Abstract
Objectives:
Critically ill children frequently receive plasma and platelet transfusions. We sought to determine evidence-based recommendations, and when evidence was insufficient, we developed expert-based consensus statements about decision-making for plasma and platelet transfusions in critically ill pediatric patients.
Design:
Systematic review and consensus conference series involving multidisciplinary, international experts in hemostasis, and plasma/platelet transfusion in critically ill infants and children (Transfusion Anemia EXpertise Initiative – Control/Avoidance of Bleeding [TAXI-CAB]).
Setting:
Not applicable.
Patients:
Children admitted to a pediatric intensive care unit (PICU) at risk of bleeding and receipt of plasma and/or platelet transfusions.
Interventions:
None
Measurements and Main Results:
A panel of 29 experts in methodology, transfusion and implementation science from five countries and nine pediatric subspecialties completed a systematic review and participated in a virtual consensus conference series to develop recommendations. The search included MEDLINE, EMBASE, and Cochrane Library databases, from inception to December 2020, using a combination of subject heading terms and text words for concepts of plasma and platelet transfusion in critically ill children. Four graded recommendations and forty-nine consensus expert statements were developed using modified RAND/UCLA and GRADE methodology. We focused on 8 sub-populations of critical illness (1, severe trauma, intracranial hemorrhage or traumatic brain injury; 2, cardiopulmonary bypass surgery; 3, extracorporeal membrane oxygenation; 4, oncologic diagnosis or hematopoietic stem cell transplantation; 5, acute liver failure or liver transplantation; 6, non-cardiac surgery; 7, invasive procedures outside the operating room; 8, sepsis and/or disseminated intravascular coagulation) as well as laboratory assays and selection/processing of plasma and platelet components. In total, we came to consensus on 4 recommendations, 5 good practice statements, and 44 consensus-based statements. These results were further developed into consensus-based clinical decision trees for plasma and platelet transfusion in critically ill pediatric patients.
Conclusions:
The TAXI-CAB program provides expert-based consensus for pediatric intensivists for the administration of plasma and/or platelet transfusions in critically ill pediatric patients. There is a pressing need for primary research to provide more evidence to guide practitioners.
MeSH Terms: platelet transfusion, plasma, hemostasis, critical illness, child, thrombocytopenia, coagulopathy
INTRODUCTION
Recent observational studies estimate that 10–15% of children admitted to a pediatric intensive care unit (PICU) have at least one episode of clinically relevant bleeding [1,2]. Critical illness increases risk of bleeding due to organ dysfunction, including bone marrow suppression, hepatic insufficiency and consumptive or dilutional coagulopathy. In addition, critically ill children often receive antiplatelet and anticoagulant medications, such as heparin, non-steroidal anti-inflammatory drugs, or milrinone, and can frequently undergo invasive procedures that increase the risk of bleeding. Bleeding and blood transfusion have been independently associated with adverse clinical outcomes including longer length of mechanical ventilation, increased nosocomial infections, and higher mortality [1,3].
As a consequence, platelet and plasma transfusions are commonly administered in pediatric critical care, when clinically significant bleeding is present or expected or to prevent bleeding complications. Not only may the transfusion of these blood components be ineffective in the prevention/treatment of bleeding at times, but they are associated with an increased risk of morbidity and mortality, including, but not limited to, bacterial contamination, transfusion related acute lung injury (TRALI), transfusion associated circulatory overload (TACO), and transfusion related immunomodulation (TRIM) [4–10]. Recent epidemiologic studies have demonstrated that over two-thirds of these transfusions are administered as prophylaxis to non-bleeding children [11,12]. Even when transfusions are driven by institutional or national guidelines, the specific laboratory thresholds that trigger a transfusion of platelets or plasma vary widely among clinicians and are not based on rigorously obtained data [11,12]. Given the significant risks, guidelines may be useful in to help clinicians to weigh the risks and benefits of transfusion for their patients. Recommendations for the transfusion of plasma and platelets have been developed by several groups, including the American Association of Blood Banks [13], British Committee for Standards in Haematology [14–17], Australian National Blood Authority [18], and the Italian Society of Transfusion Medicine and Immunohaematology [19]. However, these guidelines were not developed specifically in critically ill children and the resulting recommendations may not be applicable to this population.
To address this gap in our understanding of plasma and platelet transfusions in critically ill children, the Transfusion and Anemia eXpertise Initiative – Control/Avoidance of Bleeding (TAXI-CAB) was formed through the Pediatric Critical Care Blood Research Network (BloodNet) of the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network. We sought to determine evidence-based recommendations, but found that evidence was largely insufficient, and consequently we primarily developed expert consensus statements for plasma and platelet transfusions in critically ill children.
METHODS
The methodology for TAXI-CAB was modeled from the TAXI program that has been previously described [20]. Briefly, experts were chosen based on publication history in pediatric hemostasis and/or plasma and platelet transfusions, in conjunction with clinical practice and experience. Efforts were made to include a diverse set of contributors in terms of race, ethnicity and gender. Conflicts were vetted by an independent office. Twenty-nine experts from five countries representing pediatric specialists in intensive care, cardiac intensive care, anesthesiology, emergency medicine, surgery, cardiothoracic surgery, neurosurgery, hematology/oncology, and transfusion medicine participated over an 18-month period. Two information specialists and two experts in methodology and implementation science participated. The experts participated in a systematic review and two virtual consensus conferences. Subtopics were defined for investigation. Subgroups of participants were formed to review the literature pertinent to the topic and present draft recommendations to the entire group for consideration. We focused on 8 sub-populations of critical illness (1, severe trauma, intracranial hemorrhage or traumatic brain injury; 2, cardiopulmonary bypass surgery; 3, extracorporeal membrane oxygenation; 4, oncologic diagnosis or hematopoietic stem cell transplantation; 5, acute liver failure or liver transplantation; 6, non-cardiac surgery; 7, invasive procedures outside the operating room; 8, sepsis and/or disseminated intravascular coagulation) as well as laboratory assays and selection/processing of plasma and platelet components.
We performed comprehensive searches in the following databases: Ovid MEDLINE® (1946 to December 9, 2019), Ovid EMBASE (1974 to December 9, 2019), and Cochrane Library (Wiley). A combination of relevant subject headings and text words were used to identify plasma and platelet transfusion in critically ill pediatric patients. No language, publication date or article type restrictions were applied in the original searches. Conference proceedings were included. Searches were repeated on December 16, 2020 to check for new studies. For articles selected for inclusion, reference lists and citing articles were pulled from Scopus (Elsevier) and screened. Database search results were duplicated and screened using Covidence systematic review software. The full search strategy for Ovid MEDLINE is available in Supplemental Digital Content (see Table S1). Electronic searches and study retrieval were conducted with assistance from the Samuel J Wood Library at Weill Cornell Medicine, New York, NY. Studies were eligible for inclusion if the study population was composed of critically ill children (term infants >1 month of age up to <18 years with the exception of those undergoing CPB surgery or ECMO which included neonates > 36 weeks of gestation), with evaluation of plasma and/or platelet transfusion(s) strategies to prevent or treat bleeding. Studies were excluded if they were 1) not related to indications for plasma and/or platelet transfusion in critically ill pediatric patients within each group’s disease(s) of interest; 2) focused on plasmapheresis; 3) adult-only study population (defined as all patients ≥18 years, or mixed pediatric and adult population if it was not possible to separate data for patients <18 years); 4) animal-only studies; 5) not original data (i.e., reviews, editorials, commentaries, meeting proceedings); 6) case report or case series with sample size ≤10; 7) abstract only; or 8) language: not in English. Systematic reviews were conducted in accordance with the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) statement for observational studies [21], the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement for randomized controlled trials [22] and instructions from the Cochrane Collaboration (www.cochrane.org). The initial search was reviewed by the five members of the executive committee (MEN, OK, SLV, STB, RIP) and abstracts were removed which were not in English or did not pertain to transfusion. The remaining abstracts were then passed on to each sub-group for review (see the Supplement to this issue of the Journal references 23–28), each subgroup developing (1) good practice statements, (2) expert consensus statements and (3) recommendations according to the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) methodology when feasible [29–32]. The strength of the recommendations was based on the level of available evidence. Good practice and expert consensus statements were developed when the quality of studies was poor or lacking and could not be graded. The hierarchy of language used in the statements and recommendations is described in Table 1.
