Abstract
Electronic cigarettes (e-cigarettes) are battery powered electronic nicotine delivery systems that use a propylene glycol/vegetable glycerin base to deliver vaporized nicotine and flavorings to the body. E-cigarettes became commercially available without evidence regarding their risks, long-term safety, or efficacy for smoking cessation. Recent clinical trials suggest that e-cigarette use with counseling may be effective in reducing cigarette, but not nicotine-dependence. However, meta-analyses of observational studies demonstrate that e-cigarette use is not associated with smoking cessation. Cardiovascular studies reported sympathetic activation, vascular stiffening, and endothelial dysfunction, which are associated with adverse cardiovascular events. To date, the majority of pulmonary clinical trials in e-cigarette users utilized standard spirometry as the primary outcome measure, reporting no change in lung function. However, acute and chronic studies reported increased biomarkers of pulmonary disease in e-cigarette users. While these studies were conducted in adult subjects, >30% of high school-age adolescents reported e-cigarette use. The effects of e-cigarette use on cardiopulmonary endpoints in adolescents and young adults remains unstudied. Due to adverse clinical findings and associations between e-cigarette use and increased incidence of respiratory diseases in people who have never smoked, large longitudinal studies are needed to understand the risk profile of e-cigarettes. Consistent with the Centers for Disease Control and Prevention recommendations, clinicians should monitor the health risks of e-cigarette use, discourage nonsmokers and adolescents from using e-cigarettes, and discourage smokers from engaging in dual use without cigarette reduction or cessation.
Keywords: e-cigarette, cardiac, pulmonary, cessation
Introduction
Electronic cigarettes (e-cigarettes; EC) are a new and developing electronic nicotine delivery system that became commercially available in 2004. The long-term cardiopulmonary effects of e-cigarettes remain poorly understood. Thus, maintaining up-to-date knowledge on e-cigarettes is a current challenge for clinicians, especially since e-cigarettes continue to change in engineering design and chemical composition and are often released commercially without clinical safety or smoking cessation efficacy data. E-cigarettes are battery-powered heating elements connected to a tank or reservoir which contains a mixture of nicotine, flavors, and other chemicals dissolved in a propylene glycol/vegetable glycerin vehicle of varying ratios (Figure 1).1 The first two generations of e-cigarettes were inefficient at increasing plasma nicotine compared to combustible tobacco products. In contrast, high-powered third generation devices achieved similar plasma nicotine levels and pharmacokinetics as tobacco cigarettes (TC).2 Fourth generation EC devices (e.g. JUUL, PuffBar) switched from freebase nicotine to nicotine salt and benzoic acid in order to compensate for their decreased nicotine delivery efficiency, resulting in a much higher delivery of nicotine.3 Prior research has indicated that less e-liquid is consumed when using devices with higher nicotine content.2 Therefore, more efficient nicotine delivery could reduce overall exposure to EC aerosol; however, this has not been directly studied in fourth generation devices relative to older models. Fourth generation EC devices are now readily available in both rechargeable and disposable forms with limited regulations. However, the impact of EC on cardiopulmonary health is incompletely understood.
Figure 1: First to Fourth Generation e-Cigarette Devices.
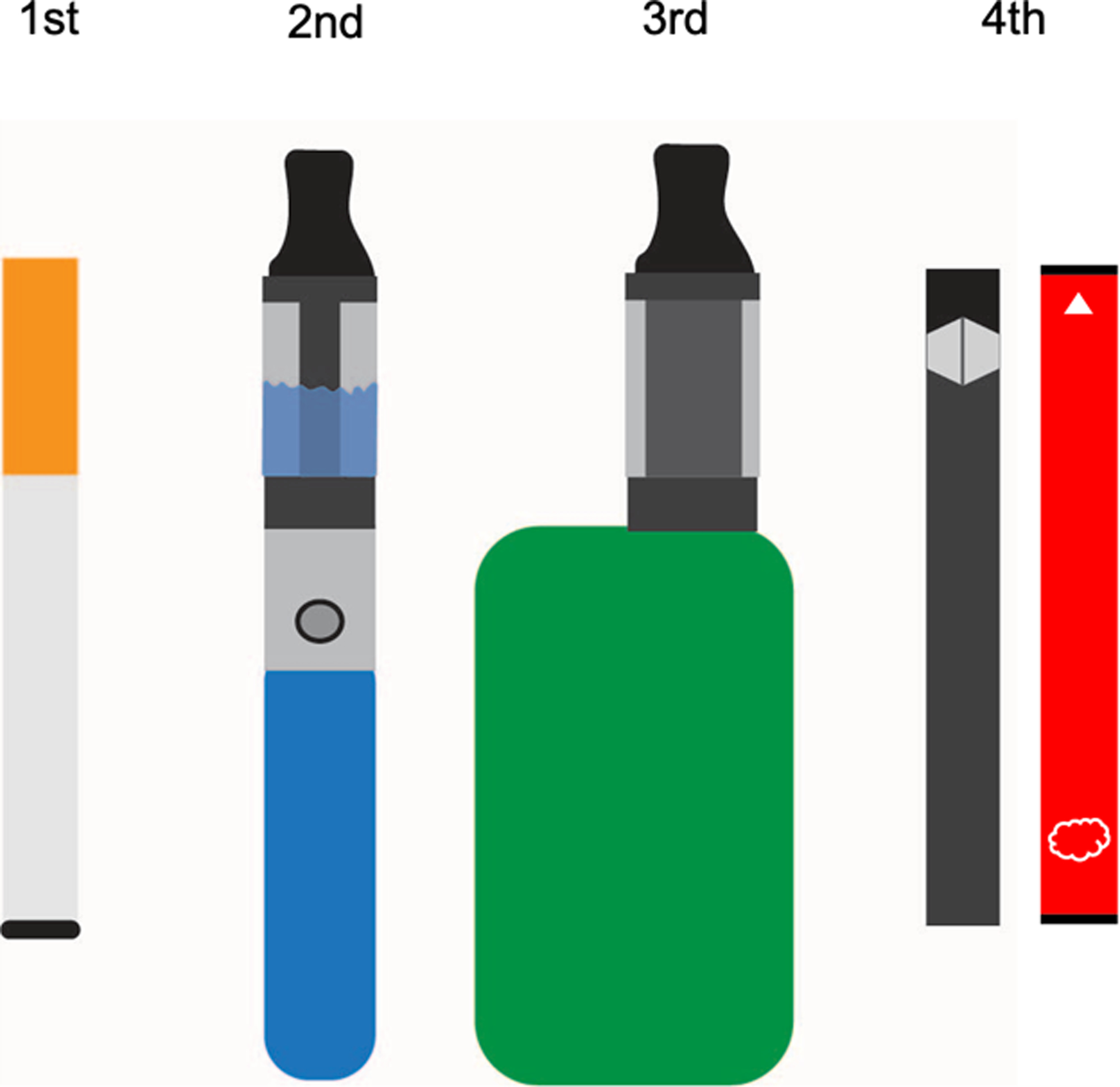
From left to right: first generation (“cig a-likes”), second generation (“vape pens”), third generation (“tanks” and “mods”), and fourth generation (“pod mods”) e-cigarette devices. First and second generation e-cigarette devices were tubular and inefficient in nicotine delivery when compared to combustible tobacco products. Third generation devices were customizable and contained larger tanks with larger, higher voltage batteries and were comparable to conventional cigarettes in nicotine delivery efficiency. Fourth generation (e.g. JUUL, PuffBar) devices have smaller tanks (pods) and batteries with decreased nicotine delivery efficiency. However, fourth generation e-cigarettes compensated for lost efficiency by switching from freebase nicotine (used in first-third generation devices) to higher concentrations of nicotine salt, along with benzoic acid. These devices are available in reusable (left) and disposable (right) forms.
The Unresolved Issues
First, it has been suggested that e-cigarettes improve smoking cessations rates (this notion has been contested and is discussed in detail later in this review). Therefore, if e-cigarettes are to be used as a transition to stop tobacco cigarette use, understanding the relative harms of e-cigarette use versus tobacco cigarette smoking is vital for clinicians considering e-cigarettes as a potential treatment option for current smokers. On the other hand, the absolute harms of e-cigarettes, including their potential to cause nicotine addiction and encourage cigarette smoking, must be considered when discussing health risks with non-tobacco cigarette smoking patients.
Second, and critically important, the use of e-cigarettes has increased in adolescents4 (+14.4% from 2017–2019, n = 4,513 12th graders) and young adults5 (+2.5% from 2014–2018, n = 1,857 aged 18–24). In 2019, self-reported past 30-day use of e-cigarettes was 27.5% in adolescents (grades 9–12, n = 10,097), and 10.5% in early adolescents (grades 6–8, n = 8,837).6 Notably, the perception of reduced harm associated with e-cigarette aerosol inhalation (vaping) compared to traditional cigarette smoking has contributed to their uptake by people who have never smoked (never-smokers);7 however, smoking cessation is not a primary reason for vaping among youth and young adults.8 Rather, a meta-analysis of longitudinal studies showed that e-cigarette use in adolescents and young adults is associated with subsequent cigarette smoking initiation (OR = 3.62 [95% CI: 2.42–5.41]).9 Over $110 million was spent on e-cigarette advertising in 2018,10 which may have contributed to the increased uptake of e-cigarettes.11 Youth may be influenced by such promotions and develop a receptive attitude toward product use.12–14 Following a congressional hearing in 2019, the FDA issued a warning regarding youth-facing advertisement strategies employed by JUUL Labs. Subsequently in 2020, the US Food and Drug Administration (FDA) passed legislation to reduce sales of e-cigarettes to underage (less than 18 years old) users, banning the sale of most fourth generation flavored cartridge pods.15 However, this legislation did not apply to disposable e-cigarettes, which became widespread in the months following. The AHA’s positions on EC regulation and long-term goals can be found in this statement.16
For clinicians, clarity is required regarding the potential clinical utility of e-cigarettes to aide with tobacco cigarette use, and the potential public health crisis predicated on an increase in youth/young adult nicotine addiction and transition to tobacco cigarette use.17 As devices continue to evolve and become more palatable, efficient, sleek, and easy to use, smokers may be more likely to transition away from traditional cigarettes. However, these attractive features coincide with youth appeal. Clinicians should balance these perspectives when making individual decisions with their patients and when discussing these devices in health education settings and on media platforms, all of which can affect public opinion and discourse. To assist clinicians in making these decisions, this review will examine the epidemiologic data of e-cigarette use, review the current state of knowledge on the efficacy of e-cigarettes as a smoking cessation tool, and examine the impact of e-cigarettes on cardiopulmonary health.
Search Strategy
PubMed and the Cochrane Central Register of Controlled Trials were searched for English-language studies related to e-cigarettes from January 2012 through November 2020. PubMed “article type” filters were applied to search for controlled clinical trials, randomized controlled trials, meta-analyses, and systematic reviews (see supplement). This search resulted in 384 results which were screened by the authors. Articles were then selected with consideration given to the general medical readership. Reviewing the references of screened articles identified additional observational studies. Additionally, a select few clinical studies, observational studies, and laboratory studies that were not represented in our searches were incorporated (see supplement). Separate, smaller searches were used to identify epidemiological studies and studies related to the E-cigarette or Vaping product use-Associated Lung Injury (EVALI).
