Abstract
Cardiovascular outcome trials revealed cardiovascular benefits for type 2 diabetes mellitus patients when treated with long‐acting glucagon‐like peptide‐1 (GLP‐1) receptor agonists. In the last decade, major advances were made characterising the physiological effects of GLP‐1 and its action on numerous targets including brain, liver, kidney, heart and blood vessels. However, the effects of GLP‐1 and receptor agonists, and the GLP‐1 receptor on the cardiovascular system have not been fully elucidated. We compare results from cardiovascular outcome trials of GLP‐1 receptor agonists and review pleiotropic clinical and preclinical data concerning cardiovascular protection beyond glycaemic control. We address current knowledge on GLP‐1 and receptor agonist actions on the heart, vasculature, inflammatory cells and platelets, and discuss evidence for GLP‐1 receptor‐dependent versus independent effects secondary of GLP‐1 metabolites. We conclude that the favourable cardiovascular profile of GLP‐1 receptor agonists might expand their therapeutic use for treating cardiovascular disease even in non‐diabetic populations.
LINKED ARTICLES
This article is part of a themed issue on GLP1 receptor ligands (BJP 75th Anniversary). To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v179.4/issuetoc
Keywords: cardiovascular outcome trial, cardiovascular protection, diabetes mellitus, GLP‐1 metabolites, glucagon‐like peptide‐1 receptor
Abbreviations
- AMPK
AMP‐activated protein kinase
- ANP
atrial natriuretic peptide
- Apoe
apolipoprotein E
- CANTOS
Canakinumab Anti‐inflammatory Thrombosis Outcome Study
- CCL20
chemokine (C‐C motif) ligand 20
- CD4+
cluster of differentiation 4
- CI
confidence interval
- CM
cardiomyocyte
- creatine kinase‐MB
creatine kinase‐muscle‐brain type
- CREB
cAMP response element‐binding protein
- CRP
C‐reactive protein
- CV
cardiovascular
- CVD
cardiovascular disease
- EC
endothelial cell
- ELIXA
Evaluation of Lixisenatide in Acute Coronary Syndrome
- eNOS
endothelial NOS
- FDA
US Food and Drug Administration
- FIGHT and LIVE
Functional Impact of GLP‐1 for Heart Failure Treatment (FIGHT) Effect of Liraglutide on Left Ventricular Function in Stable Chronic Heart Failure Patients with and without Diabetes (LIVE)
- GLP‐1
glucagon‐like peptide‐1 [GLP‐1(7–36)amide and GLP‐1(7–37)]
- ICAM‐1
intercellular adhesion molecule 1
- LEADER
Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results
- L‐NAME
L‐NG‐nitroarginine methyl ester
- L‐NNA
L‐NG‐nitroarginine
- LV
left ventricular
- LVDP
LV developed pressure
- LVEF
LV ejection fraction
- MACE
major adverse cardiovascular event
- MI
myocardial infarction
- MMP‐9
matrix metallopeptidase 9
- NO
nitric oxide
- REWIND
Researching Cardiovascular Events with a Weekly Incretin in Diabetes (dulaglutide trial)
- sAC
soluble adenylyl cyclase
- SAVOR‐TIMI 53
Saxagliptin Assessment of Vascular Outcomes Recorded (SAVOR)–Thrombolysis in Myocardial Infarction (TIMI)
- sGC
soluble guanylate cyclase
- SGLT2
sodium‐glucose cotransporter 2
- SUSTAIN‐6
semaglutide trial
- T2DM
type 2 diabetes mellitus
- TECOS
Trial Evaluating Cardiovascular Outcomes with Sitagliptin
- tmAC
transmembrane adenylyl cyclase
- TPM
transcripts per million
- VCAM‐1
vascular cell adhesion molecule 1
1. INTRODUCTION
Glucagon‐like peptide‐1 (GLP‐1) is a gut hormone secreted from enteroendocrine cells in the intestine. It derives from posttranslational processing of proglucagon and exerts various metabolic actions on multiple organs and tissues through a family B G‐protein‐coupled receptor (GPCR), the GLP‐1 receptor. In 1987, Jens Holst (Denmark) and Joel Habener (USA) first described and identified the native peptides GLP‐1 (7–36, amide extended and 7–37, glycine extended, collectively referred as GLP‐1) as secretory products from enteroendocrine cells in the gut of mammals (Holst et al., 1987; Mojsov et al., 1987). Further preclinical and clinical studies demonstrated that GLP‐1 is a potent insulinotropic agent in healthy rodents and humans with type 2 diabetes mellitus (T2DM). Due to the fact that GLP‐1 lowered plasma blood glucose in subjects with persistent hyperglycaemia, it has become the most extensively studied gut‐derived hormone (Nauck, Kleine, et al., 1993). GLP‐1 stimulates glucose‐dependent insulin secretion from beta cells in the pancreas, reduces glucagon secretion from alpha cells and slows down gastric emptying, which prohibits postprandial hyperglycaemia (Nauck, Heimesaat, et al., 1993; Wettergren et al., 1993). However, a pharmaceutical use of GLP‐1 as a new treatment option in T2DM has been complicated by the fact that it is rapidly proteolytically degraded by the ubiquitous protease dipeptidyl peptidase‐4 (DPP‐4) (see Figure 1). Therefore, GLP‐1 receptor agonists resistant to the proteolytic inactivation by DPP‐4 (e.g. exendin‐4, liraglutide and semaglutide) or inhibitors of DPP‐4 (e.g. sitagliptin and linagliptin) were developed and are nowadays established guideline therapy of T2DM.
FIGURE 1.
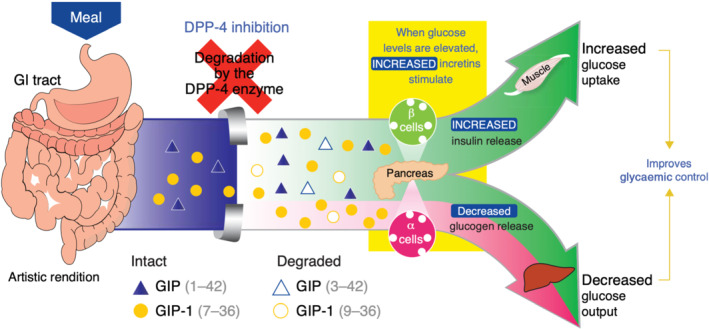
Glucagon like‐peptide‐1 (GLP‐1) and glucose‐dependent insulinotropic peptide (GIP) release after food uptake and degradation by dipeptidyl peptidase‐4 (DPP‐4). From (Herman et al., 2007) with permission. © 2007 American Society for Clinical Pharmacology and Therapeutics
The treatment of T2DM is of utmost importance since diabetic health complications are associated with 1.5 million global deaths per year plus an additional 2.2 million deaths by an indirect increase of risk factors that mostly comprise cardiovascular (CV) events such as myocardial infarction (MI) or stroke (Disease et al., 2018). Accordingly, negative CV side effects are a major risk for diabetic individuals, which is supported by a meta‐analysis reporting a hazard ratio of 3.42 (95% confidence interval [CI]: 2.23–5.23) for CV mortality of diabetic individuals (Nakagami et al., 2006). In order to obtain approval of GLP‐1 receptor agonists as a new treatment option of T2DM from the US Food and Drug Administration (FDA), clinical studies had to be conducted to test the CV safety compared with standard care and placebo in the target population resulting in a number of prominent cardiovascular outcome trials. The typical primary endpoint was the occurrence of a major adverse cardiovascular event (MACE: time to the first event of either CV death or non‐fatal MI or stroke). Among all GLP‐1receptor agonists that underwent the approval process, four (albiglutide, dulaglutide, semaglutide and liraglutide) overachieved the study goal. They were not only safe, but they significantly reduced MACE in diabetic patients at risk or with established cardiovascular disease (CVD) (Table 1). These promising results from the cardiovascular outcome trials still challenge the scientific field to better understand the mechanisms behind these outstanding beneficial effects. One important point is certainly a modification of CV risk factors by GLP‐1 therapy. Blood pressure reduction, weight loss and improved blood lipids are likely to contribute to the observed favourable CV outcomes. However, the fact that GLP‐1 receptor agonists have anti‐inflammatory and anti‐oxidant effects and other direct effects on cells and tissues of the CV system (e.g. epigenetic changes) should not be underestimated.
TABLE 1.
Major safety endpoints of selected GLP‐1 agonists tested in cardiovascular outcome trials
| Cardiovascular outcome trials of selected GLP‐1 agonists | ||||||
|---|---|---|---|---|---|---|
| Trial | ELIXA | LEADER | SUSTAIN‐6 | EXSCEL | REWIND | HARMONY |
| Lixisenatide | Liraglutide | Semaglutide | Exenatide | Dulaglutide | Albiglutide | |
| Primary outcome | 1.02 (0.89–1.17) P < .001 for noninferiority P = .81 for superiority CV death, MI, stroke | 0.87 (0.78–0.97) P < .001 for noninferiority P = .01 for superiority CV death, MI, stroke | 0.74 (0.58–0.95) P < .001 for noninferiority P = .02 for superiority CV death, MI, stroke | 0.91 (0.83–1.00) P < .001 for noninferiority P = .06 for superiority CV death, MI, stroke | 0.88 (0.79–0.99) P = .026 for superiority CV death, MI, stroke, UA | 0.78 (0.68–0.90) P < .0001 for noninferiority P = .0006 for superiority CV death, MI, stroke |
| Secondary outcome | 1.0 (0.90–1.11) P = .96 CV death, MI, stroke, UA, HF hosp., revascul. | 0.88 (0.81–0.96) P = .005 CV death, MI, stroke, UA or HF hosp., revascul. | 0.74 (0.62–0.89) P = .002 CV death, MI, stroke, UA or HF hosp., revascul. | n/a | n/a | 0.78 (0.69–0.90) P = .0005 CV death, MI, stroke, urgent revascul. For UA, individual components of the primary endpoint, CV death/hospital admission because of heart failure |
| CV death | 0.98 (0.78–1.22) P = .85 | 0.78 (0.66–0.93) P = .007 | 0.98 (0.65–1.48) P = .92 | 0.88 (0.76–1.02) | 0.91 (0.78–1.06) P = .21 | 0.93 (0.73–1.19) P = .578 |
| All cause‐death | 0.94 (0.78–1.13) P = .5 | 0.85 (0.74–0.97) P = .02 | 1.05 (0.74–1.50) P = .79 | 0.86 (0.77–0.97) | 0.90 (0.80–1.01) P = .067 | 0.95 (0.79–1.16) P = .644 |
| HF hospitalisation | 0.96 (0.75–1.23) P = .75 | 0.87 (0.73–1.05) P = .14 | 1.11 (0.77–1.61) P = .57 | 0.94 (0.78–1.13) | 0.93 (0.77–1.12) a P = .46 | 0.85 (0.70–1.04) b P = .113 |
| Reduction of glycated haemoglobin | −0.27 pps (−0.31 to −0.22) P < .001 | −0.40 pps (−0.45 to −0.34) | 0.5 mg semaglutide: −0.7 pps 1 mg semaglutide: −1.0 pps | −0.53% (−0.57 to −0.50) P < .001 | −0.61% (−0.65 to −0.58) P < .0001 | Difference at 8 months: −0.63% (−0.69 to −0.58) difference at 16 months: −0.52% (−0.58 to −0.45) |
Note: GLP‐1 receptor agonists revealing a CV benefit relative to standard care are marked in bold.
Abbreviations: CV death, cardiovascular death; HF, heart failure; hosp., hospitalisation; MI, myocardial infarction; n/a, not available; pps, percentage points; revascul., revisualisation; UA, unstable angina.
Hospital admission for heart failure or urgent visit.
Composite of death from cardiovascular causes or hospital admission for heart failure, ELIXA trial (Pfeffer et al., 2015), LEADER trial (Marso, Daniels, et al., 2016), SUSTAIN‐6 trial (Marso, Bain, et al., 2016), EXSCEL trial (Holman et al., 2017), REWIND trial (Gerstein et al., 2019), HARMONY trial (Hernandez et al., 2018).
GLP‐1 binds to the GLP‐1 receptor, a GPCR that stimulates the adenylyl cyclase pathway in pancreatic cells, resulting in insulin synthesis and subsequent release to the bloodstream (Drucker et al., 1987). Thereby, GLP‐1 and GLP‐1 receptor agonists primarily exert their effects on blood glucose control of T2DM patients. Today, plenty of studies have shown that GLP‐1 acts on several organs and tissues. In some cases, it is not clear whether these effects are directly mediated via the GLP‐1 receptor, but the GLP‐1 receptor has been shown to be expressed on many cell types, also in the CV system. Figure 2 provides information on the transcripts per million (TPM) for GLP‐1 receptor gene (GLP1R) expression in human tissues located in the CV system (blood, heart and vessels). As a reference, the highest expression can be found in the pancreas with 4.7 TPM, whereas 1.7 TPM have been detected in the atrial appendage of the heart and no transcripts in aortic tissue.
FIGURE 2.
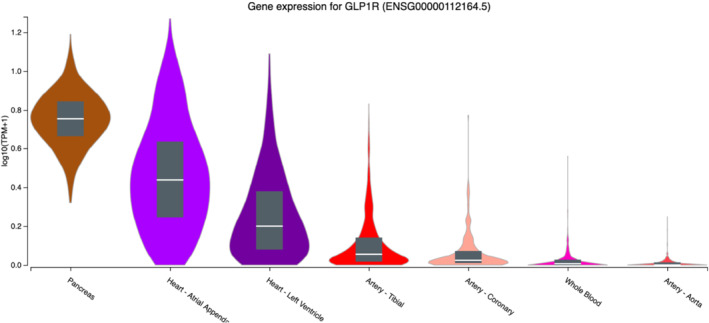
Transcripts per million (TPM) of the GLP1R gene in selected tissues related to the cardiovascular system (from GTEx Portal https://gtexportal.org/home/gene/GLP1R on 04/01/2021). The Genotype‐Tissue Expression (GTEx) project was supported by the Common Fund of the Office of the Director of the National Institutes of Health, and by NCI, NHGRI, NHLBI, NIDA, NIMH and NINDS). ©2020 The Broad Institute of MIT and Harvard
Besides the various expression patterns in rodent models and humans, the mechanistic exploration of direct/indirect CV effects of GLP‐1 is complicated by the fact that many GLP‐1 receptor antibodies and antisera exhibit suboptimal sensitivity and a lack of specificity (Panjwani et al., 2013).
In the present review, we will summarise the recent mechanistic evidence for CV protective effects of GLP‐1 receptor agonists, beyond glycaemic control. Special focus will be laid on pathways activated by direct GLP‐1 receptor interaction and the evidence given for predominant cell types within the CV system susceptible to GLP‐1 receptor activation.
