Abstract
Background
Hearing loss is highly prevalent among older adults with cognitive impairment and may exacerbate neuropsychiatric symptoms and affect interactions with others. Though audiometry is the gold standard for measuring hearing, it is not always used in research or clinical settings focused on the care of individuals with cognitive impairment. Subjective assessments of hearing, both self- and proxy-rated, are widespread but may not adequately capture the presence of hearing loss as compared to audiometry. This study investigates the concordance between subjective and objective hearing assessments among older adults with and without cognitive impairment and evaluates factors associated with concordance.
Methods
Participants were a subset of the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS), a prospective cohort study representing four US communities with adjudicated cognitive diagnoses and audiometric data, totaling to 3,326 self-rated and 520 proxy-rated hearing assessments. Sensitivity and specificity were calculated, and multivariable logistic regression estimated the magnitude of the association between the concordance of hearing assessments and variables of interest.
Results
Sensitivity and specificity for self-rated hearing status was 71.2% and 85.9% among cognitively normal older adults, 61.1% and 84.9% among persons with MCI, and 52.6% and 81.2% among persons with dementia. For proxy-rated hearing, sensitivity and specificity were 65.7% and 83.3% for persons with MCI and 73.3% and 60.3% for persons with dementia. Female sex was positively associated with concordance for self-rated hearing assessments.
Conclusions
The low sensitivity of self- and proxy-rated hearing assessments compared to audiometry suggests that hearing loss among older adults with cognitive impairment may go underreported and unaddressed in subjective assessments. Clinicians and researchers should recognize the limitations of using self- and proxy-rated hearing assessments as measures of hearing status and incorporate objective audiometric evaluation whenever possible.
Keywords: Hearing loss, dementia, cognitive impairment, audiometry, hearing care
INTRODUCTION
Hearing loss is highly prevalent among older adults with Alzheimer’s disease and related dementias (ADRD) with estimates ranging from 60% to over 90%, at or higher than estimates of hearing loss in older adults without cognitive impairment, ranging from 45% to 65%.1–7 Hearing loss is independently associated with depression, poor physical functioning, social isolation, poor quality of life, and prolonged hospitalizations among older adults.8–12 Hearing loss may be associated with additional burdens for older adults with cognitive impairment, such as an increased number and severity of neuropsychiatric symptoms, and can interfere with daily interactions.1,13,14 Identification of those with hearing loss is important to the function and well-being of older persons.
Clinicians and researchers often rely on proxies for information. Approximately 75% of Medicare beneficiaries with cognitive impairment use proxies as their sole source of providing information.15 This reliance increases with age.13,16 The diversity of roles (e.g., caregiver, spouse, child), duration of relationships, and frequency of visits can vary among proxies. Few studies have evaluated the validity of proxy reports for assessing hearing status among older adults.
Clinicians often use subjective assessments to determine whether patients would benefit from referral for further audiometric assessment. Previous studies have examined the concordance of subjective hearing assessments with audiometric assessments among cognitively normal individuals, with sensitivity and specificity estimates ranging from 30–80%.17,18 Multiple factors have been found to affect the association between subjective and objective hearing status, including sex, age, race/ethnicity, and education.19 However, the accuracy of self- or proxy-rated assessments for audiometric hearing loss among older adults with cognitive impairment is unknown. One small study has suggested that older adults with probable mild cognitive impairment (MCI) are able to self-rate their hearing appropriately.20 Older adults with ADRD or MCI face additional challenges due to increased communication impairment, and current evidence supports that care partners and health professionals often underestimate the presence of communication difficulties due to hearing loss.3,21–23 Several of the studies that examined communication barriers due to hearing loss among individuals with cognitive have been limited to small clinic-based samples3,24 or employ subjective hearing assessments.23
To better characterize the effect of cognitive impairment on subjective hearing assessments, we assessed the concordance of self- and proxy-rated hearing assessments with audiometric data among community-dwelling participants, with and without cognitive impairment. Additionally, we examined the factors associated with concordance between self- or proxy-reported hearing status and objective audiometric hearing status.
METHODS
Study Population
The Atherosclerosis Risk in Communities (ARIC) study is a community-based prospective cohort study of 15,792 men and women aged 45 to 64 years in 1987–1989 recruited from four communities in the United States: Forsyth County, North Carolina; Jackson, Mississippi; Minneapolis suburbs, Minnesota; and Washington County, Maryland. Participants were examined every three years until 1996–1998 (visits 1–4), with additional visits in 2011–2013 (visit 5), 2016–2017 (visit 6), and 2018–2019 (visit 7).25 The cohort continues to be followed. Participants underwent neurocognitive testing, informant interviews, and adjudicated review to define cognitive status (cognitively normal, MCI, or dementia) beginning at visit 5.26 For our study, participants were a subset of the ARIC-NCS cohort who received audiometric testing (visit 6), neurocognitive testing (visit 6), and had complete covariate data (visits 1, 5, 6). Details of the neurocognitive testing protocol within the ARIC-NCS cohort are described elsewhere.26 Briefly, neurocognitive testing was conducted using assessments for cognitive domains in memory, psychomotor speed/executive functioning, language, and visuospatial. Neurocognitive testing also included informant interviews with all participant proxies either in-person or over the phone by certified staff members. Among the 4,003 participants at visit 6, 3,971 completed neurocognitive testing and 3,628 completed audiometric testing. Among the 3,605 participants with both neurocognitive and audiometric testing, 279 were missing complete covariate data. A total of 3,326 participants were included in the analytical cohort.
Audiometric Assessment
Objective hearing loss was defined according to the speech-frequency pure-tone average (PTA) of hearing thresholds at 0.5, 1, 2, and 4kHz in the better-hearing ear. The severity of hearing loss was categorized into binary and ordinal variables based on the 2020 World Health Organization criteria: “normal hearing” ≤ 25 dB, “any hearing loss” ≥ 25 dB, “mild hearing loss” = 25.0 – 39.9 dB, “moderate hearing loss” 40 – 59.9 dB or “severe hearing loss” ≥ 60.0 dB.27 We employed the prior World Health Organization’s criteria to aid in comparability with previously published work that utilizes data from the ARIC cohort.28–30 Categories of individuals with moderate or greater hearing loss were collapsed together to account for the small number with severe-to-profound hearing loss (n=111).
