Abstract
In recent years, drinking water-associated pathogens that can cause infections in immunocompromised or otherwise susceptible individuals (henceforth referred to as DWPI), sometimes referred to as opportunistic pathogens or opportunistic premise plumbing pathogens, have received considerable attention. DWPI research has largely been conducted by experts focusing on specific microorganisms or within silos of expertise. The resulting mitigation approaches optimized for a single microorganism may have unintended consequences and trade-offs for other DWPI or other interests (e.g., energy costs and conservation). For example, the ecological and epidemiological issues characteristic of Legionella pneumophila diverge from those relevant for Mycobacterium avium and other nontuberculous mycobacteria. Recent advances in understanding DWPI as part of a complex microbial ecosystem inhabiting drinking water systems continues to reveal additional challenges: namely, how can all microorganisms of concern be managed simultaneously? In order to protect public health, we must take a more holistic approach in all aspects of the field, including basic research, monitoring methods, risk-based mitigation techniques, and policy. A holistic approach will (i) target multiple microorganisms simultaneously, (ii) involve experts across several disciplines, and (iii) communicate results across disciplines and more broadly, proactively addressing source water-to-customer system management.
Keywords: Pathogens, drinking water, building plumbing, Opportunistic premise plumbing pathogens (OPPs), Legionella, nontuberculous mycobacteria
Graphical Abstract
1. INTRODUCTION
Since the identification of Legionnaires’ Disease following the 1976 American Legion convention, the field of research on drinking water-associated pathogens that predominantly cause infections in immunocompromised individuals (DWPI) has exploded. New DWPI continue to be identified (e.g., a 2018 Balamuthia mandrillaria infection tentatively linked to tap water (Piper et al., 2018)) at the same time that our understanding of the diverse ecological system within pipes is expanding (Douterelo et al., 2019; Hull et al., 2019). Today, infections associated with DWPI are estimated to cost the US economy $2.39 billion annually (Collier et al., 2021), and the incidence of several DWPI continues to increase (Adjemian et al., 2012; Billinger et al., 2009; CDC, 2011; Collier et al., 2021; Marras et al., 2007; Prevots et al., 2010; Prussin et al., 2017).
Many microorganisms could be considered DWPI. While there is no perfect term for these organisms, there are several criteria for grouping them under this umbrella. Here we focus on bacteria and amoebae that are (1) adapted to grow in drinking water systems, particularly within building water systems and water fixtures (e.g., cold and hot water lines, heaters, faucets, fountains) and (2) frequently cause disease in susceptible populations (e.g., at risk individuals, older people, people with immune compromise and/or lung disease). We consider that the characteristics of susceptible populations vary and that infections sometimes occur in individuals that appear to be healthy. While present naturally in the environment (surface water, soil), DWPI typically are not present in high concentrations in finished water leaving the drinking water treatment plant, but multiply within the distribution network and especially in building water systems, often within biofilms. While all DWPI are capable of causing disease, the risk of becoming infected is influenced by various factors including the microorganism’s virulence, exposure dose, and the susceptibility of the exposed population. Here, we focus on DWPI that typically pose a lesser threat to healthy individuals compared to those with underlying health conditions or susceptibilities. Moreover, common routes of exposure to DWPI are typically through non-ingestion (e.g., aspiration or inhalation of aerosols generated from showering or other exposures) rather than ingestion (drinking) routes. Although fecal pathogens are occasionally found in drinking water systems, we exclude traditional fecal-oral pathogens, which cause gastrointestinal disease after ingestion, as these microorganisms tend not to proliferate or regrow in municipally-treated drinking water (Ashbolt, 2015). Some so-called “true” or “frank” pathogens can also grow within drinking water systems and cause disease through non-ingestion routes of exposure (e.g., Naegleria fowleri), although this is less common. The entire list of organisms we consider to be DWPI can be found in Table 1. Much of our discussion focuses on three exemplary bacterial groups that represent a significant disease burden (Collier et al., 2021), have distinct physiologies, and have the most research available: Legionella pneumophila, Mycobacterium avium and other nontuberculous mycobacteria (NTM), and Pseudomonas aeruginosa.
Table 1.
Drinking water-associated pathogens (DWPIs) that can cause infections in immunocompromised individuals and associated diseases. DWPIs are defined as bacteria and amoebae that are (1) suited to grow in drinking water systems, particularly within building water systems and water fixtures, and (2) frequently cause disease in susceptible populations.
| Organism | Disease | Infection incidence | Direct Healthcare cost4 | Deaths/year4 | Reportable |
|---|---|---|---|---|---|
| Legionella pneumophila ߆ | Legionellosis: Legionnaires’ Disease and Pontiac Fever | 8,000–50,000/year1 | 402 million | 995 | CDC (national)5 |
| Nontuberculous Mycobacterium (NTM) ߆§; Mycobacterium avium complex (MAC) ߆ & abscessus complex (MAB) ߆ | NTM lung disease, nodular bronchiectasis; skin and soft tissue infection | 86,244/year2 | 1.53 billion | 3,800 | NTM-certain states w/ disease and/or positive isolate * |
| Pseudomonas aeruginosa ß®§ | Otis externa ® | 51,000/year (healthcare associated only)3 | 564 million | 219 | Certain states with antibiotic resistance ** |
| Pseudomonas pneumonia † | 453 million | 730 | |||
| Pseudomonas septicemia § | 214 million | 695 |
Other DWPI: Other Legionella species (e.g., L. longbeachae, micdadei, bozemanii, L. dumoffi) – ߆; Burkholderia cepacian complex – ß; Achromobacter – ß; Stenotrophomonas maltophila – ß, Acinetobacter baumannii – ß, Sphingomonas paucimobili – ß, Aeromonas hydrophila – ß, Hartmanella (Vermamoeba) – Æ, Acanthamoeba – Æ Ø, Naeglaria fowleri – Æ Ø€, – Balamuthia mandrillaris Æ Ø
References 1(Prussin et al., 2017) 2 (Strollo et al., 2015) 3(CDC, 2019d) 4(Collier et al., 2021) 5(CDC, 2019b)
Reportable states *Oregon, Nevada, New Mexico, Nebraska, Missouri, Mississippi, Wisconsin, Ohio, Virginia, New Jersey, and Maryland **Minnesota, Maryland, Wisconsin, Utah, New York, Tennessee, Washington, and Michigan (CDC, 2021d, 2021e; Minnesota Department of Health, 2019b); Florida as part of electronic laboratory reporting surveillance (Florida Health, 2016)
Symbol Key
Organism type: ß = Bacterium; Æ =Amoeba
Disease type/exposure: † = Pulmonary through inhalation or aspiration of aerosols; § = skin or soft tissue infection through dermal exposure ® = Ear infection; Ø = Granulomatous Amebic Encephalitis (GAE) through intranasal exposure like irrigation; € = Eye infection
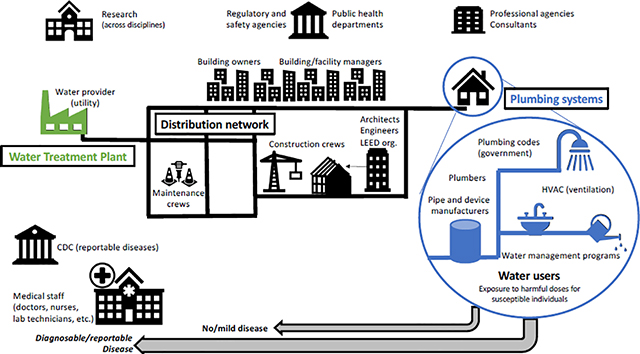
To combat DWPI disease, emerging guidance documents attempt to synthesize research knowledge into risk management strategies(Julien et al., 2020a; Singh et al., 2020). In 2015, the first industry standard for drinking water system management in buildings was released (ASHRAE, 2015; Rhoads et al., 2014) and many resources are now available to help develop what are generally known as water safety plans (ASHRAE, 2020a, 2018; CDC, 2021a). Mitigation efforts are often targeted at healthcare facilities, as many DWPI infections are nosocomial (healthcare- or hospital-acquired) due, in part to the high density of vulnerable individuals. The Veterans Affairs Office first issued a directive concerning Legionella spp. prevention in healthcare facilities in 2014 (VHA, 2014); updated in 2021 (VHA, 2021), and all hospitals receiving Medicare/Medicaid funding are now required to have building water management plans ((CMS, 2017), updated in 2018 (CMS, 2018)). Unfortunately, the content of these plans is not specifically mandated (Ambrose et al., 2021).
Regulations are a step in the right direction, but have shortcomings (Bope et al., 2018) and vary globally, as illustrated by L. pneumophila standards and guidelines (NASEM, 2019; Van Kenhove et al., 2019b). For example, although the ASHRAE standard was a substantial forward in taking some action, it does not address residential (home) systems, despite the epidemiological link between building drinking water systems and infection by DWPI (Lande et al., 2019; Pedro-Botet et al., 2002; Stout et al., 1992; Yu et al., 2004). In fact, 96% of Legionnaires’ disease cases in the U.S. are of unknown etiology, and it is suspected that sporadic residential exposure is a substantial source (CDC, 2011). It is difficult to measure the success of interventions, especially given the consistent increase in incidence of Legionnaires’ disease (i.e., 5.5 times increase in incidence of reported cases in the U.S. from 2000 to 2017 (CDC, 2018)), with reporting potentially due to factors such as increased awareness and testing in addition to an actual increase in disease incidence or prevalence. Moreover, environmental testing data (i.e., water samples) are not mandated to be centrally reported in the U.S. (CDC, 2019a), even for Legionella spp.. While the maximum contaminant level goal (MCLG) for Legionella spp. has long been zero (US EPA, 2015), regular direct monitoring in drinking water systems is not required in the U.S. In contrast, other countries (e.g., Germany) have required environmental monitoring for both L. pneumophila and P. aeruginosa (NASEM, 2019). Perhaps because Legionella spp. is associated with the most reported data and strictest regulations, available guidance has focused on Legionella spp., but guidance may be at the expense of other DWPI in certain situations. For example, NTM is thought to be at least as important to the US disease burden as Legionella spp.(Collier et al., 2021; Daley and Salfinger, 2016). A complicating factor but also a potential motivation for holistic approaches is that clinically meaningful antibiotic resistance (AR) often complicates treatment for infections by DWPI such as Legionella spp. (De Giglio et al., 2015; Shadoud et al., 2015), NTM (van Ingen et al., 2012), and P. aeruginosa (Smith et al., 2016).
1.1. Justification and Scope
In considering the fifty-plus years of research progress and mitigation efforts for DWPIs, it has become clear that a more holistic approach is needed to unite and strengthen efforts taking place across numerous fields and disciplines. “Holistic”, in this context, can be defined as considering multiple parts as interconnected and part of a whole, rather than as disconnected components. The current approach can be segmented both by discipline, and by specific microorganism of interest. Moreover, decision processes must balance many interests, including other health outcomes, cost, and energy. Additional local considerations and boundaries of water jurisdictions further segment approaches to manage DWPI.
First, while experts trained in various disciplines are making advances to understanding DWPI behavior, there is a lack of means to efficiently and effectively share findings across disciplines, in part due to terminology unique to each field (Table 2). Often, literature reviews focus on either clinical disease (Johnson and Odell, 2014), microorganism fate and transport (Falkinham, 2018), or persistence in drinking water systems (Dowdell et al., 2019). DWPI research from these disciplines often occurs in parallel fields without sufficient connection and tends to neglect translational work that bridges research and practice. For example, due to limited resources, epidemiological outbreak investigations may not aim to link clinical and environmental samples (e.g., the majority or patients diagnosed with Legionellosis in 2016–2017 in the US did not have an exposure source (healthcare setting, travel history, or senior living facility) identified (CDC, 2020a) – noting that person-to-person spread is considered negligible (Correia et al., 2016).) Both research and field investigations will benefit from increased communication.
Table 2.
Terms often used in the field with potential confusion between fields
| Term | Definition and usage in different contexts | |
|---|---|---|
| Types of water in plumbing | Potable | Water used for drinking and/or food preparation, most often cold water. In some fields, potable is inclusive of hot water |
| Non-potable water | Water unsuitable for drinking purposes but used for other activities, including irrigation, industrial uses, or other non-drinking purposes. (WateReuse, 2016) | |
| Drinking water | A term sometimes used to distinguish water identified strictly for drinking purposes (e.g., providing water to water fountains/drinking fountains/bubblers) and/or food preparation. In the US, piped drinking water may be used for non-potable purposes, as it is also routed to toilets and water heaters in most cases. This is sometimes a different system than the cold water piped to other water outlets in commercial buildings. | |
| Components of plumbing | (Hot) Water heater | A closed vessel in which water is heated by the combustion of fuels, electricity, or any other source and is withdrawn for use external to the system at pressures not exceeding 160 psig [1100 kPa (gage)], including the apparatus by which heat is generated, and all controls and devices necessary to prevent water temperatures from exceeding 210°F (99°C). A water heating (and often storage) device that provides piped hot water for consumptive uses like showering, hand-washing, dishes, and laundry. Some models are tankless. Traditional models are powered by gas or electricity. (ASHRAE, 2020b) |
| Boiler | A closed, pressure vessel that uses fuel or electricity for heating water or other fluids to supply steam or hot water for heating, humidification, or other applications. This is a more versatile water heating device that may provide water for consumptive uses, or for heating the building. (ASHRAE, 2020b) | |
| Water line/pipe | Colloquially used to refer to any water or wastewater pipe. Hot water line and hot water pipe system are interchangeable. | |
| Bacterial life cycles | Inactivation | The interruption of growth metabolism and loss of a microorganism’s ability to reproduce under suitable conditions; VBNC microorganisms represent one gray area for measurement of inactivation and microorganisms may recover from this state. The indicators we use to measure inactivation of organisms are often limited with issues related to quantification of living microbes, ability of microbes to resuscitate. Due to imperfect quantification on both ends of this spectrum (culture-based methods will underquantify viable organisms and molecular-based methods will quantify both dead and live organisms), all methods have variable detection limits that may also miss a fraction of viable organisms. |
| Persistence | The precise definition of this term varies depending on the subfield. Environmental persistence refers to how well a microorganism or chemical substance is transported (a catch-all term that includes processes of removal, stress, biodegradation, and growth and death processes for biologics) in response to environmental conditions (Boethling et al. 2009). It is typically evaluated using a plot of occurrence (e.g. ln(C/C0) for first-order inactivation or growth) over time. Medically, persistent infections refer to infections that remain relevant in the host for long periods of time despite treatment (Fisher et al., 2017). | |
| Antimicrobial resistance | The microorganism possesses physiological traits, intrinsic or horizontally acquired, that allow them to survive exposure to antibiotics, antimicrobials, and disinfecting agents. Resistance is operationally defined as an increase in the minimum inhibitory concentration (MIC) in response to exposure to an antibiotic. | |
| Decay | In biology, refers to the process of decomposition by which dead organic substances are broken down into simpler organic or inorganic matter. In chemistry, derived from radioactive decay, i.e., a reference to a chemical “half life” or time for the substance to reduce by a factor of 50% assuming a first-order practice. Informally, the term is often applied to refer to a decrease in a chemical species or bacterial population, however this term should not be used to refer to microorganism inactivation or death (Balaban et al., 2019). | |
| Disinfection | The process of inactivating pathogenic microorganisms. Primary disinfection is a common component of primary treatment of drinking water designed to inactivate microbial pathogens; secondary disinfection is used to maintain water quality achieved at the treatment plant throughout the distribution system up to the tap (WHO, 2017). | |
| Kill/dead | A state of an organism where resuscitation of metabolic activity is no longer possible; the end stage of the bacterial life cycle that occurs after all physiological processes have run their course (Colwell, 2000; Rice and Bayles, 2008) | |
| VBNC (viable but nonculturable) | Bacteria that are in a state of very low metabolic activity and do not divide due to environmental stress, and cannot be grown on standard culture media. However, the bacteria are alive and have the ability to become culturable once resuscitated (Li et al., 2014). | |
| Organism type | Indicator | Traditionally, indicator microorganisms are not necessarily pathogenic but are members of the intestinal flora of warm-blooded animals used to suggest the presence of pathogens or fecal contamination (see Bonde’s criteria (Bonde, 1966)) (National Research Council, 2004). However, an indicator can be any proxy or sentinel for a particular outcome or process (Berg, 1978). |
| Pathogen | Any microorganism capable of causing damage in a host. It is noted that there are concerns raised with this term due to the existence of pathogenic and non-pathogenic states (Pirofski and Casadevall, 2012). | |
| Opportunistic pathogen (OP) | A microorganism that can become pathogenic following a perturbation to their host (e.g. disease, wound, medication, prior infection, immunodeficiency, and ageing) (Brown et al., 2012), or in some cases that potentially can infect healthy individuals at higher doses. In drinking water contexts, they are often contrasted with fecal pathogens, by being capable of growth in the distribution system and by non-ingestion routes of exposure. See notes on “pathogen” above. | |
| Drinking water-associated pathogens that can cause infections in immunocompro mised individuals (DWPI) | Term proposed by this paper to be used to refer to a specific group of bacteria and amoebae that are (1) suited to grow in drinking water systems, particularly within building water system and (2) frequently cause disease in susceptible populations (e.g., people with immune compromise and/or lung disease), though the characteristics of susceptible populations can vary, and infections can still occur in healthy individuals. While present naturally in the environment (surface water, soil), DWPI typically (i) are not present in high quantities in finished water leaving the drinking water treatment plant, and (ii) multiply within building water system and/or the distribution network, often within biofilms. These organisms may cause exposure outside of “drinking water”, but the term is used to distinguish problems oligotrophic “drinking” water, from specific issues encountered in other water supply systems like heating/cooling systems and gray water systems. | |
| Pipe system | Plumbing, Building Plumbing, Premise Plumbing, Premises Plumbing Building water system | Plumbing is the pipes, tanks, fittings, and other devices that convey water within a building. Plumbing refers to both the supply side and waste side (drinking and wastewater). Premise plumbing is a term adopted to include plumbing and the service line leading to the building. A more grammatically correct term would be “premises” plumbing. Plumbing on the supply side may refer to both drinking water systems, that carry potable water to places where it can be consumed, as well as pipe systems that convey water for other purposes (e.g., heating and cooling systems, fire protection systems). |
| Service line | The pipe that conveys water from the source of water for the building (i.e., municipal water system or well) into the building. The service line often has split ownership at the water meter between water provider and building owner. | |
| Distribution system, distribution network | The pipes used to carry water between a water or wastewater source and the user or end-stage purpose (e.g. resulting in intentional reuse or disposal to a water body and de-facto exposure). Often this refers to the buried pipes that convey water from a water treatment plant throughout the service area to individual buildings. | |
| Engineered water system | General term for any infrastructure created by humans used to store and/or convey water. For example, the term is used to refer to both the distribution system and plumbing. | |
| Water Treatment | Treatment | Any biological, chemical, and/or physical process applied to water or wastewater that is designed to remove or otherwise reduce the risk from unwanted substances such as contaminants to improve quality for its desired purpose. |
| Finished water | Water that has passed through a water treatment plant, such that all the treatment processes are completed, or finished. This water is ready to be delivered to consumers. | |
| Polishing | A treatment stage placed at the end of other treatment to bring the water to a more highly conditioned and more perfect state, e.g., a mixed bed of ion-exchange media installed as the final treatment step in the deionization process to remove last traces of undesirable ions. (McTigue and Symons, 2010) | |
| Risk | Hazard | A substance or situation to which exposure would result in the ability to cause harm to humans or other organisms. |
| Risk | The probability of an adverse outcome from exposure to a hazard; a function of both hazard and exposure. This definition can be broader across domains and applications and typically includes some acknowledgement of uncertainty and associated consequences of a particular scenario. The risk for pathogens can be quantified using quantitative microbial risk assessment (QMRA). | |
| Disease terms | Median infectious dose (ID50) or median affected dose (N50) | Descriptive parameters of a dose response relationship that describes the dose (number of microorganisms which a test subject is exposed to via a given route) at which a particular response or health endpoint (infection, illness, or death) is observed in 50% of the test subjects (animals, humans, cells, etc.). The term “infectious dose” or “minimal infective dose” is an imprecise descriptor colloquially used by the medical community to refer to the dose of microorganism required to establish an infection in the host and should be avoided when discussing risk. |
| Dose response relationship | A mathematical function which describes the quantitative relationship between the dose of a microorganism (number of microorganisms which a test subject is exposed to via a given route) associated with the probability of a particular response or health endpoint (infection, illness, or death) in the test subjects. | |
| Health endpoint, outcome, or other sequelae | An outcome of a particular exposure event; this might be infection, illness, or death for microbes; for chemical exposures these can be carcinogenic or non-carcinogenic outcomes. The endpoint is typically specified in a dose-response experiment (e.g., shedding in feces, positive colonization sites, lesions in the lung, cancer, asthma, etc.) and can be continuous (on a numeric scale), quantal (number of positive or negative responses), or categorical (mild, moderate, etc.), but quantal data is typically used for microbial risk assessment dose response modeling. Valuation of a risk outcome is typically related to what endpoint is specified and therefore “meaningful” endpoints are typically chosen based on data availability and relevance to the risk scenario and/or disease process of interest. Definition of endpoint depends on the application (e.g., human health vs. ecological). Sequela represent longer-term impacts or outcomes following a microbial colonization and/or infection and resulting processes. | |
| Colonization | Colonization can occur in the context of the environmental system (e.g., colonizing a plumbing system biofilm in an environmental science and engineering context) or within the human body (at a target organ such as the lungs in a health risk context). Colonization indicates that a microorganism is able to establish itself and grow at a particular location or portal of entry (e.g., a target organ or pipe). In the medical context, colonization does not necessitate interaction between the host and organism, therefore it may be asymptomatic and does not necessarily indicate clinical expression or immune response (Dani, 2014). | |
| Infection | Survival and initiation of infectious processes and/or immune response in a host resulting from interplay between host and pathogen, whether or not it develops into a disease (Dani, 2014). | |
| (Infectious) disease | The development of an illness (presenting signs and symptoms) caused by a specific infectious agent or its toxic product that results from transmission of that agent or its product from its source to a susceptible host. | |
| Contaminated | The presence of a microorganism or substance that is hazardous to human health if exposure were to occur, or hazardous to other organisms depending on the context. This term is more often used in the engineering field to describe engineered or environmental systems (e.g., rivers, water). |
Second, and perhaps more importantly, disciplinary teams often focus on one microorganism due to logistical limitations and available methods, as reflected in literature reviews that focus on one microorganism (Bédard et al., 2016; Falkinham, 2018, 2009; NASEM, 2019). Coinfections or polymicrobial infections with DWPIs are possible (Naito et al., 2020; Dev and Ashbolt, 2020) but data regarding co-infection within the same species of DWPI or with multiple DWPIs, as well as regarding the ability for a DWPI infection to serve as a pre-disposing factor for other infectious agents is a data gap. Some reviews of microbial ecology (Berry et al., 2006; Proctor and Hammes, 2015a), or broad scoping reviews (Falkinham et al., 2015) can take multiple pathogens into consideration, but this does not necessarily translate to specific interventions designed for practitioners. With most guidance directed at individual DWPI, we have noted examples of incompatible recommendations. For instance, adding free chlorine at the building level can be effective in reducing concentrations of L. pneumophila, but might increase NTM occurrence in building water system (Moore et al., 2006; Rhoads et al., 2017b). Similarly, the use of monochloramines may offer enhanced diffusion into biofilms for pathogen inactivation, but as a weaker oxidant will prove less effective against NTM (Revetta et al., 2013). The authors have noted a lack of studies that simultaneously tackle the full suite of DWPI. Even clinical or plumbing management recommendations targeted at a single microorganism type may fail to take into account host micro-eukaryotes (Thomas et al., 2010) that may be critical for pathogen recovery (Dey et al., 2019a) and strain variability within specific groups, such as that exemplified by the wide variation in virulence and health outcomes as a result of infection with different species within the Mycobacterium genus (Tortoli, 2014). Future recommendations should consider the impact on the full suite of DWPI.