Table 1.
Hierarchy Used in Statements and Recommendations
| Certainty → | CARRY OUT OR DO | DO NOT DO | Certainty → |
| Is recommended | |||
| Should be selected / used | |||
| We suggest | |||
| Is suggested | |||
| Might be considered | May not be beneficial | ||
| Uncertain whether there is any benefit | Unlikely to benefit | ||
| Insufficient evidence | Insufficient evidence |
The consideration of certain laboratory assays and definitions were discussed prior to the systematic review. While the international normalized ratio (INR) is validated as a measure of the anticoagulant intensity of vitamin-K antagonist therapy, it is not predictive of bleeding. Due to the lack of data using other relevant measures of coagulopathy, INR values were incorporated into the expert consensus statements. However, the group recognized these limitations, and, for the purposes of the expert consensus statements, considered that the terms such as INR and Prothrombin time ratio (PTR) are used interchangeably. Likewise, as bleeding can be difficult to define precisely, experts used the Bleeding Assessment Scale in critically Ill Children (BASIC) definition of bleeding in critically ill children to differentiate minimal, moderate and severe bleeding as described in Table 2 [33]. Finally, given the several ways in which plasma can be prepared, we have chosen to use the term plasma to refer to fresh plasma, fresh frozen plasma and Frozen Plasma 24 (FP24).
Table 2.
Bleeding Assessment Scale in critically Ill Children (BASIC)
| Any of the following criteria define severe bleeding: |
|
| All of the following criteria must be present to define moderate bleeding: |
|
| Any of the following criteria define minimal bleeding: |
|
During the first online consensus conference, the recommendations from each group were discussed and debated. Using the Research and Development/University of California, Los Angeles (UCLA) Appropriateness Method [34], the recommendations were scored anonymously using an online tool (Survey Monkey, San Mateo, CA). Using a 9-point Likert scale, ratings of 1–3 indicated disagreement, 4–6 represented neutrality and 7–9 represented agreement. Expert consensus agreement was defined a priori as 80% of the experts rating the recommendation a 7, 8, or 9. If the statements did not reach expert consensus agreement, they were returned to the sub-groups for consideration of revision and resubmission to voting, or removal from further consideration.
During the second online consensus meeting, the final recommendations were discussed, research priorities were debated, and clinical decision trees were developed.
RESULTS
The TAXI-CAB collaboration has reached agreement on four evidence-based recommendations, 5 good practice statements, and 44 expert consensus statements. Each of these are presented with the proportion of experts agreeing, and the median (interquartile range, IQR) of voting scores. All recommendations pertain to infants, children, adolescents and, where noted, neonates. Clinical decision trees were developed to guide plasma and platelet transfusions in critically ill pediatric patients and are presented in Figures 1 and 2.
Figure 1.
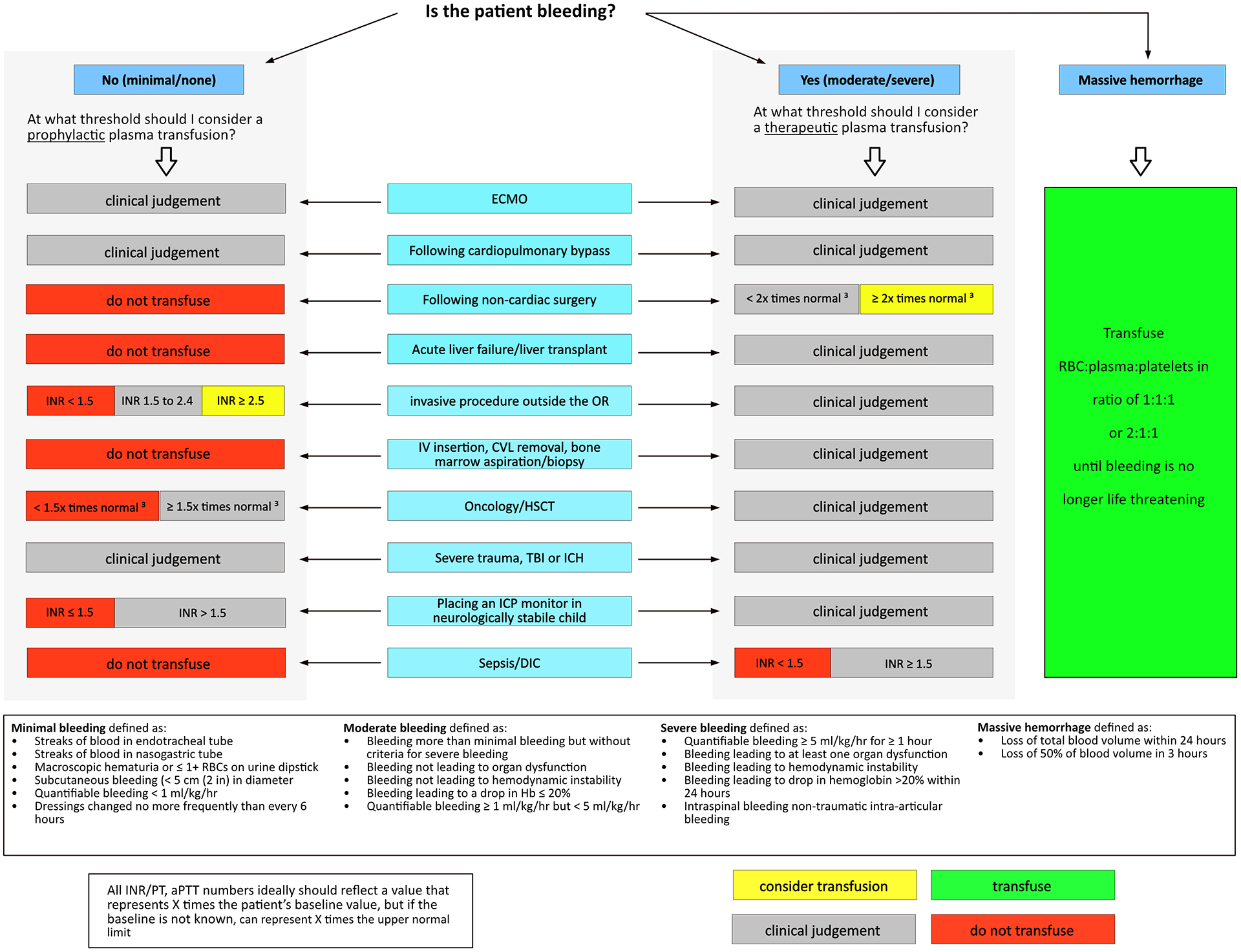
Clinical Decision Tree to Aid Prescription of Plasma in Critically Ill Children
Figure 2.
