Abstract
Nucleic acid (NA)-containing damage- and pathogen-associated molecular patterns (DAMPs and PAMPs, respectively) are implicated in numerous pathological conditions from infectious diseases to autoimmune disorders. Nucleic acid-binding polymers, including polyamidoamine (PAMAM) dendrimers, have demonstrated anti-inflammatory properties when administered to neutralize DAMPs/PAMPs. The PAMAM G3 variant has been shown to have beneficial effects in a cutaneous lupus erythematosus (CLE) murine model and improve survival of mice challenged with influenza. Unfortunately, the narrow therapeutic window of cationic PAMAM dendrimers makes their clinical development challenging. An alternative nucleic acid-binding polymer that has been evaluated in humans is a linear β-cyclodextrin-containing polymer (CDP). CDP’s characteristics prompted us to evaluate its anti-inflammatory potential in CLE autoimmune and influenza infectious disease mouse models. We report that CDP effectively inhibits NA-containing DAMP-mediated activation of Toll-like receptors (TLRs) in cell culture, improves healing in lupus mice, and does not immunocompromise treated animals upon influenza infection but improves survival even when administered 3 days after infection. Finally, as anticipated, we observe limited toxicity in animals treated with CDP compared with PAMAM G3. Thus, CDP is a new anti-inflammatory agent that may be readily translated to the clinic to combat diseases associated with pathological NA-containing DAMPs/PAMPs.
Keywords: nucleic acid binding polymer, DAMPs and PAMPs, influenza, lupus, pattern recognition receptors
Graphical abstract
Damage- and pathogen-associated molecular patterns (DAMPs and PAMPs, respectively) are implicated in numerous pathologies from infectious diseases to autoimmune disorders. We report that a β-cyclodextrin-containing polymer (CDP) inhibits DAMP/PAMP activation of innate immune receptors and improves healing in lupus mice and survival following influenza challenge with minimal toxicity. CDP is a new anti-inflammatory agent.
Introduction
Influenza infection remains one of the most common and clinically important respiratory infections in the world. Recent global estimates indicate that between 290,000 and 645,000 deaths occur annually as a result of seasonal influenza.1 Unchecked acute inflammation that does not resolve remains one of the hallmarks of severe influenza infection, and recent attention has turned to developing approaches to effectively control such pathological inflammation. Many recent studies have highlighted the importance of damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) as key mediators of such inflammation and associated pathobiology (reviewed in Gong et al.2 and Newton et al.3). Because several DAMPs and PAMPs are RNA or DNA or molecules bound to nucleic acids (e.g., histones or HMGB1), many groups, including our own, have started to explore the ability of nucleic acid-binding polymers to capture and neutralize proinflammatory nucleic acid-containing DAMPs and PAMPs in vivo.4 Because these nucleic acid-binding polymers, including polyamidoamine (PAMAM) dendrimers, have been used previously for delivery of small interfering RNA (siRNA) and other charged molecules for therapeutic applications,4,5 much has been determined regarding the manufacturing, pharmacology, and toxicology of such compounds.
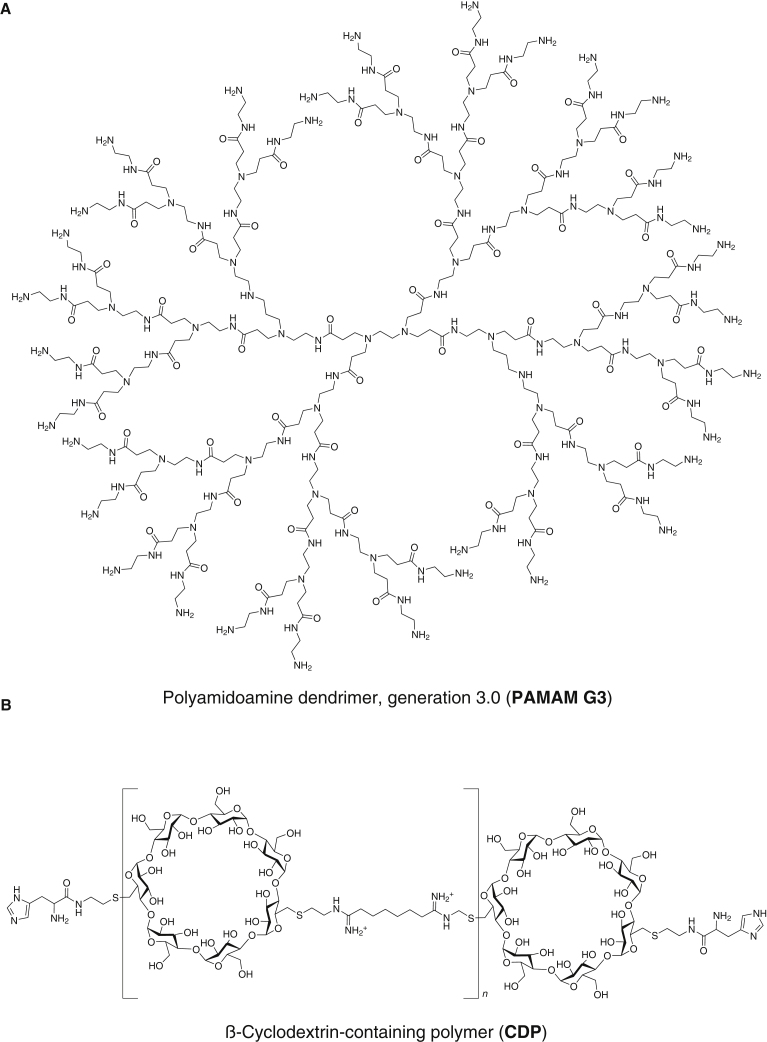
Many variants of PAMAM have been synthesized, and some have been shown to have anti-inflammatory properties when administered to capture nucleic acids in vivo.4,6, 7, 8 Specifically, the amine-terminated cationic generation 3 variant, designated PAMAM G3 (Figure 1A), has been shown to inhibit nucleic acid-containing DAMP-mediated activation of Toll-like receptors (TLRs) in cell culture assays and reduce inflammation and associated pathologies in in vivo models of inflammatory shock and cancer metastasis.4,9, 10, 11 These anti-inflammatory properties appear to be due to binding and sequestration of nucleic acids or nucleic acid-containing DAMPs/PAMPs, which can activate TLRs or other pattern recognition receptors (PRRs). Thus, moderation of the innate immune response and any associated detrimental inflammation may assist in treatment of several conditions.
Figure 1.
Polymer chemical structures
(A) PAMAM dendrimer, amine-terminated cationic generation 3 variant (PAMAM G3). (B) Imidazole-terminated linear β-cyclodextrin-containing polymer (CDP).
