Abstract
Background
Diabetes, hypertension, and cardiovascular disease (CVD) are modifiable lifestyle-related cardiometabolic conditions associated with dementia. Yet, little is known regarding these associations among American Indian and Alaska Native (AI/AN) people. Thus, we examined the association of diabetes, hypertension, and CVD with all-cause dementia among AI/ANs aged 65 years and older.
Method
This was a cross-sectional analysis of the Indian Health Service Improving Health Care Delivery Data Project. Our study population was a 1:1 matched sample of 4 074 AI/ANs aged 65 years and older and Indian Health Service active users during fiscal year 2013. We employed International Classification of Diseases, Ninth Revision, Clinical Modification diagnostic codes for all-cause dementia, hypertension, and CVD. Diabetes was measured with a validated algorithm to identify adults with diabetes that uses diagnoses, laboratory test results, and medication criteria.
Results
Multivariable analyses revealed that diabetes and CVD were associated with increased odds of all-cause dementia and hypertension was not. Cardiovascular disease types associated with all-cause dementia differed with cerebrovascular disease having the strongest association. Analyses stratified by gender revealed that diabetes and CVD were associated with increased odds of all-cause dementia for women and only CVD was associated with all-cause dementia for men.
Conclusions
Training and support of primary care clinicians, addressing cultural considerations, and ensuring inclusion of AI/ANs in research are steps that could help meet AI/AN people’s needs. Our findings underscore to the importance of improved management and control of diabetes and CVD, which may lead to the prevention of dementia among older AI/ANs.
Keywords: Cardiovascular disease, Diabetes, Hypertension
The population of older American Indian and Alaska Native (AI/AN) people is increasing rapidly. Between 2012 and 2050, the number of AI/ANs aged 65 years and older will increase nearly 4-fold, and almost 8-fold among those aged 85 years and older (1). Given that the risk of developing dementia increases quickly with age (2), this condition will become a growing challenge for older AI/ANs, their families, and local systems of care. However, to date, few studies have examined all-cause dementia in this population. Among the 2014 Medicare Fee-for-Service beneficiaries aged 65 years and older, 10.5% of AI/ANs met criteria for all-cause dementia compared to 11.3% of Whites (3); but these rates were not age-adjusted to account for the shorter life expectancy at birth of Native people compared to Whites (71 vs 78 years, respectively) (4). Meanwhile, 2 other studies that examined Kaiser Permanente Northern California members found that AI/ANs suffer from substantial dementia disparities. These studies’ results showed that AI/ANs had the second highest dementia incidence (5) and the second shortest median survival among all race and ethnic groups aged 60 years and older (6).
There is no known cure for Alzheimer’s disease and related dementias (ADRDs). However, diabetes, hypertension, and cardiovascular disease (CVD) are modifiable lifestyle-related cardiometabolic conditions associated with ADRD. Mounting evidence led the Alzheimer’s Association to conclude that effective management of these cardiometabolic conditions along with their risk factors can reduce cognitive decline and may even reduce the likelihood of dementia (7). Diabetes, hypertension, and CVD disproportionately affect AI/ANs. American Indian and Alaska Native people have the highest rates of diabetes in the United States (8). Age-adjusted prevalence of hypertension characterizes 30.0% of AI/ANs compared to 23.6% of Whites (9) and AI/ANs are plagued by higher CVD incidence and CVD-related mortality than any other race and ethnic group (10).
Despite the emerging research on ADRD in other populations, little is known about the association between diabetes, hypertension, and CVD with all-cause dementia among AI/ANs, and how these relationships may vary by gender. Considering the projected increase in the number of older AI/ANs and their disproportionate burden of diabetes, hypertension, and CVD, we need to better understand the relationship between these cardiometabolic conditions and all-cause dementia in this population. Accordingly, our study examined the association of diabetes, hypertension, and CVD with all-cause dementia among AI/ANs with dementia matched with controls who used the Indian Health Service (IHS) and tribal health services.
Method
Indian Health Service Data Project
About a third of AI/ANs obtain health care through services funded by the IHS, which includes hospitals, clinics, and health programs operated by the federal government, tribal organizations, and urban Indian health programs. Known collectively as I/T/Us, they serve approximately 2.3 million AI/ANs (11). We used data from the IHS Improving Health Care Delivery Data Project (IHS Data Project) data infrastructure that houses health status, service use, and treatment cost data for over 640 000 AI/ANs, representing nearly 30% of AI/ANs who use IHS services (12).
The IHS Data Project includes data for a purposeful sample of AI/ANs who lived in 15 IHS Service Units (henceforth referred to as project sites), which are IHS geographic classifications located throughout the United States. Project site data are representative of AI/ANs by age and gender who lived in the site and the data for the 15 project sites combined are comparable to the national IHS service population in terms of age and gender. One project site is in the East, 4 in the Northern Plains, 2 in the Southern Plains, 5 in the Southwest, 2 in the Pacific Coast, and 1 in Alaska. Two-thirds of our study population resided in counties classified as metropolitan. In our project sites, the vast majority of health service use was at IHS and tribal health facilities so we refer to the providers as I/T instead of I/T/U providers. This data infrastructure is a synthesis of existing electronic health record data from multiple IHS platforms for 7 years (FY2007–2013), including registration, demographic, and I/T service use data from the National Data Warehouse, and non-I/T provided but I/T paid service use data from Purchased and Referred Care services. Greater detail on this data infrastructure is reported elsewhere (12).
Project personnel have partnered with IHS and the tribal organizations that participate in the IHS Data Project. This collaboration takes place through the project’s Collaborative Network and a process to obtain approvals from the IHS institutional review board, tribal institutional review boards, tribal councils, and/or tribal authorities in addition to the university’s institutional review board.