Subjective Hearing Assessments
For self-rated hearing assessments, participants were asked to “best describe [their] hearing” in both ears without the use of a hearing aid. Responses were recorded from the self-rated better-hearing ear as excellent, good, a little trouble, moderate trouble, a lot of trouble, or deaf. To condense these responses, participants with excellent (n=439) and good (n=1141) responses were grouped as “excellent to good”, while those with moderate trouble (n=556), a lot of trouble (n=371) or deaf (n=31) were grouped as “moderate trouble or greater”. Exploratory analyses of the distribution of self-rated responses over PTAs informed our definition of concordance. Concordance was defined as “a little trouble” or more for those with mild audiometric hearing loss, “moderate trouble” or more for those with moderate or greater audiometric hearing loss, and “excellent to good” for those without audiometric hearing loss.
For proxy-rated hearing assessments, informants characterized the participants’ hearing as whether they had “significant hearing difficulties that interfere with daily communication.” Responses were recorded as yes or no. The ARIC-NCS protocol does not specify whether proxies should consider hearing aid use when characterizing the participant’s hearing.
Covariates
Covariates included demographic characteristics for sex (male or female), self-identified race/ethnicity (white or Black), age, education (less than high school, high school or equivalent, at least some college), and income at visit 1 (< $25,000, $25,000–49,999, ≥ $50,000). Health-related covariates included the global cognitive function z-score, while current hearing aid user status was included as a potential confounding variable. For proxy-rated hearing analyses, informant-related covariates included the informant relationship, duration, and frequency of interaction with the participant. Additional demographic information, such as age and sex, for proxies are unavailable.
Statistical Analysis
Descriptive statistics were used to describe demographic and clinical characteristics stratified by cognitive status. Comparisons were calculated using Pearson’s chi-squared tests or ANOVA. Empirical cumulative distribution functions were used to display the distribution of participants with self-rated hearing by cognition level over their audiometric hearing status. Sensitivity and specificity of self-reported hearing assessments were calculated within three strata (cognitively normal, MCI, and dementia), while those of proxy-reported hearing assessments were calculated within two (MCI and dementia). For self-rated assessments, participants with moderate or greater audiometric hearing loss were considered to truly have hearing loss, while self-ratings of “moderate trouble” or greater were considered test positives. Sensitivity was calculated as the proportion of participants with audiometric hearing loss that endorsed “moderate trouble” or greater, while specificity was the proportion of participants without audiometric hearing loss that endorsed “excellent,” “good,” or “a little trouble” with hearing. For proxy-rated assessments, “yes” responses were defined as test positives. Multivariable logistic regression was used to estimate the magnitude of the association between the concordance of hearing assessments and the variables of interest. The regression model was adjusted for demographic- and health-related factors, including global cognitive function scores, pure tone averages, age, sex, education, income, and current hearing aid use. Statistical analysis was performed using STATA statistical software (version 15.1, StataCorp).
RESULTS
Demographic and hearing-related characteristics of the study participants who provided self-rated hearing status are presented in Table 1, stratified by cognitive status (also see Supplemental Table S1). Overall, participants with dementia were older, less educated, had lower income, and worse hearing (or higher PTAs) than those who were cognitively normal. Figure 1 displays the empirical cumulative distributions for participants at different cognitive levels stratified by their hearing self-rating. Among participants who self-rated their hearing as “excellent” or “good,” an increased severity of hearing loss was noted among those with dementia. Differences between cognitive groups diminished with increasing severity of their hearing self-rating (also see Supplemental Figure S1). The sensitivity and specificity of self-rated hearing assessments are displayed in Table 2. Sensitivity declined with increasing cognitive impairment, with sensitivities decreasing from cognitively normal (71.2%, 95% CI: 67.5%, 74.7%) to MCI (61.1%, 95% CI: 53.7%, 68.0%) to dementia (52.6%, 95% CI: 40.8%, 64.2%).
Table 1.
Demographic and hearing-related characteristics of participants with audiometric testing and self-reported hearing (n = 3326)
| Characteristics, % (n) | Cognitive Status | ||||
|---|---|---|---|---|---|
| Normal (n = 2525) | MCI (n = 608) | Dementia (n = 193) | All (n = 3326) | p-value3 | |
| Age at visit 6, years | |||||
| Average age (SD)1 | 79.4 (4.5) | 80.4 (4.7) | 82.7 (4.9) | 79.8 (4.7) | <0.001 |
| 70–74 | 17.2 (434) | 11.5 (70) | 5.2 (10) | 15.5 (514) | <0.001 |
| 75–79 | 44.4 (1122) | 41.9 (255) | 28.5 (55) | 43.1 (1432) | |
| ≥ 80 | 38.4 (969) | 46.6 (283) | 66.3 (128) | 41.5 (1380) | |
| Female | 60.1 (1518) | 56.9 (346) | 52.3 (101) | 59.1 (1965) | 0.05 |
| Black, % | 22.7 (573) | 17.6 (107) | 28.5 (55) | 22.1 (735) | 0.002 |
| Education | |||||
| Less than high school | 10.6 (267) | 9.4 (57) | 32.1 (62) | 11.6 (386) | <0.001 |
| High school, GED, or vocational school | 42.5 (1074) | 37.8 (230) | 38.3 (74) | 41.4 (1378) | |
| At least some college | 46.9 (1184) | 52.8 (321) | 29.5 (57) | 47.0 (1562) | |
| Income at visit 1 | |||||
| < 25,000 | 22.7 (572) | 21.9 (133) | 36.3 (70) | 23.3 (775) | <0.001 |
| 25,000 – 49,999 | 41.0 (1035) | 42.8 (260) | 36.3 (70) | 41.0 (1365) | |
| ≥ 50,000 | 36.4 (918) | 35.4 (215) | 27.5 (53) | 35.7 (1186) | |
| Degree of hearing loss by PTA | |||||
| PTA in better hearing ear in dB HL, (SD)1 | 32.2 (13.5) | 34.6 (14.0) | 38.4 (15.1) | 33.0 (13.8) | <0.001 |
| Any hearing loss | 64.6 (1630) | 71.4 (434) | 81.9 (158) | 66.8 (2222) | <0.001 |
| No hearing loss | 35.5 (895) | 28.6 (174) | 18.1 (35) | 33.2 (1104) | <0.001 |
| Mild hearing loss | 39.5 (998) | 40.1 (244) | 42.5 (82) | 39.8 (1324) | |
| Moderate or greater hearing loss | 25.0 (632) | 31.3 (190) | 39.4 (76) | 27.0 (898) | |
| Self-reported hearing loss2 | |||||
| Excellent to good | 47.9 (1209) | 47.0 (286) | 44.0 (85) | 47.5 (1580) | 0.81 |
| A little trouble | 23.7 (599) | 23.5 (143) | 23.8 (46) | 23.7 (788) | |
| Moderate or greater | 28.4 (717) | 29.4 (179) | 32.1 (62) | 28.8 (958) | |
| Current hearing aid user | 19.5 (493) | 20.9 (127) | 21.8 (42) | 19.9 (662) | 0.60 |
Significant p-values are designated in bold. Abbreviations: MCI = mild cognitive impairment; PTA = pure tone average, dB HL = decibels in hearing level.