Previously, we observed that PAMAM G3 treatment has beneficial effects during (1) acute inflammation following influenza infection, a setting where RNA-based PAMPs are thought to be key drivers of inflammation, as well as (2) chronic inflammation associated with autoimmune diseases such as lupus, a setting where DNA-based DAMPs are strong drivers. We used two complementary disease models to assess the potential broad utility of PAMAM G3 treatment. Utilizing a tape stripping model that mimics cutaneous lupus erythematosus (CLE), CLE-prone mice treated locally with PAMAM G3 recovered better from skin damage than control-treated animals.7 We then assessed whether PAMAM G3 treatment in lupus-prone mice suppressed the adaptive immune response to viral challenge and discovered that the recovery of mice infected with PR8 influenza improved with such treatment. In further studies, standard C57BL/6J mice infected with a lethal dose of influenza and treated with PAMAM G3 showed decreased weight loss and increased survival compared with control mice.7 These observations indicate that regulating the innate immune response by modulating the activation of TLRs does not necessarily compromise an animal’s overall immune response and can improve recovery from infection. Moreover, they suggest that such treatment may prove beneficial for treating diseases associated with acute and chronic inflammation. However, two major caveats of that study must be addressed to determine whether this strategy may actually be beneficial for limiting pathological inflammation and its consequences following viral infection or to combat autoimmunity in affected individuals. First, the nucleic acid scavenger PAMAM G3 was present coincident with influenza infection; it is unclear whether this approach can be therapeutically useful when therapy is initiated after infection. Second, the known cytotoxicity and consequent narrow therapeutic window of PAMAM G3 limits its potential usefulness as a treatment (reviewed in Janaszewska et al.12), particularly in severely ill virus-infected individuals or at high local doses in the skin in those with CLE. Here we attempt to address these two issues to determine whether a polymer-based DAMP/PAMP scavenger strategy has potential merit for translation into the clinic.
An alternative nucleic acid-binding polymer to PAMAM G3 is a linear β-cyclodextrin-containing polymer (CDP; Figure 1B).13, 14, 15 This imidazole-terminated CDP is the first polymer designed specifically to bind to nucleic acids and have low toxicity in vitro and in vivo.13, 14, 15 CDP has been assessed extensively for toxicity and is well tolerated in mice, non-human primates, and humans.16 Furthermore, because CDP has been used as a component of a nanoparticle delivery system of siRNA for human therapeutic use,14,17,18 it has been synthesized under good manufacturing practice conditions at clinical scale. Here we evaluate CDP’s ability to serve as a nucleic acid DAMP/PAMP scavenger that limits pathological inflammation when delivered locally following cutaneous injury of lupus-prone mice and systemically when administered coincident with or several days after influenza challenge of mice.
Results
CDP inhibits TLR activation by DNA and RNA agonists
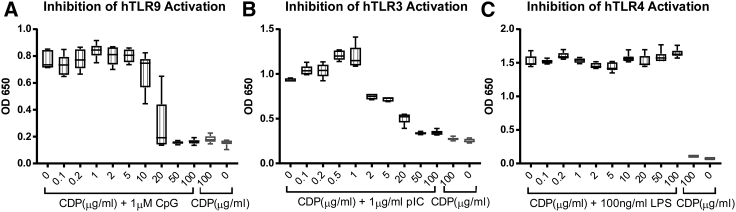
We first assessed the ability of CDP in a dose-dependent manner to inhibit TLR activation in cell culture. HEK-Blue TLR reporter cells are useful tools for assessing interactions of inhibitory molecules upon the activation of different TLRs by their respective agonists. As shown by the data in Figure 2, CDP can block activation of human TLR9 (hTLR9) and hTLR3 by their prototypic DNA and RNA agonists CpG and poly(I:C), respectively. Dose-dependent inhibition can be seen when these cells are incubated with such nucleic acid-based activators and increasing concentrations of CDP, achieving a half maximal inhibitory concentration (IC50) of 26.6 μg/mL in hTLR9 and 11.6 μg/mL in hTLR3 and limiting activation to background levels at doses over 50 μg/mL. In contrast, CDP did not inhibit activation of hTLR4 by the non-nucleic acid ligand lipopolysaccharide (LPS) at these concentrations. Moreover, addition of CDP without an agonist had no effect on signaling in these assays and did not affect cell viability. Therefore, CDP is able to selectively inhibit activation of nucleic acid-sensing TLRs by RNA and DNA ligands in cell culture in a dose-dependent manner.
Figure 2.
CDP effectively inhibits hTLR9 and hTLR3 activation by nucleic acids in a cell culture reporter assay
(A) HEK-Blue hTLR9 cells were incubated with or without the TLR9 agonist CpG ODN2006 (1μM) and increasing concentrations of CDP. CDP effectively inhibits TLR9 activation with an IC50 value of 26.6 μg/mL, returning to baseline when added at 50 μg/mL and above. (B) HEK-Blue hTLR3 cells were incubated with or without the TLR3 agonist high-molecular-weight (HMW) poly(I:C) (1 μg/mL) and increasing concentrations of CDP. CDP effectively inhibits TLR3 activation with an IC50 value of 11.6 μg/mL, returning to baseline at 20 μg/mL and above. (C) HEK-Blue hTLR4 cells were incubated with or without the TLR4 agonist lipopolysaccharide (LPS; 100 ng/mL) and increasing concentrations of CDP. CDP did not affect LPS activation of hTLR4. Whiskers represent max and min of replicates.
Local delivery of CDP assists recovery following cutaneous injury in lupus mice
Next, we sought to determine whether local administration of CDP to animals would be well tolerated and counteracts nucleic acid DAMP-mediated inflammation. Therefore, we evaluated CDP in the aforementioned murine autoimmune model of dermal injury and subsequent inflammation that mimics human CLE. Lupus-prone new zealand black white hybrid model (NZBW F1) mice have high levels of antinuclear antibodies and pro-inflammatory cytokines and develop hyperinflamed cutaneous lesions following skin injury by tape stripping. Previously, we observed that injured mice treated with subcutaneous (s.c.) injection of PAMAM G3 (20 mg/kg) healed more effectively than mice treated with saline.7 However, at higher doses of PAMAM G3, we often observed injection site-associated dermal toxicity in treated animals (L.K., L.B.O., and R.E.R., unpublished data). Therefore, we wanted to test whether local CDP treatment has beneficial effects on wound healing and inflammation in this dermal damage-induced autoimmune model.