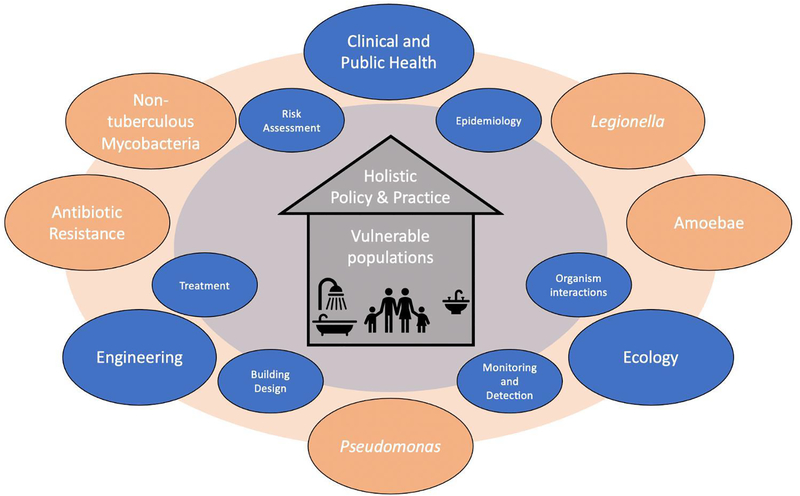
In this paper, we outline similarities and differences among DWPI that can lead to conflicting guidance and present research needs that could help with the development of a necessary consensus across disciplines. We consider these similarities and differences across three main categories: clinical and public health, microbial ecology, and engineering controls. We highlight several opportunities to encourage research to be more cross-disciplinary and to increase the translation of research findings into practice. We present this commentary to advocate for a more holistic approach to DWPI research, legislative action, building codes, monitoring efforts, and professional practice to reduce the burden of disease associated with DWPI. The authors represent a diverse background of primary disciplines and organisms of interest (Table S1) to emphasize the importance of cross-disciplinary cooperation.
2. CLINICAL MEDICINE & PUBLIC HEALTH
While clinical medicine focuses on the treatment and outcomes of individual patients, the field of public health addresses the prevention of disease and promotion of health at the population level. We have chosen to discuss them together here as both focus on human health. The overlap in research and practice. DWPI have long been the focus of both clinical and public health research; however, in some cases, disparate goals have resulted in a lack of alignment among disease surveillance and reporting, clinical diagnosis, and assessment of DWPI risks. Multiple DWPI hazards exist, and the ability to detect DWPI-associated diseases and to predict their impacts varies. Holistically assessing these factors and impacts is nontrivial, especially as exposures may relate to episodic and complex phenomena at play in plumbing systems.
2.1. Reporting
In the U.S., Legionellosis is currently the only nationally notifiable group of diseases caused by DWPIs to the Centers for Disease Control and Prevention (CDC) (CDC, 2019b). NTM infections and positive isolates are required to be reported in some states (Table 1). The wide variation among NTM reporting requirements has resulted in calls for a national reporting requirement for NTM (Daley and Salfinger, 2016; Donohue, 2018; Winthrop et al., 2017). Certain infections with DWPI may meet criteria for reporting in the CDC National Healthcare Safety Network for healthcare-associated infections, such as Pseudomonas-related blood stream infections. Drug-resistant P. aeruginosa infections are tracked by CDC through its Antibiotic Resistance (AR) Laboratory Network, however the AR lab network is “not a surveillance network and does not represent testing of every isolate in every state” (CDC, 2019c). Certain states such as Minnesota and Wisconsin require reporting of free-living amoebic infections, including Acanthamoeba spp., N. fowleri, Balamuthia spp., and Sappinia spp. (Minnesota Department of Health, 2019a; Wisconsin Department of Health Services, 2018), and primary amoebic encephalitis caused by N. fowleri is state-reportable in Florida, Texas, and Louisiana (CDC, 2017). This patchwork of surveillance and notification requirements, often motivated by high-profile outbreaks, highlights the need for unified DWPI associated disease reporting to fully assess disease burdens. Even so, mild infections often go undiagnosed or untreated, while healthcare-associated infections are also likely underestimated (Perkins et al., 2019; Spagnolo et al., 2016). Overall, reported cases are the “tip of the iceberg” (WHO-Europe, 2019; Yang et al., 2012).
2.2. Diagnosis and epidemiologic case definitions
An epidemiological case definition is “a set of standard criteria for classifying whether a person has a particular disease, syndrome, or other health conditions” (CDC, 2012a). Some of these definitions have been developed into national standards that are comparable across clinical settings and locations. A standardized case definition is an important step toward making large-scale comparisons of DWPI. Case definition criteria can be more limited during an outbreak investigation, and the strictness of the definition depends on the purposes for classifying the occurrence of a disease (CDC, 2012a). A case definition is not the same as a clinical diagnostic criterion, where the former is used to aid an epidemiologic investigation and the latter is used to make treatment decisions for individual patients (LaMorte, 2016). Clinicians’ ability to diagnose DWPI diseases vary, but most approaches are based on culture or molecular analysis of the pathogen in bodily fluids, or observation of antibodies to a particular infection.
With regard to standardized case definitions, Legionnaires’ disease has a clear case definition (CDC, 2021b), but a standard case definition for Pontiac fever, which is generally considered to be a milder form of legionellosis without pneumonia, has only recently been established (CSTE, 2019, 2010, 2005; Tossa et al., 2006). Similarly, case definitions have only recently been standardized for free-living amoebae (CDC, 2012b) and NTM (CSTE, 2017; Oregon Health Authority, 2018) infections, while P. aeruginosa infections are diagnosed via bacterial culture as a primary or secondary bloodstream infection; secondary bloodstream infections are not reportable as Laboratory Confirmed Bloodstream Infections to the CDC National Healthcare Safety Network (CDC, 2020b). Other case definition criteria are used for antibiotic-resistant P. aeruginosa infections (Minnesota Department of Health, 2019b; Walters et al., 2019). For M. avium and other NTM, the American Thoracic Society/Infectious Disease Society of America and the British Thoracic Society define a confirmed case as one with NTM grown from two separate sputum samples, or a single positive sample from a broncoalveolar lavage or a lung biopsy (Griffith et al., 2007; Haworth et al., 2017). In addition, the Council of State and Territorial Epidemiologists (CSTE) has recently published a case definition for extrapulmonary NTM (CSTE, 2017; Oregon Health Authority, 2018). Granulomatous amoebic encephalitis (GAE, i.e. amoebic infection of the central nervous system) is often diagnosable, but rarely before it becomes fatal (Reed et al., 1997; Sell et al., 1997). Factors including (i) extended disease progression, especially for NTM which may have a progression over years (Field et al., 2004; Lewis et al., 1960), (ii) lack of identification needed to reach a diagnosis or treatment (e.g. otitis externa or “swimmer’s ear” can be treated without identifying the causative organism as P. aeruginosa), and (iii) lack of clinical awareness (Alaga et al., 2018) can obscure connections between environmental exposure and disease (Johnson and Odell, 2014; Provoost et al., 2018). The use of medical decision trees, tools with visual descriptions of a series of diagnostic tools and subsequent outcomes and downstream decisions to aid in arriving at a diagnosis, have been long-employed in the medical literature (Detsky et al. 1997). Such tools would be beneficial in the DWPI context for codifying information that could distinguish between different DWPI disease outcomes, as well as to highlight gaps where additional information could be collected to clinicians in differentiating between DWPI infections and elucidating causal pathways(Detsky et al., 1997).
Testing is critical for diagnosis, but convenience, reproducibility, and accuracy (sensitivity, or the probability of correctly identifying a positive case of disease, and specificity, the probability of correctly testing negative in the absence of disease) of available testing technologies varies by DWPI, and the compatibility of environmental and clinical tests varies due to conflicting goals, as discussed below. Taken together, these challenges for diagnosis amplify the underreporting of DWPI disease. Accurate reporting will require (1) rapid, reproducible, and precise testing methodologies for DWPI diseases, (2) diagnostic protocols that include clear decision trees for routine inclusion of DWPI in clinical workups, and (3) national reporting requirements and databases for DWPI.
Epidemiological investigations offer a clear opportunity for cross-disciplinary work. Clinical medicine can identify disease (and report it), while public health specialists will look for environmental sources of exposure to link to the outcome. This requires timely cooperation and communication so that environments can be sampled close to the exposure window. Linkage typically requires work at the subspecies level and should look for co-infections with other DWPI. Engineers can also contribute to epidemiological studies by identifying key exposure points that can be added to epidemiological surveys of exposure history (e.g., do you live near a water feature?). Successful quantification of both exposure and outcomes can help validate risk models.
2.3. An Example of Holistic Thinking: Risk Assessment and Management
In assessing risks from DWPI, a traditional approach may focus on risks of a single pathogen, epidemiological observations and studies, or even costs associated with treating a single disease. However, a combination of metrics would be needed to holistically evaluate and prioritize the impacts, costs, and tradeoffs of DWPI in different contexts, and substantial value judgements would be necessary when communicating these types of analyses for legislative and preventative efforts. Further, complexity is added when competing objectives of many stakeholders (e.g., energy costs, liabilities, and management objectives unrelated to DWPI) must be considered. In this section, we describe how the current approach to risk assessment includes aspects of a holistic approach and can be further improved to incorporate metrics from other disciplines.
The prevalence of infections by DWPI varies (Table 1), although some rank-order differences have been estimated: NTM (up to 41.3 per 100,000) > Legionnaires’ disease (up to 1.36 per 100,000) > P. aeruginosa > Acanthamoeba > N. fowleri (Falkinham et al., 2015; Strollo et al., 2015). Furthermore, disease prognoses vary greatly; for example, N. fowleri associated meningoencephalitis has a >95% case fatality rate (Wang et al., 2018), while the case fatality rate for healthcare-associated Legionnaires’ disease is up to 25% in some susceptible populations (CDC, 2021c). Quantitative microbial risk assessment (QMRA) may be useful for prioritization, as it aims to understand underlying mechanisms, relative contributions of processes and modeling rare outcomes, among other attributes bridging environmental concentrations to outcomes of interest via dose-response models (Haas et al., 2014; World Health Organization, 2016). QMRA is especially useful as a companion tool to traditional epidemiological methods which can necessitate large studies in order to observe potentially small effect size. Dose-response models used in QMRA are available for quantifying relationships between exposure and the probability of an outcome for DWPI (Table S2). A growing body of QMRA literature addresses Legionella spp. (Hamilton and Haas, 2016), NTM (Hamilton et al., 2017; Rice et al., 2005), P. aeruginosa (Dean and Mitchell, 2020a; Rasheduzzaman et al., 2019), and N. fowleri (Dean et al., 2019; Rasheduzzaman et al., 2019), but important limitations remain as many variables must be considered. For example, the likelihood of infection for a DWPI is not only dependent on the species/strain of microorganism, the virulence of the microorganism at a given time, and host characteristics (e.g., immune status/age/demographics), but also on the fate and transport of the microorganism in the environment, and exposure route and dose. For example, men over 55 years of age are at higher risk for L. pneumophila while NTM tends to disproportionately infect slender, taller, post-menopausal women (Chalmers et al., 2018; Kartalija et al., 2013). New dose-response models will be useful for microbial ecologists, water quality engineers, and ultimately legislators seeking to identify risk-based monitoring targets for environmental control of these organisms. Such efforts to derive risk-based concentration monitoring targets have already begun for Acanthamoeba spp. (Dean and Mitchell, 2020b), P. aeruginosa and N. fowleri (Rasheduzzaman et al., 2019) and L. pneumophila (Hamilton et al., 2019).
Risk prioritization could also be assigned based on cost, but tracking disease and quantifying costs of illness are challenging (Adam et al., 2017). Multiple approaches are available in the economic, risk, decision analysis, and policy analysis literature to inform decisions that take into account multiple pathogen considerations (e.g. (Mitchell-Blackwood et al., 2011)). These approaches not only consider the direct losses (e.g., hospitalization costs, Table 1, (Collier et al., 2021)), but also secondary considerations such as non-hospitalized infections (i.e., missed work, lowered productivity) and mortality when allocating scarce resources (Institute of Medicine, 2009; National Research Council, 1990). For example, Corso et al. (2003) identified healthcare costs and productivity losses from the 1993 cryptosporidiosis outbreak in Milwaukee, Wisconsin using costs from categories of people with mild, moderate, and severe illness using a retrospective analysis, arriving at an estimate of $96.2 million in losses (2003 US dollars) (Corso et al., 2003). In addition to direct costs, disease has many indirect costs, including loss of trust in the safety of municipal drinking water. In considering costs, the expense of trade-offs, including alternative risks and costs of preventative measures should be considered. For example, raising the temperature of a water heater can decrease risk of DWPI infection, but will increase the risk of scalding and energy costs. Such analyses would improve if all DWPI were reportable, noting that cryptosporidiosis did not become a nationally notifiable disease until 1995 after the catastrophic Milwaukee outbreak (CDC, 1995).
The level of “acceptable” risk is typically dependent upon the hazard, consequences, and ability to control exposure (NASEM, 2019). Drinking water efforts for policy standardization have typically relied on health-risk based target benchmarks such as one in 10,000 infections per person per year (Lim and Jiang, 2013; Regli et al., 1991). Per-exposure versus annual risks are often chosen as a decision basis depending on whether exposures are continuous or short term, leading to different monitoring approaches, with annual-based approaches focusing less on short-term (hazardous event) variations (NASEM, 2019; Signor and Ashbolt, 2009). The choice of an acceptable risk value, like one in 10,000, is a policy decision and may be too stringent in certain situations (Haas, 1996; Lim and Jiang, 2013). Other endpoint metrics are available such as the disability adjusted life year (DALY) to measure the health impacts of a disease incorporating both fatal and non-fatal outcomes (a 10−6 DALY per person per year is often used as a reference point) (Sinclair et al., 2015; WHO, 2015). A DALY value is available for Legionellosis (van Lier et al., 2016), however, this value is location- and context-specific. Regardless of the quantification method used, there is no such thing as “zero risk” (De Keuckelaere et al., 2015).
Research needs for DWPI risk assessment and prioritization can be summarized as follows: (1) an increased understanding of infectivity, including new dose response models for emerging DWPI for various exposure routes (e.g. aspiration), endpoints, susceptibilities, (2) the impact of underlying human characteristics and behavioral factors (e.g. smoking, use of tap water for non-recommended uses such as neti-pots, contact lenses, and medical uses, occupational (co)exposures of host populations and impact on risk), (3) knowledge of virulent types amongst each of the DWPI species to aid monitoring, (4) improved exposure information for aerosol exposures as well as non-potable uses such as cooling towers, healthcare equipment, dental equipment, and other known sources of infection, (5) consideration of multiple costs and risk tradeoffs (e.g. increasing water heater temperatures to inactivate Legionella spp. results in higher energy costs), and (6) careful consideration of risk benchmark metrics in various decision- making processes. The insurance industry (Section 4.5), may provide an opportunity to address risks and consider costs across multiple sectors.
2.4. Proposed Approach
Clinicians, public health practitioners, and other health researchers who wish to participate in a holistic approach to DWPI can embrace several strategies. Recognizing that these proposed approaches are not always easy to implement, they are ordered from most to least readily achievable.
Educate at-risk individuals about their personal risk factors, and provide them with the resources to protect their water systems and modify behavior to minimize personal risk.
Timely studies across public health and medicine that link exposures and disease outcomes/outbreaks, improving linkages among professionals in the medical and environmental space for this purpose. Following a case investigation, communicate with water providers and building managers about potential risks and strategies for future disease prevention, regardless of whether positive environmental samples were collected, in order to increase awareness of potential problems.
Report every instance of DWPI to a centralized database (Box 1).
Make reporting of all DWPI mandatory, rather than just some pathogens, and to the extent possible, match clinical cases with environmental samples within linked reporting databases.
Using risk assessment to systematically identify and prioritize research gaps, understand tradeoffs, and improve modeling efforts toward predicting and preventing rather than reacting to DWPI illness
Rapid, more granular public health data collection for DWPI-associated illnesses and expansion beyond Legionella spp., which also considers people presenting with symptoms associated with DWPI for further testing and diagnosis
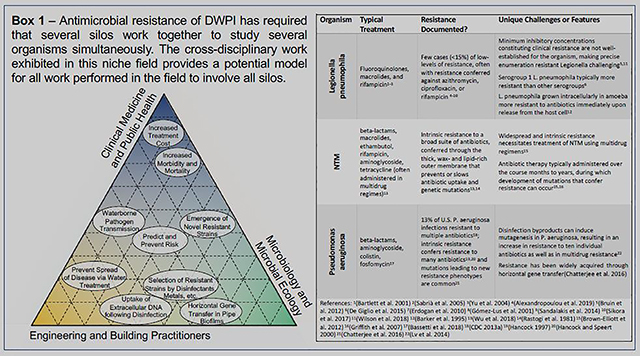
Box 1.
Various groups are responsible for water safety regarding drinking water-associated pathogens that can cause infections in immunocompromised individuals. Acronyms used: CDC = centers for disease control; HVAC = heating, ventilation and air conditioning Citations in Box 1 [for the purpose of citation managers only..]: 1(Bartlett et al., 2000) 2 (Sabrià et al., 2005) 3 (Yu et al., 2004) 4 (Alexandropoulou et al., 2019) 5 (Bruin et al., 2012) 6 (De Giglio et al., 2015) 7 (Erdogan et al., 2010) 8 (Gómez-Lus et al., 2001) 9(Sandalakis et al., 2014) 10 (Sikora et al., 2017) 11(Wilson et al., 2018) 12(Barker et al., 1995) 13(Wu et al., 2018) 14(Rastogi et al., 1981) 15(Brown-Elliott et al., 2012) 16(Griffith et al., 2007) 17(Bassetti et al., 2018) 18(CDC, 2013) 19(Hancock, 1997) 20(Hancock and Speert, 2000) 21(Chatterjee et al., 2016) 22(Lv et al., 2014)
3. MICROBIOLOGY AND MICROBIAL ECOLOGY
Each DWPI has a unique set of ideal environmental conditions for proliferation. These conditions generally overlap to allow for growth within the environment, drinking water systems, and the human body, but clear differences in niches have emerged. In studying microorganisms in isolated laboratory conditions, important discoveries are made, but studies can also be designed to emulate realistic conditions to inform complex cross-species interactions, as well as actionable engineering strategies and policies.
