Abstract
The Systolic Blood Pressure Intervention Trial (SPRINT) results have influenced clinical practice but have also generated discussion regarding the validity, generalizability and importance of the findings. Following the SPRINT primary results manuscript in 2015, additional results and analyses of the data have addressed these concerns. The primary objective of this manuscript is to respond to key questions that have been raised.
The Systolic Blood Pressure Intervention Trial (SPRINT) demonstrated the effectiveness of treating SBP to levels well below those previously recommended in US and European BP guidelines.1 The SPRINT results have informed guideline committees in recommending treatment to SBP targets lower than previously advised.2–4 However, some of the SPRINT findings have generated discussion, and questions have been raised regarding their application in clinical practice. These include generalizability, validity of the outcome measures (especially heart failure), the methods used for event ascertainment, the effect size of the intervention benefit, and safety and tolerability of the <120 mmHg target.5, 6 Following the SPRINT primary results publication in 2015, additional reports from SPRINT have addressed many of these concerns. This manuscript reviews evidence for validity and potential clinical implications of the SPRINT findings.
Keywords: heart failure, controlled clinical trial, myocardial infarction, hypertension, blood pressure, cardiovascular disease
SPRINT Design Elements
SPRINT recruited 9,361 adults ≥50 years who were at increased risk for cardiovascular disease (CVD) and had an average SBP 130–180 mmHg. They were randomized to a SBP treatment goal of either <120 mmHg (Intensive) or <140 mmHg (Standard) (Table S1).1, 7–9 In order to maintain SBP separation between randomized groups, the protocol included a provision to taper treatment in the Standard group for SBP <135 on two consecutive visits or any single visit with SBP <130.
The trial was conducted at 102 clinical practice sites in the United States, including Puerto Rico. Clinical outcomes were assessed at specified follow-up visits using a prospective randomized open-blinded end point (PROBE) design and adjudicated by experienced, trained physicians blinded to treatment assignment using a well-documented pre-specified process. A detailed protocol and manual of procedures (MOP) were developed prior to starting the trial.8 The primary outcome was a CVD composite of myocardial infarction, stroke, acute coronary syndrome (ACS), acute decompensated heart failure (ADHF), and CVD death. Antihypertensive drug treatment regimens were recommended based on best evidence from clinical trials, but the ultimate choice of therapy was made by the physician site investigators.
Generalizability of BP Measurements in SPRINT
Critics have asserted that the SPRINT BP measurement method was different from what was used in previous BP treatment trials and have questioned the generalizability of SPRINT BP values to routine clinical practice.5 A primary goal of BP measurement in SPRINT was to standardize readings across the trial and to obtain accurate estimates of the BP level.
Procedures for measurement of BP in SPRINT were generally consistent with existing recommendations and similar to those used in other clinical outcome trials.10 Each clinical site was provided an Omron 907XL automatic oscillometric BP measurement device. Oscillometric BP measurement devices were used in at least 11 hypertension treatment trials prior to SPRINT.10 Some questioned the validity of BP readings in SPRINT due to reports that they were obtained with staff absent from the room. This was postulated to result in substantially lower BP values than those obtained in other trials or clinical practice.5, 11–14 However, the SPRINT protocol did not specify whether staff should be present or absent during BP measurement, and the SPRINT MOP recommended but did not require clinic staff to be out of the room during the rest period prior to BP measurement.
Because the details of BP measurements in SPRINT became a focus of attention, clinics were surveyed immediately after completing their study closeout visits to inquire whether site staff were usually in (attended measurements) or out of the room (unattended measurements) during the rest period and/or during BP measurement. Staff presence or absence was not associated with significant differences in levels of BP, major study outcomes, or safety events (Table S2).15 At least six other reports have concluded that staff attendance, per se, has limited effect on BP estimation with none reporting a SBP difference ≥2 mmHg.16–18 In a randomized controlled trial that mimicked the procedures used in SPRINT, the average differences in attended compared to unattended SBP and DBP values were 1.5 and 0 mmHg, respectively.19
In clinical practice, BP is often measured with little attention to quality control and may overestimate average SBP by as much as 10–15 mmHg compared to values obtained using guideline recommended methods that have been employed in most landmark antihypertensive drug treatment trials.20 In some patients, however, routine clinic values are lower compared to guideline recommended measurements. There is no accurate means to estimate the “true” level of BP using poor quality measurements. These findings underscore the importance of BP measurement using the methods recommended in guidelines2, 21 to derive the benefits obtained in clinical trials. However, staff attendance or absence, per se, does not appear to be a major factor in accuracy of BP estimation.
SUMMARY OF SPRINT FINDINGS
Cardiovascular Outcomes
Achieved median and mean BPs overall and in the pre-specified and other subgroups of interest are provided in Table 1 and Table S3. During trial follow-up, the average achieved median SBPs in the Intensive and Standard groups, respectively, were 119.2 and 135.8 mmHg after the 6-month drug titration period. In the 2015 SPRINT main results report, the primary outcome and all-cause mortality were 25% (p<0.001) and 27% (p=0.003) lower in the Intensive compared to Standard group.1 This included a 43% reduction (p=0.005) for CV death and 38% reduction (p=0.002) for ADHF. Results from a subsequent publication, with 56 additional adjudicated primary outcome events, are shown in Table 2.22
Table 1.