Disease Epidemiology
Epidemiological studies on vaping, including meta-analyses, have been performed (Table 1). Associations were reported between e-cigarette use and higher incidence of asthma (OR range = 1.39–3.41 [95% CI range: 1.15–6.49]),18,19 respiratory disease (OR range = 1.31–2.58 [95% CI range: 1.03–4.89]),20–22 COVID-19 (OR = 5.05 [95% CI: 1.82–13.96]),23 wheeze (OR = 1.67 [95% CI: 1.23–2.15]),24 and myocardial infarction (OR = 1.79 [95% CI: 1.20–2.66]).25 Furthermore, dual use of e-cigarettes and cigarettes has been associated with higher rates of cardiovascular disease (OR = 1.36 [95% CI: 1.18–1.56])26, and cardiovascular risk factors, including metabolic syndrome (OR = 1.57 [95% CI: 1.03–2.40])27 versus sole cigarette users. Smoking cessation aided by e-cigarettes or other noncombustible nicotine/tobacco products was recently shown to put quitters at increased risk of cardiovascular disease relative to those who quit without use of these alternatives (HR = 1.31 [95% CI: 1.01–1.70]).28 Due to the novelty of e-cigarettes and the rapidly changing market, these associations (excluding respiratory disease) are derived solely from cross-sectional studies and will require longitudinal surveillance for validation.
Table 1:
E-cigarette Disease epidemiology
| Design/Location, Yearref | Patient population, No. | Findings |
|---|---|---|
| Respiratory Symptoms and Disease | ||
| Asthma | ||
| Cross-sectional analysis/South Korea, 201618 | N = 35,904 high school students | Prevalence rates of asthmatics was 3.9% in current e-cigarette users, 2.2% in ‘former e-cigarette users’, and 1.7% in ‘never e-cigarette users’. The AOR for asthma for ‘current e-cigarette’ users was 3.41 (95% CI: 1.79–6.49). |
| Cross-Sectional analysis/US, 201919 | N = 402,822 never combustible cigarette smoker adults (> 18 years old) Current e-cigarette users, 3,103 (0.8%). 8.5% had asthma Median age group of current e-cigarette users was 18–24 years | Current e-cigarette use was associated with higher odds of self-reported asthma compared to never e- cigarette users (OR= 1.39; 95% CI: 1.15–1.68). |
| Chronic Bronchitis (chronic cough, phlegm, wheeze or bronchitis) | ||
| Cross-sectional study/California, US, 201720 | N = 2,086 youth (11th and 12th grade) | Risk of bronchitic symptoms was higher among past users (OR= 1.85; 95% CI: 1.37–2.49), and current users (OR= 2.02; 95% CI; 1.42–2.88), compared with never-users, |
| Cross-Sectional analysis/Hawaii, US, 201921 | N = 8,087, mean age 55 years old | E-cigarette use was associated with chronic pulmonary disorder (AOR= 2.58; 95% CI 1.36–4.89, p<0.01). |
| Other respiratory symptoms | ||
| Longitudinal analysis/US, 202022 | N = 46,000 ages 12 and older | Associations between former e-cigarette use (AOR= 1.31; 95% CI: 1.07–1.60) or current e-cigarette use (AOR= 1.29; 95% CI: 1.03–1.61) and respiratory disease. Current combustible tobacco smoking (AOR= 2.56; 95% CI: 1.92–3.41) was also associated with having respiratory disease. |
| Cross-sectional study/US, 202024 | N = 28,171 adults, 641 (1.2%) current exclusive e-cigarette users, 8525 (16.6%) were current exclusive smokers, 1106 (2.0%) were dual users, 17 899 (80.2%) were nonusers | Incidence of wheezing and related respiratory symptoms was higher in current e-cigarette users (AOR= 1.67; 95% CI: 1.23–2.15) compared with nonusers. Current e-cigarette users had lower risk of wheezing and related respiratory symptoms compared with current smokers (AOR= 0.68; 95% CI: 0.53–0.87). |
| Cardiovascular Disease | ||
| Cross-sectional analysis/US, 201825 | N = 69,725 adults (>18 years) | Daily e-cigarette use was independently associated with higher odds of having had a myocardial infarction (OR= 1.79; 95% CI: 1.20–2.66, p=0.004) as was daily conventional cigarette smoking (OR= 2.72; 95% CI: 2.29–3.24). |
| Cross-sectional analysis/US, 201926 | N = 449,092 adults (>18 years) | No association between e-cigarette use and cardiovascular disease. Dual use was significantly associated with higher rates of cardiovascular disease vs smoking alone (OR = 1.35; 95% CI: 1.18–1.56). |
Table Legend: OR = odds ratio, AOR = adjusted odds ratio, CI = confidence interval
E-Cigarettes as a Potential Smoking Cessation Tool - Table 2.
Table 2:
Findings from e-cigarette Smoking Cessation Studies
| Design, Location, Yearref | Sample Size/Patient Population | Device Generation | Findings |
|---|---|---|---|
| Meta-analysis, 202029 | 50 studies representing 12,430 patients. 26/50 were randomized clinical trials. |
N/A | Use of electronic cigarettes with nicotine improves smoking cessation rates compared to electronic cigarettes without nicotine (RR = 1.71 [95% CI: 1.00 to 2.92]) and nicotine replacement therapies (NRTs) (RR = 1.69 [95% CI: 1.25 to 2.27]). |
| Meta-analysis, 202137 | 64 studies. 9/64 were randomized clinical trials. | N/A | In clinical trials, use of electronic cigarettes improves smoking cessation rates (OR = 1.529 [95% CI: 1.158 to 2.019]) compared to conventional therapies. However, observational study data showed that e-cigarette use as a consumer product was not associated with smoking cessation in adult smokers (OR = 0.947 [95% CI: 0.772 to 1.160), nor in a subset of adult smokers who were motivated to quit smoking (OR = 0.851 [95% CI: 0.684 to 1.057). |
| Meta-analysis, 201635 | 20 studies representing 40,815 individuals. 2/20 were randomized clinical trials with 757 patients combined | N/A | The odds of quitting cigarettes were 28% lower (OR = 0.72 [95% CI: 0.57 to 0.91]) in those who used e-cigarettes compared to those who did not. This data was driven by observational studies, as the two clinical trials collectively showed no change in cessation rates. |
| Meta-analysis, 201734 | 12 studies representing 14,122 individuals. 3/12 were randomized clinical trials with 1,007 patients combined | N/A | The odds of quitting cigarettes were 26% lower (OR = 0.74 [95% CI: 0.55 to 1.0]) in those who used e-cigarettes compared to those who did not. Results were of low certainty, however, based on the Grading of Recommendations Assessment, Development and Evaluation. approach |
| Randomized clinical trial, United Kingdom, 201931 | 886 patients from stop-smoking services in the UK. Largely middle-aged smokers, median age 41. | 2nd | 1-year smoking abstinence rates were significantly higher (18%) for the e-cigarette group compared to 9.9% in the nicotine replacement therapy group (RR = 1.83 [95% CI: 1.30–2.58]). Patients in the e-cigarette group were more likely to still be using e-cigarettes (80%) than subjects who were treated with NRT (9%) after 1 year. 25% of participants in the e-cigarette group became dual users. |
| Randomized clinical trial, Canada, 202033 | 376 patients with a moderate to strong desire to attempt to quit. Mean age 52. | 2nd | 12-week smoking abstinence rates were significantly higher for the nicotine e-cigarette group with counseling (21.9%) vs counseling alone (9.1%). Abstinence rates for the nicotine-free e-cigarette group with counseling were not significantly higher (17.3%) than counseling alone. |
| Randomized intervention trial, United States, 201836 | 6,006 smokers employed by 54 US companies. Median age 44. | Unclear | 6-month smoking abstinence rates were not significantly different in the e-cigarette group (1.0%) with standard care (free motivational text messaging and information about the benefits of cessation) versus the nicotine replacement therapy group (0.5%) with standard care nor standard care alone (0.1%). |
Evaluating the efficacy of e-cigarette use as a smoking cessation strategy is of utmost importance to clinicians. Clinical trials have lagged behind the rapidly evolving e-cigarette market, limiting the bulk of data to outdated first- and second-generation devices. In 2020, a Cochrane Database Systematic Review meta-analysis of 50 clinical trials and intervention studies by Hartmann-Boyce et al.29 (total n = 12,430) concluded that there is moderate-certainty evidence that electronic cigarettes with nicotine increase smoking cessation rates compared to electronic cigarettes without nicotine (RR = 1.71 [95% CI: 1.00 to 2.92]) and nicotine replacement therapies (NRTs) (RR = 1.69 [95% CI: 1.25 to 2.27]).29 The most recent update to this e-cigarette Cochrane Systematic Review now includes 56 studies and 12,804 people.30 The authors conclude with moderate confidence that nicotine e-cigarettes probably do help people to stop smoking for at least six months and probably work better than nicotine replacement therapy and nicotine-free e-cigarettes. However, they also stress the need for more reliable evidence to be confident about the effects of newer e-cigarettes that have greater nicotine delivery.
The duration of cessation is a critical factor that has been examined in other studies. A UK-based trial achieved 1-year smoking abstinence rates of 18% and 9.9% for those provided with second generation e-cigarettes or their choice of standard nicotine replacement therapies (NRTs), respectively (n = 886).31 All patients also received counseling for a minimum of 4 weeks. Importantly, while e-cigarettes were effective smoking cessation aids, these subjects were much more likely to still be using e-cigarettes (80%) than subjects who were treated with NRTs (9%) after 1 year. Further, 25% of participants in the e-cigarette group became dual users, although dual users in the e-cigarette group experienced greater reductions in cigarette use than dual NRT/cigarette users. These caveats have led other groups to argue against e-cigarette use as first-line treatment option, due to the lack of comparable efficacy in nicotine-cessation and the potential for e-cigarette-induced harm during or after cigarette cessation.32 A recent Canadian trial (n = 376) using second generation devices found that providing a nicotine-containing e-cigarette with counseling vs counseling alone significantly increased smoking abstinence rates at 12 weeks (21.9% vs 9.1%, respectively), while nicotine-free e-cigarettes did not (17.3%). However, this study did not include an NRT comparison group, and early termination limited statistical power of the trial.33
Outside of clinical settings, the effects of vaping on smoking cessation seem less favorable.34–37 The most recent meta-analysis that included observational studies regarding e-cigarette use and smoking cessation was published in 2021 and utilized studies published through January 2020.37 This meta-analysis included 55 observational studies and 9 randomized clinical trials. Analysis of clinical trial data corroborated the Hartmann-Boyce et al. review, showing an association between e-cigarette use and smoking cessation (OR = 1.53 [95% CI: 1.16 to 2.02]). However, the observational study data showed that e-cigarette use was not associated with smoking cessation in adult smokers (OR = 0.95; [95% CI: 0.77 to 1.16), nor in a subset of adult smokers who were motivated to quit smoking (OR = 0.85; [95% CI: 0.68 to 1.06). Similar to these meta observational study data, a randomized intervention trial of smokers (n = 6,006) employed by 54 companies found that providing free e-cigarettes alongside standard care (free motivational text messaging and information about the benefits of cessation) was not more effective than other free cessation aids with standard care nor standard care alone.36 Unlike other randomized trials that provided evidence for e-cigarettes as effective smoking cessation aids,31,33 this study was not carried out in a clinical setting and participants did not have access to in-person behavioral support.
