Abstract
Accelerated postnatal growth is a potentially modifiable risk factor for future obesity. To study how specific breast milk components contribute to early growth and obesity risk, we quantified one-carbon metabolism-related metabolites in human breast milk and found an inverse association between milk betaine content and infant growth. This association was replicated in an independent and geographically distinct cohort. To determine the potential role of milk betaine in modulating offspring obesity risk, we performed maternal betaine supplementation experiments in mice. Higher betaine intake during lactation increased milk betaine content in dams and led to lower adiposity and improved glucose homeostasis throughout adulthood in mouse offspring. These effects were accompanied by a transient increase in Akkermansia spp. abundance in the gut during early life and a long-lasting increase in intestinal goblet cell number. The link between breast milk betaine and Akkermansia abundance in the gut was also observed in humans, as infants exposed to higher milk betaine content during breastfeeding showed higher fecal Akkermansia muciniphila abundance. Furthermore, administration of A. muciniphila to mouse pups during the lactation period partially replicated the effects of maternal breast milk betaine, including increased intestinal goblet cell number, lower adiposity, and improved glucose homeostasis during adulthood. These data demonstrate a link between breast milk betaine content and long-term metabolic health of offspring.
INTRODUCTION
Extensive research in humans and animal models indicates that a sensitive period of growth and development both in utero and during early infancy strongly influences life-course health outcomes (1). A number of perinatal factors, including maternal nutritional status and gestational weight gain, postnatal growth trajectories, feeding patterns, and early-life intestinal microbiota modulate future risk of obesity (2–5). Among the risk factors, accelerated growth rate during the first months of life has been consistently associated with higher risk of future obesity and metabolic disease (5–8).
Metabolites from the one-carbon metabolism pool have long been recognized as critical nutrients for infant growth and development (9–11). Choline is in high demand during early growth, and its concentration is especially elevated in milk (11, 12). It can be used for phosphatidylcholine biosynthesis or oxidized to betaine. Betaine is a trimethylated derivative of glycine readily available in the diet (13, 14). Its circulating concentration is inversely associated with body mass index (BMI) and diabetes risk in adult subjects (15–17). Betaine acts as a potent osmolyte regulating cellular volume and protecting against external stress in multiple organisms, from bacteria to mammals (13, 14, 18). In mammals, betaine is primarily metabolized in the liver, transferring a methyl group to homocysteine to sequentially produce methionine, S-adenosylmethionine (SAM), S-adenosylhomocysteine (SAH), and homocysteine in the methionine cycle (19).
Maternal status of one-carbon metabolites during pregnancy has been linked to offspring health outcomes. For instance, lower maternal plasma folate concentration during gestation has been associated with higher offspring obesity risk (10), and lower maternal circulating betaine concentration during the third trimester of pregnancy has been correlated with higher infant weight and adiposity at birth (20). However, despite their importance for infant growth and development, metabolic profiling of one-carbon–related intermediates in breast milk has not been performed, and whether milk content of these metabolites plays a role in modulating infant growth and obesity risk remains unknown. To address these gaps in knowledge, the present study sought to (i) investigate potential associations of milk one-carbon metabolites with infant early growth and (ii) test whether maternal supplementation with these metabolites modulated growth, body composition, and glucose homeostasis into adulthood in a murine model.
RESULTS
Breast milk betaine content is inversely associated with infant growth
To determine whether milk one-carbon metabolites are associated with early growth, we studied a U.S.-based cohort of 34 exclusively breastfeeding mother-infant dyads from the Oklahoma area (21) (cohort I, subject characteristics in Table 1). We quantified choline, betaine, methionine, SAM, SAH, and cystathionine concentrations in human breast milk samples obtained 1 month after birth (Table 1) and examined potential associations of these metabolites with infant growth rate, assessed by changes in weight-for-length z score from birth to 1 month. We observed a significant inverse association between weight-for-length z score change and milk betaine concentration (P = 0.015; cohort I, Table 2). This association was still significant after further adjustment for maternal variables known to affect infant growth, including prepregnancy maternal BMI, gestational weight gain, and delivery method (P = 0.028; cohort I, Table 2).
Table 1.
Demographic and metabolic characteristics of mother-infant dyads from cohorts I and II.Values represent means and SD.
| Cohort I | Cohort II | |
|---|---|---|
| Mother | n = 34 | n = 109 |
| Age (years) | 29.1 ± 5.1 | 32.8 ± 6.2 |
| Prepregnancy BMI (kg/m2) |
27.3 ± 7.1 | 23.5 ± 3.8 |
| Gestational weight gain (kg)* | 12.0 ± 7.4 | 12.1 ± 4.7 |
| Infant | ||
| Birth | n = 34 | n = 109 |
| Gender (female/male) | 15/19 | 60/49 |
| Gestational age (weeks) | 39.6 ± 1.2 | 39.6 ± 1.1 |
| Weight (kg) | 3.53 ± 0.48 | 3.35 ± 0.44 |
| Length (cm) | 51.6 ± 2.3 | 50.1 ± 2.2 |
| Weight-for-length z score | −0.59 ± 1.2 | −0.20 ± 1.15 |
| 1 month | n = 34 | n = 109 |
| Weight (kg) | 4.67 ± 0.71 | 4.23 ± 0.57 |
| Length (cm) | 55.8 ± 2.1 | 54.5 ± 2.3 |
| Weight-for-length z score | −0.36 ± 1.04 | −0.50 ± 1.17 |
| 12 months | n = 107 | |
| Weight (kg) | - | 9.46 ± 1.04 |
| Length (cm) | - | 74.8 ± 2.9 |
| Weight-for-length z score | - | 0.18 ± 0.87 |
| Breast milk metabolites | n = 34 | n = 109 |
| SAM (nM) | 1469 ± 436 | 2034 ± 649 |
| SAH (nM) | 216 ± 81 | 327 ± 184 |
| Methionine (|iM) | 4.39 ± 2.69 | 6.74 ± 12.36 |
| Cystathionine (nM) | 44.9 ± 34.3 | 129.6 ± 75.7 |
| Betaine (|iM) | 3.12 ± 2.65 | 5.33 ± 6.13 |
| Choline (|iM) | 97 ± 49 | 165 ± 115 |
n = 32 for gestational weight gain data in cohort I.
Table 2.
Multivariate regression between human breast milk metabolite concentrations and infant growth.Least-square regression models were applied to assess the correlation between milk metabolite concentrations and change in weight-for-length z score from birth to 1 month or 12 months of age. B, size effect estimate; CI, confidence interval; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine; Met, methionine; Cysta, cystathionine; Bet, betaine; Cho, choline. Bold font indicates P < 0.05.
| Cohort I | Cohort II | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight-for-length z score 1 month | Weight-for-length z score 1 month | Weight-for-length z score 12 months | ||||||||||
| Adjusted* | Adjusted† | Adjusted* | Adjusted† | Adjusted* | Adjusted† | |||||||
| n = 34 | n = 32 | n = 109 | n = 109 | n = 107 | n = 107 | |||||||
| B (CI 95%) | P | B (CI 95%) | P | B (CI 95%) | P | B (CI 95%) | P | B (CI 95%) | P | B (CI 95%) | P | |
| SAM | 0.09 (−1.08 to 1.25) | 0.879 | −0.12 (−1.35 to 1.11) | 0.841 | 0.52 (−0.03 to 1.07) | 0.066 | 0.55 (0.01 to 1.10) | 0.047 | 0.07 (−0.39 to 0.53) | 0.765 | 0.01 (−0.45 to 0.48) | 0.964 |
| SAH | −0.95 (−1.91 to 0.01) | 0.053 | −0.88 (−1.86 to 0.10) | 0.077 | 0.10 (−0.20 to 0.39) | 0.525 | 0.12 (−0.17 to 0.42) | 0.407 | −0.02 (−0.26 to 0.22) | 0.848 | −0.06 (−0.31 to 0.18) | 0.609 |
| Met | 0.10 (−0.59 to 0.79) | 0.772 | 0.39 (−0.36 to 1.14) | 0.298 | −0.57 (−0.87 to −0.26) | 0.001 | −0.51 (−0.81 to−0.21) | 0.001 | −0.22 (−0.47 to 0.04) | 0.094 | −0.18 (−0.44 to 0.08) | 0.182 |
| Cysta | −0.33 (−0.68 to 0.02) | 0.061 | −0.35 (−0.71 to 0.02) | −0.35 (−0.71 to 0.02) | 0.061 | 0.341 | −0.22 (−0.59 to 0.16) | 0.253 | −0.18 (−0.48 to 0.13) | 0.258 | −0.17 (−0.48 to 0.14) | 0.266 |
| Bet | −0.66 (−1.18 to −0.14) | 0.015 | −0.60 (−1.14 to −0.07) | 0.028 | −0.56 (−0.91 to −0.21) | 0.002 | −0.47 (−0.83 to −0.11) | 0.010 | −0.38 (−0.67 to −0.1) | 0.009 | −0.34 –0.64 to −0.04) | 0.026 |
| Cho | −0.23 (−0.92 to 0.46) | 0.503 | −0.26 (−1.03 to 0.51) | 0.497 | −0.28 (−0.67 to 0.10) | 0.148 | −0.23 (−0.61 to 0.15) | 0.237 | −0.21 (−0.53 to 0.11) | 0.190 | −0.17 (−0.49 to 0.15) | 0.287 |
Adjusted for gestational age and weight-for-length z score at birth.
Adjusted for gestational age, weight-for-length z score at birth, prepregnancy BMI, gestational weight gain, and mode of birth.
