Abstract
Background:
The correlation between visual emphysema patterns and subsequent progression of disease may provide a way to enrich a study population for treatment trials of emphysema.
Purpose:
To evaluate the potential relationship between emphysema visual subtypes and progression of emphysema and gas trapping.
Materials and Methods:
Current and former smokers with and without chronic obstructive pulmonary disease (COPD) enrolled in the prospective Genetic Epidemiology of COPD (COPDGene) study (ClinicalTrials.gov identifier: NCT02445183) between 2008 and 2011 had their Fleischner Society visual CT scores assessed at baseline, quantitative inspiratory, and expiratory CT and at 5 years. They also underwent pulmonary function testing at baseline CT and at 5 years. The dependent variables were inspiratory lung density at 15th percentile (adjusted for lung volume) as a measure of emphysema and percentage of lung volume with attenuation less than −856 HU at expiratory CT as a measure of air trapping. Statistical analysis used a linear mixed model, adjusted for age, height, sex, race, smoking status, and scanner make.
Results:
A total of 4166 participants (mean age, 60 years ± 9 [standard deviation]; 2091 [50%] men) were evaluated. In participants with COPD (1655 participants, 40%), those with visual presence of mild, moderate, and confluent emphysema at baseline CT showed a mean decline in lung density of 4.6 g/L ± 1.1 (P < .001), 6.7 g/L ± 1.1 (P < .001), and 6.4 g/L ± 1.2 (P < .001), respectively, compared with 2.4 g/L ± 1.3 (P < .001) for those with trace emphysema. For participants without COPD, those with visual presence of mild and moderate emphysema at baseline CT showed a mean decline in lung density of 3.6 g/L ± 1.0 (P < .001) and 3.1 g/L ± 1.6 (P < .001), respectively, compared with 1.8 g/L ± 1.0 (P < .001) for those with trace emphysema.
Conclusion:
The pattern of parenchymal emphysema at baseline CT was an independent predictor of subsequent progression of emphysema in participants who are current or former cigarette smokers with and without chronic obstructive pulmonary disease.
Summary
In current and former smokers with and without chronic obstructive pulmonary disease, the Fleischner Society visual pattern of emphysema at CT helped predict progression of emphysema and gas trapping.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines chronic obstructive pulmonary disease (COPD) as a common, preventable, and treatable disease characterized by persistent respiratory symptoms and airflow limitation. The COPD airflow limitation is physiologically defined by expiratory airflow obstruction with a postbronchodilator ratio of forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) less than 0.7 (1). However, the disease is heterogeneous, with a variety of phenotypes demonstrated with CT and pathologic findings, including emphysema, chronic bronchitis, and nonemphysematous small airway obstruction (2–4). CT is increasingly used in patients with COPD to assess the presence, pattern, and severity of emphysema and to identify concomitant diseases—particularly lung cancer. Quantitative CT imaging is also used to help quantify the features of COPD, specifically emphysema, air trapping, and airway abnormality (5–8). Quantitative CT has also been used to help demonstrate the efficacy of treatment for emphysema related to α-1 antitrypsin deficiency and would potentially be an important end point in trials of treatment for smoking-related emphysema (9). A recent white paper from the Fleischner Society classified the patterns of emphysema severity; parenchymal emphysema was classified as trace centrilobular emphysema (CLE), mild CLE, moderate CLE, confluent emphysema, or advanced destructive emphysema (10). Paraseptal emphysema (PSE) was classified as mild or substantial. Considering that a panlobular pattern can be confused with an advanced destructive pattern, the term panlobular emphysema is preferably reserved for patients with known α-1 antitrypsin deficiency (10).
Factors associated with progression of quantitative emphysema in patients with COPD include current smoking, female sex, and an upper lobe distribution (11,12). To our knowledge, no study has assessed the relationship between visually assessed severity of emphysema and subsequent progression of emphysema. Also, little is known about the factors that influence progression of emphysema and air trapping in cigarette smokers without COPD. We hypothesized that the presence of confluent or advanced destructive emphysema would be associated with more rapid progression of emphysema and gas trapping. The purpose of this study was to evaluate the potential relationship between emphysema visual subtypes and progression of emphysema and gas trapping.
Materials and Methods
This cohort study was based on data collected from the Genetic Epidemiology of COPD (COPDGene) study (funded by the National Heart, Lung, and Blood Institute, ClinicalTrials.gov identifier: NCT02445183); a longitudinal multicenter investigation mainly focused on the genetic epidemiologic characteristics of COPD (13). In 21 centers in the United States, 10,192 participants, including smokers and nonsmokers without COPD as control participants, were recruited between 2008 and 2011. The research protocol was approved by institutional review boards. The study was compliant with the Health Insurance Portability and Accountability Act, written informed consent was obtained (13). Data generated or analyzed during the study are available from the corresponding author by request.
Participants
For our study, we included 4995 participants from the cohort who completed a second visit approximately 5 years after the first visit. All participants were either current or former smokers with at least 10 pack-years of exposure to smoking and who self-identified as either non-Hispanic African American or non-Hispanic White. From the initial group of participants, participants were excluded on the basis of the following criteria: (a) missing smoking history, (b) smoking status change, (c) no usable pulmonary function test data, and (d) more than 180 days between pulmonary function test and CT (Fig 1). As was found in a previous study (14), the excluded participants differed from those included in the study in a few respects, including younger age, higher proportion of African-American participants, and higher proportion of current smokers (Table E1 [online]). However, the baseline spirometric parameters and GOLD stage distribution were similar. In this previous study, 3171 participants had been previously reported (14). The previous study addressed the association between visual emphysema patterns and risk of mortality, whereas in our study, we examined the progression of emphysema and air trapping.