Represented as a continuous variable with the standard deviation in parenthesis.
In the self-reported better hearing ear
A one-way ANOVA was used for continuous data, while the χ2 test was used for categorical data.
Figure 1.
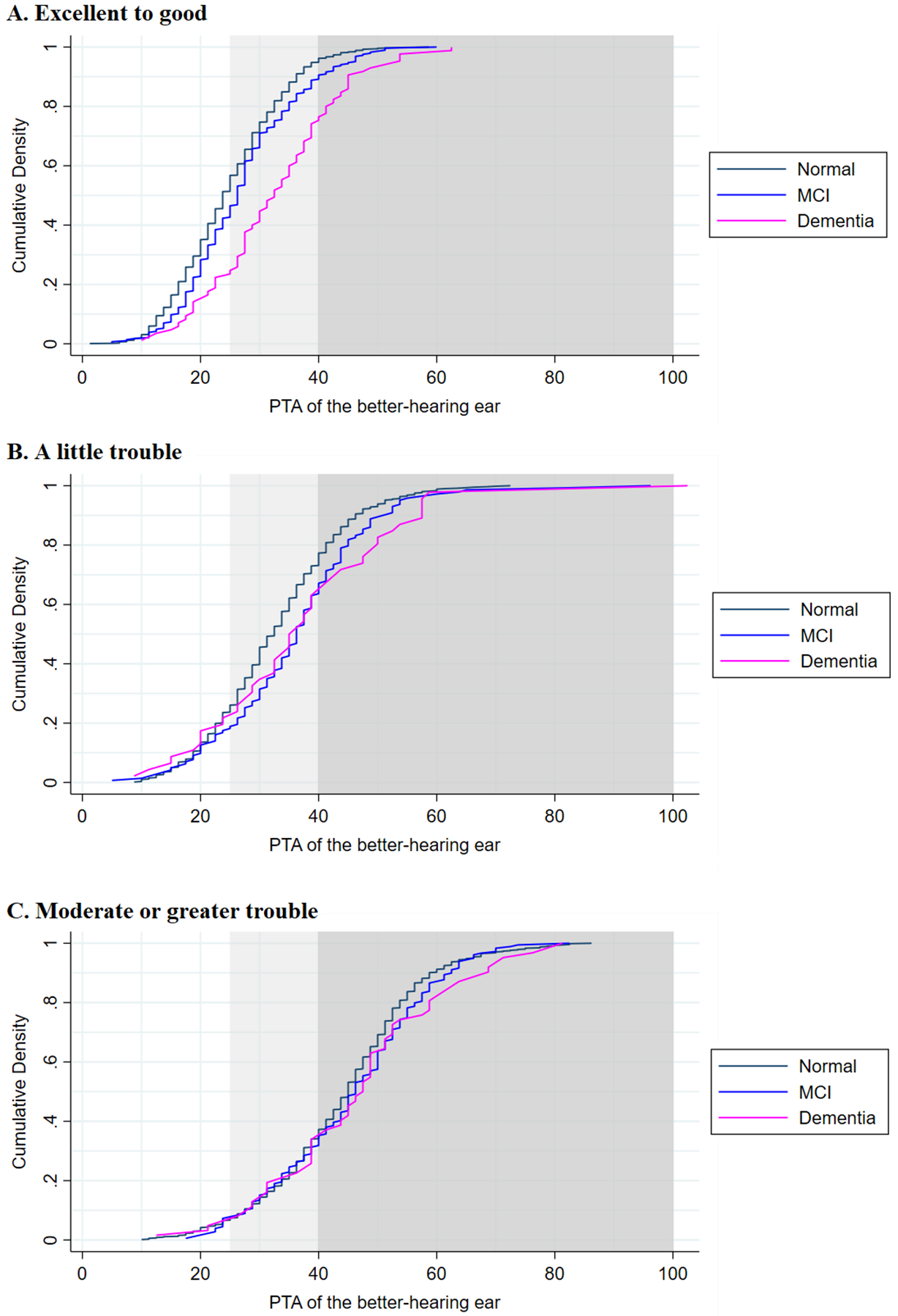
Empirical cumulative distribution functions of audiometric hearing status by cognition level over self-rated hearing. Light gray denotes the start of audiometric hearing loss, while dark gray indicates moderate or greater audiometric hearing loss.
Table 2.
Sensitivity and specificity of self- and proxy-rated hearing stratified by cognition
| Normal (n = 2525) | MCI (n = 608) | Dementia (n = 193) | ||
|---|---|---|---|---|
| Self-rated hearing | Sensitivity (95% CI) | 71.2 (67.5, 74.7) | 61.1 (53.7, 68.0) | 52.6 (40.8, 64.2) |
| Specificity (95% CI) | 85.9 (84.2, 87.4) | 84.9 (81.1, 88.2) | 81.2 (72.9, 87.8) | |
| Proxy-rated hearing | Sensitivity (95% CI) | - | 65.7 (57.0, 73.7) | 73.3 (54.1, 87.7) |
| Specificity (95% CI) | - | 83.3 (78.5, 87.3) | 60.3 (47.2, 72.4) |
All participants with a better-hearing ear pure tone average > 40 dB were considered to have hearing loss. Test positives for self-rated hearing were defined as those who endorsed moderate or greater trouble hearing, while those for proxy-rated hearing responded affirmatively to “significant hearing difficulties that interfere with daily communication.”
