Abstract
Aims
Desmoplakin (DSP) cardiomyopathy is an increasingly recognized form of arrhythmogenic cardiomyopathy. With a genotype-specific approach, we characterized the diagnosis, natural history, and risk for ventricular arrhythmia and heart failure in DSP cardiomyopathy.
Methods and results
We followed 91 individuals [45 probands, 34% male, median age 27.5 years (interquartile interval 20.0–43.9)] with pathogenic or likely pathogenic DSP variants for a median of 4.3 years. Regarding the ventricular involvement, left predominance was most common (n = 22, 28%) followed by bi-ventricular in 12 (15%) and right predominance in 5 (6%). Myocardial injury (chest pain, elevated troponin, normal coronary angiogram) occurred in 20 (22%) individuals. Incidence rates of sustained ventricular arrhythmia and heart failure (ventricular dysfunction ± symptoms) were 5.9 [95% confidence interval (CI): 3.9–9.1] and 6.7 (95% CI: 4.5–9.8) per 100 person-years, respectively. In univariate regression, myocardial injury was associated with sustained ventricular arrhythmia [hazard ratio (HR) 2.53, 95% CI: 1.05–6.11] and heart failure (HR 7.53, 95% CI: 3.10–18.26). After adjustment, left ventricular ejection fraction <35% and right ventricular dysfunction were prognostic for sustained ventricular arrhythmia while proband status and myocardial injury were prognostic for heart failure (all P < 0.05). The sensitivity of the arrhythmogenic right ventricular cardiomyopathy Task Force Criteria in diagnosing left dominant disease was 0.73; 5/22 (23%) of patients with sustained ventricular arrhythmias did not meet these criteria.
Conclusion
DSP cardiomyopathy affects both ventricles and carries high risk for ventricular arrhythmia and heart failure. Myocardial injury is associated with worse disease outcomes. Both diagnosis and risk stratification of DSP cardiomyopathy need refinement.
Keywords: Desmoplakin, Arrhythmogenic cardiomyopathy, Myocardial injury, Ventricular arrhythmia, Heart failure
Graphical Abstract
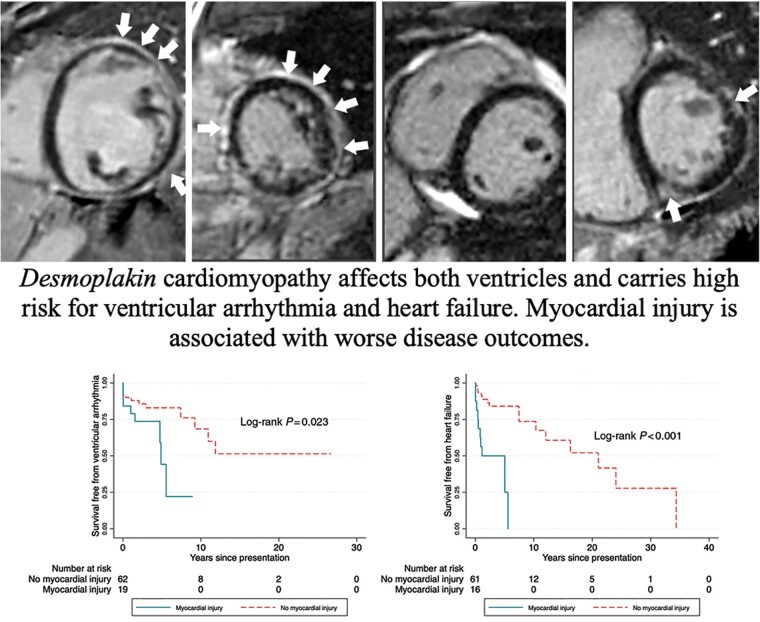
LGE was indicated by the white arrowheads. LGE, late gadolinium enhancement; LVEDVi, indexed left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; RVEDVi, indexed right ventricular end-diastolic volume; RVEF, right ventricular ejection fraction.
What’s new?
Desmoplakin cardiomyopathy affects both ventricles and carries high risk for ventricular arrhythmia and heart failure.
Myocardial injury is prospectively associated with worse disease outcomes among desmoplakin carriers.
Reduced left and right ventricular systolic dysfunction was prognostic for sustained ventricular arrhythmia, while proband status and myocardial injury were prognostic for heart failure among desmoplakin carriers.
The sensitivity of the arrhythmogenic right ventricular cardiomyopathy Task Force Criteria in diagnosing desmoplakin cardiomyopathy is limited.
Introduction
Desmoplakin (DSP) is the most abundant protein component of desmosomes in the heart and skin. It maintains cell adhesion by anchoring intermediate filaments to desmosomal plaques.1 Pathogenic/likely pathogenic (P/LP) DSP variants are identified in 3–15% of patients with arrhythmogenic right ventricular cardiomyopathy (ARVC).2 They are also associated with familial dilated cardiomyopathy.3 However, DSP variant carriers are under-represented in previous studies of ARVC and of dilated cardiomyopathy.4
Case reports and small case series of cardiac involvement in individuals with P/LP DSP variants started to emerge in the early 2000s.5 We previously reported that DSP variants were more commonly associated with left ventricular involvement, heart failure, and sudden death than PKP2-associated ARVC.6 Recently, a multicentre North American cohort of 107 patients with pathogenic DSP variants was compared to patients with PKP2 variants,7 and confirmed DSP cardiomyopathy is a distinct form of arrhythmogenic cardiomyopathy (ACM). DSP cardiomyopathy is different from classical ARVC because it tends to disproportionately involve the left ventricle and appears to present frequently with myocardial injury with chest pain and troponin elevation.7,8
While we have identified risk factors for worse clinical outcomes in ARVC9,10 risk stratification in DSP cardiomyopathy is largely unknown. Likewise, it is uncertain whether myocardial injury portends a more severe course in DSP cardiomyopathy. Finally, the performance of Task Force Criteria (TFC) developed for ARVC/ACM in DSP cardiomyopathy is unclear.