Figure 1:
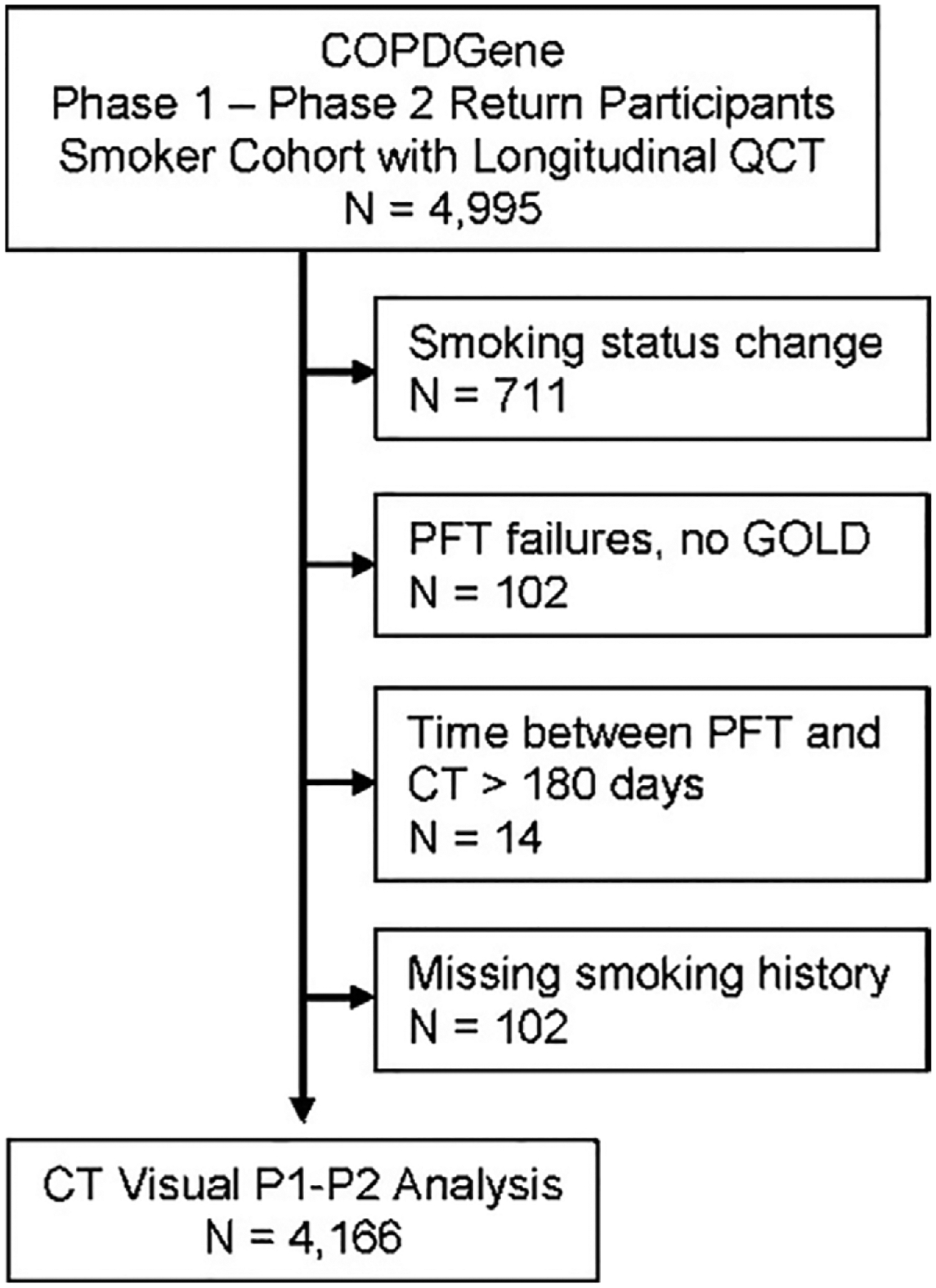
Flowchart of study population consort. COPDGene = genetic epidemiology of chronic pulmonary obstructive disease, GOLD = Global Initiative for Chronic Obstructive Lung Disease, PFT = pulmonary function test, P1 = phase 1, P2 = phase 2, QCT = quantitative CT.
Clinical Evaluation
Standardized functional and clinical parameters were collected. Functional parameters were assessed with spirometric measurements using a standardized protocol (13). Respiratory function was respectively estimated at spirometry with FEV1 and the FEV1/FVC ratio. Participants were classified according to the GOLD classification system (1), and participants with an unclassified pattern (preserved FEV1/FVC ratio and reduced FEV1 expressed as a percentage of predicted values) were defined as Preserved Ratio Impaired Spirometry (15). In addition, bronchodilator responsiveness and 6-minute walk test results using standard techniques were evaluated (16). Data issued from clinical evaluation of each participant, including age, height, weight, sex, race, smoking status, evaluation of respiratory symptoms (St George Respiratory Questionnaire score) (17), dyspnea score (modified Medical Research Council dyspnea score) (18), self-reported history of exacerbations in the previous year to enrollment, and symptoms of chronic bronchitis were collected.
Visual Analysis
The method of visual assessment has been previously described (14). In brief, visual analyses of emphysema were conducted by four trained research analysts according to the Fleischner Society classification system (Fig 2). Each scan was evaluated by two trained analysts; discordances between analysts were adjudicated by a thoracic radiologist.
Figure 2:
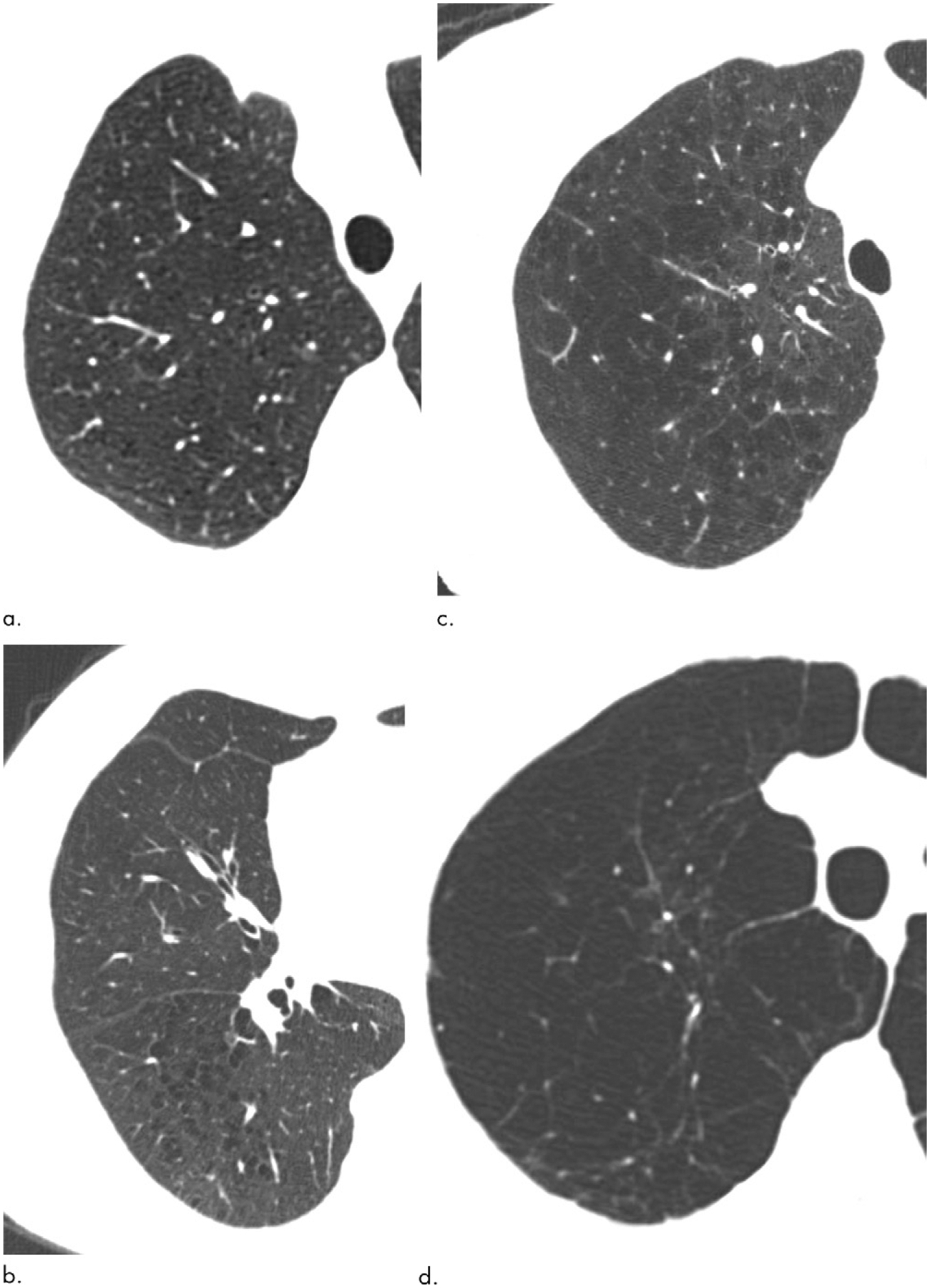
Axial CT images show major elements of Fleischner Society scoring system for parenchymal emphysema. (a) Mild centrilobular emphysema (involving less than 5% of lung lobe). (b) Moderate centrilobular emphysema (involving more than 5% of lung zone). (c) Confluent emphysema. (d) Advanced destructive emphysema.