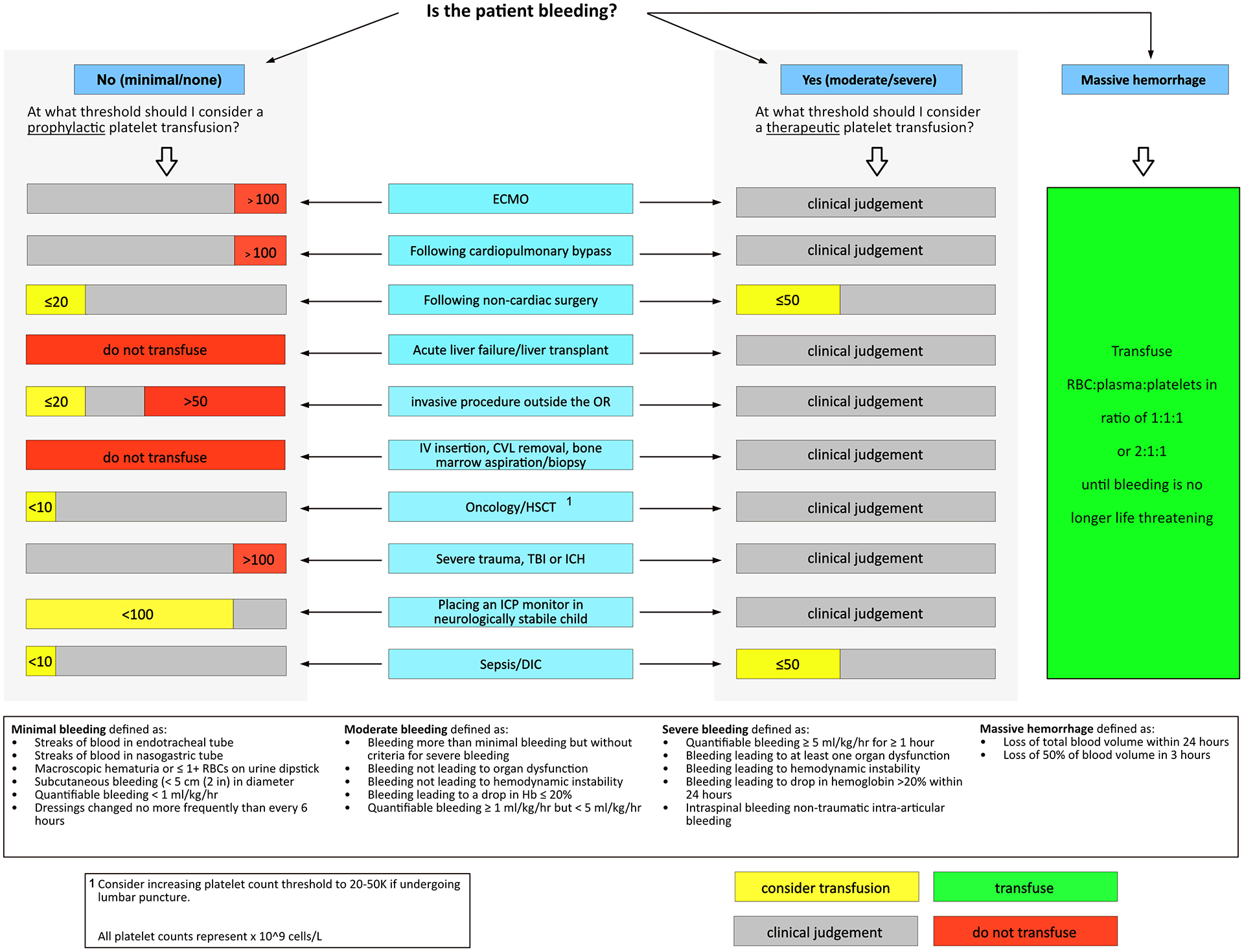
Clinical Decision Tree to Aid Prescription of Platelets in Critically Ill Children
Good Practice Statements applicable to all critically ill children
When deciding to transfuse plasma and/or platelets to a critically ill pediatric patient, we suggest monitoring not only hemostasis (e.g. monitoring coagulation system dysfunction and the platelet count), but also assessing the overall clinical context (e.g. symptoms, signs of bleeding, physiological markers, other laboratory results like platelet dysfunction) and the risk, benefits, and alternatives to plasma and platelet transfusion. Consensus Panel Expertise, 88% Agreement (n=24), Median 8, IQR 7.25–9.
In critically ill pediatric patients, the use of plasma as a volume expander may not be beneficial. Consensus Panel Expertise, 88% Agreement (n=24), Median 8, IQR 8–9.
In critically ill pediatric patients, taking measures to minimize the number of donor exposures might be considered. Consensus Panel Expertise, 88% Agreement (n=24), Median 9, IQR 7.25–9.
Laboratory Assays Used to Prescribe Plasma and Platelet Transfusions (see reference 23)
Expert Consensus Statements
-
1.1.
In critically ill pediatric patients, there is insufficient evidence to recommend a specific laboratory test as a threshold or as a target for platelet transfusion for prophylactic or therapeutic indications. Consensus Panel Expertise, 92% Agreement (n=24), Median 8, IQR 7–9.
Though there have been recent advances in assays of platelet function testing, studies have not been rigorously evaluated to determine the need for platelet transfusion; consequently, the majority of platelet transfusions are prescribed based on platelet count alone. Until such tests are validated, practitioners are encouraged to consider both the presence of thrombocytopenia, and other risk factors, such as presence of severe bleeding, severity of illness, trajectory of illness (deteriorating or recovering), hemodynamic instability, and similar conditions when considering platelet transfusion.
-
1.2
In critically ill pediatric patients, there is insufficient evidence to recommend a specific laboratory test as a threshold or as a target for plasma transfusion for prophylactic or therapeutic indications. Consensus Panel Expertise, 96% Agreement (n=24), Median 8.5, IQR 7.25–9.
Prothrombin time (PT) and activated partial thromboplastin time (aPTT) are often used as an estimation of coagulation efficiency. Similarly, viscoelastic testing (VET), such as thromboelastometry (ROTEM™) or thromboelastography (TEG™), measure various parameters regarding clot formation. However, studies evaluating traditional and viscoelastic test parameters in critically ill children in the evaluation of bleeding are inconclusive, and results should be used to guide plasma transfusion in combination with the clinical scenario.
Indications for Plasma or Platelet Transfusions in Critically Ill Children with Severe Trauma, ICH or TBI (see reference 24)
Good Practice Statement
-
2.1.
In critically ill pediatric patients with severe trauma, moderate to severe TBI or non-traumatic ICH, we suggest close monitoring of bleeding, including evaluation of potential expansion of ICH, and serial evaluation of the coagulation system. Consensus Panel Expertise, 83% Agreement (n=24), Median 8, IQR 7–9.
There is an absence of high-quality data to guide best plasma and platelet transfusion strategies in pediatric patients with severe trauma, moderate to severe TBI or non-traumatic ICH. Nevertheless, we suggest close monitoring with serial evaluation of the coagulation system, including standard coagulation tests – PT/INR, aPTT, fibrinogen and/or platelet count - in pediatric patients with severe trauma, moderate to severe TBI or non-traumatic ICH. In some instances, TEG or ROTEM can be considered to guide decision making.
Expert Consensus Statements
-
2.2.
In critically ill pediatric patients with severe trauma, moderate to severe TBI or in critically ill children with non-traumatic ICH, there is insufficient evidence to make any recommendation regarding specific indications or transfusion strategies to direct plasma or platelet transfusion. Consensus Panel Expertise, 83% Agreement (n=24), Median 8, IQR 7–9.
-
2.3.
In critically injured pediatric patients in hemorrhagic shock following trauma, a resuscitation strategy of red blood cells (RBCs), plasma, and platelets in ratios between 2:1:1 to 1:1:1 might be considered. Consensus Panel Expertise, 90% Agreement (n=21), Median 8, IQR 7.5–9.
Massive transfusion (MT) is required in critically injured pediatric patients in hemorrhagic shock following trauma. MT can be delivered by the transfusion of RBC, plasma and platelets in a set ratio or the transfusion of whole blood. Despite the lack of strong pediatric evidence, early replacement therapy with ratios between 1:1:1 or 2:1:1 for RBC/plasma/platelets might be considered.
-
2.4.
In critically ill pediatric patients with severe trauma, moderate to severe TBI or non-traumatic ICH, viscoelastic testing (e.g. TEG, ROTEM) might be considered as an adjunct to standard laboratory hemostatic testing to inform decisions regarding the transfusion of plasma and/or platelets. Consensus Panel Expertise, 95% Agreement (n=22), Median 8.5, IQR 7.75–9.
Within minutes of injury, a high proportion of patients severely injured and in hemorrhagic shock develop a clotting abnormality termed Trauma Induced Coagulopathy (TIC). While there are no convincing data on which coagulation studies constitute most informative monitoring in patients with severe trauma, moderate to severe TBI or non-traumatic ICH, standard laboratory hemostatic testing and VET might be considered.
-
2.5.
In a neurologically stable critically ill pediatric patient with severe trauma, moderate or severe TBI and/or ICH, platelet transfusion if the platelet count is > 100×109/L (100,000/mm3) may not be beneficial. Consensus Panel Expertise, 91% Agreement (n=23), Median 9, IQR 8–9.
While the threshold below which platelet transfusion should be given is not clear, in a child with stable Glasgow coma score, intracranial pressure (ICP), cerebral perfusion pressure and in whom there are no increased seizures, platelet transfusion administered when the platelet count is > 100×109/L may not be beneficial and has the potential to harm.
-
2.6.
