Abstract
Introduction
The mRNA vaccine, mRNA-1273/TAK-919, encodes the prefusion-stabilised spike protein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). We report interim results of the first study evaluating safety and immunogenicity of mRNA-1273 in healthy Japanese participants.
Methods
This phase 1/2, randomised, observer-blind, placebo-controlled trial, conducted in Japan (two sites), enrolled healthy adults aged ≥ 20 years with no prior exposure to investigational coronavirus vaccines/treatments, and no known history/risk of SARS-CoV-2 infection. Participants were stratified by age (< 65/≥ 65 years) and randomised to receive two doses of 100 μg mRNA-1273 or placebo administered as intramuscular injections 28 days apart. Primary outcomes were safety and immunogenicity assessed by anti-SARS-CoV-2-spike protein-binding antibody level (bAb). A secondary outcome was SARS-CoV-2 neutralising antibody (nAb) response.
Results
Participants were enrolled between 21 January and 3 February 2021, and 200 were randomised: mRNA-1273, n = 150 (< 65 years, n = 100; ≥ 65 years, n = 50); placebo, n = 50 (< 65 years, n = 40; ≥ 65 years, n = 10). Solicited adverse events (AEs) through 7 days after each vaccination occurred in 144/150 (96%) and 19/50 (38%) participants in the mRNA-1273 and placebo arms, respectively. In the mRNA-1273 arm, injection-site pain, myalgia and fatigue were the most frequently reported solicited AEs after each vaccination, irrespective of age. Robust immune responses occurred with mRNA-1273 (n = 147) with a bAb geometric mean fold rise (95% confidence interval [CI]) from baseline of 1009 (865, 1177) and a nAb of 21.7 (19.8, 23.8) at day 57. Seroconversion rates (95% CI) for bAb and nAb were both 100% (97.5, 100) at day 57. No such response occurred with placebo (n = 49).
Conclusion
Two doses of 100 μg mRNA-1273 given 28 days apart demonstrated an acceptable safety profile and induced significant anti-SARS-CoV-2 immune responses in a Japanese population aged ≥ 20 years. Funding: Takeda Pharmaceutical Company Limited and Japan Agency for Medical Research and Development (AMED). ClinicalTrials.gov: NCT04677660.
Abbreviations: AE, adverse event; bAb, binding antibody; CI, confidence interval; COVID-19, coronavirus disease 2019; ELISA, enzyme-linked immunosorbent assay; FAS, full analysis set; GMFR, geometric mean fold rise; GMT, geometric mean titre; LLOQ, lower limit of quantitation; MAAE, medically attended adverse events; MN, microneutralisation; nAb, neutralising antibody; PPS, per protocol set; S-2P, SARS-CoV-2 spike protein; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SAE, serious adverse event; SAS, safety analysis set; SCR, seroconversion rate; ULOQ, upper limit of quantification
Keywords: SARS-CoV-2, COVID-19, mRNA-1273, Vaccine, Safety, Immunogenicity
1. Introduction
As of August 2021, there have been over 205 million confirmed cases of the novel coronavirus disease 2019 (COVID-19) and over 4.3 million deaths attributed to the disease worldwide [13]. In Japan, there have been over 1 million confirmed cases of COVID-19 with over 15,000 deaths reported [14]. To date, more than 6.5 billion vaccine doses have been administered worldwide [13].
mRNA-1273 is a lipid-nanoparticle encapsulated mRNA vaccine encoding a pre-fusion stabilised form of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein (S-2P). To date, preclinical and clinical evaluations have demonstrated that mRNA-1273 is well tolerated, is immunogenic, and drives a robust SARS-CoV-2-specific antibody and T-cell response [1], [2], [3], [4], [5], [6]. In a recent phase 3 study of 30,420 volunteers in the USA, two doses of 100 μg mRNA-1273 were shown to be 94.1% effective (95% confidence interval [CI]: 89.3, 96.8; P < 0.001 vs placebo) at preventing COVID-19 illness, including severe disease [3].
At the time of writing, mRNA-1273 is currently being used as part of the COVID-19 vaccination programme in several countries. In December 2020, the USA Food and Drug Administration issued emergency-use authorisation for mRNA-1273 for the prevention of COVID-19 for individuals ≥ 18 years of age, with the UK also granting approval in January 2021 [7], [15]. In Japan, mRNA-1273 received special approval in May 2021 [16].
As part of the vaccine registration process in Japan, we conducted a phase 1/2 study of mRNA-1273 (development code in Japan: TAK-919) in healthy Japanese adults (aged ≥ 20 years) to evaluate the safety and immunogenicity of two doses of 100 μg mRNA-1273, given 28 days apart. The participants are to be followed up for 12 months after the second dose. Herein, we report results from the primary data analysis at the 4-week follow-up after the second dose (up to day 57), ahead of completion of the 12-month follow-up period. This is the first report of the safety and immunogenicity of mRNA-1273 in healthy Japanese adults aged ≥ 20 years.
2. Methods and materials
2.1. Study design and participants
This was a phase 1/2 randomised, observer-blind, placebo-controlled trial, conducted at two centres in Japan, to evaluate the safety and immunogenicity of two doses of mRNA-1273 (TAK-919) in Japanese adults (ClinicalTrials.gov, NCT04677660). The study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and the International Conference on Harmonisation Harmonised Tripartite Guideline for Good Clinical Practice and all applicable regulations. An institutional review board, which covered both participating sites, approved the study protocol. All participants provided written informed consent.
Eligible participants were Japanese adults aged ≥ 20 years who were in good health as determined by medical history, physical examination (including vital signs and laboratory tests) and clinical judgement of the investigator. Participants were excluded if they received any other SARS-CoV-2 vaccines or other experimental novel coronavirus vaccine, had been in close contact with anyone known to have COVID-19 within 30 days before study vaccination, or were positive for SARS-CoV-2 infection before the trial. The trial design was developed in accordance with the guidance from the Pharmaceuticals and Medical Devices Agency [17].