The apparent dichotomy between the success of clinical trials and lack of efficacy in observational studies may be due in part to the behavioral support and counseling provided during clinical trials. Other variables that may contribute to variable quit rates include nicotine concentration and users’ prior experience with vaping, which have both been shown to be significant predictors of success.38,39 While these referenced studies did not directly assess smoking cessation or nicotine cessation, their findings suggest that training naïve e-cigarette users to properly use their e-cigarettes could improve initial efficacy following clinical recommendations and/or device provision.
Based in part by the evidence reviewed here, the U.S. Preventative Services Task Force has concluded that the data are insufficient to recommend e-cigarettes for smoking cessation in adults.40 In the 2020 Smoking Cessation: A report of the Surgeon General a comparable conclusion was reached that the evidence is inadequate to infer that e-cigarettes increase smoking cessation.41 However, the report suggested that the use of e-cigarettes containing nicotine was associated with increased smoking cessation compared with the use of nicotine-free e-cigarettes. E-cigarettes are not currently approved by the FDA as an aid for quitting tobacco cigarette use. Outside of clinical studies, the use of e-cigarettes for smoking cessation is not supported and should not be recommended to patients. Smoking cessation trials using third and fourth generation e-cigarettes that deliver greater amounts of nicotine are needed; studies should include both smoking cessation and nicotine cessation as e-cigarette efficacy endpoints.
Pathophysiology
Clinical study issues
To evaluate the acute effects of vaping, most of the clinical trials assessed in this review used randomized, crossover designs with significant washout periods. Since e-cigarette research is still in its early stages, long-term prospective cohort studies evaluating the risks of chronic use are unavailable. Further, the development of new e-cigarettes has outpaced the research. The bulk of studies have examined use of first- or second-generation e-cigarettes (see Figure 1), which differ significantly from modern devices in terms of electronic power, nicotine strength, and popularity. The results of trials with these devices may not translate nor be relevant to newer devices that can deliver higher amounts of nicotine. Due to the novelty of e-cigarettes, clinical guidelines and decision-making must be informed by clinical, observational, and laboratory studies.
E-cigarette Impact on the Cardiovascular System - Table 3
Table 3:
Effects of e-cigarette use on the cardiovascular system
| Acute EC Use, Clinical Trials | ||||||
|---|---|---|---|---|---|---|
| Condition | Biomarker | Change | Device Generation | Nicotine Concentration (mg/mL) | Flavor | Sample Size (n) |
| Sympathetic Effects | HR | ↑42–47 | 1st,2nd,3rd | 0,1.5,3,18,24, Several, Unclear | None, Tobacco, Several | 15,20,23,25,30 |
| No Change44,46,48 | 1st,2nd,3rd | 0,12,Several | None, Several, Unclear | 23,30,70 | ||
| Systolic BP | ↑42–46 | 1st,2nd,3rd | 0,1.5,3,24, Several, Unclear | None, Tobacco, Several | 15,20,23,25,30 | |
| No Change44,47, 48 | 1st,2nd | 0,12,18, Several | Tobacco, Several, Unclear | 20,23,70 | ||
| Diastolic BP | ↑43–45 | 1st,2nd,3rd | 0,3,Several, Unclear | None, Tobacco, Several | 20,23,25 | |
| No Change42,46–48 | 2nd,3rd | 0,12,18,24 | None, Tobacco, Unclear | 15,20,30,70 | ||
| Tone | Sympathetic ↑49 | 2nd | 12 | Strawberry | 33 | |
| HRV | ↓105 | 1st (2nd hand exposure) | 1.8 | None | 5 | |
| Vascular Stiffness | PWV | ↑42,43,48 | 2nd,3rd | 0,3,12,24 | None, Tobacco, Unclear | 15,25,70 |
| No Change43 | 3rd | 0 | None | 25 | ||
| AIx | ↑42,43,48 | 2nd,3rd | 0,3,12,24 | None, Tobacco, Unclear | 15,25,70 | |
| No Change43 | 3rd | 0 | None | 25 | ||
| Endothelial Function | NO Bioavailability | ↑45,61 | 2nd,Unclear | Unclear45,61 | Tobacco45, 61 | 20,40 |
| FMD | ↓45,55,61 | 1st,2nd, Unclear | 0,Unclear | Tobacco, Unclear | 20,31,40 | |
| Endothelial Damage | EPCs | ↑64 | 2nd | 12 | None | 16 |
| Endothelial Microvesicles | ↑63 | 3rd | 19 | None | 17 | |
| No Change47,63, 64 | 2nd,3rd | 0, 12, 18 | None, Tobacco | 16,17,20 | ||
| Oxidative Stress | MPO | ↑43 | 3rd | 3 | None | 25 |
| No change43 | 3rd | 0 | None | 25 | ||
| H2O2 | ↑45 | 2nd | Unclear | Tobacco | 20 | |
| sNOX2-dp | ↑45,61 | 2nd,Unclear | Unclear45,61 | Tobacco45,61 | 20,40 | |
| 8-iso-PGF2α | ↑45,61 | 2nd,Unclear | Unclear45,61 | Tobacco45,61 | 20,40 | |
| MDA | ↑48 | 2nd | 0, 12 | Unclear | 70 | |
| LDL-Ox, PON1, HOI | No Change49 | 2nd | 12 | Strawberry | 33 | |
| Thrombotic Effects | Platelet Aggregation | ↑70 | Unclear | Unclear | Tobacco | 40 |
| sP-selectin | ↑45,70 | 2nd,Unclear | Unclear45,70 | Tobacco45,70 | 20,40 | |
| sCD-40L | ↑45,70 | 2nd,Unclear | Unclear45,70 | Tobacco45,70 | 20,40 | |
| Platelet- derived Microvesicles | ↑47,63 | 2nd,3rd | 18,19 | None, Tobacco | 17,20 | |
| No Change63 | 3rd | 0 | None | 17 | ||
| Myocardial Effects | Systolic & Diastolic Function | No Change72 | 2nd | 11 | None | 36 |
| Observational Studies | ||||||
| Condition | Biomarker | Change | Subject Characteristics | |||
| Sympathetic Effects | HR | No Change58 | E-cigarette users (n =36), cigarette smokers(n =285), (both >4 days/week) vs nonuser controls (n =94) | |||
| Systolic BP | ||||||
| Diastolic BP | ||||||
| Tone | Sympathetic↑50 | Habitual e-cigarette users (n = 16) (>1year) vs nonuser controls (n = 18) | ||||
| Vascular Stiffness | PWV | No Change58 | E-cigarette users (n =36), cigarette smokers(n =285), (both >4 days/week) vs nonuser controls (n =94) | |||
| AIx | ↑*58 | |||||
| Oxidative Stress | LDL-Ox | ↑50 | Habitual e-cigarette users (n = 16) (>1year) vs nonuser controls (n = 18) | |||
| PON1 | No Change50 | |||||
| HOI | No Change50 | |||||
| 8-iso-PGF2α | No Change68 | E-cigarette users (n = 261) vs nonuser controls (n = 2191) | ||||
Table Legend: HR = Heart Rate, BP = Blood Pressure, HRV = Heart Rate Variability, AIx = Augmentation Index, NO = Nitric Oxide, EPC = Endothelial Progenitor Cell, MPO = myeloperoxidase, sNOX2-dp = soluble NOX2-derived peptide, MDA = malondialdehyde, 8-iso-PGF2α = 8-iso-prostaglandin F2α, LDL-Ox = low-density lipoprotein oxidation, PON1 = paraoxonase 1, HOI = high‐density lipoprotein antioxidant index, sP-selectin = soluble P-selectin, sCD-40L = soluble CD-40 ligand, 8-iso-PGF2α = urinary 8-isoprostane, hr = hour, d = day, wk = week
Vertical bars (|) separate references for each comma separated term in the respective box. Reference group for all studies were either baseline measures or within-subjects sham vaping/smoking control.
significant difference across groups via ANOVA, post-hoc of e-cigarette vs nonuser control not provided
Effects of Vaping on Sympathetic Activation of the Cardiovascular System
The effects of vaping on heart rate and blood pressure have been extensively studied. Despite using different e-cigarette device types (generations 1–3), nicotine concentrations, and instructions for user inhalation, 5/7 studies found that vaping with nicotine caused significant acute increases in both heart rate and blood pressure (n = 15–70)42–48. Only one study demonstrated significant increases during nicotine-free vaping, and this effect did not persist long after the vaping session.43 Electrocardiogram measurements revealed that vaping shifted heart rate variability toward sympathetic predominance.49,50 Collectively, these results suggested that use of nicotine-containing e-cigarettes caused sympathetic activation of the cardiovascular system, which could pose long-term health risks for chronic users, and/or exacerbate pre-existing cardiopulmonary conditions.51,52
Effects of Vaping on Vascular Health
Arterial stiffening has been validated as a reliable, independent predictor of adverse cardiovascular outcomes and all-cause mortality.53 Arterial stiffening contributes to the development of heart failure and is closely linked to atherosclerosis pathogenesis.54 The rate at which pressure waves propagate through arteries (pulse wave velocity, PWV) is a clinically relevant indicator of arterial stiffness. Several studies showed that acute e-cigarette use increased PWV, with increases ranging from 0.19 m/s to 0.80 m/s (n = 15–70).42,43,48,55 Significant increases in PWV that were both nicotine-dependent (n = 35)48 and nicotine-independent (n = 35, n = 31) were detected.48,55 Due to the rapid timeframe of these trials, increases in PWV are likely the result of sympathetic modulation of smooth muscle tone, and/or endothelial dysfunction (discussed below), as opposed to vascular remodeling.56,57 A 2020 cross-sectional study (n = 36 e-cigarette users, 285 smokers, and 94 nonuser controls) found that e-cigarette and cigarette users showed no difference in adjusted PWV vs nonusers.58 However, elsewhere, cigarette use has been associated with increased PWV,59 highlighting the need for more long-term observational cohort studies with e-cigarette users. Therefore, the effects of chronic vaping on clinically relevant cardiovascular stiffness endpoints remain speculative and will require further study.