Study Population
The study population included a 1:1 matched sample of 4 074 AI/ANs (2 037 persons with dementia and 2 037 controls) who lived in one of the project sites and were IHS active users between October 1, 2012 and September 30, 2013 (FY2013). An active user is someone who used services at least once during the specified fiscal year or the preceding 2 fiscal years. The sample was created by matching FY2013 active users aged 65 years and older who had dementia to similarly aged FY2013 active users without dementia based on birth year (±1 year), gender, and project site. The 2 037 dementia patients were identified as those who had at least one qualifying International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic code for all-cause dementia during FY2007–2013.
Measures
FY2013 data on age, gender, project site, health coverage, and health status were extracted from the IHS Data Project data infrastructure. Health coverage measures included Medicare, Medicaid, private insurance, and no coverage other than access to I/T services. We employed ICD-9-CM diagnostic codes, recorded in the National Data Warehouse and Purchased and Referred Care inpatient and outpatient service use records, supplemented by blood sugar values and medication data, to identify diabetes, hypertension, and CVD. The qualifying ICD-9-CM codes used to identify dementia included those for Alzheimer’s disease and vascular, Lewy body, frontotemporal, alcohol-induced, and other types of dementia that were used in a recent Medicare study (13). We used a validated algorithm to identify adults with diabetes that uses diagnoses, laboratory test results, and medication criteria (14). Sightlines DxCG Risk Solutions software groups ICD-9-CM codes into Diagnostic Cost Groups, which are employed by the federal government and private insurers, to identify conditions patients have. We used this software to identify adults with hypertension and one or more types of CVD (15). The types of CVD included ischemic heart disease (coronary artery disease such as acute myocardial infarction and coronary atherosclerosis), congestive heart failure or other related heart conditions (heart valve and pericardial conditions and cardiac arrhythmias), cerebrovascular disease (stroke and poststroke paralysis), and vascular disease (peripheral atherosclerosis and thrombosis/phlebitis). We also created 4 additional CVD measures, 1 for each type of CVD.
Statistical Analyses
The prevalence of diabetes, hypertension, and CVD among adults with and without dementia, overall and within each age group, was compared using McNemar’s tests for paired data. Pearson’s chi-squared tests compared the prevalence of these conditions by gender as independent samples. To assess the association between cardiometabolic conditions with dementia, we used multiple variable conditional logistic regressions for matched data. We ran 2 alternative final regression models with diabetes, hypertension, and CVD included in both models: one model included CVD as an overall binary measure and the second model included the 4 CVD types. We also included health coverage information in both models to control for financial access to services, other than I/T services. Although using a matched case control design could mitigate/eliminate the strong confounding effects of age for the association of interest, this design reduced the available sample size substantially. To evaluate the potential impact of the study design on the findings, we conducted a sensitivity analysis using data from all FY2013 active users aged 65 years and older, with age included as a covariate in the multivariable logistic regressions. The relationships of diabetes, hypertension, and CVD with dementia in this analysis were very similar to those based on matched data and thus we only present the results based on matched data. We used SAS software version 9.4 for variable construction and statistical analyses (16).
Results
We identified 2 088 AI/ANs aged 65 years and older with all-cause dementia. We matched 2 037 (97.6%) of these adults with adults without all-cause dementia. Data for the 51 unmatched adults with dementia, for whom no adult of similar age and gender without dementia could be found at their site, were excluded from the study. Table 1 presents the distribution of the characteristics on which the 2 samples were matched, as well as the distribution of their health coverage, diabetes, hypertension, and CVD. Approximately 61% of AI/ANs with dementia were women and the majority resided in the Southwest or Southern Plains. Close to one-quarter of persons with dementia were aged 65–74 years, 44.3% were aged 75–84 years, and 32.7% were aged 85 years and older.
Table 1.
Study Population Characteristics by Dementia Status
| With Dementia (n = 2 037) | Without Dementia (n = 2 037) | |||
|---|---|---|---|---|
| n | % | n | % | |
| Gender | ||||
| Male | 799 | 39.2 | 799 | 39.2 |
| Female | 1 238 | 60.8 | 1 238 | 60.8 |
| Age (y) | ||||
| 65–69 | 188 | 9.2 | 190 | 9.3 |
| 70–74 | 283 | 13.9 | 287 | 14.1 |
| 75–79 | 409 | 20.1 | 411 | 20.2 |
| 80–84 | 492 | 24.2 | 498 | 24.5 |
| 85–89 | 387 | 19.0 | 381 | 18.7 |
| ≥90 | 278 | 13.7 | 270 | 13.3 |
| Region | ||||
| East | 95 | 4.7 | 95 | 4.7 |
| Southern Plains | 593 | 29.1 | 593 | 29.1 |
| Southwest | 690 | 33.9 | 690 | 33.9 |
| Pacific Coast | 42 | 2.1 | 42 | 2.1 |
| Alaska | 437 | 21.5 | 437 | 21.5 |
| Northern Plains | 180 | 8.8 | 180 | 8.8 |
| Health coverage | ||||
| Medicaid | 379 | 18.6 | 207 | 10.2*** |
| Medicare | 1 957 | 96.1 | 1 926 | 94.6* |
| Private | 102 | 5.0 | 145 | 7.1** |
| None | 51 | 2.5 | 66 | 3.2 |
| Health conditions | ||||
| Diabetes | 948 | 46.5 | 819 | 40.2*** |
| Hypertension | 1 442 | 70.8 | 1 334 | 65.5*** |
| CVD | 1 217 | 59.7 | 920 | 45.2*** |
| CVD types | ||||
| Ischemic heart disease | 537 | 26.4 | 395 | 19.4*** |
| Congestive heart failure | 836 | 41.0 | 633 | 31.1*** |
| Vascular disease | 461 | 22.6 | 313 | 15.4*** |
| Cerebrovascular disease | 357 | 17.5 | 158 | 7.8*** |
Notes: CVD = cardiovascular disease.