An analysis of possible associations between multiple factors and the concordance of self-rated hearing with audiometric hearing status is shown in Table 3. Concordance was positively associated with global cognitive function (OR: 1.14, 95% CI: 1.03, 1.27, p = 0.01) and female sex (OR: 1.23, 95% CI: 1.06, 1.44, p = 0.01). Concordance was negatively associated with increasing audiometric hearing loss (0.98, 95% CI: 0.97, 0.99, p < 0.001).
Table 3.
Factors associated with concordance of self-rated hearing loss with audiometric hearing loss
| Odds Ratio (95% CI) | p-value | |
|---|---|---|
| Global cognitive function z-score | 1.14 (1.03, 1.27) | 0.01 |
| Pure tone average | 0.98 (0.97, 0.99) | <0.001 |
| Age at visit 6, years | 1.00 (0.98, 1.02) | 0.98 |
| Female | 1.23 (1.06, 1.44) | 0.01 |
| Black | 1.14 (0.92, 1.40) | 0.24 |
| Education | ||
| Less than high school | REF | REF |
| High school, GED, or vocational school | 1.20 (0.94, 1.53) | 0.15 |
| At least some college | 1.18 (0.91, 1.52) | 0.22 |
| Income at visit 1 | ||
| < 25,000 | REF | REF |
| 25,000 – 49,999 | 0.96 (0.79, 1.17) | 0.70 |
| ≥ 50,000 | 0.94 (0.75, 1.18) | 0.59 |
| Current hearing aid user | 3.70 (2.95, 4.67) | <0.001 |
Significant p-values are designated in bold.
We then characterized the relationship between proxy-rated hearing status and objective audiometry between those with MCI or dementia. Proxy ratings were completed for 520 participants with either MCI or dementia. Overall, participants with dementia were older, more likely to self-identify as Black, have lower education, and a higher level of proxy-rated hearing loss (Supplemental Table S2). The calculated sensitivity and specificity of the proxy-rated assessments are shown in Table 2. The low sensitivity of proxy-rated hearing loss was seen among those with MCI (65.7%, 95% CI: 57.0%, 73.7%) and dementia (73.3%, 95% CI: 54.1%, 87.7%) group, while higher specificity was observed in the MCI group (83.3%, 95% CI: 78.5%, 87.7%) compared to those with dementia (60.3%, 95% CI: 47.2%, 72.4%). The predicted probabilities of positive proxy ratings are shown in Figure 2. Proxies for participants with dementia generally reported hearing loss at PTAs approximately 10 dB worse than those for participants with MCI.
Figure 2.
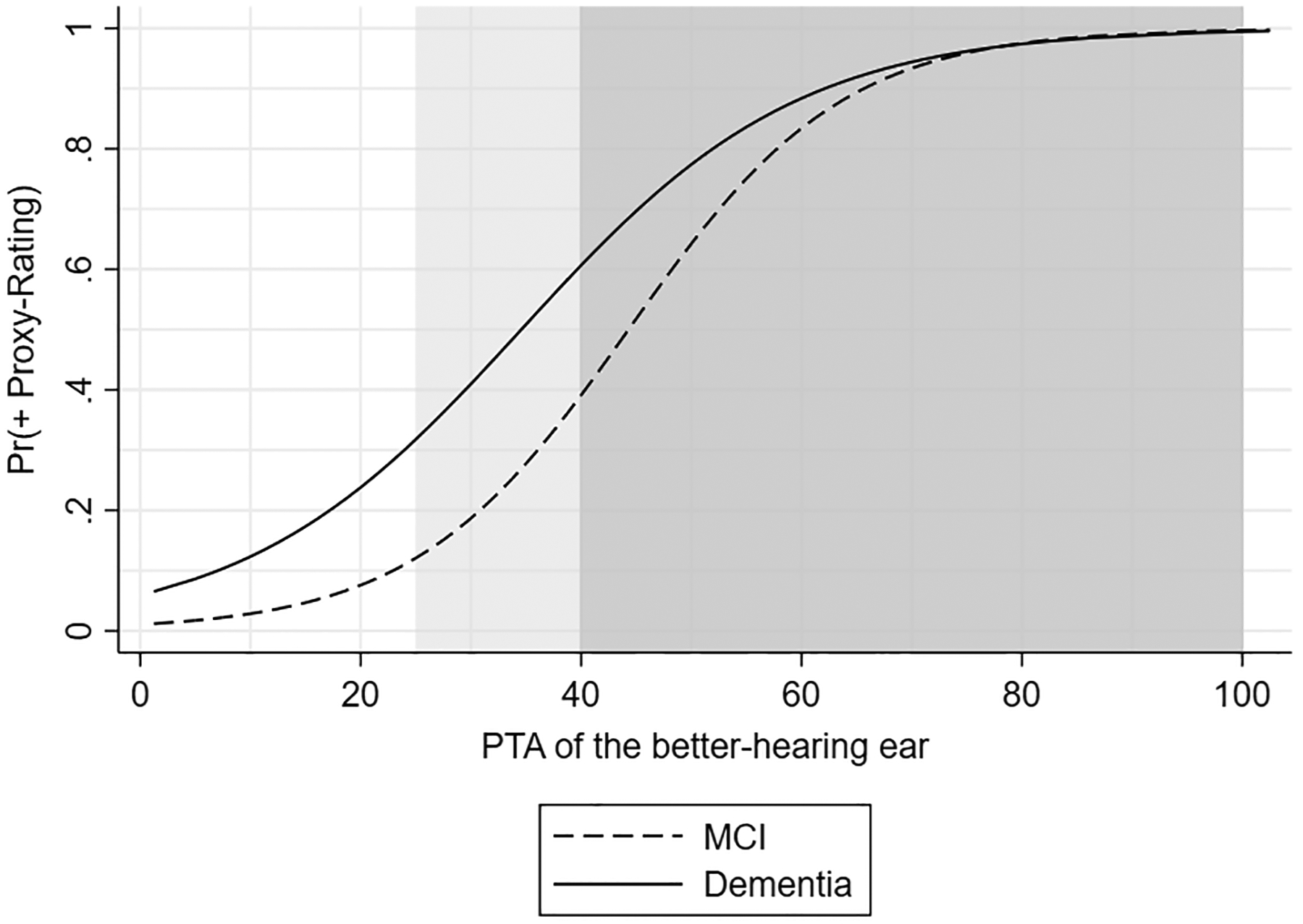
Predicted probabilities of positive proxy-rated hearing by cognition level
We also described factors associated with concordance of proxy-rated hearing assessments with audiometric hearing status. Global cognitive function was not significantly associated with concordance (OR: 1.25, 95% CI: 0.82, 1.91), though better concordance was seen with better audiometric hearing (OR: 0.97, 95% CI: 0.95, 0.99, p < 0.01). Demographic and informant-related characteristics, such as relationship to the participant, duration of relationship, and frequency of visits, were not associated with concordance.