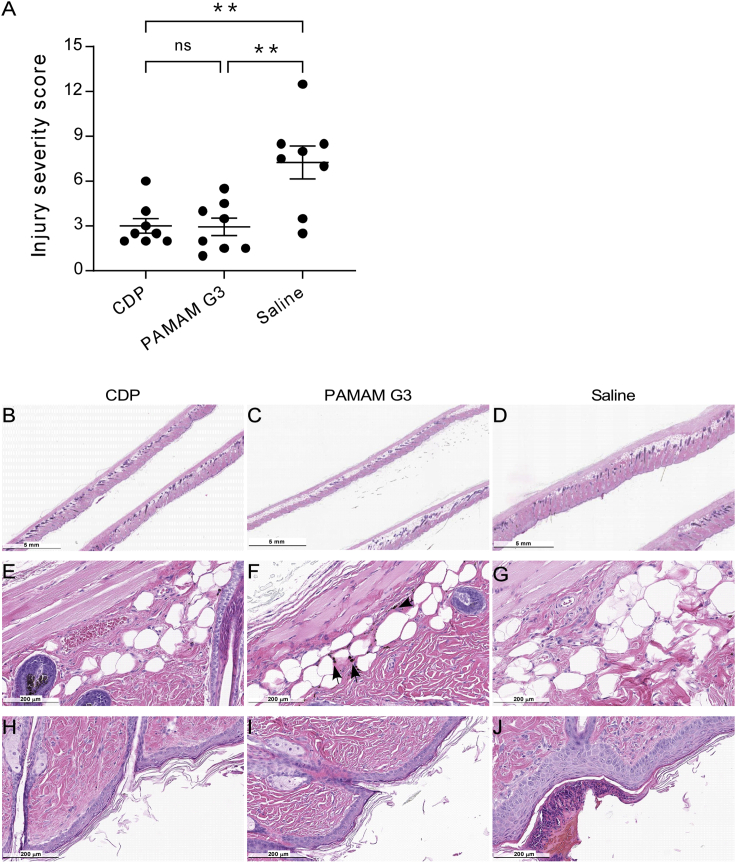
To test this possibility, skin lesions were formed on the backs of NZBW F1 mice by tape stripping (Figure S1S). These mice were then treated every 3 days after injury with sterile saline, PAMAM G3 (20 mg/kg) in saline, or CDP (20 mg/kg) in saline by s.c. injection. As shown in Figure 3A, animals that received CDP or PAMAM G3 had significantly lower injury severity scores than saline-treated mice on day 14 after injury, as scored by a blinded pathologist. Overall skin lesion healing was improved similarly in mice treated with either nucleic acid-binding polymer, as indicated by reduced thickening of the skin by H&E histopathology of the damaged and then healed region of skin (Figures 3B–3D). Reduced panicular inflammation was observed as well as reduced immune cell infiltration compared with saline-treated mice (Figures 3E–3G). PAMAM G3 and CDP-treated mice also had reduced thickness of the epidermis and fewer ulcerations than mice treated with saline (Figures 3H–3J). In PAMAM G3-treated mice, dark inclusion bodies were observed in the s.c. space of many of the sections, possibly representing polymer aggregation (Figure 3F). Such inclusion bodies were not seen in mice treated with CDP (Figure 3E), and no dose-limiting toxicity was observed. Thus, locally delivered CDP treatment improves wound healing by reducing pathological inflammation in this murine model of cutaneous autoimmunity.
Figure 3.
Recovery from skin injury in a murine model of CLE is improved with nucleic acid-containing DAMP scavenger treatment
(A) Animals that received CDP and PAMAM G3 had a significantly lower score in injury severity. See Materials and methods for a description of scoring. Data represents mean ± standard error of the mean, ns represents a p ≥ 0.05, ∗∗ represents a p ≤ 0.01. (B–D) Recovery of skin lesions was improved in mice treated with (B) CDP and (C) PAMAM G3 compared with (D) saline, as evident by reduced thickening of the skin. (E–G) At higher magnification, reduction of panicular inflammation is observed with reduced immune cell infiltration in (E) CDP- and (F) PAMAM G3- compared with (G) saline-treated mice. (H–J) Fewer ulcerations and reduced pathologic thickening of the epidermis was observed in (H) CDP- and (I) PAMAM G3-treated mice than mice treated with (J) saline. Note the inclusion bodies found in PAMAM G3-treated mice (arrows, F), which do not appear in CDP- or saline-treated animals (E and G, respectively). Representative clinical photos of mice injury during treatment are shown in Figure S1.
Systemic CDP treatment improves outcomes in mice challenged with influenza
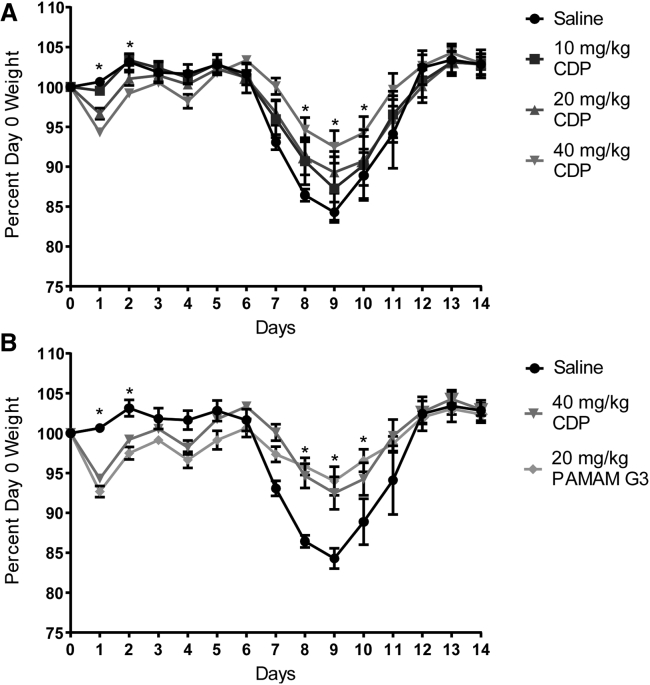
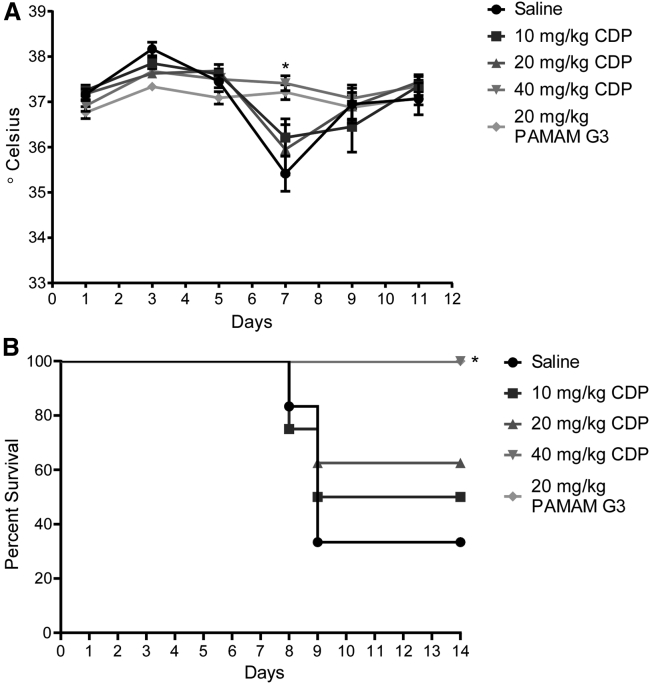
Because CDP appears to be well tolerated and effective at limiting local inflammation, we next sought to determine whether systemic delivery would limit morbidity and mortality when treated animals are challenged with a proinflammatory respiratory virus. To examine the potential benefits of CDP in an in vivo model, we utilized the well-characterized mouse-adapted influenza A H1N1 strain A/Puerto Rico/8/34 (PR8).19 Mice were challenged intranasally at the mouse lethal dose 50 (LD50; measured as a weight loss of more than 15%) and saline or CDP administered by intraperitoneal (i.p.) injection starting at the time of infection to determine whether the presence and persistence of circulating CDP would improve survival and recovery. Mice were treated with saline or CDP at 10, 20 or 40 mg/kg every 3 days for a total of four dosings (day 0, 3, 6, and 9). Mice were monitored for body weight over the course of the 14-day study (Figure 4). Improvement over saline treatment was observed with all doses of CDP, but weight loss was reduced significantly in animals treated with 40 mg/kg CDP (Figure 4A). Concurrently, a group of mice were treated with PAMAM G3 as a positive control at 20 mg/kg;7 PAMAM G3 at 40 mg/kg is not well tolerated (L.B.O. and R.E.R., unpublished data). As shown in Figure 4B, when we directly compared the CDP (40 mg/kg) treatment group with the PAMAM G3-positive control group (20 mg/kg), similar maintenance of body weight throughout the study was achieved by both nucleic acid-containing DAMP/PAMP scavengers in influenza-challenged animals.