If an ICP monitoring device must be inserted in a neurologically deteriorating critically ill pediatric patient with TBI and/or ICH, platelet transfusion might be considered if the platelet count is < 100 × 109/L (100,000/mm3). Consensus Panel Expertise, 86% Agreement (n=22), Median 7.5, IQR 7–9.
This guidance is based solely on expert opinion alone with strong agreement. No studies have addressed this question in children and at this time, the American Society of Neurosurgery and the American Society of Anesthesiology have no recommendations for threshold platelet count levels for placement of an ICP monitor.
-
2.7.
If an ICP monitoring device must be inserted in a neurologically deteriorating critically ill pediatric patient with TBI and/or ICH, plasma transfusion if the INR is ≤ 1.5 may not be beneficial. Consensus Panel Expertise, 87% Agreement (n=23), Median 8, IQR 8–9.
There are no formal recommendations from neurological societies related to plasma transfusion prior to ICP monitor placement. Small observational studies have noted no increased risk of hemorrhage with ICP placement when the INR ≤ 1.5.
Indications for Plasma or Platelet Transfusions in Critically Ill Children Following CPB Surgery (see reference 25)
Clinical Recommendations
-
3.1.
In neonatal and pediatric patients undergoing cardiac surgery with CPB, VET might be considered as an adjunct to standard hemostatic testing (in the operating room after rewarming and protamine, and in the intensive care unit) to inform decisions regarding prophylactic and therapeutic transfusions of plasma and/or platelets. Weak recommendation, Moderate quality level of evidence (2B), 82% Agreement (n=22), Median 8, IQR 7–9.
Intra operative VET is increasingly used for anticoagulation monitoring during CPB and to guide the choice of blood product transfusion post-operatively. Monitoring coagulation with point of care VET during CPB with intuition specific criteria, may generate sufficient evidence to develop patient specific recommendations for transfusion. VET-guided protocols might be considered in maximizing hemostasis, reducing the amount of wasted blood products, institutional costs, and associated complications.
-
3.2.
In neonatal and pediatric patients undergoing cardiac surgery with CPB, the development of institution-specific transfusion algorithms for post-bypass (after rewarming and protamine, and in intensive care unit) transfusion management might be considered to reduce individual case and global overall blood product usage. Weak recommendation, Moderate quality level of evidence (2B), 96% Agreement (n=23), Median 8, IQR 8–9.
In some settings, the implementation of transfusion algorithms for children following CPB have been shown to decrease total transfusions without an increase in bleeding. Additionally, the use of transfusion algorithms in this patient population has been independently associated with reduced mortality.
Expert Consensus Statement
-
3.3.
In neonatal and pediatric patients undergoing cardiac surgery, there is insufficient evidence to make a recommendation regarding specific indications or transfusion strategies to direct plasma or platelet transfusion. Consensus Panel Expertise, 87% Agreement (n=23), Median 8, IQR 8–9.
While transfusion algorithms have decreased transfusion exposure, specific thresholds at which to transfuse are unclear. Universally accepted bleeding definitions for CPB necessary to evaluate management strategies are not available. In addition, limitations and challenges include patient factors such as age/gestational age, pre-existing conditions, or end-organ compromise; hemostatic alterations due to CPB; and operative characteristics such as surgical technique variability and surgical hemostatic control.
Indications for Plasma or Platelet Transfusions in Critically Ill Children Supported by Extracorporeal Membrane Oxygenation (ECMO) (see reference 25)
Good Practice Statement
-
4.1.
In critically ill neonatal and pediatric patients on ECMO, we suggest measuring platelet counts and coagulation system dysfunction before all platelet and plasma transfusions, unless the patient experiences life-threatening bleeding. Consensus Panel Expertise, 92% Agreement (n=24), Median 9, IQR 7.25–9.
In the absence of clinically significant bleeding and/or laboratory results predictive of bleeding and response to transfusion products, transfusion of platelets and/or plasma may expose patients to the risks of transfusion without potential benefit. Therefore, we suggest that laboratory assays be used to help direct the decision to transfuse.
Expert Consensus Statements
-
4.2.
In critically ill neonatal and pediatric patients on ECMO, there is insufficient evidence to make a recommendation regarding specific indications or transfusion strategies to direct plasma or platelet transfusion. Consensus Panel Expertise, 96% Agreement (n=23), Median 8, IQR 8–9.
Platelet transfusions are potentially harmful and should be avoided when possible. However, the optimal thresholds at which platelets should be administered have not been determined. Likewise, data to inform decisions regarding plasma transfusions in neonates and children supported by ECMO are lacking. The decisions are complex, as anticoagulation strategies, hemostatic monitoring and plasma transfusion practices vary across institutions.
-
4.3.
In critically ill neonatal and pediatric patients on ECMO, prophylactic platelet transfusion in the absence of clinically significant bleeding is unlikely to benefit patients if the platelet count is >100 × 109/L (100,000/mm3). Consensus Panel Expertise, 91% Agreement (n=23), Median 9, IQR 8–9.
The majority of studies report arbitrary platelet transfusion thresholds of 50–100 ×109/L to guide platelet transfusion during neonatal and pediatric ECMO support. Considering the morbidities associated with platelet transfusions, prophylactic platelet transfusions above this threshold are unlikely to benefit patients and may even be harmful.
Indications for Plasma and Platelet Transfusions in Critically Ill Children with an Underlying Oncologic Diagnosis or Following HSCT (see reference 26).
Expert Consensus Statements
-
5.1.
In critically ill neonatal or pediatric oncology/HSCT patients, there is insufficient evidence to make a recommendation regarding specific indications or transfusion strategies to direct plasma or platelet transfusion. Consensus Panel Expertise, 95% Agreement (n=22), Median 8, IQR 7.75–9.
-
5.2.
In critically ill pediatric oncology/HSCT patients, prophylactic platelet transfusions might be considered for a platelet count < 10 × 109/L (10,000/mm3). Consensus Panel Expertise, 100% Agreement (n=22), Median 8, IQR 7–9.
There are few studies that provide evidence about prophylactic platelet indications in children with leukemia and none in critically ill oncology children. This expert consensus statement is extrapolated from studies in adults and non-critically ill children.
-
5.3
In critically ill pediatric oncology/HSCT patients, therapeutic platelet transfusions might be considered for moderate or severe bleeding. Consensus Panel Expertise, 82% Agreement (n=22), Median 8, IQR 7–9.
This expert consensus statement took into consideration the higher rates of clinically significant bleeding seen in pediatric oncology patients as compared with adults, the results of randomized controlled trials (RCTs) performed in pediatric and adult patients and guidance provided from international guidelines. While platelet transfusions might be considered in bleeding patients, a specific pre-transfusion platelet threshold is not supported by data.
-
5.4.
In critically ill pediatric oncology/HSCT patients, prophylactic plasma transfusions for minor coagulopathy (INR ≤ 1.5, aPTT ≤ 1.5) may not be beneficial unless performing surgery in a critical site (orbit, brain, facial nerve, spinal column, etc.). Consensus Panel Expertise, 86% Agreement (n=22), Median 8, IQR 7–9.
Given the risks and limited evidence of benefit, prophylactic plasma transfusions for minor coagulopathy may not be beneficial to patients. The risks of pre-surgical plasma transfusions need to be weighed against the child’s underlying co-morbidities, severity of illness, etiology of the coagulopathy, type of surgical procedure, risk of bleeding, and additional hemostatic measures.
Indications for Plasma or Platelet Transfusions in Critically Ill Children with Acute Liver Failure or Following Liver Transplantation (see reference 26)
Expert Consensus Statements
-
6.1.
In critically ill pediatric patients with acute liver failure and/or following liver transplantation, there is insufficient evidence to make a recommendation regarding the specific indications or transfusion strategies to direct plasma or platelet transfusion. Consensus Panel Expertise, 96% Agreement (n=23), Median 8, IQR 8–9.
-
6.2.
In critically ill pediatric patients with acute liver failure and/or following liver transplantation, prophylactic plasma and platelet transfusions may not be beneficial in the absence of moderate or severe bleeding. Consensus Panel Expertise, 87% Agreement (n=23), Median 8, IQR 7–9.
The expert consensus that plasma transfusions may not be beneficial when used prophylactically to prevent bleeding in pediatric patients with acute liver failure or post liver transplantation is supported by limited data as they do not decrease the overall plasma pro-thrombotic profile and may increase the risk of postoperative thrombosis. To date, no studies have specifically assessed use of platelet transfusions in this population.