DISCUSSION
In this multi-site community-based study, we investigated the sensitivity and specificity of subjective hearing assessments (self- and proxy-rated) among 3,326 community-dwelling older adults with and without cognitive impairment as well as factors associated with concordance between subjective and objective hearing assessments. Both self- and proxy-rated hearing assessments were poor predictors of audiometric hearing status among all participants, particularly among individuals with dementia, regardless of the proxy’s relationship with the participant. The overall low sensitivity of self- and proxy-rated assessments suggests that subjective measures of hearing underreport the true prevalence of hearing loss. In addition, we found that the sensitivity of self- and proxy-ratings were lowest among older adults with cognitive impairment, which further underestimates hearing loss in this vulnerable population.
Other studies in the general older adult population have also noted the unreliability of self-report as compared to audiometry in assessing hearing status.3,17–19,22,24,31 Our study observed improved concordance between self-reported hearing and hearing status with better hearing and female sex. Previous studies among older adults in general have found demographic characteristics as significant factors in predicting concordance, including age, sex, race/ethnicity, education, and occupation.17–19,32 For self-rated assessments, our finding of improved concordance among females is congruent with a prior study among older adults.19 The influence of sex varies with hearing-related research, particularly given the variability in representation of females within epidemiological cohorts. Within the ARIC-NCS cohort included in this study, females make up >50% of the cohort due to survival bias. Overall, the low sensitivity observed demonstrates that many older adults with hearing loss do not provide an accurate self-assessment of their hearing status and thus may underestimate the true prevalence of hearing loss.
The poor sensitivity of proxy-rated hearing assessments seen in our study presents evidence for questioning the use of proxy ratings as a measure of hearing status among older adults with cognitive impairment. In contrast, proxy respondents for older adults in general have reported comparable levels of agreement with the participant for functional assessments, while proxies for persons with dementia tend to describe more functional impairment.15 One study has noted that the concordance of older adults in general with their proxies on hearing status was 49%.33 Despite this tendency, our study demonstrates that proxies inadequately capture moderate hearing loss when asked for significant hearing difficulties faced by individuals with MCI or dementia. Despite exploring the potential factors influencing the concordance of proxy-rated hearing assessments with audiometry, no demographic or proxy-related factors, such as relationship, duration, and frequency were statistically significant. Additionally, this study did not include clinicians or other healthcare providers as proxies for the participants. Though the relationship of the proxy was not significant for family members or friends, whether the concordance of proxy ratings differs when healthcare professionals serve as proxies is unknown. One study among nursing home residents without dementia has shown that nurses’ assessments of residents’ hearing handicaps were less useful than audiometry with self-assessments of hearing handicap for guiding aural rehabilitation.22 Hospital staff also exhibited low sensitivity for hearing loss among older adults, with only 30% of nurses correctly identifying whether their patient had hearing loss.32
There are several limitations of our study. We employ a previous version of the grading criteria from the World Health Organization rather than the most recent criteria from 2021, which varies by 5 dB.34 While the criteria employed in our analyses does not reflect the latest recommendations from the World Health Organization, the analyses allow for comparison with prior work, including within the ARIC cohort.17,28–30,35 Self-rated hearing assessments were provided as multiple subjective responses and converted into a binary variable when calculating the sensitivity and specificity by the investigators rather than the participant. However, dichotomizing the self-rated assessments also enabled comparisons to other studies that have used similar or identical methods.17,19 This limitation also applies to the measure of proxy-rated hearing utilized in this study. Other forms of proxy-rated hearing may provide improved sensitivity and specificity, but data are currently lacking. The audiometric data available within the ARIC-NCS dataset also only includes air conduction pure tone audiometry and does not include word recognition testing, limiting the available information regarding participants’ hearing status. Additionally, we recognize that this study focuses primarily on hearing status rather than hearing or communication function, which is a meaningful and multifaceted construct, particularly in terms of viewing hearing loss within the broader context of aging and the holistic care of older adults, as highlighted by the World Health Organization’s Integrated Care for Older People (ICOPE).36 Furthermore, function, such as self-perceived hearing difficulties, is an important predictor of hearing care behaviors, such as adoption of hearing aids.37–39 There are also variables that were not included in these analyses that may influence subjective assessments of hearing status, such as the degree of asymmetry, vision, and other comorbidities, and is an area of needed research.35,40–43 The generalizability of the cohort to the older adult population of the United States is also limited, as the participants from the four US communities was lacking in racial/ethnic minority representation beyond African American participants. Additionally, given that ARIC participants needed to survive and be healthy enough to attend visit 6, they likely do not represent many older adults in the general US population, particularly those with cognitive impairment, and so our results may also not be generalizable to those individuals.