We also studied an independent European-based cohort with 109 exclusively breastfeeding mother-infant dyads from the Spanish-Mediterranean area (22), with infant anthropometric data obtained at birth, and at 1 and 12 months of age (cohort II, subject characteristics in Table 1). One-carbon metabolites were also quantified in breast milk samples obtained 1 month after birth (cohort II, Table 2). The association between milk betaine concentration and change in weight-for-length z score at 1 month after birth was replicated in this cohort (cohort II, Table 2). Furthermore, the longitudinal data from the replication cohort showed that milk betaine content at 1 month after birth was also inversely associated with weight-for-length z score change at 12 months of age (cohort II, Table 2), indicating that the correlation persisted through the first year of life. In this cohort, we also observed associations of infant weight-for-length z score change at 1 month with methionine and SAM concentrations (neither were observed in cohort I), but these associations were lost at 12 months (cohort II, Table 2). Betaine was the only metabolite whose association with weight-for-length z score change persisted until 12 months of age (Table 2). No association was observed for milk betaine content with change in body length z score or head circumference in either cohort (table S1).
Dietary betaine transfer into breast milk and decreased adiposity in young mice
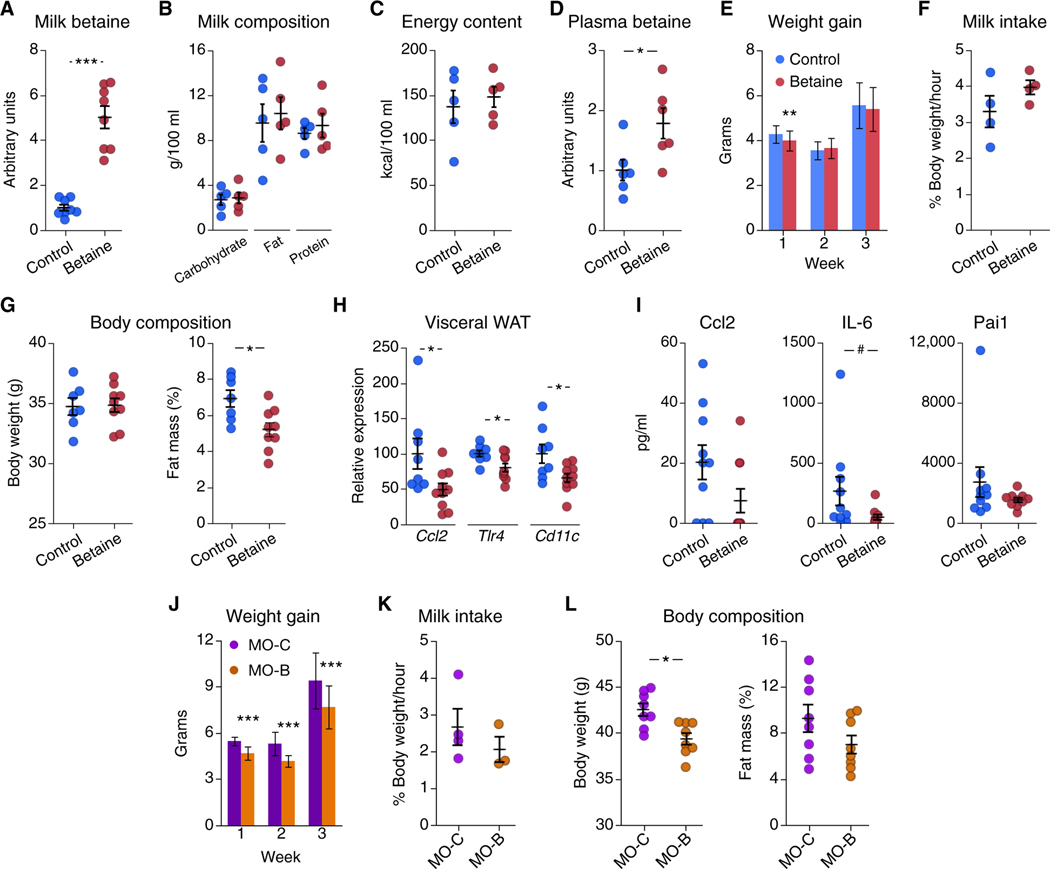
To determine whether betaine plays a role in postnatal growth and future obesity, we performed maternal supplementation experiments in mice during lactation. For this purpose, dams were randomly assigned to control or treatment (1% betaine) groups the first day after delivery. Milk samples were collected at day 14 from a subset of dams to determine betaine content. Supplementation increased milk betaine content by fivefold (Fig. 1A) without altering macronutrient composition (Fig. 1B) or energy content (Fig. 1C). The increase in milk betaine content translated into elevated plasma betaine concentration in suckling 14-day-old offspring (Fig. 1D). We next examined the effects of maternal betaine supplementation on offspring growth during the lactation period. Supplementation induced a modest decrease in offspring weight gain during the first week of lactation (Fig. 1E). No differences in milk intake were observed between groups (Fig. 1F). Body composition analysis at 6 weeks of age revealed decreased fat mass in betaine-exposed mice compared to control mice (Fig. 1G). These changes in adiposity were accompanied by decreased mRNA expression of immune markers C-C motif ligand 2 (Ccl2, also known as MCP-1), Toll-like receptor 4 (Tlr4), and integrin subunit alpha X (Cd11c) in visceral white adipose tissue (vWAT) (Fig. 1H); expression of the proinflammatory marker Ccl2 was also lower in brown adipose tissue (BAT), liver, and skeletal muscle (fig. S1A). Supplementation decreased circulating concentrations of interleukin-6 (IL-6), whereas Ccl2 and plasminogen activator inhibitor-1 (PAI-1) did not show a significant change (Fig. 1I). Betaine has been shown to induce hepatic Fgf21 expression in adult mice in a diet-induced obesity context (16); however, we observed no effect of maternal betaine administration on offspring hepatic Fgf21 expression at either 2 or 6 weeks of age (fig. S1B). There were no differences in other parameters including glucose tolerance and uncoupling protein 1 (Ucp1) expression in BAT (fig. S1, C and D). Similarly, oxygen consumption, respiratory exchange ratio, physical activity, or food intake did not differ between groups at this age (fig. S2, A to D).
Fig. 1. Maternal betaine supplementation decreases adiposity in mouse offspring.
(A) Shown are betaine relative concentrations in breast milk from control (blue) and betaine-treated (red) dams (n = 8 per group). (B and C) Shown is dam milk macronutrient composition (B) and energy content (C) (n = 5 per group). (D) Shown is plasma relative concentration of betaine in 2-week-old mice born to control dams (blue) or dams administered betaine (red) (n = 6 per group). (E) Shown is offspring weight gain during lactation (n = 36 per group, means ± SD). (F) Shown is milk intake at 1 week of age (pups grouped in litters, with n = 4 litters per group). (G) Body composition measured by MRI of 6-week-old offspring of control dams (n = 7, blue) or betaine-treated dams (n = 9, red) is shown. (H) mRNA expression of vWAT proinflammatory markers in 6-week-old male mice (n = 8 to 10 per group) is presented. (I) Plasma concentrations of proinflammatory markers Ccl2, IL-6, and Pai1 in 6-week-old male mice (n = 10 per group) are presented. (J) Shown is weight gain (means ± SD) during each week of lactation of offspring from obese control dams (MO-C, purple, n = 19) or betaine-treated obese dams (MO-B, orange, n = 21). (K) Shown is milk intake at 1 week of age for pups born to obese control dams (MO-C, purple) or betaine-treated obese dams (MO-B, orange) (pups grouped in litters, with n = 3 to 4 litters per group). (L) Shown is body composition in 6-week-old mice born to obese control dams (MO-C, purple) or betaine-treated obese dams (MO-B, orange) (n = 8 per group). Unless otherwise stated, data are means ± SEM. *, t test P < 0.05; **, t test P < 0.01; ***, P < 0.005; #, Mann-Whitney U test P < 0.05.
We next studied the effects of betaine supplementation in a maternal obesity model, which leads to increased offspring early growth and higher adiposity during adulthood in rodents (23). For this purpose, diet-induced obese dams were randomly assigned to control (MO-C) or betaine-treated (MO-B) groups at delivery. Maternal obesity increased weight gain during lactation compared with normal-weight dams (5.5 ± 0.1 g versus 4.3 ± 0.1 g during week 1 after birth for MO-C and control groups in Fig. 1, J and E). In this context of accelerated growth due to maternal obesity, betaine supplementation decreased offspring weight gain throughout the lactation period compared to offspring from control obese dams (Fig. 1J), with no differences in milk intake (Fig. 1K). At 6 weeks of age, changes in adiposity in offspring of control obese dams (MO-C) and betaine-treated obese dams (MO-B) did not reach statistical difference (Fig. 1L).