Median Follow-Up Systolic Blood Pressures (SBPs) and Median Follow-Up SBPs After 6 Months
| Subgroup | Baseline SBP | Median F/U SBPs |
Median F/U SBP Δ vs Standard SBP | Median F/U SBP after 6 Months |
Median F/U SBP Δ vs Standard SBP after 6 Months | ||||
|---|---|---|---|---|---|---|---|---|---|
| Intensive | Standard | Intensive | standard | Intensive | standard | ||||
| Overall | 4678 | 4683 | 138.0 | 120.5 | 135.1 | −14.6 | 119.2 | 135.8 | −16.6 |
| Pre-Specified | |||||||||
| age<75 | 3361 | 3364 | 138.0 | 120.0 | 135.0 | −15.0 | 118.5 | 135.6 | −17.1 |
| age>=75 | 1317 | 1319 | 140.0 | 122.4 | 135.5 | −13.1 | 121.2 | 136.2 | −15.0 |
| Male | 2994 | 3035 | 138.0 | 120.5 | 135.0 | −14.5 | 119.0 | 135.6 | −16.6 |
| Female | 1684 | 1648 | 140.0 | 120.6 | 135.4 | −14.8 | 119.3 | 136.0 | −16.7 |
| Black | 1454 | 1493 | 138.5 | 120.8 | 135.6 | −14.7 | 119.4 | 136.2 | −16.9 |
| Nonblack | 3224 | 3190 | 138.0 | 120.4 | 135.0 | −14.6 | 119.1 | 135.5 | −16.4 |
| Prior CKD | 1329 | 1316 | 138.0 | 121.7 | 135.4 | −13.7 | 120.7 | 136.2 | −15.5 |
| No Prior CKD | 3349 | 3367 | 139.0 | 120.1 | 135.1 | −15.0 | 118.6 | 135.6 | −17.0 |
| Prior CVD | 940 | 937 | 138.0 | 120.7 | 134.7 | −14.1 | 119.4 | 135.5 | −16.1 |
| No Prior CVD | 3738 | 3746 | 139.0 | 120.5 | 135.2 | −14.7 | 119.1 | 135.8 | −16.7 |
| Not Pre-Specified | |||||||||
| Fit | 178 | 196 | 138.0 | 119.9 | 135.7 | −15.8 | 118.9 | 136.3 | −17.4 |
| Less Fit | 659 | 680 | 140.0 | 122.5 | 135.2 | −12.7 | 121.4 | 136.0 | −14.6 |
| Frail | 474 | 434 | 143.0 | 123.4 | 135.9 | −12.5 | 121.8 | 136.4 | −14.6 |
| Non-Hispanic White | 2698 | 2701 | 138.0 | 120.6 | 134.9 | −14.2 | 119.3 | 135.4 | −16.2 |
| Non-Hispanic Black | 1379 | 1423 | 138.0 | 121.0 | 135.5 | −14.5 | 119.5 | 136.2 | −16.7 |
| Hispanic | 503 | 481 | 139.0 | 118.8 | 135.3 | −16.5 | 117.7 | 135.9 | −18.2 |
| Prior Metabolic Syndrome | 1825 | 1870 | 137.0 | 119.7 | 134.8 | −15.1 | 118.4 | 135.2 | −16.8 |
| No Prior Metabolic Syndrome | 2812 | 2755 | 139.0 | 121.1 | 135.4 | −14.4 | 119.7 | 136.1 | −16.5 |
| Pre-diabetes | 1941 | 1957 | 138.0 | 120.3 | 135.2 | −14.9 | 119.0 | 135.7 | −16.7 |
| Normal | 2721 | 2704 | 139.0 | 120.7 | 135.1 | −14.4 | 119.2 | 135.8 | −16.5 |
Median follow-up (F/U) SBP readings (mmHg): medians of 1M, 2M, 3M and quarterly SBP protocol visit readings through to the last visit occurring on or before end of trial intervention (August 20, 2015). Median F/U SBP readings only after 6 months: medians of the quarterly SBP protocol visit readings occurring after and not including the 6-month protocol visit through to the last visit occurring on or before end of trial intervention.
Table 2.
Clinical Outcomes
| Overall | Intensive | Standard | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | Outcome | Events | %/yr (95% CI) |
Events | %/yr (95% CI) |
Events | %/yr (95% CI) | HR (95% CI) |
P |
| All | Primary outcome (any of A-E) | 618 | 2.08 (1.92, 2.25) |
264 | 1.77 (1.57, 2.00) |
354 | 2.40 (2.16, 2.66) |
0.73 (0.63, 0.86) |
<0.001 |
| All | (A) All MI | 242 | 0.80 (0.71, 0.91) |
102 | 0.68 (0.56, 0.82) |
140 | 0.93 (0.79, 1.10) |
0.72 (0.56, 0.93) |
0.01 |
| All | (B) Non-MI ACS | 83 | 0.27 (0.22, 0.34) |
42 | 0.28 (0.20, 0.37) |
41 | 0.27 (0.20, 0.37) |
1.02 (0.66, 1.57) |
0.93 |
| All | (C) All stroke | 147 | 0.49 (0.41, 0.57) |
69 | 0.45 (0.36, 0.58) |
78 | 0.52 (0.41, 0.64) |
0.89 (0.64, 1.23) |
0.48 |
| All | (D) All HF | 173 | 0.57 (0.49, 0.66) |
68 | 0.45 (0.35, 0.57) |
105 | 0.70 (0.58, 0.84) |
0.63 (0.46, 0.86) |
0.003 |
| All | (E) CVD death | 112 | 0.37 (0.31, 0.44) |
41 | 0.27 (0.20, 0.36) |
71 | 0.47 (0.37, 0.59) |
0.57 (0.39, 0.84) |
0.004 |
| All | Non-fatal MI | 240 | 0.80 (0.70, 0.90) |
101 | 0.67 (0.55, 0.81) |
139 | 0.93 (0.78, 1.09) |
0.72 (0.56, 0.93) |
0.01 |
| All | Non-fatal stroke | 144 | 0.48 (0.40, 0.56) |
68 | 0.45 (0.35, 0.57) |
76 | 0.50 (0.40, 0.63) |
0.90 (0.65, 1.25) |
0.53 |
| All | Non-fatal heart failure | 167 | 0.55 (0.47, 0.64) |
66 | 0.43 (0.34, 0.55) |
101 | 0.67 (0.55, 0.81) |
0.64 (0.47, 0.87) |
0.004 |
| All | All deaths | 378 | 1.24 (1.12, 1.37) |
163 | 1.06 (0.91, 1.24) |
215 | 1.41 (1.23, 1.61) |
0.75 (0.61, 0.92) |
0.006 |
| All | Primary or death | 844 | 2.84 (2.65, 3.03) |
370 | 2.47 (2.24, 2.74) |
474 | 3.20 (2.93, 3.50) |
0.77 (0.67, 0.88) |
<0.001 |
| CKD | Primary CKD outcome (any of F-H) | 33 | 0.38 (0.27, 0.54) |
17 | 0.39 (0.24, 0.63) |
16 | 0.37 (0.23, 0.61) |
1.02 (0.51, 2.05) |
0.95 |
| CKD | (F) 50% reduction in eGFR (2x, ≥90 days apart) | 24 | 0.28 (0.19, 0.42) |
12 | 0.28 (0.16, 0.49) |
12 | 0.28 (0.16, 0.49) |
0.97 (0.43, 2.20) |
0.95 |
| CKD | (G) Dialysis | 17 | 0.20 (0.12, 0.32) |
7 | 0.16 (0.08, 0.34) |
10 | 0.23 (0.13, 0.43) |
0.65 (0.23, 1.70) |
0.38 |
| CKD | (H) Kidney transplant | 0 | - | 0 | - | 0 | - | - | |
| CKD | Incident albuminuria (CKD) | 149 | 4.74 (4.04, 5.57) |
64 | 3.93 (3.08, 5.03) |
85 | 5.61 (4.54, 6.94) |
0.71 (0.50, 1.00) |
0.05 |
| Non-CKD | Secondary CKD outcome (non-CKD subsample, any of I-K) | 189 | 0.88 (0.76, 1.02) |
148 | 1.39 (1.18, 1.63) |
41 | 0.38 (0.28, 0.51) |
3.67 (2.62, 5.26) |
<0.001 |
| Non-CKD | (I) 30% reduction in eGFR and <60 (2x, >=90 days apart) | 189 | 0.88 (0.76, 1.02) |
148 | 1.39 (1.18, 1.63) |
41 | 0.38 (0.28, 0.51) |
3.67 (2.62, 5.26) |
<0.001 |
| Non-CKD | (J) Dialysis | 0 | - | 0 | - | 0 | - | - | |
| Non-CKD | (K) Kidney transplant | 0 | - | 0 | - | 0 | - | - | |
| Non-CKD | Incident albuminuria (non-CKD) | 326 | 2.90 (2.60, 3.23) |
142 | 2.54 (2.16, 3.00) |
184 | 3.25 (2.82, 3.76) |
0.77 (0.62, 0.96) |
0.02 |
Final results of pre-specified primary and secondary outcomes from SPRINT. Lewis CE, Fine LJ, Beddhu S, et al. Final cardiovascular and mortality results of a randomized trial of intensive versus standard blood pressure control. N Engl J Med. 2021;384:1921–30
In this analysis, the primary outcome and all-cause mortality were 27% and 25% lower in the Intensive compared to Standard group, respectively. A significant reduction was evident even when ADHF was excluded from the primary outcome.22 The reduction in primary outcome events in the Intensive group was seen in the pre-specified subgroups defined by categories of age, gender, levels of baseline SBP presence or absence of CVD history or CKD, and Black or non-Black race (Fig 1). In the 2,636 non-institutionalized participants who were aged ≥75 years at baseline, the benefits were similar, resulting in prevention of primary outcome events and all-cause mortality for one in every 28 and 41 Intensive participants, respectively. 9In post hoc analyses, the findings were similar in Hispanics, frail older adults, and in those with metabolic syndrome or prediabetes (Figs 1,2). Meta-analyses of trials that have compared random assignment to different levels of BP provide similar results, whether or not the SPRINT results are included.23–26
Figure 1.