3.1. Key Microbial Physiological Considerations
Fundamental microbiology studies have revealed several innate physiological and structural traits that might predict where DWPI are likely to be found in the environment. Hydrophobicity and surface charge are important factors in determining the partitioning of cells/aggregates in a drinking water system (e.g., planktonic vs. surface microlayer vs. biofilm) and the likelihood of aerosolization (Falkinham, 2018; Parker et al., 1983). Many DWPI are hydrophobic and prefer to grow on surfaces in biofilms (Falkinham, 2009). NTM are especially notable given their waxy, “acid-fast” cell walls, which make them particularly hydrophobic and readily aerosolized (Falkinham, 2018; Parker et al., 1983). Surface charge may also dictate ecological distribution and infectivity (Steed and Falkinham, 2006; Stormer and Falkinham, 1989; van Oss et al., 1975). For example, differences in the electrophoretic mobility among L. pneumophila serogroups likely contributed to differences in serogroup behaviors (Buse et al., 2018). In contrast, several M. avium complex species exhibited less variability in electrophoretic mobility (Lytle et al., 2004). It is also important to recognize the way DWPI may be presented in exposure scenarios, such as packaged within amoebal vesicles, trophozoites, or cysts (Shaheen and Ashbolt, 2018). Further understanding of these differences is critical to identifying likely hotspots and explaining disease patterns.
DWPI growth rates vary considerably, impacting incubation periods between exposure and symptom onset, preferred growth location, and ideal culture detection methods. While doubling times and growth conditions can vary within a species, generally the growth rates for M. avium. and Pseudomonas spp. Are generally low (Sharaby et al., 2017; van der Kooij et al., 1982). These growth rates are lower in the drinking water environment than in clinical or laboratory settings due to oligotrophic conditions, this can mean that culture-based recovery of environmental DWPI isolates can require >10 days (Lalancette et al., 2017). In this field, growth is considered rapid if organisms are detectable in less than 7 days. Specific strains and species also exhibit wide variation. For example, Mycobacterium malmoense requires 8–12 weeks for culture detection, while M. abscessus and other “rapidly growing” NTM can be detected in <7 days) (Bartlett et al., 2000). Caution should be taken in extrapolating conclusions regarding growth rates as a slow growth rate does not necessarily imply slow metabolism; mycobacterial increase in numbers is slow because a great deal of energy is expended in synthesizing the long-chain (C60-C80) lipids that comprise the outer membrane (Brennan and Nikaido, 1995). It is critical to understand these basic pure culture behaviors in order to (1) understand microbial incubation periods and colonization patterns across ecosystems, (2) develop robust cost-effective methods (Box 2), and (3) design engineering solutions (e.g., intermittent flush, heat shock) for multiple DWPI simultaneously.
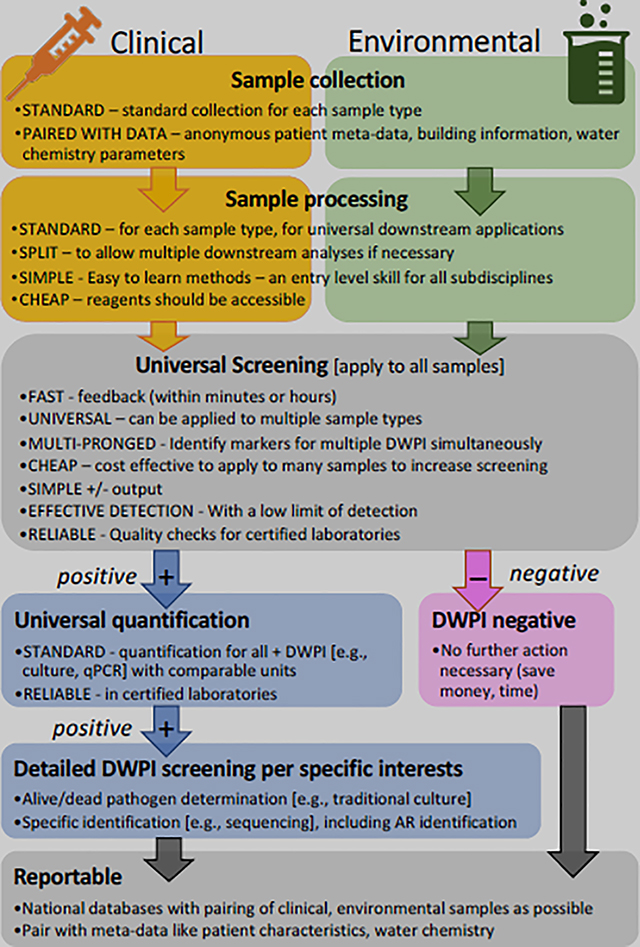
Box 2. Proposed ideal method workflow that incorporates a universal screening step and reporting of all results to centralized databases.
Many DWPI survive stress due to harsh conditions in potable water (e.g., low nutrients, temperature, and exposure to chlorine residuals) by resorting to a dormant or protective state. This includes the poorly understood viable but nonculturable (VBNC) state (Alleron et al., 2008; Dopp et al., 2017; Li et al., 2014) and survival within protozoa, which has been observed for many bacterial DWPI (Thomas et al., 2010). These survival mechanisms can result in false non-detects for culture-based methods and sometimes molecular methods (e.g., when amoebae are not lysed effectively). Importantly, these seemingly non-active microorganisms are still capable of infection (Dey et al., 2019b), with several studies showing the reactivation of DWPI after they are exposed to a less stressful environment (Dietersdorfer et al., 2018), such as within the human body. The importance of detecting viability varies across stakeholders (Section 3.5), but improved methods to quickly detect and differentiate between different physiological states would be widely beneficial across disciplines.
3.2. Environmental Conditions
DWPI respond to a variety of physio-chemical factors, including temperature, disinfectant residual type and concentration, oxygen levels, and other water chemistry characteristics. Many of these factors have been studied with direct implications for engineering solutions, indicating cooperation between these disciplines. Care must be taken in interpreting results from different scales of experiments, that can range from pure culture to studies of full-scale buildings. As studies often focus on only one DWPI or limited environmental factors, it is difficult to determine the applicability of findings (1) across DWPI, (2) in different systems, or (3) at different scales of study. Still, some differences in niche seem to emerge amongst DWPI.
Ideal temperatures for most DWPI are similar (28–35 °C), but growth can occur outside this range. For example, P. aeruginosa can grow between 10 and 42 °C (Brown, 1957). While most DWPI are considered inactive at temperatures greater than 55 to 60 °C, their recovery after exposure to higher temperatures (e.g., 63 °C for L. pneumophila (Borella et al., 2004) and 65 °C for NTM (Merkal and Crawford, 1979)) may indicate they are present in the VBNC state (i.e., VBNC L. pneumophila after exposure to 70 °C (Allegra et al., 2008)) or exhibit other survival mechanisms under such conditions. Conclusions about responses to temperature extremes are especially difficult to scale-up to engineered water systems because in situ temperatures are difficult to measure at various scales (e.g. within a biofilm or at a pipe wall versus at the center of the water column), and survival may further be aided by biofilms, protozoa, and areas not exposed to maximum or minimum system temperatures. As temperature may allow for niche differentiation, this presents an opportunity for further multi-DWPI studies
The chemical characteristics of drinking water vary based on source (e.g., groundwater vs. surface water), residual disinfectant type (i.e., chloramine vs. free chlorine), and specific building parameters (i.e., pipe material), all of which individually and collectively influence the growth and survival of DWPI and their hosts. As all of these water chemistry factors can differ across studies, it is difficult to (1) isolate factors of concern, and (2) compare results for different DWPI. Comparison of disinfectant efficacy is especially difficult. Even for a single organism, Ct (disinfection concentration (C) × time (t) [mg*min/L]) inactivation values can vary based on strain, material type, presence of biofilm, pH, and/or temperature (Buse et al., 2019; Dowdell et al., 2019). Still, each DWPI also appears to demonstrate slightly different tolerances towards chloramine and free chlorine; chloramine has a strong effect on L. pneumophila and P. aeruginosa, but is less effective towards inactivating NTM (Dowdell et al., 2019; Lytle et al., 2021; Moore et al., 2006; Rhoads et al., 2017b). M. avium complex and P. aeruginosa typically exhibit high resistance to both disinfectants (Grobe et al., 2001; Taylor et al., 2000). To allow more accurate conclusions regarding disinfectants’ effects on DWPI, it is critical to (1) fully report all water chemistry variables, (2) study multiple DWPIs within the same experimental framework.
The concentrations of metal ions can also impact growth and survival of DWPI, but are not always considered in studies at multiple scales. Iron, typically originating from iron pipes, may be a critical nutrient for Legionella spp., NTM, and P. aeruginosa growth, although in different quantities. Copper, originating from copper-silver ionization systems or from copper pipes, is generally considered to have antimicrobial properties (Cachafeiro et al., 2007; Landeen et al., 1989; Lin et al., 1996; Liu et al., 1994). Furthermore, copper has also been linked to VBNC induction in P. aeruginosa (Bédard et al., 2014; Dopp et al., 2017; Dwidjosiswojo et al., 2011). Still, these results are difficult to scale to engineering solutions, especially considering the variability of metal concentrations both spatially and temporally within building water systems and changes in metal chemistry throughout the lifetime of a building (Brazeau and Edwards, 2013; Rhoads et al., 2017b; Salehi et al., 2020). For example, while the use of copper pipes has been suggested as a control mechanism for L. pneumophila (Learbuch et al., 2019; Rogers et al., 1994), the effects on L. pneumophila and other DWPI might be short-lived, pH dependent, and modified in the presence of biofilm (Buse et al., 2017; Elguindi et al., 2009; Proctor et al., 2017; Rhoads et al., 2017b). Under certain conditions, the presence of copper may even selectively encourage the growth of L. pneumophila (Proctor et al., 2017; Rhoads et al., 2017b), by changing the pipe microbiome (Buse et al., 2014). Elucidating the impact of metals on the broader microbiome under varied water chemistry conditions will allow better understanding of conflicting findings in full-scale systems.
Each DWPI also has a unique set of nutrient and oxygen requirements, with some DWPI surviving under extreme conditions that may provide competitive advantages. The M. avium complex is considered microaerobic, growing in as little as 6% oxygen, and surviving in anaerobic conditions (Lewis and Falkinham, 2015). P. aeruginosa is generally considered aerobic, but can grow anaerobically utilizing nitrate as a terminal electron acceptor (Palmer et al., 2007). L. pneumophila, on the other hand, is an obligate aerobe, requires amino acids produced by other microorganisms, and can grow necrotrophically (Temmerman et al., 2006). Amoebae similarly rely on other microorganisms, digesting them via phagocytosis (Samba-Louaka et al., 2019). Better understanding of this niche differentiation will assist in finding mitigation strategies that are effective for mitigating the full suite of DWPI.
3.3. DWPI Interactions
When DWPI are studied together, relationships (parasitic, symbiotic and competitive) are uncovered that would not be seen in monoculture studies. Many DWPI, including Legionella spp. and NTM can be phagocytized by free-living protozoa, and amoebae (Delafont et al., 2018), which facilitates replication, offers protection, and enhances virulence (Buse and Ashbolt, 2011; Cateau et al., 2011; Cirillo et al., 1997). Amoebic relationships may account for the preference of L. pneumophila in biofilms with high cell densities (van der Kooij et al., 2017) versus the preference of Pseudomonas spp. and Mycobacterium spp. in biofilms with low cell densities (Proctor et al., 2018, 2016). Some of these protozoan hosts are also DWPI themselves (e.g., Acanthamoeba). P. aeruginosa can suppress the growth of L. pneumophila (Kimura et al., 2009) and may prevent their attachment to simple biofilms (Stewart et al., 2012), likely through the production of bacteriocin-like substances that suppress competitors (Guerrieri et al., 2008). Also, there appears to be preference by amoeba for prey that are not amoeba-resisting (Shaheen and Ashbolt, 2021). In continuously chlorinated cooling towers, Legionella spp. and Pseudomonas spp. had negative associations due to direct or indirect relationships with hosts (Paranjape et al., 2020). In addition, DWPI can compete with one another and other drinking water bacteria for uptake by amoebic hosts, potentially affecting inactivation and virulence (Berry et al., 2010; Cirillo et al., 1997; Declerck et al., 2005; Thomas and Ashbolt, 2011). It may be possible to leverage these findings to eliminate DWPI from water systems.
3.4. An Example of Holistic Thinking: Antibiotic Resistance
The differentiation of niches amongst DWPI is especially well exemplified with respect to antibiotic resistance (AR) (Box 1). The resistance of microorganisms to antibiotics has been widely studied across multiple disciplines and multiple DWPI, embodying key aspects of the proposed holistic approach. Epidemiologists and clinicians traditionally study AR with respect to identifying sources, limiting their spread, and determining the best treatment approaches. Ecologists study environmental niches conducive to growth of AR organisms, conditions that select for resistant strains, and microenvironments favorable to the transfer of AR genes via cell-to-cell via horizontal gene transfer. Concerns regarding AR are emerging for engineers, who have studies how engineered systems can serve as barriers to the spread of AR or, conversely, can inadvertently promote dissemination of AR. Identifying solutions to these pressing and unconventional challenges necessitates collaboration among researchers across disciplines, but holistic solutions are needed given that each organism presents unique challenges. Holistic consideration of these challenges by engineers, ecologists, and epidemiologists working in collaboration is necessary for parallel control of these disparate AR-DWPIs in water systems.
3.5. An Example of Holistic Thinking: Method Development
The methods used to measure DWPI by different disciplines often create a barrier because each field has different goals (Table 3). A public health specialist or epidemiologist requires in-depth identification (e.g., serotyping, sequence typing) to trace outbreaks, while a physician focuses on information pertinent to diagnosis and treatment (e.g., symptoms may be enough for some diseases/organisms, while complete typing is needed for others). Thus, positive diagnostic tests (e.g., urinary antigen tests) may not be paired with more in-depth analysis (e.g., sputum culturing, water testing) on a routine basis. Environmental monitoring may also use culture-based techniques, but clinical culture media are often not suitable to recover nutrient-starved DWPI cells typically found in drinking water. A microbial ecologist may only be interested in the presence of a microorganism, and thus DNA-based molecular methods may be adequate (i.e., composite capture of viable, VBNC, and dead cells). The meaning of the unit gene copy per volume is not immediately relatable to quantities reported from traditional culture methods (i.e., colony forming units (CFU) per unit volume), and relative abundance (e.g., percent abundance from amplicon sequencing data) does not directly translate into the absolute concentrations needed for risk assessment. A monitoring program requires relatively quick and easy methods for live- or revivable (VBNC)-pathogen detection and emerging methods for achieving this report often rely on non-traditional units of measurement (e.g., most probable number (MPN) per unit volume). Other engineers need immediate indicators for a change in physicochemical, nutrient, or total bacterial conditions and may be particularly interested in methods designed to denote hydraulic changes rather than direct correlations with human pathogens. Even if these do not relate directly to DWPI growth or survival, they could indicate a failure in treatment or hydraulic issues and need for corrective action. Building owners may not be interested in finding definitive evidence of DWPI because of legal liability and may prefer more general water quality metrics that could be indicative of conditions conducive to DWPI growth (e.g., temperature, chlorine concentration).
Table 3.
Features of methods used to detect and quantify drinking water-associated pathogens that can cause infections in immunocompromised individuals (DWPI). Molecular methods are abbreviated as follows: polymerase chain reaction (PCR), quantitative-PCR (qPCR) and digital PCR (dPCR).
| Method | What reaction/substance does it measure? | Live/dead? | Sample type (typical) | Description of method | Specificity of target | Enable type matching? § | Primary question it answers | Questions it cannot answer | Sector used by | ISO approved? | Time required | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urinary antigen test | Antigen | Y | Urine, bodily fluids | Confirms presence/absence of antigen in sample | Serogroup (only for L. pneumophila SG1) | N | Is or was the human body fighting this organism recently? | Was the body fighting it before, or a different strain of it? | Health | N * | Minutes | |
| Culture | Growth | Y | Sputum, Water (sometimes filtered), tissue | Quantifies growth in specific nutrient rich conditions. | Specificity varies, typically species. | Y – must do prompt-ly | Can the organism grow right now under specific conditions? | Could the organism grow before, later, or under more realistic conditions? Is the organisms in a VBNC state? | Health, Ecology, Engineering | Y (N for NTM) | 7+ days | |
| PCR, qPCR, ddPCR | DNA-based | DNA (in all cells) | N | Water (filtered), swabs | PCR – confirms presence/absence of a sequence. qPCR and dPCR – quantify a sequence. | Specificity varies typically genus, species or indicator genes. | Y *** | Is or was a gene present in the sample, and how much, absolutely? | Is it capable of infection/the thing that caused infection? (for DNA – is it alive?) | Ecology, Engineering, [Health as secondary method] | Y‡ | 8–24 hrs |
| RNA-based | RNA (only in live cells) | Y | N | |||||||||
| PMA/EMA based | DNA [live/dead stained] | Y** | N | 8–24 hrs** | ||||||||
| ‘omics methods (e.g., amplicon sequencing, metagenomics, shotgun sequencing) | DNA (in all cells) | N | Water (filtered), swabs | Identifies relative abundance of sequences in communities. For taxonomic profiling or genome assembly. | Varies: Amplicon sequencing – resolution to genus level, metagenomics to species and in some cases strain level. | Y*** | Is or was a gene present in the sample, and how much, relatively? How does occurrence of several DWPI compare? | Is it capable of infection/the thing that caused infection? (for DNA – is it alive?) | Ecology | N | 1–2 wks | |
| RNA (only in live cells) | Y | |||||||||||
| DNA [live/de ad stained] | Y** | |||||||||||
| Pseudalert | Growth | Y | Water | Most probable number based on growth in chambered trays | Species – P. aeruginosa only | Y – must do prompt-ly | Can the organism grow right now under specific conditions? | Could the organism grow before, later, or under more realistic conditions? Is the organisms VBNC? | Engineering | Y | 1 day | |
| Legiolert | Growth | Y | Species – L. pneumophila only | N | 7 days | |||||||
| Adenosine tri-phosphate (ATP) | Chemical cycled by living cells | Y | Drinking water | Quantifies ‘total activity’ of microbes | Non-specific – total bacterial measures**** | N | Are cells generally active? More so than usual? | Is there a specific organism of concern present? Total growth not consistently linked with any DWPI, but can indicate general temporal/spatial conditions conducive to microbial growth. | Engineering, Ecology (primarily in research now) | N | Seconds | |
| Flow cytometry | Nucleic acids within cells | Y | Drinking water | Quantifies number of cells (total or intact) | N | How many cells are there? (How many are intact?) | N | Mins. | ||||
| Heterotrophic Plate Counts | Growth | Y | Drinking water (sometimes filtered) | Quantifies growth of heterotrophs in relatively nutrient rich conditions. | N | Can heterotro phs grow right now under specific conditions? | Engineering (regulated drinking water method) | Y | 2–3 days | |||
As detection methods – none of these can alone be used for definitively determining the exact source of a disease. Typically, a more in-depth method must be applied to determine sequence, type, or serogroup matching.
ISO does not typically approve diagnostic tests. This is CDC approved, however.
Success of PMA and EMA based methods for differentiating between live and dead cells is debated.
Extracted nucleic acids (RNA, DNA, or EMA/PMA differentiated DNA) used w/metagenome sequencing can identify more in depth characteristics. This is typically expensive.
Flow cytometry measures have been developed specifically for Legionella, involving a immunomagnetic separation pre-processing step – but have not been widely validated.
In 2019, ISO approved a molecular method for Legionella and Legionella pneumophila.
All available methods have benefits and drawbacks, and a single method cannot address all questions. At the same time, financial, time, and expertise limitations will not typically allow for application of all methods in all circumstances. Better understanding of the differences and relative benefits of information provided by these methods can allow for (i) communication between disciplines, and (ii) better design of studies that will translate more effectively across disciplines. Similarly, convenient environmental sampling methods could be designed with compatibility for downstream processing like sequence-based typing (Mercante and Winchell, 2015; Scaturro et al., 2005) or whole genome sequencing (Haworth et al., 2017) to allow for links with healthcare cases. Using select methods in concert can substantially increase the value of studies (e.g., allowing interpretation of various units of measurement), but requires pooling of resources.
