.4) | |
| Iderberg H et al, 2018, [47] 32 | Swespine registry | National spine surgery registry (covers ≥80% of surgical procedures for degenerative lumbar spine disorders), Sweden. Mailed follow-up questionnaires | Patients who underwent surgery for LSS on 1 or 2 adjacent levels during 2008-2012 Analysis of predictors of surgical outcome |
No information on case mix variables or without 1-year follow-up data | Surgery (decompression, mostly without fusion) | 12 | 7643 (47) |
66.2 |
| Knutsson B et al, 2013, [92] 33 | Age ≥50 years; surgery for LSS 01-2006 to 06-2008. Analysis on influence of obesity (BMI groups: <25, 25-30, >30kg/m2) | No 2-year follow-up; invalid weight/height measures; invalid personal identification number; <50 years | Surgery | 24 | 2633 (43) |
68.67 (8.3) | ||
| Sanden B et al, 2011, [44] 34 | Age ≥50 years; diagnosis of central LSS; undergoing surgery before 10-2006. Analysis smoking/no smoking | Invalid personal identification number; age <50 years; <2-year follow-up | Surgery | 24 | 4555 (56) |
67.3 (8.55) | ||
| Strömqvist F et al, 2011, [93] 35 | Surgery for central LSS with/without root canal stenosis. Analysis on incidence of dural lesions | Isolated lateral spinal stenosis | Surgery (decompression) | 12 | 2875 (n.r) |
N.r. | ||
| Paulsen R et al, 2018, [42] 36 | DaneSpine registry | DaneSpine database, 3 regional centers in Denmark: Middelfart, Køge, Silkeborg Hospitals, Denmark. Mailed follow-up questionnaires | Symptomatic and confirmed (MRI) LSS; undergoing surgery between 2009 and 2014 | Previous spine surgery; concomitant fusion | Surgery (posterior decompression) | 12 | 1983 (53) |
66.6 (11.1) |
| Bouras T et al, 2010, [45] 37 | Mixed methods (baseline chart review, prospective follow-up interview) | Clinic of Neurosurgery at Evangelismos Hospital (4 surgeons), Athens, Greece | Patients aged ≥65 years undergoing laminectomy without fusion for LSS within 1999-2004 | Predominant back pain as preoperative symptom and/or imaging findings implying probable spinal instability or discopathy | Surgery (decompression) | Mean 61 | 125 (55) |
71.3 |
| Keorochana G et al, 2011, [94] 38 | Single center prospective observational study | Department of Orthopedics, Ramathibodi Hospital, Bangkok, Thailand. Database for baseline data, mailed follow-up questionnaires | Patients with symptomatic and confirmed LSS (CT, CT-myelography, or MRI) undergoing surgery; limitation of the functional activities; back, buttock and/or leg pain | Previous surgery for spinal stenosis; not able to complete questionnaires | Surgery (decompression and instrumented fusion) | Mean 2.6 | 158 (82) |
60.3 (range 34-87) |
| Kim HJ et al, 2015, [49] 39 | Single center prospective observational study | Spine Center, Seoul National University College of Medicine and Seoul National University Bundang Hospital, Republic of Korea. Baseline chart review, follow-up structured questionnaires | Age 40-80 years, symptomatic and confirmed (MRI) LSS; undergoing spine surgery between 06-2012 and 04-2013; ≥1 symptom(s): walking intolerance due to neurogenic claudication, pain/numbness/tingling sensation in the buttocks and lower extremities, motor weakness, bladder/bowel dysfunction | History of major psychiatric disorders or peripheral vascular disease; concurrent serious medical condition such as sepsis or cancer | Surgery (decompression with/without fusion) all performed by 1 surgeon | 12 | 157 (66) |
65.7 (9.6) |
| Miyamoto H et al, 2008, [95] 40 | Single center prospective observational study | Department of Orthopedic Surgery, National Hospital Kobe Medical Center, Japan. Baseline clinical assessment, follow-up questionnaires | Patients with LSS who underwent extended in-hospital conservative treatment between 1982 and 1998 after non-surgical outpatient treatments failed | Lumbar disc herniation; osteoarthritis of the knee/hip; spondylolysis; traumatic spinal deformity; cerebrovascular diseases; dementia; previous surgery | Non-surgical treatment (in-bed pelvic traction, body cast in lumbar spine, epidural block, selective nerve root block) | Minimum 60, mean 95 (range 60-216) | 120 (42) |
63.6 (8.2) |
| Hara N et al, 2010, [96] 41 | Single center prospective observational study | Department of Orthopedic Surgery, University hospital of Tokyo, Japan. Clinical baseline and follow-up assessment and questionnaires | Surgery for symptomatic and confirmed LSS (plain radiographs, MRI/myelography followed by contrast-enhanced CT scan); leg pain/numbness and/or gait disturbance with no response to conservative therapy ≥3 months | Severe spinal deformity; spondylolysis; post-traumatic stenosis or re-stenosis after prior decompression surgery | Surgery (decompression) | 24 | 89 (37) |
66.3 (11.2) |
| Kim HJ et al, 2008, [97] 42 | Single center retrospective chart review | Orthopedic Surgery Unit (2 surgeons), Yonsei University, Seoul, Korea. Hospital records and national health insurance data | Spine surgery for LSS between January 1997 and June 2006 | N.r. | Surgery (decompression with/without fusion) | Min 12 | 1015 (63) |
60 (n.r.) |
| Yaldiz C et al, 2015, [98] 43 | Single center retrospective chart review | Neurosurgery units of 2 university hospitals, Turkey. Chart review and clinical follow-up visit | Surgery for degenerative LSS between 01-2013 and 01-2014; ≥2 levels of laminectomy and facetectomy | N.r. | Surgery (posterior stabilization) | 1 | 540 (28) |
56.45 (9.81) |
| Gepstein R et al, 2006, [99] 44.1 | Single center retrospective chart review and follow-up interview | Spinal Care Unit, Sapir Medical Center, Kfar-Saba, Israel. Database including baseline in-person interview (structured questionnaire) and follow-up telephone interview | Age ≥65 years; surgery for degenerative LSS between 1990 and 2000 | Patients in whom fusion procedures were performed; spondylolisthesis | Surgery (decompression and/or discectomy) | Mean 41.6 | 298 (51) |
71.4 (5.4) |
| Gepstein R et al, 2004, [57] 44.2 | Subgroup analysis: influence of obesity | Mean 44.8 | 298 (51) |
71.4 (5.4) | ||||
| Shabat S et al, 2011, [100] 44.3 | Subgroup analysis: revision surgery | Mean 64 | 357 (n.r.) |
72 | ||||
| Shabat S et al, 2005, [101] 44.4 | Subgroup analysis: gender | Mean 66 (range 12-125) | 367 (48) |
71.42 (5.4) | ||||
| Arinzon Z et al, 2004, [39] 44.5 | Subgroup analysis: comparison of diabetic/non-diabetic patients | N.r. | Mean 41 | 124 (48) |
71 (4.8) | |||
| Arinzon Z et al, 2003, [102] 44.6 | Subgroup analysis: influence of age on surgical outcome | N.r. | Mean 42.2 | 283 (40) |
73.6 (3.1) | |||
| Nanjo Y et al, 2013, [103] 45 | Multicenter retrospective chart review | Six orthopedic surgery units, Japan | Age >40 years; confirmed LSS (physical and radiographic examination); undergoing decompression surgery between 2006-2010 | Age ≤40 years; previous surgery for LSS or locomotor disease ≤1 year; hemodialysis; lumbar disc herniation; spondylolysis; | Surgery (decompression without fusion) | Mean 14, range 6-60) | 241 (40) |
72.2 (range 45-93) |
| rheumatoid arthritis; psychiatric disease; vertebral fracture; scoliosis ≥10°; ≥3mm spondylolisthesis/≥10° instability or ≥4mm/≥20° | ||||||||
| Kong C et al, 2019, [56] 46 | Single center retrospective chart review | Department of Orthopedics, Beijing Xuanwu Hospital, Capital Medical University, Beijing, China | Patients >70 years with main diagnosis lumbar stenosis with instability +/- spondylolisthesis or scoliosis. | Previous lumbar surgery, malignancy, infection, or trauma | Surgery (posterior arthrodesis with pedicle screw fixation) | N.r. | 215 (63.7) | 75.7 (4.6) |
| Minamide A et al, 2017, [104] 47 | Single center retrospective chart review | Department of Orthopedic Surgery, Wakayama, Japan | Surgery for symptomatic (neurogenic claudication and/or radicular leg pain with associated neurologic signs) and confirmed (MRI) LSS after failed conservative treatment for ≥3 months | Cobb angle <10°; missing data (socio-demographic, clinical, or imaging studies); prior spine surgery or trauma; intraoperative complication; incomplete follow-up data | Surgery: microendoscopic laminectomy (MEL) or microendoscopic foraminotomy (MEF) | Minimum 24 | 122 (53) |
70.4 (8.0) |
| Choi J et al, 2017, [105] 48 | Single center retrospective chart review | Department of Neurosurgery, Kyung Hee University Hospital, Seoul, Korea | Age ≥70 years; posterior lumbar fusion with pedicle screw fixation for degenerative LSS | Fusion surgery for disease; decompression surgery; spinal tumors, trauma, or infections | Surgery (posterior lumbar fusion with pedicle screw fixation) | 132 | 116 (57) |
74.3 |
| Lee CK et al, 2018, [61] 49 | Insurance claims database analysis | Korean National Health Insurance System (KNHIS) on all national in-/outpatient data, South Korea | Cases with LSS diagnosis codes in KNHIS database, 2005 - 2007 | Cases where LSS diagnostic code was registered only once or twice; <50 years old; previous lumbar spine surgery | Undefined surgery or conservative treatment | 96 | 14298 (68) |
64 (8.5) |
| Kim C et al, 2013, [60] 50 | Cases with procedure codes for lumbar spine surgery and disease codes for LSS (international classification of disease 10th edition (ICD-10) and health insurance review and assessment agency (HIRA)) in 2003 | Lumbar surgery in the preceding 5 years; age ≤20 years; concomitant disease (fracture, neoplasm, infection), spondylolisthesis | Surgery (decompression or fusion) | Minimum 60 | 11027 (56) |
57.3 (11.8) | ||
| Yamada K et al, 2018, [106] 51 | Mixed methods (chart review preoperative data, cross-sectional survey) | Department of Orthopedic Surgery, Wajokai Eniwa Hospital, Osaka, Japan (4 spine surgeons) | Age >50 years; surgery for symptomatic and confirmed (MRI) LSS, 2002-2010; symptoms of neurogenic claudication, intolerable leg pain/numbness despite conservative treatment, severe muscle weakness or bladder/bowel dysfunction | Prior spinal surgery, vertebral fracture, spinal malignant neoplasm, spinal infection; age ≤50 years; lack of radiographs | Surgery (decompression alone or with fusion) | Mean 8.6 years (SD 2.0) | 1063 (47) |
66.6 (7.7) |
Abbreviations: SD, standard deviation; N.r., not reported; IQR, interquartile range.
Follow-up duration ranged from hospital discharge [35] to 180 months [36],sample sizes ranged from 101 [24] to 471’215 [35] subjects, and mean age from 53 [22] to 83 [37] years. The treatment type was surgical in 43 studies (84.3%), conservative in three (5.9%), both conservative and surgical in five studies (9.8%). Due to heterogeneous reporting of outcomes and comorbidities, only 37 of 72 publications had in total 170 arms suitable for subgroup analyses.
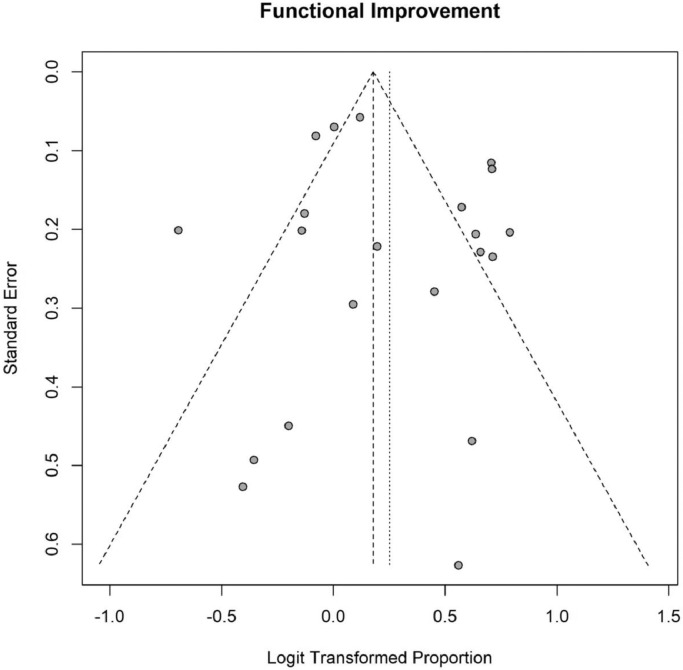
The quality was high in two RCTs (100%) and acceptable in the other studies (Appendix 3). No study was excluded due to a high risk of bias. Visual inspection of the funnel plot (Appendix 4) was symmetrical.
Studies reported different sets of comorbidities and the prevalence of comorbidities varied widely (Appendix 5, 6): diabetes (7.8% to 37.1%), cardiovascular disease (43.1% to 59.9%), lung disease (1.7% to 26.1%), nonspecific musculoskeletal disorders (1.8% to 55.9%), osteoporosis (6.2% to 35.6%). Neurological diseases (excluded in 16 studies (31.4%)) had a low prevalence (2.1% to 8.0%).
Predictors for satisfaction
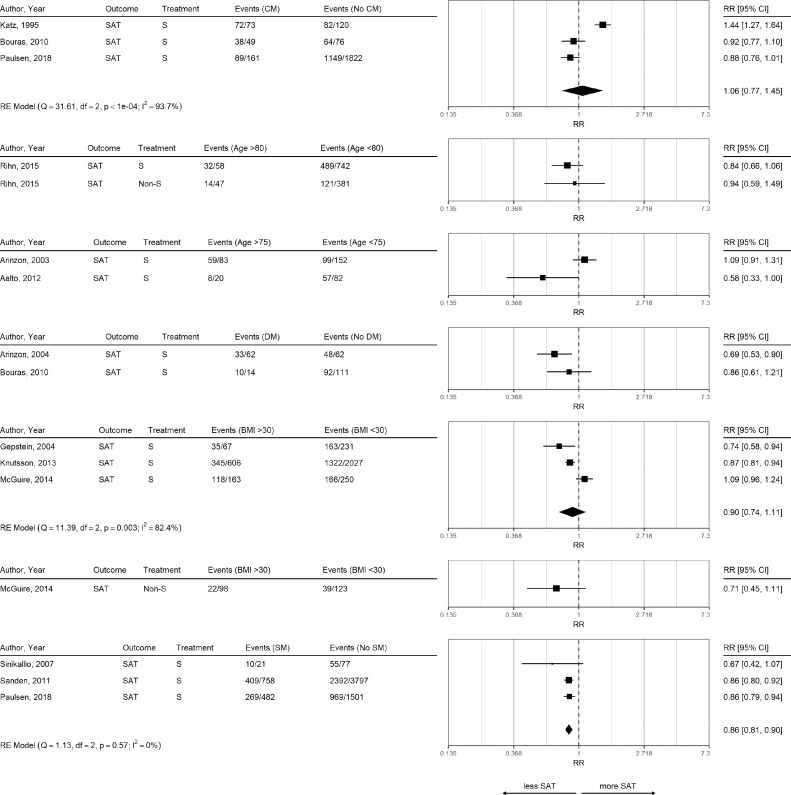
Table 2 provides an overview of studies reporting results for satisfaction, and symptoms and functional improvement. Although one study reported higher satisfaction in patients with comorbidities compared to patients without comorbidities [38], there was no evidence for an association (RR 1.06, 95% CI 0.77 to 1.45; Figure 2).
Table 2.
Association of comorbidities with function, symptoms and satisfaction.
| Comorbidity | Function | Evidence strength | Symptoms | Evidence strength | Satisfaction | Evidence strength | |||
|---|---|---|---|---|---|---|---|---|---|
| sign.:n | n.s.:n | for/against association | sign.:n | n.s.:n | for/against association | sign.:n | n.s.: n | for/against association | |
| Comorbidities, comorbidity measures (CM) | 3 | 5 | weak against | 4 | 2 | weak for | 1 | 3 | weak against |
| Previous spine surgery | 3 | 3 | conflicting | 1 | 3 | weak against | 1 | 0 | weak for |
| Symptom duration | 3 | 4 | weak against | 0 | 4 | strong against | 1 | 2 | weak against |
| Body weight | 1 | 4 | strong against | 0 | 1 | weak against | 0 | 2 | weak against |
| Obesity | 1 | 2 | weak against | 0 | 2 | weak against | 1 | 3 | weak against |
| Hypertension | 0 | 0 | no evidence | 0 | 0 | no evidence | 0 | 0 | no evidence |
| Diabetes | 1 | 3 | weak against | 1 | 2 | weak against | 1 | 1 | conflicting |
| Smoking | 2 | 5 | strong against | 0 | 2 | weak against | 2 | 2 | conflicting |
| Cardiovascular disease | 1 | 1 | conflicting | 2 | 0 | weak for | 1 | 1 | conflicting |
| Lung disease | 0 | 1 | weak against | 0 | 1 | weak against | 0 | 0 | no evidence |
| Neurologic disease | 0 | 1 | weak against | 0 | 1 | weak against | 1 | 0 | weak for |
| Rheumatologic disease | 1 | 2 | weak against | 0 | 1 | weak against | 0 | 2 | weak against |
| Depression | 4 | 3 | weak for | 4 | 2 | weak for | 1 | 1 | conflicting |
| Anxiety, fear avoidance beliefs (FAB) | 0 | 2 | weak against | 1 | 0 | weak for | 0 | 0 | no evidence |
| Cancer | 0 | 0 | no evidence | 0 | 0 | no evidence | 1 | 0 | weak for |
| Kidney disease | 0 | 0 | no evidence | 0 | 0 | no evidence | 0 | 0 | no evidence |
| Age | |||||||||
| Continuous | 1 | 10 | strong against | 0 | 8 | strong against | 1 | 4 | strong against |
| Categorical | 1 | 5 | strong against | 1 | 3 | weak against | 1 | 1 | conflicting |
Abbreviations: n, number of studies; sign., significant; n.s., not significant.