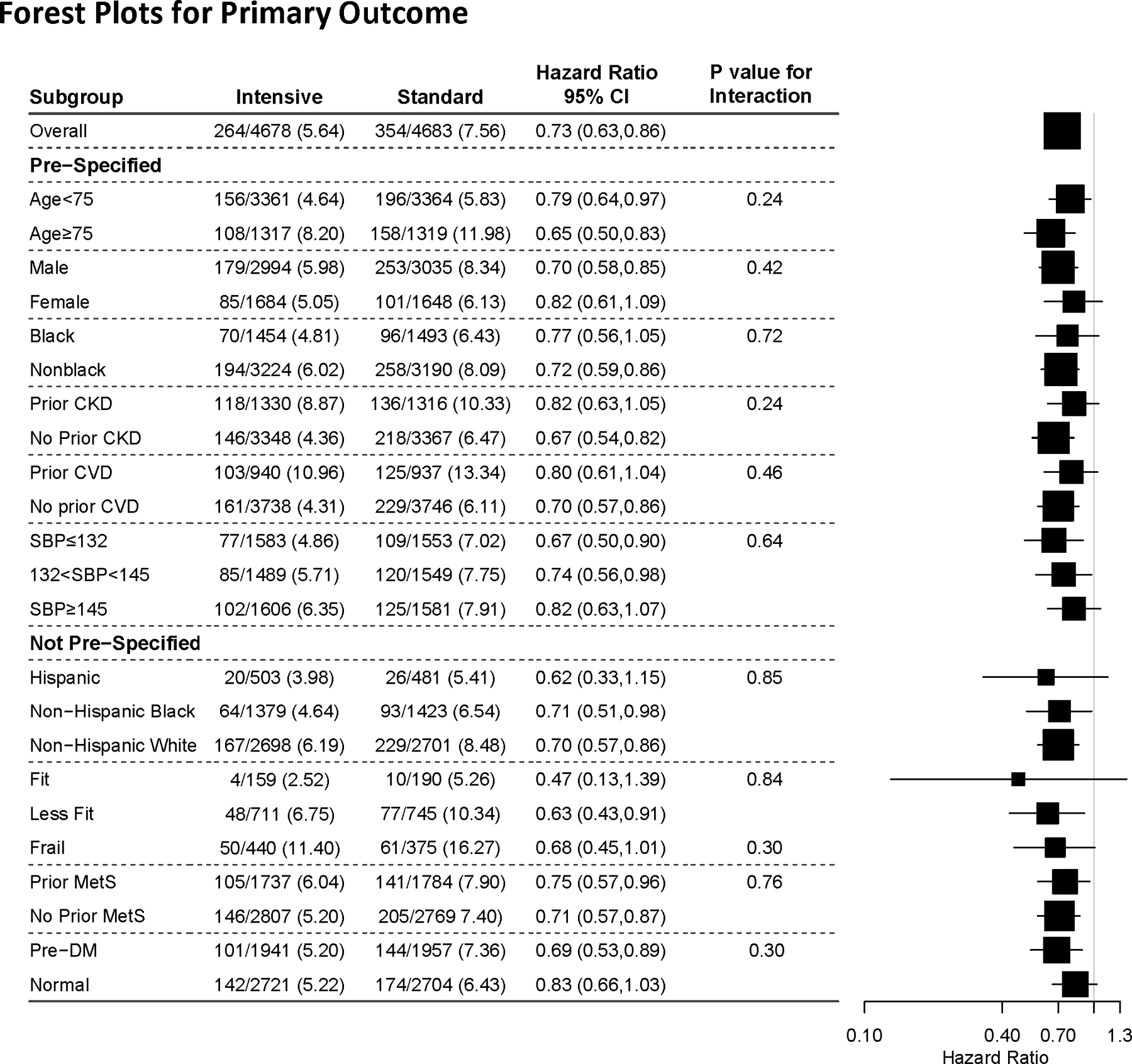
Primary outcome in prespecified (age, sex, race, and history of cardiovascular disease [CVD] and CKD)22 and post hoc (Hispanic ethnicity, frailty, metabolic syndrome, and prediabetes) subgroups.7,9,22,40,46,47
Figure 2.