Depletion of vasodilatory nitric oxide is a widely accepted driver of endothelial and vascular dysfunction.60 Several studies reported significant decreases (27–31%) in nitric oxide bioavailability or nitric oxide metabolite concentrations in patient plasma after acute vaping (n = 10–40).45,61,62 Accordingly, studies also demonstrated reductions in vasodilatory function after acute e-cigarette use.43,45,55,61 Reductions in stimulated nitric oxide production from e-cigarette user endothelial cells were also observed in a cross-sectional observational study; however, no differences in baseline FMD were reported between groups (n = 36 e-cigarette users, 285 smokers, and 94 nonuser controls).58 In addition to reductions in endothelial function, acutely elevated biomarkers of endothelial damage have been observed in patient serum after vaping (n = 10–40).61,63–65
Oxidative stress underlies multiple cardiovascular disorders and is known to deplete nitric oxide and induce endothelial cell damage.66,67 Clinical and observational trials of acute e-cigarette inhalation ± nicotine found significant increases in biomarkers of oxidative stress (n = 10–70).43,45,48,50,61,62
Overall, vaping acutely altered the human vasculature, likely as a result of sympathetic modulation and oxidative stress. While the long-term consequences of e-cigarette use on vascular health remain to be determined, there are reasonable grounds for concern that chronic vaping may impair vascular function in never-smokers. However, when compared to traditional cigarette smoking, some studies found that acute vaping caused less pronounced effects on vascular function and oxidative stress (n = 20–70).45,48,61 Additionally, a recent large scale observational study (n = 2191 nonusers, 261 e-cigarette users, 3261 smokers, and 1417 dual users) found that both dual use and exclusive cigarette use were significantly associated with higher levels of inflammatory and oxidative stress biomarkers in blood, while exclusive e-cigarette use was not.68 Further, a 2019 trial (n = 114) found that switching from traditional cigarettes to a first generation e-cigarette for one month led to a clinically significant improvement in FMD (1.49% [95% CI: 0.93 to 2.04%]) and a significant decrease in PWV (−0.53 [95% CI: −0.95 to −0.11]).69 Overall, these findings suggest that from a vascular perspective, exclusive vaping has a profile of reduced harm when compared to cigarette smoking.
Associations Between Vaping and Pro-Thrombotic Biomarkers
To date, studies have indicated that vaping acutely induces platelet aggregation and activation.45,47,63,70,71 These studies generally found that e-cigarettes have less pronounced effects on biomarkers of platelet activation and aggregation than traditional cigarettes.45,70 Considering the importance of platelet activation in cardiovascular disease states including thrombosis, atherosclerosis, and myocardial infarction, the potential consequences of chronic e-cigarette use on this aspect of cardiovascular physiology requires further study.
Assessment of Myocardial Health in E-cigarette users
Over time, traditional cigarette smoking increases the risk of cardiovascular diseases including hypertension, atherosclerosis, and heart failure, suggesting that these outcomes should be monitored in chronic e-cigarette users.51,52 Survey data showed that daily e-cigarette use was independently associated with higher odds of myocardial infarction (OR = 1.79 [95% CI: 1.20–2.66], n = 69,725).25 While this study is limited by its cross-sectional design and requires longitudinal studies for validation, the findings underscore the importance of monitoring for secondary myocardial alterations that can occur following chronic pulmonary and vascular changes. To date, only one study has investigated the potential acute effects of e-cigarette use on myocardial function in adult smokers using echocardiography and reported no changes (n = 36).72 However, acute/short term studies are not likely to induce cardiac remodeling, and thus are not a proxy for long-term studies. Critically, clinical trials and prospective studies investigating the effects of e-cigarette use on myocardial function have not yet been performed.
In summary, a shift in cardiac autonomic balance towards increased sympathetic drive,73 increased oxidative stress,74 increased vascular stiffness,53 and endothelial dysfunction75 all associate with increased cardiovascular morbidity and mortality. However, the evidence reviewed above regarding the cardiovascular effect of e-cigarettes is based on studies of small sample sizes, with limited clinical follow-up. Large scale studies designed to dissect the mechanisms by which e-cigarettes convey potential cardiovascular harm are needed to fully assess the safety of e-cigarettes. The current 2019 ACC/AHA guideline on primary prevention of cardiovascular disease recommend that clinicians ask all adults about e-cigarette use.76 Critically, e-cigarettes are not recommended for tobacco cessation treatment.
E-cigarette Impact on the Respiratory System-Table 4
Table 4:
Effects of e-cigarette use on the respiratory system
| Design, yearref | Patient population | Device Type/Study Duration | Findings |
|---|---|---|---|
| Acute Studies Assessing Lung Function | |||
| Double-blinded crossover study, 2019106 | n = 17 occasional smokers, 6 males, mean age 26 | 3rd generation | Acute increase in flow resistance, indicating obstruction of the conducting airways. |
| Randomized Clinical Trial, 201894 | n = 23 occasional smokers, 16 males, mean age 23 | 3rd generation | Tissue hypoxia and lower airway injury. |
| Laboratory-based study, 201580 | n = 20 smokers (10) and nonsmokers (10), mean age 39 | 1st generation | No immediate adverse effects of nicotine-free vaping on non-smokers and only small effects on FEV1 and FEF25 in smokers. |
| Laboratory-based study, 201285 | n = 30 smokers, 14 men, mean age 35 | 1st generation | Increased respiratory impedance and resistance, likely indicative of airway bronchoconstriction. |
| Repeated-measures controlled study, 2013107 | n = 30 smokers (15) and never-smokers (15), 16 males, age 18–57 | 2st generation | 1-hour tobacco smoking, but not e-cigarette vaping transiently reduced lung function. |
| Crossover and placebo-controlled trial, 201782 | n = 20 (healthy, 20–37 years) and 10 (asthmatic, 21–40 years) | Unclear | 1-hour vaping session of propylene glycol/glycerol did not significantly affect pulmonary functions in healthy or asthmatic subjects. |
| Acute Studies Assessing Lung Health Biomarkers and Omics Approaches | |||
| Single-blind within-subjects study, 201993 | n = 25 smokers, 18 males, mean age 23 | 3rd generation | Increased biomarker of airway epithelial injury (serum CC16), sustained decrement in transcutaneous oxygen tension and impaired arterial oxygen tension. |
| Randomized, investigator-blinded, three-period crossover study, 202046 | n = 30 male e-cigarette users who were former smokers, mean age 38 | 3rd generation | Decreased lung inflammation, increased biomarker of airway epithelial injury (serum CC16), decreased transcutaneous O2 tension. |
| Pilot clinical trial, 2020108 | n = 30 never smokers, male and females, age 21–30 | 3rd generation; 4-week intervention period | E-cigarette use and inhalation correlated with change in cell counts (macrophages, and lymphocytes) and cytokines (IL-8, IL-13, and TNF-α). No significant changes in mRNA or miRNA gene expression. |
| Cohort Studies, 201865 | n = 10 adult never-smokers (≥ 21 years), 5 females | 1st generation | Altered transcriptomes of small airway epithelium and alveolar macrophages for all subjects following inhalation of e-cigarette with nicotine. |
| Observational Studies | |||
| Observational, cross-sectional study; sputum collection, 201895 | n = 44 adults (≥ 18 years); 14 current cigarette smokers, 15 current e-cigarette users, and 15 never-smokers | N/A | Increases in aldehyde-detoxification and oxidative stress-related proteins associated with cigarette smoke. Innate defense proteins associated with COPD, including MUC5AC, were elevated in e-cigarette users. Increases in neutrophil granulocyte-related and NET-related proteins. |
| Observational, cross-sectional study; research bronchoscopies, 201884, 201977 | n = 41–42 healthy, adults (>21 years), 19–20 female | N/A | Vaper airways appeared friable and erythematous. 113 uniquely altered proteins in e-cigarette users airways. MUC5AC elevated in e-cigarette users. Neutrophil elastase, MMP-2, and MMP-9 activities and protein levels were equally elevated in both e-cigarette users’ and smokers’ BAL relative to nonsmokers. |
| E-cigarette Assisted Cessation Trials | |||
| Randomized controlled trial, 201988 | n = 263 smokers, 111 males, mean age 47 | 1st generation; 3-month cessation trial | No changes in standard spirometry. |
| Randomized controlled trial, 201786 | n = 105 smokers, 68 males, mean age 38 | 1st generation; 5 day cessation trial | No changes in standard spirometry. |
| Randomized controlled trial, 201687 | n = 130, 190 males, mean age 44 | 1st generation; 1 year cessation trial | No changes in most standard spirometry measures, improvement in FEF25–75% among quitters. |
Table Legend: CC16 = club cell protein-16, FEF25 = forced expiratory flow at 25%, IL = interleukin, TNF-α = tumor necrosis factor alpha, mRNA = messenger RNA, miRNA = microRNA, FeNO = fractional exhaled nitric oxide, eCO = exhaled carbon monoxide, MUC5AC = Mucin 5AC, NET = neutrophil extracellular trap, MMP = matrix metalloprotease, BAL = bronchoalveolar lavage
Pharmacokinetics have not yet been performed on e-cigarette constituents in the lung. However, it is estimated that nicotine levels are ~200 times higher in the lungs than at peak systemic levels after both smoking and vaping.77 Chronic tobacco smoking damages the lung’s ultrastructure and erodes innate immunity, leading to higher incidence of chronic obstructive pulmonary disease (COPD) and lung cancer. While it is not known whether vaping will produce lung disease, such signs (i.e. bronchitis, alveolar damage, decreased immunity to infection, wheeze etc.) are potential symptoms to be monitored in chronic e-cigarette users. Regarding lung function, differences between non-smokers and smokers are relatively small at young ages (i.e. 20–40 years) but become more significant over time (at 40–69 years of age).78 Thus, care must be taken when interpreting lung function measurements following e-cigarette use, and the timeframes over which changes in lung function were captured must be factored into the analysis. Based on this review of the literature (Table 4), it appears that the majority of published clinical trials on e-cigarettes have not been of sufficient duration to detect significant differences in lung function between groups.
Effects of Vaping on Lung Function
Smoking cessation has been shown to halt the decline in lung function caused by combustible tobacco product use, but does not restore lung function.79 Whether or not e-cigarette use is less harmful than conventional cigarette smoking is controversial (Table 4). Randomized clinical trials of acute and chronic e-cigarette use have shown variable results, with some showing reductions in lung function measured via spirometry80,81, and others showing no change.77,82–84 Using impulse oscillometry, which measures both small and large airway resistance and is more sensitive than standard spirometry, Vardavas et al. found elevated impedance in smokers after acute vape sessions (n = 30).85 Due to the rapid timeframe, these changes were likely due to airway smooth muscle contraction (bronchoconstriction) rather than to lung damage.
In one study, there was no change in spirometry five days after switching from smoking to vaping of first generation e-cigarettes (n = 105).86 Similarly, cigarette users who switched to vaping first generation e-cigarettes saw no improvement after 12 months (n = 183),87 and in a randomized controlled trial where smokers were provided with either e-cigarettes or a nicotine-free cigarette substitute, no changes were detected between the two groups after 3 months (n = 263).88 Unfortunately, these two studies did not include control groups who continued to smoke cigarettes. Collectively, switching from smoking to vaping did not provide short-term benefits; however, longer trials with appropriate control arms are needed to determine if switching from smoking to vaping can improve lung function.
The above studies were all performed on healthy non-smokers or smokers. However, a number of individuals who have asthma, COPD, or other diseases also use e-cigarettes or switch from smoking to e-cigarettes.89 While it is known that smoking cessation is beneficial and mitigates chronic declines in lung function,90 a 24-month longitudinal study found that switching from smoking to vaping had no effect on spirometry in asthmatic smokers (n = 18).91 Analysis of two observational cohorts (COPDGene, n = 3536; SPIROMICS, n = 1060) found that e-cigarette use was not associated with improved lung function in COPD patients, and instead showed higher prevalence of both chronic bronchitis and acute exacerbations.92 This was a longitudinal study; however, it was observational in nature and more studies are needed to validate or refute this finding. Collectively, these data indicated that switching from smoking combustible tobacco to vaping does not improve lung function/respiratory health in patients with pre-existing lung conditions.