2. CLINICAL EVIDENCE FOR CV PROTECTIVE EFFECTS OF GLP‐1 RECEPTOR AGONISTS
In the past, several glucose‐lowering drugs were approved by the FDA based on relatively short trials, which focused on glucose‐lowering effects in a healthy study population. The latter circumstances and the fact that many human subjects suffering from T2DM are at increased risk for CVD or even accumulate several CV risk factors, raised the suspicion of several approved glucose‐lowering drugs, such as the insulin‐sensitiser rosiglitazone, to increase the risk for adverse CV events (Nissen et al., 2005; Nissen & Wolski, 2007). Thus, since 2008, the approval of new anti‐diabetic drugs by the FDA requires evidence for CV safety. Therefore, several clinical trials are now available for DPP‐4 inhibitors, sodium‐glucose cotransporter 2 (SGLT2) inhibitors and GLP‐1 receptor agonists, all of which were designed to test CV safety but not superiority to standard care therapy of T2DM. Inhibition of DPP‐4 by saxagliptin, alogliptin, or sitagliptin in the clinical trials SAVOR‐TIMI 53 (Scirica et al., 2013), EXAMINE (alogliptin trial White et al., 2013) and TECOS (J. B. Green et al., 2015), respectively, did not increase the risk of CV death, MI or stroke in patients suffering from T2DM and at high risk for CV events. The studies were able to confirm the CV safety of DPP‐4 inhibition, but no additional or even beneficial effect on the CV outcome of T2DM patients was found. Unlike DPP‐4 inhibitors, SGLT2‐inhibitors and some of the investigated GLP‐1 receptor agonists did demonstrate an additive effect on top of standard care in human subjects with T2DM and reduced the onset of MACE in these patients. This body of evidence led to the recommendation of the European Society of Cardiology (ESC) to treat T2DM patients with CVD with an SGLT2‐inhibitor or a GLP‐1 receptor agonist on class 1, level A in their recent guidelines for the treatment of chronic coronary syndrome (Knuuti et al., 2020). Other societies such as the American Diabetes Association (ADA) recommend treatment of patients at high‐risk for CVD with a GLP‐1 receptor agonist (American Diabetes Association, 2020). According to this guideline, patients suffering from heart failure or chronic kidney disease should be treated with an SGLT2 inhibitor. Since the latter have a diuretic effect, heart failure seems to be a reasonable indication, but it remains questionable whether ischemic‐cardiomyopathy can always be clearly differentiated from heart failure by other reasons. In many cases, there might be an overlap of CVD and heart failure. On the other hand, there are still more conservative societies such as the National Institute for Health and Care Excellence (NICE, UK), which still recommend initial treatment with classical diabetic therapy such as metformin independent of the patient's CV risk.
2.1. Cardiovascular outcome trials of GLP‐1 receptor agonists
Today, several GLP‐receptor agonists are approved for the treatment of T2DM (exenatide since 2005, liraglutide since 2010, lixisenatide since 2016, albiglutide since 2014 but withdrawn from the market for economic reasons, dulaglutide since 2014 and semaglutide since 2017). CV benefits are proven for dulaglutide (REWIND), albiglutide (HARMONY OUTCOMES), semaglutide (SUSTAIN‐6) and liraglutide (LEADER) (see Table 1).
In the LEADER trial, 9340 patients underwent randomisation and the mean follow‐up was 3.8 years. Treatment of T2DM with liraglutide additionally to standard care reduced the risk for CV death, MI or stroke by 13% (hazard ratio, 0.87; 95% CI, 0.78–0.97; P < .001 for noninferiority; P = .01 for superiority). All‐cause mortality and death from CV causes were both significantly reduced by treatment with liraglutide. The study demonstrated that treatment with liraglutide directly reduces the rates of CV death (4.7% vs. 6.0%; hazard ratio, 0.78; 95% CI, 0.66–0.93), MI (hazard ratio, 0.86; 95% CI, 0.73–1.00; P = .046) and stroke (hazard ratio, 0.86; 95% CI, 0.71–1.06). The study included patients with an increased risk for CVD, as subjects were older than 50 years with at least one CV coexisting condition (coronary heart disease, cerebrovascular disease, peripheral vascular disease, chronic kidney disease of stage 3 or greater, or chronic heart failure of New York Heart Association class II or III). Besides, a majority of the patients (81.3%) already had an established CVD and the average duration of diabetes was 12.8 years with a mean glycated haemoglobin level of 8.7% (Marso, Daniels, et al., 2016). The results on CV events in the study are promising; however, no beneficial effects on hospitalisation for heart failure were found (Marso, Daniels, et al., 2016).
Unlike liraglutide, which has to be administered subcutaneously daily, weekly subcutaneously or even oral administration (Husain et al., 2019) in the future is sufficient for the GLP‐1 receptor agonist semaglutide. A clinical study to ensure CV safety was also carried out for this GLP‐1 receptor agonist. The SUSTAIN‐6 trial exceeded expectations as the investigation of 3297 T2DM patients at increased risk for CVD (83% with established CVD) revealed that semaglutide is superior to standard therapy with regard to the CV endpoint. Similar to liraglutide, semaglutide decreased the combined primary composite outcome (CV death, nonfatal MI or stroke) (6.6% vs. 8.9%; hazard ratio, 0.74; 95% CI, 0.58–0.95). Semaglutide did not reduce death by CV causes and reduction of the primary endpoint was therefore driven by the reduction of nonfatal MI (2.9% vs 3.9%) and stroke (1.6% vs 2.7%). As well as semaglutide, dulaglutide has to be administered once weekly. The REWIND study demonstrated dulaglutide to reduce the primary composite outcome by 12% (hazard ratio 0.88; 95% CI, 0.79–0.99; P = .026) compared to standard care (Gerstein et al., 2019). All‐cause mortality did not differ between groups. Another GLP‐1 receptor agonist with demonstrated CV superiority is albiglutide. The HARMONY trial enrolled 9436 patients and compared standard care therapy with additional albiglutide application in T2DM patients. In line with the other mentioned trials, it has been shown that albiglutide reduces CV events (Hernandez et al., 2018). However, in 2018, albiglutide was withdrawn from the market due to economic reasons.
CV safety was demonstrated for all approved GLP‐1 receptor agonists (Table 1), but the reason why only the mentioned four GLP‐1 receptor agonists (liraglutide, semaglutide, dulaglutide and albiglutide) showed CV protective effects are not fully understood. One explanation might be the differences in the pharmacology of the drugs. Liraglutide and semaglutide significantly reduced CV events by 13% (Marso, Daniels, et al., 2016) and 26% (Marso, Bain, et al., 2016), respectively, but lixisenatide (ELIXA) did not have any effect on the CV outcome (hazard ratio, 1.02; 95% CI, 0.89–1.17) (Pfeffer et al., 2015). Contrary to liraglutide and semaglutide, the half‐life of lixisenatide is shorter (t 1/2 = 3 h) (Andersen et al., 2018), which could explain why this GLP‐1 receptor agonist is not in favour compared to long‐acting GLP‐1 receptor agonists regarding the CV outcome (Meier et al., 2015). Besides, it could also be that differences in study design contribute to the different study results, for example, the ELIXA trial only included patients with a recent acute coronary syndrome. It should be mentioned that there are also differences between the GLP‐1 receptor agonists that have demonstrated an improved CV outcome. In the clinical trials investigating liraglutide and albiglutide, reduction of MACE was mainly driven by a reduction of MI, whereas dulaglutide and semaglutide predominantly reduced stroke (for review, see Drucker, 2018b). Table 1 summarises the results of cardiovascular outcome trials for selected GLP‐1 receptor agonists and highlights the ones that demonstrated superiority relative to standard care therapy.
The mechanisms of CV protection can not only be explained by improved glycaemic control since the achieved HbA1c reduction was relatively small (mostly <0.7%; see Table 1) and optimisation of hyperglycaemia usually takes a time to benefit the primary CV outcome as demonstrated in UKPDS trial (a 15% reduction in MI emerged over 10 years) (Holman et al., 2008). Furthermore, GLP‐1 recetor receptor agonists also improve CV risk factors such as blood pressure (Andreadis et al., 2018), body weight (Andreadis et al., 2018) and hyperlipoproteinaemia (Hermansen et al., 2013), which might explain the protective effects on the CV system. Direct and indirect effects of GLP‐1 receptor agonists on different organs and tissues involved in the pathogenesis of CVD have been described, that might explain the superiority of incretin mimetics in treatment of T2DM as a CV risk factor. In the following, we will discuss these “nondiabetic” effects from a clinical and preclinical perspective.
2.2. GLP‐1 receptor agonists in CVD and arterial hypertension
RNAseq analyses of human tissue have revealed the presence of GLP1R mRNA in atrial, left ventricular (LV), arterial (coronary and tibial) but not aortic tissue (Figure 2; https://www.gtexportal.org/home/gene/GLP1R#gene-transcript-browser-block). However, the presence of a functional GLP‐1 receptor in human coronary arteries is not clearly established (Drucker, 2016). The cardiovascular outcome trials investigated T2DM patients with either MI in their medical history or at high risk for MI and showed that they can reduce the onset of such events. In the acute setting of MI, it has been observed that 72‐h infusion of GLP‐1 peptide improves post‐MI left ventricular ejection fraction (LVEF) and global wall motion indices, independently of diabetes in the medical history of the patients (Nikolaidis et al., 2004). A larger trial with more than 170 patients with acute MI investigated the effect of the GLP‐1 receptor agonist exenatide. Patients received standard care including percutaneous coronary intervention and an exenatide infusion 15 min before and maintained for 6 h after intervention. In this study, acute infusion of exenatide reduced infarct size after MI (Lonborg et al., 2012). In another study, it has been reported that, besides infarction size and improved LVEF, also the releases of creatine kinase‐MB and troponin I were significantly reduced after exenatide application (Woo et al., 2013). Furthermore, also liraglutide treatment reduced the infarction size and improved LVEF fraction after percutaneous intervention in ST‐segment elevation and non‐ST‐segment elevation MI (Chen et al., 2015; Chen, Chen, et al., 2016; Chen, Shen, et al., 2016). One explanation for these findings might be a protective effect of GLP‐1 on ischaemia‐induced LV dysfunction. In a small study, it has been shown that GLP‐1 peptide (GLP‐1(7–36)amide) improves stunning and LV dysfunction after balloon occlusion of the left anterior descending artery, without any change in myocardial glucose extraction (McCormick et al., 2015). On the other hand, exenatide was shown to improve glucose uptake by the myocardium in T2DM patients (Gejl et al., 2012). Endothelial dysfunction correlates with the risk for CV events and can also be found in acute MI. Intravenous GLP‐1 augmented endothelial function and the GLP‐1 receptor agonist liraglutide improved acetylcholine‐induced vasodilation (Nandy et al., 2014; Nystrom et al., 2004). The potential mechanisms for the improved endothelial function will be discussed in more detail in Section 3.
Inflammation is an important driver and part of the pathophysiology of CVD and atherosclerosis. The Canakinumab Anti‐inflammatory Thrombosis Outcome Study (CANTOS) trial demonstrated that suppression of the IL‐1β pathway by a specific antibody can reduce the onset of CV events in a similar observational period as GLP‐1 receptor agonists in their trials (SUSTAIN‐6, LEADER) (Ridker et al., 2017). However, in the CANTOS trial, the suppression of the inflammatory system led to an increase of infections, which abrogated the beneficial effect of reduced CV events on the all‐cause mortality. GLP‐1 and GLP‐1 receptor agonists also have anti‐inflammatory effects, that might explain their protective effects on the CV system. Exenatide and liraglutide reduced C‐reactive protein (CRP) levels in humans by 61% and 23%, respectively (Bunck et al., 2010; Plutzky et al., 2009). Furthermore, reduction of TNF‐α, IL‐6 and IL‐1β levels have been observed, reflecting the anti‐inflammatory effects of this class of drugs (Chaudhuri et al., 2012; Hogan et al., 2014).
Arterial hypertension is one of the most important risk factors for CVD. Clinical trials for almost all GLP‐ receptor agonists, except lixisenatide, demonstrated a systolic blood pressure lowering effect with an average of 2–3 mm Hg (Robinson et al., 2013; Sun et al., 2015), whereas the diastolic blood pressure is less affected. In experimental animal studies, a link between GLP‐1 receptor signalling, atrial natriuretic peptide (ANP) and renal sodium excretion has been proposed. This interaction has not been clearly shown for humans. In the FIGHT and LIVE study, which investigated the effect of liraglutide in patients with heart failure, no difference in ANP levels were found. Furthermore, liraglutide did not lead to greater post‐hospitalisation clinical stability in patients recently hospitalised with heart failure and reduced LVEF, which might be driven by GLP‐1 receptor agonist‐mediated heart rate increase (Liang & Gu, 2020; Margulies et al., 2016). Nevertheless, in healthy, obese and T2DM patients, treatment with GLP‐1 receptor agonists induces natriuresis, which might contribute to blood pressure reduction (Lovshin et al., 2015). On the other hand, elevated nitric oxide (NO) bioavailability by reduced oxidative stress and induction of endothelial NOS (eNOS) could also explain the blood pressure reduction.
3. THE GLP‐1 RECEPTOR AND ITS MECHANISTIC ROLE IN CV PROTECTION
The GLP‐receptor was first isolated by expression cloning from a rat pancreatic islet cDNA library and belongs to the class B family of GPCR (Thorens, 1992). Accordingly, GLP‐1 receptor predominantly signals through the Gs alpha subunit (Gsα), activating the transmembrane adenylyl cyclase (tmAC), thereby upregulating intracellular cyclic AMP (cAMP) that subsequently targets protein kinase A (PKA) or Epac (exchange protein activated by cAMP) (Donnelly, 2012; Graaf et al., 2016).
An important and still not completely answered question is whether all of the actions attributed to native GLP‐1 and GLP‐1 receptor agonists depend on GLP‐1 receptor activity or possibly involve GLP‐1 receptor‐independent actions (see Section 4). Hence, precise tissue and cell type localisation of the GLP‐1 receptor is important for mechanistic interpretations of the CV actions of GLP‐1 and GLP‐1 receptor agonists, which can be almost exclusively investigated in preclinical models (Figure 3).
FIGURE 3.
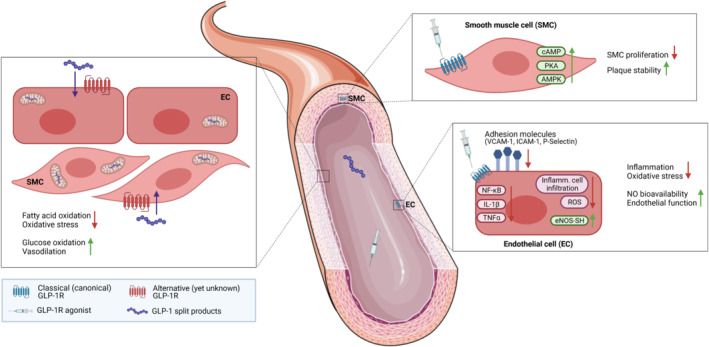
Proposed cellular targets and protective effects of GLP‐1 receptor agonists (GLP‐1RAs) and GLP‐1 split products in the vasculature. The benefits are likely mediated by a combination of GLP‐1 receptor‐dependent mechanisms on endothelial and smooth muscle cells (ECs, SMCs) and GLP‐1 receptor‐independent actions of GLP‐1 metabolites. The existence of a second, yet not discovered, GLP‐1 receptor has been proposed. The figure was created with BioRender (https://biorender.com) and by using elements from Servier Medical Art (https://smart.servier.com), which is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/)
3.1. GLP‐1 receptor and the heart
Current evidence from several species restricts GLP‐1 receptor expression to the cardiac atrium. In mice, reporter gene expression using GLP1R fluorescent labelling localised the GLP‐1 receptor to atrial cardiomyocytes (CMs) (Richards et al., 2014), whereas a study in primates and humans limited cardiac GLP‐1 receptor expression to the sinoatrial node (Pyke et al., 2014). Though, (low level) GLP‐1 receptor expression remains possible in non‐CM cell types within the ventricles (nerves, fibroblasts and immune cells) and was reported in vascular smooth muscle cells (VSMCs) within ventricular blood vessels (see Section 3.2). Furthermore, context‐dependent translational control of ventricular GLP‐1 receptor activity cannot be ruled out (Nauck et al., 2017; Ussher & Drucker, 2014).