The investment in test method development and availability varies considerably by DWPI. Culture-based and culture-independent methods have been developed for the detection and quantification of most DWPI, but their convenience and standardization vary. Easy-to-use environmental detection tests, like Pseudalert® and Legiolert®, target single pathogen types. For L. pneumophila, a simple urinary antigen test is available for clinical testing (albeit only for the detection of serogroup 1). While convenient, the urinary antigen test results in the underestimation of other disease-causing serogroups or species (e.g., L. longbeachae) (Mercante and Winchell, 2015) and does not produce isolates for disease tracking. More rapid serologic tests based on enzyme immunoassays are under development for NTM, but are not commonly employed (Aksamit et al., 2014; Haworth et al., 2017). While culture-based methods generally require specialized expertise, the CDC administers a control program for L. pneumophila: the ELITE program (Environmental Legionella Isolation Techniques Evaluation). Development of easier, faster methods is needed across to address all DWPI, as is the development of quality control programs. As it is believed that amoeba and protozoa may play important roles in DWPI persistence in drinking water systems, a method to identify DWPIs that have been resident in those protists (e.g., DNA modification) would be valuable. Moreover, faster standardized methods would allow for the testing of multiple organisms at once, especially in environmental samples. Currently, measuring all DWPI in a sample can be burdensome, even for research projects. A possible workflow for an “ideal universal method”, which considers multiple DWPIs, multiple field-specific interests, as well as the real-world constraints of time and cost, is proposed in Box 2.
3.6. Proposed Approach:
Microbial ecologists, who primarily participate in research on the bench scale, can embrace several strategies to make their research more useful from a holistic perspective.
Rapid, and well documented, well quality-controlled, clearly reporting sampling strategies that are deliberately designed to answer research questions relevant to cross-cutting objectives in health, microbiology, and engineering.
Identify methods to distinguish virulent (from avirulent) DWPI species to allow monitoring of DWPIs that are of greatest public health significance.
Given the need for CT values for DWPI for informing engineering interventions and complexities of comparing values derived from piecemeal experimental studies, a systematic review and meta-analysis of kinetic parameters (e.g., inactivation and growth under different temperature, disinfectant residual, water chemistry, and temporal conditions) for DWPIs is needed for identifying research gaps and data needs.
Include and measure multiple DWPIs in research projects, so that the potential effect of altered environmental conditions (e.g., temperature, disinfectant residual levels) can be understood for all DWPI within comparable conditions. Comparison of conditions across existing published studies is difficult.
Conduct mixed community experiments under conditions representative of real systems (alongside studying full-scale drinking water systems, which are more difficult to control). Complementary pure culture, mixed culture and full scale experiments will allows the field to build on basic understanding and move towards applicable engineering solutions.
Work with other scientific fields to elucidate transmission dynamics of multiple DWPI between media. It would be useful to understand the fluxes from biofilm to water, water to aerosol, aerosol to inhalation, and inhalation to infection under multiple water physio-chemical scenarios. This would require accurate and comparable quantification of organisms in multiple media (see methods below).
Develop methods that can (i) easily be used for multiple DWPI detection and quantification, and (ii) be used on multiple media (bodily fluids, water, biofilm) with a single understandable metric (Box 2) and (iii) move toward near real-time DWPI detection.
Harmonize environmental measurements with clinical approaches for understanding source-to-receptor exposures along the full exposure continuum.
Track the development of DWPI-AR within studies by routinely incorporating AR-tracking methods (e.g., qPCR) (Box 1). This is done in some studies (e.g., Cocuzza et al., 2021; Sikora et al., 2017; Vaz-Moreira et al., 2012) already, but efforts can be expanded.
4. ENGINEERING & OTHER BUILDING PRACTITIONERS
Since DWPI were first identified, water quality engineers have made efforts to reduce their proliferation and eliminate them from water systems, with many interventions focused on Legionella pneumophila alone. Initial interventions adapted methods used at the treatment plant (i.e., adding traditional disinfectants), and could only be applied by trained operators and building managers. The field has since expanded to an examination of building and plumbing system design itself, with maintenance-free strategies introduced at the design-stage by architects and engineers. Borrowing from the field of industrial hygiene, hazard removal or employment of maintenance-free engineering controls is preferable to relying upon building operation habits or having individuals responsible for reducing their own exposure (CDC and NIOSH, 2015). Nonetheless, implementation of many engineering strategies for pathogen control will involve multiple professions (e.g., architects, plumbers, building code officials and building managers), requiring effective communication.
4.1. Applying Disinfectant
Since loss of disinfectant residual is associated with microbial growth (Berry et al., 2006; Proctor and Hammes, 2015b), boosting the concentrations of disinfectants either in the distribution system or in the plumbing of large buildings is a promising DWPI engineering control. While there are many reported successes of on-site disinfectant systems, there are trade-offs between disinfectant types (e.g., efficacy discussed above, disinfection byproducts, and corrosion of materials (Giovanardi et al., 2020) and differential selection of one DWPI over another. Chloramine residuals persist longer and have greater effect on biofilms than chlorine in distribution systems, but their relative efficacies can be reversed in building water system under conditions that accelerate chloramine decay (Zhang et al., 2009). In comparing samples from buildings where either chlorine or chloramine were applied in the supply’s distribution system, L. pneumophila was detected with similar frequency with both disinfectants, while certain NTM were detected more often in chloraminated systems (Donohue et al., 2019). In the distribution system, the choice of disinfectant type is often driven by concerns other than DWPI, including disinfectant byproduct formation.
Applying disinfectant at the building level can provide additional control, but an important nuance is how disinfectant is applied. Chloramine addition at the building level is able to control Legionella spp., P. aeruginosa and NTM when applied correctly (Duda et al., 2014; Lytle et al., 2021). However, in cooling towers, continuous chlorine treatment appeared to suppress Legionella spp. while promoting Pseudomonas spp. (Paranjape et al., 2020). The choice of oxidant (e.g., chloramine, chlorine, or chlorine dioxide) can also influence the specific species or strains in a system (Marchesi et al., 2016), therefore, the choice of residual disinfectant and delivery mechanism may select for certain DWPI species or strains. The choice of disinfectant type at this level is often driven by cost, experience, and maintenance concerns. Additionally, adding on-site disinfectant still may not protect all distal ends, can be expensive, and may result in a regulatory burden (additional testing and permits (SDWA, 1996)), deterring some building owners from preventative action. Ultimately, an effective disinfectant-based solution, if achievable, will rely heavily on building design features, upstream water quality, and importantly, competent operators, which may not be feasible in all buildings.
Non-traditional disinfection approaches like ultraviolet (UV)-irradiation, ozonation, and copper-silver ionization have been applied in buildings (Lin et al., 2011). Neither UV nor ozone provide a strong residual, and Mycobacterium spp. and Legionella spp. have been shown to survive in full-scale ozone contactors (Kotlarz et al., 2018). Copper-silver ionization generally is effective for reducing culturable L. pneumophila (Lin et al., 2011); however, its effectiveness is highly dependent on chemical water quality characteristics (Lin et al., 2002), and resistance can develop over time (June and Dziewulski, 2018), thus, vigilant maintenance is required (Office of Inspector General, 2013). Copper-silver ionization has not been tested extensively for other DWPI, and may be less effective against NTM (Huang et al., 2008; June and Dziewulski, 2018; Kusnetsov et al., 2001). Overall, the effectiveness of non-traditional disinfection approaches against all DWPI, as well as the skill and capability of system operators must be taken into consideration for long-term successful treatment. Different types of filters and devices (UV, UV-LED) installed at the point of use are available commercially and can be installed at faucets and showerheads to prevent exposure to bacteria and DWPI (NASEM, 2019). Water quality (e.g., turbidity) will affect their efficacy and the duration of their use before breakthrough. Manufacturers test products using ISO 22196:2011, which involves incubation of pure cultures of bacteria on the antimicrobial surface found inside the showerhead (ISO, 2011) and may not represent realistic conditions. Nonetheless, successful implementation of such devices has been observed. Since DWPI can also grow in point-of-use filters (Chaidez and Gerba, 2004; Rodgers et al., 1999), frequent replacement is often recommended, requiring vigilant building operators. Recent product improvements incorporate biocides and include inactivation technologies that extend the life of these devices. Other filters, like granular activated carbon filters may create an ideal niche for L. pneumophila and NTM (Dai et al., 2018; Rodgers et al., 1999; Wu et al., 2017). To ensure public safety, validation of these devices under varied realistic scenarios with multiple DWPI is needed.
4.2. Temperature Control
In applying findings about temperature (discussed above) to full-scale systems, there are several strategies to keep water out of ideal growth ranges. In large hot water systems, recirculation helps maintain elevated temperatures capable of inactivating DWPI throughout the system. Maintaining temperatures > 60 °C in the water heater and > 55 °C at distal points of the system is recommended for prevention of L. pneumophila growth (Bédard et al., 2015a; Darelid et al., 2002; NASEM, 2019). However, some studies indicate that recirculation at improper (i.e. too low) temperatures can increase L. pneumophila densities, presumably due to heat losses prolonging the time within the optimal growth conditions (Rhoads et al. 2015). Similarly, increasing pipe insulation seems beneficial, in that hot water can stay hotter, but sometimes adding insulation can increase the time of ideal Legionella spp. growth temperatures during stagnation in hot water lines (Bédard et al., 2015a; Van Kenhove et al., 2019a). While cold water systems are not typically the focus for Legionella spp. research, toilets can also transmit L. pneumophila (Couturier et al., 2020), and ideal growth temperature can be achieved in cold water systems with co-location of hot/cold pipes or where warm ambient temperatures are consistently present. Other DWPI including P. aeruginosa (Bédard et al., 2015b) are more often studied in cold water systems. Studies are needed regarding efficacy of optimizing temperature designs against non-Legionella DWPIs and the prevalence of all DWPIs in cold water systems.
One-time or periodic treatment can also be applied to remediate potential problems (e.g., recommissioning of buildings after long stagnation or disuse (Proctor et al., 2020)). Heat-shock methods have found some success for L. pneumophila control, but recovery, recolonization, and development of resistance or acclimation are common (Allegra et al., 2011; Farhat et al., 2010; Ji et al., 2018; Temmerman et al., 2006), likely attributable to dissipation of temperature in distal ends, VBNC protective states, or even the ability of L. pneumophila to subsist necrotrophically on other cells killed by the intervention. Biofilm “cleaning methods” (e.g., ice slurry) are also available commercially, but without independent verification. Hyperchlorination or shock chlorination, i.e., temporarily increasing disinfectant concentrations, has also been employed, but mitigation has been short lived and damage to pipe materials is a concern (Lin et al., 2011; Proctor et al., 2020). The recommended strength (i.e., dose of chlorine, temperature), duration, and frequency of these treatments must consider the varied DWPI growth rates, physiology and resistance to treatment, as well as specific characteristics of treated buildings.
4.3. Building Design Features
Many aspects of plumbing design are dictated in building code, but most codes were not developed with microbial quality or DWPI growth in mind. Especially with changing trends in water conservation, an update to these building codes is warranted. For example, despite lower water flows, pipe diameter requirements remain unchanged since 1940 Hunter design codes (Hunter, 1940), resulting in over-sized pipes, long stagnation times, and low linear flowrates, which are hypothesized to influence biofilm and water quality (Julien et al., 2020b). On the other hand, smaller pipes increase surface-area-to-volume ratios, increasing the influence of biofilm on bulk water microbial quality. Competing objectives will make it challenging to achieve consensus for updated codes.
Decisions made during the design and construction of a building can have consequences for water flow patterns, temperature, and residence time of water in that building for its entire lifetime. In particular, buildings are not designed with “microbially-informed” parameters in mind. In Germany, the length of distal ends is restricted to 3 m to limit Legionella spp. growth (NASEM, 2019). Furthermore, by placing the toilet at the end of a trunk-and-branch line, water flow can be maximized through the pipes. Even the orientation of pipes (up or down from the pipe to the fixture) has implications on temperature and hence DWPI growth due to induction of convective mixing (Rhoads et al., 2015). Minimizing the water system footprint by reducing the length of distal pipes, reducing the number of fixtures, and installing water fixtures close together (i.e., minimizing wet walls) would help simplify investigations of DWPI colonization, remove de facto dead-ends, lower construction costs, and decrease energy needs for hot water, but achieving these collective goals requires communication between many silos of expertise.
In most plumbing designs, distal ends still present a challenge since water treatment will only affect the distal fixture by opening that particular fixture during treatment. To address this, some new plumbing designs and management protocols either automatically drain pipes or direct manual draining of pipes to dryness between uses. As NTM, P. aeruginosa, and fungi thrive in such semi- moist environments (e.g., such as showerheads (Gebert et al., 2018; Hageskal et al., 2009; Novak Babič et al., 2017), tap aerators, and sink drains (Walker and Moore, 2015)), these changes have the potential to alter microbial ecology within plumbing. NTM are particularly resistant to desiccation (Archuleta et al., 2002). Electronic non-touch faucets were designed, in part, to reduce microbial contamination problems from faucets functioning as fomites, yet are associated with colonization and growth of many DWPI (Bédard et al., 2016; Kotsanas et al., 2008; Livni et al., 2008; Sydnor et al., 2012). Due to these unintended consequences, new plumbing designs should be thoroughly tested for risk of colonization internally by multiple DWPI as well as for their ability to serve as fomites on external-facing surfaces.
The propensity of pipe material to support DWPI survival, persistence and growth are often studied. Iron or copper can have positive (iron), negative (copper-silver), or mixed (copper) effects on DWPI growth (Buse et al., 2014; Haig et al., 2020; Lin et al., 2002, 1996). Corrosion can also accelerate decay of disinfectant residual (Nguyen et al., 2012; Rhoads et al., 2017a), reducing stress on all DWPI. Many plastic pipes leach assimilable organic carbon (Bucheli-Witschel et al., 2012; van der Kooij, 1992), a nutrient for growth of many DWPIs as they are heterotrophs. The type and amount of carbon leaching varies considerably amongst plastics, and can affect microbial communities and DWPI (Proctor et al., 2016). One limitation in pipe material research is the duration of studies, as most are limited to a few days, weeks, or months of pipe use. The hydrophobicity of pipe materials may have an influence on initial attachment (Simões et al., 2007), and copper may have anti-microbial effects (Learbuch et al., 2019; Rogers et al., 1994), but the long-term effects are questionable (Buse et al., 2014; Gião et al., 2015; Proctor et al., 2017; Rhoads et al., 2017b). Case studies can offer insights into long-term effects of plumbing design, but interpretation must consider multiple confounding variables. Consensus on which pipe material to use when or where will require rigorous long-term controlled and field-validation studies.
Commissioning, the final stage of building construction, can also impact water quality for the lifetime of the building. Careful hydraulic balancing (i.e., calibrating pressures) helps maintain proper temperatures in both hot and cold water systems, if done properly (NASEM, 2019). While some standards for the commissioning process exist, they do not necessarily consider water quality. Often, water is allowed to stagnate in pipes for weeks before buildings are occupied (Inkinen et al., 2014), allowing time for biofilm formation. ASHRAE suggests considering shock-chlorination after four weeks of stagnation (ASHRAE, 2018). According to a survey of the many professional groups involved, professional responsibility for the commissioning phase is unclear, often resulting in missed steps and minimal review (Potts and Wall, 2002). While the building owner is responsible for water quality, many people influence it before they take possession of the property.
4.4. Building Maintenance
Day-to-day, decisions regarding building water system can drastically affect DWPI growth. Some of these, such as local stagnation at a fixture, are difficult to control, but others, like water heater settings, are being incorporated into building water management plans aimed at curbing DWPI.
Long stagnation times are generally thought to permit DWPI growth, e.g., due to reduced chlorine residual. Studies of green buildings with water-saving devices that elevate water age often find DWPI (Rhoads et al., 2016) and samples taken after longer stagnation have higher microbial densities (Haig et al., 2020, 2018; Lautenschlager et al., 2010). Increased water age is also associated with a change in microbial community composition (Ji et al., 2017; Ling et al., 2018; Proctor et al., 2018), and even the type of NTM found (Haig et al., 2018). The community shift may indicate the importance of biofilm interactions with the water phase. The speed of biofilm-water interactions may account for the need for high frequency flushes to protect against L. pneumophila in some buildings (e.g., every 2 hours (Totaro et al., 2018)). However, flushing will not remove biofilms, and evidence suggests that increasing the flushing frequency may even cause greater net biofilm growth due to the delivery of fresh nutrients (Rhoads et al., 2015). It should be stressed that flushing after abnormally long stagnation times (e.g., upon return to a second home), may remove the immediate exposure threat from DWPI (Proctor et al., 2020). Even so, it is difficult to reach consensus amongst experts regarding best flushing practices (Proctor et al., 2020), and the direct relationship between stagnation and Legionella spp. growth is questioned (Rhoads and Hammes, 2021).
Depending on the system size and responsiveness changing water heater settings is a relatively simple way to control DWPI, however, scalding concerns and energy conservation efforts have resulted in low recommended temperatures (e.g., 48 °C/120 °F). Such settings put hot water delivery pipes within the ideal temperature range of DWPI. Thermostatic mixing valves can be installed close to the point of use to allow higher temperatures and are recommended (NASEM, 2019), but they also have potential issues and require maintenance (Proctor et al., 2020). Instantaneous tankless water heaters may also allow temperature control while alleviating energy concerns (Brazeau and Edwards, 2013). Most research on water heater settings have focused on L. pneumophila (NASEM, 2019). Research considering the use of temperature to control other DWPIs is needed. Moreover, cost-benefit analysis to weigh health, scalding, and energy concerns are needed from multidisciplinary teams.
Water management plans vary widely but are all aimed at understanding and improving water quality. Plans should identify potential hazards (e.g., aerosol-producing water features, low-use fixtures) and characterize the water system (e.g., water heater operation, connections to other systems), which may be beyond the scope of information routinely available to building operators or feasible to collect on a routine basis. Control measures might be suggested, including physical controls, temperature management, disinfectant control, and pathogen testing. To date, plans sporadically include best-practices like cleaning showerheads and aerators, flushing hot water tanks, and minimizing aerosol exposure through increased ventilation in bathrooms (Masters et al., 2018). While success of water management plans is challenging to measure, simply having a water management plan may not be sufficient to improve water quality (van der Lugt et al., 2019). Moving forward, creators of water management plans likely require more guidance, training, site-specific information and access to specific expertise to develop and execute an effective document. Conflicting advice and interests (e.g., liability with testing) also influence the effectiveness of water management plans. Most water management plans are targeted at L. pneumophila prevention, including those required by the Centers for Medicare and Medicaid Services (CMS, 2018). Such a focus may be promoting practices that unintentionally create ideal conditions for other DWPI as was shown following the substitution of monochloramine for chlorine and the disappearance of Legionella spp., but an increase in Mycobacterium spp. numbers (Pryor et al., 2004). Moreover, maintenance of other water systems (e.g., cooling towers) might be overlooked in these plans, but likely have a strong effect on disease.
4.5. An Example of Holistic Thinking: Creating Proactive Incentives
Implementation of engineering solutions and legislation after the discovery of a problem in the field is typically reactive, rather than proactive, and may not consider multiple hazards or unintended consequences of action. For example, legislation was enacted nationwide for drinking water in response to the 1993 Cryptosporidium outbreak (Gostin et al., 2000), and for cooling towers in New York City response to a 2015 Legionnaire’s outbreak (Bassett and Balter, 2017). Even in communication of risk, there is a clear lack of multi-hazard consideration. Communication materials are available to inform the public on steps they can take to make decisions (e.g., individual risk factors and behaviors) in order to manage DWPI, emphasizing that this is a shared responsibility between utilities, facilities managers, and individuals (Masters et al., 2018); however, most of the guidance available is for preventing risks of Legionella spp. at the exclusion of other water quality issues (Singh et al., 2020). The benefits of awareness and having a water management plan are not entirely conclusive (Clopper et al., 2021; van der Lugt et al., 2019), perhaps due to a variety in plan content and follow-through. However, a recent study suggested that 81% of L. pneumophila fatalities occurred in facilities without a water management plan (Clopper et al., 2021). In a survey of Minnesota hospitals, the majority of building managers expressed interest in getting assistance to set up a water management plan (Danila et al., 2018). More research to target the effective elements of water management plans will assist in creating effective plans.
A potential solution to raise awareness, and move towards more effective proactive solutions, like building water management plans, could be to incorporate building water maintenance into health and building insurance programs. This approach would take into consideration potential future savings on health care costs (due to lowered infection rates), and building owners’ legal fees (due to lowered liability in case of infection). These plans can incentivize preventative actions, and even offset the energy, institutional, or testing costs associated with those preventative actions.
4.6. Proposed Approach
DWPI can be considered over the entire lifetime of the building, from design through everyday maintenance. The implementation of engineering solutions requires many non-engineering strategies.