*p < .05. **p < .01. ***p < .001.
While close to 95% of adults, regardless of dementia status, had Medicare coverage, a larger percentage of adults with dementia had Medicaid coverage (18.6%) than adults without dementia (10.2%, p < .001). Five percent of adults with dementia and 7.1% of adults without dementia had private insurance coverage (p < .01). Only a small percentage of adults (~3%) with or without dementia had no health coverage other than IHS access.
Adults with dementia had a higher prevalence of diabetes, hypertension, and CVD than those without dementia. The prevalence of diabetes was 46.5% among adults with dementia and 40.2% among adults without dementia (p < .001). The prevalence of hypertension was 70.8% among adults with dementia and 65.5% among adults without dementia (p < .001). For CVD, the prevalence was 59.7% among adults with dementia and 45.2% among adults without dementia (p < .001).
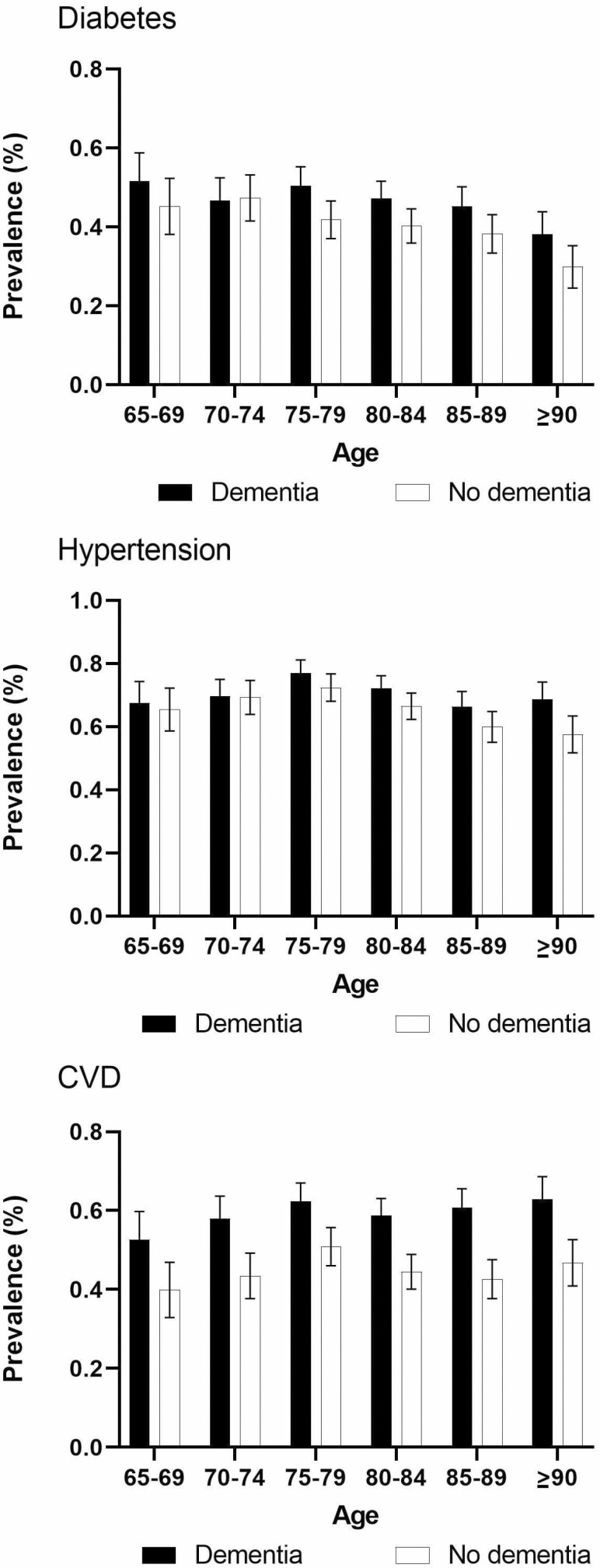
The prevalence of diabetes among adults with dementia was higher than the prevalence among those without dementia in adults aged 75 years and older (Figure 1). The prevalence of CVD among those with dementia was significantly higher than that among those without dementia in every age group. The differences in the prevalence of hypertension between the 2 populations were only significant among those aged 90 years and older.
Figure 1.
Prevalence of diabetes, hypertension, and cardiovascular disease by age and dementia status.
As shown in Table 2, there were no significant differences by gender among persons with dementia as well as persons without dementia with respect to diabetes or hypertension prevalence. Yet, among persons with dementia, men had a higher prevalence of CVD than women (65.1% vs. 56.3%, respectively, p < .001). Among the 4 types of CVD, men with dementia had a significantly higher prevalence of ischemic heart disease, congestive heart failure, and vascular disease than women. Among adults without dementia, while men also had significantly higher prevalence of CVD than women, only the prevalence of ischemic heart disease was significantly higher among men among the 4 CVD types.
Table 2.