The Johns Hopkins ARVC/ACM registry prospectively enrolls patients and family members with definite or probable ARVC as well as those with P/LP desmosomal variants regardless of diagnosis. From this registry, we identified patients with P/LP DSP variants and aimed to (i) describe their clinical characteristics including incidence of sustained ventricular arrhythmias and heart failure; (ii) identify risk factors associated with sustained ventricular arrhythmias and heart failure; and (iii) evaluate how the TFC performs in diagnosis and prognostication of DSP cardiomyopathy.
Methods
Patient selection
We included patients and family members who carried a P/LP DSP variant (Class 4 or 5). The source population is the Johns Hopkins ARVC/ACM registry. From 1999 to 2021, the registry has recruited 981 individuals among whom more than 95% were genotyped. Pathogenicity of variants were adjudicated according to the joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association of Molecular Pathology11 with ARVC-specific modifications as previously described.12 DSP variants were identified primarily through commercial genetic testing. Genotype of cases who presented at autopsy was based on direct sequencing by molecular autopsy or in the case of obligate carriers inferred based on position in the pedigree.
This study complies with the Declaration of Helsinki. All patients provided written informed consent. The Johns Hopkins School of Medicine Institutional Review Board approved the study and participants provided written informed consent.
Clinical phenotypes
Medical history was obtained by review of medical records, clinical evaluation, and patient interview. Genetic counsellors with expertise in ARVC/ACM evaluated family history and constructed 3-generation pedigrees.
The study had two primary endpoints: (i) first sustained ventricular arrhythmia and (ii) heart failure. First sustained ventricular arrhythmia was a composite of spontaneous sustained ventricular tachycardia, aborted sudden cardiac death, or appropriate implantable cardioverter-defibrillator therapy for a sustained ventricular arrhythmia as previously described.13 Sustained ventricular arrhythmias were adjudicated by electrocardiograms, medical records, and device-stored electrocardiograms. Device settings were at the discretion of the treating physician. Heart failure was defined as the presence of structural abnormalities [left and/or right ventricular (RV) dysfunction] and/or at least one heart failure sign or symptom. Heart failure signs and symptoms included shortness of breath at rest, dyspnoea on exertion, orthopnoea, paroxysmal nocturnal dyspnoea, lower extremity oedema, abdominal swelling/ascites, fatigue, S3 summation gallop, jugular venous distention, and rales.9 Structural abnormalities were evaluated based on the transthoracic echocardiogram and/or cardiac magnetic resonance (CMR) report. Specifically, left ventricular systolic dysfunction was defined as left ventricular ejection fraction (LVEF) <50%, and severe dysfunction as LVEF <35%. Left ventricular dilation was defined as end-diastolic volume by CMR >214 mL or left ventricular diastolic diameter >58 mm in males and end-diastolic volume by CMR >178 mL or diastolic diameter >52 mm in females depending on the availability of CMR and echocardiogram.14,15 Right ventricular dysfunction was defined as RV ejection fraction on CMR ≤45% or visually reduced RV systolic function on transthoracic echocardiogram. Right ventricular dilation was indexed RV end-diastolic volume >100 mL/m2 (male) and >90 mL/m2 (female).14,15 Heart failure outcomes were adjudicated from clinical documentation in the electronic medical records. Every study participant was chart reviewed by a senior cardiology fellow (W.W.) and adjudicated as having heart failure or not. New York Heart Association class was assigned if heart failure was present. Among the heart failure cases, 20% were randomly selected and confirmed by a heart failure specialist (N.A.G.). The clinical documentation was either from Johns Hopkins Hospital or referring institutions. They were obtained by dedicated research personnel, scanned, and systemically reviewed by staff and research fellows.
Myocardial injury was defined as chest pain, serum cardiac troponin elevation greater than upper limit of normal as per local laboratory reference ranges, and the absence of obstructive coronary disease on coronary angiogram.7
Lastly, individuals were assessed as to whether they met the 2010 TFC for ARVC.16 Morphologic phenotype was classified as dominant left, dominant right, or biventricular, or as having normal function as per recently proposed criteria.17
Statistical analysis
Continuous variables are described using mean and standard deviation or median and interquartile interval for normal and skewed distribution data, respectively. Categorical variables are described as frequency and percentage. Baseline characteristics between probands and family members were compared by the Chi-squared test or Kruskal–Wallis test for categorical variables, and t-test or Wilcoxon Rank-Sum test for continuous variables.
Incidence rates for clinical outcomes were calculated. Cox regression was used to assess the associations between risk factors and clinical endpoints. Time zero was set at the time of first clinical presentation. Time of censoring was either the time of the endpoints (sustained ventricular arrhythmia or heart failure, respectively) or time of transplant or last follow-up, whichever came first. Univariate analyses were performed first. Variables of interest in the Cox models included age, sex, proband status, syncope at presentation, number of inverted T waves, non-sustained ventricular tachycardia, LVEF, RV function, late gadolinium enhancement (LGE),18 number of premature ventricular complexes (PVCs), and myocardial injury. These variables were chosen based on literature showing association with sustained ventricular arrhythmia10 and/or heart failure9 in ARVC or biological plausibility. Limited by the number of events, a parsimonious model (only variables with P < 0.1 in univariate analyses) was built to adjust for confounders. Event-free survival was plotted with the Kaplan–Meier survival curves and compared by log-rank test.
Statistical analyses were performed using Stata/IC 14.2. A two-sided P-value of <0.05 was considered statistically significant.
Results
Baseline characteristics
This study included 91 individuals with P/LP DSP variants (Table 1). Half (n = 45) were probands and one-third (n = 31) were male. Participants were from 45 families. Median numbers recruited from each family was 2 (range 1–5). Median age at presentation was 27.5 (interquartile interval: 20.0, 43.9) years. Nine (10%) individuals presented with sudden death and were diagnosed on autopsy. Eight (9%) came to medical attention after cascade testing due to positive family history. Patient genotypes are listed in Supplementary material online, Table S1. All but two patients had a single heterozygous P/LP DSP variant. One patient had two DSP P/LP variants in cis and one had an MYBPC3 deletion in addition to a DSP variant. Variants were predominantly premature terminating variants (n = 65, 71%).