Figure 4.
Treatment with CDP is beneficial for maintaining weight and recovery of PR8-infected mice
(A) Mice treated with 10, 20, or 40 mg/kg CDP compared with weights of the saline control group, represented as a percentage of their day 0 weight. (B) Mice treated with 20 mg/kg PAMAM G3 compared with the 40 mg/kg CDP and saline control groups from above. A comparison of mice treated with 40 mg/kg CDP and 20 mg/kg PAMAM shows comparable maintenance of weight throughout the study. A two-way mixed ANOVA was run to determine the effect of PAMAM G3 and doses of CDP versus saline over time on weight following influenza infection over time. Data in graphs are mean ± standard error of the mean; n = 8. There was a statistically significant interaction between treatment and time on weight; F (56, 450) = 3.189, p < 0.0001. Post hoc analysis via Tukey’s multiple comparisons test revealed that CDP treated mice at 40 mg/kg were significantly heavier than saline-treated mice on day 8 (mean difference (diff), 1.66 g; p = 0.046), day 9 (mean diff, 1.86 g; p = 0.021), and day 10 (mean diff, 1.84 g; p = 0.048) after infection. Similarly, Tukey’s multiple comparisons test revealed that PAMAM G3-treated mice at 20 mg/kg were significantly heavier than saline-treated mice on day 9 (mean diff, 2.18 g; p = 0.010) and day 10 (mean diff, 2.24 g; p = 0.034) after infection. An asterisk represents significant difference between one or more of the treatment groups compared with saline treatment.
Another metric to assess disease and recovery from viral infection is maintenance of body temperature after PR8 challenge. Unlike humans, mice do not develop a fever but become hypothermic when infected with influenza. As shown by Figure 5A, infected mice treated with saline had a drastic dip in body temperature by day 7 after infection. Treatment with CDP (40 mg/kg) prevented this body temperature change (Figure 5A). Similarly, survival, as defined by weight maintained above 85% of the starting weight, showed improvement with treatment (Figure 5B). Mice treated with all doses of CDP trended toward increased survival compared with the saline group, with CDP (40 mg/kg) treatment significantly maintaining body weight and improving survival, similar to control PAMAM G3-treated animals.
Figure 5.
Treatment with CDP improved maintenance of body temperature and survival based on a 15% loss of body weight metric
(A) Temperature as measured by rectal probe over the course of treatment. A two-way mixed ANOVA was run to determine the effect of PAMAM G3 and several doses of CDP versus saline over time on temperature following influenza infection over time. Data in the graph are mean ± standard error of the mean. There was a statistically significant interaction between treatment and time on temperature; F (20, 163) = 3.997, p < 0.0001. Post hoc analysis via Tukey’s multiple comparisons test revealed that CDP-treated mice at 40 mg/kg and PAMAM G3-treated mice at 20 mg/kg had significantly higher temperatures compared with saline-treated mice on day 7 after infection (CDP: 1.97°C, p = 0.014; PAMAM G3: 1.80°C, p = 0.024). (B) Survival of mice based on a 15% weight loss cutoff. There was a significant increase in survival, determined using the Mantel-Cox method, between saline-treated mice versus mice treated with 40 mg/kg CDP or mice treated with 20 mg/kg PAMAM G3 (p = 0.0082).
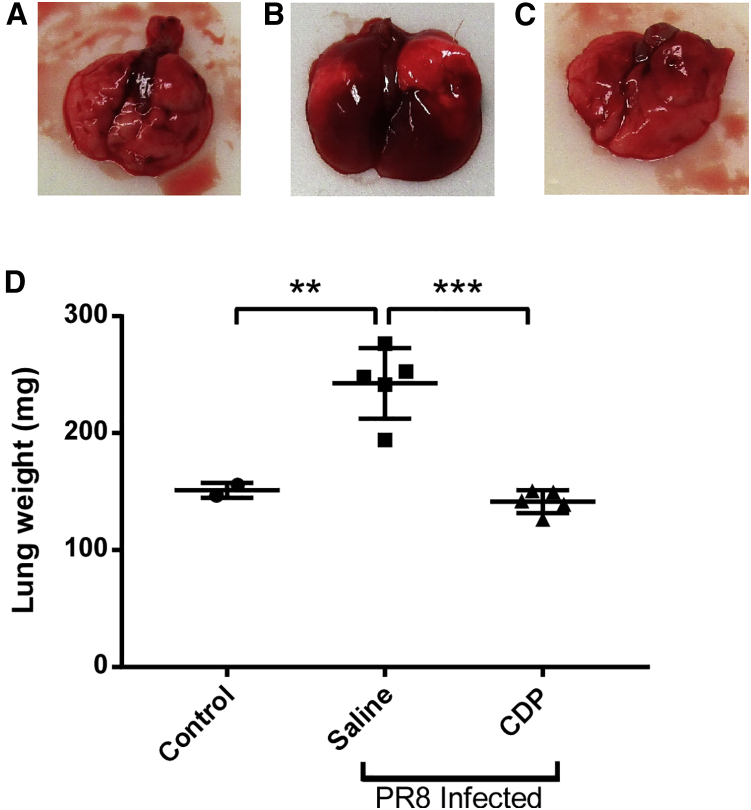
To further assess CDP’s effects on influenza symptoms and inflammation, we examined the lungs of infected mice on 7 days after infection. Day 7 was chosen because it is typically the day when mice exhibit the largest drop in temperature throughout the course of the infection and when weight loss is first noticeable. Mice were challenged with PR8 at the LD50 and treated with CDP or saline by i.p. injection on days 0, 3, and 6. On day 7, the lungs were removed, examined by gross pathology, and weighed (Figure 6). As can be seen in the representative images in Figures 6A–6C, the lungs of mice treated with saline were edematous, engorged, and red, whereas the lungs of mice treated with CDP were pink, smaller, and visually similar to uninfected healthy controls. This difference was reinforced by the weights of the removed lungs (Figure 6D). CDP-treated mice maintained normal lung weight compared with uninfected mice, in sharp contrast to the significantly heavier lungs from mice challenged with influenza and treated with saline. The difference can also be seen microscopically.