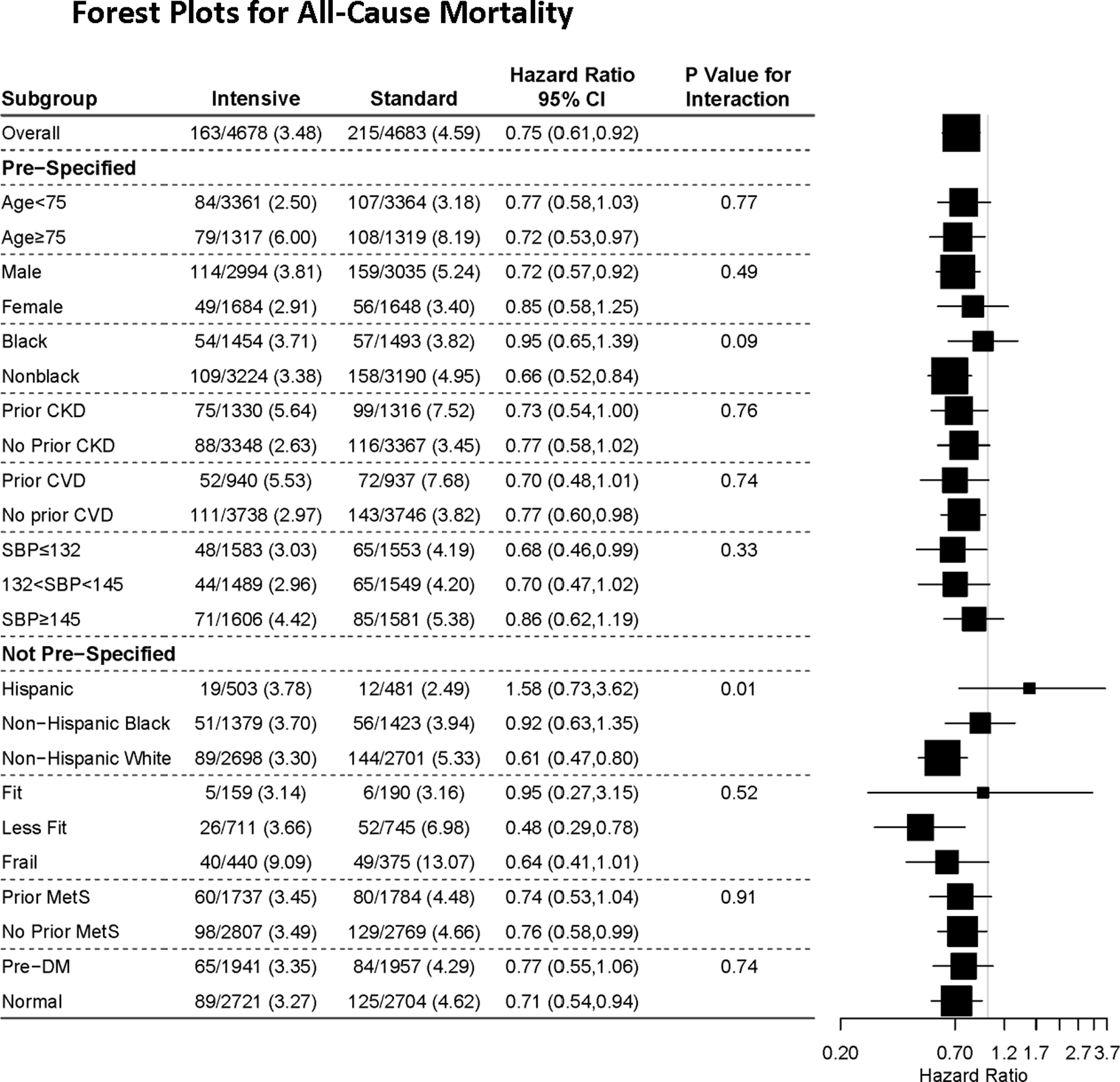
All-cause mortality outcome in prespecified (age, sex, race, and history of cardiovascular disease [CVD] and CKD)22 and post hoc (Hispanic ethnicity, frailty, metabolic syndrome, and prediabetes) subgroups. 7,9,22,40,46,47
Validity of the Heart Failure Findings
The 38% reduction in ADHF in those randomized to Intensive compared to Standard therapy has also been questioned.27 The diagnosis of ADHF in SPRINT was based on rigorous, objective criteria that required either a hospitalization or emergency department visit which necessitated intravenous therapy (diuretic or inotropic agents) for a clinical syndrome that presented with multiple signs and symptoms consistent with ADHF.28 In addition, the ADHF designation required adjudication by a committee of experienced clinicians who were blinded to the participant’s randomization and followed standardized procedures outlined in the MOP. In multivariable analysis, participants who developed ADHF during the trial had a 27-fold higher risk of CVD death, a 16-fold higher risk of myocardial infarction, and a 10-fold increased risk of death from any cause compared with those who did not develop ADHF ( Table S4).28 The beneficial effect of BP reduction on heart failure in SPRINT was consistent with experience in prior trials, including 64%, 50%, and 36% reductions in the HYVET,29 SHEP,30 and Syst-Eur31 trials, respectively. Thus, the SPRINT ADHF result was neither unexpected nor a “soft” outcome as suggested by some observers. 27
It has also been suggested that the ADHF benefit in SPRINT resulted from differential use of diuretics and masking of underlying heart failure.27 Only 11 of the 391 participants in whom diuretics were withdrawn at the baseline visit developed ADHF, and this occurred ≤1 month after diuretic withdrawal in only one participant. This represents approximately 6% of the 173 participants who developed ADHF, and an analysis excluding these participants had minimal effect on the estimate of benefit for ADHF prevention in the Intensive group.32, 33 At the final follow-up visit, 68% of the Intensive and 43% of the Standard group were being treated with a diuretic. Diuretic use during the trial was not a significant predictor of ADHF (HR, 0.96 [0.66–1.40], P=0.83).32 Diuretic use was more, rather than less, prevalent in participants who developed ADHF regardless of treatment arms than in those without ADHF, and most ADHF events occurred in participants who were already on a prescribed diuretic at the time of their first ADHF diagnosis (Fig S2). Furthermore, separation of ADHF rates between the two treatment groups began after 6 months of follow-up and appeared to increase throughout the trial rather than shortly after medication titration.28
Brain Outcomes
Stroke was not significantly reduced in SPRINT (HR:0.89 (0.64, 1.23), but the trial was not powered to assess differences in individual components of the primary outcome. The stroke hazard ratio confidence interval was wide and included a possible 36% reduction in risk (Table 2). In the ACCORD BP trial, stroke was reduced by 41%. However, Kaplan-Meier curves for the stroke outcome did not begin to separate until after 3 years of treatment; this duration is noteworthy since SPRINT was stopped after a median of 3.3 years.34
Despite previous concerns about the potential for adverse cognitive effects,35 intensive BP treatment resulted in a significant reduction in mild cognitive impairment during the trial and a composite of mild cognitive impairment and probable dementia during combined trial and post-trial follow-up (Table 3).36 Additionally, during a median follow-up of 3.97 years, an MRI sub-study conducted in 670 SPRINT participants reported significantly less progression of cerebral small vessel ischemic disease, as indicated by dense white matter lesions, in the Intensive compared to Standard group.37 Similar findings were observed in the ACCORD BP trial and post-trial follow-up analysis, and in the INFINITY trial.38, 39 In SPRINT and ACCORD, a small though significant reduction in total brain volume was noted with intensive treatment, but the clinical significance of this finding is uncertain.
Table 3.