Assessment of Lung Damage in E-cigarette users
In a series of randomized clinical trials, an increase in the lung damage biomarker club cell protein-16 was detected in serum following acute exposure to e-cigarettes ± nicotine (n = 30, 25, 23).46,93,94 Just 2 hours after e-cigarette exposure, 71 genes were significantly altered in airway biopsies and 27 genes were altered in primary alveolar macrophages, suggesting that vaping can elicit rapid responses in the lungs.65 The effects of long-term vaping on pulmonary gene/protein expression remain understudied. However, proteomic analysis indicated that more proteins were uniquely changed in e-cigarette users’ sputum (66) than in smokers’ sputum (29), relative to healthy non-smokers (n = 15 e-cigarette users, 14 smokers, and 15 nonsmoker controls).95 A number of innate defense proteins were altered.95 Similarly, neutrophil elastase and matrix metalloproteases, which predispose lungs to damage, were equally elevated in smokers’ and e-cigarette users’ bronchoalveolar lavage (BAL) samples (n = 14 per group).77 Proteomic analysis of bronchial epithelial brush biopsies identified uniquely altered proteins in e-cigarette users’ lungs including mucins, calcium signaling-related proteins and xenobiotic metabolizing proteins (n = 10 e-cigarette users, 13 smokers, 18 nonuser controls).84 These studies also found comparable increases in MUC5AC mucin levels in bronchial brush biopsies and sputum from both e-cigarette users and smokers relative to non-smokers.84,95 Increases in expression levels of gel-forming mucins such as MUC5AC are predictive of COPD disease severity.96 Interestingly, pulmonary function was normal across all groups,84,95 which underscores the need for more sensitive assays beyond spirometry to assess lung function in healthy e-cigarette users. Lung imaging using computed tomography (CT) or other techniques may be useful in this regard but has not been tested in healthy e-cigarette users.
E-cigarette or Vaping product use-Associated Lung Injury
E-cigarette or Vaping product use-Associated Lung Injury (EVALI) is a point-source epidemic, multi-organ syndrome characterized by lung injury, gastrointestinal and other symptoms. CT scans of EVALI patients’ lungs showed diffuse ground glass opacities, diffuse alveolar damage, interlobular septal thickening, and scarring of lower lobes, with sparing of the periphery.97 EVALI increased from a weekly mean of 4 visits per 1 million in January, 2017 to 116 visits per 1 million in autumn, 2019.98 As of February 20, 2020, a total of 2,880 EVALI cases99 and 68 EVALI-associated deaths were reported in the US;100 current data collection has been ceased due to the COVID-19 pandemic; however, physicians should still inquire about tetrahydrocannabinol (THC) history in presenting patients. 82–86% of patients reported using THC-containing products while 14% used nicotine-based e-liquids but did not report THC use (n = 2,668).99 One report found that vitamin E acetate was present in the BAL of 48/51 cases of EVALI across 16 US states101. Vitamin E acetate is commonly used as a thickening agent for THC vaping products and vitamin E acetate exposure induced inflammation and increased the number of lipid-laden macrophages in mice that was characteristic of EVALI.102 These findings suggest that vape products containing vitamin E acetate are likely to be the causative agents of most EVALI symptoms and may also synergize with other harmful additives. Other harmful constituents found in e-liquids are trace metals such as Cd, Si, Cu, Ni, and Pb, although their contribution to EVALI remains uncertain they may pose as potential risks to e-cigarette users103,104. Due to challenges in diagnosis, the CDC approach for patients with suspected EVALI begins by examination of symptoms and assessment of e-cigarette or vape history and use within 90 days. THC history should also be assessed, but it is important to note that not all e-cigarette users may recognize this name and for some patients, slang names for THC should be tried (e.g. dab etc.).
Limitations and Future Directions
This review has several limitations. First, clinical trials examining the effects of vaping on cardiopulmonary health are heterogeneous and many were conducted over too short a timeframe to fully address the clinically important questions related to morbidity and mortality. Larger longitudinal studies with clinical follow-up will be crucial to determine the effects of e-cigarette use on cardiovascular health and inform guidelines for use of e-cigarettes as a smoking cessation tool. Second, studies assessing the effects of e-cigarettes on lung function were too short of a duration. Future longitudinal studies are needed to determine the effects of e-cigarette use on lung function and to assess whether lung function decline is halted when converting from conventional cigarettes to e-cigarettes. Third, the majority of studies enrolled adult smokers as opposed to younger e-cigarette users, who make up a sizeable majority of new users. Adult smokers have the potential confounder of pre-existing cardiovascular and pulmonary disease. Critically, the impact of vaping on adolescents and young adults requires further evaluation. Lastly, our search criteria were limited to English-language studies and may have not included relevant publications. In addition, knowledge gaps exist with a lack of prospective studies assessing the effects of e-cigarettes on clinical cardiovascular outcomes.
Conclusions
Observational epidemiological studies have associated vaping with higher incidence of pulmonary disease and myocardial infarction, and acute studies investigating pulmonary and cardiovascular biomarkers suggest tissue damage and compromised vascular function. Although these findings are largely limited to cross-sectional studies and short-term clinical trials, current evidence of absolute harm signals that e-cigarettes could compromise cardiovascular and respiratory health over time. Several studies assessing relative harm suggest reduced harm for vaping compared to smoking; however, harm reduction has not been noted for all outcome measures studied (e.g. spirometry), and the extent of reduction in harm when smokers switch to electronic cigarettes is uncertain and requires further study. While e-cigarettes may facilitate smoking cessation, they are not associated with a reduction in nicotine use dependency and may lead to dual use of e-cigarettes and cigarettes. Clinicians should ask about and document e-cigarette use to enable the assessment of the health risks of e-cigarette use. Clinicians should also discourage nonsmokers and adolescents from using e-cigarettes and discourage smokers from engaging in dual use without cigarette reduction or cessation (Table 5).
Table 5.
Considerations for Clinicians
|
Supplementary Material
Figure 2. Overall Schematic.
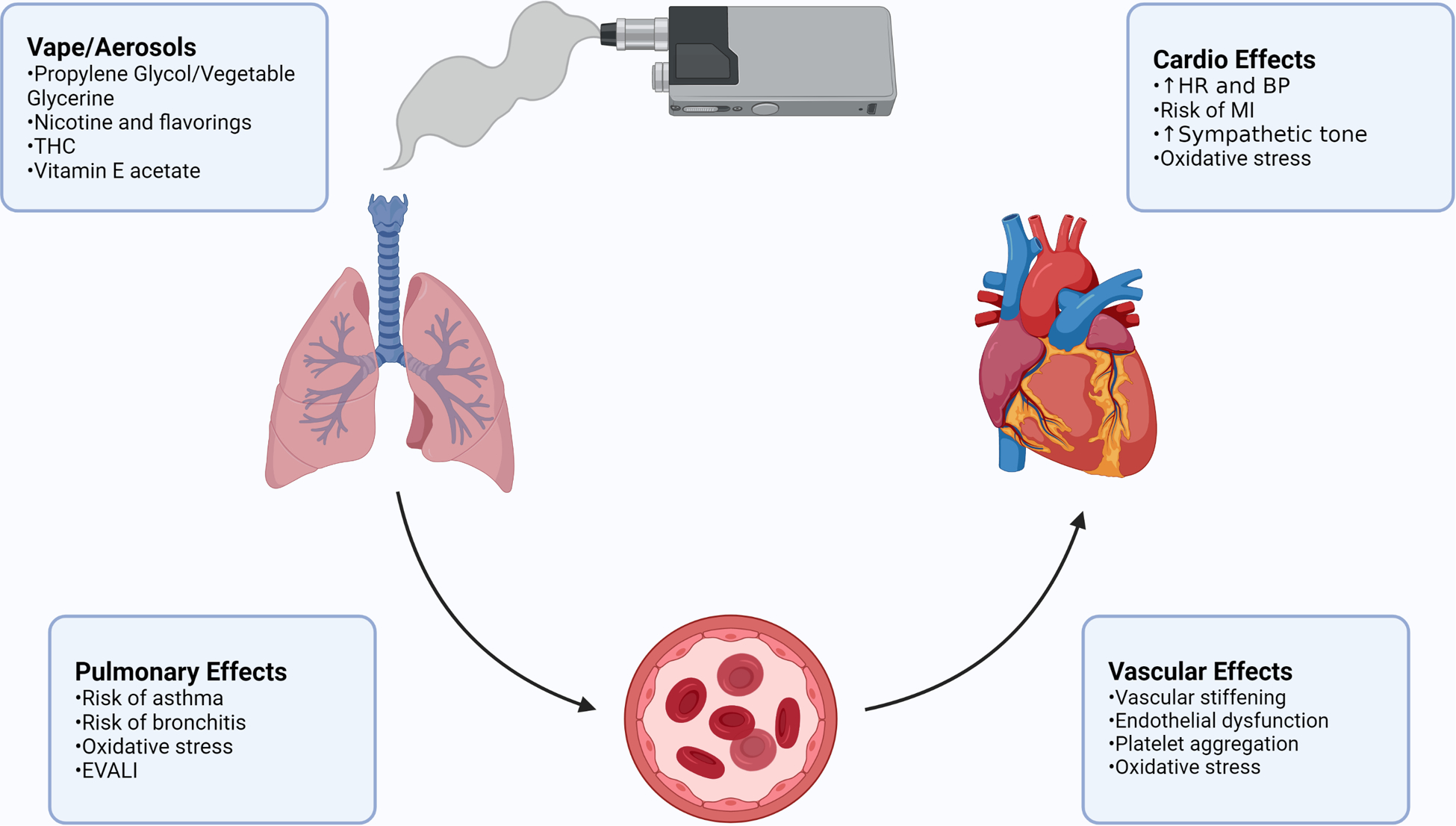
Schematic representing the cardiopulmonary effects of various aerosols from e-cigarette vapor, and potential concerns for clinicians. Created with BioRender.com
Acknowledgements
We thank Dr. Erin Worthington for critical insight into treating EVALI patients and Daniel N Lesman and Guozhen Xie for editorial guidance.
Funding Sources
Funded by grant #20YVNR35490079 from the American Heart Association, U01OH012056 from the Centers for Disease Control and Prevention, and HL139348/AG057046 from the NIH to LEW, and HL135642/HL153698 from the NIH to RT. RJG is the James Hay and Ruth Jansson Wilson Professor in Cardiology and recipient of the Robert J. Anthony Fund for Cardiovascular Disease Research at The Ohio State University Wexner Medical Center.
Non-standard Abbreviations and Acronyms
- e-cigarettes; EC
Electronic Cigarettes
- TC
Tobacco Cigarettes
- EVALI
E-cigarette or Vaping product use-Associated Lung Injury
- NRTs
Nicotine Replacement Therapies
- PWV
Pulse Wave Velocity
- BAL
Bronchoalveolar Lavage
- THC
Tetrahydrocannabinol
Footnotes
Disclosures
All authors have nothing to disclose.