GLP‐1 and GLP‐1 receptor agonists potently reduce blood pressure, as shown in several experimental models of hypertension (Hirata et al., 2009; Laugero et al., 2009; Yu et al., 2003). One mechanism responsible for the anti‐hypertensive effect of GLP‐1 receptor agonists in rodents was revealed by M. Kim et al. (2013), who demonstrated lowered systolic and diastolic blood pressure in angiotensin II‐treated male C57BL/6J mice with liraglutide, actions that were dependent on direct GLP‐1 receptor activation on atrial CMs mediating ANP secretion. In addition to that, we recently identified a vascular target, specifically the endothelial GLP‐1 receptor, to control NO bioavailability, contributing to the anti‐hypertensive effects in murine arterial hypertension (see Section 3.2). It can be speculated that this mechanism might be also relevant in humans since GLP‐1 receptor agonists robustly lower blood pressure in diabetic individuals (see Section 2.2), yet consistent reports of ANP release upon treatment in humans are lacking (Li et al., 2014; Lovshin et al., 2015). Together with the fact that GLP‐1 receptor expression in the human heart is restricted to the sinoatrial node, growing evidence links reductions in blood pressure to cardiac‐independent GLP‐1 receptor activation.
Preclinical studies in mice, rats, pigs and dogs uniformly demonstrated that GLP‐1, GLP‐1 receptor agonists and DPP‐4 inhibitors exert salutary effects in experimental models of acute MI by reducing the area of necrosis (Bose et al., 2005; Ihara et al., 2015; Noyan‐Ashraf et al., 2009; Sauve et al., 2010; Timmers et al., 2009) (extensively reviewed in Drucker, 2016; Ussher & Drucker, 2014). Furthermore, therapeutic benefits of native GLP‐1 and GLP‐1 receptor agonists were described in experimental models of ventricular dysfunction and heart failure (Liu et al., 2010; Poornima et al., 2008; Vyas et al., 2011; Withaar et al., 2020). Of note, even though cardioprotection is unquestioned with GLP‐1 receptor agonist treatment starting prior to induction of ischaemia, the beneficial effects of GLP‐1 receptor agonism after cardiac ischaemia are disputed, with preclinical studies describing different outcomes (Ussher et al., 2014; Wohlfart et al., 2013). This contrasts human studies that mainly report positive results with GLP‐1 receptor agonist therapy concomitant or following ischemic injury (see Section 2.2). Treatment in both models was associated with sustained or improved LV performance. As ventricular CMs do not express the GLP‐1 receptor, direct (GLP‐1 receptor‐dependent) cardiac protection would involve atrial GLP‐1 receptor activity. However, Ussher et al. demonstrated that the endogenous CM GLP‐1 receptor is dispensable for GLP‐1 receptor‐mediated cardioprotection. Mice with a selective knockdown of the atrial GLP‐1 receptor (Glp1r CM−/− ) were neither prone to enhanced mortality or adverse LV remodelling nor were the cardioprotective actions of liraglutide abolished in Glp1r CM−/− mice following left anterior descending (left anterior descending) coronary artery occlusion (Ussher et al., 2014). Thus, current data suggests that GLP‐1 receptor‐dependent cardioprotection in vivo results from indirect mechanisms. Indeed, several animal studies report an effect on cardiac fuel metabolism. Ban et al. (2008) showed GLP‐1 administration to increase cardiac glucose uptake in a myocardial ischaemia–reperfusion injury model in mice. In rodent models of ischaemia–reperfusion injury and diabetic cardiomyopathy, native GLP‐1 and GLP‐1 receptor agonists (albiglutide and liraglutide, respectively) enhanced glucose uptake and utilisation in the heart, which was associated with an energetic shift from fatty acid to enhanced glucose oxidation (Almutairi et al., 2021; Aravindhan et al., 2015; Bao et al., 2011). These metabolic profiles were accompanied by augmented coronary blood flow and improved contractile function. Although it is intuitive to attribute these regional changes in metabolism to indirect GLP‐1 receptor‐mediated effects on glycaemic control, other studies showed that the insulinotropic effects of GLP‐1 do not solely account for the observed effects. In dogs with dilated cardiomyopathy, GLP‐1(7–36)amide increased myocardial glucose uptake and improved LV performance, effects that were mimicked by the DPP‐4‐degradation product GLP‐1(9–36)amide (Nikolaidis et al., 2005). Numerous publications since then confirmed CV effects of truncated GLP‐1 peptides, likely by targeting CMs and/or endothelial cells (ECs) (see Section 4). However, there is also evidence for a vascular target in the heart depending on GLP‐1 receptor signalling, as studies demonstrated increased myocardial blood flow in diabetic patients with DPP‐4‐resistant GLP‐1 receptor receptor agonist exenatide (Gejl et al., 2012) and immediate cardioprotective action of GLP‐1 receptor agonist lixisenatide in isolated perfused hearts subjected to ischaemia–reperfusion injury (Wohlfart et al., 2013).
GLP‐1 receptor agonists exert a positive chronotropic effect in both rodents and humans, independently of metabolic parameters (Drucker, 2018a). The relative increase in heart rate has been correlated to the magnitude and duration of GLP‐1 receptor engagement (Baggio et al., 2017; Lorenz et al., 2017). Mechanistic studies in Glp1r CM−/− mice showed reductions in basal heart rate (Ussher et al., 2014) and attenuated chronotropic responses to liraglutide in vivo, but neither native GLP‐1 nor lixisenatide increased heart rate in perfused hearts ex vivo (Baggio et al., 2017). Hence, atrial GLP‐1 receptor activation on CMs contributes to the control of heart rate in mice but does not function in a heart‐autonomous manner, relying on inputs from the sympathetic and parasympathetic nervous system. This is also confirmed by the fact that GLP‐1 receptor activation effectively opposed the effects of β‐adrenoceptor stimulation on cardiac ventricular excitability and reduced ventricular arrhythmic potential. These actions on the ventricular myocardium were indirect (mediated by acetylcholine and nitric oxide), which might be also explained by stimulation of GLP‐1receptor bearing cardiac parasympathetic neurons (Ang et al., 2018). The indirect or remote mechanism of cardioprotection by GLP‐1 receptor activation is also evident in ischemic preconditioning. Herein, vagal nerves are required and mediated cardioprotection by a mechanism involving M3 muscarinic receptors (Basalay et al., 2016). The latter mechanism has also partly been demonstrated in humans, since remote ischemic conditioning protected against endothelial ischaemia–reperfusion injury in healthy males. The protective effect of remote ischemic conditioning was abolished by infusion of exendin(9–39), which might indicate an involvement of the GLP‐1 receptor (Verouhis et al., 2019). It seems likely that the GLP‐1 receptor‐dependent increase in heart rate in humans can be attributed to GLP‐1 receptor activity in the sinoatrial node in a similar way, even though it has not been causally studied. The possible functional consequences and risks (e.g. enhanced coronary blood flow and tachyarrhythmia) of prolonged increases in heart rate with GLP‐1 receptor agonist treatment are still uncertain (Nauck et al., 2017; Ussher & Drucker, 2014) and need further clarification, particularly in patients with manifest heart failure (Khan et al., 2020) (see also Section 2).
3.2. GLP‐1 receptor and the vessel
Several studies reported positive GLP‐1 receptor protein expression in human endothelial cell (EC) lines, including human umbilical vein ECs (HUVECs) and human aortic ECs (HAECs), though the detection of the authentic endogenous GLP‐1 receptor remains uncertain given the lack of specificity of many of the used GLP‐1 receptor antibodies (Pujadas & Drucker, 2016). Additionally, conclusive evidence of GLP‐1 receptor expression in ECs within intact blood vessels from specific vascular beds is to date missing (Drucker, 2018a; Nauck et al., 2017). With regard to vascular smooth muscle cells in the CV system, GLP‐1 receptor expression was demonstrated in vascular smooth muscle cells within ventricular blood vessels (Richards et al., 2014) and the thoracic artery (Kimura et al., 2017) from mice, whereas no GLP1R mRNA transcripts were detected in coronary artery vascular smooth muscle cells (and ECs) from human heart samples (Baggio et al., 2018). Conversely, GLP‐1 receptor expression was found in some vascular smooth muscle cells of renal arterioles in rodents and humans (Drucker, 2018a).
A number of in vitro studies demonstrated beneficial actions of native GLP‐1 and GLP‐1 receptor agonists on human umbilical vein ECs and human aortic ECs, effects that were associated with reductions of endothelial inflammation and oxidative stress as well as activation of the AMPK/Akt/eNOS pathway increasing NO production (Pujadas & Drucker, 2016). In preclinical studies, first indications of a vascular target by GLP‐1 receptor signalling emerged from reports of direct cardioprotective actions of GLP‐1 receptor agonists in ischaemia–reperfusion injury experiments ex vivo (Wohlfart et al., 2013; see Section 3.1) and increased myocardial, mesenteric or muscle microvascular blood flow by GLP‐1 treatment (Ban et al., 2008; Chai et al., 2012; Dokken et al., 2010). However, a direct vascular mechanism for the latter changes has not been fully unravelled yet since the improved blood flow might also result from increases in heart rate with GLP‐1 receptor activation (see Section 3.1) or involve a neural target (Cabou et al., 2011). In studies with native GLP‐1, the effect on blood vessels may be partially ascribed to GLP‐1 metabolites. In line with that, a direct vasodilatory effect was shown for GLP‐1(9–36)amide (see Section 4) but not for GLP‐1 receptor agonists, both ex vivo (M. Kim et al., 2013) and in vivo (Moberly et al., 2012).
Nevertheless, we recently identified the endothelial GLP‐1 receptor to mediate CV protection by GLP‐1 receptor agonist liraglutide in experimental arterial hypertension, independently of glycaemic control (Helmstadter et al., 2020). In a mechanistic study using male C57BL/6J mice treated with angiotensin II, we showed that liraglutide suppressed the angiotensin II‐induced inflammatory cascade in the vascular wall by downregulating NF‐κB signalling, leading to reduced expression of adhesion molecules (VCAM‐1, ICAM‐1, P‐selectin) on the surface of ECs. Consequently, fewer inflammatory cells (particularly inflammatory monocytes and neutrophils) infiltrated into the vascular wall, diminishing vascular oxidative stress. As a result, the redox‐sensitive enzyme eNOS was prevented from uncoupling, a known complication in hypertension, thus restoring NO bioavailability (Helmstadter et al., 2020). Given the importance of NO in maintaining a healthy vascular tone and reactivity, liraglutide‐treated mice were protected from an angiotensin II‐induced endothelial dysfunction and high blood pressure (see Figure 3). Importantly, the vascular protective effects were abolished in global Glp1r −/− and mice with selective disruption of GLP‐1 receptor expression in (pan‐) ECs (GLP1R ec −/− ). The anti‐inflammatory and anti‐oxidant properties of liraglutide also strongly mitigated vascular fibrosis and cardiac hypertrophy in angiotensin II‐treated wild type mice (Helmstadter et al., 2020), indicating that an endothelial target of GLP‐1 receptor activation indirectly contributes to GLP‐1 receptor‐dependent protection of the heart. Furthermore, in vitro studies demonstrated anti‐oxidant and anti‐proliferative actions of GLP‐1 receptor agonists on vascular smooth muscle cells (Pujadas & Drucker, 2016). In accordance with that, Jojima et al. (2017) reported suppressed atherosclerotic lesions by liraglutide treatment in high‐fat diet fed Apoe −/− mice, actions that were assigned to liraglutide‐induced AMPK signalling in vascular smooth muscle cells, thus inhibiting their proliferation (see Figure 3). The above‐mentioned mechanisms are relevant not only for protection of the heart from ischemic events by GLP‐1 signalling but also for the brain. Augmented blood flow and/or vascular anti‐inflammatory actions also reduce infarct volume and improve functional outcome (for review, see (Basalay et al., 2020; Maskery et al., 2021).
In conclusion, even though GLP‐1 receptor expression in ECs and vascular smooth muscle cells within major blood vessels is still debated, numerous studies demonstrated protective actions of GLP‐1 receptor agonists on vascular cells in vitro, ex vivo and in vivo. We recently identified the GLP‐1 receptor expressed on ECs to play an indispensable role in CV protection by liraglutide in angiotensin II‐induced murine hypertension, by conveying anti‐inflammatory and anti‐oxidant actions, thereby controlling NO bioavailability (Helmstadter et al., 2020) Thus, growing evidence suggests that the anti‐hypertensive and anti‐atherogenic effects of GLP‐1 receptor agonists reported in preclinical and cardiovascular outcome trials studies may be attributed to their actions on vascular/ECs.
3.3. GLP‐1 receptor and inflammatory cells
The CV benefits of liraglutide and semaglutide reported in the LEADER and SUSTAIN‐6 trial have been attributed to the reduction of atherosclerosis‐driven events by anti‐inflammatory mechanisms (Kaul, 2017; Marso, Bain, et al., 2016; Marso, Daniels, et al., 2016) (see also Section 2).
Reduced cardiac and vascular inflammation has been well documented for GLP‐1 and pharmacological GLP‐1 receptor agonists in several rodent and human studies (for detailed review, see Drucker, 2016). In preclinical studies, native GLP‐1, exendin‐4 and liraglutide attenuated the development of atherosclerosis and cardiac dysfunction in mice, actions that were associated with reduced vascular monocyte adhesion and/or macrophage infiltration within blood vessels (Arakawa et al., 2010; Nagashima et al., 2011; Noyan‐Ashraf et al., 2013). Our group demonstrated that the GLP‐1 receptor agonist liraglutide lowers vascular and systemic inflammation in a lipopolysaccharide‐induced sepsis model which improves survival, effects that were absent in septic Glp1r −/− mice (Steven et al., 2015, 2017). Besides, GLP‐1 and GLP‐1 receptor agonist exenatide exert anti‐oxidant actions, as shown by reduced formation of ROS in human umbilical vein ECs (R. Wang et al., 2015) and human monocytes/macrophages (Chaudhuri et al., 2012). Additionally, GLP‐1 receptor agonists (exenatide, liraglutide) and DPP‐4 inhibitor (sitagliptin) were reported to decrease the production of proinflammatory cytokines such as TNFα, IL‐1β and IL‐6 in human mononuclear cells (Chaudhuri et al., 2012; Hogan et al., 2014; Makdissi et al., 2012) and to significantly reduce plasma levels of the inflammatory biomarker CRP (Nauck et al., 2017). The endogenous anti‐inflammatory role of GLP‐1 is furthermore underlined by the fact that inflammatory signals regulate its synthesis, secretion and action (Drucker, 2016).