Table 1.
Characteristics of study population
| Overall (N = 91) | Family member, N = 46 (51%) | Proband, N = 45 (49%) | P-value | |
|---|---|---|---|---|
| Age at presentation, median (IQI) | 27.5 (20.0, 43.9) | 26.4 (17.1, 37.0) | 30.0 (22.7, 47.0) | 0.100 |
| Male | 31 (34%) | 17 (37%) | 14 (31%) | 0.56 |
| Type of presentation | ||||
| Abnormal test result | 32 (36%) | 23 (51%) | 9 (20%) | 0.002 |
| Resuscitated cardiac arrest | 4 (4%) | 1 (2%) | 3 (7%) | |
| Sudden cardiac death | 9 (10%) | 0 (0%) | 9 (20%) | |
| Symptomatic and livinga | 36 (40%) | 16 (36%) | 20 (45%) | |
| Myocardial injury | 20 (22%) | 9 (20%) | 11 (24%) | 0.57 |
| T-wave inversions | ||||
| V1-2 | 30 (33%) | 14 (30%) | 16 (36%) | 0.60 |
| V1-3 | 18 (20%) | 6 (13%) | 12 (27%) | 0.10 |
| V4-6 | 23 (25%) | 7 (15%) | 16 (36%) | 0.026 |
| I and aVL | 7 (9%) | 2 (5%) | 5 (14%) | 0.14 |
| Low voltage on electrocardiogram | 12 (15%) | 5 (11%) | 7 (19%) | 0.31 |
| PVCs >500/24 h | 44 (48%) | 20 (43%) | 24 (53%) | 0.35 |
| LVEF, mean (SD) | 49.1 (12.7) | 53.8 (10.8) | 43.3 (12.4) | <0.001 |
| LVEF <50% | 34 (42%) | 11 (25%) | 23 (64%) | <0.001 |
| LVEF <35% | 12 (15%) | 2 (5%) | 10 (28%) | 0.004 |
| LV dilation | 27 (31%) | 7 (16%) | 20 (47%) | 0.002 |
| Late gadolinium enhancement | 32 (49%) | 14 (38%) | 18 (64%) | 0.035 |
| RV dysfunction | 17 (21%) | 6 (14%) | 11 (31%) | 0.066 |
| RV dilation | 27 (34%) | 12 (27%) | 15 (43%) | 0.15 |
| Ventricular involvement | ||||
| Normal ventricular function | 41 (51%) | 32 (73%) | 9 (25%) | <0.001 |
| Left predominance | 22 (28%) | 6 (14%) | 16 (44%) | |
| Right predominance | 5 (6%) | 1 (2%) | 4 (11%) | |
| Bi-ventricular | 12 (15%) | 5 (11%) | 7 (19%) | |
| Implantable cardioverter-defibrillator | 45 (49%) | 15 (33%) | 30 (67%) | 0.001 |
All participants were Caucasian.
IQI: interquartile interval; LVEF, left ventricular ejection fraction; PVC, premature ventricular complex; RV: right ventricle; SD, standard deviation.
Symptomatic and living including those presenting with sustained ventricular tachycardia and cardiac syncope.
Table 1 also shows the electrocardiogram, Holter, and imaging data at last follow-up. Specifically, 23 (25%) individuals had T-wave inversions in V4–V6 and 7 (9%) in I and aVL. Forty-four (48%) individuals had >500 PVCs in 24 h (median 24-h PVC count 408, interquartile interval: 0–2951). LVEF was 49.1% ± 12.7%, with 12 (15%) individuals having LVEF <35%. Right ventricular dysfunction was noted in 17 (21%) individuals.
One-fifth (n = 20) experienced myocardial injury at a median age of 27.5 (interquartile interval: 18.9, 42.6). Thirteen (65%) individuals had myocardial injury as the initial presentation. The troponin level was available in 13 individuals (8 with troponin I, 5 with unknown troponin type). The median troponin I level was 5.0 nanograms per millilitre (range 0.2–65.0) (Supplementary material online, Table S2). Results of inflammatory markers (erythrocyte sedimentation rate and C-reactive protein) were available in only two patients and were normal. Four individuals had two episodes of myocardial injury. Two patients underwent fluorodeoxyglucose–positron emission tomography scan which revealed evidence of inflammation.