#Evidence strength defined as follows; strong: 3 or more studies difference (no effect vs. significant effect), weak: difference of 1-2 studies, conflicting: equal number of studies with or without an effect.
Fig. 2.
Risk Ratio for Satisfaction in Different Subgroups.
Risk ratios for outcome satisfaction (SAT) and different subgroup comparisons in surgical and non-surgical treatment (S and Non-S). Meta-analyses were performed only if at least three studies with the same outcome and treatment were available. Subgroup abbreviations: comorbidity (CM), diabetes mellitus (DM), smoking (SM).
Older age (>80 years and >75 years) did not influence satisfaction in five studies, whereas one study showed an association of younger age with more satisfaction. Diabetes was associated with lower satisfaction in one study [39], but not in another study [40].
There was a (not significant) trend in obese patients towards less satisfaction after surgery (RR 0.90, 95% CI 0.74 to 1.11), which was comparable for non-surgical treatments in one study [41]. Smoking was associated with less satisfaction in all three studies with an overall RR of 0.86 (95% CI 0.81 to 0.90). Whereas heterogeneity was very high in studies using comorbidity measures ( 93.7%) and BMI ( 82.4%), heterogeneity was 0% for smoking.
One study assessed the influence of previous lumbar surgery and found a higher satisfaction in patients without previous lumbar operation (odds ratio (OR) 3.65, 95% CI 1.13 to 11.79) [40].In a registry study, patients with neurologic disease and cancer were less satisfied with surgery [42]. Depression was associated with lower satisfaction rate in one study [43] but not in another study [38]. Findings from individual studies are summarized in Appendix 7.
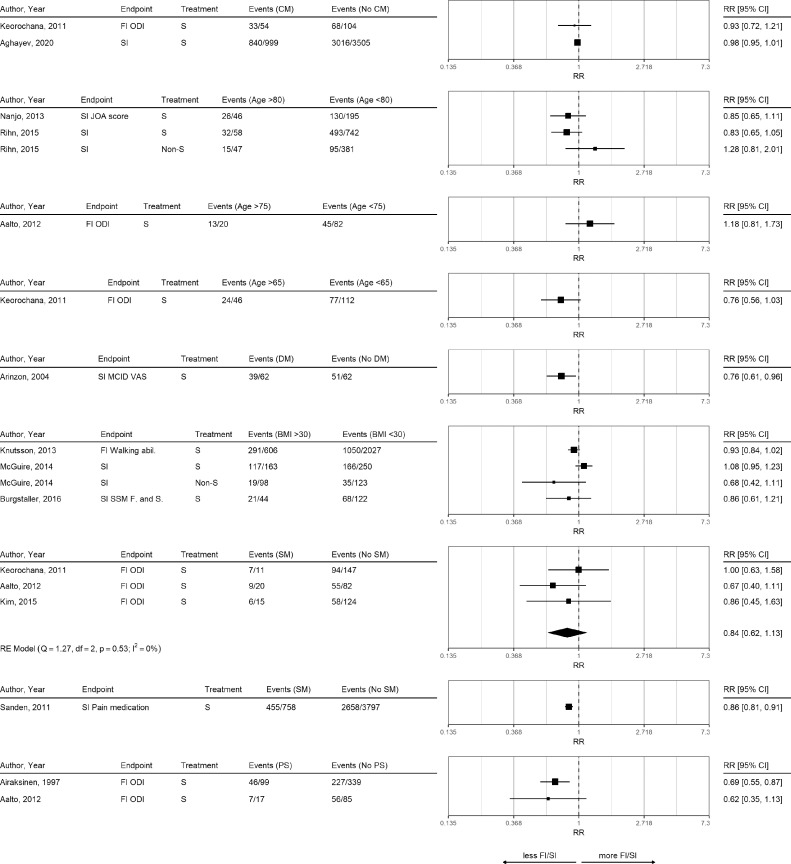
Predictors for functional and symptoms improvement
Only a limited number of studies assessed clinically relevant functional improvement and provided sufficient information to perform subgroup analyses (Figure 3). Patients with comorbidities seemed to have comparable functional improvement (Figure 3, Table 2) compared to patients without comorbidities. Findings for symptoms improvement showed a weak association of comorbidities with less improvement (Table 2). Most studies were performed using data from the Eurospine registry [23,33,34]. Higher ASA scores were associated with lower improvement rates (Core Outcome Measures Index (COMI) sum-score) [23,33] and global outcome [34].
Fig. 3.
Risk Ratio for Functional Improvement and Symptoms Improvement.
Risk ratios for outcomes functional improvement (FI) and symptoms improvement (SI) comparing different subgroups in surgical and non-surgical treatment (S and Non-S). Meta-analyses were performed only if at least three studies with the same outcome and treatment were available. Subgroup abbreviations: comorbidity (CM), diabetes mellitus (DM), smoking (SM), previous surgery (PS).
Older age and obesity were in most studies not associated with worse symptoms and functional improvement. Based on one study [39], diabetes was associated with less clinical meaningful improvement in symptoms (RR 0.76, 95% CI 0.61 to 0.96). Smoking was not associated with functional improvement (RR 0.84, 95% CI 0.62 to 1.13, 0%). One study reported that patients who smoked less needed more additional pain medication [44].
Findings for previous spine surgery were conflicting. Whereas three studies found no influence on the Oswestry Disability Index (ODI) [24,40,45], three other studies found less functional improvement [22,46,47]. Previous lumbar surgery was associated with less functional improvement at one year (Spinal Stenosis Measure (SSM) function) [46], more disability (ODI) [47], and good functional outcome (ODI at 4.3 years) [22].
Less cardiovascular comorbidity was only associated with less symptoms at two years [27]. Other factors with conflicting findings on functional improvement based on a few studies were symptom duration, obesity, and rheumatologic disease (see Table 2).
Whereas patients with depression had less functional improvement in four studies of moderate quality and small sample size [27,[48], [49], [50]], this contrasted with three other studies without evidence for depression to influence function [45,46,51]. Particularly the high quality Lumbar Epidural Steroid Injections for Spinal Stenosis trial (LESS) including 400 patients found no evidence that baseline depression scores would influence improvement in the Roland Morris Questionnaire (RMQ) at six weeks [51]. Baseline depression scores seemed to be associated with less symptoms improvement in most studies [46,48,50,52]. Although baseline fear avoidance beliefs (FAB) were not associated with functional improvement [51,53], persisting FAB was associated with less symptoms improvement [53].
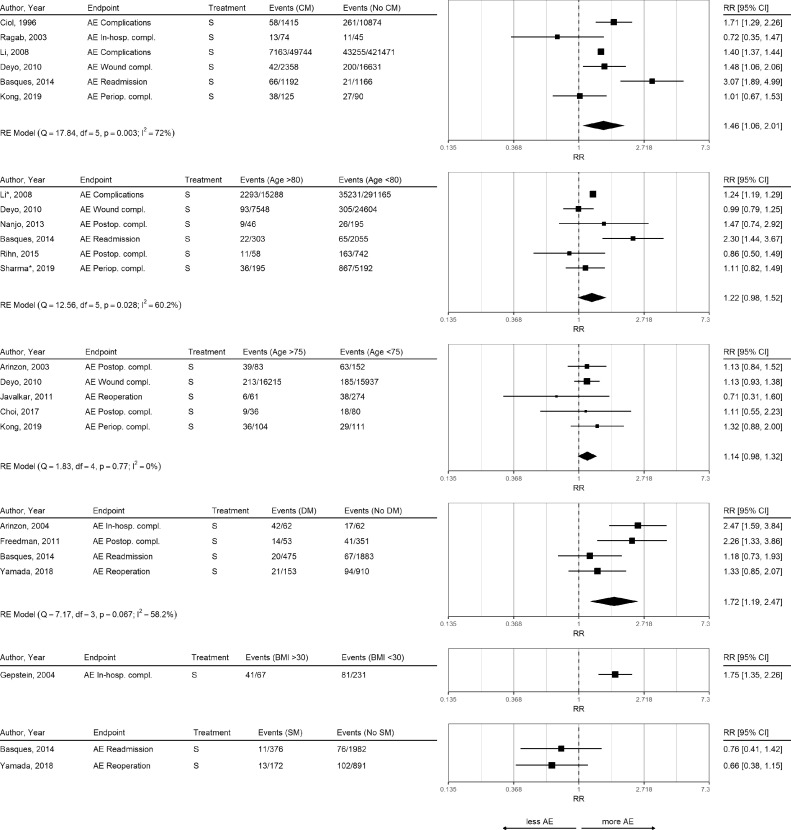
Predictors for adverse events (AE)
Overall, 13 studies reported AE (Figure 4). Comorbidities were associated with an increased risk for postoperative complications (RR 1.46, 95% CI 1.06 to 2.01, 72%). Patients with comorbidities showed higher rates of overall complications, wound complications, and hospital readmissions.
Fig. 4.
Risk Ratio for Adverse Events in Different Subgroups.
Risk ratios for outcome adverse events (AE) and different subgroup comparisons in surgical treatment (S). A* in the author column indicates that this study had a different age split. Li* and Sharma* were split at age 85 and 90, respectively. Meta-analyses were performed only if at least three studies with the same outcome and treatment were available. Subgroup abbreviations: binary comorbidity measure (CM), diabetes mellitus (DM), smoking (SM).
There was a non-significant trend that older age was associated with an increased risk for complications (age >80 years: RR 1.22, 95%CI 0.98 to 1.52). Diabetes was associated with an increased risk for AE (RR 1.72, 95% CI 1.19 to 2.47) mainly due to increased postoperative and in-hospital complication rates, but not with postoperative wound infections (Appendix 7).
Obesity was associated with an increased risk for surgical site infections [54], and in-hospital complications in one study [41] but not in two other studies [55,56]. Smoking did not influence the risk for AE. Congestive heart failure was associated with increased in-hospital complications [57], and 90-day readmission rate [54]. Ischemic heart disease was associated with an increased risk for in-hospital perioperative complications [57] and surgical site infection [54]. Evidence for the influence of previous spine surgery was conflicting.
Discussion
This synthesis of 51 studies revealed an increased risk for adverse events (AE) in patients with comorbidities or higher comorbidity burden compared to patients without comorbidities. Comorbidities did not influence satisfaction, and improvement in function and pain after surgery. Older age alone did not affect satisfaction, symptoms and functional improvement, or the risk of AE. Diabetes was associated with a higher risk for AE and less symptoms improvement with conflicting influence on satisfaction. Other factors that may be associated with less satisfaction were smoking, previous spine surgery, neurological disease, and active cancer disease. There is some indication that patients with depressive symptoms may experience less symptoms improvement.
Discussion in context of the literature
Current disease specific treatment guidelines such as the North American Spine Society (NASS) guideline [58] offer only limited guidance on how comorbidities should be considered in the treatment decision. In addition to one study [39] included in the NASS guideline [58] four additional studies identified in this review confirmed an increased risk for AE in patients with diabetes compared to non-diabetic patients [57,[59], [60], [61]].
In the SPORT trial patients with diabetes had an increased rate of postoperative complications [59]. In patients undergoing surgery, diabetes did not influence functional and symptoms improvement, and satisfaction [59]. In the current systematic review we observed less symptoms improvement in diabetic patients. One reason for this finding may be that lower extremity symptoms due to LSS may sometimes be difficult to distinguish from diabetic peripheral neuropathy. However, the overall prevalence of diabetes in the studies was low and ranged from 4 to 37% and two studies excluded diabetic patients. Therefore, the full extent of long-term diabetes and diabetic peripheral neuropathy on symptoms improvement may be underestimated.
Further, symptoms due to undiagnosed peripheral arterial disease in patients with diabetes may also reduce the efficacy of surgery for LSS. The prevalence of diagnosed peripheral arterial disease in the studies included in the systematic review was very low (2-11%) and three studies excluded patients with the diagnosis.
We observed conflicting findings for previous spine surgery. Whereas in three studies previous spine surgery did not influence the improvement of function [24,40,45], three other studies observed less functional improvement [22,46,47]. One explanation may be that the proportion of postoperative perineural fibrosis and/or arachnoiditis varies among different study populations [62].
Other spine surgeries (e.g. disc herniation [63]) guidelines discuss an increased risk of preoperative depression, older age, and longer symptom duration with poorer outcomes.
A systematic review published in 2006 [28] assessed preoperative predictors and found cardiovascular disease, depression and higher comorbidity burden to be negative predictors for treatment outcomes after LSS surgery [28]. The conclusion was mainly based on one study [27], which was also included in our review. Despite the frequency of the disease, we identified only one additional study that found in patients with coronary artery disease or heart failure a decreased symptoms improvement [46]. Further, three studies reported an increased rate of AE in patients with cardiovascular disease [54,57,61]. Therefore, cardiovascular disease may be an important factor to consider in the treatment decision.
A systematic review assessed the influence of preoperative depression on treatment outcome in LSS and found a negative influence [64]. For the current review, ten additional studies with a sample size of more than 100 patients were available. Although there was some indication that depression may have a negative impact on symptoms improvement [46,48,50,52], it remains a matter of debate whether preoperative depression is causal or a result of the functional limitation. Two studies observed that depressive symptoms improve with global improvement after spine surgery [65,66], which may indicate that preoperative assessment of depression alone may not be sufficient to fully assess the influence of depression on treatment outcome.
Strengths and limitations
Although we used rigorous and standardized methods to identify all relevant studies, there are several limitations that need to be discussed. Despite a considerable number of studies available for the analysis, only data from 37 studies could be used for the meta-analysis. The findings of the meta-analysis are therefore of exploratory nature and additional studies should provide high-quality evidence to support or refute the findings.
Further, reporting of comorbidities and outcomes was very heterogeneous and not comparable between the studies. Although we aimed to analyze the influence of comorbidities on non-surgical and surgical treatments, only limited number of studies for non-surgical treatments were available. Therefore, the influence of comorbidities on non-surgical treatments remains unclear.
Finally, comorbidities may influence treatment outcome depending on the surgical technique used. Due to the limited number of studies that assessed comorbidities and the limited information on the surgical techniques (e.g. open surgery vs. minimal-invasive surgery) that were used, we were unable to address this aspect.
Implications for research
Future studies should report comorbidities of patients in a standardized fashion. In addition, the influence of diabetic peripheral neuropathy and peripheral arterial disease on the treatment outcome in patients undergoing surgery for symptomatic LSS should be assessed. Further, the influence of comorbidities should be assessed for different surgical techniques (e.g. obesity may influence open surgery but not minimally invasive approaches). To assess the impact of comorbidities on treatment outcome, studies need to have sufficient power to assess the treatment effect in subgroups. Further, study outcome assessments should be standardized and comparable. Future studies should assess whether systematic management or improvement of comorbidities preoperatively may influence potential negative factors.
Implications for clinical practice
There was no evidence that age alone influences surgical outcomes for symptomatic LSS. In clinical practice, modifiable prognostic factors that may result in worse treatment outcomes when untreated should be identified and considered. Relevant and potentially modifiable factors identified in this systematic review include diabetes, cardiovascular disease, and smoking. Further, depression and psychological factors may, if they persist, negatively influence treatment outcome [53].
Conclusion
In patients with LSS and comorbidities (particularly diabetes), a higher risk for AE should be considered in the treatment decision. Older age alone does not expose to an increased risk for AE. Elderly patients undergoing surgery for LSS were equally likely to experience functional and symptoms improvement, and to be satisfied.
FDA device/drug status
Not applicable.
Funding
None.
Author's disclosures
AB: Nothing to disclose. AS: Nothing to disclose. MW: Nothing to disclose. UH: Nothing to disclose. LH: Nothing to disclose. ERB: Nothing to disclose. JS: Nothing to disclose. FB: Nothing to disclose.
Affirmation of authorship
All authors had access to the data and a role in writing this manuscript. MW, AB, AS designed the study. AS and AB performed the independent literature screening, data extraction and quality assessment. MW, AB, AS, LH, UH analyzed the data. The first draft of the article was written by MW, AB, AS and revised by ERB, FB, JS, LH, and UH. All authors approved the final version of the article.
Declarations of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Dr. Sabine Klein, Main Library, University of Zurich, for helping us to carry out the literature search.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.xnsj.2021.100072.
Appendix 1. Search strategy
Table A1.