2.2. Randomisation and blinding
Randomisation was undertaken according to randomisation tables generated by sponsor randomisation personnel or an unblinded designee. Participants were randomised (3:1) to the mRNA-1273 arm or placebo arm and were stratified by age: ≥ 20 years to < 65 years (mRNA-1273, n = 100; placebo, n = 40); ≥ 65 years (mRNA-1273, n = 50; placebo, n = 10).
As an observer-blinded trial, the participants, data collectors (e.g. investigators) and data evaluators were blinded to the trial-group assignment. Designated site personnel had access to the trial-group assignments to manage and prepare the trial vaccines but were not involved in data collection or safety evaluation after vaccine administration. Once day 57 data from all participants were collected, the database was locked, and the trial was unblinded (switched to an open-label study). Participants were informed about the vaccination assignment (mRNA-1273 or placebo) and reconsent was obtained for them to continue the study. Any participants who received an approved SARS-CoV-2 vaccine while enrolled on the trial (either during the double-blind phase up to the database lock of day 57 or the open-label phase after the database lock) were terminated from the trial.
2.3. Trial procedures
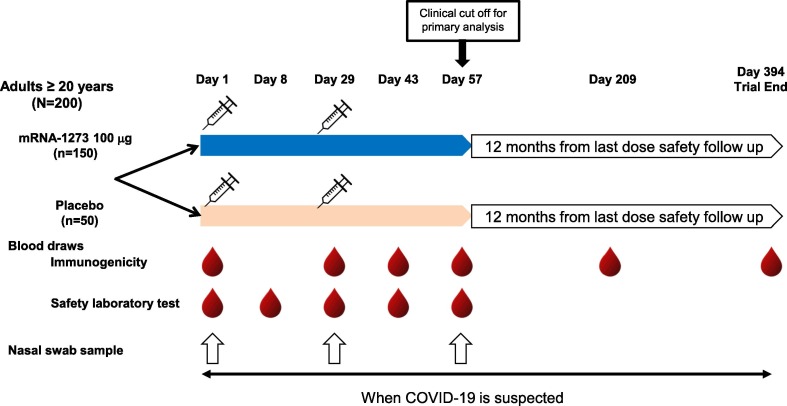
Participants received deltoid intramuscular injection (injection volume, 0.5 mL; needle diameter, 25G; needle length, 1 in. [2.54 cm]) of either two doses of 100 µg mRNA-1273 vaccine or two doses of saline placebo on day 1 and day 29 (28 ± 3 days after the first vaccination; Fig. 1 ). Additional information regarding the mRNA-1273 vaccine is provided in the Supplementary Information. All participants were to be followed up with in-person visits on day 1 (day of first dose), day 8 (7 + 3 days post-first dose), day 29 (28 + 3 days post-first dose), day 43 (14 + 3 days post-second dose), day 57 (28 + 3 days post-second dose), day 209 (180 ± 7 days post-second dose), and day 394 (365 ± 14 days post-second dose).
Fig. 1.
Schematic of trial design. COVID-19 = coronavirus disease 2019, N/n = number of participants.
2.4. Safety assessments
For 7 days after each injection (including the day of injection), participants self-reported oral body temperature and solicited local and systemic adverse events (AEs) in an eDiary. Solicited local AEs were defined as injection-site pain, erythema/redness, swelling, induration, and axillary swelling or tenderness ipsilateral to the side of injection. Solicited systemic AEs were headache, fatigue, myalgia, arthralgia, nausea/vomiting, chills, and fever. Data regarding unsolicited AEs were collected for 28 days after each injection (day of injection and 27 subsequent days). Solicited AEs occurring between 7 and 27 days after injection were classified as unsolicited AEs. In addition, all participants were to be followed for serious AEs (SAEs), medically attended AEs (MAAEs), and AEs leading to trial withdrawal or discontinuation from informed consent throughout the study (day 1 to day 394). The Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials [18] was used in this study, with minor modifications for solicited AEs. Blood samples for safety laboratory tests were taken on days 1, 8, 29, 43 and 57, with standard haematology and blood chemistry tests performed. At prespecified time points (days 1, 29, and 57), and where clinical symptoms of COVID-19 were suspected, participants were tested for SARS-CoV-2 infection by polymerase chain reaction test.
2.5. Immunogenicity assessments
Immunogenicity was to be assessed in blood samples collected on day 1 (before injection) and on days 29 (before injection), 43, 57, 209 and 394. The assay methods used have been previously published [4]. Briefly, SARS-CoV-2-binding antibody (bAb) levels were measured by an enzyme-linked immunosorbent assay (ELISA) specific to S-2P (collaborative development with PPD Laboratories, Richmond, VA, USA). Interpolation from an 11-point dilution of a commercial anti-S-2P monoclonal antibody (clone CR3022, Rockland, Inc, Limerick, PA, USA) was used to determine the serum-derived anti-S-2P antibody concentration (AU/mL). Serum neutralising antibody (nAb) titres against SARS-CoV-2 were measured using a live virus microneutralisation (MN) assay based on an in situ ELISA readout. The assay was conducted in accordance with Battelle laboratory protocols and qualified critical reagents (Columbus, Ohio, USA). The 50% MN titre (MN50) was reported for each sample. While the assays were qualified in the previous study [6], both bAb and nAb assays were fully validated at initiation for this study.
2.6. Outcome measures
In the present study, the primary safety outcome measures were: solicited AEs through 7 days after each vaccination; unsolicited AEs through 28 days after each vaccination; MAAEs, SAEs, AEs leading to discontinuation of vaccination and AEs leading to participant withdrawal from the trial through day 57; and proportion of participants with SARS-CoV-2 infection through day 57. The primary immunogenicity outcome measure was serum bAb levels against SARS-CoV-2 on day 57. The key secondary immunogenicity outcome measures were serum bAb levels and serum nAb titres against SARS-CoV-2 throughout the follow-up period (except for serum bAb level on day 57, which was the primary outcome measure). Only data to day 57 are available to date.