Whether direct GLP‐1 receptor signalling on immune cells or other cell types and/or GLP‐1 receptor‐independent actions (see Section 4) are responsible for the anti‐inflammatory effects of GLP‐1 is to date not fully elucidated. Even though the GLP‐1 receptor has been detected in circulating lymphocytes from thymus, spleen and bone marrow in mice (Hadjiyanni et al., 2010), the expression is limited to very low levels and is even more debated for murine monocytes and macrophages where GLP1R mRNA has been inconsistently reported (Kodera et al., 2011; Panjwani et al., 2013). In contrast, GLP‐1 receptor expression is assured and enriched on duodenal Brunner glands and intestinal intraepithelial lymphocytes (IELs) of the gastrointestinal system (Drucker, 2018b). In the former, liraglutide was shown to upregulate genes encoding barrier protective molecules such as IL‐33, mucin 5b and CCL20, thereby attenuating inflammatory bowel disease in mice (Bang‐Berthelsen et al., 2016). The activation of the GLP‐1 receptor on intestinal intraepithelial lymphocytes within the immune system has been related to local modulation of the intestinal inflammatory responses (Yusta et al., 2015). GLP‐1 receptor agonist exendin‐4 stimulated cAMP accumulation in intraepithelial lymphocytes, which suppressed the production of proinflammatory cytokines and led to an improved gut barrier function, an effect absent in Glp1r −/− mice. Although intestinal inflammation and alterations in intestinal integrity are associated with CVD (Witkowski et al., 2020), direct activation of the GLP‐1 receptor on gut immune cells has not been linked to reductions of cardiac or vascular inflammation (Drucker, 2018b). However, intraepithelial lymphocytes (integrin β7+) were recently shown to act as gatekeeper that limit the bioavailability of GLP‐1 (He et al., 2019). Due to their Glp1r high expression, intraepithelial lymphocytes residing in the small intestine bind thus capture secreted GLP‐1 from neighbouring enteroendocrine L cells and regulate systemically available GLP‐1. Consequently, integrin β7 −/− mice that lack natural intraepithelial lymphocytes had increased GLP‐1 plasma levels that were responsible for the protection from diet high in fat, sugar and salt‐induced CVD (He et al., 2019).
Elevated GLP‐1 plasma levels were also found in critically ill septic patients but, in this case, shown to correlate with the severity of illness and mortality of patients (Lebherz et al., 2017). The authors hypothesise that the GLP‐1 elevation is not causal for the increase of mortality but rather reflects a counterbalancing response of the massively activated the immune system. Hence, the ‘rectifying’ actions of GLP‐1 seem to depend on the severity of the inflammatory state. It cannot be excluded though that GLP‐1 receptor expression is upregulated in immune cells during inflammatory or atherosclerotic conditions (Drucker, 2018b). Yet, we recently demonstrated that the GLP‐1 receptor on myeloid cells is not needed for the anti‐inflammatory properties of GLP‐1 receptor agonist liraglutide in a murine model of arterial hypertension, as the beneficial CV effects of liraglutide persisted in mice with a myelomonocytic cell‐specific deletion of the GLP‐1 receptor (GLP1R my −/− ) (Helmstadter et al., 2020). Instead, we could highlight the importance of the endothelial GLP‐1 receptor for conveying vascular anti‐inflammatory actions leading to cardioprotection (see Section 3.2).
3.4. GLP‐1 receptor and thrombocytes
Current evidence associates GLP‐1 receptor stimulation with an inhibitory effect on platelets. Several preclinical studies demonstrated anti‐aggregatory effects on murine and human platelets ex vivo (Barale et al., 2017; Cameron‐Vendrig et al., 2016; Sternkopf et al., 2020; Steven et al., 2017) and reduced clot formation in rodents in vivo (Cameron‐Vendrig et al., 2016). However, the expression of the GLP‐1 receptor on platelets is debated, including its direct relevance for anti‐thrombotic events. Murine platelets were shown to express GLP1R mRNA (Sternkopf et al., 2020), albeit with low abundance, whereas data on GLP‐1 receptor expression on human platelets is inconsistent, with some studies confirming it on mRNA level (BLUEPRINT Consortium. BLUEPRINT progenitors, 2014; Cameron‐Vendrig et al., 2016) and some not (Burkhart et al., 2012; Schmidt et al., 2018; Sternkopf et al., 2020). Nonetheless, positive GLP‐1 receptor protein expression was demonstrated for both murine and human platelets (Barale et al., 2017; Steven et al., 2017), though the validity of the GLP‐ 1 receptor antibodies used in these studies remains uncertain.
The first indication of a direct/platelet GLP‐‐mediated inhibition of platelet aggregation was given by Cameron et al., who reported a dose‐dependent intracellular cAMP response to native GLP‐1 and GLP‐1 receptor agonist exenatide in the human megakaryocyte cell line MEG‐01 (Cameron‐Vendrig et al., 2016). Our group showed an increase of vasodilator‐stimulated phosphoprotein (VASP‐Ser157) phosphorylation in murine and human platelets when treated with the GLP‐1 receptor agonist exenatide (Steven et al., 2017). This effect was accompanied with increased cAMP levels and phosphorylation of PKA substrate (human platelets), whereas the effect was abolished in platelets from Glp1r −/− mice.
Furthermore, exenatide did not inhibit ex vivo thrombus formation in eNOS −/− mice (Cameron‐Vendrig et al., 2016). Even though the platelet‐inhibitory effect of NO (by activating the sGC/cGMP/PKG pathway) is undisputed (G. R. Wang et al., 1998), a GLP‐1 receptor‐dependent increase in NO has been shown in vascular cells (Ding & Zhang, 2012) but not in platelets and platelet eNOS expression is to date controversial (Gambaryan & Tsikas, 2015; Jia et al., 2016). Nevertheless, one proposed mechanism for the anti‐aggregatory effects of GLP‐1 receptor agonists includes GLP‐1 receptor activation on platelets, thereby inducing platelet eNOS (via cAMP/PKA/phospho‐eNOS at Ser1177) and platelet‐derived NO conveying the anti‐thrombotic effects (Jia et al., 2016).
Platelets of patients with CVD exhibit impaired responsiveness to NO (“platelet NO resistance”), induced by oxidative stress. Contributing factors involve enhanced clearance of NO by superoxide, oxidation thus inactivation of the soluble GC and uncoupling of the redox‐sensitive eNOS enzyme (Rajendran & Chirkov, 2008). Hence, the anti‐oxidant actions of GLP‐1 and GLP‐1 receptor agonists represent another possible platelet inhibitory mechanism. Indeed, GLP‐1 and GLP‐1 receptor agonist liraglutide reduced arachidonic acid‐induced oxidative stress within platelets, which was associated with increased NO‐anti‐aggregatory effects (Barale et al., 2017). As GLP‐1(9–36)amide produced the same effects, the authors attribute this mechanism to be GLP‐1 receptor independent, though. We recently identified GLP‐1 receptor activation on ECs to directly reduce oxidative stress and control NO bioavailability in the vasculature of hypertensive mice treated with liraglutide (see Section 3.2) (Helmstadter et al., 2020). The contribution of non‐bone‐marrow‐derived cell types, such as the endothelium, to the anti‐aggregatory and anti‐thrombotic effects of GLP‐1 was already suggested by Cameron‐Vendrig et al. (2016), as the anti‐thrombotic effects of exenatide were attenuated but not completely abrogated in mice transplanted with GLP‐1 receptor‐deficient bone marrow. Of note, in a recent study from Sternkopf et al. (2020), the authors identified native GLP‐1 to suppress thrombus formation under physiological flow conditions, yet without a main relevance of a platelet GLP‐1 receptor. As this study was performed ex vivo, this does not exclude the involvement of the GLP‐1 receptor on other cell types (e.g., the endothelial GLP‐1 receptor).
4. EVIDENCE FOR GLP‐1 RECEPTOR INDEPENDENT EFFECTS ON THE CV SYSTEM
Increasing evidence collected from in vivo, ex vivo and in vitro studies indicates that the degradation products of GLP‐1(7–36)amide may exert their own CV protective effects independent of the known GLP‐1 receptor. On the one hand, this might explain effects on cells and tissues in which the expression of a functional GLP‐1 receptor is questionable; on the other hand, it raises the question whether a second, yet not explored, GLP‐1 receptor exists (see Figure 3). However, the importance of GLP‐1 degradation products for the beneficial effects of GLP‐1 receptor agonists in clinical studies is questionable and needs further research since they are only minimal degradable.
GLP‐1(1–37) derives from the tissue‐specific posttranslational processing of proglucagon and is itself post‐translationally cleaved of six amino acids at the N‐terminus to native GLP‐1(7–37). In humans, in approximately 80% of GLP‐1(7–37) the C‐terminal glycine is removed and the penultimate arginine is then amidated by the peptidyl‐glycine alpha‐amidating monooxygenase, whereas in rodents the glycine‐extended form is predominant (50–60%) (Deacon, 2004; Orskov et al., 1994), leading to two insulinotropic forms of GLP‐1s that are able to bind to the GLP‐1 receptor (GLP‐1(7–37) and GLP‐1(7–36)amide, both referred as GLP‐1, if not specified). The circulation time of active GLP‐1 with a plasma half‐life of 1–2 min is very short since both peptides are quickly N‐terminally degraded by ubiquitous DPP‐4 to the truncated forms GLP‐1(9–37) and GLP‐1(9–36)amide (Kieffer et al., 1995). The latter are unable to activate or even partly antagonise the GLP‐1 receptor and therefore have no (or low) insulinotropic properties. Neutral endopeptidases (NEP 24.11, also neprilysin) further enzymatically cleave the peptides at internal C‐terminal sites producing GLP‐1(28–37), GLP‐1(28–36)amide, GLP‐1(32–37) and GLP‐1(32–36)amide (Guglielmi & Sbraccia, 2017).
The pharmacological approach to make use of the insulinotropic effects of GLP‐1 was to prevent the rapid degradation by engineering peptides, which are more stable or even resistant to enzymatic cleavage. Liraglutide is an acylated GLP‐1 receptor agonist derived from human GLP‐1(7–37) with a plasma half‐life of 13 h and is metabolised by DPP‐4 producing a N‐truncated form, which could take an active part in GLP‐1 receptor‐independent pathways mediated by GLP‐1 metabolites (Malm‐Erjefalt et al., 2010). On the other hand, exendin‐4 discovered from Gila monster venom has a nine‐residue C‐terminal extension unlike human GLP‐1 and shares only 53% structural homology with it, leading to degradation resistance against DPP‐4 (Yap & Misuan, 2019). Semaglutide shares 94% structural homology with native GLP‐1 but has reduced susceptibility to DPP‐4 degradation due to its modifications leading to a plasma half‐life of approximately 1 week (Jensen et al., 2017). Therefore, exendin‐4 and semaglutide should not exert the same GLP‐1‐independent effects as liraglutide if those are mediated by GLP‐1 metabolites binding to a still unknown receptor.
It has been shown that 48 h of continuous intravenous infusion of dogs with either GLP‐1(7–36)amide or GLP‐1(9–36)amide exhibit similar improvement of LV performance and myocardial glucose uptake after pacing‐induced dilated cardiomyopathy (Nikolaidis et al., 2005). Sonne et al. (2008) demonstrated in an isolated rat hearts ischaemia–reperfusion injury model improved LV performance after 45‐min global no‐flow ischaemia, followed by 120‐min reperfusion, whereby exendin‐4 or GLP‐1(9–36)amide were added in the first 15 min. Interestingly, this effect was not reflected by the infarct size; only exendin‐4 showed infarct‐size limiting effects. Ban et al. used an ischaemia–reperfusion injury model with isolated wild type (WT) and Glp1r −/− mouse hearts administered with GLP‐1 or GLP‐1(9–36)amide, either 20 min before or after 30‐min global no‐flow ischaemia. Pretreatment with GLP‐1 in both WT and Glp1r −/− significantly improved recovery of LV developed pressure (LVDP) and CM survival, indicated by reduced lactate dehydrogenase (LDH) release. Paradoxically, pretreatment in WT with GLP‐1(9–36)amide showed no improvement of LVDP recovery or CM viability. But post‐treatment with GLP‐1(9–36)amide again showed the mentioned cardioprotective effects to a lesser extent (Ban et al., 2008). On the contrary, Ossum et al. (2009) observed no infarct‐size limiting effects and no improved recovery of LVDP of GLP‐1(9–36)amide in contrast to GLP‐1 in an ischaemia–reperfusion injury model with isolated rat hearts. Additionally, in vitro experiments GLP‐1(9–36)amide and exendin‐4 showed direct cytoprotective effects in mouse neonatal ventricular CMs exposed to stimulated ischaemia–reperfusion injury models, decreased LDH release and caspase‐3 activity. In normoxic CM cultures, treatment with GLP‐1(9–36)amide and exendin‐4 increased phosphorylation of prosurvival kinases PKB/Akt, ERK1/2 and CREB (cAMP response element‐binding protein) as well as cAMP formation in a dose‐dependent manner.
Permanent ligation of the left anterior descending artery following 14 days of s.c. infusion of either GLP‐1(28–36)amide, a scrambled amino acid sequence of GLP‐1(28–36)amide or GLP‐1 showed that GLP‐1(28–36)amide and GLP‐1 equally and significantly reduced infarct size compared to scrambled control. Additionally, using an ischaemia–reperfusion injury model with 20‐min GLP‐1(28–36)amide pretreatment showed concentration‐dependent LVDP recovery improvement and reduced infarct size in isolated WT and Glp1 −/− mouse hearts. In this study, it has been revealed that in primary coronary artery smooth muscle cells and coronary artery ECs cytoprotective effects of GLP‐1(28–36) are sAC dependent, following intracellular cAMP accumulation. In affinity pull‐down experiments biotinylated, but still functional, GLP‐1(28–36)amide bound mitochondrial trifunctional protein subunit α which is involved in fatty acid beta‐oxidation was used. GLP‐1(28–36)amide is involved in bioenergetic pathways and probably shifts the substrate preference from fatty acid to glycolysis by inhibiting mitochondrial trifunctional protein subunit α (Siraj et al., 2020) (see Figure 3). Cardiac effects of GLP‐1 metabolites in ischaemia–reperfusion injury‐ and MI‐models are not completely consistent throughout the literature but still strongly suggest that the protective effects of GLP‐1 are at least partly mediated by an unknown GLP‐1 receptor‐independent effect of GLP‐1(9–36)amide and GLP‐1(28–36)amide possibly involving pro‐survival signalling via Akt and ERK1/2.
Apart from cardioprotective effects, a number of ex vivo and in vitro studies also demonstrated vascular actions of GLP‐1 metabolites. B. D. Green et al. (2008) showed in vascular reactivity studies a concentration‐dependent relaxation of isolated rat aortas treated with either GLP‐1(7–36)amide, GLP‐1(9–36)amide, exendin‐4 and, surprisingly, exendin(9–39). Vascular action of GLP‐1 metabolites was also under investigation in the already mentioned studies from Ban et al. They observed an increased cGMP release into coronary venous effluent of ex vivo normoxic hearts treated with GLP‐1 or GLP‐1(9–36)amide, indicating NO/cGMP‐dependent vasodilatory effects. GLP‐1 and GLP‐1(9–36)amide, but not exendin‐4 showed equivalent vasodilatory response on mesenteric artery from WT and Glp1r −/− . Addition of the DPP‐4 inhibitor sitagliptin, which prevents degradation to GLP‐1(9–36)amide, reduced the vasodilatory effects of GLP‐1. To assess the participation of the L‐arginine‐NO pathway, mesenteric arteries were preincubated with the NOS Inhibitor L‐NNA (L‐NG‐nitroarginine). Vasodilatory actions of GLP‐1 and GLP‐1(9–36)amide were completely abolished (Ban et al., 2008). Additionally, in human aortic ECs, GLP‐1(9–36)amide, but not exendin‐4, showed cytoprotective effects in ischaemia–reperfusion injury models (hypoxia‐reoxygenation or H2O2), which were again abolished by pretreatment with NOS inhibitor L‐NAME (L‐NG‐nitroarginine methyl ester) (Ban et al., 2010). In addition to the vasodilatory effects, the studies imply that GLP‐1 metabolites also seem to reduce vascular inflammation and oxidative stress. First of all, in vitro pretreatment with GLP‐1(9–36)amide significantly reduced chemokine‐induced migration of isolated human CD4+ lymphocytes. GLP‐1(9–36)amide inhibits this early step of atherogenesis by inhibition of the PI3‐kinase pathway independent of cAMP and GLP‐1 receptor signalling (Liberman et al., 2013). Burgmaier et al. demonstrated that overexpression of GLP‐1(9–37) or GLP‐1(28–37) stabilises atherosclerotic lesions in Apoe −/− mice but do not reduce the formation. Increased plaque stability in the aortic root is mediated by significantly reduced macrophage infiltration, MMP‐9 content and a subsequent increase in collagen I + II and fibrous cap thickness (Burgmaier et al., 2013). Finally, it was also shown that GLP‐1(9–36)amide is able to disrupt the ROS‐generating feedback loop, therefore normalising persistent overproduction of ROS in human aortic ECs and in mice induced by transient exposure to high glucose (Giacco et al., 2015).