Maternal betaine supplementation improves offspring long-term metabolic health
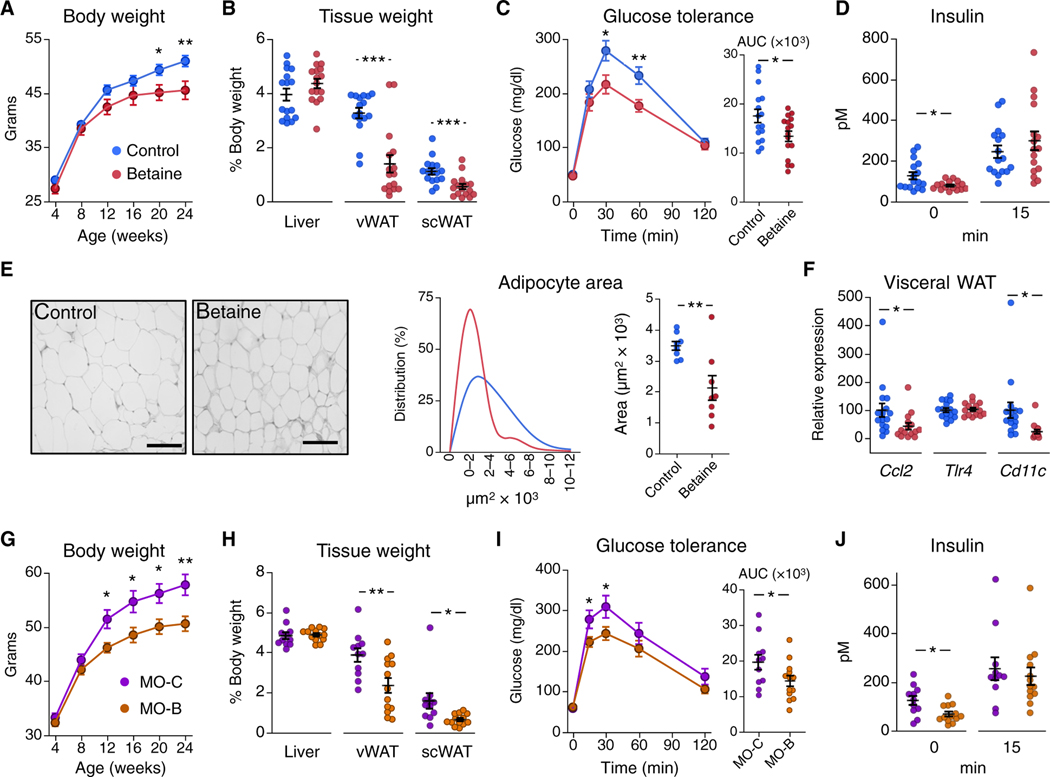
To determine whether the early-life intervention had long-lasting effects, mice were monitored until adulthood. Offspring from betaine-treated dams on a chow diet showed lower body weight during adulthood (Fig. 2A). These mice showed decreased vWAT and sub-cutaneous WAT (scWAT) weight, with no changes in liver weight at 24 weeks of age compared to control mice (Fig. 2B). These effects were accompanied by improved glucose tolerance (Fig. 2C) and de creased fasting insulin concentrations (Fig. 2D). Consistent with lower adiposity, adipocyte size (Fig. 2E) and proinflammatory markers Ccl2 and Cd11c were decreased in vWAT from betaine-exposed mice (Fig. 2F). We observed similar long-term effects of betaine supplementation during lactation in the maternal obesity mouse model, with improvements in body weight (Fig. 2G), adiposity (Fig. 2H), glucose tolerance (Fig. 2I), and fasting insulin concentration (Fig. 2J) in offspring born to betaine-treated obese dams (MO-B) compared to offspring born to control obese dams (MO-C).
Fig. 2. Maternal betaine supplementation improves mouse offspring long-term metabolic health.
(A to F) Offspring from control (blue) or betaine-treated (red) dams were fed a chow diet after weaning (n = 16 per group) and multiple parameters were measured. (A) Body weight throughout adulthood is shown. (B) Weight of liver, visceral WAT (vWAT), and subcutaneous WAT (scWAT) at euthanasia (24 weeks of age) are shown. (C and D) Shown is a glucose tolerance test (C) and insulin concentrations at 0 and 15 min after glucose load (D) in 20-week-old mouse offspring. (E) Shown is vWAT adipocyte area (scale bars, 100 μm), distribution, and average cell area in 24-week-old mice (n = 8 per group). (F) mRNA expression of proinflammatory markers in vWAT from control (blue) and betaine-treated (red) 24-week-old mouse off-spring (n = 15 to 16 per group) is shown. (G to J) Offspring from obese control dams (MO-C, n = 11, purple) and betaine-treated obese dams (MO-B, n = 13, orange) were fed a chow diet after weaning and then multiple parameters were measured. (G) Body weight was monitored until adulthood. (H) Liver, vWAT, and scWAT weights at euthanasia (24 weeks of age) were measured. (I) Glucose tolerance and (J) insulin concentrations at 0 and 15 min after glucose load were measured in 20-week-old off-spring. AUC, area under the curve. Data are means ± SEM. *, t test P < 0.05; **, P < 0.01; ***, P < 0.005.
Given that postnatal nutrition is a major determinant of the infant gut microbiota and changes in early-life intestinal bacteria can modulate long-term adiposity in mice (4, 24–26), we tested whether gut microbiota contributed to betaine’s long-term effects. For this purpose, we coadministered antibiotics to dams during lactation to disrupt the offspring’s gut microbiome. Dams were randomly assigned to four groups receiving betaine (1% betaine), antibiotics [ampicillin (1 g/liter) and neomycin (0.5 g/liter)], antibiotics and betaine, or no supplements; offspring were fed a chow diet after weaning and monitored until adulthood (fig. S3A). Maternal antibiotic administration disrupted the offspring gut microbiota, as shown by the decrease in bacterial content in cecal samples from 2-week-old suckling mice (fig. S3B). Maternal antibiotic administration during lactation abolished long-term betaine-induced effects on body weight, adiposity, glucose tolerance, and vWAT immune markers Ccl2 and Cd11c (fig. S3, C to F).
Maternal betaine transiently increases offspring Akkermansia spp. abundance during early life
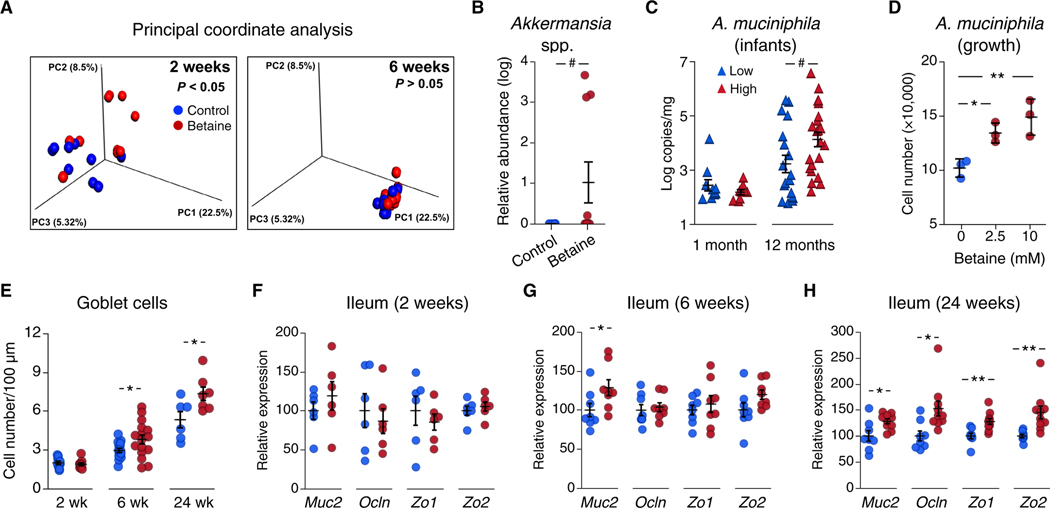
To determine whether breast milk betaine shapes the offspring gut microbiota, we analyzed cecal samples from dams, as well as 2-and 6-week-old offspring. Although betaine supplementation did not modify maternal gut microbiota composition (fig. S4, A and B), principal coordinate analysis (PCoA) of UniFrac distances showed differential microbial community composition in 2-week-old offspring across groups (n = 10 mice from 8 different litters per group; Fig. 3A, left); these changes were transient, as no differences were observed in 6-week-old mice (n = 10 from 6 different litters per group; Fig. 3A, right). Alpha diversity (measured as Chao1 and Shannon indices) did not differ between groups (fig. S4C). We applied linear discriminant analysis (LDA) effect size (LEfSe) to identify differentially abundant bacteria at 2 weeks of age and found that Akkermansia spp. was the only group altered (fig. S4, D and E), with higher relative abundance in the betaine-treated compared to the control group (Fig. 3B).
Fig. 3. Maternal betaine supplementation increases Akkermansia abundance in neonatal mice.
(A) PCoA of unweighted UniFrac distances for the cecal microbiota from 2-and 6-week-old offspring of betaine-treated dams (red) or control dams (blue) is presented; P value was assessed by the Adonis test (n = 10 per group). (B) Relative abundance of Akkermansia spp. in cecal samples from 2-week-old mice is shown as log(x + 1) values. (C) A. muciniphila copy number per mg of fecal sample in 1-month old (n = 19) and 12-month-old (n = 40) human infants is shown. Infants were categorized into low-exposure (blue triangles) and high-exposure (red triangles) groups based on milk betaine concentration median values (4.1 μM). (D) A. muciniphila was grown in vitro in the absence (blue) or presence (red) of 2.5 or 10 mM betaine until reaching the stationary phase (25 hours of growth); cell number was quantified by qPCR (n = 3, means ± SD; one-way ANOVA with post hoc Dunnett’s test). (E) Shown is goblet cell number in ileum sections from 2-week-old (n = 10 per group), 6-week-old (n = 16 per group), and 24-week-old (n = 6 to 7 per group) offspring born to control (blue) and betaine-treated (red) dams. (F to H) Shown are ileal mRNA concentrations of four intestinal barrier markers Muc2, Ocln, Zo1, and Zo2 in mouse offspring at 2 weeks (n = 10 per group), (G) 6 weeks (n = 8 per group), and (H) 24 weeks of age (n = 8 to 10 per group). Unless otherwise stated, data are means ± SEM. *, Student’s t test P < 0.05; **, P < 0.01; #, Mann-Whitney U test P < 0.05.