Differences in Prevalence of Diabetes, Hypertension, and CVD by Dementia Status and Gender
| With Dementia | Without Dementia | |||||||
|---|---|---|---|---|---|---|---|---|
| Females | Males | Females | Males | |||||
| n | % | n | % | n | % | n | % | |
| Diabetes | 569 | 46.0 | 379 | 47.4 | 483 | 39.0 | 336 | 42.1 |
| Hypertension | 889 | 71.8 | 553 | 69.2 | 815 | 65.8 | 519 | 65.0 |
| CVD | 697 | 56.3 | 520 | 65.1*** | 526 | 42.5 | 394 | 49.3** |
| CVD with diabetes | 373 | 65.6 | 279 | 73.6** | 253 | 52.4 | 219 | 65.2** |
| CVD without diabetes | 324 | 48.4 | 241 | 57.4** | 273 | 36.2 | 175 | 37.8 |
| CVD type | ||||||||
| Ischemic heart disease | 279 | 22.5 | 258 | 32.3*** | 183 | 14.8 | 212 | 26.5*** |
| Congestive heart failure | 480 | 38.8 | 356 | 44.6** | 371 | 30.0 | 262 | 32.8 |
| Vascular disease | 256 | 20.7 | 205 | 25.7** | 182 | 14.7 | 131 | 16.4 |
| Cerebrovascular disease | 204 | 16.5 | 153 | 19.2 | 93 | 7.5 | 65 | 8.1 |
Notes: CVD = cardiovascular disease.
**p < .01. ***p < .001.
Table 3 presents results for 2 multiple variable conditional logistic regressions that examined the associations between the cardiometabolic conditions and all-cause dementia. In Model 1 that adjusted for health coverage and the other cardiometabolic conditions with the overall binary CVD measure, the odds of dementia were 17% (odds ratio [OR] = 1.17, 95% CI: 1.02, 1.35) higher among those with diabetes than that among those without diabetes. Cardiovascular disease was also associated with higher odds of dementia (OR = 1.79; 95% CI: 1.55, 2.07). After controlling for CVD and diabetes, the association between hypertension and dementia was insignificant. The relationships between these 3 conditions and dementia were similar among women. However, among men, only CVD was significantly associated with higher odds of dementia.
Table 3.
Conditional Logistic Regression Models’ Results for the Association Between Diabetes, Hypertension, and CVD With Dementia
| Model 1a | Model 2b | |||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| All adults | ||||
| Diabetes vs no diabetes | 1.17 | 1.02, 1.35* | 1.16 | 1.00, 1.33* |
| Hypertension vs no hypertension | 0.96 | 0.83, 1.12 | 0.98 | 0.84, 1.14 |
| CVD | 1.79 | 1.55, 2.07*** | — | — |
| CVD type | ||||
| Ischemic heart disease | — | — | 1.20 | 1.01, 1.43* |
| Congestive heart failure | — | — | 1.25 | 1.08, 1.46** |
| Vascular disease | — | — | 1.28 | 1.07, 1.53** |
| Cerebrovascular disease | — | — | 2.21 | 1.78, 2.74*** |
| Females | ||||
| Diabetes vs no diabetes | 1.20 | 1.00, 1.44* | 1.19 | 0.99, 1.42 |
| Hypertension vs no hypertension | 1.05 | 0.87, 1.27 | 1.05 | 0.87, 1.28 |
| CVD | 1.71 | 1.43, 2.05*** | — | — |
| CVD type | ||||
| Ischemic heart disease | — | — | 1.42 | 1.12, 1.79** |
| Congestive heart failure | — | — | 1.18 | 0.97, 1.44 |
| Vascular disease | — | — | 1.18 | 0.93, 1.49 |
| Cerebrovascular disease | — | — | 1.99 | 1.51, 2.61*** |
| Males | ||||
| Diabetes vs no diabetes | 1.13 | 0.90, 1.41 | 1.12 | 0.89, 1.41 |
| Hypertension vs no hypertension | 0.82 | 0.64, 1.07 | 0.88 | 0.68, 1.13 |
| CVD | 1.94 | 1.52, 2.48*** | — | — |
| CVD type | ||||
| Ischemic heart disease | — | — | 0.96 | 0.73, 1.26 |
| Congestive heart failure | — | — | 1.37 | 1.07, 1.75* |
| Vascular disease | — | — | 1.42 | 1.07, 1.89* |
| Cerebrovascular disease | — | — | 2.74 | 1.91, 3.93*** |
Notes: CI = confidence interval; CVD = cardiovascular disease; OR = odds ratio.
aAdjusted for health care coverage, diabetes, hypertension, and CVD. bAdjusted for health care coverage, diabetes, hypertension, and the 4 CVD types.
*p < .05. **p < .01. ***p < .001.
Model 2 differed in that it included the 4 different types of CVD rather than the overall binary CVD measure. The odds of dementia among adults with ischemic heart disease were 20% higher than that among adults without this condition (OR = 1.20, 95% CI: 1.01, 1.43). Adults with congestive heart failure had 25% higher odds of dementia (OR = 1.25, 95% CI: 1.08, 1.46) than those without this condition and the association between dementia and vascular disease was similar (OR = 1.28; 95% CI: 1.07, 1.53). The OR of dementia for adults with versus without cerebrovascular disease was 2.21 (95% CI: 1.78, 2.74).
The relationship between types of CVD and dementia differed by gender. Among women, the odds of dementia were significantly higher for those with ischemic heart disease and with cerebrovascular disease. Neither congestive heart failure nor vascular disease was associated with dementia among women. The association between ischemic heart disease and dementia among men was not statistically significant. Yet, the odds of dementia were significantly higher among men with congestive heart failure, vascular disease, and cerebrovascular disease.
Discussion
In this large sample of AI/ANs, diabetes and CVD overall were associated with greater odds of having dementia compared to people without these cardiometabolic conditions. Also, all 4 CVD types were associated with increased odds of dementia with cerebrovascular disease having the strongest association. We did not find an association between hypertension and dementia. Research has demonstrated that persons with diabetes have an increased risk for dementia and the evidence has continued to mount (17–19). A meta-analysis of longitudinal studies reported a 51% increased risk of any dementia among patients with diabetes (17); while another population-based study reported that newly diagnosed diabetes was associated with 16% higher risk of dementia (18). An examination of Kaiser Permanente Northern California plan members with type 2 diabetes found that dementia risk was highest among AI/ANs and African Americans compared to Whites, Latinos, and Asians with Asians having the lowest risk (19). Another study that examined AI/ANs who used the Banner Health system found that a diabetes diagnosis was significantly associated with an increased risk of having an ADRD diagnosis (20). Here, we found diabetes was associated with a 16% higher odds of all-cause dementia in our sample.