5. CONCLUSION
Overnutrition, lack of exercise and the consumption of highly processed food all contribute to an increasing prevalence of T2DM, which severely restricts not only life expectancy but also the quality of life (GBD 2013 Mortality and Causes of Death Collaborators, 2015). Worldwide the prevalence of diabetes mellitus was 9.3% (463 million people) in 2019, rising to 10.2% (578 million) by 2030 and 10.9% (700 million) by 2045 (Saeedi et al., 2019). Diabetes mellitus is associated with a high global mortality burden mostly comprising CV events such as MI or stroke (Disease et al., 2018). Accordingly, new treatment options are urgently needed. GLP‐1 receptor agonists represent a relatively new class of drugs used for the treatment of T2DM. They are not only able to improve hyperglycaemia of diabetic patients but also directly influence in addition other significant risk factors for CVD such as high blood pressure, dyslipidaemia or obesity. These properties explain at least in part the positive results of the CV outcome studies for liraglutide, semaglutide, dulaglutide and albiglutide, all of which showed not only CV safety but also an improvement in CV prognosis. Although the GLP‐1 receptor‐dependent effects of these compounds were well characterised, GLP‐1 receptor‐independent, pleiotropic effects on the CV system need much more investigation, especially regarding their signalling pathways (Nauck et al., 2017). GLP‐1 receptor‐independent pathways obviously include anti‐inflammatory and anti‐oxidant effects as well as direct vasodilatory and thus cardioprotective effects. The protective properties of GLP‐1 and GLP‐1 receptor agonists should be investigated in detail using modern omics techniques, for example, to reveal epigenetic, metabolomics and proteomic changes to explain the observed beneficial effects, especially as the knowledge of GLP‐1 receptor expression levels in different cell types is hampered by the limited specificity of commercially available antibodies.
GLP‐1 receptor agonists represent indeed a milestone in the treatment of diabetic patients with CVD and it is very likely that with the detection of additional GLP‐1 signalling pathways (independent of glucose lowering effects), this class of drug becomes relevant for the treatment of nonalcoholic steatohepatitis (Newsome et al., 2020), neuroinflammatory diseases (Y. K. Kim et al., 2020) or even prevention of CVD independent of T2DM.
5.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in the IUPHAR/BPS Guide to PHARMACOLOGY http://www.guidetopharmacology.org and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019).
AUTHOR CONTRIBUTIONS
J.H., K.K., L.K., A.D. and S.S. wrote the manuscript by contribution with sections according to her/his own expertise. S.S. provided the outline of the manuscript; A.D. and T.M. critically revised the manuscript. All authors have read and approved the final manuscript.
CONFLICT OF INTEREST
S.S. received lecture honorary by Novo Nordisk.
DATA SHARING INFORMATION
Data sharing is not applicable—no new data generated.
ACKNOWLEDGEMENTS
This study was supported by a grant of the German Research Foundation (DFG STE2528/2‐1) to S. Steven, who holds a Virchow‐Fellowship from the Center of Thrombosis and Hemostasis (Mainz, Germany) funded by the Federal Ministry of Education and Research (BMBF 01EO1003). K. Keppeler receives a PhD stipend of the TransMed PhD Program at the University Medical Center Mainz. L. Küster holds a MD stipend of the Robert‐Müller foundation at the University Medical Center Mainz. We thank the Mainz Heart Foundation for continuous support. T. Münzel is a Principal Investigator of the DZHK (German Center for Cardiovascular Research), Partner Site Rhine‐Main, Mainz, Germany.
Open access funding enabled and organized by Projekt DEAL.
Helmstädter, J. , Keppeler, K. , Küster, L. , Münzel, T. , Daiber, A. , & Steven, S. (2022). Glucagon‐like peptide‐1 (GLP‐1) receptor agonists and their cardiovascular benefits—The role of the GLP‐1 receptor. British Journal of Pharmacology, 179(4), 659–676. 10.1111/bph.15462
[Correction added on 4 August 2021, after first online publication: Funding Statement has been added in this current version.]
Funding information Federal Ministry of Education and Research, Grant/Award Number: BMBF 01EO1003; German Research Foundation, Grant/Award Number: DFG STE2528/2‐1
REFERENCES
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Sharman, J. L. , Southan, C. , Davies, J. A. , & CGTP Collaborators . (2019). The Concise Guide to PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176(Suppl 1), S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almutairi, M. , Gopal, K. , Greenwell, A. A. , Young, A. , Gill, R. , Aburasayn, H. , al Batran, R. , Chahade, J. J. , Gandhi, M. , Eaton, F. , Mailloux, R. J. , & Ussher, J. R. (2021). The GLP‐1 receptor agonist liraglutide increases myocardial glucose oxidation rates via indirect mechanisms and mitigates experimental diabetic cardiomyopathy. The Canadian Journal of Cardiology, 37(1), 140–150. 10.1016/j.cjca.2020.02.098 [DOI] [PubMed] [Google Scholar]
- American Diabetes Association . (2020). 9. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes‐2020. Diabetes Care, 43(Suppl 1), S98–S110. 10.2337/dc20-S009 [DOI] [PubMed] [Google Scholar]
- Andersen, A. , Lund, A. , Knop, F. K. , & Vilsboll, T. (2018). Glucagon‐like peptide 1 in health and disease. Nature Reviews. Endocrinology, 14(7), 390–403. 10.1038/s41574-018-0016-2 [DOI] [PubMed] [Google Scholar]
- Andreadis, P. , Karagiannis, T. , Malandris, K. , Avgerinos, I. , Liakos, A. , Manolopoulos, A. , Bekiari, E. , Matthews, D. R. , & Tsapas, A. (2018). Semaglutide for type 2 diabetes mellitus: A systematic review and meta‐analysis. Diabetes, Obesity & Metabolism, 20(9), 2255–2263. 10.1111/dom.13361 [DOI] [PubMed] [Google Scholar]
- Ang, R. , Mastitskaya, S. , Hosford, P. S. , Basalay, M. , Specterman, M. , Aziz, Q. , Li, Y. , Orini, M. , Taggart, P. , Lambiase, P. D. , Gourine, A. , Tinker, A. , & Gourine, A. V. (2018). Modulation of cardiac ventricular excitability by GLP‐1 (glucagon‐like peptide‐1). Circulation. Arrhythmia and Electrophysiology, 11(10), e006740. 10.1161/CIRCEP.118.006740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa, M. , Mita, T. , Azuma, K. , Ebato, C. , Goto, H. , Nomiyama, T. , Fujitani, Y. , Hirose, T. , Kawamori, R. , & Watada, H. (2010). Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon‐like peptide‐1 receptor agonist, exendin‐4. Diabetes, 59(4), 1030–1037. 10.2337/db09-1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravindhan, K. , Bao, W. , Harpel, M. R. , Willette, R. N. , Lepore, J. J. , & Jucker, B. M. (2015). Cardioprotection resulting from glucagon‐like peptide‐1 administration involves shifting metabolic substrate utilization to increase energy efficiency in the rat heart. PLoS One, 10(6), e0130894. 10.1371/journal.pone.0130894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio, L. L. , Ussher, J. R. , McLean, B. A. , Cao, X. , Kabir, M. G. , Mulvihill, E. E. , Mighiu, A. S. , Zhang, H. , Ludwig, A. , Seeley, R. J. , Heximer, S. P. , & Drucker, D. J. (2017). The autonomic nervous system and cardiac GLP‐1 receptors control heart rate in mice. Molecular MetabolismMolecular Metabolism, 6(11), 1339–1349. 10.1016/j.molmet.2017.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio, L. L. , Yusta, B. , Mulvihill, E. E. , Cao, X. , Streutker, C. J. , Butany, J. , Cappola, T. P. , Margulies, K. B. , & Drucker, D. J. (2018). GLP‐1 receptor expression within the human heart. Endocrinology, 159(4), 1570–1584. 10.1210/en.2018-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban, K. , Kim, K. H. , Cho, C. K. , Sauve, M. , Diamandis, E. P. , Backx, P. H. , Drucker, D. J. , & Husain, M. (2010). Glucagon‐like peptide (GLP)‐1(9–36)amide‐mediated cytoprotection is blocked by exendin(9‐39) yet does not require the known GLP‐1 receptor. Endocrinology, 151(4), 1520–1531. 10.1210/en.2009-1197 [DOI] [PubMed] [Google Scholar]
- Ban, K. , Noyan‐Ashraf, M. H. , Hoefer, J. , Bolz, S. S. , Drucker, D. J. , & Husain, M. (2008). Cardioprotective and vasodilatory actions of glucagon‐like peptide 1 receptor are mediated through both glucagon‐like peptide 1 receptor‐dependent and ‐independent pathways. Circulation, 117(18), 2340–2350. 10.1161/CIRCULATIONAHA.107.739938 [DOI] [PubMed] [Google Scholar]
- Bang‐Berthelsen, C. H. , Holm, T. L. , Pyke, C. , Simonsen, L. , Sokilde, R. , Pociot, F. , Heller, R. S. , Folkersen, L. , Kvist, P. H. , Jackerott, M. , & Frederiksen, K. S. (2016). GLP‐1 induces barrier protective expression in Brunner's glands and regulates colonic inflammation. Inflammatory Bowel Diseases, 22(9), 2078–2097. 10.1097/MIB.0000000000000847 [DOI] [PubMed] [Google Scholar]
- Bao, W. , Aravindhan, K. , Alsaid, H. , Chendrimada, T. , Szapacs, M. , Citerone, D. R. , Harpel, M. R. , Willette, R. N. , Lepore, J. J. , & Jucker, B. M. (2011). Albiglutide, a long lasting glucagon‐like peptide‐1 analog, protects the rat heart against ischemia/reperfusion injury: Evidence for improving cardiac metabolic efficiency. PLoS One, 6(8), e23570. 10.1371/journal.pone.0023570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barale, C. , Buracco, S. , Cavalot, F. , Frascaroli, C. , Guerrasio, A. , & Russo, I. (2017). Glucagon‐like peptide 1‐related peptides increase nitric oxide effects to reduce platelet activation. Thrombosis and Haemostasis, 117(6), 1115–1128. 10.1160/TH16-07-0586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basalay, M. V. , Davidson, S. M. , & Yellon, D. M. (2020). Can glucagon‐like peptide‐1 (GLP‐1) analogues make neuroprotection a reality? Neural Regeneration Research, 15(10), 1852–1853. 10.4103/1673-5374.280313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basalay, M. V. , Mastitskaya, S. , Mrochek, A. , Ackland, G. L. , Del Arroyo, A. G. , Sanchez, J. , Sjoquist, P. O. , Pernow, J. , Gourine, A. V. , & Gourine, A. (2016). Glucagon‐like peptide‐1 (GLP‐1) mediates cardioprotection by remote ischaemic conditioning. Cardiovascular Research, 112(3), 669–676. 10.1093/cvr/cvw216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLUEPRINT Consortium. BLUEPRINT progenitors . (2014). Retrieved from http://dcc.blueprint-epigenome.eu/#/home
- Bose, A. K. , Mocanu, M. M. , Carr, R. D. , Brand, C. L. , & Yellon, D. M. (2005). Glucagon‐like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes, 54(1), 146–151. 10.2337/diabetes.54.1.146 [DOI] [PubMed] [Google Scholar]
- Bunck, M. C. , Diamant, M. , Eliasson, B. , Corner, A. , Shaginian, R. M. , Heine, R. J. , Taskinen, M. R. , Yki‐Jarvinen, H. , & Smith, U. (2010). Exenatide affects circulating cardiovascular risk biomarkers independently of changes in body composition. Diabetes Care, 33(8), 1734–1737. 10.2337/dc09-2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgmaier, M. , Liberman, A. , Mollmann, J. , Kahles, F. , Reith, S. , Lebherz, C. , Marx, N. , & Lehrke, M. (2013). Glucagon‐like peptide‐1 (GLP‐1) and its split products GLP‐1(9‐37) and GLP‐1(28‐37) stabilize atherosclerotic lesions in ApoE(−)/(−) mice. Atherosclerosis, 231(2), 427–435. 10.1016/j.atherosclerosis.2013.08.033 [DOI] [PubMed] [Google Scholar]
- Burkhart, J. M. , Vaudel, M. , Gambaryan, S. , Radau, S. , Walter, U. , Martens, L. , Geiger, J. , Sickmann, A. , & Zahedi, R. P. (2012). The first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathways. Blood, 120(15), e73–e82. 10.1182/blood-2012-04-416594 [DOI] [PubMed] [Google Scholar]
- Cabou, C. , Vachoux, C. , Campistron, G. , Drucker, D. J. , & Burcelin, R. (2011). Brain GLP‐1 signaling regulates femoral artery blood flow and insulin sensitivity through hypothalamic PKC‐delta. Diabetes, 60(9), 2245–2256. 10.2337/db11-0464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron‐Vendrig, A. , Reheman, A. , Siraj, M. A. , Xu, X. R. , Wang, Y. , Lei, X. , Afroze, T. , Shikatani, E. , el‐Mounayri, O. , Noyan, H. , Weissleder, R. , Ni, H. , & Husain, M. (2016). Glucagon‐like peptide 1 receptor activation attenuates platelet aggregation and thrombosis. Diabetes, 65(6), 1714–1723. 10.2337/db15-1141 [DOI] [PubMed] [Google Scholar]
- Chai, W. , Dong, Z. , Wang, N. , Wang, W. , Tao, L. , Cao, W. , & Liu, Z. (2012). Glucagon‐like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide‐dependent mechanism. Diabetes, 61(4), 888–896. 10.2337/db11-1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri, A. , Ghanim, H. , Vora, M. , Sia, C. L. , Korzeniewski, K. , Dhindsa, S. , Makdissi, A. , & Dandona, P. (2012). Exenatide exerts a potent antiinflammatory effect. The Journal of Clinical Endocrinology and Metabolism, 97(1), 198–207. 10.1210/jc.2011-1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W. R. , Chen, Y. D. , Tian, F. , Yang, N. , Cheng, L. Q. , Hu, S. Y. , Wang, J. , Yang, J. J. , Wang, S. F. , & Gu, X. F. (2016). Effects of Liraglutide on reperfusion Injury in patients with ST‐segment‐elevation myocardial infarction. Circulation. Cardiovascular Imaging, 9(12), e005146. 10.1161/CIRCIMAGING.116.005146 [DOI] [PubMed] [Google Scholar]