Figure 6.
Influenza-induced lung edema and lung weight increase are limited by treatment with CDP
(A–C) Lungs of naive uninfected mice and mice infected with PR8 and treated with (B) saline or (C) CDP (10 mg/kg). (D) Lung weights (mg) from uninfected control mice compared with PR8-challenged mice treated with saline or CDP (10 mg/kg). Data represents mean ± standard error of the mean, ∗∗ represents a p ≤ 0.01, ∗∗∗ represents a p ≤ 0.001.
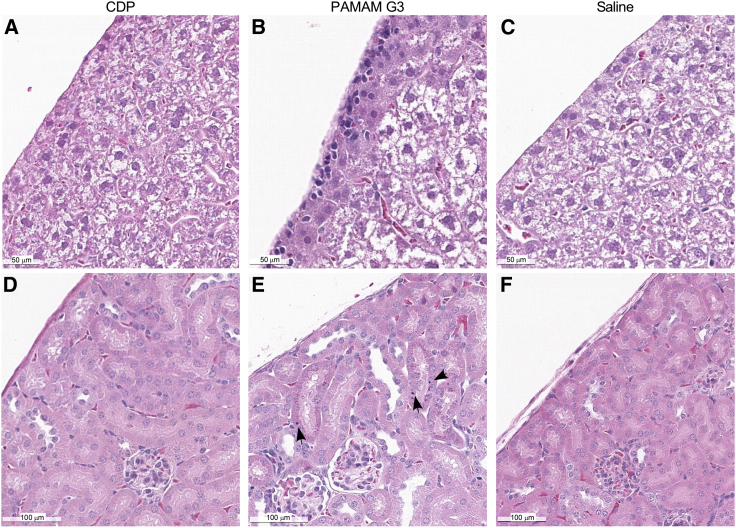
Because the efficacies of CDP (40 mg/kg) and PAMAM G3 (20 mg/kg) in protecting mice from the LD50 challenge of influenza were similar (Figure 5B), we next compared the two for any pathology associated with polymer treatment alone. Infection-naive C57Bl6 mice received 4 doses of CDP (40 mg/kg), PAMAM G3 (20 mg/kg), or saline on days 0, 3, 6, and 9 to match the dose regimen of the influenza challenge studies and then sacrificed on day 10. As shown in Figure 7, the histology of the livers and kidneys from these mice revealed that PAMAM G3 treatment induced hepatic inflammation (Figure 7B) and inclusion bodies in the nephrons (Figure 7E). In contrast, no such pathology was observed in animals treated with CDP (Figures 7A and 7D). In addition, serum collected at the time of sacrifice, 24 h after the fourth dose of polymer, was also evaluated. Although most markers of liver damage and kidney function remained within the normal range, mice treated with PAMAM G3 had noticeably higher levels of alanine aminotransferase, aspartate transaminase, blood urea nitrogen, and creatinine than mice that received saline or CDP (Figure S2). These results indicate that systemic administration of CDP can limit influenza-induced morbidity and mortality in mice (Figures 4, 5, and 6) with fewer effects on the liver and kidneys than PAMAM G3 (Figure 7).
Figure 7.
Organ toxicity is less evident by histology in CDP-treated mice compared with PAMAM G3-treated mice
(A and B) I.p. administration of CDP (40 mg/kg, A) does not induce hepatic surface inflammation as seen in PAMAM G3-treated (20 mg/kg, B) mice. (C) The livers from CDP-treated animals appear similar to those of saline-treated mice. (D–F) Kidneys from CDP treated animals (D) do not have inclusion bodies in murine nephrons, which are seen in PAMAM G3-treated mice (E), but appear similar to those of saline-treated control animals (F).
CDP is effective as a therapeutic agent and improves outcome in influenza-infected mice
Although our results indicate that treatment with CDP can reduce the morbidity and mortality associated with influenza infection when the polymer is present at the time of infection, for clinical translation, it is important to determine whether such an improvement in outcome can be achieved when the therapeutic agent is administered several days after infection. We therefore examined CDP’s effectiveness in controlling or reducing symptoms of influenza in mice when administered 3 days after infection. Mice were challenged with PR8 at the LD50 and split into a saline group and two groups receiving CDP (40 mg/kg), with one group starting treatment on the day of infection (prophylactic treatment group) as a positive control and the second group starting treatment 3 days after infection (therapeutic treatment group). Mice treated with the delayed/therapeutic dosing regimen received saline on day 0 and CDP on days 3, 6, and 9 after infection.
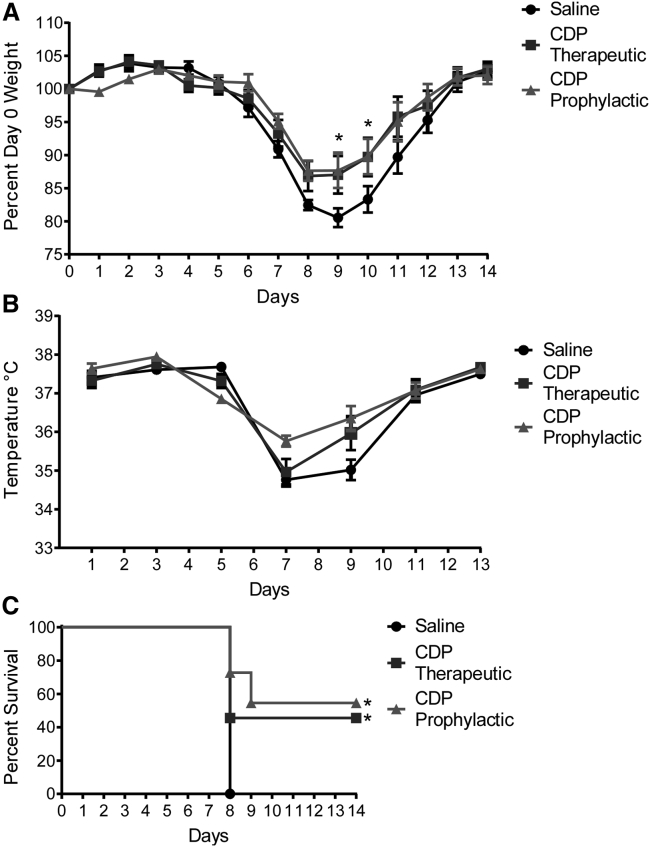
After viral challenge, the prophylactic and therapeutic CDP treatment groups of mice maintained their body weight significantly better than saline-treated animals (Figure 8A). Although the therapeutic CDP regimen was not as effective at maintaining body temperature compared with the prophylactic CDP group at early time points, the therapeutic group recovered body temperature more quickly than saline-treated mice (Figure 8B). Most importantly, survival based on less than 15% weight loss was significantly better in the CDP therapeutic and prophylactic treatment groups compared with saline-treated animals (Figure 8C). These data indicate that CDP can be administered 3 days after influenza infection and still significantly improve outcome in virally challenged mice.