Medline – with Epub Ahead of Print, In-process, Other Non-Indexed Citations.
| Step | Search strategy | References |
|---|---|---|
| 1 | exp Spinal Stenosis/ or ((spinal* or spine or lumbar or root or foraminal) adj3 stenosis).ti,ab. | 8966 |
| 2 | exp Diabetes Mellitus/ or exp Hypertension/ or exp Heart Diseases/ or exp Cardiomyopathies/ or exp Cerebrovascular Disorders/ or exp Asthma/ or exp Pulmonary Disease, Chronic Obstructive/ or exp Hyperlipidemias/ or exp Hypercholesterolemia/ or exp Thyroid Diseases/ or exp Arthritis, Rheumatoid/ or exp Mental Disorders/ or exp Neoplasms/ or exp Kidney Diseases/ or exp Liver Diseases/ or exp Osteoporosis/ or (diabet* or hypertens* or "high blood pressure" or arrythmia*).ti,ab. or ((heart or cardiac or cardiovascular or coronary) adj3 (disease* or disorder* or failure)).ti,ab. Or ((cerebrovascular or vascular or carotoid* or arter*) adj3 (disorder* or disease*)).ti,ab. or ((mental or anxiety or mood or psychological or sleep) adj3 (disease* or disorder*)).ti,ab. or (depression or schizophren* or psychos* or addiction*).ti,ab. or ((kidney* or renal) adj3 (disease* or disorder* or failure)).ti,ab. or (liver adj3 (disease* or disorder*)).ti,ab. or (asthma* or hyperlipidem* or hypercholesterolemia* or hypertriglyceridemia* or "rheumatoid arthritis" or neoplasm* or cancer* or osteoporosis).ti,ab. | 8366814 |
| 3 | (((coocur* or co-ocur* or coexist* or co-exist* or multipl*) adj3 (disease* or ill* or care or condition* or disorder* or health* or medication* or symptom* or syndrom*)) or chronic).ti,ab. | 1142402 |
| 4 | 2 and 3 | 622591 |
| 5 | exp Comorbidity/ or exp Chronic Disease/ or exp "Severity of Illness Index"/ or exp risk factors/ or (comorbid* or co-morbid* or multimorbid* or multi-morbid* or multidisease* or multi-disease*).ti,ab. or (multiple adj3 (ill* or disease* or condition* or syndrom* or disorder*)).ti,ab. or (chronic* adj3 (disease* or ill* or care or condition* or disorder* or health* or medication* or syndrom* or symptom*)).ti,ab. or "severity of illness index".ti,ab. or "risk factor*".ti,ab. | 1868160 |
| 6 | 4 or 5 | 2160985 |
| 7 | 1 and 6 | 1217 |
| 8 | 7 not (animals not humans).sh. | 1195 |
| 9 | 8 not ((exp child/ or exp infant/ or exp adolescent/) not exp adult/) | 1182 |
Table A2.
Embase.
| Step | Search strategy | References |
|---|---|---|
| 1 | 'lumbar spinal stenosis'/exp OR (((spinal* OR spine OR lumbar OR root OR foraminal) NEAR/3 stenosis):ti,ab) | 9969 |
| 2 | 'diabetes mellitus'/exp OR 'hypertension'/exp OR 'heart disease'/exp OR 'myocardial disease'/exp OR 'cerebrovascular disease'/de OR 'asthma'/exp OR 'chronic obstructive lung disease'/exp OR 'hyperlipidemia'/exp OR 'hypercholesterolemia'/exp OR 'thyroid disease'/exp OR 'rheumatoid arthritis'/exp OR 'mental disease'/exp OR 'neoplasm'/exp OR 'kidney disease'/exp OR 'liver disease'/exp OR 'osteoporosis'/exp OR diabet*:ti,ab OR hypertens*:ti,ab OR 'high blood pressure':ti,ab OR arrythmia*:ti,ab OR (((heart OR cardiac OR cardiovascular OR coronary) NEAR/3 (disease* OR disorder* OR failure)):ti,ab) OR (((cerebrovascular OR vascular OR carotoid* OR arter*) NEAR/3 (disorder* OR disease*)):ti,ab) OR (((mental OR anxiety OR mood OR psychological OR sleep) NEAR/3 (disease* OR disorder*)):ti,ab) OR depression:ti,ab OR schizophren*:ti,ab OR psychos*:ti,ab OR addiction*:ti,ab OR (((kidney* OR renal) NEAR/3 (disease* OR disorder* OR failure)):ti,ab) OR ((liver NEAR/3 (disease* OR disorder*)):ti,ab) OR asthma*:ti,ab OR hyperlipidem*:ti,ab OR hypercholesterolemia*:ti,ab OR hypertriglyceridemia*:ti,ab OR 'rheumatoid arthritis':ti,ab OR neoplasm*:ti,ab OR cancer*:ti,ab OR osteoporosis:ti,ab | 11835749 |
| 3 | (((coocur* OR 'co ocur*' OR coexist* OR 'co exist*' OR multipl*) NEAR/3 (disease* OR ill* OR care OR condition* OR disorder* OR health* OR medication* OR symptom* OR syndrom*)):ti,ab) OR chronic:ti,ab | 1612598 |
| 4 | #2 AND #3 | 998846 |
| 5 | 'comorbidity'/exp OR 'chronic disease'/exp OR 'severity of illness index'/exp OR 'risk factor'/exp OR comorbid*:ti,ab OR 'co morbid*':ti,ab OR multimorbid*:ti,ab OR 'multi morbid*':ti,ab OR multidisease*:ti,ab OR 'multi disease*':ti,ab OR ((multiple NEAR/3 (ill* OR disease* OR condition* OR syndrom* OR disorder*)):ti,ab) OR ((chronic* NEAR/3 (disease* OR ill* OR care OR condition* OR disorder* OR health* OR medication* OR syndrom* OR symptom*)):ti,ab) OR 'severity of illness index':ti,ab OR 'risk factor*':ti,ab | 2093321 |
| 6 | #4 OR #5 | 2621159 |
| 7 | #1 AND #6 | 1278 |
| 8 | #7 NOT ( [animals]/lim NOT [humans]/lim) | 1263 |
| 9 | #8 NOT (( [infant]/lim OR [child]/lim OR [adolescent]/lim) NOT ( [adult]/lim OR [aged]/lim)) | 1255 |
| 10 | #9 NOT [conference abstract]/lim | 863 |
Appendix 2. Description of Comorbidity Measures (CM)
The extracted comorbidity measures were: American Society of Anesthesiologists score (ASA, range 0-5), Cumulative Illness Rating Scale (CIRS, range 0-52 or 0-56), Charlson Comorbidity Index (CCI, range 0-31), Functional Comorbidity Index (FCI, range 0-18), Gagne score (range -2-26), and the Elixhauser index (0-≥3).
Appendix 3. Quality Assessed with the Scottish Intercollegiate Guidelines Network (SIGN) Methodology Checklist
Assessment criteria: High quality (++): yes in ≥50% items and <1 item as “no”, Acceptable quality (+): yes in <50% items and ≤50% items “no”. Retrospective and single cohort studies were assigned to the acceptable (+) quality due to their weaker study design. Low quality (-): no in >50% items or concerns by reviewers about a high risk of bias (Tables A3 and A4).
Table A3.
Quality of RCTs.
| Internal validity | Overall assessment | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Author | Year | 1.1 | 1.2 | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 | 1.8 | 1.9 | 1.10 | 2.1 | 2.2 | 2.3 |
| 1.1- | LESS Trial | 2014- | Y | Y | Y | Y | Y | Y | Y | 3.5% | Y | CS | ++ | Y | Y |
| 1.3 | (Lumbar Epidural injections for Spinal Stenosis trial) | 2018 | |||||||||||||
| 2.1- | SPORT (Spine Patient Outcomes Research Trial) | 2011- | Y | Y | CS | DA | Y | Y | Y | DA | Y | CS | ++ | Y | Y |
| 2.5 | randomized arm | 2016 | |||||||||||||
Abbreviations: ID, identification number; Y, yes; N, no; CS, can't say; DA, does not apply; ++, high quality; +, acceptable; 0, low quality.
1.1 The study addresses an appropriate and clearly focused question.
1.2 The assignment of subjects to treatment groups is randomized.
1.3 An adequate concealment method is used.
1.4 The design keeps subjects and investigators "blind" about treatment allocation.
1.5 The treatment and control groups are similar at the start of the trial.
1.6 The only difference between groups is the treatment under investigation.
1.7 All relevant outcomes are measured in a standard, valid and reliable way.
1.8 What percentage of the individuals or clusters recruited into each treatment arm of the study dropped out before the study was completed?.
1.9 All the subjects are analyzed in the groups to which they were randomly allocated (often referred to as intention to treat analysis).
1.10 Where the study is carried out at more than one site, results are comparable for all sites.
2.1 How well was the study done to minimize bias?.
2.2 Taking into account clinical considerations, your evaluation of the methodology used, and the statistical power of the study, are you certain that the overall effect is due to the study intervention?.
2.3 Are the results of this study directly applicable to the patient group targeted in this guideline?.
Table A4.
Quality of cohort studies.
| Internal validity | Overall assessment | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Focused question | Selection of subjects | Assessment | Confounding | Statistical analysis | Risk of bias | Clinical judgment | Applicability | ||||||||||||
| ID | Author | Year | 1.1 | 1.2 | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 | 1.8 | 1.9 | 1.10 | 1.11 | 1.12 | 1.13 | 1.14 | 2.1 | 2.2 | 2.3 |
| 2.1- | SPORT (Spine Patient Outcomes Research Trial) observational arm | 2011- | Y | Y | DA | DA | DA | DA | Y | DA | CS | Y | Y | N | Y | Y | + | Y | Y |
| 2.5 | 2016 | ||||||||||||||||||
| 3 | Atlas SJ et al. | 2005 | Y | Y | DA | DA | 21% | Y | Y | N | Y | Y | Y | CS | Y | Y | + | Y | Y |
| 4.1- | Katz JN et al. | 1995 | Y | DA | DA | DA | 27% | Y | Y | Y | Y | Y | Y | Y | Y | N | + | Y | Y |
| 4.2 | 1999 | ||||||||||||||||||
| 5 | Herron L et al. | 1991 | Y | DA | DA | DA | 8% | DA | Y | DA | N | CS | CS | N | N | N | + | CS | Y |
| 6 | Ilyas H et al. | 2019 | Y | DA | DA | DA | DA | DA | Y | DA | CS | Y | DA | DA | Y | Y | + | Y | Y |
| 7 | Lubelski D et al. | 2015 | Y | DA | DA | DA | DA | DA | Y | DA | N | Y | Y | DA | Y | Y | + | Y | Y |
| 8 | Javalkar V et al. | 2010 | Y | DA | DA | DA | DA | DA | Y | DA | N | Y | DA | DA | Y | Y | + | CS | Y |
| 9 | Movassaghi K et al. | 2019 | Y | DA | DA | DA | DA | DA | Y | DA | CS | Y | N | DA | Y | Y | + | Y | Y |
| 10 | Ragab A et al. | 2003 | Y | DA | DA | DA | DA | DA | Y | DA | N | Y | CS | DA | CS | N | + | N | Y |
| 11 | Li G et al. | 2008 | Y | DA | DA | DA | DA | DA | Y | DA | N | Y | DA | DA | Y | Y | + | Y | Y |
| 12 | Deyo R et al. | 2010 | Y | DA | DA | DA | DA | DA | Y | DA | CS | Y | DA | DA | Y | Y | + | Y | Y |
| 13 | Drazin D et al. | 2017 | Y | CS | DA | DA | DA | DA | Y | CS | Y | Y | DA | DA | Y | Y | + | Y | Y |
| 14 | Ciol M et al. | 1996 | Y | DA | DA | DA | DA | DA | Y | DA | CS | Y | DA | DA | Y | Y | + | Y | Y |
| 15 | Lad S et al. | 2013 | Y | Y | N | DA | DA | DA | Y | DA | Y | Y | DA | DA | Y | Y | + | Y | Y |
| 16 | Sharma M et al. | 2019 | Y | DA | DA | DA | DA | DA | Y | DA | CS | DA | DA | Y | Y | + | Y | Y | |
| 17 | Basques B et al. | 2014 | Y | DA | DA | DA | DA | DA | Y | DA | CS | Y | DA | DA | Y | N | + | Y | Y |
| 18 | Merrill R et al. | 2018 | Y | Y | N | DA | DA | DA | Y | DA | Y | Y | Y | DA | Y | N | + | Y | Y |
| 20.1-20.6 | LSOS (Lumbar Stenosis Outcome Study) | 2015-2019 | Y | DA | DA | DA | DA | DA | Y | DA | Y | Y | Y | N | Y | Y | + | Y | Y |
| 21.1-21.5 | Aalto T et al., Sinikallio S et al., Tuomainen I et al., Pakarinen M et al. | 2007-2018 | Y | DA | DA | DA | DA | DA | Y | DA | Y | Y | Y | N | Y | Y | + | Y | Y |
| 22 | Airaksinen O et al. | 1997 | Y | DA | DA | DA | 11% | DA | Y | DA | Y | Y | Y | N | Y | N | + | CS | Y |
| 23 | Jakola A et al. | 2010 | Y | DA | DA | DA | 1% | DA | Y | DA | Y | Y | Y | N | Y | Y | + | CS | Y |
| 24 | Guigui P et al. | 2002 | Y | DA | DA | DA | 0,3% | DA | Y | DA | CS | Y | Y | N | Y | N | + | CS | Y |
| 25 | Ferrero E et al. | 2018 | Y | DA | DA | DA | 0% | N | Y | DA | N | Y | Y | CS | Y | N | + | Y | Y |
| 26 | Papavero L et al. | 2009 | Y | Y | N | DA | 0% | N | Y | N | Y | CS | Y | N | Y | N | + | CS | N |
| 27 | Costa F et al. | 2007 | Y | DA | DA | DA | DA | DA | CS | DA | CS | CS | N | DA | Y | Y | + | CS | Y |
| 28.1-28.2 | Rillardon L et al., Lenoir T et al. | 2003-2008 | Y | DA | DA | DA | DA | DA | Y | DA | N | Y | Y | DA | CS | N | + | CS | Y |
| 29 | Aghayev E et al. | 2019 | Y | Y | DA | DA | 0% | DA | Y | DA | CS | Y | Y | N | Y | Y | + | Y | Y |
| 30 | Sobottke R et al. | 2017 | Y | Y | Y | DA | 54% | DA | Y | DA | Y | Y | Y | N | Y | Y | + | Y | Y |
| 31 | Kleinstück F et al. | 2009 | Y | DA | DA | DA | 0% | DA | Y | DA | CS | Y | Y | N | Y | Y | + | Y | Y |
| 32 | Iderberg H et al. | 2018 | Y | DA | DA | DA | 40% | DA | Y | DA | CS | Y | Y | CS | Y | Y | + | Y | Y |
| 33 | Knutsson B et al. | 2013 | Y | Y | N | DA | 43% | Y | Y | N | Y | Y | CS | N | Y | Y | + | Y | Y |
| 34 | Sanden B et al. | 2011 | Y | Y | N | DA | 29% | Y | Y | N | Y | Y | CS | N | Y | Y | + | Y | Y |
| 35 | Strömqvist F et al. | 2011 | Y | DA | DA | DA | 22% | DA | CS | DA | N | Y | DA | N | Y | Y | + | Y | Y |
| 36 | Paulsen R et al. | 2018 | Y | DA | DA | DA | 22% | Y | Y | DA | N | Y | Y | N | Y | Y | + | Y | Y |
| 37 | Bouras T et al. | 2010 | Y | DA | DA | DA | 31% | DA | Y | DA | Y | Y | Y | N | Y | N | + | Y | Y |
| 38 | Keorochana G et al. | 2011 | Y | DA | DA | DA | 34% | DA | Y | Y | Y | Y | Y | Y | Y | Y | + | Y | Y |
| 39 | Kim HJ et al. | 2015 | Y | DA | DA | DA | 11% | DA | Y | DA | Y | Y | Y | N | Y | Y | + | Y | Y |
| 40 | Miyamoto H et al. | 2008 | Y | DA | DA | DA | 30% | DA | Y | DA | CS | Y | Y | N | Y | Y | + | Y | Y |
| 41 | Hara N et al. | 2010 | Y | DA | DA | DA | 18% | DA | Y | DA | CS | Y | Y | N | N | Y | + | Y | Y |
| 42 | Kim HJ et al. | 2008 | Y | DA | DA | DA | DA | DA | Y | DA | Y | Y | DA | DA | Y | Y | + | Y | Y |
| 43 | Yaldiz C et al. | 2015 | Y | DA | DA | DA | DA | DA | Y | DA | N | Y | DA | DA | CS | Y | + | Y | Y |
| 44.1-44.6 | Gepstein R et al., Shabat S et al., Arinzon Z et al. | 2003-2011 | Y | Y | DA | DA | DA | DA | Y | DA | CS | Y | DA | DA | Y | N | + | Y | Y |
| 45 | Nanjo Y et al. | 2013 | Y | Y | DA | DA | DA | DA | Y | DA | N | CS | Y | DA | Y | N | + | Y | Y |
| 46 | Kong C et al. | 2019 | Y | DA | DA | DA | DA | DA | CS | DA | Y | Y | DA | DA | Y | Y | + | Y | Y |
| 47 | Minamide A et al. | 2017 | Y | DA | DA | DA | DA | DA | Y | DA | Y | CS | Y | DA | CS | N | + | Y | Y |
| 48 | Choi J et al. | 2017 | Y | Y | DA | DA | DA | DA | Y | N | CS | Y | DA | DA | N | N | + | CS | Y |
| 49 | Lee CK et al. | 2018 | Y | Y | DA | DA | DA | DA | Y | N | CS | Y | DA | DA | Y | Y | + | Y | Y |
| 50 | Kim C et al. | 2013 | Y | DA | DA | DA | DA | DA | Y | DA | CS | Y | Y | DA | Y | Y | + | Y | Y |
| 51 | Yamada K et al. | 2018 | Y | DA | DA | DA | 48% | DA | Y | DA | Y | Y | Y | DA | Y | Y | + | Y | Y |
Abbreviations: ID, identification number; Y, yes; N, no; CS, can't say; DA, does not apply; ++, high quality; +, acceptable; 0, low quality.
1.1 The study addresses an appropriate and clearly focused question.
1.2 The two groups being studied are selected from source populations that are comparable in all respects other than the factor under investigation.
1.3 The study indicates how many of the people asked to take part did so, in each of the groups being studied.
1.4 The likelihood that some eligible subjects might have the outcome at the time of enrolment is assessed and taken into account in the analysis.
1.5 What percentage of individuals or clusters recruited into each arm of the study dropped out before the study was completed? Only in prospective studies.
1.6 Comparison is made between full participants and those lost to follow-up, by exposure status.
1.7 The outcomes are clearly defined.
1.8 The assessment of outcome is made blind to exposure status.
1.9 Where blinding was not possible, there is some recognition that knowledge of exposure status could have influenced the assessment of outcome.