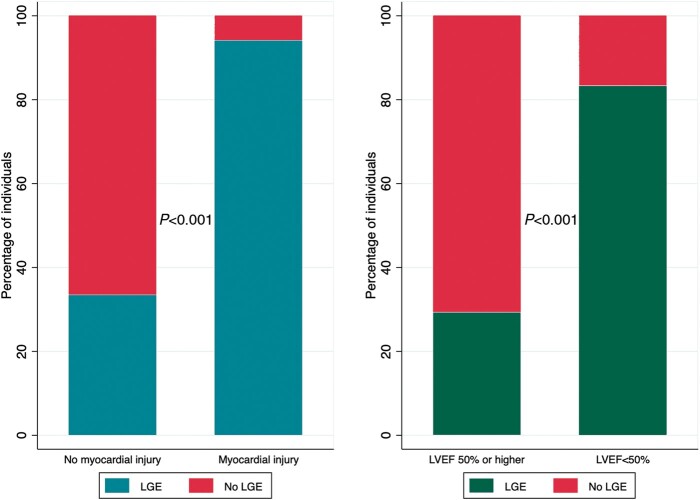
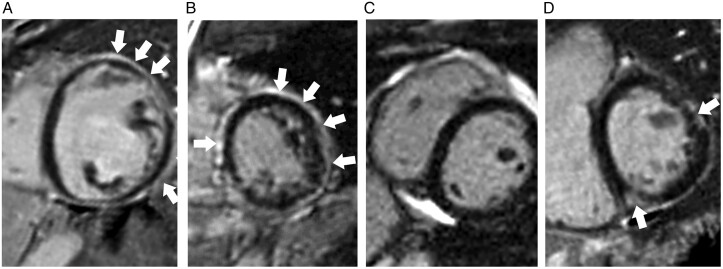
Sixty-four individuals had CMR, on which the LVEF was 53.1% ± 11.3%, left ventricular end-diastolic volume indexed by body surface area was 86.6 ± 22.0 ml/m2, RV ejection fraction was 48.9% ± 11.5%, and RV end-diastolic volume indexed by body surface area was 88.4 ± 20.9 mL/m2. Thirty-two (50.8%) had LGE. The majority had a sub-epicardial pattern (n = 21, 72%), followed by mid-myocardial (n = 14, 48%), and other (1 sub-endocardial, 3 patchy). Location wise, LGE of the left ventricle was seen in the anterior wall in 12 (38%) patients, lateral wall in 15 (47%) patients, septal wall in 18 (56%) patients, inferior wall in 17 (53%) patients, and apex in 6 (19%) patients. As in Figure 1, LGE was more common in individuals with myocardial injury (n = 20) than those without (94% vs. 33%, P < 0.001) and LVEF <50% than those with LVEF higher than 50% (83% vs. 29% P < 0.001). CMR examples demonstrating patterns of ventricular involvement and LGE are presented in Figure 2.
Figure 1.
Late gadolinium enhancement by history of myocardial injury and left ventricular function. LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction.
Figure 2.
Examples of desmoplakin cardiomyopathy on cardiac magnetic resonance. (A) Left dominant: left ventricle is dilated and dysfunctional (LVEF = 25% and LVEDVi = 188 mL/m2. The RV is normal. Extensive subendocardial LGE in the left ventricle at mid cavity. (B) Biventricular involvement: both left and right ventricular function was reduced (LVEF 49% and RVEF 43%). Extensive subepicardial and mid-wall LGE in a non-ischaemic pattern throughout the entire circumference of the left ventricle at mid-cavity. (C) Right dominant: left ventricle is normal but RVEF is reduced (34%) although RVEDVi remains normal (79 mL/m2) No evidence of LGE. (D) Normal ventricular function but LGE present.
As shown in Table 1, family members were less symptomatic, had less lateral T-wave inversions, better ventricular function, and less LGE on CMR compared to probands. However, the prevalence of myocardial injury and PVC burden were similar between probands and family members.
A defibrillator was implanted in 45 individuals, 17 (38%) being placed within 6 months of the first clinical presentation. Twenty-eight (62%) devices were implanted for primary prevention.
At the last follow-up, 13 (17%) participants were on diuretics, 42 (55%) on beta blockers, 28 (37%) on angiotensin-converting enzyme inhibitors, and 12 (16%) on anti-arrhythmic medication (9 sotalol, 2 amiodarone, 1 dofetilide).
Clinical outcomes and risk stratification
Excluding the 9 individuals whose initial presentation was sudden death and described in the Supplementary material online, Table S3, 82 individuals were followed for a median of 4.3 years (interquartile interval: 2.2–8.1). During follow-up, there were three (3.6%) heart transplants (at ages age 42, 46, and 54) but no deaths (Table 2).
Table 2.
Clinical outcomes in follow-up
| Overall (N = 82)a | Family member (N = 46) | Proband (N = 36) | P-value | |
|---|---|---|---|---|
| Sustained ventricular arrhythmia | 22 (27%) | 6 (13%) | 16 (44%) | 0.001 |
| Ventricular fibrillation | 14 (17%) | 3 (7%) | 11 (31%) | |
| Ventricular tachycardia | 5 (6%) | 1 (2%) | 4 (11%) | |
| Appropriate ICD shock or anti-tachycardia therapy for sustained ventricular arrhythmia | 10 (12%) | 2 (4%) | 8 (22%) | |
| Heart failure | 31 (38%) | 10 (22%) | 21 (58%) | <0.001 |
| NYHA I | 14 (45%) | 6 (60%) | 8 (38%) | |
| NYHA II | 13 (42%) | 3 (30%) | 10 (48%) | |
| NYHA III | 4 (13%) | 1 (10%) | 3 (14%) | |
| Heart transplant | 3(4%) | 0 (0%) | 3 (8%) | 0.05 |
ICD, implantable cardioverter-defibrillator; NYHA, New York Heart Association.
Excluding the nine individuals whose initial presentation was sudden death.
During the follow-up, sustained ventricular arrhythmia occurred in 22 (27% out of 82) individuals, including ventricular fibrillation/resuscitated cardiac arrest (n = 14), sustained ventricular tachycardia (n = 5), and appropriate defibrillator shock or anti-tachycardia therapy for sustained ventricular arrhythmia (n = 10). The incidence rate of sustained ventricular arrhythmia was 5.9 [95% confidence interval (CI): 3.9–9.1] per 100 person-years. The median age at first sustained ventricular arrhythmia was 32.6 (interquartile interval: 21.8–51.6) years.
In univariable analyses, proband status [hazard ratio (HR) 3.29, 95% CI: 1.30–8.31], LVEF <35% (HR 3.21, 95% CI: 1.43–7.21), RV dysfunction (HR 3.52, 95% CI: 1.32–9.38), and myocardial injury (HR 2.82, 95% CI: 1.13–7.03) were associated with sustained ventricular arrhythmia. In multivariable analyses, LVEF <35% (HR 3.45, 95% CI: 1.42–8.39) and RV dysfunction (HR 3.98, 95% CI: 1.46–10.88) were independently associated with sustained ventricular arrhythmia, while proband status and myocardial injury did not reach statistical significance (Table 3).
Table 3.