Incidence of Probable Dementia and Mild Cognitive Impairment by Treatment Group*
| Treatment Group | |||||
|---|---|---|---|---|---|
| Intensive | Standard | ||||
| Outcomes | # with Outcomes/ Person-Years | Cases/ 1000 person-years | # with Outcomes/ Person-Years | Cases/ 1000 person-years | Hazard Ratio (95% CI)a P value |
| Probable Dementia | 149 / 20,569 | 7.2 | 176 / 20,378 | 8.6 | 0.83 (0.67 – 1.04) 0.10 |
| Mild Cognitive Impairment | 287 / 19,690 | 14.6 | 352 / 19,281 | 18.3 | 0.81 (0.69 – 0.95) 0.007 |
| Composite of Mild Cognitive Impairment or Probable Dementia | 402 / 19,873 | 20.2 | 468 / 19,488 | 24.1 | 0.85 (0.74 – 0.97) 0.01 |
Includes data during trial treatment intervention ending August 20, 2015 and that collected during extended observational follow-up between October 2017 and July 2018. Williamson JD, et al. JAMA. 2019;321(6):553–561.
Renal Outcomes:
No difference in the primary kidney disease composite outcome of 50% reduction in estimated glomerular disease rate (eGFR) or end stage renal disease (ESRD) in those with baseline CKD defined as eGFR< 60 ml/min/1.73 m2 was noted between the two treatment groups (Table 2), but there was limited power for these events. There was a slight average decrease in eGFR in the Intensive arm during the initial six months of therapy in those with (Fig S1) and without CKD (eGFR treatment group difference of 3.3 ml/min/1.).40, 41 In the Standard arm, a modest acute increase in eGFR in the CKD subgroup and no eGFR change in the non-CKD subgroup was noted. There was no relationship between the early eGFR decrease and CVD outcomes.42 An acute decline in eGFR has been noted during more intensive antihypertensive treatment in other trials that randomized participants to different BP targets, especially with more preserved renal function.43, 44 This acute decline in eGFR in the Intensive arm has been attributed to a reversible hemodynamic effect of antihypertensive drug therapy on the renal microcirculation.45 In both those with and without CKD at baseline, a small annual decline in eGFR of similar magnitude was consistent with the anticipated effects of aging on kidney function, was seen in the two treatment groups after the six month visit. However, the rate of decline was slightly higher in the Intensive than in the Standard arm in the CKD subgroup. In those without CKD at baseline, incident CKD defined as a ≥30% decrease in eGFR and confirmed eGFR ≤60 ml/min/1.73m2 during follow-up occurred in 4.2% of the Intensive and 1.2% of the Standard groups, but none of the participants developed ESRD and <10% had a decrease in eGFR ≥50% at their final visit.40 40 Incident albuminuria was significantly less common in the Intensive compared to the Standard group both in those with and without CKD at baseline (Table 2).
Implications of SPRINT for patients with Diabetes Mellitus
SPRINT excluded patients with diabetes, but demonstrated similar treatment benefits in participants with or without pre-diabetes or the metabolic syndrome at baseline.46, 47 In addition, analyses comparing the effects of intensive and standard BP treatment in ACCORD participants who received standard glycemic therapy have identified benefits similar to those seen in SPRINT.48–51 Likewise, following discontinuation of the intensive glycemic intervention in the ACCORD BP trial, a pattern of CVD benefit similar to that seen in SPRINT was identified.51 Thus, SPRINT provides supportive but not definitive evidence on the effect of intensive BP treatment in patients with diabetes.
The J-curve Hypothesis: DBP Reduction and SPRINT Outcomes
J- and U-shaped relationships between DBP and CVD have been identified in some cohort studies52 and on-treatment analyses of antihypertensive drug treatment trials,53–55
influencing treatment recommendations in at least one BP guideline.21 A fundamentally important question with these J-curve reports is whether the lower BP was the cause or consequence of CVD. A SPRINT on-treatment analysis also identified a U-shaped relationship between baseline DBP and the primary CVD composite outcome as well as all-cause mortality in both the Intensive and Standard groups.56 However, despite the greater risk of CVD events in those with a lower baseline DBP in both randomized groups, in randomized comparisons both the primary outcome and all-cause mortality were significantly less common in the Intensive compared with the Standard group across all five quintiles of DBP with no suggestion of hazard ratio heterogeneity. These results fail to support an increase in absolute risk based on level of achieved DBP during treatment of hypertension.
Adverse Effects of Intensive Treatment in SPRINT
Detailed procedures for collecting adverse effects, including serious adverse effects (SAEs) were specified in the trial protocol and MOP, particularly related to hypotension, syncope, falls, and acute kidney injury. The Medical Dictionary for Regulatory Activities (MedDRA) was used to classify SAEs. A SPRINT-specific standardized MedDRA with queries for syncope, hypotension, and falls was developed to capture preferred terms under these headings. Adverse events, unlike clinical outcomes, could be collected at any time during the trial. There was no significant difference in overall SAEs between the Intensive and Standard groups (HR=1.04, P=0.25), including in those >75 years old at baseline (HR=1.00, P=0.93).1, 9, 57 Hypotension, syncope, and falls occurred in 1.7%, 1.8%, and 2.2% of the SPRINT participants, respectively.57, 58 Compared to the Standard group, the Intensive group had a greater risk of SAE associated with hypotension (2.4% vs. 1.4%, HR=1.67, P=0.002), but the corresponding differences were not significant for syncope (2.3% vs. 1.7%, HR=1.32, P=0.07) or injurious falls (2.2 vs. 2.3%, HR=0.98, P=0.90). For all three outcomes, the hazard ratio for an SAE was higher and nominally significant in the subgroups with baseline CKD or frailty. Baseline age ≥75 years was also associated with a significantly higher risk of self or provider reported syncope, hypotension, and falls, but not for hospital associated injurious falls. However, there was no age-by-treatment interaction for any of these SAE outcomes.57 There was no difference in patient reported health-related quality of life measurements, even in participants over 75 years of age.59
Acute Kidney Injury (AKI) or Acute Renal Failure (ARF)
AKI or ARF, defined as a hospital admission diagnosis, occurrence during a hospitalization or reported in the hospital discharge summary as a primary or main secondary diagnosis, occurred more often in the Intensive (3.8%) compared with the Standard (2.3%) group (HR=1.66, P<0.001).60 Of those with adjudicated AKI or ARF events, 78% of participants in the Intensive group and 77% in the Standard group had only KDIGO Stage 1 or 2 severity (identical in those with or without CKD at baseline) and by the end of trial follow-up, 90.4% of the AKI events in the Intensive group and 86.9% in Standard group had completely resolved (serum creatinine within 20% of the participant’s baseline value). Partial resolution (creatinine within 30% of the baseline value) was seen in an additional 4.8% of Intensive and 4.0% of Standard group participants with AKI.