- Chen, W. R. , Hu, S. Y. , Chen, Y. D. , Zhang, Y. , Qian, G. , Wang, J. , Yang, J. J. , Wang, Z. F. , Tian, F. , & Ning, Q. X. (2015). Effects of liraglutide on left ventricular function in patients with ST‐segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. American Heart Journal, 170(5), 845–854. 10.1016/j.ahj.2015.07.014 [DOI] [PubMed] [Google Scholar]
- Chen, W. R. , Shen, X. Q. , Zhang, Y. , Chen, Y. D. , Hu, S. Y. , Qian, G. , Wang, J. , Yang, J. J. , Wang, Z. F. , & Tian, F. (2016). Effects of liraglutide on left ventricular function in patients with non‐ST‐segment elevation myocardial infarction. Endocrine, 52(3), 516–526. 10.1007/s12020-015-0798-0 [DOI] [PubMed] [Google Scholar]
- Deacon, C. F. (2004). Circulation and degradation of GIP and GLP‐1. Hormone and Metabolic Research, 36(11–12), 761–765. 10.1055/s-2004-826160 [DOI] [PubMed] [Google Scholar]
- Ding, L. , & Zhang, J. (2012). Glucagon‐like peptide‐1 activates endothelial nitric oxide synthase in human umbilical vein endothelial cells. Acta Pharmacologica Sinica, 33(1), 75–81. 10.1038/aps.2011.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disease, G. B. D. , Injury, I. , & Prevalence, C. (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet, 392(10159), 1789–1858. 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokken, B. B. , Hilwig, W. R. , Teachey, M. K. , Panchal, R. A. , Hubner, K. , Allen, D. , Rogers, D. C. , & Kern, K. B. (2010). Glucagon‐like peptide‐1 (GLP‐1) attenuates post‐resuscitation myocardial microcirculatory dysfunction. Resuscitation, 81(6), 755–760. 10.1016/j.resuscitation.2010.01.031 [DOI] [PubMed] [Google Scholar]
- Donnelly, D. (2012). The structure and function of the glucagon‐like peptide‐1 receptor and its ligands. British Journal of Pharmacology, 166(1), 27–41. 10.1111/j.1476-5381.2011.01687.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker, D. J. (2016). The cardiovascular biology of glucagon‐like peptide‐1. Cell Metabolism, 24(1), 15–30. 10.1016/j.cmet.2016.06.009 [DOI] [PubMed] [Google Scholar]
- Drucker, D. J. (2018a). The ascending GLP‐1 road from clinical safety to reduction of cardiovascular complications. Diabetes, 67(9), 1710–1719. 10.2337/dbi18-0008 [DOI] [PubMed] [Google Scholar]
- Drucker, D. J. (2018b). Mechanisms of action and therapeutic application of glucagon‐like peptide‐1. Cell Metabolism, 27(4), 740–756. 10.1016/j.cmet.2018.03.001 [DOI] [PubMed] [Google Scholar]
- Drucker, D. J. , Philippe, J. , Mojsov, S. , Chick, W. L. , & Habener, J. F. (1987). Glucagon‐like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proceedings of the National Academy of Sciences of the United States of America, 84(10), 3434–3438. 10.1073/pnas.84.10.3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambaryan, S. , & Tsikas, D. (2015). A review and discussion of platelet nitric oxide and nitric oxide synthase: Do blood platelets produce nitric oxide from L‐arginine or nitrite? Amino Acids, 47(9), 1779–1793. 10.1007/s00726-015-1986-1 [DOI] [PubMed] [Google Scholar]
- GBD 2013 Mortality and Causes of Death Collaborators . (2015). Global, regional, and national age‐sex specific all‐cause and cause‐specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet, 385(9963), 117–171. 10.1016/S0140-6736(14)61682-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gejl, M. , Sondergaard, H. M. , Stecher, C. , Bibby, B. M. , Moller, N. , Botker, H. E. , Hansen, S. B. , Gjedde, A. , Rungby, J. , & Brock, B. (2012). Exenatide alters myocardial glucose transport and uptake depending on insulin resistance and increases myocardial blood flow in patients with type 2 diabetes. The Journal of Clinical Endocrinology and Metabolism, 97(7), E1165–E1169. 10.1210/jc.2011-3456 [DOI] [PubMed] [Google Scholar]
- Gerstein, H. C. , Colhoun, H. M. , Dagenais, G. R. , Diaz, R. , Lakshmanan, M. , Pais, P. , Probstfield, J. , Riesmeyer, J. S. , Riddle, M. C. , Rydén, L. , & Xavier, D. (2019). Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double‐blind, randomised placebo‐controlled trial. Lancet, 394(10193), 121–130. 10.1016/S0140-6736(19)31149-3 [DOI] [PubMed] [Google Scholar]
- Giacco, F. , Du, X. , Carratu, A. , Gerfen, G. J. , D'Apolito, M. , Giardino, I. , Rasola, A. , Marin, O. , Divakaruni, A. S. , Murphy, A. N. , & Shah, M. S. (2015). GLP‐1 cleavage product reverses persistent ROS generation after transient hyperglycemia by disrupting an ROS‐generating feedback loop. Diabetes, 64(9), 3273–3284. 10.2337/db15-0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graaf, C. , Donnelly, D. , Wootten, D. , Lau, J. , Sexton, P. M. , Miller, L. J. , Ahn, J. M. , Liao, J. , Fletcher, M. M. , Yang, D. , & Brown, A. J. (2016). Glucagon‐like peptide‐1 and its class B G protein‐coupled receptors: A long march to therapeutic successes. Pharmacological Reviews, 68(4), 954–1013. 10.1124/pr.115.011395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, B. D. , Hand, K. V. , Dougan, J. E. , McDonnell, B. M. , Cassidy, R. S. , & Grieve, D. J. (2008). GLP‐1 and related peptides cause concentration‐dependent relaxation of rat aorta through a pathway involving KATP and cAMP. Archives of Biochemistry and Biophysics, 478(2), 136–142. 10.1016/j.abb.2008.08.001 [DOI] [PubMed] [Google Scholar]
- Green, J. B. , Bethel, M. A. , Armstrong, P. W. , Buse, J. B. , Engel, S. S. , Garg, J. , Josse, R. , Kaufman, K. D. , Koglin, J. , Korn, S. , & Lachin, J. M. (2015). Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. The New England Journal of Medicine, 373(3), 232–242. 10.1056/NEJMoa1501352 [DOI] [PubMed] [Google Scholar]
- Guglielmi, V. , & Sbraccia, P. (2017). GLP‐1 receptor independent pathways: Emerging beneficial effects of GLP‐1 breakdown products. Eating and Weight Disorders, 22(2), 231–240. 10.1007/s40519-016-0352-y [DOI] [PubMed] [Google Scholar]
- Hadjiyanni, I. , Siminovitch, K. A. , Danska, J. S. , & Drucker, D. J. (2010). Glucagon‐like peptide‐1 receptor signalling selectively regulates murine lymphocyte proliferation and maintenance of peripheral regulatory T cells. Diabetologia, 53(4), 730–740. 10.1007/s00125-009-1643-x [DOI] [PubMed] [Google Scholar]
- He, S. , Kahles, F. , Rattik, S. , Nairz, M. , McAlpine, C. S. , Anzai, A. , Selgrade, D. , Fenn, A. M. , Chan, C. T. , Mindur, J. E. , Valet, C. , Poller, W. C. , Halle, L. , Rotllan, N. , Iwamoto, Y. , Wojtkiewicz, G. R. , Weissleder, R. , Libby, P. , Fernández‐Hernando, C. , … Swirski, F. K. (2019). Gut intraepithelial T cells calibrate metabolism and accelerate cardiovascular disease. Nature, 566(7742), 115–119. 10.1038/s41586-018-0849-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstadter, J. , Frenis, K. , Filippou, K. , Grill, A. , Dib, M. , Kalinovic, S. , Pawelke, F. , Kus, K. , Kröller‐Schön, S. , Oelze, M. , & Chlopicki, S. (2020). Endothelial GLP‐1 (glucagon‐like Peptide‐1) receptor mediates cardiovascular protection by liraglutide in mice with experimental arterial hypertension. Arteriosclerosis, Thrombosis, and Vascular Biology, 40(1), 145–158. 10.1161/atv.0000615456.97862.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman, G. A. , Stein, P. P. , Thornberry, N. A. , & Wagner, J. A. (2007). Dipeptidyl peptidase‐4 inhibitors for the treatment of type 2 diabetes: Focus on sitagliptin. Clinical Pharmacology and Therapeutics, 81(5), 761–767. 10.1038/sj.clpt.6100167 [DOI] [PubMed] [Google Scholar]
- Hermansen, K. , Baekdal, T. A. , During, M. , Pietraszek, A. , Mortensen, L. S. , Jorgensen, H. , & Flint, A. (2013). Liraglutide suppresses postprandial triglyceride and apolipoprotein B48 elevations after a fat‐rich meal in patients with type 2 diabetes: A randomized, double‐blind, placebo‐controlled, cross‐over trial. Diabetes, Obesity & Metabolism, 15(11), 1040–1048. 10.1111/dom.12133 [DOI] [PubMed] [Google Scholar]
- Hernandez, A. F. , Green, J. B. , Janmohamed, S. , D'Agostino, R. B. Sr. , Granger, C. B. , Jones, N. P. , Leiter, L. A. , Rosenberg, A. E. , Sigmon, K. N. , Somerville, M. C. , & Thorpe, K. M. (2018). Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (harmony outcomes): A double‐blind, randomised placebo‐controlled trial. Lancet, 392(10157), 1519–1529. 10.1016/S0140-6736(18)32261-X [DOI] [PubMed] [Google Scholar]
- Hirata, K. , Kume, S. , Araki, S. , Sakaguchi, M. , Chin‐Kanasaki, M. , Isshiki, K. , Sugimoto, T. , Nishiyama, A. , Koya, D. , Haneda, M. , Kashiwagi, A. , & Uzu, T. (2009). Exendin‐4 has an anti‐hypertensive effect in salt‐sensitive mice model. Biochemical and Biophysical Research Communications, 380(1), 44–49. 10.1016/j.bbrc.2009.01.003 [DOI] [PubMed] [Google Scholar]
- Hogan, A. E. , Gaoatswe, G. , Lynch, L. , Corrigan, M. A. , Woods, C. , O'Connell, J. , & O'Shea, D. (2014). Glucagon‐like peptide 1 analogue therapy directly modulates innate immune‐mediated inflammation in individuals with type 2 diabetes mellitus. Diabetologia, 57(4), 781–784. 10.1007/s00125-013-3145-0 [DOI] [PubMed] [Google Scholar]
- Holman, R. R. , Bethel, M. A. , Mentz, R. J. , Thompson, V. P. , Lokhnygina, Y. , Buse, J. B. , Chan, J. C. , Choi, J. , Gustavson, S. M. , Iqbal, N. , & Maggioni, A. P. (2017). Effects of once‐weekly exenatide on cardiovascular outcomes in type 2 diabetes. The New England Journal of Medicine, 377(13), 1228–1239. 10.1056/NEJMoa1612917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman, R. R. , Paul, S. K. , Bethel, M. A. , Matthews, D. R. , & Neil, H. A. (2008). 10‐year follow‐up of intensive glucose control in type 2 diabetes. The New England Journal of Medicine, 359(15), 1577–1589. 10.1056/NEJMoa0806470 [DOI] [PubMed] [Google Scholar]
- Holst, J. J. , Orskov, C. , Nielsen, O. V. , & Schwartz, T. W. (1987). Truncated glucagon‐like peptide I, an insulin‐releasing hormone from the distal gut. FEBS Letters, 211(2), 169–174. 10.1016/0014-5793(87)81430-8 [DOI] [PubMed] [Google Scholar]
- Husain, M. , Birkenfeld, A. L. , Donsmark, M. , Dungan, K. , Eliaschewitz, F. G. , Franco, D. R. , Jeppesen, O. K. , Lingvay, I. , Mosenzon, O. , Pedersen, S. D. , & Tack, C. J. (2019). Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. The New England Journal of Medicine, 381(9), 841–851. 10.1056/NEJMoa1901118 [DOI] [PubMed] [Google Scholar]
- Ihara, M. , Asanuma, H. , Yamazaki, S. , Kato, H. , Asano, Y. , Shinozaki, Y. , Mori, H. , Minamino, T. , Asakura, M. , Sugimachi, M. , Mochizuki, N. , & Kitakaze, M. (2015). An interaction between glucagon‐like peptide‐1 and adenosine contributes to cardioprotection of a dipeptidyl peptidase 4 inhibitor from myocardial ischemia‐reperfusion injury. American Journal of Physiology. Heart and Circulatory Physiology, 308(10), H1287–H1297. 10.1152/ajpheart.00835.2014 [DOI] [PubMed] [Google Scholar]
- Jensen, L. , Helleberg, H. , Roffel, A. , van Lier, J. J. , Bjornsdottir, I. , Pedersen, P. J. , Rowe, E. , Karsbøl, J. D. , & Pedersen, M. L. (2017). Absorption, metabolism and excretion of the GLP‐1 analogue semaglutide in humans and nonclinical species. European Journal of Pharmaceutical Sciences, 104, 31–41. 10.1016/j.ejps.2017.03.020 [DOI] [PubMed] [Google Scholar]
- Jia, G. , Aroor, A. R. , & Sowers, J. R. (2016). Glucagon‐like peptide 1 receptor activation and platelet function: Beyond glycemic control. Diabetes, 65(6), 1487–1489. 10.2337/dbi16-0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jojima, T. , Uchida, K. , Akimoto, K. , Tomotsune, T. , Yanagi, K. , Iijima, T. , Suzuki, K. , Kasai, K. , & Aso, Y. (2017). Liraglutide, a GLP‐1 receptor agonist, inhibits vascular smooth muscle cell proliferation by enhancing AMP‐activated protein kinase and cell cycle regulation, and delays atherosclerosis in ApoE deficient mice. Atherosclerosis, 261, 44–51. 10.1016/j.atherosclerosis.2017.04.001 [DOI] [PubMed] [Google Scholar]
- Kaul, S. (2017). Mitigating cardiovascular risk in type 2 diabetes with antidiabetes drugs: A review of principal cardiovascular outcome results of EMPA‐REG OUTCOME, LEADER, and SUSTAIN‐6 trials. Diabetes Care, 40(7), 821–831. 10.2337/dc17-0291 [DOI] [PubMed] [Google Scholar]
- Khan, M. S. , Fonarow, G. C. , McGuire, Hernandez, A. F. , Vaduganathan, M. , Rosenstock, J. , Handelsman, Y. , Verma, S. , Anker, S. D. , McMurray, & Kosiborod, M. N. (2020). Glucagon‐like peptide 1 receptor agonists and heart failure: The need for further evidence generation and practice guidelines optimization. Circulation, 142(12), 1205–1218. 10.1161/CIRCULATIONAHA.120.045888 [DOI] [PubMed] [Google Scholar]
- Kieffer, T. J. , McIntosh, C. H. , & Pederson, R. A. (1995). Degradation of glucose‐dependent insulinotropic polypeptide and truncated glucagon‐like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology, 136(8), 3585–3596. 10.1210/endo.136.8.7628397 [DOI] [PubMed] [Google Scholar]
- Kim, M. , Platt, M. J. , Shibasaki, T. , Quaggin, S. E. , Backx, P. H. , Seino, S. , Simpson, J. A. , & Drucker, D. J. (2013). GLP‐1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nature Medicine, 19(5), 567–575. 10.1038/nm.3128 [DOI] [PubMed] [Google Scholar]