2.7. Statistical analysis
The trial planned to enrol 200 participants, 150 in the mRNA-1273 arm and 50 in the placebo arm. No formal statistical power calculations were performed to determine the sample size. The sample size for the trial was considered sufficient with a 95% probability of observing at least one AE with a 2% event rate in the mRNA-1273 arm. The interim analysis was performed for safety and immunogenicity after all participants had completed the day 57 visit (data cut-off date: 31 March 2021). The full analysis set (FAS) and safety analysis set (SAS) each included all randomised participants who received at least one treatment dose, and the per protocol set (PPS) included participants in the FAS who received two treatment doses, had evaluable immunogenicity data and had no significant protocol deviations. All safety summaries were presented by treatment group based on the SAS, and unsolicited AEs were coded using the Medical Dictionary for Regulatory Activities dictionary version 24.0. For the immunogenicity endpoints, geometric mean titre (GMT), geometric mean fold rises (GMFR), and seroconversion rate (SCR; defined as percentage of participants with a change from below the lower limit of quantification [LLOQ, 1.0 AU/mL for bAb and 159.79 for nAb] to equal to or above LLOQ, or a ≥ 4-fold rise from baseline), and their respective 95% CIs were analysed in the PPS. Antibody values reported as below LLOQ were replaced by 0.5 × LLOQ. Values that were greater than the upper limit of quantification (ULOQ; 2052 AU/mL for bAb and 11173.11 for nAb) were replaced by the ULOQ. Sub-analyses by age groups (≥ 20 years to < 65 years or ≥ 65 years) and sex (male or female) were performed for solicited AEs, unsolicited AEs and immunogenicity analyses. All analyses were conducted using SAS version 9.4.
3. Results
3.1. Participants
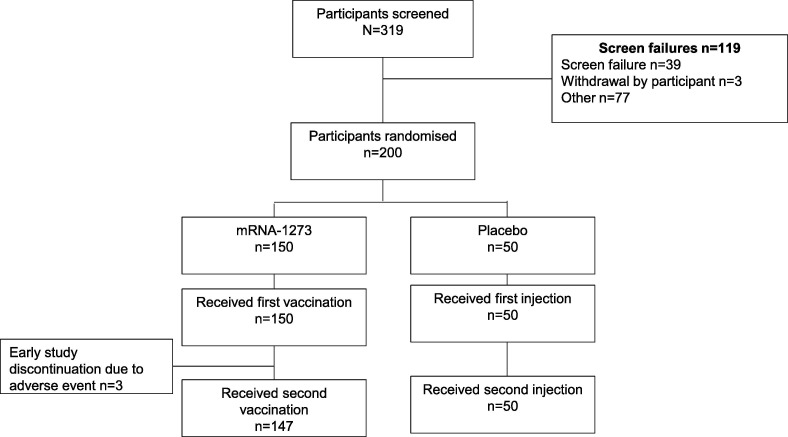
A total of 319 participants were screened between 21 January 2021 and 3 February 2021, and 200 participants were randomised (150 participants in the mRNA-1273 arm and 50 participants in the placebo arm; Fig. 2 ). In the mRNA-1273 arm, 147/150 (98.0%) participants received both doses of vaccine. Three participants (2.0%) did not receive a second vaccination in the mRNA-1273 arm due to AEs (injection-site rash in 2 participants; injection-site pain, fatigue, arthralgia, headache and axillary pain in 1 participant). Despite not receiving their second vaccinations, the 3 participants in the mRNA-1273 arm agreed to continue with safety assessments during the follow-up period. All participants received both injections in the placebo arm and no participants discontinued from the study by 31 March 2021, the data cut-off date for this interim analysis. All 200 participants were included in the SAS and FAS; and 147 of 150 (98.0%) participants were included in the PPS from the mRNA-1273 arm and 49 of 50 (98.0%) participants from the placebo arm. In total, 4 participants were excluded from the PPS: 3 in the mRNA-1273 arm because they did not receive a second vaccination and 1 in the placebo arm because their day 57 visit could not be completed by the data cut-off date.
Fig. 2.
Participant disposition.
The baseline characteristics were generally balanced across the mRNA-1273 and placebo arms and in each age group (Table 1 ). In the mRNA-1273 and placebo arms, respectively, the mean ages were 53.3 (range 20–77) and 52.4 (range 20–76) years; 85/150 (56.7%) and 27/50 (54.0%) of participants were male, and all participants were Japanese. Overall, 60 participants (30.0%) were aged ≥ 65 years (50/150 [33.3%] in the mRNA-1273 arm; 10/50 [20.0%] in the placebo arm).
Table 1.
Background demographics (safety analysis set).
| mRNA-1273 |
Placebo |
|||||
|---|---|---|---|---|---|---|
| Total (N = 150) |
≥20 to <65 years (N = 100) |
≥65 years (N = 50) |
Total (N = 50) |
≥20 to <65 years (N = 40) |
≥65 years (N = 10) |
|
| Age years, mean (range) | 53.3 (20–77) | 45.1 (20–64) | 69.6 (65–77) | 52.4 (20–76) | 48.2 (20–64) | 69.5 (67–76) |
| Sex, n (%) | ||||||
| Male | 85 (56.7) | 54 (54.0) | 31 (62.0) | 27 (54.0) | 21 (52.5) | 6 (60.0) |
| Female | 65 (43.3) | 46 (46.0) | 19 (38.0) | 23 (46.0) | 19 (47.5) | 4 (40.0) |
| Race, n (%) | ||||||
| Japanese | 150 (100) | 100 (100) | 50 (100) | 50 (100) | 40 (100) | 10 (100) |
| Other | 0 | 0 | 0 | 0 | 0 | 0 |
| BMI kg/m2, mean (SD) | 22.39 (2.65) | 22.12 (2.66) | 22.94 (2.57) | 23.35 (2.60) | 23.36 (2.72) | 23.30 (2.17) |
BMI = body mass index, SD = standard deviation.
Percentages are based on the number of randomised participants.