-
6.3.
In critically ill pediatric patients with acute liver failure and/or following liver transplantation, restrictive plasma and platelet transfusion strategies might be considered including not treating a laboratory number alone, but taking into consideration the overall clinical hemostatic status of the infant or child. Consensus Panel Expertise, 96% Agreement (n=23), Median 8, IQR 7–9.
In liver failure or following liver transplantation, the hemostatic system is “rebalanced” as both procoagulant and anticoagulant factor levels are abnormal. Standard laboratory assays may be abnormal but do not predict bleeding. Retrospective data suggest that restrictive plasma transfusion strategies might be considered.
Indication for Plasma or Platelet Transfusions in Critically Ill Children Following Non-Cardiac Surgery (see reference 27)
Expert Consensus Statements
-
7.1.
In critically ill pediatric patients who have undergone non-cardiac surgery and have no active or minimal bleeding, there is insufficient evidence to make a recommendation regarding specific indications or transfusion strategies to direct plasma or platelet transfusion. Consensus Panel Expertise, 100% Agreement (n=24), Median 8, IQR 8–9.
-
7.2.
In critically ill pediatric patients who have undergone non-cardiac surgery and have moderate bleeding, there is insufficient evidence to make a recommendation regarding specific indications or transfusion strategies to direct plasma or platelet transfusion. Consensus Panel Expertise, 96% Agreement (n=24), Median 8, IQR 7.25–9.
-
7.3.
In critically ill pediatric patients who have undergone non-cardiac surgery and who are not actively bleeding or have minimal bleeding, routine coagulation testing may not be beneficial. Consensus Panel Expertise, 87% Agreement (n=23), Median 8, IQR 8–9.
Studies have demonstrated that neither preoperative bleeding history nor routine laboratory screening with tests that assess in vitro time to clot (e.g. PT/INR, aPTT) are reliable predictors of postoperative bleeding.
-
7.4.
In critically ill pediatric patients who have undergone non-cardiac surgery and who are not actively bleeding or have minimal bleeding, if any coagulation testing is done and an abnormality is noticed, prophylactic plasma transfusion based solely on abnormal PT, INR or aPTT values may not be beneficial and we encourage formal evaluation by hematology or transfusion medicine in order to ascertain the cause. Consensus Panel Expertise, 86% Agreement (n=22), Median 8, IQR 7–9.
Prophylactic plasma transfusion based solely on abnormal coagulation parameters may result in overuse of plasma and may not be beneficial.
-
7.5.
In critically ill pediatric patients who have undergone non-cardiac surgery and who have moderate bleeding, coagulation testing might be considered to ascertain the likely etiology of bleeding and to permit appropriate targeted intervention. Consensus Panel Expertise, 87% Agreement (n=23), Median 8, IQR 7–9.
If bleeding and abnormal coagulation tests persist in a post-surgical patient with moderate bleeding, coagulation testing might be considered, and etiology of bleeding should be promptly investigated and treated.
-
7.6.
In critically ill pediatric patients who have undergone non-cardiac surgery and who have moderate bleeding, plasma transfusion might be considered to correct abnormal PT, INR or aPTT values that are ≥2.0 times the reference value (as compared to baseline for patient if available or upper limit of normal for local institution). The risk of plasma transfusion should be balanced against the clinical condition, timing from surgery, type of surgery, site of bleeding and the risks associated, pattern or trajectory of bleeding, as well as consideration of coagulation parameters such as plasma fibrinogen, platelet count and viscoelastic monitoring results. In addition to clinical evaluation for bleeding at the surgical site, we suggest formal evaluation by experts in hematology or transfusion medicine if the etiology of bleeding is unclear. Consensus Panel Expertise, 87% Agreement (n=23), Median 8, IQR 8–9.
The primary indication for plasma products is repletion of coagulation factors in the context of abnormal hemostasis associated with clinically relevant bleeding. Plasma might be considered only if the benefit of correcting abnormal hemostasis (reduction of bleeding) is judged to outweigh the risks of transfusion and a safer alternative is unavailable. Plasma products are only partially effective to replete coagulation factors, have short duration of action, may contribute to morbidity.
-
7.7.
In critically ill pediatric patients who have undergone non-cardiac surgery and have no active or minimal bleeding, platelet transfusion might be considered when the platelet count is ≤ 20 × 109/L (20,000/mm3). The risk of platelet transfusion should be balanced against the clinical condition, timing from surgery, type of surgery and potential risk of bleeding. Consensus Panel Expertise, 96% Agreement (n=23), Median 8, IQR 7–9.
-
7.8.
In critically ill pediatric patients who have undergone non-cardiac surgery and have moderate bleeding, platelet transfusion might be considered when the platelet count is ≤ 50 × 109/L (50,000/mm3). The risk of platelet transfusion should be balanced against the clinical condition, timing from surgery, type of surgery, pattern or trajectory of bleeding, site of bleeding and associated risk, other coagulation parameters. Consensus Panel Expertise, 96% Agreement (n=23), Median 8, IQR 7–9.
Clinical judgement is warranted when one assesses the need for platelet transfusion in the context of postoperative bleeding. Factors to consider include whether there is an acquired or inherited platelet function defect, coagulopathy involving platelet consumption such as DIC, or bleeding at the surgical site. Generally, a patient with a platelet count >50 ×109/L does not require treatment unless additional issues that might impair hemostasis and cause further blood loss are present and/or expected.
Indications for Plasma and Platelet Transfusions in Critically Ill Children Undergoing Invasive Procedures Outside of the Operating Room (see reference 27)
Expert Consensus Statements
-
8.1.
In critically ill pediatric patients undergoing invasive procedures outside of the operating room, there is insufficient evidence to make a recommendation regarding specific indications or transfusion strategies to direct plasma or platelet transfusion. Consensus Panel Expertise, 100% Agreement (n=22), Median 8, IQR 8–9.
-
8.2.
In critically ill pediatric patients undergoing invasive procedures outside of the operating room (e.g., central line insertion, liver biopsy), plasma transfusion may not be beneficial if the INR is ≤ 1.5. Consensus Panel Expertise, 91% Agreement (n=22), Median 9, IQR 8–9.
Observational studies of both adults and children have shown no appreciable differences in INR measurements pre and post plasma transfusion when the pre-transfusion INR is ≤ 1.5.
-
8.3.
In critically ill pediatric patients undergoing invasive procedures outside of the operating room, it is uncertain whether there is any benefit of plasma transfusions in correcting an INR between 1.6 and 2.4, and the risks of the plasma transfusion should be balanced against the clinical condition, the presence of bleeding symptoms, other coagulation abnormalities, the type of procedure, the risks associated with procedure, and the risk of bleeding. Consensus Panel Expertise, 85% Agreement (n=20), Median 8, IQR 8–9.
Studies in adults and children, including critically ill children, have demonstrated that plasma transfusions have only very small, if any, effect on standard coagulation testing for patients with mild coagulopathies, including those with INR values as high as 2.5. The clinical context must be considered before plasma is administered.
-
8.4.
In pediatric patients undergoing invasive procedures outside of the operating room in whom the INR is > 2.5, plasma transfusion might be considered, but should be balanced against the risks of the plasma transfusion and the clinical context. Consensus Panel Expertise, 91% Agreement (n=22), Median 8, IQR 7.75–9.
Plasma transfusion may shorten the INR when it is > 2.5 and therefore might be considered in these cases. However, the clinical context, especially the degree of invasiveness and associated risk of bleeding with the procedure, should be considered.
-
8.5.
In critically ill pediatric patients undergoing invasive procedures outside of the operating room, prophylactic platelet transfusions may not be beneficial when the platelet count is > 50 × 109/L (50,000/mm3). Consensus Panel Expertise, 83% Agreement (n=23), Median 8, IQR 7–9.
The majority of studies examining thresholds for platelet transfusion have focused on children with liver failure undergoing liver biopsy or children with oncologic diagnoses undergoing lumbar puncture. There are limited data for children undergoing central line placement in the PICU.
-
8.6.