- Kim, Y. K. , Kim, O. Y. , & Song, J. (2020). Alleviation of depression by glucagon‐like peptide 1 through the regulation of neuroinflammation, neurotransmitters, neurogenesis, and synaptic function. Frontiers in Pharmacology, 11, 1270. 10.3389/fphar.2020.01270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, T. , Obata, A. , Shimoda, M. , Okauchi, S. , Hirukawa, H. , Kohara, K. , Kinoshita, T. , Nogami, Y. , Nakanishi, S. , Mune, T. , Kaku, K. , & Kaneto, H. (2017). Decreased glucagon‐like peptide 1 receptor expression in endothelial and smooth muscle cells in diabetic db/db mice: TCF7L2 is a possible regulator of the vascular glucagon‐like peptide 1 receptor. Diabetes & Vascular Disease Research, 14(6), 540–548. 10.1177/1479164117725898 [DOI] [PubMed] [Google Scholar]
- Knuuti, J. , Wijns, W. , Saraste, A. , Capodanno, D. , Barbato, E. , Funck‐Brentano, C. , Prescott, E. , Storey, R. F. , Deaton, C. , Cuisset, T. , & Agewall, S. (2020). 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. European Heart Journal, 41(3), 407–477. 10.1093/eurheartj/ehz425 [DOI] [PubMed] [Google Scholar]
- Kodera, R. , Shikata, K. , Kataoka, H. U. , Takatsuka, T. , Miyamoto, S. , Sasaki, M. , Kajitani, N. , Nishishita, S. , Sarai, K. , Hirota, D. , Sato, C. , Ogawa, D. , & Makino, H. (2011). Glucagon‐like peptide‐1 receptor agonist ameliorates renal injury through its anti‐inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia, 54(4), 965–978. 10.1007/s00125-010-2028-x [DOI] [PubMed] [Google Scholar]
- Laugero, K. D. , Stonehouse, A. H. , Guss, S. , Landry, J. , Vu, C. , & Parkes, D. G. (2009). Exenatide improves hypertension in a rat model of the metabolic syndrome. Metabolic Syndrome and Related Disorders, 7(4), 327–334. 10.1089/met.2008.0095 [DOI] [PubMed] [Google Scholar]
- Lebherz, C. , Schlieper, G. , Mollmann, J. , Kahles, F. , Schwarz, M. , Brunsing, J. , Dimkovic, N. , Koch, A. , Trautwein, C. , Flöge, J. , & Marx, N. (2017). GLP‐1 levels predict mortality in patients with critical illness as well as end‐stage renal disease. The American Journal of Medicine, 130(7), 833–841 e833. 10.1016/j.amjmed.2017.03.010 [DOI] [PubMed] [Google Scholar]
- Li, C. J. , Yu, Q. , Yu, P. , Yu, T. L. , Zhang, Q. M. , Lu, S. , & Yu, D. M. (2014). Changes in liraglutide‐induced body composition are related to modifications in plasma cardiac natriuretic peptides levels in obese type 2 diabetic patients. Cardiovascular Diabetology, 13, 36. 10.1186/1475-2840-13-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, B. , & Gu, N. (2020). Liraglutide in the treatment of heart failure: Insight from FIGHT and LIVE. Cardiovascular Diabetology, 19(1), 106. 10.1186/s12933-020-01088-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman, A. , Esser, M. , Marx, N. , & Burgmaier, M. (2013). Glucagon‐like peptide‐1(9–36) inhibits chemokine‐induced migration of human CD4‐positive lymphocytes. PLoS One, 8(3), e58445. 10.1371/journal.pone.0058445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Q. , Anderson, C. , Broyde, A. , Polizzi, C. , Fernandez, R. , Baron, A. , & Parkes, D. G. (2010). Glucagon‐like peptide‐1 and the exenatide analogue AC3174 improve cardiac function, cardiac remodeling, and survival in rats with chronic heart failure. Cardiovascular Diabetology, 9, 76. 10.1186/1475-2840-9-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonborg, J. , Vejlstrup, N. , Kelbaek, H. , Botker, H. E. , Kim, W. Y. , Mathiasen, A. B. , Jørgensen, E. , Helqvist, S. , Saunamäki, K. , Clemmensen, P. , & Holmvang, L. (2012). Exenatide reduces reperfusion injury in patients with ST‐segment elevation myocardial infarction. European Heart Journal, 33(12), 1491–1499. 10.1093/eurheartj/ehr309 [DOI] [PubMed] [Google Scholar]
- Lorenz, M. , Lawson, F. , Owens, D. , Raccah, D. , Roy‐Duval, C. , Lehmann, A. , Perfetti, R. , & Blonde, L. (2017). Differential effects of glucagon‐like peptide‐1 receptor agonists on heart rate. Cardiovascular Diabetology, 16(1), 6. 10.1186/s12933-016-0490-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovshin, J. A. , Barnie, A. , DeAlmeida, A. , Logan, A. , Zinman, B. , & Drucker, D. J. (2015). Liraglutide promotes natriuresis but does not increase circulating levels of atrial natriuretic peptide in hypertensive subjects with type 2 diabetes. Diabetes Care, 38(1), 132–139. 10.2337/dc14-1958 [DOI] [PubMed] [Google Scholar]
- Makdissi, A. , Ghanim, H. , Vora, M. , Green, K. , Abuaysheh, S. , Chaudhuri, A. , Dhindsa, S. , & Dandona, P. (2012). Sitagliptin exerts an antinflammatory action. The Journal of Clinical Endocrinology and Metabolism, 97(9), 3333–3341. 10.1210/jc.2012-1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malm‐Erjefalt, M. , Bjornsdottir, I. , Vanggaard, J. , Helleberg, H. , Larsen, U. , Oosterhuis, B. , van Lier, J. J. , Zdravkovic, M. , & Olsen, A. K. (2010). Metabolism and excretion of the once‐daily human glucagon‐like peptide‐1 analog liraglutide in healthy male subjects and its in vitro degradation by dipeptidyl peptidase IV and neutral endopeptidase. Drug Metabolism and Disposition, 38(11), 1944–1953. 10.1124/dmd.110.034066 [DOI] [PubMed] [Google Scholar]
- Margulies, K. B. , Hernandez, A. F. , Redfield, M. M. , Givertz, M. M. , Oliveira, G. H. , Cole, R. , Mann, D. L. , Whellan, D. J. , Kiernan, M. S. , Felker, G. M. , & McNulty (2016). Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: A randomized clinical trial. JAMA, 316(5), 500–508. 10.1001/jama.2016.10260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marso, S. P. , Bain, S. C. , Consoli, A. , Eliaschewitz, F. G. , Jodar, E. , Leiter, L. A. , Lingvay, I. , Rosenstock, J. , Seufert, J. , Warren, M. L. , & Woo, V. (2016). Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. The New England Journal of Medicine, 375(19), 1834–1844. 10.1056/NEJMoa1607141 [DOI] [PubMed] [Google Scholar]
- Marso, S. P. , Daniels, G. H. , Brown‐Frandsen, K. , Kristensen, P. , Mann, J. F. , Nauck, M. A. , Nissen, S. E. , Pocock, S. , Poulter, N. R. , Ravn, L. S. , & Steinberg, W. M. (2016). Liraglutide and cardiovascular outcomes in type 2 diabetes. The New England Journal of Medicine, 375(4), 311–322. 10.1056/NEJMoa1603827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskery, M. P. , Holscher, C. , Jones, S. P. , Price, C. I. , Strain, W. D. , Watkins, C. L. , Werring, D. J. , & Emsley, H. C. (2021). Glucagon‐like peptide‐1 receptor agonists as neuroprotective agents for ischemic stroke: A systematic scoping review. Journal of Cerebral Blood Flow and Metabolism, 41(1), 14–30. 10.1177/0271678X20952011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick, L. M. , Hoole, S. P. , White, P. A. , Read, P. A. , Axell, R. G. , Clarke, S. J. , O'Sullivan, M. , West, N. E. J. , & Dutka, D. P. (2015). Pre‐treatment with glucagon‐like Peptide‐1 protects against ischemic left ventricular dysfunction and stunning without a detected difference in myocardial substrate utilization. JACC. Cardiovascular Interventions, 8(2), 292–301. 10.1016/j.jcin.2014.09.014 [DOI] [PubMed] [Google Scholar]
- Meier, J. J. , Rosenstock, J. , Hincelin‐Mery, A. , Roy‐Duval, C. , Delfolie, A. , Coester, H. V. , Menge, B. A. , Forst, T. , & Kapitza, C. (2015). Contrasting effects of lixisenatide and liraglutide on postprandial glycemic control, gastric emptying, and safety parameters in patients with type 2 diabetes on optimized insulin glargine with or without metformin: A randomized, open‐label trial. Diabetes Care, 38(7), 1263–1273. 10.2337/dc14-1984 [DOI] [PubMed] [Google Scholar]
- Moberly, S. P. , Berwick, Z. C. , Kohr, M. , Svendsen, M. , Mather, K. J. , & Tune, J. D. (2012). Intracoronary glucagon‐like peptide 1 preferentially augments glucose uptake in ischemic myocardium independent of changes in coronary flow. Experimental Biology and Medicine (Maywood, N.J.), 237(3), 334–342. 10.1258/ebm.2011.011288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojsov, S. , Weir, G. C. , & Habener, J. F. (1987). Insulinotropin: Glucagon‐like peptide I (7–37) co‐encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. The Journal of Clinical Investigation, 79(2), 616–619. 10.1172/JCI112855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima, M. , Watanabe, T. , Terasaki, M. , Tomoyasu, M. , Nohtomi, K. , Kim‐Kaneyama, J. , Miyazaki, A. , & Hirano, T. (2011). Native incretins prevent the development of atherosclerotic lesions in apolipoprotein E knockout mice. Diabetologia, 54(10), 2649–2659. 10.1007/s00125-011-2241-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagami, T. , Qiao, Q. , Tuomilehto, J. , Balkau, B. , Tajima, N. , Hu, G. , & Borch‐Johnsen, K. (2006). Screen‐detected diabetes, hypertension and hypercholesterolemia as predictors of cardiovascular mortality in five populations of Asian origin: The DECODA study. European Journal of Cardiovascular Prevention and Rehabilitation, 13(4), 555–561. 10.1097/01.hjr.0000183916.28354.69 [DOI] [PubMed] [Google Scholar]
- Nandy, D. , Johnson, C. , Basu, R. , Joyner, M. , Brett, J. , Svendsen, C. B. , & Basu, A. (2014). The effect of liraglutide on endothelial function in patients with type 2 diabetes. Diabetes & Vascular Disease Research, 11(6), 419–430. 10.1177/1479164114547358 [DOI] [PubMed] [Google Scholar]
- Nauck, M. A. , Heimesaat, M. M. , Orskov, C. , Holst, J. J. , Ebert, R. , & Creutzfeldt, W. (1993). Preserved incretin activity of glucagon‐like peptide 1 [7–36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type‐2 diabetes mellitus. The Journal of Clinical Investigation, 91(1), 301–307. 10.1172/JCI116186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauck, M. A. , Kleine, N. , Orskov, C. , Holst, J. J. , Willms, B. , & Creutzfeldt, W. (1993). Normalization of fasting hyperglycaemia by exogenous glucagon‐like peptide 1 (7–36 amide) in type 2 (non‐insulin‐dependent) diabetic patients. Diabetologia, 36(8), 741–744. 10.1007/BF00401145 [DOI] [PubMed] [Google Scholar]
- Nauck, M. A. , Meier, J. J. , Cavender, M. A. , Abd El Aziz, M. , & Drucker, D. J. (2017). Cardiovascular actions and clinical outcomes with glucagon‐like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors. Circulation, 136(9), 849–870. 10.1161/CIRCULATIONAHA.117.028136 [DOI] [PubMed] [Google Scholar]
- Newsome, P. N. , Buchholtz, K. , Cusi, K. , Linder, M. , Okanoue, T. , Ratziu, V. , Sanyal, A. J. , Sejling, A. S. , & Harrison, S. A. (2020). A placebo‐controlled trial of subcutaneous semaglutide in nonalcoholic Steatohepatitis. The New England Journal of Medicine, 384, 1113–1124. 10.1056/NEJMoa2028395 [DOI] [PubMed] [Google Scholar]
- Nikolaidis, L. A. , Elahi, D. , Shen, Y. T. , & Shannon, R. P. (2005). Active metabolite of GLP‐1 mediates myocardial glucose uptake and improves left ventricular performance in conscious dogs with dilated cardiomyopathy. American Journal of Physiology. Heart and Circulatory Physiology, 289(6), H2401–H2408. 10.1152/ajpheart.00347.2005 [DOI] [PubMed] [Google Scholar]
- Nikolaidis, L. A. , Mankad, S. , Sokos, G. G. , Miske, G. , Shah, A. , Elahi, D. , & Shannon, R. P. (2004). Effects of glucagon‐like peptide‐1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation, 109(8), 962–965. 10.1161/01.CIR.0000120505.91348.58 [DOI] [PubMed] [Google Scholar]
- Nissen, S. E. , & Wolski, K. (2007). Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. The New England Journal of Medicine, 356(24), 2457–2471. 10.1056/NEJMoa072761 [DOI] [PubMed] [Google Scholar]
- Nissen, S. E. , Wolski, K. , & Topol, E. J. (2005). Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA, 294(20), 2581–2586. 10.1001/jama.294.20.joc50147 [DOI] [PubMed] [Google Scholar]
- Noyan‐Ashraf, M. H. , Momen, M. A. , Ban, K. , Sadi, A. M. , Zhou, Y. Q. , Riazi, A. M. , Baggio, L. L. , Henkelman, R. M. , Husain, M. , & Drucker, D. J. (2009). GLP‐1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes, 58(4), 975–983. 10.2337/db08-1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyan‐Ashraf, M. H. , Shikatani, E. A. , Schuiki, I. , Mukovozov, I. , Wu, J. , Li, R. K. , Volchuk, A. , Robinson, L. A. , Billia, F. , Drucker, D. J. , & Husain, M. (2013). A glucagon‐like peptide‐1 analog reverses the molecular pathology and cardiac dysfunction of a mouse model of obesity. Circulation, 127(1), 74–85. 10.1161/CIRCULATIONAHA.112.091215 [DOI] [PubMed] [Google Scholar]
- Nystrom, T. , Gutniak, M. K. , Zhang, Q. , Zhang, F. , Holst, J. J. , Ahren, B. , & Sjoholm, A. (2004). Effects of glucagon‐like peptide‐1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. American Journal of Physiology. Endocrinology and Metabolism, 287(6), E1209–E1215. 10.1152/ajpendo.00237.2004 [DOI] [PubMed] [Google Scholar]
- Orskov, C. , Rabenhoj, L. , Wettergren, A. , Kofod, H. , & Holst, J. J. (1994). Tissue and plasma concentrations of amidated and glycine‐extended glucagon‐like peptide I in humans. Diabetes, 43(4), 535–539. 10.2337/diab.43.4.535 [DOI] [PubMed] [Google Scholar]