The exact mechanism by which diabetes increases dementia risk is unknown but factors that increase one’s risk include the length in which one has had diabetes, having complications associated with diabetes and insulin use (21). These factors could serve as a means for clinics and tribal communities to focus on brain health by integrating both prevention and effective control of diabetes to prevent dementia through community education, diabetes educator-focused training, and public health approaches.
Although no published studies have examined the association of CVD with dementia among AI/ANs, research with other racial/ethnic groups has revealed that a history of stroke was associated with approximately 2 times the risk of incidence dementia (22). American Indian and Alaska Native people have the highest prevalence of stroke of any racial/ethnic group (23) and thus might be at especially high risk of developing dementia due to cerebrovascular disease. Two recent reviews on the association of CVD types with ADRD have found that a history of coronary heart disease, heart failure, atrial fibrillation, and severe atherosclerosis are all associated with an increased risk of ADRD (24,25). Both reviews only found and included a few eligible studies and called for additional high-quality studies to confirm and further delineate the relationships between different CVD subtypes and dementia risk. Research with other racial and ethnic groups suggests that effective CVD management can reduce the risk of cognitive decline and may reduce dementia risk (7). However, additional work is warranted to improve our understanding of the types of CVD that are more likely to negatively impact cognitive health, to identify ways to moderate this association, and to further clarify racial/ethnic as well as gender differences.
We did not find a significant association between hypertension and dementia while controlling for diabetes and CVD. Our findings are contrary to another study of AI/ANs, which found a significant association between hypertension and ADRD while adjusting for diabetes and hyperlipidemia (20). Overall, prior research presents a mixed picture with respect to the association of hypertension and dementia, which varies by patient age and time of hypertension onset. Hypertension has been associated with poorer cognitive functioning among those aged 65–74 years; yet among individuals aged 75 years and older, hypertension appears to be associated with better cognitive functioning (26). Moreover, compared to persons without hypertension, those with hypertension onset between ages 80 and 89 years had lower dementia risk and those with hypertension onset at 90 years and older had the lowest dementia risk (27). In contrast, other research has found that both low and high blood pressure were associated with poorer cognitive performance among persons aged 70 years and older (28). The cross-sectional nature of our data precluded considering the role of age at hypertension onset although this question needs to be examined in future analyses of the longitudinal data. Also, it is possible, however, that our study population has overall good hypertension management.
Regarding the association between diabetes and dementia, we found that the association was present for women but not for men. Cardiovascular disease overall and cerebrovascular disease were associated with dementia for both males and females. However, ischemic heart disease was positively associated with dementia only among females, while congestive heart failure and vascular disease were positively associated with dementia only among males. Meanwhile, hypertension was not associated with dementia for either males or females. These findings may be due in part to gender differences in age of CVD onset, type, and mortality. Additional attention to such differences could improve our understanding of modifiable risk factors and treatment needs. A recent review concluded that gender differences in risk factors for dementia have occasioned only modest interest, which led to recommendations for future research priorities (29). Two priorities include considering the degree to which gender differences in dementia may be related to differences in associated risk factors and comorbidities among racial/ethnic subgroups.
Given the well-known disparities experienced by AI/ANs for diabetes, hypertension, and CVD, our findings highlight the importance of preventing or improving the management of these cardiometabolic conditions in this population for dementia-related sequalae. Theoretically, more than a third of all-cause dementia cases may be preventable (30). While obesity is a major risk factor for diabetes and CVD, geriatric obesity can be complex. For instance, a meta-analysis found that mid-life obesity is associated with increased ADRD risk, while obesity in persons aged 65 years and older was associated with a reduced ADRD risk (31). Thus, lifestyle interventions geared toward young and mid-life adults that have proven effectiveness at reducing the risk of diabetes should be emphasized to prevent dementia later in life (30). Currently, the American Diabetes Association recommends, as part of lifestyle management, that older adults engage in dietary changes, participate in physical activity for those that have the capacity to do so safely, and modest weight loss of 5%–7% (32).
Our study has several limitations. First, the data informing these analyses were cross-sectional preventing any assessment of causality. Future efforts will employ longitudinal data, extracted, assembled, and linked at the individual level from this data set, to assess the associations suggested here. Second, as with other electronic health record-based studies, assuming dementia via diagnostic codes likely underestimates prevalence. This is particularly true for a service delivery system with resource constraints that serves AI/ANs who reside in rural areas with limited access to specialists (33). Third, while these data allowed us to examine a large number of AI/ANs, we did not have the detail that would have been available from medical record reviews or other types of health assessments. In particular, education and income were unavailable and therefore could not be adjusted in our regression models. Yet, given the major potential confounders were already included in our models, adjusting additional covariates likely would not substantially change our parameter estimates.