Orthostatic Hypotension (OH)
OH (defined as a decrease in SBP ≥20 mmHg or diastolic BP ≥10 mmHg one minute after standing from a seated position) was not a SPRINT exclusion criterion, but individuals with a standing SBP <110 mmHg were not enrolled.61 OH was present in 7% of the SPRINT participants at baseline and was more frequent in those with a higher seated SBP.62 During a mean follow-up of 3 years, OH events were noted in 18.5% of the participants and were more common in the Standard (5.7%) compared to Intensive (5.0%) group. In both treatment groups combined, OH was not associated with CVD events (primary outcome: HR=1.06; 95%CI: 0.78 to 1.44) or any secondary outcome.62 Moreover, OH was not associated with syncope, electrolyte abnormalities, injurious falls, or AKI/ARF. However, OH was associated with hypotension-related hospitalizations or emergency department visits (HR=1.77; 1.11 to 2.82) and bradycardia (HR=1.94; 1.19 to 3.15), but these associations did not differ by BP treatment goal.
Balancing Benefits and Risks of Intensive Treatment
Overall, there was no significant difference in SAEs between the two treatment groups at any age. The SAEs that were significantly more common during Intensive treatment did not lead to an overall increase in major morbidity or mortality. Unlike clinical outcomes which were ascertained only at quarterly protocol visits, SAEs could be reported at any visit including prn visits for BP control and except for the AKI/ARF events were not adjudicated. There were approximately 8% more study visits in the Intensive compared with the Standard group, mostly related to achieving the BP targets, which added opportunities to report adverse events but not trial outcomes ( Table S5). Although some authors have done so,63, 64 the SPRINT hard outcome benefits, including prevention of major CVD and all-cause mortality, and the softer potential adverse events should not be weighted equally. Finally, neither patient-reported health-related quality of life measurements nor gait speed differed by randomized treatment assignment, even in participants over 75 years of age.59
Summary/Conclusion
The SPRINT findings indicate that more intensive BP reduction yields substantial health benefits that outweigh the risks of adverse events. The SPRINT design and methods were based on best practices in the conduct of clinical trials, and the trial results were consistent with external evidence. The ability to generalize the SPRINT results to clinical practice requires accurate assessment of BP and evidence of high CV risk. These requirements are common to generalization of other landmark BP treatment trials.
Supplementary Material
Acknowledgements:
Jackson T Wright Jr, Paul K Whelton, Karen C Johnson, Joni K Snyder, David M Reboussin, William C Cushman, Jeff D Williamson, Nicholas M Pajewski, Alfred K Cheung, Cora E Lewis, Suzanne Oparil, Michael V Rocco, Srinivasan Beddhu, Lawrence J Fine, Jeffrey A Cutler, Walter T Ambrosius, Mahboob Rahman, Carolyn H Still, Zhengyi Chen, Curtis Tatsuoka, for the SPRINT Research Group.
Sources of Funding:
The Systolic Blood Pressure Intervention Trial was funded with Federal funds from the National Institutes of Health (NIH), including the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute on Aging (NIA), and the National Institute of Neurological Disorders and Stroke (NINDS), under Contract Numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, HHSN268200900049C, and Inter-Agency Agreement Number A-HL-13–002-001. It was also supported in part with resources and use of facilities through the Department of Veterans Affairs. The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals International, Inc. All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing and revision were carried out by the authors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the U.S. Department of Veterans Affairs, or the United States Government.
We also acknowledge the support from the following CTSAs funded by NCATS: CWRU: UL1TR000439, OSU: UL1RR025755, U Penn: UL1RR024134& UL1TR000003, Boston: UL1RR025771, Stanford: UL1TR000093, Tufts: UL1RR025752, UL1TR000073 & UL1TR001064, University of Illinois: UL1TR000050, University of Pittsburgh: UL1TR000005, UT Southwestern: 9U54TR000017–06, University of Utah: UL1TR000105–05, Vanderbilt University: UL1 TR000445, George Washington University: UL1TR000075, University of CA, Davis: UL1 TR000002, University of Florida: UL1 TR000064, University of Michigan: UL1TR000433, Tulane University: P30GM103337 COBRE Award NIGMS, Wake Forest University: UL1TR001420. Dr. Whelton was supported by a Centers for Research Excellence grant from the National Institute of General Medical Sciences (P20GM109036).
Footnotes
SPRINT Research Group is listed in Supplement
Disclosures: J.Wright reports a consulting fee from Medtronic; W. Cushman a grant/contract from Eli Lilly & Co; and S Oparil stock options in CinCor Pharma, Inc.
References:
- 1.Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. Hypertension 2018;71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 3.Nerenberg KA, Zarnke KB, Leung AA, Dasgupta K, Butalia S, McBrien K, Harris KC, Nakhla M, Cloutier L, Gelfer M, et al. Hypertension Canada’s 2018 Guidelines for Diagnosis, Risk Assessment, Prevention, and Treatment of Hypertension in Adults and Children. Can J Cardiol 2018;34:506–525. [DOI] [PubMed] [Google Scholar]
- 4.Gabb GM, Mangoni AA, Anderson CS, Cowley D, Dowden JS, Golledge J, Hankey GJ, Howes FS, Leckie L, Perkovic V, Schlaich M, Zwar NA, Medley TL and Arnolda L. Guideline for the diagnosis and management of hypertension in adults - 2016. Med J Aust 2016;205:85–9. [DOI] [PubMed] [Google Scholar]
- 5.Kjeldsen SE, Lund-Johansen P, Nilsson PM and Mancia G. Unattended Blood Pressure Measurements in the Systolic Blood Pressure Intervention Trial: Implications for Entry and Achieved Blood Pressure Values Compared With Other Trials. Hypertension 2016;67:808–12. [DOI] [PubMed] [Google Scholar]
- 6.Ortiz E and James PA. Let’s Not SPRINT to Judgment About New Blood Pressure Goals. Ann Intern Med 2016;165:889–890. [DOI] [PubMed] [Google Scholar]
- 7.Still CH, Rodriguez CJ, Wright JT, Craven TE, Bress AP, Chertow GM, Whelton PK, Whittle JC, Freedman BI, Johnson KC, et al. Clinical Outcomes by Race and Ethnicity in the Systolic Blood Pressure Intervention Trial (SPRINT): A Randomized Clinical Trial. Am J Hypertens 2018;31:97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, Fine LJ, Goff DC Jr., Johnson KC, Killeen AA, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials 2014;11:532–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, Fine LJ, Haley WE, Hawfield AT, Ix JH, et al. Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged >/=75 Years: A Randomized Clinical Trial. JAMA 2016;315:2673–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giorgini P, Weder AB, Jackson EA and Brook RD. A review of blood pressure measurement protocols among hypertension trials: implications for “evidence-based” clinical practice. J Amer Soc Hypertension 2014;8:670–676. [DOI] [PubMed] [Google Scholar]