- Ossum, A. , van Deurs, U. , Engstrom, T. , Jensen, J. S. , & Treiman, M. (2009). The cardioprotective and inotropic components of the postconditioning effects of GLP‐1 and GLP‐1(9–36)a in an isolated rat heart. Pharmacological Research, 60(5), 411–417. 10.1016/j.phrs.2009.06.004 [DOI] [PubMed] [Google Scholar]
- Panjwani, N. , Mulvihill, E. E. , Longuet, C. , Yusta, B. , Campbell, J. E. , Brown, T. J. , Streutker, C. , Holland, D. , Cao, X. , Baggio, L. L. , & Drucker, D. J. (2013). GLP‐1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE(−/−) mice. Endocrinology, 154(1), 127–139. 10.1210/en.2012-1937 [DOI] [PubMed] [Google Scholar]
- Pfeffer, M. A. , Claggett, B. , Diaz, R. , Dickstein, K. , Gerstein, H. C. , Kober, L. V. , Lawson, F. C. , Ping, L. , Wei, X. , Lewis, E. F. , & Maggioni, A. P. (2015). Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. The New England Journal of Medicine, 373(23), 2247–2257. 10.1056/NEJMoa1509225 [DOI] [PubMed] [Google Scholar]
- Plutzky, J. , Garber, A. , Falahati, A. , Toft, A. D. , & Poulter, N. R. (2009). Reductions in lipids and CV risk markers in patients with type 2 diabetes treated with liraglutide: A meta‐analysis. Canadian Journal of Diabetes, 33(3), 209–210. 10.1016/S1499-2671(09)33072-5 [DOI] [PubMed] [Google Scholar]
- Poornima, I. , Brown, S. B. , Bhashyam, S. , Parikh, P. , Bolukoglu, H. , & Shannon, R. P. (2008). Chronic glucagon‐like peptide‐1 infusion sustains left ventricular systolic function and prolongs survival in the spontaneously hypertensive, heart failure‐prone rat. Circulation. Heart Failure, 1(3), 153–160. 10.1161/CIRCHEARTFAILURE.108.766402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujadas, G. , & Drucker, D. J. (2016). Vascular biology of glucagon receptor superfamily peptides: Mechanistic and clinical relevance. Endocrine Reviews, 37(6), 554–583. 10.1210/er.2016-1078 [DOI] [PubMed] [Google Scholar]
- Pyke, C. , Heller, R. S. , Kirk, R. K. , Orskov, C. , Reedtz‐Runge, S. , Kaastrup, P. , Hvelplund, A. , Bardram, L. , Calatayud, D. , & Knudsen, L. B. (2014). GLP‐1 receptor localization in monkey and human tissue: Novel distribution revealed with extensively validated monoclonal antibody. Endocrinology, 155(4), 1280–1290. 10.1210/en.2013-1934 [DOI] [PubMed] [Google Scholar]
- Rajendran, S. , & Chirkov, Y. Y. (2008). Platelet hyperaggregability: Impaired responsiveness to nitric oxide (“platelet NO resistance”) as a therapeutic target. Cardiovascular Drugs and Therapy, 22(3), 193–203. 10.1007/s10557-008-6098-7 [DOI] [PubMed] [Google Scholar]
- Richards, P. , Parker, H. E. , Adriaenssens, A. E. , Hodgson, J. M. , Cork, S. C. , Trapp, S. , Gribble, F. M. , & Reimann, F. (2014). Identification and characterization of GLP‐1 receptor‐expressing cells using a new transgenic mouse model. Diabetes, 63(4), 1224–1233. 10.2337/db13-1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker, P. M. , Everett, B. M. , Thuren, T. , MacFadyen, Chang, W. H. , Ballantyne, C. , Fonseca, F. , Nicolau, J. , Koenig, W. , Anker, S. D. , & Kastelein, J. J. (2017). Antiinflammatory therapy with canakinumab for atherosclerotic disease. The New England Journal of Medicine, 377(12), 1119–1131. 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- Robinson, L. E. , Holt, T. A. , Rees, K. , Randeva, H. S. , & O'Hare, J. P. (2013). Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: Systematic review and meta‐analysis. BMJ Open, 3(1). 10.1136/bmjopen-2012-001986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeedi, P. , Petersohn, I. , Salpea, P. , Malanda, B. , Karuranga, S. , Unwin, N. , Colagiuri, S. , Guariguata, L. , Motala, A. A. , Ogurtsova, K. , & Shaw, J. E. (2019). Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Research and Clinical Practice, 157, 107843. 10.1016/j.diabres.2019.107843 [DOI] [PubMed] [Google Scholar]
- Sauve, M. , Ban, K. , Momen, M. A. , Zhou, Y. Q. , Henkelman, R. M. , Husain, M. , & Drucker, D. J. (2010). Genetic deletion or pharmacological inhibition of dipeptidyl peptidase‐4 improves cardiovascular outcomes after myocardial infarction in mice. Diabetes, 59(4), 1063–1073. 10.2337/db09-0955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, T. , Samaras, P. , Frejno, M. , Gessulat, S. , Barnert, M. , Kienegger, H. , Krcmar, H. , Schlegl, J. , Ehrlich, H. C. , Aiche, S. , Kuster, B. , & Wilhelm, M. (2018). ProteomicsDB. Nucleic Acids Research, 46(D1), D1271–D1281. 10.1093/nar/gkx1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scirica, B. M. , Bhatt, D. L. , Braunwald, E. , Steg, P. G. , Davidson, J. , Hirshberg, B. , Ohman, P. , Frederich, R. , Wiviott, S. D. , Hoffman, E. B. , & Cavender, M. A. (2013). Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. The New England Journal of Medicine, 369(14), 1317–1326. 10.1056/NEJMoa1307684 [DOI] [PubMed] [Google Scholar]
- Siraj, M. A. , Mundil, D. , Beca, S. , Momen, A. , Shikatani, E. A. , Afroze, T. , Sun, X. , Liu, Y. , Ghaffari, S. , Lee, W. , Wheeler, M. B. , Keller, G. , Backx, P. , & Husain, M. (2020). Cardioprotective GLP‐1 metabolite prevents ischemic cardiac injury by inhibiting mitochondrial trifunctional protein‐alpha. The Journal of Clinical Investigation, 130(3), 1392–1404. 10.1172/JCI99934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonne, D. P. , Engstrom, T. , & Treiman, M. (2008). Protective effects of GLP‐1 analogues exendin‐4 and GLP‐1(9–36) amide against ischemia‐reperfusion injury in rat heart. Regulatory Peptides, 146(1–3), 243–249. 10.1016/j.regpep.2007.10.001 [DOI] [PubMed] [Google Scholar]
- Sternkopf, M. , Nagy, M. , Baaten, C. , Kuijpers, M. J. E. , Tullemans, B. M. E. , Wirth, J. , Theelen, W. , Mastenbroek, T. G. , Lehrke, M. , Winnerling, B. , & Baerts, L. (2020). Native, intact glucagon‐like peptide 1 is a natural suppressor of thrombus growth under physiological flow conditions. Arteriosclerosis, Thrombosis, and Vascular Biology, 40(3), e65–e77. 10.1161/ATVBAHA.119.313645 [DOI] [PubMed] [Google Scholar]
- Steven, S. , Hausding, M. , Kroller‐Schon, S. , Mader, M. , Mikhed, Y. , Stamm, P. , Zinßius, E. , Pfeffer, A. , Welschof, P. , Agdauletova, S. , & Sudowe, S. (2015). Gliptin and GLP‐1 analog treatment improves survival and vascular inflammation/dysfunction in animals with lipopolysaccharide‐induced endotoxemia. Basic Research in Cardiology, 110(2), 6. 10.1007/s00395-015-0465-x [DOI] [PubMed] [Google Scholar]
- Steven, S. , Jurk, K. , Kopp, M. , Kroller‐Schon, S. , Mikhed, Y. , Schwierczek, K. , Roohani, S. , Kashani, F. , Oelze, M. , Klein, T. , & Tokalov, S. (2017). Glucagon‐like peptide‐1 receptor signalling reduces microvascular thrombosis, nitro‐oxidative stress and platelet activation in endotoxaemic mice. British Journal of Pharmacology, 174(12), 1620–1632. 10.1111/bph.13549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, F. , Wu, S. , Guo, S. , Yu, K. , Yang, Z. , Li, L. , Zhang, Y. , Quan, X. , Ji, L. , & Zhan, S. (2015). Impact of GLP‐1 receptor agonists on blood pressure, heart rate and hypertension among patients with type 2 diabetes: A systematic review and network meta‐analysis. Diabetes Research and Clinical Practice, 110(1), 26–37. 10.1016/j.diabres.2015.07.015 [DOI] [PubMed] [Google Scholar]
- Thorens, B. (1992). Expression cloning of the pancreatic beta cell receptor for the gluco‐incretin hormone glucagon‐like peptide 1. Proceedings of the National Academy of Sciences of the United States of America, 89(18), 8641–8645. 10.1073/pnas.89.18.8641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers, L. , Henriques, J. P. , de Kleijn, D. P. , Devries, J. H. , Kemperman, H. , Steendijk, P. , Verlaan, C. W. , Kerver, M. , Piek, J. J. , Doevendans, P. A. , & Pasterkamp, G. (2009). Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. Journal of the American College of Cardiology, 53(6), 501–510. 10.1016/j.jacc.2008.10.033 [DOI] [PubMed] [Google Scholar]
- Ussher, J. R. , Baggio, L. L. , Campbell, J. E. , Mulvihill, E. E. , Kim, M. , Kabir, M. G. , Cao, X. , Baranek, B. M. , Stoffers, D. A. , Seeley, R. J. , & Drucker, D. J. (2014). Inactivation of the cardiomyocyte glucagon‐like peptide‐1 receptor (GLP‐1R) unmasks cardiomyocyte‐independent GLP‐1R‐mediated cardioprotection. Molecular Metabolism, 3(5), 507–517. 10.1016/j.molmet.2014.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ussher, J. R. , & Drucker, D. J. (2014). Cardiovascular actions of incretin‐based therapies. Circulation Research, 114(11), 1788–1803. 10.1161/CIRCRESAHA.114.301958 [DOI] [PubMed] [Google Scholar]
- Verouhis, D. , Saleh, N. , Settergren, M. , Sorensson, P. , Gourine, A. , & Pernow, J. (2019). Remote ischemic conditioning protects against endothelial ischemia‐reperfusion injury via a glucagon‐like peptide‐1 receptor‐mediated mechanism in humans. International Journal of Cardiology, 274, 40–44. 10.1016/j.ijcard.2018.09.061 [DOI] [PubMed] [Google Scholar]
- Vyas, A. K. , Yang, K. C. , Woo, D. , Tzekov, A. , Kovacs, A. , Jay, P. Y. , & Hruz, P. W. (2011). Exenatide improves glucose homeostasis and prolongs survival in a murine model of dilated cardiomyopathy. PLoS One, 6(2), e17178. 10.1371/journal.pone.0017178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, G. R. , Zhu, Y. , Halushka, P. V. , Lincoln, T. M. , & Mendelsohn, M. E. (1998). Mechanism of platelet inhibition by nitric oxide: In vivo phosphorylation of thromboxane receptor by cyclic GMP‐dependent protein kinase. Proceedings of the National Academy of Sciences of the United States of America, 95(9), 4888–4893. 10.1073/pnas.95.9.4888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, R. , Lu, L. , Guo, Y. , Lin, F. , Chen, H. , Chen, W. , & Chen, M. (2015). Effect of glucagon‐like peptide‐1 on high‐glucose‐induced oxidative stress and cell apoptosis in human endothelial cells and its underlying mechanism. Journal of Cardiovascular Pharmacology, 66(2), 135–140. 10.1097/FJC.0000000000000255 [DOI] [PubMed] [Google Scholar]
- Wettergren, A. , Schjoldager, B. , Mortensen, P. E. , Myhre, J. , Christiansen, J. , & Holst, J. J. (1993). Truncated GLP‐1 (proglucagon 78–107‐amide) inhibits gastric and pancreatic functions in man. Digestive Diseases and Sciences, 38(4), 665–673. 10.1007/BF01316798 [DOI] [PubMed] [Google Scholar]
- White, W. B. , Cannon, C. P. , Heller, S. R. , Nissen, S. E. , Bergenstal, R. M. , Bakris, G. L. , Perez, A. T. , Fleck, P. R. , Mehta, C. R. , Kupfer, S. , & Wilson, C. (2013). Alogliptin after acute coronary syndrome in patients with type 2 diabetes. The New England Journal of Medicine, 369(14), 1327–1335. 10.1056/NEJMoa1305889 [DOI] [PubMed] [Google Scholar]
- Withaar, C. , Meems, L. M. G. , Markousis‐Mavrogenis, G. , Boogerd, C. J. , Sillje, H. H. W. , Schouten, E. M. , Dokter, M. M. , Voors, A. A. , Westenbrink, B. D. , Lam, C. S. , & de Boer, R. A. (2020). The effects of liraglutide and dapagliflozin on cardiac function and structure in a multi‐hit mouse model of heart failure with preserved ejection fraction. Cardiovascular Research, cvaa256. 10.1093/cvr/cvaa256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski, M. , Weeks, T. L. , & Hazen, S. L. (2020). Gut microbiota and cardiovascular disease. Circulation Research, 127(4), 553–570. 10.1161/CIRCRESAHA.120.316242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfart, P. , Linz, W. , Hubschle, T. , Linz, D. , Huber, J. , Hess, S. , Crowther, D. , Werner, U. , & Ruetten, H. (2013). Cardioprotective effects of lixisenatide in rat myocardial ischemia‐reperfusion injury studies. Journal of Translational Medicine, 11, 84. 10.1186/1479-5876-11-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo, J. S. , Kim, W. , Ha, S. J. , Kim, J. B. , Kim, S. J. , Kim, W. S. , Seon, H. J. , & Kim, K. S. (2013). Cardioprotective effects of exenatide in patients with ST‐segment‐elevation myocardial infarction undergoing primary percutaneous coronary intervention: Results of exenatide myocardial protection in revascularization study. Arteriosclerosis, Thrombosis, and Vascular Biology, 33(9), 2252–2260. 10.1161/ATVBAHA.113.301586 [DOI] [PubMed] [Google Scholar]
- Yap, M. K. K. , & Misuan, N. (2019). Exendin‐4 from Heloderma suspectum venom: From discovery to its latest application as type II diabetes combatant. Basic & Clinical Pharmacology & Toxicology, 124(5), 513–527. 10.1111/bcpt.13169 [DOI] [PubMed] [Google Scholar]
- Yu, M. , Moreno, C. , Hoagland, K. M. , Dahly, A. , Ditter, K. , Mistry, M. , & Roman, R. J. (2003). Antihypertensive effect of glucagon‐like peptide 1 in Dahl salt‐sensitive rats. Journal of Hypertension, 21(6), 1125–1135. 10.1097/00004872-200306000-00012 [DOI] [PubMed] [Google Scholar]
- Yusta, B. , Baggio, L. L. , Koehler, J. , Holland, D. , Cao, X. , Pinnell, L. J. , Johnson‐Henry, K. C. , Yeung, W. , Surette, M. G. , Bang, K. W. A. , Sherman, P. M. , & Drucker, D. J. (2015). GLP‐1R agonists modulate enteric immune responses through the intestinal intraepithelial lymphocyte GLP‐1R. Diabetes, 64(7), 2537–2549. 10.2337/db14-1577 [DOI] [PubMed] [Google Scholar]