1.10 The method of assessment of exposure is reliable.
1.11 Evidence from other sources is used to demonstrate that the method of outcome assessment is valid and reliable.
1.12 Exposure level or prognostic factor is assessed more than once.
1.13 The main potential confounders are identified and taken into account in the design and analysis.
1.14 Confidence intervals are provided.
2.1 How well was the study done to minimize the risk of bias or confounding?.
2.2 Taking into account clinical considerations, your evaluation of the methodology used, and the statistical power of the study, do you think there is clear evidence of an association between exposure and outcome?.
2.3 Are the results of this study directly applicable to the patient group targeted in this guideline?.
Appendix 4. Funnel Plot for Functional Improvement
Fig. A1.
Appendix 5. Prevalence of Comorbidities
| Comorbidities | Randomized controlled trials | Observational studies | Retrospective, insurance database studies | Registries | Mixed methods |
|---|---|---|---|---|---|
| Prevalence (%) | |||||
| Previous spine surgery | - | 9.0 - 25.0 [24,40,46,75,87] | 4.9 - 6.8 [8,36,102] | 17.0 - 19.4 [23,47,92,93] | 6.6 - 22.6 [22,45] |
| Symptom duration, years: mean (SD)/median [IQR] | - | 15.8 (13.9) [43] | 1.4, [103] 1.6 (1.7) [77] |
- | 3.3 [106] |
| - | 1 (0.8) [49] | 11.2 [55] | - | 7 [22] | |
| - | 1.3 [75] | - | - | - | |
| - | 2.1 [0-42], 1.9 [0-13]a[24] |
- | - | - | |
| Specified symptom duration, n (%) | 105 (26.3)b[51] | 182 (60.7),c[46] 194 (87.4)d[82] |
- | 3983 (52.1)e, 3009 (39.4)f[47] | - |
| Obesity (BMI>30) | 41.2 - 43.6 [41,68] | 15.8 -54.9 [24,40,46,84,89] | 7.0 - 49.8 [54,55,57,80,101] | 23.0 [92] | - |
| Hypertension | 43.6 - 45.9 [41,59,[69], [70], [71]] | - | 7.8 - 71.6 [56,61,78,80,98,103,105] | - | - |
| Diabetes | 7.8 - 22.5 [41,51,59,[67], [68], [69], [70], [71]] | 10.8 - 14.5 [40,53,82] | 4.2 - 37.1 [[54], [55], [56],60,61,77,78,80,98,101,103,105] | - | 3.0 - 14.4 [22,45,106] |
| Smoking | 7.5 - 14.3 [41,51,59,67,[69], [70], [71]] | 7.0 - 24.7 [24,40,43,46,48,49,82,94] | 8.1 - 61.6 [54,77,80] | 11.7 - 24.3 [42,44,47,92,93] | 16.2 [106] |
| Cardiovascular disease | |||||
| All, not specified | - | 59.9 [27] | 43.1 [61] | - | 6.6 [22] |
| Arrhythmia | - | - | 3.3 – 4.2 [56,103] | - | - |
| Coronary artery disease (CAD) | - | 5.7 - 7.3 [53,83] | 7.1 – 23.6 [54,56,103,105] | - | - |
| Heart failure | - | 5.0 - 6.0 [53,83] | 2.1 - 10.3 [54,60,103] | - | - |
| CAD/heart failure | - | 6.3 [46] | - | - | - |
| CAD/heart disease | - | - | 17.8 [78] | - | - |
| Heart disease | 19.6 - 26.3 [41,59,[69], [70], [71]] | - | 4.8 [80] | 4.4 [42] | - |
| Comorbidities | Randomized controlled trials | Observational studies | Retrospective, insurance database studies | Registries | Mixed methods |
|---|---|---|---|---|---|
| Previous myocardial infarction | - | - | 3.4 - 8.1 [60,78] | - | - |
| Aortic aneurysm | - | - | 1.2 [103] | - | - |
| Stroke/cardiovascular disease (CVD) | 2.1 [59] | - | 7.5 - 48.2 [56,60,61,103,105] | - | - |
| Peripheral vascular disease (PVD) | 6.0 - 6.1 [41,59] | - | 5.9 - 11.3 [39,60,78,101] | - | 2.1 [22] |
| Lung disease | 7.6 - 7.7 [41,59] | 5.6 - 15.7 [46,53,83] | 1.7 - 26.1 [8,54,56,60,78,80,103,105] | - | 3.9 [22] |
| Neurologic disease | 2.1 [41,59] | - | - | 2.4 [42] | 8.0 [22] |
| Parkinson's disease (PD)/peripheral neuropathy | - | 2.3 - 11.1 [46,53,83] | - | - | - |
| Dementia/hemi-/paraplegia | - | - | 2.9 [60] | - | - |
| PD/Alzheimer disease /hearing loss | - | - | 16.3 [54] | - | - |
| Rheumatologic disease | |||||
| Musculoskeletal disorder, not specified | 51.0 - 55.9 [41,59,[69], [70], [71]] | 44.1 [27] | 13.3 [60] | - | 1.8 [22] |
| Osteoporosis | 6.2 - 10.4 [59,[69], [70], [71]] | - | 35.6 [60] | - | - |
| Osteoarthritis (OA) | - | - | 6.8 - 20.2 [39,78,101] | - | |
| Knee OA | - | 4.9 - 15.8 [40,53,83] | - | - | 4.8 [22] |
| Hip OA | - | 2.0 - 13.7 [40,53,83] | - | - | 2.7 [22] |
| Hip/knee OA | - | 1.0 - 35.0 [40,46] | - | - | - |
| Rheumatoid arthritis | - | 2.0 [40] | 0.8 [78] | - | - |
| Pseudogout | - | - | 0.8 [78] | - | - |
| Diffuse idiopathic skeletal hyperostosis (DISH) | - | - | 0.8 [78] | - | - |
| Psychiatric disease | . | ||||
| Depression | 11.0 - 53.4 [41,59,[68], [69], [70], [71]] | 17.9 - 27.3 [46,53,82] | 5.7 – 7.3 [39,101] | - | 2.7 [45] |
| Anxiety | 4.8 - 38.1 [59,68] | 17.1-17.6 [53,82] | - | - | - |
| Drug addiction | 0.3 [59] | - | - | - | - |
| Cancer | 7.7 [59] | - | 4.6 - 9.3 [60,78,103,105] | 1.3 [42] | - |
| Kidney disease | 4.6 [41,59] | - | 0.8 - 9.4 [56,60,61,103,105] | - | - |
| Urologic disease | |||||
| Prostate hypertrophy | - | - | 2.5 – 17.7 [54,103] | - | - |
| Gastrointestinal disease | |||||
| Gastric disease | 20.6 - 22.2 [41,59,[69], [70], [71]] | - | 42.4 [60] | - | - |
| Intestinal disease | 10.4 - 13.7 [41,59,[69], [70], [71]] | - | 1.7 [78] | - | - |
| Liver disease | 1.6 [41,59] | - | 31.2 [60] | - | - |
| Endocrinologic disease | |||||
| Hypothyroidism | - | - | 0.8 [78] | - | - |
| Acquired immune deficiency syndrome (AIDS) | - | - | 0.1 [60] | - | - |
| Ophthalmologic disease | |||||
| Glaucoma | - | - | 0.8 [78] | ||
| Other diseases | 29.7 - 78.5g [41,[69], [70], [71]] | - | - | - | 8.0h,22 |
Abbreviations: SD, standard deviation; IQR, interquartile range; n, number of patients
Value for back pain and leg pain respectively;
symptom duration >5 years;
symptom duration >6 months;
symptom duration >3 months;
duration of back pain >2 years;
duration of leg pain >2 years;
other diseases related to stroke, cancer, fibromyalgia, chronic fatigue syndrome, posttraumatic stress disorder, alcohol consumption, drug addiction, lung, liver, kidney, blood vessel, nervous system, migraine or anxiety;
other diseases than hip or knee joint arthrosis, peripheral vascular disease, diabetes, cardiovascular, pulmonary, neurologic or rheumatic disease
Appendix 6. Prevalence of Comorbidity Measures (CM)
| Comorbidity measures (CM) | Observational studies | Retrospective, insurance database studies | Registries | Mixed methods |
|---|---|---|---|---|
| Mean (SD) or prevalence n (%) | ||||
| American Society of Anesthesiologists (ASA 0-5) | 2.23 [89] | 2.27 (range 1-3.5) [105] | - | - |
| Cumulative Illness Rating Scale (CIRS, 0-52 or 0-56) | 9.3 (3.8), [82] 3.0 (range 0-10.9), [87] 4.5 (3.4) [88] |
4.8 (4.9), [91] 4.8 (4) [36] |
- | - |
| Charlson Comorbidity Index (CCI, 0-31) | 1.2 (1.2) [88] | - | - | - |
| Functional Comorbidity Index (FCI, 0-18) | 3.2 (1.3) [88] | - | - | - |
| Gagne score (-2-26) | - | 0.48 (1.41) [55] | - | |
| Comorbidities/patient | 2 (3) [88] | 2.85 (1.84), [57,99] 2.78 (1.58), [101] 2.55 (1.11), [39] 1.75 (range 0-4) [105] |
- | - |
| CIRS (0-56), median [range] | 8 [4.8] [84] | - | - | - |
| Comorbidities/patient, median [range] | 5.5 [0.7] [48] | - | - | - |
| Patients with ASA score ≥3 | 29 (28.7), [24] 25 (8) [87] |
74 (62.7), [78] 1192 (50.6), [80] 33 (11), [57,99,101] 23 (18.5), [39] 19 (6.7), [102] 58 (27.6), [77] 125 (58.1) [56] |
1135 (23.8), [23] 106 (48), [33] 999 (22.2) [34] |
- |
| Patients with CCI or modified CCI (mCCI or Quan Comorbidity score, 0-31 or 0-3+) score ≥3 | - | 2358 (7.3), [8] 1756 (6.1), [17] 343 (1.2) [79] |
- | - |
| Patients with CCI ≥2 | - | - | - | 72 (39.6) [45] |
| Patients with Elixhauser index ≥3 | 751 (13.94) [37] | |||
| Patients with any comorbid illnesses | 59 (60.8), [74] 54 (34.2) [94] |
8338 (75.6) [60] | 2544 (33.3) [47] | - |
| Patients with ≥3 comorbidities | 74 (38.1) [38] | 49744 (10.6), [35] 138 (48.8), [102] 78 (36.3) [56] |
- | - |
| Patients with ≥5 comorbidities | 55 (53.9) [40] | - | - | - |
Abbreviations: SD, standard deviation; n (%), number of patients (%)
Appendix 7. Predictors of outcomes
Table A5.
Predictors for satisfaction.
| Comorbidity | Significant predictors | Ref. | Not significant predictors | Ref. |
|---|---|---|---|---|
| Comorbidities, Comorbidity measures (CM) | Worse Cumulative Illness Rating Scale (CIRS, 0-56) associated with dissatisfaction (very/somewhat, 4-point scale) at 6 months: adjusted (adj.) beta 0.08 (p=0.03) | 4.2 [38] | Univariate, <5 comorbidities: no association with satisfaction (totally cured/condition has considerably improved, 7-point scale) at 2 years | 21.1 [40] |
| Less overall comorbidity associated with satisfaction at 2 years: univariate significant (sign.) Spearman correlation (r=0.23) | 4.1 [27] | Univariate, comorbid illnesses not associated with satisfaction with current state (delighted/pleased/mostly satisfied, 7-point scale) at 10 years | 3 [74] | |
| Logistic regression (log.), higher American Society of Anesthesiologists (ASA, range 1-6) class and number of comorbidities: no association with less satisfaction (very/somewhat dissatisfied, 4-point scale) at a mean of 3.5 years | 44.1 [99] | |||
| Age | <75 years associated with satisfaction (totally cured/condition has considerably improved, 7-point scale) at 2 years: adj. odds ratio (OR) 4.03 (95% confidence interval (CI) 1.35 to 12.02) | 21.1 [40] | Univariate: no association with satisfaction with current state (delighted/pleased/mostly satisfied, 7-point scale) at 10 years | 3 [74] |
| Younger age associated with dissatisfaction (surgery total failure/condition slightly improved, 7-point scale) at 3 months: adj. OR 0.93 (95% CI 0.88 to 0.99) | 21.2 [43] | Univariate: no association with satisfaction at 2 years | 4.1 [27] | |
| Multivariate: no association with dissatisfaction (very/somewhat, 4-point scale) at 6 months | 4.2 [38] | |||
| Multivariate: no association with dissatisfaction (3-point scale) at 1 year | 36 [42] | |||
| Log. regression, older age: no association with less satisfaction (very/somewhat dissatisfied, 4-point scale) at a mean of 3.5 years | 44.1 [99] | |||
| <80 versus (vs.) ≥80 years: older age not associated with less satisfaction after surgery | 20.6 [20] | |||
| Previous spine surgery | No previous lumbar operation associated with satisfaction (totally cured/condition has considerably improved, 7-point scale) at 2 years: adj. OR 3.65 (95% CI 1.13 to 11.79) | 21.1 [40] | - | - |
| Symptom duration | Duration of leg pain >2 years associated with dissatisfaction (3-point scale) at 1 year, adj. OR 2.46 (95% CI 1.01 to 5.97) | 36 [42] | Univariate, length of current episode >6 months: no association with satisfaction with current state (delighted/pleased/mostly satisfied, 7-point scale) at 10 years | 3 [74] |
| Multivariate, duration of back pain (<3 or 3-12 months or 1-2 or >2 years): no association with dissatisfaction (3-point scale) at 1 year | Bivariate, pain <1 year (reference) vs. >1 year: no association with satisfaction | 9 [77] | ||
| Body weight | Compared to normal weight, obesity (body mass index (BMI) ≥30) associated with dissatisfaction (unsatisfied/uncertain with surgery, 3-point scale) at 2 years: adj. OR 1.73 (95% CI 1.36 to 2.19) | 33 [92] | Log. regression: no association with satisfaction (very/somewhat, 4-point scale) at a mean of 3.5 years | 44.1 [99] |
| Multivariate: no association with dissatisfaction (3-point scale) at 1 year | 36 [42] | |||
| Univariate, BMI <30: no association with satisfaction (totally cured/condition has considerably improved, 7-point scale) at 2 years | 21.1 [40] | |||
| BMI <24.9 vs. 25-29.9 vs. ≥30: obesity not associated with dissatisfaction (very/somewhat, 4-point scale) at a mean of 3.7 years | 44.2 [57] | |||
| BMI <30 vs. ≥30 to <35 vs. ≥35, surgical/non-surgical group: obesity not associated with dissatisfaction at 4 years | 2.4 [41] | |||
| Diabetes | Significant difference in satisfaction (very/somewhat satisfied, 4-point scale) between patients with/without diabetes at a mean of 3.4 years: diabetes: 53%, no-diabetes: 78% (p=0.0067) | 44.5 [39] | Univariate, type 2: no association with satisfaction (totally cured/condition has considerably improved, 7-point scale) at 2 years | 21.1 [40] |
| Smoking | Smoking associated with dissatisfaction (3-point scale) at 1 year: adj. OR 1.61 (95% CI 1.19 to 2.17) | 36 [42] | Univariate, never smoking: no association with satisfaction (totally cured/condition has considerably improved, 7-point scale) at 2 years | 21.1 [40] |
| Smoking associated with dissatisfaction at 2 years: adj. OR 1.79 (95% CI 1.51 to 2.12) | 34 [44] | Multivariate, cigarette use (current or quit in <6 months): no association with satisfaction with current state (delighted/pleased/mostly satisfied, 7-point scale) at 10 years | 3 [74] | |
| Cardiovascular disease | Less cardiovascular comorbidity associated with satisfaction at 2 years: adj. beta 3.7 (p=0.0002) | 4.1 [27] | Multivariate, cardiac disease: no association with dissatisfaction (3-point scale) at 1 year | 36 [42] |
| Neurologic disease | Neurological disease associated with dissatisfaction (3-point scale) at 1 year: adj. OR 2.05 (95% CI 1.00 to 4.20) | 36 [42] | - | - |
| Rheumatologic disease | - | - | Univariate, no comorbidity affecting walking: no association with satisfaction (totally cured/condition has considerably improved, 7-point scale) at 2 years | 21.1 [40] |
| Univariate, less musculoskeletal comorbidity: no association with satisfaction at 2 years | 4.1 [27] | |||
| Psychiatric disease | Depression associated with dissatisfaction (surgery total failure/condition slightly improved, 7-point scale) at 3 months: adj. OR 1.18 (95% CI 1.03 to 1.34) | 21.2 [43] | Multivariate, depression: no association with dissatisfaction (very/somewhat, 4-point scale) at 6 months | 4.2 [38] |
| Better mental health (3-item depression scale) associated univariate significant (Spearman correlation r=0.25), multivariate not significant with satisfaction at 2 years | 4.1 [27] | |||
| Cancer | Cancer disease associated with dissatisfaction (3-point scale) at 1 year: adj. OR 3.75 (95% CI 1.58 to 8.89) | 36 [42] | - | - |
Table A6.