We next asked whether breast milk betaine content was linked to Akkermansia abundance in humans. To answer this question, we determined the absolute concentration of Akkermansia muciniphila by quantitative polymerase chain reaction (qPCR) analysis in a subset of infants from cohort II from whom fecal samples were available (83 at 1 month and 91 at 12 months of age). Infants were categorized into “low” and “high” betaine exposure groups on the basis of the median values of breast milk betaine concentrations of their mothers. As expected from previous studies (27), A. muciniphila prevalence was low during early life and was detected only in 19 infants at 1 month of age, whereas it was detected in 40 infants at 12 months of age (table S2). Although there were no differences in prevalence between low and high betaine exposure groups (table S2), A. muciniphila absolute concentration was higher in 12-month-old infants that were exposed to higher milk betaine during breastfeeding compared to those exposed to lower milk betaine content (Fig. 3C). Furthermore, A. muciniphila abundance at 12 months showed a direct correlation to milk betaine concentrations when analyzed as a continuous variable (Spearman’s rho = 0.35, P = 0.026), but its correlation to weight-for-length z score change from birth to 12 months of age did not reach statistical significance (Spearman’s rho = −0.25, P = 0.112).
We then tested whether betaine could modulate Akkermansia growth by determining the effect of different betaine concentrations on in vitro bacterial growth under anaerobic conditions. Presence of betaine in the culture media (2.5 and 10 mM) resulted in a modest but significant increase in A. muciniphila growth in vitro (one-way analysis of variance (ANOVA) P = 0.008; Fig. 3D). We performed similar experiments with other bacterial species typically present in the infant gut microbiota, including Escherichia coli (phylum Proteobacteria), Lactobacillus johnsonni (phylum Firmicutes), and Bifidobacterium longum (phylum Actinobacteria). We observed no effects of betaine on the growth of these bacteria in vitro (fig. S5).
Akkermansia spp. abundance has been tightly linked to lower gut inflammation in mice, which is partly due to an increase in intestinal goblet cell number and improved gut barrier function (28–31). Thus, we next performed a histological analysis of the mouse ileum. Ileal villi height and crypt depth did not differ between groups at 2, 6, or 24 weeks of age (fig. S6A). Ileal goblet cell number showed no differences during breastfeeding in 2-week-old mice but was higher in mice at 6 weeks of age in the betaine-exposed group (Fig. 3E). The effect of the early-life intervention on ileal goblet cells was maintained throughout adulthood, as 24-week-old mice born to betaine-treated dams still showed higher goblet cell number than did controls (Fig. 3E). Intestinal goblet cells are responsible for mucin 2 (Muc2) production to maintain the gut mucosal barrier. Ileal Muc2 expression paralleled the increase in goblet cells, with no major differences observed in 2-week-old mice between groups (Fig. 3F) and higher Muc2 expression in 6-week-old betaine-exposed mice compared to controls (Fig. 3G); other gut barrier markers including occludin (Ocln), tight-junction protein 1 (Zo1), and tight-junction protein 2 (Zo2) were not changed at either 2 weeks (Fig. 3F) or 6 weeks of age (Fig. 3G). Expression of Muc2, Ocln, Zo1, and Zo2 genes was increased at 24 weeks of age (Fig. 3H). Alterations in the early-life gut microbiota can also modulate the development of the immune system by altering T cell differentiation and affecting long-term metabolic health (26). Thus, we analyzed whether master transcription factors that drive CD4+ T cell differentiation into the different T cell subsets [T helper 1 cell (TH1), TH2, TH17, and regulatory T cell] were altered by maternal betaine supplementation. We observed no differences in mouse ileal gene expression of markers Tbx21, Gata3, Rorc, and Foxp3 at 2, 6, or 24 weeks of age (fig. S6B).
Early-life A. muciniphila exposure improves long-term metabolic health in mice
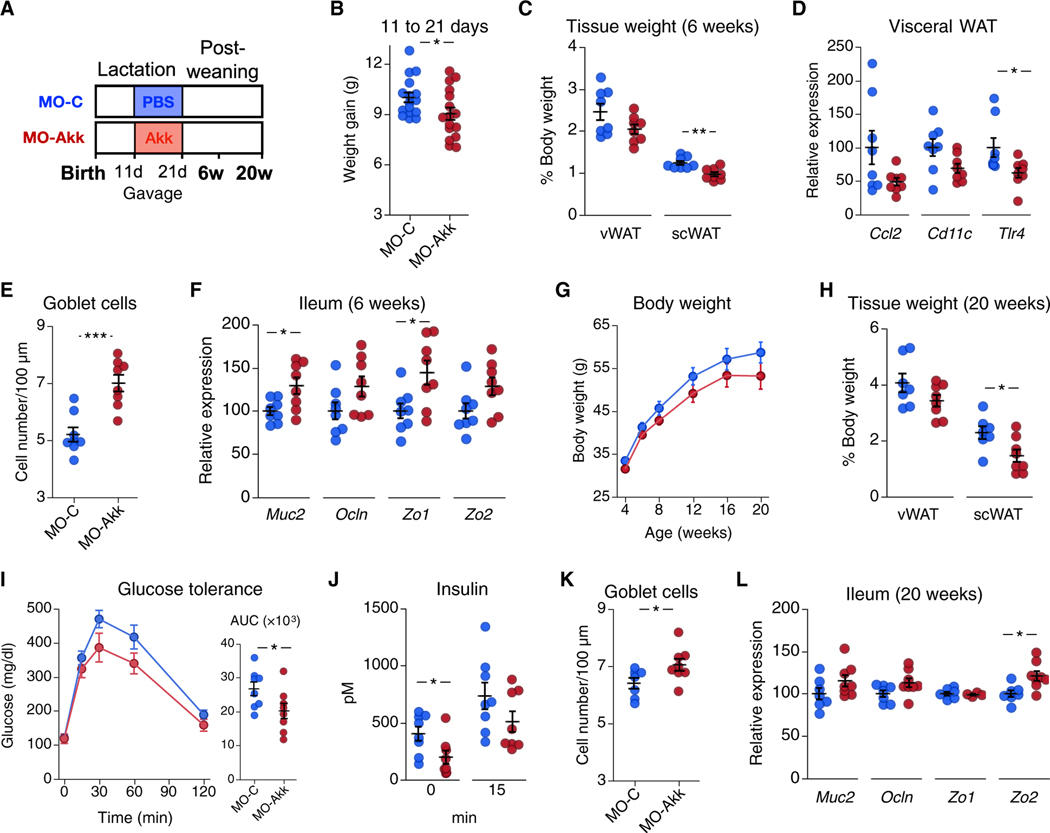
To test whether exposure to Akkermansia spp. during early life replicated maternal betaine effects, we administered A. muciniphila to pups in a mouse model of maternal obesity. For this experiment, we used pasteurized A. muciniphila (pAkk) as it has been shown to exert similar effects on metabolic parameters as the live form when administered to humans or mice (28, 32). Eleven-day-old offspring from diet-induced obese dams were administered pAkk (MO-Akk group) or phosphate-buffered saline (PBS) as a vehicle control (MO-C group) by gavage three times per week until weaning (Fig. 4A). pAkk administration induced a 10% decrease in weight gain over the treatment period compared to the control group (Fig. 4B). At 6 weeks of age, these mice showed lower scWAT weight (Fig. 4C). Tlr4 expression was decreased in vWAT in pAkk-treated mice, whereas changes in Ccl2 and Cd11c did not reach statistical significance (Fig. 4D). Early-life pAkk exposure resulted in higher ileal goblet cell number at 6 weeks of age (Fig. 4E), in parallel to increased expression of Muc2 and Zo1 (Fig. 4F). To study the long-term effects of this early-life intervention, we monitored the mice until adulthood. Although no difference in body weight over time was observed in pAkk-treated mice (Fig. 4G), 20-week-old mice showed decreased scWAT weight (Fig. 4H), as well as improved glucose tolerance (Fig. 4I) and fasting insulin concentrations (Fig. 4J) compared to controls. Although slightly weaker, the effect of early-life pAkk exposure on the mouse ileum was sustained over time, as ileal goblet cell number (Fig. 4K) and expression of the gut barrier marker Zo2 were increased in adult mice (Fig. 4L).
Fig. 4. Early-life A. muciniphila exposure improves long-term metabolic health in mice.
(A) Eleven-day-old male mice born to obese dams were administered PBS as control (MO-C, n = 16, blue) or pAkk (MO-Akk, n = 16, red) thrice weekly until day 21 and were then euthanized at 6 or 20 weeks of age (n = 8 per group for each time point).(B) Shown is weight gain for mouse offspring through days 11 to 21 (n = 16 per group). (C and D) Shown is vWAT and scWAT weight (C) and vWAT mRNA expression (D) in mouse offspring at 6 weeks of age (n = 8 per group). (E) Goblet cell number and (F) mRNA expression of intestinal barrier markers Muc2, Ocln, Zo1, and Zo2 in ileum of mouse offspring at 6 weeks of age (n = 8 per group) are shown. (G) Body weight was monitored until adulthood (n = 8 per group). (H) vWAT and scWAT weight in mice at 20 weeks of age is shown (n = 7 to 8 per group). (I) Glucose tolerance and (J) insulin concentrations at 0 and 15 min after glucose load in mice at 18 weeks of age (n = 8 per group) are shown. (K) Goblet cell number and (L) mRNA expression of intestinal barrier markers Muc2, Ocln, Zo1, and Zo2 in ileum of mice at 20 weeks of age (n = 7 to 8 per group) are shown. AUC, area under the curve. Data are means ± SEM. *, t test P < 0.05; **, P < 0.01; ***, P < 0.005.
DISCUSSION
Breastfeeding is considered the gold standard of early nutrition and is regarded as a window of opportunity for early-life preventive interventions (33). In this study, we quantified one-carbon–related metabolites in human milk to shed more light into the potential role of these metabolites in infant growth and obesity risk. Among these metabolites, betaine was associated with changes in weight-for-length z score during the first month of life in both the discovery and the replication cohorts. It is worth noting that these cohorts are based in two geographically different regions, Oklahoma (United States) and the Spanish Mediterranean area (Europe), with distinct dietary and lifestyle habits. Furthermore, data from the second cohort showed that the association between milk betaine content and growth was still present in 12-month-old infants. Although studies investigating breast milk composition and infant health are sparse (34), a small number of other metabolites and other components have been identified associated with growth. Differences in milk oligosaccharide diversity and hormone concentrations (insulin and leptin) have been correlated with infant growth, adiposity, and gut microbiota composition (21, 25, 35). Furthermore, we recently reported the milk metabolome associated with maternal obesity, with only adenine correlating with both maternal BMI and infant weight status (36).