References
- 1.Zare S, Nemati M, Zheng Y. A systematic review of consumer preference for e-cigarette attributes: Flavor, nicotine strength, and type. PLoS One. 2018;13:e0194145. doi: 10.1371/journal.pone.0194145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagener TL, Floyd EL, Stepanov I, Driskill LM, Frank SG, Meier E, Leavens EL, Tackett AP, Molina N, Queimado L. Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tob Control. 2017;26:e23–e28. doi: 10.1136/tobaccocontrol-2016-053041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrington-Trimis JL, Leventhal AM. Adolescents’ Use of “Pod Mod” E-Cigarettes — Urgent Concerns. N Engl J Med. 2018;379:1099–1102. doi: 10.1056/NEJMp1805758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miech R, Johnston L, O’Malley PM, Bachman JG, Patrick ME. Trends in Adolescent Vaping, 2017–2019. N Engl J Med. 2019;381:1490–1491. doi: 10.1056/NEJMc1910739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai H, Leventhal AM. Prevalence of e-Cigarette Use among Adults in the United States, 2014–2018. JAMA. 2019;322:1824–1827. doi: 10.1001/jama.2019.15331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cullen KA, Gentzke AS, Sawdey MD, Chang JT, Anic GM, Wang TW, Creamer MLR, Jamal A, Ambrose BK, King BA. e-Cigarette Use among Youth in the United States, 2019. JAMA. 2019;322:2095–2103. doi: 10.1001/jama.2019.18387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartwell G, Thomas S, Egan M, Gilmore A, Petticrew M. E-cigarettes and equity: A systematic review of differences in awareness and use between sociodemographic groups. Tob Control. 2017;26:e85–e91. doi: 10.1136/tobaccocontrol-2016-053222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Department of Health U, Services H, for Disease Control C, Center for Chronic Disease Prevention N, Promotion H, on Smoking O. E-Cigarette Use Among Youth and Young Adults: A Report of the Surgeon General [Internet]. 2016. [cited 20210 Jan 2];1–298. Available from: https://e-cigarettes.surgeongeneral.gov/documents/2016_sgr_full_report_non-508.pdf
- 9.Soneji S, Barrington-Trimis JL, Wills TA, Leventhal AM, Unger JB, Gibson LA, Yang JW, Primack BA, Andrews JA, Miech RA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults a systematic review and meta-analysis. JAMA Pediatr. 2017;171:788–797. doi: 10.1001/jamapediatrics.2017.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali FRM, Marynak KL, Kim Y, Binns S, Emery SL, Gomez Y, King BA. E-cigarette advertising expenditures in the United States, 2014–2018. Tob Control. 2020;29:e124–e126. doi: 10.1136/tobaccocontrol-2019-055424. [DOI] [PubMed] [Google Scholar]
- 11.Kimber C, Frings D, Cox S, Albery I, Dawkins L. The effects of the European e-cigarette health warnings and comparative health messages on non-smokers’ and smokers’ risk perceptions and behavioural intentions. BMC Public Health. 2018;18:1259. doi: 10.1186/s12889-018-6161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Padon AA, Lochbuehler K, Maloney EK, Cappella JN. A randomized trial of the effect of youth appealing e-cigarette advertising on susceptibility to use e-cigarettes among youth. Nicotine Tob Res. 2018;20:954–961. doi: 10.1093/ntr/ntx155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim M, Popova L, Halpern-Felsher B, Ling PM. Effects of e-Cigarette Advertisements on Adolescents’ Perceptions of Cigarettes. Health Commun. 2019;34:290–297. doi: 10.1080/10410236.2017.1407230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Booth P, Albery IP, Cox S, Frings D. Survey of the effect of viewing an online e-cigarette advertisement on attitudes towards cigarette and e-cigarette use in adults located in the UK and USA: A cross-sectional study. BMJ Open. 2019;9:e027525. doi: 10.1136/bmjopen-2018-027525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.FDA finalizes enforcement policy on unauthorized flavored cartridge-based e-cigarettes that appeal to children, including fruit and mint | FDA [Internet]. FDA News Release. 2020. [cited 20210 Jan 2]. ;Available from: https://www.fda.gov/news-events/press-announcements/fda-finalizes-enforcement-policy-unauthorized-flavored-cartridge-based-e-cigarettes-appeal-children [Google Scholar]
- 16.Bhatnagar A, Whitsel LP, Blaha MJ, Huffman MD, Krishan-Sarin S, Maa J, Rigotti N, Robertson RM, Warner JJ. New and Emerging Tobacco Products and the Nicotine Endgame: The Role of Robust Regulation and Comprehensive Tobacco Control and Prevention: A Presidential Advisory From the American Heart Association. Circulation. 2019;139:e937–e958. doi: 10.1161/CIR.0000000000000669. [DOI] [PubMed] [Google Scholar]
- 17.Public Health Consequences of E-Cigarettes [Internet]. Washington, D.C.: National Academies Press; 2018. doi: 10.17226/24952 [DOI] [PubMed] [Google Scholar]
- 18.Cho JH, Paik SY. Association between electronic cigarette use and asthma among high school students in South Korea. PLoS One. 2016;11:e0151022. doi: 10.1371/journal.pone.0151022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osei AD, Mirbolouk M, Orimoloye OA, Dzaye O, Uddin SMI, Dardari ZA, Defilippis AP, Bhatnagar A, Blaha MJ. The association between e-cigarette use and asthma among never combustible cigarette smokers: Behavioral risk factor surveillance system (BRFSS) 2016 & 2017. BMC Pulm Med. 2019;19:180. doi: 10.1186/s12890-019-0950-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McConnell R, Barrington-Trimis JL, Wang K, Urman R, Hong H, Unger J, Samet J, Leventhal A, Berhane K. Electronic cigarette use and respiratory symptoms in adolescents. Am J Respir Crit Care Med. 2017;195:1043–1049. doi: 10.1164/rccm.201604-0804OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wills TA, Pagano I, Williams RJ, Tam EK. E-cigarette use and respiratory disorder in an adult sample. Drug Alcohol Depend. 2019;194:363–370. doi: 10.1016/j.drugalcdep.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhatta DN, Glantz SA. Association of E-Cigarette Use With Respiratory Disease Among Adults: A Longitudinal Analysis. Am J Prev Med. 2020;58:182–190. doi: 10.1016/j.amepre.2019.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaiha SM, Cheng J, Halpern-Felsher B. Association Between Youth Smoking, Electronic Cigarette Use, and COVID-19. J Adolesc Health. 2020;67:519–523. doi: 10.1016/j.jadohealth.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li D, Sundar IK, McIntosh S, Ossip DJ, Goniewicz ML, O’Connor RJ, Rahman I. Association of smoking and electronic cigarette use with wheezing and related respiratory symptoms in adults: Cross-sectional results from the Population Assessment of Tobacco and Health (PATH) study, wave 2. Tob Control. 2020;29:140–147. doi: 10.1136/tobaccocontrol-2018-054694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alzahrani T, Pena I, Temesgen N, Glantz SA. Association Between Electronic Cigarette Use and Myocardial Infarction. Am J Prev Med. 2018;55:455–461. doi: 10.1016/j.amepre.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osei AD, Mirbolouk M, Orimoloye OA, Dzaye O, Uddin SMI, Benjamin EJ, Hall ME, DeFilippis AP, Stokes A, Bhatnagar A, et al. Association Between E-Cigarette Use and Cardiovascular Disease Among Never and Current Combustible-Cigarette Smokers. Am J Med. 2019;132:949–954.e2. doi: 10.1016/j.amjmed.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Kim CY, Paek YJ, Seo HG, Cheong YS, Lee CM, Park SM, Park DW, Lee K. Dual use of electronic and conventional cigarettes is associated with higher cardiovascular risk factors in Korean men. Sci Rep. 2020;10:5612. doi: 10.1038/s41598-020-62545-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi S, Lee K, Park SM. Combined Associations of Changes in Noncombustible Nicotine or Tobacco Product and Combustible Cigarette Use Habits With Subsequent Short-Term Cardiovascular Disease Risk Among South Korean Men: A Nationwide Cohort Study. Circulation. 2021. doi: 10.1161/CIRCULATIONAHA.121.054967. [DOI] [PubMed] [Google Scholar]
- 29.Hartmann-Boyce J, McRobbie H, Lindson N, Bullen C, Begh R, Theodoulou A, Notley C, Rigotti NA, Turner T, Butler AR, et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev. 2020;10:CD010216. doi: 10.1002/14651858.CD010216.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartmann-Boyce J, McRobbie H, Lindson N, Bullen C, Begh R, Theodoulou A, Notley C, Rigotti NA, Turner T, Butler AR, et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst. Rev. 2021;4:CD010216. doi: 10.1002/14651858.CD010216.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hajek P, Phillips-Waller A, Przulj D, Pesola F, Smith KM, Bisal N, Li J, Parrott S, Sasieni P, Dawkins L, et al. A randomized trial of E-cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019;380:629–637. doi: 10.1056/NEJMoa1808779. [DOI] [PubMed] [Google Scholar]
- 32.Stein JH, Korcarz CE. E-Cigarettes versus Nicotine-Replacement Therapy for Smoking Cessation. N Engl J Med. 2019;380:1973–1974. doi: 10.1056/NEJMc1903758. [DOI] [PubMed] [Google Scholar]
- 33.Eisenberg MJ, Hébert-Losier A, Windle SB, Greenspoon T, Brandys T, Fülöp T, Nguyen T, Elkouri S, Montigny M, Wilderman I, et al. Effect of e-Cigarettes Plus Counseling vs Counseling Alone on Smoking Cessation. JAMA. 2020;324:1844–1854. doi: 10.1001/jama.2020.18889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El Dib R, Suzumura EA, Akl EA, Gomaa H, Agarwal A, Chang Y, Prasad M, Ashoorion V, Heels-Ansdell D, Maziak W, et al. Electronic nicotine delivery systems and/or electronic non-nicotine delivery systems for tobacco smoking cessation or reduction: A systematic review and meta-analysis. BMJ Open. 2017;7:e012680. doi: 10.1136/bmjopen-2016-012680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalkhoran S, Glantz SA. E-cigarettes and smoking cessation in real-world and clinical settings: A systematic review and meta-analysis. Lancet Respir Med. 2016;4:116–128. doi: 10.1016/S2213-2600(15)00521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halpern SD, Harhay MO, Saulsgive K, Brophy C, Troxel AB, Volp KG. A pragmatic trial of e-cigarettes, incentives, and drugs for smoking cessation. N Engl J Med. 2018;378:2302–2310. doi: 10.1056/NEJMsa1715757. [DOI] [PubMed] [Google Scholar]