Risk factors for sustained ventricular arrhythmia
| Hazard ratio (95% confidence interval) |
||
|---|---|---|
| Unadjusted | Adjusted | |
| Age at presentation | 1.00 (0.97–1.03) | |
| Male | 0.84 (0.32–2.21) | |
| Proband | 3.29 (1.30–8.31) | 2.09 (0.72–6.05) |
| Number of inverted T waves | 1.08 (0.90–1.28) | |
| Non-sustained VT | 0.91 (0.38–2.17) | |
| LVEF <35% | 3.21 (1.43–7.21) | 3.45 (1.42–8.39) |
| RV dysfunction | 3.52 (1.32–9.38) | 3.98 (1.46–10.88) |
| Myocardial injury | 2.82 (1.13–7.03) | 2.12 (0.81–5.57) |
| Late gadolinium enhancement | 2.23 (0.70–7.11) | |
| PVCs >500/24 ha | 0.91 (0.38–2.17) | |
Age at presentation was also characterized as categorical in tertile.
Adjusted for proband status, LVEF <35%, RV dysfunction, and myocardial injury.
LVEF, left ventricular ejection fraction; PVC, premature ventricular complex; RV, right ventricle; VT, ventricular tachycardia.
PVC was also characterized as binary with a different cut-off (>1000/24 h vs. rest) and as continuous with the logarithm of 24-h PVC count. Neither reached statistical significance.
High PVC burden was not associated with sustained ventricular arrhythmia. We treated PVC burden both as dichotomous (>500/24 h vs. rest; >1000/24 h vs. rest) and as continuous with the logarithm of 24-h PVC count. Only three individuals had syncope (none had sustained ventricular arrhythmia). Therefore, syncope was not tested in the Cox model.
We next examined the heart failure outcome. Thirty-one (38%) developed heart failure at median age of 38.0 (interquartile interval: 25.7–48.1) years, of whom 17 (21%) had at least New York Heart Association Class II symptoms. The incidence rate of heart failure was 6.7 (95% CI: 4.5–9.8) per 100 person-years.
Proband status (HR 3.21, 95% CI: 1.37–7.52) and myocardial injury (HR 6.44, 95% CI: 2.61–15.90) were associated with development of heart failure in univariable analyses. In multivariable analyses, both associations remained statistically significant (Table 4).
Table 4.
Risk factors for heart failure
| Hazard ratio (95% confidence interval) |
||
|---|---|---|
| Unadjusted | Adjusted | |
| Age at presentation | 1.01 (0.99–1.04) | |
| Male | 0.74 (0.30–1.83) | |
| Proband | 3.21 (1.37–7.52) | 2.81 (1.16–6.78) |
| Number of inverted T waves | 1.13 (0.90–1.44) | |
| Myocardial injury | 6.44 (2.61–15.90) | 5.67 (2.38–13.48) |
| Late gadolinium enhancement | 2.58 (0.93–7.20) | 0.85 (0.29–2.54) |
| PVCs >500/24 ha | 1.93 (0.85–4.39) | |
Age at presentation was also characterized as categorical in tertile.
Adjusted for proband status, myocardial injury, and late gadolinium enhancement.
PVC was also characterized as binary with a different cut-off (>1000/24 h vs. rest) and as continuous with the logarithm of 24-h PVC count. Neither reached statistical significance.
PVC, premature ventricular complex.
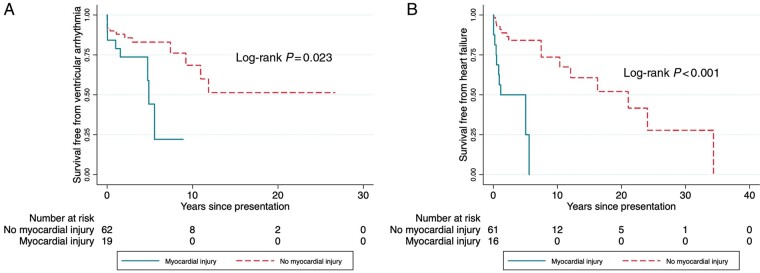
Figure 3 shows the Kaplan–Meier curves of survival free from sustained ventricular arrhythmia and heart failure by the presence or absence of myocardial injury.
Figure 3.
Kaplan–Meier curves of survival free from sustained ventricular arrhythmia and heart failure by the presence of myocardial injury.
We also limited the analyses to probands only. LVEF <35% (HR 3.92, 95% CI: 1.42–10.80) was independently associated with ventricular arrhythmia in probands. Myocardial injury (HR 5.35, 95% CI: 2.06–13.90) remained independently associated with heart failure (Supplementary material online, Tables S4 and S5).
Phenotype by the Task Force Criteria for ARVC
At baseline, 46 (51%) met the TFC for ARVC. The details of TFC designations are in Supplementary material online, Table S6.
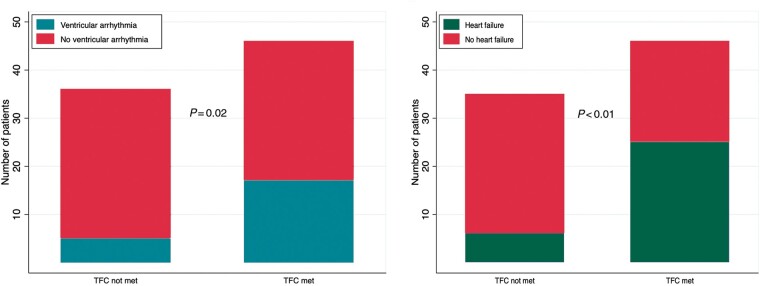
As shown in Figure 4, more individuals who met the TFC had sustained ventricular arrhythmia (36% vs. 14%) and heart failure (54% vs. 17%) than individuals who did not (P < 0.05). New York Heart Association Class II or worse symptoms were reported by 26% (n = 12) of individuals who met the TFC and 14% (n = 5) of individuals who did not (P = 0.20).
Figure 4.