Our data from both cohorts indicated an association between lower milk betaine content and higher infant growth during the first months of life. Lower maternal betaine status during pregnancy has been correlated with increased infant birth weight and adiposity (20), and maternal betaine supplementation during gestation resulted in lower fetal adiposity in a mouse study (37). Together with our results on human breast milk, these data support a link between lower maternal betaine status during pregnancy and breastfeeding and increased fetal and postnatal growth. Accelerated growth during early infancy (as early as the first weeks of life) has been consistently associated with obesity years later (5–8, 38, 39). Therefore, our results suggest a link between lower milk betaine content and higher childhood obesity risk.
Betaine is naturally present in the diet and can also be derived endogenously from choline. A human study showed that providing a choline supplement during lactation increased both plasma and milk betaine concentrations (12), suggesting that dietary choline may modulate milk betaine concentration. Furthermore, our experiments in mice showed that maternal betaine supplementation increased milk content of this metabolite, indicating that milk betaine content can be readily modulated by dietary intake. Betaine is abundant in whole grains and some vegetables and scarce in Western dietary patterns (40). Further studies are necessary to determine whether consumption of betaine-rich or choline-rich foods determines milk betaine status.
Despite the fact that early growth trajectories are largely divergent between rodents and humans, our supplementation experiments in mice paralleled the association between milk betaine content and early growth observed in the human studies. Moreover, early-life exposure to betaine had long-lasting metabolic consequences in mice, decreasing adiposity and improving glucose homeostasis during adulthood. A number of animal models support a link between nutritional challenges during early development, growth trajectories, and long-term metabolic risk. For instance, low birth weight or neonatal overfeeding result in accelerated growth, leading to obesity and metabolic dysregulation during adulthood (41). Conversely, decreasing early growth by postnatal undernutrition proved beneficial to prevent future obesity (42). Betaine has long been used as a feed additive in weaned livestock animals due to its carcass-modifier properties, increasing lean versus fat mass ratio (43); in this context, our results indicate that betaine supplementation during the suckling period might suffice for decreasing long-term adiposity.
The early-life gut microbiome has recently emerged as an important determinant of long-term disease risk (26, 33, 44, 45). Mode of delivery and early nutrition are major determinants in shaping the infant intestinal microbiome. In particular, breast milk stimulates the proliferation of a balanced gut microbiome, the development of a healthy intestine, and maturation of the immune system (44, 45). The transition from milk to solid food induces broad changes in nutrient availability and metabolism, as well as in the gut microbiota composition and ileal epithelial structure. Microbial dysbiosis during infancy has been associated with a number of diseases years later, including obesity (4, 24–26, 44, 46, 47). For instance, antibiotic use during infancy has been associated with childhood obesity (4, 47), and maternal low-dose antibiotic administration during lactation in mice led to overweight offspring (26).
Our results suggest that the betaine-induced effects on metabolic health are, at least in part, mediated by the early-life gut microbiota. We showed that maternal antibiotic coadministration blunted betaine-induced improvements in offspring metabolic health; however, we recognize that antibiotics induce broad changes in the gut microbiome and exert a wide range of effects on the host, including alterations in intestinal permeability and adiposity that could be independent of betaine’s mechanism of action (26). Furthermore, we observed that maternal betaine supplementation transiently increased Akkermansia spp. abundance in the intestines of offspring during early life and that betaine was capable of enhancing A. muciniphila growth in vitro. Data from our human cohort further strengthened the link between milk betaine and this microorganism, as exposure to higher milk betaine content during breastfeeding resulted in higher A. muciniphila abundance in 12-month-old infants. Finally, administration of pAkk to mouse pups during the lactation period partially recapitulated maternal betaine effects. Together, these data suggest a role for this microorganism in mediating betaine’s effects. Betaine also acts as a methyl group donor in the methionine cycle, ultimately providing SAM and increasing glutathione availability (16, 19). Thus, additional mechanisms related to oxidative stress, epigenetic changes, or phosphatidylcholine biosynthesis may also contribute to betaine-induced improvements in metabolic health.
We have considered different mechanisms by which maternal betaine could modulate offspring intestinal Akkermansia spp. abundance. Betaine supplementation did not alter milk macronutrient composition or energy content, although it could modify other components, including hormones or immune system elements, that may affect bacterial communities in the offspring. However, our in vitro experiments showing enhanced A. muciniphila growth in the presence of betaine support the hypothesis of a direct effect of betaine on this microorganism. Owing to its dipolar zwitterion structure and osmoprotectant properties, bacteria readily use betaine as protection against external stress (18). Thus, betaine could be metabolized or used as an osmolyte by Akkermansia. Betaine was not equally effective in modulating growth of other bacteria, suggesting some specificity toward Akkermansia. However, growth characteristics of a single bacterium in vitro can differ in the presence of other bacteria or under different conditions, where symbiotic or competitive interactions can greatly determine growth dynamics of specific bacteria. In this regard, it is worth noting that betaine supplementation increased Akkermansia spp. abundance in mouse pups but not in dams, suggesting a context-dependent effect of betaine. The structure and diversity of the gut microbiota varies greatly between newborns and adults, and the early-life gut microbiota could potentially provide a more favorable environment for Akkermansia growth in the presence of betaine. Furthermore, Akkermansia might also be a constituent of breast tissue microbiota in humans (48) and betaine could increase its abundance in breast tissue or milk, ultimately affecting the offspring gut microbiota. Thus, further experiments would be needed to decipher the exact mechanisms by which betaine increases A. muciniphila growth and whether it can exert this effect in different environments.
Akkermansia has received much attention, mostly due to reports showing that decreased abundance is associated with obesity and metabolic disorders in both humans and animal models, suggesting a beneficial role for this bacterial species (28–30, 49). Furthermore, a recent report linked A. muciniphila to increased life span in a mouse model of accelerated aging (31). Conversely, other studies have reported increased Akkermansia abundance in patients with neurological disorders, including multiple sclerosis, Parkinson’s disease, and Alzheimer’s disease (50–53). These data highlight the notion that interactions between microbes and host are highly dependent on multiple factors (environment, genetic background, and type of disease) and the specific effects of Akkermansia might also be context dependent (54, 55).
A number of studies have shown that daily administration of A. muciniphila to adult mice decreases body weight gain and adiposity and improves glucose homeostasis in both diet-induced obese mice (28–30) and lean chow diet–fed mice (56). Depommier et al. (32) recently reported results from a clinical trial in which administration of A. muciniphila improved insulin sensitivity in human subjects with obesity. Administration of pAkk to either mice or humans has shown similar effects on metabolic parameters as the live form (28, 32), indicating that these effects are independent of bacterial metabolism. Administration of an Akkermansia-specific extracellular membrane protein (Amuc_1100) to mice recapitulated most of the effects of the live bacteria (28). Our results show that administration of pAkk to pups during early life partially recapitulated betaine’s effects, including lower early weight gain. Although the mechanistic link between Akkermansia and growth remains unclear, lower Akkermansia abundance has been associated with shorter breast-feeding duration and increased weight gain in children (4). Moreover, an increase in Akkermansia has been correlated with slower early weight gain in a mouse model of postnatal undernutrition (57). Together with these studies, our data add to the evidence that Akkermansia abundance during early life may be linked to postnatal growth.
Several studies indicate that the beneficial effects of A. muciniphila are mediated, at least in part, by improving the intestinal barrier function. Daily administration of A. muciniphila to adult obese mice for 4 to 5 weeks increased ileal goblet cell number and expression of intestinal barrier markers, leading to decreased systemic inflammation and improved metabolic health (28 30).Our data show that, in parallel to transiently increasing Akkermansia abundance, maternal betaine supplementation induced a long-lasting increase in offspring ileal goblet cell number and expression of intestinal barrier markers, decreased systemic inflammation and adiposity, and improved glucose homeostasis. The increase in goblet cells was observed at 6 weeks of age but not during lactation. The reason for this could be that the effect on goblet cell number might only be evident after weaning or, alternatively, that 2 weeks of maternal betaine supple mentation was able to increase Akkermansia abundance in offspring but was not sufficient to induce the response in goblet cells yet. Furthermore, pAkk administration during lactation led to a lasting increase in intestinal goblet cell number after weaning, in parallel to improvements in adiposity and glucose homeostasis during adulthood.
We observed a strong short-term effect of early-life pAkk administration on goblet cells and intestinal barrier markers; however, its long-term effects were more modest than with betaine supple mentation. Although these data might suggest the existence of addi tional mechanisms mediating betaine action, it is worth noting that pAkk administration was started at day 11 after birth because gavage was not feasible in smaller pups. The first 10 days of life consti tute an important developmental window, and it is plausible that pAkk exposure during this crucial period of time would trigger an even more robust long-term effect. The neonatal period offers a window of opportunity in which important processes including the development and maturation of the gut microbiota and intestinal barrier, imprinting of the immune system, or the growth trajectory may influence health outcomes across the life span (8, 33, 44, 45). Exposure to elevated milk insulin and leptin in infants during the first weeks of life has been proposed to exert beneficial effects by improving the intestinal barrier function (25). It is intriguing to speculate that modulating intestinal development and barrier function during this critical developmental window may have a role in programming life-course metabolic health.