Encourage “microbially-informed design” of buildings through mechanisms such as LEED or national organizations
Incorporate education about all DWPI into implementation of engineering solutions, such that architects, construction professionals, building owners, managers, and maintenance personnel have greater understanding of the significance of the work. This can include educating building tenants about personal risk factors for DWPI.
Improving communication/education across disciplines e.g., regarding plumbing operation /codes /fixture designs among non-engineering communities and consideration of public health concerns by engineering community at the design phase
Encourage building owners’ and renters’ insurance to include partial coverage of building water quality to incentivize preventative actions.
Include previously developed water management plans in the sale of buildings, and require water testing as an inspection element, so that new tenants are aware of potential issues and address them. If testing isn’t required, disclosure of previous water issues and testing, if conducted, should be required.
Create a database of building testing data for all DWPI to collect existing data and encourage greater investigations into large data trends. This can include a more standardized reporting procedure for building size, sample type, and engineering solutions currently in place.
Incorporate AR tracing into studies of real buildings, including epidemiological investigations of outbreaks to better understand how widespread AR is within engineered drinking water systems (Box 1). Investigations of engineering controls for AR can also be scaled up and tested in the field.
5. CONCLUSIONS
Clearly, key similarities exist among DWPIs: exposure routes are similar and various niches in plumbing systems provide ideal growth conditions, often with similar temperature ranges for growth, slow growth, and preference for the biofilm phase. However, differences in DWPI physiology and response to the culmination of various conditions in building water system (e.g., resistance factors, competition) can result in different susceptibilities to each DWPI among individuals and populations, while mitigation efforts are also not universally effective against each DWPI.
The most well-studied DWPI across many fields is undoubtedly L. pneumophila. While awareness and treatment of this microorganism is a positive step towards improving plumbing design and operation and better protecting public health and safer plumbing, ignoring other DWPIs could result in unintended consequences and increase the burden on the healthcare system. Strategies to reduce L. pneumophila tend to reduce overall biofilm concentration and increase disinfectant residual, and thus may create conditions ideal for NTM (Pryor et al., 2004) proliferation. The attention placed on L. pneumophila has supported development of rapid and easy diagnostic and environmental measuring techniques, which in turn has led to substantial advancement of understanding of L. pneumophila in both clinical and environmental realms. Our understanding of other microorganisms would benefit from similar development of rapid tests, which can be motivated with financial and legislative incentives.
Ultimately, strategies to holistically protect the public from pathogens capable of growth in drinking water will involve the consideration of all DWPIs together and across all disciplines. For example, more DWPI associated diseases should be reportable and microorganisms should be studied concurrently in buildings to better inform risk models. While a single solution that creates a “zero-risk” plumbing system is not possible, increased cooperation and multiple DWPI consideration will allow for informed balancing of risk and consequences.
Supplementary Material
Figure 1.
Various groups are responsible for water safety regarding drinking water-associated pathogens that can cause infections in immunocompromised individuals. The professions and backgrounds of individuals within these groups may cross discipline silo boundaries or exist entirely outside of the disciplines – necessitating outreach on multiple fronts. Acronyms used: CDC = centers for disease control; HVAC = heating, ventilation, and air conditioning
Highlights.
Drinking water pathogen research often lacks cross-disciplinary communication
Differences in pathogen behavior and management should be considered holistically
Discipline-specific methods, vocabulary, and goals hinder cross-disciplinary work
Comprehensive messaging and research regarding these pathogens is recommended
ACKNOWLEDGMENTS
This paper was initiated by SJH and CP, with CP, EG, KAH, and SJH contributing equally to writing. The work was supported in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (National Institutes of Health, Bethesda, MD, USA), the University of Pittsburgh’s Central Development Research Fund and the Water Research Foundation (Award 5721). The sponsors had no role in the decision to submit this work for publication.
ACRONYMS USED
- AR
antibiotic resistance
- CDC
Centers for Disease Control and Prevention
- CFU
colony forming units
- CSTE
Council of State and Territorial Epidemiologists
- DALY
disability adjusted life year
- DWPI
drinking water-associated pathogens that can cause infections in immunocompromised individuals
- GAE
granulomatous amoebic encephalitis
- MAC
Mycobacterium avium complex
- MCLG
maximum contaminant level goal
- MPN
most probable number
- NTM
nontuberculous mycobacteria
- QMRA
quantitative microbial risk assessment
- UV
ultraviolet
- VBNC
viable but nonculturable
Footnotes
Declaration of Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adam EA, Collier SA, Fullerton KE, Gargano JW, Beach MJ, 2017. Prevalence and direct costs of emergency department visits and hospitalizations for selected diseases that can be transmitted by water, United States. J. Water Health 15, 673–683. 10.2166/wh.2017.083 [DOI] [PubMed] [Google Scholar]
- Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR, 2012. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am. J. Respir. Crit. Care Med. 185, 881–886. 10.1164/rccm.201111-2016OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksamit TR, Philley JV, Griffith DE, 2014. Nontuberculous mycobacterial (NTM) lung disease: the top ten essentials. Respir. Med. 108, 417–425. 10.1016/j.rmed.2013.09.014 [DOI] [PubMed] [Google Scholar]
- Alaga KC, Konja JM, Salim A, Levine P, Smith S, Zervos MJ, Kilgore P, 2018. 675. Current Physician Knowledge, Attitudes, and Clinical Practice Regarding Legionnaires’ Disease in the Aftermath of the Flint Water Crisis in Genesee County, Michigan. Open Forum Infect. Dis. 5, S244. 10.1093/ofid/ofy210.682 [DOI] [Google Scholar]
- Alexandropoulou I, Parasidis T, Konstantinidis T, Panopoulou M, Constantinidis TC, 2019. A Proactive Environmental Approach for Preventing Legionellosis in Infants: Water Sampling and Antibiotic Resistance Monitoring, a 3-Years Survey Program. Healthc. Basel Switz. 7. 10.3390/healthcare7010039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegra S, Berger F, Berthelot P, Grattard F, Pozzetto B, Riffard S, 2008. Use of flow cytometry to monitor Legionella viability. Appl. Environ. Microbiol. 74, 7813–7816. 10.1128/AEM.01364-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegra S, Grattard F, Girardot F, Riffard S, Pozzetto B, Berthelot P, 2011. Longitudinal evaluation of the efficacy of heat treatment procedures against Legionella spp. in hospital water systems by using a flow cytometric assay. Appl. Environ. Microbiol. 77, 1268–1275. 10.1128/AEM.02225-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alleron L, Merlet N, Lacombe C, Frère J, 2008. Long-term survival of Legionella pneumophila in the viable but nonculturable state after monochloramine treatment. Curr. Microbiol. 57, 497–502. 10.1007/s00284-008-9275-9 [DOI] [PubMed] [Google Scholar]
- Ambrose M, Roselle GA, Kralovic SM, Gamage SD, 2021. Healthcare-Associated Legionella Disease: A Multi-Year Assessment of Exposure Settings in a National Healthcare System in the United States. Microorganisms 9, 264. 10.3390/microorganisms9020264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archuleta RJ, Mullens P, Primm TP, 2002. The relationship of temperature to desiccation and starvation tolerance of the Mycobacterium avium complex. Arch. Microbiol. 178, 311–314. 10.1007/s00203-002-0455-x [DOI] [PubMed] [Google Scholar]
- Ashbolt NJ, 2015. Microbial Contamination of Drinking Water and Human Health from Community Water Systems. Curr. Environ. Health Rep. 2, 95–106. 10.1007/s40572-014-0037-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASHRAE, 2020a. Guideline 12–2020 -- Managing the Risk of Legionellosis Associated with Building Water Systems [WWW Document]. Am. Soc. Heat. Refrig. Air-Cond. Eng. URL https://www.techstreet.com/ashrae/standards/guideline-12-2020-managing-the-risk-of-legionellosis-associated-with-building-water-systems?product_id=2111422 (accessed 4.28.21).
- ASHRAE, 2020b. ASHRAE Terminology [WWW Document]. Am. Soc. Heat. Refrig. Air-Cond. Eng. URL https://www.ashrae.org/technical-resources/authoring-tools/terminology (accessed 4.28.21).
- ASHRAE, 2018. ASHRAE 188–2018. Legionellosis: Risk Management for Building Water Systems [WWW Document]. Am. Soc. Heat. Refrig. Air-Cond. Eng. URL https://www.techstreet.com/ashrae/standards/ashrae-188-2018?gateway_code=ashrae&product_id=2020895 (accessed 4.28.21).
- ASHRAE, 2015. ANSI/ASHRAE Standard 188–2015. Legionellosis: Risk Management for Building Water Systems. (Standard.). American Society of Heating, Refrigerating and Air-Conditioning Engineers. [Google Scholar]
- Balaban NQ, Helaine S, Lewis K, Ackermann M, Aldridge B, Andersson DI, Brynildsen MP, Bumann D, Camilli A, Collins JJ, Dehio C, Fortune S, Ghigo J-M, Hardt W-D, Harms A, Heinemann M, Hung DT, Jenal U, Levin BR, Michiels J, Storz G, Tan M-W, Tenson T, Van Melderen L, Zinkernagel A, 2019. Definitions and guidelines for research on antibiotic persistence. Nat. Rev. Microbiol. 17, 441–448. 10.1038/s41579-019-0196-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J, Scaife H, Brown MR, 1995. Intraphagocytic growth induces an antibiotic-resistant phenotype of Legionella pneumophila. Antimicrob. Agents Chemother. 39, 2684–2688. 10.1128/aac.39.12.2684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett JG, Dowell SF, Mandell LA, File TM, Musher DM, Fine MJ, 2000. Practice guidelines for the management of community-acquired pneumonia in adults. Infectious Diseases Society of America. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 31, 347–382. 10.1086/313954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett MT, Balter S, 2017. Regulating Cooling Towers to Prevent Outbreaks of Legionnaires’ Disease. Public Health Rep. 132, 133–135. 10.1177/0033354916689612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassetti M, Vena A, Croxatto A, Righi E, Guery B, 2018. How to manage Pseudomonas aeruginosa infections. Drugs Context 7, 212527. 10.7573/dic.212527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bédard E, Charron D, Lalancette C, Déziel E, Prévost M, 2014. Recovery of Pseudomonas aeruginosa culturability following copper- and chlorine-induced stress. FEMS Microbiol. Lett. 356, 226–234. 10.1111/1574-6968.12494 [DOI] [PubMed] [Google Scholar]
- Bédard E, Fey S, Charron D, Lalancette C, Cantin P, Dolcé P, Laferrière C, Déziel E, Prévost M, 2015a. Temperature diagnostic to identify high risk areas and optimize Legionella pneumophila surveillance in hot water distribution systems. Water Res. 71, 244–256. 10.1016/j.watres.2015.01.006 [DOI] [PubMed] [Google Scholar]
- Bédard E, Laferrière C, Charron D, Lalancette C, Renaud C, Desmarais N, Déziel E, Prévost M, 2015b. Post-Outbreak Investigation of Pseudomonas aeruginosa Faucet Contamination by Quantitative Polymerase Chain Reaction and Environmental Factors Affecting Positivity. Infect. Control Hosp. Epidemiol. 36, 1337–1343. 10.1017/ice.2015.168 [DOI] [PubMed] [Google Scholar]
- Bédard E, Prévost M, Déziel E, 2016. Pseudomonas aeruginosa in premise plumbing of large buildings. MicrobiologyOpen 5, 937–956. 10.1002/mbo3.391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg G, 1978. Indicators of viruses in water and food. Ann Arbor Science, Ann Arbor, Mich. [Google Scholar]
- Berry D, Horn M, Xi C, Raskin L, 2010. Mycobacterium avium infections of Acanthamoeba strains: host strain variability, grazing-acquired infections, and altered dynamics of inactivation with monochloramine. Appl. Environ. Microbiol. 76, 6685–6688. 10.1128/AEM.00644-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry D, Xi C, Raskin L, 2006. Microbial ecology of drinking water distribution systems. Curr. Opin. Biotechnol. 17, 297–302. 10.1016/j.copbio.2006.05.007 [DOI] [PubMed] [Google Scholar]
- Billinger ME, Olivier KN, Viboud C, de Oca RM, Steiner C, Holland SM, Prevots DR, 2009. Nontuberculous mycobacteria-associated lung disease in hospitalized persons, United States, 1998–2005. Emerg. Infect. Dis. 15, 1562–1569. 10.3201/eid1510.090196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonde GJ, 1966. Bacteriological methods for estimation of water pollution. Health Lab. Sci. 3, 124–128. [PubMed] [Google Scholar]
- Bope A, Weir MH, Pruden A, Morowitz M, Mitchell J, Dannemiller KC, 2018. Translating research to policy at the NCSE 2017 symposium “Microbiology of the Built Environment: Implications for Health and Design.” Microbiome 6, 160. 10.1186/s40168-018-0552-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borella P, Montagna MT, Romano-Spica V, Stampi S, Stancanelli G, Triassi M, Neglia R, Marchesi I, Fantuzzi G, Tatò D, Napoli C, Quaranta G, Laurenti P, Leoni E, De Luca G, Ossi C, Moro M, Ribera D’Alcalà G, 2004. Legionella infection risk from domestic hot water. Emerg. Infect. Dis. 10, 457–464. 10.3201/eid1003.020707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazeau RH, Edwards MA, 2013. Role of Hot Water System Design on Factors Influential to Pathogen Regrowth: Temperature, Chlorine Residual, Hydrogen Evolution, and Sediment. Environ. Eng. Sci. 30, 617–627. 10.1089/ees.2012.0514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PJ, Nikaido H, 1995. The envelope of mycobacteria. Annu. Rev. Biochem. 64, 29–63. 10.1146/annurev.bi.64.070195.000333 [DOI] [PubMed] [Google Scholar]
- Brown AD, 1957. Some general properties of a psychrophilic pseudomonad: the effects of temperature on some of these properties and the utilization of glucose by this organism and Pscudomonas aeruginosa. J. Gen. Microbiol. 17, 640–648. 10.1099/00221287-17-3-640 [DOI] [PubMed] [Google Scholar]
- Brown SP, Cornforth DM, Mideo N, 2012. Evolution of virulence in opportunistic pathogens: generalism, plasticity, and control. Trends Microbiol. 20, 336–342. 10.1016/j.tim.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Elliott BA, Nash KA, Wallace RJ, 2012. Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontuberculous mycobacteria. Clin. Microbiol. Rev. 25, 545–582. 10.1128/CMR.05030-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruin JP, Ijzerman EPF, den Boer JW, Mouton JW, Diederen BMW, 2012. Wild-type MIC distribution and epidemiological cut-off values in clinical Legionella pneumophila serogroup 1 isolates. Diagn. Microbiol. Infect. Dis. 72, 103–108. 10.1016/j.diagmicrobio.2011.09.016 [DOI] [PubMed] [Google Scholar]
- Bucheli-Witschel M, Kötzsch S, Darr S, Widler R, Egli T, 2012. A new method to assess the influence of migration from polymeric materials on the biostability of drinking water. Water Res. 46, 4246–4260. 10.1016/j.watres.2012.05.008 [DOI] [PubMed] [Google Scholar]
- Buse HY, Ashbolt NJ, 2011. Differential growth of Legionella pneumophila strains within a range of amoebae at various temperatures associated with in-premise plumbing. Lett. Appl. Microbiol. 53, 217–224. 10.1111/j.1472-765X.2011.03094.x [DOI] [PubMed] [Google Scholar]
- Buse HY, Hoelle JM, Muhlen C, Lytle DA, 2018. Electrophoretic mobility of Legionella pneumophila serogroups 1 to 14. FEMS Microbiol. Lett. 365. 10.1093/femsle/fny067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse HY, Morris J, Struewing B, Szabo IT, J.G., 2019. Chlorine and Monochloramine Disinfection of Legionella pneumophila Colonizing Copper and Polyvinyl Chloride Drinking Water Biofilms. Appl. Environ. Microbiol. 85, e02956–18. 10.1128/AEM.02956-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse HY, Ji P, Gomez-Alvarez V, Pruden A, Edwards MA, Ashbolt NJ, 2017. Effect of temperature and colonization of Legionella pneumophila and Vermamoeba vermiformis on bacterial community composition of copper drinking water biofilms. Microb. Biotechnol. 10, 773–788. 10.1111/1751-7915.12457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse HY, Lu J, Lu X, Mou X, Ashbolt NJ, 2014. Microbial diversities (16S and 18S rRNA gene pyrosequencing) and environmental pathogens within drinking water biofilms grown on the common premise plumbing materials unplasticized polyvinylchloride and copper. FEMS Microbiol. Ecol. 88, 280–295. 10.1111/1574-6941.12294 [DOI] [PubMed] [Google Scholar]
- Cachafeiro SP, Naveira IM, García IG, 2007. Is copper-silver ionisation safe and effective in controlling legionella? J. Hosp. Infect. 67, 209–216. 10.1016/j.jhin.2007.07.017 [DOI] [PubMed] [Google Scholar]
- Cateau E, Verdon J, Fernandez B, Hechard Y, Rodier M-H, 2011. Acanthamoeba sp. promotes the survival and growth of Acinetobacter baumanii. FEMS Microbiol. Lett. 319, 19–25. 10.1111/j.1574-6968.2011.02261.x [DOI] [PubMed] [Google Scholar]
- CDC, 2021a. Legionella: Toolkit: Developing a Water Management Program to Reduce Legionella Growth and Spread in Buildings. A Practical Guide to Implementing Industry Standards [WWW Document]. URL https://www.cdc.gov/legionella/wmp/toolkit/index.html (accessed 4.28.21).
- CDC, 2021b. Legionnaires Disease Case Definitions | CDC; [WWW Document]. URL https://www.cdc.gov/legionella/health-depts/surv-reporting/case-definitions.html (accessed 4.28.21). [Google Scholar]
- CDC, 2021c. Legionnaires Disease: Clinical Features | CDC; [WWW Document]. URL https://www.cdc.gov/legionella/clinicians/clinical-features.html (accessed 4.28.21). [Google Scholar]
- CDC, 2021d. How to Participate in CDC’s AR Lab Network: Lab Testing [WWW Document]. Cent. Dis. Control Prev. URL https://www.cdc.gov/drugresistance/laboratories/AR-lab-network-testing-details.html (accessed 4.28.21).
- CDC, 2021e. About the AR Lab Network [WWW Document]. Cent. Dis. Control Prev. URL https://www.cdc.gov/drugresistance/laboratories.html (accessed 4.28.21).
- CDC, 2020a. Legionnaire’s Disease Surveillance Summary Report, United States 2016–2017 50. [Google Scholar]
- CDC, 2020b. Current HAI Progress Report | HAI | CDC; [WWW Document]. URL https://www.cdc.gov/hai/data/portal/progress-report.html (accessed 4.28.21). [Google Scholar]
- CDC, 2019a. National Outbreak Reporting System (NORS) | CDC; [WWW Document]. URL https://www.cdc.gov/nors/index.html (accessed 4.28.21). [Google Scholar]
- CDC, 2019b. Nationally Notifiable Diseases | Healthy Water | CDC; [WWW Document]. URL https://www.cdc.gov/healthywater/statistics/surveillance/notifiable.html (accessed 4.28.21). [Google Scholar]
- CDC, 2019c. Tracking Carbapenem-Resistant Pseudomonas aeruginosa | HAI | CDC; [WWW Document]. URL https://www.cdc.gov/hai/organisms/pseudomonas/tracking.html (accessed 4.28.21). [Google Scholar]
- CDC, 2019d. Pseudomonas aeruginosa Infection | HAI | CDC; [WWW Document]. URL https://www.cdc.gov/hai/organisms/pseudomonas.html (accessed 4.28.21). [Google Scholar]
- CDC, 2018. Notifiable Infectious Diseases and Conditions Data Tables | NNDSS; [WWW Document]. URL https://wwwn.cdc.gov/nndss/infectious-tables.html (accessed 4.28.21). [Google Scholar]
- CDC, 2017. Policy & Recommendations | Naegleria fowleri | CDC; [WWW Document]. URL https://www.cdc.gov/parasites/naegleria/policy.html (accessed 4.28.21). [Google Scholar]
- CDC, 2013. Antibiotic Resistance Threats in the US, 2013.
- CDC, 2012a. Principles of Epidemiology | Lesson 1 - Section 5 [WWW Document]. URL https://www.cdc.gov/csels/dsepd/ss1978/lesson1/section5.html (accessed 4.28.21).
- CDC, 2012b. Free-living Amebae Infections | 2012 Case Definition [WWW Document]. URL /nndss/conditions/free-living-amebae-infections/case-definition/2012/ (accessed 4.28.21).