Quantitative CT Analysis
CT scans were acquired with multidetector CT scanners (11). For this report, we considered volumetric CT scans obtained with full inspiration (200 mAs, 120 kVp) and passive expiration (50 mAs, 120 kVp). CT settings are detailed in the COPDGene study CT protocol (13). The CT images were reconstructed in contiguous submillimeter-thickness axial sections using a medium-sharp resolution reconstruction algorithm. We used 3D Slicer software (version 4.2; https://www.slicer.org/) to perform a quantitative analysis of extent of emphysema. Several previous studies suggested 15th-percentile lung density as a reliable index to assess the progression of emphysema at inspiratory CT at total lung capacity (19,20). Stoel et al (21) advanced the concept of volume correction of this lung density measure for longitudinal evaluation of emphysema. Thus, for our study, emphysema was quantified by calculating volume adjusted lung density at the 15th percentile (12,21). It was calculated as follows: adjusted lung density = [(Perc15+1000) × (CT lung volume/predicted functional total lung capacity)].
Statistical Analysis
κ statistics for the presence of emphysema and weighted κ coefficients for severity levels of CLE and PSE were calculated for each pair of analysts to assess interobserver agreement (22). The values of κ and weighted κ coefficients can range from +1 (almost perfect agreement) to −1, where zero represents the amount of agreement that can be expected from random chance (23). Linear mixed models were used to fit adjusted lung density and percentage of lung volume with attenuation less than −856 HU as a function of time, presence or absence of visual emphysema, their interaction, and the following covariables: (a) age, (b) height, (c) sex, (d) race, and (e) smoking status (current smokers or not current smokers). Random intercepts were included for study center, scanner model, and participant. The random intercept for participants accounted for the two measures within participants. Models were stratified according to the Fleischner Society emphysema classification and GOLD staging. The analyses were performed with SAS statistical software (version 9.4; SAS Institute), and P = .05 was used to determine a statistically significant difference.
Results
Participant Characteristics
Of 4995 participants who completed a second visit approximately 5 years after the first visit, 829 participants were excluded because of 711 smoking status changes, 102 had no usable pulmonary function test data, 14 had intervals of more than 180 days between pulmonary function test and CT, and two were missing smoking history (Fig 1). After exclusions, 4166 participants, including 2091 (50%) men were evaluated. Ages ranged from 39 to 81 years (mean age, 60 years ± 9 [standard deviation]), and 3011 (72%) of the participants were identified as non-Hispanic White. At baseline, 1778 (43%) participants reported continuous smoking, and 1655 (40%) were diagnosed with COPD.
Interobserver Agreement Values
The interobserver agreement values among the analysts reading in consensus were all good to excellent as shown in Table 1. The mean κ and weighted κ values for presence and severity of CLE were 0.82 (95% CI: 0.80, 0.84) and 0.76 (95% CI: 0.74, 0.77), respectively. The mean κ value for presence of PSE was 0.72 (95% CI: 0.69, 0.75). Discordant readings for presence of CLE occurred in 373 of 4166 participants (9%), whereas discordant readings for CLE grade occurred in 1386 of 4166 participants (33%). Discordant readings for substantial versus absent or mild PSE occurred in 359 of 4166 participants (9%). Discordant readings were adjudicated by a thoracic radiologist.
Table 1:
Observer Agreement for Visual CT Features
| Observer Sets | No. of Participants | Presence or Absence of Parenchymal Emphysema | Emphysema Grade | Presence or Absence of Paraseptal Emphysema |
|---|---|---|---|---|
| Observer 1 vs observer 2 | 1826 | 0.83 (0.80, 0.86) | 0.80 (0.78, 0.82) | 0.61 (0.55, 0.66) |
| Observer 2 vs observer 3 | 1431 | 0.80 (0.77, 0.83) | 0.69 (0.67, 0.72) | 0.80 (0.76, 0.83) |
| Observer 2 vs observer 4 | 750 | 0.83 (0.79, 0.87) | 0.75 (0.72, 0.78) | 0.68 (0.62, 0.74) |
| Observer 3 vs observer 4 | 757 | 0.79 (0.74, 0.83) | 0.70 (0.67, 0.73) | 0.77 (0.72, 0.82) |
| Overall | 4166 | 0.82 (0.80, 0.84) | 0.76 (0.74, 0.77) | 0.72 (0.69, 0.75) |
Note.—Unless otherwise noted, data are κ values, with 95% CIs in parentheses.
Participants with CLE Patterns
After visual analysis of emphysema patterns, the CLE pattern was found in 2525 of 4166 participants (61%), and the PSE pattern was found in 1737 participants (42%).
In participants with the CLE pattern, 1583 participants (63%) were graded as trace or mild, 547 (22%) as moderate, 290 (12%) as confluent, and 105 (4%) as advanced destructive (AD) (Table 2). An increase in severity of CLE pattern was associated with older age (from 59 years [trace] to 66 years [AD], P < .001), lower weight (from 86 kg [trace] to 75 kg [AD], P < .001), and a higher proportion of non-Hispanic White participants (from 67% of non-Hispanic White participants [trace] to 88% of non-Hispanic White participants [AD], P < .001). Those with higher grades of emphysema had a greater smoking history (from 40 pack-years [trace] to 58 pack-years [AD], P < .001), and a lower prevalence of current smokers (from 52% of current smokers [trace] to 11% of current smokers [AD], P < .001) have also been observed in high grades of emphysema (Table 3).