Predictors for functional improvement.
| Comorbidity | Significant predictors | Ref. | Not significant predictors | Ref. |
|---|---|---|---|---|
| Comorbidities, Comorbidity measures (CM) | Higher comorbidity score (Elixhauser§) associated with more disability (Oswetry disability index (ODI), range 0-100) at 1 year: adjusted (adj.) beta 2.04 (95% confidence interval (CI) 1.55 to 2.61) | 32 [47] | Logistic (log.) regression, no previously diagnosed comorbidity: no association with good outcome (ODI <40) at mean 4.3 years | 22 [22] |
| Less overall comorbidity associated with greater walking capacity at 2 years: univariate significant (sign.) Spearman correlation (r=0.33) with "able to walk 1 mile" | 4.1 [27] | Univariate: no association between <5 comorbidities and good improvement in disability (>30% ODI improvement) at 2 years | 21.1 [40] | |
| Higher Cumulative Illness Rating Scale (CIRS, 0-52 or 0-56) associated with poor functional outcome (less Self-administered Beaujon Questionnaire score (SABQ, range 0-100)) at 1 year: adj. beta -0.94 (p=0.001) | 25 [88] | Univariate, associated diseases: no association with failed clinical improvement (ODI <15% improvement) at a mean of 2.64 years | 38 [94] | |
| Log. regression, higher American Society of Anesthesiology (ASA, range 1-6) score (dichotomous: ASA 1 and 2 versus (vs.) ASA 3 and 4): no association with less improvement in disability (ODI) at 1 year | 23 [24] | |||
| Univariate, Charlson Comorbidity Index (CCI, 0-3+) >1: no association with less disability (ODI) improvement at mean 5.1 years | 37 [45] | |||
| Age | Older age associated with more disability (ODI) at 1 year: adj. beta 0.15 (95% CI 0.11 to 0.19) | 32 [47] | No association with poor functional outcome (less SABQ) at 1 year | 25 [88] |
| >65 years associated with failed improvement (ODI <15% improvement) at a mean of 2.64 years: adj. OR 2.16 (95% CI 1.02 to 4.57) | 38 [94] | Multivariate: no association with an unfavorable surgical outcome at 1 year (ODI >22) | 39 [49] | |
| Univariate: no association with disability (ODI) at mean 5.1 years | 37 [45] | |||
| Log. regression: no association with recovery rate <30% (based on Japanese Orthopedic Association (JOA) scoring system (0-29 or -6-23)) at 2 years | 47 [104] | |||
| Log. regression: no association with hindrance to activities of daily living (based on JOA score) at mean 95 months | 40 [95] | |||
| No association with more disability (ODI) at 10 years | 21.3 [48] | |||
| No association with less improvement in disability (Roland Morris Questionnaire (RMQ, range 0-24)) at 6 weeks | 1.1 [51] | |||
| Univariate: no association with walking capacity (“able to walk 1 mile”) at 2 years | 4.1 [27] | |||
| No association with good outcome (ODI <40) at a mean of 4.3 years | 22 [22] | |||
| No association with less improvement in disability (ODI) at 1 year | 23 [24] | |||
| Univariate: no association aged <75 years with >30% ODI improvement at 2 years | 21.1 [40] | |||
| Age ≥75 years: no association with functional improvement (SSM function, Spinal Stenosis Measure for disability (minimal clinically important difference (MCID) = ≥0.52 points, range 1-4) at 1 year | 20.2 [46] | |||
| <65 vs. 65-75 vs. >75 years: no association with less improvement of Neurogenic Claudication Outcome Score (NCOS, range 0-72) at 1 year | 26 [89] | |||
| <80 vs. ≥80 years: no association with a poor/fair recovery ratio (JOA score) at mean 14 months | 45 [103] | |||
| <80 vs. ≥80 years, surgical and non-surgical treatment: age not associated with ODI improvement at 4 years | 2.5 [71] | |||
| Previous spine surgery | Any previous lumbar surgery associated with less functional improvement at 1 year: regression analysis log OR -0.99 (95%CI -1.95 to -0.02), SSM function | 20.2 [46] | Log. regression, any previous back surgery: no association with less improvement in disability (ODI) at 1 year | 23 [24] |
| Any previous surgery associated with more disability (ODI) at 1 year: adj. beta 6.41 (95% CI 5.32 to 7.61) | 32 [47] | Univariate, any previous lumbar surgery: no association with less improvement in disability (ODI) at a mean of 5.1 years | 37 [45] | |
| No previous surgery associated with good outcome (ODI <40) at mean 4.3 years: log. regression OR 2.4 (95% CI not reported) | 22 [22] | Multivariate, no previous lumbar operation: no association with good improvement in ODI (>30% improvement) at 2 years | 21.1 [40] | |
| Symptom duration | Back pain for 1-2 years associated with more disability (ODI) at 1 year: adj. beta 3.50 (95% CI 1.56 to 5.27) | 32 [47] | Symptom duration ≥6 months: no association with less functional improvement at 1 year (SSM function) | 20.2 [46] |
| Longer duration of leg pain associated with less improvement in disability (ODI) at 1 year: log. regression beta 0.46 (p=<0.001) | 23 [24] | Univariate, symptom duration: no association with unfavorable surgical outcome at 1 year (ODI >22) | 39 [49] | |
| Compared to buttock/leg/hip pain duration <3 months, longer duration associated with less improvement in disability (RMDQ) at 6 weeks: 3-12 months adj. mean difference -0.7 (95% CI -2.3 to 1.0), 1-5 years adj. mean difference 1.6 (95% CI -0.2 to 3.3), >5 years adj. mean difference 0.6 (95% CI -1.2 to 2.3) (p=0.03) | 1.1 [51] | Multivariate, comparison pain <1 year (reference) vs. >1year: no association with less functional improvement (ODI) at 2 years | 9 [77] | |
| Multivariate, preoperative symptom duration: no association with bad functional score# at mean 10 years | 28.1 [91] | |||
| Body weight | BMI <30 vs. ≥30 to <35 vs. ≥35, non-surgical group: obesity associated with less improvement in disability (ODI) at 4 years: <30: mean -12.7 (standard deviation (SD) 1.7), ≥30 to <35: mean -2.3 (SD 2.6), ≥35: mean -6.4 (SD 3.4) (p=0.003) BMI <30 vs. ≥30 to <35 vs. ≥35, surgical group: obesity no association with less improvement in disability (ODI) at 4 years |
2.4 [41] | Multivariate, higher BMI: no association with poor functional outcome (less SABQ) at 1 year | 25 [88] |
| Higher BMI associated with less functional improvement at 1 year: regression analysis log. OR -0.96 (95% CI -1.63 to -0.28), SSM function. | 20.2 [46] | Multivariate: no association with an unfavorable surgical outcome (ODI >22) at 1 year | 39 [49] | |
| BMI <25 vs. 25 to <30 and ≥30, obesity: no association with less functional improvement (SSM function) at 1 year | 20.4 [84] | No association with less improvement in disability (RMQ) at 6 weeks | 1.1 [51] | |
| Log. regression: no association with less improvement in disability (ODI) at 1 year | 23 [24] | |||
| Univariate, BMI <30: no association with good improvement in ODI (>30% improvement) at 2 years | 21.1 [40] | |||
| Diabetes | Patients with diabetes: more disability compared to patients without diabetes in non-surgical group at 4 years: diabetes mean -2.6 (SD 3.5), no-diabetes mean -10.2 (SD 1.4) (p=0.044). Diabetes vs. no-diabetes, surgical group: diabetes no association with less improvement in disability (ODI) at 4 years | 2.3 [59] | No association between diabetes on insulin and less improvement in disability (RMQ) at 6 weeks | 1.1 [51] |
| Univariate: no association between diabetes and improvement in disability (ODI) at a mean of 5.1 years | 37 [45] | |||
| Univariate: no association between diabetes and good improvement in disability (>30% ODI improvement) at 2 years | 21.1 [40] | |||
| Smoking | Non-smoking associated with good improvement in disability (ODI >30%) at 2 years: adj. OR 3.47 (95% CI 1.09 to 11.03) | 21.1 [40] | Univariate, history of smoking: no association with failed improvement in disability (ODI improvement <15%) at mean 2.64 years | 38 [94] |
| Smoking associated with more disability (ODI) at 1 year: adj. beta 4.72 (95% CI 3.73 to 5.61) | 32 [47] | Univariate: no association with unfavorable surgical outcome (ODI >22) at 1 year | 39 [49] | |
| Log. regression: no association with less improvement in disability (ODI) at 1 year | 23 [24] | |||
| No association with less functional improvement (SSM function) at 1 year | 20.2 [46] | |||
| No association with less improvement in disability (RMQ) at 6 weeks | 1.1 [51] | |||
| Cardiovascular disease | Less cardiovascular comorbidity associated with better walking capacity at 2 years: adj. beta 2.7 (p=0.008) with "able to walk 1 mile" | 4.1 [27] | Coronary heart disease or heart insufficiency: no association with less functional improvement (SSM function) at 1 year | 20.2 [46] |
| Lung disease | - | - | Asthma/Chronic Obstructive Pulmonary disease (COPD): no association with less functional improvement (SSM function) at 1 year | 20.2 [46] |
| Neurologic disease | - | - | Parkinson's disease or peripheral neuropathy: no association with less functional improvement (SSM function) at 1 year | 20.2 [46] |
| Rheumatologic disease | Cox-/gonarthrosis associated with less functional improvement at 1 year: regression analysis log OR -0.71 (95%CI -1.36 to -0.06), SSM function | 20.2 [46] | Univariate, less musculoskeletal comorbidity: no association with greater walking capacity "able to walk 1 mile" at 2 years | 4.1 [27] |
| Univariate, no comorbidity affecting walking: no association with good improvement in disability (>30% ODI improvement) at 2 years | 21.1 [40] | |||
| Psychiatric disease | Higher depressive burden (higher Beck depression inventory (BDI), range 0-63) associated with more disability (ODI) at 10 years: adj. beta 0.25 (95%CI 0.18 to 0.33) | 21.3 [48] | Depression: no association with less functional improvement (SSM function) at 1 year | 20.2 [46] |
| Less depression associated with greater walking capacity at 2 years: univariate sign. Spearman correlation (r=0.19) with "able to walk 1 mile" | 4.1 [27] | Log. regression, high Fear Avoidance Beliefs Questionnaire Physical activity (FABQ-P, range 0-24) baseline, high FABQ-P at 6 months and persistent high FABQ-P at baseline and at 6 months: no association with less clinical meaningful improvement at 1 year | 20.5 [53] | |
| Higher depression scores associated with less functional improvement in Patient Reported Outcomes Measurement Information System (PROMIS) physical function: F (1, 109) = 6.11 (p=0.015) and higher depression scores associated with less functional improvement in disability (ODI): F (1, 98) = 28.59 (p=<0.0001) | 18 [50] | Greater depression (Personal Health Questionnaire depression scale (PHQ-8), range 0-24), more anxiety (Generalized Anxiety Disorder scale (GAD-7), range 0-21)), more pain catastrophizing (Pain Catastrophizing Scale (PCS, range 0-52)) and more FABQ-PA not associated with less improvement in disability (RMQ) at 6 weeks | 1.1 [51] | |
| Higher depression score (total Pain Sensitivity Questionnaire (PSQ, range 0-10)) associated with unfavorable outcome (ODI >22) at 1 year: adj. OR 1.29 (95% CI 1.03 to 1.62) | 39 [49] | Univariate, depression: no association with less disability (ODI) improvement at a mean of 5.1 years | 37 [45] |
Bad functional score, based on global/lumbar/radicular pain and signs of radicular ischemia (range 0-100, 0 = very bad function, 100 = very good function)
Elixhauser comorbidity score (0-30), method for measuring patient comorbidity based on diagnosis codes in administrative data (includes mental disorders, drug and alcohol abuse, obesity, coagulopathy)
Table A7.
Predictors for symptoms and global improvement.
| Comorbidity | Significant Predictors | Ref. | Not significant predictors | Ref. |
|---|---|---|---|---|
| Comorbidities, Comorbidity measures (CM) | Less overall comorbidity associated with less symptom severity at 2 years: univariate significant (sign.) Spearman correlation (r=0.27) with “no severe pain” | 4.1 [27] | Univariate, Charlson Comorbidity Index (CCI, range 0-3+) >1: no association with less pain (Visual Analogue Scales (VAS)) improvement at a mean of 5.1 years | 37 [45] |
| Compared to American Society of Anesthesiology (ASA, range 1-6) score 1, other ASA scores associated with lower Core Outcome Measure Index (COMI, range 8-40) sum-score improvement (in ≥1 domain) at mean 1.3 years: ASA 2: adjusted (adj.) odds ratio (OR) 0.86 (95% confidence interval (CI) 0.72 to 1.03), ASA >2: adj. OR 0.69 (95% CI 0.56 to 0.85) | 30 [23] | Logistic (log.) regression, higher ASA score (dichotomous ASA 1 and 2 versus (vs.) ASA 3 and 4): no association with less EuroQol 5 Dimensions Questionnaire (EQ-5D, 0-100); EuroQol Visual Analogue Scales (VAS, 0-100); at 1 year | 23 [24] | |
| Higher ASA score (≥3 vs. 1) associated with negative global outcome in patients with predominant back pain at 1.3 year: OR 1.76 (1.15-2.70) | 29 [34] | |||
| Multivariate, higher ASA score (≥3 vs. 1): no association with negative global outcome in patients with predominant leg pain at 1.3 year | ||||
| Lower comorbidity (ASA score) associated with more improvement in COMI sum-score at 1 year: beta 0.619 (95% CI 0.06 to 1.18) | 31 [33] | |||
| Multivariate, lower comorbidity (ASA score): no association with good outcome (surgery helped a lot/helped, 5-point scale) at 1 year | ||||
| Age | <80 vs. ≥80 years, surgical group: older age associated with less improvement in Short Form 36 Questionnaire subscale bodily pain (SF-36 bodily pain, 0-100) sub-score at 4 years: <80: mean 28 (standard deviation (SD) 0.6), ≥80: 21.3 (2.2), p=0.004 | 2.5 [71] | Multivariate: no association with good outcome (surgery helped a lot/ helped, 5-point scale) and COMI sum-score improvement at 1 year | 31 [33] |
| <80 vs. ≥80 years, non-surgical group: older age not associated with less improvement in SF-36 bodily pain sub-score at 4 years | ||||
| Univariate: no association with less pain (VAS) improvement at a mean of 5.1 years | 37 [45] | |||
| Univariate: no association with improvement of pain (VAS) at a mean of 2.5 years | 27 [90] | |||
| Univariate: no association with less symptom severity (“no severe pain”) at 2 years | 4.1 [27] | |||
| Multivariate, age (per 1 year higher): no association with less EQ-5D improvement (any or ≥0.1 points improvement) | 7 [52] | |||
| No association with poor outcome for back/leg pain (improvement VAS (0-100) <25%) | 5 [75] | |||
| Log. regression: no association with leg pain/numbness and gait disturbance (Japanese Orthopedic Association (JOA, 0-17) score 0-1) at 2 years | 41 [96] | |||
| Log. regression, older age: no association with less EQ-5D total score/EQ-VAS improvement at 1 year | 23 [24] | |||
| In all age groups significant back pain improvement (graphic rating scale (GRS) ≥2 points) at a mean of 1.3 years: 20-64 years: adj. OR 1.38 (95% CI 1.33 to 1.44), 65-74 years: adj. OR 1.44 (95% CI 1.38 to 1.50), ≥75 years: adj. OR 1.50 (95% CI 1.43 to 1.57) | 30 [23] | |||
| <65 vs. 65-75 vs. >75 years: older age not associated with less pain (VAS) reduction at 1 year | 26 [89] | |||
| <80 vs. ≥80 years: older age not associated with less improvement in Spinal Stenosis Measure for pain (SSM symptom severity, minimal clinically important difference (MCID) ≥0.48 points, range 1-5); ≥0.5 point at 1 year | 20.6 [20] | |||
| Previous spine surgery | After a mean of 1.3 years, ≥1 previous spine surgery compared to no previous surgery is associated with: - lower COMI sum-score ≥1 domain improvement: 1 previous surgery adj. OR 0.73 (95% CI 0.61 to 0.87), >1 previous surgery adj. OR 0.55 (95% CI 0.41-0.74) | 30 [23] | No association between any lumbar surgery and less pain (VAS) improvement at a mean of 5.1 years | 37 [45] |
| - less back pain improvement (GRS ≥2 points): 1 previous surgery adj. OR 0.75 (95% CI 0.62 to 0.90), >1 previous surgery adj. OR 0.72 (95% CI 0.53-0.98) | No association between any lumbar surgery and less symptom severity improvement (SSM symptoms) at 1 year | 20.2 [46] | ||
| - less leg pain improvement (GRS ≥2 points): 1 previous surgery adj. OR 0.72 (95% CI 0.60 to 0.86), >1 previous surgery adj. OR 0.58 (95% CI 0.43-0.78) | Log. regression: no association between any back surgery and less EQ-5D total score/EQ-VAS improvement at 1 year | 23 [24] | ||
| Symptom duration | - | - | Symptom duration ≥6 months: no association with less improvement in symptom severity (SSM symptoms) at 1 year | 20.2 [46] |
| No association of symptom duration and poor outcome for back and leg pain (VAS (0-100) improvement ≤25%) at a mean of 3.5 years | 5 [75] | |||
| Log. regression, longer duration of back and leg pain: no association with less EQ-5D total score/EQ-VAS improvement at 1 year | 23 [24] | |||
| Multivariate, duration of pain <1 year (reference) vs. > 1 year not associated with less improvement in VAS back/leg pain at 2 years | 9 [77] | |||
| Body weight | - | - | Log. regression: no association with less EQ-5D total score/EQ-VAS improvement at 1 year | 23 [24] |
| No association between obesity (body mass index (BMI) ≥30) and less symptom severity improvement (SSM symptoms) at 1 year | 20.2 [46] | |||
| No association between obesity (BMI ≥30)/overweight (BMI 25 to <30) compared to BMI <25 in symptom severity improvement (SSM symptoms) at 1 year | 20.4 [84] | |||
| BMI <30 vs. ≥30 to <35 vs. ≥35, surgical and non-surgical group: obesity not associated with less improvement in SF-36 bodily pain sub-score at 4 years | 2.4 [41] | |||
| Diabetes | Diabetes vs. no-diabetes: diabetes associated with less decrease of ≥4 points of VAS: diabetes 63%, no-diabetes 83% x2= 5.57, p=0.018 | 44.5 [39] | Univariate, diabetes: no association with less pain (VAS) improvement at a mean of 5.1 years | 37 [45] |
| Diabetes vs. no-diabetes, surgical and non-surgical group: diabetes not associated with less improvement in SF-36 bodily pain sub-score at 4 years | 2.3 [59] | |||
| Smoking | Smokers vs. non-smokers: smokers used more frequently analgesics at 2 years after surgery: OR 1.86 (95% CI 1.55 to 2.23) | 34 [44] | No association with less improvement in symptom severity (SSM symptoms) at 1 year | 20.2 [46] |
| Log. regression: no association with less EQ-5D total score/EQ-VAS improvement at 1 year | 23 [24] | |||
| Cardiovascular disease | Coronary heart disease or heart insufficiency associated with less symptom severity improvement at 1 year: regression analysis, log OR -1.21 (95% CI -2.35 to -0.09), SSM symptoms | 20.2 [46] | - | |
| Less cardiovascular comorbidity associated with less symptom severity (“no severe pain”) at 2 years: adj. beta 2.6 (p=0.01) with "able to walk 1 mile" | 4.1 [27] | |||
| Lung disease | - | - | Asthma/Chronic Obstructive Pulmonary Disease (COPD): no association with less improvement in symptom severity (SSM symptoms) at 1 year | 20.2 [46] |
| Neurologic disease | - | - | Parkinson's disease/peripheral neuropathy or hip-/knee arthritis: no association with less improvement in symptom severity (SSM symptoms) at 1 year | 20.2 [46] |
| Rheumatologic disease | - | - | Univariate, less musculoskeletal comorbidity: no association with less symptom severity (“no severe pain”) at 2 years | 4.1 [27] |
| Psychiatric disease | Greater depression score (per one-unit increase in Personal Health Questionnaire depression scale (PHQ-9, 0-24); -) associated with lower probability of any improvement in EQ-5D: adj. OR 0.95 (95% CI 0.90 to 0.998) and of MCID EQ-5D ≥0.1-point improvement: adj. OR 0.92 (95% CI 0.87 to 0.98) at mean 6 months | 7 [52] | Univariate, depression: no association with less pain (VAS) improvement at a mean of 5.1 years | 37 [45] |
| Higher depression scores associated with less depression improvement in Patient-Reported Outcomes Measurement Information System (PROMIS) depression: F (1, 109) = 148.3 (p=<0.0001) and higher depression scores associated with less pain score improvement in PROMIS pain: F (1, 109) = 23.36 (p=<0.0001) | 18 [50] | Multivariate, better mental health (3-item depression scale): no association with less symptom severity (“no severe pain”) at 2 years | 4.1 [27] | |
| Higher depression score (Hospital Anxiety and Depression Scale (HADS, range 0-21) ≥8 points) associated with less improvement in symptom severity at 1 year: log OR -1.09 (95% CI -1.87 to -0.31), SSM symptoms | 20.2 [46] | |||
| Higher Fear Avoidance Beliefs Questionnaire Physical activity (FABQ-P, 0-24) (at 6 months): less clinical meaningful improvement at 1 year: multiple log. regression, adj. OR 0.46 (95% CI 0.24-0.91) for SSM symptoms | 20.5 [53] | |||
| Persistent higher FABQ-P (at baseline and 6 months): less clinical meaningful improvement at 1 year: multiple log. regression, adj. OR 0.34 (95% CI 0.16-0.73) for SSM symptoms | ||||
| FABQ-P high (baseline) not associated with less clinical meaningful improvement (SSM symptoms) at 1 year | ||||
| Higher depressive burden (higher Beck depression inventory (BDI, range 0.63)) associated with higher pain scores (VAS (0-100)) at 10 years: mixed model, sign. beta 0.19 (95% CI 0.07 to 0.31) | 21.3 [48] |
.