Other study limitations pertain to the nature of the IHS Data Project. We did not have data for services from non-I/T providers not paid for by the Purchased and Referred Care program such as some specialty services. Moreover, access to other services varied across project sites. Sites also varied by the types of services provided, Purchased and Referred Care service use, and data completeness. Sites that provided more specialty outpatient and inpatient services and Purchased and Referred Care funding may have had data that are more complete on diagnosed conditions. Second, data for AI/AN residents in long-term care facilities are not included in the IHS Data Project except for very rare exceptions. Hence, we likely undercounted the treated prevalence of dementia, and underestimated its association with other chronic conditions such as diabetes and CVD. Data regarding AI/ANs with dementia in nursing homes are limited. Yet, an examination of the FY2003 Minimum Data Set found that 0.7% of nursing home admissions were AI/ANs and AI/ANs had the highest percentage of nursing home residents who were cognitively intact (34). However, it is suggested that subsequent efforts will want to expand study samples to include AI/ANs residing in long-term care facilities as well as independently to further examine the associations of cardiometabolic conditions and ADRD. Lastly, although our results are generalizable to adults who lived in the 15 project sites, representing a large proportion of AI/ANs eligible for I/T health services, our findings may not reflect the health status of AI/ANs who live elsewhere or who do not obtain health services from I/T providers. This also includes the limited generalizability of our results to users of urban Indian health clinics.
Despite these limitations, our study has significant strengths. First, the IHS Data Project includes a large number of older AI/ANs from geographically diverse sites across the United States, representing approximately 30% of all the IHS service users. Second, this is one of the first studies that used the electronic health record data to characterize the association between potential risk factors and diagnosed dementia among AI/ANs, who are at high risk of diabetes and CVD. The availability of multiple diagnosed health conditions is a strong advantage of these data as well as different subtypes of CVD can be identified relatively accurately.
Our study adds to the small, but growing body of dementia research with AI/ANs. American Indian and Alaska Native people are living longer than ever before (4) and older AI/ANs are rapidly increasing in numbers (1). Our study focused on older AI/ANs who obtained services from I/T providers who may have insufficient resources for diagnosing and treating patients with dementia. The per capita spending of IHS was $3 851 in fiscal year 2017 (11), which is markedly lower than the U.S. general population per capita spending ($10 739) during 2017 (35). Another challenge is the limited availability and difficulty accessing long-term care in tribal communities (36). The Amended Indian Health Care Improvement Plan Act of 2010 addressed long-term care needs of older AI/ANs. While many AI/AN communities are working to address those needs, the unmet need is substantial (37). For example, 16.3% of AI/AN Medicaid aged enrollees who accessed IHS services used institutional and community-based long-term care services in 2012; this compares to 41.6% of non-Hispanic White Medicaid aged enrollees and this disparity increased with age (38). Indian Health Service resources are further compromised by provider shortages and by community-level factors such as low household incomes and rural landscapes that compromise service use and health (33).
Although there are notable barriers to addressing dementia among older AI/ANs, there are some promising developments. For instance, FY2021 federal appropriations to IHS for the first time included $5M specifically for dementia care. The appropriations recommend that $1M of this allocation goes toward awareness campaigns, $2M to develop a quarterly curriculum for primary care providers, and $2M for pilot programs to increase early detection and supportive caregiver services (39). In addition, a number of tribal communities are engaged in innovative approaches to meet dementia-related needs of their members (40). For example, the Pyramid Lake Paiute Tribe used grant funding to implement the Dementia Friendly America process. The objectives of their project are to facilitate educational opportunities as well as peer support among persons living with dementia and their caregivers, increase community awareness on the early signs of dementia, educate the community about how to support loved ones with dementia, and provide respite services for caregivers. Several tribes in Wisconsin have implemented state-funded dementia specialist programs. The specialists provide general education about dementia in their respective tribes, perform member assessments, link caregivers with information and services, and lead gatherings that provide persons with dementia socialization opportunities.
We have several specific suggestions for providers. First, providers should adhere to diagnosis guidelines. For instance, the American Diabetes Association recommends that all overweight adults with risk factors and all adults aged 45 years and older should be screened for prediabetes and diabetes every 1–3 years (41). Second, providers should use the risk of developing cognitive impairment to motivate patients to engage in treatment plans regarding their cardiometabolic management. Third, providers should look for cognitive impairment with respect to any signs of changes in memory or thinking. In 2019, the American Academy of Neurology recommended annual cognitive screening guidelines for older adults (42). Although in 2020, the U.S. Preventive Services Task Force found insufficient evidence to screen for cognitive disorders, up to 67% of patients with dementia have not received a diagnosis (43,44).
In addition to these actionable steps for providers, there are several other outstanding needs that would aid in our response to related care needs of AI/ANs, including ADRD training and support of primary care clinicians and other health care workers, addressing cultural considerations, and ensuring inclusion of AI/ANs in future research. As the AI/AN population ages, the likelihood they will experience ADRD increases and their health care needs will become more complex. Our findings underscore the critical importance of improved management and control of diabetes and CVD, which may lead to the prevention of dementia among older AI/ANs.
Funding
This work was supported by the Indian Health Service and the National Institute on Health’s National Institute of General Medical Sciences through the Native American Research Centers for Health (U261IHS0078 to R.T.G.), the National Institutes of Health’s National Institute on Aging (R01AG061189 to L. J. and J.O.; P30AG15292 to S.M.M.), and the National Institutes of Health’s National Institute of Diabetes and Digestive and Kidney Diseases (R18DK114757 to J.O.; P30DK092923 to S.M.M.). Funding for the development of the data infrastructure was supported by the Patient-Centered Outcomes Research Institute (AD-1304-6451 to J.O.) and Agency for Healthcare Research and Quality (290-2006-00020-I to J.O.). The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of these organizations.
Conflict of Interest
None declared.
Acknowledgments
The data used in this secondary analysis stem from a project, known as the Indian Health Service (IHS) Health Care Delivery Data Project, which includes information for many American Indian and Alaska Native communities. This work was conducted with the guidance and advice of IHS and tribal health program colleagues, as well as members of the project’s Steering, Project Site, and patient committees. Members of tribal and IHS institutional review boards, tribal Councils, and tribal Authorities educate us about the health concerns they have for their tribal members and how they hope this project will inform their work. This project relies on their support and approval.