Table 2:
Baseline Characteristics of Participants according to Visual Grades of Emphysema
| Visual Grades | Absent | Trace | Mild | Moderate | Confluent | ADE | P Value* |
|---|---|---|---|---|---|---|---|
| No. of participants (n = 4166) | 1641 (39) | 771 (19) | 812 (20) | 547 (13) | 290 (7) | 105 (3) | |
| Demographic Information and Race | |||||||
| Age (y) | 58 ± 9 | 59 ± 9 | 60 ± 9 | 63 ± 8 | 65 ± 7 | 66 ± 7 | <.001 |
| Height (cm)† | 170 ± 9 | 170 ± 10 | 170 ± 10 | 169 ± 10 | 168 ± 10 | 169 ± 10 | .004 |
| Weight (kg)† | 88 ± 20 | 86 ± 20 | 81 ± 18 | 79 ± 17 | 77 ± 18 | 75 ± 17 | <.001 |
| BMI (kg/m2)† | 31 ± 6 | 30 ± 6 | 28 ± 6 | 28 ± 5 | 27 ± 5 | 26 ± 5 | <.001 |
| No. of men | 776 (47) | 405 (53) | 435 (54) | 277 (51) | 142 (49) | 56 (53) | .04 |
| No. of non-Hispanic White participants | 1206 (74) | 517 (67) | 566 (70) | 388 (71) | 241 (83) | 92 (88) | <.001 |
| No. of African-American participants | 435 (27) | 254 (33) | 246 (30) | 159 (29) | 49 (17) | 13 (12) | <.001 |
| Smoking status | |||||||
| No. of current smokers | 645 (39) | 397 (52) | 429 (53) | 247 (45) | 49 (17) | 11 (11) | <.001 |
| Smoking history (pack-year)† | 35 ± 20 | 40 ± 22 | 46 ± 23 | 51 ± 24 | 56 ± 29 | 58 ± 27 | <.001 |
| Comorbidities | |||||||
| No. of exacerbations in previous year‡ | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (1) | 0 (1) | <.001 |
| No. of participants with chronic bronchitis | 192 (12) | 109 (14) | 152 (19) | 116 (21) | 59 (20) | 14 (13) | |
| Spirometry | |||||||
| FEV1 (predicted %)† | 90 ± 17 | 84 ± 19 | 80 ± 21 | 70 ± 23 | 57 ± 23 | 46 ± 22 | <.001 |
| FEV1-to-FVC ratio† | 0.8 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 | <.001 |
| GOLD stage | |||||||
| No. of participants with PRISm | 247 (15) | 132 (17) | 80 (10) | 31 (6) | 5 (2) | 0 (0) | |
| No. of participants with GOLD 0 | 1126 (69) | 408 (53) | 347 (43) | 119 (22) | 15 (5) | 1 (1) | |
| No. of participants with GOLD 1 | 90 (6) | 71 (9) | 98 (12) | 86 (16) | 34 (12) | 6 (6) | |
| No. of participants with GOLD 2 | 148 (9) | 115 (15) | 207 (26) | 187 (34) | 108 (37) | 33 (31) | |
| No. of participants with GOLD 3 | 26 (2) | 44 (6) | 70 (9) | 109 (20) | 102 (35) | 35 (33) | |
| No. of participants with GOLD 4 | 4 (0) | 1 (0) | 10 (1) | 15 (3) | 26 (9) | 30 (29) | |
| 6-minute walk (ft)† | 1516 ± 357 | 1441 ± 368 | 1426 ± 343 | 1362 ± 358 | 1291 ± 352 | 1198 ± 337 | <.001 |
| MMRC‡ | 0 (1) | 0 (2) | 1 (2) | 1 (3) | 2 (2) | 3 (2) | <.001 |
| SGRQ‡ | 9 (22) | 15 (27) | 17 (32) | 26 (34) | 33 (28) | 36 (24) | <.001 |
| Emphysema (%LAA–950insp)† | 2.4 ± 3.2 | 2.5 ± 3.7 | 3.8 ± 5.1 | 8.1 ± 7.4 | 19.2 ± 10.6 | 30.5 ± 10.9 | <.001 |
| Gas trapping (%LAA–856exp)† | 11.7 ± 9.9 | 13.6 ± 11.9 | 19.1 ± 14.9 | 29.0 ± 18.1 | 42.5 ± 18.5 | 55.4 ± 14.1 | <.001 |
Note.—Except where indicated, data are numbers of patients, with percentages in parentheses. ADE = advanced destructive emphysema, BMI = body mass index, FEV1 = force expiratory volume in 1 second, FVC = forced vital capacity, GOLD = Global Initiative for Chronic Obstructive Lung Disease, MMRC = Modified Medical Research Council, %LAA−950insp = percentage of lung volume with attenuation less than −950 HU at inspiration, %LAA−856exp = percentage of lung volume with attenuation less than −856 HU at expiration, PRISm = preserved ratio impaired spirometry, SGRQ = St George’s Respiratory Questionnaire.
P values are for differences across emphysema grades, calculated with the χ2 test for categoric variables and with the F test from analysis of variance for continuous variables. The Kruskall-Wallis test was used for variables displayed as medians and interquartile ranges.
Numbers are means ± standard deviations.
Numbers are medians, with interquartile ranges in parentheses.