Sustained ventricular arrhythmia and heart failure symptoms by diagnosis per arrhythmogenic right ventricular cardiomyopathy Task Force Criteria. TFC: Task Force Criteria.
However, the TFC did not identify all affected individuals or individuals who experienced clinically significant events. For individuals with LVEF <50%, the sensitivity of TFC was 0.79, specificity 0.59, positive predictive value 0.59, and negative predictive value 0.79. Among individuals with left dominant disease, 73% (16 out of 22) met the TFC. All (5 out of 5) individuals with right dominant disease and 92% (11 out of 12) with bi-ventricular disease met the TFC.
Five individuals did not meet the TFC but had sustained ventricular arrhythmia. Their average age of presentation was 25.0 (±19.6) years and average age of sustained ventricular arrhythmia was 32.5 (±19.4) years. Four (80%) were women and three (60%) had experienced myocardial injury. Two (40%) had LVEF <35%, none had RV dysfunction, and two (40%) had LGE on CMR.
Discussion
Main findings
We leveraged our large ARVC/ACM registry to characterize 91 individuals with P/LP DSP variants with the aim of informing genotype-specific diagnosis, clinical event risk stratification, and ultimately patient management. We showed that these individuals are at high risk for sustained ventricular arrhythmia and heart failure. Myocardial injury was present in one-fifth of the cohort, and importantly myocardial injury was shown to be prospectively associated with heart failure and sustained ventricular arrhythmia. Also, reduced ventricular function (left and/or right) was independently associated with sustained ventricular arrhythmia. Lastly, our results suggest the TFC has modest sensitivity in diagnosis and limited prognostic value for DSP cardiomyopathy, with a significant proportion of individuals with both sustained ventricular arrhythmia and heart failure not meeting TFC.
Prior studies of DSP cardiomyopathy
DSP was the second gene identified in ARVC after plakoglobin (JUP) when homozygous carriers were described to have Carvajal syndrome.19 Shortly after, with widespread use of genetic testing in patients with suspected ACM, case reports of cardiac involvement in individuals with DSP variants started to emerge from early 2000s.5 In contrast to classic ARVC, DSP cardiomyopathy commonly involves the left ventricle, appears to have worse disease outcomes and may have female predominance.7 Because the 2010 TFC was designed to diagnose patients with predominantly RV involvement,16 it has been recognized that the TFC tend to underdiagnosed ACM in DSP carriers. Also, myocardial injury appears to cluster in DSP cardiomyopathy20 and was hypothesized to play a role in disease progression. However, prior studies were limited by small case numbers and short follow-up. In this context, we report our findings from the Johns Hopkins ARVC/ACM registry.
Clinical features of DSP cardiomyopathy
In accordance with prior reports, we observed left ventricular fibrosis (LGE on CMR) and dysfunction, frequent PVCs, and myocardial injury with chest pain and troponin elevation as the cardinal features of DSP cardiomyopathy. Also, among this mixed group of probands and family members, the event rates for sustained ventricular arrhythmia and heart failure were strikingly high (both approximately 6 events per 100 person-year). Family members and probands had similar prevalence of myocardial injury and PVC burden. Furthermore, more than one-third of the family members had LGE on CMR. This is in line with the observation that DSP variants could be associated with worse clinical outcomes than PKP2 variants.4,7 During follow-up, probands were more likely to develop heart failure and ventricular arrhythmia. After multivariable adjustment, proband status was prospectively associated with heart failure but not ventricular arrhythmia.
Myocardial injury in DSP cardiomyopathy
Acute myocardial injury has recently been reported as an important feature of DSP cardiomyopathy.7 In our study population, 22% had documented myocardial injury and 49% had LGE suggestive of myocardial fibrosis on CMR. Of the subset with myocardial injury, more than 90% had LGE on CMR. The difference in prevalence between LGE and documented myocardial injury (49–22% = 28%) may represent minor episode(s) of myocardial injury which was asymptomatic or caused mild symptoms not enough to trigger medical evaluation. Both the ‘silent’ episodes and the symptomatic episodes mimicking myocarditis could contribute to scar formation and decline of left ventricular function which are substrate for sustained ventricular arrhythmia and heart failure symptoms. This was also supported by the observation that myocardial injury (at median age 28 years) preceded sustained ventricular arrhythmia (at 33 years) and heart failure (at 38 years).
At this point, it remains unclear what triggers myocardial injury (although intense exercise is hypothesized to play a role) and which factors (genetic and/or environmental) determine the severity of the episodes.
Sustained ventricular arrhythmia in DSP cardiomyopathy
In our study, LVEF <35% and RV dysfunction were independently associated with higher risk of sustained ventricular arrhythmia. Unlike typical prior ARVC cohorts, we did not see difference by gender. High PVC burden is a risk factor for sustained ventricular arrhythmia in ARVC.10 However, it was not associated with an increased risk of sustained ventricular arrhythmia in our study, which echoes the recent report by Smith et al.7 On the other hand, our study additionally found RV dysfunction to be associated with sustained ventricular arrhythmia. One difference here is that our association was tested in a prospective fashion (HR) while it was cross-sectional (odds ratio) by Smith et al.
Since sudden death is devastating to the patient and the family, it is worth looking at the absolute risk of sustained ventricular arrhythmia. Among individuals with LVEF >50%, 12% (n = 5) had sustained ventricular arrhythmia. This risk in itself would merit consideration of an implantable defibrillator. Overall, risk stratification among DSP cardiomyopathy remains crude.
Heart failure in DSP cardiomyopathy
Heart failure symptoms were common in our study. DSP cardiomyopathy appears to affect both left and right ventricle indiscriminately with a progressive nature. Myocardial injury precedes and is associated with the development of heart failure. Aside from myocardial injury and proband status, we did not identify other risk factors for heart failure.