There are several limitations to our study including that maternal diet during breastfeeding was not controlled and might have affected milk metabolite concentrations. Furthermore, milk metabolites were determined at a single time point (1 month after birth), where-as milk composition is dynamic and may change over time. In the animal study, our data do not rule out the possibility that betaine supplementation might have altered other compounds that could potentially exert effects on the mouse offspring gut microbiota and long-term metabolic health. Last, betaine bioavailability and tissue distribution (for instance, transfer into breast milk) might differ between mice and humans. Despite these shortcomings, the association between milk betaine content and infant growth was robust and sustained adjustment for confounding variables in two independent and geographically different cohorts. Furthermore, our data demonstrate a link between breast milk betaine content and off-spring long-term metabolic health. The implications of this study might go beyond breastfeeding, as formula-fed infants are at higher risk of accelerated early growth and childhood obesity and might benefit from revisiting one-carbon metabolite concentrations in artificial milk. In summary, this study highlights the breastfeeding period as an important window of opportunity for early interventions and suggests that modulating betaine intake during lactation may hold promise as a strategy for childhood obesity prevention.
MATERIALS AND METHODS
Study design
The study was designed to investigate potential associations of milk one-carbon metabolites with infant early growth in humans and in mice. For the human studies, we analyzed breast milk samples from a discovery cohort (n = 34, cohort I) and a replication cohort (n = 109, cohort II). These cohorts were part of two ongoing studies and had both breast milk samples and infant growth data available. Subject number was determined by the number of breast milk samples and mother-infant dyads available in both cohorts. For the mouse studies, we investigated whether maternal supplementation with betaine exclusively during the lactation period had an impact on growth and long-term metabolic health of offspring. For this purpose, male and female mice were mated to obtain progeny. At delivery, dams were randomly assigned to control or treatment groups and offspring were monitored until adulthood. For all experiments, litters were adjusted at birth to eight pups, removing the heaviest and lightest mice to minimize growth variability (58). Two offspring mice were retired from experiments because of lesions, as established in the animal protocols, and were euthanized; one was from the MO-C group in the pasteurized Akkermansia administration experiment (Fig. 4) at 20 weeks of age and the other was from the betaine group in the antibiotic supplementation experiment (fig. S3) at 16 weeks of age. All animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Barcelona.
Human studies
Cohort I: This human cohort was previously reported (21). The study was approved by institutional review board, and informed consent was obtained (ClinicalTrials.gov NCT02535637). Briefly, exclusively breastfeeding mothers and infants arrived between 8:00 and 10:00 a.m. on the campus of the University of Oklahoma Health Sciences (Oklahoma City, OK, USA). The mother was encouraged to empty the entire breast using a hospital-grade breast pump. Cohort II: A prospective mother-infant birth cohort from the Spanish-Mediterranean area was also analyzed (22). The study was approved by the Ethics committees (ref. 2018/0024 at the Hospital Universitario y Politécnico La Fe), and informed consent was obtained from all participants (ClinicalTrials.gov NCT03552939). Women were enrolled at the end of pregnancy and families were followed up during the first year of life. Participants with singleton pregnancy and exclusive breastfeeding for at least 3 months were included in this study. For the standardized milk collection, breast skin was cleaned with 0.5% chlorhexidine solution and first drops were discarded. Breast milk samples were collected during the morning at 1 month postpartum by use of sterile hospital-grade breast pump. Infant weight and length were measured from birth up to 12 months of age. Infant fecal samples were also collected at 1 and 12 months. For both cohorts, infant anthropometric measure-ments were collected by trained staff using standard methods. Weight-for-length z scores were calculated using the World Health Organization (WHO) child growth tables, with the WHO Anthro software (www.who.int/childgrowth/software/en/).
Mouse studies
Mice were housed on a 12-hour light-dark cycle with free access to food and water. Male and female ICR-CD1 mice (Envigo) were mated to obtain progeny, and male offspring was monitored until adulthood. For maternal obesity studies, 6-week-old females were fed a high-fat diet with 45% kcal from fat (D12451, Research Diets) for 8 weeks before mating, and the same diet was maintained through pregnancy and lactation. In both models, betaine (1% w/v; ref. 61962, Sigma-Aldrich) was administered to dams in water during the lactation period. For the antibiotic coadministration experiment, ampicilin (1 g/liter) and neomycin (0.5 g/liter) (Sigma-Aldrich) were administered to the dams in water during the lactation period. After weaning, mice were fed 2014 Teklad diet (Envigo) in all experiments. Milk intake was assessed by separating 6-day-old pups from their mothers for 2 hours and returning them to their cages for a 1-hour feeding period. Pup body weight was measured before and after the feeding, and the difference in weight indicated the amount of milk intake, expressed as grams per initial body weight per hour. Individual data for pups from a single litter were pooled, and one point per litter was used for the analysis. Milk samples (100 to 300 μl) were collected manually under anesthesia at day 14 after birth, after separating dams from pups for 4 hours. Body composition at 6 weeks of age was determined by using a 7.0 T Bruker Biospect MRI system (Bruker Medical Gmbh). Indirect calorimetry was measured in metabolic cages (PhenoMaster/LabMaster, TSE Systems GmbH, Germany); O2, CO2, food intake, and locomotor activity were monitored for 48 hours, and data were analyzed using two-way ANOVA for repeated measures. Intraperitoneal glucose (1.5 g/kg) tolerance test was performed after a 16-hour fast. Mice were euthanized between 9 and 11 a.m.
Biochemical analyses
One-carbon metabolites (choline, betaine, methionine, SAM, SAH, and cystathionine) in human breast milk samples were determined using stable-isotope dilution liquid chromatography–electrospray ionization–tandem mass spectrometry as previously described (59). For mouse milk, relative betaine concentrations were determined by liquid chromatography (Acquity UPLC BEH HILIC column, Waters) coupled to mass spectrometry (QqQ/MS 6490, Agilent). Mouse milk samples were diluted 1:3 before macronutrient analysis by mid-infrared spectroscopy in a Miris Analyzer (Miris AB). Plasma insulin was determined by enzyme-linked immunosorbent assay (Millipore). Plasma Ccl2, IL-6, and PAI-1 were measured using the Milliplex Map (EMD Millipore).
Gene expression and histological analysis
Total tissue RNA was isolated from tissues with TRI Reagent (Sigma-Aldrich) and complementary DNA (cDNA) obtained with a highcapacity cDNA kit (Applied Biosystems). qPCR was performed with SYBR green (Takara Bio), using Hprt as housekeeping gene. Primer sequences are provided in table S3. Tissues were fixed by immersion in formalin, paraffin embedded, and sections stained with hematoxylin and eosin. For WAT, adipocyte area was measured in 30 random microscopic fields per animal (10 to 20 cells per field). For ileum, cross-sectional cuts were stained with Periodic acid–Schiff method. Ten villi per mouse from five to six sections were analyzed. Goblet cell number per villus was determined and expressed as number of cells per 100 μm. Analyses were performed using ImageJ by a researcher blinded to sample ID.
Gut microbiota analysis
Cecal content from dams, 2-and 6-week-old mice from control and betaine-treated groups was collected and stored at −80°C until bacterial DNA was isolated using the PowerSoil DNA Isolation Kit (MOBIO Laboratories). Microbial composition was analyzed using Illumina MiSeq System to sequence the V3-V4 region of the 16S ribosomal RNA gene following Illumina recommendations, obtaining an average of 24,833 reads per sample. Paired-end reads were processed with QIIME v1.9 performing demultiplexing and quality filtering, operational taxonomic unit (OTU) picking and taxonomic assignment from phyla to species level. Taxonomic assignment was performed using closed reference OTU picking protocol with Greengenes database (http://greengenes.lbl.gov). Unweighted UniFrac distances were used to perform the PCoA and compare community structure. Alpha diversity (Chao1 and Shannon indexes) was also determined. Only taxa with a minimum relative abundance of 0.1% in at least one of the samples were analyzed. LEfSe algorithm (60) was used to identify differences in relative abundance.
In vitro evaluation of microbial growth
Assays for the evaluation of the influence of betaine in A. muciniphila growth were performed with cells grown in BHI:MRS-C (10 ml per replicate) broth at 37°C in anaerobic chamber (Whitley A35 anaerobic workstation). Cells were inoculated in BHI:MRS-C broth supplemented with different betaine concentrations (2.5 and 10 mM). Medium without betaine supplementation was included as a control. Cells were incubated anaerobically at 37°C until reaching stationary phase. Total DNA was extracted from cell pellets obtained from 1 ml of culture media using the “QIAamp DNA Mini Kit” (Qiagen Inc.) according to the manufacturer’s manual. The total amount of A. muciniphila was determined by qPCR using specific primers for the species, as previously described (27), with the aid of SYBR Green PCR Master Mix (Applied Biosystems). Standard curve was constructed with DNA coming from 10-fold diluted standardized DSM 22959T fresh cells. Lactobacillus johnsonii (BPL130) and B. longum (CECT7347) were anaerobically grown in MRS medium with cysteine and E. coli [American Type Culture Collection (ATCC) 11303] aerobically in LB medium at 37°C. After 17 hours, cultures were harvested, washed, standardized with McFarland standard, and resuspended in MRS medium for lactobacilli and bifidobacteria and LB for E. coli, containing the different concentrations of betaine. Cell suspensions were incubated at 37°C until reaching stationary phase in a microplate reader (Multiskan Ascent) and number of cells per milliliter were determined by optical density at stationary phase (600 nm). All assays were performed in triplicate.
A. muciniphila qPCR analysis in infant fecal samples
Sample collection, storage, and DNA extraction in cohort II were described elsewhere (22). Available infant fecal DNA samples were used to quantify the presence and abundance of A. muciniphila. Specific A. muciniphila qPCR was performed in a LightCycler 480 real-time PCR System (Roche Technologies, Basel, Switzerland) as previously reported (27). A melting curve analysis was made after amplification to distinguish the targeted PCR. Standard curve was created using serial 10-fold dilutions of A. muciniphila pure culture DNA corresponding to 102 to 1010 gene fragment numbers. The bacterial concentration of each sample was calculated by comparing the threshold cycle values obtained from the standard curve, and expressed as log number of copies per milligram of fecal sample.