Concomitant medications (used on or after day 1) reported in ≥ 2 participants were paracetamol (80 [53.3%] participants), apronal/caffeine/ibuprofen (3 [2.0%] participants), febuxostat, loxoprofen sodium dihydrate, camphor/diphenhydramine/enoxolone/levomenthol/thymol, amlodipine besilate, rebamipide, zinc oxide, and atorvastatin calcium (2 [1.3%] participants each) in the mRNA-1273 arm, and paracetamol and fluticasone (2 [4.0%] participants) in the placebo arm. The smoking status of participants was not collected.
3.2. Safety
3.2.1. Solicited AEs
The incidences of solicited local and systemic AEs generally occurred at higher frequencies in participants who received mRNA-1273 than placebo (overall incidence after any injection, 144/150 [96.0%] vs 19/50 [38.0%] participants; Table 2 ).
Table 2.
Solicited adverse events within 7 days after injections (safety analysis set).
| First injection |
Second injection |
Any injection |
||||
|---|---|---|---|---|---|---|
| n (%) | mRNA-1273 (N = 150) |
Placebo (N = 50) |
mRNA-1273 (N = 147) |
Placebo (N = 50) |
mRNA-1273 (N = 150) |
Placebo (N = 50) |
| Any solicited adverse event | 138 (92.0) | 12 (24.0) | 135 (91.8) | 11 (22.0) | 144 (96.0) | 19 (38.0) |
| Maximum severity | ||||||
| Grade 1 | 106 (70.7) | 11 (22.0) | 42 (28.6) | 10 (20.0) | 44 (29.3) | 17 (34.0) |
| Grade 2 | 26 (17.3) | 0 | 47 (32.0) | 1 (2.0) | 51 (34.0) | 1 (2.0) |
| Grade 3 | 6 (4.0) | 1 (2.0) | 45 (30.6) | 0 | 48 (32.0) | 1 (2.0) |
| Grade 4 | 0 | 0 | 1 (0.7) | 0 | 1 (0.7) | 0 |
In the mRNA-1273 arm, injection-site pain was the most frequently reported solicited local AE in all age groups and after any vaccination, occurring in 138/150 (92.0%) participants (vs 4/50 [8.0%] in the placebo arm). The most frequently reported solicited systemic AEs across all age groups and after any vaccination were fatigue (96/150 [64.0%]), myalgia (91/150 [60.7%]), chills (77/150 [51.3%]), headache (73/150 [48.7%]), and fever (60/150 [40.0%]).
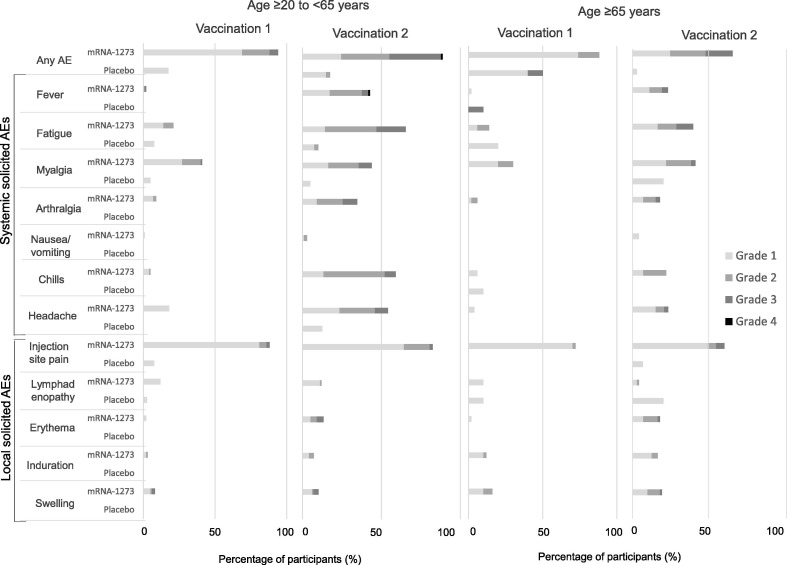
Solicited local and systemic AEs through day 7 were mainly mild (grade 1) or moderate (grade 2) in severity in both arms after the first and second injections (Fig. 3 A; Table 2). In the mRNA-1273 arm, the incidence of grade 2 or 3 solicited AEs increased after the second vaccination, particularly for systemic AEs. After the second vaccination, the incidence of grade 3 or 4 solicited AEs was 31.3% (46/147 participants) versus 4.0% (6/150 participants) after the first vaccination, in the mRNA-1273 arm. One grade 4 fever was reported in 1 (0.7%) participant after the second vaccination in the mRNA-1273 arm. Solicited AEs generally resolved within 1–3 days in both arms: the median duration of solicited local and systemic AEs was 3 days (range, 1–12 days) and 2 days (1–9 days) in the mRNA-1273 arm, respectively, and 1 day (1–3 days) for both in the placebo arm.
Fig. 3.
Local and systemic solicited adverse events within 7 days after injections in A) all age groups and B) by age group. AE = adverse event.
The incidence of solicited local or systemic AEs was similar irrespective of participant age in the mRNA-1273 arm: solicited local AEs were reported in 92/100 (92.0%) participants aged ≥ 20 years to < 65 years versus 46/50 (92.0%) participants aged ≥ 65 years, and solicited systemic AEs were reported in 84/100 (84.0%) participants aged ≥ 20 years to < 65 years and 44/50 (88.0%) participants aged ≥ 65 years (Fig. 3 B).
The incidence of solicited local and systemic AEs was slightly higher in females than in males, in the mRNA-1273 arm: solicited local AEs were reported in 61/65 (93.8%) females versus 77/85 (90.6%) males and solicited systemic AEs were reported in 60/65 (92.3%) females versus 68/85 (80.0%) males.