Table 3:
Baseline Characteristics of Participants according to Visual Grades of Paraseptal Emphysema
| Visual Grades | Absent | Mild | Substantial | P Value* |
|---|---|---|---|---|
| No. of participants (n = 4166) | 2429 (58) | 1010 (24) | 727 (18) | |
| Demographic information and race | ||||
| Age (y) | 61 ± 9 | 59 ± 9 | 60 ± 9 | <.001 |
| Height (cm)† | 169 ± 10 | 171 ± 10 | 173 ± 9 | <.001 |
| Weight (kg)† | 85 ± 20 | 85 ± 20 | 81 ± 18 | <.001 |
| BMI (kg/m2)† | 30 ± 6 | 29 ± 6 | 27 ± 5 | <.001 |
| No. of men | 1025 (42) | 562 (56) | 503 (69) | <.001 |
| No. of non-Hispanic White participants | 1873 (77) | 661 (65) | 478 (66) | <.001 |
| No. of African-American participants | 556 (23) | 349 (35) | 249 (34) | <.001 |
| Smoking status | ||||
| No. of current smokers | 833 (34) | 560 (55) | 386 (53) | <.001 |
| Smoking history (pack-year)† | 39 ± 22 | 45 ± 24 | 49 ± 26 | <.001 |
| Comorbidities | ||||
| No. of exacerbations in previous year‡ | 0(0) | 0(0) | 0(0) | <.001 |
| No. of participants with chronic bronchitis | 304 (13) | 173 (17) | 165 (23) | |
| Spirometry | ||||
| FEV1 (predicted %)† | 83 ± 22 | 81 ± 22 | 74 ± 24 | <.001 |
| FEV1-to-FVC ratio† | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.2 | <.001 |
| GOLD stage | ||||
| No. of participants with PRISm | 311 (13) | 123 (12) | 61 (8) | |
| No. of participants with GOLD 0 | 1312 (54) | 471 (47) | 233 (32) | |
| No. of participants with GOLD 1 | 182 (8) | 106 (11) | 97 (13) | |
| No. of participants with GOLD 2 | 383 (16) | 213 (21) | 202 (28) | |
| No. of participants with GOLD 3 | 191 (8) | 85 (8) | 110 (15) | |
| No. of participants with GOLD 4 | 50 (2) | 12 (1) | 24 (3) | |
| 6-minute walk (ft)† | 1465 ± 370 | 1419 ± 354 | 1394 ± 350 | <.001 |
| MMRC‡ | 0 (2) | 0 (2) | 1 (3) | <.001 |
| SGRQ‡ | 13 (27) | 18 (31) | 25 (32) | <.001 |
| Emphysema (%LAA–950insp)† | 4.8 ± 7.7 | 3.8 ± 5.7 | 9.3 ± 10.6 | <.001 |
| Gas trapping (%LAA–856exp)† | 17.6 ± 16.3 | 17.0 ± 15.3 | 27.1 ± 20.0 | <.001 |
Note.—Except where indicated, data are numbers of patients, with percentages in parentheses. ADE = advanced destructive emphysema, BMI = body mass index, FEV1 = force expiratory volume in 1 second, FVC = forced vital capacity, GOLD = Global Initiative for Chronic Obstructive Lung Disease, MMRC = Modified Medical Research Council, %LAA−950insp = percentage of lung volume with attenuation less than −950 HU at inspiration, %LAA−856exp = percentage of lung volume with attenuation less than −856 HU at expiration, PRISm = preserved ratio impaired spirometry, SGRQ = St George’s Respiratory Questionnaire.
P values are for differences across emphysema grades, calculated with the χ2 test for categoric variables and with the F test from analysis of variance for continuous variables. The Kruskall-Wallis test was used for variables displayed as medians and interquartile ranges.
Numbers are means ± standard deviations.
Numbers are medians, with interquartile ranges in parentheses.
Participants with PSE Pattern
In participants with the PSE pattern, 1010 participants (58%) were graded as mild, and 727 participants (42%) were graded as substantial (Table 3). Visual presence of PSE was related to a lower proportion of non-Hispanic White participants (77% of non-Hispanic White participants [absent], 65% of non-Hispanic White participants [trace], and 66% of non-Hispanic White participants[substantial], P < .001) and more persistent current smokers (34% of current smokers [absent], 54% of current smokers [trace], and 53% of current smokers [substantial], P < .001) with a higher smoking history (39.1 pack-years [absent], 45.2 pack-years [trace], and 49.3 pack-years [substantial], P < .001). Greater severity of visual PSE pattern was more likely related to lower weight (85 kg [trace] and 81 kg [substantial], P < .001) and male sex (56% men [trace] 69% men [AD], P < .001). No consistent age differences (59 years [trace] and 60 years [substantial], P < .001) were noted between the groups.
Severe Emphysema Patterns in Both CLE and PSE
In both CLE and PSE, severe emphysema patterns were associated with more frequent exacerbations in the year before enrollment. They were also associated with increased GOLD stage (CLE, from 0% of participants [trace] to 29% of participants with GOLD 4 [AD], P < .001) (PSE, from 1% of participants [trace] to 3% of participants with GOLD 4 [substantial], P < .001), airflow obstruction worsening (CLE, from 84% [trace] to 46% predicted FEV1 [AD], P < .001) (PSE, from 81% [trace] to 74% [substantial], P < .001), and reduction in 6-minute walk test distance (CLE, from 1441 ft [trace] to 1198 ft [AD], P < .001) (PSE, from 1419 ft [trace] to 1394 ft [substantial], P < .001). Additionally, visual patterns of emphysema were well matched with quantitation of emphysema (3% [trace], 4% [mild], 8% [moderate], 19% [confluent], and 31% of lung volume with attenuation less than −950insp [AD]) and gas trapping (14% [trace], 19% [mild], 29% [moderate], 43% [confluent], and 55% of lung volume with attenuation less than −856exp [AD]). Increased grade of emphysema was associated with increased respiratory symptoms measured by the Modified Medical Research Council (mean score, 1 [trace] vs 2 [AD]) and St George’s Respiratory Questionnaire (mean score, 21 [trace] vs 41 [AD]) scores.
Changes in Lung Density for Participants with and without COPD
In participants with COPD, those with parenchymal emphysema at baseline CT showed a decline in lung density of −5.1 g/L (95% CI: −6.0, −4.1; P < .001) compared with −0.1 g/L (95% CI: −1.4, 1.3; P = .92) for those without parenchymal emphysema. For participants without COPD, corresponding values were −2.6 g/L (95% CI: −3.4, −1.8; P < .001) and −0.2 g/L (95% CI: −0.9, 0.6, P = .64) (Table 4). Similar findings were identified for PSE (Table 4). The corresponding values for percentage of lung volume with attenuation less than −856 HU are given in Table 5.
Table 4:
Longitudinal Change in Volume-adjusted Lung Density, Stratified according to Presence or Absence of COPD and according to Presence or Absence of Parenchymal and Paraseptal Emphysema
| COPD (n = 1655) | No COPD (n = 2511) | |||
|---|---|---|---|---|
| Presence or Absence of Emphysema | Mean Change (g/L) | P Value | Mean Change (g/L) | P Value |
| Parenchymal emphysema | ||||
| No emphysema at baseline | −0.1 (−1.4, 1.3) | .92 | −0.2 (−0.9, 0.6) | .64 |
| Emphysema at baseline | −5.1 (−6.0, −4.1) | <.001 | −2.6 (−3.4, −1.8) | <.001 |
| Paraseptal emphysema | ||||
| No paraseptal emphysema at baseline | −3.0 (−4.0, −2.1) | <.001 | −0.9 (−1.6, −0.3) | .005 |
| Paraseptal emphysema at baseline | −6.0 (−7.1, −4.8) | <.001 | −3.5 (−4.7, −2.3) | <.001 |
Note.—Models are adjusted for age, height, sex, race, and smoking status. Progressions differed by extent of emphysema (P < .001) for each group (COPD or no COPD) and emphysema classification variable. Numbers in parentheses are 95% CIs. COPD = chronic obstructive pulmonary disease.