- 37.Wang RJ, Bhadriraju S, Glantz SA. E-Cigarette Use and Adult Cigarette Smoking Cessation: A Meta-Analysis. Am J Public Health. 2021;111:230–246. doi: 10.2105/AJPH.2020.305999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hiler M, Breland A, Spindle T, Maloney S, Lipato T, Karaoghlanian N, Shihadeh A, Lopez A, Ramôa C, Eissenberg T. Electronic cigarette user plasma nicotine concentration, puff topography, heart rate, and subjective effects: Influence of liquid nicotine concentration and user experience. Exp Clin Psychopharmacol. 2017;25:380–392. doi: 10.1037/pha0000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith TT, Wahlquist AE, Heckman BW, Cummings MK, Carpenter MJ. Impact of E-cigarette Sampling on Cigarette Dependence and Reinforcement Value. Nicotine Tob Res. 2020;22:297–301. doi: 10.1093/ntr/nty258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krist AH, Davidson KW, Mangione CM, Barry MJ, Cabana M, Caughey AB, Donahue K, Doubeni CA, Epling JW, Kubik M, et al. Interventions for Tobacco Smoking Cessation in Adults, Including Pregnant Persons. JAMA. 2021;325:265–279. doi: 10.1001/jama.2020.25019. [DOI] [PubMed] [Google Scholar]
- 41.Department of Health U, Services H, for Disease Control C, Center for Chronic Disease Prevention N, Promotion H, on Smoking O. Smoking Cessation: A Report of the Surgeon General. 2020. [cited 2021 Jan 2];1–700. Available from: https://www.hhs.gov/sites/default/files/2020-cessation-sgr-full-report.pdf [Google Scholar]
- 42.Franzen KF, Willig J, Cayo Talavera S, Meusel M, Sayk F, Reppel M, Dalhoff K, Mortensen K, Droemann D. E-cigarettes and cigarettes worsen peripheral and central hemodynamics as well as arterial stiffness: A randomized, double-blinded pilot study. Vasc Med. 2018;23:419–425. doi: 10.1177/1358863X18779694. [DOI] [PubMed] [Google Scholar]
- 43.Chaumont M, De Becker B, Zaher W, Culié A, Deprez G, Mélot C, Reyé F, Van Antwerpen P, Delporte C, Debbas N, et al. Differential Effects of E-Cigarette on Microvascular Endothelial Function, Arterial Stiffness and Oxidative Stress: A Randomized Crossover Trial. Sci Rep. 2018;8:10378. doi: 10.1038/s41598-018-28723-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan XS, D’Ruiz C. Effects of using electronic cigarettes on nicotine delivery and cardiovascular function in comparison with regular cigarettes. Regul Toxicol Pharmacol. 2015;71:24–34. doi: 10.1016/j.yrtph.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 45.Biondi-Zoccai G, Sciarretta S, Bullen C, Nocella C, Violi F, Loffredo L, Pignatelli P, Perri L, Peruzzi M, Marullo AGM, et al. Acute Effects of Heat-Not-Burn, Electronic Vaping, and Traditional Tobacco Combustion Cigarettes: The Sapienza University of Rome-Vascular Assessment of Proatherosclerotic Effects of Smoking (SUR-VAPES) 2 Randomized Trial. J Am Heart Assoc. 2019;8:e010455. doi: 10.1161/JAHA.118.010455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chaumont M, Tagliatti V, Channan EM, Colet JM, Bernard A, Morra S, Deprez G, van Muylem A, Debbas N, Schaefer T, et al. Short halt in vaping modifies cardiorespiratory parameters and urine metabolome: A randomized trial. Am J Physiol Lung Cell Mol Physiol. 2020;318:L331–L344. doi: 10.1152/ajplung.00268.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kerr DMI, Brooksbank KJM, Taylor RG, Pinel K, Rios FJ, Touyz RM, Delles C. Acute effects of electronic and tobacco cigarettes on vascular and respiratory function in healthy volunteers. J Hypertens. 2018;37:154–166. doi: 10.1097/HJH.0000000000001890. [DOI] [PubMed] [Google Scholar]
- 48.Ikonomidis I, Vlastos D, Kourea K, Kostelli G, Varoudi M, Pavlidis G, Efentakis P, Triantafyllidi H, Parissis J, Andreadou I, et al. Electronic Cigarette Smoking Increases Arterial Stiffness and Oxidative Stress to a Lesser Extent Than a Single Conventional Cigarette. Circulation. 2018;137:303–306. doi: 10.1161/CIRCULATIONAHA.117.029153. [DOI] [PubMed] [Google Scholar]
- 49.Moheimani RS, Bhetraratana M, Peters KM, Yang BK, Yin F, Gornbein J, Araujo JA, Middlekauff HR. Sympathomimetic Effects of Acute E-Cigarette Use: Role of Nicotine and Non-Nicotine Constituents. J Am Heart Assoc. 2017;6:e006579. doi: 10.1161/JAHA.117.006579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moheimani RS, Bhetraratana M, Yin F, Peters KM, Gornbein J, Araujo JA, Middlekauff HR. Increased cardiac sympathetic activity and oxidative stress in habitual electronic cigarette users: Implications for cardiovascular risk. JAMA Cardiol. 2017;2:278–285. doi: 10.1001/jamacardio.2016.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andreas S The association of cardiovascular autonomic dysfunction and the prediction of COPD can be explained by neurohumoral activation. Eur Respir J. 2018;51:1800737. doi: 10.1183/13993003.00737-2018. [DOI] [PubMed] [Google Scholar]
- 52.Zhang DY, Anderson AS. The Sympathetic Nervous System and Heart Failure. Cardiol Clin. 2014;32:33–45, vii. doi: 10.1016/j.ccl.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of Cardiovascular Events and All-Cause Mortality With Arterial Stiffness. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 54.Lyle AN, Raaz U. Killing me unsoftly: Causes and mechanisms of arterial stiffness. Arterioscler Thromb Vasc Biol. 2017;37:e1–e11. doi: 10.1161/ATVBAHA.116.308563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caporale A, Langham MC, Guo W, Johncola A, Chatterjee S, Wehrli FW. Acute effects of electronic cigarette aerosol inhalation on vascular function detected at quantitative MRI. Radiology. 2019;293:97–106. doi: 10.1148/radiol.2019190562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 57.Tsioufis C, Dimitriadis K. Sympathetic System–Related Artery Stiffness. Hypertension. 2019;73:975–976. doi: 10.1161/HYPERTENSIONAHA.119.12571. [DOI] [PubMed] [Google Scholar]
- 58.Fetterman JL, Keith RJ, Palmisano JN, McGlasson KL, Weisbrod RM, Majid S, Bastin R, Stathos MM, Stokes AC, Robertson RM, et al. Alterations in Vascular Function Associated With the Use of Combustible and Electronic Cigarettes. J Am Heart Assoc. 2020;9:e014570. doi: 10.1161/JAHA.119.014570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Doonan RJ, Hausvater A, Scallan C, Mikhailidis DP, Pilote L, Daskalopoulou SS. The effect of smoking on arterial stiffness. Hypertens Res. 2010;33:398–410. doi: 10.1038/hr.2010.25. [DOI] [PubMed] [Google Scholar]
- 60.Farah C, Michel LYM, Balligand JL. Nitric oxide signalling in cardiovascular health and disease. Nat Rev Cardiol. 2018;15:292–316. doi: 10.1038/nrcardio.2017.224. [DOI] [PubMed] [Google Scholar]
- 61.Carnevale R, Sciarretta S, Violi F, Nocella C, Loffredo L, Perri L, Peruzzi M, Marullo AGM, De Falco E, Chimenti I, et al. Acute Impact of Tobacco vs Electronic Cigarette Smoking on Oxidative Stress and Vascular Function. Chest. 2016;150:606–612. doi: 10.1016/j.chest.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 62.Chatterjee S, Tao JQ, Johncola A, Guo W, Caporale A, Langham MC, Wehrli FW. Acute exposure to e-cigarettes causes inflammation and pulmonary endothelial oxidative stress in nonsmoking, healthy young subjects. Am J Physiol Lung Cell Mol Physiol. 2019;317:L155–L166. doi: 10.1152/ajplung.00110.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mobarrez F, Antoniewicz L, Hedman L, Bosson JA, Lundbäck M. Electronic cigarettes containing nicotine increase endothelial and platelet derived extracellular vesicles in healthy volunteers. Atherosclerosis. 2020;301:93–100. doi: 10.1016/j.atherosclerosis.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 64.Antoniewicz L, Bosson JA, Kuhl J, Abdel-Halim SM, Kiessling A, Mobarrez F, Lundbäck M. Electronic cigarettes increase endothelial progenitor cells in the blood of healthy volunteers. Atherosclerosis. 2016;255:179–185. doi: 10.1016/j.atherosclerosis.2016.09.064. [DOI] [PubMed] [Google Scholar]
- 65.Staudt MR, Salit J, Kaner RJ, Hollmann C, Crystal RG. Altered lung biology of healthy never smokers following acute inhalation of E-cigarettes. Respir Res. 2018;19:78. doi: 10.1186/s12931-018-0778-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hill MA, Sowers J, Sutliff R, Sena CM, Leandro A, Azul L, Seiça R, Perry G. Vascular Oxidative Stress: Impact and Therapeutic Approaches. Front Physiol. 2018;9:1668. doi: 10.3389/fphys.2018.01668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pierini D, Bryan NS. Nitric oxide availability as a marker of oxidative stress. Methods Mol Biol. 2015;1208:63–71. doi: 10.1007/978-1-4939-1441-8_5. [DOI] [PubMed] [Google Scholar]
- 68.Stokes AC, Xie W, Anna Wilson ME, Hanqi Yang M, Olusola Orimoloye BA, Alyssa Harlow MF, Jessica Fetterman ML, DeFilippis AP, Benjamin EJ, Rose Marie Robertson S, et al. Association of Cigarette and Electronic Cigarette Use Patterns With Levels of Inflammatory and Oxidative Stress Biomarkers Among US Adults Population Assessment of Tobacco and Health Study. Circulation. 2021;143:869–871. doi: 10.1161/CIRCULATIONAHA.120.051551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.George J, Hussain M, Vadiveloo T, Ireland S, Hopkinson P, Struthers AD, Donnan PT, Khan F, Lang CC. Cardiovascular Effects of Switching From Tobacco Cigarettes to Electronic Cigarettes. J Am Coll Cardiol. 2019;74:3112–3120. doi: 10.1016/j.jacc.2019.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nocella C, Biondi-Zoccai G, Sciarretta S, Peruzzi M, Pagano F, Loffredo L, Pignatelli P, Bullen C, Frati G, Carnevale R. Impact of Tobacco Versus Electronic Cigarette Smoking on Platelet Function. Am J Cardiol. 2018;122:1477–1481. doi: 10.1016/j.amjcard.2018.07.029. [DOI] [PubMed] [Google Scholar]
- 71.Hom S, Chen L, Wang T, Ghebrehiwet B, Yin W, Rubenstein DA. Platelet activation, adhesion, inflammation, and aggregation potential are altered in the presence of electronic cigarette extracts of variable nicotine concentrations. Platelets. 2016;27:694–702. doi: 10.3109/09537104.2016.1158403. [DOI] [PubMed] [Google Scholar]
- 72.Farsalinos KE, Tsiapras D, Kyrzopoulos S, Savvopoulou M, Voudris V. Acute effects of using an electronic nicotine-delivery device (electronic cigarette) on myocardial function: Comparison with the effects of regular cigarettes. BMC Cardiovasc Disord. 2014;14. doi: 10.1186/1471-2261-14-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsuji H, Larson MG, Venditti FJ, Manders ES, Evans JC, Feldman CL, Levy D. Impact of Reduced Heart Rate Variability on Risk for Cardiac Events. Circulation. 1996;94:2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 74.Csordas A, Bernhard D. The biology behind the atherothrombotic effects of cigarette smoke. Nat Rev Cardiol. 2013;10:219–230. doi: 10.1038/nrcardio.2013.8. [DOI] [PubMed] [Google Scholar]