In the mRNA-1273 arm, injection-site pain was the most frequently reported solicited local AE after both the first and second vaccination and in both age groups (≥ 20 years to < 65 years, 88/100 [88.0%] and 81/98 [82.7%] participants, respectively; ≥ 65 years, 36/50 [72.0%] and 44/49 [89.8%] participants, respectively). After the first vaccination, induration and swelling in the mRNA-1273 arm were more frequently reported in older participants (6/50 [12.0%] and 8/50 [16.0%] participants, respectively) than in younger participants (3/100 [3.0%] and 8/100 [8.0%] participants, respectively). After the second vaccination, erythema, induration and swelling were more frequently reported in older participants (13/49 [26.5%], 12/49 [24.5%] and 14/49 [28.6%] participants, respectively) than in younger participants (13/98 [13.3%], 7/98 [7.1%], and 10/98 [10.2%] participants, respectively; Fig. 3).
Myalgia was the most frequently reported solicited systemic AE in the mRNA-1273 arm after the first vaccination, irrespective of age: age ≥ 20 years to < 65 years (41/100 [41.0%] participants); age ≥ 65 years (15/50 [30.0%] participants). After the second vaccination, fatigue was most frequently reported among younger participants (64/98 [65.3%] participants) followed by chills (58/98 [59.2%]) and headache (53/98 [54.1%]), while myalgia was the most frequent systemic AE in older participants (30/49 [61.2%] participants) followed by fatigue (29/49 [59.2%]), fever and headache (both, 17/49 [34.7%]). Solicited systemic AEs occurred more frequently after the second vaccination in the mRNA-1273 arm in both age groups (Fig. 3).
3.2.2. Unsolicited AEs
Unsolicited AEs were reported in 45/150 (30.0%) participants who received mRNA-1273 and 11/50 (22.0%) participants who received placebo (Table 3 ). Of these, treatment-related unsolicited AEs were reported in 27/150 (18.0%) participants in the mRNA-1273 arm and 1/50 (2.0%) in the placebo arm. The most frequently reported unsolicited AEs in the 28 days after any vaccination in the mRNA-1273 arm included injection-site pruritus (9/150 [6.0%] participants), nasopharyngitis and headache (each, 4/150 [2.7%] participants). Treatment-related unsolicited AEs (≥ 2 participants) reported in the 28 days after any vaccination in the mRNA-1273 arm included injection-site pruritus (9/150 [6.0%] participants), chest discomfort, injection-site rash, injection-site warmth, pollakiuria, and oropharyngeal pain (each, 2/150 [1.3%] participants; data not shown). No grade ≥ 3 unsolicited AEs were reported in either arm.
Table 3.
Summary of unsolicited adverse events by treatment arm and injections (safety analysis set).
| First injection |
Second injection |
Any injection |
||||
|---|---|---|---|---|---|---|
| n (%) | mRNA-1273 (N = 150) |
Placebo (N = 50) |
mRNA-1273 (N = 147) |
Placebo (N = 50) |
mRNA-1273 (N = 150) |
Placebo (N = 50) |
| Any unsolicited adverse event | 31 (20.7) | 9 (18.0) | 27 (18.4) | 7 (14.0) | 45 (30.0) | 11 (22.0) |
| Maximum severity | ||||||
| Grade 1 | 21 (14.0) | 8 (16.0) | 21 (14.3) | 3 (6.0) | 29 (19.3) | 7 (14.0) |
| Grade 2 | 10 (6.7) | 1 (2.0) | 6 (4.1) | 4 (8.0) | 16 (10.7) | 4 (8.0) |
| Grade ≥ 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Relationship to intervention | ||||||
| Not related | 21 (14.0) | 8 (16.0) | 7 (4.8) | 7 (14.0) | 18 (12.0) | 10 (20.0) |
| Related | 10 (6.7) | 1 (2.0) | 20 (13.6) | 0 | 27 (18.0) | 1 (2.0) |
Delayed local reactions (defined as local reactions that occurred more than 8 days after receipt of mRNA-1273) were observed in 8 participants (10 events: injection-site rash [2 events], injection-site pruritus [3 events], axillary pain, lymphadenopathy, injection-site erythema, injection-site warmth and eczema [1 event each]). All delayed local reactions were grade 1 or grade 2 in severity, occurred after the first dose and resolved within the follow-up period. Time from the first dose to the onset of delayed local reactions ranged from 8 to 22 days; events lasted for 2–15 days. Two participants who experienced injection-site rash discontinued the second dose at the discretion of the investigator; no repeat events were reported in the 6 participants who received the second dose.
MAAEs were reported in 7/150 (4.7%) participants who received mRNA-1273 and in 1/50 (2.0%) participant who received placebo. The only MAAE reported by ≥ 2 participants in the mRNA-1273 arm was dental caries. No noteworthy differences by age group (≥20 to <65 years or ≥65 years) were observed in the unsolicited AE profile across the arms. No deaths, SAEs or AEs leading to a participant's withdrawal from study occurred in the 28 days after any injections. AEs leading to discontinuation from vaccination were reported in 3 participants in the mRNA-1273 arm. No SARS-CoV-2-infected participants were reported through to the data cut-off date.
There were no clinically meaningful changes in safety clinical laboratory tests in either groups. In the mRNA-1273 arm, alanine aminotransferase increased, and aspartate aminotransferase increased were reported in 1 participant each (0.7%) as unsolicited AEs. These events were grade 1 in severity, resolved, and were considered unrelated to treatment (data not shown).
3.3. Immunogenicity
Two doses of 100 μg mRNA-1273 given 28 days apart resulted in the induction of bAb for S-2P in all 147 (100%) participants from baseline by day 57, in the primary immunogenicity analysis. No such induction of bAb was observed in the placebo group (Table 4 ). The GMT of serum bAb in the mRNA-1273 arm at baseline was low (0.81 AU/mL [95% CI: 0.70, 0.93]), and 813 AU/mL (95% CI: 759, 871) on day 57; GMFR and SCR on day 57 were 1009 (95% CI: 865, 1177) and 100% (95% CI: 97.5, 100), respectively.
Table 4.