Figure 8.
A therapeutic dosing regimen of CDP in influenza-infected mice aids recovery
(A) Weight of influenza-infected mice treated with saline or CDP (40 mg/kg) under a therapeutic dosing regimen (starting on day 3 after infection) or a prophylactic regimen (starting on the day of infection), represented as a percentage of their day 0 weight. A two-way repeated-measures ANOVA was run to determine the effect of therapeutic versus prophylactic treatment with CDP versus saline over time on weight following influenza infection. Data in the graph are mean ± standard error of the mean; n = 11. There was a statistically significant interaction between treatment and time on weight; F (28, 406) = 2.334, p = 0.0002, partial η2 = 0.0283. (B) Temperature measured by rectal probe over the course of the study. Post hoc analysis via Tukey’s multiple comparisons test revealed that prophylactic regimen CDP-treated mice had a significantly increased temperature compared with saline-treated mice on days 7 and 9 after infection. Therapeutically treated mice did not see a significant improvement over saline. (C) Survival of mice based on a 15% weight loss cutoff. There was significantly improved survival, determined using the Mantel-Cox method, between saline-treated mice and mice treated therapeutically with CDP (p = 0.0171) as well as mice treated with the prophylactic regimen (p = 0.0008).
Discussion
Inflammation plays an important role in developing an immune response to pathogens or other external irritants. When unchecked, however, persistent or excessive inflammation can result in a number of harmful responses, including autoimmune diseases like systemic lupus erythematosus (SLE)20 or infection-induced cytokine storm.21,22 Because TLRs have been implicated in such pathological inflammation, much attention is currently focused on controlling their activity. For example, it has been shown previously that mice with the Tlr3 gene deleted have an advantage over wild-type mice in recovery from influenza infection.23 Similarly, inhibition of TLR2, TLR4, TLR7, and TLR9 by a peptide inhibitor has been reported to improve survival of influenza-infected mice.24 We recently reported that lupus-prone animals treated with the nucleic acid scavenger PAMAM G3 are resistant to lethal viral challenge7 and that PAMAM G3 can capture anti-DNA immune complexes present in samples from individuals with lupus ex vivo.25 Unfortunately, we and others have observed that certain cationic PAMAM variants are associated with significant toxicity in vivo. The cytotoxicity of larger generations of PAMAM has been well documented.26 Although we showed that PAMAM G3 has a therapeutic window that allows us to see a therapeutic benefit in these and other mouse models, we observed negative effects with prolonged dosing, such as lesions on the liver, thickening of the liver, and general body weight loss. Moreover, in our study, some models and strains of mice were less tolerant of PAMAM G3 treatment than others. A less cytotoxic therapy is strongly preferred for translation and safe clinical use in a diverse human population.
Our study suggest that CDP has anti-inflammatory properties that may be useful for reducing symptoms of infection as well as controlling chronic inflammation. Interestingly, monomethyl-β-cyclodextrin has been shown to deplete viral membrane cholesterol and reduce infectivity of the flu virus in vitro.27 In vivo studies in a PR8 influenza model have demonstrated a therapeutic effect with a sulfonated derivative, but the derivative did not deplete cholesterol from the viral membrane.28 Thus, the utility of the monomer may potentially increase the therapeutic potential of CDP over other cationic polymers in treatment of influenza. We hope to further elucidate potential contributions of the monomer to the therapeutic benefits of polymeric CDP in future studies.
Other nucleic acid-binding polymers, including PAMAM G3, have been utilized to reduce inflammation in a number of models, including rheumatoid arthritis,29 sepsis,11 scarring,7 and chemical exposure.30 The wide potential utility of such polymers as anti-inflammatory therapeutic agents makes them an attractive class of molecules for further study and clinical development. The effect of these polymers on the innate and adaptive immune response, including their potential to modulate those responses in terms of cytokine and chemokine levels in these models is of great interest. Several reports indicate that PAMAM G3 or related nucleic acid scavenger treatment results in reduced levels of inflammatory cytokines and chemokines, including tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), IL-10, IL-1β, and interferon α (IFN-α) in several murine models of acute and chronic inflammation.9,11,31 Thus, inhibition of TLR activation appears to have broad anti-inflammatory effects. However, whether such reductions are the direct cause or a result of reduced pathologies in such animal models has not been fully elucidated. More definitive studies will likely require careful time-course experiments that employ sophisticated, high-resolution molecular analyses such as single-cell RNA sequencing. The toxicity of these polymers needs to be considered when determining which to pursue for clinical evaluation and potential therapeutic use.
Our initial toxicity evaluation of CDP as a nucleic acid DAMP/PAMP scavenger corroborates previous reports16,32 that indicate that CDP is considerably less toxic and likely to be more clinically translatable than PAMAM G3 or other related nucleic acid binding polymers. Overall, CDP is as therapeutically effective as PAMAM G3 at similar doses in an influenza infection model as well as a model of CLE. The reduced toxicity of CDP should allow increased dosing, which may further improve outcomes, as we have yet to establish its maximum tolerated dose in these models. Most importantly, CDP has the ability to improve morbidity and mortality in mice treated 3 days after lethal influenza challenge. This result suggests that CDP therapy may be a beneficial strategy to treat individuals after influenza infection and improve their outcome. Increasing the dose of CDP may also improve the therapeutic efficacy of nucleic acid scavengers for treatment of individuals with autoimmune diseases or respiratory viral diseases that are associated with uncontrolled inflammation, such as coronavirus disease 2019 (COVID-19).22
Materials and methods
Polymers
PAMAM G3 was purchased in a PBS solution from Dendritech (Midland, MI). CDP was prepared as described previously15 and shipped from Caltech to Duke University as a dry powder. CDP was formulated in saline as described for in vitro and in vivo studies.