Table 5:
Longitudinal Change in Percentage of Lung Volume with Attenuation Less than −856 HU, Stratified according to Presence or Absence of COPD and according to Presence or Absence of Parenchymal and Paraseptal Emphysema
| Presence or Absence of Emphysema | COPD (n = 1655) | No COPD (n = 2511) | ||
|---|---|---|---|---|
| Mean Change (%LAA–856) | P Value | Mean Change (%LAA–856) | P Value | |
| Parenchymal emphysema | ||||
| No emphysema at baseline | 0.1 (−1.3, 1.5) | 0.92 | −0.6 (−1.0, −0.1) | .02 |
| Emphysema at baseline | 3.9 (3.0, 4.7) | <.001 | 0.8 (0.3, 1.3) | .003 |
| Paraseptal emphysema | ||||
| No paraseptal emphysema at baseline | 2.5 (1.6, 3.4) | <.001 | −0.2 (−0.6, 0.2) | .41 |
| Paraseptal emphysema at baseline | 3.9 (2.7, 5.0) | <.001 | 0.6 (−0.3, 1.4) | .22 |
Note.—Models are adjusted for age, height, sex, race, and smoking status. Progressions differed by extent of emphysema (P < .001) for each group (COPD or no COPD) and emphysema classification variable. Numbers in parentheses are 95% CIs. COPD = chronic obstructive pulmonary disease, %LAA−856 = percentage of lung volume with attenuation less than −856 HU.
The results were slightly different between racial groups. In participants with COPD and visual emphysema, African American participants showed a mean decline in lung density of 6.7 g/L (95% CI: 5.5, 8.0) compared with 4.6 g/L (95% CI: 3.7, 5.6) for non-Hispanic White participants (P < .001) for comparison.
When progression was stratified by emphysema classification, the decrease in lung density over 5 years in participants with COPD was −0.3 g/L (95% CI: −1.5, 1.0; P = .64) for those who had no CLE. Participants with trace, mild, and moderate CLE showed a decrease in lung density of −2.4 g/L (95% CI: −3.7, −1.1; P < .001), −4.6 g/L (95% CI: −5.7, −3.5: P < .001), and −6.7 g/L (95% CI: −7.7, −5.6; P < .001). The decreases for confluent and advanced emphysema were −6.4 g/L (95% CI: −7.6, −5.2; P < .001) and −6.2 g/L (95% CI: −8.0, −4.5; P < .001), respectively. The changes in lung density between the groups in participants with and without COPD are reported in Table 6. The corresponding results for each GOLD group are reported in Table 7.
Table 6:
Longitudinal Change in Volume-adjusted Lung Density, Stratified according to Presence or Absence of COPD and according to Parenchymal Emphysema Grades
| Parenchymal Emphysema Grade | COPD (n = 1655) | No COPD (n = 2511) | ||
|---|---|---|---|---|
| Mean Change (g/L) | P Value | Mean Change (g/L) | P Value | |
| Absent | −0.3 (−1.5, 1.0) | .64 | −0.3 (−1.0, 0.4) | .42 |
| Trace | −2.4 (−3.7, −1.1) | <.001 | −1.8 (−2.8, −0.9) | <.001 |
| Mild | −4.6 (−5.7, −3.5) | <.001 | −3.6 (−4.7, −2.6) | <.001 |
| Moderate | −6.7 (−7.7, −5.6) | <.001 | −3.1 (−4.7, −1.5) | <.001 |
| Confluent | −6.4 (−7.6, −5.2) | <.001 | −4.0 (−8.1, 0.2) | .06 |
| ADE | −6.2 (−8.0, −4.5) | <.001 | −17.6 (−35.9, 0.8) | .06 |
Note.—Models are adjusted for age, height, sex, race, and smoking status. Progressions differed by extent of emphysema (P < .001) for each group (COPD or no COPD) and emphysema classification variable. Numbers in parentheses are 95% CIs. ADE = advanced destructive emphysema, COPD = chronic obstructive pulmonary disease.
Table 7:
Longitudinal Change in Volume-adjusted Lung Density, Stratified according to GOLD Groups and according to Parenchymal Emphysema Grades
| GOLD 0 | GOLD 1 | GOLD 2 | GOLD 3 | GOLD 4 | PRISm | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parenchymal emphysema | Mean change (g/L) | P value | Mean change (g/L) | P value | Mean change (g/L) | P value | Mean change (g/L) | P value | Mean change (g/L) | P value | Mean change (g/L) | P value |
| No | −0.2 (−1.0, 0.6) | .69 | −2.5 (−4.8, −0.1) | .04 | 1.2 (−0.5, 3.0) | .17 | 0.5 (−2.7, 3.8) | .75 | 5.1 (−2.7, 12.9) | .200 | −0.9 (−2.8, 0.9) | .33 |
| Trace | −1.7 (−2.8, − 0.7) | .001 | −3.6 (−6.0, −1.1) | .005 | −1.3 (−3.3, 0.6) | .18 | −2.4 (−5.1, 0.3) | .08 | −7.3 (−21.7, 7.1) | .32 | −2.0 (−4.2, 0.2) | .08 |
| Mild | −3.7 (−4.8, −2.6) | <.001 | −3.5 (−5.8, −1.3) | .002 | −4.2 (−5.8, −2.7) | <.001 | −6.4 (−8.6, −4.1) | <.001 | −2.4 (−7.6, 2.9) | .38 | −3.2 (−5.8, −0.6) | .02 |
| Moderate | −3.0 (−4.7, −1.2) | <.001 | −4.8 (−7.1, −2.6) | <.001 | −7.0 (−8.6, −5.3) | <.001 | −7.2 (−9.2, −5.2) | <.001 | −7.6 (−12.0, −3.1) | .001 | −3.2 (−7.0, 0.6) | .10 |
| Confluent | −3.7 (−8.4, 0.9) | .12 | −7.2 (−10.4, −4.0) | <.001 | −5.8 (−7.7, −3.8) | <.001 | −6.7 (−8.7, −4.7) | <.001 | −5.4 (−9.3, −1.5) | .007 | −5.9 (−15.0, 3.1) | .20 |
| ADE | −17.2 (−35.1, 0.8) | .06 | −5.9 (−13.2, 1.4) | .11 | −7.2 (−10.5, −4.0) | <.001 | −6.2 (−9.1, −3.4) | <.001 | −4.6 (−8.1, −1.1) | .01 | Nonest | Nonest |
Note.— Models are adjusted for age, height, sex, race, and smoking status. Progressions differed by extent of emphysema (P < .001) for each group (chronic obstructive pulmonary disease or no chronic obstructive pulmonary disease) and emphysema classification variable. Numbers in parentheses are 95% CIs. ADE = advanced destructive emphysema, GOLD = Global Initiative for Chronic Obstructive Lung Disease, Nonest = nonestimable, PRISm = preserved ratio impaired spirometry.