Humoral immunogenicity responses to mRNA-1273 by age group (per protocol data set).
| mRNA-1273 |
Placebo |
|||||
|---|---|---|---|---|---|---|
| Total (N = 147) |
≥20 to < 65 years (N = 98) |
≥65 years (N = 49) |
Total (N = 49) |
≥20 to <65 years (N = 39) |
≥65 years (N = 10) |
|
| Serum binding antibody titres,aAU/mL | ||||||
| Baseline | ||||||
| GMT (95% CI) | 0.81 (0.70, 0.93) | 0.78 (0.66, 0.93) | 0.86 (0.66, 1.12) | 0.67 (0.58, 0.77) | 0.67 (0.57, 0.79) | 0.66 (0.48, 0.91) |
| Day 29 | ||||||
| GMT (95% CI) | 141 (125, 159) | 153 (133, 176) | 119 (95, 149) | 0.66 (0.58, 0.75) | 0.66 (0.56, 0.76) | 0.67 (0.48, 0.93) |
| GMFR (95% CI) | 175 (149, 205) | 196 (165, 232) | 139 (99, 196) | 0.98 (0.90, 1.08) | 0.98 (0.87, 1.10) | 1.01 (0.94, 1.08) |
| Seroconversion, n (%) | 147 (100) | 98 (100) | 49 (100) | 3 (6.1) | 3 (7.7) | 0 |
| SCR (95% CIb) | 100 (97.5, 100) | 100 (96.3, 100) | 100 (92.7, 100) | 6.1 (1.3, 16.9) | 7.7 (1.6, 20.9) | 0 (0.0, 30.8) |
| Day 43 | ||||||
| GMT (95% CI) | 1022 (941, 1110) | 1024 (931, 1126) | 1017 (863, 1199) | 0.62 (0.55, 0.70) | 0.62 (0.53, 0.71) | 0.64 (0.48, 0.86) |
| GMFR (95% CI) | 1268 (1073, 1498) | 1311 (1080, 1592) | 1187 (857, 1643) | 0.93 (0.87, 1.00) | 0.92 (0.84, 1.00) | 0.98 (0.94, 1.01) |
| Seroconversion, n (%) | 147 (100) | 98 (100) | 49 (100) | 1 (2.0) | 1 (2.6) | 0 |
| SCR (95% CIb) | 100 (97.5, 100) | 100 (96.3, 100) | 100 (92.7, 100) | 2.0 (0.1, 10.9) | 2.6 (0.1, 13.5) | 0 (0.0, 30.8) |
| Day 57 | ||||||
| GMT (95% CI) | 813 (759, 871) | 811 (750, 876) | 818 (711, 941) | 0.60 (0.53, 0.68) | 0.59 (0.52, 0.67) | 0.66 (0.48, 0.91) |
| GMFR (95% CI) | 1009 (865, 1177) | 1038 (867, 1242) | 955 (707, 1289) | 0.90 (0.83, 0.98) | 0.88 (0.79, 0.98) | 1.00 (0.93, 1.07) |
| Seroconversion, n (%) | 147 (100) | 97 (100)d | 49 (100) | 1 (2.0) | 1 (2.6) | 0 |
| SCR (95% CIb) | 100 (97.5, 100) | 100 (96.3, 100) | 100 (92.7, 100) | 2.0 (0.1, 10.9) | 2.6 (0.1, 13.5) | 0 (0.0, 30.8) |
| Neutralising antibody titres,cMN50 | ||||||
| Baseline | ||||||
| GMT (95% CI) | 79.9 (79.9, 79.9)e | 79.9 (79.9, 79.9)e | 79.9 (79.9, 79.9)e | 79.9 (79.9, 79.9)e | 79.9 (79.9, 79.9)e | 79.9 (79.9, 79.9)e |
| Day 57 | ||||||
| GMT (95% CI) | 1731 (1579, 1898) | 1727 (1549, 1927) | 1738 (1460, 2070) | 79.9 (79.9, 79.9)e | 79.9 (79.9, 79.9)e | 79.9 (79.9, 79.9)e |
| GMFR (95% CI) | 21.7 (19.8, 23.8) | 21.6 (19.4, 24.1) | 21.8 (18.3, 25.9) | 1.0 (1.0, 1.0)e | 1.0 (1.0, 1.0)e | 1.0 (1.0, 1.0)e |
| Seroconversion, n (%) | 146 (100) | 97 (100)d | 49 (100) | 0 | 0 | 0 |
| SCR (95% CIb) | 100 (97.5, 100) | 100 (96.3, 100) | 100 (92.7, 100) | 0 (0.0, 7.3) | 0 (0.0, 9.0) | 0 (0.0, 30.8) |
Titre values measured as below LLOQ are imputed to a value that is half of the LLOQ. Titre values measured as above ULOQ are imputed at the ULOQ value.
AU = arbitrary unit, bAb = binding antibody, CI = confidence interval, GMFR = geometric mean fold rise, GMT = geometric mean titre, IgG = immunoglobulin G, LLOQ = lower limit of quantification, MN50 = 50% microneutralisation titre, nAb = neutralising antibody, SARS-CoV-2 = severe acute respiratory syndrome coronavirus-2, SCR = seroconversion rate, ULOQ = upper limit of quantification.
Serum bAb against SARS-CoV-2 as measured by ligand-binding assay specific to the SARS-CoV-2 S-protein (VAC65 Spike IgG Antibody [LLOQ: 1 AU/mL, ULOQ: 2052 AU/mL]).
Two-sided 95% Clopper-Pearson CI for proportions within each treatment group.
Serum nAb against SARS-CoV-2 as measured by assay specific to wild-type virus (MN50 [LLOQ: 159.79, ULOQ: 11,173.11]).
A value of “NR“ (not reportable) in a participant at day 57 in the TAK-919 arm was excluded from the analysis.
Less than LLOQ.
For SCR, defined as percentage of participants with a change from below the LLOQ to equal to or above LLOQ, or a ≥ 4-fold rise from baseline, the placebo arm showed a 2.0% (95% CI: 0.1, 10.9) SCR on day 57 (Table 4). However, the proportion of participants in the placebo arm with a ≥ 4-fold rise was 0% (95% CI: 0.0, 7.3). In contrast, in the mRNA-1273 arm, the proportion of participants with a ≥ 4-fold rise was 100% (95% CI: 96.2, 100).