- CDC, 2011. Legionellosis --- United States, 2000−−2009. Weekly August 19, 2011 / 60(32);1083–1086 [WWW Document]. URL https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6032a3.htm (accessed 4.28.21). [PubMed] [Google Scholar]
- CDC, 1995. Assessing the public health threat associated with waterborne cryptosporidiosis : report of a workshop. MMWR [WWW Document]. URL https://stacks.cdc.gov/view/cdc/6628 (accessed 4.28.21). [PubMed] [Google Scholar]
- CDC, NIOSH, 2015. Hierarchy of Controls.CDC (Centers for Disease Control and Prevention). NIOSH (National Institute for Occupational Safety and Health). [WWW Document]. URL https://www.cdc.gov/niosh/topics/hierarchy/default.html (accessed 4.28.21). [Google Scholar]
- Chaidez C, Gerba C, 2004. Comparison of the microbiologic quality of point-of-use (POU)-treated water and tap water. Int. J. Environ. Health Res. 14, 253–260. 10.1080/09603120410001725595 [DOI] [PubMed] [Google Scholar]
- Chalmers JD, Aksamit T, Carvalho ACC, Rendon A, Franco I, 2018. Non-tuberculous mycobacterial pulmonary infections. Pulmonology, World TB day 2018: targeting tuberculosis 24, 120–131. 10.1016/j.pulmoe.2017.12.005 [DOI] [Google Scholar]
- Chatterjee M, Anju CP, Biswas L, Anil Kumar V, Gopi Mohan C, Biswas R, 2016. Antibiotic resistance in Pseudomonas aeruginosa and alternative therapeutic options. Int. J. Med. Microbiol. IJMM 306, 48–58. 10.1016/j.ijmm.2015.11.004 [DOI] [PubMed] [Google Scholar]
- Cirillo JD, Falkow S, Tompkins LS, Bermudez LE, 1997. Interaction of Mycobacterium avium with environmental amoebae enhances virulence. Infect. Immun. 65, 3759–3767. 10.1128/IAI.65.9.3759-3767.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clopper BR, Kunz JM, Salandy SW, Smith JC, Hubbard BC, Sarisky JP, 2021. A Methodology for Classifying Root Causes of Outbreaks of Legionnaires’ Disease: Deficiencies in Environmental Control and Water Management. Microorganisms 9, E89. 10.3390/microorganisms9010089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CMS, 2018. Centers for Medicare and Medicaid Services. SUBJ: Requirement to Reduce Legionella Risk in Healthcare Facility Water Systems to Prevent Cases and Outbreaks of Legionnaires’ Disease (LD). *Revised to Clarify Expectations for Providers, Accrediting Organizations, and Surveyors*. [Google Scholar]
- CMS, 2017. Centers for Medicare and Medicaid Services. SUBJ: Requirement to Reduce Legionella Risk in Healthcare Facility Water Systems to Prevent Cases and Outbreaks of Legionnaires’ Disease (LD). [Google Scholar]
- Cocuzza CE, Martinelli M, Perdoni F, Giubbi C, Vinetti MEA, Calaresu E, Frugoni S, Scaturro M, Ricci ML, Musumeci R, 2021. Antibiotic Susceptibility of Environmental Legionella pneumophila Strains Isolated in Northern Italy. Int. J. Environ. Res. Public. Health 18, 9352. 10.3390/ijerph18179352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier SA, Deng L, Adam EA, Benedict KM, Beshearse EM, Blackstock AJ, Bruce BB, Derado G, Edens C, Fullerton KE, Gargano JW, Geissler AL, Hall AJ, Havelaar AH, Hill VR, Hoekstra RM, Reddy SC, Scallan E, Stokes EK, Yoder JS, Beach MJ, 2021. Estimate of Burden and Direct Healthcare Cost of Infectious Waterborne Disease in the United States. Emerg. Infect. Dis. 27, 140–149. 10.3201/eid2701.190676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell RR, 2000. Bacterial Death Revisited, in: Colwell RR, Grimes DJ (Eds.), Nonculturable Microorganisms in the Environment. Springer US, Boston, MA, pp. 325–342. 10.1007/978-1-4757-0271-2_18 [DOI] [Google Scholar]
- Correia AM, Ferreira JS, Borges V, Nunes A, Gomes B, Capucho R, Gonçalves J, Antunes DM, Almeida S, Mendes A, Guerreiro M, Sampaio DA, Vieira L, Machado J, Simões MJ, Gonçalves P, Gomes JP, 2016. Probable Person-to-Person Transmission of Legionnaires’ Disease. N. Engl. J. Med. 374, 497–498. 10.1056/NEJMc1505356 [DOI] [PubMed] [Google Scholar]
- Corso PS, Kramer MH, Blair KA, Addiss DG, Davis JP, Haddix AC, 2003. Cost of illness in the 1993 waterborne Cryptosporidium outbreak, Milwaukee, Wisconsin. Emerg. Infect. Dis. 9, 426–431. 10.3201/eid0904.020417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couturier J, Ginevra C, Nesa D, Adam M, Gouot C, Descours G, Campèse C, Battipaglia G, Brissot E, Beraud L, Ranc A-G, Jarraud S, Barbut F, 2020. Transmission of Legionnaires’ Disease through Toilet Flushing. Emerg. Infect. Dis. 26, 1526–1528. 10.3201/eid2607.190941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CSTE, 2019. Council of State and Territorial Epidemiologists. Position Statement Title: Revision to the Case Definition for National Legionellosis Surveillance. Position statement 19-ID-04. [Google Scholar]
- CSTE, 2017. Council of State and Territorial Epidemiologists. Operational Guidance for Position Statement 17 ID 07: Standardized Case Definition for Extrapulmonary Nontuberculous Mycobacteria Infections. 5. [Google Scholar]
- CSTE, 2010. Council of State and Territorial Epidemiologists. Title: Public Health Reporting and National Notification for Legionellosis, Position statement 09-ID-45.
- CSTE, 2005. Council of State and Territorial Epidemiologists. Position Statement Title: Strengthening surveillance for travel-associated legionellosis and revised case definitions for legionellosis. Position statement 05-ID-01. [Google Scholar]
- Dai D, Proctor CR, Williams K, Edwards MA, Pruden A, 2018. Mediation of effects of biofiltration on bacterial regrowth, Legionella pneumophila, and the microbial community structure under hot water plumbing conditions. Environ. Sci. Water Res. Technol. 4, 183–194. 10.1039/C7EW00301C [DOI] [Google Scholar]
- Daley C, Salfinger M, 2016. Has the Time Come to Require Nontuberculous Mycobacteria Reporting?
- Dani A, 2014. Colonization and infection. Cent. Eur. J. Urol. 67, 86–87. 10.5173/ceju.2014.01.art19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danila RN, Koranteng N, Como-Sabetti KJ, Robinson TJ, Laine ES, 2018. Hospital Water Management Programs for Legionella Prevention, Minnesota, 2017. Infect. Control Hosp. Epidemiol. 39, 336–338. 10.1017/ice.2017.310 [DOI] [PubMed] [Google Scholar]
- Darelid J, Löfgren S, Malmvall B-E, 2002. Control of nosocomial Legionnaires’ disease by keeping the circulating hot water temperature above 55 degrees C: experience from a 10-year surveillance programme in a district general hospital. J. Hosp. Infect. 50, 213–219. 10.1053/jhin.2002.1185 [DOI] [PubMed] [Google Scholar]
- De Giglio O, Napoli C, Lovero G, Diella G, Rutigliano S, Caggiano G, Montagna MT, 2015. Antibiotic susceptibility of Legionella pneumophila strains isolated from hospital water systems in Southern Italy. Environ. Res. 142, 586–590. 10.1016/j.envres.2015.08.013 [DOI] [PubMed] [Google Scholar]
- De Keuckelaere A, Jacxsens L, Amoah P, Medema G, McClure P, Jaykus L-A, Uyttendaele M, 2015. Zero Risk Does Not Exist: Lessons Learned from Microbial Risk Assessment Related to Use of Water and Safety of Fresh Produce. Compr. Rev. Food Sci. Food Saf. 14, 387–410. 10.1111/1541-4337.12140 [DOI] [Google Scholar]
- Dean K, Mitchell J, 2020a. A dose response model for the inhalation route of exposure to P. aeruginosa. Microb. Risk Anal. 15, 100115. 10.1016/j.mran.2020.100115 [DOI] [Google Scholar]
- Dean K, Mitchell J, 2020b. Reverse QMRA for Pseudomonas aeruginosa in Premise Plumbing to Inform Risk Management. J. Environ. Eng. 146, 04019120. 10.1061/(ASCE)EE.1943-7870.0001641 [DOI] [Google Scholar]
- Dean K, Weir MH, Mitchell J, 2019. Development of a dose-response model for Naegleria fowleri. J. Water Health 17, 63–71. 10.2166/wh.2018.181 [DOI] [PubMed] [Google Scholar]
- Declerck P, Behets J, Delaedt Y, Margineanu A, Lammertyn E, Ollevier F, 2005. Impact of non-Legionella bacteria on the uptake and intracellular replication of Legionella pneumophila in Acanthamoeba castellanii and Naegleria lovaniensis. Microb. Ecol. 50, 536–549. 10.1007/s00248-005-0258-0 [DOI] [PubMed] [Google Scholar]
- Delafont V, Rodier M-H, Maisonneuve E, Cateau E, 2018. Vermamoeba vermiformis: a Free-Living Amoeba of Interest. Microb. Ecol. 76, 991–1001. 10.1007/s00248-018-1199-8 [DOI] [PubMed] [Google Scholar]
- Detsky AS, Naglie G, Krahn MD, Redelmeier DA, Naimark D, 1997. Primer on medical decision analysis: Part 2--Building a tree. Med. Decis. Mak. Int. J. Soc. Med. Decis. Mak. 17, 126–135. 10.1177/0272989X9701700202 [DOI] [PubMed] [Google Scholar]
- Dey R, Mount H, Ensminger AW, Tyrrell GJ, Ward LP, Ashbolt NJ, 2019a. Isolation of Legionella pneumophila by Co-culture with Local Ameba, Canada. Emerg. Infect. Dis. J. - CDC 25. 10.3201/eid2511.190522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey R, Rieger AM, Stephens C, Ashbolt NJ, 2019b. Interactions of Pseudomonas aeruginosa with Acanthamoeba polyphaga Observed by Imaging Flow Cytometry. Cytom. Part J. Int. Soc. Anal. Cytol. 95, 555–564. 10.1002/cyto.a.23768 [DOI] [PubMed] [Google Scholar]
- Dietersdorfer E, Kirschner A, Schrammel B, Ohradanova-Repic A, Stockinger H, Sommer R, Walochnik J, Cervero-Aragó S, 2018. Starved viable but non-culturable (VBNC) Legionella strains can infect and replicate in amoebae and human macrophages. Water Res. 141, 428–438. 10.1016/j.watres.2018.01.058 [DOI] [PubMed] [Google Scholar]
- Donohue MJ, 2018. Increasing nontuberculous mycobacteria reporting rates and species diversity identified in clinical laboratory reports. BMC Infect. Dis. 18, 163. 10.1186/s12879-018-3043-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue MJ, Vesper S, Mistry J, Donohue JM, 2019. Impact of Chlorine and Chloramine on the Detection and Quantification of Legionella pneumophila and Mycobacterium Species. Appl. Environ. Microbiol. 85. 10.1128/AEM.01942-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopp E, Richard J, Dwidjosiswojo Z, Simon A, Wingender J, 2017. Influence of the copper-induced viable but non-culturable state on the toxicity of Pseudomonas aeruginosa towards human bronchial epithelial cells in vitro. Int. J. Hyg. Environ. Health 220, 1363–1369. 10.1016/j.ijheh.2017.09.007 [DOI] [PubMed] [Google Scholar]
- Douterelo I, Sharpe RL, Husband S, Fish KE, Boxall JB, 2019. Understanding microbial ecology to improve management of drinking water distribution systems. WIREs Water 6, e01325. 10.1002/wat2.1325 [DOI] [Google Scholar]
- Dowdell K, Haig S-J, Caverly LJ, Shen Y, LiPuma JJ, Raskin L, 2019. Nontuberculous mycobacteria in drinking water systems - the challenges of characterization and risk mitigation. Curr. Opin. Biotechnol. 57, 127–136. 10.1016/j.copbio.2019.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda S, Kandiah S, Stout JE, Baron JL, Yassin M, Fabrizio M, Ferrelli J, Hariri R, Wagener MM, Goepfert J, Bond J, Hannigan J, Rogers D, 2014. Evaluation of a new monochloramine generation system for controlling Legionella in building hot water systems. Infect. Control Hosp. Epidemiol. 35, 1356–1363. 10.1086/678418 [DOI] [PubMed] [Google Scholar]
- Dwidjosiswojo Z, Richard J, Moritz MM, Dopp E, Flemming H-C, Wingender J, 2011. Influence of copper ions on the viability and cytotoxicity of Pseudomonas aeruginosa under conditions relevant to drinking water environments. Int. J. Hyg. Environ. Health 214, 485–492. 10.1016/j.ijheh.2011.06.004 [DOI] [PubMed] [Google Scholar]
- Elguindi J, Wagner J, Rensing C, 2009. Genes involved in copper resistance influence survival of Pseudomonas aeruginosa on copper surfaces. J. Appl. Microbiol. 106, 1448–1455. 10.1111/j.1365-2672.2009.04148.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdogan H, Can F, Demirbilek M, Timurkaynak F, Arslan H, 2010. In vitro activity of antimicrobial agents against Legionella isolated from environmental water systems: first results from Turkey. Environ. Monit. Assess. 171, 487–491. 10.1007/s10661-009-1293-y [DOI] [PubMed] [Google Scholar]
- Falkinham JO, 2018. Mycobacterium avium complex: Adherence as a way of life. AIMS Microbiol. 4, 428–438. 10.3934/microbiol.2018.3.428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkinham JO, 2009. Surrounded by mycobacteria: nontuberculous mycobacteria in the human environment. J. Appl. Microbiol. 107, 356–367. 10.1111/j.1365-2672.2009.04161.x [DOI] [PubMed] [Google Scholar]
- Falkinham JO, Hilborn ED, Arduino MJ, Pruden A, Edwards MA, 2015. Epidemiology and Ecology of Opportunistic Premise Plumbing Pathogens: Legionella pneumophila, Mycobacterium avium, and Pseudomonas aeruginosa. Environ. Health Perspect. 123, 749–758. 10.1289/ehp.1408692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhat M, Trouilhé M-C, Briand E, Moletta-Denat M, Robine E, Frère J, 2010. Development of a pilot-scale 1 for Legionella elimination in biofilm in hot water network: heat shock treatment evaluation. J. Appl. Microbiol. 108, 1073–1082. 10.1111/j.1365-2672.2009.04541.x [DOI] [PubMed] [Google Scholar]
- Field SK, Fisher D, Cowie RL, 2004. Mycobacterium avium complex pulmonary disease in patients without HIV infection. Chest 126, 566–581. 10.1378/chest.126.2.566 [DOI] [PubMed] [Google Scholar]
- Fisher RA, Gollan B, Helaine S, 2017. Persistent bacterial infections and persister cells. Nat. Rev. Microbiol. 15, 453–464. 10.1038/nrmicro.2017.42 [DOI] [PubMed] [Google Scholar]
- Florida Health, 2016. Section 5 Antimicrobial Resistance Surveillance.