Mice
Female C57BL/6J mice (stock number 000664) and male NZBW F1/J mice (stock number 100008) were obtained from The Jackson Laboratory and housed in a barrier BSL2 facility at Duke University. All studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Duke University Institutional Animal Care and Use Committee (protocols A019-19-01 and A201-18-08).
TLR activation assays
HEK-Blue hTLR3, hTLR4, and hTLR9 were purchased from InvivoGen. Cells were maintained in culture according to the manufacturer’s instructions. Agonists (high-molecular-weight [HMW] poly(I:C), CpG, and LPS for hTLR3, hTLR9, and hTLR4, respectively), were purchased from InvivoGen and resuspended according to the manufacturer’s instructions. Cells were plated for activation assays in 96-well plates at 50,000 cells per well and allowed to settle overnight. The medium was removed, and fresh medium, including control agonists and treatment, was added and allowed to incubate at 37°C for 18–24 h. After incubation, 40 μL of supernatant was removed from each well to a fresh 96-well plate. Quantiblue (InvivoGen) was resuspended according to the manufacturer’s instructions, and 160 μL was added to the wells and incubated for 3 h at 37°C. The absorbance of the wells was read at 650 nm on a SpectraMax i3 plate reader (Molecular Devices).
Tape stripping
Tape stripping was performed on the dorsal caudal area of the mice after shaving and application of depilatory cream (Nair), 40 strokes from 10 total standard-size bandages. Saline, PAMAM G3 (20 mg/kg, Dendritech), or CDP (20 mg/kg) was administered via 4 small-volume (less than 50 μL) s.c. injections around the site of injury at the time of tape stripping and every 3 days after injury. Tape stripping and injections were performed under isoflurane anesthesia delivered by nose cone. Animals were sacrificed 14 days following injury. All skin samples were then harvested and fixed in 10% (v/v) formalin for future histological analysis.
Skin lesion scoring was conducted in a blinded fashion by a trained veterinarian pathologist as described previously.7 Briefly, skin samples were collected, fixed in 10% (v/v) formalin, paraffin embedded (FFPE), and further processed for H&E staining. The five parameters of epidermal thickness, degree of ulceration, intraepithelial inflammation, dermal inflammation, and panniculus inflammation were assessed and graded on a scale from 0–3: 0, normal skin architecture; 1, mild inflammation with slight hyperplasia; 2, moderate inflammation with noticeable hyperplasia; 3, severe inflammation with marked hyperplasia. All parameters were scored separately and summed to reach a total disease score.
PR8 infection
A mouse-adapted virus strain, PR8, was obtained from Charles River Laboratories. Nine-week-old mice were anesthetized with vaporized isoflurane. The virus was administered intranasally in a total volume of 40 μL of sterile pharmaceutical-grade saline. Mice were treated with i.p. injection with PAMAM G3, CDP, or saline every 3 days starting on day 0 or day 3. Polymer solutions and saline were sterile filtered using a 0.20-μm syringe filter prior to injection. Mice were monitored and weighed daily and sacrificed when weight loss was greater than 25% of the starting body weight. Temperature was monitored every other day by rectal probe starting on day 1.
Gross pathology and serum collection after polymer-only treatment
Mice were treated on days 0, 3, 6, and 9 with PAMAM G3, CDP, or saline and sacrificed on day 10, with blood for serum obtained by an inferior vena cava bleed.
Acknowledgments
This work was supported in part by National Institutes of Health (NIH) grants U19AI067798 (to B.A.S.), R01AR073935 (to B.A.S.), and T32GM007171 (to L.B.O.).
Author contributions
L.K., L.B.O., and B.A.S. conceived and designed the experiments. L.K., L.B.O., and R.E.R. performed and analyzed the experiments. J.I.E. analyzed and scored histology. D.L. prepared the CDP polymer. L.K., L.B.O., R.E.R., S.K.N., M.E.D., and B.A.S. interpreted the data. L.K. and B.A.S. wrote the manuscript. L.B.O., R.E.R., D.L., S.K.N., and M.E.D. edited the manuscript. M.E.D. and B.A.S. acquired funding.
Declaration of interests
Duke University has applied for patents on the strategy to reduce inflammation via nucleic acid scavengers.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2021.10.003.
Supplemental information
References
- 1.Iuliano A.D., Roguski K.M., Chang H.H., Muscatello D.J., Palekar R., Tempia S., Cohen C., Gran J.M., Schanzer D., Cowling B.J., et al. Global Seasonal Influenza-associated Mortality Collaborator Network Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391:1285–1300. doi: 10.1016/S0140-6736(17)33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gong T., Liu L., Jiang W., Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020;20:95–112. doi: 10.1038/s41577-019-0215-7. [DOI] [PubMed] [Google Scholar]
- 3.Newton A.H., Cardani A., Braciale T.J. The host immune response in respiratory virus infection: balancing virus clearance and immunopathology. Semin. Immunopathol. 2016;38:471–482. doi: 10.1007/s00281-016-0558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee J., Sohn J.W., Zhang Y., Leong K.W., Pisetsky D., Sullenger B.A. Nucleic acid-binding polymers as anti-inflammatory agents. Proc. Natl. Acad. Sci. USA. 2011;108:14055–14060. doi: 10.1073/pnas.1105777108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morille M., Passirani C., Vonarbourg A., Clavreul A., Benoit J.P. Progress in developing cationic vectors for non-viral systemic gene therapy against cancer. Biomaterials. 2008;29:3477–3496. doi: 10.1016/j.biomaterials.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 6.Holl E.K., Shumansky K.L., Pitoc G., Ramsburg E., Sullenger B.A. Nucleic acid scavenging polymers inhibit extracellular DNA-mediated innate immune activation without inhibiting anti-viral responses. PLoS ONE. 2013;8:e69413. doi: 10.1371/journal.pone.0069413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holl E.K., Shumansky K.L., Borst L.B., Burnette A.D., Sample C.J., Ramsburg E.A., Sullenger B.A. Scavenging nucleic acid debris to combat autoimmunity and infectious disease. Proc. Natl. Acad. Sci. USA. 2016;113:9728–9733. doi: 10.1073/pnas.1607011113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang Y., Han Y., Liu L., Shen W., Zhang H., Wang Y., Cui X., Wang Y., Liu G., Qi R. Protective effects and mechanisms of G5 PAMAM dendrimers against acute pancreatitis induced by caerulein in mice. Biomacromolecules. 2015;16:174–182. doi: 10.1021/bm501390d. [DOI] [PubMed] [Google Scholar]