- 11.Messerli FH, Bangalore S and Kjeldsen SE. Letter by Messerli et al Regarding Article, “The Implications of Blood Pressure Measurement Methods on Treatment Targets for Blood Pressure”. Circulation 2017;135:e45–e46. [DOI] [PubMed] [Google Scholar]
- 12.Bakris G. Response by Bakris to Letter Regarding Article, “The Implications of Blood Pressure Measurement Methods on Treatment Targets for Blood Pressure”. Circulation 2017;135:e47. [DOI] [PubMed] [Google Scholar]
- 13.Schiffrin EL, Calhoun DA and Flack JM. SPRINT Proves that Lower Is Better for Nondiabetic High-Risk Patients, but at a Price. Am J Hypertens 2016;29:2–4. [DOI] [PubMed] [Google Scholar]
- 14.Myers MG. A Short History of Automated Office Blood Pressure - 15 Years to SPRINT. J Clin Hypertens (Greenwich) 2016;18:721–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson KC, Whelton PK, Cushman WC, Cutler JA, Evans GW, Snyder JK, Ambrosius WT, Beddhu S, Cheung AK, Fine LJ, et al. Blood Pressure Measurement in SPRINT (Systolic Blood Pressure Intervention Trial). Hypertension 2018;71:848–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stergiou G, Kollias A, Parati G and O’Brien E. Office Blood Pressure Measurement: The Weak Cornerstone of Hypertension Diagnosis. Hypertension 2018;71:813–815. [DOI] [PubMed] [Google Scholar]
- 17.Graves JW, Nash C, Burger K, Bailey K and Sheps SG. Clinical decision-making in hypertension using an automated (BpTRU) measurement device. J Hum Hypertens 2003;17:823–7. [DOI] [PubMed] [Google Scholar]
- 18.Kollias A, Stambolliu E, Kyriakoulis KG, Gravvani A and Stergiou GS. Unattended versus attended automated office blood pressure: Systematic review and meta-analysis of studies using the same methodology for both methods. J Clin Hypertens (Greenwich) 2019;21:148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauer F, Seibert FS, Rohn B, Bauer KAR, Rolshoven E, Babel N and Westhoff TH. Attended Versus Unattended Blood Pressure Measurement in a Real Life Setting. Hypertension 2018;71:243–249. [DOI] [PubMed] [Google Scholar]
- 20.Powers BJ, Olsen MK, Smith VA, Woolson RF, Bosworth HB and Oddone EZ. Measuring blood pressure for decision making and quality reporting: where and how many measures? Ann Intern Med 2011;154:781–8, W-289–90. [DOI] [PubMed] [Google Scholar]
- 21.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 22.SPRINT Research Group, Lewis CE, Fine LJ, Beddhu S, Cheung AK, Cushman WC, Cutler JA, Evans GW, Johnson KC, Kitzman DW, et al. Final Report of a Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med 2021;384:1921–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie X, Atkins E, Lv J, Bennett A, Neal B, Ninomiya T, Woodward M, MacMahon S, Turnbull F, Hillis GS, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. The Lancet 2016;387:435–443. [DOI] [PubMed] [Google Scholar]
- 24.Bundy JD, Li C, Stuchlik P and et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: A systematic review and network meta-analysis. JAMA Cardiology 2017;2:775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verdecchia P, Angeli F, Gentile G and Reboldi G. More Versus Less Intensive Blood Pressure-Lowering Strategy: Cumulative Evidence and Trial Sequential Analysis. Hypertension 2016;68:642–53. [DOI] [PubMed] [Google Scholar]
- 26.Bangalore S, Toklu B, Gianos E, Schwartzbard A, Weintraub H, Ogedegbe G and Messerli FH. Optimal Systolic Blood Pressure Target After SPRINT: Insights from a Network Meta-Analysis of Randomized Trials. Am J Med 2017;130:707–719.e8. [DOI] [PubMed] [Google Scholar]
- 27.Kjeldsen SE, Narkiewicz K, Hedner T and Mancia G. The SPRINT study: Outcome may be driven by difference in diuretic treatment demasking heart failure and study design may support systolic blood pressure target below 140 mmHg rather than below 120 mmHg. Blood Press 2016;25:63–6. [DOI] [PubMed] [Google Scholar]
- 28.Upadhya B, Rocco M, Lewis CE, Oparil S, Lovato LC, Cushman WC, Bates JT, Bello NA, Aurigemma G, Fine LJ, et al. Effect of Intensive Blood Pressure Treatment on Heart Failure Events in the Systolic Blood Pressure Reduction Intervention Trial. Circ Heart Fail 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008;358:1887–1898. [DOI] [PubMed] [Google Scholar]
- 30.SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension:final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991;265:3255–3264. [PubMed] [Google Scholar]
- 31.Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhager WH, Bulpitt CJ, de Leeuw PW, Dollery CT, Fletcher AE, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997;350:757–764. [DOI] [PubMed] [Google Scholar]
- 32.Upadhya B, Lovato LC, Rocco M, Lewis CE, Oparil S, Cushman WC, Kostis JB, Rodriguez CJ, Cho ME, Cloud LW, et al. Heart Failure Prevention in Older Patients Using Intensive Blood Pressure Reduction: Potential Role of Diuretics. JACC Heart Fail 2019;7:1032–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raby K, Rocco M, Oparil S, Gilbert ON and Upadhya B. Heart Failure Primary Prevention: What Does SPRINT Add?: Recent Advances in Hypertension. Hypertension 2021;77:1804–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cushman WC, Evans GW, Byington RP, Goff DC, Grimm RH, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, et al. Effects of intensive blood pressure control in type 2 diabetes. New Engl J Med 2010;362:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tadic M, Cuspidi C and Hering D. Hypertension and cognitive dysfunction in elderly: blood pressure management for this global burden. BMC Cardiovasc Disord 2016;16:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.SPRINT MIND Investigators for the SPRINT Research Group. Effect of Intensive vs Standard Blood Pressure Control on Probable Dementia: A Randomized Clinical Trial. JAMA 2019;321:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.SPRINT MIND Investigators for the SPRINT Research Group. Association of Intensive vs Standard Blood Pressure Control With Cerebral White Matter Lesions. JAMA 2019;322:524–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murray AM, Hsu FC, Williamson JD, Bryan RN, Gerstein HC, Sullivan MD, Miller ME, Leng I, Lovato LL, Launer LJ, et al. ACCORDION MIND: results of the observational extension of the ACCORD MIND randomised trial. Diabetologia 2017;60:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White WB, Wakefield DB, Moscufo N, Guttmann CRG, Kaplan RF, Bohannon RW, Fellows D, Hall CB and Wolfson L. Effects of Intensive Versus Standard Ambulatory Blood Pressure Control on Cerebrovascular Outcomes in Older People (INFINITY). Circulation 2019;140:1626–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beddhu S, Rocco MV, Toto R and et al. Effects of intensive systolic blood pressure control on kidney and cardiovascular outcomes in persons without kidney disease: A secondary analysis of a randomized trial. Ann Intern Med 2017;167:375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheung AK, Rahman M, Reboussin DM, Craven TE, Greene T, Kimmel PL, Cushman WC, Hawfield AT, Johnson KC, Lewis CE, et al. Effects of Intensive BP Control in CKD. J Am Soc Nephrol 2017;28: 2812–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beddhu S, Shen J, Cheung AK, Kimmel PL, Chertow GM, Wei G, Boucher RE, Chonchol M, Arman F, et al. Implications of Early Decline in eGFR due to Intensive BP Control for Cardiovascular Outcomes in SPRINT. J Am Soc Nephrol 2019;30:1523–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW and Striker G. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group [see comments]. N Engl J Med 1994;330:877–884. [DOI] [PubMed] [Google Scholar]
- 44.Wright JT Jr., Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 2002;288:2421–2431. [DOI] [PubMed] [Google Scholar]
- 45.Peralta CA, McClure LA, Scherzer R, Odden MC, White CL, Shlipak M, Benavente O and Pergola P. Effect of Intensive Versus Usual Blood Pressure Control on Kidney Function Among Individuals With Prior Lacunar Stroke: A Post Hoc Analysis of the Secondary Prevention of Small Subcortical Strokes (SPS3) Randomized Trial. Circulation 2016;133:584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bress AP, King JB, Kreider KE, Beddhu S, Simmons DL, Cheung AK, Zhang Y, Doumas M, Nord J, Sweeney ME, et al. Effect of Intensive Versus Standard Blood Pressure Treatment According to Baseline Prediabetes Status: A Post Hoc Analysis of a Randomized Trial. Diabetes Care 2017;40:1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dungan KM, Craven TE, Soe K, Wright JT Jr, Basile J, Haley WE, Kressin NR, Uzma R, Tamariz L, Whittle J, et al. Influence of metabolic syndrome and race on the relationship between intensive blood pressure control and cardiovascular outcomes in the SPRINT cohort. Diabetes, Obesity Metab 2018;20:629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cushman WC, Whelton PK, Fine LJ, Wright JT, Reboussin DM, Johnson KC, Oparil S for the SPRINT Research Group. SPRINT Trial Results: Latest News in Hypertension Management. Hypertension 2016;67:263–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perkovic V and Rodgers A. Redefining Blood-Pressure Targets--SPRINT Starts the Marathon. N Engl J Med 2015;373:2175–2178. [DOI] [PubMed] [Google Scholar]
- 50.Buckley LF, Dixon DL, Wohlford GF, Wijesinghe DS, Baker WL and Van Tassell BW. Intensive Versus Standard Blood Pressure Control in SPRINT-Eligible Participants of the ACCORD-BP Trial. Diabetes Care 2017. [DOI] [PubMed] [Google Scholar]
- 51.Beddhu S, Chertow GM, Greene T, Whelton PK, Ambrosius WT, Cheung AK, Cutler J, Fine L, Boucher R, Wei G, et al. Effects of Intensive Systolic Blood Pressure Lowering on Cardiovascular Events and Mortality in Patients With Type 2 Diabetes Mellitus on Standard Glycemic Control and in Those Without Diabetes Mellitus: Reconciling Results From ACCORD BP and SPRINT. J Am Heart Assoc 2018;7:e009326. DOI: 10.1161/JAHA.118.009326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vidal-Petiot E, Ford I, Greenlaw N, Ferrari R, Fox KM, Tardif JC, Tendera M, Tavazzi L, Bhatt DL, Steg PG et al. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet 2016;388:2142–2152. [DOI] [PubMed] [Google Scholar]
- 53.Cruickshank JM, Thorp JM and Zacharias FJ. Benefits and potential harm of lowering high blood pressure. Lancet 1987;1:581–4. [DOI] [PubMed] [Google Scholar]
- 54.Messerli FH, Mancia G, Conti CR, Hewkin AC, Kupfer S, Champion A, Kolloch R, Benetos A and Pepine CJ. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med 2006;144:884–893. [DOI] [PubMed] [Google Scholar]
- 55.Bohm M, Schumacher H, Teo KK, Lonn EM, Mahfoud F, Mann JFE, Mancia G, Redon J, Schmieder RE, et al. Achieved blood pressure and cardiovascular outcomes in high-risk patients: results from ONTARGET and TRANSCEND trials. Lancet 2017;389:2226–2237. [DOI] [PubMed] [Google Scholar]
- 56.Beddhu S, Chertow GM, Cheung AK, Cushman WC, Rahman M, Greene T, Wei G, Campbell RC, Conroy M, Freedman BI, et al. Influence of Baseline Diastolic Blood Pressure on Effects of Intensive Compared With Standard Blood Pressure Control. Circulation 2018;137:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sink KM, Evans GW, Shorr RI, Bates JT, Berlowitz D, Conroy MB, Felton DM, Gure T, Johnson KC, Kitzman D, et al. Syncope, Hypotension, and Falls in the Treatment of Hypertension: Results from the Randomized Clinical Systolic Blood Pressure Intervention Trial. J Am Geriatr Soc 2018;66:679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A and Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016;387:957–967. [DOI] [PubMed] [Google Scholar]
- 59.Berlowitz DR, Foy CG, Kazis LE, Bolin LP, Conroy MB, Fitzpatrick P, Gure TR, Kimmel PL, Kirchner K, Morisky DE, et al. Effect of Intensive Blood-Pressure Treatment on Patient-Reported Outcomes. N Engl J Med 2017;377:733–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rocco MV, Sink KM, Lovato LC, Wolfgram DF, Wiegmann TB, Wall BM, Umanath K, Rahbari-Oskoui F, Porter AC, Pisoni R, et al. Effects of Intensive Blood Pressure Treatment on Acute Kidney Injury Events in the Systolic Blood Pressure Intervention Trial (SPRINT). Am J Kidney Dis 2018;71:352–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Townsend RR, Chang TI, Cohen DL, Cushman WC, Evans GW, Glasser SP, Haley WE, Olney C, Oparil S, Del Pinto R, Pisoni R, Taylor AA, Umanath K, Wright JT and Yeboah J. Orthostatic changes in systolic blood pressure among SPRINT participants at baseline. J Am Soc Hypertens 2016;10:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Juraschek SP, Taylor AA, Wright JT Jr., Evtableans GW, Miller ER 3rd, Plante TB, Cushman WC, Gure TR, Haley WE, Moinuddin I, Nord J, et al. Orthostatic Hypotension, Cardiovascular Outcomes, and Adverse Events: Results From SPRINT. Hypertension 2020;75:660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bangalore S, Toklu B, Gianos E, Schwartzbard A, Weintraub H, Ogedegbe G and Messerli FH. Optimal Systolic Blood Pressure Target After SPRINT: Insights from a Network Meta-Analysis of Randomized Trials. Am J Med 2017;130:707–719 e8. [DOI] [PubMed] [Google Scholar]
- 64.Phillips RA, Xu J, Peterson LE, Arnold RM, Diamond JA and Schussheim AE. Impact of Cardiovascular Risk on the Relative Benefit and Harm of Intensive Treatment of Hypertension. J Am Coll Cardiol 2018;71:1601–1610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