In the mRNA-1273 arm, anti-S-2P bAb levels increased by day 29 (28 days after first vaccination; GMT 141 AU/mL [95% CI: 125, 159]) and then further increased by day 43 (14 days after the second vaccination; GMT 1022 AU/mL [95% CI: 941, 1110]); thereafter, bAb levels remained elevated through day 57 (Table 4). The increase in bAb level was considerably greater after the second vaccination than after the first vaccination. Neutralising activity by nAb for wild-type virus was undetectable (less than LLOQ) at baseline in all participants; after the second vaccination (day 57) participants had a GMT of 1731 (95% CI: 1579, 1898), GMFR (calculated by imputing the baseline values as 79.9) of 21.7 (95% CI: 19.8, 23.8) and SCR of 100% (95% CI: 97.5, 100.0) in the mRNA-1273 arm. No increase of nAb was observed with placebo.
Immunogenicity responses were similar between age groups. On day 57, the GMT in bAb for S-2P in participants aged ≥ 20 years to < 65 years in the mRNA-1273 arm was 811 AU/mL (95% CI: 750, 876) versus 818 AU/mL (95% CI: 711, 941) in participants aged ≥ 65 years. The nAb titre values at day 57 were also similar irrespective of age (GMT 1727 [95% CI: 1549, 1927] and 1738 [95% CI: 1460, 2070], respectively). No significant differences in results were observed between the FAS and PPS.
Immunogenicity responses were numerically greater among female versus male participants. On day 57, the GMT in bAb for S-2P was 935 AU/mL (95% CI: 848, 1031) in female participants versus 732 AU/mL (95% CI: 669, 801) in male participants in the mRNA-1273 arm; the GMT of nAb titre values at day 57 was 1839 (95% CI: 1589, 2128) versus 1654 (95% CI: 1467, 1864), respectively.
4. Discussion
This was the first phase 1/2 study to assess the safety and immunogenicity of mRNA-1273 in healthy Japanese adults aged 20 years or older. Two doses of 100 μg mRNA-1273 administered 28 days apart elicited robust immune responses and an acceptable safety profile that was in line with US phase 2 and 3 mRNA-1273 studies [3], [4]. Based on the data from the present study and the US data, mRNA-1273 was approved for emergency use in Japan in May 2021 [16].
Local and systemic AEs were predominantly grade 1–2 in severity, generally resolved within 3 days, and occurred at a similar incidence, irrespective of age. While we must be careful in our assessment due to the difference in sample size between the studies, the incidence rate of some solicited AEs, including grade 3 and grade 4 fever, tended to be higher in this phase 1/2 study in a Japanese population compared with the phase 3 study in the USA. The incidence rates of any grade, grade 3 and grade 4 fever post-second vaccination were 40.1% (59/147 participants), 4.8% (7/147 participants), and 0.7% (1/147 participants) in the present study versus 17.4% (1806/10,352 participants), 1.6% (168/10,352 participants), and <0.1% (10/10,352 participants) in the US phase 3 study [[3], [19]]. However, the majority of solicited AEs in the mRNA-1273 group were grade 1–2 in severity and generally resolved within a few days in both studies. The difference in incidence rate of solicited AEs might be due to the same vaccine dose being administered to participants in Japan and the USA irrespective of mean body mass, which was higher among the US participants [8]. In the present study, mean (standard deviation) body mass index was 22.39 (± 2.65) kg/m2 in the mRNA-1273 arm whereas in the US study participants had a mean body mass index of 29.3 (± 6.9) kg/m2 [3]. No specific concerns for the Japanese population were raised with regard to the unsolicited AEs identified. Hence, there was no clinically significant difference in safety profile between the Japanese phase 1/2 and US phase 3 study.
Delayed-onset local reactions (defined as those with an onset on or after day 8) after either the first or second dose of mRNA-1273 were reported in both the US phase 3 study (rate after first dose, 0.8% [244/30,420 participants]; rate after second dose 0.2% [68/30,420 participants]) and in a subsequent case series [3], [9]. In the present study, we observed 8 relevant cases as described in the results, all of which were grade 1–2 in severity and resolved within the follow-up periods. The exact mechanism underlying delayed-onset local reactions is not known; however, delayed-type or T-cell-mediated hypersensitivity is hypothesised [9]. Although such delayed reactions may be worrisome for people receiving the vaccine, neither delayed hypersensitivity reaction nor local injection-site reactions are contraindications to future doses [10], and administration of the second vaccine dose is considered prudent given the robust acquired immune response we observed post-second dose.
In the present study, Japanese adults who received two doses of 100 μg of mRNA-1273 given 28 days apart induced robust immune response for both bAb and nAb against the recombinant S-2P or wild-type virus by day 57, with GMFRs > 1000 (bAb) and >20 (nAb), respectively. In the present study, anti-S-2P bAb and nAb were produced in response to both doses of mRNA-1273 within 28 days (day 29) after the first vaccination, rising to peak titres by 14 days after the second vaccination (day 43) and then remaining elevated to day 57. SCR was 100% at day 57. A similar response pattern and time course was reported in a phase 2 study of mRNA-1273 conducted in healthy adults in the USA [4].
While vaccine efficacy (prevention of symptomatic virus infection) was not investigated in the present study, the immunogenicity findings and response patterns observed were similar to those observed in the phase 2 [4] and phase 3 (unpublished data) US studies, so we anticipate that mRNA-1273 would achieve similar vaccine efficacy in Japanese populations. In a phase 3 US study of 30,420 volunteers, of whom 2.2% had evidence of SARS-CoV-2 infection (serologic or virologic, or both) at baseline, mRNA-1273 (100 μg) vaccine efficacy was shown to be 94.1% (95% CI: 89.3, 96.8; P < 0.001) [3]. These findings further support the use of mRNA-1273 in the Japanese population.