- 9.Holl E.K., Frazier V., Landa K., Boczkowski D., Sullenger B., Nair S.K. Controlling cancer-induced inflammation with a nucleic acid scavenger prevents lung metastasis in murine models of breast cancer. Mol. Ther. 2021;29:1772–1781. doi: 10.1016/j.ymthe.2020.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naqvi I., Gunaratne R., McDade J.E., Moreno A., Rempel R.E., Rouse D.C., Herrera S.G., Pisetsky D.S., Lee J., White R.R., Sullenger B.A. Polymer-Mediated Inhibition of Pro-invasive Nucleic Acid DAMPs and Microvesicles Limits Pancreatic Cancer Metastasis. Mol. Ther. 2018;26:1020–1031. doi: 10.1016/j.ymthe.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawulieti J., Sun M., Zhao Y., Shao D., Yan H., Lao Y.H., Hu H., Cui L., Lv X., Liu F., et al. Treatment of severe sepsis with nanoparticulate cell-free DNA scavengers. Sci. Adv. 2020;6:eaay7148. doi: 10.1126/sciadv.aay7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janaszewska A., Lazniewska J., Trzepiński P., Marcinkowska M., Klajnert-Maculewicz B. Cytotoxicity of Dendrimers. Biomolecules. 2019;9:330. doi: 10.3390/biom9080330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez H., Hwang S.J., Davis M.E. New class of polymers for the delivery of macromolecular therapeutics. Bioconjug. Chem. 1999;10:1068–1074. doi: 10.1021/bc990072j. [DOI] [PubMed] [Google Scholar]
- 14.Davis M.E. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol. Pharm. 2009;6:659–668. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 15.Davis M.E., Pun S.H., Bellocq N.C., Reineke T.M., Popielarski S.R., Mishra S., Heidel J.D. Self-assembling nucleic acid delivery vehicles via linear, water-soluble, cyclodextrin-containing polymers. Curr. Med. Chem. 2004;11:179–197. doi: 10.2174/0929867043456179. [DOI] [PubMed] [Google Scholar]
- 16.Heidel J.D., Schluep T. Cyclodextrin-containing polymers: versatile platforms of drug delivery materials. J. Drug Deliv. 2012;2012:262731. doi: 10.1155/2012/262731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis M.E., Zuckerman J.E., Choi C.H., Seligson D., Tolcher A., Alabi C.A., Yen Y., Heidel J.D., Ribas A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zuckerman J.E., Gritli I., Tolcher A., Heidel J.D., Lim D., Morgan R., Chmielowski B., Ribas A., Davis M.E., Yen Y. Correlating animal and human phase Ia/Ib clinical data with CALAA-01, a targeted, polymer-based nanoparticle containing siRNA. Proc. Natl. Acad. Sci. USA. 2014;111:11449–11454. doi: 10.1073/pnas.1411393111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGee C.E., Sample C.J., Kilburg-Basnyat B., Gabor K.A., Fessler M.B., Gowdy K.M. Influenza-Mediated Lung Infection Models. Methods Mol. Biol. 2019;1960:191–205. doi: 10.1007/978-1-4939-9167-9_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muñoz L.E., Janko C., Schulze C., Schorn C., Sarter K., Schett G., Herrmann M. Autoimmunity and chronic inflammation - two clearance-related steps in the etiopathogenesis of SLE. Autoimmun. Rev. 2010;10:38–42. doi: 10.1016/j.autrev.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Zheng B.J., Chan K.W., Lin Y.P., Zhao G.Y., Chan C., Zhang H.J., Chen H.L., Wong S.S., Lau S.K., Woo P.C., et al. Delayed antiviral plus immunomodulator treatment still reduces mortality in mice infected by high inoculum of influenza A/H5N1 virus. Proc. Natl. Acad. Sci. USA. 2008;105:8091–8096. doi: 10.1073/pnas.0711942105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henderson L.A., Canna S.W., Schulert G.S., Volpi S., Lee P.Y., Kernan K.F., Caricchio R., Mahmud S., Hazen M.M., Halyabar O., et al. On the Alert for Cytokine Storm: Immunopathology in COVID-19. Arthritis Rheumatol. 2020;72:1059–1063. doi: 10.1002/art.41285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Goffic R., Balloy V., Lagranderie M., Alexopoulou L., Escriou N., Flavell R., Chignard M., Si-Tahar M. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2006;2:e53. doi: 10.1371/journal.ppat.0020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piao W., Shirey K.A., Ru L.W., Lai W., Szmacinski H., Snyder G.A., Sundberg E.J., Lakowicz J.R., Vogel S.N., Toshchakov V.Y. A Decoy Peptide that Disrupts TIRAP Recruitment to TLRs Is Protective in a Murine Model of Influenza. Cell Rep. 2015;11:1941–1952. doi: 10.1016/j.celrep.2015.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stearns N.A., Lee J., Leong K.W., Sullenger B.A., Pisetsky D.S. The inhibition of anti-DNA binding to DNA by nucleic acid binding polymers. PLoS ONE. 2012;7:e40862. doi: 10.1371/journal.pone.0040862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malik N., Wiwattanapatapee R., Klopsch R., Lorenz K., Frey H., Weener J.W., Meijer E.W., Paulus W., Duncan R. Dendrimers: relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. J. Control. Release. 2000;65:133–148. doi: 10.1016/s0168-3659(99)00246-1. [DOI] [PubMed] [Google Scholar]
- 27.Sun X., Whittaker G.R. Role for influenza virus envelope cholesterol in virus entry and infection. J. Virol. 2003;77:12543–12551. doi: 10.1128/JVI.77.23.12543-12551.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goncharova E.P., Kostyro Y.A., Ivanov A.V., Zenkova M.A. A Novel Sulfonated Derivative of β-Cyclodextrin Effectively Inhibits Influenza A Virus Infection in vitro and in vivo. Acta Naturae. 2019;11:20–30. doi: 10.32607/20758251-2019-11-3-20-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng B., Liang H., Li Y., Dong C., Shen J., Mao H.Q., Leong K.W., Chen Y., Liu L. Tuned Cationic Dendronized Polymer: Molecular Scavenger for Rheumatoid Arthritis Treatment. Angew. Chem. Int. Ed. Engl. 2019;58:4254–4258. doi: 10.1002/anie.201813362. [DOI] [PubMed] [Google Scholar]
- 30.Mariappan N., Husain M., Zafar I., Singh V., Smithson K.G., Crowe D.R., Pittet J.F., Ahmad S., Ahmad A. Extracellular nucleic acid scavenging rescues rats from sulfur mustard analog-induced lung injury and mortality. Arch. Toxicol. 2020;94:1321–1334. doi: 10.1007/s00204-020-02699-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang H., Peng B., Dong C., Liu L., Mao J., Wei S., Wang X., Xu H., Shen J., Mao H.Q., et al. Cationic nanoparticle as an inhibitor of cell-free DNA-induced inflammation. Nat. Commun. 2018;9:4291. doi: 10.1038/s41467-018-06603-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis M.E., Brewster M.E. Cyclodextrin-based pharmaceutics: past, present and future. Nat. Rev. Drug Discov. 2004;3:1023–1035. doi: 10.1038/nrd1576. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.