Author Contributions
R.T.G. wrote the manuscript and secured funding; B.W. contributed to the discussion and reviewed/edited the manuscript; L.J. contributed to the manuscript and provided statistical supervision; L.G. and M.R. analyzed the data; M.M.C. reviewed the manuscript and results; S.M.M. reviewed/edited the manuscript; and J.O. wrote the methods, reviewed/edited the manuscript, and secured funding.
References
- 1. Ortman JM, Velkoff VA, Hogan H.. An Aging Nation: The Older Population in the United States. Report Number P25-1140. U.S. Department of Commerce, Economics and Statistics Administration, U.S. Census Bureau; May 2014. [Google Scholar]
- 2. Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0b013e31828726f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matthews KA, Xu W, Gaglioti AH, et al. . Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged ≥65 years. Alzheimers Dement. 2019;15:17–24. doi: 10.1016/j.jalz.2018.06.3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arias E, Xu J, Jim MA. Period life tables for the non-Hispanic American Indian and Alaska Native population, 2007–2009. Am J Public Health. 2014;14:S312–S319. doi: 10.2105/AJPH.2013.301635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12:216–224. doi: 10.1016/j.jalz.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mayeda ER, Glymour MM, Quesenberry CP, Johnson JK, Pérez-Stable EJ, Whitmer RA. Survival after dementia diagnosis in five racial/ethnic groups. Alzheimers Dement. 2017;13:761–769. doi: 10.1016/j.jalz.2016.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement. 2015;11:718–726. doi: 10.1016/j.jalz.2015.05.016 [DOI] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention. National Diabetes Statistics Report 2020: Estimates of Diabetes and Its Burden in the United States. Atlanta, GA: U.S. Department of Health and Human Services; 2017. [Google Scholar]
- 9. Schiller JS, Lucas JW, Ward BW, Peregoy JA. Summary health statistics for U.S. adults: National Health Interview Survey, 2010. Vital Health Stat. 2012;10:1–207. [PubMed] [Google Scholar]
- 10. Veazie M, Ayala C, Schieb L, Dai S, Henderson JA, Cho P. Trends and disparities in heart disease mortality among American Indians/Alaska Natives, 1990–2009. Am J Public Health. 2014;104:S359–S367. doi: 10.2105/AJPH.2013.301715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. U.S. Department of Health and Human Services, Indian Health Service. IHS Year 2016 Profile Based on 2000–2016 Data—Numbers Are Approximate. U.S. Department of Health and Human Services; 2016. https://www.ihs.gov/newsroom/factsheets/ihsyear2016profile/. Accessed October 20, 2016. [Google Scholar]
- 12. O’Connell J, Guh S, Ouellet J, et al. . ARRA ACTION: Comparative Effectiveness of Health Care Delivery Systems for American Indians and Alaska Natives Using Enhanced Data Infrastructure: Final Report. Rockville, MD: Agency for Healthcare Research and Quality; 2014. [Google Scholar]
- 13. Goodman RA, Lochner KA, Thambisetty M, Wingo TS, Posner SF, Ling SM. Prevalence of dementia subtypes in United States Medicare fee-for-service beneficiaries, 2011–2013. Alzheimers Dement. 2017;13:28–37. doi: 10.1016/j.jalz.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Raebel MA, Schroeder EB, Goodrich GK, et al. . Validating type 1 and type 2 diabetes mellitus in the Mini-Sentinel Distributed Database using the SUrveillance PREvention, and ManagEment of Diabetes Mellitus (SUPREME-DM) DataLink. 2016. https://www.sentinelinitiative.org/sentinel/methods/validating-type-1-and-type-2-diabetes-mellitus-mini-sentinel-distributed-database. Accessed January 14, 2019.
- 15.Verisk Health, Inc. Sightlines DxCG Risk Solutions. Version 4.0.1. 2011. https://www.verisk.com
- 16. SAS Software [Computer Program]. Version 9.4. Cary, NC: SAS Institute, Inc; 2013. [Google Scholar]
- 17. Gudala K, Bansal D, Schifano F, Bhansali A. Diabetes mellitus and risk of dementia: a meta-analysis of prospective observational studies. J Diabetes Investig. 2013;4:640–650. doi: 10.1111/jdi.12087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Haroon NN, Austin PC, Shah BR, Wu J, Gill SS, Booth GL. Risk of dementia in seniors with newly diagnosed diabetes: a population-based study. Diabetes Care. 2015;38:1868–1875. doi: 10.2337/dc15-0491 [DOI] [PubMed] [Google Scholar]
- 19. Mayeda ER, Karter AJ, Huang ES, Moffet HH, Haan MN, Whitmer RA. Racial/ethnic differences in dementia risk among older type 2 diabetic patients: the diabetes and aging study. Diabetes Care. 2014;37:1009–1015. doi: 10.2337/dc13-0215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carty CL, Noonan C, Muller C, et al. . Risk factors for Alzheimer’s disease and related dementia diagnoses in American Indians. Ethn Dis. 2020;30:671–680. doi: 10.18865/ed.30.4.671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cholerton B, Baker LD, Montine TJ, Craft S. Type 2 diabetes, cognition, and dementia in older adults: toward a precision health approach. Diabetes Spectr. 2016;29:210–219. doi: 10.2337/ds16-0041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Savva GM, Stephan BC; Alzheimer’s Society Vascular Dementia Systematic Review Group . Epidemiological studies of the effect of stroke on incident dementia: a systematic review. Stroke. 2010;41:e41–e46. doi: 10.1161/STROKEAHA.109.559880 [DOI] [PubMed] [Google Scholar]