- Gebert MJ, Delgado-Baquerizo M, Oliverio AM, Webster TM, Nichols LM, Honda JR, Chan ED, Adjemian J, Dunn RR, Fierer N, 2018. Ecological Analyses of Mycobacteria in Showerhead Biofilms and Their Relevance to Human Health. mBio 9. 10.1128/mBio.01614-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gião MS, Wilks SA, Keevil CW, 2015. Influence of copper surfaces on biofilm formation by Legionella pneumophila in potable water. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 28, 329–339. 10.1007/s10534-015-9835-y [DOI] [PubMed] [Google Scholar]
- Giovanardi R, Bononi M, Messori M, Bargellini A, Paduano S, Borella P, Marchesi I, 2020. Corrosion resistance of commonly used plumbing materials for water distribution systems exposed to disinfection treatments. Corros. Eng. Sci. Technol. 55, 224–231. 10.1080/1478422X.2020.1721806 [DOI] [Google Scholar]
- Gómez-Lus R, Adrián F, del Campo R, Gómez-Lus P, Sánchez S, García C, Rubio MC, 2001. Comparative in vitro bacteriostatic and bactericidal activity of trovafloxacin, levofloxacin and moxifloxacin against clinical and environmental isolates of Legionella spp. Int. J. Antimicrob. Agents 18, 49–54. 10.1016/s0924-8579(01)00339-9 [DOI] [PubMed] [Google Scholar]
- Gostin LO, Lazzarini Z, Neslund VS, Osterholm MT, 2000. Water quality laws and waterborne diseases: Cryptosporidium and other emerging pathogens. Am. J. Public Health 90, 847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ, Winthrop K, ATS Mycobacterial Diseases Subcommittee, American Thoracic Society, Infectious Disease Society of America, 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 175, 367–416. 10.1164/rccm.200604-571ST [DOI] [PubMed] [Google Scholar]
- Grobe S, Wingender J, Flemming HC, 2001. Capability of mucoid Pseudomonas aeruginosa to survive in chlorinated water. Int. J. Hyg. Environ. Health 204, 139–142. 10.1078/1438-4639-00085 [DOI] [PubMed] [Google Scholar]
- Guerrieri E, Bondi M, Sabia C, de Niederhäusern S, Borella P, Messi P, 2008. Effect of bacterial interference on biofilm development by Legionella pneumophila. Curr. Microbiol. 57, 532–536. 10.1007/s00284-008-9237-2 [DOI] [PubMed] [Google Scholar]
- Haas CN, 1996. Acceptable Microbial Risk. J. AWWA 88, 8–8. 10.1002/j.1551-8833.1996.tb06652.x [DOI] [Google Scholar]
- Haas CN, Rose JB, Gerba CP, 2014. Quantitative Microbial Risk Assessment, 2nd edition. ed. John Wiley & Sons, Inc. [Google Scholar]
- Hageskal G, Lima N, Skaar I, 2009. The study of fungi in drinking water. Mycol. Res. 113, 165–172. 10.1016/j.mycres.2008.10.002 [DOI] [PubMed] [Google Scholar]
- Haig S-J, Kotlarz N, Kalikin LM, Chen T, Guikema S, LiPuma JJ, Raskin L, 2020. Emerging investigator series: bacterial opportunistic pathogen gene markers in municipal drinking water are associated with distribution system and household plumbing characteristics. Environ. Sci. Water Res. Technol. 6, 3032–3043. 10.1039/D0EW00723D [DOI] [Google Scholar]
- Haig S-J, Kotlarz N, LiPuma JJ, Raskin L, 2018. A High-Throughput Approach for Identification of Nontuberculous Mycobacteria in Drinking Water Reveals Relationship between Water Age and Mycobacterium avium. mBio 9. 10.1128/mBio.02354-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton KA, Ahmed W, Toze S, Haas CN, 2017. Human health risks for Legionella and Mycobacterium avium complex (MAC) from potable and non-potable uses of roof-harvested rainwater. Water Res. 119, 288–303. 10.1016/j.watres.2017.04.004 [DOI] [PubMed] [Google Scholar]
- Hamilton KA, Haas CN, 2016. Critical review of mathematical approaches for quantitative microbial risk assessment (QMRA) of Legionella in engineered water systems: research gaps and a new framework. Environ. Sci. Water Res. Technol. 2, 599–613. 10.1039/C6EW00023A [DOI] [Google Scholar]
- Hamilton KA, Hamilton MT, Johnson W, Jjemba P, Bukhari Z, LeChevallier M, Haas CN, Gurian PL, 2019. Risk-Based Critical Concentrations of Legionella pneumophila for Indoor Residential Water Uses. Environ. Sci. Technol. 53, 4528–4541. 10.1021/acs.est.8b03000 [DOI] [PubMed] [Google Scholar]
- Hancock RE, 1997. The bacterial outer membrane as a drug barrier. Trends Microbiol. 5, 37–42. 10.1016/S0966-842X(97)81773-8 [DOI] [PubMed] [Google Scholar]
- Hancock REW, Speert DP, 2000. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and impact on treatment. Drug Resist. Updat. Rev. Comment. Antimicrob. Anticancer Chemother. 3, 247–255. 10.1054/drup.2000.0152 [DOI] [PubMed] [Google Scholar]
- Haworth CS, Banks J, Capstick T, Fisher AJ, Gorsuch T, Laurenson IF, Leitch A, Loebinger MR, Milburn HJ, Nightingale M, Ormerod P, Shingadia D, Smith D, Whitehead N, Wilson R, Floto RA, 2017. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax 72, ii1–ii64. 10.1136/thoraxjnl-2017-210927 [DOI] [PubMed] [Google Scholar]
- Huang H-I, Shih H-Y, Lee C-M, Yang TC, Lay J-J, Lin YE, 2008. In vitro efficacy of copper and silver ions in eradicating Pseudomonas aeruginosa, Stenotrophomonas maltophilia and Acinetobacter baumannii: implications for on-site disinfection for hospital infection control. Water Res. 42, 73–80. 10.1016/j.watres.2007.07.003 [DOI] [PubMed] [Google Scholar]
- Hull NM, Ling F, Pinto AJ, Albertsen M, Jang HG, Hong P-Y, Konstantinidis KT, LeChevallier M, Colwell RR, Liu W-T, 2019. Drinking Water Microbiome Project: Is it Time? Trends Microbiol. 27, 670–677. 10.1016/j.tim.2019.03.011 [DOI] [PubMed] [Google Scholar]
- Hunter RB, 1940. Methods of estimating loads in plumbing systems. US Dep. Commer. Natl. Bur. Stand. [Google Scholar]
- Inkinen J, Kaunisto T, Pursiainen A, Miettinen IT, Kusnetsov J, Riihinen K, Keinänen-Toivola MM, 2014. Drinking water quality and formation of biofilms in an office building during its first year of operation, a full scale study. Water Res. 49, 83–91. 10.1016/j.watres.2013.11.013 [DOI] [PubMed] [Google Scholar]
- Institute of Medicine, 2009. Institute of Medicine (US) Roundtable on Environmental Health Sciences, Research, and Medicine. Environmental Health Sciences Decision Making: Risk Management, Evidence, and Ethics - Workshop Summary, The National Academies Collection: Reports funded by National Institutes of Health. National Academies Press (US), Washington (DC). [PubMed] [Google Scholar]
- ISO, 2011. ISO 22196:2011. International Organization for Standardization. Measurement of antibacterial activity on plastics and other non-porous surfaces. Reviewed 2016. [WWW Document]. ISO. URL https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/05/44/54431.html (accessed 4.27.21). [Google Scholar]
- Ji P, Rhoads WJ, Edwards MA, Pruden A, 2018. Effect of heat shock on hot water plumbing microbiota and Legionella pneumophila control. Microbiome 6, 30. 10.1186/s40168-018-0406-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji P, Rhoads WJ, Edwards MA, Pruden A, 2017. Impact of water heater temperature setting and water use frequency on the building plumbing microbiome. ISME J. 11, 1318–1330. 10.1038/ismej.2017.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MM, Odell JA, 2014. Nontuberculous mycobacterial pulmonary infections. J. Thorac. Dis. 6, 210–220. 10.3978/j.issn.2072-1439.2013.12.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien R, Dreelin E, Whelton AJ, Lee J, Aw TG, Dean K, Mitchell J, 2020a. Knowledge gaps and risks associated with premise plumbing drinking water quality. AWWA Water Sci. 2, e1177. 10.1002/aws2.1177 [DOI] [Google Scholar]
- Julien R, Dreelin E, Whelton AJ, Lee J, Aw TG, Dean K, Mitchell J, 2020b. Knowledge gaps and risks associated with premise plumbing drinking water quality. AWWA Water Sci. 2, e1177. 10.1002/aws2.1177 [DOI] [Google Scholar]
- June SG, Dziewulski DM, 2018. Copper and Silver Biocidal Mechanisms, Resistance Strategies, and Efficacy for Legionella Control. J. AWWA 110, E13–E35. 10.1002/awwa.1144 [DOI] [Google Scholar]
- Kartalija M, Ovrutsky AR, Bryan CL, Pott GB, Fantuzzi G, Thomas J, Strand MJ, Bai X, Ramamoorthy P, Rothman MS, Nagabhushanam V, McDermott M, Levin AR, Frazer-Abel A, Giclas PC, Korner J, Iseman MD, Shapiro L, Chan ED, 2013. Patients with nontuberculous mycobacterial lung disease exhibit unique body and immune phenotypes. Am. J. Respir. Crit. Care Med. 187, 197–205. 10.1164/rccm.201206-1035OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Tateda K, Ishii Y, Horikawa M, Miyairi S, Gotoh N, Ishiguro M, Yamaguchi K, 2009. Pseudomonas aeruginosa Las quorum sensing autoinducer suppresses growth and biofilm production in Legionella species. Microbiol. Read. Engl. 155, 1934–1939. 10.1099/mic.0.026641-0 [DOI] [PubMed] [Google Scholar]
- Kotlarz N, Rockey N, Olson TM, Haig S-J, Sanford L, LiPuma JJ, Raskin L, 2018. Biofilms in Full-Scale Drinking Water Ozone Contactors Contribute Viable Bacteria to Ozonated Water. Environ. Sci. Technol. 52, 2618–2628. 10.1021/acs.est.7b04212 [DOI] [PubMed] [Google Scholar]
- Kotsanas D, Brett J, Kidd TJ, Stuart RL, Korman TM, 2008. Disinfection of Burkholderia cepacia complex from non-touch taps in a neonatal nursery. J. Perinat. Med. 36, 235–239. 10.1515/JPM.2008.038 [DOI] [PubMed] [Google Scholar]
- Kusnetsov J, Iivanainen E, Elomaa N, Zacheus O, Martikainen PJ, 2001. Copper and silver ions more effective against legionellae than against mycobacteria in a hospital warm water system. Water Res. 35, 4217–4225. 10.1016/s0043-1354(01)00124-5 [DOI] [PubMed] [Google Scholar]
- Lalancette C, Charron D, Laferrière C, Dolcé P, Déziel E, Prévost M, Bédard E, 2017. Hospital Drains as Reservoirs of Pseudomonas aeruginosa: Multiple-Locus Variable-Number of Tandem Repeats Analysis Genotypes Recovered from Faucets, Sink Surfaces and Patients. Pathog. Basel Switz. 6. 10.3390/pathogens6030036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMorte WW, 2016. Step 3: Establish a Case Definition; Identify Cases [WWW Document]. Boston Univ. Sch. Public Health. URL https://sphweb.bumc.bu.edu/otlt/mph-modules/ph/outbreak/Outbreak4.html (accessed 4.27.21). [Google Scholar]
- Lande L, Alexander DC, Wallace RJ, Kwait R, Iakhiaeva E, Williams M, Cameron ADS, Olshefsky S, Devon R, Vasireddy R, Peterson DD, Falkinham JO, 2019. Mycobacterium avium in Community and Household Water, Suburban Philadelphia, Pennsylvania, USA, 2010–2012. Emerg. Infect. Dis. 25, 473–481. 10.3201/eid2503.180336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landeen LK, Yahya MT, Gerba CP, 1989. Efficacy of copper and silver ions and reduced levels of free chlorine in inactivation of Legionella pneumophila. Appl. Environ. Microbiol. 55, 3045–3050. 10.1128/AEM.55.12.3045-3050.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenschlager K, Boon N, Wang Y, Egli T, Hammes F, 2010. Overnight stagnation of drinking water in household taps induces microbial growth and changes in community composition. Water Res. 44, 4868–4877. 10.1016/j.watres.2010.07.032 [DOI] [PubMed] [Google Scholar]
- Learbuch KLG, Lut MC, Liu G, Smidt H, van der Wielen PWJJ, 2019. Legionella growth potential of drinking water produced by a reverse osmosis pilot plant. Water Res. 157, 55–63. 10.1016/j.watres.2019.03.037 [DOI] [PubMed] [Google Scholar]
- Lewis AGJ, Lasche EM, Armstrong AL, Dunbar FP, 1960. A clinical study of the chronic lung disease due to nonphotochromogenic acid-fast bacilli. Ann. Intern. Med. 53, 273–285. 10.7326/0003-4819-53-2-273 [DOI] [PubMed] [Google Scholar]
- Lewis AH, Falkinham JO, 2015. Microaerobic growth and anaerobic survival of Mycobacterium avium, Mycobacterium intracellulare and Mycobacterium scrofulaceum. Int. J. Mycobacteriology 4, 25–30. 10.1016/j.ijmyco.2014.11.066 [DOI] [PubMed] [Google Scholar]
- Li L, Mendis N, Trigui H, Oliver JD, Faucher SP, 2014. The importance of the viable but non-culturable state in human bacterial pathogens. Front. Microbiol. 5, 258. 10.3389/fmicb.2014.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim K-Y, Jiang SC, 2013. Reevaluation of health risk benchmark for sustainable water practice through risk analysis of rooftop-harvested rainwater. Water Res. 47, 7273–7286. 10.1016/j.watres.2013.09.059 [DOI] [PubMed] [Google Scholar]
- Lin YE, Stout JE, Yu VL, 2011. Controlling Legionella in hospital drinking water: an evidence-based review of disinfection methods. Infect. Control Hosp. Epidemiol. 32, 166–173. 10.1086/657934 [DOI] [PubMed] [Google Scholar]
- Lin Y-SE, Vidic RD, Stout JE, Yu VL, 2002. Negative effect of high pH on biocidal efficacy of copper and silver ions in controlling Legionella pneumophila. Appl. Environ. Microbiol. 68, 2711–2715. 10.1128/aem.68.6.2711-2715.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y-SE, Vidic RD, Stout JE, Yu VL, 1996. Individual and combined effects of copper and silver ions on inactivation of Legionella pneumophila. Water Res. 30, 1905–1913. 10.1016/0043-1354(96)00077-2 [DOI] [Google Scholar]
- Ling F, Whitaker R, LeChevallier MW, Liu W-T, 2018. Drinking water microbiome assembly induced by water stagnation. ISME J. 12, 1520–1531. 10.1038/s41396-018-0101-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Stout JE, Tedesco L, Boldin M, Hwang C, Diven WF, Yu VL, 1994. Controlled evaluation of copper-silver ionization in eradicating Legionella pneumophila from a hospital water distribution system. J. Infect. Dis. 169, 919–922. 10.1093/infdis/169.4.919 [DOI] [PubMed] [Google Scholar]
- Livni G, Yaniv I, Samra Z, Kaufman L, Solter E, Ashkenazi S, Levy I, 2008. Outbreak of Mycobacterium mucogenicum bacteraemia due to contaminated water supply in a paediatric haematology-oncology department. J. Hosp. Infect. 70, 253–258. 10.1016/j.jhin.2008.07.016 [DOI] [PubMed] [Google Scholar]
- Lv L, Jiang T, Zhang S, Yu X, 2014. Exposure to mutagenic disinfection byproducts leads to increase of antibiotic resistance in Pseudomonas aeruginosa. Environ. Sci. Technol. 48, 8188–8195. 10.1021/es501646n [DOI] [PubMed] [Google Scholar]
- Lytle D, Frietch C, Covert T, 2004. Electrophoretic Mobility of Mycobacterium avium Complex Organisms. Appl. Environ. Microbiol. 70, 5667–5671. 10.1128/AEM.70.9.5667-5671.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytle DA, Pfaller S, Muhlen C, Struewing I, Triantafyllidou S, White C, Hayes S, King D, Lu J, 2021. A comprehensive evaluation of monochloramine disinfection on water quality, Legionella and other important microorganisms in a hospital. Water Res. 189, 116656. 10.1016/j.watres.2020.116656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi I, Ferranti G, Mansi A, Marcelloni AM, Proietto AR, Saini N, Borella P, Bargellini A, 2016. Control of Legionella Contamination and Risk of Corrosion in Hospital Water Networks following Various Disinfection Procedures. Appl. Environ. Microbiol. 82, 2959–2965. 10.1128/AEM.03873-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marras TK, Chedore P, Ying AM, Jamieson F, 2007. Isolation prevalence of pulmonary non-tuberculous mycobacteria in Ontario, 1997 2003. Thorax 62, 661–666. 10.1136/thx.2006.070797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters S, Clancy JL, Villegas S, LeChevallier MW, Bukhari Z, 2018. Customer Messaging on Opportunistic Pathogens in Plumbing Systems. The Water Research Foundation. Water Res Found Proj #4664. [Google Scholar]
- McTigue N, Symons JM, 2010. The Water Dictionary: A Comprehensive Reference of Water Terminology, Second Edition. AWWA-American Water Works Association. [Google Scholar]
- Mercante JW, Winchell JM, 2015. Current and emerging Legionella diagnostics for laboratory and outbreak investigations. Clin. Microbiol. Rev. 28, 95–133. 10.1128/CMR.00029-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkal RS, Crawford JA, 1979. Heat inactivation of Mycobacterium avium-Mycobacterium intracellulare complex organisms in aqueous suspension. Appl. Environ. Microbiol. 38, 827–830. 10.1128/AEM.38.5.827-830.1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnesota Department of Health, 2019a. Reporting free-living amebic infection (including at least: Acanthamoeba spp., Naegleria fowleri, Balamuthia spp., Sappinia spp) - Minnesota Dept. of Health [WWW Document]. URL https://www.health.state.mn.us/diseases/reportable/flamebic.html (accessed 4.28.21).
- Minnesota Department of Health, 2019b. Carbapenem Resistant Pseudomonas aeruginosa (CRPA) Information for Health Professionals [WWW Document]. URL https://www.health.state.mn.us/diseases/crpa/hcp.html (accessed 4.28.21).
- Mitchell-Blackwood J, Gurian PL, O’Donnell C, 2011. Finding Risk-Based Switchover Points for Response Decisions for Environmental Exposure to Bacillus anthracis. Hum. Ecol. Risk Assess. Int. J. 17, 489–509. 10.1080/10807039.2011.552401 [DOI] [Google Scholar]
- Moore MR, Pryor M, Fields B, Lucas C, Phelan M, Besser RE, 2006. Introduction of monochloramine into a municipal water system: impact on colonization of buildings by Legionella spp. Appl. Environ. Microbiol. 72, 378–383. 10.1128/AEM.72.1.378-383.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NASEM, 2019. National Academies of Sciences and Engineering. Management of Legionella in Water Systems. National Academies Press (US), Washington (DC). 10.17226/25474 [DOI] [PubMed] [Google Scholar]
- National Research Council, 2004. National Research Council (US) Committee on Indicators for Waterborne Pathogens. Indicators for Waterborne Pathogens - PubMed. 10.17226/11010 [DOI] [Google Scholar]
- National Research Council, 1990. Valuing Health Risks, Costs, and Benefits for Environmental Decision Making: Report of a Conference. National Academies Press (US), Washington (DC). [PubMed] [Google Scholar]
- Nguyen C, Elfland C, Edwards M, 2012. Impact of advanced water conservation features and new copper pipe on rapid chloramine decay and microbial regrowth. Water Res. 46, 611–621. 10.1016/j.watres.2011.11.006 [DOI] [PubMed] [Google Scholar]
- Novak Babič M, Gunde-Cimerman N, Vargha M, Tischner Z, Magyar D, Veríssimo C, Sabino R, Viegas C, Meyer W, Brandão J, 2017. Fungal Contaminants in Drinking Water Regulation? A Tale of Ecology, Exposure, Purification and Clinical Relevance. Int. J. Environ. Res. Public. Health 14. 10.3390/ijerph14060636 [DOI] [Google Scholar]
- Office of Inspector General, 2013. Healthcare Inspection: Legioinnaires’ Disease at the VA Pittsburgh Healthcare System, Pittsburgh, Pennsylvania. VA Office of Inspector General, Washington (DC). [Google Scholar]
- Oregon Health Authority, 2018. Oregon Health Authority : Nontuberculous Mycobacterial Disease (NTM) – Extrapulmonary : Diseases A to Z : State of Oregon [WWW Document]. URL https://www.oregon.gov/oha/PH/DISEASESCONDITIONS/DISEASESAZ/Pages/nontuberculosis-mycobacterial-disease.aspx (accessed 4.28.21).