Table A8.
Predictors for adverse events (AE), mortality, and other outcomes.
| Comorbidity | Significant predictors | Ref. | Not significant predictors | Ref. |
|---|---|---|---|---|
| Comorbidities, Comorbidity measures (CM) | Systemic disease (hypertension, diabetes or both) associated with higher postoperative wound infection rate: adjusted (adj.) odds ratio (OR) 3.99 (95% confidence interval (CI) 2.02 to 7.86) | 43 [98] | Compared to American Society of Anesthesiology (ASA, range 1-6) groups <3, ASA groups ≥3 not associated with more complicationsd during hospitalization | 10 [78] |
| Compared to 0 comorbidity, 2-3 comorbidities associated with higher in-hospital complication ratee: 2 comorbidities adj. OR 1.2 (95% CI 1.1 to 1.2), ≥3 comorbidities adj. OR 1.5 (95% CI 1.4 to 1.6) | 11 [35] | Multivariate, number of comorbidities: no association with reoperation rate 15 years after first surgery | 28.2 [36] | |
| Multivariate, compared to 0 comorbidity, 1 comorbidity not associated with higher in-hospital complication ratee | ||||
| Gagne comorbidity score£ (1-year increment): association with more 30-days complicationsc: adj. OR 1.25 (95% CI 1.02 to 1.29) | 13 [55] | |||
| Quan comorbidity score# ≥1 (ref. Quan 0) associated with more cardiopulmonary/stroke complications or mortality <30 days: Quan 1 adj. OR 1.27 (95% CI 1.09 to 1.47); Quan 2 adj. OR 1.62 (95% CI 1.33 to 1.97); Quan 3 adj. OR 1.78 (95% CI 1.34 to 2.36); Quan 4 adj. OR 2.14 (95% CI 1.46 to 3.16); Quan 4 adj. OR 3.14 (95% CI 2.17 to 4.55) | 12 [8] | |||
| Comorbidity score§ ≥1 (ref. 0) associated with any complications < 3 years after surgery: score 1: OR 1.31 (95% CI 1.06 to 1.61), 2: OR 1.48 (95% CI 1.13 to 1.94), 3: OR 1.63 (95% CI 1.22 to 2.19) | 14 [17] | |||
| ASA score 3 or 4 associated with increased length of stay: adj. beta 0.32 (95% CI 0.09 to 0.54), and with readmission <30 days after surgery: adj. beta 2.63 (95% CI 1.54 to 4.49) | 17 [80] | |||
| Compared to 0 comorbidity, adverse outcome (death) is associated with the presence of ≥2 comorbidities within hospitalization: 2 comorbidities adj. OR 2.3 (95% CI 1.5 to 3.6) and ≥3 comorbidities adj. OR 2.1 (95% CI 1.3 to 3.6) | 11 [35] | |||
| Multivariate, compared to 0 comorbidity, adverse outcome (death) not associated with the presence of 1 comorbidity within hospitalization | ||||
| Presence of comorbidities associated with overall reoperation rate up to 6 years: adj. hazard ratio (HR) 1.37 (95% CI 1.20 to 1.55), and with reoperation at specific time periods: early (<90 days): adj. HR 1.25 (95% CI 1.01 to 1.54), short-term (91-365 days): adj. HR 1.85 (95% CI 1.32 to 2.58), and midterm (1-6 years): adj. HR 1.30 (95% CI 1.09 to 1.55) | 50 [60] | |||
| Age | High age associated with higher occurrence of perioperative dural lesion: logistic (log.) regression significant (sign.) OR 1.03 (95% CI 1.01 to 1.04) | 35 [93] | 70-75 versus (vs.) >75 years: older age not associated with higher postoperative complication rateg | 48 [105] |
| Age (1-year increment) associated with more 30-days complicationsc: adj. OR 1.02 (95% CI 1.01 to 1.03) | 13 [55] | <80 vs. ≥80 years: older age not associated with more postoperative complicationsh | 45 [103] | |
| Compared to 18-44 years, ≥65 years associated with a higher in-hospital complication ratee: 65-84 years adj. OR 1.8 (95% CI 1.5 to 2.1), >85 years adj. OR 2.3 (95% CI 1.9 to 2.7) | 11 [35] | <80 vs. ≥80 years: older age not associated with more postoperative complicationsi | 2.5 [71] | |
| Multivariate, compared to 18-44 years, 45-64 years not associated with higher in-hospital complication ratee | 80-89 vs. >90 years: older age not associated with more complications during hospitalizationj | 16 [37] | ||
| Compared to 66-70 years, 71-74, 75-79 and ≥80 years associated with more cardiopulmonary/stroke complications or mortality <30 days 71-74 years adj. OR 1.13 (95% CI 0.94 to 1.37), 75-79 years adj. OR 1.36 (95% CI 1.14 to 1.62), >80 years adj. OR 1.70 (95% CI 1.43 to 2.04) | 12 [8] | Multivariate, no association with time to reoperation until a mean of 8.6 years | 51 [106] | |
| Compared to 65-69 years, 75-79 and ≥80 years associated with any postoperative complicationsf up to 3 years: 75-79 years OR 1.37 (95% CI 1.06 to 1.76), ≥80 years: OR 2.05 (95% CI 1.58 to 2.67) | 14 [17] | Multivariate, no association with reoperation rate up to 15 years | 28.2 [36] | |
| Compared to 65-69 years, age 70-74 years not associated with any postoperative complicationsf up to 3 years | Multivariate, no association with reoperation rate up to 8 years | 2.2 [70] | ||
| 60-69 vs. 70-79 vs. ≥80 years: 60-69 and 70-79 years associated with increase of length of stay after surgery: 60-69 years beta 0.29 (95% CI 0.01 to 0.57), 70-79 beta 0.50 (95% CI 0.21 to 0.78) | 17 [80] | Multivariate, no association with 90-day readmission | 6 [54] | |
| Age ≥80 years associated with readmission <30 days after surgery: OR 2.09 (95% CI 1.05 to 4.14) | Multivariate, compared to ≥80 years, younger age not associated with reoperation rate (at undefined time) | 8 [76] | ||
| Age 60-69 vs. 70-79 vs. ≥80 years: ≥80 years not associated with increase of length of stay after surgery (beta 0.92 (95% CI 0.55 to 1.29)) | 80-89 vs. >90 years: older age not associated with reoperations <12 months post-discharge | 16 [37] | ||
| Age 60-69 and 70-79 years: no association with readmission <30 days (60-69 years OR 0.85 (95% CI 0.45 to 1.58), 70-79 years OR 0.79 (95% CI 0.42 to 1.49) | ||||
| Age >70 years associated with reoperation rate at midterm (1-6 years): adj. HR 1.87 (95% CI 1.07 to 3.27) | 50 [60] | |||
| Multivariate, age >70 years: no association with overall (<6 years), early (<90 days) and short-term (90-365 days) reoperation rate | ||||
| Higher age (per year) associated with shorter time until surgery since start of membrane stabilizing agent (MSA) treatment: adj. HR 1.02 (95% CI 1.00 to 1.04) | 7 [52] | |||
| Multivariate, higher age (per year) not associated with need for surgery <1 year since start of MSA treatment | ||||
| >85 years associated with adverse outcome (death) within hospitalization: adj. OR 8.7 (95% CI 2.0 to 38.5) | 11 [35] | |||
| Multivariate, compared to 18-44 years, 45-84 years not associated with adverse outcome (death) within hospitalization | ||||
| Older age associated with higher 10-year survival rate: adj. HR 1.09 (95% CI 1.04 to 1.13) | 42 [97] | |||
| Previous spine surgery | Any previous operation associated with occurrence of perioperative dural lesion: log. regression sign. OR 0.70 (95% CI 0.50 to 0.98) | 35 [93] | Multivariate, any previous spine surgery: no association with cardiopulmonary, stroke complications, or mortality <30 days | 12 [8] |
| Symptom duration | - | - | Log. regression, duration of low back/leg pain (not specified): no association with occurrence of perioperative dural lesion | 35 [93] |
| Bivariate, pain <1 year (reference) vs. pain >1 year: no association with reoperation rates | 9 [77] | |||
| Body weight | Morbid obesity associated with surgical site infection: log. regression OR 6.99 (95% CI 2.65-22.03), (p<0.001) | 6 [54] | Multivariate, obesity: no association with 30-days complicationsc | 13 [55] |
| Body mass index (BMI) <30 vs. 30 to <35 vs. ≥35: BMI 30 to <35 associated with no postoperative complication up to 8 weeks after surgery: BMI <30: 85% vs. 30 to <35: 95% vs. ≥35: 83% (p=0.02) | 2.4 [41] | Binary logistic analysis, BMI >24.32 not associated with more perioperative complications (life-threatening/minor) | 46 [56] | |
| BMI <30 vs. 30 to <35 vs. ≥35: obesity not associated with postoperative complicationsb up to 8 weeks after surgery | ||||
| Obesity (BMI ≥30) associated with increased length of stay after surgery: adj. beta 0.58 (95% CI 0.26 to 0.90), and with readmission <30 days after surgery: adj. beta 3.38 (95% CI 1.36 to 8.36) | 17 [80] | Multivariate, no association with time to reoperation | 51 [106] | |
| Hypertension | - | - | Log. regression, hypertension: no association with in-hospital perioperative complicationsa | 44.2 [57] |
| Hypertension: no association with increased length of stay after surgery (multivariate) and readmission <30 days (bivariate) | 17 [80] | |||
| Multivariate, no association with mortality up to 8 years | 49 [61] | |||
| Univariate, no association with reoperation rate up to 8 years | 2.2 [70] | |||
| Diabetes | Diabetes vs. no-diabetes: diabetes associated with more “other” complications (not specified) <8 weeks after surgery: diabetes 13%, no-diabetes 4% (p=0.021) | 2.3 [59] | Multivariate: no association with 30-days complicationsc | 13 [55] |
| Diabetes vs. no-diabetes: no-diabetes associated with no complication <8 weeks after surgery: diabetes 74%, no-diabetes 90% (p=0.002) | Multivariate, treated diabetes: no association with time to reoperation | 51 [106] | ||
| Diabetes vs. no-diabetes: diabetes not associated with postoperative wound infections/hematoma <8 weeks after surgery | No association with increased length of stay after surgery (multivariate) and readmission <30 days (bivariate) | 17 [80] | ||
| Diabetes vs. no-diabetes: diabetes associated with more in-hospital perioperative complicationsa: diabetes 67%, no-diabetes 28% (p<0.0001) | 44.5 [39] | Univariate, no association with reoperation rate up to 8 years | 2.2 [70] | |
| Diabetes mellitus associated with in-hospital perioperative complicationsa: log. regression sign. OR 1.85 (95% CI 1.03 ± 3.33) | 44.2 [57] | |||
| Diabetes associated with short-term reoperation rate (91-365 days): adj. HR 1.32 (95% CI 1.02 to 1.70), and with midterm reoperation rate (1-6 years): adj. HR 1.21 (95% CI 1.04 to 1.41) | 50 [60] | |||
| Multivariate, no association with early reoperation rate (<90 days) | ||||
| Diabetes associated with mortality up to 8 years after surgery: adj. HR 1.12 (95% CI 1.0 to 1.25) | 49 [61] | |||
| Diabetes vs. no-diabetes: diabetes associated with increased length of stay (days): diabetes: mean 34.9 vs. no-diabetes: 30.7 (p=0.0008) | ||||
| Smoking | Smoking associated with occurrence of perioperative dural lesion: logistic regression, sign. OR 0.70 (95% CI 0.49 to 0.999) | 35 [93] | No association with increased length of stay after surgery (multivariate) and readmission <30 days (bivariate) | 17 [80] |
| Multivariate, no association with time to reoperation | 51 [106] | |||
| Univariate, no association with reoperation rate up to 8 years | 2.2 [70] | |||
| Cardiovascular disease | Congestive heart failure associated with in-hospital perioperative complicationsa: log. regression OR 2.96 (95% CI 1.55 ± 5.66) | 44.2 [57] | Univariate, heart problem: no association with reoperation up to 8 years | 2.2 [70] |
| Ischemic heart disease associated with in-hospital perioperative complicationsa: log. regression, sign. OR 2.02 (95% CI 1.22 ± 3.35) | Anemia (hematocrit <36) associated with increased length of stay after surgery: adj. beta 0.65 (95% CI 0.28 to 1.02) | 17 [80] | ||
| Log. regression, peripheral vascular disease: no association with in-hospital perioperative complicationsa | Bivariate, heart disease: no association with increased length of stay after surgery and readmission <30 days | |||
| History of coronary artery disease associated with surgical site infection: log. regression, sign. OR 2.26 (95% CI 1.31-3.84), p=0.003 | 6 [54] | Bivariate, anemia (hematocrit <36): no association with readmission <30 days | ||
| Cardiovascular disease associated with mortality up to 8 years after surgery: adj. HR 1.64 (95% CI 1.48 to 1.82) | 49 [61] | |||
| History of congestive heart failure associated with 90-day readmission OR 3.03 (95%CI 1.69-5.28), p<0.001 | 6 [54] | |||
| Lung disease | Pulmonary disease associated with in-hospital perioperative complicationsa: log. regression, sign. OR 2.63 (95% CI 1.42 ± 4.87) | 44.2 [57] | Pulmonary disease: no association with increased length of stay (bivariate) after surgery and readmission <30 days after surgery (multivariate) | 17 [80] |
| Neurologic disease | Cerebrovascular disease associated with mortality up to 8 years after surgery: adj. HR 1.23 (95% CI 1.11 to 1.37) | 49 [61] | - | - |
| Rheumatologic disease | Osteoarthritis associated with reoperation rate after a mean of 5.3 years: linear regression sign. beta 0.14 (p<0.001) | 44.3 [100] | Univariate, osteoporosis/joint problem: no association with reoperation up to 8 years | 2.2 [70] |
| Psychiatric disease | - | - | Multivariate, greater depression (higher Personal Health Questionnaire depression scale (PHQ-9, range 0-24)) not associated with shorter time until surgery or need for surgery <1 year since start of MSA treatment | 7 [52] |
| Univariate, depression: no association with reoperation rate <8 years | 2.2 [70] | |||
| Kidney disease | Chronic kidney disease associated with mortality up to 8 years after surgery: adj. HR 2.83 (95% CI 2.51 to 3.19) | 49 [61] | - | - |
Gagne score (-2–26);
Charlson comorbidity score based on coexisting conditions (range 0–3+);
Quan comorbidity score (adapted Charlson comorbidity score) based on comorbid conditions in any hospitalization during the previous year (range 0–3+);
urinary complications (retention, infection, incontinence), exacerbation of congestive heart failure/chronic obstructive pulmonary disease, atrial fibrillation, wound infection, delirium, unstable angina, depression, cerebrovascular accident, gastrointestinal bleeding, hypotension39 and in-hospital death; [57]
wound infections, wound hematoma and other complications;
renal, cardiac, neurological, pulmonary, deep vein thrombosis, pulmonary embolism, infection;
dural tears, confusion, pseudogout, ileus, hypotension, wound infection, urinary retention, dehiscence;
neurologic, pulmonary, thromboembolic, cardiac, urinary, renal, hemorrhage/hematoma complicating a procedure, fluid/electrolyte abnormalities;
cardiopulmonary, vascular, infectious;
infection, hematoma, pneumonia, urinary tract infection, delirium, cerebrovascular accident, acute renal failure, neutropenia;
cerebrospinal fluid leakage, delirium, epidural hematoma, infection, dysuria, insufficiency fracture, wound problem, ileus, acute cholecystitis, aortic aneurysm, deep venous thrombosis;
wound complications (infections, hematoma, dehiscence), nerve root injury or other complications;
cardiac, general neurological, pulmonary, renal, deep vein thrombosis or pulmonary embolism, infection, wound infection
Appendix H. Supplementary materials
References
- 1.Katz JN, Harris MB. Clinical practice. Lumbar spinal stenosis. N Engl J Med. 2008;358(8):818–825. doi: 10.1056/NEJMcp0708097. (In eng) [DOI] [PubMed] [Google Scholar]
- 2.Lurie J, Tomkins-Lane C. Management of lumbar spinal stenosis. BMJ (Clinical research ed) 2016;352:h6234. doi: 10.1136/bmj.h6234. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atlas SJ, Delitto A. Spinal stenosis: surgical versus nonsurgical treatment. Clin Orthop 2006;443:198-207. (Research Support, N.I.H., Extramural Review) (In English) (http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=med5&AN=16462443). [DOI] [PubMed]
- 4.Lin S-I, Lin R-M, Huang L-W. Disability in patients with degenerative lumbar spinal stenosis. Arch Phys Med Rehabil. 2006;87(9):1250–1256. doi: 10.1016/j.apmr.2006.05.021. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med5&NEWS=N&AN=16935063 [DOI] [PubMed] [Google Scholar]