A. muciniphila administration to mice
A. muciniphila strain DSM 22959T (ATCC BAA-835) was initially grown in a previously described mucin-based medium (61). Subsequent cultures and assays were done in BHI:MRS-C medium consisted in BHI [80% (v/v); Oxoid, Basingstoke, UK], MRS [20% (v/v); Oxoid]: [80%:20% (v/v); Oxoid] supplemented with 0.10% (w/v) cysteine (Sigma-Aldrich). Cultures were incubated at 37°C in anaerobic chamber [H2/CO2/N2 10%/10%/80% (v/v)]. Counts were obtained on BHI:MRS-C agar (Oxoid) incubated in anaerobic chamber at 37°C for 48 hours. Pasteurized pellet was prepared following (28), with minor modifications. Briefly, cells grown on BHI:MRS-C broth were washed with PBS and concentrated under anaerobic conditions. Pellets were pasteurized at 70°C during 30 min and frozen immediately at −80°C. Cells obtained were quantified by qPCR using specific primers (table S3). A dose of 108 colony-forming units in PBS (or the equivalent volume of PBS for controls) was administered by gavage to suckling pups three times per week starting on day 11 until weaning (day 21).
Statistical analysis
Demographic and physiologic characteristics of the human participants are shown as means and SD. Normality was tested using the Shapiro-Wilk test and nonnormal distributed variables were log transformed before further analysis. Associations between variables were assessed by standard least squares multivariate linear regression with changes in weight-for-length z score as the dependent variable and log-transformed milk metabolite concentrations as an independent variable, adjusting for potential confounding factors including gestational age, weight-for-length z score at birth, maternal prepregnancy BMI, gestational weight gain, and birth method where indicated. For mouse experiments, unless otherwise stated, data are presented as means and SEM. Two-tailed t test, Mann-Whitney U test (for non-normal distribution), or one-way ANOVA (for more than two groups) were applied to analyze differences between groups. A P < 0.05 was considered statistically significant. Statistical analyses were implemented in JMP v14 (SAS Institute Inc., Cary, NC). For figures showing composite data, individual data points are provided in data file S1.
Supplementary Material
Fig. S1. Effect of maternal betaine administration on young mouse offspring.
Fig. S2. Effects of maternal betaine administration on mouse offspring energy homeostasis.
Fig. S3. Effects of maternal antibiotic coadministration on offspring long-term metabolic health in mice.
Fig. S4. Effect of betaine administration on the maternal and offspring gut microbiome in mice.
Fig. S5. Effect of betaine on bacterial growth in vitro.
Fig. S6. Effect of maternal betaine supplementation on mouse ileum histology and gene expression.
Table S1. No association between milk betaine concentration and change in human infant body length z score and head circumference.
Table S2. Prevalence of A. muciniphila in human infants exposed to low and high breast milk betaine content.
Table S3. Primer sequences for qPCR analyses.
Data file S1. Individual level data for all figures.
Acknowledgments
Funding: This work was supported by grants from Ajinomoto Innovation Alliance Program (Ajinomoto 2015-2017) to CL, EFSD/Lilly programme to C.L., and the Spanish Government (SAF-2017-88005-R to C.L. and BFU2017-89336-R to M.V.). D.S.-I. and J.C.J.-C. are Miguel Servet investigators (Carlos III National Institute of Health, Spain). The MILK Study is supported by NIH grant R01-HD080444 to D.A.F. and E.W.D. Mead Johnson Nutrition provided financial support to D.A.F. for breast milk collection, but had no role in study design, study results, or manuscript content. M.C.C. and I.G.-M. acknowledge support from European Research Council under the European Union’s Horizon 2020 research and innovation programme (ERC Starting Grant 639226).
Footnotes
Competing interests: M.R.-K., J.C.J.-C., and C.L. are coinventors on patent application 17382270.1-1466 entitled “Betaine for the prevention of obesity,” filed in the European Patent Office (May 12, 2017). E.C. is an employee of Biópolis S.L.-ADM (Spain). The other authors declare that they have no competing interests.
Data and materials availability: All data associated with this study are available in the manuscript or the Supplementary Materials. The metagenome data have been deposited in the SRA (sequence read archive) database of NCBI (National Centre for Biotechnology Information), with accession number PRJNA670842.
REFERENCES AND NOTES
- 1.Gluckman PD, Hanson MA, Cooper C, Thornburg KL, Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 359, 61–73 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heerman WJ, Bian A, Shintani A, Barkin SL, Interaction between maternal prepregnancy body mass index and gestational weight gain shapes infant growth. Acad. Pediatr 14, 463–470 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan J, Liu L, Zhu Y, Huang G, Wang PP, The association between breastfeeding and childhood obesity: A meta-analysis. BMC Public Health 14, 1267 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korpela K, Salonen A, Virta LJ, Kekkonen RA, de Vos WM, Association of early-life antibiotic use and protective effects of breastfeeding: Role of the intestinal microbiota. JAMA Pediatr. 170, 750–757 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Andersen LG, Holst C, Michaelsen KF, Baker JL, Sørensen TI, Weight and weight gain during early infancy predict childhood obesity: A case-cohort study. Int. J. Obes 36, 1306–1311 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Taveras EM, Rifas-Shiman SL, Belfort MB, Kleinman KP, Oken E, Gillman MW, Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics 123, 1177–1183 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aris IM, Rifas-Shiman SL, Li L-J, Yang S, Belfort MB, Thompson J, Hivert M-F, Patel R, Martin RM, Kramer MS, Oken E, Association of weight for length vs body mass index during the first 2 years of life with cardiometabolic risk in early adolescence. JAMA Netw. Open 1, e182460 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leunissen RW, Kerkhof GF, Stijnen T, Hokken-Koelega A, Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA 301, 2234–2242 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Hibbard BM, Hibbard ED, Jeffcoate TN, Folic acid and reproduction. Acta Obstet. Gynecol. Scand 44, 375–400 (1965). [DOI] [PubMed] [Google Scholar]
- 10.Wang G, Hu FB, Mistry KB, Zhang C, Ren F, Huo Y, Paige D, Bartell T, Hong X, Caruso D, Ji Z, Chen Z, Ji Y, Pearson C, Ji H, Zuckerman B, Cheng TL, Wang X, Association between maternal prepregnancy body mass index and plasma folate concentrations with child metabolic health. JAMA Pediatr. 170, e160845 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeisel SH, Choline: Critical role during fetal development and dietary requirements in adults. Annu. Rev. Nutr 26, 229–250 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer LM, da Costa KA, Galanko J, Sha W, Stephenson B, Vick J, Zeisel SH, Choline intake and genetic polymorphisms influence choline metabolite concentrations in human breast milk and plasma. Am. J. Clin. Nutr 92, 336–346 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craig SA, Betaine in human nutrition. Am. J. Clin. Nutr 80, 539–549 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Lever M, Slow S, The clinical significance of betaine, an osmolyte with a key role in methyl group metabolism. Clin. Biochem 43, 732–744 (2010). [DOI] [PubMed] [Google Scholar]
- 15.Konstantinova SV, Tell GS, Vollset SE, Nygard O, Bleie O, Ueland PM, Divergent associations of plasma choline and betaine with components of metabolic syndrome in middle age and elderly men and women. J. Nutr 138, 914–920 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Ejaz A, Martinez-Guino L, Goldfine AB, Ribas-Aulinas F, De Nigris V, Ribo S, Gonzalez-Franquesa PM Garcia-Roves, Li E, Dreyfuss JM, Gall W, Kim JK,Bottiglieri T, Villarroya F, Gerszten RE, Patti ME, Lerin C, Dietary betaine supplementation increases Fgf21 levels to improve glucose homeostasis and reduce hepatic lipid accumulation in mice. Diabetes 65, 902–912 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walford GA, Ma Y, Clish C, Florez JC, Wang TJ, Gerszten RE; Diabetes Prevention Program Research Group, Metabolite profiles of diabetes incidence and intervention response in the diabetes prevention program. Diabetes 65, 1424–1433 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Rudulier D, Strom AR, Dandekar AM, Smith LT, Valentine RC, Molecular biology of osmoregulation. Science 224, 1064–1068 (1984). [DOI] [PubMed] [Google Scholar]
- 19.Mato JM, Martínez-Chantar ML, Lu SC, Methionine metabolism and liver disease. Annu. Rev. Nutr 28, 273–293 (2008). [DOI] [PubMed] [Google Scholar]