Diagnosis of DSP cardiomyopathy
The TFC were developed to diagnose patients with predominantly RV involvement and are insensitive in diagnosing DSP cardiomyopathy.7 In our data, 44% of probands had left predominant disease. When the disease was left dominant, the TFC missed one in every 4 cases (sensitivity 0.73). Among the six individuals with left-dominant disease but not meeting TFC, 2 (33%) individuals developed sustained ventricular arrhythmia and 5 (85%) developed heart failure. For individuals with LVEF <50% (which we believe is a clear marker of myocardial involvement), the TFC missed one in every 5 cases (sensitivity 0.79). On the contrary, the TFC performed well when the disease was right dominant (sensitivity 100%). Also, the TFC does not include myocardial injury which is a feature of DSP cardiomyopathy.
Over 25% individuals who experienced sustained ventricular arrhythmia or heart failure did not meet the TFC. In our cohort, the individuals who had sustained ventricular arrhythmia but did not meet TFC were more likely to be women with reduced LVEF and myocardial injury but normal RV function.
Therefore, the TFC should not be solely relied on in diagnosing DSP cardiomyopathy. Myocardial fibrosis, ventricular dysfunction (commonly left, right and bi-ventricular also possible), frequent sustained ventricular arrhythmia and PVC, and episodic myocardial injury, should raise suspicion for the diagnosis. Currently, there is no consensus on diagnosis and further work with institutional collaboration will be required to establish the optimal diagnostic criteria for DSP cardiomyopathy.
These results highlight the importance of genetic testing for not only patients with suspected ARVC, but also cases of dilated cardiomyopathy with frequent ventricular arrhythmia and patients with recurrent or young-onset idiopathic myocarditis. Such testing performed in a centre with multidisciplinary expertise will enhance early diagnosis and facilitate genotype-specific management.
Limitations
As an inherent limitation in studies of rare disease, our study population was from a referral centre, which may contribute to the high event risk observed and affect the generalizability of the findings to non-referral settings. Due to the registry nature of the data, missingness of data was inevitable. Because the missingness was not significant in our data, available case analysis approach was used, which may affect the results of the findings. Second, exercise recommendation is relevant in this population. However, our study does not have the power to comment on whether exercise is associated with worse outcomes in DSP cardiomyopathy. Third, we did not include a control group with other genotypes as this manuscript was aimed to focus on DSP carriers. However, in addition to the recent report by Smith et al.,7 we also had already compared the clinical courses of DSP carriers to PKP2 carriers.6 Also, the incidence of appropriate implantable cardioverter-defibrillator therapies could be influenced by individual programming decisions, which may overestimate incidence of life-threatening arrhythmia. The imaging parameters were abstracted from study reports instead of reviewing the images, which limits the inter-observer reliability of the measurements. Numbers of individuals from the same family were not evenly distributed (18 individuals from 18 different families while 20 other individuals from 4 families). Together with the overall small sample size and the number of variables included in the models, we could not utilize the random-effect models clustering on family to fully account for the relatedness. Although we addressed this by (i) adjusting for pedigree and (ii) limiting analyses to probands only, the relatedness of individuals within the cohort remains a methodological limitation. Future larger studies are needed to address this.
Conclusions
In this study, we characterized diagnosis, clinical course, and risk stratification in individuals with P/LP DSP variants to inform genotype-directed care. These individuals are at high risk for cardiac arrest, sustained ventricular arrhythmia, and heart failure. DSP cardiomyopathy affects both ventricles. Chest pain with troponin elevation occurs frequently and is prospectively associated with worse clinical outcomes. Low LVEF and RV dysfunction are independently associated with sustained ventricular arrhythmia while proband status and myocardial injury were prognostic for heart failure. Through large collaborative studies, there is considerable potential for developing diagnostic criteria, refining risk stratification, and enhancing genotype-specific management.
Supplementary material
Supplementary material is available at Europace online.
Supplementary Material
Acknowledgements
The authors are grateful to the patients and families who made this work possible.
Funding
This work is supported by a grant from the Fondation Leducq’ (HC) and UL1 TR0030968 from the NCATS which supports the Johns Hopkins Institute for Clinical and Translational Research. The Johns Hopkins ARVD/C Program is supported by the Leonie-Wild Foundation, the Leyla Erkan Family Fund for ARVD Research, the Dr Francis P. Chiramonte Private Foundation, the Dr Satish, Rupal, and Robin Shah ARVD Fund at Johns Hopkins, the Bogle Foundation, the Healing Hearts Foundation, the Campanella family, the Patrick J. Harrison Family, the Peter French Memorial Foundation, and the Wilmerding Endowments.
Conflict of interest: H.C. is a consultant for Medtronic Inc. and Abbott. H.C. receives research support from Boston Scientific Corp. C.T. and C.A.J. receive salary support from this grant. H.T. receives research support from Abbott. B.M. is a consultant for MyGeneCounsel. All remaining authors have declared no conflicts of interest.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Contributor Information
Weijia Wang, Division of Cardiology, Department of Medicine, Johns Hopkins University, Johns Hopkins Hospital, Blalock 545, 600 North Wolfe Street, Baltimore, MD 21287, USA; Division of Cardiology, Department of Medicine, University of Massachusetts Medical School, Worcester, MA, USA.
Brittney Murray, Division of Cardiology, Department of Medicine, Johns Hopkins University, Johns Hopkins Hospital, Blalock 545, 600 North Wolfe Street, Baltimore, MD 21287, USA.
Crystal Tichnell, Division of Cardiology, Department of Medicine, Johns Hopkins University, Johns Hopkins Hospital, Blalock 545, 600 North Wolfe Street, Baltimore, MD 21287, USA.
Nisha A Gilotra, Division of Cardiology, Department of Medicine, Johns Hopkins University, Johns Hopkins Hospital, Blalock 545, 600 North Wolfe Street, Baltimore, MD 21287, USA.
Stefan L Zimmerman, Division of Cardiology, Department of Medicine, Johns Hopkins University, Johns Hopkins Hospital, Blalock 545, 600 North Wolfe Street, Baltimore, MD 21287, USA.