Taken together, our findings have important implications for both researchers and clinicians working with older adults with cognitive impairment. For researchers, studies using subjective hearing assessments to approximate audiometric hearing loss likely introduce bias into their hearing-related findings, which may result in an inaccurate estimation of true associations. One study observed that using self-rated hearing instead of audiometric hearing may underestimate associations with objective functional outcomes while overestimating subjective outcomes.44 In using subjective hearing assessments, researchers may find participant responses more closely reflect the perception of hearing loss, which can be influenced by participant characteristics such as cognitive impairment, rather than audiometric hearing loss. Self-rated assessments may thus be more representative of function, such as word comprehension, and may not align with status. We have attempted to mitigate this limitation from the use of empirical cumulative distribution functions of the different response categories when aligning these responses with hearing status. Despite these limitations, we suggest that the clinicians’ use of subjective assessments may lead to late identification of hearing loss and delays in addressing hearing loss, such as through the incorporation of communication strategies or provision of amplification. Ensuring access to effective communication among individuals aging with MCI or ADRD may serve as secondary and tertiary prevention strategies in either slowing the progression of cognitive changes or limiting the effects of ADRD, such as neuropsychiatric symptoms, among those aging with MCI or ADRD and hearing loss, respectively.
Potential solutions to address these concerns, especially for populations with cognitive impairment, include the adoption of mobile hearing screening technologies. Several studies have demonstrated that tablet-based audiometry can be reliably performed in older adults with cognitive impairment.45–48 While the reliability of self-administered audiometric testing has been shown to be limited for MMSE scores lower than 26, technician-administered hearing threshold testing using a tablet-based, portable audiometer (typically performed with 5 to 10 minutes of testing time) successfully identified hearing status among those with more cognitive impairment.48 The test-retest reliability of audiometric assessments among participants with mild dementia has also been found comparable to that among cognitively normal participants, further suggesting that accurate measures of hearing are obtainable in this population.49 A systematic review has also found that audiometry can be used to evaluate adults with mild, moderate, and severe dementia, though completion rates ranged from 56–59%.50 Implementation of these technologies in research and clinical settings may provide more reliable screening for older adults.
CONCLUSION
This multi-site community-based cohort study provides evidence for the poor predictability of subjective assessments, both self- and proxy-rated, for audiometric hearing loss in older adults with and without cognitive impairment. With a growing understanding of the potential negative effects of hearing loss on older adults with cognitive impairment, reliable methods are needed to identify hearing loss among patients and research participants. Researchers should exercise caution in the use of self- and proxy-rated hearing assessments to represent audiometric hearing as the findings may bias associations. For clinicians, the use of self- and proxy-rated hearing assessments may delay the identification and management of hearing loss among their patients, who may already face increasing functional and communication difficulties with concurrent sensory and cognitive impairment.23 Improving the detection of hearing loss may be an important, but frequently missed, opportunity to enhance the well-being of older persons as well as focus attention on the need for accessing hearing treatment.
Supplementary Material
Key Points:
When self-rating hearing, older adults have lower sensitivity and specificity with increasing cognitive impairment.
Sensitivity and specificity were low in proxy ratings of hearing.
Why does this matter?
Subjective hearing assessments may underreport hearing loss among older adults with cognitive impairment.
ACKNOWLEDGMENTS
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). Neurocognitive data is collected by U01 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, 2U01HL096917 from the NIH (NHLBI, NINDS, NIA and NIDCD), and with previous brain MRI examinations funded by R01-HL70825 from the NHLBI. The authors thank the staff and participants of the ARIC study for their important contributions.
Funding:
This study was supported in-part by funding from the National Institutes of Health (NIA K23AG059900, CLN; NIA K01AG054693, JAD; NIA K23AG065443, NSR). The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). Neurocognitive data is collected by U01 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, 2U01HL096917 from the NIH (NHLBI, NINDS, NIA and NIDCD), and with previous brain MRI examinations funded by R01-HL70825 from the NHLBI.
Conflict of Interest:
JFB has equity and future royalties from MiDiagnostics. NSR reports nonfinancial relationships as a scientific advisor to Shoebox, Inc and Good Machine Studio. FRL reports being a consultant to Frequency Therapeutics, speaker honoraria from Caption Call, and being the director of a research center funded in part by a philanthropic gift from Cochlear Ltd to the Johns Hopkins Bloomberg School of Public Health. FRL and CLN are board members of the nonprofit Access HEARS. CLN is a member of the board of directors for the Hearing Loss Association of America. All other authors report no relevant disclosures.
Sponsor’s Role:
This article was supported by grants from the National Institute on Aging (NIA K23AG059900, CLN; NIA K01AG054693, JAD; NIA K23AG065443, NSR). The National Institute on Aging had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
REFERENCES