- 5.Rampersaud YR, Ravi B, Lewis SJ, et al. Assessment of health-related quality of life after surgical treatment of focal symptomatic spinal stenosis compared with osteoarthritis of the hip or knee. Spine J. 2008;8(2):296–304. doi: 10.1016/j.spinee.2007.05.003. (In eng) [DOI] [PubMed] [Google Scholar]
- 6.Otani K, Kikuchi S, Yabuki S, et al. Lumbar spinal stenosis has a negative impact on quality of life compared with other comorbidities: an epidemiological cross-sectional study of 1862 community-dwelling individuals. ScientificWorldJournal. 2013;2013 doi: 10.1155/2013/590652. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bays A. Prevalence of comorbidities and their influence on the treatment outcome in symptomatic lumbar spinal stenosis. A protocol of a systematic review. 2018 doi: 10.1016/j.xnsj.2021.100072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010;303(13):1259–1265. doi: 10.1001/jama.2010.338. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deyo RA. Treatment of lumbar spinal stenosis: a balancing act. Spine J. 2010;10(7):625–627. doi: 10.1016/j.spinee.2010.05.006. (In eng) [DOI] [PubMed] [Google Scholar]
- 10.Sengupta DK, Herkowitz HN. Lumbar spinal stenosis. Treatment strategies and indications for surgery. 2003;1(2):281–295. doi: 10.1016/s0030-5898(02)00069-x. [Review] [69 refs] [DOI] [PubMed] [Google Scholar]
- 11.Watters WC, 3rd, Baisden J, Gilbert TJ, et al. Degenerative lumbar spinal stenosis: an evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spinal stenosis. Spine J. 2008;8(2):305–310. doi: 10.1016/j.spinee.2007.10.033. (In eng) [DOI] [PubMed] [Google Scholar]
- 12.Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonoperative treatment for lumbar spinal stenosis four-year results of the Spine Patient Outcomes Research Trial. Spine (Phila Pa 1976) 2010;35(14):1329–1338. doi: 10.1097/BRS.0b013e3181e0f04d. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shabat S, Arinzon Z, Gepstein R, Folman Y. Long-term follow-up of revision decompressive lumbar spinal surgery in elderly patients. 2011;1(3):142-5. [DOI] [PubMed]
- 14.Marengoni A, Angleman S, Meinow B, et al. Coexisting chronic conditions in the older population: Variation by health indicators. European journal of internal medicine. 2016;31:29–34. doi: 10.1016/j.ejim.2016.02.014. (In eng) [DOI] [PubMed] [Google Scholar]
- 15.Foulongne E, Derrey S, Ould Slimane M, et al. Lumbar spinal stenosis: which predictive factors of favorable functional results after decompressive laminectomy? Neurochirurgie. 2013;59(1):23–29. doi: 10.1016/j.neuchi.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Katz JN, Lipson SJ, Larson MG, McInnes JM, Fossel AH, Liang MH. The outcome of decompressive laminectomy for degenerative lumbar stenosis. The Journal of bone and joint surgery American. 1991;73(6):809–816. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med3&NEWS=N&AN=2071616 [PubMed] [Google Scholar]
- 17.Ciol MA, Deyo RA, Howell E, Kreif S. An assessment of surgery for spinal stenosis: time trends, geographic variations, complications, and reoperations. J Am Geriatr Soc. 1996;44(3):285–290. doi: 10.1111/j.1532-5415.1996.tb00915.x. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=med4&AN=8600197 (In English) [DOI] [PubMed] [Google Scholar]
- 18.Deyo RA, Hickam D, Duckart JP, Piedra M. Complications after surgery for lumbar stenosis in a veteran population. Spine. 2013;38(19):1695–1702. doi: 10.1097/BRS.0b013e31829f65c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sobottke R, Aghayev E, Roder C, Eysel P, Delank SK, Zweig T. Predictors of surgical, general and follow-up complications in lumbar spinal stenosis relative to patient age as emerged from the Spine Tango Registry. Eur Spine J. 2012;21(3):411–417. doi: 10.1007/s00586-011-2016-y. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ulrich NH, Kleinstuck F, Woernle CM, et al. Clinical outcome in lumbar decompression surgery for spinal canal stenosis in the aged population: a prospective Swiss multicenter cohort study. Spine (Phila Pa 1976) 2015;40(6):415–422. doi: 10.1097/brs.0000000000000765. (In eng) [DOI] [PubMed] [Google Scholar]
- 21.Athiviraham A, Wali ZA, Yen D. Predictive factors influencing clinical outcome with operative management of lumbar spinal stenosis. The spine journal : official journal of the. North American Spine Society. 2011;11(7):613–617. doi: 10.1016/j.spinee.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Airaksinen O, Herno A, Turunen V, Saari T, Suomlainen O. Surgical outcome of 438 patients treated surgically for lumbar spinal stenosis. Spine. 1997;22(19):2278–2282. doi: 10.1097/00007632-199710010-00016. http://sfx.metabib.ch/sfx_locater?sid=Entrez:PubMed&id=pmid:9346149 [DOI] [PubMed] [Google Scholar]
- 23.Sobottke R, Herren C, Siewe J, Mannion AF, Roder C, Aghayev E. Predictors of improvement in quality of life and pain relief in lumbar spinal stenosis relative to patient age: a study based on the Spine Tango registry. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society 2017;26(2):462-472. DOI: https://dx.doi.org/10.1007/s00586-015-4078-8. [DOI] [PubMed]
- 24.Jakola AS, Sorlie A, Gulati S, Nygaard OP, Lydersen S, Solberg T. Clinical outcomes and safety assessment in elderly patients undergoing decompressive laminectomy for lumbar spinal stenosis: a prospective study. BMC surgery. 2010;10:34. doi: 10.1186/1471-2482-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galiano K, Obwegeser AA, Gabl MV, Bauer R, Twerdy K. Long-term outcome of laminectomy for spinal stenosis in octogenarians. Spine. 2005;30(3):332–335. doi: 10.1097/01.brs.0000152381.20719.50. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med5&NEWS=N&AN=15682015 [DOI] [PubMed] [Google Scholar]
- 26.Shabat S, Arinzon Z, Folman Y, et al. Long-term outcome of decompressive surgery for lumbar spinal stenosis in octogenarians. Eur Spine J. 2008;17(2):193–198. doi: 10.1007/s00586-007-0514-8. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katz JN, Stucki G, Lipson SJ, Fossel AH, Grobler LJ, Weinstein JN. Predictors of surgical outcome in degenerative lumbar spinal stenosis. Spine. 1999;24(21):2229–2233. doi: 10.1097/00007632-199911010-00010. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med4&NEWS=N&AN=10562989 [DOI] [PubMed] [Google Scholar]
- 28.Aalto TJ, Malmivaara A, Kovacs F, et al. Preoperative predictors for postoperative clinical outcome in lumbar spinal stenosis: systematic review. Spine. 2006;31(18):E648–E663. doi: 10.1097/01.brs.0000231727.88477.da. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med5&NEWS=N&AN=16915081 [DOI] [PubMed] [Google Scholar]
- 29.Katz JN, Lipson SJ, Chang LC, Levine SA, Fossel AH, Liang MH. Seven- to 10-year outcome of decompressive surgery for degenerative lumbar spinal stenosis. Spine. 1996;21(1):92–98. doi: 10.1097/00007632-199601010-00022. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med4&NEWS=N&AN=9122770 [DOI] [PubMed] [Google Scholar]
- 30.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clinical research ed) 2009;339 doi: 10.1136/bmj.b2535. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scottish Intercollegiate Guidelines Network (SIGN). SIGN 50: a guideline developer's handbook. Edinburgh: SIGN. (http://www.sign.ac.uk).
- 32.Core Team R. R Foundation for Statistical Computing; Vienna, Austria: 2018. R: A Language and Environment for Statistical Computing.https://www.R-project.org/ URL. [Google Scholar]
- 33.Kleinstuck FS, Grob D, Lattig F, et al. The influence of preoperative back pain on the outcome of lumbar decompression surgery. Spine. 2009;34(11):1198–1203. doi: 10.1097/BRS.0b013e31819fcf35. [DOI] [PubMed] [Google Scholar]
- 34.Aghayev E, Mannion AF, Fekete T, et al. Risk factors for negative global treatment outcomes in lumbar spinal stenosis surgery: a mixed effects model analysis of data from an international spine registry. World Neurosurg. 2019 doi: 10.1016/j.wneu.2019.12.147. (In eng) [DOI] [PubMed] [Google Scholar]
- 35.Li G, Patil CG, Lad SP, Ho C, Tian W, Boakye M. Effects of age and comorbidities on complication rates and adverse outcomes after lumbar laminectomy in elderly patients. Spine. 2008;33(11):1250–1255. doi: 10.1097/BRS.0b013e3181714a44. [DOI] [PubMed] [Google Scholar]
- 36.Lenoir T, Dauzac C, Rillardon L, Guigui P. [Long-term survival analysis after surgical management for degenerative lumbar stenosis] Etude a long terme du risque de reintervention apres traitement chirurgical d'une stenose canalaire lombaire. 2008;94(5):464–471. doi: 10.1016/j.rco.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Sharma M, Dietz N, Ugiliweneza B, Wang D, Drazin D, Boakye M. Differences in clinical outcomes and health care utilization between octogenarians and nonagenarians following decompression for lumbar spinal stenosis. A market scan analysis. Clin Neurol Neurosurg. 2019;182:63–69. doi: 10.1016/j.clineuro.2019.04.031. (Article) (In English) [DOI] [PubMed] [Google Scholar]
- 38.Katz JN, Lipson SJ, Brick GW, et al. Clinical correlates of patient satisfaction after laminectomy for degenerative lumbar spinal stenosis. Spine. 1995;20(10):1155–1160. doi: 10.1097/00007632-199505150-00008. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med3&NEWS=N&AN=7638658 [DOI] [PubMed] [Google Scholar]
- 39.Arinzon Z, Adunsky A, Fidelman Z, Gepstein R., Outcomes of decompression surgery for lumbar spinal stenosis in elderly diabetic patients European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the. Cervical Spine Research Society. 2004;13(1):32–37. doi: 10.1007/s00586-003-0643-7. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med5&NEWS=N&AN=14614597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aalto T, Sinikallio S, Kroger H, et al. Preoperative predictors for good postoperative satisfaction and functional outcome in lumbar spinal stenosis surgery–a prospective observational study with a two-year follow-up. Scandinavian journal of surgery : SJS : official organ for the Finnish Surgical Society and the Scandinavian Surgical Society. 2012;101(4):255–260. doi: 10.1177/145749691210100406. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med7&NEWS=N&AN=23238500 [DOI] [PubMed] [Google Scholar]
- 41.McGuire KJ, Khaleel MA, Rihn JA, Lurie JD, Zhao W, Weinstein JN. The effect of high obesity on outcomes of treatment for lumbar spinal conditions: subgroup analysis of the spine patient outcomes research trial. Spine. 2014;39(23):1975–1980. doi: 10.1097/BRS.0000000000000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paulsen RT, Bouknaitir JB, Fruensgaard S, Carreon L, Andersen M. Prognostic Factors for Satisfaction After Decompression Surgery for Lumbar Spinal Stenosis. Neurosurgery. 2018;82(5):645–651. doi: 10.1093/neuros/nyx298. [DOI] [PubMed] [Google Scholar]
- 43.Sinikallio S, Aalto T, Airaksinen O, et al. Lumbar spinal stenosis patients are satisfied with short-term results of surgery - younger age, symptom severity, disability and depression decrease satisfaction. Disabil Rehabil. 2007;29(7):537–544. doi: 10.1080/09638280600902646. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med5&NEWS=N&AN=17453974 [DOI] [PubMed] [Google Scholar]
- 44.Sanden B, Forsth P, Michaelsson K. Smokers show less improvement than nonsmokers two years after surgery for lumbar spinal stenosis: a study of 4555 patients from the Swedish spine register. Spine. 2011;36(13):1059–1064. doi: 10.1097/BRS.0b013e3181e92b36. [DOI] [PubMed] [Google Scholar]
- 45.Bouras T, Stranjalis G, Loufardaki M, Sourtzis I, Stavrinou LC, Sakas DE. Predictors of long-term outcome in an elderly group after laminectomy for lumbar stenosis. J Neurosurg Spine. 2010;13(3):329–334. doi: 10.3171/2010.3.SPINE09487. [DOI] [PubMed] [Google Scholar]
- 46.Held UB, Jakob M., Wertli Maria M., Pichierri Giuseppe, Winklhofer Sebastian, Brunner Florian, et al. Prognostic function to estimate the probability of meaningful clinical improvement after surgery - Results of a prospective multicenter observational cohort study on patients with lumbar spinal stenosis. PLoS One. 2018;13:11. doi: 10.1371/journal.pone.0207126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iderberg H, Willers C, Borgstrom F, et al. Predicting clinical outcome and length of sick leave after surgery for lumbar spinal stenosis in Sweden: a multi-register evaluation. Eur Spine J. 2018;03:03. doi: 10.1007/s00586-018-5842-3. https://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=medp&AN=30511244 [DOI] [PubMed] [Google Scholar]
- 48.Tuomainen I, Pakarinen M, Aalto T, et al. Depression is associated with the long-term outcome of lumbar spinal stenosis surgery: a 10-year follow-up study. Spine J. 2018;18(3):458–463. doi: 10.1016/j.spinee.2017.08.228. (In eng) [DOI] [PubMed] [Google Scholar]
- 49.Kim H-J, Park J-W, Kang K-T, et al. Determination of the Optimal Cutoff Values for Pain Sensitivity Questionnaire Scores and the Oswestry Disability Index for Favorable Surgical Outcomes in Subjects With Lumbar Spinal Stenosis. Spine. 2015;40(20):E1110–E1116. doi: 10.1097/BRS.0000000000001023. [DOI] [PubMed] [Google Scholar]
- 50.Merrill RK, Zebala LP, Peters C, Qureshi SA, McAnany SJ. Impact of Depression on Patient-Reported Outcome Measures after Lumbar Spine Decompression. Spine. 2018;43(6):434–439. doi: 10.1097/BRS.0000000000002329. (Article) (In English) [DOI] [PubMed] [Google Scholar]
- 51.Turner JAC, Bryan A., Standaert Christopher J., Heagerty Patrick J., Jarvik Jeffrey G., Deyo Richard A., Wasan Ajay D., Nedeljkovic Srdjan S., Friedly Janna L. Can patient characteristics predict benefit from epidural corticosteroid injections for lumbar spinal stenosis symptoms? The spine journal : official journal of the North American Spine Society. 2015;15:2319–2331. doi: 10.1016/j.spinee.2015.06.050. [DOI] [PubMed] [Google Scholar]
- 52.Lubelski D, Thompson NR, Agrawal B, et al. Prediction of quality of life improvements in patients with lumbar stenosis following use of membrane stabilizing agents. Clin Neurol Neurosurg. 2015;139:234–240. doi: 10.1016/j.clineuro.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 53.Burgstaller JM, Wertli MM, Steurer J, Kessels AGH, Held U, Gramke HF. The Influence of Pre- and Postoperative Fear Avoidance Beliefs on Postoperative Pain and Disability in Patients with Lumbar Spinal Stenosis. Spine. 2017;42(7):E425–E432. doi: 10.1097/BRS.0000000000001845. (Article) (In English) [DOI] [PubMed] [Google Scholar]
- 54.Ilyas H, Golubovsky JL, Chen J, Winkelman RD, Mroz TE, Steinmetz MP. Risk factors for 90-day reoperation and readmission after lumbar surgery for lumbar spinal stenosis. 2019;31(1):20. (In English). doi: 10.3171/2019.1.Spine18878. [DOI] [PubMed]
- 55.Drazin D, Shweikeh F, Lagman C, Ugiliweneza B, Boakye M. Racial Disparities in Elderly Patients Receiving Lumbar Spinal Stenosis Surgery. Global spine journal. 2017;7(2):162–169. doi: 10.1177/2192568217694012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kong C, Li X, Sun X, Ding J, Guo M, Lu S. Complications in Elderly Patients Undergoing Lumbar Arthrodesis for Spinal Stenosis. World Neurosurg. 2019;132:e949–e955. doi: 10.1016/j.wneu.2019.06.147. (In eng) [DOI] [PubMed] [Google Scholar]