- Palmer KL, Brown SA, Whiteley M, 2007. Membrane-bound nitrate reductase is required for anaerobic growth in cystic fibrosis sputum. J. Bacteriol. 189, 4449–4455. 10.1128/JB.00162-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranjape K, Bédard É, Whyte LG, Ronholm J, Prévost M, Faucher SP, 2020. Presence of Legionella spp. in cooling towers: the role of microbial diversity, Pseudomonas, and continuous chlorine application. Water Res. 169, 115252. 10.1016/j.watres.2019.115252 [DOI] [PubMed] [Google Scholar]
- Parker BC, Ford MA, Gruft H, Falkinham JO, 1983. Epidemiology of infection by nontuberculous mycobacteria. IV. Preferential aerosolization of Mycobacterium intracellulare from natural waters. Am. Rev. Respir. Dis. 128, 652–656. 10.1164/arrd.1983.128.4.652 [DOI] [PubMed] [Google Scholar]
- Pedro-Botet ML, Stout JE, Yu VL, 2002. Legionnaires’ disease contracted from patient homes: the coming of the third plague? Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 21, 699–705. 10.1007/s10096-002-0813-2 [DOI] [PubMed] [Google Scholar]
- Perkins KM, Reddy SC, Fagan R, Arduino MJ, Perz JF, 2019. Investigation of healthcare infection risks from water-related organisms: Summary of CDC consultations, 2014–2017. Infect. Control Hosp. Epidemiol. 40, 621–626. 10.1017/ice.2019.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper KJ, Foster H, Susanto D, Maree CL, Thornton SD, Cobbs CS, 2018. Fatal Balamuthia mandrillaris brain infection associated with improper nasal lavage. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 77, 18–22. 10.1016/j.ijid.2018.09.013 [DOI] [PubMed] [Google Scholar]
- Pirofski L, Casadevall A, 2012. Q and A: What is a pathogen? A question that begs the point. BMC Biol. 10, 6. 10.1186/1741-7007-10-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts K, Wall M, 2002. Managing the commissioning of building services. Eng. Constr. Archit. Manag. 9, 336–344. 10.1046/j.1365-232X.2002.00246.x [DOI] [Google Scholar]
- Prevots DR, Shaw PA, Strickland D, Jackson LA, Raebel MA, Blosky MA, Montes de Oca R, Shea YR, Seitz AE, Holland SM, Olivier KN, 2010. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am. J. Respir. Crit. Care Med. 182, 970–976. 10.1164/rccm.201002-0310OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor CR, Dai D, Edwards MA, Pruden A, 2017. Interactive effects of temperature, organic carbon, and pipe material on microbiota composition and Legionella pneumophila in hot water plumbing systems. Microbiome 5, 130. 10.1186/s40168-017-0348-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor CR, Gächter M, Kötzsch S, Rölli F, Sigrist R, Walser J-C, Hammes F, 2016. Biofilms in shower hoses – choice of pipe material influences bacterial growth and communities. Environ. Sci. Water Res. Technol. 2, 670–682. 10.1039/C6EW00016A [DOI] [Google Scholar]
- Proctor CR, Hammes F, 2015a. Drinking water microbiology--from measurement to management. Curr. Opin. Biotechnol. 33, 87–94. 10.1016/j.copbio.2014.12.014 [DOI] [PubMed] [Google Scholar]
- Proctor CR, Hammes F, 2015b. Drinking water microbiology--from measurement to management. Curr. Opin. Biotechnol. 33, 87–94. 10.1016/j.copbio.2014.12.014 [DOI] [PubMed] [Google Scholar]
- Proctor CR, Reimann M, Vriens B, Hammes F, 2018. Biofilms in shower hoses. Water Res. 131, 274–286. 10.1016/j.watres.2017.12.027 [DOI] [PubMed] [Google Scholar]
- Proctor CR, Rhoads WJ, Keane T, Salehi M, Hamilton K, Pieper KJ, Cwiertny DM, Prévost M, Whelton AJ, 2020. Considerations for Large Building Water Quality after Extended Stagnation. AWWA Water Sci. e1186. 10.1002/aws2.1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provoost J, Valour F, Gamondes D, Roux S, Freymond N, Perrot E, Souquet P-J, Kiakouama-Maleka L, Chidiac C, Lina G, Dumitrescu O, Sénéchal A, Ader F, 2018. A retrospective study of factors associated with treatment decision for nontuberculous mycobacterial lung disease in adults without altered systemic immunity. BMC Infect. Dis. 18, 659. 10.1186/s12879-018-3559-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prussin AJ, Schwake DO, Marr LC, 2017. Ten Questions Concerning the Aerosolization and Transmission of Legionella in the Built Environment. Build. Environ. 123, 684–695. 10.1016/j.buildenv.2017.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor M, Springthorpe S, Riffard S, Brooks T, Huo Y, Davis G, Sattar SA, 2004. Investigation of opportunistic pathogens in municipal drinking water under different supply and treatment regimes. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 50, 83–90. [PubMed] [Google Scholar]
- Rasheduzzaman M, Singh R, Haas CN, Tolofari D, Yassaghi H, Hamilton KA, Yang Z, Gurian PL, 2019. Reverse QMRA as a decision support tool: Setting acceptable concentration limits for Pseudomonas aeruginosa and Naegleria fowleri. Water Switz. 11, 1850. 10.3390/w11091850 [DOI] [Google Scholar]
- Rastogi N, Frehel C, Ryter A, Ohayon H, Lesourd M, David HL, 1981. Multiple drug resistance in Mycobacterium avium: is the wall architecture responsible for exclusion of antimicrobial agents? Antimicrob. Agents Chemother. 20, 666–677. 10.1128/aac.20.5.666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed RP, Cooke-Yarborough CM, Jaquiery AL, Grimwood K, Kemp AS, Su JC, Forsyth JR, 1997. Fatal granulomatous amoebic encephalitis caused by Balamuthia mandrillaris. Med. J. Aust. 167, 82–84. 10.5694/j.1326-5377.1997.tb138785.x [DOI] [PubMed] [Google Scholar]
- Regli S, Rose JB, Haas CN, Gerba CP, 1991. Modeling the Risk From Giardia and Viruses in Drinking Water. J. AWWA 83, 76–84. 10.1002/j.1551-8833.1991.tb07252.x [DOI] [Google Scholar]
- Revetta RP, Gomez-Alvarez V, Gerke TL, Curioso C, Santo Domingo JW, Ashbolt NJ, 2013. Establishment and early succession of bacterial communities in monochloramine-treated drinking water biofilms. FEMS Microbiol. Ecol. 86, 404–414. 10.1111/1574-6941.12170 [DOI] [PubMed] [Google Scholar]
- Rhoads WJ, Garner E, Ji P, Zhu N, Parks J, Schwake DO, Pruden A, Edwards MA, 2017a. Distribution System Operational Deficiencies Coincide with Reported Legionnaires’ Disease Clusters in Flint, Michigan. Environ. Sci. Technol. 51, 11986–11995. 10.1021/acs.est.7b01589 [DOI] [PubMed] [Google Scholar]
- Rhoads WJ, Hammes F, 2021. Growth of Legionella during COVID-19 lockdown stagnation. Environ. Sci. Water Res. Technol. 7, 10–15. 10.1039/D0EW00819B [DOI] [Google Scholar]
- Rhoads WJ, Ji P, Pruden A, Edwards MA, 2015. Water heater temperature set point and water use patterns influence Legionella pneumophila and associated microorganisms at the tap. Microbiome 3, 67. 10.1186/s40168-015-0134-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads WJ, Pruden A, Edwards MA, 2017b. Interactive Effects of Corrosion, Copper, and Chloramines on Legionella and Mycobacteria in Hot Water Plumbing. Environ. Sci. Technol. 51, 7065–7075. 10.1021/acs.est.6b05616 [DOI] [PubMed] [Google Scholar]
- Rhoads WJ, Pruden A, Edwards MA, 2016. Survey of green building water systems reveals elevated water age and water quality concerns. Environ. Sci. Water Res. Technol. 2, 164–173. 10.1039/C5EW00221D [DOI] [Google Scholar]
- Rhoads WJ, Pruden A, Edwards MA, 2014. Anticipating challenges with in-building disinfection for control of opportunistic pathogens. Water Environ. Res. Res. Publ. Water Environ. Fed. 86, 540–549. [DOI] [PubMed] [Google Scholar]
- Rice G, Wright JM, Boutin B, Swartout J, Rodgers P, Niemuth N, Broder M, 2005. Estimating the frequency of tap-water exposures to Mycobacterium avium complex in the U.S. population with advanced AIDS. J. Toxicol. Environ. Health A 68, 1033–1047. 10.1080/15287390590912630 [DOI] [PubMed] [Google Scholar]
- Rice KC, Bayles KW, 2008. Molecular control of bacterial death and lysis. Microbiol. Mol. Biol. Rev. MMBR 72, 85–109, table of contents. 10.1128/MMBR.00030-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers MR, Blackstone BJ, Reyes AL, Covert TC, 1999. Colonisation of point of use water filters by silver resistant non-tuberculous mycobacteria. J. Clin. Pathol. 52, 629. 10.1136/jcp.52.8.629a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Dowsett AB, Dennis PJ, Lee JV, Keevil CW, 1994. Influence of Plumbing Materials on Biofilm Formation and Growth of Legionella pneumophila in Potable Water Systems. Appl. Environ. Microbiol. 60, 1842–1851. 10.1128/AEM.60.6.1842-1851.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabrià M, Pedro-Botet ML, Gómez J, Roig J, Vilaseca B, Sopena N, Baños V, Legionnaires Disease Therapy Group, 2005. Fluoroquinolones vs macrolides in the treatment of Legionnaires disease. Chest 128, 1401–1405. 10.1378/chest.128.3.1401 [DOI] [PubMed] [Google Scholar]
- Salehi M, Odimayomi T, Ra K, Ley C, Julien R, Nejadhashemi AP, Hernandez-Suarez JS, Mitchell J, Shah AD, Whelton A, 2020. An investigation of spatial and temporal drinking water quality variation in green residential plumbing. Build. Environ. 169, 106566. 10.1016/j.buildenv.2019.106566 [DOI] [Google Scholar]
- Samba-Louaka A, Delafont V, Rodier M-H, Cateau E, Héchard Y, 2019. Free-living amoebae and squatters in the wild: ecological and molecular features. FEMS Microbiol. Rev. 43, 415–434. 10.1093/femsre/fuz011 [DOI] [PubMed] [Google Scholar]
- Sandalakis V, Chochlakis D, Goniotakis I, Tselentis Y, Psaroulaki A, 2014. Minimum inhibitory concentration distribution in environmental Legionella spp. isolates. J. Water Health 12, 678–685. 10.2166/wh.2014.217 [DOI] [PubMed] [Google Scholar]
- Scaturro M, Losardo M, De Ponte G, Ricci ML, 2005. Comparison of three molecular methods used for subtyping of Legionella pneumophila strains isolated during an epidemic of Legionellosis in Rome. J. Clin. Microbiol. 43, 5348–5350. 10.1128/JCM.43.10.5348-4350.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SDWA, 1996. The Safe Drinking Water Act. 42 USC §§300f-300j-26.
- Sell JJ, Rupp FW, Orrison WW, 1997. Granulomatous amebic encephalitis caused by acanthamoeba. Neuroradiology 39, 434–436. 10.1007/s002340050440 [DOI] [PubMed] [Google Scholar]
- Shadoud L, Almahmoud I, Jarraud S, Etienne J, Larrat S, Schwebel C, Timsit J-F, Schneider D, Maurin M, 2015. Hidden Selection of Bacterial Resistance to Fluoroquinolones In Vivo: The Case of Legionella pneumophila and Humans. EBioMedicine 2, 1179–1185. 10.1016/j.ebiom.2015.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen M, Ashbolt NJ, 2021. Differential Bacterial Predation by Free-Living Amoebae May Result in Blooms of Legionella in Drinking Water Systems. Microorganisms 9, 174. 10.3390/microorganisms9010174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen M, Ashbolt NJ, 2018. Free-Living Amoebae Supporting Intracellular Growth May Produce Vesicle-Bound Respirable Doses of Legionella Within Drinking Water Systems. Expo. Health 10, 201–209. 10.1007/s12403-017-0255-9 [DOI] [Google Scholar]
- Sharaby Y, Rodríguez-Martínez S, Oks O, Pecellin M, Mizrahi H, Peretz A, Brettar I, Höfle MG, Halpern M, 2017. Temperature-Dependent Growth Modeling of Environmental and Clinical Legionella pneumophila Multilocus Variable-Number Tandem-Repeat Analysis (MLVA) Genotypes. Appl. Environ. Microbiol. 83. 10.1128/AEM.03295-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signor RS, Ashbolt NJ, 2009. Comparing probabilistic microbial risk assessments for drinking water against daily rather than annualised infection probability targets. J. Water Health 7, 535–543. 10.2166/wh.2009.101 [DOI] [PubMed] [Google Scholar]
- Sikora A, Gładysz I, Kozioł-Montewka M, Wójtowicz-Bobin M, Stańczak T, Matuszewska R, Krogulska B, 2017. Assessment of antibiotic susceptibility of Legionella pneumophila isolated from water systems in Poland. Ann. Agric. Environ. Med. AAEM 24, 66–69. 10.5604/12321966.1234048 [DOI] [PubMed] [Google Scholar]
- Simões LC, Simões M, Oliveira R, Vieira MJ, 2007. Potential of the adhesion of bacteria isolated from drinking water to materials. J. Basic Microbiol. 47, 174–183. 10.1002/jobm.200610224 [DOI] [PubMed] [Google Scholar]
- Sinclair M, O’Toole J, Gibney K, Leder K, 2015. Evolution of regulatory targets for drinking water quality. J. Water Health 13, 413–426. 10.2166/wh.2014.242 [DOI] [PubMed] [Google Scholar]
- Singh R, Hamilton KA, Rasheduzzaman M, Yang Z, Kar S, Fasnacht A, Masters SV, Gurian PL, 2020. Managing Water Quality in Premise Plumbing: Subject Matter Experts’ Perspectives and a Systematic Review of Guidance Documents. Water 12, 347. 10.3390/w12020347 [DOI] [Google Scholar]
- Smith DJ, Ramsay KA, Yerkovich ST, Reid DW, Wainwright CE, Grimwood K, Bell SC, Kidd TJ, 2016. Pseudomonas aeruginosa antibiotic resistance in Australian cystic fibrosis centres. Respirol. Carlton Vic 21, 329–337. 10.1111/resp.12714 [DOI] [PubMed] [Google Scholar]
- Spagnolo AM, Orlando P, Perdelli F, Cristina ML, 2016. Hospital water and prevention of waterborne infections. Rev. Med. Microbiol. 27, 25–32. 10.1097/MRM.0000000000000060 [DOI] [Google Scholar]
- Steed KA, Falkinham JO, 2006. Effect of growth in biofilms on chlorine susceptibility of Mycobacterium avium and Mycobacterium intracellulare. Appl. Environ. Microbiol. 72, 4007–4011. 10.1128/AEM.02573-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CR, Muthye V, Cianciotto NP, 2012. Legionella pneumophila persists within biofilms formed by Klebsiella pneumoniae, Flavobacterium sp., and Pseudomonas fluorescens under dynamic flow conditions. PloS One 7, e50560. 10.1371/journal.pone.0050560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stormer RS, Falkinham JO, 1989. Differences in antimicrobial susceptibility of pigmented and unpigmented colonial variants of Mycobacterium avium. J. Clin. Microbiol. 27, 2459–2465. 10.1128/JCM.27.11.2459-2465.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout JE, Yu VL, Muraca P, Joly J, Troup N, Tompkins LS, 1992. Potable water as a cause of sporadic cases of community-acquired legionnaires’ disease. N. Engl. J. Med. 326, 151–155. 10.1056/NEJM199201163260302 [DOI] [PubMed] [Google Scholar]
- Strollo SE, Adjemian J, Adjemian MK, Prevots DR, 2015. The Burden of Pulmonary Nontuberculous Mycobacterial Disease in the United States. Ann. Am. Thorac. Soc. 12, 1458–1464. 10.1513/AnnalsATS.201503-173OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydnor ERM, Bova G, Gimburg A, Cosgrove SE, Perl TM, Maragakis LL, 2012. Electronic-eye faucets: Legionella species contamination in healthcare settings. Infect. Control Hosp. Epidemiol. 33, 235–240. 10.1086/664047 [DOI] [PubMed] [Google Scholar]
- Taylor RH, Falkinham JO, Norton CD, LeChevallier MW, 2000. Chlorine, chloramine, chlorine dioxide, and ozone susceptibility of Mycobacterium avium. Appl. Environ. Microbiol. 66, 1702–1705. 10.1128/aem.66.4.1702-1705.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temmerman R, Vervaeren H, Noseda B, Boon N, Verstraete W, 2006. Necrotrophic growth of Legionella pneumophila. Appl. Environ. Microbiol. 72, 4323–4328. 10.1128/AEM.00070-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JM, Ashbolt NJ, 2011. Do free-living amoebae in treated drinking water systems present an emerging health risk? Environ. Sci. Technol. 45, 860–869. 10.1021/es102876y [DOI] [PubMed] [Google Scholar]
- Thomas V, McDonnell G, Denyer SP, Maillard J-Y, 2010. Free-living amoebae and their intracellular pathogenic microorganisms: risks for water quality. FEMS Microbiol. Rev. 34, 231–259. 10.1111/j.1574-6976.2009.00190.x [DOI] [PubMed] [Google Scholar]
- Tortoli E, 2014. Microbiological features and clinical relevance of new species of the genus Mycobacterium. Clin. Microbiol. Rev. 27, 727–752. 10.1128/CMR.00035-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tossa P, Deloge-Abarkan M, Zmirou-Navier D, Hartemann P, Mathieu L, 2006. Pontiac fever: an operational definition for epidemiological studies. BMC Public Health 6, 112. 10.1186/1471-2458-6-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totaro M, Valentini P, Costa AL, Giorgi S, Casini B, Baggiani A, 2018. Rate of Legionella pneumophila colonization in hospital hot water network after time flow taps installation. J. Hosp. Infect. 98, 60–63. 10.1016/j.jhin.2017.08.021 [DOI] [PubMed] [Google Scholar]
- US EPA, 2015. National Primary Drinking Water Regulations [WWW Document]. US EPA. URL https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations (accessed 4.28.21). [Google Scholar]
- van der Kooij D, 1992. Assimilable Organic Carbon as an Indicator of Bacterial Regrowth. J. Am. Water Works Assoc. 84, 57–65. [Google Scholar]
- van der Kooij D, Bakker GL, Italiaander R, Veenendaal HR, Wullings BA, 2017. Biofilm Composition and Threshold Concentration for Growth of Legionella pneumophila on Surfaces Exposed to Flowing Warm Tap Water without Disinfectant. Appl. Environ. Microbiol. 83. 10.1128/AEM.02737-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooij D, Oranje JP, Hijnen WA, 1982. Growth of Pseudomonas aeruginosa in tap water in relation to utilization of substrates at concentrations of a few micrograms per liter. Appl. Environ. Microbiol. 44, 1086–1095. 10.1128/AEM.44.5.1086-1095.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lugt W, Euser SM, Bruin JP, den Boer JW, Yzerman EPF, 2019. Wide-scale study of 206 buildings in the Netherlands from 2011 to 2015 to determine the effect of drinking water management plans on the presence of Legionella spp. Water Res. 161, 581–589. 10.1016/j.watres.2019.06.043 [DOI] [PubMed] [Google Scholar]
- van Ingen J, Boeree MJ, van Soolingen D, Mouton JW, 2012. Resistance mechanisms and drug susceptibility testing of nontuberculous mycobacteria. Drug Resist. Updat. Rev. Comment. Antimicrob. Anticancer Chemother. 15, 149–161. 10.1016/j.drup.2012.04.001 [DOI] [PubMed] [Google Scholar]
- Van Kenhove E, Backer LD, Janssens A, Laverge J, 2019a. Simulation of Legionella concentration in domestic hot water: comparison of pipe and boiler models. J. Build. Perform. Simul. 12, 595–619. 10.1080/19401493.2019.1583286 [DOI] [Google Scholar]
- Van Kenhove E, Dinne K, Janssens A, Laverge J, 2019b. Overview and comparison of Legionella regulations worldwide. Am. J. Infect. Control 47, 968–978. 10.1016/j.ajic.2018.10.006 [DOI] [PubMed] [Google Scholar]
- van Lier A, McDonald SA, Bouwknegt M, EPI group, Kretzschmar ME, Havelaar AH, Mangen M-JJ, Wallinga J, de Melker HE, 2016. Disease Burden of 32 Infectious Diseases in the Netherlands, 2007–2011. PloS One 11, e0153106. 10.1371/journal.pone.0153106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oss CJ, Gillman C, Neumann AW, 1975. Phagocytic Engulfment and Cell Adhesiveness as Cellular Surface Phenomena. Marcel Dekker, Inc, New York. [Google Scholar]
- Vaz-Moreira I, Nunes OC, Manaia CM, 2012. Diversity and antibiotic resistance in Pseudomonas spp. from drinking water. Sci. Total Environ. 426, 366–374. 10.1016/j.scitotenv.2012.03.046 [DOI] [PubMed] [Google Scholar]
- VHA, 2021. Veterans Health Administration (VHA) Directive 1061. Prevention of Healthcare-Associated Legionella Disease and Scald Injury from Water Systems.
- VHA, 2014. Veterans Health Administration (VHA) Directive 1061. Prevention of Healthcare-Associated Legionella Disease and Scald Injury from Potable Water Distribution Systems.
- Walker J, Moore G, 2015. Pseudomonas aeruginosa in hospital water systems: biofilms, guidelines, and practicalities. J. Hosp. Infect. 89, 324–327. 10.1016/j.jhin.2014.11.019 [DOI] [PubMed] [Google Scholar]
- Walters MS, Grass JE, Bulens SN, Hancock EB, Phipps EC, Muleta D, Mounsey J, Kainer MA, Concannon C, Dumyati G, Bower C, Jacob J, Cassidy PM, Beldavs Z, Culbreath K, Phillips WE, Hardy DJ, Vargas RL, Oethinger M, Ansari U, Stanton R, Albrecht V, Halpin AL, Karlsson M, Rasheed JK, Kallen A, 2019. Carbapenem-Resistant Pseudomonas aeruginosa at US Emerging Infections Program Sites, 2015. Emerg. Infect. Dis. 25, 1281–1288. 10.3201/eid2507.181200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Li J, Ji J, Yang L, Chen L, Zhou R, Yang Y, Zheng H, Yuan J, Li L, Bi Y, Gao GF, Ma J, Liu Y, 2018. A case of Naegleria fowleri related primary amoebic meningoencephalitis in China diagnosed by next-generation sequencing. BMC Infect. Dis. 18, 349. 10.1186/s12879-018-3261-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- WateReuse, 2016. Glossary - WateReuse Association. WateReuse Assoc. Engage Educ. Advocate. URL https://watereuse.org/educate/water-reuse-101/glossary/ (accessed 4.28.21). [Google Scholar]
- WHO, 2017. Water Sanitation and Health. Water Quality Guidelines. 4th ed. Chapter 3 - Inactivation [WWW Document]. URL https://www.who.int/water_sanitation_health/water-quality/guidelines/en/watreatpath3.pdf [Google Scholar]
- WHO, 2015. WHO. Global health risks [WWW Document]. WHO. URL https://www.who.int/healthinfo/global_burden_disease/global_health_risks/en/ (accessed 4.28.21). [Google Scholar]
- WHO-Europe, 2019. 23 million people falling ill from unsafe food each year in Europe is just the tip of the iceberg [WWW Document]. URL https://www.euro.who.int/en/media-centre/sections/press-releases/2019/23-million-people-falling-ill-from-unsafe-food-each-year-in-europe-is-just-the-tip-of-the-iceberg (accessed 4.28.21).
- Wilson RE, Hill RLR, Chalker VJ, Mentasti M, Ready D, 2018. Antibiotic susceptibility of Legionella pneumophila strains isolated in England and Wales 2007–17. J. Antimicrob. Chemother. 73, 2757–2761. 10.1093/jac/dky253 [DOI] [PubMed] [Google Scholar]
- Winthrop KL, Henkle E, Walker A, Cassidy M, Hedberg K, Schafer S, 2017. On the Reportability of Nontuberculous Mycobacterial Disease to Public Health Authorities. Ann. Am. Thorac. Soc. 14, 314–317. 10.1513/AnnalsATS.201610-802PS [DOI] [PubMed] [Google Scholar]
- Wisconsin Department of Health Services, 2018. Communicable Disease Case Reporting and Investigation Protocol: Free-Living Ameba Infections [WWW Document]. Wis. Dep. Health Serv. URL https://www.dhs.wisconsin.gov/library/p-02253.htm (accessed 4.28.21). [Google Scholar]
- World Health Organization, 2016. Quantitative Microbial Risk Assessment: Application for Water Safety Management. World Health Organization. [Google Scholar]
- Wu C-C, Ghosh S, Martin KJ, Pinto AJ, Denef VJ, Olson TM, Love NG, 2017. The microbial colonization of activated carbon block point-of-use (PoU) filters with and without chlorinated phenol disinfection by-products. Environ. Sci. Water Res. Technol. 3, 830–843. 10.1039/C7EW00134G [DOI] [Google Scholar]
- Wu M-L, Aziz DB, Dartois V, Dick T, 2018. NTM drug discovery: status, gaps and the way forward. Drug Discov. Today 23, 1502–1519. 10.1016/j.drudis.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, LeJeune J, Alsdorf D, Lu B, Shum CK, Liang S, 2012. Global distribution of outbreaks of water-associated infectious diseases. PLoS Negl. Trop. Dis. 6, e1483. 10.1371/journal.pntd.0001483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu VL, Greenberg RN, Zadeikis N, Stout JE, Khashab MM, Olson WH, Tennenberg AM, 2004. Levofloxacin efficacy in the treatment of community-acquired legionellosis. Chest 125, 2135–2139. 10.1378/chest.125.6.2135 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Love N, Edwards M, 2009. Nitrification in Drinking Water Systems. Crit. Rev. Environ. Sci. Technol. 39, 153–208. 10.1080/10643380701631739 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.