In critically ill pediatric patients undergoing invasive procedures outside of the operating room, it is uncertain whether there is any benefit of prophylactic platelet transfusion when the platelet count is between 20 and 50 × 109/L (20–50,000/mm3). The risks of the platelet transfusion should be balanced against the clinical condition, the presence of bleeding symptoms, any other coagulation abnormalities, the procedure, the risks associated with procedure, and the risk of bleeding. Consensus Panel Expertise, 86% Agreement (n=22), Median 8 (IQR 7–9).
As the benefits of platelet transfusions in these scenarios are unknown, the clinical context, including the technique being used to perform the procedure (such as the use of ultrasound) must inform the decision to transfuse.
-
8.7.
In critically ill pediatric patients undergoing invasive procedures outside of the operating room, platelet transfusion might be considered when the platelet count is ≤ 20 × 109/L (20,000/mm3). Consensus Panel Expertise, 87% Agreement (n=23), Median 8, IQR 7–9.
Though no pediatric studies have examined this threshold, the AABB suggests platelet transfusion for adults who are undergoing central line placement when the platelet count is ≤ 20 × 109/L (20,000/mm3) [27].
-
8.8.
In critically ill pediatric patients, prophylactic platelet transfusion may not be beneficial prior to minor procedures such as peripheral intravenous cannula insertion, central line catheter removal, bone marrow aspirate and bone marrow biopsy. Consensus Panel Expertise, 85% Agreement (n=20), Median 7, IQR 7–8.75.
A variety of procedures are performed in the PICU that have very low bleeding risk. Prophylactic platelet transfusion may not benefit these patients and may be associated with harm.
-
8.9.
In critically ill pediatric patients undergoing elective lumbar punctures, it is uncertain whether there is any benefit of prophylactic platelet transfusion when the platelet count is 20 to 50 ×109/L (20–50,000/mm3). The risks of the platelet transfusion should be balanced against the clinical condition, the presence of bleeding symptoms, other coagulation abnormalities, the procedure, the risks associated with procedure, and the risk of bleeding. Consensus Panel Expertise, 90%Agreement (n=20), Median 8, IQR 7–8.75.
Most of the data exploring this question have focused on children with oncologic diagnoses. A recent observational study of over 2000 adults and children showed no difference in complications when platelet count ≥ or < 50 ×109/L [27]. However, the thresholds for platelet transfusion were not established. Other patient factors, and the experience of the operator, should be considered.
-
8.10
In critically ill pediatric patients undergoing elective lumbar puncture, prophylactic platelet transfusion might be considered when the platelet count is <20 × 109/L (20,000/mm3). Consensus Panel Expertise, 84% Agreement (n=19), Median 8, IQR 7–9.
The risk of traumatic tap and, more importantly, spinal hematoma, increases when lumbar puncture is performed in the setting of severe thrombocytopenia. British guidelines support consideration of platelet transfusion for lumbar puncture when platelet count is <20 × 109/L [27].
Indications for Plasma or Platelet Transfusion in Critically Ill Children with Sepsis and/or DIC (see reference 26)
Expert Consensus Statements
-
9.1.
In critically ill pediatric patients with sepsis and/or DIC, there is insufficient evidence to make a recommendation regarding specific indications or transfusion strategies to direct plasma or platelet transfusion. Consensus Panel Expertise, 95% Agreement (n=20), Median 8, IQR 8–9.
-
9.2
In critically ill pediatric patients with sepsis and/or DIC, in the absence of moderate or severe bleeding, prophylactic plasma transfusion may not be beneficial. Consensus Panel Expertise, 83% Agreement (n=23), Median 8, IQR 8–9.
Observational studies suggest an independent association between plasma transfusions and increased mortality and morbidity [26]. Although these studies did not specifically evaluate children with sepsis, decisions to transfuse plasma to children must take into account the risk of bleeding along with the risk of transfusion.
-
9.3.
In critically ill pediatric patients with sepsis and/or DIC and moderate bleeding, plasma transfusion may not be beneficial if the INR is ≤ 1.5. Consensus Panel Expertise, 96% Agreement (n=23), Median 8, IQR 8–9.
In a large cohort of critically ill children, no measurable effect of plasma was detected when the INR was between 0.5 and 1.4, although patients with sepsis/DIC were not evaluated separately [26]. Plasma may not be beneficial children with sepsis and/or DIC with moderate bleeding and mild coagulopathies.
-
9.4.
In critically ill pediatric patients with sepsis and/or DIC, in the absence of moderate or severe bleeding, platelet transfusion might be considered when the platelet count is <10 × 109/L (10,000/mm3). Consensus Panel Expertise, 87% Agreement (n=23), Median 8, IQR 7–9.
Platelets are consumed during DIC, with both bleeding and thrombosis being associated with the thrombocytopenia. Consequently, decisions to transfuse platelets must be made with caution. This guidance is drawn from evidence in children with oncologic diagnoses.
-
9.5.
In critically ill pediatric patients with sepsis and/or DIC and moderate bleeding, platelet transfusion might be considered when the platelet count is <50 × 109/L (50,000/mm3). Consensus Panel Expertise, 87% Agreement (n=23), Median 8, IQR 7–9.
Similar to the above expert consensus statement, there are no pediatric studies examining thresholds for platelet transfusion in children with sepsis and/or DIC with clinically relevant bleeding. This suggestion is consequently based on adult data.
Selection and Processing of Plasma and Platelet Components for Transfusion in Critically Ill Children (see reference 23)
Clinical Recommendations
-
10.1.
In critically ill neonatal and pediatric patients, the use of leukocyte reduced cellular blood components is recommended. Strong recommendation, Low quality pediatric evidence (1C), 96% Agreement (n=23), Median 9, IQR 8–9.
Leukocyte reduction for cellular blood products has become standard in transfusion practice due to decreased alloimmunization, fewer febrile reactions and decreased cytomegalovirus (CMV) transmission.
-
10.2.
In a critically ill RhD negative neonate, infant or child in need of a platelet transfusion, RhD positive platelets should be used if an RhD negative platelet component is not available. Strong recommendation, Low quality pediatric evidence (1C), 95% Agreement (n=20), Median 8, IQR 8–9.
Clinical studies have found the risk of RhD alloimmunization due to platelet transfusion to be very low. The transfusion of RhD positive platelets, especially apheresis products, to RhD negative patients who are bleeding or have met clinical or laboratory criteria for a platelet transfusion, appears to be safe, and are appropriate for transfusion particularly if platelet supply is limited.
Expert Consensus Statements
-
10.3.
When considering pathogen reduction and selecting products for plasma transfusion, products may be selected that balance risk of transfusion-transmitted infection, hemostatic effects, and clinical outcomes, as well as feasibility. Consensus Panel Expertise, 92% Agreement (n=24), Median 9, IQR 8–9.
Methods have been developed to decrease the risk of pathogen transmission in pooled plasma products. Observational data suggests pathogen reduced plasma may be independently associated with lower risk of mortality [23], but the hemostatic effects of pathogen reduction on plasma have not been studied in children. The method used for pathogen reduction may be based on local availability.
-
10.4.
When considering pathogen reduction and selecting products for platelet transfusion, products may be selected that balance risk of transfusion-transmitted infection, hemostatic effects, and clinical outcomes, as well as feasibility. Consensus Panel Expertise, 87% Agreement (n=23), Median 9, IQR 8–9.
Similar methods have been developed to decrease the risk of pathogen transmission related to room temperature storage of platelets. However, the hemostatic efficacy is unclear.
-
10.5.
When a critically ill pediatric patient has persistently poor platelet count increments following platelet transfusion, a clinical and laboratory assessment for platelet refractoriness is suggested to elucidate the cause. Consensus Panel Expertise, 96% Agreement (n=23), Median 9, IQR 8–9.
Platelet transfusion refractoriness (PTR) is generally defined as persistently insufficient post-transfusion platelet count increments following platelet transfusion from random donors. PTR can be due to either immune or non-immune causes, with non-immune causes common. HLA-matching of platelet transfusions can be done if immune causes are identified.