- 23. Fang J, Shaw KM, George MG. Prevalence of stroke—United States, 2006–2010. MMWR Morb Mortal Wkly Rep. 2012;61:379–382. [PubMed] [Google Scholar]
- 24. Stefanidis KB, Askew CD, Greaves K, Summers MJ. The effect of non-stroke cardiovascular disease states on risk for cognitive decline and dementia: a systematic and meta-analytic review. Neuropsychol Rev. 2018;28:1–15. doi: 10.1007/s11065-017-9359-z [DOI] [PubMed] [Google Scholar]
- 25. Wolters FJ, Segufa RA, Darweesh SKL, et al. . Coronary heart disease, heart failure, and the risk of dementia: a systematic review and meta-analysis. Alzheimers Dement. 2018;14:1493–1504. doi: 10.1016/j.jalz.2018.01.007 [DOI] [PubMed] [Google Scholar]
- 26. Euser SM, van Bemmel T, Schram MT, et al. . The effect of age on the association between blood pressure and cognitive function later in life. J Am Geriatr Soc. 2009;57:1232–1237. doi: 10.1111/j.1532-5415.2009.02264.x [DOI] [PubMed] [Google Scholar]
- 27. Corrada MM, Hayden KM, Paganini-Hill A, et al. . Age of onset of hypertension and risk of dementia in the oldest-old: the 90+ Study. Alzheimers Dement. 2017;13:103–110. doi: 10.1016/j.jalz.2016.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thorvaldsson V, Skoog I, Hofer SM, et al. . Nonlinear blood pressure effects on cognition in old age: separating between-person and within-person associations. Psychol Aging. 2012;27:375–383. doi: 10.1037/a0025631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nebel RA, Aggarwal NT, Barnes LL, et al. . Understanding the impact of sex and gender in Alzheimer’s disease: a call to action. Alzheimers Dement. 2018;14:1171–1183. doi: 10.1016/j.jalz.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Livingston G, Sommerlad A, Orgeta V, et al. . Dementia prevention, intervention, and care. Lancet. 2017;390:2673–2734. doi: 10.1016/S0140-6736(17)31363-6 [DOI] [PubMed] [Google Scholar]
- 31. Qu Y, Hu HY, Ou YN, et al. . Association of body mass index with risk of cognitive impairment and dementia: a systematic review and meta-analysis of prospective studies. Neurosci Biobehav Rev. 2020;115:189–198. doi: 10.1016/j.neubiorev.2020.05.012 [DOI] [PubMed] [Google Scholar]
- 32. American Diabetes Association. 12. Older adults: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(suppl. 1):S168–S179. doi: 10.2337/dc21-s012 [DOI] [PubMed] [Google Scholar]
- 33. U.S. Government Accountability Office. Indian Health Service: Agency Faces Ongoing Challenges Filling Provider Vacancies: Report to Congressional Requesters. GAO-18-580. U.S. Government Accountability Office; August 2018. [Google Scholar]
- 34. Buchanan RJ, Rosenthal M, Graber DR, Wang S, Kim MS. Racial and ethnic comparisons of nursing home residents at admission. J Am Med Dir Assoc. 2008;9:568–579. doi: 10.1016/j.jamda.2008.04.012 [DOI] [PubMed] [Google Scholar]
- 35. Martin AB, Hartman M, Washington B, Catlin A. National health care spending in 2017: growth slows to post-Great-Recession rates; share of GDP stabilizes. Health Affairs. 2018;38:96–106. doi: 10.1377/hlthaff.2018.05085 [DOI] [PubMed] [Google Scholar]
- 36. Goins RT, Bogart A, Roubideaux Y. Service provider perceptions of long-term care access in American Indian and Alaska Native communities. J Health Care Poor Underserved. 2010;21:1340–1353. doi: 10.1353/hpu.2010.0934 [DOI] [PubMed] [Google Scholar]
- 37. Heisler EJ. The Indian Health Care Improvement Act Reauthorization and Extension as Enacted by the ACA: Detailed Summary and Timeline (R41630). Congressional Research Service; 2011. [Google Scholar]
- 38. O’Connell J, Rockell J, LeBeau M.. Overview of Medicaid and American Indians and Alaska Natives: 2012. Report from the Centers for Medicare and Medicaid Data Project; 2017. [Google Scholar]
- 39. Department of the Interior, Environment, and Related Agencies Appropriations Bill, 2021, 116th Congress. HR Rep No. 116-448. https://www.congress.gov/116/crpt/hrpt448/CRPT-116hrpt448.pdf. Accessed March 3, 2021.
- 40. Alzheimer’s Association and Centers for Disease Control and Prevention. Healthy Brain Initiative, Road Map for Indian Country. Chicago, IL: Alzheimer’s Association; 2019. [Google Scholar]
- 41. American Diabetes Association. 8. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(suppl. 1):S100–S110. doi: 10.2337/dc21-S008 [DOI] [PubMed] [Google Scholar]
- 42. Foster NL, Bondi MW, Das R, et al. . Quality improvement in neurology: mild cognitive impairment quality measurement set. Neurology. 2019;93:705–713. doi: 10.1212/WNL.0000000000008259 [DOI] [PubMed] [Google Scholar]
- 43. Valcour VG, Masaki KH, Curb JD, Blanchette PL. The detection of dementia in the primary care setting. Arch Intern Med. 2000;160:2964–2968. doi: 10.1001/archinte.160.19.2964 [DOI] [PubMed] [Google Scholar]
- 44. Tilly E, Thyrian JR, Hetel J, et al. . Rates of formal diagnosis of dementia in primary care: the effect of screening. Alzheimers Dement. 2015;42:451–458. doi: 10.1016/j.dadm.2014.11.007 [DOI] [Google Scholar]