- 1.Lin FR, Ferrucci L, Metter EJ, An Y, Zonderman AB, Resnick SM. Hearing loss and cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology. 2011;25(6):763–770. doi: 10.1037/a0024238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nirmalasari O, Mamo SK, Nieman CL, et al. Age-related hearing loss in older adults with cognitive impairment. Int Psychogeriatr. 2017;29(1):115–121. doi: 10.1017/S1041610216001459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gold M, Lightfoot LA, Hnath-Chisolm T. Hearing loss in a memory disorders clinic. A specially vulnerable population. Arch Neurol. 1996;53(9):922–928. doi: 10.1001/archneur.1996.00550090134019 [DOI] [PubMed] [Google Scholar]
- 4.Lin FR, Thorpe R, Gordon-Salant S, Ferrucci L. Hearing loss prevalence and risk factors among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2011;66(5):582–590. doi: 10.1093/gerona/glr002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cruickshanks KJ, Wiley TL, Tweed TS, et al. Prevalence of hearing loss in older adults in Beaver Dam, Wisconsin. The Epidemiology of Hearing Loss Study. Am J Epidemiol. 1998;148(9):879–886. doi: 10.1093/oxfordjournals.aje.a009713 [DOI] [PubMed] [Google Scholar]
- 6.Lin FR, Yaffe K, Xia J, et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med. 2013;173(4):293–299. doi: 10.1001/jamainternmed.2013.1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nieman CL, Deal JA, Betz J, et al. Hearing loss and hearing care disparities among older adults with cognitive impairment: Epidemiology: Sensory impairment and cognition. Alzheimer’s & Dementia. 2020;16(S10). doi: 10.1002/alz.041465 [DOI] [Google Scholar]
- 8.Li C-M, Zhang X, Hoffman HJ, Cotch MF, Themann CL, Wilson MR. Hearing impairment associated with depression in US adults, National Health and Nutrition Examination Survey 2005–2010. JAMA Otolaryngol Head Neck Surg. 2014;140(4):293–302. doi: 10.1001/jamaoto.2014.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen DS, Genther DJ, Betz J, Lin FR. Association between hearing impairment and self-reported difficulty in physical functioning. J Am Geriatr Soc. 2014;62(5):850–856. doi: 10.1111/jgs.12800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinstein BE, Ventry IM. Hearing impairment and social isolation in the elderly. J Speech Hear Res. 1982;25(4):593–599. doi: 10.1044/jshr.2504.593 [DOI] [PubMed] [Google Scholar]
- 11.Dalton DS, Cruickshanks KJ, Klein BEK, Klein R, Wiley TL, Nondahl DM. The impact of hearing loss on quality of life in older adults. Gerontologist. 2003;43(5):661–668. doi: 10.1093/geront/43.5.661 [DOI] [PubMed] [Google Scholar]
- 12.Genther DJ, Frick KD, Chen D, Betz J, Lin FR. Association of hearing loss with hospitalization and burden of disease in older adults. JAMA. 2013;309(22):2322–2324. doi: 10.1001/jama.2013.5912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mamo SK, Oh E, Lin FR. Enhancing Communication in Adults with Dementia and Age-Related Hearing Loss. Semin Hear. 2017;38(2):177–183. doi: 10.1055/s-0037-1601573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim AS, Garcia Morales EE, Amjad H, et al. Association of Hearing Loss With Neuropsychiatric Symptoms in Older Adults With Cognitive Impairment. Am J Geriatr Psychiatry. Published online October 14, 2020. doi: 10.1016/j.jagp.2020.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neumann PJ, Araki SS, Gutterman EM. The use of proxy respondents in studies of older adults: lessons, challenges, and opportunities. J Am Geriatr Soc. 2000;48(12):1646–1654. doi: 10.1111/j.1532-5415.2000.tb03877.x [DOI] [PubMed] [Google Scholar]
- 16.Johnson M, Lin F. Communication Difficulty and Relevant Interventions in Mild Cognitive Impairment: Implications for Neuroplasticity. Top Geriatr Rehabil. 2014;30(1):18–34. doi: 10.1097/TGR.0000000000000001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiely KM, Gopinath B, Mitchell P, Browning CJ, Anstey KJ. Evaluating a dichotomized measure of self-reported hearing loss against gold standard audiometry: prevalence estimates and age bias in a pooled national data set. J Aging Health. 2012;24(3):439–458. doi: 10.1177/0898264311425088 [DOI] [PubMed] [Google Scholar]
- 18.Kim SY, Kim H-J, Kim M-S, Park B, Kim J-H, Choi HG. Discrepancy between self-assessed hearing status and measured audiometric evaluation. PLoS ONE. 2017;12(8):e0182718. doi: 10.1371/journal.pone.0182718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamil RJ, Genther DJ, Lin FR. Factors associated with the accuracy of subjective assessments of hearing impairment. Ear Hear. 2015;36(1):164–167. doi: 10.1097/AUD.0000000000000075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fausto BA, Badana ANS, Arnold ML, Lister JJ, Edwards JD. Comparison of Subjective and Objective Measures of Hearing, Auditory Processing, and Cognition Among Older Adults With and Without Mild Cognitive Impairment. J Speech Lang Hear Res. 2018;61(4):945–956. doi: 10.1044/2017_JSLHR-H-17-0263 [DOI] [PubMed] [Google Scholar]
- 21.Slaughter SE, Hopper T, Ickert C, Erin DF. Identification of hearing loss among residents with dementia: perceptions of health care aides. Geriatr Nurs. 2014;35(6):434–440. doi: 10.1016/j.gerinurse.2014.07.001 [DOI] [PubMed] [Google Scholar]
- 22.Garahan MB, Waller JA, Houghton M, Tisdale WA, Runge CF. Hearing Loss Prevalence and Management in Nursing Home Residents. Journal of the American Geriatrics Society. 1992;40(2):130–134. doi: 10.1111/j.1532-5415.1992.tb01932.x [DOI] [PubMed] [Google Scholar]
- 23.Guthrie DM, Davidson JGS, Williams N, et al. Combined impairments in vision, hearing and cognition are associated with greater levels of functional and communication difficulties than cognitive impairment alone: Analysis of interRAI data for home care and long-term care recipients in Ontario. Bowen M, ed. PLoS ONE. 2018;13(2):e0192971. doi: 10.1371/journal.pone.0192971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voeks SK, Gallagher CM, Langer EH, Drinka PJ. Self-reported hearing difficulty and audiometric thresholds in nursing home residents. J Fam Pract. 1993;36(1):54–58. [PubMed] [Google Scholar]
- 25.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 26.Knopman DS, Gottesman RF, Sharrett AR, et al. Mild cognitive impairment and dementia prevalence: The Atherosclerosis Risk in Communities Neurocognitive Study. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring. 2016;2(1):1–11. doi: 10.1016/j.dadm.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO | Grades of hearing impairment. Accessed December 20, 2020. http://www.who.int/pbd/deafness/hearing_impairment_grades/en