- 75.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: A meta-analysis. Int J Cardiovasc Imaging. 2010;26:631–640. doi: 10.1007/s10554-010-9616-1. [DOI] [PubMed] [Google Scholar]
- 76.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;140:e596–e646. doi: 10.1016/j.jacc.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ghosh A, Coakley RD, Ghio AJ, Muhlebach MS, Esther CR, Alexis NE, Tarran R. Chronic E-cigarette use increases neutrophil elastase and matrix metalloprotease levels in the lung. Am J Respir Crit Care Med. 2019;200:1392–1401. doi: 10.1164/rccm.201903-0615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leem AY, Park B, Kim YS, Chang J, Won S, Jung JY. Longitudinal decline in lung function: a community-based cohort study in Korea. Sci Rep. 2019;9:13614. doi: 10.1038/s41598-019-49598-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Willemse BWM, Postma DS, Timens W, ten Hacken NHT. The impact of smoking cessation on respiratory symptoms, lung function, airway hyperresponsiveness and inflammation. Eur Respir J. 2004;23:464–476. doi: 10.1183/09031936.04.00012704. [DOI] [PubMed] [Google Scholar]
- 80.Ferrari M, Zanasi A, Nardi E, Morselli Labate AM, Ceriana P, Balestrino A, Pisani L, Corcione N, Nava S. Short-term effects of a nicotine-free e-cigarette compared to a traditional cigarette in smokers and non-smokers. BMC Pulm Med. 2015;15:120. doi: 10.1186/s12890-015-0106-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meo SA, Ansary MA, Barayan FR, Almusallam AS, Almehaid AM, Alarifi NS, Alsohaibani TA, Zia I. Electronic Cigarettes: Impact on Lung Function and Fractional Exhaled Nitric Oxide Among Healthy Adults. Am J Mens Health. 2019;13:1557988318806073. doi: 10.1177/1557988318806073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boulay M-È, Henry C, Bossé Y, Boulet L-P, Morissette MC. Acute effects of nicotine-free and flavour-free electronic cigarette use on lung functions in healthy and asthmatic individuals. Respir Res. 2017;18:33. doi: 10.1186/s12931-017-0518-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Polosa R, Cibella F, Caponnetto P, Maglia M, Prosperini U, Russo C, Tashkin D. Health impact of E-cigarettes: A prospective 3.5-year study of regular daily users who have never smoked. Sci Rep. 2017;7:13825. doi: 10.1038/s41598-017-14043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ghosh A, Coakley RC, Mascenik T, Rowell TR, Davis ES, Rogers K, Webster MJ, Dang H, Herring LE, Sassano MF, et al. Chronic e-cigarette exposure alters the human bronchial epithelial proteome. Am J Respir Crit Care Med. 2018;198:67–76. doi: 10.1164/rccm.201710-2033OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vardavas CI, Anagnostopoulos N, Kougias M, Evangelopoulou V, Connolly GN, Behrakis PK. Short-term Pulmonary Effects of Using an Electronic Cigarette. Chest. 2012;141:1400–1406. doi: 10.1378/chest.11-2443. [DOI] [PubMed] [Google Scholar]
- 86.D’Ruiz CD, O’Connell G, Graff DW, Yan XS. Measurement of cardiovascular and pulmonary function endpoints and other physiological effects following partial or complete substitution of cigarettes with electronic cigarettes in adult smokers. Regul Toxicol Pharmacol. 2017;87:36–53. doi: 10.1016/j.yrtph.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 87.Cibella F, Campagna D, Caponnetto P, Amaradio MD, Caruso M, Russo C, Cockcroft DW, Polosa R. Lung function and respiratory symptoms in a randomized smoking cessation trial of electronic cigarettes. Clin Sci (Lond). 2016;130:1929–1937. doi: 10.1042/CS20160268. [DOI] [PubMed] [Google Scholar]
- 88.Veldheer S, Yingst J, Midya V, Hummer B, Lester C, Krebs N, Hrabovsky S, Wilhelm A, Liao J, Yen MS, et al. Pulmonary and other health effects of electronic cigarette use among adult smokers participating in a randomized controlled smoking reduction trial. Addict Behav. 2019;91:95–101. doi: 10.1016/j.addbeh.2018.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Deshpande M, Bromann S, Arnoldi J. Electronic cigarette use among adults with asthma: 2014–2017 National Health Interview Survey. Res Soc Adm Pharm. 2020;16:202–207. doi: 10.1016/j.sapharm.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 90.Lei S, Li M, Duan W, Peng C, Chen P, Wu S. The long-term outcomes of tobacco control strategies based on the cognitive intervention for smoking cessation in COPD patients. Respir Med. 2020;172:106155. doi: 10.1016/j.rmed.2020.106155. [DOI] [PubMed] [Google Scholar]
- 91.Polosa R, Morjaria JB, Caponnetto P, Caruso M, Campagna D, Amaradio MD, Ciampi G, Russo C, Fisichella A. Persisting Long Term Benefits of Smoking Abstinence and Reduction in Asthmatic Smokers Who Have Switched to Electronic Cigarettes. Discov Med. 2016;21:99–108. [PubMed] [Google Scholar]
- 92.Bowler RP, Hansel NN, Jacobson S, Graham Barr R, Make BJ, Han MLK, O’Neal WK, Oelsner EC, Casaburi R, Barjaktarevic I, et al. Electronic Cigarette Use in US Adults at Risk for or with COPD: Analysis from Two Observational Cohorts. J Gen Intern Med. 2017;32:1315–1322. doi: 10.1007/s11606-017-4150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chaumont M, van de Borne P, Bernard A, Van Muylem A, Deprez G, Ullmo J, Starczewska E, Briki R, de Hemptinne Q, Zaher W, et al. Fourth generation e-cigarette vaping induces transient lung inflammation and gas exchange disturbances: Results from two randomized clinical trials. Am J Physiol Lung Cell Mol Physiol. 2019;316:L705–L719. doi: 10.1152/ajplung.00492.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chaumont M, Bernard A, Pochet S, Mélot C, El Khattabi C, Reye F, Boudjeltia KZ, Van Antwerpen P, Delporte C, Van De Borne P. High-wattage e-cigarettes induce tissue hypoxia and lower airway injury: A randomized clinical trial. Am J Respir Crit Care Med. 2018;198:123–126. doi: 10.1164/rccm.201711-2198LE. [DOI] [PubMed] [Google Scholar]
- 95.Reidel B, Radicioni G, Clapp PW, Ford AA, Abdelwahab S, Rebuli ME, Haridass P, Alexis NE, Jaspers I, Kesimer M. E-cigarette use causes a unique innate immune response in the lung, involving increased neutrophilic activation and altered mucin secretion. Am J Respir Crit Care Med. 2018;197:492–501. doi: 10.1164/rccm.201708-1590OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kesimer M, Ford AA, Ceppe A, Radicioni G, Cao R, Davis CW, Doerschuk CM, Alexis NE, Anderson WH, Henderson AG, et al. Airway mucin concentration as a marker of chronic bronchitis. N Engl J Med. 2017;377:911–922. doi: 10.1056/NEJMoa1701632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kalininskiy A, Bach CT, Nacca NE, Ginsberg G, Marraffa J, Navarette KA, McGraw MD, Croft DP. E-cigarette, or vaping, product use associated lung injury (EVALI): case series and diagnostic approach. Lancet Respir Med. 2019;7:1017–1026. doi: 10.1016/S2213-2600(19)30415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hartnett KP, Kite-Powell A, Patel MT, Haag BL, Sheppard MJ, Dias TP, King BA, Melstrom PC, Ritchey MD, Stein Z, et al. Syndromic Surveillance for E-Cigarette, or Vaping, Product Use–Associated Lung Injury. N Engl J Med. 2020;382:766–772. doi: 10.1056/NEJMsr1915313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Krishnasamy VP, Hallowell BD, Ko JY, Board A, Hartnett KP, Salvatore PP, Danielson M, Kite-Powell A, Twentyman E, Kim L, et al. Update: Characteristics of a Nationwide Outbreak of E-cigarette, or Vaping, Product Use–Associated Lung Injury — United States, August 2019–January 2020. MMWR Morb Mortal Wkly Rep. 2020;69:90–94. doi: 10.15585/mmwr.mm6903e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ellington S, Salvatore PP, Ko J, Danielson M, Kim L, Cyrus A, Wallace M, Board A, Krishnasamy V, King BA, et al. Update: Product, Substance-Use, and Demographic Characteristics of Hospitalized Patients in a Nationwide Outbreak of E-cigarette, or Vaping, Product Use–Associated Lung Injury — United States, August 2019–January 2020. MMWR Morb Mortal Wkly Rep. 2020;69:44–49. doi: 10.15585/mmwr.mm6902e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Blount BC, Karwowski MP, Shields PG, Morel-Espinosa M, Valentin-Blasini L, Gardner M, Braselton M, Brosius CR, Caron KT, Chambers D, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382:697–705. doi: 10.1056/NEJMoa1916433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bhat TA, Kalathil SG, Bogner PN, Blount BC, Goniewicz ML, Thanavala YM. An animal model of inhaled Vitamin E acetate and EVALI-like lung injury. N Engl J Med. 2020;382:1175–1177. doi: 10.1056/NEJMc2000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Muthumalage T, Friedman MR, McGraw MD, Ginsberg G, Friedman AE, Rahman I. Chemical Constituents Involved in E-Cigarette, or Vaping Product Use-Associated Lung Injury (EVALI). Toxics. 2020;8:25. doi: 10.3390/toxics8020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aldy K, Cao DJ, McGetrick M, Willcutts D, Verbeck G, de Silva I, Hsu S. Severe e-cigarette, or vaping, product use associated lung injury requiring venovenous extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2020;385–388. doi: 10.1097/PCC.0000000000002264. [DOI] [PubMed] [Google Scholar]
- 105.Lee M-S, Rees VW, Koutrakis P, Wolfson JM, Son Y-S, Lawrence J, Christiani DC. Cardiac Autonomic Effects of Secondhand Exposure to Nicotine from Electronic Cigarettes. Environ Epidemiol. 2019;3:e033. doi: 10.1097/EE9.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Antoniewicz L, Brynedal A, Hedman L, Lundbäck M, Bosson JA. Acute Effects of Electronic Cigarette Inhalation on the Vasculature and the Conducting Airways. Cardiovasc Toxicol. 2019;19:441–450. doi: 10.1007/s12012-019-09516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Flouris AD, Chorti MS, Poulianiti KP, Jamurtas AZ, Kostikas K, Tzatzarakis MN, Wallace Hayes A, Tsatsaki AM, Koutedakis Y. Acute impact of active and passive electronic cigarette smoking on serum cotinine and lung function. Inhal Toxicol. 2013;25:91–101. doi: 10.3109/08958378.2012.758197. [DOI] [PubMed] [Google Scholar]
- 108.Song MA, Reisinger SA, Freudenheim JL, Brasky TM, Mathé EA, McElroy JP, Nickerson QA, Weng DY, Wewers MD, Shields PG. Effects of electronic cigarette constituents on the human lung: A pilot clinical trial. Cancer Prev Res (Phila). 2020;13:145–152. doi: 10.1158/1940-6207.CAPR-19-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