- 57.Gepstein R, Shabat S, Arinzon ZH, Berner Y, Catz A, Folman Y. Does obesity affect the results of lumbar decompressive spinal surgery in the elderly? Clin Orthop Relat Res. 2004;(426):138–144. doi: 10.1097/01.blo.0000141901.23322.98. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med5&NEWS=N&AN=15346065 [DOI] [PubMed] [Google Scholar]
- 58.North American Spine Society . North American Spine Society; 2011. Evidence-Based Clinical Guidelines for Multidisciplinary Spine Care, Diagnosis and Treatment of Degenerative Lumbar Spinal Stenosis.https://www.spine.org/Portals/0/Assets/Downloads/ResearchClinicalCare/Guidelines/LumbarStenosis.pdf Available from. accessed December 15th, 2019. [Google Scholar]
- 59.Freedman MK, Hilibrand AS, Blood EA, et al. The impact of diabetes on the outcomes of surgical and nonsurgical treatment of patients in the spine patient outcomes research trial. Spine. 2011;36(4):290–307. doi: 10.1097/BRS.0b013e3181ef9d8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim CH, Chung CK, Park CS, et al. Reoperation rate after surgery for lumbar spinal stenosis without spondylolisthesis: A nationwide cohort study. Spine Journal. 2013;13(10):1230–1237. doi: 10.1016/j.spinee.2013.06.069. (Article) (In English) [DOI] [PubMed] [Google Scholar]
- 61.Lee CK, Choi SK, Shin DA, et al. Influence of diabetes mellitus on patients with lumbar spinal stenosis: A nationwide population-based study. PLoS One. 2019;14(3) doi: 10.1371/journal.pone.0213858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Slipman CW, Shin CH, Patel RK, et al. Etiologies of Failed Back Surgery Syndrome. Pain Medicine. 2002;3(3):200–214. doi: 10.1046/j.1526-4637.2002.02033.x. [DOI] [PubMed] [Google Scholar]
- 63.North American Spine Society . North American Spine Society; 2012. Evidence-Based Clinical Guidelines for Multidisciplinary Spine Care, Diagnosis and Treatment of Lumbar Disc Herniation with Radiculopathy. https://www.spine.org/Portals/0/Assets/Downloads/ResearchClinicalCare/Guidelines/LumbarDiscHerniation.pdf. [Google Scholar]
- 64.McKillop AB, Carroll LJ, Battie MC. Depression as a prognostic factor of lumbar spinal stenosis: a systematic review. Spine J. 2014;14(5):837–846. doi: 10.1016/j.spinee.2013.09.052. (In eng) [DOI] [PubMed] [Google Scholar]
- 65.Falavigna A, Righesso O, Teles AR, et al. Responsiveness of depression and its influence on surgical outcomes of lumbar degenerative diseases. [DOI] [PubMed]
- 66.Havakeshian S, Mannion AF. Negative beliefs and psychological disturbance in spine surgery patients: a cause or consequence of a poor treatment outcome? Eur Spine J. 2013;22(12):2827–2835. doi: 10.1007/s00586-013-2822-5. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Friedly JLC, Bryan A., Turner Judith A., Heagerty Patrick J., Deyo Richard A., Sullivan Sean D., Bauer Zoya, Bresnahan Bryan W., Avins Andrew L., Nedeljkovic Srdjan S., Nerenz David R., Standaert Christopher, Kessler Larry, Akuthota Venu, Annaswamy Thiru, Chen Allen, Diehn Felix, Firtch William, Gerges Frederic J., Gilligan Christopher, Goldberg Harley, Kennedy David J., Mandel Shlomo, Tyburski Mark, Sanders William, Sibell David, Smuck Matthew, Wasan Ajay, Won Lawrence, Jarvik Jeffrey G. A Randomized Trial of Epidural Glucocorticoid Injections for Spinal Stenosis. New England Journald of Medicine. 2014;371:1. doi: 10.1056/NEJMoa1313265. [DOI] [PubMed] [Google Scholar]
- 68.Friedly JL, Comstock BA, Heagerty PJ, et al. Systemic effects of epidural steroid injections for spinal stenosis. Pain. 2018;159(5):876–883. doi: 10.1097/j.pain.0000000000001158. [DOI] [PubMed] [Google Scholar]
- 69.Lurie JD, Tosteson TD, Tosteson A, et al. Long-term outcomes of lumbar spinal stenosis: eight-year results of the Spine Patient Outcomes Research Trial (SPORT) Spine (Phila Pa 1976) 2015;40(2):63–76. doi: 10.1097/brs.0000000000000731. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gerling MC, Leven D, Passias PG, et al. Risk Factors for Reoperation in Patients Treated Surgically for Lumbar Stenosis: A Subanalysis of the 8-year Data From the SPORT Trial. Spine. 2016;41(10):901–909. doi: 10.1097/BRS.0000000000001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rihn JA, Hilibrand AS, Zhao W, et al. Effectiveness of surgery for lumbar stenosis and degenerative spondylolisthesis in the octogenarian population: analysis of the Spine Patient Outcomes Research Trial (SPORT) data. J Bone Joint Surg Am. 2015;97(3):177–185. doi: 10.2106/JBJS.N.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rihn JA, Radcliff K, Hilibrand AS, et al. Does obesity affect outcomes of treatment for lumbar stenosis and degenerative spondylolisthesis? Analysis of the Spine Patient Outcomes Research Trial (SPORT) Spine. 2012;37(23):1933–1946. doi: 10.1097/BRS.0b013e31825e21b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Radcliff KE, Rihn J, Hilibrand A, et al. Does the duration of symptoms in patients with spinal stenosis and degenerative spondylolisthesis affect outcomes?: Analysis of the spine outcomes research trial. Spine. 2011;36(25):2197–2210. doi: 10.1097/BRS.0b013e3182341edf. (Article) (In English) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Atlas SJ, Keller RB, Wu YA, Deyo RA, Singer DE. Long-term outcomes of surgical and nonsurgical management of lumbar spinal stenosis: 8 to 10 year results from the maine lumbar spine study. Spine (Phila Pa 1976) 2005;30(8):936–943. doi: 10.1097/01.brs.0000158953.57966.c0. (In eng) [DOI] [PubMed] [Google Scholar]
- 75.Herron LD, Mangelsdorf C. Lumbar spinal stenosis: results of surgical treatment. J Spinal Disord. 1991;4(1):26–33. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med3&NEWS=N&AN=1807528 [PubMed] [Google Scholar]
- 76.Javalkar V, Cardenas R, Tawfik TA, et al. Reoperations after surgery for lumbar spinal stenosis. World Neurosurg. 2011;75(5–6):737–742. doi: 10.1016/j.wneu.2010.10.043. [DOI] [PubMed] [Google Scholar]
- 77.Movassaghi K, Basques BA, Louie PK, et al. The Duration of Symptoms Does Not Impact Clinical Outcomes Following Lumbar Decompression Surgery. Spine. 2019;44(5):305–308. doi: 10.1097/BRS.0000000000002818. (Article) (In English) [DOI] [PubMed] [Google Scholar]
- 78.Ragab AA, Fye MA, Bohlman HH. Surgery of the lumbar spine for spinal stenosis in 118 patients 70 years of age or older. Spine (03622436) 2003;28(4):348–353. doi: 10.1097/01.BRS.0000048494.66599.DF. http://search.ebscohost.com/login.aspx?direct=true&db=c8h&AN=106704747&site=ehost-live [DOI] [PubMed] [Google Scholar]
- 79.Lad SP, Huang KT, Bagley JH, et al. Disparities in the outcomes of lumbar spinal stenosis surgery based on insurance status. Spine. 2013;38(13):1119–1127. doi: 10.1097/BRS.0b013e318287f04e. [DOI] [PubMed] [Google Scholar]
- 80.Basques BA, Varthi AG, Golinvaux NS, Bohl DD, Grauer JN. Patient characteristics associated with increased postoperative length of stay and readmission after elective laminectomy for lumbar spinal stenosis. Spine. 2014;39(10):833–840. doi: 10.1097/BRS.0000000000000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Adogwa O, Parker SL, Shau DN, et al. Preoperative Zung Depression Scale predicts outcome after revision lumbar surgery for adjacent segment disease, recurrent stenosis, and pseudarthrosis. The spine journal : official journal of the North American Spine Society 2012;12(3):179-85. doi: 10.1016/j.spinee.2011.08.014. [DOI] [PubMed]
- 82.Held U, Steurer J, Pichierri G, et al. What is the treatment effect of surgery compared with nonoperative treatment in patients with lumbar spinal stenosis at 1-year follow-up? J Neurosurg Spine. 2019:1–9. doi: 10.3171/2019.1.Spine181098. (In eng) [DOI] [PubMed] [Google Scholar]
- 83.Fekete T, Woernle C, Mannion AF, et al. The Effect of Epidural Steroid Injection on Postoperative Outcome in Patients From the Lumbar Spinal Stenosis Outcome Study. Spine. 2015;40(16):1303–1310. doi: 10.1097/BRS.0000000000000969. [DOI] [PubMed] [Google Scholar]
- 84.Burgstaller JM, Held U, Brunner F, et al. The Impact of Obesity on the Outcome of Decompression Surgery in Degenerative Lumbar Spinal Canal Stenosis: Analysis of the Lumbar Spinal Outcome Study (LSOS): A Swiss Prospective Multicenter Cohort Study. Spine (Phila Pa 1976) 2016;41(1):82–89. doi: 10.1097/brs.0000000000001128. (In eng) [DOI] [PubMed] [Google Scholar]
- 85.Pakarinen M, Vanhanen S, Sinikallio S, et al. Depressive burden is associated with a poorer surgical outcome among lumbar spinal stenosis patients: a 5-year follow-up study. Spine J. 2014;14(10):2392–2396. doi: 10.1016/j.spinee.2014.01.047. (In eng) [DOI] [PubMed] [Google Scholar]
- 86.Sinikallio S, Aalto T, Airaksinen O, Lehto SM, Kroger H, Viinamaki H. Depression is associated with a poorer outcome of lumbar spinal stenosis surgery: a two-year prospective follow-up study. Spine (Phila Pa 1976) 2011;36(8):677–682. doi: 10.1097/BRS.0b013e3181dcaf4a. (In eng) [DOI] [PubMed] [Google Scholar]
- 87.Guigui P, Cardinne L, Rillardon L, Morais T, Vuillemin A, Deburge A. [Per- and postoperative complications of surgical treatment of lumbar spinal stenosis. Prospective study of 306 patients] Complications per et postoperatoires du traitement chirurgical des stenoses lombaires Etude prospective d'une serie de 306 patients. 2002;88(7):669–677. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med4&NEWS=N&AN=12457112 [PubMed] [Google Scholar]
- 88.Ferrero E, Lonjon G, Bouyer B, Sabourin M, Ould-Slimane M, Guigui P. Influence of comorbidities on patients reported outcomes in degenerative lumbar spinal stenosis. Orthop Traumatol Surg Res. 2018;104(7):1031–1036. doi: 10.1016/j.otsr.2018.07.012. (In eng) [DOI] [PubMed] [Google Scholar]
- 89.Papavero L, Thiel M, Fritzsche E, Kunze C, Westphal M, Kothe R. Lumbar spinal stenosis: prognostic factors for bilateral microsurgical decompression using a unilateral approach. Neurosurgery. 2009;65(6 Suppl):182. doi: 10.1227/01.NEU.0000341906.65696.08. https://dx.doi.org/ -discussion187. [DOI] [PubMed] [Google Scholar]
- 90.Costa F, Sassi M, Cardia A, et al. Degenerative lumbar spinal stenosis: analysis of results in a series of 374 patients treated with unilateral laminotomy for bilateral microdecompression. J Neurosurg Spine. 2007;7(6):579–586. doi: 10.3171/SPI-07/12/579. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med5&NEWS=N&AN=18074681 [DOI] [PubMed] [Google Scholar]
- 91.Rillardon L, Guigui P, Veil-Picard A, Slulittel H, Deburge A. [Long-term results of surgical treatment of lumbar spinal stenosis] Resultats a long terme du traitement chirurgical des stenoses lombaires. 2003;89(7):621–631. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med4&NEWS=N&AN=14699308 [PubMed] [Google Scholar]
- 92.Knutsson B, Michaelsson K, Sanden B. Obesity is associated with inferior results after surgery for lumbar spinal stenosis: a study of 2633 patients from the Swedish spine register. Spine. 2013;38(5):435–441. doi: 10.1097/BRS.0b013e318270b243. [DOI] [PubMed] [Google Scholar]
- 93.Stromqvist F, Jonsson B, Stromqvist B. Swedish Society of Spinal S. Dural lesions in decompression for lumbar spinal stenosis: incidence, risk factors and effect on outcome. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2012;21(5):825–828. doi: 10.1007/s00586-011-2101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Keorochana G, Laohacharoensombat W, Wajanavisit W, Chanplakorn P, Woratanarat P, Chatchaipun P. Functional outcome after decompression and instrumented arthrodesis in degenerative lumbar spinal stenosis: factors influencing unsuccessful outcome change. J Med Assoc Thai. 2011;94(12):1487–1494. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med7&NEWS=N&AN=22295737 [PubMed] [Google Scholar]
- 95.Miyamoto H, Sumi M, Uno K, Tadokoro K, Mizuno K. Clinical outcome of nonoperative treatment for lumbar spinal stenosis, and predictive factors relating to prognosis, in a 5-year minimum follow-up. J Spinal Disord Tech. 2008;21(8):563–568. doi: 10.1097/BSD.0b013e31815d896c. [DOI] [PubMed] [Google Scholar]
- 96.Hara N, Oka H, Yamazaki T, et al. Predictors of residual symptoms in lower extremities after decompression surgery on lumbar spinal stenosis. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the. Cerv Spine Res Soc. 2010;19(11):1849–1854. doi: 10.1007/s00586-010-1374-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim HJ, Lee HM, Kim HS, et al. Life expectancy after lumbar spine surgery: One- to eleven-year follow-up of 1015 patients. Spine. 2008;33(19):2116–2121. doi: 10.1097/BRS.0b013e31817e1022. (Article) (In English) [DOI] [PubMed] [Google Scholar]
- 98.Yaldiz C, Yaldiz M, Ceylan N, et al. Retrospective, Demographic, and Clinical Investigation of the Causes of Postoperative Infection in Patients With Lumbar Spinal Stenosis Who Underwent Posterior Stabilization. Medicine (Baltimore) 2015;94(29):e1177. doi: 10.1097/MD.0000000000001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gepstein R, Arinzon Z, Adunsky A, Folman Y. Decompression surgery for lumbar spinal stenosis in the elderly: preoperative expectations and postoperative satisfaction. Spinal Cord. 2006;44(7):427–431. doi: 10.1038/sj.sc.3101857. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med5&NEWS=N&AN=16304562 [DOI] [PubMed] [Google Scholar]
- 100.Shabat S, Arinzon Z, Gepstein R, Folman Y. Long-term follow-up of revision decompressive lumbar spinal surgery in elderly patients. J Spinal Disord Tech. 2011;24(3):142–145. doi: 10.1097/BSD.0b013e3181de4b61. [DOI] [PubMed] [Google Scholar]
- 101.Shabat S, Folman Y, Arinzon Z, Adunsky A, Catz A, Gepstein R. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the. Cervical Spine Res Soc. 2005;14(10):1027–1032. doi: 10.1007/s00586-004-0808-z. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med5&NEWS=N&AN=15912353 [DOI] [PubMed] [Google Scholar]
- 102.Arinzon ZH, Fredman B, Zohar E, et al. Surgical management of spinal stenosis: a comparison of immediate and long term outcome in two geriatric patient populations. Arch Gerontol Geriatr. 2003;36(3):273–279. doi: 10.1016/s0167-4943(02)00172-3. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med4&NEWS=N&AN=12849082 [DOI] [PubMed] [Google Scholar]
- 103.Nanjo Y, Nagashima H, Dokai T, et al. Clinical features and surgical outcomes of lumbar spinal stenosis in patients aged 80 years or older: a multi-center retrospective study. Arch Orthop Trauma Surg. 2013;133(9):1243–1248. doi: 10.1007/s00402-013-1808-4. [DOI] [PubMed] [Google Scholar]
- 104.Minamide A, Yoshida M, Iwahashi H, et al. Minimally invasive decompression surgery for lumbar spinal stenosis with degenerative scoliosis: Predictive factors of radiographic and clinical outcomes. J Orthopaed Sci : Off J Japanese Orthopaed Assoc. 2017;22(3):377–383. doi: 10.1016/j.jos.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 105.Choi JM, Choi MK, Kim SB. Perioperative Results and Complications after Posterior Lumbar Interbody Fusion for Spinal Stenosis in Geriatric Patients over than 70 Years Old. J Korean Neurosurg Soc. 2017;60(6):684–690. doi: 10.3340/jkns.2017.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yamada K, Satoh S, Abe Y, Yanagibashi Y, Hyakumachi T, Masuda T. Diffuse Idiopathic Skeletal Hyperostosis Extended to the Lumbar Segment is a Risk Factor of Reoperation in Patients Treated Surgically for Lumbar Stenosis. Spine. 2018 doi: 10.1097/BRS.0000000000002618. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.