DISCUSSION
The TAXI-CAB program, the second phase following the TAXI RBC transfusion guidelines, has been able to determine by systematic assessment of the literature that in regard to platelet or plasma transfusion there is insufficient evidence to support the use of a specific laboratory test as a threshold or as a target for prophylactic or therapeutic transfusion. Furthermore, in none of the eight clinical settings that were reviewed (i.e., 1, severe trauma, ICH or TBI; 2, CPB surgery; 3, ECMO; 4, oncologic diagnosis or HSCT; 5, acute liver failure or liver transplantation; 6, non-cardiac surgery; 7, invasive procedures outside the operating room; 8, sepsis and/or DIC) could we find sufficient evidence to support specific indications for transfusion strategies to direct use of plasma or platelet transfusion. However, we were able to make four evidence-based recommendations: two of these with strong recommendations albeit based on low quality pediatric evidence for laboratory processing/selection practice, and two with weak recommendation based on moderate quality evidence for post-CPB surgery care. The panel of 29 experts were also able to use the systematic literature to develop and agree on 5 good practice statements and 44 expert consensus statements. In contrast to TAXI, our extensive systematic review identified very few studies of high quality related to plasma and/or platelet transfusion strategies in our population of interest. Therefore, a supplement in Pediatric Critical Care Medicine also includes work on research priorities for plasma and platelet transfusion strategies in critically ill children (28).
Similar to TAXI, which concluded that hemoglobin level alone should not drive the decision to transfuse RBCs [35], our search demonstrated that the commonly used coagulation parameters, INR, aPTT and platelet count, are not sufficient, either alone or in combination, to guide the prescription of plasma and platelet transfusions in critically ill children. Our experts agree that more emphasis should be given to the overall clinical hemostatic status of the patient such as bleeding signs and physiological markers, alongside other laboratory results such as fibrinogen, a key factor determining overall hemostatic competence. Though not routinely available nor standardized in the pediatric setting, VET and measures of platelet function may also aid the clinician in identifying those situations where a transfusion of platelets and/or plasma may be more likely to be beneficial or can be safely withheld.
Whereas total platelet count, INR and/or aPTT are often incorporated into the clinical decision trees as support to withhold transfusion and use restrictive transfusion practices, one must use caution not to interpret them inversely (i.e., transfuse below these thresholds). The listed values were, for the most part, not determined by rigorous testing [36]. Clinicians must also consider that, in many circumstances transfusion of plasma and/or platelets may cause harm without benefit when administered above the stated values. While INR and/or PT ratios were used to describe coagulation abnormalities in our patient population, they may not be the ideal assays to guide plasma transfusion. Similarly, while recommendations for platelet transfusion are based on platelet count, platelet dysfunction is likely to be present in some critically ill pediatric patients. However, the prevalence and magnitude of platelet dysfunction in this population is unknown. Platelet dysfunction or acquired von Willebrand syndrome may result from blood circulation through mechanical circuitry (such as CPB, ECMO, ventricular assist devices, or hemofiltration), renal failure or pharmacologic agents (such as anti-platelet agents, non-steroidal anti-inflammatories, or milrinone) [37]. Platelet aggregation studies may provide additional information in children who are at high risk of bleeding from platelet dysfunction; in those children, total platelet count should not be the sole indication for platelet transfusion [38]. Clinicians must consider the etiology of the thrombocytopenia and/or coagulopathy when deciding to transfuse.
Similar systematic reviews with guideline development have been published. Hematology/transfusion task forces in both the United Kingdom and Australia have addressed plasma and platelet transfusion guidelines for children [14, 17, 18]. TAXI-CAB recommendations and consensus statements differ from the previous published guidelines in that they are focused solely on critically ill children and particular clinical scenarios these children face, as well as being up to date with current literature. In some clinical settings, such as children with DIC or those undergoing central line placement, the expert opinion-based thresholds for transfusion in TAXI-CAB are similar to those previously recommended. However, TAXI-CAB addresses one of the most transfused patient populations, those supported by mechanical circulatory devices such as ECMO [39] and includes bleeding definitions developed particularly for critically ill children.
Whereas TAXI-CAB recommendations and consensus statements were developed with the primary focus on pediatric critical care medicine, we included specialists who practice in the operating room, emergency room and general pediatric units so that the suggestions may be considered outside of the PICU. Other factors certainly play a role in the care of children outside of the PICU, such as the ability to obtain laboratory testing in a timely fashion in the operating room, or the ability to follow-up a patient upon discharge from a general pediatric unit. However, the TAXI-CAB recommendations and consensus statements may be extrapolated and considered in the care of children outside of the PICU.
The clinical decision trees summarize each of the statements from the group. The articles pertaining to the specific clinical subgroups in the supplement to this journal provide detailed explanation and justification for each statement [23–28]. In addition, the clinical decision trees are intended to serve as the basis for the development of clinical decision support tools within an electronic medical record. Though we attempted to identify all sub-groups of critically ill children who may be at risk of receiving plasma or platelet transfusions, there will be children whose clinical scenario falls outside of those outlined here.
The TAXI-CAB expert consensus document was formulated using a strong methodology, included a diverse group of experts and incorporated BASIC, a validated definition of bleeding created for critically ill children [33]. However, the findings and statement do have limitations. As noted in many of the expert consensus statements, the body of evidence for plasma and platelet transfusions in critically ill children is limited. In nearly all groups, the evidence was not appropriate for applying GRADE methodology. Research priorities have been outlined in a separate paper to help address some of these gaps [28]. The definition of critical illness is limited; admission to the PICU may not denote critical illness. In addition, the population of critically ill children is extremely heterogeneous, and one document may not suffice to address all of the possible pathologies present. Though we did search neonatal literature, the expert consensus statements are not intended to apply to pre-term neonates. We did not include children with hemoglobinopathies, congenital bleeding or clotting disorders, inherited platelet abnormalities, thrombophilia or solid organ transplant recipients (other than liver transplants) in our search. We did not review literature or make statements for critically ill children undergoing pheresis procedures, including those with conditions such as thrombocytopenia associated multi-organ failure (TAMOF). While our aim was to provide expert consensus statements that can be implemented across all settings, we recognize that blood component therapy, particularly a sufficient supply of platelets, may be difficult to maintain and therefore these recommendations may not be suitable for resource limited areas. Hemostasis in critically ill children is complex and there are many interventions, both blood component-based, such as cryoprecipitate or fibrinogen concentrates, and pharmacologic measures, such as antifibrinolytics, that are not addressed by the TAXI-CAB program. Such work will be addressed in subsequent phases of the program.
CONCLUSIONS
The TAXI-CAB program aimed to provide guidance and recommendations for the prescription of plasma and platelet transfusions in critically ill infants and children. Even though there is insufficient literature to provide clinicians with such a document, our expert panel has reached agreement on many consensus statements. These statements are summarized in clinical decision trees about specific patient populations who may be bleeding or at risk of bleeding and may require hemostatic interventions.
Supplementary Material
ACKNOWLEDGMENTS
We thank all members of TAXI-CAB for their support, especially during the Coronavirus Disease 2019 pandemic. In addition, we thank the Chaire Héma-Québec-Bayer en médecine transfusionnelle de l’Université de Montréal, AABB, the Society for the Advancement of Blood Management, the Network for the Advancement of Patient Blood Management, Haemostasis and Thrombosis, the International Society of Blood Transfusion, the Society for Critical Care Medicine and the American Association of Neurological Surgeons for their support.
Financial Support:
The Transfusion and Anemia EXpertise Initiative – Control/Avoidance of Bleeding (TAXI-CAB) was supported, in part, by the National Institutes of Health National Heart, Lung and Blood Institute under award number R13 HL154544-01.