Nevertheless, given the continued evolution of the SARS-CoV-2 virus, it is vital that further research is conducted to assess the efficacy of 100 μg mRNA-1273 against variants [11], [12], and to examine safety and efficacy in paediatric populations. We acknowledge several limitations of our study. Because of the precautionary nature of phase 1/2 studies, our study was restricted by its small sample size, and the efficacy of the vaccine as prophylaxis for COVID-19 was not assessed. Moreover, the study included only healthy adult participants aged ≥ 20 years, so caution should be used when extrapolating results to populations with comorbid diseases that impact immune response, or in paediatric populations.
5. Conclusion
Two doses of 100 μg mRNA-1273 given 28 days apart demonstrated an acceptable safety profile and induced significant anti-SARS-CoV-2 immune responses in a Japanese population aged 20 years and older.
Data sharing statement
The datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participants' data supporting the results of the completed study, will be made available after the publication of the final study results within 3 months from initial request to researchers who provide a methodologically sound proposal. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection and requirements for consent and anonymisation.
Criteria for authorship
All listed authors meet the criteria for authorship set forth by the International Committee for Medical Journal Editors (ICMJE). Taisei Masuda : study conception and design, acquisition of data, data analysis and interpretation, drafting and revision of manuscript; Kyoko Murakami: data analysis and interpretation, revision of manuscript; Kenkichi Sugiura: study conception and design, data analysis and interpretation, drafting and revision of manuscript; Sho Sakui: study conception and design, data analysis and interpretation, drafting and revision of manuscript; Ron P. Schuring: acquisition of data, drafting of manuscript; Mitsuhiro Mori: study conception and design, data analysis and interpretation, drafting and revision of manuscript. All authors have read and approved the final manuscript and agree for it to be published.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: All authors are employees of Takeda Pharmaceutical Company Ltd with the exception of RS, who is an employee of Takeda Pharmaceutical International Ag.
Acknowledgments
Acknowledgements
The authors would like to thank the study participants, the investigators, Dr Ryuzo Hanada (Souseikai Sumida Hospital, Tokyo, Japan) and Dr Miwa Haranaka (Souseikai PS Clinic, Fukuoka, Japan), and staff at the study sites for their valued contribution to this study. Medical writing assistance was provided by Fumiko Shimizu and Emma Donadieu of MIMS Co., Ltd., sponsored by Takeda Pharmaceutical Company Limited, in compliance with Good Publication Practice 3 ethical guidelines (Battisti, et al. Ann Intern Med 2015; 163: 461–464).
Funding
This study was funded by Takeda Pharmaceutical Company Limited and Japan Agency for Medical Research and Development (AMED) [grant number: JP21nf0101621].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.02.030.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Ali K., Berman G., Zhou H., Deng W., Faughnan V., Coronado-Voges M., et al. Evaluation of mRNA-1273 SARS-CoV-2 vaccine in adolescents. N Engl J Med. 2021;385(24):2241–2251. doi: 10.1056/NEJMoa2109522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson E.J., Rouphael N.G., Widge A.T., Jackson L.A., Roberts P.C., Makhene M., et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu L., McPhee R., Huang W., Bennett H., Pajon R., Nestorova B., et al. A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine. Vaccine. 2021;39(20):2791–2799. doi: 10.1016/j.vaccine.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corbett K.S., Flynn B., Foulds K.E., Francica J.R., Boyoglu-Barnum S., Werner A.P., et al. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N Engl J Med. 2020;383(16):1544–1555. doi: 10.1056/NEJMoa2024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., et al. An mRNA vaccine against SARS-CoV-2 - preliminary report. N Engl J Med. 2020;383(20):1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahase E. Covid-19: UK approves Moderna vaccine to be given as two doses 28 days apart. BMJ. 2021;372:n74. doi: 10.1136/bmj.n74. [DOI] [PubMed] [Google Scholar]
- 8.Iguacel I., Maldonado A.L., Ruiz-Cabello A.L., Casaus M., Moreno L.A., Martínez-Jarreta B. Association between COVID-19 vaccine side effects and body mass index in Spain. Vaccines (Basel) 2021;9(11):1321. doi: 10.3390/vaccines9111321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blumenthal K.G., Freeman E.E., Saff R.R., Robinson L.B., Wolfson A.R., Foreman R.K., et al. Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2. N Engl J Med. 2021;384(13):1273–1277. doi: 10.1056/NEJMc2102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelso J.M., Greenhawt M.J., Li J.T., Nicklas R.A., Bernstein D.I., Blessing-Moore J., et al. Adverse reactions to vaccines practice parameter 2012 update. J Allergy Clin Immunol. 2012;130(1):25–43. doi: 10.1016/j.jaci.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Chung H., He S., Nasreen S., Sundaram M.E., Buchan S.A., Wilson S.E., et al. Effectiveness of BNT162b2 and mRNA-1273 covid-19 vaccines against symptomatic SARS-CoV-2 infection and severe covid-19 outcomes in Ontario, Canada: test negative design study. BMJ. 2021;374:n1943. doi: 10.1136/bmj.n1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chemaitelly H., Yassine H.M., Benslimane F.M., Al Khatib H.A., Tang P., Hasan M.R., et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med. 2021;27(9):1614–1621. doi: 10.1038/s41591-021-01446-y. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. COVID-19 statistics 2021: Global.
- 14.World Health Organization. COVID-19 statistics 2021: Japan.
- 15.US Food and Drug Administration. Moderna COVID-19 Vaccine.
- 16.Pharmaceuticals and Medical Devices Agency (PMDA). Approved medical products for COVID-19.
- 17.02 September 2020. Pharmaceuticals and Medical Devices Agency (PMDA). Principles for the evaluation of vaccines against the novel coronavirus SARS-CoV-2.
- 18.September 2007. U.S. Food and Drug Administration. Guidance for industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials.
- 19.December 17, 2020. Vaccines and Related Biological Products Advisory Committee December 17, 2020 Meeting Announcement.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.