Discussion
Our study showed that smokers with visible parenchymal emphysema at CT showed progression of emphysema at 5 years (mean decline in lung density of −5.1 g/L for those with chronic obstructive pulmonary disease [COPD] and −2.6 g/L for those without COPD), whereas those without visible emphysema did not show progression. The presence of paraseptal emphysema was similarly associated with progressive decline in lung density (mean decline in lung density of −6.0 g/L for those with COPD and −3.5 g/L for those without COPD). Increasing visual grade of parenchymal emphysema at baseline CT was generally associated with an increasing rate of progression of emphysema in all stages of the Global Initiative for Chronic Obstructive Lung Disease COPD classification.
A previous COPDGene study showed that the visual presence and pattern of emphysema were important predictors of mortality in cigarette smokers with and without COPD (14). Also, emphysema of varying degree was present in about 44% of smokers without spirometric abnormality (14). In a study of 4211 COPDGene participants followed up over 5 years, Pompe et al showed that adjusted lung density decreased in all GOLD groups; the decline was more marked in those with more advanced GOLD stages (24). Additionally, the severity of expiratory air trapping increased in those with COPD. In smokers without spirometric evidence of COPD, we recently showed that the presence of visual emphysema is a predictor of progressive airflow obstruction and emphysema progression (25). The current study expands on this body of information by including participants with COPD stratified according to GOLD groups. It demonstrates that the visual presence of emphysema at baseline CT in these groups is also associated with emphysema progression and gas trapping compared with those without visible emphysema. Additionally, we showed that, for participants with visual emphysema at baseline CT with or without COPD, progression of emphysema appears to be greater in African-American participants than in White participants. Our finding supports the increasing awareness that symptoms and progressive structural abnormalities are common in individuals with and without COPD (26,27). An important implication is that trials of emphysema treatment could be enriched by selective inclusion of patients with visible emphysema at CT.
A previous study (6) showed that percentage of lung volume with attenuation less than −856 HU correlates strongly with measures of airflow obstruction such as FEV1 expressed as a percentage of predicted values and FEV1/FVC ratio (r = 0.77 and 0.84, respectively, P < .0001 for both). Our study extended previous studies and showed that participants with emphysema also had greater progression of air trapping than those without emphysema, as measured by the percentage of lung volume with attenuation less than −856 HU. This indicated that the progression of emphysema in these participants was associated with increased airflow obstruction. Because the percentage of lung volume with attenuation less than −856 HU reflects air trapping due to emphysema and small airway disease, it is not possible to determine how much of the progressive air trapping is due to progressive small airway obstruction in addition to progressive emphysema.
Previous studies of emphysema progression have not separated patients according to emphysema subtypes (paraseptal vs centrilobular). Although our findings should be interpreted with caution as PSE and CLE may occur together, our study showed PSE is associated with a higher rate of progression of emphysema compared with parenchymal emphysema in participants with and without COPD. The subpleural cysts of PSE, which are usually near the lung apices, might enlarge more rapidly when exposed to greater transpleural pressure than areas of parenchymal emphysema. Progressive enlargement of paraseptal emphysematous cysts may culminate in development of bullae, which compress normal lung tissue (28). PSE adjacent to the trachea may also contribute to expiratory central airway collapse (29).
In individuals with COPD, greater severity of visual parenchymal emphysema at baseline CT was associated with greater lung density decline, except for confluent and advanced destructive emphysema. The lower apparent effect of baseline confluent and advanced destructive emphysema might be because of the higher mortality in these two groups. But it may also suggest a slower rate of progression of emphysema in those with already well-established disease, in keeping with previous observations showing moderate COPD (GOLD stage II) is associated with a greater rate of lung function decline compared with stage III and IV (30–34). Our results showed the predictive effect of emphysema patterns on the adjusted lung density decline is also identifiable in each GOLD group; the five-point grading of parenchymal emphysema is associated with subsequent progression of emphysema, independent of the baseline GOLD stage. This suggests visual emphysema behaves as an independent and sensitive parameter to predict changes, even in those with early stages of COPD.
Our study had limitations. First, because PSE and CLE can occur together, it is difficult to determine the independent progression of these two entities. We considered a separate analysis of participants with mixed PSE and CLE but found it challenging because of the confounding effect of disease severity. Second, our study did not address chronic bronchitis or small airway obstruction, which are other important and potentially treatable components of COPD. In the future, analysis of the interaction between visual emphysema score and severity of functional small airway disease as predictors of progression would be of interest (30).
In conclusion, the presence of visible parenchymal or paraseptal emphysema at CT in current or former smokers is an important predictor of subsequent progression of emphysema. The Fleischner Society emphysema grading system may be useful as a simple prognostic marker in smokers, with or without chronic obstructive pulmonary disease and may have value in therapeutic trials and in clinical practice.
Supplementary Material
Key Results.
In 1655 study participants with chronic obstructive pulmonary disease (COPD) and 2511 participants without COPD, those with visually identified emphysema at baseline CT showed declines in lung density of 5.1 g/L (P < .001) and 2.6 g/L (P < .001), respectively.
The presence of paraseptal emphysema helped predict a more rapid progression of emphysema than parenchymal emphysema (P < .001).
Fleischner Society visual patterns of emphysema helped predict progression of emphysema in all stages of Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification (eg, GOLD 1, from −3.5 g/L [mild], P = .002 to −7.2 g/L [confluent], P < .001).
Acknowledgment:
B.E.K. gives special thanks to Pierre-Alain Gevenois for providing endless support.
The Genetic Epidemiology of COPD (COPDGene) study is supported by the COPD Foundation through contributions made to an industry advisory board representing AstraZeneca, Boehringer Ingelheim, Novartis, Pfizer, Siemens, Sunovion, and GlaxoSmithKline.
Disclosures of Conflicts of Interest:
B.E.K. disclosed no relevant relationships. M.J.S. disclosed no relevant relationships. D.B. disclosed no relevant relationships. S.H. Activities related to the present article: institution received grant from the National Heart, Lung, and Blood Institute. Activities not related to the present article: disclosed no relevant relationships. Other relationships: has U.S. patent application pending. J.P.C. Activities related to the present article: institution received grant from the COPD Foundation. Activities not related to the present article: holds stock/stock options in Thirona. Other relationships: disclosed no relevant relationships. E.M.v.R. Activities related to the present article: institution received grant from Thirona. Activities not related to the present article: holds stock/stock options in Thirona. Other relationships: disclosed no relevant relationships. D.A.L. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant for Parexel Imaging, Boehringer Ingelheim, Veracyte, Daiichi Sankyo, and Astra-Zeneca; receives payment for lectures, including service on speakers bureaus, from Boehringer Ingelheim. Other relationships: has U.S. patent application pending.