Copyright Form Disclosure:
Drs. Nellis, Bateman, Bembea, and Russell received support for article research from the National Institutes of Health. Dr. Bembea’s institution received funding from the National Institute of Neurological Disorders and Stroke (R01NS106292), the National Institute of Child Health and Human Development, and Grifols Investigator Sponsored Research Grant. Dr. Nishijima received funding from BMS. Dr. S Ibla received funding from Cheisi Pharmaceuticals. Dr. Haas received funding from Octapharma. Dr. Goel received funding from the National Heart, Lung, and Blood Institute and Rigel Pharmaceuticals. Dr. Crighton disclosed that she is employed by Royal Children’s Hospital of Melbourne, Australia and that she was the Australian and New Zealand Society of Blood Transfusion President. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.White LJ, Fredericks R, Mannarino CN, Janofsky S, Faustino EVS. Epidemiology of bleeding in critically ill children. J Pediatr 2017, 184:114–119. [DOI] [PubMed] [Google Scholar]
- 2.Greenway T, Eysenbach L, Shabanova V, Faustino EVS. Bayesian analysis of the epidemiology of bleeding in critically ill children. J Critical Care 2021; 63:133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalton HJ, Garcia-Filion P, Holubkov R, et al. Association of bleeding and thrombosis with outcome in extracorporeal life support. Pediatr Crit Care Med 2015, 16:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karam O, Lacroix J, Robitaille N, Rimensberger PC, Tucci M. Association between plasma transfusions and clinical outcome in critically ill children: a prospective observational study. Vox Sang 2013, 104:342–349. [DOI] [PubMed] [Google Scholar]
- 5.Kleinman S, Chan P, Robillard P. Risks associated with transfusion of cellular blood components in Canada. Transf Med Rev 2003, 17:120–162. [DOI] [PubMed] [Google Scholar]
- 6.Murphy EL, Kwaan N, Looney MR, et al. Risk factors and outcomes in transfusion-associated circulatory overload. Am J Med 2013, 126: 357.e329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pandey S, Vyas G. Adverse effects of plasma transfusion. Transfusion 2012, 52 Suppl 1:65s–79s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez P, Salmi LR, Follea G, et al. Determinants of transfusion-associated bacterial contamination: results of the French BACTHEM Case-Control Study. Transfusion 2001, 41:862–872. [DOI] [PubMed] [Google Scholar]
- 9.Toy P, Gajic O, Bacchetti P, et al. Transfusion-related acute lung injury: incidence and risk factors. Blood 2012, 119:1757–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muszynski JA, Spinella PC, Cholette JM, et al. Transfusion-related immunomodulation: review of the literature and implications for pediatric critical illness. Transfusion 2017, 57:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karam O, Demaret P, Shefler A, et al. Indications and effects of plasma transfusions in critically ill children. Am J Respir Crit Care Med 2015, 191:1395–1402. [DOI] [PubMed] [Google Scholar]
- 12.Nellis ME, Karam O, Mauer E, et al. Platelet transfusion practices in critically ill children. Crit Care Med 2018, 46:1309–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufman RM, Djulbegovic B, Gernsheimer T, et al. ; AABB. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2015; 162:205–13. [DOI] [PubMed] [Google Scholar]
- 14.New HV, Berryman J, Bolton-Maggs PH, et al. Guidelines on transfusion for fetuses, neonates and older children. Br J Haematol 2016, 175:784–828. [DOI] [PubMed] [Google Scholar]
- 15.Green L, Bolton-Maggs P, Beattie C, et al. British Society of Haematology Guidelines on the spectrum of fresh frozen plasma and cryoprecipitate products: their handling and use in various patient groups in the absence of major bleeding. Br J Haematol 2018, 181:54–67. [DOI] [PubMed] [Google Scholar]
- 16.O’Shaughnessy DF, Atterbury C, Bolton Maggs P, et al. Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryosupernatant. Br J Haematol 2004, 126:11–28. [DOI] [PubMed] [Google Scholar]
- 17.New HV, Stanworth SJ, Gottstein R, et al. British Society for Haematology Guidelines on transfusion for fetuses, neonates and older children. Br J Haematol 2016; 175:784–828. Addendum August 2020. Br J Haematol 2020. [DOI] [PubMed] [Google Scholar]
- 18.National Blood Authority Australia. Patient Blood Management Guidelines: Module 6 - Neonatal and Paediatrics. Available at: https://www.blood.gov.au/pbm-module-6. Accessed August 5, 2021.
- 19.Liumbruno G, Bennardello F, Lattanzio A, Piccoli P, Rossetti G. Recommendations for the transfusion of plasma and platelets. Trasfusione del Sangue 2009, 7:132–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bembea MM, Valentine SL, Bateman ST, e al. The Pediatric Critical Care Transfusion and Anemia Expertise Initiative Consensus Conference Methodology. Pediatr Crit Care Med 2018, 19(9S Suppl 1): S93–s97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 22.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin epidemiol 2009, 62: e1–34. [DOI] [PubMed] [Google Scholar]
- 23.Delaney M, Karam O, Lieberman L, et al. What laboratory tests and physiologic triggers should guide the decision to administer a platelet or plasma transfusion in critically ill children and what product attributes are optimal to guide specific product selection? From the Transfusion and Anemia EXpertise Initiative – Control/Avoidance of Bleeding (TAXI-CAB). Pediatr Crit Care Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russell R, Bauer DF, Goobie SM, et al. Plasma and platelet transfusion strategies in children following severe trauma, traumatic brain injury and/or intracranial hemorrhage: From the Transfusion and Anemia Expertise Initiative – Control/Avoidance of Bleeding (TAXI-CAB). Pediatr Crit Care Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cholette JM, Muszynski JA, Ibla JC, et al. Plasma and platelet transfusion strategies in neonates and children undergoing cardiac surgery with cardiopulmonary bypass or neonates and children supported by extracorporeal membrane oxygenation: From the Transfusion and Anemia Expertise Initiative – Control/Avoidance of Bleeding (TAXI-CAB). Pediatr Crit Care Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lieberman L, Karam O, Stanworth SJ, et al. Plasma and Platelet Transfusion Strategies in Critically Ill Children with Malignancy, Acute Liver Failure and/or Liver Transplantation, or Sepsis: From the Transfusion and Anemia Expertise Initiative – Control/Avoidance of Bleeding (TAXI-CAB). Pediatr Crit Care Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tucci M, Crighton G, Goobie SM, et al. Plasma and platelet transfusion strategies in critically ill children following non-cardiac surgery and critically ill children undergoing invasive procedures outside the operating room: From the Transfusion and Anemia EXpertise Initiative – Control/Avoidance of Bleeding (TAXI-CAB). Pediatr Crit Care Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nellis ME, Remy KE, Lacroix J, et al. Research Priorities for Plasma and Platelet Transfusion Strategies in Critically Ill Children: From the Transfusion and Anemia EXpertise Initiative – Control/Avoidance of Bleeding (TAXI-CAB). Pediatr Crit Care Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayden JA, van der Windt DA, Cartwright JL, et al. Assessing bias in studies of prognostic factors. Ann Intern Med 2013; 158:280–286. [DOI] [PubMed] [Google Scholar]
- 30.Guyatt GH, Oxman AD, Kunz R, et al. Going from evidence to recommendations. BMJ (Clinical research ed) 2008, 336:1049–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schunemann HJ. What is “quality of evidence” and why is it important to clinicians? BMJ (Clinical research ed) 2008, 336:995–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical research ed) 2008, 336:924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nellis ME, Tucci M, Lacroix J, et al. Bleeding Assessment Scale in Critically Ill Children (BASIC): Physician-driven diagnostic criteria for bleeding severity. Crit Care Med 2019, 47:1766–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fitch K, Bernstein S, Aguilar M. The RAND/UCLA Appropriateness Method User’s Manual. In. Arlington, VA; 2001. [Google Scholar]
- 35.Doctor A, Cholette JM, Remy KE, et al. Recommendations on RBC transfusion in general critically ill children based on hemoglobin and/or physiologic thresholds from the Pediatric Critical Care Transfusion and Anemia Expertise Initiative. Pediatr Crit Care Med 2018, 19(9S Suppl 1): S98–s113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Callum JL and Dzik WH. The Use of Blood Components Prior to Invasive Bedside Procedures: a Critical Appraisal. In: Mintz PD ed. Transfusion Therapy: Clinical Principles and Practice. 3rd ed. Bethesda MD: AABB Press, 2011: 1–53. [Google Scholar]
- 37.Zwifelhofer NMJ, Bercovitz RS, Cole R, et al. Platelet Function Changes during Neonatal Cardiopulmonary Bypass Surgery: Mechanistic Basis and Lack of Correlation with Excessive Bleeding. Thromb Haemost 2020. Jan; 120:94–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanous O, Steinberg Shemer O, et al. Evaluating platelet function disorders in children with bleeding tendency - A single center study. Platelets 2017. Nov; 28:676–681. [DOI] [PubMed] [Google Scholar]
- 39.Karam O, Goel R, Dalton H, Nellis ME. Epidemiology of hemostatic transfusions in children supported by extracorporeal membrane oxygenation. Crit Care Med 2020, 48: e698–e705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