- 20.van Lee L, Tint MT, Aris IM, Quah PL, Fortier MV, Lee YS, Yap FK, Saw SM, Godfrey KM, Gluckman PD, Chong YS, Kramer MS, Chong MF, Prospective associations of maternal betaine status with offspring weight and body composition at birth: The Growing Up in Singapore Towards Healthy Outcomes (GUSTO) cohort study. Am. J. Clin. Nutr 104, 1327–1333 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Fields DA, Demerath EW, Relationship of insulin, glucose, leptin, IL-6 and TNF-a in human breast milk with infant growth and body composition. Pediatr. Obes 7, 304–312 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Mantrana I, Alcantara C, Selma-Royo M, Boix-Amoros A, Dzidic M, Gimeno-Alcaniz J, Ubeda-Sansano I, Sorribes-Monrabal I, Escuriet R, Gil-Raga F, Parra-Llorca A, Martinez-Costa C, Collado MC; MAMI team MAMI: A birth cohort focused on maternalinfant microbiota during early life. BMC Pediatr. 19, 140 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howie GJ, Sloboda DM, Kamal T, Vickers MH, Maternal nutritional history predicts obesity in adult offspring independent of postnatal diet. J. Physiol 587, 905–915 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bokulich NA, Chung J, Battaglia T, Henderson N, Jay M, Li H, Lieber AD, Wu F, Perez-Perez GI, Chen Y, Schweizer W, Zheng X, Contreras M, Dominguez-Bello MG, Blaser MJ, Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med 8, 343ra382 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemas DJ, Young BE, Baker PR, Tomczik AC, Soderborg TK, Hernandez TL, de la Houssaye BA, Robertson CE, Rudolph MC, Ir D, Patinkin ZW, Krebs NF, Santorico SA, Weir T, Barbour LA, Frank DN, Friedman JE, Alterations in human milk leptin and insulin are associated with early changes in the infant intestinal microbiome. Am. J. Clin. Nutr 103, 1291–1300 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, Kim SG, Li H, Gao Z, Mahana D, Zárate-Rodriguez JG, Rogers AB, Robine N, Loke P, Blaser MJ, Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 158, 705–721 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collado MC, Derrien M, Isolauri E, de Vos WM, Salminen S, Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl. Environ. Microbiol 73, 7767–7770 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, Chilloux J, Ottman N, Duparc T, Lichtenstein L, Myridakis A, Delzenne NM, Klievink J, Bhattacharjee A, van der Ark KC, Aalvink S, Martinez LO, Dumas ME, Maiter D, Loumaye A, Hermans MP, Thissen JP, Belzer C, de Vos WM, Cani PD, A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med 23, 107–113 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Shin N-R, Lee J-C, Lee H-Y, Kim M-S, Whon TW, Lee M-S, Bae J-W, An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 63, 727–735 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD, Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. U.S.A 110, 9066–9071 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bárcena C, Valdés-Mas R, Mayoral P, Garabaya C, Durand S, Rodríguez F, Fernández-García MT, Salazar N, Nogacka AM, Garatachea N, Bossut N, Aprahamian F, Lucia A, Kroemer G, Freije JMP, Quirós PM, López-Otín C, Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat. Med 25, 1234–1242 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, Falony G, Raes J, Maiter D, Delzenne NM, de Barsy M, Loumaye A, Hermans MP, Thissen J-P, de Vos WM, Cani PD, Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med 25, 1096–1103 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soderborg TK, Borengasser SJ, Barbour LA, Friedman JE, Microbial transmission from mothers with obesity or diabetes to infants: An innovative opportunity to interrupt a vicious cycle. Diabetologia 59, 895–906 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eriksen KG, Christensen SH, Lind MV, Michaelsen KF, Human milk composition and infant growth. Curr. Opin. Clin. Nutr. Metab. Care 21, 200–206 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Alderete TL, Autran C, Brekke BE, Knight R, Bode L, Goran MI, Fields DA, Associations between human milk oligosaccharides and infant body composition in the first 6 mo of life. Am. J. Clin. Nutr 102, 1381–1388 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Isganaitis E, Venditti S, Matthews TJ, Lerin C, Demerath EW, Fields DA, Maternal obesity and the human milk metabolome: Associations with infant body composition and postnatal weight gain. Am. J. Clin. Nutr 110, 111–120 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joselit Y, Nanobashvili K, Jack-Roberts C, Greenwald E, Malysheva OV, Caudill MA, Saxena X. Jiang, Maternal betaine supplementation affects fetal growth and lipid metabolism of high-fat fed mice in a temporal-specific manner. Nutr. Diabetes 8, 41 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stettler N, Stallings VA, Troxel AB, Zhao J, Schinnar R, Nelson SE, Ziegler EE, Strom BL, Weight gain in the first week of life and overweight in adulthood: A cohort study of European American subjects fed infant formula. Circulation 111, 1897–1903 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Singhal A, Cole TJ, Fewtrell M, Deanfield J, Lucas A, Is slower early growth beneficial for long-term cardiovascular health? Circulation 109, 1108–1113 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Konstantinova SV, Tell GS, Vollset SE, Ulvik A, Drevon CA, Ueland PM, Dietary patterns, food groups, and nutrients as predictors of plasma choline and betaine in middle-aged and elderly men and women. Am. J. Clin. Nutr 88, 1663–1669 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Pentinat T, Ramon-Krauel M, Cebria J, Diaz R, Jimenez-Chillaron JC, Transgenerational inheritance of glucose intolerance in a mouse model of neonatal overnutrition. Endocrinology 151, 5617–5623 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Jimenez-Chillaron JC, Hernandez-Valencia M, Lightner A, Faucette RR, Reamer C, Przybyla R, Ruest S, Barry K, Otis JP, Patti ME, Reductions in caloric intake and early postnatal growth prevent glucose intolerance and obesity associated with low birthweight. Diabetologia 49, 1974–1984 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Eklund M, Bauer E, Wamatu J, Mosenthin R, Potential nutritional and physiological functions of betaine in livestock. Nutr. Res. Rev 18, 31–48 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Arrieta M-C, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B, The intestinal microbiome in early life: Health and disease. Front. Immunol 5, 427 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al Nabhani Z, Eberl G, Imprinting of the immune system by the microbiota early in life. Mucosal Immunol. 13, 183–189 (2020). [DOI] [PubMed] [Google Scholar]
- 46.Forbes JD, Azad MB, Vehling L, Tun HM, Konya TB, Guttman DS, Field CJ, Lefebvre D, Sears MR, Becker AB, Mandhane PJ, Turvey SE, Moraes TJ, Subbarao P, Scott JA, Kozyrskyj AL; Canadian Healthy Infant Longitudinal Development (CHILD) Study Investigators, Association of exposure to formula in the hospital and subsequent infant feeding practices with gut microbiota and risk of overweight in the first year of life. JAMA Pediatr. 172, e181161 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bailey L, Forrest CB, Zhang P, Richards TM, Livshits A, DeRusso PA, Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 168, 1063–1069 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Urbaniak C, Cummins J, Brackstone M, Macklaim JM, Gloor GB, Baban CK, Scott L, O’Hanlon DM, Burton JP, Francis KP, Tangney M, Reid G, Microbiota of human breast tissue. Appl. Environ. Microbiol 80, 3007–3014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L, Consortium M-O, Dumas M-E, Rizkalla SW, Doré J, Cani PD, Clément K, Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 65, 426–436 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Berer K, Gerdes LA, Cekanaviciute E, Jia X, Xiao L, Xia Z, Liu C, Klotz L, Stauffer U, Baranzini SE, Kümpfel T, Hohlfeld R, Krishnamoorthy G, Wekerle H, Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc. Natl. Acad. Sci 114, 10719–10724 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cekanaviciute E, Yoo BB, Runia TF, Debelius JW, Singh S, Nelson CA, Kanner R, Bencosme Y, Lee YK, Hauser SL, Crabtree-Hartman E, Sand IK, Gacias M, Zhu Y, Casaccia P, Cree BAC, Knight R, Mazmanian SK, Baranzini SE, Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc. Natl. Acad. Sci 114, 10713–10718 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC, Carlsson CM, Asthana S, Zetterberg H, Blennow K, Bendlin BB, Rey FE, Gut microbiome alterations in Alzheimer’s disease. Sci. Rep 7, 13537 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heintz-Buschart A, Pandey U, Wicke T, Sixel-Döring F, Janzen A, Sittig-Wiegand E, Trenkwalder C, Oertel WH, Mollenhauer B, Wilmes P, The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov. Disord 33, 88–98 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cirstea M, Radisavljevic N, Finlay BB, Good bug, bad bug: Breaking through microbial stereotypes. Cell Host Microbe 23, 10–13 (2018). [DOI] [PubMed] [Google Scholar]
- 55.Ansaldo E, Slayden LC, Ching KL, Koch MA, Wolf NK, Plichta DR, Brown EM, Graham DB, Xavier RJ, Moon JJ, Barton GM, Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science 364, 1179–1184 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao S, Liu W, Wang J, Shi J, Sun Y, Wang W, Ning G, Liu R, Hong J, Akkermansia muciniphila improves metabolic profiles by reducing inflammation in chow diet-fed mice. J. Mol. Endocrinol 58, 1–14 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Preidis GA, Ajami NJ, Wong MC, Bessard BC, Conner ME, Petrosino JF, Microbial-derived metabolites reflect an altered intestinal microbiota during catch-up growth in undernourished neonatal mice. J. Nutr 146, 940–948 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parra-Vargas M, Ramon-Krauel M, Lerin C, Jimenez-Chillaron JC, Size does matter: Litter size strongly determines adult metabolism in rodents. Cell Metab. 32, 334–340 (2020). [DOI] [PubMed] [Google Scholar]
- 59.Inoue-Choi M, Nelson HH, Robien K, Arning E, Bottiglieri T, Koh W-P, Yuan J-M, One-carbon metabolism nutrient status and plasma S-adenosylmethionine concentrations in middle-aged and older Chinese in Singapore. Int. J. Mol. Epidemiol. Genet 3, 160–173 (2012). [PMC free article] [PubMed] [Google Scholar]
- 60.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C, Metagenomic biomarker discovery and explanation. Genome Biol. 12, R60 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Derrien M, Vaughan EE, Plugge CM, de Vos WM, Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. System. Evol. Microbiol 54, 1469–1476 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Effect of maternal betaine administration on young mouse offspring.
Fig. S2. Effects of maternal betaine administration on mouse offspring energy homeostasis.
Fig. S3. Effects of maternal antibiotic coadministration on offspring long-term metabolic health in mice.
Fig. S4. Effect of betaine administration on the maternal and offspring gut microbiome in mice.
Fig. S5. Effect of betaine on bacterial growth in vitro.
Fig. S6. Effect of maternal betaine supplementation on mouse ileum histology and gene expression.
Table S1. No association between milk betaine concentration and change in human infant body length z score and head circumference.
Table S2. Prevalence of A. muciniphila in human infants exposed to low and high breast milk betaine content.
Table S3. Primer sequences for qPCR analyses.
Data file S1. Individual level data for all figures.