Abbreviations
- AD
advanced destructive
- CLE
centrilobular emphysema
- COPD
chronic obstructive pulmonary disease
- COPDGene
genetic epidemiology of COPD
- FEV1
forced expiratory lung volume in 1 second
- FVC
forced vital capacity
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- PSE
paraseptal emphysema
Footnotes
Online supplemental material is available for this article.
References
- 1.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med 2017;195(5):557–582. [DOI] [PubMed] [Google Scholar]
- 2.Friedlander AL, Lynch D, Dyar LA, Bowler RP. Phenotypes of chronic obstructive pulmonary disease. COPD 2007;4(4):355–384. [DOI] [PubMed] [Google Scholar]
- 3.Segal LN, Martinez FJ. Chronic obstructive pulmonary disease subpopulations and phenotyping. J Allergy Clin Immunol 2018;141(6):1961–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fletcher C, Peto R. The natural history of chronic airflow obstruction. BMJ 1977;1(6077):1645–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madani A, Zanen J, de Maertelaer V, Gevenois PA. Pulmonary emphysema: objective quantification at multi-detector row CT--comparison with macroscopic and microscopic morphometry. Radiology 2006;238(3):1036–1043. [DOI] [PubMed] [Google Scholar]
- 6.Schroeder JD, McKenzie AS, Zach JA, et al. Relationships between airflow obstruction and quantitative CT measurements of emphysema, air trapping, and airways in subjects with and without chronic obstructive pulmonary disease. AJR Am J Roentgenol 2013;201(3):W460–W470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coxson HO, Rogers RM, Whittall KP, et al. A quantification of the lung surface area in emphysema using computed tomography. Am J Respir Crit Care Med 1999;159(3):851–856. [DOI] [PubMed] [Google Scholar]
- 8.Hackx M, Bankier AA, Gevenois PA. Chronic obstructive pulmonary disease: CT quantification of airways disease. Radiology 2012;265(1):34–48. [DOI] [PubMed] [Google Scholar]
- 9.Chapman KR, Burdon JG, Piitulainen E, et al. Intravenous augmentation treatment and lung density in severe α1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial. Lancet 2015;386(9991):360–368. [DOI] [PubMed] [Google Scholar]
- 10.Lynch DA, Austin JH, Hogg JC, et al. CT-Definable Subtypes of Chronic Obstructive Pulmonary Disease: A Statement of the Fleischner Society. Radiology 2015;277(1):192–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boueiz A, Chang Y, Cho MH, et al. Lobar Emphysema Distribution Is Associated With 5-Year Radiological Disease Progression. Chest 2018;153(1):65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coxson HO, Dirksen A, Edwards LD, et al. The presence and progression of emphysema in COPD as determined by CT scanning and biomarker expression: a prospective analysis from the ECLIPSE study. Lancet Respir Med 2013;1(2):129–136. [DOI] [PubMed] [Google Scholar]
- 13.Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD 2010;7(1):32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lynch DA, Moore CM, Wilson C, et al. CT-based Visual Classification of Emphysema: Association with Mortality in the COPDGene Study. Radiology 2018;288(3):859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wan ES, Castaldi PJ, Cho MH, et al. Epidemiology, genetics, and subtyping of preserved ratio impaired spirometry (PRISm) in COPDGene. Respir Res 2014;15(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166(1):111–117 [Published correction appears in Am J Respir Crit Care Med 2016;193(10):1185.]. [DOI] [PubMed] [Google Scholar]
- 17.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis 1992;145(6):1321–1327. [DOI] [PubMed] [Google Scholar]
- 18.Standardized Questionaries on Respiratory Symptoms. Br Med J 1960;2(5213):1665. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2098438/.13688719 [Google Scholar]
- 19.Shaker SB, Dirksen A, Laursen LC, Skovgaard LT, Holstein-Rathlou NH. Volume adjustment of lung density by computed tomography scans in patients with emphysema. Acta Radiol 2004;45(4):417–423. [DOI] [PubMed] [Google Scholar]
- 20.Parr DG, Stoel BC, Stolk J, Stockley RA. Validation of computed tomographic lung densitometry for monitoring emphysema in alpha1-antitrypsin deficiency. Thorax 2006;61(6):485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoel BC, Putter H, Bakker ME, et al. Volume correction in computed tomography densitometry for follow-up studies on pulmonary emphysema. Proc Am Thorac Soc 2008;5(9):919–924. [DOI] [PubMed] [Google Scholar]
- 22.Kundel HL, Polansky M. Measurement of observer agreement. Radiology 2003;228(2):303–308. [DOI] [PubMed] [Google Scholar]
- 23.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33(1):159–174. [PubMed] [Google Scholar]
- 24.Pompe E, Strand M, van Rikxoort EM, et al. Five-year Progression of Emphysema and Air Trapping at CT in Smokers with and Those without Chronic Obstructive Pulmonary Disease: Results from the COPDGene Study. Radiology 2020;295(1):218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh AS, Strand M, Pratte K, et al. Clinical significance of visual emphysema in smokers with normal spirometry. Radiology (in press). [Google Scholar]
- 26.Woodruff PG, Barr RG, Bleecker E, et al. Clinical Significance of Symptoms in Smokers with Preserved Pulmonary Function. N Engl J Med 2016;374(19):1811–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Regan EA, Lynch DA, Curran-Everett D, et al. Clinical and Radiologic Disease in Smokers With Normal Spirometry. JAMA Intern Med 2015;175(9):1539–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma N, Justaniah AM, Kanne JP, Gurney JW, Mohammed TL. Vanishing lung syndrome (giant bullous emphysema): CT findings in 7 patients and a literature review. J Thorac Imaging 2009;24(3):227–230. [DOI] [PubMed] [Google Scholar]
- 29.Copeland CR, Nath H, Terry NLJ, et al. Paratracheal Paraseptal Emphysema and Expiratory Central Airway Collapse in Smokers. Ann Am Thorac Soc 2018;15(4):479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhatt SP, Soler X, Wang X, et al. Association between Functional Small Airway Disease and FEV1 Decline in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2016;194(2):178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casanova C, de Torres JP, Aguirre-Jaíme A, et al. The progression of chronic obstructive pulmonary disease is heterogeneous: the experience of the BODE cohort. Am J Respir Crit Care Med 2011;184(9):1015–1021. [DOI] [PubMed] [Google Scholar]
- 32.Agusti A, Edwards LD, Celli B, et al. Characteristics, stability and outcomes of the 2011 GOLD COPD groups in the ECLIPSE cohort. Eur Respir J 2013;42(3):636–646. [DOI] [PubMed] [Google Scholar]
- 33.Kim J, Yoon HI, Oh YM, et al. Lung function decline rates according to GOLD group in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2015;10:1819–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diaz AA, Strand M, Coxson HO, et al. Disease Severity Dependence of the Longitudinal Association Between CT Lung Density and Lung Function in Smokers. Chest 2018;153(3):638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