Alessio Gasperetti, Division of Cardiology, Department of Medicine, Johns Hopkins University, Johns Hopkins Hospital, Blalock 545, 600 North Wolfe Street, Baltimore, MD 21287, USA.
Paul Scheel, Division of Cardiology, Department of Medicine, Johns Hopkins University, Johns Hopkins Hospital, Blalock 545, 600 North Wolfe Street, Baltimore, MD 21287, USA.
Harikrishna Tandri, Division of Cardiology, Department of Medicine, Johns Hopkins University, Johns Hopkins Hospital, Blalock 545, 600 North Wolfe Street, Baltimore, MD 21287, USA.
Cynthia A James, Division of Cardiology, Department of Medicine, Johns Hopkins University, Johns Hopkins Hospital, Blalock 545, 600 North Wolfe Street, Baltimore, MD 21287, USA.
References
- 1. Norgett EE, Hatsell SJ, Carvajal-Huerta L, Cabezas JC, Common J, Purkis PE et al. Recessive mutation in desmoplakin disrupts desmoplakin–intermediate filament interactions and causes dilated cardiomyopathy, woolly hair and keratoderma. Hum Mol Genet 2000;9:2761–6. [DOI] [PubMed] [Google Scholar]
- 2. Wang W, James CA, Calkins H. Diagnostic and therapeutic strategies for arrhythmogenic right ventricular dysplasia/cardiomyopathy patient. Europace 2019;21:9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jordan E, Peterson L, Ai T, Asatryan B, Bronicki L, Brown E et al. An evidence-based assessment of genes in dilated cardiomyopathy. Circulation 2021;144(1):7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Groeneweg JA, Bhonsale A, James CA, Riele AS, Dooijes D, Tichnell C et al. Clinical presentation, long-term follow-up, and outcomes of 1001 arrhythmogenic right ventricular dysplasia/cardiomyopathy patients and family members. Circ Cardiovasc Genet 2015;8:437–46. [DOI] [PubMed] [Google Scholar]
- 5. Norman M, Simpson M, Mogensen J, Shaw A, Hughes S, Syrris P et al. Novel mutation in desmoplakin causes arrhythmogenic left ventricular cardiomyopathy. Circulation 2005;112:636–42. [DOI] [PubMed] [Google Scholar]
- 6. Bhonsale A, Groeneweg JA, James CA, Dooijes D, Tichnell C, Jongbloed JDH et al. Impact of genotype on clinical course in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated mutation carriers. Eur Heart J 2015;36:847–55. [DOI] [PubMed] [Google Scholar]
- 7. Smith ED, Lakdawala NK, Papoutsidakis N, Aubert G, Mazzanti A, McCanta AC et al. Desmoplakin cardiomyopathy, a fibrotic and inflammatory form of cardiomyopathy distinct from typical dilated or arrhythmogenic right ventricular cardiomyopathy. Circulation 2020;141:1872–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scheel PJ, Murray B, Tichnell C, James CA, Tandri H, Calkins H et al. Arrhythmogenic right ventricular cardiomyopathy presenting as clinical myocarditis in women. Am J Cardiol 2021;145:128–34. [DOI] [PubMed] [Google Scholar]
- 9. Gilotra NA, Bhonsale A, James CA, Te Riele ASJ, Murray B, Tichnell C et al. Heart failure is common and under-recognized in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circ Heart Fail 2017;10:e003819. [DOI] [PubMed] [Google Scholar]
- 10. Cadrin-Tourigny J, Bosman LP, Nozza A, Wang W, Tadros R, Bhonsale A et al. A new prediction model for ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy. Eur Heart J 2019;40:1850–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J et al. ; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Lint Freyja HM, Brittney M, Crystal T, Rob Z, Nuria A, Lekanne Deprez Ronald H et al. Arrhythmogenic right ventricular cardiomyopathy-associated desmosomal variants are rarely de novo. Circ Genomic Precis Med 2019;12:e002467. [DOI] [PubMed] [Google Scholar]
- 13. Weijia W, Gabriela O, Crystal T, Brittney M, Jane C, Oliver M et al. Impact of exercise restriction on arrhythmic risk among patients with arrhythmogenic right ventricular cardiomyopathy. J Am Heart Assoc 2018;7:e008843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 15. Kawel-Boehm N, Maceira A, Valsangiacomo-Buechel ER, Vogel-Claussen J, Turkbey EB, Williams R et al. Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson 2015;17:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 2010;121:1533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Corrado D, Marra MP, Zorzi A, Beffagna G, Cipriani A, Lazzari MD et al. Diagnosis of arrhythmogenic cardiomyopathy: the Padua criteria. Int J Cardiol 2020;319:106–14. [DOI] [PubMed] [Google Scholar]
- 18. Di Marco A, Anguera I, Schmitt M, Klem I, Neilan TG, White JA et al. Late gadolinium enhancement and the risk for ventricular arrhythmias or sudden death in dilated cardiomyopathy: systematic review and meta-analysis. JACC Heart Fail 2017;5:28–38. [DOI] [PubMed] [Google Scholar]
- 19. Protonotarios N, Tsatsopoulou A. Naxos disease and Carvajal syndrome: cardiocutaneous disorders that highlight the pathogenesis and broaden the spectrum of arrhythmogenic right ventricular cardiomyopathy. Cardiovasc Pathol 2004;13:185–94. [DOI] [PubMed] [Google Scholar]
- 20. Lopez-Ayala JM, Pastor-Quirante F, Gonzalez-Carrillo J, Lopez-Cuenca D, Sanchez-Munoz JJ, Oliva-Sandoval MJ et al. Genetics of myocarditis in arrhythmogenic right ventricular dysplasia. Heart Rhythm 2015;12:766–73. [DOI] [PubMed] [Google Scholar]
- 21.Jordan E, Peterson L, Ai T, Asatryan B, Bronicki L, Brown E et al. An evidence-based assessment of genes in dilated cardiomyopathy. Circulation. 2021;144(1):7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.