- 28.Deal JA, Sharrett AR, Albert MS, et al. Hearing Impairment and Cognitive Decline: A Pilot Study Conducted Within the Atherosclerosis Risk in Communities Neurocognitive Study. American Journal of Epidemiology. 2015;181(9):680–690. doi: 10.1093/aje/kwu333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nichols E, Deal JA, Swenor BK, et al. Assessing Bias in Cognitive Testing for Older Adults with Sensory Impairment: An Analysis of Differential Item Functioning in the Baltimore Longitudinal Study on Aging (BLSA) and the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). J Int Neuropsychol Soc. Published online April 26, 2021:1–12. doi: 10.1017/S1355617721000400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mamo SK, Reed NS, Sharrett AR, et al. Relationship Between Domain-Specific Cognitive Function and Speech-in-Noise Performance in Older Adults: The Atherosclerosis Risk in Communities Hearing Pilot Study. Am J Audiol. 2019;28(4):1006–1014. doi: 10.1044/2019_AJA-19-00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hannula S, Bloigu R, Majamaa K, Sorri M, Mäki-Torkko E. Self-Reported Hearing Problems among Older Adults: Prevalence and Comparison to Measured Hearing Impairment. j am acad audiol. 2011;22(8):550–559. doi: 10.3766/jaaa.22.8.7 [DOI] [PubMed] [Google Scholar]
- 32.Mormer E, Bubb KJ, Alrawashdeh M, Cipkala-Gaffin JA. Hearing Loss and Communication Among Hospitalized Older Adults: Prevalence and Recognition. J Gerontol Nurs. 2020;46(6):34–42. doi: 10.3928/00989134-20200316-03 [DOI] [PubMed] [Google Scholar]
- 33.The Medical Research Council Cognitive Function and Ageing Studyb. Survey into health problems of elderly people: a comparison of self-report with proxy information. International Journal of Epidemiology. 2000;29(4):684–697. doi: 10.1093/ije/29.4.684 [DOI] [PubMed] [Google Scholar]
- 34.World Report on Hearing. Accessed October 1, 2021. https://www.who.int/teams/noncommunicable-diseases/sensory-functions-disability-and-rehabilitation/highlighting-priorities-for-ear-and-hearing-care
- 35.Gopinath B, Schneider J, McMahon CM, Burlutsky G, Leeder SR, Mitchell P. Dual Sensory Impairment in Older Adults Increases the Risk of Mortality: A Population-Based Study. Chao L, ed. PLoS ONE. 2013;8(3):e55054. doi: 10.1371/journal.pone.0055054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Integrated care for older people: guidelines on community-level interventions to manage declines in intrinsic capacity. Accessed October 10, 2021. https://www.who.int/publications/i/item/9789241550109 [PubMed]
- 37.Ng JH-Y, Loke AY. Determinants of hearing-aid adoption and use among the elderly: a systematic review. Int J Audiol. 2015;54(5):291–300. doi: 10.3109/14992027.2014.966922 [DOI] [PubMed] [Google Scholar]
- 38.Hickson L, Meyer C, Lovelock K, Lampert M, Khan A. Factors associated with success with hearing aids in older adults. International Journal of Audiology. 2014;53(sup1):S18–S27. doi: 10.3109/14992027.2013.860488 [DOI] [PubMed] [Google Scholar]
- 39.Stark P, Hickson L. Outcomes of hearing aid fitting for older people with hearing impairment and their significant others. International Journal of Audiology. 2004;43(7):390–398. doi: 10.1080/14992020400050050 [DOI] [PubMed] [Google Scholar]
- 40.von Gablenz P, Otto-Sobotka F, Holube I. Adjusting Expectations: Hearing Abilities in a Population-Based Sample Using an SSQ Short Form. Trends in Hearing. 2018;22:233121651878483. doi: 10.1177/2331216518784837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hämäläinen A, Pichora-Fuller MK, Wittich W, Phillips NA, Mick P. Self-report Measures of Hearing and Vision in Older Adults Participating in the Canadian Longitudinal Study of Aging are Explained by Behavioral Sensory Measures, Demographic, and Social Factors. Ear & Hearing. 2021;42(4):814–831. doi: 10.1097/AUD.0000000000000992 [DOI] [PubMed] [Google Scholar]
- 42.Vannson N, James C, Fraysse B, et al. Quality of Life and Auditory Performance in Adults with Asymmetric Hearing Loss. Audiol Neurotol. 2015;20(Suppl. 1):38–43. doi: 10.1159/000380746 [DOI] [PubMed] [Google Scholar]
- 43.Ketterer MC, Knopke S, Häußler SM, et al. Asymmetric hearing loss and the benefit of cochlear implantation regarding speech perception, tinnitus burden and psychological comorbidities: a prospective follow-up study. Eur Arch Otorhinolaryngol. 2018;275(11):2683–2693. doi: 10.1007/s00405-018-5135-9 [DOI] [PubMed] [Google Scholar]
- 44.Choi JS, Betz J, Deal J, et al. A Comparison of Self-Report and Audiometric Measures of Hearing and Their Associations With Functional Outcomes in Older Adults. J Aging Health. 2016;28(5):890–910. doi: 10.1177/0898264315614006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bastianelli M, Mark AE, McAfee A, Schramm D, Lefrançois R, Bromwich M. Adult validation of a self-administered tablet audiometer. J Otolaryngol Head Neck Surg. 2019;48(1):59. doi: 10.1186/s40463-019-0385-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saliba J, Al-Reefi M, Carriere JS, Verma N, Provencal C, Rappaport JM. Accuracy of Mobile-Based Audiometry in the Evaluation of Hearing Loss in Quiet and Noisy Environments. Otolaryngol Head Neck Surg. 2017;156(4):706–711. doi: 10.1177/0194599816683663 [DOI] [PubMed] [Google Scholar]
- 47.Thompson GP, Sladen DP, Borst BJH, Still OL. Accuracy of a Tablet Audiometer for Measuring Behavioral Hearing Thresholds in a Clinical Population. Otolaryngol Head Neck Surg. 2015;153(5):838–842. doi: 10.1177/0194599815593737 [DOI] [PubMed] [Google Scholar]
- 48.Pletnikova A, Reed NS, Amjad H, et al. Identification of Hearing Loss in Individuals With Cognitive Impairment Using Portable Tablet Audiometer. Perspectives of the ASHA Special Interest Groups. 2019;4(5):947–953. doi: 10.1044/2019_PERS-SIG8-2018-0018 [DOI] [Google Scholar]
- 49.McClannahan KS, Chiu Y-F, Sommers MS, Peelle JE. Test-Retest Reliability of Audiometric Assessment in Individuals With Mild Dementia. JAMA Otolaryngol Head Neck Surg. 2021;147(5):442–449. doi: 10.1001/jamaoto.2021.0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bott A, Meyer C, Hickson L, Pachana NA. Can adults living with dementia complete pure-tone audiometry? A systematic review. International Journal of Audiology. 2019;58(4):185–192. doi: 10.1080/14992027.2018.1550687 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


